FALSE000141168500014116852024-09-232024-09-23
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
PURSUANT TO SECTION 13 OR 15(d) of the SECURITIES EXCHANGE ACT OF 1934
Date of Report (Date of earliest event reported): March 3, 2025
Vistagen Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| | | | | | | | |
| Nevada | 000-54014 | 20-5093315 |
(State or other jurisdiction of incorporation) | (Commission File Number) | (IRS Employer Identification Number) |
343 Allerton Ave.
South San Francisco, California 94080
(Address of principal executive offices)
(650) 577-3600
(Registrant’s telephone number, including area code)
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a -12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d -2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e -4(c))
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | |
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| Common Stock, par value $0.001 per share | VTGN | Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (17 CFR 230.405) or Rule 12b-2 of the Securities Exchange Act of 1934 (17 CFR 240.12b-2)
Emerging Growth Company o
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act o
Item 8.01 Other Events
On March 3, 2025, Vistagen Therapeutics, Inc. (the “Company”) presented at the 45th Annual TD Cowen Healthcare Conference. A copy of the slide presentation used during the TD Cowen Healthcare Conference is attached to this Current Report on Form 8-K as Exhibit 99.1.
On March 4, 2025, the Company announced that it will participate at the Stifel 2025 Virtual CNS Forum on March 18, 2025. A copy of the press release issued by the Company is attached hereto as Exhibit 99.2.
On February 13, 2025, John Cesario and David Preka (the “Plaintiffs”) filed a civil action (the “Complaint”) pro se (i.e., acting on their own behalf rather than through an attorney) against the Company and its Board of Directors (the “Board”), certain of its executive officers, professional services and financial advisors, and industry analysts in the United States District Court for the Northern District of California (Case No. 4:25-cv-01510). The Plaintiffs seek compensatory and punitive damages, as well as fees and costs. The Complaint alleges violations of Section 10(b) of the Securities Exchange Act of 1934, as amended, and Rule 10b-5 promulgated thereunder, and Sections 11 and 17(a) of the Securities Act of 1933, as amended, along with other causes of action. The Plaintiffs allege, among other things, that the Company, and certain of its executive officers, made misleading statements and material omissions in various public disclosures concerning clinical trials for certain of the Company’s product candidates, which they allege caused plaintiffs to incur compensable losses. The Complaint also alleges that the Board, certain of the Company’s professional and financial advisors, and industry analysts aided and abetted the alleged wrongful conduct. The Company believes all allegations asserted in the Complaint are wholly without merit, and intends to defend them vigorously.
Item 9.01 Financial Statements and Exhibits
(d)Exhibits Index
| | | | | | | | |
| Exhibit No. | | Description |
| | | |
| 99.1 | | |
| 99.2 | | |
| 104 | | Cover Page Interactive Data File (embedded within the Inline XBRL document) |
Signatures
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | | | | |
| | Vistagen Therapeutics, Inc. |
| | |
| Date: March 6, 2025 | By: | /s/ Shawn K. Singh |
| | | Shawn K. Singh
President and Chief Executive Officer |

Nasdaq: VTGN Pioneering neuroscience with nose-to-brain neurocircuitry March 3, 2025 TD Cowen 45th Annual Health Care Conference

Forward-looking Statements 2 This presentation contains certain forward-looking statements that are within the meaning of federal securities laws. These forward-looking statements involve known and unknown risks that are difficult to predict and include all matters that are not historical facts. In some cases, you can identify forward-looking statements by the use of words such as “may,” “could,” “expect,” “project,” “outlook,” “strategy,” “intend,” “plan,” “seek,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “strive,” “goal,” “continue,” “likely,” “will,” “would” and variations of these terms and similar expressions, or the negative of these terms or similar expressions. Such forward-looking statements are necessarily based upon estimates and assumptions that, while considered reasonable by Vistagen Therapeutics, Inc. (Vistagen or the Company) and its management, are inherently uncertain. As with all pharmaceutical products, there are substantial risks and uncertainties in the process of development and commercialization and actual results or developments may differ materially from those projected or implied in these forward-looking statements. There can be no guarantee that any of the Company’s product candidates will successfully complete ongoing or future clinical trials within estimated timelines or at all, receive regulatory approval or be commercially successful, or that the Company will be able to successfully replicate the result of past studies of its product candidates. Other factors that may cause such a difference include, without limitation, risks and uncertainties relating to conducting and/or completing ongoing and planned nonclinical studies and clinical trials, including PALISADE-3 and PALISADE-4, as currently expected or at all; the timing of completion of preclinical studies and clinical trials and related preparatory work required to apply for an maintain regulatory approval for any of the Company’s product candidates; launching planned clinical trials for any of our product candidates; the Company’s submission of a new drug application (NDA) to the U.S. FDA for any product candidate, including fasedienol; the ability of any clinical trial information submitted by the Company to the U.S. FDA to support a NDA; the Company’s dependence on third-party collaborators for the development, regulatory approval, and/or commercialization of products and other aspects of our business, which are outside of our full control; risks associated with current and potential future healthcare reforms; the scope and enforceability of the Company’s patents, including patents related to Vistagen’s pherine product candidates and AV-101; fluctuating costs of materials and other resources and services required to conduct Vistagen’s ongoing and/or planned clinical and non-clinical trials; market conditions; the impact of general economic, industry or political conditions in the United States or internationally; and other technical and unexpected hurdles in the development, manufacture and commercialization of Vistagen’s product candidates. These risks are more fully discussed in the section entitled “Risk Factors” in Vistagen’s Annual Report on Form 10-K for the fiscal year ended March 31, 2024, and Quarterly Report on Form 10-Q for the period ended December 31, 2024, as well as discussions of potential risks, uncertainties, and other important factors in our other filings with the U.S. Securities and Exchange Commission (SEC). The Company’s SEC filings are available on the SEC’s website at www.sec.gov. Given these uncertainties, you should not place undue reliance on these forward-looking statements, which apply only as of the date of this presentation and should not be relied upon as representing the Company’s views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements other than as may be required by law. If the Company does update one or more forward-looking statements, no inference should be made that we will make additional updates with respect to those or other forward-looking statements. Be aware that our development and commercialization plans may change at any time, without publ ic notice, based on the kinds of risk factors described above. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. These data involve numerous assumptions and limitations, and you are cautioned not to give undue weight to such estimates and data.

3 Highlights - Funded registration-directed Phase 3 program in Social Anxiety Disorder underway - Multi-billion-dollar peak sales potential across several high prevalence indications - Partnering opportunities in multiple indications and territories - Harnessing the therapeutic power and potential of nose-to-brain neurocircuitry - Developing a new class of non-systemic intranasal product candidates called “pherines” - Five clinical-stage pherine product candidates with positive results

Pherines Harnessing the power and potential of nose-to-brain neurocircuitry
Pherines 5 A new class of intranasal neuroscience product candidates - Rapidly activate nose-to-brain neurocircuits affecting multiple high-prevalence indications - Non-systemic MOAs are distinguished from all FDA-approved drugs for target indications - Microgram-level dosing without binding to neurons in the brain - Favorable and differentiated safety data observed in all clinical trials completed to date
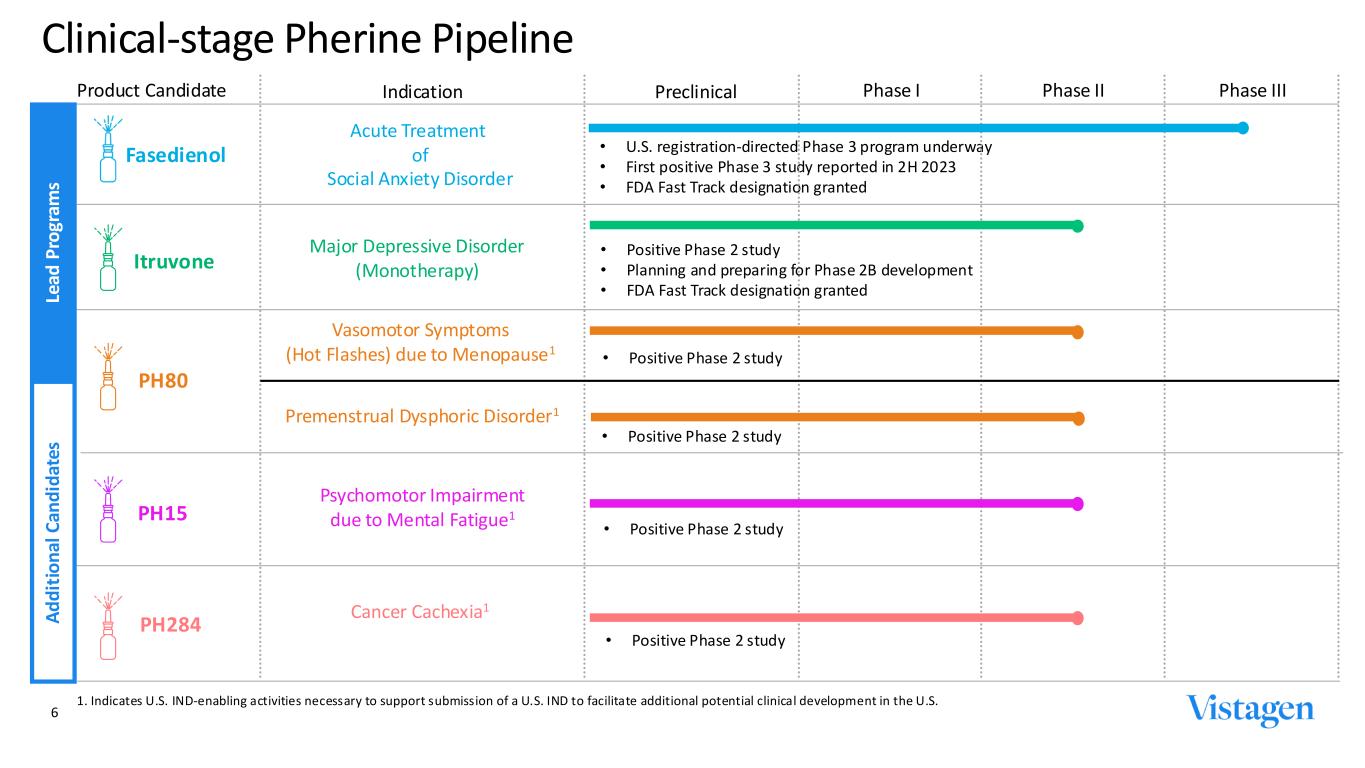
Product Candidate Indication Preclinical Phase I Phase II Phase III Fasedienol Itruvone Acute Treatment of Social Anxiety Disorder Major Depressive Disorder (Monotherapy) • U.S. registration-directed Phase 3 program underway • First positive Phase 3 study reported in 2H 2023 • FDA Fast Track designation granted • Positive Phase 2 study • Planning and preparing for Phase 2B development • FDA Fast Track designation granted PH80 PH15 PH284 Vasomotor Symptoms (Hot Flashes) due to Menopause1 Psychomotor Impairment due to Mental Fatigue1 Cancer Cachexia1 Clinical-stage Pherine Pipeline 6 1. Indicates U.S. IND-enabling activities necessary to support submission of a U.S. IND to facilitate additional potential clinical development in the U.S. Premenstrual Dysphoric Disorder1 • Positive Phase 2 study Le ad P ro gr am s A d d it io n al C an d id at es • Positive Phase 2 study • Positive Phase 2 study • Positive Phase 2 study

Acute Treatment of Social Anxiety Disorder Fasedienol
Social Anxiety Disorder (SAD) 8 Presenting at work or school Eating/drinking in front of others Public speaking Making a phone call Emotional Symptoms • Overwhelming fear • Surges of anxiety • Extreme self-consciousness • Isolation leading to depression Source: ADAA Social Anxiety Brochure 2021 Chronic mental health disorder, onset often in adolescence, characterized by: Meeting new people Interviewing for a job Debilitating emotional and physical symptoms in everyday social and performance situations Physical Symptoms • Blushing / Sweating • Trembling • Nausea • Fast heartbeat / Chest discomfort • Shortness of breath / Dizziness
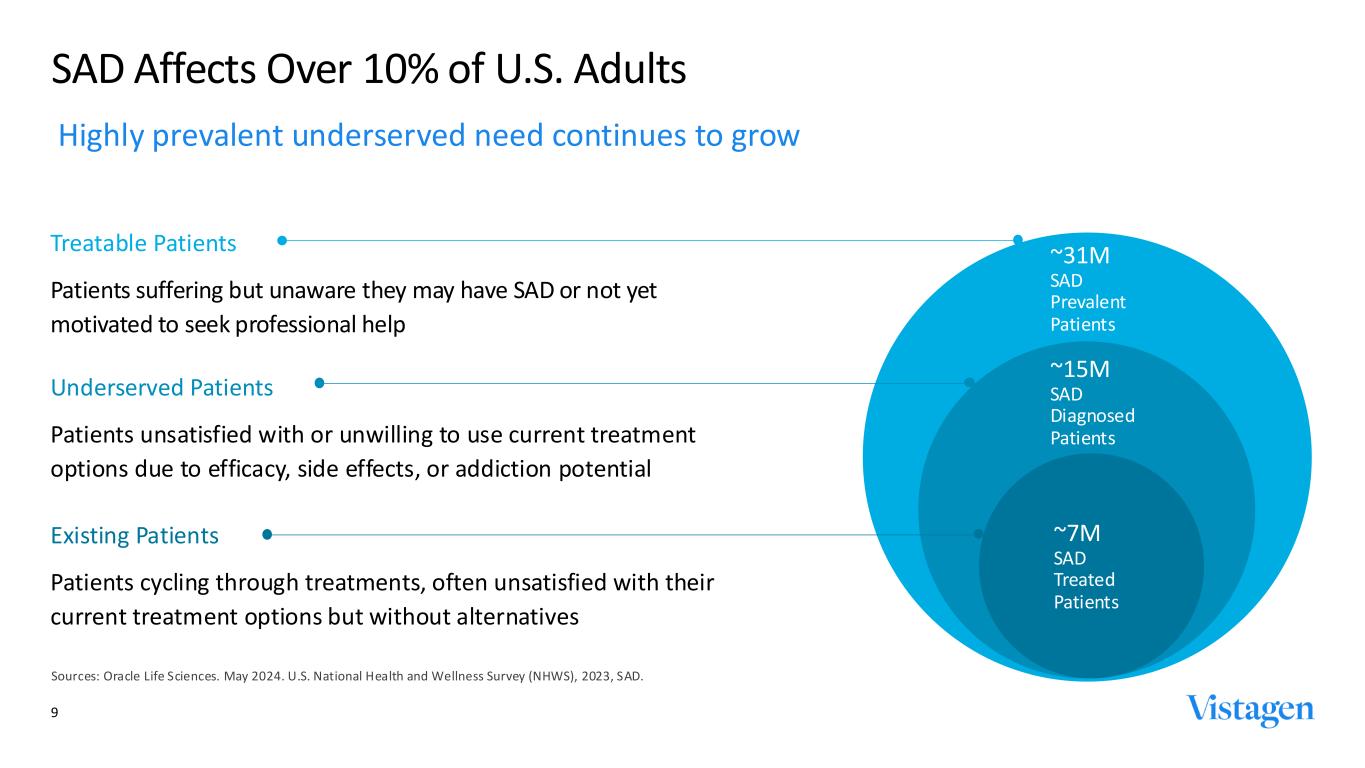
~31M SAD Prevalent Patients ~15M SAD Diagnosed Patients ~7M SAD Treated Patients Treatable Patients Patients suffering but unaware they may have SAD or not yet motivated to seek professional help 9 SAD Affects Over 10% of U.S. Adults Sources: Oracle Life Sciences. May 2024. U.S. National Health and Wellness Survey (NHWS), 2023, SAD. Existing Patients Patients cycling through treatments, often unsatisfied with their current treatment options but without alternatives Underserved Patients Patients unsatisfied with or unwilling to use current treatment options due to efficacy, side effects, or addiction potential Highly prevalent underserved need continues to grow
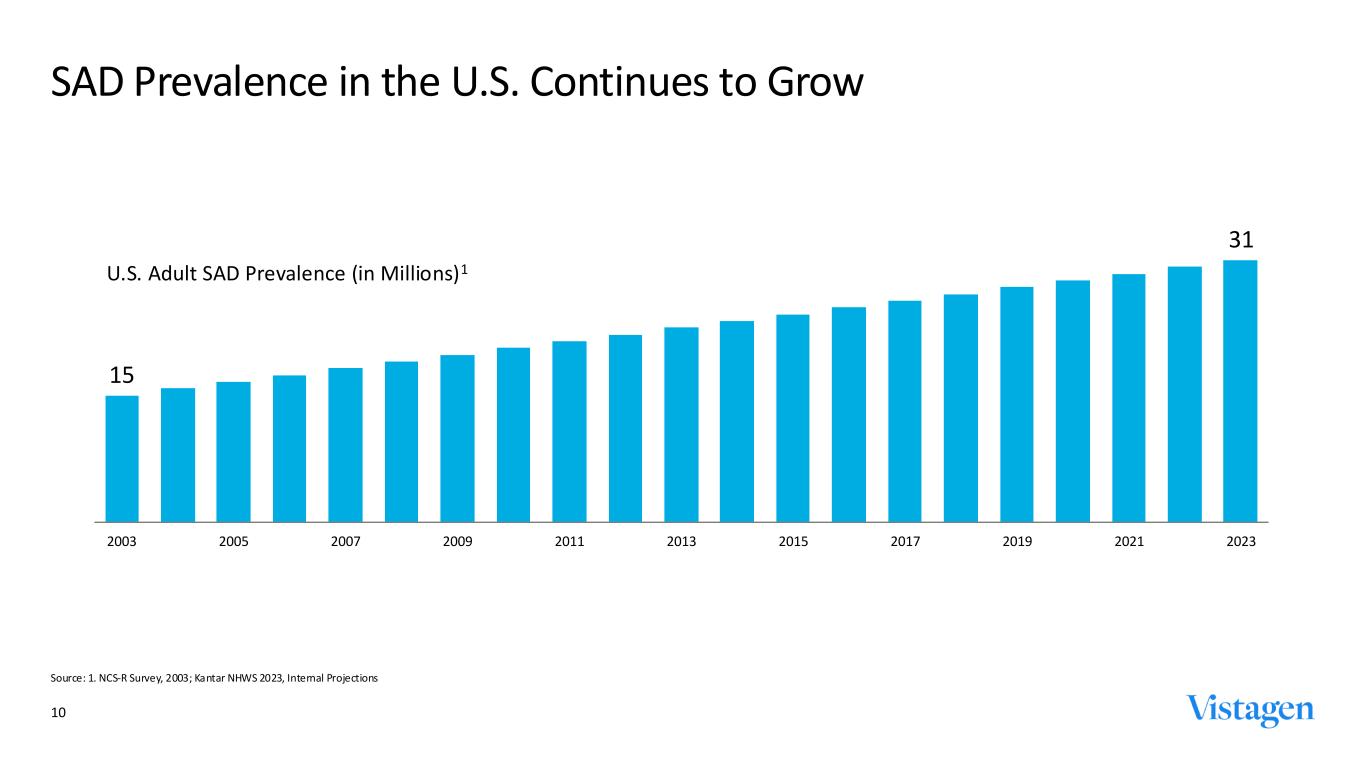
10 SAD Prevalence in the U.S. Continues to Grow Source: 1. NCS-R Survey, 2003; Kantar NHWS 2023, Internal Projections 15 31 2003 2005 2007 2009 2011 2013 2015 2017 2019 2021 2023 U.S. Adult SAD Prevalence (in Millions)1
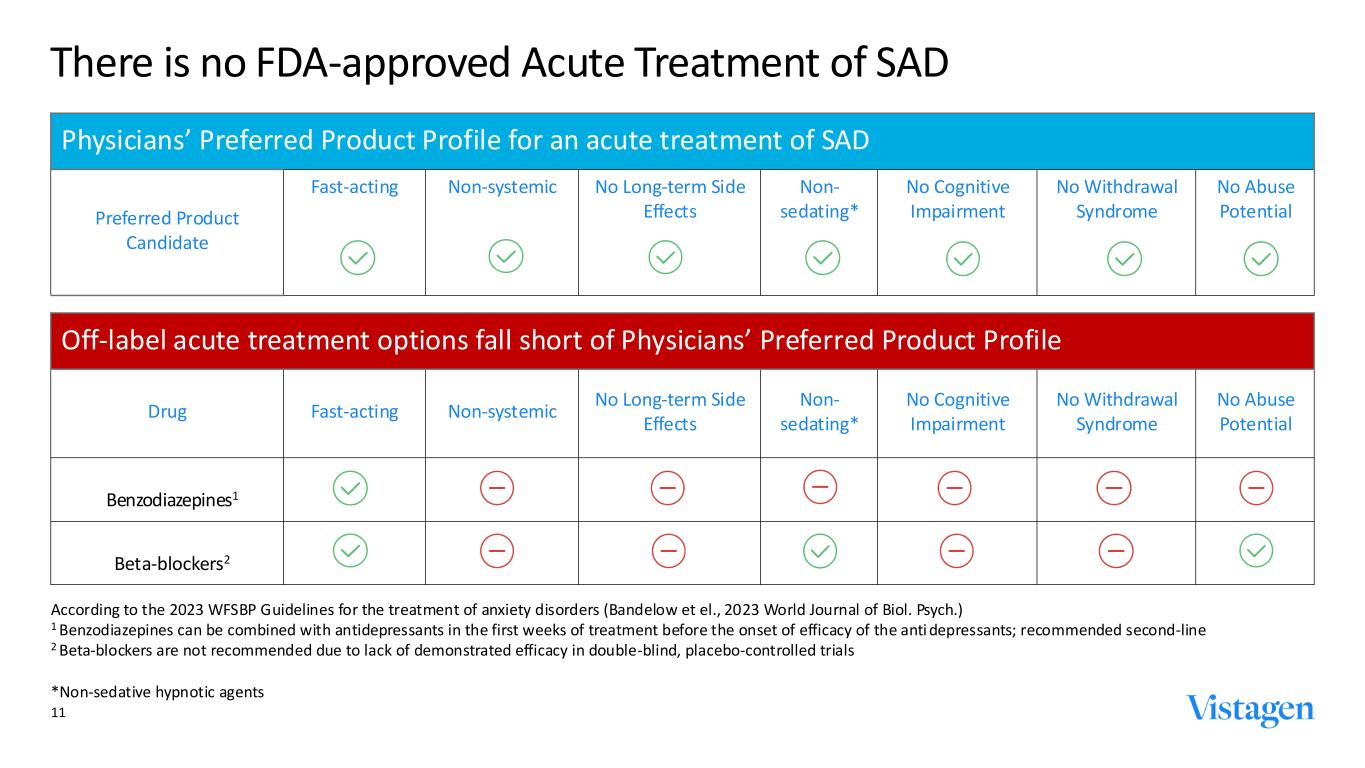
11 There is no FDA-approved Acute Treatment of SAD Off-label acute treatment options fall short of Physicians’ Preferred Product Profile Drug Fast-acting Non-systemic No Long-term Side Effects Non- sedating* No Cognitive Impairment No Withdrawal Syndrome No Abuse Potential Benzodiazepines1 Beta-blockers2 According to the 2023 WFSBP Guidelines for the treatment of anxiety disorders (Bandelow et el., 2023 World Journal of Biol. Psych.) 1 Benzodiazepines can be combined with antidepressants in the first weeks of treatment before the onset of efficacy of the anti depressants; recommended second-line 2 Beta-blockers are not recommended due to lack of demonstrated efficacy in double-blind, placebo-controlled trials *Non-sedative hypnotic agents Physicians’ Preferred Product Profile for an acute treatment of SAD Preferred Product Candidate Fast-acting Non-systemic No Long-term Side Effects Non- sedating* No Cognitive Impairment No Withdrawal Syndrome No Abuse Potential

Fasedienol Brings New Optimism for SAD Patients ‐ Rapid-onset efficacy and differentiated safety ‐ Potential to be the first FDA-approved acute treatment of SAD ‐ Patient-tailored administration, as needed, up to several times a day ‐ No observed systemic absorption or binding to neurons in the brain ‐ Not a ”benzo” - does not potentiate GABA or bind to abuse liability receptors ‐ Favorable tolerability profile, no evidence of abuse liability potential ‐ Multi-billion-dollar U.S. peak sales potential ‐ FDA Fast Track designation granted 12
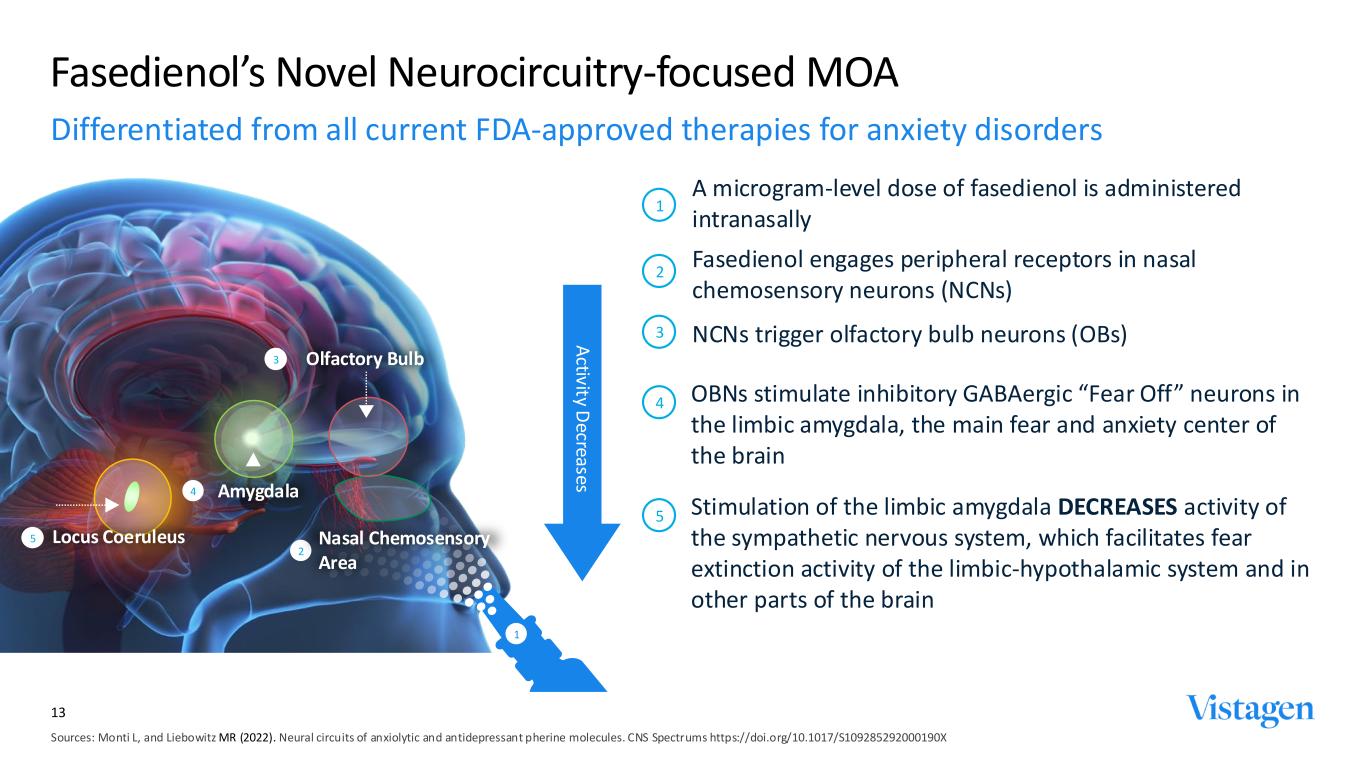
Sources: Monti L, and Liebowitz MR (2022). Neural circuits of anxiolytic and antidepressant pherine molecules. CNS Spectrums https://doi.org/10.1017/S109285292000190X Fasedienol’s Novel Neurocircuitry-focused MOA A microgram-level dose of fasedienol is administered intranasally 1 A ctivity D ecreases 5 Stimulation of the limbic amygdala DECREASES activity of the sympathetic nervous system, which facilitates fear extinction activity of the limbic-hypothalamic system and in other parts of the brain Locus Coeruleus5 4 OBNs stimulate inhibitory GABAergic “Fear Off” neurons in the limbic amygdala, the main fear and anxiety center of the brain 2 Fasedienol engages peripheral receptors in nasal chemosensory neurons (NCNs) 3 NCNs trigger olfactory bulb neurons (OBs) 1 Amygdala4 2 Nasal Chemosensory Area 3 Olfactory Bulb 13 Differentiated from all current FDA-approved therapies for anxiety disorders
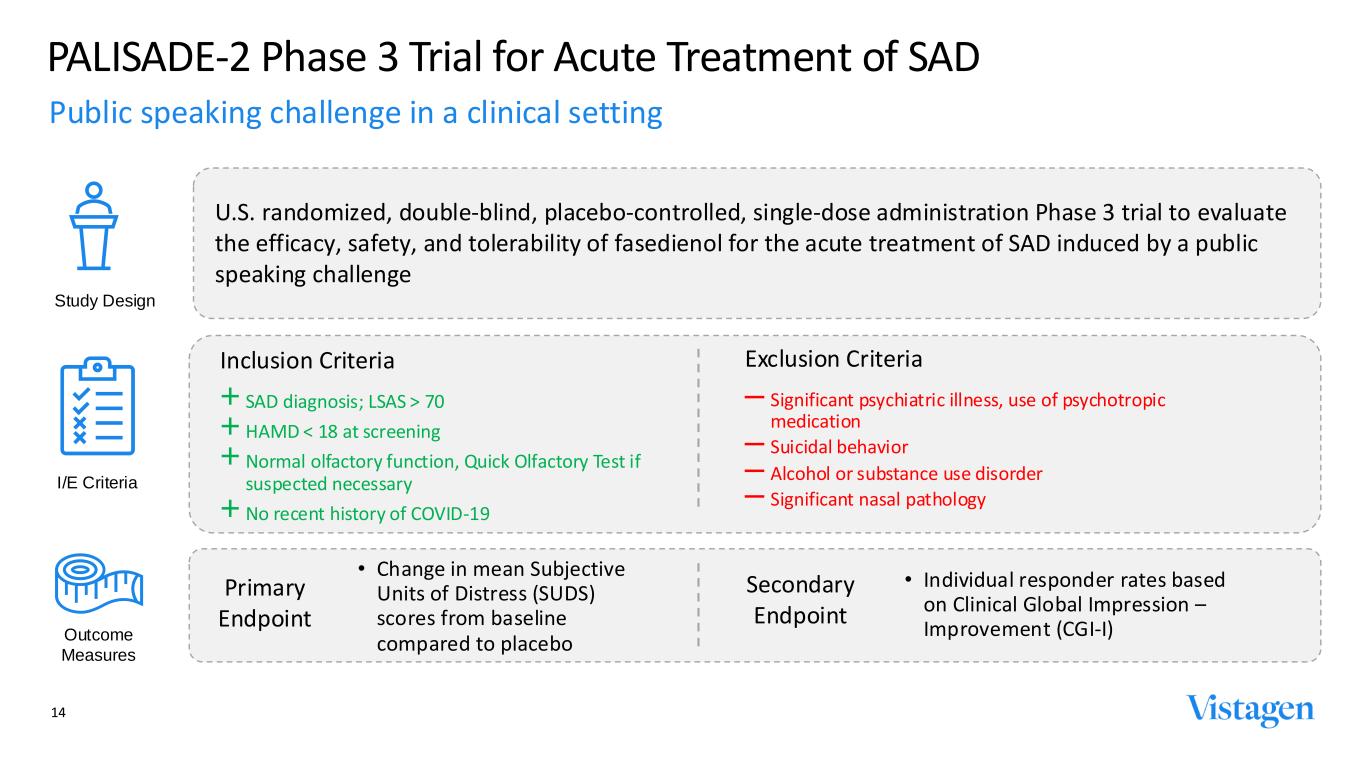
14 14 PALISADE-2 Phase 3 Trial for Acute Treatment of SAD : A VISIONARY APPROACH TO MENTAL HEALTH CARE U.S. randomized, double-blind, placebo-controlled, single-dose administration Phase 3 trial to evaluate the efficacy, safety, and tolerability of fasedienol for the acute treatment of SAD induced by a public speaking challenge Study Design I/E Criteria Outcome Measures • Individual responder rates based on Clinical Global Impression – Improvement (CGI-I) • Change in mean Subjective Units of Distress (SUDS) scores from baseline compared to placebo Primary Endpoint Secondary Endpoint Public speaking challenge in a clinical setting Inclusion Criteria + SAD diagnosis; LSAS > 70 + HAMD < 18 at screening + Normal olfactory function, Quick Olfactory Test if suspected necessary + No recent history of COVID-19 Exclusion Criteria ‒ Significant psychiatric illness, use of psychotropic medication ‒ Suicidal behavior ‒ Alcohol or substance use disorder ‒ Significant nasal pathology
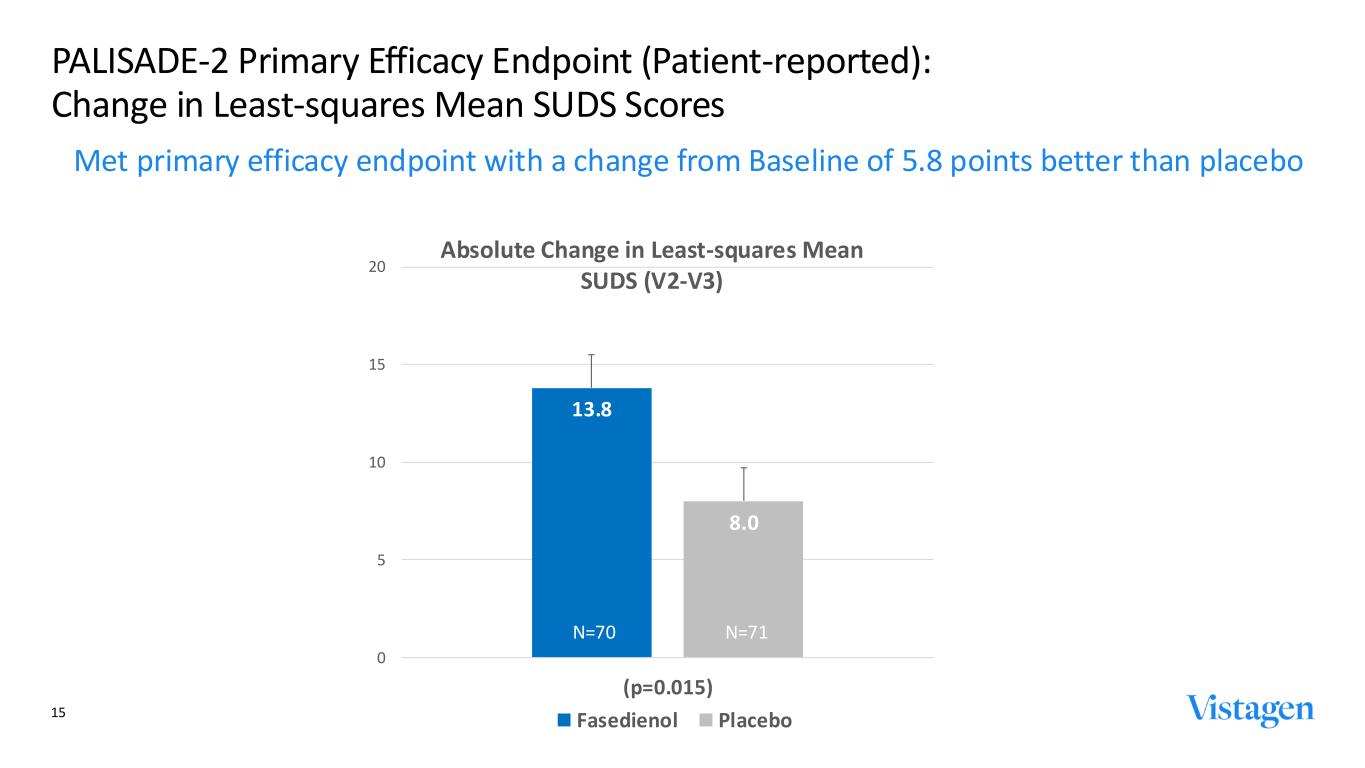
13.8 8.0 0 5 10 15 20 (p=0.015) Absolute Change in Least-squares Mean SUDS (V2-V3) Fasedienol Placebo PALISADE-2 Primary Efficacy Endpoint (Patient-reported): Change in Least-squares Mean SUDS Scores Met primary efficacy endpoint with a change from Baseline of 5.8 points better than placebo 15 N=70 N=71
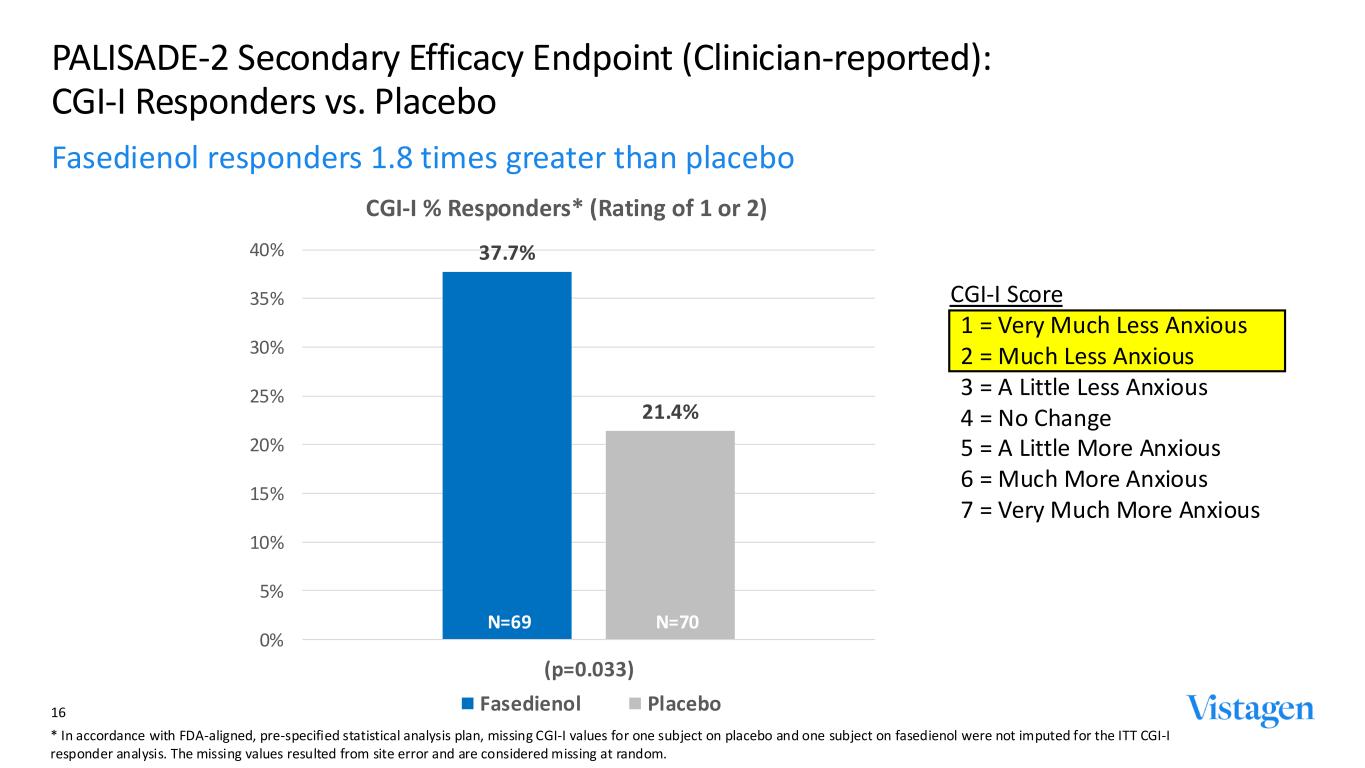
CGI-I Score 1 = Very Much Less Anxious 2 = Much Less Anxious 3 = A Little Less Anxious 4 = No Change 5 = A Little More Anxious 6 = Much More Anxious 7 = Very Much More Anxious 37.7% 21.4% 0% 5% 10% 15% 20% 25% 30% 35% 40% (p=0.033) CGI-I % Responders* (Rating of 1 or 2) Fasedienol Placebo PALISADE-2 Secondary Efficacy Endpoint (Clinician-reported): CGI-I Responders vs. Placebo Fasedienol responders 1.8 times greater than placebo 16 N=69 N=70 * In accordance with FDA-aligned, pre-specified statistical analysis plan, missing CGI-I values for one subject on placebo and one subject on fasedienol were not imputed for the ITT CGI-I responder analysis. The missing values resulted from site error and are considered missing at random.
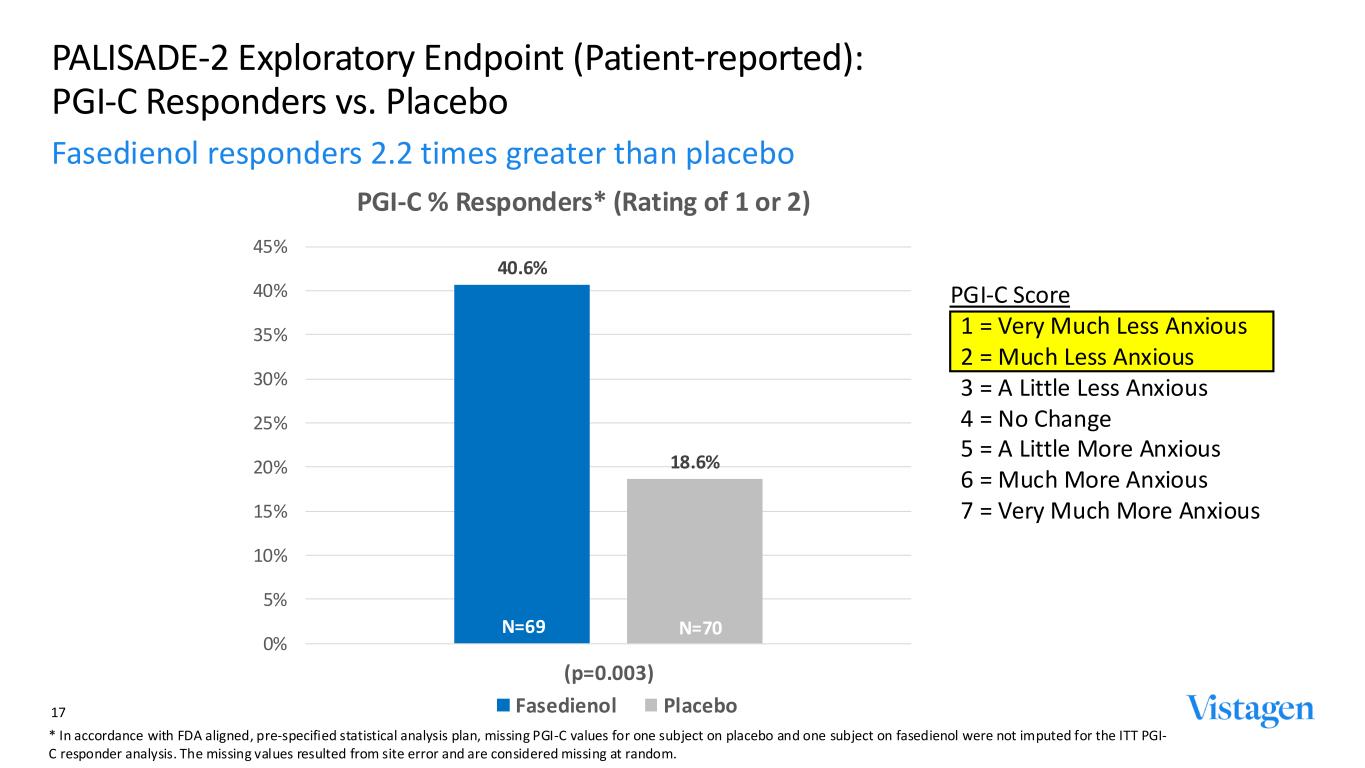
PGI-C Score 1 = Very Much Less Anxious 2 = Much Less Anxious 3 = A Little Less Anxious 4 = No Change 5 = A Little More Anxious 6 = Much More Anxious 7 = Very Much More Anxious 40.6% 18.6% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% (p=0.003) PGI-C % Responders* (Rating of 1 or 2) Fasedienol Placebo PALISADE-2 Exploratory Endpoint (Patient-reported): PGI-C Responders vs. Placebo Fasedienol responders 2.2 times greater than placebo 17 N=69 N=70 * In accordance with FDA aligned, pre-specified statistical analysis plan, missing PGI-C values for one subject on placebo and one subject on fasedienol were not imputed for the ITT PGI- C responder analysis. The missing values resulted from site error and are considered missing at random.
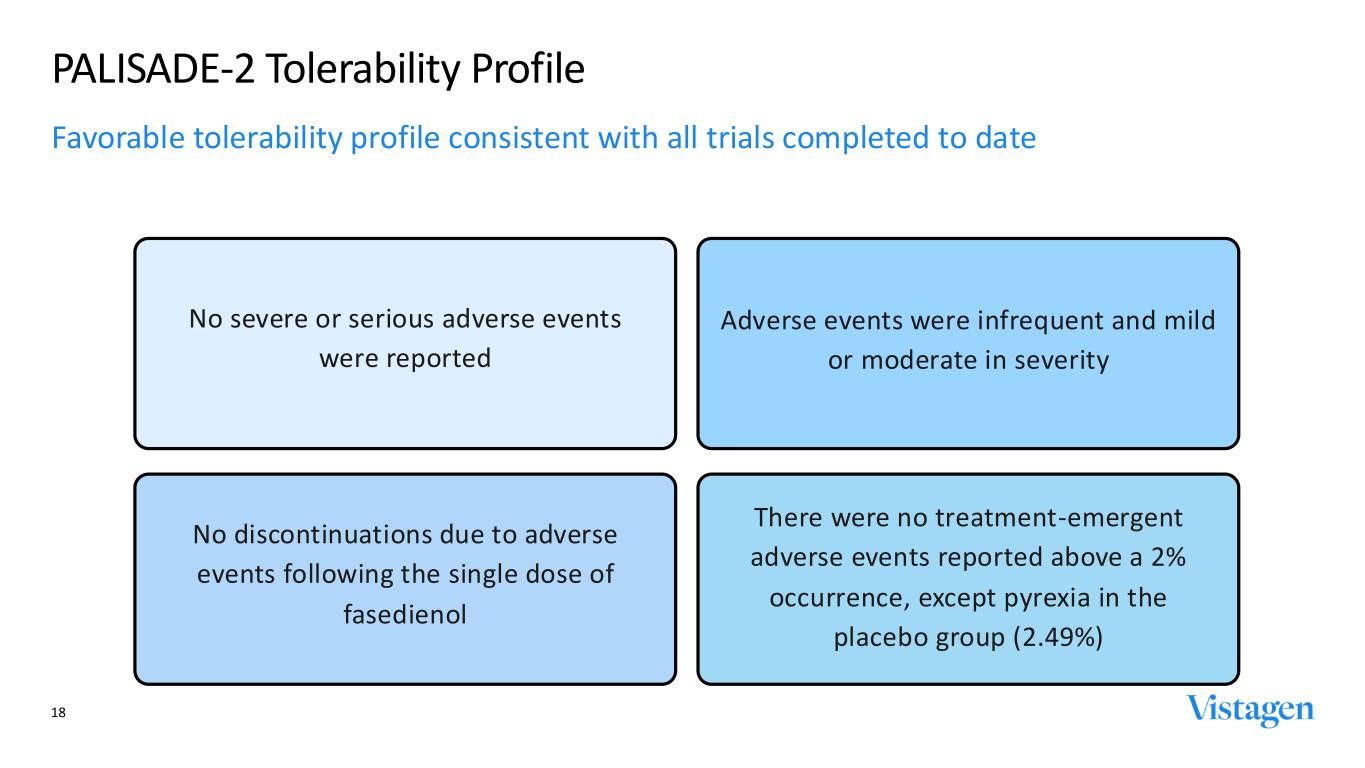
No severe or serious adverse events were reported No discontinuations due to adverse events following the single dose of fasedienol Adverse events were infrequent and mild or moderate in severity There were no treatment-emergent adverse events reported above a 2% occurrence, except pyrexia in the placebo group (2.49%) PALISADE-2 Tolerability Profile Favorable tolerability profile consistent with all trials completed to date 18
19 Fasedienol U.S. Registration-directed Phase 3 Program To complement the positive PALISADE-2 Phase 3 trial, Vistagen is conducting two ongoing PALISADE Phase 3 studies as part of its U.S. registration-directed Phase 3 program for the acute treatment of SAD PALISADE-3 and PALISADE-4 Phase 3 Trials with Open-label Extension (OLE) Design: Phase 3 Acute Treatment Public Speaking Challenge similar to PALISADE-2 Potential OLE: Up to 12 months Target enrollment: Approximately 236 randomized in each study Estimated top-line data readouts: 2025 Vistagen believes either PALISADE-3 or PALISADE-4, if successful, together with PALISADE-2, may establish substantial evidence of the effectiveness of fasedienol in support of a potential U.S. NDA submission to the FDA for the acute treatment of Social Anxiety Disorder

‐ No mask-wearing during the public speaking challenges ‐ Recurring in-person training of clinical site personnel ‐ Expanded subject eligibility review at screening ‐ Increased surveillance by Vistagen clinical site-facing staff, reduced reliance on CRO ‐ Treatment administration by clinical site healthcare provider ‐ No symptoms of Covid or recent nasal swabs 20 PALISADE-3 and PALISADE-4 Study Enhancements Designed to drive high-quality enrollment, increase surveillance of rigorous adherence to the study protocol, and limit variability
Major Depressive Disorder Itruvone
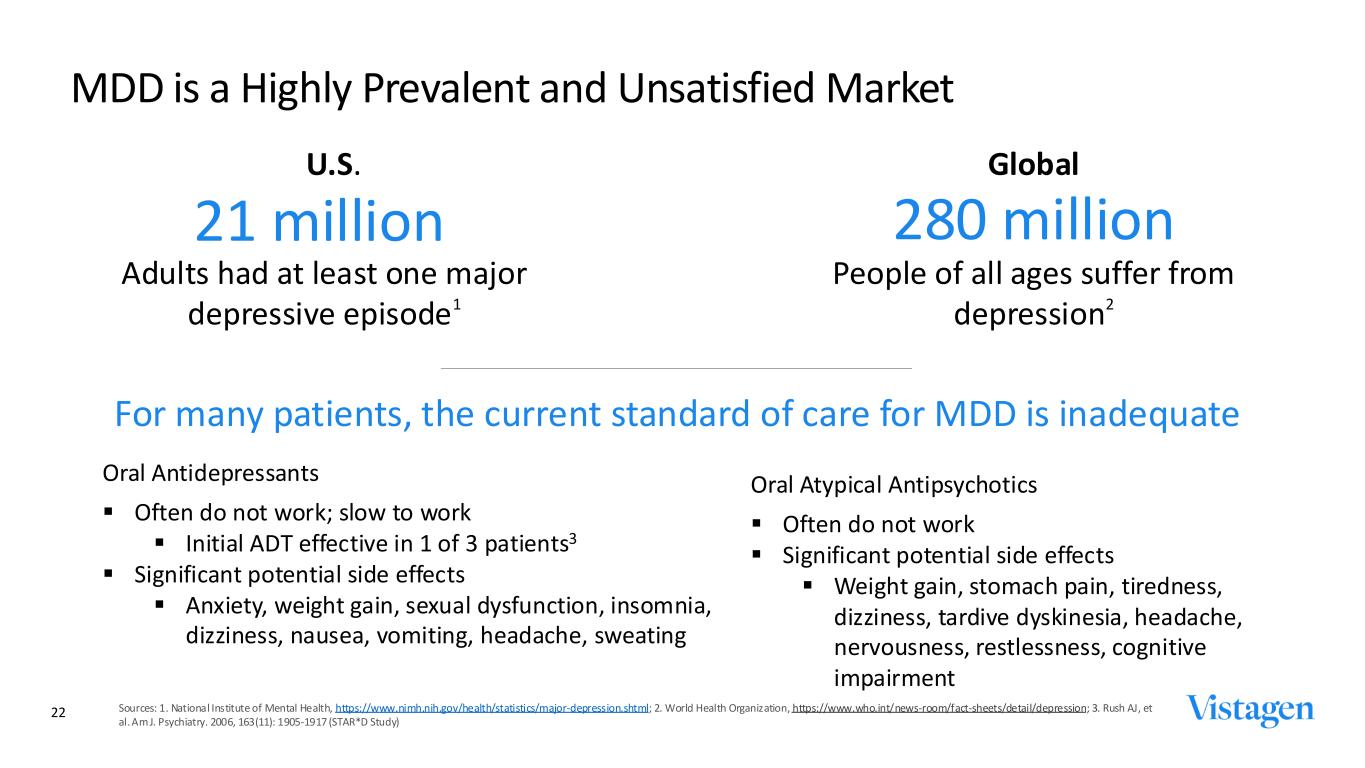
MDD is a Highly Prevalent and Unsatisfied Market Sources: 1. National Institute of Mental Health, https://www.nimh.nih.gov/health/statistics/major-depression.shtml; 2. World Health Organization, https://www.who.int/news-room/fact-sheets/detail/depression; 3. Rush AJ, et al. Am J. Psychiatry. 2006, 163(11): 1905-1917 (STAR*D Study) For many patients, the current standard of care for MDD is inadequate Oral Antidepressants ▪ Often do not work; slow to work ▪ Initial ADT effective in 1 of 3 patients3 ▪ Significant potential side effects ▪ Anxiety, weight gain, sexual dysfunction, insomnia, dizziness, nausea, vomiting, headache, sweating Oral Atypical Antipsychotics ▪ Often do not work ▪ Significant potential side effects ▪ Weight gain, stomach pain, tiredness, dizziness, tardive dyskinesia, headache, nervousness, restlessness, cognitive impairment 21 million 280 million U.S. Global Adults had at least one major depressive episode1 People of all ages suffer from depression2 22
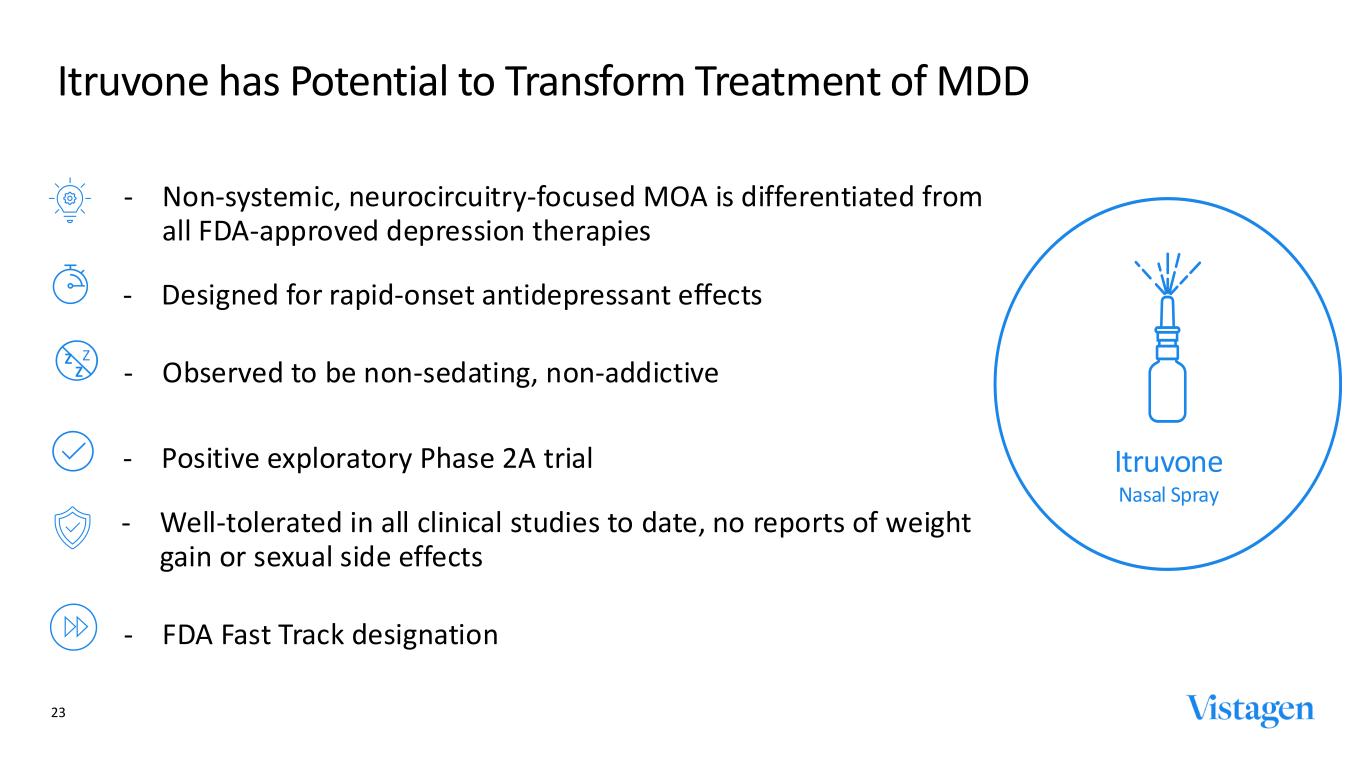
Itruvone has Potential to Transform Treatment of MDD ‐ Non-systemic, neurocircuitry-focused MOA is differentiated from all FDA-approved depression therapies ‐ Positive exploratory Phase 2A trial ‐ Designed for rapid-onset antidepressant effects ‐ Observed to be non-sedating, non-addictive ‐ Well-tolerated in all clinical studies to date, no reports of weight gain or sexual side effects ‐ FDA Fast Track designation Itruvone Nasal Spray Z Z Z 23
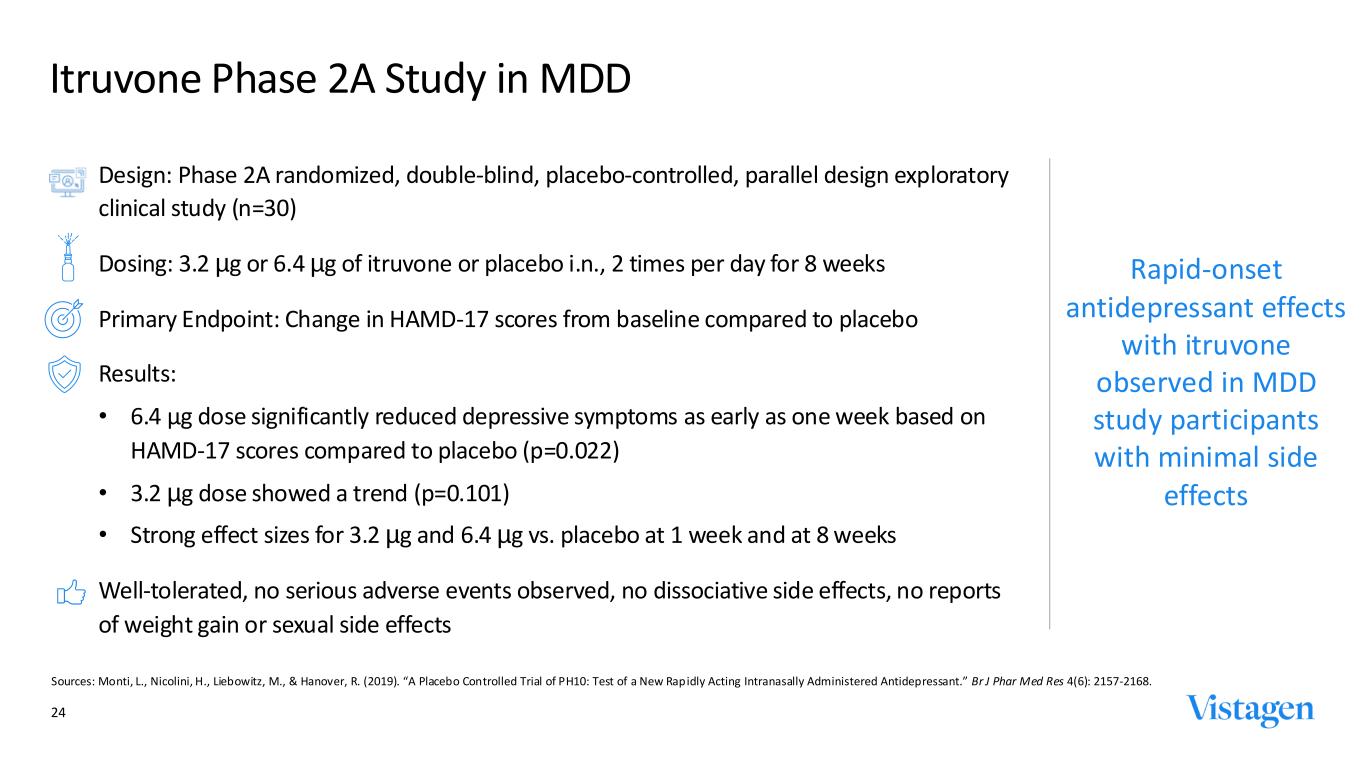
Rapid-onset antidepressant effects with itruvone observed in MDD study participants with minimal side effects Design: Phase 2A randomized, double-blind, placebo-controlled, parallel design exploratory clinical study (n=30) Dosing: 3.2 µg or 6.4 µg of itruvone or placebo i.n., 2 times per day for 8 weeks Primary Endpoint: Change in HAMD-17 scores from baseline compared to placebo Results: • 6.4 µg dose significantly reduced depressive symptoms as early as one week based on HAMD-17 scores compared to placebo (p=0.022) • 3.2 µg dose showed a trend (p=0.101) • Strong effect sizes for 3.2 µg and 6.4 µg vs. placebo at 1 week and at 8 weeks Well-tolerated, no serious adverse events observed, no dissociative side effects, no reports of weight gain or sexual side effects 24 Itruvone Phase 2A Study in MDD Sources: Monti, L., Nicolini, H., Liebowitz, M., & Hanover, R. (2019). “A Placebo Controlled Trial of PH10: Test of a New Rap idly Acting Intranasally Administered Antidepressant.” Br J Phar Med Res 4(6): 2157-2168.
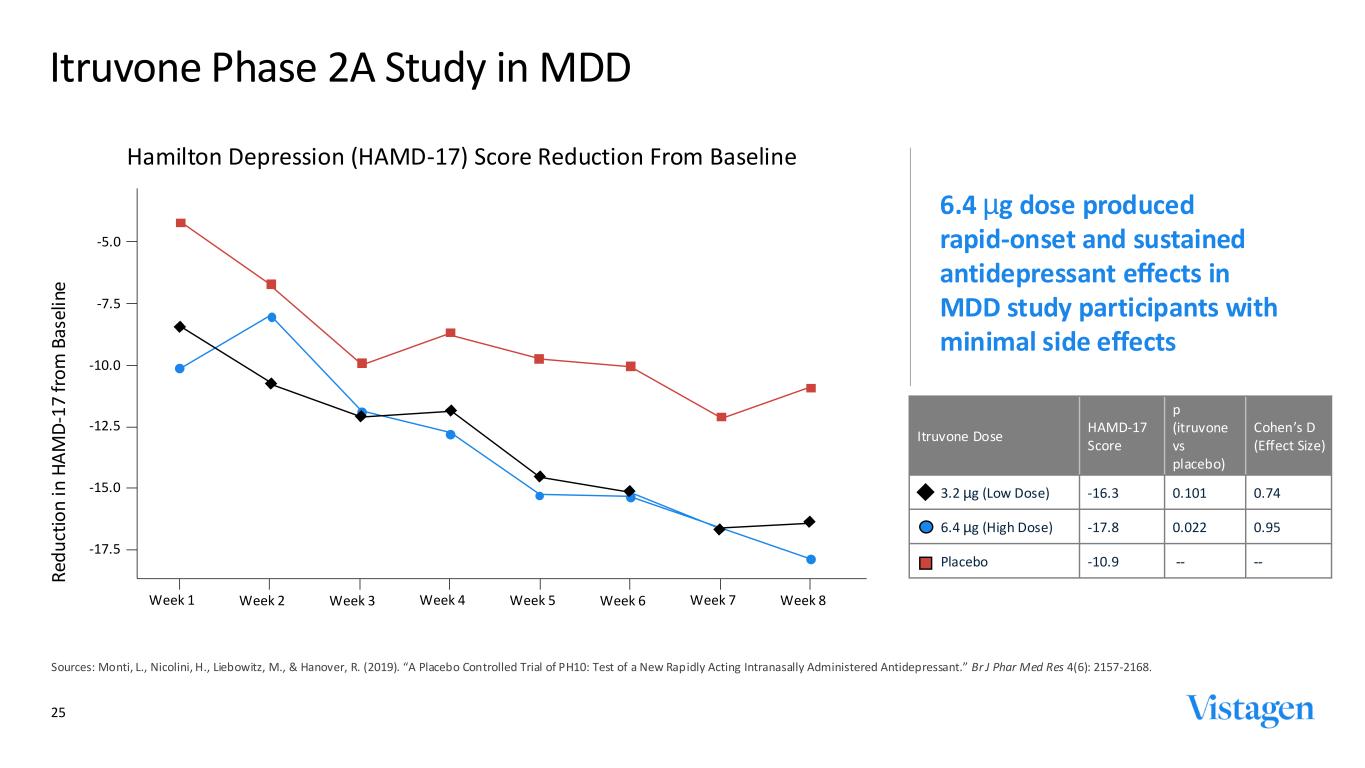
25 Itruvone Phase 2A Study in MDD Sources: Monti, L., Nicolini, H., Liebowitz, M., & Hanover, R. (2019). “A Placebo Controlled Trial of PH10: Test of a New Rap idly Acting Intranasally Administered Antidepressant.” Br J Phar Med Res 4(6): 2157-2168. Hamilton Depression (HAMD-17) Score Reduction From Baseline Itruvone Dose HAMD-17 Score p (itruvone vs placebo) Cohen’s D (Effect Size) 3.2 µg (Low Dose) -16.3 0.101 0.74 6.4 µg (High Dose) -17.8 0.022 0.95 Placebo -10.9 -- -- -5.0 -7.5 -10.0 -12.5 -15.0 -17.5 Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Week 7 Week 8 R ed u ct io n in H A M D -1 7 fr o m B as el in e 6.4 µg dose produced rapid-onset and sustained antidepressant effects in MDD study participants with minimal side effects

26 Itruvone Phase 2B Clinical Plan* Planning and preparation for Phase 2B development of itruvone as a non-systemic monotherapy for MDD is underway ‐ Potential Design: U.S. randomized, double-blind, placebo-controlled, parallel study in male and female subjects (18 to 65 years old) with a confirmed diagnosis of moderate to severe MDD ‐ Outpatient self-administration of 6.4 µg (3.2 µg twice daily) itruvone nasal spray over a 6- week period ‐ Potential Primary Efficacy Endpoint: Change from Baseline to Day 42 in the HAMD-17 Rating Scale *Potential initiation of this Phase 2B study is subject to FDA feedback and strategic considerations
Vasomotor Symptoms (Hot Flashes) due to Menopause PH80
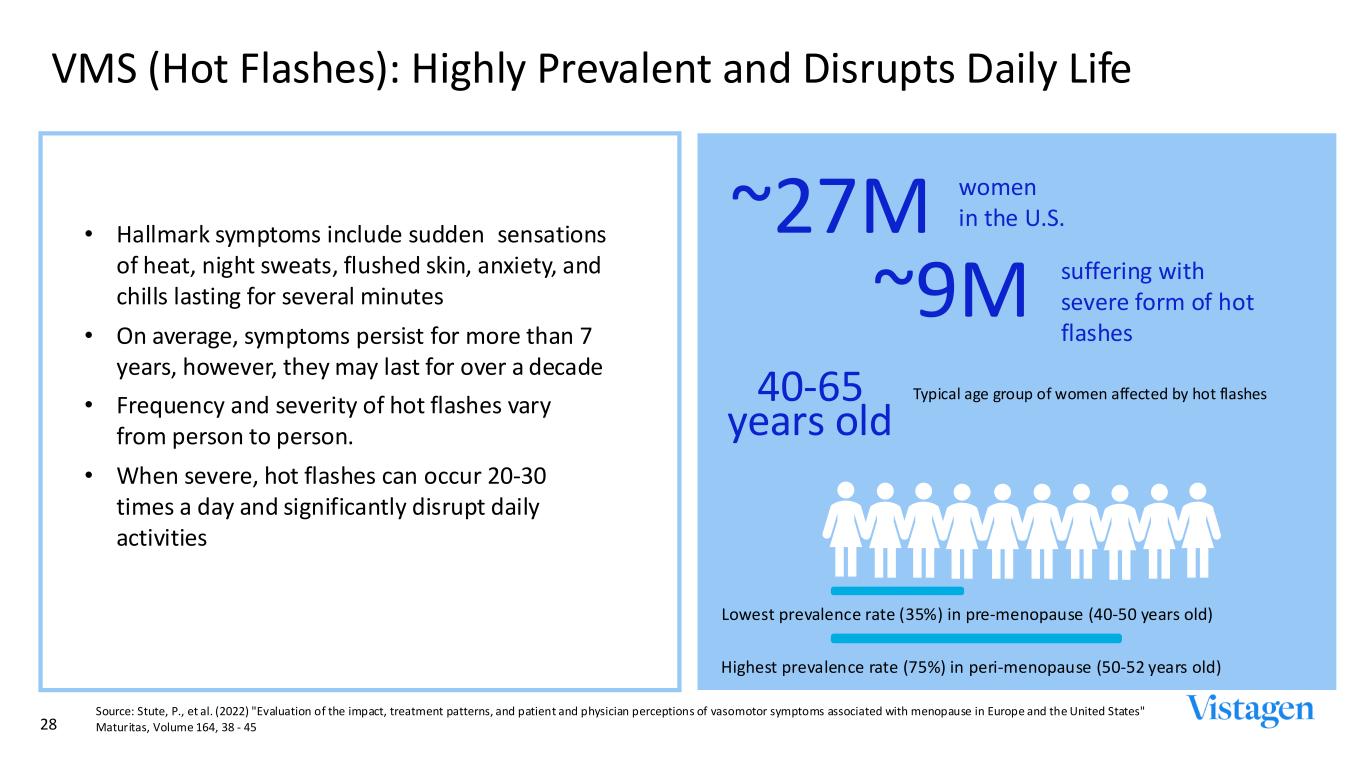
VMS (Hot Flashes): Highly Prevalent and Disrupts Daily Life 28 • Hallmark symptoms include sudden sensations of heat, night sweats, flushed skin, anxiety, and chills lasting for several minutes • On average, symptoms persist for more than 7 years, however, they may last for over a decade • Frequency and severity of hot flashes vary from person to person. • When severe, hot flashes can occur 20-30 times a day and significantly disrupt daily activities Highest prevalence rate (75%) in peri-menopause (50-52 years old) ~27M women in the U.S. ~9M suffering with severe form of hot flashes Typical age group of women affected by hot flashes40-65 years old Lowest prevalence rate (35%) in pre-menopause (40-50 years old) Source: Stute, P., et al. (2022) "Evaluation of the impact, treatment patterns, and patient and physician perceptions of vasomotor symptoms associated with menopause in Europe and the United States" Maturitas, Volume 164, 38 - 45
PH80 potential to transform treatment of VMS (Hot Flashes) 29 PH80 Nasal Spray ‐ Neurocircuitry-focused MOA differentiated from all approved treatments ‐ Non-hormonal and non-systemic ‐ Rapid-onset potential to be taken as-needed to provide relief in the moment ‐ Potential for differentiated safety and tolerability advantages over currently approved systemic hormonal and NK3 therapies ‐ Positive exploratory Phase 2A study (n=36); IND-enabling program to facilitate further Phase 2 development underway
PH80 Phase 2A Study in Menopausal Hot Flashes 30 Objective: Proof-of-principle evaluation of PH80 efficacy and tolerability for the management of vasomotor symptoms (hot flashes) due to menopause Study Details: Randomized, double-blind, placebo-controlled, Phase 2A study. Participants self- administered PH80 (3.2 µg/dose) or placebo for 4 weeks up to 4 times daily with a dose at night if needed (up to 16 µg/day). Participants were followed up weekly during the treatment period Outcome Measures: Daily ratings of the Number, Severity, Disruption in function (Bother), and Sweating associated with daily hot flashes, PGI-C, CGI-I, Safety, and Tolerability Results: PH80 showed statistically and clinically significant improvement vs. placebo in the number and severity of hot flashes while also significantly reducing participant-reported disruption in function and sweating associated with hot flashes Participants: Menopausal women aged 45-60 (n=36) with ≥ 8 hot flashes of moderate to severe intensity per day on average for 1 week (≈ 56/week)
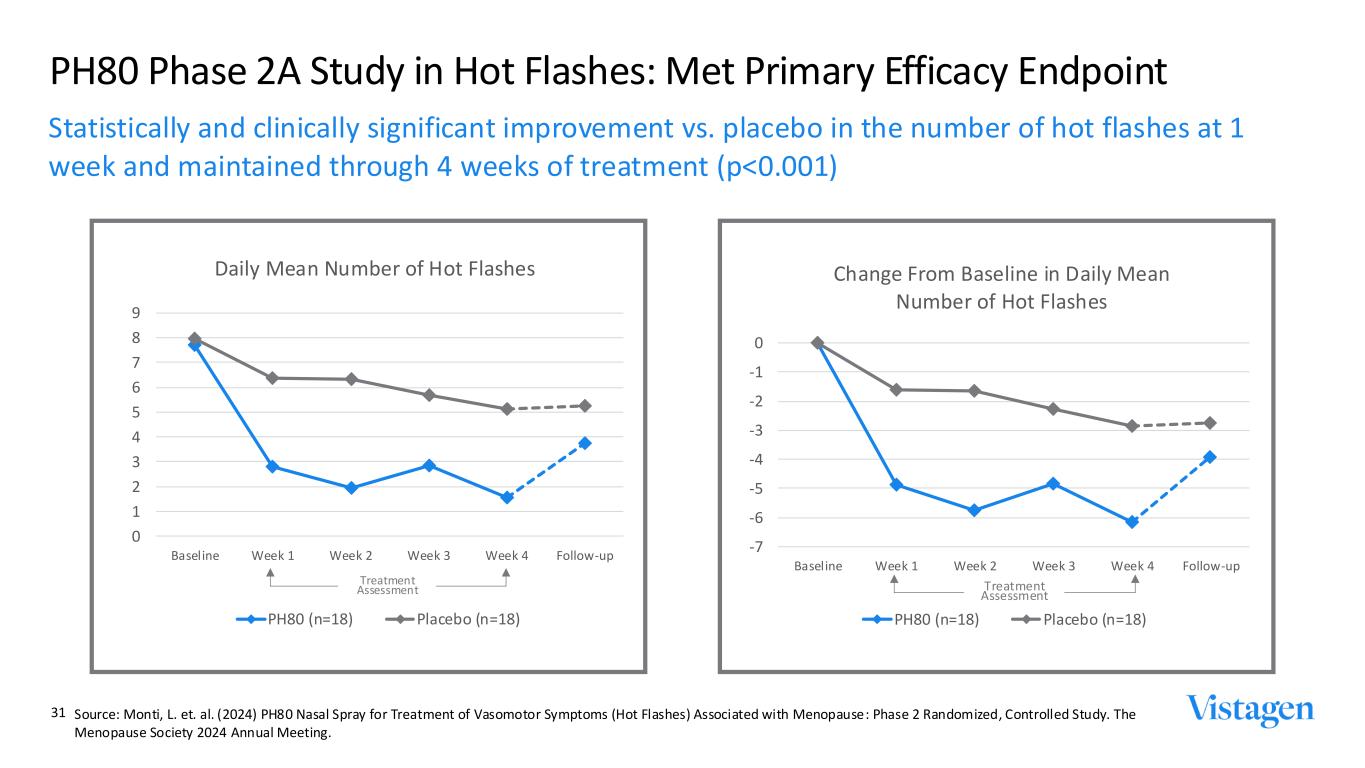
PH80 Phase 2A Study in Hot Flashes: Met Primary Efficacy Endpoint Statistically and clinically significant improvement vs. placebo in the number of hot flashes at 1 week and maintained through 4 weeks of treatment (p<0.001) 0 1 2 3 4 5 6 7 8 9 Baseline Week 1 Week 2 Week 3 Week 4 Follow-up Daily Mean Number of Hot Flashes PH80 (n=18) Placebo (n=18) -7 -6 -5 -4 -3 -2 -1 0 Baseline Week 1 Week 2 Week 3 Week 4 Follow-up Change From Baseline in Daily Mean Number of Hot Flashes PH80 (n=18) Placebo (n=18) Treatment Assessment Treatment Assessment 31 Source: Monti, L. et. al. (2024) PH80 Nasal Spray for Treatment of Vasomotor Symptoms (Hot Flashes) Associated with Menopause: Phase 2 Randomized, Controlled Study. The Menopause Society 2024 Annual Meeting.
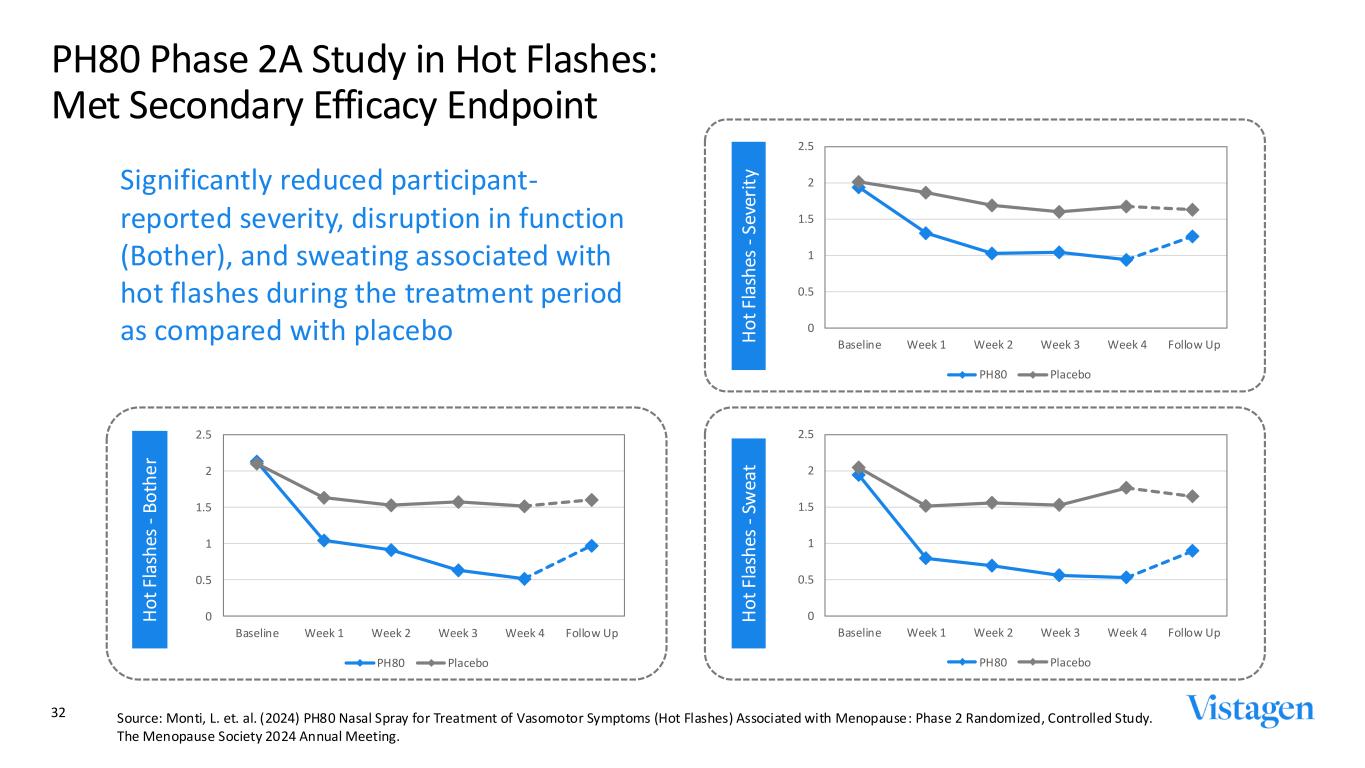
PH80 Phase 2A Study in Hot Flashes: Met Secondary Efficacy Endpoint H o t Fl as h es - B o th e r H o t Fl as h es - Sw ea t H o t Fl as h es - S ev er it y 0 0.5 1 1.5 2 2.5 Baseline Week 1 Week 2 Week 3 Week 4 Follow Up PH80 Placebo 0 0.5 1 1.5 2 2.5 Baseline Week 1 Week 2 Week 3 Week 4 Follow Up PH80 Placebo 0 0.5 1 1.5 2 2.5 Baseline Week 1 Week 2 Week 3 Week 4 Follow Up PH80 Placebo Significantly reduced participant- reported severity, disruption in function (Bother), and sweating associated with hot flashes during the treatment period as compared with placebo 32 Source: Monti, L. et. al. (2024) PH80 Nasal Spray for Treatment of Vasomotor Symptoms (Hot Flashes) Associated with Menopause: Phase 2 Randomized, Controlled Study. The Menopause Society 2024 Annual Meeting.

33 Distinguished Clinical and Regulatory Advisors Maurizio Fava, M.D. Professor of Psychiatry, Harvard Medical School; Director, Division of Clinical Research, Massachusetts General Hospital (MGH) Research Institute; and Executive Vice Chair of the Department of Psychiatry Sanjay Mathew, M.D. Vice Chair for Research and Professor of Psychiatry and Behavioral Sciences at Baylor College of Medicine; Staff Psychiatrist at the Michael E. DeBakey VA Medical Center Thomas Laughren, M.D. Director (retired), U.S. Food and Drug Administration (FDA) Division of Psychiatry Products, Office of New Drugs, Center for Drug Evaluation and Research (CDER) Gerard Sanacora, Ph.D., M.D. Professor of Psychiatry, Yale School of Medicine; Director, Yale Depression Research Program; Co-Director, Yale-New Haven Hospital Interventional Psychiatry Service Michael Liebowitz, M.D. Former Columbia University psychiatrist, director and founder of the Anxiety Disorders Clinic at the New York State Psychiatric Institute; current Managing Director of The Medical Research Network LLC Mark Wallace, M.D. Professor of Clinical Anesthesiology, Chair of the Division of Pain Medicine, Medical Director and Director at the University of California, San Diego VistaGen: A VISIONARY APPROACH TO MENTAL HEALTH CARE Representing premier institutions and deep neuroscience and regulatory expertise

Nasdaq: VTGN Thank you
EXHIBIT 99.2
Vistagen to Participate in Stifel 2025 Virtual CNS Forum
SOUTH SAN FRANCISCO, Calif, March 4, 2025 - Vistagen (Nasdaq: VTGN), a clinical-stage biopharmaceutical company pioneering neuroscience with nose-to-brain neurocircuitry to develop and commercialize a new class of intranasal product candidates called pherines, today announced that management will participate in Stifel’s 2025 Virtual CNS Forum.
Vistagen’s President and Chief Executive Officer, Shawn Singh, will participate in a fireside chat presentation on Tuesday, March 18, 2025, at 12 p.m. Eastern Time. A live webcast will be accessible through the “Events” page in the “Investors” section of the Company’s website at www.Vistagen.com. A replay of the webcast will be archived and available following the event.
About Vistagen
Headquartered in South San Francisco, CA, Vistagen (Nasdaq: VTGN) is a clinical-stage biopharmaceutical company leveraging a deep understanding of nose-to-brain neurocircuitry to develop and commercialize a broad and diverse pipeline of clinical-stage product candidates from a new class of intranasal therapies called pherines.
Pherines specifically and selectively bind to peripheral receptors in human nasal chemosensory neurons, which activate olfactory bulb-to-brain neurocircuits without requiring systemic absorption or uptake into the brain to achieve desired therapeutic benefits and differentiated safety. Vistagen’s neuroscience pipeline also includes an oral prodrug with potential to impact certain neurological conditions involving the NMDA receptor. Vistagen is passionate about developing transformative treatment options to improve the lives of individuals underserved by the current standard of care for multiple highly prevalent indications, including social anxiety disorder, major depressive disorder, and vasomotor symptoms (hot flashes) associated with menopause. Connect at www.Vistagen.com.
Investor Inquiries:
Mark A. McPartland
markmcp@vistagen.com
Media Inquiries:
Michelle P. Wellington
mwellington@vistagen.com
v3.25.0.1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14a
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
{
"version": "2.2",
"instance": {
"vtgn-20250303.htm": {
"nsprefix": "vtgn",
"nsuri": "http://www.vistagen.com/20250303",
"dts": {
"inline": {
"local": [
"vtgn-20250303.htm"
]
},
"schema": {
"local": [
"vtgn-20250303.xsd"
],
"remote": [
"http://www.xbrl.org/2003/xbrl-instance-2003-12-31.xsd",
"http://www.xbrl.org/2003/xbrl-linkbase-2003-12-31.xsd",
"http://www.xbrl.org/2003/xl-2003-12-31.xsd",
"http://www.xbrl.org/2003/xlink-2003-12-31.xsd",
"http://www.xbrl.org/2005/xbrldt-2005.xsd",
"http://www.xbrl.org/lrr/role/negated-2009-12-16.xsd",
"http://www.xbrl.org/lrr/role/net-2009-12-16.xsd",
"https://www.xbrl.org/dtr/type/2022-03-31/types.xsd",
"https://xbrl.sec.gov/dei/2024/dei-2024.xsd"
]
},
"labelLink": {
"local": [
"vtgn-20250303_lab.xml"
]
},
"presentationLink": {
"local": [
"vtgn-20250303_pre.xml"
]
}
},
"keyStandard": 22,
"keyCustom": 0,
"axisStandard": 0,
"axisCustom": 0,
"memberStandard": 0,
"memberCustom": 0,
"hidden": {
"total": 2,
"http://xbrl.sec.gov/dei/2024": 2
},
"contextCount": 1,
"entityCount": 1,
"segmentCount": 0,
"elementCount": 23,
"unitCount": 0,
"baseTaxonomies": {
"http://xbrl.sec.gov/dei/2024": 22
},
"report": {
"R1": {
"role": "http://www.vistagen.com/role/Cover",
"longName": "0000001 - Document - Cover",
"shortName": "Cover",
"isDefault": "true",
"groupType": "document",
"subGroupType": "",
"menuCat": "Cover",
"order": "1",
"firstAnchor": {
"contextRef": "c-1",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "vtgn-20250303.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "vtgn-20250303.htm",
"first": true,
"unique": true
}
}
},
"tag": {
"dei_AmendmentFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "AmendmentFlag",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Amendment Flag",
"label": "Amendment Flag",
"documentation": "Boolean flag that is true when the XBRL content amends previously-filed or accepted submission."
}
}
},
"auth_ref": []
},
"dei_CityAreaCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "CityAreaCode",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "City Area Code",
"label": "City Area Code",
"documentation": "Area code of city"
}
}
},
"auth_ref": []
},
"dei_CoverAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "CoverAbstract",
"lang": {
"en-us": {
"role": {
"label": "Cover [Abstract]",
"documentation": "Cover page."
}
}
},
"auth_ref": []
},
"dei_DocumentPeriodEndDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentPeriodEndDate",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Period End Date",
"label": "Document Period End Date",
"documentation": "For the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD."
}
}
},
"auth_ref": []
},
"dei_DocumentType": {
"xbrltype": "submissionTypeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentType",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Type",
"label": "Document Type",
"documentation": "The type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'."
}
}
},
"auth_ref": []
},
"dei_EntityAddressAddressLine1": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressAddressLine1",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, Address Line One",
"label": "Entity Address, Address Line One",
"documentation": "Address Line 1 such as Attn, Building Name, Street Name"
}
}
},
"auth_ref": []
},
"dei_EntityAddressCityOrTown": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressCityOrTown",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, City or Town",
"label": "Entity Address, City or Town",
"documentation": "Name of the City or Town"
}
}
},
"auth_ref": []
},
"dei_EntityAddressPostalZipCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressPostalZipCode",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, Postal Zip Code",
"label": "Entity Address, Postal Zip Code",
"documentation": "Code for the postal or zip code"
}
}
},
"auth_ref": []
},
"dei_EntityAddressStateOrProvince": {
"xbrltype": "stateOrProvinceItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressStateOrProvince",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, State or Province",
"label": "Entity Address, State or Province",
"documentation": "Name of the state or province."
}
}
},
"auth_ref": []
},
"dei_EntityCentralIndexKey": {
"xbrltype": "centralIndexKeyItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityCentralIndexKey",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Central Index Key",
"label": "Entity Central Index Key",
"documentation": "A unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK."
}
}
},
"auth_ref": [
"r1"
]
},
"dei_EntityEmergingGrowthCompany": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityEmergingGrowthCompany",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Emerging Growth Company",
"label": "Entity Emerging Growth Company",
"documentation": "Indicate if registrant meets the emerging growth company criteria."
}
}
},
"auth_ref": [
"r1"
]
},
"dei_EntityFileNumber": {
"xbrltype": "fileNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityFileNumber",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity File Number",
"label": "Entity File Number",
"documentation": "Commission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen."
}
}
},
"auth_ref": []
},
"dei_EntityIncorporationStateCountryCode": {
"xbrltype": "edgarStateCountryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityIncorporationStateCountryCode",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Incorporation, State or Country Code",
"label": "Entity Incorporation, State or Country Code",
"documentation": "Two-character EDGAR code representing the state or country of incorporation."
}
}
},
"auth_ref": []
},
"dei_EntityRegistrantName": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityRegistrantName",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Registrant Name",
"label": "Entity Registrant Name",
"documentation": "The exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC."
}
}
},
"auth_ref": [
"r1"
]
},
"dei_EntityTaxIdentificationNumber": {
"xbrltype": "employerIdItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityTaxIdentificationNumber",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Tax Identification Number",
"label": "Entity Tax Identification Number",
"documentation": "The Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS."
}
}
},
"auth_ref": [
"r1"
]
},
"dei_LocalPhoneNumber": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "LocalPhoneNumber",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Local Phone Number",
"label": "Local Phone Number",
"documentation": "Local phone number for entity."
}
}
},
"auth_ref": []
},
"dei_PreCommencementIssuerTenderOffer": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "PreCommencementIssuerTenderOffer",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Pre-commencement Issuer Tender Offer",
"label": "Pre-commencement Issuer Tender Offer",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act."
}
}
},
"auth_ref": [
"r3"
]
},
"dei_PreCommencementTenderOffer": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "PreCommencementTenderOffer",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Pre-commencement Tender Offer",
"label": "Pre-commencement Tender Offer",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act."
}
}
},
"auth_ref": [
"r5"
]
},
"dei_Security12bTitle": {
"xbrltype": "securityTitleItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "Security12bTitle",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Title of 12(b) Security",
"label": "Title of 12(b) Security",
"documentation": "Title of a 12(b) registered security."
}
}
},
"auth_ref": [
"r0"
]
},
"dei_SecurityExchangeName": {
"xbrltype": "edgarExchangeCodeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "SecurityExchangeName",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Security Exchange Name",
"label": "Security Exchange Name",
"documentation": "Name of the Exchange on which a security is registered."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_SolicitingMaterial": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "SolicitingMaterial",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Soliciting Material",
"label": "Soliciting Material",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act."
}
}
},
"auth_ref": [
"r4"
]
},
"dei_TradingSymbol": {
"xbrltype": "tradingSymbolItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "TradingSymbol",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Trading Symbol",
"label": "Trading Symbol",
"documentation": "Trading symbol of an instrument as listed on an exchange."
}
}
},
"auth_ref": []
},
"dei_WrittenCommunications": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "WrittenCommunications",
"presentation": [
"http://www.vistagen.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Written Communications",
"label": "Written Communications",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act."
}
}
},
"auth_ref": [
"r6"
]
}
}
}
},
"std_ref": {
"r0": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b"
},
"r1": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b-2"
},
"r2": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "d1-1"
},
"r3": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "13e",
"Subsection": "4c"
},
"r4": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "14a",
"Subsection": "12"
},
"r5": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "14d",
"Subsection": "2b"
},
"r6": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Securities Act",
"Number": "230",
"Section": "425"
}
}
}




































































