
As filed with the Securities and Exchange Commission on April 26, 2022
Registration Statement No. 333-____
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
*HEMOGLOBIN OXYGEN THERAPEUTICS LLC
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 46-5395558 | ||
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
674 Souder Road
Souderton, PA 18964
(267) 382-0064
(Address and telephone number of registrant’s principal executive offices)
Igor Serov
Chief Financial Officer
Hemoglobin Oxygen Therapeutics Inc.
674 Souder Road
Souderton, PA 18964
(267) 382-0064
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
David Ficksman, Esq. William P. Hubbard, Esq. TroyGould PC 1801 Century Park East, 16th Floor Los Angeles, CA 90067 Tel.: (310) 553-4441 |
Thomas J. Poletti, Esq. Veronica Lah, Esq. Manatt, Phelps & Phillips, LLP 695 Town Center Drive, 14th Floor Costa Mesa, CA 92646 Tel.: (714) 371-2500 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | Non-accelerated filer ☒ | Smaller reporting company ☒ | |||
| Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. ☐
* Prior to the closing of the offering to which this registration statement relates, Hemoglobin Oxygen Therapeutics LLC intends to convert into a Delaware corporation pursuant to a statutory conversion, and will change its name to Hemoglobin Oxygen Therapeutics Inc.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
Hemoglobin Oxygen Therapeutics LLC, the registrant whose name appears on the cover of this registration statement, is a Delaware limited liability company. Immediately prior to the closing of the offering to which this Registration Statement relates, Hemoglobin Oxygen Therapeutics LLC will convert into a Delaware corporation pursuant to a statutory conversion and change its name to Hemoglobin Oxygen Therapeutics Inc. as described in the section “Corporate Conversion” of the accompanying prospectus. As a result of the Corporate Conversion, all holders of membership interests and options exercisable for membership interests of Hemoglobin Oxygen Therapeutics LLC will become holders of shares of common stock and options to purchase common stock of Hemoglobin Oxygen Therapeutics Inc.
References in the accompanying prospectus to our capitalization and other matters pertaining to our common equity relate to the capitalization and common equity of Hemoglobin Oxygen Therapeutics Inc. after giving effect to the Corporate Conversion. However, the Financial Statements and summary historical financial data included in the accompanying prospectus are those of Hemoglobin Oxygen Therapeutics LLC and do not give effect to the Corporate Conversion. Shares of common stock of Hemoglobin Oxygen Therapeutics Inc. are being offered by this prospectus. The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell, nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| 2 |
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell, nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated April 26, 2022
PRELIMINARY PROSPECTUS
___________Shares of Common Stock
HEMOGLOBIN OXYGEN THERAPEUTICS LLC
Common Stock
|
This is an initial public offering of shares of our Common Stock. Prior to this offering, there has been no public market for our Common Stock. It is currently estimated that the initial public offering price per share will be between $___ and $___. We have applied for listing of our common stock on The Nasdaq Capital Market.
The actual public offering price per share will be determined between us and the underwriters at the time of pricing and may be at a discount to the current market price. Therefore, the assumed public offering price used throughout this prospectus may not be indicative of the final offering price.
Investing in our securities involves risks. See “Risk Factors” beginning on page 14.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Price to the public | $ | $ | ||||||
| Underwriting discounts and commissions | $ | $ | ||||||
| Proceeds to us (before expenses)(1) | $ | $ | ||||||
| (1) | Does not include a non-accountable expense allowance equal to 1% of the gross proceeds of this offering payable to the underwriters, or the reimbursement of certain expenses of the underwriters. We refer you to “Underwriting” beginning on page 103 of this prospectus for additional information regarding underwriting compensation. |
We have granted the underwriters the option for a period of 45 days to purchase up to an additional ___ shares of common stock at the initial public offering price, less underwriting discounts and commissions, solely to cover over-allotments, if any.
The underwriter expects to deliver the shares on or about _____, 2022.
WestPark Capital, Inc.
Prospectus dated _______, 2022
| 3 |
TABLE OF CONTENTS
We have not, and the underwriters have not, authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We take no responsibility for and can provide no assurance as to the reliability of, any other information that others may give to you.
You should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. The selling stockholders are offering to sell and seeking offers to buy our common stock only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of these securities.
All trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the ® and TM symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
| 4 |
The following summary highlights selected information contained elsewhere in this prospectus and is qualified in its entirety by the more detailed information and financial statements included elsewhere in this prospectus. It does not contain all the information that may be important to you and your investment decision. You should carefully read this entire prospectus, including the matters set forth under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our financial statements and related notes included elsewhere in this prospectus. In this prospectus, unless context requires otherwise, references to “we,” “us,” “our,” “HBO2 Therapeutics” or the “Company” refer prior to the Corporate Conversion discussed herein, to Hemoglobin Oxygen Therapeutics LLC, and after the Corporate Conversion, to Hemoglobin Oxygen Therapeutics Inc. References in this prospectus to our capitalization and other matters pertaining to our common equity relate to the capitalization and common equity of Hemoglobin Oxygen Therapeutics Inc. after giving effect to the Corporate Conversion. However, the Financial Statements and summary historical financial data included in this prospectus are those of Hemoglobin Oxygen Therapeutics LLC and do not give effect to the Corporate Conversion. The terms (A) “IPO Price” means the initial public offering price of the shares offered by means of this prospectus and (B) “Debt Conversion” refer to the conversion of the following principal amount of convertible indebtedness, plus interest accrued and unpaid interest where indicated below, immediately prior to the effective date of this offering assuming an IPO Price of $____per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (i) $7,917,000 convertible into an estimated _____shares of common stock and (ii) $2,000,000 due on demand bearing no interest convertible into an estimated _____shares of common stock (a ___35% discount to the IPO Price). (iii) $486,000 due on demand bearing no interest convertible into an estimated _____shares of common stock (a 25% discount to the IPO Price).Unless otherwise indicated, all share and per share information gives effect to the “Debt Conversion”.
Company Overview
Certain Definitions
Throughout this document, the below terms shall have the following meanings:
| ● | “Indication” means a specific use of a product, such as the treatment of a specific condition or use in a specific procedure. | |
| ● | “Our product” means any product we have developed that has been approved for certain indications and markets. | |
| ● | “Product candidate” means any of our products that are under development, either for an initial approval, or development of an existing product for a new indication. |
Description of Our Operations, Principal Activities and Key Factors
We are a biotechnology company focused on the development, manufacture, and commercialization of a proprietary technology platform for oxygen-carrying solutions that address critical unmet medical needs in both human and veterinary medicine. Using our patents, proprietary technology and know-how, we develop and sell products that, under certain conditions, are a possible alternative to blood transfusions to deliver oxygen to organs and tissues. Through numerous clinical studies, our products have been shown to address many of the current limitations that exist for blood transfusion. These include the limited shelf life of blood, logistical challenges of typing/cross-matching, delays in testing for pathogens, and blood shortages during times of high need and/or insufficient donations. We have significant experience gained through extensive pre-clinical and clinical testing of these products, which have a well-understood and extensively documented safety and efficacy profile.
| 5 |
Management believes that our products are the only hemoglobin-based oxygen carriers (HBOCs) which have received marketing authorizations anywhere in the world. These products were acquired from OPK Biotech LLC in 2014, which had previously purchased them from Biopure Corporation in 2009. Our current products are:
| ● | Hemopure ® (hemoglobin glutamer -250 (bovine); HBOC-201) – for human use; and | |
| ● | Oxyglobin® (hemoglobin glutamer -200 (bovine) HBOC-301) - for veterinary use. |
Hemopure and Oxyglobin are sterile medicinal products administered via intravenous or arterial infusion that deliver oxygen to organs and tissues that could provide a potential alternative to blood transfusion. Our sterile veterinary and human products have been widely investigated in pre-clinical models, clinical trials and real-world clinical experience for more than 20 years and described in at least 250 peer-reviewed publications in the scientific literature and reference texts.
Hemopure, our human product, can address some of the logistical limitations of blood transfusions. The potential benefits are a reduction in both mortality and morbidity while offering several strategic advantages, such as universal compatibility, room temperature storage, off-the-shelf availability, readiness for use (supplied in sterile IV infusion bags) and a three-year shelf life. Clinical data suggests that Hemopure could provide a life-saving option for patients. In markets where Hemopure is not yet authorized or approved, approval(s) for Hemopure would be pursued for an indication when suitable blood transfusion is indicated, but not readily available, or an option.
Oxyglobin is the only oxygen-carrying solution approved by the US Food and Drug Administration (FDA), the European Medicines Agency (“EMA”) and the British Veterinary Medicines Directorate (“VMD”) to treat all-cause canine anemia.
We are also pursuing a medical device indication for Hemopure under the name ZK1. ZK1 is intended to act as an additive oxygen carrier solution during ex-vivo, sub-sub normothermic and normothermic machine perfusion of organs to recondition and assess organ viability prior to transplantation.
Product Portfolio Characteristics
The key characteristics of our product portfolio are:
| ● | Sterile, ultra-purified, oxygen-carrying solutions for intravenous or intra-arterial infusion, for the purpose of increasing total Hb concentration | |
| ● | No type and crossmatch required, and compatible with all blood types | |
| ● | Ready to use, requiring no reconstitution | |
| ● | Stable for at least three years at 2° to 30° Celsius | |
| ● | No refrigeration required | |
| ● | Carry oxygen at low perfusion pressure through constricted or partially blocked blood vessels to areas of the body that allogenic red blood cells (RBCs) cannot reach due to their larger size |
Markets
Our immediate focus is to relaunch Hemopure and Oxyglobin under approved indications in those jurisdictions where they already have marketing authorizations. The clinical development of ZK1 and Hemopure product candidates will continue in parallel, targeting markets for future approvals.
| 6 |
Human Transfusion Market
Hemopure is approved in South Africa to eliminate, delay, or reduce the need for RBC s in adult surgical patients. Hemopure was also approved in the Russian Federation for the treatment of acute, all-cause anemia. When the situation normalizes in that country and sanctions are lifted, we will evaluate reactivating the license and conclude a distribution agreement.
In the United States, Hemopure is under clinical development for the treatment of life-threatening anemia when blood transfusion is unavailable, or otherwise indicated but is not an option.
Human Organ Perfusion System Market
Clinical development of ZK1 for use in sub-normothermic and normothermic organ perfusion, without the use of traditional blood products is ongoing. Under this application ZK1 would not be administered to the patient, but instead used outside the body, as an additive to perfusion solutions for the ex-vivo machine perfusion of donor organs prior to transplantation. As an ancillary medicinal product used outside the body as one component of a machine perfusion system, ZK1 would be evaluated under a medical device application.
Multiple scientific journal articles describing the use of ZK1 as an adjunct to ex-vivo machine perfusion of donor organs prior to transplantation have generated considerable interest amongst the organ transplantation community resulting in preclinical and clinical research, to evaluate donor organ resuscitation and viability at several academic hospitals in the US and Europe.
Veterinary Market
Oxyglobin is the trademark name for our veterinary drug, the only oxygen carrying solution to be approved in the United States, the European Union and the United Kingdom to treat all cause anemia in dogs. Oxyglobin has additionally been used by veterinarians in Canada through Health Canada’s Emergency Drug Release (EDR) program. We plan to distribute Oxyglobin in North America through an exclusive distribution agreement with Dechra Veterinary Products, a global veterinary pharmaceutical company, and in Europe through regional distributors specializing in the veterinary critical care market.
Under the US Department of Defense Joint Theater Trauma System (“JTTS”) Clinical Practice Guideline for emergency treatment of military working dogs (“MWD”) that sustained combat related injuries, Oxyglobin was used as a resuscitative fluid to stabilize military working dogs (“MWD”) after combat-related injuries, in preparation for removal by MEDEVAC transport to veterinarians. The guideline was first published in April 2011 for non-veterinarians (i.e., human trauma surgeons and medics).
Veterinarians have limited treatment options when it comes to treating anemia in other species, as finding a healthy same species blood donor is not always practical. Oxyglobin’s long shelf life, and the ability to provide oxygen carrying capacity while stabilizing a patient in order to treat the underlying condition, led to practitioners using the product off-label in a variety of different species. The off-label use of Oxyglobin has been extensively published in peer reviewed literature, and veterinary reference texts. Examples of other species in which Oxyglobin has been used are feline, equine, avian, and exotics. Though still less common, avian and exotic pets are becoming increasingly popular companion animal choices, and therefore require veterinary care. Once Oxyglobin is relaunched in the canine market, we will seek to expand its use, to treat non-domestic animals in captivity, such as those zoos, aquariums or wild animal sanctuaries. This would involve filing a request for MUMS (Minor Use and Minor Species) drug designation. This permits the use of a drug in minor species, or for use in major species afflicted with uncommon diseases or conditions (minor uses).
| 7 |
Risks Associated with Our Business
Our business is subject to a number of risks of which you should be aware of before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. Primarily, our manufacturing facility is under construction to accommodate the additional process steps resulting from consolidation of the two other facilities. In order to do so, we must refit our newly constructed facility in Souderton, Pennsylvania with the equipment necessary for manufacturing and packaging, which has been relocated from facilities in Cambridge, Massachusetts and Ontario, Canada. Our ability to initially start manufacturing our products and generating revenues is substantially dependent upon the successful completion of the construction and re-fitting of our Souderton facility. Until it is complete, and the relevant cGMP certification is received, we will not be able release and commercially distribute any product. The proceeds of the Offering will be insufficient to fully complete the relaunch of our products into the market. We will require approximately $15 million of additional monies to complete this process. If we do not successfully raise such monies through public or private equity or debt financings or other sources, such as strategic collaborations or license and development agreements, the facility cannot be completed and we will be unable to produce and commercially distribute any product.
In addition to the risks associated with our facility, some of the risks involved with the offering include the following:
| ● | We have incurred substantial losses since our inception and anticipate that we will continue to incur substantial and increasing losses for the foreseeable future. | |
| ● | We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our product development or commercialization efforts. | |
| ● | We currently have very limited revenues. We may never generate significant revenues or achieve profitability. | |
| ● | We expect to continue to incur significant operating and non-operating expenses, which may make it difficult for us to secure sufficient financing. Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements for the year ended December 31, 2021 with respect to this uncertainty. | |
| ● | It is possible that we may face substantial competition caused by others discovering, developing or commercializing products before or more successfully than we do. | |
| ● | If we are unable to successfully develop and commercialize our product candidates or experience significant delays in doing so, our business may be materially harmed. | |
| ● | Our success relies on third-party suppliers and manufacturers. Any failure by such third parties, including, but not limited to, failure to successfully perform and comply with regulatory requirements, could negatively impact our business and our ability to develop and market our product candidate, and our business could be substantially harmed. | |
| ● | Our future success is dependent on the regulatory approval of our product candidates. | |
| ● | Our business may be adversely affected by the ongoing coronavirus pandemic. | |
| ● | Business interruptions could adversely affect future operations, revenues, and financial condition, and may increase our cost of doing business. | |
| ● | We may infringe the intellectual property rights of others, which may prevent or delay our product development efforts. | |
| ● | Our intellectual property may not be sufficient to protect our products from competition. |
| 8 |
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, enacted in April 2012, and we will remain an emerging growth company until the earliest to occur of: the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities held by non-affiliates; the issuance, in any three-year period, by us of more than $1.0 billion in non-convertible debt securities; and the last day of the fiscal year ending after the fifth anniversary of our initial public offering. For so long as we remain an emerging growth company, we are permitted and intend to rely on certain exemptions from various public company reporting requirements, including not being required to have our internal control over financial reporting audited by our independent registered public accounting firm pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and any golden parachute payments not previously approved. In particular, in this prospectus, we have provided only two years of audited financial statements and have not included all of the executive compensation-related information that would be required if we were not an emerging growth company. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of some accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of the extended transition period to comply with new or revised accounting standards and to adopt certain of the reduced disclosure requirements available to emerging growth companies. As a result, we will not be subject to the same implementation timing for new or revised accounting standards as other public companies that are not emerging growth companies which may make comparison of our financial statements to those of other public companies more difficult. As a result of this election, the information that we provide in this prospectus may be different than the information you may receive from other public companies in which you hold equity interests.
We are also a smaller reporting company as defined in the Securities Exchange Act of 1934, as amended. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as (i) the market value of our voting and non-voting common stock held by non-affiliates is less than $250 million measured on the last business day of our second fiscal quarter or (ii) our annual revenue is less than $100 million during the most recently completed fiscal year and the market value of our voting and non-voting common stock held by non-affiliates is less than $700 million measured on the last business day of our second fiscal quarter. Specifically, as a smaller reporting company, we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and have reduced disclosure obligations regarding executive compensation, and, similar to emerging growth companies, if we are a smaller reporting company with less than $100 million in annual revenue, we would not be required to obtain an attestation report on internal control over financial reporting issued by our independent registered public accounting firm.
Corporate Information
We were formed as a Delaware limited liability company in February 2014. Prior to the closing of this offering, Hemoglobin Oxygen Therapeutics LLC intends to convert into a Delaware corporation pursuant to a statutory conversion, and will change its name to Hemoglobin Oxygen Therapeutics Inc. See “Corporate Conversion.”
| 9 |
Hemoglobin Oxygen Therapeutics LLC was organized in February 2014 in connection with the acquisition of OPK Biotech LLC through a Chapter 7 bankruptcy auction. The assets we acquired from OPK Biotech LLC consisted of equipment, intellectual property, finished product inventory and the capital stock of companies incorporated in the Netherlands and South Africa. OPK Biotech had in turn acquired certain assets from Biopure Corporation and intellectual property from Northfield Laboratories. Our principal executive offices are located at 674 Souder Road, Souderton, PA 18964 and our telephone number is (267) 382-0064. Our website address is www.hbo2therapeutics.com. The information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information contained on, or that can be accessed through, our website as part of this prospectus or in deciding whether to invest in our common stock.
Product History
Biopure Corporation developed a veterinary (Oxyglobin) and a human (Hemopure) product and by the end of 1990’s received its veterinary product approval by the FDA (US) and the EMA (EU). The expensive production process, however, did not allow Biopure to sell the veterinary product at a profit and the Company continued its pursuit of human approval. Biopure went public in the early 2000s and traded at a market capitalization in excess of a billion dollars. The total funds spent by Biopure on the product development during its existence was around $900 million.
In the early-2000s, an ill-designed Phase 3 clinical trial protocol followed by several managerial missteps led to difficulties, and Biopure was sold in 2009 to OPK Biotech (beneficial owner Sergey Pugachev). The new owner invested an additional $100 million to improve the production process resulting in significant reductions in the cost of producing the company’s products. In 2011, Mr. Pugachev became involved in litigation with the Russian government which resulted in the expropriation of all his Russian assets. The beneficial owner’s financial difficulties in Russia and inability to financially support the company led to conflict with the management of OPK Biotech, resulting in OPK Biotech management departure from the Company in early 2013. In 2014, creditors of OPK Biotech initiated a Chapter 7 bankruptcy process, which eventually resulted in the seizure of OPK assets and the sale of such assets through an auction.
Hemoglobin Oxygen Therapeutics LLC was formed in 2014 with the sole purpose of buying the assets of OPK Biotech. HbO2 Therapeutics participated in that auction and won the bid. After purchasing those assets in late 2014, HbO2 Therapeutics elected to consolidate three production facilities and house the whole process under one roof in Souderton, PA. Currently all of the equipment is waiting to be assembled in the newly erected building in Souderton, pending the proceeds of this Offering.
| 10 |
THE OFFERING
| Shares offered by us | ___ shares. | |
| Common stock outstanding prior to this offering | ___ shares | |
| Common stock to be outstanding immediately after this offering | _______ shares ( _______shares if the underwriters exercise their over-allotment option in full) | |
| Option to purchase additional shares | The underwriters have an option for a period of 45 days to purchase up to an additional ____shares of our common stock and/or Warrants to purchase up to ___ additional shares of our common stock (equal to 15% of the number of shares of common stock sold in the offering), from us in any combination thereof. | |
| Use of proceeds | We estimate that the net proceeds from this offering will be approximately $___, or approximately $____if the underwriters exercise their over-allotment option in full, at an assumed public offering price of $___ per share, after deducting the underwriting discounts and commissions, the non-accountable expense allowance payable to the underwriters, and estimated offering expenses payable by us. We intend to use approximately $20 million of the net proceeds from this offering toward reassembling our facility in Souderton, Pennsylvania and the balance for general corporate expenses and working capital. See “Use of Proceeds” for a more complete description of the intended use of proceeds from this offering. | |
| Lock-up agreements | Our executive officers, directors and shareholders have agreed with the underwriters not to sell, transfer or dispose of any shares or similar securities for a period of 180 days after the date of this prospectus. For additional information regarding our arrangement with the underwriters, please see “Underwriting.” | |
| Risk factors | See “Risk Factors” on page 14 and other information included in this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. | |
| Proposed Nasdaq Capital Market symbol | HOTI |
The number of shares of common stock outstanding is based on _____shares of our common stock outstanding as of December 31, 2021 after giving effect to (i) the Corporate Conversion to occur immediately prior to the effective date of this offering and (ii) the issuance of _____ shares of common stock issuable upon conversion of convertible and other indebtedness immediately prior to the effective date of this offering assuming an initial public offering price of $____per share, which is the midpoint of the price range set forth on the cover page of this prospectus (the “Debt Conversion”) and excludes as of such date:
| ● | 922,011 and [ ] shares of common stock issuable upon exercise of the outstanding common stock warrant at an exercise price of $2.75 and $[ ] per common share, respectively. | |
| ● | [ ] shares of common stock reserved for future grants pursuant to our 2022 Equity Incentive Plan. | |
| ● | [ ] shares of common stock issuable upon exercise of warrants to be issued to the underwriters as part of this offering at an exercise price of $[ ] per common share (120% of the assumed public offering price of $[ ] per share). |
Except as otherwise indicated herein, all information in this prospectus assumes or gives effect to:
| ● | [ ] shares of common stock issuable upon conversion of our outstanding convertible notes in an aggregate principal amount of $6,930,000. | |
| ● | [ ] shares of common stock issuable upon conversion of debt held by our facilities’ landlord in an aggregate principal amount of $2,000,000 | |
| ● | [ ] shares of common stock issuable upon conversion of debt held by the engineering firm building our facilities, in an aggregate principal amount of $486,000. | |
| ● | The retroactive effect of the conversion of our former limited liability company to a C corporation, to be effected on consummation of the offering. | |
| ● | No exercise by the underwriters of their option to purchase an additional [ ] shares of common stock. |
| 11 |
Financial Statement Presentation
The financial statements for the years ended December 31, 2021 and 2020, represent the operations of Hemoglobin Oxygen Therapeutics LLC. In connection with the closing of this offering, Hemoglobin Oxygen Therapeutics LLC will complete a Corporate Conversion into a Delaware corporation pursuant to a statutory conversion, and will change its name to Hemoglobin Oxygen Therapeutics Inc. All holders of membership interests of Hemoglobin Oxygen Therapeutics LLC will become holders of shares of common stock of Hemoglobin Oxygen Therapeutics Inc., as described under the heading “Corporate Conversion.” In this prospectus, we refer to all transactions related to our conversion to a corporation as the Corporate Conversion. We expect that the Corporate Conversion will not have a material effect on our financial statements.
| 12 |
Selected Summary Consolidated Financial Data
The following tables set forth selected consolidated summary financial data as of the dates and for the periods indicated. We have derived the summary statement of operations data for the years ended December 31, 2021 and 2020 from our audited financial statements included elsewhere in this prospectus. The summary statements of operations data for the years ended December 31, 2021 and 2020 and the summary balance sheet data as of December 31, 2021 have been derived from our audited financial statements included elsewhere in this prospectus. The following summary financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes and other information included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results to be expected in the future.
| Consolidated Statement of Operations Data: | ||||||||
Years Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenues | $ | - | $ | 35,470 | ||||
| Operating expenses | ||||||||
| Research and development | 768,621 | 571,738 | ||||||
| General and administrative | 2,668,311 | 2,673,445 | ||||||
| Total operating expenses | 3,436,932 | 3,245,183 | ||||||
| Loss from operations | (3,436,932 | ) | (3,209,713 | ) | ||||
| Other Income and expense | ||||||||
| Interest expense | (681,518 | ) | (379,862 | ) | ||||
| Gain on paycheck protection program loan forgiveness | 206,200 | - | ||||||
| Loss on extinguishment of debt | (2,235,770 | ) | - | |||||
| Gain on foreign currency translation | - | 1,699 | ||||||
| Gain on change in fair value of warrant liability | 21,782 | 1,862 | ||||||
| Total other expense | (2,689,306 | ) | (376,301 | ) | ||||
| Net loss | $ | (6,126,238 | ) | $ | (3,586,014 | ) | ||
| Loss per unit – basic and diluted | $ | (33.16 | ) | $ | (19.65 | ) | ||
| Weighted average units outstanding – basic and diluted | 184,763 | 182,451 | ||||||
| Balance Sheet Data | ||||||||||||
As of December 31, 2021 (Pro Forma and Pro Forma As Adjusted is unaudited) | ||||||||||||
| Actual | Pro Forma 1 | Pro Forma As Adjusted 2, | ||||||||||
| Cash | $ | 443,895 | $ | 443,895 | $ | - | ||||||
| Total assets | $ | 6,034,988 | $ | 6,034,988 | $ | - | ||||||
| Total liabilities | $ | 15,284,136 | $ | 7,159,411 | $ | - | ||||||
| Total members’ deficit | $ | (9,249,148 | ) | $ | - | $ | - | |||||
| Total stockholders’ deficit | $ | - | $ | (1,124,423 | ) | $ | - | |||||
1 As presented in the unaudited pro forma financial information included within this prospectus, pro forma gives effect to (1) the Corporate Conversion; (2) the conversion of the following principal amount of convertible indebtedness, plus interest accrued and unpaid interest where indicated below, immediately prior to the effective date of this offering assuming an IPO Price of $____per share, which is the midpoint of the price range set forth on the cover page of this prospectus, (i) $6,930,000 with maturity dates ranging from July 2022 to September 2025, bearing interest at rates ranging from 5% to 10% per annum convertible into an estimated _____shares of common stock at varying conversion prices from $2.75 to $5.49 and (ii) $2,000,000 due on demand bearing [no] interest convertible into an estimated _____shares of common stock (a 25% discount to the IPO Price). (iii) $486,000 due on demand bearing [no]interest convertible into an estimated _____shares of common stock (a 25% discount to the IPO Price)
2 As will be presented in the section labeled “Capitalization” included within this Prospectus, pro forma as adjusted amounts reflect the sale of shares of our common stock in this offering at the assumed public offering of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each $1.00 increase (decrease) in the assumed public offering price of $ per share , which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash, additional paid-in capital, total stockholders’ (deficit) equity and total capitalization by approximately $ million, assuming that the number of shares by us, as set forth on the cover page of this Prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 1 million shares the number of shares offered by us would increase (decrease) the pro forma as adjusted amount of each of cash, additional paid-in capital, total stockholders’ (deficit) equity and total capitalization by approximately $ million, assuming that the assumed public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and the estimated offering expenses payable by us. Additionally pro forma as adjusted includes $20 million of proceeds from the offering in total assets and total stockholders’ equity (deficit) related to the reassembly of the company’s facility in Souderton utilizing proceeds of this offering.
| 13 |
An investment in our common stock involves a high degree of risk. Before making an investment decision, you should give careful consideration to the following risk factors, in addition to the other information included in this prospectus, including our financial statements and related notes, before deciding whether to invest in shares of our common stock. The occurrence of any of the adverse developments described in the following risk factors could materially and adversely harm our business, financial condition, results of operations or prospects. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to Our Financial Position and Capital Needs
We have incurred significant losses since our inception and anticipate that we will continue to incur losses for the foreseeable future. We have generated very limited revenues since our inception, which may make it difficult for you to assess our future viability.
We are not profitable and have incurred losses in each year since our founding in February 2014, following the acquisition of our assets from OPK Biotech LLC, which in turn acquired them from Biopure Corporation. Our net losses for the years ended December 31, 2021 and 2020 were $6.3 million and $3.6 million, respectively. As of December 31, 2021, we had a members’ deficit of $9.4 million. We have financed our operations primarily through the sale of equity securities and convertible debt. We continue to incur significant expenses related to our ongoing operations and expect to incur losses for the foreseeable future. We anticipate these losses will increase as we:
| ● | build and refit our Souderton facility; | |
| ● | obtain the requisite regulatory approvals for the new facility, which will enable the relaunch of Oxyglobin, and reintroduction of Hemopure in the applicable approved markets; | |
| ● | continue to advance the development and pursue approvals for ZK-1, as well as commercialization of Oxyglobin, Hemopure and ZK1; | |
| ● | expand the Oxyglobin markets and obtain additional regulatory and marketing approvals into new indications and new countries; | |
| ● | continue the development of Oxyglobin and Hemopure for unapproved indications and markets; and | |
| ● | expand our organization to support our research, development and commercialization activities and our operations as a public company. |
We have generated very limited revenues from product sales since we became the successor of Biopure and OPK Biotech, because our manufacturing facility is not currently operational. We may never be able to complete the construction or retrofit of our manufacturing facility or relaunch or commercialize our products or any product candidates to any significant degree or achieve profitability. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Our failure to achieve sustained profitability would depress the value of our Company and could impair our ability to raise capital, expand our business, diversify our research and development pipeline, market our products or product candidates we may identify and pursue, if approved, or continue our operations. Our prior losses, combined with expected future losses, have had and will continue to have an adverse effect on our stockholders’ equity and working capital.
Our management has concluded that factors raise substantial doubt about our ability to continue as a going concern and our independent registered public accounting firm has included an explanatory paragraph relating to our ability to continue as a going concern in its report on our audited financial statements included in this Registration Document.
Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements for the year ended December 31, 2021 with respect to this uncertainty. Our ability to continue as a going concern will require us to obtain additional funding. We believe that the net proceeds from this Offering, together with our existing cash, will enable us to fund our operating expenses and debt service payments through April 2023. However, we will need approximately $15 million in additional funds to complete the reassembly, validation and certification of the Souderton, Pennsylvania facility. We have based these estimates on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. If we are unable to raise capital when needed or on acceptable terms, we would be forced to delay, or discontinue the further development and commercialization efforts of one or more of our products and/or product candidates or may be forced to reduce or terminate our operations.
| 14 |
Our ability to start manufacturing our products and generating revenues is dependent upon the successful completion of the construction and re-fitting of our Souderton facility. While we plan on using a significant portion of the proceeds of this offering towards the construction and re-fitting of the facility, the proceeds of this offering will not be sufficient to complete this process. We will require approximately $15 million of additional monies to complete this process. If we do not successfully raise such monies, the facility cannot be completed, and we will be unable to produce and commercially distribute any product.
In order to start manufacturing our products and generating revenues, we must successfully complete the construction and re-fitting of our Souderton facility with the equipment necessary for manufacturing and packaging, which has been relocated from facilities in Cambridge, Massachusetts and Ontario, Canada and obtain the relevant cGMP certification. While we plan on using a significant portion of the proceeds of this offering towards the construction and re-fitting of the facility, the proceeds of this offering will not be sufficient to complete this process. We will require approximately $15 million of additional monies to complete this process. We will need to seek such additional funds through public or private equity or debt financings or other sources, such as strategic collaborations or license and development agreements. If we do not successfully raise such monies, the facility cannot be completed, and we will be unable to produce and commercially distribute any product.
Such construction and re-fitting may also be subject to unforeseen delays and cost overruns which would negatively impact our business and results of operations. The construction and refit are also subject to our ability to hire sub-contractors with sufficient availability and obtain and comply with regulatory or other licenses and permits. If the construction of the Souderton facility is delayed or is not completed, we will not be able to manufacture our products and generate any material revenues, which would adversely affect our ability to operate and continue our business.
Once we begin conducting all of our manufacturing operations at the single facility in Souderton, Pennsylvania, any interruption in operations at such facility could result in our inability to satisfy demand for our approved products. Despite our efforts to safeguard this facility, including acquiring insurance on commercially reasonable terms, adopting environmental health and safety protocols and utilizing off-site storage of computer data, a number of factors could damage or destroy our manufacturing equipment or our inventory of component supplies or finished goods, cause substantial delays in our operations, result in the loss of key information, and cause us to incur additional expenses, including:
● relocation expense;
● operating restrictions, partial suspension or total shutdown of production imposed by regulatory authorities;
● equipment malfunctions or failures;
● technology malfunctions;
● work stoppages;
● damage to or destruction of the facility due to natural disasters or other events; and
● regional or local power shortages.
| 15 |
We will require additional funding to achieve our business goals. If we are unable to obtain this funding when needed and on acceptable terms, we would be forced to delay, limit or terminate our product development efforts or other operations. Raising additional capital may subject us to unfavorable terms, cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our approved products, product candidates, and technologies.
As mentioned above, we plan on using a significant portion of the proceeds of this offering towards the construction and re-fitting of our Souderton facility. The proceeds of this offering will not be sufficient to complete this process and we require approximately $15 million of additional monies to complete this process.
In addition, we are currently developing new indications for Oxyglobin and Hemopure, and ZK1. Developing and commercializing biopharmaceutical products and medical devices is expensive and time-consuming, and we expect our research, development and manufacturing expenses to increase substantially in connection with our ongoing activities. As of December 31, 2021, we had negative working capital of $13.5 million and capital resources consisting of cash of approximately $0.4 million. Because the outcome of any clinical development and regulatory approval process is highly uncertain, we cannot reasonably estimate the actual capital amounts necessary to successfully complete the development, regulatory approval process and commercialization of ZK1 and new indications of Oxyglobin and Hemopure.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to commercialize our approved products and develop and commercialize ZK1 or new indications of Oxyglobin and Hemopure and any other product candidates that we may identify and pursue. Moreover, such financing may result in dilution to stockholders, imposition of debt covenants and repayment obligations, or other restrictions that may affect our business. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit or terminate the commercialization or our approved products or the development of one or more of our programs for new indications of Oxyglobin and Hemopure and our product candidates or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially and adversely affect our business, prospects, financial condition and results of operations.
Risks Related to the Development and Regulatory Approval of our Product Candidates or New Indications of our Approved Products
Any biopharmaceutical products or medical devices advanced into clinical development are subject to extensive regulation, which can be costly and time consuming, cause unanticipated delays or prevent the receipt of the required approvals to commercialize such product or device.
The clinical development, manufacturing, labeling, storage, record-keeping, advertising, promotion, import, export, marketing and distribution of biopharmaceutical products are subject to extensive regulation by the FDA in the U.S. and by comparable health authorities in foreign markets. In the U.S., the FDA must approve the new facility and products manufactured there, as complying with Good Manufacturing Practice (‘cGMP’). The process of obtaining approval is expensive, often takes many years and can vary substantially based upon the type, complexity and novelty of the proposed new indications, with respect to Oxyglobin and Hemopure, or the product candidate involved. In addition to the significant clinical testing requirements, our ability to obtain marketing approval for these proposed new indications, with respect to Oxyglobin and Hemopure, or the product candidates depends on obtaining the final results of required non-clinical testing, including characterization of the manufactured components of such products and validation of our manufacturing processes. The FDA may determine that our manufacturing processes, testing procedures or facilities are insufficient to justify approval. Approval policies or regulations may change, and the FDA has substantial discretion in the approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. Despite the time and expense invested in clinical development of product candidates, regulatory approval is never guaranteed.
| 16 |
The FDA or another regulatory agency can delay, limit or deny approval of a proposed new indication, with respect to Oxyglobin or Hemopure, or a product candidate for many reasons, including, but not limited to:
| ● | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of clinical trials; | |
| ● | we may be unable to demonstrate to the satisfaction of the FDA that a proposed new indication, with respect to Oxyglobin and Hemopure, or a product candidate is safe and effective for any indication; | |
| ● | the FDA may not accept clinical data from trials which are conducted by individual investigators or in countries where the standard of care is potentially different from the U.S.; | |
| ● | the results of clinical trials may not meet the level of statistical significance required by the FDA for approval; | |
| ● | we may be unable to demonstrate that a proposed new indication, with respect to Oxyglobin and Hemopure, or a product candidate’s clinical and other benefits outweigh its safety risks; | |
| ● | the FDA may disagree with our interpretation of data from preclinical studies or clinical trials; | |
| ● | the FDA may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we or our collaborators contract for clinical and commercial supplies; or | |
| ● | the approval policies or regulations of the FDA may significantly change in a manner rendering our clinical data insufficient for approval. |
With respect to foreign markets, approval procedures vary among countries and, in addition to the aforementioned risks, can involve additional product testing, administrative review periods and agreements with pricing authorities. Any delay in obtaining, or inability to obtain, applicable regulatory approvals with respect to new indications and new markets could prevent us from commercializing any proposed new indications, with respect to Oxyglobin and Hemopure, or our product candidates.
Any proposed new indication with respect to Oxyglobin and Hemopure, or other product candidates that we advance into clinical trials, may cause unacceptable adverse events or have other properties that may delay or prevent their regulatory approval or commercialization or limit their commercial potential.
Unacceptable adverse events caused by any proposed new indication, with respect to Oxyglobin and Hemopure, or other product candidates that we advance into clinical trials, could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in the denial of regulatory approval by the FDA or other regulatory authorities for any or all targeted indications and markets. This, in turn, could prevent us from commercializing the affected proposed new indication, with respect to Oxyglobin and Hemopure, or product candidate in such indication or market and generating revenues from its sale. Hemopure remains under clinical development, and we cannot know the extent of any unexpected adverse events in future clinical trial patients. Any unforeseen safety issues with a product candidate could prevent and/or delay the regulatory approval process.
Delays in the commencement of clinical trials could result in increased costs and delay our ability to pursue regulatory approval.
The commencement of clinical trials can be delayed for a variety of reasons, including delays in:
| ● | obtaining regulatory clearance to commence a clinical trial; | |
| ● | identifying, recruiting and training suitable clinical investigators; |
| 17 |
| ● | reaching mutually agreeable contract terms for research agreements with large institutional sites; | |
| ● | maintaining adequate levels of clinical trial supplies (including investigational drug products); | |
| ● | obtaining an IRB or ethics committee approval to conduct a clinical trial at a prospective site; | |
| ● | identifying, recruiting and enrolling patients to participate in a clinical trial; and | |
| ● | early withdrawal of clinical trial participants for any reason, related to product safety issues or otherwise. |
Any delays in the commencement of clinical trials will delay our ability to pursue regulatory approval for any proposed new indications. In addition, many of the factors that cause, or lead to, a delay in the commencement of clinical trials may also ultimately lead to the denial of regulatory approval of a proposed new indication with respect to Oxyglobin and Hemopure, or a product candidate.
Suspensions or delays in the completion of clinical testing could result in increased costs to us and delay or prevent our ability to complete development of that product or generate product revenues.
Once a clinical trial has begun, patient recruitment and enrollment may be slower than we anticipate. Clinical trials may also be delayed as a result of ambiguous or negative interim results or difficulties in obtaining sufficient quantities of product manufactured in accordance with regulatory requirements. Further, a clinical trial may be modified, suspended or terminated by us, an Institutional Review Board (IRB), a hospital ethics committee, data safety monitoring oversight committee, or any regulatory authority due to a number of factors, including:
| ● | protocol non-compliance | |
| ● | violations of Good Clinical Practice (GCP) by us, investigator sites, or vendors contracted on our behalf | |
| ● | stopping rules contained in the protocol; | |
| ● | unforeseen safety issues or any determination that the clinical trial presents unacceptable health risks; and | |
| ● | lack of adequate funding to continue the clinical trial. |
Any changes in the current regulatory requirements and guidance also may occur, and we may need to amend clinical trial protocols to reflect these changes. Amendments may require us to resubmit clinical trial protocols to IRBs and regulatory authorities for additional review, which may impact the costs, timing and the likelihood of a successful completion of a clinical trial. If we experience delays in the completion of, or if we must suspend or terminate, any clinical trial with respect to a proposed new indication or any product candidate, our ability to obtain regulatory approval will be delayed, and the commercial prospects, if any, for the proposed new indication, or approval may suffer as a result. In addition, many of these factors may also ultimately lead to the denial of regulatory approval.
We may expend our limited resources to pursue a proposed new indication or approval for a product or product candidate and fail to capitalize on product candidates or indications for which there may be a greater likelihood of success.
Because we have limited financial and managerial resources, we may forego or delay pursuit of opportunities with new indications, with respect to Oxyglobin and Hemopure, or other product candidates for which there may be a greater likelihood of success or may prove to have greater commercial potential. Research programs to identify new product candidates or pursue alternative indications for approved products require substantial technical, financial and administrative support. We may never successfully develop any proposed new indication, with respect to Oxyglobin and Hemopure, or any product candidates.
| 18 |
We may incur substantial product liability or indemnification claims relating to the clinical testing of Oxyglobin and Hemopure for any proposed new indication or our product candidates.
We face an inherent risk of product liability exposure related to the testing of Oxyglobin and Hemopure for any proposed new indication or our product candidates in human clinical trials, and claims could be brought against us if use or misuse of Oxyglobin and Hemopure for any proposed new indication or one of our product candidates causes, or merely appears to have caused, personal injury or death. While we have and intend to maintain product liability insurance relating to our clinical trials, our coverage may not be sufficient to cover claims that may be made, and we may be unable to maintain such insurance. Any claims, regardless of their merit, could severely harm our financial condition, strain our management and other resources or destroy the prospects for commercialization of the product which is the subject of any such claim. We are unable to predict if we will be able to obtain or maintain product liability insurance for any products that may be approved for marketing. Additionally, it is expected that we will need to enter into various agreements where we indemnify third parties for certain claims relating to the testing of Oxyglobin and Hemopure for any proposed new indication or our product candidates. These indemnification obligations may require us to pay significant sums of money for claims that are covered by these indemnifications.
Our research and development activities could be affected or delayed as a result of possible restrictions on animal testing.
Certain laws and regulations require us to test our product candidates on animals before initiating clinical trials involving human patients. Animal testing activities have been the subject of controversy and adverse publicity. Animal rights groups and other organizations and individuals have attempted to stop animal testing activities by pressing for legislation and regulation in these areas and by disrupting these activities through protests and other means. To the extent the activities of these groups are successful, our research and development activities may be interrupted, delayed or become more expensive.
We may find it difficult to enroll patients in our clinical trials which could delay or prevent the start of clinical trials for our product candidates.
Identifying and qualifying patients to participate in clinical trials of Oxyglobin and Hemopure for any proposed new indication or our product candidates is essential to our success. The timing of our clinical trials depends in part on the rate at which we can recruit patients to participate in clinical trials of Oxyglobin and Hemopure for any proposed new indication or our product candidates, and we may experience delays in our clinical trials if we encounter difficulties in enrollment. If we experience delays in our clinical trials, the timeline for obtaining regulatory approval of Oxyglobin and Hemopure for any proposed new indication or our product candidates will most likely be delayed.
Many factors may affect our ability to identify, enroll and maintain qualified patients, including the following:
| ● | eligibility criteria of our ongoing and planned clinical trials with specific characteristics appropriate for inclusion in our clinical trials; | |
| ● | design of the clinical trial; | |
| ● | size and nature of the patient population; | |
| ● | patients’ perceptions as to risks and benefits of the proposed new indication of Oxyglobin and Hemopure or the product candidate under study and the participation in a clinical trial generally in relation to other available therapies; | |
| ● | the availability and efficacy of competing therapies and clinical trials; | |
| ● | pendency of other trials underway in the same patient population; | |
| ● | willingness of physicians to participate in our planned clinical trials; |
| 19 |
| ● | severity of the disease under investigation; | |
| ● | proximity of patients to clinical sites; | |
| ● | patients who do not complete the trials for personal reasons; and | |
| ● | issues with Contract Research Organizations (“CROs”) and/or with other vendors that handle our clinical trials. |
We may not be able to initiate or continue to support clinical trials for any proposed new indication or of our product candidates, for one or more applications, or any future proposed new indication, with respect to Oxyglobin and Hemopure, or product candidates if we are unable to locate and enroll a sufficient number of eligible participants in these trials as required by the FDA or other regulatory authorities. Even if we are able to enroll a sufficient number of patients in our clinical trials, if the pace of enrollment is slower than we expect, the development costs for Oxyglobin and Hemopure for any proposed new indication or our product candidates may increase and the completion of our trials may be delayed, or our trials could become too expensive to complete.
If we experience delays in the completion of, or termination of, any clinical trials of Oxyglobin and Hemopure for any proposed new indication or our product candidates, the commercial prospects of these could be harmed, and our ability to generate product revenue therefrom could be delayed or prevented. In addition, any delays in completing our clinical trials would likely increase our overall costs, impair development and jeopardize our ability to obtain regulatory approval relative to our current plans. Any of these occurrences may harm our business, financial condition, and prospects significantly.
Even if we successfully complete the construction and re-fitting of our Souderton facility, if the manufacturing facility does not meet regulatory requirements or, once operational, is unable to meet our supply demands, our business will be harmed.
We are involved in the preparation of therapeutics for clinical trials or commercial sale and are therefore subject to extensive regulation. These regulations govern manufacturing processes and procedures, including recordkeeping, and the implementation and operation of quality systems to control and assure the quality of investigational products and products approved for sale. Poor control of production processes can lead to the introduction of contaminants or to inadvertent changes in the properties or stability of our products. Our failure to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, suspension of production, seizures or recalls of products or marketed drugs, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect clinical or commercial supplies of our products.
We must supply all necessary documentation in support of a Biologics License Application (“BLA”) or Marketing Authorisation Application (“MAA”) on a timely basis and must adhere to Good Laboratory Practices (“cGLP”) and Good Manufacturing Practices (“cGMP”) regulations enforced by the FDA and other regulatory agencies through their facilities inspection program. In addition, the regulatory authorities may, at any time, audit or inspect our manufacturing facility involved with the preparation of our products or our product or the associated quality systems for compliance with the regulations applicable to the activities being conducted. If our facility does not pass a pre-approval plant inspection, regulatory approval of the products may not be granted or may be substantially delayed until any violations are corrected to the satisfaction of the regulatory authority, if ever.
The regulatory authorities also may, at any time following approval of one of our products for sale, audit our manufacturing facility, if completed. If any such inspection or audit identifies a failure to comply with applicable regulations or if a violation of our product specifications or applicable regulations occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly and/or time consuming for us to implement, and that may include the temporary or permanent suspension of commercial sales or the temporary or permanent closure of our sole manufacturing facility. Any such remedial measures imposed upon us could materially harm our business.
| 20 |
Risks associated with operating in foreign countries could materially adversely affect our product development.
We may conduct future studies in countries outside of the United States. Consequently, we may be subject to risks related to operating in foreign countries. Risks associated with conducting operations in foreign countries include:
| ● | differing regulatory requirements for device approvals and regulation of approved devices in foreign countries; more stringent privacy requirements for data to be supplied to our operations in the U.S., e.g., General Data Protection Regulation in the European Union; | |
| ● | unexpected changes in tariffs, trade barriers and regulatory requirements; economic weakness, including inflation, or political instability in particular foreign economies and markets; compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; foreign taxes, including withholding of payroll taxes; | |
| ● | differing payor reimbursement regimes, governmental payors or patient self-pay systems and price controls; | |
| ● | foreign currency fluctuations, which could result in increased operating expenses or reduced revenues, and other obligations incident to doing business or operating in another country; | |
| ● | workforce uncertainty in countries where labor unrest is more common than in the U.S.; | |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and | |
| ● | business interruptions resulting from geopolitical actions, including war and terrorism. |
Failure to obtain regulatory approval in international jurisdictions would prevent our product candidates from being marketed abroad.
In addition to regulations in the U.S., to market and sell Oxyglobin and Hemopure for any proposed new indication or our product candidates in the European Union, United Kingdom, many Asian countries and other jurisdictions, we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the U.S. does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. We may not be able to obtain approvals from regulatory authorities outside the U.S. on a timely basis, if at all. Clinical trials accepted in one country may not be accepted by regulatory authorities in other countries. In addition, many countries outside the U.S. require that a product be approved for reimbursement before it can be approved for sale in that country. A product candidate that has been approved for sale in a particular country may not receive reimbursement approval in that country.
We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our products in any additional markets. If we are unable to obtain approval of Oxyglobin and Hemopure for any proposed new indication or any of our current product candidates or any future product candidates we may pursue by regulatory authorities in the European Union, United Kingdom, Asia or elsewhere, the commercial prospects of that product may be significantly diminished, our business prospects could decline and this could materially adversely affect our business, results of operations and financial condition.
| 21 |
Even though our current primary indications of Oxyglobin and Hemopure received regulatory approval, they may still face future development and regulatory difficulties.
Even though we have obtained regulatory approval for certain indications for Oxyglobin and Hemopure in certain markets, and if we obtain approval of any product candidate or of any new indications of Oxyglobin and Hemopure in other markets, that approval is and/or would be subject to ongoing requirements by the FDA and comparable foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, adverse event reporting, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-marketing information. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance by us and/or our Contract Manufacturing Organizations (“CMOs”) and CROs for any post-approval clinical trials that we may conduct. The safety profile of any product after approval continues to be closely monitored by the FDA and comparable foreign regulatory authorities. If the FDA or comparable foreign regulatory authorities become aware of new safety information after a product’s approval, they may require labeling changes or establishment of a risk evaluation and mitigation strategy, impose significant restrictions on such product’s indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance.
In addition, manufacturers of devices and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with Current Good Manufacturing Practice, Good Clinical Practice, and other regulations. If we or a regulatory agency discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we or our productor at the manufacturing facilities for our product fail to comply with applicable regulatory requirements, a regulatory agency may:
| ● | issue warning letters or untitled letters; | |
| ● | mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; | |
| ● | require us to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; | |
| ● | seek an injunction or impose civil or criminal penalties or monetary fines; | |
| ● | suspend or withdraw regulatory approval; | |
| ● | suspend any ongoing clinical trials; | |
| ● | refuse to approve pending applications or supplements to applications filed by us; | |
| ● | suspend or impose restrictions on operations, including costly new manufacturing requirements; or | |
| ● | seize or detain products, refuse to permit the import or export of products, or require us to initiate a product recall. |
The occurrence of any event or penalty described above may inhibit our ability to successfully commercialize our product and generate revenues.
Advertising and promotion of any product that obtains approval in the U.S. is heavily scrutinized by the FDA, the Department of Justice, the Office of Inspector General of Health and Human Services, state attorneys general, members of Congress and the public. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved label. Additionally, advertising and promotion of any product that obtains approval outside of the U.S. is heavily scrutinized by comparable foreign regulatory authorities. Violations, including actual or alleged promotion of our product for unapproved or off-label uses, are subject to enforcement letters, inquiries and investigations, and civil and criminal sanctions by the FDA, as well as prosecution under the federal False Claims Act. Any actual or alleged failure to comply with labeling and promotion requirements may have a negative impact on our business.
| 22 |
Risks Related to Our Dependence on Third Parties
If independent research parties perform in an unsatisfactory manner, it may harm our business.
We have relied upon and plan to continue to rely upon our independent investigators at multiple academic medical institutions, and US military, with whom we have agreements for the supply and use of our products in their laboratories and clinical research. These third parties are responsible for ensuring that their studies and trials are conducted in accordance with the applicable protocols, legal, regulatory, and scientific standards and are required to comply, where appropriate, with Good Clinical Practice (“cGCP”), and Good Laboratory Practices (“cGLP”), which are regulations and guidelines enforced by the FDA, the competent authorities of the member states of the European Economic Area, (“EEA”), and comparable foreign regulatory authorities. Regulatory authorities enforce these regulations through periodic inspections of study sponsors, principal investigators, study sites, and other contractors. If our independent research partners fail to comply with applicable regulations, these clinical trials may be delayed or terminated and may cause delay in clinical development of targeted indications, which would impair our ability to sell our products for such indications.
Our manufacturing facility is expected to be fully automated and dependent on software and other information technology to function properly. Failure to protect our information technology infrastructure against cyber-based attacks, network security breaches or data corruption could materially disrupt our operations and adversely affect our business and operating results.
The efficient operation of our business depends on our information technology systems. If we are able to refit the facility and resume production, we will rely on our information technology systems to effectively manage our manufacturing capacity, sales and marketing data, accounting and financial functions, inventory management, product development tasks, clinical data, customer service and technical support functions. Our information technology systems are vulnerable to damage or interruption from earthquakes, fires, floods and other natural disasters; terrorist attacks; cyber-based attacks; attacks by computer viruses or hackers; power losses, computer system or data network failures; security breaches and data corruption. Federal, state and international laws and regulations, such as the European Union’s General Data Protection Regulation, (“GDPR”), which took effect in May 2018, can expose us to enforcement actions and investigations by regulatory authorities, and potentially result in regulatory penalties and significant legal liability, if our information technology security efforts fail. In addition, our software systems include cloud-based applications that are hosted by third-party service providers with security and information technology systems subject to similar risks. The failure of either our or our service providers’ information technology could disrupt our entire operation or result in decreased sales, increased overhead costs and product shortages, all of which could materially and adversely affect our business, financial condition, operating results, cash flows and prospects.
Our main raw material is bovine blood, which we intend to obtain from a single supplier located near our Souderton facility. If that supplier performs in an unsatisfactory manner, this may harm our business.
The success of our business depends in part on our ability to cost-effectively and efficiently manufacture products on a commercial scale. While we intend to manufacture all of our products at our Souderton facility, we will rely on suppliers to acquire the necessary raw materials needed for production.
We intend to obtain bovine hemoglobin from one abattoir of animals raised in several states of the United States. We cannot predict the future effect, if any, on us of the spread of bovine spongiform encephalopathy (“mad cow” disease) in the United States. Any quarantine affecting herds that supply us or a shutdown of the abattoir that we use could have a material adverse effect on us, as it would have to find, validate and obtain regulatory approval of new sources of supply.
Replacing suppliers for key materials and components could result in unexpected delays and expenses.
We intend to obtain some key materials, including membranes and chemicals, bags for packaging, and services from sole-source suppliers. All of these materials are commercially available elsewhere. If such materials were no longer available at a reasonable cost from existing suppliers, we would need to purchase substitute materials from new suppliers. If a new supplier must be located, the substitute or replacement materials or facilities would need to be tested for equivalency. Such equivalency tests could significantly delay product development, or delay or limit commercial sales of approved products and cause us to incur additional expense.
| 23 |
The establishment of new supply relationships involves also numerous uncertainties, including those relating to payment terms, supply adequacy of supplying capacity, quality control and timeliness of delivery. For some products we may seek to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of those products. We may face significant competition as well as risks in seeking and maintaining appropriate suppliers.
Risks Related to our Intellectual Property
If we are unable to obtain and maintain sufficient intellectual property protection for our products or product candidates or if the scope of the intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours. Our pending patent applications may be challenged, thus impairing our ability to protect our products or product candidates.
Our success notably depends on our ability to obtain and maintain protection of the intellectual property covering our products, product candidates and technology, particularly patents. Obtaining and enforcing biopharmaceutical patents is costly, time consuming and complex. It is also possible that we will fail to identify patentable aspects of our research and development programs or that we will not have the right to control the preparation, filing and prosecution of patent applications.
Our patent protecting the technology embodied in Oxyglobin and Hemopure expired on February 28, 2022. We believe that the expiry of this patent will not have a material adverse effect on our prospects or strategy given that (i) our proprietary technology is protected by a combination of trade secrets, know-how and trademarks and (ii) in view of the complexities of the processing of a biological material of animal origin, it is unlikely that competitors could manufacture product similar to Oxyglobin or Hemopure simply by reverse engineering the final product. However, as noted, we cannot be certain that we will be able to obtain and maintain sufficient intellectual property protection for Oxyglobin, Hemopure or ZK1, that the scope of protection obtained will be sufficiently broad, or that our competitors will not develop and commercialize products similar or identical to our products or product candidates (e.g., by circumventing our patent by developing similar or alternative products in a non-infringing manner).
We may not be aware of all third-party intellectual property rights potentially relating to our products or product candidates (since publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing or, in some cases, not at all). Any of our pending patent applications may become involved in opposition, derivation, reexamination, inter parties review, post-grant review or interference proceedings. Any new patents, if our applications therefor have been approved, may be challenged (as to inventorship, scope, validity or enforceability) in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated, or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical products, or limit the duration of the patent protection of our products or product candidates. As a result, the ownership, inventorship, issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain.
The laws of foreign countries may not protect our rights to the same extent as the laws of the United States, or vice versa, and changes in patent policy and rules could impair our ability to protect our products or product candidates and increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patent.
Any such event could have a material adverse effect on our business.
| 24 |
If we are not able to prevent disclosure of our trade secrets, know-how or other proprietary information (“Confidential Information”), the value of our technology and products could be significantly diminished, which may have a material adverse effect on our business.
We have a policy of requiring our consultants, contract personnel, advisers, institutions and third-party partners to enter into confidentiality agreements and our employees to enter into invention and non-disclosure agreements. However, no assurance can be given that we have entered into appropriate agreements with all parties that have had access to our Confidential Information or that such agreements will provide meaningful protection of Confidential Information in the event of any unauthorized use or disclosure of information. In addition, despite such contractual provisions, (i) the need to share trade secrets and other confidential information, (ii) the possibility for any of our employees, consultants, contract personnel or third-party partners, either accidentally or through willful misconduct, to disclose Confidential Information and (iii) possible breaches of our physical or electronic security systems, increase the risks that our trade secrets become known by our competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. A competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have a material adverse effect on our business. Any action to enforce our rights against any misappropriation or unauthorized use and/or disclosure of Confidential Information is likely to be time-consuming and expensive, and may ultimately be unsuccessful, or may result in a remedy that is not commercially valuable.
We may become subject to claims alleging infringement of third parties’ patents and other intellectual property rights, which may result in costly litigation and could result in us having to pay substantial damages or limit our ability to commercialize our products.
Our commercial success depends upon our ability, and the ability of any third party with which we may partner, to develop product candidates and to manufacture, market and sell our approved products and use our patent-protected technologies without infringing the patents of third parties. There is considerable patent litigation in the biotechnology and pharmaceutical industries. As the biopharmaceutical industry expands and more patents are issued, we face greater risk that there may be patents issued to third parties that relate to our products and technology of which we are not aware or that we must challenge to continue our operations as currently contemplated.
Our current and any future approved products may infringe upon or may be alleged to infringe upon existing patents or patents that may be granted to third parties in the future Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our technologies, our products or the use of our products. As a result, we may become party to, or threatened with, future adversarial proceedings or litigation regarding patents with respect to our products and technology.
If we are sued for patent infringement, we would need to demonstrate that our products or methods either do not infringe upon the patent claims of the relevant patent or that the patent claims are invalid, and we may not be able to do this. If we are found to infringe upon a third party’s patent, we could be required to obtain a license from such third party to continue commercializing and marketing our products and technology or we may elect to enter into such a license in order to settle litigation or in order to resolve disputes prior to litigation. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we are able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us and could require us to make substantial royalty payments. We could also be forced, including by court order, to cease commercializing the infringing technology or products. A finding of infringement could prevent us from commercializing our products or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similarly negative impact on our business. Any such claims are likely to be expensive to defend, and some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Moreover, even if we are successful in defending any infringement proceedings, we may incur substantial costs and divert management’s time and attention in doing so, which could materially adversely affect our business, results of operations or financial condition.
We may be involved in lawsuits to protect or enforce our patent or other intellectual property rights, which may result in costly litigation.
Competitors may infringe upon our patent or other intellectual property rights. Although we are not currently involved in any litigation, if we initiate infringement legal proceedings, the defendant could counterclaim that the patent covering our product is invalid and/or unenforceable. The outcome following legal assertions of invalidity and unenforceability is unpredictable. Our defense of litigation may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs or enter into development partnerships that would help us commercialize our products or bring our product candidates to market.
| 25 |
Risks Relating to Commercializing of our Current Approved Products and Future Product Candidates
While we have marketing approvals for Oxyglobin and Hemopure for certain indications in certain jurisdictions, the commercial success of our products will depend upon the degree of market acceptance by clinicians, patients, third-party payors and others in the veterinary and medical community.
While we have marketing approvals for some of our products in certain jurisdictions, if our products and product candidates do not achieve an adequate level of acceptance, we may not generate significant revenue from product sales and we may not become profitable. Before granting reimbursement approval, healthcare payors may require us to demonstrate that our products, in addition to treating the target indications, also provide incremental health benefits and/or economic benefit to the payor compared to standard fluid therapy. Our efforts to educate the medical community and third-party payors about the benefits of our products may require significant resources and may never be successful. The degree of market acceptance of our products and product candidates, if approved for commercial sale, will depend on a number of factors, including:
| ● | the efficacy and potential advantages compared to alternative treatments; | |
| ● | our ability to offer our products for sale at competitive prices; | |
| ● | the convenience and ease of administration compared to alternative treatments, including future alternative treatments; | |
| ● | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; | |
| ● | the availability of products and their ability to meet market demand, including a reliable supply for long-term daily treatment; | |
| ● | the strength of marketing and distribution support; | |
| ● | the availability of third-party coverage and adequate reimbursement; | |
| ● | the clinical indications for which the product is approved; | |
| ● | the prevalence and severity of any side effects and overall safety profile; and | |
| ● | any restrictions on the use of our products together with other medications. |
We must continue to educate surgeons, transplant centers and private payors and demonstrate the potential merits of ZK1 compared with cold storage or new competing technologies. Even if ZK1 receives regulatory approval, surgeons, transplant centers and private payors may require additional clinical data prior to adopting or maintaining coverage of ZK1.
Directors of transplant programs are key decision-makers in the adoption of novel medical devices used in organ transplantation. An important part of our commercialization efforts is to educate transplant center program directors and other surgeons on the potential relative merits of ZK1. Our success depends on effectively marketing and educating program directors and other surgeons about the benefits of ZK1. Acceptance of ZK1 also depends on educating program directors, other surgeons and private payors as to the distinctive characteristics, perceived medical and economic benefits, safety and ease of use and cost-effectiveness of ZK1. If program directors, other surgeons and private payors do not find our body of published clinical evidence and data compelling or wish to wait for additional studies, they may choose not to use or provide coverage and reimbursement for our products.
| 26 |
Our long-term growth depends on our ability to expand into new indications for Oxyglobin and Hemopure and develop the next generation of product candidates.
Our long-term growth depends on our ability to expand the potential uses for Oxyglobin and Hemopure and seek to develop new product candidates. Developing such new or modified products is expensive and time-consuming and diverts management’s attention away from current operations. The success of any new product offering, or product enhancements will depend on several factors, including our ability to:
| ● | properly identify and anticipate clinician and patient needs; | |
| ● | develop and introduce new products and product modifications in a timely manner; | |
| ● | avoid infringing upon, misappropriating or otherwise violating the intellectual property rights of third parties; | |
| ● | demonstrate the safety and efficacy of new products and product modifications; | |
| ● | obtain necessary regulatory clearances or approvals; | |
| ● | comply with regulations regarding the marketing of new products or product modifications | |
| ● | provide adequate training to potential users; | |
| ● | receive adequate coverage and reimbursement for procedures performed with our products; and | |
| ● | develop an effective sales and marketing effort. |
If we are not successful in expanding our indications of Oxyglobin and Hemopure and developing the next generation of product candidates, our ability to increase our revenue would be impaired, which could materially and adversely affect our business, financial condition, operating results, cash flows and prospects.
We may be unsuccessful in commercializing our approved products if we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell such products.
Although some of our employees have experience with commercializing products while employed at other companies, we as a company have no experience selling and marketing our approved products and we currently have no marketing or sales organization. To successfully commercialize any approved products that may result from our development programs, we will need to rely on third-party distributors. We currently plan to sell Oxyglobin in North America through our exclusive distributor Dechra Veterinary Products, and in the European Union and elsewhere, through other regional distributors. We will also rely on blood banks and other third-party distributors for Hemopure, and on perfusion machine manufacturers for ZK1. If our product candidates receive regulatory approval, we intend to establish an internal sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize our products in major markets, which will be expensive, difficult and time consuming. Any failure or delay in the development of these distribution capabilities would adversely impact the commercialization of our products.
If our future collaborators do not commit sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we will be unable to generate sufficient product revenue to sustain our business.
| 27 |
The insurance coverage and reimbursement status of newly approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for new or current products could limit our ability to market those products and decrease our ability to generate revenue.
Our target patient populations are small, and accordingly the pricing, coverage and reimbursement of our approved products, and our product candidates, if approved, must be adequate to support our commercial infrastructure. Sales of our approved products will depend substantially, both domestically and abroad, on the extent to which the costs of our approved products will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers and other third-party payors. Our per-patient prices must be sufficient to recover our development and manufacturing costs and potentially achieve profitability. Accordingly, the availability and extent of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments, such as ours, assuming approval. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our approved products. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services (“CMS”), an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicaid or Medicare. However, the practices and requirements relating to the payment of rebates by drug manufacturers for Medicaid purchases are determined by each state, and in some cases, if a company does not enter into a rebate agreement, its Medicaid sales will be subjected to a “prior authorization” procedure that requires state agency approval to qualify a doctor’s prescription for reimbursement.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada, and other countries has and will continue to put pressure on the pricing and usage of our approved products and any product candidates, if approved. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems are substantially lower than in the United States. Other countries allow companies to fix their own prices for medicines but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our products. Accordingly, in markets outside the United States, the reimbursement for our approved products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenues and profits.
Moreover, increasing efforts by governmental and third-party payors, in the United States and abroad, to cap or reduce healthcare costs may cause such organizations to limit both coverage and levels of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our products. We have in the past and expect to continue to experience pricing pressures in connection with the sale of our approved products, and any product candidates, if approved, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative and political changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, drug prices are under significant scrutiny in the markets in which our products may be sold, and drug pricing and other healthcare costs continue to be subject to intense political and social pressures which we anticipate will continue and escalate on a global basis. As a result, our business and reputation may be harmed, our stock price may be adversely impacted and experience periods of volatility, we may have difficulty raising funds and our results of operations may be adversely impacted.
Risks Related to Our Business Operations
Our future success depends on our ability to retain key employees, consultants and advisors and to attract, retain and motivate qualified personnel.
We are highly dependent on the management, research and development, clinical, financial and business development expertise of our executive officers, as well as the other members of our scientific and clinical teams. Although we have employment agreements with each of our executive officers, each of them may terminate their employment with us at any time. We do not maintain “key person” insurance for any of our executives or employees.
| 28 |
Recruiting and retaining qualified scientific and clinical personnel and scaling up for commercialization, sales and marketing personnel, will also be critical to our success. The loss of the services of our executive officers or other key employees could impede the achievement of our research, development and commercialization objectives and seriously harm our ability to successfully implement our business strategy. Furthermore, replacing executive officers and key employees may be difficult and may take an extended period of time because of the limited number of individuals in our industry with the breadth of skills and experience required to successfully develop, gain regulatory approval for and commercialize our approved products and product candidates. Competition to hire qualified personnel in our industry and geographic market is intense, and we may be unable to hire, train, retain or motivate these key personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. Furthermore, to the extent we hire personnel from competitors, we may be subject to allegations that they have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. If we are unable to continue to attract and retain high quality personnel, our ability to pursue our growth strategy will be limited.
Failure to comply with anti-bribery, anti-corruption, and anti-money laundering laws, including the Foreign Corrupt Practices Act (“FCPA”), as well as export control laws, customs laws, sanctions laws and other laws governing our operations could result in civil or criminal penalties, other remedial measures and legal expenses
As we grow our international presence, we are increasingly exposed to trade and economic sanctions and other restrictions imposed by the United States, the European Union and other governments and organizations. The U.S. Departments of Justice, Commerce, State and U.S. Treasury and other federal agencies and authorities have a broad range of civil and criminal penalties they may seek to impose against corporations and individuals for violations of economic sanctions laws, export control laws, the FCPA and other federal statutes and regulations, including those established by the Office of Foreign Assets Control (“OFAC”). In addition, the Bribery Act prohibits both domestic and international bribery, as well as bribery across both private and public sectors, where business or personnel engaged by it have a connection with the U.K. An organization with that connection and that “fails to prevent bribery” by anyone associated with the organization can be found guilty under the Bribery Act unless the organization can establish the defense of having implemented “adequate procedures” to prevent bribery. Under these laws and regulations, as well as other anti-corruption laws, anti-money laundering laws, export control laws, customs laws, sanctions laws and other laws governing our operations, various government agencies may require export licenses, may seek to impose modifications to business practices, including cessation of business activities in sanctioned countries or with sanctioned persons or entities and modifications to compliance programs, which may increase company’s exposure to fines, penalties and other sanctions. A violation of these laws or regulations would negatively affect our business, financial condition and results of operations. Due to sales of our products to government or government-affiliated entities, we may be exposed to heightened risk of potential violations of the FCPA, the Bribery Act, or other relevant law.
We have implemented policies and procedures designed to ensure compliance by us and our directors, officers, employees, representatives, consultants and agents with the FCPA, OFAC restrictions, the Bribery Act and other export control, anti-corruption, anti-money-laundering and anti- terrorism laws and regulations. We cannot assure you, however, that our policies and procedures are or will be sufficient or that directors, officers, employees, representatives, consultants and agents have not engaged and will not engage in conduct for which we may be held responsible, nor can we assure you that our business partners have not engaged and will not engage in conduct that could materially affect their ability to perform their contractual obligations to us or even result in our being held liable for such conduct. Violations of the FCPA, OFAC restrictions, the Bribery Act or other export control, anti-corruption, anti-money laundering and anti-terrorism laws or regulations may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could have a material adverse effect on our business, financial condition and results of operations.
| 29 |
There are product liability risks specifically related to ZKI.
If approved and commercialized, our medical device, ZK1, may be deemed to be defectively designed, manufactured or labeled, and we could face substantial and costly litigation by transplant centers that purchase or use ZK1 or by their patients or others claiming damages on their behalf. Because ZK1 represents a novel approach to organ transplantation, a patient or transplant center may choose to name us as a party to a lawsuit relating to the use of ZK1 in connection with a planned or completed transplant procedure regardless of whether ZK1 caused or contributed to a serious adverse event or death of a patient. Any claim, whether or not we are ultimately successful, could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us.
Improper use, marketing or promotion of our approved products, or product candidates, if approved, or off-label use of our approved products, or product candidates, if approved, may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
Oxyglobin has been approved by the FDA in the United States, the EMA in the European Union, and VMD in the United Kingdom, and our promotional materials and training methods must comply with regulatory requirements in the countries where they are sold. Our distributors do to not promote Oxyglobin for uses outside of the approved indications for use, known as “off-label uses.” We cannot, however, prevent customers from using Oxyglobin off-label. The use of Oxyglobin for indications other than those approved by the FDA or approved by any foreign regulatory body may lead to adverse effects, which could harm our reputation in the marketplace among surgeons and patients.
If the FDA or any foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance or imposition of an untitled letter, which is used for violators that do not necessitate a warning letter, injunction, seizure, civil fine or criminal penalties.
Misuse of our products could harm our reputation in the marketplace, lead to product liability lawsuits, or otherwise harm our business.
Surgeons may misuse Hemopure or use improper techniques if they are not adequately trained, potentially leading to unsatisfactory patient outcomes, patient injuries, negative publicity and an increased risk of product liability. If Hemopure is misused or used with improper technique, we may become subject to costly litigation by our customers or their patients.
Legislative or regulatory reforms in the United States or other jurisdictions may make it more difficult and costly for us to obtain regulatory clearances or approvals for our product candidates or to manufacture, market or distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in the U.S. Congress that could significantly change the statutory provisions governing the regulation of veterinary and human health pharmaceuticals and medical devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain approval for, manufacture, market or distribute our products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping.
The European Parliament has adopted the Medical Devices Regulation, the European Union Medical Devices Directive and the Active Implantable Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, regulations would be directly applicable, (i.e., without the need for adoption of EEA member state laws implementing them) in all EEA member states and are intended to eliminate current differences in the regulation of medical devices among EEA member states. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation.
| 30 |
The new regulations, among other things:
| ● | strengthen the rules on placing devices on the market and reinforce surveillance once they are available; | |
| ● | establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; | |
| ● | improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; | |
| ● | set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the European Union; and | |
| ● | strengthen rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
Our products may be affected by these rules, which may mean longer or more burdensome assessment of our products. These modifications may have an effect on the way we conduct our business in the EEA.
We recognize that our products will have to be re-certified under the Medical Devices Regulation and we are in the process of updating internal procedures to ensure compliance with the new Medical Devices Regulation and have added international regulatory personnel to assist with the transition.
In addition, there are significant concerns associated with whether EU Notified Bodies will be able to re-certify all devices in their care in time. If we do not manage to re-certify our products under this new regulation or cannot rely on the transitional provisions, we may have to take our products off the EU market until this is the case.
Failure to maintain an ethical and inclusive corporate culture, or damage to our reputation, could have a material adverse effect on our business.
We strive to create a culture in which our employees act with integrity, treat each other with respect and consider themselves empowered to report suspected misconduct. Our ability to attract and retain a high-quality workforce depends upon our commitment to a diverse and inclusive environment, along with our perceived trustworthiness and ethics. Allegations of misconduct by employees, particularly leaders, erode trust and confidence and cause reputational damage. Negative public opinion can result from actual or alleged conduct by the Company or those currently or formerly associated with the Company. Issues can arise in any number of circumstances, including employment-related offenses such as workplace harassment and discrimination, regulatory noncompliance, and failure to properly use and protect data and systems, as well as from actions taken by regulators or others in response to such conduct. Addressing allegations of misconduct detracts focus from business operations and is expensive.
We may encounter difficulties in managing our expected level of growth, which could disrupt our operations.
As our development progresses, we expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of research, drug development, regulatory affairs and, potentially, sales, marketing and distribution. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
| 31 |
Our business may be adversely affected by the ongoing coronavirus pandemic.
The outbreak of the novel coronavirus (COVID-19) has evolved into a global pandemic. The coronavirus has spread to many regions of the world. Many research facilities around the world were closed for a prolonged period of time, and some of our clinical research and development work suffered delays. The extent to which the coronavirus impacts our business and operating results will depend on future developments that are highly uncertain and cannot be accurately predicted, including new information that may emerge concerning the coronavirus and the actions to contain the coronavirus or treat its impact, among others.
As a result of the continuing spread of the coronavirus, our business operations could be delayed or interrupted. For instance, our clinical trials may be affected by the pandemic. Site initiation, participant recruitment and enrollment, and study monitoring and data analysis may be paused or delayed due to changes in hospital or university policies, federal, state or local regulations, prioritization of hospital resources toward pandemic efforts, or other reasons related to the pandemic. If the coronavirus continues to spread, some participants and clinical investigators may not be able to comply with clinical trial protocols. For example, quarantines or other travel limitations (whether voluntary or required) may impede participant movement, affect sponsor access to study sites, or interrupt healthcare services, and we may be unable to conduct our clinical trials. Further, if the spread of the coronavirus pandemic continues and our operations are adversely impacted, we risk a delay, default and/or non-performance under existing agreements which may increase our costs. These cost increases may not be fully recoverable or adequately covered by insurance.
Infections and deaths related to the pandemic may disrupt the United States’ healthcare and healthcare regulatory systems. Such disruptions could divert healthcare resources away from, or materially delay FDA review and/or approval with respect to, our clinical trials. It is unknown how long these disruptions could continue, were they to occur. Any elongation or de-prioritization of our clinical trials or delay in regulatory review resulting from such disruptions could materially affect the development and study of our product candidates.
We currently utilize third parties to, among other things, manufacture raw materials. If any third-party in the supply chain for materials used in the production of our product candidates are adversely impacted by restrictions resulting from the coronavirus outbreak, our supply chain may be disrupted, limiting our ability to manufacture our product candidates for our clinical trials and research and development operations.
As a result of the shelter-in-place order and other mandated local travel restrictions, our employees conducting research and development or manufacturing activities may not be able to access their laboratory or manufacturing space which may result in our core activities being significantly limited or curtailed, possibly for an extended period of time.
While the potential economic impact brought by and the duration of the pandemic may be difficult to assess or predict, it has already caused, and is likely to result in further, significant disruption of global financial markets, which may reduce our ability to access capital either at all or on favorable terms. In addition, a recession, depression or other sustained adverse market event resulting from the spread of the coronavirus could materially and adversely affect our business and the value of our common stock.
The ultimate impact of the current pandemic, or any other health epidemic, is highly uncertain and subject to change. We do not yet know the full extent of new variants, potential delays or impacts on our business, our clinical trials, our research programs, healthcare systems or the global economy as a whole. However, these effects could have a material impact on our operations, and we will continue to monitor the situation closely.
Significant disruptions of information technology systems, computer system failures or breaches of information security could adversely affect our business.
We rely to a large extent upon sophisticated information technology systems to operate our business. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including, but not limited to, personal information and intellectual property). The size and complexity of our information technology and information security systems, and those of our third-party vendors with whom we may contract, make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from malicious attacks by third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage and market manipulation) and expertise. While we intend to invest in the protection of data and information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches.
| 32 |
Our internal computer systems, and those of our CROs, our CMOs, and other business vendors on which we may rely, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, fire, terrorism, war and telecommunication and electrical failures. We exercise little or no control over these third parties, which increases our vulnerability to problems with their systems. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs. Any interruption or breach in our systems could adversely affect our business operations or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business and reputational harm to us or allow third parties to gain material, inside information that they use to trade in our securities. For example, the loss of clinical trial data from completed or ongoing clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability, the further development of our current products and future product candidates could be delayed and our business could be otherwise adversely affected.
Risks Related to Healthcare Compliance Regulations
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings. If we or they are unable to comply with these provisions, we may become subject to civil and criminal investigations and proceedings that could have a material adverse effect on our business, financial condition and prospects.
Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of approved products and of any product candidates for which we obtain regulatory approval. Our current and future arrangements with healthcare providers, healthcare entities, third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we research, develop and will market, sell and distribute our approved products. As a pharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, federal and state healthcare laws and regulations pertaining to fraud and abuse and patients’ rights are applicable to our business. Restrictions under applicable federal and state healthcare laws and regulations that may affect our ability to operate include the following:
| ● | the federal healthcare Anti-Kickback Statute which prohibits, among other things, individuals and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid; | |
| ● | federal civil and criminal false claims laws, including the federal False Claims Act that can be enforced through civil whistleblower or qui tam actions, and civil monetary penalty laws, prohibit individuals or entities from knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment or approval that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; | |
| ● | the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”) which imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information on entities subject to the law, such as certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, and their respective business associates that perform services for them that involve the creation, use, maintenance or disclosure of, individually identifiable health information; |
| 33 |
| ● | the federal physician sunshine requirements under the Affordable Care Act (“ACA”) which requires certain manufacturers of devices, biologics and medical supplies, with certain exceptions, to report annually to Health and Human Services (“HHS”) information related to payments and other transfers of value to physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations; | |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; some state laws which require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers, marketing expenditures or pricing information; and certain state and local laws which require the registration of pharmaceutical sales representatives; and | |
| ● | state and foreign laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and often are not pre-empted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, disgorgement, exclusion from government funded healthcare programs, such as Medicare and Medicaid, integrity oversight and reporting obligations, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not comply with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Privacy Provisions of HIPAA
HIPAA, among other things, protects the privacy and security of individually identifiable health information by limiting its use and disclosure. HIPAA directly regulates “covered entities” (healthcare providers, insurers and clearinghouses) and indirectly regulates “business associates” with respect to the privacy of patients’ medical information. All entities that receive and process protected health information are required to adopt certain procedures to safeguard the security of that information. It is uncertain whether we would be deemed to be a covered entity under HIPAA, and it is unlikely that we, based on our current business model, would be a business associate. Nevertheless, we may be contractually required to physically safeguard the integrity and security of any patient information that we receive, store, create or transmit. If we fail to adhere to our contractual commitments, then certain of our contract counterparties may be subject to civil monetary penalties and this could adversely affect our ability to market our approved products. If we are deemed to be a vendor, under the Health Information Technology for Economic and Clinical Health Act, enacted as part of the American Recovery and Reinvestment Act of 2009, then we will be obligated to adopt various security measures. We may also be subject to state and foreign privacy laws under which breaches could lead to substantial fines and liability.
| 34 |
Risks Related to Owning our Common Stock and This Offering
Internal control deficiencies have historically been identified that constituted material weaknesses in our internal control over financial reporting. If we fail to implement and maintain effective internal controls over financial reporting, we may be unable to accurately or timely report our financial condition or results of operations, which may adversely affect our business.
In connection with the audit of the Company’s consolidated financial statements for the years ended December 31, 2021 and 2020, the Company identified material weaknesses in internal control over financial reporting. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. Management has determined that the Company had the following material weaknesses in its internal control over financial reporting:
Control Environment, Risk Assessment, and Monitoring
Management did not maintain appropriately designed entity-level controls impacting the control environment, risk assessment procedures, and effective monitoring controls to prevent or detect material misstatements to the consolidated financial statements. These deficiencies were attributed to a lack of structure and responsibility, insufficient number of qualified resources, and inadequate oversight and accountability over internal controls.
Control Activities and Information and Communication
These material weaknesses contributed to the following additional material weaknesses with certain business processes and the information technology environment:
| ● | Management did not maintain appropriately designed information technology general controls in the areas of user access, and segregation of duties related to certain information technology systems that support the Company’s financial reporting process. | |
| ● | Management did not design and maintain effective controls over the financial close process. Specifically, management did not identify controls over the timely review of accounts to support its financial statements, and did not implement controls around the review of accrued expenses. |
Management intends to implement additional controls to address the material weaknesses, including:
| ● | Restricting user access and dedicating personnel, including management, to specific controls to improve the control environment; | |
| ● | Continue to hire qualified staff and outside resources to segregate key functions within the Company’s financial and information technology processes | |
| ● | Enhancing and expanding policies and procedures over the performance of user access reviews, change management, and the monitoring of segregation of duties; | |
| ● | Developing a training program and educating control owners concerning the principles and requirements of each control related to user access, change management, and segregation of duties within IT systems impacting financial reporting; | |
| ● | Reassessing and formalizing the design of certain accounting and information technology policies relating to security and change management controls; | |
| ● | Continuing to enhance and formalize the Company’s accounting, business operations, and information technology policies, procedures, and controls to achieve complete, accurate, and timely; financial accounting, reporting and disclosures; | |
| ● | Developing internal controls documentation, including comprehensive accounting policies and procedures over certain key financial processes and related disclosures; and |
| 35 |
While Management believes that these efforts will improve the Company’s internal control over financial reporting, the implementation of these procedures has not yet been implemented and will require validation and testing of the design and operating effectiveness of internal controls over a sustained period of financial reporting cycles. Management cannot be certain that these measures will successfully remediate the material weaknesses, or that other material weaknesses and control deficiencies will not be discovered in the future.
Management has not completed an assessment of the effectiveness of internal control over financial reporting and the Company’s independent registered public accounting firm has not conducted an audit of the Company’s internal control over financial reporting. Evaluation of the Company’s internal controls over financial reporting may identify additional material weaknesses. The identification of a material weakness in the Company’s internal controls or the failure to remediate existing material weaknesses in internal controls may cause the Company to be unable to report financial information on a timely basis and thereby subject the Company to adverse regulatory consequences, including sanctions by the SEC or violations of Nasdaq rules. There also could be a negative reaction in the financial markets due to a loss of investor confidence in the Company and the reliability of its financial statements. This could have a material adverse effect on business, financial condition and results of operations and could also to a decline in the price of the Company’s common stock.
Management is not currently required to comply with the SEC’s rules Section 404 of Sarbanes-Oxley and are therefore not required to make a formal assessment of the effectiveness of the Company’s internal control over financial reporting for that purpose. Upon becoming a public company, Management will be required to comply with the SEC’s rules implementing Sections 302 and 404 of Sarbanes-Oxley, which will require the Company’s management to certify financial and other information in the quarterly and annual reports and provide an annual management report on the effectiveness of the Company’s internal control over financial reporting. Though Management will be required to disclose material changes made to internal controls and procedures on a quarterly basis, Management will not be required to make the first annual assessment of the Company’s internal control over financial reporting pursuant to Section 404 until the year following the first annual report Management is required to file with the SEC. To comply with the requirements of being a public company, Management will need to implement additional internal controls, reporting systems and procedures and hire additional accounting and finance staff. For as long as Management is an “emerging growth company” under the JOBS Act, the Company’s independent registered public accounting firm will not be required to attest to the effectiveness of internal control over financial reporting pursuant to Section 404. Management could be an “emerging growth company” for up to five years. An independent assessment of the effectiveness of the Company’s internal control over financial reporting could detect problems that the management assessment might not identify. Undetected material weaknesses in the Company’s internal control over financial reporting could lead to financial statement restatements and require the Company to incur the expense of remediation.
If we fail to establish and maintain an effective system of integrated internal controls, we may not be able to report our financial results accurately, which could have a material adverse effect on our business, financial condition and results of operations.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that will need to be evaluated frequently. Section 404 of the Sarbanes-Oxley Act requires public companies to conduct an annual review and evaluation of their internal controls and requires attestations of the effectiveness of internal controls by independent auditors. We would be required to perform the annual review and evaluation of our internal controls no later than for fiscal 2027. We initially expect to qualify as an emerging growth company, and thus, we would be exempt from the auditors’ attestation requirement until such time as we no longer qualify as an emerging growth company. Regardless of whether we qualify as an emerging growth company, we will still need to implement substantial control systems and procedures in order to satisfy the reporting requirements under the Exchange Act and applicable Nasdaq requirements, among other items. Establishing these internal controls will be costly and may divert management’s attention.
Evaluation by us of our internal controls over financial reporting may identify material weaknesses that may cause us to be unable to report our financial information on a timely basis and thereby subject us to adverse regulatory consequences, including sanctions by the SEC or violations of Nasdaq rules. There also could be a negative reaction in the financial markets due to a loss of investor confidence in us and the reliability of our financial statements. Confidence in the reliability of our financial statements also could suffer if we or our independent registered public accounting firm were to report a material weakness in our internal controls over financial reporting. This could have a material adverse effect on our business, financial condition and results of operations and could also lead to a decline in the price of our common stock.
| 36 |
Financial reporting obligations of being a public company in the U.S. are expensive and time-consuming, and our management will be required to devote substantial time to compliance matters.
As a publicly traded company we will incur significant additional legal, accounting and other expenses. The obligations of being a public company in the U.S. will require significant expenditures and will place significant demands on our management and other personnel, including costs resulting from public company reporting obligations under the Exchange Act and the rules and regulations regarding corporate governance practices, including those under the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, and the listing requirements of the stock exchange on which our securities are listed. These rules require the establishment and maintenance of effective disclosure and financial controls and procedures, internal control over financial reporting and changes in corporate governance practices, among many other complex rules that are often difficult to implement, monitor and maintain compliance with. Moreover, despite recent reforms made possible by the JOBS Act, the reporting requirements, rules, and regulations will make some activities more time-consuming and costly, particularly after we are no longer an “emerging growth company.” In addition, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance. Our management and other personnel will need to devote a substantial amount of time to ensure that we comply with all of these requirements and to keep pace with new regulations, otherwise we may fall out of compliance and risk becoming subject to litigation or being delisted, among other potential problems.
The market price of our Common Stock may be volatile.
The market price of our Common Stock may be highly volatile. Some of the factors that may materially affect the market price of our Common Stock are beyond our control, such as changes in financial estimates by industry and securities analysts, conditions or trends in the industry in which we operate or sales of our Common Stock. These factors may materially adversely affect the market price of our Common Stock, regardless of our performance. In addition, public stock markets have experienced extreme price and trading volume volatility. This volatility has significantly affected the market prices of securities of many companies for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our Common Stock.
We may issue more shares in a future financing or pursuant to existing agreements which will result in substantial dilution.
Our Certificate of Incorporation authorizes the issuance of a maximum of 95,000,000 shares of Common Stock and a maximum of 5,000,000 shares of Preferred Stock. Any future merger or acquisition effected by us would result in the issuance of additional securities without stockholder approval and the substantial dilution in the percentage of our Common Stock held by our then existing stockholders. Moreover, the Common Stock issued in any such merger or acquisition transaction may be valued on an arbitrary or non-arm’s-length basis by our management, resulting in an additional reduction in the percentage of Common Stock held by our then existing stockholders. Additionally, we expect to seek additional financing in order to provide working capital to the operating business. Our Board of Directors has the power to issue any or all of such authorized but unissued shares without stockholder approval. To the extent that additional shares of Common Stock or Preferred Stock are issued in connection with and following a business combination or otherwise, dilution to the interests of our stockholders will occur and the rights of the holders of Common Stock might be materially and adversely affected.
Our Board of Directors is authorized to issue Preferred Stock without obtaining shareholder approval.
Our Certificate of Incorporation authorizes the issuance of up to 5,000,000 shares of Preferred Stock with designations, rights and preferences determined from time to time by the Board of Directors. Accordingly, our Board of Directors is empowered, without stockholder approval, to issue Preferred Stock with dividend, liquidation, conversion, voting, or other rights which could adversely affect the voting power or other rights of the holders of the Common Stock. In the event of issuance, the Preferred Stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of the Company. Although we have no present intention to issue any shares of Preferred Stock, there can be no assurance that the Company will not do so in the future.
| 37 |
An active trading market for our common stock may not develop, and you may not be able to sell your common stock at or above the public offering price.
Prior to the consummation of this offering, there has been no public market for our common stock. An active trading market for shares of our common stock may never develop or be sustained following this offering. If an active trading market does not develop, you may have difficulty selling your shares of common stock at an attractive price, or at all. The price for our Shares in this offering will be determined by negotiations between us and the underwriters, and it may not be indicative of prices that will prevail in the open market following this offering. Consequently, you may not be able to sell your common stock at or above the public offering price or at any other price or at the time that you would like to sell. An inactive market may also impair our ability to raise capital by selling our common stock, and it may impair our ability to attract and motivate our employees through equity incentive awards and our ability to acquire other companies, products or technologies by using our common stock as consideration.
The price of our common stock may fluctuate substantially.
You should consider an investment in our common stock to be risky, and you should invest in our Shares only if you can withstand a significant loss and wide fluctuations in the market value of your investment. We expect that revenue from sales, if any, will fluctuate significantly and our future annual expenses as a percentage of our revenue may be significantly different from those we have recorded in the past. Our financial results may fluctuate from period to period due to a number of factors, including the timing of completion of our Souderton facility. After the completion of the facility, factors affecting the fluctuation of our financial results include the timing of the production and sale of Oxyglobin and Hemopure, the successful development and commercialization of new indications of Hemopure and ZK1, and foreign currency exchange rates.
Other factors that may cause the market price of our common stock to fluctuate, in addition to the other risks mentioned in this “Risk Factors” section and elsewhere in this prospectus, are:
| ● | sale of our common stock by our stockholders, executives, and directors and our stockholders whose shares are being registered in this offering; | |
| ● | volatility and limitations in trading volumes of our shares of common stock; | |
| ● | our ability to obtain financings to conduct and complete research and development activities including, but not limited to, our clinical trials, and other business activities; | |
| ● | possible delays in the expected recognition of revenue due to lengthy and sometimes unpredictable sales timelines; | |
| ● | the timing and success of introductions of new products by us or our competitors or any other change in the competitive dynamics of our industry, including consolidation among competitors, customers or strategic partners; | |
| ● | network outages or security breaches; | |
| ● | our ability to attract new customers; | |
| ● | our ability to secure resources and the necessary personnel to conduct clinical trials on our desired schedule; | |
| ● | commencement, enrollment or results of our clinical trials for our product candidate or any future clinical trials we may conduct; |
| 38 |
| ● | changes in the development status of our product candidates; | |
| ● | any delays or adverse developments or perceived adverse developments with respect to the FDA’s review of our planned preclinical and clinical trials; | |
| ● | any delay in our submission for studies or product approvals or adverse regulatory decisions, including failure to receive regulatory approval for our product candidates; | |
| ● | unanticipated safety concerns related to the use of our products or product candidates; | |
| ● | failures to meet external expectations or management guidance; | |
| ● | changes in our capital structure or dividend policy, future issuances of securities, sales of large blocks of common stock by our stockholders; | |
| ● | our cash position; | |
| ● | announcements and events surrounding financing efforts, including debt and equity securities; | |
| ● | our inability to enter into new markets or develop new products; | |
| ● | reputational issues; | |
| ● | competition from existing technologies and products or new technologies and products that may emerge; | |
| ● | announcements of acquisitions, partnerships, collaborations, joint ventures, new products, capital commitments, or other events by us or our competitors; | |
| ● | changes in general economic, political and market conditions in or any of the regions in which we conduct our business; | |
| ● | changes in industry conditions or perceptions; | |
| ● | changes in valuations of similar companies or groups of companies; | |
| ● | analyst research reports, recommendation and changes in recommendations, price targets, and withdrawals of coverage; | |
| ● | departures and additions of key personnel; | |
| ● | disputes and litigations related to intellectual properties, proprietary rights, and contractual obligations; | |
| ● | changes in applicable laws, rules, regulations, or accounting practices and other dynamics; and | |
| ● | other events or factors, many of which may be out of our control. |
In addition, if the market for stocks in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
| 39 |
A sale or perceived sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
All of our executive officers and directors and all of our stockholders and warrant holders have agreed not to sell shares of our common stock for a period of 180 days following this offering, subject to extension under specified circumstances. See “Underwriting.” Common stock subject to these lock-up agreements will become eligible for sale in the public market upon expiration of these lock-up agreements, subject to limitations imposed by Rule 144 under the Securities Act of 1933, as amended. If our stockholders sell substantial amounts of our common stock in the public market, the market price of our common stock could fall. Moreover, the perceived risk of this potential dilution could cause stockholders to attempt to sell their shares and investors to short our common stock. These sales also may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
Market and economic conditions may negatively impact our business, financial condition and share price.
Concerns over medical epidemics, energy costs, geopolitical issues such as the conflict between Russia and Ukraine, the U.S. mortgage market, unstable global credit markets and financial conditions, and volatile oil prices have led to periods of significant economic instability, diminished liquidity and credit availability, declines in consumer confidence and discretionary spending, diminished expectations for the global economy and expectations of slower global economic growth, increased unemployment rates, and increased credit defaults in recent years. Our general business strategy may be adversely affected by any such economic downturns (including the current downturn related to the current COVID-19 pandemic), volatile business environments and continued unstable or unpredictable economic and market conditions. If these conditions continue to deteriorate or do not improve, it may make any necessary debt or equity financing more difficult to complete, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance, and share price and could require us to delay or abandon development or commercialization plans.
If securities or industry analysts do not publish research or reports, or publish unfavorable research or reports about our business, our stock price and trading volume may decline.
The trading market for our common stock will rely in part on the research and reports that industry or financial analysts publish about us, our business, our markets and our competitors. We do not control these analysts. If securities analysts do not cover our common stock after the closing of this offering, the lack of research coverage may adversely affect the market price of our common stock. Furthermore, if one or more of the analysts who do cover us downgrade our stock or if those analysts issue other unfavorable commentary about us or our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our stock could decrease, which in turn could cause our stock price or trading volume to decline and may also impair our ability to expand our business with existing customers and attract new customers.
Because certain of our stockholders control a significant number of shares of our common stock, they may have effective control over actions requiring stockholder approval.
Following this offering, ___ will directly or indirectly beneficially own approximately __% of our outstanding shares of common stock. As a result, ___, would have the ability to control the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. Accordingly, this concentration of ownership might harm the market price of our common stock by:
| ● | delaying, deferring or preventing a change in corporate control; | |
| ● | impeding a merger, consolidation, takeover or other business combination involving us; or | |
| ● | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
| 40 |
You will incur immediate dilution as a result of this offering.
If you purchase common stock in this offering, you will pay more for your shares than the net tangible book value of your shares. As a result, you will incur immediate dilution of $___ per share, representing the difference between the assumed public offering price of $__ per share and our estimated as adjusted net tangible book value as of December 31, 2021, of $___ per share. Accordingly, should we be liquidated at our book value, you would not receive the full amount of your investment.
We do not intend to pay cash dividends on our shares of common stock so any returns will be limited to the value of our shares.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to the increase, if any, of our share price.
We may be at risk of securities class action litigation.
We may be at risk of securities class action litigation. In the past, medical device, biotechnology and pharmaceutical companies have experienced significant stock price volatility, particularly when associated with binary events such as clinical trials and product approvals. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business and results in a decline in the market price of our common stock.
Our Certificate of Incorporation and our Bylaws, and Delaware law may have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price to decline.
Our Certificate of Incorporation and our Bylaws, and Delaware law could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. Upon consummation of this offering, we will be authorized to issue up to 5,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our Board of Directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our Certificate of Incorporation and our Bylaws and Delaware law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. Such provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, the certificate of incorporation and bylaws and Delaware law, as applicable, among other things:
| ● | provide the board of directors with the ability to alter the bylaws without stockholder approval; | |
| ● | place limitations on the removal of directors; | |
| ● | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings; and | |
| ● | provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum. |
General Risks
Our ability to consummate this offering might be affected due to the uncertainty resulting from a war, civil unrest, political and economic instability, including the Russian invasion in Ukraine.
| 41 |
INFORMATION REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that involve risks and uncertainties. You should not place undue reliance on these forward-looking statements. All statements other than statements of historical facts contained in this prospectus are forward-looking statements. The forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. In some cases, you can identify these forward-looking statements by terms such as “anticipate,” “believe,” “continue,” “could,” “depends,” “estimate,” “expects,” “intend,” “may,” “ongoing,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms or other similar expressions, although not all forward-looking statements contain those words. We have based these forward-looking statements on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short- and long-term business operations and objectives, and financial needs. These forward-looking statements include, but are not limited to, statements concerning the following:
| ● | our projected financial position and estimated cash burn rate; | |
| ● | our estimates regarding expenses, future revenues and capital requirements; | |
| ● | our ability to continue as a going concern; | |
| ● | our need to raise substantial additional capital to fund our operations; | |
| ● | the success, cost and timing of our clinical trials; | |
| ● | our dependence on third parties in the conduct of our clinical trials; | |
| ● | our ability to obtain the necessary regulatory approvals to market and commercialize our approved products and product candidates; | |
| ● | the ultimate impact of the current coronavirus pandemic, or any other health epidemic, on our business, our clinical trials, our research programs, healthcare systems or the global economy as a whole; | |
| ● | the potential that results of preclinical and clinical trials indicate that product candidates we may seek to develop are unsafe or ineffective; | |
| ● | the results of market research conducted by us or others; | |
| ● | our ability to obtain and maintain intellectual property protection for our approved products and any product candidates; | |
| ● | our ability to protect our intellectual property rights and the potential for us to incur substantial costs from lawsuits to enforce or protect our intellectual property rights; | |
| ● | the possibility that a third party may claim we or our third-party licensors have infringed, misappropriated or otherwise violated their intellectual property rights and that we may incur substantial costs and be required to devote substantial time defending against claims against us; | |
| ● | our reliance on third-party suppliers and manufacturers; | |
| ● | the success of competing therapies and products that are or become available; |
| 42 |
| ● | our ability to expand our organization to accommodate potential growth and our ability to retain and attract key personnel; | |
| ● | the potential for us to incur substantial costs resulting from product liability lawsuits against us and the potential for these product liability lawsuits to cause us to limit our commercialization of our approved products or any product candidate, if approved; | |
| ● | market acceptance of our approved products or any product candidate, if approved, the size and growth of the potential markets for our approved products or any product candidate, if approved that we may seek to develop, and our ability to serve those markets; and | |
| ● | the successful development of our commercialization capabilities, including sales and marketing capabilities. |
These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus to conform these statements to actual results or to changes in our expectations.
You should read this prospectus and the documents that we reference in this prospectus and have filed with the SEC as exhibits to the registration statement of which this prospectus is a part with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect
This prospectus contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this prospectus from our own research as well as from industry and general publications, surveys and studies conducted by third parties. This data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty, including those discussed in “Risk Factors.” We caution you not to give undue weight to such projections, assumptions and estimates. Further, industry and general publications, studies and surveys generally state that they have been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. While we believe that these publications, studies and surveys are reliable, we have not independently verified the data contained in them. In addition, while we believe that the results and estimates from our internal research are reliable, such results and estimates have not been verified by any independent source.
| 43 |
We estimate that the net proceeds from our issuance and sale of our Common Stock in this offering will be approximately $____, based on an assumed public offering price of $__ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters exercise their over-allotment option to purchase additional shares in full, we estimate that the net proceeds from this offering will be approximately $___.
We intend to use the net proceeds as follows: at least $20 million toward reassembly of the company’s facility in Souderton, Pennsylvania with the remaining proceeds for general corporate expenses and working capital.
A $1.00 increase or decrease in the assumed public offering price of $___ per share would increase or decrease the net proceeds from this offering by approximately $___, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus remains the same and after deducting the estimated underwriting discounts and commissions and non-accountable expense allowance payable to the underwriters.
This expected use of the net proceeds from this offering and our existing cash represents our intentions based upon our current plans, financial condition and business conditions. The proceeds of the Offering will be insufficient to fully complete the relaunch of our products into the market. We will require approximately $15 million of additional monies to complete this process. We will need to seek such additional funds through public or private equity or debt financings or other sources, such as strategic collaborations or license and development agreements. If we do not successfully raise such monies, the facility cannot be completed and we will be unable to produce and commercially distribute any product. Further, predicting the cost necessary to develop a product candidate can be difficult and the amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development and commercialization efforts, the status of and results from clinical trials, any collaborations that we may enter into with third parties for our products and product candidates and any unforeseen cash needs. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering and our existing cash.
In the ordinary course of our business, we expect to from time to time evaluate the acquisition of, investment in or in-license of complementary products, technologies or businesses, and we could use a portion of the net proceeds from this offering for such activities. We currently do not have any agreements, arrangements or commitments with respect to any potential acquisition, investment or license.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including short-term, investment-grade, interest-bearing instruments and government securities.
We have never paid or declared any cash dividends on our common stock, and we do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain all available funds and any future earnings to fund the development and expansion of our business. Any future determination to pay dividends will be at the discretion of our board of directors and will depend upon a number of factors, including our results of operations, financial condition, future prospects, contractual restrictions, restrictions imposed by applicable law and other factors our board of directors deems relevant.
CORPORATE CONVERSION
We currently operate as a Delaware limited liability company under the name Hemoglobin Oxygen Therapeutics LLC. Prior to the closing of this offering, Hemoglobin Oxygen Therapeutics LLC will convert into a Delaware corporation pursuant to a statutory conversion, and will change its name to Hemoglobin Oxygen Therapeutics Inc. In order to consummate the corporate conversion, a certificate of conversion will be filed with the Secretary of State of the State of Delaware. In this prospectus, we refer to all transactions related to our conversion to a corporation as the Corporate Conversion.
As part of the Corporate Conversion, based on the assumed initial offering price of $[ ] , which is the midpoint of the price range set forth on the cover page of this prospectus, all of our outstanding membership interests will be converted into an aggregate of shares of our common stock [and existing options to purchase membership interests will be converted into options to purchase an aggregate of shares of our common stock, for the same aggregate purchase price and upon the same terms and conditions].
| 44 |
In connection with the Corporate Conversion, Hemoglobin Oxygen Therapeutics Inc. will continue to hold all property and assets of Hemoglobin Oxygen Therapeutics LLC and will assume all of the debts and obligations of Hemoglobin Oxygen Therapeutics LLC. Hemoglobin Oxygen Therapeutics Inc. will be governed by a certificate of incorporation filed with the Secretary of State of the State of Delaware and bylaws, the material portions of which are described under the heading “Description of Capital Stock.” On the effective date of the Corporate Conversion, the members of the board of managers of Hemoglobin Oxygen Therapeutics LLC will become the initial members of Hemoglobin Oxygen Therapeutics Inc.’s board of directors, and as such are referred to throughout this prospectus as “Directors” rather than “Managers” and the officers of Hemoglobin Oxygen Therapeutics LLC will become the officers of Hemoglobin Oxygen Therapeutics Inc.
References in this prospectus to our capitalization and other matters pertaining to our equity prior to the Corporate Conversion relate to the capitalization and equity of Hemoglobin Oxygen Therapeutics LLC, and after the Corporate Conversion, to Hemoglobin Oxygen Therapeutics Inc. The financial statements included elsewhere in this prospectus are those of Hemoglobin Oxygen Therapeutics LLC. We expect that the Corporate Conversion will not have a material effect on our financial statements.
The purpose of the Corporate Conversion is to reorganize our structure so that the entity that is offering our common stock to the public in this offering is a Delaware corporation rather than a Delaware limited liability company, and so that our existing investors will own our common stock rather than equity interests in a limited liability company.
The following table sets forth our actual, pro forma, and pro forma as adjusted capitalization as of December 31, 2021,
| Year Ended December 31, 2021 | ||||||||||||
Actual (a) |
Pro Forma (unaudited) (b) | Pro Forma As Adjusted (unaudited) (c) | ||||||||||
| Cash | $ | 443,895 | $ | 443,895 | $ | - | ||||||
| Members’ equity/(deficit): | ||||||||||||
| Membership interests: membership interests issued and outstanding, actual; no membership interests issued or outstanding for, pro forma as adjusted | (9,249,148 | ) | - | - | ||||||||
| Stockholders’ equity: | ||||||||||||
| Common stock, $0.001 par value per share; no shares authorized, issued and ____ shares authorized pro forma, pro forma, as adjusted, and ____ and ____ shares issued and outstanding, pro forma and pro forma, as adjusted. | - | 18,477 | - | |||||||||
| Additional paid-in capital | - | - | - | |||||||||
| Accumulated deficit | - | (1,142,900 | ) | - | ||||||||
| Total equity (deficit) | (9,249,148 | ) | (1,124,423 | ) | - | |||||||
| Total capitalization | $ | (8,805,253 | ) | $ | (680,528 | ) | $ | - | ||||
| (a) | on an actual basis; |
| 45 |
| (b) | on a pro forma basis to give effect to the Corporate Conversion and the Debt Conversion, based on the assumed initial public offering price of $ [ ] per share, which is the midpoint of the price range set forth on the cover page of this prospectus; and illustrated in the unaudited pro forma financial information included within this Prospectus. | |
| (c) | on a pro forma as adjusted basis to give further effect to our issuance and sale of shares of our common stock in this offering at an assumed initial public offering price of $ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us and therefore providing net proceeds of approximately $20 million.. |
The pro forma as adjusted information below is illustrative only, and our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this information in conjunction with our financial statements and the related notes included elsewhere in this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Corporate Conversion” sections and other financial information contained in this prospectus.
| (1) | In connection with the Corporate Conversion, all membership interests will be reduced to zero to reflect the elimination of all outstanding units and other interests in Hemoglobin Oxygen Therapeutics LLC and corresponding adjustments will be reflected as common stock and additional paid-in capital. The pro forma and pro forma as adjusted information is illustrative only. |
| (2) | Each $1.00 increase (decrease) in the assumed initial public offering price of $ [ ] per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash, total equity and total capitalization by $[ ] million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of 0.1 million shares in the number of shares offered by us at the assumed initial public offering price per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted amount of each of cash, total equity and total capitalization by approximately $ [ ] million. |
The number of shares of common stock on a pro forma and pro forma as adjusted basis set forth in the table above is based on _____shares of our common stock outstanding as of December 31, 2021 after giving effect to (i) the Corporate Conversion to occur immediately prior to the effective date of this offering and (ii) the issuance of _____ shares of common stock issuable upon conversion of convertible and other indebtedness immediately prior to the effective date of this offering assuming an initial public offering price of $____per share, which is the midpoint of the price range set forth on the cover page of this prospectus (the “Debt Conversion”) and excludes as of such date:
| ● | 922,011 and [ ] shares of common stock issuable upon exercise of the outstanding common stock warrant at an exercise price of $2.75 and $[ ] per common share, respectively. |
| 46 |
| ● | [ ] shares of common stock reserved for future grants pursuant to our 2022 Equity Incentive Plan. |
| ● | [ ] shares of common stock issuable upon exercise of warrants to be issued to the underwriters as part of this offering at an exercise price of $[ ] per common share (120% of the assumed public offering price of $[ ] per share). |
| ● | No exercise by the underwriters of their option to purchase an additional __________shares of common stock. |
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the assumed initial public offering price of $ per share (the mid-point of the range appearing on the front cover of this prospectus) and the pro forma as adjusted net tangible book value per share of our common stock immediately upon the consummation of this offering. Pro forma net tangible book value per share represents the book value of our tangible assets less the book value of our products or any product candidate, if approved;
After giving effect to the Corporate Conversion and the Debt Conversion, as of December 31, 2021, we had a pro forma historical net tangible book value of $(1,121,687), or $(0.01 ) per share of common stock, based on ___ shares of common stock deemed outstanding pro forma as of December 31, 2021.
After giving effect to the issuance and sale of ___ shares of our common stock in this offering at an assumed public offering price of $___ per share, and after deducting estimated underwriting discounts and commissions, the non-accountable expense allowance payable to the underwriters, and estimated offering costs payable by us, our pro forma as adjusted net tangible book value as of [December 31, 2021] would have been $____, or $___ per share. This represents an immediate increase in pro forma as adjusted net tangible book value per share of $___ to existing stockholders and immediate dilution of $___ in pro forma as adjusted net tangible book value per share to new investors purchasing common stock in this offering. Dilution per share to new investors is determined by subtracting pro forma as adjusted net tangible book value per share after this offering from the assumed public offering price per share paid by new investors. The following table illustrates this dilution on a per share basis:
| Assumed combined public offering price per share | $ | |||||||
| Historical net tangible book value per share as of December 31, 2021 | (0.01 | ) | ||||||
| Increase in historical net tangible book value per share attributable to this offering | $ | |||||||
| As adjusted net tangible book value per share as of December 31, 2021 after this offering | ||||||||
| Dilution in as adjusted net tangible book value per share to new investors | $ |
The dilution information discussed above is illustrative only and will change based on the actual public offering price and other terms of this offering determined at pricing. A $1.00 increase in the assumed public offering price of $___ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would increase our pro forma net tangible book value after this offering by approximately $____ per share and the dilution to new investors purchasing common stock in this offering by approximately $___ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discount and commissions and the non-accountable expense allowance payable to the underwriters. A $1.00 decrease in the assumed public offering price of $___ per share, which is the midpoint of the price range set forth on the cover page of this prospectus, would decrease our pro forma net tangible book value after this offering by approximately $___ per share and the dilution to new investors purchasing common stock in this offering by approximately $___ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discount and commissions and the non-accountable expense allowance payable to the underwriters.
| 47 |
An increase of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase our pro forma net tangible book value after this offering by approximately $___ per share and decrease the dilution to new investors purchasing common stock in this offering by approximately $___ per share, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and the non-accountable expense allowance payable to the underwriters. A decrease of 500,000 shares in the number of shares offered by us, as set forth on the cover page of this prospectus, would decrease our pro forma net tangible book value after this offering by approximately $___ per share and increase the dilution to new investors purchasing common stock in this offering by approximately $___ per share, assuming no change in the assumed public offering price per share and after deducting estimated underwriting discounts and commissions and the non-accountable expense allowance payable to the underwriters.
If the underwriters exercise their option to purchase additional shares in full, the as adjusted net tangible book value per share after giving effect to the offering would be $___ per share. This represents an increase in as adjusted net tangible book value of $___ per share to existing stockholders and dilution in as adjusted net tangible book value of $___ per share to new investors.
The following table sets forth as of December 31, 2021, on a pro forma combined basis to reflect the Corporate Conversion and the Debt Conversion, the number of shares of common stock, the total price and average price per share paid to us by our existing holders and the new investors, before deducting estimated offering and acquisition expenses payable by us, using the assumed public offering price of $___ per share (the mid-point of the range appearing on the front cover of this prospectus).
| Shares Purchased | Total Consideration | Average | ||||||||||||||||||
| Number | Percent | Number | Percent | Price | ||||||||||||||||
| Existing stockholders | $ | $ | ||||||||||||||||||
| New investors in this offering | $ | 30,000,000 | $ | |||||||||||||||||
| Total capitalization | 100.0 | % | 100.0 | % | $ | |||||||||||||||
The number of shares of common stock outstanding after this offering excludes:
| ● | 922,011 and [ ] shares of common stock issuable upon exercise of the outstanding common stock warrant at an exercise price of $2.75 and $[ ] per common share, respectively. |
| ● | [ ] shares of common stock reserved for future grants pursuant to our 2022 Equity Incentive Plan. | |
| ● | [ ] shares of common stock issuable upon exercise of warrants to be issued to the underwriters as part of this offering at an exercise price of $[ ] per common share (120% of the assumed public offering price of $[ ] per share). |
To the extent that stock options are exercised, or we issue additional shares of common stock in the future, there will be further dilution to investors participating in this offering. In addition, if we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
| 48 |
UNAUDITED PRO FORMA FINANCIAL INFORMATION
Defined terms included below have the same meaning as terms defined and included elsewhere in this prospectus.
Introduction
The following unaudited pro forma financial information is provided to you to aid in your analysis of the financial aspects of the Corporate Conversion and Debt Conversion as defined elsewhere in this prospectus. The following unaudited pro forma financial information has been prepared in accordance with Article 11 of Regulation S-X as amended by the final rule, Release No. 33-10786.
The following unaudited pro forma balance sheet of Hemoglobin Oxygen Therapeutics LLC (“HbO2”) as of December 31, 2021 after giving effect to the Corporate Conversion and Debt Conversion and related adjustments described in the accompanying notes. See the accompanying notes to the unaudited pro forma financial information for a discussion of assumptions made.
The unaudited pro forma financial information has been developed from and should be read in conjunction with:
| ● | the accompanying notes to the unaudited pro forma financial statements; | |
| ● | HbO2’s audited consolidated financial statements as of and for the year ended December 31, 2021 and the related notes, which are included elsewhere in this prospectus; | |
| ● | other information related to HbO2 included elsewhere in this prospectus, including sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and other financial information. |
Basis of Pro Forma Presentation
The unaudited pro forma balance sheet and statement of operations have presented for illustrative purposes only and do not necessarily reflect what HbO2’s financial condition would have been had the Corporate Conversion and Debt Conversion occurred on the date indicated. Further, the unaudited pro forma financial information also may not be useful in predicting the future financial condition of HbO2. The actual financial position may differ significantly from the pro forma amounts reflected herein due to a variety of factors. The unaudited pro forma adjustments represent management’s estimates based on information available as of the date of this filing and is subject to change as additional information becomes available and analyses are performed. However, Management believes that the assumptions provide a reasonable basis for presenting the significant effects of the Pro Forma Transactions as contemplated and that the pro forma adjustments give appropriate effect to those assumptions and are properly applied in the unaudited pro forma balance sheet and pro forma statement of operations. These pro forma statements do not reflect the use of proceeds from this offering.
| 49 |
| As of December 31, 2021 | ||||||||||||
| Historical | Pro Forma Adjustments | Pro Forma as Converted | ||||||||||
| Assets | ||||||||||||
| Current Assets | ||||||||||||
| Cash and cash equivalents | $ | 443,895 | $ | - | $ | 443,895 | ||||||
| Prepaid expenses and other current assets | 141,401 | 141,401 | ||||||||||
| Total Current Assets | 585,296 | - | 585,296 | |||||||||
| Property and equipment, net | 4,477,719 | - | 4,477,719 | |||||||||
| Operating right-of-use asset | 932,103 | - | 932,103 | |||||||||
| Deposits | 39,870 | - | 39,870 | |||||||||
| Total Assets | $ | 6,034,988 | $ | - | $ | 6,034,988 | ||||||
| Liabilities and Equity/(Deficit) | ||||||||||||
| Current Liabilities | ||||||||||||
| Accounts payable | $ | 1,729,575 | $ | (486,000 | )(A) | $ | 1,243,575 | |||||
| Accrued expenses and other current liabilities | 2,770,051 | (566,749 | )(B) | 2,203,302 | ||||||||
| Convertible notes payable, current | 3,880,000 | (3,880,000 | )(C) | - | ||||||||
| Convertible notes payable, current – related party | 390,000 | (390,000 | )(C) | - | ||||||||
| Payable to landlord | 3,152,111 | (2,000,000 | )(D) | 1,152,111 | ||||||||
| Operating lease liability, current | 695,633 | - | 695,633 | |||||||||
| Accrued interest, current – related party | 202,622 | (202,622 | )(E) | - | ||||||||
| Deferred revenue | 378,637 | - | 378,637 | |||||||||
| Warrant liability | 888,674 | - | 888,674 | |||||||||
| Total Current Liabilities | 14,087,303 | (7,525,371 | ) | 6,561,932 | ||||||||
| Convertible notes payable, net | 395,071 | (395,071 | )(C) | - | ||||||||
| Convertible notes payable, net – related party | 100,000 | (100,000 | )(C) | - | ||||||||
| Notes payable – related party | 300,485 | - | 300,485 | |||||||||
| Accrued interest, related party | 32,174 | (29,438 | )(E) | 2,736 | ||||||||
| Accrued interest | 74,845 | (74,845 | )(B) | - | ||||||||
| Operating lease liability | 294,258 | - | 294,258 | |||||||||
| Total Liabilities | 15,284,136 | (8,124,725 | ) | 7,159,411 | ||||||||
| Equity/Deficit | ||||||||||||
| Members’ equity/(deficit) | (9,249,148 | ) | 9,249,148 | (G) | - | |||||||
| Common stock | - | 18,477 | (F)(G) | 18,477 | ||||||||
| Additional paid in capital | - | - | (A)(C)(D) (E) | - | ||||||||
| - | - | |||||||||||
| Accumulated deficit | - | (1,142,900 | )(A)(B)(C)(D)(E)(G) | (1,142,900 | ) | |||||||
| Total Equity/(Deficit) | (9,249,148 | ) | 8,124,725 | (1,124,423 | ) | |||||||
| Total Liabilities and Equity/(Deficit) | $ | 6,034,988 | $ | - | $ | 6,034,988 | ||||||
| 50 |
| For the Year Ended December 31, 2021 | ||||||||||||
| Historical | Pro Forma Adjustments | Pro Forma as Converted | ||||||||||
| Revenues | $ | - | $ | - | $ | - | ||||||
| Operating expenses | ||||||||||||
| Research and development | 768,621 | - | 768,621 | |||||||||
| General and administrative | 2,668,311 | - | 2,668,311 | |||||||||
| Total operating expenses | 3,436,932 | - | 3,436,932 | |||||||||
| Loss from operations | (3,436,932 | ) | - | (3,436,932 | ) | |||||||
| Other income (expense) | ||||||||||||
| Interest expense | (681,518 | ) | (737,032 | )(H) | (1,418,550 | ) | ||||||
| Gain on paycheck protection program loan forgiveness | 206,200 | - | 206,200 | |||||||||
| Loss on extinguishment of debt | (2,235,770 | ) | (1,238,923 | )(I) | (3,474,693 | ) | ||||||
| Gain on change in fair value of warrant liability | 21,782 | - | 21,782 | |||||||||
| Total other expense | (2,689,306 | ) | (1,975,955 | ) | (4,665,261 | ) | ||||||
| Income tax benefit (expense) | - | - | (J) | - | ||||||||
| Net loss | $ | (6,126,238 | ) | $ | (1,975,955 | ) | $ | (8,102,193 | ) | |||
| Weighted average shares outstanding, basic and diluted | (F) | 184,763,000 | ||||||||||
| Loss / Pro Forma loss per share | $ | (0.03 | ) | |||||||||
| 51 |
Notes to the Unaudited Pro Forma Financial Statements
Note 1—Basis of Presentation
We currently operate as a Delaware limited liability company under the name Hemoglobin Oxygen Therapeutics LLC. Prior to the closing of this offering, Hemoglobin Oxygen Therapeutics LLC (“HbO2”) will convert into a Delaware corporation pursuant to a statutory conversion, and will change its name to Hemoglobin Oxygen Therapeutics Inc. In order to consummate the corporate conversion, a certificate of conversion will be filed with the Secretary of State of the State of Delaware. In this prospectus, we refer to all transactions related to our conversion to a corporation as the Corporate Conversion.
As part of the Corporate Conversion, all the Company’s outstanding membership interests will be converted into shares of common stock. Hemoglobin Oxygen Therapeutics Inc. will continue to hold all property and assets of Hemoglobin Oxygen Therapeutics LLC and will assume all of the debts and obligations of Hemoglobin Oxygen Therapeutics LLC. Hemoglobin Oxygen Therapeutics Inc. will be governed by a certificate of incorporation filed with the Secretary of State of the State of Delaware and bylaws, the material portions of which are described under the heading “Description of Capital Stock.” On the effective date of the Corporate Conversion, the members of the board of managers of Hemoglobin Oxygen Therapeutics LLC will become the initial members of Hemoglobin Oxygen Therapeutics Inc.’s board of directors, and as such are referred to throughout this prospectus as “Directors” rather than “Managers” and the officers of Hemoglobin Oxygen Therapeutics LLC will become the officers of Hemoglobin Oxygen Therapeutics Inc.
In connection with the closing of the Offering, the Company will convert all outstanding convertible notes, amounts due to the landlord and amounts payable to an engineering firm into shares of common stock of Hemoglobin Oxygen Therapeutics Inc.
Management has made significant estimates and assumptions in its determination of the pro forma adjustments. As the unaudited pro forma financial information has been prepared based on these preliminary estimates, the final amounts recorded may differ materially from the information presented. The unaudited pro forma financial information does not give effect to any anticipated synergies, operating efficiencies, tax savings, or cost savings that may be associated with the merger.
The pro forma balance sheet and statement of operations do not reflect the proceeds from the offering.
The unaudited pro forma balance sheet as of December 31, 2021 assumes that the Corporate Conversion and Debt Conversion occurred on December 31, 2021. The unaudited pro forma statement of operations for the year ended December 31, 2021 assumes that the Corporate Conversion, Debt Conversion, including the amounts payable to the landlord and engineering firm, occurred on January 1, 2021.
The unaudited pro forma balance sheet and statement of operations as of and for the year ended December 31, 2021 has been prepared using, and should be read in conjunction with, Hemoglobin Oxygen Therapeutics LLC’s audited consolidated financial statements as of and for the year ended December 31, 2021, and the related notes, included elsewhere in this prospectus.
Note 2 —Transaction Accounting Adjustments
The following describes the transaction accounting adjustments included in the unaudited pro forma financial statements presented herein, as of and for the year ended December 31, 2021.
Adjustments to Unaudited Pro Forma Balance Sheet as of December 31, 2021
The transaction accounting adjustments included in the unaudited pro forma balance sheet as of December 31, 2021 are as follows:
| (A) | To reflect the settlement of $486,000 of accounts payable to engineering firm through the issuance of common stock. |
| 52 |
| (B) | To reflect $641,594 of accrued interest that was reversed to reflect conversion as of December 31, 2021 as outstanding accrued interest is forfeited upon conversion of convertible notes payable. As this interest was not related party, it is reflected as a reduction of accumulated deficit. | |
| (C) | To reflect the conversion of $4,765,071 in short- and long-term convertible notes payable through the issuance of common stock as of December 31, 2021, as presented in Exhibits 10.10 through 10.17 to this Prospectus. | |
| (D) | To reflect the settlement of $2,000,000 in amounts payable to the landlord through the issuance of common stock as of December 31, 2021. | |
| (E) | To reflect $232,060 of accrued interest – related party that was reversed to reflect conversion as of December 31, 2021 and recorded to additional paid in capital due to the related party relationship with the note holders. | |
| (F) | For this illustration, it is anticipated each outstanding LLC member unit at December 31, 2021 will be converted into 100 shares of common stock with a par value of $0.001 per share. | |
| (G) | Represents the conversion of the Company’s members’ deficit into stockholders’ deficit, as effected by the Corporate Conversion, and the equity impact of the Debt Conversion. The members’ deficit balance of $9,249,148, offset by the settlement of accounts payable to the engineering firm described in (A), the conversion of convertible notes payable described in (C), the settlement of amounts payable to the landlord described in (D), and accrued interest – related party described in (E) results in a net deficiency within additional paid-in capital. Since additional paid-in capital cannot be presented as a deficiency, additional paid-in capital was reduced to $0 and the net deficiency was reclassified to accumulated deficit. |
Adjustments to Unaudited Pro Forma Statement of Operations for the Year Ended December 31, 2021
| (H) | To reflect the increase in interest expense from writing off the unamortized debt discount on the convertible notes payable and the commitment fee expense to interest expense in the amounts of $741,791 and $637,259, respectively, upon conversion on January 1, 2021, offset by the removal of interest expense on convertible notes in the amount of $262,503 and the amortization of the debt discount of $276,862 and amortization of the commitment fee expense of $102,653, that was recorded for the year ended December 31, 2021. | |
| (I) | To reflect $1,076,923 of pro forma loss to be recorded on the settlement of $2,000,000 of the landlord payable through the issuance of common stock at a 35% discount as if it had occurred on January 1, 2021 and a $162,000 loss on the settlement of $486,000 that was due to an engineering firm through the issuance of common stock at a 25% discount as if it had occurred on January 1, 2021. | |
| (J) | Reflects the estimated income tax benefit (expense) associated with our pro forma results of operations assuming our earnings had been subject to federal and state income tax as a sub-chapter C corporation using a combined federal and state tax rate of approximately 30.99% based on the estimated US federal income tax and state tax rate during the year ended December 31, 2021. With recurring losses for the years presented, any tax asset or benefit arising from net operating losses would be fully reserved with a valuation allowance, therefore zero tax benefit (expense) has been reflected. |
| 53 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
This management’s discussion and analysis (“MD&A”) of the financial condition and results of operations of Hemoglobin Oxygen Therapeutics LLC and its subsidiaries (“HbO2 Therapeutics”, the “Company”, “us”, “our” or “we”) is supplemental to, and should be read in conjunction with, the “Summary Historical Financial Information of HbO2 Therapeutics” section of this filing and HbO2 Therapeutics information, the discussion in this section contains forward-looking statements that reflect our plans, estimates, and beliefs that involve risks and uncertainties. Actual future results could differ materially from those discussed below for many reasons, including those set forth under the “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” section and elsewhere in this prospectus.
HbO2 Therapeutics’ financial statements are prepared in accordance with generally accepted accounting principles in the United States of America (“GAAP”).
Objective
The objective of the Management Discussion and Analysis is to detail material information, events, uncertainties and factors impacting the Company and provide investors an understanding from “Management’s perspective”.
Company Overview
We are a biotechnology company focused on the development, manufacture, and commercialization of a proprietary technology platform for oxygen-carrying solutions that address critical unmet medical needs in both human and veterinary medicine. Using our patent, proprietary technology and know-how, we develop and sell products that, under certain conditions, are a possible alternative to blood transfusions to deliver oxygen to organs and tissues. Through numerous clinical studies, our products have been shown to address many of the current limitations that exist for blood transfusion. These include the limited shelf life of blood, logistical challenges of typing/cross-matching, delays in testing for pathogens, and blood shortages during times of high need and/or insufficient donations. We have significant experience gained through extensive pre-clinical and clinical testing of these products, which have a well-understood and extensively documented safety and efficacy profile.
| ● | Management believes that our products are the only hemoglobin-based oxygen carriers (HBOCs) which have received marketing authorizations approved anywhere in the world. These products were acquired from OPK Biotech LLC in 2014, which had previously purchased them from Biopure Corporation in 2009. Our current products are: Hemopure ® (hemoglobin glutamer -250 (bovine); HBOC-201) – for human use; and | |
| ● | Oxyglobin® (hemoglobin glutamer -200 (bovine) HBOC-301) – for veterinary use. |
Hemopure and Oxyglobin are sterile medicinal products administered via intravenous or arterial infusion that deliver oxygen to organs and tissues, that could provide a potential alternative to blood transfusion. Our sterile veterinary and human products have been widely investigated in pre-clinical models, clinical trials and real-world clinical experience for more than 20 years and described in at least 250 peer-reviewed publications in the scientific literature and reference texts.
Hemopure, our human product, can address some of the logistical limitations of blood transfusions. The potential benefits are a reduction in both mortality and morbidity while offering several strategic advantages, such as universal compatibility, room temperature storage, off-the-shelf availability, readiness for use (supplied in sterile IV infusion bags) and a three-year shelf life. Clinical data suggests that when blood transfusion is not an option, Hemopure could provide a life-saving option for patients. In markets where Hemopure is not yet authorized / or approved, approval(s) for Hemopure would be pursued for an indication when suitable blood transfusion is indicated, but not readily available, or an option.
Oxyglobin is the only oxygen-carrying solution approved by the FDA, the European Medicines Agency (“EMA”) and the British Veterinary Medicines Directorate (“VMD”) to treat all-cause canine anemia.
| 54 |
We are also pursuing a medical device indication for Hemopure under the name ZK1. ZK1 is intended to act as an additive oxygen carrier solution during ex vivo sub-sub normothermic and normothermic machine perfusion of organs to recondition and assess organ viability prior to transplantation.
We have reached an agreement with all convertible debt holders to convert their loans into equity at the time of the offering, including the outstanding convertible debt principal amount is $6,930,000 as of December 31, 2021. We also reached an agreement on January 3, 2022 with the landlord of our facility to convert $2,000,000 of the outstanding debt into equity at the time of the offering at a 35% discount to the offering price. He will also receive an option to convert an additional $1,000,000 of the outstanding debt into equity during the 18 months following the offering. The total outstanding debt to the landlord as of December 31, 2021 is approximately $3,200,000.
On January 7, 2022, we reached an agreement with the engineering firm building out our production facility to convert $486,000 of the outstanding debt into equity at the time of the offering at a 25% discount to the offering price, which is our total debt to the engineering firm as of December 31, 2021.
Key Factors Affecting Our Performance
Retrofit of our Manufacturing Facility
Our ability to start manufacturing our products and generating revenues is dependent upon the successful completion of the construction and re-fitting of our Souderton facility. While we plan on using the proceeds of this offering towards the construction and re-fitting of the facility, the proceeds of this offering will not be sufficient to complete this process. As such, the proceeds of the Offering will be insufficient to fully complete the relaunch of our products into the market. We will require approximately $15 million of additional monies to complete this process. We will need to seek such additional funds through public or private equity or debt financings or other sources, such as strategic collaborations or license and development agreements. If we do not successfully raise such monies, the facility cannot be completed and we will be unable to produce and commercially distribute any product.
Regulation
The production and marketing of some of our products and ongoing research and development activities will be subject to extensive regulation by numerous governmental authorities governing the production of approved products and product candidates in the United States. Prior to marketing in the United States, any product candidate developed by us must undergo rigorous preclinical (animal) and clinical (human) testing and an extensive regulatory approval process implemented by the FDA under the Federal Food, Drug, and Cosmetic Act. There can be no assurance that we will not encounter problems in producing these products or in clinical trials that will cause us or the FDA to delay or suspend the clinical trials.
Intellectual Property Protection
Our success will depend in part on our ability to obtain patents and product license rights, maintain trade secrets, and operate without infringing on the proprietary rights of others, both in the United States and other countries. There can be no assurance that patents issued to or licensed by us will not be challenged, rendered unenforceable, invalidated, or circumvented, or that the rights granted thereunder will provide proprietary protection or competitive advantages to us.
Impact of COVID-19
On March 27, 2020, the World Health Organization declared the global outbreak of COVID-19 to be a pandemic. The COVID-19 global pandemic has been unprecedented and unpredictable and is likely to continue to result in significant national and global economic disruption, which may adversely affect the Company’s business. The COVID-19 pandemic has delayed a trial for Hemopure the Company had planned in South Africa in 2020, which is now currently planned to take place in 2022. Certain labs/plants had to close their doors in response to COVID-19 regulations in 2020, which resulted in overall lower salary costs and some delays in research and development. Further, as a result of COVID-19 shutdowns during 2020, the Company’s spending on outside services, projects or operations was reduced. Based on the Company’s current assessment, it does not expect any material impact on its long-term strategic plans, its operations, or its liquidity due to the worldwide spread of the COVID-19 virus. However, the Company is actively monitoring this situation and the possible effects on its financial condition, liquidity, operations, suppliers, and industry.
| 55 |
On March 27, 2020, the “Coronavirus Aid, Relief, and Economic Security (“CARES”) Act was signed into law to provide certain aid and stimulus to the U.S. Economy. The CARES Act, among other things, includes provisions relating to refundable payroll tax credits, deferment of employer side social security payments, net operating loss carryback periods, alternative minimum tax credit refunds, modifications to the net interest deduction limitations, increased limitations on qualified charitable contributions, and technical corrections to tax depreciation methods for qualified improvement property. The CARES Act also allocated funds for the U.S. Small Business Administration’s (“SBA”) Paycheck Protection Program (“PPP”) loans that are forgivable in certain situations to promote continued employment.
We qualified as a small business under the CARES Act and submitted a loan application with a qualified lender for funding under the PPP. Our PPP Loan was approved on May 5, 2020 for $206,200. The PPP Loan was eligible to be forgiven provided that we satisfied certain conditions and upon the approval by the lender and the SBA and was fully forgiven in the first quarter of 2021 by the SBA and the lender. As a result, the Company recognized a gain on paycheck protection program loan forgiveness of $206,200 within other income (expense) in the consolidated statement of operations for the year ended December 31, 2021.
Public Company Costs
Our common shares will be registered with the SEC and listed on The Nasdaq Capital Market, which will require us to hire additional personnel and implement public company procedures and processes. We expect to incur additional annual expenses as a public company for internal controls compliance and public company reporting obligations, directors’ and officers’ liability insurance, director fees and additional internal and external accounting and legal and administrative resources, including increased audit and legal fees.
Components of Results of Operations
The following describes the components of revenue and expenses that are reflected in our consolidated statements of operations:
Revenue
Our revenue through contracts with customers relate to the selling of hemoglobin-based oxygen carriers (“HBOCs”), which represent single performance obligations that are satisfied when control of the product passes to the customer. We consider the timing of right to payment, transfer of risk and rewards, transfer of title, transfer of physical possession and customer acceptance when determining when control transfers to the customer. As a result, revenue is recognized at a point in time upon product shipment. Shipping and handling activities are accounted for as activities to fulfill a promise to transfer a product to a customer and as such, costs incurred are recorded when the related revenue is recognized. Payment for products is due within a limited time period after shipment or delivery, typically within 30 calendar days of the respective invoice dates.
Revenue is measured as the amount of consideration we expect to receive in exchange for transferring goods. Amounts billed and due from our customers are classified as accounts receivable, less allowance for doubtful accounts on the balance sheet. We record a contract asset when it has a right to payment from a customer that is conditioned on events that have occurred other than the passage of time. We also record a contract liability when customers prepay but we have not yet satisfied our performance obligation. The Company had deferred revenue of $378,637 as of December 31, 2021 and 2020 As of December 31, 2020, deferred revenue is classified as a current liability as all remaining performance obligations are expected to be fulfilled in 2022.
Research and Development Costs
We record research and development activities conducted by third-party service providers, which include the conduct of preclinical studies and clinical trials, and contract manufacturing activities, to research and development expense as incurred.
| 56 |
General and Administrative
General and administrative expenses are recorded in the period which they are incurred and include salaries, benefits, finance, other administrative services, information technology expenses, outside services, legal and accounting services, and other costs required to support our operations.
Interest Expense
Our interest expense is related to convertible promissory notes totaling $2,500,000 that will be converted as of the closing of this Offering. There was an increase in the average outstanding balance of these notes in 2021 over 2020, and therefore, interest expense was higher. We recorded a Convertible Note debt discount to account for the beneficial conversion features on convertible notes issued in the year ended December 31, 2020, as well as the 1st and 2nd Warrant issued in conjunction with the $2,400,000 Convertible Note. The Convertible Note debt discounts are being accreted to non-cash interest expense over the life of each associated Convertible Note or will be accreted in full upon conversion of the corresponding Convertible Note into Member Units. If the holder chooses to convert, they forego the related accrued interest; the conversion is for principal only. If the note is not converted, the principal plus accrued interest is due on the maturity date.
On January 5, 2021 the Company entered into amendments to three convertible notes totaling $2,500,000 with the same lender. Under the amendments, the conversion price of the loans was decreased from $548.81 to $274.40. The Company accounted for the amendments as debt extinguishments under ASC 470-50 resulting in a loss on extinguishment of debt of $2,235,770 which included the fair value of the embedded conversion option. As the new debt was issued at a substantial premium, the fair value of the full premium of $2,235,770 was recorded as additional paid-in capital.
Contractual interest expense on the Convertible Notes Payable for the years ended December 31, 2021 and 2020 was $302,003 and $261,002 respectively. Non-cash interest expense related to the amortization of debt discount for the years ended December 31, 2021 and 2020 was $276,862 and $76,689 respectively. The remaining unamortized debt discount as of December 31, 2021 of $2,164,929 million will be amortized over 3.7 years. Accrued interest expense on the Convertible Notes as of December 31, 2021 and 2020 was $876,391 and $574,388 respectively. The effective interest rate on the Convertible Notes for the years ended December 31, 2021 and 2020, was 10.10% and 6.96%, respectively.
| 57 |
Results of Operations for the Years Ended December 31, 2021 and 2020
The following table summarizes our results of operations for the years ended December 31, 2021 and 2020:
Year Ended 2021 | Year Ended 2020 | Dollar Change | Percent Change | |||||||||||||
| Revenue | $ | - | $ | 35,470 | $ | (35,470 | ) | (100.0 | )% | |||||||
| Operating expenses: | ||||||||||||||||
| Research and development | 768,621 | 571,738 | 196,883 | 34.4 | % | |||||||||||
| General and administrative | 2,668,311 | 2,673,445 | (5,134 | ) | (0.2 | )% | ||||||||||
| Total operating expenses | 3,436,932 | 3,245,183 | 191,749 | 5.9 | % | |||||||||||
| Loss from operations | (3,574,524 | ) | (3,209,713 | ) | (227,219 | ) | 7.1 | % | ||||||||
| Interest expense | (681,518 | ) | (379,862 | ) | (301,656 | ) | 79.4 | % | ||||||||
| Gain on paycheck protection program loan forgiveness | 206,200 | - | 206,200 | 100.0 | % | |||||||||||
| Loss on extinguishment of debt | (2,235,770 | ) | - | (2,235,770 | ) | (100.0 | )% | |||||||||
| Gain on foreign current translation | - | 1,699 | (1,699 | ) | (100.0 | )% | ||||||||||
| (Loss) gain on change in fair value of warrant liability | 21,782 | 1,862 | 19,920 | 1,069.8 | % | |||||||||||
| Total other expense, net | (2,689,306 | ) | (376,301 | ) | (2,313,005 | ) | (614.7 | )% | ||||||||
| Net loss | $ | (6,126,238 | ) | $ | (3,586,014 | ) | $ | (2,540,224 | ) | (70.8 | )% | |||||
Revenue
There were two sales of product from expired shelf-life inventory during the year that ended December 31 2020. Revenues decreased by 100% or $35,470 during the year ending December 2021 as our production facility remained non-operational.
Research and Development
Our research and development expenses increased 34.4% between the years ended December 31, 2021 and 2020 as a result of additional spending as certain plants/labs that had to close their doors in response to COVID-19 regulations during 2020, which resulted in overall lower R&D costs. In 2021, we were able to resume our work on new and existing product development and incurred higher costs, which were consistent with our pre-COVID annual spending.
General and Administrative
General and administrative expenses remained steady with a decrease of 0.2% between the years ended December 31, 2021 and 2020 as a result of a relatively fixed cost corporate structure, as well as continued cost containment measures where applicable. The Company also incurred expenses related to their proposed initial public offering which were capitalized during the year ended December 31, 2021.
Interest Expense
Interest expense increased 79.4% between the years ended December 31, 2021 and 2020 as a result of a higher average outstanding debt balance during 2021 including additional debt discounts that are accreted to interest expense.
Gain on Paycheck Protection Program Loan Forgiveness
In May 2020, the Company obtained a loan (“PPP Loan”) in the amount of $206,200. In January 2021, the PPP loan was fully forgiven by SBA and lender. As a result, the Company recognized a gain on paycheck protection program loan forgiveness of $206,200 within other income (expense) in 2021.
Loss on Extinguishment of Debt
On January 5, 2021 the Company entered into amendments to three convertible notes totaling $2,500,000 with the same lender. Under the amendments, the conversion price of the loans was decreased from $548.81 to $274.40. The Company accounted for the amendments as debt extinguishments resulting in a loss on extinguishment of debt of $2,235,770, which included the fair value of the embedded conversion option.
Change in Fair Value of Warrant Liability
The Company accounts for warrants issued in conjunction with financial instruments as liability-classified instruments. Changes in the estimated fair value of the warrants are recognized as non-cash gains or losses on the consolidated statements of operations.
| 58 |
Critical Accounting Policies and Estimates
We believe the following accounting policies are most critical in understanding and evaluating this management discussion and analysis:
Revenue Recognition
Revenue is recognized when control of the goods and services provided is transferred to the Company’s customers and in an amount that reflects the consideration the Company expects to be entitled to in exchange for those good and services using the following steps: 1) identification of the contract, or contracts with a customer, 2) identification of performance obligations in the contract, 3) determination of the transaction price, 4) allocation of the transaction price to the performance obligations in the contract and 5) recognition of revenue when or as the Company satisfies the performance obligation(s).
The Company’s contracts with customers are comprised primarily of contractual agreement paperwork (i.e., purchase orders) and verbal orders. The Company accounts for a contract when it has approval and commitment from both parties, the rights of the parties and payment terms are identified, the contract has commercial substance and collectability of consideration is probable. These contracts with customers relate to the selling of hemoglobin-based oxygen carriers (“HBOCs”), which represent single performance obligations that are satisfied when control of the product passes to the customer. The Company considers the timing of right to payment, transfer of risk and rewards, transfer of title, transfer of physical possession and customer acceptance when determining when control transfers to the customer. As a result, revenue is recognized at a point in time upon product shipment. Shipping and handling activities are accounted for as activities to fulfill a promise to transfer a product to a customer and, as such, applicable costs incurred are recorded when the related revenue is recognized. Payment for products is due within a limited time period after shipment or delivery, typically within 30 calendar days of the respective invoice date. The Company does not generally offer extended payment terms.
Revenue is measured as the amount of consideration the Company expects to receive in exchange for transferring goods. Amounts billed and due from the Company’s customers are classified as accounts receivable, less allowance for doubtful accounts on the consolidated balance sheet. During 2021, the Company had no revenue. The Company’s 2020 revenue consisted solely of sales of Hemopure in South Africa.
The Company records a contract asset when it has a right to payment from a customer that is conditioned on events that have occurred other than the passage of time. The Company records a contract liability when customers prepay but the Company has not yet satisfied its performance obligation. The Company had $378,637 of deferred revenue as of December 31, 2021 and 2020, which is classified as a current liability as of December 31, 2021 as all remaining performance obligations are expected to be fulfilled in 2022. The Company did not have any material contract assets as of December 31, 2021 and 2020.
Convertible Debt
We have entered into several convertible notes payable with interest rates varying from 5-10%. A discount is recorded to the convertible notes for the intrinsic value of the conversion options embedded in debt instruments based upon the differences between the fair value of the underlying member unit at the commitment date of the note and the effective conversion price embedded in the note. Debt discounts under these arrangements are amortized to noncash interest expense using the effective interest method over the term of the related debt to their date of maturity. If the note becomes convertible only upon the occurrence of a future event outside our control, or, is convertible from inception, but contains conversion terms that change upon the occurrence of a future event, then any contingent beneficial conversion feature is measured and recognized when the triggering event occurs, and contingency has been resolved. All of our convertible debt will be converted into equity at the closing of the Offering upon terms which vary depending on the holder of the debt. See “Capitalization”.
| 59 |
Warrants
The Company accounts for warrants issued in conjunction with financial instruments as either equity-classified or liability-classified instruments based on an assessment of the warrants’ specific terms and applicable authoritative guidance in FASB ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”) and Topic 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgement, is conducted at the time of warrant issuance and as of each subsequent quarterly reporting end date while the warrants are outstanding.
For issued or modified warrants that do not meet all of the criteria for equity classification, the warrants are required to be recorded as liabilities at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as non-cash gain or loss on the statement of operations. The fair value of the warrants at issuance and at December 31, 2021 and 2020 was estimated using a Black Scholes option pricing model.
Liquidity and Capital Resources
Sources of Liquidity
To date, we have financed our operations primarily through issuance of convertible debt. In the near term, we expect to continue to increase investing activities as we build out our operational facility. Ultimate cash inflow to the Company from the common stock offering will depend on the per share offering price and number of shares sold.
Funding Our Operations
The ability to raise funds through equity or debt is subject to many risks and uncertainties and, even if we were successful, future equity issuances would result in dilution to our existing stockholders and any future debt or debt securities may contain covenants that may limit our operations or ability to enter into certain transactions.
We estimate that we will need approximately $35 million to complete the construction, re-fitting, automation process update, validation and certification of our Souderton facility. As the proceeds of the Offering will be insufficient to fully complete this process, we will require approximately $15 million of additional monies. Our ability to generate sales to fund operations is subject to a number of uncertainties, including regulatory, financial and business risks, many of which are beyond our control. If we are unable to generate sufficient revenue from our sales, or when needed, to secure additional sources of funding; it may be necessary to significantly reduce our current rate of spending or explore other strategic alternatives.
Cash and Cash Flows
Our principal uses of cash since the Company’s inception in 2014 have been funding our operations and investing in capital expenditures. The following table presents cash and cash provided by (used in) operating, investing and financing activities during the years ended December 31, 2021 and 2020:
| Year Ended December 31, 2021 | Year Ended December 31, 2020 | |||||||
| Beginning balance of cash | $ | 357,169 | $ | 75,823 | ||||
| Net cash used in operating activities | (1,913,759 | ) | (931,252 | ) | ||||
| Net cash used in investing activities | - | (50,040 | ) | |||||
| Net cash provided by financing activities | 2,000,485 | 1,262,638 | ||||||
| Ending balance of cash | $ | 443,895 | $ | 357,169 | ||||
| 60 |
The following is a summary of our cash flows from operating, investing and financing activities for the years ended December 31, 2021 and 2020:
Operating Activities
During the year ended December 31, 2021, our cash used in operations of $1,913,759 consisted primarily of the Company’s net loss of $6,126,238, offset by non-cash expenses of $2,985,198 mainly associated with the loss on extinguishment of debt and right of use asset amortization and $1,227,281 in positive changes in operating assets and liabilities.
During the year ended December 31, 2020, our cash used in operations of $931,252 consisted of the Company’s net loss of $3,586,014, offset by non-cash expenses of $663,832 mainly associated with right of use asset amortization and non-cash interest expense and positive changes in operating assets and liabilities of $1,990,930.
Investing Activities
During the year ended December 31, 2020, our investing activities involved the purchase of $50,040 in property and equipment. There were no investing activities during the year ended December 31, 2021.
Financing Activities
During the years ended December 31, 2021 and 2020, our cash flows provided by financing activities entailed proceeds from the issuance of convertible notes payable of $1,700,000 and $1,056,438, respectively, proceeds of $300,485 from the issuance of notes payable in 2021, and proceeds from the Paycheck Protection Program loan of $206,200 in 2020.
Operating and Capital Expenditure Requirements
Our ability to start manufacturing our products and generating revenues is dependent upon the successful completion of the construction and re-fitting of our Souderton facility. Until it is complete, and the relevant cGMP certification is received, we will not be able to release and commercially distribute any product. The proceeds of the Offering will be insufficient to fully complete the relaunch of our products into the market. We will require approximately $15 million of additional monies to complete this process. We are seeking such additional funds through public or private equity or debt financings or other sources, such as strategic collaborations or license and development agreements. We believe our existing cash, cash equivalents, together with cash provided by operations and funding from this equity issuance, will be sufficient to meet our needs, other than those required to complete the construction and re-fitting of our facility, for at least the next 12 months. Our future capital requirements will depend on many factors including our revenue growth rate, the timing and extent of spending to support further sales and marketing and research and development efforts, the timing and extent of additional capital expenditures to invest in the expansion of our existing facilities. We may enter into arrangements in the future to acquire or invest in complementary businesses, services and technologies, including intellectual property rights. We may be required to seek additional equity or debt financing. If additional financing is required from outside sources, we may not be able to raise it on terms acceptable to us or at all. If we are unable to raise additional capital when desired, our business, results of operations and financial condition would be materially and adversely affected.
Going Concern and Liquidity
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. We have experienced net losses and negative cash flows from operating activities since inception and expect to continue to incur net losses into the foreseeable future. We have an members’ deficit of approximately $9,249,000 at December 31, 2021, incurred a net loss of approximately $6,126,000 for the year ended December 31, 2021 and used approximately $1,914,000 of net cash for operating activities during the year ended December 31, 2021. To date, these operating losses have been funded primarily from outside sources of invested capital through the issuance of convertible promissory notes, notes payable and sale of member units. At December 31, 2021, we had cash on hand of approximately $444,000. We expect operating losses and negative cash flows to continue for the foreseeable future as we continue to incur costs related to research and development and commercialization efforts. Management has prepared cash flow forecasts which indicate that based on our expected operating losses and negative cash flows, there is substantial doubt about the Company’s ability to continue as a going concern within twelve months after the date these consolidated financial statements are available for issuance.
| 61 |
Our ability to continue as a going concern is dependent upon its ability to raise additional funding. Management plans to raise additional capital to fulfill our operating and capital requirements for the next twelve months. In 2021, we raised an additional approximately $1,700,000 through the issuance of convertible notes. In 2021, we raised an additional approximately $300,000 through note payable issuances. In 2022, we plan to fund our operating losses and capital funding needs primarily through an initial public offering, and/or through additional debt and equity financings. These financing and capital raising options may not be able to be successfully achieved and may not be available on a timely basis or on terms acceptable to us. If we are not able to secure adequate additional funding in a timely manner or on favorable terms, we may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, or suspend or curtail planned programs. Any of these actions could have a material adverse effect on our business, results of operations and future prospects.
The accompanying consolidated financial statements do not reflect any adjustments that might result if we are unable to continue as a going concern. Since inception, we have been engaged in organizational activities, including raising capital and research and development activities. We have not generated any substantial revenues and has not yet achieved profitable operations, nor have we ever generated positive cash flows from operations. There is no assurance that profitable operations, if achieved, could be sustained on a continuing basis. Further, our future operations are dependent on the success of our efforts to complete and re-fit our manufacturing facility and to raise additional capital and the market acceptance of our products. There can be no assurance that these efforts will be successful.
Emerging Growth Company
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, which we refer to as the JOBS Act. We would cease to be an emerging growth company upon the earliest of: (i) the last day of the first fiscal year in which our annual gross revenues are $1.07 billion or more; (ii) the end of any fiscal year in which the market value of our common stock held by non-affiliates exceeded $700.0 million as of the end of the second quarter of that fiscal year; or (iii) the date on which we have, during the previous three-year period, issued more than $1.07 billion in non-convertible debt securities. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. We have elected to take advantage of these reduced disclosure obligations and may elect to take advantage of other reduced reporting obligations in the future.
For so long as HbO2 Therapeutics is an emerging growth company, we will not be required to:
| ● | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (i.e., an auditor discussion and analysis); | |
| ● | have an auditor report on our internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; | |
| ● | submit certain executive compensation matters to shareholder advisory votes, such as “say-on-pay,” “say-on-frequency” and pay ratio; and | |
| ● | disclose certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the CEO’s compensation to median employee compensation. |
In addition, Section 107 of the JOBS Act also provides that an EGC can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. As a result, the Company may adopt new or revised accounting standard by the date private companies are required to comply.
| 62 |
Description of Our Operations, Principal Activities and Key Factors
We are a biotechnology company focused on the development, manufacture, and commercialization of a proprietary technology platform for oxygen-carrying solutions that address critical unmet medical needs in both human and veterinary medicine. Using our patent, proprietary technology and know-how, we develop and sell products that, under certain conditions, are a possible alternative to blood transfusions to deliver oxygen to organs and tissues. Through numerous clinical studies, our products have been shown to address many of the current limitations that exist for blood transfusion. These include the limited shelf life of blood, logistical challenges of typing/cross-matching, delays in testing for pathogens, and blood shortages during times of high need and/or insufficient donations. We have significant experience gained through extensive pre-clinical and clinical testing of these products, which have a well-understood and extensively documented safety and efficacy profile.
Management believes that our products are the only hemoglobin-based oxygen carriers (HBOCs) which have received marketing authorizations approved anywhere in the world. These products were acquired from OPK Biotech LLC in 2014, which had previously purchased them from Biopure Corporation in 2009. Our current products are:
| ● | Hemopure ® (hemoglobin glutamer -250 (bovine); HBOC-201) – for human use; and | |
| ● | Oxyglobin® (hemoglobin glutamer -200 (bovine) HBOC-301) – for veterinary use. |
Hemopure and Oxyglobin are sterile medicinal products administered via intravenous or arterial infusion that deliver oxygen to organs and tissues, that could provide a potential alternative to blood transfusion. Our sterile veterinary and human products have been widely investigated in pre-clinical models, clinical trials and real-world clinical experience for more than 20 years and described in at least 250 peer-reviewed publications in the scientific literature and reference texts.
Hemopure, our human product, can address some of the logistical limitations of blood transfusions. The potential benefits are a reduction in both mortality and morbidity while offering several strategic advantages, such as universal compatibility, room temperature storage, off-the-shelf availability, readiness for use (supplied in sterile IV infusion bags) and a three-year shelf life. Clinical data suggests that when blood transfusion is not an option, Hemopure could provide a life-saving option for patients. In markets where Hemopure is not yet authorized / or approved, approval(s) for Hemopure would be pursued for an indication when suitable blood transfusion is indicated, but not readily available, or an option.
Oxyglobin is the only oxygen-carrying solution approved by the FDA, the European Medicines Agency (EMA) and the British Veterinary Medicines Directorate (VMD) to treat all-cause canine anemia.
We are also pursuing a medical device indication for Hemopure under the name ZK1. ZK1 is intended to act as an additive oxygen carrier solution during ex vivo sub-sub normothermic and normothermic machine perfusion of organs to recondition and assess organ viability prior to transplantation. Markets
We are focusing on three main markets: the veterinary transfusion market for Oxyglobin, the human transfusion market for Hemopure and the organ perfusion system market for ZK1.
Veterinary Transfusion Market
Oxyglobin is the only oxygen-carrying solution approved by the US Food and Drug Administration (FDA), the e European Medicines Agency (EMA) and the British Veterinary Medicines Directorate (VMD) to treat all-cause canine anemia. We plan to distribute this product in North America through an exclusive distribution agreement with Dechra Veterinary Products, a global veterinary pharmaceutical company, and in Europe through regional distributors that specialize in the veterinary critical care market.
| 63 |
Human Transfusion Market
Hemopure is approved in South Africa, where this product is indicated for the purpose of eliminating, delaying or reducing the need for allogenic red blood cells (RBCs). Hemopure was also approved in the Russian Federation for the treatment of acute, all-cause anemia. When the situation normalizes in that Russia and sanctions are lifted, we will evaluate the state of affairs and conclude a distribution agreement. In the United States, Hemopure is under clinical development for the treatment of life-threatening anemia when blood transfusion is indicated but is not an option.
Organ Perfusion System Market
Clinical development of ZK1 for use in sub-normothermic and normothermic organ perfusion, without the use of traditional blood products is ongoing. Under this application ZK1 would not be administered to the patient, but instead used outside the body, as an additive to perfusion solutions for the ex-vivo machine perfusion of donor organs prior to transplantation. As an ancillary medicinal product used outside the body as one component of a machine perfusion system, ZK1 would be evaluated under a medical device application.
ZK1 research and findings thus far have generated significant interest from the organ transplant community, culminating in research collaboration agreements with several major academic medical centers in both North America and Europe.
Description of Our Business
Our products are used to deliver oxygen to organs and tissues. Oxyglobin is indicated for the treatment of canine anemia and Hemopure is in clinical development for the treatment of anemia in humans when blood or blood products are not an option. ZK1 is under development for organ perfusion, preservation and refurbishment prior to re-attachment (replantation) or transplantation. Each of these products and indications are discussed in further detail below.
Hemopure: an Oxygen Therapeutic Addressing Certain Red Blood Cell Limitations
Oxygen is indispensable to all living tissues in animals and humans. Under normal conditions, hemoglobin, a native protein contained inside circulating red blood cells (“RBCs”), binds with oxygen from the lungs and delivers it to organs and tissues.

Blood loss, inadequate RBC production, excessive destruction of RBCs or blood vessel obstruction can compromise the delivery of oxygen to the body’s tissues. Oxygen deprivation, even for a few minutes, can result in cell damage, organ dysfunction and, if prolonged, death.
| 64 |
Anemia is a systemic deficiency of RBCs associated with blood loss (e.g., from injury or surgery) or certain medical conditions. Ischemia is insufficient blood flow to an organ(s) or tissue(s) due to blockage or constriction of blood vessels (such as heart attacks, strokes and certain medical procedures), or to insufficient cardiac output. Both anemia and ischemia can compromise tissue oxygenation.
RBC transfusion is the standard therapy for anemia, but it comes with serious limitations including limited shelf life, refrigeration requirements, cross matching for blood types, inflammation and risk of transmissible disease. HBOCs, which are sometimes referred to as blood substitutes, carry oxygen and can thus address some of these issues faced with RBC transfusion.
HBOCs as a class of products can be derived from human or animal blood and administered intravenously or intra-arterially to increase oxygen transport to the body’s tissues. They do not treat or cure the underlying causes of anemia but can provide an oxygen bridge to support life sustaining metabolic function until a safe RBC concentration is achieved, either by production of new RBCs or transfusion of suitable blood when it becomes available.
We believe there are many possible applications for our products. Anemia, ischemia, hemorrhagic trauma, wound healing, thalassemia, sickle cell crisis and organ perfusion are just a few of the clinical conditions that could benefit from our technology.
Production Process
At our inception, we made a business decision to consolidate our three manufacturing sites at a single location in Souderton, PA, the site previously responsible for just the initial production steps. This Souderton facility is currently undergoing an expansion and renovation to accommodate all the production steps and is not operational. Included in the OPK Biotech acquisition was an inventory of Hemopure manufactured in the previous manufacturer’s cGMP certified facility. This finished product inventory is maintained in secure, environmentally monitored and controlled conditions. Further, to assure the ongoing safety, purity, and potency of inventory on hand, we have implemented a stability program that mandates release testing of all batches every six months. All testing is conducted using validated analytical equipment by appropriately trained personnel in accordance with our quality control standard operating procedures (“SOP”s).
The inventory on hand is being utilized as follows:
| ● | To treat patients with life threatening anemia who require a transfusion, but for whom receiving blood or blood products is not an option. In the US, these patients receive the product through the FDA’s expanded access program, or, in the case of an overseas patient, that country’s regulatory equivalent. | |
| ● | Sufficient inventory held in reserve for a pre-hospital trauma trial in South Africa. | |
| ● | To supply the ongoing investigation and development of ZK1 as an oxygen carrier component in organ perfusion solutions. |
Prior to the release and distribution of any newly manufactured product, our Souderton facility must be in full compliance with current good manufacturing practice (cGMP), and the European Directorate for the Quality of Medicines & Healthcare (EDQM) that certifies compliance with the European Pharmacopoeia general chapter 5.2.8 ‘Minimizing the risk of transmitting animal spongiform encephalopathy agents via medicinal products’. The manufacturing process involves:
| ● | Sourcing hemoglobin from bovine blood derived from USDA-inspected cattle having traceability from birth and released for human consumption, | |
| ● | Extraction, purification, and the chemical cross-linking of hemoglobin prior to formulation in a modified lactated Ringer’s solution, | |
| ● | Sterile filling of finished product bags, enclosed inside a transparent oxygen barrier overwrap. |
The human and veterinary product are manufactured under one quality system using the same raw material, personnel and process equipment. Other than packaging and unit volume, the only difference between the two products is molecular weight distribution, with an average molecular weight (MW) of 200 kD for Oxyglobin and 250 kD for Hemopure/ZK1, achieved through a final fractionation step.
| 65 |
Both
products consist of chemically stabilized hemoglobin polymers that are smaller and release oxygen more readily than RBCs and are solubilized
in solutions that are less viscous than blood. The favorable oxygen release properties of Oxyglobin and Hemopure are
attributable to their lower affinity for oxygen compared to RBC hemoglobin as indicated by p50 (a measure of oxygen affinity).
Product Portfolio Characteristics
The key characteristics of Oxyglobin and Hemopure are:
| ● | Sterile, ultra-purified, oxygen-carrying solutions for intravenous or intra-arterial infusion, for the purpose of increasing total Hb concentration | |
| ● | No type and, crossmatch required, and compatible with all blood types | |
| ● | Ready to use, requiring no reconstitution | |
| ● | Stable for at least three years at 2° to 30° Celsius | |
| ● | No refrigeration required | |
| ● | Carry oxygen at low perfusion pressure through constricted or partially blocked blood vessels to areas of the body that RBCs cannot reach due to their larger size |
Oxyglobin
Oxyglobin is the trademark name for our veterinary product, the only oxygen therapeutic approved in both the United States and European Union to treat anemia in dogs. A number of scientific publications and veterinary reference texts also discuss the off-label use of Oxyglobin in other species. Examples include cats, horses, and even exotics. Although often overlooked due to unfamiliarity, or it simply not being practical, exotic animals with blood loss can suffer from anemia and require transfusion as would a typical domestic animal. Furthermore, many of the same practices (monitoring, transfusion rate guidelines, etc.) used in cat and dog blood transfusions can be used in other species. Once Oxyglobin is relaunched in the canine market, we will seek to expand its use, to treat non-domestic animals in captivity, such as those zoos, aquariums or wild animal sanctuaries. This would involve filing a request for MUMS (Minor Use and Minor Species) drug designation. This permits the use of a drug in minor species, or for use in major species afflicted with uncommon diseases or conditions (minor uses).
Biopure Corporation commercialized Oxyglobin from 1999 until 2008, when production was shut down as part of the Company’s bankruptcy proceeding. In 2013, after revalidation of manufacturing facility updates and process improvements, OPK Biotech resumed commercial distribution of Oxyglobin in Europe. Between the end of 2012 and 2014, when the owner of OPK Biotech ceased funding operations, and the facility was shut down, a total of 4,282 units of Oxyglobin were sold to the European distributor.
Under the US Department of Defense Joint Theater Trauma System (“JTTS”) Clinical Practice Guideline for emergency treatment of military working dogs (“MWD”) that sustained combat related injuries, Oxyglobin was used as a resuscitative fluid to stabilize MWD after combat-related injuries, in preparation for removal by MEDEVAC transport to veterinarians. The guideline was first published in April 2011 for non-veterinarians (i.e., human trauma surgeons and medics). The US military currently utilizes around 2,300 workings dogs with another 1448 canine teams deployed by Homeland Security across the US.
A National Stock Number (“NSN”) is a unique item identifier applied to items repeatedly procured, stocked, stored, issued, and used throughout the US Federal supply system. The NSN for Oxyglobin is NSN 6509-01-9159. We believe that significant demand exists for Oxyglobin, given the dearth of other anemia treatment options. As soon as possible after completion of this offering, we, with our distributor Dechra International, plans to contact customers and veterinary colleges that purchased Oxyglobin in the past in the United States and advise them that the product is expected to become available for distribution as soon as GMP certification of the manufacturing facility is obtained, expected to occur in 2023.
| 66 |
While we believe that many veterinarians are aware of the benefits of Oxyglobin, they had to endure an uncertain and inconsistent supply of product by predecessor companies. We intend to demonstrate our ability to provide an uninterrupted supply of Oxyglobin as well as the product’s competitive advantages as it re-enters the market. We believe that the use of Oxyglobin can optimize current transfusion and fluid therapy practice. We believe that the benefits of not having to cross match blood, ease of use and long shelf life will again convince veterinarians to restore Oxyglobin as a treatment option of choice.
In Europe, we intend to pursue a similar strategy and is currently engaged in dialogs with several veterinary distributors regarding a product relaunch, and future commercial distribution in the European Union and the United Kingdom.
Hemopure
Hemopure is the trademark name for our human product. Originally developed and positioned as an alternative to RBC transfusion, the product has been evaluated in 22 clinical trials to date, primarily focusing on surgical anemia. These trials enrolled 1,512 patients, of whom 836 received Hemopure. The Company is currently in clinical development for pre-hospital and in-hospital indications when blood is not an option for patients requiring a transfusion.
We believe that due to its unique properties, Hemopure is able to address some of the limitations of blood transfusions. The potential benefits are a reduction in both mortality and morbidity while offering several strategic advantages, such as universal compatibility, room temperature storage, off-the-shelf availability, readiness for use (supplied in sterile IV infusion bags) and a three-year shelf life. Clinical data suggests that when blood is not an option, Hemopure provides a life-saving option for patients. Upon receiving authorization, the Company intends to market Hemopure for indications when suitable blood is not readily available or an option, as illustrated below:
Hemopure Approvals for Human Use
| ● | In South Africa: The product has received marketing authorization for eliminating, reducing or delaying the need for allogeneic RBC transfusions in surgical patients who are acutely anemic. Due to local distribution issues, Hemopure was only briefly marketed during 2007. During the brief period that Hemopure was commercially available, the product received strong support from the local medical community, including specialist medical societies and the South African armed forces. We believe that Hemopure continues to have strong support from the South African medical community and armed forces. We consider South Africa to have a strategic value due to its utility as a testing ground to gain valuable information about how doctors understand and use Hemopure, enabling us to plan for larger markets and other potential indications. We are currently in dialog with the South African National Blood Service (SANBS) regarding potential distribution of Hemopure in the country. | |
| ● | In Russia: In October 2010, the Russian Federation Ministry of Health approved Hemopure for the treatment of all-cause acute anemia. Lack of a suitable distribution partner at that time precluded marketing of the product. In December 2015, HbO2 Therapeutics decided not to renew its Russian license for Hemopure. In 2020, we entered into discussions with two large distribution groups in the Russian Federation, aiming to reactivate the license and conclude a distribution agreement. When the situation normalizes in that country and sanctions are lifted, we will evaluate reactivating the license and conclude a distribution agreement. |
| 67 |
Expanded Access
Expanded access is a regulatory pathway for patients with an immediately life-threatening condition or serious disease/condition to gain access to an investigational drug, for treatment outside of a clinical trial when no comparable or satisfactory alternative therapy options are available.
The FDA has authorized the use of Hemopure in US hospitals under two categories of Expanded Access. They are:
| ● | Emergency single patient investigational new drug (IND) | |
| This is sometimes also referred to as compassionate use. Approvals under this category are granted on a case-by-case basis to a physician from any institution, who may have a patient they deem to have met the criteria for expanded access use. Shipments of Hemopure under this category are also on a per patient basis, i.e., hospitals are not permitted to maintain any inventory of the product, and upon conclusion of the case, all products must be accounted for and returned to us. | ||
| ● | Intermediate sized population IND | |
| In this category there are nine academic medical centers where multiple patients received Hemopure, each under their own single patient IND. The logistical and administrative burden of filing multiple individual patient INDs is significant, and the shipping of product on a per patient basis introduces further delays in treatment for already critical patients due to potential in transit delays. | ||
| The intermediate sized population IND is intended for just such circumstances. These nine medical centers have now implemented FDA and IRB approved protocols to treat patients with Hemopure. Under these protocols they also able to consent, enroll and treat eligible patients immediately because each center now maintains an inventory of Hemopure. These intermediate sized population INDs are subject to procedures for safety, annual, and other IND application reporting that are similar to those for investigational IND applications. The requirements for mandatory IND Application Safety Reporting are also the same as for all other IND applications. |
Medical centers currently enrolling under intermediate sized population INDs are:
| ● | University of Michigan Medical Center | |
| ● | Johns Hopkins Medical Center | |
| ● | Duke University Medical Center | |
| ● | University of New Mexico | |
| ● | University of Miami - Jackson Memorial Hospital | |
| ● | Englewood Hospital & Medical Center | |
| ● | University of Florida at Gainesville | |
| ● | University of Pittsburg Medical Center | |
| ● | Vanderbilt University Medical Center |
In May 2017, the Company, members of its advisory board and a senior representative of the US Department of Defense (“DoD”) met with FDA’s senior management and review team to discuss a path forward for the clinical development of Hemopure. During the ensuing discussion, FDA proposed that we submit a proposal for a phase III clinical trial for the use of Hemopure in hospitalized patients who require a transfusion, but for whom blood, and blood products are not an option. We are now engaged in a dialog with FDA regarding design of the proposed trial. Our proposal that existing Expanded Access investigator-sponsored INDs be merged into a single IND for a pivotal trial, is currently being prepared for consideration by FDA.
| 68 |
US Department of Defense Pre-Hospital Trauma Indication
The FDA has acknowledged that given the relatively short pre-hospital (ambulance, medevac) transit times from the scene of a trauma to healthcare facility, conducting a pre-hospital study in the US would be difficult.
In September 2018, the US Department of Defense awarded an $8.2 million contract to Stellenbosch University in Cape Town, South Africa to coordinate a multi-center prehospital clinical trial to evaluate use of Hemopure on its own and in conjunction with freeze-dried plasma in trauma victims. The FDA is fully aware of the South African trial and has representation on the DoD appointed study advisory board.
ZK1: Donor organ perfusion prior to transplantation and limb perfusion prior to replantation.
Despite significant improvements in patient outcomes after liver transplantation, many severely ill patients die while awaiting an available organ for transplantation. Organ shortage is due not only to an inadequate number of available organs, but also the inability to adequately assess and evaluate these organs prior to transplantation. In recent years, ex-vivo machine perfusion of the liver, both hypothermic (“HMP”) and normothermic (“NMP”), have emerged as useful techniques for improved organ preservation prior to transplantation and assessment of organs. Recent studies have shown the superiority of ex-vivo normothermic machine perfusion (NMP) over traditional cold static storage (“SCS”) which is fraught by poor oxygenation, ischemia/reperfusion injury, loss of cellular energy stores and histologically demonstrable deterioration of organ structure. While both HMP and NMP have shown great promise to mitigate ischemia-reperfusion injury associated with the procurement and organ preservation process, NMP provides a means to regenerate organ energy stores and prolong storage time. Most importantly NMP, by virtue of perfusion at physiologic temperature, provides a means of donor organ viability testing not afforded by HMP and SCS. Generally, quality and quantity of bile formed, and secreted, hepatic artery and portal vein flows and certain biomarkers serve as indicators of potential liver graft function.
In order for ex-vivo organs to function at physiologic temperature, NMP, unlike HMP and SCS, requires perfusion with an oxygen carrier, along with nutrients in the organ perfusate to support organ metabolism. Recently, a major liver transplant center demonstrated the benefits of perfusing livers via a protocol involving initial HMP perfusion followed by NMP. Liver surgeons at the Queen Elizabeth Hospital, Birmingham, UK, perfusing discarded human livers via HMP using a saline solution, followed by NMP using blood, observed superior metabolic recovery, decreased inflammation and decreased oxidative stress compared to livers perfused by NMP alone.
While use of RBCs during NMP has provided a platform for pre-transplant organ assessment, especially with marginal livers, at temperatures below normal body temperature, blood becomes more viscous and RBCs become more fragile and disintegrate, impairing organ perfusion. This fact complicates multi-temperature organ perfusion that must be conducted using different perfusates (e.g., saline during HMP and a hemoglobin-based perfusate during NMP). The viscosity of ZK1 is affected little at lower temperatures and, as a cell-free oxygen carrier, RBC damage is obviated, making ZK1 preferable for organ perfusion protocols that include an HMP phase.
We have been collaborating with leading transplant surgeons, enabling them to use ZK1 as an oxygen carrier additive to organ perfusion solutions for different organs. This has allowed surgeons to perfuse, recondition and evaluate the viability of discarded human organs. Using this strategy with ZK1 in a clinical trial, liver transplant surgeons at the University Medical Center Groningen in the Netherlands extended the Birmingham team’s results by perfusing 16 human livers, previously rejected by all Netherland transplant centers, with Hemopure under an HMP+NMP protocol. This protocol enabled refurbishment and qualification for transplantation of 11 (69%) livers that would have otherwise been lost, increasing the number of available livers at this center by 20% during the course of the trial. All transplant recipients experienced a normal recovery. This study made possible the world’s first discarded liver transplantation and represents a key step toward meeting the overwhelming demand for donor organs. The benefits include a reduction in the long wait patients must endure before their organ transplantation, thereby improving their quality of life.
Given that ZK1 is an ex vivo solution, we intend to apply for market approval under a device label, which is typically more expedient when compared to the ordinary approval process in the United States and Europe.
Our Manufacturing Facilities
The active pharmaceutical ingredient (API) in our products is polymerized bovine hemoglobin, formulated in a vehicle of modified Lactated Ringer’s solution. The bulk drug substance for Oxyglobin and Hemopure is hemoglobin glutamer–200 (bovine), and hemoglobin glutamer–250 (bovine), respectively.
| 69 |
The Manufacturing Process
Overall, product formulation and the manufacturing process flow will remain unchanged in the new manufacturing facility. There are five main steps to manufacture our products
| ● | Collection of Starting Material (Citrated Bovine Blood) | |
| The collection process is a manual operation designed to harvest bovine whole blood from controlled animals at slaughter. The collected whole blood is mixed with citrate solution, an anticoagulant, and transported to our facility for processing. | ||
| ● | Manufacture of Cell Free Purified Hemoglobin Intermediate (C-500) | |
| Red blood cells are washed via diafiltration, separated via centrifugation, processed through stage 1 and 2 ultrafiltration, final filtration and temporarily stored. | ||
| ● | Manufacture of Purified Deoxygenated Hemoglobin Intermediate (C-800) | |
| The C-500 intermediate is purified/separated via ion exchange chromatography, concentrated, deoxygenated, diafiltered into storage solution and processed through a final filtration to create C-800 bulk. | ||
| ● | Manufacture of Bulk Drug Substance (Polymerized Bovine Hemoglobin in a Modified Lactated Ringer’s Solution) | |
| In the final manufacturing process steps for Oxyglobin and Hemopure, C-800 is polymerized via reaction with glutaraldehyde, concentrated, diafiltered with borate, quenched with borohydride, diafiltered into lactated ringer solution and processed via final filtration of pooled sublots. The only manufacturing process difference between Hemopure and Oxyglobin is that Hemopure has an additional fractionation step prior to the filtration and pooling of sublots. | ||
| ● | Sterile Fill Finish | |
| The pooled bulk drug substance stored under nitrogen to maintain a deoxygenated state is transferred under constant nitrogen pressure through sterilizing-grade filters to the automated aseptic bag filling machine. Final product bags are sealed and stored in individual nitrogen-purged overwraps. |
Prior to relocating all phases of the manufacturing process to the Souderton, Pennsylvania facility, only steps 1 and 2 were performed in Souderton. The C-500 intermediate product was then transported in refrigerated trucks to Cambridge, Massachusetts, where steps 3 and 4 could commence through completion of the bulk drug substance before the bulk drug substance was trucked to Toronto, Canada for sterile fill finish (step 5) and subsequently shipped back to Cambridge for storage.
| 70 |
Schematic of Manufacturing Process
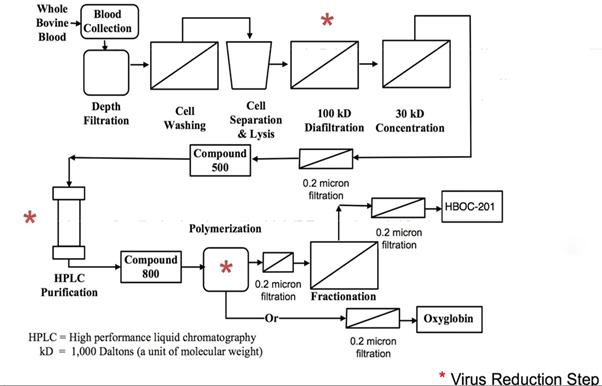
New Facility
Facility Expansion and Fit-Out
We will reinitiate communication with FDA’s Office of Pharmaceutical Quality in the Pennsylvania area and request a face-to-face meeting to review our manufacturing process design, controls, and strategy. Obtaining early input from FDA process and facility reviewers is critical to long term success.
All utilities will be sized appropriately to support the added capacity. The existing Souderton facility, comprised of an 18,000 sq ft production area, will be insufficient to accommodate all stages of the Oxyglobin and Hemopure manufacturing process. The following is a high-level overview of the changes to be made to our existing Souderton facility with the substantial portion of the proceeds of this offering:
| ● | Add ~34,000 sq. ft of production space. | |
| ● | Expand warehouse and utilities | |
| ● | Expand quality control (“QC”) laboratories, including the addition of a sterility lab for onsite sterility testing. An expanded QC lab is needed to accommodate the additional microbiology and chemistry lab equipment transferred from the former Cambridge facility. Upgrades to the lab equipment will be performed for analytical equipment deemed out of date where maintenance as well as performance may be compromised or unavailable. |
| 71 |
| ● | Locate manufacturing equipment in the appropriate clean room environments. With the addition of the fill-finish suite, all processing areas will be designed to ensure appropriate segregation of each intermediate and clean areas. | |
| ● | Add office space to bring full support services to the production facility. |
We expect that the upgraded facility will have a validated annual production capacity of 150,000 Hemopure units or 600,000 Oxyglobin units. This capacity can be used for any combination of Oxyglobin and Hemopure units. Total company head count will increase to realize this manufacturing output and provide support for related activities including quality control, quality assurance, product shipping and facilities maintenance.
In addition, an option on an adjacent plot of land has been secured for the construction of a new plant in 2026-2028 when we expect we may need to expand further our production capacity. The construction of this new production plant could provide an additional annual capacity of 500,000 units of Hemopure.
Stages of Construction
Certain stages of the construction for the upgraded facility are already complete. The first phase of production (base material collection, hemoglobin extraction and initial stages of purification) has been installed and is ready to be initiated. Building permits for the second phase of production, as well as laboratory and office space, have been acquired. Engineering building designs are complete, and utilities allocation has been achieved. The new building is constructed adjacent to the existing facility.
From the prepared cash flow projections and project delivery schedule, we believe the following deliverables should be achieved using the initial $20 million of proceeds from this offering in approximately 10 months:
| ● | All internal detail design and Issue for Construction Documents could be completed; | |
| ● | Building permit for internal fit-out could be obtained; | |
| ● | All preconstruction efforts could be completed for procurement of Long Lead equipment; | |
| ● | All major central plant equipment supporting environmental conditions and process support equipment could be ordered fabricated and delivered and 75% rigged into place; | |
| ● | Rough-in of all overhead MEP throughout facility; and | |
| ● | Structural steel reinforcement and equipment platforms/dunnage steel could be fabricated, delivered and installed. |
Thereafter, additional work will remain to be completed which we estimate will require approximately $15 million and take approximately eight additional months to complete. The remaining 25% central and process support equipment will need to be installed. Building Automation and Process Automation systems will need to be updated. Start-up and commissioning of central support systems will need to be completed, as will the start-up and commissioning of process support equipment. Lastly, validation of all systems and environments will need to be completed. Commissioning and validation are discussed in further detail below.
| 72 |
Manufacturing Facility Commissioning, Qualification and Validation
Because there has been a complete shutdown of operations and relocation of the manufacturing equipment, the entire facility, if and when completed, will go through a full commissioning, qualification and validation of the utilities, unit operations, methods, procedure and finally the validation of the manufacturing process. The objective of our validation program is to verify that manufacturing systems have been suitably designed and are fit for their intended use through verification of their associated critical aspects and to demonstrate control of the critical process parameters such that there is a high degree of assurance that manufacturing processes consistently produce products meeting attributes related to identity, strength, quality, purity, and potency. The program is founded upon the key concepts described in ASTM E2500-133 and the FDA’s recommended three (3) stages of process validation in the product lifecycle of Stage 1 – Process Design, Stage 2 – Process Qualification, and Stage 3 – Continued Process Verification4.
A validation master plan will detail the rationale and methodology approach of the validation process and control execution of qualification and validation activities. The master plan will include pre-approved plans, protocols, procedures, lot records and forms. At the completion of the validation process a minimum of three batches (of each product) will be placed on stability testing. If the facility is completed, all aspects of the facility restart will be conducted in accordance with cGMP regulations and subject to full inspection and approval by US and European regulatory authorities to achieve GMP certification prior to the release of either product for distribution or use. Validation of the production line and manufacturing process will require up to nine months after installation of the Phase II manufacturing equipment. [c]GMP certification is anticipated in 2023.
Addressable Markets
Worldwide Blood Shortage
Veterinary Markets
In 2020, the global animal health products market was valued at $45.4 billion and is expected to have a compound annual growth rate of 9% to 2028 North America has the largest market share at approximately 34% and is expected to maintain its dominant position until 2028, due to the presence of a well-established pharmaceutical and veterinary health care industry, according to ‘Animal Health Market Size, Share & Trends Analysis Report’ by Grand View Research published in August 20215. Europe is expected to be the second largest market share holder in the animal health market due to prevalence of zoonotic diseases and awareness among pet owners.
In the United States alone, there are an estimated 108 million dogs and 79 million cats6. Within the canine population, there are an estimated 500,000 service dogs (approximately 10,000 of which are guide dogs)7. This represents a key targeted population due to the high standard of care levied on these animals.
| Average Expenditure per | ||||||||||||
Number of Pets – US7 | Number of Pets – Europe | Surgical Visit | Routine Visit | |||||||||
| Dogs | 108 million | 89 million8 | $ | 474 | $ | 257 | ||||||
| Cats | 79 million | 110 million9 | $ | 245 | $ | 182 | ||||||
We have approvals for Oxyglobin in the United States and European Union. Upon completion of our manufacturing facility, the necessary regulatory filings and inspections by the Competent Authorities will be necessary for both jurisdictions as part of the cGMP certification process. This will allow us to resume commercial distribution in both markets while pursuing registrations in Canada and Australia which have 7.6 and 5.8 million dogs, respectively and pursue other significant markets including Brazil and India, which has one of the fastest growing pet populations in the world.
3 ASTM E2500-13, Standard Guide for Specification, Design, and Verification of Pharmaceutical and Biopharmaceutical Manufacturing Systems and Equipment, ASTM International, www.astm.org.
4 FDA (CDER)Process Validation: General Principles and Practices. Guidance for Industry. January 2011.
5 ‘Animal Health Market Size, Share & Trends Analysis Report By Animal Type (Production, Companion), By Product (Pharmaceuticals, Diagnostics), By Distribution Channel, By End-use, By Region, And Segment Forecasts, 2021-2028’ published August 2021
6 https://bestfriends.org/no-kill-2025/animal-welfare-statistics#households
7 https://www.hepper.com/service-dog-statistics-and-facts/
8 https://www.statista.com/statistics/414956/dog-population-european-union-eu-by-country/
9 https://www.statista.com/statistics/516041/cat-population-europe-europe/
| 73 |
Over the last two decades, technological advances have resulted in veterinarians being able to treat the previously untreatable, and animals are living longer. The increased need for transfusions has led to the growth of animal blood banks in both the US and Europe. Even with the existence of animal blood bank, there are multiple reports of shortages in both regions.:
Canine blood banks are struggling to keep up with the demand from veterinarians for companion animal blood. In the United States commercial blood banks provide 30%-40% of needed blood products10. Cross matching, cold storage and spoilage of blood products add to animal blood bank limitations in treating animal anemia.
The inadequacy of existing alternatives should be considered against the backdrop of a growing transfusion market. Based on an average transfusion rate in animal populations of 1.5%, the Company estimates the current need for canine transfusions is 1.3 million annually and 1.4 million in cats in the United States, with similar numbers in Europe.
As a readily accessible alternative to animal blood, we believe that Oxyglobin can meet the supply-demand gap in the growing animal transfusion market.
Human Market
Sources of blood for transfusion in humans include stored supplies of donated blood (allogenic) and recipient’s pre-donated (autologous) blood. In many countries, donations do not reach the critical WHO full-support threshold of 30 units per 1,000 population in high-income countries as illustrated below.
Whole blood donations Per 1000 Population, 2013
10 American Veterinary Medical Association, 22 December 2021 https://www.avma.org/news/animal-blood-shortage-deepens-during-pandemic
| 74 |
Distribution of Countries by Number of Whole Blood Donations per 1,000 Population, 201311
This, along with seasonal variations in donor participation, the limited shelf life of blood and logistical challenges of typing and cross-matching, can result in blood shortages during times of high demand.
In addition, there is a growing demand for blood in developing countries to treat injury and for accidents, as well as an increasing number of children affected by diseases that lead to severe anemia. In industrialized countries, technological advances have led to new medical treatments and procedures that require significant amounts of blood.
This increased demand for blood donations have varying geography-dependent etiologies, ranging from contamination of blood by new pathogens to more successful treatments of patients with blood diseases and different types of cancer. In 2014 blood shortage was estimated to be approximately 40 million units annually, focused primarily in developing countries due to the lack of robust infrastructure for blood collection, storage, distribution and the high-discard rate of collected blood.

11 World Health Organization, “Global status report on blood safety and availability”, 2016-2017
| 75 |
Regional Distribution of Population and Blood Donations
by WHO Region and World Bank Income Group, 2013
In 2019, this trend remains relevant with more than 40% of the 117 million blood donations collected globally coming from high-income countries, home to less than 20% of the world’s population. Consequently, 80% of the world’s population does not meet their needs through their own blood collections.12
Even high-income countries experience frequent low blood supplies. As an example, on May 14, 2019, the American Red Cross faced a critical shortage of type O blood and urged eligible donors to give promptly. The type O blood supply available for emergency rooms had fallen to less than a two-day supply, meaning only six units of type O blood where available for every 100,000 people compared to a typical supply of 14 units per 100,000 individuals. Such low blood supplies typically result in elective surgery delays and may prove more serious if coincident with a major natural or man-made disaster. And COVID-19 pandemic exacerbated the problem. Current demand is higher than in a normal year. But supply, which comes from volunteer donors, is lower than in typical times.
According to the South African National Blood Service (SANBS), annual blood donations run at approximately 813,000 units, whereas the actual transfusion need is closer to 1.7 million units in South Africa to meet the World Health Organization (“WHO”) threshold of 30 units per 1,000 people, generating a shortage of 887,000 units of blood.
In Russia, the average rate of blood donation observed is 14 donations per 1,000 population annually, (i.e., 2 million blood units collected) far from reaching the WHO threshold, which would require about 4.3 million units of blood per year. 13
Worldwide Donor Organ Shortage
Organ transplantation is the preferred therapy for most patients with end-stage organ failure since both survival and quality of life are superior in organ recipients compared to similar patients without transplantation. As outcomes of transplantation of organs such as kidney and livers have improved, the number of transplant candidates listed for donor transplantation has increased dramatically, leading to high levels of organ shortage. Organ transplantation saves thousands of lives annually, but the shortage of donors, and consequently of organs, is a major limiting factor to optimizing the benefits of this strategy.
12 https://www.who.int/news-room/fact-sheets/detail/blood-safety-and-availability
13 Gift of Life Fund, https://podari-zhizn.ru/ru/give-help/stat-donorom/chasto-zadavaemye-voprosy/razve-donorskoi-krovi-ne-hvataet
| 76 |
In the United States:
| ● | 107,000 persons are on the national transplant waiting list as of May 2021 and every 9 minutes another person is added to the list14 | |
| ● | 39,000 transplants were performed in 2020, including 22,817 transplants of kidneys, 8,906 livers and 3,658 hearts according to the US Government information on Organ Donation and Transplantation, | |
| ● | 17 people die each day waiting for a transplant. |
Source : Organ procurement and transplantation network optn.transplant.hrsa.gov and OPTN/SRTR Annual Report
In the European Union:
| ● | More than 58,000 patients were on the waiting list as of December 2019. Almost one person is added to the waiting list every 10 minutes15, | |
| ● | Approximately 34,285 organ transplantations, out of which 21,235 kidneys, 7,900 livers and 2,269 hearts, were performed in 201816, | |
| ● | On average, 19 people die every day while waiting for an organ transplant17. |
14 https://www.americantransplantfoundation.org/about-transplant/facts-and-myths/
15 Nature, ‘Organ Donation and transplantation: a multi-stakeholder call for action’ by Vanholder et al, 5 May 2021 https://www.nature.com/articles/s41581-021-00425-3
16 Nature, ‘Organ Donation and transplantation: a multi-stakeholder call for action’ by Vanholder et al, 5 May 2021 https://www.nature.com/articles/s41581-021-00425-3
17 Nature, ‘Organ Donation and transplantation: a multi-stakeholder call for action’ by Vanholder et al, 5 May 2021 https://www.nature.com/articles/s41581-021-00425-3
| 77 |
The persistent significant gap between the number of people on the waiting list and the number of donors and transplants is stressed by the World Health Organization as a universal challenge and urges all countries to seek self-sufficiency in their organ procurement programs. The critical shortage of organs is emphasized by stringent criteria for donations and the short life of available organs whereas the selection and distribution process last approximately 40 hours.
A variety of approaches are being implemented to expand the organ donor pool including live donations, increases in deceased donor donations, split organ donations and greater utilization of expanded criteria donors such as expanding the types of donors to older populations.
Similar trends to the previous discussion on ZK1 on donor liver preservation are beginning in transplantation medicine for other organ types. Kidney transplant surgeons have recently demonstrated that ZK1-based perfusate functions as an excellent alternative to RBC-based perfusate in isolated pig kidneys during sub-sub normothermic perfusion. In fact, ZK1 protected kidneys better than perfusion with blood or saline based on indicators of renal histology and function. Other investigators have similarly found that a perfusate containing ZK1 yielded superior tissue energy stores, blood flow and renal function in rat kidneys compared to a standard care hemoglobin-free saline solution.
Other investigators have shown that at physiological perfusion temperatures, pig limbs perfused with a ZK1-based perfusate retained contractile function, electrophysiological activity and histological integrity for much longer durations (greater than 12 hours) than were limbs stored under standard care conditions (static cold saline solution). Still other surgical teams are beginning to explore the potential benefits of perfusing donor lungs and hearts with ZK1-based perfusate.
All organ perfusion investigators cite the same limitations of RBC-based perfusion including constraint to normothermic perfusion, limited RBC availability, need for cross-matching, mechanical hemolysis, risk of transmissible disease and activation of pro-inflammatory proteins. By contrast, ZK1 is characterized by perfusion temperature flexibility, low immunogenicity, no risk of hemolytic reactions or transmissible disease and enhanced convective and diffusive oxygen delivery. In this context, the Company believes that there exists a large untapped opportunity for ZK1 to support a campaign to increase procurement and qualification of organs previously considered marginal or declined for transplant.
Prehospital
Civilian and Military Trauma and Medical Readiness
Hemorrhagic shock is a form of hypovolemic shock in which severe blood loss leads to inadequate oxygen supply at the cellular level. If hemorrhage continues unchecked, mortality quickly follows. Hemorrhagic shock is still a leading cause of preventable death after severe trauma in both military and civilian settings.
Mortality from hemorrhage represents a significant problem worldwide, with an estimated 1.9 million deaths per year globally, of which 1.5 million results from trauma18. Strategies to improve morbidity and mortality associated with trauma include measures that enable early diagnosis, rapid hemorrhage control, short scene time and the early deployment of ambulances to rapidly transport patients to advanced medical and surgical assistance.
Patients who experience excessive bleeding before arriving at hospital have for many years been resuscitated with hemoglobin-free physiological saline or colloidal solutions because blood and blood products are often unavailable at civilian injury scenes and in far-forward military geographies. Although these solutions can replace lost blood, they cannot carry oxygen and can interrupt the natural clotting process in injured blood vessels. The key management strategy is to arrest bleeding and replace lost blood volume and oxygen-carrying capacity via transfusion. Early identification of the severity of the hemorrhage and coagulopathy can expedite blood product administration and hemostatic interventions if blood products are available. The use of blood products in the prehospital environment has been encouraged as recent studies have concluded that early hemorrhage control and targeted hemostatic resuscitation with balanced blood product administration improves outcomes following hemorrhagic shock. In the United States, 25.3% of the Helicopter Emergency Medical Services (“HEMS”) programs now carry blood for use in prehospital hemorrhagic shock. However, with storage and logistic challenges, the opportunities for ground Emergency Medical Services (“EMS”) administration of blood products are still limited. Hemopure could provide a valuable alternative in such situations because it can provide trauma victims who urgently need restoration of oxygen delivery, a lifesaving “oxygen bridge” before arrival at hospital, potentially saving patients who might otherwise die.
18 Cannon, J.W., Hemorrhagic Shock. New England Journal of Medicine, 2018. 378(4): p. 370-379.
| 78 |
In the military field, as part of the studies being done in South Africa by the U.S. Department of Defense, Hemopure will be evaluated for inclusion in standard soldiers’ backpacks because 90% of soldiers who die from battle injuries never make it to the field hospital. A majority die from hemorrhage and could be saved with proper blood or HBOC transfusion. However, blood transfusion is rarely practical in the field because stored blood requires refrigeration, has a limited shelf life and is blood-type specific.
For example, in Operation “Iraqi Freedom”, 68% of the military fatalities suffered from a severe hemorrhage as part of their injuries which is estimated to have caused between 1,300 and 2,000 military deaths. There are about 2.2 million active military personnel in the United States of which approximately one million could be involved in field operations at any given time.
During the Balkan War, the US military shipped approximately 5,000 units of blood to the region, of which only 73 units were used, with the balance being discarded after it expired. An HBOC, characterized by no refrigeration requirement, long shelf life, universal acceptance and no transmissible disease potential could have been used more efficiently and excess supply shipped to the next destination for later use.
Finally, medical readiness is an integral part of disaster planning. Currently, the US national strategic stockpiles for disaster preparedness do not store blood for transfusion because of its short shelf-life and cold storage requirement. The pre-deployment of Hemopure presents an alternative strategy to prepare for a major disaster and its potential for demand-side shocks to the blood distribution system because Hemopure could meet the key functions of stored blood without the limitations that render stored blood impractical.
The Company estimates that the worldwide potential market for disaster preparedness could reach up to 350,000 units of Hemopure annually. Similar estimates have been made to address annual, worldwide US military applications. The worldwide potential market for both of these applications could add up to $525 million.
When Blood is Not an Option to Patients
In certain cases, even when available, blood may not be an option to particular patients to address medical need.
Jehovah’s Witnesses
There are approximately 8.3 million Jehovah’s Witnesses worldwide according to the 2017 Yearbook of Jehovah’s Witnesses, with an estimated 1.2 million in the United States and about 1.6 million in Europe.
The religious beliefs of Jehovah’s Witnesses regarding blood lead them to refuse:
| ● | the administration of heterologous blood, | |
| ● | the administration of certain blood-derived products such as red blood cells, white blood cells, platelets and plasma, | |
| ● | certain blood saving techniques such as blood donation or storage for autotransfusion, as soon as the physical contact between the patient and his or her blood is disrupted. |
In recent years, the governing organization of Jehovah’s Witnesses has left it to the conscience of each member to accept certain blood fractions such as albumin, immunoglobulins or antithrombin III and coagulation factors, particularly in preparations for hemophiliacs. Conventional vascular filling and hemodilution products (crystalloids, synthetic colloids (dextrans, gelatins, hydroxyethyl starch)) are also typically accepted.
| 79 |

Source: Religious and Ethical Position on medical therapy and related matters, JW.org
However, these options are only possible in the strict context of scheduled surgery under certain defined conditions, whereas in the context of an emergency such as trauma or severe anemias, it is necessary to use therapeutic alternatives to the transfusion of RBCs. The care of Jehovah’s Witness patients in an emergency setting leads to a specialized approach to minimize blood loss and optimize efficient oxygen transport to the organs. However, such measures are not always sufficient to avoid anemia-related morbidity and death.
We believe that Hemopure is an alternative that meets the requirements of JW’s Governing Body and could ensure the effective treatment by medical personnel in emergency situations for patients who refuse blood transfusions.
According to Jehovah’s Witnesses Watchtower Society, the numbers of members in the United States and Western Europe are very similar with approximately 1.2 million members in each region. It seems statistically reasonable to consider that the population of Jehovah’s Witnesses is subject to the same 2% transfusion rate per year as the average world population.
Auto Immunized Patients
Autoimmune Hemolytic Anemia (AIHA) is a serious disorder which occurs when antibodies directed against a person’s own red blood cells cause them to lyse (disintegrate), leading to potentially life-threatening anemia as a result of an insufficient number of oxygen-carrying red blood cells in circulation.
This is a rare event, estimated to affect one to three people per 100,000 annually in the general population with a lower rate of occurrence in Western Europe (1.3 people per 100,000) than in the United States (2.8 people per 100,000) Autoimmune Hemolytic Anemia affects males and females with a slight predominance of cases in females (60%) People of any age, including children, may develop the disease, although it is more common among adults, with a peak incidence between 60-70 years In the United States and Western Europe, the number of patients with AIHA is estimated at around 10,000 people respectively.
Management of an AIHA patient is often done in a relatively urgent setting, as this condition sometimes develops rapidly and with intensity, resulting in life-threatening anemia. Overall adult mortality in this context is between 8% and 15% in accordance with a series of recently published reports19
19 Alberto Zanella and Wilma Barcellini, Treatment of Autoimmune Hemolytic anemia, Haematologica, 2014 Oct; 99(10): 1547-1554 doi:10.3324/haemotol.2014.114561
| 80 |
A rare and more serious form of AIHA, called Evans Syndrome, may be associated (simultaneously or separately) with thrombocytopenia (deficiency of platelets in the blood) and/or autoimmune neutropenia (deficiency of neutrophil granulocytes or polynuclear cells in the blood). The Evan’s syndrome rate of occurrence in patients with AIHA is evaluated at 37-73%.
Treatment and management of AIHA depends on the type of hemolysis and usually involves use of folic acid, corticosteroids, rituximab and intravenous immunoglobulin G. Blood transfusions should be avoided as the risk of transfused RBC lysis (breakage) is high, worsening symptoms and potentially leading to cardiovascular, renal, and other complications.
In the United States and Western Europe, approximately 30,000 patients are diagnosed with AIHA annually (including patients with Evans syndrome), of whom 9,000 require transfusion.
Alloimmunized Patients
Blood transfusion plays a key role in the treatment of patients affected with hemoglobinopathies. Insufficient blood supplies and difficulties in finding immunologically compatible blood for patients who are repeatedly transfused are largely responsible for the morbidity and mortality in thalassemic and sickle cell disease syndrome (SCD).
Thalassemias are inherited blood disorders. People with thalassemia are not able to produce enough hemoglobin. Low RBC hemoglobin content can cause systemic anemia. Organs then become starved for oxygen, are unable to function properly and may sustain irreversible damage. There are two major types of thalassemia, alpha and beta, which are named for the two protein chains in normal hemoglobin. Alpha and beta thalassemia have both mild and severe forms. Transfusion therapies in severe thalassemia patients aim to maintain hemoglobin levels above 9-10 g/dL, which is usually achieved via administration of packed RBCs (pRBCs, a routinely used blood product comprised primarily red blood cells) requiring approximately 30-40 units of pRBCs per year in a 70 kg patient.
An acute sickle cell crisis is characterized by distortion of the red blood cells from a smooth, donut-shape into a crescent or half-moon shape. The distorted RBCs can block small blood vessels, impairing blood flow. This condition leads to shortened RBC survival and anemia. Poor blood oxygen levels and blood vessel blockages in people with SCD can lead to recurring acute pain syndromes, severe bacterial infections and necrosis (tissue death). The frequency of sickle cell crisis varies from country to country and is particularly common among those whose ancestors derive from Sub-Saharan Africa. In the United States, the exact number of people living with SCD is unknown but estimated at approximately 100,000 individuals According to the European Medicines Agency, SCD affected 2.6 per 10,000 people in the European Union in 2017.
Sickle cell anemia is typically treated by transfusing normal RBCs to achieve a minimal safe hemoglobin concentration. The severity of sickle cell anemia and transfusion requirements vary widely among SCD patients.
For all patients, blood transfusions carry the risk of immune reactions, protein-mediated inflammation, transmissible disease and iron overload. Transfusion of a patient with a blood component from another individual will introduce unknown antigens, thereby initiating an immunization process that produces “irregular” alloantibodies (alloimmunization). Infusion of blood products from a donor who is not compatible with the recipient’s blood type can result in dramatic transfusion reactions. A test that identifies the recipient’s blood type before transfusion is essential to avoid transfusion reactions. However, conducting a blood-type test in emergency situations is difficult and may be impossible. For patients who need repeated transfusions, the formation of a new red cell alloantibodies is unpredictable. Indeed, in patients who have been previously transfused or pregnant, the alloimmunization rate has been reported to be approximately 1–3% in the general hospital population, 5% or more in multiply-transfused patients and multiparous females, and can be greater than 20% in patients with transfusion-dependent diseases, such as sickle cell anemia or thalassemia. Hemopure contains no RBC-related antigens and, therefore, elicits very few immune reactions, even after repeated transfusions.
The number of alloimmunized patients in the United States and the European Union is estimated at approximately 65,000 in each geography (including SCD and Thalassemia), 40% of whom are expected to benefit from transfusions.
Important Events
Company Background
Founded in February 2014, we are the successor-in-interest to technology that was first developed by the US-based Biopure Corporation, and its successor, OPK Biotech LLC. The technology has been developed over a 25-year period at a cost of approximately $1 billion dollars. This is in addition to over $25.9 million invested by the US Naval Medical Research Center (NMRC) and US Army.
In July 2009, Biopure ceased operations and sold its assets to OPK Biotech. Concurrently, OPK Biotech acquired HBOC-related intellectual property from Northfield Laboratories in 2010. Thereafter, we acquired the assets and intellectual property of OPK Biotech in August 2014. To date, we have raised approximately $23 million through the issuance of member units and convertible notes payable to support our operations.
| 81 |
Investments
We purchased the assets of OPK Biotech through a Chapter 7 bankruptcy auction. The assets consisted of equipment, intellectual property, finished product inventory and two companies incorporated in the Netherlands (OPK Biotech Netherlands BV) and South Africa (OPK Biotech SA Pty Ltd), herein referred to as the “Subsidiaries”.
Each of the Subsidiaries was set up as a holding vehicle for local registration purposes (OPK Biotech Netherlands BV to hold the European approval for marketing of Oxyglobin and OPK Biotech SA Pty Ltd to hold the South African approval for marketing of Hemopure) in accordance with regulatory requirements. This is currently the only function of those companies. All business negotiations and agreements are conducted by HbO2 Therapeutics. Neither Subsidiary has premises, employees or bank accounts. Subsidiaries’ expenses are paid directly by the US parent company.
Resumption of Oxyglobin sales in Europe and Hemopure in South Africa will trigger implementation of agreements between the parent company and its subsidiaries to pay part of the profits received from those markets to those subsidiaries, most probably in a form of license payment.
Environmental Matters
We are subject to a broad range of federal, foreign, state and local laws and regulations relating to pollution and the protection of the environment. These laws regulate, among other things, the discharge of materials into the environment, the handling and disposal of wastes, remediation of contaminated sites and other matters relating to worker and consumer health, and safety and to the protection of the environment. Noncompliance with these regulations can result in significant fines or penalties or limitations on our operations. Some of these laws impose strict, and in certain circumstances, joint and several liability for costs to remediate contaminated sites on owners and operators, as well as persons who arrange to send regulated materials to such sites. In addition, in our manufacturing processes we use substances regulated under U.S. and European environmental laws.
Intellectual Property
Patents, trademarks, and other intellectual property rights are important in the pharmaceutical industry in which we operate. We own intellectual property protection (purchased from its predecessor) for the technology embodied in its Oxyglobin and Hemopure products. Our patent, patent applications and other intellectual property related matters are managed in collaboration with its patent and trademark counsels. While the patent protecting the composition of Oxyglobin and Hemopure have expired or will soon expire, we may, from time to time, file patent applications for inventions covering combinations based on its products or new applications of and uses for its products that may be relevant to future business.
Intellectual Property Landscape
Our proprietary technology is protected by a combination of trade secrets, patents and trademarks. The most important component of our intellectual property is the manufacturing technology to produce its products at industrial scale. In view of the complexities of processing a biological material of animal origin, and in contrast to other products such as small molecule pharmaceuticals, or even larger proteins of biologics, we believe it is unlikely that competitors could manufacture a same or similar product by reverse engineering product starting with the final product.
Patent and Patent Applications
Our patent and patent applications are comprised of those first filed by its predecessors in the United States, with corresponding applications filed later in other countries of interest to our business, e.g., Europe, Canada, New Zealand and where deemed appropriate. The selection of countries in which such patent applications were pursued was based, in part, on assessments of the commercial importance of such future markets.
We recently filed two patent applications which are still pending. Securing a patent typically involves negotiation between the Company and the governmental authority that issues the patent (e.g., the United States Patent and Trademark Office (“USPTO”) in the United States). In the course of such negotiation, the examining authority may initially reject the patent application claims, for example, based on its interpretation of prior art, and, from time to time, may issue a “final” ruling rejecting certain patent application claims. We, in conjunction with its patent attorneys in the pertinent jurisdiction, may modify or delete claims, or accept suggested claim amendments offered by the examining authority, to secure issuance of a patent. Alternatively, we may continue to pursue the same or similar patent application claims by way of a continuation application, a request for continued examination, or a divisional application, depending upon the applicable jurisdiction.
| 82 |
Patent applications are generally kept secret until published by the USPTO. In some cases, the application claims filed in a patent application may include claims of various scope or directed to different inventions and, after interaction with the relevant patent examining authority, we may elect to file divisional applications, continuation applications, or other types of applications to pursue patents of varied scope.
The term of a US patent is generally 20 years from the earliest effective filing date claimed by the patent application, subject to patent term adjustment resulting from USPTO delay during examination, patent term extension as a result of regulatory delay in approval of a product embodying the patented invention, and payment of applicable maintenance fees. The actual protection afforded by a patent outside the United States, which can vary from country to country, depends upon the type of patent, the scope of its coverage and the availability of legal remedies in the country. All the Company’s issued patents have expired. The nature of our expired patents and our new patent applications are indicated in the table below.
| Country | Patent Title | Expiry Date | First Priority Date | Patent Number | ||||
| USA | Purification of red blood cells by separation and diafiltration
|
February 28, 2022 | February 28, 2022 | 7,001,715 | ||||
| International | Partially Oxidized Hemoglobin-Based Oxygen Carrier as a Treatment for Cyanide with or without Carbon Monoxide Poisoning
|
September 29, 2020 | PCT Application No.: PCT/US2021/052617 | |||||
| International | Hemoglobin Substitute Mixtures including Reconstituted Plasma and Platelets and their manufacture and use | July 2, 2019 | PCT Application/US2020/040698 on the National Stage -5114.1002-001
|
Additionally, we have submitted two provisional patent applications and a PCT patent application for two recent inventions as described below. If patents are approved for these pending applications, the issued patents will be valid until July 2, 2039 and September 29, 2040 (20 years from the respective priority dates).
| 1) | A provisional patent application (62/869,825) was filed July 2, 2019, entitled “Resuscitation Products Providing Robust Oxygen Delivery And Hemostasis By Reconstituting Freeze-Dried Plasma And Freeze-Dried Platelets With HBOC-201”. This application was filed jointly with collaborators at the US Army Institute of Surgical Research and will protect the use of Hemopure in one or more combination products rapidly formulated at the time of need in the field to treat severe anemia, hypovolemia and dilutional or consumptive coagulopathy. | |
| 2) | A PCT non-provisional patent application (PCT/US20/40698) was filed July 2, 2020, entitled “Resuscitation Products Providing Robust Oxygen Delivery and Hemostasis By Reconstituting Freeze-Dried Plasma and Freeze-Dried Platelets With HBOC-201”. This application was filed jointly with collaborators at the US Army Institute of Surgical Research and will protect the use of Hemopure in one or more combination products rapidly formulated at the time of need in the field to treat severe anemia, hypovolemia and dilutional or consumptive coagulopathy. The International Searching Authority has completed a search for related intellectual property and issued a written opinion that supports the industrial applicability of all claims and the novelty of several claims in this patent application. However, the opinion also identified prior art that challenges inventive steps in this application. | |
| 3) | A provisional patent application (US 63/084,887) was filed September 29, 2020, entitled “Partially oxidized hemoglobin-based oxygen carrier as a treatment for cyanide poisoning with or without carbon monoxide poisoning”. This application will protect the use of Hemopure and mixtures of Hemopure and partially oxidized Hemopure as an antidote to treat cyanide poisoning with or without simultaneous carbon monoxide poisoning. |
Confidential Information and Trade Secrets
The success of our business depends, in part, on maintenance of confidential information and trade secrets, generally referred to as proprietary information. We have implemented procedures to maintain the confidentiality of its proprietary information. Employees enter into confidentiality agreements with us and, where appropriate, a confidentiality agreement is executed before confidential information is revealed. Confidentiality provisions are also present in consulting agreements and supplier agreements in certain cases where the consultant or supplier may be exposed to confidential information.
| 83 |
Trademarks
The Company has registered trademarks of the product names Hemopure and Oxyglobin the US with the USPTO, in the European Union and other territories. The list of trademarks and trademark applications owned the Company is below:
| Docket No. | Trademark | Country | Status | Application # | Date Filed | Registration # | Registration Date | |||||||
1161.0007-000 |
HEMOPURE |
USA |
Registered |
73/712,949 |
Feb 23, 1988 |
1509885 |
Oct 25, 1988 | |||||||
1161.0007-001 |
HEMOPURE |
European Union |
Registered |
001956093 |
Nov 9, 2000 |
001956093 |
Nov 9, 2000 | |||||||
1161.0007-003 |
HEMOPURE |
Japan |
Registered |
2002052743 |
Jun 25, 2002 |
4816902 |
Nov 12, 2004 | |||||||
1161.0007-004 |
HEMOPURE |
South Africa |
Registered |
2002/08043 |
Jun 6, 2002 |
200208043 |
Oct 30, 2006 | |||||||
1161.0007-005 |
HEMOPURE |
Norway |
Registered |
200600408 |
Jan 11, 2006 |
241142 |
Sep 30, 2007 | |||||||
1161.0007-006 |
HEMOPURE |
Switzerland |
Registered |
502252006 |
Jan 11, 2006 |
548591 |
Aug 24, 2006 | |||||||
1161.0007-007 |
HEMOPURE |
Australia |
Registered |
1107628 |
Apr 5, 2006 |
1107628 |
Nov 14, 2006 | |||||||
1161.0007-010 |
HEMOPURE |
Russian Federation |
Registered |
2006709162 |
Apr 11, 2006 |
329444 |
Jul 11, 2007 | |||||||
1161.0007-011 |
HEMOPURE |
Canada |
Registered |
1,593,151 |
Sep 7, 2012 |
TMA1004050 |
Aug 31, 2018 | |||||||
1161.0009-000 |
OXYGLOBIN |
USA |
Registered |
73/712,948 |
Feb 23, 1988 |
1507754 |
Oct 11, 1988 | |||||||
1161.0009-001 |
OXYGLOBIN |
Japan |
Registered |
16673/98 |
Feb 27, 1998 |
4303177 |
Aug 6, 1999 | |||||||
1161.0009-002 |
OXYGLOBIN |
European Union |
Registered |
001956192 |
Nov 9, 2000 |
001956192 |
Nov 9, 2000 | |||||||
1161.0009-003 |
OXYGLOBIN |
Japan |
Registered |
2002052745 |
Jun 25, 2002 |
4654007 |
Mar 14, 2003 | |||||||
1161.0009-004 |
OXYGLOBIN |
South Africa |
Registered |
200208045 |
Jun 6, 2002 |
200208045 |
May 11, 2007 | |||||||
1161.0009-005 |
OXYGLOBIN |
Australia |
Registered |
1107629 |
Apr 5, 2006 |
1107629 |
Nov 14, 2006 | |||||||
1161.0009-006 |
OXYGLOBIN |
Norway |
Registered |
200603631 |
Apr 7, 2006 |
235373 |
Oct 5, 2006 | |||||||
1161.0009-007 |
OXYGLOBIN |
Switzerland |
Registered |
53237/2006 |
Apr 10, 2006 |
546435 |
Apr 10, 2006 | |||||||
1161.0009-009 |
OXYGLOBIN |
Brazil |
Registered |
840263627 |
Sep 12, 2012 |
840263627 |
Dec 12, 2017 | |||||||
1161.0088-001 |
BIOPURE (EXPANDED) |
European Union |
Registered |
001368273 |
Oct 28, 1999 |
001368273 |
Oct 28, 1999 | |||||||
1161.0104-002 |
OXYGEN THERAPEUTICS |
Switzerland |
Registered |
142692000 |
Dec 1, 2000 |
497388 |
Dec 1, 2000 | |||||||
1161.0105-001 |
OXYCOLLOID |
Japan |
Registered |
2002052747 |
Jun 25, 2002 |
4654009 |
Mar 14, 2003 |
| 84 |
| Docket No. | Trademark | Country | Status | Application # | Date Filed | Registration # | Registration Date | |||||||
1161.0106-001 |
OXYGEN BRIDGE |
European Union |
Registered |
002063402 |
Jan 19, 2001 |
002063402 |
Jan 19, 2001 | |||||||
1161.0106-002 |
OXYGEN BRIDGE |
Japan |
Registered |
2002052748 |
Jun 25, 2002 |
4654010 |
Mar 14, 2003 | |||||||
1161.0106-003 |
OXYGEN BRIDGE |
South Africa |
Registered |
2002/08047 |
Jun 6, 2002 |
200208047 |
Jul 28, 2006 | |||||||
1161.0127-001 |
HEMO2PURE |
European Union |
Registered |
002810158 |
Jul 24, 2002 |
002810158 |
Jul 24, 2002 | |||||||
1161.0127-002 |
HEMO2PURE |
Japan |
Registered |
2002064203 |
Jul 30, 2002 |
4816904 |
Nov 12, 2004 | |||||||
1161.0127-004 |
HEMO2PURE |
Hong Kong |
Registered |
18622/2002 |
Jul 16, 2002 |
20041304053 |
Jul 16, 2002 | |||||||
1161.0127-006 |
HEMO2PURE |
South Africa |
Registered |
2002/18608 |
Nov 26, 2002 |
200218608 |
Nov 26, 2002 | |||||||
1161.0128-001 |
OXYBRIJ |
European Union |
Registered |
003012978 |
Jan 15, 2003 |
003012978 |
Jan 15, 2003 | |||||||
1161.0128-002 |
OXYBRIJ |
Australia |
Registered |
950315 |
Apr 11, 2003 |
950315 |
Apr 11, 2003 | |||||||
1161.0128-004 |
OXYBRIJ |
Hong Kong |
Registered |
300005570 |
Apr 11, 2003 |
300005570 |
Apr 11, 2003 | |||||||
1161.0128-007 |
OXYBRIJ |
Singapore |
Registered |
T03/05350I |
Apr 15, 2003 |
T0305350I |
Apr 15, 2003 | |||||||
1161.0134-000 |
HBOC-201 |
United States of America |
Registered |
78/176,543 |
Oct 21, 2002 |
3086047 |
Apr 25, 2006 | |||||||
1161.0134-001 |
HBOC-201 |
China |
Registered |
3398178 |
Dec 9, 2002 |
3398178 |
Aug 7, 2004 | |||||||
1161.0134-002 |
HBOC-201 |
European Union |
Registered |
002958015 |
Nov 27, 2002 |
002958015 |
Nov 27, 2002 | |||||||
1161.0134-004 |
HBOC-201 |
Japan |
Registered |
2002101001 |
Nov 29, 2002 |
4803811 |
Sep 17, 2004 | |||||||
1161.0134-010 |
HBOC-201 |
Norway |
Registered |
200603616 |
Apr 7, 2006 |
235324 |
Oct 4, 2006 | |||||||
1161.0134-011 |
HBOC-201 |
Russian Federation |
Registered |
2006709163 |
Apr 11, 2006 |
338390 |
Nov 30, 2007 | |||||||
1161.0134-012 |
HBOC-201 |
Switzerland |
Registered |
53236/2006 |
Apr 10, 2006 |
551693 |
Apr 10, 2006 | |||||||
1161.0150-000 |
OXYGEN BRIDGE (EXPANDED) |
United States of America |
Registered |
78/847,457 |
Mar 28, 2006 |
3396256 |
Mar 11, 2008 | |||||||
1161.0150-003 |
OXYGEN BRIDGE (EXPANDED) |
Switzerland |
Registered |
53238/2006 |
Apr 10, 2006 |
546436 |
Apr 10, 2006 |
| 85 |
Trademark registrations are usually granted for a period of ten years and can be renewed indefinitely. Some countries require proof of use in order to maintain the associated trademark rights. In other countries, registrations remain valid unless a third party with an interest therein files an action to have the trademark declared lapsed owing to lack of use.
Domain names
We own seven domain names including those composed of our product names. Domain names are generally renewable every year or every two years.
| ● | biopure.com | |
| ● | opkbiotech.com | |
| ● | hbo2therapeutics.com | |
| ● | hemopure.com | |
| ● | oxyglobin.com | |
| ● | hemopure.net | |
| ● | hemopure.co.za |
Litigation
We are not currently involved in any dispute pertaining to its intellectual property rights, either as a plaintiff or a defendant.
Government Regulation
Governmental authorities in the US, the European Union (“EU”) and elsewhere in the world extensively regulate the testing, manufacturing, labelling, advertising, promotion, export and marketing of drugs and biologics for humans and animals. In the United States, the FDA, an agency within the US Department of Health and Human Services (“DHHS”), serves this function under the authority of the Federal Food Drug and Cosmetic Act (“FFDCA”) and its implementing regulations. The FDA consists of the Office of the Commissioner and four directorates overseeing the core functions of the agency: Medical Products and Tobacco, Foods and Veterinary Medicine, Global Regulatory Operations and Policy, and Operations. In the EU, drugs for veterinary use are regulated in the EU-directive Dir. 2001/82/EC (as amended), together with a number of ancillary Directives and Regulations. They may – just like drugs for human use – be authorized in a Centralized procedure performed by the EMA, or in the Decentralized Procedure, the Mutual Recognition Procedure or the National Procedure by regulatory bodies in the Member States. The Centralized procedure is governed by the EU-regulation Reg. (EC) 726/2004 and is mandatory for a variety of drugs (e.g., drugs derived from biotechnology, monoclonal antibodies, veterinary drugs for use as performance enhancers and orphan drugs). Furthermore, the centralized procedure may be used on a voluntary basis for any new active ingredient which was not authorized in the community when the Regulation entered into force, and for drugs that constitute a significant therapeutic, scientific or technical innovation.
The regulation of drugs for humans and animals aligns with one of the main purposes of these regulatory authorities – to protect the human and animal population from the potential impact of unsafe and possibly dangerous unapproved drug products. As such, in the US, the FDA is focused, as it relates to drugs, on assuring the safety, effectiveness and security of human and veterinary drugs. Within the FDA, veterinary drugs are regulated through the Center for Veterinary Medicine (“CVM”), and human drugs are regulated through the Center for Drug Evaluation and Research (“CDER”).
| 86 |
The EMA was created in 1995 (then named European Agency for the Evaluation of Medicinal Products, “EMEA”) to establish a centralized European drug authorization to exist beside the national drug authorization procedures that are still available in the EU Member States. Within the EMA, drugs for human use are evaluated by the Committee for Medicinal Products for Human Use (“CHMP”) and veterinary drugs are evaluated by the Committee for Medicinal Products for Veterinary Use (“CVMP”). The CVMP consists of members from the 28 EU Member States plus Norway and Iceland.
Any oxygen therapeutic product administered to human patients or companion animals is regulated as drug or biologic drug and requires regulatory approvals before it is commercialized. Our oxygen therapeutic products, for humans and animals, are subject to such regulation.
In order to gain approval (in the US) and marketing authorization (in the EU), governmental authorities require satisfactory completion of an FDA or EMA inspection of the manufacturing facilities at which the product is made to assess compliance with current Good Manufacturing Practices (cGMP) and applicable quality standards. These inspections include elaborate testing, control, documentation and other quality assurance procedures. Both jurisdictions require that the process for approval of a drug and requirements after approval adhere to Good Current Practice (“cGXP”), which includes current good clinical practice (cGCP), current good laboratory practice (cGLP), and current good pharmacovigilance practice (cGVP) and cGMP.
The standards to assure that only quality products are being provided to animals in the US and EU are relatively similar. While the FDA generally inspects manufacturing facilities once every two years, the FDA can choose to inspect any facility used in the manufacture or manufacturing process of the company’s products at any time in the event the FDA has questions or concerns about quality, manufacturing, labelling, adherence to advertising and promotion guidelines or for other reasons. Additionally, modifications and other changes to a manufacturing process and facility relocation are subject to review and inspection to ensure continued compliance with cGMP quality standards. Accordingly, on the expansion of the Company’s Souderton Facility, we are subject to review and inspection to ensure such continued compliance with cGMP quality standards. The FDA can withdraw approval at any time from the manufacturing facilities if the FDA determines that the product is not being manufactured in accordance with cGMP such that the safety, effectiveness and quality of the product cannot be ascertained.
Besides the market authorization for the particular drug, any company that manufactures drugs within the EU has to obtain a manufacturing license from the local regulatory body in the Member State where the manufacturing takes place. National authorities perform inspections of manufacturing license holders to ensure that they are adhering to the principles and guidelines of good manufacturing practice (GMP). We do not currently manufacture within the EU, and this is expected to be solely undertaken at the Souderton Facility in the United States. Similar to the FDA framework, modifications and other changes to a manufacturing process and facility relocation are subject to review and inspection to ensure continued compliance with GMP quality standards. Accordingly, on the expansion of the Company’s Souderton Facility, the Company is likely to seek certain variances and is subject to review and inspection to ensure such continued compliance with GMP quality standards.
The EMA coordinates inspections requested by the CVMP in connection with the assessment of market authorization applications or matters referred to these committees to verify compliance with the principles of GMP, GCP, GLP and GVP. Under EU law, all manufacturers and importers of pharmaceutical products located in the European Economic Area (EEA) must hold a manufacturing authorization. These authorizations are supervised by medicines regulatory authorities in Member States, which are responsible for issuing authorizations for activities taking place in their territories.
For products imported from countries outside the EU, it has to be verified that the manufacturer conforms to standards of GMP equivalent to those in force in the EU, unless the country has negotiated an appropriate agreement with the EU establishing mutual recognition of GMP inspections. As for the Mutual Recognition Agreement with the US, this has been concluded but is not in operation20 .. The supervisory authority for manufacturing sites in countries outside the EU is the regulatory authority that issues the manufacturing authorization to the importer.
20(https://www.ema.europa.eu/en/documents/procedural-steps-after/oxyglobin-epar-procedural-steps-taken-scientific-information-after-authorisation_en.pdf).
| 87 |
The EMA’s Manufacturing and Quality Compliance Section coordinates GMP inspections of manufacturing sites connected to centrally authorized human and veterinary medicines. If a drug is manufactured in the EU, the market authorization holder has to take account of technical and scientific progress and to make variations to the manufacturing process if required. Such variations have to be approved by the competent authority. The market authorization holder has to have at his disposal an appropriately qualified person responsible for pharmacovigilance.
With respect to its veterinary oxygen therapeutic prescription product, Oxyglobin, we have received regulatory approval and marketing authorization from the FDA and European Commission to market this product to veterinarians for the treatment of canine anemia. Currently both FDA and European Commission authorizations are indefinite and have no expiry date. Oxyglobin was approved by the FDA under New Animal Drug Application (“NADA”) #141-067 12 January 1998, and the FDA approved a change in the sponsor name for the product from Biopure Corporation, the original sponsor, to OPK Biotech, LLC in January 2011. In early 2015, the FDA acknowledged that the sponsor of Oxyglobin was changed from OPK Biotech, LLC to Hemoglobin Oxygen Therapeutics LLC. In the US, the critical standards for evaluation of new animal drugs are safety (human food supply, target animal, the environment overall and to the user); effectiveness, as demonstrated by studies with the proposed new animal drug; a quality manufactured product; and a properly labelled product. A brief overview of the approval process in the US through the FDA’s CVM is as follows:
| ● | The drug sponsor collects information about the safety and effectiveness of a new animal drug. | |
| ● | The sponsor may need to conduct studies to get this information. For any studies that are performed, the sponsor analyses the results. | |
| ● | Based on the collected information, including any study results, the sponsor decides if there is enough proof that the drug is safe and effective to meet the requirements for approval. | |
| ● | The sponsor submits a New Animal Drug Application (NADA) to CVM. The NADA includes all the information about the drug and the proposed label. | |
| ● | A team of CVM personnel, including veterinarians, animal scientists, biostatisticians, chemists, microbiologists, pharmacologists, and toxicologists, reviews the NADA. If the center’s team agrees with the sponsor’s conclusion that the drug is safe and effective if it is used according to the proposed label, the NADA is approved, and the drug sponsor can legally sell the drug. |
Additionally, in the US, under the Animal Medicinal Drug Use Clarification Act (“AMDUCA”) of 1994, veterinarians may prescribe our approved drug for extra-label uses under certain conditions. “Extra-label” means the actual or intended use of a drug by or under the order of a veterinarian in a manner that is not in accordance with the approval labelling for the drug. Deviations from the label include, but are not limited to, use in a species not listed in the labelling; use for indications not listed in the labelling; use at dosage levels, frequencies or routes of administration other than those in the labelling; and deviation from the labelled withdrawal time based on these different uses. While veterinarians may prescribe our product for extra-label uses, we may not promote our products for extra-label uses. If the FDA determines that any of our marketing activities constitute promotion of an extra-label use, we could be subject to regulatory enforcement, including seizure of any misbranded or mislabeled drugs, and civil or criminal penalties, any of which could have an adverse impact on our reputation and expose us to potential liability.
In the EU, the market authorization for Oxyglobin is in the name of our wholly owned subsidiary, OPK Biotech Netherlands, BV, due to the EU legal requirement that the marketing authorization be held by an EU based entity. Oxyglobin’s market authorizations were granted in 1999 under the authorization numbers EU/2/99/015/003 and EU/2/99/015/004. The authorizations are valid throughout the EU and the EEA and were, after the first renewal in 2004, granted for an indefinite period.
| 88 |
To obtain any type of market authorization in the EU or a Member State, the drug’s efficacy and safety have to be established. In most cases, this requires the performance of pre-clinical and clinical trials. Companies wishing to obtain a marketing authorization for a drug for veterinary use need to provide the EMA with an application that includes a comprehensive package of data (the dossier) establishing that the medicine meets the required quality, safety and efficacy standards; the safety of the medicine with regard to people coming into contact with the medicine and to the environment must also be demonstrated. Upon submission of the complete dossier, the validation procedure takes up to 210 days. This time can be interrupted to allow applicants to prepare responses to questions from the CVMP. For each product that has to be evaluated, the CVMP appoints two rapporteurs to lead and coordinate the evaluation. The EMA committees prepare the drug assessment report, but the market authorization itself is granted by the European Commission. At the end of the procedure, the CVMP issues a scientific opinion on whether or not the medicine should be authorized. This opinion is submitted to the European Commission that grants the market authorization within 67 days from receipt of the CVMP opinion.
Any EU market authorization for medicinal products is subject to a so-called sunset clause, i.e., the market authorization expires after three years if the product is not placed on the market, or, if the product was originally placed on the market, if marketing was paused for at least three years. We have requested and received an exemption to the sunset clause from the European Union. We intend to continue marketing Oxyglobin®.
Since the UK voted to leave the European Union, they are also no longer a part of the EU medicines framework. The resulting impact to Oxyglobin is that a stand-alone marketing authorization for Great Britain was issued (Authorization # Vm 17408/5000). This Authorization is valid indefinitely unless, within five years of granting it, any issues related to pharmacovigilance could, result in an expiration after five years, unless a further renewal is applied for.
Even a valid market authorization may be modified, suspended or withdrawn if the risk-benefit ratio of the drug is no longer positive or in cases of incompliance with legal requirements. Furthermore, it may be necessary to obtain variations of a market authorization in accordance with variation procedures set forth in Reg. (EC) 1234/2008. As shown in the Oxyglobin summary of procedural steps),
https://www.ema.europa.eu/en/documents/procedural-steps-after/oxyglobin-epar-procedural-steps-taken-scientific-information-after-authorization_en.pdf.
Oxyglobin has been subject to several variation procedures in recent years in connection with a number of changes including manufacturing processes, supplier arrangements, facility changes and pharmacovigilance. Each of the variations was approved.
With respect to its human oxygen therapeutic product, we have received market authorization (registration no. 34/30.4/0261) from the South African Health Products Regulatory Authority (SAHPRA), which at the time was the Medicines Control Council (“MCC”) . The Hemopure approval in South Africa tis for the treatment of adult peri-operative anemia. Because of local regulatory requirements, the marketing authorization is in the name of Company’s wholly owned subsidiary, OPK Biotech South Africa (Pty) Ltd. Although SAHPRA reserves the right to inspect the US manufacturing facilities for cGMP compliance, mutual recognition agreements are in place, and both EU and US cGMP inspections are considered valid. SAHPRA will instead conduct an inspection of the US manufacturing documentation, copies of which need to be maintained onsite at the local subsidiary office The Company has also received authorization from the Russian Ministry of Health to market Hemopure (registration #LP-000011) for the treatment of anemia in the Russian Federation. In 2020, the company had discussions with two large distribution groups in the Russian Federation. When the situation normalizes in that country and sanctions are lifted, we will evaluate reactivating the license and conclude a distribution agreement.
Our manufacturing facility in Souderton, Pennsylvania, if and when completed, will have to be inspected and GMP certified by the FDA or EMA prior to any product release for distribution. In 2020 the Center for Veterinary Medicines (“CVM”) announced their decision to include veterinary pharmaceuticals as part of the FDA/EU Mutual Recognition Agreement (“MRA”) for pharmaceutical Good Manufacturing Practice inspections. here is also precedent, that the human and veterinary product do not require separate inspections. The products are manufactured under the same quality system, using the same process (with the addition of one final step for Hemopure), raw materials, personnel, equipment and are subject to the same manufacturing requirements. Both products share the same nominal Hb concentration and Hb oxygen affinity (p50). Oxyglobin and HBOC-201 active pharmaceutical ingredients are physically distinguished only by a moderate difference in their Hb polymer molecular weight distribution (MWD), such that the average MW of Hemopure is 250 kD, compared to Oxyglobin’s 200 kD average MWD.
| 89 |
Other Regulation
In addition to regulations enforced by the FDA, we are also subject to U.S. regulation under the Controlled Substances Act, the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and other present and potential future federal, state, local or similar foreign regulations. Our research and development may involve the controlled use of hazardous materials, chemicals and radioactive compounds. Although we believe that its safety procedures for handling and disposing of such materials comply with the standards prescribed by state and federal regulations, the risk of accidental contamination or injury from these materials cannot be completely eliminated. In the event of any accident, we could be held liable for any damages that result and any such liability could exceed our resources
Employees and Human Capital
As of the date hereof, we have 10 full-time employees. We have relied and plan on continuing to rely on independent organizations, advisors and consultants to perform certain services for us, including handling substantially all aspects of regulatory approval, clinical management, manufacturing, marketing, financial reporting and sales. Such services may not always be available to us on a timely basis or at costs that we can afford. Our future performance will depend in part on our ability to successfully integrate newly hired officers and to engage and retain consultants, as well as our ability to develop an effective working relationship with our management and consultants.
The following table and biographical summaries set forth information, including principal occupation and business experience, about our directors and executive officers as of the date of this prospectus:
| Name | Age | Position | ||
| Zafiris G. Zafirelis | 76 | Chief Executive Officer, Director | ||
| Igor Serov | 50 | Chief Financial Officer, Director | ||
| Joseph Rappold, M.D. | 60 | Chief Medical Officer | ||
| Brian Dawson | 57 | Senior Director of Process Development | ||
| Gregory P. Dube, Ph.D. | 67 | Senior Director of Pharmacology | ||
| Melissa Zafirelis | 50 | Director of Regulatory Affairs and Clinical Operations | ||
| Anthony E. Pusateri, Ph.D. | 60 | Director Nominee | ||
| Richard Kilsby | 70 | Director Nominee | ||
| Leslie S.T. Fang, MD PhD | 76 | Director Nominee |
The appointment of Mr. Joseph Rappold is conditioned on the completion of this offering. After the completion of the offering Mr. Gregory Dube will be transferred from the consultancy agreement to the full-time employment contract.
Zafiris G. Zafirelis, Chief Executive Officer
Mr. Zafirelis co-founded the Company in February 2014. From October 2009 to January 2013, Mr. Zafirelis was Chief Operating Officer of OPK Biotech LLC, the predecessor-in-interest to the Company. From July 2004 to September 2009, he was President and Chief Executive Officer of Biopure Corporation, the predecessor-in-interest to OPK Biotech. Prior to rejoining Biopure in 2004, Mr. Zafirelis was Director of Product Applications was June 1988 to July 1995, at BioPure Corporation where he led development of Oxyglobin. Subsequently Mr. Zafirelis served as entrepreneur-in-residence at Medical Science Partners (August 1995-December 1995), cofounder and CEO of Cardiac Assist Technologies (January 1996 – May 2002) now part of LivaNova, a global medical technology company, and CEO of Medquest Medical Products (June 2002-May 2004) acquired by WorldHeart Inc (subsequently acquired by HeartWare, now part of Medtronic). Previously he served as managing director of Millipore South Africa and held various managerial positions at American Cyanamid (South Africa). Mr. Zafirelis has a South African Diploma in Chemistry, Graduate Diploma in Chemistry from the University of Rhodesia and MBA from the University of Southern California. He has also completed the Harvard Business School program “Making Corporate Boards More Effective” (ISS accredited).
| 90 |
Igor Serov, Chief Financial Officer
In April 2014, Mr. Serov co-founded the Company. From April 2012 to April 2014, he was a managing director of Sberbank, the largest Russian bank. From January 2009 to April 2011, he was Chief Executive Officer of United Industrial Corporation, a multi-industrial holding company based in Moscow, Russia. From July 2006 to October 2008, he was managing director of Renaissance Capital. Prior to that date, he held management positions with various financial institutions in London and New York. Mr. Serov has a BA in Mathematics and Economics from Connecticut College.
Joseph Rappold, MD, Chief Medical Officer
Dr. Rappold CAPT, USN (Ret) is Professor of Surgery at Tufts University, and the Chief of Acute Care Surgery and the Trauma Medical Director at Maine Medical Center. He retired from the US Navy after 30 years of active-duty service during which he served a series of six combat deployments to both Iraq and Afghanistan commanding a variety of facilities including all US personnel at the British Field Hospital at Camp Bastion. He has published multiple articles and book chapters and has received a variety of unit and personal awards including the Bronze Star for valor in combat and the Defense Meritorious Service Medal.
Brian Dawson, Senior Director of Process Development
Mr. Dawson has over 25 years of experience working with the development and commercialization of Hemopure and Oxyglobin. Brian started his career with Biopure in 1989 and held several positions in manufacturing operations offering broad qualifications in all facets of production, maintenance, calibration, supply chain, process engineering, validation, environmental control, anaerobic aseptic filling, packaging, microbiology and capacity management. The scope of Mr. Dawson’s experience encompasses preclinical research and development phase through product approval and marketing. He is successful in streamlining production processes, accelerating performance, reducing costs, and meeting time-critical industry demands. Brian was Director of Operations for both Biopure and OPK Biotech LLC and has a BA from North Adams State College (Massachusetts College of Liberal Arts).
Gregory P. Dube, PhD., Senior Director of Pharmacology
Mr. Dube has an extensive background in preclinical research, clinical research, and drug research and development. He earned his BS in Biology at Northeastern University and his PhD in Pharmacology and Cell Biophysics at University of Cincinnati, College of Medicine. He has authored numerous industry related articles and abstracts.
Melissa Zafirelis, Director of Regulatory Affairs and Clinical Operations
Ms. Zafirelis has over 13 years of clinical development experience in contract research organizations (CROs), pharmaceutical, medical device, biotechnology firms and clinical trial software companies. She has a BA from University of Massachusetts and a MBA from the University of Southern California, Marshall School of Business.
Anthony E. Pusateri, Ph.D., Director Nominee
Dr. Anthony Pusateri is chief scientist at Leidos, a major US defense, aviation, information technology and biomedical research company. His 30-year career in biomedical R&D has included a broad range of positions in the pharmaceutical industry and government. He has served on or led product development teams that resulted in numerous products advancing into clinical phase development (some still active) or attaining FDA approval. He has held discovery research and product development positions with international pharmaceutical companies (Novo Nordisk and Pharming Health Care) where he focused on products related to hematology and hemorrhage control. As the manager of the US Department of Defense Hemorrhage and Resuscitation Research and Development Program, he oversaw major R&D programs that led to significant advances in the areas of blood transfusion and other emergency resuscitative procedures and had responsibility for annual budgets exceeding $100 million. He also served as director of research and chief scientific officer at the United States Army Institute of Surgical Research, with responsibility for staff and facilities conducting laboratory, animal, and clinical research programs, and annual R&D budgets over $40 million. Dr. Pusateri has been involved in the design and oversight of national and international multicenter clinical trials, and has remained active in research, with over 100 research publications. He earned his B.S. from the University of Illinois, M.S. from Iowa State University, and Ph.D. from Purdue University. Dr. Pusateri is also a retired Army Reserve officer.
| 91 |
Richard Kilsby, Director Nominee
Mr. Kilsby has held non-executive and executive roles in both publicly listed and private companies in industries ranging from financial services to technology. He was non-executive Chairman for 10 years of 888 Holdings Plc - a provider of global online gaming. Most recently he was Chairman of Telit Communications PLC - a leading supplier of IOT modules and associated services. He has also been a non-executive director at Tullet Prebon, Impact Holdings Plc and Collins Stewart Plc and has acted as an Independent Monitor for the SEC. From 1998 to 2002 Mr. Kilsby was Chief Executive/Vice Chairman of Tradepoint / virt-x - the Swiss SMI exchange. From 1995 to 1998 he was executive director at the London Stock Exchange responsible for secondary market regulation and introducing the order driven market and associated stamp duty changes to London. He was also the Competent Authority for Listing, a role which was subsequently taken on by the FSA (“Financial Services Authority”) in the United Kingdom.
Dr. Leslie Fang, Director Nominee
Dr. Leslie S.T. Fang, MD PhD, did his medical training at Harvard Medical School and his residency at the Massachusetts General Hospital where he was Chief Resident. A clinician and teacher, he has an international concierge practice in Internal Medicine and Nephrology. He is the recipient of the John R. Gallagher III and Katherine A. Gallagher Endowed Chair, Clinical Excellence in Nephrology, and former Firm Chief of the Walter Bauer Firm in the department of Medicine at the Massachusetts General Hospital, Harvard Medical School. A highly acclaimed teacher with multiple teaching awards from Harvard Medical School and Massachusetts General Hospital, he served on the Faculty Council of Harvard Medical School for 7 years and was Chairman of the Internship Selection Committee at the Massachusetts General Hospital for 23 years. He is the Executive Chairman of the Fang Foundation with a mission to enhance the quality of life of men and women on the front line of medical care and Chairman of the Board for Games on Autism Research (“GOFAR”).
Director Terms; Qualifications
Members of our board of directors serve until the next annual meeting of stockholders, or until their successors have been duly elected.
When considering whether directors and nominees have the experience, qualifications, attributes and skills to enable the board of directors to satisfy its oversight responsibilities effectively in light of the Company’s business and structure, the board of directors focuses primarily on the industry and transactional experience, and other background, in addition to any unique skills or attributes associated with a director.
Family Relationships with Board of Directors
None.
Board of Directors and Corporate Governance
Concurrently with the closing of this offering, our Board of Directors will consist of 5 members, consisting of Zafiris G. Zafirelis, Igor Serov, Anthony E. Pusateri, Richard Kilsby and Leslie Fang.
Board Committees
Our Board of Directors has appointed an audit committee, governance committee and compensation committee.
| 92 |
Audit Committee
The audit committee is responsible for overseeing: (i) our accounting and reporting practices and compliance with legal and regulatory requirements regarding such accounting and reporting practices; (ii) the quality and integrity of our financial statements; (iii) our internal control and compliance programs; (iv) our independent auditors’ qualifications and independence and (v) the performance of our independent auditors and our internal audit function. In so doing, the audit committee maintains free and open means of communication between our directors, internal auditors and management.
Concurrently with the closing of this offering, the Audit Committee will consist of Dr. Anthony E. Pusateri, Mr. Richard Kilsby, and Dr. Leslie Fang, with Mr. Richard Kilsby acting as Chairman and the Audit Committee financial expert.
Compensation Committee
The compensation committee is responsible for reviewing and approving the compensation of our executive officers and directors and our performance plans and other compensation plans. The compensation committee makes recommendations to our Board of Directors in connection with such compensation and performance plans.
Concurrently with the closing of this offering, the Compensation Committee will consist of Dr. Anthony E. Pusateri, Mr. Richard Kilsby, and Dr. Leslie Fang, with Dr. Leslie Fang acting as Chairman.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for (i) identifying, screening and reviewing individuals qualified to serve as directors (consistent with criteria approved by our Board of Directors) and recommending to our Board candidates for nomination for election at the annual meeting of shareholders or to fill board vacancies or newly created directorships; (ii) developing and recommending to our Board of Directors and overseeing the implementation of our corporate governance guidelines (if any); (iii) overseeing evaluations of our Board of Directors and (iv) recommending to our Board of Directors candidates for appointment to board committees.
Concurrently with the closing of this offering, the Nominating and Corporate Governance Committee will consist of Dr. Anthony E. Pusateri, Mr. Richard Kilsby, and Dr. Leslie Fang, with Dr. Anthony E. Pusateri acting as Chairman.
Code of Ethics
The Company adopted a formal code of ethics within the meaning of Item 406 of Regulation S-K promulgated under the Securities Act, that applies to our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions and that that establishes, among other things, procedures for handling actual or apparent conflicts of interest. Our Code of Ethics is available at our website, www.hbo2therapeutics.com.
Indemnification Agreements
We have entered into indemnification agreements for our directors and executive officers (“Indemnification Agreement”). The Indemnification Agreement provides for indemnification against expenses, judgments, fines and penalties actually and reasonably incurred by an indemnitee in connection with threatened, pending or completed actions, suits or other proceedings, subject to certain limitations. The Indemnification Agreement also provides for the advancement of expenses in connection with a proceeding prior to a final, non-appealable judgment or other adjudication, provided that the indemnitee provides an undertaking to repay to us any amounts advanced if the indemnitee is ultimately found not to be entitled to indemnification by us. The Indemnification Agreement sets forth procedures for making and responding to a request for indemnification or advancement of expenses, as well as dispute resolution procedures that will apply to any dispute between us and an indemnitee arising under the Indemnification Agreement.
| 93 |
The table below summarizes the compensation earned for services rendered to us in all capacities, for the years indicated, by its named executive officers:
| Name and Principal Position | Year | Salary Paid in Cash | Accrued Salary | Total Compensation | ||||||||||||
| Zafiris G. Zafirelis, Chief Executive Officer | 2021 | $ | 100,000 | , | $ | 100,000 | $ | 200,000 | ||||||||
| 2020 | $ | 77,808 | $ | 122,193 | $ | 200,001 | ||||||||||
| Igor Serov, Chief Financial Officer | 2021 | $ | 100,000 | $ | 100,000 | $ | 200,000 | |||||||||
| 2020 | $ | 77,808 | $ | 122,193 | $ | 200,001 | ||||||||||
We have entered into at-will employment agreements with each of Zafiris G. Zafirelis, Igor Serov, and Joseph Rappold with annual compensation of $350,000 with the possibility of an annual bonus, which will commence once this Company is listed on NASDAQ. In the event of a termination of employment without cause, we are obligated to make a severance payment of $350,000. Messrs. Zafirelis, Serov, and Rappold may also be eligible to be granted equity awards under our 2022 Equity Incentive Plan as may be determined by the Compensation Committee.
We have entered into an at-will employment agreement with Brian Dawson in the position of Senior Director of Process Development, with annual compensation of $180,000 with a possibility of an annual bonus. In the event of a termination of employment without cause, we are obligated to make a severance payment of $180,000. Mr. Dawson may also be eligible to be granted equity awards under our 2022 Equity Incentive Plan as may be determined by the Compensation Committee.
Director Compensation
None of the directors received any compensation during the year ended December 31, 2021.
The Board adopted a Non-Employee Director Compensation Policy (the “Director Compensation Policy”) as following:
Annual Cash Compensation
Once this offering is effective, the Non-Employee Director will receive the cash compensation set forth below for service on the Board. The annual cash compensation amounts will be payable in equal quarterly installments, in arrears following the end of each quarter in which the service occurred, pro-rated for any partial months of service. All annual cash fees are vested upon payment.
| 1. | Annual Board Service Retainer: |
| a. | All Non-Employee Directors other than the Board Chair: $30,000 | |
| b. | Non-Employee Director who is the Board Chair:: Currently held by Employee Director |
| 2. | Annual Committee Chair Service Retainer (in addition to Annual Board Service Retainer): |
| a. | Chairman of the Audit Committee: $20,000 | |
| b. | Chairman of the Compensation Committee: $5,000 | |
| c. | Chairman of the Corporate Governance Committee: $5,000 |
| 94 |
Equity Compensation
Equity awards will be granted under a 2022 Equity Incentive Plan or any successor equity incentive plan (the “Plan”). All stock options granted under this Director Compensation Policy will be Nonstatutory Stock Options (as defined in the Plan), with a term of ten years from the date of grant and an exercise price per share equal to 100% of the Fair Market Value (as defined in the Plan) of the underlying common stock of the Company (“Common Stock”) on the date of grant.
(i) Initial Grant for New Directors. Without any further action of the Board, each person who, after the Effective Date, is elected or appointed for the first time to be a Non-Employee Director will automatically, upon the date of his or her initial election or appointment to be a Non-Employee Director, be granted a Nonstatutory Stock Option to purchase shares of Common Stock (the “Initial Grant”), regardless of when such person is elected or appointed to the Board. Each Initial Grant will fully vest on the date of the annual meeting of the stockholders of the Company (“Annual Meeting”) next following the Initial Grant.
(ii) Annual Grant. Without any further action of the Board, at the close of business on the date of each Annual Meeting following the Effective Date, each person who is then a Non-Employee Director will automatically be granted a Nonstatutory Stock Option to purchase a number of shares of Common Stock having an Option Value (calculated on the date of grant) of $50,000 (the “Annual Grant”). Each Annual Grant will vest in a series of four (4) successive equal quarterly installments over the one-year period measured from the date of grant.
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
Except for employment arrangements which are described under “executive compensation,” since January 1, 2020 there has not been, nor is there currently proposed, any transaction in which we are or were a participant, the amount involved exceeds the lesser of $120,000 or 1% of the average of the total assets at December 31, 2021 and 2020, and any of our directors, executive officers, holders of more than 5% of our common stock or any immediate family member of any of the foregoing had or will have a direct or indirect material interest.
Review, Approval or Ratification of Transactions with Related Persons
Due to the small size of our Company, we do not at this time have a formal written policy regarding the review of related party transactions and rely on our full Board of Directors to review, approve or ratify such transactions and identify and prevent conflicts of interest. Our Board of Directors reviews any such transaction in light of the particular affiliation and interest of any involved director, officer or other employee or stockholder and, if applicable, any such person’s affiliates or immediate family members. Management aims to present transactions to our Board of Directors for approval before they are entered into or, if that is not possible, for ratification after the transaction has occurred. If our Board of Directors finds that a conflict of interest exists, then it will determine the appropriate action or remedial action, if any. Our Board of Directors approves or ratifies a transaction if it determines that the transaction is consistent with our best interests and the best interest of our stockholders.
Director Independence
As of the closing of this offering, our Board of Directors will consist of five members: Zafiris G. Zafirelis, Igor Serov, Anthony E. Pusateri, Richard Kilsby and Leslie Fang. Our Board of Directors undertook a review of the composition of our Board of Directors and the independence of each director. Based upon information requested from and provided by each director concerning their background, employment and affiliations, including family relationships, our Board of Directors has determined that Anthony E. Pusateri, Richard Kilsby and Leslie Fang qualify as “independent” as that term is defined by NASDAQ Listing Rule 5605(a) (2).
| 95 |
SECURITY OWNERSHIP OF BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding beneficial ownership of our common stock as of the date of this prospectus by (i) each person (or group of affiliated persons) who is known by us to own more than five percent (5%) of the outstanding shares of our common stock, (ii) each director and executive officer, and (iii) all of our directors, executive officers and director nominees as a group. As of the date of this prospectus, there were 18,476,291 shares of our common stock issued and outstanding. Except as otherwise indicated, the persons listed below have sole voting and investment power with respect to all shares of our common stock owned by them, except to the extent that power may be shared with a spouse.
Percentage ownership of our common stock before this offering is based on [ ] shares of common stock outstanding as of December 31, 2021 after giving effect to the Corporate Conversion and the Debt Conversion. Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock that such person currently owns or has the right to acquire within 60 days of the date of this prospectus. With respect to options and warrants, this would include options and warrants that are currently exercisable within 60 days. With respect to convertible securities, this would include securities that are currently convertible within 60 days.
Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such stockholders. Unless otherwise indicated, the address for each director and executive officer listed is: c/o Hemoglobin Oxygen Therapeutics Inc., 674 Souder Rd, Souderton, PA 18964.
| Name of Beneficial Owner or Identity of Group | Title of Class | Shares21 | Percentage | Post Offering Shares | Post Offering Percentage | |||||||||||||
| 5% or greater stockholders: | ||||||||||||||||||
| Peritimos Investments Limited 85 Spyrou Kyprianou 4004 Limassol, Cypress | Common Stock | 6,733,251 | 36.44 | % | ||||||||||||||
| Ivanka Kowalski 8115/128 Na Hrebenkach Prague, Czech Republic 15000 | Common Stock | 4,485,731 | 24.28 | % | ||||||||||||||
| Executive Officers and Directors: | ||||||||||||||||||
| Zafiris Zafirelis 71 Harris Avenue, Needham, MA 02192 | Common Stock | 4,258,209 | 23.05 | % | ||||||||||||||
| Igor Serov 63 Westerly Road, Weston, MA 02493 | Common Stock | 2,026,373 | 10.97 | % | ||||||||||||||
| Richard Kilsby Avenue Des Planches 21B Montreux, Switzerland | Common Stock | 66,600 | 0.36 | % | ||||||||||||||
Total Officers and Directors as a Group (3 persons) | Common Stock | 6,351,182 | 34. 37% | |||||||||||||||
21 Based on 18,476,291 issued and outstanding shares.
| 96 |
General
After giving effect to the Corporate Conversion and upon completion of this offering, our authorized capital stock will consist of 95,000,000 shares of common stock, par value $0.001 per share, and 5,000,000 shares of preferred stock, par value $0.001 per share.
After giving effect to the Corporate Conversion to be effected on the consummation of the offering, as of March 31, 2022, there were 26 holders of record of our common stock, ____ shares of common stock issued and outstanding and no shares of Preferred Stock issued and outstanding.
The following description of our capital stock and provisions of our Certificate of Incorporation and Bylaws to be effective upon the completion of this offering is only a summary. You should also refer to our Certificate of Incorporation, a copy of which is filed as an exhibit to the registration statement of which this prospectus is a part, and our Bylaws, a copy of which is filed as an exhibit to the registration statement of which this prospectus is a part.
Common Stock
We are authorized to issue up to a total of 95,000,000 shares of common stock, par value $0.001 per share. Holders of our common stock are entitled to one vote for each share held on all matters submitted to a vote of our stockholders. Holders of our common stock have no cumulative voting rights.
Further, holders of our common stock have no pre-emptive or conversion rights or other subscription rights. Upon our liquidation, dissolution or winding-up, holders of our common stock are entitled to share in all assets remaining after payment of all liabilities and the liquidation preferences of any of our outstanding shares of preferred stock. Subject to preferences that may be applicable to any outstanding shares of preferred stock, holders of our common stock are entitled to receive dividends, if any, as may be declared from time to time by our board of directors out of our assets which are legally available. Each outstanding share of our common stock is, and all shares of common stock to be issued in this offering when they are paid for will be, fully paid and non-assessable.
The holders of a majority of the shares of our capital stock, represented in person or by proxy, are necessary to constitute a quorum for the transaction of business at any meeting. If a quorum is present, an action by stockholders entitled to vote on a matter is approved if the number of votes cast in favor of the action exceeds the number of votes cast in opposition to the action, with the exception of the election of directors, which requires a plurality of the votes cast.
Preferred Stock
Our Board of Directors will have the authority, without further action by the stockholders, to issue up to 5,000,000 shares of preferred stock in one or more series and to fix the designations, powers, preferences, privileges, and relative participating, optional, or special rights as well as the qualifications, limitations, or restrictions of the preferred stock, including dividend rights, conversion rights, voting rights, terms of redemption, and liquidation preferences, any or all of which may be greater than the rights of the common stock. Our board of directors, without stockholder approval, will be able to issue convertible preferred stock with voting, conversion, or other rights that could adversely affect the voting power and other rights of the holders of common stock. Preferred stock could be issued quickly with terms calculated to delay or prevent a change of control or make removal of management more difficult. Additionally, the issuance of preferred stock may have the effect of decreasing the market price of our common stock and may adversely affect the voting and other rights of the holders of common stock. At present, we have no plans to issue any shares of preferred stock following this offering.
Options
We intend to sell or issue restricted shares of common stock or to grant incentive stock options or non-qualified stock options, stock appreciation rights, and restricted stock unit awards for the purchase of shares of common stock to employees, members of the Board of Directors and under an equity incentive plan that will be offered in 2022. As of December 31, 2021, there were no compensatory options outstanding to purchase shares of our common stock. All options to our officers, directors and other employees will be issued pursuant to the 2022 Equity Incentive Plan.
| 97 |
Anti-Takeover Provisions of Delaware Law, our Certificate of Incorporation and our Amended and Restated Bylaws
Delaware Law
We are governed by the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly traded Delaware corporation from engaging in a business combination with an interested stockholder for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A business combination includes mergers, asset sales or other transactions resulting in a financial benefit to the stockholder. An interested stockholder is a person who, together with affiliates and associates, owns (or within three years, did own) 15% or more of the corporation’s voting stock, subject to certain exceptions. The statute could have the effect of delaying, deferring or preventing a change in control of our Company.
Board of Directors Vacancies
Our Certificate of Incorporation and Bylaws authorize only our board of directors to fill vacant directorships. In addition, the number of directors constituting our board of directors may be set only by resolution of the majority of the incumbent directors.
Stockholder Action; Special Meeting of Stockholders
Our Certificate of Incorporation and Bylaws provide that our stockholders may take action by written consent. Our Certificate of Incorporation and Amended and Bylaws further provide that special meetings of our stockholders may be called by a majority of the board of directors, the Chief Executive Officer, or the Chairman of the board of directors.
Advance Notice Requirements for Stockholder Proposals and Director Nominations
Our Bylaws provide that stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election as directors at our annual meeting of stockholders, must provide timely notice of their intent in writing. To be timely, a stockholder’s notice must be delivered to the secretary at our principal executive offices not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding year’s annual meeting; provided, however, that in the event the date of the annual meeting is more than 30 days before or more than 60 days after such anniversary date, or if no annual meeting was held in the preceding year, notice by the stockholder to be timely must be so delivered not earlier than the close of business on the 120th day prior to such annual meeting and not later than the close of business on the later of the 90th day prior to such annual meeting or the 10th day following the day on which a public announcement of the date of such meeting is first made by us. These provisions may preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders.
Authorized but Unissued Shares
Our authorized but unissued shares of common stock and preferred stock are available for future issuance without stockholder approval and may be utilized for a variety of corporate purposes, including future public offerings to raise additional capital, corporate acquisitions and employee benefit plans. The existence of authorized but unissued and unreserved common stock and preferred stock could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise. If we issue such shares without stockholder approval and in violation of limitations imposed by The Nasdaq Capital Market or any stock exchange on which our stock may then be trading, our stock could be delisted.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is ____________.
Stock Market Listing
We have applied to have our shares of common stock listed for trading on The Nasdaq Capital Market under the symbol “HOTI”
| 98 |
DESCRIPTION OF SECURITIES WE ARE OFFERING
Common Stock
We are offering the Shares of our Common Stock at the public offering price of $___ per Share. The material terms and provisions of our common stock are described under the caption “Description of Our Capital Stock” in this prospectus.
Representative’s Warrants
Please see “Underwriting — Underwriters’ Warrants” for a description of the warrants we have agreed to issue to the representative of the underwriters in this offering, subject to the completion of the offering. We expect to enter into a warrant agreement in respect of the representative’s warrants prior to the closing of this offering.
MATERIAL
U.S. FEDERAL INCOME TAX CONSEQUENCES TO
NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a summary of the material U.S. federal income tax consequences to non-U.S. holders (as defined below) of the ownership and disposition of our common stock but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended (“Internal Revenue Code”) Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in U.S. federal income tax consequences different from those set forth below. No ruling on the U.S. federal, state, or local tax considerations relevant to our operations or to the purchase, ownership or disposition of our shares, has been requested from the IRS or other tax authority. No assurance can be given that the IRS would not assert, or that a court would not sustain, a position contrary to any of the tax consequences described below.
This summary also does not address the tax considerations arising under the laws of any non-U.S., state or local jurisdiction, or under U.S. federal gift and estate tax laws, except to the limited extent set forth below. In addition, this discussion does not address tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
| ● | banks, insurance companies or other financial institutions, regulated investment companies or real estate investment trusts; | |
| ● | persons subject to the alternative minimum tax or Medicare contribution tax on net investment income; | |
| ● | tax-exempt organizations or governmental organizations; | |
| ● | controlled foreign corporations, passive foreign investment companies and corporations that accumulate earnings to avoid U.S. federal income tax; | |
| ● | brokers or dealers in securities or currencies; | |
| ● | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; | |
| ● | persons that own, or are deemed to own, more than five percent of our capital stock (except to the extent specifically set forth below); | |
| ● | U.S. expatriates and certain former citizens or long-term residents of the U.S.; | |
| ● | partnerships or entities classified as partnerships for U.S. federal income tax purposes or other pass-through entities (and investors therein); |
| 99 |
| ● | persons who hold our common stock as a position in a hedging transaction, “straddle,” “conversion transaction” or other risk reduction transaction or integrated investment; | |
| ● | persons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; | |
| ● | persons who do not hold our common stock as a capital asset within the meaning of Section 1221 of the Internal Revenue Code; or | |
| ● | persons deemed to sell our common stock under the constructive sale provisions of the Internal Revenue Code. |
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our common stock arising under the U.S. federal estate or gift tax rules or under the laws of any state, local, non-U.S., or other taxing jurisdiction or under any applicable tax treaty.
Non-U.S. Holder Defined
For purposes of this discussion, you are a non-U.S. holder (other than a partnership) if you are any holder other than:
| ● | an individual citizen or resident of the U.S. (for U.S. federal income tax purposes); | |
| ● | a corporation or other entity taxable as a corporation created or organized in the U.S. or under the laws of the U.S., any state thereof, or the District of Columbia, or other entity treated as such for U.S. federal income tax purposes; | |
| ● | an estate whose income is subject to U.S. federal income tax regardless of its source; or | |
| ● | a trust (x) whose administration is subject to the primary supervision of a U.S. court and which has one or more “U.S. persons” (within the meaning of Section 7701(a)(30) of the Internal Revenue Code) who have the authority to control all substantial decisions of the trust or (y) which has made a valid election to be treated as a U.S. person. |
In addition, if a partnership or entity classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
Distributions
As described in “Dividend Policy,” we have never declared or paid cash dividends on our common stock and do not anticipate paying any dividends on our common stock in the foreseeable future. However, if we do make distributions on our common stock, those payments will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of stock as described below under “— Gain on Disposition of Common Stock.”
Subject to the discussion below on effectively connected income, backup withholding and foreign accounts, any dividend paid to you generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, you must provide us with an IRS Form W-8BEN, IRS Form W-8BEN-E or other appropriate version of IRS Form W-8 certifying qualification for the reduced rate. A non-U.S. holder of shares of our common stock eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, which then will be required to provide certification to us or our paying agent, either directly or through other intermediaries.
| 100 |
Dividends received by you that are effectively connected with your conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by you in the U.S.) are generally exempt from such withholding tax. In order to obtain this exemption, you must provide us with an IRS Form W-8ECI or other applicable IRS Form W-8 properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. In addition, if you are a corporate non-U.S. holder, dividends you receive that are effectively connected with your conduct of a U.S. trade or business may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty. You should consult your tax advisor regarding any applicable tax treaties that may provide for different rules.
Gain on Disposition of our Shares
Subject to the discussion below regarding backup withholding and foreign accounts, you generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
| ● | the gain is effectively connected with your conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment maintained by you in the U.S.); | |
| ● | you are a non-resident alien individual who is present in the U.S. for a period or periods aggregating 183 days or more during the taxable year in which the sale or disposition occurs and certain other conditions are met; or | |
| ● | our common stock constitutes a U.S. real property interest by reason of our status as a “U.S. real property holding corporation,” (“USRPHC”), for U.S. federal income tax purposes at any time within the shorter of (i) the five-year period preceding your disposition of our common stock, or (ii) your holding period for our common stock. |
We believe that we are not currently and will not become a USRPHC for U.S. federal income tax purposes, and the remainder of this discussion so assumes. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as U.S. real property interests only if you actually or constructively hold more than five percent of such regularly traded common stock at any time during the shorter of the five-year period preceding your disposition of, or your holding period for, our common stock.
If you are a non-U.S. holder described in the first bullet above, you will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and a corporate non-U.S. holder described in the first bullet above also may be subject to the branch profits tax at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty. If you are an individual non-U.S. holder described in the second bullet above, you will be required to pay a flat 30% tax (or such lower rate specified by an applicable income tax treaty) on the gain derived from the sale, which gain may be offset by U.S. source capital losses for the year (provided you have timely filed U.S. federal income tax returns with respect to such losses). You should consult any applicable income tax or other treaties that may provide for different rules.
Federal Estate Tax
Our common stock beneficially owned by an individual who is not a citizen or resident of the U.S. (as defined for U.S. federal estate tax purposes) at the time of their death will generally be includable in the decedent’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax treaty provides otherwise. The test for whether an individual is a resident of the U.S. for U.S. federal estate tax purposes differs from the test used for U.S. federal income tax purposes. Some individuals, therefore, may be non-U.S. holders for U.S. federal income tax purposes, but not for U.S. federal estate tax purposes, and vice versa.
| 101 |
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
Payments of dividends or of proceeds on the disposition of stock made to you may be subject to information reporting and backup withholding at a current rate of 28% unless you establish an exemption, for example, by properly certifying your non-U.S. status on an IRS Form W-8BEN, IRS Form W-8BEN-E or another appropriate version of IRS Form W-8.
Backup withholding is not an additional tax; rather, the U.S. federal income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
Foreign Account Tax Compliance
The Foreign Account Tax Compliance Act (“FATCA”), imposes withholding tax at a rate of 30% on dividends on and gross proceeds from the sale or other disposition of our common stock paid to “foreign financial institutions” (as specially defined under these rules), unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding the U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners) or otherwise establishes an exemption. FATCA also generally imposes a U.S. federal withholding tax of 30% on dividends on and gross proceeds from the sale or other disposition of our common stock paid to a “non-financial foreign entity” (as specially defined for purposes of these rules) unless such entity provides the withholding agent with a certification identifying certain substantial direct and indirect U.S. owners of the entity, certifies that there are none or otherwise establishes an exemption. The withholding provisions under FATCA generally apply to dividends on our common stock, and under current transition rules, are expected to apply with respect to the gross proceeds from the sale or other disposition of our common stock on or after January 1, 2019. An intergovernmental agreement between the U.S. and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their tax advisors regarding the possible implications of this legislation on their investment in our common stock.
Each prospective investor should consult its tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, including the consequences of any proposed change in applicable laws.
| 102 |
WestPark Capital, Inc. is acting as the representative of the underwriters of this offering. Under the terms of an underwriting agreement, which is filed as an exhibit to the registration statement, each of the underwriters named below has severally agreed to purchase from us the respective number of shares of common stock shown opposite its name below:
| Underwriters | Number
of Shares |
|||
| WestPark Capital, Inc. | ||||
The underwriting agreement provides that the underwriters’ obligation to purchase Shares depends on the satisfaction of the conditions contained in the underwriting agreement including:
| ● | the representations and warranties made by us to the underwriters are true; | |
| ● | there is no material change in our business or the financial markets; and | |
| ● | we deliver customary closing documents to the underwriters. |
Commissions and Expenses
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Share | Total with no Over-Allotment |
Total with Over-Allotment |
||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discount (7.0%)(1) | $ | $ | $ | |||||||||
| Non-accountable expense allowance (1.0%) | $ | $ | $ | |||||||||
| Proceeds, before expenses, to us | $ | $ | $ | |||||||||
| (1) | 3% in the case of investors referred by the Company. |
As of December 21, 2021, we have paid an advance of $22,500 to the representative and anticipate an additional $22,500 to be advanced prior to the placement, which will be applied against actual out-of-pocket accountable expenses and reimbursed to us to the extent any portion thereof is not actually incurred in compliance with Federal Industry Regulatory Authority (“FINRA”) Rule 5110(f)(2)(C).
The underwriter proposes to offer the Shares directly to the public at the public offering price on the cover of this prospectus and to selected dealers, which may include the underwriter, at such offering price less a selling concession not in excess of $0.___ per share.
The expenses of this offering that are payable by us are estimated to be approximately $____ (which excludes estimated underwriting discounts and commissions and the non-accountable expense allowance payable to the underwriters). We will be responsible for all of the underwriters expenses related to this offering, including filing fees and communication expenses for the registration of the shares, all filing fees associated with the review of this offering by FINRA, fees and expenses relating to the listing of the shares of common stock and Warrants on The Nasdaq Capital Market, fees relating to background checks (up to a maximum of $5,000), fees relating to the registration, qualification or exemptions of the shares under securities laws of foreign jurisdictions, cost of making and printing the underwriting documents, cost and expenses of a public relations firm, cost of preparing, printing and delivering stock certificates, fees and expenses of the transfer agent, and fees and expenses of our legal counsel, road show expenses for this offering, and fees and expenses of the underwriters legal counsel. The maximum amount of fees, costs and expenses incurred by the underwriters that we shall be responsible for may not exceed $125,000.
| 103 |
Option to Purchase Additional Securities
We have granted the underwriters an option exercisable for 45 days after the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of (i) ___ shares of common stock sold in this offering) from us in any combination thereof to cover over allotments, if any, at the public offering price, less underwriting discounts and commissions and the non-accountable expense allowance payable to the underwriters. To the extent that this option is exercised, each underwriter will be obligated, subject to certain conditions, to purchase its pro rata portion of these additional shares or Warrants based on the underwriter’s percentage underwriting commitment in this offering as indicated in the table at the beginning of this Underwriting Section.
Lock-Up Agreements
All of our directors, executive officers and shareholders have agreed that, for a period of 180 days after the date of this prospectus and subject to certain limited exceptions, we and they will not, directly or indirectly, without the prior written consent of WestPark Capital, Inc. (i) offer for sale, sell, pledge, or otherwise dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the disposition by any person at any time in the future of) any shares of common stock (including, without limitation, shares of common stock that may be deemed to be beneficially owned by us or them in accordance with the rules and regulations of the SEC and shares of common stock that may be issued upon exercise of any options or warrants) or securities convertible into or exercisable or exchangeable for common stock, (ii) enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of common stock, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of common stock or other securities, in cash or otherwise, (iii) make any demand for or exercise any right or file or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any of our other securities, or (iv) publicly disclose the intention to do any of the foregoing.
WestPark Capital, Inc., in its sole discretion, may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time. When determining whether or not to release common stock and other securities from lock-up agreements, WestPark Capital, Inc. will consider, among other factors, the holder’s reasons for requesting the release, the number of shares of common stock and other securities for which the release is being requested and market conditions at the time.
Underwriter Warrants
We have also agreed to issue to the underwriters or their designees at the closing of this offering, warrants (the “Underwriters’ Warrants”) to purchase an aggregate of ___ shares of common stock (10% of the number of shares sold in the offering, excluding the over-allotment option). The Underwriters’ Warrants will be exercisable at any time and from time to time, in whole or in part, during a period commencing six months from the effective date of this offering and expiring five years from the effective date of the offering. The Underwriters’ Warrants will be exercisable at a price equal to 120% of the public offering price per share of common stock and such warrants shall be exercisable on a cash basis, provided that if a registration statement registering the common stock underlying the Underwriters’ Warrants is not effective, the Underwriters’ Warrants may be exercised on a cashless basis. The Underwriters’ Warrants have been deemed compensation by FINRA and are, therefore, subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The underwriters or their permitted assignees under this Rule 5110(g)(1) shall not sell, transfer, assign, pledge or hypothecate the Underwriters’ Warrants, nor engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the Underwriters’ Warrants, for a period of 180 days from the effective date of the offering, except that they may be assigned, in whole or in part, as specifically set forth in the underwriting agreement. The Underwriters’ Warrants will provide for customary anti-dilution provisions (for stock dividends, splits and recapitalizations and the like) consistent with FINRA Rule 5110, and the number of shares underlying the Underwriters’ Warrants shall be reduced, or the exercise price increased, if necessary, to comply with FINRA rules or regulations. Further, the Underwriters’ Warrants will provide for a one-time demand registration right and unlimited piggyback rights. The Underwriters’ Warrants and underlying shares are included in this prospectus.
Offering Price Determination
The actual offering price of the Shares we are offering will be negotiated between us and the underwriters.
| 104 |
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
Stabilization, Short Positions and Penalty Bids
The underwriters may engage in stabilizing transactions, short sales and purchases to cover positions created by short sales, and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of the common stock, in accordance with Regulation M under the Exchange Act:
| ● | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. | |
| ● | A short position involves a sale by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase in the offering, which creates the syndicate short position. This short position may be either a covered short position or a naked short position. In a covered short position, the number of shares involved in the sales made by the underwriters in excess of the number of shares they are obligated to purchase is not greater than the number of shares that they may purchase by exercising their option to purchase additional shares. In a naked short position, the number of shares involved is greater than the number of shares in their option to purchase additional shares. The underwriters may close out any short position by either exercising their option to purchase additional shares and/or purchasing shares in the open market. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through their option to purchase additional shares. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. | |
| ● | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. | |
| ● | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result, the price of the common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on The Nasdaq Capital Market or otherwise and, if commenced, may be discontinued at any time.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the common stock. In addition, neither we nor any of the underwriters make any representation that the underwriters will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Electronic Distribution
A prospectus in electronic format may be made available on the Internet sites or through other online services maintained by one or more of the underwriters and/or selling group members participating in this offering, or by their affiliates. In those cases, prospective investors may view offering terms online and, depending upon the particular underwriter or selling group member, prospective investors may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations.
Other than the prospectus in electronic format, the information on any underwriter’s or selling group member’s web site and any information contained in any other web site maintained by an underwriter or selling group member is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter or selling group member in its capacity as underwriter or selling group member and should not be relied upon by investors.
| 105 |
Listing on The Nasdaq Capital Market
We have applied to have our common stock listed on The Nasdaq Capital Market under the symbol “HOTI” subject to notice of issuance.
Discretionary Sales
The underwriters have informed us that they do not expect to sell more than 5% of the common stock in the aggregate to accounts over which they exercise discretionary authority.
Other Relationships
Certain of the underwriters and their affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they may in the future receive customary fees.
Selling Restrictions
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Notice to Prospective Investors in the European Economic Area and the United Kingdom
In relation to each Member State of the European Economic Area and the United Kingdom (each a “Relevant State”), no shares have been offered or will be offered pursuant to the offering to the public in that Relevant State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that offers of shares may be made to the public in that Relevant State at any time under the following exemptions under the Prospectus Regulation:
| (a) | to any legal entity which is a qualified investor as defined under the Prospectus Regulation; |
| (b) | to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation), subject to obtaining the prior consent of the underwriters; or |
| (c) | in any other circumstances falling within Article 1(4) of the Prospectus Regulation, |
provided that no such offer of shares shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation and each person who initially acquires any shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and us that it is a “qualified investor” within the meaning of Article 2(e) of the Prospectus Regulation. In the case of any shares being offered to a financial intermediary as that term is used in the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any shares to the public other than their offer or resale in a Relevant State to qualified investors as so defined or in circumstances in which the prior consent of the underwriters have been obtained to each such proposed offer or resale.
| 106 |
For the purposes of this provision, the expression an “offer to the public” in relation to shares in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
Notice to Prospective Investors in the United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Regulation) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the shares in the United Kingdom within the meaning of the Financial Services and Markets Act 2000.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (the “SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document does not constitute a prospectus within the meaning of and has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares, or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA (the “FINMA”), and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (the “CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in France
This prospectus (including any amendment, supplement or replacement thereto) is not being distributed in the context of a public offering in France within the meaning of Article L. 411-1 of the French Monetary and Financial Code (Code monétaire et financier). This prospectus has not been and will not be submitted to the French Autorité des marchés financiers (the “AMF”) for approval in France and accordingly may not and will not be distributed to the public in France.
Pursuant to Article 211-3 of the AMF General Regulation, French residents are hereby informed that:
| 1. | the transaction does not require a prospectus to be submitted for approval to the AMF; | |
| 2. | persons or entities referred to in Point 2°, Section II of Article L. 411-2 of the Monetary and Financial Code may take part in the transaction solely for their own account, as provided in Articles D. 411-1, D. 734-1, D. 744-1, D. 754-1 and D. 764-1 of the Monetary and Financial Code; and | |
| 3. | the financial instruments thus acquired cannot be distributed directly or indirectly to the public otherwise than in accordance with Articles L. 411-1, L. 411-2, L. 412-1 and L. 621-8 to L. 621-8-3 of the Monetary and Financial Code. |
| 107 |
This prospectus is not to be further distributed or reproduced (in whole or in part) in France by the recipients of this prospectus. This prospectus has been distributed on the understanding that such recipients will only participate in the issue or sale of our common stock for their own account and undertake not to transfer, directly or indirectly, our common stock to the public in France, other than in compliance with all applicable laws and regulations and in particular with Articles L. 411-1 and L. 411-2 of the French Monetary and Financial Code.
Notice to Prospective Investors in Germany
Our common stock may be offered and sold in the Federal Republic of Germany only in compliance with the Prospectus Regulation, the Commission Delegated Regulations (EU) 2019/979 and (EU) 2019/980, each as of March 14, 2019 and the German Securities Prospectus Act (Wertpapierprospektgesetz), as amended, or any other laws applicable in Germany governing the issue, offering and sale of securities. This prospectus has not been approved under the Prospectus Regulation and, accordingly, our common stock may not be offered publicly in the Federal Republic of Germany. Our common stock will only be offered in the Federal Republic of Germany in reliance on an exemption from the requirement to publish an approved securities prospectus under the Prospectus Regulation. Any resale of our common stock in Germany may only be made in accordance with the Prospectus Regulation and other applicable laws.
Notice to Prospective Investors in Hong Kong
The shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (the “SFO”) of Hong Kong and any rules made thereunder; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong) (the “CO”) or which do not constitute an offer to the public within the meaning of the CO. No advertisement, invitation or document relating to the shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the SFO and any rules made thereunder.
Notice to Prospective Investors in China
This prospectus will not be circulated or distributed in the PRC and the shares will not be offered or sold and will not be offered or sold to any person for re-offering or resale directly or indirectly to any residents of the PRC except pursuant to any applicable laws and regulations of the PRC. Neither this prospectus nor any advertisement or other offering material may be distributed or published in the PRC, except under circumstances that will result in compliance with applicable laws and regulations.
The validity of the issuance of the common stock offered by us in this offering will be passed upon for us TroyGould PC, Los Angeles, California. Certain legal matters in connection with this offering will be passed upon for the underwriters by Manatt, Phelps & Phillips, LLP, Costa Mesa, California.
The consolidated financial statements as of December 31, 2021 and 2020 and for the years then ended included in this Prospectus and in the Registration Statement have been so included in reliance on the report of BDO USA, LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting, appearing elsewhere herein. The report on the consolidated financial statements contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Securities and Exchange Commission a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus. This prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. For further information pertaining to us and our common stock, reference is made to the registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
The registration statement is available at the Securities and Exchange Commission’s website at www.sec.gov. The registration statement, including all exhibits and amendments to the registration statement, has been filed electronically with the Securities and Exchange Commission. we will become subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934, as amended, and, accordingly, will be required to file annual reports containing financial statements audited by an independent public accounting firm, quarterly reports containing unaudited financial data, current reports, proxy statements and other information with the Securities and Exchange Commission. You will be able to inspect and copy such periodic reports, proxy statements and other information at the website of the Securities and Exchange Commission referred to above
| 108 |
Hemoglobin Oxygen Therapeutics LLC and Subsidiaries
Table of Contents
| F-1 |
Report of Independent Registered Public Accounting Firm
To the Members and Board of Managers
Hemoglobin Oxygen Therapeutics LLC
Souderton, Pennsylvania
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Hemoglobin Oxygen Therapeutics LLC and its subsidiaries (the “Company”) as of December 31, 2021 and 2020, the related consolidated statements of operations, changes in members’ deficit, and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020 and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations since its inception and has a working capital deficiency and members’ deficit as of December 31, 2021. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BDO USA, LLP
We have served as the Company’s auditor since 2019.
Philadelphia, Pennsylvania
April 25, 2022
| F-2 |
Consolidated Financial Statements
Hemoglobin Oxygen Therapeutics LLC
Consolidated Balance Sheets
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash | $ | 443,895 | $ | 357,169 | ||||
| Deferred loan commitment fee, current | — | 134,160 | ||||||
| Prepaid expenses and other current assets | 141,401 | 9,591 | ||||||
| Total Current Assets | 585,296 | 500,920 | ||||||
| Property and equipment | 4,477,719 | 4,477,719 | ||||||
| Deferred loan commitment fee | — | 503,099 | ||||||
| Operating right-of-use asset | 932,103 | 1,529,999 | ||||||
| Deposits | 39,870 | 46,023 | ||||||
| Total Assets | $ | 6,034,988 | $ | 7,057,760 | ||||
| Liabilities and Members’ Deficit | ||||||||
| Current Liabilities | ||||||||
| Convertible notes payable, current | $ | 3,880,000 | $ | 1,000,000 | ||||
| Convertible notes payable, current – related party | 390,000 | 200,000 | ||||||
| Paycheck protection program loan payable, current | — | 137,467 | ||||||
| Warrant liability | 888,674 | 241,556 | ||||||
| Accounts payable | 1,729,575 | 1,644,571 | ||||||
| Related party payable to landlord | 3,152,111 | 2,305,808 | ||||||
| Operating lease liability, current | 695,633 | 637,697 | ||||||
| Accrued interest, current – related parties | 202,622 | 103,288 | ||||||
| Deferred revenue | 378,637 | — | ||||||
| Accrued expenses and other current liabilities | 2,770,051 | 1,564,675 | ||||||
| Total Current Liabilities | 14,087,303 | 7,835,062 | ||||||
| Convertible notes payable, net | 395,071 | 2,998,209 | ||||||
| Convertible notes payable, net, related party | 100,000 | 290,000 | ||||||
| Notes payable – related party | 300,485 | — | ||||||
| Paycheck protection program loan payable | — | 68,733 | ||||||
| Accrued interest – related party | 32,174 | 89,273 | ||||||
| Accrued interest | 74,845 | 263,129 | ||||||
| Operating lease liability | 294,258 | 989,891 | ||||||
| Deferred revenue | — | 378,637 | ||||||
| Total Liabilities | 15,284,136 | 12,912,934 | ||||||
| Commitments and Contingencies (See Note 10) | ||||||||
| Members’ Deficit | ||||||||
| Members’ deficit | (9,249,148 | ) | (5,855,174 | ) | ||||
| Total Members’ Deficit | (9,249,148 | ) | (5,855,174 | ) | ||||
| Total Liabilities and Members’ Deficit | $ | 6,034,988 | $ | 7,057,760 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
Hemoglobin Oxygen Therapeutics LLC
Consolidated Statements of Operations
For the Years Ended December 31, | ||||||||
| 2021 | 2020 | |||||||
| Revenue | $ | — | $ | 35,470 | ||||
| Operating expenses | ||||||||
| Research and development | 768,621 | 571,738 | ||||||
| General and administrative | 2,668,311 | 2,673,445 | ||||||
| Total Operating Expenses | 3,436,932 | 3,245,183 | ||||||
| Loss from Operations | (3,436,932 | ) | (3,209,713 | ) | ||||
| Other Income and (Expense) | ||||||||
| Interest expense | (681,518 | ) | (379,862 | ) | ||||
| Gain on paycheck protection program loan forgiveness | 206,200 | — | ||||||
| Loss on extinguishment of debt | (2,235,770 | ) | — | |||||
| Gain on foreign currency transaction | — | 1,699 | ||||||
| Gain on change in fair value of warrant liability | 21,782 | 1,862 | ||||||
| Total Other Expense | (2,689,306 | ) | (376,301 | ) | ||||
| Net Loss | $ | (6,126,238 | ) | $ | (3,586,014 | ) | ||
| Net Loss per Unit | ||||||||
| Basic and diluted | $ | (33.16 | ) | $ | (19.65 | ) | ||
| Weighted-average Units Outstanding | ||||||||
| Basic and diluted | 184,763 | 182,451 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
Hemoglobin Oxygen Therapeutics LLC
Consolidated Statements of Changes in Members’ Deficit
| Members’ Deficit | ||||||||
| Units | Amount | |||||||
| Balance, December 31, 2019 | 182,213 | (4,511,663 | ) | |||||
| Beneficial conversion feature on convertible notes payable | — | 274,806 | ||||||
| Warrant issued in conjunction with convertible notes payable | — | 933,703 | ||||||
| Issuance of Member Units from notes payable conversion | 2,550 | 1,033,994 | ||||||
| Net loss | — | (3,586,014 | ) | |||||
| Balance, December 31, 2020 | 184,763 | $ | (5,855,174 | ) | ||||
| Beneficial conversion feature on convertible notes payable | — | 496,494 | ||||||
| Debt premium associated with fair value of amended convertible note issuance | — | 2,235,770 | ||||||
| Net loss | — | (6,126,238 | ) | |||||
| Balance, December 31, 2021 | 184,763 | $ | (9,249,148 | ) | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
Hemoglobin Oxygen Therapeutics LLC
Consolidated Statements of Cash Flows
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2021 | 2020 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (6,126,238 | ) | $ | (3,586,014 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Non-cash interest expense | 379,514 | 118,860 | ||||||
| Gain on change in fair value of warrant liability | (21,782 | ) | (1,862 | ) | ||||
| Gain on paycheck protection program loan forgiveness | (206,200 | ) | — | |||||
| Loss on extinguishment of debt | 2,235,770 | — | ||||||
| Operating right-of-use asset amortization | 597,896 | 546,834 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | (131,810 | ) | (9,591 | ) | ||||
| Long-term deposits | 6,153 | — | ||||||
| Accounts Payable | 85,004 | 219,087 | ||||||
| Payable to landlord | 846,303 | 896,143 | ||||||
| Accrued expenses, other current liabilities and accrued interest | 1,059,328 | 1,092,586 | ||||||
| Operating lease liability | (637,697 | ) | (585,932 | ) | ||||
| Deferred revenue | — | 378,637 | ||||||
| Net cash used in operating activities | (1,913,759 | ) | (931,252 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of property and equipment | — | (50,040 | ) | |||||
| Net cash used in investing activities | — | (50,040 | ) | |||||
| Cash flows from financing activities: | ||||||||
| Proceeds from issuance of convertible notes payable | 1,700,000 | 1,056,438 | ||||||
| Proceeds from issuance of notes payable | 300,485 | — | ||||||
| Proceeds from Paycheck Protection Program loan | — | 206,200 | ||||||
| Net cash provided by financing activities | 2,000,485 | 1,262,638 | ||||||
| Net increase in cash | 86,726 | 281,346 | ||||||
| Cash at beginning of year | 357,169 | 75,823 | ||||||
| Cash at end of year | $ | 443,895 | $ | 357,169 | ||||
| Schedule of non-cash financing activities | ||||||||
| Debt premium associated with amendments to convertible notes payable | $ | 2,235,770 | $ | — | ||||
| Issuance of Member Units from notes payable conversion | $ | — | $ | 991,103 | ||||
| Accrued interest reclassified to equity from notes payable conversion | $ | — | $ | 42,891 | ||||
| Warrant issued as loan commitment fee associated with convertible notes payable | $ | — | $ | 933,703 | ||||
| Warrant issued in conjunction with convertible notes payable | $ | 668,900 | $ | 243,418 | ||||
| Beneficial conversion feature on convertible notes payable | $ | 496,494 | $ | 274,806 | ||||
| Deferred loan commitment fee reclassified to debt discount | $ | 534,607 | $ | 254,272 | ||||
| Right-of-use asset and lease liability associated with operating lease | $ | — | $ | 147,904 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
| 1. | Significant Accounting Policies |
Description of Business
Hemoglobin Oxygen Therapeutics LLC (“Hemoglobin” or, the “Company”) was incorporated in Delaware, USA on February 18, 2014, as a private limited liability company. The Company and its subsidiaries are developers of hemoglobin-based solutions that are administered intravenously to increase the oxygen carrying capacity of blood when blood is not available. The Company has two products, Hemopure, for human use, and Oxyglobin, for veterinary use. Although the Company’s product Hemopure is not currently approved by the U.S. Food and Drug Administration (“FDA”), it is allowed under the FDA’s Expanded Access Program (“EAP”), also known as compassionate use. It is currently approved in South Africa for human use. The Company’s Oxyglobin product is currently approved by both the FDA and the European Commission for veterinary use. The Company also has wholly-owned subsidiaries in the Netherlands and South Africa.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. The Company has experienced net losses and negative cash flows from operating activities since its inception and expects to continue to incur net losses into the foreseeable future. The Company had a members’ deficit of $9,249,148 at December 31, 2021, incurred a net loss of $6,126,238 for the year ended December 31, 2021 and used $1,913,759 of net cash for operating activities during the year ended December 31, 2021. To date, these operating losses have been funded primarily from outside sources of invested capital through the issuance of convertible promissory notes, and sale of its member units. At December 31, 2021, the Company had cash on hand of approximately $444,000. Management expects operating losses and negative cash flows to continue for the foreseeable future as the Company continues to incur costs related to research and development and commercialization efforts. Management has prepared cash flow forecasts which indicate that based on the Company’s expected operating losses and negative cash flows, there is substantial doubt about the Company’s ability to continue as a going concern within twelve months after the date these consolidated financial statements are available to be issued.
The Company’s ability to continue as a going concern is dependent upon its ability to raise additional funding. Management plans to raise additional capital to fulfill its operating and capital requirements for the next twelve months. In 2021, the Company raised additional funds of approximately $300,000 through the issuance of notes payable (See Note 7) and drew down an aggregate of approximately $1,700,000 from its 2020 convertible notes facility (See Note 6). In 2022, the Company plans to fund its operating losses and capital funding needs primarily through an initial public offering (“IPO”), and/or through additional debt financings and equity offerings. In 2022 to date, the Company has raised an additional $100,000 in notes payable and $200,000 in convertible notes. These financing and capital raising options may not be able to be successfully achieved and may not be available on a timely basis or on terms acceptable to the Company. If the Company is not able to secure adequate additional funding in a timely manner or on favorable terms, the Company may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, or suspend or curtail planned operating activities. Any of these actions could have a material adverse effect on the Company’s business, results of operations and future prospects.
The accompanying consolidated financial statements do not reflect any adjustments that might result if the Company is unable to continue as a going concern. Since inception, the Company has been engaged in organizational activities, including raising capital and research and development activities. The Company has not generated any substantial revenues and has not yet achieved profitable operations, nor has it ever generated positive cash flows from operations. There is no assurance that profitable operations, if achieved, could be sustained on a continuing basis. Further, the Company’s future operations are dependent on the success of the Company’s efforts to raise additional capital and the market acceptance of the Company’s products. There can be no assurance that these efforts will be successful.
| F-7 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
COVID-19 Business Impact
On March 27, 2020, the World Health Organization declared the global outbreak of COVID-19 to be a pandemic. The COVID-19 global pandemic has been unprecedented and unpredictable and is likely to continue to result in significant national and global economic disruption, which may adversely affect the Company’s business. The COVID-19 pandemic has delayed a trial for Hemopure the Company had planned in South Africa in 2020, which is now currently planned to take place in 2022. Certain labs/plants had to close their doors in response to COVID-19 regulations in 2020, which resulted in overall lower salary costs and some delays in research and development. Further, as a result of COVID-19 shutdowns during 2020, the Company’s spending on outside services, projects or operations was reduced. Based on the Company’s current assessment, it does not expect any material impact on its long-term strategic plans, its operations, or its liquidity due to the worldwide spread of the COVID-19 virus. However, the Company is actively monitoring this situation and the possible effects on its financial condition, liquidity, operations, suppliers, and industry.
Basis of Presentation
The accounting and reporting policies of the Company are in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The Company’s reporting currency is the U.S. Dollar.
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Hemoglobin Oxygen Therapeutics LLC (the “Parent”), and its wholly-owned subsidiaries, OPK Biotech Netherlands BV (“OPK-BV”) and OPK Biotech South Africa (Proprietary) Limited (“OPK-SA”) (collectively, the “Subsidiaries”). Each of the Subsidiaries was set up as a holding vehicle for local registrations: the Dutch company for Oxyglobin European Union (“EU”) approval and the South Africa company for Hemopure South African approval, in accordance with their local regulatory requirements. This is currently the only function of those companies. All business negotiations and agreements are conducted and executed by the U.S. parent company. Neither subsidiary has premises, employees, or bank accounts. All subsidiaries’ expenses are paid directly by the U.S. parent company. The Parent and the Subsidiaries are collectively referred to as the “Company.” All significant inter-company balances and transactions have been eliminated in consolidation.
Foreign Currency Transactions
The financial statements of OPK-BV and OPK-SA are translated into U.S. dollars based on the determination that the local currency is the functional currency. All assets and liabilities of OPK-BV’s and OPK-SA’s foreign operations are translated into U.S. dollars at year-end exchange rates. Equity accounts are translated at historical exchange rates. Income statement accounts are translated at the weighted average exchange rate prevailing during the year. Translation adjustments resulting from differences in exchange rates, when material, are reflected as a component of other comprehensive income (loss). Foreign currency gains and losses resulting from transactions denominated in foreign currencies, including intercompany transactions, are reflected in other (expense) income in the accompanying consolidated statements of operations. Since OPK-BV and OPK-SA do not hold material assets and liabilities and have limited transaction activity, such foreign currency activity is not presented separately in the accompanying consolidated statements of operations, as the amounts are not material.
| F-8 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make certain estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, management evaluates its estimates, including but not limited to those related to: (1) the valuation of the Company’s Member Units, (2) the recognition and disclosure of contingent liabilities, (3) the useful lives and recoverability of long-lived assets, (4) assumptions used to determine the fair value of financial instruments (warrants) and (5) accrued expenses. These estimates are based on historical data and experience, as well as various relevant assumptions that management believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Such estimates often require the selection of appropriate valuation methodologies and models, and significant judgment in evaluating ranges of assumptions and financial inputs. Although the Company believes the estimates and assumptions used in determining the recorded amounts in the accompanying consolidated financial statements are reasonable, actual results may differ from those estimates under different assumptions or circumstances.
Emerging Growth Company
As an emerging growth company (“EGC”), the Jumpstart Our Business Startups Act (“JOBS Act”) allows the Company to delay adoption of new or revised accounting pronouncements applicable to public companies until such pronouncements are applicable to private companies. The Company has elected to use the extended transition period under the JOBS Act until such time the company is not considered to be an EGC. The adoption dates are discussed below in Note 1 to reflect this election.
Concentration of Credit Risk
Financial instruments which potentially subject the Company to concentration of credit risk consist primarily of cash deposits. The Company maintains deposits in high-quality federally insured financial institutions in excess of federally insured limits. Management believes that the Company is not exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held. The Company has not experienced any losses on deposits since inception.
Fair Value of Financial Instruments
The accounting guidance defines fair value, establishes a consistent framework for measuring fair value, and expands disclosure for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis. Fair value is defined as an exit price representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, the accounting guidance establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
Level 1: Observable inputs, such as quoted prices in active markets
Level 2: Inputs, other than the quoted prices in active markets that are observable either directly or indirectly
Level 3: Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions which reflect those that a market participant would use
| F-9 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
Financial assets and liabilities are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement requires judgment and may affect the valuation of fair value assets and liabilities and their placement within the fair value hierarchy levels.
In determining the fair value of its financial instruments, the Company considers the source of observable market data inputs, liquidity of the instrument, the credit risk of the counterparty to the contract, and its risk of nonperformance. In the case where fair value is not observable for items subject to fair value measurements, the Company applies valuation techniques deemed the most appropriate under the U.S. GAAP guidance based on the nature of the assets and liabilities being measured.
The carrying amounts of accounts payable and accrued liabilities represent reasonable estimates of their fair value because of the short maturity of these items. The carrying amounts of the Company’s notes payable for the year ended December 31, 2021 approximate fair value. The fair value of the Company’s convertible notes of $11,231,156 and $8,756,421 as of December 31, 2021 and 2020, respectively, was determined based upon Level 3 fair value measurements. To derive the measurements, the total business enterprise value was first determined using an income-based discounted cash flow (“DCF”) valuation method. Inputs to the DCF valuation model included management’s long-range cash flow forecast in conjunction with a discount rate derived based upon a capital asset pricing model (“CAPM”) formula. A hybrid method using a combination of the probability weighted expected return model (“PWERM”) and the option pricing model (“OPM”) was then utilized to allocate the total enterprise value to derive the fair value of the Company’s convertible notes.
Revenue Recognition
Revenue is recognized when control of the goods and services provided is transferred to the Company’s customers and in an amount that reflects the consideration the Company expects to be entitled to in exchange for those good and services using the following steps: 1) identification of the contract, or contracts with a customer, 2) identification of performance obligations in the contract, 3) determination of the transaction price, 4) allocation of the transaction price to the performance obligations in the contract and 5) recognition of revenue when or as the Company satisfies the performance obligation(s).
In order to identify the performance obligations in a contract with a customer, the Company must assess the promised goods or services in the contract and identify each promised good or service that is distinct. A performance obligation meets the Financial Accounting Standards Board’s (“FASB”) Accounting Standards Codification (“ASC”) Topic 606 – Revenue from Contracts with Customers definition of a distinct good or service if both of the following criteria are met: The customer can benefit from the good or service either on its own or together with other resources that are readily available to it (i.e., the good or service is capable of being distinct), and the Company’s promise to transfer the good or service to the customer is separately identifiable from other promises in the contract (i.e., the promise to transfer the good or service is distinct within the context of the contract). If a good or service is not distinct, the good or service is combined with other promised goods or services until a bundle of goods or services is identified that is distinct.
| F-10 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer. The consideration promised in a contract with a customer may include fixed amounts, variable amounts, or both.
Variable consideration is included in the transaction price only to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. The transaction price is allocated to each performance obligation on a relative standalone selling price basis. The transaction price allocated to each performance obligation is recognized when that performance obligation is satisfied, at a point in time or over time as appropriate. The Company’s current customer contracts do not contain terms such as product discounts that result in variable consideration.
The Company’s contracts with customers are comprised primarily of contractual agreement paperwork (i.e., purchase orders) and verbal orders. The Company accounts for a contract when it has approval and commitment from both parties, the rights of the parties and payment terms are identified, the contract has commercial substance and collectability of consideration is probable. These contracts with customers relate to the selling of hemoglobin-based oxygen carriers (“HBOCs”), which represent single performance obligations that are satisfied when control of the product passes to the customer. The Company considers the timing of right to payment, transfer of risk and rewards, transfer of title, transfer of physical possession and customer acceptance when determining when control transfers to the customer. As a result, revenue is recognized at a point in time upon product shipment. Shipping and handling activities are accounted for as activities to fulfill a promise to transfer a product to a customer and, as such, applicable costs incurred are recorded when the related revenue is recognized. Payment for products is due within a limited time period after shipment or delivery, typically within 30 calendar days of the respective invoice date. The Company does not generally offer extended payment terms.
Revenue is measured as the amount of consideration the Company expects to receive in exchange for transferring goods. Amounts billed and due from the Company’s customers are classified as accounts receivable, less allowance for doubtful accounts on the consolidated balance sheet. The Company had no revenue in 2021. The Company’s 2020 revenues consisted solely of sales of Hemopure in South Africa.
The Company records a contract asset when it has a right to payment from a customer that is conditioned on events that have occurred other than the passage of time. The Company did not have any contract assets as of December 31, 2021 and 2020. The Company records a contract liability when customers prepay but the Company has not yet satisfied its performance obligation. The Company had contract liabilities of $378,637 as of December 31, 2021 and 2020, that was recorded as deferred revenue on the consolidated balance sheets. The contract liability relates to the Hemopure trial planned in South Africa that was delayed due to COVID-19. All performance obligations associated with the deferred revenue balance are expected to be met in 2022.
| F-11 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
Offering Costs
Offering costs of $121,591 associated with the pending public offering, consisting principally of professional, printing, filing, regulatory and other costs, have been deferred and are reported in prepaid expenses and other current assets on the consolidated balance sheet as of December 31, 2021. These offering costs will be recorded as a reduction in proceeds raised from the public offering, as a charge to Members’ Deficit, at the time of closing.
Property and Equipment, Net
Property and equipment, which consist of various equipment and leasehold improvements, are stated at cost less accumulated depreciation. All property and equipment held by the Company as of December 31, 2021 and 2020 have yet to be put into service. Depreciation, once the property and equipment are put into service, will be calculated using the straight-line method over the estimated useful lives of the assets, as follows.
| Asset Category | Useful Life | |
| Manufacturing and Utilities Equipment | 15 Years | |
| Lab Equipment | 10 Years |
Impairment of Long-lived Assets
It is required that long-lived assets, including property, plant and equipment with finite lives, be reviewed for possible impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of the asset to future undiscounted net cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. The Company did not record any impairment charges for the years ended December 31, 2021 and 2020.
Research and Development Costs
The Company records research and development activities conducted by third-party service providers, including preclinical studies and clinical trials, and contract manufacturing activities, to research and development expense as incurred. The Company is required to estimate the amount of services provided but not yet invoiced and include these expenses in accrued expenses in the consolidated balance sheets and within research and development expenses in the consolidated statements of operations. These expenses are a significant component of the Company’s research and development expenses and require significant estimates and judgments. The Company accrues for these expenses based on factors such as estimates of the work completed and in accordance with agreements established with its third-party service providers. As actual expenses become known, the Company adjusts its accrued expenses. The Company has not experienced any material differences between accrued research and development expenses and actual research and development expenses incurred. For the years ended December 31, 2021 and 2020, the Company’s research and development costs consist mainly of the salaries of employees.
Beneficial Conversion Feature of Convertible Debt
The Company accounts for convertible debt in accordance with the guidelines established by ASC 470-20, Debt with Conversion and Other Options. The beneficial conversion feature (“BCF”) of convertible debt is normally characterized as the convertible portion or feature of certain debt that provide a rate of conversion that is below market value or in-the-money when issued. The Company records a discount to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying member unit at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are accreted to non-cash interest expense using the effective interest rate method over the term of the related debt to their date of maturity. If a security or instrument becomes convertible only upon the occurrence of a future event outside the control of the Company, or, is convertible from inception, but contains conversion terms that change upon the occurrence of a future event, then any contingent BCF is measured and recognized when the triggering event occurs, and contingency has been resolved.
| F-12 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
Warrants
The Company accounts for warrants issued in conjunction with financial instruments as either equity-classified or liability-classified instruments based on an assessment of the warrants’ specific terms and applicable authoritative guidance in FASB ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”) and Topic 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability pursuant to ASC 480, and whether the warrants meet all the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own ordinary shares and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgement, is conducted at the time of warrant issuance and as of each subsequent quarterly reporting end date while the warrants are outstanding.
For issued or modified warrants that do not meet all of the criteria for equity classification, the warrants are required to be recorded as liabilities at their initial fair value on the date of issuance, and each balance sheet date thereafter. Changes in the estimated fair value of the warrants are recognized as non-cash gains or losses on the consolidated statements of operations.
Commitment Fee
The Company accounts for fees paid to secure a firm commitment from a lender for a specified maximum amount over a specified period of time in accordance with FASB Topic ASC 470, Debt (“ASC 470”). If the Company does not immediately draw down all the capital available to them, the deferred initial up-front commitment fees paid represent the benefit of being able to access capital over the contractual term, and therefore, meet the definition of an asset and are recorded as a deferred commitment fee which is amortized on a straight-line basis to non-cash interest expense over the entire term of the arrangement. When the Company draws down on the capital available to them, a portion of the unamortized costs related to the respective draw down is then presented as a direct deduction from the carrying value of the debt when drawn and accreted to non-cash interest expense over the term of the loan using the effective interest rate method.
Loss Contingencies
The Company recognizes a liability with regard to loss contingencies when it believes it is probable a liability has occurred, and the amount can be reasonably estimated. If some amount within a range of losses appears at the time to be a better estimate than any other amount within the range, the Company accrues that amount. When no amount within the range is a better estimate than any other amount, the Company accrues the minimum amount in the range. The Company has not recorded any such contingent liabilities as of December 31, 2021 and 2020.
| F-13 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
Income Taxes
No provision for federal income taxes is presented in these consolidated financial statements as the Company is a limited liability company that has elected to be taxed as a partnership and, accordingly, the Company’s taxable income is allocated to its members for income tax reporting purposes. However, in certain circumstances, the Company may be required to pay income taxes to states. For the years ended December 31, 2021 and 2020, the Company was subject to entity level taxes in certain states; however, the Company recorded no related liability, given its overall operating loss position. The net difference between the tax basis and the reported amounts of the Company’s assets and liabilities was approximately $4,065,000 and $2,486,000 for the years ended December 31, 2021 and 2020 respectively, which primarily relate to fixed assets and accrued expenses.
When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the positions taken or the amount of the positions that would be ultimately sustained. The benefit of a tax position is recognized in the consolidated financial statements in the period during which, based on all available evidence, it is more likely than not that the position would be sustained upon examination, including the resolution of appeals or litigation process, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more likely than not recognition threshold are measured as the largest amount of tax benefit that is more than 50% likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying consolidated balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination. Interest and penalties associated with unrecognized tax benefits are classified as additional income taxes in the consolidated statements of operations.
The Company has not recognized any liabilities for uncertain tax positions or unrecognized benefits as of December 31, 2021 and 2020.
The Company is open to examination for tax years ended December 31, 2021, 2020 and 2019. There are currently no open tax examinations by income taxing authorities.
Segments
The Company operates as a single operating segment and a single reportable segment. The Company’s chief operating decision maker, its Chief Executive Officer, reviews financial information on a consolidated basis for the purposes of allocating resources and evaluating financial performance.
Earnings (Loss) per Unit
Under the LLC structure, earnings (loss) of the Company are to be distributed to unitholders based on their proportionate share of the underlying equity, and, as a result, earnings (loss) per unit (“EPU”) has been presented in the accompanying consolidated statements of operations. Basic EPU is computed by dividing net income (loss) by the weighted average number of member units outstanding for the years presented. Diluted EPU is computed by dividing net income (loss) by the weighted average of all potentially dilutive member units that were outstanding for the years presented. For the purposes of determining common unit equivalents for the diluted EPU calculation, the Company uses the if-converted method for calculating the common stock equivalent units associated with its outstanding convertible notes payable. The treasury stock method is used to determine common unit equivalents for outstanding warrants.
| F-14 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
Basic and Diluted EPU are the same for both years ended December 31, 2021 and 2020 as all common unit equivalents as associated with convertible notes payable and warrants were determined to be antidilutive. As of December 31, 2021 and 2020, notes payable convertible into 22,448 and 11,698 member units, respectively, were not included in computing diluted EPU because their effects were antidilutive. Warrants convertible into 9,220 and 6,122 member units as of December 31, 2021 and 2020, respectively, were not included in computing diluted EPU because their effects were anti-dilutive.
Member Unit Valuation
Due to the absence of an active market for the Company’s member units, the Company utilized methodologies in accordance ASC Topic 820: Fair Value Measurements and Disclosures, and the AICPA Accounting and Valuation Guide for the Valuation of Privately-Held-Company Equity Securities Issued as Compensation, to estimate the fair value of its member units. The fair value of our member units is determined by the board. Given the absence of a public trading market, the board considered numerous objective and subjective factors to determine the fair value of our member units. These factors included, but are not limited to: (i) contemporaneous third-party valuations of member units; (ii) the rights, preferences and privileges of convertible debt relative to member units; (iii) the lack of marketability of member units; (iv) stage and development of the Company’s business; (v) general economic conditions; and (vi) the likelihood of achieving a liquidity event, such as an initial public offering (“IPO”) or sale of the Company, given prevailing market conditions.
Recently Adopted and Issued Accounting Pronouncements
Adopted
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurement (Topic 820) - Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”), which is designed to improve the effectiveness of disclosures by removing, modifying, and adding disclosures related to fair value measurements. This amendment is effective for fiscal years beginning after December 15, 2019. The Company adopted the provisions of this update for its December 31, 2020 consolidated financial statements. Adopting this update did not have a material effect on the Company’s consolidated financial statements.
Issued and Not Yet Adopted
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (“ASC 326”). ASC 326 provides more decision-useful information about the expected credit losses on financial instruments and other commitments to extend credit held by a reporting entity at each reporting date. ASC 326 was originally effective for annual reporting periods beginning after December 15, 2019. Following the release of ASU 2019-10 in November 2019, ASC 326 is effective for private companies for annual reporting periods beginning after December 15, 2022. The provisions of this guidance are to be applied using a modified-retrospective approach. The Company is currently evaluating the impact of adoption of this guidance on its consolidated financial statements.
| F-15 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
In August 2020, the FASB issued ASU No. 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current U.S. GAAP and also simplifies the diluted earnings per share (“EPS”) calculation in certain areas. Under the new guidance there will be no separate accounting for embedded conversion features. It removes certain settlement conditions that are required for equity contracts to qualify for the derivative scope exception. ASU 2020-06 is effective for fiscal years beginning after December 15, 2023. Early adoption is permitted. The Company is currently evaluating the impact that the adoption of this guidance may have on the Company’s consolidated financial statements.
| 2. | Property and Equipment |
Property and equipment consisted of the following at December 31:
| 2021 | 2020 | |||||||
| Manufacturing and Utilities Equipment | $ | 2,724,958 | $ | 2,724,958 | ||||
| Lab Equipment | 25,500 | 25,500 | ||||||
| Construction-in-Process | 1,727,261 | 1,727,261 | ||||||
| Property and equipment | $ | 4,477,719 | $ | 4,477,719 | ||||
The property and equipment has not yet been placed in service. The fair value of the property and equipment is in excess of its carrying value, and therefore no impairment was recognized in the fiscal years ending December 31, 2021 and 2020.
| 3. | Leases |
The Company accounts for its lease arrangements in accordance with Topic 842 (“ASC 842”). Under the ASC 842, the Company determines if an arrangement is or contains a lease at inception. The Company has lease agreements for its corporate offices and warehouses which were determined to be operating leases.
Operating lease right-of-use (“ROU”) assets and operating lease liabilities are recognized based on the present value of future minimum payments over the lease term at commencement date. The Company has elected the practical expedient to not separate lease and non-lease components. As the Company’s leases do not provide an implicit rate, the Company used the incremental borrowing rate based on the information available at commencement date in determining the present value of future payments. The operating lease ROU asset also includes any lease payments made and excludes lease incentives and initial direct costs incurred. Lease expense for minimum lease payments is recognized on a straight-line basis over the lease term.
Leases with an initial term of 12 months or less are not recorded on the balance sheet as the Company has elected to apply the short-term lease exemption. The Company recognizes lease expense for these leases on a straight-line basis over the lease term.
| F-16 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
The Company has a lease agreement for its manufacturing facility together with all personal property, fixtures and equipment in the facility with a related party (see Note 11), which was previously amended to add additional space. The amended lease agreement commenced in September 2017 and terms in May 2023 with the option to renew the lease for four extended terms, the first being for an additional six years, and the remaining for five years each. The amended lease agreement entailed six months of free rent, which is being recognized on a straight-line basis over the term of the amended lease agreement. After the free rent period, the monthly lease payment amount was $58,319. Simultaneously, the Company entered into a Security Agreement offering as collateral, all personal property, equipment, machinery, and fixtures, and any other systems within the leased premises. The Company did not include the renewal options in the lease term as the renewal periods are dependent upon the Company completing the build out of the facility, which is dependent upon raising additional funding. The Company accounted for the lease as an operating lease, resulting in recording a lease liability of $2,550,821 and ROU asset of $2,374,199. A rate commensurate with assets of a similar term of 8.85%, as estimated by management, was used to discount the future payments on the lease to their present value.
The Company has a lease agreement for office space with a lease term originally ending in February 2020. The lease payments begin at $3,963 per month with an increase on an annual basis of five percent. The lease provided the Company with the option to renew for a three-year period, however the renewal period was not included in the original lease term as it was not reasonably certain that the option to renew the lease would be exercised. The Company accounted for the lease as an operating lease and recorded a lease liability and ROU asset of $58,545. A rate commensurate with asset of a similar term of 5%, as estimated by management, was used to discount the future payments on the lease to their present value.
On February 27, 2020, the Company entered into a lease extension for the Waltham office space, extending the term for a period of three years commencing on March 1, 2020 and ending on February 28, 2023. The lease payments begin at $4,341 per month with an increase on an annual basis of two percent. The lease contains an option to renew for a three-year period that the Company is not reasonably certain to exercise given their financial condition. The Company has accounted for the lease as an operating lease, resulting in a lease liability and ROU asset of $147,904 as of the lease commencement date. A rate commensurate with assets of a similar term of 5%, as estimated by management, was used to discount the future payments on the lease to their present value.
Lease asset and lease liabilities are as follows:
| December 31, | December 30, | |||||||||
| Lease Classification | Classification | 2021 | 2020 | |||||||
| Assets | ||||||||||
| Long-term | ||||||||||
| Operating | Long-term assets | $ | 932,103 | $ | 1,529,999 | |||||
| Total right-of-use assets | $ | 932,103 | $ | 1,529,999 | ||||||
| Liabilities | ||||||||||
| Current | ||||||||||
| Operating | Current liabilities | $ | 695,633 | $ | 637,697 | |||||
| Noncurrent | ||||||||||
| Operating | Long-term lease liability | 294,258 | 989,891 | |||||||
| Total lease liabilities | $ | 989,891 | $ | 1,627,588 | ||||||
| F-17 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
The components of lease expense were as follows:
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Operating lease expense | $ | 713,067 | $ | 712,823 | ||||
| Short-term lease expense | 105,600 | 105,600 | ||||||
| Total lease expense | $ | 818,667 | $ | 818,423 | ||||
Other information related to leases was as follows:
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Weighted average remaining lease term (in years): | ||||||||
| Operating leases | 1.4 | 2.4 | ||||||
| Weighted average discount rate: | ||||||||
| Operating leases | 8.6 | % | 8.6 | % | ||||
| December 31, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Cash paid for amounts included in measurement of lease liabilities: | ||||||||
| Operating cash flows from operating leases | $ | 73,037 | $ | 52,097 | ||||
| ROU assets obtained in exchange for lease obligations: | ||||||||
| Operating leases | $ | — | $ | 147,904 | ||||
Future minimum lease payments under non-cancellable leases as of December 31, 2021 were as follows:
| Year | ||||
| 2022 | $ | 754,002 | ||
| 2023 | 300,655 | |||
| Total future minimum lease payments | 1,054,657 | |||
| Less: imputed interest | (64,766 | ) | ||
| Total | 989,891 | |||
| Less: lease liability, current | (695,633 | ) | ||
| Total long-term lease liability | $ | 294,258 | ||
The Company is currently not making rental payments on its manufacturing facility. Amounts for unpaid rent due are included within Payable to Landlord on the consolidated balance sheets (See Note 11). The Company notes that the landlord chose not to exercise his option to deliver the Company a notice of default and chose to exercise his option to cure the default on behalf of the company. Under the lease agreement, if the landlord cures a default on behalf of the Company, the Company shall reimburse the landlord upon demand for all costs incurred in curing the default. In 2022, the Company came to an agreement with the landlord to have amounts owed to him converted into equity upon a public offering (See Note 12). Upon an event of default, the landlord has the right to terminate the lease. Additionally, all personal property, equipment, machinery and fixtures placed upon or installed within the facility the Company is renting including any specific systems used in the Company’s trade or business shall become the sole and exclusive property of the Landlord.
| F-18 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
| 4. | Accrued Expenses and Other Current Liabilities |
Accrued expenses and other current liabilities consisted of the following at December 31:
| 2021 | 2020 | |||||||
| Accrued payroll | $ | 1,335,345 | $ | 963,291 | ||||
| Accrued interest | 566,749 | 118,699 | ||||||
| Accrued professional fees | 598,416 | 255,139 | ||||||
| Other accrued expenses | 269,541 | 227,546 | ||||||
| Total accrued expenses and other current liabilities | $ | 2,770,051 | $ | 1,564,675 | ||||
In January 2020, the Company reached an agreement with all employees to defer certain amounts of their salaries at different rates. The Company plans to repay all accrued amounts upon completion of an IPO.
| 5. | Paycheck Protection Program Loan |
On March 27, 2020, the Coronavirus Aid, Relief and Economic Security (“CARES”) Act was enacted and signed into law to provide certain aid and stimulus to the U.S. economy.
On May 5, 2020, the Company obtained a loan (“PPP Loan”) in the amount of $206,200. The PPP Loan bore fixed interest at 1.00% per annum, which began accruing from the date of the loan, and was scheduled to mature on May 5, 2022. The PPP Loan was unsecured and guaranteed by the Small Business Association (“SBA”). The PPP loan was eligible to be forgiven provided the Company satisfied certain conditions and upon approval by the lender and the SBA. The PPP Loan provided for the deferral of payments until the SBA had determined the forgiveness amount, at which time, any remaining PPP Loan amount required equal monthly payments of principal plus accrued interest in an amount sufficient to repay the remaining PPP Loan balance by the maturity date. As of December 31, 2020, the outstanding balance of the PPP Loan amounted to $206,200, which was classified as a liability and was included in current and long-term PPP loan payable in the accompanying Consolidated Balance Sheet. In October 2020, the Company submitted the PPP loan forgiveness application to the lender. On January 26, 2021, the PPP loan was fully forgiven by SBA and lender. As a result, the Company recognized a gain on paycheck protection program loan forgiveness of $206,200 within other income (expense) in the consolidated statement of operations for the year ended December 31, 2021.
| 6. | Convertible Notes Payable |
During 2015 and 2016, the Company entered into convertible note agreements (“2015-2016 Convertible Notes”) with third parties for aggregate borrowings of $1,150,000 with various maturity dates, of which $550,000 remain outstanding as of December 31, 2021 and 2020. The 2015-2016 Convertible Notes accrue interest at various rates ranging from 2% to 10% per annum, with principal and interest due upon maturity. The 2015-2016 Convertible Notes are convertible into Member Units at a price of $362.21 per unit as specified in the table below.
| F-19 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
During 2018, the Company entered into convertible note agreements (“2018 Convertible Notes”) with third parties for aggregate borrowings of $1,884,665 with various maturity dates. The 2018 Convertible Notes accrue interest at various rates ranging from 2% to 12% per annum, with principal and interest due upon maturity. The 2018 Convertible Notes are convertible into Member Units at a price of either $362.21 or $548.81 per unit as specified in the table below.
During 2019, the Company entered into convertible note agreements (“2019 Convertible Notes”) with third parties for aggregate borrowings of $2,730,000 with various maturity dates. The 2019 Convertible Notes accrue interest at 5% per annum with principal and interest due upon maturity. The 2019 Convertible Notes are convertible into Member Units at a price of $548.81 as specified in the table below.
During 2020, the Company entered into convertible note agreements (“2020 Convertible Notes”) with third parties for aggregate borrowings up to $2,756,438 with various maturity dates, which included a $2,400,000 convertible note facility (the “$2,400,000 Convertible Note Facility”) issued on September 21, 2020 that allows the Company to draw down amounts in tranches as needed through September 2022. The Company drew down $700,000 of the $2,400,000 Convertible Note in 2020. The 2020 Convertible Notes accrue interest at 5% per annum with principal and interest due upon maturity. The 2020 Convertible Notes are convertible into Member Units at a price of either $548.81 or $274.40 as specified in the table below. A total of $196,438 of 2020 Convertible Note borrowings were converted in November 2020.
During 2021, the Company drew down the remaining $1,700,000 available on the $2,400,000 Convertible Note Facility. No additional convertible notes were issued in 2021.
| F-20 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
Below is a summary of convertible notes payable issuances (collectively, the “Convertible Notes”), by issuance date, for Convertible Notes outstanding as of December 31, 2021 and 2020:
| December 31, | ||||||||||||||||||||
| 2021 | 2020 | |||||||||||||||||||
| Issuance Date | Interest Rate | Conversion Price | Principal Amount | Principal Amount | Maturity Date | |||||||||||||||
| Nov 4, 2015 | 10 | % | $ | 362.21 | $ | 200,000 | $ | 200,000 | On Demand | |||||||||||
| Dec 15, 2015 | 10 | % | 362.21 | 50,000 | 50,000 | Dec 15, 2022 | ||||||||||||||
| Jan 4, 2016 | 5 | % | 362.21 | 100,000 | 100,000 | Jan 4, 2022 | ||||||||||||||
| Jan 20, 2016 | 5 | % | 362.21 | 50,000 | 50,000 | Jan 20, 2023 | ||||||||||||||
| Feb 5, 2016 | 5 | % | 362.21 | 100,000 | 100,000 | Aug 5, 2022 | ||||||||||||||
| Mar 8, 2016 | 5 | % | 362.21 | 50,000 | 50,000 | Mar 8, 2023 | ||||||||||||||
| Jul 20, 2018 | 5 | % | 362.21 | 40,000 | 40,000 | Jul 20, 2022 | ||||||||||||||
| Aug 6, 2018 | 5 | % | 362.21 | 50,000 | 50,000 | Aug 6, 2022 | ||||||||||||||
| Aug 16, 2018 | 5 | % | 548.81 | 500,000 | 500,000 | Aug 16, 2022 | ||||||||||||||
| Aug 21, 2018 | 5 | % | 548.81 | 500,000 | 500,000 | Aug 21, 2022 | ||||||||||||||
| Jan 15, 2019 | 5 | % | $ | 274.40 | $ | 2,000,000 | $ | 2,000,000 | Jan 15, 2022 | |||||||||||
| May 8, 2019 | 5 | % | 548.81 | 230,000 | 230,000 | May 8, 2022 | ||||||||||||||
| Oct 8, 2019 | 5 | % | 274.40 | 100,000 | 100,000 | Oct 8, 2022 | ||||||||||||||
| Nov 5, 2019 | 5 | % | 274.40 | 400,000 | 400,000 | Nov 5, 2022 | ||||||||||||||
| Feb 4, 2020 | 5 | % | $ | 274.40 | $ | 40,000 | $ | 40,000 | Feb 4, 2023 | |||||||||||
| Mar 9, 2020 | 5 | % | 274.40 | 20,000 | 20,000 | Mar 9, 2023 | ||||||||||||||
| Apr 6, 2020 | 5 | % | 274.40 | 20,000 | 20,000 | Apr 6, 2023 | ||||||||||||||
| Apr 30, 2020 | 5 | % | 274.40 | 20,000 | 20,000 | Apr 30, 2023 | ||||||||||||||
| Aug 5, 2020 | 5 | % | 274.40 | 30,000 | 30,000 | Aug 5, 2023 | ||||||||||||||
| Sep 1, 2020 | 5 | % | 274.40 | 30,000 | 30,000 | Sep 1, 2023 | ||||||||||||||
| Sep 21, 2020* | 5 | % | 274.40 | 700,000 | 700,000 | Sep 21, 2025 | ||||||||||||||
| Sep 21, 2020** | 5 | % | $ | 274.40 | $ | 1,700,000 | $ | — | Sep 21, 2025 | |||||||||||
| Total convertible notes | $ | 6,930,000 | $ | 5,230,000 | ||||||||||||||||
| Less: unamortized note discount | (2,164,929 | ) | (741,791 | ) | ||||||||||||||||
| Total | 4,765,071 | 4,488,209 | ||||||||||||||||||
| Less: current portion | (4,270,000 | ) | (1,200,000 | ) | ||||||||||||||||
| Total long-term convertible notes | $ | 495,071 | $ | 3,288,209 | ||||||||||||||||
*This represents the 2020 draw downs on the $2,400,000 Convertible Note facility. The Company drew down $100,000, $100,000, $100,000 and $400,000 on this facility on September 21, 2020, October 22, 2020, November 20, 2020 and December 14, 2020, respectively.
**This represents the 2021 draw downs on the $2,400,000 Convertible Note facility. The Company drew down $100,000, $100,000, $100,000, $100,000, $100,000, $100,000, $100,000, $400,000, $300,000, and $300,000 on this facility on April 12, 2021, May 19, 2021, June 17, 2021, July 19, 2021, August 19, 2021, September 20, 2021, October 18, 2021, October 20, 2021, November 15,2021, and December 14, 2021, respectively.
The 2015-2016, 2018, 2019, and 2020 Convertible Notes remain in force until an investment document is signed by both the lender and the Company, as borrower, converting the Convertible Notes Payable into Member Units, or until the Convertible Notes Payable are repaid. If the holder chooses to convert, they forgo the related accrued interest; the conversion is for the principal only. The Notes can be converted until the earlier of i) the Company raising the next round of financing and starting the construction of its production facility or ii) the maturity or repayment of the underlying loan. If the Convertible Note is not converted, the principal plus interest are due on the maturity date.
| F-21 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
The Company may prepay amounts outstanding under the Convertible Notes Payable in whole or in part on any date, with 15 or 30-days’ notice in writing, as stated in the applicable agreement. Prepayments must include accrued interest.
The Convertible Notes Payable do not contain any provisions regarding collateral or debt covenants. In November 2020, a total of $991,103 in principal of Convertible Notes Payable, including $196,438 of 2020 Convertible Note borrowings, were converted into 2,550 Member Units resulting in an increase in Members’ capital of $991,103, a reclass of $42,891 of forfeited accrued interest to equity and recognition of $16,823 of non-cash interest expense related to unamortized debt discount on the related notes.
On October 20, 2020, the Company entered into an amendment to nine convertible notes with a certain third-party lender. The amended convertible notes consisted of the October 8, 2019 note in the amount of $100,000, the November 5, 2019 note in the amount of $400,000, the February 4, 2020 note in the amount of $40,000, the March 9, 2020 note in the amount of $20,000, the April 6, 2020 note in the amount of $20,000, the April 30, 2020 note in the amount of $20,000, the August 5, 2020 note in the amount of $30,000, the September 1, 2020 note in the amount of $30,000, and September 21,2020 note in the amount of $2,400,000. Under the amendment, the provision in all of the notes providing the lender the right to request an early repayment of the note in twelve months from the date of the note if the Company does not start construction of its production facility by that date and requiring the Company to make payment within thirty days of the request was considered null and void. No other terms or conditions of these notes were amended.
On January 5, 2021 the Company entered into amendments to three convertible notes with the same lender. The amended convertible notes consisted of the January 15, 2019 note in the amount of $2,000,000, the October 8, 2019 note in the amount of $100,000 and the November 5, 2019 note in the amount of $400,000. Under the amendments, the conversion price of these notes was decreased from $548.81 per unit to $274.40 per unit. No other terms or conditions of these notes were amended. The Company accounted for the amendments as debt extinguishments under ASC 470-50 resulting in a loss on extinguishment of debt of $2,235,770, which included the fair value of the embedded conversion option. As the new debt was issued at a substantial premium, the fair value of the full premium of $2,235,770 was recorded within Members’ deficit.
Contractual interest expense on the Convertible Notes Payable for the years ended December 31, 2021 and 2020 was $302,003 and $261,002 respectively. Non-cash interest expense related to the amortization of debt discount for the years ended December 31, 2021 and 2020 was $276,862 and $76,689, respectively. The remaining unamortized debt discount as of December 31, 2021 of $2,164,929 will be amortized 3.7 years. Accrued interest expense on the Convertible Notes as of December 31, 2021 and 2020 was $876,391 and $574,388, respectively. The effective interest rate on the Convertible Notes for the years ended December 31, 2021 and 2020, was 10.10% and 6.96%, respectively.
| F-22 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
As of December 31, 2020, future principal payments through maturity are as follows:
| Years ending December 31, | ||||
| 2022 | $ | 4,270,000 | ||
| 2023 | 260,000 | |||
| 2024 | — | |||
| 2025 | 2,400,000 | |||
| Total | $ | 6,930,000 | ||
Warrant issuances in connection with $2,400,000 Convertible Note Facility
In connection with the $2,400,000 Convertible Note Facility, the Company issued two options (the “Warrants”) to the same third party to purchase additional Member Units. Both options can be exercised until the earlier of i) 365 days from the closing date of the next round of the Company’s financing, provided that the amount raised is at least $20 million or ii) the maturity or repayment of the underlying Loan.
The first option (the “1st Warrant”) is to purchase additional Member Units of the Company at a price of $274.40 per unit in the aggregate amount of $1,330,000 for a total of 4,847 additional units. As the option is available to the lender upon the signing of the agreement and the $2,400,000 Convertible Note Facility allows for delayed draw down, the Company accounted for the option as a deferred loan commitment fee recorded at fair value in the amount of $933,703 with the offset to members’ deficit as it was determined to be equity classified. The deferred loan commitment fee is being amortized over the $2,400,000 Convertible Note Facility access period. For the years ended December 31, 2021 and 2020, $102,652 and $42,171, respectively, of non-cash interest expense was recognized related to the amortization of the deferred loan commitment fee. At each draw down date, a proportionate amount of the unamortized deferred loan commitment fee is being reclassified to debt discount on Convertible Note Payable and accreted to non-cash interest expense over the term of the $2,400,000 Convertible Note Facility draw down period, or will be fully accreted upon conversion of the corresponding $2,400,000 Convertible Note Facility into Member Units. For the years ended December 31, 2021 and 2020, $534,607 and $254,272, respectively, of the deferred loan commitment fee was reclassified to debt discount on Convertible Note Payable.
The fair value of the 1st Warrant, representing a commitment fee at the date of issuance was determined via the Black Scholes option pricing model, which assumes the volatility rate, risk-free rate, expected dividend yield, and expected term.
The assumptions used to measure the fair value of the 1st Warrant as of its September 21, 2020 issuance date was as follows:
| September 21, 2020 | ||||
| Volatility rate | 55.0 | % | ||
| Risk-free rate | 0.27 | % | ||
| Expected dividend yield | 0.0 | % | ||
| Expected term | 5.0 years | |||
The second option (the “2nd Warrant”) is to purchase additional Member Units of the Company at a price of $274.40 per unit in the amount equal to 50% of the outstanding loan amount under the $2,400,000 Convertible Note Facility at the time of option exercise. As the option is for a variable number of shares for a fixed price, the Company has accounted for it as a warrant liability under ASC Topic 480 Distinguishing Liabilities from Equity that is to be remeasured at fair value on a recurring basis. At each draw down date, the Company has recorded a warrant liability at fair value with a corresponding offset to Convertible Note Payable debt discount. The Convertible Note Payable debt discount is being accreted to non-cash interest expense over the term of the Convertible Note Facility or will be accreted in full upon conversion of the corresponding Convertible Note Facility to Member Units. The Convertible Note Payable debt discount recorded for the 2nd Warrant the years ended December 31, 2021 and 2020 was $668,900 and $243,418, respectively. As of December 31, 2021 and 2020, the fair value of the warrant liability was determined to be $888,674 and $241,556, respectively, with $21,782 and $1,862, respectively, recorded as a gain on change in fair value of warrant liability in the consolidated statement of operations. See Note 8 for further disclosure concerning the Company’s determination of the fair value of the 2nd Warrant.
| F-23 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
The following is a summary of the warrant activity for the years ended December 31, 2021 and 2020:
Number of Warrant Units |
Weighted-Average Exercise Price | |||||||
| Outstanding, December 31, 2019 | - | $ | - | |||||
| Granted | 6,122 | 274.40 | ||||||
| Outstanding, December 31, 2020 | 6,122 | 274.40 | ||||||
| Granted | 3,098 | 274.40 | ||||||
| Outstanding, December 31, 2021 | 9,220 | $ | 274.40 | |||||
Number of Warrant Units Outstanding |
Exercise Price |
Expiration Date | ||||||||
| 1st Warrant | 4,847 | $ | 274.40 | September 21, 2025 | ||||||
| 2nd Warrant | 4,373 | $ | 274.40 | September 21, 2025 | ||||||
| Total Warrants | 9,220 | |||||||||
Beneficial Conversion Features
For the years ended December 31, 2021 and 2020, the Company recorded a Convertible Note Payable debt discount of $496,494 and $202,309, respectively, to account for the $2,400,000 Convertible Notes Facility’s BCF. The Convertible Note Payable debt discount resulting from the BCF is being accreted to interest expense over the term of the $2,400,000 Convertible Note Facility or will be accreted in full upon conversion of the corresponding Convertible Notes Payable into Member Units. In connection with the draw downs of the $2,400,000 Convertible Note Facility, the BCF recognized was limited by the amount allocated to the convertible instrument. The excess of the fair value of the instrument that the holder would receive at conversion over the proceeds received is equal to $1,227,492.
The Company recorded a Convertible Note Payable debt discount of $72,497 to account for the BCFs on other convertible notes issued in the year ended December 31, 2020. The Convertible Note Payable debt discount is being accreted to non-cash interest expense over the term of each associated Convertible Note or will be accreted in full upon conversion of the corresponding Convertible Note Payable into Member Units.
| F-24 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
| 7. | Notes Payable |
During 2021, the Company issued promissory notes (“2021 Notes Payable”) to third parties for aggregate borrowings of $337,566, with various maturity dates, which included a $205,000 note payable (the “$205,000 Note Payable Facility”) issued on August 10, 2021, that allows the Company to draw down amounts in tranches as needed. The Company drew down $167,919 of the $205,000 Note Payable Facility as of December 31, 2021. All 2021 Notes Payable accrue interest at 2% per annum. The principal plus accrued interest are due on the maturity date.
As of December 31, 2021, future minimum principal payments required under the 2021 Notes Payable are as follows:
| Years ending December 31, | ||||
| 2022 | $ | — | ||
| 2023 | — | |||
| 2024 | 300,485 | |||
| Total | $ | 300,485 | ||
| 8. | Fair Value Hierarchy |
As of December 31, 2021, warrant liability measured at fair value on a recurring basis is as follows:
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Warrant liability | $ | — | $ | — | $ | 888,674 | $ | 888,674 | ||||||||
As of December 31, 2020, warrant liability measured at fair value on a recurring basis is as follows:
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Warrant liability | $ | — | $ | — | $ | 241,556 | $ | 241,556 | ||||||||
There were no assets or liabilities measured at fair value on a nonrecurring basis at December 31, 2021 and 2020.
Fair value of the warrant liability is determined on a recurring basis based on appraisals by an independent valuation firm.
The following table presents information as of December 31, 2021 and 2020 about significant unobservable inputs (Level 3) used in the valuation of liabilities measured at fair value on a recurring basis:
| Financial Instrument | December 31, 2021 Fair Value | December 31, 2020 Fair Value | Valuation Technique | Significant Unobservable Inputs | ||||||||
| Warrant Liability | $ | 888,674 | $ | 241,556 | Black Scholes | Expected volatility, fair value of Member Units | ||||||
The fair value of the warrant liability (see Note 6) at the date of issuance was determined via the Black Scholes option pricing model, which assumes the volatility rate, risk-free rate, expected dividend yield, and expected term. Since the Company’s units are not publicly traded and its units are rarely traded privately, expected volatility is estimated based on the average historical volatility of similar entities with publicly traded shares. The risk-free rate for the expected term of the warrants is based on the U.S. Treasury yield curve at the date of grant. The expected term represents the remaining contractual term of the warrants as of the valuation date.
| F-25 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
The assumptions used to measure the fair value of the warrant liability as of original issuance dates and as of December 31, 2021 and 2020 were as follows:
| 2021 | December 31, 2021 | |||||||
| Volatility rate | 55% to 60 | % | 55.0 | % | ||||
| Risk-free rate | 0.63% to 1.08 | % | 1.08 | % | ||||
| Expected dividend yield | 0.0 | % | 0.0 | % | ||||
| Expected term | 3.77 to 4.48 years | 3.73 years | ||||||
| 2020 | December 31, 2020 | |||||||
| Volatility rate | 55.0 | % | 55.0 | % | ||||
| Risk-free rate | 0.27% to 0.37 | % | 0.33 | % | ||||
| Expected dividend yield | 0.0 | % | 0.0 | % | ||||
| Expected term | 4.77 to 5.00 years | 4.73 years | ||||||
A summary of the changes in the Company’s warrant liability measured at fair value using significant unobservable inputs (Level 3) as of and for the year ended December 31,2021 and 2020 is as follows:
| Warrant liability, December 31, 2019 | $ | — | ||
| Warrant liabilities issued | 243,418 | |||
| Change in fair value | (1,862 | ) | ||
| Warrant liability, December 31, 2020 | $ | 241,556 | ||
| Warrant liabilities issued | 668,900 | |||
| Change in fair value | (21,782 | ) | ||
| Warrant liability, December 31, 2021 | $ | 888,674 |
The Company recognized a gain on the change in fair value of the warrant liability for the years ended December 31, 2021 and 2020 of 21,782 and $1,862, respectively.
| 9. | Members’ Equity (Deficit) |
Based upon the Company’s Amended and Restated Operating Agreement (“Operating Agreement”), dated August 29, 2016, the Company has authorized a single class of units designated as Member Units (“MUs”).
MUs are issued at the discretion of the Company and the Board of Managers in accordance with the Company’s Operating Agreement. Each MU shall carry the right to cast one vote per unit on any matter to be approved by the Members, such that each Member is entitled to a number of votes equal to the number of Units held by such Member.
MUs are not transferable without the written approval of the Board of Managers, with certain specified exceptions, as defined in the Operating Agreement.
In accordance with the Company’s Operating Agreement, net profits and net losses of the Company from operations for any year or other fiscal period shall be allocated among the Members in accordance with their Percentage Interests, as defined. Upon liquidation of the Company, Distributable Cash and net proceeds shall be distributed to the Members, at such times and in such amounts as the Board of Managers may approve, provided that all such distributions shall be distributed to and among the Members in accordance with their respective Percentage Interests.
| F-26 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
| 10. | Commitments and Contingencies |
Litigation
From time to time, the Company may be a party to various claims in the normal course of business. Legal fees and other costs associated with such actions are expensed as incurred. The Company assesses, in conjunction with its legal counsel, the need to record a liability for litigation and contingencies. Reserve estimates are recorded when and if it is determined that a loss related matter is both probable and reasonably estimable. As of December 31, 2021 and 2020, the Company had no pending or threatened litigation.
Indemnifications
In the ordinary course of business, the Company enters into various agreements with partners, suppliers, and vendors containing standard indemnification provisions. The Company’s indemnification obligations under such provisions are typically in effect from the date of execution of the applicable agreement through the end of the applicable statute of limitations. Pursuant to these provisions, the Company may be obligated to indemnify such parties for losses or claims suffered or incurred in connection with its service, breach of representations or covenants, intellectual property infringement or other claims made against such parties. These provisions may limit the time within which an indemnification claim can be made. It is not possible to determine the maximum potential amount under these indemnification obligations due to the limited history of prior indemnification claims and unique facts and circumstances involved in each particular agreement. To date, the Company has not incurred any material costs as a result of such indemnifications and has not accrued any liabilities related to such obligations in these consolidated financial statements.
| 11. | Related Party Transactions |
Jerry Kowalski was the Company’s CEO from the Company’s formation until May of 2018 when he passed away. Both his wife Ivanka Kowalski and his brother Kevin Kowalski are members of the Company and hold the following convertible notes payable:
| ● | In 2015 and 2016, Ivanka extended $200,000 and $100,000, respectively, in the form of convertible notes accruing interest at rates ranging from 5% to 10% per annum with a conversion option for 828 units. These notes are still outstanding as of December 31, 2021. | |
| ● | In 2016 and 2018, Kevin extended $100,000 and $40,000, respectively, in the form of convertible notes accruing interest at 5% per annum with a conversion option for 387 units. These notes are still outstanding as of December 31, 2021. | |
| ● | After Jerry Kowalski’s passing, all of his equity holdings were transferred to Ivanka Kowalski’s name. Ivanka’s current member holdings are 44,857.31 units. |
Igor Serov has been the CFO and a member of the Company since the Company’s formation. In 2015, Igor Serov extended $50,000 to the Company in the form of convertible notes accruing interest at 10% per annum with a conversion option for 138 member units. This note is still outstanding as of December 31, 2021.
| F-27 |
Hemoglobin Oxygen Therapeutics LLC
Notes to Consolidated Financial Statements
The Company has certain executive employment contracts in place with Zaf Zafirelis (CEO) and Igor Serov (CFO), which specify terms and conditions of employment, including severance to be paid upon termination of employment from the Company by the Company without Cause, for which the Company shall pay an amount equal to the greater of (i) each executive’s annual base salary in effect immediately prior to termination and (ii) $200,000 (“Severance”), each as defined.
In 2016, Peritimos Investments Ltd. (“Peritimos”) acquired 72,885 member units in exchange for $10,000,000. In 2018, Peritimos extended $794,655 to the Company in the form of convertible notes that accrue interest at rates ranging from 2% to 12% per annum with a conversion option for 2,192 member units. Additionally, in 2020, Peritimos extended an additional $196,438 to the Company in the form of convertible notes that accrue interest at 5% per annum with a conversion option for 358 member units. On November 27, 2020, Peritimos converted all of their outstanding convertible notes into 2,550 member units. In 2021, Peritimos extended $337,566 to the Company in the form of promissory notes, with various maturity dates that accrue interest at 2%. The principal plus accrued interest is due on the maturity date (see Note 7).
Jerry Spears, a Member of the Company, is the landlord for the manufacturing facility lease together with all personal property, fixtures and equipment on the property by the Company. Rent expense incurred on real estate and equipment leased from Mr. Spears was $659,835 for the years ended December 31, 2021 and 2020. Amounts owed to Mr. Spears for unpaid rents was $2,442,723, and $1,762,893 as of December 31, 2021 and 2020, respectively. From time to time, Mr. Spears paid other expenses for the Company such as property taxes, construction in process, other rent, equipment, and utilities. Expense incurred for such items paid by Mr. Spears amounted to $166,473 and $196,319 for the years ended December 31, 2021 and 2020, respectively. Amounts owed to Mr. Spears for other expense he paid for on behalf of the company was $709,389 and $542,916 as of December 31, 2021 and 2020, respectively. These amounts remain owed to Mr. Spears and are included within Payable to Landlord in the consolidated balance sheets.
| 12. | Subsequent Events |
Management has evaluated subsequent events through April 25, 2022, which is the date these consolidated financial statements were available to be issued. All material subsequent events that have occurred since December 31, 2021 that require disclosure in these consolidated financial statements are included below:
In January 2022, the Company reached an agreement with all convertible debt holders to convert their loans into equity of the Company at the time of the Company’s anticipated offering. The Company also reached an agreement with Jerry Spears to convert $2,000,000 of outstanding payable to him into equity at the time of the Company’s offering at a 35% discount. Mr. Spears will also have the option to convert an additional $1,000,000 of the outstanding payable into equity at the same discount during the 18 months following the Company’s offering. Additionally, the Company reached an agreement with the engineering firm building out their production facility to convert $486,000 of the outstanding payable to them into equity at the time of the Company’s offering at a 25% discount.
On January 15, 2022, the Company reached an agreement with one of their lenders to extend the maturity date of a convertible note payable due January 15, 2022 by one year. No other terms of the loan were amended.
On March 1, 2022, the Company entered into a note payable agreement for $100,000 with an interest rate of 5% per annum. The note matures on March 1, 2023. The principal plus accrued interest are due on the maturity date.
On March 21, 2022, the Company entered into a convertible note agreement for $200,000 with an interest rate of 5% per annum. The note matures on March 21, 2023. The principal plus accrued interest are due on the maturity date. The principal of the note automatically converts upon an initial public offering to shares of common stock issued in the public offering at a discount of 25%. Any accrued interest on the note will be extinguished upon the conversion of the principal in a public offering. Additionally, at the closing of the initial public offering, the Company shall grant the lender warrants to purchase shares up to the amount of $200,000. The warrants are exercisable for one year from the effective date of the registration statement at a price equal to 100% of the offering price.
On April 22, 2022, the Company entered into a convertible note agreement for $200,000 with an interest rate of 5% per annum. The note matures on April 22, 2023. The principal plus accrued interest are due on the maturity date. The principal of the note automatically converts upon an initial public offering to shares of common stock issued in the public offering at a discount of 25%. Any accrued interest on the note will be extinguished upon the conversion of the principal in a public offering. Additionally, at the closing of the initial public offering, the Company shall grant the lender warrants to purchase shares up to the amount of $200,000. The warrants are exercisable for one year from the effective date of the registration statement at a price equal to 100% of the offering price.
| F-28 |
HEMOGLOBIN OXYGEN THERAPEUTICS INC.
Common Stock
Prospectus
, 2022
WestPark Capital, Inc.
PART II — INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth all expenses, other than the underwriting discounts and commissions, payable by the registrant in connection with the sale of the securities being registered. All the amounts shown are estimates except the SEC registration fee and the FINRA filing fee.
| Amount to be paid | ||||
| SEC registration fee | $ | 2,781 | ||
| FINRA filing fee | $ | |||
| The Nasdaq Capital Market initial listing fee | $ | 90,000 | ||
| Transfer agent and registrar fees | $ | * | ||
| Accounting fees and expenses | $ | * | ||
| Legal fees and expenses | $ | * | ||
| Printing and engraving expenses | $ | * | ||
| Miscellaneous | $ | * | ||
| Total | $ | * | ||
* To be provided by amendment
| II-1 |
Item 14. Indemnification of Directors and Officers
Section 102 of the Delaware General Corporation Law (“DGCL”) permits a corporation to eliminate the personal liability of directors of a corporation to the corporation or its stockholders for monetary damages for a breach of fiduciary duty as a director, except where the director breached his duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our Amended and Restated Certificate of Incorporation provides that no director of the Company shall be personally liable to it or its stockholders for monetary damages for any breach of fiduciary duty as a director, notwithstanding any provision of law imposing such liability, except to the extent that the DGCL prohibits the elimination or limitation of liability of directors for breaches of fiduciary duty.
Section 145 of the DGCL provides that a corporation has the power to indemnify a director, officer, employee, or agent of the corporation, or a person serving at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise in related capacities against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with an action, suit or proceeding to which he was or is a party or is threatened to be made a party to any threatened, ending or completed action, suit or proceeding by reason of such position, if such person acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful, except that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
Our Certificate of Incorporation and Bylaws provide indemnification for our directors and officers to the fullest extent permitted by the DGCL. We will indemnify each person who was or is a party or threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of us) by reason of the fact that he or she is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise (all such persons being referred to as an “Indemnitee”), or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding and any appeal therefrom, if such Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, and, with respect to any criminal action or proceeding, he or she had no reasonable cause to believe his or her conduct was unlawful. Our Certificate of Incorporation and Amended and Restated Bylaws provide that we will indemnify any Indemnitee who was or is a party to an action or suit by or in the right of us to procure a judgment in our favor by reason of the fact that the Indemnitee is or was, or has agreed to become, a director or officer, or is or was serving, or has agreed to serve, at our request as a director, officer, partner, employee or trustee of, or in a similar capacity with, another corporation, partnership, joint venture, trust or other enterprise, or by reason of any action alleged to have been taken or omitted in such capacity, against all expenses (including attorneys’ fees) and, to the extent permitted by law, amounts paid in settlement actually and reasonably incurred in connection with such action, suit or proceeding, and any appeal therefrom, if the Indemnitee acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, our best interests, except that no indemnification shall be made with respect to any claim, issue or matter as to which such person shall have been adjudged to be liable to us, unless a court determines that, despite such adjudication but in view of all of the circumstances, he or she is entitled to indemnification of such expenses. Notwithstanding the foregoing, to the extent that any Indemnitee has been successful, on the merits or otherwise, he or she will be indemnified by us against all expenses (including attorneys’ fees) actually and reasonably incurred in connection therewith. Expenses must be advanced to an Indemnitee under certain circumstances.
As of the date of this prospectus, we have entered into separate indemnification agreements with each of our directors and executive officers. Each indemnification agreement provides, among other things, for indemnification to the fullest extent permitted by law and our Certificate of Incorporation against any and all expenses, judgments, fines, penalties and amounts paid in settlement of any claim. The indemnification agreements provide for the advancement or payment of all expenses to the indemnitee and for the reimbursement to us if it is found that such indemnitee is not entitled to such indemnification.
In addition, upon consummation of this offering, we intend to obtain a general liability insurance policy that covers certain liabilities of directors and officers of our corporation arising out of claims based on acts or omissions in their capacities as directors or officers.
In any underwriting agreement we enter into in connection with the sale of common stock being registered hereby, the underwriters will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us within the meaning of the Securities Act against certain liabilities.
| II-2 |
Item 15. Recent Sales of Unregistered Securities
LLC Participation Units
Issuance Date | Investor | Amount | Number of Units | |||||||
| November 20, 2020 | Peritimos Investments Ltd. | $ | 991,103 | 2,550 | ||||||
| (Convertible debt conversion) | ||||||||||
Convertible Debt Issuance
Issuance Date | Investor | Amount | Drawn in Tranches | |||||||
| January 15, 2019 | OVA International Investment LLC | $ | 2,000,000 | $ | 1,000,000 | |||||
| April 29, 2019 | 500,000 | |||||||||
| August 2, 2019 | 500,000 | |||||||||
| May 8, 2019 | Chris DiPaolo | 230,000 | ||||||||
| October 8, 2019 | OVA International Investment LLC | 100,000 | ||||||||
| November 5, 2019 | OVA International Investment LLC | 400,000 | ||||||||
| February 4, 2020 | OVA International Investment LLC | 40,000 | ||||||||
| February 13, 2020 | Michael Spektor | 48,023 | ||||||||
| March 9, 2020 | OVA International Investment LLC | 20,000 | ||||||||
| March 12, 2020 | Michael Spektor | 70,050 | 43,350 | |||||||
| April 21, 2020 | 26,700 | |||||||||
| April 6, 2020 | OVA International Investment LLC | 20,000 | ||||||||
| April 30, 2020 | OVA International Investment LLC | 20,000 | ||||||||
| August 5, 2020 | OVA International Investment LLC | 30,000 | ||||||||
| August 10, 2020 | Michael Spektor | 78,365 | 34,918 | |||||||
| September 11, 2020 | 23,325 | |||||||||
| October 6,2020 | 20,122 | |||||||||
| September 1, 2020 | OVA International Investment LLC | 30,000 | ||||||||
| September 21, 2020 | OVA International Investment LLC | 2,400,000 | 100,000 | |||||||
| October 22, 2020 | 100,000 | |||||||||
| November 20, 2020 | 100,000 | |||||||||
| December 14, 2020 | 400,000 | |||||||||
| April 12, 2021 | 100,000 | |||||||||
| May 19, 2021 | 100,000 | |||||||||
| June 17, 2021 | 100,000 | |||||||||
| July 12, 2021 | 100,000 | |||||||||
| August 19, 2021 | 100,000 | |||||||||
| September 20, 2021 | 100,000 | |||||||||
| October 18, 2021 | 100,000 | |||||||||
| October 20, 2021 | 400,000 | |||||||||
| November 15, 2021 | 300,000 | |||||||||
| December 14, 2021 | 300,000 | |||||||||
The above securities were issued pursuant to an exemption from the registration provisions of the Securities Act of 1933 as amended under Section 4(a)(2) thereof.
| II-3 |
Item 16. Exhibits and Financial Statement Schedules
EXHIBIT INDEX
| II-4 |
| 10.17 | Debt Conversion Agreement, dated as of January 3, 2022 between the Company and Igor Serov* | |
| 23.1 | Consent of BDO USA, LLP* | |
| 23.2 | Consent of TroyGould PC (Included in Exhibit 5.1)** | |
| 24.1 | Power of Attorney (included on signature page of this Form S-1)* | |
| 99.1 | Consent of Director Nominee* | |
| 99.2 | Consent of Director Nominee* | |
| 99.3 | Consent of Director Nominee* | |
| 107 | Exhibit Filing Fee | |
| 101.INS | XBRL Instance Document* | |
| 101.SCH | XBRL Taxonomy Extension Schema Document* | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document* | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document* | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document* | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document* |
| * | Filed Herewith | |
| ** | To be filed by amendment |
Financial Statement Schedules
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings
| (a) | The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser. | |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the Company has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment the Company of expenses incurred or paid by a director, officer or controlling person of the Company in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Company will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction, the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. | |
| (c) | The undersigned hereby further undertakes that: |
| (1) | For purposes of determining any liability under the Securities Act the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by Hemoglobin Oxygen Therapeutics pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. | |
| (2) | For the purpose of determining any liability under the Securities Act each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| II-5 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized in the Town of Souderton, State of Pennsylvania, on the 26 day of April 2022.
| HEMOGLOBIN OXYGEN THERAPEUTICS LLC | ||
| By: | /s/ Zafiris F. Zafirelis | |
| Name: | Zafiris F. Zafirelis | |
| Title: | Chief Executive Officer | |
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Zafiris Zafirelis and Igor Serov, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him and in his name, place, and stead, in any and all capacities, to sign any and all amendments to this Registration Statement on Form S-1 and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1934, this S-1 has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Zafiris F. Zafirelis | Chief Executive Officer and Director | April 26, 2022 | ||
| Zafiris F. Zafirelis |
(Principal Executive Officer) |
|||
| /s/ Igor Serov | Chief Financial Officer and Director (Principal | April 26, 2022 | ||
| Igor Serov |
Financial Officer and Principal Accounting Officer) |
|||
| /s/ Anthony E. Pusateri | Director Nominee | April 26, 2022 | ||
| Anthony E. Pusateri | ||||
| /s/ Richard Kilsby | Director Nominee | April 26, 2022 | ||
| Richard Kilsby | ||||
| /s/ Leslie Fang | Director Nominee | April 26, 2022 | ||
| Leslie Fang |
| II-6 |
Exhibit 10.1
HEMOGLOBIN OXYGEN THERAPEUTICS, INC.
January 10, 2022
Zafiris G. Zafirelis
71 Harris Avenue
Needham, MA 02492
RE: Agreement of Employment
Dear Zaf:
We are pleased to offer you continued employment as the full-time position of Chief Executive Officer (“CEO”) of Hemoglobin Oxygen Therapeutics, LLC (the “Company”) as of January 10, 2022. As you know, the Company plans to convert to a C corporation (“Newco”), and upon such conversion, the reference to “Company” herein shall be to Newco. This offer is contingent upon your agreement to execute the Company’s Nondisclosure, Noncompetition and Assignment of Developments Agreement (the “Nondisclosure Agreement”), a copy of which is attached hereto as Exhibit A. This letter agreement (the “Agreement”) summarizes the initial terms of your continued employment.
1. Position and Responsibilities. Upon the terms and conditions herein set forth, you agree to continue to serve as CEO of the Company. In such capacity, you agree to perform the duties and responsibilities required by this position. You shall devote your business time and efforts to the performance of your duties hereunder. You will perform the duties of the CEO and as such shall be responsible for aspects of management, research and development, manufacturing, finance, corporate structure, capital structure, employment matters, and all other aspects of the Company’s business customarily required of a CEO or as so directed by the Company’s Board of Directors (the “Board”). Additionally, subject to stockholder ratification and the charter documents of the Company, you shall be a member of the Board. You shall exercise such powers and comply with and perform, faithfully and to the best of your ability, such directions and duties in relation to the business and affairs of the Company as may from time to time be vested in or requested of you.
If requested by the Board you shall become and remain a director of any subsidiary of the Company as the Board may from time to time request. As a director, you shall at all times comply with the applicable governing documents of the subsidiary of which you are a director.
You shall resign from your position as a director of a subsidiary and/or as a director of the Company on request of the Board in accordance with the applicable Bylaws or immediately upon termination of your employment howsoever arising. You hereby irrevocably appoint each of the Company and the Company’s secretary to be your attorney to execute any such resignation document and generally to use your name for the purpose of giving the Company or its nominee the full benefit of this provision.
You shall perform your services under this Agreement at such locations as may be reasonably required by the Company or the Board. Your primary location of employment will be the Boston, Massachusetts area, but you will be expected to travel from time to time as directed by the Company or the Board, and in particular to the Company’s facility in Souderton, Pennsylvania.
| 1 |
You shall be subject to all policies, codes and rules of the Company in effect from time to time, including but not limited to policies relating to sick leave and holidays, disciplinary and grievance matters, code of ethics, and insider trading, and you agree to comply with such policies.
2. Compensation, Bonuses and Other Benefits. During the term of this Agreement, the Company shall pay you, as compensation for your performance of your duties and obligations hereunder, the following:
(a) Your annual base salary shall be at an initial rate of $350,000, less applicable withholdings, and shall be payable in conformity with the Company’s customary payroll practices, as such practices shall be established or modified from time to time.
(b) During your employment, you may be eligible to receive an annual bonus at the sole discretion of the Board (or a committee thereof), based on the Board’s (or a committee’s) assessment of relevant factors including but not limited to your personal performance, the performance of the Company, and the availability of cash. No bonus under this paragraph shall be payable to you with respect to any calendar year during which your employment is terminated for cause. For the avoidance of doubt, payment of an annual bonus in any one or more years shall not create an entitlement for you to be paid a bonus in any subsequent years, nor will it interfere with the Company’s discretion in any way with respect to the bonus.
(c) You shall be eligible to participate, to the extent applicable, in all employee benefit plans of the Company in effect from time to time, including but not limited to the Company’s health insurance program, disability insurance program (if applicable), and any applicable incentive plan (an “Incentive Plan”). The Company will provide additional information about the health insurance program, disability insurance program (if applicable), and the Incentive Plan upon request. Your participation in the Company’s employee benefit plans shall be subject to (i) the terms of the applicable plan documents, (ii) generally applicable Company policies, and (iii) the discretion of the Company and/or the Board or any administrative or other committee provided for in or contemplated by such plan. The Company’s current plans and policies shall govern all other benefits. The Company may alter, modify, add to, or delete its employee benefit plans at any time as the Company and/or the Board, in their sole judgment, determines to be appropriate. The Company does not currently offer a pension plan benefit.
(d) You shall be eligible to accrue vacation time at the rate of 1.67 days of vacation per full month worked, up to a maximum of 20 days of vacation per calendar year. You may not carry over any unused vacation time from year to year without prior written approval, and you are not entitled to payment in lieu of vacation time. However, you will be paid for accrued but unused vacation time during the year in which your employment terminates.
(e) The Company shall pay or reimburse all reasonable business expenses incurred or paid by you in the performance of your responsibilities hereunder in accordance with the Company’s prevailing policy and practice relating to reimbursements as established, modified or amended from time to time. You must provide substantiation and documentation of these expenses to the Company in order to receive reimbursement.
| 2 |
(f) The Company shall have the right to deduct and withhold from any amounts payable hereunder or pursuant to any other agreements or arrangements between the Company (and any of its affiliates) and you, any federal, state, local, foreign or other taxes (“Taxes”) that the Company determines should be withheld with respect to such payments. In the event that the Company does not withhold the proper amount of Taxes from any such payments, you will make a prompt payment, on demand and in cash, to the Company of the amount under-withheld.
4. Employment Term and Severance.
(a) You and the Company understand and agree that your employment is as an employee at will, and that you may resign, or the Company may terminate your employment, at any time and for any or for no reason. Nothing in this Agreement shall be construed to alter the at-will nature of your employment, nor shall anything in this Agreement be construed as providing you with a definite term of employment.
(b) In the event your employment with the Company terminates for any reason (including death or Disability (as hereinafter defined)), the Company shall pay to you any base salary including accrued but unused vacation pay, expense reimbursements, compensation and benefits under any applicable plan, and any and all benefits and other similar amounts, accrued but unpaid as of the date of termination. In addition, upon termination of your employment with the Company by the Company without Cause (as defined below) (but excluding death or Disability), contingent upon your execution, your personal representative’s execution and delivery of the Release (as defined below), the Company shall pay to you an amount equal to the greater of (i) your annual base salary in effect immediately prior to termination and (ii) $350,000 (“Severance”), to be paid over a like number of months consistent with the Company’s normal payroll schedule; provided, however, (x) payment of any Severance is contingent upon execution and delivery to the Company of the Release and such Release being effective and not revoked by the end of the sixtieth (60th) day following your date of termination and (y) that in the event of your material breach of the Nondisclosure Agreement, which breach, if reasonably susceptible to cure, has not been cured to the satisfaction of the Company within ten (10) business days of your receipt of written notice of such breach, then the Company’s obligation to pay Severance shall terminate and be of no further force or effect. The payment of Severance shall commence on the sixtieth (60th) day after your date of termination; provided that the Release is effective on such date. Your rights to any Severance shall constitute your sole remedy in the event of termination of your employment. For purposes of this Agreement, your termination of employment shall mean your “separation from service” within the meaning of Treasury Regulation Section 1.409(A)-1(h).
(c) “Cause” shall mean (i) your failure or refusal to render services to the Company in accordance with your obligations under this Agreement; (ii) a determination by the Board that you have refused to follow the lawful instructions of the Board; (iii) gross negligence, dishonesty or breach of fiduciary duty; (iv) the commission of an act of fraud, embezzlement, misappropriation of any money or other assets or property (whether tangible or intangible), deliberate disregard of the rules or policies of the Company, or the commission of any other action which injures the Company which is done not in good faith and without reasonable belief that your action was in the best interest of the Company; (v) being charged with a felony or crime of moral turpitude, which in the determination of the Board after reasonable investigation, may injury the Company or its reputation; or (vi) breach of this Agreement, the Nondisclosure Agreement or any other agreement executed by you in connection with your employment; provided that with respect to the items set forth in clauses (i), (ii), (iii) or (vi), to the extent reasonably curable, you have not cured such reason for Cause within ten (10) business days after a written demand for substantial performance is delivered to you by the Company which specifically identifies the manner in which the Company believes it has the right to terminate you for Cause. For purposes of this Subsection, no act or failure to act on your part shall be deemed “willful” unless done or omitted to be done by you not in good faith and without reasonable belief that your action or omission was in the best interest of the Company.
| 3 |
(d) “Disability” You shall be deemed to have a Disability for purposes of this Agreement either (i) if you are deemed disabled for purposes of any group or individual disability policy paid for by the Company and at the time in effect, or (ii) if, in the good faith judgment of the Board, you are substantially unable to perform your duties under this Agreement for more than ninety (90) days, whether or not consecutive, in any twelve (12) month period, by reason of a physical or mental illness or injury.
(e) “Release” shall mean a waiver and release (including confidentiality and non- disparagement provisions), based on the Company’s standard form or other reasonable form, of any and all claims you may have against the Company and its parents, subsidiaries, affiliates, predecessors and successors, as well as each of their past, present and future stockholders, directors, officers, employees, consultants, representatives, attorneys, insurers, agents, assigns, any other legal entity describing the organizations or through which any of them conducts business, and their employee benefits plans and trustees, fiduciaries, and administrators of those plans.
(f) Payments of Severance to you shall be bifurcated into two portions, consisting of the portion, if any, that includes the maximum amount of the payments that does not constitute “nonqualified deferred compensation” within the meaning of Section 409A of the Code, and the portion, if any, that includes the excess of the total payments that does constitute nonqualified deferred compensation. Payments hereunder shall first be made from the portion that does not consist of nonqualified deferred compensation until such portion is exhausted and then shall be made from the portion that does constitute nonqualified deferred compensation. Notwithstanding the foregoing, if you are a “specified employee” as defined in Section 409A(a)(3)(B)(i) of the Code, the commencement of the delivery of the portion that constitutes nonqualified deferred compensation will be delayed to the date that is 6 months and one day after your termination of employment (the “Earliest Payment Date”). Any payments that are delayed pursuant to the preceding sentence shall be paid pro rata during the period beginning on the Earliest Payment Date and ending on the date that is 6 months following the Earliest Payment Date. The determination of whether, and the extent to which, any of the payments to be made to you hereunder are nonqualified deferred compensation shall be made after the application of all applicable exclusions under Treasury Reg. § 1.409A-1(b)(9). Any payments that are intended to qualify for the exclusion for separation pay due to involuntary separation from service set forth in Treasury Regulation Section 1.409A-1(b)(9)(iii) must be paid no later than the last day of the second taxable year of you following the taxable year of you in which your termination of employment occurs.
(g) This Agreement is intended to comply with the provisions of Section 409A and the Agreement shall, to the extent practicable, be construed in accordance therewith. Terms defined in the Agreement shall have the meanings given such terms under Section 409A if and to the extent required in order to comply with Section 409A. No payments to be made under this Agreement may be accelerated or deferred except as specifically permitted under Section 409A. In the event that the Agreement shall be deemed not to comply with Section 409A, then neither the Company, the Company’s Board s nor its or their designees or agents shall be liable to you or other person for actions, decisions or determinations made in good faith.
| 4 |
(h) Neither the Company nor you shall have the right to accelerate or defer the delivery of any payments except to the extent specifically permitted or required by Section 409A.
5. Disability. The Company intends to provide disability insurance. You agree to abide by the terms of such insurance policy and any policy of the Company applicable to disabled employees in effect from time to time.
6. Survival of Certain Provisions. Provisions of this Agreement shall survive any termination of employment or termination or expiration of this Agreement if so provided herein or if necessary or desirable to fully accomplish the purposes of such provision. Without limiting the foregoing, your obligations under the Nondisclosure Agreement expressly survive any termination of employment or termination or expiration of this Agreement. The obligation of the Company to make payments to you or on your behalf under Section 4 hereof is expressly conditioned upon your continued full performance of the obligations under the terms of the Nondisclosure Agreement executed herewith between you and the Company.
7. Consent and Waiver by Third Parties. You hereby represent that you have obtained all waivers and/or consents from third parties which are necessary to enable you to enjoy employment with the Company on the terms and conditions set forth herein and to execute and perform this Agreement without being in conflict with any other agreement, obligation or understanding with any such third party. You represent that you are not bound by any agreement or any other existing or previous business relationship which conflicts with, or may conflict with, the performance of your obligations hereunder or prevent the full performance of your duties and obligations hereunder.
8. Governing Law. This Agreement, the employment relationship contemplated herein and any claim arising from such relationship, whether or not arising under this Agreement, shall be governed by and construed in accordance with the internal laws of Massachusetts, without giving effect to the principles of choice of law or conflicts of laws. Any claims or legal actions by one party against the other arising out of the relationship between the parties contemplated herein (whether or not arising under this Agreement) shall be commenced or maintained in any state or federal court located in Delaware, and you and the Company each hereby submit to the jurisdiction and venue of any such court.
9. Severability. In case any one or more of the provisions contained in this Agreement or the other agreements executed in connection with the transactions contemplated hereby for any reason shall be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other provision of this Agreement or such other agreements, but this Agreement or such other agreements, as the case may be, shall be construed and reformed to the maximum extent permitted by law.
10. Waivers and Modifications. No waiver by the Company of any breach by you of any provision hereof shall be deemed to be a waiver of any later or other breach thereof or as a waiver of any other provision of this Agreement. This Agreement and its terms may not be waived, changed, discharged or terminated orally or by any course of dealing between the parties, but only by an instrument in writing signed by the party against whom any waiver, change, discharge or termination is sought. No modification or waiver by the Company shall be effective without the consent of the Board or its designee.
| 5 |
11. Assignment. You acknowledge that the services to be rendered by you hereunder are unique and personal in nature. Accordingly, you may not assign any of your rights or delegate any of your duties or obligations under this Agreement. The rights and obligations of the Company under this Agreement may be assigned by the Company and shall inure to the benefit of, and shall be binding upon, the successors and assigns of the Company.
12. Acknowledgments. You hereby acknowledge and recognize that the enforcement of any of the provisions in this Agreement and the Nondisclosure Agreement executed herewith may potentially interfere with your ability to pursue a proper livelihood. You represent that you are knowledgeable about the business of the Company. You recognize and agree that the enforcement of the Nondisclosure Agreement is necessary to ensure the preservation, protection and continuity of the Company’s business, trade secrets and goodwill. You agree that, due to the proprietary nature of the Company’s business, the restrictions set forth in the Nondisclosure Agreement are reasonable as to time and scope.
13. Entire Agreement. This Agreement and the Nondisclosure Agreement executed herewith, constitute the entire understanding of the parties relating to the subject matter hereof and supersede and cancel all agreements relating to the subject matter hereof, whether written or oral, made prior to the date hereof between you and the Company or any of its affiliates.
14. Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed to be an original, but all of which together shall constitute one and the same instrument. If the foregoing accurately sets forth our agreement, please indicate by signing and returning to us both copies of this Agreement and the enclosed Nondisclosure Agreement. We will return fully- executed copies of both agreements for your records.
Very truly yours,
| HEMOGLOBIN OXYGEN THERAPEUTICS, LLC. | Accepted and Agreed: | |||
| By: | By: | |||
| Name: | Igor Serov | Name: | Zafiris G. Zafirelis | |
| Title: | CFO | Date: | January 10, 2022 | |
| 6 |
EXHIBIT A
NONDISCLOSURE, NONCOMPETITION AND
ASSIGNMENT OF DEVELOPMENTS AGREEMENT
This Nondisclosure, Noncompetition and Assignment of Developments Agreement (this “Nondisclosure Agreement”) is made as of this 10 day of January, 2022 by and between Zafiris G. Zafirelis, an individual, and Hemoglobin Oxygen Therapeutics, LLC. a corporation with offices at 674 Souder Road, Souderton PA 18964, USA (the “Company”). Upon conversion of the Company to a C corporation (“Newco”), references herein to the Company shall be to Newco.
WHEREAS, the Company’s business is conducted throughout the world and its reputation and goodwill are an integral part of its business success;
WHEREAS, my position with the Company shall require that I be trusted with extensive confidential and trade secret information of the Company and that I develop a thorough and comprehensive knowledge of details of the Company’s business, including, but not limited to, information relating to development, inventions, processes, financial and strategic planning, marketing, distribution and licensing of the Company’s products and services. The Company will not provide, and will not agree to continue to provide, me with this information unless I provide the necessary assurances and commitments to protect this information and the Company’s line of business as more fully set forth herein; and
WHEREAS, as an additional material inducement for me to enter into this Nondisclosure Agreement, the Company shall provide me with the Employment Agreement, which is ancillary to the Nondisclosure Agreement and is to be executed in connection herewith (the “Employment Agreement”), which includes eligibility for certain severance benefits as set forth therein.
NOW, THEREFORE, in consideration for the Company providing me with the Employment Agreement, and in consideration for my continued employment by the Company, subject to the policies, procedures and practices that the Company may establish from time to time, the compensation I shall receive from the Company during my employment and other good and valuable consideration, and as a material inducement for the Company to disclose to me in connection with its business certain confidential, proprietary and/or trade secret information described below, and intending to be legally bound, I hereby agree as follows:
1. Best Efforts. During the period of my employment by the Company, I shall devote my full time and best efforts to the business of the Company during normal and other customary business hours, and I shall not engage in any other business activity, whether or not such business activity is pursued for gain, profit, or other pecuniary advantage, which may tend to (i) interfere with the performance of any job duties and responsibilities assigned to me by the Company, (ii) create a conflict of interest, or (iii) be competitive with the business activities of the Company; provided that I may, to the extent not otherwise prohibited by this Agreement (including without limitation Section 8), devote such amount of time as does not interfere or compete with the performance of my duties under the Employment Agreement to any one or more of the following activities: (i) investing my personal assets in the record or beneficial ownership of five percent (5%) or less of the outstanding publicly traded capital stock of any entity; or (ii) engaging in charitable activities, including serving on the Boards of Directors of charitable organizations.
2. Nondisclosure Obligation. I shall not at any time, whether during or after the termination of my employment, reveal to any person or entity any Confidential Information, except to employees, authorized agents, and professional advisors of the Company who need to know such Confidential Information for the purposes of their employment or affiliation with the Company and other third parties, or as otherwise authorized by the Company. The term “Confidential Information” shall include any information concerning the organization, business or finances of the Company or of any third party that the Company is under an obligation to keep confidential or that is maintained by the Company as confidential. Such Confidential Information shall include, but is not limited to, trade secrets, proprietary or confidential information respecting the identity of the Company’s customers; the identity of distributors and suppliers of the Company; the identity of representatives responsible for entering into contracts with the Company; specific customer, distributor and supplier needs and requirements; the details of contracts and proposals between the Company and its customers, distributors and suppliers; selling and marketing strategies, prices, costs and profit margins; the names, addresses and other contact information of purchasing agents, vendors or other entities; purchasing techniques, methods, procedures and processes; manufacturing and production techniques, methods, procedures and processes; other techniques, methodologies and processes used by the Company in the conduct of its business; techniques, methods, procedures, know-how, blueprints, engineering data, prototypes and technical specifications; computer data, software, software codes, computer models, works of authorship, research projects, data processing and other programs; production and manufacturing equipment and operating practices; information with respect to products and product formulae, designs, plans for future business, new business, products or other developments; new or innovative ideas, customer proposals, marketing plans and ideas, and future developments or strategies; information pertaining to research and development, acquisitions or divestitures, marketing and sales, cost cutting, revenue generation, or other matters concerning the Company’s planning and strategy; personnel information, and other nonpublic financial and other information of the Company disclosed to or known by me as a consequence of or through my employment (or other service relationship) with the Company (including information conceived, originated, discovered or developed by me), which information is not generally known in the relevant trade or industry or public knowledge. I shall keep confidential all matters entrusted to me and shall not use or attempt to use any Confidential Information except as may be required in the ordinary course of performing my duties as an employee of the Company, nor shall I use any Confidential Information in any manner which may injure or cause loss or may be calculated to injure or cause loss to the Company, whether directly or indirectly.
3. Company Property. I agree that during my employment, I shall not make, use or permit to be used any Company Property otherwise than for the benefit of the Company. The term “Company Property” shall include all notes, memoranda, reports, lists, cost sheets, records, drawings, designs, sketches, specifications, blue prints, prototypes, estimates, databases, software programs, software code, data, proprietary processes, computers, cellular telephones, pagers, credit and/or calling cards, keys, access cards, documentation, automobiles or other equipment or materials of any nature and in any form, whether written, printed, electronic or in digital format or otherwise, relating to any matter within the scope of the business of the Company or concerning any of its dealings or affairs and any other Company property in my possession, custody or control. I further agree that I shall not, after the termination of my employment, use or permit others to use any such Company Property. I acknowledge and agree that all Company Property shall be and remain the sole and exclusive property of the Company. Immediately upon the termination of my employment, I shall deliver all Company Property in my possession, and all copies thereof, to the Company.
| 2 |
4. Assignment of Developments.
(a) If at any time during my employment, I shall (either alone or with others) make, conceive, create, certify, discover, invent or reduce to practice any Development (as defined below) that (i) relates to the business of the Company or any customer of or supplier to the Company or any of the products or services being developed, certified, manufactured or sold by the Company or which may be used in relation therewith; or (ii) results from tasks assigned to me by the Company; or (iii) results from the use of premises or personal property (whether tangible or intangible) owned, leased or contracted for by the Company, then all such Developments and the benefits thereof are and shall immediately become the sole and absolute property of the Company and its assigns, as works made for hire or otherwise. The term “Development” shall mean any invention, modification, discovery, design, development, improvement, process, software program, work of authorship, documentation, formula, data, technique, know-how, trade secret, certification or intellectual property right whatsoever or any interest therein (whether or not patentable or registrable under copyright, trademark or similar statutes). I shall promptly disclose to the Company (or any person(s) designated by it) each such Development. I hereby assign all rights (including, but not limited to, rights to inventions, moral rights, patentable subject matter, copyrights and trademarks) I may have or may acquire in any Development and all benefits and/or rights resulting therefrom to the Company and its assigns without further compensation and shall communicate, without cost or delay, and without disclosing to others the same, all available information relating thereto (with all necessary plans and models) to the Company.
(b) I recognize that Developments or Confidential Information relating to my activities while working for the Company and conceived or made by me, alone or with others, within three (3) months after termination of my employment may have been conceived in significant part while employed by the Company. Accordingly, I agree that such Developments and Confidential Information shall be presumed to have been conceived during my employment with the Company and are to be assigned to the Company unless and until I have established the contrary.
5. Further Assurances. I shall, during my employment and at any time thereafter, at the request and cost of the Company, promptly sign, execute, make and do all such deeds, documents, acts and things as the Company and its duly authorized officers may reasonably require:
(a) to apply for, obtain, register and vest in the name of the Company alone (unless the Company otherwise directs) patents, copyrights, trademarks or other analogous protection in any country throughout the world relating to a Development and when so obtained or vested to renew and restore the same; and
(b) to defend any judicial, opposition or other proceedings with respect to such applications and any judicial, opposition or other proceeding, petition or application for revocation of any such patent, copyright, trademark, certifications or other analogous protection.
If the Company is unable, after reasonable effort, to secure my signature on any application for patent, copyright, trademark or other analogous protection or other documents regarding any legal protection relating to a Development, whether because of my physical or mental incapacity or for any other reason whatsoever, I hereby irrevocably designate and appoint the Company and its duly authorized officers and agents as my agent and attorney-in-fact, to act for and in my behalf and stead to execute and file any such application or applications or other documents and to do all other lawfully permitted acts to further the prosecution and issuance of patent, copyright or trademark registrations or any other legal protection thereon with the same legal force and effect as if executed by me.
| 3 |
6. Nonsolicitation of Customers. During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for my termination, I shall not, directly or indirectly, alone or as an owner, member, manager, partner, officer, employee, director, investor, lender, consultant or independent contractor of any entity, call upon, solicit, divert or take away or attempt to divert or take away or do business with, either for myself or for any other person or entity, any current or prospective customers or accounts of the Company that I had contact with, for whom I performed services on behalf of the Company, or about whom I obtained Confidential Information through the Company for the purpose of competing (i.e., by providing the Products and Services, as defined below), whether directly or indirectly, nor shall I make known to any other person or entity, either directly or indirectly, the names and addresses of and other pertinent information relating to any such customers or accounts, or any Confidential Information relating to any of them.
7. Non-solicitation of suppliers. During the period of my employment with the Company and for 12 months following the termination of my employment, regardless of the reasons for my termination, I shall not do or attempt to do anything which causes or may cause any entity which has supplied products or services to the Company to cease, alter or materially to reduce its supplies to the Company or alter its terms of business with and to the detriment of the Company.
8. Noncompetition. During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for my termination, I shall not, anywhere where the Company conducts business directly or indirectly, alone or as an owner, member, manager, partner, officer, agent, employee, director, investor, lender, consultant or independent contractor of any entity, (a) accept employment or establish any other relationship with any business that is in competition, whether directly or indirectly, with the products or services being created, certified, developed, manufactured or planning to be manufactured, marketed or planning to be marketed, distributed or planning to be distributed, or sold or planning to be sold by the Company at the termination of my employment (collectively, the “Products and Services”), or (b) engage in any business or activity that is in competition with the Products and Services. For purposes of this Section, Products and Services shall include, without limitation any products or services substantially similar to the products and services designed, conceived, marketed, distributed or developed by the Company at the time of the termination of my employment. Notwithstanding the foregoing, the record or beneficial ownership by me of five (5) percent (5%) or less of the outstanding publicly traded capital stock of any entity shall not be deemed, in and of itself, to be in violation of this Section 8.
9. Nonsolicitation of Employees.
(a) During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for the termination, I will not, in any manner, directly or indirectly, hire or engage, or assist any company or business organization by which I am employed or which is directly or indirectly controlled by me to hire or engage, any person who is or was employed by the Company (or is or was an agent, representative, contractor, project consultant or consultant of the Company) at the time of my termination or during the period of twelve (12) months prior thereto.
| 4 |
(b) During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for the termination, I will not, in any manner, directly or indirectly, solicit, recruit or induce, or assist any company or business organization by which I am employed or which is directly or indirectly controlled by me to solicit, recruit or induce, any person who is or was employed by the Company (or is or was an agent, representative, contractor, project consultant or consultant of the Company) at the time of my termination or during the period of twelve (12) months prior thereto, to leave his or her employment, relationship or engagement with the Company.
11. Nondisparagement. I agree that at all times during and after my employment with the Company, regardless of the reason for my termination, I shall not disparage the Company, its products, services, agents or employees.
12. Employment Status. I understand that this Nondisclosure Agreement does not constitute an implied or written employment contract.
13. Severability. I hereby agree that each provision and the subparts of each provision herein shall be treated as separate and independent clauses, and the unenforceability of any one clause shall in no way impair the enforceability of any of the other clauses of the Nondisclosure Agreement. Moreover, if one or more of the provisions contained in this Nondisclosure Agreement shall for any reason be held to be excessively broad as to scope, activity, subject or otherwise so as to be unenforceable at law, such provision or provisions shall be construed by the appropriate judicial body by limiting or reducing it or them, so as to be enforceable to the maximum extent compatible with the applicable law as it shall then appear. I hereby further agree that the language of all parts of this Nondisclosure Agreement shall in all cases be construed as a whole according to its fair meaning and not strictly for or against either of the parties.
14. Amendments; Waiver. Any amendment to or modification of this Nondisclosure Agreement, or any waiver of any provision hereof, shall be in writing and signed by the Company and me. Any waiver by the Company of a breach of any provision of this Nondisclosure Agreement shall not operate or be construed as a waiver of any subsequent breach of such provision or any other provision hereof.
15. Survival. This Nondisclosure Agreement shall be effective as of the date entered below. My obligations under this Nondisclosure Agreement shall survive the termination of my employment regardless of the reason for or the manner of such termination and shall be binding upon my heirs, executors, administrators and legal representatives.
16. Assignment. The Company shall have the right to assign this Nondisclosure Agreement to its successors and assigns, and all covenants and agreements hereunder shall inure to the benefit of and be enforceable by said successors or assigns. However, I may not assign this Nondisclosure Agreement.
| 5 |
17. Notification. I agree and acknowledge that during my employment with the Company and for a period of twelve (12) months following the termination of my employment with Company for any reason, I shall inform each new employer I may have, prior to accepting employment, of the existence of this Nondisclosure Agreement, and I shall provide each such employer with a copy of this Nondisclosure Agreement. I also agree and acknowledge that the Company has the right to independently contact any potential or actual future employer of mine to notify the future employer of my obligations under this Nondisclosure Agreement and provide such future employer with a copy of this Nondisclosure Agreement. The Company also shall be entitled to notify such actual or potential future employer of the Company’s understanding of the requirements of this Nondisclosure Agreement and what steps, if any, the Company intends to take to insure compliance with or enforcement of this Nondisclosure Agreement.
18. Representations.
(a) I represent that my employment with the Company and my performance of all of the terms of this Nondisclosure Agreement do not and will not breach any agreement to keep in confidence proprietary information acquired by me in confidence or in trust prior to my employment by the Company. I have not entered into, and I shall not enter into, any agreement either written or oral in conflict herewith.
(b) I further agree that any breach of this Nondisclosure Agreement by me will cause irreparable damage to the Company and that in the event of such breach the Company shall have, in addition to any and all remedies of law, the right to an injunction, specific performance or other equitable relief to prevent the violation of my obligations hereunder. The Company may apply for such injunctive relief in any court of competent jurisdiction without the necessity of posting any bond or other security.
19. Governing Law; Forum Selection Clause. This Nondisclosure Agreement and any claims arising out of this Nondisclosure Agreement (or any other claims arising out of the relationship between the parties) shall be governed by and construed in accordance with the laws of Massachusetts and shall in all respects be interpreted, enforced and governed under the internal and domestic laws thereof, without giving effect to the principles of conflicts of laws of Massachusetts. Any claims or legal actions by one party against the other shall be commenced and maintained in any state or federal court located in Massachusetts and I and the Company each hereby submit to the jurisdiction and venue of any such court.
20. Entire Agreement. This Nondisclosure Agreement and the Employment Agreement executed in connection herewith, set forth the complete, sole and entire agreement between the parties on the subject matter herein and supersede any and all other agreements, negotiations, discussions, proposals, or understandings, whether oral or written, previously entered into, discussed or considered by the parties on the subject matter herein.
***************
| 6 |
IN WITNESS WHEREOF, the undersigned have executed this Nondisclosure Agreement as of the date first set forth above.
| HEMOGLOBIN OXYGEN THERAPEUTICS, LLC | ||||
| By: | By: | |||
| Name: | Igor Serov | Name: | Zafiris G. Zafirelis | |
| Its: | CFO | |||
| 7 |
Exhibit 10.2
HEMOGLOBIN OXYGEN THERAPEUTICS, INC.
January 10, 2022
Igor Serov
63 Westerly Road
Weston, MA 02493
| RE: | Agreement of Employment |
Dear Igor:
We are pleased to offer you continued employment as the full-time position of Chief Financial Officer (“CFO”) of Hemoglobin Oxygen Therapeutics, LLC (the “Company”) as of January 10, 2022. As you know, the Company plans to convert to a C corporation (“Newco”), and upon such conversion, the reference to “Company” herein shall be to Newco. This offer is contingent upon your agreement to execute the Company’s Nondisclosure, Noncompetition and Assignment of Developments Agreement (the “Nondisclosure Agreement”), a copy of which is attached hereto as Exhibit A. This letter agreement (the “Agreement”) summarizes the initial terms of your continued employment.
1. Position and Responsibilities. Upon the terms and conditions herein set forth, you agree to continue to serve as CFO of the Company. In such capacity, you agree to perform the duties and responsibilities required by this position. You shall devote your business time and efforts to the performance of your duties hereunder. You will perform the duties of the CFO and as such shall be responsible for aspects of management, finance, taxation, corporate structure, capital structure, employment matters, and all other aspects of the Company’s business customarily required of a CFO or as so directed by the Company’s Board of Directors (the “Board”). Additionally, subject to stockholder ratification and the charter documents of the Company, you shall be a member of the Board. You shall exercise such powers and comply with and perform, faithfully and to the best of your ability, such directions and duties in relation to the business and affairs of the Company as may from time to time be vested in or requested of you.
If requested by the Board you shall become and remain a director of any subsidiary of the Company as the Board may from time to time request. As a director, you shall at all times comply with the applicable governing documents of the subsidiary of which you are a director.
You shall resign from your position as a director of a subsidiary and/or as a director of the Company on request of the Board in accordance with the applicable Bylaws or immediately upon termination of your employment howsoever arising. You hereby irrevocably appoint each of the Company and the Company’s secretary to be your attorney to execute any such resignation document and generally to use your name for the purpose of giving the Company or its nominee the full benefit of this provision.
You shall perform your services under this Agreement at such locations as may be reasonably required by the Company or the Board. Your primary location of employment will be the Boston, Massachusetts area, but you will be expected to travel from time to time as directed by the Company or the Board, and in particular to the Company’s facility in Souderton, Pennsylvania.
| 1 |
You shall be subject to all policies, codes and rules of the Company in effect from time to time, including but not limited to policies relating to sick leave and holidays, disciplinary and grievance matters, code of ethics, and insider trading, and you agree to comply with such policies.
2. Compensation, Bonuses and Other Benefits. During the term of this Agreement, the Company shall pay you, as compensation for your performance of your duties and obligations hereunder, the following:
(a) Your annual base salary shall be at an initial rate of $350,000, less applicable withholdings, and shall be payable in conformity with the Company’s customary payroll practices, as such practices shall be established or modified from time to time.
(b) During your employment, you may be eligible to receive an annual bonus at the sole discretion of the Board (or a committee thereof), based on the Board’s (or a committee’s) assessment of relevant factors including but not limited to your personal performance, the performance of the Company, and the availability of cash. No bonus under this paragraph shall be payable to you with respect to any calendar year during which your employment is terminated for cause. For the avoidance of doubt, payment of an annual bonus in any one or more years shall not create an entitlement for you to be paid a bonus in any subsequent years, nor will it interfere with the Company’s discretion in any way with respect to the bonus.
(c) You shall be eligible to participate, to the extent applicable, in all employee benefit plans of the Company in effect from time to time, including but not limited to the Company’s health insurance program, disability insurance program (if applicable), and any applicable incentive plan (an “Incentive Plan”). The Company will provide additional information about the health insurance program, disability insurance program (if applicable), and the Incentive Plan upon request. Your participation in the Company’s employee benefit plans shall be subject to (i) the terms of the applicable plan documents, (ii) generally applicable Company policies, and (iii) the discretion of the Company and/or the Board or any administrative or other committee provided for in or contemplated by such plan. The Company’s current plans and policies shall govern all other benefits. The Company may alter, modify, add to, or delete its employee benefit plans at any time as the Company and/or the Board, in their sole judgment, determines to be appropriate. The Company does not currently offer a pension plan benefit.
(d) You shall be eligible to accrue vacation time at the rate of 1.67 days of vacation per full month worked, up to a maximum of 20 days of vacation per calendar year. You may not carry over any unused vacation time from year to year without prior written approval, and you are not entitled to payment in lieu of vacation time. However, you will be paid for accrued but unused vacation time during the year in which your employment terminates.
(e) The Company shall pay or reimburse all reasonable business expenses incurred or paid by you in the performance of your responsibilities hereunder in accordance with the Company’s prevailing policy and practice relating to reimbursements as established, modified or amended from time to time. You must provide substantiation and documentation of these expenses to the Company in order to receive reimbursement.
(f) The Company shall have the right to deduct and withhold from any amounts payable hereunder or pursuant to any other agreements or arrangements between the Company (and any of its affiliates) and you, any federal, state, local, foreign or other taxes (“Taxes”) that the Company determines should be withheld with respect to such payments. In the event that the Company does not withhold the proper amount of Taxes from any such payments, you will make a prompt payment, on demand and in cash, to the Company of the amount under-withheld.
| 2 |
| 4. | Employment Term and Severance. |
(a) You and the Company understand and agree that your employment is as an employee at will, and that you may resign, or the Company may terminate your employment, at any time and for any or for no reason. Nothing in this Agreement shall be construed to alter the at-will nature of your employment, nor shall anything in this Agreement be construed as providing you with a definite term of employment.
(b) In the event your employment with the Company terminates for any reason (including death or Disability (as hereinafter defined)), the Company shall pay to you any base salary including accrued but unused vacation pay, expense reimbursements, compensation and benefits under any applicable plan, and any and all benefits and other similar amounts, accrued but unpaid as of the date of termination. In addition, upon termination of your employment with the Company by the Company without Cause (as defined below) (but excluding death or Disability), contingent upon your execution, your personal representative’s execution and delivery of the Release (as defined below), the Company shall pay to you an amount equal to the greater of (i) your annual base salary in effect immediately prior to termination and (ii) $350,000 (“Severance”), to be paid over a like number of months consistent with the Company’s normal payroll schedule; provided, however, (x) payment of any Severance is contingent upon execution and delivery to the Company of the Release and such Release being effective and not revoked by the end of the sixtieth (60th) day following your date of termination and (y) that in the event of your material breach of the Nondisclosure Agreement, which breach, if reasonably susceptible to cure, has not been cured to the satisfaction of the Company within ten (10) business days of your receipt of written notice of such breach, then the Company’s obligation to pay Severance shall terminate and be of no further force or effect. The payment of Severance shall commence on the sixtieth (60th) day after your date of termination; provided that the Release is effective on such date. Your rights to any Severance shall constitute your sole remedy in the event of termination of your employment. For purposes of this Agreement, your termination of employment shall mean your “separation from service” within the meaning of Treasury Regulation Section 1.409(A)-1(h).
(c) “Cause” shall mean (i) your failure or refusal to render services to the Company in accordance with your obligations under this Agreement; (ii) a determination by the Board that you have refused to follow the lawful instructions of the Board; (iii) gross negligence, dishonesty or breach of fiduciary duty; (iv) the commission of an act of fraud, embezzlement, misappropriation of any money or other assets or property (whether tangible or intangible), deliberate disregard of the rules or policies of the Company, or the commission of any other action which injures the Company which is done not in good faith and without reasonable belief that your action was in the best interest of the Company; (v) being charged with a felony or crime of moral turpitude, which in the determination of the Board after reasonable investigation, may injury the Company or its reputation; or (vi) breach of this Agreement, the Nondisclosure Agreement or any other agreement executed by you in connection with your employment; provided that with respect to the items set forth in clauses (i), (ii), (iii) or (vi), to the extent reasonably curable, you have not cured such reason for Cause within ten (10) business days after a written demand for substantial performance is delivered to you by the Company which specifically identifies the manner in which the Company believes it has the right to terminate you for Cause. For purposes of this Subsection, no act or failure to act on your part shall be deemed “willful” unless done or omitted to be done by you not in good faith and without reasonable belief that your action or omission was in the best interest of the Company.
| 3 |
(d) “Disability” You shall be deemed to have a Disability for purposes of this Agreement either (i) if you are deemed disabled for purposes of any group or individual disability policy paid for by the Company and at the time in effect, or (ii) if, in the good faith judgment of the Board, you are substantially unable to perform your duties under this Agreement for more than ninety (90) days, whether or not consecutive, in any twelve (12) month period, by reason of a physical or mental illness or injury.
(e) “Release” shall mean a waiver and release (including confidentiality and non-disparagement provisions), based on the Company’s standard form or other reasonable form, of any and all claims you may have against the Company and its parents, subsidiaries, affiliates, predecessors and successors, as well as each of their past, present and future stockholders, directors, officers, employees, consultants, representatives, attorneys, insurers, agents, assigns, any other legal entity describing the organizations or through which any of them conducts business, and their employee benefits plans and trustees, fiduciaries, and administrators of those plans.
(f) Payments of Severance to you shall be bifurcated into two portions, consisting of the portion, if any, that includes the maximum amount of the payments that does not constitute “nonqualified deferred compensation” within the meaning of Section 409A of the Code, and the portion, if any, that includes the excess of the total payments that does constitute nonqualified deferred compensation. Payments hereunder shall first be made from the portion that does not consist of nonqualified deferred compensation until such portion is exhausted and then shall be made from the portion that does constitute nonqualified deferred compensation. Notwithstanding the foregoing, if you are a “specified employee” as defined in Section 409A(a)(3)(B)(i) of the Code, the commencement of the delivery of the portion that constitutes nonqualified deferred compensation will be delayed to the date that is 6 months and one day after your termination of employment (the “Earliest Payment Date”). Any payments that are delayed pursuant to the preceding sentence shall be paid pro rata during the period beginning on the Earliest Payment Date and ending on the date that is 6 months following the Earliest Payment Date. The determination of whether, and the extent to which, any of the payments to be made to you hereunder are nonqualified deferred compensation shall be made after the application of all applicable exclusions under Treasury Reg. § 1.409A- 1(b)(9). Any payments that are intended to qualify for the exclusion for separation pay due to involuntary separation from service set forth in Treasury Regulation Section 1.409A-1(b)(9)(iii) must be paid no later than the last day of the second taxable year of you following the taxable year of you in which your termination of employment occurs.
(g) This Agreement is intended to comply with the provisions of Section 409A and the Agreement shall, to the extent practicable, be construed in accordance therewith. Terms defined in the Agreement shall have the meanings given such terms under Section 409A if and to the extent required in order to comply with Section 409A. No payments to be made under this Agreement may be accelerated or deferred except as specifically permitted under Section 409A. In the event that the Agreement shall be deemed not to comply with Section 409A, then neither the Company, the Company’s Board s nor its or their designees or agents shall be liable to you or other person for actions, decisions or determinations made in good faith.
(h) Neither the Company nor you shall have the right to accelerate or defer the delivery of any payments except to the extent specifically permitted or required by Section 409A.
| 4 |
5. Disability. The Company intends to provide disability insurance. You agree to abide by the terms of such insurance policy and any policy of the Company applicable to disabled employees in effect from time to time.
6. Survival of Certain Provisions. Provisions of this Agreement shall survive any termination of employment or termination or expiration of this Agreement if so provided herein or if necessary or desirable to fully accomplish the purposes of such provision. Without limiting the foregoing, your obligations under the Nondisclosure Agreement expressly survive any termination of employment or termination or expiration of this Agreement. The obligation of the Company to make payments to you or on your behalf under Section 4 hereof is expressly conditioned upon your continued full performance of the obligations under the terms of the Nondisclosure Agreement executed herewith between you and the Company.
7. Consent and Waiver by Third Parties. You hereby represent that you have obtained all waivers and/or consents from third parties which are necessary to enable you to enjoy employment with the Company on the terms and conditions set forth herein and to execute and perform this Agreement without being in conflict with any other agreement, obligation or understanding with any such third party. You represent that you are not bound by any agreement or any other existing or previous business relationship which conflicts with, or may conflict with, the performance of your obligations hereunder or prevent the full performance of your duties and obligations hereunder.
8. Governing Law. This Agreement, the employment relationship contemplated herein and any claim arising from such relationship, whether or not arising under this Agreement, shall be governed by and construed in accordance with the internal laws of Massachusetts, without giving effect to the principles of choice of law or conflicts of laws. Any claims or legal actions by one party against the other arising out of the relationship between the parties contemplated herein (whether or not arising under this Agreement) shall be commenced or maintained in any state or federal court located in Delaware, and you and the Company each hereby submit to the jurisdiction and venue of any such court.
9. Severability. In case any one or more of the provisions contained in this Agreement or the other agreements executed in connection with the transactions contemplated hereby for any reason shall be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other provision of this Agreement or such other agreements, but this Agreement or such other agreements, as the case may be, shall be construed and reformed to the maximum extent permitted by law.
10. Waivers and Modifications. No waiver by the Company of any breach by you of any provision hereof shall be deemed to be a waiver of any later or other breach thereof or as a waiver of any other provision of this Agreement. This Agreement and its terms may not be waived, changed, discharged or terminated orally or by any course of dealing between the parties, but only by an instrument in writing signed by the party against whom any waiver, change, discharge or termination is sought. No modification or waiver by the Company shall be effective without the consent of the Board or its designee.
11. Assignment. You acknowledge that the services to be rendered by you hereunder are unique and personal in nature. Accordingly, you may not assign any of your rights or delegate any of your duties or obligations under this Agreement. The rights and obligations of the Company under this Agreement may be assigned by the Company and shall inure to the benefit of, and shall be binding upon, the successors and assigns of the Company.
| 5 |
12. Acknowledgments. You hereby acknowledge and recognize that the enforcement of any of the provisions in this Agreement and the Nondisclosure Agreement executed herewith may potentially interfere with your ability to pursue a proper livelihood. You represent that you are knowledgeable about the business of the Company. You recognize and agree that the enforcement of the Nondisclosure Agreement is necessary to ensure the preservation, protection and continuity of the Company’s business, trade secrets and goodwill. You agree that, due to the proprietary nature of the Company’s business, the restrictions set forth in the Nondisclosure Agreement are reasonable as to time and scope.
13. Entire Agreement. This Agreement and the Nondisclosure Agreement executed herewith, constitute the entire understanding of the parties relating to the subject matter hereof and supersede and cancel all agreements relating to the subject matter hereof, whether written or oral, made prior to the date hereof between you and the Company or any of its affiliates.
14. Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed to be an original, but all of which together shall constitute one and the same instrument. If the foregoing accurately sets forth our agreement, please indicate by signing and returning to us both copies of this Agreement and the enclosed Nondisclosure Agreement. We will return fully- executed copies of both agreements for your records.
Very truly yours,
| HEMOGLOBIN OXYGEN THERAPEUTICS, LLC. | Accepted and Agreed: | ||
| By: |  |
By: |  |
| Name: | Zafiris G. Zafirelis | Name: | Igor Serov |
| Title: | CEO | Date: | January 10, 2022 |
| 6 |
EXHIBIT A
NONDISCLOSURE, NONCOMPETITION AND
ASSIGNMENT OF DEVELOPMENTS AGREEMENT
This Nondisclosure, Noncompetition and Assignment of Developments Agreement (this “Nondisclosure Agreement”) is made as of this ___ day of January, 2022 by and between Igor Serov, an individual, and Hemoglobin Oxygen Therapeutics, LLC. a corporation with offices at 674 Souder Road, Souderton PA 18964, USA (the “Company”).Upon conversion of the Company to a C corporation (“Newco”), references herein to the Company shall be to Newco.
WHEREAS, the Company’s business is conducted throughout the world and its reputation and goodwill are an integral part of its business success;
WHEREAS, my position with the Company shall require that I be trusted with extensive confidential and trade secret information of the Company and that I develop a thorough and comprehensive knowledge of details of the Company’s business, including, but not limited to, information relating to development, inventions, processes, financial and strategic planning, marketing, distribution and licensing of the Company’s products and services. The Company will not provide, and will not agree to continue to provide, me with this information unless I provide the necessary assurances and commitments to protect this information and the Company’s line of business as more fully set forth herein; and
WHEREAS, as an additional material inducement for me to enter into this Nondisclosure Agreement, the Company shall provide me with the Employment Agreement, which is ancillary to the Nondisclosure Agreement and is to be executed in connection herewith (the “Employment Agreement”), which includes eligibility for certain severance benefits as set forth therein.
NOW, THEREFORE, in consideration for the Company providing me with the Employment Agreement, and in consideration for my continued employment by the Company, subject to the policies, procedures and practices that the Company may establish from time to time, the compensation I shall receive from the Company during my employment and other good and valuable consideration, and as a material inducement for the Company to disclose to me in connection with its business certain confidential, proprietary and/or trade secret information described below, and intending to be legally bound, I hereby agree as follows:
1. Best Efforts. During the period of my employment by the Company, I shall devote my full time and best efforts to the business of the Company during normal and other customary business hours, and I shall not engage in any other business activity, whether or not such business activity is pursued for gain, profit, or other pecuniary advantage, which may tend to (i) interfere with the performance of any job duties and responsibilities assigned to me by the Company, (ii) create a conflict of interest, or (iii) be competitive with the business activities of the Company; provided that I may, to the extent not otherwise prohibited by this Agreement (including without limitation Section 8), devote such amount of time as does not interfere or compete with the performance of my duties under the Employment Agreement to any one or more of the following activities: (i) investing my personal assets in the record or beneficial ownership of five percent (5%) or less of the outstanding publicly traded capital stock of any entity; or (ii) engaging in charitable activities, including serving on the Boards of Directors of charitable organizations.
2. Nondisclosure Obligation. I shall not at any time, whether during or after the termination of my employment, reveal to any person or entity any Confidential Information, except to employees, authorized agents, and professional advisors of the Company who need to know such Confidential Information for the purposes of their employment or affiliation with the Company and other third parties, or as otherwise authorized by the Company. The term “Confidential Information” shall include any information concerning the organization, business or finances of the Company or of any third party that the Company is under an obligation to keep confidential or that is maintained by the Company as confidential. Such Confidential Information shall include, but is not limited to, trade secrets, proprietary or confidential information respecting the identity of the Company’s customers; the identity of distributors and suppliers of the Company; the identity of representatives responsible for entering into contracts with the Company; specific customer, distributor and supplier needs and requirements; the details of contracts and proposals between the Company and its customers, distributors and suppliers; selling and marketing strategies, prices, costs and profit margins; the names, addresses and other contact information of purchasing agents, vendors or other entities; purchasing techniques, methods, procedures and processes; manufacturing and production techniques, methods, procedures and processes; other techniques, methodologies and processes used by the Company in the conduct of its business; techniques, methods, procedures, know-how, blueprints, engineering data, prototypes and technical specifications; computer data, software, software codes, computer models, works of authorship, research projects, data processing and other programs; production and manufacturing equipment and operating practices; information with respect to products and product formulae, designs, plans for future business, new business, products or other developments; new or innovative ideas, customer proposals, marketing plans and ideas, and future developments or strategies; information pertaining to research and development, acquisitions or divestitures, marketing and sales, cost cutting, revenue generation, or other matters concerning the Company’s planning and strategy; personnel information, and other nonpublic financial and other information of the Company disclosed to or known by me as a consequence of or through my employment (or other service relationship) with the Company (including information conceived, originated, discovered or developed by me), which information is not generally known in the relevant trade or industry or public knowledge. I shall keep confidential all matters entrusted to me and shall not use or attempt to use any Confidential Information except as may be required in the ordinary course of performing my duties as an employee of the Company, nor shall I use any Confidential Information in any manner which may injure or cause loss or may be calculated to injure or cause loss to the Company, whether directly or indirectly.
3. Company Property. I agree that during my employment, I shall not make, use or permit to be used any Company Property otherwise than for the benefit of the Company. The term “Company Property” shall include all notes, memoranda, reports, lists, cost sheets, records, drawings, designs, sketches, specifications, blue prints, prototypes, estimates, databases, software programs, software code, data, proprietary processes, computers, cellular telephones, pagers, credit and/or calling cards, keys, access cards, documentation, automobiles or other equipment or materials of any nature and in any form, whether written, printed, electronic or in digital format or otherwise, relating to any matter within the scope of the business of the Company or concerning any of its dealings or affairs and any other Company property in my possession, custody or control. I further agree that I shall not, after the termination of my employment, use or permit others to use any such Company Property. I acknowledge and agree that all Company Property shall be and remain the sole and exclusive property of the Company. Immediately upon the termination of my employment, I shall deliver all Company Property in my possession, and all copies thereof, to the Company.
| 2 |
| 4. | Assignment of Developments. |
(a) If at any time during my employment, I shall (either alone or with others) make, conceive, create, certify, discover, invent or reduce to practice any Development (as defined below) that
(í) relates to the business of the Company or any customer of or supplier to the Company or any of the products or services being developed, certified, manufactured or sold by the Company or which may be used in relation therewith; or (ii) results from tasks assigned to me by the Company; or (iii) results from the use of premises or personal property (whether tangible or intangible) owned, leased or contracted for by the Company, then all such Developments and the benefits thereof are and shall immediately become the sole and absolute property of the Company and its assigns, as works made for hire or otherwise. The term “Development” shall mean any invention, modification, discovery, design, development, improvement, process, software program, work of authorship, documentation, formula, data, technique, know-how, trade secret, certification or intellectual property right whatsoever or any interest therein (whether or not patentable or registrable under copyright, trademark or similar statutes). I shall promptly disclose to the Company (or any person(s) designated by it) each such Development. I hereby assign all rights (including, but not limited to, rights to inventions, moral rights, patentable subject matter, copyrights and trademarks) I may have or may acquire in any Development and all benefits and/or rights resulting therefrom to the Company and its assigns without further compensation and shall communicate, without cost or delay, and without disclosing to others the same, all available information relating thereto (with all necessary plans and models) to the Company.
(b) I recognize that Developments or Confidential Information relating to my activities while working for the Company and conceived or made by me, alone or with others, within three (3) months after termination of my employment may have been conceived in significant part while employed by the Company. Accordingly, I agree that such Developments and Confidential Information shall be presumed to have been conceived during my employment with the Company and are to be assigned to the Company unless and until I have established the contrary.
5. Further Assurances. I shall, during my employment and at any time thereafter, at the request and cost of the Company, promptly sign, execute, make and do all such deeds, documents, acts and things as the Company and its duly authorized officers may reasonably require:
(a) to apply for, obtain, register and vest in the name of the Company alone (unless the Company otherwise directs) patents, copyrights, trademarks or other analogous protection in any country throughout the world relating to a Development and when so obtained or vested to renew and restore the same; and
(b) to defend any judicial, opposition or other proceedings with respect to such applications and any judicial, opposition or other proceeding, petition or application for revocation of any such patent, copyright, trademark, certifications or other analogous protection.
If the Company is unable, after reasonable effort, to secure my signature on any application for patent, copyright, trademark or other analogous protection or other documents regarding any legal protection relating to a Development, whether because of my physical or mental incapacity or for any other reason whatsoever, I hereby irrevocably designate and appoint the Company and its duly authorized officers and agents as my agent and attorney-in-fact, to act for and in my behalf and stead to execute and file any such application or applications or other documents and to do all other lawfully permitted acts to further the prosecution and issuance of patent, copyright or trademark registrations or any other legal protection thereon with the same legal force and effect as if executed by me.
| 3 |
6. Nonsolicitation of Customers. During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for my termination, I shall not, directly or indirectly, alone or as an owner, member, manager, partner, officer, employee, director, investor, lender, consultant or independent contractor of any entity, call upon, solicit, divert or take away or attempt to divert or take away or do business with, either for myself or for any other person or entity, any current or prospective customers or accounts of the Company that I had contact with, for whom I performed services on behalf of the Company, or about whom I obtained Confidential Information through the Company for the purpose of competing (i.e., by providing the Products and Services, as defined below), whether directly or indirectly, nor shall I make known to any other person or entity, either directly or indirectly, the names and addresses of and other pertinent information relating to any such customers or accounts, or any Confidential Information relating to any of them.
7. Non-solicitation of suppliers. During the period of my employment with the Company and for 12 months following the termination of my employment, regardless of the reasons for my termination, I shall not do or attempt to do anything which causes or may cause any entity which has supplied products or services to the Company to cease, alter or materially to reduce its supplies to the Company or alter its terms of business with and to the detriment of the Company.
8. Noncompetition. During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for my termination, I shall not, anywhere where the Company conducts business directly or indirectly, alone or as an owner, member, manager, partner, officer, agent, employee, director, investor, lender, consultant or independent contractor of any entity, (a) accept employment or establish any other relationship with any business that is in competition, whether directly or indirectly, with the products or services being created, certified, developed, manufactured or planning to be manufactured, marketed or planning to be marketed, distributed or planning to be distributed, or sold or planning to be sold by the Company at the termination of my employment (collectively, the “Products and Services”), or (b) engage in any business or activity that is in competition with the Products and Services. For purposes of this Section, Products and Services shall include, without limitation any products or services substantially similar to the products and services designed, conceived, marketed, distributed or developed by the Company at the time of the termination of my employment. Notwithstanding the foregoing, the record or beneficial ownership by me of five (5) percent (5%) or less of the outstanding publicly traded capital stock of any entity shall not be deemed, in and of itself, to be in violation of this Section 8.
| 9. | Nonsolicitation of Employees. |
(a) During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for the termination, I will not, in any manner, directly or indirectly, hire or engage, or assist any company or business organization by which I am employed or which is directly or indirectly controlled by me to hire or engage, any person who is or was employed by the Company (or is or was an agent, representative, contractor, project consultant or consultant of the Company) at the time of my termination or during the period of twelve (12) months prior thereto.
| 4 |
(b) During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for the termination, I will not, in any manner, directly or indirectly, solicit, recruit or induce, or assist any company or business organization by which I am employed or which is directly or indirectly controlled by me to solicit, recruit or induce, any person who is or was employed by the Company (or is or was an agent, representative, contractor, project consultant or consultant of the Company) at the time of my termination or during the period of twelve (12) months prior thereto, to leave his or her employment, relationship or engagement with the Company.
11. Nondisparagement. I agree that at all times during and after my employment with the Company, regardless of the reason for my termination, I shall not disparage the Company, its products, services, agents or employees.
12. Employment Status. I understand that this Nondisclosure Agreement does not constitute an implied or written employment contract.
13. Severability. I hereby agree that each provision and the subparts of each provision herein shall be treated as separate and independent clauses, and the unenforceability of any one clause shall in no way impair the enforceability of any of the other clauses of the Nondisclosure Agreement. Moreover, if one or more of the provisions contained in this Nondisclosure Agreement shall for any reason be held to be excessively broad as to scope, activity, subject or otherwise so as to be unenforceable at law, such provision or provisions shall be construed by the appropriate judicial body by limiting or reducing it or them, so as to be enforceable to the maximum extent compatible with the applicable law as it shall then appear. I hereby further agree that the language of all parts of this Nondisclosure Agreement shall in all cases be construed as a whole according to its fair meaning and not strictly for or against either of the parties.
14. Amendments; Waiver. Any amendment to or modification of this Nondisclosure Agreement, or any waiver of any provision hereof, shall be in writing and signed by the Company and me. Any waiver by the Company of a breach of any provision of this Nondisclosure Agreement shall not operate or be construed as a waiver of any subsequent breach of such provision or any other provision hereof.
15. Survival. This Nondisclosure Agreement shall be effective as of the date entered below. My obligations under this Nondisclosure Agreement shall survive the termination of my employment regardless of the reason for or the manner of such termination and shall be binding upon my heirs, executors, administrators and legal representatives.
16. Assignment. The Company shall have the right to assign this Nondisclosure Agreement to its successors and assigns, and all covenants and agreements hereunder shall inure to the benefit of and be enforceable by said successors or assigns. However, I may not assign this Nondisclosure Agreement.
17. Notification. I agree and acknowledge that during my employment with the Company and for a period of twelve (12) months following the termination of my employment with Company for any reason, I shall inform each new employer I may have, prior to accepting employment, of the existence of this Nondisclosure Agreement, and I shall provide each such employer with a copy of this Nondisclosure Agreement. I also agree and acknowledge that the Company has the right to independently contact any potential or actual future employer of mine to notify the future employer of my obligations under this Nondisclosure Agreement and provide such future employer with a copy of this Nondisclosure Agreement. The Company also shall be entitled to notify such actual or potential future employer of the Company’s understanding of the requirements of this Nondisclosure Agreement and what steps, if any, the Company intends to take to insure compliance with or enforcement of this Nondisclosure Agreement.
| 5 |
| 18. | Representations. |
(a) I represent that my employment with the Company and my performance of all of the terms of this Nondisclosure Agreement do not and will not breach any agreement to keep in confidence proprietary information acquired by me in confidence or in trust prior to my employment by the Company. I have not entered into, and I shall not enter into, any agreement either written or oral in conflict herewith.
(b) I further agree that any breach of this Nondisclosure Agreement by me will cause irreparable damage to the Company and that in the event of such breach the Company shall have, in addition to any and all remedies of law, the right to an injunction, specific performance or other equitable relief to prevent the violation of my obligations hereunder. The Company may apply for such injunctive relief in any court of competent jurisdiction without the necessity of posting any bond or other security.
19. Governing Law; Forum Selection Clause. This Nondisclosure Agreement and any claims arising out of this Nondisclosure Agreement (or any other claims arising out of the relationship between the parties) shall be governed by and construed in accordance with the laws of Massachusetts and shall in all respects be interpreted, enforced and governed under the internal and domestic laws thereof, without giving effect to the principles of conflicts of laws of Massachusetts. Any claims or legal actions by one party against the other shall be commenced and maintained in any state or federal court located in Massachusetts and I and the Company each hereby submit to the jurisdiction and venue of any such court.
20. Entire Agreement. This Nondisclosure Agreement and the Employment Agreement executed in connection herewith, set forth the complete, sole and entire agreement between the parties on the subject matter herein and supersede any and all other agreements, negotiations, discussions, proposals, or understandings, whether oral or written, previously entered into, discussed or considered by the parties on the subject matter herein.
***************
| 6 |
IN WITNESS WHEREOF, the undersigned have executed this Nondisclosure Agreement as of the date first set forth above.
| HEMOGLOBIN OXYGEN THERAPEUTICS, LLC | |||
| By: |  |
By: |  |
| Name: | Zafiris G. Zafirelis | Name: | Igor Serov |
| Its: | CEO | ||
| 7 |
Exhibit 10.3
HEMOGLOBIN OXYGEN THERAPEUTICS, INC.
January 31, 2022
Joseph Rappold
[Street address]
Portland, ME [Zip]
RE: Agreement of Employment
Dear Joe:
We are pleased to offer you continued employment as the full-time position of Chief Medical Officer (“CMO”) of Hemoglobin Oxygen Therapeutics, LLC (the “Company”) as of August 1, 2022. As you know, the Company plans to convert to a C corporation (“Newco”), and upon such conversion, the reference to “Company” herein shall be to Newco. This offer is contingent upon the Company successfully closing its initial public offering, and your agreement to execute the Company’s Nondisclosure, Noncompetition and Assignment of Developments Agreement (the “Nondisclosure Agreement”), a copy of which is attached hereto as Exhibit A. This letter agreement (the “Agreement”) summarizes the initial terms of your continued employment.
1. Position and Responsibilities. Upon the terms and conditions herein set forth, you agree to continue to serve as CMO of the Company. In such capacity, you agree to perform the duties and responsibilities required by this position. You shall devote your business time and efforts to the performance of your duties hereunder. You will perform the duties of the CMO and as such shall be responsible for aspects of management, clinical development, product research and development, corporate structure, and all other aspects of the Company’s business customarily required of a CMO or as so directed by the Company’s Chief Executive Officer.
You shall resign from your position as a director of a subsidiary and/or as a director of the Company on request of the Board in accordance with the applicable Bylaws or immediately upon termination of your employment howsoever arising. You hereby irrevocably appoint each of the Company and the Company’s secretary to be your attorney to execute any such resignation document and generally to use your name for the purpose of giving the Company or its nominee the full benefit of this provision.
You shall perform your services under this Agreement at such locations as may be reasonably required by the Company or the Board. Your primary location of employment will be the Boston, Massachusetts area, but you will be expected to travel from time to time as directed by the Company CEO, and in particular to Clinical Sites and the Company’s facility in Souderton, Pennsylvania.
You shall be subject to all policies, codes and rules of the Company in effect from time to time, including but not limited to policies relating to sick leave and holidays, disciplinary and grievance matters, code of ethics, and insider trading, and you agree to comply with such policies.
| 1 |
2. Compensation, Bonuses and Other Benefits. During the term of this Agreement, the Company shall pay you, as compensation for your performance of your duties and obligations hereunder, the following:
(a) Your annual base salary shall be at an initial rate of $350,000, less applicable withholdings, and shall be payable in conformity with the Company’s customary payroll practices, as such practices shall be established or modified from time to time.
(b) During your employment, you may be eligible to receive an annual bonus at the sole discretion of the Board (or a committee thereof), based on the Board’s (or a committee’s) assessment of relevant factors including but not limited to your personal performance, the performance of the Company, and the availability of cash. No bonus under this paragraph shall be payable to you with respect to any calendar year during which your employment is terminated for cause. For the avoidance of doubt, payment of an annual bonus in any one or more years shall not create an entitlement for you to be paid a bonus in any subsequent years, nor will it interfere with the Company’s discretion in any way with respect to the bonus.
(c) You shall be eligible to participate, to the extent applicable, in all employee benefit plans of the Company in effect from time to time, including but not limited to the Company’s health insurance program, disability insurance program (if applicable), and any applicable incentive plan (an “Incentive Plan”). The Company will provide additional information about the health insurance program, disability insurance program (if applicable), and the Incentive Plan upon request. Your participation in the Company’s employee benefit plans shall be subject to (i) the terms of the applicable plan documents, (ii) generally applicable Company policies, and (iii) the discretion of the Company and/or the Board or any administrative or other committee provided for in or contemplated by such plan. The Company’s current plans and policies shall govern all other benefits. The Company may alter, modify, add to, or delete its employee benefit plans at any time as the Company and/or the Board, in their sole judgment, determines to be appropriate. The Company does not currently offer a pension plan benefit.
(d) You shall be eligible to accrue vacation time at the rate of 1.67 days of vacation per full month worked, up to a maximum of 20 days of vacation per calendar year. You may not carry over any unused vacation time from year to year without prior written approval, and you are not entitled to payment in lieu of vacation time. However, you will be paid for accrued but unused vacation time during the year in which your employment terminates.
(e) The Company shall pay or reimburse all reasonable business expenses incurred or paid by you in the performance of your responsibilities hereunder in accordance with the Company’s prevailing policy and practice relating to reimbursements as established, modified or amended from time to time. You must provide substantiation and documentation of these expenses to the Company in order to receive reimbursement.
(f) The Company shall have the right to deduct and withhold from any amounts payable hereunder or pursuant to any other agreements or arrangements between the Company (and any of its affiliates) and you, any federal, state, local, foreign or other taxes (“Taxes”) that the Company determines should be withheld with respect to such payments. In the event that the Company does not withhold the proper amount of Taxes from any such payments, you will make a prompt payment, on demand and in cash, to the Company of the amount under-withheld.
| 2 |
4. Employment Term and Severance.
(a) You and the Company understand and agree that your employment is as an employee at will, and that you may resign, or the Company may terminate your employment, at any time and for any or for no reason. Nothing in this Agreement shall be construed to alter the at-will nature of your employment, nor shall anything in this Agreement be construed as providing you with a definite term of employment.
(b) In the event your employment with the Company terminates for any reason (including death or Disability (as hereinafter defined)), the Company shall pay to you any base salary including accrued but unused vacation pay, expense reimbursements, compensation and benefits under any applicable plan, and any and all benefits and other similar amounts, accrued but unpaid as of the date of termination. In addition, upon termination of your employment with the Company by the Company without Cause (as defined below) (but excluding death or Disability), contingent upon your execution, your personal representative’s execution and delivery of the Release (as defined below), the Company shall pay to you an amount equal to the greater of (i) your annual base salary in effect immediately prior to termination and (ii) $350,000 (“Severance”), to be paid over a like number of months consistent with the Company’s normal payroll schedule; provided, however, (x) payment of any Severance is contingent upon execution and delivery to the Company of the Release and such Release being effective and not revoked by the end of the sixtieth (60th) day following your date of termination and (y) that in the event of your material breach of the Nondisclosure Agreement, which breach, if reasonably susceptible to cure, has not been cured to the satisfaction of the Company within ten (10) business days of your receipt of written notice of such breach, then the Company’s obligation to pay Severance shall terminate and be of no further force or effect. The payment of Severance shall commence on the sixtieth (60th) day after your date of termination; provided that the Release is effective on such date. Your rights to any Severance shall constitute your sole remedy in the event of termination of your employment. For purposes of this Agreement, your termination of employment shall mean your “separation from service” within the meaning of Treasury Regulation Section 1.409(A)-1(h).
(c) “Cause” shall mean (i) your failure or refusal to render services to the Company in accordance with your obligations under this Agreement; (ii) a determination by the Board that you have refused to follow the lawful instructions of the Board; (iii) gross negligence, dishonesty or breach of fiduciary duty; (iv) the commission of an act of fraud, embezzlement, misappropriation of any money or other assets or property (whether tangible or intangible), deliberate disregard of the rules or policies of the Company, or the commission of any other action which injures the Company which is done not in good faith and without reasonable belief that your action was in the best interest of the Company; (v) being charged with a felony or crime of moral turpitude, which in the determination of the Board after reasonable investigation, may injury the Company or its reputation; or (vi) breach of this Agreement, the Nondisclosure Agreement or any other agreement executed by you in connection with your employment; provided that with respect to the items set forth in clauses (i), (ii), (iii) or (vi), to the extent reasonably curable, you have not cured such reason for Cause within ten (10) business days after a written demand for substantial performance is delivered to you by the Company which specifically identifies the manner in which the Company believes it has the right to terminate you for Cause. For purposes of this Subsection, no act or failure to act on your part shall be deemed “willful” unless done or omitted to be done by you not in good faith and without reasonable belief that your action or omission was in the best interest of the Company.
| 3 |
(d) “Disability” You shall be deemed to have a Disability for purposes of this Agreement either (i) if you are deemed disabled for purposes of any group or individual disability policy paid for by the Company and at the time in effect, or (ii) if, in the good faith judgment of the Board, you are substantially unable to perform your duties under this Agreement for more than ninety (90) days, whether or not consecutive, in any twelve (12) month period, by reason of a physical or mental illness or injury.
(e) “Release” shall mean a waiver and release (including confidentiality and non- disparagement provisions), based on the Company’s standard form or other reasonable form, of any and all claims you may have against the Company and its parents, subsidiaries, affiliates, predecessors and successors, as well as each of their past, present and future stockholders, directors, officers, employees, consultants, representatives, attorneys, insurers, agents, assigns, any other legal entity describing the organizations or through which any of them conducts business, and their employee benefits plans and trustees, fiduciaries, and administrators of those plans.
(f) Payments of Severance to you shall be bifurcated into two portions, consisting of the portion, if any, that includes the maximum amount of the payments that does not constitute “nonqualified deferred compensation” within the meaning of Section 409A of the Code, and the portion, if any, that includes the excess of the total payments that does constitute nonqualified deferred compensation. Payments hereunder shall first be made from the portion that does not consist of nonqualified deferred compensation until such portion is exhausted and then shall be made from the portion that does constitute nonqualified deferred compensation. Notwithstanding the foregoing, if you are a “specified employee” as defined in Section 409A(a)(3)(B)(i) of the Code, the commencement of the delivery of the portion that constitutes nonqualified deferred compensation will be delayed to the date that is 6 months and one day after your termination of employment (the “Earliest Payment Date”). Any payments that are delayed pursuant to the preceding sentence shall be paid pro rata during the period beginning on the Earliest Payment Date and ending on the date that is 6 months following the Earliest Payment Date. The determination of whether, and the extent to which, any of the payments to be made to you hereunder are nonqualified deferred compensation shall be made after the application of all applicable exclusions under Treasury Reg. § 1.409A-1(b)(9). Any payments that are intended to qualify for the exclusion for separation pay due to involuntary separation from service set forth in Treasury Regulation Section 1.409A-1(b)(9)(iii) must be paid no later than the last day of the second taxable year of you following the taxable year of you in which your termination of employment occurs.
(g) This Agreement is intended to comply with the provisions of Section 409A and the Agreement shall, to the extent practicable, be construed in accordance therewith. Terms defined in the Agreement shall have the meanings given such terms under Section 409A if and to the extent required in order to comply with Section 409A. No payments to be made under this Agreement may be accelerated or deferred except as specifically permitted under Section 409A. In the event that the Agreement shall be deemed not to comply with Section 409A, then neither the Company, the Company’s Board s nor its or their designees or agents shall be liable to you or other person for actions, decisions or determinations made in good faith.
(h) Neither the Company nor you shall have the right to accelerate or defer the delivery of any payments except to the extent specifically permitted or required by Section 409A.
5. Disability. The Company intends to provide disability insurance. You agree to abide by the terms of such insurance policy and any policy of the Company applicable to disabled employees in effect from time to time.
| 4 |
6. Survival of Certain Provisions. Provisions of this Agreement shall survive any termination of employment or termination or expiration of this Agreement if so provided herein or if necessary or desirable to fully accomplish the purposes of such provision. Without limiting the foregoing, your obligations under the Nondisclosure Agreement expressly survive any termination of employment or termination or expiration of this Agreement. The obligation of the Company to make payments to you or on your behalf under Section 4 hereof is expressly conditioned upon your continued full performance of the obligations under the terms of the Nondisclosure Agreement executed herewith between you and the Company.
7. Consent and Waiver by Third Parties. You hereby represent that you have obtained all waivers and/or consents from third parties which are necessary to enable you to enjoy employment with the Company on the terms and conditions set forth herein and to execute and perform this Agreement without being in conflict with any other agreement, obligation or understanding with any such third party. You represent that you are not bound by any agreement or any other existing or previous business relationship which conflicts with, or may conflict with, the performance of your obligations hereunder or prevent the full performance of your duties and obligations hereunder.
8. Governing Law. This Agreement, the employment relationship contemplated herein and any claim arising from such relationship, whether or not arising under this Agreement, shall be governed by and construed in accordance with the internal laws of Massachusetts, without giving effect to the principles of choice of law or conflicts of laws. Any claims or legal actions by one party against the other arising out of the relationship between the parties contemplated herein (whether or not arising under this Agreement) shall be commenced or maintained in any state or federal court located in Delaware, and you and the Company each hereby submit to the jurisdiction and venue of any such court.
9. Severability. In case any one or more of the provisions contained in this Agreement or the other agreements executed in connection with the transactions contemplated hereby for any reason shall be held to be invalid, illegal or unenforceable in any respect, such invalidity, illegality or unenforceability shall not affect any other provision of this Agreement or such other agreements, but this Agreement or such other agreements, as the case may be, shall be construed and reformed to the maximum extent permitted by law.
10. Waivers and Modifications. No waiver by the Company of any breach by you of any provision hereof shall be deemed to be a waiver of any later or other breach thereof or as a waiver of any other provision of this Agreement. This Agreement and its terms may not be waived, changed, discharged or terminated orally or by any course of dealing between the parties, but only by an instrument in writing signed by the party against whom any waiver, change, discharge or termination is sought. No modification or waiver by the Company shall be effective without the consent of the Board or its designee.
11. Assignment. You acknowledge that the services to be rendered by you hereunder are unique and personal in nature. Accordingly, you may not assign any of your rights or delegate any of your duties or obligations under this Agreement. The rights and obligations of the Company under this Agreement may be assigned by the Company and shall inure to the benefit of, and shall be binding upon, the successors and assigns of the Company.
| 5 |
12. Acknowledgments. You hereby acknowledge and recognize that the enforcement of any of the provisions in this Agreement and the Nondisclosure Agreement executed herewith may potentially interfere with your ability to pursue a proper livelihood. You represent that you are knowledgeable about the business of the Company. You recognize and agree that the enforcement of the Nondisclosure Agreement is necessary to ensure the preservation, protection and continuity of the Company’s business, trade secrets and goodwill. You agree that, due to the proprietary nature of the Company’s business, the restrictions set forth in the Nondisclosure Agreement are reasonable as to time and scope.
13. Entire Agreement. This Agreement and the Nondisclosure Agreement executed herewith, constitute the entire understanding of the parties relating to the subject matter hereof and supersede and cancel all agreements relating to the subject matter hereof, whether written or oral, made prior to the date hereof between you and the Company or any of its affiliates.
14. Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed to be an original, but all of which together shall constitute one and the same instrument. If the foregoing accurately sets forth our agreement, please indicate by signing and returning to us both copies of this Agreement and the enclosed Nondisclosure Agreement. We will return fully- executed copies of both agreements for your records.
Very truly yours,
| HEMOGLOBIN OXYGEN THERAPEUTICS, LLC. | Accepted and Agreed: | |||
| By: | By: | |||
| Name: | Zafiris G. Zafirelis | Name: | Joseph Rappold | |
| Title: | CEO | Date: | 26 Mar 2022 | |
| 6 |
EXHIBIT A
NONDISCLOSURE,
NONCOMPETITION AND
ASSIGNMENT OF DEVELOPMENTS AGREEMENT
This Nondisclosure, Noncompetition and Assignment of Developments Agreement (this “Nondisclosure Agreement”) is made as of this 31 day of January, 2022 by and between Joseph Rappold, an individual, and Hemoglobin Oxygen Therapeutics, LLC. a corporation with offices at 674 Souder Road, Souderton PA 18964, USA (the “Company”). Upon conversion of the Company to a C corporation (“Newco”), references herein to the Company shall be to Newco.
WHEREAS, the Company’s business is conducted throughout the world and its reputation and goodwill are an integral part of its business success;
WHEREAS, my position with the Company shall require that I be trusted with extensive confidential and trade secret information of the Company and that I develop a thorough and comprehensive knowledge of details of the Company’s business, including, but not limited to, information relating to development, inventions, processes, financial and strategic planning, marketing, distribution and licensing of the Company’s products and services. The Company will not provide, and will not agree to continue to provide, me with this information unless I provide the necessary assurances and commitments to protect this information and the Company’s line of business as more fully set forth herein; and
WHEREAS, as an additional material inducement for me to enter into this Nondisclosure Agreement, the Company shall provide me with the Employment Agreement, which is ancillary to the Nondisclosure Agreement and is to be executed in connection herewith (the “Employment Agreement”), which includes eligibility for certain severance benefits as set forth therein.
NOW, THEREFORE, in consideration for the Company providing me with the Employment Agreement, and in consideration for my continued employment by the Company, subject to the policies, procedures and practices that the Company may establish from time to time, the compensation I shall receive from the Company during my employment and other good and valuable consideration, and as a material inducement for the Company to disclose to me in connection with its business certain confidential, proprietary and/or trade secret information described below, and intending to be legally bound, I hereby agree as follows:
1. Best Efforts. During the period of my employment by the Company, I shall devote my full time and best efforts to the business of the Company during normal and other customary business hours, and I shall not engage in any other business activity, whether or not such business activity is pursued for gain, profit, or other pecuniary advantage, which may tend to (i) interfere with the performance of any job duties and responsibilities assigned to me by the Company, (ii) create a conflict of interest, or (iii) be competitive with the business activities of the Company; provided that I may, to the extent not otherwise prohibited by this Agreement (including without limitation Section 8), devote such amount of time as does not interfere or compete with the performance of my duties under the Employment Agreement to any one or more of the following activities: (i) investing my personal assets in the record or beneficial ownership of five percent (5%) or less of the outstanding publicly traded capital stock of any entity; or (ii) engaging in charitable activities, including serving on the Boards of Directors of charitable organizations.
2. Nondisclosure Obligation. I shall not at any time, whether during or after the termination of my employment, reveal to any person or entity any Confidential Information, except to employees, authorized agents, and professional advisors of the Company who need to know such Confidential Information for the purposes of their employment or affiliation with the Company and other third parties, or as otherwise authorized by the Company. The term “Confidential Information” shall include any information concerning the organization, business or finances of the Company or of any third party that the Company is under an obligation to keep confidential or that is maintained by the Company as confidential. Such Confidential Information shall include, but is not limited to, trade secrets, proprietary or confidential information respecting the identity of the Company’s customers; the identity of distributors and suppliers of the Company; the identity of representatives responsible for entering into contracts with the Company; specific customer, distributor and supplier needs and requirements; the details of contracts and proposals between the Company and its customers, distributors and suppliers; selling and marketing strategies, prices, costs and profit margins; the names, addresses and other contact information of purchasing agents, vendors or other entities; purchasing techniques, methods, procedures and processes; manufacturing and production techniques, methods, procedures and processes; other techniques, methodologies and processes used by the Company in the conduct of its business; techniques, methods, procedures, know-how, blueprints, engineering data, prototypes and technical specifications; computer data, software, software codes, computer models, works of authorship, research projects, data processing and other programs; production and manufacturing equipment and operating practices; information with respect to products and product formulae, designs, plans for future business, new business, products or other developments; new or innovative ideas, customer proposals, marketing plans and ideas, and future developments or strategies; information pertaining to research and development, acquisitions or divestitures, marketing and sales, cost cutting, revenue generation, or other matters concerning the Company’s planning and strategy; personnel information, and other nonpublic financial and other information of the Company disclosed to or known by me as a consequence of or through my employment (or other service relationship) with the Company (including information conceived, originated, discovered or developed by me), which information is not generally known in the relevant trade or industry or public knowledge. I shall keep confidential all matters entrusted to me and shall not use or attempt to use any Confidential Information except as may be required in the ordinary course of performing my duties as an employee of the Company, nor shall I use any Confidential Information in any manner which may injure or cause loss or may be calculated to injure or cause loss to the Company, whether directly or indirectly.
3. Company Property. I agree that during my employment, I shall not make, use or permit to be used any Company Property otherwise than for the benefit of the Company. The term “Company Property” shall include all notes, memoranda, reports, lists, cost sheets, records, drawings, designs, sketches, specifications, blue prints, prototypes, estimates, databases, software programs, software code, data, proprietary processes, computers, cellular telephones, pagers, credit and/or calling cards, keys, access cards, documentation, automobiles or other equipment or materials of any nature and in any form, whether written, printed, electronic or in digital format or otherwise, relating to any matter within the scope of the business of the Company or concerning any of its dealings or affairs and any other Company property in my possession, custody or control. I further agree that I shall not, after the termination of my employment, use or permit others to use any such Company Property. I acknowledge and agree that all Company Property shall be and remain the sole and exclusive property of the Company. Immediately upon the termination of my employment, I shall deliver all Company Property in my possession, and all copies thereof, to the Company.
| 2 |
4. Assignment of Developments.
(a) If at any time during my employment, I shall (either alone or with others) make, conceive, create, certify, discover, invent or reduce to practice any Development (as defined below) that (i) relates to the business of the Company or any customer of or supplier to the Company or any of the products or services being developed, certified, manufactured or sold by the Company or which may be used in relation therewith; or (ii) results from tasks assigned to me by the Company; or (iii) results from the use of premises or personal property (whether tangible or intangible) owned, leased or contracted for by the Company, then all such Developments and the benefits thereof are and shall immediately become the sole and absolute property of the Company and its assigns, as works made for hire or otherwise. The term “Development” shall mean any invention, modification, discovery, design, development, improvement, process, software program, work of authorship, documentation, formula, data, technique, know-how, trade secret, certification or intellectual property right whatsoever or any interest therein (whether or not patentable or registrable under copyright, trademark or similar statutes). I shall promptly disclose to the Company (or any person(s) designated by it) each such Development. I hereby assign all rights (including, but not limited to, rights to inventions, moral rights, patentable subject matter, copyrights and trademarks) I may have or may acquire in any Development and all benefits and/or rights resulting therefrom to the Company and its assigns without further compensation and shall communicate, without cost or delay, and without disclosing to others the same, all available information relating thereto (with all necessary plans and models) to the Company.
(b) I recognize that Developments or Confidential Information relating to my activities while working for the Company and conceived or made by me, alone or with others, within three (3) months after termination of my employment may have been conceived in significant part while employed by the Company. Accordingly, I agree that such Developments and Confidential Information shall be presumed to have been conceived during my employment with the Company and are to be assigned to the Company unless and until I have established the contrary.
5. Further Assurances. I shall, during my employment and at any time thereafter, at the request and cost of the Company, promptly sign, execute, make and do all such deeds, documents, acts and things as the Company and its duly authorized officers may reasonably require:
(a) to apply for, obtain, register and vest in the name of the Company alone (unless the Company otherwise directs) patents, copyrights, trademarks or other analogous protection in any country throughout the world relating to a Development and when so obtained or vested to renew and restore the same; and
(b) to defend any judicial, opposition or other proceedings with respect to such applications and any judicial, opposition or other proceeding, petition or application for revocation of any such patent, copyright, trademark, certifications or other analogous protection.
If the Company is unable, after reasonable effort, to secure my signature on any application for patent, copyright, trademark or other analogous protection or other documents regarding any legal protection relating to a Development, whether because of my physical or mental incapacity or for any other reason whatsoever, I hereby irrevocably designate and appoint the Company and its duly authorized officers and agents as my agent and attorney-in-fact, to act for and in my behalf and stead to execute and file any such application or applications or other documents and to do all other lawfully permitted acts to further the prosecution and issuance of patent, copyright or trademark registrations or any other legal protection thereon with the same legal force and effect as if executed by me.
| 3 |
6. Nonsolicitation of Customers. During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for my termination, I shall not, directly or indirectly, alone or as an owner, member, manager, partner, officer, employee, director, investor, lender, consultant or independent contractor of any entity, call upon, solicit, divert or take away or attempt to divert or take away or do business with, either for myself or for any other person or entity, any current or prospective customers or accounts of the Company that I had contact with, for whom I performed services on behalf of the Company, or about whom I obtained Confidential Information through the Company for the purpose of competing (i.e., by providing the Products and Services, as defined below), whether directly or indirectly, nor shall I make known to any other person or entity, either directly or indirectly, the names and addresses of and other pertinent information relating to any such customers or accounts, or any Confidential Information relating to any of them.
7. Non-solicitation of suppliers. During the period of my employment with the Company and for 12 months following the termination of my employment, regardless of the reasons for my termination, I shall not do or attempt to do anything which causes or may cause any entity which has supplied products or services to the Company to cease, alter or materially to reduce its supplies to the Company or alter its terms of business with and to the detriment of the Company.
8. Noncompetition. During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for my termination, I shall not, anywhere where the Company conducts business directly or indirectly, alone or as an owner, member, manager, partner, officer, agent, employee, director, investor, lender, consultant or independent contractor of any entity, (a) accept employment or establish any other relationship with any business that is in competition, whether directly or indirectly, with the products or services being created, certified, developed, manufactured or planning to be manufactured, marketed or planning to be marketed, distributed or planning to be distributed, or sold or planning to be sold by the Company at the termination of my employment (collectively, the “Products and Services”), or (b) engage in any business or activity that is in competition with the Products and Services. For purposes of this Section, Products and Services shall include, without limitation any products or services substantially similar to the products and services designed, conceived, marketed, distributed or developed by the Company at the time of the termination of my employment. Notwithstanding the foregoing, the record or beneficial ownership by me of five (5) percent (5%) or less of the outstanding publicly traded capital stock of any entity shall not be deemed, in and of itself, to be in violation of this Section 8.
9. Nonsolicitation of Employees.
(a) During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for the termination, I will not, in any manner, directly or indirectly, hire or engage, or assist any company or business organization by which I am employed or which is directly or indirectly controlled by me to hire or engage, any person who is or was employed by the Company (or is or was an agent, representative, contractor, project consultant or consultant of the Company) at the time of my termination or during the period of twelve (12) months prior thereto.
| 4 |
(b) During the period of my employment by the Company and for twelve (12) months following the termination of my employment, regardless of the reasons for the termination, I will not, in any manner, directly or indirectly, solicit, recruit or induce, or assist any company or business organization by which I am employed or which is directly or indirectly controlled by me to solicit, recruit or induce, any person who is or was employed by the Company (or is or was an agent, representative, contractor, project consultant or consultant of the Company) at the time of my termination or during the period of twelve (12) months prior thereto, to leave his or her employment, relationship or engagement with the Company.
11. Nondisparagement. I agree that at all times during and after my employment with the Company, regardless of the reason for my termination, I shall not disparage the Company, its products, services, agents or employees.
12. Employment Status. I understand that this Nondisclosure Agreement does not constitute an implied or written employment contract.
13. Severability. I hereby agree that each provision and the subparts of each provision herein shall be treated as separate and independent clauses, and the unenforceability of any one clause shall in no way impair the enforceability of any of the other clauses of the Nondisclosure Agreement. Moreover, if one or more of the provisions contained in this Nondisclosure Agreement shall for any reason be held to be excessively broad as to scope, activity, subject or otherwise so as to be unenforceable at law, such provision or provisions shall be construed by the appropriate judicial body by limiting or reducing it or them, so as to be enforceable to the maximum extent compatible with the applicable law as it shall then appear. I hereby further agree that the language of all parts of this Nondisclosure Agreement shall in all cases be construed as a whole according to its fair meaning and not strictly for or against either of the parties.
14. Amendments; Waiver. Any amendment to or modification of this Nondisclosure Agreement, or any waiver of any provision hereof, shall be in writing and signed by the Company and me. Any waiver by the Company of a breach of any provision of this Nondisclosure Agreement shall not operate or be construed as a waiver of any subsequent breach of such provision or any other provision hereof.
15. Survival. This Nondisclosure Agreement shall be effective as of the date entered below. My obligations under this Nondisclosure Agreement shall survive the termination of my employment regardless of the reason for or the manner of such termination and shall be binding upon my heirs, executors, administrators and legal representatives.
16. Assignment. The Company shall have the right to assign this Nondisclosure Agreement to its successors and assigns, and all covenants and agreements hereunder shall inure to the benefit of and be enforceable by said successors or assigns. However, I may not assign this Nondisclosure Agreement.
| 5 |
17. Notification. I agree and acknowledge that during my employment with the Company and for a period of twelve (12) months following the termination of my employment with Company for any reason, I shall inform each new employer I may have, prior to accepting employment, of the existence of this Nondisclosure Agreement, and I shall provide each such employer with a copy of this Nondisclosure Agreement. I also agree and acknowledge that the Company has the right to independently contact any potential or actual future employer of mine to notify the future employer of my obligations under this Nondisclosure Agreement and provide such future employer with a copy of this Nondisclosure Agreement. The Company also shall be entitled to notify such actual or potential future employer of the Company’s understanding of the requirements of this Nondisclosure Agreement and what steps, if any, the Company intends to take to insure compliance with or enforcement of this Nondisclosure Agreement.
18. Representations.
(a) I represent that my employment with the Company and my performance of all of the terms of this Nondisclosure Agreement do not and will not breach any agreement to keep in confidence proprietary information acquired by me in confidence or in trust prior to my employment by the Company. I have not entered into, and I shall not enter into, any agreement either written or oral in conflict herewith.
(b) I further agree that any breach of this Nondisclosure Agreement by me will cause irreparable damage to the Company and that in the event of such breach the Company shall have, in addition to any and all remedies of law, the right to an injunction, specific performance or other equitable relief to prevent the violation of my obligations hereunder. The Company may apply for such injunctive relief in any court of competent jurisdiction without the necessity of posting any bond or other security.
19. Governing Law; Forum Selection Clause. This Nondisclosure Agreement and any claims arising out of this Nondisclosure Agreement (or any other claims arising out of the relationship between the parties) shall be governed by and construed in accordance with the laws of Massachusetts and shall in all respects be interpreted, enforced and governed under the internal and domestic laws thereof, without giving effect to the principles of conflicts of laws of Massachusetts. Any claims or legal actions by one party against the other shall be commenced and maintained in any state or federal court located in Massachusetts and I and the Company each hereby submit to the jurisdiction and venue of any such court.
20. Entire Agreement. This Nondisclosure Agreement and the Employment Agreement executed in connection herewith, set forth the complete, sole and entire agreement between the parties on the subject matter herein and supersede any and all other agreements, negotiations, discussions, proposals, or understandings, whether oral or written, previously entered into, discussed or considered by the parties on the subject matter herein.
***************
| 6 |
IN WITNESS WHEREOF, the undersigned have executed this Nondisclosure Agreement as of the date first set forth above.
| HEMOGLOBIN OXYGEN THERAPEUTICS, LLC | ||||
| By: | By: | |||
| Name: | Zafiris G. Zafirelis | Name: | Joseph Rappold | |
| Its: | CEO | |||
| 7 |
Exhibit 10.4

674 Souder Rd, Souderton, PA 18964
Melissa Zafirelis
32 Maple Street
Waltham, MA 02452
Dear Melissa,
I am pleased to offer you the position of Director of Regulatory Affairs and Clinical Operations with Hemoglobin Oxygen Therapeutics LLC, on the following terms;
As Director of Regulatory Affairs you will be based at the company’s Dedham MA office and report to the Company’s CEO. As part of your duties you may be required to travel from time to time.
Your starting gross salary will be $144,000 per annum, less all applicable withholdings, payable on a bi-weekly basis. Your commencement date will be July 1st 2014. You will accrue vacation time on a pro rata basis at a rate of 20 days per 12 months. During your first year of employment you may carry into the following year up to 10 days of vacation time. Subsequently, fifteen days of vacation time must be used during the year that it is accrued and a maximum of 5 days may be carried over from year to year. You will be entitled to participate in Company benefit plans and programs in effect from time to time for employees at your level, including group medical and dental insurance. As a founding member of the Company’s senior management team you will also be entitled to participate in the Company’s Stock Option Plan. Details of these benefit plans will announced as soon as these become finalized.
During the course of your employment, you will be required to comply with the Company’s standard employee policies and practices.
In accepting this position, you confirm that you are under no other employment obligation, nor bound by any other employment-related agreement. You also confirm that you employment with the Company will be at “will” which means that it may be terminated either you or the Company at any time and for any reason, with or without cause subject to the additional provisions of this paragraph. If the Company terminates you employment without cause, you will continue to be paid, for a period of 3 months, your base salary at the rate in effect at the time of such termination.
The company is required to verify your identity and authorization to work in the United States and your employment is contingent upon the Company’s receipt of acceptable documentation and completion of Form I-9.

674 Souder Rd, Souderton, PA 18964
This letter, along with the Employee Obligations Agreement referred to above sets forth the terms of your employment with the Company and supersedes any prior representations or agreements whether written or oral. This letter may not be modified OF amended except by: a written document signed hy the CEO of the Company and by you. This letter shall be governed by the laws of the State of Pennsylvania without regard to conflicts of law principles.
Please sign and date this letter and return to me to indicate your acceptance of this position, Welcome to the Hemoglobin Oxygen Therapeutics, LLC team.
Sincerely,
| HEMOGLOBIN OXYGEN THERAPEUTICS LLC. | |
| MELISSA ZAFIRELIS | |
| Date____________________________________________ |
Exhibit 10.5
Hemoglobin Oxygen Therapeutics, LLC
September 1, 2014
Brian Dawson
9 Jordan Circle
Braintree, MA 02184
Dear Brian,
I am pleased to offer you the position of Sr. Director of Process Development with Hemoglobin Oxygen Therapeutics LLC, on the following terms;
As Sr. Director of Process Development you will be based at the company’s Dedham, MA facility and report to the Company’s President. As part of your duties you are required to travel to the Souderton, PA facility.
Your starting gross salary will be $180,000 per annum, less all applicable withholdings, payable on a bi-weekly basis. Your commencement date will be September 1, 2014. You will receive a sign on bonus equivalent to one month’s salary. You will receive a second sign on bonus equivalent to one month’s salary within 6 months. You will accrue vacation time on a pro rata basis at a rate of 20 days per 12 months. During your first year of employment you may carry into the following year up to 10 days of vacation time. Subsequently, fifteen days of vacation time must be used during the year that it is accrued and a maximum of 5 days may be carried over from year to year. You will be entitled to participate in Company benefit plans and programs in effect from time to time for employees at your level, including group medical and dental insurance. As a founding member of the Company’s senior management team you will also be entitled to participate in the Company’s Stock Option Plan. Details of these benefit plans will announced as soon as these become finalized.
During the course of your employment, you will be required to comply with the Company’s standard employee policies and practices, in effect from time to time.
In accepting this position, you confirm that you are under no other employment obligation, nor bound by any other employment-related agreement. You also confirm that you employment with the Company will be at “will” which means that it may be terminated either you or the Company at any time and for any reason, with or without cause subject to the additional provisions of this paragraph. If the Company terminates your employment without cause, you will continue to be paid, for a period of 6 months, your base salary at the rate in effect at the time of such termination. After 5 years this will increase to 1 years severance.
674 Souder Rd, Souderton, PA 18964
The company is required to verify your identity and authorization to work in the United States and your employment is contingent upon the Company’s receipt of acceptable documentation and completion of Form I-9.
This letter, along with the Employee Obligations Agreement referred to above sets forth the terms of your employment with the Company and supersedes any prior representations or agreements whether written or oral. This letter may not be modified or amended except by a written document signed by the president or CEO of the Company and by you. This letter shall be governed by the laws of the State of Pennsylvania without regard to conflicts of law principles.
Please sign and date this letter and return to me to indicate your acceptance of this position. Welcome to the Hemoglobin Oxygen Therapeutics LLC team.
Sincerely,
Zaf & Jerry
| Hemoglobin Oxygen Therapeutics LLC | |
| Date____________________________________________ | |
| Brian Dawson | |
| Date____________________________________________ |
674 Souder Rd, Souderton, PA 18964
Exhibit 10.7













Exhibit 10.8

TABLE OF CONTENTS
| I. | PROJECT SUMMARY | 2 |
| II. | BASIS OF SYSTEM CRITERIA | 3 |
| 1.0 | General | 3 |
| 2.0 | Site/Civil | 4 |
| 3.0 | Concrete | 5 |
| 4.0 | Masonry | 6 |
| 5.0 | Structural / Steel | 6 |
| 6.0 | Carpentry. | 8 |
| 7.0 | Thermal and Moisture Protection | 8 |
| 8.0 | Doors and Windows | 9 |
| 9.0 | Finishes | 11 |
| 10.0 | Specialties | 13 |
| 11.0 | Equipment | 15 |
| 12.0 | Furnishings | 16 |
| 13.0 | Special Construction | 17 |
| 14.0 | Conveying Systems | 18 |
| 15.0 | Mechanical | 19 |
| 16.0 | Electrical | 56 |
| 17.0 | PROCESS | 64 |
| III. | DESIGN/BUILD PROJECT DISCUSSION | 81 |
| IV. | CLARIFICATIONS | 88 |
| V. | ESTIMATE | 99 |
| VI. | COMMERCIAL TERMS | 99 |
| VII. | MILESTONE SCHEDULE | 100 |
| VIII. | ATTACHMENTS | 100 |
5110 Campus Drive ● Suite 110 Plymouth Meeting, PA 19462
Phone: 610.941.1001 ● Fax 610.941.0110 ● www. protecsinc.com
| 1 |

| I. | PROJECT SUMMARY |
Hemoglobin Oxygen Therapeutics, LLC (HbO2) has acquired the technology and IP to manufacture bovine derived Hemoglobin from a former company that filed for bankruptcy. The Hemoglobin manufacturing and packaging processes were previously performed at three separate facility located in Pennsylvania, Massachusetts and Canada. HbO2 is relocating the entire manufacturing, packaging and warehousing operations to its Pennsylvania facility located at 674 Souder Road in Souderton, PA. The landlord has built a building expansion of approximately 34,000 SF that was complete in July of 2017.
At the request of HbO2, PROTECS has developed a scope of work and a Guaranteed Maximum Price ($GMP) for the design, construction and commissioning of the planned fit-out of the building shell expansion. The fit-out will include the construction of new office space, laboratory space, chemical storage, cGMP manufacturing and packaging, warehousing, locker rooms, bathrooms, supporting electrical/mechanical space and the associated utilities. The intent of the project is for HbO2 to relocate the manufacturing, packaging and warehousing operations to the Souderton facility. HbO2’s program is as follows:
| Description | Area (SF) | |||
| Office | 10,800 | SF | ||
| Cleanroom ISO 8 | 12,100 | SF | ||
| Cleanroom ISO 7 | 600 | SF | ||
| Utility/Support Space | 13,600 | SF | ||
| Warehouse | 2,700 | SF | ||
| Laboratory | 2,500 | SF | ||
| Equipment Platform | 4,700 | SF | ||
| Total | 47,000 | SF | ||
The overall schedule for the project will require release of the $GMP within two days to enable subcontractor and material release for completion in September 2018. A schedule outlining the overall project timeline including all stages of engineering, permit approval process, construction activities, commissioning and validation for the project is also attached.
PROTECS is prepared to assist HbO2 with moving forward on this project. Based on the preliminary information and the proposed design and construction discussed, PROTECS recommends HbO2 continue with the methodology below to deliver this project. We believe proceeding in the fashion outlined below will lead to the safest, most cost effective and timely delivery of this project
| ● | Complete the project design and issue Bulletin #1 drawings and P&IDs. | |
| ● | Confirm pricing and release specific subcontractors/vendors critical to the project schedule. | |
| ● | Mobilize to site and commence construction activities. | |
| ● | Construction management, scheduling, and cost control for all labor and materials managed at the site by PROTECS personnel. | |
| ● | Commissioning and start-up. | |
| ● | Occupancy permit will be obtained by PROTECS. | |
| ● | Occupancy by HbO2. | |
| ● | Validation facility and processes by HbO2. |
A summary description of the estimate basis follows:
www.protecsinc.com
| 2 |

| II. | BASIS OF SYSTEM CRITERIA |
| 1.0 | General |
The intent of this project is to renovate the existing empty shell to provide for a mixed-use laboratory, office, manufacturing, packaging and warehouse operations at the Souderton, PA facility. The current building is mixed use occupancy with use group F-2 Factory and use group S-2 Low Hazard Storage – Non-Separated Mixed use. A detailed code review and hazardous material code review is included on the drawings.
The building will include the following occupancy classifications:
| ● | F-2 - Factory | |
| ● | S-2 – Low Hazard Storage | |
| ● | B – Business Group |
The existing building is a steel and concrete structure.
The design criteria employed on the project is defined below and will be in accordance with the requirements of the following codes and standards:
| ● | International Building Code (IBC 2009) | |
| ● | International Fire Code (IFC 2009) | |
| ● | International Plumbing Code (IPC 2009) | |
| ● | International Mechanical Code (IMC 2009) | |
| ● | International Energy Conservation Code (IECC 2009) | |
| ● | NFPA (Applicable) National Fire Protection Association | |
| ● | NFPA 70 National Electrical Code | |
| ● | NFPA 72 – National Fire Alarm Code (NFAC) | |
| ● | NFPA 70E – Standard for Electrical Safety Requirement for Employee Workplaces | |
| ● | NFPA 110 – Standard for Emergency and Standby Power Systems | |
| ● | ADA Title II Accessibility Guidelines | |
| ● | ASHRAE American Society of Heating Refrigeration and Air Conditioning Engineers | |
| ● | SMACNA Sheet Metal and Air Conditioning Contractors National Association | |
| ● | ASME American Society of Mechanical Engineers | |
| ● | OSHA | |
| ● | ASCE as noted below | |
| ● | AISC as noted below |
www.protecsinc.com
| 3 |

| ● | ASTM as noted below | |
| ● | ACI as noted below | |
| ● | ANSI (Applicable) American National Standards Institute | |
| ● | ASSE American Society of Sanitary Engineers | |
| ● | AWWA American Water Works Association | |
| ● | CAGI Compressed Air and Gas Institute | |
| ● | CISPI Cast Iron Soil Pipe Institute | |
| ● | CGA Compressed Gas Association | |
| ● | Regulatory and validation requirements related to the facility design, construction and operations. Applicable sections of the following: |
| ○ | FDA CFR Guidelines for GMP operations in the United States | |
| ○ | European Commission’s EU Guidelines to Good Manufacturing Practices for Medicinal Products for Human and Veterinary Use, Annex 1 |
| 2.0 | Site/Civil |
| 2.1 | Overview |
There will be no site improvements required for the project. The new building and all associated site/civil activities will be completed by HbO2 as part of the building shell construction project.
The site benchmark will be determined during detailed design as provided by the M&W Group.
The project will have to conform the local zoning requirements which were completed by the M&W Group.
| 2.2 | Site Grading |
Site grading will be limited to the liquid nitrogen pad.
| 2.3 | Site Utilities – Electric, Natural Gas, Water, Sewer, Storm Drainage |
The natural gas service was upgraded by PECO and the service will serve the new and existing systems. The electrical service will be upgraded to meet the project requirements. PPL will replace the existing pad mount transformer and set the new transformer on the existing vault. A new termination cabinet, required by PPL, will be provided to feed the new and existing switchboards.
www.protecsinc.com
| 4 |

| 2.6 | Parking and Roadways |
There are no parking and/or roadway modifications required. The parking lot wearing course and line stripping will be completed by the landlord after the completion of the project.
| 2.7 | Dock Area |
The new dock area and shipping / receiving will be completed by HbO2 as part of the building shell construction.
| 2.8 | Fencing |
A galvanized chain link fence with access gate and man gate will be provided at the bulk nitrogen tank pad.
| 3.0 | Concrete |
The concrete slab for the building expansion loading dock area is included as part of the building shell construction.
A new concrete slab will be provided for the building expansion (except the loading dock area to be poured as part of the building shell construction).
A new concrete slab will be provided for the new second floor office area.
Reinforcement of the concrete slab as required for the process equipment will be provided.
The existing concrete floor will be cut and patched to accommodate new underground piping. The patchwork will include a vapor barrier and reinforcing to “dowel-in” to the existing slab to remain. This work is based on the existing concrete slab floor being 6” thick.
Interior concrete housekeeping pads will be provided for the new electrical gear and mechanical equipment in Utilities #181 and #184. Inertia bases will be provided directly onto the floor structure for pumps as required.
A new exterior concrete pad will be provided for the liquid nitrogen tank.
Miscellaneous concrete pads and curbs will be provided for architectural features such as the Serum Receiving wash-down area.
www.protecsinc.com
| 5 |

| 3.1 | Concrete |
| a. | Footings (ACI 301): Normal weight mix, 4,000 psi compressive strength at 28 days | |
| b. | Slab on grade (ACI 360): 6 inches thick, normal weight mix, 4,000 psi compressive strength at 28 days | |
| c. | Housekeeping Pads: 6 inches thick, normal weight mix, 4,000 psi compressive strength at 28 days (existing slab to remain) |
| 4.0 | Masonry |
Masonry work will be limited to new elevator hoistway walls and cutting/patching existing masonry for new openings. New openings will include the following:
| ● | Door openings from the existing building to the new expansion | |
| ● | Mechanical penetrations from the existing building to the new expansion | |
| ● | Door openings in the existing building |
The new elevator hoistway walls will be constructed of 8” CMU and will be grouted solid.
| 5.0 | Structural / Steel |
The renovation project will require structural steel dunnage for roof top equipment, structural reinforcement and framing for roof top equipment, a second floor cat walk with metal grating for access to equipment requiring maintenance, two stair towers, SS platforms for process tank access and a new second floor office area.
The second floor office area will be 1 ½” – 20 gauge composite metal deck with 2 ½” normal weight concrete slab (4” total thickness).
The existing process equipment platforms relocated from the Cambridge, MA facility will not be re-used. New process equipment platforms with stair / ladder access and diamond plate decking will be provided. The new platforms will be constructed of stainless steel with a #4 Mill finish for access to the process equipment. The platforms will be provided with the process skid assemblies and final assembled during the skid installation.
Two steel pan stair towers will be provided as required for egress / access to the second floor. Roof access from the stair towers will not be provided. The stair tower construction will be open to the second floor level, but closed at the first floor for security. The construction will not be rated for fire separation due to exceptions in the code.
www.protecsinc.com
| 6 |

Steel framing will be provided for new roof top equipment at the same elevation as the existing roof framing.
Steel dunnage will be provided to support the roof top chillers and generator equipment. The chiller dunnage will allow for 2’-0” clear space between the finished roof and the bottom of steel to allow for access to equipment and roof drains. The dunnage will be bolted connections with the steel being shop primed and painted. (See alternate price for all exposed structural steel dunnage to be hot- dipped galvanized).
Supplemental framing steel will be required as additional support around new penetrations exceeding one square foot. This angle framing will be shop primed and installed with any field welds brushed and painted after installation. All other steel work will be performed using materials in accordance with the following:
The structural design will be in accordance with the requirements of the following codes and standards:
| 5.1 | Gravity Live Loads (Code Minimum) |
| a. | Slab on grade: | 200 pounds per square foot | |
| b. | Second floor: | 65 (50 office + 15 for partitions) pounds per square foot | |
| c. | Second floor cat walk (Main): | 40 pounds per square foot | |
| d. | Second floor cat walk (Egress): | 60 pounds per square foot | |
| e. | Roof level: | 20 pounds per square foot plus the actual weight of mechanical equipment |
| 5.2 | Wind Loads (Code Minimum) |
| a. | Basic wind velocity (per ASCE 7-05) = 90 mph | |
| b. | Exposure category = C | |
| c. | Wind importance factor = 1.0 |
| 5.3 | Seismic Loads (Code Minimum) |
| a. | Spectral response acceleration at short period, Ss = 0.276g | |
| b. | Spectral response acceleration at 1 second period, S1 = 0.062g | |
| c. | Seismic Occupancy Category = II | |
| d. | Site class = C | |
| e. | Seismic Design Category = B |
www.protecsinc.com
| 7 |

| 5.4 | Structural Steel |
| a. | Wide flange members: ASTM A992 | |
| b. | Angle, channel, plates, and rods: ASTM A36 | |
| c. | Hollow structural shapes: ASTM A500, Grade B | |
| d. | Structural pipe: ASTM A53, Type E, Grade B | |
| e. | Structural bolts: ASTM A325-N |
| 5.5 | Metal Deck |
| a. | 1-1/2” deep x 20 gauge composite metal floor deck (galvanized) | |
| b. | 1-1/2” deep x 22 gauge wide rib metal roof deck (galvanized) |
| 6.0 | Carpentry |
| 6.1 | Blocking |
Wood grounds and nailers will be provided for equipment and roof supports as required. Wood in contact with masonry and roof nailers will be treated in accordance with AWPA C2 requirements, and will be mechanically fastened securely to substrate. The use of exposed wood will be prohibited in any interior areas. Wood used for blocking will be fire retardant treated.
| 6.2 | Millwork |
Millwork will primarily consist of plastic laminate over pressboard in the restroom areas. A solid surface countertop will be provided in the CNC restrooms. Painted wood trim will be provided at the top of the wall surrounding Stair 1. Plastic laminate window sills will be provided at most of the exterior windows in the addition.
| 7.0 | Thermal and Moisture Protection |
| 7.1 | Roofing |
All roof materials will be designed as an assembly in order to maintain a single source roof warranty. All roof flashing is per SMACNA’s standard flashing details, including the proposed building expansion joints.
Roof work related to the fit out project will be limited to new penetrations and equipment curbs in the existing roof. For existing roof area requiring cutting and patching, roof flashing, cutting, and patching will be provided to conform to the existing manufacturer’s warranty requirements per the manufacturer’s standard details. This will be provided to maintain the existing warranty and bond, if any on the existing roof.
www.protecsinc.com
| 8 |

| 7.2 | Sealants |
All joints will be sealed with an appropriate sealant and backer as required. At the building interior, all products will be FDA approved and bacteria inhibiting. Interior building joints and penetrations in fire rated wall construction to be fire stopped with an appropriate UL assembly. Exterior masonry wall assemblies will receive approved block filler as required for moisture protection.
All exterior joints will be sealed watertight.
| 7.3 | Fireproofing |
Fireproofing is not required.
| 7.4 | Firestopping |
Fire caulking as manufactured by Hilti or other similar manufacturers will be the primary material used for this. Where there are multiple penetrations an assembly will be prepared in consultation with the architect and engineer.
| 8.0 | Doors and Windows |
| 8.1 | Hollow Metal Doors |
Doors will be constructed of hollow metal with vision lights where indicated and be mounted in hollow metal frames. The hollow metal frames will be knock down wrap around type in all non-process areas. Exterior egress doors are existing. Where doors are installed in rated walls, UL fire rated doors, frames and hardware will be installed. Doors will have a painted finish over a factory prime coat.
Hollow metal frames will be 18 gauge for interior and 14 gauge for exterior. Interior frames will be cold rolled steel and exterior frames will be galvanized steel. Hollow metal doors will be 20 gauge for interior and 16 gauge for exterior, primed gray. Interior doors will be cold rolled steel and honeycomb core and exterior doors will be galvanized steel with polyurethane (insulated) core.
All doors will be a standard 7’-0” height unless otherwise noted.
| 8.2 | Wood Doors |
Doors in the office areas will be wood doors and will be mounted in hollow metal frames. The frames will be knock down wrap around type. Hollow metal frames will be 16 gauge. Interior frames will be cold rolled steel and exterior frames will be galvanized steel.
Doors will be a pre-finished stain grade. Wood doors will be Mohawk Commercial Series finished to one of Mohawk’s standard colors or approved equal.
www.protecsinc.com
| 9 |

| 8.3 | Hardware |
General door hardware will be ADA compliant. Locksets will be cylindrical type, heavy duty. Electrified hardware will be installed where security devices are shown. Double doors will include flush bolts on the inactive leaf. A typical door will receive 1 ½ pair hinges.
Fire rated doors will be equipped with surface mounted overhead closers. All doors other than office doors and cleanroom doors will be equipped with surface mounted overhead closers. Cleanroom doors will be equipped with concealed closers.
Door hardware will be Hager hinges or approved equal; Schlage “ND” series locks or approved equal; Falcon DL-24/25 series panic hardware or approved equal; Schlage M450 series magnetic locks or approved equal; Von Duprin 6000 series electric strikes or approved equal; LCN 4010/4110/4020 series closers or approved equal; Hager manual flush-bolts, floor and wall stops, armor plates and weather- stripping products or approved equals; Von Duprin power supplies or approved equal. Hardware are US26D, US32D and 689 finishes. Construction keying for doors will be provided. Final keying will be completed by HbO2.
| 8.4 | Vision Panels |
Rated fire glass will be provided in all interior doors that carry a rating label and are programmed as requiring vision panels. Tempered safety glass will be provided in other glazing applications in hollow metal frames. Exterior hollow metal doors are existing.
Door lites will typically be ¼” thick clear tempered safety glass and 24”x32” in size, 5”x 30” (100 si) for rated lites. Door lites within the cleanroom suites will be finished flush with the door. The glass will be caulked in place and gasketed. The frames for these windows will be hollow metal, welded. Where both sides of the window are clean, the glass will be flush on both sides. Where only one side is clean, the non-cleanroom side will not be flush glass.
| 8.5 | Special Doors |
Where special modular cleanroom partitions are used the doors will be integral to the system. Door will be constructed of powder coated aluminum with vision lights where indicated and will be mounted in aluminum frames.
| 8.6 | Storefront |
There are no storefront systems required.
www.protecsinc.com
| 10 |

| 8.7 | High Speed Roll Up Doors |
Fabric type high speed roll up doors (HSRUD) with wall based activation switches or overhead pull strings and vision panels will be provided as indicated on the plans. The HSRUDs will ensure a tight seal to maintain space pressurization in the cleanroom areas.
Note: Refer to contract drawings for door type and locations.
| 9.0 | Finishes |
| 9.1 | Partitions |
Gypsum wall board (GWB) partitions will be constructed of cold-formed metal studs of appropriate size and gauge for interior furred-out and utility chase walls and will be finished in standard gypsum board. In wet locations moisture resistant type gypsum board will be used. Deep-leg deflection tracks will be provided at the tops of light gauge steel partitions that are built to the underside of the deck above to alleviate crushing and deformation. The layers of GWB and thickness will be governed by the rating of the partition as applicable.
All finished gypsum wallboard within the laboratories, corridors, warehouse and office areas will have a level 4 finish.
Ceramic tile walls up to four feet will be provided within the new bathrooms.
A prefabricated cleanroom wall system as manufactured by Dagard will be used for interior walls within the cleanroom area. The cleanroom wall panels and accessories have been purchased by HbO2 and will be integrated into the project design. The full thickness walls will be typically 4-3/4”, nominally, consisting of 151/256” (15mm) liner panels fastened directly over metal studs or hat track channel of the appropriate size and gauge. The wall finish will be a PVC coating with solvent welded seams. The walls will have integral coved corners. This same wall system will be used to construct a “dust alcove” in the Weigh Room in in lieu of installing a piece of equipment.
Note: Refer to contract drawings identifying wall partition type for each room.
| 9.2 | Ceilings |
The ceiling systems will include GWB ceilings, suspended grid systems, prefabricated cleanroom ceiling systems and open structure areas.
The GWB ceiling will include GWB panels screwed to suspended GWB grid system.
www.protecsinc.com
| 11 |

The office area ceiling tiles will sit in a 2’ by 4’ inverted tee ceiling grid system suspended by wire from the overhead structure. The office area tiles will be a second look type tile.
The lab area ceiling tiles will sit in a 2’ by 4’ inverted tee ceiling grid system suspended by wire from the overhead structure. The ceiling tiles will be mylar or vinyl coated composite tiles.
The cleanroom ceilings will be 2’ x 4’ modular suspended ceiling system as manufactured by Dagard. The walls will have integral coved corners. The ceiling will NOT be walkable and access to equipment above the cleanrooms will be from a calk walk system.
Note: Refer to contract drawings identifying ceiling type for each room.
| 9.3 | Finishes |
| 9.3.1 | Floor Coatings / Coverings |
The epoxy flooring will be a troweled on epoxy coating similar to that provided by Stonhard, Cornerstone, Sherwin Williams or approved equal. The epoxy flooring system will be similar to the Stonclad GS system with a Stonkote GS4 topcoat or approved equal. This coating will be 3/16” thick with a slip resistant finish. The epoxy flooring system should be resistant to the chemicals indicated by HbO2.
The coving will match the epoxy floor and will be a hospital type integral cove to 4” up the wall.
The vinyl composite tile will be 12” x 12” x 1/8” thick with a vinyl cove base. The VCT will be similar to Armstrong Standard Excelon or approved equal.
The polished concrete floors will be a pigmented finish or clear finish depending on the location.
Wood look luxury vinyl flooring system similar to the Venue Wood product by Tandus Centiva will be provided in the office area.
Ceramic tile floors will be provided within the bathrooms (not including production area bathrooms). 12”x24” Dal Tile San Michele glazed paver tile or approved equal with 3”x12” bullnose cap will be provided.
Note: Refer to contract drawings identifying flooring type for each room.
www.protecsinc.com
| 12 |

| 9.3.2 | Wall and GWB Ceiling Coatings / Coverings |
The epoxy paint will be a water-borne epoxy paint. On new walls, two coats will be provided over a primer coat. On existing walls one coat will be provided over the existing coat. On block walls a coating of block filler will be provided before painting.
The latex paint will be a water based paint. On new walls, two coats will be provided over a primer coat. On existing walls one coat will be provided over the existing coat. On block walls a coating of block filler will be provided before painting.
Accent walls will be provided in the following areas:
| ● | Office Area |
| ● | Entrance / Lobby Area |
Note: Refer to contract drawings identifying wall finish for each room.
| 9.3.3 | Special Coatings |
There are no special coatings.
| 10.0 | Specialties |
| 10.1 | Fire Extinguisher Accessories |
HbO2 will furnish 10 lb portable dry chemical type fire extinguishers for mounting in cabinets or on hooks provided under this scope of work.
Fully or Semi-recessed painted steel fire extinguisher cabinets with vertical duo panel doors will be provided to house HbO2 supplied ten-pound multi-purpose dry chemical portable fire extinguishers at intervals to satisfy building code requirements.
| 10.2 | Toilet Partitions / Accessories |
The toilet rooms will be provided with accessories including grab bars, mirrors, dispensers for toilet tissue, soap, paper towels, and feminine products, and waste disposal units. This includes floor mounted, powder coated metal toilet partitions in the office area and ceiling hung, powder coated metal toilet partitions in the production area. Urinal screens are provided. The appropriate ADA compliant accessories are included within these items.
| 10.3 | Laboratory Casework |
Refer to Division 12.
www.protecsinc.com
| 13 |

| 10.4 | Screens |
Specialty screens are not required.
| 10.5 | Warehouse Racks |
The warehouse racking system will be furnished and installed by HbO2. The contract drawings will indicate locations for reference and coordination only.
The warehouse has been designed to accommodate 28 pallet positions (maximum 2400lbs per pallet position on floor level, maximum 1500lbs per pallet on the 2nd level, maximum 500lbs on the top level) per level with an initial height of 3-tiers (54” clear between tiers). The current concrete slab is assumed to be 6”. Any increase in height above the three tiers will require evaluation of the existing slab and warehouse racking system.
The QA/Quarantine has been designed to accommodate 12 pallet positions (maximum 2500lbs per pallet position on floor level, maximum 2500lbs per pallet on the 2nd level) with an initial height of 2-tiers (54” clear between tiers). The concrete slab for this area of the fit out will be designed for the loads indicated above. Any increase in height above the two tiers will require evaluation of the slab design and warehouse racking system.
| 10.6 | Wall Bumper / Corner Guards |
4”x 4” x 96” SS Corner Guards will be provided.
A wall mounted wall protection system (cart protection) has been provided as an alternate.
Note: Refer to contract drawings identifying corner guard and wall bumper locations.
| 10.7 | Interior Room Signage |
Interior room signage except required by code will be completed by HbO2.
| 10.8 | Gowning Room Accessories |
Gowning room accessories (i.e. mirrors, hooks, dispensers, etc…) will be furnished and installed by HbO2. The gowning room accessories layout will be included on the project drawings and indicated as “by owner”. Blocking for wall mounted items will be based on the locations indicated.
www.protecsinc.com
| 14 |

| 10.9 | Fork Truck |
A Raymond 31 Easi Reach forklift will be provided by HbO2. Power for the lift battery charger will be provided in the location indicated on the drawings.
| 10.10 | Lockers / Benches |
Lockers will be provided within locker rooms. Lockers will be double tier and approximately 12”W x 18”D x 72”H. Lockers will be provided with sloped tops with a “Z” base. Key and/or combination pad locks will be by HbO2. The lockers will be numbered sequentially starting at #1.
Benches will be provided within locker rooms and personnel airlocks as indicated on the drawings. Benches will be anchored into the floor with SS anchors to allow for benches to be removable.
| 10.11 | Bug Light |
Bug lights will be indicated on the project drawings and will furnished and installed by HbO2. Power for the bug lights will be provided at the following locations:
| ● | Entrance Vestibule | |
| ● | Loading Dock | |
| ● | Serum Receiving | |
| ● | Exterior Doors In Utilities Corridor |
| 11.0 | Equipment |
| 11.1 | Fume Hoods |
See Division 12 for details.
| 11.2 | Loading Dock Equipment |
Loading dock equipment is existing.
| 11.3 | Process Equipment |
See division 17.0 for details.
| 11.4 | Lab Equipment |
Lab equipment, refrigerators, freezers, bench top autoclaves and incubators will be by HbO2.
| 11.5 | Overhead Projector |
The overhead projectors will be furnished by HbO2. Installation and electrical hook up will be provided.
www.protecsinc.com
| 15 |

| 11.6 | Wash Down Equipment |
The wash down equipment for the Serum receiving will be relocated from its location in the warehouse.
| 11.7 | Labeling Equipment |
The labeling equipment (labeling machine, label counter, and overwrap machine) will be furnished and installed by HbO2.
| 11.8 | Lab Glass Washer |
A lab glass washer will be provided in the lab area and will be served by the lab RODI water system. The glass washer will be qualified, but not validated and a GMP washer is not required.
| 11.9 | Fork Truck |
A Raymond 31 Easi Reach forklift will be provided by HbO2. Power for the lift battery charger will be provided in the location indicated on the drawings.
| 12.0 | Furnishings |
| 12.1 | Laboratory Casework |
Used laboratory metal casework and new epoxy countertops will be provided in the laboratories and final packaging. The used casework will have the exterior electrostatically painted in place after installation. The casework layout will be determined based on cabinet availability and will be reviewed with HbO2 prior to installation.
The casework will be 30” deep (including service chase) and 36” high. A peg board will be provided in the Glass Wash room.
| 12.2 | Fume Hoods |
A 4’ Benchtop fume hood with an epoxy work surface and appropriate metal base cabinets will be provided in the weigh room.
ASHRAE 110 testing of fume hoods is not included.
www.protecsinc.com
| 16 |

| 12.3 | Bio-Safety Cabinets |
A Bio-Safety cabinet (recirculating type) will be furnished and installed by HbO2 in the new Micro Lab.
| 12.4 | Dispensing Hood |
See section 9.0
| 12.5 | Pass-Thru’s |
A 24”W x 24”H x 24” L ID material pass through, constructed with 16 gauge 304 SS, with surface mounted SS frame doors with ¼” tempered glass window, continuous hinges, ¼ turn chrome plated handles, and a mechanical interlock will be provided between the Following:
| ● | Filling room and Labeling and Overwrap room. | |
| ● | Filling room and Aseptic Waste Out room. | |
| ● | Filling room and Aseptic Equipment Prep room. |
| 12.6 | Office Furnishings |
Office furnishings, including but not limited to the reception desk, computers, copiers, IT equipment, cubicles, desks and chairs, shredders, book cases and conference tables, will be provided and installed by HbO2.
| 12.7 | Process Area / Warehouse Furnishings |
Process area furnishings, including but not limited to desks/chairs, rolling tool boxes, shelving, SS work tables, computers/printers, storage racks, rolling racks, rolling tables, rolling cages, file cabinets (Fire King), metro racks, scales, check weight stations, integrity tester, peg boards and flammable storage cabinets, will be provided and installed by HbO2.
| 12.8 | Gas Cylinders and Storage Cages / Racks |
Gas cylinders and storage cages / racks will be provided and installed by HbO2.
| 12.9 | Window Treatments |
Except for the electrical room where privacy film will be applied to the existing windows, window treatments will be provided and installed by HbO2.
| 12.10 | Office Furniture |
Office furniture including workstations/cubicles will be provided and installed by HbO2. PROTECS will provide power to the cubicles for final connection by HbO2. Locations for power connections will be shown on the contract drawings based on a generic furniture layout unless HbO2 provides specific furniture selections and power connection requirements. Power connections will be configured as whips unless HbO2 provides specific furniture selections.
| 13.0 | Special Construction |
| 13.1 | Leak Detection |
Low level oxygen sensors will be provided by HbO2 if required.
| 13.2 | Rigging |
Rigging will be required for new steel and mechanical/electrical equipment.
| 13.3 | Rigging (Owner Process Equipment) |
See division 17 for details.
| 13.4 | Cold Storage Room (Relocated) |
The existing cold room will be relocated to its new location.
| 13.5 | Cold Storage Room (New) |
A new cold storage room will be provided per the below criteria:
| (a) | Performance: | |
| (i) | Temperature Performance | |
| 1. | Design Temperature Setpoint: +5 degrees C +/-3C | |
| 2. | Maximum temperature uniformity between any two points: +/- 2 degree C horizontal and +/- 3 degrees C vertical. | |
| 3. | Design Humidity Setpoint: No humidity control. | |
| 4. | System Capacity: Refrigeration system shall be capable of maintaining specifications under the following conditions: | |
| a. | Temperature Range Surrounding Room: 18 to 28 degrees C. | |
| b. | Ambient Temperature at Air-Cooled Condensing Units: 120 degrees F. | |
| c. | Man Door Openings: Six (6) per hour for an average duration of 15 seconds. | |
| d. | Personnel: Two (2) person per hour for an average duration of 10 minutes. | |
| e. | Environmental Room Load: Lighting. | |
| f. | Materials: One ambient temperature pallet of material (200lbs) will be loaded into the cold room per day. |
www.protecsinc.com
| 17 |

| (ii) | Noise: Room shall generate no more than 70 dBA at any point across room at 6 feet above floor. |
| 1. | All mechanical and electrical equipment shall generate no more than 75 dBA within 3 feet of unit in any direction. | |
| (iii) | Structural: | |
| 1. | Dead Loads: Self-weight of all elements and attached finishes. | |
| 2. | Seismic Loads: Per applicable codes | |
| a. | General: Connections shall be positive anchorage without consideration for frictional resistance. |
| (b) | Backup System: Provide Cold Storage Room with a 100 percent redundant refrigeration system (including condensers, compressors, and evaporator coils) and blower system, with these systems having automatic switchover during normal operation. | |
| (c) | Lighting: Lighting output shall be a minimum of 50 foot-candles at a measured 40 inches above floor based on empty space. | |
| (d) | Room Size (interior dimensions): 8’-0” wide x 12’-6” long x 8’-0” high. | |
| (e) | Doors: 1 man door (4’-0” x 7’-0”) | |
| (f) | Floor: Cold Box will sit on the existing concrete slab. | |
| (g) | Electrical Requirements: |
| (i) | Control Panels: 120/208 V, 3 phase, 60 Hz, 3 wire. | |
| 1. | Provide unit-mounted transformer at control panel for all internal connections. | |
| (ii) | Condensing/Compressor Units: 460 V, 3 phase, 60 Hz | |
| (iii) | Lights: Single point connection to control panel power supply. | |
| (iv) | Electrical Receptacles: 120 V, 20 amp GFCI. | |
| (h) | Condensing Equipment: To be located on top of the cold room |
| 14.0 | Conveying Systems |
| 14.1 | Elevator |
A two-stop hydraulic machine-room-less (MRL) elevator with a 5000lbs capacity will be provided. The basis of design is the Otis HydroFit, and the shaft and pit requirements will be based on this unit. The elevator pit is not included as part of the building shell construction. The pit will be included in the fit out project to allow coordination with and confirmation of the manufacturer’s requirements.
www.protecsinc.com
| 18 |

| 15.0 | Mechanical |
| 15.1 | Introduction |
The project will require new HVAC systems to support the fit out. The intent of this work is to duplicate/supplement the existing systems approach by adding specific new items of equipment.
Accordingly, the new HVAC equipment that will be added is summarized below (Refer to equipment list for sizing):
| ● | Plant steam boiler | |
| ● | Roof / Wall mounted exhaust fans | |
| ● | Roof top air-handling units | |
| ● | Humidifiers | |
| ● | CRAC Units | |
| ● | VAVs / FTUs | |
| ● | Electric Duct Heaters | |
| ● | HEPA Filters | |
| ● | Unit Heaters | |
| ● | Louvers |
The work associated with these components will include the procurement, receipt, rigging, installation, piping or duct connection to the existing main, electrical connection, installation of new control devices, start-up, testing, commissioning, and turn-over.
The equipment will be provided with direct drives where possible and variable frequency (VFD’s) drives or ECM variable speed motors where required.
A mechanical “cat walk” will be provided above the production cleanroom to provide access to equipment requiring service and access to controlling valves, dampers, etc…
The balance of this section describes the approach for the project’s mechanical systems.
www.protecsinc.com
| 19 |

| 15.2 | Outside Design Conditions |
The summer outdoor design conditions used will be the ASHRAE 0.4% dry bulb temperature and mean coincident wet bulb for Souderton, PA. The ASHRAE 0.4% numbers are exceeded for approximately 35 hours each summer in a typical year. The winter design conditions will be the ASHRAE 99.6% dry bulb temperature and outdoor design conditions will be as follows:
| Area | Outdoor Design Conditions | |||
| Summer | Winter | |||
| Cleanrooms | 93°F db/75°F wb | 13.8°F/4.3 grains | ||
| Laboratories | 93°F db/75°F wb | 13.8°F/4.3 grains | ||
| Warehouse | 93°F db/75°F wb | 13.8°F/4.3 grains | ||
| Office | 93°F db/75°F wb | 13.8°F/4.3 grains | ||
| Mechanical and other Ventilated spaces | 93°F db/75°F wb | 13.8°F/4.3 grains | ||
The elevation of the site is 600 ft.
www.protecsinc.com
| 20 |

| 15.3 | Interior Design Conditions |
The design temperatures for the interior zones are outlined below (these are design ranges, validation ranges, if applicable, shall be broader):
| AHU Number | AHU Zone Name | Temperature | Relative Humidity | |||
|
AHU-06 |
Filling Area - Rooms: 168, 168A, 168B, 168C) |
65°F +/- 3°F |
30% - 70% RH | |||
|
AHU-04 & AHU-05 |
Process Area – Rooms: 157, 164, 165, 167, 169, 169A, 170, 171, 174 |
68°F +/- 3°F |
30% - 70% RH | |||
| AHU-04 & AHU-05 | Process Area – Rooms: 172, 173, 173A, 175 | 68°F +/- 3°F | 30% - 70% RH | |||
| AHU-04 & AHU-05 | Process Area – Rooms: 149, 156, 162A, 163A, 176, 176A, 180 |
68°F +/- 3°F |
30% - 70% RH | |||
| AHU-08 | Office Area – Rooms: 160, 160B, 161, 161D, 162, 163, 177, 179, 179, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 220 |
Heating: 70°F +/- 5°F Cooling: 75°F +/- 5°F |
Summer: <65% Winter: Uncontrolled | |||
| AHU-08 | Support & Utility Space – Rooms 161A, 161C, 181, 182 | Heating: 70°F +/- 5°F Cooling: 75°F +/- 5°F |
Summer: <65% Winter: Uncontrolled | |||
| AHU-08 | Metrology Lab – Room 161B | Heating: 70°F +/- 5°F Cooling: 75°F +/- 5°F |
Summer: <65% Winter: Uncontrolled | |||
| AHU-08 | Chemical Storage – Room 183 | 70°F +/- 5°F | Summer: <65% Winter: Uncontrolled | |||
AHU-09 and Existing AHU-02 |
Warehouse – Rooms 139, 148, 150, 151, 153 |
75 Heating: 70°F +/- 5°F Cooling: 75°F +/- 5°F |
Uncontrolled | |||
| AHU-09 | Final Packaging Room 155 | 68°F +/- 3°F | 30% - 70% RH | |||
| AHU-09 | Laboratories – Rooms 105A, 105, 118, 121, 123 |
70°F +/- 3°F | 30% - 70% RH | |||
| SF-4.1 & SF-4.2 | Utilities – Room 184 | Summer: <100F Winter: >60F |
Uncontrolled | |||
| AHU-07 | Micro Lab – Room 119 |
70°F +/- 3°F | 30% - 70% RH |
www.protecsinc.com
| 21 |

NOTE: AHU tags noted above and below reflect the total unit count for the building including existing and are all noted here as AHUs. The drawings depict the new units as Roof Top Units (RTUs). See the below table for summary:
| Unit Tag | Area Served | |
| AHU-1 | Existing Process Area Unit | |
| AHU-2 | Existing Lab Area Interior Unit | |
| AHU-3 | Existing Office Area Exterior Unit | |
| AHU-4 (RTU-1) | New Process Area Unit | |
| AHU-5 (RTU-2) | New Process Area Unit | |
| AHU-6 (RTU-3) | New Fill Suite Unit | |
| AHU-7 (RTU-4) | New Micro Lab Unit | |
| AHU-8 (RTU-5) | New Office and Support Area Unit | |
| AHU-9 (RTU-6) | New Renovation Area Unit |
NOTE: The relative humidity set points will be: Winter – 40% RH, Summer – 60% RH.
Humidity control for process and fill areas will be provided by a gas fired humidification boilers. Humidity control for the QC Labs will be provided by electro-steam humidifiers with their own dispersion manifold located in supply ductwork to support the zones indicated. The humidifiers will be controlled with wall mounted humidity sensors and will connected to the building BAS.
Dehumidification will be accomplished through the AHUs.
For both humidification and dehumidification Fill Room #168 was selected as the controlling sensor location for AHU-06. The other rooms are generally expected to be in the range; however, conditions may vary as they will be following the lead of the system to satisfy the critical room, Fill Room #168. Each functional room or group of rooms will have its own temperature control zone.
For both humidification and dehumidification GMP Production #168 was selected as the controlling sensor location for AHU-04 and AHU-05. The other rooms are generally expected to be in the range; however, conditions may vary as they will be following the lead of the system to satisfy the critical room, GMP Production Room #170. Each functional room or group of rooms will have its own temperature control zone. Additional process related room humidifiers will be provided as noted above for rooms with winter humidification control.
Temperature control for the building will be provided by electric duct heaters to support the zones indicated.
www.protecsinc.com
| 22 |

Note: Refer to contract drawings identifying temperature and humidity zones and selected controlling sensor locations.
Room Classifications / Design Air Change Rates
The design air change rates for the classified interior zones are outlined below (these are design ranges, validation ranges, if applicable, shall be broader):
| Room | ISO / EU Classification | Particulate Count/Size | Design Air Change Rate or FPM | |||
Filling Area - Rooms: 168, 168A, 168B, 168C) |
ISO 7 / Grade B | 352,000 > 0.5micron, 83,200 > 1 micron, 2,930 > 5 micron |
50 ACH | |||
Process Area Zone – Rooms: 157, 164, 165, 167, 169, 169A, 170, 171, 174, 172, 173, 173A, 175 |
ISO 8 / Grade C |
3,520,000 > 0.5 micron, 832,000/ >1 micron, 29,300 >5 micron |
20 ACH | |||
Process Area – Rooms: 149, 156, 162A, 163A, 176, 176A, 180 |
CNC / Grade D |
3,520,000 > 0.5 micron, 832,000/ >1 micron, 29,300 >5 micron |
20 ACH | |||
Office Area – Rooms: 160, 160B, 161, 161D, 162, 163, 177, 179, 179, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 220 |
N/A | N/A | N/A | |||
Support & Utility Space – Rooms 161A, 161C, 181, 182 |
N/A | N/A | N/A | |||
Metrology Lab – Room 161B |
N/A | N/A | 12 ACH | |||
| Chemical Storage – Room 183 |
N/A |
N/A |
N/A | |||
Warehouse – Rooms 139, 148, 150, 151, 153 |
N/A |
N/A |
N/A | |||
Final Packaging - Room 155 |
N/A | N/A | 12 ACH | |||
Laboratories – Rooms 105A, 105, 118, 121, 123 |
N/A |
N/A |
12 ACH | |||
Utilities – Room 184 |
N/A | N/A | N/A | |||
Micro Lab – Room 119 |
ISO 7 |
352,000 > 0.5micron, 83,200 > 1 micron, 2,930 > 5 micron |
50 ACH |
| 15.4 | Ventilation Air |
Ventilation air will be as required by the 2009 International Mechanical Code and ASHRAE 62-2001 or as required for pressurization and makeup air. Unless noted in the tables above, the supply air rates will be based on heating load and/or exhaust air requirements, whichever is greater. Outdoor air supply rates will be based on minimum dilution ventilation requirements for occupant comfort, occupant density, pressurization criteria, contamination control, and/or exhaust air requirements.
| 15.5 | Internal Load Criteria |
Internal heat gains from lighting, people and electric-powered or other heat generating equipment were evaluated using the following criteria.
Occupancy loads are based on one person within Column Cleaning (Room #175), one person within Equipment Staging/COP (Room #173A), one person within Commodity/Equipment Prep (Room #172), two people within Materials Exchange Clean Side (Room #169), two people within Weigh Room (Room #171), six people within GMP Production Room (Room #170), Fill Room (Room #168), four people within Labeling & Overwrap (Room #167) and one person within an office/cubicle.
www.protecsinc.com
| 23 |

| Item / Category | Sensible Heat Gain |
Latent Heat Gain | ||
| People: | ||||
| Cleanrooms | 275 | 475 | ||
| Laboratories | 275 | 275 | ||
| Warehouse | 275 | 275 | ||
| Office | 275 | 275 | ||
| Mechanical & Utility Spaces | 275 | 275 | ||
| Lighting: | ||||
Heat Gain Watts/Sq. Ft. | ||||
| Cleanrooms | 1.0 | |||
| Laboratories | 1.0 | |||
| Warehouse | 1.0 | |||
| Office | 1.0 | |||
| Mechanical & Utility Spaces | 1.0 | |||
| Equipment: | ||||
Heat Gain Watts/Sq. Ft. | ||||
| Cleanrooms | 5.0 | |||
| Laboratories | 5.0 | |||
| Warehouse | 5.0 | |||
| Office | 5.0 | |||
| Mechanical & Utility Spaces | 5.0 | |||
| GMP Production Room 170 | 8.5 | |||
| Equipment Staging / C.O.P Rm | 15.55 | |||
| Column Cleaning / Janitor Room | 19.65 | |||
Where provided, actual equipment loads will be used.
www.protecsinc.com
| 24 |

| 15.6 | Exterior Loads Criteria |
Based on recommended procedures and methods as described in ASHRAE 2012 Fundamentals.
| 15.7 | Acoustical Criteria |
The mechanical systems will be designed to limit the impact of noise in the occupied area to acceptable levels as required by applicable codes. These NC ratings are “background” ratings (i.e. they are exclusive of noise generated by occupants and their equipment, such as reactors, freezers and biosafety cabinets). These values are measured in the center of the room, 3 feet above finished floor and, for laboratories, a minimum of 5 feet away from all fume hoods with the fume hood sash closed.
| 15.8 | Expansion and Redundancy Considerations |
There are no expansion or redundancy considerations; however, specific critical equipment will be connected to back-up power.
| 15.9 | Mechanical Systems Seismic Restraint Criteria |
Mechanical systems will be seismically restrained per minimum code requirements. All mechanical systems will be assigned an importance factor of 1.0.
| 15.10 | Systems Description (Air-side) |
15.10.1 Supply Air Systems
Supply air will be delivered to the space via air handling units. A summary listing of the air handling unit components is listed below and followed by a table identifying the preliminary configuration for the air handling units on the project:
All units will be double wall unless indicated otherwise. The basis for the unit manufacturer is Aaon, Daikin, Liebert, Trane, Carrier, or approved equal.
www.protecsinc.com
| 25 |

| AIR HANDLING UNIT SECTION TABLE | |||||||||||||||||||||||
| COMPONENT | AHU TYPE | ENTRY | CHEMICAL FILTER | PREFILTER | INTERMEDIATE FILTER | PREHEAT | COOLING | FAN | REHEAT | FINAL FILTER | INTEGRAL HUMIDIFIER | ||||||||||||
| AHU-04 | Outdoor | Mix | No | 30% | 95% | Gas | DX | AF-P | Yes | No | Yes | ||||||||||||
| AHU-05 | Outdoor | Mix | No | 30% | 95% | Gas | DX | AF-P | Yes | No | Yes | ||||||||||||
| AHU-06 | Outdoor | Mix | No | 30% | 95% | Gas | DX | AF-P | Yes | No | Yes | ||||||||||||
| AHU-07 | Outdoor | Mix | No | 30% | 95% | Gas | DX | AF-P | Yes | No | No | ||||||||||||
| AHU-08 | Outdoor | Mix | No | 30% | 95% | Gas | DX | AF-P | Yes | No | No | ||||||||||||
| AHU-09 | Outdoor | Mix | No | 30% | 95% | Gas | DX | AF-P | Yes | No | No | ||||||||||||
| CRU-1 / CU-1 | CRAC | Mix | No | 30% | N/A | KW | DX | FC-C | No | No | Yes | ||||||||||||
CRU-2 /CU-2 |
CRAC | Mix | No | 30% | N/A | KW | DX | FC-C | No | No | No | ||||||||||||
AHU Type
Outdoor – The unit will be an outdoor air handling unit type with a sloped roof, insulation, 2” double wall casing with R13 foam injected insulation, exterior of unit is painted, no thru-metal construction, full perimeter base rail, outside air inlet with rain hood and filter magnehelic gauges.
CRAC – The unit will be a computer room air conditioning unit as commonly available from one of the CRAC manufacturers listed above. The unit will be a direct-expansion cooling type. The condensing unit will be mounted outdoors on a curb. The air handling unit section will be mounted indoors.
Entry
Mix – The outside air or make-up air enters the unit and mixes with the return air in this section. The section has an exterior louver and dampers on both entry points. The dampers are adjusted and set manually.
www.protecsinc.com
| 26 |

Filter Sections (Pre-Filter, Final Filter)
30% - A two-inch ASHRAE 30%, MERV 8 pre-filter will be provided. The filter will fit within the rack in the unit and is accessible via the section access door. The filters will slide in and out of the rack or the filters will clip in and out of the rack. If a higher efficiency filter is provided immediately downstream from this filter, both filters will be integral in the rack.
95% - A four-inch ASHRAE 95%, MERV 14 filter will be provided after or with an integral pre-filter noted above. The filters will be cartridge type. The filter will fit within the rack in the unit and is accessible via the section access door. The filters will slide in and out of the rack or the filters will clip in and out of the rack.
Pre-heat
Natural Gas – 10:1 or better modulating indirect fired natural gas burner and heat exchanger constructed of 300 series stainless steel.
KW - An electric resistance section will provide pre-heat. The electric heating section will have an SCR control to modulate the output.
Cooling
DX - A direct-expansion (DX as indicated in the table) coil will provide cooling. The unit will have a variable speed compressor or digital scroll compressor to provide good capacity control. The coil will have an integral drain pan for condensate. The pan will be sloped to the unit exterior with a pipe connection. The coil will be coated with Husky Coil Coat BGF-865 or manufacturer recommended equal for resistance to cleaning chemicals.
Hot Gas Reheat Coil
Utilizes the refrigerant hot gas during dehumidification mode to reheat the air to minimize the use of the zone electric duct heaters.
Fan
AF-P – An open plenum or plug fan. This fan section has a septum wall, bell- mouthed inlet, and an open fan wheel enclosed by a wire metal screen for protection. Fans will be direct drive with VFD. Multiple plenum fans in a single array will be utilized for reliability and service ease.
FC-C – A scroll housed centrifugal fan with a forward curved blade. The fan is belt driven.
www.protecsinc.com
| 27 |

Humidifier
A humidifier dispersion manifold is provided within the AHU to distribute steam to the respective zone. Steam is supplied from a gas fired steam humidifiers. The humidifier will utilize a space located humidity sensor.
15.10.2 Air System Requirements
Based on revised preliminary calculations, the projected supply airflows, zoning and type of unit are as follows:
| EQUIPMENT NO. | AREAS SERVED | APPROXIMATE MAX. SUPPLY/RETURN CFM | APPROXIMATE MINIMUM OA CFM | AIR DEVICE(S) | ||||
| AHU-04 | Process Area | 28,400 | 7,100 | HEPAs | ||||
| AHU-05 | Process Area |
28,400 | 7,100 | HEPAs | ||||
| AHU-06 | Filling | 7,500 | 2,500 | HEPAs | ||||
| AHU-07 | Micro Lab | 1,200 | 430 | HEPAs | ||||
| AHU-08 | Office Addition |
33,000 | 3,000 | Diffusers / Grilles | ||||
| AHU-09 | Laboratory & Warehouse Renovation Areas | 8,000 | 2,000 | Diffusers / Grilles | ||||
| CRU-1 / CU-1 | Automation Control |
3,750 | 0 | Diffusers / Grilles | ||||
| CRU-2 / CU-2 | Server Room |
885 | 0 | Diffusers / Grilles |
Refer to the Air Handling Unit zone maps for segregation of areas along with pressurization plans.
www.protecsinc.com
| 28 |

The supply and return ductwork distribution system will be composed of a network of galvanized steel sheet metal duct mains, sub-mains, laterals, and branches. Dampers will be provided at branch take-offs and diffuser take-offs to facilitate balancing. The ductwork will be constructed to SMACNA standards with respect to proper metal gauge, reinforcement, fitting construction, longitudinal seams, transverse seams and sealing. Unless not allowed by code, flexible ductwork will be used to connect to ceiling mounted air distribution devices. All supply and return sheet metal ductwork in unconditioned spaces will be insulated with a 1.5-inch thick fiberglass wrap.
All production and warehouse ductwork will be delivered cleaned with ends covered and stored on site per SMACNA Duct Cleanliness for New Construction Guidelines for Advanced Level. The supply ductwork will have a pressure class of +3” W.C. to the VAVs/FTU/Duct Heaters and a pressure class of +2” W.C. to the diffusers. The the return/exhaust ductwork will have a pressure class of -2” W.C. or -3” W.C as required.
Stainless steel ductwork will be provided at the drain exhaust hoods, humidifier manifolds, and at short radius low wall exhaust grilles in processing rooms.
Air will be supplied to the space at the top of the room through room side replaceable HEPAs and extracted from the space through low wall exhaust grilles in each production room. In the offices and laboratories, air will be supplied and returned via ceiling mounted diffusers.
The production areas will be designed for a room pressurization of 0.05” w.g between all rooms. Validation requirements should have a broader range (0.03” w.g.).
www.protecsinc.com
| 29 |

15.10.3 System Operation
| AIR HANDLING ZONE CONTROL | ||||||||||||||||
| COMPONENT | ENABLED | DISTRIBUTION (S / R) | ZONE HEATING MEANS | HUMIDIFIER | FLOW MEASURE | FILTER CHECK | FAN CONTROL | NOTES | ||||||||
| AHU- 04 | BAS | HB/HB | KW | Unit | N/A | M | VFD | Fan Speed Set at TAB | ||||||||
AHU- 05 |
BAS | HB/HB | KW | Unit | N/A | M | VFD | Fan Speed Set at TAB | ||||||||
| AHU- 06 | BAS | HB/HB | KW | Unit | N/A | M | VFD | Fan Speed Set at TAB | ||||||||
AHU- 07 |
BAS | HB/HB | KW | ES | N/A | M | VFD | |||||||||
| AHU- 08 | BAS | VAV | KW | N/A | DP | M | VFD | |||||||||
AHU- 09 |
BAS | VAV | KW | ES | DP | M | VFD | |||||||||
CRU-1 / CU-1 |
HOA | 1 Zone | Unit | Unit | N/A | N/A | HB | |||||||||
CRU-2 / CU-2 |
HOA | 1 Zone | Unit | N/A | N/A | N/A | HB | |||||||||
Enabled – This indicates how the unit is enabled – see key below:
BAS – The unit is enabled through the BAS once all safety / status switches are confirmed.
HOA – The unit is enabled through the HOA switch on the starter or VFD and will run after internal safeties wired through the start contacts are made.
Distribution (Supply / Return) – This indicates how the air is distributed – see key below:
1 Zone – This indicates the unit is a single zone constant volume unit. The air flow will be at a constant volume with the supply air temperature controlled by a sensor located in the zone, zone return air duct, or zone exhaust duct.
www.protecsinc.com
| 30 |

HB – This indicates the unit will be hard balanced via dampers to a specific set point with zone reheat coils. The unit will condition the supply air to a temperature as dictated by the duct mounted temperature sensor. Zone temperature control is accomplished via feedback from the zone sensors to the reheat coils. Multiple rooms will be included per zone and will be controlled to a critical room control. On a fall in temperature below the set point, the reheat coil will be energized to satisfy space temperature. Where the HB is indicated on the return side, this indicates that the return will be hard balanced to a specific set point.
VAV – Variable Air Volume Terminal units with integral heating coils will be utilized as the means to meet the required energy code.
Zone Heating Means – This indicates the heating medium for a zone – see key below:
KW – This indicates the heating coil in the duct or at the VAV or CV box is an electric coil. The coil will have an integral disconnect and air flow switch. The coil will be SCR controlled.
UNIT – This indicates the heating is provided at the unit.
HUMIDIFIER – This indicates the humidifying means for the air handling system – see key below:
ES – An electro-steam humidifier unit with its own dispersion unit will provide humidification to suit the zones as indicated. Electric immersion heaters convert domestic water to steam for distribution in the ductwork via a duct mounted dispersion manifold. The humidifier will have an automatic fill and drain cycle and an integral control panel for these types of safeties. The humidifier controls will include the zone sensor, an airflow switch, and a high limit sensor. The humidifier control will be via the BAS.
UNIT – This indicates the humidification is provided at the unit.
Flow Measure – This indicates if there is a flow measurement device to measure or control the flow of supply air.
DP – This indicates that a differential pressure switch will be used to confirm air flow in the system via a pressure sensing device mounted in the duct. It is used to sense if the unit is on or off. If used in an automatic mode, this sensor can be set to a specific point and provide feedback to the control system to control the fan speed.
www.protecsinc.com
| 31 |

Filter Check – This indicates if there is a means provided to check the differential pressure across the unit mounted filters – see key below:
M – This indicates a magnehelic gauge will be installed across the filters to indicate the pressure drop.
Fan Control – This indicates how the supply or supply and return fans on the system are controlled. – see key below:
VFD – This indicates that the VFD can provide automatic control based on feedback from an air flow monitor or differential pressure switch. If there is not air flow monitor or pressure sensor, then fan speed will be adjusted manually.
MVFD – This indicates the fan speed will be adjusted manually through the VFD.
HB – This indicates the fan speed will be adjusted during balancing by installing the sheaves to meet the design criteria.
15.10.4 Automatic Temperature Controls and Monitoring
The existing Siemens Building Automation System (BAS) will be expanded to provide the HVAC temperature and humidity controls.
This system will utilize feedback sensor input with microprocessor controllers to allow for independent operation of the HVAC systems. All actuators and operators will be electronic, and of the proportionally modulating type with feedback capabilities. All control components and end devices located within cGMP spaces will be of industrial type, high accuracy, factory calibrated and field adjustable/calibrateable. Standard BAS controllers and instrumentation will be supplied where possible. The below is a general controls approach for all non-process controlled systems:
| 1. | Cleanroom Area |
| a. | RTU-1/RTU-2 (Correct Tag – AHU-4/AHU-5) |
| i. | DAT control (Sensor Factory Installed In Unit) (DAT set point: 55F) | |
| ii. | Supply fan control (manually set VFD) | |
| iii. | Return fan control (manually set VFD) |
www.protecsinc.com
| 32 |

| iv. | (Dehumidification set point 60%RH) (2 RH sensors in GMP Production#170, 1 RH sensor Weigh Room #171, 1 RH sensor Commodity Prep #172) Wall mounted, wash down/cleanroom rated sensors will be furnished and installed by BAS subcontractor and connected to BAS. BAS to send highest RH reading to RTU. False readings will be disregarded. | |
| v. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points and control inputs) | |
| vi. | Supply & Return Smoke detectors to be provided with AHU and factory installed. | |
| vii. | Supply and return air smoke isolation damper to be furnished by sheet metal subcontractor and wired to unit controller. Isolation damper will be controlled by AHU controller. | |
| viii. | No economizer. |
| b. | GFH-1/H-1, GFH-2/H-2 |
| i. | (2 RH sensors in GMP Production#170, 1 RH sensor Weigh Room #171, 1 RH sensor Commodity Prep #172) Wall mounted, wash down/cleanroom rated sensors will be furnished and installed by BAS subcontractor and connected to BAS. BAS to send RH reading furthest from set point to each humidifier. False readings will be disregarded. Both humidifiers will be controlled by same signal. | |
| ii. | Duct high limit sensor (sensor purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) | |
| iii. | Air proving switch (switch purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) | |
| iv. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points and control inputs) (Humidification Set Point 40%RH) |
www.protecsinc.com
| 33 |

| c. | EHC-149, EHC-164, EHC-165, EHC-167, EHC-169, EHC- 170-A, EHC-170-B, EHC-170-C, EHC-170-D, EHC-171, EHC-172, EHC-173, EHC-173A, EHC-175, EHC-156, EHC- 176 |
| i. | Temperature Sensors (Furnished & installed in space by BAS subcontractor, wall mounted near monitoring system temp sensor, wash down/cleanroom rated sensor, calibratable, 1 sensors per duct heater, +/-1F accuracy by BAS Subcontractor) | |
| ii. | Duct Heaters purchased with SCR controls. Duct heater output commanded through BAS. (Duct heater set point 68F, set point will be entered in BAS) | |
| iii. | Air proving switch included integral with the duct heater. |
| 2. | Filling Cleanroom Area |
| d. | RTU-3 (Correct Tag – AHU-6) |
| i. | DAT control (Sensor Factory Installed In Unit) (DAT set point: 55F) | |
| ii. | Supply fan control (manually set VFD) | |
| iii. | Return fan control (manually set VFD) | |
| iv. | Dehumidification set point 60%RH (1) wall mounted wash down/cleanroom rated sensor will be furnished and installed by BAS subcontractor and connected to BAS. BAS will send space RH reading to RTU through Bacnet. | |
| v. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points, input values) | |
| vi. | Supply & Return Smoke detectors to be provided with AHU and factory installed. | |
| vii. | No economizer. |
| e. | GFH-3/H-3 |
| i. | (1) Wall mounted wash down/cleanroom rated sensor will be furnished and installed by BAS subcontractor and connected to BAS. BAS will send space RH reading to RTU through Bacnet. | |
| ii. | Duct high limit sensor (sensor purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) | |
| iii. | Air proving switch (switch purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) | |
| iv. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points, send control value) (Humidification Set Point 40%RH) |
www.protecsinc.com
| 34 |

| v. | EHC-168, EHC-168B | |
| vi. | Temperature Sensors (Furnished & installed in space by BAS subcontractor, wall mounted near monitoring system temp sensor, wash down rated sensor, 1 sensors per duct heater, +/-1F accuracy) | |
| vii. | Duct Heaters purchased with SCR controls. Duct heater output commanded through BAS. (Duct heater set point 65F, set point will be entered in BAS) | |
| viii. | Air proving switch included integral with the duct heater. |
| 3. | MicroLab |
| f. | RTU-4 (Correct Tag – AHU-7) |
| i. | DAT control (Sensor Factory Installed In Unit) (DAT Set Point: 55F) | |
| ii. | Supply fan control (manually set ECM Motor) | |
| iii. | Dehumidification set point 60%RH (1) wall mounted sensor located near monitoring system will be furnished and installed by BAS subcontractor and connected to BAS. BAS to send value to RTU through Bacnet. | |
| iv. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points and control value) | |
| v. | Duct smoke detector to be furnished and wired by FA subcontractor, installed in ductwork by sheet metal subcontractor | |
| vi. | No economizer. |
| g. | HUM-1 (Generation Unit to be Located in Lab Water Room #143) |
| i. | (1) Wall mounted sensor will be furnished and installed by BAS subcontractor and connected to BAS. BAS to send RH reading to the humidifier. | |
| ii. | Duct high limit sensor (sensor purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) | |
| iii. | Air proving switch (switch purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) | |
| iv. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points) (Humidification Set Point 40%RH) |
www.protecsinc.com
| 35 |

| h. | EHC-119 |
| i. | Temperature Sensor (Furnished & installed in space, wall mounted near monitoring system temp sensor, 1 sensors per duct heater, +/-1F accuracy by BAS Subcontractor) | |
| ii. | Duct Heaters purchased with SCR controls. Duct heater output commanded through BAS. (Duct heater set point 70F, set point will be entered in BAS) | |
| iii. | Air proving switch included integral with the duct heater. |
| 4. | Office Area |
| i. | RTU-5 (Correct Tag – AHU-8) |
| i. | DAT control (Sensor Factory Installed In Unit) (DAT Set Point: 55F) | |
| ii. | Supply fan control (Duct static pressure sensor purchased with RTU, installed In supply ductwork & wired to unit controller by sheet metal subcontractor) | |
| iii. | Return fan control (Building pressure sensor purchased with RTU, installed in ceiling and wired to unit controller by sheet metal subcontractor) | |
| iv. | Dehumidification control (sensor purchased with RTU, Installed In Return Air Ductwork and wired to unit controller by sheet metal subcontractor) (Dehumidification set point 55%RH) | |
| v. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points) |
| vi. | Supply & Return Smoke detectors to be provided with AHU and factory installed. | |
| vii. | Economizer (Enthalpy control, factory installed sensor) |
| b) | VAV-1, VAV-2, VAV-2, VAV-3, VAV-4, VAV-5, VAV-6, VAV- 7, VAV-8, VAV-9, VAV-10, VAV-11, VAV-12, VAV-15, VAV- 16, VAV-107 |
| i. | Temperature Sensor (Furnished & Installed in space, wall mounted, 1 per heater, sensor +/-2F accuracy by BAS Subcontractor)(VAV with no heater shall be controlled by shared sensor in space) | |
| ii. | VAV Heaters Command by BAS. (Duct heater set point 70F) | |
| iii. | Air proving switch furnished with VAV. | |
| iv. | VAV Controls provided by BAS Subcontractor. |
www.protecsinc.com
| 36 |

| c) | VAV-M-1, VAV-M-2 |
| i. | Constant Volume, controls by BAS subcontractor. |
| d) | FTU-1, FTU-2, FTU-3, FTU-4, FTU-5, FTU-7, FTU-8, FTU-9, FTU-10, FTU-11, FTU-12, FTU-13, FTU-14, FTU-15 |
| i. | Temperature Sensor (Furnished & Installed in space, wall mounted, 1 per heating sensor +/-2F accuracy by BAS Subcontractor) | |
| ii. | FTU Heaters Command by BAS. (Duct heater set point 70F) | |
| iii. | Air proving switch furnished with FTU. | |
| iv. | FTU Controls provided by BAS Subcontractor. |
| 5. | Lab Area |
| b. | RTU-6 (Correct Tag – AHU-9) |
| i. | DAT control (Sensor Factory Installed In Unit) (DAT Set Point: 55F) | |
| ii. | Supply fan control (Duct static pressure sensor purchased with RTU, installed In supply ductwork & wired to unit controller by sheet metal subcontractor) | |
| iii. | Return fan control (Building pressure sensor purchased with RTU, installed in ceiling and wired to unit controller by sheet metal subcontractor) | |
| iv. | Dehumidification control (sensor purchased with RTU, Installed In Return Air Ductwork and wired to unit controller by sheet metal subcontractor) (Dehumidification set point 55%RH) | |
| v. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points) | |
| vi. | Supply & Return Smoke detectors to be provided with AHU and factory installed. | |
| vii. | Economizer (Enthalpy control, factory installed sensor) |
| c. | HUM-2 (Generation Unit to be Located in Lab Water Room #143) |
| i. | (1) Wall mounted sensor will be furnished and installed by BAS subcontractor and connected to BAS. BAS to send RH reading to the humidifier. | |
| ii. | Duct high limit sensor (sensor purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) |
www.protecsinc.com
| 37 |

| iii. | Air proving switch (switch purchased with humidifier, installed in supply ductwork and wired to humidifier controller by sheet metal subcontractor) | |
| iv. | BacNET card for interface to BAS System (BAS to monitor unit function and input set points and space reading) (Humidification Set Point 40%RH) |
| d. | VAV-139, VAV-143, VAV-123, VAV-118, VAV-121, VAV- 105, VAV-150, VAV-148, VAV-156 |
| i. | Temperature Sensor (Furnished & Installed in space, wall mounted, 1 per heater, sensor +/-2F accuracy by BAS Subcontractor) | |
| ii. | VAV Heaters Command by BAS. (Duct heater set point 70F) | |
| iii. | Air proving switch furnished with VAV. | |
| iv. | VAV Controls provided by BAS Subcontractor. |
| 6. | Boiler B-1 |
| e. | Stand Alone factory controls. | |
| f. | Boiler Trouble Alarm tied into BAS | |
| g. | Feed water Trouble Alarm tied into BAS |
| 7. | EF-1, EF-2, EF-3, EF-6, EF-8, EF-9, EF-10, EF-11, EF-12 |
| h. | Hard Balanced (Manually Set ECM) | |
| i. | EFs Status tied into BAS System (Line voltage/current sensor furnished, installed and wired by BAS subcontractor) |
| 8. | EF-5 |
| j. | Hard Balanced (Manually Set ECM) | |
| k. | Not connected to BAS |
| 9. | EF-7 |
| l. | Hard Balanced (Manually Set ECM) |
| m. | EFs Status tied into BAS System (Line voltage/current sensor furnished, installed and wired by BAS subcontractor) | |
| n. | Hydrogen Gas Alarm tied into BAS System (Hydrogen sensor furnished, installed and wired by BAS subcontractor) |
| 10. | SF-4-1, SF-4-2 |
| o. | Stand Alone Controls – Control Sensors Furnished and Installed by Sheet Metal Subcontractor. |
| 11. | Electric Cabinet Unit Heaters (CUH-1, CUH-2, CUH-3) |
| p. | Stand Alone Controls – Control Sensors Furnished and Installed by Sheet Metal Subcontractor. |
www.protecsinc.com
| 38 |

| 12. | Condensate Pumps (CP-1, CP-2) |
| q. | Stand Alone Controls – Control Sensors Furnished and Installed by Sheet Metal Subcontractor. |
| 13. | NG Unit Heater (GUH-1) |
| r. | Stand Alone Controls – Control Sensors Furnished and Installed by Sheet Metal Subcontractor. |
| 14. | NG Unit Heater (GUH-2) (Roof Mounted) |
| s. | Stand Alone Controls – Control Sensors Furnished and Installed by Sheet Metal Subcontractor. BAS subcontractor to connect to MAU to send an enable command that is tied to the generator operation. MAU to run only when normal power is lost. |
| 15. | Electric Unit Heaters (EUH 1-1, EUH 1-2) |
| t. | Stand Alone Controls – Control Sensors Furnished and Installed by Sheet Metal Subcontractor. |
| 16. | CRAC Units (CRU-1/CU-1) |
| u. | Stand Alone Controls – Control Sensors Furnished and Installed by Sheet Metal Subcontractor. | |
| v. | BAS Connection through BacNET interface. (Monitoring & Alarming by BAS Only) |
| 17. | Chilled Water System |
| w. | Stand alone existing chiller controls shall be utilized to operate chiller to maintain temperature. Piping subcontractor to furnish and wire temperature controlling sensors. | |
| x. | BAS subcontractor shall furnish control sensors and valves for installation by piping subcontractor. Control valves and sensor will be wired by BAS Subcontractor. | |
| y. | Chilled Water system pumps and associated VFDs shall be furnished by Piping subcontractor and will be wired to BAS system by BAS subcontractor for control by BAS system. | |
| z. | Use point control valves shall be furnished by Process Automation Controls subcontractor for installation by piping subcontractor. Process Automation Control devices and sensors will be wired by Process Automation subcontractor. |
| 18. | Waste Neutralization |
| aa. | Stand alone controls. Plumbing subcontractor shall receive and install controls purchased with waste neutralization system. | |
| bb. | Trouble Alarm tied into BAS (Wiring by BAS Subcontractor) |
| 19. | Generator |
| cc. | Stand Alone controls. Controls furnished with generator, installed and wired by Electrical subcontractor. |
www.protecsinc.com
| 39 |

| dd. | Trouble Alarm tied into BAS (Wiring by BAS Subcontractor) | |
| ee. | Run status tied to BAS for control of MAU. |
| 20. | Bulk Nitrogen |
| ff. | Stand Alone Controls furnished and installed by Owner’s vendor. | |
| gg. | Trouble Alarm tied into BAS (Wiring by BAS Subcontractor) |
| 21. | High Pressure Nitrogen |
| hh. | Pressure Alarm sensor furnished by BAS subcontractor, installed by plumbing subcontractor and wired by BAS subcontractor. |
| 22. | Air Compressor |
| ii. | Stand Alone controls. Controls furnished with Air Compressor and installed and wired to compressor controller by plumbing subcontractor. | |
| jj. | Trouble Alarm tied into BAS (Wiring by BAS Subcontractor) |
| 23. | VAC |
| kk. | Stand Alone controls. Controls furnished with Vacuums and installed and wired to vacuum controller by plumbing subcontractor. | |
| ll. | Trouble Alarm tied into BAS (Wiring by BAS Subcontractor) |
| 24. | Cold Boxes |
| mm. | Stand Alone controls furnished and installed by Cold Box subcontractor. No connection to BAS system. |
| 25. | Existing Loading Dock (FN-8-1, FN-8-2) |
BAS subcontractor will extend existing controls to new locations and modify as required for separate system operation. Existing pneumatic controls to be re-used to the greatest extent possible.
A separate building monitoring system that will be CFR 21, Part 11 compliant will be provided. This system will be separate from the BAS system. The monitoring system will monitor temperature, humidity and pressure in classified areas as indicated on the contract drawings.
| 15.11 | Systems Description – Exhaust and Supply Fans |
15.11.1 Toilet Areas (EF-5)
The toilet areas will be served by spun aluminum mushroom cap exhaust fans with integral backdraft dampers. These will be connected to the toilet areas via a galvanized duct system and exhaust air grilles.
www.protecsinc.com
| 40 |

15.11.2 Chemical Storage Exhaust (EF-2)
An exhaust fan will be provided in a utility fan set arrangement, upblast discharge with an exhaust stack. The fan will be mounted on rails on the roof. The fan will be similar to those manufactured by Twin City, Greenheck, Peerless or approved equal. The fan will run at a constant speed. There is not a redundant fan in the system. The exhaust ducts for this system will be for room exhaust only. The exhaust duct will be galvanized steel sheet metal duct constructed to a 2.0” pressure classification.
15.11.3 Fumehood Exhaust (EF-1)
An exhaust fan will be provided in a utility fan set arrangement, upblast discharge with an exhaust stack. The fan will be mounted on rails on the roof. The fan will be similar to those manufactured by Twin City, Greenheck, Peerless or approved equal. The fan will run at a constant speed. There is not a redundant fan in the system. The exhaust ducts for this system will be for room exhaust only. The exhaust duct will be galvanized steel sheet metal duct constructed to a 2.0” pressure classification.
15.11.4 Column Clean Rm. 175 (EF-3)
An exhaust fan will be provided in a utility fan set arrangement, upblast discharge with an exhaust stack. The fan will be mounted on rails on the roof. The fan will be similar to those manufactured by Twin City, Greenheck, Peerless or approved equal. The fan will run at a constant speed. There is not a redundant fan in the system. The exhaust ducts for this system will be for room exhaust only. The exhaust duct will be galvanized steel sheet metal duct constructed to a 2.0” pressure classification.
15.11.5 Utility Room Rm 184 (EF-4.1 & 4.2)
Regular aluminum, curb mounted supply fans with inlet filters and integral backdraft dampers will be utilized. Fans will be thermostatically controlled for summer time ventilation to maintain temperature.
15.11.6 Process Room Mechanical Chases (EF-6)
A spun aluminum, curb mounted exhaust fan with integral backdraft dampers will be utilized to remove the moist air associated with the CIP waste vapor removal. The fan will run constantly and be of the upblast discharge arrangement.
15.11.7 Battery Charging Station (EF-7)
A spun aluminum, curb mounted exhaust fan with integral backdraft dampers will be utilized to remove the hydrogen generated during lift truck battery recharging. The fan will run constantly and be of the upblast discharge arrangement.
www.protecsinc.com
| 41 |

15.11.8 COP Room (EF-8)
A spun aluminum, curb mounted exhaust fan with integral backdraft dampers will be utilized to help remove all residual moisture and particles associated with the COP operations. The fan will run constantly and be of the upblast discharge arrangement.
15.11.9 Weigh Room General Exhaust Fan (EF-9)
A spun aluminum, curb mounted exhaust fan with integral backdraft dampers will be utilized to help remove particles associated with the weighing operations and prevent return to the main AHU. The fan will run constantly and be of the upblast discharge arrangement. Fan will connect to the built in slot hood installed in the dispensing enclosure.
15.11.10 COP 177A (EF-11)
A spun aluminum, wall mounted exhaust fan with integral backdraft dampers will be utilized to help remove all residual moisture and particles associated with the COP operations. The fan will run constantly.
15.11.11 QC Lab Suite General Exhaust Fan (EF-12)
A new utility set exhaust fan similar to existing installed on the existing fan support dunnage serving the expanded QC Lab Suite.
15.11.12 Column Packing Exhaust Arm Exhaust Fan (EF-10)
Relocated axial fan and exhaust hood/arm system discharging through roof with a stack to 10’ above the roof.
| 15.12 | Central Utility System Descriptions |
The following plant utility systems will provide full capacity for the new Manufacturing Suites:
| ● | Plant Steam and Condensate System (Tied Into Existing System) |
A detailed description of the systems follows:
15.12.1 Chilled Water System
See division 17.0 for details.
15.12.2 Plant Steam and Condensate System
The existing 80 HP boiler will remain.
www.protecsinc.com
| 42 |

One (1) new 50 HP gas fired, Low-pressure boiler will be provided. The boiler will be packaged fire tube type, including a forced draft, natural gas fired power burner and control panel. The boiler package will be provided with all code required safety devices; controls, fuel train and flex connections. The system will tie into a common distribution header with the existing to serve the process equipment.
15.12.2.2 System Operation
The new boiler will be controlled by factory provided stand-alone controls. A trouble alarm will be connected to the plant BAS system.
15.12.2.3 Steam and Condensate Distribution System
The system will utilize two-way modulating control valves. Isolation valves, coil valve rig by-passes, strainers, vents, drip legs, traps, expansion provisions, and temperature and pressure read-out devices will be included in this piping. Gate valves with union bonnets will be used in all piping 2” and smaller, OS&Y gate valves will be used in all piping 2 ½” and larger. Isolation valves will be provided in all branch/take-off lines. All valves will be tagged and piping runs labeled at twenty-foot intervals and all changes in direction. The condensate piping will be schedule 80.
The steam piping material 2 ½” and larger will be carbon steel schedule 40 with welded fittings. Piping 2” and smaller will be schedule 40 carbon steel with screw fittings. Screw fittings will not be used over the production area; socket weld or butt weld pipe and fittings will be used.
All steam piping will be insulated with fiberglass and have a pre-formed ASJ cover. Where this insulation is exposed to a cleanroom air stream; the jacket will be covered with PVC or epoxy painted. Ball joints or expansion joints will be used in conjunction with expansion loops in the piping to control expansion. Roller hangers will be used where appropriate.
www.protecsinc.com
| 43 |

| 15.13 | PLUMBING |
This section is dedicated to the description of the proposed Plumbing/Laboratory and Process Utilities Piping Systems for the project. Systems will be designed in accordance with the latest applicable codes, standards and authorities having jurisdiction. The balance of this section discusses the extension of the water systems to serve the process equipment and the new compressed air, vacuum, bulk nitrogen system and process waste.
Plumbing/Laboratory and Process Systems:
| ● | Domestic Cold Water | |
| ● | Domestic Hot water | |
| ● | Laboratory/Process Cold Water (Non-Potable Cold Water) | |
| ● | Laboratory/Process Hot Water (Non-Potable Hot Water) | |
| ● | Tepid water for safety shower eyewash stations | |
| ● | House nitrogen | |
| ● | Clean dry air | |
| ● | Natural gas piping | |
| ● | DI water | |
| ● | Vacuum | |
| ● | Sanitary drainage | |
| ● | Process Waste System |
The following sections describe the approach for the project’s plumbing, laboratory and process utility piping systems.
15.13.1 General
| ● | Sleeves will be provided for piping penetrations at walls, floors, and roofs of mechanical areas. Floor sleeves will extend above finished floor in exposed areas and will be flush with floor when concealed in walls. | |
| ● | The new plumbing fixtures, equipment and systems will be installed and located as shown on the architectural plans with ADA compliance as indicated. | |
| ● | All piping penetrations through fire rated walls and floors will be caulked and sealed with a fire-stopped material to maintain the fire rating integrity. | |
| ● | All utility services will be valved for branch-to-header connections and at all points of use and to allow isolation of equipment for maintenance and modifications. Access will be provided to the valves. | |
| ● | The design criteria that will be used for water calculations are 66 psig static pressure and 63 psig residual pressure at 1,280 GPM. | |
| ● | The design criteria that will be used for the natural gas system is 25-99 psig street pressure with a pressure reducing valve supplying 5 psig into the building. |
www.protecsinc.com
| 44 |

15.13.1.1 Seismic Restraint Criteria
| ● | Piping systems will be seismically restrained per minimum code requirements (see Structural Section). Any systems that require restraint and the applicable industry standards will be identified. |
15.13.1.2 Maintainability Criteria
| ● | A primary goal of the new facility is to isolate the routine maintenance access to laboratory and process support systems from lab/process primary circulation. Access to all terminal devices serving laboratory and process spaces in the new facility will be located outside of the lab/process envelope. |
15.13.1.3 Expansion Criteria
| ● | No provisions will be made for future expansion in the new facility. |
15.13.2 Systems Description
15.13.2.1 Water Systems
| ● | The domestic water system will be supplied from a connection to the existing water main service located in the service entrance mechanical room. The domestic water system will supply cold-water to plumbing fixtures, equipment and the laboratory/process water system. | |
| ● | The water service will be designed to provide water to the building’s fixtures and equipment at a minimum pressure of 40 psig at the most remote point. Maximum pressure will not exceed 80 psig at a fixture and distribution and flow velocity will not exceed 8 fps. The buildings service main size is anticipated to be 4” diameter. Pending final water pressure data, it is not anticipated that a water booster system will not be required. | |
| ● | The building’s water system is isolated from the municipal water system by a reduced pressure zone backflow preventer assembly. The domestic and process water systems located downstream of the water meter will each incorporate their own backflow preventers sized at 100% of the design load. The process water systems will be designed to be a dedicated building system that will be isolated from the domestic water system. The lab/process cold water supply will be a non-potable water system supplying cold water to the laboratory and process water consuming fixtures and equipment. The non-potable water system will also supply the lab/process non-potable hot water system. |
www.protecsinc.com
| 45 |

| ● | A new domestic electric hot water heater will be provided for the fit- out. Domestic hot water will be generated at 140°F and distributed at 120°F. The domestic hot water system will incorporate a master digital type thermostatic mixing valve that will reduce water temperatures to the desired system distribution temperature of 120°F. The hot water heater will be located within the Janitors Closet 161A. The 120°F water system is anticipated to be a re-circulated system, which will be returned back to the generation heater. | |
| ● | All safety shower and eye wash equipment will be supplied with tepid water from the existing emergency safety thermostatic mixing valve located in utility room 125. |
All water piping will be distributed in type “L” copper tubing, hard drawn, ASTM B88, with 95/5tin antimony silver soldered joints on piping 2 ½” and smaller and grooved coupling with rolled grooved joints on piping 3” and larger. Valves will be two piece full port ball valves on piping 3” and OS&Y gate valves on piping 4” and larger. All piping will be insulated using fiberglass insulation. A PVC exterior jacket will be applied to all domestic water piping when exposed to view in finished areas and exterior building areas.
15.13.2.2 Drainage
This project will require modifications to the existing facility sanitary and process drainage systems.
Storm Drainage
| ● | This project will not require modifications to the existing facility storm drainage system. |
Sanitary
| ● | The sanitary waste and vent system will be extended to new domestic fixtures and equipment locations to support the renovations. Plumbing fixtures will be drained by gravity through soil, waste and vent stacks, building drains and building sewers to the building main sanitary sewer. | |
| ● | All fixtures including trench drains and floor drains will be trapped and vented to atmosphere. Vents will be extended through the roof. Sanitary waste will not be treated. | |
| ● | Waterless trap primers (trap guards) will be provided at floor drains and trenches not having daily water flow. |
www.protecsinc.com
| 46 |

| ● | The sanitary waste system will be designed to maintain a minimum velocity of 2 fps. The sanitary vent system will be designed so that the differential pressure at any point in the building does not exceed 1 inch water column. | |
| ● | Floor drains will not be provided within immediate location of emergency showers. | |
| ● | Below ground sanitary waste and vent piping will be Schedule 40 PVC DWV with drainage pattern fittings and solvent cement joints. Above ground sanitary waste and vent piping will be Schedule 40 PVC DWV with drainage pattern fittings and solvent cement joints. | |
| ● | Sanitary piping receiving heating boiler condensate discharge and loading dock washdown shall be ductile iron pipe and DWV fittings meeting AWWA C110/A21.10 and push on joints ASTM A 746. |
Process Waste
| ● | Floor drains in the process area and process equipment will be drained to a separate process waste system. The process waste system will drain through dedicated waste and vent stacks, building drains and building sewers to the facility process waste system. The process waste will be run by gravity to a sump basin where it will be pumped to a central neutralization system. | |
| ● | The process waste system will be designed to maintain a minimum velocity of 2 fps. The process vent system will be designed so that the differential pressure at any point in the building does not exceed 1 inch water column. | |
| ● | A pH monitor with chart recorder will be located where the process waste main discharges from the neutralization equipment. The neutralization system will have a “trouble” alarm tied into the facility BAS system. | |
| ● | All waste stream discharge temperatures will be controlled and discharged at <140°F prior to discharging to the below ground waste system. | |
| ● | Floor drains located in the process area will be fitted with solid type 304 stainless steel tops incorporating neoprene rubber gaskets continuous around perimeter of solid top. Removal of the solid tops shall be accomplished by a hand held suction device. Floor drains located in ventilated shafts that receive drain down discharge from GMP areas will be open with no installed top. | |
| ● | Below ground process waste and vent piping will be Schedule 40 CPVC DWV with drainage pattern fittings and solvent cement joints. Above ground process waste and vent piping will be Schedule 40 CPVC DWV with drainage pattern fittings and solvent cement joints. |
www.protecsinc.com
| 47 |

| ● | Process waste discharge rates from the C-800 and C-900 CMP areas is based on a combined instantaneous flow rate of 32 gallons per minute (GPM) based on gravity flow. |
Neutralization System
| ● | C-500 process waste neutralization system is existing and will remain unchanged. | |
| ● | The facility will be fitted with a new neutralization skid equipped to handle 32 GPM peak flow and adjust pH prior to discharging to the municipal street sewer. | |
| ● | The neutralization system will incorporate a two (2) tank system that will be programmed to balance the pH settings between tanks with the final pH discharge set between 6-9. The tanks shall be provided for a chemical neutralization maintaining a discharge pH range between 6-9 with both acid and caustic injection. The neutralization system shall be equipped with a sampling port prior to the final connection to the sanitary sewer system. The neutralization system shall be an automatic system with self regulating caustic and acid injection pumps adjusting pH ranges that will be acceptable to the municipal waste water treatment plant. PH shall be monitored between each storage tank and final discharge running trap. | |
| ● | The acid and caustic supply lines will be installed in an open ended containment pipe that will be drained back to the neutralization tanks. The intent of the containment pipe is to prevent line breakage and reconnection of tank supplies from coming in contact with personnel. | |
| ● | The neutralization system main control panel will be provided with a common trouble alarm that will be monitored and supervised by the building BAS system. | |
| ● | The process gravity waste system shall be collected by a single underslab fiberglass vertical basin with a steel top with individual pump removal access hatches. The steel top shall be anchored and gasket to the finished floor. The basin will incorporate duplex stainless steel pumps that will be sized to handle flow and pressure discharged from the process equipment. | |
| ● | The sump basin stainless steel pumps will pump waste effluent to the neutralization stage one tank where it will begin to neutralize the effluent. |
www.protecsinc.com
| 48 |

| ● | The waste water sump pumps will be controlled for both lead/lag/emergency on (two pumps running) and automatic telemetry that will be controlled by a main pump control panel. | |
| ● | The interior basin effluent level system will be controlled by a float system that will control pump on/off, emergency on (pump No.2) and high water alarm signals. The alarm signals will be remote to the pump control panel. |
15.13.2.3 Plumbing Fixtures and Equipment
| ● | All plumbing fixtures will be selected in accordance with water conservation codes and ADA standards. | |
| ● | Water Closets: 1.28 gpf water-saver, vitreous china wall-hung siphon jet type. Flush valve will be battery powered sensor operated. | |
| ● | Urinals: 0.125 gpf water-saver, vitreous wall-hung washout type flush valves. Flush valve will be battery powered sensor operated. | |
| ● | Lavatories: Wall-hung or countertop type units, with 1 gpm restricted flow faucets receiving separate hot and cold water supplies. Faucets will be battery operated. The faucet operation will have a maximum water flow duration of 30 seconds giving a flow usage of 0.17 gallons. | |
| ● | Break Room Sinks: Countertop type units, with 2.5 gpm restricted flow faucets. | |
| ● | Equipment Staging / COP Scullery Sink: Floor mounted, 24-inch x 36-inch x 17-inch deep single bowl with two 24-inch integral drainboards, stainless steel sinks with no integral faucets (water supplied via WFI drop). | |
| ● | Commodity / Equipment Prep Scullery Sink: Floor mounted, 24-inch x 24- inch x 17-inch deep single bowl with two 24-inch integral drainboards, stainless steel sinks with no integral faucets (water supplied via WFI drop). | |
| ● | Aseptic Equipment Prep Scullery Sink: Floor mounted, 20-inch x 20- inch x17-inch deep single bowl with right side 24-inch integral drainboard, stainless steel sinks with ½ gpm restricted flow hand- operated faucets with wrist blade handles and gooseneck spout. | |
| ● | Mop Receptors: Terrazzo type with wall mounted hose-end type faucet, complete with vacuum breaker and pail hook. | |
| ● | Safety Shower & Eyewash: Speakman SE-1200 or 1255. Floor drains will be provided at locations were underground work is already being done. | |
| ● | Drinking Foutains: Wall hung electric water cooler, barrier free for handicapped use. |
www.protecsinc.com
| 49 |

15.13.2.4 Compressed Air
| ● | The existing reciprocating compressor will be demolished. | |
| ● | The existing 90 scfm compressor and dryer will remain. | |
| ● | A new air system will provide 225 scfm of compressed air. The new system will be tied into a distribution manifold to supplement the existing compressor and extend from the new manifold to all use points. Compressed air will be generated at 110 psig to provide air at building use points at the pressure required. | |
| ● | The compressed air system will be located in the utility space. The compressed air system will consist of an air-cooled, two stage, oil- free rotary screw compressor, duplex pre-filters, simplex final filter, existing receiver tank (390 gal), desiccant dryer and associated controls. Compressed air will be filtered and dried to a –40 degree F pressure dew point. Air will then be filtered through an after filter and a final filter with a 0.2 micron removal rating. | |
| ● | All compressed air filters at generation will be sized to handle 100% of the daily calculated load of 225scfm. | |
| ● | Compressed air piping shall be delivered to the rooms at 100 psi. | |
| ● | Piping will run above the 1st floor ceiling and drop down to equipment locations along the wall, terminating at a quarter turn ball valve. | |
| ● | Piping material will be type “L” hard drawn copper with nitrogen purged brazed joints. Joints will be brazed (BcuP Series brazing) without flux, while being brazed all joints will be continuously purged with an inert gas to prevent the formation of scale. Valves will two-piece full port ball valves. All piping, fittings, valves, and accessories will be provided cleaned for oxygen service. | |
| ● | Type 316L stainless steel piping with swage lock compression fitting will be provided within the GMP process areas. | |
| ● | Compressed air piping system will be sized based on 1 scfm per outlet or the outlet requirements. Diversity factors will be applied to laboratory outlets as indicated below. |
www.protecsinc.com
| 50 |

Table 2 Compressed Air System Diversity Factors | ||||||
Number of Outlets |
Diversity Factor |
Minimum Flow (scfm) |
Empirical Formula for Flowrate (scfm) | |||
| 1-5 | 1.00 | 0 | No. of Outlets*1 | |||
| 6-12 | 0.80 | 5 | 5+(No. of Outlets-5)*5/7 | |||
| 13-33 | 0.60 | 10 | 10+(No. of Outlets-12)*10/21 | |||
| 34-80 | 0.50 | 20 | 20+(No. of Outlets-33)*20/47 | |||
| 81-150 | 0.40 | 40 | 40+(No. of Outlets-80)*20/70 | |||
| 151-315 | 0.35 | 60 | 60+(No. of Outlets-150)*50/165 | |||
| 316-565 | 0.30 | 110 | 110+(No. of Outlets-315)*60/250 | |||
| ● | The piping system will be sized to limit pressure drop across the system to a maximum of 10% of the pressure regulator pressure. | |
| ● | The compressed air equipment will be controlled by the stand alone main control panel and will be provided with a common trouble alarm that will be monitored and supervised by the building BAS system. |
15.13.2.5 Nitrogen
| ● | A liquid nitrogen tank and ambient vaporizer will be provided by a contracted vendor. The equipment will be located at the exterior of the building. The new tank and equipment will provide 7,000 scfh at 45 psig. Gaseous nitrogen service will be from the new liquid storage tank system. The liquid storage tank, vaporizer and associated components will be leased equipment. | |
| ● | The nitrogen service to the facility will be fitted with duplex particulate filters each rated for 1 micron of filtration located at the exterior next to the ambient vaporizers. All piping will be sloped and trapped at low points for accumulation of condensation. | |
| ● | The nitrogen system distribution pressure to all equipment and points of use shall be set to deliver pressures at 40 psig. | |
| ● | Higher pressure nitrogen for the GPM area HPLC operation is provided from local gas cylinders and changeover manifolds provided by the owner. | |
| ● | Nitrogen will be filtered through a final sterile filter rated for 0.2 micron removal rating. All point of use outlets and GMP area equipment will be fitted with point of use sterile filers. | |
| ● | Piping will run above the first-floor ceiling and drop down to equipment locations along the wall, terminating at a quarter turn ball valve. | |
| ● | Nitrogen piping will be ASTM B-819 Type “L” piping with brazed joints. Joints will be brazed (BcuP Series brazing) without flux, while being brazed all joints will be continuously purged with an inert gas to prevent the formation of scale. |
www.protecsinc.com
| 51 |

| ● | Type 316L stainless steel piping with swage lock compression fitting will be provided within the GMP process areas. | |
| ● | Valves will be 2 piece full port ball valves. | |
| ● | All piping, fittings, valves, and accessories will be provided cleaned for oxygen service. | |
| ● | A 0.2 micron filter will be provided at each point of use. | |
| ● | The nitrogen system will be designed to distribute low pressure nitrogen. Pressure will be reduced as necessary at points-of-use. The system will be sized based upon a load of 1 scfm per outlet and the total number of connected outlets connected to the system. Any point loads for specific equipment will be added to the outlet load after any diversity factors are applied. The diversity factors indicated below will be used for determining the load for outlets. |
Table 2 Nitrogen System Diversity Factors | ||||||
Number of Outlets |
Diversity Factor |
Minimum Flow (scfm) |
Empirical Formula for Flowrate (scfm) | |||
| 1-5 | 1.00 | 0 | No. of Outlets*1 | |||
| 6-12 | 0.80 | 5 | 5+(No. of Outlets-5)*5/7 | |||
| 13-33 | 0.60 | 10 | 10+(No. of Outlets-12)*10/21 | |||
| 34-80 | 0.50 | 20 | 20+(No. of Outlets-33)*20/47 | |||
| 81-150 | 0.40 | 40 | 40+(No. of Outlets-80)*20/70 | |||
| 151-315 | 0.35 | 60 | 60+(No. of Outlets-150)*50/165 | |||
| ● | The piping system will be sized to limit pressure drop across the system to a maximum of 10% of the pressure regulator pressure. | |
| ● | The nitrogen generation equipment will be monitored by the vendor for tank fill and tank pressure set points. |
15.13.2.6 Natural Gas
| ● | The existing natural gas service is supplied by PECO. The street main located in Souder Road fluctuates pressures between 25-99 psig. The street main has built in capability for all future needs in the area. | |
| ● | The building existing incoming gas main is sized at 2 inches with pressures fluctuating between 25-99 psig. |
www.protecsinc.com
| 52 |

| ● | The building existing 2 inch gas main will remain in place and supply all building gas requirements for existing building needs and all new building expansions. | |
| ● | The gas meter and main pressure regulator was replaced to handle the new building gas loads and capacities. The new pressure regulator was set to deliver 5 psig gas pressure to the building. | |
| ● | The existing building pressure regulator located in the boiler room will remain as designed and pressures will be maintained to match the existing equipment requirements. | |
| ● | The new expansion will be fitted with one main pressure regulator assembly that will reduce the incoming 5 psig gas pressure down to 2 psig. The 2 psig gas main will be distributed to local pressure regulators throughout the building for gas pressure control to equipment. | |
| ● | The emergency electrical generator will incorporate its own pressure regulator assembly located at the exterior of the building. The incoming 5 psig gas pressure will be reduced down to 14” wc. Exterior signage will be provided above the main shut-off valve for the gas supply to the emergency electrical generator. | |
| ● | Natural gas will be distributed in schedule 40 black steel, Grade B piping with wrench operated plug valves. High-pressure piping (greater than 1/2 psig) and all piping 2-1/2” and larger will have butt- welded joints. Low pressure piping (1/2” psig or less) which is 2” and smaller and exposed to view, will have threaded joints. All concealed piping in walls or above ceiling will have butt-welded joints. | |
| ● | All gas piping materials, components and installation will comply with NFPA 54 “National Fuel Gas Code”. |
15.13.2.7 Vacuum
| ● | A vacuum system will be provided to all laboratory and process areas as programmed. | |
| ● | Two (2) 150 acfm @ 26” Hg vacuum pumps will be relocated to the new facility. The vacuum pumps will be tied together into a common header to service the expansion use requirements for Filling, C-800 and C-900. | |
| ● | Piping will run above the ceiling and drop down to equipment locations along the wall, terminating at a quarter turn ball valve. | |
| ● | Vacuum piping will be ASTM B-819 Type “L” piping with brazed joints. Joints will be brazed (BcuP Series brazing) without flux, while being brazed all joints will be continuously purged with an inert gas to prevent the formation of scale. |
www.protecsinc.com
| 53 |

| ● | Type 316L stainless steel piping with swage lock compression fitting will be provided within the GMP process areas. | |
| ● | Valves will be 2 piece full port ball valves. | |
| ● | All piping, fittings, valves, and accessories will be provided cleaned for oxygen service. | |
| ● | Laboratory vacuum piping system will be sized based on 0.5 scfm per outlet plus any flow required for individual pieces of equipment. Diversity factors will be applied to laboratory outlets as indicated below. |
Table 2 Laboratory Vacuum System Diversity Factors | ||||||
| Number of Outlets | Diversity Factor | Minimum Flow (scfm) |
Empirical Formula for Flowrate (scfm) | |||
| 1-5 | 1.00 | 0 | No. of Inlets*0.5 | |||
| 6-12 | 0.80 | 2.5 | (5+(No. of Inlets-5)*5/7)*0.5 | |||
| 13-33 | 0.60 | 5 | (10+(No. of Inlets-12)*10/21)*0.5 | |||
| 34-80 | 0.50 | 10 | (20+(No. of Inlets-33)*20/47)*0.5 | |||
| 81-150 | 0.40 | 20 | (40+(No. of Inlets-80)*20/70)*0.5 | |||
| 151-315 | 0.35 | 30 | (60+(No. of Outlets-150)*50/165) *0.5 | |||
| 316-565 | 0.30 | 55 | (110+(No. of Outlets-315)*60/250) *0.5 | |||
| ● | The piping system will be sized to limit pressure drop across the system to maximum of 5 inches of mercury vacuum. All piping will be sloped and trapped at low points for the accumulation of condensation. | |
| ● | The laboratory vacuum system will not be designed to handle highly corrosive or hazardous applications. | |
| ● | Minimum vacuum pressure to all use points is sized for 19” Hg. | |
| ● | The vacuum system is designed and based on 50 scfm (252 acfm) at 19” at all equipment and outlet usage points with a pump inlet pressure of 26” Hg. |
www.protecsinc.com
| 54 |

15.13.2.8 RODI Lab Water System – See Division 17
| 15.13 | FIRE PROTECTION |
| 15.13.1 | Introduction |
The project will require new fire protection systems to support the renovated area. The intent of this work is to provide a new sprinkler system for the new building extension from the existing underground combined service main. The new areas will be fully sprinklered.
The codes, standards, and regulations cited in Section 1 will be used and referred to for the design of this project.
The balance of this section describes the approach for the project’s fire protection systems.
| 15.13.2 | Systems Description and Criteria |
The city water feeds into the mechanical room as an independent feed for fire water. The 8” line enters into the building and is distributed to the facility. A new 6” branch line will be taken from the main to the expansion area. The design criteria will be a hydraulically calculated automatic wet sprinkler system, ordinary hazard, with a density as determined by code for specific interior room areas and applications as noted below.
Water Flow Data Required (Available per HbO2 provided data)
Static PSI = 66
Residual PSI = 63
Flow GPM = 1,280
Hazard Occupancy and Design Criteria
Ordinary Hazard # – Wet type sprinkler system
| ● | 0.15 GPM per SF over most remote 1500 SF | |
| ● | 130 SF max. head spacing |
Lay-In/Hard Ceilings:
White concealed sprinkler head
135-170°F temperature rating,
Lay-In/Hard Ceilings: (Clean Areas):
White sealed, concealed sprinkler head
135-170°F temperature rating,
Exposed Ceiling Areas:
Brass Finish, Upright Sprinkler Head 135-170°F Temperature Rating,
Warehouse Area:
Empty pallets are not allowed to be stored in racks and solid piled wood pallets are not permitted to be stored more than 8 ft. high.
An Early Suppression Fast Response (ESFR) type sprinklers or in-rack sprinklers are not required. Materials will not be stored higher than 12’-0” above finished floor.
Wet pipe sprinkler piping will be schedule 40 black steel with cut grooved couplings or schedule 10 with roll grooved couplings in sizes 2” and larger and threaded joints and fittings in sizes 1 ½” and smaller.
www.protecsinc.com
| 55 |

| 16.0 | Electrical |
| 16.1 | Introduction |
The proposed expansion will require an extension from the existing electrical service at the facility to power the expansion. An owner furnished backup generator will be installed and a new UPS will be provided for backup power to specific equipment and systems.
A description of the systems follows:
| 16.2 | Electrical Service |
The existing building electrical service will be will be upgraded to support the expansion. The existing pad mount transformer will be replaced by PPL and a new termination cabinet will be provided to re-feed the existing switchgear and feed the new switchgear required for the project.
| 16.3 | Power Distribution |
All mechanical system motors, where possible, will be operated at 480Volt/3- Phase. All process system motors, where possible, will be operated at 480Volt/3- Phase. Some mixers and other process systems are rated for 355V at 50hz. VFDs will be programmed to convert the frequency and voltage to run the motors properly on 480V/60hz house power. The VFDs serving all process equipment shall be equipped with Modbus TCO interface modules with communication wiring to skid and automation control panels as required. All motors will either be fed from either individual starters or variable frequency drives (VFD). These starters and VFDs will be fed from the 480V distribution power panels located in the second floor platform space. In general, the motor controllers will be NEMA- Rated, combination-starter type, with motor circuit protectors and solid- state (electronic) overload protection. Each skid panel will have a Hand-Off-Automatic (HOA) switch, green “run” pilot light, and control power transformer with 100% spare capacity. A minimum of two normally open and two normally closed auxiliary contacts will be provided with each starter. Other auxiliary contacts for miscellaneous controls will be provided as required. VFDs serving equipment in the process areas will be located on the catwalk or upper level corridor.
480V distribution panels will be installed to serve HVAC and process equipment and miscellaneous process receptacle and lighting panels at 208Y/120V. These receptacles panels will be fed via step-down transformers.
All lighting fixtures will be powered at 277V. New 480Y/277V lighting panels will be installed throughout the facility to distribute power to the lighting fixtures. These lighting panels will be fed from 480 Volt distribution panels located within the areas they serve.
www.protecsinc.com
| 56 |

208Y/120V receptacle panels will be installed throughout the facility as required to feed receptacle devices and miscellaneous equipment requiring 208 or 120 Volts. These panels will be fed from appropriately sized step-down transformers.
All panels will be designed to be full-rated for short circuit protection. Buses and wiring shall be designed with copper as the basis of design.
Panel and ATS short circuit ratings shall be confirmed by the required Short Circuit Studies, life safety breaker coordination shall be confirmed by the required Coordination Studies, and new equipment warning labels shall be confirmed by the required Arc Flash Studies.
All step-down transformers will be specified to have a 150° C temperature rise over a 40° C ambient and a 220° C insulation system.
| 16.4 | Emergency Power |
An owner furnished outdoor 625 kW, 480 Volt natural gas back up generator will be installed to provide back-up emergency power. The generator will be located on the roof. The emergency generator will be designed in accordance with NFPA 110. Emergency power will be distributed to a new life safety automatic transfer switch (ATS) and to the existing ATS which will be re-designated as a legally required & optional standby ATS. Both switches will be located in the interstitial space of the existing facility. Life safety loads will be redistributed to segregated life safety power distribution via new EM1 and EL1 panels adjacent to the new life safety ATS.
Miscellaneous 480 Volt and 208Y/120 Volt emergency power and receptacle panels will be installed throughout the facility to distribute emergency power. In addition, 480Y/277V emergency lighting panels will be installed to feed emergency lighting at 277V.
The generator will serve the following loads:
Life Safety:
| ● | Emergency lighting and exit signs | |
| ● | Access control system | |
| ● | Fire alarm system |
Legally Required & Option Standby:
| ● | Existing Souderton Facility Back-up EMCC equipment |
www.protecsinc.com
| 57 |

| ● | Filling Area HVAC | |
| ● | Building Automation System (BAS) | |
| ● | Process Automation System | |
| ● | C-800 Process Equipment – Excluding Chillers (See Process Equipment Matrix) | |
| ● | C-900 Process Equipment – Excluding Chillers (See Process Equipment Matrix) | |
| ● | Buffer Prep Process Equipment (See Process Equipment Matrix) | |
| ● | Filling Equipment (See Process Equipment Matrix) | |
| ● | Filling Equipment RABS (See Process Equipment Matrix) | |
| ● | Process Equipment Utilities (See Process Equipment Matrix) | |
| ● | Waste Neutralization System | |
| ● | CFR 21 Part 11 Compliant Monitoring System | |
| ● | Relocated Walk in Refrigerator | |
| ● | New Walk in Refrigerator | |
| ● | Chemical Room Exhaust Fan | |
| ● | Chemical Room HVAC | |
| ● | Uninterruptible power supply system | |
| ● | Security system | |
| ● | Freezer, Lab #105 | |
| ● | Refrigerators (1), Lab #105 | |
| ● | Incubators (2), Lab #105 | |
| ● | Biosafety Hood (1), Lab #119 | |
| ● | QC Refrigerator (2), Lab #121 | |
| ● | QC Freezer (1), Lab #121 | |
| ● | Low Temperature Refrigerator (1), Lab #121 | |
| ● | Refrigerator (Samples & Glute) (1), Rm. #169 | |
| ● | Weigh Roof Dispensing Exhaust Fan (EF-9) | |
| ● | 4’ Fume Hood & Associated Exhaust Fan (1), Rm. #171 | |
| ● | Fumehood Pump (1), Rm. #171 | |
| ● | Server Equipment (120V, 20A Circuit), Rm. #214 |
A remote shut-off pushbutton will be located in the electrical switchgear room. A remote generator annunciator panel will be installed at central control room.
An additional automatic transfer switch (ATS) will be added for standby equipment and located in the new electrical room 161C for proximity to equipment in the new addition which require generator backup.
www.protecsinc.com
| 58 |

| 16.5 | Uninterruptible Power Supply |
A rack-mounted sealed battery 30 KW UPS system, will be provided to support specific critical equipment and systems. The existing UPS will continue to serve loads in C-500 and as originally connected. 15 minutes of runtime will be provided.
The UPS will serve the following loads:
| ● | Process Automation System | |
| ● | Building Automation System (BAS) | |
| ● | Access Control System | |
| ● | CFR 21 Part 11 Compliant Monitoring System | |
| ● | Security System | |
| ● | Server Equipment (120V, 20A Circuit in Servers Room 214) |
| 16.6 | Surge Suppression Device |
A surge protection device will not be required for this project – considered in main building gear by others.
| 16.7 | Lighting |
Light fixtures and lighting levels throughout the building will be as follows:
| AREA | FIXTURE TYPE LAMP/LENS | FOOTCANDLE LEVEL (At Task) | ||
| Corridors | Fluorescent/Parabolic | 30-40 | ||
| Office | Fluorescent/Prismatic | 40-50 | ||
| Laboratories | Fluorescent/Prismatic | 50-60 | ||
| Cleanrooms | Fluorescent/Wet | 50-60 | ||
| Mechanical Spaces | Fluorescent/Industrial | 30-40 | ||
| Electrical Rooms | Fluorescent/Industrial | 40-45 | ||
| Warehouse | Fluorescent or LED / Aisle Lighter | 30-40 |
Standard fluorescent troffers with parabolic lenses will be installed in the office, conference rooms, and office support areas. Fluorescent troffers with prismatic lenses will be provided in lockers rooms, lab spaces, and lab support areas. Chain- hung 1’x4’ industrial fixtures with 10-15% uplight component will be installed in utility areas, mechanical spaces, electrical rooms and the maintenance shop. All fluorescent fixtures will be provided with energy-saving 28 watt T-5 and 24W T5HO, 3500K lamps and electronic ballasts.
www.protecsinc.com
| 59 |

Triple gasketed cleanroom type fixtures with powder coated or stainless steel frames will be installed in the cleanroom suites, airlocks, and corridors to maintain air pressurization and cleanliness.
Pendant mounted high-bay aisle lighter type fixtures will be provided throughout the warehouse to allow for adequate lighting levels on the vertical surfaces. Low- bay fixtures will be installed in the shipping and receiving loading dock areas.
New low wattage LED type exit signs will be installed throughout the new building extension as required by local and national codes.
Wall toggle switches will be installed for control of lighting in process areas. Wall or ceiling occupancy sensors will be installed to control lighting in areas other than process areas. Certain fixtures within the facility will connect to the emergency generator and serve as emergency lighting in the event of a power failure. These fixtures will be controlled from circuit breakers in the emergency lighting panels from where they are fed. These fixtures will serve as night lighting for the facility. Circuit breakers controlling fixtures will be switching duty rated breakers. Light switches in lab areas will be specified with gasketed covers to protect against moisture during cleaning.
| 16.8 | Site Lighting |
Site lighting is existing to remain. Two new pole lights for the parking lot expansion will be provided as part of the building shell construction project.
| 16.9 | Receptacles |
Convenience receptacles will be provided throughout the facility such that the distance to a receptacle from any location in the building will be no more than 50 feet.
Rectangular shaped offices will be provided with a duplex receptacle on at least three walls. The receptacle on the wall near the office occupant’s desk will be a quadraplex receptacle (double duplex) to provide outlets for computer equipment as well as miscellaneous office equipment such as pencil sharpeners, task lights, etc.
Special receptacles at 208V, 230V or 480 V will be provided to serve specific equipment requiring voltages other than 120V. All receptacle devices located in process rooms will be provided with neoprene gasketing and weatherproof coverplates to maintain cleanliness and air pressurization while protecting against moisture during cleaning. All receptacles in all lab areas are to be GFI protected.
www.protecsinc.com
| 60 |

The labs will be provided with a pre-wired, surface mounted, dual channel, raceway or wiremold system. This system will contain both receptacle and tele/data devices segregated by means of a divider. Tele/data wiring and devices will be provided as noted in the Telecommunications discussion below. Spacing of receptacle and tele/data devices will be determined based upon the layout and utility requirements of the laboratory equipment. All 120V receptacles in the lab area and where within 6’-0” of a sink will be ground-fault interrupter type receptacles.
| 16.10 | Basic Materials and Methods |
In general, raceways will be as follows:
Outdoors
| 1. | Feeders (exposed) – Rigid Galvanized Steel. | |
| 2. | Feeders (concealed) – Rigid Galvanized Steel. | |
| 3. | Connections to motors and vibrating equipment – Liquid Tight flexible metal conduit. | |
| 4. | Concrete encased – Schedule 40 PVC rigid non-metallic conduit. | |
| 5. | Boxes and enclosures – NEMA 3R. |
Indoors
| 1. | Feeders & branch circuits (exposed outside of CNC and ISO classified spaces) – Electrical Metallic Tubing (EMT). | |
| 2. | Feeders & branch circuits (exposed in CNC and ISO classified spaces) – PVC coated EMT and LFMC. | |
| 3. | Feeders (damp or wet locations) – Rigid Galvanized Steel. | |
| 4. | Lighting and receptacle branch circuits – Electrical Metallic Tubing. | |
| 5. | Exposed conduits in lab rooms –Rigid Galvanized Steel. | |
| 6. | Connections to motors and vibrating equipment – Flexible metal conduit in dry area and liquid tight flexible metal conduit in wet areas. | |
| 7. | MC cable or flexible metal conduit to overcome building obstructions and between junction boxes and lighting fixtures with a maximum of 6 foot length. MC cable will also be used for receptacle branch circuits in hollow stud partitions except for homeruns and room interconnection runs, which will be run in EMT. | |
| 8. | Boxes and enclosures – NEMA 1 in dry area and NEMA 4 in damp or wet areas. | |
| 9. | Boxes and enclosures – NEMA 3R in CNC and ISO classified spaces. | |
| 10. | Hazardous Locations – Rigid Galvanized Steel. |
www.protecsinc.com
| 61 |

| 11. | Sealed fittings will be provided for all conduits penetrating into hazardous locations. |
| 16.11 | Grounding |
The existing grounding system for the building will remain as-is. All new equipment will be grounded in accordance with the National Electric Code (NEC) Article 250. Included in the project cost are separate equipment grounding conductors which will be sized and installed in all raceways in accordance with the NEC.
Grounding requirements are to be determined pending the hydrogen generation calculation and evaluation.
| 16.12 | Lightning Protection System |
New roof top equipment will be connected to the existing lightning protection system. Existing system to be recertified at construction completion to current level.
| 16.13 | Fire Alarm System |
The existing building fire alarm system has been upgraded to a new fire alarm system as part of the building shell construction project which has been confirmed to have adequate capacity for quantity of new devices. Devices will be provided as required by applicable national and local codes. As a minimum, the system will consist of the following:
| a. | Manual pull stations will be located at each exit from the facility and at exits from others floors of the building. Pull stations will be located with a maximum travel distance of 200 feet between stations. | |
| b. | Combination horn-strobes and strobes will be located in the corridors, toilet rooms and common spaces. Horn-strobes will also be located in all Process and Manufacturing Rooms. | |
| c. | Ionization-type duct smoke detectors will be located as required for supply and return fans. The duct detectors will be hard wired to their corresponding fan motor starters or VFDs and to the fire alarm main control panel. | |
| d. | Smoke detectors will be provided in electrical rooms, mechanical rooms, warehouse, storage areas, tele/data closets, and on equipment platforms. | |
| e. | A fire alarm annunciator panel will be located in the lobby. | |
| f. | Fire Alarm extender panels will be provided as required. |
The fire alarm system will be interfaced with the security and access control systems to release all egress doors in the event of a building alarm. In addition, the fire alarm system will interface with the building automation system.
www.protecsinc.com
| 62 |

The electrical contractor will provide back boxes with empty conduit and pull wire to above nearest accessible ceiling. The fire alarm vendor will furnish, install, and wire all new fire alarm devices.
| 16.14 | Security System |
By HbO2.
| 16.15 | Telecommunications |
The telephone and data scope of work will be completed by HbO2. Conduit drops with pull strings and boxes at use points will be provided by PROTECS.
Tele/Data drops required for systems installed by PROTECS will be identified for installation by HbO2’s tele/data subcontractor.
| 16.16 | Public Address System |
Not applicable
| 16.17 | Access Control (Pressurization) |
The airlock doors within the cleanroom area will be interlocked to allow for only one door to be open at a time within a given airlock.
The existing card reader system will be expanded to include new exterior doors and specific interior doors for access control.
Mag Locks or electrified hardware will be used for interlocking and card access.
www.protecsinc.com
| 63 |

| 17.0 | PROCESS |
| 17.1 | EXECUTIVE SUMMARY |
Hemoglobin Oxygen (HbO2) Therapeutics is expanding its existing Souderton, PA facility to incorporate the C-800 and C-900 processes being relocated from the Cambridge, MA facility and the aseptic filling operations that were previously performed in Canada.
Manufactured products are hemoglobin based oxygen carrying solutions, Hemopure (intended for human use) and Oxyglobin (intended for veterinary use). The renovated Souderton, PA facility is designed to perform separation, purification and polymerization of a hemoglobin based oxygen carrying solution and aseptic filling and packaging. The Souderton, PA facility was previously designed to perform the first step of the manufacturing process, the separation of source material into a stable intermediate (C-500). A previously existing Cambridge, MA facility was designed to perform the final two steps of the manufacturing process, the purification of the cell free hemoglobin to a purified deoxygenated hemoglobin (C-800) and the polymerization of purified hemoglobin to the final product (C-900). The C-800 and C-900 processes occurring at the Cambridge, MA facility will be relocated to the Souderton, PA expansion area. In addition, new aseptic filling and packaging process previously performed by a CMO will be added to the Souderton, PA expansion area under a Restricted Area Barrier System (RABS).
The purpose of this section is to detail the process operations which will take place in the Souderton, PA facility expansion and the process and clean utility equipment that will be installed to support the new intended operation of the facility.
The facility is designed to meet the requirements of North American and European regulatory requirements.
| 64 |

| 17.2 | PROCESS |
| 17.2.1 | General |
The Souderton, PA facility currently designed to complete the initial C-500 process will be expanded to incorporate the final C-800 and C-900 processes previously executed in the Cambridge, MA facility. In addition, aseptic filling and packaging operations will be added to the facility.
| 17.2.2 | Product Description |
There are two drug products that are derived from the source material:
| ● | Hemopure (intended for human use) | |
| ● | Oxyglobin (intended for veterinary use) |
Hemopure is a hemoglobin based oxygen carrying solutions used as an alternative to blood transfusions for surgical anemic patients and compassionate use. Oxyglobin is the veterinary equivalent to Hemopure. The difference between the two solutions is the additional step of removing the low molecular weight fraction after polymerization required for the Hemopure product. The products will be packaged in sterile IV bags for injection.
The products are planned to serve the European and US markets. Therefore, the facility will require compliance with current Good Manufacturing Practices in North America and Europe.
| 17.2.3 | Plant capacity |
The facility will be designed for the production of 50,000 units of Hemopure per year (200,000 units of Oxyglobin). The design is based on the following assumptions:
| Table 1: | Oxyglobin | Hemopure | ||||
| Available weeks: | 46 | 46 | Weeks / year | |||
| Work hours per day (2 x 12 hr shift): | 24 | 24 | Hours / day | |||
| Work days per week: | 7 | 7 | Days / week | |||
| Batch working volume (up to): | 769 | 384 | L | |||
| Unit volume: | 0.125 | 0.250 | L | |||
| Number of units (up to): | 5226 | 1307 | Units / batch |
The C500 stage of the process produces a 1300L batch of C500 intermediate product. The C800 stage of the process produces an 1100L batch of C800 intermediate product. This volume of C800 intermediate product is then split into three sub-batches for the C900 stage of the process which produces a final batch volume of up to 769L of Oxyglobin or 384L of Hemopure product. The chromatography phase of the C800 process was determined to be the rate limiting operation taking a total of 5 days to complete.
www.protecsinc.com
| 65 |

| 17.2.4 | Manufacturing |
The manufacturing of the hemoglobin based oxygen carrying solution products consist of the following main procedures:
| ● | Blood Collection | |
| ● | Separation (C-500) | |
| ● | Purification (C-800) | |
| ● | Polymerization (C-900) | |
| ● | Aseptic Filling / Packaging |
Additional supporting procedures include:
| ● | Component / equipment washing, preparation and sterilization. |
| 17.2.4.1 | Blood Collection |
Bovine whole blood (i.e. the raw material) is collected from a large volume abattoir. The whole blood arrives at the facility in individual pre-sanitized semi- closed, 20L single use bags. The collection bags are pre-dosed with sodium citrate solution, an anti-coagulant, at the Souderton, PA facility. The filled bags are placed on ice during transport to the facility.
| 17.2.4.2 | Separation (C-500) |
The C-500 separation area receives source material and converts it to a stable intermediate (C-500). There are three unit operations taking place within the separation area which are all conducted in closed systems.
The three unit operations are:
| ● | Cell Wash | |
| ● | Separation | |
| ● | Ultrafiltration |
During transfer from the single use bags to the cell wash tank, macro-contaminants are removed by pumping citrated bovine blood through a 50 µ strainer and 60 µ depth filter. During the cell washing step, plasma proteins are removed and the solution is recirculated across a 0.45 µ hollow fiber filter while saline is continuously added.
Washed cells are then pumped into a separation centrifuge spinning at approximately 13,500 x G which separates the heavy phase containing red blood cells from the light phase containing the white blood cells. Mechanical lysing of the red blood cells occurs while the cells are discharged from the separator and strike the wall of the collection pan.
The heavy phase is then transferred to the ultrafiltration system to remove cell wall debris and micro contaminants falling outside the range of 100,000D molecular weight cut-off (MWCO) to 30,000D MWCO. Ultrafiltration occurs in two phases.
www.protecsinc.com
| 66 |

Phase I uses diafiltration with purified water across 100 kD hallow fiber filters. Phase II of ultrafiltration uses recirculation across a 30 kD filter.
C-500 is pumped through a 0.50 µ pre-filter and 0.20 µ final filter to either a hold tank or sterilized, single use bulk bags. In bags, C-500 is held in cold storage (2- 10ºC) for up to 28 days. In jacketed hold tank, C-500 is kept at 2-10 ºC until ready to transfer to purification.
| 17.2.4.3 | Purification (C-800) |
The C-800 purification area receives the cell-free hemoglobin intermediate (C- 500) and converts it to purified deoxygenated hemoglobin (C-800). There are four processes taking place within the purification area.
The four processes are:
| ● | High Performance Liquid Chromatography (HPLC) | |
| ● | Concentration | |
| ● | Deoxygenation | |
| ● | Diafiltration |
The C-500 is received and transferred to the chromatography feed tank to initiate the purification process by HPLC. The chromatographic cycle consists of injecting the hemoglobin onto the column, developing a pH gradient, collecting the product, column wash, and column re-equilibration with load buffer. The pH gradient is made by mixing two buffers (Buffer A and Buffer B) to decrease the pH of the columns to rid of unbound and loosely bound non-hemoglobin components. The pH is reduced again to release the hemoglobin that is then diverted to a concentration tank. A high salt buffer (Buffer C) is used to elute tightly bound non- hemoglobin components. The columns are then flushed with a high pH buffer (Buffer A) to re-equilibrate for another injection.
The purified hemoglobin from the chromatography columns is collected in the concentration tank where it is continuously concentrated using a 30,000D MWCO ultrafilter. The concentrated hemoglobin is then pumped to a deoxygenation tank where the hemoglobin solution is deoxygenated by recirculating it past a 0.05 µ phase transfer membrane. Filtered nitrogen gas is passed on the opposite side of the membrane in a counter-current direction to strip the oxygen out of the hemoglobin solution.
The deoxygenated, concentrated hemoglobin solution is then stabilized through diafiltration. The solution is exchanged with three volumes of deoxygenated Compound-800 Storage Solution by recirculating it across a 30,000D MWCO ultrafilter in diafiltration mode.
The final deoxygenated hemoglobin product (C-800) is transferred through a 1 µ pre-filter and a 0.20 µ filter before collecting in one of two sanitized, agitated C-800 batch holding tanks. These nitrogen blanketed tanks hold the C-800 product at 17-22º until ready for transfer to polymerization.
www.protecsinc.com
| 67 |

| 17.2.4.4 | Polymerization (C-900) |
The C-900 polymerization area receives purified hemoglobin (C-800) and converts it to the final product (C-900). There are four processes taking place within the polymerization area.
The four processes are:
| ● | Activation (polymerization) | |
| ● | Stabilization | |
| ● | Equilibration | |
| ● | Fractionation (Hemopure only) |
Deoxygenated hemoglobin is polymerized with the addition of glutaraldehyde. The reaction conditions are carefully controlled. They include glutaraldehyde addition rate, solution temperature, and mixing rate.
The polymerized hemoglobin is concentrated and diafiltered with borate buffer to adjust the pH by recirculation through 30,000D MCWO ultrafilters. The reaction products are stabilized with the addition of sodium borohydride.
During the addition of sodium borohydride, the polymerized hemoglobin solution is recirculated across a phase transfer membrane to minimize levels of hydrogen gas and maintain the deoxygenated state. It is then concentrated and diafiltered with Diafiltration Solution A to achieve equilibration.
To produce Hemopure, the polymerized product is then transferred to the fractionation tank where the low molecular weight fraction is removed using a 70,000D MWCO ultrafiltration process by diafiltration with Diafiltration Solution C. This product is then concentrated with a 30,000D MCWO ultrafilter.
The polymerized hemoglobin solution is then transferred through a series of three filters: a 0.50 µ pre-filter, a 0.20 µ sterile filter, and a 0.10 µ final sterile filter before collecting into sterilized pooling tanks held under a nitrogen blanket at 15- 30º.
| 17.2.4.5 | Filling / Packaging |
The product is kept in the pooling tanks for up to 10 days or until release testing results are obtained. The product is then The filling will be done under ISO class 5 Restricted Access Barrier Space (RABS), located in a ISO class 7 background. The final fill process consists of the semi-automated filling equipment which was used to fill the I.V. bags at the previous contract manufacturer’s site. An operator is required to load the empty IV bags. The automated filling equipment prints the batch number, opens sterile I.V. bags, vacuums the inside of the empty I.V. bag, fills it with a pre- set dose of product, permanently seals the bags, cuts the piece above the weld, and unloads the I.V. bags. I.V bags are two different sizes: 250mL and 500mL, with fill targets of 60mL, 125mL, 250mL. Filling line is cleaned in place and sterilized in place (CIP/SIP)
www.protecsinc.com
| 68 |

After filling the product is transferred out of the filling room to the labeling and overwrap room where the product is inspected including leak testing, seal integrity testing and weight checks. The product is next labeled and then inserted into clear overwrap pouches, purged with nitrogen and sealed under a vacuum. A checkweigher will be used to verify the weights of filled I.V. bags throughout the filling operation (samples only - IPC). The product is then inspected including leak testing, seal integrity testing and weight checks before transferring to product storage. Once an order if filled the product is removed from storage, individually boxed, labeled, crated and transferred to the warehouse for shipping.
The filling process including set up, product fill and cleaning will be targeted to a one-week duration. Maximum fill time will be based on product. Target fill time will be up to 12 hours over the span of two shifts.
| 17.2.4.6 | Component / equipment washing, preparation and sterilization |
A new cGMP autoclave and clean parts wash room are required to support the filling operation.
| 17.2.5 | Process equipment |
| 17.2.5.1 | Separation (C-500) |
| 17.2.5.1.1 | Citrate Buffer |
The citrate buffer equipment consists of an agitated 300 L tank (T-150) to pre-fill 20L single use bags with sodium citrate solution (anticoagulant) for bovine whole blood collection and transfer.
This system is pre-existing and will not be modified.
| 17.2.5.1.2 | Saline Buffer |
The saline buffer equipment consists of an agitated 4000 L make-up tank (T-170) and 2500 L loop tank (T-171) used to infuse saline solution into cell wash steps during recirculation across cell wash filters.
This system is pre-existing and will not be modified.
| 17.2.5.1.3 | Cell Wash |
The cell wash equipment consists of an agitated 1200 L cell wash tank (T- 201) used to remove macro-contaminants and plasma proteins.
This system is pre-existing and will not be modified.
www.protecsinc.com
| 69 |

| 17.2.5.1.4 | Separation |
The separation equipment consists of a Separation Centrifuge (CT-301) used separate the heavy phase containing red blood cells from the light phase containing white blood cells.
This system is pre-existing and will not be modified.
| 17.2.5.1.5 | Stage 1 Ultrafiltration |
The stage 1 ultrafiltration equipment consists of a 900 L Stage 1 Ultrafiltration tank (T-510) which uses diafiltration across a 100 kD filter.
This system is pre-existing and will not be modified.
| 17.2.5.1.6 | Stage 2 Ultrafiltration |
The stage 2 ultrafiltration equipment consists of a 2000 L Stage 2 Ultrafiltration tank (T-511) which recirculates across a 30 kD filter.
This system is pre-existing and will not be modified.
| 17.2.5.2 | Purification (C-800) |
| 17.2.5.2.1 | Radial Chromatography |
The radial chromatography equipment consists of a nitrogen blanketed 1400 L feed tank (T-601) to an eight column High Performance Liquid Chromatography (HPLC) system using radial compression chromatographic media columns containing silica-based anion media.
This system is a pre-existing system being relocated. The feed tank, along with a vent filter and nitrogen filter, will be repurposed from an existing tank (T-806C). A new agitator will be added to the feed tank.
The new agitator will have the following characteristics:
| ● | Stainless steel 316L, surface finish of 20Ra µin | |
| ● | Variable Speed | |
| ● | Bottom Mounted Mag Drive |
| 17.2.5.2.2 | Buffer Prep and Distribution |
The buffer prep equipment consists of four agitated 1500 L make-up tanks (T-610, T-620A, T-620B, and T-640), a 1200 L make-up tank (T-650), two 1500 L loop tanks (T-611 ad T-621), a 1400 L loop tank (T-651), a new 1500 L loop tank (T-641) and an agitated 1500 L C-800 storage buffer make-up tank (T-630) to be used throughout the purification process.
This system is a pre-existing system being relocated. The new 1500L loop tank (T-641) will replace the previously existing 1100L loop tank and be modeled after the additional 1500L buffer loop tanks (T-611 and T-621).
www.protecsinc.com
| 70 |

The new Buffer C Loop Tank will have the following characteristics:
| ● | Stainless steel 316L, internal surface finish of 20Ra µin and Electropolished (BPE SF-4) | |
| ● | Working volume: 1500 L | |
| ● | Design pressure: 50 PSIG & F.V. | |
| ● | Chilled water jacket | |
| ● | Top connections: |
| ○ | Manhole | |
| ○ | Product inlet port | |
| ○ | Vent filter | |
| ○ | Sprayball |
| ● | Bottom connections: |
| ○ | Product outlet |
| 17.2.5.2.3 | Concentration |
The concentration equipment consists of a 500 L concentration tank (T- 801) continuously recirculating product across a 30 kD filter.
This system is a pre-existing system being relocated and will not be modified.
| 17.2.5.2.4 | Deoxygenation and Diafiltration |
The deoxygenation and diafiltration equipment consists of an agitated 500 L* deoxygenation tank (T-805) uses a 0.05 µ phase transfer membrane with nitrogen passes on the opposite side in a countercurrent direction for deoxygenation and uses a 30 kD filter in diafiltration mode to exchanges three volumes of C-800 storage solution for stabilization.
This system is a pre-existing system being relocated. The deoxygenation tank will be repurposed from an existing tank (previously T-601). A new agitator will be added to the deoxygenation tank.
The new agitator will have the following characteristics:
| ● | Stainless steel 316L, surface finish of 20Ra µin | |
| ● | Variable Speed | |
| ● | Bottom Mounted Mag Drive |
| 17.2.5.2.5 | Batching Tanks |
The batching tank equipment consists of two agitated 1400 L batch tanks (T-806A and T-806B) maintaining C-800 product at a temperature of 17- 22ºC under a nitrogen blanket until ready for polymerization.
This system is a pre-existing system being relocated. A new agitator will be added to each batch tank.
www.protecsinc.com
| 71 |

| The new agitators will have the following characteristics: | |||||
| ● | Stainless steel 316L, surface finish of 20Ra µin | ||||
| 17.2.5.3 | Polymerization (C-900) | ||||
| 17.2.5.3.1 DeOxy WFI and Borate | |||||
| The deoxy WFI and borate equipment consists of an agitated 2000 L make-up tank (T-960) used during polymerization for stabilization. | |||||
| This system is a pre-existing system being relocated and will not be modified. | |||||
| 17.2.5.3.2 DFA and DFC | |||||
| The DFA and DFC equipment consists of three agitated 2000 L make-up tanks (T-970, T-980A and T-980B) and two 2000L loop tanks (T-971 and T-981) used during polymerization for equilibration and fractionation. | |||||
| This system is a pre-existing system being relocated and will not be modified. | |||||
| 17.2.5.3.3 Polymerization Reactor | |||||
| The polymerization reactor equipment consists of a double agitated 1100 L reactor (R-952) which recirculates through a 30 kD polymerization filter and a 0.05 µ phase transfer membrane with nitrogen. | |||||
| This system is a pre-existing system being relocated. The reactor, including the two agitators, will be new. | |||||
| The new reactor will have the following characteristics: | |||||
| ● | Stainless steel 316L, internal surface finish of 15Ra µin and Electropolished (BPE SF-4) | ||||
| ● | Working volume: 1100 L | ||||
| ● | Design pressure: 60 PSIG & F.V. | ||||
| ● | Chilled water and warm water jacket | ||||
| ● | Top connections: | ||||
| ○ | Product transfer port | ||||
| ○ | Vent filter | ||||
| ○ | N2 filter | ||||
| ○ | Connection for agitator | ||||
| ○ | Cleaning machine | ||||
| ● | Bottom connections: | ||||
| ○ | Product outlet | ||||
| ○ | Connection for agitator | ||||
www.protecsinc.com
| 72 |

| ● | Top Agitator | |||
| ○ | Stainless steel 316L, surface finish of 15Ra µin and Electropolished (BPE SF-4) | |||
| ○ | Top mounted | |||
| ○ | Large diameter impeller | |||
| ○ | Slow speed | |||
| ● | Bottom Agitator | |||
| ○ | Stainless steel 316L, surface finish of 15Ra µin and Electropolished (BPE SF-4) | |||
| ○ | Bottom mounted | |||
| ○ | Mag Drive | |||
| 17.2.5.3.4 Reactor Activation, Deactivation and Neutralization | ||
| The reactor activation, deactivation and neutralization equipment consists of an agitated 300 L activation tank (T-951), an agitated 150 L quench tank (T-953) and a 300L Off Gas Tank used during polymerization for activation and neutralization. | ||
| This system is a pre-existing system being relocated. All three tanks will be purchased new. | ||
| The new activation tank will have the following characteristics: |
| ● | Stainless steel 316L, internal surface finish of 15Ra µin and Electropolished (BPE SF-4) | ||
| ● | Working volume: 300 L | ||
| ● | Design pressure: 50 PSIG & F.V. | ||
| ● | Chilled water jacket | ||
| ● | Top connections: | ||
| ○ | Manhole | ||
| ○ | DeOxy WFI port | ||
| ○ | Vent filter | ||
| ○ | N2 filter | ||
| ○ | Sprayball | ||
| ● | Bottom connections: | ||
| ○ | Product outlet | ||
| ○ | Connection for agitator | ||
| ● | Agitator | ||
| ○ | Stainless steel 316L, surface finish of 15Ra and Electropolished (BPE SF-4) | ||
| ○ | Bottom mounted | ||
| ○ | VS | ||
www.protecsinc.com
| 73 |

| The new quench solution tank will have the following characteristics: | ||||
| ● | Stainless steel 316L, internal surface finish of 15Ra µin and Electropolished (BPE SF-4) | |||
| ● | Working volume: 150 L | |||
| ● | Design pressure: 50 PSIG & F.V. | |||
| ● | Top connections: | |||
| ○ | Manhole | |||
| ○ | DeOxy WFI port | |||
| ○ | Vent filter | |||
| ○ | N2 filter | |||
| ○ | Sprayball | |||
| ● | Bottom connections: | |||
| ○ | Product outlet | |||
| ○ | Connection for agitator | |||
| ● | Agitator | |||
| ○ | Stainless steel 316L, surface finish of 15Ra µin and Electropolished (BPE SF-4) | |||
| ○ | Bottom mounted | |||
| ○ | VS | |||
| The new off gas tank will have the following characteristics: | |||
| ● | Glass lined Carbon Steel Tank with Epoxy Paint | ||
| ● | Working volume: 300 L | ||
| ● | Design pressure: 15 PSIG & F.V. | ||
| ● | Top connections: | ||
| ○ | Product inlet port | ||
| ○ | Vent port | ||
| ○ | N2 port | ||
| ● | Bottom connections: | ||
| ○ | Product outlet | ||
| 17.2.5.3.5 Fractionation |
The fractionation equipment consists of an agitated 700 L fractionation tank (T-957) using 70 kD fractionation filters and a 30 kD concentration filter.
This system is pre-existing system being relocated and will not be modified.
| 17.2.5.3.6 Filtrate Recovery |
The filtrate recovery equipment consists of a 700 L filtrate recovery tank (T-958) using 30 kD filtrate recovery filter.
This system is pre-existing system and will not be installed in this project. A space holder has been used for this future use on the layout.
www.protecsinc.com
| 74 |

| 17.2.5.3.7 Final Filtration and Pooling |
The final filtration equipment consists of a 0.5 µ pre-filter, a 0.2 µ sterile filter and a 0.1 µ final sterile filter to obtain sterility. The pooling equipment consists of two agitated 1400 L bulk holding tanks (T-991A and future T-991B) maintaining C-900 product at a temperature of 15- 30ºC under a nitrogen blanket until ready for filling.
This system is pre-existing system being relocated. The new bulk holding tank (T-991B) will be new, but will be purchased at a later date for future use and modeled after T-991A.
For future use: The new bulk holding tank will have the following characteristics:
| ● | Stainless steel 316L, internal surface finish of 15Ra µin and Electropolished (BPE SF-4) | ||
| ● | Working volume: 1400 L | ||
| ● | Design pressure: 50 PSIG & F.V. | ||
| ● | Chilled water jacket at 100 PSIG | ||
| ● | Top connections: | ||
| ○ | Product transfer port | ||
| ○ | Vent filter | ||
| ○ | N2 filter | ||
| ○ | Connection for agitator | ||
| ○ | Sprayball | ||
| ● | Bottom connections: | ||
| ○ | Product outlet | ||
| ● | Agitator | ||
| ○ | Stainless steel 316L, surface finish of 15Ra µin and Electropolished (BPE SF-4) | ||
| ○ | Top mounted | ||
| 17.2.5.4 Filling / Packaging |
The product is transferred from the pooling tank through 0.1 µ sterile filters to the filling machine. This is accomplished using a combination of sterilized piping and single-use equipment. The location of the pooled bulk holding tanks will be evaluated in relation to the location of the filling operation. This will be done to minimize potential for product losses and process complexity. In addition, the requirements and practice of pre-use, post-sterilization integrity testing of the product sterilizing filters will be evaluated per a risk assessment. If pre-use option is chosen by the client, a filter integrity test apparatus (bubble point test) will be used prior to sterile filtration of each batch to ensure that the filter is not defective and will not compromise the sterility of the product.*
Aseptic filling of sterile product into ready to use I.V. bags will be performed within a Restricted Access Barrier System (RABS) under an ISO class 5 (grade A) environment located immediately within an ISO class 7 (grade B) background.
www.protecsinc.com
| 75 |

The semi-automatic filling machine previously used at the contract manufacture’s site will be relocated to the HbO2 facility. The filling machine will print the batch number, open the bag, vacuum the inside of the empty bag, fill it with a pre-set dose of sterile product, permanently seal the bag, cuts the piece above the weld, and unload the I.V. bag. The filling machine must accommodate bags of two different sizes (250mL and 500mL).
After filling, the filled I.V. bags are transferred out of the filling room to the labeling and overwrap room where the product is inspected including leak testing, seal integrity testing and weight checks. The product is then labeled and then inserted into clear overwrap pouches, purged with nitrogen and sealed under a vacuum. A checkweigher will be used to verify the weights of the filled I.V. bags throughout the filling operation (samples only – IPC). It is then transferred to product storage. Once an order is filled the product is removed from storage, individually boxed, labeled, crated and transferred to the warehouse for shipping.
Filter shall be either located under RABS or aseptic connections in non-sterile environments could be achieved using single- use aseptic connectors from the sterile side of the filter to the filling line product inlet. These connectors are designed for sterile transfer as well as limiting the risk of human error. The connections are tool-less. They are composed of male and female plugs, pre- sterilized in autoclave or gamma irradiated.
Several models of aseptic connectors are available on the market:
| ● | Sartorius-stedim: Opta Connector | |
| ● | Pall: Kleenpak Connector | |
| ● | Cole-Parmer: Asepti-Quik Connector | |
| ● | Millipore: Lynx S2S Connector |
| 17.2.5.4.1 Part wash room |
Parts provided to the aseptic filling room will be cleaned by in the parts wash area located in the equipment preparation room. This will allow the cleaning of compounding and filling components.
| 17.2.5.4.1 Autoclave |
Parts provided to the aseptic filling room will be sterilized using an autoclave located in the equipment preparation room. This autoclave will allow sterilization of components required to support filling within the RABS.
The autoclave has the following characteristics:
| ● | Double-door (pass-through) | |
| ● | Chamber size: 1 ft (approximatively) |
| ● | cGMP design – Pharmaceutical grade | ||
| ● | Model: TBD | ||
| ● | Cycles: | ||
| ○ | Wrapped goods with vacuum drying | ||
| ○ | Hard goods with fast exhaust | ||
www.protecsinc.com
| 76 |
.

| 17.3 PROCESS UTILITES |
| 17.3.1 Scope of work | ||
| This section describes the process utilities required to support manufacturing operations for the HbO2 expansion project. | ||
| The following items are included in process utilities scope: |
| ● | Water for Injection (WFI) | |
| ● | Clean Steam | |
| ● | Chilled water or glycol | |
| ● | Clean in Place (CIP) and Clean out of Place (COP) |
| New mechanical items required for this expansion project scope: |
| ● | New WFI system to service C-500,C-800 and C-900 operations | |
| ● | Relocated Clean Steam generator | |
| ● | Relocated CIP and COP | |
| ● | New Chilled Water system to service C-800 and C-900 operations |
| Additional items included in this project scope: |
| ● | Clean utilities piping (WFI, clean steam, process chilled water/glycol) |
| 17.3.2 Design criteria |
Clean and plant utilities are based on the utility requirements for the previous facilities. Clean utilities will be provided utilizing sanitary piping appropriate for the system.
| 17.3.3 Clean utilities |
| 17.3.3.1 WFI | |
A new WFI system will be installed to support all the process activities (including C-500). The generation system with an output of 25 gpm will supply the existing storage vessel in utility room 125. This new generation system is under HbO2 responsibility. The current distribution will be intercept to fill a new 20 000L storage tank to support C-800/900 operations. Both cold and hot WFI will be available for the process. The new WFI system will include provisions for periodic in-place heat or chemical system sanitization.
The distribution piping needs to be Stainless steel 316L, surface finish of 15Ra µin and Electropolished (BPE SF-4).
RO reject is estimated at over 90,000 gallons (340,000 L) per week (>40% total process effluent at full capacity, >60% total process effluent when C-500, C-800 and C-900 are run sequentially).
| 17.3.3.2 Purified Water (PW) |
The HPW tank and distribution system for C-500 operations is a pre-existing system and will be modified to store WFI and supply the new storage vessel. The current vessel and distribution will not be modified to match the new piping specification requirements (15Ra µin and Electropolished).
www.protecsinc.com
| 77 |

A new WFI tank, cooler and distribution system for C-800 and C-900 operations is needed to service all C-800 buffers, and all CIP and COP fluids for C-800 and C-900. WFI is also the feed water for the clean steam generators.
The new WFI system is to be maintained and distributed at 80ºC with a small cold WFI distribution loop to support commodity prep activities serviced by a cooling heat exchanger to a set temperature (20 to 30ºC). The new WFI system should contain a 20,000 L tank with a capacity of 60 lpm and a distribution of 300 lpm.
| 17.3.3.3 RODI Water |
The RODI water will be provided for lab use only and will not meet USP requirements. This new system is under HbO2 responsibility.
The distribution piping will be polypropylene IR plus fusion. The loop will supply four faucets and one glasswasher.
| 17.3.3.4 Plant Steam |
See Division 15.0 for details.
| 17.3.3.5 Clean Steam |
The clean steam generator and distribution system for C-500 operations is a pre- existing system and will not be modified.
A similar clean steam system is required for process equipment in C-800 and C- 900 area. A 1,000 lb/hr generator is being relocated from the Cambridge, MA facility. The new header will be connected to the existing C-500 distribution header which will be expanded so that both generators are available to service the entire plant. The verification of the existing CS generators is under HbO2 responsibility and the assumption is that the equipment will be functional.
| 17.3.3.6 Chilled Glycol |
The chilled glycol system for C-500 operations is a pre-existing system and will not be modified.
The chilled water system for C-800 and C-900 operations will be required. The Cambridge, MA facility’s system consisted of a thermal storage reservoir and two chillers. One 200 ton (700 kW) chiller is used to make the ice circulated through the ice tanks and a 1,500 gallon (5,700 L) reservoir. The second 200 ton (700 kW) chiller is used to cool the returned chilled water before it re-enters the ice tanks.
The new chilled water system consists into two 200 ton chillers to meet the process requirements (C-800/900).
www.protecsinc.com
| 78 |

| 17.3.3.7 CIP and COP |
The CIP system for C-500 operations is a pre-existing system and will not be modified.
Two CIP systems will be relocated from the Cambridge facility, one for C-800 operations and one for C-900 operations. The single tank system uses saline, sodium hydroxide and phosphoric acid wash fluids. It may service any equipment as the CIP fluids are not returned. The CIP tank is used to make up the required fluid at its specified temperature and transfer it to the equipment. The CIP fluids are circulated by either process or CIP pumps located near the equipment and discharged to local process waste drains. Line circuits are washed once through with flow directly from the CIP tank if they cannot be combined with an equipment circuit.
The COP system for C-500 operations has two COP skids and a parts washer located in a designated wash area. This is a pre-existing system and will not be modified.
Two COP skids will be relocated from the Cambridge facility to service the C-800 and C-900 equipment. COP is used to clean the various filters from diafiltration and fractionation. The equipment is moved into a designated COP room and connected to the COP equipment with flex hoses.
One COP skid will be reinstalled in the room 177A to clean the fractionation filter. This equipment will be a field installation.
| 17.3.3.8 Insulation |
Process and clean utilities piping need to be insulated for personal protection and energy conservation. Here are the general specifications for the insulation.
| ● | Insulation shall be chloride and asbestos free | |
| ● | Insulation shall not support fungi or bacteria growth | |
| ● | Insulation shall be smooth surface | |
| ● | Insulation shall be an easy-to-clean surface free of cracks and cavities that can withstand harsh cleaning agents |
| 17.3.3.9 Process Waste Treatment |
See division 15 for details.
www.protecsinc.com
| 79 |

| 17.4 SKID ASSEMBLY |
17.4.1 General description
In order to expedite the project and reduce cost and provide a design build standard of performance for the C-800 and C-900 manufacturing trains, PROTECS and LaPorte will work closely with the selected skid manufacturer to develop all final required design documentation and work through all FAT requirements and PRTOECS will take chain of custody ownership of the skids, shipping, receiving, rigging, installation and SAT in alignment with the overall project delivery schedule.
An inventory was performed of existing equipment and parts against the process P&IDs to identify components that are existing and components that must be ordered new. The new components will be ordered by a single source.
All the product contact piping needs to be Stainless steel 316L, surface finish of 15Ra µin and Electropolished (BPE SF-4). The skid frame and platform need to be stainless steel 304 with clean and smooth surface finish to allow a proper cleaning and sanitization.
| 17.5 PROCESS AUTOMATION |
The automation strategy for the Souderton, PA facility expansion will include a Process Control System (PCS) which will provide supervisory control/data acquisition for skid mounted equipment with vendor supplied PLC control and direct control of off skid equipment. Each piece will be equipped with the level of automation it requires. Environmental monitoring and data acquisition functionality will be incorporated into the PCS system for environmental monitoring instruments within the GMP spaces.
The results of an automation assessment conclude that, primarily due to hardware obsolescence, the Bailey automation system should be upgraded to modern hardware. Three process control units (PCU) from Cambridge and one in Souderton should be migrated per manufacturer’s guidelines. Similarly, the pneumatic control panels from both Cambridge and Souderton should be replaced with modern hardware because the existing hardware is no longer manufactured or supported.
The upgraded DCS and Festo hardware will rely on modern, network-based communication and co-exist on a single, unified plant network. Process skids from the Cambridge, MA facility will include junction boxes which house the upgraded pneumatic hardware. Souderton pneumatic panels will be upgraded in their existing enclosures.
Networking hardware, servers, and thick/thin clients will be included to support typical activities including HMI control/monitoring, process data historian, and alarm reporting. Programming, including migrations, will be included.
To leverage the network infrastructure, all variable frequency drives (VFDs) are recommended to be upgraded or replaced with models that can support network- based control from the PCS.
The PLC-based control systems in two COP skids from Cambridge, two COP skids in Souderton, and the CIP skid in Souderton should be upgraded due to hardware obsolescence. In all cases, the PLC and HMI should be upgraded to modern hardware. The existing program will be migrated to support the new hardware.
www.protecsinc.com
| 80 |

| III. | DESIGN/BUILD PROJECT DISCUSSION |
| 1.0 | General |
| 1.1 | Staged Design Services: We have concluded the preliminary stage of our design services that are represented by this document. This effort was used to solicit competitive market prices from key vendors and subcontractors for the work on this project. The effort to complete the project design will entail the final design development for construction issue documentation (Bulletin #1 due to permit requirements for IFC drawings) (confirmation of work to ensure proper system and component sizing and to provide construction administration through the course of the work). | |
PROTECS believes we can complete this work by September 2018. We have also planned for the strategic release of packages to allow for early procurement of materials and vendors’ services that must be pre-fabricated to lessen the amount of time in the field. Our design team will remain intimately involved with the field construction throughout the balance of the work including commissioning. | ||
| 1.2 | Project Safety: We will implement a vigorous safety program and coordinate all field activities with site safety goals as top priority. Our jobs run smoothly and safely as a result of our total commitment to field safety management. We will conduct weekly safety meetings and our field staff will routinely monitor field operations with a keen eye toward total adherence to safe practices. PROTECS routinely reviews the client site requirements and incorporates them into the comprehensive PROTECS plan. This plan will also be coordinated with HbO2. | |
| 1.3 | Subcontractors: A list of pre-selected approved trade contractors that PROTECS has previously worked with and/or are qualified for the work through extensive experience with this type of facility were contacted by PROTECS to develop our pricing for this work. All parties are highly capable and well-staffed organizations that can meet both the HbO2’s and PROTECS’ needs and requirements. Additionally, subcontractors/vendors at the request of HbO2 and the landlord have also been contacted to develop the pricing for this work. | |
| 1.4 | Construction Management: This project demands efficient execution and thorough planning. Our logistics plan calls for management of subcontractors led by a project manager from PROTECS who is supported by a project engineer and superintendent as well as home office planning and cost control staff. Through these efforts by a single source, HbO2 will realize coordination benefits. |
www.protecsinc.com
| 81 |

| Along with the selection of talented subcontractors, successful implementation must include an aggressive expedition and scheduling program. We will establish short-term schedules, hold frequent coordination meetings with subcontractors regarding scheduling, quality, and safety and establish a very detailed progress measurement system that will allow for confident tracking and control. | ||
| Our team is familiar with your facility, the personnel we have been introduced to, the demands of your market place, and the area trade contractors. With our personnel on site full-time, we will maintain the right management team on your project at all times. | ||
| 1.6 | Purchasing/Expediting: We propose to undertake an aggressive pre- purchase and expediting program in order to meet the scheduled activities. We have outlined this in our schedule. We believe the key facets to a successful timely delivery of this project will center around the early release of the process skid assembly scope, major mechanical and electrical equipment (i.e. boiler, AHUs, waste neutralization system) and key subcontractors. | |
| 1.7 | Permits: Our office has been in contact with Franconia Township and the permit applications were submitted and permit approvals were recently received. We can present them with a single point of contact for both the design and construction questions they may have since our team is integral within our office. | |
| 1.8 | Cost Control: We will establish a detailed cost control budget for the project and will work closely with major subcontractors to develop a coordinated cost reporting system. We will carefully measure engineering and field progress and deploy our change control program for timely identification of potential issues for team discussion. We will clearly define status, progress, and projected final totals in a meaningful way. We will support any cost saving/value engineering opportunities that arise to assure HbO2 that a cost-efficient facility is being provided that meets immediate occupancy needs, as well as accommodates longer-term objectives where prudent and possible. Our expert cost engineering capabilities will pay a vital role in the cost control program for the project. |
www.protecsinc.com
| 82 |

| 2.0 | SCOPE OF SERVICES | |
| PROJECT MANAGEMENT | ||
| 2.1 | The work will be broken down by construction packages that best fit HbO2’s needs. PROTECS will procure the project in packages according to client production needs, relocation dates, permitting requirements, design constraints, and equipment delivery requirements. | |
| 2.2 | PROTECS monitors cost through the use of several systems. Change requests (CR) are issued immediately upon identification of changes in the overall project scope. This allows our clients the ability to react to changes and make decisions based on cost and schedule impacts. Likewise, PROTECS uses a system for Potential Change Orders (PCOs) to track the subcontractor changes. We also monitor and track the progress of the project against our detailed CSI coded cost breakout and the project schedule in support of project invoices to HbO2 and from subcontractors. | |
| 2.3 | Time Management and Project Scheduling is the key tool for project success. A Project Milestone Schedule has been provided with this quotation. At the start of the project, PROTECS will prepare and link all activities required for the design, procurement, and construction of the project. The schedule is an active document to provide the team with the plan for a successful project. Changes are incorporated and evaluated and progress will be tracked on a weekly basis. Once the baseline schedule is developed, a Critical Path schedule will be established. Any deviation to the Critical Path Method will be evaluated and work around plans provided. The schedule will be maintained in MS Project. | |
| 2.4 | Risk Management is a function of good communication. The PROTECS Project Manager will work closely and communicate with HbO2 to identify and evaluate risk. PROTECS typically works with its clients to identify client business plans to enable review of design and procurement construction activities for potential risk. PROTECS will assist to evaluate these issues and manage the subsequent activities accordingly. | |
| 2.5 | Project Reporting and Control Systems are provided to meet the client and project needs. As previously discussed, cost and schedule are monitored and updated regularly. PROTECS will conduct client project review meetings on a bi-weekly (weekly, when necessary) basis and site contractor review meetings on a weekly basis. PROTECS also conducts in-house design coordination meetings on a weekly basis (through the design phase) in addition to drawing reviews and constructability reviews that are conducted as needed throughout the project. The frequencies of meetings are adjusted to meet the project needs.
|
www.protecsinc.com
| 83 |

| 2.6 | Document Management is initiated on a project specific basis. The Project Lead Sheet will contain the drawing and revision dates for the project. Specifications are logged by number and date. All distributions are issued and accompanied by a transmittal, containing the description, revision dated, and distribution. | |
| 2.7 | Configuration and Change Management are managed through our CR and PCO (work order) documents. Changes in construction packages are controlled through the issuing of bulletins and all contractor questions are written on a standard Request for Information format. | |
| 2.8 | Records Management. All project related documents are filed in the project file, which is regulated through a standard filing system. Documents are issued via a transmittal and correspondence is coded. Logs are kept electronically and backed up daily. | |
| 2.9 | Project Coordination. All PROTECS services for this project are delivered through one office; this allows for easy access and for a smooth flow of information. The project group is linked via an email system and can easily access common software through the company computer system. Formal meetings are organized with an agenda and minutes are kept and issued. All issues are surfaced, distributed, and resolved in an efficient manner. The aforementioned activities of scheduling and cost control are additional tools used to coordinate and prioritize the activities performed by the project team. | |
| 2.10 | Project Lists will be provided and are typical for: |
| Drawing List | ||
| Specification Log | ||
| Document Distribution List | ||
| Shop Drawings / Expediting Log | ||
| PCO Log | ||
| CR Log | ||
| Additional lists will be provided as the project requires. |
www.protecsinc.com
| 84 |

| 3.0 | FINAL ENGINEERING AND DESIGN |
| 3.1 | Detailed Design & Construction Documentation Phase | |
| Listed below is a summary description of the anticipated design deliverables to complete the design through the construction documentation phase of the work: | ||
| 1. | Develop and issue pre-purchase specifications for long lead equipment and respond to questions and clarifications. | ||
| 2. | Final Construction Drawings. | ||
| 3. | Required project meetings. | ||
| 4. | Subcontractor assistance. | ||
| ● | Respond to questions / clarifications from contractors | ||
| ● | Technical evaluation of work scopes | ||
| 3.2 | Construction Phase (Construction Administration) | |
| Listed below is a summary of anticipated deliverables by the PROTECS’ architect and engineer to support the construction phase effort of the project: | ||
| 1. | Shop drawing reviews. | |
| 2. | Respond to Requests for Information (RFIs). | |
| 3. | Conflict resolution during construction. | |
| 4. | Periodic construction site visits by each discipline. We have assumed key design engineers will visit at various stages of construction. | |
| 5. | Prepare field observation reports and punch lists by each discipline. | |
| 6. | Assist in commissioning of systems by providing general guidance. | |
| 7. | Review commissioning report. |
| 3.3 | Record Drawings | |
| Listed below is a summary of the anticipated deliverables by PROTECS in support of the project close-out: | ||
| 1. | Review subcontractor as-built drawings. | |
| 2. | Architectural, Structural, Mechanical, Plumbing, Process, and Electrical Deliverables. |
The anticipated drawing list is made up of all the drawings attached at the end of this document.
www.protecsinc.com
| 85 |

| 4.0 | PROCUREMENT, CONTRACT ADMINISTRATION AND CONSTRUCTION MANAGEMENT |
| 4.1 | Procurement |
| 1. | Develop and issue pre-purchase specifications for long-lead equipment. | |
| 2. | Provide early bid packages to confirm work scopes in accordance with approved design documents. | |
| 3. | Provide purchase orders for owner-selected long-lead equipment. |
| 4.2 | Contract Administration |
| 1. | Work scope walk-through meetings. | |
| 2. | Respond to questions/clarifications from bidders/contractors (RFI’s). | |
| 3. | Evaluation of bids. | |
| 4. | Awarding contracts. | |
| 5. | Shop drawing reviews. | |
| 6. | Periodic construction site visits. | |
| 7. | Punch listing. | |
| 8. | Assist in commissioning of systems by providing general guidance. | |
| 9. | Review commissioning report. |
| 4.3 | Construction Management |
| 1. | Full time on-site supervision. | |
| 2. | Preparation and monitoring of project schedules. | |
| 3. | Safety standard enforcement. | |
| 4. | Coordination of owner furnished items. | |
| 5. | Site cleanliness. | |
| 6. | Preparing and maintaining cost accounting procedures that track costs to date and costs to complete. | |
| 7. | Expediting long lead equipment. | |
| 8. | Monitoring subcontractor progress cost and change requests. | |
| 9. | Reviewing major discipline scopes and pricing versus budget with HbO2. | |
| 10. | Subcontractor coordination. | |
| 11. | Quality Assurance. | |
| 12. | Job Site Office. | |
| 13. | Shutdown Coordination. | |
| 14. | Estimating of alternatives or scope change requests. | |
| 15. | Constructability Review. | |
| 16. | Pre-purchase of Equipment. | |
| 17. | Contract Administration. |
www.protecsinc.com
| 86 |

| 5.0 | COMMISSIONING AND QUALIFICATION SERVICES |
| 5.1 | Introduction | |
The Commissioning and Validation Master Plans (VMP) will be drafted/developed by HbO2 for the project to define the approaches to be taken for commissioning, qualification and validation of the facility, equipment and utility systems related to HbO2’s project. The facility will be designed to comply with all current industry, regulatory and HbO2’s requirements. To date, PROTECS has used the previous validation/qualification documentation to develop the design and this proposal. A project Validation Master Plan (master plan) is required for the project to define the approaches to be taken for the commissioning, qualification, and validation of the facility, equipment, and utility systems related to HbO2’s project.
|
| 5.2 | Scope | |
| PROTECS will provide equipment start-up services for new equipment and provide it’s standard pre-functional and functional commissioning documentation for the major equipment installed including the following equipment: |
| ● | AHUs, | |
| ● | Humidifiers | |
| ● | Air compressor | |
| ● | Boiler | |
| ● | Generator | |
| ● | Chillers | |
| ● | Waste Neutralization System |
www.protecsinc.com
| 87 |

| IV. | CLARIFICATIONS |
| Following is a summary listing of the qualifications, clarifications, and assumptions we have made in developing this document. |
| 1.0 | General |
| 1.1 | This proposal and all contents of our offer expressed herein are confidential and privileged cannot be disclosed to parties outside of PROTECS and HbO2 Therapeutics without the specific written permission of PROTECS. Dissemination, distribution, or copying of this document or any portion thereof by any party other than PROTECS is strictly prohibited. | |
| 1.2 | HbO2 will be responsible for equipment and building system maintenance and control upon completion of start-up and training. | |
| 1.3 | All documents will be prepared in accordance with PROTECS standards. | |
| 1.4 | All documents delivered electronically as part of the final submittal will be in AutoCAD 2013, Microsoft Project, Microsoft Office, or Adobe PDF formats. | |
| 1.5 | Vendor documents will be furnished in the usual publication formats provided by the vendor. | |
| 1.6 | Redline As-built drawings will be provided based on subcontractor mark-ups. The P&ID drawings will be updated in CAD to incorporate contractor mark-ups. | |
| 1.7 | Tax costs in this proposal have been excluded based on HbO2 providing the appropriate PA state tax form for PROTECS to file with vendors and subcontractors. | |
| 1.8 | All unit costs in the $GMP identified as “ALLOW” represent an allowance for that particular line item that is the maximum financial responsibility of PROTECS within the $GMP. Any costs for any line item with unit cost of “ALLOW” in excess of the indicated amount will be in addition to the $GMP. | |
| 1.9 | The proposal is based on the work schedule attached hereto and milestone dates identified therein. | |
| 1.10 | The contingency is exclusively meant to pay for all the cost associated with estimating variances, work associated with further design development to meet the basis of design criteria, etc. The purpose of the contingency, however, is not to pay for additional work requests or scope adjustments by HbO2 or cost associated with force majeure (i.e., Acts of God, labor strife, strikes, etc. or field conditions). | |
| 1.11 | Our proposal is based on mutually agreed terms based on an AIA 191 Owner/Design-Builder contract document. | |
| 1.12 | PROTECS standard insurance coverage will be acceptable for the project. | |
| 1.13 | Payment and performance bonds have been excluded. | |
| 1.14 | Builders Risk Insurance will be provided by HbO2. | |
| 1.15 | One hard copy and one digital copy of as-built drawings and IOM manuals will be provided as part of this scope of work. |
www.protecsinc.com
| 88 |

| 1.16 | PROTECS’ standard one-year warranty on labor and materials is provided. Additional warranty requirements beyond these terms have not been included at this time. | |
| 1.17 | The order of precedence for the documents provided as part of this proposal is $GMP Clarifications/exclusions within this document, $GMP Basis of System Criteria, then the issue for construction drawings. | |
| 1.18 | This $GMP is made up of multiple project costs that contain individual line items, all of which comprise this $GMP. It is understood that no guarantee exists on any single item of work, and that dollar savings on a particular trade discipline may be transferred to cover possible overruns of another. | |
| 1.19 | This proposal is based on being paid for materials stored off site. | |
| 1.20 | General conditions include costs associated with the supervision and management of permitting, procurement, and construction activities throughout the duration of the project. The proposal is based on the work schedule attached hereto and milestone dates identified therein. Any work associated with General Conditions extending beyond the schedule indicated due to revisions directed by the owner are excluded. | |
| 1.21 | Fee is shown as a percentage of costs only for the overall presentation of the costs. It remains to be a fixed amount for the given scope whether there are cost savings on the project or otherwise. The fee for Owner requested additional work will be calculated at the same percentage basis. If the project scope increases, the fee and overhead also increase either at the same percentage rate as the shown in the $GMP breakdown or on the basis of requirements dictated by the project schedule and job conditions-whichever is applicable. |
| 2.0 | Design |
| 2.1 | The layout of the space and equipment is based on the drawings attached in the Appendix of this proposal. | |
| 2.2 | The layout and design is based on the equipment matrix dated February 27, 2017. | |
| 2.3 | We have based our efforts on the conceptual information and utility information provided by HbO2. We have done our best to estimate discrepancies or missing components in the information. These will be sorted through before the drawings can be finalized. | |
| 2.4 | Other chemicals will be provided by HbO2 and will be stored in appropriate cabinets, provided by HbO2, suited for the purpose of storing such chemicals per NFPA code requirements. | |
| 2.5 | Refer to the Hazardous Material Information BOSC902 drawing dated February 27, 2017 for the chemicals and quantities anticipated for the facility. | |
| 2.6 | Adequate and approved water supply to support the demand of the potable water system and sanitary water is understood to be available at the site. The pressure fluctuation of this system must be evaluated against the selected equipment requirements during design development. |
www.protecsinc.com
| 89 |

| 2.7 | Adequate and approved water supply to support the demand of the DI water system is understood to be available at the site. The pressure fluctuation of this system must be evaluated against the selected equipment requirements during design development. | |
| 2.8 | Environmental engineering, air permits, and/or other discharge permits are not included. | |
| 2.9 | The initial layout of the facility provides for cGMP compliance. | |
| 2.10 | The cleanroom area construction materials specified are resistant to the chemicals identified by HbO2 during the design process. PROTECS shall not be responsible for damages caused by improper use, changes to the cleaning procedures or use of chemicals not identified by HbO2 during the design process. | |
| 2.11 | This proposal and fees presented herein are based on PROTECS providing full design build services for the project. Should PROTECS provide design services only, the cost for these services will have to be increased accordingly. | |
| 2.12 | This proposal includes a complete design package as required to obtain permitting approval from the local governing authorities, Franconia Township, PA, and full time on-site PROTECS supervision and coordination of all trades and subcontractors performing the work. | |
| 2.13 | Health and Safety reviews for HAZOPs are not included. | |
| 2.14 | An as-built basis of system criteria or basis of design is not included. | |
| 2.15 | The services under this scope will be provided with the diligence and a standard of care consistent with and equal to the prevailing practices of similar professional organizations providing similar services within this industry and geographical area. | |
| 2.16 | PROTECS cannot be held responsible for schedule impact due to failure of local building officials to approve building permit by date indicated on the Project Schedule. PROTECS is not responsible for schedule impact due to failure of code officials to provide inspections of installed work in a reasonable period of time. | |
| 2.17 | The design and construction is based on the known national code requirements. Any requirements above and beyond these standards that may be required by the Owner’s insurance provider or by the local authority having jurisdiction (that are not published at the time the design was commenced) are not included as part of this work. | |
| 2.18 | The site is not in a flood zone. | |
| 2.19 | The engineering services for this project are offered on a lump sum basis and include the credit for the Permit to $GMP services. | |
| 2.20 | The engineering fee is fixed based on the $GMP proposal submitted on September 11, 2017. Additional engineering fees will not be added to the Process Automation and Process Materials procurement scopes that will be added into the project as long as they do not exceed the allowance amounts carried in the September 11, 2017 $GMP estimate. |
www.protecsinc.com
| 90 |

| 3.0 | Scope – Trade / Site | |
| The items below are meant to clarify or amplify the scope of the work. Although they are listed by division, they apply to the project as a whole (i.e., they are not limited to the work of any specific trade). | ||
| General | ||
| 3.1.1 | This proposal is solely based upon the material and quantity descriptions identified in this proposal and associated cost breakdown. | |
| 3.1.2 | The proposal is based on working over a period of 8 hours/day, between 7:00 AM and 5:00 PM during the week, Monday through Friday unless stated otherwise. The normal workday for the project site will be 7:00 AM to 3:30 PM. | |
| 3.1.3 | The estimate is based on receiving all construction areas clean and clear on the agreed-upon dates. The cleaning and clearing of the spaces of owner furniture prior to construction is not included as part of this estimate; however, we can provide services to assist your office in coordinating this work as an optional cost. | |
| 3.1.4 | We have not included any future allowances for cost impacts or escalation in regard to the current issues with shortages of copper, concrete, steel, and related products, materials, and equipment (steel, reinforcing bar, conduit, ductwork, piping, HVAC equipment, electrical gear, etc.). Any cost impacts related to this issue will be addressed as an added cost to the project scope, as necessary. | |
| 3.1.5 | We have allowed for a separate construction site office for this project within the existing building space including office space for PROTECS project team and with telephone and data lines available from within the building. PROTECS will have full use of the conference room and (3) offices throughout the construction activities. An alternate is provided for a separate onsite construction office if these spaces cannot be provided to PROTECS. | |
| 3.1.6 | This scope is based on gaining access to the building on October 2, 2017. | |
| 3.1.7 | Delays in the project start date will cause corresponding delays in the project substantial completion date. | |
| 3.1.8 | PROTECS has accounted for covering HbO2’s site specific orientation and safety training coincident with our safety and site orientation provided to our materialmen working on site and that HbO2’s orientation and safety issues can be covered within a thirty minute time frame. | |
| 3.1.9 | The budget reflects that the project is to be built by ‘merit shop’ labor. Specific trades will utilize union labor as required for the cleanroom wall panel installation. | |
www.protecsinc.com
| 91 |

| 3.1.10 | PROTECS will coordinate any work that will require the shutdown of existing switches, valves, etc. within the facility. HbO2 will provide staff personnel to close/open existing valves and switches that are required during this work. | |
| 3.1.11 | All utility work is included to only five feet outside the building line except the electrical service upgrade which extends to the existing pad mount transformer location. | |
| 3.1.12 | All existing utilities that are being connected to have been maintained, are functional, and are not clogged, blocked, etc. No remedial work will be required for these existing systems. | |
| 3.1.13 | See the site logistics plan for access, laydown, storage, temporary protection and dumpster locations. | |
| 3.1.14 | The Conex boxes onsite will be available for PROTECS use for storage of materials. | |
| 3.1.15 | It is assumed that all existing systems are operational. HbO2 will be responsible for re-starting all existing equipment and systems. PROTECS can assist with this work if required at additional cost. | |
| 3.1.16 | All owner furnished equipment/materials will be located at the site or in the warehouse located at 300 Enterprise Lane, Colmar, PA. | |
| 3.1.17 | Vendors warranties are pass through warranties. |
| Site | ||
| 3.2.1 | Construction debris will be removed from the site by containers provided under this scope. | |
| 3.2.2 | Temporary protection will be provided as depicted on the site logistics plan. | |
| 3.2.3 | Subcontractor parking will be onsite in the site’s parking lot. The parking lot wearing layer and stripping will be completed by others after completion of construction. | |
| 3.2.4 | The soil bearing capacity was based on the Geotechnical report (File No. 14-90554- 02) dated January 21, 2015 prepared by Gilmore & Associates. | |
| 3.2.5 | The pricing is based on being able to use the material excavated for the project as backfill material around the foundations, cut/fill operations, and site grade adjustments. | |
| Concrete | ||
| 3.3.1 | It is assumed the existing concrete slab is no greater than 6” thick. | |
| Masonry | ||
| 3.4.1 | Intentionally left blank. | |
| Structural | ||
| 3.5.1 | An AISC certified erector will not be required for steel erection. 3rd Party steel inspections will be performed. | |
www.protecsinc.com
| 92 |

| Carpentry | ||
| 3.6.1 | Intentionally left blank. | |
| Thermal and Moisture Protection | ||
| 3.7.1 | Non penetrating pipe and duct supports will be provided where possible. | |
| Doors and Windows | ||
| 3.8.1 | It is assumed the exterior doors have been prepped to receive the card reader hardware. | |
| Finishes | ||
| 3.9.1 | The epoxy flooring system specified is based on cart traffic and hand trucking on rubber and plastic wheels with a maximum load of no more than 1,000 lbs. | |
| 3.9.2 | Colors at all products are manufacturer’s standard unless indicated otherwise. PROTECS has allowed for a calcium chloride moisture test in the area. PROTECS excludes testing the substrate for hydrostatic and/or osmotic pressure prior to installation of epoxy floor. | |
| 3.9.3 | Bottled gases such as nitrogen and associated panels will be provided by HbO2. | |
| 3.9.4 | EIFS removal to the backer board or CMU block has been included. Replacement of the backer board or mold remediation have not been included, but can be provided at additional cost. | |
| 3.9.5 | It is understood that HbO2 will provide the necessary Dagard cleanroom wall panels and associated hardware for installation. The Dagard cleanroom wall panel system will be stored local (within 20 min) to the jobsite and transportation of the materials from this location to the jobsite is included. New materials and hardware for the ceilings, doors and cleanroom windows will be provided by PROTECS. Additional materials required for a complete installation can be provided at additional costs if materials are not provided by HbO2. | |
| 3.9.6 | The polished concrete flooring will show the existing concrete aggregate and the condition of the concrete will determine the final finish. No provisions are included to replace existing concrete. | |
| Specialties | ||
| 3.10.1 | The wall protection will be limited to SS corner guards. SS wall protection has been provided as an alternate. | |
| 3.10.2 | All cleanroom gowning, garments, and accessories will be furnished by HbO2 as a basis for this proposal. | |
| 3.10.3 | Lockers will be installed on “Z” bases in lieu of a concrete pad. |
www.protecsinc.com
| 93 |

| Equipment | ||
| 3.11.1 | The process equipment provided by HbO2 will arrive at the site in time to be installed in coordination with the construction of the production area. | |
| 3.11.2 | Procurement, unloading, rigging, installation, commissioning and validation of any HbO2 equipment have not been included unless specifically identified elsewhere in this document. | |
| Furnishings | ||
| 3.12.1 | The lab casework for the project will be used with new epoxy tops. | |
| 3.12.2 | Fumehood certification is not included. | |
| Special Construction | ||
| 3.13.1 | The rigging of the roof top equipment for the project can be done from the parking lot adjacent to the project work area during normal working hours. | |
| 3.13.2 | The existing cold box to be relocated is assumed to be in good working order and repairs/replacement of equipment/parts is not included. The existing wall and ceiling panels are to be re-used and replacement of damaged panels is not included, but can be provided for additional cost. | |
| Conveying Systems | ||
| 3.14.1 | Intentionally left blank | |
| Mechanical | ||
| 3.15.1 | Adequate and approved water supply to support the demand of the fire water, potable water, and sanitary water is understood to be available at the site. | |
| 3.15.2 | Temporary heating and cooling will be done using the new HVAC equipment. | |
| 3.15.3 | Safety showers are not connected to a drainage system. | |
| 3.15.4 | Standard Flexible ductwork with R-6 insulation factor will be provided. | |
| 3.15.5 | SS 316 tubing with Swagelok compression fitting will be provided for connection to process equipment in the production space for N2, VAC and Compressed Air. | |
| 3.15.6 | HEPA integrity testing will be based on the factory tests. HEPA filter certification and room certification testing will be completed by HbO2. | |
| 3.15.7 | The HbO2 furnished chillers are assumed to meet the performance specifications provided to PROTECS. Modifications/repairs to the chillers are not included, but can be provided at additional cost. | |
| 3.15.8 | The lab RODI water system will be furnished and installed by HbO2. Distribution piping to use points as indicated will be provided. | |
| 3.15.9 | Polyisocyanurate insulation with a PVC jacket will be provided in the cleanroom areas for the chilled/warm glycol piping. | |
| 3.15.10 | Standard validation documentation for execution by HbO2 will be provided with the CFR 21 Part 11 compliant monitoring system. Custom validation documentation is not included, but can be provided at additional cost. | |
www.protecsinc.com
| 94 |

| 3.15.11 | HEPA filter module housings will be of aluminum construction (housing is located above ceiling and is not exposed to the cleanroom) and the cleanroom exposed perforated grille and perimeter trim will be 304 SS. | |
| 3.15.12 | BAS graphics will be created or modified to depict the new control systems. | |
| 3.15.13 | BAS standard point and program checkout is included. Loop checks and field calibration are not included. |
| Electrical | ||
| 3.16.1 | We have allowed for MC cable for lighting and power branch circuit conduit and wire within designed spaces. | |
| 3.16.2 | HbO2 will pay all utility company charges for permanent or temporary services including consumption. | |
| 3.16.3 | All underground electrical conduit to be PVC schedule 40. | |
| 3.16.4 | Proposal and pricing for the electrical work is based on Copper wire pricing received at the time of the proposal. Our suppliers will not guarantee wire pricing beyond a one day period. PROTECS reserves the right to adjust the project proposal pricing shown to reflect any increases in copper wire proportional to the quantities in the project and the base price of copper current at the time wire can be purchased. | |
| 3.16.5 | Individual fixture support wires for air devices and light fixtures are not included. | |
| 3.16.6 | The HbO2 furnished generator is assumed to include the equipment and performance criteria noted on the reviewed generator submittal. Modifications and repairs to the generator are not included, but can be provided at additional cost. | |
| 3.16.7 | The VFDs to be provided carry a 5 kAIC rating with a non-barrier bypass. The 75 kAIC rating and barrier bypass can be provided at additional cost. | |
| Process | ||
| 3.17.1 | PROTECS will direct and coordinate LaProte’s efforts in a design build manner for the skid assemble scope of work. | |
| 3.17.2 | Passivation is not included, but will be required. The Passivation scope can be provided at additional cost. | |
| 3.17.3 | No spare parts are included in the scope. | |
| 3.17.4 | The purchase of required software and license if necessary is by HbO2 | |
| 3.17.5 | No shop calibration will be performed prior to final site installation for existing equipment. Existing instruments are assumed to be suitable for calibration. | |
| 3.17.6 | If the materials procurement scope is removed from the scope of this proposal, the costs expended to date will be removed from the credit amount to HbO2. | |
| 3.17.7 | No guarantee is provided on the process performance of the skid (i.e. product yield, etc.) | |
| 3.17.8 | SOPs and Batch Records are by HbO2. | |
www.protecsinc.com
| 95 |

| 3.17.9 | It is assumed that all HbO2 furnished materials and equipment are in good working order and have the necessary documentation (model and serial number, material certificates and surface finishes, weld log, calibration certificates as applicable). If material and surface finish certificates are missing for existing equipment, an inspection can be performed at additional cost. | |
| 3.17.10 | Warranty does not extend to equipment or parts provided by others and installed under this proposal. | |
| 3.17.11 | The demolition of the existing Process RODI water system and installation of the new WFI Generation system will be completed by HbO2. | |
| 3.17.12 | Validation writing and execution is by HbO2. | |
| 3.17.13 | FAT/SAT writing and execution is included and availability of HbO2 personnel is required for input/review in a timely manner. | |
| 3.17.14 | IQ/OQ is by HbO2. | |
| 3.17.15 | The filling equipment will be furnished and installed by HbO2. The recommended modifications will be completed by HbO2. The particulate monitoring system that is required is to be provided by HbO2. The risk assessment and design of the monitoring system can be provided at additional cost. | |
| 3.17.16 | The Process Automation Engineering is included, but the installation costs (including wiring and field calibration) are not included. The Process Automation installation scope can be provided at additional cost. | |
| 3.17.17 | The Process System Components Procurement (Skid, Non-Skid & Automation) costs are not included and will be addressed once a final parts purchase list has been developed and agreed to by HbO2, PROTECS & LaPorte. | |
| Commissioning / Qualification | ||
| 3.18.1 | Any required gas, water or environmental microbiological sampling will be performed by HbO2. | |
| 3.18.2 | All sampling materials will be obtained by HbO2. | |
| 3.18.3 | The approach to control system testing will be complete functional testing. Code review is not included in the scope of work. PROTECS can provide code review, if requested by HbO2. § 21 CFR Part 11 compliance assessments for the equipment PLCs / control systems are not included in the scope of work. PROTECS can quote this separately after reviewing the systems and documentation provided by the vendor. | |
| 3.18.4 | Commissioning costs include all of the new facility equipment only and do not include any existing or process systems. Commissioning costs include costs associated with the applicable subcontractor’s standard commissioning procedures. Commissioning procedures, guidelines, and forms required by the owner are excluded. Validation is also excluded. | |
| 3.18.5 | The writing of operations and maintenance SOPs is by HbO2. | |
www.protecsinc.com
| 96 |

| 3.18.6 | The development of URS is by HbO2 and will need to match the design. Design changes due to URS requirements are not included. | |
| 3.18.7 | No warranties cover fitness for use, merchantability or purpose / use. |
| 4.0 | Exclusions | |
| Work not included: | ||
| 4.1.1 | Builder’s risk insurance and associated deductible. | |
| 4.1.2 | LEED requirements. | |
| 4.1.3 | Building permitting and expediting. We have assumed HbO2 will directly pay all state and local permit fees. We will process and file for local permits on HbO2’s behalf. | |
| 4.1.4 | Payment and performance bond. | |
| 4.1.5 | Utility company consumption charges. | |
| 4.1.6 | Rock excavation. | |
| 4.1.7 | Office furnishings. | |
| 4.1.8 | Snow and ice removal. | |
| 4.1.9 | Premium time / acceleration of schedule. | |
| 4.1.10 | Room identification and signage. | |
| 4.1.11 | Excavation, except at new foundations and plumbing trenches. | |
| 4.1.12 | Handling, removal, and disposal of toxic or hazardous materials in or within the soil is not part of this work. These materials will have to be addressed if and when encountered during the course of the work. | |
| 4.1.13 | Site security, exterior lighting, etc. during the course of construction. | |
| 4.1.14 | Painting of piping, conduit, and insulation. Manufacturer’s standard painting will be provided on equipment and will be touched up in the field if necessary due to installation damage. | |
| 4.1.15 | Seismic hangers and/or earthquake bracing. | |
| 4.1.16 | Spray fireproofing of the steel structure. | |
| 4.1.17 | Cleanroom furniture,workstations or accessories. | |
| 4.1.18 | A dedicated fire watch service during the construction process. | |
| 4.1.19 | Site security services. | |
| 4.1.20 | Civil, environmental, equipment and process engineering. | |
| 4.1.21 | Planning board documents and/or meetings | |
| 4.1.22 | Waste permits, waste discharge permits, air permits, and other discharge permits. | |
| 4.1.23 | Field calibration. | |
| 4.1.24 | Window treatment | |
| 4.1.25 | Roof and equipment screening. | |
| 4.1.26 | Water meter, sewer, or assessment fees. | |
| 4.1.27 | Laboratory hoods. | |
| 4.1.28 | Biosafety cabinets. | |
| 4.1.29 | Room particulate and pressure testing. |
www.protecsinc.com
| 97 |

| 4.1.30 | Explosion venting. | |
| 4.1.31 | Utility company (i.e., POWER COMPANY, GAS COMPANY, TELE/DATA COMPANY, etc.) installation charges, usage charges or other fees. HbO2 to negotiate these items and related charges, costs, or fees directly with the utility company. | |
| 4.1.32 | Special electrical systems (i.e., speakers, Muzak, PA, paging, intercom, clocks, sound masking, security, cameras, monitors, card readers, TV cables, TV antennas, AV, etc.). | |
| 4.1.33 | Special grounding systems, ground grids, ground loops, ground rods, grounding risers, or other special “power quality” systems. | |
| 4.1.34 | Warehouse racks. | |
| 4.1.35 | Revisions to existing systems affected by relocated equipment from those systems. | |
| 4.1.36 | Temporary fencing for “lay-down” areas or site storage areas. | |
| 4.1.37 | Trade remobilization other than what is indicated in the project schedule and associated staging and phasing plan. | |
| 4.1.38 | Sloping or leveling of the existing floor. | |
| 4.1.39 | Site filter challenge tests. | |
| 4.1.40 | Ultrasonic testing. | |
| 4.1.41 | Slab x-rays. | |
| 4.1.42 | Temporary sanitary facilities. | |
| 4.1.43 | Subsurface investigation. | |
| 4.1.44 | Water meter, sewer, and assessment fees | |
| 4.1.45 | Permanent fire extinguishers. | |
| 4.1.46 | Factory / Shop inspections and tests. | |
| 4.1.47 | Landscaping. | |
| 4.1.48 | X-ray tests on welds. | |
| 4.1.49 | Repair to existing insulation that is damaged prior to this work. | |
| 4.1.50 | Warranty or guarantee of existing systems. | |
| 4.1.51 | A crane hoist for service and maintenance will not be provided. |
www.protecsinc.com
| 98 |

| V. | ESTIMATE |
| VI. | COMMERCIAL TERMS |
PROTECS proposes to provide professional engineering and construction management services to implement the project as described herein for a Guaranteed Maximum Price ($GMP) of Twenty Four Million Six Hundred Forty Eight Thousand Two Hundred Forty Eight Dollars ($24,648,248). A breakdown for these costs in CSI format is provided within the attached estimate. This price is based on proceeding with the work in accordance with the terms and conditions of the AIA 191 Part 2 Design/Build Agreement previously agreed to. Once this proposal is signed an Exhibit will be provided to amend this proposal to the Agreement as indicated therein.
Work can commence immediately upon receiving written authorization to proceed (i.e., a contract) or a purchase order and a ten (10%) percent down payment. Our services will be invoiced monthly per the agreement and are payable net 20 days.
This proposal is valid for a period of thirty (30) days from the date of the proposal. If the services of PROTECS do not commence, or are stopped for a period of thirty (30) days through no act or fault of PROTECS, PROTECS may elect to renegotiate the terms of this agreement to reflect changes in project scope, schedule, and fee schedules.
Any changes made to this proposal do not constitute an agreement. All changes must be approved by both parties in writing. If you accept this proposal, please sign one copy and return it to PROTECS.
| PROTECS | ACCEPTED BY: | ||
| PROPOSAL No. 1074PPA17.1 Rev. 1 | Hemoglobin Oxygen Therapeutics, LLC. | ||
 |
|||
| Christopher R. DiPaolo | |||
| President | Date: | ||
www.protecsinc.com
| 99 |

| VII. | MILESTONE SCHEDULE |
| VIII. | ATTACHMENTS |
| 8.1 | HbO2 – Facility Expansion Documents List dated September 8, 2017 (9 Pages) | |
Note: Documents Previously Issued to HbO2 are indicated on the documents list, but are not attached. |
| 8.2 | Site Logistics Plan dated July 19, 2017 (1 Page) |
www.protecsinc.com
| 100 |

2017 Standard Terms and Conditions
Consulting Services
This document represents the standard terms and conditions for the PROTECS proposal to which this document is attached. These terms and conditions shall apply to the work unless specifically stated otherwise in the clarification section or commercial terms section of the proposal. These terms and conditions shall supersede blanket terms in seller/purchaser agreements, such as “the terms and conditions of this purchase order or contract shall render all previous terms, conditions, etc. null and void”, unless the specific paragraphs of this document are referenced. These terms and conditions shall also apply to authorizations issued by purchaser to seller on purchaser’s letterhead and/or purchase order.
|
1. CONTROLLING DOCUMENT The acceptance of Purchaser’s order is expressly made conditional on Purchaser’s assent to the terms and conditions set forth herein, and PROTECS (“Seller”) agrees to furnish the covered thereby only upon these terms and conditions. This document constitutes the entire agreement of the parties with respect to the subject matter hereof and supersedes all prior understandings and agreements. No term or condition of Purchaser’s order inconsistent with the terms and conditions hereof; and no term, condition, statement or representation not contained herein, shall be binding on Seller as a warranty or otherwise. No wavier, alteration, or modification of any of the provisions hereof, shall be binding on Seller unless made in writing signed by an officer of Seller.
2. PRICES AND TAXES Prices shown are for delivery of goods/services, F.O.B. point of shipment. Any manufacturer’s tax, sales tax, use tax, excise tax, custom, inspection or testing fee, or any other tax, fee or charge of any nature whatsoever imposed by governmental authority, on or measured by the transaction between Seller and Purchaser shall be paid by Purchaser in addition to the prices quoted or invoiced. In the event Seller is required to pay any such tax, fee, or charge, Purchaser shall within ten (10) business days reimburse Seller therefore. Delinquent payments shall be subject to a carrying charge with interest terms below.
3. TERMS AND METHOD OF PAYMENT Where Seller has extended credit to Purchaser, the terms of payment shall be net ten (10) days from date of invoice. RE: Services-Services will be invoiced on a percentage complete basis and are due upon receipt. Seller reserves the right to invoice on a periodic basis as required to facilitate the work. If, in the judgment of Seller, the financial condition of Purchaser at any time does not justify continuance of production of shipment upon the terms of payment specified, Seller may require full or partial payment in advance.
4. DELIVERY AND DELAY Delivery of goods or services to a carrier at Seller’s plant or other loading point shall constitute delivery to Purchaser. Seller shall not be liable for any loss or damage as a result of any delay due to any cause beyond Seller’s control including, without limitation: an Act of God, act of Purchaser, fire, theft, accident, slowdown, strike, riot, embargo, governmental act, regulation or request, delays of common carriers, or other similar causes. In case of any such delay, the date of delivery shall be extended for a period equal to the time lost by cause of the delay. In no event shall Seller’s liability for any other delay exceed the sales price to Purchaser. Seller shall not be liable for any special, contingent, indirect or consequential damages (including anticipated profits) resulting from delay.
5. STOP WORK OR CANCELLATION In the event of a request to stop work or to cancel the whole or part of any order, Purchaser shall pay Seller for: (a) Any and all work that has been completed. (b) For all other work in process and all materials and supplies procured or for which definite commitments have been made by Seller in connection with the order. Purchaser shall pay Seller all of Seller’s costs and expenses.
6. TOOLING AND DESIGNS All specifications, drawings, designs, data, information, methods, descriptions, programs, software, software source code, ideas and/or inventions made, used, conceived, developed or acquired by Seller incident to its performance hereunder and all patent, trade-secret, know-how, copyright or other proprietary rights therein, shall be exclusive property of Seller and no part of the purchase price hereunder shall be deemed applicable to the foregoing items.
7. INFRINGEMENT CLAIMS Proposals are offered for the sole and private use of Client’s name and is in response to solicitation for our services. The ideas, suggestions and intellectual contents remain the property of PROTECS and should not be shared with other unauthorized individuals or outside organizations without the written permission of PROTECS. |
Purchaser shall indemnify Seller for the costs of defending any suit or proceeding (including attorneys’ fees, witness fees, judgment or settlement) brought against Seller for infringement if the claimed infringement has resulted from Seller’s compliance with Purchaser’s designs, specifications, and instructions or by reason of the alterations, application or use of the goods by Purchaser or any other party.
8. JURISDICTION LAW The agreement hereunder shall be governed by, and its terms constructed in accordance with, the laws of the State of Pennsylvania.
9. ARBITRATION Any controversy or claim arising out of or relating to this Contract, or the breach thereof, shall be settled by arbitration held in Montgomery County, Pennsylvania, in accordance with the rules of the American Arbitration Association, and judgment upon the award rendered by the Arbitrator(s) may be entered in any Court having jurisdiction thereof.
10. INTEREST Payments are due upon receipt unless noted otherwise in the commercial terms section of a Sellers proposal. Payments over thirty (30) days late shall accrue an interest charge at the rate of prime plus one percent. Prime is the prime interest rate as published in the Wall Street Journal on the first business day the payment is due and shall be adjustable on a monthly basis over the period the balance due remains an open seller receivable.
11. ADDITIONAL SERVICES As time is of the essence situations/issues may arise where the Purchaser will require additional services not covered in Sellers proposal. Seller will provide these services on a time and material basis in accordance with Seller’s current published rate sheet. The additional services will be provided on a continuous basis so the impact to the project will be minimized. Approval of these services on the Purchasers behalf shall be considered tacit in nature. As completed Seller will issue a scope adjustment notice for record purposes only. Seller reserves the right to refrain from providing these additional services for any cause.
12. HIRING In accepting or authorizing this proposal, Purchaser represents they will not hire Seller’s personnel, directly or indirectly, during the period Seller is performing services for Purchaser and for ninety (90) consecutive calendar days thereafter. Should Purchaser hire Seller’s personnel, direct or non-direct, Seller shall be entitled to a “Finders Fee” of 25% of the employee’s first year salary, payable in accordance with paragraph 3 upon the hire date of Seller’s employee by Purchaser.
13. REIMBURSABLES Purchaser agrees to pay Seller for all reimbursable cost in addition to the fee addressed in the proposal. Reimbursable costs include but are not limited to travel, airfare, rail fare, car fees, meals; lodging, tolls, copies of documents and drawings, plots of documents and drawings, phone calls; photographs, etc. A 1.05 times multiplier will be added to the costs of the reimbursables.
14.NON-SOLICITATION During the term of the contract with PROTECS, and for a period of one (1) year thereafter, the client shall not, directly or indirectly, without the prior written consent of the PROTECS, solicit or induce any employee or consultant of PROTECS to leave the employ or service of PROTECS or hire, retain, employ, or engage for any purpose any employee or consultant of PROTECS; however, such covenant shall be subject to PROTECS remaining in operation for the restrictive period defined hereunder. When PROTECS provides written consent, 25% finder’s fee will be charged to the client. |
www.protecsinc.com
| 101 |
Exhibit 10.10
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022 by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and OVA International Investment LLC, a Washington limited liability company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of five million sixty thousand dollars ($5,060,000) (the “Advances”) evidenced by loan agreements. | |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). | |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). | |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of five million sixty thousand dollars ($5,060,000), for shares of the Common Stock of Newco at a conversion rate of $2.744 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of one million eight hundred forty four thousand and twenty three (1,844,023) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 2.744. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
| - 2 - |
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Igor Serov | |
| Title: | CFO | |
| OVA International Investments LLC: | ||
| By: | ||
| Name: | Oleg Orlovskii | |
| Title: | President | |
| - 3 - |
Exhibit 10.11
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022, by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and the undersigned creditor of the Company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of five hundred thousand dollars ($500,000.00) (the “Advances”) evidenced by a loan agreement. | |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). | |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). | |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of five hundred thousand dollars ($500,000.00), for shares of the Common Stock of Newco at a conversion rate of $5.4881 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of ninety one thousand one hundred and six (91,106) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 5.4881. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
[ SIGNATURE PAGE FOLLOWS ]
| - 2 - |
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Igor Serov | |
| Title: | CFO | |
| Purchaser: | Igor Nikitin | |
| By: |
| - 3 - |
Exhibit 10.12
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022, by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and the undersigned creditor of the Company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of five hundred thousand dollars ($500,000.00) (the “Advances”) evidenced by a loan agreement. | |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). | |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). | |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of five hundred thousand dollars ($500,000.00), for shares of the Common Stock of Newco at a conversion rate of $5.4881 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of ninety one thousand one hundred and six (91,106) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 5.4881. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
[ SIGNATURE PAGE FOLLOWS ]
| - 2 - |
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Igor Serov | |
| Title: | CFO | |
| Purchaser: | Maxim Dashtiev | |
| By: |
| - 3 - |
Exhibit 10.13
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022, by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and the undersigned creditor of the Company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of three hundred thousand dollars ($300,000.00) (the “Advances”) evidenced by a loan agreement and promissory notes. | |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). | |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). | |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of three hundred thousand dollars ($300,000.00), for shares of the Common Stock of Newco at a conversion rate of $3.6221 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of eighty two thousand eight hundred and twenty five (82,825) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 3.6221. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
| - 2 - |
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Igor Serov | |
| Title: | CFO | |
| Purchaser: | Ivanka Kowalski | |
| By: |
| - 3 - |
Exhibit 10.14
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022, by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and the undersigned creditor of the Company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of two hundred thirty thousand dollars ($230,000.00) (the “Advances”) evidenced by a loan agreement. | |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). | |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). | |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of two hundred thirty thousand dollars ($230,000.00), for shares of the Common Stock of Newco at a conversion rate of $5.4881 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of forty one thousand nine hundred and nine (41,909) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 5.4881. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
[ SIGNATURE PAGE FOLLOWS ]
| - 2 - |
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Igor Serov | |
| Title: | CFO | |
| Purchaser: | Christopher R. DiPaolo | |
| By: |
| - 3 - |
Exhibit 10.15
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022, by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and the undersigned creditor of the Company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of one hundred fifty thousand dollars ($150,000.00) (the “Advances”) evidenced by promissory notes. | |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). | |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). | |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of one hundred fifty thousand dollars ($150,000.00), for shares of the Common Stock of Newco at a conversion rate of $3.6221 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of forty one thousand four hundred and twelve (41,412) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 3.6221. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
[ SIGNATURE PAGE FOLLOWS ]
| - 2 - |
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Igor Serov | |
| Title: | CFO | |
| Purchaser: | Richard Kilsby | |
| By: |
| - 3 - |
Exhibit 10.16
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022, by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and the undersigned creditor of the Company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of one hundred forty thousand dollars ($140,000.00) (the “Advances”) evidenced by promissory notes. | |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). | |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). | |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of one hundred forty thousand dollars ($140,000.00), for shares of the Common Stock of Newco at a conversion rate of $3.6221 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of thirty eight thousand six hundred and fifty two (38,652) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 3.6221. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
[ SIGNATURE PAGE FOLLOWS ]
| - 2 - |
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Igor Serov | |
| Title: | CFO | |
| Purchaser: | Kevin Kowalski | |
| By: |
| - 3 - |
Exhibit 10.17
DEBT CONVERSION AGREEMENT
THIS DEBT CONVERSION AGREEMENT (this “Agreement”) is made and entered into as of January 3, 2022, by and between Hemoglobin Oxygen Therapeutics, LLC., a Delaware limited liability company (the “Company”), and the undersigned creditor of the Company (“Purchaser”).
RECITALS
| A. | Purchaser has made advances to the Company in the aggregate amount of fifty thousand dollars ($50,000.00) (the “Advances”) evidenced by a promissory note. |
| B. | The Company plans to convert to a Delaware C corporation (“Newco”) after January 3, 2022, on the basis of one hundred shares of Common Stock of Newco for each limited liability unit of the Company (the “Restructuring”). |
| C. | The Company after the Restructuring plans to engage in an underwritten initial public offering of shares of its Common Stock (the “Public Offering”). |
| D. | On the terms and subject to the conditions of this Agreement, Purchaser desires to convert the Advances in the amount of fifty thousand dollars ($50,000.00), for shares of the Common Stock of Newco at a conversion rate of $3.6221 per share. |
NOW, THEREFORE, with reference to the foregoing facts, the Company and the Purchaser agree as follows:
AGREEMENT
1. Conversion of Advances. The Company hereby agrees that Newco shall issue to Purchaser an aggregate of thirteen thousand eight hundred and four (13,804) shares (the “Shares”) of Common Stock of Newco, and the Purchaser hereby agrees to convert the Advances into the Shares. The number of Shares has been determined based upon dividing the outstanding Advances by 3.6221. The Company agrees that Newco will instruct its transfer agent to issue the Shares to Purchaser promptly upon closing of the Public Offering. It is understood that any accrued interest on the Advances shall be extinguished as soon as the Advances will be converted to Shares at the closing of the Public Offering. The conversion of the Advances shall occur concurrently with the closing of the Public Offering and is conditioned thereon.
2. Representations and Warranties of the Purchaser. Purchaser hereby represents and warrants to, and agrees with, the Company as follows:
2.1 Purchaser understands that: (a) the Shares will not registered under the Securities Act of 1933, as amended (the “Securities Act”), or any state securities laws; (b) the issuance and sale of the Shares is intended to be exempt from registration under the Securities Act, by virtue of Section 4(2) thereof and the provisions of Regulation D promulgated thereunder, based, in part, upon the representations, warranties and agreements of the Purchaser contained in this Agreement.
2.2 Purchaser will be acquiring the Shares solely for the Purchaser’s own account for investment and not with a view to resale or distribution thereof, in whole or in part.
2.3 Purchaser must bear the substantial economic risks of the investment in the Shares indefinitely, because none of the Shares may be sold, assigned, transferred, hypothecated or otherwise encumbered or disposed of unless subsequently registered under the Securities Act and applicable state securities laws or any exemption from such registration is available. Legends shall be placed on the Shares to the effect that they have not been registered under the Securities Act or applicable state securities laws. In addition, appropriate notations thereof will be made in Newco’s books, and stop transfer instructions will be placed with the transfer agent of the Shares.
2.4 Purchaser has adequate means of providing for such Purchaser’s current financial needs and foreseeable contingencies and has no need for liquidity of the investment in the Shares for an indefinite period of time.
2.5 Purchaser understands that an investment in the shares involves a high degree of risk.
2.6 Purchaser is an “accredited investor” under Regulation D under the Securities Act.
3. Miscellaneous
3.1 This Agreement constitutes the entire agreement between Purchaser and the Company with respect to the subject matter hereof and supersedes all prior oral or written agreements and understandings, if any, relating to the subject matter hereof. The terms and provisions of this Agreement may be waived, or consent for the departure therefrom granted, only by a written document executed by the party entitled to the benefits of such terms or provisions.
3.2 Purchaser’s representations and warranties made in this Agreement shall survive the execution and delivery hereof and delivery of the Shares.
3.3 This Agreement may be executed in one or more counterparts each of which shall be deemed an original, but all of which shall together constitute one and the same instrument.
3.4 Each provision of this Agreement shall be considered separable and if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable law such invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
3.5 This Agreement shall be governed by and construed in accordance with the laws of the State of Delaware relating to contracts entered into and to be performed wholly within such State.
3.6 Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
[ SIGNATURE PAGE FOLLOWS ]
| - 2 - |
| Hemoglobin Oxygen Therapeutics, LLC. | ||
| By: | ||
| Name: | Zafiris Zafirelis | |
| Title: | CEO | |
| Purchaser: | Igor Serov | |
| By: | ||
| - 3 - |
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
Hemoglobin Oxygen Therapeutics LLC
Souderton, Pennsylvania
We hereby consent to the use in the Prospectus constituting a part of this Registration Statement of our report dated April 25, 2022, relating to the consolidated financial statements of Hemoglobin Oxygen Therapeutics LLC, which is contained in that Prospectus. Our report contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
We also consent to the reference to us under the caption “Experts” in the Prospectus.
/s/ BDO USA, LLP
Philadelphia, Pennsylvania
April 25, 2022
Exhibit 99.1
Consent of Director Nominee
Pursuant to Rule 438 promulgated under the Securities Act of 1933, as amended (the “Securities Act”), I hereby consent to my being named in the Registration Statement on Form S-1 of Hemoglobin Oxygen Therapeutics LLC, and all amendments thereto (the “Registration Statement”) and any related prospectus filed pursuant to Rule 424 promulgated under the Securities Act, as a nominee to become a director of Hemoglobin Oxygen Therapeutics Inc. upon the incorporation of Hemoglobin Oxygen Therapeutics Inc. and to the filing of this consent as an exhibit to the Registration Statement.
| /s/ Leslie Fang | ||
| Name: | Leslie Fang |
Exhibit 99.2
Consent of Director Nominee
Pursuant to Rule 438 promulgated under the Securities Act of 1933, as amended (the “Securities Act”), I hereby consent to my being named in the Registration Statement on Form S-1 of Hemoglobin Oxygen Therapeutics LLC, and all amendments thereto (the “Registration Statement”) and any related prospectus filed pursuant to Rule 424 promulgated under the Securities Act, as a nominee to become a director of Hemoglobin Oxygen Therapeutics Inc. upon the incorporation of Hemoglobin Oxygen Therapeutics Inc. and to the filing of this consent as an exhibit to the Registration Statement.
| /s/ Anthony E. Pusateri | ||
| Name: | Anthony E. Pusateri | |
Exhibit 99.3
Consent of Director Nominee
Pursuant to Rule 438 promulgated under the Securities Act of 1933, as amended (the “Securities Act”), I hereby consent to my being named in the Registration Statement on Form S-1 of Hemoglobin Oxygen Therapeutics LLC, and all amendments thereto (the “Registration Statement”) and any related prospectus filed pursuant to Rule 424 promulgated under the Securities Act, as a nominee to become a director of Hemoglobin Oxygen Therapeutics Inc. upon the incorporation of Hemoglobin Oxygen Therapeutics Inc. and to the filing of this consent as an exhibit to the Registration Statement.
| /s/ Richard Kilsby | ||
| Name: | Richard Kilsby | |
Exhibit 107
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(5) | Amount of Registration Fee(2) | ||||||
| Shares of Common Stock, par value $0.0001 per share | ||||||||
| Warrants to purchase common stock to be issued to the Underwriters(3)(4) | $ | — | $ | — | ||||
| Common stock issuable upon exercise of warrants to purchase common stock to be issued to the Underwriters(3)(4) | $ | $ | ||||||
| Total: | $ | 30,000,000 | $ | 2,781 | ||||
| (1) | Estimated solely for the purpose of computing the amount of the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended. Includes shares of common stock that the underwriters have the option to purchase to cover over-allotments, if any. |
| (2) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price of the securities registered hereunder to be sold by the registrant. |
| (3) | We have agreed to issue to the underwriters, upon closing of this offering, warrants to purchase 6% of the number of shares of common stock sold in this offering. Resales of shares of common stock issuable upon exercise of the underwriters’ warrants are being similarly registered on a delayed or continuous basis. We have calculated the proposed maximum aggregate offering price of the common stock underlying the underwriters’ warrants by assuming that such warrants are exercisable at a price per share equal to 120% of the price per share sold in this offering. |
| (4) | No fee required pursuant to Rule 457(g). |
| (5) | Pursuant to Rule 416 under the Securities Act, there is also being registered hereby such indeterminate number of additional shares of common stock of the Registrant as may be issued or issuable because of stock splits, stock dividends, stock distributions, and similar transactions. |