 ®
®Annual report pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934. For the fiscal year ended | |||||
| Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. For the transition period from __________ to __________ | |||||
 ®
®| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||
| Title of each class | Trading Symbol | Name of each exchange on which registered | ||||||
| Item | Description | Page | ||||||||||||
Report of Independent Registered Public Accounting Firm (PCAOB ID | ||||||||||||||

















| Location Country or State | Square Feet (in thousands) | |||||||
| Connecticut | 1,138 | |||||||
| Puerto Rico | 811 | |||||||
| Mexico | 762 | |||||||
| China | 735 | |||||||
| Minnesota | 623 | |||||||
| Italy | 485 | |||||||
| Ireland | 446 | |||||||
| Dominican Republic | 304 | |||||||
| Arizona | 294 | |||||||
| Switzerland | 283 | |||||||
| France | 268 | |||||||
| Colorado | 259 | |||||||
| Florida | 255 | |||||||
| California | 210 | |||||||
| Fiscal Period | Total Number of Shares Purchased | Average Price Paid per Share | Total Number of Shares Purchased as a Part of Publicly Announced Program | Maximum Approximate Dollar Value of Shares that may yet be Purchased Under the Program | ||||||||||||||||||||||
| 1/29/2022-2/25/2022 | 1,130,750 | $ | 103.16 | 1,130,750 | $ | 4,234,214,099 | ||||||||||||||||||||
| 2/26/2022-4/1/2022 | 6,141,716 | 107.85 | 6,141,716 | 3,571,839,180 | ||||||||||||||||||||||
| 4/2/2022-4/29/2022 | 5,627,112 | 110.47 | 5,627,112 | 2,950,215,113 | ||||||||||||||||||||||
| Total | 12,899,578 | $ | 108.58 | 12,899,578 | $ | 2,950,215,113 | ||||||||||||||||||||
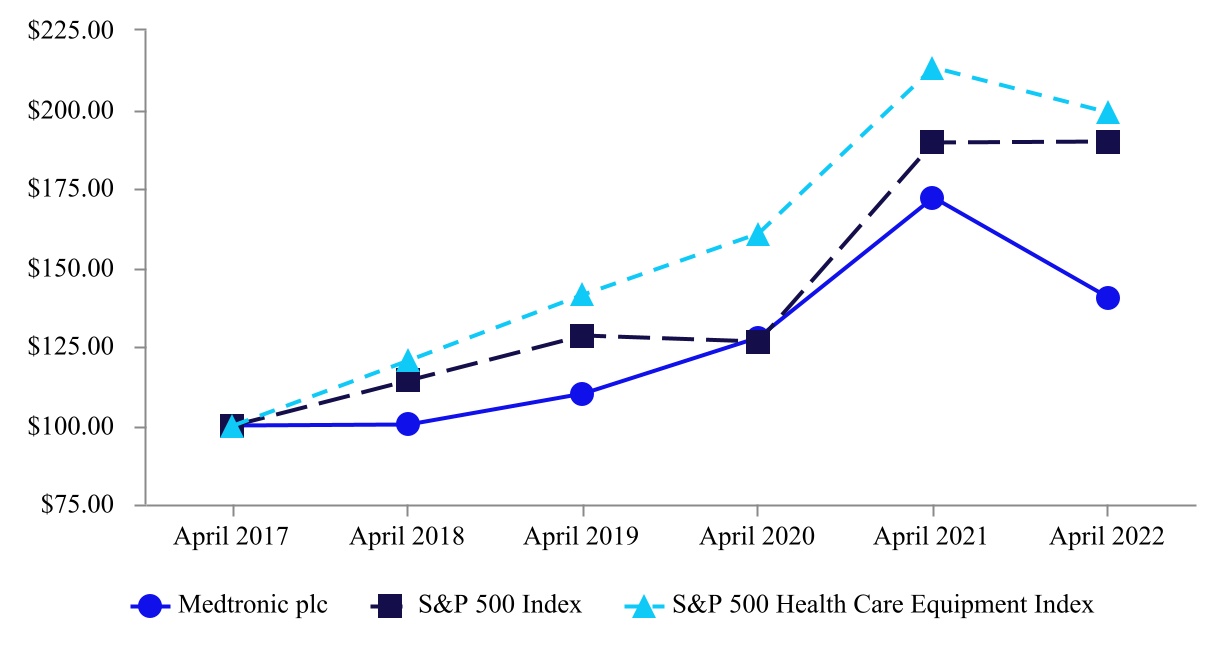
| Company/Index | April 2017 | April 2018 | April 2019 | April 2020 | April 2021 | April 2022 | ||||||||||||||||||||||||||||||||
| Medtronic plc | $ | 100.00 | $ | 100.06 | $ | 109.91 | $ | 127.67 | $ | 172.10 | $ | 140.27 | ||||||||||||||||||||||||||
| S&P 500 Index | 100.00 | 114.20 | 128.28 | 126.28 | 189.28 | 189.68 | ||||||||||||||||||||||||||||||||
| S&P 500 Health Care Equipment Index | 100.00 | 120.35 | 141.25 | 160.75 | 213.16 | 198.87 | ||||||||||||||||||||||||||||||||
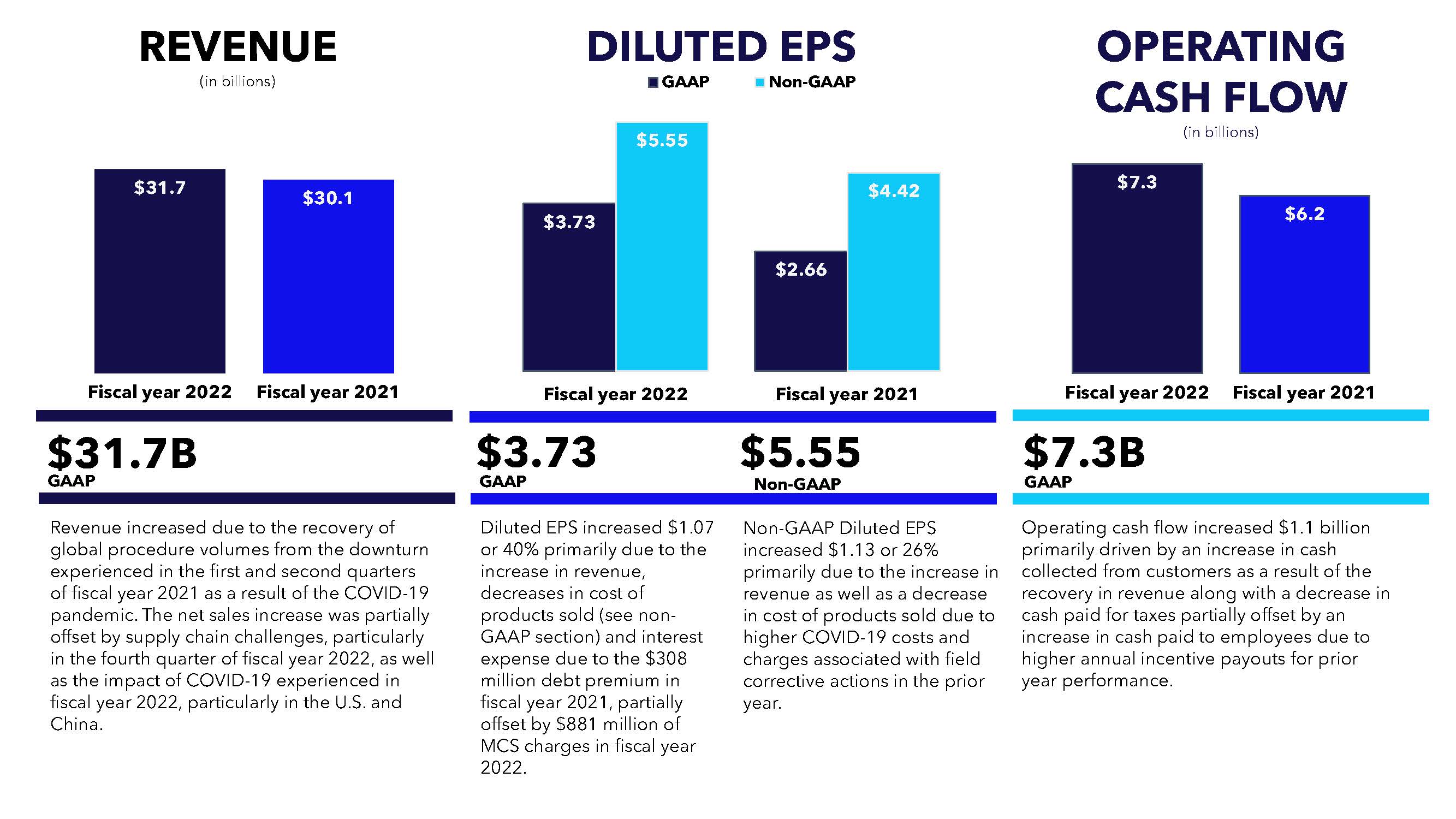
| Fiscal year ended April 29, 2022 | |||||||||||||||||||||||||||||
| (in millions, except per share data) | Income Before Income Taxes | Income Tax Provision (Benefit) | Net Income Attributable to Medtronic | Diluted EPS | Effective Tax Rate | ||||||||||||||||||||||||
| GAAP | $ | 5,517 | $ | 456 | $ | 5,039 | $ | 3.73 | 8.3 | % | |||||||||||||||||||
| Non-GAAP Adjustments: | |||||||||||||||||||||||||||||
Restructuring and associated costs (1) | 335 | 54 | 281 | 0.21 | 16.1 | ||||||||||||||||||||||||
Acquisition-related items (2) | (43) | 5 | (48) | (0.04) | (11.6) | ||||||||||||||||||||||||
| Certain litigation charges | 95 | 17 | 78 | 0.06 | 17.9 | ||||||||||||||||||||||||
(Gain)/loss on minority investments (3) | (12) | — | (9) | (0.01) | — | ||||||||||||||||||||||||
Medical device regulations (4) | 102 | 16 | 86 | 0.06 | 15.7 | ||||||||||||||||||||||||
| Amortization of intangible assets | 1,733 | 266 | 1,467 | 1.09 | 15.3 | ||||||||||||||||||||||||
MCS impairment / costs (5) | 881 | 220 | 661 | 0.49 | 25.0 | ||||||||||||||||||||||||
Certain tax adjustments, net (6) | — | 50 | (50) | (0.04) | — | ||||||||||||||||||||||||
| Non-GAAP | $ | 8,609 | $ | 1,084 | $ | 7,505 | $ | 5.55 | 12.6 | % | |||||||||||||||||||
| Fiscal year ended April 30, 2021 | |||||||||||||||||||||||||||||
| (in millions, except per share data) | Income Before Income Taxes | Income Tax (Benefit) Provision | Net Income Attributable to Medtronic | Diluted EPS | Effective Tax Rate | ||||||||||||||||||||||||
| GAAP | $ | 3,895 | $ | 265 | $ | 3,606 | $ | 2.66 | 6.8 | % | |||||||||||||||||||
| Non-GAAP Adjustments: | |||||||||||||||||||||||||||||
Restructuring and associated costs (1) | 617 | 128 | 489 | 0.36 | 20.7 | ||||||||||||||||||||||||
Acquisition-related items (2) | (15) | (20) | 4 | — | 126.7 | ||||||||||||||||||||||||
| Certain litigation charges | 118 | 23 | 95 | 0.07 | 19.5 | ||||||||||||||||||||||||
(Gain)/loss on minority investments (3) | (61) | — | (57) | (0.04) | — | ||||||||||||||||||||||||
Impairment charges (7) | 76 | 7 | 68 | 0.05 | 10.5 | ||||||||||||||||||||||||
Medical device regulations (4) | 83 | 15 | 68 | 0.05 | 18.1 | ||||||||||||||||||||||||
Debt tender premium and other charges (8) | 308 | 60 | 248 | 0.18 | 19.5 | ||||||||||||||||||||||||
| Amortization of intangible assets | 1,783 | 283 | 1,500 | 1.11 | 15.9 | ||||||||||||||||||||||||
Certain tax adjustments, net (9) | — | 41 | (41) | (0.03) | — | ||||||||||||||||||||||||
| Non-GAAP | $ | 6,804 | $ | 802 | $ | 5,980 | $ | 4.42 | 11.8 | % | |||||||||||||||||||
| Fiscal Year | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Net cash provided by operating activities | $ | 7,346 | $ | 6,240 | |||||||
| Additions to property, plant, and equipment | (1,368) | (1,355) | |||||||||
| Free cash flow | $ | 5,978 | $ | 4,885 | |||||||
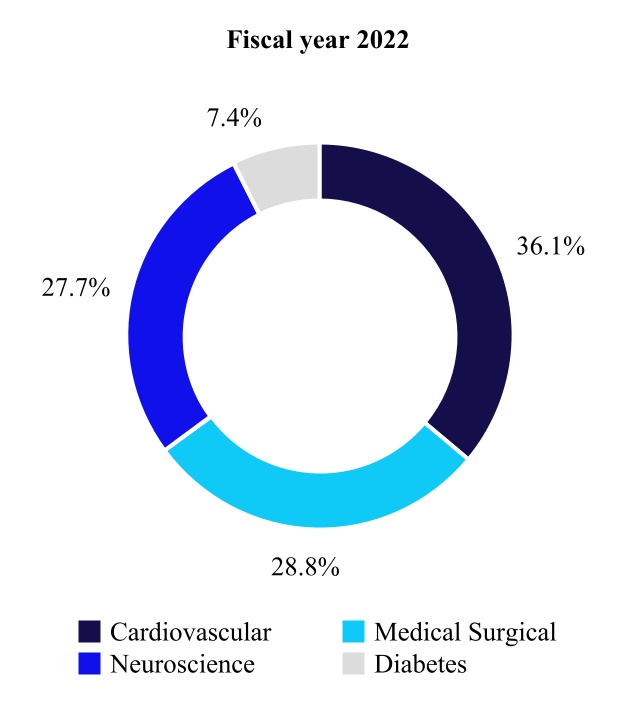
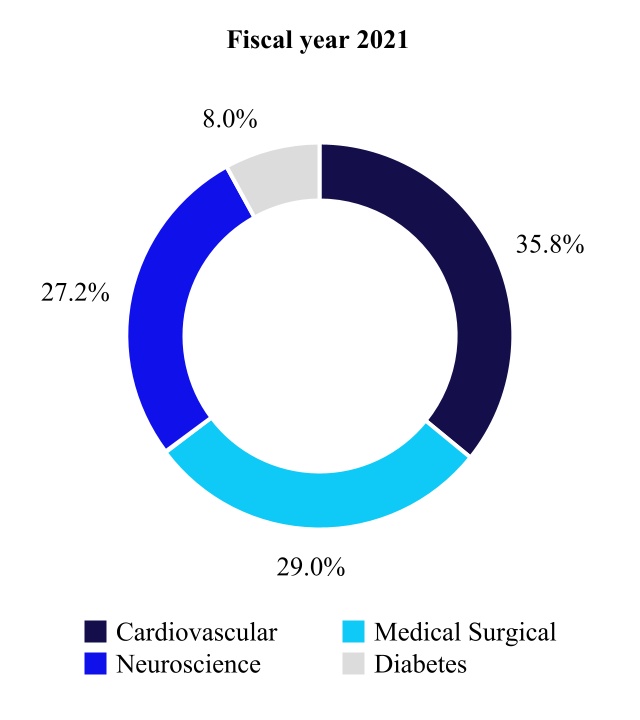
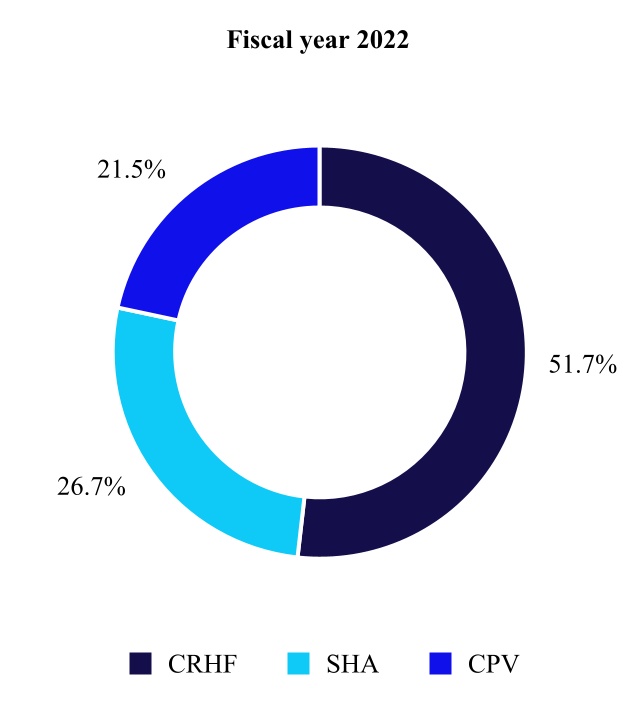
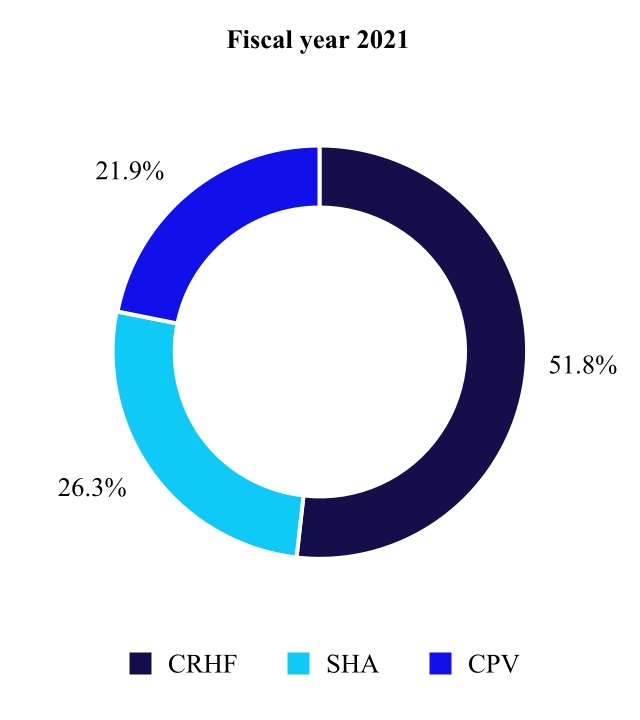
| Net Sales by Fiscal Year | Percent Change | ||||||||||||||||
| (in millions) | 2022 | 2021 | |||||||||||||||
| Cardiac Rhythm & Heart Failure | $ | 5,908 | $ | 5,584 | 6 | % | |||||||||||
| Structural Heart & Aortic | 3,055 | 2,834 | 8 | ||||||||||||||
| Coronary & Peripheral Vascular | 2,460 | 2,354 | 5 | ||||||||||||||
| Cardiovascular | 11,423 | 10,772 | 6 | ||||||||||||||
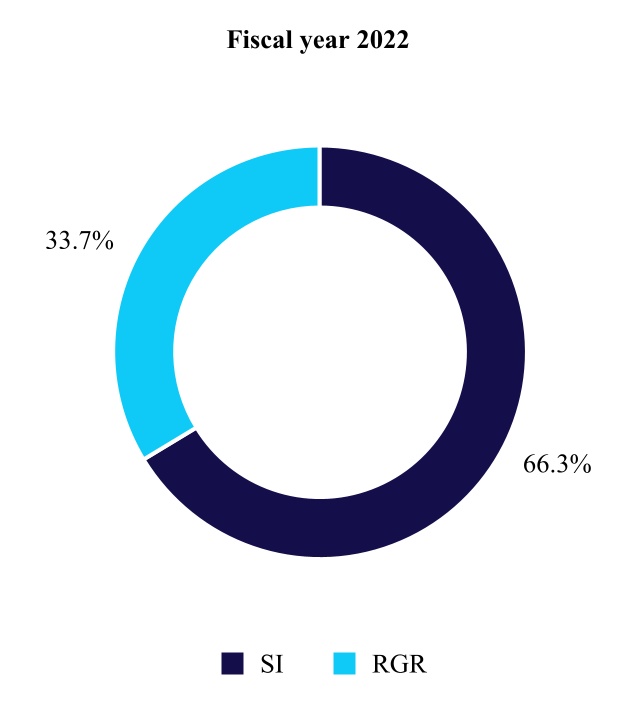
| Surgical Innovations | 6,060 | 5,438 | 11 | ||||||||||||||
| Respiratory, Gastrointestinal, & Renal | 3,081 | 3,298 | (7) | ||||||||||||||
| Medical Surgical | 9,141 | 8,737 | 5 | ||||||||||||||
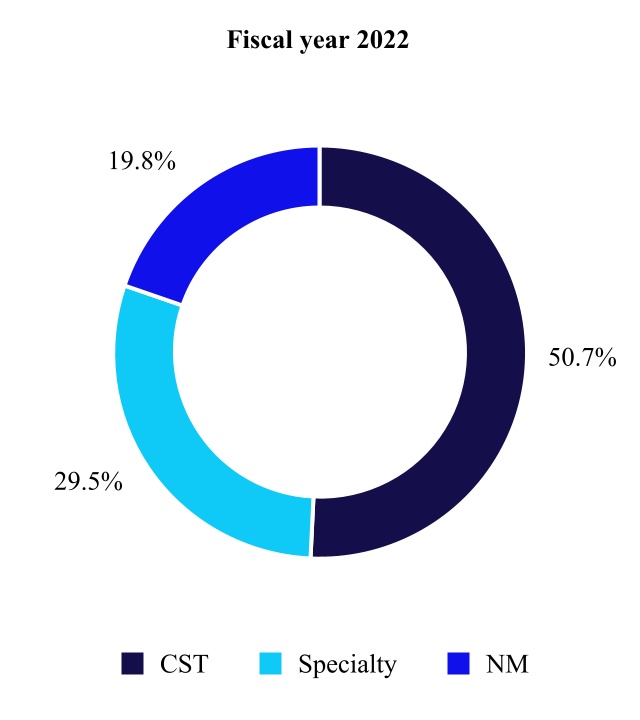
| Cranial & Spinal Technologies | 4,456 | 4,288 | 4 | ||||||||||||||
| Specialty Therapies | 2,592 | 2,307 | 12 | ||||||||||||||
| Neuromodulation | 1,735 | 1,601 | 8 | ||||||||||||||
| Neuroscience | 8,784 | 8,195 | 7 | ||||||||||||||
| Diabetes | 2,338 | 2,413 | (3) | ||||||||||||||
| Total | $ | 31,686 | $ | 30,117 | 5 | % | |||||||||||
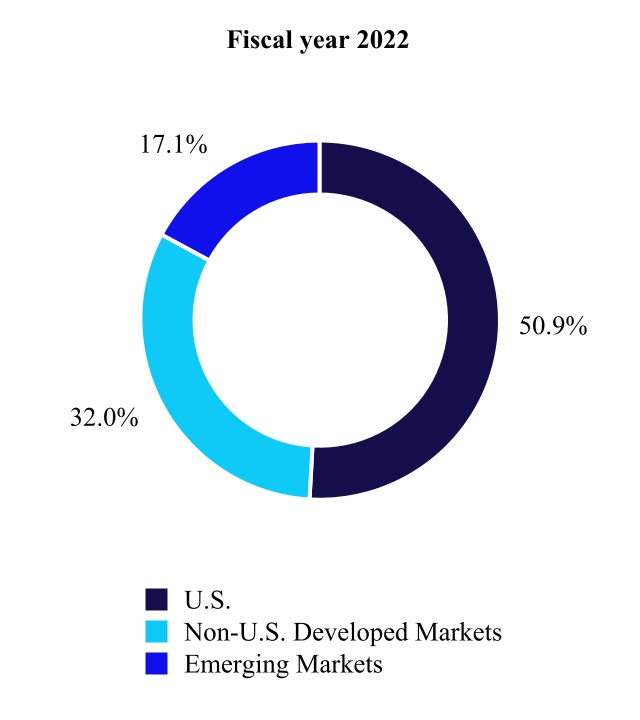
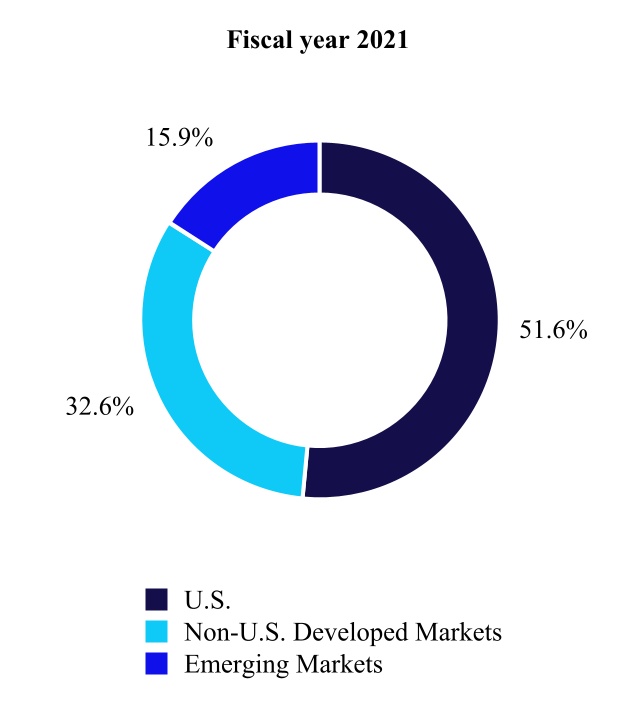
U.S.(1) | Non-U.S. Developed Markets(2) | Emerging Markets(3) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| (in millions) | Fiscal Year 2022 | Fiscal Year 2021 | % Change | Fiscal Year 2022 | Fiscal Year 2021 | % Change | Fiscal Year 2022 | Fiscal Year 2021 | % Change | ||||||||||||||||||||||||||||||||||||||||||||
| Cardiovascular | $ | 5,545 | $ | 5,248 | 6 | % | $ | 3,866 | $ | 3,752 | 3 | % | $ | 2,012 | $ | 1,773 | 13 | % | |||||||||||||||||||||||||||||||||||
| Medical Surgical | 3,862 | 3,650 | 6 | 3,373 | 3,320 | 2 | 1,905 | 1,766 | 8 | ||||||||||||||||||||||||||||||||||||||||||||
| Neuroscience | 5,753 | 5,456 | 5 | 1,801 | 1,724 | 4 | 1,229 | 1,015 | 21 | ||||||||||||||||||||||||||||||||||||||||||||
| Diabetes | 974 | 1,171 | (17) | 1,085 | 1,019 | 6 | 279 | 222 | 26 | ||||||||||||||||||||||||||||||||||||||||||||
| Total | $ | 16,135 | $ | 15,526 | 4 | % | $ | 10,126 | $ | 9,815 | 3 | % | $ | 5,426 | $ | 4,777 | 14 | % | |||||||||||||||||||||||||||||||||||


| Fiscal Year | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Amortization of intangible assets | $ | 1,733 | $ | 1,783 | |||||||
| Restructuring charges, net | 60 | 293 | |||||||||
| Certain litigation charges | 95 | 118 | |||||||||
| Other operating expense, net | 862 | 315 | |||||||||
| Other non-operating income, net | (318) | (336) | |||||||||
| Interest expense | 553 | 925 | |||||||||
| Fiscal Year | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Income tax provision (benefit) | $ | 456 | $ | 265 | |||||||
| Income before income taxes | 5,517 | 3,895 | |||||||||
| Effective tax rate | 8.3 | % | 6.8 | % | |||||||
| Non-GAAP income tax provision | $ | 1,084 | $ | 802 | |||||||
| Non-GAAP income before income taxes | 8,609 | 6,804 | |||||||||
| Non-GAAP Nominal Tax Rate | 12.6 | % | 11.8 | % | |||||||
| Difference between the effective tax rate and Non-GAAP Nominal Tax Rate | 4.3 | % | 5.0 | % | |||||||
| Fiscal Year | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Cash provided by (used in): | |||||||||||
| Operating activities | $ | 7,346 | $ | 6,240 | |||||||
| Investing activities | (1,659) | (2,866) | |||||||||
| Financing activities | (5,336) | (4,136) | |||||||||
| Effect of exchange rate changes on cash and cash equivalents | (231) | 215 | |||||||||
| Net change in cash and cash equivalents | $ | 121 | $ | (547) | |||||||
Agency Rating (1) | ||||||||||||||
| April 29, 2022 | April 30, 2021 | |||||||||||||
| Standard & Poor's Ratings Services | ||||||||||||||
| Long-term debt | A | A | ||||||||||||
| Short-term debt | A-1 | A-1 | ||||||||||||
| Moody's Investors Service | ||||||||||||||
| Long-term debt | A3 | A3 | ||||||||||||
| Short-term debt | P-2 | P-2 | ||||||||||||
| Maturity by Fiscal Year | ||||||||||||||||||||||||||||||||||||||||||||
| (in millions) | Total | 2023 | 2024 | 2025 | 2026 | 2027 | Thereafter | |||||||||||||||||||||||||||||||||||||
| Contractual obligations related to off-balance sheet arrangements: | ||||||||||||||||||||||||||||||||||||||||||||
Commitments to fund minority investments, milestone payments, and royalty obligations(1) | $ | 233 | $ | 95 | $ | 54 | $ | 30 | $ | 18 | $ | 18 | $ | 19 | ||||||||||||||||||||||||||||||
Interest payments(2) | 6,902 | 466 | 460 | 460 | 394 | 391 | 4,732 | |||||||||||||||||||||||||||||||||||||
Other(3) | 995 | 445 | 235 | 121 | 66 | 34 | 94 | |||||||||||||||||||||||||||||||||||||
Contractual obligations reflected in the balance sheet(4): | ||||||||||||||||||||||||||||||||||||||||||||
Debt obligations(5) | $ | 24,275 | $ | 3,744 | $ | 6 | $ | 1,895 | $ | 2,133 | $ | 1,969 | $ | 14,528 | ||||||||||||||||||||||||||||||
| Operating leases | 976 | 213 | 164 | 130 | 103 | 82 | 284 | |||||||||||||||||||||||||||||||||||||
Contingent consideration(6) | 119 | 35 | 49 | 33 | 1 | — | — | |||||||||||||||||||||||||||||||||||||
Tax obligations(7) | 1,496 | 176 | 330 | 440 | 550 | — | — | |||||||||||||||||||||||||||||||||||||
| (in millions) | Medtronic & Medtronic Luxco Senior Notes (1) | CIFSA Senior Notes (2) | |||||||||
| Net sales | $ | 2,063 | $ | — | |||||||
| Operating profit | 469 | (5) | |||||||||
| Loss before income taxes | (518) | (974) | |||||||||
| Net loss attributable to Medtronic | (529) | (1,005) | |||||||||
| (in millions) | Medtronic & Medtronic Luxco Senior Notes (1) | CIFSA Senior Notes (2) | |||||||||
Total current assets(3) | $ | 20,767 | $ | 6,881 | |||||||
Total noncurrent assets(4) | 12,099 | 8,293 | |||||||||
Total current liabilities(5) | 32,647 | 24,302 | |||||||||
Total noncurrent liabilities(6) | 50,542 | 60,292 | |||||||||
| Noncontrolling interests | 171 | 171 | |||||||||
| Increase (decrease) | ||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | ||||||||||||
| 10% appreciation in the U.S. dollar | $ | 903 | $ | 995 | ||||||||||
| 10% depreciation in the U.S. dollar | (903) | (995) | ||||||||||||
| Increase (decrease) | ||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | ||||||||||||
| 10 basis point increase in interest rates | $ | 53 | $ | 21 | ||||||||||
| 10 basis point decrease in interest rates | (53) | (21) | ||||||||||||
/s/ | ||
| June 23, 2022 | ||
| Fiscal Year | |||||||||||||||||
| (in millions, except per share data) | 2022 | 2021 | 2020 | ||||||||||||||
| Net sales | $ | $ | $ | ||||||||||||||
| Costs and expenses: | |||||||||||||||||
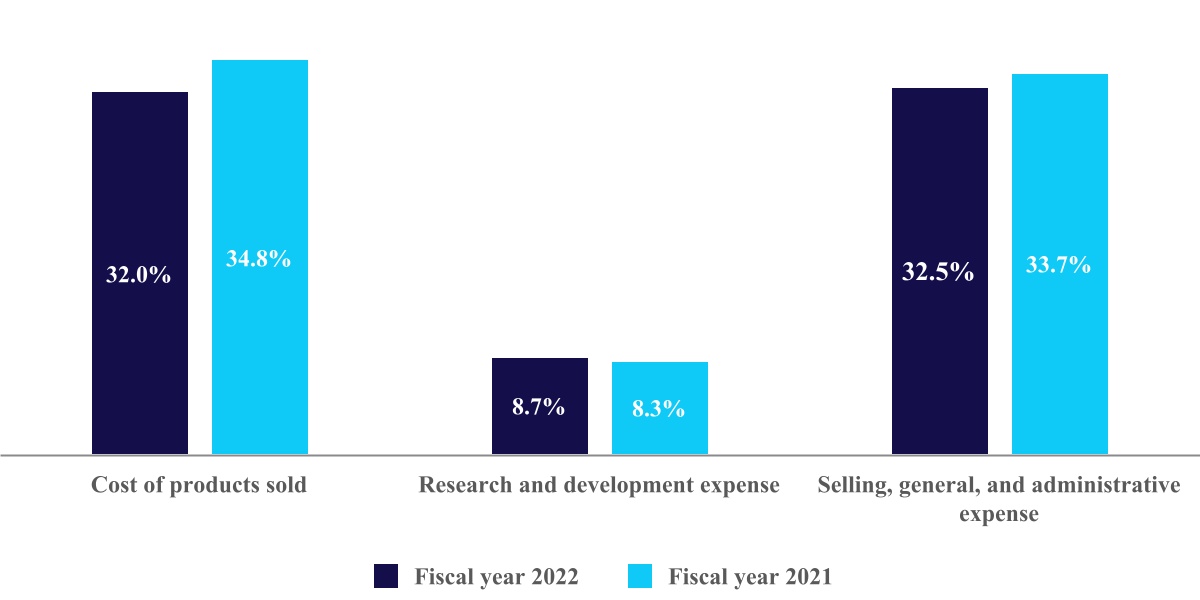
| Cost of products sold, excluding amortization of intangible assets | |||||||||||||||||
| Research and development expense | |||||||||||||||||
| Selling, general, and administrative expense | |||||||||||||||||
| Amortization of intangible assets | |||||||||||||||||
| Restructuring charges, net | |||||||||||||||||
| Certain litigation charges | |||||||||||||||||
| Other operating expense, net | |||||||||||||||||
| Operating profit | |||||||||||||||||
| Other non-operating income, net | ( | ( | ( | ||||||||||||||
| Interest expense | |||||||||||||||||
| Income before income taxes | |||||||||||||||||
| Income tax provision (benefit) | ( | ||||||||||||||||
| Net income | |||||||||||||||||
| Net income attributable to noncontrolling interests | ( | ( | ( | ||||||||||||||
| Net income attributable to Medtronic | $ | $ | $ | ||||||||||||||
| Basic earnings per share | $ | $ | $ | ||||||||||||||
| Diluted earnings per share | $ | $ | $ | ||||||||||||||
| Basic weighted average shares outstanding | |||||||||||||||||
| Diluted weighted average shares outstanding | |||||||||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Net income | $ | $ | $ | ||||||||||||||
| Other comprehensive income (loss), net of tax: | |||||||||||||||||
| Unrealized (loss) gain on investment securities | ( | ||||||||||||||||
| Translation adjustment | ( | ( | |||||||||||||||
| Net investment hedge | ( | ||||||||||||||||
| Net change in retirement obligations | ( | ||||||||||||||||
| Unrealized (loss) gain on cash flow hedges | ( | ||||||||||||||||
| Other comprehensive income (loss) | ( | ||||||||||||||||
| Comprehensive income including noncontrolling interests | |||||||||||||||||
| Comprehensive income attributable to noncontrolling interests | ( | ( | ( | ||||||||||||||
| Comprehensive income attributable to Medtronic | $ | $ | $ | ||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | ||||||||||||
| ASSETS | ||||||||||||||
| Current assets: | ||||||||||||||
| Cash and cash equivalents | $ | $ | ||||||||||||
| Investments | ||||||||||||||
Accounts receivable, less allowances and credit losses of $ | ||||||||||||||
| Inventories, net | ||||||||||||||
| Other current assets | ||||||||||||||
| Total current assets | ||||||||||||||
| Property, plant, and equipment, net | ||||||||||||||
| Goodwill | ||||||||||||||
| Other intangible assets, net | ||||||||||||||
| Tax assets | ||||||||||||||
| Other assets | ||||||||||||||
| Total assets | $ | $ | ||||||||||||
| LIABILITIES AND EQUITY | ||||||||||||||
| Current liabilities: | ||||||||||||||
| Current debt obligations | $ | $ | ||||||||||||
| Accounts payable | ||||||||||||||
| Accrued compensation | ||||||||||||||
| Accrued income taxes | ||||||||||||||
| Other accrued expenses | ||||||||||||||
| Total current liabilities | ||||||||||||||
| Long-term debt | ||||||||||||||
| Accrued compensation and retirement benefits | ||||||||||||||
| Accrued income taxes | ||||||||||||||
| Deferred tax liabilities | ||||||||||||||
| Other liabilities | ||||||||||||||
| Total liabilities | ||||||||||||||
| Commitments and contingencies (Notes 3, 16, and 18) | ||||||||||||||
| Shareholders’ equity: | ||||||||||||||
Ordinary shares— par value $ | ||||||||||||||
| Additional paid-in capital | ||||||||||||||
| Retained earnings | ||||||||||||||
| Accumulated other comprehensive loss | ( | ( | ||||||||||||
| Total shareholders’ equity | ||||||||||||||
| Noncontrolling interests | ||||||||||||||
| Total equity | ||||||||||||||
| Total liabilities and equity | $ | $ | ||||||||||||
| Ordinary Shares | Additional Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Loss | Total Shareholders’ Equity | Noncontrolling Interests | Total Equity | ||||||||||||||||||||||||||||||||||||||||||||
| (in millions, except per share data) | Number | Par Value | ||||||||||||||||||||||||||||||||||||||||||||||||
| April 26, 2019 | $ | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss | — | — | — | — | ( | ( | ( | ( | ||||||||||||||||||||||||||||||||||||||||||
Dividends to shareholders ($ | — | — | — | ( | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Issuance of shares under stock purchase and award plans | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Repurchase of ordinary shares | ( | — | ( | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Changes to noncontrolling ownership interests | — | — | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||||
| — | — | — | ( | — | ( | — | ( | |||||||||||||||||||||||||||||||||||||||||||
| April 24, 2020 | $ | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive income | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
Dividends to shareholders ($ | — | — | — | ( | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Issuance of shares under stock purchase and award plans | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Repurchase of ordinary shares | ( | — | ( | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Changes to noncontrolling ownership interests | — | — | ( | — | — | ( | ( | |||||||||||||||||||||||||||||||||||||||||||
| — | — | — | ( | — | ( | — | ( | |||||||||||||||||||||||||||||||||||||||||||
| April 30, 2021 | $ | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||||||||||||||||
| Net income | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive income | — | — | — | — | ( | |||||||||||||||||||||||||||||||||||||||||||||
Dividends to shareholders ($ | — | — | — | ( | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Issuance of shares under stock purchase and award plans | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||
| Repurchase of ordinary shares | ( | — | ( | — | — | ( | — | ( | ||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||||||
| Changes to noncontrolling ownership interests | — | — | — | — | ( | ( | ||||||||||||||||||||||||||||||||||||||||||||
| April 29, 2022 | $ | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||||||||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Operating Activities: | |||||||||||||||||
| Net income | $ | $ | $ | ||||||||||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Provision for credit losses | |||||||||||||||||
| Deferred income taxes | ( | ( | ( | ||||||||||||||
| Stock-based compensation | |||||||||||||||||
| Loss on debt extinguishment | |||||||||||||||||
| Asset impairment charges | |||||||||||||||||
| Other, net | |||||||||||||||||
| Change in operating assets and liabilities, net of acquisitions and divestitures: | |||||||||||||||||
| Accounts receivable, net | ( | ( | |||||||||||||||
| Inventories, net | ( | ( | |||||||||||||||
| Accounts payable and accrued liabilities | ( | ||||||||||||||||
| Other operating assets and liabilities | ( | ( | ( | ||||||||||||||
| Net cash provided by operating activities | |||||||||||||||||
| Investing Activities: | |||||||||||||||||
| Acquisitions, net of cash acquired | ( | ( | ( | ||||||||||||||
| Additions to property, plant, and equipment | ( | ( | ( | ||||||||||||||
| Purchases of investments | ( | ( | ( | ||||||||||||||
| Sales and maturities of investments | |||||||||||||||||
| Other investing activities, net | ( | ( | ( | ||||||||||||||
| Net cash used in investing activities | ( | ( | ( | ||||||||||||||
| Financing Activities: | |||||||||||||||||
| Change in current debt obligations, net | ( | ( | |||||||||||||||
| Proceeds from short-term borrowings (maturities greater than 90 days) | |||||||||||||||||
| Repayments from short-term borrowings (maturities greater than 90 days) | ( | ||||||||||||||||
| Issuance of long-term debt | |||||||||||||||||
| Payments on long-term debt | ( | ( | ( | ||||||||||||||
| Dividends to shareholders | ( | ( | ( | ||||||||||||||
| Issuance of ordinary shares | |||||||||||||||||
| Repurchase of ordinary shares | ( | ( | ( | ||||||||||||||
| Other financing activities | ( | ( | |||||||||||||||
| Net cash used in financing activities | ( | ( | ( | ||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | ( | ( | |||||||||||||||
| Net change in cash and cash equivalents | ( | ( | |||||||||||||||
| Cash and cash equivalents at beginning of period | |||||||||||||||||
| Cash and cash equivalents at end of period | $ | $ | $ | ||||||||||||||
| Supplemental Cash Flow Information | |||||||||||||||||
| Cash paid for: | |||||||||||||||||
| Income taxes | $ | $ | $ | ||||||||||||||
| Interest | |||||||||||||||||
| Net Sales by Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Cardiac Rhythm & Heart Failure | $ | $ | $ | ||||||||||||||
| Structural Heart & Aortic | |||||||||||||||||
| Coronary & Peripheral Vascular | |||||||||||||||||
| Cardiovascular | |||||||||||||||||
| Surgical Innovations | |||||||||||||||||
| Respiratory, Gastrointestinal, & Renal | |||||||||||||||||
| Medical Surgical | |||||||||||||||||
| Cranial & Spinal Technologies | |||||||||||||||||
| Specialty Therapies | |||||||||||||||||
| Neuromodulation | |||||||||||||||||
| Neuroscience | |||||||||||||||||
| Diabetes | |||||||||||||||||
| Total | $ | $ | $ | ||||||||||||||
U.S.(1) | Non-U.S. Developed Markets(2) | Emerging Markets(3) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| (in millions) | Fiscal Year 2022 | Fiscal Year 2021 | Fiscal Year 2020 | Fiscal Year 2022 | Fiscal Year 2021 | Fiscal Year 2020 | Fiscal Year 2022 | Fiscal Year 2021 | Fiscal Year 2020 | ||||||||||||||||||||||||||||||||||||||||||||
| Cardiovascular | $ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||||||||||||||||||||
| Medical Surgical | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Neuroscience | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Diabetes | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Total | $ | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||||||||||||||||||||
| Fiscal Year | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Beginning Balance | $ | $ | |||||||||
| Purchase price contingent consideration | |||||||||||
| Purchase price allocation adjustments | |||||||||||
| Payments | ( | ( | |||||||||
| Change in fair value | ( | ||||||||||
| Ending Balance | $ | $ | |||||||||
| (in millions) | Fair Value at April 29, 2022 | Unobservable Input | Range | Weighted Average (1) | ||||||||||||||||||||||
| Discount rate | ||||||||||||||||||||||||||
| Revenue and other performance-based payments | $ | Probability of payment | ||||||||||||||||||||||||
| Projected fiscal year of payment | 2023 - 2027 | 2025 | ||||||||||||||||||||||||
| Discount rate | ||||||||||||||||||||||||||
| Product development and other milestone-based payments | $ | Probability of payment | ||||||||||||||||||||||||
| Projected fiscal year of payment | 2023 - 2024 | 2024 | ||||||||||||||||||||||||
| (in millions) | Employee Termination Benefits | Associated Costs(1) | Asset Write-downs | Other Costs | Total | ||||||||||||||||||||||||
| April 26, 2019 | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Charges | |||||||||||||||||||||||||||||
| Cash payments | ( | ( | ( | ( | |||||||||||||||||||||||||
| Settled non-cash | ( | ( | |||||||||||||||||||||||||||
Accrual adjustments(2) | ( | ( | ( | ||||||||||||||||||||||||||
| April 24, 2020 | |||||||||||||||||||||||||||||
| Charges | |||||||||||||||||||||||||||||
| Cash payments | ( | ( | ( | ( | |||||||||||||||||||||||||
Accrual adjustments(2) | ( | ( | ( | ||||||||||||||||||||||||||
| April 30, 2021 | |||||||||||||||||||||||||||||
| Charges | |||||||||||||||||||||||||||||
| Cash payments | ( | ( | ( | ||||||||||||||||||||||||||
Accrual adjustments(2) | ( | ( | |||||||||||||||||||||||||||
| April 29, 2022 | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| April 29, 2022 | |||||||||||||||||||||||||||||||||||
| Valuation | Balance Sheet Classification | ||||||||||||||||||||||||||||||||||
| (in millions) | Cost | Unrealized Gains | Unrealized Losses | Fair Value | Investments | Other Assets | |||||||||||||||||||||||||||||
| Level 1: | |||||||||||||||||||||||||||||||||||
| U.S. government and agency securities | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||
| Level 2: | |||||||||||||||||||||||||||||||||||
| Corporate debt securities | ( | ||||||||||||||||||||||||||||||||||
| U.S. government and agency securities | ( | ||||||||||||||||||||||||||||||||||
| Mortgage-backed securities | ( | ||||||||||||||||||||||||||||||||||
| Non-U.S. government and agency securities | |||||||||||||||||||||||||||||||||||
| Certificates of deposit | |||||||||||||||||||||||||||||||||||
| Other asset-backed securities | ( | ||||||||||||||||||||||||||||||||||
| Total Level 2 | ( | ||||||||||||||||||||||||||||||||||
| Level 3: | |||||||||||||||||||||||||||||||||||
| Auction rate securities | ( | ||||||||||||||||||||||||||||||||||
| Total available-for-sale debt securities | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||
| April 30, 2021 | |||||||||||||||||||||||||||||||||||
| Valuation | Balance Sheet Classification | ||||||||||||||||||||||||||||||||||
| (in millions) | Cost | Unrealized Gains | Unrealized Losses | Fair Value | Investments | Other Assets | |||||||||||||||||||||||||||||
| Level 1: | |||||||||||||||||||||||||||||||||||
| U.S. government and agency securities | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||
| Level 2: | |||||||||||||||||||||||||||||||||||
| Corporate debt securities | ( | ||||||||||||||||||||||||||||||||||
| U.S. government and agency securities | ( | ||||||||||||||||||||||||||||||||||
| Mortgage-backed securities | ( | ||||||||||||||||||||||||||||||||||
| Non-U.S. government and agency securities | |||||||||||||||||||||||||||||||||||
| Certificates of deposit | |||||||||||||||||||||||||||||||||||
| Other asset-backed securities | ( | ||||||||||||||||||||||||||||||||||
| Debt funds | |||||||||||||||||||||||||||||||||||
| Total Level 2 | ( | ||||||||||||||||||||||||||||||||||
| Level 3: | |||||||||||||||||||||||||||||||||||
| Auction rate securities | ( | ||||||||||||||||||||||||||||||||||
| Total available-for-sale debt securities | $ | $ | $ | ( | $ | $ | $ | ||||||||||||||||||||||||||||
| April 29, 2022 | |||||||||||||||||||||||
| Less than 12 months | More than 12 months | ||||||||||||||||||||||
| (in millions) | Fair Value | Unrealized Losses | Fair Value | Unrealized Losses | |||||||||||||||||||
| U.S. government and agency securities | $ | $ | $ | $ | ( | ||||||||||||||||||
| Corporate debt securities | ( | ( | |||||||||||||||||||||
| Mortgage-backed securities | ( | ||||||||||||||||||||||
| Other asset-backed securities | ( | ||||||||||||||||||||||
| Auction rate securities | ( | ||||||||||||||||||||||
| Total | $ | $ | ( | $ | $ | ( | |||||||||||||||||
| April 30, 2021 | |||||||||||||||||||||||
| Less than 12 months | More than 12 months | ||||||||||||||||||||||
| (in millions) | Fair Value | Unrealized Losses | Fair Value | Unrealized Losses | |||||||||||||||||||
| U.S. government and agency securities | $ | $ | ( | $ | $ | ||||||||||||||||||
| Corporate debt securities | ( | ||||||||||||||||||||||
| Mortgage-backed securities | ( | ||||||||||||||||||||||
| Other asset-backed securities | ( | ||||||||||||||||||||||
| Auction rate securities | ( | ||||||||||||||||||||||
| Total | $ | $ | ( | $ | $ | ( | |||||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | April 24, 2020 | ||||||||||||||
| Proceeds from sales and maturities | $ | $ | $ | ||||||||||||||
| Gross realized gains | |||||||||||||||||
| Gross realized losses | ( | ( | ( | ||||||||||||||
| (in millions) | April 29, 2022 | ||||
| Due in one year or less | $ | ||||
| Due after one year through five years | |||||
| Due after five years through ten years | |||||
| Due after ten years | |||||
| Total debt securities | $ | ||||
| (in millions) | April 29, 2022 | April 30, 2021 | ||||||||||||
| Investments with readily determinable fair value (marketable equity securities) | $ | $ | ||||||||||||
| Investments without readily determinable fair values | ||||||||||||||
| Equity method and other investments | ||||||||||||||
| Total equity and other investments | $ | $ | ||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | April 24, 2020 | |||||||||||||||||
| Proceeds from sales | $ | $ | $ | |||||||||||||||||
| Gross gains | ||||||||||||||||||||
| Gross losses | ( | ( | ( | |||||||||||||||||
| Impairment losses recognized | ( | ( | ( | |||||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | |||||||||
| Bank borrowings | $ | $ | |||||||||
| Finance lease obligations | |||||||||||
| $ | $ | ||||||||||
| April 29, 2022 | April 30, 2021 | ||||||||||||||||||||||||||||
| (in millions, except interest rates) | Maturity by Fiscal Year | Amount | Effective Interest Rate | Amount | Effective Interest Rate | ||||||||||||||||||||||||
| 2023 | $ | % | $ | % | |||||||||||||||||||||||||
| 2023 | |||||||||||||||||||||||||||||
| 2023 | |||||||||||||||||||||||||||||
| 2025 | |||||||||||||||||||||||||||||
| 2026 | |||||||||||||||||||||||||||||
| 2026 | |||||||||||||||||||||||||||||
| 2027 | |||||||||||||||||||||||||||||
| 2027 | |||||||||||||||||||||||||||||
| 2029 | |||||||||||||||||||||||||||||
| 2031 | |||||||||||||||||||||||||||||
| 2032 | |||||||||||||||||||||||||||||
| 2033 | |||||||||||||||||||||||||||||
| 2035 | |||||||||||||||||||||||||||||
| 2038 | |||||||||||||||||||||||||||||
| 2039 | |||||||||||||||||||||||||||||
| 2039 | |||||||||||||||||||||||||||||
| 2040 | |||||||||||||||||||||||||||||
| 2040 | |||||||||||||||||||||||||||||
| 2041 | |||||||||||||||||||||||||||||
| 2042 | |||||||||||||||||||||||||||||
| 2043 | |||||||||||||||||||||||||||||
| 2044 | |||||||||||||||||||||||||||||
| 2045 | |||||||||||||||||||||||||||||
| 2050 | |||||||||||||||||||||||||||||
| 2051 | |||||||||||||||||||||||||||||
| 2023-2035 | |||||||||||||||||||||||||||||
| Debt discount, net | 2023-2051 | ( | — | ( | — | ||||||||||||||||||||||||
| Deferred financing costs | 2023-2051 | ( | — | ( | — | ||||||||||||||||||||||||
| Long-term debt | $ | $ | |||||||||||||||||||||||||||
| (in millions) | |||||
| 2023 | $ | ||||
| 2024 | |||||
| 2025 | |||||
| 2026 | |||||
| 2027 | |||||
| Thereafter | |||||
| Total | $ | ||||
| (Gain) Loss Recognized in Accumulated Other Comprehensive Income | (Gain) Loss Reclassified into Income | ||||||||||||||||||||||||||||||||||||||||
| Fiscal Year | Fiscal Year | Location of (Gain) Loss in Income Statement | |||||||||||||||||||||||||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | 2022 | 2021 | 2020 | |||||||||||||||||||||||||||||||||||
| Cash flow hedges | |||||||||||||||||||||||||||||||||||||||||
| Currency exchange rate contracts | $ | ( | $ | $ | ( | $ | ( | $ | ( | $ | ( | Other operating expense, net | |||||||||||||||||||||||||||||
| Currency exchange rate contracts | Cost of products sold | ||||||||||||||||||||||||||||||||||||||||
| Net investment hedges | ( | ( | ( | Other operating expense, net | |||||||||||||||||||||||||||||||||||||
| Total | $ | ( | $ | $ | ( | $ | ( | $ | ( | $ | ( | ||||||||||||||||||||||||||||||
| (Gain) Loss Recognized in Income | |||||||||||||||||||||||
| Fiscal Year | Location of (Gain) Loss in Income Statement | ||||||||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||||||||
| Derivatives not designated as hedging instruments | |||||||||||||||||||||||
| Currency exchange rate contracts | $ | ( | $ | $ | ( | Other operating expense, net | |||||||||||||||||
| Total return swaps | ( | Other operating expense, net | |||||||||||||||||||||
| Total | $ | ( | $ | $ | ( | ||||||||||||||||||
| Fair Value - Assets | Fair Value - Liabilities | ||||||||||||||||||||||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | Balance Sheet Classification | April 29, 2022 | April 30, 2021 | Balance Sheet Classification | |||||||||||||||||||||||||||||
| Derivatives designated as hedging instruments | |||||||||||||||||||||||||||||||||||
| Currency exchange rate contracts | $ | $ | Other current assets | $ | $ | Other accrued expenses | |||||||||||||||||||||||||||||
| Currency exchange rate contracts | Other assets | Other liabilities | |||||||||||||||||||||||||||||||||
| Total derivatives designated as hedging instruments | |||||||||||||||||||||||||||||||||||
| Derivatives not designated as hedging instruments | |||||||||||||||||||||||||||||||||||
| Currency exchange rate contracts | Other current assets | Other accrued expenses | |||||||||||||||||||||||||||||||||
| Total return swaps | Other current assets | Other accrued expenses | |||||||||||||||||||||||||||||||||
| Total derivatives not designated as hedging instruments | |||||||||||||||||||||||||||||||||||
| Total derivatives | $ | $ | $ | $ | |||||||||||||||||||||||||||||||
| April 29, 2022 | April 30, 2021 | ||||||||||||||||||||||
| (in millions) | Level 1 | Level 2 | Level 1 | Level 2 | |||||||||||||||||||
| Derivative assets | $ | $ | $ | $ | |||||||||||||||||||
| Derivative liabilities | |||||||||||||||||||||||
| April 29, 2022 | ||||||||||||||||||||||||||
| Gross Amount Not Offset on the Balance Sheet | ||||||||||||||||||||||||||
| (in millions) | Gross Amount of Recognized Assets (Liabilities) | Financial Instruments | Cash Collateral (Received) Posted | Net Amount | ||||||||||||||||||||||
| Derivative assets: | ||||||||||||||||||||||||||
| Currency exchange rate contracts | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||
| Derivative liabilities: | ||||||||||||||||||||||||||
| Currency exchange rate contracts | ( | |||||||||||||||||||||||||
| Total return swaps | ( | ( | ||||||||||||||||||||||||
| ( | ( | |||||||||||||||||||||||||
| Total | $ | $ | $ | ( | $ | |||||||||||||||||||||
| April 30, 2021 | ||||||||||||||||||||||||||
| Gross Amount Not Offset on the Balance Sheet | ||||||||||||||||||||||||||
| (in millions) | Gross Amount of Recognized Assets (Liabilities) | Financial Instruments | Cash Collateral (Received) Posted | Net Amount | ||||||||||||||||||||||
| Derivative assets: | ||||||||||||||||||||||||||
| Currency exchange rate contracts | $ | $ | ( | $ | $ | |||||||||||||||||||||
| Total return swaps | ||||||||||||||||||||||||||
| ( | ||||||||||||||||||||||||||
| Derivative liabilities: | ||||||||||||||||||||||||||
| Currency exchange rate contracts | ( | ( | ||||||||||||||||||||||||
| Total | $ | ( | $ | $ | $ | ( | ||||||||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | |||||||||
| Finished goods | $ | $ | |||||||||
| Work-in-process | |||||||||||
| Raw materials | |||||||||||
| Total | $ | $ | |||||||||
| (in millions) | Cardiovascular | Medical Surgical | Neuroscience | Diabetes | Total | ||||||||||||||||||||||||
| April 24, 2020 | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Goodwill as a result of acquisitions | |||||||||||||||||||||||||||||
| Purchase accounting adjustments | ( | ( | ( | ( | |||||||||||||||||||||||||
| Currency translation and other | |||||||||||||||||||||||||||||
| April 30, 2021 | |||||||||||||||||||||||||||||
| Goodwill as a result of acquisitions | |||||||||||||||||||||||||||||
| Purchase accounting adjustments | ( | ||||||||||||||||||||||||||||
| Currency translation and other | ( | ( | ( | ( | ( | ||||||||||||||||||||||||
| April 29, 2022 | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| April 29, 2022 | April 30, 2021 | ||||||||||||||||||||||
| (in millions) | Gross Carrying Amount | Accumulated Amortization | Gross Carrying Amount | Accumulated Amortization | |||||||||||||||||||
| Definite-lived: | |||||||||||||||||||||||
| Customer-related | $ | $ | ( | $ | $ | ( | |||||||||||||||||
| Purchased technology and patents | ( | ( | |||||||||||||||||||||
| Trademarks and tradenames | ( | ( | |||||||||||||||||||||
| Other | ( | ( | |||||||||||||||||||||
| Total | $ | $ | ( | $ | $ | ( | |||||||||||||||||
| Indefinite-lived: | |||||||||||||||||||||||
| IPR&D | $ | $ | — | $ | $ | — | |||||||||||||||||
| (in millions) | Amortization Expense | ||||
| 2023 | $ | ||||
| 2024 | |||||
| 2025 | |||||
| 2026 | |||||
| 2027 | |||||
| (in millions) | April 29, 2022 | April 30, 2021 | Estimated Useful Lives (in years) | ||||||||||||||
| Equipment | $ | $ | Generally | ||||||||||||||
| Computer software | Up to | ||||||||||||||||
| Land and land improvements | Up to | ||||||||||||||||
| Buildings and leasehold improvements | Up to | ||||||||||||||||
| Construction in progress | — | ||||||||||||||||
| Property, plant, and equipment | |||||||||||||||||
| Less: Accumulated depreciation | ( | ( | |||||||||||||||
| Property, plant, and equipment, net | $ | $ | |||||||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Stock options | $ | $ | $ | ||||||||||||||
| Restricted stock | |||||||||||||||||
| Performance share units | |||||||||||||||||
| Employee stock purchase plan | |||||||||||||||||
| Total stock-based compensation expense | $ | $ | $ | ||||||||||||||
| Cost of products sold | $ | $ | $ | ||||||||||||||
| Research and development expense | |||||||||||||||||
| Selling, general, and administrative expense | |||||||||||||||||
| Total stock-based compensation expense | |||||||||||||||||
| Income tax benefits | ( | ( | ( | ||||||||||||||
| Total stock-based compensation expense, net of tax | $ | $ | $ | ||||||||||||||
| Fiscal Year | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| Weighted average fair value of options granted | $ | $ | $ | ||||||||||||||
| Assumptions used: | |||||||||||||||||
| Expected life (years) | |||||||||||||||||
| Risk-free interest rate | % | % | % | ||||||||||||||
| Volatility | % | % | % | ||||||||||||||
| Dividend yield | % | % | % | ||||||||||||||
| Options (in thousands) | Wtd. Avg. Exercise Price | Wtd. Avg. Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in millions) | ||||||||||||||||||||
Outstanding at April 30, 2021 | $ | ||||||||||||||||||||||
| Granted | |||||||||||||||||||||||
| Exercised | ( | ||||||||||||||||||||||
| Expired/Forfeited | ( | ||||||||||||||||||||||
Outstanding at April 29, 2022 | $ | ||||||||||||||||||||||
Expected to vest at April 29, 2022 | |||||||||||||||||||||||
Exercisable at April 29, 2022 | |||||||||||||||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Cash proceeds from options exercised | $ | $ | $ | ||||||||||||||
| Intrinsic value of options exercised | |||||||||||||||||
| Tax benefit related to options exercised | |||||||||||||||||
| Units (in thousands) | Wtd. Avg. Grant Price | ||||||||||
Nonvested at April 30, 2021 | $ | ||||||||||
| Granted | |||||||||||
| Vested | ( | ||||||||||
| Forfeited | ( | ||||||||||
Nonvested at April 29, 2022 | |||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions, except per share data) | 2022 | 2021 | 2020 | ||||||||||||||
| Weighted-average grant-date fair value per restricted stock | $ | $ | $ | ||||||||||||||
| Fair value of restricted stock vested | |||||||||||||||||
| Tax benefit related to restricted stock vested | |||||||||||||||||
| Units (in thousands) | Wtd. Avg. Grant Price | ||||||||||
Nonvested at April 30, 2021 | $ | ||||||||||
| Granted | |||||||||||
| Forfeited | ( | ||||||||||
Nonvested at April 29, 2022 | |||||||||||
| Fiscal Year | |||||||||||
| (in millions, except per share data) | 2022 | 2021 | |||||||||
| Weighted-average grant-date fair value per performance share units | $ | $ | |||||||||
| Fair value of performance share units vested | |||||||||||
| Tax benefit related to performance share units vested | |||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| U.S. | $ | $ | ( | $ | |||||||||||||
| International | |||||||||||||||||
| Income before income taxes | $ | $ | $ | ||||||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Current tax expense: | |||||||||||||||||
| U.S. | $ | $ | $ | ||||||||||||||
| International | |||||||||||||||||
| Total current tax expense | |||||||||||||||||
| Deferred tax (benefit) expense: | |||||||||||||||||
| U.S. | ( | ( | ( | ||||||||||||||
| International | ( | ( | |||||||||||||||
| Net deferred tax benefit | ( | ( | ( | ||||||||||||||
Income tax provision (benefit) | $ | $ | $ | ( | |||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021(1) | |||||||||
| Deferred tax assets: | |||||||||||
| Intangible assets | $ | $ | |||||||||
| Net operating loss, capital loss, and credit carryforwards | |||||||||||
| Capitalization of research and development | |||||||||||
| Other accrued liabilities | |||||||||||
| Accrued compensation | |||||||||||
| Pension and post-retirement benefits | |||||||||||
| Stock-based compensation | |||||||||||
| Inventory | |||||||||||
| Lease obligations | |||||||||||
| Federal and state benefit on uncertain tax positions | |||||||||||
| Interest limitation | |||||||||||
| Other | |||||||||||
| Gross deferred tax assets | |||||||||||
| Valuation allowance | ( | ( | |||||||||
| Total deferred tax assets | |||||||||||
| Deferred tax liabilities: | |||||||||||
| Intangible assets | ( | ( | |||||||||
| Realized loss on derivative financial instruments | ( | ( | |||||||||
| Right of use leases | ( | ( | |||||||||
| Unrealized gain on available-for-sale securities and derivative financial instruments | ( | ||||||||||
| Accumulated depreciation | ( | ( | |||||||||
| Outside basis difference of subsidiaries | ( | ( | |||||||||
| Other | ( | ( | |||||||||
| Total deferred tax liabilities | ( | ( | |||||||||
| Prepaid income taxes | |||||||||||
| Income tax receivables | |||||||||||
| Tax assets, net | $ | $ | |||||||||
| Reported as (after valuation allowance and jurisdictional netting): | |||||||||||
| Other current assets | $ | $ | |||||||||
| Tax assets | |||||||||||
| Deferred tax liabilities | ( | ( | |||||||||
| Tax assets, net | $ | $ | |||||||||
| Fiscal Year | |||||||||||||||||
| 2022 | 2021 | 2020 | |||||||||||||||
| U.S. federal statutory tax rate | % | % | % | ||||||||||||||
| Increase (decrease) in tax rate resulting from: | |||||||||||||||||
| U.S. state taxes, net of federal tax benefit | ( | ||||||||||||||||
| Research and development credit | ( | ( | ( | ||||||||||||||
| Puerto Rico excise tax | ( | ( | ( | ||||||||||||||
| International | ( | ( | ( | ||||||||||||||
| Stock based compensation | ( | ( | ( | ||||||||||||||
| Interest on uncertain tax positions | |||||||||||||||||
| Base erosion anti-abuse tax | |||||||||||||||||
| Foreign derived intangible income benefit | ( | ( | ( | ||||||||||||||
| Certain tax adjustments | ( | ( | ( | ||||||||||||||
| Legal entity restructuring | |||||||||||||||||
| U.S. tax on foreign earnings | |||||||||||||||||
| Other, net | ( | ||||||||||||||||
| Effective tax rate | % | % | ( | % | |||||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Gross unrecognized tax benefits at beginning of fiscal year | $ | $ | $ | ||||||||||||||
| Gross increases: | |||||||||||||||||
| Prior year tax positions | |||||||||||||||||
| Current year tax positions | |||||||||||||||||
| Gross decreases: | |||||||||||||||||
| Prior year tax positions | ( | ( | ( | ||||||||||||||
| Settlements | ( | ( | ( | ||||||||||||||
| Statute of limitation lapses | ( | ( | ( | ||||||||||||||
| Gross unrecognized tax benefits at end of fiscal year | |||||||||||||||||
| Cash advance paid to taxing authorities | ( | ( | ( | ||||||||||||||
| Gross unrecognized tax benefits at end of fiscal year, net of cash advance | $ | $ | $ | ||||||||||||||
| Jurisdiction | Earliest Year Open | |||||||
| United States - federal and state | 2005 | |||||||
| Australia | 2018 | |||||||
| Brazil | 2017 | |||||||
| Canada | 2013 | |||||||
| China | 2015 | |||||||
| Costa Rica | 2018 | |||||||
| Dominican Republic | 2019 | |||||||
| France | 2019 | |||||||
| Germany | 2014 | |||||||
| India | 2002 | |||||||
| Ireland | 2012 | |||||||
| Israel | 2010 | |||||||
| Italy | 2005 | |||||||
| Japan | 2018 | |||||||
| Korea | 2017 | |||||||
| Luxembourg | 2017 | |||||||
| Mexico | 2017 | |||||||
| Puerto Rico | 2011 | |||||||
| Singapore | 2016 | |||||||
| Switzerland | 2010 | |||||||
| United Kingdom | 2017 | |||||||
| Fiscal Year | |||||||||||||||||
| (in millions, except per share data) | 2022 | 2021 | 2020 | ||||||||||||||
| Numerator: | |||||||||||||||||
| Net income attributable to ordinary shareholders | $ | $ | $ | ||||||||||||||
| Denominator: | |||||||||||||||||
| Basic – weighted average shares outstanding | |||||||||||||||||
| Effect of dilutive securities: | |||||||||||||||||
| Employee stock options | |||||||||||||||||
| Employee restricted stock units | |||||||||||||||||
| Other | |||||||||||||||||
| Diluted – weighted average shares outstanding | |||||||||||||||||
| Basic earnings per share | $ | $ | $ | ||||||||||||||
| Diluted earnings per share | $ | $ | $ | ||||||||||||||
| U.S. Pension Benefits | Non-U.S. Pension Benefits | ||||||||||||||||||||||
| Fiscal Year | Fiscal Year | ||||||||||||||||||||||
| (in millions) | 2022 | 2021 | 2022 | 2021 | |||||||||||||||||||
| Accumulated benefit obligation at end of year: | $ | $ | $ | $ | |||||||||||||||||||
| Change in projected benefit obligation: | |||||||||||||||||||||||
| Projected benefit obligation at beginning of year | $ | $ | $ | $ | |||||||||||||||||||
| Service cost | |||||||||||||||||||||||
| Interest cost | |||||||||||||||||||||||
| Employee contributions | |||||||||||||||||||||||
| Plan curtailments and settlements | ( | ( | |||||||||||||||||||||
Actuarial (gain) loss(1) | ( | ( | |||||||||||||||||||||
| Benefits paid | ( | ( | ( | ( | |||||||||||||||||||
| Special termination benefits | |||||||||||||||||||||||
| Currency exchange rate changes and other | ( | ||||||||||||||||||||||
| Projected benefit obligation at end of year | $ | $ | $ | $ | |||||||||||||||||||
| Change in plan assets: | |||||||||||||||||||||||
| Fair value of plan assets at beginning of year | $ | $ | $ | $ | |||||||||||||||||||
| Actual return on plan assets | ( | ||||||||||||||||||||||
| Employer contributions | |||||||||||||||||||||||
| Employee contributions | |||||||||||||||||||||||
| Plan settlements | ( | ( | |||||||||||||||||||||
| Benefits paid | ( | ( | ( | ( | |||||||||||||||||||
| Currency exchange rate changes and other | ( | ||||||||||||||||||||||
| Fair value of plan assets at end of year | $ | $ | $ | $ | |||||||||||||||||||
| Funded status at end of year: | |||||||||||||||||||||||
| Fair value of plan assets | $ | $ | $ | $ | |||||||||||||||||||
| Benefit obligations | |||||||||||||||||||||||
| Over (under) funded status of the plans | ( | ( | ( | ||||||||||||||||||||
| Recognized asset (liability) | $ | $ | ( | $ | ( | $ | ( | ||||||||||||||||
| Amounts recognized on the consolidated balance sheets consist of: | |||||||||||||||||||||||
| Non-current assets | $ | $ | $ | $ | |||||||||||||||||||
| Current liabilities | ( | ( | ( | ( | |||||||||||||||||||
| Non-current liabilities | ( | ( | ( | ( | |||||||||||||||||||
| Recognized asset (liability) | $ | $ | ( | $ | ( | $ | ( | ||||||||||||||||
| Amounts recognized in accumulated other comprehensive loss: | |||||||||||||||||||||||
| Prior service cost (credit) | $ | $ | $ | ( | $ | ( | |||||||||||||||||
| Net actuarial loss | |||||||||||||||||||||||
| Ending balance | $ | $ | $ | $ | |||||||||||||||||||
| Fiscal Year | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Accumulated benefit obligation | $ | $ | |||||||||
| Projected benefit obligation | |||||||||||
| Plan assets at fair value | |||||||||||
| Fiscal Year | |||||||||||
| (in millions) | 2022 | 2021 | |||||||||
| Projected benefit obligation | $ | $ | |||||||||
| Plan assets at fair value | |||||||||||
| U.S. Pension Benefits | Non-U.S. Pension Benefits | ||||||||||||||||||||||||||||||||||
| Fiscal Year | Fiscal Year | ||||||||||||||||||||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | 2022 | 2021 | 2020 | |||||||||||||||||||||||||||||
| Service cost | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||
| Interest cost | |||||||||||||||||||||||||||||||||||
| Expected return on plan assets | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Amortization of prior service cost | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Amortization of net actuarial loss | |||||||||||||||||||||||||||||||||||
| Settlement and curtailment (gain) loss | ( | ||||||||||||||||||||||||||||||||||
| Special termination benefits | |||||||||||||||||||||||||||||||||||
| Net periodic benefit cost | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||
| (in millions) | U.S. Pension Benefits | Non-U.S. Pension Benefits | |||||||||
| Net actuarial gain | $ | ( | $ | ( | |||||||
| Amortization of prior service credit | |||||||||||
| Amortization and settlement recognition of actuarial loss | ( | ( | |||||||||
| Effect of exchange rates | ( | ||||||||||
| Total recognized in other comprehensive income | ( | ( | |||||||||
| Total recognized in net periodic benefit cost and other comprehensive income | $ | ( | $ | ( | |||||||
| U.S. Pension Benefits | Non-U.S. Pension Benefits | ||||||||||||||||||||||||||||||||||
| Fiscal Year | Fiscal Year | ||||||||||||||||||||||||||||||||||
| 2022 | 2021 | 2020 | 2022 | 2021 | 2020 | ||||||||||||||||||||||||||||||
| Critical assumptions – projected benefit obligation: | |||||||||||||||||||||||||||||||||||
| Discount rate | |||||||||||||||||||||||||||||||||||
| Rate of compensation increase | % | % | % | % | % | % | |||||||||||||||||||||||||||||
| Critical assumptions – net periodic benefit cost: | |||||||||||||||||||||||||||||||||||
Discount rate – benefit obligation | |||||||||||||||||||||||||||||||||||
Discount rate – service cost | |||||||||||||||||||||||||||||||||||
Discount rate – interest cost | |||||||||||||||||||||||||||||||||||
| Expected return on plan assets | % | % | % | % | % | ||||||||||||||||||||||||||||||
| Rate of compensation increase | % | % | % | % | % | ||||||||||||||||||||||||||||||
| U.S. Plans | Target Allocation | Actual Allocation | |||||||||||||||
April 29, 2022 | April 29, 2022 | April 30, 2021 | |||||||||||||||
| Asset Category: | |||||||||||||||||
| Equity securities | % | % | % | ||||||||||||||
| Debt securities | |||||||||||||||||
| Other | |||||||||||||||||
| Total | % | % | % | ||||||||||||||
| Fair Value at | |||||||||||||||||||||||||||||
| Fair Value Measurements Using Inputs Considered as | Investments Measured at Net Asset Value | ||||||||||||||||||||||||||||
| (in millions) | April 29, 2022 | Level 1 | Level 2 | Level 3 | |||||||||||||||||||||||||
| Short-term investments | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Mutual funds | |||||||||||||||||||||||||||||
| Equity commingled trusts | |||||||||||||||||||||||||||||
| Fixed income commingled trusts | |||||||||||||||||||||||||||||
| Partnership units | |||||||||||||||||||||||||||||
| $ | $ | $ | $ | $ | |||||||||||||||||||||||||
| Fair Value at | Fair Value Measurements Using Inputs Considered as | Investments Measured at Net Asset Value | |||||||||||||||||||||||||||
| (in millions) | April 30, 2021 | Level 1 | Level 2 | Level 3 | |||||||||||||||||||||||||
| Short-term investments | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Mutual funds | |||||||||||||||||||||||||||||
| Equity commingled trusts | |||||||||||||||||||||||||||||
| Fixed income commingled trusts | |||||||||||||||||||||||||||||
| Partnership units | |||||||||||||||||||||||||||||
| $ | $ | $ | $ | $ | |||||||||||||||||||||||||
| (in millions) | Partnership Units | ||||
April 24, 2020 | $ | ||||
| Total realized gains, net | |||||
| Total unrealized gains, net | |||||
| Purchases and sales, net | |||||
April 30, 2021 | |||||
| Total realized gains, net | |||||
| Total unrealized gains, net | |||||
| Purchases and sales, net | |||||
April 29, 2022 | $ | ||||
| Fair Value at | Fair Value Measurements Using Inputs Considered as | Investments Measured at Net Asset Value | |||||||||||||||||||||||||||
| (in millions) | April 29, 2022 | Level 1 | Level 2 | Level 3 | |||||||||||||||||||||||||
| Registered investment companies | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Insurance contracts | — | ||||||||||||||||||||||||||||
| $ | $ | $ | $ | $ | |||||||||||||||||||||||||
| Fair Value at | Fair Value Measurements Using Inputs Considered as | Investments Measured at Net Asset Value | |||||||||||||||||||||||||||
| (in millions) | April 30, 2021 | Level 1 | Level 2 | Level 3 | |||||||||||||||||||||||||
| Registered investment companies | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Insurance contracts | — | ||||||||||||||||||||||||||||
| $ | $ | $ | $ | $ | |||||||||||||||||||||||||
| (in millions) | Gross Payments | ||||||||||
| Fiscal Year | U.S. Pension Benefits | Non-U.S. Pension Benefits | |||||||||
| 2023 | $ | $ | |||||||||
| 2024 | |||||||||||
| 2025 | |||||||||||
| 2026 | |||||||||||
| 2027 | |||||||||||
| 2028 – 2032 | |||||||||||
| Total | $ | $ | |||||||||
| (in millions) | Balance Sheet Classification | April 29, 2022 | April 30, 2021 | ||||||||||||||
| Right-of-use assets | $ | $ | |||||||||||||||
| Current liability | |||||||||||||||||
| Non-current liability | |||||||||||||||||
| April 29, 2022 | April 30, 2021 | April 24, 2020 | ||||||||||||||||||
| Weighted-average remaining lease term | | |||||||||||||||||||
| Weighted-average discount rate | ||||||||||||||||||||
| Fiscal Year | ||||||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | |||||||||||||||||
| Operating lease cost | $ | $ | $ | |||||||||||||||||
| Short-term lease cost | ||||||||||||||||||||
| Total operating lease cost | $ | $ | $ | |||||||||||||||||
| Fiscal Year | ||||||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | |||||||||||||||||
| Cash paid for amounts included in the measurement of operating lease liabilities | $ | $ | $ | |||||||||||||||||
| Right-of-use assets obtained in exchange for operating lease liabilities | ||||||||||||||||||||
| (in millions) Fiscal Year | Operating Leases | |||||||
| 2023 | $ | |||||||
| 2024 | ||||||||
| 2025 | ||||||||
| 2026 | ||||||||
| 2027 | ||||||||
| Thereafter | ||||||||
| Total expected lease payments | ||||||||
| Less: Imputed interest | ( | |||||||
| Total lease liability | $ | |||||||
| (in millions) | Unrealized (Loss) Gain on Investment Securities | Cumulative Translation Adjustments | Net Investment Hedges | Net Change in Retirement Obligations | Unrealized (Loss) Gain on Cash Flow Hedges | Total Accumulated Other Comprehensive (Loss) Income | |||||||||||||||||||||||||||||
| April 26, 2019 | $ | ( | $ | ( | $ | ( | $ | ( | $ | $ | ( | ||||||||||||||||||||||||
| Other comprehensive income (loss) before reclassifications | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Reclassifications | ( | ( | |||||||||||||||||||||||||||||||||
| Other comprehensive income (loss) | ( | ( | ( | ||||||||||||||||||||||||||||||||
| April 24, 2020 | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Other comprehensive income (loss) before reclassifications | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Reclassifications | |||||||||||||||||||||||||||||||||||
| Other comprehensive income (loss) | ( | ( | |||||||||||||||||||||||||||||||||
| April 30, 2021 | ( | ( | ( | ( | ( | ||||||||||||||||||||||||||||||
| Other comprehensive income (loss) before reclassifications | ( | ( | |||||||||||||||||||||||||||||||||
| Reclassifications | ( | ||||||||||||||||||||||||||||||||||
| Other comprehensive income (loss) | ( | ( | |||||||||||||||||||||||||||||||||
| April 29, 2022 | $ | ( | $ | ( | $ | $ | ( | $ | $ | ( | |||||||||||||||||||||||||
| Fiscal Year | |||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | ||||||||||||||
| Cardiovascular | $ | $ | $ | ||||||||||||||
| Medical Surgical | |||||||||||||||||
| Neuroscience | |||||||||||||||||
| Diabetes | |||||||||||||||||
| Segment operating profit | |||||||||||||||||
| Interest expense | ( | ( | ( | ||||||||||||||
| Other non-operating income, net | |||||||||||||||||
| Amortization of intangible assets | ( | ( | ( | ||||||||||||||
| Corporate | ( | ( | ( | ||||||||||||||
| Centralized distribution costs | ( | ( | ( | ||||||||||||||
| Restructuring and associated costs | ( | ( | ( | ||||||||||||||
| Acquisition-related items | ( | ||||||||||||||||
| Certain litigation charges | ( | ( | ( | ||||||||||||||
| Impairment charges | ( | ||||||||||||||||
| MCS impairment / costs | ( | ||||||||||||||||
| IPR&D charges | ( | ( | ( | ||||||||||||||
| Exit of businesses | ( | ||||||||||||||||
| Debt tender premium and other charges | |||||||||||||||||
| Medical device regulations | ( | ( | ( | ||||||||||||||
| Contribution to Medtronic Foundation | ( | ||||||||||||||||
| Income before income taxes | $ | $ | $ | ||||||||||||||
| Total Assets | Depreciation Expense | ||||||||||||||||||||||||||||
| (in millions) | April 29, 2022 | April 30, 2021 | 2022 | 2021 | 2020 | ||||||||||||||||||||||||
| Cardiovascular | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Medical Surgical | |||||||||||||||||||||||||||||
| Neuroscience | |||||||||||||||||||||||||||||
| Diabetes | |||||||||||||||||||||||||||||
| Segments | |||||||||||||||||||||||||||||
| Corporate | |||||||||||||||||||||||||||||
| Total | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Net sales | Property, plant, and equipment, net | ||||||||||||||||||||||||||||
| (in millions) | 2022 | 2021 | 2020 | April 29, 2022 | April 30, 2021 | ||||||||||||||||||||||||
| Ireland | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| United States | |||||||||||||||||||||||||||||
| Rest of world | |||||||||||||||||||||||||||||
| Total other countries, excluding Ireland | |||||||||||||||||||||||||||||
| Total | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Name | Age | Position with the Company | ||||||||||||
| Geoffrey S. Martha | 52 | Chairman and Chief Executive Officer | ||||||||||||
| Ivan K. Fong | 60 | Executive Vice President, General Counsel and Corporate Secretary of the Company | ||||||||||||
| Karen L. Parkhill | 56 | Executive Vice President and Chief Financial Officer | ||||||||||||
| Carol A. Surface | 56 | Executive Vice President and Chief Human Resources Officer | ||||||||||||
| Robert ten Hoedt | 61 | Executive Vice President and President, EMEA Region, President, APAC Region | ||||||||||||
| Robert J. White | 59 | Executive Vice President and President, Medical Surgical Portfolio | ||||||||||||
| John Liddicoat, M.D. | 58 | Executive Vice President and President, Americas Region | ||||||||||||
| Sean Salmon | 57 | Executive Vice President and President, Diabetes Operating Unit, President, Cardiovascular Portfolio | ||||||||||||
| Brett Wall | 57 | Executive Vice President and President, Neuroscience Portfolio | ||||||||||||
| (a) | 1. Financial Statement Schedules | ||||
Schedule II. Valuation and Qualifying Accounts — years ended April 29, 2022, April 30, 2021, and April 24, 2020. | |||||
| Additions | Deductions | ||||||||||||||||||||||||||||
| Balance at Beginning of Fiscal Year | Charges to Income | Charges to Other Accounts | Other Changes (Debit) Credit | Balance at End of Fiscal Year | |||||||||||||||||||||||||
| Allowance for doubtful accounts: | |||||||||||||||||||||||||||||
| Fiscal year ended April 29, 2022 | $ | $ | $ | $ | ( | (a) | $ | ||||||||||||||||||||||
| Fiscal year ended April 30, 2021 | ( | (a) | |||||||||||||||||||||||||||
| Fiscal year ended April 24, 2020 | ( | (a) | |||||||||||||||||||||||||||
| Inventory reserve: | |||||||||||||||||||||||||||||
| Fiscal year ended April 29, 2022 | $ | $ | $ | $ | ( | (b) | $ | ||||||||||||||||||||||
| Fiscal year ended April 30, 2021 | ( | (b) | |||||||||||||||||||||||||||
| Fiscal year ended April 24, 2020 | ( | (b) | |||||||||||||||||||||||||||
| Deferred tax valuation allowance: | |||||||||||||||||||||||||||||
| Fiscal year ended April 29, 2022 | $ | $ | $ | ( | (e) | $ | ( | (d) | $ | ||||||||||||||||||||
| Fiscal year ended April 30, 2021 | (e) | ( | (d) | ||||||||||||||||||||||||||
| Fiscal year ended April 24, 2020 | ( | (c) | ( | (d) | |||||||||||||||||||||||||
| ( | (e) | ||||||||||||||||||||||||||||
| (a) Primarily consists of uncollectible accounts written off, less recoveries. | |||||
| (b) Primarily reflects utilization of the inventory reserve. | |||||
| (c) Reflects the impact from acquisitions and amounts recognized in accumulated other comprehensive income/loss. | |||||
| (d) Primarily reflects carryover attribute utilization and expiration. | |||||
| (e) Primarily reflects the effects of currency fluctuations. | |||||
| All other schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto. | |||||||||||||||||
| 2. Exhibits | |||||||||||||||||
| Exhibit No. | Description | ||||||||||
| 3.1 | |||||||||||
| 3.2 | |||||||||||
| 4.1 | |||||||||||
| 4.2 | |||||||||||
| 4.3 | |||||||||||
| 4.4 | |||||||||||
| 4.5 | |||||||||||
| 4.6 | |||||||||||
| 4.7 | |||||||||||
| 4.8 | |||||||||||
| 4.9 | |||||||||||
| 4.10 | |||||||||||
| 4.11 | |||||||||||
| 4.12 | |||||||||||
| 4.13 | |||||||||||
| 4.14 | |||||||||||
| 4.15 | |||||||||||
| 4.16 | |||||||||||
| 4.17 | |||||||||||
| 4.18 | |||||||||||
| 4.19 | |||||||||||
| 4.20 | |||||||||||
| 4.21 | |||||||||||
| 4.22 | |||||||||||
| 4.23 | |||||||||||
| 4.24 | |||||||||||
| #4.25 | |||||||||||
| 10.1 | |||||||||||
| 10.2 | |||||||||||
| 10.3 | |||||||||||
| 10.4 | |||||||||||
| 10.5 | |||||||||||
| *10.6 | |||||||||||
| *10.7 | |||||||||||
| *10.8 | |||||||||||
| *10.9 | |||||||||||
| *10.10 | |||||||||||
| *10.11 | |||||||||||
| *10.12 | |||||||||||
| *10.13 | |||||||||||
| *10.14 | |||||||||||
| *10.15 | |||||||||||
| *10.16 | |||||||||||
| *10.17 | |||||||||||
| *10.18 | |||||||||||
| *10.19 | |||||||||||
| *10.20 | |||||||||||
| *10.21 | |||||||||||
| *10.22 | |||||||||||
| *10.23 | |||||||||||
| *10.24 | |||||||||||
| *10.25 | |||||||||||
| *10.26 | |||||||||||
| *10.27 | |||||||||||
| *10.28 | |||||||||||
| *10.29 | |||||||||||
| *10.30 | |||||||||||
| *10.31 | |||||||||||
| *10.32 | |||||||||||
| *10.33 | |||||||||||
| *10.34 | |||||||||||
| *10.35 | |||||||||||
| *10.36 | |||||||||||
| *10.37 | |||||||||||
| *10.38 | |||||||||||
| *10.39 | |||||||||||
| *10.40 | |||||||||||
| *10.41 | |||||||||||
| *10.42 | |||||||||||
| *10.43 | |||||||||||
| *10.44 | |||||||||||
| *10.45 | |||||||||||
| *10.46 | |||||||||||
| *10.47 | |||||||||||
| *10.48 | |||||||||||
| *10.49 | |||||||||||
| *10.50 | |||||||||||
| *10.51 | |||||||||||
| *10.52 | |||||||||||
| *10.53 | |||||||||||
| *10.54 | |||||||||||
| *10.55 | |||||||||||
| *10.56 | |||||||||||
| *10.57 | |||||||||||
| *10.58 | |||||||||||
| *10.59 | |||||||||||
| *10.60 | |||||||||||
| *10.61 | |||||||||||
| *10.62 | |||||||||||
| *10.63 | |||||||||||
| *10.64 | |||||||||||
| *10.65 | |||||||||||
| *10.66 | |||||||||||
| *10.67 | |||||||||||
| *10.68 | |||||||||||
| *10.69 | |||||||||||
| *10.70 | |||||||||||
| *10.71 | |||||||||||
| *10.72 | |||||||||||
| *10.73 | |||||||||||
| *10.74 | |||||||||||
| *10.75 | |||||||||||
| 10.76 | |||||||||||
| *10.77 | |||||||||||
| *10.78 | |||||||||||
| *10.79 | |||||||||||
| *10.80 | |||||||||||
| *10.81 | |||||||||||
| *10.82 | |||||||||||
| #21 | |||||||||||
| #22 | |||||||||||
| #23 | |||||||||||
| #24 | |||||||||||
| #31.1 | |||||||||||
| #31.2 | |||||||||||
| #32.1 | |||||||||||
| #32.2 | |||||||||||
| #101.SCH | XBRL Taxonomy Extension Schema Document | ||||||||||
| #101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | ||||||||||
| #101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | ||||||||||
| #101.LAB | XBRL Taxonomy Extension Label Linkbase Document | ||||||||||
| #101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | ||||||||||
| #104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | ||||||||||
| *Exhibits that are management contracts or compensatory plans or arrangements. | ||||||||
| #Filed herewith | ||||||||
| Medtronic plc | ||||||||
| Dated: June 23, 2022 | By: | /s/ Geoffrey S. Martha | ||||||
| Geoffrey S. Martha | ||||||||
| Chairman and Chief Executive Officer | ||||||||
| Medtronic plc | ||||||||
| Dated: June 23, 2022 | By: | /s/ Geoffrey S. Martha | ||||||
| Geoffrey S. Martha | ||||||||
| Chairman and Chief Executive Officer | ||||||||
| (Principal Executive Officer) | ||||||||
| Dated: June 23, 2022 | By: | /s/ Karen L. Parkhill | ||||||
| Karen L. Parkhill | ||||||||
| Executive Vice President and Chief Financial Officer | ||||||||
| (Principal Financial Officer) | ||||||||
| Dated: June 23, 2022 | By: | /s/ Jennifer M. Kirk | ||||||
| Jennifer M. Kirk | ||||||||
| Global Controller and Chief Accounting Officer | ||||||||
| (Principal Accounting Officer) | ||||||||
| Directors | ||||||||
| Richard H. Anderson* | ||||||||
| Craig Arnold* | ||||||||
| Scott C. Donnelly* | ||||||||
| Andrea J. Goldsmith, PH.D.* | ||||||||
| Randall J. Hogan,* | ||||||||
| Kevin E. Lofton* | ||||||||
| Geoffrey S. Martha | ||||||||
| Elizabeth G. Nabel, M.D.* | ||||||||
| Denise M. O’Leary* | ||||||||
| Kendall J. Powell* | ||||||||
| Dated: June 23, 2022 | By: | /s/ Ivan K. Fong | ||||||
| Ivan K. Fong | ||||||||
| (a) | amending the objects or memorandum of association of Medtronic; | |||||||
| (b) | amending the Articles of Association; | |||||||
| (c) | approving a change of name of Medtronic; | |||||||
| (a) | a court-approved scheme of arrangement under the Irish Companies Act. A scheme of arrangement with shareholders requires a court order from the Irish High Court and the approval of a majority in number representing 75% in value of each class of shareholder present and voting in person or by proxy at a meeting called to approve the scheme; | |||||||
| (b) | through a tender or takeover offer by a third party for all of the shares of Medtronic. Where the holders of 80% or more of Medtronic’s shares have accepted an offer for their shares in Medtronic, the remaining shareholders may also be statutorily required to transfer their shares. If the bidder does not exercise its “squeeze out” right, then the non-accepting shareholders also have a statutory right to require the bidder to acquire their shares on the same terms. If shares of Medtronic were to be listed on the Irish Stock Exchange or another regulated stock exchange in the European Union, the “squeeze out” threshold would be increased to 90%; and | |||||||
| (c) | by way of a merger with a company incorporated in the European Economic Area (“EEA”) under the EU Cross-Border Mergers Directive (EU) 2017/1132 or with another Irish company under the Irish Companies Act. Such a merger must be approved by a special resolution. Shareholders also may be entitled to have their shares acquired for cash. See the section entitled “Description of Share Capital—Appraisal Rights” | |||||||
| (a) | in the event of an offer, all holders of securities of the target company should be afforded equivalent treatment and, if a person acquires control of a company, the other holders of securities must be protected; | |||||||
| (b) | the holders of the securities of the target company must have sufficient time and information to enable them to reach a properly informed decision on the offer; where it advises the holders of securities, the board of the target company must give its views on the effects of implementation of the offer on employment, conditions of employment and the locations of the target company’s places of business; | |||||||
| (c) | the board of the target company must act in the interests of the company as a whole and must not deny the holders of securities the opportunity to decide on the merits of the offer; | |||||||
| (d) | false markets must not be created in the securities of the target company, the bidder or of any other company concerned by the offer in such a way that the rise or fall of the prices of the securities becomes artificial and the normal functioning of the markets is distorted; | |||||||
| (e) | a bidder must announce an offer only after ensuring that he or she can fulfill in full, any cash consideration, if such is offered, and after taking all reasonable measures to secure the implementation of any other type of consideration; | |||||||
| (f) | a target company must not be hindered in the conduct of its affairs for longer than is reasonable by an offer for its securities; and | |||||||
| (g) | a substantial acquisition of securities (whether such acquisition is to be effected by one transaction or a series of transactions) shall take place only at an acceptable speed and shall be subject to adequate and timely disclosure. | |||||||
| (a) | the action is approved by Medtronic’s shareholders at a general meeting; or | ||||||||||
| (b) | the Irish Takeover Panel has given its consent, where: | ||||||||||
| (i) | it is satisfied the action would not constitute frustrating action; | ||||||||||
| (ii) | Medtronic shareholders that hold 50% of the voting rights state in writing that they approve the proposed action and would vote in favor of it at a general meeting; | ||||||||||
| (iii) | the action is taken in accordance with a contract entered into prior to the announcement of the offer; or | ||||||||||
| (iv) | the decision to take such action was made before the announcement of the offer and either has been at least partially implemented or is in the ordinary course of business. | ||||||||||
| Series | Maturity | Interest Rate | Interest Payment Dates | Record Dates | ||||||||||
| 2022 notes | December 2, 2022 | 0.00% | December 2 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 0.375% 2023 notes | March 7, 2023 | 0.375% | March 7 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 0.000% 2023 notes | March 15, 2023 | 0.00% | March 15 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 0.25% 2025 notes | July 2, 2025 | 0.250% | July 2 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 0.000% 2025 notes | October 15, 2025 | 0.00% | October 15 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 2027 notes | March 7, 2027 | 1.125% | March 7 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 2028 notes | October 15, 2025 | 0.375% | October 15 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 1.625% 2031 notes | March 7, 2031 | 1.625% | March 7 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 1.000% 2031 notes | July 2, 2031 | 1.000% | July 2 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 2032 notes | October 15, 2032 | 0.750% | October 15 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 2.250% 2039 notes | March 7, 2039 | 2.250% | March 7 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 1.500% 2039 notes | July 2, 2039 | 1.500% | July 2 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 2040 notes | October 15, 2040 | 1.375% | October 15 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 2049 notes | July 2, 2049 | 1.750% | July 2 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| 2050 notes | October 15, 2050 | 1.625% | October 15 | Close of business on the business day immediately preceding the interest payment date. | ||||||||||
| • | 100% of the principal amount of the fixed rate notes of the applicable series being redeemed; and | |||||||
| • | the sum, as determined by a Quotation Agent (defined below), of the present values of the Remaining Scheduled Payments (as defined below) of principal and interest on the notes of such series to be redeemed (excluding any portion of such payments of interest accrued as of the date of redemption and assuming, in the case of the 2022 notes, the 0.375% 2023 notes, the 0.25% 2025 notes, the 0.000% 2025 notes, the 2027 notes, the 2028 notes, the 1.625% 2031 notes, the 1.000% 2031 notes, the 2032 notes, the 2.250% 2039 notes, the 1.500% 2039 notes, the 2040 notes, the 2049 notes, and the 2050 notes, that such notes matured on the applicable Par Call Date), discounted to the redemption date on an annual basis (ACTUAL/ACTUAL(ICMA)) at the Comparable Bond Rate (defined below), plus 15 basis points, in the case of the 2022 notes, the 0.375 2023 notes, the 0.000% 2023 notes, the 0.25% 2025 notes, and the 0.000% 2025 notes, 20 basis points, in the case of the 2027 notes, the 2028 notes, the 1.000% 2031 notes, and the 2032 notes, 25 basis points, in the case of the 1.625% 2031 notes, the 1.500% 2039 notes and the 2049 notes, and 30 basis points, in the case of the 2.250% 2039 notes the 2040 notes, and the 2050 notes; | |||||||
| • | failure to pay any interest on the notes of that series when due and payable and such failure continues for 30 days; | |||||||
| • | failure to pay principal of or any premium on the notes of that series at its maturity, acceleration, redemption or otherwise; | |||||||
| • | failure to perform or the breach of any other covenant or warranty in the Indenture applicable to such series and such failure continues for 60 days after written notice as provided in such Indenture; | |||||||
| • | failure to pay principal when due at maturity or a default that results in the acceleration of maturity of Medtronic plc’s or any Restricted Subsidiary’s (defined below) indebtedness for borrowed money in an aggregate amount of $150 million or more; | |||||||
| • | Medtronic plc’s or Medtronic, Inc.’s guarantee ceases to be in full force and effect or is declared to be null and void and unenforceable or such guarantee is found to be invalid or Medtronic plc or Medtronic, Inc. denies its liability under its guarantee (other than by reason of release of a Guarantor in accordance with the terms of the Indenture); | |||||||
| • | certain events in bankruptcy, insolvency, examinership or reorganization, voluntary or involuntary, relating to Medtronic Luxco, Medtronic or Medtronic, Inc.; and | |||||||
| • | any other event of default provided with respect to notes of such series. | |||||||
| • | to evidence the succession of another person to us or any guarantor and the assumption by any such successor of our or such Guarantor’s covenants under the Indenture and in the notes; | |||||||
| • | to add to our covenants or the covenants applicable to any guarantor for the benefit of the holders or to surrender any right or power in the Indenture conferred upon us or any Guarantor; | |||||||
| • | to add any additional events of default for the benefit of the holders; | |||||||
| • | to secure the notes or any related guarantee; | |||||||
| • | to evidence and provide for the acceptance of appointment hereunder by a successor trustee; | |||||||
| • | to cure any ambiguity, to correct or supplement any provision in the Indenture or in any supplemental indenture which may be inconsistent with any other provision of such indenture or supplemental indenture, or to make any other provisions with respect to matters or questions arising under the Indenture; provided such action shall not adversely affect the interests of the holders in any material respect; | |||||||
| • | to conform the Indenture or any supplemental indenture to the description of the notes set forth in any prospectus or prospectus supplement related to such series of notes; | |||||||
| • | to comply with the requirements of the SEC in order to effect or maintain the qualifications of the indenture under the Trust Indenture Act of 1939, as amended (the “Trust Indenture Act”); | |||||||
| • | to add to or change any of the provisions of the Indenture to such extent as shall be necessary to permit or facilitate the issuance of notes in bearer form or to facilitate the issuance of notes in uncertificated form; | |||||||
| • | to provide for the issuance and establish the forms or terms and conditions of notes of any series as permitted by the Indenture; | |||||||
| • | to add or release a Guarantor as permitted by the Indenture; or | |||||||
| • | to comply with the rules of any applicable securities depositary. | |||||||
| (1) | the common depositary notifies us that it is unwilling, unable or no longer qualified to continue as depositary for the global notes and we fail to appoint a successor depositary within 90 calendar days; | ||||
| (2) | Medtronic Luxco, at its option, notifies the trustee in writing that it elects to cause the issuance of certificated notes; or | ||||
| (3) | there has occurred and is continuing an Event of Default with respect to the notes. | ||||
Medtronic plc and Subsidiaries | ||||||||||||||
At April 29, 2022 | ||||||||||||||
| Company | Jurisdiction of Formation | ||||
| 2074417 Alberta ULC | Canada | ||||
| A&E Products de Honduras S.A. | Honduras | ||||
| A&E Products do Brasil Ltda. | Brazil | ||||
| A&E Products Group, Inc. | Delaware | ||||
| Advanced Medical Technologies GmbH | Germany | ||||
| AI Biomed Corp | California | ||||
| Aircraft Medical Ltd. | United Kingdom | ||||
| Airox | France | ||||
| Airox, Inc. | Delaware | ||||
| Arterial Vascular Engineering Canada, Company | Canada | ||||
| Arterial Vascular Engineering UK Limited | United Kingdom | ||||
| Auto Suture do Brasil Ltda. | Brazil | ||||
| Auto Suture Holdings Pty Ltd | Australia | ||||
| AV Medical Technologies Ltd. | Israel | ||||
| Avenu Medical, Inc. | Delaware | ||||
| Batts LLC | Delaware | ||||
| Batts, Inc. | Delaware | ||||
| Beacon Endoscopic LLC | Delaware | ||||
| Bellco Do Brasil | Brazil | ||||
| Bellco Hoxen Medical (Hong Kong) Co. Limited | Hong Kong | ||||
| Bellco Hoxen Medical (Shanghai) Co. Ltd. | China | ||||
| Bellco S.r.l. | Italy | ||||
| Between Investeringsgroep B.V. | Netherlands | ||||
| Biostar Biomedikal Mühendislik Anonim Sirketi | Turkey | ||||
| Bo Yao (Shanghai) Medical Device Co. Ltd. | China | ||||
| CardioInsight Technologies Inc. | Delaware | ||||
| Carlisle Philippines, Inc. | Philippines | ||||
| Carmel Biosensors Ltd. | Israel | ||||
| CCI Istanbul Teknolojik Hizmetler Limited Sirketi | Turkey | ||||
| Changzhou Kangdi Medical Stapler Co., Ltd. | China | ||||
| Changzhou Kanghui Medical Innovation Co., Ltd. | China | ||||
| CircuLite, Inc. | Delaware | ||||
| Clearum GmbH | Germany | ||||
| Comercial Kendall (Chile) Limitada | Chile | ||||
| Companion Medical, Inc. | Delaware | ||||
| Corporativo Dayton, S. de R.L. de C.V. | Mexico | ||||
| Covidien (China) Medical Devices Technology Co., Ltd. | China | ||||
| Covidien (Gibraltar) Limited | Gibraltar | ||||
| Covidien (Shanghai) Management Consulting Co., Ltd. | China | ||||
| Covidien Adhesives Italia Srl | Italy | ||||
| Covidien AG | Switzerland | ||||
| Covidien Argentina S.A. | Argentina | ||||
| Covidien Australia Pty Ltd | Australia | ||||
| Covidien Canada Holdings LLC | Delaware | ||||
| Covidien Caribbean, Inc. | Delaware | ||||
| Covidien Delaware VI Corp. | Delaware | ||||
| Covidien Deutschland GmbH | Germany | ||||
| Covidien Eurasia LLC | Russia | ||||
| Covidien France Holdings, Inc. | Connecticut | ||||
| Covidien Group Holdings Limited | Bermuda | ||||
| Covidien Group S.à.r.l. | Luxembourg | ||||
| Covidien Healthcare International Trading (Shanghai) Co., Ltd. | China | ||||
| Covidien Holding Inc. | Delaware | ||||
| Covidien Holdings International Corporation | Delaware | ||||
| Covidien Holdings Ireland Limited | Ireland | ||||
| Covidien Holdings S.à.r.l. | Luxembourg | ||||
| Covidien International (US) Holdings A, LLC | Delaware | ||||
| Covidien International Finance S.A. | Luxembourg | ||||
| Covidien International S.à.r.l. | Luxembourg | ||||
| Covidien Israel Holdings Ltd | Israel | ||||
| Covidien Israel Investments Ltd | Israel | ||||
| Covidien Israel Surgical Research Ltd | Israel | ||||
| Covidien Japan, Inc. | Japan | ||||
| Covidien Unlimited Company | Ireland | ||||
| Covidien llc | Delaware | ||||
| Covidien LP | Delaware | ||||
| Covidien Manufacturing Grenoble | France | ||||
| Covidien Medical Products (Shanghai) Manufacturing L.L.C. | China | ||||
| Covidien Peru S.A. | Peru | ||||
| Covidien Philippines, Inc. | Philippines | ||||
| Covidien Private Limited | Singapore | ||||
| Covidien Pty Limited | Australia | ||||
| Covidien Sales LLC | Delaware | ||||
| Covidien Services Europe Limited | Ireland | ||||
| Covidien Swiss Holding GmbH | Switzerland | ||||
| Covidien UK Holding Ltd | United Kingdom | ||||
| Covidien Uruguay S.A. | Uruguay | ||||
| Covidien US Holdings, Inc. | Delaware | ||||
| Covidien Ventures Ltd. | Bermuda | ||||
| Davis & Geck Caribe Limited | Cayman Islands | ||||
| Diabeter Nederland B.V. | Netherlands | ||||
| Digital Surgery Limited | United Kingdom | ||||
| Epix Therapeutics, Inc. | Delaware | ||||
| ev3 Australia Pty Limited | Australia | ||||
| Ev3, Inc. | Delaware | ||||
| First Lafayette Holdings LLC | Delaware | ||||
| Floreane Medical Implants | France | ||||
| GC Holdings, Inc. | Delaware | ||||
| Georgia Packaging, LLC | Delaware | ||||
| Given Imaging (Los Angeles) LLC | California | ||||
| Given Imaging B.V. | Netherlands | ||||
| Given Imaging Ltd. | Israel | ||||
| Given Imaging Pty Limited | Australia | ||||
| Given Imaging Vietnam Co., Ltd. | Vietnam | ||||
| Given Imaging, Inc. | Delaware | ||||
| Graphic Controls (Barbados), Ltd. | Barbados | ||||
| Haemopharm Biofluids S.r.l. | Italy | ||||
| HeartWare InternationalLLC | Delaware | ||||
| HeartWare, Inc. | Delaware | ||||
| HET Systems, LLC | Delaware | ||||
| IHS LLC | Russia | ||||
| IHS Argentina SA | Argentina | ||||
| IHS Health Services Egypt LLC | Egypt | ||||
| IHS Health Services Pakistan (Private) Limited | Pakistan | ||||
| IHS Health Services Lebanon Sarl | Lebanon | ||||
| IHS Managed Services SAS | Colombia | ||||
| IHS Saglik Hizmetleri Ltd STI | Turkey | ||||
| India Medtronic Private Limited | India | ||||
| Integrated Health Solutions Chile S.A. | Chile | ||||
| Integrated Health Solutions International Sàrl | Switzerland | ||||
| Integrated Health Solutions Pty Ltd | Australia | ||||
| Invatec S.p.A. | Italy | ||||
| Invatec Technology Center GmbH | Switzerland | ||||
| Kendall de Mexico, S.A. de C.V. | Mexico | ||||
| Kendall de Venezuela, C.A. | Venezuela | ||||
| Kendall Innovadores en Cuidados al Paciente S.A. | Costa Rica | ||||
| KLHC, Inc. | Delaware | ||||
| Kendall S.A. | Panama | ||||
| KMS Colon, Panama, S.A. | Panama | ||||
| KMS Montevideo, Uruguay, S.A. | Uruguay | ||||
| Klue, Inc. | Delaware | ||||
| Kyphon South Africa (Proprietary) Ltd. | South Africa | ||||
| Laboratoire Soludia SAS | France | ||||
| Life Design Systems, Inc. | Wisconsin | ||||
| M-Smart Electronic Equipment Trading (Shanghai) Co. Ltd. | China | ||||
| Makani II Unlimited Company | Ireland | ||||
| Mallinckrodt DAR Srl | Italy | ||||
| Mallinckrodt Holdings B.V. | Netherlands | ||||
| Mallinckrodt Holdings, LLC | Delaware | ||||
| Mallinckrodt Medical Unlimited Company | Ireland | ||||
| Mallinckrodt US LLC | Delaware | ||||
| Marblehead Medical LLC | Minnesota | ||||
| Mazor Robotics Ltd. | Israel | ||||
| MDT Hungária Kereskedelmi Korlátolt Felelősségű Társaság | Hungary | ||||
| MDT Turkey Finansal Danışmanlık Limited Şirketi | Turkey | ||||
| Medefield Pty Limited | Australia | ||||
| Medical Education Y. K. | Japan | ||||
| Medical Medtronic Nigeria Limited | Nigeria | ||||
| Medicrea Australia Pty Ltd. | Australia | ||||
| Medicrea International | France | ||||
| Medicrea Technologies UK Limited | United Kingdom | ||||
| Medicrea USA, Corp | Delaware | ||||
| Medina Medical LLC | Delaware | ||||
| Medina Medical, Inc. | Delaware | ||||
| Medinse S. de R.L. de C.V. | Mexico | ||||
| Medtronic – Sequoia (Cayman) Innovation Investment Management Partners, Ltd | Cayman Islands | ||||
| Medtronic (Africa) (Proprietary) Limited | South Africa | ||||
| Medtronic (Changzhou) Medical Devices Technology Co., Ltd. | China | ||||
| Medtronic (Chengdu) Management Consulting Co., Ltd. | China | ||||
| Medtronic (Schweiz) A.G. (Medtronic (Suisse) S.A.) | Switzerland | ||||
| Medtronic (Shanghai) Ltd. | China | ||||
| Medtronic (Shanghai) Management Co. Ltd. | China | ||||
| Medtronic (Taiwan) Ltd. | Taiwan | ||||
| Medtronic (Thailand) Limited | Thailand | ||||
| Medtronic Ablation Frontiers LLC | Delaware | ||||
| Medtronic Adriatic d.o.o. | Croatia | ||||
| Medtronic Advanced Energy LLC | Delaware | ||||
| Medtronic Advanced Energy Luxembourg S.à.r.l. | Luxembourg | ||||
| Medtronic AF Luxembourg S.à r.l. | Luxembourg | ||||
| Medtronic AG | Switzerland | ||||
| Medtronic Aktiebolag | Sweden | ||||
| Medtronic Angiolink, Inc. | Delaware | ||||
| Medtronic Ardian LLC | Delaware | ||||
| Medtronic Ardian Luxembourg S.à.r.l. | Luxembourg | ||||
| Medtronic ATS Medical, Inc. | Minnesota | ||||
| Medtronic Australasia Pty Ltd | Australia | ||||
| Medtronic B.V. | Netherlands | ||||
| Medtronic Bakken Research Center B.V. | Netherlands | ||||
| Medtronic Bangladesh Pvt. Ltd. | Bangladesh | ||||
| Medtronic Belgium S.A./N.V. | Belgium | ||||
| Medtronic BioPharma B.V. | Netherlands | ||||
| Medtronic BioPharma Sàrl | Switzerland | ||||
| Medtronic Bulgaria EOOD | Bulgaria | ||||
| Medtronic Canada ULC | Canada | ||||
| Medtronic Care Management Services, LLC | Minnesota | ||||
| Medtronic Cash Pool LLC | Massachusetts | ||||
| Medtronic Changzhou Medical Device Co., Ltd. | China | ||||
| Medtronic China Kanghui Holdings | Cayman Islands | ||||
| Medtronic China Venture Fund (Cayman), L.P. | Cayman Islands | ||||
| Medtronic China, LLC. | Minnesota | ||||
| Medtronic Colombia S.A. | Colombia | ||||
| Medtronic Comercial Ltda. | Brazil | ||||
| Medtronic Communities Foundation | Minnesota | ||||
| Medtronic CoreValve LLC | Delaware | ||||
| Medtronic CryoCath LP | Canada | ||||
| Medtronic CV Luxembourg S.à.r.l. | Luxembourg | ||||
| Medtronic Czechia s.r.o. | Czech Republic | ||||
| Medtronic Danmark A/S | Denmark | ||||
| Medtronic Diabetes (Chengdu) Co., Ltd. | China | ||||
| Medtronic do Brasil Ltda. | Brazil | ||||
| Medtronic Dominican Republic S.A.S. | Dominican Republic | ||||
| Medtronic Dominicana (Manufactura), S.A. | Dominican Republic | ||||
| Medtronic Egypt LLC | Egypt | ||||
| Medtronic Empalme S. de R.L. de C.V. | Mexico | ||||
| Medtronic Engineering and Innovation Center Private Limited | India | ||||
| Medtronic EuropeLimited | Malta | ||||
| Medtronic Europe Sàrl | Switzerland | ||||
| Medtronic Fabrication | France | ||||
| Medtronic Finance Holdings ULC | Cayman Islands | ||||
| Medtronic Finance Hungary Kft. | Hungary | ||||
| Medtronic Finland Oy | Finland | ||||
| Medtronic France | France | ||||
| Medtronic GmbH | Germany | ||||
| Medtronic Global Health Foundation | Minnesota | ||||
| Medtronic Global Holdings GP S.à r.l. | Luxembourg | ||||
| Medtronic Global Holdings S.C.A. | Luxembourg | ||||
| Medtronic Group Holding, Inc. | Minnesota | ||||
| Medtronic Hellas Medical Devicea Commercial Single Member S.A. | Greece | ||||
| Medtronic Holding B.V. | Netherlands | ||||
| Medtronic Holding Company Sàrl | Switzerland | ||||
| Medtronic Holding Hungary Kft. | Hungary | ||||
| Medtronic Holding Switzerland G.m.b.H. | Switzerland | ||||
| Medtronic Holding, Inc. | Minnesota | ||||
| Medtronic Holdings France | France | ||||
| Medtronic Holdings Sàrl | Luxembourg | ||||
| Medtronic Hong Kong Medical Limited | Hong Kong | ||||
| Medtronic Hungaria Kereskedelmi Kft | Hungary | ||||
| Medtronic Ibérica S.A. | Spain | ||||
| Medtronic Innovation Center (Israel) Ltd | Israel | ||||
| Medtronic Integrated Health Solutions LLC | Minnesota | ||||
| Medtronic International Holding LLC | Minnesota | ||||
| Medtronic International Investment LLC | Minnesota | ||||
| Medtronic International Technology, Inc. | Minnesota | ||||
| Medtronic International Trading Holding LLC | Delaware | ||||
| Medtronic International Trading Pte. Ltd. | Singapore | ||||
| Medtronic International Trading Sàrl | Switzerland | ||||
| Medtronic International Trading, Inc. | Minnesota | ||||
| Medtronic International, Ltd. | Delaware | ||||
| Medtronic Interventional Vascular, Inc. | Massachusetts | ||||
| Medtronic Invatec LLC | Delaware | ||||
| Medtronic IP Holding International Luxembourg S.à.r.l. | Luxembourg | ||||
| Medtronic Ireland Limited | Ireland | ||||
| Medtronic Ireland Manufacturing Unlimited Company | Ireland | ||||
| Medtronic Italia S.p.A. | Italy | ||||
| Medtronic Japan Co., Ltd. | Japan | ||||
| Medtronic Jolife LLC | Delaware | ||||
| Medtronic Kazakhstan Limited Liability Partnership | Kazakhstan | ||||
| Medtronic KL Holdings LLC | Minnesota | ||||
| Medtronic Korea Ltd. | South Korea | ||||
| Medtronic Labs PBC | Delaware | ||||
| Medtronic Lateral, Inc. | Delaware | ||||
| Medtronic Latin America, Inc. | Minnesota | ||||
| Medtronic Limited | United Kingdom | ||||
| Medtronic LLC | Russia | ||||
| Medtronic Logistics LLC | Minnesota | ||||
| Medtronic Luxembourg Global Holdings S.à r.l. | Luxembourg | ||||
| Medtronic Malaysia Sdn. Bhd. | Malaysia | ||||
| Medtronic Medical CR S de RL | Costa Rica | ||||
| Medtronic Medical Device (Chengdu) Co., Ltd. | China | ||||
| Medtronic Medikal Teknoloji Ticaret Limited Sirketi | Turkey | ||||
| Medtronic Mediterranean Offshore SAL | Lebanon | ||||
| Medtronic META FZ-LLC | United Arab Emirates | ||||
| Medtronic Mexico S. de R.L. de C.V. | Mexico | ||||
| Medtronic MiniMed, Inc. | Delaware | ||||
| Medtronic Mlab Management Co., Ltd | China | ||||
| Medtronic Monitoring, Inc. | Delaware | ||||
| Medtronic Navigation Israel Ltd. | Israel | ||||
| Medtronic Navigation, Inc. | Delaware | ||||
| Medtronic New Zealand Limited | New Zealand | ||||
| Medtronic Norge AS | Norway | ||||
| Medtronic Oesterreich G.m.b.H. | Austria | ||||
| Medtronic Pacific Trading, Inc. | Minnesota | ||||
| Medtronic Pakistan (Private) Limited | Pakistan | ||||
| Medtronic Philippines, Inc. | Philippines | ||||
| Medtronic Poland Sp. z o.o. | Poland | ||||
| Medtronic Poland Finance Sp.z.o.o. | Poland | ||||
| Medtronic Portugal, Lda | Portugal | ||||
| Medtronic PS Medical, Inc. | California | ||||
| Medtronic Puerto Rico Operations Co. | Cayman Islands | ||||
| Medtronic RCS Holding GmbH | Switzerland | ||||
| Medtronic Romania SRL | Romania | ||||
| Medtronic S. de R.L. de C.V. | Mexico | ||||
| Medtronic S.A.I.C. | Argentina | ||||
| Medtronic Saudi Arabia Company | Saudi Arabia | ||||
| Medtronic Servicios S. de R.L. de C.V. | Mexico | ||||
| Medtronic SG, LLC | Delaware | ||||
| Medtronic Shared Services Americas S.A.S. | Colombia | ||||
| Medtronic Shared Services SRL | Costa Rica | ||||
| Medtronic Singapore Operations Pte. Ltd. | Singapore | ||||
| Medtronic Slovakia s.r.o. | Slovakia | ||||
| Medtronic Sofamor Danek Co., Ltd. | Japan | ||||
| Medtronic Sofamor Danek Deggendorf GmbH | Germany | ||||
| Medtronic Sofamor Danek South Africa (Proprietary) Limited | South Africa | ||||
| Medtronic Sofamor Danek USA, Inc. | Tennessee | ||||
| Medtronic Sofamor Danek, Inc. | Indiana | ||||
| Medtronic Srbija d.o.o. Beograd-Novi Beograd | Serbia | ||||
| Medtronic Sweden Finance AB | Sweden | ||||
| Medtronic Trading Ltd. | Israel | ||||
| Medtronic Trading NL BV | Netherlands | ||||
| Medtronic Ukraine Limited Liability Company | Ukraine | ||||
| Medtronic USA, Inc. | Minnesota | ||||
| Medtronic Vascular Galway Unlimited Company | Ireland | ||||
| Medtronic Vascular Holdings Unlimited Company | Ireland | ||||
| Medtronic Vascular, Inc. | Delaware | ||||
| Medtronic Ventor Technologies Ltd. | Israel | ||||
| Medtronic Vietnam Company Limited | Vietnam | ||||
| Medtronic VT, LLC | Delaware | ||||
| Medtronic World Trade Corporation | Minnesota | ||||
| Medtronic Xomed, Inc. | Delaware | ||||
| Medtronic Xomed LLC | Delaware | ||||
| Medtronic, Inc. | Minnesota | ||||
| Medtronic, trgovina z medicinsko tehnologijo in opremo d.o.o. | Slovenia | ||||
| Micro Therapeutics, Inc. | Maryland | ||||
| MiniMed Distribution Corp. | Delaware | ||||
| MiniMed Pty Ltd | Australia | ||||
| MMJ, S.A. de C.V. | Mexico | ||||
| MSCH LLC | Delaware | ||||
| N.G.C. Medical Srl | Italy | ||||
| NayaMed International Sàrl | Switzerland | ||||
| Nederelandse Obesitas Kliniek Zuid B.V. | Netherlands | ||||
| N.O.K. Nederlandse Obesitas Kliniek B.V. | Netherlands | ||||
| Nederlandse Obesitas Kliniek West B.V. | Netherlands | ||||
| Nederlandse Obesitas kliniek Zeeland B.V. | Netherlands | ||||
| Nellcor Puritan Bennett Ireland Holdings Unlimited Company | Ireland | ||||
| Nellcor Puritan Bennett Ireland Unlimited Company | Ireland | ||||
| Nellcor Puritan Bennett LLC | Delaware | ||||
| Nellcor Puritan Bennett Mexico, S.A. de C.V. | Mexico | ||||
| New Wave Surgical, LLC | Delaware | ||||
| NeuroRadial Technologies, Inc. | Delaware | ||||
| Nurtino Health Ltd | Israel | ||||
| Obesitas International B.V. | Netherlands | ||||
| Obesitas Nederland B.V. | Netherlands | ||||
| Old Colony State Insurance Company | Vermont | ||||
| Oridion Capnography, Inc. | California | ||||
| Oridion Medical 1987 Ltd. | Israel | ||||
| Oridion Systems Ltd. | Israel | ||||
| Osteotech, Inc. | Delaware | ||||
| Panmedica Pty Limited | Australia | ||||
| Plastics Holding Corporation | Nevada | ||||
| Polyken Technologies Europe, Inc. | Delaware | ||||
| Polysuture Industria e Comercio Ltda. | Brazil | ||||
| PT. Covidien Indonesia | Indonesia | ||||
| PT Medtronic Indonesia | Indonesia | ||||
| PTB International LLC | Delaware | ||||
| Quro Obesity Center Poly Clinic LLC | United Arab Emirates | ||||
| Raychem Tecnologias, S. de R.L. de C.V. | Mexico | ||||
| RCS Japan G.K. | Japan | ||||
| Renal Care Solutions Ireland Limited | Ireland | ||||
| Retail Group de Mexico S.A. de C.V. | Mexico | ||||
| Reverse Medical LLC | Delaware | ||||
| RF Surgical Systems LLC | Minnesota | ||||
| RIST Neurovascular, Inc. | Delaware | ||||
| Sanatis GmbH | Germany | ||||
| Sapheon LLC | California | ||||
| Setagon, Inc. | Delaware | ||||
| Shanghai Medtronic Zhikang Medical Devices Co., Ltd. | China | ||||
| Shanghai MyanCor Medical Ltd | China | ||||
| Sherwood Medical Company I | Delaware | ||||
| Sherwood Medical Industries Pty Ltd | Australia | ||||
| Societe De Fabrication de Material Orthopedique En Abrege Sofamor | France | ||||
| Sofradim Production | France | ||||
| SonarMed Inc. | Delaware | ||||
| Sophono, Inc. | Colorado | ||||
| SpinalGraft Technologies, LLC | Tennessee | ||||
| superDimension, Inc. | Delaware | ||||
| Suzhou Medtronic Venture Capital Partnership Enterprise (L.P.) | China | ||||
| Suzhou Medtronic-Sequoia Innovation Investment Management Co., Ltd. | China | ||||
| Suzhou Mei Zhong Capital Investment Management Co., Ltd. | China | ||||
| THC Holdings Limited | Thailand | ||||
| Titan Spine, Inc. | Delaware | ||||
| Tissue Science Laboratories Limited | United Kingdom | ||||
| Twelve Australia Pty Ltd | Australia | ||||
| Twelve, Inc. | Delaware | ||||
| U.S.S.C. Puerto Rico (NY), Inc. | New York | ||||
| U.S.S.C. Puerto Rico, Inc. | Cayman Islands | ||||
| United States Surgical Corporation | Delaware | ||||
| USSC FSC, Inc. | Barbados | ||||
| USSC Medical GmbH | Germany | ||||
| Valera Holdings S.à.r.l. | Luxembourg | ||||
| Valleylab Holding Corporation | Delaware | ||||
| Vascular Medcure, Inc. | Delaware | ||||
| Visionsense Corp. | Delaware | ||||
| Visionsense Ltd. | Israel | ||||
| Visualase, Inc. | Delaware | ||||
| Vitatron Holding B.V. | Netherlands | ||||
| VNUS Medical Technologies II, Inc. | Delaware | ||||
| Warsaw Orthopedic Inc. | Indiana | ||||
| WEM Equipamentos Electronicos Ltda. | Brazil | ||||
| World Heart Corporation | Delaware | ||||
| Zephyr Technology LLC | Delaware | ||||
| Zorginitiatieven B.V. | Netherlands | ||||
Registered Senior Notes Issued Under | Issuer | Guarantors | ||||||
Indenture, dated as of October 22, 2007 | Covidien International Finance S.A. | Covidien Ltd., Covidien plc, Medtronic plc and Medtronic Global Holdings, S.C.A. | ||||||
Indenture, dated as of March 12, 2009 | Medtronic, Inc. | Medtronic plc and Medtronic Global Holdings, S.C.A. | ||||||
Indenture, dated as of December 10, 2014 | Medtronic, Inc. | Medtronic plc and Medtronic Global Holdings S.C.A. | ||||||
Senior Indenture, dated as of March 28, 2017 | Medtronic Global Holdings S.C.A. | Medtronic plc and Medtronic, Inc. | ||||||
/s/ PricewaterhouseCoopers LLP | ||
| Minneapolis, Minnesota | ||
| June 23, 2022 | ||
| /s/ Richard H. Anderson | /s/ Kevin E. Lofton | |||||||
| Richard H. Anderson | Kevin E. Lofton | |||||||
| /s/ Craig Arnold | /s/ Geoffrey S. Martha | |||||||
| Craig Arnold | Geoffrey S. Martha | |||||||
| /s/ Scott C. Donnelly | /s/ Elizabeth G. Nabel, M.D. | |||||||
| Scott C. Donnelly | Elizabeth G. Nabel, M.D. | |||||||
| /s/ Andrea Goldsmith, PH.D. | /s/ Denise M. O’Leary | |||||||
| Andrea Goldsmith, PH.D. | Denise M. O’Leary | |||||||
| /s/ Randall J. Hogan | /s/ Kendall J. Powell | |||||||
| Randall J. Hogan | Kendall J. Powell | |||||||
| June 23, 2022 | /s/ Geoffrey S. Martha | ||||
| Geoffrey S. Martha | |||||
| Chief Executive Officer | |||||
| June 23, 2022 | /s/ Karen L. Parkhill | ||||
| Karen L. Parkhill | |||||
| Executive Vice President and | |||||
| Chief Financial Officer | |||||
| June 23, 2022 | /s/ Geoffrey S. Martha | ||||
Geoffrey S. Martha | |||||
Chief Executive Officer | |||||
| June 23, 2022 | /s/ Karen L. Parkhill | ||||
Karen L. Parkhill | |||||
| Executive Vice President and | |||||
| Chief Financial Officer | |||||
Audit Information |
12 Months Ended |
|---|---|
Apr. 29, 2022 | |
| Audit Information [Abstract] | |
| Auditor Firm ID | 238 |
| Auditor Name | PricewaterhouseCoopers LLP |
| Auditor Location | Minneapolis, Minnesota |
Consolidated Statements of Comprehensive Income - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Statement of Comprehensive Income [Abstract] | |||
| Net income | $ 5,062 | $ 3,630 | $ 4,806 |
| Other comprehensive income (loss), net of tax: | |||
| Unrealized (loss) gain on investment securities | (301) | 92 | 45 |
| Translation adjustment | (2,086) | 1,699 | (829) |
| Net investment hedge | 2,299 | (1,694) | 405 |
| Net change in retirement obligations | 574 | 505 | (544) |
| Unrealized (loss) gain on cash flow hedges | 727 | (519) | 72 |
| Other comprehensive income (loss) | 1,213 | 83 | (851) |
| Comprehensive income including noncontrolling interests | 6,274 | 3,713 | 3,955 |
| Comprehensive income attributable to noncontrolling interests | (16) | (32) | (15) |
| Comprehensive income attributable to Medtronic | $ 6,258 | $ 3,681 | $ 3,940 |
Consolidated Balance Sheets (Parenthetical) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Statement of Financial Position [Abstract] | ||
| Allowances and credit losses | $ 230 | $ 241 |
| Common stock, par value (in dollars per share) | $ 0.0001 | $ 0.0001 |
| Common stock, authorized (in shares) | 2,600,000,000 | 2,600,000,000 |
| Common stock, issued (in shares) | 1,330,743,395 | 1,345,400,671 |
| Common stock, outstanding (in shares) | 1,330,743,395 | 1,345,400,671 |
Consolidated Statements of Equity (Parenthetical) - $ / shares |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Statement of Stockholders' Equity [Abstract] | |||
| Dividends to shareholders (usd per share) | $ 2.52 | $ 2.32 | $ 2.16 |
Consolidated Statements of Cash Flows - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Operating Activities: | |||
| Net income | $ 5,062 | $ 3,630 | $ 4,806 |
| Adjustments to reconcile net income to net cash provided by operating activities: | |||
| Depreciation and amortization | 2,707 | 2,702 | 2,663 |
| Provision for credit losses | 58 | 128 | 99 |
| Deferred income taxes | (604) | (422) | (1,315) |
| Stock-based compensation | 359 | 344 | 297 |
| Loss on debt extinguishment | 0 | 308 | 406 |
| Asset impairment charges | 515 | 0 | 0 |
| Other, net | 138 | 251 | 217 |
| Change in operating assets and liabilities, net of acquisitions and divestitures: | |||
| Accounts receivable, net | (477) | (761) | 1,291 |
| Inventories, net | (560) | 78 | (577) |
| Accounts payable and accrued liabilities | 213 | 531 | (44) |
| Other operating assets and liabilities | (65) | (549) | (609) |
| Net cash provided by operating activities | 7,346 | 6,240 | 7,234 |
| Investing Activities: | |||
| Acquisitions, net of cash acquired | (91) | (994) | (488) |
| Additions to property, plant, and equipment | (1,368) | (1,355) | (1,213) |
| Purchases of investments | (9,882) | (11,808) | (11,039) |
| Sales and maturities of investments | 9,692 | 11,345 | 9,574 |
| Other investing activities, net | (10) | (54) | (37) |
| Net cash used in investing activities | (1,659) | (2,866) | (3,203) |
| Financing Activities: | |||
| Change in current debt obligations, net | 0 | (311) | (17) |
| Proceeds from short-term borrowings (maturities greater than 90 days) | 0 | 2,789 | 0 |
| Repayments from short-term borrowings (maturities greater than 90 days) | 0 | (2,853) | 0 |
| Issuance of long-term debt | 0 | 7,172 | 5,568 |
| Payments on long-term debt | (1) | (7,367) | (6,110) |
| Dividends to shareholders | (3,383) | (3,120) | (2,894) |
| Issuance of ordinary shares | 429 | 474 | 662 |
| Repurchase of ordinary shares | (2,544) | (652) | (1,326) |
| Other financing activities | 163 | (268) | (81) |
| Net cash used in financing activities | (5,336) | (4,136) | (4,198) |
| Effect of exchange rate changes on cash and cash equivalents | (231) | 215 | (86) |
| Net change in cash and cash equivalents | 121 | (547) | (253) |
| Cash and cash equivalents at beginning of period | 3,593 | 4,140 | 4,393 |
| Cash and cash equivalents at end of period | 3,714 | 3,593 | 4,140 |
| Cash paid for: | |||
| Income taxes | 996 | 1,250 | 878 |
| Interest | $ 540 | $ 582 | $ 643 |
Summary of Significant Accounting Policies |
12 Months Ended |
|---|---|
Apr. 29, 2022 | |
| Accounting Policies [Abstract] | |
| Summary of Significant Accounting Policies | Summary of Significant Accounting Policies Nature of Operations Medtronic plc (Medtronic or the Company) is the leading global healthcare technology company– alleviating pain, restoring health, and extending life for millions of people around the world. The Company provides innovative products and therapies to serve healthcare systems, physicians, clinicians, and patients. Medtronic was founded in 1949 and is headquartered in Dublin, Ireland. Principles of Consolidation The consolidated financial statements include the accounts of Medtronic plc, its wholly-owned subsidiaries, entities for which the Company has a controlling financial interest, and variable interest entities for which the Company is the primary beneficiary. Intercompany transactions and balances have been fully eliminated in consolidation. Certain reclassifications have been made to prior year financial statements to conform to classifications used in the current year. Amounts reported in millions within this annual report are computed based on the amounts in thousands, and therefore, the sum of the components may not equal the total amount reported in millions due to rounding. Additionally, certain columns and rows within tables may not sum due to rounding. Use of Estimates The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States (U.S.) (U.S. GAAP) requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Estimates are used when accounting for items such as income taxes, contingencies, intangible asset, and liability valuations. Actual results may or may not differ from those estimates. COVID-19 has had, and may continue to have, an adverse effect on our business, results of operations, financial condition, and cash flows, and its future impacts remain uncertain and unpredictable. The Company has considered the disruptions caused by COVID-19 and has assessed the potential impact on certain accounting estimates including, but not limited to, the allowance for doubtful accounts, inventory reserves, return reserves, the valuation of goodwill, intangible assets, other long-lived assets, investments and contingent consideration, as of April 29, 2022 and through the date of this report. There was not a material impact to accounting estimates associated with the Company’s consolidated financial statements as of and for each of the three fiscal years ended April 29, 2022, April 30, 2021, and April 24, 2020. Fiscal Year-End The Company utilizes a 52/53-week fiscal year, ending the last Friday in April, for the presentation of its consolidated financial statements and related notes thereto at April 29, 2022 and April 30, 2021 and for each of the three fiscal years ended April 29, 2022 (fiscal year 2022), April 30, 2021 (fiscal year 2021), and April 24, 2020 (fiscal year 2020). Fiscal year 2021 was a 53-week year, with the extra week having occurred in the first fiscal month of the first quarter. Cash Equivalents The Company considers highly liquid investments with maturities of three months or less from the date of purchase to be cash equivalents. These investments are carried at cost, which approximates fair value. Investments The Company invests in marketable debt and equity securities, investments that do not have readily determinable fair values, and investments accounted for under the equity method. Marketable debt securities are classified and accounted for as available-for-sale. These investments are recorded at fair value in the consolidated balance sheets. The change in fair value for available-for-sale securities is recorded, net of taxes, as a component of accumulated other comprehensive loss on the consolidated balance sheets. The Company determines the appropriate classification of its investments in marketable debt securities at the time of purchase and reevaluates such determinations at each balance sheet date. The classification of marketable debt securities as current or long-term is based on the nature of the securities and the availability for use in current operations consistent with the Company's management of its capital structure and liquidity. Certain of the Company’s investments in marketable equity securities and other securities are long-term, strategic investments in companies that are in various stages of development and are included in other assets on the consolidated balance sheets. Marketable equity securities are recorded at fair value in the consolidated balance sheets. The change in fair value of marketable equity securities is recognized within other non-operating income, net in the consolidated statements of income. At each reporting period, the Company makes a qualitative assessment considering impairment indicators to evaluate whether the investment is impaired. Equity securities accounted for under the equity method are initially recorded at the amount of the Company’s investment and are adjusted each period for the Company’s share of the investee’s income or loss and dividends paid. Securities accounted for under the equity method are reviewed quarterly for changes in circumstance or the occurrence of events that suggest other than temporary impairment has occurred. Accounts Receivable and Allowance for Doubtful Accounts and Credit Losses The Company grants credit to customers in the normal course of business and maintains an allowance for doubtful accounts for potential credit losses. When evaluating allowances for doubtful accounts, the Company considers various factors, including historical experience and customer-specific information. Uncollectible accounts are written-off against the allowance when it is deemed that a customer account is uncollectible. Inventories Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. The Company reduces the carrying value of inventories for items that are potentially excess, obsolete, or slow-moving based on changes in customer demand, technology developments, or other economic factors. Property, Plant, and Equipment Property, plant, and equipment is stated at cost and depreciated over the useful lives of the assets using the straight-line method. Additions and improvements that extend the lives of the assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. The Company assesses property, plant, and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of property, plant, and equipment asset groupings may not be recoverable. The cost of interest that is incurred in connection with significant ongoing construction projects is capitalized using a weighted average interest rate. These costs are included in property, plant, and equipment and amortized over the useful life of the related asset. Upon retirement or disposal of property, plant, and equipment, the costs and related amounts of accumulated depreciation or amortization are eliminated from the asset and accumulated depreciation accounts. The difference, if any, between the net asset value and the proceeds, is recognized in earnings. Goodwill and Intangible Assets Goodwill is the excess of the purchase price over the estimated fair value of net assets of acquired businesses. The Company assesses goodwill for impairment annually in the third quarter of the fiscal year and whenever an event occurs or circumstances change that would indicate the carrying amount may be impaired. Impairment testing for goodwill is performed at a reporting unit level. The test for impairment of goodwill requires the Company to make several estimates related to projected future cash flows to determine the fair value of the goodwill reporting units. The Company calculates the excess of each reporting unit's fair value over its carrying amount, including goodwill, utilizing a discounted cash flow analysis. Internal operational budgets and long-range strategic plans are used as a basis for the cash flow analysis. The Company also utilizes assumptions for working capital, capital expenditures, and terminal growth rates. The discount rate applied to the cash flow analysis is based on the weighted average cost of capital (“WACC”) for each reporting unit. An impairment is recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit. Intangible assets include patents, trademarks, tradenames, customer relationships, purchased technology, and in-process research and development (IPR&D). Intangible assets with a definite life are amortized on a straight-line basis with estimated useful lives typically ranging from to 20 years. Amortization is recognized within amortization of intangible assets in the consolidated statements of income. Intangible assets with a definite life are tested for impairment whenever events or changes in circumstances indicate that the carrying amount of an intangible asset (asset group) may not be recoverable. When events or changes in circumstances indicate that the carrying amount of an intangible asset may not be recoverable, the Company calculates the excess of an intangible asset's carrying value over its undiscounted future cash flows. If the carrying value is not recoverable, an impairment is recognized based on the amount by which the carrying value exceeds the fair value. The fair value of an intangible asset (asset group) is estimated by utilizing a discounted cash flow analysis. Acquired IPR&D represents the fair value assigned to those research and development projects that were acquired in a business combination for which the related products have not received regulatory approval and have no alternative future use. IPR&D is capitalized at its fair value as an indefinite-lived intangible asset, and any development costs incurred after the acquisition are expensed as incurred. The fair value of IPR&D is determined by estimating the future cash flows of each project and discounting the net cash flows back to their present values. Upon achieving regulatory approval or commercial viability for the related product, the indefinite-lived intangible asset is accounted for as a definite-lived asset and is amortized on a straight-line basis over the estimated useful life. If the project is not completed or is terminated or abandoned, the Company may have an impairment related to the IPR&D, which is charged to expense. Indefinite-lived intangible assets are tested for impairment annually in the third quarter of the fiscal year and whenever events or changes in circumstances indicate that the carrying amount may be impaired. Impairment is calculated as the excess of the asset’s carrying value over its fair value. Fair value is generally determined using a discounted future cash flow analysis. IPR&D with no alternative future use acquired outside of a business combination is expensed immediately. Contingent Consideration Certain of the Company’s business combinations involve potential payment of future consideration that is contingent upon the achievement of certain product development milestones and/or contingent on the acquired business reaching certain performance milestones. The Company records contingent consideration at fair value at the date of acquisition based on the consideration expected to be transferred, estimated as the probability-weighted future cash flows, discounted back to present value. The fair value of contingent consideration is measured using projected payment dates, discount rates, probabilities of payment, and projected revenues (for revenue-based considerations). Projected revenues are based on the Company’s most recent internal operational budgets and long-range strategic plans. The discount rate used is determined at the time of measurement in accordance with accepted valuation methodologies. Changes in projected revenues, probabilities of payment, discount rates, and projected payment dates may result in adjustments to the fair value measurements. Contingent consideration is remeasured each reporting period using Level 3 inputs, and the change in fair value, including accretion for the passage of time, is recognized as income or expense within other operating expense, net in the consolidated statements of income. Contingent consideration payments made soon after the acquisition date are classified as investing activities in the consolidated statements of cash flows. Contingent consideration payments not made soon after the acquisition date that are related to the acquisition date fair value are reported as financing activities in the consolidated statements of cash flows, and amounts paid in excess of the original acquisition date fair value are reported as operating activities in the consolidated statements of cash flows. Self-Insurance The Company self-insures the majority of its insurable risks, including medical and dental costs, disability coverage, physical loss to property, business interruptions, workers’ compensation, comprehensive general, and product liability. Insurance coverage is obtained for risks required to be insured by law or contract. The Company uses claims data and historical experience, as applicable, to estimate liabilities associated with the exposures that the Company has self-insured. Retirement Benefit Plan Assumptions The Company sponsors various retirement benefit plans, including defined benefit pension plans, post-retirement medical plans, defined contribution savings plans, and termination indemnity plans, covering substantially all U.S. employees and many employees outside the U.S. See Note 15 for assumptions used in determining pension and post-retirement benefit costs and liabilities. Derivatives The Company recognizes all derivative financial instruments in its consolidated financial statements at fair value in accordance with authoritative guidance on derivatives and hedging, and presents assets and liabilities associated with derivative financial instruments on a gross basis in the consolidated financial statements. For derivative instruments that are designated and qualify as hedging instruments, the hedging instrument must be designated as a fair value hedge or a cash flow hedge, based upon the exposure being hedged. See Note 7 for more information on the Company's derivative instruments and hedging programs. Fair Value Measurements The Company follows the authoritative guidance on fair value measurements and disclosures with respect to assets and liabilities that are measured at fair value on both a recurring and nonrecurring basis. Fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The authoritative guidance also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability, based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The hierarchy is broken down into three levels defined as follows: •Level 1 - Inputs are quoted prices in active markets for identical assets or liabilities. •Level 2 - Inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly. •Level 3 - Inputs are unobservable for the asset or liability. Financial assets that are classified as Level 1 securities include highly liquid government bonds within U.S. government and agency securities and marketable equity securities for which quoted market prices are available. In addition, the Company classifies currency forward contracts as Level 1 since they are valued using quoted market prices in active markets which have identical assets or liabilities. The valuation for most fixed maturity securities are classified as Level 2. Financial assets that are classified as Level 2 include corporate debt securities, government and agency securities, other asset-backed securities, certificate of deposits, debt funds, and mortgage-backed securities whose value is determined using inputs that are observable in the market or may be derived principally from, or corroborated by, observable market data such as pricing for similar securities, recently executed transactions, cash flow models with yield curves, and benchmark securities. In addition, total return swaps are included in Level 2 as the Company uses inputs other than quoted prices that are observable for the asset. The Level 2 derivative instruments are primarily valued using standard calculations and models that use readily observable market data as their basis. Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies, or similar techniques, and at least one significant model assumption or input is unobservable. Financial assets that are classified as Level 3 include certain investment securities for which there is limited market activity such that the determination of fair value requires significant judgment or estimation, and auction rate securities. With the exception of auction rate securities, these securities are valued using third-party pricing sources that incorporate transaction details such as contractual terms, maturity, timing, and amount of expected future cash flows, as well as assumptions about liquidity and credit valuation adjustments by market participants. The fair value of auction rate securities is estimated by the Company using a discounted cash flow model, which incorporates significant unobservable inputs. The significant unobservable inputs used in the fair value measurement of the Company’s auction rate securities are years to principal recovery and the illiquidity premium that is incorporated into the discount rate. For goodwill, other intangible assets, and IPR&D, inputs used in the fair value analysis fall within Level 3 of the fair value hierarchy due to the use of significant unobservable inputs to determine fair value. Certain investments for which the fair value is measured using the net asset value per share (or its equivalent) practical expedient are excluded from the fair value hierarchy. Financial assets for which the fair value is measured using the net asset value per share practical expedient include certain debt funds, equity and fixed income commingled trusts, and registered investment companies. Revenue Recognition The Company sells its products through direct sales representatives and independent distributors. Additionally, a portion of the Company's revenue is generated from consignment inventory maintained at hospitals. The Company recognizes revenue when control is transferred to the customer. For products sold through direct sales representatives and independent distributors, control is transferred upon shipment or upon delivery, based on the contract terms and legal requirements. For consignment inventory, control is transferred when the product is used or implanted. Payment terms vary depending on the country of sale, type of customer, and type of product. If a contract contains more than one performance obligation, the transaction price is allocated to each performance obligation based on relative standalone selling price. Shipping and handling is treated as a fulfillment activity rather than a promised service, and therefore, is not considered a performance obligation. Taxes assessed by a governmental authority that are both imposed on, and concurrent with, a specific revenue producing transaction and collected by the Company from customers (for example, sales, use, value added, and some excise taxes) are not included in revenue. For contracts that have an original duration of one year or less, the Company uses the practical expedient applicable to such contracts and does not adjust the transaction price for the time value of money. The amount of revenue recognized reflects sales rebates and returns, which are estimated based on sales terms, historical experience, and trend analysis. In estimating rebates, the Company considers the lag time between the point of sale and the payment of the rebate claim, the stated rebate rates, and other relevant information. The Company records adjustments to rebates and returns reserves as increases or decreases of revenue. The Company records a deferred revenue liability if a customer pays consideration before the Company transfers a good or service to the customer. Deferred revenue primarily represents remote monitoring services and equipment maintenance, for which consideration is received at the same time as consideration for the device or equipment. Revenue related to remote monitoring services and equipment maintenance is recognized over the service period as time elapses. Shipping and Handling Shipping and handling costs incurred to physically move product from the Company's premises to the customer's premises are recognized in selling, general, and administrative expense in the consolidated statements of income and were $354 million, $308 million, and $347 million in fiscal years 2022, 2021, and 2020, respectively. Other shipping and handling costs incurred to store, move, and prepare products for shipment are recognized in cost of products sold in the consolidated statements of income. Research and Development Research and development costs are expensed when incurred. Research and development costs include costs of research, engineering, and technical activities to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include pre-approval regulatory and clinical trial expenses. Contingencies The Company records a liability in the consolidated financial statements for loss contingencies when a loss is known or considered probable, and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. Income Taxes The Company has deferred taxes that arise as a result of the different treatment of transactions for U.S. GAAP and income tax accounting, known as temporary differences. The Company records the tax effect of these temporary differences as deferred tax assets and deferred tax liabilities. Deferred tax assets generally represent items that may be used as a tax deduction or credit in a tax return in future years for which the Company has already recognized the tax benefit in the consolidated statements of income. The Company establishes valuation allowances for deferred tax assets when the amount of expected future taxable income is not likely to support the use of the deduction or credit. Deferred tax liabilities generally represent tax expense for which payment has been deferred or expense has already been taken as a deduction on the Company’s tax return but has not yet been recognized as an expense in the consolidated statements of income. See Footnote 13 for more information on the Company's uncertain tax positions and tax policies. Other Operating Expense, Net Other operating expense, net primarily includes royalty income and expense, currency remeasurement and derivative gains and losses, Puerto Rico excise taxes, changes in fair value of contingent consideration, changes in amounts accrued for certain contingent liabilities for a past acquisition, charges related to the June 2021 decision to stop the distribution and sale of Medtronic's HVAD System within the Mechanical Circulatory Support Operating Unit (MCS) (MCS charges), impairment charges, and income from funded research and development arrangements. Other Non-Operating Income, Net Other non-operating income, net includes the non-service component of net periodic pension and post-retirement benefit cost, investment gains and losses, and interest income. Currency Translation Assets and liabilities of non-U.S. dollar functional currency entities are translated to U.S. dollars at period-end exchange rates, and the currency impacts arising from the translation of the assets and liabilities are recorded as a cumulative translation adjustment, a component of accumulated other comprehensive loss, on the consolidated balance sheets. Elements of the consolidated statements of income are translated at the average monthly currency exchange rates in effect during the period. Currency transaction gains and losses are included in other operating expense, net in the consolidated statements of income. Stock-Based Compensation The Company measures stock-based compensation expense at the grant date based on the fair value of the award and recognizes the compensation expense over the requisite service period, which is generally the vesting period. The amount of stock-based compensation expense recognized during a period is based on the portion of the awards that are expected to vest. The Company estimates pre-vesting forfeitures at the time of grant and revises the estimates in subsequent periods. Recently Adopted Accounting Standards Current Expected Credit Losses In June 2016, the Financial Accounting Standards Board (FASB) issued guidance changing the methodology to be used to measure credit losses for certain financial instruments and financial assets, including trade receivables. The new methodology requires the recognition of an allowance that reflects the current estimate of credit losses expected to be incurred over the life of the financial asset. The Company adopted this guidance using the modified retrospective method in the first quarter of fiscal year 2021. The adoption of this guidance did not have a material impact to the Company’s consolidated financial statements. Leases In February 2016, the FASB issued guidance which requires lessees to recognize right-of-use assets and lease liabilities on the balance sheet. This guidance also requires additional qualitative and quantitative lease related disclosures in the notes to the consolidated financial statements. The Company adopted this guidance using the modified retrospective method in the first quarter of fiscal year 2020. During the implementation, the Company elected the package of practical expedients available under the transition guidance that allowed an entity not to reassess whether any expired or existing contracts are or contain leases, the classification for any expired or existing leases or any initial direct costs for existing leases. Further, the Company made accounting policy elections to not apply the recognition requirements to short-term leases and to account for lease and nonlease components as a single lease component. The adoption of this guidance resulted in the recognition of right-of-use assets and lease liabilities in an amount of approximately $1.0 billion, an immaterial cumulative-effect adjustment to retained earnings as of April 27, 2019, and expansion of lease related disclosures. The adoption of this guidance did not have a material impact on the Company's consolidated statements of income or consolidated statements of cash flows.
|
Revenue |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenue from Contract with Customer [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenue | Revenue The Company's revenues are principally derived from device-based medical therapies and services related to cardiac rhythm disorders, cardiovascular disease, renal disease, neurological disorders and diseases, spinal conditions and musculoskeletal trauma, chronic pain, urological and digestive disorders, ear, nose, and throat conditions, and diabetes conditions as well as advanced and general surgical care products, respiratory and monitoring solutions, and neurological surgery technologies. The Company's primary customers include healthcare systems, clinics, third-party healthcare providers, distributors, and other institutions, including governmental healthcare programs and group purchasing organizations. The table below illustrates net sales by segment and division for fiscal years 2022, 2021, and 2020:
The table below includes net sales by market geography and segment for fiscal years 2022, 2021, and 2020:
(1)U.S. includes the United States and U.S. territories. (2)Non-U.S. developed markets include Japan, Australia, New Zealand, Korea, Canada, and the countries of Western Europe. (3)Emerging markets include the countries of the Middle East, Africa, Latin America, Eastern Europe, and the countries of Asia that are not included in the non-U.S. developed markets, as defined above. At April 29, 2022, $981 million of rebates were classified as other accrued expenses, and $548 million of rebates were classified as a reduction of accounts receivable in the consolidated balance sheet. At April 30, 2021, $906 million of rebates were classified as other accrued expenses, and $485 million of rebates were classified as a reduction of accounts receivable in the consolidated balance sheet. During fiscal year 2022, adjustments to rebate and return reserves recognized in revenue that were included in the rebate and return reserves at the beginning of the period were not material. Deferred Revenue and Remaining Performance Obligations Deferred revenue at April 29, 2022 and April 30, 2021 was $399 million and $368 million, respectively. At April 29, 2022 and April 30, 2021, $305 million and $276 million was included in other accrued expenses, respectively, and $94 million and $93 million was included in other liabilities, respectively. During the fiscal year ended April 29, 2022, the Company recognized $243 million of revenue that was included in deferred revenue as of April 30, 2021. Remaining performance obligations include goods and services that have not yet been delivered or provided under existing, noncancellable contracts with minimum purchase commitments. At April 29, 2022, the estimated revenue expected to be recognized in future periods related to unsatisfied performance obligations for executed contracts with an original duration of one year or more was approximately $925 million. The Company expects to recognize revenue on the majority of these remaining performance obligations over the next three years.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acquisitions |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Business Combination and Asset Acquisition [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Acquisitions | Acquisitions The Company had acquisitions during fiscal years 2022 and 2021 that were accounted for as business combinations. The assets and liabilities of businesses acquired were recorded and consolidated on the acquisition date at their respective fair values. Goodwill resulting from business combinations is largely attributable to future yet to be defined technologies, new customer relationships, existing workforce of the acquired businesses, and synergies expected to arise after the Company's acquisition of these businesses. The pro forma impact of acquisitions during fiscal years 2022 and 2021 was not significant, either individually or in the aggregate, to the consolidated results of the Company. The results of operations of acquired businesses have been included in the Company’s consolidated statements of income since the date each business was acquired. Purchase price allocation adjustments for fiscal years 2022 and 2021 business combinations were not significant. Fiscal Year 2022 The acquisition date fair value of net assets acquired during fiscal year 2022 was $125 million, consisting of $154 million of assets acquired and $29 million of liabilities assumed. Based upon preliminary valuations, assets acquired were primarily comprised of $50 million of technology-based intangible assets with estimated useful lives ranging from 15 to 16 years, and $80 million of goodwill. The goodwill is not deductible for tax purposes. The Company recognized $31 million of contingent consideration liabilities in connection with business combinations during fiscal year 2022, which are comprised of revenue and regulatory milestone-based payments. Fiscal Year 2021 The acquisition date fair value of net assets acquired during fiscal year 2021 was $1.2 billion, consisting of $1.4 billion of assets acquired and $161 million of liabilities assumed. Based upon final valuations, assets acquired were primarily comprised of $417 million of technology-based intangible assets and $13 million of customer-related intangible assets with estimated useful lives ranging from 8 to 15 years, and $816 million of goodwill. The goodwill is not deductible for tax purposes. The Company recognized $253 million of contingent consideration liabilities in connection with business combinations during fiscal year 2021, which are comprised of revenue and regulatory milestone-based payments. Additionally, the Company recognized a gain of $132 million related to a change in amounts accrued for certain contingent liabilities from a past acquisition. The benefit was recognized in other operating expense, net in the consolidated statements of income as the purchase accounting was finalized in fiscal year 2020. Subsequent Acquisitions Subsequent to fiscal year 2022, on May 13, 2022, the Company's Neuroscience segment acquired Intersect ENT, a global ear, nose, and throat (ENT) medical technology leader. The acquisition expands Medtronic's portfolio of products used during ENT procedures and, combined with the Company's navigation, powered instruments, and existing tissue health products, will offer a broader suite of solutions to assist surgeons treating patients who suffer from chronic rhinosinusitis (CRS). Total consideration for the transaction, in which the Company acquired all outstanding shares of Intersect ENT for $28.25 per share, was approximately $1.2 billion. The transaction will be accounted for as a business combination using the acquisition method of accounting. This requires, among other things, that assets acquired and liabilities assumed be recognized at their fair values as of the acquisition date. Due to the limited amount of time since the acquisition date and the significant limitations on access to Intersect ENT information prior to the acquisition date the preliminary acquisition valuation for the business combination is incomplete at this time. As a result, the Company is unable to provide the amounts recognized as of the acquisition date for the major classes of assets acquired and liabilities assumed, including the information required for valuation of intangible assets and goodwill. We will include such disclosures in our Form 10-Q for the quarter ending July 29, 2022. Acquired In-Process Research & Development (IPR&D) IPR&D with no alternative future use acquired outside of a business combination is expensed immediately. During fiscal year 2022, the Company acquired $101 million of IPR&D in connection with asset acquisitions of technology not approved by regulators, which was recognized in research and development expense in the consolidated statements of income. During fiscal year 2021, IPR&D acquired in connection with asset acquisitions was not significant. Contingent Consideration Certain of the Company’s business combinations involve potential payment of future consideration that is contingent upon the achievement of certain product development milestones and/or contingent on the acquired business reaching certain performance milestones. A liability is recorded for the estimated fair value of the contingent consideration on the acquisition date. The fair value of the contingent consideration is remeasured at each reporting period, and the change in fair value is recognized within other operating expense, net in the consolidated statements of income. The fair value of contingent consideration at April 29, 2022 and April 30, 2021 was $119 million and $270 million, respectively. At April 29, 2022, $35 million was recorded in other accrued expenses, and $84 million was recorded in other liabilities on the consolidated balance sheets. At April 30, 2021, $78 million was reflected in other accrued expenses, and $192 million was reflected in other liabilities on the consolidated balance sheets. The following table provides a reconciliation of the beginning and ending balances of contingent consideration:
The recurring Level 3 fair value measurements of contingent consideration for which a liability is recorded include the following significant unobservable inputs:
(1) Unobservable inputs were weighted by the relative fair value of the contingent consideration liability. For projected fiscal year of payment, the amount represents the median of the inputs and is not a weighted average.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Restructuring Charges |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Restructuring and Related Activities [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Restructuring Charges | Restructuring Charges Enterprise Excellence In the third quarter of fiscal year 2018, the Company announced its Enterprise Excellence restructuring program, which was designed to leverage the Company's global size and scale, as well as enhance the customer and employee experience, with a focus on three objectives: global operations, functional optimization, and commercial optimization. Since inception, the Company has incurred pre-tax exit and disposal costs and other costs, across all segments, of $1.6 billion in connection with the Enterprise Excellence program. In total, the Company estimates it will recognize approximately $1.8 billion of exit and disposal costs and other costs related to the program by the end of fiscal year 2023. The remaining charges are costs associated with the restructuring program, such as salaries and benefits for employees supporting the program, including program management and transition teams, and strategic and operational consulting services related to the three objectives of the program. These charges are recognized within restructuring charges, net, cost of products sold, and selling, general, and administrative expense in the consolidated statements of income. For fiscal years 2022, 2021 and 2020, the Company recognized net charges of $259 million, $349 million, and $441 million, respectively, of which $116 million, $128 million, and $155 million, respectively, were recognized within cost of products sold, and $112 million, $169 million, and $168 million, respectively, were recognized within selling, general, and administrative expense in the consolidated statements of income. Simplification In the first quarter of fiscal year 2021, the Company initiated the Simplification restructuring program, designed to make the Company a more nimble and competitive organization focused on accelerating innovation, enhancing the customer experience, driving revenue growth, and winning market share, while also more efficiently and effectively leveraging the enterprise scale. Since inception, the Company has incurred pre-tax exit and disposal costs and other costs, across all segments, of $349 million in connection with the program. In total, the Company estimates it will recognize approximately $450 million of exit and disposal costs and other costs related to the Simplification program by the end of fiscal year 2023. The remaining charges are costs associated with the restructuring program, such as salaries for employees supporting the program and consulting expenses. These charges are recognized within restructuring charges, net, cost of products sold, and selling, general, and administrative expense in the consolidated statements of income. For fiscal years 2022 and 2021, the Company recognized net charges of $82 million and $268 million, respectively, of which $45 million and $27 million were recognized within selling, general, and administrative expense in the consolidated statements of income. The net charges for fiscal year 2021 included $97 million of incremental defined benefit pension and post-retirement related expenses for employees that accepted voluntary early retirement packages and are not included in the table below, as they are associated with costs that are accounted for under the pension and post-retirement rules. See Note 15 for further discussion on these charges. The following table summarizes the activity related to the restructuring programs noted above for fiscal years 2022, 2021, and 2020:
(1)Associated costs include costs incurred as a direct result of the restructuring program, such as salaries for employees supporting the program and consulting expenses. (2)Accrual adjustments relate to certain employees identified for termination finding other positions within the Company or contract terminations being settled for less than originally estimated. Mechanical Circulatory Support (MCS) On June 3, 2021, the Company announced the decision to stop the distribution and sale of the Medtronic HVAD System in light of a growing body of observational clinical comparisons indicating a lower frequency of neurological adverse events and mortality with another circulatory support device available to patients compared to the HVAD system. In connection with this decision, the Company recorded charges of $726 million (MCS charges) within the Cardiovascular segment during the first quarter of fiscal year 2022, including $58 million recognized in costs of products sold and $668 million recognized within other operating expense, net in the consolidated statement of income. The charges included $515 million of non-cash impairments and write-downs primarily related to $409 million of intangible asset impairments and $58 million of inventory write-downs. The Company also recorded charges of $211 million for commitments and obligations associated with the decision, which included charges for patient support obligations, restructuring, and other associated costs. During the fourth quarter of fiscal year 2022, the Company recorded additional charges of $155 million within other operating expense, net primarily related to incremental commitments and obligations associated with the exit of the business. As of April 29, 2022, accruals were recorded in the consolidated balance sheet for these obligations, with $82 million reflected in other accrued expenses and $152 million recorded in other liabilities. Medtronic remains committed to serving the needs of the approximately 3,500 patients currently implanted with the HVAD system.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Financial Instruments |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Investments [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Financial Instruments | Financial Instruments Debt Securities The Company holds investments in marketable debt securities that are classified and accounted for as available-for-sale and are remeasured on a recurring basis. The following tables summarize the Company's investments in available-for-sale debt securities by significant investment category and the related consolidated balance sheet classification at April 29, 2022 and April 30, 2021:
The amortized cost of debt securities excludes accrued interest, which is reported in other current assets in the consolidated balance sheets. The following tables present the gross unrealized losses and fair values of the Company’s available-for-sale debt securities that have been in a continuous unrealized loss position deemed to be temporary, aggregated by investment category at April 29, 2022 and April 30, 2021:
The Company reviews the fair value hierarchy classification on a quarterly basis. Changes in the ability to observe valuation inputs may result in a reclassification of levels for certain securities within the fair value hierarchy. There were no transfers into or out of Level 3 during the fiscal years ended April 29, 2022 and April 30, 2021. When a determination is made to classify an asset or liability within Level 3, the determination is based upon the significance of the unobservable inputs to the overall fair value measurement. Activity related to the Company’s available-for-sale debt securities portfolio is as follows:
During the fiscal year ended April 30, 2021, the Company had proceeds from maturities of investments classified as held to maturity of $911 million. The April 29, 2022 balance of available-for-sale debt securities by contractual maturity is shown in the following table. Within the table, maturities of mortgage-backed securities have been allocated based upon timing of estimated cash flows assuming no change in the current interest rate environment. Actual maturities may differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties.
Equity Securities, Equity Method Investments, and Other Investments The Company holds investments in equity securities with readily determinable fair values, equity investments without readily determinable fair values, investments accounted for under the equity method, and other investments. Equity securities with readily determinable fair values are included in Level 1 of the fair value hierarchy, as they are measured using quoted market prices. Equity method investments and investments without readily determinable fair values are included within Level 3 of the fair value hierarchy due to the use of significant unobservable inputs to determine fair value. To determine the fair value of these investments, the Company uses all pertinent financial information available related to the investees, including financial statements, market participant valuations from recent and proposed equity offerings, and other third-party data. The following table summarizes the Company's equity and other investments at April 29, 2022 and April 30, 2021, which are classified as other assets in the consolidated balance sheets:
The table below includes activity related to the Company’s portfolio of equity and other investments. Gains and losses on equity and other investments are recognized in other non-operating income, net in the consolidated statements of income.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Financing Arrangements |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Debt Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Financing Arrangements | Financing Arrangements Current debt obligations consisted of the following:
Bank Borrowings Outstanding bank borrowings at April 29, 2022 and April 30, 2021 were not significant. Commercial Paper On January 26, 2015, Medtronic Global Holdings S.C.A. (Medtronic Luxco), an entity organized under the laws of Luxembourg, entered into various agreements pursuant to which Medtronic Luxco may issue United States Dollar-denominated unsecured commercial paper notes (the 2015 CP Program) on a private placement basis, and on January 31, 2020 Medtronic Luxco entered into various agreements pursuant to which Medtronic Luxco may issue Euro-denominated unsecured commercial paper notes (the 2020 CP Program) on a private placement basis. The Maximum aggregate amount outstanding at any time under the 2015 CP Program and the 2020 CP Program together may not exceed the equivalent of $3.5 billion. The Company and Medtronic, Inc. have guaranteed the obligations of Medtronic Luxco under the 2015 CP Program and the 2020 CP Program. There was no commercial paper outstanding at April 29, 2022 and April 30, 2021, or during fiscal year 2021. During fiscal year 2022, the weighted average original maturity of the commercial paper outstanding was approximately fifteen days and the weighted average interest rate was 0.70 percent. The issuance of commercial paper reduces the amount of credit available under the Company's existing credit facility, defined below. Line of Credit On December 12, 2021, Medtronic Luxco, as borrower, entered into an amendment to its amended and restated credit agreement (Credit Facility), by and among Medtronic, Medtronic, Inc., Medtronic Luxco, the lenders from time to time party thereto, and Bank of America, N.A., as administrative agent and issuing bank, extending the maturity date of the Credit Facility to December 2026 and removing the cap on the number of extension options available. The Credit Facility provides for a $3.5 billion five-year unsecured revolving credit facility (Credit Facility). At each anniversary date of the Credit Facility we can request a one-year extension of the maturity date. The Credit Facility provides the Company with the ability to increase its borrowing capacity by an additional $1.0 billion at any time during the term of the agreement. The Company and Medtronic, Inc. have guaranteed the obligations of the borrowers under the Credit Facility, and Medtronic Luxco will also guarantee the obligations of any designated borrower. The Credit Facility includes a multi-currency borrowing feature for certain specified foreign currencies. At April 29, 2022 and April 30, 2021, no amounts were outstanding under the Credit Facility. Interest rates on advances on the Credit Facility are determined by a pricing matrix based on the Company’s long-term debt ratings, assigned by Standard & Poor’s Ratings Services and Moody’s Investors Service. Facility fees are payable on the Credit Facility and are determined in the same manner as the interest rates. The Company is in compliance with all covenants related to the Credit Facility. The Company's long-term debt obligations consisted of the following:
Senior Notes The Company has outstanding unsecured senior obligations, described as senior notes in the tables above (collectively, the Senior Notes). The Senior Notes rank equally with all other unsecured and unsubordinated indebtedness of the Company. The Company is in compliance with all covenants related to the Seniors Notes. In June 2019, Medtronic Luxco issued six tranches of Euro-denominated Senior Notes with an aggregate principal of €5.0 billion, with maturities ranging from fiscal year 2021 to fiscal year 2050, resulting in cash proceeds of approximately $5.6 billion, net of discounts and issuance costs. The Company used the net proceeds of the offering to fund the cash tender offer and early redemption of $5.2 billion of Medtronic Inc., CIFSA, and Medtronic Luxco Senior Notes for $5.6 billion of total consideration. The Company recognized a loss on debt extinguishment of $413 million in fiscal year 2020, which primarily included cash premiums and accelerated amortization of deferred financing costs and debt discounts and premiums. The loss was recognized in interest expense in the consolidated statement of income. In September 2020, Medtronic Global Holdings S.C.A. (Medtronic Luxco) issued an additional six tranches of Euro-denominated Senior Notes with an aggregate principal of €6.3 billion, with maturities ranging from fiscal year 2023 to fiscal year 2051, resulting in cash proceeds of approximately $7.2 billion, net of discounts and issuance costs. The Company used the net proceeds of the offering to fund the early redemption of $4.3 billion of Medtronic Inc. and CIFSA Senior Notes and €1.5 billion of Medtronic Luxco Senior Notes for $6.3 billion of total consideration in October 2020. Additionally, the Company used the proceeds to repay its €750 million floating rate senior notes at maturity in March 2021. The Company recognized a loss on debt extinguishment of $308 million in fiscal year 2021, which primarily included cash premiums and accelerated amortization of deferred financing costs and debt discounts and premiums. The loss was recognized in interest expense in the consolidated statement of income. The Euro-denominated debt issued in June 2019 and September 2020 is designated as a net investment hedge of certain of the Company's European operations. Refer to Note 7 for additional information regarding the net investment hedge. Term Loan Agreements On May 12, 2020, Medtronic Luxco entered into a term loan agreement (Fiscal 2021 Loan Agreement) by and among Medtronic Luxco, Medtronic plc, Medtronic, Inc., and Mizuho Bank, Ltd. as administrative agent and as lender. The Fiscal 2021 Loan Agreement provided an unsecured term loan in an aggregate principal amount of up to ¥300 billion, with a term of six months and the option to extend for an additional six months at Medtronic Luxco’s option. On May 13, 2020, Medtronic Luxco borrowed the entire amount of the term loan under the Fiscal 2021 Loan Agreement. The Japanese Yen-denominated debt was designated as a net investment hedge for certain of the Company's Japanese operations. Borrowings under the Fiscal 2021 Loan Agreement carried interest at the TIBOR Rate (as defined in the Fiscal 2021 Loan Agreement) plus a margin of 0.50% per annum. Medtronic plc and Medtronic, Inc. guaranteed the obligations of Medtronic Luxco under the Fiscal 2021 Loan Agreement. On November 12, 2020, the Company exercised its option to extend the term loan for an additional six months. During the fourth quarter of fiscal year 2021, the Company de-designated the Yen-denominated debt as a net investment hedge and repaid the term loan in full, including interest. Subsequent to fiscal year 2022, on May 2, 2022, Medtronic Luxco entered into a term loan agreement (Fiscal 2023 Loan Agreement) by and among Medtronic Luxco, Medtronic plc, Medtronic, Inc., and Mizuho Bank, Ltd. as administrative agent and as lender. The Fiscal 2023 Loan Agreement provides an unsecured term loan in an aggregate principal amount of up to ¥300 billion with a term of 364 days. Borrowings under the Fiscal 2023 Loan Agreement bear interest at the TIBOR Rate (as defined in the Fiscal 2023 Loan Agreement) plus a margin of 0.40% per annum. Medtronic plc and Medtronic, Inc. have guaranteed the obligations of Medtronic Luxco under the Fiscal 2023 Loan Agreement. In May and June 2022, Medtronic Luxco borrowed an aggregate of ¥297 billion, or approximately $2.3 billion, of the term loan, under the Fiscal 2023 Loan Agreement. The Company used the net proceeds of the borrowings to fund the early redemption of $1.9 billion of Medtronic Inc.'s 3.500% Senior Notes due 2025 for $1.9 billion of total consideration, and $368 million of Medtronic Luxco's 3.350% Senior Notes due 2027 for $376 million of total consideration. The Company will recognize a total loss on debt extinguishment of $53 million in the quarter ended July 29, 2022, which primarily includes cash premiums and accelerated amortization of deferred financing costs and debt discounts and premiums. The loss will be recognized in interest expense in the consolidated statements of income. Contractual maturities of debt for the next five fiscal years and thereafter, excluding deferred financing costs and debt discount, net, are as follows:
Financial Instruments Not Measured at Fair Value At April 29, 2022, the estimated fair value of the Company’s Senior Notes was $22.9 billion compared to a principal value of $24.2 billion. At April 30, 2021 the estimated fair value was $28.6 billion compared to a principal value of $26.5 billion. The fair value was estimated using quoted market prices for the publicly registered Senior Notes, which are classified as Level 2 within the fair value hierarchy. The fair values and principal values consider the terms of the related debt and exclude the impacts of debt discounts and hedging activity.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Derivatives and Currency Exchange Risk Management |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Derivative Instruments and Hedging Activities Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Derivatives and Currency Exchange Risk Management | Derivatives and Currency Exchange Risk Management The Company uses operational and economic hedges, including currency exchange rate derivative contracts and interest rate derivative instruments, to manage the impact of currency exchange and interest rate changes on earnings and cash flows. In addition, the Company uses cross currency interest rate swaps to manage currency risk related to certain debt. In order to minimize earnings and cash flow volatility resulting from currency exchange rate changes, the Company enters into derivative instruments, principally forward currency exchange rate contracts. These contracts are designed to hedge anticipated foreign currency transactions and changes in the value of specific assets and liabilities. At inception of the contract, the derivative is designated as either a freestanding derivative or a cash flow hedge. Currencies of our derivative instruments include the Euro, Japanese Yen, Chinese Yuan, and others. The Company does not enter into currency exchange rate derivative contracts for speculative purposes. The gross notional amount of all currency exchange rate derivative instruments outstanding was $13.8 billion and $14.7 billion at April 29, 2022 and April 30, 2021, respectively. The Company also uses derivative and non-derivative instruments to manage the impact of currency exchange rate changes on net investments in foreign currency-denominated operations. The information that follows explains the various types of derivatives and financial instruments used by the Company, reasons the Company uses such instruments, and the impact such instruments have on the Company’s consolidated balance sheets and statements of income. Freestanding Derivative Contracts Freestanding derivative contracts are primarily used to offset the Company’s exposure to the change in value of specific foreign-currency-denominated assets and liabilities, and to offset variability of cash flows associated with forecasted transactions denominated in foreign currencies. The gross notional amount of the Company's freestanding currency exchange rate contracts outstanding at April 29, 2022 and April 30, 2021 was $4.9 billion and $5.7 billion, respectively. The Company's freestanding currency exchange rate contracts are not designated as hedges, and therefore, changes in the value of these contracts are recognized in earnings, thereby offsetting the current earnings effect of the related change in value of foreign-currency-denominated assets, liabilities, and cash flows. The Company also uses total return swaps to hedge the liability of a non-qualified, deferred compensation plan. The gross notional amount of the Company's total return swaps outstanding at April 29, 2022 and April 30, 2021 was $226 million and $243 million, respectively. The Company's total return swaps are not designated as hedges, and therefore, changes in the value of these instruments are recognized in earnings. The cash flows related to the Company's freestanding derivative contracts are reported as operating or financing activities, depending on the nature of the underlying hedged item, in the consolidated statements of cash flows. Cash Flow Hedges Forward contracts designated as cash flow hedges are designed to hedge the variability of cash flows associated with forecasted transactions denominated in a foreign currency that will take place in the future. The gross notional amount of these contracts, designated as cash flow hedges, outstanding at April 29, 2022 and April 30, 2021 was $8.8 billion and $9.0 billion, respectively, and will mature within the subsequent three-year period. For derivative instruments that are designated and qualify as a cash flow hedge, the gain or loss on the derivative instrument is reported as a component of accumulated other comprehensive loss. The gain or loss on the derivative instrument is reclassified into earnings and is included in other operating expense, net or cost of products sold in the consolidated statements of income in the same period or periods during which the hedged transaction affects earnings. Amounts excluded from the measurement of hedge effectiveness are recognized in earnings in the current period. The cash flows related to all of the Company's derivative instruments designated as cash flow hedges are reported as operating activities in the consolidated statements of cash flows. No components of the hedge contracts were excluded in the measurement of hedge effectiveness, and no forward contracts designated as cash flow hedges were derecognized or discontinued during fiscal years 2022, 2021, or 2020. At April 29, 2022 and April 30, 2021, the Company had $474 million in after-tax unrealized gains and $253 million in after-tax unrealized losses, respectively, associated with cash flow hedging instruments recorded in accumulated other comprehensive loss. The Company expects that $368 million of after-tax net unrealized gains at April 29, 2022 will be recognized in the consolidated statements of income over the next 12 months. Net Investment Hedges The Company has designated Euro-denominated debt as a net investment hedge of certain of its European operations to manage the exposure to currency and exchange rate movements for foreign currency-denominated net investments in foreign operations. At April 29, 2022, the Company had €16.0 billion, or $17.0 billion, of outstanding Euro-denominated debt designated as a hedge of its net investment in certain of its European operations, which will mature in fiscal year 2023 through fiscal year 2051. In February 2021, the Company de-designated ¥300 billion of outstanding Yen-denominated debt previously designated as a net investment hedge and concurrently entered into freestanding forward derivative contracts with a total notional value of ¥300 billion, or approximately $2.9 billion. These forward contracts were not designated as hedges. The Company used the proceeds from these forward derivative contracts to repay the ¥300 billion of Yen-denominated debt in conjunction with the maturity of these forward contracts in March and April of 2021. For instruments that are designated and qualify as net investment hedges, the gains or losses are reported as a component of accumulated other comprehensive loss. The gains or losses are reclassified into earnings upon a liquidation event or deconsolidation of the foreign subsidiary. Amounts excluded from the assessment of effectiveness are recognized in other operating expense, net. The cash flows related to the Company's derivative instruments designated as net investment hedges are reported as investing activities in the consolidated statements of cash flows. At April 29, 2022 and April 30, 2021, the Company had $841 million in after-tax unrealized gains and $1.5 billion in after-tax unrealized losses associated with net investment hedges recorded in accumulated other comprehensive loss, respectively. The Company does not expect any of the after-tax unrealized gains at April 29, 2022 to be recognized in the consolidated statements of income over the next 12 months. The Company did not recognize any gains or losses during fiscal years 2022, 2021, or 2020 on instruments that no longer qualify as net investment hedges. Gains and Losses on Hedging Instruments and Derivatives not Designated as Hedging Instruments The amount of the gains and losses on our hedging instruments and the classification of those gains and losses within our consolidated financial statements for fiscal years 2022, 2021, and 2020 were as follows:
The amount of the gains and losses on our derivative instruments not designated as hedging instruments and the classification of those gains and losses within our consolidated financial statements for fiscal years 2022, 2021, and 2020 were as follows:
Balance Sheet Presentation The following tables summarize the balance sheet classification and fair value of derivative instruments included in the consolidated balance sheets at April 29, 2022 and April 30, 2021. The fair value amounts are presented on a gross basis, and are segregated between derivatives that are designated and qualify as hedging instruments and those that are not designated and do not qualify as hedging instruments, and are further segregated by type of contract within those two categories.
The Company has elected to present the fair value of derivative assets and liabilities within the consolidated balance sheets on a gross basis, even when derivative transactions are subject to master netting arrangements and may otherwise qualify for net presentation. The cash flows related to collateral posted and received are reported gross as investing and financing activities, respectively, in the consolidated statements of cash flows. The following tables provide information as if the Company had elected to offset the asset and liability balances of derivative instruments, netted in accordance with various criteria as stipulated by the terms of the master netting arrangements with each of the counterparties. Derivatives not subject to master netting arrangements are not eligible for net presentation.
Concentrations of Credit Risk Financial instruments, which potentially subject the Company to significant concentrations of credit risk, consist principally of interest-bearing investments, forward exchange derivative contracts, and trade accounts receivable. Global concentrations of credit risk with respect to trade accounts receivable are limited due to the large number of customers and their dispersion across many geographic areas. The Company monitors the creditworthiness of its customers to which it grants credit terms in the normal course of business. The Company maintains cash and cash equivalents, investments, and certain other financial instruments (including currency exchange rate and interest rate derivative contracts) with various major financial institutions. The Company performs periodic evaluations of the relative credit standings of these financial institutions and limits the amount of credit exposure with any one institution. In addition, the Company has collateral credit agreements with its primary derivatives counterparties. Under these agreements, either party is required to post eligible collateral when the market value of transactions covered by the agreement exceeds specific thresholds, thus limiting credit exposure for both parties. As of April 29, 2022, the Company received net cash collateral of $254 million from its counterparties. As of April 30, 2021, the Company posted net cash collateral of $46 million to its counterparties. Cash collateral posted is recorded as a reduction in cash and cash equivalents, with the offset recorded as an increase in other current assets in the consolidated balance sheets. Cash collateral received is recorded as an increase in cash and cash equivalents with the offset recorded in other accrued expenses in the consolidated balance sheets.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Inventories |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inventory Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inventories | Inventories Inventory balances, net of reserves, were as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Goodwill and Other Intangible Assets |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Goodwill and Intangible Assets Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Goodwill and Other Intangible Assets | Goodwill and Other Intangible Assets Goodwill The following table presents the changes in the carrying amount of goodwill by segment:
The Company did not recognize any goodwill impairments during fiscal years 2022, 2021, or 2020. Intangible Assets The following table presents the gross carrying amount and accumulated amortization of intangible assets:
During fiscal year 2022, the Company recognized $409 million of definite-lived intangible asset impairment charges in connection with MCS within the Cardiovascular Portfolio. The intangible asset impairment charge primarily related to purchased technology and patents. Refer to Note 4 Restructuring Charges for additional information on what led to the impairment. During fiscal year 2021, the Company recognized $30 million of definite-lived intangible asset charges in connection with the abandonment of certain intangible assets within the Neuroscience segment. During fiscal year 2020, the Company recognized $37 million of definite-lived intangible asset charges, including $33 million and $4 million recognized in connection with business exits in the Neuroscience and Cardiovascular segments, respectively. Definite-lived intangible asset charges are recognized in other operating expense, net in the consolidated statements of income. Indefinite-lived intangible asset impairment charges were not significant for fiscal year 2022. During fiscal year 2021, the Company recognized $45 million of indefinite-lived intangible asset impairment charges related to the abandonment of certain IPR&D projects in the Neuroscience segment. During fiscal year 2020, the Company recognized $35 million of indefinite-lived intangible asset impairment charges, including $25 million relating to a partial impairment of an IPR&D project within the Neuroscience segment and $10 million in connection with the discontinuation of an IPR&D project within the Cardiovascular segment. Indefinite-lived intangible asset impairment charges are recognized in other operating expense, net in the consolidated statements of income. Due to the nature of IPR&D projects, the Company may experience future delays or failures to obtain regulatory approvals to conduct clinical trials, failures of such clinical trials, delays or failures to obtain required market clearances, other failures to achieve a commercially viable product, or the discontinuation of certain projects, and as a result, may recognize impairment losses in the future. Amortization Expense Intangible asset amortization expense was $1.7 billion for fiscal year 2022 and $1.8 billion for fiscal years 2021 and 2020. Estimated aggregate amortization expense by fiscal year based on the current carrying value and remaining estimated useful lives of definite-lived intangible assets at April 29, 2022, excluding any possible future amortization associated with acquired IPR&D which has not met technological feasibility, is as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Property, Plant, and Equipment |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Property, Plant and Equipment [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Property, Plant, and Equipment | Property, Plant, and Equipment Property, plant, and equipment balances and corresponding estimated useful lives were as follows:
Depreciation expense of $974 million, $919 million, and $907 million was recognized in fiscal years 2022, 2021, and 2020, respectively.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Shareholders' Equity |
12 Months Ended |
|---|---|
Apr. 29, 2022 | |
| Stockholders' Equity Note [Abstract] | |
| Shareholders' Equity | Shareholders’ Equity Share Capital Medtronic plc is authorized to issue 2.6 billion Ordinary Shares, $0.0001 par value; 40 thousand Euro Deferred Shares, €1.00 par value; 127.5 million Preferred Shares, $0.20 par value; and 500 thousand A Preferred Shares, $1.00 par value. Euro Deferred Shares The authorized share capital of the Company includes 40 thousand Euro Deferred Shares, with a par value of €1.00 per share. At April 29, 2022, no Euro Deferred Shares were issued or outstanding. Preferred Shares The authorized share capital of the Company includes 127.5 million of Preferred Shares, with a par value of $0.20 per share. At April 29, 2022, no Preferred Shares were issued or outstanding. A Preferred Shares The authorized share capital of the Company includes 500 thousand A Preferred Shares, with a par value of $1.00 per share. During the third quarter of fiscal year 2022 the Company redeemed the previously outstanding 1,872 A Preferred Shares for $0.075 million. At April 29, 2022, no A Preferred Shares were outstanding. Dividends The timing, declaration, and payment of future dividends to holders of the Company's ordinary shares falls within the discretion of the Company's Board of Directors and depends upon many factors, including the statutory requirements of Irish law, the Company's earnings and financial condition, the capital requirements of the Company's businesses, industry practice and any other factors the Board of Directors deems relevant. Ordinary Share Repurchase Program Shares are repurchased on occasion to support the Company’s stock-based compensation programs and to return capital to shareholders. During fiscal years 2022 and 2021, the Company repurchased approximately 22 million and 4 million shares, respectively, at an average price of $113.11 and $126.80, respectively. In March 2019, the Company's Board of Directors authorized $6.0 billion for repurchase of the Company's ordinary shares. There is no specific time-period associated with these repurchase authorizations. At April 29, 2022, the Company had used $3.0 billion of the $6.0 billion authorized under the repurchase program, leaving approximately $3.0 billion available for future repurchases. The Company accounts for repurchases of ordinary shares using the par value method and shares repurchased are cancelled.
|
Stock Purchase and Award Plans |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Share-based Payment Arrangement [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock Purchase and Award Plans | Stock Purchase and Award Plans In fiscal year 2022, the Company granted stock awards under the Medtronic plc 2013 Plan (2013 Plan) and the 2021 Medtronic plc Long Term Incentive Plan (2021 Plan). The 2021 Plan was approved by the Company's shareholders on December 9, 2021, and provides for a maximum of 115 million ordinary shares to be issued, in addition to the 14 million ordinary shares previously approved for issuance under the 2013 Plan. The 2021 Plan provides for the grant of non-qualified and incentive stock options, stock appreciation rights, restricted stock, restricted stock units, performance awards, and other stock and cash-based awards. At April 29, 2022, there were approximately 127 million shares available for future grants under the 2021 Plan. Stock-Based Compensation Expense The following table presents the components and classification of stock-based compensation expense recognized for stock options, restricted stock, performance share units, and employee stock purchase plan (ESPP) in fiscal years 2022, 2021, and 2020:
Stock Options Options are granted at the exercise price, which is equal to the closing price of the Company’s ordinary shares on the grant date. The majority of the Company’s options are non-qualified options with a 10-year life and a 4-year ratable vesting term. The Company uses the Black-Scholes option pricing model (Black-Scholes model) to determine the fair value of stock options at the grant date. The fair value of stock options under the Black-Scholes model requires management to make assumptions regarding projected employee stock option exercise behaviors, risk-free interest rates, volatility of the Company’s stock price, and expected dividends. The following table provides the weighted average fair value of options granted to employees and the related assumptions used in the Black-Scholes model:
The following table summarizes stock option activity during fiscal year 2022:
The following table summarizes the total cash received from the issuance of new shares upon stock option award exercises, the total intrinsic value of options exercised, and the related tax benefit during fiscal years 2022, 2021, and 2020:
Unrecognized compensation expense related to outstanding stock options at April 29, 2022 was $90 million and is expected to be recognized over a weighted average period of 2.5 years. Restricted Stock Restricted stock units are expensed over the vesting period and are subject to forfeiture if employment terminates prior to the lapse of the restrictions. The expense recognized for restricted stock units is equal to the grant date fair value, which is equal to the closing stock price on the date of grant. Restricted stock units either have a 4-year ratable vesting term or cliff vest after three years. The Company also grants shares of performance-based restricted stock units that typically cliff vest after three years only if the Company has also achieved certain performance objectives. Performance awards are expensed over the performance period based on the probability of achieving the performance objectives. Restricted stock units are not considered issued or outstanding ordinary shares of the Company. Dividend equivalent units are accumulated on restricted stock units during the vesting period. The following table summarizes restricted stock activity during fiscal year 2022:
The following table summarizes the weighted-average grant date fair value of restricted stock granted, total fair value of restricted stock vested and related tax benefit during fiscal years 2022, 2021, and 2020:
Unrecognized compensation expense related to restricted stock as of April 29, 2022 was $316 million and is expected to be recognized over a weighted average period of 2.5 years. Performance Share Units Beginning in fiscal year 2021, the Company granted performance share units to officers and key employees. Performance share units typically cliff vest after three years. The awards include three metrics: relative total shareholder return (rTSR), revenue growth, and return on investor capital (ROIC). rTSR is considered a market condition metric, and the expense is determined at the grant date and will not be adjusted even if the market condition is not met. Revenue growth and ROIC are considered performance metrics, and the expense is recorded over the performance period, which will be reassessed each reporting period based on the probability of achieving the various performance conditions. The number of shares earned at the end of the three-year period will vary, based on only actual performance, from 0% to 200% of the target number of performance share units granted. Performance share units are subject to forfeiture if employment terminates prior to the lapse of the restrictions. Performance share units are not considered issued or outstanding ordinary shares of the Company. Dividend equivalent units are accumulated on performance share units for each component of the award during the vesting period. The Company calculates the fair value of the performance share units for each component individually. The fair value of the rTSR metric will be determined using the Monte Carlo valuation model. The fair value of the revenue growth and ROIC metrics are equal to the closing stock price on the grant date. The following table summarizes performance share unit activity during fiscal year 2022:
The following table summarizes the weighted-average grant date fair value of performance share units granted, total fair value of performance share units vested and related tax benefit during fiscal year 2022 and 2021:
Unrecognized compensation expense related to performance share units as of April 29, 2022 was $84 million and is expected to be recognized over a weighted average period of 1.9 years. Employees Stock Purchase Plan The Medtronic plc Amended and Restated 2014 Employees Stock Purchase Plan allows participating employees to purchase the Company's ordinary shares at a discount through payroll deductions. The expense recognized for shares purchased under the Company’s ESPP is equal to the 15 percent discount the employee receives. Employees purchased 2 million shares at an average price of $98.75 per share in fiscal year 2022. At April 29, 2022, approximately 7 million ordinary shares were available for future purchase under the ESPP.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Income Taxes |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income Tax Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income Taxes | Income Taxes The income tax provision (benefit) is based on income before income taxes reported for financial statement purposes. The components of income before income taxes, based on tax jurisdiction, are as follows:
The income tax provision (benefit) consists of the following:
Tax assets (liabilities), shown before jurisdictional netting of deferred tax assets (liabilities), are comprised of the following:
(1)Certain prior year amounts have been reclassified to conform to current year presentation No deferred taxes have been provided on the approximately $79.3 billion and $74.2 billion of undistributed earnings of the Company’s subsidiaries at April 29, 2022 and April 30, 2021, respectively, since these earnings have been, and under current plans will continue to be, permanently reinvested in these subsidiaries. Due to the number of legal entities and jurisdictions involved, the complexity of the legal entity structure of the Company, and the complexity of the tax laws in the relevant jurisdictions, the Company believes it is not practicable to estimate, within any reasonable range, the amount of additional taxes which may be payable upon distribution of these undistributed earnings. At April 29, 2022, the Company had approximately $25.4 billion of net operating loss carryforwards in certain non-U.S. jurisdictions, of which $20.0 billion have no expiration, and the remaining $5.4 billion will expire during fiscal years 2023 through 2042. Included in these net operating loss carryforwards are $18.6 billion of net operating losses related to a subsidiary of the Company, substantially all of which were recorded in fiscal year 2008 as a result of the receipt of a favorable tax ruling from certain non-U.S. taxing authorities. The Company has recorded a full valuation allowance against these net operating losses, as management does not believe that it is more likely than not that these net operating losses will be utilized. Certain of the remaining non-U.S. net operating loss carryforwards of $6.8 billion have a valuation allowance recorded against the carryforwards, as management does not believe that it is more likely than not that these net operating losses will be utilized. At April 29, 2022, the Company had $222 million of U.S. federal net operating loss carryforwards, of which $47 million have no expiration. The remaining loss carryforwards will expire during fiscal years 2023 through 2036. For U.S. state purposes, the Company had $1.4 billion of net operating loss carryforwards at April 29, 2022, $72 million of which have no expiration. The remaining U.S. state loss carryforwards will expire during fiscal years 2023 through 2042. At April 29, 2022, the Company also had $254 million of tax credits available to reduce future income taxes payable, of which $120 million have no expiration. The remaining credits will expire during fiscal years 2023 through 2042. The Company has established valuation allowances of $6.6 billion and $5.8 billion at April 29, 2022 and April 30, 2021, respectively, primarily related to the uncertainty of the utilization of certain deferred tax assets which are primarily comprised of tax loss and credit carryforwards in various jurisdictions. The increase in the valuation allowance during fiscal year 2022 is primarily related to the step up in tax basis for Swiss Cantonal purposes, the generation of certain net operating losses and the effects of currency fluctuations. These valuation allowances would result in a reduction to the income tax provision in the consolidated statements of income if they are ultimately not required. The Company’s effective income tax rate varied from the U.S. federal statutory tax rate as follows:
During fiscal year 2022, the net benefit from certain tax adjustments of $50 million, recognized in income tax provision (benefit) in the consolidated statement of income, included the following: •A benefit of $82 million associated with a step up in tax basis for Swiss Cantonal purposes. •A benefit of $82 million related to a change in tax rates on intangible assets. •A cost of $47 million associated with the amortization of the previously established deferred tax assets from intercompany intellectual property transactions. •A cost of $41 million associated with a change in the Company’s permanent reinvestment assertion on certain historical earnings. •A net cost of $26 million primarily associated with an intercompany sale of assets. During fiscal year 2021, the net benefit from certain tax adjustments of $41 million, recognized in income tax provision (benefit) in the consolidated statement of income, included the following: •A net benefit of $106 million associated with the resolution of an audit at the IRS Appellate level for fiscal years 2012, 2013, and 2014. The issues resolved relate to the utilization of certain net operating losses and the allocation of income between Medtronic, Inc. and its wholly owned subsidiary operating in Puerto Rico for businesses that are not the subject of the U.S. Tax Court Case for fiscal years 2005 and 2006. •A net cost of $73 million related to a tax basis adjustment of previously established deferred tax assets from intercompany intellectual property transactions. The cumulative amount of deferred tax benefit previously recognized from intercompany intellectual property transactions and recorded as Certain Tax Adjustments is $1.5 billion. The corresponding deferred tax assets will be amortized over a period of approximately 20 years. •A cost of $50 million associated with the amortization of the previously established deferred tax assets from intercompany intellectual property transactions. •A net cost of $25 million associated with an internal restructuring and intercompany sale of assets. •A benefit of $83 million related to the capitalization of certain research and development costs for U.S. income tax purposes and the establishment of a deferred tax asset at the U.S. federal statutory tax rate. During fiscal year 2020, the net benefit from certain tax adjustments of $1.2 billion, recognized in income tax provision (benefit) in the consolidated statement of income, included the following: •A net benefit of $63 million related to the finalization of certain state tax impacts from U.S. Tax Reform, and the issuance of certain final U.S. Treasury Regulations associated with U.S. Tax Reform. The primary impact of these regulations resulted in the Company re-establishing its permanently reinvested assertion on certain foreign earnings and reversing the previously accrued tax liability. This benefit was partially offset by additional tax associated with a previously executed internal reorganization of certain foreign subsidiaries. •A benefit of $252 million related to tax legislative changes in Switzerland, which abolished certain preferential tax regimes the Company benefited from and replaced them with a new set of internationally accepted measures. The legislation provided for higher effective tax rates but allowed for a transitional period whereby an amortizable asset was created for Swiss federal income tax purposes that will be amortized and deducted over a 10-year period. •A benefit of $658 million related to the release of a valuation allowance previously recorded against certain net operating losses. Luxembourg enacted tax legislation during the year requiring the Company to reassess the realizability of certain net operating losses. The Company evaluated both the positive and negative evidence and released valuation allowance equal to the expected benefit from the utilization of certain net operating losses in connection with a planned intercompany sale of intellectual property. •A benefit of $269 million associated with the intercompany sale of intellectual property and the establishment of a deferred tax asset. Currently, the Company’s operations in Puerto Rico, Singapore, Dominican Republic, Costa Rica, and China have various tax holidays and tax incentive grants. The tax reductions as compared to the local statutory rate favorably impacted earnings by $248 million, $301 million, and $231 million in fiscal years 2022, 2021, and 2020, respectively, and diluted earnings per share by $0.18, $0.22, and $0.17, in fiscal years 2022, 2021, and 2020, respectively. The tax holidays are conditional upon the Company meeting certain thresholds required under statutory law. The tax incentive grants, unless extended, will expire between fiscal years 2023 and 2034. The Company’s historical practice has been to renew, extend, or obtain new tax incentive grants upon expiration of existing tax incentive grants. If the Company is not able to renew, extend, or obtain new tax incentive grants, the expiration of existing tax incentive grants could have a material impact on the Company’s financial results in future periods. The tax incentive grants which expired during fiscal year 2022 did not have a material impact on the Company's consolidated financial statements. The Company had $1.7 billion, $1.7 billion, and $1.9 billion of gross unrecognized tax benefits at April 29, 2022, April 30, 2021, and April 24, 2020, respectively. A reconciliation of the beginning and ending amount of unrecognized tax benefits for fiscal years 2022, 2021, and 2020 is as follows:
If all of the Company’s unrecognized tax benefits at April 29, 2022, April 30, 2021, and April 24, 2020 were recognized, $1.6 billion, $1.6 billion, and $1.8 billion would impact the Company’s effective tax rate, respectively. Although the Company believes that it has adequately provided for liabilities resulting from tax assessments by taxing authorities, positions taken by these tax authorities could have a material impact on the Company’s effective tax rate in future periods. The Company has recorded gross unrecognized tax benefits, net of cash advance, of $787 million as a noncurrent liability. The Company estimates that within the next 12 months it is reasonably possible that its uncertain tax positions excluding interest, could decrease by as much as $15 million, net as a result of statute of limitation lapses. The Company recognizes interest and penalties related to income tax matters in income tax provision (benefit) in the consolidated statements of income and records the liability in the current or noncurrent accrued income taxes in the consolidated balance sheets, as appropriate. The Company had $117 million, $99 million, and $225 million of accrued gross interest and penalties at April 29, 2022, April 30, 2021, and April 24, 2020, respectively. During fiscal years 2022, 2021, and 2020, the Company recognized gross interest expense of $17 million, income of $44 million, and expense of $53 million, respectively, in income tax provision (benefit) in the consolidated statements of income. The Company reserves for uncertain tax positions related to unresolved matters with the IRS and other taxing authorities. These reserves are subject to a high degree of estimation and management judgment. Resolution of these significant unresolved matters, or positions taken by the IRS or other tax authorities during future tax audits, could have a material impact on the Company’s financial results in future periods. The Company continues to believe that its reserves for uncertain tax positions are appropriate and that it has meritorious defenses for its tax filings and will vigorously defend them during the audit process, appellate process, and through litigation in courts, as necessary. The major tax jurisdictions where the Company conducts business which remain subject to examination are as follows:
See Note 18 for additional information regarding the status of current tax audits and proceedings.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Earnings Per Share |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Earnings Per Share [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Earnings Per Share | Earnings Per Share Basic earnings per share is computed based on the weighted average number of ordinary shares outstanding. Diluted earnings per share is computed based on the weighted number of ordinary shares outstanding, increased by the number of additional shares that would have been outstanding had the potentially dilutive ordinary shares been issued, and reduced by the number of shares the Company could have repurchased with the proceeds from issuance of the potentially dilutive shares. Potentially dilutive ordinary shares include stock-based awards granted under stock-based compensation plans and shares committed to be purchased under the employee stock purchase plan. The table below sets forth the computation of basic and diluted earnings per share:
The calculation of weighted average diluted shares outstanding excludes options to purchase approximately 5 million ordinary shares in fiscal year 2022 and 4 million ordinary shares in fiscal years 2021 and 2020 because their effect would have been anti-dilutive on the Company’s earnings per share.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Retirement Benefit Plans |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Retirement Benefits [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Retirement Benefit Plans | Retirement Benefit Plans The Company sponsors various retirement benefit plans, including defined benefit pension plans, post-retirement medical plans, defined contribution savings plans, and termination indemnity plans, covering substantially all U.S. employees and many employees outside the U.S. The net expense related to these plans was $459 million, $668 million, and $467 million in fiscal years 2022, 2021, and 2020, respectively. In the U.S., the Company maintains qualified pension plans designed to provide guaranteed minimum retirement benefits to all eligible U.S. participants. Pension coverage for non-U.S. employees is provided, to the extent deemed appropriate, through separate plans. In addition to the benefits provided under the qualified pension plan, retirement benefits associated with wages in excess of the IRS allowable limits are provided to certain employees under a non-qualified plan. U.S. and Puerto Rico employees are also eligible to receive a medical benefit component, in addition to normal retirement benefits, through the Company’s post-retirement benefits. At April 29, 2022 and April 30, 2021, the funded status of the Company’s benefit plans was $74 million overfunded and $705 million underfunded, respectively. During fiscal year 2021, as part of the Simplification restructuring program, the Company offered certain eligible U.S. employees voluntary early retirement packages, resulting in incremental expense of $97 million recognized. Of this amount, $73 million related to U.S. pension benefits, $11 million related to defined contribution plans, $11 million related to U.S. post-retirement benefits, and $2 million related to cash payments and administrative fees. See Note 4 for additional information on the Simplification restructuring program. As of April 24, 2020, the Company announced the freezing of U.S. pension benefits beginning in 2027. Employees will continue to earn benefits as required by the plan until April 30, 2027, after which date benefits will no longer be earned and employees will earn benefits under a new defined contribution structure. The Company recognized curtailment benefits of $94 million in fiscal year 2020 as a result of this change. Defined Benefit Pension Plans The change in benefit obligation and funded status of the Company’s U.S. and Non-U.S. pension benefits are as follows:
(1)Actuarial gains and losses result from changes in actuarial assumptions (such as changes in the discount rate and revised mortality rates). The actuarial gain in fiscal year 2022 was primarily related to increases in discount rates. The actuarial loss in fiscal year 2021 was primarily related to decreases in discount rates. In certain countries outside the U.S., fully funding pension plans is not a common practice, as funding provides no income tax benefit. Consequently, certain pension plans were partially funded at April 29, 2022 and April 30, 2021. U.S. and non-U.S. pension plans with accumulated benefit obligations in excess of plan assets consist of the following:
U.S. and non-U.S. pension plans with projected benefit obligations in excess of plan assets consist of the following:
The net periodic benefit cost of the plans includes the following components:
The other changes in plan assets and projected benefit obligations recognized in other comprehensive income for fiscal year 2022 are as follows:
The actuarial assumptions are as follows:
The Company utilizes a full yield curve approach methodology to estimate the service and interest cost components of net periodic pension cost and net periodic post-retirement benefit cost for the Company’s pension and other post-retirement benefits. The full yield curve approach applies specific spot rates along the yield curve to their underlying projected cash flows in estimation of the cost components. The current yield curves represent high quality, long-term fixed income instruments. The expected long-term rate of return on plan assets assumptions are determined using a building block approach, considering historical averages and real returns of each asset class. In certain countries, where historical returns are not meaningful, consideration is given to local market expectations of long-term returns. Retirement Benefit Plan Investment Strategy The Company sponsors trusts that hold the assets for U.S. pension plans and other U.S. post-retirement benefit plans, primarily retiree medical benefits. For investment purposes, the Medtronic U.S. pension and other U.S. post-retirement benefit plans employ similar investment strategies with different asset allocation targets. The Company has a Qualified Plan Committee (the Plan Committee) that sets investment guidelines for U.S. pension plans and other U.S. post-retirement benefit plans with the assistance of external consultants. These guidelines are established based on market conditions, risk tolerance, funding requirements, and expected benefit payments. The Plan Committee also oversees the investment allocation process, selects the investment managers, and monitors asset performance. As pension liabilities are long-term in nature, the Company employs a long-term total return approach to maximize the long-term rate of return on plan assets for a prudent level of risk. An annual analysis on the risk versus the return of the investment portfolio is conducted to justify the expected long-term rate of return assumption. The investment portfolios contain a diversified allocation of investment categories, including equities, fixed income securities, hedge funds, and private equity. Securities are also diversified in terms of domestic and international, short- and long-term, growth and value styles, large cap and small cap stocks, and active and passive management. Outside the U.S., pension plan assets are typically managed by decentralized fiduciary committees. There is significant variation in policy asset allocation from country to country. Local regulations, funding rules, and financial and tax considerations are part of the funding and investment allocation process in each country. The weighted average target asset allocations at April 29, 2022 for the plans are 41% equity securities, 33% debt securities, and 26% other. The plans did not hold any investments in the Company’s ordinary shares at April 29, 2022 or April 30, 2021. The Company’s U.S. plans target asset allocations at April 29, 2022, compared to the U.S. plans actual asset allocations at April 29, 2022 and April 30, 2021 by asset category, are as follows:
Strong performance on equity securities during the fiscal year resulted in asset allocations different than targets. Management expects to move the allocations closer to target over the intermediate term. Retirement Benefit Plan Asset Fair Values The following is a description of the valuation methodologies used for retirement benefit plan assets measured at fair value: Short-term investments: Valued at the closing price reported in the active markets in which the individual security is traded. Mutual funds: Comprised of investments in equity and fixed income securities held in pooled investment vehicles. The valuations of mutual funds are based on the respective net asset values which are determined by the fund daily at market close. The net asset values are calculated based on the valuation of the underlying assets which are determined using observable inputs. The net asset values are publicly reported. Equity commingled trusts: Comprised of investments in equity securities held in pooled investment vehicles. The valuations of equity commingled trusts are based on the respective net asset values which are determined by the fund daily at market close. The net asset values are calculated based on the valuation of the underlying assets which are determined using observable inputs. The net asset values are not publicly reported, and funds are valued at the net asset value practical expedient. Fixed income commingled trusts: Comprised of investments in fixed income securities held in pooled investment vehicles. The valuations of fixed income commingled trusts are based on the respective net asset values which are determined by the fund daily at market close. The net asset values are calculated based on the valuation of the underlying assets which are determined using observable inputs. The net asset values are not publicly reported, and funds are valued at the net asset value practical expedient. Partnership units: Valued based on the year-end net asset values of the underlying partnerships. The net asset values of the partnerships are based on the fair values of the underlying investments of the partnerships. Quoted market prices are used to value the underlying investments of the partnerships, where the partnerships consist of the investment pools which invest primarily in common stocks. Partnership units include partnerships, private equity investments, and real asset investments. Partnerships primarily include long/short equity and absolute return strategies. These investments may be redeemed monthly with notice periods ranging from 45 to 95 days. At April 29, 2022, there are no funds in the process of liquidation. Private equity investments consist of common stock and debt instruments of private companies. For private equity funds, the sum of the unfunded commitments at April 29, 2022 is $204 million, and the estimated liquidation period of these funds is expected to be to 15 years. Real asset investments consist of commodities, derivatives, Real Estate Investment Trusts, and illiquid real estate holdings. These investments have redemption and liquidation periods ranging from 30 days to 10 years. At April 29, 2022, there are no real estate investments in the process of liquidation. Valuation procedures are utilized to arrive at fair value if a quoted market price is not available for a partnership investment. Registered investment companies: Valued at net asset values which are not publicly reported. The net asset values are calculated based on the valuation of the underlying assets. The underlying assets are valued at the quoted market prices of shares held by the plan at year-end in the active market on which the individual securities are traded. Insurance contracts: Comprised of investments in collective (group) insurance contracts, consisting of individual insurance policies. The policyholder is the employer, and each member is the owner/beneficiary of their individual insurance policy. These policies are a part of the insurance company’s general portfolio and participate in the insurer’s profit-sharing policy on an excess yield basis. The methods described above may produce fair values that may not be indicative of net realizable value or reflective of future fair values. Furthermore, while the Company believes its valuation methodologies are appropriate and consistent with other market participants, the use of different methodologies or assumptions to determine fair value of certain financial instruments could result in a different fair value measurement at the reporting date. The following tables provide information by level for the retirement benefit plan assets that are measured at fair value, as defined by U.S. GAAP. Certain investments for which the fair value is measured using the net asset value per share (or its equivalent) practical expedient are not presented within the fair value hierarchy. The fair value amounts presented for these investments are intended to permit reconciliation to the total fair value of plan assets at April 29, 2022 and April 30, 2021. U.S. Pension Benefits
The following tables provide a reconciliation of the beginning and ending balances of U.S. pension benefit assets measured at fair value that used significant unobservable inputs (Level 3):
Non-U.S. Pension Benefits
Non-U.S. pension benefit assets that are valued using significant unobservable inputs (Level 3) was $43 million and $49 million as of April 29, 2022 and April 30, 2021, respectively. The decrease in the fair value of the assets was due to insurance contracts being sold. There were no transfers into or out of Level 3 for both the U.S. and non-U.S. pension plans during the fiscal years ended April 29, 2022 and April 30, 2021. Retirement Benefit Plan Funding It is the Company’s policy to fund retirement costs within the limits of allowable tax deductions. During fiscal year 2022, the Company made discretionary contributions of approximately $24 million to the U.S. pension plan. Internationally, the Company contributed approximately $70 million for pension benefits during fiscal year 2022. The Company anticipates that it will make contributions of $21 million and $52 million to its U.S. pension benefit plans and non-U.S. pension benefit plans, respectively, in fiscal year 2023. Based on the guidelines under the U.S. Employee Retirement Income Security Act of 1974 and the various guidelines which govern the plans outside the U.S., the majority of anticipated fiscal year 2023 contributions will be discretionary. The Company believes that pension assets, returns on invested pension assets, and Company contributions will be able to meet its pension and other post-retirement obligations in the future. Retiree benefit payments, which reflect expected future service, are anticipated to be paid as follows:
Post-retirement Benefit Plans The net periodic benefit cost associated with the Company’s post-retirement benefit plans was income of $20 million, $6 million, and $15 million in fiscal years 2022, 2021, and 2020, respectively. The Company’s projected benefit obligation for all post-retirement benefit plans was $276 million and $337 million at April 29, 2022 and April 30, 2021, respectively. The Company’s fair value of plan assets for all post-retirement benefit plans was $325 million and $345 million at April 29, 2022 and April 30, 2021, respectively. The post-retirement benefit plan assets at both April 29, 2022 and April 30, 2021 primarily comprised of equity and fixed commingled trusts, consistent with the U.S. retirement benefit plan assets outlined in the fair value leveling tables above. Defined Contribution Savings Plans The Company has defined contribution savings plans that cover substantially all U.S. employees and certain non-U.S. employees. The general purpose of these plans is to provide additional financial security during retirement by providing employees with an incentive to make regular savings. Company contributions to the plans are based on employee contributions and Company performance. Expense recognized under these plans was $403 million, $495 million, and $376 million in fiscal years 2022, 2021, and 2020, respectively. Effective May 1, 2005, the Company froze participation in the original defined benefit pension plan in the U.S. and implemented two new plans: an additional defined benefit pension plan, the Personal Pension Account (PPA), and a new defined contribution plan, the Personal Investment Account (PIA). Employees in the U.S. hired on or after May 1, 2005 but before January 1, 2016 had the option to participate in either the PPA or the PIA. Participants in the PPA receive an annual allocation of their salary and bonus on which they will receive an annual guaranteed rate of return, which is based on the ten-year Treasury bond rate. Participants in the PIA also receive an annual allocation of their salary and bonus; however, they are allowed to determine how to invest their funds among identified fund alternatives. The cost associated with the PPA is included in U.S. Pension Benefits in the tables presented earlier. The defined contribution cost associated with the PIA was approximately $48 million, $50 million, and $52 million in fiscal years 2022, 2021, and 2020, respectively. Effective January 1, 2016, the Company froze participation in the existing defined benefit (PPA) and contribution (PIA) pension plans in the U.S. and implemented a new form of benefit under the existing defined contribution plan for legacy Covidien employees and employees in the U.S. hired on or after January 1, 2016 or rehired after July 1, 2020. Participants in the Medtronic Core Contribution (MCC) also receive an annual allocation of their salary and bonus and are allowed to determine how to invest their funds among identified fund alternatives. The defined contribution cost associated with the MCC was approximately $83 million, $73 million, and $66 million and in fiscal years 2022, 2021, and 2020, respectively.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Leases |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leases [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leases | Leases The Company leases office, manufacturing, and research facilities and warehouses, as well as transportation, data processing, and other equipment. The Company determines whether a contract is a lease or contains a lease at inception date. Upon commencement, the Company recognizes a right-of-use asset and lease liability. Right-of-use assets represent the Company's right to use the underlying asset for the lease term. Lease liabilities are the Company's obligation to make the lease payments arising from a lease. As the Company’s leases typically do not provide an implicit rate, the Company’s lease liabilities are measured on a discounted basis using the Company's incremental borrowing rate. Lease terms used in the recognition of right-of-use assets and lease liabilities include only options to extend the lease that are reasonably certain to be exercised. Additionally, lease terms underlying the right-of-use assets and lease liabilities consider terminations that are reasonably certain to be executed. The Company's lease agreements include leases that have both lease and associated nonlease components. The Company has elected to account for lease components and the associated nonlease components as a single lease component. The consolidated balance sheets do not include recognized assets or liabilities for leases that, at the commencement date, have a term of twelve months or less and do not include an option to purchase the underlying asset that is reasonably certain to be exercised. The Company recognizes such leases in the consolidated statements of income on a straight-line basis over the lease term. Additionally, the Company recognizes variable lease payments not included in its lease liabilities in the period in which the obligation for those payments is incurred. Variable lease payments for fiscal year 2022, 2021, and 2020 were not material. The Company's lease agreements include leases accounted for as operating leases and those accounted for as finance leases. The right-of-use assets, lease liabilities, lease costs, cash flows, and lease maturities associated with the Company's finance leases were not material to the consolidated financial statements at April 29, 2022 or April 30, 2021 or for fiscal year 2022, 2021 and 2020. Finance lease right-of-use assets are included in property, plant, and equipment, net, and finance lease liabilities are included in current debt obligations and long-term debt on the consolidated balance sheets. The following table summarizes the balance sheet classification of the Company's operating leases and amounts of the right-of-use assets and lease liabilities at April 29, 2022 and April 30, 2021:
The following table summarizes the weighted-average remaining lease term and weighted-average discount rate for the Company's operating leases at April 29, 2022, April 30, 2021, and April 24, 2020:
The following table summarizes the components of total operating lease cost for fiscal year 2022, 2021, and 2020:
The following table summarizes the cash paid for amounts included in the measurement of operating lease liabilities and right-of-use assets obtained in exchange for operating lease liabilities for fiscal year 2022, 2021, and 2020:
The following table summarizes the maturities of the Company's operating leases at April 29, 2022:
The Company makes certain products available to customers under lease arrangements, including arrangements whereby equipment is placed with customers who then purchase consumable products to accompany the use of the equipment. Income arising from arrangements where the Company is the lessor is recognized within net sales in the consolidated statements of income and the Company's net investments in sales-type leases are included in other current assets and other assets in the consolidated balance sheets. Lessor income and the related assets and lease maturities were not material to the consolidated financial statements at or for the fiscal year ended April 29, 2022 and April 30, 2021.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Accumulated Other Comprehensive Loss |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Equity [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accumulated Other Comprehensive Loss | Accumulated Other Comprehensive Loss The following table provides changes in AOCI, net of tax and by component:
The income tax on gains and losses on investment securities in other comprehensive income before reclassifications during fiscal years 2022, 2021, and 2020 was a benefit of $51 million, an expense of $31 million and a benefit of $13 million, respectively. During fiscal years 2022, 2021, and 2020, realized gains and losses on investment securities reclassified from AOCI were reduced by income taxes of $1 million, $2 million and $3 million, respectively. When realized, gains and losses on investment securities reclassified from AOCI are recognized within other non-operating income, net. Refer to Note 5 for additional information. During fiscal years 2022, 2021, and 2020, the income tax on cumulative translation adjustment was a benefit of $8 million, an expense of $7 million, and a benefit of $9 million, respectively. During fiscal years 2022, 2021, and 2020, there were no tax impacts on net investment hedges. Refer to Note 7 for additional information. The net change in retirement obligations in other comprehensive income includes amortization of net actuarial losses included in net periodic benefit cost. The income tax on the net change in retirement obligations in other comprehensive income before reclassifications during fiscal years 2022, 2021, and 2020 resulted in an expense of $134 million and $115 million, and a benefit of $159 million, respectively. During fiscal years 2022, 2021, and 2020, the gains and losses on defined benefit and pension items reclassified from AOCI were reduced by income taxes of $20 million, $16 million, and $12 million, respectively. When realized, net gains and losses on defined benefit and pension items reclassified from AOCI are recognized within other non-operating income, net. Refer to Note 15 for additional information. The income tax on unrealized gains and losses on cash flow hedges in other comprehensive income before reclassifications during fiscal years 2022, 2021, and 2020 was an expense of $152 million, a benefit of $87 million, and an expense of $88 million, respectively. Amounts reclassified from AOCI related to cash flow hedges included income taxes of $26 million, $14 million, and $80 million for fiscal years 2022, 2021, and 2020, respectively. When realized, gains and losses on currency exchange rate contracts reclassified from AOCI are recognized within other operating expense, net or cost of products sold. Refer to Note 7 for additional information.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Commitments and Contingencies |
12 Months Ended |
|---|---|
Apr. 29, 2022 | |
| Commitments and Contingencies Disclosure [Abstract] | |
| Commitments and Contingencies | Commitments and Contingencies Legal Matters The Company and its affiliates are involved in a number of legal actions from time to time involving product liability, employment, intellectual property and commercial disputes, shareholder related matters, environmental proceedings, tax disputes, and governmental proceedings and investigations, including those described below. With respect to governmental proceedings and investigations, like other companies in our industry, the Company is subject to extensive regulation by national, state, and local governmental agencies in the United States and in other jurisdictions in which the Company and its affiliates operate. As a result, interaction with governmental agencies is ongoing. The Company’s standard practice is to cooperate with regulators and investigators in responding to inquiries. The outcomes of legal actions are not within the Company’s complete control and may not be known for prolonged periods of time. In some actions, the enforcement agencies or private claimants seek damages, as well as other civil or criminal remedies (including injunctions barring the sale of products that are the subject of the proceeding), that could require significant expenditures, result in lost revenues, or limit the Company's ability to conduct business in the applicable jurisdictions. The Company records a liability in the consolidated financial statements on an undiscounted basis for loss contingencies related to legal actions when a loss is known or considered probable and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. When determining the estimated loss or range of loss, significant judgment is required. Estimates of probable losses resulting from litigation and governmental proceedings involving the Company are inherently difficult to predict, particularly when the matters are in early procedural stages with incomplete scientific facts or legal discovery, involve unsubstantiated or indeterminate claims for damages, potentially involve penalties, fines or punitive damages, or could result in a change in business practice. The Company classifies certain specified litigation charges and gains related to significant legal matters as certain litigation charges in the consolidated statements of income. During fiscal years 2022, 2021, and 2020, the Company recognized $95 million, $118 million, and $313 million, respectively, of additional certain litigation charges. At April 29, 2022 and April 30, 2021, total accrued litigation charges were approximately $0.3 billion and $0.4 billion, respectively. The ultimate cost to the Company with respect to accrued litigation could be materially different than the amount of the current estimates and accruals and could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows. The Company includes accrued litigation in other accrued expenses and other liabilities on the consolidated balance sheets. While it is not possible to predict the outcome for most of the legal matters discussed below, the Company believes it is possible that the costs associated with these matters could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows. Product Liability Matters Pelvic Mesh Litigation The Company is currently involved in litigation in various state and federal courts against manufacturers of pelvic mesh products alleging personal injuries resulting from the implantation of those products. Two subsidiaries of Covidien supplied pelvic mesh products to one of the manufacturers, C.R. Bard (Bard), named in the litigation. The litigation includes a federal multi-district litigation in the U.S. District Court for the Northern District of West Virginia and cases in various state courts and jurisdictions outside the U.S. Generally, complaints allege design and manufacturing claims, failure to warn, breach of warranty, fraud, violations of state consumer protection laws and loss of consortium claims. In fiscal year 2016, Bard paid the Company $121 million towards the settlement of 11,000 of these claims. In May 2017, the agreement with Bard was amended to extend the terms to apply to up to an additional 5,000 claims. That agreement does not resolve the dispute between the Company and Bard with respect to claims that do not settle, if any. As part of the agreement, the Company and Bard agreed to dismiss without prejudice their pending litigation with respect to Bard’s obligation to defend and indemnify the Company. The Company estimates law firms representing approximately 16,200 claimants have asserted or may assert claims involving products manufactured by Covidien’s subsidiaries. As of June 1, 2022, the Company had reached agreements to settle approximately 15,900 of these claims. The Company's accrued expenses for this matter are included within accrued litigation as discussed above. Hernia Mesh Litigation Starting in fiscal year 2020, plaintiffs began filing lawsuits against certain subsidiaries of the Company in U.S. state and federal courts alleging personal injury from hernia mesh products sold by those subsidiaries. The majority of the pending cases are in Massachusetts state court, where they have been consolidated before a single judge. As of June 6, 2022, subsidiaries of the Company have been named as defendants in lawsuits filed on behalf of approximately 5,900 individual plaintiffs, and certain plaintiffs’ law firms have advised the Company that they may file additional cases in the future. On June 6, 2022, the Judicial Panel on Multidistrict Litigation transferred 83 actions involving the Company’s hernia mesh to a federal Multidistrict Litigation in the U.S. District Court for the District of Massachusetts for pretrial proceedings. The pending lawsuits relate almost entirely to hernia mesh products that have not been subject to recalls, withdrawals, or other adverse regulatory action. The Company has not recorded an expense related to damages in connection with these matters because any potential loss is not currently probable and reasonably estimable. Additionally, the Company is unable to reasonably estimate the range of loss, if any, that may result from these matters. Environmental Proceedings The Company is involved in various stages of investigation and cleanup related to environmental remediation matters at a number of sites. These projects relate to a variety of activities, including removal of solvents, metals and other hazardous substances from soil and groundwater. The ultimate cost of site cleanup and timing of future cash flows is difficult to predict given uncertainties regarding the extent of the required cleanup, the interpretation of applicable laws and regulations, and alternative cleanup methods. The Company is a successor to a company which owned and operated a chemical manufacturing facility in Orrington, Maine from 1967 until 1982, and is responsible for the costs of completing an environmental site investigation as required by the Maine Department of Environmental Protection (MDEP). MDEP served a compliance order on Mallinckrodt LLC and U.S. Surgical Corporation, subsidiaries of Covidien, in December 2008, which included a directive to remove a significant volume of soils at the site. After a hearing on the compliance order before the Maine Board of Environmental Protection (Maine Board) to challenge the terms of the compliance order, the Maine Board modified the MDEP order and issued a final order requiring removal of two landfills, capping of the remaining three landfills, installation of a groundwater extraction system and long-term monitoring of the site and the three remaining landfills. The Company has proceeded with remediation in accordance with the MDEP order as modified by the Maine Board order. Since the early 2000s, the Company or its predecessors have also been involved in a lawsuit filed in the U.S. District Court for the District of Maine by the Natural Resources Defense Council and the Maine People’s Alliance. Plaintiffs sought an injunction requiring the Company's predecessor to conduct extensive studies of mercury contamination of the Penobscot River and Bay and options for remediating such contamination, and to perform appropriate remedial activities, if necessary. Following a trial in March 2002, the court held that conditions in the Penobscot River and Bay may pose an imminent and substantial endangerment and that the Company’s predecessor was liable for the cost of performing a study of the River and Bay. Following a second trial in June 2014, the court ordered that further engineering study and engineering design work was needed to determine the nature and extent of remediation in the Penobscot River and Bay. The court also appointed an engineering firm to conduct such studies and issue a report on potential remediation alternatives. In connection with these proceedings, reports have been produced including a variety of cost estimates for a variety of potential remedial options. In March 2021, the parties notified the court that they had agreed on a settlement in principle of all issues in this matter. Finalization of the proposed settlement remains subject to court approval. The Company's accrued expenses for environmental proceedings are included within accrued litigation as discussed above. Income Taxes In March 2009, the IRS issued its audit report on Medtronic, Inc. for fiscal years 2005 and 2006. Medtronic, Inc. reached agreement with the IRS on some, but not all matters related to these fiscal years. The remaining unresolved issue for fiscal years 2005 and 2006 relates to the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico, which is one of the Company's key manufacturing sites. The U.S. Tax Court reviewed this dispute, and in June 2016, issued an opinion with respect to the allocation of income between the parties for fiscal years 2005 and 2006. The Tax Court generally rejected the IRS’s position, but also made certain modifications to the Medtronic, Inc. tax returns as filed. In April 2017, the IRS filed a Notice of Appeal to the U.S. Court of Appeals for the Eighth Circuit regarding the Tax Court opinion. Oral argument for the Appeal occurred in March 2018. The Court of Appeals issued its opinion in August 2018 and remanded the case back to the Tax Court for additional factual findings. The Tax Court trial relating to the issues remanded by the Court of Appeals concluded during June 2021. The parties are awaiting the Tax Court decision, which will remain subject to appeal by either party upon its issuance. The IRS has issued its audit reports on Medtronic, Inc. for fiscal years 2007 through 2016. Medtronic, Inc. and the IRS have reached agreement on all significant issues except for the allocation of income between Medtronic, Inc. and its wholly-owned subsidiary operating in Puerto Rico for the businesses that are the subject of the U.S. Tax Court matter for fiscal years 2005 and 2006. Medtronic, Inc.’s fiscal years 2017, 2018, and 2019 U.S. federal income tax returns are currently being audited by the IRS. Covidien LP (a wholly owned subsidiary of Medtronic plc) has either reached agreement with the IRS or the statute of limitations has lapsed on its U.S. federal income tax returns through fiscal year 2018. Although it is not possible to predict the outcome for most of the income tax matters discussed above, the Company believes it is possible that charges associated with these matters could have a material adverse impact on the Company’s consolidated earnings, financial position, and/or cash flows. Refer to Note 13 for additional discussion of income taxes. Guarantees In the normal course of business, the Company and/or its affiliates periodically enter into agreements that require one or more of the Company and/or its affiliates to indemnify customers or suppliers for specific risks, such as claims for injury or property damage arising as a result of the Company or its affiliates’ products, the negligence of the Company's personnel, or claims alleging that the Company's products infringe on third-party patents or other intellectual property. The Company also offers warranties on various products. The Company’s maximum exposure under these guarantees is unable to be estimated. Historically, the Company has not experienced significant losses on these types of guarantees. The Company believes the ultimate resolution of the above guarantees is not expected to have a material effect on the Company’s consolidated earnings, financial position, and/or cash flows.
|
Segment and Geographic Information |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segment Reporting [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segment and Geographic Information | Segment and Geographic Information There were no changes to the reportable segments during the fiscal year ended April 29, 2022. The Company's four principal operating and reportable segments are as follows: Cardiovascular Portfolio, Neuroscience Portfolio, Medical Surgical Portfolio, and Diabetes Operating Unit. The Company's management has chosen to organize the entity based upon therapy solutions provided by each segment. The four principal segments are strategic businesses that are managed separately, as each one develops and manufactures products and provides services oriented toward targeted therapy solutions. The primary products and services from which the Cardiovascular Portfolio segment derives its revenues include products for the diagnosis, treatment, and management of cardiac rhythm disorders and cardiovascular disease, as well as services to diagnose, treat, and manage heart and vascular-related disorders and diseases. The primary products and services from which the Medical Surgical Portfolio segment derives its revenues include those focused on diseases of the respiratory system, gastrointestinal tract, renal system, lungs, pelvic region, kidneys, obesity, and other preventable complications. The primary products and services from which the Neuroscience Portfolio segment derives its revenues include those focused on neurostimulation therapies and drug delivery systems for the treatment of chronic pain, as well as various areas of the spine and brain, along with pelvic health and conditions of the ear, nose, and throat. The primary products from which the Diabetes Operating Unit segment derives its revenues include those focused on diabetes management, including insulin pumps, continuous glucose monitoring systems, smart insulin pens, and insulin pump consumables. Segment disclosures are on a performance basis, consistent with internal management reporting. Net sales of the Company's segments include end-customer revenues from the sale of products the segment develops, manufactures, and distributes. Refer to Note 2 for discussion on net sales by segment. There are certain corporate and centralized expenses that are not allocated to the segments. The Company's management evaluates the performance of the segments and allocates resources based on net sales and segment operating profit. Segment operating profit represents income before income taxes, excluding interest expense, amortization of intangible assets, centralized distribution costs, non-operating income or expense items, certain corporate charges, and other items not allocated to the segments. The accounting policies of the segments are the same as those described in Note 1. Certain depreciable assets may be recorded by one segment, while the depreciation expense is allocated to another segment. The allocation of depreciation expense is based on the proportion of the assets used by each segment. Segment Operating Profit
Total Assets and Depreciation Expense
Geographic Information Net sales are attributed to the country based on the location of the customer taking possession of the products or in which the services are rendered. Geographic property, plant, and equipment are attributed to the country based on the physical location of the assets. The following table presents net sales for fiscal years 2022, 2021, and 2020, and property, plant, and equipment, net at April 29, 2022 and April 30, 2021 for the Company's country of domicile, countries with significant concentrations, and all other countries:
No single customer represented over 10 percent of the Company’s consolidated net sales in fiscal years 2022, 2021, or 2020.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Subsequent Events |
12 Months Ended |
|---|---|
Apr. 29, 2022 | |
| Subsequent Events [Abstract] | |
| Subsequent Events | Subsequent Events On May 25, 2022, the Company and DaVita Inc. (“DaVita”) entered into a definitive agreement with the intent to form a new, independent kidney care-focused medical device company (“NewCo”) with equal equity ownership. The transaction is expected to close in calendar year 2023, subject to customary regulatory approvals and closing conditions. Medtronic is contributing its entire Renal Care Solutions business (“RCS”) to NewCo. RCS is part of the Respiratory, Gastrointestinal, and Renal division in the Company’s Medical Surgical portfolio, and had revenue of $325 million in fiscal year 2022. |
Schedule II |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SEC Schedule, 12-09, Valuation and Qualifying Accounts [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule II - Valuation and Qualifying Accounts | MEDTRONIC PLC AND SUBSIDIARIES SCHEDULE II – VALUATION AND QUALIFYING ACCOUNTS (in millions)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Summary of Significant Accounting Policies (Policies) |
12 Months Ended |
|---|---|
Apr. 29, 2022 | |
| Accounting Policies [Abstract] | |
| Principles of Consolidation | The consolidated financial statements include the accounts of Medtronic plc, its wholly-owned subsidiaries, entities for which the Company has a controlling financial interest, and variable interest entities for which the Company is the primary beneficiary. Intercompany transactions and balances have been fully eliminated in consolidation. |
| Reclassifications | Certain reclassifications have been made to prior year financial statements to conform to classifications used in the current year. |
| Use of Estimates | The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States (U.S.) (U.S. GAAP) requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Estimates are used when accounting for items such as income taxes, contingencies, intangible asset, and liability valuations. Actual results may or may not differ from those estimates. |
| Fiscal Year-End | The Company utilizes a 52/53-week fiscal year, ending the last Friday in April, for the presentation of its consolidated financial statements and related notes thereto at April 29, 2022 and April 30, 2021 and for each of the three fiscal years ended April 29, 2022 (fiscal year 2022), April 30, 2021 (fiscal year 2021), and April 24, 2020 (fiscal year 2020). Fiscal year 2021 was a 53-week year, with the extra week having occurred in the first fiscal month of the first quarter. |
| Cash Equivalents | The Company considers highly liquid investments with maturities of three months or less from the date of purchase to be cash equivalents. These investments are carried at cost, which approximates fair value. |
| Investments | The Company invests in marketable debt and equity securities, investments that do not have readily determinable fair values, and investments accounted for under the equity method.Marketable debt securities are classified and accounted for as available-for-sale. These investments are recorded at fair value in the consolidated balance sheets. The change in fair value for available-for-sale securities is recorded, net of taxes, as a component of accumulated other comprehensive loss on the consolidated balance sheets. The Company determines the appropriate classification of its investments in marketable debt securities at the time of purchase and reevaluates such determinations at each balance sheet date. The classification of marketable debt securities as current or long-term is based on the nature of the securities and the availability for use in current operations consistent with the Company's management of its capital structure and liquidity.Certain of the Company’s investments in marketable equity securities and other securities are long-term, strategic investments in companies that are in various stages of development and are included in other assets on the consolidated balance sheets. Marketable equity securities are recorded at fair value in the consolidated balance sheets. The change in fair value of marketable equity securities is recognized within other non-operating income, net in the consolidated statements of income. At each reporting period, the Company makes a qualitative assessment considering impairment indicators to evaluate whether the investment is impaired. Equity securities accounted for under the equity method are initially recorded at the amount of the Company’s investment and are adjusted each period for the Company’s share of the investee’s income or loss and dividends paid. Securities accounted for under the equity method are reviewed quarterly for changes in circumstance or the occurrence of events that suggest other than temporary impairment has occurred. |
| Accounts Receivable and Allowance for Doubtful Accounts and Credit Losses | The Company grants credit to customers in the normal course of business and maintains an allowance for doubtful accounts for potential credit losses. When evaluating allowances for doubtful accounts, the Company considers various factors, including historical experience and customer-specific information. Uncollectible accounts are written-off against the allowance when it is deemed that a customer account is uncollectible. |
| Inventories | Inventories are stated at the lower of cost or net realizable value, with cost determined on a first-in, first-out basis. The Company reduces the carrying value of inventories for items that are potentially excess, obsolete, or slow-moving based on changes in customer demand, technology developments, or other economic factors. |
| Property, Plant, and Equipment | Property, plant, and equipment is stated at cost and depreciated over the useful lives of the assets using the straight-line method. Additions and improvements that extend the lives of the assets are capitalized, while expenditures for repairs and maintenance are expensed as incurred. The Company assesses property, plant, and equipment for impairment whenever events or changes in circumstances indicate that the carrying amount of property, plant, and equipment asset groupings may not be recoverable. The cost of interest that is incurred in connection with significant ongoing construction projects is capitalized using a weighted average interest rate. These costs are included in property, plant, and equipment and amortized over the useful life of the related asset. Upon retirement or disposal of property, plant, and equipment, the costs and related amounts of accumulated depreciation or amortization are eliminated from the asset and accumulated depreciation accounts. The difference, if any, between the net asset value and the proceeds, is recognized in earnings. |
| Goodwill | Goodwill is the excess of the purchase price over the estimated fair value of net assets of acquired businesses. The Company assesses goodwill for impairment annually in the third quarter of the fiscal year and whenever an event occurs or circumstances change that would indicate the carrying amount may be impaired. Impairment testing for goodwill is performed at a reporting unit level. The test for impairment of goodwill requires the Company to make several estimates related to projected future cash flows to determine the fair value of the goodwill reporting units. The Company calculates the excess of each reporting unit's fair value over its carrying amount, including goodwill, utilizing a discounted cash flow analysis. Internal operational budgets and long-range strategic plans are used as a basis for the cash flow analysis. The Company also utilizes assumptions for working capital, capital expenditures, and terminal growth rates. The discount rate applied to the cash flow analysis is based on the weighted average cost of capital (“WACC”) for each reporting unit. An impairment is recognized when the carrying amount of the reporting unit’s net assets exceeds the estimated fair value of the reporting unit. |
| Intangible Assets | Intangible assets include patents, trademarks, tradenames, customer relationships, purchased technology, and in-process research and development (IPR&D). Intangible assets with a definite life are amortized on a straight-line basis with estimated useful lives typically ranging from to 20 years. Amortization is recognized within amortization of intangible assets in the consolidated statements of income. Intangible assets with a definite life are tested for impairment whenever events or changes in circumstances indicate that the carrying amount of an intangible asset (asset group) may not be recoverable. When events or changes in circumstances indicate that the carrying amount of an intangible asset may not be recoverable, the Company calculates the excess of an intangible asset's carrying value over its undiscounted future cash flows. If the carrying value is not recoverable, an impairment is recognized based on the amount by which the carrying value exceeds the fair value. The fair value of an intangible asset (asset group) is estimated by utilizing a discounted cash flow analysis. |
| IPR&D | Acquired IPR&D represents the fair value assigned to those research and development projects that were acquired in a business combination for which the related products have not received regulatory approval and have no alternative future use. IPR&D is capitalized at its fair value as an indefinite-lived intangible asset, and any development costs incurred after the acquisition are expensed as incurred. The fair value of IPR&D is determined by estimating the future cash flows of each project and discounting the net cash flows back to their present values. Upon achieving regulatory approval or commercial viability for the related product, the indefinite-lived intangible asset is accounted for as a definite-lived asset and is amortized on a straight-line basis over the estimated useful life. If the project is not completed or is terminated or abandoned, the Company may have an impairment related to the IPR&D, which is charged to expense. Indefinite-lived intangible assets are tested for impairment annually in the third quarter of the fiscal year and whenever events or changes in circumstances indicate that the carrying amount may be impaired. Impairment is calculated as the excess of the asset’s carrying value over its fair value. Fair value is generally determined using a discounted future cash flow analysis. IPR&D with no alternative future use acquired outside of a business combination is expensed immediately. |
| Contingent Consideration | Certain of the Company’s business combinations involve potential payment of future consideration that is contingent upon the achievement of certain product development milestones and/or contingent on the acquired business reaching certain performance milestones. The Company records contingent consideration at fair value at the date of acquisition based on the consideration expected to be transferred, estimated as the probability-weighted future cash flows, discounted back to present value. The fair value of contingent consideration is measured using projected payment dates, discount rates, probabilities of payment, and projected revenues (for revenue-based considerations). Projected revenues are based on the Company’s most recent internal operational budgets and long-range strategic plans. The discount rate used is determined at the time of measurement in accordance with accepted valuation methodologies. Changes in projected revenues, probabilities of payment, discount rates, and projected payment dates may result in adjustments to the fair value measurements. Contingent consideration is remeasured each reporting period using Level 3 inputs, and the change in fair value, including accretion for the passage of time, is recognized as income or expense within other operating expense, net in the consolidated statements of income. Contingent consideration payments made soon after the acquisition date are classified as investing activities in the consolidated statements of cash flows. Contingent consideration payments not made soon after the acquisition date that are related to the acquisition date fair value are reported as financing activities in the consolidated statements of cash flows, and amounts paid in excess of the original acquisition date fair value are reported as operating activities in the consolidated statements of cash flows. |
| Self-Insurance | The Company self-insures the majority of its insurable risks, including medical and dental costs, disability coverage, physical loss to property, business interruptions, workers’ compensation, comprehensive general, and product liability. Insurance coverage is obtained for risks required to be insured by law or contract. The Company uses claims data and historical experience, as applicable, to estimate liabilities associated with the exposures that the Company has self-insured. |
| Retirement Benefit Plan Assumptions | The Company sponsors various retirement benefit plans, including defined benefit pension plans, post-retirement medical plans, defined contribution savings plans, and termination indemnity plans, covering substantially all U.S. employees and many employees outside the U.S. See Note 15 for assumptions used in determining pension and post-retirement benefit costs and liabilities. |
| Derivatives | The Company recognizes all derivative financial instruments in its consolidated financial statements at fair value in accordance with authoritative guidance on derivatives and hedging, and presents assets and liabilities associated with derivative financial instruments on a gross basis in the consolidated financial statements. For derivative instruments that are designated and qualify as hedging instruments, the hedging instrument must be designated as a fair value hedge or a cash flow hedge, based upon the exposure being hedged. See Note 7 for more information on the Company's derivative instruments and hedging programs. |
| Fair Value Measurements | The Company follows the authoritative guidance on fair value measurements and disclosures with respect to assets and liabilities that are measured at fair value on both a recurring and nonrecurring basis. Fair value is defined as the exit price, or the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants as of the measurement date. The authoritative guidance also establishes a hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs market participants would use in valuing the asset or liability, based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors market participants would use in valuing the asset or liability developed based upon the best information available in the circumstances. The categorization of financial assets and financial liabilities within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The hierarchy is broken down into three levels defined as follows: •Level 1 - Inputs are quoted prices in active markets for identical assets or liabilities. •Level 2 - Inputs include quoted prices for similar assets or liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs (other than quoted prices) that are observable for the asset or liability, either directly or indirectly. •Level 3 - Inputs are unobservable for the asset or liability. Financial assets that are classified as Level 1 securities include highly liquid government bonds within U.S. government and agency securities and marketable equity securities for which quoted market prices are available. In addition, the Company classifies currency forward contracts as Level 1 since they are valued using quoted market prices in active markets which have identical assets or liabilities. The valuation for most fixed maturity securities are classified as Level 2. Financial assets that are classified as Level 2 include corporate debt securities, government and agency securities, other asset-backed securities, certificate of deposits, debt funds, and mortgage-backed securities whose value is determined using inputs that are observable in the market or may be derived principally from, or corroborated by, observable market data such as pricing for similar securities, recently executed transactions, cash flow models with yield curves, and benchmark securities. In addition, total return swaps are included in Level 2 as the Company uses inputs other than quoted prices that are observable for the asset. The Level 2 derivative instruments are primarily valued using standard calculations and models that use readily observable market data as their basis. Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies, or similar techniques, and at least one significant model assumption or input is unobservable. Financial assets that are classified as Level 3 include certain investment securities for which there is limited market activity such that the determination of fair value requires significant judgment or estimation, and auction rate securities. With the exception of auction rate securities, these securities are valued using third-party pricing sources that incorporate transaction details such as contractual terms, maturity, timing, and amount of expected future cash flows, as well as assumptions about liquidity and credit valuation adjustments by market participants. The fair value of auction rate securities is estimated by the Company using a discounted cash flow model, which incorporates significant unobservable inputs. The significant unobservable inputs used in the fair value measurement of the Company’s auction rate securities are years to principal recovery and the illiquidity premium that is incorporated into the discount rate. For goodwill, other intangible assets, and IPR&D, inputs used in the fair value analysis fall within Level 3 of the fair value hierarchy due to the use of significant unobservable inputs to determine fair value. Certain investments for which the fair value is measured using the net asset value per share (or its equivalent) practical expedient are excluded from the fair value hierarchy. Financial assets for which the fair value is measured using the net asset value per share practical expedient include certain debt funds, equity and fixed income commingled trusts, and registered investment companies.
|
| Revenue Recognition and Shipping and Handling | The Company sells its products through direct sales representatives and independent distributors. Additionally, a portion of the Company's revenue is generated from consignment inventory maintained at hospitals. The Company recognizes revenue when control is transferred to the customer. For products sold through direct sales representatives and independent distributors, control is transferred upon shipment or upon delivery, based on the contract terms and legal requirements. For consignment inventory, control is transferred when the product is used or implanted. Payment terms vary depending on the country of sale, type of customer, and type of product. If a contract contains more than one performance obligation, the transaction price is allocated to each performance obligation based on relative standalone selling price. Shipping and handling is treated as a fulfillment activity rather than a promised service, and therefore, is not considered a performance obligation. Taxes assessed by a governmental authority that are both imposed on, and concurrent with, a specific revenue producing transaction and collected by the Company from customers (for example, sales, use, value added, and some excise taxes) are not included in revenue. For contracts that have an original duration of one year or less, the Company uses the practical expedient applicable to such contracts and does not adjust the transaction price for the time value of money. The amount of revenue recognized reflects sales rebates and returns, which are estimated based on sales terms, historical experience, and trend analysis. In estimating rebates, the Company considers the lag time between the point of sale and the payment of the rebate claim, the stated rebate rates, and other relevant information. The Company records adjustments to rebates and returns reserves as increases or decreases of revenue. The Company records a deferred revenue liability if a customer pays consideration before the Company transfers a good or service to the customer. Deferred revenue primarily represents remote monitoring services and equipment maintenance, for which consideration is received at the same time as consideration for the device or equipment. Revenue related to remote monitoring services and equipment maintenance is recognized over the service period as time elapses. Shipping and handling costs incurred to physically move product from the Company's premises to the customer's premises are recognized in selling, general, and administrative expense in the consolidated statements of income and were $354 million, $308 million, and $347 million in fiscal years 2022, 2021, and 2020, respectively. Other shipping and handling costs incurred to store, move, and prepare products for shipment are recognized in cost of products sold in the consolidated statements of income.
|
| Research and Development | Research and development costs are expensed when incurred. Research and development costs include costs of research, engineering, and technical activities to develop a new product or service or make significant improvement to an existing product or manufacturing process. Research and development costs also include pre-approval regulatory and clinical trial expenses. |
| Contingencies | The Company records a liability in the consolidated financial statements for loss contingencies when a loss is known or considered probable, and the amount may be reasonably estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and may be reasonably estimated, the estimated loss or range of loss is disclosed. |
| Income Taxes | The Company has deferred taxes that arise as a result of the different treatment of transactions for U.S. GAAP and income tax accounting, known as temporary differences. The Company records the tax effect of these temporary differences as deferred tax assets and deferred tax liabilities. Deferred tax assets generally represent items that may be used as a tax deduction or credit in a tax return in future years for which the Company has already recognized the tax benefit in the consolidated statements of income. The Company establishes valuation allowances for deferred tax assets when the amount of expected future taxable income is not likely to support the use of the deduction or credit. Deferred tax liabilities generally represent tax expense for which payment has been deferred or expense has already been taken as a deduction on the Company’s tax return but has not yet been recognized as an expense in the consolidated statements of income. |
| Other Operating Expense, Net | Other operating expense, net primarily includes royalty income and expense, currency remeasurement and derivative gains and losses, Puerto Rico excise taxes, changes in fair value of contingent consideration, changes in amounts accrued for certain contingent liabilities for a past acquisition, charges related to the June 2021 decision to stop the distribution and sale of Medtronic's HVAD System within the Mechanical Circulatory Support Operating Unit (MCS) (MCS charges), impairment charges, and income from funded research and development arrangements. |
| Other Non-Operating Income, Net | Other non-operating income, net includes the non-service component of net periodic pension and post-retirement benefit cost, investment gains and losses, and interest income. |
| Currency Translation | Assets and liabilities of non-U.S. dollar functional currency entities are translated to U.S. dollars at period-end exchange rates, and the currency impacts arising from the translation of the assets and liabilities are recorded as a cumulative translation adjustment, a component of accumulated other comprehensive loss, on the consolidated balance sheets. Elements of the consolidated statements of income are translated at the average monthly currency exchange rates in effect during the period. Currency transaction gains and losses are included in other operating expense, net in the consolidated statements of income. |
| Stock-Based Compensation | The Company measures stock-based compensation expense at the grant date based on the fair value of the award and recognizes the compensation expense over the requisite service period, which is generally the vesting period. The amount of stock-based compensation expense recognized during a period is based on the portion of the awards that are expected to vest. The Company estimates pre-vesting forfeitures at the time of grant and revises the estimates in subsequent periods. |
| Recently Adopted Accounting Standards | Current Expected Credit Losses In June 2016, the Financial Accounting Standards Board (FASB) issued guidance changing the methodology to be used to measure credit losses for certain financial instruments and financial assets, including trade receivables. The new methodology requires the recognition of an allowance that reflects the current estimate of credit losses expected to be incurred over the life of the financial asset. The Company adopted this guidance using the modified retrospective method in the first quarter of fiscal year 2021. The adoption of this guidance did not have a material impact to the Company’s consolidated financial statements. Leases In February 2016, the FASB issued guidance which requires lessees to recognize right-of-use assets and lease liabilities on the balance sheet. This guidance also requires additional qualitative and quantitative lease related disclosures in the notes to the consolidated financial statements. The Company adopted this guidance using the modified retrospective method in the first quarter of fiscal year 2020. During the implementation, the Company elected the package of practical expedients available under the transition guidance that allowed an entity not to reassess whether any expired or existing contracts are or contain leases, the classification for any expired or existing leases or any initial direct costs for existing leases. Further, the Company made accounting policy elections to not apply the recognition requirements to short-term leases and to account for lease and nonlease components as a single lease component. The adoption of this guidance resulted in the recognition of right-of-use assets and lease liabilities in an amount of approximately $1.0 billion, an immaterial cumulative-effect adjustment to retained earnings as of April 27, 2019, and expansion of lease related disclosures. The adoption of this guidance did not have a material impact on the Company's consolidated statements of income or consolidated statements of cash flows.
|
| Earnings Per Share | Basic earnings per share is computed based on the weighted average number of ordinary shares outstanding. Diluted earnings per share is computed based on the weighted number of ordinary shares outstanding, increased by the number of additional shares that would have been outstanding had the potentially dilutive ordinary shares been issued, and reduced by the number of shares the Company could have repurchased with the proceeds from issuance of the potentially dilutive shares. Potentially dilutive ordinary shares include stock-based awards granted under stock-based compensation plans and shares committed to be purchased under the employee stock purchase plan. |
Revenue (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenue from Contract with Customer [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Disaggregation of Revenue | The table below illustrates net sales by segment and division for fiscal years 2022, 2021, and 2020:
The table below includes net sales by market geography and segment for fiscal years 2022, 2021, and 2020:
(1)U.S. includes the United States and U.S. territories. (2)Non-U.S. developed markets include Japan, Australia, New Zealand, Korea, Canada, and the countries of Western Europe. (3)Emerging markets include the countries of the Middle East, Africa, Latin America, Eastern Europe, and the countries of Asia that are not included in the non-U.S. developed markets, as defined above.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acquisitions (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Business Combination and Asset Acquisition [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Reconciliation of Beginning and Ending Balances of Contingent Consideration Associated with Acquisitions | The following table provides a reconciliation of the beginning and ending balances of contingent consideration:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Significant Unobservable Inputs | The recurring Level 3 fair value measurements of contingent consideration for which a liability is recorded include the following significant unobservable inputs:
(1) Unobservable inputs were weighted by the relative fair value of the contingent consideration liability. For projected fiscal year of payment, the amount represents the median of the inputs and is not a weighted average.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Restructuring Charges (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Restructuring and Related Activities [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Restructuring Reserve | The following table summarizes the activity related to the restructuring programs noted above for fiscal years 2022, 2021, and 2020:
(1)Associated costs include costs incurred as a direct result of the restructuring program, such as salaries for employees supporting the program and consulting expenses. (2)Accrual adjustments relate to certain employees identified for termination finding other positions within the Company or contract terminations being settled for less than originally estimated.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Financial Instruments (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Investments [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Investments by Category and Related Balance Sheet Presentation | The following tables summarize the Company's investments in available-for-sale debt securities by significant investment category and the related consolidated balance sheet classification at April 29, 2022 and April 30, 2021:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Gross Unrealized Losses and Fair Values of Available-for-sale Securities that Have Been in a Continuous Unrealized Loss Position Deemed to be Temporary, Aggregated by Investment Category | The following tables present the gross unrealized losses and fair values of the Company’s available-for-sale debt securities that have been in a continuous unrealized loss position deemed to be temporary, aggregated by investment category at April 29, 2022 and April 30, 2021:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Activity Related to the Company's Available for Sale Securities Portfolio | Activity related to the Company’s available-for-sale debt securities portfolio is as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Available-for-sale Debt Securities Contractual Maturities | The April 29, 2022 balance of available-for-sale debt securities by contractual maturity is shown in the following table. Within the table, maturities of mortgage-backed securities have been allocated based upon timing of estimated cash flows assuming no change in the current interest rate environment. Actual maturities may differ from contractual maturities because the issuers of the securities may have the right to prepay obligations without prepayment penalties.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Equity and Other Investments | The following table summarizes the Company's equity and other investments at April 29, 2022 and April 30, 2021, which are classified as other assets in the consolidated balance sheets:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Activity Related to the Company's Equity and Other Investments Portfolio | The table below includes activity related to the Company’s portfolio of equity and other investments. Gains and losses on equity and other investments are recognized in other non-operating income, net in the consolidated statements of income.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Financing Arrangements (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Debt Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Current Debt Obligations | Current debt obligations consisted of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Long-term Debt | The Company's long-term debt obligations consisted of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Maturities of Long-term Debt | Contractual maturities of debt for the next five fiscal years and thereafter, excluding deferred financing costs and debt discount, net, are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Derivatives and Currency Exchange Risk Management (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Derivative Instruments and Hedging Activities Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of (Gain) Loss on Derivative Instruments | The amount of the gains and losses on our hedging instruments and the classification of those gains and losses within our consolidated financial statements for fiscal years 2022, 2021, and 2020 were as follows:
The amount of the gains and losses on our derivative instruments not designated as hedging instruments and the classification of those gains and losses within our consolidated financial statements for fiscal years 2022, 2021, and 2020 were as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Classification and Fair Value Amounts of Derivative Instruments in Balance Sheets | The following tables summarize the balance sheet classification and fair value of derivative instruments included in the consolidated balance sheets at April 29, 2022 and April 30, 2021. The fair value amounts are presented on a gross basis, and are segregated between derivatives that are designated and qualify as hedging instruments and those that are not designated and do not qualify as hedging instruments, and are further segregated by type of contract within those two categories.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Derivative Assets and Liabilities Measured at Fair Value on a Recurring Basis | The following table provides information by level for the derivative assets and liabilities that are measured at fair value on a recurring basis:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Offsetting Assets |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Offsetting Liabilities |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Inventories (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inventory Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Inventory Balances | Inventory balances, net of reserves, were as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Goodwill and Other Intangible Assets (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Goodwill and Intangible Assets Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Goodwill | The following table presents the changes in the carrying amount of goodwill by segment:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Finite-Lived Intangible Assets by Major Class | The following table presents the gross carrying amount and accumulated amortization of intangible assets:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Indefinite-Lived Intangible Assets by Major Class | The following table presents the gross carrying amount and accumulated amortization of intangible assets:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Finite-Lived Intangible Assets, Future Amortization Expense | Estimated aggregate amortization expense by fiscal year based on the current carrying value and remaining estimated useful lives of definite-lived intangible assets at April 29, 2022, excluding any possible future amortization associated with acquired IPR&D which has not met technological feasibility, is as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Property, Plant, and Equipment (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Property, Plant and Equipment [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Property, Plant and Equipment Balances and Corresponding Lives | Property, plant, and equipment balances and corresponding estimated useful lives were as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stock Purchase and Award Plans (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Share-based Payment Arrangement [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Stock-Based Compensation Expense | The following table presents the components and classification of stock-based compensation expense recognized for stock options, restricted stock, performance share units, and employee stock purchase plan (ESPP) in fiscal years 2022, 2021, and 2020:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Stock Options Valuation Assumptions | The following table provides the weighted average fair value of options granted to employees and the related assumptions used in the Black-Scholes model:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Stock Options Activity | The following table summarizes stock option activity during fiscal year 2022:
The following table summarizes the total cash received from the issuance of new shares upon stock option award exercises, the total intrinsic value of options exercised, and the related tax benefit during fiscal years 2022, 2021, and 2020:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Restricted Stock Activity | The following table summarizes restricted stock activity during fiscal year 2022:
The following table summarizes the weighted-average grant date fair value of restricted stock granted, total fair value of restricted stock vested and related tax benefit during fiscal years 2022, 2021, and 2020:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Performance Share Unit Activity | The following table summarizes performance share unit activity during fiscal year 2022:
The following table summarizes the weighted-average grant date fair value of performance share units granted, total fair value of performance share units vested and related tax benefit during fiscal year 2022 and 2021:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Income Taxes (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income Tax Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of Income Before Income Taxes, Based on Jurisdiction | The components of income before income taxes, based on tax jurisdiction, are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income Tax (Benefit) Provision | The income tax provision (benefit) consists of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Deferred Tax Assets and Liabilities | Tax assets (liabilities), shown before jurisdictional netting of deferred tax assets (liabilities), are comprised of the following:
(1)Certain prior year amounts have been reclassified to conform to current year presentation
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Effective Income Tax Rate Reconciliation | The Company’s effective income tax rate varied from the U.S. federal statutory tax rate as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reconciliation of Beginning and Ending Amount of Unrecognized Tax Benefits | A reconciliation of the beginning and ending amount of unrecognized tax benefits for fiscal years 2022, 2021, and 2020 is as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Major Tax Jurisdictions Which Remain Subject to Examination | The major tax jurisdictions where the Company conducts business which remain subject to examination are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Earnings Per Share (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Earnings Per Share [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Computation of Basic and Diluted Earnings Per Share | The table below sets forth the computation of basic and diluted earnings per share:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Retirement Benefit Plans (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Retirement Benefits [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Defined Benefit Plans | The change in benefit obligation and funded status of the Company’s U.S. and Non-U.S. pension benefits are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Accumulated Benefit Obligations in Excess of Fair Value of Plan Assets | U.S. and non-U.S. pension plans with accumulated benefit obligations in excess of plan assets consist of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Benefit Obligations in Excess of Fair Value of Plan Assets | U.S. and non-U.S. pension plans with projected benefit obligations in excess of plan assets consist of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Net Benefit Costs | The net periodic benefit cost of the plans includes the following components:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Defined Benefit Plan Amounts Recognized in Other Comprehensive Income (Loss) | The other changes in plan assets and projected benefit obligations recognized in other comprehensive income for fiscal year 2022 are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Assumptions Used | The actuarial assumptions are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Allocation of Plan Assets | The Company’s U.S. plans target asset allocations at April 29, 2022, compared to the U.S. plans actual asset allocations at April 29, 2022 and April 30, 2021 by asset category, are as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value Measurements, Retirement Benefit Plan Assets | The following tables provide information by level for the retirement benefit plan assets that are measured at fair value, as defined by U.S. GAAP. Certain investments for which the fair value is measured using the net asset value per share (or its equivalent) practical expedient are not presented within the fair value hierarchy. The fair value amounts presented for these investments are intended to permit reconciliation to the total fair value of plan assets at April 29, 2022 and April 30, 2021. U.S. Pension Benefits
Non-U.S. Pension Benefits
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Retirement Benefit Plan Assets, Unobservable Input Reconciliation | The following tables provide a reconciliation of the beginning and ending balances of U.S. pension benefit assets measured at fair value that used significant unobservable inputs (Level 3):
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Expected Benefit Payments | Retiree benefit payments, which reflect expected future service, are anticipated to be paid as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Leases (Tables) |
12 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leases [Abstract] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Balance Sheet Classification of Operating Leases and Amounts of Right-of-Use Assets and Lease Liabilities | The following table summarizes the balance sheet classification of the Company's operating leases and amounts of the right-of-use assets and lease liabilities at April 29, 2022 and April 30, 2021:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Components of Total Operating Lease Cost and Supplemental Lease Information | The following table summarizes the weighted-average remaining lease term and weighted-average discount rate for the Company's operating leases at April 29, 2022, April 30, 2021, and April 24, 2020:
The following table summarizes the components of total operating lease cost for fiscal year 2022, 2021, and 2020:
The following table summarizes the cash paid for amounts included in the measurement of operating lease liabilities and right-of-use assets obtained in exchange for operating lease liabilities for fiscal year 2022, 2021, and 2020:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Maturities of Operating Leases | The following table summarizes the maturities of the Company's operating leases at April 29, 2022:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Accumulated Other Comprehensive Loss (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Equity [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Accumulated Other Comprehensive Loss | The following table provides changes in AOCI, net of tax and by component:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Segment and Geographic Information (Tables) |
12 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Apr. 29, 2022 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segment Reporting [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income From Operations Before Income Taxes by Reportable Segment and Reconciliation to Consolidated | Segment Operating Profit
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reconciliation of Assets and Depreciation Expense from Segments to Consolidated | Total Assets and Depreciation Expense
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Net Sales to External Customers and Property, Plant, and Equipment, Net, by Geographical Region | The following table presents net sales for fiscal years 2022, 2021, and 2020, and property, plant, and equipment, net at April 29, 2022 and April 30, 2021 for the Company's country of domicile, countries with significant concentrations, and all other countries:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Summary of Significant Accounting Policies (Details) - USD ($) $ in Millions |
12 Months Ended | |||
|---|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
Apr. 27, 2019 |
|
| Shipping and Handling | ||||
| Cost of products sold, excluding amortization of intangible assets | $ 10,145 | $ 10,483 | $ 9,424 | |
| New Accounting Standards | ||||
| Right-of-use assets | 854 | 998 | $ 1,000 | |
| Lease liability | 871 | $ 1,000 | ||
| Shipping and Handling | ||||
| Shipping and Handling | ||||
| Cost of products sold, excluding amortization of intangible assets | $ 354 | $ 308 | $ 347 | |
| Minimum | ||||
| Intangible Assets | ||||
| Intangible assets, estimated useful life | 3 years | |||
| Maximum | ||||
| Intangible Assets | ||||
| Intangible assets, estimated useful life | 20 years | |||
Revenue - Disaggregation of Net Sales by Segment and Division (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Disaggregation of Revenue [Line Items] | |||
| Net sales | $ 31,686 | $ 30,117 | $ 28,913 |
| Cardiovascular | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 11,423 | 10,772 | 10,468 |
| Cardiovascular | Cardiac Rhythm & Heart Failure | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 5,908 | 5,584 | 5,141 |
| Cardiovascular | Structural Heart & Aortic | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 3,055 | 2,834 | 2,842 |
| Cardiovascular | Coronary & Peripheral Vascular | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 2,460 | 2,354 | 2,486 |
| Medical Surgical | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 9,141 | 8,737 | 8,352 |
| Medical Surgical | Surgical Innovations | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 6,060 | 5,438 | 5,513 |
| Medical Surgical | Respiratory, Gastrointestinal, & Renal | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 3,081 | 3,298 | 2,839 |
| Neuroscience | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 8,784 | 8,195 | 7,725 |
| Neuroscience | Cranial & Spinal Technologies | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 4,456 | 4,288 | 4,082 |
| Neuroscience | Specialty Therapies | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 2,592 | 2,307 | 2,147 |
| Neuroscience | Neuromodulation | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 1,735 | 1,601 | 1,497 |
| Diabetes | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | $ 2,338 | $ 2,413 | $ 2,368 |
Revenue - Disaggregation of Net Sales by Market Geography for Each Segment (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Disaggregation of Revenue [Line Items] | |||
| Net sales | $ 31,686 | $ 30,117 | $ 28,913 |
| U.S. | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 16,135 | 15,526 | 14,919 |
| Non-U.S. Developed Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 10,126 | 9,815 | 9,287 |
| Emerging Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 5,426 | 4,777 | 4,707 |
| Cardiovascular | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 11,423 | 10,772 | 10,468 |
| Cardiovascular | U.S. | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 5,545 | 5,248 | 5,062 |
| Cardiovascular | Non-U.S. Developed Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 3,866 | 3,752 | 3,519 |
| Cardiovascular | Emerging Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 2,012 | 1,773 | 1,887 |
| Medical Surgical | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 9,141 | 8,737 | 8,352 |
| Medical Surgical | U.S. | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 3,862 | 3,650 | 3,532 |
| Medical Surgical | Non-U.S. Developed Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 3,373 | 3,320 | 3,169 |
| Medical Surgical | Emerging Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 1,905 | 1,766 | 1,651 |
| Neuroscience | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 8,784 | 8,195 | 7,725 |
| Neuroscience | U.S. | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 5,753 | 5,456 | 5,122 |
| Neuroscience | Non-U.S. Developed Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 1,801 | 1,724 | 1,659 |
| Neuroscience | Emerging Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 1,229 | 1,015 | 945 |
| Diabetes | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 2,338 | 2,413 | 2,368 |
| Diabetes | U.S. | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 974 | 1,171 | 1,204 |
| Diabetes | Non-U.S. Developed Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | 1,085 | 1,019 | 940 |
| Diabetes | Emerging Markets | |||
| Disaggregation of Revenue [Line Items] | |||
| Net sales | $ 279 | $ 222 | $ 224 |
Revenue - Narrative (Details) - USD ($) $ in Millions |
12 Months Ended | |
|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
|
| Disaggregation of Revenue [Line Items] | ||
| Deferred revenue | $ 399 | $ 368 |
| Revenue recognized that was previously included in deferred revenue | 243 | |
| Estimated revenue expected to be recognized in future periods related to unsatisfied performance obligations | $ 925 | |
| Period over which remaining performance obligations are expected to be recognized as revenue | three years | |
| Other accrued expenses | ||
| Disaggregation of Revenue [Line Items] | ||
| Rebate obligations | $ 981 | 906 |
| Deferred revenue | 305 | 276 |
| Reduction of accounts receivable | ||
| Disaggregation of Revenue [Line Items] | ||
| Rebate obligations | 548 | 485 |
| Other liabilities | ||
| Disaggregation of Revenue [Line Items] | ||
| Deferred revenue | $ 94 | $ 93 |
Acquisitions - Additional Information (Details) - USD ($) $ / shares in Units, $ in Millions |
12 Months Ended | |||
|---|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
May 13, 2022 |
Apr. 24, 2020 |
|
| Business Acquisition [Line Items] | ||||
| Goodwill | $ 40,502 | $ 41,961 | $ 39,841 | |
| Fair value of contingent consideration | 119 | 270 | $ 280 | |
| In Process Research Development | ||||
| Business Acquisition [Line Items] | ||||
| Acquired in-process research & development | 101 | |||
| Other accrued expenses | ||||
| Business Acquisition [Line Items] | ||||
| Fair value of contingent consideration | 35 | 78 | ||
| Other liabilities | ||||
| Business Acquisition [Line Items] | ||||
| Fair value of contingent consideration | $ 84 | 192 | ||
| Minimum | ||||
| Business Acquisition [Line Items] | ||||
| Estimated useful life | 3 years | |||
| Maximum | ||||
| Business Acquisition [Line Items] | ||||
| Estimated useful life | 20 years | |||
| All Business Acquisitions | ||||
| Business Acquisition [Line Items] | ||||
| Fair value of net assets acquired | $ 125 | 1,200 | ||
| Assets acquired | 154 | 1,400 | ||
| Liabilities assumed | 29 | 161 | ||
| Goodwill | 80 | 816 | ||
| Contingent consideration liabilities | 31 | 253 | ||
| Gain related to change in amounts accrued for certain contingent liabilities for recent acquisition | 132 | |||
| All Business Acquisitions | Technology-Based Intangible Assets | ||||
| Business Acquisition [Line Items] | ||||
| Intangible assets acquired | $ 50 | $ 417 | ||
| All Business Acquisitions | Technology-Based Intangible Assets | Minimum | ||||
| Business Acquisition [Line Items] | ||||
| Estimated useful life | 15 years | 8 years | ||
| All Business Acquisitions | Technology-Based Intangible Assets | Maximum | ||||
| Business Acquisition [Line Items] | ||||
| Estimated useful life | 16 years | 15 years | ||
| All Business Acquisitions | Customer-Related Intangible Assets | ||||
| Business Acquisition [Line Items] | ||||
| Intangible assets acquired | $ 13 | |||
| All Business Acquisitions | Customer-Related Intangible Assets | Minimum | ||||
| Business Acquisition [Line Items] | ||||
| Estimated useful life | 8 years | |||
| All Business Acquisitions | Customer-Related Intangible Assets | Maximum | ||||
| Business Acquisition [Line Items] | ||||
| Estimated useful life | 15 years | |||
| Intersect ENT | Subsequent Event | ||||
| Business Acquisition [Line Items] | ||||
| Acquisition of outstanding shares (in dollars per share) | $ 28.25 | |||
| Total consideration | $ 1,200 | |||
Acquisitions - Contingent Consideration (Details) $ in Millions |
12 Months Ended | |
|---|---|---|
|
Apr. 29, 2022
USD ($)
|
Apr. 30, 2021
USD ($)
|
|
| Reconciliation of Beginning and Ending Balances of Contingent Milestone Payments Associated with Acquisitions | ||
| Beginning Balance | $ 270 | $ 280 |
| Purchase price contingent consideration | 31 | 253 |
| Purchase price allocation adjustments | 7 | 0 |
| Payments | (86) | (299) |
| Change in fair value | (103) | 36 |
| Ending Balance | 119 | 270 |
| Fair Value Measurement Inputs and Valuation Techniques [Line Items] | ||
| Contingent consideration | 119 | $ 270 |
| Fair Value, Measurements, Recurring | Level 3 | Revenue and other performance-based payments | ||
| Reconciliation of Beginning and Ending Balances of Contingent Milestone Payments Associated with Acquisitions | ||
| Ending Balance | 104 | |
| Fair Value Measurement Inputs and Valuation Techniques [Line Items] | ||
| Contingent consideration | $ 104 | |
| Fair Value, Measurements, Recurring | Level 3 | Revenue and other performance-based payments | Discount Rate | Minimum | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 0.112 | |
| Fair Value, Measurements, Recurring | Level 3 | Revenue and other performance-based payments | Discount Rate | Maximum | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 0.272 | |
| Fair Value, Measurements, Recurring | Level 3 | Revenue and other performance-based payments | Discount Rate | Weighted Average | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 0.146 | |
| Fair Value, Measurements, Recurring | Level 3 | Revenue and other performance-based payments | Probability of Payment | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 100 | |
| Fair Value, Measurements, Recurring | Level 3 | Revenue and other performance-based payments | Probability of Payment | Weighted Average | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 1 | |
| Fair Value, Measurements, Recurring | Level 3 | Product development and other milestone-based payments | ||
| Reconciliation of Beginning and Ending Balances of Contingent Milestone Payments Associated with Acquisitions | ||
| Ending Balance | $ 15 | |
| Fair Value Measurement Inputs and Valuation Techniques [Line Items] | ||
| Contingent consideration | $ 15 | |
| Fair Value, Measurements, Recurring | Level 3 | Product development and other milestone-based payments | Discount Rate | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 0.055 | |
| Fair Value, Measurements, Recurring | Level 3 | Product development and other milestone-based payments | Discount Rate | Weighted Average | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 0.055 | |
| Fair Value, Measurements, Recurring | Level 3 | Product development and other milestone-based payments | Probability of Payment | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 1 | |
| Fair Value, Measurements, Recurring | Level 3 | Product development and other milestone-based payments | Probability of Payment | Weighted Average | ||
| Fair Value Inputs | ||
| Contingent consideration, significant unobservable inputs | 1 | |
Restructuring Charges - Additional Information (Details) $ in Millions |
3 Months Ended | 12 Months Ended | |||
|---|---|---|---|---|---|
|
Apr. 29, 2022
USD ($)
patient
|
Jul. 30, 2021
USD ($)
|
Apr. 29, 2022
USD ($)
patient
|
Apr. 30, 2021
USD ($)
|
Apr. 24, 2020
USD ($)
|
|
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring charges, net | $ 60 | $ 293 | $ 118 | ||
| Incremental expense related to acceptance of voluntary early retirement packages | 97 | ||||
| Impairment of finite-lived intangible assets | 37 | ||||
| Number of mechanical circulatory support patients | patient | 3,500 | 3,500 | |||
| Cardiovascular | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring write down and impairment provisions | $ 726 | ||||
| Asset impairment charges and inventory write down | 515 | ||||
| Impairment of finite-lived intangible assets | 409 | 4 | |||
| Inventory write-down | 58 | ||||
| Other restructuring costs | 211 | ||||
| Business exit costs | $ 155 | ||||
| Restructuring reserve, current | 82 | $ 82 | |||
| Restructuring reserve, noncurrent | 152 | 152 | |||
| Cost of products sold | Cardiovascular | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring write down and impairment provisions | 58 | ||||
| Other operating expense, net | Cardiovascular | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Business exit costs | $ 668 | ||||
| Enterprise Excellence | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring charges, net | 259 | 349 | 441 | ||
| Enterprise Excellence | Cost of products sold | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring charges, net | 116 | 128 | 155 | ||
| Enterprise Excellence | Selling, general, and administrative expense | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring charges, net | 112 | 169 | $ 168 | ||
| Enterprise Excellence | Pre-tax exit and disposal costs | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Pre-tax exit, disposal costs and other costs | 1,600 | 1,600 | |||
| Enterprise Excellence | Pre-tax exit and disposal costs | Maximum | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Estimated expected restructuring costs | 1,800 | 1,800 | |||
| Simplification | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring charges, net | 82 | 268 | |||
| Incremental expense related to acceptance of voluntary early retirement packages | 97 | ||||
| Simplification | Selling, general, and administrative expense | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Restructuring charges, net | 45 | $ 27 | |||
| Simplification | Pre-tax exit and disposal costs | |||||
| Restructuring Cost and Reserve [Line Items] | |||||
| Pre-tax exit, disposal costs and other costs | 349 | 349 | |||
| Estimated expected restructuring costs | $ 450 | $ 450 | |||
Restructuring Charges (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Changes in Restructuring Reserves | |||
| Beginning balance | $ 146 | $ 112 | $ 122 |
| Charges | 354 | 539 | 462 |
| Cash payments | (378) | (486) | (427) |
| Settled non-cash | (24) | ||
| Accrual adjustments | (13) | (19) | (21) |
| Ending balance | 110 | 146 | 112 |
| Employee Termination Benefits | |||
| Changes in Restructuring Reserves | |||
| Beginning balance | 123 | 89 | 101 |
| Charges | 80 | 213 | 129 |
| Cash payments | (109) | (162) | (128) |
| Settled non-cash | 0 | ||
| Accrual adjustments | (13) | (17) | (13) |
| Ending balance | 81 | 123 | 89 |
| Associated Costs | |||
| Changes in Restructuring Reserves | |||
| Beginning balance | 22 | 19 | 9 |
| Charges | 274 | 322 | 300 |
| Cash payments | (269) | (319) | (290) |
| Settled non-cash | 0 | ||
| Accrual adjustments | 0 | 0 | 0 |
| Ending balance | 27 | 22 | 19 |
| Asset Write-downs | |||
| Changes in Restructuring Reserves | |||
| Beginning balance | 0 | 0 | 0 |
| Charges | 0 | 0 | 24 |
| Cash payments | 0 | 0 | 0 |
| Settled non-cash | (24) | ||
| Accrual adjustments | 0 | 0 | 0 |
| Ending balance | 0 | 0 | 0 |
| Other Costs | |||
| Changes in Restructuring Reserves | |||
| Beginning balance | 1 | 4 | 12 |
| Charges | 0 | 4 | 9 |
| Cash payments | 0 | (5) | (9) |
| Settled non-cash | 0 | ||
| Accrual adjustments | 0 | (2) | (8) |
| Ending balance | $ 1 | $ 1 | $ 4 |
Financial Instruments - Investments by Category and Related Balance Sheet Presentation (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Schedule of Investments [Line Items] | ||
| Cost | $ 7,131 | $ 7,144 |
| Unrealized Gains | 5 | 155 |
| Unrealized Losses | (245) | (42) |
| Fair Value | 6,893 | 7,257 |
| Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 6,859 | 7,224 |
| Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 33 | 33 |
| Level 1 | U.S. government and agency securities | ||
| Schedule of Investments [Line Items] | ||
| Cost | 533 | 505 |
| Unrealized Gains | 1 | 26 |
| Unrealized Losses | (15) | (3) |
| Fair Value | 518 | 528 |
| Level 1 | U.S. government and agency securities | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 518 | 528 |
| Level 1 | U.S. government and agency securities | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 2 | ||
| Schedule of Investments [Line Items] | ||
| Cost | 6,563 | 6,603 |
| Unrealized Gains | 4 | 129 |
| Unrealized Losses | (227) | (36) |
| Fair Value | 6,341 | 6,696 |
| Level 2 | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 6,341 | 6,696 |
| Level 2 | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 2 | U.S. government and agency securities | ||
| Schedule of Investments [Line Items] | ||
| Cost | 910 | 810 |
| Unrealized Gains | 0 | 0 |
| Unrealized Losses | (41) | (7) |
| Fair Value | 869 | 804 |
| Level 2 | U.S. government and agency securities | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 869 | 804 |
| Level 2 | U.S. government and agency securities | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 2 | Corporate debt securities | ||
| Schedule of Investments [Line Items] | ||
| Cost | 4,457 | 4,557 |
| Unrealized Gains | 4 | 103 |
| Unrealized Losses | (140) | (13) |
| Fair Value | 4,321 | 4,647 |
| Level 2 | Corporate debt securities | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 4,321 | 4,647 |
| Level 2 | Corporate debt securities | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 2 | Mortgage-backed securities | ||
| Schedule of Investments [Line Items] | ||
| Cost | 592 | 645 |
| Unrealized Gains | 0 | 21 |
| Unrealized Losses | (35) | (16) |
| Fair Value | 558 | 650 |
| Level 2 | Mortgage-backed securities | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 558 | 650 |
| Level 2 | Mortgage-backed securities | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 2 | Non-U.S. government and agency securities | ||
| Schedule of Investments [Line Items] | ||
| Cost | 17 | 31 |
| Unrealized Gains | 0 | 1 |
| Unrealized Losses | 0 | 0 |
| Fair Value | 17 | 33 |
| Level 2 | Non-U.S. government and agency securities | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 17 | 33 |
| Level 2 | Non-U.S. government and agency securities | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 2 | Certificates of deposit | ||
| Schedule of Investments [Line Items] | ||
| Cost | 20 | 19 |
| Unrealized Gains | 0 | 0 |
| Unrealized Losses | 0 | 0 |
| Fair Value | 20 | 19 |
| Level 2 | Certificates of deposit | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 20 | 19 |
| Level 2 | Certificates of deposit | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | |
| Level 2 | Other asset-backed securities | ||
| Schedule of Investments [Line Items] | ||
| Cost | 567 | 534 |
| Unrealized Gains | 0 | 4 |
| Unrealized Losses | (11) | (1) |
| Fair Value | 556 | 537 |
| Level 2 | Other asset-backed securities | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 556 | 537 |
| Level 2 | Other asset-backed securities | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 2 | Debt funds | ||
| Schedule of Investments [Line Items] | ||
| Cost | 7 | |
| Unrealized Gains | 0 | |
| Unrealized Losses | 0 | |
| Fair Value | 7 | |
| Level 2 | Debt funds | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 7 | |
| Level 2 | Debt funds | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | |
| Level 3 | Auction rate securities | ||
| Schedule of Investments [Line Items] | ||
| Cost | 36 | 36 |
| Unrealized Gains | 0 | 0 |
| Unrealized Losses | (3) | (3) |
| Fair Value | 33 | 33 |
| Level 3 | Auction rate securities | Investments | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | 0 | 0 |
| Level 3 | Auction rate securities | Other Assets | ||
| Schedule of Investments [Line Items] | ||
| Fair Value | $ 33 | $ 33 |
Financial Instruments - AFS in Continuous Loss Position (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Fair Value | ||
| Less than 12 months | $ 222 | $ 946 |
| More than 12 months | 5,004 | 4,423 |
| Unrealized Losses | ||
| Less than 12 months | (1) | (10) |
| More than 12 months | (244) | (32) |
| U.S. government and agency securities | ||
| Fair Value | ||
| Less than 12 months | 0 | 946 |
| More than 12 months | 945 | 0 |
| Unrealized Losses | ||
| Less than 12 months | 0 | (10) |
| More than 12 months | (56) | 0 |
| Corporate debt securities | ||
| Fair Value | ||
| Less than 12 months | 222 | 0 |
| More than 12 months | 2,993 | 3,209 |
| Unrealized Losses | ||
| Less than 12 months | (1) | 0 |
| More than 12 months | (139) | (13) |
| Mortgage-backed securities | ||
| Fair Value | ||
| Less than 12 months | 0 | 0 |
| More than 12 months | 507 | 650 |
| Unrealized Losses | ||
| Less than 12 months | 0 | 0 |
| More than 12 months | (35) | (16) |
| Other asset-backed securities | ||
| Fair Value | ||
| Less than 12 months | 0 | 0 |
| More than 12 months | 526 | 531 |
| Unrealized Losses | ||
| Less than 12 months | 0 | 0 |
| More than 12 months | (11) | (1) |
| Auction rate securities | ||
| Fair Value | ||
| Less than 12 months | 0 | 0 |
| More than 12 months | 33 | 33 |
| Unrealized Losses | ||
| Less than 12 months | 0 | 0 |
| More than 12 months | $ (3) | $ (3) |
Financial Instruments - Additional Information (Details) - USD ($) $ in Millions |
12 Months Ended | |
|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
|
| Schedule of Investments [Line Items] | ||
| Proceeds from maturities of investments classified as held to maturity | $ 911 | |
| Equity Investments | ||
| Schedule of Investments [Line Items] | ||
| Net unrealized gains (losses) on equity and other investments still held | $ 8 | $ 63 |
Financial Instruments - Activity Related to the Company's Debt Securities Portfolio and Available-for-sale Debt Securities Contractual Maturities (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| AFS Gross Realized Gain (Loss) | |||
| Proceeds from sales and maturities | $ 9,611 | $ 10,420 | $ 9,559 |
| Gross realized gains | 15 | 15 | 25 |
| Gross realized losses | (18) | (14) | $ (22) |
| AFS Debt Maturities | |||
| Due in one year or less | 1,501 | ||
| Due after one year through five years | 3,465 | ||
| Due after five years through ten years | 1,271 | ||
| Due after ten years | 656 | ||
| Total debt securities | $ 6,893 | $ 7,257 | |
Financial Instruments - Summary of Equity and Other Investments (Details) - Other Assets - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Schedule of Equity Method Investments [Line Items] | ||
| Investments with readily determinable fair value (marketable equity securities) | $ 64 | $ 74 |
| Investments without readily determinable fair values | 732 | 537 |
| Equity method and other investments | 85 | 76 |
| Total equity and other investments | $ 881 | $ 687 |
Financial Instruments - Activity Related to the Company's Investment Portfolio, Equity and Other Investments (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Marketable Securities [Line Items] | |||
| Proceeds from sales | $ 9,692 | $ 11,345 | $ 9,574 |
| Equity Investments | |||
| Marketable Securities [Line Items] | |||
| Proceeds from sales | 81 | 13 | 15 |
| Gross gains | 99 | 68 | 17 |
| Gross losses | (52) | (3) | (30) |
| Impairment losses recognized | $ (17) | $ (4) | $ (4) |
Financing Arrangements - Current Debt Obligations (Details) - USD ($) $ in Millions |
12 Months Ended | |
|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
|
| Debt [Line Items] | ||
| Finance Lease, Liability, Current, Statement of Financial Position [Extensible Enumeration] | Current debt obligations | Current debt obligations |
| Finance lease obligations | $ 6 | $ 9 |
| Current debt obligations | $ 3,742 | 11 |
| 0.375 percent four-year 2019 senior notes | ||
| Debt [Line Items] | ||
| Stated interest rate | 0.375% | |
| 0.000 percent two-year 2020 senior notes | ||
| Debt [Line Items] | ||
| Stated interest rate | 0.00% | |
| Debt term | 2 years | |
| Senior Notes | 0.000 percent three-year 2019 senior notes | ||
| Debt [Line Items] | ||
| Stated interest rate | 0.00% | |
| Debt term | 3 years | |
| Current maturities of long-term debt | $ 798 | 0 |
| Senior Notes | 0.375 percent four-year 2019 senior notes | ||
| Debt [Line Items] | ||
| Debt term | 4 years | |
| Current maturities of long-term debt | $ 1,596 | 0 |
| Senior Notes | 0.000 percent two-year 2020 senior notes | ||
| Debt [Line Items] | ||
| Current maturities of long-term debt | 1,330 | 0 |
| Bank borrowings | ||
| Debt [Line Items] | ||
| Bank borrowings | $ 12 | $ 2 |
Financing Arrangements - Additional Information (Details) |
1 Months Ended | 12 Months Ended | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Jul. 29, 2022
USD ($)
|
May 02, 2022
USD ($)
|
Dec. 12, 2020
USD ($)
|
Nov. 12, 2020 |
May 12, 2020
JPY (¥)
|
Dec. 12, 2018
extenstion
|
Mar. 31, 2021
EUR (€)
|
Oct. 31, 2020
USD ($)
|
Oct. 31, 2020
EUR (€)
|
Sep. 30, 2020
USD ($)
|
Jun. 30, 2019
USD ($)
|
Apr. 29, 2022
USD ($)
|
Apr. 30, 2021
USD ($)
|
Apr. 24, 2020
USD ($)
|
Jun. 30, 2022
USD ($)
|
Jun. 30, 2022
JPY (¥)
|
May 31, 2022
USD ($)
|
May 31, 2022
JPY (¥)
|
May 02, 2022
JPY (¥)
|
Sep. 30, 2020
EUR (€)
tranche
|
Jun. 30, 2019
EUR (€)
tranche
|
|
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Amount of current debt obligations outstanding | $ 3,742,000,000 | $ 11,000,000 | |||||||||||||||||||
| Principal value | 24,200,000,000 | 26,500,000,000 | |||||||||||||||||||
| Cash proceeds | 0 | 7,172,000,000 | $ 5,568,000,000 | ||||||||||||||||||
| Loss on debt extinguishment | 0 | 308,000,000 | $ 406,000,000 | ||||||||||||||||||
| Estimated fair value of senior notes | 22,900,000,000 | 28,600,000,000 | |||||||||||||||||||
| Term Loan Agreements | Medtronic Luxco | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Debt term | 6 months | ||||||||||||||||||||
| Principal value | ¥ | ¥ 300,000,000,000 | ||||||||||||||||||||
| Additional extension option, term of extension | 6 months | 6 months | |||||||||||||||||||
| Term Loan Agreements | Medtronic Luxco | Subsequent Event | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Debt term | 364 days | ||||||||||||||||||||
| Principal value | $ 2,300,000,000 | ¥ 297,000,000,000 | $ 2,300,000,000 | ¥ 297,000,000,000 | ¥ 300,000,000,000 | ||||||||||||||||
| Term Loan Agreements | Medtronic Luxco | TIBOR Rate | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Margin added per annum | 0.50% | ||||||||||||||||||||
| Term Loan Agreements | Medtronic Luxco | TIBOR Rate | Subsequent Event | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Margin added per annum | 0.40% | ||||||||||||||||||||
| $3.5 Billion Revolving Credit Facility | Credit Facility | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Maximum borrowing capacity | $ 3,500,000,000 | ||||||||||||||||||||
| Debt term | 5 years | ||||||||||||||||||||
| Line of credit, number of extension options | extenstion | 2 | ||||||||||||||||||||
| Length of extension from maturity date | 1 year | ||||||||||||||||||||
| Additional borrowing capacity | $ 1,000,000,000 | ||||||||||||||||||||
| Committed line of credit outstanding | 0 | 0 | |||||||||||||||||||
| 2019 Senior Notes | Senior Notes | Medtronic Luxco | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Number of tranches | tranche | 6 | ||||||||||||||||||||
| Principal value | € | € 5,000,000,000 | ||||||||||||||||||||
| Cash proceeds | $ 5,600,000,000 | ||||||||||||||||||||
| Medtronic Inc, CIFSA, and Medtronic Luxco Senior Notes | Senior Notes | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Extinguishment of debt | 5,200,000,000 | ||||||||||||||||||||
| Redemption of senior notes, consideration | $ 6,300,000,000 | $ 5,600,000,000 | |||||||||||||||||||
| Loss on debt extinguishment | 308,000,000 | 413,000,000 | |||||||||||||||||||
| Medtronic Inc, CIFSA, and Medtronic Luxco Senior Notes | Senior Notes | Subsequent Event | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Loss on debt extinguishment | $ 53,000,000 | ||||||||||||||||||||
| Medtronic Inc. and CIFSA Senior Notes | Senior Notes | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Extinguishment of debt | $ 4,300,000,000 | ||||||||||||||||||||
| Medtronic Luxco Senior Notes | Senior Notes | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Extinguishment of debt | € | € 750,000,000 | € 1,500,000,000 | |||||||||||||||||||
| Medtronic Luxco Senior Notes | Senior Notes | Subsequent Event | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Extinguishment of debt | $ 368,000,000 | ||||||||||||||||||||
| Redemption of senior notes, consideration | 376,000,000 | ||||||||||||||||||||
| Medtronic Inc Senior Notes | Senior Notes | Subsequent Event | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Extinguishment of debt | $ 1,900,000,000 | ||||||||||||||||||||
| Senior Notes 2020 | Senior Notes | Medtronic Luxco | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Number of tranches | tranche | 6 | ||||||||||||||||||||
| Principal value | € | € 6,300,000,000 | ||||||||||||||||||||
| Cash proceeds | $ 7,200,000,000 | ||||||||||||||||||||
| Commercial Paper | |||||||||||||||||||||
| Debt Instrument [Line Items] | |||||||||||||||||||||
| Commercial paper, maximum borrowing capacity | 3,500,000,000 | ||||||||||||||||||||
| Amount of current debt obligations outstanding | $ 0 | $ 0 | |||||||||||||||||||
| Weighted average original maturity | 15 days | ||||||||||||||||||||
| Weighted average interest rate | 0.70% | ||||||||||||||||||||
Financing Arrangements - Long-term Debt (Details) - USD ($) $ in Millions |
12 Months Ended | |
|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
|
| Debt Instrument [Line Items] | ||
| Finance lease obligations | $ 56 | $ 62 |
| Debt discount, net | (52) | (75) |
| Deferred financing costs | (109) | (125) |
| Long-term debt | $ 20,372 | $ 26,378 |
| Effective Interest Rate | 9.15% | 9.29% |
| Finance Lease, Liability, Noncurrent, Statement of Financial Position [Extensible Enumeration] | Long-term debt | Long-term debt |
| Senior Notes | 0.000 percent three-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 0.00% | |
| Debt term | 3 years | |
| Amount | $ 0 | $ 907 |
| Effective Interest Rate | 0.00% | 0.08% |
| Senior Notes | 0.375 percent four-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 0.375% | |
| Debt term | 4 years | |
| Amount | $ 0 | $ 1,813 |
| Effective Interest Rate | 0.00% | 0.55% |
| Senior Notes | 0.000 percent two-year 2020 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 0.00% | |
| Debt term | 2 years | |
| Amount | $ 0 | $ 1,511 |
| Effective Interest Rate | 0.00% | 0.12% |
| Senior Notes | 3.500 percent ten-year 2015 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 3.50% | |
| Debt term | 10 years | |
| Amount | $ 1,890 | $ 1,890 |
| Effective Interest Rate | 3.74% | 3.74% |
| Senior Notes | 0.250 percent six-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 0.25% | |
| Debt term | 6 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 0.45% | 0.43% |
| Senior Notes | 0.000 percent five-year 2020 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 0.00% | |
| Debt term | 5 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 0.25% | 0.22% |
| Senior Notes | 1.125 percent eight-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 1.125% | |
| Debt term | 8 years | |
| Amount | $ 1,596 | $ 1,813 |
| Effective Interest Rate | 1.26% | 1.24% |
| Senior Notes | 3.350 percent ten-year 2017 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 3.35% | |
| Debt term | 10 years | |
| Amount | $ 368 | $ 368 |
| Effective Interest Rate | 3.53% | 3.53% |
| Senior Notes | 0.375 percent eight-year 2020 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 0.375% | |
| Debt term | 8 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 0.52% | 0.51% |
| Senior Notes | 1.625 percent twelve-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 1.625% | |
| Debt term | 12 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 1.75% | 1.74% |
| Senior Notes | 1.000 percent twelve-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 1.00% | |
| Debt term | 12 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 1.06% | 1.05% |
| Senior Notes | 0.750 percent twelve-year 2020 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 0.75% | |
| Debt term | 12 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 0.81% | 0.81% |
| Senior Notes | 4.375 percent twenty-year 2015 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 4.375% | |
| Debt term | 20 years | |
| Amount | $ 1,932 | $ 1,932 |
| Effective Interest Rate | 4.47% | 4.47% |
| Senior Notes | 6.550 percent thirty-year 2007 CIFSA senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 6.55% | |
| Debt term | 30 years | |
| Amount | $ 253 | $ 253 |
| Effective Interest Rate | 4.67% | 4.67% |
| Senior Notes | 2.250 percent twenty-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 2.25% | |
| Debt term | 20 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 2.35% | 2.34% |
| Senior Notes | 6.500 percent thirty-year 2009 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 6.50% | |
| Debt term | 30 years | |
| Amount | $ 158 | $ 158 |
| Effective Interest Rate | 6.56% | 6.56% |
| Senior Notes | 1.500 percent twenty-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 1.50% | |
| Debt term | 20 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 1.59% | 1.58% |
| Senior Notes | 5.550 percent thirty-year 2010 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 5.55% | |
| Debt term | 30 years | |
| Amount | $ 224 | $ 224 |
| Effective Interest Rate | 5.58% | 5.58% |
| Senior Notes | 1.375 percent twenty-year 2020 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 1.375% | |
| Debt term | 20 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 1.47% | 1.46% |
| Senior Notes | 4.500 percent thirty-year 2012 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 4.50% | |
| Debt term | 30 years | |
| Amount | $ 105 | $ 105 |
| Effective Interest Rate | 4.54% | 4.54% |
| Senior Notes | 4.000 percent thirty-year 2013 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 4.00% | |
| Debt term | 30 years | |
| Amount | $ 305 | $ 305 |
| Effective Interest Rate | 4.09% | 4.09% |
| Senior Notes | 4.625 percent thirty-year 2014 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 4.625% | |
| Debt term | 30 years | |
| Amount | $ 127 | $ 127 |
| Effective Interest Rate | 4.67% | 4.67% |
| Senior Notes | 4.625 percent thirty-year 2015 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 4.625% | |
| Debt term | 30 years | |
| Amount | $ 1,813 | $ 1,813 |
| Effective Interest Rate | 4.69% | 4.69% |
| Senior Notes | 1.750 percent thirty-year 2019 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 1.75% | |
| Debt term | 30 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 1.88% | 1.87% |
| Senior Notes | 1.625 percent thirty-year 2020 senior notes | ||
| Debt Instrument [Line Items] | ||
| Stated interest rate | 1.625% | |
| Debt term | 30 years | |
| Amount | $ 1,064 | $ 1,209 |
| Effective Interest Rate | 1.76% | 1.75% |
Financing Arrangements - Long-term Debt Maturities (Details) $ in Millions |
Apr. 29, 2022
USD ($)
|
|---|---|
| Long-term Debt, Fiscal Year Maturity | |
| 2023 | $ 3,744 |
| 2024 | 6 |
| 2025 | 1,895 |
| 2026 | 2,133 |
| 2027 | 1,969 |
| Thereafter | 14,528 |
| Total | $ 24,275 |
Derivatives and Currency Exchange Risk Management - Narrative (Details) € in Billions |
12 Months Ended | |||||
|---|---|---|---|---|---|---|
|
Apr. 29, 2022
USD ($)
|
Apr. 30, 2021
USD ($)
|
Apr. 24, 2020
USD ($)
|
Apr. 29, 2022
EUR (€)
|
Feb. 28, 2021
USD ($)
|
Feb. 28, 2021
JPY (¥)
|
|
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| After-tax net unrealized gains (losses) | $ 474,000,000 | $ (253,000,000) | ||||
| Cash flow hedge unrealized gains to be reclassified over the next twelve months | 368,000,000 | |||||
| After-tax net unrealized gains (losses) associated with net investment hedging instruments recorded in AOCI | 841,000,000 | (1,500,000,000) | ||||
| Net investment hedge unrealized gains or losses to be reclassified over the next twelve months, net of tax | 0 | |||||
| Gains (losses) on firm commitments that no longer qualify as net investment hedges | 0 | 0 | $ 0 | |||
| Net cash collateral received | 254,000,000 | (46,000,000) | ||||
| Term Loan Agreements | Medtronic Luxco | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Outstanding debt | ¥ | ¥ 300,000,000,000 | |||||
| Currency exchange rate contracts | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Gross notional amount | 13,800,000,000 | 14,700,000,000 | ||||
| Currency exchange rate contracts | Derivatives not designated as hedging instruments | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Gross notional amount | 4,900,000,000 | 5,700,000,000 | ||||
| Currency exchange rate contracts | Derivatives designated as hedging instruments | Cash flow hedges | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Gross notional amount | $ 8,800,000,000 | 9,000,000,000 | ||||
| Maximum remaining maturity of foreign currency derivatives | 3 years | |||||
| Currency exchange rate contracts | Derivatives designated as hedging instruments | Net investment hedges | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Gross notional amount | $ 17,000,000,000 | € 16.0 | ||||
| Currency exchange rate contracts | Derivatives designated as hedging instruments | Net investment hedges | Dedesignation of hedge | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Gross notional amount | ¥ | 300,000,000,000 | |||||
| Total return swaps | Derivatives not designated as hedging instruments | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Gross notional amount | $ 226,000,000 | $ 243,000,000 | ||||
| Forward Contracts | Derivatives not designated as hedging instruments | ||||||
| Derivative Instruments and Hedging Activities Disclosures [Line Items] | ||||||
| Gross notional amount | $ 2,900,000,000 | ¥ 300,000,000,000 | ||||
Derivatives and Currency Exchange Risk Management - Derivative (Gains) Losses Designated as Hedging Instruments (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Derivative Instruments, (Gain) Loss [Line Items] | |||
| Total | $ (3,234) | $ 2,321 | $ (802) |
| Total | (83) | (2) | (344) |
| Other operating expense, net | |||
| Derivative Instruments, (Gain) Loss [Line Items] | |||
| Net investment hedges | (2,299) | 1,694 | (405) |
| Net investment hedges | 0 | 0 | (9) |
| Currency exchange rate contracts | Other operating expense, net | |||
| Derivative Instruments, (Gain) Loss [Line Items] | |||
| (Gain) Loss Recognized in Accumulated Other Comprehensive Income | (953) | 519 | (397) |
| (Gain) Loss Reclassified into Income | (144) | (17) | (335) |
| Currency exchange rate contracts | Cost of products sold | |||
| Derivative Instruments, (Gain) Loss [Line Items] | |||
| (Gain) Loss Recognized in Accumulated Other Comprehensive Income | 18 | 108 | 0 |
| (Gain) Loss Reclassified into Income | $ 61 | $ 15 | $ 0 |
Derivatives and Currency Exchange Risk Management - Gains and Losses on Derivatives Not Designated as Hedging Instruments (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Derivative Instruments, (Gain) Loss [Line Items] | |||
| Derivative instruments not designated as hedging instruments, (gain) loss, net | $ (53) | $ 166 | $ (126) |
| Other operating expense, net | Currency exchange rate contracts | |||
| Derivative Instruments, (Gain) Loss [Line Items] | |||
| Derivative instruments not designated as hedging instruments, (gain) loss, net | (54) | 247 | (133) |
| Other operating expense, net | Total return swaps | |||
| Derivative Instruments, (Gain) Loss [Line Items] | |||
| Derivative instruments not designated as hedging instruments, (gain) loss, net | $ 1 | $ (81) | $ 7 |
Derivatives and Currency Exchange Risk Management - Classification and Fair Value Amounts of Derivative Instruments in Balance Sheets (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | $ 695 | $ 102 |
| Fair Value - Liabilities | 129 | 296 |
| Currency exchange rate contracts | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 695 | 85 |
| Fair Value - Liabilities | 109 | 296 |
| Total return swaps | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 18 | |
| Fair Value - Liabilities | 20 | |
| Derivatives designated as hedging instruments | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 649 | 70 |
| Fair Value - Liabilities | 60 | 285 |
| Derivatives designated as hedging instruments | Currency exchange rate contracts | Other current assets | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 481 | 49 |
| Derivatives designated as hedging instruments | Currency exchange rate contracts | Other assets | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 168 | 22 |
| Derivatives designated as hedging instruments | Currency exchange rate contracts | Other accrued expenses | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Liabilities | 43 | 190 |
| Derivatives designated as hedging instruments | Currency exchange rate contracts | Other liabilities | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Liabilities | 16 | 94 |
| Derivatives not designated as hedging instruments | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 46 | 32 |
| Fair Value - Liabilities | 69 | 11 |
| Derivatives not designated as hedging instruments | Currency exchange rate contracts | Other current assets | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 46 | 14 |
| Derivatives not designated as hedging instruments | Currency exchange rate contracts | Other accrued expenses | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Liabilities | 49 | 11 |
| Derivatives not designated as hedging instruments | Total return swaps | Other current assets | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Assets | 0 | 18 |
| Derivatives not designated as hedging instruments | Total return swaps | Other accrued expenses | ||
| Derivatives, Fair Value [Line Items] | ||
| Fair Value - Liabilities | $ 20 | $ 0 |
Derivatives and Currency Exchange Risk Management - Derivative Assets and Liabilities Measured at Fair Value on a Recurring Basis (Details) - Fair Value, Measurements, Recurring - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Level 1 | ||
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] | ||
| Derivative assets | $ 695 | $ 85 |
| Derivative liabilities | 0 | 296 |
| Level 2 | ||
| Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items] | ||
| Derivative assets | 109 | 18 |
| Derivative liabilities | $ 20 | $ 0 |
Derivatives and Currency Exchange Risk Management - Offsetting Assets and Liabilities (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Derivative assets: | ||
| Gross Amount of Recognized Assets (Liabilities) | $ 695 | $ 102 |
| Gross Amount Not Offset on the Balance Sheet, Financial Instruments | (83) | |
| Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted | 0 | |
| Net Amount | 19 | |
| Derivative liabilities: | ||
| Gross Amount of Recognized Assets (Liabilities) | (129) | (296) |
| Gross Amount Not Offset on the Balance Sheet, Financial Instruments | 109 | |
| Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted | 0 | |
| Net Amount | (20) | |
| Total | ||
| Gross Amount of Recognized Assets (Liabilities) | 566 | (194) |
| Gross Amount Not Offset on the Balance Sheet, Financial Instruments | 0 | 0 |
| Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted | (254) | 46 |
| Net Amount | 312 | (148) |
| Currency exchange rate contracts | ||
| Derivative assets: | ||
| Gross Amount of Recognized Assets (Liabilities) | 695 | 85 |
| Gross Amount Not Offset on the Balance Sheet, Financial Instruments | (109) | (83) |
| Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted | (254) | 0 |
| Net Amount | 332 | 1 |
| Derivative liabilities: | ||
| Gross Amount of Recognized Assets (Liabilities) | (109) | (296) |
| Gross Amount Not Offset on the Balance Sheet, Financial Instruments | 109 | 83 |
| Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted | 0 | 46 |
| Net Amount | 0 | (167) |
| Total return swaps | ||
| Derivative assets: | ||
| Gross Amount of Recognized Assets (Liabilities) | 18 | |
| Gross Amount Not Offset on the Balance Sheet, Financial Instruments | 0 | |
| Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted | 0 | |
| Net Amount | $ 18 | |
| Derivative liabilities: | ||
| Gross Amount of Recognized Assets (Liabilities) | (20) | |
| Gross Amount Not Offset on the Balance Sheet, Financial Instruments | 0 | |
| Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted | 0 | |
| Net Amount | $ (20) |
Inventories (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Inventory Disclosure [Abstract] | ||
| Finished goods | $ 3,070 | $ 2,906 |
| Work-in-process | 682 | 611 |
| Raw materials | 864 | 796 |
| Total | $ 4,616 | $ 4,313 |
Goodwill and Other Intangible Assets - Changes in Goodwill (Details) - USD ($) |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Goodwill [Roll Forward] | |||
| Goodwill, beginning balance | $ 41,961,000,000 | $ 39,841,000,000 | |
| Goodwill as a result of acquisitions | 80,000,000 | 816,000,000 | |
| Purchase accounting adjustments | 25,000,000 | (8,000,000) | |
| Currency translation and other | (1,563,000,000) | 1,312,000,000 | |
| Goodwill, ending balance | 40,502,000,000 | 41,961,000,000 | $ 39,841,000,000 |
| Goodwill impairment | 0 | 0 | 0 |
| Cardiovascular | |||
| Goodwill [Roll Forward] | |||
| Goodwill, beginning balance | 7,209,000,000 | 6,831,000,000 | |
| Goodwill as a result of acquisitions | 55,000,000 | 248,000,000 | |
| Purchase accounting adjustments | 21,000,000 | (2,000,000) | |
| Currency translation and other | (125,000,000) | 132,000,000 | |
| Goodwill, ending balance | 7,160,000,000 | 7,209,000,000 | 6,831,000,000 |
| Medical Surgical | |||
| Goodwill [Roll Forward] | |||
| Goodwill, beginning balance | 21,195,000,000 | 20,176,000,000 | |
| Goodwill as a result of acquisitions | 0 | 12,000,000 | |
| Purchase accounting adjustments | 3,000,000 | (5,000,000) | |
| Currency translation and other | (1,241,000,000) | 1,012,000,000 | |
| Goodwill, ending balance | 19,957,000,000 | 21,195,000,000 | 20,176,000,000 |
| Neuroscience | |||
| Goodwill [Roll Forward] | |||
| Goodwill, beginning balance | 11,300,000,000 | 10,920,000,000 | |
| Goodwill as a result of acquisitions | 26,000,000 | 210,000,000 | |
| Purchase accounting adjustments | 3,000,000 | 3,000,000 | |
| Currency translation and other | (196,000,000) | 167,000,000 | |
| Goodwill, ending balance | 11,132,000,000 | 11,300,000,000 | 10,920,000,000 |
| Diabetes | |||
| Goodwill [Roll Forward] | |||
| Goodwill, beginning balance | 2,257,000,000 | 1,914,000,000 | |
| Goodwill as a result of acquisitions | 0 | 346,000,000 | |
| Purchase accounting adjustments | (2,000,000) | (4,000,000) | |
| Currency translation and other | (1,000,000) | 1,000,000 | |
| Goodwill, ending balance | $ 2,254,000,000 | $ 2,257,000,000 | $ 1,914,000,000 |
Goodwill and Other Intangible Assets - Intangible Assets (Details) - USD ($) $ in Millions |
3 Months Ended | 12 Months Ended | ||
|---|---|---|---|---|
Jul. 30, 2021 |
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Finite-Lived Intangible Assets [Line Items] | ||||
| Gross Carrying Amount | $ 28,308 | $ 28,879 | ||
| Accumulated Amortization | (13,006) | (11,533) | ||
| Impairment of finite-lived intangible assets | $ (37) | |||
| Impairment of indefinite-lived intangible assets | 35 | |||
| Neuroscience | ||||
| Finite-Lived Intangible Assets [Line Items] | ||||
| Impairment of finite-lived intangible assets | (30) | (33) | ||
| Impairment of indefinite-lived intangible assets | 0 | 45 | 25 | |
| Cardiovascular | ||||
| Finite-Lived Intangible Assets [Line Items] | ||||
| Impairment of finite-lived intangible assets | $ (409) | (4) | ||
| Impairment of indefinite-lived intangible assets | $ 10 | |||
| IPR&D | ||||
| Finite-Lived Intangible Assets [Line Items] | ||||
| Indefinite-lived Intangible Assets | 293 | 394 | ||
| Customer-related | ||||
| Finite-Lived Intangible Assets [Line Items] | ||||
| Gross Carrying Amount | 16,953 | 17,036 | ||
| Accumulated Amortization | (7,005) | (6,058) | ||
| Purchased technology and patents | ||||
| Finite-Lived Intangible Assets [Line Items] | ||||
| Gross Carrying Amount | 10,802 | 11,286 | ||
| Accumulated Amortization | (5,667) | (5,156) | ||
| Trademarks and tradenames | ||||
| Finite-Lived Intangible Assets [Line Items] | ||||
| Gross Carrying Amount | 473 | 475 | ||
| Accumulated Amortization | (266) | (251) | ||
| Other | ||||
| Finite-Lived Intangible Assets [Line Items] | ||||
| Gross Carrying Amount | 80 | 82 | ||
| Accumulated Amortization | $ (69) | $ (68) | ||
Goodwill and Other Intangible Assets - Amortization Expense (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Goodwill and Intangible Assets Disclosure [Abstract] | |||
| Amortization expense | $ 1,733 | $ 1,783 | $ 1,756 |
| 2023 | 1,659 | ||
| 2024 | 1,624 | ||
| 2025 | 1,602 | ||
| 2026 | 1,588 | ||
| 2027 | $ 1,564 | ||
Property, Plant, and Equipment (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment | $ 13,365 | $ 12,700 | |
| Less: Accumulated depreciation | (7,952) | (7,479) | |
| Property, plant, and equipment, net | 5,413 | 5,221 | |
| Depreciation expense | $ 974 | 919 | $ 907 |
| Equipment | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment, useful life | 7 years | ||
| Property, plant, and equipment | $ 6,489 | 6,308 | |
| Equipment | Minimum | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment, useful life | 2 years | ||
| Equipment | Maximum | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment, useful life | 15 years | ||
| Computer software | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment | $ 2,617 | 2,346 | |
| Computer software | Maximum | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment, useful life | 5 years | ||
| Land and land improvements | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment | $ 170 | 178 | |
| Land and land improvements | Maximum | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment, useful life | 20 years | ||
| Buildings and leasehold improvements | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment | $ 2,351 | 2,370 | |
| Buildings and leasehold improvements | Maximum | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment, useful life | 40 years | ||
| Construction in progress | |||
| Property, Plant and Equipment [Line Items] | |||
| Property, plant, and equipment | $ 1,737 | $ 1,498 | |
Shareholders' Equity (Details) |
12 Months Ended | 38 Months Ended | ||||
|---|---|---|---|---|---|---|
|
Apr. 29, 2022
€ / shares
$ / shares
shares
|
Apr. 30, 2021
$ / shares
shares
|
Apr. 29, 2022
USD ($)
|
Apr. 29, 2022
USD ($)
$ / shares
shares
|
Jan. 28, 2022
shares
|
Mar. 31, 2019
USD ($)
|
|
| Class of Stock [Line Items] | ||||||
| Common stock, authorized (in shares) | 2,600,000,000 | 2,600,000,000 | ||||
| Common stock, par value (in dollars per share) | $ / shares | $ 0.0001 | $ 0.0001 | ||||
| Deferred stock, shares authorized (in shares) | 40,000 | |||||
| Deferred stock, par value (in euros per share) | € / shares | € 1.00 | |||||
| Preferred stock, authorized (in shares) | 127,500,000 | |||||
| Preferred stock, par value (in dollars per share) | $ / shares | $ 0.20 | |||||
| Preferred stock, outstanding (in shares) | 0 | |||||
| Deferred stock, issued (in shares) | 0 | |||||
| Deferred stock, outstanding (in shares) | 0 | |||||
| Preferred stock, issued (in shares) | 0 | |||||
| Shares repurchased (in shares) | 22,000,000 | 4,000,000 | ||||
| Average repurchase price (in dollars per share) | $ / shares | € 113.11 | $ 126.80 | ||||
| Amount authorized for repurchase | $ | $ 6,000,000,000 | |||||
| Amount repurchased | $ | $ 3,000,000,000 | |||||
| Amount available for future repurchases | $ | $ 3,000,000,000 | |||||
| Series A Preferred Shares | ||||||
| Class of Stock [Line Items] | ||||||
| Preferred stock, authorized (in shares) | 500,000 | |||||
| Preferred stock, par value (in dollars per share) | $ / shares | $ 1.00 | |||||
| Preferred stock, outstanding (in shares) | 0 | 1,872 | ||||
| Preferred stock, value, outstanding | $ | $ 75,000.000 | |||||
Stock Purchase and Award Plans - Additional Information (Details) - USD ($) $ / shares in Units, $ in Millions |
12 Months Ended | |
|---|---|---|
Apr. 29, 2022 |
Dec. 09, 2021 |
|
| Stock options | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Expiration period | 10 years | |
| Vesting period | 4 years | |
| Unrecognized compensation expense related to outstanding stock options | $ 90 | |
| Weighted average period over which unrecognized compensation is expected to be recognized | 2 years 6 months | |
| Restricted stock | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Weighted average period over which unrecognized compensation is expected to be recognized | 2 years 6 months | |
| Unrecognized compensation expense related to restricted stock awards | $ 316 | |
| Restricted stock units | Minimum | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Vesting period | 3 years | |
| Restricted stock units | Maximum | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Vesting period | 4 years | |
| Performance-based restricted stock awards | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Vesting period | 3 years | |
| Performance share units | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Vesting period | 3 years | |
| Weighted average period over which unrecognized compensation is expected to be recognized | 1 year 10 months 24 days | |
| Unrecognized compensation expense related to restricted stock awards | $ 84 | |
| Performance share units | Minimum | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Vesting percentage | 0.00% | |
| Performance share units | Maximum | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Vesting percentage | 200.00% | |
| Employee stock purchase plan | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| The discount rate from market value on purchase date | 15.00% | |
| Shares purchased by employees (in shares) | 2,000,000 | |
| Average purchase price (in dollars per share) | $ 98.75 | |
| Shares available for future purchase (in shares) | 7,000,000 | |
| 2013 Plan | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Issuance of ordinary shares (in shares) | 14,000,000 | |
| 2021 Plan | ||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Issuance of ordinary shares (in shares) | 115,000,000 | |
| Shares available for future grants (in shares) | 127,000,000 |
Stock Purchase and Award Plans - Stock-based Compensation Expense (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | $ 359 | $ 344 | $ 297 |
| Income tax benefits | (62) | (59) | (51) |
| Total stock-based compensation expense, net of tax | 297 | 285 | 246 |
| Cost of products sold | |||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | 36 | 35 | 28 |
| Research and development expense | |||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | 40 | 38 | 36 |
| Selling, general, and administrative expense | |||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | 283 | 272 | 233 |
| Stock options | |||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | 70 | 72 | 61 |
| Restricted stock | |||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | 184 | 185 | 205 |
| Performance share units | |||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | 66 | 49 | 0 |
| Employee stock purchase plan | |||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |||
| Total stock-based compensation expense | $ 39 | $ 38 | $ 31 |
Stock Purchase and Award Plans - Valuation Assumptions (Details) - $ / shares |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Share-based Payment Arrangement [Abstract] | |||
| Weighted average fair value of options granted (in dollars per share) | $ 22.83 | $ 16.15 | $ 15.49 |
| Assumptions used: | |||
| Expected life (years) | 6 years | 6 years | 6 years 1 month 6 days |
| Risk-free interest rate | 0.90% | 0.33% | 1.88% |
| Volatility | 23.04% | 24.17% | 17.97% |
| Dividend yield | 1.95% | 2.36% | 2.09% |
Stock Purchase and Award Plans - Stock Options Activity (Details) - Stock options - USD ($) $ / shares in Units, shares in Thousands, $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Options | |||
| Outstanding at beginning of period (in shares) | 27,972 | ||
| Granted (in shares) | 4,153 | ||
| Exercised (in shares) | (3,222) | ||
| Expired/Forfeited (in shares) | (641) | ||
| Outstanding at end of period (in shares) | 28,263 | 27,972 | |
| Options, Expected to vest (in shares) | 8,818 | ||
| Options, Exercisable (in shares) | 18,804 | ||
| Wtd. Avg. Exercise Price | |||
| Outstanding at beginning of period (in dollars per share) | $ 84.38 | ||
| Granted (in dollars per share) | 129.03 | ||
| Exercised (in dollars per share) | 70.52 | ||
| Expired/Forfeited (in dollars per share) | 107.42 | ||
| Outstanding at end of period (in dollars per share) | 92.00 | $ 84.38 | |
| Weighted Average Exercise Price, Expected to vest (in dollars per share) | 110.27 | ||
| Weighted Average Exercise Price, Exercisable (in dollars per share) | $ 82.62 | ||
| Additional Disclosures | |||
| Weighted Average Remaining Contractual Term, Outstanding | 5 years 6 months | ||
| Weighted Average Remaining Contractual Term, Expected to vest | 8 years 4 months 24 days | ||
| Weighted Average Remaining Contractual Term, Exercisable | 4 years | ||
| Aggregate Intrinsic Value, Outstanding | $ 450 | ||
| Aggregate Intrinsic Value, Expected to vest | 35 | ||
| Aggregate Intrinsic Value, Exercisable | 414 | ||
| Cash proceeds from options exercised | 209 | $ 277 | $ 484 |
| Intrinsic value of options exercised | 174 | 205 | 349 |
| Tax benefit related to options exercised | $ 40 | $ 47 | $ 75 |
Stock Purchase and Award Plans - Restricted Stock Activity (Details) - Restricted stock - USD ($) $ / shares in Units, shares in Thousands, $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Units | |||
| Nonvested at beginning of period (in shares) | 5,980 | ||
| Granted (in shares) | 1,935 | ||
| Vested (in shares) | (2,089) | ||
| Forfeited (in shares) | (456) | ||
| Nonvested at end of period (in shares) | 5,370 | 5,980 | |
| Wtd. Avg. Grant Price | |||
| Nonvested at beginning of period (in dollars per share) | $ 97.66 | ||
| Granted (in dollars per share) | 127.47 | $ 99.48 | $ 103.52 |
| Vested (in dollars per share) | 93.05 | ||
| Forfeited (in dollars per share) | 107.53 | ||
| Nonvested at end of period (in dollars per share) | $ 108.92 | $ 97.66 | |
| Fair value of restricted stock vested | $ 194 | $ 280 | $ 242 |
| Tax benefit related to restricted stock vested | $ 52 | $ 65 | $ 62 |
Stock Purchase and Award Plans - Performance Share Unit Activity (Details) - Performance share units - USD ($) $ / shares in Units, shares in Thousands, $ in Millions |
12 Months Ended | |
|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
|
| Units | ||
| Nonvested at beginning of period (in shares) | 828 | |
| Granted (in shares) | 831 | |
| Forfeited (in shares) | (78) | |
| Nonvested at end of period (in shares) | 1,581 | 828 |
| Wtd. Avg. Grant Price | ||
| Nonvested at beginning of period (in dollars per share) | $ 129.05 | |
| Granted (in dollars per share) | 149.16 | $ 129.04 |
| Forfeited (in dollars per share) | 138.31 | |
| Nonvested at end of period (in dollars per share) | $ 138.95 | $ 129.05 |
| Fair value of restricted stock vested | $ 0 | $ 0 |
| Tax benefit related to restricted stock vested | $ 0 | $ 0 |
Income Taxes - Components of Income Before Income Taxes, Based on Jurisdiction (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Income Tax Disclosure [Abstract] | |||
| U.S. | $ 436 | $ (358) | $ 466 |
| International | 5,081 | 4,253 | 3,589 |
| Income before income taxes | $ 5,517 | $ 3,895 | $ 4,055 |
Income Taxes - Income Tax (Benefit) Provision (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Current tax expense: | |||
| U.S. | $ 467 | $ 287 | $ 151 |
| International | 599 | 439 | 375 |
| Total current tax expense | 1,066 | 726 | 526 |
| Deferred tax (benefit) expense: | |||
| U.S. | (402) | (625) | (138) |
| International | (209) | 165 | (1,139) |
| Net deferred tax benefit | (611) | (461) | (1,277) |
| Income tax provision (benefit) | $ 456 | $ 265 | $ (751) |
Income Taxes - Schedule of Deferred Tax Assets and Liabilities (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
|---|---|---|
| Deferred tax assets: | ||
| Intangible assets | $ 2,334 | $ 1,536 |
| Net operating loss, capital loss, and credit carryforwards | 5,982 | 6,114 |
| Capitalization of research and development | 597 | 408 |
| Other accrued liabilities | 483 | 442 |
| Accrued compensation | 332 | 411 |
| Pension and post-retirement benefits | 66 | 234 |
| Stock-based compensation | 146 | 132 |
| Inventory | 146 | 164 |
| Lease obligations | 92 | 106 |
| Federal and state benefit on uncertain tax positions | 60 | 55 |
| Interest limitation | 386 | 352 |
| Other | 374 | 336 |
| Gross deferred tax assets | 10,998 | 10,290 |
| Valuation allowance | (6,583) | (5,822) |
| Valuation allowance | 4,415 | 4,468 |
| Deferred tax liabilities: | ||
| Intangible assets | (1,488) | (1,856) |
| Realized loss on derivative financial instruments | (66) | (75) |
| Right of use leases | (89) | (102) |
| Unrealized gain on available-for-sale securities and derivative financial instruments | 0 | (16) |
| Accumulated depreciation | (121) | (151) |
| Outside basis difference of subsidiaries | (129) | (101) |
| Other | (70) | (81) |
| Total deferred tax liabilities | (1,963) | (2,382) |
| Prepaid income taxes | 474 | 458 |
| Income tax receivables | 358 | 353 |
| Tax assets, net | 3,284 | 2,897 |
| Reported as (after valuation allowance and jurisdictional netting): | ||
| Other current assets | 765 | 756 |
| Tax assets | 3,403 | 3,169 |
| Deferred tax liabilities | $ (884) | $ (1,028) |
Income Taxes - Additional Information (Details) - USD ($) $ / shares in Units, $ in Millions |
12 Months Ended | |||
|---|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
Apr. 26, 2019 |
|
| Operating Loss Carryforwards [Line Items] | ||||
| Undistributed earnings from non-U.S. subsidiaries | $ 79,300 | $ 74,200 | ||
| Tax credit carryforward | 254 | |||
| Tax credit carryforward, no expiration | 120 | |||
| Valuation allowance | 6,583 | 5,822 | ||
| Net benefit from certain tax adjustments | 50 | 41 | $ 1,200 | |
| Benefit associated with tax basis for Swiss Cantonal | 82 | 106 | ||
| Tax expense (benefit) related to tax basis adjustment, intercompany intellectual property transactions | (82) | 73 | ||
| Tax expense associated with the amortization of the previously established deferred tax assets | 47 | 50 | ||
| Tax expense related to internal restructuring and intercompany sale of assets | 41 | |||
| Intercompany sale of assets | 26 | 25 | ||
| Deferred tax assets, goodwill and intangible assets | $ 1,500 | |||
| Amortization period of deferred tax asset | 20 years | |||
| Tax benefit related to capitalization of research and development costs | $ 83 | |||
| Benefit from U.S. tax reform | 63 | |||
| Benefit related to tax legislative changes in Switzerland | 252 | |||
| Benefit related to release of valuation allowance | 658 | |||
| Tax benefit from intercompany sale of intellectual property | 269 | |||
| Gross unrecognized tax benefits | 1,661 | 1,668 | 1,862 | $ 1,836 |
| Unrecognized tax benefits that would impact effective tax rate | 1,600 | 1,600 | 1,800 | |
| Gross unrecognized tax benefits, net of cash advance, noncurrent liability | 787 | |||
| Estimated reasonably possible decrease in uncertain tax positions excluding interest over the next 12 months | 15 | |||
| Accrued income tax penalties and interest | 117 | 99 | 225 | |
| Gross interest (income) expense | 17 | (44) | $ 53 | |
| Amortizable Asset Created for Swiss Federal Income Tax Purposes | ||||
| Operating Loss Carryforwards [Line Items] | ||||
| Period over which amortization asset will be amortized and deducted | 10 years | |||
| Non-U.S. Tax Authorities | ||||
| Operating Loss Carryforwards [Line Items] | ||||
| Net operating loss carryforwards | 25,400 | |||
| Net operating loss carryforwards, no expiration | 20,000 | |||
| Net operating loss carryforwards, expiring in future years | 5,400 | |||
| Net operating loss carryforwards, valuation allowance | 6,800 | |||
| Tax reductions from tax holiday | $ 248 | $ 301 | $ 231 | |
| Impact on diluted earnings per share (in dollars per share) | $ 0.18 | $ 0.22 | $ 0.17 | |
| Non-U.S. Tax Authorities | Subsidiaries | ||||
| Operating Loss Carryforwards [Line Items] | ||||
| Net operating loss carryforwards | $ 18,600 | |||
| U.S. Tax Authority | ||||
| Operating Loss Carryforwards [Line Items] | ||||
| Net operating loss carryforwards | 222 | |||
| Net operating loss carryforwards, no expiration | 47 | |||
| State and Local Tax Authorities | ||||
| Operating Loss Carryforwards [Line Items] | ||||
| Net operating loss carryforwards, no expiration | 72 | |||
| Operating loss carryforwards | $ 1,400 | |||
Income Taxes - Effective Income Tax Rate Reconciliation (Details) |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Income Tax Disclosure [Abstract] | |||
| U.S. federal statutory tax rate | 21.00% | 21.00% | 21.00% |
| Increase (decrease) in tax rate resulting from: | |||
| U.S. state taxes, net of federal tax benefit | 0.20% | (1.10%) | 0.50% |
| Research and development credit | (1.30%) | (2.30%) | (2.10%) |
| Puerto Rico excise tax | (1.10%) | (2.00%) | (1.50%) |
| International | (11.20%) | (12.60%) | (10.00%) |
| Stock based compensation | (0.80%) | (0.80%) | (1.50%) |
| Interest on uncertain tax positions | 0.50% | 0.90% | 1.30% |
| Base erosion anti-abuse tax | 0.90% | 0.50% | 2.60% |
| Foreign derived intangible income benefit | (1.00%) | (1.90%) | (1.20%) |
| Certain tax adjustments | (0.90%) | (1.00%) | (30.80%) |
| Legal entity restructuring | 0.00% | 1.80% | 0.00% |
| U.S. tax on foreign earnings | 2.20% | 3.40% | 2.80% |
| Other, net | (0.20%) | 0.90% | 0.40% |
| Effective tax rate | 8.30% | 6.80% | (18.50%) |
Income Taxes - Reconciliation of Beginning and Ending Amount of Unrecognized Tax Benefits (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Reconciliation of Unrecognized Tax Benefits, Excluding Amounts Pertaining to Examined Tax Returns [Roll Forward] | |||
| Gross unrecognized tax benefits at beginning of fiscal year | $ 1,668 | $ 1,862 | $ 1,836 |
| Gross increases: | |||
| Prior year tax positions | 1 | 88 | 12 |
| Current year tax positions | 40 | 62 | 55 |
| Gross decreases: | |||
| Prior year tax positions | (29) | (106) | (9) |
| Settlements | (8) | (216) | (5) |
| Statute of limitation lapses | (11) | (21) | (27) |
| Gross unrecognized tax benefits at end of fiscal year | 1,661 | 1,668 | 1,862 |
| Cash advance paid to taxing authorities | (859) | (859) | (859) |
| Gross unrecognized tax benefits at end of fiscal year, net of cash advance | $ 802 | $ 809 | $ 1,003 |
Earnings Per Share (Details) - USD ($) $ / shares in Units, shares in Millions, $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Numerator: | |||
| Net income attributable to ordinary shareholders | $ 5,039 | $ 3,606 | $ 4,789 |
| Denominator: | |||
| Basic - weighted average shares outstanding (in shares) | 1,342.4 | 1,344.9 | 1,340.7 |
| Effect of dilutive securities: | |||
| Employee stock options (in shares) | 6.6 | 6.6 | 7.2 |
| Employee restricted stock units (in shares) | 1.6 | 2.1 | 2.8 |
| Other (in shares) | 0.8 | 0.5 | 0.4 |
| Diluted – weighted average shares outstanding (in shares) | 1,351.4 | 1,354.0 | 1,351.1 |
| Basic earnings per share (in dollars per share) | $ 3.75 | $ 2.68 | $ 3.57 |
| Diluted earnings per share (in dollars per share) | $ 3.73 | $ 2.66 | $ 3.54 |
| Stock options | |||
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] | |||
| Number of shares excluded from computation of earnings per share (in shares) | 5.0 | 4.0 | 4.0 |
Retirement Benefit Plans - Additional Information (Details) $ in Millions |
12 Months Ended | |||
|---|---|---|---|---|
|
Apr. 29, 2022
USD ($)
fund
|
Apr. 30, 2021
USD ($)
|
Apr. 24, 2020
USD ($)
|
May 01, 2005
plan
|
|
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Cost of retirement benefit plans | $ 459 | $ 668 | $ 467 | |
| Net underfunded status of the plans | $ (74) | 705 | ||
| Incremental expense related to acceptance of voluntary early retirement packages | 97 | |||
| Cost of providing special and contractual termination benefits | 11 | |||
| Cash payments and administrative expense related to providing special and contractual termination benefits | 2 | |||
| Number of funds in process of liquidation | fund | 0 | |||
| Expense under defined contribution plans | $ 403 | 495 | 376 | |
| Treasury bond rate of guaranteed rate of return | 10 years | |||
| Personal Investment Account | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Expense under defined contribution plans | $ 48 | 50 | 52 | |
| Medtronic Core Contribution | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Expense under defined contribution plans | $ 83 | 73 | 66 | |
| Partnerships | Minimum | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Range of notice period | 45 days | |||
| Partnerships | Maximum | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Range of notice period | 95 days | |||
| Private equity fund | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Private equity funds, unfunded commitments | $ 204 | |||
| Private equity funds, estimated minimum liquidation period | 1 year | |||
| Private equity funds, estimated maximum liquidation period | 15 years | |||
| Real asset investments | Minimum | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Real asset investments, estimated liquidation and redemption period | 30 days | |||
| Real asset investments | Maximum | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Real asset investments, estimated liquidation and redemption period | 10 years | |||
| Real estate investments | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Number of funds in process of liquidation | fund | 0 | |||
| U.S. | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Number of new plans created | plan | 2 | |||
| Pension benefits | U.S. | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Net underfunded status of the plans | $ (33) | 319 | ||
| Special termination benefits | 0 | 73 | 0 | |
| Curtailment benefits recognized | $ 0 | 0 | 94 | |
| Target allocations | 100.00% | |||
| Post-retirement benefit plans, fair value of plan assets | $ 3,559 | 3,660 | 2,982 | |
| Employer contributions | 24 | 95 | ||
| Estimated future employer contributions in next fiscal year | 21 | |||
| Post-retirement benefit plans, net periodic benefit cost, income | (39) | (116) | (64) | |
| Post-retirement benefit plans, benefit obligations | 3,526 | 3,979 | 3,723 | |
| Pension benefits | U.S. | Level 3 | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Post-retirement benefit plans, fair value of plan assets | $ 1,011 | 860 | ||
| Pension benefits | U.S. | Equity | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Target allocations | 34.00% | |||
| Pension benefits | U.S. | Debt | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Target allocations | 51.00% | |||
| Pension benefits | U.S. | Other | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Target allocations | 15.00% | |||
| Pension benefits | Non-U.S. | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Net underfunded status of the plans | $ 8 | 394 | ||
| Special termination benefits | 0 | 0 | 0 | |
| Curtailment benefits recognized | 11 | 4 | ||
| Post-retirement benefit plans, fair value of plan assets | 1,732 | 1,900 | 1,404 | |
| Employer contributions | 70 | 149 | ||
| Estimated future employer contributions in next fiscal year | 52 | |||
| Post-retirement benefit plans, net periodic benefit cost, income | (37) | (64) | (42) | |
| Post-retirement benefit plans, benefit obligations | 1,740 | 2,294 | 2,024 | |
| Pension benefits | Non-U.S. | Level 3 | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Post-retirement benefit plans, fair value of plan assets | $ 43 | 49 | ||
| Pension benefits | Non-U.S. | Equity | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Target allocations | 41.00% | |||
| Pension benefits | Non-U.S. | Debt | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Target allocations | 33.00% | |||
| Pension benefits | Non-U.S. | Other | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Target allocations | 26.00% | |||
| Other post-retirement benefits | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Post-retirement benefit plans, fair value of plan assets | $ 325 | 345 | ||
| Post-retirement benefit plans, net periodic benefit cost, income | 20 | 6 | $ 15 | |
| Post-retirement benefit plans, benefit obligations | $ 276 | 337 | ||
| Other post-retirement benefits | U.S. | ||||
| Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items] | ||||
| Special termination benefits | $ 11 | |||
Retirement Benefit Plans - Change in Benefit Obligation and Funded Status (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Funded status at end of year: | |||
| Over (under) funded status of the plans | $ 74 | $ (705) | |
| Amounts recognized on the consolidated balance sheets consist of: | |||
| Non-current liabilities | (1,113) | (1,557) | |
| Pension benefits | |||
| Plans with accumulated benefit obligations in excess of plan assets | |||
| Accumulated benefit obligation | 830 | 5,089 | |
| Projected benefit obligation | 880 | 5,198 | |
| Plan assets at fair value | 356 | 4,561 | |
| Plans with projected benefit obligations in excess of plan assets | |||
| Projected benefit obligation | 907 | 5,921 | |
| Plan assets at fair value | 379 | 5,159 | |
| U.S. Pension Benefits | Pension benefits | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Accumulated benefit obligation at end of year | 3,396 | 3,786 | |
| Change in projected benefit obligation: | |||
| Projected benefit obligation at beginning of year | 3,979 | 3,723 | |
| Service cost | 98 | 106 | $ 106 |
| Interest cost | 102 | 109 | 126 |
| Employee contributions | 0 | 0 | |
| Plan curtailments and settlements | 0 | 0 | (94) |
| Actuarial (gain) loss | (513) | 99 | |
| Benefits paid | (141) | (129) | |
| Special termination benefits | 0 | 73 | |
| Currency exchange rate changes and other | 0 | 0 | |
| Projected benefit obligation at end of year | 3,526 | 3,979 | 3,723 |
| Change in plan assets: | |||
| Fair value of plan assets at beginning of year | 3,660 | 2,982 | |
| Actual return on plan assets | 15 | 715 | |
| Employer contributions | 24 | 95 | |
| Employee contributions | 0 | 0 | |
| Plan settlements | 0 | 0 | |
| Benefits paid | (141) | (129) | |
| Currency exchange rate changes and other | 0 | 0 | |
| Fair value of plan assets at end of year | 3,559 | 3,660 | 2,982 |
| Funded status at end of year: | |||
| Fair value of plan assets | 3,559 | 3,660 | 2,982 |
| Benefit obligations | 3,526 | 3,979 | 3,723 |
| Over (under) funded status of the plans | 33 | (319) | |
| Recognized asset (liability) | 33 | (319) | |
| Amounts recognized on the consolidated balance sheets consist of: | |||
| Non-current assets | 313 | 110 | |
| Current liabilities | (21) | (20) | |
| Non-current liabilities | (259) | (408) | |
| Recognized asset (liability) | 33 | (319) | |
| Amounts recognized in accumulated other comprehensive loss: | |||
| Prior service cost (credit) | 0 | 0 | |
| Net actuarial loss | 854 | 1,220 | |
| Ending balance | 854 | 1,220 | |
| Non-U.S. Pension Benefits | Pension benefits | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Accumulated benefit obligation at end of year | 1,638 | 2,035 | |
| Change in projected benefit obligation: | |||
| Projected benefit obligation at beginning of year | 2,294 | 2,024 | |
| Service cost | 64 | 70 | 59 |
| Interest cost | 26 | 28 | 28 |
| Employee contributions | 12 | 12 | |
| Plan curtailments and settlements | (11) | (4) | |
| Actuarial (gain) loss | (394) | 6 | |
| Benefits paid | (48) | (41) | |
| Special termination benefits | 0 | 0 | |
| Currency exchange rate changes and other | (203) | 200 | |
| Projected benefit obligation at end of year | 1,740 | 2,294 | 2,024 |
| Change in plan assets: | |||
| Fair value of plan assets at beginning of year | 1,900 | 1,404 | |
| Actual return on plan assets | (12) | 232 | |
| Employer contributions | 70 | 149 | |
| Employee contributions | 12 | 12 | |
| Plan settlements | (1) | (4) | |
| Benefits paid | (48) | (41) | |
| Currency exchange rate changes and other | (188) | 149 | |
| Fair value of plan assets at end of year | 1,732 | 1,900 | 1,404 |
| Funded status at end of year: | |||
| Fair value of plan assets | 1,732 | 1,900 | 1,404 |
| Benefit obligations | 1,740 | 2,294 | $ 2,024 |
| Over (under) funded status of the plans | (8) | (394) | |
| Recognized asset (liability) | (8) | (394) | |
| Amounts recognized on the consolidated balance sheets consist of: | |||
| Non-current assets | 240 | 48 | |
| Current liabilities | (6) | (6) | |
| Non-current liabilities | (242) | (436) | |
| Recognized asset (liability) | (8) | (394) | |
| Amounts recognized in accumulated other comprehensive loss: | |||
| Prior service cost (credit) | (4) | (6) | |
| Net actuarial loss | 161 | 530 | |
| Ending balance | $ 157 | $ 524 | |
Retirement Benefit Plans - Net Periodic Cost and AOCI (Details) - Pension benefits - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| U.S. Pension Benefits | |||
| Net Periodic Benefit Cost | |||
| Service cost | $ 98 | $ 106 | $ 106 |
| Interest cost | 102 | 109 | 126 |
| Expected return on plan assets | (226) | (242) | (225) |
| Amortization of prior service cost | 0 | 1 | 1 |
| Amortization of net actuarial loss | 64 | 69 | 56 |
| Settlement and curtailment (gain) loss | 0 | 0 | 0 |
| Special termination benefits | 0 | 73 | 0 |
| Net periodic benefit cost | 39 | 116 | 64 |
| Amounts Recognized in AOCI | |||
| Net actuarial gain | (303) | ||
| Amortization of prior service credit | 0 | ||
| Amortization and settlement recognition of actuarial loss | (64) | ||
| Effect of exchange rates | 0 | ||
| Total recognized in other comprehensive income | (367) | ||
| Total recognized in net periodic benefit cost and other comprehensive income | (328) | ||
| Non-U.S. Pension Benefits | |||
| Net Periodic Benefit Cost | |||
| Service cost | 64 | 70 | 59 |
| Interest cost | 26 | 28 | 28 |
| Expected return on plan assets | (64) | (59) | (58) |
| Amortization of prior service cost | (1) | (1) | (1) |
| Amortization of net actuarial loss | 22 | 25 | 14 |
| Settlement and curtailment (gain) loss | (10) | 1 | 0 |
| Special termination benefits | 0 | 0 | 0 |
| Net periodic benefit cost | 37 | $ 64 | $ 42 |
| Amounts Recognized in AOCI | |||
| Net actuarial gain | (317) | ||
| Amortization of prior service credit | 1 | ||
| Amortization and settlement recognition of actuarial loss | (22) | ||
| Effect of exchange rates | (29) | ||
| Total recognized in other comprehensive income | (367) | ||
| Total recognized in net periodic benefit cost and other comprehensive income | $ (331) | ||
Retirement Benefit Plans - Actuarial Assumptions and Plan Assets Target Allocations (Details) - Pension benefits |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| U.S. Pension Benefits | |||
| Critical assumptions – projected benefit obligation: | |||
| Rate of compensation increase | 4.83% | 4.83% | 3.90% |
| Critical assumptions – net periodic benefit cost: | |||
| Expected return on plan assets | 7.50% | 7.90% | |
| Rate of compensation increase | 3.90% | 3.90% | |
| Plan Assets Target Allocations | |||
| Target Allocation | 100.00% | ||
| Actual Allocation | 100.00% | 100.00% | |
| U.S. Pension Benefits | Equity securities | |||
| Plan Assets Target Allocations | |||
| Target Allocation | 34.00% | ||
| Actual Allocation | 36.00% | 39.00% | |
| U.S. Pension Benefits | Debt securities | |||
| Plan Assets Target Allocations | |||
| Target Allocation | 51.00% | ||
| Actual Allocation | 45.00% | 32.00% | |
| U.S. Pension Benefits | Other | |||
| Plan Assets Target Allocations | |||
| Target Allocation | 15.00% | ||
| Actual Allocation | 19.00% | 29.00% | |
| U.S. Pension Benefits | Minimum | |||
| Critical assumptions – projected benefit obligation: | |||
| Discount rate | 4.23% | 2.80% | 3.10% |
| Critical assumptions – net periodic benefit cost: | |||
| Discount rate – benefit obligation | 2.80% | 3.10% | 3.90% |
| Discount rate – service cost | 2.50% | 2.60% | 3.70% |
| Discount rate – interest cost | 2.08% | 2.80% | 3.50% |
| Expected return on plan assets | 5.60% | ||
| Rate of compensation increase | 3.90% | ||
| U.S. Pension Benefits | Maximum | |||
| Critical assumptions – projected benefit obligation: | |||
| Discount rate | 4.48% | 3.50% | 3.70% |
| Critical assumptions – net periodic benefit cost: | |||
| Discount rate – benefit obligation | 3.46% | 3.70% | 4.30% |
| Discount rate – service cost | 3.51% | 3.90% | 4.00% |
| Discount rate – interest cost | 2.87% | 3.20% | 4.30% |
| Expected return on plan assets | 7.40% | ||
| Rate of compensation increase | 4.83% | ||
| Non-U.S. Pension Benefits | |||
| Critical assumptions – projected benefit obligation: | |||
| Rate of compensation increase | 2.70% | 2.90% | 2.91% |
| Critical assumptions – net periodic benefit cost: | |||
| Expected return on plan assets | 3.67% | 3.78% | 4.19% |
| Rate of compensation increase | 2.90% | 2.91% | 2.87% |
| Non-U.S. Pension Benefits | Equity securities | |||
| Plan Assets Target Allocations | |||
| Target Allocation | 41.00% | ||
| Non-U.S. Pension Benefits | Debt securities | |||
| Plan Assets Target Allocations | |||
| Target Allocation | 33.00% | ||
| Non-U.S. Pension Benefits | Other | |||
| Plan Assets Target Allocations | |||
| Target Allocation | 26.00% | ||
| Non-U.S. Pension Benefits | Minimum | |||
| Critical assumptions – projected benefit obligation: | |||
| Discount rate | 0.60% | 0.30% | 0.30% |
| Critical assumptions – net periodic benefit cost: | |||
| Discount rate – benefit obligation | 0.25% | 0.30% | 0.40% |
| Discount rate – service cost | 0.24% | 0.30% | 0.40% |
| Discount rate – interest cost | 0.08% | 0.30% | 0.40% |
| Non-U.S. Pension Benefits | Maximum | |||
| Critical assumptions – projected benefit obligation: | |||
| Discount rate | 25.40% | 13.30% | 13.30% |
| Critical assumptions – net periodic benefit cost: | |||
| Discount rate – benefit obligation | 12.80% | 13.90% | 13.90% |
| Discount rate – service cost | 12.80% | 13.90% | 13.90% |
| Discount rate – interest cost | 12.80% | 13.90% | 13.90% |
Retirement Benefit Plans - Fair Value Measurement (Details) - Pension benefits - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| U.S. Pension Benefits | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | $ 3,559 | $ 3,660 | $ 2,982 |
| U.S. Pension Benefits | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 197 | 331 | |
| U.S. Pension Benefits | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,011 | 860 | |
| U.S. Pension Benefits | Level 3 | Partnership units | |||
| Reconciliation of Retirement Benefit Plan Assets Measured at Fair Value Using Significant Unobservable Inputs | |||
| Beginning balance | 860 | 625 | |
| Total realized gains, net | 28 | 8 | |
| Total unrealized gains, net | 72 | 89 | |
| Purchases and sales, net | 51 | 139 | |
| Ending balance | 1,011 | 860 | |
| U.S. Pension Benefits | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 2,350 | 2,470 | |
| U.S. Pension Benefits | Short-term investments | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 73 | 232 | |
| U.S. Pension Benefits | Short-term investments | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 73 | 232 | |
| U.S. Pension Benefits | Short-term investments | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Short-term investments | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Short-term investments | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Mutual funds | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 125 | 99 | |
| U.S. Pension Benefits | Mutual funds | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 125 | 99 | |
| U.S. Pension Benefits | Mutual funds | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Mutual funds | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Mutual funds | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Equity commingled trusts | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,281 | 1,420 | |
| U.S. Pension Benefits | Equity commingled trusts | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Equity commingled trusts | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Equity commingled trusts | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Equity commingled trusts | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,281 | 1,420 | |
| U.S. Pension Benefits | Fixed income commingled trusts | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,069 | 1,050 | |
| U.S. Pension Benefits | Fixed income commingled trusts | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Fixed income commingled trusts | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Fixed income commingled trusts | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Fixed income commingled trusts | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,069 | 1,050 | |
| U.S. Pension Benefits | Partnership units | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,011 | 860 | |
| U.S. Pension Benefits | Partnership units | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Partnership units | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| U.S. Pension Benefits | Partnership units | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,011 | 860 | |
| U.S. Pension Benefits | Partnership units | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,732 | 1,900 | $ 1,404 |
| Non-U.S. Pension Benefits | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 43 | 49 | |
| Non-U.S. Pension Benefits | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,689 | 1,850 | |
| Non-U.S. Pension Benefits | Registered investment companies | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,689 | 1,850 | |
| Non-U.S. Pension Benefits | Registered investment companies | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | Registered investment companies | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | Registered investment companies | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | Registered investment companies | Investments measured at net asset value | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 1,689 | 1,850 | |
| Non-U.S. Pension Benefits | Insurance contracts | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 43 | 49 | |
| Non-U.S. Pension Benefits | Insurance contracts | Level 1 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | Insurance contracts | Level 2 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | 0 | 0 | |
| Non-U.S. Pension Benefits | Insurance contracts | Level 3 | |||
| Defined Benefit Plan Disclosure [Line items] | |||
| Fair value of plan assets | $ 43 | $ 49 | |
Retirement Benefit Plans - Future Benefit Payments (Details) - Pension benefits $ in Millions |
Apr. 29, 2022
USD ($)
|
|---|---|
| U.S. Pension Benefits | |
| Estimated Future Benefit Payments | |
| 2023 | $ 150 |
| 2024 | 160 |
| 2025 | 172 |
| 2026 | 182 |
| 2027 | 193 |
| 2028 – 2032 | 1,110 |
| Total | 1,966 |
| Non-U.S. Pension Benefits | |
| Estimated Future Benefit Payments | |
| 2023 | 61 |
| 2024 | 55 |
| 2025 | 59 |
| 2026 | 59 |
| 2027 | 65 |
| 2028 – 2032 | 367 |
| Total | $ 666 |
Leases - Balance Sheet Classification and Amounts of Right-of-Use Assets and Lease Liabilities (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 27, 2019 |
|---|---|---|---|
| Leases [Abstract] | |||
| Right-of-use assets | $ 854 | $ 998 | $ 1,000 |
| Balance Sheet Classification, Other assets | Other assets | Other assets | Other assets |
| Current liability | $ 167 | $ 186 | |
| Balance Sheet Classification, Other accrued expenses | Other accrued expenses | Other accrued expenses | |
| Non-current liability | $ 703 | $ 829 | |
| Balance Sheet Classification, Other liabilities | Other liabilities | Other liabilities | Other liabilities |
Leases - Weighted-Average Remaining Lease Term and Weighted-Average Discount Rate for Operating Leases (Details) |
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|---|---|---|---|
| Leases [Abstract] | |||
| Weighted-average remaining lease term | 7 years 3 months 18 days | 7 years 6 months | 7 years 2 months 12 days |
| Weighted-average discount rate | 2.00% | 2.30% | 3.00% |
Leases - Components of Total Operating Lease Cost (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Leases [Abstract] | |||
| Operating lease cost | $ 195 | $ 216 | $ 223 |
| Short-term lease cost | 65 | 35 | 46 |
| Total operating lease cost | $ 260 | $ 251 | $ 269 |
Leases - Supplemental Cash Flow Information (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Leases [Abstract] | |||
| Cash paid for amounts included in the measurement of operating lease liabilities | $ 174 | $ 216 | $ 221 |
| Right-of-use assets obtained in exchange for operating lease liabilities | $ 78 | $ 230 | $ 174 |
Leases - Maturities of Operating Leases (Details) - USD ($) $ in Millions |
Apr. 29, 2022 |
Apr. 27, 2019 |
|---|---|---|
| Leases [Abstract] | ||
| 2023 | $ 213 | |
| 2024 | 164 | |
| 2025 | 130 | |
| 2026 | 103 | |
| 2027 | 82 | |
| Thereafter | 284 | |
| Total expected lease payments | 976 | |
| Less: Imputed interest | (105) | |
| Total lease liability | $ 871 | $ 1,000 |
Accumulated Other Comprehensive Loss (Details) - USD ($) |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | $ 51,602,000,000 | $ 50,872,000,000 | $ 50,212,000,000 |
| Other comprehensive income (loss) | 1,213,000,000 | 83,000,000 | (851,000,000) |
| Ending balance | 52,722,000,000 | 51,602,000,000 | 50,872,000,000 |
| Accumulated other comprehensive loss | (2,265,000,000) | (3,485,000,000) | |
| Total Accumulated Other Comprehensive (Loss) Income | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | (3,485,000,000) | (3,560,000,000) | (2,711,000,000) |
| Other comprehensive income (loss) before reclassifications | 1,210,000,000 | (20,000,000) | (666,000,000) |
| Reclassifications | 9,000,000 | 95,000,000 | (183,000,000) |
| Other comprehensive income (loss) | 1,219,000,000 | 75,000,000 | (849,000,000) |
| Ending balance | (2,265,000,000) | (3,485,000,000) | (3,560,000,000) |
| Unrealized (Loss) Gain on Investment Securities | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | 92,000,000 | 0 | (45,000,000) |
| Other comprehensive income (loss) before reclassifications | (304,000,000) | 92,000,000 | 43,000,000 |
| Reclassifications | 3,000,000 | 0 | 2,000,000 |
| Other comprehensive income (loss) | (301,000,000) | 92,000,000 | 45,000,000 |
| Ending balance | (209,000,000) | 92,000,000 | 0 |
| Other comprehensive income (loss), tax expense (benefit) | (51,000,000) | 31,000,000 | (13,000,000) |
| Reclassifications from AOCI, tax expense (benefit) | (1,000,000) | 2,000,000 | (3,000,000) |
| Cumulative Translation Adjustments | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | (519,000,000) | (2,210,000,000) | (1,383,000,000) |
| Other comprehensive income (loss) before reclassifications | (2,080,000,000) | 1,691,000,000 | (827,000,000) |
| Reclassifications | 0 | 0 | 0 |
| Other comprehensive income (loss) | (2,080,000,000) | 1,691,000,000 | (827,000,000) |
| Ending balance | (2,599,000,000) | (519,000,000) | (2,210,000,000) |
| Other comprehensive income (loss), tax expense (benefit) | (8,000,000) | 7,000,000 | (9,000,000) |
| Net Investment Hedges | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | (1,458,000,000) | ||
| Other comprehensive income (loss) before reclassifications | 2,299,000,000 | (1,694,000,000) | |
| Reclassifications | 0 | 0 | |
| Other comprehensive income (loss) | 2,299,000,000 | (1,694,000,000) | |
| Ending balance | 841,000,000 | (1,458,000,000) | |
| Other comprehensive income (loss), tax expense (benefit) | 0 | 0 | |
| Net Investment Hedges | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | 236,000,000 | (169,000,000) | |
| Other comprehensive income (loss) before reclassifications | 405,000,000 | ||
| Reclassifications | 0 | ||
| Other comprehensive income (loss) | 405,000,000 | ||
| Ending balance | 236,000,000 | ||
| Other comprehensive income (loss), tax expense (benefit) | 0 | ||
| Net Change in Retirement Obligations | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | (1,347,000,000) | (1,852,000,000) | (1,308,000,000) |
| Other comprehensive income (loss) before reclassifications | 514,000,000 | 432,000,000 | (596,000,000) |
| Reclassifications | 60,000,000 | 73,000,000 | 52,000,000 |
| Other comprehensive income (loss) | 574,000,000 | 505,000,000 | (544,000,000) |
| Ending balance | (773,000,000) | (1,347,000,000) | (1,852,000,000) |
| Other comprehensive income (loss), tax expense (benefit) | 134,000,000 | 115,000,000 | (159,000,000) |
| Reclassifications from AOCI, tax expense (benefit) | (20,000,000) | (16,000,000) | (12,000,000) |
| Unrealized (Loss) Gain on Cash Flow Hedges | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | (253,000,000) | ||
| Other comprehensive income (loss) before reclassifications | 781,000,000 | (541,000,000) | |
| Reclassifications | (54,000,000) | 22,000,000 | |
| Other comprehensive income (loss) | 727,000,000 | (519,000,000) | |
| Ending balance | 474,000,000 | (253,000,000) | |
| Other comprehensive income (loss), tax expense (benefit) | 152,000,000 | (87,000,000) | |
| Reclassifications from AOCI, tax expense (benefit) | $ 26,000,000 | 14,000,000 | |
| Unrealized (Loss) Gain on Cash Flow Hedges | |||
| AOCI Attributable to Parent, Net of Tax [Roll Forward] | |||
| Beginning balance | $ 266,000,000 | 194,000,000 | |
| Other comprehensive income (loss) before reclassifications | 309,000,000 | ||
| Reclassifications | (237,000,000) | ||
| Other comprehensive income (loss) | 72,000,000 | ||
| Ending balance | 266,000,000 | ||
| Other comprehensive income (loss), tax expense (benefit) | 88,000,000 | ||
| Reclassifications from AOCI, tax expense (benefit) | $ 80,000,000 | ||
Commitments and Contingencies (Details) $ in Millions |
1 Months Ended | 12 Months Ended | |||||
|---|---|---|---|---|---|---|---|
|
Jun. 06, 2022
plaintiff
claim
|
Jun. 01, 2022
claim
|
May 31, 2017
claim
|
Apr. 29, 2022
USD ($)
subsidiary
landfill
claimant
manufacturer
|
Apr. 30, 2021
USD ($)
|
Apr. 24, 2020
USD ($)
|
Apr. 28, 2017
USD ($)
claim
|
|
| Loss Contingencies [Line Items] | |||||||
| Certain litigation charges | $ | $ 95 | $ 118 | $ 313 | ||||
| Accrued litigations charges | $ | $ 300 | $ 400 | |||||
| Orrington, Maine Chemical Manufacturing Facility | |||||||
| Loss Contingencies [Line Items] | |||||||
| Number of landfills requiring removal (in landfills) | landfill | 2 | ||||||
| Number of landfills requiring capping (in landfills) | landfill | 3 | ||||||
| Pelvic Mesh Litigation | Damages from product defects | |||||||
| Loss Contingencies [Line Items] | |||||||
| Number of subsidiaries which supplied pelvic mesh to manufacturer (in subsidiaries) | subsidiary | 2 | ||||||
| Number of manufacturers (in manufacturers) | manufacturer | 1 | ||||||
| Settlement consideration received | $ | $ 121 | ||||||
| Number of claims settled (in claims) | claim | 5,000 | 11,000 | |||||
| Number of claimants (in claimants) | claimant | 16,200 | ||||||
| Pelvic Mesh Litigation | Damages from product defects | Subsequent Event | |||||||
| Loss Contingencies [Line Items] | |||||||
| Number of claims settled (in claims) | claim | 15,900 | ||||||
| Hernia Mesh Litigation | Subsequent Event | Pending litigation | |||||||
| Loss Contingencies [Line Items] | |||||||
| Number of claimants (in claimants) | claim | 83 | ||||||
| Number of plaintiffs (in plaintiffs) | plaintiff | 5,900 | ||||||
Segment and Geographic Information - Additional Information (Details) |
12 Months Ended |
|---|---|
|
Apr. 29, 2022
segment
| |
| Segment Reporting [Abstract] | |
| Number of operating segments | 4 |
| Number of reporting segments | 4 |
Segment and Geographic Information - Income From Operations Before Income Taxes by Reportable Segment and Reconciliation to Consolidated (Details) - USD ($) $ in Millions |
3 Months Ended | 12 Months Ended | |||
|---|---|---|---|---|---|
Apr. 29, 2022 |
Jul. 30, 2021 |
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Segment Reporting Information [Line Items] | |||||
| Segment operating profit | $ 5,752 | $ 4,484 | $ 4,791 | ||
| Other non-operating income, net | 318 | 336 | 356 | ||
| Amortization of intangible assets | (1,733) | (1,783) | (1,756) | ||
| Certain litigation charges | (95) | (118) | (313) | ||
| Impairment charges | (515) | 0 | 0 | ||
| Income before income taxes | 5,517 | 3,895 | 4,055 | ||
| Cardiovascular | |||||
| Segment Reporting Information [Line Items] | |||||
| MCS impairment / costs | $ (726) | ||||
| Exit of businesses | $ (155) | ||||
| Reportable segments | |||||
| Segment Reporting Information [Line Items] | |||||
| Segment operating profit | 12,432 | 10,632 | 10,224 | ||
| Reportable segments | Cardiovascular | |||||
| Segment Reporting Information [Line Items] | |||||
| Segment operating profit | 4,512 | 3,850 | 3,719 | ||
| Reportable segments | Medical Surgical | |||||
| Segment Reporting Information [Line Items] | |||||
| Segment operating profit | 3,572 | 3,021 | 3,044 | ||
| Reportable segments | Neuroscience | |||||
| Segment Reporting Information [Line Items] | |||||
| Segment operating profit | 3,765 | 3,162 | 2,915 | ||
| Reportable segments | Diabetes | |||||
| Segment Reporting Information [Line Items] | |||||
| Segment operating profit | 583 | 598 | 546 | ||
| Segment reconciling items | |||||
| Segment Reporting Information [Line Items] | |||||
| Interest expense | (553) | (925) | (1,092) | ||
| Other non-operating income, net | 318 | 336 | 356 | ||
| Amortization of intangible assets | (1,733) | (1,783) | (1,756) | ||
| Corporate | (1,724) | (1,577) | (1,239) | ||
| Centralized distribution costs | (1,752) | (1,877) | (1,420) | ||
| Restructuring and associated costs | (335) | (617) | (441) | ||
| Acquisition-related items | 43 | 15 | (66) | ||
| Certain litigation charges | (95) | (118) | (313) | ||
| Impairment charges | 0 | (76) | 0 | ||
| MCS impairment / costs | (881) | 0 | 0 | ||
| IPR&D charges | (101) | (31) | (25) | ||
| Exit of businesses | 0 | 0 | (52) | ||
| Debt tender premium and other charges | 0 | 0 | 7 | ||
| Medical device regulations | (102) | (83) | (48) | ||
| Contribution to Medtronic Foundation | $ 0 | $ 0 | $ (80) | ||
Segment and Geographic Information - Reconciliation of Assets and Depreciation Expense from Segments to Consolidated (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Segment Reporting Information [Line Items] | |||
| Total Assets | $ 90,981 | $ 93,083 | |
| Depreciation Expense | 974 | 919 | $ 907 |
| Reportable segments | |||
| Segment Reporting Information [Line Items] | |||
| Total Assets | 72,144 | 75,168 | |
| Depreciation Expense | 746 | 696 | 675 |
| Reportable segments | Cardiovascular | |||
| Segment Reporting Information [Line Items] | |||
| Total Assets | 14,490 | 15,027 | |
| Depreciation Expense | 214 | 212 | 210 |
| Reportable segments | Medical Surgical | |||
| Segment Reporting Information [Line Items] | |||
| Total Assets | 36,940 | 39,319 | |
| Depreciation Expense | 200 | 195 | 194 |
| Reportable segments | Neuroscience | |||
| Segment Reporting Information [Line Items] | |||
| Total Assets | 16,917 | 17,151 | |
| Depreciation Expense | 265 | 236 | 233 |
| Reportable segments | Diabetes | |||
| Segment Reporting Information [Line Items] | |||
| Total Assets | 3,797 | 3,671 | |
| Depreciation Expense | 67 | 53 | 38 |
| Corporate | |||
| Segment Reporting Information [Line Items] | |||
| Total Assets | 18,837 | 17,915 | |
| Depreciation Expense | $ 228 | $ 223 | $ 232 |
Segment and Geographic Information - Schedule of Net Sales to External Customers and Property, Plant, and Equipment, Net, by Geographic Region (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Revenues from External Customers and Long-Lived Assets [Line Items] | |||
| Net sales | $ 31,686 | $ 30,117 | $ 28,913 |
| Property, plant, and equipment, net | 5,413 | 5,221 | |
| Ireland | |||
| Revenues from External Customers and Long-Lived Assets [Line Items] | |||
| Net sales | 101 | 100 | 85 |
| Property, plant, and equipment, net | 177 | 170 | |
| Total other countries, excluding Ireland | |||
| Revenues from External Customers and Long-Lived Assets [Line Items] | |||
| Net sales | 31,585 | 30,017 | 28,828 |
| Property, plant, and equipment, net | 5,236 | 5,051 | |
| United States | |||
| Revenues from External Customers and Long-Lived Assets [Line Items] | |||
| Net sales | 16,135 | 15,526 | 14,919 |
| Property, plant, and equipment, net | 3,821 | 3,688 | |
| Rest of world | |||
| Revenues from External Customers and Long-Lived Assets [Line Items] | |||
| Net sales | 15,450 | 14,491 | $ 13,909 |
| Property, plant, and equipment, net | $ 1,415 | $ 1,363 | |
Subsequent Events (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| Subsequent Event [Line Items] | |||
| Revenue | $ 31,686 | $ 30,117 | $ 28,913 |
| Medical Surgical | |||
| Subsequent Event [Line Items] | |||
| Revenue | 9,141 | $ 8,737 | $ 8,352 |
| NewCo | Medical Surgical | |||
| Subsequent Event [Line Items] | |||
| Revenue | $ 325 | ||
Schedule II (Details) - USD ($) $ in Millions |
12 Months Ended | ||
|---|---|---|---|
Apr. 29, 2022 |
Apr. 30, 2021 |
Apr. 24, 2020 |
|
| SEC Schedule, 12-09, Movement in Valuation Allowances and Reserves [Roll Forward] | |||
| Other Changes (Debit) Credit | $ (187) | ||
| Allowance for Doubtful Accounts | |||
| SEC Schedule, 12-09, Movement in Valuation Allowances and Reserves [Roll Forward] | |||
| Balance at Beginning of Fiscal Year | $ 241 | $ 208 | 190 |
| Charges to Income | 58 | 128 | 99 |
| Charges to Other Accounts | 0 | 0 | 0 |
| Other Changes (Debit) Credit | (69) | (95) | (81) |
| Balance at End of Fiscal Year | 230 | 241 | 208 |
| Inventory Reserve | |||
| SEC Schedule, 12-09, Movement in Valuation Allowances and Reserves [Roll Forward] | |||
| Balance at Beginning of Fiscal Year | 629 | 544 | 521 |
| Charges to Income | 156 | 483 | 282 |
| Charges to Other Accounts | 0 | 0 | 0 |
| Other Changes (Debit) Credit | (157) | (398) | (259) |
| Balance at End of Fiscal Year | 628 | 629 | 544 |
| Deferred Tax Valuation Allowance | |||
| SEC Schedule, 12-09, Movement in Valuation Allowances and Reserves [Roll Forward] | |||
| Balance at Beginning of Fiscal Year | 5,822 | 5,482 | 6,300 |
| Charges to Income | 884 | 342 | 119 |
| Charges to Other Accounts | (19) | 170 | (6) |
| Other Changes (Debit) Credit | (103) | (172) | (744) |
| Balance at End of Fiscal Year | $ 6,583 | $ 5,822 | $ 5,482 |
| Label | Element | Value |
|---|---|---|
| Accounting Standards Update [Extensible Enumeration] | us-gaap_AccountingStandardsUpdateExtensibleList | Accounting Standards Update 2016-13 [Member] |
{
"instance": {
"mdt-20220429.htm": {
"axisCustom": 0,
"axisStandard": 49,
"contextCount": 843,
"dts": {
"calculationLink": {
"local": [
"mdt-20220429_cal.xml"
]
},
"definitionLink": {
"local": [
"mdt-20220429_def.xml"
]
},
"inline": {
"local": [
"mdt-20220429.htm"
]
},
"labelLink": {
"local": [
"mdt-20220429_lab.xml"
]
},
"presentationLink": {
"local": [
"mdt-20220429_pre.xml"
]
},
"schema": {
"local": [
"mdt-20220429.xsd"
],
"remote": [
"http://www.xbrl.org/2003/xbrl-instance-2003-12-31.xsd",
"http://www.xbrl.org/2003/xbrl-linkbase-2003-12-31.xsd",
"http://www.xbrl.org/2003/xl-2003-12-31.xsd",
"http://www.xbrl.org/2003/xlink-2003-12-31.xsd",
"http://www.xbrl.org/2005/xbrldt-2005.xsd",
"http://www.xbrl.org/2006/ref-2006-02-27.xsd",
"http://www.xbrl.org/lrr/role/negated-2009-12-16.xsd",
"http://www.xbrl.org/lrr/role/net-2009-12-16.xsd",
"http://www.xbrl.org/lrr/role/reference-2009-12-16.xsd",
"https://www.xbrl.org/2020/extensible-enumerations-2.0.xsd",
"https://www.xbrl.org/dtr/type/2020-01-21/types.xsd",
"https://xbrl.fasb.org/srt/2021/elts/srt-2021-01-31.xsd",
"https://xbrl.fasb.org/srt/2021/elts/srt-roles-2021-01-31.xsd",
"https://xbrl.fasb.org/srt/2021/elts/srt-types-2021-01-31.xsd",
"https://xbrl.fasb.org/us-gaap/2021/elts/us-gaap-2021-01-31.xsd",
"https://xbrl.fasb.org/us-gaap/2021/elts/us-roles-2021-01-31.xsd",
"https://xbrl.fasb.org/us-gaap/2021/elts/us-types-2021-01-31.xsd",
"https://xbrl.sec.gov/country/2021/country-2021.xsd",
"https://xbrl.sec.gov/dei/2021q4/dei-2021q4.xsd"
]
}
},
"elementCount": 1121,
"entityCount": 1,
"hidden": {
"http://fasb.org/us-gaap/2021-01-31": 43,
"http://www.medtronic.com/20220429": 2,
"http://xbrl.sec.gov/dei/2021q4": 6,
"total": 51
},
"keyCustom": 96,
"keyStandard": 575,
"memberCustom": 100,
"memberStandard": 94,
"nsprefix": "mdt",
"nsuri": "http://www.medtronic.com/20220429",
"report": {
"R1": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "dei:DocumentType",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "document",
"isDefault": "true",
"longName": "000010001 - Document - Cover",
"role": "http://www.medtronic.com/role/Cover",
"shortName": "Cover",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "dei:DocumentType",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R10": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SignificantAccountingPoliciesTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210011001 - Disclosure - Summary of Significant Accounting Policies",
"role": "http://www.medtronic.com/role/SummaryofSignificantAccountingPolicies",
"shortName": "Summary of Significant Accounting Policies",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SignificantAccountingPoliciesTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R100": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingLeasePayments",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240844053 - Disclosure - Leases - Supplemental Cash Flow Information (Details)",
"role": "http://www.medtronic.com/role/LeasesSupplementalCashFlowInformationDetails",
"shortName": "Leases - Supplemental Cash Flow Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingLeasePayments",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R101": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LesseeOperatingLeaseLiabilityMaturityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240854054 - Disclosure - Leases - Maturities of Operating Leases (Details)",
"role": "http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails",
"shortName": "Leases - Maturities of Operating Leases (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LesseeOperatingLeaseLiabilityMaturityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R102": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i8362330ce180425da2583cdfcef52524_I20210430",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240884055 - Disclosure - Accumulated Other Comprehensive Loss (Details)",
"role": "http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails",
"shortName": "Accumulated Other Comprehensive Loss (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfAccumulatedOtherComprehensiveIncomeLossTableTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ic481d4932af24468bbfeb62b868f3529_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:OtherComprehensiveIncomeLossBeforeReclassificationsNetOfTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R103": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:LossContingencyAccrualProvision",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240904056 - Disclosure - Commitments and Contingencies (Details)",
"role": "http://www.medtronic.com/role/CommitmentsandContingenciesDetails",
"shortName": "Commitments and Contingencies (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-8",
"lang": "en-US",
"name": "us-gaap:LossContingencyAccrualAtCarryingValue",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R104": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "INF",
"first": true,
"lang": "en-US",
"name": "us-gaap:NumberOfOperatingSegments",
"reportCount": 1,
"unique": true,
"unitRef": "segment",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240934057 - Disclosure - Segment and Geographic Information - Additional Information (Details)",
"role": "http://www.medtronic.com/role/SegmentandGeographicInformationAdditionalInformationDetails",
"shortName": "Segment and Geographic Information - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "INF",
"first": true,
"lang": "en-US",
"name": "us-gaap:NumberOfOperatingSegments",
"reportCount": 1,
"unique": true,
"unitRef": "segment",
"xsiNil": "false"
}
},
"R105": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingIncomeLoss",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240944058 - Disclosure - Segment and Geographic Information - Income From Operations Before Income Taxes by Reportable Segment and Reconciliation to Consolidated (Details)",
"role": "http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"shortName": "Segment and Geographic Information - Income From Operations Before Income Taxes by Reportable Segment and Reconciliation to Consolidated (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ReconciliationOfOperatingProfitLossFromSegmentsToConsolidatedTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "if4a9202fbd8e4ddbb60c18d2e1c62c2b_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:OperatingIncomeLoss",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R106": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ReconciliationOfAssetsFromSegmentToConsolidatedTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:Assets",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240954059 - Disclosure - Segment and Geographic Information - Reconciliation of Assets and Depreciation Expense from Segments to Consolidated (Details)",
"role": "http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails",
"shortName": "Segment and Geographic Information - Reconciliation of Assets and Depreciation Expense from Segments to Consolidated (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ReconciliationOfAssetsFromSegmentToConsolidatedTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ia24b2716630d4f35afce51bef90147e8_I20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:Assets",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R107": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240964060 - Disclosure - Segment and Geographic Information - Schedule of Net Sales to External Customers and Property, Plant, and Equipment, Net, by Geographic Region (Details)",
"role": "http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails",
"shortName": "Segment and Geographic Information - Schedule of Net Sales to External Customers and Property, Plant, and Equipment, Net, by Geographic Region (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfRevenuesFromExternalCustomersAndLongLivedAssetsByGeographicalAreasTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:PropertyPlantAndEquipmentNet",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R108": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240984061 - Disclosure - Subsequent Events (Details)",
"role": "http://www.medtronic.com/role/SubsequentEventsDetails",
"shortName": "Subsequent Events (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i68f6b29ccdd841339d8ca6156a84ea80_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R109": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"srt:ScheduleOfValuationAndQualifyingAccountsDisclosureTextBlock",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ifc5d58ddf31a4bef96f9419b19cdf72a_D20190427-20200424",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ValuationAllowancesAndReservesDeductions",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "241004062 - Disclosure - Schedule II (Details)",
"role": "http://www.medtronic.com/role/ScheduleIIDetails",
"shortName": "Schedule II (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"srt:ScheduleOfValuationAndQualifyingAccountsDisclosureTextBlock",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ifc5d58ddf31a4bef96f9419b19cdf72a_D20190427-20200424",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ValuationAllowancesAndReservesDeductions",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R11": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210041002 - Disclosure - Revenue",
"role": "http://www.medtronic.com/role/Revenue",
"shortName": "Revenue",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R12": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:BusinessCombinationDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210091003 - Disclosure - Acquisitions",
"role": "http://www.medtronic.com/role/Acquisitions",
"shortName": "Acquisitions",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:BusinessCombinationDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R13": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:RestructuringAndRelatedActivitiesDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210131004 - Disclosure - Restructuring Charges",
"role": "http://www.medtronic.com/role/RestructuringCharges",
"shortName": "Restructuring Charges",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:RestructuringAndRelatedActivitiesDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R14": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:FinancialInstrumentsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210171005 - Disclosure - Financial Instruments",
"role": "http://www.medtronic.com/role/FinancialInstruments",
"shortName": "Financial Instruments",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:FinancialInstrumentsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R15": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210251006 - Disclosure - Financing Arrangements",
"role": "http://www.medtronic.com/role/FinancingArrangements",
"shortName": "Financing Arrangements",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R16": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeInstrumentsAndHedgingActivitiesDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210311007 - Disclosure - Derivatives and Currency Exchange Risk Management",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagement",
"shortName": "Derivatives and Currency Exchange Risk Management",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeInstrumentsAndHedgingActivitiesDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R17": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:InventoryDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210391008 - Disclosure - Inventories",
"role": "http://www.medtronic.com/role/Inventories",
"shortName": "Inventories",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:InventoryDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R18": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:GoodwillAndIntangibleAssetsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210421009 - Disclosure - Goodwill and Other Intangible Assets",
"role": "http://www.medtronic.com/role/GoodwillandOtherIntangibleAssets",
"shortName": "Goodwill and Other Intangible Assets",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:GoodwillAndIntangibleAssetsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R19": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:PropertyPlantAndEquipmentDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210471010 - Disclosure - Property, Plant, and Equipment",
"role": "http://www.medtronic.com/role/PropertyPlantandEquipment",
"shortName": "Property, Plant, and Equipment",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:PropertyPlantAndEquipmentDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R2": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "dei:AuditorFirmId",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "document",
"isDefault": "false",
"longName": "000020002 - Document - Audit Information",
"role": "http://www.medtronic.com/role/AuditInformation",
"shortName": "Audit Information",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "dei:AuditorFirmId",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R20": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:StockholdersEquityNoteDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210501011 - Disclosure - Shareholders' Equity",
"role": "http://www.medtronic.com/role/ShareholdersEquity",
"shortName": "Shareholders' Equity",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:StockholdersEquityNoteDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R21": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210521012 - Disclosure - Stock Purchase and Award Plans",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlans",
"shortName": "Stock Purchase and Award Plans",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R22": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:IncomeTaxDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210601013 - Disclosure - Income Taxes",
"role": "http://www.medtronic.com/role/IncomeTaxes",
"shortName": "Income Taxes",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:IncomeTaxDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R23": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:EarningsPerShareTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210681014 - Disclosure - Earnings Per Share",
"role": "http://www.medtronic.com/role/EarningsPerShare",
"shortName": "Earnings Per Share",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:EarningsPerShareTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R24": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:PensionAndOtherPostretirementBenefitsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210711015 - Disclosure - Retirement Benefit Plans",
"role": "http://www.medtronic.com/role/RetirementBenefitPlans",
"shortName": "Retirement Benefit Plans",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:PensionAndOtherPostretirementBenefitsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R25": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:LesseeOperatingLeasesTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210791016 - Disclosure - Leases",
"role": "http://www.medtronic.com/role/Leases",
"shortName": "Leases",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:LesseeOperatingLeasesTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R26": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ComprehensiveIncomeNoteTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210861017 - Disclosure - Accumulated Other Comprehensive Loss",
"role": "http://www.medtronic.com/role/AccumulatedOtherComprehensiveLoss",
"shortName": "Accumulated Other Comprehensive Loss",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ComprehensiveIncomeNoteTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R27": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:LossContingencyDisclosures",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210891018 - Disclosure - Commitments and Contingencies",
"role": "http://www.medtronic.com/role/CommitmentsandContingencies",
"shortName": "Commitments and Contingencies",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:LossContingencyDisclosures",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R28": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SegmentReportingDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210911019 - Disclosure - Segment and Geographic Information",
"role": "http://www.medtronic.com/role/SegmentandGeographicInformation",
"shortName": "Segment and Geographic Information",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SegmentReportingDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R29": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SubsequentEventsTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210971020 - Disclosure - Subsequent Events",
"role": "http://www.medtronic.com/role/SubsequentEvents",
"shortName": "Subsequent Events",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SubsequentEventsTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R3": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "statement",
"isDefault": "false",
"longName": "100010003 - Statement - Consolidated Statements of Income",
"role": "http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"shortName": "Consolidated Statements of Income",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:ResearchAndDevelopmentExpenseExcludingAcquiredInProcessCost",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R30": {
"firstAnchor": {
"ancestors": [
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "srt:ScheduleOfValuationAndQualifyingAccountsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "210991021 - Disclosure - Schedule II",
"role": "http://www.medtronic.com/role/ScheduleII",
"shortName": "Schedule II",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "srt:ScheduleOfValuationAndQualifyingAccountsDisclosureTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R31": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ConsolidationPolicyTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "220022001 - Disclosure - Summary of Significant Accounting Policies (Policies)",
"role": "http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies",
"shortName": "Summary of Significant Accounting Policies (Policies)",
"subGroupType": "policies",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ConsolidationPolicyTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R32": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DisaggregationOfRevenueTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230053001 - Disclosure - Revenue (Tables)",
"role": "http://www.medtronic.com/role/RevenueTables",
"shortName": "Revenue (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DisaggregationOfRevenueTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R33": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfBusinessAcquisitionsByAcquisitionContingentConsiderationTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230103002 - Disclosure - Acquisitions (Tables)",
"role": "http://www.medtronic.com/role/AcquisitionsTables",
"shortName": "Acquisitions (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfBusinessAcquisitionsByAcquisitionContingentConsiderationTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R34": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfRestructuringAndRelatedCostsTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230143003 - Disclosure - Restructuring Charges (Tables)",
"role": "http://www.medtronic.com/role/RestructuringChargesTables",
"shortName": "Restructuring Charges (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfRestructuringAndRelatedCostsTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R35": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtSecuritiesAvailableForSaleTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230183004 - Disclosure - Financial Instruments (Tables)",
"role": "http://www.medtronic.com/role/FinancialInstrumentsTables",
"shortName": "Financial Instruments (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtSecuritiesAvailableForSaleTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R36": {
"firstAnchor": {
"ancestors": [
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfShortTermDebtTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230263005 - Disclosure - Financing Arrangements (Tables)",
"role": "http://www.medtronic.com/role/FinancingArrangementsTables",
"shortName": "Financing Arrangements (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfShortTermDebtTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R37": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230323006 - Disclosure - Derivatives and Currency Exchange Risk Management (Tables)",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementTables",
"shortName": "Derivatives and Currency Exchange Risk Management (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R38": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfInventoryCurrentTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230403007 - Disclosure - Inventories (Tables)",
"role": "http://www.medtronic.com/role/InventoriesTables",
"shortName": "Inventories (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfInventoryCurrentTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R39": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfGoodwillTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230433008 - Disclosure - Goodwill and Other Intangible Assets (Tables)",
"role": "http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsTables",
"shortName": "Goodwill and Other Intangible Assets (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfGoodwillTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R4": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ProfitLoss",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "statement",
"isDefault": "false",
"longName": "100020004 - Statement - Consolidated Statements of Comprehensive Income",
"role": "http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome",
"shortName": "Consolidated Statements of Comprehensive Income",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:OtherComprehensiveIncomeUnrealizedHoldingGainLossOnSecuritiesArisingDuringPeriodNetOfTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R40": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:PropertyPlantAndEquipmentTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230483009 - Disclosure - Property, Plant, and Equipment (Tables)",
"role": "http://www.medtronic.com/role/PropertyPlantandEquipmentTables",
"shortName": "Property, Plant, and Equipment (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:PropertyPlantAndEquipmentTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R41": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230533010 - Disclosure - Stock Purchase and Award Plans (Tables)",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlansTables",
"shortName": "Stock Purchase and Award Plans (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R42": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfIncomeBeforeIncomeTaxDomesticAndForeignTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230613011 - Disclosure - Income Taxes (Tables)",
"role": "http://www.medtronic.com/role/IncomeTaxesTables",
"shortName": "Income Taxes (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfIncomeBeforeIncomeTaxDomesticAndForeignTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R43": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230693012 - Disclosure - Earnings Per Share (Tables)",
"role": "http://www.medtronic.com/role/EarningsPerShareTables",
"shortName": "Earnings Per Share (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R44": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfDefinedBenefitPlansDisclosuresTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230723013 - Disclosure - Retirement Benefit Plans (Tables)",
"role": "http://www.medtronic.com/role/RetirementBenefitPlansTables",
"shortName": "Retirement Benefit Plans (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfDefinedBenefitPlansDisclosuresTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R45": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "mdt:AssetsAndLiabilitiesLesseeTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230803014 - Disclosure - Leases (Tables)",
"role": "http://www.medtronic.com/role/LeasesTables",
"shortName": "Leases (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "mdt:AssetsAndLiabilitiesLesseeTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R46": {
"firstAnchor": {
"ancestors": [
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfAccumulatedOtherComprehensiveIncomeLossTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230873015 - Disclosure - Accumulated Other Comprehensive Loss (Tables)",
"role": "http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossTables",
"shortName": "Accumulated Other Comprehensive Loss (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ScheduleOfAccumulatedOtherComprehensiveIncomeLossTableTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R47": {
"firstAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ReconciliationOfOperatingProfitLossFromSegmentsToConsolidatedTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "230923016 - Disclosure - Segment and Geographic Information (Tables)",
"role": "http://www.medtronic.com/role/SegmentandGeographicInformationTables",
"shortName": "Segment and Geographic Information (Tables)",
"subGroupType": "tables",
"uniqueAnchor": {
"ancestors": [
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:ReconciliationOfOperatingProfitLossFromSegmentsToConsolidatedTextBlock",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R48": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:CostOfGoodsAndServicesSold",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240034001 - Disclosure - Summary of Significant Accounting Policies (Details)",
"role": "http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails",
"shortName": "Summary of Significant Accounting Policies (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i4a01f87a94b6457eb39023a7f66dd136_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:CostOfGoodsAndServicesSold",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R49": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240064002 - Disclosure - Revenue - Disaggregation of Net Sales by Segment and Division (Details)",
"role": "http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"shortName": "Revenue - Disaggregation of Net Sales by Segment and Division (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:DisaggregationOfRevenueTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ifd0a3d2a80594860ad5cf7c3488d5cdd_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R5": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:CashAndCashEquivalentsAtCarryingValue",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "statement",
"isDefault": "false",
"longName": "100030005 - Statement - Consolidated Balance Sheets",
"role": "http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"shortName": "Consolidated Balance Sheets",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:CashAndCashEquivalentsAtCarryingValue",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R50": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240074003 - Disclosure - Revenue - Disaggregation of Net Sales by Market Geography for Each Segment (Details)",
"role": "http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"shortName": "Revenue - Disaggregation of Net Sales by Market Geography for Each Segment (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:DisaggregationOfRevenueTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ic8780f7ba9e14346a6a65c72ae219078_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R51": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ContractWithCustomerLiability",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240084004 - Disclosure - Revenue - Narrative (Details)",
"role": "http://www.medtronic.com/role/RevenueNarrativeDetails",
"shortName": "Revenue - Narrative (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ContractWithCustomerLiability",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R52": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfGoodwillTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:Goodwill",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240114005 - Disclosure - Acquisitions - Additional Information (Details)",
"role": "http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"shortName": "Acquisitions - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ia102707fbbfe4345bb45fcf4165321b4_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:AssetAcquisitionConsiderationTransferred",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R53": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfBusinessAcquisitionsByAcquisitionContingentConsiderationTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i8362330ce180425da2583cdfcef52524_I20210430",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:BusinessCombinationContingentConsiderationLiability",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240124006 - Disclosure - Acquisitions - Contingent Consideration (Details)",
"role": "http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"shortName": "Acquisitions - Contingent Consideration (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfBusinessAcquisitionsByAcquisitionContingentConsiderationTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "mdt:BusinessAcquisitionPriceContingentConsideration",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R54": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "mdt:RestructuringChargesNetOfRestructuringReversalsIncludingCostOfProductSoldImpact",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240154007 - Disclosure - Restructuring Charges - Additional Information (Details)",
"role": "http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"shortName": "Restructuring Charges - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i87129e63aa104425b671b2e2f9122889_D20210501-20210730",
"decimals": "-6",
"lang": "en-US",
"name": "mdt:AssetImpairmentChargesAndInventoryWriteDown",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R55": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfRestructuringAndRelatedCostsTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i8362330ce180425da2583cdfcef52524_I20210430",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:RestructuringReserve",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240164008 - Disclosure - Restructuring Charges (Details)",
"role": "http://www.medtronic.com/role/RestructuringChargesDetails",
"shortName": "Restructuring Charges (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfRestructuringAndRelatedCostsTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i1aa626fafe594bf5bf718c04583d00b2_I20190426",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:RestructuringReserve",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R56": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtSecuritiesAvailableForSaleAmortizedCostExcludingAccruedInterestAfterAllowanceForCreditLoss",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240194009 - Disclosure - Financial Instruments - Investments by Category and Related Balance Sheet Presentation (Details)",
"role": "http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"shortName": "Financial Instruments - Investments by Category and Related Balance Sheet Presentation (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtSecuritiesAvailableForSaleAmortizedCostExcludingAccruedInterestAfterAllowanceForCreditLoss",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R57": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240204010 - Disclosure - Financial Instruments - AFS in Continuous Loss Position (Details)",
"role": "http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"shortName": "Financial Instruments - AFS in Continuous Loss Position (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R58": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ProceedsFromMaturitiesPrepaymentsAndCallsOfHeldToMaturitySecurities",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240214011 - Disclosure - Financial Instruments - Additional Information (Details)",
"role": "http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails",
"shortName": "Financial Instruments - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ProceedsFromMaturitiesPrepaymentsAndCallsOfHeldToMaturitySecurities",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R59": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:GainLossOnInvestmentsTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ProceedsFromSaleAndMaturityOfAvailableForSaleSecurities",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240224012 - Disclosure - Financial Instruments - Activity Related to the Company's Debt Securities Portfolio and Available-for-sale Debt Securities Contractual Maturities (Details)",
"role": "http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails",
"shortName": "Financial Instruments - Activity Related to the Company's Debt Securities Portfolio and Available-for-sale Debt Securities Contractual Maturities (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:GainLossOnInvestmentsTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ProceedsFromSaleAndMaturityOfAvailableForSaleSecurities",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R6": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:AllowanceForDoubtfulAccountsReceivableCurrent",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "statement",
"isDefault": "false",
"longName": "100040006 - Statement - Consolidated Balance Sheets (Parenthetical)",
"role": "http://www.medtronic.com/role/ConsolidatedBalanceSheetsParenthetical",
"shortName": "Consolidated Balance Sheets (Parenthetical)",
"subGroupType": "parenthetical",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:AllowanceForDoubtfulAccountsReceivableCurrent",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R60": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"mdt:EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestmentsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ib0a9d7071581480e838c3ff820de7ef7_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:EquitySecuritiesFvNi",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240234013 - Disclosure - Financial Instruments - Summary of Equity and Other Investments (Details)",
"role": "http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails",
"shortName": "Financial Instruments - Summary of Equity and Other Investments (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"mdt:EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestmentsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ib0a9d7071581480e838c3ff820de7ef7_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:EquitySecuritiesFvNi",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R61": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ProceedsFromSaleMaturityAndCollectionsOfInvestments",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240244014 - Disclosure - Financial Instruments - Activity Related to the Company's Investment Portfolio, Equity and Other Investments (Details)",
"role": "http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails",
"shortName": "Financial Instruments - Activity Related to the Company's Investment Portfolio, Equity and Other Investments (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"mdt:ScheduleofInvestmentsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i6ffffdd393d849f6ba1eaab54ae7a99e_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:ProceedsFromSaleMaturityAndCollectionsOfInvestments",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R62": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfShortTermDebtTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:FinanceLeaseLiabilityCurrent",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240274015 - Disclosure - Financing Arrangements - Current Debt Obligations (Details)",
"role": "http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"shortName": "Financing Arrangements - Current Debt Obligations (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfShortTermDebtTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:FinanceLeaseLiabilityCurrent",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R63": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DebtCurrent",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240284016 - Disclosure - Financing Arrangements - Additional Information (Details)",
"role": "http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"shortName": "Financing Arrangements - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "INF",
"lang": "en-US",
"name": "us-gaap:DebtInstrumentFaceAmount",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R64": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDebtInstrumentsTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:FinanceLeaseLiabilityNoncurrent",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240294017 - Disclosure - Financing Arrangements - Long-term Debt (Details)",
"role": "http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails",
"shortName": "Financing Arrangements - Long-term Debt (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDebtInstrumentsTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:FinanceLeaseLiabilityNoncurrent",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R65": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfMaturitiesOfLongTermDebtTableTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:LongTermDebtMaturitiesRepaymentsOfPrincipalInNextTwelveMonths",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240304018 - Disclosure - Financing Arrangements - Long-term Debt Maturities (Details)",
"role": "http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails",
"shortName": "Financing Arrangements - Long-term Debt Maturities (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfMaturitiesOfLongTermDebtTableTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:LongTermDebtMaturitiesRepaymentsOfPrincipalInNextTwelveMonths",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R66": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "mdt:UnrealizedGainLossOnInterestRateCashFlowHedgesAccumulatedOtherComprehensiveIncomeLossNetOfTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240334019 - Disclosure - Derivatives and Currency Exchange Risk Management - Narrative (Details)",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"shortName": "Derivatives and Currency Exchange Risk Management - Narrative (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "mdt:UnrealizedGainLossOnInterestRateCashFlowHedgesAccumulatedOtherComprehensiveIncomeLossNetOfTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R67": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "mdt:OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossBeforeReclassificationAndTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240344020 - Disclosure - Derivatives and Currency Exchange Risk Management - Derivative (Gains) Losses Designated as Hedging Instruments (Details)",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"shortName": "Derivatives and Currency Exchange Risk Management - Derivative (Gains) Losses Designated as Hedging Instruments (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "mdt:OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossBeforeReclassificationAndTax",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R68": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeInstrumentsNotDesignatedAsHedgingInstrumentsGainLossNet",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240354021 - Disclosure - Derivatives and Currency Exchange Risk Management - Gains and Losses on Derivatives Not Designated as Hedging Instruments (Details)",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"shortName": "Derivatives and Currency Exchange Risk Management - Gains and Losses on Derivatives Not Designated as Hedging Instruments (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeInstrumentsNotDesignatedAsHedgingInstrumentsGainLossNet",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R69": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeFairValueOfDerivativeAsset",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240364022 - Disclosure - Derivatives and Currency Exchange Risk Management - Classification and Fair Value Amounts of Derivative Instruments in Balance Sheets (Details)",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"shortName": "Derivatives and Currency Exchange Risk Management - Classification and Fair Value Amounts of Derivative Instruments in Balance Sheets (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ib47d37ac89e843279da722060b5fc138_I20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:DerivativeFairValueOfDerivativeAsset",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R7": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i2a0df28ce15c4f849a2c2f1136a84ed8_I20190426",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:CommonStockSharesOutstanding",
"reportCount": 1,
"unique": true,
"unitRef": "shares",
"xsiNil": "false"
},
"groupType": "statement",
"isDefault": "false",
"longName": "100050007 - Statement - Consolidated Statements of Equity",
"role": "http://www.medtronic.com/role/ConsolidatedStatementsofEquity",
"shortName": "Consolidated Statements of Equity",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i2a0df28ce15c4f849a2c2f1136a84ed8_I20190426",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:CommonStockSharesOutstanding",
"reportCount": 1,
"unique": true,
"unitRef": "shares",
"xsiNil": "false"
}
},
"R70": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i97c5c08b0dff4487986afc18702024a1_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeAssets",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240374023 - Disclosure - Derivatives and Currency Exchange Risk Management - Derivative Assets and Liabilities Measured at Fair Value on a Recurring Basis (Details)",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails",
"shortName": "Derivatives and Currency Exchange Risk Management - Derivative Assets and Liabilities Measured at Fair Value on a Recurring Basis (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i97c5c08b0dff4487986afc18702024a1_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeAssets",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R71": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DerivativeFairValueOfDerivativeAsset",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240384024 - Disclosure - Derivatives and Currency Exchange Risk Management - Offsetting Assets and Liabilities (Details)",
"role": "http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails",
"shortName": "Derivatives and Currency Exchange Risk Management - Offsetting Assets and Liabilities (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:OffsettingLiabilitiesTableTextBlock",
"us-gaap:OffsettingAssetsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i8362330ce180425da2583cdfcef52524_I20210430",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:DerivativeAssetNotOffsetPolicyElectionDeduction",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R72": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfInventoryCurrentTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:InventoryFinishedGoodsNetOfReserves",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240414025 - Disclosure - Inventories (Details)",
"role": "http://www.medtronic.com/role/InventoriesDetails",
"shortName": "Inventories (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfInventoryCurrentTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:InventoryFinishedGoodsNetOfReserves",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R73": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i8362330ce180425da2583cdfcef52524_I20210430",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:Goodwill",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240444026 - Disclosure - Goodwill and Other Intangible Assets - Changes in Goodwill (Details)",
"role": "http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails",
"shortName": "Goodwill and Other Intangible Assets - Changes in Goodwill (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfGoodwillTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:GoodwillAcquiredDuringPeriod",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R74": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfIndefiniteLivedIntangibleAssetsTableTextBlock",
"us-gaap:ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:FiniteLivedIntangibleAssetsGross",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240454027 - Disclosure - Goodwill and Other Intangible Assets - Intangible Assets (Details)",
"role": "http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"shortName": "Goodwill and Other Intangible Assets - Intangible Assets (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfIndefiniteLivedIntangibleAssetsTableTextBlock",
"us-gaap:ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:FiniteLivedIntangibleAssetsGross",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R75": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:AmortizationOfIntangibleAssets",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240464028 - Disclosure - Goodwill and Other Intangible Assets - Amortization Expense (Details)",
"role": "http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsAmortizationExpenseDetails",
"shortName": "Goodwill and Other Intangible Assets - Amortization Expense (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:FiniteLivedIntangibleAssetsAmortizationExpenseNextTwelveMonths",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R76": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:PropertyPlantAndEquipmentTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetBeforeAccumulatedDepreciationAndAmortization",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240494029 - Disclosure - Property, Plant, and Equipment (Details)",
"role": "http://www.medtronic.com/role/PropertyPlantandEquipmentDetails",
"shortName": "Property, Plant, and Equipment (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:PropertyPlantAndEquipmentTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetBeforeAccumulatedDepreciationAndAmortization",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R77": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i8362330ce180425da2583cdfcef52524_I20210430",
"decimals": "INF",
"first": true,
"lang": "en-US",
"name": "us-gaap:CommonStockSharesAuthorized",
"reportCount": 1,
"unitRef": "shares",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240514030 - Disclosure - Shareholders' Equity (Details)",
"role": "http://www.medtronic.com/role/ShareholdersEquityDetails",
"shortName": "Shareholders' Equity (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "INF",
"lang": "en-US",
"name": "mdt:DeferredStockSharesAuthorized",
"reportCount": 1,
"unique": true,
"unitRef": "shares",
"xsiNil": "false"
}
},
"R78": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ibad7796dc70b439ba17b01eee35c154b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240544031 - Disclosure - Stock Purchase and Award Plans - Additional Information (Details)",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"shortName": "Stock Purchase and Award Plans - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ibad7796dc70b439ba17b01eee35c154b_D20210501-20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R79": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:AllocatedShareBasedCompensationExpense",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240554032 - Disclosure - Stock Purchase and Award Plans - Stock-based Compensation Expense (Details)",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails",
"shortName": "Stock Purchase and Award Plans - Stock-based Compensation Expense (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:AllocatedShareBasedCompensationExpense",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R8": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "INF",
"first": true,
"lang": "en-US",
"name": "us-gaap:CommonStockDividendsPerShareDeclared",
"reportCount": 1,
"unique": true,
"unitRef": "usdPerShare",
"xsiNil": "false"
},
"groupType": "statement",
"isDefault": "false",
"longName": "100060008 - Statement - Consolidated Statements of Equity (Parenthetical)",
"role": "http://www.medtronic.com/role/ConsolidatedStatementsofEquityParenthetical",
"shortName": "Consolidated Statements of Equity (Parenthetical)",
"subGroupType": "parenthetical",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "INF",
"first": true,
"lang": "en-US",
"name": "us-gaap:CommonStockDividendsPerShareDeclared",
"reportCount": 1,
"unique": true,
"unitRef": "usdPerShare",
"xsiNil": "false"
}
},
"R80": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "2",
"first": true,
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue",
"reportCount": 1,
"unique": true,
"unitRef": "usdPerShare",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240564033 - Disclosure - Stock Purchase and Award Plans - Valuation Assumptions (Details)",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlansValuationAssumptionsDetails",
"shortName": "Stock Purchase and Award Plans - Valuation Assumptions (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "2",
"first": true,
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue",
"reportCount": 1,
"unique": true,
"unitRef": "usdPerShare",
"xsiNil": "false"
}
},
"R81": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92df1c8e3dee47e19e0f3e813ce0f243_I20210430",
"decimals": "-3",
"first": true,
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber",
"reportCount": 1,
"unitRef": "shares",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240574034 - Disclosure - Stock Purchase and Award Plans - Stock Options Activity (Details)",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails",
"shortName": "Stock Purchase and Award Plans - Stock Options Activity (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "ibad7796dc70b439ba17b01eee35c154b_D20210501-20220429",
"decimals": "-3",
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross",
"reportCount": 1,
"unique": true,
"unitRef": "shares",
"xsiNil": "false"
}
},
"R82": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i43a4a2f4d9fa4104846a5d462d7df86f_I20210430",
"decimals": "-3",
"first": true,
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber",
"reportCount": 1,
"unitRef": "shares",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240584035 - Disclosure - Stock Purchase and Award Plans - Restricted Stock Activity (Details)",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails",
"shortName": "Stock Purchase and Award Plans - Restricted Stock Activity (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i52c9dc93ae4e4185a6bc5b971e91ebb2_D20210501-20220429",
"decimals": "-3",
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod",
"reportCount": 1,
"unique": true,
"unitRef": "shares",
"xsiNil": "false"
}
},
"R83": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ShareBasedCompensationPerformanceSharesAwardUnvestedActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "iba5035e1d4b64bd7a2a96040e952d5d6_I20210430",
"decimals": "-3",
"first": true,
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber",
"reportCount": 1,
"unitRef": "shares",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240594036 - Disclosure - Stock Purchase and Award Plans - Performance Share Unit Activity (Details)",
"role": "http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"shortName": "Stock Purchase and Award Plans - Performance Share Unit Activity (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ShareBasedCompensationPerformanceSharesAwardUnvestedActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i37d7e688d2884e5cada26b32f67fbecb_D20210501-20220429",
"decimals": "-3",
"lang": "en-US",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod",
"reportCount": 1,
"unique": true,
"unitRef": "shares",
"xsiNil": "false"
}
},
"R84": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:IncomeLossFromContinuingOperationsBeforeIncomeTaxesDomestic",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240624037 - Disclosure - Income Taxes - Components of Income Before Income Taxes, Based on Jurisdiction (Details)",
"role": "http://www.medtronic.com/role/IncomeTaxesComponentsofIncomeBeforeIncomeTaxesBasedonJurisdictionDetails",
"shortName": "Income Taxes - Components of Income Before Income Taxes, Based on Jurisdiction (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:IncomeLossFromContinuingOperationsBeforeIncomeTaxesDomestic",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R85": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:CurrentFederalTaxExpenseBenefit",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240634038 - Disclosure - Income Taxes - Income Tax (Benefit) Provision (Details)",
"role": "http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails",
"shortName": "Income Taxes - Income Tax (Benefit) Provision (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:CurrentFederalTaxExpenseBenefit",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R86": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DeferredTaxAssetsGoodwillAndIntangibleAssets",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240644039 - Disclosure - Income Taxes - Schedule of Deferred Tax Assets and Liabilities (Details)",
"role": "http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails",
"shortName": "Income Taxes - Schedule of Deferred Tax Assets and Liabilities (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DeferredTaxAssetsGoodwillAndIntangibleAssets",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R87": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-8",
"first": true,
"lang": "en-US",
"name": "us-gaap:UndistributedEarningsOfForeignSubsidiaries",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240654040 - Disclosure - Income Taxes - Additional Information (Details)",
"role": "http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails",
"shortName": "Income Taxes - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-8",
"first": true,
"lang": "en-US",
"name": "us-gaap:UndistributedEarningsOfForeignSubsidiaries",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R88": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "INF",
"first": true,
"lang": "en-US",
"name": "us-gaap:EffectiveIncomeTaxRateReconciliationAtFederalStatutoryIncomeTaxRate",
"reportCount": 1,
"unique": true,
"unitRef": "number",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240664041 - Disclosure - Income Taxes - Effective Income Tax Rate Reconciliation (Details)",
"role": "http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails",
"shortName": "Income Taxes - Effective Income Tax Rate Reconciliation (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "INF",
"first": true,
"lang": "en-US",
"name": "us-gaap:EffectiveIncomeTaxRateReconciliationAtFederalStatutoryIncomeTaxRate",
"reportCount": 1,
"unique": true,
"unitRef": "number",
"xsiNil": "false"
}
},
"R89": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i8362330ce180425da2583cdfcef52524_I20210430",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:UnrecognizedTaxBenefits",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240674042 - Disclosure - Income Taxes - Reconciliation of Beginning and Ending Amount of Unrecognized Tax Benefits (Details)",
"role": "http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails",
"shortName": "Income Taxes - Reconciliation of Beginning and Ending Amount of Unrecognized Tax Benefits (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:UnrecognizedTaxBenefitsIncreasesResultingFromPriorPeriodTaxPositions",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R9": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:ProfitLoss",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "statement",
"isDefault": "false",
"longName": "100070009 - Statement - Consolidated Statements of Cash Flows",
"role": "http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows",
"shortName": "Consolidated Statements of Cash Flows",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:DepreciationDepletionAndAmortization",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R90": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:NetIncomeLoss",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240704043 - Disclosure - Earnings Per Share (Details)",
"role": "http://www.medtronic.com/role/EarningsPerShareDetails",
"shortName": "Earnings Per Share (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-5",
"lang": "en-US",
"name": "us-gaap:IncrementalCommonSharesAttributableToShareBasedPaymentArrangements",
"reportCount": 1,
"unique": true,
"unitRef": "shares",
"xsiNil": "false"
}
},
"R91": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:PensionAndOtherPostretirementBenefitExpense",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240734044 - Disclosure - Retirement Benefit Plans - Additional Information (Details)",
"role": "http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"shortName": "Retirement Benefit Plans - Additional Information (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:PensionAndOtherPostretirementBenefitExpense",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R92": {
"firstAnchor": {
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanFundedStatusOfPlan",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240744045 - Disclosure - Retirement Benefit Plans - Change in Benefit Obligation and Funded Status (Details)",
"role": "http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"shortName": "Retirement Benefit Plans - Change in Benefit Obligation and Funded Status (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i588282f8b2d34f0792cd8328652db2e6_I20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAggregateAccumulatedBenefitObligation",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R93": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"ix:continuation",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "id8df7deb9ba140f7b733686f6b6a057f_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanServiceCost",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240754046 - Disclosure - Retirement Benefit Plans - Net Periodic Cost and AOCI (Details)",
"role": "http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails",
"shortName": "Retirement Benefit Plans - Net Periodic Cost and AOCI (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"us-gaap:ScheduleOfNetBenefitCostsTableTextBlock",
"div",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "id8df7deb9ba140f7b733686f6b6a057f_D20210501-20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanExpectedReturnOnPlanAssets",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R94": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfAssumptionsUsedTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "id8ee90868e1b452e839a41d7b5b0017e_I20220429",
"decimals": "4",
"first": true,
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanAssumptionsUsedCalculatingBenefitObligationRateOfCompensationIncrease",
"reportCount": 1,
"unique": true,
"unitRef": "number",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240764047 - Disclosure - Retirement Benefit Plans - Actuarial Assumptions and Plan Assets Target Allocations (Details)",
"role": "http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"shortName": "Retirement Benefit Plans - Actuarial Assumptions and Plan Assets Target Allocations (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfAssumptionsUsedTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "id8ee90868e1b452e839a41d7b5b0017e_I20220429",
"decimals": "4",
"first": true,
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanAssumptionsUsedCalculatingBenefitObligationRateOfCompensationIncrease",
"reportCount": 1,
"unique": true,
"unitRef": "number",
"xsiNil": "false"
}
},
"R95": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"mdt:FairValueMeasurementsRetirementBenefitPlanAssetsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "id8ee90868e1b452e839a41d7b5b0017e_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanFairValueOfPlanAssets",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240774048 - Disclosure - Retirement Benefit Plans - Fair Value Measurement (Details)",
"role": "http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"shortName": "Retirement Benefit Plans - Fair Value Measurement (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"mdt:FairValueMeasurementsRetirementBenefitPlanAssetsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "if48c2510b55a409ca84c770b93792a0a_I20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanFairValueOfPlanAssets",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R96": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfExpectedBenefitPaymentsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "id8ee90868e1b452e839a41d7b5b0017e_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanExpectedFutureBenefitPaymentsNextTwelveMonths",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240784049 - Disclosure - Retirement Benefit Plans - Future Benefit Payments (Details)",
"role": "http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"shortName": "Retirement Benefit Plans - Future Benefit Payments (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfExpectedBenefitPaymentsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "id8ee90868e1b452e839a41d7b5b0017e_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:DefinedBenefitPlanExpectedFutureBenefitPaymentsNextTwelveMonths",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R97": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"mdt:AssetsAndLiabilitiesLesseeTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingLeaseRightOfUseAsset",
"reportCount": 1,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240814050 - Disclosure - Leases - Balance Sheet Classification and Amounts of Right-of-Use Assets and Lease Liabilities (Details)",
"role": "http://www.medtronic.com/role/LeasesBalanceSheetClassificationandAmountsofRightofUseAssetsandLeaseLiabilitiesDetails",
"shortName": "Leases - Balance Sheet Classification and Amounts of Right-of-Use Assets and Lease Liabilities (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"mdt:AssetsAndLiabilitiesLesseeTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": "-6",
"lang": "en-US",
"name": "us-gaap:OperatingLeaseLiabilityCurrent",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R98": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingLeaseWeightedAverageRemainingLeaseTerm1",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240824051 - Disclosure - Leases - Weighted-Average Remaining Lease Term and Weighted-Average Discount Rate for Operating Leases (Details)",
"role": "http://www.medtronic.com/role/LeasesWeightedAverageRemainingLeaseTermandWeightedAverageDiscountRateforOperatingLeasesDetails",
"shortName": "Leases - Weighted-Average Remaining Lease Term and Weighted-Average Discount Rate for Operating Leases (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i92869655957340aea391088638261188_I20220429",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingLeaseWeightedAverageRemainingLeaseTerm1",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
},
"R99": {
"firstAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingLeaseCost",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
},
"groupType": "disclosure",
"isDefault": "false",
"longName": "240834052 - Disclosure - Leases - Components of Total Operating Lease Cost (Details)",
"role": "http://www.medtronic.com/role/LeasesComponentsofTotalOperatingLeaseCostDetails",
"shortName": "Leases - Components of Total Operating Lease Cost (Details)",
"subGroupType": "details",
"uniqueAnchor": {
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"baseRef": "mdt-20220429.htm",
"contextRef": "i650e9c87a6bd4aadadfd9c4b48d1313b_D20210501-20220429",
"decimals": "-6",
"first": true,
"lang": "en-US",
"name": "us-gaap:OperatingLeaseCost",
"reportCount": 1,
"unique": true,
"unitRef": "usd",
"xsiNil": "false"
}
},
"R9999": {
"firstAnchor": null,
"groupType": "",
"isDefault": "false",
"longName": "Uncategorized Items - mdt-20220429.htm",
"role": "http://xbrl.sec.gov/role/uncategorizedFacts",
"shortName": "Uncategorized Items - mdt-20220429.htm",
"subGroupType": "",
"uniqueAnchor": null
}
},
"segmentCount": 200,
"tag": {
"country_IE": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "IRELAND",
"terseLabel": "Ireland"
}
}
},
"localname": "IE",
"nsuri": "http://xbrl.sec.gov/country/2021",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "domainItemType"
},
"country_US": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "UNITED STATES",
"netLabel": "United States",
"terseLabel": "U.S. Pension Benefits",
"verboseLabel": "U.S."
}
}
},
"localname": "US",
"nsuri": "http://xbrl.sec.gov/country/2021",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "domainItemType"
},
"dei_AmendmentFlag": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the XBRL content amends previously-filed or accepted submission.",
"label": "Amendment Flag",
"terseLabel": "Amendment Flag"
}
}
},
"localname": "AmendmentFlag",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "booleanItemType"
},
"dei_AuditorFirmId": {
"auth_ref": [
"r1033",
"r1034",
"r1035"
],
"lang": {
"en-us": {
"role": {
"documentation": "PCAOB issued Audit Firm Identifier",
"label": "Auditor Firm ID",
"terseLabel": "Auditor Firm ID"
}
}
},
"localname": "AuditorFirmId",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/AuditInformation"
],
"xbrltype": "nonemptySequenceNumberItemType"
},
"dei_AuditorLocation": {
"auth_ref": [
"r1033",
"r1034",
"r1035"
],
"lang": {
"en-us": {
"role": {
"label": "Auditor Location",
"terseLabel": "Auditor Location"
}
}
},
"localname": "AuditorLocation",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/AuditInformation"
],
"xbrltype": "internationalNameItemType"
},
"dei_AuditorName": {
"auth_ref": [
"r1033",
"r1034",
"r1035"
],
"lang": {
"en-us": {
"role": {
"label": "Auditor Name",
"terseLabel": "Auditor Name"
}
}
},
"localname": "AuditorName",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/AuditInformation"
],
"xbrltype": "internationalNameItemType"
},
"dei_CityAreaCode": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Area code of city",
"label": "City Area Code",
"terseLabel": "City Area Code"
}
}
},
"localname": "CityAreaCode",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "normalizedStringItemType"
},
"dei_CountryRegion": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Region code of country",
"label": "Country Region",
"terseLabel": "Country Region"
}
}
},
"localname": "CountryRegion",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "normalizedStringItemType"
},
"dei_CoverAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Cover page.",
"label": "Cover [Abstract]"
}
}
},
"localname": "CoverAbstract",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"xbrltype": "stringItemType"
},
"dei_CurrentFiscalYearEndDate": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "End date of current fiscal year in the format --MM-DD.",
"label": "Current Fiscal Year End Date",
"terseLabel": "Current Fiscal Year End Date"
}
}
},
"localname": "CurrentFiscalYearEndDate",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "gMonthDayItemType"
},
"dei_DocumentAnnualReport": {
"auth_ref": [
"r1033",
"r1034",
"r1035"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true only for a form used as an annual report.",
"label": "Document Annual Report",
"terseLabel": "Document Annual Report"
}
}
},
"localname": "DocumentAnnualReport",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "booleanItemType"
},
"dei_DocumentFiscalPeriodFocus": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Fiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY.",
"label": "Document Fiscal Period Focus",
"terseLabel": "Document Fiscal Period Focus"
}
}
},
"localname": "DocumentFiscalPeriodFocus",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "fiscalPeriodItemType"
},
"dei_DocumentFiscalYearFocus": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "This is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006.",
"label": "Document Fiscal Year Focus",
"terseLabel": "Document Fiscal Year Focus"
}
}
},
"localname": "DocumentFiscalYearFocus",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "gYearItemType"
},
"dei_DocumentPeriodEndDate": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "For the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.",
"label": "Document Period End Date",
"terseLabel": "Document Period End Date"
}
}
},
"localname": "DocumentPeriodEndDate",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "dateItemType"
},
"dei_DocumentTransitionReport": {
"auth_ref": [
"r1036"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true only for a form used as a transition report.",
"label": "Document Transition Report",
"terseLabel": "Document Transition Report"
}
}
},
"localname": "DocumentTransitionReport",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "booleanItemType"
},
"dei_DocumentType": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "The type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.",
"label": "Document Type",
"terseLabel": "Document Type"
}
}
},
"localname": "DocumentType",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "submissionTypeItemType"
},
"dei_DocumentsIncorporatedByReferenceTextBlock": {
"auth_ref": [
"r1031"
],
"lang": {
"en-us": {
"role": {
"documentation": "Documents incorporated by reference.",
"label": "Documents Incorporated by Reference [Text Block]",
"terseLabel": "Documents Incorporated by Reference"
}
}
},
"localname": "DocumentsIncorporatedByReferenceTextBlock",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "textBlockItemType"
},
"dei_EntitiesTable": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Container to assemble all relevant information about each entity associated with the document instance",
"label": "Entities [Table]",
"terseLabel": "Entities [Table]"
}
}
},
"localname": "EntitiesTable",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "stringItemType"
},
"dei_EntityAddressAddressLine1": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Address Line 1 such as Attn, Building Name, Street Name",
"label": "Entity Address, Address Line One",
"terseLabel": "Entity Address, Address Line One"
}
}
},
"localname": "EntityAddressAddressLine1",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityAddressCityOrTown": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Name of the City or Town",
"label": "Entity Address, City or Town",
"terseLabel": "Entity Address, City or Town"
}
}
},
"localname": "EntityAddressCityOrTown",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityAddressCountry": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "ISO 3166-1 alpha-2 country code.",
"label": "Entity Address, Country",
"terseLabel": "Entity Address, Country"
}
}
},
"localname": "EntityAddressCountry",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "countryCodeItemType"
},
"dei_EntityAddressPostalZipCode": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Code for the postal or zip code",
"label": "Entity Address, Postal Zip Code",
"terseLabel": "Entity Address, Postal Zip Code"
}
}
},
"localname": "EntityAddressPostalZipCode",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityCentralIndexKey": {
"auth_ref": [
"r1030"
],
"lang": {
"en-us": {
"role": {
"documentation": "A unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK.",
"label": "Entity Central Index Key",
"terseLabel": "Entity Central Index Key"
}
}
},
"localname": "EntityCentralIndexKey",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "centralIndexKeyItemType"
},
"dei_EntityCommonStockSharesOutstanding": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument.",
"label": "Entity Common Stock, Shares Outstanding",
"terseLabel": "Entity Common Stock, Shares Outstanding"
}
}
},
"localname": "EntityCommonStockSharesOutstanding",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "sharesItemType"
},
"dei_EntityCurrentReportingStatus": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure.",
"label": "Entity Current Reporting Status",
"terseLabel": "Entity Current Reporting Status"
}
}
},
"localname": "EntityCurrentReportingStatus",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "yesNoItemType"
},
"dei_EntityDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "All the names of the entities being reported upon in a document. Any legal structure used to conduct activities or to hold assets. Some examples of such structures are corporations, partnerships, limited liability companies, grantor trusts, and other trusts. This item does not include business and geographical segments which are included in the geographical or business segments domains.",
"label": "Entity [Domain]",
"terseLabel": "Entity [Domain]"
}
}
},
"localname": "EntityDomain",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"dei_EntityEmergingGrowthCompany": {
"auth_ref": [
"r1030"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate if registrant meets the emerging growth company criteria.",
"label": "Entity Emerging Growth Company",
"terseLabel": "Entity Emerging Growth Company"
}
}
},
"localname": "EntityEmergingGrowthCompany",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "booleanItemType"
},
"dei_EntityFileNumber": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Commission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.",
"label": "Entity File Number",
"terseLabel": "Entity File Number"
}
}
},
"localname": "EntityFileNumber",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "fileNumberItemType"
},
"dei_EntityFilerCategory": {
"auth_ref": [
"r1030"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure.",
"label": "Entity Filer Category",
"terseLabel": "Entity Filer Category"
}
}
},
"localname": "EntityFilerCategory",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "filerCategoryItemType"
},
"dei_EntityIncorporationStateCountryCode": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Two-character EDGAR code representing the state or country of incorporation.",
"label": "Entity Incorporation, State or Country Code",
"terseLabel": "Entity Incorporation, State or Country Code"
}
}
},
"localname": "EntityIncorporationStateCountryCode",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "edgarStateCountryItemType"
},
"dei_EntityInformationLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Entity Information [Line Items]",
"terseLabel": "Entity Information [Line Items]"
}
}
},
"localname": "EntityInformationLineItems",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "stringItemType"
},
"dei_EntityInteractiveDataCurrent": {
"auth_ref": [
"r1044"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).",
"label": "Entity Interactive Data Current",
"terseLabel": "Entity Interactive Data Current"
}
}
},
"localname": "EntityInteractiveDataCurrent",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "yesNoItemType"
},
"dei_EntityPublicFloat": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter.",
"label": "Entity Public Float",
"terseLabel": "Entity Public Float"
}
}
},
"localname": "EntityPublicFloat",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "monetaryItemType"
},
"dei_EntityRegistrantName": {
"auth_ref": [
"r1030"
],
"lang": {
"en-us": {
"role": {
"documentation": "The exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC.",
"label": "Entity Registrant Name",
"terseLabel": "Entity Registrant Name"
}
}
},
"localname": "EntityRegistrantName",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityShellCompany": {
"auth_ref": [
"r1030"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act.",
"label": "Entity Shell Company",
"terseLabel": "Entity Shell Company"
}
}
},
"localname": "EntityShellCompany",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "booleanItemType"
},
"dei_EntitySmallBusiness": {
"auth_ref": [
"r1030"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicates that the company is a Smaller Reporting Company (SRC).",
"label": "Entity Small Business",
"terseLabel": "Entity Small Business"
}
}
},
"localname": "EntitySmallBusiness",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "booleanItemType"
},
"dei_EntityTaxIdentificationNumber": {
"auth_ref": [
"r1030"
],
"lang": {
"en-us": {
"role": {
"documentation": "The Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS.",
"label": "Entity Tax Identification Number",
"terseLabel": "Entity Tax Identification Number"
}
}
},
"localname": "EntityTaxIdentificationNumber",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "employerIdItemType"
},
"dei_EntityVoluntaryFilers": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.",
"label": "Entity Voluntary Filers",
"terseLabel": "Entity Voluntary Filers"
}
}
},
"localname": "EntityVoluntaryFilers",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "yesNoItemType"
},
"dei_EntityWellKnownSeasonedIssuer": {
"auth_ref": [
"r1068"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A.",
"label": "Entity Well-known Seasoned Issuer",
"terseLabel": "Entity Well-known Seasoned Issuer"
}
}
},
"localname": "EntityWellKnownSeasonedIssuer",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "yesNoItemType"
},
"dei_IcfrAuditorAttestationFlag": {
"auth_ref": [
"r1033",
"r1034",
"r1035"
],
"lang": {
"en-us": {
"role": {
"label": "ICFR Auditor Attestation Flag",
"terseLabel": "ICFR Auditor Attestation Flag"
}
}
},
"localname": "IcfrAuditorAttestationFlag",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "booleanItemType"
},
"dei_LegalEntityAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "The set of legal entities associated with a report.",
"label": "Legal Entity [Axis]",
"terseLabel": "Legal Entity [Axis]"
}
}
},
"localname": "LegalEntityAxis",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"dei_LocalPhoneNumber": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Local phone number for entity.",
"label": "Local Phone Number",
"terseLabel": "Local Phone Number"
}
}
},
"localname": "LocalPhoneNumber",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "normalizedStringItemType"
},
"dei_Security12bTitle": {
"auth_ref": [
"r1029"
],
"lang": {
"en-us": {
"role": {
"documentation": "Title of a 12(b) registered security.",
"label": "Title of 12(b) Security",
"terseLabel": "Title of 12(b) Security"
}
}
},
"localname": "Security12bTitle",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "securityTitleItemType"
},
"dei_SecurityExchangeName": {
"auth_ref": [
"r1032"
],
"lang": {
"en-us": {
"role": {
"documentation": "Name of the Exchange on which a security is registered.",
"label": "Security Exchange Name",
"terseLabel": "Security Exchange Name"
}
}
},
"localname": "SecurityExchangeName",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "edgarExchangeCodeItemType"
},
"dei_TradingSymbol": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Trading symbol of an instrument as listed on an exchange.",
"label": "Trading Symbol",
"terseLabel": "Trading Symbol"
}
}
},
"localname": "TradingSymbol",
"nsuri": "http://xbrl.sec.gov/dei/2021q4",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "tradingSymbolItemType"
},
"mdt_AccumulatedGainLossNetNetInvestmentHedgeParentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated Gain (Loss), Net, Net Investment Hedge, Parent [Member]",
"label": "Accumulated Gain (Loss), Net, Net Investment Hedge, Parent [Member]",
"terseLabel": "Net Investment Hedges"
}
}
},
"localname": "AccumulatedGainLossNetNetInvestmentHedgeParentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "domainItemType"
},
"mdt_AcquisitionRelatedGainsCharges": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 12.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The following items are included within acquisition-related items: The change in the fair value of the contingent milestone consideration for the reporting period, certain-related acquisition costs, such as banker fees, legal fees, severance, and other professional fees, and impairment losses of recently acquired in-process research and development (IPR&D).",
"label": "Acquisition Related (Gains) Charges",
"negatedLabel": "Acquisition-related items"
}
}
},
"localname": "AcquisitionRelatedGainsCharges",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_AllBusinessAcquisitionsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "All Business Acquisitions [Member]",
"label": "All Business Acquisitions [Member]",
"verboseLabel": "All Business Acquisitions"
}
}
},
"localname": "AllBusinessAcquisitionsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_AmendedandRestatedRevolvingCreditAgreementMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Amended and Restated Revolving Credit Agreement [Member]",
"label": "Amended and Restated Revolving Credit Agreement [Member]",
"terseLabel": "$3.5 Billion Revolving Credit Facility"
}
}
},
"localname": "AmendedandRestatedRevolvingCreditAgreementMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_AmortizableAssetCreatedForSwissFederalIncomeTaxPurposesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Amortizable Asset Created for Swiss Federal Income Tax Purposes",
"label": "Amortizable Asset Created for Swiss Federal Income Tax Purposes [Member]",
"terseLabel": "Amortizable Asset Created for Swiss Federal Income Tax Purposes"
}
}
},
"localname": "AmortizableAssetCreatedForSwissFederalIncomeTaxPurposesMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_AssetImpairmentChargesAndInventoryWriteDown": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "AssetImpairment charges and inventory write down",
"label": "AssetImpairment charges and inventory write down",
"terseLabel": "Asset impairment charges and inventory write down"
}
}
},
"localname": "AssetImpairmentChargesAndInventoryWriteDown",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_AssetWriteDownsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Asset Write Downs",
"label": "Asset Write-downs [Member]",
"terseLabel": "Asset Write-downs"
}
}
},
"localname": "AssetWriteDownsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"mdt_AssetsAndLiabilitiesLesseeTableTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Assets And Liabilities, Lessee",
"label": "Assets And Liabilities, Lessee [Table Text Block]",
"terseLabel": "Schedule of Balance Sheet Classification of Operating Leases and Amounts of Right-of-Use Assets and Lease Liabilities"
}
}
},
"localname": "AssetsAndLiabilitiesLesseeTableTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/LeasesTables"
],
"xbrltype": "textBlockItemType"
},
"mdt_AssociatedCostsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Associated Costs [Member]",
"label": "Associated Costs [Member]",
"terseLabel": "Associated Costs"
}
}
},
"localname": "AssociatedCostsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"mdt_AuditInformationAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Audit Information",
"label": "Audit Information [Abstract]"
}
}
},
"localname": "AuditInformationAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"xbrltype": "stringItemType"
},
"mdt_BuildingsAndLeaseholdImprovementsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Facility held for productive use including, but not limited to, office, production, storage and distribution facilities and any addition or improvement to assets held under a lease arrangement (including addition or improvement to assets held by lessee under an operating lease arrangement).",
"label": "Buildings and Leasehold Improvements [Member]",
"terseLabel": "Buildings and leasehold improvements"
}
}
},
"localname": "BuildingsAndLeaseholdImprovementsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "domainItemType"
},
"mdt_BusinessAcquisitionContingentConsiderationAtFairValueRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Business Acquisition, Contingent Consideration, at Fair Value [Roll Forward]",
"terseLabel": "Reconciliation of Beginning and Ending Balances of Contingent Milestone Payments Associated with Acquisitions"
}
}
},
"localname": "BusinessAcquisitionContingentConsiderationAtFairValueRollForward",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "stringItemType"
},
"mdt_BusinessAcquisitionContingentConsiderationMilestonePayment": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Contingent milestone payments in business acquisition transactions.",
"label": "Business Acquisition, Contingent Consideration, Milestone Payment",
"negatedLabel": "Payments"
}
}
},
"localname": "BusinessAcquisitionContingentConsiderationMilestonePayment",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_BusinessAcquisitionPriceContingentConsideration": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "BusinessAcquisitionPriceContingentConsideration",
"label": "BusinessAcquisitionPriceContingentConsideration",
"terseLabel": "Purchase price contingent consideration"
}
}
},
"localname": "BusinessAcquisitionPriceContingentConsideration",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_BusinessAcquisitionPurchasePriceContingentConsideration": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of contingent consideration arrangement resulting from business combinations.",
"label": "Business Acquisition, Purchase Price Contingent Consideration",
"terseLabel": "Purchase price allocation adjustments"
}
}
},
"localname": "BusinessAcquisitionPurchasePriceContingentConsideration",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedAssetsIncludingGoodwill": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails": {
"order": 1.0,
"parentTag": "us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredGoodwillAndLiabilitiesAssumedNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Business Combination, Recognized Identifiable Assets Acquired And Liabilities Assumed, Assets, Including Goodwill",
"label": "Business Combination, Recognized Identifiable Assets Acquired And Liabilities Assumed, Assets, Including Goodwill",
"terseLabel": "Assets acquired"
}
}
},
"localname": "BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedAssetsIncludingGoodwill",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_CardiacRhythmandHeartFailureDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Cardiac Rhythm and Heart Failure Division [Member]",
"label": "Cardiac Rhythm and Heart Failure Division [Member]",
"terseLabel": "Cardiac Rhythm & Heart Failure"
}
}
},
"localname": "CardiacRhythmandHeartFailureDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_CardiovascularMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Cardiovascular",
"label": "Cardiovascular [Member]",
"terseLabel": "Cardiovascular",
"verboseLabel": "Cardiovascular"
}
}
},
"localname": "CardiovascularMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "domainItemType"
},
"mdt_CashAdvancePaidinConnectionWithProposedSettlements": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Cash Advance Paid in Connection With Proposed Settlements",
"label": "Cash Advance Paid in Connection With Proposed Settlements",
"negatedTerseLabel": "Cash advance paid to taxing authorities"
}
}
},
"localname": "CashAdvancePaidinConnectionWithProposedSettlements",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_CentralizedDistributionCosts": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 14.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Centralized Distribution Costs",
"label": "Centralized Distribution Costs",
"negatedTerseLabel": "Centralized distribution costs"
}
}
},
"localname": "CentralizedDistributionCosts",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_CommercialPaperMaximumBorrowingCapacity": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Commercial Paper, Maximum Borrowing Capacity",
"label": "Commercial Paper, Maximum Borrowing Capacity",
"terseLabel": "Commercial paper, maximum borrowing capacity"
}
}
},
"localname": "CommercialPaperMaximumBorrowingCapacity",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_ComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Before tax amount, net of reclassifications, of pension and other postretirement benefit plans (gain) loss included in comprehensive income.",
"label": "Comprehensive Income (Loss), Pension and Other Postretirement Benefit Plans, Adjustment, before Tax",
"terseLabel": "Total recognized in net periodic benefit cost and other comprehensive income"
}
}
},
"localname": "ComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_ContingentConsiderationPolicyTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Describes an entity's accounting policy for contingent consideration.",
"label": "Contingent Consideration [Policy Text Block]",
"terseLabel": "Contingent Consideration"
}
}
},
"localname": "ContingentConsiderationPolicyTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"mdt_CoronaryAndPeripheralVascularDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Coronary And Peripheral Vascular Division [Member]",
"label": "Coronary And Peripheral Vascular Division [Member]",
"terseLabel": "Coronary & Peripheral Vascular"
}
}
},
"localname": "CoronaryAndPeripheralVascularDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_CorporateOverhead": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 13.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Corporate overhead expenses",
"label": "Corporate Overhead",
"negatedTerseLabel": "Corporate"
}
}
},
"localname": "CorporateOverhead",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_CranialAndSpinalTechnologiesDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Cranial And Spinal Technologies Division [Member]",
"label": "Cranial And Spinal Technologies Division [Member]",
"terseLabel": "Cranial & Spinal Technologies"
}
}
},
"localname": "CranialAndSpinalTechnologiesDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_DebtFundsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Debt Funds",
"label": "Debt Funds [Member]",
"terseLabel": "Debt funds"
}
}
},
"localname": "DebtFundsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_DebtInstrumentExtensionOptionTermOfExtension": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Debt Instrument, Extension Option, Term Of Extension",
"label": "Debt Instrument, Extension Option, Term Of Extension",
"terseLabel": "Additional extension option, term of extension"
}
}
},
"localname": "DebtInstrumentExtensionOptionTermOfExtension",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_DebtInstrumentNumberofTranches": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Debt Instrument, Number of Tranches",
"label": "Debt Instrument, Number of Tranches",
"terseLabel": "Number of tranches"
}
}
},
"localname": "DebtInstrumentNumberofTranches",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "integerItemType"
},
"mdt_DebtLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Debt",
"label": "Debt [Line Items]",
"terseLabel": "Debt [Line Items]"
}
}
},
"localname": "DebtLineItems",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "stringItemType"
},
"mdt_DebtSecuritiesAvailableforsaleUnrealizedLossPositionFairValueAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Debt Securities, Available-for-sale, Unrealized Loss Position, Fair Value [Abstract]",
"label": "Debt Securities, Available-for-sale, Unrealized Loss Position, Fair Value [Abstract]",
"terseLabel": "Fair Value"
}
}
},
"localname": "DebtSecuritiesAvailableforsaleUnrealizedLossPositionFairValueAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "stringItemType"
},
"mdt_DebtTenderPremiumandOtherCharges": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 7.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Debt Tender Premium and Other Charges",
"label": "Debt Tender Premium and Other Charges",
"terseLabel": "Debt tender premium and other charges"
}
}
},
"localname": "DebtTenderPremiumandOtherCharges",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredStockParorStatedValuePerShare": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Stock, Par or Stated Value Per Share",
"label": "Deferred Stock, Par or Stated Value Per Share",
"terseLabel": "Deferred stock, par value (in euros per share)"
}
}
},
"localname": "DeferredStockParorStatedValuePerShare",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "perShareItemType"
},
"mdt_DeferredStockSharesAuthorized": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Stock, Shares Authorized",
"label": "Deferred Stock, Shares Authorized",
"terseLabel": "Deferred stock, shares authorized (in shares)"
}
}
},
"localname": "DeferredStockSharesAuthorized",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"mdt_DeferredStockSharesIssued": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Stock, Shares Issued",
"label": "Deferred Stock, Shares Issued",
"terseLabel": "Deferred stock, issued (in shares)"
}
}
},
"localname": "DeferredStockSharesIssued",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"mdt_DeferredStockSharesOutstanding": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Stock, Shares Outstanding",
"label": "Deferred Stock, Shares Outstanding",
"terseLabel": "Deferred stock, outstanding (in shares)"
}
}
},
"localname": "DeferredStockSharesOutstanding",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"mdt_DeferredTaxAssetAmortizationPeriod": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Asset, Amortization Period",
"label": "Deferred Tax Asset, Amortization Period",
"terseLabel": "Amortization period of deferred tax asset"
}
}
},
"localname": "DeferredTaxAssetAmortizationPeriod",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_DeferredTaxAssetsAndLiabilitiesBalanceSheetReportedAmountAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Deferred Tax Assets and Liabilities, Balance Sheet, Reported Amount [Abstract]",
"terseLabel": "Reported as (after valuation allowance and jurisdictional netting):"
}
}
},
"localname": "DeferredTaxAssetsAndLiabilitiesBalanceSheetReportedAmountAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"mdt_DeferredTaxAssetsGoodwillAndIntangibleAssetsPreviouslyRecognized": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Assets, Goodwill and Intangible Assets, Previously Recognized",
"label": "Deferred Tax Assets, Goodwill and Intangible Assets, Previously Recognized",
"terseLabel": "Deferred tax assets, goodwill and intangible assets"
}
}
},
"localname": "DeferredTaxAssetsGoodwillAndIntangibleAssetsPreviouslyRecognized",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsInterestLimitation": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 10.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Assets, Interest Limitation",
"label": "Deferred Tax Assets, Interest Limitation",
"terseLabel": "Interest limitation"
}
}
},
"localname": "DeferredTaxAssetsInterestLimitation",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsLeaseObligations": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 11.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Assets, Lease Obligations",
"label": "Deferred Tax Assets, Lease Obligations",
"terseLabel": "Lease obligations"
}
}
},
"localname": "DeferredTaxAssetsLeaseObligations",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsNetofValuationAllowanceOther": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_DeferredTaxAssetsLiabilitiesNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Assets, Net of Valuation Allowance, Other",
"label": "Deferred Tax Assets, Net of Valuation Allowance, Other",
"verboseLabel": "Other current assets"
}
}
},
"localname": "DeferredTaxAssetsNetofValuationAllowanceOther",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsOperatingAndCapitalLossesAndTaxCreditCarryforwards": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss, capital loss and tax credit carryforwards.",
"label": "Deferred Tax Assets, Operating and Capital Losses, and Tax Credit Carryforwards",
"terseLabel": "Net operating loss, capital loss, and credit carryforwards"
}
}
},
"localname": "DeferredTaxAssetsOperatingAndCapitalLossesAndTaxCreditCarryforwards",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsResearchAndDevelopmentCapitalization": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 12.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Assets, Research and Development Capitalization",
"label": "Deferred Tax Assets, Research and Development Capitalization",
"terseLabel": "Capitalization of research and development"
}
}
},
"localname": "DeferredTaxAssetsResearchAndDevelopmentCapitalization",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsTaxCreditCarryforwardsNotSubjecttoExpiration": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Assets, Tax Credit Carryforwards, Not Subject to Expiration",
"label": "Deferred Tax Assets, Tax Credit Carryforwards, Not Subject to Expiration",
"terseLabel": "Tax credit carryforward, no expiration"
}
}
},
"localname": "DeferredTaxAssetsTaxCreditCarryforwardsNotSubjecttoExpiration",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsPostretirementBenefitsAndPensions": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 5.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from pension and postretirement benefits.",
"label": "Deferred Tax Assets, Tax Deferred Expense, Compensation and Benefits, Postretirement Benefits and Pensions",
"terseLabel": "Pension and post-retirement benefits"
}
}
},
"localname": "DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsPostretirementBenefitsAndPensions",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxAssetsTaxDeferredExpenseFederalAndStateBenefitOnUncertainTaxPositions": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 9.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The tax effect as of the balance sheet date of the amount of estimated future tax deductions arising from the federal and state benefit on uncertain tax positions, which can only be deducted for tax purposes when actual costs are incurred, and which can only be realized if sufficient tax-basis income is generated in future periods to enable the deduction to be taken.",
"label": "Deferred Tax Assets Tax Deferred Expense Federal And State Benefit On Uncertain Tax Positions",
"terseLabel": "Federal and state benefit on uncertain tax positions"
}
}
},
"localname": "DeferredTaxAssetsTaxDeferredExpenseFederalAndStateBenefitOnUncertainTaxPositions",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxLiabilitiesInvestmentInSubsidiaries": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 5.0,
"parentTag": "us-gaap_DeferredIncomeTaxLiabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Liabilities, Investment In Subsidiaries",
"label": "Deferred Tax Liabilities, Investment In Subsidiaries",
"negatedLabel": "Outside basis difference of subsidiaries"
}
}
},
"localname": "DeferredTaxLiabilitiesInvestmentInSubsidiaries",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DeferredTaxLiabilitiesUnrealizedGainsOnTradingSecuritiesAndDerivatives": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 7.0,
"parentTag": "us-gaap_DeferredIncomeTaxLiabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Deferred Tax Liabilities, Unrealized Gains On Trading Securities And Derivatives",
"label": "Deferred Tax Liabilities, Unrealized Gains On Trading Securities And Derivatives",
"negatedTerseLabel": "Unrealized gain on available-for-sale securities and derivative financial instruments"
}
}
},
"localname": "DeferredTaxLiabilitiesUnrealizedGainsOnTradingSecuritiesAndDerivatives",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DefinedBenefitAndContributionPlanCashPaymentsAndAdministrativeExpenseRelatedToProvidingSpecialAndContractualTerminationBenefits": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Defined Benefit And Contribution Plan, Cash Payments And Administrative Expense Related To Providing Special And Contractual Termination Benefits",
"label": "Defined Benefit And Contribution Plan, Cash Payments And Administrative Expense Related To Providing Special And Contractual Termination Benefits",
"terseLabel": "Cash payments and administrative expense related to providing special and contractual termination benefits"
}
}
},
"localname": "DefinedBenefitAndContributionPlanCashPaymentsAndAdministrativeExpenseRelatedToProvidingSpecialAndContractualTerminationBenefits",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DefinedBenefitAndContributionPlanCostOfProvidingSpecialAndContractualTerminationBenefits": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Defined Benefit And Contribution Plan, Cost of Providing Special and Contractual Termination Benefits",
"label": "Defined Benefit And Contribution Plan, Cost of Providing Special and Contractual Termination Benefits",
"terseLabel": "Incremental expense related to acceptance of voluntary early retirement packages"
}
}
},
"localname": "DefinedBenefitAndContributionPlanCostOfProvidingSpecialAndContractualTerminationBenefits",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostInterestCostDiscountRate": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Defined Benefit Plan, Assumptions Used Calculating Net Periodic Benefit Cost, Interest Cost, Discount Rate",
"label": "Defined Benefit Plan, Assumptions Used Calculating Net Periodic Benefit Cost, Interest Cost, Discount Rate",
"terseLabel": "Discount rate \u2013 interest cost"
}
}
},
"localname": "DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostInterestCostDiscountRate",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"mdt_DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostServiceCostDiscountRate": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Defined Benefit Plan, Assumptions Used Calculating Net Periodic Benefit Cost, Service Cost, Discount Rate",
"label": "Defined Benefit Plan, Assumptions Used Calculating Net Periodic Benefit Cost, Service Cost, Discount Rate",
"terseLabel": "Discount rate \u2013 service cost"
}
}
},
"localname": "DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostServiceCostDiscountRate",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"mdt_DefinedBenefitPlanCurtailmentsandSettlementsBenefitObligation": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Defined Benefit Plan, Curtailments and Settlements, Benefit Obligation",
"label": "Defined Benefit Plan, Curtailments and Settlements, Benefit Obligation",
"negatedLabel": "Plan curtailments and settlements",
"terseLabel": "Curtailment benefits recognized"
}
}
},
"localname": "DefinedBenefitPlanCurtailmentsandSettlementsBenefitObligation",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DefinedBenefitPlanExpectedFutureBenefitPayments": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of benefits expected to be paid in years following the latest fiscal year from a defined benefit plan.",
"label": "Defined Benefit Plan, Expected Future Benefit Payments",
"totalLabel": "Total"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureBenefitPayments",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DefinedBenefitPlanInvestmentsEstimatedLiquidationAndRedemptionPeriod": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Defined Benefit Plan Investments Estimated Liquidation and Redemption Period",
"label": "Defined Benefit Plan Investments Estimated Liquidation and Redemption Period",
"terseLabel": "Real asset investments, estimated liquidation and redemption period"
}
}
},
"localname": "DefinedBenefitPlanInvestmentsEstimatedLiquidationAndRedemptionPeriod",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_DefinedBenefitPlanInvestmentsEstimatedLiquidationPeriodMaximum": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Estimated maximum liquidation period for investments.",
"label": "Defined Benefit Plan, Investments, Estimated Liquidation Period, Maximum",
"terseLabel": "Private equity funds, estimated maximum liquidation period"
}
}
},
"localname": "DefinedBenefitPlanInvestmentsEstimatedLiquidationPeriodMaximum",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_DefinedBenefitPlanInvestmentsEstimatedLiquidationPeriodMinimum": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Estimated minimum liquidation period for investments.",
"label": "Defined Benefit Plan, Investments, Estimated Liquidation Period, Minimum",
"terseLabel": "Private equity funds, estimated minimum liquidation period"
}
}
},
"localname": "DefinedBenefitPlanInvestmentsEstimatedLiquidationPeriodMinimum",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_DefinedBenefitPlanInvestmentsUnfundedCommitments": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The sum of unfunded commitments for investments that the Company's retirement plans are invested in.",
"label": "Defined Benefit Plan, Investments, Unfunded Commitments",
"terseLabel": "Private equity funds, unfunded commitments"
}
}
},
"localname": "DefinedBenefitPlanInvestmentsUnfundedCommitments",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DefinedBenefitPlanNumberOfNewPlansCreated": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Defined Benefit Plan, Number of New Plans Created",
"label": "Defined Benefit Plan, Number of New Plans Created",
"terseLabel": "Number of new plans created"
}
}
},
"localname": "DefinedBenefitPlanNumberOfNewPlansCreated",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "integerItemType"
},
"mdt_DefinedContributionPlanCostOfProvidingSpecialAndContractualTerminationBenefits": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Defined Contribution Plan, Cost Of Providing Special And Contractual Termination Benefits",
"label": "Defined Contribution Plan, Cost Of Providing Special And Contractual Termination Benefits",
"terseLabel": "Cost of providing special and contractual termination benefits"
}
}
},
"localname": "DefinedContributionPlanCostOfProvidingSpecialAndContractualTerminationBenefits",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DefinedContributionPlanTreasuryBondRateOfGuaranteedRateOfReturn": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Defined Contribution Plan, Treasury Bond Rate of Guaranteed Rate of Return",
"label": "Defined Contribution Plan, Treasury Bond Rate of Guaranteed Rate of Return",
"terseLabel": "Treasury bond rate of guaranteed rate of return"
}
}
},
"localname": "DefinedContributionPlanTreasuryBondRateOfGuaranteedRateOfReturn",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_DerivativeAssetLiabilityAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Derivative Asset (Liability) [Abstract]",
"label": "Derivative Asset (Liability) [Abstract]",
"terseLabel": "Total"
}
}
},
"localname": "DerivativeAssetLiabilityAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"mdt_DerivativeAssetLiabilityFairValueGrossAssetLiability": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_DerivativeFairValueAmountOffsetAgainstCollateralNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Derivative Asset (Liability), Fair Value, Gross Asset (Liability)",
"label": "Derivative Asset (Liability), Fair Value, Gross Asset (Liability)",
"totalLabel": "Gross Amount of Recognized Assets (Liabilities)"
}
}
},
"localname": "DerivativeAssetLiabilityFairValueGrossAssetLiability",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DerivativeAssetsandLiabilitiesNetCollateralObligationtoReturnCash": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_DerivativeFairValueAmountOffsetAgainstCollateralNet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Derivative Assets and Liabilities, Net, Collateral, Obligation to Return Cash",
"label": "Derivative Assets and Liabilities, Net, Collateral, Obligation to Return Cash",
"negatedTotalLabel": "Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted",
"terseLabel": "Net cash collateral received"
}
}
},
"localname": "DerivativeAssetsandLiabilitiesNetCollateralObligationtoReturnCash",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DerivativeAssetsandLiabilitiesNetNotOffsetPolicyElectionDeduction": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DerivativeFairValueAmountOffsetAgainstCollateralNet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Derivative Assets and Liabilities, Net, Not Offset, Policy Election Deduction",
"label": "Derivative Assets and Liabilities, Net, Not Offset, Policy Election Deduction",
"negatedTotalLabel": "Gross Amount Not Offset on the Balance Sheet, Financial Instruments"
}
}
},
"localname": "DerivativeAssetsandLiabilitiesNetNotOffsetPolicyElectionDeduction",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_DiabetesOperatingUnitMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Diabetes Operating Unit",
"label": "Diabetes Operating Unit [Member]",
"terseLabel": "Diabetes",
"verboseLabel": "Diabetes"
}
}
},
"localname": "DiabetesOperatingUnitMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "domainItemType"
},
"mdt_EffectiveIncomeTaxRateReconciliationBaseErosionAndAntiAbuseTaxPercent": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 2.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Effective Income Tax Rate Reconciliation, Base Erosion And Anti-Abuse Tax, Percent",
"label": "Effective Income Tax Rate Reconciliation, Base Erosion And Anti-Abuse Tax, Percent",
"terseLabel": "Base erosion anti-abuse tax"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationBaseErosionAndAntiAbuseTaxPercent",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"mdt_EffectiveIncomeTaxRateReconciliationDeductionForeignDerivedIntangibleIncomePercent": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 8.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": -1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Effective Income Tax Rate Reconciliation, Deduction, Foreign-Derived Intangible Income, Percent",
"label": "Effective Income Tax Rate Reconciliation, Deduction, Foreign-Derived Intangible Income, Percent",
"negatedTerseLabel": "Foreign derived intangible income benefit"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationDeductionForeignDerivedIntangibleIncomePercent",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"mdt_EffectiveIncomeTaxRateReconciliationInterestOnUncertainTaxPositionsPercent": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 3.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Effective Income Tax Rate Reconciliation, Interest On Uncertain Tax Positions, Percent",
"label": "Effective Income Tax Rate Reconciliation, Interest On Uncertain Tax Positions, Percent",
"terseLabel": "Interest on uncertain tax positions"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationInterestOnUncertainTaxPositionsPercent",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"mdt_EffectiveIncomeTaxRateReconciliationNondeductibleExpensePermanentReinvestmentAssertion": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Effective Income Tax Rate Reconciliation, Nondeductible Expense, Permanent Reinvestment Assertion",
"label": "Effective Income Tax Rate Reconciliation, Nondeductible Expense, Permanent Reinvestment Assertion",
"terseLabel": "Tax expense related to internal restructuring and intercompany sale of assets"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationNondeductibleExpensePermanentReinvestmentAssertion",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_EffectiveIncomeTaxRateReconciliationPuertoRicoExciseTax": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 1.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": -1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "The portion of the difference between the effective income tax rate and domestic federal statutory income tax rate attributable to Puerto Rico excise tax from intercompany purchases.",
"label": "Effective Income Tax Rate Reconciliation, Puerto Rico Excise Tax",
"negatedTerseLabel": "Puerto Rico excise tax"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationPuertoRicoExciseTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"mdt_EffectiveIncomeTaxRateReconciliationTaxBasisAdjustmentOfIntangibleAssetsAmount": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Effective Income Tax Rate Reconciliation, Tax Basis Adjustment of Intangible Assets, Amount",
"label": "Effective Income Tax Rate Reconciliation, Tax Basis Adjustment of Intangible Assets, Amount",
"terseLabel": "Tax expense (benefit) related to tax basis adjustment, intercompany intellectual property transactions"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationTaxBasisAdjustmentOfIntangibleAssetsAmount",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_EmployeesStockPurchasePlanMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Employees Stock Purchase Plan [Member]",
"terseLabel": "Employee stock purchase plan"
}
}
},
"localname": "EmployeesStockPurchasePlanMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"mdt_EnterpriseExcellenceMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Enterprise Excellence [Member]",
"label": "Enterprise Excellence [Member]",
"terseLabel": "Enterprise Excellence"
}
}
},
"localname": "EnterpriseExcellenceMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"mdt_EquityInvestmentsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Equity Investments [Member]",
"label": "Equity Investments [Member]",
"verboseLabel": "Equity Investments"
}
}
},
"localname": "EquityInvestmentsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails",
"http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestments": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Equity Securities, FV-NI, Equity Securities Without Readily Determinable Fair Value, and Equity Method Investments",
"label": "Equity Securities, FV-NI, Equity Securities Without Readily Determinable Fair Value, and Equity Method Investments",
"totalLabel": "Total equity and other investments"
}
}
},
"localname": "EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestments",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestmentsTableTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Equity Securities, FV-NI, Equity Securities Without Readily Determinable Fair Value, and Equity Method Investments [Table Text Block]",
"label": "Equity Securities, FV-NI, Equity Securities Without Readily Determinable Fair Value, and Equity Method Investments [Table Text Block]",
"terseLabel": "Schedule of Equity and Other Investments"
}
}
},
"localname": "EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestmentsTableTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsTables"
],
"xbrltype": "textBlockItemType"
},
"mdt_FairValueMeasurementsRetirementBenefitPlanAssetsTableTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure related to retirement benefit plan assets that are measured at fair value in periods after initial recognition. Disclosures include, but are not limited to: (a) the fair value measurements recorded and the reasons for the measurements and (b) the level within the fair value hierarchy in which the fair value measurements are categorized in their entirety (levels 1, 2, 3) as well as transfers between levels 1 and 2.",
"label": "Fair Value Measurements, Retirement Benefit Plan Assets [Table Text Block]",
"terseLabel": "Fair Value Measurements, Retirement Benefit Plan Assets"
}
}
},
"localname": "FairValueMeasurementsRetirementBenefitPlanAssetsTableTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"mdt_FairValueRetirementBenefitPlanAssetsUnobservableInputReconciliationTableTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the fair value measurement of retirement benefit plan assets using significant unobservable inputs (Level 3), a reconciliation of the beginning and ending balances, separately presenting changes during the period attributable to the following: (1) total gains or losses for the period (realized and unrealized), segregating those gains or losses included in earnings (or changes in net assets) and gains or losses recognized in other comprehensive income (loss), and a description of where those gains or losses included in earnings (or changes in net assets) are reported in the statement of income (or activities); (2) purchases, sales, issues, and settlements (each type disclosed separately); and (3) transfers in and transfers out of Level 3 (for example, transfers due to changes in the observability of significant inputs), by class of asset.",
"label": "Fair Value, Retirement Benefit Plan Assets, Unobservable Input Reconciliation [Table Text Block]",
"terseLabel": "Schedule of Retirement Benefit Plan Assets, Unobservable Input Reconciliation"
}
}
},
"localname": "FairValueRetirementBenefitPlanAssetsUnobservableInputReconciliationTableTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"mdt_GainLossfromHedgedFirmCommitmentNotQualifyingasNetInvestmentHedgeNet": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Gain (Loss) from Hedged Firm Commitment Not Qualifying as Net Investment Hedge, Net",
"label": "Gain (Loss) from Hedged Firm Commitment Not Qualifying as Net Investment Hedge, Net",
"terseLabel": "Gains (losses) on firm commitments that no longer qualify as net investment hedges"
}
}
},
"localname": "GainLossfromHedgedFirmCommitmentNotQualifyingasNetInvestmentHedgeNet",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_GainonInvestments": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Gain on Investments",
"label": "Gain on Investments",
"terseLabel": "Gross gains"
}
}
},
"localname": "GainonInvestments",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_HerniaMeshLitigationMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Hernia Mesh Litigation",
"label": "Hernia Mesh Litigation [Member]",
"terseLabel": "Hernia Mesh Litigation"
}
}
},
"localname": "HerniaMeshLitigationMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"mdt_ImpairmentofInProcessResearchandDevelopment": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 5.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Impairment of In Process Research and Development",
"label": "Impairment of In Process Research and Development",
"negatedTerseLabel": "IPR&D charges"
}
}
},
"localname": "ImpairmentofInProcessResearchandDevelopment",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_InProcessResearchDevelopmentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "In Process Research Development",
"label": "In Process Research Development [Member]",
"terseLabel": "In Process Research Development"
}
}
},
"localname": "InProcessResearchDevelopmentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_IncomeTaxesandInterestPaidAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Income Taxes and Interest Paid [Abstract]",
"label": "Income Taxes and Interest Paid [Abstract]",
"terseLabel": "Cash paid for:"
}
}
},
"localname": "IncomeTaxesandInterestPaidAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "stringItemType"
},
"mdt_IncreaseDecreaseInTaxRateAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Increase Decrease In Tax Rate [Abstract]",
"terseLabel": "Increase (decrease) in tax rate resulting from:"
}
}
},
"localname": "IncreaseDecreaseInTaxRateAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "stringItemType"
},
"mdt_IncrementalCommonSharesAttributabletoDilutiveEffectofOtherShares": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/EarningsPerShareDetails": {
"order": 4.0,
"parentTag": "us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Incremental Common Shares Attributable to Dilutive Effect of Other Shares",
"label": "Incremental Common Shares Attributable to Dilutive Effect of Other Shares",
"terseLabel": "Other (in shares)"
}
}
},
"localname": "IncrementalCommonSharesAttributabletoDilutiveEffectofOtherShares",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "sharesItemType"
},
"mdt_InsuranceContractMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Insurance Contract [Member]",
"terseLabel": "Insurance contracts"
}
}
},
"localname": "InsuranceContractMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"mdt_IntersectENTMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Intersect ENT",
"label": "Intersect ENT [Member]",
"terseLabel": "Intersect ENT"
}
}
},
"localname": "IntersectENTMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_InvestmentsInvestmentRedemptionNoticePeriodRange": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "States the range of notice requirement (for example, 30 to 60 days) the entity is required to deliver before it can redeem its investment, or portion thereof, by major category.",
"label": "Investments, Investment Redemption, Notice Period, Range",
"terseLabel": "Range of notice period"
}
}
},
"localname": "InvestmentsInvestmentRedemptionNoticePeriodRange",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_InvestmentsNumberofInvestmentsInProcessofLiquidation": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Investments, Number of Investments In Process of Liquidation",
"label": "Investments, Number of Investments In Process of Liquidation",
"terseLabel": "Number of funds in process of liquidation"
}
}
},
"localname": "InvestmentsNumberofInvestmentsInProcessofLiquidation",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "integerItemType"
},
"mdt_JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Japan, Australia, New Zealand, Korea, Canada, and Western Europe [Member]",
"label": "Japan, Australia, New Zealand, Korea, Canada, and Western Europe [Member]",
"terseLabel": "Non-U.S. Developed Markets"
}
}
},
"localname": "JapanAustraliaNewZealandKoreaCanadaandWesternEuropeMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails"
],
"xbrltype": "domainItemType"
},
"mdt_LineOfCreditFacilityAdditionalBorrowingCapacity": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Additional borrowing capacity under the credit facility without consideration of any current restrictions on the amount that could be borrowed or the amounts currently outstanding under the facility.",
"label": "Line of Credit Facility, Additional Borrowing Capacity",
"terseLabel": "Additional borrowing capacity"
}
}
},
"localname": "LineOfCreditFacilityAdditionalBorrowingCapacity",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_LineOfCreditFacilityLengthOfExtension": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line of Credit Facility, Length of Extension",
"label": "Line of Credit Facility, Length of Extension",
"terseLabel": "Length of extension from maturity date"
}
}
},
"localname": "LineOfCreditFacilityLengthOfExtension",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_LineofCreditNumberofExtensionOptions": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line of Credit, Number of Extension Options",
"label": "Line of Credit, Number of Extension Options",
"terseLabel": "Line of credit, number of extension options"
}
}
},
"localname": "LineofCreditNumberofExtensionOptions",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "integerItemType"
},
"mdt_LossContingencyNumberofManufacturers": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Loss Contingency, Number of Manufacturers",
"label": "Loss Contingency, Number of Manufacturers",
"terseLabel": "Number of manufacturers (in manufacturers)"
}
}
},
"localname": "LossContingencyNumberofManufacturers",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "integerItemType"
},
"mdt_LossContingencyNumberofSubsidiaries": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Loss Contingency, Number of Subsidiaries",
"label": "Loss Contingency, Number of Subsidiaries",
"terseLabel": "Number of subsidiaries which supplied pelvic mesh to manufacturer (in subsidiaries)"
}
}
},
"localname": "LossContingencyNumberofSubsidiaries",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "integerItemType"
},
"mdt_LossonInvestments": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Loss on Investments",
"label": "Loss on Investments",
"negatedTerseLabel": "Gross losses"
}
}
},
"localname": "LossonInvestments",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_MeasurementInputProbabilityofPaymentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Measurement Input, Probability of Payment [Member]",
"label": "Measurement Input, Probability of Payment [Member]",
"terseLabel": "Probability of Payment"
}
}
},
"localname": "MeasurementInputProbabilityofPaymentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MedicalDeviceRegulations": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 4.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Medical Device Regulations",
"label": "Medical Device Regulations",
"negatedTerseLabel": "Medical device regulations"
}
}
},
"localname": "MedicalDeviceRegulations",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_MedicalSurgicalPortfolioMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Medical Surgical Portfolio",
"label": "Medical Surgical Portfolio [Member]",
"terseLabel": "Medical Surgical",
"verboseLabel": "Medical Surgical"
}
}
},
"localname": "MedicalSurgicalPortfolioMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails",
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MedtronicCoreContributionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Medtronic Core Contribution [Member]",
"label": "Medtronic Core Contribution [Member]",
"terseLabel": "Medtronic Core Contribution"
}
}
},
"localname": "MedtronicCoreContributionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MedtronicIncAndCIFSASeniorNotesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Medtronic Inc. And CIFSA Senior Notes",
"label": "Medtronic Inc. And CIFSA Senior Notes [Member]",
"terseLabel": "Medtronic Inc. and CIFSA Senior Notes"
}
}
},
"localname": "MedtronicIncAndCIFSASeniorNotesMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MedtronicIncCIFSAandMedtronicLuxcoSeniorNotesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Medtronic Inc, CIFSA, and Medtronic Luxco Senior Notes [Member]",
"label": "Medtronic Inc, CIFSA, and Medtronic Luxco Senior Notes [Member]",
"terseLabel": "Medtronic Inc, CIFSA, and Medtronic Luxco Senior Notes"
}
}
},
"localname": "MedtronicIncCIFSAandMedtronicLuxcoSeniorNotesMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MedtronicIncSeniorNotesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Medtronic Inc Senior Notes",
"label": "Medtronic Inc Senior Notes [Member]",
"terseLabel": "Medtronic Inc Senior Notes"
}
}
},
"localname": "MedtronicIncSeniorNotesMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MedtronicLuxcoMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Medtronic Luxco [Member]",
"label": "Medtronic Luxco [Member]",
"terseLabel": "Medtronic Luxco"
}
}
},
"localname": "MedtronicLuxcoMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MedtronicLuxcoSeniorNotesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Medtronic Luxco Senior Notes",
"label": "Medtronic Luxco Senior Notes [Member]",
"terseLabel": "Medtronic Luxco Senior Notes"
}
}
},
"localname": "MedtronicLuxcoSeniorNotesMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Middle East, Africa, Latin America, Eastern Europe, and Asia, Excluding Japan and Korea [Member]",
"label": "Middle East, Africa, Latin America, Eastern Europe, and Asia, Excluding Japan and Korea [Member]",
"terseLabel": "Emerging Markets"
}
}
},
"localname": "MiddleEastAfricaLatinAmericaEasternEuropeandAsiaExcludingJapanandKoreaMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails"
],
"xbrltype": "domainItemType"
},
"mdt_NetInvestmentHedgeGainLosstobeReclassifiedWithinTwelveMonths": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Net Investment Hedge Gain (Loss) to be Reclassified Within Twelve Months",
"label": "Net Investment Hedge Gain (Loss) to be Reclassified Within Twelve Months",
"terseLabel": "Net investment hedge unrealized gains or losses to be reclassified over the next twelve months, net of tax"
}
}
},
"localname": "NetInvestmentHedgeGainLosstobeReclassifiedWithinTwelveMonths",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_NeuromodulationDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Neuromodulation Division [Member]",
"label": "Neuromodulation Division [Member]",
"terseLabel": "Neuromodulation"
}
}
},
"localname": "NeuromodulationDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_NeurosciencePortfolioMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Neuroscience Portfolio",
"label": "Neuroscience Portfolio [Member]",
"terseLabel": "Neuroscience",
"verboseLabel": "Neuroscience"
}
}
},
"localname": "NeurosciencePortfolioMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "domainItemType"
},
"mdt_NewCoMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "NewCo",
"label": "NewCo [Member]",
"terseLabel": "NewCo"
}
}
},
"localname": "NewCoMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "domainItemType"
},
"mdt_NumberOfMechanicalCirculatorySupportPatients": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Number Of Mechanical Circulatory Support Patients",
"label": "Number Of Mechanical Circulatory Support Patients",
"terseLabel": "Number of mechanical circulatory support patients"
}
}
},
"localname": "NumberOfMechanicalCirculatorySupportPatients",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "integerItemType"
},
"mdt_OffsettingAssetsandLiabilitiesLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "[Line Items] for Offsetting Assets and Liabilities [Table]",
"label": "Offsetting Assets and Liabilities [Line Items]",
"terseLabel": "Offsetting Assets and Liabilities [Line Items]"
}
}
},
"localname": "OffsettingAssetsandLiabilitiesLineItems",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"mdt_OffsettingAssetsandLiabilitiesTable": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Offsetting Assets and Liabilities [Table]",
"label": "Offsetting Assets and Liabilities [Table]",
"terseLabel": "Offsetting Assets and Liabilities [Table]"
}
}
},
"localname": "OffsettingAssetsandLiabilitiesTable",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"mdt_OrringtonMaineChemicalManufacturingFacilityMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Orrington, Maine Chemical Manufacturing Facility [Member]",
"label": "Orrington, Maine Chemical Manufacturing Facility [Member]",
"terseLabel": "Orrington, Maine Chemical Manufacturing Facility"
}
}
},
"localname": "OrringtonMaineChemicalManufacturingFacilityMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"mdt_OtherAccruedExpensesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Other Accrued Expenses",
"label": "Other Accrued Expenses [Member]",
"terseLabel": "Other accrued expenses"
}
}
},
"localname": "OtherAccruedExpensesMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"mdt_OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossBeforeReclassificationAndTax": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Other Comprehensive Income (Loss), Cash Flow Hedge and Net Investment Hedge, Gain (Loss), before Reclassification and Tax",
"label": "Other Comprehensive Income (Loss), Cash Flow Hedge and Net Investment Hedge, Gain (Loss), before Reclassification and Tax",
"negatedTotalLabel": "Total"
}
}
},
"localname": "OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossBeforeReclassificationAndTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossReclassificationBeforeTax": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossReclassificationBeforeTax",
"label": "OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossReclassificationBeforeTax",
"negatedTotalLabel": "Total"
}
}
},
"localname": "OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossReclassificationBeforeTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansForeignExchangeAdjustment": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 4.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Foreign exchange adjustment to pension and other postretirement benefit plans (gain) loss included in accumulated other comprehensive income.",
"label": "Other Comprehensive Income (Loss), Pension and Other Postretirement Benefit Plans, Foreign Exchange Adjustment",
"terseLabel": "Effect of exchange rates"
}
}
},
"localname": "OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansForeignExchangeAdjustment",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_OtherComprehensiveIncomeLossReclassificationPensionAndOtherPostretirementBenefitPlansNetGainLossRecognizedInNetPeriodicBenefitCostBeforeTax": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 3.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "2012 Element. Before tax amount of the income statement impact of the reclassification adjustment for actuarial gain (loss) recognized as a component of net periodic benefit cost.",
"label": "Other Comprehensive Income (Loss), Reclassification, Pension and Other Postretirement Benefit Plans, Net Gain (Loss) Recognized in Net Periodic Benefit Cost, before Tax",
"negatedTerseLabel": "Amortization and settlement recognition of actuarial loss"
}
}
},
"localname": "OtherComprehensiveIncomeLossReclassificationPensionAndOtherPostretirementBenefitPlansNetGainLossRecognizedInNetPeriodicBenefitCostBeforeTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_OtherComprehensiveIncomeLossUnrealizedGainLossOnDerivativesArisingDuringPeriodNetInvestmentHedgeNetOfTax": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 3.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossNetOfTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Other Comprehensive Income (Loss), Unrealized Gain (Loss) On Derivatives Arising During Period, Net Investment Hedge, Net Of Tax",
"label": "Other Comprehensive Income (Loss), Unrealized Gain (Loss) On Derivatives Arising During Period, Net Investment Hedge, Net Of Tax",
"terseLabel": "Net investment hedge"
}
}
},
"localname": "OtherComprehensiveIncomeLossUnrealizedGainLossOnDerivativesArisingDuringPeriodNetInvestmentHedgeNetOfTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"mdt_OtherNonoperatingIncomeExpenseNetPolicyTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Other Nonoperating Income (Expense), Net [Policy Text Block]",
"label": "Other Nonoperating Income (Expense), Net [Policy Text Block]",
"terseLabel": "Other Non-Operating Income, Net"
}
}
},
"localname": "OtherNonoperatingIncomeExpenseNetPolicyTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"mdt_OtherOperatingIncomeExpenseNetPolicyTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Other Operating Income (Expense), Net [Policy Text Block]",
"label": "Other Operating Income (Expense), Net [Policy Text Block]",
"terseLabel": "Other Operating Expense, Net"
}
}
},
"localname": "OtherOperatingIncomeExpenseNetPolicyTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"mdt_OtherPlanAssetsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Other categories of plan assets not specified in the taxonomy.",
"label": "Other Plan Assets [Member]",
"terseLabel": "Other"
}
}
},
"localname": "OtherPlanAssetsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_PartnershipUnitsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Partnership units include partnerships, private equity investments, and real asset investments.",
"label": "Partnership Units [Member]",
"terseLabel": "Partnership units"
}
}
},
"localname": "PartnershipUnitsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"mdt_PartnershipsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Partnerships primarily include long/short equity and absolute return strategies.",
"label": "Partnerships [Member]",
"terseLabel": "Partnerships"
}
}
},
"localname": "PartnershipsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_PelvicMeshLitigationMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Pelvic Mesh Litigation [Member]",
"label": "Pelvic Mesh Litigation [Member]",
"terseLabel": "Pelvic Mesh Litigation"
}
}
},
"localname": "PelvicMeshLitigationMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"mdt_PerformanceBasedRestrictedStockAwardMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Performance-Based Restricted Stock Award [Member]",
"label": "Performance-Based Restricted Stock Award [Member]",
"terseLabel": "Performance-based restricted stock awards"
}
}
},
"localname": "PerformanceBasedRestrictedStockAwardMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_PersonalInvestmentAccountMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Personal Investment Account [Member]",
"label": "Personal Investment Account [Member]",
"terseLabel": "Personal Investment Account"
}
}
},
"localname": "PersonalInvestmentAccountMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_PreTaxExitandDisposalCostsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Pre Tax Exit and Disposal Costs [Member]",
"label": "Pre Tax Exit and Disposal Costs [Member]",
"terseLabel": "Pre-tax exit and disposal costs"
}
}
},
"localname": "PreTaxExitandDisposalCostsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_ProductDevelopmentAndOtherMilestoneBasedPaymentsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A milestone in achieving certain product development target.",
"label": "Product Development And Other Milestone-Based Payments [Member]",
"terseLabel": "Product development and other milestone-based payments"
}
}
},
"localname": "ProductDevelopmentAndOtherMilestoneBasedPaymentsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_PurchasedTechnologyAndPatentsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Purchased technology and patents.",
"label": "Purchased Technology and Patents [Member]",
"terseLabel": "Purchased technology and patents"
}
}
},
"localname": "PurchasedTechnologyAndPatentsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "domainItemType"
},
"mdt_RealAssetInvestmentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Real asset investment consist of commodities, derivatives, Real Estate Investment Trusts, and illiquid real estate holdings.",
"label": "Real Asset Investment [Member]",
"terseLabel": "Real asset investments"
}
}
},
"localname": "RealAssetInvestmentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_RegisteredInvestmentCompanyMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Registered Investment Company [Member]",
"terseLabel": "Registered investment companies"
}
}
},
"localname": "RegisteredInvestmentCompanyMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"mdt_RespiratoryGastrointestinalAndRenalDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Respiratory, Gastrointestinal, And Renal Division [Member]",
"label": "Respiratory, Gastrointestinal, And Renal Division [Member]",
"terseLabel": "Respiratory, Gastrointestinal, & Renal"
}
}
},
"localname": "RespiratoryGastrointestinalAndRenalDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_RestructuringChargesNetOfRestructuringReversalsIncludingCostOfProductSoldImpact": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 6.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of expenses, net of reversals, associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses related to discontinued operation or an asset retirement obligation.",
"label": "Restructuring Charges, Net of Restructuring Reversals Including Cost of Product Sold Impact",
"terseLabel": "Restructuring charges, net"
}
}
},
"localname": "RestructuringChargesNetOfRestructuringReversalsIncludingCostOfProductSoldImpact",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_RestructuringChargesNetofReversalsIncludingCostofProductSoldImpact": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 15.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Restructuring Charges, Net of Reversals Including Cost of Product Sold Impact",
"label": "Restructuring Charges, Net of Reversals Including Cost of Product Sold Impact",
"negatedLabel": "Restructuring and associated costs"
}
}
},
"localname": "RestructuringChargesNetofReversalsIncludingCostofProductSoldImpact",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_RestructuringWriteDownAndImpairmentProvisions": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 16.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Restructuring Write Down And Impairment Provisions",
"label": "Restructuring Write Down And Impairment Provisions",
"negatedLabel": "MCS impairment / costs",
"terseLabel": "Restructuring write down and impairment provisions"
}
}
},
"localname": "RestructuringWriteDownAndImpairmentProvisions",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_RevenueAndOtherPerformanceBasedPaymentsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A milestone in achieving certain revenue target.",
"label": "Revenue And Other Performance-Based Payments [Member]",
"terseLabel": "Revenue and other performance-based payments"
}
}
},
"localname": "RevenueAndOtherPerformanceBasedPaymentsMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_ScheduleOfDebtTable": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule Of Debt",
"label": "Schedule Of Debt [Table]",
"terseLabel": "Schedule Of Debt [Table]"
}
}
},
"localname": "ScheduleOfDebtTable",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "stringItemType"
},
"mdt_ScheduleOfShareBasedCompensationEmployeeStockPurchasePlanAveragePurchasePrice": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Average purchase price of stock purchased under employee stock purchase plan.",
"label": "Schedule of Share-based Compensation, Employee Stock Purchase Plan, Average Purchase Price",
"terseLabel": "Average purchase price (in dollars per share)"
}
}
},
"localname": "ScheduleOfShareBasedCompensationEmployeeStockPurchasePlanAveragePurchasePrice",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "perShareItemType"
},
"mdt_ScheduleOfShareBasedCompensationEmployeeStockPurchasePlanSharesAvailableForFuturePurchase": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Number of shares available for future purchase under employee stock purchase plan.",
"label": "Schedule of Share-based Compensation, Employee Stock Purchase Plan, Shares Available for Future Purchase",
"terseLabel": "Shares available for future purchase (in shares)"
}
}
},
"localname": "ScheduleOfShareBasedCompensationEmployeeStockPurchasePlanSharesAvailableForFuturePurchase",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "sharesItemType"
},
"mdt_ScheduleOfShareBasedCompensationEmployeeStockPurchasePlanSharesPurchased": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Number of shares by employees under employee stock purchase plans.",
"label": "Schedule of Share-based Compensation, Employee Stock Purchase Plan, Shares Purchased",
"terseLabel": "Shares purchased by employees (in shares)"
}
}
},
"localname": "ScheduleOfShareBasedCompensationEmployeeStockPurchasePlanSharesPurchased",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "sharesItemType"
},
"mdt_ScheduleofInvestmentsTableTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule of Investments [Table Text Block]",
"label": "Schedule of Investments [Table Text Block]",
"terseLabel": "Schedule of Activity Related to the Company's Equity and Other Investments Portfolio"
}
}
},
"localname": "ScheduleofInvestmentsTableTextBlock",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsTables"
],
"xbrltype": "textBlockItemType"
},
"mdt_SeniorNotes2009Due2039Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "2039 Senior Notes - unsecured debt not collateralized by pledge, mortgage or other lien in the entity's assets.",
"label": "Senior Notes 2009 Due 2039 [Member]",
"terseLabel": "6.500 percent thirty-year 2009 senior notes"
}
}
},
"localname": "SeniorNotes2009Due2039Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2010Due2040Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "2040 Senior Notes - unsecured debt not collateralized by pledge, mortgage or other lien in the entity's assets.",
"label": "Senior Notes 2010 Due 2040 [Member]",
"terseLabel": "5.550 percent thirty-year 2010 senior notes"
}
}
},
"localname": "SeniorNotes2010Due2040Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2012Due2042Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "2042 Senior Notes - unsecured debt not collateralized by pledge, mortgage or other lien in the entity's assets.",
"label": "Senior Notes 2012 Due 2042 [Member]",
"terseLabel": "4.500 percent thirty-year 2012 senior notes"
}
}
},
"localname": "SeniorNotes2012Due2042Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2013Due2043Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "2043 Senior Notes - unsecured debt not collateralized by pledge, mortgage or other lien in the entity's assets.",
"label": "Senior Notes 2013 Due 2043 [Member]",
"terseLabel": "4.000 percent thirty-year 2013 senior notes"
}
}
},
"localname": "SeniorNotes2013Due2043Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2014Due2044Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "2044 Senior Notes - unsecured debt not collateralized by pledge, mortgage or other lien in the entity's assets.",
"label": "Senior Notes 2014 Due 2044 [Member]",
"terseLabel": "4.625 percent thirty-year 2014 senior notes"
}
}
},
"localname": "SeniorNotes2014Due2044Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2015Due2025Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2015 Due 2025 [Member]",
"label": "Senior Notes 2015 Due 2025 [Member]",
"terseLabel": "3.500 percent ten-year 2015 senior notes"
}
}
},
"localname": "SeniorNotes2015Due2025Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2015Due2035Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2015 Due 2035 [Member]",
"label": "Senior Notes 2015 Due 2035 [Member]",
"terseLabel": "4.375 percent twenty-year 2015 senior notes"
}
}
},
"localname": "SeniorNotes2015Due2035Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2015Due2045Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2015 Due 2045 [Member]",
"label": "Senior Notes 2015 Due 2045 [Member]",
"terseLabel": "4.625 percent thirty-year 2015 senior notes"
}
}
},
"localname": "SeniorNotes2015Due2045Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2017Due2027Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2017 Due 2027 [Member]",
"label": "Senior Notes 2017 Due 2027 [Member]",
"terseLabel": "3.350 percent ten-year 2017 senior notes"
}
}
},
"localname": "SeniorNotes2017Due2027Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2021FloatingMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2021 Floating [Member]",
"label": "Senior Notes 2019 Due 2021 Floating [Member]",
"terseLabel": "0.375 percent four-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2021FloatingMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2021Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2021 [Member]",
"label": "Senior Notes 2019 Due 2021 [Member]",
"terseLabel": "0.000 percent three-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2021Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2022Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2022 [Member]",
"label": "Senior Notes 2019 Due 2022 [Member]",
"terseLabel": "0.00% Senior Notes due 2022"
}
}
},
"localname": "SeniorNotes2019Due2022Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due20230.000PercentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2023, 0.000 Percent [Member]",
"label": "Senior Notes 2019 Due 2023, 0.000 Percent [Member]",
"terseLabel": "0.000 percent three-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due20230.000PercentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due20230.375PercentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2023, 0.375 Percent [Member]",
"label": "Senior Notes 2019 Due 2023, 0.375 Percent [Member]",
"terseLabel": "0.375% Senior Notes due 2023"
}
}
},
"localname": "SeniorNotes2019Due20230.375PercentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2023Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2023 [Member]",
"label": "Senior Notes 2019 Due 2023 [Member]",
"terseLabel": "0.375 percent four-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2023Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2025Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2025 [Member]",
"label": "Senior Notes 2019 Due 2025 [Member]",
"terseLabel": "0.25% Senior Notes due 2025"
}
}
},
"localname": "SeniorNotes2019Due2025Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2026Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2026 [Member]",
"label": "Senior Notes 2019 Due 2026 [Member]",
"terseLabel": "0.250 percent six-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2026Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2027Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2027 [Member]",
"label": "Senior Notes 2019 Due 2027 [Member]",
"terseLabel": "1.125 percent eight-year 2019 senior notes",
"verboseLabel": "1.125% Senior Notes due 2027"
}
}
},
"localname": "SeniorNotes2019Due2027Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due20311.00PercentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2031, 1.00 Percent [Member]",
"label": "Senior Notes 2019 Due 2031, 1.00 Percent [Member]",
"terseLabel": "1.00% Senior Notes due 2031"
}
}
},
"localname": "SeniorNotes2019Due20311.00PercentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due20311.625PercentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2031, 1.625 Percent [Member]",
"label": "Senior Notes 2019 Due 2031, 1.625 Percent [Member]",
"terseLabel": "1.625% Senior Notes due 2031"
}
}
},
"localname": "SeniorNotes2019Due20311.625PercentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2031Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2031 [Member]",
"label": "Senior Notes 2019 Due 2031 [Member]",
"terseLabel": "1.625 percent twelve-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2031Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2032Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2032 [Member]",
"label": "Senior Notes 2019 Due 2032 [Member]",
"terseLabel": "1.000 percent twelve-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2032Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due20391.50PercentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2039, 1.50 Percent [Member]",
"label": "Senior Notes 2019 Due 2039, 1.50 Percent [Member]",
"terseLabel": "1.50% Senior Notes due 2039"
}
}
},
"localname": "SeniorNotes2019Due20391.50PercentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due20392.250PercentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2039, 2.250 Percent [Member]",
"label": "Senior Notes 2019 Due 2039, 2.250 Percent [Member]",
"terseLabel": "2.250% Senior Notes due 2039"
}
}
},
"localname": "SeniorNotes2019Due20392.250PercentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2039Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2039 [Member]",
"label": "Senior Notes 2019 Due 2039 [Member]",
"terseLabel": "2.250 percent twenty-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2039Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2040Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2040 [Member]",
"label": "Senior Notes 2019 Due 2040 [Member]",
"terseLabel": "1.500 percent twenty-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2040Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2049Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2049 [Member]",
"label": "Senior Notes 2019 Due 2049 [Member]",
"terseLabel": "1.75% Senior Notes due 2049"
}
}
},
"localname": "SeniorNotes2019Due2049Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Due2050Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 Due 2050 [Member]",
"label": "Senior Notes 2019 Due 2050 [Member]",
"terseLabel": "1.750 percent thirty-year 2019 senior notes"
}
}
},
"localname": "SeniorNotes2019Due2050Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2019Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2019 [Member]",
"label": "Senior Notes 2019 [Member]",
"terseLabel": "2019 Senior Notes"
}
}
},
"localname": "SeniorNotes2019Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due20230000PercentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2023, 0.000 Percent",
"label": "Senior Notes 2020 Due 2023, 0.000 Percent [Member]",
"terseLabel": "0.000 percent two-year 2020 senior notes"
}
}
},
"localname": "SeniorNotes2020Due20230000PercentMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2023Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2023",
"label": "Senior Notes 2020 Due 2023 [Member]",
"terseLabel": "0.000% Senior Notes due 2023",
"verboseLabel": "0.000 percent two-year 2020 senior notes"
}
}
},
"localname": "SeniorNotes2020Due2023Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2025Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2025",
"label": "Senior Notes 2020 Due 2025 [Member]",
"terseLabel": "0.000% Senior Notes due 2025"
}
}
},
"localname": "SeniorNotes2020Due2025Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2026Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2026",
"label": "Senior Notes 2020 Due 2026 [Member]",
"terseLabel": "0.000 percent five-year 2020 senior notes"
}
}
},
"localname": "SeniorNotes2020Due2026Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2028Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2028",
"label": "Senior Notes 2020 Due 2028 [Member]",
"terseLabel": "0.375% Senior Notes due 2028"
}
}
},
"localname": "SeniorNotes2020Due2028Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2029Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2029",
"label": "Senior Notes 2020 Due 2029 [Member]",
"terseLabel": "0.375 percent eight-year 2020 senior notes"
}
}
},
"localname": "SeniorNotes2020Due2029Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2032Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2032",
"label": "Senior Notes 2020 Due 2032 [Member]",
"terseLabel": "0.750% Senior Notes due 2032"
}
}
},
"localname": "SeniorNotes2020Due2032Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2033Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2033",
"label": "Senior Notes 2020 Due 2033 [Member]",
"terseLabel": "0.750 percent twelve-year 2020 senior notes"
}
}
},
"localname": "SeniorNotes2020Due2033Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2040Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2040",
"label": "Senior Notes 2020 Due 2040 [Member]",
"terseLabel": "1.375% Senior Notes due 2040"
}
}
},
"localname": "SeniorNotes2020Due2040Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2041Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2041",
"label": "Senior Notes 2020 Due 2041 [Member]",
"terseLabel": "1.375 percent twenty-year 2020 senior notes"
}
}
},
"localname": "SeniorNotes2020Due2041Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2050Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2050",
"label": "Senior Notes 2020 Due 2050 [Member]",
"terseLabel": "1.625% Senior Notes due 2050"
}
}
},
"localname": "SeniorNotes2020Due2050Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Due2051Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020 Due 2051",
"label": "Senior Notes 2020 Due 2051 [Member]",
"terseLabel": "1.625 percent thirty-year 2020 senior notes"
}
}
},
"localname": "SeniorNotes2020Due2051Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotes2020Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes 2020",
"label": "Senior Notes 2020 [Member]",
"terseLabel": "Senior Notes 2020"
}
}
},
"localname": "SeniorNotes2020Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SeniorNotesCIFSA2007Due2038Member": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Senior Notes CIFSA 2007 Due 2038 [Member]",
"label": "Senior Notes CIFSA 2007 Due 2038 [Member]",
"terseLabel": "6.550 percent thirty-year 2007 CIFSA senior notes"
}
}
},
"localname": "SeniorNotesCIFSA2007Due2038Member",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"mdt_ShortTermDebtWeightedAverageOriginalMaturity": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "The weighted average original maturity of short-term debt outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.",
"label": "Short-term debt, Weighted Average Original Maturity",
"terseLabel": "Weighted average original maturity"
}
}
},
"localname": "ShortTermDebtWeightedAverageOriginalMaturity",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"mdt_SimplificationRestructuringProgramMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Simplification Restructuring Program",
"label": "Simplification Restructuring Program [Member]",
"terseLabel": "Simplification"
}
}
},
"localname": "SimplificationRestructuringProgramMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SiteContingencyNumberofLandfillsRequiringCapping": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Site Contingency, Number of Landfills Requiring Capping",
"label": "Site Contingency, Number of Landfills Requiring Capping",
"terseLabel": "Number of landfills requiring capping (in landfills)"
}
}
},
"localname": "SiteContingencyNumberofLandfillsRequiringCapping",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "integerItemType"
},
"mdt_SiteContingencyNumberofLandfillsRequiringRemoval": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Site Contingency, Number of Landfills Requiring Removal",
"label": "Site Contingency, Number of Landfills Requiring Removal",
"terseLabel": "Number of landfills requiring removal (in landfills)"
}
}
},
"localname": "SiteContingencyNumberofLandfillsRequiringRemoval",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "integerItemType"
},
"mdt_SpecialChargesGains": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 1.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Special Charges (Gains)",
"label": "Special Charges (Gains)",
"negatedTerseLabel": "Contribution to Medtronic Foundation"
}
}
},
"localname": "SpecialChargesGains",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_SpecialtyTherapiesDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Specialty Therapies Division [Member]",
"label": "Specialty Therapies Division [Member]",
"terseLabel": "Specialty Therapies"
}
}
},
"localname": "SpecialtyTherapiesDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_StockRepurchaseProgramAveragePurchasePrice": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Average purchase price of common shares under a stock repurchase plan.",
"label": "Stock Repurchase Program, Average Purchase Price",
"terseLabel": "Average repurchase price (in dollars per share)"
}
}
},
"localname": "StockRepurchaseProgramAveragePurchasePrice",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "perShareItemType"
},
"mdt_StructuralHeartAndAorticDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Structural Heart And Aortic Division [Member]",
"label": "Structural Heart And Aortic Division [Member]",
"terseLabel": "Structural Heart & Aortic"
}
}
},
"localname": "StructuralHeartAndAorticDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_SurgicalInnovationsDivisionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Surgical Innovations Division [Member]",
"label": "Surgical Innovations Division [Member]",
"terseLabel": "Surgical Innovations"
}
}
},
"localname": "SurgicalInnovationsDivisionMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_TaxExpenseBenefitAssociatedWithSaleofIntellectualProperty": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Tax Expense (Benefit) Associated With Sale of Intellectual Property",
"label": "Tax Expense (Benefit) Associated With Sale of Intellectual Property",
"negatedLabel": "Tax benefit from intercompany sale of intellectual property"
}
}
},
"localname": "TaxExpenseBenefitAssociatedWithSaleofIntellectualProperty",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_TokyoInterBankOfferedRateTIBORMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tokyo Inter-bank Offered Rate (TIBOR)",
"label": "Tokyo Inter-bank Offered Rate (TIBOR) [Member]",
"terseLabel": "TIBOR Rate"
}
}
},
"localname": "TokyoInterBankOfferedRateTIBORMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_TotalOtherCountriesExcludingIrelandMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Total Other Countries, Excluding Ireland [Member]",
"label": "Total Other Countries, Excluding Ireland [Member]",
"terseLabel": "Total other countries, excluding Ireland"
}
}
},
"localname": "TotalOtherCountriesExcludingIrelandMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_TotalOtherCountriesExcludingUnitedStatesandIrelandMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Total Other Countries, Excluding United States and Ireland [Member]",
"label": "Total Other Countries, Excluding United States and Ireland [Member]",
"terseLabel": "Rest of world"
}
}
},
"localname": "TotalOtherCountriesExcludingUnitedStatesandIrelandMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "domainItemType"
},
"mdt_TwoThousandTwentyOneStockAwardAndIncentivePlanMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Two Thousand Twenty One Stock Award and Incentive Plan",
"label": "Two Thousand Twenty One Stock Award and Incentive Plan [Member]",
"terseLabel": "2021 Plan"
}
}
},
"localname": "TwoThousandTwentyOneStockAwardAndIncentivePlanMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_TwoThousandandThirteenStockAwardandIncentivePlanMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Two Thousand and Thirteen Stock Award and Incentive Plan",
"label": "Two Thousand and Thirteen Stock Award and Incentive Plan [Member]",
"verboseLabel": "2013 Plan"
}
}
},
"localname": "TwoThousandandThirteenStockAwardandIncentivePlanMember",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"mdt_UnrealizedGainLossOnInterestRateCashFlowHedgesAccumulatedOtherComprehensiveIncomeLossNetOfTax": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of unrealized gain (loss), net of tax as of the balance sheet date, related to the increase or decrease in fair value of interest rate derivatives designated as cash flow hedging instruments, which was recorded in accumulated other comprehensive income to the extent that the cash flow hedge was determined to be effective.",
"label": "Unrealized Gain (Loss) on Interest Rate Cash Flow Hedges, Accumulated Other Comprehensive Income (Loss), Net of Tax",
"terseLabel": "After-tax net unrealized gains (losses)"
}
}
},
"localname": "UnrealizedGainLossOnInterestRateCashFlowHedgesAccumulatedOtherComprehensiveIncomeLossNetOfTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_UnrealizedGainLossonNetInvestmentHedgesAccumulatedOtherComprehensiveIncomeLossNetofTax": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Unrealized Gain (Loss) on Net Investment Hedges, Accumulated Other Comprehensive Income (Loss), Net of Tax",
"label": "Unrealized Gain (Loss) on Net Investment Hedges, Accumulated Other Comprehensive Income (Loss), Net of Tax",
"verboseLabel": "After-tax net unrealized gains (losses) associated with net investment hedging instruments recorded in AOCI"
}
}
},
"localname": "UnrealizedGainLossonNetInvestmentHedgesAccumulatedOtherComprehensiveIncomeLossNetofTax",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"mdt_UnrecognizedTaxBenefitsGrossDecreasesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Unrecognized Tax Benefits Gross Decreases [Abstract]",
"terseLabel": "Gross decreases:"
}
}
},
"localname": "UnrecognizedTaxBenefitsGrossDecreasesAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "stringItemType"
},
"mdt_UnrecognizedTaxBenefitsGrossIncreasesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "-- None. No documentation exists for this element. --",
"label": "Unrecognized Tax Benefits Gross Increases [Abstract]",
"terseLabel": "Gross increases:"
}
}
},
"localname": "UnrecognizedTaxBenefitsGrossIncreasesAbstract",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "stringItemType"
},
"mdt_UnrecognizedTaxBenefitsNetofCashAdvancePaid": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Unrecognized Tax Benefits, Net of Cash Advance Paid",
"label": "Unrecognized Tax Benefits, Net of Cash Advance Paid",
"terseLabel": "Gross unrecognized tax benefits at end of fiscal year, net of cash advance"
}
}
},
"localname": "UnrecognizedTaxBenefitsNetofCashAdvancePaid",
"nsuri": "http://www.medtronic.com/20220429",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"srt_ConsolidatedEntitiesAxis": {
"auth_ref": [
"r170",
"r404",
"r409",
"r417",
"r758",
"r759",
"r766",
"r767",
"r884",
"r1026",
"r1046",
"r1057",
"r1066",
"r1067"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by consolidated entity or group of entities.",
"label": "Consolidated Entities [Axis]",
"terseLabel": "Consolidated Entities [Axis]"
}
}
},
"localname": "ConsolidatedEntitiesAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"srt_ConsolidatedEntitiesDomain": {
"auth_ref": [
"r170",
"r404",
"r409",
"r417",
"r758",
"r759",
"r766",
"r767",
"r884",
"r1026",
"r1046",
"r1057",
"r1066",
"r1067"
],
"lang": {
"en-us": {
"role": {
"documentation": "Entity or group of entities consolidated into reporting entity.",
"label": "Consolidated Entities [Domain]",
"terseLabel": "Consolidated Entities [Domain]"
}
}
},
"localname": "ConsolidatedEntitiesDomain",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"srt_ConsolidationItemsAxis": {
"auth_ref": [
"r170",
"r234",
"r247",
"r248",
"r249",
"r250",
"r252",
"r254",
"r258",
"r404",
"r405",
"r406",
"r407",
"r408",
"r409",
"r411",
"r412",
"r414",
"r416",
"r417",
"r1057",
"r1058",
"r1059",
"r1060",
"r1061",
"r1062",
"r1063",
"r1064",
"r1065",
"r1066",
"r1067"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by components, eliminations, non-segment corporate-level activity and reconciling items used in consolidating a parent entity and its subsidiaries or its operating segments.",
"label": "Consolidation Items [Axis]",
"terseLabel": "Consolidation Items [Axis]"
}
}
},
"localname": "ConsolidationItemsAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "stringItemType"
},
"srt_ConsolidationItemsDomain": {
"auth_ref": [
"r170",
"r234",
"r247",
"r248",
"r249",
"r250",
"r252",
"r254",
"r258",
"r404",
"r405",
"r406",
"r407",
"r408",
"r409",
"r411",
"r412",
"r414",
"r416",
"r417",
"r1057",
"r1058",
"r1059",
"r1060",
"r1061",
"r1062",
"r1063",
"r1064",
"r1065",
"r1066",
"r1067"
],
"lang": {
"en-us": {
"role": {
"documentation": "Components, elimination, non-segment corporate-level activity and reconciling items used in consolidating a parent entity and its subsidiaries or its operating segments.",
"label": "Consolidation Items [Domain]",
"terseLabel": "Consolidation Items [Domain]"
}
}
},
"localname": "ConsolidationItemsDomain",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "domainItemType"
},
"srt_CumulativeEffectPeriodOfAdoptionAdjustmentMember": {
"auth_ref": [
"r2",
"r183",
"r191",
"r197",
"r307",
"r669",
"r670",
"r671",
"r718",
"r719",
"r810",
"r813",
"r815",
"r816",
"r1071"
],
"lang": {
"en-us": {
"role": {
"documentation": "Increase (decrease) to financial statements for cumulative-effect adjustment in period of adoption of amendment to accounting standards.",
"label": "Cumulative Effect, Period of Adoption, Adjustment [Member]",
"terseLabel": "Cumulative effect of change in accounting principle"
}
}
},
"localname": "CumulativeEffectPeriodOfAdoptionAdjustmentMember",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"srt_CumulativeEffectPeriodOfAdoptionAxis": {
"auth_ref": [
"r2",
"r183",
"r191",
"r197",
"r307",
"r669",
"r670",
"r671",
"r718",
"r719",
"r810",
"r813",
"r815",
"r816",
"r1071"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by cumulative-effect adjustment to financial statements in period of adoption of amendment to accounting standards.",
"label": "Cumulative Effect, Period of Adoption [Axis]",
"terseLabel": "Cumulative Effect, Period of Adoption [Axis]"
}
}
},
"localname": "CumulativeEffectPeriodOfAdoptionAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "stringItemType"
},
"srt_CumulativeEffectPeriodOfAdoptionDomain": {
"auth_ref": [
"r2",
"r183",
"r191",
"r197",
"r307",
"r669",
"r670",
"r671",
"r718",
"r719",
"r810",
"r813",
"r815",
"r816",
"r1071"
],
"lang": {
"en-us": {
"role": {
"documentation": "Cumulative-effect adjustment to financial statements in period of adoption of amendment to accounting standards.",
"label": "Cumulative Effect, Period of Adoption [Domain]",
"terseLabel": "Cumulative Effect, Period of Adoption [Domain]"
}
}
},
"localname": "CumulativeEffectPeriodOfAdoptionDomain",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"srt_LitigationCaseAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of judicial proceeding, alternative dispute resolution or claim.",
"label": "Litigation Case [Axis]",
"terseLabel": "Litigation Case [Axis]"
}
}
},
"localname": "LitigationCaseAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "stringItemType"
},
"srt_LitigationCaseTypeDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Judicial proceeding, alternative dispute resolution or claim. For example, but not limited to, name of case, category of litigation, or other differentiating information.",
"label": "Litigation Case [Domain]",
"terseLabel": "Litigation Case Type [Domain]"
}
}
},
"localname": "LitigationCaseTypeDomain",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"srt_MaximumMember": {
"auth_ref": [
"r422",
"r462",
"r615",
"r625",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r923",
"r988",
"r991",
"r1027",
"r1028"
],
"lang": {
"en-us": {
"role": {
"documentation": "Upper limit of the provided range.",
"label": "Maximum [Member]",
"terseLabel": "Maximum"
}
}
},
"localname": "MaximumMember",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "domainItemType"
},
"srt_MinimumMember": {
"auth_ref": [
"r422",
"r462",
"r615",
"r625",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r923",
"r988",
"r991",
"r1027",
"r1028"
],
"lang": {
"en-us": {
"role": {
"documentation": "Lower limit of the provided range.",
"label": "Minimum [Member]",
"terseLabel": "Minimum"
}
}
},
"localname": "MinimumMember",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "domainItemType"
},
"srt_ProductOrServiceAxis": {
"auth_ref": [
"r261",
"r505",
"r510",
"r925",
"r987",
"r989"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by product and service, or group of similar products and similar services.",
"label": "Product and Service [Axis]",
"terseLabel": "Product and Service [Axis]"
}
}
},
"localname": "ProductOrServiceAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "stringItemType"
},
"srt_ProductsAndServicesDomain": {
"auth_ref": [
"r261",
"r505",
"r510",
"r925",
"r987",
"r989"
],
"lang": {
"en-us": {
"role": {
"documentation": "Product or service, or a group of similar products or similar services.",
"label": "Product and Service [Domain]",
"terseLabel": "Product and Service [Domain]"
}
}
},
"localname": "ProductsAndServicesDomain",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "domainItemType"
},
"srt_RangeAxis": {
"auth_ref": [
"r422",
"r462",
"r548",
"r615",
"r625",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r923",
"r988",
"r991",
"r1027",
"r1028"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by statistical measurement. Includes, but is not limited to, minimum, maximum, weighted average, arithmetic average, and median.",
"label": "Statistical Measurement [Axis]",
"terseLabel": "Statistical Measurement [Axis]"
}
}
},
"localname": "RangeAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "stringItemType"
},
"srt_RangeMember": {
"auth_ref": [
"r422",
"r462",
"r548",
"r615",
"r625",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r923",
"r988",
"r991",
"r1027",
"r1028"
],
"lang": {
"en-us": {
"role": {
"documentation": "Statistical measurement. Includes, but is not limited to, minimum, maximum, weighted average, arithmetic average, and median.",
"label": "Statistical Measurement [Domain]",
"terseLabel": "Statistical Measurement [Domain]"
}
}
},
"localname": "RangeMember",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "domainItemType"
},
"srt_ScenarioUnspecifiedDomain": {
"auth_ref": [
"r192",
"r197",
"r620"
],
"lang": {
"en-us": {
"role": {
"documentation": "Scenario reported, distinguishing information from actual fact. Includes, but is not limited to, pro forma and forecast. Excludes actual facts.",
"label": "Scenario [Domain]",
"terseLabel": "Scenario [Domain]"
}
}
},
"localname": "ScenarioUnspecifiedDomain",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"srt_ScheduleOfValuationAndQualifyingAccountsDisclosureTextBlock": {
"auth_ref": [
"r178",
"r1056"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for valuation and qualifying accounts and reserves.",
"label": "SEC Schedule, 12-09, Schedule of Valuation and Qualifying Accounts Disclosure [Text Block]",
"terseLabel": "Schedule II - Valuation and Qualifying Accounts"
}
}
},
"localname": "ScheduleOfValuationAndQualifyingAccountsDisclosureTextBlock",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleII"
],
"xbrltype": "textBlockItemType"
},
"srt_SegmentGeographicalDomain": {
"auth_ref": [
"r262",
"r263",
"r505",
"r511",
"r990",
"r1017",
"r1018",
"r1019",
"r1020",
"r1021",
"r1022",
"r1023",
"r1024",
"r1025",
"r1045",
"r1048",
"r1049",
"r1050",
"r1051",
"r1052",
"r1053",
"r1054",
"r1055"
],
"lang": {
"en-us": {
"role": {
"documentation": "Geographical area.",
"label": "Geographical [Domain]",
"terseLabel": "Geographical [Domain]"
}
}
},
"localname": "SegmentGeographicalDomain",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "domainItemType"
},
"srt_StatementGeographicalAxis": {
"auth_ref": [
"r262",
"r263",
"r505",
"r511",
"r990",
"r1011",
"r1017",
"r1018",
"r1019",
"r1020",
"r1021",
"r1022",
"r1023",
"r1024",
"r1025",
"r1045",
"r1047"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by geographical components.",
"label": "Geographical [Axis]",
"terseLabel": "Geographical [Axis]"
}
}
},
"localname": "StatementGeographicalAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "stringItemType"
},
"srt_StatementScenarioAxis": {
"auth_ref": [
"r192",
"r197",
"r386",
"r620",
"r890"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by scenario reported, distinguishing information from actual fact. Includes, but is not limited to, pro forma and forecast. Excludes actual facts.",
"label": "Scenario [Axis]",
"terseLabel": "Scenario [Axis]"
}
}
},
"localname": "StatementScenarioAxis",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"srt_SubsidiariesMember": {
"auth_ref": [
"r576",
"r881",
"r882",
"r883"
],
"lang": {
"en-us": {
"role": {
"documentation": "Entity owned or controlled by another entity.",
"label": "Subsidiaries [Member]",
"terseLabel": "Subsidiaries"
}
}
},
"localname": "SubsidiariesMember",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"srt_ValuationAndQualifyingAccountsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "SEC Schedule, 12-09, Valuation and Qualifying Accounts [Abstract]",
"terseLabel": "SEC Schedule, 12-09, Valuation and Qualifying Accounts [Abstract]"
}
}
},
"localname": "ValuationAndQualifyingAccountsAbstract",
"nsuri": "http://fasb.org/srt/2021-01-31",
"xbrltype": "stringItemType"
},
"srt_ValuationAndQualifyingAccountsDisclosureLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "SEC Schedule, 12-09, Valuation and Qualifying Accounts Disclosure [Line Items]",
"terseLabel": "SEC Schedule, 12-09, Valuation and Qualifying Accounts Disclosure [Line Items]"
}
}
},
"localname": "ValuationAndQualifyingAccountsDisclosureLineItems",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "stringItemType"
},
"srt_ValuationAndQualifyingAccountsDisclosureTable": {
"auth_ref": [
"r171",
"r172",
"r173",
"r176",
"r177",
"r1056"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of valuation and qualifying accounts and reserves.",
"label": "SEC Schedule, 12-09, Valuation and Qualifying Accounts Disclosure [Table]",
"terseLabel": "SEC Schedule, 12-09, Valuation and Qualifying Accounts Disclosure [Table]"
}
}
},
"localname": "ValuationAndQualifyingAccountsDisclosureTable",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "stringItemType"
},
"srt_WeightedAverageMember": {
"auth_ref": [
"r898",
"r900",
"r903",
"r1027",
"r1028"
],
"lang": {
"en-us": {
"role": {
"documentation": "Average of a range of values, calculated with consideration of proportional relevance.",
"label": "Weighted Average [Member]",
"terseLabel": "Weighted Average"
}
}
},
"localname": "WeightedAverageMember",
"nsuri": "http://fasb.org/srt/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AOCIAttributableToParentNetOfTaxRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "AOCI Attributable to Parent, Net of Tax [Roll Forward]",
"terseLabel": "AOCI Attributable to Parent, Net of Tax [Roll Forward]"
}
}
},
"localname": "AOCIAttributableToParentNetOfTaxRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AccountingPoliciesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Accounting Policies [Abstract]",
"terseLabel": "Accounting Policies [Abstract]"
}
}
},
"localname": "AccountingPoliciesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_AccountingStandardsUpdateExtensibleList": {
"auth_ref": [
"r0",
"r1",
"r2",
"r3",
"r4",
"r184",
"r185",
"r186",
"r187",
"r277",
"r278",
"r304",
"r305",
"r306",
"r307",
"r308",
"r309",
"r403",
"r665",
"r666",
"r667",
"r668",
"r669",
"r670",
"r671",
"r672",
"r718",
"r719",
"r807",
"r808",
"r809",
"r810",
"r811",
"r812",
"r813",
"r814",
"r815",
"r816",
"r817",
"r836",
"r837",
"r838",
"r839",
"r840",
"r841",
"r842",
"r843",
"r880",
"r992",
"r993",
"r994",
"r995",
"r996",
"r997",
"r998",
"r999",
"r1000",
"r1001",
"r1002",
"r1003",
"r1069",
"r1070",
"r1071",
"r1072",
"r1073"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicates amendment to accounting standards.",
"label": "Accounting Standards Update [Extensible Enumeration]",
"terseLabel": "Accounting Standards Update [Extensible Enumeration]"
}
}
},
"localname": "AccountingStandardsUpdateExtensibleList",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "enumerationSetItemType"
},
"us-gaap_AccountsPayableCurrent": {
"auth_ref": [
"r55",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 3.0,
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer).",
"label": "Accounts Payable, Current",
"terseLabel": "Accounts payable"
}
}
},
"localname": "AccountsPayableCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AccountsReceivableMember": {
"auth_ref": [
"r1012"
],
"lang": {
"en-us": {
"role": {
"documentation": "Due from customers or clients for goods or services that have been delivered or sold.",
"label": "Accounts Receivable [Member]",
"terseLabel": "Reduction of accounts receivable"
}
}
},
"localname": "AccountsReceivableMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AccountsReceivableNetCurrent": {
"auth_ref": [
"r10",
"r34",
"r267",
"r268"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 3.0,
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current.",
"label": "Accounts Receivable, after Allowance for Credit Loss, Current",
"terseLabel": "Accounts receivable, less allowances and credit losses of $230 and $241, respectively"
}
}
},
"localname": "AccountsReceivableNetCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AccruedIncomeTaxesCurrent": {
"auth_ref": [
"r26",
"r936",
"r969"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 5.0,
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount as of the balance sheet date of the unpaid sum of the known and estimated amounts payable to satisfy all currently due domestic and foreign income tax obligations.",
"label": "Accrued Income Taxes, Current",
"terseLabel": "Accrued income taxes"
}
}
},
"localname": "AccruedIncomeTaxesCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AccruedIncomeTaxesNoncurrent": {
"auth_ref": [
"r28",
"r936",
"r969"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 2.0,
"parentTag": "us-gaap_Liabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount as of the balance sheet date of the unpaid sum of the known and estimated amounts payable to satisfy all domestic and foreign income tax obligations due beyond one year or the operating cycle, whichever is longer. Alternate captions include income taxes payable, noncurrent.",
"label": "Accrued Income Taxes, Noncurrent",
"terseLabel": "Accrued income taxes"
}
}
},
"localname": "AccruedIncomeTaxesNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AccumulatedDefinedBenefitPlansAdjustmentMember": {
"auth_ref": [
"r94",
"r100",
"r110",
"r111",
"r112",
"r765"
],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated other comprehensive (income) loss related to defined benefit plans attributable to the parent.",
"label": "Accumulated Defined Benefit Plans Adjustment Attributable to Parent [Member]",
"terseLabel": "Net Change in Retirement Obligations"
}
}
},
"localname": "AccumulatedDefinedBenefitPlansAdjustmentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AccumulatedGainLossNetCashFlowHedgeParentMember": {
"auth_ref": [
"r100",
"r110",
"r111",
"r112",
"r113",
"r764"
],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated other comprehensive income (loss) from gain (loss) of derivative instrument designated and qualifying as cash flow hedge included in assessment of hedge effectiveness, attributable to parent.",
"label": "Accumulated Gain (Loss), Net, Cash Flow Hedge, Parent [Member]",
"terseLabel": "Unrealized (Loss) Gain on Cash Flow Hedges"
}
}
},
"localname": "AccumulatedGainLossNetCashFlowHedgeParentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember": {
"auth_ref": [
"r89",
"r100",
"r764"
],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated other comprehensive income (loss) resulting from gain (loss) from derivative instruments designated and qualifying as the effective portion of cash flow hedges, attributable to the parent.",
"label": "Accumulated Net Gain (Loss) from Cash Flow Hedges Attributable to Parent [Member]",
"terseLabel": "Unrealized (Loss) Gain on Cash Flow Hedges"
}
}
},
"localname": "AccumulatedNetGainLossFromDesignatedOrQualifyingCashFlowHedgesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AccumulatedNetUnrealizedInvestmentGainLossMember": {
"auth_ref": [
"r90",
"r91",
"r92",
"r100",
"r110",
"r111",
"r112"
],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated unrealized gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), attributable to parent.",
"label": "AOCI, Accumulated Gain (Loss), Debt Securities, Available-for-sale, Parent [Member]",
"terseLabel": "Unrealized (Loss) Gain on Investment Securities"
}
}
},
"localname": "AccumulatedNetUnrealizedInvestmentGainLossMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AccumulatedOtherComprehensiveIncomeLossLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Accumulated Other Comprehensive Income (Loss) [Line Items]",
"terseLabel": "Accumulated Other Comprehensive Income (Loss) [Line Items]"
}
}
},
"localname": "AccumulatedOtherComprehensiveIncomeLossLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax": {
"auth_ref": [
"r37",
"r97",
"r99",
"r100",
"r972",
"r999",
"r1003"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 4.0,
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated change in equity from transactions and other events and circumstances from non-owner sources, net of tax effect, at period end. Excludes Net Income (Loss), and accumulated changes in equity from transactions resulting from investments by owners and distributions to owners. Includes foreign currency translation items, certain pension adjustments, unrealized gains and losses on certain investments in debt and equity securities, other than temporary impairment (OTTI) losses related to factors other than credit losses on available-for-sale and held-to-maturity debt securities that an entity does not intend to sell and it is not more likely than not that the entity will be required to sell before recovery of the amortized cost basis, as well as changes in the fair value of derivatives related to the effective portion of a designated cash flow hedge.",
"label": "Accumulated Other Comprehensive Income (Loss), Net of Tax",
"terseLabel": "Accumulated other comprehensive loss"
}
}
},
"localname": "AccumulatedOtherComprehensiveIncomeLossNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails",
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AccumulatedOtherComprehensiveIncomeLossTable": {
"auth_ref": [
"r110",
"r111",
"r848",
"r849",
"r850",
"r851",
"r852",
"r854"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about components of accumulated other comprehensive income (loss).",
"label": "Accumulated Other Comprehensive Income (Loss) [Table]",
"terseLabel": "Accumulated Other Comprehensive Income (Loss) [Table]"
}
}
},
"localname": "AccumulatedOtherComprehensiveIncomeLossTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AccumulatedOtherComprehensiveIncomeMember": {
"auth_ref": [
"r96",
"r100",
"r110",
"r111",
"r112",
"r180",
"r181",
"r182",
"r765",
"r994",
"r995",
"r1073"
],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated increase (decrease) in equity from transactions and other events and circumstances from non-owner sources, attributable to the parent. Excludes net income (loss), and accumulated changes in equity from transactions resulting from investments by owners and distributions to owners.",
"label": "AOCI Attributable to Parent [Member]",
"terseLabel": "Accumulated Other Comprehensive Loss",
"verboseLabel": "Total Accumulated Other Comprehensive (Loss) Income"
}
}
},
"localname": "AccumulatedOtherComprehensiveIncomeMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails",
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"us-gaap_AccumulatedTranslationAdjustmentMember": {
"auth_ref": [
"r87",
"r100",
"r110",
"r111",
"r112",
"r765",
"r849",
"r850",
"r851",
"r852",
"r854"
],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated other comprehensive income (loss) resulting from foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature, attributable to the parent.",
"label": "Accumulated Foreign Currency Adjustment Attributable to Parent [Member]",
"terseLabel": "Cumulative Translation Adjustments"
}
}
},
"localname": "AccumulatedTranslationAdjustmentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AdditionalPaidInCapital": {
"auth_ref": [
"r35",
"r672",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 2.0,
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock.",
"label": "Additional Paid in Capital",
"terseLabel": "Additional paid-in capital"
}
}
},
"localname": "AdditionalPaidInCapital",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AdditionalPaidInCapitalMember": {
"auth_ref": [
"r180",
"r181",
"r182",
"r669",
"r670",
"r671",
"r815"
],
"lang": {
"en-us": {
"role": {
"documentation": "Excess of issue price over par or stated value of the entity's capital stock and amounts received from other transactions involving the entity's stock or stockholders.",
"label": "Additional Paid-in Capital [Member]",
"terseLabel": "Additional Paid-in Capital"
}
}
},
"localname": "AdditionalPaidInCapitalMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"us-gaap_AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue": {
"auth_ref": [
"r627",
"r629",
"r675",
"r676"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase to additional paid-in capital (APIC) for recognition of cost for award under share-based payment arrangement.",
"label": "APIC, Share-based Payment Arrangement, Increase for Cost Recognition",
"terseLabel": "Stock-based compensation"
}
}
},
"localname": "AdjustmentsToAdditionalPaidInCapitalSharebasedCompensationRequisiteServicePeriodRecognitionValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Adjustments to Reconcile Net Income (Loss) to Cash Provided by (Used in) Operating Activities [Abstract]",
"terseLabel": "Adjustments to reconcile net income to net cash provided by operating activities:"
}
}
},
"localname": "AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "stringItemType"
},
"us-gaap_AllocatedShareBasedCompensationExpense": {
"auth_ref": [
"r629",
"r661",
"r674"
],
"calculation": {
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails": {
"order": 1.0,
"parentTag": "us-gaap_AllocatedShareBasedCompensationExpenseNetOfTax",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of expense for award under share-based payment arrangement. Excludes amount capitalized.",
"label": "Share-based Payment Arrangement, Expense",
"verboseLabel": "Total stock-based compensation expense"
}
}
},
"localname": "AllocatedShareBasedCompensationExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AllocatedShareBasedCompensationExpenseNetOfTax": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after tax, of expense for award under share-based payment arrangement.",
"label": "Share-based Payment Arrangement, Expense, after Tax",
"totalLabel": "Total stock-based compensation expense, net of tax"
}
}
},
"localname": "AllocatedShareBasedCompensationExpenseNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AllowanceForCreditLossMember": {
"auth_ref": [
"r171",
"r172",
"r173",
"r176",
"r177"
],
"lang": {
"en-us": {
"role": {
"documentation": "Allowance for credit loss from right to consideration in exchange for good or service transferred to customer when right is conditioned on something other than passage of time.",
"label": "SEC Schedule, 12-09, Allowance, Credit Loss [Member]",
"terseLabel": "Allowance for Doubtful Accounts"
}
}
},
"localname": "AllowanceForCreditLossMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AllowanceForDoubtfulAccountsReceivableCurrent": {
"auth_ref": [
"r41",
"r273",
"r310"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of allowance for credit loss on accounts receivable, classified as current.",
"label": "Accounts Receivable, Allowance for Credit Loss, Current",
"terseLabel": "Allowances and credit losses"
}
}
},
"localname": "AllowanceForDoubtfulAccountsReceivableCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheetsParenthetical"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AmortizationOfIntangibleAssets": {
"auth_ref": [
"r148",
"r341",
"r349"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 5.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": -1.0
},
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 3.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The aggregate expense charged against earnings to allocate the cost of intangible assets (nonphysical assets not used in production) in a systematic and rational manner to the periods expected to benefit from such assets. As a noncash expense, this element is added back to net income when calculating cash provided by or used in operations using the indirect method.",
"label": "Amortization of Intangible Assets",
"negatedTerseLabel": "Amortization of intangible assets",
"netLabel": "Amortization expense",
"terseLabel": "Amortization of intangible assets"
}
}
},
"localname": "AmortizationOfIntangibleAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsAmortizationExpenseDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount": {
"auth_ref": [
"r211"
],
"lang": {
"en-us": {
"role": {
"documentation": "Securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented.",
"label": "Antidilutive Securities Excluded from Computation of Earnings Per Share, Amount",
"terseLabel": "Number of shares excluded from computation of earnings per share (in shares)"
}
}
},
"localname": "AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis": {
"auth_ref": [
"r211"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of antidilutive security.",
"label": "Antidilutive Securities [Axis]",
"terseLabel": "Antidilutive Securities [Axis]"
}
}
},
"localname": "AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items]",
"terseLabel": "Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items]"
}
}
},
"localname": "AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AntidilutiveSecuritiesNameDomain": {
"auth_ref": [
"r211"
],
"lang": {
"en-us": {
"role": {
"documentation": "Incremental common shares attributable to securities that were not included in diluted earnings per share (EPS) because to do so would increase EPS amounts or decrease loss per share amounts for the period presented.",
"label": "Antidilutive Securities, Name [Domain]",
"terseLabel": "Antidilutive Securities, Name [Domain]"
}
}
},
"localname": "AntidilutiveSecuritiesNameDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AociDerivativeQualifyingAsHedgeExcludedComponentParentMember": {
"auth_ref": [
"r100",
"r110",
"r111",
"r112",
"r805"
],
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated other comprehensive income (loss) from increase (decrease) in value of excluded component of derivative hedge, attributable to parent.",
"label": "AOCI, Derivative Qualifying as Hedge, Excluded Component, Parent [Member]",
"terseLabel": "Net Investment Hedges"
}
}
},
"localname": "AociDerivativeQualifyingAsHedgeExcludedComponentParentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AssetAcquisitionAxis": {
"auth_ref": [
"r746"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by asset acquisition.",
"label": "Asset Acquisition [Axis]",
"terseLabel": "Asset Acquisition [Axis]"
}
}
},
"localname": "AssetAcquisitionAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AssetAcquisitionConsiderationTransferred": {
"auth_ref": [
"r747",
"r748",
"r749",
"r750"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of consideration transferred in asset acquisition. Includes, but is not limited to, cash, liability incurred by acquirer, and equity interest issued by acquirer.",
"label": "Asset Acquisition, Consideration Transferred",
"terseLabel": "Acquired in-process research & development"
}
}
},
"localname": "AssetAcquisitionConsiderationTransferred",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AssetAcquisitionDomain": {
"auth_ref": [
"r746"
],
"lang": {
"en-us": {
"role": {
"documentation": "Asset acquisition.",
"label": "Asset Acquisition [Domain]",
"terseLabel": "Asset Acquisition [Domain]"
}
}
},
"localname": "AssetAcquisitionDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AssetBackedSecuritiesMember": {
"auth_ref": [
"r291",
"r549"
],
"lang": {
"en-us": {
"role": {
"documentation": "Securities that are primarily serviced by the cash flows of a discrete pool of receivables or other financial assets for example, but not limited to, credit card receivables, car loans, recreational vehicle loans, and mobile home loans.",
"label": "Asset-backed Securities [Member]",
"terseLabel": "Other asset-backed securities"
}
}
},
"localname": "AssetBackedSecuritiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AssetImpairmentCharges": {
"auth_ref": [
"r148",
"r356"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 10.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0
},
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 8.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of write-down of assets recognized in the income statement. Includes, but is not limited to, losses from tangible assets, intangible assets and goodwill.",
"label": "Asset Impairment Charges",
"negatedTerseLabel": "Impairment charges",
"terseLabel": "Asset impairment charges"
}
}
},
"localname": "AssetImpairmentCharges",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_Assets": {
"auth_ref": [
"r163",
"r242",
"r249",
"r256",
"r303",
"r404",
"r405",
"r406",
"r408",
"r409",
"r410",
"r411",
"r413",
"r415",
"r417",
"r418",
"r758",
"r766",
"r835",
"r885",
"r887",
"r934",
"r968"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Sum of the carrying amounts as of the balance sheet date of all assets that are recognized. Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events.",
"label": "Assets",
"terseLabel": "Total Assets",
"totalLabel": "Total assets"
}
}
},
"localname": "Assets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AssetsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Assets [Abstract]",
"terseLabel": "ASSETS"
}
}
},
"localname": "AssetsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "stringItemType"
},
"us-gaap_AssetsCurrent": {
"auth_ref": [
"r12",
"r14",
"r73",
"r163",
"r303",
"r404",
"r405",
"r406",
"r408",
"r409",
"r410",
"r411",
"r413",
"r415",
"r417",
"r418",
"r758",
"r766",
"r835",
"r885",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 5.0,
"parentTag": "us-gaap_Assets",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Sum of the carrying amounts as of the balance sheet date of all assets that are expected to be realized in cash, sold, or consumed within one year (or the normal operating cycle, if longer). Assets are probable future economic benefits obtained or controlled by an entity as a result of past transactions or events.",
"label": "Assets, Current",
"totalLabel": "Total current assets"
}
}
},
"localname": "AssetsCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AssetsCurrentAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Assets, Current [Abstract]",
"terseLabel": "Current assets:"
}
}
},
"localname": "AssetsCurrentAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "stringItemType"
},
"us-gaap_AuctionRateSecuritiesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Debt instrument securities (for example, but not limited to, corporate or municipal bonds) that typically have long-term nominal maturities for which the interest rate is reset through an auction process.",
"label": "Auction Rate Securities [Member]",
"terseLabel": "Auction rate securities"
}
}
},
"localname": "AuctionRateSecuritiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax": {
"auth_ref": [
"r284"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails": {
"order": 1.0,
"parentTag": "us-gaap_DebtSecuritiesAvailableForSaleAmortizedCostExcludingAccruedInterestAfterAllowanceForCreditLoss",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of unrealized gain in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-sale, Accumulated Gross Unrealized Gain, before Tax",
"terseLabel": "Unrealized Gains"
}
}
},
"localname": "AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax": {
"auth_ref": [
"r285"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails": {
"order": 2.0,
"parentTag": "us-gaap_DebtSecuritiesAvailableForSaleAmortizedCostExcludingAccruedInterestAfterAllowanceForCreditLoss",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of unrealized loss in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-sale, Accumulated Gross Unrealized Loss, before Tax",
"negatedTerseLabel": "Unrealized Losses"
}
}
},
"localname": "AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AvailableForSaleSecuritiesContinuousUnrealizedLossPositionAccumulatedLossAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Debt Securities, Available-for-sale, Unrealized Loss Position, Accumulated Loss [Abstract]",
"terseLabel": "Unrealized Losses"
}
}
},
"localname": "AvailableForSaleSecuritiesContinuousUnrealizedLossPositionAccumulatedLossAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AvailableForSaleSecuritiesDebtMaturitiesAfterFiveThroughTenYearsFairValue": {
"auth_ref": [
"r286",
"r289",
"r957"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_AvailableForSaleSecuritiesDebtSecurities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), with single maturity date and allocated without single maturity date, maturing in sixth through tenth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Debt Securities, Available-for-Sale, Fair Value, Maturity, Allocated and Single Maturity Date, after Year 5 Through 10",
"terseLabel": "Due after five years through ten years"
}
}
},
"localname": "AvailableForSaleSecuritiesDebtMaturitiesAfterFiveThroughTenYearsFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AvailableForSaleSecuritiesDebtMaturitiesAfterOneThroughFiveYearsFairValue": {
"auth_ref": [
"r286",
"r288",
"r956"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_AvailableForSaleSecuritiesDebtSecurities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), with single maturity date and allocated without single maturity date, maturing in second through fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Debt Securities, Available-for-Sale, Fair Value, Maturity, Allocated and Single Maturity Date, after Year One Through Five",
"terseLabel": "Due after one year through five years"
}
}
},
"localname": "AvailableForSaleSecuritiesDebtMaturitiesAfterOneThroughFiveYearsFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AvailableForSaleSecuritiesDebtMaturitiesAfterTenYearsFairValue": {
"auth_ref": [
"r286",
"r290",
"r958"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails": {
"order": 4.0,
"parentTag": "us-gaap_AvailableForSaleSecuritiesDebtSecurities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), with single maturity date and allocated without single maturity date, maturing after tenth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Debt Securities, Available-for-Sale, Fair Value, Maturity, Allocated and Single Maturity Date, after Year 10",
"terseLabel": "Due after ten years"
}
}
},
"localname": "AvailableForSaleSecuritiesDebtMaturitiesAfterTenYearsFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AvailableForSaleSecuritiesDebtMaturitiesFairValueAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Debt Securities, Available-for-sale, Fair Value, Fiscal Year Maturity [Abstract]",
"terseLabel": "AFS Debt Maturities"
}
}
},
"localname": "AvailableForSaleSecuritiesDebtMaturitiesFairValueAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AvailableForSaleSecuritiesDebtMaturitiesWithinOneYearFairValue": {
"auth_ref": [
"r286",
"r287",
"r955"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_AvailableForSaleSecuritiesDebtSecurities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), with single maturity date and allocated without single maturity date, maturing in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Debt Securities, Available-for-Sale, Fair Value, Maturity, Allocated and Single Maturity Date, Year One",
"terseLabel": "Due in one year or less"
}
}
},
"localname": "AvailableForSaleSecuritiesDebtMaturitiesWithinOneYearFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AvailableForSaleSecuritiesDebtSecurities": {
"auth_ref": [
"r279",
"r283",
"r317",
"r943"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails": {
"order": 3.0,
"parentTag": "us-gaap_DebtSecuritiesAvailableForSaleAmortizedCostExcludingAccruedInterestAfterAllowanceForCreditLoss",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-sale",
"terseLabel": "Fair Value",
"totalLabel": "Total debt securities"
}
}
},
"localname": "AvailableForSaleSecuritiesDebtSecurities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_AvailableForSaleSecuritiesGrossRealizedGainLossAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Debt Securities, Available-for-sale, Realized Gain (Loss) [Abstract]",
"terseLabel": "AFS Gross Realized Gain (Loss)"
}
}
},
"localname": "AvailableForSaleSecuritiesGrossRealizedGainLossAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_AwardTypeAxis": {
"auth_ref": [
"r631",
"r663"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of award under share-based payment arrangement.",
"label": "Award Type [Axis]",
"terseLabel": "Award Type [Axis]"
}
}
},
"localname": "AwardTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_BalanceSheetLocationAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by location on balance sheet (statement of financial position).",
"label": "Balance Sheet Location [Axis]",
"terseLabel": "Balance Sheet Location [Axis]"
}
}
},
"localname": "BalanceSheetLocationAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails",
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_BalanceSheetLocationDomain": {
"auth_ref": [
"r780",
"r785"
],
"lang": {
"en-us": {
"role": {
"documentation": "Location in the balance sheet (statement of financial position).",
"label": "Balance Sheet Location [Domain]",
"terseLabel": "Balance Sheet Location [Domain]"
}
}
},
"localname": "BalanceSheetLocationDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails",
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_BusinessAcquisitionAcquireeDomain": {
"auth_ref": [
"r607",
"r621"
],
"lang": {
"en-us": {
"role": {
"documentation": "Identification of the acquiree in a material business combination (or series of individually immaterial business combinations), which may include the name or other type of identification of the acquiree.",
"label": "Business Acquisition, Acquiree [Domain]",
"terseLabel": "Business Acquisition, Acquiree [Domain]"
}
}
},
"localname": "BusinessAcquisitionAcquireeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_BusinessAcquisitionAxis": {
"auth_ref": [
"r607",
"r621",
"r734",
"r735"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by business combination or series of individually immaterial business combinations.",
"label": "Business Acquisition [Axis]",
"terseLabel": "Business Acquisition [Axis]"
}
}
},
"localname": "BusinessAcquisitionAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_BusinessAcquisitionCostOfAcquiredEntityTransactionCosts": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of direct costs of the business combination including legal, accounting, and other costs incurred to consummate the business acquisition.",
"label": "Business Acquisition, Transaction Costs",
"terseLabel": "Total consideration"
}
}
},
"localname": "BusinessAcquisitionCostOfAcquiredEntityTransactionCosts",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessAcquisitionLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Business Acquisition [Line Items]",
"terseLabel": "Business Acquisition [Line Items]"
}
}
},
"localname": "BusinessAcquisitionLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_BusinessAcquisitionSharePrice": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Price of a single share of a number of saleable stocks paid or offered to be paid in a business combination.",
"label": "Business Acquisition, Share Price",
"terseLabel": "Acquisition of outstanding shares (in dollars per share)"
}
}
},
"localname": "BusinessAcquisitionSharePrice",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_BusinessCombinationAndAssetAcquisitionAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Business Combination and Asset Acquisition [Abstract]"
}
}
},
"localname": "BusinessCombinationAndAssetAcquisitionAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_BusinessCombinationContingentConsiderationArrangementsChangeInAmountOfContingentConsiderationLiability1": {
"auth_ref": [
"r147",
"r745"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase (decrease) in the value of a contingent consideration liability, including, but not limited to, differences arising upon settlement.",
"label": "Business Combination, Contingent Consideration Arrangements, Change in Amount of Contingent Consideration, Liability",
"negatedTerseLabel": "Change in fair value"
}
}
},
"localname": "BusinessCombinationContingentConsiderationArrangementsChangeInAmountOfContingentConsiderationLiability1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessCombinationContingentConsiderationLiability": {
"auth_ref": [
"r742",
"r743",
"r744"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liability recognized arising from contingent consideration in a business combination.",
"label": "Business Combination, Contingent Consideration, Liability",
"periodEndLabel": "Ending Balance",
"periodStartLabel": "Beginning Balance",
"terseLabel": "Contingent consideration",
"verboseLabel": "Fair value of contingent consideration"
}
}
},
"localname": "BusinessCombinationContingentConsiderationLiability",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessCombinationContingentConsiderationLiabilityMeasurementInput": {
"auth_ref": [
"r825"
],
"lang": {
"en-us": {
"role": {
"documentation": "Value of input used to measure contingent consideration liability from business combination.",
"label": "Business Combination, Contingent Consideration, Liability, Measurement Input",
"terseLabel": "Contingent consideration, significant unobservable inputs"
}
}
},
"localname": "BusinessCombinationContingentConsiderationLiabilityMeasurementInput",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "decimalItemType"
},
"us-gaap_BusinessCombinationDisclosureTextBlock": {
"auth_ref": [
"r751"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for a business combination (or series of individually immaterial business combinations) completed during the period, including background, timing, and recognized assets and liabilities. The disclosure may include leverage buyout transactions (as applicable).",
"label": "Business Combination Disclosure [Text Block]",
"terseLabel": "Acquisitions"
}
}
},
"localname": "BusinessCombinationDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/Acquisitions"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_BusinessCombinationProvisionalInformationInitialAccountingIncompleteAdjustmentsRelatedToPreviousPeriod": {
"auth_ref": [
"r738"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase (decrease) in income that would have been recognized in previous periods if the adjustments to provisional amounts were recognized as of the acquisition date.",
"label": "Business Combination, Provisional Information, Initial Accounting Incomplete, Adjustments Related to Previous Period",
"terseLabel": "Gain related to change in amounts accrued for certain contingent liabilities for recent acquisition"
}
}
},
"localname": "BusinessCombinationProvisionalInformationInitialAccountingIncompleteAdjustmentsRelatedToPreviousPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedContingentLiability": {
"auth_ref": [
"r736",
"r737"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount of liability arising from an inherited contingency (as defined) which has been recognized as of the acquisition date.",
"label": "Business Combination, Recognized Identifiable Assets Acquired and Liabilities Assumed, Contingent Liability",
"terseLabel": "Contingent consideration liabilities"
}
}
},
"localname": "BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedContingentLiability",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedIntangibles": {
"auth_ref": [
"r736",
"r737"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount of identifiable intangible assets recognized as of the acquisition date.",
"label": "Business Combination, Recognized Identifiable Assets Acquired and Liabilities Assumed, Finite-Lived Intangibles",
"terseLabel": "Intangible assets acquired"
}
}
},
"localname": "BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedIntangibles",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedLiabilities": {
"auth_ref": [
"r737"
],
"calculation": {
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails": {
"order": 2.0,
"parentTag": "us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredGoodwillAndLiabilitiesAssumedNet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liabilities assumed at the acquisition date.",
"label": "Business Combination, Recognized Identifiable Assets Acquired and Liabilities Assumed, Liabilities",
"terseLabel": "Liabilities assumed"
}
}
},
"localname": "BusinessCombinationRecognizedIdentifiableAssetsAcquiredAndLiabilitiesAssumedLiabilities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessCombinationRecognizedIdentifiableAssetsAcquiredGoodwillAndLiabilitiesAssumedNet": {
"auth_ref": [
"r737"
],
"calculation": {
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount recognized for assets, including goodwill, in excess of (less than) the aggregate liabilities assumed.",
"label": "Business Combination, Recognized Identifiable Assets Acquired, Goodwill, and Liabilities Assumed, Net",
"totalLabel": "Fair value of net assets acquired"
}
}
},
"localname": "BusinessCombinationRecognizedIdentifiableAssetsAcquiredGoodwillAndLiabilitiesAssumedNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_BusinessExitCosts1": {
"auth_ref": [
"r148"
],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 6.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of expenses associated with exit or disposal activities pursuant to an authorized plan. Includes, but is not limited to, one-time termination benefits, termination of an operating lease or other contract, consolidating or closing facilities, and relocating employees, and termination benefits associated with an ongoing benefit arrangement. Excludes expenses associated with special or contractual termination benefits, a discontinued operation or an asset retirement obligation.",
"label": "Business Exit Costs",
"negatedTerseLabel": "Exit of businesses",
"terseLabel": "Business exit costs"
}
}
},
"localname": "BusinessExitCosts1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CashAndCashEquivalentsAtCarryingValue": {
"auth_ref": [
"r8",
"r49",
"r150"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 2.0,
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation.",
"label": "Cash and Cash Equivalents, at Carrying Value",
"terseLabel": "Cash and cash equivalents"
}
}
},
"localname": "CashAndCashEquivalentsAtCarryingValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CashAndCashEquivalentsPolicyTextBlock": {
"auth_ref": [
"r19",
"r152"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value.",
"label": "Cash and Cash Equivalents, Policy [Policy Text Block]",
"terseLabel": "Cash Equivalents"
}
}
},
"localname": "CashAndCashEquivalentsPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents": {
"auth_ref": [
"r144",
"r150",
"r154"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage. Excludes amount for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates.",
"label": "Cash, Cash Equivalents, Restricted Cash and Restricted Cash Equivalents",
"periodEndLabel": "Cash and cash equivalents at end of period",
"periodStartLabel": "Cash and cash equivalents at beginning of period"
}
}
},
"localname": "CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect": {
"auth_ref": [
"r144",
"r846"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates.",
"label": "Cash, Cash Equivalents, Restricted Cash and Restricted Cash Equivalents, Period Increase (Decrease), Including Exchange Rate Effect",
"totalLabel": "Net change in cash and cash equivalents"
}
}
},
"localname": "CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CashFlowHedgeGainLossToBeReclassifiedWithinTwelveMonths": {
"auth_ref": [
"r804"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The estimated net amount of existing gains or losses on cash flow hedges at the reporting date expected to be reclassified to earnings within the next 12 months.",
"label": "Cash Flow Hedge Gain (Loss) to be Reclassified within Twelve Months",
"terseLabel": "Cash flow hedge unrealized gains to be reclassified over the next twelve months"
}
}
},
"localname": "CashFlowHedgeGainLossToBeReclassifiedWithinTwelveMonths",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CashFlowHedgingMember": {
"auth_ref": [
"r776"
],
"lang": {
"en-us": {
"role": {
"documentation": "Hedge of the exposure to variability in the cash flows of a recognized asset or liability, or of a forecasted transaction, that is attributable to a particular risk.",
"label": "Cash Flow Hedging [Member]",
"terseLabel": "Cash flow hedges"
}
}
},
"localname": "CashFlowHedgingMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_CertificatesOfDepositMember": {
"auth_ref": [
"r960"
],
"lang": {
"en-us": {
"role": {
"documentation": "Short to medium-term investment available at banks and savings and loan institutions where a customer agrees to lend money to the institution for a certain amount of time and is paid a predetermined rate of interest. Certificates of deposit (CD) are typically Federal Deposit Insurance Corporation (FDIC) insured.",
"label": "Certificates of Deposit [Member]",
"terseLabel": "Certificates of deposit"
}
}
},
"localname": "CertificatesOfDepositMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ClassOfStockDomain": {
"auth_ref": [
"r160",
"r163",
"r200",
"r204",
"r205",
"r207",
"r210",
"r219",
"r220",
"r221",
"r303",
"r404",
"r409",
"r410",
"r411",
"r417",
"r418",
"r460",
"r461",
"r465",
"r469",
"r835",
"r1037"
],
"lang": {
"en-us": {
"role": {
"documentation": "Share of stock differentiated by the voting rights the holder receives. Examples include, but are not limited to, common stock, redeemable preferred stock, nonredeemable preferred stock, and convertible stock.",
"label": "Class of Stock [Domain]",
"terseLabel": "Class of Stock [Domain]"
}
}
},
"localname": "ClassOfStockDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/Cover",
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ClassOfStockLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Class of Stock [Line Items]",
"terseLabel": "Class of Stock [Line Items]"
}
}
},
"localname": "ClassOfStockLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_CommercialPaperMember": {
"auth_ref": [
"r400"
],
"lang": {
"en-us": {
"role": {
"documentation": "Unsecured promissory note (generally negotiable) that provides institutions with short-term funds.",
"label": "Commercial Paper [Member]",
"terseLabel": "Commercial Paper"
}
}
},
"localname": "CommercialPaperMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_CommitmentsAndContingencies": {
"auth_ref": [
"r66",
"r385",
"r944",
"r977"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 2.0,
"parentTag": "us-gaap_LiabilitiesAndStockholdersEquity",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Represents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur.",
"label": "Commitments and Contingencies",
"terseLabel": "Commitments and contingencies (Notes 3, 16, and 18)"
}
}
},
"localname": "CommitmentsAndContingencies",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CommitmentsAndContingenciesDisclosureAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Commitments and Contingencies Disclosure [Abstract]",
"terseLabel": "Commitments and Contingencies Disclosure [Abstract]"
}
}
},
"localname": "CommitmentsAndContingenciesDisclosureAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_CommitmentsAndContingenciesPolicyTextBlock": {
"auth_ref": [
"r399",
"r1013"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for commitments and contingencies, which may include policies for recognizing and measuring loss and gain contingencies.",
"label": "Commitments and Contingencies, Policy [Policy Text Block]",
"terseLabel": "Contingencies"
}
}
},
"localname": "CommitmentsAndContingenciesPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_CommonStockDividendsPerShareDeclared": {
"auth_ref": [
"r483"
],
"lang": {
"en-us": {
"role": {
"documentation": "Aggregate dividends declared during the period for each share of common stock outstanding.",
"label": "Common Stock, Dividends, Per Share, Declared",
"terseLabel": "Dividends to shareholders (usd per share)"
}
}
},
"localname": "CommonStockDividendsPerShareDeclared",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquityParenthetical"
],
"xbrltype": "perShareItemType"
},
"us-gaap_CommonStockMember": {
"auth_ref": [
"r180",
"r181",
"r815"
],
"lang": {
"en-us": {
"role": {
"documentation": "Stock that is subordinate to all other stock of the issuer.",
"label": "Common Stock [Member]",
"terseLabel": "Ordinary Shares",
"verboseLabel": "Ordinary shares, par value $0.0001 per share"
}
}
},
"localname": "CommonStockMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity",
"http://www.medtronic.com/role/Cover"
],
"xbrltype": "domainItemType"
},
"us-gaap_CommonStockParOrStatedValuePerShare": {
"auth_ref": [
"r33"
],
"lang": {
"en-us": {
"role": {
"documentation": "Face amount or stated value per share of common stock.",
"label": "Common Stock, Par or Stated Value Per Share",
"terseLabel": "Common stock, par value (in dollars per share)"
}
}
},
"localname": "CommonStockParOrStatedValuePerShare",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheetsParenthetical",
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_CommonStockSharesAuthorized": {
"auth_ref": [
"r33"
],
"lang": {
"en-us": {
"role": {
"documentation": "The maximum number of common shares permitted to be issued by an entity's charter and bylaws.",
"label": "Common Stock, Shares Authorized",
"terseLabel": "Common stock, authorized (in shares)"
}
}
},
"localname": "CommonStockSharesAuthorized",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheetsParenthetical",
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_CommonStockSharesIssued": {
"auth_ref": [
"r33"
],
"lang": {
"en-us": {
"role": {
"documentation": "Total number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury.",
"label": "Common Stock, Shares, Issued",
"terseLabel": "Common stock, issued (in shares)"
}
}
},
"localname": "CommonStockSharesIssued",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheetsParenthetical"
],
"xbrltype": "sharesItemType"
},
"us-gaap_CommonStockSharesOutstanding": {
"auth_ref": [
"r33",
"r476"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of shares of common stock outstanding. Common stock represent the ownership interest in a corporation.",
"label": "Common Stock, Shares, Outstanding",
"periodEndLabel": "Ending balance (in shares)",
"periodStartLabel": "Beginning balance (in shares)",
"terseLabel": "Common stock, outstanding (in shares)"
}
}
},
"localname": "CommonStockSharesOutstanding",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheetsParenthetical",
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "sharesItemType"
},
"us-gaap_CommonStockValue": {
"auth_ref": [
"r33",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 1.0,
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Aggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity.",
"label": "Common Stock, Value, Issued",
"terseLabel": "Ordinary shares\u2014 par value $0.0001, 2.6 billion shares authorized, 1,330,743,395 and 1,345,400,671 shares issued and outstanding, respectively"
}
}
},
"localname": "CommonStockValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CompensationAndRetirementDisclosureAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Retirement Benefits [Abstract]",
"terseLabel": "Retirement Benefits [Abstract]"
}
}
},
"localname": "CompensationAndRetirementDisclosureAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_ComponentsOfDeferredTaxAssetsAndLiabilitiesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Components of Deferred Tax Assets and Liabilities [Abstract]",
"terseLabel": "Components of deferred tax assets/(Liabilities):"
}
}
},
"localname": "ComponentsOfDeferredTaxAssetsAndLiabilitiesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ComprehensiveIncomeNetOfTax": {
"auth_ref": [
"r105",
"r107",
"r108",
"r121",
"r950",
"r982"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners.",
"label": "Comprehensive Income (Loss), Net of Tax, Attributable to Parent",
"totalLabel": "Comprehensive income attributable to Medtronic"
}
}
},
"localname": "ComprehensiveIncomeNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ComprehensiveIncomeNetOfTaxAttributableToNoncontrollingInterest": {
"auth_ref": [
"r105",
"r107",
"r120",
"r756",
"r757",
"r770",
"r949",
"r981"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 2.0,
"parentTag": "us-gaap_ComprehensiveIncomeNetOfTax",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income (loss) and other comprehensive income (loss), attributable to noncontrolling interests. Excludes changes in equity resulting from investments by owners and distributions to owners.",
"label": "Comprehensive Income (Loss), Net of Tax, Attributable to Noncontrolling Interest",
"negatedLabel": "Comprehensive income attributable to noncontrolling interests"
}
}
},
"localname": "ComprehensiveIncomeNetOfTaxAttributableToNoncontrollingInterest",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ComprehensiveIncomeNetOfTaxIncludingPortionAttributableToNoncontrollingInterest": {
"auth_ref": [
"r105",
"r107",
"r119",
"r755",
"r770",
"r948",
"r980"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 1.0,
"parentTag": "us-gaap_ComprehensiveIncomeNetOfTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income. Excludes changes in equity resulting from investments by owners and distributions to owners.",
"label": "Comprehensive Income (Loss), Net of Tax, Including Portion Attributable to Noncontrolling Interest",
"totalLabel": "Comprehensive income including noncontrolling interests"
}
}
},
"localname": "ComprehensiveIncomeNetOfTaxIncludingPortionAttributableToNoncontrollingInterest",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ComprehensiveIncomeNoteTextBlock": {
"auth_ref": [
"r118",
"r128",
"r947",
"r979"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for comprehensive income, which includes, but is not limited to, 1) the amount of income tax expense or benefit allocated to each component of other comprehensive income, including reclassification adjustments, 2) the reclassification adjustments for each classification of other comprehensive income and 3) the ending accumulated balances for each component of comprehensive income.",
"label": "Comprehensive Income (Loss) Note [Text Block]",
"terseLabel": "Accumulated Other Comprehensive Loss"
}
}
},
"localname": "ComprehensiveIncomeNoteTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLoss"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ComputerSoftwareIntangibleAssetMember": {
"auth_ref": [
"r342",
"r347",
"r741"
],
"lang": {
"en-us": {
"role": {
"documentation": "Collection of computer programs and related data that provide instructions to a computer, for example, but not limited to, application program, control module or operating system, that perform one or more particular functions or tasks.",
"label": "Computer Software, Intangible Asset [Member]",
"terseLabel": "Computer software"
}
}
},
"localname": "ComputerSoftwareIntangibleAssetMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ConsolidationPolicyTextBlock": {
"auth_ref": [
"r156",
"r760"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary.",
"label": "Consolidation, Policy [Policy Text Block]",
"terseLabel": "Principles of Consolidation"
}
}
},
"localname": "ConsolidationPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ConstructionInProgressMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Structure or a modification to a structure under construction. Includes recently completed structures or modifications to structures that have not been placed into service.",
"label": "Construction in Progress [Member]",
"terseLabel": "Construction in progress"
}
}
},
"localname": "ConstructionInProgressMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ContingentConsiderationByTypeAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of contingent consideration.",
"label": "Contingent Consideration by Type [Axis]",
"terseLabel": "Contingent Consideration by Type [Axis]"
}
}
},
"localname": "ContingentConsiderationByTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ContingentConsiderationTypeDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Description of contingent payment arrangement.",
"label": "Contingent Consideration Type [Domain]",
"terseLabel": "Contingent Consideration Type [Domain]"
}
}
},
"localname": "ContingentConsiderationTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ContractWithCustomerLiability": {
"auth_ref": [
"r485",
"r486",
"r506"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of obligation to transfer good or service to customer for which consideration has been received or is receivable.",
"label": "Contract with Customer, Liability",
"terseLabel": "Deferred revenue"
}
}
},
"localname": "ContractWithCustomerLiability",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ContractWithCustomerLiabilityRevenueRecognized": {
"auth_ref": [
"r507"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of revenue recognized that was previously included in balance of obligation to transfer good or service to customer for which consideration from customer has been received or is due.",
"label": "Contract with Customer, Liability, Revenue Recognized",
"terseLabel": "Revenue recognized that was previously included in deferred revenue"
}
}
},
"localname": "ContractWithCustomerLiabilityRevenueRecognized",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ContractWithCustomerRefundLiabilityCurrent": {
"auth_ref": [
"r509"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liability for consideration received or receivable from customer which is not included in transaction price, when consideration is expected to be refunded to customer, classified as current.",
"label": "Contract with Customer, Refund Liability, Current",
"terseLabel": "Rebate obligations"
}
}
},
"localname": "ContractWithCustomerRefundLiabilityCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CorporateDebtSecuritiesMember": {
"auth_ref": [
"r549",
"r598",
"r1005"
],
"lang": {
"en-us": {
"role": {
"documentation": "Debt securities issued by domestic or foreign corporate business, banks and other entities with a promise of repayment.",
"label": "Corporate Debt Securities [Member]",
"terseLabel": "Corporate debt securities"
}
}
},
"localname": "CorporateDebtSecuritiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_CorporateNonSegmentMember": {
"auth_ref": [
"r247",
"r248",
"r249",
"r250",
"r252",
"r258",
"r260"
],
"lang": {
"en-us": {
"role": {
"documentation": "Corporate headquarters or functional department that may not earn revenues or may earn revenues that are only incidental to the activities of the entity and is not considered an operating segment.",
"label": "Corporate, Non-Segment [Member]",
"terseLabel": "Corporate"
}
}
},
"localname": "CorporateNonSegmentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_CostOfGoodsAndServicesSold": {
"auth_ref": [
"r125",
"r925"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 2.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The aggregate costs related to goods produced and sold and services rendered by an entity during the reporting period. This excludes costs incurred during the reporting period related to financial services rendered and other revenue generating activities.",
"label": "Cost of Goods and Services Sold",
"terseLabel": "Cost of products sold, excluding amortization of intangible assets"
}
}
},
"localname": "CostOfGoodsAndServicesSold",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CostOfSalesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Primary financial statement caption encompassing cost of sales.",
"label": "Cost of Sales [Member]",
"terseLabel": "Cost of products sold",
"verboseLabel": "Cost of products sold"
}
}
},
"localname": "CostOfSalesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_CostsAndExpensesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Costs and Expenses [Abstract]",
"terseLabel": "Costs and expenses:"
}
}
},
"localname": "CostsAndExpensesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome"
],
"xbrltype": "stringItemType"
},
"us-gaap_CreditFacilityAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of credit facility. Credit facilities provide capital to borrowers without the need to structure a loan for each borrowing.",
"label": "Credit Facility [Axis]",
"terseLabel": "Credit Facility [Axis]"
}
}
},
"localname": "CreditFacilityAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_CreditFacilityDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Type of credit facility. Credit facilities provide capital to borrowers without the need to structure a loan for each borrowing.",
"label": "Credit Facility [Domain]",
"terseLabel": "Credit Facility [Domain]"
}
}
},
"localname": "CreditFacilityDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_CurrentFederalTaxExpenseBenefit": {
"auth_ref": [
"r164",
"r712",
"r722"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails": {
"order": 1.0,
"parentTag": "us-gaap_CurrentIncomeTaxExpenseBenefit",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of current federal tax expense (benefit) pertaining to income (loss) from continuing operations.",
"label": "Current Federal Tax Expense (Benefit)",
"terseLabel": "U.S."
}
}
},
"localname": "CurrentFederalTaxExpenseBenefit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CurrentForeignTaxExpenseBenefit": {
"auth_ref": [
"r164",
"r712"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails": {
"order": 2.0,
"parentTag": "us-gaap_CurrentIncomeTaxExpenseBenefit",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of current foreign income tax expense (benefit) pertaining to income (loss) from continuing operations.",
"label": "Current Foreign Tax Expense (Benefit)",
"terseLabel": "International"
}
}
},
"localname": "CurrentForeignTaxExpenseBenefit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CurrentIncomeTaxExpenseBenefit": {
"auth_ref": [
"r164",
"r712",
"r722",
"r724"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails": {
"order": 1.0,
"parentTag": "us-gaap_IncomeTaxExpenseBenefit",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of current income tax expense (benefit) pertaining to taxable income (loss) from continuing operations.",
"label": "Current Income Tax Expense (Benefit)",
"totalLabel": "Total current tax expense"
}
}
},
"localname": "CurrentIncomeTaxExpenseBenefit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_CurrentIncomeTaxExpenseBenefitContinuingOperationsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Current Income Tax Expense (Benefit), Continuing Operations [Abstract]",
"terseLabel": "Current tax expense:"
}
}
},
"localname": "CurrentIncomeTaxExpenseBenefitContinuingOperationsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_CustomerRelatedIntangibleAssetsMember": {
"auth_ref": [
"r739"
],
"lang": {
"en-us": {
"role": {
"documentation": "Customer-related asset, including, but not limited to, customer lists, and noncontractual customer relationships.",
"label": "Customer-Related Intangible Assets [Member]",
"terseLabel": "Customer-related",
"verboseLabel": "Customer-Related Intangible Assets"
}
}
},
"localname": "CustomerRelatedIntangibleAssetsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DamagesFromProductDefectsMember": {
"auth_ref": [
"r393"
],
"lang": {
"en-us": {
"role": {
"documentation": "The risk of loss arises with respect to product defects and recalls, or improperly performed services which actually or allegedly resulted in damages suffered by the injured party, excluding major product liability matters.",
"label": "Damages from Product Defects [Member]",
"terseLabel": "Damages from product defects"
}
}
},
"localname": "DamagesFromProductDefectsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DebtCurrent": {
"auth_ref": [
"r59"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 2.0,
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0
},
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of short-term debt and current maturity of long-term debt and capital lease obligations due within one year or the normal operating cycle, if longer.",
"label": "Debt, Current",
"terseLabel": "Current debt obligations",
"totalLabel": "Current debt obligations",
"verboseLabel": "Amount of current debt obligations outstanding"
}
}
},
"localname": "DebtCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtDisclosureAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Debt Disclosure [Abstract]",
"terseLabel": "Debt Disclosure [Abstract]"
}
}
},
"localname": "DebtDisclosureAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_DebtDisclosureTextBlock": {
"auth_ref": [
"r159",
"r425",
"r426",
"r427",
"r428",
"r429",
"r430",
"r431",
"r436",
"r443",
"r444",
"r446",
"r456"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for information about short-term and long-term debt arrangements, which includes amounts of borrowings under each line of credit, note payable, commercial paper issue, bonds indenture, debenture issue, own-share lending arrangements and any other contractual agreement to repay funds, and about the underlying arrangements, rationale for a classification as long-term, including repayment terms, interest rates, collateral provided, restrictions on use of assets and activities, whether or not in compliance with debt covenants, and other matters important to users of the financial statements, such as the effects of refinancing and noncompliance with debt covenants.",
"label": "Debt Disclosure [Text Block]",
"terseLabel": "Financing Arrangements"
}
}
},
"localname": "DebtDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangements"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_DebtInstrumentAxis": {
"auth_ref": [
"r24",
"r26",
"r27",
"r162",
"r170",
"r419",
"r420",
"r421",
"r422",
"r423",
"r424",
"r426",
"r432",
"r433",
"r434",
"r435",
"r437",
"r438",
"r439",
"r440",
"r441",
"r442",
"r450",
"r451",
"r452",
"r453",
"r862",
"r935",
"r938",
"r965"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of debt instrument, including, but not limited to, draws against credit facilities.",
"label": "Debt Instrument [Axis]",
"terseLabel": "Debt Instrument [Axis]"
}
}
},
"localname": "DebtInstrumentAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DebtInstrumentBasisSpreadOnVariableRate1": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Percentage points added to the reference rate to compute the variable rate on the debt instrument.",
"label": "Debt Instrument, Basis Spread on Variable Rate",
"terseLabel": "Margin added per annum"
}
}
},
"localname": "DebtInstrumentBasisSpreadOnVariableRate1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DebtInstrumentCarryingAmount": {
"auth_ref": [
"r27",
"r447",
"r938",
"r965"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before unamortized (discount) premium and debt issuance costs, of long-term debt. Includes, but is not limited to, notes payable, bonds payable, commercial loans, mortgage loans, convertible debt, subordinated debt and other types of debt.",
"label": "Long-term Debt, Gross",
"totalLabel": "Total"
}
}
},
"localname": "DebtInstrumentCarryingAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtInstrumentFaceAmount": {
"auth_ref": [
"r419",
"r450",
"r451",
"r859",
"r862",
"r863"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Face (par) amount of debt instrument at time of issuance.",
"label": "Debt Instrument, Face Amount",
"verboseLabel": "Principal value"
}
}
},
"localname": "DebtInstrumentFaceAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtInstrumentInterestRateEffectivePercentage": {
"auth_ref": [
"r63",
"r449",
"r859",
"r862"
],
"lang": {
"en-us": {
"role": {
"documentation": "Effective interest rate for the funds borrowed under the debt agreement considering interest compounding and original issue discount or premium.",
"label": "Debt Instrument, Interest Rate, Effective Percentage",
"terseLabel": "Effective Interest Rate"
}
}
},
"localname": "DebtInstrumentInterestRateEffectivePercentage",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DebtInstrumentInterestRateStatedPercentage": {
"auth_ref": [
"r63",
"r420"
],
"lang": {
"en-us": {
"role": {
"documentation": "Contractual interest rate for funds borrowed, under the debt agreement.",
"label": "Debt Instrument, Interest Rate, Stated Percentage",
"verboseLabel": "Stated interest rate"
}
}
},
"localname": "DebtInstrumentInterestRateStatedPercentage",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DebtInstrumentLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Debt Instrument [Line Items]",
"terseLabel": "Debt Instrument [Line Items]"
}
}
},
"localname": "DebtInstrumentLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DebtInstrumentNameDomain": {
"auth_ref": [
"r64",
"r162",
"r170",
"r419",
"r420",
"r421",
"r422",
"r423",
"r424",
"r426",
"r432",
"r433",
"r434",
"r435",
"r437",
"r438",
"r439",
"r440",
"r441",
"r442",
"r450",
"r451",
"r452",
"r453",
"r862"
],
"lang": {
"en-us": {
"role": {
"documentation": "The name for the particular debt instrument or borrowing that distinguishes it from other debt instruments or borrowings, including draws against credit facilities.",
"label": "Debt Instrument, Name [Domain]",
"terseLabel": "Debt Instrument, Name [Domain]"
}
}
},
"localname": "DebtInstrumentNameDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DebtInstrumentTable": {
"auth_ref": [
"r64",
"r162",
"r170",
"r419",
"r420",
"r421",
"r422",
"r423",
"r424",
"r426",
"r432",
"r433",
"r434",
"r435",
"r437",
"r438",
"r439",
"r440",
"r441",
"r442",
"r445",
"r450",
"r451",
"r452",
"r453",
"r477",
"r480",
"r481",
"r482",
"r858",
"r859",
"r862",
"r863",
"r961"
],
"lang": {
"en-us": {
"role": {
"documentation": "A table or schedule providing information pertaining to long-term debt instruments or arrangements, including identification, terms, features, collateral requirements and other information necessary to a fair presentation. These are debt arrangements that originally required repayment more than twelve months after issuance or greater than the normal operating cycle of the company, if longer.",
"label": "Schedule of Long-term Debt Instruments [Table]",
"terseLabel": "Schedule of Long-term Debt Instruments [Table]"
}
}
},
"localname": "DebtInstrumentTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DebtInstrumentTerm": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Period of time between issuance and maturity of debt instrument, in PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.",
"label": "Debt Instrument, Term",
"terseLabel": "Debt term"
}
}
},
"localname": "DebtInstrumentTerm",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_DebtInstrumentUnamortizedDiscountPremiumNet": {
"auth_ref": [
"r432",
"r858",
"r859",
"r860",
"r861",
"r863"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails": {
"order": 1.0,
"parentTag": "us-gaap_LongTermDebtAndCapitalLeaseObligations",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after accumulated amortization, of debt discount (premium).",
"label": "Debt Instrument, Unamortized Discount (Premium), Net",
"negatedTerseLabel": "Debt discount, net"
}
}
},
"localname": "DebtInstrumentUnamortizedDiscountPremiumNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleAmortizedCostExcludingAccruedInterestAfterAllowanceForCreditLoss": {
"auth_ref": [
"r317"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amortized cost excluding accrued interest, after allowance for credit loss, of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-Sale, Amortized Cost, Excluding Accrued Interest, after Allowance for Credit Loss",
"totalLabel": "Cost"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleAmortizedCostExcludingAccruedInterestAfterAllowanceForCreditLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLonger": {
"auth_ref": [
"r294",
"r319",
"r322"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for more than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset.",
"label": "Debt Securities, Available-for-sale, Continuous Unrealized Loss Position, 12 Months or Longer",
"terseLabel": "More than 12 months"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLonger",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerAccumulatedLoss": {
"auth_ref": [
"r294",
"r319"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for 12 months or longer, without allowance for credit loss. Includes beneficial interest in securitized financial asset.",
"label": "Debt Securities, Available-for-sale, Continuous Unrealized Loss Position, 12 Months or Longer, Accumulated Loss",
"negatedTerseLabel": "More than 12 months"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerAccumulatedLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months": {
"auth_ref": [
"r294",
"r319",
"r322"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset.",
"label": "Debt Securities, Available-for-sale, Continuous Unrealized Loss Position, Less than 12 Months",
"terseLabel": "Less than 12 months"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12MonthsAccumulatedLoss": {
"auth_ref": [
"r294",
"r319"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset.",
"label": "Debt Securities, Available-for-sale, Continuous Unrealized Loss Position, Less than 12 Months, Accumulated Loss",
"negatedTerseLabel": "Less than 12 months"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12MonthsAccumulatedLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleRealizedGain": {
"auth_ref": [
"r295"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of realized gain on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-sale, Realized Gain",
"terseLabel": "Gross realized gains"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleRealizedGain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleRealizedLoss": {
"auth_ref": [
"r295"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of realized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-sale, Realized Loss",
"negatedLabel": "Gross realized losses"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleRealizedLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleTable": {
"auth_ref": [
"r296"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-sale [Table]",
"terseLabel": "Debt Securities, Available-for-sale [Table]"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleTableTextBlock": {
"auth_ref": [
"r296"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Debt Securities, Available-for-sale [Table Text Block]",
"terseLabel": "Schedule of Investments by Category and Related Balance Sheet Presentation"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock": {
"auth_ref": [
"r293",
"r318",
"r322"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of fair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position, without allowance for credit loss. Includes beneficial interest in securitized financial asset.",
"label": "Debt Securities, Available-for-sale, Unrealized Loss Position, Fair Value [Table Text Block]",
"terseLabel": "Schedule of Gross Unrealized Losses and Fair Values of Available-for-sale Securities that Have Been in a Continuous Unrealized Loss Position Deemed to be Temporary, Aggregated by Investment Category"
}
}
},
"localname": "DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_DecreaseInUnrecognizedTaxBenefitsIsReasonablyPossible": {
"auth_ref": [
"r690"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of decrease reasonably possible in the next twelve months for the unrecognized tax benefit.",
"label": "Decrease in Unrecognized Tax Benefits is Reasonably Possible",
"terseLabel": "Estimated reasonably possible decrease in uncertain tax positions excluding interest over the next 12 months"
}
}
},
"localname": "DecreaseInUnrecognizedTaxBenefitsIsReasonablyPossible",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredCompensationLiabilityCurrent": {
"auth_ref": [
"r518",
"r519"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 4.0,
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Aggregate carrying value as of the balance sheet date of the liabilities for all deferred compensation arrangements payable within one year (or the operating cycle, if longer). Represents currently earned compensation under compensation arrangements that is not actually paid until a later date.",
"label": "Deferred Compensation Liability, Current",
"terseLabel": "Accrued compensation"
}
}
},
"localname": "DeferredCompensationLiabilityCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredFederalIncomeTaxExpenseBenefit": {
"auth_ref": [
"r164",
"r713",
"r722"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails": {
"order": 1.0,
"parentTag": "us-gaap_DeferredIncomeTaxExpenseBenefit",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred federal income tax expense (benefit) pertaining to income (loss) from continuing operations.",
"label": "Deferred Federal Income Tax Expense (Benefit)",
"terseLabel": "U.S."
}
}
},
"localname": "DeferredFederalIncomeTaxExpenseBenefit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredFinanceCostsNet": {
"auth_ref": [
"r54",
"r432",
"r860"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails": {
"order": 2.0,
"parentTag": "us-gaap_LongTermDebtAndCapitalLeaseObligations",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after accumulated amortization, of debt issuance costs. Includes, but is not limited to, legal, accounting, underwriting, printing, and registration costs.",
"label": "Debt Issuance Costs, Net",
"negatedTerseLabel": "Deferred financing costs"
}
}
},
"localname": "DeferredFinanceCostsNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredForeignIncomeTaxExpenseBenefit": {
"auth_ref": [
"r164",
"r713",
"r722"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails": {
"order": 2.0,
"parentTag": "us-gaap_DeferredIncomeTaxExpenseBenefit",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred foreign income tax expense (benefit) pertaining to income (loss) from continuing operations.",
"label": "Deferred Foreign Income Tax Expense (Benefit)",
"terseLabel": "International"
}
}
},
"localname": "DeferredForeignIncomeTaxExpenseBenefit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredIncomeTaxAssetsNet": {
"auth_ref": [
"r684",
"r685"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 6.0,
"parentTag": "us-gaap_Assets",
"weight": 1.0
},
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DeferredTaxAssetsLiabilitiesNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, with jurisdictional netting.",
"label": "Deferred Income Tax Assets, Net",
"terseLabel": "Tax assets",
"verboseLabel": "Tax assets"
}
}
},
"localname": "DeferredIncomeTaxAssetsNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredIncomeTaxExpenseBenefit": {
"auth_ref": [
"r148",
"r164",
"r713",
"r722",
"r723",
"r724"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails": {
"order": 2.0,
"parentTag": "us-gaap_IncomeTaxExpenseBenefit",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred income tax expense (benefit) pertaining to income (loss) from continuing operations.",
"label": "Deferred Income Tax Expense (Benefit)",
"totalLabel": "Net deferred tax benefit"
}
}
},
"localname": "DeferredIncomeTaxExpenseBenefit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredIncomeTaxExpenseBenefitContinuingOperationsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Deferred Income Tax Expense (Benefit), Continuing Operations [Abstract]",
"terseLabel": "Deferred tax (benefit) expense:"
}
}
},
"localname": "DeferredIncomeTaxExpenseBenefitContinuingOperationsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DeferredIncomeTaxLiabilities": {
"auth_ref": [
"r29",
"r30",
"r702",
"r937",
"r964"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails_1": {
"order": 1.0,
"parentTag": "us-gaap_DeferredTaxAssetsLiabilitiesNet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred tax liability attributable to taxable temporary differences.",
"label": "Deferred Tax Liabilities, Gross",
"negatedTotalLabel": "Total deferred tax liabilities"
}
}
},
"localname": "DeferredIncomeTaxLiabilities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredIncomeTaxLiabilitiesNet": {
"auth_ref": [
"r684",
"r685"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 5.0,
"parentTag": "us-gaap_Liabilities",
"weight": 1.0
},
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_DeferredTaxAssetsLiabilitiesNet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after deferred tax asset, of deferred tax liability attributable to taxable differences with jurisdictional netting.",
"label": "Deferred Income Tax Liabilities, Net",
"negatedTerseLabel": "Deferred tax liabilities",
"terseLabel": "Deferred tax liabilities"
}
}
},
"localname": "DeferredIncomeTaxLiabilitiesNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredIncomeTaxesAndTaxCredits": {
"auth_ref": [
"r149"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 9.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred income tax expense (benefit) and income tax credits.",
"label": "Deferred Income Taxes and Tax Credits",
"terseLabel": "Deferred income taxes"
}
}
},
"localname": "DeferredIncomeTaxesAndTaxCredits",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsGoodwillAndIntangibleAssets": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from intangible assets including goodwill.",
"label": "Deferred Tax Assets, Goodwill and Intangible Assets",
"terseLabel": "Intangible assets"
}
}
},
"localname": "DeferredTaxAssetsGoodwillAndIntangibleAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsGross": {
"auth_ref": [
"r703"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_DeferredTaxAssetsNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards.",
"label": "Deferred Tax Assets, Gross",
"totalLabel": "Gross deferred tax assets"
}
}
},
"localname": "DeferredTaxAssetsGross",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsInventory": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 8.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from inventory.",
"label": "Deferred Tax Assets, Inventory",
"terseLabel": "Inventory"
}
}
},
"localname": "DeferredTaxAssetsInventory",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsLiabilitiesNet": {
"auth_ref": [
"r705"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails_1": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after allocation of valuation allowances and deferred tax liability, of deferred tax asset attributable to deductible differences and carryforwards, without jurisdictional netting.",
"label": "Deferred Tax Assets, Net",
"totalLabel": "Tax assets, net"
}
}
},
"localname": "DeferredTaxAssetsLiabilitiesNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsNet": {
"auth_ref": [
"r705"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails_1": {
"order": 4.0,
"parentTag": "us-gaap_DeferredTaxAssetsLiabilitiesNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards.",
"label": "Deferred Tax Assets, Net of Valuation Allowance",
"totalLabel": "Valuation allowance"
}
}
},
"localname": "DeferredTaxAssetsNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsNetOfValuationAllowanceAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Deferred Tax Assets, Net of Valuation Allowance [Abstract]",
"terseLabel": "Deferred tax assets:"
}
}
},
"localname": "DeferredTaxAssetsNetOfValuationAllowanceAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DeferredTaxAssetsOperatingLossCarryforwards": {
"auth_ref": [
"r710",
"r711"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards.",
"label": "Deferred Tax Assets, Operating Loss Carryforwards",
"terseLabel": "Operating loss carryforwards"
}
}
},
"localname": "DeferredTaxAssetsOperatingLossCarryforwards",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsOperatingLossCarryforwardsNotSubjectToExpiration": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards that are not subject to expiration dates.",
"label": "Deferred Tax Assets, Operating Loss Carryforwards, Not Subject to Expiration",
"terseLabel": "Net operating loss carryforwards, no expiration"
}
}
},
"localname": "DeferredTaxAssetsOperatingLossCarryforwardsNotSubjectToExpiration",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsOperatingLossCarryforwardsSubjectToExpiration": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards that are subject to expiration dates.",
"label": "Deferred Tax Assets, Operating Loss Carryforwards, Subject to Expiration",
"terseLabel": "Net operating loss carryforwards, expiring in future years"
}
}
},
"localname": "DeferredTaxAssetsOperatingLossCarryforwardsSubjectToExpiration",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsOther": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 7.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before allocation of valuation allowance, of deferred tax asset attributable to deductible temporary differences, classified as other.",
"label": "Deferred Tax Assets, Other",
"terseLabel": "Other"
}
}
},
"localname": "DeferredTaxAssetsOther",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsEmployeeCompensation": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 4.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from employee compensation.",
"label": "Deferred Tax Assets, Tax Deferred Expense, Compensation and Benefits, Employee Compensation",
"terseLabel": "Accrued compensation"
}
}
},
"localname": "DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsEmployeeCompensation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 6.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from share-based compensation.",
"label": "Deferred Tax Assets, Tax Deferred Expense, Compensation and Benefits, Share-based Compensation Cost",
"terseLabel": "Stock-based compensation"
}
}
},
"localname": "DeferredTaxAssetsTaxDeferredExpenseCompensationAndBenefitsShareBasedCompensationCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsTaxDeferredExpenseReservesAndAccrualsAccruedLiabilities": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_DeferredTaxAssetsGross",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from accrued liabilities.",
"label": "Deferred Tax Assets, Tax Deferred Expense, Reserves and Accruals, Accrued Liabilities",
"terseLabel": "Other accrued liabilities"
}
}
},
"localname": "DeferredTaxAssetsTaxDeferredExpenseReservesAndAccrualsAccruedLiabilities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxAssetsValuationAllowance": {
"auth_ref": [
"r704"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DeferredTaxAssetsNet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized.",
"label": "Deferred Tax Assets, Valuation Allowance",
"negatedLabel": "Valuation allowance",
"terseLabel": "Valuation allowance"
}
}
},
"localname": "DeferredTaxAssetsValuationAllowance",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails",
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxLiabilitiesDerivatives": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DeferredIncomeTaxLiabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred tax liability attributable to taxable temporary differences from derivatives.",
"label": "Deferred Tax Liabilities, Derivatives",
"negatedLabel": "Realized loss on derivative financial instruments"
}
}
},
"localname": "DeferredTaxLiabilitiesDerivatives",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxLiabilitiesGoodwillAndIntangibleAssetsIntangibleAssets": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_DeferredIncomeTaxLiabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred tax liability attributable to taxable temporary differences from intangible assets other than goodwill.",
"label": "Deferred Tax Liabilities, Intangible Assets",
"negatedLabel": "Intangible assets"
}
}
},
"localname": "DeferredTaxLiabilitiesGoodwillAndIntangibleAssetsIntangibleAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxLiabilitiesLeasingArrangements": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 6.0,
"parentTag": "us-gaap_DeferredIncomeTaxLiabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred tax liability attributable to taxable temporary differences from leasing arrangements.",
"label": "Deferred Tax Liabilities, Leasing Arrangements",
"negatedTerseLabel": "Right of use leases"
}
}
},
"localname": "DeferredTaxLiabilitiesLeasingArrangements",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxLiabilitiesNetAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Deferred Tax Liabilities, Net [Abstract]",
"terseLabel": "Deferred tax liabilities:"
}
}
},
"localname": "DeferredTaxLiabilitiesNetAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DeferredTaxLiabilitiesOther": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_DeferredIncomeTaxLiabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred tax liability attributable to taxable temporary differences classified as other.",
"label": "Deferred Tax Liabilities, Other",
"negatedLabel": "Other"
}
}
},
"localname": "DeferredTaxLiabilitiesOther",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DeferredTaxLiabilitiesPropertyPlantAndEquipment": {
"auth_ref": [
"r710",
"r711"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails": {
"order": 4.0,
"parentTag": "us-gaap_DeferredIncomeTaxLiabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of deferred tax liability attributable to taxable temporary differences from property, plant, and equipment.",
"label": "Deferred Tax Liabilities, Property, Plant and Equipment",
"negatedLabel": "Accumulated depreciation"
}
}
},
"localname": "DeferredTaxLiabilitiesPropertyPlantAndEquipment",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAccumulatedBenefitObligation": {
"auth_ref": [
"r557"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of actuarial present value of benefits attributed to employee service rendered, excluding assumptions about future compensation level.",
"label": "Defined Benefit Plan, Accumulated Benefit Obligation",
"terseLabel": "Accumulated benefit obligation at end of year"
}
}
},
"localname": "DefinedBenefitPlanAccumulatedBenefitObligation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeBeforeTax": {
"auth_ref": [
"r95",
"r100",
"r569"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of accumulated other comprehensive (income) loss for defined benefit plan, that has not been recognized in net periodic benefit cost (credit).",
"label": "Defined Benefit Plan, Accumulated Other Comprehensive (Income) Loss, before Tax",
"totalLabel": "Ending balance"
}
}
},
"localname": "DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeBeforeTaxAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Accumulated Other Comprehensive (Income) Loss, before Tax [Abstract]",
"terseLabel": "Amounts recognized in accumulated other comprehensive loss:"
}
}
},
"localname": "DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeBeforeTaxAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeNetGainsLossesBeforeTax": {
"auth_ref": [
"r100",
"r569"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": 2.0,
"parentTag": "us-gaap_DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeBeforeTax",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of accumulated other comprehensive income (loss) for gain (loss) of defined benefit plan, that has not been recognized in net periodic benefit (cost) credit.",
"label": "Defined Benefit Plan, Accumulated Other Comprehensive Income (Loss), Gain (Loss), before Tax",
"negatedTerseLabel": "Net actuarial loss"
}
}
},
"localname": "DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeNetGainsLossesBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeNetPriorServiceCostCreditBeforeTax": {
"auth_ref": [
"r100",
"r569"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": 1.0,
"parentTag": "us-gaap_DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeBeforeTax",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of accumulated other comprehensive (income) loss for cost (credit) of benefit change attributable to participants' prior service from plan amendment or plan initiation of defined benefit plan, that has not been recognized in net periodic benefit cost (credit).",
"label": "Defined Benefit Plan, Accumulated Other Comprehensive (Income) Loss, Prior Service Cost (Credit), before Tax",
"terseLabel": "Prior service cost (credit)"
}
}
},
"localname": "DefinedBenefitPlanAccumulatedOtherComprehensiveIncomeNetPriorServiceCostCreditBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanActualReturnOnPlanAssets": {
"auth_ref": [
"r538",
"r598"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase (decrease) in plan assets of defined benefit plan from actual return (loss) determined by change in fair value of plan assets adjusted for contributions, benefit payments, and other expenses.",
"label": "Defined Benefit Plan, Plan Assets, Increase (Decrease) for Actual Return (Loss)",
"terseLabel": "Actual return on plan assets"
}
}
},
"localname": "DefinedBenefitPlanActualReturnOnPlanAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanActuarialGainLoss": {
"auth_ref": [
"r531"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of gain (loss) from change in actuarial assumptions which (increases) decreases benefit obligation of defined benefit plan. Assumptions include, but are not limited to, interest, mortality, employee turnover, salary, and temporary deviation from substantive plan.",
"label": "Defined Benefit Plan, Benefit Obligation, Actuarial Gain (Loss)",
"negatedTerseLabel": "Actuarial (gain) loss"
}
}
},
"localname": "DefinedBenefitPlanActuarialGainLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAmortizationOfGainsLosses": {
"auth_ref": [
"r524",
"r564",
"r592",
"r598",
"r599"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 5.0,
"parentTag": "us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of gain (loss) recognized in net periodic benefit (cost) credit of defined benefit plan.",
"label": "Defined Benefit Plan, Amortization of Gain (Loss)",
"negatedLabel": "Amortization of net actuarial loss"
}
}
},
"localname": "DefinedBenefitPlanAmortizationOfGainsLosses",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAmortizationOfPriorServiceCostCredit": {
"auth_ref": [
"r524",
"r565",
"r593",
"r598",
"r599"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 4.0,
"parentTag": "us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of prior service cost (credit) recognized in net periodic benefit cost (credit) of defined benefit plan.",
"label": "Defined Benefit Plan, Amortization of Prior Service Cost (Credit)",
"terseLabel": "Amortization of prior service cost"
}
}
},
"localname": "DefinedBenefitPlanAmortizationOfPriorServiceCostCredit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAmountsRecognizedInBalanceSheet": {
"auth_ref": [
"r522",
"r546"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of asset (liability), recognized in statement of financial position, for defined benefit pension and other postretirement plans.",
"label": "Defined Benefit Plan, Amounts for Asset (Liability) Recognized in Statement of Financial Position",
"totalLabel": "Recognized asset (liability)"
}
}
},
"localname": "DefinedBenefitPlanAmountsRecognizedInBalanceSheet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAmountsRecognizedInBalanceSheetAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Amounts for Asset (Liability) Recognized in Statement of Financial Position [Abstract]",
"terseLabel": "Amounts recognized on the consolidated balance sheets consist of:"
}
}
},
"localname": "DefinedBenefitPlanAmountsRecognizedInBalanceSheetAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanAmountsRecognizedInOtherComprehensiveIncomeAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Amounts Recognized in Other Comprehensive Income (Loss) [Abstract]",
"terseLabel": "Amounts Recognized in AOCI"
}
}
},
"localname": "DefinedBenefitPlanAmountsRecognizedInOtherComprehensiveIncomeAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanAssetsForPlanBenefitsNoncurrent": {
"auth_ref": [
"r22",
"r522",
"r523",
"r546",
"r598",
"r933",
"r967"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": 1.0,
"parentTag": "us-gaap_DefinedBenefitPlanAmountsRecognizedInBalanceSheet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of asset, recognized in statement of financial position, for overfunded defined benefit pension and other postretirement plans.",
"label": "Assets for Plan Benefits, Defined Benefit Plan",
"terseLabel": "Non-current assets"
}
}
},
"localname": "DefinedBenefitPlanAssetsForPlanBenefitsNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanAssetsTargetAllocationsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Plan Assets, Allocations [Abstract]",
"terseLabel": "Plan Assets Target Allocations"
}
}
},
"localname": "DefinedBenefitPlanAssetsTargetAllocationsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanAssumptionsUsedCalculatingBenefitObligationDiscountRate": {
"auth_ref": [
"r571"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average rate for present value of future retirement benefits cash flows, used to determine benefit obligation of defined benefit plan.",
"label": "Defined Benefit Plan, Assumptions Used Calculating Benefit Obligation, Discount Rate",
"terseLabel": "Discount rate"
}
}
},
"localname": "DefinedBenefitPlanAssumptionsUsedCalculatingBenefitObligationDiscountRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DefinedBenefitPlanAssumptionsUsedCalculatingBenefitObligationRateOfCompensationIncrease": {
"auth_ref": [
"r572"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average rate increase of compensation, used to determine benefit obligation of defined benefit plan. Plan includes, but is not limited to, pay-related defined benefit plan.",
"label": "Defined Benefit Plan, Assumptions Used Calculating Benefit Obligation, Rate of Compensation Increase",
"terseLabel": "Rate of compensation increase"
}
}
},
"localname": "DefinedBenefitPlanAssumptionsUsedCalculatingBenefitObligationRateOfCompensationIncrease",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostDiscountRate": {
"auth_ref": [
"r571"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average rate for present value of future retirement benefits cash flows, used to determine net periodic benefit cost of defined benefit plan.",
"label": "Defined Benefit Plan, Assumptions Used Calculating Net Periodic Benefit Cost, Discount Rate",
"terseLabel": "Discount rate \u2013 benefit obligation"
}
}
},
"localname": "DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostDiscountRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostExpectedLongTermReturnOnAssets": {
"auth_ref": [
"r573",
"r597"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average rate of return on plan assets, reflecting average rate of earnings expected on existing plan assets and expected contributions, used to determine net periodic benefit cost of defined benefit plan.",
"label": "Defined Benefit Plan, Assumptions Used Calculating Net Periodic Benefit Cost, Expected Long-term Rate of Return on Plan Assets",
"terseLabel": "Expected return on plan assets"
}
}
},
"localname": "DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostExpectedLongTermReturnOnAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostRateOfCompensationIncrease": {
"auth_ref": [
"r572"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average rate of compensation increase used to determine net periodic benefit cost of defined benefit plan. Plan includes, but is not limited to, pay-related defined benefit plan.",
"label": "Defined Benefit Plan, Assumptions Used Calculating Net Periodic Benefit Cost, Rate of Compensation Increase",
"terseLabel": "Rate of compensation increase"
}
}
},
"localname": "DefinedBenefitPlanAssumptionsUsedCalculatingNetPeriodicBenefitCostRateOfCompensationIncrease",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DefinedBenefitPlanBenefitObligation": {
"auth_ref": [
"r526"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": 2.0,
"parentTag": "us-gaap_DefinedBenefitPlanFundedStatusOfPlan",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of actuarial present value of benefits attributed to service rendered by employee for defined benefit plan.",
"label": "Defined Benefit Plan, Benefit Obligation",
"periodEndLabel": "Projected benefit obligation at end of year",
"periodStartLabel": "Projected benefit obligation at beginning of year",
"terseLabel": "Benefit obligations",
"verboseLabel": "Post-retirement benefit plans, benefit obligations"
}
}
},
"localname": "DefinedBenefitPlanBenefitObligation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanBenefitObligationBenefitsPaid": {
"auth_ref": [
"r533",
"r604"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of payment to participant of defined benefit plan which decreases benefit obligation. For pension plan, payment includes, but is not limited to, pension benefits and death benefits. For other postretirement plan, payment includes, but is not limited to, prescription drug benefits, health care benefits, life insurance benefits, and legal, educational and advisory services.",
"label": "Defined Benefit Plan, Benefit Obligation, Benefits Paid",
"negatedLabel": "Benefits paid"
}
}
},
"localname": "DefinedBenefitPlanBenefitObligationBenefitsPaid",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanBenefitObligationContributionsByPlanParticipant": {
"auth_ref": [
"r530"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of contributions received by defined benefit plan from participant which increase benefit obligation.",
"label": "Defined Benefit Plan, Benefit Obligation, Contributions by Plan Participant",
"terseLabel": "Employee contributions"
}
}
},
"localname": "DefinedBenefitPlanBenefitObligationContributionsByPlanParticipant",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanByPlanAssetCategoriesAxis": {
"auth_ref": [
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r576",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by defined benefit plan asset investment.",
"label": "Defined Benefit Plan, Plan Assets, Category [Axis]",
"terseLabel": "Defined Benefit Plan, Plan Assets, Category [Axis]"
}
}
},
"localname": "DefinedBenefitPlanByPlanAssetCategoriesAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanChangeInBenefitObligationRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Defined Benefit Plan, Change in Benefit Obligation [Roll Forward]",
"terseLabel": "Change in projected benefit obligation:"
}
}
},
"localname": "DefinedBenefitPlanChangeInBenefitObligationRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanChangeInFairValueOfPlanAssetsRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Defined Benefit Plan, Change in Fair Value of Plan Assets [Roll Forward]",
"terseLabel": "Change in plan assets:"
}
}
},
"localname": "DefinedBenefitPlanChangeInFairValueOfPlanAssetsRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanContributionsByEmployer": {
"auth_ref": [
"r540",
"r549",
"r551",
"r596",
"r598",
"r599"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of contribution received by defined benefit plan from employer which increases plan assets.",
"label": "Defined Benefit Plan, Plan Assets, Contributions by Employer",
"terseLabel": "Employer contributions"
}
}
},
"localname": "DefinedBenefitPlanContributionsByEmployer",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanCostOfProvidingSpecialOrContractualTerminationBenefitRecognizedDuringPeriod": {
"auth_ref": [
"r579"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 7.0,
"parentTag": "us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cost of providing special or contractual termination benefits payable from defined benefit plan.",
"label": "Defined Benefit Plan, Cost of Providing Special and Contractual Termination Benefits",
"terseLabel": "Special termination benefits"
}
}
},
"localname": "DefinedBenefitPlanCostOfProvidingSpecialOrContractualTerminationBenefitRecognizedDuringPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanDebtSecurityMember": {
"auth_ref": [
"r549"
],
"lang": {
"en-us": {
"role": {
"documentation": "Debt instrument issued by corporation, government and governmental agency, municipality, and other institution; in which defined benefit plan asset is invested.",
"label": "Defined Benefit Plan, Debt Security [Member]",
"terseLabel": "Debt securities",
"verboseLabel": "Debt"
}
}
},
"localname": "DefinedBenefitPlanDebtSecurityMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DefinedBenefitPlanDisclosureLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Defined Benefit Plan Disclosure [Line Items]",
"terseLabel": "Defined Benefit Plan Disclosure [Line items]"
}
}
},
"localname": "DefinedBenefitPlanDisclosureLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanEquitySecuritiesMember": {
"auth_ref": [
"r549",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Security representing ownership in corporation or other legal entity for which ownership is represented by share of stock, in which defined benefit plan asset is invested. Includes, but is not limited to, common stock, preferred stock, convertible security, stock right and stock warrant.",
"label": "Defined Benefit Plan, Equity Securities [Member]",
"terseLabel": "Equity securities",
"verboseLabel": "Equity"
}
}
},
"localname": "DefinedBenefitPlanEquitySecuritiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DefinedBenefitPlanEstimatedFutureBenefitPaymentsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Expected Future Benefit Payment [Abstract]",
"terseLabel": "Estimated Future Benefit Payments"
}
}
},
"localname": "DefinedBenefitPlanEstimatedFutureBenefitPaymentsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanExpectedFutureBenefitPaymentsFiveFiscalYearsThereafter": {
"auth_ref": [
"r558"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails": {
"order": 6.0,
"parentTag": "mdt_DefinedBenefitPlanExpectedFutureBenefitPayments",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of benefit for defined benefit plan expected to be paid in five fiscal years after fifth fiscal year following current fiscal year.",
"label": "Defined Benefit Plan, Expected Future Benefit Payment, after Year Five for Next Five Years",
"terseLabel": "2028 \u2013 2032"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureBenefitPaymentsFiveFiscalYearsThereafter",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanExpectedFutureBenefitPaymentsNextTwelveMonths": {
"auth_ref": [
"r558"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails": {
"order": 1.0,
"parentTag": "mdt_DefinedBenefitPlanExpectedFutureBenefitPayments",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of benefit for defined benefit plan expected to be paid in next fiscal year following current fiscal year.",
"label": "Defined Benefit Plan, Expected Future Benefit Payment, Year One",
"terseLabel": "2023"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureBenefitPaymentsNextTwelveMonths",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanExpectedFutureBenefitPaymentsYearFive": {
"auth_ref": [
"r558"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails": {
"order": 5.0,
"parentTag": "mdt_DefinedBenefitPlanExpectedFutureBenefitPayments",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of benefit for defined benefit plan expected to be paid in fifth fiscal year following current fiscal year.",
"label": "Defined Benefit Plan, Expected Future Benefit Payment, Year Five",
"terseLabel": "2027"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureBenefitPaymentsYearFive",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanExpectedFutureBenefitPaymentsYearFour": {
"auth_ref": [
"r558"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails": {
"order": 4.0,
"parentTag": "mdt_DefinedBenefitPlanExpectedFutureBenefitPayments",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of benefit for defined benefit plan expected to be paid in fourth fiscal year following current fiscal year.",
"label": "Defined Benefit Plan, Expected Future Benefit Payment, Year Four",
"terseLabel": "2026"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureBenefitPaymentsYearFour",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanExpectedFutureBenefitPaymentsYearThree": {
"auth_ref": [
"r558"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails": {
"order": 3.0,
"parentTag": "mdt_DefinedBenefitPlanExpectedFutureBenefitPayments",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of benefit for defined benefit plan expected to be paid in third fiscal year following current fiscal year.",
"label": "Defined Benefit Plan, Expected Future Benefit Payment, Year Three",
"terseLabel": "2025"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureBenefitPaymentsYearThree",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanExpectedFutureBenefitPaymentsYearTwo": {
"auth_ref": [
"r558"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails": {
"order": 2.0,
"parentTag": "mdt_DefinedBenefitPlanExpectedFutureBenefitPayments",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of benefit for defined benefit plan expected to be paid in second fiscal year following current fiscal year.",
"label": "Defined Benefit Plan, Expected Future Benefit Payment, Year Two",
"terseLabel": "2024"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureBenefitPaymentsYearTwo",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanExpectedFutureEmployerContributionsNextFiscalYear": {
"auth_ref": [
"r559",
"r599"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of contribution expected to be received by defined benefit plan from employer in next fiscal year following current fiscal year.",
"label": "Defined Benefit Plan, Expected Future Employer Contributions, Next Fiscal Year",
"terseLabel": "Estimated future employer contributions in next fiscal year"
}
}
},
"localname": "DefinedBenefitPlanExpectedFutureEmployerContributionsNextFiscalYear",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanExpectedReturnOnPlanAssets": {
"auth_ref": [
"r524",
"r563",
"r591",
"r598",
"r599"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 3.0,
"parentTag": "us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of expected return (loss) recognized in net periodic benefit (cost) credit, calculated based on expected long-term rate of return and market-related value of plan assets of defined benefit plan.",
"label": "Defined Benefit Plan, Expected Return (Loss) on Plan Assets",
"negatedLabel": "Expected return on plan assets"
}
}
},
"localname": "DefinedBenefitPlanExpectedReturnOnPlanAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanFairValueOfPlanAssets": {
"auth_ref": [
"r537",
"r549",
"r551",
"r552",
"r598"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": 1.0,
"parentTag": "us-gaap_DefinedBenefitPlanFundedStatusOfPlan",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of asset segregated and restricted to provide benefit under defined benefit plan. Asset includes, but is not limited to, stock, bond, other investment, earning from investment, and contribution by employer and employee.",
"label": "Defined Benefit Plan, Plan Assets, Amount",
"periodEndLabel": "Fair value of plan assets at end of year",
"periodStartLabel": "Fair value of plan assets at beginning of year",
"terseLabel": "Fair value of plan assets",
"verboseLabel": "Post-retirement benefit plans, fair value of plan assets"
}
}
},
"localname": "DefinedBenefitPlanFairValueOfPlanAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanForeignCurrencyExchangeRateChangesBenefitObligation": {
"auth_ref": [
"r532"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of foreign currency translation gain (loss) which (increases) decreases benefit obligation of defined benefit plan.",
"label": "Defined Benefit Plan, Benefit Obligation, Foreign Currency Translation Gain (Loss)",
"terseLabel": "Currency exchange rate changes and other"
}
}
},
"localname": "DefinedBenefitPlanForeignCurrencyExchangeRateChangesBenefitObligation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanFundedStatusOfPlan": {
"auth_ref": [
"r522",
"r546",
"r598"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of funded (unfunded) status of defined benefit plan, measured as difference between fair value of plan assets and benefit obligation. Includes, but is not limited to, overfunded (underfunded) status.",
"label": "Defined Benefit Plan, Funded (Unfunded) Status of Plan",
"negatedTerseLabel": "Net underfunded status of the plans",
"totalLabel": "Over (under) funded status of the plans"
}
}
},
"localname": "DefinedBenefitPlanFundedStatusOfPlan",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanFundedStatusOfPlanAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Funded (Unfunded) Status of Plan [Abstract]",
"terseLabel": "Funded status at end of year:"
}
}
},
"localname": "DefinedBenefitPlanFundedStatusOfPlanAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanInterestCost": {
"auth_ref": [
"r524",
"r529",
"r562",
"r590",
"r598",
"r599"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 2.0,
"parentTag": "us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cost recognized for passage of time related to defined benefit plan.",
"label": "Defined Benefit Plan, Interest Cost",
"terseLabel": "Interest cost"
}
}
},
"localname": "DefinedBenefitPlanInterestCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost": {
"auth_ref": [
"r560",
"r588",
"r598",
"r599"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of net periodic benefit cost (credit) for defined benefit plan.",
"label": "Defined Benefit Plan, Net Periodic Benefit Cost (Credit)",
"negatedLabel": "Post-retirement benefit plans, net periodic benefit cost, income",
"totalLabel": "Net periodic benefit cost"
}
}
},
"localname": "DefinedBenefitPlanNetPeriodicBenefitCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanNetPeriodicBenefitCostAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Net Periodic Benefit Cost (Credit) [Abstract]",
"terseLabel": "Net Periodic Benefit Cost"
}
}
},
"localname": "DefinedBenefitPlanNetPeriodicBenefitCostAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanPensionPlanWithProjectedBenefitObligationInExcessOfPlanAssetsPlanAssets": {
"auth_ref": [
"r584",
"r598"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of plan asset for defined benefit pension plan with projected benefit obligation in excess of plan assets.",
"label": "Defined Benefit Plan, Pension Plan with Projected Benefit Obligation in Excess of Plan Assets, Plan Assets",
"terseLabel": "Plan assets at fair value"
}
}
},
"localname": "DefinedBenefitPlanPensionPlanWithProjectedBenefitObligationInExcessOfPlanAssetsPlanAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPensionPlanWithProjectedBenefitObligationInExcessOfPlanAssetsProjectedBenefitObligation": {
"auth_ref": [
"r584",
"r598"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of projected benefit obligation for defined benefit pension plan with projected benefit obligation in excess of plan assets.",
"label": "Defined Benefit Plan, Pension Plan with Projected Benefit Obligation in Excess of Plan Assets, Projected Benefit Obligation",
"terseLabel": "Projected benefit obligation"
}
}
},
"localname": "DefinedBenefitPlanPensionPlanWithProjectedBenefitObligationInExcessOfPlanAssetsProjectedBenefitObligation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Plan with Accumulated Benefit Obligation in Excess of Plan Assets [Abstract]",
"terseLabel": "Plans with accumulated benefit obligations in excess of plan assets"
}
}
},
"localname": "DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAggregateAccumulatedBenefitObligation": {
"auth_ref": [
"r584",
"r585",
"r598"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of accumulated benefit obligation for defined benefit plan with accumulated benefit obligation in excess of plan assets.",
"label": "Defined Benefit Plan, Plan with Accumulated Benefit Obligation in Excess of Plan Assets, Accumulated Benefit Obligation",
"terseLabel": "Accumulated benefit obligation"
}
}
},
"localname": "DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAggregateAccumulatedBenefitObligation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAggregateFairValueOfPlanAssets": {
"auth_ref": [
"r584",
"r585",
"r598"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of plan asset for defined benefit plan with accumulated benefit obligation in excess of plan assets.",
"label": "Defined Benefit Plan, Plan with Accumulated Benefit Obligation in Excess of Plan Assets, Plan Assets",
"terseLabel": "Plan assets at fair value"
}
}
},
"localname": "DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAggregateFairValueOfPlanAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAggregateProjectedBenefitObligation": {
"auth_ref": [
"r584"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of projected benefit obligation for defined benefit pension plan with accumulated benefit obligation in excess of plan assets.",
"label": "Defined Benefit Plan, Pension Plan with Accumulated Benefit Obligation in Excess of Plan Assets, Projected Benefit Obligation",
"terseLabel": "Projected benefit obligation"
}
}
},
"localname": "DefinedBenefitPlanPensionPlansWithAccumulatedBenefitObligationsInExcessOfPlanAssetsAggregateProjectedBenefitObligation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPlanAssetsBenefitsPaid": {
"auth_ref": [
"r542",
"r604"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of payment to participant under defined benefit plan which decreases plan assets. For pension plan, payment includes, but is not limited to, pension benefits and death benefits. For other postretirement plan, payment includes, but is not limited to, prescription drug benefits, health care benefits, life insurance benefits, and legal, educational and advisory services.",
"label": "Defined Benefit Plan, Plan Assets, Benefits Paid",
"negatedLabel": "Benefits paid"
}
}
},
"localname": "DefinedBenefitPlanPlanAssetsBenefitsPaid",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPlanAssetsContributionsByPlanParticipant": {
"auth_ref": [
"r541"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of contributions received by defined benefit plan from participant which increases plan assets.",
"label": "Defined Benefit Plan, Plan Assets, Contributions by Plan Participant",
"terseLabel": "Employee contributions"
}
}
},
"localname": "DefinedBenefitPlanPlanAssetsContributionsByPlanParticipant",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPlanAssetsForeignCurrencyTranslationGainLoss": {
"auth_ref": [
"r539"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of foreign currency translation gain (loss) which increases (decreases) plan assets of defined benefit plan.",
"label": "Defined Benefit Plan, Plan Assets, Foreign Currency Translation Gain (Loss)",
"terseLabel": "Currency exchange rate changes and other"
}
}
},
"localname": "DefinedBenefitPlanPlanAssetsForeignCurrencyTranslationGainLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanPlanAssetsTargetAllocationPercentage": {
"auth_ref": [
"r548",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of target investment allocation to total plan assets. Includes, but is not limited to, percentage on weighted-average basis if more than one plan.",
"label": "Defined Benefit Plan, Plan Assets, Target Allocation, Percentage",
"terseLabel": "Target Allocation",
"verboseLabel": "Target allocations"
}
}
},
"localname": "DefinedBenefitPlanPlanAssetsTargetAllocationPercentage",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DefinedBenefitPlanPlansWithBenefitObligationsInExcessOfPlanAssetsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Pension Plan with Project Benefit Obligation in Excess of Plan Assets [Abstract]",
"terseLabel": "Plans with projected benefit obligations in excess of plan assets"
}
}
},
"localname": "DefinedBenefitPlanPlansWithBenefitObligationsInExcessOfPlanAssetsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanRealEstateMember": {
"auth_ref": [
"r549",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Property composed of building, land and land improvement; in which defined benefit plan asset is invested.",
"label": "Defined Benefit Plan, Real Estate [Member]",
"terseLabel": "Real estate investments"
}
}
},
"localname": "DefinedBenefitPlanRealEstateMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DefinedBenefitPlanRecognizedNetGainLossDueToSettlements1": {
"auth_ref": [
"r525",
"r567",
"r595"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 6.0,
"parentTag": "us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of gain (loss) recognized in net periodic benefit (cost) credit from irrevocable action relieving primary responsibility for benefit obligation and eliminating risk related to obligation and assets used to effect settlement.",
"label": "Defined Benefit Plan, Net Periodic Benefit Cost (Credit), Gain (Loss) Due to Settlement",
"negatedLabel": "Settlement and curtailment (gain) loss"
}
}
},
"localname": "DefinedBenefitPlanRecognizedNetGainLossDueToSettlements1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanServiceCost": {
"auth_ref": [
"r527",
"r561",
"r589",
"r598",
"r599"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 1.0,
"parentTag": "us-gaap_DefinedBenefitPlanNetPeriodicBenefitCost",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cost for actuarial present value of benefits attributed to service rendered by employee for defined benefit plan.",
"label": "Defined Benefit Plan, Service Cost",
"terseLabel": "Service cost"
}
}
},
"localname": "DefinedBenefitPlanServiceCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanSettlementsPlanAssets": {
"auth_ref": [
"r545"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of payment, which decreases plan assets of defined benefit plan, for irrevocable action relieving primary responsibility for benefit obligation and eliminating risk for obligation and assets used to effect settlement. Transaction constituting settlement includes, but is not limited to, making lump-sum cash payment to participant in exchange for their rights to receive specified benefits and purchasing nonparticipating annuity contract.",
"label": "Defined Benefit Plan, Plan Assets, Payment for Settlement",
"negatedTerseLabel": "Plan settlements"
}
}
},
"localname": "DefinedBenefitPlanSettlementsPlanAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanSpecialTerminationBenefits": {
"auth_ref": [
"r528"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase in benefit obligation for benefits provided to employees payable from defined benefit plan or payable upon retirement.",
"label": "Defined Benefit Plan, Benefit Obligation, Special and Contractual Termination Benefits",
"terseLabel": "Special termination benefits"
}
}
},
"localname": "DefinedBenefitPlanSpecialTerminationBenefits",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DefinedBenefitPlanWeightedAverageAssetAllocations": {
"auth_ref": [
"r548"
],
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of actual investment allocation to total plan assets. Includes, but is not limited to, percentage on weighted-average basis if more than one plan.",
"label": "Defined Benefit Plan, Plan Assets, Actual Allocation, Percentage",
"terseLabel": "Actual Allocation"
}
}
},
"localname": "DefinedBenefitPlanWeightedAverageAssetAllocations",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_DefinedBenefitPlanWeightedAverageAssumptionsUsedInCalculatingBenefitObligationAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Weighted Average Assumptions Used in Calculating Benefit Obligation [Abstract]",
"terseLabel": "Critical assumptions \u2013 projected benefit obligation:"
}
}
},
"localname": "DefinedBenefitPlanWeightedAverageAssumptionsUsedInCalculatingBenefitObligationAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlanWeightedAverageAssumptionsUsedInCalculatingNetPeriodicBenefitCostAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Defined Benefit Plan, Weighted Average Assumptions Used in Calculating Net Periodic Benefit Cost [Abstract]",
"terseLabel": "Critical assumptions \u2013 net periodic benefit cost:"
}
}
},
"localname": "DefinedBenefitPlanWeightedAverageAssumptionsUsedInCalculatingNetPeriodicBenefitCostAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlansAndOtherPostretirementBenefitPlansDisclosuresTable": {
"auth_ref": [
"r582",
"r583",
"r586",
"r587",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosures and provisions pertaining to defined benefit pension plans or other postretirement defined benefit plans. The arrangements are generally based on terms and conditions stipulated by the entity, and which contain a promise by the employer to pay certain amounts or awards at designated future dates, including a period after retirement, upon compliance with stipulated requirements. Excludes disclosures pertaining to defined contribution plans.",
"label": "Defined Benefit Plans and Other Postretirement Benefit Plans Disclosures [Table]",
"terseLabel": "Defined Benefit Plans and Other Postretirement Benefit Plans Disclosures [Table]"
}
}
},
"localname": "DefinedBenefitPlansAndOtherPostretirementBenefitPlansDisclosuresTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedBenefitPlansAndOtherPostretirementBenefitPlansTableTextBlockLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items]",
"terseLabel": "Defined Benefit Plans and Other Postretirement Benefit Plans Table Text Block [Line Items]"
}
}
},
"localname": "DefinedBenefitPlansAndOtherPostretirementBenefitPlansTableTextBlockLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DefinedContributionPlanCostRecognized": {
"auth_ref": [
"r605"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cost for defined contribution plan.",
"label": "Defined Contribution Plan, Cost",
"terseLabel": "Expense under defined contribution plans"
}
}
},
"localname": "DefinedContributionPlanCostRecognized",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_Depreciation": {
"auth_ref": [
"r148",
"r357"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount of expense recognized in the current period that reflects the allocation of the cost of tangible assets over the assets' useful lives. Includes production and non-production related depreciation.",
"label": "Depreciation",
"terseLabel": "Depreciation expense",
"verboseLabel": "Depreciation Expense"
}
}
},
"localname": "Depreciation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DepreciationDepletionAndAmortization": {
"auth_ref": [
"r148",
"r237"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 8.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets.",
"label": "Depreciation, Depletion and Amortization",
"terseLabel": "Depreciation and amortization"
}
}
},
"localname": "DepreciationDepletionAndAmortization",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeAssetNotOffsetPolicyElectionDeduction": {
"auth_ref": [
"r78",
"r83"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "mdt_DerivativeAssetsandLiabilitiesNetNotOffsetPolicyElectionDeduction",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of financial asset or other contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset, elected not to be offset, deducted from derivative assets.",
"label": "Derivative Asset, Not Offset, Policy Election Deduction",
"negatedLabel": "Gross Amount Not Offset on the Balance Sheet, Financial Instruments"
}
}
},
"localname": "DerivativeAssetNotOffsetPolicyElectionDeduction",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeAssets": {
"auth_ref": [
"r77",
"r82",
"r85",
"r834"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value, after the effects of master netting arrangements, of a financial asset or other contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset. Includes assets not subject to a master netting arrangement and not elected to be offset.",
"label": "Derivative Asset",
"terseLabel": "Derivative assets"
}
}
},
"localname": "DerivativeAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeAssetsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Derivative Asset [Abstract]",
"terseLabel": "Derivative assets:"
}
}
},
"localname": "DerivativeAssetsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeCollateralObligationToReturnCash": {
"auth_ref": [
"r79",
"r83",
"r84",
"r800"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DerivativeFairValueOfDerivativeAssetAmountOffsetAgainstCollateral",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of obligation to return cash collateral under master netting arrangements that have not been offset against derivative assets.",
"label": "Derivative, Collateral, Obligation to Return Cash",
"negatedLabel": "Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted"
}
}
},
"localname": "DerivativeCollateralObligationToReturnCash",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeCollateralRightToReclaimCash": {
"auth_ref": [
"r79",
"r83",
"r84",
"r800"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_DerivativeFairValueOfDerivativeLiabilityAmountOffsetAgainstCollateral",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of right to receive cash collateral under master netting arrangements that have not been offset against derivative liabilities.",
"label": "Derivative, Collateral, Right to Reclaim Cash",
"terseLabel": "Gross Amount Not Offset on the Balance Sheet, Cash Collateral (Received) Posted"
}
}
},
"localname": "DerivativeCollateralRightToReclaimCash",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeContractTypeDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Financial instrument or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset.",
"label": "Derivative Contract [Domain]",
"terseLabel": "Derivative Contract [Domain]"
}
}
},
"localname": "DerivativeContractTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DerivativeFairValueAmountOffsetAgainstCollateralNet": {
"auth_ref": [
"r799"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails_1": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The net amount as of the balance sheet date of the fair value of derivative assets and derivative liabilities that in accordance with the entity's accounting policy were offset against collateral under a master netting arrangement.",
"label": "Derivative, Fair Value, Amount Offset Against Collateral, Net",
"totalLabel": "Net Amount"
}
}
},
"localname": "DerivativeFairValueAmountOffsetAgainstCollateralNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeFairValueOfDerivativeAsset": {
"auth_ref": [
"r76",
"r85",
"r86",
"r783",
"r905"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_DerivativeFairValueOfDerivativeAssetAmountOffsetAgainstCollateral",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value, before effects of master netting arrangements, of a financial asset or other contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset. Includes assets elected not to be offset. Excludes assets not subject to a master netting arrangement.",
"label": "Derivative Asset, Fair Value, Gross Asset",
"terseLabel": "Fair Value - Assets",
"verboseLabel": "Gross Amount of Recognized Assets (Liabilities)"
}
}
},
"localname": "DerivativeFairValueOfDerivativeAsset",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeFairValueOfDerivativeAssetAmountOffsetAgainstCollateral": {
"auth_ref": [
"r83",
"r799"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails_1": {
"order": 1.0,
"parentTag": "us-gaap_DerivativeFairValueAmountOffsetAgainstCollateralNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value, after effects of master netting arrangements, of financial asset or other contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset offset against an obligation to return collateral. Includes assets not subject to a master netting arrangement and not elected to be offset.",
"label": "Derivative Asset, Fair Value, Amount Offset Against Collateral",
"totalLabel": "Net Amount"
}
}
},
"localname": "DerivativeFairValueOfDerivativeAssetAmountOffsetAgainstCollateral",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeFairValueOfDerivativeLiability": {
"auth_ref": [
"r76",
"r85",
"r86",
"r783",
"r905"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DerivativeFairValueOfDerivativeLiabilityAmountOffsetAgainstCollateral",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value, before effects of master netting arrangements, of a financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset. Includes liabilities elected not to be offset. Excludes liabilities not subject to a master netting arrangement.",
"label": "Derivative Liability, Fair Value, Gross Liability",
"negatedTerseLabel": "Gross Amount of Recognized Assets (Liabilities)",
"terseLabel": "Fair Value - Liabilities"
}
}
},
"localname": "DerivativeFairValueOfDerivativeLiability",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeFairValueOfDerivativeLiabilityAmountOffsetAgainstCollateral": {
"auth_ref": [
"r83",
"r799"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails_1": {
"order": 2.0,
"parentTag": "us-gaap_DerivativeFairValueAmountOffsetAgainstCollateralNet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value, after effects of master netting arrangements, of financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset offset against the right to receive collateral. Includes liabilities not subject to a master netting arrangement and not elected to be offset.",
"label": "Derivative Liability, Fair Value, Amount Offset Against Collateral",
"negatedTotalLabel": "Net Amount"
}
}
},
"localname": "DerivativeFairValueOfDerivativeLiabilityAmountOffsetAgainstCollateral",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeInstrumentRiskAxis": {
"auth_ref": [
"r85",
"r781",
"r784",
"r790",
"r796"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of derivative contract.",
"label": "Derivative Instrument [Axis]",
"terseLabel": "Derivative Instrument [Axis]"
}
}
},
"localname": "DerivativeInstrumentRiskAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeInstrumentsAndHedgingActivitiesDisclosureAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Derivative Instruments and Hedging Activities Disclosure [Abstract]",
"terseLabel": "Derivative Instruments and Hedging Activities Disclosure [Abstract]"
}
}
},
"localname": "DerivativeInstrumentsAndHedgingActivitiesDisclosureAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeInstrumentsAndHedgingActivitiesDisclosureTextBlock": {
"auth_ref": [
"r806",
"r818"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for derivative instruments and hedging activities including, but not limited to, risk management strategies, non-hedging derivative instruments, assets, liabilities, revenue and expenses, and methodologies and assumptions used in determining the amounts.",
"label": "Derivative Instruments and Hedging Activities Disclosure [Text Block]",
"terseLabel": "Derivatives and Currency Exchange Risk Management"
}
}
},
"localname": "DerivativeInstrumentsAndHedgingActivitiesDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagement"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_DerivativeInstrumentsAndHedgingActivitiesDisclosuresLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Derivative Instruments and Hedging Activities Disclosures [Line Items]",
"terseLabel": "Derivative Instruments and Hedging Activities Disclosures [Line Items]"
}
}
},
"localname": "DerivativeInstrumentsAndHedgingActivitiesDisclosuresLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeInstrumentsAndHedgingActivitiesDisclosuresTable": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about derivatives and hedging activities.",
"label": "Derivative Instruments and Hedging Activities Disclosures [Table]",
"terseLabel": "Derivative Instruments and Hedging Activities Disclosures [Table]"
}
}
},
"localname": "DerivativeInstrumentsAndHedgingActivitiesDisclosuresTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeInstrumentsGainLossByHedgingRelationshipAxis": {
"auth_ref": [
"r778",
"r781",
"r790"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of hedging relationship.",
"label": "Hedging Relationship [Axis]",
"terseLabel": "Hedging Relationship [Axis]"
}
}
},
"localname": "DerivativeInstrumentsGainLossByHedgingRelationshipAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeInstrumentsGainLossByHedgingRelationshipByIncomeStatementLocationByDerivativeInstrumentRiskTable": {
"auth_ref": [
"r778",
"r781",
"r790",
"r796",
"r797",
"r801",
"r803"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about the location and amount of derivative instruments and nonderivative instruments designated as hedging instruments reported before netting adjustments, and the amount of gain (loss) on derivative instruments and nonderivative instruments designated and qualified as hedging instruments.",
"label": "Derivative Instruments, Gain (Loss) [Table]",
"terseLabel": "Derivative Instruments, (Gain) Loss [Table]"
}
}
},
"localname": "DerivativeInstrumentsGainLossByHedgingRelationshipByIncomeStatementLocationByDerivativeInstrumentRiskTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeInstrumentsGainLossLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Derivative Instruments, Gain (Loss) [Line Items]",
"terseLabel": "Derivative Instruments, (Gain) Loss [Line Items]"
}
}
},
"localname": "DerivativeInstrumentsGainLossLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeInstrumentsNotDesignatedAsHedgingInstrumentsGainLossNet": {
"auth_ref": [
"r789",
"r791"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of realized and unrealized gain (loss) of derivative instruments not designated or qualifying as hedging instruments.",
"label": "Derivative Instruments Not Designated as Hedging Instruments, Gain (Loss), Net",
"negatedTerseLabel": "Derivative instruments not designated as hedging instruments, (gain) loss, net"
}
}
},
"localname": "DerivativeInstrumentsNotDesignatedAsHedgingInstrumentsGainLossNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeLiabilities": {
"auth_ref": [
"r77",
"r82",
"r85",
"r834"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value, after the effects of master netting arrangements, of a financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset. Includes liabilities not subject to a master netting arrangement and not elected to be offset.",
"label": "Derivative Liability",
"terseLabel": "Derivative liabilities"
}
}
},
"localname": "DerivativeLiabilities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeLiabilitiesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Derivative Liability [Abstract]",
"terseLabel": "Derivative liabilities:"
}
}
},
"localname": "DerivativeLiabilitiesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativeLiabilityNotOffsetPolicyElectionDeduction": {
"auth_ref": [
"r78",
"r83"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_DerivativeFairValueOfDerivativeLiabilityAmountOffsetAgainstCollateral",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of financial liability or contract with one or more underlyings, notional amount or payment provision or both, and the contract can be net settled by means outside the contract or delivery of an asset, elected not to be offset, deducted from derivative liabilities.",
"label": "Derivative Liability, Not Offset, Policy Election Deduction",
"terseLabel": "Gross Amount Not Offset on the Balance Sheet, Financial Instruments"
}
}
},
"localname": "DerivativeLiabilityNotOffsetPolicyElectionDeduction",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativeNotionalAmount": {
"auth_ref": [
"r773",
"r775"
],
"lang": {
"en-us": {
"role": {
"documentation": "Nominal or face amount used to calculate payment on derivative.",
"label": "Derivative, Notional Amount",
"terseLabel": "Gross notional amount"
}
}
},
"localname": "DerivativeNotionalAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DerivativesFairValueLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Derivatives, Fair Value [Line Items]",
"terseLabel": "Derivatives, Fair Value [Line Items]"
}
}
},
"localname": "DerivativesFairValueLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DerivativesPolicyTextBlock": {
"auth_ref": [
"r169",
"r772",
"r774",
"r778",
"r779",
"r798"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for its derivative instruments and hedging activities.",
"label": "Derivatives, Policy [Policy Text Block]",
"terseLabel": "Derivatives"
}
}
},
"localname": "DerivativesPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_DesignatedAsHedgingInstrumentMember": {
"auth_ref": [
"r778"
],
"lang": {
"en-us": {
"role": {
"documentation": "Derivative instrument designated as hedging instrument under Generally Accepted Accounting Principles (GAAP).",
"label": "Designated as Hedging Instrument [Member]",
"terseLabel": "Derivatives designated as hedging instruments"
}
}
},
"localname": "DesignatedAsHedgingInstrumentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_DisaggregationOfRevenueLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Disaggregation of Revenue [Line Items]",
"terseLabel": "Disaggregation of Revenue [Line Items]"
}
}
},
"localname": "DisaggregationOfRevenueLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DisaggregationOfRevenueTable": {
"auth_ref": [
"r505",
"r510",
"r511",
"r512",
"r513",
"r514",
"r515",
"r516"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about disaggregation of revenue into categories depicting how nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factor.",
"label": "Disaggregation of Revenue [Table]",
"terseLabel": "Disaggregation of Revenue [Table]"
}
}
},
"localname": "DisaggregationOfRevenueTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_DisaggregationOfRevenueTableTextBlock": {
"auth_ref": [
"r505"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of disaggregation of revenue into categories depicting how nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factor.",
"label": "Disaggregation of Revenue [Table Text Block]",
"terseLabel": "Disaggregation of Revenue"
}
}
},
"localname": "DisaggregationOfRevenueTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock": {
"auth_ref": [
"r677"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for share-based payment arrangement.",
"label": "Share-based Payment Arrangement [Text Block]",
"terseLabel": "Stock Purchase and Award Plans"
}
}
},
"localname": "DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlans"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_DisclosureOfCompensationRelatedCostsSharebasedPaymentsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Share-based Payment Arrangement [Abstract]",
"terseLabel": "Share-based Payment Arrangement [Abstract]"
}
}
},
"localname": "DisclosureOfCompensationRelatedCostsSharebasedPaymentsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_DividendsCommonStockCash": {
"auth_ref": [
"r483"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of paid and unpaid common stock dividends declared with the form of settlement in cash.",
"label": "Dividends, Common Stock, Cash",
"negatedLabel": "Dividends to shareholders"
}
}
},
"localname": "DividendsCommonStockCash",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_DomesticCountryMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Designated tax department of the government that is entitled to levy and collect income taxes from the entity in its country of domicile.",
"label": "Domestic Tax Authority [Member]",
"terseLabel": "U.S. Tax Authority"
}
}
},
"localname": "DomesticCountryMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_EarningsPerShareAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Earnings Per Share [Abstract]",
"terseLabel": "Earnings Per Share [Abstract]"
}
}
},
"localname": "EarningsPerShareAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_EarningsPerShareBasic": {
"auth_ref": [
"r122",
"r189",
"r190",
"r191",
"r192",
"r193",
"r198",
"r200",
"r207",
"r209",
"r210",
"r214",
"r215",
"r816",
"r817",
"r951",
"r983"
],
"lang": {
"en-us": {
"role": {
"documentation": "The amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period.",
"label": "Earnings Per Share, Basic",
"terseLabel": "Basic earnings per share (in dollars per share)"
}
}
},
"localname": "EarningsPerShareBasic",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_EarningsPerShareDiluted": {
"auth_ref": [
"r122",
"r189",
"r190",
"r191",
"r192",
"r193",
"r200",
"r207",
"r209",
"r210",
"r214",
"r215",
"r816",
"r817",
"r951",
"r983"
],
"lang": {
"en-us": {
"role": {
"documentation": "The amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period.",
"label": "Earnings Per Share, Diluted",
"terseLabel": "Diluted earnings per share (in dollars per share)"
}
}
},
"localname": "EarningsPerShareDiluted",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_EarningsPerSharePolicyTextBlock": {
"auth_ref": [
"r211",
"r212"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements.",
"label": "Earnings Per Share, Policy [Policy Text Block]",
"terseLabel": "Earnings Per Share"
}
}
},
"localname": "EarningsPerSharePolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_EarningsPerShareTextBlock": {
"auth_ref": [
"r211",
"r212",
"r213",
"r216"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for earnings per share.",
"label": "Earnings Per Share [Text Block]",
"terseLabel": "Earnings Per Share"
}
}
},
"localname": "EarningsPerShareTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShare"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents": {
"auth_ref": [
"r846"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 4.0,
"parentTag": "us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase (decrease) from effect of exchange rate changes on cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; held in foreign currencies. Excludes amounts for disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates.",
"label": "Effect of Exchange Rate on Cash, Cash Equivalents, Restricted Cash and Restricted Cash Equivalents",
"terseLabel": "Effect of exchange rate changes on cash and cash equivalents"
}
}
},
"localname": "EffectOfExchangeRateOnCashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EffectiveIncomeTaxRateContinuingOperations": {
"auth_ref": [
"r687"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of current income tax expense (benefit) and deferred income tax expense (benefit) pertaining to continuing operations.",
"label": "Effective Income Tax Rate Reconciliation, Percent",
"totalLabel": "Effective tax rate"
}
}
},
"localname": "EffectiveIncomeTaxRateContinuingOperations",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationAtFederalStatutoryIncomeTaxRate": {
"auth_ref": [
"r166",
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 9.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of domestic federal statutory tax rate applicable to pretax income (loss).",
"label": "Effective Income Tax Rate Reconciliation, at Federal Statutory Income Tax Rate, Percent",
"terseLabel": "U.S. federal statutory tax rate"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationAtFederalStatutoryIncomeTaxRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationForeignIncomeTaxRateDifferential": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 7.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations applicable to statutory income tax expense (benefit) outside of the country of domicile.",
"label": "Effective Income Tax Rate Reconciliation, Foreign Income Tax Rate Differential, Percent",
"terseLabel": "International"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationForeignIncomeTaxRateDifferential",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationNondeductibleExpenseOther": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 10.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to other nondeductible expenses.",
"label": "Effective Income Tax Rate Reconciliation, Nondeductible Expense, Other, Percent",
"terseLabel": "Certain tax adjustments"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationNondeductibleExpenseOther",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationNondeductibleExpenseRestructuringCharges": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 13.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to restructuring charges.",
"label": "Effective Income Tax Rate Reconciliation, Nondeductible Expense, Restructuring Charges, Percent",
"terseLabel": "Legal entity restructuring"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationNondeductibleExpenseRestructuringCharges",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationNondeductibleExpenseShareBasedCompensationCost": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 4.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying domestic federal statutory income tax rate to pretax income (loss) from continuing operation, attributable to nondeductible expense for share-based payment arrangement.",
"label": "Effective Income Tax Rate Reconciliation, Nondeductible Expense, Share-based Payment Arrangement, Percent",
"terseLabel": "Stock based compensation"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationNondeductibleExpenseShareBasedCompensationCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationOtherAdjustments": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 6.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to other adjustments.",
"label": "Effective Income Tax Rate Reconciliation, Other Adjustments, Percent",
"terseLabel": "Other, net"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationOtherAdjustments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationRepatriationOfForeignEarnings": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 5.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to the repatriation of foreign earnings.",
"label": "Effective Income Tax Rate Reconciliation, Repatriation of Foreign Earnings, Percent",
"terseLabel": "U.S. tax on foreign earnings"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationRepatriationOfForeignEarnings",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationStateAndLocalIncomeTaxes": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 12.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations applicable to state and local income tax expense (benefit), net of federal tax expense (benefit).",
"label": "Effective Income Tax Rate Reconciliation, State and Local Income Taxes, Percent",
"terseLabel": "U.S. state taxes, net of federal tax benefit"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationStateAndLocalIncomeTaxes",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationTaxCreditsResearch": {
"auth_ref": [
"r687",
"r726"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails": {
"order": 11.0,
"parentTag": "us-gaap_EffectiveIncomeTaxRateContinuingOperations",
"weight": -1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to research tax credit.",
"label": "Effective Income Tax Rate Reconciliation, Tax Credit, Research, Percent",
"negatedLabel": "Research and development credit"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationTaxCreditsResearch",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesEffectiveIncomeTaxRateReconciliationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_EffectiveIncomeTaxRateReconciliationTaxCutsAndJobsActOf2017Amount": {
"auth_ref": [
"r687"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of reported income tax expense (benefit) in excess of (less than) expected income tax expense (benefit) computed by applying domestic federal statutory income tax rate to pretax income (loss) from continuing operations, attributable to Tax Cuts and Jobs Act.",
"label": "Effective Income Tax Rate Reconciliation, Tax Cuts and Jobs Act, Amount",
"negatedLabel": "Benefit from U.S. tax reform"
}
}
},
"localname": "EffectiveIncomeTaxRateReconciliationTaxCutsAndJobsActOf2017Amount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1": {
"auth_ref": [
"r662"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days.",
"label": "Share-based Payment Arrangement, Nonvested Award, Cost Not yet Recognized, Period for Recognition",
"terseLabel": "Weighted average period over which unrecognized compensation is expected to be recognized"
}
}
},
"localname": "EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedShareBasedAwardsOtherThanOptions": {
"auth_ref": [
"r662"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cost to be recognized for nonvested award under share-based payment arrangement. Excludes share and unit options.",
"label": "Share-based Payment Arrangement, Nonvested Award, Excluding Option, Cost Not yet Recognized, Amount",
"terseLabel": "Unrecognized compensation expense related to restricted stock awards"
}
}
},
"localname": "EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedShareBasedAwardsOtherThanOptions",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions": {
"auth_ref": [
"r662"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cost to be recognized for option under share-based payment arrangement.",
"label": "Share-based Payment Arrangement, Nonvested Award, Option, Cost Not yet Recognized, Amount",
"terseLabel": "Unrecognized compensation expense related to outstanding stock options"
}
}
},
"localname": "EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EmployeeServiceShareBasedCompensationTaxBenefitFromCompensationExpense": {
"auth_ref": [
"r661"
],
"calculation": {
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails": {
"order": 2.0,
"parentTag": "us-gaap_AllocatedShareBasedCompensationExpenseNetOfTax",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of tax benefit for recognition of expense of award under share-based payment arrangement.",
"label": "Share-based Payment Arrangement, Expense, Tax Benefit",
"negatedLabel": "Income tax benefits"
}
}
},
"localname": "EmployeeServiceShareBasedCompensationTaxBenefitFromCompensationExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EmployeeServiceShareBasedCompensationTaxBenefitFromExerciseOfStockOptions": {
"auth_ref": [
"r664"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of tax benefit from exercise of option under share-based payment arrangement.",
"label": "Share-based Payment Arrangement, Exercise of Option, Tax Benefit",
"terseLabel": "Tax benefit related to options exercised",
"verboseLabel": "Tax benefit related to restricted stock vested"
}
}
},
"localname": "EmployeeServiceShareBasedCompensationTaxBenefitFromExerciseOfStockOptions",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EmployeeSeveranceMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Termination of an employee associated with exit from or disposal of business activities or restructurings pursuant to a plan.",
"label": "Employee Severance [Member]",
"terseLabel": "Employee Termination Benefits"
}
}
},
"localname": "EmployeeSeveranceMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_EmployeeStockOptionMember": {
"auth_ref": [
"r659"
],
"lang": {
"en-us": {
"role": {
"documentation": "Share-based payment arrangement granting right, subject to vesting and other restrictions, to purchase or sell certain number of shares at predetermined price for specified period of time.",
"label": "Share-based Payment Arrangement, Option [Member]",
"terseLabel": "Stock options"
}
}
},
"localname": "EmployeeStockOptionMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_EnvironmentalRemediationSiteAxis": {
"auth_ref": [
"r364",
"r365",
"r366",
"r367",
"r394"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by location or named area designated for environmental remediation.",
"label": "Environmental Remediation Site [Axis]",
"terseLabel": "Environmental Remediation Site [Axis]"
}
}
},
"localname": "EnvironmentalRemediationSiteAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_EnvironmentalRemediationSiteDomain": {
"auth_ref": [
"r364"
],
"lang": {
"en-us": {
"role": {
"documentation": "Location or named area designated for environmental remediation.",
"label": "Environmental Remediation Site [Domain]",
"terseLabel": "Environmental Remediation Site [Domain]"
}
}
},
"localname": "EnvironmentalRemediationSiteDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_EquipmentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tangible personal property used to produce goods and services.",
"label": "Equipment [Member]",
"terseLabel": "Equipment"
}
}
},
"localname": "EquipmentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_EquityAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Equity [Abstract]"
}
}
},
"localname": "EquityAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_EquityComponentDomain": {
"auth_ref": [
"r2",
"r110",
"r111",
"r112",
"r180",
"r181",
"r182",
"r185",
"r194",
"r196",
"r218",
"r307",
"r476",
"r483",
"r669",
"r670",
"r671",
"r718",
"r719",
"r815",
"r848",
"r849",
"r850",
"r851",
"r852",
"r854",
"r994",
"r995",
"r996",
"r1073"
],
"lang": {
"en-us": {
"role": {
"documentation": "Components of equity are the parts of the total Equity balance including that which is allocated to common, preferred, treasury stock, retained earnings, etc.",
"label": "Equity Component [Domain]",
"terseLabel": "Equity Component [Domain]"
}
}
},
"localname": "EquityComponentDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails",
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"us-gaap_EquityFundsMember": {
"auth_ref": [
"r549"
],
"lang": {
"en-us": {
"role": {
"documentation": "An investment that pools funds from many investors to invest in a combination of underlying investments, primarily equity investments.",
"label": "Equity Funds [Member]",
"terseLabel": "Equity commingled trusts"
}
}
},
"localname": "EquityFundsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_EquityMethodInvestments": {
"auth_ref": [
"r50",
"r243",
"r300"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails": {
"order": 2.0,
"parentTag": "mdt_EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestments",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "This item represents the carrying amount on the entity's balance sheet of its investment in common stock of an equity method investee. This is not an indicator of the fair value of the investment, rather it is the initial cost adjusted for the entity's share of earnings and losses of the investee, adjusted for any distributions (dividends) and other than temporary impairment (OTTI) losses recognized.",
"label": "Equity Method Investments",
"terseLabel": "Equity method and other investments"
}
}
},
"localname": "EquityMethodInvestments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EquitySecuritiesFvNi": {
"auth_ref": [
"r833"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails": {
"order": 3.0,
"parentTag": "mdt_EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestments",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of investment in equity security measured at fair value with change in fair value recognized in net income (FV-NI), classified as current.",
"label": "Equity Securities, FV-NI, Current",
"terseLabel": "Investments with readily determinable fair value (marketable equity securities)"
}
}
},
"localname": "EquitySecuritiesFvNi",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_EquitySecuritiesWithoutReadilyDeterminableFairValueAmount": {
"auth_ref": [
"r297"
],
"calculation": {
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails": {
"order": 1.0,
"parentTag": "mdt_EquitySecuritiesFVNIEquitySecuritiesWithoutReadilyDeterminableFairValueandEquityMethodInvestments",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of investment in equity security without readily determinable fair value.",
"label": "Equity Securities without Readily Determinable Fair Value, Amount",
"terseLabel": "Investments without readily determinable fair values"
}
}
},
"localname": "EquitySecuritiesWithoutReadilyDeterminableFairValueAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ExtinguishmentOfDebtAmount": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Gross amount of debt extinguished.",
"label": "Extinguishment of Debt, Amount",
"terseLabel": "Extinguishment of debt"
}
}
},
"localname": "ExtinguishmentOfDebtAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items]",
"terseLabel": "Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items]"
}
}
},
"localname": "FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisTable": {
"auth_ref": [
"r820",
"r821",
"r822",
"r831"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about asset and liability measured at fair value on recurring and nonrecurring basis.",
"label": "Fair Value, Recurring and Nonrecurring [Table]",
"terseLabel": "Fair Value, Recurring and Nonrecurring [Table]"
}
}
},
"localname": "FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Fair Value Measurement Inputs and Valuation Techniques [Line Items]",
"terseLabel": "Fair Value Measurement Inputs and Valuation Techniques [Line Items]"
}
}
},
"localname": "FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesTable": {
"auth_ref": [
"r823"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about input and valuation technique used to measure fair value and change in valuation approach and technique for each separate class of asset and liability measured on recurring and nonrecurring basis.",
"label": "Fair Value Measurement Inputs and Valuation Techniques [Table]",
"terseLabel": "Fair Value Measurement Inputs and Valuation Techniques [Table]"
}
}
},
"localname": "FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesTableTextBlock": {
"auth_ref": [
"r823"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of input and valuation technique used to measure fair value and change in valuation approach and technique for each separate class of asset and liability measured on recurring and nonrecurring basis.",
"label": "Fair Value Measurement Inputs and Valuation Techniques [Table Text Block]",
"terseLabel": "Schedule of Significant Unobservable Inputs"
}
}
},
"localname": "FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_FairValueAssetsMeasuredOnRecurringBasisUnobservableInputReconciliationByAssetClassDomain": {
"auth_ref": [
"r826"
],
"lang": {
"en-us": {
"role": {
"documentation": "Class of asset.",
"label": "Asset Class [Domain]",
"terseLabel": "Asset Class [Domain]"
}
}
},
"localname": "FairValueAssetsMeasuredOnRecurringBasisUnobservableInputReconciliationByAssetClassDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValueAssetsMeasuredOnRecurringBasisUnobservableInputReconciliationCalculationRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Fair Value, Assets Measured on Recurring Basis, Unobservable Input Reconciliation, Calculation [Roll Forward]",
"terseLabel": "Reconciliation of Retirement Benefit Plan Assets Measured at Fair Value Using Significant Unobservable Inputs"
}
}
},
"localname": "FairValueAssetsMeasuredOnRecurringBasisUnobservableInputReconciliationCalculationRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueByAssetClassAxis": {
"auth_ref": [
"r820",
"r831"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by class of asset.",
"label": "Asset Class [Axis]",
"terseLabel": "Asset Class [Axis]"
}
}
},
"localname": "FairValueByAssetClassAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueByFairValueHierarchyLevelAxis": {
"auth_ref": [
"r434",
"r450",
"r451",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r598",
"r821",
"r895",
"r896",
"r897"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by level within fair value hierarchy and fair value measured at net asset value per share as practical expedient.",
"label": "Fair Value Hierarchy and NAV [Axis]",
"terseLabel": "Fair Value Hierarchy and NAV [Axis]"
}
}
},
"localname": "FairValueByFairValueHierarchyLevelAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueByMeasurementFrequencyAxis": {
"auth_ref": [
"r820",
"r821",
"r824",
"r825",
"r832"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by measurement frequency.",
"label": "Measurement Frequency [Axis]",
"terseLabel": "Measurement Frequency [Axis]"
}
}
},
"localname": "FairValueByMeasurementFrequencyAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueInputsLevel1Member": {
"auth_ref": [
"r434",
"r549",
"r551",
"r556",
"r598",
"r821",
"r895"
],
"lang": {
"en-us": {
"role": {
"documentation": "Quoted prices in active markets for identical assets or liabilities that the reporting entity can access at the measurement date.",
"label": "Fair Value, Inputs, Level 1 [Member]",
"terseLabel": "Level 1"
}
}
},
"localname": "FairValueInputsLevel1Member",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValueInputsLevel2Member": {
"auth_ref": [
"r434",
"r450",
"r451",
"r549",
"r551",
"r556",
"r598",
"r821",
"r896"
],
"lang": {
"en-us": {
"role": {
"documentation": "Inputs other than quoted prices included within level 1 that are observable for an asset or liability, either directly or indirectly, including, but not limited to, quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in inactive markets.",
"label": "Fair Value, Inputs, Level 2 [Member]",
"terseLabel": "Level 2"
}
}
},
"localname": "FairValueInputsLevel2Member",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValueInputsLevel3Member": {
"auth_ref": [
"r434",
"r450",
"r451",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r598",
"r821",
"r897"
],
"lang": {
"en-us": {
"role": {
"documentation": "Unobservable inputs that reflect the entity's own assumption about the assumptions market participants would use in pricing.",
"label": "Fair Value, Inputs, Level 3 [Member]",
"terseLabel": "Level 3"
}
}
},
"localname": "FairValueInputsLevel3Member",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValueInputsQuantitativeInformationAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Fair Value Measurement Inputs and Valuation Techniques [Abstract]",
"terseLabel": "Fair Value Inputs"
}
}
},
"localname": "FairValueInputsQuantitativeInformationAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FairValueMeasuredAtNetAssetValuePerShareMember": {
"auth_ref": [
"r551",
"r819",
"r832"
],
"lang": {
"en-us": {
"role": {
"documentation": "Fair value measured at net asset value per share as practical expedient.",
"label": "Fair Value Measured at Net Asset Value Per Share [Member]",
"terseLabel": "Investments measured at net asset value"
}
}
},
"localname": "FairValueMeasuredAtNetAssetValuePerShareMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValueMeasurementFrequencyDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Measurement frequency.",
"label": "Measurement Frequency [Domain]",
"terseLabel": "Measurement Frequency [Domain]"
}
}
},
"localname": "FairValueMeasurementFrequencyDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValueMeasurementPolicyPolicyTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for fair value measurements of financial and non-financial assets, liabilities and instruments classified in shareholders' equity. Disclosures include, but are not limited to, how an entity that manages a group of financial assets and liabilities on the basis of its net exposure measures the fair value of those assets and liabilities.",
"label": "Fair Value Measurement, Policy [Policy Text Block]",
"terseLabel": "Fair Value Measurements"
}
}
},
"localname": "FairValueMeasurementPolicyPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetGainLossIncludedInEarnings1": {
"auth_ref": [
"r827"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of gain (loss) recognized in income from asset measured at fair value on recurring basis using unobservable input (level 3).",
"label": "Fair Value, Measurement with Unobservable Inputs Reconciliation, Recurring Basis, Asset, Gain (Loss) Included in Earnings",
"terseLabel": "Total realized gains, net"
}
}
},
"localname": "FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetGainLossIncludedInEarnings1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetGainLossIncludedInOtherComprehensiveIncomeLoss": {
"auth_ref": [
"r828"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of gain (loss) recognized in other comprehensive income (OCI) from asset measured at fair value on recurring basis using unobservable input (level 3).",
"label": "Fair Value, Measurement with Unobservable Inputs Reconciliation, Recurring Basis, Asset, Gain (Loss) Included in Other Comprehensive Income (Loss)",
"terseLabel": "Total unrealized gains, net"
}
}
},
"localname": "FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetGainLossIncludedInOtherComprehensiveIncomeLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetPurchasesSalesIssuancesSettlements": {
"auth_ref": [
"r829"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of purchases, (sales), issuances and (settlements) of financial instrument classified as an asset measured using unobservable inputs that reflect the entity's own assumption about the assumptions market participants would use in pricing.",
"label": "Fair Value, Measurement with Unobservable Inputs Reconciliation, Recurring Basis, Asset, Purchases, (Sales), Issuances, (Settlements)",
"terseLabel": "Purchases and sales, net"
}
}
},
"localname": "FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetPurchasesSalesIssuancesSettlements",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetValue": {
"auth_ref": [
"r826"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of financial instrument classified as an asset measured using unobservable inputs that reflect the entity's own assumption about the assumptions market participants would use in pricing.",
"label": "Fair Value, Measurement with Unobservable Inputs Reconciliation, Recurring Basis, Asset Value",
"periodEndLabel": "Ending balance",
"periodStartLabel": "Beginning balance"
}
}
},
"localname": "FairValueMeasurementWithUnobservableInputsReconciliationRecurringBasisAssetValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FairValueMeasurementsFairValueHierarchyDomain": {
"auth_ref": [
"r434",
"r450",
"r451",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r598",
"r895",
"r896",
"r897"
],
"lang": {
"en-us": {
"role": {
"documentation": "Categories used to prioritize the inputs to valuation techniques to measure fair value.",
"label": "Fair Value Hierarchy and NAV [Domain]",
"terseLabel": "Fair Value Hierarchy and NAV [Domain]"
}
}
},
"localname": "FairValueMeasurementsFairValueHierarchyDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValueMeasurementsRecurringMember": {
"auth_ref": [
"r830",
"r832"
],
"lang": {
"en-us": {
"role": {
"documentation": "Frequent fair value measurement. Includes, but is not limited to, fair value adjustment for impairment of asset, liability or equity, frequently measured at fair value.",
"label": "Fair Value, Recurring [Member]",
"terseLabel": "Fair Value, Measurements, Recurring"
}
}
},
"localname": "FairValueMeasurementsRecurringMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeAssetsandLiabilitiesMeasuredatFairValueonaRecurringBasisDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FairValuesDerivativesBalanceSheetLocationByDerivativeContractTypeByHedgingDesignationTable": {
"auth_ref": [
"r780",
"r786",
"r801"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule that discloses the location and fair value amounts of derivative instruments (and nonderivative instruments that are designated and qualify as hedging instruments) reported in the statement of financial position.",
"label": "Fair Values Derivatives, Balance Sheet Location, by Derivative Contract Type [Table]",
"terseLabel": "Fair Values Derivatives, Balance Sheet Location, by Derivative Contract Type [Table]"
}
}
},
"localname": "FairValuesDerivativesBalanceSheetLocationByDerivativeContractTypeByHedgingDesignationTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FinanceLeaseLiabilityCurrent": {
"auth_ref": [
"r865"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails": {
"order": 3.0,
"parentTag": "us-gaap_DebtCurrent",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Present value of lessee's discounted obligation for lease payments from finance lease, classified as current.",
"label": "Finance Lease, Liability, Current",
"terseLabel": "Finance lease obligations"
}
}
},
"localname": "FinanceLeaseLiabilityCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FinanceLeaseLiabilityCurrentStatementOfFinancialPositionExtensibleList": {
"auth_ref": [
"r866"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicates line item in statement of financial position that includes current finance lease liability.",
"label": "Finance Lease, Liability, Current, Statement of Financial Position [Extensible Enumeration]",
"terseLabel": "Finance Lease, Liability, Current, Statement of Financial Position [Extensible Enumeration]"
}
}
},
"localname": "FinanceLeaseLiabilityCurrentStatementOfFinancialPositionExtensibleList",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "enumerationSetItemType"
},
"us-gaap_FinanceLeaseLiabilityNoncurrent": {
"auth_ref": [
"r865"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails": {
"order": 4.0,
"parentTag": "us-gaap_LongTermDebtAndCapitalLeaseObligations",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Present value of lessee's discounted obligation for lease payments from finance lease, classified as noncurrent.",
"label": "Finance Lease, Liability, Noncurrent",
"terseLabel": "Finance lease obligations"
}
}
},
"localname": "FinanceLeaseLiabilityNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FinanceLeaseLiabilityNoncurrentStatementOfFinancialPositionExtensibleList": {
"auth_ref": [
"r866"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicates line item in statement of financial position that includes noncurrent finance lease liability.",
"label": "Finance Lease, Liability, Noncurrent, Statement of Financial Position [Extensible Enumeration]",
"terseLabel": "Finance Lease, Liability, Noncurrent, Statement of Financial Position [Extensible Enumeration]"
}
}
},
"localname": "FinanceLeaseLiabilityNoncurrentStatementOfFinancialPositionExtensibleList",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "enumerationSetItemType"
},
"us-gaap_FinancialInstrumentAxis": {
"auth_ref": [
"r291",
"r292",
"r297",
"r298",
"r299",
"r311",
"r313",
"r314",
"r315",
"r316",
"r318",
"r320",
"r321",
"r322",
"r445",
"r474",
"r806",
"r892",
"r893",
"r894",
"r895",
"r896",
"r897",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r904",
"r906",
"r907",
"r908",
"r909",
"r910",
"r911",
"r912",
"r913",
"r914",
"r915",
"r916",
"r917",
"r918",
"r919",
"r920",
"r921",
"r922",
"r1037",
"r1038",
"r1039",
"r1040",
"r1041",
"r1042",
"r1043"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of financial instrument.",
"label": "Financial Instrument [Axis]",
"terseLabel": "Financial Instrument [Axis]"
}
}
},
"localname": "FinancialInstrumentAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails",
"http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FinancialInstrumentsDisclosureTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for financial instruments. This disclosure includes, but is not limited to, fair value measurements of short and long term marketable securities, international currencies forward contracts, and auction rate securities. Financial instruments may include hedging and non-hedging currency exchange instruments, derivatives, securitizations and securities available for sale at fair value. Also included are investment results, realized and unrealized gains and losses as well as impairments and risk management disclosures.",
"label": "Financial Instruments Disclosure [Text Block]",
"terseLabel": "Financial Instruments"
}
}
},
"localname": "FinancialInstrumentsDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstruments"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_FiniteLivedIntangibleAssetUsefulLife": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Useful life of finite-lived intangible assets, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.",
"label": "Finite-Lived Intangible Asset, Useful Life",
"netLabel": "Period over which amortization asset will be amortized and deducted",
"terseLabel": "Intangible assets, estimated useful life",
"verboseLabel": "Estimated useful life"
}
}
},
"localname": "FiniteLivedIntangibleAssetUsefulLife",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsAccumulatedAmortization": {
"auth_ref": [
"r348"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Accumulated amount of amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life.",
"label": "Finite-Lived Intangible Assets, Accumulated Amortization",
"negatedLabel": "Accumulated Amortization"
}
}
},
"localname": "FiniteLivedIntangibleAssetsAccumulatedAmortization",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseNextTwelveMonths": {
"auth_ref": [
"r350"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Finite-Lived Intangible Asset, Expected Amortization, Year One",
"terseLabel": "2023"
}
}
},
"localname": "FiniteLivedIntangibleAssetsAmortizationExpenseNextTwelveMonths",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsAmortizationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFive": {
"auth_ref": [
"r350"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Finite-Lived Intangible Asset, Expected Amortization, Year Five",
"terseLabel": "2027"
}
}
},
"localname": "FiniteLivedIntangibleAssetsAmortizationExpenseYearFive",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsAmortizationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearFour": {
"auth_ref": [
"r350"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Finite-Lived Intangible Asset, Expected Amortization, Year Four",
"terseLabel": "2026"
}
}
},
"localname": "FiniteLivedIntangibleAssetsAmortizationExpenseYearFour",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsAmortizationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearThree": {
"auth_ref": [
"r350"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Finite-Lived Intangible Asset, Expected Amortization, Year Three",
"terseLabel": "2025"
}
}
},
"localname": "FiniteLivedIntangibleAssetsAmortizationExpenseYearThree",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsAmortizationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsAmortizationExpenseYearTwo": {
"auth_ref": [
"r350"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of amortization for assets, excluding financial assets and goodwill, lacking physical substance with finite life expected to be recognized in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Finite-Lived Intangible Asset, Expected Amortization, Year Two",
"terseLabel": "2024"
}
}
},
"localname": "FiniteLivedIntangibleAssetsAmortizationExpenseYearTwo",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsAmortizationExpenseDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsByMajorClassAxis": {
"auth_ref": [
"r342",
"r344",
"r348",
"r352",
"r926",
"r930"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by major type or class of finite-lived intangible assets.",
"label": "Finite-Lived Intangible Assets by Major Class [Axis]",
"terseLabel": "Finite-Lived Intangible Assets by Major Class [Axis]"
}
}
},
"localname": "FiniteLivedIntangibleAssetsByMajorClassAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsGross": {
"auth_ref": [
"r348",
"r930"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount before amortization of assets, excluding financial assets and goodwill, lacking physical substance with a finite life.",
"label": "Finite-Lived Intangible Assets, Gross",
"terseLabel": "Gross Carrying Amount"
}
}
},
"localname": "FiniteLivedIntangibleAssetsGross",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Finite-Lived Intangible Assets [Line Items]",
"terseLabel": "Finite-Lived Intangible Assets [Line Items]"
}
}
},
"localname": "FiniteLivedIntangibleAssetsLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_FiniteLivedIntangibleAssetsMajorClassNameDomain": {
"auth_ref": [
"r342",
"r347"
],
"lang": {
"en-us": {
"role": {
"documentation": "The major class of finite-lived intangible asset (for example, patents, trademarks, copyrights, etc.) A major class is composed of intangible assets that can be grouped together because they are similar, either by their nature or by their use in the operations of a company.",
"label": "Finite-Lived Intangible Assets, Major Class Name [Domain]",
"terseLabel": "Finite-Lived Intangible Assets, Major Class Name [Domain]"
}
}
},
"localname": "FiniteLivedIntangibleAssetsMajorClassNameDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_FiscalPeriod": {
"auth_ref": [
"r752"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for determining an entity's fiscal year or other fiscal period. This disclosure may include identification of the fiscal period end-date, the length of the fiscal period, any reporting period lag between the entity and its subsidiaries, or equity investees. If a reporting lag exists, the closing date of the entity having a different period end is generally noted, along with an explanation of the necessity for using different closing dates. Any intervening events that materially affect the entity's financial position or results of operations are generally also disclosed.",
"label": "Fiscal Period, Policy [Policy Text Block]",
"terseLabel": "Fiscal Year-End"
}
}
},
"localname": "FiscalPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_FixedIncomeFundsMember": {
"auth_ref": [
"r549"
],
"lang": {
"en-us": {
"role": {
"documentation": "Investment that pools funds from investors to invest in a combination of underlying investments, primarily fixed income investments.",
"label": "Fixed Income Funds [Member]",
"terseLabel": "Fixed income commingled trusts"
}
}
},
"localname": "FixedIncomeFundsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ForeignCountryMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Designated tax departments of governments entitled to levy and collect income taxes from the entity outside the entity's country of domicile.",
"label": "Foreign Tax Authority [Member]",
"terseLabel": "Non-U.S. Tax Authorities"
}
}
},
"localname": "ForeignCountryMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock": {
"auth_ref": [
"r856"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for (1) transactions denominated in a currency other than the reporting enterprise's functional currency, (2) translating foreign currency financial statements that are incorporated into the financial statements of the reporting enterprise by consolidation, combination, or the equity method of accounting, and (3) remeasurement of the financial statements of a foreign reporting enterprise in a hyperinflationary economy.",
"label": "Foreign Currency Transactions and Translations Policy [Policy Text Block]",
"terseLabel": "Currency Translation"
}
}
},
"localname": "ForeignCurrencyTransactionsAndTranslationsPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ForeignExchangeContractMember": {
"auth_ref": [
"r85",
"r549",
"r795"
],
"lang": {
"en-us": {
"role": {
"documentation": "Derivative instrument whose primary underlying risk is tied to foreign exchange rates.",
"label": "Foreign Exchange Contract [Member]",
"terseLabel": "Currency exchange rate contracts"
}
}
},
"localname": "ForeignExchangeContractMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ForeignGovernmentDebtSecuritiesMember": {
"auth_ref": [
"r549",
"r1005"
],
"lang": {
"en-us": {
"role": {
"documentation": "Debt security issued by government not domiciled in United States of America (US).",
"label": "Debt Security, Government, Non-US [Member]",
"terseLabel": "Non-U.S. government and agency securities"
}
}
},
"localname": "ForeignGovernmentDebtSecuritiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ForeignPlanMember": {
"auth_ref": [
"r606",
"r610",
"r624"
],
"lang": {
"en-us": {
"role": {
"documentation": "Location of employer sponsoring plan, designed to provide retirement benefits, not determined as principal place of business. Includes, but is not limited to, defined benefit and defined contribution plans.",
"label": "Foreign Plan [Member]",
"terseLabel": "Non-U.S. Pension Benefits",
"verboseLabel": "Non-U.S."
}
}
},
"localname": "ForeignPlanMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ForwardContractsMember": {
"auth_ref": [
"r794"
],
"lang": {
"en-us": {
"role": {
"documentation": "Contracts negotiated between two parties to purchase and sell a specific quantity of a financial instrument, foreign currency, or commodity at a price specified at origination of the contract, with delivery and settlement at a specified future date.",
"label": "Forward Contracts [Member]",
"terseLabel": "Forward Contracts"
}
}
},
"localname": "ForwardContractsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_GainLossOnInvestmentsTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of realized and unrealized gain (loss) on investment in security.",
"label": "Gain (Loss) on Securities [Table Text Block]",
"terseLabel": "Schedule of Activity Related to the Company's Available for Sale Securities Portfolio"
}
}
},
"localname": "GainLossOnInvestmentsTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_GainsLossesOnExtinguishmentOfDebt": {
"auth_ref": [
"r148",
"r454",
"r455"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 7.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Difference between the fair value of payments made and the carrying amount of debt which is extinguished prior to maturity.",
"label": "Gain (Loss) on Extinguishment of Debt",
"negatedTerseLabel": "Loss on debt extinguishment"
}
}
},
"localname": "GainsLossesOnExtinguishmentOfDebt",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_Goodwill": {
"auth_ref": [
"r330",
"r332",
"r887",
"r932"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 2.0,
"parentTag": "us-gaap_Assets",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after accumulated impairment loss of an asset representing future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized.",
"label": "Goodwill",
"periodEndLabel": "Goodwill, ending balance",
"periodStartLabel": "Goodwill, beginning balance",
"terseLabel": "Goodwill"
}
}
},
"localname": "Goodwill",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_GoodwillAcquiredDuringPeriod": {
"auth_ref": [
"r333"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase in asset representing future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized resulting from a business combination.",
"label": "Goodwill, Acquired During Period",
"terseLabel": "Goodwill as a result of acquisitions"
}
}
},
"localname": "GoodwillAcquiredDuringPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_GoodwillAndIntangibleAssetsDisclosureAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Goodwill and Intangible Assets Disclosure [Abstract]",
"terseLabel": "Goodwill and Intangible Assets Disclosure [Abstract]"
}
}
},
"localname": "GoodwillAndIntangibleAssetsDisclosureAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_GoodwillAndIntangibleAssetsDisclosureTextBlock": {
"auth_ref": [
"r355"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for goodwill and intangible assets.",
"label": "Goodwill and Intangible Assets Disclosure [Text Block]",
"terseLabel": "Goodwill and Other Intangible Assets"
}
}
},
"localname": "GoodwillAndIntangibleAssetsDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssets"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_GoodwillAndIntangibleAssetsGoodwillPolicy": {
"auth_ref": [
"r337"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for goodwill. This accounting policy also may address how an entity assesses and measures impairment of goodwill, how reporting units are determined, how goodwill is allocated to such units, and how the fair values of the reporting units are determined.",
"label": "Goodwill and Intangible Assets, Goodwill, Policy [Policy Text Block]",
"terseLabel": "Goodwill"
}
}
},
"localname": "GoodwillAndIntangibleAssetsGoodwillPolicy",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_GoodwillAndIntangibleAssetsIntangibleAssetsPolicy": {
"auth_ref": [
"r345"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for intangible assets. This accounting policy may address both intangible assets subject to amortization and those that are not. The following also may be disclosed: (1) a description of intangible assets (2) the estimated useful lives of those assets (3) the amortization method used (4) how the entity assesses and measures impairment of such assets (5) how future cash flows are estimated (6) how the fair values of such asset are determined.",
"label": "Goodwill and Intangible Assets, Intangible Assets, Policy [Policy Text Block]",
"terseLabel": "Intangible Assets"
}
}
},
"localname": "GoodwillAndIntangibleAssetsIntangibleAssetsPolicy",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_GoodwillForeignCurrencyTranslationGainLoss": {
"auth_ref": [
"r335"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of foreign currency translation gain (loss) which increases (decreases) an asset representing future economic benefits from other assets acquired in a business combination that are not individually identified and separately recognized.",
"label": "Goodwill, Foreign Currency Translation Gain (Loss)",
"terseLabel": "Currency translation and other"
}
}
},
"localname": "GoodwillForeignCurrencyTranslationGainLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_GoodwillImpairmentLoss": {
"auth_ref": [
"r148",
"r331",
"r334",
"r338"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of loss from the write-down of an asset representing the future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized.",
"label": "Goodwill, Impairment Loss",
"terseLabel": "Goodwill impairment"
}
}
},
"localname": "GoodwillImpairmentLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_GoodwillLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Goodwill [Line Items]",
"terseLabel": "Goodwill [Line Items]"
}
}
},
"localname": "GoodwillLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_GoodwillPurchaseAccountingAdjustments": {
"auth_ref": [
"r336",
"r733"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase (decrease) from adjustments after acquisition date under purchase accounting of an asset representing the future economic benefits arising from other assets acquired in a business combination that are not individually identified and separately recognized.",
"label": "Goodwill, Purchase Accounting Adjustments",
"terseLabel": "Purchase accounting adjustments"
}
}
},
"localname": "GoodwillPurchaseAccountingAdjustments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_GoodwillRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Goodwill [Roll Forward]",
"terseLabel": "Goodwill [Roll Forward]"
}
}
},
"localname": "GoodwillRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_HedgingDesignationAxis": {
"auth_ref": [
"r778",
"r797"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by designation of purpose of derivative instrument.",
"label": "Hedging Designation [Axis]",
"terseLabel": "Hedging Designation [Axis]"
}
}
},
"localname": "HedgingDesignationAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_HedgingDesignationDomain": {
"auth_ref": [
"r778"
],
"lang": {
"en-us": {
"role": {
"documentation": "Designation of purpose of derivative instrument.",
"label": "Hedging Designation [Domain]",
"terseLabel": "Hedging Designation [Domain]"
}
}
},
"localname": "HedgingDesignationDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_HedgingRelationshipDomain": {
"auth_ref": [
"r778"
],
"lang": {
"en-us": {
"role": {
"documentation": "Nature or intent of a hedge.",
"label": "Hedging Relationship [Domain]",
"terseLabel": "Hedging Relationship [Domain]"
}
}
},
"localname": "HedgingRelationshipDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ImpairmentOfIntangibleAssetsFinitelived": {
"auth_ref": [
"r148",
"r353"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount of impairment loss recognized in the period resulting from the write-down of the carrying amount of a finite-lived intangible asset to fair value.",
"label": "Impairment of Intangible Assets, Finite-lived",
"negatedTerseLabel": "Impairment of finite-lived intangible assets",
"terseLabel": "Impairment of finite-lived intangible assets"
}
}
},
"localname": "ImpairmentOfIntangibleAssetsFinitelived",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ImpairmentOfIntangibleAssetsIndefinitelivedExcludingGoodwill": {
"auth_ref": [
"r148",
"r353"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of impairment loss resulting from write-down of assets, excluding financial assets and goodwill, lacking physical substance and having a projected indefinite period of benefit to fair value.",
"label": "Impairment of Intangible Assets, Indefinite-lived (Excluding Goodwill)",
"terseLabel": "Impairment of indefinite-lived intangible assets"
}
}
},
"localname": "ImpairmentOfIntangibleAssetsIndefinitelivedExcludingGoodwill",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ImpairmentOfInvestments": {
"auth_ref": [
"r281"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount by which the fair value of an investment is less than the amortized cost basis or carrying amount of that investment at the balance sheet date and the decline in fair value is deemed to be other than temporary, before considering whether or not such amount is recognized in earnings or other comprehensive income.",
"label": "Other than Temporary Impairment Losses, Investments",
"negatedTerseLabel": "Impairment losses recognized"
}
}
},
"localname": "ImpairmentOfInvestments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InProcessResearchAndDevelopmentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "In process investigation of new knowledge useful in developing new product or service or new process or technique or improvement to existing product or process, and translation of knowledge into plan or design for new product or process or for improvement to existing product or process.",
"label": "In Process Research and Development [Member]",
"terseLabel": "IPR&D"
}
}
},
"localname": "InProcessResearchAndDevelopmentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_InProcessResearchAndDevelopmentPolicy": {
"auth_ref": [
"r53",
"r679"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for costs assigned to identifiable tangible and intangible assets of an acquired entity to be used in the research and development activities of the combined enterprise. An entity also may disclose the appraisal method or significant assumptions used to value acquired research and development assets.",
"label": "In Process Research and Development, Policy [Policy Text Block]",
"terseLabel": "IPR&D"
}
}
},
"localname": "InProcessResearchAndDevelopmentPolicy",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesDomestic": {
"auth_ref": [
"r165",
"r725"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesComponentsofIncomeBeforeIncomeTaxesBasedonJurisdictionDetails": {
"order": 1.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The portion of earnings or loss from continuing operations before income taxes that is attributable to domestic operations.",
"label": "Income (Loss) from Continuing Operations before Income Taxes, Domestic",
"terseLabel": "U.S."
}
}
},
"localname": "IncomeLossFromContinuingOperationsBeforeIncomeTaxesDomestic",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesComponentsofIncomeBeforeIncomeTaxesBasedonJurisdictionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest": {
"auth_ref": [
"r116",
"r242",
"r248",
"r252",
"r255",
"r258",
"r931",
"r945",
"r953",
"r985"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 1.0,
"parentTag": "us-gaap_ProfitLoss",
"weight": 1.0
},
"http://www.medtronic.com/role/IncomeTaxesComponentsofIncomeBeforeIncomeTaxesBasedonJurisdictionDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of income (loss) from continuing operations, including income (loss) from equity method investments, before deduction of income tax expense (benefit), and income (loss) attributable to noncontrolling interest.",
"label": "Income (Loss) from Continuing Operations before Income Taxes, Noncontrolling Interest",
"totalLabel": "Income before income taxes"
}
}
},
"localname": "IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/IncomeTaxesComponentsofIncomeBeforeIncomeTaxesBasedonJurisdictionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesForeign": {
"auth_ref": [
"r165",
"r725"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesComponentsofIncomeBeforeIncomeTaxesBasedonJurisdictionDetails": {
"order": 2.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The portion of earnings or loss from continuing operations before income taxes that is attributable to foreign operations, which is defined as Income or Loss generated from operations located outside the entity's country of domicile.",
"label": "Income (Loss) from Continuing Operations before Income Taxes, Foreign",
"terseLabel": "International"
}
}
},
"localname": "IncomeLossFromContinuingOperationsBeforeIncomeTaxesForeign",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesComponentsofIncomeBeforeIncomeTaxesBasedonJurisdictionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeStatementAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Income Statement [Abstract]",
"terseLabel": "Income Statement [Abstract]"
}
}
},
"localname": "IncomeStatementAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_IncomeStatementLocationAxis": {
"auth_ref": [
"r361",
"r372"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by location in the income statement.",
"label": "Income Statement Location [Axis]",
"terseLabel": "Income Statement Location [Axis]"
}
}
},
"localname": "IncomeStatementLocationAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_IncomeStatementLocationDomain": {
"auth_ref": [
"r372"
],
"lang": {
"en-us": {
"role": {
"documentation": "Location in the income statement.",
"label": "Income Statement Location [Domain]",
"terseLabel": "Income Statement Location [Domain]"
}
}
},
"localname": "IncomeStatementLocationDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_IncomeTaxAuthorityAxis": {
"auth_ref": [
"r691"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by tax jurisdiction.",
"label": "Income Tax Authority [Axis]",
"terseLabel": "Income Tax Authority [Axis]"
}
}
},
"localname": "IncomeTaxAuthorityAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_IncomeTaxAuthorityDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Agency, division or body classification that levies income taxes, examines tax returns for compliance, or grants exemptions from or makes other decisions pertaining to income taxes.",
"label": "Income Tax Authority [Domain]",
"terseLabel": "Income Tax Authority [Domain]"
}
}
},
"localname": "IncomeTaxAuthorityDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_IncomeTaxDisclosureAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Income Tax Disclosure [Abstract]",
"terseLabel": "Income Tax Disclosure [Abstract]"
}
}
},
"localname": "IncomeTaxDisclosureAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_IncomeTaxDisclosureTextBlock": {
"auth_ref": [
"r166",
"r688",
"r700",
"r707",
"r720",
"r727",
"r729",
"r730",
"r732"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for income taxes. Disclosures may include net deferred tax liability or asset recognized in an enterprise's statement of financial position, net change during the year in the total valuation allowance, approximate tax effect of each type of temporary difference and carryforward that gives rise to a significant portion of deferred tax liabilities and deferred tax assets, utilization of a tax carryback, and tax uncertainties information.",
"label": "Income Tax Disclosure [Text Block]",
"terseLabel": "Income Taxes"
}
}
},
"localname": "IncomeTaxDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxes"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_IncomeTaxExaminationInterestExpense": {
"auth_ref": [
"r689"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount of estimated interest recognized in the period arising from income tax examinations.",
"label": "Income Tax Examination, Interest Expense",
"terseLabel": "Gross interest (income) expense"
}
}
},
"localname": "IncomeTaxExaminationInterestExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxExpenseBenefit": {
"auth_ref": [
"r167",
"r195",
"r196",
"r240",
"r686",
"r721",
"r728",
"r986"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 2.0,
"parentTag": "us-gaap_ProfitLoss",
"weight": -1.0
},
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of current income tax expense (benefit) and deferred income tax expense (benefit) pertaining to continuing operations.",
"label": "Income Tax Expense (Benefit)",
"terseLabel": "Income tax provision (benefit)",
"totalLabel": "Income tax provision (benefit)"
}
}
},
"localname": "IncomeTaxExpenseBenefit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/IncomeTaxesIncomeTaxBenefitProvisionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxHolidayIncomeTaxBenefitsPerShare": {
"auth_ref": [
"r727"
],
"lang": {
"en-us": {
"role": {
"documentation": "Per share amount effect of the income tax benefit resulting from the income tax holidays granted by taxing jurisdictions.",
"label": "Income Tax Holiday, Income Tax Benefits Per Share",
"terseLabel": "Impact on diluted earnings per share (in dollars per share)"
}
}
},
"localname": "IncomeTaxHolidayIncomeTaxBenefitsPerShare",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_IncomeTaxPolicyTextBlock": {
"auth_ref": [
"r109",
"r682",
"r683",
"r700",
"r701",
"r706",
"r714"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements.",
"label": "Income Tax, Policy [Policy Text Block]",
"terseLabel": "Income Taxes"
}
}
},
"localname": "IncomeTaxPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_IncomeTaxReceivable": {
"auth_ref": [
"r48",
"r963"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails_1": {
"order": 3.0,
"parentTag": "us-gaap_DeferredTaxAssetsLiabilitiesNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount as of the balance sheet date of income taxes previously overpaid to tax authorities (such as U.S. Federal, state and local tax authorities) representing refunds of overpayments or recoveries based on agreed-upon resolutions of disputes. Also called income tax refund receivable.",
"label": "Income Taxes Receivable",
"terseLabel": "Income tax receivables"
}
}
},
"localname": "IncomeTaxReceivable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationChangeInDeferredTaxAssetsValuationAllowance": {
"auth_ref": [
"r687"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to increase (decrease) in the valuation allowance for deferred tax assets.",
"label": "Effective Income Tax Rate Reconciliation, Change in Deferred Tax Assets Valuation Allowance, Amount",
"negatedTerseLabel": "Benefit related to release of valuation allowance"
}
}
},
"localname": "IncomeTaxReconciliationChangeInDeferredTaxAssetsValuationAllowance",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationDispositionOfAssets": {
"auth_ref": [
"r687"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying domestic federal statutory income tax rates to pretax income (loss) from continuing operations, attributable to disposition of asset. Includes, but is not limited to, intra-entity transfer of asset other than inventory.",
"label": "Effective Income Tax Rate Reconciliation, Disposition of Asset, Amount",
"terseLabel": "Intercompany sale of assets"
}
}
},
"localname": "IncomeTaxReconciliationDispositionOfAssets",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationNondeductibleExpenseAmortization": {
"auth_ref": [
"r687"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to nondeductible amortization.",
"label": "Effective Income Tax Rate Reconciliation, Nondeductible Expense, Amortization, Amount",
"terseLabel": "Tax expense associated with the amortization of the previously established deferred tax assets"
}
}
},
"localname": "IncomeTaxReconciliationNondeductibleExpenseAmortization",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationOtherReconcilingItems": {
"auth_ref": [
"r687"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to tax exempt income, equity in earnings (loss) of an unconsolidated subsidiary, minority noncontrolling interest income (loss), tax holiday, disposition of a business, disposition of an asset, repatriation of foreign earnings, repatriation of foreign earnings jobs creation act of 2004, increase (decrease) in enacted tax rate, prior year income taxes, increase (decrease) in deferred tax asset valuation allowance, and other adjustments.",
"label": "Effective Income Tax Rate Reconciliation, Other Reconciling Items, Amount",
"negatedTerseLabel": "Net benefit from certain tax adjustments"
}
}
},
"localname": "IncomeTaxReconciliationOtherReconcilingItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationTaxContingenciesForeign": {
"auth_ref": [
"r687"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to increase (decrease) in foreign income tax contingency.",
"label": "Effective Income Tax Rate Reconciliation, Tax Contingency, Foreign, Amount",
"negatedTerseLabel": "Benefit related to tax legislative changes in Switzerland"
}
}
},
"localname": "IncomeTaxReconciliationTaxContingenciesForeign",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationTaxCreditsResearch": {
"auth_ref": [
"r687"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to research tax credit.",
"label": "Effective Income Tax Rate Reconciliation, Tax Credit, Research, Amount",
"negatedLabel": "Tax benefit related to capitalization of research and development costs"
}
}
},
"localname": "IncomeTaxReconciliationTaxCreditsResearch",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationTaxHolidays": {
"auth_ref": [
"r687"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to income exempt from income tax because of a tax holiday.",
"label": "Effective Income Tax Rate Reconciliation, Tax Holiday, Amount",
"terseLabel": "Tax reductions from tax holiday"
}
}
},
"localname": "IncomeTaxReconciliationTaxHolidays",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxReconciliationTaxSettlements": {
"auth_ref": [
"r687"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the difference between reported income tax expense (benefit) and expected income tax expense (benefit) computed by applying the domestic federal statutory income tax rates to pretax income (loss) from continuing operations attributable to income tax settlements. Including, but not limited to, domestic tax settlement, foreign tax settlement, state and local tax settlement, and other tax settlements.",
"label": "Effective Income Tax Rate Reconciliation, Tax Settlement, Amount",
"negatedLabel": "Benefit associated with tax basis for Swiss Cantonal"
}
}
},
"localname": "IncomeTaxReconciliationTaxSettlements",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncomeTaxesPaidNet": {
"auth_ref": [
"r153"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount of cash paid during the current period to foreign, federal, state, and local authorities as taxes on income, net of any cash received during the current period as refunds for the overpayment of taxes.",
"label": "Income Taxes Paid, Net",
"terseLabel": "Income taxes"
}
}
},
"localname": "IncomeTaxesPaidNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncreaseDecreaseInAccountsAndNotesReceivable": {
"auth_ref": [
"r147"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 6.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The increase (decrease) during the reporting period of the sum of amounts due within one year (or one business cycle) from customers for the credit sale of goods and services; and from note holders for outstanding loans.",
"label": "Increase (Decrease) in Accounts and Notes Receivable",
"negatedLabel": "Accounts receivable, net"
}
}
},
"localname": "IncreaseDecreaseInAccountsAndNotesReceivable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities": {
"auth_ref": [
"r147"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 12.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid.",
"label": "Increase (Decrease) in Accounts Payable and Accrued Liabilities",
"terseLabel": "Accounts payable and accrued liabilities"
}
}
},
"localname": "IncreaseDecreaseInAccountsPayableAndAccruedLiabilities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncreaseDecreaseInInventories": {
"auth_ref": [
"r147"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 2.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The increase (decrease) during the reporting period in the aggregate value of all inventory held by the reporting entity, associated with underlying transactions that are classified as operating activities.",
"label": "Increase (Decrease) in Inventories",
"negatedLabel": "Inventories, net"
}
}
},
"localname": "IncreaseDecreaseInInventories",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncreaseDecreaseInOperatingCapitalAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Increase (Decrease) in Operating Capital [Abstract]",
"terseLabel": "Change in operating assets and liabilities, net of acquisitions and divestitures:"
}
}
},
"localname": "IncreaseDecreaseInOperatingCapitalAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "stringItemType"
},
"us-gaap_IncreaseDecreaseInOtherOperatingCapitalNet": {
"auth_ref": [
"r147"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 5.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase (decrease) in operating assets after deduction of operating liabilities classified as other.",
"label": "Increase (Decrease) in Other Operating Assets and Liabilities, Net",
"negatedLabel": "Other operating assets and liabilities"
}
}
},
"localname": "IncreaseDecreaseInOtherOperatingCapitalNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IncreaseDecreaseInStockholdersEquityRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Increase (Decrease) in Stockholders' Equity [Roll Forward]",
"terseLabel": "Increase (Decrease) in Stockholders' Equity [Roll Forward]"
}
}
},
"localname": "IncreaseDecreaseInStockholdersEquityRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "stringItemType"
},
"us-gaap_IncrementalCommonSharesAttributableToNonvestedSharesWithForfeitableDividends": {
"auth_ref": [
"r201",
"r202",
"r203",
"r208",
"r210"
],
"calculation": {
"http://www.medtronic.com/role/EarningsPerShareDetails": {
"order": 3.0,
"parentTag": "us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Number of additional shares included in diluted EPS for potentially dilutive effect of nonvested equity-based payment award containing forfeitable rights to dividends or dividend equivalents, whether paid or unpaid.",
"label": "Incremental Common Shares Attributable to Dilutive Effect of Nonvested Shares with Forfeitable Dividends",
"terseLabel": "Employee restricted stock units (in shares)"
}
}
},
"localname": "IncrementalCommonSharesAttributableToNonvestedSharesWithForfeitableDividends",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_IncrementalCommonSharesAttributableToShareBasedPaymentArrangements": {
"auth_ref": [
"r201",
"r202",
"r203",
"r210"
],
"calculation": {
"http://www.medtronic.com/role/EarningsPerShareDetails": {
"order": 2.0,
"parentTag": "us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Additional shares included in the calculation of diluted EPS as a result of the potentially dilutive effect of share based payment arrangements using the treasury stock method.",
"label": "Incremental Common Shares Attributable to Dilutive Effect of Share-based Payment Arrangements",
"terseLabel": "Employee stock options (in shares)"
}
}
},
"localname": "IncrementalCommonSharesAttributableToShareBasedPaymentArrangements",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_IndefiniteLivedIntangibleAssetsByMajorClassAxis": {
"auth_ref": [
"r343",
"r351"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type or class of assets, excluding financial assets and goodwill, lacking physical substance and having a projected indefinite period of benefit.",
"label": "Indefinite-lived Intangible Assets [Axis]",
"terseLabel": "Indefinite-lived Intangible Assets [Axis]"
}
}
},
"localname": "IndefiniteLivedIntangibleAssetsByMajorClassAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_IndefiniteLivedIntangibleAssetsExcludingGoodwill": {
"auth_ref": [
"r351"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of assets, excluding financial assets and goodwill, lacking physical substance and having a projected indefinite period of benefit.",
"label": "Indefinite-lived Intangible Assets (Excluding Goodwill)",
"terseLabel": "Indefinite-lived Intangible Assets"
}
}
},
"localname": "IndefiniteLivedIntangibleAssetsExcludingGoodwill",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IndefiniteLivedIntangibleAssetsMajorClassNameDomain": {
"auth_ref": [
"r343",
"r351"
],
"lang": {
"en-us": {
"role": {
"documentation": "The major class of indefinite-lived intangible asset (for example, trade names, etc. but not all-inclusive), excluding goodwill. A major class is composed of intangible assets that can be grouped together because they are similar, either by their nature or by their use in the operations of the company.",
"label": "Indefinite-lived Intangible Assets, Major Class Name [Domain]",
"terseLabel": "Indefinite-lived Intangible Assets, Major Class Name [Domain]"
}
}
},
"localname": "IndefiniteLivedIntangibleAssetsMajorClassNameDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_IntangibleAssetsNetExcludingGoodwill": {
"auth_ref": [
"r340",
"r346"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 4.0,
"parentTag": "us-gaap_Assets",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Sum of the carrying amounts of all intangible assets, excluding goodwill, as of the balance sheet date, net of accumulated amortization and impairment charges.",
"label": "Intangible Assets, Net (Excluding Goodwill)",
"terseLabel": "Other intangible assets, net"
}
}
},
"localname": "IntangibleAssetsNetExcludingGoodwill",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_IntangibleAssetsNetExcludingGoodwillAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Intangible Assets, Net (Excluding Goodwill) [Abstract]",
"terseLabel": "Intangible Assets"
}
}
},
"localname": "IntangibleAssetsNetExcludingGoodwillAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_InterestExpense": {
"auth_ref": [
"r114",
"r236",
"r857",
"r860",
"r952"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 3.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of the cost of borrowed funds accounted for as interest expense.",
"label": "Interest Expense",
"terseLabel": "Interest expense"
}
}
},
"localname": "InterestExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InterestIncomeExpenseNonoperatingNet": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 10.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The net amount of nonoperating interest income (expense).",
"label": "Interest Income (Expense), Nonoperating, Net",
"negatedLabel": "Interest expense"
}
}
},
"localname": "InterestIncomeExpenseNonoperatingNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InterestPaidNet": {
"auth_ref": [
"r141",
"r145",
"r153"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount.",
"label": "Interest Paid, Excluding Capitalized Interest, Operating Activities",
"terseLabel": "Interest"
}
}
},
"localname": "InterestPaidNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InventoryDisclosureAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Inventory Disclosure [Abstract]",
"terseLabel": "Inventory Disclosure [Abstract]"
}
}
},
"localname": "InventoryDisclosureAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_InventoryDisclosureTextBlock": {
"auth_ref": [
"r327"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for inventory. Includes, but is not limited to, the basis of stating inventory, the method of determining inventory cost, the classes of inventory, and the nature of the cost elements included in inventory.",
"label": "Inventory Disclosure [Text Block]",
"terseLabel": "Inventories"
}
}
},
"localname": "InventoryDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/Inventories"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_InventoryFinishedGoodsNetOfReserves": {
"auth_ref": [
"r42",
"r326"
],
"calculation": {
"http://www.medtronic.com/role/InventoriesDetails": {
"order": 1.0,
"parentTag": "us-gaap_InventoryNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount, net of valuation reserves and adjustments, as of the balance sheet date of merchandise or goods held by the company that are readily available for sale.",
"label": "Inventory, Finished Goods, Net of Reserves",
"terseLabel": "Finished goods"
}
}
},
"localname": "InventoryFinishedGoodsNetOfReserves",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/InventoriesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InventoryNet": {
"auth_ref": [
"r9",
"r70",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 4.0,
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0
},
"http://www.medtronic.com/role/InventoriesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after valuation and LIFO reserves of inventory expected to be sold, or consumed within one year or operating cycle, if longer.",
"label": "Inventory, Net",
"terseLabel": "Inventories, net",
"totalLabel": "Total"
}
}
},
"localname": "InventoryNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/InventoriesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InventoryPolicyTextBlock": {
"auth_ref": [
"r18",
"r71",
"r157",
"r217",
"r323",
"r325",
"r327",
"r924"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of inventory accounting policy for inventory classes, including, but not limited to, basis for determining inventory amounts, methods by which amounts are added and removed from inventory classes, loss recognition on impairment of inventories, and situations in which inventories are stated above cost.",
"label": "Inventory, Policy [Policy Text Block]",
"terseLabel": "Inventories"
}
}
},
"localname": "InventoryPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_InventoryRawMaterialsNetOfReserves": {
"auth_ref": [
"r44",
"r326"
],
"calculation": {
"http://www.medtronic.com/role/InventoriesDetails": {
"order": 3.0,
"parentTag": "us-gaap_InventoryNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount, net of valuation reserves and adjustments, as of the balance sheet date of unprocessed items to be consumed in the manufacturing or production process.",
"label": "Inventory, Raw Materials, Net of Reserves",
"terseLabel": "Raw materials"
}
}
},
"localname": "InventoryRawMaterialsNetOfReserves",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/InventoriesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InventoryValuationReserveMember": {
"auth_ref": [
"r171",
"r172",
"r173",
"r176",
"r177"
],
"lang": {
"en-us": {
"role": {
"documentation": "Reserve to reduce inventory to lower of cost or net realizable value.",
"label": "SEC Schedule, 12-09, Reserve, Inventory [Member]",
"terseLabel": "Inventory Reserve"
}
}
},
"localname": "InventoryValuationReserveMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_InventoryWorkInProcessNetOfReserves": {
"auth_ref": [
"r43",
"r326"
],
"calculation": {
"http://www.medtronic.com/role/InventoriesDetails": {
"order": 2.0,
"parentTag": "us-gaap_InventoryNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount, net of reserves and adjustments, as of the balance sheet date of merchandise or goods which are partially completed. This inventory is generally comprised of raw materials, labor and factory overhead costs, which require further materials, labor and overhead to be converted into finished goods, and which generally require the use of estimates to determine percentage complete and pricing.",
"label": "Inventory, Work in Process, Net of Reserves",
"terseLabel": "Work-in-process"
}
}
},
"localname": "InventoryWorkInProcessNetOfReserves",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/InventoriesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InventoryWriteDown": {
"auth_ref": [
"r324"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of loss from reductions in inventory due to subsequent measurement adjustments, including, but not limited to, physical deterioration, obsolescence, or changes in price levels.",
"label": "Inventory Write-down",
"terseLabel": "Inventory write-down"
}
}
},
"localname": "InventoryWriteDown",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_InvestmentPolicyTextBlock": {
"auth_ref": [
"r301",
"r984"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for investment in financial asset.",
"label": "Investment, Policy [Policy Text Block]",
"terseLabel": "Investments"
}
}
},
"localname": "InvestmentPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_InvestmentsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Investments [Abstract]",
"terseLabel": "Investments [Abstract]"
}
}
},
"localname": "InvestmentsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_InvestmentsClassifiedByContractualMaturityDateTableTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of maturities of an entity's investments as well as any other information pertinent to the investments.",
"label": "Investments Classified by Contractual Maturity Date [Table Text Block]",
"terseLabel": "Schedule of Available-for-sale Debt Securities Contractual Maturities"
}
}
},
"localname": "InvestmentsClassifiedByContractualMaturityDateTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_InvestmentsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Assets held for their financial return, rather than for the entity's operations.",
"label": "Investments [Member]",
"terseLabel": "Investments"
}
}
},
"localname": "InvestmentsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_LandAndLandImprovementsMember": {
"auth_ref": [
"r15"
],
"lang": {
"en-us": {
"role": {
"documentation": "Real estate held and assets that are an addition or improvement to real estate held.",
"label": "Land and Land Improvements [Member]",
"terseLabel": "Land and land improvements"
}
}
},
"localname": "LandAndLandImprovementsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_LeaseCost": {
"auth_ref": [
"r876",
"r878"
],
"calculation": {
"http://www.medtronic.com/role/LeasesComponentsofTotalOperatingLeaseCostDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lease cost recognized by lessee for lease contract.",
"label": "Lease, Cost",
"totalLabel": "Total operating lease cost"
}
}
},
"localname": "LeaseCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesComponentsofTotalOperatingLeaseCostDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LeaseCostTableTextBlock": {
"auth_ref": [
"r876"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income.",
"label": "Lease, Cost [Table Text Block]",
"terseLabel": "Schedule of Components of Total Operating Lease Cost and Supplemental Lease Information"
}
}
},
"localname": "LeaseCostTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_LeasesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Leases [Abstract]",
"terseLabel": "Leases [Abstract]"
}
}
},
"localname": "LeasesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock": {
"auth_ref": [
"r877"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position.",
"label": "Lessee, Operating Lease, Liability, Maturity [Table Text Block]",
"terseLabel": "Schedule of Maturities of Operating Leases"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityMaturityTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails_1": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease.",
"label": "Lessee, Operating Lease, Liability, to be Paid",
"totalLabel": "Total expected lease payments"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityPaymentsDue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFive": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails_1": {
"order": 6.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease due after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Lessee, Operating Lease, Liability, to be Paid, after Year Five",
"terseLabel": "Thereafter"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueAfterYearFive",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails_1": {
"order": 1.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year One",
"terseLabel": "2023"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFive": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails_1": {
"order": 5.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year Five",
"terseLabel": "2027"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueYearFive",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails_1": {
"order": 4.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year Four",
"terseLabel": "2026"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueYearFour",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails_1": {
"order": 3.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year Three",
"terseLabel": "2025"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueYearThree",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails_1": {
"order": 2.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year Two",
"terseLabel": "2024"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueYearTwo",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount": {
"auth_ref": [
"r877"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails": {
"order": 1.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease.",
"label": "Lessee, Operating Lease, Liability, Undiscounted Excess Amount",
"negatedLabel": "Less: Imputed interest"
}
}
},
"localname": "LesseeOperatingLeaseLiabilityUndiscountedExcessAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LesseeOperatingLeasesTextBlock": {
"auth_ref": [
"r879"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability.",
"label": "Lessee, Operating Leases [Text Block]",
"terseLabel": "Leases"
}
}
},
"localname": "LesseeOperatingLeasesTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/Leases"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_Liabilities": {
"auth_ref": [
"r60",
"r163",
"r250",
"r303",
"r404",
"r405",
"r406",
"r409",
"r410",
"r411",
"r413",
"r415",
"r417",
"r418",
"r759",
"r766",
"r767",
"r835",
"r885",
"r886"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 1.0,
"parentTag": "us-gaap_LiabilitiesAndStockholdersEquity",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Sum of the carrying amounts as of the balance sheet date of all liabilities that are recognized. Liabilities are probable future sacrifices of economic benefits arising from present obligations of an entity to transfer assets or provide services to other entities in the future.",
"label": "Liabilities",
"totalLabel": "Total liabilities"
}
}
},
"localname": "Liabilities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LiabilitiesAndStockholdersEquity": {
"auth_ref": [
"r40",
"r163",
"r303",
"r835",
"r887",
"r940",
"r975"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any.",
"label": "Liabilities and Equity",
"totalLabel": "Total liabilities and equity"
}
}
},
"localname": "LiabilitiesAndStockholdersEquity",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LiabilitiesAndStockholdersEquityAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Liabilities and Equity [Abstract]",
"terseLabel": "LIABILITIES AND EQUITY"
}
}
},
"localname": "LiabilitiesAndStockholdersEquityAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "stringItemType"
},
"us-gaap_LiabilitiesCurrent": {
"auth_ref": [
"r62",
"r163",
"r303",
"r404",
"r405",
"r406",
"r409",
"r410",
"r411",
"r413",
"r415",
"r417",
"r418",
"r759",
"r766",
"r767",
"r835",
"r885",
"r886",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 1.0,
"parentTag": "us-gaap_Liabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Total obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer.",
"label": "Liabilities, Current",
"totalLabel": "Total current liabilities"
}
}
},
"localname": "LiabilitiesCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LiabilitiesCurrentAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Liabilities, Current [Abstract]",
"terseLabel": "Current liabilities:"
}
}
},
"localname": "LiabilitiesCurrentAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "stringItemType"
},
"us-gaap_LiabilityForUncertainTaxPositionsNoncurrent": {
"auth_ref": [
"r65"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount recognized for uncertainty in income taxes classified as noncurrent.",
"label": "Liability for Uncertainty in Income Taxes, Noncurrent",
"terseLabel": "Gross unrecognized tax benefits, net of cash advance, noncurrent liability"
}
}
},
"localname": "LiabilityForUncertainTaxPositionsNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LineOfCredit": {
"auth_ref": [
"r27",
"r938",
"r965"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The carrying value as of the balance sheet date of the current and noncurrent portions of long-term obligations drawn from a line of credit, which is a bank's commitment to make loans up to a specific amount. Examples of items that might be included in the application of this element may consist of letters of credit, standby letters of credit, and revolving credit arrangements, under which borrowings can be made up to a maximum amount as of any point in time conditional on satisfaction of specified terms before, as of and after the date of drawdowns on the line. Includes short-term obligations that would normally be classified as current liabilities but for which (a) postbalance sheet date issuance of a long term obligation to refinance the short term obligation on a long term basis, or (b) the enterprise has entered into a financing agreement that clearly permits the enterprise to refinance the short-term obligation on a long term basis and the following conditions are met (1) the agreement does not expire within 1 year and is not cancelable by the lender except for violation of an objectively determinable provision, (2) no violation exists at the BS date, and (3) the lender has entered into the financing agreement is expected to be financially capable of honoring the agreement.",
"label": "Long-term Line of Credit",
"terseLabel": "Committed line of credit outstanding"
}
}
},
"localname": "LineOfCredit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LineOfCreditFacilityMaximumBorrowingCapacity": {
"auth_ref": [
"r57"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Maximum borrowing capacity under the credit facility without consideration of any current restrictions on the amount that could be borrowed or the amounts currently outstanding under the facility.",
"label": "Line of Credit Facility, Maximum Borrowing Capacity",
"terseLabel": "Maximum borrowing capacity"
}
}
},
"localname": "LineOfCreditFacilityMaximumBorrowingCapacity",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LineOfCreditMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A contractual arrangement with a lender under which borrowings can be made up to a specific amount at any point in time, and under which borrowings outstanding may be either short-term or long-term, depending upon the particulars.",
"label": "Line of Credit [Member]",
"terseLabel": "Credit Facility"
}
}
},
"localname": "LineOfCreditMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_LitigationSettlementAmountAwardedFromOtherParty": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount awarded from other party in judgment or settlement of litigation.",
"label": "Litigation Settlement, Amount Awarded from Other Party",
"terseLabel": "Settlement consideration received"
}
}
},
"localname": "LitigationSettlementAmountAwardedFromOtherParty",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LitigationStatusAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by status of pending, threatened, or settled litigation.",
"label": "Litigation Status [Axis]",
"terseLabel": "Litigation Status [Axis]"
}
}
},
"localname": "LitigationStatusAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_LitigationStatusDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Status of pending, threatened, or settled litigation.",
"label": "Litigation Status [Domain]",
"terseLabel": "Litigation Status [Domain]"
}
}
},
"localname": "LitigationStatusDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_LongTermDebt": {
"auth_ref": [
"r27",
"r433",
"r448",
"r450",
"r451",
"r938",
"r971"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after unamortized (discount) premium and debt issuance costs, of long-term debt. Includes, but not limited to, notes payable, bonds payable, debentures, mortgage loans and commercial paper. Excludes capital lease obligations.",
"label": "Long-term Debt",
"terseLabel": "Outstanding debt"
}
}
},
"localname": "LongTermDebt",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtAndCapitalLeaseObligations": {
"auth_ref": [
"r27"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 6.0,
"parentTag": "us-gaap_Liabilities",
"weight": 1.0
},
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of long-term debt and lease obligation, classified as noncurrent.",
"label": "Long-term Debt and Lease Obligation",
"terseLabel": "Long-term debt",
"totalLabel": "Long-term debt"
}
}
},
"localname": "LongTermDebtAndCapitalLeaseObligations",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtByMaturityAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Long-term Debt, Fiscal Year Maturity [Abstract]",
"terseLabel": "Long-term Debt, Fiscal Year Maturity"
}
}
},
"localname": "LongTermDebtByMaturityAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_LongTermDebtCurrent": {
"auth_ref": [
"r59"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails": {
"order": 1.0,
"parentTag": "us-gaap_DebtCurrent",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after unamortized (discount) premium and debt issuance costs, of long-term debt, classified as current. Includes, but not limited to, notes payable, bonds payable, debentures, mortgage loans and commercial paper. Excludes capital lease obligations.",
"label": "Long-term Debt, Current Maturities",
"verboseLabel": "Current maturities of long-term debt"
}
}
},
"localname": "LongTermDebtCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtFairValue": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The fair value amount of long-term debt whether such amount is presented as a separate caption or as a parenthetical disclosure. Additionally, this element may be used in connection with the fair value disclosures required in the footnote disclosures to the financial statements. The element may be used in both the balance sheet and disclosure in the same submission.",
"label": "Long-term Debt, Fair Value",
"terseLabel": "Estimated fair value of senior notes"
}
}
},
"localname": "LongTermDebtFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalAfterYearFive": {
"auth_ref": [
"r170",
"r401",
"r438"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails": {
"order": 6.0,
"parentTag": "us-gaap_DebtInstrumentCarryingAmount",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing after fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Long-Term Debt, Maturity, after Year Five",
"terseLabel": "Thereafter"
}
}
},
"localname": "LongTermDebtMaturitiesRepaymentsOfPrincipalAfterYearFive",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInNextTwelveMonths": {
"auth_ref": [
"r170",
"r401",
"r438"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails": {
"order": 1.0,
"parentTag": "us-gaap_DebtInstrumentCarryingAmount",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Long-Term Debt, Maturity, Year One",
"terseLabel": "2023"
}
}
},
"localname": "LongTermDebtMaturitiesRepaymentsOfPrincipalInNextTwelveMonths",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearFive": {
"auth_ref": [
"r170",
"r401",
"r438"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails": {
"order": 5.0,
"parentTag": "us-gaap_DebtInstrumentCarryingAmount",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in fifth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Long-Term Debt, Maturity, Year Five",
"terseLabel": "2027"
}
}
},
"localname": "LongTermDebtMaturitiesRepaymentsOfPrincipalInYearFive",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearFour": {
"auth_ref": [
"r170",
"r401",
"r438"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails": {
"order": 4.0,
"parentTag": "us-gaap_DebtInstrumentCarryingAmount",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Long-Term Debt, Maturity, Year Four",
"terseLabel": "2026"
}
}
},
"localname": "LongTermDebtMaturitiesRepaymentsOfPrincipalInYearFour",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearThree": {
"auth_ref": [
"r170",
"r401",
"r438"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails": {
"order": 3.0,
"parentTag": "us-gaap_DebtInstrumentCarryingAmount",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Long-Term Debt, Maturity, Year Three",
"terseLabel": "2025"
}
}
},
"localname": "LongTermDebtMaturitiesRepaymentsOfPrincipalInYearThree",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearTwo": {
"auth_ref": [
"r170",
"r401",
"r438"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails": {
"order": 2.0,
"parentTag": "us-gaap_DebtInstrumentCarryingAmount",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach).",
"label": "Long-Term Debt, Maturity, Year Two",
"terseLabel": "2024"
}
}
},
"localname": "LongTermDebtMaturitiesRepaymentsOfPrincipalInYearTwo",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongTermDebtNoncurrent": {
"auth_ref": [
"r64"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails": {
"order": 3.0,
"parentTag": "us-gaap_LongTermDebtAndCapitalLeaseObligations",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after unamortized (discount) premium and debt issuance costs of long-term debt classified as noncurrent and excluding amounts to be repaid within one year or the normal operating cycle, if longer. Includes, but not limited to, notes payable, bonds payable, debentures, mortgage loans and commercial paper. Excludes capital lease obligations.",
"label": "Long-term Debt, Excluding Current Maturities",
"verboseLabel": "Amount"
}
}
},
"localname": "LongTermDebtNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LongtermDebtTypeAxis": {
"auth_ref": [
"r64"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of long-term debt.",
"label": "Long-term Debt, Type [Axis]",
"terseLabel": "Long-term Debt, Type [Axis]"
}
}
},
"localname": "LongtermDebtTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_LongtermDebtTypeDomain": {
"auth_ref": [
"r64",
"r402"
],
"lang": {
"en-us": {
"role": {
"documentation": "Type of long-term debt arrangement, such as notes, line of credit, commercial paper, asset-based financing, project financing, letter of credit financing. These are debt arrangements that originally required repayment more than twelve months after issuance or greater than the normal operating cycle of the company, if longer.",
"label": "Long-term Debt, Type [Domain]",
"terseLabel": "Long-term Debt, Type [Domain]"
}
}
},
"localname": "LongtermDebtTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_LossContingenciesByNatureOfContingencyAxis": {
"auth_ref": [
"r385",
"r386",
"r387",
"r389",
"r390",
"r391",
"r392",
"r397",
"r398"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of existing condition, situation, or set of circumstances involving uncertainty as to possible loss to an enterprise that will ultimately be resolved when one or more future events occur or fail to occur.",
"label": "Loss Contingency Nature [Axis]",
"terseLabel": "Loss Contingency Nature [Axis]"
}
}
},
"localname": "LossContingenciesByNatureOfContingencyAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_LossContingenciesLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Loss Contingencies [Line Items]",
"verboseLabel": "Loss Contingencies [Line Items]"
}
}
},
"localname": "LossContingenciesLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_LossContingenciesTable": {
"auth_ref": [
"r385",
"r386",
"r387",
"r389",
"r390",
"r391",
"r392",
"r397",
"r398"
],
"lang": {
"en-us": {
"role": {
"documentation": "Discloses the specific components (such as the nature, name, and date) of the loss contingency and gives an estimate of the possible loss or range of loss, or states that a reasonable estimate cannot be made. Excludes environmental contingencies, warranties and unconditional purchase obligations.",
"label": "Loss Contingencies [Table]",
"terseLabel": "Loss Contingencies [Table]"
}
}
},
"localname": "LossContingenciesTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_LossContingencyAccrualAtCarryingValue": {
"auth_ref": [
"r385"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of loss contingency liability.",
"label": "Loss Contingency Accrual",
"terseLabel": "Accrued litigations charges"
}
}
},
"localname": "LossContingencyAccrualAtCarryingValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LossContingencyAccrualProvision": {
"auth_ref": [
"r385"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 7.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": -1.0
},
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 9.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount charged against operating income increasing loss contingency liability, after adjustments to reduce previously estimated charges.",
"label": "Loss Contingency Accrual, Provision",
"negatedTerseLabel": "Certain litigation charges",
"terseLabel": "Certain litigation charges"
}
}
},
"localname": "LossContingencyAccrualProvision",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails",
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_LossContingencyClaimsSettledNumber": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Number of claims settled.",
"label": "Loss Contingency, Claims Settled, Number",
"terseLabel": "Number of claims settled (in claims)"
}
}
},
"localname": "LossContingencyClaimsSettledNumber",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "integerItemType"
},
"us-gaap_LossContingencyDisclosures": {
"auth_ref": [
"r395",
"r396"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for loss and gain contingencies. Describes any existing condition, situation, or set of circumstances involving uncertainty as of the balance sheet date (or prior to issuance of the financial statements) as to a probable or reasonably possible loss incurred by an entity that will ultimately be resolved when one or more future events occur or fail to occur, and typically discloses the amount of loss recorded or a range of possible loss, or an assertion that no reasonable estimate can be made.",
"label": "Contingencies Disclosure [Text Block]",
"terseLabel": "Commitments and Contingencies"
}
}
},
"localname": "LossContingencyDisclosures",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingencies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_LossContingencyNatureDomain": {
"auth_ref": [
"r385",
"r386",
"r387",
"r389",
"r390",
"r391",
"r392",
"r397",
"r398"
],
"lang": {
"en-us": {
"role": {
"documentation": "An existing condition, situation, or set of circumstances involving uncertainty as to possible loss to an enterprise that will ultimately be resolved when one or more future events occur or fail to occur. Resolution of the uncertainty may confirm the incurrence of a loss or impairment of an asset or the incurrence of a liability.",
"label": "Loss Contingency, Nature [Domain]",
"terseLabel": "Loss Contingency, Nature [Domain]"
}
}
},
"localname": "LossContingencyNatureDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_LossContingencyNumberOfPlaintiffs": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Number of plaintiffs that have filed claims pertaining to a loss contingency.",
"label": "Loss Contingency, Number of Plaintiffs",
"terseLabel": "Number of plaintiffs (in plaintiffs)"
}
}
},
"localname": "LossContingencyNumberOfPlaintiffs",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "integerItemType"
},
"us-gaap_LossContingencyPendingClaimsNumber": {
"auth_ref": [
"r388"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of pending claims pertaining to a loss contingency.",
"label": "Loss Contingency, Pending Claims, Number",
"terseLabel": "Number of claimants (in claimants)"
}
}
},
"localname": "LossContingencyPendingClaimsNumber",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "integerItemType"
},
"us-gaap_MarketableSecuritiesLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Marketable Securities [Line Items]",
"terseLabel": "Marketable Securities [Line Items]"
}
}
},
"localname": "MarketableSecuritiesLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_MarketableSecuritiesTable": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about investment in marketable security.",
"label": "Marketable Securities [Table]",
"terseLabel": "Marketable Securities [Table]"
}
}
},
"localname": "MarketableSecuritiesTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_MaterialReconcilingItemsMember": {
"auth_ref": [
"r252"
],
"lang": {
"en-us": {
"role": {
"documentation": "Items used in reconciling reportable segments' amounts to consolidated amount. Excludes corporate-level activity.",
"label": "Segment Reconciling Items [Member]",
"terseLabel": "Segment reconciling items"
}
}
},
"localname": "MaterialReconcilingItemsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_MaximumRemainingMaturityOfForeignCurrencyDerivatives1": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Maximum amount of time remaining before foreign currency exchange rate derivatives mature or expire, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.",
"label": "Maximum Remaining Maturity of Foreign Currency Derivatives",
"terseLabel": "Maximum remaining maturity of foreign currency derivatives"
}
}
},
"localname": "MaximumRemainingMaturityOfForeignCurrencyDerivatives1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_MeasurementInputDiscountRateMember": {
"auth_ref": [
"r823"
],
"lang": {
"en-us": {
"role": {
"documentation": "Measurement input using interest rate to determine present value of future cash flows.",
"label": "Measurement Input, Discount Rate [Member]",
"terseLabel": "Discount Rate"
}
}
},
"localname": "MeasurementInputDiscountRateMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_MeasurementInputTypeAxis": {
"auth_ref": [
"r823"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of measurement input used to determine value of asset and liability.",
"label": "Measurement Input Type [Axis]",
"terseLabel": "Measurement Input Type [Axis]"
}
}
},
"localname": "MeasurementInputTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_MeasurementInputTypeDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Measurement input used to determine value of asset and liability.",
"label": "Measurement Input Type [Domain]",
"terseLabel": "Measurement Input Type [Domain]"
}
}
},
"localname": "MeasurementInputTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsContingentConsiderationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_MediumTermNotesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Debt instruments with maturities ranging from five to ten years.",
"label": "Medium-term Notes [Member]",
"terseLabel": "Term Loan Agreements"
}
}
},
"localname": "MediumTermNotesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_MinorityInterest": {
"auth_ref": [
"r69",
"r163",
"r303",
"r404",
"r409",
"r410",
"r411",
"r417",
"r418",
"r835",
"r939",
"r974"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 2.0,
"parentTag": "us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Total of all stockholders' equity (deficit) items, net of receivables from officers, directors, owners, and affiliates of the entity which is directly or indirectly attributable to that ownership interest in subsidiary equity which is not attributable to the parent (that is, noncontrolling interest, previously referred to as minority interest).",
"label": "Stockholders' Equity Attributable to Noncontrolling Interest",
"terseLabel": "Noncontrolling interests"
}
}
},
"localname": "MinorityInterest",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_MinorityInterestPeriodIncreaseDecrease": {
"auth_ref": [
"r754"
],
"lang": {
"en-us": {
"role": {
"documentation": "Net Increase or Decrease in balance of noncontrolling interest in the subsidiary during the reporting period.",
"label": "Noncontrolling Interest, Period Increase (Decrease)",
"terseLabel": "Changes to noncontrolling ownership interests"
}
}
},
"localname": "MinorityInterestPeriodIncreaseDecrease",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_MortgageBackedSecuritiesMember": {
"auth_ref": [
"r282",
"r549",
"r551",
"r598",
"r1005"
],
"lang": {
"en-us": {
"role": {
"documentation": "Securities collateralized by mortgage loans.",
"label": "Collateralized Mortgage Backed Securities [Member]",
"terseLabel": "Mortgage-backed securities"
}
}
},
"localname": "MortgageBackedSecuritiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_MovementInValuationAllowancesAndReservesRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "SEC Schedule, 12-09, Movement in Valuation Allowances and Reserves [Roll Forward]",
"terseLabel": "SEC Schedule, 12-09, Movement in Valuation Allowances and Reserves [Roll Forward]"
}
}
},
"localname": "MovementInValuationAllowancesAndReservesRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_MutualFundMember": {
"auth_ref": [
"r549"
],
"lang": {
"en-us": {
"role": {
"documentation": "Regulated investment instrument that pools funds from multiple investors to invest principally in a portfolio of securities and money market instruments to match the investment objective.",
"label": "Mutual Fund [Member]",
"terseLabel": "Mutual funds"
}
}
},
"localname": "MutualFundMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_NetCashProvidedByUsedInFinancingActivities": {
"auth_ref": [
"r144"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 3.0,
"parentTag": "us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit.",
"label": "Net Cash Provided by (Used in) Financing Activities",
"totalLabel": "Net cash used in financing activities"
}
}
},
"localname": "NetCashProvidedByUsedInFinancingActivities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Net Cash Provided by (Used in) Financing Activities [Abstract]",
"terseLabel": "Financing Activities:"
}
}
},
"localname": "NetCashProvidedByUsedInFinancingActivitiesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "stringItemType"
},
"us-gaap_NetCashProvidedByUsedInInvestingActivities": {
"auth_ref": [
"r144"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 2.0,
"parentTag": "us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash inflow (outflow) from investing activities, including discontinued operations. Investing activity cash flows include making and collecting loans and acquiring and disposing of debt or equity instruments and property, plant, and equipment and other productive assets.",
"label": "Net Cash Provided by (Used in) Investing Activities",
"totalLabel": "Net cash used in investing activities"
}
}
},
"localname": "NetCashProvidedByUsedInInvestingActivities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Net Cash Provided by (Used in) Investing Activities [Abstract]",
"terseLabel": "Investing Activities:"
}
}
},
"localname": "NetCashProvidedByUsedInInvestingActivitiesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "stringItemType"
},
"us-gaap_NetCashProvidedByUsedInOperatingActivities": {
"auth_ref": [
"r144",
"r146",
"r149"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 1.0,
"parentTag": "us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities.",
"label": "Net Cash Provided by (Used in) Operating Activities",
"totalLabel": "Net cash provided by operating activities"
}
}
},
"localname": "NetCashProvidedByUsedInOperatingActivities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Net Cash Provided by (Used in) Operating Activities [Abstract]",
"terseLabel": "Operating Activities:"
}
}
},
"localname": "NetCashProvidedByUsedInOperatingActivitiesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "stringItemType"
},
"us-gaap_NetIncomeLoss": {
"auth_ref": [
"r7",
"r103",
"r106",
"r112",
"r117",
"r149",
"r163",
"r184",
"r189",
"r190",
"r191",
"r192",
"r195",
"r196",
"r206",
"r242",
"r248",
"r252",
"r255",
"r258",
"r303",
"r404",
"r405",
"r406",
"r409",
"r410",
"r411",
"r413",
"r415",
"r417",
"r418",
"r817",
"r835",
"r946",
"r978"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The portion of profit or loss for the period, net of income taxes, which is attributable to the parent.",
"label": "Net Income (Loss) Attributable to Parent",
"terseLabel": "Net income attributable to ordinary shareholders",
"totalLabel": "Net income attributable to Medtronic"
}
}
},
"localname": "NetIncomeLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_NetIncomeLossAttributableToNoncontrollingInterest": {
"auth_ref": [
"r103",
"r106",
"r112",
"r195",
"r196",
"r762",
"r769"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 2.0,
"parentTag": "us-gaap_NetIncomeLoss",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of Net Income (Loss) attributable to noncontrolling interest.",
"label": "Net Income (Loss) Attributable to Noncontrolling Interest",
"negatedTerseLabel": "Net income attributable to noncontrolling interests"
}
}
},
"localname": "NetIncomeLossAttributableToNoncontrollingInterest",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_NetIncomeLossAvailableToCommonStockholdersBasicAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Net Income (Loss) Available to Common Stockholders, Basic [Abstract]",
"terseLabel": "Numerator:"
}
}
},
"localname": "NetIncomeLossAvailableToCommonStockholdersBasicAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_NetInvestmentHedgingMember": {
"auth_ref": [
"r777"
],
"lang": {
"en-us": {
"role": {
"documentation": "Hedges of a net investment in a foreign operation.",
"label": "Net Investment Hedging [Member]",
"terseLabel": "Net investment hedges"
}
}
},
"localname": "NetInvestmentHedgingMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_NewAccountingPronouncementsAndChangesInAccountingPrinciplesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Accounting Standards Update and Change in Accounting Principle [Abstract]",
"terseLabel": "New Accounting Standards"
}
}
},
"localname": "NewAccountingPronouncementsAndChangesInAccountingPrinciplesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_NewAccountingPronouncementsOrChangeInAccountingPrincipleLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "New Accounting Pronouncements or Change in Accounting Principle [Line Items]",
"terseLabel": "New Accounting Pronouncements or Change in Accounting Principle [Line Items]"
}
}
},
"localname": "NewAccountingPronouncementsOrChangeInAccountingPrincipleLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_NewAccountingPronouncementsOrChangeInAccountingPrincipleTable": {
"auth_ref": [
"r0",
"r1",
"r2",
"r3",
"r4",
"r183",
"r184",
"r185",
"r186",
"r187",
"r188",
"r191",
"r197",
"r214",
"r277",
"r278",
"r304",
"r305",
"r306",
"r307",
"r308",
"r309",
"r403",
"r665",
"r666",
"r667",
"r668",
"r669",
"r670",
"r671",
"r672",
"r716",
"r717",
"r718",
"r719",
"r807",
"r808",
"r809",
"r810",
"r811",
"r812",
"r813",
"r814",
"r815",
"r816",
"r817",
"r836",
"r837",
"r838",
"r839",
"r840",
"r841",
"r842",
"r843",
"r880",
"r927",
"r928",
"r929",
"r992",
"r993",
"r994",
"r995",
"r996",
"r997",
"r998",
"r999",
"r1000",
"r1001",
"r1002",
"r1003",
"r1069",
"r1070",
"r1071",
"r1072",
"r1073"
],
"lang": {
"en-us": {
"role": {
"documentation": "Summarization of the changes in an accounting principle or a new accounting pronouncement, including the line items affected by the change and the financial effects of the change on those particular line items.",
"label": "Accounting Standards Update and Change in Accounting Principle [Table]",
"terseLabel": "New Accounting Pronouncements or Change in Accounting Principle [Table]"
}
}
},
"localname": "NewAccountingPronouncementsOrChangeInAccountingPrincipleTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.",
"label": "New Accounting Pronouncements, Policy [Policy Text Block]",
"terseLabel": "Recently Adopted Accounting Standards"
}
}
},
"localname": "NewAccountingPronouncementsPolicyPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_NoncontrollingInterestMember": {
"auth_ref": [
"r180",
"r181",
"r182",
"r483",
"r753"
],
"lang": {
"en-us": {
"role": {
"documentation": "This element represents that portion of equity (net assets) in a subsidiary not attributable, directly or indirectly, to the parent. A noncontrolling interest is sometimes called a minority interest.",
"label": "Noncontrolling Interest [Member]",
"terseLabel": "Noncontrolling Interests"
}
}
},
"localname": "NoncontrollingInterestMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"us-gaap_NondesignatedMember": {
"auth_ref": [
"r778"
],
"lang": {
"en-us": {
"role": {
"documentation": "Derivative instrument not designated as hedging instrument under Generally Accepted Accounting Principles (GAAP).",
"label": "Not Designated as Hedging Instrument [Member]",
"terseLabel": "Derivatives not designated as hedging instruments"
}
}
},
"localname": "NondesignatedMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_NotesPayableToBanksMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A written promise to pay a note to a bank.",
"label": "Notes Payable to Banks [Member]",
"terseLabel": "Bank borrowings"
}
}
},
"localname": "NotesPayableToBanksMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_NumberOfOperatingSegments": {
"auth_ref": [
"r230"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of operating segments. An operating segment is a component of an enterprise: (a) that engages in business activities from which it may earn revenues and incur expenses (including revenues and expenses relating to transactions with other components of the same enterprise), (b) whose operating results are regularly reviewed by the enterprise's chief operating decision maker to make decisions about resources to be allocated to the segment and assess its performance, and (c) for which discrete financial information is available. An operating segment may engage in business activities for which it has yet to earn revenues, for example, start-up operations may be operating segments before earning revenues.",
"label": "Number of Operating Segments",
"terseLabel": "Number of operating segments"
}
}
},
"localname": "NumberOfOperatingSegments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationAdditionalInformationDetails"
],
"xbrltype": "integerItemType"
},
"us-gaap_NumberOfReportableSegments": {
"auth_ref": [
"r230"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of segments reported by the entity. A reportable segment is a component of an entity for which there is an accounting requirement to report separate financial information on that component in the entity's financial statements.",
"label": "Number of Reportable Segments",
"terseLabel": "Number of reporting segments"
}
}
},
"localname": "NumberOfReportableSegments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationAdditionalInformationDetails"
],
"xbrltype": "integerItemType"
},
"us-gaap_OffsettingAssetsTableTextBlock": {
"auth_ref": [
"r80",
"r81"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of derivative and other financial assets that are subject to offsetting, including master netting arrangements.",
"label": "Offsetting Assets [Table Text Block]",
"terseLabel": "Schedule of Offsetting Assets"
}
}
},
"localname": "OffsettingAssetsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_OffsettingLiabilitiesTableTextBlock": {
"auth_ref": [
"r80",
"r81"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of derivative and other financial liabilities that are subject to offsetting, including master netting arrangements.",
"label": "Offsetting Liabilities [Table Text Block]",
"terseLabel": "Schedule of Offsetting Liabilities"
}
}
},
"localname": "OffsettingLiabilitiesTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_OperatingIncomeLoss": {
"auth_ref": [
"r242",
"r248",
"r252",
"r255",
"r258"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 2.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
},
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 2.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The net result for the period of deducting operating expenses from operating revenues.",
"label": "Operating Income (Loss)",
"terseLabel": "Segment operating profit",
"totalLabel": "Operating profit"
}
}
},
"localname": "OperatingIncomeLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLeaseCost": {
"auth_ref": [
"r870",
"r878"
],
"calculation": {
"http://www.medtronic.com/role/LeasesComponentsofTotalOperatingLeaseCostDetails": {
"order": 1.0,
"parentTag": "us-gaap_LeaseCost",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability.",
"label": "Operating Lease, Cost",
"terseLabel": "Operating lease cost"
}
}
},
"localname": "OperatingLeaseCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesComponentsofTotalOperatingLeaseCostDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLeaseLiability": {
"auth_ref": [
"r865"
],
"calculation": {
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails": {
"order": 2.0,
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Present value of lessee's discounted obligation for lease payments from operating lease.",
"label": "Operating Lease, Liability",
"terseLabel": "Total lease liability",
"verboseLabel": "Lease liability"
}
}
},
"localname": "OperatingLeaseLiability",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesMaturitiesofOperatingLeasesDetails",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLeaseLiabilityCurrent": {
"auth_ref": [
"r865"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Present value of lessee's discounted obligation for lease payments from operating lease, classified as current.",
"label": "Operating Lease, Liability, Current",
"terseLabel": "Current liability"
}
}
},
"localname": "OperatingLeaseLiabilityCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesBalanceSheetClassificationandAmountsofRightofUseAssetsandLeaseLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLeaseLiabilityCurrentStatementOfFinancialPositionExtensibleList": {
"auth_ref": [
"r866"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicates line item in statement of financial position that includes current operating lease liability.",
"label": "Operating Lease, Liability, Current, Statement of Financial Position [Extensible Enumeration]",
"terseLabel": "Balance Sheet Classification, Other accrued expenses"
}
}
},
"localname": "OperatingLeaseLiabilityCurrentStatementOfFinancialPositionExtensibleList",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesBalanceSheetClassificationandAmountsofRightofUseAssetsandLeaseLiabilitiesDetails"
],
"xbrltype": "enumerationSetItemType"
},
"us-gaap_OperatingLeaseLiabilityNoncurrent": {
"auth_ref": [
"r865"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Present value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent.",
"label": "Operating Lease, Liability, Noncurrent",
"terseLabel": "Non-current liability"
}
}
},
"localname": "OperatingLeaseLiabilityNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesBalanceSheetClassificationandAmountsofRightofUseAssetsandLeaseLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLeaseLiabilityNoncurrentStatementOfFinancialPositionExtensibleList": {
"auth_ref": [
"r866"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicates line item in statement of financial position that includes noncurrent operating lease liability.",
"label": "Operating Lease, Liability, Noncurrent, Statement of Financial Position [Extensible Enumeration]",
"terseLabel": "Balance Sheet Classification, Other liabilities"
}
}
},
"localname": "OperatingLeaseLiabilityNoncurrentStatementOfFinancialPositionExtensibleList",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesBalanceSheetClassificationandAmountsofRightofUseAssetsandLeaseLiabilitiesDetails"
],
"xbrltype": "enumerationSetItemType"
},
"us-gaap_OperatingLeasePayments": {
"auth_ref": [
"r868",
"r872"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use.",
"label": "Operating Lease, Payments",
"terseLabel": "Cash paid for amounts included in the measurement of operating lease liabilities"
}
}
},
"localname": "OperatingLeasePayments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesSupplementalCashFlowInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLeaseRightOfUseAsset": {
"auth_ref": [
"r864"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of lessee's right to use underlying asset under operating lease.",
"label": "Operating Lease, Right-of-Use Asset",
"terseLabel": "Right-of-use assets"
}
}
},
"localname": "OperatingLeaseRightOfUseAsset",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesBalanceSheetClassificationandAmountsofRightofUseAssetsandLeaseLiabilitiesDetails",
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLeaseRightOfUseAssetStatementOfFinancialPositionExtensibleList": {
"auth_ref": [
"r866"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicates line item in statement of financial position that includes operating lease right-of-use asset.",
"label": "Operating Lease, Right-of-Use Asset, Statement of Financial Position [Extensible Enumeration]",
"terseLabel": "Balance Sheet Classification, Other assets"
}
}
},
"localname": "OperatingLeaseRightOfUseAssetStatementOfFinancialPositionExtensibleList",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesBalanceSheetClassificationandAmountsofRightofUseAssetsandLeaseLiabilitiesDetails"
],
"xbrltype": "enumerationSetItemType"
},
"us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent": {
"auth_ref": [
"r875",
"r878"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average discount rate for operating lease calculated at point in time.",
"label": "Operating Lease, Weighted Average Discount Rate, Percent",
"terseLabel": "Weighted-average discount rate"
}
}
},
"localname": "OperatingLeaseWeightedAverageDiscountRatePercent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesWeightedAverageRemainingLeaseTermandWeightedAverageDiscountRateforOperatingLeasesDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1": {
"auth_ref": [
"r874",
"r878"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days.",
"label": "Operating Lease, Weighted Average Remaining Lease Term",
"terseLabel": "Weighted-average remaining lease term"
}
}
},
"localname": "OperatingLeaseWeightedAverageRemainingLeaseTerm1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesWeightedAverageRemainingLeaseTermandWeightedAverageDiscountRateforOperatingLeasesDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_OperatingLossCarryforwards": {
"auth_ref": [
"r708"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of operating loss carryforward, before tax effects, available to reduce future taxable income under enacted tax laws.",
"label": "Operating Loss Carryforwards",
"terseLabel": "Net operating loss carryforwards"
}
}
},
"localname": "OperatingLossCarryforwards",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingLossCarryforwardsLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Operating Loss Carryforwards [Line Items]",
"terseLabel": "Operating Loss Carryforwards [Line Items]"
}
}
},
"localname": "OperatingLossCarryforwardsLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_OperatingLossCarryforwardsTable": {
"auth_ref": [
"r709"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule reflecting pertinent information, such as tax authority, amounts, and expiration dates, of net operating loss carryforwards, including an assessment of the likelihood of utilization.",
"label": "Operating Loss Carryforwards [Table]",
"terseLabel": "Operating Loss Carryforwards [Table]"
}
}
},
"localname": "OperatingLossCarryforwardsTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_OperatingLossCarryforwardsValuationAllowance": {
"auth_ref": [
"r704"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The portion of the valuation allowance pertaining to the deferred tax asset representing potential future taxable deductions from net operating loss carryforwards for which it is more likely than not that a tax benefit will not be realized.",
"label": "Operating Loss Carryforwards, Valuation Allowance",
"terseLabel": "Net operating loss carryforwards, valuation allowance"
}
}
},
"localname": "OperatingLossCarryforwardsValuationAllowance",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OperatingSegmentsMember": {
"auth_ref": [
"r247",
"r248",
"r249",
"r250",
"r252",
"r258"
],
"lang": {
"en-us": {
"role": {
"documentation": "Identifies components of an entity that engage in business activities from which they may earn revenue and incur expenses, including transactions with other components of the same entity.",
"label": "Operating Segments [Member]",
"terseLabel": "Reportable segments"
}
}
},
"localname": "OperatingSegmentsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_OtherAssetsCurrent": {
"auth_ref": [
"r72",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 1.0,
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of current assets classified as other.",
"label": "Other Assets, Current",
"terseLabel": "Other current assets"
}
}
},
"localname": "OtherAssetsCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherAssetsMember": {
"auth_ref": [
"r780",
"r801"
],
"lang": {
"en-us": {
"role": {
"documentation": "Primary financial statement caption encompassing other assets.",
"label": "Other Assets [Member]",
"terseLabel": "Other Assets",
"verboseLabel": "Other assets"
}
}
},
"localname": "OtherAssetsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_OtherAssetsNoncurrent": {
"auth_ref": [
"r54"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 1.0,
"parentTag": "us-gaap_Assets",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of noncurrent assets classified as other.",
"label": "Other Assets, Noncurrent",
"terseLabel": "Other assets"
}
}
},
"localname": "OtherAssetsNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationGainLossArisingDuringPeriodNetOfTax": {
"auth_ref": [
"r88",
"r97",
"r844",
"r845",
"r847"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 2.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossNetOfTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after tax, before reclassification adjustments of gain (loss) on foreign currency translation adjustments, foreign currency transactions designated and effective as economic hedges of a net investment in a foreign entity and intra-entity foreign currency transactions that are of a long-term-investment nature.",
"label": "Other Comprehensive Income (Loss), Foreign Currency Transaction and Translation Gain (Loss) Arising During Period, Net of Tax",
"terseLabel": "Translation adjustment"
}
}
},
"localname": "OtherComprehensiveIncomeForeignCurrencyTransactionAndTranslationGainLossArisingDuringPeriodNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossAmortizationAdjustmentFromAOCIPensionAndOtherPostretirementBenefitPlansForNetPriorServiceCostCreditBeforeTax": {
"auth_ref": [
"r97",
"r101",
"r102",
"r568"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 2.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of reclassification adjustment from accumulated other comprehensive (income) loss for prior service cost (credit) of defined benefit plan.",
"label": "Other Comprehensive (Income) Loss, Defined Benefit Plan, Prior Service Cost (Credit), Reclassification Adjustment from AOCI, before Tax",
"negatedTerseLabel": "Amortization of prior service credit"
}
}
},
"localname": "OtherComprehensiveIncomeLossAmortizationAdjustmentFromAOCIPensionAndOtherPostretirementBenefitPlansForNetPriorServiceCostCreditBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossBeforeReclassificationsNetOfTax": {
"auth_ref": [
"r100",
"r110",
"r111",
"r113",
"r848",
"r850",
"r854"
],
"calculation": {
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails": {
"order": 1.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossNetOfTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after tax, before reclassification adjustments of other comprehensive income (loss).",
"label": "Other Comprehensive Income (Loss), before Reclassifications, Net of Tax",
"terseLabel": "Other comprehensive income (loss) before reclassifications"
}
}
},
"localname": "OtherComprehensiveIncomeLossBeforeReclassificationsNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossBeforeReclassificationsTax": {
"auth_ref": [
"r98",
"r110"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of tax expense (benefit) allocated to other comprehensive income (loss) before reclassification adjustment from accumulated other comprehensive income (loss).",
"label": "Other Comprehensive Income (Loss) before Reclassifications, Tax",
"terseLabel": "Other comprehensive income (loss), tax expense (benefit)"
}
}
},
"localname": "OtherComprehensiveIncomeLossBeforeReclassificationsTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossCashFlowHedgeGainLossAfterReclassificationAndTax": {
"auth_ref": [
"r89",
"r97"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 5.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossNetOfTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after tax and reclassification, of gain (loss) from derivative instrument designated and qualifying as cash flow hedge included in assessment of hedge effectiveness.",
"label": "Other Comprehensive Income (Loss), Cash Flow Hedge, Gain (Loss), after Reclassification and Tax",
"terseLabel": "Unrealized (loss) gain on cash flow hedges"
}
}
},
"localname": "OtherComprehensiveIncomeLossCashFlowHedgeGainLossAfterReclassificationAndTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossCashFlowHedgeGainLossBeforeReclassificationAndTax": {
"auth_ref": [
"r89",
"r97",
"r782",
"r787",
"r802"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails": {
"order": 1.0,
"parentTag": "mdt_OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossBeforeReclassificationAndTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax and reclassification, of gain (loss) from derivative instrument designated and qualifying cash flow hedge included in assessment of hedge effectiveness.",
"label": "Other Comprehensive Income (Loss), Cash Flow Hedge, Gain (Loss), before Reclassification and Tax",
"negatedTerseLabel": "(Gain)\u00a0Loss\u00a0Recognized in Accumulated Other Comprehensive Income"
}
}
},
"localname": "OtherComprehensiveIncomeLossCashFlowHedgeGainLossBeforeReclassificationAndTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossCashFlowHedgeGainLossReclassificationBeforeTax": {
"auth_ref": [
"r97",
"r101",
"r788"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails": {
"order": 1.0,
"parentTag": "mdt_OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossReclassificationBeforeTax",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of reclassification of gain (loss) from accumulated other comprehensive income (AOCI) for derivative instrument designated and qualifying as cash flow hedge included in assessment of hedge effectiveness.",
"label": "Other Comprehensive Income (Loss), Cash Flow Hedge, Gain (Loss), Reclassification, before Tax",
"negatedTerseLabel": "(Gain) Loss Reclassified into Income"
}
}
},
"localname": "OtherComprehensiveIncomeLossCashFlowHedgeGainLossReclassificationBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossNetInvestmentHedgeGainLossBeforeReclassificationAndTax": {
"auth_ref": [
"r792"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails": {
"order": 2.0,
"parentTag": "mdt_OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossBeforeReclassificationAndTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax and reclassification, of gain (loss) from derivative designated and qualifying as net investment hedge.",
"label": "Other Comprehensive Income (Loss), Net Investment Hedge, Gain (Loss), before Reclassification and Tax",
"negatedTerseLabel": "Net investment hedges"
}
}
},
"localname": "OtherComprehensiveIncomeLossNetInvestmentHedgeGainLossBeforeReclassificationAndTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossNetInvestmentHedgeGainLossReclassificationBeforeTax": {
"auth_ref": [
"r793"
],
"calculation": {
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails": {
"order": 2.0,
"parentTag": "mdt_OtherComprehensiveIncomeLossCashFlowHedgeAndNetInvestmentHedgeGainLossReclassificationBeforeTax",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of reclassification from accumulated other comprehensive income (AOCI) for gain (loss) from derivative designated and qualifying as net investment hedge.",
"label": "Other Comprehensive Income (Loss), Net Investment Hedge, Gain (Loss), Reclassification, before Tax",
"negatedTerseLabel": "Net investment hedges"
}
}
},
"localname": "OtherComprehensiveIncomeLossNetInvestmentHedgeGainLossReclassificationBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossNetOfTax": {
"auth_ref": [
"r104",
"r107",
"r110",
"r111",
"r113",
"r118",
"r476",
"r848",
"r853",
"r854",
"r947",
"r979"
],
"calculation": {
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
},
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 2.0,
"parentTag": "us-gaap_ComprehensiveIncomeNetOfTaxIncludingPortionAttributableToNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after tax and reclassification adjustments of other comprehensive income (loss).",
"label": "Other Comprehensive Income (Loss), Net of Tax",
"terseLabel": "Other comprehensive income (loss)",
"totalLabel": "Other comprehensive income (loss)"
}
}
},
"localname": "OtherComprehensiveIncomeLossNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails",
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome",
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossNetOfTaxPeriodIncreaseDecreaseAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Other Comprehensive Income (Loss), Net of Tax [Abstract]",
"terseLabel": "Other comprehensive income (loss), net of tax:"
}
}
},
"localname": "OtherComprehensiveIncomeLossNetOfTaxPeriodIncreaseDecreaseAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "stringItemType"
},
"us-gaap_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax": {
"auth_ref": [
"r95",
"r97",
"r568",
"r598"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, after reclassification adjustment, of (increase) decrease in accumulated other comprehensive income for defined benefit plan.",
"label": "Other Comprehensive (Income) Loss, Defined Benefit Plan, after Reclassification Adjustment, before Tax",
"totalLabel": "Total recognized in other comprehensive income"
}
}
},
"localname": "OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentNetOfTax": {
"auth_ref": [
"r95",
"r97"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 4.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossNetOfTax",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after tax and reclassification adjustment, of (increase) decrease in accumulated other comprehensive income for defined benefit plan.",
"label": "Other Comprehensive (Income) Loss, Defined Benefit Plan, after Reclassification Adjustment, after Tax",
"negatedLabel": "Net change in retirement obligations"
}
}
},
"localname": "OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansNetUnamortizedGainLossArisingDuringPeriodBeforeTax": {
"auth_ref": [
"r93",
"r97",
"r568"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails": {
"order": 1.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansAdjustmentBeforeTax",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before tax, of gain (loss) for (increase) decrease in value of benefit obligation for change in actuarial assumptions and increase (decrease) in value of plan assets from experience different from that assumed of defined benefit plan, that has not been recognized in net periodic benefit (cost) credit.",
"label": "Other Comprehensive Income (Loss), Defined Benefit Plan, Gain (Loss) Arising During Period, before Tax",
"negatedTerseLabel": "Net actuarial gain"
}
}
},
"localname": "OtherComprehensiveIncomeLossPensionAndOtherPostretirementBenefitPlansNetUnamortizedGainLossArisingDuringPeriodBeforeTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherComprehensiveIncomeUnrealizedHoldingGainLossOnSecuritiesArisingDuringPeriodNetOfTax": {
"auth_ref": [
"r90",
"r97"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 1.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossNetOfTax",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after tax and before adjustment, of unrealized holding gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale). Excludes unrealized gain (loss) on investment in debt security measured at amortized cost (held-to-maturity) from transfer to available-for-sale.",
"label": "OCI, Debt Securities, Available-for-Sale, Unrealized Holding Gain (Loss), before Adjustment, after Tax",
"terseLabel": "Unrealized (loss) gain on investment securities"
}
}
},
"localname": "OtherComprehensiveIncomeUnrealizedHoldingGainLossOnSecuritiesArisingDuringPeriodNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherIntangibleAssetsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Intangible assets classified as other.",
"label": "Other Intangible Assets [Member]",
"terseLabel": "Other"
}
}
},
"localname": "OtherIntangibleAssetsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_OtherLiabilitiesCurrent": {
"auth_ref": [
"r16",
"r17",
"r61",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 1.0,
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liabilities classified as other, due within one year or the normal operating cycle, if longer.",
"label": "Other Liabilities, Current",
"terseLabel": "Other accrued expenses"
}
}
},
"localname": "OtherLiabilitiesCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherLiabilitiesMember": {
"auth_ref": [
"r780",
"r801"
],
"lang": {
"en-us": {
"role": {
"documentation": "Primary financial statement caption encompassing other liabilities.",
"label": "Other Liabilities [Member]",
"terseLabel": "Other liabilities"
}
}
},
"localname": "OtherLiabilitiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_OtherLiabilitiesNoncurrent": {
"auth_ref": [
"r65"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 3.0,
"parentTag": "us-gaap_Liabilities",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liabilities classified as other, due after one year or the normal operating cycle, if longer.",
"label": "Other Liabilities, Noncurrent",
"terseLabel": "Other liabilities"
}
}
},
"localname": "OtherLiabilitiesNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherNoncashIncomeExpense": {
"auth_ref": [
"r149"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 4.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of income (expense) included in net income that results in no cash inflow (outflow), classified as other.",
"label": "Other Noncash Income (Expense)",
"negatedTerseLabel": "Other, net"
}
}
},
"localname": "OtherNoncashIncomeExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherNonoperatingIncomeExpense": {
"auth_ref": [
"r127"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 1.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
},
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails": {
"order": 11.0,
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of income (expense) related to nonoperating activities, classified as other.",
"label": "Other Nonoperating Income (Expense)",
"negatedTerseLabel": "Other non-operating income, net",
"terseLabel": "Other non-operating income, net"
}
}
},
"localname": "OtherNonoperatingIncomeExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherOperatingIncomeExpenseMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Primary financial statement caption encompassing other operating income (expense).",
"label": "Other Operating Income (Expense) [Member]",
"terseLabel": "Other operating expense, net"
}
}
},
"localname": "OtherOperatingIncomeExpenseMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementDerivativeGainsLossesDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_OtherOperatingIncomeExpenseNet": {
"auth_ref": [],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 8.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The net amount of other operating income and expenses, the components of which are not separately disclosed on the income statement, from items that are associated with the entity's normal revenue producing operations.",
"label": "Other Operating Income (Expense), Net",
"negatedLabel": "Other operating expense, net"
}
}
},
"localname": "OtherOperatingIncomeExpenseNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherPostretirementBenefitPlansDefinedBenefitMember": {
"auth_ref": [
"r521",
"r522",
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r573",
"r574",
"r576",
"r579",
"r583",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r598",
"r599",
"r605",
"r606",
"r607",
"r608",
"r609",
"r610"
],
"lang": {
"en-us": {
"role": {
"documentation": "Plan designed to provide other postretirement benefits. Includes, but is not limited to, defined benefit and defined contribution plans. Excludes pension benefits.",
"label": "Other Postretirement Benefits Plan [Member]",
"terseLabel": "Other post-retirement benefits"
}
}
},
"localname": "OtherPostretirementBenefitPlansDefinedBenefitMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_OtherRestructuringCosts": {
"auth_ref": [
"r148"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of other expenses associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses associated with a discontinued operation or an asset retirement obligation.",
"label": "Other Restructuring Costs",
"terseLabel": "Other restructuring costs"
}
}
},
"localname": "OtherRestructuringCosts",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_OtherRestructuringMember": {
"auth_ref": [
"r370",
"r371",
"r380",
"r381"
],
"lang": {
"en-us": {
"role": {
"documentation": "Restructuring and related activities classified as other.",
"label": "Other Restructuring [Member]",
"terseLabel": "Other Costs"
}
}
},
"localname": "OtherRestructuringMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ParentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Portion of equity, or net assets, in the consolidated entity attributable, directly or indirectly, to the parent. Excludes noncontrolling interests.",
"label": "Parent [Member]",
"terseLabel": "Total Shareholders\u2019 Equity"
}
}
},
"localname": "ParentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"us-gaap_PaymentsForProceedsFromOtherInvestingActivities": {
"auth_ref": [
"r131",
"r134"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 5.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash (inflow) outflow from investing activities classified as other.",
"label": "Payments for (Proceeds from) Other Investing Activities",
"negatedLabel": "Other investing activities, net"
}
}
},
"localname": "PaymentsForProceedsFromOtherInvestingActivities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PaymentsForRepurchaseOfCommonStock": {
"auth_ref": [
"r138"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 6.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash outflow to reacquire common stock during the period.",
"label": "Payments for Repurchase of Common Stock",
"negatedLabel": "Repurchase of ordinary shares"
}
}
},
"localname": "PaymentsForRepurchaseOfCommonStock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PaymentsForRestructuring": {
"auth_ref": [
"r142",
"r371"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash payments made as the result of exit or disposal activities. Excludes payments associated with a discontinued operation or an asset retirement obligation.",
"label": "Payments for Restructuring",
"negatedLabel": "Cash payments"
}
}
},
"localname": "PaymentsForRestructuring",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PaymentsOfDividendsCommonStock": {
"auth_ref": [
"r138"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 1.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash outflow in the form of ordinary dividends to common shareholders of the parent entity.",
"label": "Payments of Ordinary Dividends, Common Stock",
"negatedLabel": "Dividends to shareholders"
}
}
},
"localname": "PaymentsOfDividendsCommonStock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PaymentsToAcquireBusinessesNetOfCashAcquired": {
"auth_ref": [
"r132"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 1.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash outflow associated with the acquisition of a business, net of the cash acquired from the purchase.",
"label": "Payments to Acquire Businesses, Net of Cash Acquired",
"negatedLabel": "Acquisitions, net of cash acquired"
}
}
},
"localname": "PaymentsToAcquireBusinessesNetOfCashAcquired",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PaymentsToAcquireInvestments": {
"auth_ref": [
"r134"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 3.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash outflow associated with the purchase of all investments (debt, security, other) during the period.",
"label": "Payments to Acquire Investments",
"negatedLabel": "Purchases of investments"
}
}
},
"localname": "PaymentsToAcquireInvestments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PaymentsToAcquirePropertyPlantAndEquipment": {
"auth_ref": [
"r133"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 4.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets.",
"label": "Payments to Acquire Property, Plant, and Equipment",
"negatedLabel": "Additions to property, plant, and equipment"
}
}
},
"localname": "PaymentsToAcquirePropertyPlantAndEquipment",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PendingLitigationMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Risk of loss associated with the outcome of pending litigation against the entity, for example, but not limited to, litigation in arbitration or within the trial process.",
"label": "Pending Litigation [Member]",
"terseLabel": "Pending litigation"
}
}
},
"localname": "PendingLitigationMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_PensionAndOtherPostretirementBenefitExpense": {
"auth_ref": [],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cost (reversal of cost) for pension and other postretirement benefits.",
"label": "Pension and Other Postretirement Benefits Cost (Reversal of Cost)",
"terseLabel": "Cost of retirement benefit plans"
}
}
},
"localname": "PensionAndOtherPostretirementBenefitExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PensionAndOtherPostretirementBenefitsDisclosureTextBlock": {
"auth_ref": [
"r548",
"r550",
"r556",
"r575",
"r577",
"r578",
"r579",
"r580",
"r581",
"r598",
"r600",
"r603",
"r605",
"r626"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for retirement benefits.",
"label": "Retirement Benefits [Text Block]",
"terseLabel": "Retirement Benefit Plans"
}
}
},
"localname": "PensionAndOtherPostretirementBenefitsDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlans"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_PensionAndOtherPostretirementDefinedBenefitPlansCurrentLiabilities": {
"auth_ref": [
"r26",
"r522",
"r523",
"r546",
"r598"
],
"calculation": {
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": 2.0,
"parentTag": "us-gaap_DefinedBenefitPlanAmountsRecognizedInBalanceSheet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liability, recognized in statement of financial position, for defined benefit pension and other postretirement plans, classified as current.",
"label": "Liability, Defined Benefit Plan, Current",
"negatedLabel": "Current liabilities"
}
}
},
"localname": "PensionAndOtherPostretirementDefinedBenefitPlansCurrentLiabilities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PensionAndOtherPostretirementDefinedBenefitPlansLiabilitiesNoncurrent": {
"auth_ref": [
"r28",
"r522",
"r523",
"r546",
"r598"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 4.0,
"parentTag": "us-gaap_Liabilities",
"weight": 1.0
},
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails": {
"order": 3.0,
"parentTag": "us-gaap_DefinedBenefitPlanAmountsRecognizedInBalanceSheet",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of liability, recognized in statement of financial position, for defined benefit pension and other postretirement plans, classified as noncurrent.",
"label": "Liability, Defined Benefit Plan, Noncurrent",
"negatedLabel": "Non-current liabilities",
"terseLabel": "Accrued compensation and retirement benefits"
}
}
},
"localname": "PensionAndOtherPostretirementDefinedBenefitPlansLiabilitiesNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PensionAndOtherPostretirementPlansPolicy": {
"auth_ref": [
"r582",
"r601",
"r602",
"r605",
"r611"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for pension and other postretirement benefit plans. This accounting policy may address (1) the types of plans sponsored by the entity, and the benefits provided by each plan (2) groups that participate in (or are covered by) each plan (3) how plan assets, liabilities and expenses are measured, including the use of any actuaries and (4) significant assumptions used by the entity to value plan assets and liabilities and how such assumptions are derived.",
"label": "Pension and Other Postretirement Plans, Policy [Policy Text Block]",
"terseLabel": "Retirement Benefit Plan Assumptions"
}
}
},
"localname": "PensionAndOtherPostretirementPlansPolicy",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_PensionPlansDefinedBenefitMember": {
"auth_ref": [
"r520",
"r522",
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r573",
"r574",
"r576",
"r579",
"r583",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r598",
"r599",
"r605",
"r606",
"r621",
"r622",
"r623",
"r624"
],
"lang": {
"en-us": {
"role": {
"documentation": "Plan designed to provide participant with pension benefits. Includes, but is not limited to, defined benefit and defined contribution plans. Excludes other postretirement benefits.",
"label": "Pension Plan [Member]",
"terseLabel": "Pension benefits"
}
}
},
"localname": "PensionPlansDefinedBenefitMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_PerformanceSharesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Share-based payment arrangement awarded for meeting performance target.",
"label": "Performance Shares [Member]",
"terseLabel": "Performance share units"
}
}
},
"localname": "PerformanceSharesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_PlanAssetCategoriesDomain": {
"auth_ref": [
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r576",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Defined benefit plan asset investment.",
"label": "Defined Benefit Plan, Plan Assets, Category [Domain]",
"terseLabel": "Defined Benefit Plan, Plan Assets, Category [Domain]"
}
}
},
"localname": "PlanAssetCategoriesDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_PlanNameAxis": {
"auth_ref": [
"r631",
"r663"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by plan name for share-based payment arrangement.",
"label": "Plan Name [Axis]",
"terseLabel": "Plan Name [Axis]"
}
}
},
"localname": "PlanNameAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_PlanNameDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Plan name for share-based payment arrangement.",
"label": "Plan Name [Domain]",
"terseLabel": "Plan Name [Domain]"
}
}
},
"localname": "PlanNameDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_PreferredStockParOrStatedValuePerShare": {
"auth_ref": [
"r32",
"r460"
],
"lang": {
"en-us": {
"role": {
"documentation": "Face amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer.",
"label": "Preferred Stock, Par or Stated Value Per Share",
"terseLabel": "Preferred stock, par value (in dollars per share)"
}
}
},
"localname": "PreferredStockParOrStatedValuePerShare",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_PreferredStockSharesAuthorized": {
"auth_ref": [
"r32"
],
"lang": {
"en-us": {
"role": {
"documentation": "The maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws.",
"label": "Preferred Stock, Shares Authorized",
"terseLabel": "Preferred stock, authorized (in shares)"
}
}
},
"localname": "PreferredStockSharesAuthorized",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_PreferredStockSharesIssued": {
"auth_ref": [
"r32",
"r460"
],
"lang": {
"en-us": {
"role": {
"documentation": "Total number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) issued to shareholders (includes related preferred shares that were issued, repurchased, and remain in the treasury). May be all or portion of the number of preferred shares authorized. Excludes preferred shares that are classified as debt.",
"label": "Preferred Stock, Shares Issued",
"terseLabel": "Preferred stock, issued (in shares)"
}
}
},
"localname": "PreferredStockSharesIssued",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_PreferredStockSharesOutstanding": {
"auth_ref": [
"r32"
],
"lang": {
"en-us": {
"role": {
"documentation": "Aggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased.",
"label": "Preferred Stock, Shares Outstanding",
"terseLabel": "Preferred stock, outstanding (in shares)"
}
}
},
"localname": "PreferredStockSharesOutstanding",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_PreferredStockValueOutstanding": {
"auth_ref": [
"r32"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Value of all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by shareholders, which is net of related treasury stock. May be all or a portion of the number of preferred shares authorized. These shares represent the ownership interest of the preferred shareholders.",
"label": "Preferred Stock, Value, Outstanding",
"terseLabel": "Preferred stock, value, outstanding"
}
}
},
"localname": "PreferredStockValueOutstanding",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PrepaidExpensesAndOtherCurrentAssetsMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Primary financial statement caption encompassing prepaid expenses and other current assets.",
"label": "Prepaid Expenses and Other Current Assets [Member]",
"terseLabel": "Other current assets"
}
}
},
"localname": "PrepaidExpensesAndOtherCurrentAssetsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_PrepaidTaxes": {
"auth_ref": [
"r11",
"r13",
"r328",
"r329"
],
"calculation": {
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails_1": {
"order": 2.0,
"parentTag": "us-gaap_DeferredTaxAssetsLiabilitiesNet",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of asset related to consideration paid in advance for income and other taxes that provide economic benefits within a future period of one year or the normal operating cycle, if longer.",
"label": "Prepaid Taxes",
"terseLabel": "Prepaid income taxes"
}
}
},
"localname": "PrepaidTaxes",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesScheduleofDeferredTaxAssetsandLiabilitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PriorPeriodReclassificationAdjustmentDescription": {
"auth_ref": [
"r5"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for reclassification affecting comparability of financial statement. Excludes amendment to accounting standards, other change in accounting principle, and correction of error.",
"label": "Reclassification, Comparability Adjustment [Policy Text Block]",
"terseLabel": "Reclassifications"
}
}
},
"localname": "PriorPeriodReclassificationAdjustmentDescription",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_PrivateEquityFundsMember": {
"auth_ref": [
"r549"
],
"lang": {
"en-us": {
"role": {
"documentation": "Investments held in private equity funds.",
"label": "Private Equity Funds [Member]",
"terseLabel": "Private equity fund"
}
}
},
"localname": "PrivateEquityFundsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ProceedsFromIssuanceOfLongTermDebt": {
"auth_ref": [
"r136"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 8.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash inflow from a debt initially having maturity due after one year or beyond the operating cycle, if longer.",
"label": "Proceeds from Issuance of Long-term Debt",
"terseLabel": "Issuance of long-term debt",
"verboseLabel": "Cash proceeds"
}
}
},
"localname": "ProceedsFromIssuanceOfLongTermDebt",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromIssuanceOrSaleOfEquity": {
"auth_ref": [
"r135"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 2.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash inflow from the issuance of common stock, preferred stock, treasury stock, stock options, and other types of equity.",
"label": "Proceeds from Issuance or Sale of Equity",
"terseLabel": "Issuance of ordinary shares"
}
}
},
"localname": "ProceedsFromIssuanceOrSaleOfEquity",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromMaturitiesPrepaymentsAndCallsOfHeldToMaturitySecurities": {
"auth_ref": [
"r130",
"r280"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash inflow associated with the maturity, prepayments and calls (requests for early payments) of debt securities designated as held-to-maturity.",
"label": "Proceeds from Maturities, Prepayments and Calls of Held-to-maturity Securities",
"terseLabel": "Proceeds from maturities of investments classified as held to maturity"
}
}
},
"localname": "ProceedsFromMaturitiesPrepaymentsAndCallsOfHeldToMaturitySecurities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromPaymentsForOtherFinancingActivities": {
"auth_ref": [
"r137",
"r140"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 5.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash inflow (outflow) from financing activities classified as other.",
"label": "Proceeds from (Payments for) Other Financing Activities",
"terseLabel": "Other financing activities"
}
}
},
"localname": "ProceedsFromPaymentsForOtherFinancingActivities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromRepaymentsOfShortTermDebtMaturingInThreeMonthsOrLess": {
"auth_ref": [
"r136",
"r139",
"r151"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 4.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash inflow from a borrowing net of the cash outflow from repayment of a borrowing having initial term of repayment within three months.",
"label": "Proceeds from (Repayments of) Short-term Debt, Maturing in Three Months or Less",
"terseLabel": "Change in current debt obligations, net"
}
}
},
"localname": "ProceedsFromRepaymentsOfShortTermDebtMaturingInThreeMonthsOrLess",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromSaleAndMaturityOfAvailableForSaleSecurities": {
"auth_ref": [
"r129",
"r130",
"r280"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash inflow from sale, maturity, prepayment and call of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale).",
"label": "Proceeds from Sale and Maturity of Debt Securities, Available-for-sale",
"terseLabel": "Proceeds from sales and maturities"
}
}
},
"localname": "ProceedsFromSaleAndMaturityOfAvailableForSaleSecurities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysDebtSecuritiesPortfolioandAvailableforsaleDebtSecuritiesContractualMaturitiesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromSaleMaturityAndCollectionsOfInvestments": {
"auth_ref": [
"r131"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 2.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash inflow associated with the sale, maturity and collection of all investments such as debt, security and so forth during the period.",
"label": "Proceeds from Sale, Maturity and Collection of Investments",
"terseLabel": "Sales and maturities of investments",
"verboseLabel": "Proceeds from sales"
}
}
},
"localname": "ProceedsFromSaleMaturityAndCollectionsOfInvestments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows",
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromShortTermDebt": {
"auth_ref": [
"r136"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 3.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash inflow from a borrowing having initial term of repayment within one year or the normal operating cycle, if longer.",
"label": "Proceeds from Short-term Debt",
"terseLabel": "Proceeds from short-term borrowings (maturities greater than 90 days)"
}
}
},
"localname": "ProceedsFromShortTermDebt",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProceedsFromStockOptionsExercised": {
"auth_ref": [
"r135",
"r664"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of cash inflow from exercise of option under share-based payment arrangement.",
"label": "Proceeds from Stock Options Exercised",
"terseLabel": "Cash proceeds from options exercised"
}
}
},
"localname": "ProceedsFromStockOptionsExercised",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ProfitLoss": {
"auth_ref": [
"r7",
"r103",
"r106",
"r112",
"r143",
"r163",
"r184",
"r195",
"r196",
"r242",
"r248",
"r252",
"r255",
"r258",
"r303",
"r404",
"r405",
"r406",
"r409",
"r410",
"r411",
"r413",
"r415",
"r417",
"r418",
"r755",
"r761",
"r763",
"r769",
"r770",
"r817",
"r835",
"r953"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 3.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0
},
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome": {
"order": 1.0,
"parentTag": "us-gaap_ComprehensiveIncomeNetOfTaxIncludingPortionAttributableToNoncontrollingInterest",
"weight": 1.0
},
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 1.0,
"parentTag": "us-gaap_NetIncomeLoss",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The consolidated profit or loss for the period, net of income taxes, including the portion attributable to the noncontrolling interest.",
"label": "Net Income (Loss), Including Portion Attributable to Noncontrolling Interest",
"terseLabel": "Net income",
"totalLabel": "Net income",
"verboseLabel": "Net income"
}
}
},
"localname": "ProfitLoss",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows",
"http://www.medtronic.com/role/ConsolidatedStatementsofComprehensiveIncome",
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity",
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PropertyPlantAndEquipmentAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Property, Plant and Equipment [Abstract]",
"terseLabel": "Property, Plant and Equipment [Abstract]"
}
}
},
"localname": "PropertyPlantAndEquipmentAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAccumulatedDepreciationAndAmortization": {
"auth_ref": [
"r359",
"r867",
"r869"
],
"calculation": {
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails": {
"order": 2.0,
"parentTag": "us-gaap_PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortization",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of accumulated depreciation and amortization from plant, property, and equipment and right-of-use asset from finance lease.",
"label": "Property, Plant, and Equipment and Finance Lease Right-of-Use Asset, Accumulated Depreciation and Amortization",
"negatedTerseLabel": "Less: Accumulated depreciation"
}
}
},
"localname": "PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAccumulatedDepreciationAndAmortization",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortization": {
"auth_ref": [
"r74",
"r360",
"r869"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 3.0,
"parentTag": "us-gaap_Assets",
"weight": 1.0
},
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, after accumulated depreciation and amortization, of property, plant, and equipment and finance lease right-of-use asset.",
"label": "Property, Plant, and Equipment and Finance Lease Right-of-Use Asset, after Accumulated Depreciation and Amortization",
"terseLabel": "Property, plant, and equipment, net",
"totalLabel": "Property, plant, and equipment, net"
}
}
},
"localname": "PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortization",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetBeforeAccumulatedDepreciationAndAmortization": {
"auth_ref": [
"r20",
"r358",
"r864"
],
"calculation": {
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails": {
"order": 1.0,
"parentTag": "us-gaap_PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetAfterAccumulatedDepreciationAndAmortization",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, before accumulated depreciation and amortization, of property, plant, and equipment and finance lease right-of-use asset.",
"label": "Property, Plant, and Equipment and Finance Lease Right-of-Use Asset, before Accumulated Depreciation and Amortization",
"terseLabel": "Property, plant, and equipment"
}
}
},
"localname": "PropertyPlantAndEquipmentAndFinanceLeaseRightOfUseAssetBeforeAccumulatedDepreciationAndAmortization",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PropertyPlantAndEquipmentByTypeAxis": {
"auth_ref": [
"r52",
"r360"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of long-lived, physical assets used to produce goods and services and not intended for resale.",
"label": "Long-Lived Tangible Asset [Axis]",
"terseLabel": "Property, Plant and Equipment, Type [Axis]"
}
}
},
"localname": "PropertyPlantAndEquipmentByTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_PropertyPlantAndEquipmentDisclosureTextBlock": {
"auth_ref": [
"r363",
"r1014",
"r1015",
"r1016"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections.",
"label": "Property, Plant and Equipment Disclosure [Text Block]",
"terseLabel": "Property, Plant, and Equipment"
}
}
},
"localname": "PropertyPlantAndEquipmentDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipment"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_PropertyPlantAndEquipmentLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Property, Plant and Equipment [Line Items]",
"terseLabel": "Property, Plant and Equipment [Line Items]"
}
}
},
"localname": "PropertyPlantAndEquipmentLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_PropertyPlantAndEquipmentNet": {
"auth_ref": [
"r20",
"r21",
"r360",
"r887",
"r959",
"r976"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures.",
"label": "Property, Plant and Equipment, Net",
"terseLabel": "Property, plant, and equipment, net"
}
}
},
"localname": "PropertyPlantAndEquipmentNet",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_PropertyPlantAndEquipmentPolicyTextBlock": {
"auth_ref": [
"r51",
"r360",
"r1014",
"r1015"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for long-lived, physical asset used in normal conduct of business and not intended for resale. Includes, but is not limited to, work of art, historical treasure, and similar asset classified as collections.",
"label": "Property, Plant and Equipment, Policy [Policy Text Block]",
"terseLabel": "Property, Plant, and Equipment"
}
}
},
"localname": "PropertyPlantAndEquipmentPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_PropertyPlantAndEquipmentTextBlock": {
"auth_ref": [
"r20",
"r360"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation.",
"label": "Property, Plant and Equipment [Table Text Block]",
"terseLabel": "Property, Plant and Equipment Balances and Corresponding Lives"
}
}
},
"localname": "PropertyPlantAndEquipmentTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_PropertyPlantAndEquipmentTypeDomain": {
"auth_ref": [
"r20",
"r358"
],
"lang": {
"en-us": {
"role": {
"documentation": "Listing of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale. Examples include land, buildings, machinery and equipment, and other types of furniture and equipment including, but not limited to, office equipment, furniture and fixtures, and computer equipment and software.",
"label": "Long-Lived Tangible Asset [Domain]",
"terseLabel": "Property, Plant and Equipment, Type [Domain]"
}
}
},
"localname": "PropertyPlantAndEquipmentTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_PropertyPlantAndEquipmentUsefulLife": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Useful life of long lived, physical assets used in the normal conduct of business and not intended for resale, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Examples include, but not limited to, land, buildings, machinery and equipment, office equipment, furniture and fixtures, and computer equipment.",
"label": "Property, Plant and Equipment, Useful Life",
"terseLabel": "Property, plant, and equipment, useful life"
}
}
},
"localname": "PropertyPlantAndEquipmentUsefulLife",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_ProvisionForDoubtfulAccounts": {
"auth_ref": [
"r123",
"r312"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 1.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of expense (reversal of expense) for expected credit loss on accounts receivable.",
"label": "Accounts Receivable, Credit Loss Expense (Reversal)",
"terseLabel": "Provision for credit losses"
}
}
},
"localname": "ProvisionForDoubtfulAccounts",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ReclassificationFromAccumulatedOtherComprehensiveIncomeCurrentPeriodNetOfTax": {
"auth_ref": [
"r100",
"r110",
"r111",
"r113",
"r848",
"r852",
"r854"
],
"calculation": {
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails": {
"order": 2.0,
"parentTag": "us-gaap_OtherComprehensiveIncomeLossNetOfTax",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount after tax of reclassification adjustments of other comprehensive income (loss).",
"label": "Reclassification from Accumulated Other Comprehensive Income, Current Period, Net of Tax",
"negatedLabel": "Reclassifications"
}
}
},
"localname": "ReclassificationFromAccumulatedOtherComprehensiveIncomeCurrentPeriodNetOfTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ReclassificationFromAociCurrentPeriodTax": {
"auth_ref": [
"r98",
"r102",
"r110"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of tax expense (benefit) of reclassification adjustment from accumulated other comprehensive income (loss).",
"label": "Reclassification from AOCI, Current Period, Tax",
"terseLabel": "Reclassifications from AOCI, tax expense (benefit)"
}
}
},
"localname": "ReclassificationFromAociCurrentPeriodTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ReconciliationOfAssetsFromSegmentToConsolidatedTextBlock": {
"auth_ref": [
"r249",
"r252"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of all significant reconciling items in the reconciliation of total assets from reportable segments to the entity's consolidated assets.",
"label": "Reconciliation of Assets from Segment to Consolidated [Table Text Block]",
"terseLabel": "Reconciliation of Assets and Depreciation Expense from Segments to Consolidated"
}
}
},
"localname": "ReconciliationOfAssetsFromSegmentToConsolidatedTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ReconciliationOfOperatingProfitLossFromSegmentsToConsolidatedTextBlock": {
"auth_ref": [
"r248",
"r252"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the reconciliation of profit (loss) from reportable segments to the consolidated income (loss) before income tax expense (benefit) and discontinued operations. Includes, but is not limited to, reconciliation after income tax if income tax is allocated to the reportable segment.",
"label": "Reconciliation of Operating Profit (Loss) from Segments to Consolidated [Table Text Block]",
"terseLabel": "Income From Operations Before Income Taxes by Reportable Segment and Reconciliation to Consolidated"
}
}
},
"localname": "ReconciliationOfOperatingProfitLossFromSegmentsToConsolidatedTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ReconciliationOfUnrecognizedTaxBenefitsExcludingAmountsPertainingToExaminedTaxReturnsRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Reconciliation of Unrecognized Tax Benefits, Excluding Amounts Pertaining to Examined Tax Returns [Roll Forward]",
"terseLabel": "Reconciliation of Unrecognized Tax Benefits, Excluding Amounts Pertaining to Examined Tax Returns [Roll Forward]"
}
}
},
"localname": "ReconciliationOfUnrecognizedTaxBenefitsExcludingAmountsPertainingToExaminedTaxReturnsRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RepaymentsOfLongTermDebt": {
"auth_ref": [
"r139"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 7.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash outflow for debt initially having maturity due after one year or beyond the normal operating cycle, if longer.",
"label": "Repayments of Long-term Debt",
"negatedLabel": "Payments on long-term debt"
}
}
},
"localname": "RepaymentsOfLongTermDebt",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RepaymentsOfSeniorDebt": {
"auth_ref": [
"r139"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash outflow for a long-term debt where the holder has highest claim on the entity's asset in case of bankruptcy or liquidation during the period.",
"label": "Repayments of Senior Debt",
"terseLabel": "Redemption of senior notes, consideration"
}
}
},
"localname": "RepaymentsOfSeniorDebt",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RepaymentsOfShortTermDebt": {
"auth_ref": [
"r139"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 9.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cash outflow for a borrowing having initial term of repayment within one year or the normal operating cycle, if longer.",
"label": "Repayments of Short-term Debt",
"negatedTerseLabel": "Repayments from short-term borrowings (maturities greater than 90 days)"
}
}
},
"localname": "RepaymentsOfShortTermDebt",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ResearchAndDevelopmentExpenseExcludingAcquiredInProcessCost": {
"auth_ref": [
"r678"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 3.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The costs incurred in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process whether intended for sale or the entity's use, during the reporting period charged to research and development projects, excluding in-process research and development acquired in a business combination consummated during the period. Excludes software research and development, which has a separate concept.",
"label": "Research and Development Expense (Excluding Acquired in Process Cost)",
"terseLabel": "Research and development expense"
}
}
},
"localname": "ResearchAndDevelopmentExpenseExcludingAcquiredInProcessCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ResearchAndDevelopmentExpenseMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Primary financial statement caption in which the reported facts about research and development expense have been included.",
"label": "Research and Development Expense [Member]",
"terseLabel": "Research and development expense"
}
}
},
"localname": "ResearchAndDevelopmentExpenseMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ResearchAndDevelopmentExpensePolicy": {
"auth_ref": [
"r678"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process.",
"label": "Research and Development Expense, Policy [Policy Text Block]",
"terseLabel": "Research and Development"
}
}
},
"localname": "ResearchAndDevelopmentExpensePolicy",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_RestrictedStockMember": {
"auth_ref": [
"r211"
],
"lang": {
"en-us": {
"role": {
"documentation": "Stock including a provision that prohibits sale or substantive sale of an equity instrument for a specified period of time or until specified performance conditions are met.",
"label": "Restricted Stock [Member]",
"terseLabel": "Restricted stock",
"verboseLabel": "Restricted stock"
}
}
},
"localname": "RestrictedStockMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_RestrictedStockUnitsRSUMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Share instrument which is convertible to stock or an equivalent amount of cash, after a specified period of time or when specified performance conditions are met.",
"label": "Restricted Stock Units (RSUs) [Member]",
"verboseLabel": "Restricted stock units"
}
}
},
"localname": "RestrictedStockUnitsRSUMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_RestructuringAndRelatedActivitiesAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Restructuring and Related Activities [Abstract]",
"terseLabel": "Restructuring and Related Activities [Abstract]"
}
}
},
"localname": "RestructuringAndRelatedActivitiesAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_RestructuringAndRelatedActivitiesDisclosureTextBlock": {
"auth_ref": [
"r369",
"r371",
"r374",
"r383",
"r384"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for restructuring and related activities. Description of restructuring activities such as exit and disposal activities, include facts and circumstances leading to the plan, the expected plan completion date, the major types of costs associated with the plan activities, total expected costs, the accrual balance at the end of the period, and the periods over which the remaining accrual will be settled.",
"label": "Restructuring and Related Activities Disclosure [Text Block]",
"terseLabel": "Restructuring Charges"
}
}
},
"localname": "RestructuringAndRelatedActivitiesDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringCharges"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_RestructuringAndRelatedCostCostIncurredToDate1": {
"auth_ref": [
"r370",
"r373",
"r380",
"r382"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of costs incurred to date for the specified restructuring cost.",
"label": "Restructuring and Related Cost, Cost Incurred to Date",
"terseLabel": "Pre-tax exit, disposal costs and other costs"
}
}
},
"localname": "RestructuringAndRelatedCostCostIncurredToDate1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RestructuringAndRelatedCostExpectedCost1": {
"auth_ref": [
"r370",
"r373",
"r380",
"r382"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount expected to be recognized in earnings for the specified restructuring cost.",
"label": "Restructuring and Related Cost, Expected Cost",
"terseLabel": "Estimated expected restructuring costs"
}
}
},
"localname": "RestructuringAndRelatedCostExpectedCost1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RestructuringCharges": {
"auth_ref": [
"r148",
"r368",
"r377",
"r380"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of expenses associated with exit or disposal activities pursuant to an authorized plan. Excludes expenses related to a discontinued operation or an asset retirement obligation.",
"label": "Restructuring Charges",
"verboseLabel": "Charges"
}
}
},
"localname": "RestructuringCharges",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RestructuringCostAndReserveAxis": {
"auth_ref": [
"r370",
"r371",
"r380",
"r381"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of restructuring cost.",
"label": "Restructuring Type [Axis]",
"terseLabel": "Restructuring Type [Axis]"
}
}
},
"localname": "RestructuringCostAndReserveAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RestructuringCostAndReserveLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Restructuring Cost and Reserve [Line Items]",
"terseLabel": "Restructuring Cost and Reserve [Line Items]"
}
}
},
"localname": "RestructuringCostAndReserveLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RestructuringPlanAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by individual restructuring plan.",
"label": "Restructuring Plan [Axis]",
"terseLabel": "Restructuring Plan [Axis]"
}
}
},
"localname": "RestructuringPlanAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RestructuringPlanDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Identification of the individual restructuring plans.",
"label": "Restructuring Plan [Domain]",
"terseLabel": "Restructuring Plan [Domain]"
}
}
},
"localname": "RestructuringPlanDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_RestructuringReserve": {
"auth_ref": [
"r371",
"r378"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount (including both current and noncurrent portions of the accrual) as of the balance sheet date pertaining to a specified type of cost associated with exit from or disposal of business activities or restructuring pursuant to a duly authorized plan.",
"label": "Restructuring Reserve",
"periodEndLabel": "Ending balance",
"periodStartLabel": "Beginning balance"
}
}
},
"localname": "RestructuringReserve",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RestructuringReserveAccrualAdjustment1": {
"auth_ref": [
"r371",
"r381"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of expense (reversal of expense) which increases (decreases) the restructuring reserve from an adjustment to a previously accrued restructuring liability.",
"label": "Restructuring Reserve, Accrual Adjustment",
"terseLabel": "Accrual adjustments"
}
}
},
"localname": "RestructuringReserveAccrualAdjustment1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RestructuringReserveCurrent": {
"auth_ref": [
"r26",
"r371",
"r381"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount as of the balance sheet date of known and estimated obligations associated with exit from or disposal of business activities or restructurings pursuant to a duly authorized plan, which are expected to be paid in the next twelve months or in the normal operating cycle if longer. Costs of such activities include those for one-time termination benefits, termination of an operating lease or other contract, consolidating or closing facilities, relocating employees, and costs associated with an ongoing benefit arrangement, but excludes costs associated with the retirement of a long-lived asset.",
"label": "Restructuring Reserve, Current",
"terseLabel": "Restructuring reserve, current"
}
}
},
"localname": "RestructuringReserveCurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RestructuringReserveNoncurrent": {
"auth_ref": [
"r75",
"r371",
"r381"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Carrying amount as of the balance sheet date of known and estimated costs associated with exit from or disposal of business activities or restructurings pursuant to a duly authorized plan, which are expected to be paid after one year or beyond the next operating cycle, if longer. Costs of such activities include those for one-time termination benefits, termination of an operating lease or other contract, consolidating or closing facilities, and relocating employees, and costs associated with an ongoing benefit arrangement, but excludes costs associated with the retirement of a long-lived asset.",
"label": "Restructuring Reserve, Noncurrent",
"terseLabel": "Restructuring reserve, noncurrent"
}
}
},
"localname": "RestructuringReserveNoncurrent",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RestructuringReserveRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Restructuring Reserve [Roll Forward]",
"terseLabel": "Changes in Restructuring Reserves"
}
}
},
"localname": "RestructuringReserveRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RestructuringReserveSettledWithoutCash2": {
"auth_ref": [
"r371",
"r381"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of decrease in the reserve for full or partial settlement through consideration other than cash.",
"label": "Restructuring Reserve, Settled without Cash",
"negatedTerseLabel": "Settled non-cash"
}
}
},
"localname": "RestructuringReserveSettledWithoutCash2",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RetainedEarningsAccumulatedDeficit": {
"auth_ref": [
"r36",
"r483",
"r672",
"r887",
"r973",
"r998",
"r1003"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 3.0,
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The cumulative amount of the reporting entity's undistributed earnings or deficit.",
"label": "Retained Earnings (Accumulated Deficit)",
"terseLabel": "Retained earnings"
}
}
},
"localname": "RetainedEarningsAccumulatedDeficit",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RetainedEarningsMember": {
"auth_ref": [
"r2",
"r180",
"r181",
"r182",
"r185",
"r194",
"r196",
"r307",
"r669",
"r670",
"r671",
"r718",
"r719",
"r815",
"r994",
"r996"
],
"lang": {
"en-us": {
"role": {
"documentation": "The cumulative amount of the reporting entity's undistributed earnings or deficit.",
"label": "Retained Earnings [Member]",
"terseLabel": "Retained Earnings"
}
}
},
"localname": "RetainedEarningsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "domainItemType"
},
"us-gaap_RetirementPlanNameAxis": {
"auth_ref": [
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r572",
"r573",
"r574",
"r576",
"r579",
"r583",
"r584",
"r585",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r612",
"r613",
"r614",
"r615",
"r616",
"r617",
"r618",
"r619",
"r620",
"r625"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by name of plan designed to provide retirement benefits. Includes, but is not limited to, legal name of defined benefit and defined contribution plans.",
"label": "Retirement Plan Name [Axis]",
"terseLabel": "Retirement Plan Name [Axis]"
}
}
},
"localname": "RetirementPlanNameAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RetirementPlanNameDomain": {
"auth_ref": [
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r572",
"r573",
"r574",
"r576",
"r579",
"r583",
"r584",
"r585",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r612",
"r613",
"r614",
"r615",
"r616",
"r617",
"r618",
"r619",
"r620",
"r625"
],
"lang": {
"en-us": {
"role": {
"documentation": "Name of plan designed to provide retirement benefits. Includes, but is not limited to, legal name of defined benefit and defined contribution plans.",
"label": "Retirement Plan Name [Domain]",
"terseLabel": "Retirement Plan Name [Domain]"
}
}
},
"localname": "RetirementPlanNameDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_RetirementPlanSponsorLocationAxis": {
"auth_ref": [
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r572",
"r573",
"r574",
"r576",
"r579",
"r584",
"r585",
"r587",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r606",
"r610",
"r624"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by location of employer sponsoring plan designed to provide retirement benefits. Includes, but is not limited to, defined benefit and defined contribution plans.",
"label": "Retirement Plan Sponsor Location [Axis]",
"terseLabel": "Retirement Plan Sponsor Location [Axis]"
}
}
},
"localname": "RetirementPlanSponsorLocationAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RetirementPlanSponsorLocationDomain": {
"auth_ref": [
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r572",
"r573",
"r574",
"r576",
"r579",
"r584",
"r585",
"r587",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r606",
"r610",
"r624"
],
"lang": {
"en-us": {
"role": {
"documentation": "Location of employer sponsoring plan designed to provide retirement benefits. Includes, but is not limited to, defined benefit and defined contribution plans.",
"label": "Retirement Plan Sponsor Location [Domain]",
"terseLabel": "Retirement Plan Sponsor Location [Domain]"
}
}
},
"localname": "RetirementPlanSponsorLocationDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_RetirementPlanTypeAxis": {
"auth_ref": [
"r520",
"r521",
"r522",
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r573",
"r574",
"r576",
"r579",
"r583",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r598",
"r599",
"r605",
"r606",
"r607",
"r608",
"r609",
"r610",
"r621",
"r622",
"r623",
"r624"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of retirement benefit plan. Includes, but is not limited to, retirement benefit arrangement for defined benefit pension and other postretirement plans, retirement benefit arrangement for defined contribution pension and other postretirement plans, and special and contractual termination benefits payable upon retirement.",
"label": "Retirement Plan Type [Axis]",
"terseLabel": "Retirement Plan Type [Axis]"
}
}
},
"localname": "RetirementPlanTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RetirementPlanTypeDomain": {
"auth_ref": [
"r520",
"r521",
"r522",
"r526",
"r527",
"r528",
"r529",
"r530",
"r531",
"r532",
"r533",
"r534",
"r535",
"r536",
"r537",
"r538",
"r539",
"r540",
"r541",
"r542",
"r543",
"r544",
"r545",
"r546",
"r548",
"r549",
"r551",
"r552",
"r553",
"r554",
"r555",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r567",
"r568",
"r569",
"r571",
"r573",
"r574",
"r576",
"r579",
"r583",
"r588",
"r589",
"r590",
"r591",
"r592",
"r593",
"r594",
"r595",
"r596",
"r597",
"r598",
"r599",
"r605",
"r606",
"r607",
"r608",
"r609",
"r610",
"r621",
"r622",
"r623",
"r624"
],
"lang": {
"en-us": {
"role": {
"documentation": "Type of plan designed to provide participants with retirement benefits. Includes, but is not limited to, retirement benefit arrangement for defined benefit pension and other postretirement plans, retirement benefit arrangement for defined contribution pension and other postretirement plans, and special and contractual termination benefits payable upon retirement.",
"label": "Retirement Plan Type [Domain]",
"terseLabel": "Retirement Plan Type [Domain]"
}
}
},
"localname": "RetirementPlanTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_RevenueFromContractWithCustomerAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Revenue from Contract with Customer [Abstract]",
"terseLabel": "Shipping and Handling",
"verboseLabel": "Shipping and Handling"
}
}
},
"localname": "RevenueFromContractWithCustomerAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax": {
"auth_ref": [
"r233",
"r234",
"r247",
"r253",
"r254",
"r261",
"r262",
"r265",
"r504",
"r505",
"r925"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 1.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise.",
"label": "Revenue from Contract with Customer, Excluding Assessed Tax",
"netLabel": "Revenue",
"terseLabel": "Net sales",
"verboseLabel": "Net sales"
}
}
},
"localname": "RevenueFromContractWithCustomerExcludingAssessedTax",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails",
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RevenueFromContractWithCustomerPolicyTextBlock": {
"auth_ref": [
"r158",
"r496",
"r497",
"r498",
"r499",
"r500",
"r501",
"r502",
"r503",
"r517"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for revenue from contract with customer.",
"label": "Revenue from Contract with Customer [Policy Text Block]",
"terseLabel": "Revenue Recognition and Shipping and Handling"
}
}
},
"localname": "RevenueFromContractWithCustomerPolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_RevenueFromContractWithCustomerTextBlock": {
"auth_ref": [
"r487",
"r488",
"r489",
"r490",
"r491",
"r492",
"r494",
"r495",
"r508",
"r517"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure of revenue from contract with customer to transfer good or service and to transfer nonfinancial asset. Includes, but is not limited to, disaggregation of revenue, credit loss recognized from contract with customer, judgment and change in judgment related to contract with customer, and asset recognized from cost incurred to obtain or fulfill contract with customer. Excludes insurance and lease contracts.",
"label": "Revenue from Contract with Customer [Text Block]",
"terseLabel": "Revenue"
}
}
},
"localname": "RevenueFromContractWithCustomerTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/Revenue"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_RevenuePerformanceObligationDescriptionOfTiming": {
"auth_ref": [
"r488"
],
"lang": {
"en-us": {
"role": {
"documentation": "Description of timing for satisfying performance obligation in contract with customer. Includes, but is not limited to, as services are rendered, and upon shipment, delivery or completion of service.",
"label": "Revenue, Performance Obligation, Description of Timing",
"terseLabel": "Period over which remaining performance obligations are expected to be recognized as revenue"
}
}
},
"localname": "RevenuePerformanceObligationDescriptionOfTiming",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RevenueRemainingPerformanceObligation": {
"auth_ref": [
"r493"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of transaction price allocated to performance obligation that has not been recognized as revenue.",
"label": "Revenue, Remaining Performance Obligation, Amount",
"terseLabel": "Estimated revenue expected to be recognized in future periods related to unsatisfied performance obligations"
}
}
},
"localname": "RevenueRemainingPerformanceObligation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueNarrativeDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_RevenuesFromExternalCustomersAndLongLivedAssetsLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Revenues from External Customers and Long-Lived Assets [Line Items]",
"terseLabel": "Revenues from External Customers and Long-Lived Assets [Line Items]"
}
}
},
"localname": "RevenuesFromExternalCustomersAndLongLivedAssetsLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability": {
"auth_ref": [
"r873",
"r878"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase in right-of-use asset obtained in exchange for operating lease liability.",
"label": "Right-of-Use Asset Obtained in Exchange for Operating Lease Liability",
"terseLabel": "Right-of-use assets obtained in exchange for operating lease liabilities"
}
}
},
"localname": "RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesSupplementalCashFlowInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ScenarioAdjustmentMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Domain member used to indicate figures that are adjustments during a period or as of a point in time. This domain member would never be expected to appear in a relationship group without the \"Scenario, Previously Reported\" Member with the same parent.",
"label": "Scenario, Adjustment [Member]",
"terseLabel": "Dedesignation of hedge"
}
}
},
"localname": "ScenarioAdjustmentMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ScheduleOfAccumulatedBenefitObligationsInExcessOfFairValueOfPlanAssetsTableTextBlock": {
"auth_ref": [
"r584",
"r585",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of benefit obligation and plan assets of defined benefit plan with accumulated benefit obligation in excess of plan assets.",
"label": "Defined Benefit Plan, Plan with Accumulated Benefit Obligation in Excess of Plan Assets [Table Text Block]",
"terseLabel": "Schedule of Accumulated Benefit Obligations in Excess of Fair Value of Plan Assets"
}
}
},
"localname": "ScheduleOfAccumulatedBenefitObligationsInExcessOfFairValueOfPlanAssetsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfAccumulatedOtherComprehensiveIncomeLossTableTextBlock": {
"auth_ref": [
"r100",
"r853",
"r854"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the components of accumulated other comprehensive income (loss).",
"label": "Schedule of Accumulated Other Comprehensive Income (Loss) [Table Text Block]",
"terseLabel": "Schedule of Accumulated Other Comprehensive Loss"
}
}
},
"localname": "ScheduleOfAccumulatedOtherComprehensiveIncomeLossTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfAllocationOfPlanAssetsTableTextBlock": {
"auth_ref": [
"r547"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the major categories of plan assets of pension plans and/or other employee benefit plans. This information may include, but is not limited to, the target allocation of plan assets, the fair value of each major category of plan assets, and the level within the fair value hierarchy in which the fair value measurements fall.",
"label": "Schedule of Allocation of Plan Assets [Table Text Block]",
"terseLabel": "Schedule of Allocation of Plan Assets"
}
}
},
"localname": "ScheduleOfAllocationOfPlanAssetsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTable": {
"auth_ref": [
"r211"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule for securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by Antidilutive Securities.",
"label": "Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share [Table]",
"terseLabel": "Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share [Table]"
}
}
},
"localname": "ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfAssumptionsUsedTableTextBlock": {
"auth_ref": [
"r570"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of assumption used to determine benefit obligation and net periodic benefit cost of defined benefit plan. Includes, but is not limited to, discount rate, rate of compensation increase, expected long-term rate of return on plan assets and interest crediting rate.",
"label": "Defined Benefit Plan, Assumptions [Table Text Block]",
"terseLabel": "Schedule of Assumptions Used"
}
}
},
"localname": "ScheduleOfAssumptionsUsedTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfAvailableForSaleSecuritiesLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Debt Securities, Available-for-sale [Line Items]",
"terseLabel": "Debt Securities, Available-for-sale [Line Items]"
}
}
},
"localname": "ScheduleOfAvailableForSaleSecuritiesLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfBenefitObligationsInExcessOfFairValueOfPlanAssetsTableTextBlock": {
"auth_ref": [
"r584",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of benefit obligation and plan assets for defined benefit pension plan with projected benefit obligation in excess of plan assets.",
"label": "Defined Benefit Plan, Plan with Projected Benefit Obligation in Excess of Plan Assets [Table Text Block]",
"terseLabel": "Schedule of Benefit Obligations in Excess of Fair Value of Plan Assets"
}
}
},
"localname": "ScheduleOfBenefitObligationsInExcessOfFairValueOfPlanAssetsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfBusinessAcquisitionsByAcquisitionContingentConsiderationTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of contingent payment arrangements including the terms that will result in payment and the accounting treatment that will be followed if such contingencies occur, including the potential impact on earnings per share if contingencies are to be settled in common stock of the entity. The description also may include the period over which amounts are expected to be paid, and changes in the amount since the previous reporting period. This also includes contingent options and commitments.",
"label": "Schedule of Business Acquisitions by Acquisition, Contingent Consideration [Table Text Block]",
"terseLabel": "Schedule of Reconciliation of Beginning and Ending Balances of Contingent Consideration Associated with Acquisitions"
}
}
},
"localname": "ScheduleOfBusinessAcquisitionsByAcquisitionContingentConsiderationTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfBusinessAcquisitionsByAcquisitionTable": {
"auth_ref": [
"r734",
"r735"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule reflecting each material business combination (or series of individually immaterial business combinations) completed during the period, including background, timing, and recognized assets and liabilities.",
"label": "Schedule of Business Acquisitions, by Acquisition [Table]",
"terseLabel": "Schedule of Business Acquisitions, by Acquisition [Table]"
}
}
},
"localname": "ScheduleOfBusinessAcquisitionsByAcquisitionTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock": {
"auth_ref": [
"r660"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of cost recognized for award under share-based payment arrangement by plan. Includes, but is not limited to, related tax benefit.",
"label": "Share-based Payment Arrangement, Cost by Plan [Table Text Block]",
"terseLabel": "Schedule of Stock-Based Compensation Expense"
}
}
},
"localname": "ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock": {
"auth_ref": [
"r714"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the components of income tax expense attributable to continuing operations for each year presented including, but not limited to: current tax expense (benefit), deferred tax expense (benefit), investment tax credits, government grants, the benefits of operating loss carryforwards, tax expense that results from allocating certain tax benefits either directly to contributed capital or to reduce goodwill or other noncurrent intangible assets of an acquired entity, adjustments of a deferred tax liability or asset for enacted changes in tax laws or rates or a change in the tax status of the entity, and adjustments of the beginning-of-the-year balances of a valuation allowance because of a change in circumstances that causes a change in judgment about the realizability of the related deferred tax asset in future years.",
"label": "Schedule of Components of Income Tax Expense (Benefit) [Table Text Block]",
"terseLabel": "Income Tax (Benefit) Provision"
}
}
},
"localname": "ScheduleOfComponentsOfIncomeTaxExpenseBenefitTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfDebtInstrumentsTextBlock": {
"auth_ref": [
"r64",
"r170",
"r450",
"r452",
"r477",
"r480",
"r481",
"r482",
"r858",
"r859",
"r863",
"r961"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of long-debt instruments or arrangements, including identification, terms, features, collateral requirements and other information necessary to a fair presentation. These are debt arrangements that originally required repayment more than twelve months after issuance or greater than the normal operating cycle of the entity, if longer.",
"label": "Schedule of Long-term Debt Instruments [Table Text Block]",
"terseLabel": "Schedule of Long-term Debt"
}
}
},
"localname": "ScheduleOfDebtInstrumentsTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock": {
"auth_ref": [
"r705"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets.",
"label": "Schedule of Deferred Tax Assets and Liabilities [Table Text Block]",
"terseLabel": "Schedule of Deferred Tax Assets and Liabilities"
}
}
},
"localname": "ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfDefinedBenefitPlanAmountsRecognizedInOtherComprehensiveIncomeLossTableTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the changes in plan assets and benefit obligations recognized in other comprehensive income (loss) during the period.",
"label": "Schedule of Defined Benefit Plan Amounts Recognized in Other Comprehensive Income (Loss) [Table Text Block]",
"terseLabel": "Schedule of Defined Benefit Plan Amounts Recognized in Other Comprehensive Income (Loss)"
}
}
},
"localname": "ScheduleOfDefinedBenefitPlanAmountsRecognizedInOtherComprehensiveIncomeLossTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfDefinedBenefitPlansDisclosuresTable": {
"auth_ref": [
"r582",
"r583",
"r586",
"r587",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosures about an individual defined benefit pension plan or an other postretirement defined benefit plan. It may be appropriate to group certain similar plans. Also includes schedule for fair value of plan assets by major categories of plan assets by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets or liabilities (Level 1), Significant other observable inputs (Level 2), and significant unobservable inputs (Level 3).",
"label": "Schedule of Defined Benefit Plans Disclosures [Table]",
"terseLabel": "Schedule of Defined Benefit Plans Disclosures [Table]"
}
}
},
"localname": "ScheduleOfDefinedBenefitPlansDisclosuresTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansActuarialAssumptionsandPlanAssetsTargetAllocationsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansChangeinBenefitObligationandFundedStatusDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansFutureBenefitPaymentsDetails",
"http://www.medtronic.com/role/RetirementBenefitPlansNetPeriodicCostandAOCIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfDefinedBenefitPlansDisclosuresTextBlock": {
"auth_ref": [
"r582",
"r583",
"r586",
"r587",
"r598"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of one or more of the entity's defined benefit pension plans or one or more other defined benefit postretirement plans, separately for pension plans and other postretirement benefit plans including the entity's schedule of fair value of plan assets for defined benefit or other postretirement plans.",
"label": "Schedule of Defined Benefit Plans Disclosures [Table Text Block]",
"terseLabel": "Schedule of Defined Benefit Plans"
}
}
},
"localname": "ScheduleOfDefinedBenefitPlansDisclosuresTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock": {
"auth_ref": [
"r781",
"r790",
"r797"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the location and amount of derivative instruments and nonderivative instruments designated as hedging instruments reported before netting adjustments, and the amount of gain (loss) on derivative instruments and nonderivative instruments designated and qualified as hedging instruments.",
"label": "Derivative Instruments, Gain (Loss) [Table Text Block]",
"terseLabel": "Schedule of (Gain) Loss on Derivative Instruments"
}
}
},
"localname": "ScheduleOfDerivativeInstrumentsGainLossInStatementOfFinancialPerformanceTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfDerivativeInstrumentsInStatementOfFinancialPositionFairValueTextBlock": {
"auth_ref": [
"r786"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the location and fair value amounts of derivative instruments (and nonderivative instruments that are designated and qualify as hedging instruments) reported in the statement of financial position.",
"label": "Schedule of Derivative Instruments in Statement of Financial Position, Fair Value [Table Text Block]",
"terseLabel": "Schedule of Classification and Fair Value Amounts of Derivative Instruments in Balance Sheets"
}
}
},
"localname": "ScheduleOfDerivativeInstrumentsInStatementOfFinancialPositionFairValueTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock": {
"auth_ref": [
"r210"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of an entity's basic and diluted earnings per share calculations, including a reconciliation of numerators and denominators of the basic and diluted per-share computations for income from continuing operations.",
"label": "Schedule of Earnings Per Share, Basic and Diluted [Table Text Block]",
"terseLabel": "Computation of Basic and Diluted Earnings Per Share"
}
}
},
"localname": "ScheduleOfEarningsPerShareBasicAndDilutedTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock": {
"auth_ref": [
"r687"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the reconciliation using percentage or dollar amounts of the reported amount of income tax expense attributable to continuing operations for the year to the amount of income tax expense that would result from applying domestic federal statutory tax rates to pretax income from continuing operations.",
"label": "Schedule of Effective Income Tax Rate Reconciliation [Table Text Block]",
"terseLabel": "Schedule of Effective Income Tax Rate Reconciliation"
}
}
},
"localname": "ScheduleOfEffectiveIncomeTaxRateReconciliationTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfEquityMethodInvestmentsLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Schedule of Equity Method Investments [Line Items]",
"terseLabel": "Schedule of Equity Method Investments [Line Items]"
}
}
},
"localname": "ScheduleOfEquityMethodInvestmentsLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfEquityMethodInvestmentsTable": {
"auth_ref": [
"r7",
"r163",
"r302",
"r303",
"r835"
],
"lang": {
"en-us": {
"role": {
"documentation": "Summarization of information required and determined to be disclosed concerning equity method investments in common stock. The summarized information includes: (a) the name of each investee or group of investees for which combined disclosure is appropriate, (2) the percentage ownership of common stock, (3) the difference, if any, between the carrying amount of an investment and the value of the underlying equity in the net assets and the accounting treatment of difference, if any, and (4) the aggregate value of each identified investment based on its quoted market price, if available.",
"label": "Schedule of Equity Method Investments [Table]",
"terseLabel": "Schedule of Equity Method Investments [Table]"
}
}
},
"localname": "ScheduleOfEquityMethodInvestmentsTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsSummaryofEquityandOtherInvestmentsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfExpectedBenefitPaymentsTableTextBlock": {
"auth_ref": [
"r558"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of benefits expected to be paid by pension plans and/or other employee benefit plans in each of the next five fiscal years and in the aggregate for the five fiscal years thereafter.",
"label": "Schedule of Expected Benefit Payments [Table Text Block]",
"terseLabel": "Schedule of Expected Benefit Payments"
}
}
},
"localname": "ScheduleOfExpectedBenefitPaymentsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock": {
"auth_ref": [
"r820",
"r821"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of assets and liabilities, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3).",
"label": "Schedule of Fair Value, Assets and Liabilities Measured on Recurring Basis [Table Text Block]",
"terseLabel": "Schedule of Derivative Assets and Liabilities Measured at Fair Value on a Recurring Basis"
}
}
},
"localname": "ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfFiniteLivedIntangibleAssetsTable": {
"auth_ref": [
"r342",
"r347",
"r926"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule of assets, excluding financial assets and goodwill, lacking physical substance with a finite life.",
"label": "Schedule of Finite-Lived Intangible Assets [Table]",
"terseLabel": "Schedule of Finite-Lived Intangible Assets [Table]"
}
}
},
"localname": "ScheduleOfFiniteLivedIntangibleAssetsTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock": {
"auth_ref": [
"r342",
"r347"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of assets, excluding financial assets and goodwill, lacking physical substance with a finite life, by either major class or business segment.",
"label": "Schedule of Finite-Lived Intangible Assets [Table Text Block]",
"terseLabel": "Schedule of Finite-Lived Intangible Assets by Major Class"
}
}
},
"localname": "ScheduleOfFiniteLivedIntangibleAssetsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfGoodwillTable": {
"auth_ref": [
"r337",
"r339"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule of goodwill and the changes during the year due to acquisition, sale, impairment or for other reasons.",
"label": "Schedule of Goodwill [Table]",
"terseLabel": "Schedule of Goodwill [Table]"
}
}
},
"localname": "ScheduleOfGoodwillTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfGoodwillTextBlock": {
"auth_ref": [
"r337",
"r339"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of goodwill by reportable segment and in total which includes a rollforward schedule.",
"label": "Schedule of Goodwill [Table Text Block]",
"terseLabel": "Schedule of Goodwill"
}
}
},
"localname": "ScheduleOfGoodwillTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfIncomeBeforeIncomeTaxDomesticAndForeignTableTextBlock": {
"auth_ref": [
"r164"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of income before income tax between domestic and foreign jurisdictions.",
"label": "Schedule of Income before Income Tax, Domestic and Foreign [Table Text Block]",
"terseLabel": "Components of Income Before Income Taxes, Based on Jurisdiction"
}
}
},
"localname": "ScheduleOfIncomeBeforeIncomeTaxDomesticAndForeignTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfIndefiniteLivedIntangibleAssetsTableTextBlock": {
"auth_ref": [
"r351",
"r354"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of assets, excluding financial assets and goodwill, lacking physical substance and exist in perpetuity, by either major class or business segment.",
"label": "Schedule of Indefinite-Lived Intangible Assets [Table Text Block]",
"terseLabel": "Schedule of Indefinite-Lived Intangible Assets by Major Class"
}
}
},
"localname": "ScheduleOfIndefiniteLivedIntangibleAssetsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfInventoryCurrentTableTextBlock": {
"auth_ref": [
"r18",
"r45",
"r46",
"r47"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the carrying amount as of the balance sheet date of merchandise, goods, commodities, or supplies held for future sale or to be used in manufacturing, servicing or production process.",
"label": "Schedule of Inventory, Current [Table Text Block]",
"terseLabel": "Inventory Balances"
}
}
},
"localname": "ScheduleOfInventoryCurrentTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/InventoriesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfInvestmentsLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Schedule of Investments [Line Items]",
"terseLabel": "Schedule of Investments [Line Items]"
}
}
},
"localname": "ScheduleOfInvestmentsLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfInvestmentsTable": {
"auth_ref": [
"r1006"
],
"lang": {
"en-us": {
"role": {
"documentation": "A container table for all schedule of investment items. It ties in the \"Legal Entity [Axis]\" to all of its contained line items.",
"label": "Schedule of Investments [Table]",
"terseLabel": "Schedule of Investments [Table]"
}
}
},
"localname": "ScheduleOfInvestmentsTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfMaturitiesOfLongTermDebtTableTextBlock": {
"auth_ref": [
"r401"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of maturity and sinking fund requirement for long-term debt.",
"label": "Schedule of Maturities of Long-term Debt [Table Text Block]",
"terseLabel": "Schedule of Maturities of Long-term Debt"
}
}
},
"localname": "ScheduleOfMaturitiesOfLongTermDebtTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfNetBenefitCostsTableTextBlock": {
"auth_ref": [
"r560"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the components of net benefit costs for pension plans and/or other employee benefit plans including service cost, interest cost, expected return on plan assets, gain (loss), prior service cost or credit, transition asset or obligation, and gain (loss) recognized due to settlements or curtailments.",
"label": "Schedule of Net Benefit Costs [Table Text Block]",
"terseLabel": "Schedule of Net Benefit Costs"
}
}
},
"localname": "ScheduleOfNetBenefitCostsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfPropertyPlantAndEquipmentTable": {
"auth_ref": [
"r52",
"r360"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation.",
"label": "Property, Plant and Equipment [Table]",
"terseLabel": "Property, Plant and Equipment [Table]"
}
}
},
"localname": "ScheduleOfPropertyPlantAndEquipmentTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/PropertyPlantandEquipmentDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfRestructuringAndRelatedCostsTable": {
"auth_ref": [
"r370",
"r371",
"r372",
"r373",
"r380",
"r381",
"r382"
],
"lang": {
"en-us": {
"role": {
"documentation": "Table presenting the description of the restructuring costs, such as the expected cost; the costs incurred during the period; the cumulative costs incurred as of the balance sheet date; the income statement caption within which the restructuring charges recognized for the period are included; and the amount of and periodic changes to an entity's restructuring reserve that occurred during the period associated with the exit from or disposal of business activities or restructurings for each major type of cost by type of restructuring.",
"label": "Schedule of Restructuring and Related Costs [Table]",
"terseLabel": "Schedule of Restructuring and Related Costs [Table]"
}
}
},
"localname": "ScheduleOfRestructuringAndRelatedCostsTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfRestructuringAndRelatedCostsTextBlock": {
"auth_ref": [
"r375",
"r376",
"r379"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of costs incurred for restructuring including, but not limited to, exit and disposal activities, remediation, implementation, integration, asset impairment, and charges against earnings from the write-down of assets.",
"label": "Restructuring and Related Costs [Table Text Block]",
"terseLabel": "Schedule of Restructuring Reserve"
}
}
},
"localname": "ScheduleOfRestructuringAndRelatedCostsTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfRevenuesFromExternalCustomersAndLongLivedAssetsByGeographicalAreasTableTextBlock": {
"auth_ref": [
"r124",
"r264"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of information concerning material long-lived assets (excluding financial instruments, customer relationships with financial institutions, mortgage and other servicing rights, deferred policy acquisition costs, and deferred taxes assets) located in identified geographic areas and/or the amount of revenue from external customers attributed to that country from which revenue is material. An entity may also provide subtotals of geographic information about groups of countries.",
"label": "Schedule of Revenue from External Customers and Long-Lived Assets, by Geographical Areas [Table Text Block]",
"terseLabel": "Schedule of Net Sales to External Customers and Property, Plant, and Equipment, Net, by Geographical Region"
}
}
},
"localname": "ScheduleOfRevenuesFromExternalCustomersAndLongLivedAssetsByGeographicalAreasTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfRevenuesFromExternalCustomersAndLongLivedAssetsTable": {
"auth_ref": [
"r115",
"r264"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule of material long-lived assets (excluding financial instruments, customer relationships with financial institutions, mortgage and other servicing rights, deferred policy acquisition costs, and deferred taxes assets) located in identified geographic areas and/or the amount of revenue from external customers attributed to that country from which revenue is material. An entity may also provide subtotals of geographic information about groups of countries.",
"label": "Schedule of Revenues from External Customers and Long-Lived Assets [Table]",
"terseLabel": "Schedule of Revenues from External Customers and Long-Lived Assets [Table]"
}
}
},
"localname": "ScheduleOfRevenuesFromExternalCustomersAndLongLivedAssetsTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationScheduleofNetSalestoExternalCustomersandPropertyPlantandEquipmentNetbyGeographicRegionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfSegmentReportingInformationBySegmentTable": {
"auth_ref": [
"r242",
"r245",
"r251",
"r337"
],
"lang": {
"en-us": {
"role": {
"documentation": "A table disclosing the profit or loss and total assets for each reportable segment of the entity. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss.",
"label": "Schedule of Segment Reporting Information, by Segment [Table]",
"terseLabel": "Schedule of Segment Reporting Information, by Segment [Table]"
}
}
},
"localname": "ScheduleOfSegmentReportingInformationBySegmentTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTable": {
"auth_ref": [
"r631",
"r663"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of information about share-based payment arrangement.",
"label": "Schedule of Share-based Compensation Arrangements by Share-based Payment Award [Table]",
"terseLabel": "Schedule of Share-based Compensation Arrangements by Share-based Payment Award [Table]"
}
}
},
"localname": "ScheduleOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock": {
"auth_ref": [
"r638"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the number and weighted-average grant date fair value for restricted stock units that were outstanding at the beginning and end of the year, and the number of restricted stock units that were granted, vested, or forfeited during the year.",
"label": "Share-based Payment Arrangement, Restricted Stock Unit, Activity [Table Text Block]",
"terseLabel": "Schedule of Restricted Stock Activity"
}
}
},
"localname": "ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock": {
"auth_ref": [
"r638",
"r649",
"r652"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value.",
"label": "Share-based Payment Arrangement, Option, Activity [Table Text Block]",
"terseLabel": "Schedule of Stock Options Activity"
}
}
},
"localname": "ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock": {
"auth_ref": [
"r654"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions.",
"label": "Schedule of Share-based Payment Award, Stock Options, Valuation Assumptions [Table Text Block]",
"terseLabel": "Schedule of Stock Options Valuation Assumptions"
}
}
},
"localname": "ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfShortTermDebtTextBlock": {
"auth_ref": [
"r58"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of short-term debt arrangements (having initial terms of repayment within one year or the normal operating cycle, if longer) including: (1) description of the short-term debt arrangement; (2) identification of the lender or type of lender; (3) repayment terms; (4) weighted average interest rate; (5) carrying amount of funds borrowed under the specified short-term debt arrangement as of the balance sheet date; (6) description of the refinancing of a short-term obligation when that obligation is excluded from current liabilities in the balance sheet; and (7) amount of a short-term obligation that has been excluded from current liabilities in the balance sheet because of a refinancing of the obligation.",
"label": "Schedule of Short-term Debt [Table Text Block]",
"terseLabel": "Schedule of Current Debt Obligations"
}
}
},
"localname": "ScheduleOfShortTermDebtTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleOfStockByClassTable": {
"auth_ref": [
"r67",
"r160",
"r219",
"r220",
"r457",
"r458",
"r459",
"r460",
"r461",
"r462",
"r463",
"r465",
"r469",
"r474",
"r477",
"r478",
"r479",
"r480",
"r481",
"r482",
"r483"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule detailing information related to equity by class of stock. Class of stock includes common, convertible, and preferred stocks which are not redeemable or redeemable solely at the option of the issuer. It also includes preferred stock with redemption features that are solely within the control of the issuer and mandatorily redeemable stock if redemption is required to occur only upon liquidation or termination of the reporting entity.",
"label": "Schedule of Stock by Class [Table]",
"terseLabel": "Schedule of Stock by Class [Table]"
}
}
},
"localname": "ScheduleOfStockByClassTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ScheduleOfUnrecognizedTaxBenefitsRollForwardTableTextBlock": {
"auth_ref": [
"r699",
"r715"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the change in unrecognized tax benefits.",
"label": "Schedule of Unrecognized Tax Benefits Roll Forward [Table Text Block]",
"terseLabel": "Reconciliation of Beginning and Ending Amount of Unrecognized Tax Benefits"
}
}
},
"localname": "ScheduleOfUnrecognizedTaxBenefitsRollForwardTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ScheduleofFiniteLivedIntangibleAssetsFutureAmortizationExpenseTableTextBlock": {
"auth_ref": [
"r347"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of the amount of amortization expense expected to be recorded in succeeding fiscal years for finite-lived intangible assets.",
"label": "Schedule of Finite-Lived Intangible Assets, Future Amortization Expense [Table Text Block]",
"terseLabel": "Schedule of Finite-Lived Intangible Assets, Future Amortization Expense"
}
}
},
"localname": "ScheduleofFiniteLivedIntangibleAssetsFutureAmortizationExpenseTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_SegmentDomain": {
"auth_ref": [
"r229",
"r233",
"r234",
"r235",
"r236",
"r237",
"r238",
"r239",
"r240",
"r241",
"r242",
"r243",
"r244",
"r247",
"r248",
"r249",
"r250",
"r252",
"r253",
"r254",
"r255",
"r256",
"r258",
"r265",
"r373",
"r382",
"r987"
],
"lang": {
"en-us": {
"role": {
"documentation": "Components of an entity that engage in business activities from which they may earn revenue and incur expenses, including transactions with other components of the same entity.",
"label": "Segments [Domain]",
"terseLabel": "Segments [Domain]"
}
}
},
"localname": "SegmentDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails",
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_SegmentReportingAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Segment Reporting [Abstract]",
"terseLabel": "Segment Reporting [Abstract]"
}
}
},
"localname": "SegmentReportingAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_SegmentReportingDisclosureTextBlock": {
"auth_ref": [
"r229",
"r231",
"r232",
"r242",
"r246",
"r252",
"r256",
"r257",
"r258",
"r259",
"r261",
"r264",
"r265",
"r266"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for reporting segments including data and tables. Reportable segments include those that meet any of the following quantitative thresholds a) it's reported revenue, including sales to external customers and intersegment sales or transfers is 10 percent or more of the combined revenue, internal and external, of all operating segments b) the absolute amount of its reported profit or loss is 10 percent or more of the greater, in absolute amount of 1) the combined reported profit of all operating segments that did not report a loss or 2) the combined reported loss of all operating segments that did report a loss c) its assets are 10 percent or more of the combined assets of all operating segments.",
"label": "Segment Reporting Disclosure [Text Block]",
"terseLabel": "Segment and Geographic Information"
}
}
},
"localname": "SegmentReportingDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformation"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_SegmentReportingInformationLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Segment Reporting Information [Line Items]",
"terseLabel": "Segment Reporting Information [Line Items]"
}
}
},
"localname": "SegmentReportingInformationLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_SelfInsuranceReservePolicyTextBlock": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for self-insurance reserves, including, but not limited to incurred but not reported reserves (IBNR).",
"label": "Self Insurance Reserve [Policy Text Block]",
"terseLabel": "Self-Insurance"
}
}
},
"localname": "SelfInsuranceReservePolicyTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_SellingGeneralAndAdministrativeExpense": {
"auth_ref": [
"r126"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome": {
"order": 4.0,
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": -1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The aggregate total costs related to selling a firm's product and services, as well as all other general and administrative expenses. Direct selling expenses (for example, credit, warranty, and advertising) are expenses that can be directly linked to the sale of specific products. Indirect selling expenses are expenses that cannot be directly linked to the sale of specific products, for example telephone expenses, Internet, and postal charges. General and administrative expenses include salaries of non-sales personnel, rent, utilities, communication, etc.",
"label": "Selling, General and Administrative Expense",
"terseLabel": "Selling, general, and administrative expense"
}
}
},
"localname": "SellingGeneralAndAdministrativeExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_SellingGeneralAndAdministrativeExpensesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Primary financial statement caption encompassing selling, general and administrative expense.",
"label": "Selling, General and Administrative Expenses [Member]",
"terseLabel": "Selling, general, and administrative expense"
}
}
},
"localname": "SellingGeneralAndAdministrativeExpensesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_SeniorNotesMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Bond that takes priority over other debt securities sold by the issuer. In the event the issuer goes bankrupt, senior debt holders receive priority for (must receive) repayment prior to (relative to) junior and unsecured (general) creditors.",
"label": "Senior Notes [Member]",
"terseLabel": "Senior Notes"
}
}
},
"localname": "SeniorNotesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails",
"http://www.medtronic.com/role/FinancingArrangementsLongtermDebtDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_SeriesAPreferredStockMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Outstanding nonredeemable series A preferred stock or outstanding series A preferred stock. Classified within stockholders' equity if nonredeemable or redeemable solely at the option of the issuer. Classified within temporary equity if redemption is outside the control of the issuer.",
"label": "Series A Preferred Stock [Member]",
"terseLabel": "Series A Preferred Shares"
}
}
},
"localname": "SeriesAPreferredStockMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ShareBasedCompensation": {
"auth_ref": [
"r147"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows": {
"order": 11.0,
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of noncash expense for share-based payment arrangement.",
"label": "Share-based Payment Arrangement, Noncash Expense",
"terseLabel": "Stock-based compensation"
}
}
},
"localname": "ShareBasedCompensation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1": {
"auth_ref": [
"r632"
],
"lang": {
"en-us": {
"role": {
"documentation": "Period over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Period",
"terseLabel": "Vesting period"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardDiscountFromMarketPricePurchaseDate": {
"auth_ref": [
"r663"
],
"lang": {
"en-us": {
"role": {
"documentation": "Discount rate from fair value on purchase date that participants pay for shares.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Discount from Market Price, Purchase Date",
"terseLabel": "The discount rate from market value on purchase date"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardDiscountFromMarketPricePurchaseDate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsAdditionalDisclosuresAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Additional Disclosures [Abstract]",
"terseLabel": "Wtd. Avg. Grant Price"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsAdditionalDisclosuresAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeitedInPeriod": {
"auth_ref": [
"r643"
],
"lang": {
"en-us": {
"role": {
"documentation": "The number of equity-based payment instruments, excluding stock (or unit) options, that were forfeited during the reporting period.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Forfeited in Period",
"negatedLabel": "Forfeited (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeitedInPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue": {
"auth_ref": [
"r648"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average fair value as of the grant date of equity-based award plans other than stock (unit) option plans that were not exercised or put into effect as a result of the occurrence of a terminating event.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Forfeitures, Weighted Average Grant Date Fair Value",
"terseLabel": "Forfeited (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod": {
"auth_ref": [
"r646"
],
"lang": {
"en-us": {
"role": {
"documentation": "The number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan).",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Grants in Period",
"terseLabel": "Granted (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue": {
"auth_ref": [
"r646"
],
"lang": {
"en-us": {
"role": {
"documentation": "The weighted average fair value at grant date for nonvested equity-based awards issued during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan).",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Grants in Period, Weighted Average Grant Date Fair Value",
"terseLabel": "Granted (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber": {
"auth_ref": [
"r645"
],
"lang": {
"en-us": {
"role": {
"documentation": "The number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Nonvested, Number",
"periodEndLabel": "Nonvested at end of period (in shares)",
"periodStartLabel": "Nonvested at beginning of period (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Nonvested, Number of Shares [Roll Forward]",
"terseLabel": "Units"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue": {
"auth_ref": [
"r645"
],
"lang": {
"en-us": {
"role": {
"documentation": "Per share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Nonvested, Weighted Average Grant Date Fair Value",
"periodEndLabel": "Nonvested at end of period (in dollars per share)",
"periodStartLabel": "Nonvested at beginning of period (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod": {
"auth_ref": [
"r647"
],
"lang": {
"en-us": {
"role": {
"documentation": "The number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Vested in Period",
"negatedLabel": "Vested (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodTotalFairValue": {
"auth_ref": [
"r651"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Fair value of share-based awards for which the grantee gained the right by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Vested in Period, Fair Value",
"terseLabel": "Fair value of restricted stock vested"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodTotalFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue": {
"auth_ref": [
"r647"
],
"lang": {
"en-us": {
"role": {
"documentation": "The weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Vested in Period, Weighted Average Grant Date Fair Value",
"terseLabel": "Vested (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsAndMethodologyAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Fair Value Assumptions and Methodology [Abstract]",
"terseLabel": "Assumptions used:"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsAndMethodologyAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansValuationAssumptionsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate": {
"auth_ref": [
"r657"
],
"lang": {
"en-us": {
"role": {
"documentation": "The estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Fair Value Assumptions, Expected Dividend Rate",
"terseLabel": "Dividend yield"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansValuationAssumptionsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate": {
"auth_ref": [
"r656"
],
"lang": {
"en-us": {
"role": {
"documentation": "The estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Fair Value Assumptions, Expected Volatility Rate",
"terseLabel": "Volatility"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansValuationAssumptionsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate": {
"auth_ref": [
"r658"
],
"lang": {
"en-us": {
"role": {
"documentation": "The risk-free interest rate assumption that is used in valuing an option on its own shares.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Fair Value Assumptions, Risk Free Interest Rate",
"terseLabel": "Risk-free interest rate"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansValuationAssumptionsDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award [Line Items]",
"terseLabel": "Share-based Compensation Arrangement by Share-based Payment Award [Line Items]"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized": {
"auth_ref": [
"r634"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of shares authorized for issuance under share-based payment arrangement.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Authorized",
"terseLabel": "Issuance of ordinary shares (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAuthorized",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant": {
"auth_ref": [
"r663"
],
"lang": {
"en-us": {
"role": {
"documentation": "The difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Number of Shares Available for Grant",
"verboseLabel": "Shares available for future grants (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAdditionalDisclosuresAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Additional Disclosures [Abstract]",
"terseLabel": "Additional Disclosures"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAdditionalDisclosuresAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber": {
"auth_ref": [
"r641"
],
"lang": {
"en-us": {
"role": {
"documentation": "The number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Exercisable, Number",
"terseLabel": "Options, Exercisable (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice": {
"auth_ref": [
"r641"
],
"lang": {
"en-us": {
"role": {
"documentation": "The weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Exercisable, Weighted Average Exercise Price",
"terseLabel": "Weighted Average Exercise Price, Exercisable (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisesInPeriodTotalIntrinsicValue": {
"auth_ref": [
"r651"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of accumulated difference between fair value of underlying shares on dates of exercise and exercise price on options exercised (or share units converted) into shares.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Exercises in Period, Intrinsic Value",
"terseLabel": "Intrinsic value of options exercised"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisesInPeriodTotalIntrinsicValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriod": {
"auth_ref": [
"r644"
],
"lang": {
"en-us": {
"role": {
"documentation": "For presentations that combine terminations, the number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan or that expired.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Forfeitures and Expirations in Period",
"negatedLabel": "Expired/Forfeited (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriodWeightedAverageExercisePrice": {
"auth_ref": [
"r644"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average price of options that were either forfeited or expired.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Forfeitures and Expirations in Period, Weighted Average Exercise Price",
"terseLabel": "Expired/Forfeited (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriodWeightedAverageExercisePrice",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Gross number of share options (or share units) granted during the period.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Gross",
"terseLabel": "Granted (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue": {
"auth_ref": [
"r650"
],
"lang": {
"en-us": {
"role": {
"documentation": "The weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Grants in Period, Weighted Average Grant Date Fair Value",
"terseLabel": "Weighted average fair value of options granted (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansValuationAssumptionsDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue": {
"auth_ref": [
"r663"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Outstanding, Intrinsic Value",
"terseLabel": "Aggregate Intrinsic Value, Outstanding"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber": {
"auth_ref": [
"r640",
"r663"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of options outstanding, including both vested and non-vested options.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Outstanding, Number",
"periodEndLabel": "Outstanding at end of period (in shares)",
"periodStartLabel": "Outstanding at beginning of period (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Outstanding [Roll Forward]",
"terseLabel": "Options"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice": {
"auth_ref": [
"r639"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Outstanding, Weighted Average Exercise Price",
"periodEndLabel": "Outstanding at end of period (in dollars per share)",
"periodStartLabel": "Outstanding at beginning of period (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Outstanding, Weighted Average Exercise Price [Abstract]",
"terseLabel": "Wtd. Avg. Exercise Price"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingAggregateIntrinsicValue": {
"auth_ref": [
"r652"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount by which current fair value of underlying stock exceeds exercise price of fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Vested and Expected to Vest, Outstanding, Aggregate Intrinsic Value",
"terseLabel": "Aggregate Intrinsic Value, Expected to vest"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingAggregateIntrinsicValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber": {
"auth_ref": [
"r653"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of fully vested and expected to vest options outstanding that can be converted into shares under option plan. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Vested and Expected to Vest, Outstanding, Number",
"terseLabel": "Options, Expected to vest (in shares)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingNumber",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice": {
"auth_ref": [
"r653"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted-average exercise price, at which grantee can acquire shares reserved for issuance, for fully vested and expected to vest options outstanding. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Vested and Expected to Vest, Outstanding, Weighted Average Exercise Price",
"terseLabel": "Weighted Average Exercise Price, Expected to vest (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageExercisePrice",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardAwardTypeAndPlanNameDomain": {
"auth_ref": [
"r628",
"r635"
],
"lang": {
"en-us": {
"role": {
"documentation": "Award under share-based payment arrangement.",
"label": "Award Type [Domain]",
"terseLabel": "Award Type [Domain]"
}
}
},
"localname": "ShareBasedCompensationArrangementsByShareBasedPaymentAwardAwardTypeAndPlanNameDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansPerformanceShareUnitActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansRestrictedStockActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails",
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockbasedCompensationExpenseDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average price at which option holders acquired shares when converting their stock options into shares.",
"label": "Share-based Compensation Arrangements by Share-based Payment Award, Options, Exercises in Period, Weighted Average Exercise Price",
"terseLabel": "Exercised (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average per share amount at which grantees can acquire shares of common stock by exercise of options.",
"label": "Share-based Compensation Arrangements by Share-based Payment Award, Options, Grants in Period, Weighted Average Exercise Price",
"terseLabel": "Granted (in dollars per share)"
}
}
},
"localname": "ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "perShareItemType"
},
"us-gaap_ShareBasedCompensationOptionAndIncentivePlansPolicy": {
"auth_ref": [
"r631",
"r636"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for award under share-based payment arrangement. Includes, but is not limited to, methodology and assumption used in measuring cost.",
"label": "Share-based Payment Arrangement [Policy Text Block]",
"terseLabel": "Stock-Based Compensation"
}
}
},
"localname": "ShareBasedCompensationOptionAndIncentivePlansPolicy",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ShareBasedCompensationPerformanceSharesAwardUnvestedActivityTableTextBlock": {
"auth_ref": [
"r637"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of number and weighted-average grant date fair value for nonvested performance shares.",
"label": "Share-based Payment Arrangement, Performance Shares, Activity [Table Text Block]",
"terseLabel": "Schedule of Performance Share Unit Activity"
}
}
},
"localname": "ShareBasedCompensationPerformanceSharesAwardUnvestedActivityTableTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsPercentage": {
"auth_ref": [
"r632"
],
"lang": {
"en-us": {
"role": {
"documentation": "Percentage of vesting of award under share-based payment arrangement.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Award Vesting Rights, Percentage",
"terseLabel": "Vesting percentage"
}
}
},
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardAwardVestingRightsPercentage",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod": {
"auth_ref": [
"r633"
],
"lang": {
"en-us": {
"role": {
"documentation": "Period from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Expiration Period",
"terseLabel": "Expiration period"
}
}
},
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansAdditionalInformationDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1": {
"auth_ref": [
"r655",
"r673"
],
"lang": {
"en-us": {
"role": {
"documentation": "Expected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Fair Value Assumptions, Expected Term",
"terseLabel": "Expected life (years)"
}
}
},
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansValuationAssumptionsDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1": {
"auth_ref": [
"r663"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Exercisable, Intrinsic Value",
"terseLabel": "Aggregate Intrinsic Value, Exercisable"
}
}
},
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1": {
"auth_ref": [
"r663"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Exercisable, Weighted Average Remaining Contractual Term",
"terseLabel": "Weighted Average Remaining Contractual Term, Exercisable"
}
}
},
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2": {
"auth_ref": [
"r653"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Outstanding, Weighted Average Remaining Contractual Term",
"terseLabel": "Weighted Average Remaining Contractual Term, Outstanding"
}
}
},
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageRemainingContractualTerm1": {
"auth_ref": [
"r653"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average remaining contractual term for fully vested and expected to vest options outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, unvested options for which requisite service period has not been rendered but that are expected to vest based on achievement of performance condition, if forfeitures are recognized when they occur.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Vested and Expected to Vest, Outstanding, Weighted Average Remaining Contractual Term",
"terseLabel": "Weighted Average Remaining Contractual Term, Expected to vest"
}
}
},
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedAndExpectedToVestOutstandingWeightedAverageRemainingContractualTerm1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "durationItemType"
},
"us-gaap_ShippingAndHandlingMember": {
"auth_ref": [
"r510"
],
"lang": {
"en-us": {
"role": {
"documentation": "Packing and transport of product.",
"label": "Shipping and Handling [Member]",
"terseLabel": "Shipping and Handling"
}
}
},
"localname": "ShippingAndHandlingMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ShortTermBorrowings": {
"auth_ref": [
"r23",
"r887",
"r935",
"r970"
],
"calculation": {
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails": {
"order": 2.0,
"parentTag": "us-gaap_DebtCurrent",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Reflects the total carrying amount as of the balance sheet date of debt having initial terms less than one year or the normal operating cycle, if longer.",
"label": "Short-term Debt",
"terseLabel": "Bank borrowings"
}
}
},
"localname": "ShortTermBorrowings",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ShortTermDebtTypeAxis": {
"auth_ref": [
"r58"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of short-term debt arrangement.",
"label": "Short-term Debt, Type [Axis]",
"terseLabel": "Short-term Debt, Type [Axis]"
}
}
},
"localname": "ShortTermDebtTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_ShortTermDebtTypeDomain": {
"auth_ref": [
"r55"
],
"lang": {
"en-us": {
"role": {
"documentation": "Type of short-term debt arrangement, such as notes, line of credit, commercial paper, asset-based financing, project financing, letter of credit financing.",
"label": "Short-term Debt, Type [Domain]",
"terseLabel": "Short-term Debt, Type [Domain]"
}
}
},
"localname": "ShortTermDebtTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancingArrangementsCurrentDebtObligationsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ShortTermDebtWeightedAverageInterestRate": {
"auth_ref": [
"r56"
],
"lang": {
"en-us": {
"role": {
"documentation": "Weighted average interest rate of short-term debt outstanding calculated at point in time.",
"label": "Short-term Debt, Weighted Average Interest Rate, at Point in Time",
"terseLabel": "Weighted average interest rate"
}
}
},
"localname": "ShortTermDebtWeightedAverageInterestRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "percentItemType"
},
"us-gaap_ShortTermInvestments": {
"auth_ref": [
"r25",
"r941",
"r942",
"r966"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 5.0,
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of investments including trading securities, available-for-sale securities, held-to-maturity securities, and short-term investments classified as other and current.",
"label": "Short-term Investments",
"terseLabel": "Investments"
}
}
},
"localname": "ShortTermInvestments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ShortTermInvestmentsMember": {
"auth_ref": [
"r1007",
"r1008",
"r1009",
"r1010"
],
"lang": {
"en-us": {
"role": {
"documentation": "Investments which are not otherwise included in another category or item that the entity has the intent to sell or dispose of within one year from the date of the balance sheet.",
"label": "Short-term Investments [Member]",
"terseLabel": "Short-term investments"
}
}
},
"localname": "ShortTermInvestmentsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RetirementBenefitPlansFairValueMeasurementDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ShortTermLeaseCost": {
"auth_ref": [
"r871",
"r878"
],
"calculation": {
"http://www.medtronic.com/role/LeasesComponentsofTotalOperatingLeaseCostDetails": {
"order": 2.0,
"parentTag": "us-gaap_LeaseCost",
"weight": 1.0
}
},
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of short-term lease cost, excluding expense for lease with term of one month or less.",
"label": "Short-term Lease, Cost",
"terseLabel": "Short-term lease cost"
}
}
},
"localname": "ShortTermLeaseCost",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/LeasesComponentsofTotalOperatingLeaseCostDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_SignificantAccountingPoliciesTextBlock": {
"auth_ref": [
"r155",
"r179"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for all significant accounting policies of the reporting entity.",
"label": "Significant Accounting Policies [Text Block]",
"terseLabel": "Summary of Significant Accounting Policies"
}
}
},
"localname": "SignificantAccountingPoliciesTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_StateAndLocalJurisdictionMember": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Designated tax department of a state or local government entitled to levy and collect income taxes from the entity.",
"label": "State and Local Jurisdiction [Member]",
"terseLabel": "State and Local Tax Authorities"
}
}
},
"localname": "StateAndLocalJurisdictionMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_StatementBusinessSegmentsAxis": {
"auth_ref": [
"r6",
"r229",
"r233",
"r234",
"r235",
"r236",
"r237",
"r238",
"r239",
"r240",
"r241",
"r242",
"r243",
"r244",
"r247",
"r248",
"r249",
"r250",
"r252",
"r253",
"r254",
"r255",
"r256",
"r258",
"r265",
"r337",
"r362",
"r373",
"r382",
"r987"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by business segments.",
"label": "Segments [Axis]",
"terseLabel": "Segments [Axis]"
}
}
},
"localname": "StatementBusinessSegmentsAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsChangesinGoodwillDetails",
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails",
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbyMarketGeographyforEachSegmentDetails",
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationIncomeFromOperationsBeforeIncomeTaxesbyReportableSegmentandReconciliationtoConsolidatedDetails",
"http://www.medtronic.com/role/SegmentandGeographicInformationReconciliationofAssetsandDepreciationExpensefromSegmentstoConsolidatedDetails",
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_StatementClassOfStockAxis": {
"auth_ref": [
"r31",
"r32",
"r33",
"r160",
"r163",
"r200",
"r204",
"r205",
"r207",
"r210",
"r219",
"r220",
"r221",
"r303",
"r404",
"r409",
"r410",
"r411",
"r417",
"r418",
"r460",
"r461",
"r465",
"r469",
"r476",
"r835",
"r1037"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by the different classes of stock of the entity.",
"label": "Class of Stock [Axis]",
"terseLabel": "Class of Stock [Axis]"
}
}
},
"localname": "StatementClassOfStockAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/Cover",
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_StatementEquityComponentsAxis": {
"auth_ref": [
"r2",
"r68",
"r110",
"r111",
"r112",
"r180",
"r181",
"r182",
"r185",
"r194",
"r196",
"r218",
"r307",
"r476",
"r483",
"r669",
"r670",
"r671",
"r718",
"r719",
"r815",
"r848",
"r849",
"r850",
"r851",
"r852",
"r854",
"r994",
"r995",
"r996",
"r1073"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by component of equity.",
"label": "Equity Components [Axis]",
"terseLabel": "Equity Components [Axis]"
}
}
},
"localname": "StatementEquityComponentsAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails",
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "stringItemType"
},
"us-gaap_StatementLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.",
"label": "Statement [Line Items]",
"terseLabel": "Statement [Line Items]"
}
}
},
"localname": "StatementLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "stringItemType"
},
"us-gaap_StatementOfCashFlowsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Statement of Cash Flows [Abstract]",
"terseLabel": "Statement of Cash Flows [Abstract]"
}
}
},
"localname": "StatementOfCashFlowsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_StatementOfFinancialPositionAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Statement of Financial Position [Abstract]",
"terseLabel": "Statement of Financial Position [Abstract]"
}
}
},
"localname": "StatementOfFinancialPositionAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_StatementOfIncomeAndComprehensiveIncomeAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Statement of Comprehensive Income [Abstract]",
"terseLabel": "Statement of Comprehensive Income [Abstract]"
}
}
},
"localname": "StatementOfIncomeAndComprehensiveIncomeAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_StatementOfStockholdersEquityAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Statement of Stockholders' Equity [Abstract]",
"terseLabel": "Statement of Stockholders' Equity [Abstract]"
}
}
},
"localname": "StatementOfStockholdersEquityAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_StatementTable": {
"auth_ref": [
"r180",
"r181",
"r182",
"r218",
"r925"
],
"lang": {
"en-us": {
"role": {
"documentation": "Schedule reflecting a Statement of Income, Statement of Cash Flows, Statement of Financial Position, Statement of Shareholders' Equity and Other Comprehensive Income, or other statement as needed.",
"label": "Statement [Table]",
"terseLabel": "Statement [Table]"
}
}
},
"localname": "StatementTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "stringItemType"
},
"us-gaap_StockIssuedDuringPeriodSharesShareBasedCompensation": {
"auth_ref": [
"r32",
"r33",
"r476",
"r483"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number, after forfeiture, of shares or units issued under share-based payment arrangement. Excludes shares or units issued under employee stock ownership plan (ESOP).",
"label": "Shares Issued, Shares, Share-based Payment Arrangement, after Forfeiture",
"terseLabel": "Issuance of shares under stock purchase and award plans (in shares)"
}
}
},
"localname": "StockIssuedDuringPeriodSharesShareBasedCompensation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "sharesItemType"
},
"us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised": {
"auth_ref": [
"r32",
"r33",
"r476",
"r483",
"r642"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of share options (or share units) exercised during the current period.",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options, Exercises in Period",
"negatedLabel": "Exercised (in shares)"
}
}
},
"localname": "StockIssuedDuringPeriodSharesStockOptionsExercised",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/StockPurchaseandAwardPlansStockOptionsActivityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_StockIssuedDuringPeriodValueShareBasedCompensation": {
"auth_ref": [
"r32",
"r33",
"r483",
"r630",
"r650"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Value, after forfeiture, of shares issued under share-based payment arrangement. Excludes employee stock ownership plan (ESOP).",
"label": "Shares Issued, Value, Share-based Payment Arrangement, after Forfeiture",
"terseLabel": "Issuance of shares under stock purchase and award plans"
}
}
},
"localname": "StockIssuedDuringPeriodValueShareBasedCompensation",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_StockRepurchaseProgramAuthorizedAmount1": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of stock repurchase plan authorized.",
"label": "Stock Repurchase Program, Authorized Amount",
"terseLabel": "Amount authorized for repurchase"
}
}
},
"localname": "StockRepurchaseProgramAuthorizedAmount1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_StockRepurchaseProgramRemainingAuthorizedRepurchaseAmount1": {
"auth_ref": [],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount remaining of a stock repurchase plan authorized.",
"label": "Stock Repurchase Program, Remaining Authorized Repurchase Amount",
"terseLabel": "Amount available for future repurchases"
}
}
},
"localname": "StockRepurchaseProgramRemainingAuthorizedRepurchaseAmount1",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_StockRepurchasedAndRetiredDuringPeriodShares": {
"auth_ref": [
"r32",
"r33",
"r476",
"r483"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of shares that have been repurchased and retired during the period.",
"label": "Stock Repurchased and Retired During Period, Shares",
"negatedLabel": "Repurchase of ordinary shares (in shares)"
}
}
},
"localname": "StockRepurchasedAndRetiredDuringPeriodShares",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "sharesItemType"
},
"us-gaap_StockRepurchasedAndRetiredDuringPeriodValue": {
"auth_ref": [
"r32",
"r33",
"r476",
"r483"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Equity impact of the value of stock that has been repurchased and retired during the period. The excess of the purchase price over par value can be charged against retained earnings (once the excess is fully allocated to additional paid in capital).",
"label": "Stock Repurchased and Retired During Period, Value",
"negatedLabel": "Repurchase of ordinary shares"
}
}
},
"localname": "StockRepurchasedAndRetiredDuringPeriodValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_StockRepurchasedDuringPeriodShares": {
"auth_ref": [
"r32",
"r33",
"r476",
"r483"
],
"lang": {
"en-us": {
"role": {
"documentation": "Number of shares that have been repurchased during the period and have not been retired and are not held in treasury. Some state laws may govern the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock.",
"label": "Stock Repurchased During Period, Shares",
"terseLabel": "Shares repurchased (in shares)"
}
}
},
"localname": "StockRepurchasedDuringPeriodShares",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_StockRepurchasedDuringPeriodValue": {
"auth_ref": [
"r32",
"r33",
"r476",
"r483"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Equity impact of the value of stock that has been repurchased during the period and has not been retired and is not held in treasury. Some state laws may mandate the circumstances under which an entity may acquire its own stock and prescribe the accounting treatment therefore. This element is used when state law does not recognize treasury stock.",
"label": "Stock Repurchased During Period, Value",
"terseLabel": "Amount repurchased"
}
}
},
"localname": "StockRepurchasedDuringPeriodValue",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquityDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_StockholdersEquity": {
"auth_ref": [
"r33",
"r38",
"r39",
"r163",
"r275",
"r303",
"r835",
"r887"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 1.0,
"parentTag": "us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Total of all stockholders' equity (deficit) items, net of receivables from officers, directors, owners, and affiliates of the entity which are attributable to the parent. The amount of the economic entity's stockholders' equity attributable to the parent excludes the amount of stockholders' equity which is allocable to that ownership interest in subsidiary equity which is not attributable to the parent (noncontrolling interest, minority interest). This excludes temporary equity and is sometimes called permanent equity.",
"label": "Stockholders' Equity Attributable to Parent",
"totalLabel": "Total shareholders\u2019 equity"
}
}
},
"localname": "StockholdersEquity",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_StockholdersEquityAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Stockholders' Equity Attributable to Parent [Abstract]",
"terseLabel": "Shareholders\u2019 equity:"
}
}
},
"localname": "StockholdersEquityAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedBalanceSheets"
],
"xbrltype": "stringItemType"
},
"us-gaap_StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest": {
"auth_ref": [
"r2",
"r3",
"r111",
"r163",
"r180",
"r181",
"r182",
"r185",
"r194",
"r303",
"r307",
"r483",
"r669",
"r670",
"r671",
"r718",
"r719",
"r753",
"r754",
"r768",
"r815",
"r835",
"r848",
"r849",
"r854",
"r995",
"r996",
"r1073"
],
"calculation": {
"http://www.medtronic.com/role/ConsolidatedBalanceSheets": {
"order": 3.0,
"parentTag": "us-gaap_LiabilitiesAndStockholdersEquity",
"weight": 1.0
}
},
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of stockholders' equity (deficit), net of receivables from officers, directors, owners, and affiliates of the entity, attributable to both the parent and noncontrolling interests. Amount excludes temporary equity. Alternate caption for the concept is permanent equity.",
"label": "Stockholders' Equity, Including Portion Attributable to Noncontrolling Interest",
"periodEndLabel": "Ending balance",
"periodStartLabel": "Beginning balance",
"totalLabel": "Total equity"
}
}
},
"localname": "StockholdersEquityIncludingPortionAttributableToNoncontrollingInterest",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AccumulatedOtherComprehensiveLossDetails",
"http://www.medtronic.com/role/ConsolidatedBalanceSheets",
"http://www.medtronic.com/role/ConsolidatedStatementsofEquity"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_StockholdersEquityNoteAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Stockholders' Equity Note [Abstract]",
"terseLabel": "Stockholders' Equity Note [Abstract]"
}
}
},
"localname": "StockholdersEquityNoteAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_StockholdersEquityNoteDisclosureTextBlock": {
"auth_ref": [
"r161",
"r461",
"r464",
"r465",
"r466",
"r467",
"r468",
"r469",
"r470",
"r471",
"r472",
"r473",
"r475",
"r483",
"r484"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for shareholders' equity comprised of portions attributable to the parent entity and noncontrolling interest, including other comprehensive income. Includes, but is not limited to, balances of common stock, preferred stock, additional paid-in capital, other capital and retained earnings, accumulated balance for each classification of other comprehensive income and amount of comprehensive income.",
"label": "Stockholders' Equity Note Disclosure [Text Block]",
"terseLabel": "Shareholders' Equity"
}
}
},
"localname": "StockholdersEquityNoteDisclosureTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ShareholdersEquity"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_SubsegmentsAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by business subsegments.",
"label": "Subsegments [Axis]",
"terseLabel": "Subsegments [Axis]"
}
}
},
"localname": "SubsegmentsAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_SubsegmentsDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Divisions of a component of an entity that engage in business activities from which they may earn revenue and incur expenses, including transactions with other components of the same entity.",
"label": "Subsegments [Domain]",
"terseLabel": "Subsegments [Domain]"
}
}
},
"localname": "SubsegmentsDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RevenueDisaggregationofNetSalesbySegmentandDivisionDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_SubsequentEventLineItems": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Detail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event.",
"label": "Subsequent Event [Line Items]",
"terseLabel": "Subsequent Event [Line Items]"
}
}
},
"localname": "SubsequentEventLineItems",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_SubsequentEventMember": {
"auth_ref": [
"r855",
"r889"
],
"lang": {
"en-us": {
"role": {
"documentation": "Identifies event that occurred after the balance sheet date but before financial statements are issued or available to be issued.",
"label": "Subsequent Event [Member]",
"terseLabel": "Subsequent Event"
}
}
},
"localname": "SubsequentEventMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_SubsequentEventTable": {
"auth_ref": [
"r855",
"r889"
],
"lang": {
"en-us": {
"role": {
"documentation": "Discloses pertinent information about one or more significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued.",
"label": "Subsequent Event [Table]",
"terseLabel": "Subsequent Event [Table]"
}
}
},
"localname": "SubsequentEventTable",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SubsequentEventsDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_SubsequentEventTypeAxis": {
"auth_ref": [
"r855",
"r889"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by event that occurred after the balance sheet date but before financial statements are issued or available to be issued.",
"label": "Subsequent Event Type [Axis]",
"terseLabel": "Subsequent Event Type [Axis]"
}
}
},
"localname": "SubsequentEventTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_SubsequentEventTypeDomain": {
"auth_ref": [
"r855",
"r889"
],
"lang": {
"en-us": {
"role": {
"documentation": "Event that occurred after the balance sheet date but before financial statements are issued or available to be issued.",
"label": "Subsequent Event Type [Domain]",
"terseLabel": "Subsequent Event Type [Domain]"
}
}
},
"localname": "SubsequentEventTypeDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails",
"http://www.medtronic.com/role/CommitmentsandContingenciesDetails",
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_SubsequentEventsAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Subsequent Events [Abstract]"
}
}
},
"localname": "SubsequentEventsAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"xbrltype": "stringItemType"
},
"us-gaap_SubsequentEventsTextBlock": {
"auth_ref": [
"r888",
"r891"
],
"lang": {
"en-us": {
"role": {
"documentation": "The entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business.",
"label": "Subsequent Events [Text Block]",
"terseLabel": "Subsequent Events"
}
}
},
"localname": "SubsequentEventsTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SubsequentEvents"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_SummaryOfIncomeTaxExaminationsTextBlock": {
"auth_ref": [
"r692",
"r715"
],
"lang": {
"en-us": {
"role": {
"documentation": "Tabular disclosure of income tax examinations that an enterprise is currently subject to or that have been completed in the current period typically including a description of the examination, the jurisdiction conducting the examination, the tax year(s) under examination, the likelihood of an unfavorable settlement, the range of possible losses, the liability recorded, the increase or decrease in the liability from the prior period, and any penalties and interest that have been recorded.",
"label": "Summary of Income Tax Examinations [Table Text Block]",
"terseLabel": "Major Tax Jurisdictions Which Remain Subject to Examination"
}
}
},
"localname": "SummaryOfIncomeTaxExaminationsTextBlock",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesTables"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_SupplementalCashFlowInformationAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Supplemental Cash Flow Information [Abstract]",
"terseLabel": "Supplemental Cash Flow Information"
}
}
},
"localname": "SupplementalCashFlowInformationAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofCashFlows"
],
"xbrltype": "stringItemType"
},
"us-gaap_TaxCreditCarryforwardAmount": {
"auth_ref": [
"r708"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "The amount of the tax credit carryforward, before tax effects, available to reduce future taxable income under enacted tax laws.",
"label": "Tax Credit Carryforward, Amount",
"terseLabel": "Tax credit carryforward"
}
}
},
"localname": "TaxCreditCarryforwardAmount",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_TechnologyBasedIntangibleAssetsMember": {
"auth_ref": [
"r739"
],
"lang": {
"en-us": {
"role": {
"documentation": "Technology-based intangible assets, including, but not limited to, patented technology, unpatented technology, and developed technology rights.",
"label": "Technology-Based Intangible Assets [Member]",
"terseLabel": "Technology-Based Intangible Assets"
}
}
},
"localname": "TechnologyBasedIntangibleAssetsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/AcquisitionsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_TotalReturnSwapMember": {
"auth_ref": [
"r771"
],
"lang": {
"en-us": {
"role": {
"documentation": "Contracts in which one party makes payments at a fixed or variable rate while the counterparty makes payments based on an asset, including the income and capital gains derived therefrom.",
"label": "Total Return Swap [Member]",
"terseLabel": "Total return swaps",
"verboseLabel": "Total return swaps"
}
}
},
"localname": "TotalReturnSwapMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementClassificationandFairValueAmountsofDerivativeInstrumentsinBalanceSheetsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementGainsandLossesonDerivativesNotDesignatedasHedgingInstrumentsDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementNarrativeDetails",
"http://www.medtronic.com/role/DerivativesandCurrencyExchangeRiskManagementOffsettingAssetsandLiabilitiesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_TradeAndOtherAccountsReceivablePolicy": {
"auth_ref": [
"r269",
"r270",
"r271",
"r272",
"r274",
"r276"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for accounts receivable.",
"label": "Accounts Receivable [Policy Text Block]",
"terseLabel": "Accounts Receivable and Allowance for Doubtful Accounts and Credit Losses"
}
}
},
"localname": "TradeAndOtherAccountsReceivablePolicy",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_TrademarksAndTradeNamesMember": {
"auth_ref": [
"r740"
],
"lang": {
"en-us": {
"role": {
"documentation": "Rights acquired through registration of a trademark to gain or protect exclusive use of a business name, symbol or other device or style, or rights either acquired through registration of a business name to gain or protect exclusive use thereof.",
"label": "Trademarks and Trade Names [Member]",
"terseLabel": "Trademarks and tradenames"
}
}
},
"localname": "TrademarksAndTradeNamesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/GoodwillandOtherIntangibleAssetsIntangibleAssetsDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_TransfersAndServicingOfFinancialInstrumentsTypesOfFinancialInstrumentsDomain": {
"auth_ref": [
"r291",
"r292",
"r297",
"r298",
"r299",
"r445",
"r474",
"r806",
"r892",
"r893",
"r894",
"r895",
"r896",
"r897",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r904",
"r906",
"r907",
"r908",
"r909",
"r910",
"r911",
"r912",
"r913",
"r914",
"r915",
"r916",
"r917",
"r918",
"r919",
"r920",
"r921",
"r922",
"r1037",
"r1038",
"r1039",
"r1040",
"r1041",
"r1042",
"r1043"
],
"lang": {
"en-us": {
"role": {
"documentation": "Instrument or contract that imposes a contractual obligation to deliver cash or another financial instrument or to exchange other financial instruments on potentially unfavorable terms and conveys a contractual right to receive cash or another financial instrument or to exchange other financial instruments on potentially favorable terms.",
"label": "Financial Instruments [Domain]",
"terseLabel": "Financial Instruments [Domain]"
}
}
},
"localname": "TransfersAndServicingOfFinancialInstrumentsTypesOfFinancialInstrumentsDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"http://www.medtronic.com/role/FinancialInstrumentsActivityRelatedtotheCompanysInvestmentPortfolioEquityandOtherInvestmentsDetails",
"http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_TypeOfRestructuringDomain": {
"auth_ref": [
"r370",
"r371",
"r380",
"r381"
],
"lang": {
"en-us": {
"role": {
"documentation": "Identification of the types of restructuring costs.",
"label": "Type of Restructuring [Domain]",
"terseLabel": "Type of Restructuring [Domain]"
}
}
},
"localname": "TypeOfRestructuringDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/RestructuringChargesAdditionalInformationDetails",
"http://www.medtronic.com/role/RestructuringChargesDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_USGovernmentAgenciesDebtSecuritiesMember": {
"auth_ref": [
"r168",
"r549",
"r954"
],
"lang": {
"en-us": {
"role": {
"documentation": "Debentures, notes, and other debt securities issued by US government agencies, for example, but not limited to, Government National Mortgage Association (GNMA or Ginnie Mae). Excludes US treasury securities and debt issued by government-sponsored Enterprises (GSEs), for example, but is not limited to, Federal Home Loan Mortgage Corporation (FHLMC or Freddie Mac), Federal National Mortgage Association (FNMA or Fannie Mae), and the Federal Home Loan Bank (FHLB).",
"label": "US Government Agencies Debt Securities [Member]",
"terseLabel": "U.S. government and agency securities"
}
}
},
"localname": "USGovernmentAgenciesDebtSecuritiesMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAFSinContinuousLossPositionDetails",
"http://www.medtronic.com/role/FinancialInstrumentsInvestmentsbyCategoryandRelatedBalanceSheetPresentationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_UndistributedEarningsOfForeignSubsidiaries": {
"auth_ref": [
"r680",
"r731",
"r962",
"r1004"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of undistributed earnings of foreign subsidiaries intended to be permanently reinvested outside the country of domicile.",
"label": "Undistributed Earnings of Foreign Subsidiaries",
"terseLabel": "Undistributed earnings from non-U.S. subsidiaries"
}
}
},
"localname": "UndistributedEarningsOfForeignSubsidiaries",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrealizedGainLossOnInvestments": {
"auth_ref": [
"r148"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of unrealized gain (loss) on investment.",
"label": "Unrealized Gain (Loss) on Investments",
"terseLabel": "Net unrealized gains (losses) on equity and other investments still held"
}
}
},
"localname": "UnrealizedGainLossOnInvestments",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancialInstrumentsAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefits": {
"auth_ref": [
"r681",
"r693"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of unrecognized tax benefits.",
"label": "Unrecognized Tax Benefits",
"periodEndLabel": "Gross unrecognized tax benefits at end of fiscal year",
"periodStartLabel": "Gross unrecognized tax benefits at beginning of fiscal year",
"terseLabel": "Gross unrecognized tax benefits"
}
}
},
"localname": "UnrecognizedTaxBenefits",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails",
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefitsDecreasesResultingFromPriorPeriodTaxPositions": {
"auth_ref": [
"r694"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of decrease in unrecognized tax benefits resulting from tax positions taken in prior period tax returns.",
"label": "Unrecognized Tax Benefits, Decrease Resulting from Prior Period Tax Positions",
"negatedLabel": "Prior year tax positions"
}
}
},
"localname": "UnrecognizedTaxBenefitsDecreasesResultingFromPriorPeriodTaxPositions",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefitsDecreasesResultingFromSettlementsWithTaxingAuthorities": {
"auth_ref": [
"r696"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of decrease in unrecognized tax benefits resulting from settlements with taxing authorities.",
"label": "Unrecognized Tax Benefits, Decrease Resulting from Settlements with Taxing Authorities",
"negatedLabel": "Settlements"
}
}
},
"localname": "UnrecognizedTaxBenefitsDecreasesResultingFromSettlementsWithTaxingAuthorities",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefitsIncomeTaxPenaltiesAndInterestAccrued": {
"auth_ref": [
"r689"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount accrued for interest on an underpayment of income taxes and penalties related to a tax position claimed or expected to be claimed in the tax return.",
"label": "Unrecognized Tax Benefits, Income Tax Penalties and Interest Accrued",
"terseLabel": "Accrued income tax penalties and interest"
}
}
},
"localname": "UnrecognizedTaxBenefitsIncomeTaxPenaltiesAndInterestAccrued",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefitsIncreasesResultingFromCurrentPeriodTaxPositions": {
"auth_ref": [
"r695"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase in unrecognized tax benefits resulting from tax positions that have been or will be taken in current period tax return.",
"label": "Unrecognized Tax Benefits, Increase Resulting from Current Period Tax Positions",
"terseLabel": "Current year tax positions"
}
}
},
"localname": "UnrecognizedTaxBenefitsIncreasesResultingFromCurrentPeriodTaxPositions",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefitsIncreasesResultingFromPriorPeriodTaxPositions": {
"auth_ref": [
"r694"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase in unrecognized tax benefits resulting from tax positions taken in prior period tax returns.",
"label": "Unrecognized Tax Benefits, Increase Resulting from Prior Period Tax Positions",
"terseLabel": "Prior year tax positions"
}
}
},
"localname": "UnrecognizedTaxBenefitsIncreasesResultingFromPriorPeriodTaxPositions",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefitsReductionsResultingFromLapseOfApplicableStatuteOfLimitations": {
"auth_ref": [
"r697"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of decrease in unrecognized tax benefits resulting from lapses of applicable statutes of limitations.",
"label": "Unrecognized Tax Benefits, Reduction Resulting from Lapse of Applicable Statute of Limitations",
"negatedLabel": "Statute of limitation lapses"
}
}
},
"localname": "UnrecognizedTaxBenefitsReductionsResultingFromLapseOfApplicableStatuteOfLimitations",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesReconciliationofBeginningandEndingAmountofUnrecognizedTaxBenefitsDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UnrecognizedTaxBenefitsThatWouldImpactEffectiveTaxRate": {
"auth_ref": [
"r698"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "The total amount of unrecognized tax benefits that, if recognized, would affect the effective tax rate.",
"label": "Unrecognized Tax Benefits that Would Impact Effective Tax Rate",
"terseLabel": "Unrecognized tax benefits that would impact effective tax rate"
}
}
},
"localname": "UnrecognizedTaxBenefitsThatWouldImpactEffectiveTaxRate",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/IncomeTaxesAdditionalInformationDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_UseOfEstimates": {
"auth_ref": [
"r222",
"r223",
"r224",
"r225",
"r226",
"r227",
"r228"
],
"lang": {
"en-us": {
"role": {
"documentation": "Disclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles.",
"label": "Use of Estimates, Policy [Policy Text Block]",
"terseLabel": "Use of Estimates"
}
}
},
"localname": "UseOfEstimates",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"xbrltype": "textBlockItemType"
},
"us-gaap_ValuationAllowanceOfDeferredTaxAssetsMember": {
"auth_ref": [
"r171",
"r172",
"r173",
"r176",
"r177"
],
"lang": {
"en-us": {
"role": {
"documentation": "Valuation allowance of deferred tax asset attributable to deductible temporary difference and carryforward.",
"label": "SEC Schedule, 12-09, Valuation Allowance, Deferred Tax Asset [Member]",
"terseLabel": "Deferred Tax Valuation Allowance"
}
}
},
"localname": "ValuationAllowanceOfDeferredTaxAssetsMember",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ValuationAllowancesAndReservesBalance": {
"auth_ref": [
"r171",
"r177"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of valuation and qualifying accounts and reserves.",
"label": "SEC Schedule, 12-09, Valuation Allowances and Reserves, Amount",
"periodEndLabel": "Balance at End of Fiscal Year",
"periodStartLabel": "Balance at Beginning of Fiscal Year"
}
}
},
"localname": "ValuationAllowancesAndReservesBalance",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ValuationAllowancesAndReservesChargedToCostAndExpense": {
"auth_ref": [
"r174"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase in valuation and qualifying accounts and reserves from charge to cost and expense.",
"label": "SEC Schedule, 12-09, Valuation Allowances and Reserves, Additions, Charge to Cost and Expense",
"terseLabel": "Charges to Income"
}
}
},
"localname": "ValuationAllowancesAndReservesChargedToCostAndExpense",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ValuationAllowancesAndReservesChargedToOtherAccounts": {
"auth_ref": [
"r175"
],
"crdr": "credit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of increase in valuation and qualifying accounts and reserves from charge to accounts other than cost and expense.",
"label": "SEC Schedule, 12-09, Valuation Allowances and Reserves, Additions, Charge to Other Account",
"terseLabel": "Charges to Other Accounts"
}
}
},
"localname": "ValuationAllowancesAndReservesChargedToOtherAccounts",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ValuationAllowancesAndReservesDeductions": {
"auth_ref": [
"r176"
],
"crdr": "debit",
"lang": {
"en-us": {
"role": {
"documentation": "Amount of decrease in valuation and qualifying accounts and reserves.",
"label": "SEC Schedule, 12-09, Valuation Allowances and Reserves, Deduction",
"negatedTerseLabel": "Other Changes (Debit) Credit"
}
}
},
"localname": "ValuationAllowancesAndReservesDeductions",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "monetaryItemType"
},
"us-gaap_ValuationAllowancesAndReservesDomain": {
"auth_ref": [
"r171",
"r172",
"r173",
"r176",
"r177"
],
"lang": {
"en-us": {
"role": {
"documentation": "Valuation and qualifying accounts and reserves.",
"label": "SEC Schedule, 12-09, Valuation Allowances and Reserves [Domain]",
"terseLabel": "SEC Schedule, 12-09, Valuation Allowances and Reserves [Domain]"
}
}
},
"localname": "ValuationAllowancesAndReservesDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_ValuationAllowancesAndReservesTypeAxis": {
"auth_ref": [
"r171",
"r172",
"r173",
"r176",
"r177"
],
"lang": {
"en-us": {
"role": {
"documentation": "Information by valuation and qualifying accounts and reserves.",
"label": "SEC Schedule, 12-09, Valuation Allowances and Reserves Type [Axis]",
"terseLabel": "SEC Schedule, 12-09, Valuation Allowances and Reserves Type [Axis]"
}
}
},
"localname": "ValuationAllowancesAndReservesTypeAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ScheduleIIDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_VariableRateAxis": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Information by type of variable rate.",
"label": "Variable Rate [Axis]",
"terseLabel": "Variable Rate [Axis]"
}
}
},
"localname": "VariableRateAxis",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_VariableRateDomain": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Interest rate that fluctuates over time as a result of an underlying benchmark interest rate or index.",
"label": "Variable Rate [Domain]",
"terseLabel": "Variable Rate [Domain]"
}
}
},
"localname": "VariableRateDomain",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/FinancingArrangementsAdditionalInformationDetails"
],
"xbrltype": "domainItemType"
},
"us-gaap_WeightedAverageNumberDilutedSharesOutstandingAdjustmentAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Incremental Weighted Average Shares Attributable to Dilutive Effect [Abstract]",
"terseLabel": "Effect of dilutive securities:"
}
}
},
"localname": "WeightedAverageNumberDilutedSharesOutstandingAdjustmentAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding": {
"auth_ref": [
"r199",
"r210"
],
"calculation": {
"http://www.medtronic.com/role/EarningsPerShareDetails": {
"order": null,
"parentTag": null,
"root": true,
"weight": null
}
},
"lang": {
"en-us": {
"role": {
"documentation": "The average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period.",
"label": "Weighted Average Number of Shares Outstanding, Diluted",
"terseLabel": "Diluted weighted average shares outstanding (in shares)",
"totalLabel": "Diluted \u2013 weighted average shares outstanding (in shares)"
}
}
},
"localname": "WeightedAverageNumberOfDilutedSharesOutstanding",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "sharesItemType"
},
"us-gaap_WeightedAverageNumberOfSharesOutstandingAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"label": "Weighted Average Number of Shares Outstanding, Diluted [Abstract]",
"terseLabel": "Denominator:"
}
}
},
"localname": "WeightedAverageNumberOfSharesOutstandingAbstract",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "stringItemType"
},
"us-gaap_WeightedAverageNumberOfSharesOutstandingBasic": {
"auth_ref": [
"r198",
"r210"
],
"calculation": {
"http://www.medtronic.com/role/EarningsPerShareDetails": {
"order": 1.0,
"parentTag": "us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding",
"weight": 1.0
}
},
"lang": {
"en-us": {
"role": {
"documentation": "Number of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period.",
"label": "Weighted Average Number of Shares Outstanding, Basic",
"terseLabel": "Basic weighted average shares outstanding (in shares)",
"verboseLabel": "Basic - weighted average shares outstanding (in shares)"
}
}
},
"localname": "WeightedAverageNumberOfSharesOutstandingBasic",
"nsuri": "http://fasb.org/us-gaap/2021-01-31",
"presentation": [
"http://www.medtronic.com/role/ConsolidatedStatementsofIncome",
"http://www.medtronic.com/role/EarningsPerShareDetails"
],
"xbrltype": "sharesItemType"
}
},
"unitCount": 19
}
},
"std_ref": {
"r0": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "105",
"URI": "http://asc.fasb.org/extlink&oid=124434974&loc=SL124442142-165695"
},
"r1": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "105",
"URI": "http://asc.fasb.org/extlink&oid=124434974&loc=SL124442142-165695"
},
"r10": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6676-107765"
},
"r100": {
"Name": "Accounting Standards Codification",
"Paragraph": "14A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669686-108580"
},
"r1000": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(g)(2)(iii)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r1001": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(g)(2)(iv)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r1002": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(h)(1)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r1003": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(h)(2)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r1004": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "740",
"Subparagraph": "(b)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=6487024&loc=d3e29054-158556"
},
"r1005": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(e)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124508989&loc=d3e19393-158473"
},
"r1006": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "320",
"Subparagraph": "(SX 210.12-12)",
"Topic": "946",
"URI": "http://asc.fasb.org/extlink&oid=122147990&loc=d3e611133-123010"
},
"r1007": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "320",
"Subparagraph": "(SX 210.12-15(Column A))",
"Topic": "946",
"URI": "http://asc.fasb.org/extlink&oid=122147990&loc=d3e611379-123010"
},
"r1008": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "320",
"Subparagraph": "(SX 210.12-15(Column B))",
"Topic": "946",
"URI": "http://asc.fasb.org/extlink&oid=122147990&loc=d3e611379-123010"
},
"r1009": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "320",
"Subparagraph": "(SX 210.12-15(Column C))",
"Topic": "946",
"URI": "http://asc.fasb.org/extlink&oid=122147990&loc=d3e611379-123010"
},
"r101": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=d3e689-108580"
},
"r1010": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "320",
"Subparagraph": "(SX 210.12-15(Column D))",
"Topic": "946",
"URI": "http://asc.fasb.org/extlink&oid=122147990&loc=d3e611379-123010"
},
"r1011": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "310",
"Subparagraph": "(SX 210.12-29(Footnote 4))",
"Topic": "948",
"URI": "http://asc.fasb.org/extlink&oid=120402547&loc=d3e617274-123014"
},
"r1012": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "310",
"Topic": "954",
"URI": "http://asc.fasb.org/extlink&oid=123364037&loc=d3e3115-115594"
},
"r1013": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "450",
"Topic": "954",
"URI": "http://asc.fasb.org/extlink&oid=6491354&loc=d3e6049-115624"
},
"r1014": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "360",
"Subparagraph": "(d)",
"Topic": "958",
"URI": "http://asc.fasb.org/extlink&oid=120429125&loc=d3e99779-112916"
},
"r1015": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "360",
"Topic": "958",
"URI": "http://asc.fasb.org/extlink&oid=120429125&loc=d3e99893-112916"
},
"r1016": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "360",
"Topic": "958",
"URI": "http://asc.fasb.org/extlink&oid=120429125&loc=SL120174063-112916"
},
"r1017": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column B))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1018": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column C))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1019": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column D))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r102": {
"Name": "Accounting Standards Codification",
"Paragraph": "17A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL34724391-108580"
},
"r1020": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column E))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1021": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column F))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1022": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column G))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1023": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column H))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1024": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Column I))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1025": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Footnote 2))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1026": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "360",
"Subparagraph": "(SX 210.12-28(Footnote 4))",
"Topic": "970",
"URI": "http://asc.fasb.org/extlink&oid=120402810&loc=d3e638233-123024"
},
"r1027": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "310",
"Subparagraph": "(c)",
"Topic": "976",
"URI": "http://asc.fasb.org/extlink&oid=6497875&loc=d3e22274-108663"
},
"r1028": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "310",
"Subparagraph": "(b)",
"Topic": "978",
"URI": "http://asc.fasb.org/extlink&oid=123360121&loc=d3e27327-108691"
},
"r1029": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "12",
"Subsection": "b"
},
"r103": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669619-108580"
},
"r1030": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "12",
"Subsection": "b-2"
},
"r1031": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "12",
"Subsection": "b-23"
},
"r1032": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "12",
"Subsection": "d1-1"
},
"r1033": {
"Name": "Form 10-K",
"Number": "249",
"Publisher": "SEC",
"Section": "310"
},
"r1034": {
"Name": "Form 20-F",
"Number": "249",
"Publisher": "SEC",
"Section": "220",
"Subsection": "f"
},
"r1035": {
"Name": "Form 40-F",
"Number": "249",
"Publisher": "SEC",
"Section": "240",
"Subsection": "f"
},
"r1036": {
"Name": "Forms 10-K, 10-Q, 20-F",
"Number": "240",
"Publisher": "SEC",
"Section": "13",
"Subsection": "a-1"
},
"r1037": {
"Name": "Regulation S-K (SK)",
"Number": "229",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "1402"
},
"r1038": {
"Name": "Regulation S-K (SK)",
"Number": "229",
"Paragraph": "(b)",
"Publisher": "SEC",
"Section": "1402",
"Subparagraph": "(1)"
},
"r1039": {
"Name": "Regulation S-K (SK)",
"Number": "229",
"Paragraph": "(b)",
"Publisher": "SEC",
"Section": "1402",
"Subparagraph": "(2)"
},
"r104": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669619-108580"
},
"r1040": {
"Name": "Regulation S-K (SK)",
"Number": "229",
"Paragraph": "(b)",
"Publisher": "SEC",
"Section": "1402",
"Subparagraph": "(3)"
},
"r1041": {
"Name": "Regulation S-K (SK)",
"Number": "229",
"Paragraph": "(c)",
"Publisher": "SEC",
"Section": "1402",
"Subparagraph": "(2)(i)"
},
"r1042": {
"Name": "Regulation S-K (SK)",
"Number": "229",
"Paragraph": "(c)",
"Publisher": "SEC",
"Section": "1402",
"Subparagraph": "(2)(ii)"
},
"r1043": {
"Name": "Regulation S-K (SK)",
"Number": "229",
"Paragraph": "(c)",
"Publisher": "SEC",
"Section": "1402",
"Subparagraph": "(2)(iii)"
},
"r1044": {
"Name": "Regulation S-T",
"Number": "232",
"Publisher": "SEC",
"Section": "405"
},
"r1045": {
"Footnote": "2",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1046": {
"Footnote": "4",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1047": {
"Footnote": "4",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Publisher": "SEC",
"Section": "12",
"Subsection": "29"
},
"r1048": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column B",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1049": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column C",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r105": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669619-108580"
},
"r1050": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column D",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1051": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column E",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1052": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column F",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1053": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column G",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1054": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column H",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1055": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "Column I",
"Publisher": "SEC",
"Section": "12",
"Subsection": "28"
},
"r1056": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Publisher": "SEC",
"Section": "12",
"Subsection": "09"
},
"r1057": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(i)",
"Subsection": "01"
},
"r1058": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(i)",
"Subsection": "02"
},
"r1059": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(ii)",
"Subsection": "01"
},
"r106": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669625-108580"
},
"r1060": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iii)",
"Subsection": "01"
},
"r1061": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iii)(A)",
"Subsection": "01"
},
"r1062": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iii)(A)",
"Subsection": "02"
},
"r1063": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iii)(B)",
"Subsection": "01"
},
"r1064": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iii)(B)",
"Subsection": "02"
},
"r1065": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iii)(C)",
"Subsection": "02"
},
"r1066": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iv)",
"Subsection": "01"
},
"r1067": {
"Name": "Regulation S-X (SX)",
"Number": "210",
"Paragraph": "(a)",
"Publisher": "SEC",
"Section": "13",
"Subparagraph": "(4)(iv)",
"Subsection": "02"
},
"r1068": {
"Name": "Securities Act",
"Number": "230",
"Publisher": "SEC",
"Section": "405"
},
"r1069": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "848"
},
"r107": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669625-108580"
},
"r1070": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)(1)",
"Topic": "848"
},
"r1071": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)(2)",
"Topic": "848"
},
"r1072": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)(3)(iii)(01)",
"Topic": "848"
},
"r1073": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)(3)(iii)(03)",
"Topic": "848"
},
"r108": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=d3e557-108580"
},
"r109": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124431353&loc=SL116659661-227067"
},
"r11": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(g)(4)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6676-107765"
},
"r110": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124431353&loc=SL124442407-227067"
},
"r111": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124431353&loc=SL124442411-227067"
},
"r112": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124431353&loc=SL124452729-227067"
},
"r113": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124507222&loc=d3e1436-108581"
},
"r114": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(210.5-03(11))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r115": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(1))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r116": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(10))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r117": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(20))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r118": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(21))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r119": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(22))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r12": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6676-107765"
},
"r120": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(23))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r121": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(24))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r122": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(25))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r123": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03(5))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r124": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03.1)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r125": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03.2(a),(d))",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r126": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03.4)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r127": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-03.9)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=123367319&loc=SL114868664-224227"
},
"r128": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "220",
"URI": "http://asc.fasb.org/topic&trid=2134417"
},
"r129": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3151-108585"
},
"r13": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6787-107765"
},
"r130": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3179-108585"
},
"r131": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3179-108585"
},
"r132": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3213-108585"
},
"r133": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3213-108585"
},
"r134": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3213-108585"
},
"r135": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3255-108585"
},
"r136": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3255-108585"
},
"r137": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3255-108585"
},
"r138": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3291-108585"
},
"r139": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3291-108585"
},
"r14": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6801-107765"
},
"r140": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3291-108585"
},
"r141": {
"Name": "Accounting Standards Codification",
"Paragraph": "17",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3367-108585"
},
"r142": {
"Name": "Accounting Standards Codification",
"Paragraph": "17",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(f)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3367-108585"
},
"r143": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3000-108585"
},
"r144": {
"Name": "Accounting Standards Codification",
"Paragraph": "24",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3521-108585"
},
"r145": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3536-108585"
},
"r146": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3536-108585"
},
"r147": {
"Name": "Accounting Standards Codification",
"Paragraph": "28",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3602-108585"
},
"r148": {
"Name": "Accounting Standards Codification",
"Paragraph": "28",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3602-108585"
},
"r149": {
"Name": "Accounting Standards Codification",
"Paragraph": "28",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3602-108585"
},
"r15": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6812-107765"
},
"r150": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3044-108585"
},
"r151": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123570139&loc=d3e3098-108585"
},
"r152": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123431023&loc=d3e4273-108586"
},
"r153": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123431023&loc=d3e4297-108586"
},
"r154": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "230",
"URI": "http://asc.fasb.org/extlink&oid=123431023&loc=SL98516268-108586"
},
"r155": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=123372394&loc=d3e18726-107790"
},
"r156": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=123372394&loc=d3e18823-107790"
},
"r157": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=123372394&loc=d3e18823-107790"
},
"r158": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=123372394&loc=d3e18823-107790"
},
"r159": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(c))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r16": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6904-107765"
},
"r160": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(d))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r161": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(e)(1))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r162": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(f))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r163": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(g)(1)(ii))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r164": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(h)(1)(Note 1))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r165": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(h)(1))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r166": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(h)(2))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r167": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(h))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r168": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(m)(1)(ii)(A))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r169": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.4-08(n))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e23780-122690"
},
"r17": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6911-107765"
},
"r170": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-04(a))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24072-122690"
},
"r171": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09(Column B))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r172": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09(Column C(1)))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r173": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09(Column C(2)))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r174": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09(Column C)(1))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r175": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09(Column C)(2))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r176": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09(Column D))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r177": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09(Column E))",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r178": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.12-09)",
"Topic": "235",
"URI": "http://asc.fasb.org/extlink&oid=120395691&loc=d3e24092-122690"
},
"r179": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "235",
"URI": "http://asc.fasb.org/topic&trid=2122369"
},
"r18": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=6361739&loc=d3e7789-107766"
},
"r180": {
"Name": "Accounting Standards Codification",
"Paragraph": "23",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124436220&loc=d3e21914-107793"
},
"r181": {
"Name": "Accounting Standards Codification",
"Paragraph": "24",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124436220&loc=d3e21930-107793"
},
"r182": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124436220&loc=d3e21711-107793"
},
"r183": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124436220&loc=d3e21728-107793"
},
"r184": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)(2)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22499-107794"
},
"r185": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)(3)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22499-107794"
},
"r186": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)(4)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22499-107794"
},
"r187": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(2)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22499-107794"
},
"r188": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22499-107794"
},
"r189": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22694-107794"
},
"r19": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(1))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r190": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22694-107794"
},
"r191": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22583-107794"
},
"r192": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22595-107794"
},
"r193": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22644-107794"
},
"r194": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22644-107794"
},
"r195": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22658-107794"
},
"r196": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=124431687&loc=d3e22663-107794"
},
"r197": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 11.M.Q2)",
"Topic": "250",
"URI": "http://asc.fasb.org/extlink&oid=122038215&loc=d3e31137-122693"
},
"r198": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1448-109256"
},
"r199": {
"Name": "Accounting Standards Codification",
"Paragraph": "16",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1505-109256"
},
"r2": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "105",
"URI": "http://asc.fasb.org/extlink&oid=124434974&loc=SL124442142-165695"
},
"r20": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(13))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r200": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1252-109256"
},
"r201": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1707-109256"
},
"r202": {
"Name": "Accounting Standards Codification",
"Paragraph": "23",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1757-109256"
},
"r203": {
"Name": "Accounting Standards Codification",
"Paragraph": "28A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1500-109256"
},
"r204": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1278-109256"
},
"r205": {
"Name": "Accounting Standards Codification",
"Paragraph": "55",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e2626-109256"
},
"r206": {
"Name": "Accounting Standards Codification",
"Paragraph": "60B",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=SL5780133-109256"
},
"r207": {
"Name": "Accounting Standards Codification",
"Paragraph": "60B",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=SL5780133-109256"
},
"r208": {
"Name": "Accounting Standards Codification",
"Paragraph": "68B",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=SL5498026-109256"
},
"r209": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125511455&loc=d3e1337-109256"
},
"r21": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(14))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r210": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=124432515&loc=d3e3550-109257"
},
"r211": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=124432515&loc=d3e3550-109257"
},
"r212": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=124432515&loc=d3e3630-109257"
},
"r213": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=109243012&loc=SL65017193-207537"
},
"r214": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125512782&loc=d3e3842-109258"
},
"r215": {
"Name": "Accounting Standards Codification",
"Paragraph": "52",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "260",
"URI": "http://asc.fasb.org/extlink&oid=125512782&loc=d3e4984-109258"
},
"r216": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "260",
"URI": "http://asc.fasb.org/topic&trid=2144383"
},
"r217": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "270",
"URI": "http://asc.fasb.org/extlink&oid=124437754&loc=d3e543-108305"
},
"r218": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "272",
"URI": "http://asc.fasb.org/extlink&oid=125520817&loc=d3e70191-108054"
},
"r219": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "272",
"URI": "http://asc.fasb.org/extlink&oid=125520817&loc=d3e70229-108054"
},
"r22": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(17))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r220": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "272",
"URI": "http://asc.fasb.org/extlink&oid=6373374&loc=d3e70434-108055"
},
"r221": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "272",
"URI": "http://asc.fasb.org/extlink&oid=6373374&loc=d3e70478-108055"
},
"r222": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "275",
"URI": "http://asc.fasb.org/extlink&oid=99393423&loc=d3e5967-108592"
},
"r223": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "275",
"URI": "http://asc.fasb.org/extlink&oid=99393423&loc=d3e5967-108592"
},
"r224": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "275",
"URI": "http://asc.fasb.org/extlink&oid=99393423&loc=d3e6161-108592"
},
"r225": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "275",
"URI": "http://asc.fasb.org/extlink&oid=99393423&loc=d3e6191-108592"
},
"r226": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "275",
"URI": "http://asc.fasb.org/extlink&oid=99393423&loc=d3e6061-108592"
},
"r227": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "275",
"URI": "http://asc.fasb.org/extlink&oid=99393423&loc=d3e6132-108592"
},
"r228": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "275",
"URI": "http://asc.fasb.org/extlink&oid=99393423&loc=d3e6143-108592"
},
"r229": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8657-108599"
},
"r23": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(19)(a))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r230": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8672-108599"
},
"r231": {
"Name": "Accounting Standards Codification",
"Paragraph": "21",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8721-108599"
},
"r232": {
"Name": "Accounting Standards Codification",
"Paragraph": "21",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8721-108599"
},
"r233": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r234": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r235": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r236": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r237": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r238": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r239": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(g)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r24": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(19))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r240": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(h)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r241": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(j)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r242": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8736-108599"
},
"r243": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8813-108599"
},
"r244": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8813-108599"
},
"r245": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8813-108599"
},
"r246": {
"Name": "Accounting Standards Codification",
"Paragraph": "26",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8844-108599"
},
"r247": {
"Name": "Accounting Standards Codification",
"Paragraph": "30",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8906-108599"
},
"r248": {
"Name": "Accounting Standards Codification",
"Paragraph": "30",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8906-108599"
},
"r249": {
"Name": "Accounting Standards Codification",
"Paragraph": "30",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8906-108599"
},
"r25": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(2))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r250": {
"Name": "Accounting Standards Codification",
"Paragraph": "30",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8906-108599"
},
"r251": {
"Name": "Accounting Standards Codification",
"Paragraph": "30",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8906-108599"
},
"r252": {
"Name": "Accounting Standards Codification",
"Paragraph": "31",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8924-108599"
},
"r253": {
"Name": "Accounting Standards Codification",
"Paragraph": "32",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8933-108599"
},
"r254": {
"Name": "Accounting Standards Codification",
"Paragraph": "32",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8933-108599"
},
"r255": {
"Name": "Accounting Standards Codification",
"Paragraph": "32",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8933-108599"
},
"r256": {
"Name": "Accounting Standards Codification",
"Paragraph": "32",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8933-108599"
},
"r257": {
"Name": "Accounting Standards Codification",
"Paragraph": "32",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8933-108599"
},
"r258": {
"Name": "Accounting Standards Codification",
"Paragraph": "32",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8933-108599"
},
"r259": {
"Name": "Accounting Standards Codification",
"Paragraph": "34",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8981-108599"
},
"r26": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(20))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r260": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e8475-108599"
},
"r261": {
"Name": "Accounting Standards Codification",
"Paragraph": "40",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e9031-108599"
},
"r262": {
"Name": "Accounting Standards Codification",
"Paragraph": "41",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e9038-108599"
},
"r263": {
"Name": "Accounting Standards Codification",
"Paragraph": "41",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e9038-108599"
},
"r264": {
"Name": "Accounting Standards Codification",
"Paragraph": "41",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e9038-108599"
},
"r265": {
"Name": "Accounting Standards Codification",
"Paragraph": "42",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "280",
"URI": "http://asc.fasb.org/extlink&oid=123359005&loc=d3e9054-108599"
},
"r266": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "280",
"URI": "http://asc.fasb.org/topic&trid=2134510"
},
"r267": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=124259787&loc=d3e4428-111522"
},
"r268": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=124259787&loc=d3e4531-111522"
},
"r269": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=123577603&loc=d3e4975-111524"
},
"r27": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(22))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r270": {
"Name": "Accounting Standards Codification",
"Paragraph": "11B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=123577603&loc=SL6953423-111524"
},
"r271": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=123577603&loc=d3e5212-111524"
},
"r272": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=123577603&loc=d3e5033-111524"
},
"r273": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=123577603&loc=d3e5074-111524"
},
"r274": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=123577603&loc=d3e5093-111524"
},
"r275": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 4.E)",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=122038336&loc=d3e74512-122707"
},
"r276": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=84159169&loc=d3e10133-111534"
},
"r277": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=124402435&loc=SL124402458-218513"
},
"r278": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "310",
"URI": "http://asc.fasb.org/extlink&oid=124402435&loc=SL124402458-218513"
},
"r279": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=124260329&loc=d3e26610-111562"
},
"r28": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(24))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r280": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=124260329&loc=d3e26853-111562"
},
"r281": {
"Name": "Accounting Standards Codification",
"Paragraph": "8A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=124260329&loc=SL6284422-111562"
},
"r282": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=SL6283291-111563"
},
"r283": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(aa)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27161-111563"
},
"r284": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27161-111563"
},
"r285": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27161-111563"
},
"r286": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27161-111563"
},
"r287": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27198-111563"
},
"r288": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27198-111563"
},
"r289": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27198-111563"
},
"r29": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(26)(a))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r290": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27198-111563"
},
"r291": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27232-111563"
},
"r292": {
"Name": "Accounting Standards Codification",
"Paragraph": "5A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=SL120269820-111563"
},
"r293": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27290-111563"
},
"r294": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27337-111563"
},
"r295": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "320",
"URI": "http://asc.fasb.org/extlink&oid=123581744&loc=d3e27357-111563"
},
"r296": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "320",
"URI": "http://asc.fasb.org/topic&trid=2196928"
},
"r297": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "321",
"URI": "http://asc.fasb.org/extlink&oid=123583765&loc=SL75117539-209714"
},
"r298": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "321",
"URI": "http://asc.fasb.org/extlink&oid=123583765&loc=SL75117539-209714"
},
"r299": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "321",
"URI": "http://asc.fasb.org/extlink&oid=123583765&loc=SL75117539-209714"
},
"r3": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "105",
"URI": "http://asc.fasb.org/extlink&oid=124434974&loc=SL124442142-165695"
},
"r30": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(26)(b))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r300": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "323",
"URI": "http://asc.fasb.org/extlink&oid=109237563&loc=d3e33749-111570"
},
"r301": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(2)",
"Topic": "323",
"URI": "http://asc.fasb.org/extlink&oid=114001798&loc=d3e33918-111571"
},
"r302": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(b)",
"Topic": "323",
"URI": "http://asc.fasb.org/extlink&oid=114001798&loc=d3e33918-111571"
},
"r303": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "323",
"URI": "http://asc.fasb.org/extlink&oid=114001798&loc=d3e33918-111571"
},
"r304": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=122640432&loc=SL121648383-210437"
},
"r305": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=122640432&loc=SL121648383-210437"
},
"r306": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=122640432&loc=SL121648383-210437"
},
"r307": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=122640432&loc=SL121648383-210437"
},
"r308": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(e)(3)",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=122640432&loc=SL121648383-210437"
},
"r309": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(e)(4)",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=122640432&loc=SL121648383-210437"
},
"r31": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(27))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r310": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124255206&loc=SL82895884-210446"
},
"r311": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124255953&loc=SL82919244-210447"
},
"r312": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124255953&loc=SL82919249-210447"
},
"r313": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124255953&loc=SL82919249-210447"
},
"r314": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124255953&loc=SL82919253-210447"
},
"r315": {
"Name": "Accounting Standards Codification",
"Paragraph": "16",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124255953&loc=SL82919258-210447"
},
"r316": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124255953&loc=SL82919230-210447"
},
"r317": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124258926&loc=SL82898722-210454"
},
"r318": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124269663&loc=SL82922888-210455"
},
"r319": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124269663&loc=SL82922890-210455"
},
"r32": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(28))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r320": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124269663&loc=SL82922895-210455"
},
"r321": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=124269663&loc=SL82922900-210455"
},
"r322": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "30",
"Topic": "326",
"URI": "http://asc.fasb.org/extlink&oid=121590138&loc=SL82922954-210456"
},
"r323": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "330",
"URI": "http://asc.fasb.org/extlink&oid=116847112&loc=d3e4492-108314"
},
"r324": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "330",
"URI": "http://asc.fasb.org/extlink&oid=116847112&loc=d3e4542-108314"
},
"r325": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "330",
"URI": "http://asc.fasb.org/extlink&oid=116847112&loc=d3e4556-108314"
},
"r326": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 5.BB)",
"Topic": "330",
"URI": "http://asc.fasb.org/extlink&oid=27011343&loc=d3e100047-122729"
},
"r327": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "330",
"URI": "http://asc.fasb.org/topic&trid=2126998"
},
"r328": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "05",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "340",
"URI": "http://asc.fasb.org/extlink&oid=123349782&loc=d3e5879-108316"
},
"r329": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "340",
"URI": "http://asc.fasb.org/extlink&oid=6387103&loc=d3e6435-108320"
},
"r33": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(29))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r330": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=99380562&loc=d3e13770-109266"
},
"r331": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=99380562&loc=d3e13777-109266"
},
"r332": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=120320667&loc=SL49117168-202975"
},
"r333": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=121556970&loc=d3e13816-109267"
},
"r334": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(e)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=121556970&loc=d3e13816-109267"
},
"r335": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(f)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=121556970&loc=d3e13816-109267"
},
"r336": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(g)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=121556970&loc=d3e13816-109267"
},
"r337": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=121556970&loc=d3e13816-109267"
},
"r338": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=121556970&loc=d3e13854-109267"
},
"r339": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=121556970&loc=d3e13854-109267"
},
"r34": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(3))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r340": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=6388964&loc=d3e16212-109274"
},
"r341": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=6388964&loc=d3e16225-109274"
},
"r342": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16265-109275"
},
"r343": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(b)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16265-109275"
},
"r344": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(d)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16265-109275"
},
"r345": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16265-109275"
},
"r346": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "((a)(1),(b))",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16323-109275"
},
"r347": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16323-109275"
},
"r348": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)(1)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16323-109275"
},
"r349": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)(2)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16323-109275"
},
"r35": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(30)(a)(1))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r350": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)(3)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16323-109275"
},
"r351": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(b)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16323-109275"
},
"r352": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(d)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16323-109275"
},
"r353": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(b)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16373-109275"
},
"r354": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(b),(d)",
"Topic": "350",
"URI": "http://asc.fasb.org/extlink&oid=66006027&loc=d3e16373-109275"
},
"r355": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "350",
"URI": "http://asc.fasb.org/topic&trid=2144416"
},
"r356": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "360",
"URI": "http://asc.fasb.org/extlink&oid=123351718&loc=d3e2420-110228"
},
"r357": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "360",
"URI": "http://asc.fasb.org/extlink&oid=6391035&loc=d3e2868-110229"
},
"r358": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "360",
"URI": "http://asc.fasb.org/extlink&oid=6391035&loc=d3e2868-110229"
},
"r359": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "360",
"URI": "http://asc.fasb.org/extlink&oid=6391035&loc=d3e2868-110229"
},
"r36": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(30)(a)(3))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r360": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "360",
"URI": "http://asc.fasb.org/extlink&oid=6391035&loc=d3e2868-110229"
},
"r361": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "360",
"URI": "http://asc.fasb.org/extlink&oid=109226691&loc=d3e2941-110230"
},
"r362": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)",
"Topic": "360",
"URI": "http://asc.fasb.org/extlink&oid=109226691&loc=d3e2941-110230"
},
"r363": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "360",
"URI": "http://asc.fasb.org/topic&trid=2155823"
},
"r364": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(d)",
"Topic": "410",
"URI": "http://asc.fasb.org/extlink&oid=6393242&loc=d3e13237-110859"
},
"r365": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "410",
"URI": "http://asc.fasb.org/extlink&oid=6393242&loc=d3e13283-110859"
},
"r366": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "410",
"URI": "http://asc.fasb.org/extlink&oid=6393242&loc=d3e13296-110859"
},
"r367": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "410",
"URI": "http://asc.fasb.org/extlink&oid=6393242&loc=d3e13207-110859"
},
"r368": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=109237686&loc=d3e17752-110868"
},
"r369": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=6394359&loc=d3e17939-110869"
},
"r37": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(30)(a)(4))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r370": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)(1)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=6394359&loc=d3e17939-110869"
},
"r371": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)(2)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=6394359&loc=d3e17939-110869"
},
"r372": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=6394359&loc=d3e17939-110869"
},
"r373": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=6394359&loc=d3e17939-110869"
},
"r374": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=6394359&loc=d3e17939-110869"
},
"r375": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=6394359&loc=d3e17939-110869"
},
"r376": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB TOPIC 5.P.3)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140864-122747"
},
"r377": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 5.P.3)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140864-122747"
},
"r378": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB TOPIC 5.P.4(b)(2))",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140904-122747"
},
"r379": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB TOPIC 5.P.4)",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140904-122747"
},
"r38": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(30))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r380": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 5.P.4(b)(1))",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140904-122747"
},
"r381": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 5.P.4(b)(2))",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140904-122747"
},
"r382": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 5.P.4(d))",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140904-122747"
},
"r383": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 5.P.4(e))",
"Topic": "420",
"URI": "http://asc.fasb.org/extlink&oid=115931487&loc=d3e140904-122747"
},
"r384": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "420",
"URI": "http://asc.fasb.org/topic&trid=2175745"
},
"r385": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14326-108349"
},
"r386": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14615-108349"
},
"r387": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14394-108349"
},
"r388": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14435-108349"
},
"r389": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14435-108349"
},
"r39": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(31))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r390": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14453-108349"
},
"r391": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14472-108349"
},
"r392": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=121557415&loc=d3e14557-108349"
},
"r393": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=116646759&loc=d3e14981-108350"
},
"r394": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "20",
"Subparagraph": "(SAB TOPIC 5.Y.Q2)",
"Topic": "450",
"URI": "http://asc.fasb.org/extlink&oid=27011672&loc=d3e149879-122751"
},
"r395": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"SubTopic": "20",
"Topic": "450",
"URI": "http://asc.fasb.org/subtopic&trid=2127163"
},
"r396": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"SubTopic": "30",
"Topic": "450",
"URI": "http://asc.fasb.org/subtopic&trid=2127197"
},
"r397": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "460",
"URI": "http://asc.fasb.org/extlink&oid=124440162&loc=d3e12021-110248"
},
"r398": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "460",
"URI": "http://asc.fasb.org/extlink&oid=124440162&loc=d3e12053-110248"
},
"r399": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "460",
"URI": "http://asc.fasb.org/extlink&oid=123368208&loc=d3e12565-110249"
},
"r4": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "105",
"URI": "http://asc.fasb.org/extlink&oid=124434974&loc=SL124442142-165695"
},
"r40": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(32))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r400": {
"Name": "Accounting Standards Codification",
"Paragraph": "12A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=99376301&loc=SL5988623-112600"
},
"r401": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123465755&loc=d3e1835-112601"
},
"r402": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123465755&loc=SL6230698-112601"
},
"r403": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S65",
"SubTopic": "10",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359872&loc=SL124427846-239511"
},
"r404": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-01(a)(4)(i))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442526-122756"
},
"r405": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-01(a)(4)(ii))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442526-122756"
},
"r406": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-01(a)(4)(iii)(A))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442526-122756"
},
"r407": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-01(a)(4)(iii)(B))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442526-122756"
},
"r408": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-01(a)(4)(iii))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442526-122756"
},
"r409": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-01(a)(4)(iv))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442526-122756"
},
"r41": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(4)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r410": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-01(a)(5))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442526-122756"
},
"r411": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(4)(i))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r412": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(4)(iii)(A)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r413": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(4)(iii)(A))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r414": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(4)(iii)(B)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r415": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(4)(iii)(B))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r416": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(4)(iii)(C))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r417": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(4)(iv))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r418": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.13-02(a)(5))",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=124359900&loc=SL124442552-122756"
},
"r419": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r42": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(6)(a)(1))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r420": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r421": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r422": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r423": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(e)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r424": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(f)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r425": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(g)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r426": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r427": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(i)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r428": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495323-112611"
},
"r429": {
"Name": "Accounting Standards Codification",
"Paragraph": "1C",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495334-112611"
},
"r43": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(6)(a)(3))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r430": {
"Name": "Accounting Standards Codification",
"Paragraph": "1C",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495334-112611"
},
"r431": {
"Name": "Accounting Standards Codification",
"Paragraph": "1C",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495334-112611"
},
"r432": {
"Name": "Accounting Standards Codification",
"Paragraph": "1D",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495340-112611"
},
"r433": {
"Name": "Accounting Standards Codification",
"Paragraph": "1D",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495340-112611"
},
"r434": {
"Name": "Accounting Standards Codification",
"Paragraph": "1D",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495340-112611"
},
"r435": {
"Name": "Accounting Standards Codification",
"Paragraph": "1E",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495348-112611"
},
"r436": {
"Name": "Accounting Standards Codification",
"Paragraph": "1E",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495348-112611"
},
"r437": {
"Name": "Accounting Standards Codification",
"Paragraph": "1E",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495348-112611"
},
"r438": {
"Name": "Accounting Standards Codification",
"Paragraph": "1E",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495348-112611"
},
"r439": {
"Name": "Accounting Standards Codification",
"Paragraph": "1F",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495355-112611"
},
"r44": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(6)(a)(4))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r440": {
"Name": "Accounting Standards Codification",
"Paragraph": "1F",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495355-112611"
},
"r441": {
"Name": "Accounting Standards Codification",
"Paragraph": "1F",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(1)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495355-112611"
},
"r442": {
"Name": "Accounting Standards Codification",
"Paragraph": "1F",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(2)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495355-112611"
},
"r443": {
"Name": "Accounting Standards Codification",
"Paragraph": "1I",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495371-112611"
},
"r444": {
"Name": "Accounting Standards Codification",
"Paragraph": "1I",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495371-112611"
},
"r445": {
"Name": "Accounting Standards Codification",
"Paragraph": "1I",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495371-112611"
},
"r446": {
"Name": "Accounting Standards Codification",
"Paragraph": "1I",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466505&loc=SL123495371-112611"
},
"r447": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(1)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466204&loc=SL6031897-161870"
},
"r448": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(3)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466204&loc=SL6031897-161870"
},
"r449": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466204&loc=SL6036836-161870"
},
"r45": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(6)(a))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r450": {
"Name": "Accounting Standards Codification",
"Paragraph": "69B",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466577&loc=SL123495735-112612"
},
"r451": {
"Name": "Accounting Standards Codification",
"Paragraph": "69C",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466577&loc=SL123495737-112612"
},
"r452": {
"Name": "Accounting Standards Codification",
"Paragraph": "69E",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466577&loc=SL123495743-112612"
},
"r453": {
"Name": "Accounting Standards Codification",
"Paragraph": "69F",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123466577&loc=SL123495745-112612"
},
"r454": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "40",
"SubTopic": "50",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123467658&loc=d3e12317-112629"
},
"r455": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "40",
"SubTopic": "50",
"Topic": "470",
"URI": "http://asc.fasb.org/extlink&oid=123467658&loc=d3e12355-112629"
},
"r456": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "470",
"URI": "http://asc.fasb.org/topic&trid=2208564"
},
"r457": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(CFRR 211.02)",
"Topic": "480",
"URI": "http://asc.fasb.org/extlink&oid=122040564&loc=d3e177068-122764"
},
"r458": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=65888546&loc=d3e21300-112643"
},
"r459": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21553-112644"
},
"r46": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(6)(b))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r460": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r461": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r462": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r463": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r464": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(g)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r465": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(h)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r466": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(i)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r467": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496158-112644"
},
"r468": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496171-112644"
},
"r469": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496171-112644"
},
"r47": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(6)(c))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r470": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496171-112644"
},
"r471": {
"Name": "Accounting Standards Codification",
"Paragraph": "16",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496180-112644"
},
"r472": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496189-112644"
},
"r473": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496189-112644"
},
"r474": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496189-112644"
},
"r475": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=SL123496189-112644"
},
"r476": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21463-112644"
},
"r477": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21475-112644"
},
"r478": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21484-112644"
},
"r479": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21488-112644"
},
"r48": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02(8))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r480": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21506-112644"
},
"r481": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21521-112644"
},
"r482": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=123467817&loc=d3e21538-112644"
},
"r483": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.3-04)",
"Topic": "505",
"URI": "http://asc.fasb.org/extlink&oid=120397183&loc=d3e187085-122770"
},
"r484": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "505",
"URI": "http://asc.fasb.org/topic&trid=2208762"
},
"r485": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123360276&loc=SL49130531-203044"
},
"r486": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123360276&loc=SL49130532-203044"
},
"r487": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130551-203045"
},
"r488": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130554-203045"
},
"r489": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130554-203045"
},
"r49": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.1)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r490": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130554-203045"
},
"r491": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130554-203045"
},
"r492": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130554-203045"
},
"r493": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130556-203045"
},
"r494": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)(2)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130556-203045"
},
"r495": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130558-203045"
},
"r496": {
"Name": "Accounting Standards Codification",
"Paragraph": "17",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130561-203045"
},
"r497": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130563-203045"
},
"r498": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130563-203045"
},
"r499": {
"Name": "Accounting Standards Codification",
"Paragraph": "19",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130564-203045"
},
"r5": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "205",
"URI": "http://asc.fasb.org/extlink&oid=124429488&loc=d3e326-107755"
},
"r50": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.12)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r500": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130566-203045"
},
"r501": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130566-203045"
},
"r502": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130566-203045"
},
"r503": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130566-203045"
},
"r504": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130543-203045"
},
"r505": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130545-203045"
},
"r506": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130549-203045"
},
"r507": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130549-203045"
},
"r508": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123351226&loc=SL49130550-203045"
},
"r509": {
"Name": "Accounting Standards Codification",
"Paragraph": "27",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130611-203046-203046"
},
"r51": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.13(a))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r510": {
"Name": "Accounting Standards Codification",
"Paragraph": "91",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130690-203046-203046"
},
"r511": {
"Name": "Accounting Standards Codification",
"Paragraph": "91",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130690-203046-203046"
},
"r512": {
"Name": "Accounting Standards Codification",
"Paragraph": "91",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130690-203046-203046"
},
"r513": {
"Name": "Accounting Standards Codification",
"Paragraph": "91",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130690-203046-203046"
},
"r514": {
"Name": "Accounting Standards Codification",
"Paragraph": "91",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130690-203046-203046"
},
"r515": {
"Name": "Accounting Standards Codification",
"Paragraph": "91",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Subparagraph": "(f)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130690-203046-203046"
},
"r516": {
"Name": "Accounting Standards Codification",
"Paragraph": "91",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Subparagraph": "(g)",
"Topic": "606",
"URI": "http://asc.fasb.org/extlink&oid=123410239&loc=SL49130690-203046-203046"
},
"r517": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "606",
"URI": "http://asc.fasb.org/topic&trid=49130388"
},
"r518": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "25",
"SubTopic": "10",
"Topic": "710",
"URI": "http://asc.fasb.org/extlink&oid=6409733&loc=d3e19512-108361"
},
"r519": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "30",
"SubTopic": "10",
"Topic": "710",
"URI": "http://asc.fasb.org/extlink&oid=6409875&loc=d3e20028-108363"
},
"r52": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.14)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r520": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "15",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "712",
"URI": "http://asc.fasb.org/extlink&oid=6410066&loc=d3e79218-111664"
},
"r521": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "15",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "712",
"URI": "http://asc.fasb.org/extlink&oid=6410066&loc=d3e79218-111664"
},
"r522": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123453770&loc=d3e1703-114919"
},
"r523": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123453770&loc=d3e1731-114919"
},
"r524": {
"Name": "Accounting Standards Codification",
"Paragraph": "3A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123453770&loc=SL108413299-114919"
},
"r525": {
"Name": "Accounting Standards Codification",
"Paragraph": "3A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123453770&loc=SL108413299-114919"
},
"r526": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r527": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r528": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(10)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r529": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r53": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.15)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r530": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(3)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r531": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(4)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r532": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(5)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r533": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(6)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r534": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(7)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r535": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(8)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r536": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(9)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r537": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r538": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r539": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r54": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.17)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r540": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(3)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r541": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(4)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r542": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(5)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r543": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(6)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r544": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(7)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r545": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(8)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r546": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r547": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(5)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r548": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(i)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r549": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(ii)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r55": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.19(a))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r550": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(iii)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r551": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(iv)(01)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r552": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(iv)(02)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r553": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(iv)(02)(A)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r554": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(iv)(02)(B)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r555": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(iv)(02)(C)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r556": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(iv)(03)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r557": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(e)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r558": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(f)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r559": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(g)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r56": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.19(b))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r560": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r561": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r562": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r563": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)(3)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r564": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)(4)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r565": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)(5)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r566": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)(6)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r567": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(h)(7)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r568": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(i)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r569": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(j)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r57": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.19(b),22(b))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r570": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(k)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r571": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(k)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r572": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(k)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r573": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(k)(3)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r574": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(k)(4)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r575": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(l)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r576": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(n)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r577": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(o)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r578": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(p)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r579": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(q)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r58": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.19)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r580": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(r)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r581": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(r)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r582": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e1928-114920"
},
"r583": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2410-114920"
},
"r584": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2417-114920"
},
"r585": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2417-114920"
},
"r586": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2417-114920"
},
"r587": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2439-114920"
},
"r588": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r589": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r59": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.19,20)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r590": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r591": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(3)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r592": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(4)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r593": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(5)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r594": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(6)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r595": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)(7)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r596": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2709-114920"
},
"r597": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123447040&loc=d3e2919-114920"
},
"r598": {
"Name": "Accounting Standards Codification",
"Paragraph": "17",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123450688&loc=d3e4179-114921"
},
"r599": {
"Name": "Accounting Standards Codification",
"Paragraph": "18",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=123450688&loc=d3e4587-114921"
},
"r6": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)",
"Topic": "205",
"URI": "http://asc.fasb.org/extlink&oid=109222650&loc=d3e1361-107760"
},
"r60": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.19-26)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r600": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "20",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=21916913&loc=d3e273930-122802"
},
"r601": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=6412939&loc=d3e15145-114933"
},
"r602": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "60",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=66047640&loc=d3e39622-114963"
},
"r603": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "60",
"Subparagraph": "(c)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=6414203&loc=d3e39689-114964"
},
"r604": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "60",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=6414203&loc=d3e39716-114964"
},
"r605": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "70",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=49170846&loc=d3e28014-114942"
},
"r606": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "35",
"SubTopic": "80",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=29639808&loc=d3e29008-114946"
},
"r607": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(a)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450702-114947"
},
"r608": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450702-114947"
},
"r609": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(c)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450702-114947"
},
"r61": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.20)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r610": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450702-114947"
},
"r611": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=d3e29149-114947"
},
"r612": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(a)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r613": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r614": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(c)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r615": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(d)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r616": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(e)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r617": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(e)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r618": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(f)(1)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r619": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(f)(2)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r62": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.21)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r620": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(f)(3)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450657-114947"
},
"r621": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(a)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450673-114947"
},
"r622": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450673-114947"
},
"r623": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(c)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450673-114947"
},
"r624": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "80",
"Subparagraph": "(b)",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=65877416&loc=SL14450691-114947"
},
"r625": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "80",
"Topic": "715",
"URI": "http://asc.fasb.org/extlink&oid=35742348&loc=SL14450788-114948"
},
"r626": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "715",
"URI": "http://asc.fasb.org/topic&trid=2235017"
},
"r627": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "35",
"SubTopic": "10",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=123468992&loc=d3e4534-113899"
},
"r628": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5047-113901"
},
"r629": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5047-113901"
},
"r63": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.22(a)(1))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r630": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5047-113901"
},
"r631": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5047-113901"
},
"r632": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(1)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r633": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r634": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(3)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r635": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a),(g)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r636": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b),(f)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r637": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r638": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r639": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)(i)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r64": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.22)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r640": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)(i)-(ii)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r641": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)(iii)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r642": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)(iv)(2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r643": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)(iv)(3)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r644": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)(iv)(3)-(4)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r645": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(2)(i)-(ii)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r646": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(2)(iii)(1)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r647": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(2)(iii)(2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r648": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(2)(iii)(3)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r649": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r65": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.24)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r650": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)(1)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r651": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)(2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r652": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r653": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)(1)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r654": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)(2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r655": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)(2)(i)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r656": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)(2)(ii)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r657": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)(2)(iii)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r658": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)(2)(iv)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r659": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(g)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r66": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.25)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r660": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(h)(1)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r661": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(h)(1)(i)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r662": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(i)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r663": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=d3e5070-113901"
},
"r664": {
"Name": "Accounting Standards Codification",
"Paragraph": "2A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=120381028&loc=SL79508275-113901"
},
"r665": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r666": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r667": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r668": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r669": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r67": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.28,29)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r670": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(f)(1)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r671": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(f)(2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r672": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(g)(2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=121322162&loc=SL121327923-165333"
},
"r673": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 14.D.2)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=122041274&loc=d3e301413-122809"
},
"r674": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 14.F)",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=122041274&loc=d3e301413-122809"
},
"r675": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=122142933&loc=d3e11149-113907"
},
"r676": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "718",
"URI": "http://asc.fasb.org/extlink&oid=122142933&loc=d3e11178-113907"
},
"r677": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "718",
"URI": "http://asc.fasb.org/topic&trid=2228938"
},
"r678": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "730",
"URI": "http://asc.fasb.org/extlink&oid=6420194&loc=d3e21568-108373"
},
"r679": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "730",
"URI": "http://asc.fasb.org/extlink&oid=6420387&loc=d3e23199-108380"
},
"r68": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.29-31)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r680": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "25",
"SubTopic": "10",
"Subparagraph": "(a)(1)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123452999&loc=d3e28200-109314"
},
"r681": {
"Name": "Accounting Standards Codification",
"Paragraph": "10B",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123427490&loc=SL37586934-109318"
},
"r682": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123427490&loc=d3e32247-109318"
},
"r683": {
"Name": "Accounting Standards Codification",
"Paragraph": "28",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123427490&loc=d3e32280-109318"
},
"r684": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123427490&loc=d3e31917-109318"
},
"r685": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123427490&loc=d3e31931-109318"
},
"r686": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32672-109319"
},
"r687": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32687-109319"
},
"r688": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32705-109319"
},
"r689": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32718-109319"
},
"r69": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.31)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r690": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)(3)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32718-109319"
},
"r691": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32718-109319"
},
"r692": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32718-109319"
},
"r693": {
"Name": "Accounting Standards Codification",
"Paragraph": "15A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=SL6600010-109319"
},
"r694": {
"Name": "Accounting Standards Codification",
"Paragraph": "15A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(1)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=SL6600010-109319"
},
"r695": {
"Name": "Accounting Standards Codification",
"Paragraph": "15A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(2)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=SL6600010-109319"
},
"r696": {
"Name": "Accounting Standards Codification",
"Paragraph": "15A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(3)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=SL6600010-109319"
},
"r697": {
"Name": "Accounting Standards Codification",
"Paragraph": "15A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(4)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=SL6600010-109319"
},
"r698": {
"Name": "Accounting Standards Codification",
"Paragraph": "15A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=SL6600010-109319"
},
"r699": {
"Name": "Accounting Standards Codification",
"Paragraph": "15A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=SL6600010-109319"
},
"r7": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "205",
"URI": "http://asc.fasb.org/extlink&oid=109222650&loc=SL51721683-107760"
},
"r70": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.6(a))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r700": {
"Name": "Accounting Standards Codification",
"Paragraph": "17",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32809-109319"
},
"r701": {
"Name": "Accounting Standards Codification",
"Paragraph": "19",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32840-109319"
},
"r702": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32537-109319"
},
"r703": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32537-109319"
},
"r704": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32537-109319"
},
"r705": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32537-109319"
},
"r706": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32847-109319"
},
"r707": {
"Name": "Accounting Standards Codification",
"Paragraph": "21",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32857-109319"
},
"r708": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32559-109319"
},
"r709": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32559-109319"
},
"r71": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.6(b))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r710": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32621-109319"
},
"r711": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32632-109319"
},
"r712": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32639-109319"
},
"r713": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32639-109319"
},
"r714": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=121826272&loc=d3e32639-109319"
},
"r715": {
"Name": "Accounting Standards Codification",
"Paragraph": "217",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=124434304&loc=d3e36027-109320"
},
"r716": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123459177&loc=SL121830611-158277"
},
"r717": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123459177&loc=SL121830611-158277"
},
"r718": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(d)(2)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123459177&loc=SL121830611-158277"
},
"r719": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(d)(3)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123459177&loc=SL121830611-158277"
},
"r72": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.8)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r720": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB TOPIC 6.I.5.Q1)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330036-122817"
},
"r721": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB TOPIC 6.I.7)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330036-122817"
},
"r722": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 6.I.7)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330036-122817"
},
"r723": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 6.I.Fact.1)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330036-122817"
},
"r724": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 6.I.Fact.2)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330036-122817"
},
"r725": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 6.I.Fact.3)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330036-122817"
},
"r726": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 6.I.Fact.4)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330036-122817"
},
"r727": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 11.C)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=122134291&loc=d3e330215-122817"
},
"r728": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=123586238&loc=d3e38679-109324"
},
"r729": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "270",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=6424409&loc=d3e44925-109338"
},
"r73": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX 210.5-02.9)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r730": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=6424122&loc=d3e41874-109331"
},
"r731": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(b)",
"Topic": "740",
"URI": "http://asc.fasb.org/extlink&oid=6424122&loc=d3e41874-109331"
},
"r732": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "740",
"URI": "http://asc.fasb.org/topic&trid=2144680"
},
"r733": {
"Name": "Accounting Standards Codification",
"Paragraph": "16",
"Publisher": "FASB",
"Section": "25",
"SubTopic": "10",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123586518&loc=d3e961-128460"
},
"r734": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=79982066&loc=d3e1392-128463"
},
"r735": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=79982066&loc=d3e1486-128463"
},
"r736": {
"Name": "Accounting Standards Codification",
"Paragraph": "37",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123455525&loc=d3e2207-128464"
},
"r737": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123413009&loc=d3e4845-128472"
},
"r738": {
"Name": "Accounting Standards Codification",
"Paragraph": "4A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123413009&loc=SL65897772-128472"
},
"r739": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123410050&loc=d3e5227-128473"
},
"r74": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SX210.5-02(13))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r740": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123410050&loc=d3e5263-128473"
},
"r741": {
"Name": "Accounting Standards Codification",
"Paragraph": "38",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123410050&loc=d3e5504-128473"
},
"r742": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "25",
"SubTopic": "30",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=6911189&loc=d3e6408-128476"
},
"r743": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "35",
"SubTopic": "30",
"Subparagraph": "(b)",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=116859824&loc=d3e6819-128478"
},
"r744": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(c)(1)",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=120321790&loc=d3e6927-128479"
},
"r745": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)(1)",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=120321790&loc=d3e7008-128479"
},
"r746": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "15",
"SubTopic": "50",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=6911878&loc=d3e8732-128492"
},
"r747": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "25",
"SubTopic": "50",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123385561&loc=d3e9135-128495"
},
"r748": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "30",
"SubTopic": "50",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123362884&loc=d3e9212-128498"
},
"r749": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "30",
"SubTopic": "50",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=123362884&loc=d3e9215-128498"
},
"r75": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "210-10-S99-1(SX 210.5-02(24))",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=120391452&loc=d3e13212-122682"
},
"r750": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "50",
"Topic": "805",
"URI": "http://asc.fasb.org/extlink&oid=6829253&loc=SL6831962-166255"
},
"r751": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "805",
"URI": "http://asc.fasb.org/topic&trid=2303972"
},
"r752": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123454820&loc=d3e5291-111683"
},
"r753": {
"Name": "Accounting Standards Codification",
"Paragraph": "15",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123454820&loc=SL4568447-111683"
},
"r754": {
"Name": "Accounting Standards Codification",
"Paragraph": "16",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123454820&loc=SL4568740-111683"
},
"r755": {
"Name": "Accounting Standards Codification",
"Paragraph": "19",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123454820&loc=SL4569616-111683"
},
"r756": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123454820&loc=SL4569643-111683"
},
"r757": {
"Name": "Accounting Standards Codification",
"Paragraph": "21",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123454820&loc=SL4613674-111683"
},
"r758": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=116870748&loc=SL6758485-165988"
},
"r759": {
"Name": "Accounting Standards Codification",
"Paragraph": "25",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=116870748&loc=SL6758485-165988"
},
"r76": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=51824906&loc=SL20225862-175312"
},
"r760": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=109239629&loc=d3e5614-111684"
},
"r761": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(1)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=109239629&loc=SL4573702-111684"
},
"r762": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(2)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=109239629&loc=SL4573702-111684"
},
"r763": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=109239629&loc=SL4573702-111684"
},
"r764": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(3)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=109239629&loc=SL4573702-111684"
},
"r765": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c),(3)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=109239629&loc=SL4573702-111684"
},
"r766": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(bb)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123419778&loc=d3e5710-111685"
},
"r767": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=123419778&loc=d3e5710-111685"
},
"r768": {
"Name": "Accounting Standards Codification",
"Paragraph": "4I",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=120409616&loc=SL4590271-111686"
},
"r769": {
"Name": "Accounting Standards Codification",
"Paragraph": "4J",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=120409616&loc=SL4591551-111686"
},
"r77": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=51824906&loc=SL20225862-175312"
},
"r770": {
"Name": "Accounting Standards Codification",
"Paragraph": "4K",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "810",
"URI": "http://asc.fasb.org/extlink&oid=120409616&loc=SL4591552-111686"
},
"r771": {
"Name": "Accounting Standards Codification",
"Paragraph": "83",
"Publisher": "FASB",
"Section": "15",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125514181&loc=d3e34841-113949"
},
"r772": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5579240-113959"
},
"r773": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5579245-113959"
},
"r774": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5579245-113959"
},
"r775": {
"Name": "Accounting Standards Codification",
"Paragraph": "1B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5580258-113959"
},
"r776": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(1)(ii)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=d3e41620-113959"
},
"r777": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(1)(iii)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=d3e41620-113959"
},
"r778": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=d3e41620-113959"
},
"r779": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=d3e41638-113959"
},
"r78": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(1)(i)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=51824906&loc=SL20225862-175312"
},
"r780": {
"Name": "Accounting Standards Codification",
"Paragraph": "4A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5618551-113959"
},
"r781": {
"Name": "Accounting Standards Codification",
"Paragraph": "4A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5618551-113959"
},
"r782": {
"Name": "Accounting Standards Codification",
"Paragraph": "4A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)(2)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5618551-113959"
},
"r783": {
"Name": "Accounting Standards Codification",
"Paragraph": "4B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a),(c)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624163-113959"
},
"r784": {
"Name": "Accounting Standards Codification",
"Paragraph": "4B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624163-113959"
},
"r785": {
"Name": "Accounting Standards Codification",
"Paragraph": "4B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624163-113959"
},
"r786": {
"Name": "Accounting Standards Codification",
"Paragraph": "4B",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624163-113959"
},
"r787": {
"Name": "Accounting Standards Codification",
"Paragraph": "4C",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624171-113959"
},
"r788": {
"Name": "Accounting Standards Codification",
"Paragraph": "4C",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624171-113959"
},
"r789": {
"Name": "Accounting Standards Codification",
"Paragraph": "4C",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624171-113959"
},
"r79": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(d)(2)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=51824906&loc=SL20225862-175312"
},
"r790": {
"Name": "Accounting Standards Codification",
"Paragraph": "4C",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624171-113959"
},
"r791": {
"Name": "Accounting Standards Codification",
"Paragraph": "4CC",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL109998890-113959"
},
"r792": {
"Name": "Accounting Standards Codification",
"Paragraph": "4CCC",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL109998896-113959"
},
"r793": {
"Name": "Accounting Standards Codification",
"Paragraph": "4CCC",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL109998896-113959"
},
"r794": {
"Name": "Accounting Standards Codification",
"Paragraph": "4D",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624177-113959"
},
"r795": {
"Name": "Accounting Standards Codification",
"Paragraph": "4D",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)(2)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624177-113959"
},
"r796": {
"Name": "Accounting Standards Codification",
"Paragraph": "4D",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624177-113959"
},
"r797": {
"Name": "Accounting Standards Codification",
"Paragraph": "4E",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=SL5624181-113959"
},
"r798": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=d3e41675-113959"
},
"r799": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=d3e41678-113959"
},
"r8": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6676-107765"
},
"r80": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=51824906&loc=SL20225862-175312"
},
"r800": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=125515794&loc=d3e41678-113959"
},
"r801": {
"Name": "Accounting Standards Codification",
"Paragraph": "182",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123421605&loc=SL5629052-113961"
},
"r802": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=121577181&loc=SL110061190-113977"
},
"r803": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "25",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=121577467&loc=d3e76258-113986"
},
"r804": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(c)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=121549185&loc=d3e80748-113994"
},
"r805": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=121549185&loc=d3e80784-113994"
},
"r806": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "40",
"Subparagraph": "(f)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123477628&loc=d3e90205-114008"
},
"r807": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(a)(1)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r808": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(a)(2)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r809": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(a)(3)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r81": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=51824906&loc=SL20225877-175312"
},
"r810": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(b)(1)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r811": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(b)(2)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r812": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(c)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r813": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(d)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r814": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(e)(2)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r815": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(e)(3)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r816": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(e)(4)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r817": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(f)",
"Topic": "815",
"URI": "http://asc.fasb.org/extlink&oid=123482062&loc=SL123482106-238011"
},
"r818": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "815",
"URI": "http://asc.fasb.org/topic&trid=2229140"
},
"r819": {
"Name": "Accounting Standards Codification",
"Paragraph": "54B",
"Publisher": "FASB",
"Section": "35",
"SubTopic": "10",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=122636397&loc=SL7495116-110257"
},
"r82": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=99393222&loc=SL20226008-175313"
},
"r820": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r821": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r822": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(bb)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r823": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(bbb)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r824": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(bbb)(1)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r825": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(bbb)(2)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r826": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r827": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r828": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(1a)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r829": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)(2)",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r83": {
"Name": "Accounting Standards Codification",
"Paragraph": "13",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=99393222&loc=SL20226016-175313"
},
"r830": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19207-110258"
},
"r831": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=d3e19279-110258"
},
"r832": {
"Name": "Accounting Standards Codification",
"Paragraph": "6A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "820",
"URI": "http://asc.fasb.org/extlink&oid=123874694&loc=SL6742756-110258"
},
"r833": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123594786&loc=SL75136599-209740"
},
"r834": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123594938&loc=d3e13433-108611"
},
"r835": {
"Name": "Accounting Standards Codification",
"Paragraph": "28",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(f)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123596393&loc=d3e14064-108612"
},
"r836": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL120254526-165497"
},
"r837": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL120254526-165497"
},
"r838": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL121967933-165497"
},
"r839": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL121967933-165497"
},
"r84": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=99393222&loc=SL20226038-175313"
},
"r840": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(c)(1)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL121967933-165497"
},
"r841": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(c)(2)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL121967933-165497"
},
"r842": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL122642865-165497"
},
"r843": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "825",
"URI": "http://asc.fasb.org/extlink&oid=123597120&loc=SL122642865-165497"
},
"r844": {
"Name": "Accounting Standards Codification",
"Paragraph": "9",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=120253306&loc=d3e28129-110885"
},
"r845": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "35",
"SubTopic": "20",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=123602790&loc=d3e30304-110892"
},
"r846": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "230",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=123444420&loc=d3e33268-110906"
},
"r847": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=118261656&loc=d3e32022-110900"
},
"r848": {
"Name": "Accounting Standards Codification",
"Paragraph": "17",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=118261656&loc=d3e32136-110900"
},
"r849": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Subparagraph": "(a)",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=118261656&loc=d3e32211-110900"
},
"r85": {
"Name": "Accounting Standards Codification",
"Paragraph": "22",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=99393222&loc=SL20226052-175313"
},
"r850": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Subparagraph": "(b)",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=118261656&loc=d3e32211-110900"
},
"r851": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Subparagraph": "(c)",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=118261656&loc=d3e32211-110900"
},
"r852": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Subparagraph": "(d)",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=118261656&loc=d3e32211-110900"
},
"r853": {
"Name": "Accounting Standards Codification",
"Paragraph": "20",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=118261656&loc=d3e32211-110900"
},
"r854": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=6450520&loc=d3e32583-110901"
},
"r855": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "830",
"URI": "http://asc.fasb.org/extlink&oid=6450520&loc=d3e32618-110901"
},
"r856": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "830",
"URI": "http://asc.fasb.org/topic&trid=2175825"
},
"r857": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "835",
"URI": "http://asc.fasb.org/extlink&oid=6450988&loc=d3e26243-108391"
},
"r858": {
"Name": "Accounting Standards Codification",
"Paragraph": "1A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "835",
"URI": "http://asc.fasb.org/extlink&oid=124435984&loc=d3e28541-108399"
},
"r859": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "835",
"URI": "http://asc.fasb.org/extlink&oid=124435984&loc=d3e28551-108399"
},
"r86": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=99393222&loc=SL20226000-175313"
},
"r860": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "835",
"URI": "http://asc.fasb.org/extlink&oid=124435984&loc=d3e28555-108399"
},
"r861": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "835",
"URI": "http://asc.fasb.org/extlink&oid=124435984&loc=d3e28567-108399"
},
"r862": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Topic": "835",
"URI": "http://asc.fasb.org/extlink&oid=124429444&loc=SL124452920-239629"
},
"r863": {
"Name": "Accounting Standards Codification",
"Paragraph": "8",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "30",
"Topic": "835",
"URI": "http://asc.fasb.org/extlink&oid=114775985&loc=d3e28878-108400"
},
"r864": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123391704&loc=SL77918627-209977"
},
"r865": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123391704&loc=SL77918627-209977"
},
"r866": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123391704&loc=SL77918631-209977"
},
"r867": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123391704&loc=SL77918638-209977"
},
"r868": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123391704&loc=SL77918643-209977"
},
"r869": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r87": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a),(b),(c)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r870": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r871": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r872": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(g)(1)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r873": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(g)(2)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r874": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(g)(3)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r875": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(g)(4)",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r876": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918686-209980"
},
"r877": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123408670&loc=SL77918701-209980"
},
"r878": {
"Name": "Accounting Standards Codification",
"Paragraph": "53",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "20",
"Topic": "842",
"URI": "http://asc.fasb.org/extlink&oid=123414884&loc=SL77918982-209971"
},
"r879": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"SubTopic": "20",
"Topic": "842",
"URI": "http://asc.fasb.org/subtopic&trid=77888251"
},
"r88": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(a-c)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r880": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "848",
"URI": "http://asc.fasb.org/extlink&oid=122150657&loc=SL122150809-237846"
},
"r881": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(c)",
"Topic": "850",
"URI": "http://asc.fasb.org/extlink&oid=6457730&loc=d3e39549-107864"
},
"r882": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "850",
"URI": "http://asc.fasb.org/extlink&oid=6457730&loc=d3e39549-107864"
},
"r883": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "850",
"URI": "http://asc.fasb.org/extlink&oid=6457730&loc=d3e39603-107864"
},
"r884": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "852",
"URI": "http://asc.fasb.org/extlink&oid=124437977&loc=d3e55792-112764"
},
"r885": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "852",
"URI": "http://asc.fasb.org/extlink&oid=124433192&loc=SL2890621-112765"
},
"r886": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "852",
"URI": "http://asc.fasb.org/extlink&oid=124433192&loc=SL2890621-112765"
},
"r887": {
"Name": "Accounting Standards Codification",
"Paragraph": "10",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "10",
"Topic": "852",
"URI": "http://asc.fasb.org/extlink&oid=84165509&loc=d3e56426-112766"
},
"r888": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(a)",
"Topic": "855",
"URI": "http://asc.fasb.org/extlink&oid=6842918&loc=SL6314017-165662"
},
"r889": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "855",
"URI": "http://asc.fasb.org/extlink&oid=6842918&loc=SL6314017-165662"
},
"r89": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(d)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r890": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Topic": "855",
"URI": "http://asc.fasb.org/extlink&oid=6842918&loc=SL6314020-165662"
},
"r891": {
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"Topic": "855",
"URI": "http://asc.fasb.org/topic&trid=2122774"
},
"r892": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(2)(i)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r893": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(2)(ii)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r894": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(3)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r895": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(bb)(1)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r896": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(bb)(2)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r897": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(bb)(3)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r898": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)(1)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r899": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)(2)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r9": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "210",
"URI": "http://asc.fasb.org/extlink&oid=124098289&loc=d3e6676-107765"
},
"r90": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(e)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r900": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)(3)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107207-111719"
},
"r901": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(1)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107314-111719"
},
"r902": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(2)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107314-111719"
},
"r903": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(b)(3)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107314-111719"
},
"r904": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=d3e107314-111719"
},
"r905": {
"Name": "Accounting Standards Codification",
"Paragraph": "4D",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Subparagraph": "(c)(2)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=121570589&loc=SL51823488-111719"
},
"r906": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "30",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=66007379&loc=d3e113888-111728"
},
"r907": {
"Name": "Accounting Standards Codification",
"Paragraph": "7",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "30",
"Subparagraph": "(a)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=109249958&loc=SL34722452-111729"
},
"r908": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(1)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122625-111746"
},
"r909": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(2)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122625-111746"
},
"r91": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(f)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r910": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(3)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122625-111746"
},
"r911": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(4)(i)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122625-111746"
},
"r912": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(1)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r913": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(2)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r914": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(3)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r915": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(4)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r916": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(5)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r917": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(6)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r918": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(a)(7)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r919": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(b)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r92": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(h)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r920": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(e)(1)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r921": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(e)(2)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r922": {
"Name": "Accounting Standards Codification",
"Paragraph": "4",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "50",
"Subparagraph": "(e)(3)",
"Topic": "860",
"URI": "http://asc.fasb.org/extlink&oid=125521744&loc=d3e122739-111746"
},
"r923": {
"Name": "Accounting Standards Codification",
"Paragraph": "6",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "10",
"Subparagraph": "(b)",
"Topic": "910",
"URI": "http://asc.fasb.org/extlink&oid=123353855&loc=SL119991595-234733"
},
"r924": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "330",
"Topic": "912",
"URI": "http://asc.fasb.org/extlink&oid=6471895&loc=d3e55923-109411"
},
"r925": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "10",
"Subparagraph": "(SAB Topic 11.L)",
"Topic": "924",
"URI": "http://asc.fasb.org/extlink&oid=6472922&loc=d3e499488-122856"
},
"r926": {
"Name": "Accounting Standards Codification",
"Paragraph": "5",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "20",
"Topic": "926",
"URI": "http://asc.fasb.org/extlink&oid=120154696&loc=d3e54445-107959"
},
"r927": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "20",
"Subparagraph": "(a)",
"Topic": "926",
"URI": "http://asc.fasb.org/extlink&oid=120154821&loc=SL120154904-197079"
},
"r928": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "20",
"Subparagraph": "(b)",
"Topic": "926",
"URI": "http://asc.fasb.org/extlink&oid=120154821&loc=SL120154904-197079"
},
"r929": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "20",
"Subparagraph": "(c)",
"Topic": "926",
"URI": "http://asc.fasb.org/extlink&oid=120154821&loc=SL120154904-197079"
},
"r93": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(i)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r930": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "340",
"Topic": "928",
"URI": "http://asc.fasb.org/extlink&oid=6473545&loc=d3e61844-108004"
},
"r931": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "25",
"SubTopic": "20",
"Topic": "940",
"URI": "http://asc.fasb.org/extlink&oid=123384075&loc=d3e41242-110953"
},
"r932": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(10)(1))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r933": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(10))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r934": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(11))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r935": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(13))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r936": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(15)(1))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r937": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(15)(2))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r938": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(16))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r939": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(22))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r94": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(i),(j),(k)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r940": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(23))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r941": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(4))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r942": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(5))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r943": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03(6))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r944": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.9-03.17)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120398452&loc=d3e534808-122878"
},
"r945": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04(15))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r946": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04(22))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r947": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04(23))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r948": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04(24))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r949": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04(25))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r95": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Subparagraph": "(i-k)",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r950": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04(26))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r951": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04(27))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r952": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.9-04.9)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399700&loc=SL114874048-224260"
},
"r953": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "235",
"Subparagraph": "(SX 210.9-05(b)(2))",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=120399901&loc=d3e537907-122884"
},
"r954": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "320",
"Subparagraph": "(b)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=123599081&loc=d3e62557-112803"
},
"r955": {
"Name": "Accounting Standards Codification",
"Paragraph": "3A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "320",
"Subparagraph": "(a)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=123599081&loc=SL120269850-112803"
},
"r956": {
"Name": "Accounting Standards Codification",
"Paragraph": "3A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "320",
"Subparagraph": "(b)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=123599081&loc=SL120269850-112803"
},
"r957": {
"Name": "Accounting Standards Codification",
"Paragraph": "3A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "320",
"Subparagraph": "(c)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=123599081&loc=SL120269850-112803"
},
"r958": {
"Name": "Accounting Standards Codification",
"Paragraph": "3A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "320",
"Subparagraph": "(d)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=123599081&loc=SL120269850-112803"
},
"r959": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "360",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=124429447&loc=SL124453093-239630"
},
"r96": {
"Name": "Accounting Standards Codification",
"Paragraph": "10A",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=SL7669646-108580"
},
"r960": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "405",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=116652737&loc=d3e64164-112818"
},
"r961": {
"Name": "Accounting Standards Codification",
"Paragraph": "3",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "470",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=123599511&loc=d3e64711-112823"
},
"r962": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "740",
"Subparagraph": "(b)",
"Topic": "942",
"URI": "http://asc.fasb.org/extlink&oid=6479915&loc=d3e66715-112838"
},
"r963": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(10))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r964": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(15)(b)(2))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r965": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(16))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r966": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(1)(g))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r967": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(10))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r968": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(12))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r969": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(15)(b)(1))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r97": {
"Name": "Accounting Standards Codification",
"Paragraph": "11",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=d3e637-108580"
},
"r970": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(16)(a)(1))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r971": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(16))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r972": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(23)(a)(3))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r973": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(23)(a)(4))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r974": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(24))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r975": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(25))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r976": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03(a)(8))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r977": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "210",
"Subparagraph": "(SX 210.7-03.(a),19)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400017&loc=d3e572229-122910"
},
"r978": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(18))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r979": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(19))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r98": {
"Name": "Accounting Standards Codification",
"Paragraph": "12",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=d3e640-108580"
},
"r980": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(20))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r981": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(21))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r982": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(22))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r983": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(23))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r984": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(3)(b))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r985": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(8))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r986": {
"Name": "Accounting Standards Codification",
"Paragraph": "1",
"Publisher": "FASB",
"Section": "S99",
"SubTopic": "220",
"Subparagraph": "(SX 210.7-04(9))",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=120400993&loc=SL114874131-224263"
},
"r987": {
"Name": "Accounting Standards Codification",
"Paragraph": "4H",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "40",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=116884468&loc=SL65671331-158438"
},
"r988": {
"Name": "Accounting Standards Codification",
"Paragraph": "7A",
"Publisher": "FASB",
"Section": "50",
"SubTopic": "40",
"Subparagraph": "(d)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124506351&loc=SL117782755-158439"
},
"r989": {
"Name": "Accounting Standards Codification",
"Paragraph": "13H",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "40",
"Subparagraph": "(a)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124504033&loc=SL117783719-158441"
},
"r99": {
"Name": "Accounting Standards Codification",
"Paragraph": "14",
"Publisher": "FASB",
"Section": "45",
"SubTopic": "10",
"Topic": "220",
"URI": "http://asc.fasb.org/extlink&oid=124509347&loc=d3e681-108580"
},
"r990": {
"Name": "Accounting Standards Codification",
"Paragraph": "13H",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "40",
"Subparagraph": "(b)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124504033&loc=SL117783719-158441"
},
"r991": {
"Name": "Accounting Standards Codification",
"Paragraph": "29F",
"Publisher": "FASB",
"Section": "55",
"SubTopic": "40",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124504033&loc=SL117819544-158441"
},
"r992": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(a)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r993": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(b)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r994": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(e)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r995": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(f)(1)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r996": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(f)(2)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r997": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(g)(1)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r998": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(g)(2)(i)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
},
"r999": {
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Publisher": "FASB",
"Section": "65",
"SubTopic": "40",
"Subparagraph": "(g)(2)(ii)",
"Topic": "944",
"URI": "http://asc.fasb.org/extlink&oid=124501264&loc=SL117420844-207641"
}
},
"version": "2.1"
}