
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
Sagimet Biosciences Inc.
(Address of principal executive offices, including zip code)
(
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trade Symbol(s) |
Name of each exchange on which registered |
| The |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging
growth company
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 2.02 | Results of Operations and Financial Condition |
On May 8, 2025, Sagimet Biosciences Inc. (the “Company”) issued a press release announcing its financial results for the quarter ended March 31, 2025. A copy of the press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information contained in this Item 2.02 (including Exhibit 99.1) is being furnished and shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section and shall not be deemed to be incorporated by reference in any filing under the Securities Act of 1933, as amended (the “Securities Act”), or the Exchange Act, except as shall be expressly set forth by specific reference in such filing.
| Item 8.01 | Other Events. |
On May 8, 2025, the Company updated information reflected in a slide presentation, which is attached as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference. Representatives of the Company will use the updated presentation in various meetings with investors from time to time.
| Item 9.01 | Financial Statements and Exhibits |
(d) Exhibits
| Exhibit
No. |
Document | |
| 99.1 | Press Release of Sagimet Biosciences Inc., dated May 8, 2025 | |
| 99.2 | Investor Presentation of Sagimet Biosciences Inc., dated May 8, 2025 | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Sagimet Biosciences Inc. | ||
| Date: May 8, 2025 | By: | /s/ David Happel |
| David Happel | ||
| Chief Executive Officer | ||
Exhibit 99.1

Sagimet Biosciences Reports First Quarter 2025 Financial Results and Provides Corporate Updates
Phase 1 clinical trial to evaluate the pharmacokinetics (PK) of a combination of denifanstat and resmetirom expected to initiate in 2H 2025; data readout expected 1H 2026
San Mateo, Calif., May 8, 2025 – Sagimet Biosciences Inc. (Nasdaq: SGMT), a clinical-stage biopharmaceutical company developing novel therapeutics targeting dysfunctional metabolic and fibrotic pathways, today reported financial results for the quarter ended March 31, 2025, and provided recent corporate updates.
“Sagimet is committed to bringing innovative therapies to MASH patients, following the successful results of our Phase 2b FASCINATE-2 clinical trial of denifanstat in MASH F2-F3 patients, particularly in more advanced F3 stage patients. In a Phase 1 clinical trial in patients with and without hepatic impairment, denifanstat exhibited similar pharmacokinetic characteristics and was well tolerated among all groups. Considering these strong Phase 1 and Phase 2 data, further development of denifanstat in MASH, including as part of a combination program, could potentially offer an opportunity to serve patient groups with the strongest need of treatment including those with stage 4 fibrosis,” said David Happel, Chief Executive Officer of Sagimet. “Building on our presentation of compelling preclinical data at 2024 EASL demonstrating the synergistic effect of a FASN inhibitor combined with resmetirom on important liver disease markers, we anticipate initiating a Phase 1 clinical trial to evaluate the PK and tolerability of a combination of denifanstat and resmetirom in the second half of 2025. If the outcome of this Phase 1 trial is positive, we will explore moving into the development of a combination product -- which we envision as a single tablet -- for patients living with MASH. We remain strongly convinced of the significant therapeutic potential associated with FASN inhibition across multiple disease states.”
Recent Corporate Highlights
| • | Pre-clinical data presented at EASL in 2024 for two mouse models of MASH showed that the combination of a FASN inhibitor (TVB-3664, a surrogate for denifanstat) and resmetirom had a synergistic effect on important liver disease markers, including improvement of NAS (NAFLD Activity Score) by histologic analysis and more robust improvement in hepatic collagen content compared to the single agents. Synergistic activity of the combination was demonstrated in the rate of histological improvement (NAS ≥2 points). The FASN inhibitor monotherapy showed 33% improvement, resmetirom monotherapy showed 25% improvement, and the combination of the two showed an 80% improvement, a level of improvement that greatly exceeds a simple addition of the activity of the two drugs. Building on this combination data, subject to consultation with regulatory authorities, Sagimet plans to initiate a Phase 1 clinical trial to evaluate the PK of a combination of denifanstat and resmetirom in the second half of 2025, with an anticipated data readout in the first half of 2026. If the outcome of this Phase 1 clinical PK trial is positive, Sagimet anticipates exploring the development of a combination product for MASH patients. |
Rohit Loomba, M.D., M.H.Sc., Professor of Medicine, Chief, Division of Gastroenterology and Hepatology, and Director, MASLD Research Center, University of California San Diego, said, “I’m excited to see Sagimet initiate development of a combination of denifanstat and resmetirom with this Phase 1 PK trial which will potentially answer important questions about the compatibility of these two molecules in humans. Results of this Phase 1 trial, if successful, could lead to further development of a combination of Sagimet’s fat synthesis inhibitor, denifanstat, with a fat oxidizer in MASH patients, potentially including those with stage 4 fibrosis.”
| • | End-of-Phase 2 interactions with the FDA were successfully completed in October 2024, supporting the advancement of denifanstat into Phase 3 in MASH. While Sagimet is operationally ready to dose patients in Phase 3 trials in F2/F3 MASH patients, it does not intend to initiate these trials until such time as it has sufficient funding to do so. Sagimet is currently exploring various alternatives to fund the ongoing development of denifanstat as a monotherapy. |
| • | Effective May 6, 2025, George Kemble, Ph.D. transitioned from his executive officer position as Executive Chairman and moved into the role of non-executive Chair of the Board. Also effective as of May 6, 2025, the Board appointed Beth Seidenberg, M.D. to serve as Lead Independent Director of the Board. Effective as of June 9, 2025, the date of Sagimet’s Annual Meeting, Merdad Parsey, M.D., Ph.D, will step off the Board. The Board thanks Dr. Parsey for his fifteen years of service to the Company as a Director. |
Publications and Presentations
| • | In May 2025, Sagimet is presenting three poster presentations featuring additional analyses from the Phase 2b FASCINATE-2 trial of denifanstat in MASH at the European Association for the Study of Liver (EASL) Congress 2025. The posters focus on antifibrotic effects of denifanstat in difficult-to-treat patients, new bile acid biomarkers to measure denifanstat response and alternative endpoints to liver biopsy such as MRI to detect patient improvement. |
| • | In February 2025, Sagimet delivered an oral presentation at the MASH Pathogenesis and Therapeutic Approaches Keystone Symposium. The presentation featured lipidomic data on improvements in polyunsaturated fatty acid triglycerides and LDL cholesterol levels in advanced fibrosis patients from the Phase 2b FASCINATE-2 trial of denifanstat in MASH, and preclinical data showing reduction of LDL and chemokines in a preclinical atherosclerosis model with a FASN inhibitor (TVB-3664, a surrogate for denifanstat). |
| • | In January 2025, Sagimet delivered an oral presentation at the 9th Annual MASH-TAG Conference highlighting the differentiated mechanism of action of the FASN inhibitor denifanstat and its observed anti-fibrotic effect in the Phase 2b FASCINATE-2 trial in F2/F3 MASH. |
Anticipated Upcoming Milestones
| • | Phase 1 clinical trial to evaluate the PK and tolerability of a combination of denifanstat and resmetirom, planned to initiate in the second half of 2025, with an anticipated data readout in the first half of 2026. |
| • | In November 2024, the Company’s license partner for China, Ascletis BioScience Co. Ltd. (Ascletis) announced completion of enrollment of 480 patients in its Phase 3 clinical trial of denifanstat for acne in China, and that it expects to announce topline results in the second quarter of 2025. |
| • | First-in-human Phase 1 clinical trial of TVB-3567 in acne expected to initiate in the second half of 2025, following the IND clearance in March 2025. |
Financial Results for the Three Months Ended March 31, 2025
| • | Cash, cash equivalents and marketable securities as of March 31, 2025, were $144.6 million. |
| • | Research and development expense for the quarter ended March 31, 2025, was $15.3 million compared to $5.3 million for the first quarter of 2024. |
| • | General and administrative expense for the quarter ended March 31, 2025, was $4.5 million, compared to $3.5 million for the first quarter of 2024. |
| • | Net loss for the quarter ended March 31, 2025, was $18.2 million compared to $6.6 million for the first quarter of 2024. |
About Sagimet Biosciences
Sagimet is a clinical-stage biopharmaceutical company developing novel fatty acid synthase (FASN) inhibitors that are designed to target dysfunctional metabolic and fibrotic pathways in diseases resulting from the overproduction of the fatty acid, palmitate. Sagimet’s lead drug candidate, denifanstat, is an oral, once-daily pill and selective FASN inhibitor in development for the treatment of metabolic dysfunction associated steatohepatitis (MASH). FASCINATE-2, a Phase 2b clinical trial of denifanstat in MASH with liver biopsy-based primary endpoints, was successfully completed with positive results. Denifanstat has been granted Breakthrough Therapy designation by the FDA for the treatment of non-cirrhotic MASH with moderate to advanced liver fibrosis (consistent with stages F2 to F3 fibrosis), and end-of-Phase 2 interactions with the FDA have been successfully completed, supporting the advancement of denifanstat into further development. Sagimet’s second FASN inhibitor, TVB-3567, a potent and selective small molecule FASN inhibitor, received IND clearance in March 2025, allowing initiation of a first-in-human Phase 1 clinical trial in acne. For additional information about Sagimet, please visit www.sagimet.com.
About MASH
Metabolic-dysfunction associated steatohepatitis (MASH) is a progressive and severe liver disease which is estimated to impact more than 115 million people worldwide, for which there is only one recently approved treatment in the United States and no currently approved treatments in Europe. In 2023, global liver disease medical societies and patient groups formalized the decision to rename non-alcoholic fatty liver disease (NAFLD) to metabolic dysfunction-associated steatotic liver disease (MASLD) and nonalcoholic steatohepatitis (NASH) to MASH. Additionally, an overarching term, steatotic liver disease (SLD), was established to capture multiple types of liver diseases associated with fat buildup in the liver. The goal of the name change was to establish an affirmative, non-stigmatizing name and diagnosis.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995. All statements contained in this press release, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding: the expected timing of the presentation of data from ongoing clinical trials, Sagimet’s clinical development plans and related anticipated development milestones, Sagimet’s cash and financial resources and expected cash runway. These statements involve known and unknown risks, uncertainties and other important factors that may cause Sagimet’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. In some cases, these statements can be identified by terms such as “may,” “might,” “will,” “should,” “expect,” “plan,” “aim,” “seek,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “forecast,” “potential” or “continue” or the negative of these terms or other similar expressions.
The forward-looking statements in this press release are only predictions. Sagimet has based these forward-looking statements largely on its current expectations and projections about future events and financial trends that Sagimet believes may affect its business, financial condition and results of operations. These forward-looking statements speak only as of the date of this press release and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond Sagimet’s control, including, among others: the clinical development and therapeutic potential of denifanstat or any other drug candidates Sagimet may develop; Sagimet’s ability to advance drug candidates into and successfully complete clinical trials within anticipated timelines; Sagimet’s relationship with Ascletis, and the success of its development efforts for denifanstat; the accuracy of Sagimet’s estimates regarding its capital requirements; and Sagimet’s ability to maintain and successfully enforce adequate intellectual property protection. These and other risks and uncertainties are described more fully in the “Risk Factors” section of Sagimet’s most recent filings with the Securities and Exchange Commission and available at www.sec.gov. You should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in these forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, Sagimet operates in a dynamic industry and economy. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that Sagimet may face. Except as required by applicable law, Sagimet does not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
Investor Contact:
Joyce Allaire
LifeSci Advisors
JAllaire@LifeSciAdvisors.com
Media Contact:
Michael Fitzhugh
LifeSci Advisors
mfitzhugh@lifescicomms.com
SAGIMET BIOSCIENCES INC.
CONDENSED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(unaudited)
(in thousands, except for share and per share amounts)
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| (unaudited) | ||||||||
| Operating expenses: | ||||||||
| Research and development | 15,342 | 5,262 | ||||||
| General and administrative | 4,523 | 3,506 | ||||||
| Total operating expenses | 19,865 | 8,768 | ||||||
| Loss from operations | (19,865 | ) | (8,768 | ) | ||||
| Total other income | 1,689 | 2,139 | ||||||
| Net loss | $ | (18,176 | ) | $ | (6,629 | ) | ||
| Net loss per share, basic and diluted | $ | (0.56 | ) | $ | (0.23 | ) | ||
| Weighted-average shares outstanding, basic and diluted | 32,195,345 | 29,039,427 | ||||||
| Net loss | $ | (18,176 | ) | $ | (6,629 | ) | ||
| Other comprehensive loss: | ||||||||
| Net unrealized loss on marketable securities | (109 | ) | (23 | ) | ||||
| Total comprehensive loss | $ | (18,285 | ) | $ | (6,652 | ) | ||
SAGIMET BIOSCIENCES INC.
CONDENSED BALANCE SHEETS
(unaudited)
(in thousands)
| As of | ||||||||
| March 31, 2025 | December 31, 2024 | |||||||
| Cash, cash equivalents and marketable securities | $ | 144,569 | $ | 158,658 | ||||
| Total assets | $ | 146,172 | $ | 160,259 | ||||
| Current liabilties | $ | 7,180 | $ | 4,454 | ||||
| Stockholders' equity | $ | 138,992 | $ | 155,805 | ||||
| Liabilities and stockholders' equity | $ | 146,172 | $ | 160,259 | ||||
Exhibit 99.2
Targeting Metabolic Dysfunction with Novel Therapies to Treat M ASH, Acne & Cancer May 2025

2 May 2025 Forward - Looking Statements and Disclaimer This presentation contains forward - looking statements within the meaning of, and made pursuant to the safe harbor provisions of, The Private Securities Litigation Reform Act of 1995 . All statements contained in this document, other than statements of historical facts or statements that relate to present facts or current conditions, including but not limited to, statements regarding possible or assumed future results of operations, business strategies, research and development plans, regulatory activities, the presentation of data from clinical trials, Sagimet’s clinical development plans and related anticipated clinical development milestones, market opportunity, competitive position and potential growth opportunities are forward - looking statements . These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward - looking statements . In some cases, you can identify forward - looking statements by terms such as “may,” “will,” “should,” “would,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “believe,” “estimate,” “predict,” “potential,” or “continue” or the negative of these terms or other similar expressions . The forward - looking statements in this presentation are only predictions . These forward - looking statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, some of which cannot be predicted or quantified and some of which are beyond our control, including, among others : the clinical development and therapeutic potential of denifanstat or any other drug candidates we may develop ; our ability to advance drug candidates into and successfully complete clinical trials , the risk the topline clinical trials may not be predictive of, and may differ from final clinical data and later - stage clinical trials ; our ability to advance drug candidates into and successfully complete clinical trials within anticipated timelines ; that unfavorable new clinical trial data may emerge in other clinical trials of our product candidates ; that clinical trial data are subject to differing interpretations and assessments, including by regulatory authorities ; our relationship with Ascletis , and the success of its development efforts for denifanstat ; the accuracy of our estimates regarding our capital requirements ; and our ability to maintain and successfully enforce adequate intellectual property protection . These and other risks and uncertainties are described more fully in the “Risk Factors” section of our most recent filings with the Securities and Exchange Commission (SEC) and available at www . sec . gov . You should not rely on these forward - looking statements as predictions of future events . The events and circumstances reflected in our forward - looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward - looking statements . Moreover, we operate in a dynamic industry and economy . New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties that we may face . Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise .

3 May 2025 Leadership Team with Proven Development and Commercialization Experience Dave Happel President & CEO >20 years of experience in executive leadership in biotech and pharma Brought multiple innovative healthcare products to the market Eduardo Martins CMO >20 years of leadership of large - scale multinational clinical trials & global teams in pharma and biotech Led clinical development team of cenicriviroc for MASH Thierry Chauche CFO >20 years of financial and operational leadership experience in finance and healthcare companies Elizabeth Rozek General Counsel >20 years of legal experience including executive leadership of legal, IP and compliance functions in biopharma and biotech Rob D’Urso Senior Vice President of New Products >20 years of US and global leadership experience in dermatology Marie O'Farrell Senior Vice President of R&D >20 years of experience in R&D and translational medicine in biopharma and biotech Successfully guided development for multiple clinical programs

4 May 2025 Sagimet at a Glance • Our lead molecule, denifanstat, is a novel fatty acid synthase (FASN) inhibitor with a differentiated MOA with the potential to target multiple underserved diseases • Strong clinical data demonstrates denifanstat’s proof of concept across multiple disease states Unique MOA: FASN Inhibition • Denifanstat directly targets the 3 key drivers of MASH (metabolic dysfunction - associated steatohepatitis) – liver fat, inflammation, and fibrosis • Successful outcome of Phase 2b trial; met both primary endp oints with significant reduction in fibrosis • Pre - clinical data demonstrated synergistic effect of combination of FASN inhibitor and resmetirom • Phase 1 clinical trial to evaluate the pharmacokinetics ථ (PK) and tolerability of a co mbination of denifanstat and resmetirom planned to initiate in 2H 2025; data readout expected 1H 2026 Denifanstat in MASH TVB - 3567 in Acne • Our follow - on FASN inhibitor, TVB 3567, received Investigational New Drug (IND) clearance in March 2025 • First - in - human Phase 1 clinical trial for development of an acne indication expected to initiate 2H 2025

5 May 2025 Strong IP, Cash Position, and Collaboration Potential • Phase 3 clinical trial in patients with moderate to severe acne vulgaris in progress in China • Topline results expected in 2Q2025 • Phase 3 clinical trial in recurrent glioblastoma multiforme (GBM) in combination with bevacizumab in progress in China • Denifanstat: • Method of use patent — 2036; potential PTE to 2041 • Composition of matter patent — 2032 • Combination of denifanstat and resmetirom: • Application filed 2024; if granted — 2044; potential PTE to 2048 • TVB - 3567: • Method of use application for TVB - 3567 for acne filed 2025; if granted — 2046 • Composition of matter patent — 2035; potential PTE to 2038 Cash Position • Nasdaq: SGMT; $144.6M of cash, cash equivalents and marketable securities as of 03/31/2025 Strategic Collaboration with Ascletis in Acne & Cancer IP Portfolio
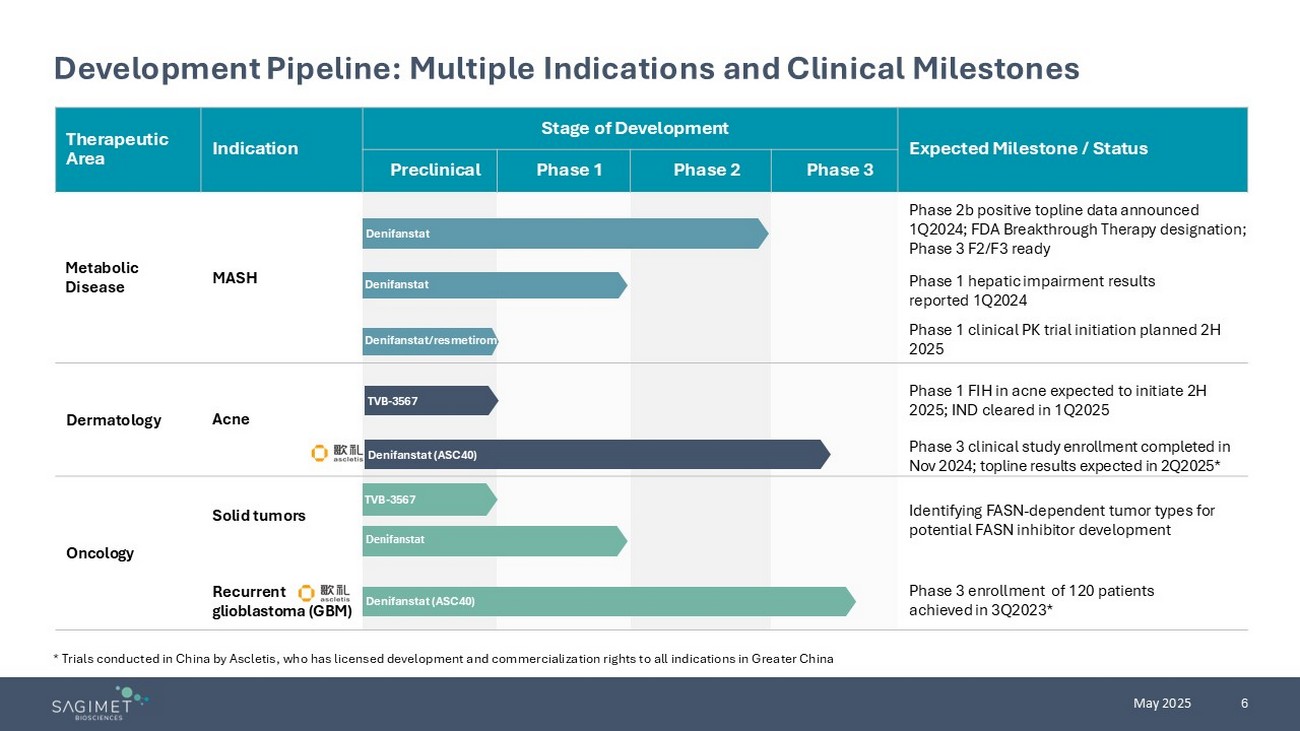
6 May 2025 Expected Milestone / Status Stage of Development Indication Therapeutic Area Phase 3 Phase 2 Phase 1 Preclinical Phase 2b positive topline data announced 1Q2024 ; FDA Breakthrough Therapy designation; Phase 3 F2/F3 ready MASH Metabolic Disease Phase 1 hepatic impairment results reported 1Q 2024 Phase 1 clinical PK trial initiation planned 2H 2025 Phase 1 FIH in acne expected to initiate 2H 2025; IND cleared in 1Q2025 Acne Dermatology Phase 3 clinical study enrollment completed in Nov 2024; topline results expected in 2Q2025* Identifying FASN - dependent tumor types for potential FASN inhibitor development Solid tumors Oncology Phase 3 enrollment of 120 patients achieved in 3Q2023 * Recurrent glioblastoma (GBM) Development Pipeline: Multiple Indications and Clinical Milestones * Trials conducted in China by Ascletis , who has licensed development and commercialization rights to all indications in Greater China Denifanstat Denifanstat TVB - 3567 Denifanstat (ASC40) TVB - 3567 Denifanstat (ASC40) Denifanstat Denifanstat/resmetirom
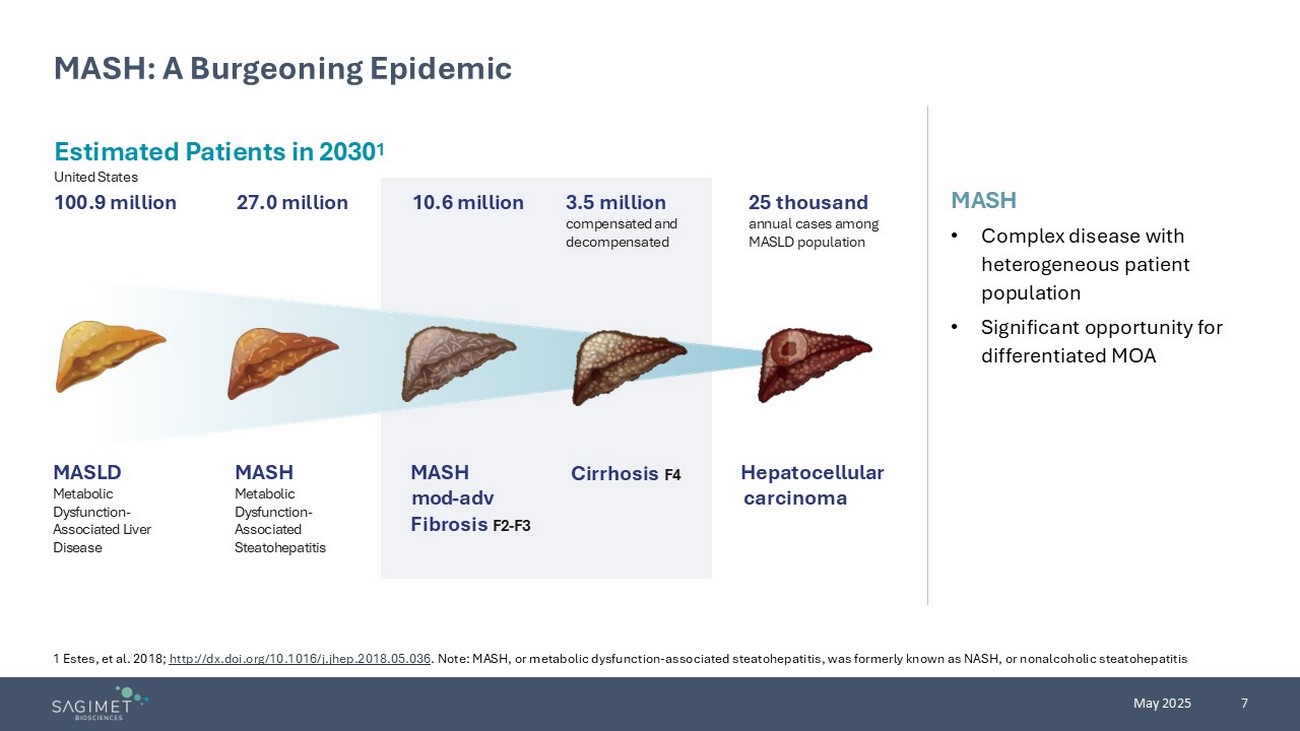
7 May 2025 MASH: A Burgeoning Epidemic 1 Estes, et al. 2018; http://dx.doi.org/10.1016/j.jhep.2018.05.036 . Note: MASH, or metabolic dysfunction - associated steatohepatitis, was formerly known as NASH, or nonalcoholic steatohepatitis Disease challenges Estimated Patients in 20 30 1 United States 100.9 million Hepatocellular carcinoma Cirrhosis F4 25 thousand annual cases among MASLD population 3.5 million compensated and decompensated 27.0 million 10.6 million MASH • Complex disease with h eterogeneous patient population • Significant opportunity for differentiated MOA MASLD Metabolic Dysfunction - Associated Liver Disease MASH Metabolic Dysfunction - Associated Steatohepatitis MASH mod - adv F ibrosis F2 - F3
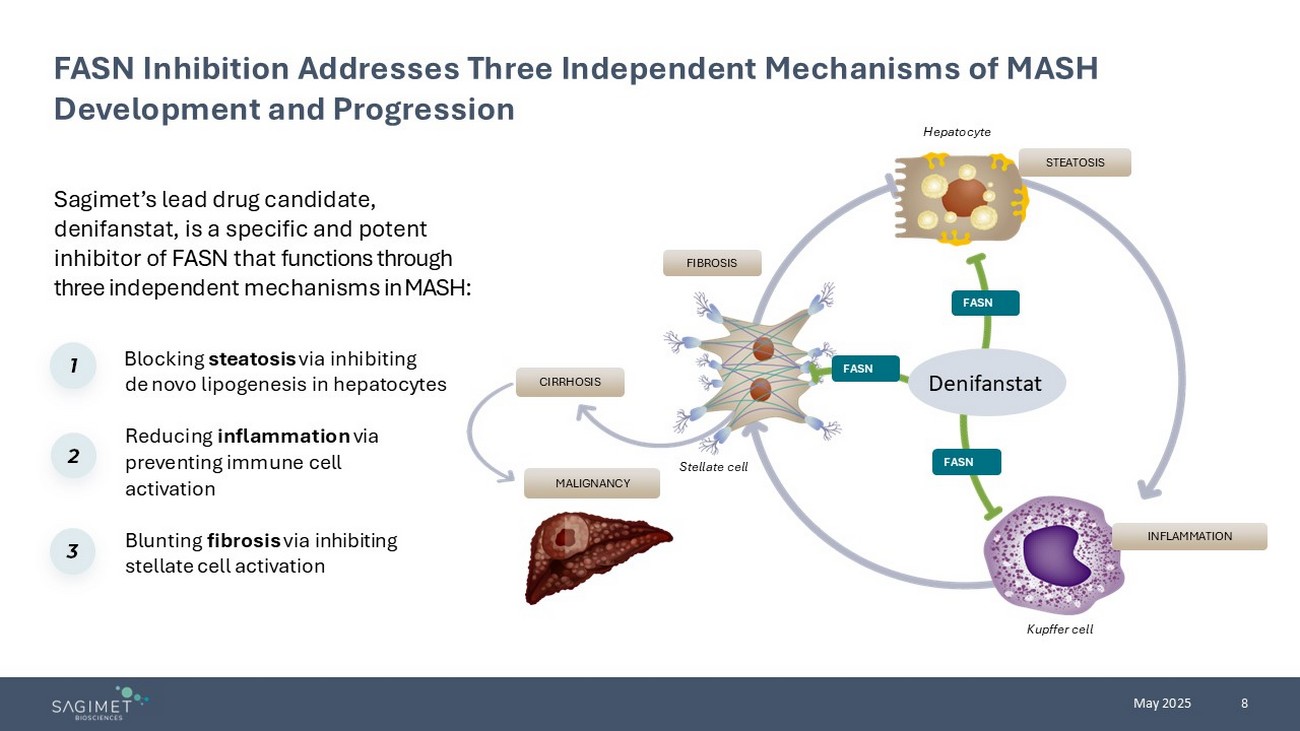
8 May 2025 Sagimet’s lead drug candidate, denifanstat, is a specific and potent inhibitor of FASN that functions through three independent mechanisms in MASH : FASN Inhibition Addresses Three Independent Mechanisms of MASH Development and Progression Blocking steatosis via inhibiting de novo lipogenesis in hepatocytes Reducing inflammation via preventing immune cell activation Blunting fibrosis via inhibiting stellate cell activation 1 2 3 INFLAMMATION FIBROSIS STEATOSIS Hepatocyte Kupffer cell Stellate cell CIRRHOSIS MALIGNANCY FASN FASN FASN Denifanstat
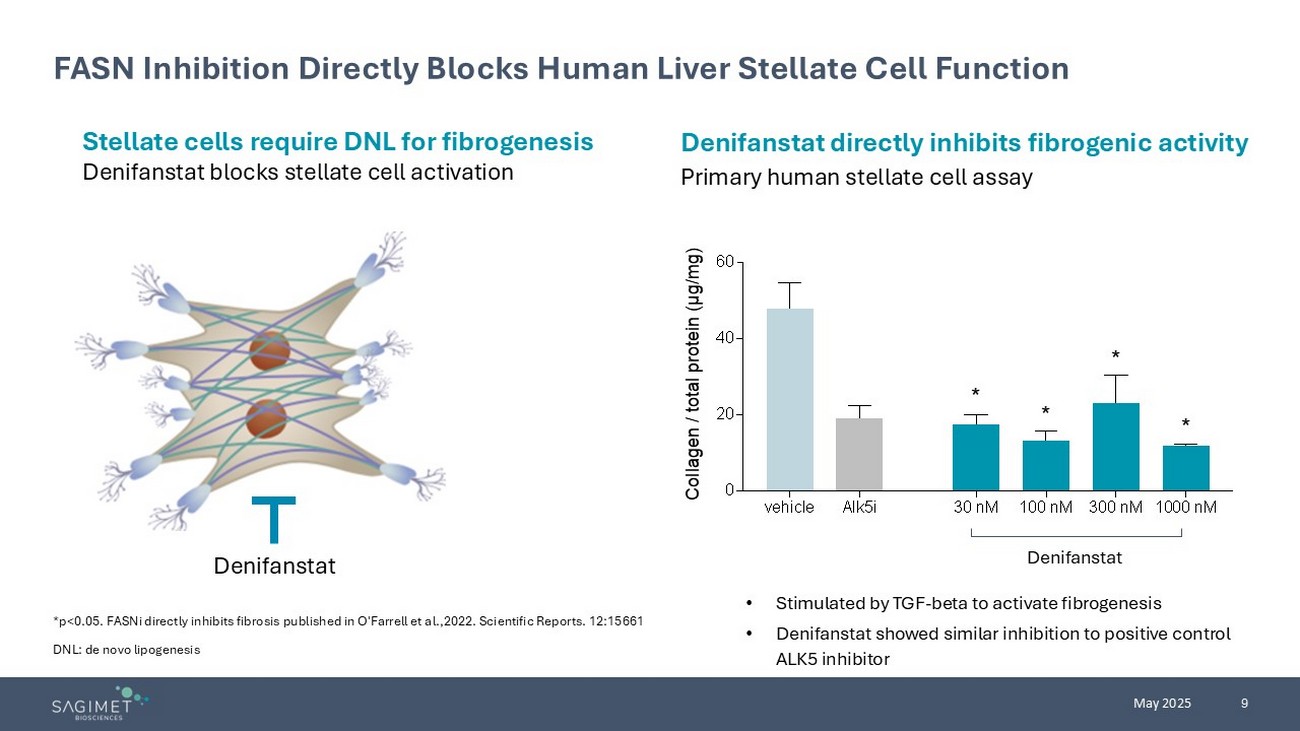
9 May 2025 FASN Inhibition Directly Blocks Human Liver Stellate Cell Function Stellate cells require DNL for fibrogenesis Denifanstat blocks stellate cell activation Denifanstat directly inhibits fibrogenic activity Primary human stellate cell assay Denifanstat • Stimulated by TGF - beta to activate fibrogenesis • Denifanstat showed similar inhibition to positive control ALK5 inhibitor *p<0.05. FASNi directly inhibits fibrosis published in O'Farrell et al.,2022. Scientific Reports. 12:15661 DNL: de novo lipogenesis vehicle Alk5i 30 nM 100 nM 300 nM 1000 nM 0 20 40 60 Collagen / total protein (μg/mg) * * * * Denifanstat
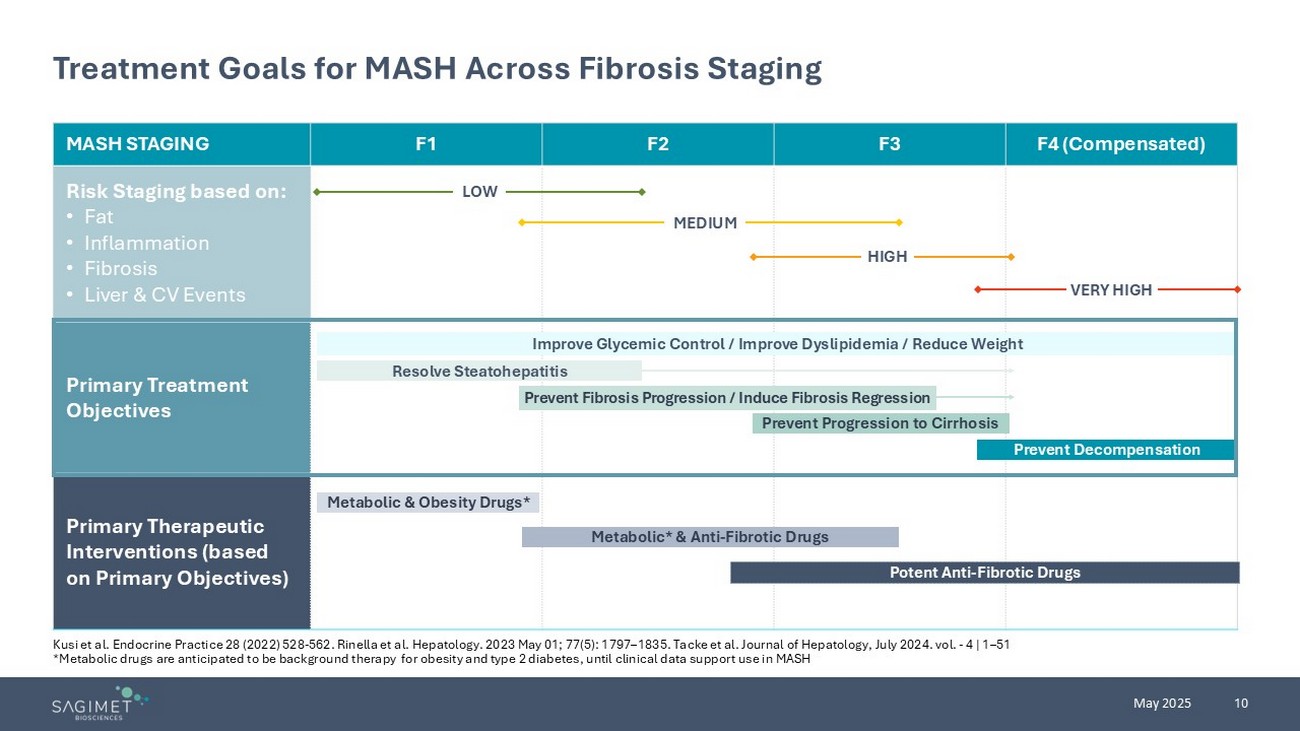
10 May 2025 Treatment Goals for MASH Across Fibrosis Staging F4 (Compensated) F3 F2 F1 MASH STAGING Risk Staging based on: • Fat • Inflammation • Fibrosis • Liver & CV Events Primary Treatment Objectives Primary Therapeutic Interventions (based on Primary Objectives) Improve Glycemic Control / Improve Dyslipidemia / Reduce Weight Resolve Steatohepatitis Prevent Progression to Cirrhosis Prevent Decompensation Metabolic & Obesity Drugs* Metabolic* & Anti - Fibrotic Drugs Potent Anti - Fibrotic Drugs Prevent Fibrosis Progression / Induce Fibrosis Regression LOW MEDIUM HIGH VERY HIGH Kusi et al. Endocrine Practice 28 (2022) 528 - 562. Rinella et al. Hepatology. 2023 May 01; 77(5): 1797 – 1835. Tacke et al. Journal of Hepatology, July 2024. vol. - 4 | 1 – 51 *Metabolic drugs are anticipated to be background therapy for obesity and type 2 diabetes, until clinical data support use i n M ASH

Strong MASH Data Creates Opportunities to Reach Advanced Patient Populations
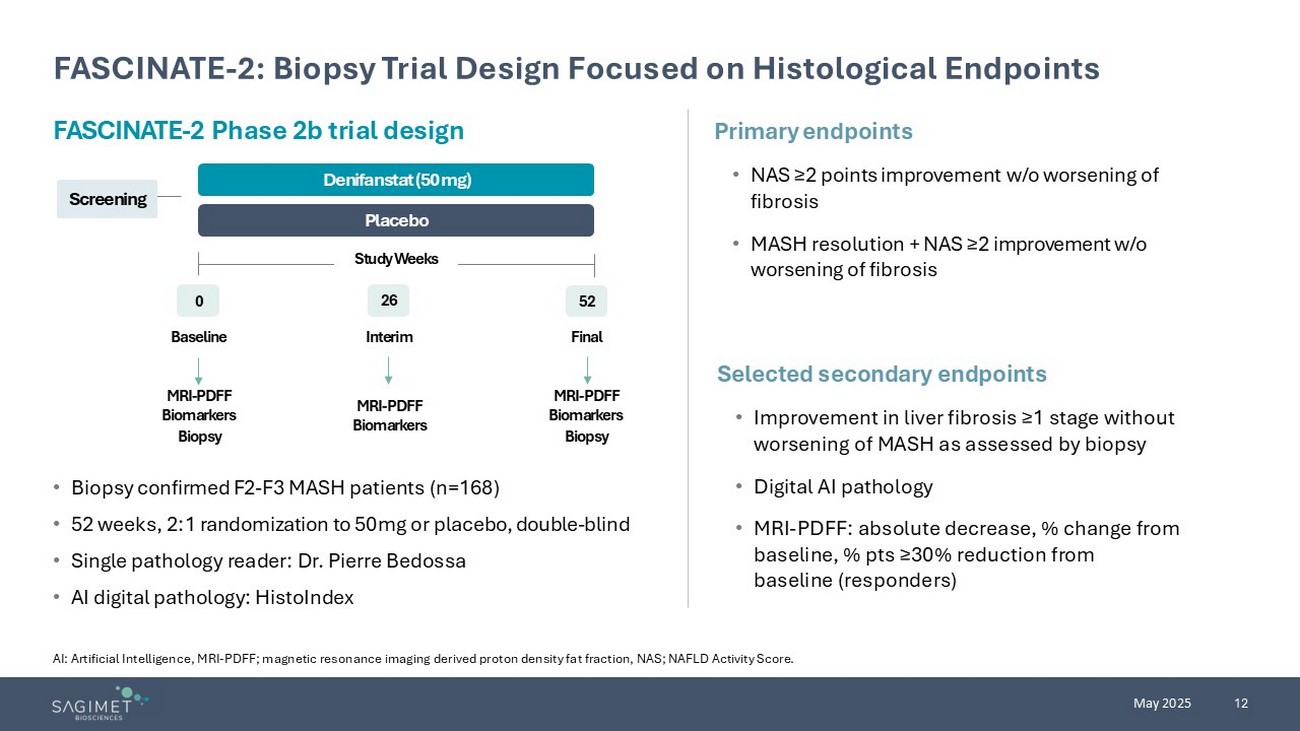
12 May 2025 FASCINATE - 2: Biopsy Trial Design Focused on Histological Endpoints AI: Artificial Intelligence, MRI - PDFF; magnetic resonance imaging derived proton density fat fraction, NAS; NAFLD Activity Score . • Biopsy confirmed F2 - F3 MASH patients (n=168) • 52 weeks, 2:1 randomization to 50mg or placebo, double - blind • Single pathology reader: Dr. Pierre Bedossa • AI digital pathology: HistoIndex Primary endpoints • NAS ≥2 points improvement w/o worsening of fibrosis • MASH resolution + NAS ≥2 improvement w/o worsening of fibrosis Selected secondary endpoints • Improvement in liver fibrosis ≥1 stage without worsening of MASH as assessed by biopsy • Digital AI pathology • MRI - PDFF: absolute decrease, % change from baseline, % pts ≥30% reduction from baseline (responders) FASCINATE - 2 Phase 2b trial design Screening Placebo Denifanstat (50 mg) 0 26 52 Study Weeks Baseline Interim Final MRI - PDFF Biomarkers Biopsy MRI - PDFF Biomarkers MRI - PDFF Biomarkers Biopsy
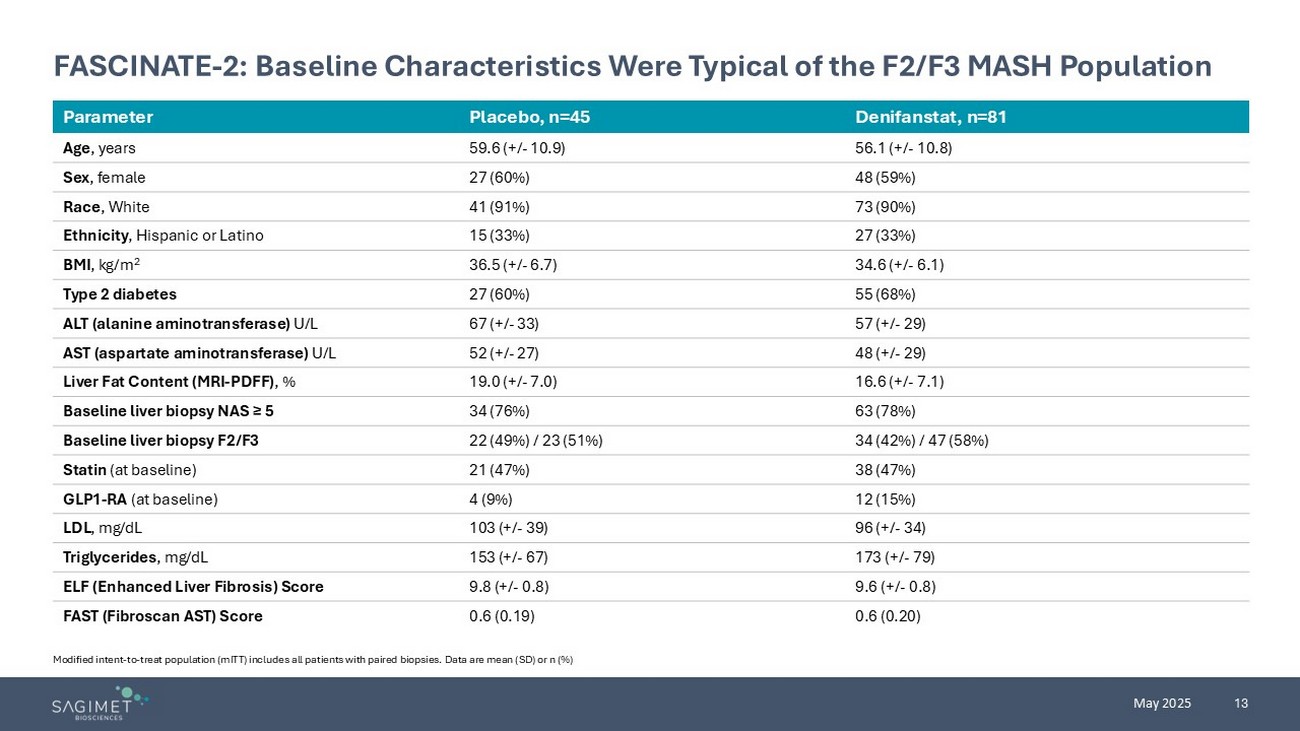
13 May 2025 FASCINATE - 2: Baseline Characteristics Were Typical of the F2/F3 MASH Population Denifanstat , n=81 Placebo, n=45 Parameter 56.1 (+/ - 10.8) 59.6 (+/ - 10.9) Age , years 48 (59%) 27 (60%) Sex , female 73 (90%) 41 (91%) Race , White 27 (33%) 15 (33%) Ethnicity , Hispanic or Latino 34.6 (+/ - 6.1) 36.5 (+/ - 6.7) BMI , kg/m 2 55 (68%) 27 (60%) Type 2 diabetes 57 (+/ - 29) 67 (+/ - 33) ALT (alanine aminotransferase) U/L 48 (+/ - 29) 52 (+/ - 27) AST (aspartate aminotransferase) U/L 16.6 (+/ - 7.1) 19.0 (+/ - 7.0) Liver Fat Content (MRI - PDFF) , % 63 (78%) 34 (76%) Baseline liver biopsy NAS ≥ 5 34 (42%) / 47 (58%) 22 (49%) / 23 (51%) Baseline liver biopsy F2/F3 38 (47%) 21 (47%) Statin (at baseline) 12 (15%) 4 (9%) GLP1 - RA (at baseline) 96 ( +/ - 34) 103 ( +/ - 39) LDL , mg/dL 173 ( +/ - 79) 153 ( +/ - 67) Triglycerides , mg/dL 9.6 ( +/ - 0.8) 9.8 ( +/ - 0.8) ELF (Enhanced Liver Fibrosis) Score 0.6 (0.20) 0.6 (0.19) FAST ( Fibroscan AST) Score Modified intent - to - treat population (mITT) includes all patients with paired biopsies. Data are mean (SD) or n (%)
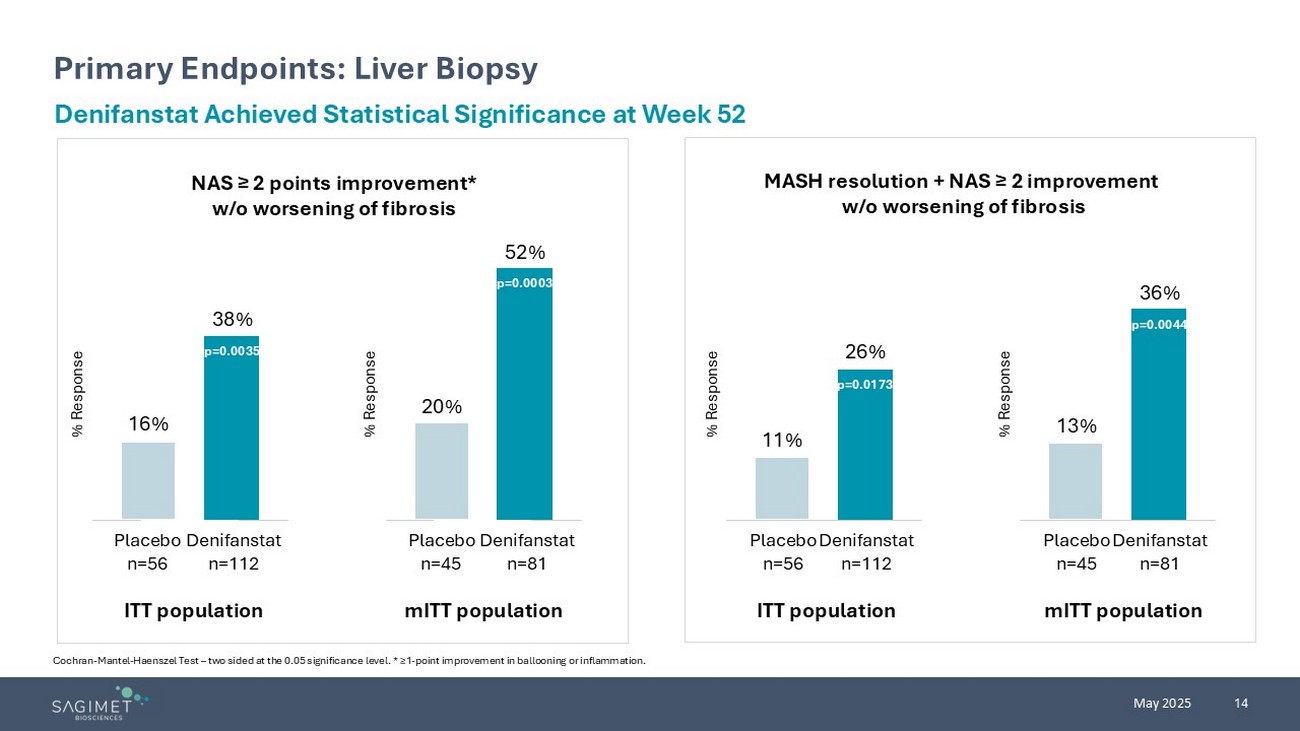
14 May 2025 Primary Endpoints: Liver Biopsy Cochran - Mantel - Haenszel Test – two sided at the 0.05 significance level. * ≥1 - point improvement in ballooning or inflammation. Denifanstat Achieved Statistical Significance at Week 52 NAS ≥ 2 points improvement* w/o worsening of fibrosis p=0.0001 MASH resolution + NAS ≥ 2 improvement w/o worsening of fibrosis ITT population mITT population Placebo n=56 Denifanstat n=112 16% 38% Placebo n=56 Denifanstat n=112 11% 26% Placebo n=45 Denifanstat n=81 20% 52% Placebo n=45 Denifanstat n=81 13% 36% ITT population mITT population % Response % Response % Response % Response p=0.0173 p=0.0044 p=0.0003 p=0.0035
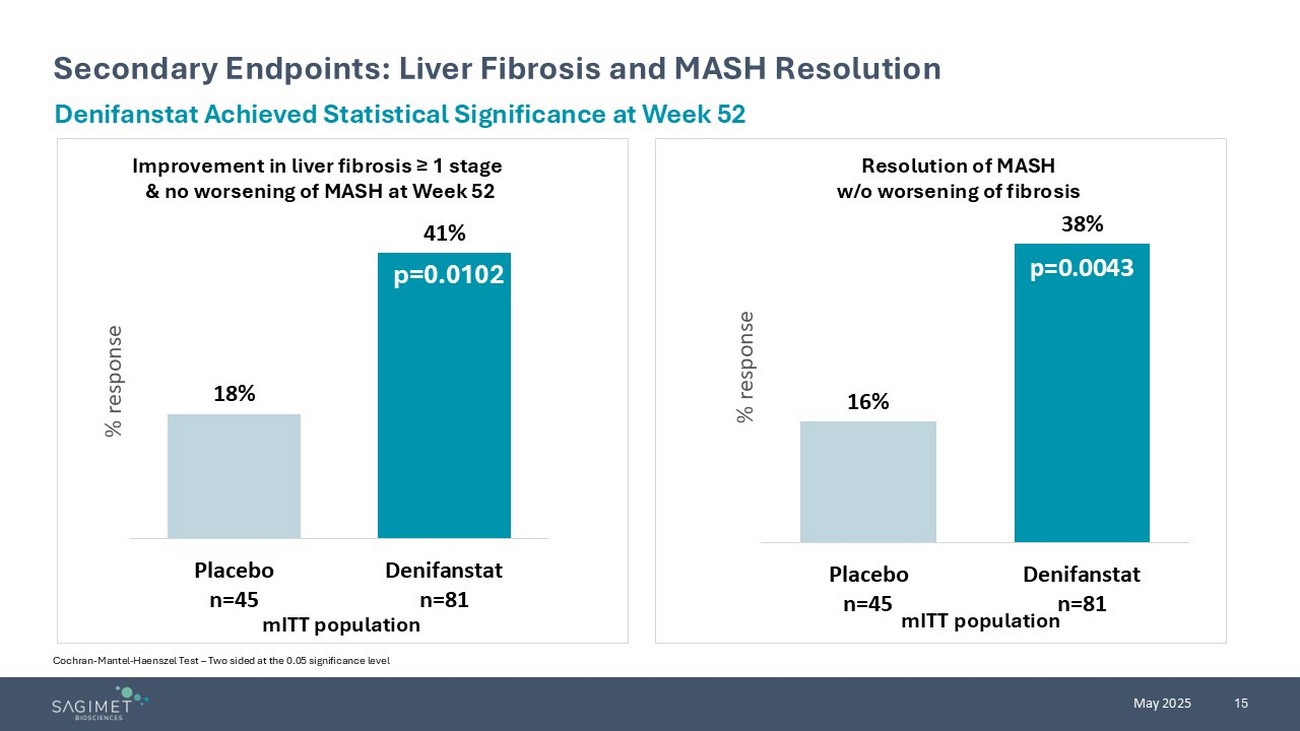
15 May 2025 Secondary Endpoints: Liver Fibrosis and MASH Resolution Cochran - Mantel - Haenszel Test – Two sided at the 0.05 significance level Denifanstat Achieved Statistical Significance at Week 52 18% 41% Placebo n=45 Denifanstat n=81 % response Improvement in liver fibrosis ≥ 1 stage & no worsening of MASH at Week 52 p=0.0102 mITT population Resolution of MASH w/o worsening of fibrosis p= 16% 38% Placebo n=45 Denifanstat n=81 % response p=0.0043 mITT population
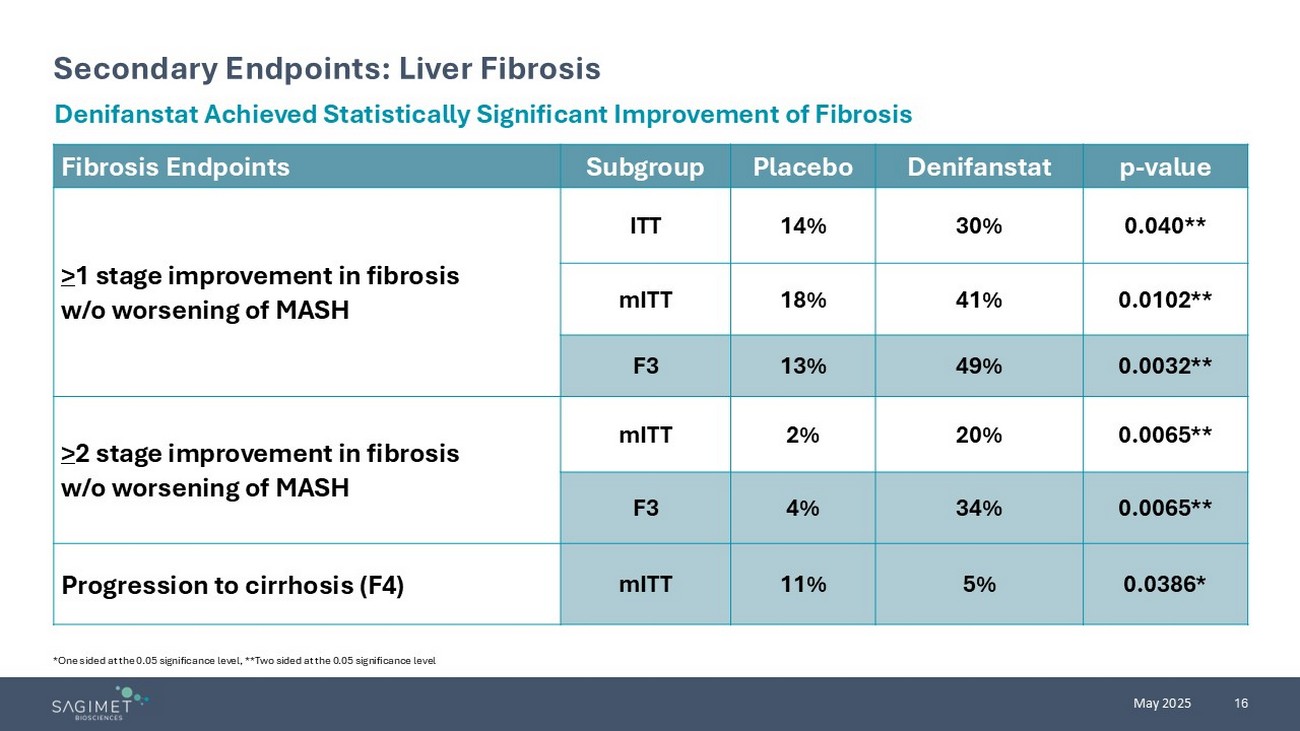
16 May 2025 Secondary Endpoints: Liver Fibrosis *One sided at the 0.05 significance level, **Two sided at the 0.05 significance level Denifanstat Achieved Statistically Significant Improvement of Fibrosis p - value Denifanstat Placebo Subgroup Fibrosis Endpoints 0.040** 30% 14% ITT > 1 stage improvement in fibrosis w/o worsening of MASH 0.0102** 41% 18% mITT 0.0032** 49% 13% F3 0.0065** 20% 2% mITT > 2 stage improvement in fibrosis w/o worsening of MASH 0.0065** 34% 4% F3 0.0386* 5% 11% mITT Progression to cirrhosis (F4)
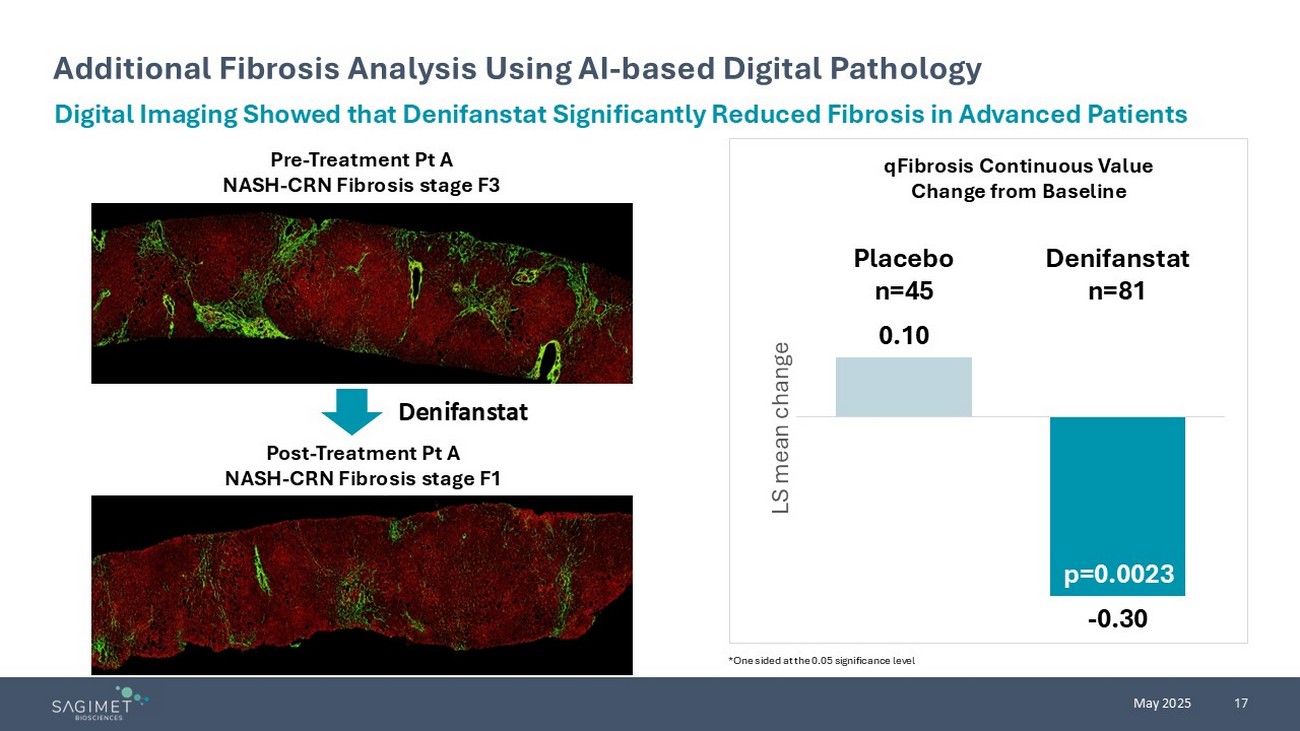
17 May 2025 Additional Fibrosis Analysis Using AI - based Digital Pathology Digital Imaging Showed that Denifanstat Significantly Reduced Fibrosis in Advanced Patients Pre - Treatment Pt A NASH - CRN Fibrosis stage F3 Post - Treatment Pt A NASH - CRN Fibrosis stage F1 Denifanstat 0.10 - 0.30 Placebo n=45 Denifanstat n=81 LS mean change qFibrosis Continuous Value Change from Baseline p=0.0023 *One sided at the 0.05 significance level
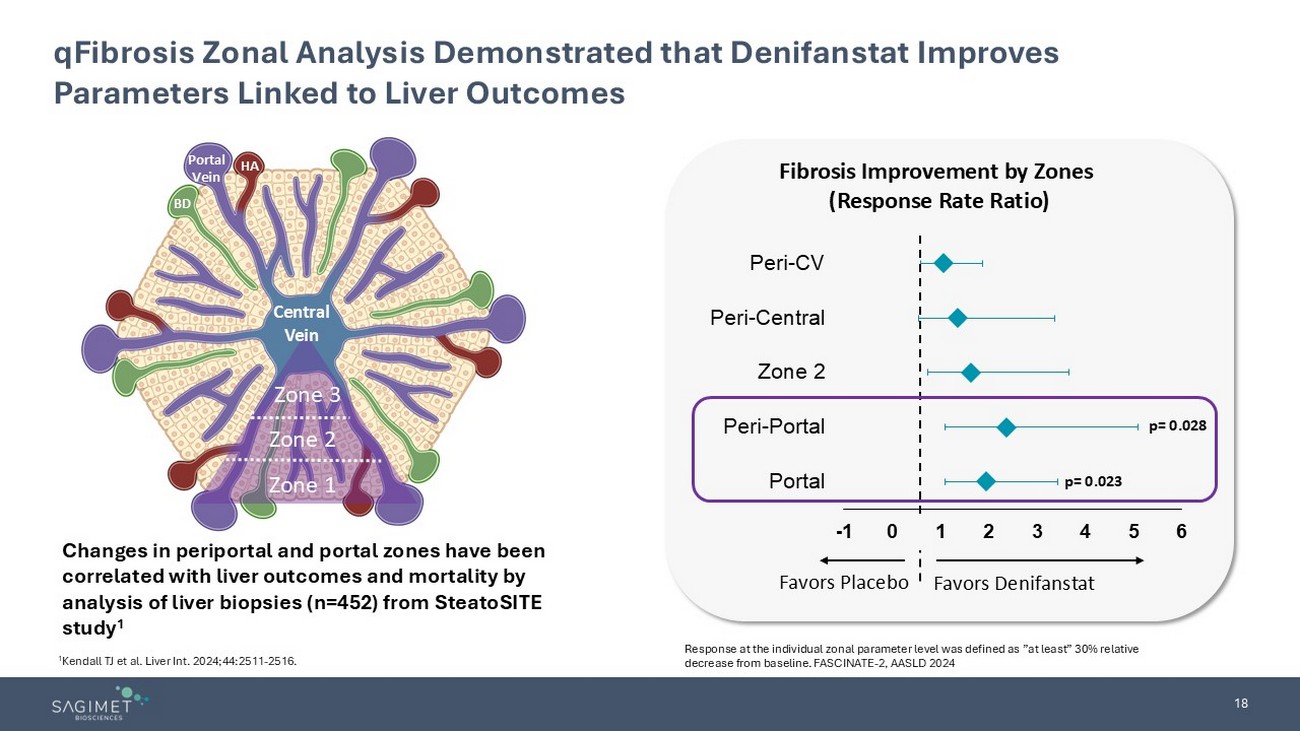
18 qFibrosis Zonal Analysis Demonstrated that Denifanstat Improves Parameters Linked to Liver Outcomes Zone 1 Zone 2 Zone 3 Central Vein Portal Vein HA BD Response at the individual zonal parameter level was defined as ”at least” 30% relative decrease from baseline. FASCINATE - 2, AASLD 2024 Fibrosis Improvement by Zones (Response Rate Ratio) -1 0 1 2 3 4 5 6 Portal Peri-Portal Zone 2 Peri-Central Peri-CV Favors Denifanstat Favors Placebo p= 0.023 p= 0.028 Changes in periportal and portal zones have been correlated with liver outcomes and mortality by analysis of liver biopsies (n=452) from SteatoSITE study 1 1 Kendall TJ et al. Liver Int. 2024;44:2511 - 2516.
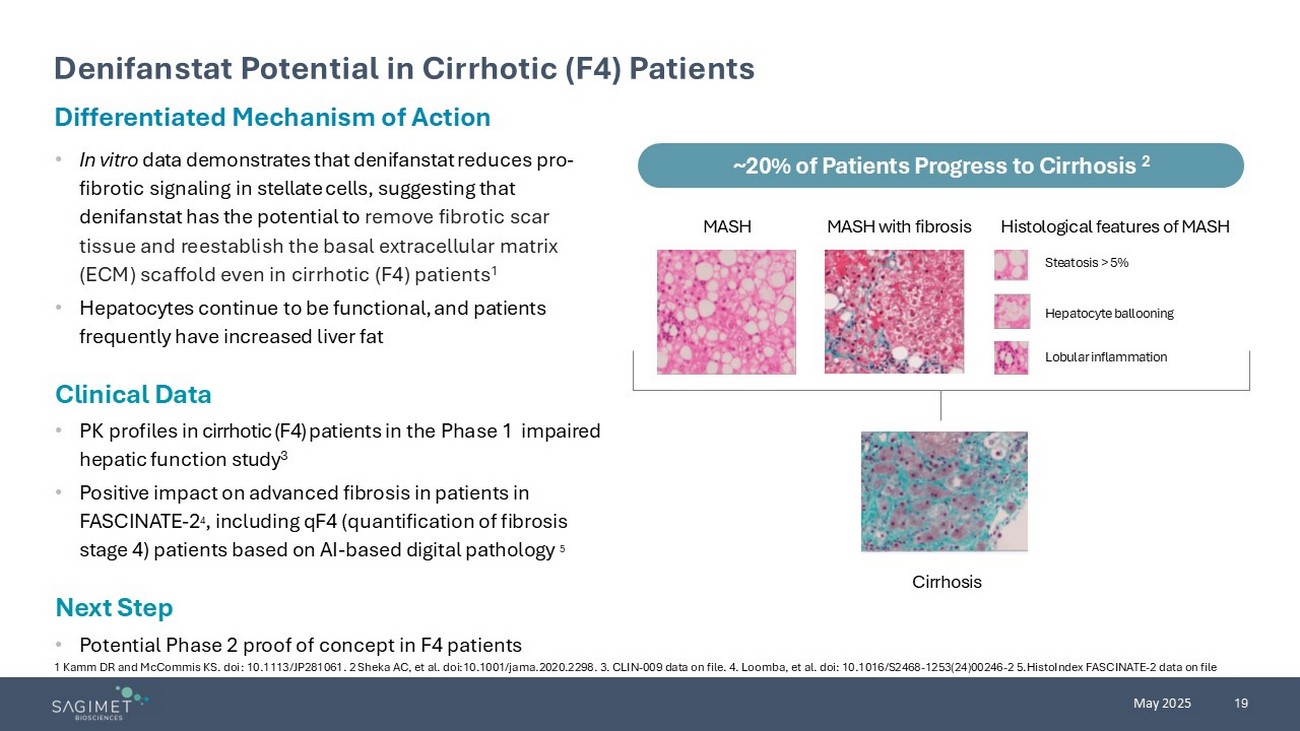
19 May 2025 1 Kamm DR and McCommis KS. doi : 10.1113/JP281061. 2 Sheka AC, et al. doi:10.1001/jama.2020.2298. 3. CLIN - 009 data on file. 4. Loomba, et al. doi : 10.1016/S2468 - 1253(24)00246 - 2 5.HistoIndex FASCINATE - 2 data on file Differentiated Mechanism of Action • In vitro data demonstrates that denifanstat reduces pro - fibrotic signaling in stellate cells , suggesting that denifanstat has the potential to remove fibrotic scar tissue and reestablish the basal extracellular matrix ( ECM ) scaffold even in cirrhotic (F4) patients 1 • Hepatocytes continue to be functional, and patients frequently have increased liver fat Clinical Data • PK profiles in cirrhotic (F4) patients in the Phase 1 impaired hepatic function study 3 • Positive impact on advanced fibrosis in patients in FASCINATE - 2 4 , including qF4 (quantification of fibrosis stage 4) patients based on AI - based digital pathology 5 Next Step • Potential Phase 2 proof of concept in F4 patients Denifanstat Potential in Cirrhotic (F4) Patients ~20% of Patients Progress to Cirrhosis 2 MASH MASH with fibrosis Histological features of MASH Steatosis > 5% Hepatocyte ballooning Lobular inflammation Cirrhosis
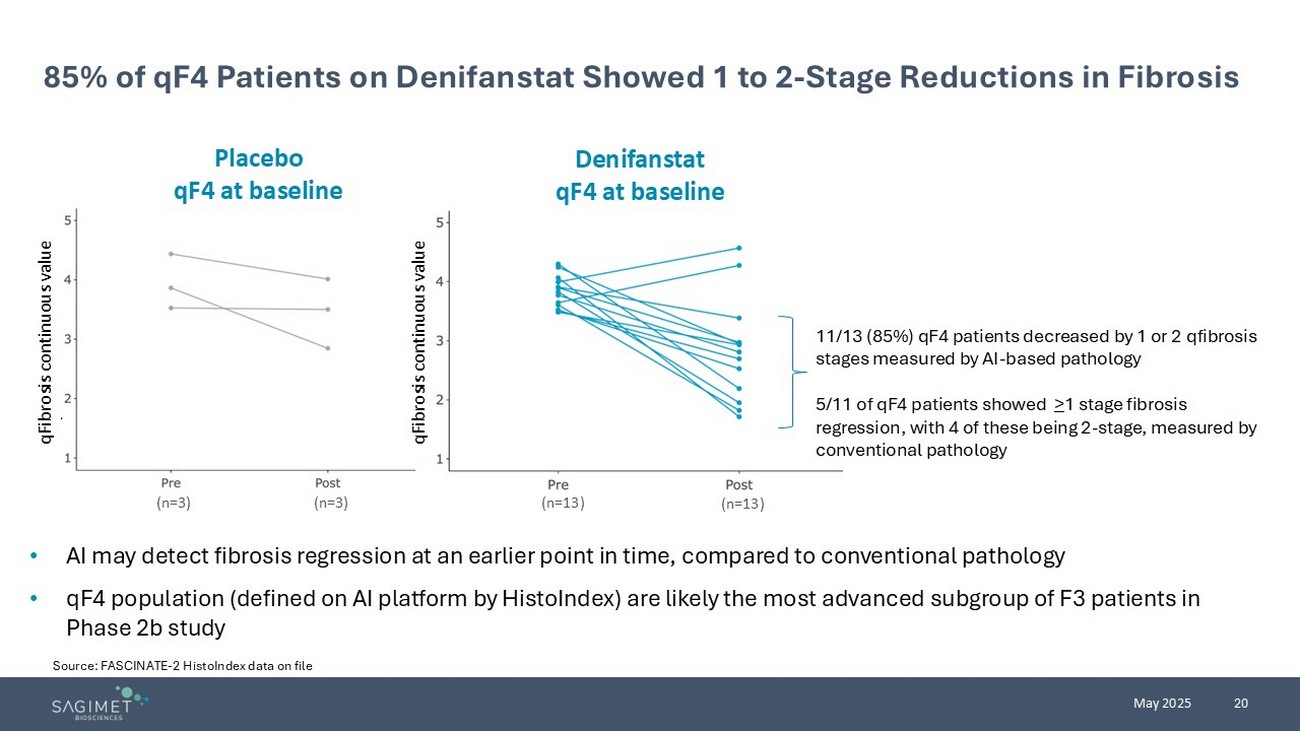
20 May 2025 Source: FASCINATE - 2 HistoIndex data on file 85% of qF4 Patients on Denifanstat Showed 1 to 2 - Stage Reductions in Fibrosis • AI may detect fibrosis regression at an earlier point in time, compared to conventional pathology • qF4 population (defined on AI platform by HistoIndex ) are likely the most advanced subgroup of F3 patients in Phase 2b study (n=3) (n=3) (n=13) (n=13) Placebo qF4 at baseline Denifanstat qF4 at baseline qFibrosis continuous value qFibrosis continuous value 11/13 (85%) qF4 patients decreased by 1 or 2 qfibrosis stages measured by AI - based pathology 5/11 of qF4 patients showed > 1 stage fibrosis regression, with 4 of these being 2 - stage, measured by conventional pathology
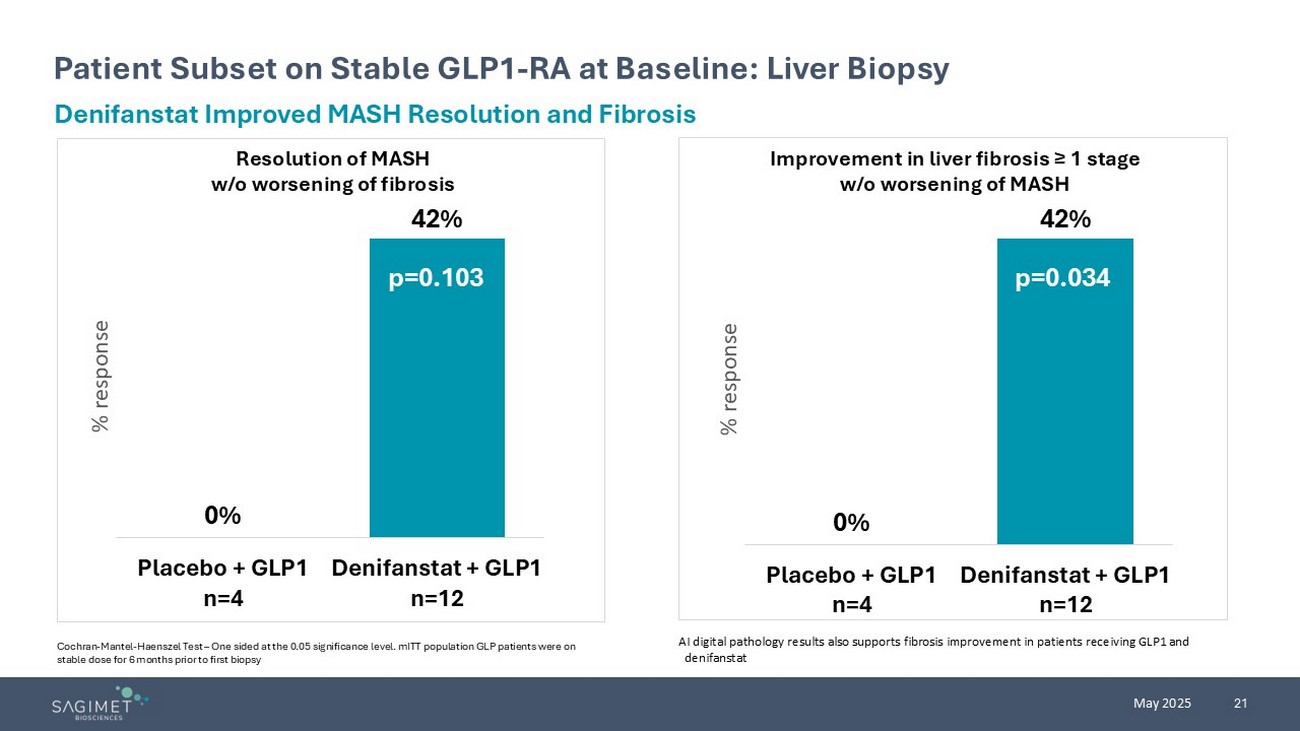
21 May 2025 Patient Subset on Stable GLP1 - RA at Baseline: Liver Biopsy Cochran - Mantel - Haenszel Test – One sided at the 0.05 significance level. mITT population GLP patients were on stable dose for 6 months prior to first biopsy Denifanstat Improved MASH Resolution and Fibrosis Resolution of MASH w/o worsening of fibrosis Improvement in liver fibrosis ≥ 1 stage w/o worsening of MASH 0% 42% Placebo + GLP1 n=4 Denifanstat + GLP1 n=12 % response 0% 42% Placebo + GLP1 n=4 Denifanstat + GLP1 n=12 % response p=0.034 p=0.103 AI digital pathology results also supports fibrosis improvement in patients receiving GLP1 and denifanstat
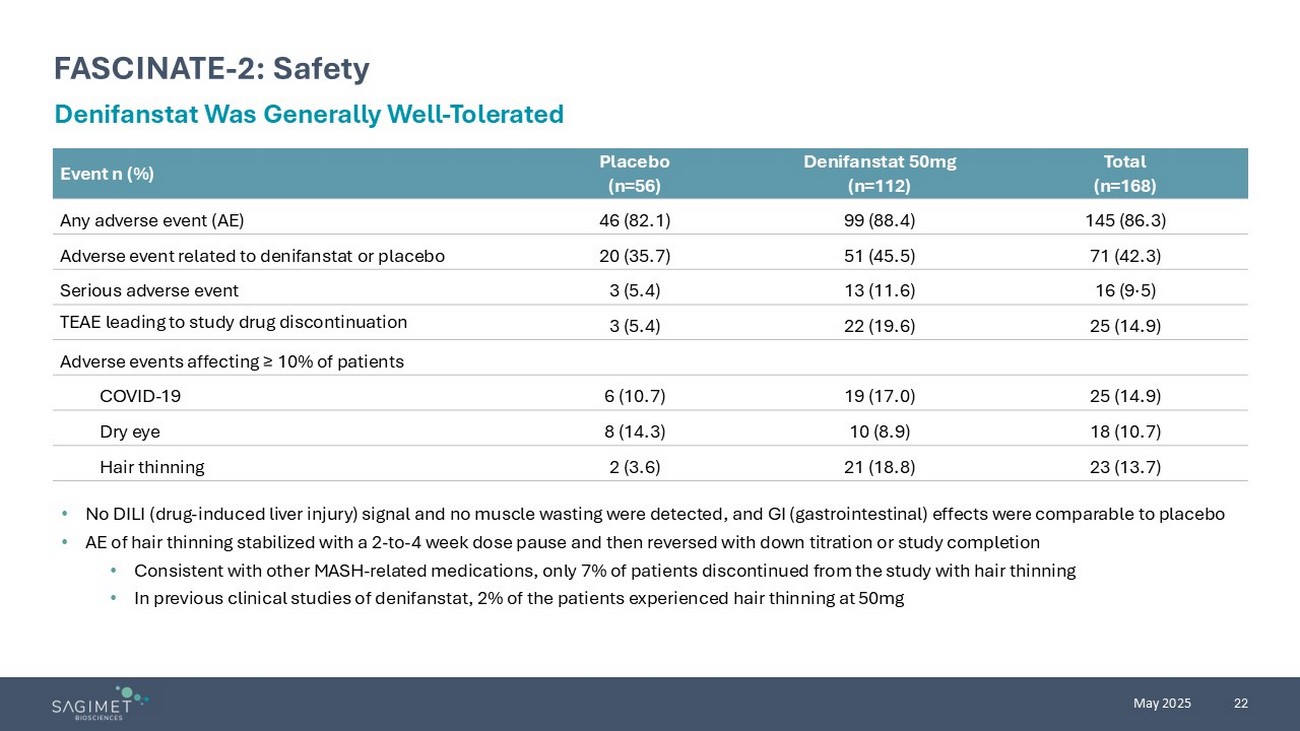
22 May 2025 FASCINATE - 2: Safety Denifanstat Was Generally Well - Tolerated • No DILI (drug - induced liver injury) signal and no muscle wasting were detected, and GI (gastrointestinal) effects were comparabl e to placebo • AE of hair thinning stabilized with a 2 - to - 4 week dose pause and then reversed with down titration or study completion • Consistent with other MASH - related medications, only 7% of patients discontinued from the study with hair thinning • In previous clinical studies of denifanstat, 2% of the patients experienced hair thinning at 50mg Total (n=168) Denifanstat 50mg (n=112) Placebo (n=56) Event n (%) 145 (86.3) 99 (88.4) 46 (82.1) Any adverse event (AE) 71 (42.3) 51 (45.5) 20 (35.7) Adverse event related to denifanstat or placebo 16 (9·5) 13 (11.6) 3 (5.4) Serious adverse event 25 (14.9) 22 (19.6) 3 (5.4) TEAE leading to study drug discontinuation Adverse events affecting ≥ 10% of patients 25 (14.9) 19 (17.0) 6 (10.7) COVID - 19 18 (10.7) 10 (8.9) 8 (14.3) Dry eye 23 (13.7) 21 (18.8) 2 (3.6) Hair thinning
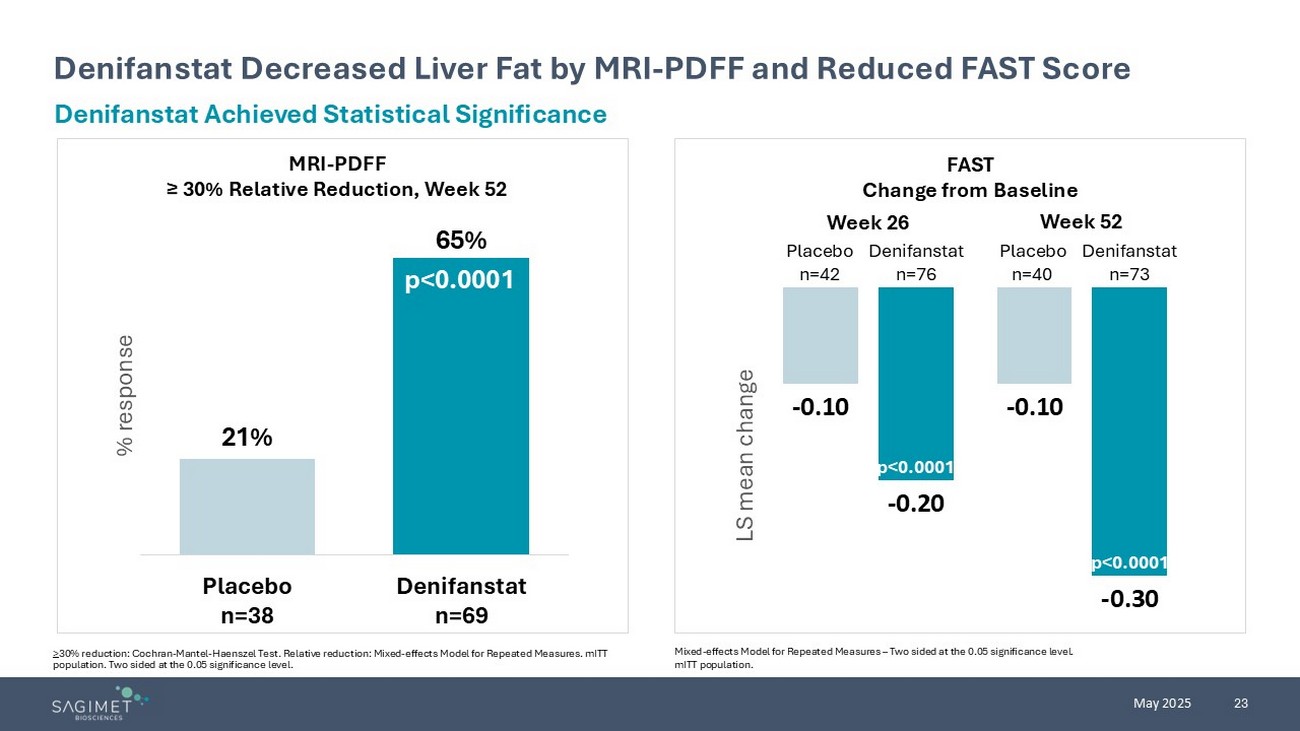
23 May 2025 Denifanstat Decreased Liver Fat by MRI - PDFF and Reduced FAST Score > 30% reduction: Cochran - Mantel - Haenszel Test. Relative reduction: Mixed - effects Model for Repeated Measures. mITT population. Two sided at the 0.05 significance level. Denifanstat Achieved Statistical Significance p<0.0001 21% 65% Placebo n=38 Denifanstat n=69 % response p<0.0001 MRI - PDFF ≥ 30% Relative Reduction, Week 52 FAST Change from Baseline - 0.10 - 0.10 - 0.20 - 0.30 LS mean change p<0.0001 p<0.0001 Week 26 Week 52 Placebo n=42 Denifanstat n=76 Placebo n=40 Denifanstat n=73 Mixed - effects Model for Repeated Measures – Two sided at the 0.05 significance level. mITT population.
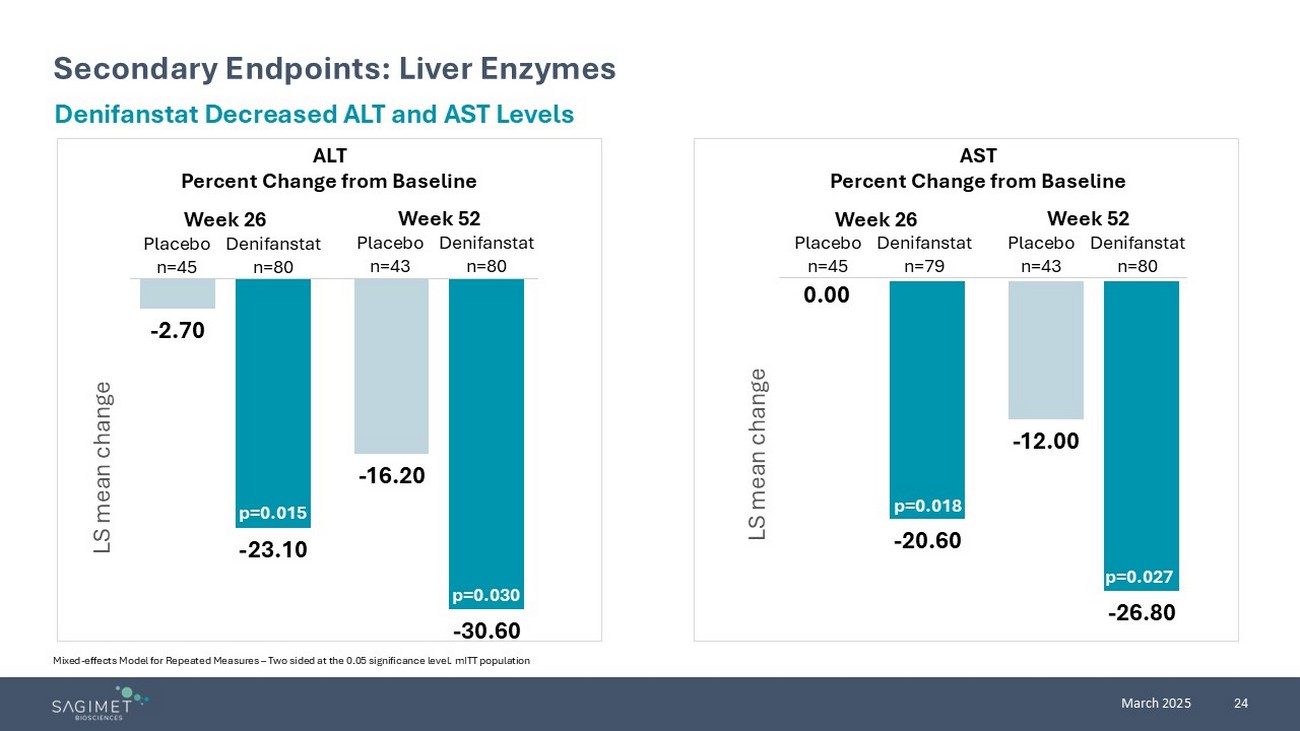
24 March 2025 Secondary Endpoints: Liver Enzymes Mixed - effects Model for Repeated Measures – Two sided at the 0.05 significance level. mITT population Denifanstat Decreased ALT and AST Levels ALT Percent Change from Baseline AST Percent Change from Baseline - 2.70 - 16.20 - 23.10 - 30.60 LS mean change p=0.015 p=0.030 0.00 - 12.00 - 20.60 - 26.80 LS mean change p=0.018 p=0.027 Week 26 Week 52 Week 26 Week 52 Placebo n=45 Denifanstat n=80 Placebo n=43 Denifanstat n=80 Placebo n=45 Denifanstat n=79 Placebo n=43 Denifanstat n=80
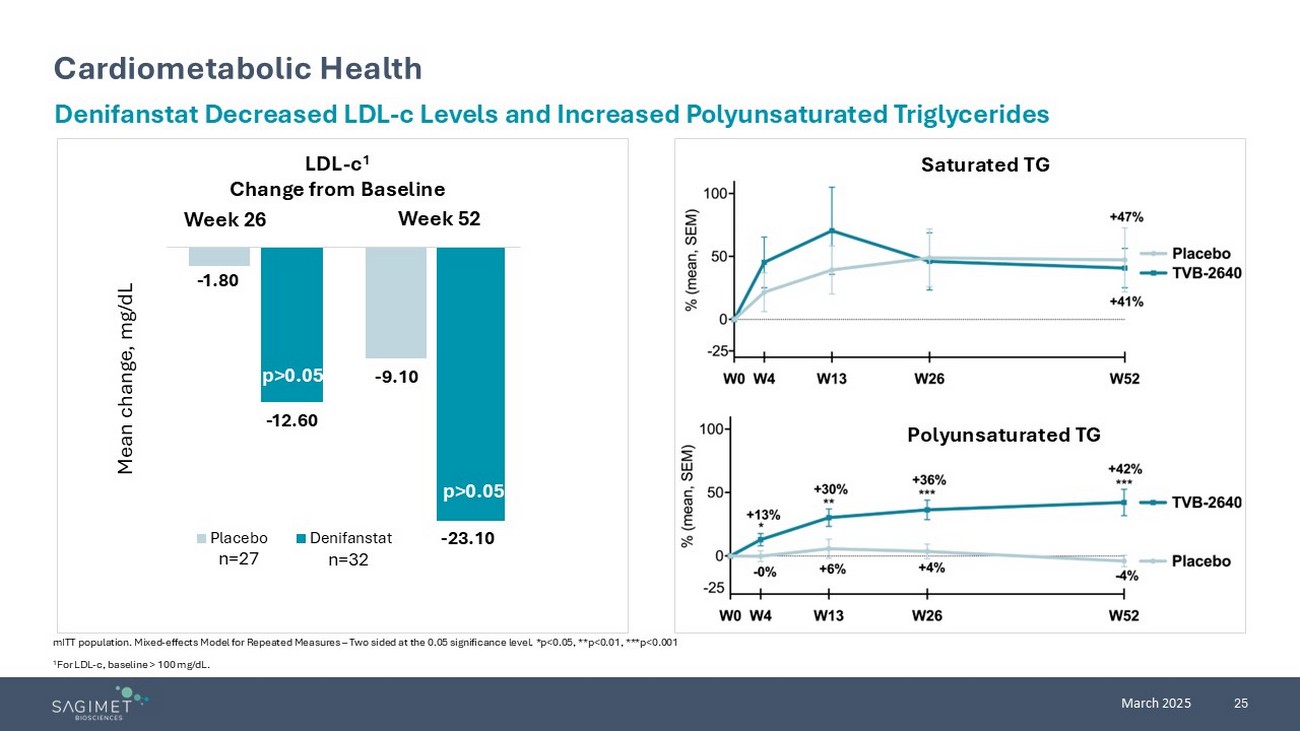
25 March 2025 Cardiometabolic Health mITT population. Mixed - effects Model for Repeated Measures – Two sided at the 0.05 significance level. *p<0.05, **p<0.01, ***p<0.001 1 For LDL - c, baseline > 100 mg/dL. Denifanstat Decreased LDL - c Levels and Increased Polyunsaturated Triglycerides LDL - c 1 Change from Baseline Saturated TG Polyunsaturated TG Week 26 Week 52 p>0.05 Mean change, mg/dL n=27 n=32 - 1.80 - 9.10 - 12.60 - 23.10 Placebo Denifanstat p>0.05 - 1.80 n=27 n=32 p>0.05
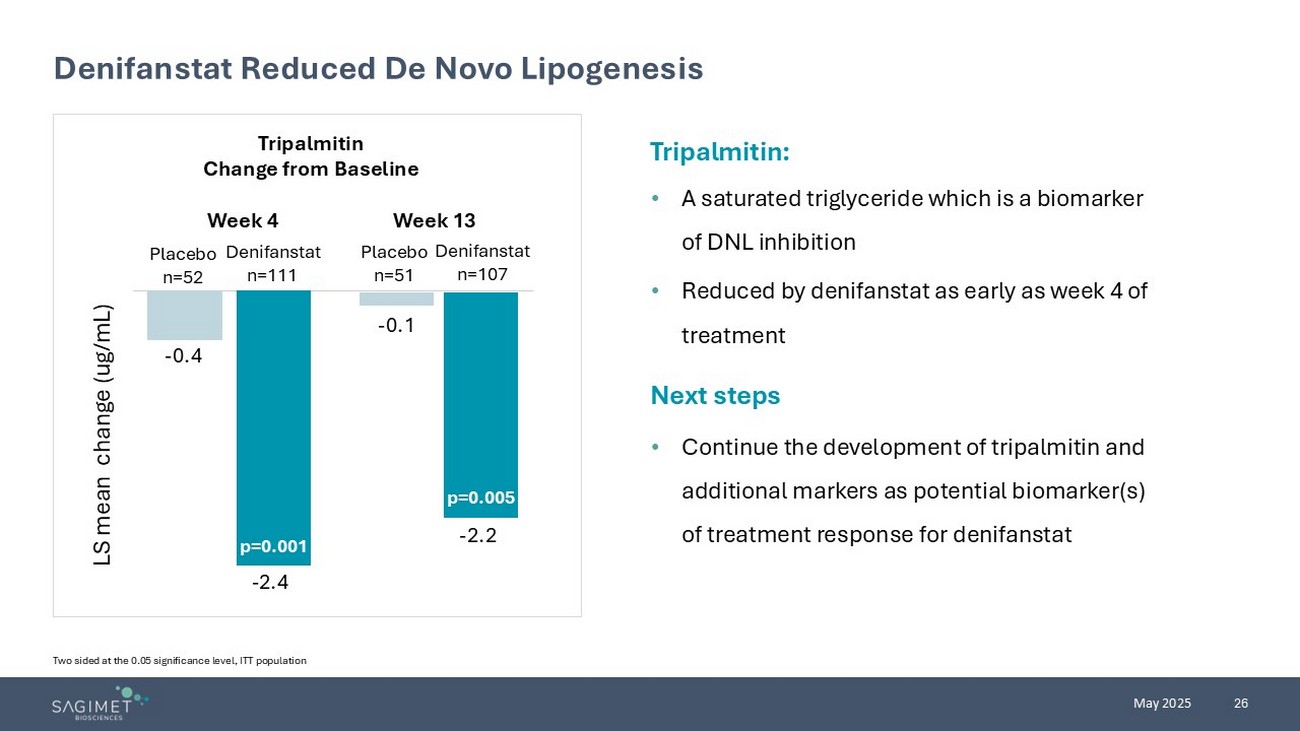
26 May 2025 Denifanstat Reduced De Novo Lipogenesis Two sided at the 0.05 significance level , ITT population Tripalmitin : • A saturated triglyceride which is a biomarker of DNL inhibition • Reduced by denifanstat as early as week 4 of treatment Next steps • Continue the development of tripalmitin and additional markers as potential biomarker(s) of treatment response for denifanstat Tripalmitin Change from Baseline Placebo n=52 Denifanstat n=111 Placebo n=51 Denifanstat n=107 - 2.2 LS mean change (ug/mL) - 2.4 - 0.4 - 0.1 P=0.005 Week 4 Week 13 p=0.001 p=0.005
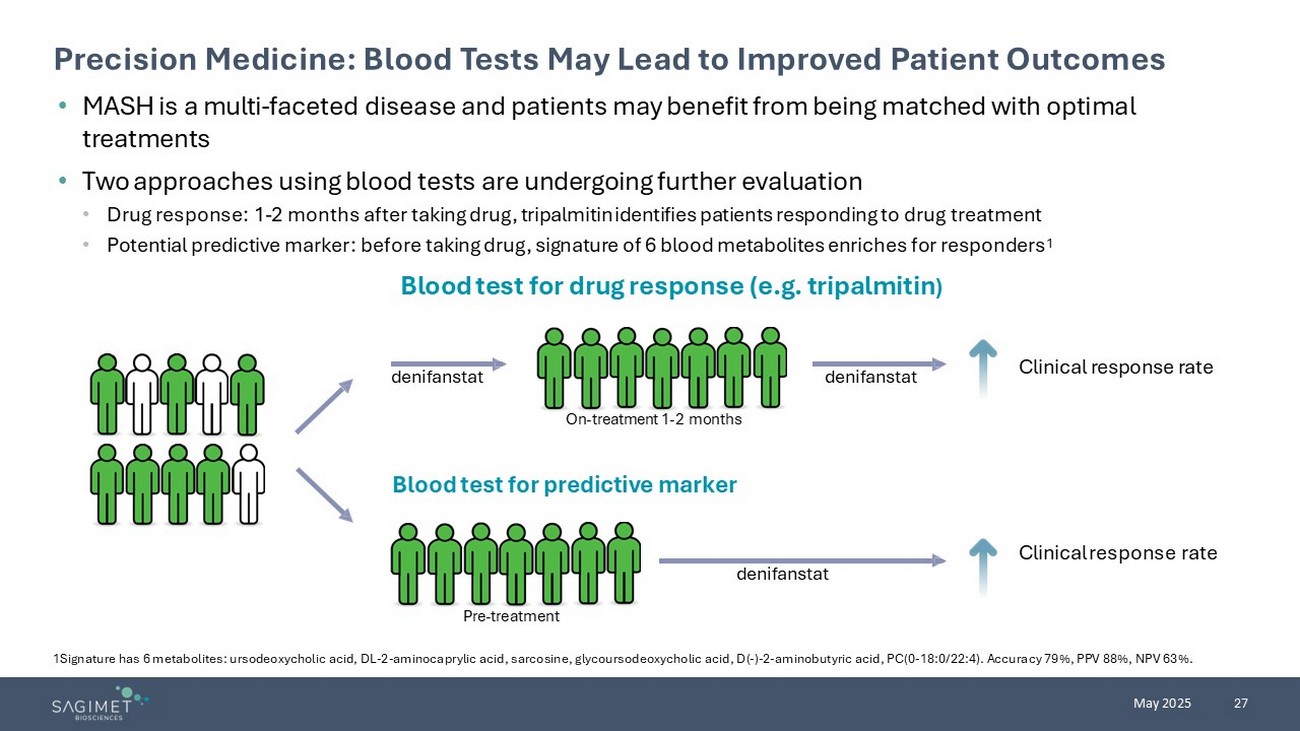
27 May 2025 Precision Medicine: Blood Tests May Lead to Improved Patient Outcomes 1Signature has 6 metabolites: ursodeoxycholic acid, DL - 2 - aminocaprylic acid, sarcosine, glycoursodeoxycholic acid, D( - ) - 2 - aminobutyric acid, PC(0 - 18:0/22:4). Accuracy 79%, PPV 88%, NPV 63%. • MASH is a multi - faceted disease and patients may benefit from being matched with optimal treatments • Two approaches using blood tests are undergoing further evaluation • Drug response: 1 - 2 months after taking drug, tripalmitin identifies patients responding to drug treatment • P otential p redictive marker: before taking drug, signature of 6 blood metabolites enriches for responders 1 Blood test for predictive marker denifanstat denifanstat denifanstat Clinical response rate On - treatment 1 - 2 months Pre - treatment Clinical response rate Blood test for drug response (e.g. tripalmitin )
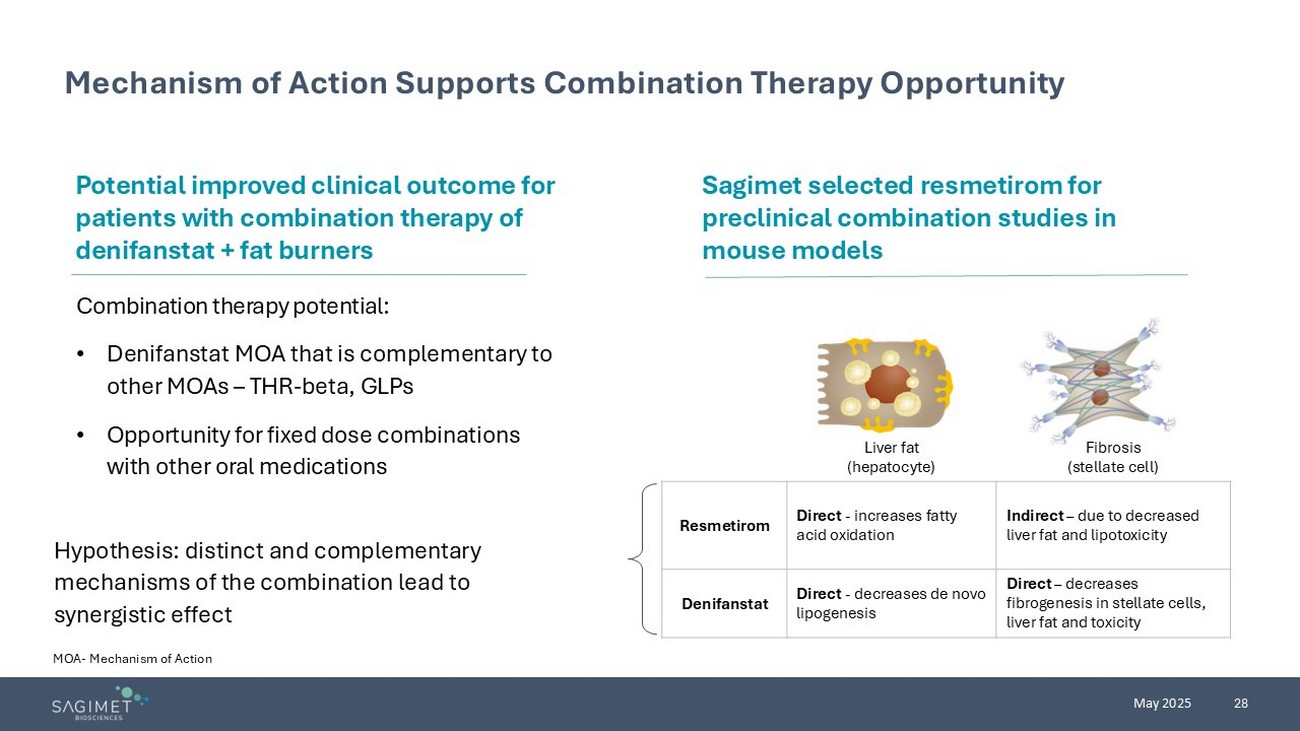
28 May 2025 Mechanism of Action Supports Combination Therapy Opportunity MOA - Mechanism of Action Combination therapy potential: • Denifanstat MOA that is complementary to other MOAs – THR - beta, GLPs • Opportunity for fixed dose combinations with other oral medications Potential improved clinical outcome for patients with combination therapy of denifanstat + fat burners Hypothesis: distinct and complementary mechanisms of the combination lead to synergistic effect Sagimet selected resmetirom for preclinical combination studies in mouse models Fibrosis (stellate cell) Liver fat (hepatocyte) Indirect – due to decreased liver fat and lipotoxicity Direct - increases fatty acid oxidation Resmetirom Direct – decreases fibrogenesis in stellate cells, liver fat and toxicity Direct - decreases de novo lipogenesis Denifanstat

29 May 2025 Phase 1 Clinical Pharmacokinetic (PK) Drug Interaction Trial Planned with Denifanstat and Resmetirom Objectives • Phase 1 clinical trial to measure pharmacokinetics of denifanstat and of resmetirom when co - administered, compared to monotherapy of each drug • Once daily oral administration as monotherapy or co - administered • Measure and compare multiple PK parameters • Assess tolerability when co - administered • Subject to consultation with regulatory authorities, plan to initiate in second half of 2025 Outcome • Define the range of dose levels of denifanstat and of resmetirom to conduct combination trials in MASH patients

FASN Inhibition Offers Potential Benefit in Multiple Indications
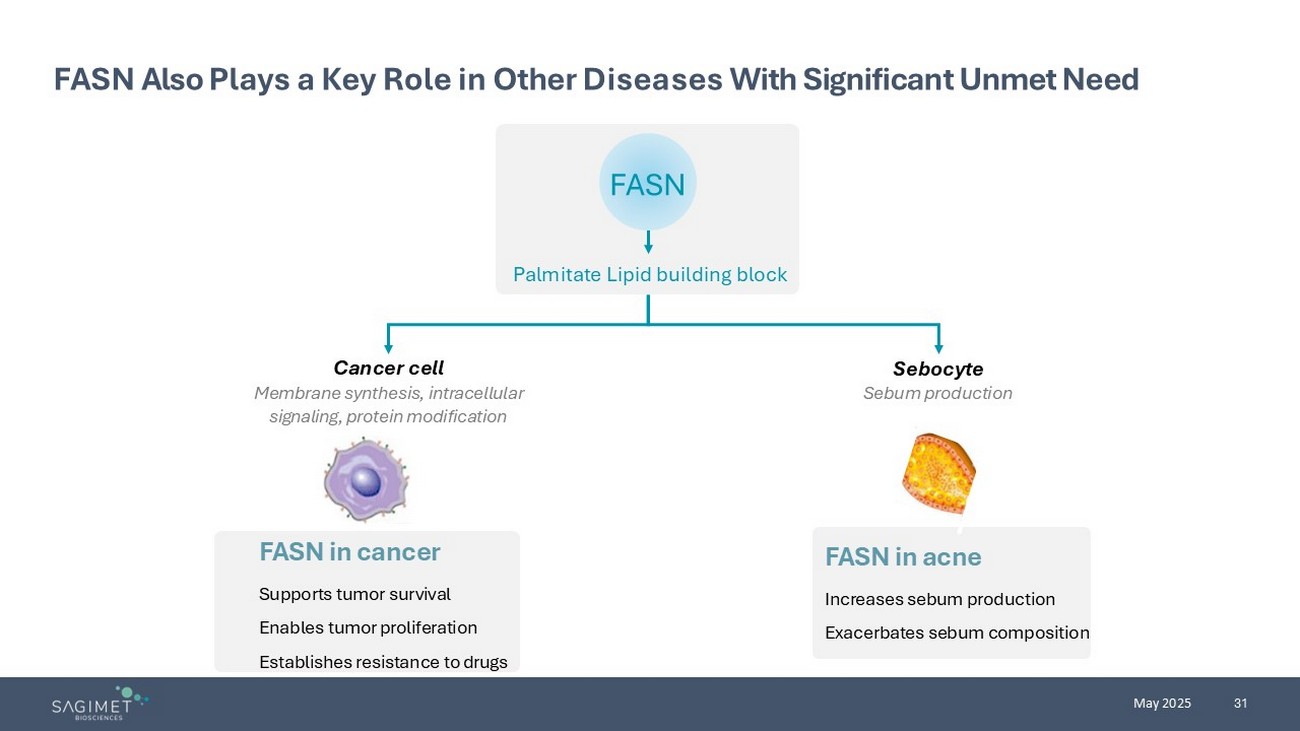
31 May 2025 FASN Also Plays a Key Role in Other Diseases With Significant Unmet Need FASN in cancer Supports t umor survival Enables t umor proliferation Establishes r esistance to drugs FASN in acne Increases sebum production Exacerbates sebum composition Cancer cell Membrane synthesis, intracellular signaling, protein modification Sebocyte Sebum production Palmitate Lipid building block FASN
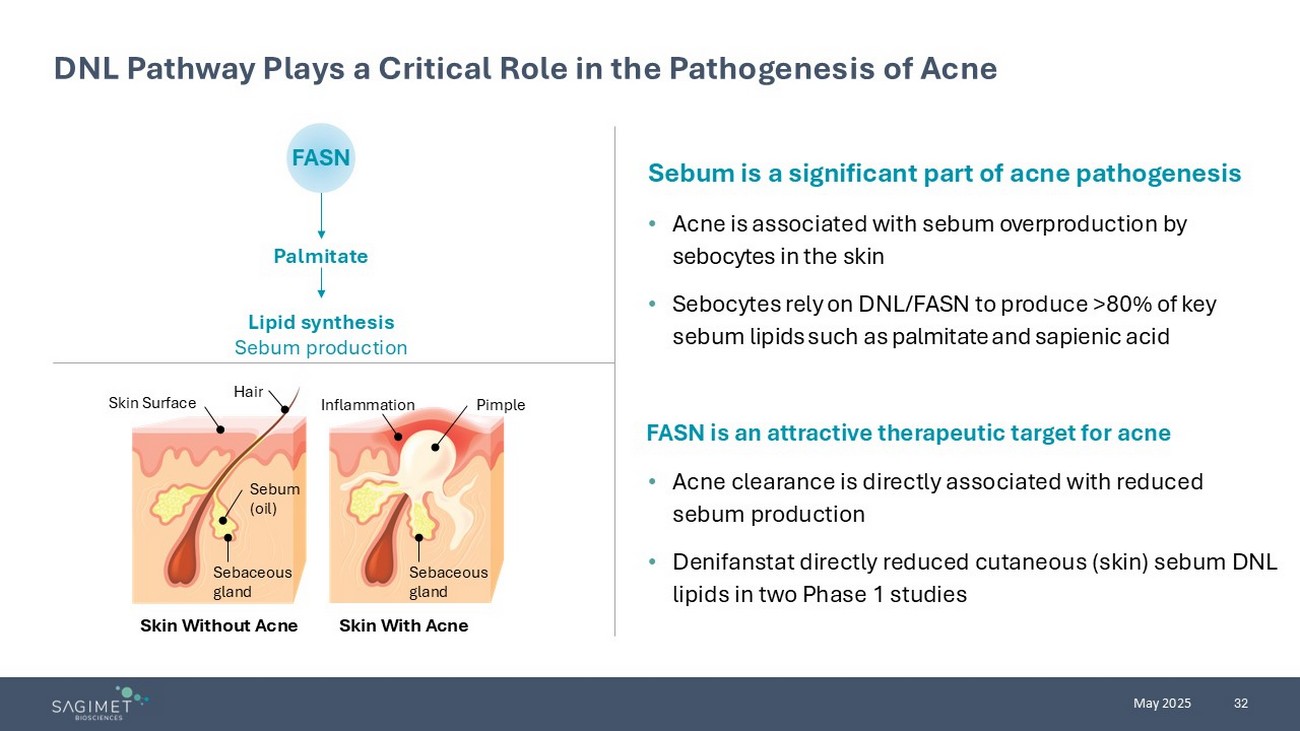
32 May 2025 DNL Pathway Plays a Critical Role in the Pathogenesis of Acne Sebum is a significant part of acne pathogenesis • Acne is associated with sebum over production by sebocytes in the skin • Sebocytes rely on DNL/FASN to produce >80% of key sebum lipids such as palmitate and sapienic acid FASN is an attractive therapeutic target for acne • Acne clearance is directly associated with reduced sebum production • Denifanstat directly reduced cutaneous (skin) sebum DNL lipids in two Phase 1 studies FASN Palmitate Lipid synthesis Sebum production Hair Skin Surface Sebum (oil) Inflammation Sebaceous gland Skin With Acne Skin Without Acne Pimple Sebaceous gland
33 May 2025 Blackheads Whiteheads Papules & Pustules Cysts & Nodules Acne US Market Overview Acne market in dermatology is large and highly aligned to a FASN inhibitor TPP value proposition 5.1 million US acne patients are treated by dermatologists annually (total US acne market is 50 million people ) 1 2 • Acne is the #1 or #2 patient concern in dermatology offices and 65%+ of patients in dermatology offices have private insurance 3 • Although acne treatments are currently available, d ermatologists are open to new therapies ( Seysara ® Tablets & Winlevi ® Cream) • T here is no cure for acne ; due to its pathology, most patients require chronic management and multiple courses for flare control annually Acne patients visiting a dermatologist are highly aligned to our TPP’s value proposition and positioning 3 • 70 % of patients presenting to dermatologists have moderate to severe disease 3 • Approximately 70% of patients have inflammatory lesions, and 16% of patients are post - menopausal women 3 1 Bickers DR, Lim HW, Margolis D, Weinstock MA, Goodman C, Faulkner E et al. The burden of skin diseases: 2004 a joint projec t o f the American Academy of Dermatology Association and the Society for Investigative Dermatology. Journal of the American Academy of Dermatology 2006;55:490 - 500 2 American Academy of Dermatology/Milliman. Burden of Skin Disease. 2017. www.aad.org/BSD 3 Sagimet market research conducted in July 2024 among 50 dermatologists, data on file
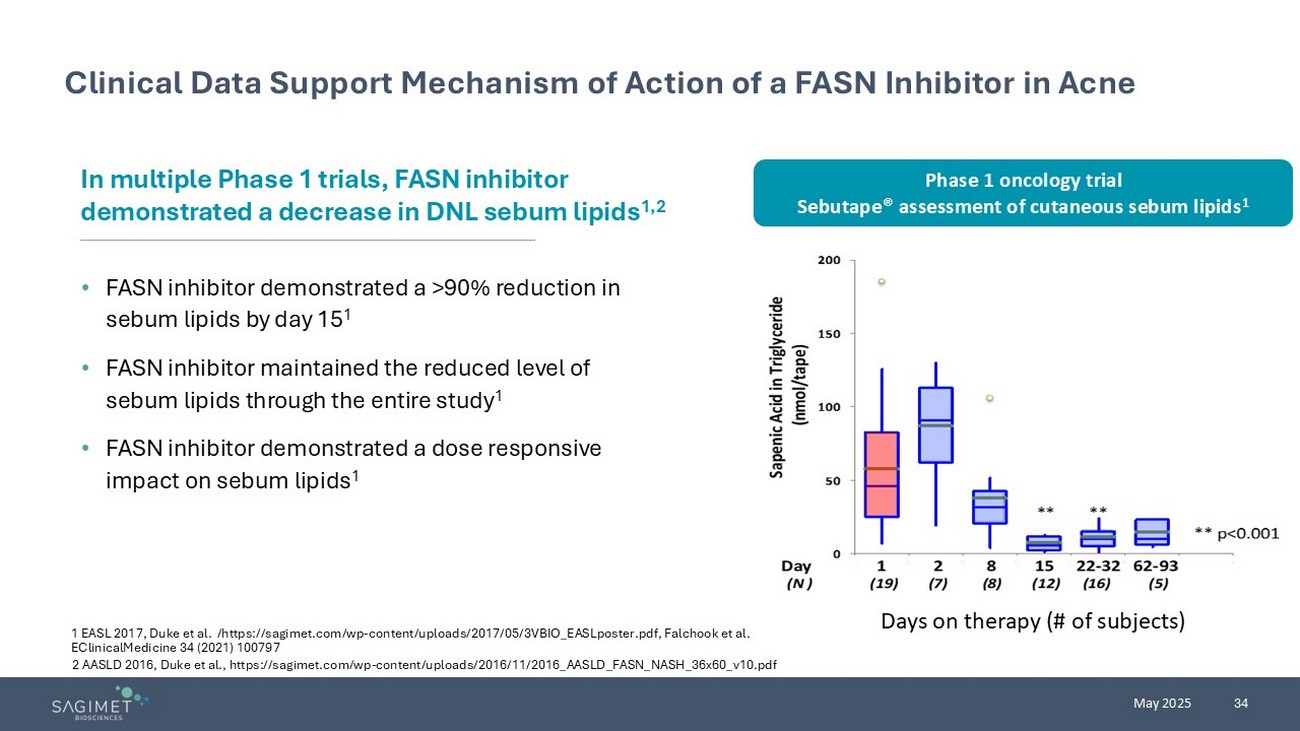
34 May 2025 Clinical Data Support Mechanism of Action of a FASN Inhibitor in Acne • FASN inhibitor demonstrated a >90% reduction in sebum lipids by day 15 1 • FASN inhibitor maintained the reduced level of sebum lipids through the entire study 1 • FASN inhibitor demonstrated a dose responsive impact on sebum lipids 1 I n multiple Phase 1 trials, FASN inhibitor demonstrated a decrease in DNL sebum lipids 1,2 1 EASL 2017, Duke et al. /https://sagimet.com/wp - content/uploads/2017/05/3VBIO_EASLposter.pdf, Falchook et al. EClinicalMedicine 34 (2021) 100797 Days on therapy (# of subjects) 2 AASLD 2016, Duke et al., https://sagimet.com/wp - content/uploads/2016/11/2016_AASLD_FASN_NASH_36x60_v10.pdf Phase 1 oncology trial Sebutape ® assessment of cutaneous sebum lipids 1
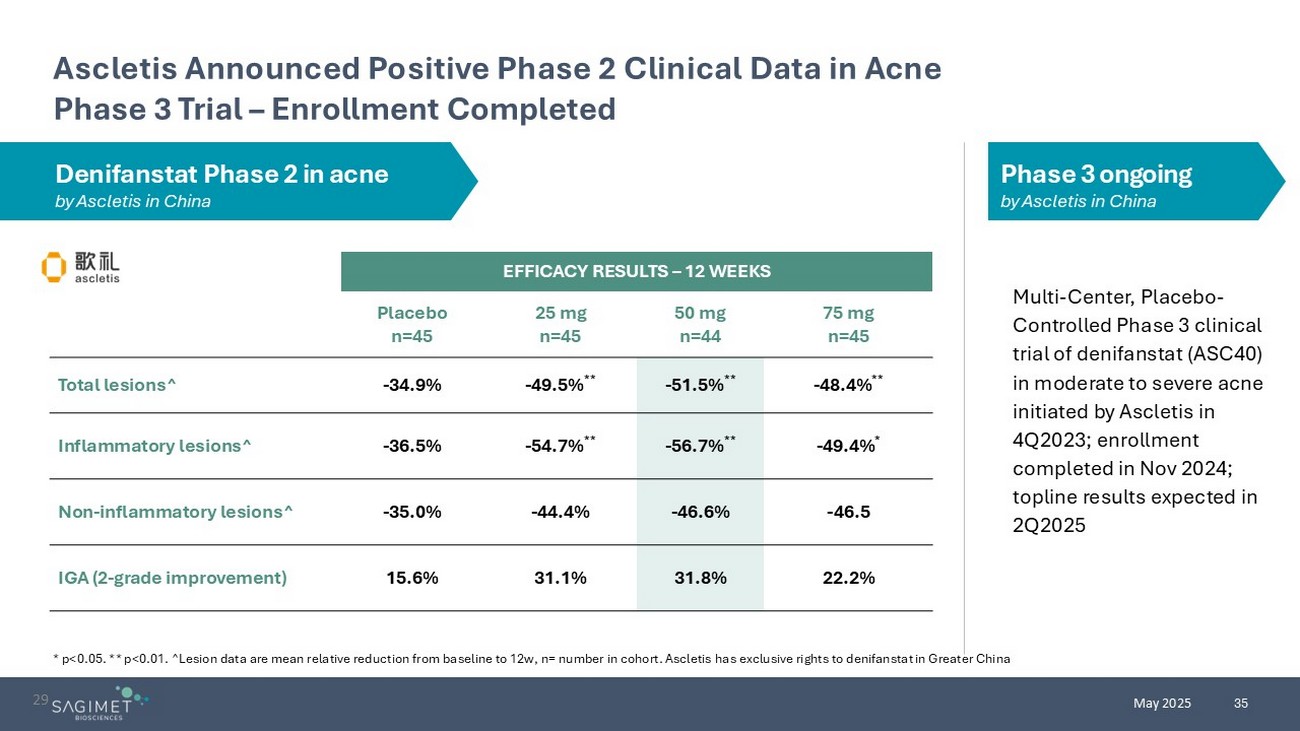
35 May 2025 EFFICACY RESULTS – 12 WEEKS 75 mg n=45 50 mg n=44 25 mg n=45 Placebo n=45 - 48.4% ** - 51.5% ** - 49.5% ** - 34.9% Total lesions^ - 49.4% * - 56.7% ** - 54.7% ** - 36.5% Inflammatory lesions^ - 46.5 - 46.6% - 44.4% - 35.0% Non - inflammatory lesions^ 22.2% 31.8% 31.1% 15.6% IGA (2 - grade improvement) Ascletis Announced Positive Phase 2 Clinical Data in Acne Phase 3 Trial – Enrollment Completed * p<0.05. ** p<0.01. ^Lesion data are mean relative reduction from baseline to 12w, n= number in cohort. Ascletis has exclusive rights to denifanstat in Greater China 2 9 Multi - Center, Placebo - Controlled Phase 3 clinical trial of denifanstat (ASC40) in moderate to severe acne initiated by Ascletis in 4Q2023; enrollment completed in Nov 2024; topline results expected in 2Q2025 Denifanstat Phase 2 in acne by Ascletis in China Phase 3 ongoing by Ascletis in China
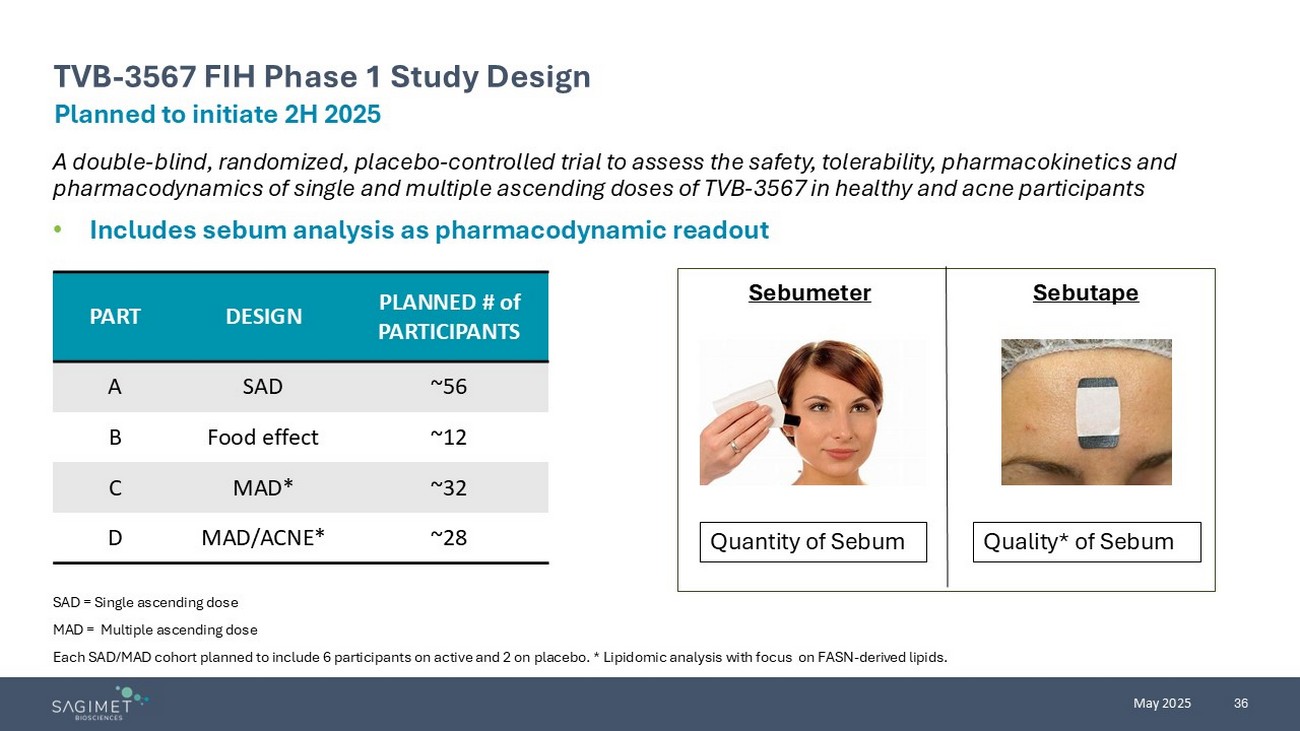
36 May 2025 A double - blind, randomized, placebo - controlled trial to assess the safety, tolerability, pharmacokinetics and pharmacodynamics of single and multiple ascending doses of TVB - 3567 in healthy and acne participants • Includes sebum analysis as pharmacodynamic readout SAD = Single ascending dose MAD = Multiple ascending dose Each SAD/MAD cohort planned to include 6 participants on active and 2 on placebo. * Lipidomic analysis with focus on FASN - deriv ed lipids. Planned to initiate 2H 2025 TVB - 3567 FIH Phase 1 Study Design Sebutape Sebumeter Quantity of Sebum Quality* of Sebum PLANNED # of PARTICIPANTS DESIGN PART ~56 SAD A ~12 Food effect B ~32 MAD* C ~28 MAD/ACNE* D
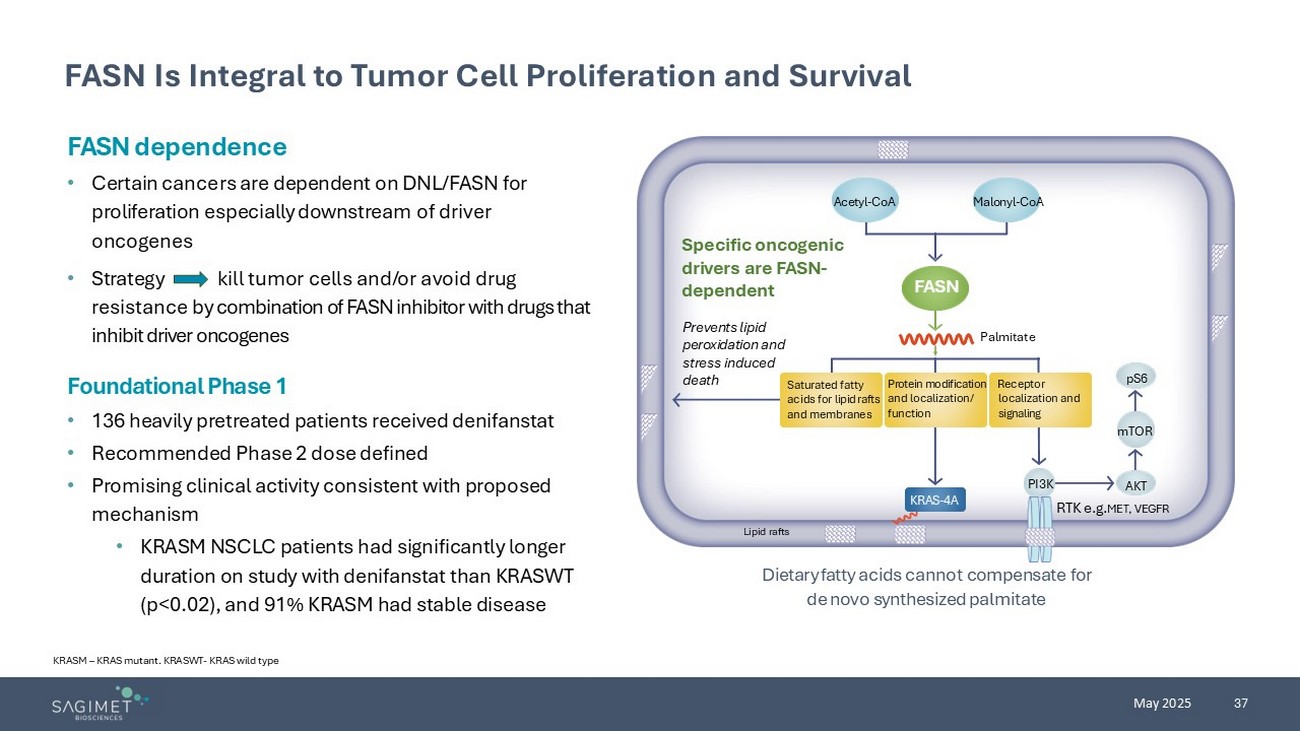
37 May 2025 FASN Is Integral to Tumor Cell Proliferation and Surviv al KRASM – KRAS mutant. KRASWT - KRAS wild type FASN dependence • Certain cancers are dependent on DNL/FASN for proliferation especially downstream of driver oncogenes • Strategy kill tumor cells and/or avoid drug resistance by combination of FASN inhibitor with drugs that inhibit driver oncogenes Dietary fatty acids cannot compensate for de novo synthesized palmitate Specific oncogenic drivers are FASN - dependent Prevents lipid peroxidation and stress induced death Palmitate RTK e.g. MET , VEGFR Saturated fatty acids for lipid rafts and membranes Protein modification and localization/ function Receptor localization and signaling Acetyl - CoA Malonyl - CoA pS6 mTOR AKT PI3K KRAS - 4A Lipid rafts FASN Foundational Phase 1 • 136 heavily pretreated patients received denifanstat • Recommended Phase 2 dose defined • Promising clinical activity consistent with proposed mechanism • KRASM NSCLC patients had significantly longer duration on study with denifanstat than KRASWT (p<0.02), and 91% KRASM had stable disease
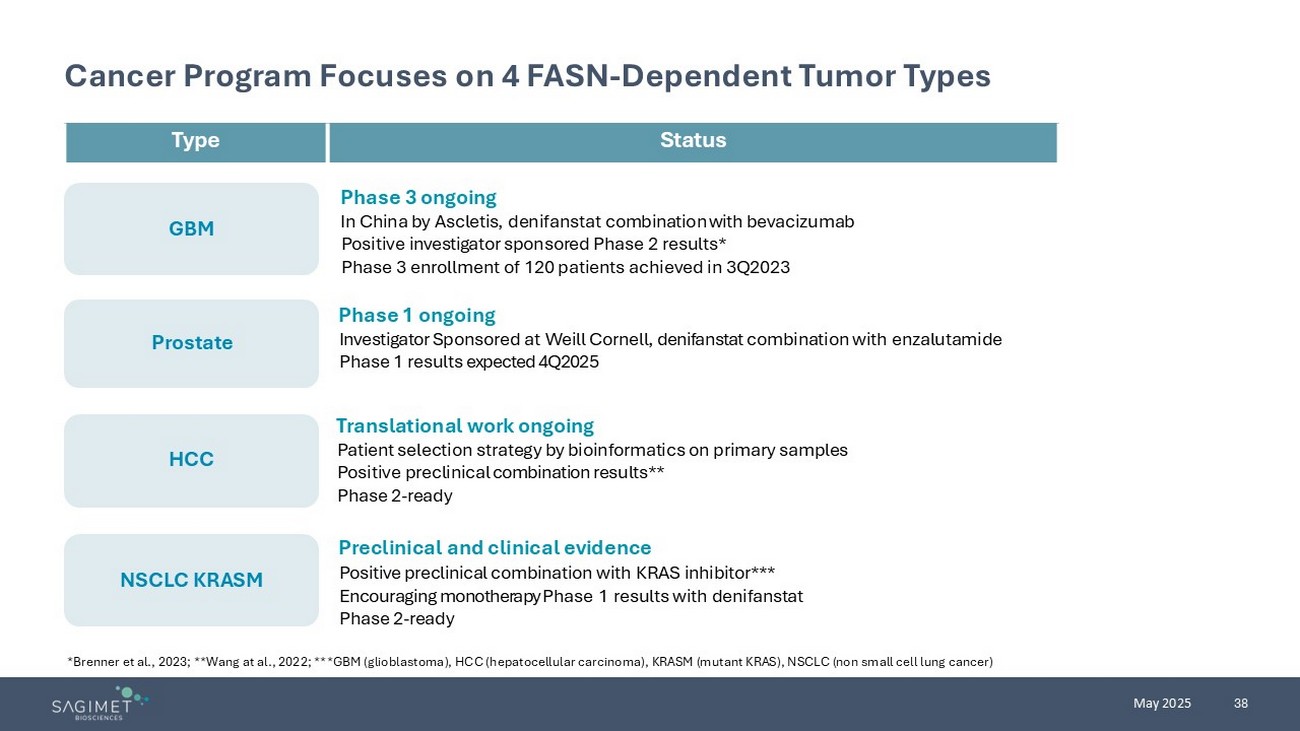
38 May 2025 GBM Prostate HCC NSCLC KRASM Cancer Program Focuses on 4 FASN - Dependent Tumor Types *Brenner et al., 2023; **Wang at al., 2022; ***GBM (glioblastoma), HCC (hepatocellular carcinoma), KRASM (mutant KRAS), NSCLC ( non small cell lung cancer) Phase 3 ongoing In China by Ascletis , denifanstat combination with bevacizumab Positive investigator sponsored Phase 2 results * Phase 3 enrollment of 120 patients achieved in 3Q2023 Status Type Phase 1 ongoing Investigator Sponsored at Weill Cornell, denifanstat combination with enzalutamide Phase 1 results expected 4Q2025 Translational work ongoing Patient selection strategy by bioinformatics on primary samples Positive preclinical combination results** Phase 2 - ready Preclinical and clinical evidence Positive preclinical combination with KRAS inhibitor*** Encouraging monotherapy Phase 1 results with denifanstat Phase 2 - ready

39 May 2025 Sagimet at a Glance • Our lead molecule, denifanstat, is a novel fatty acid synthase (FASN) inhibitor with a differentiated MOA with the potential to target multiple underserved diseases • Strong clinical data demonstrates denifanstat’s proof of concept across multiple disease states Unique MOA: FASN Inhibition • Denifanstat directly targets the 3 key drivers of MASH (metabolic dysfunction - associated steatohepatitis) – liver fat, inflammation, and fibrosis • Successful outcome of Phase 2b trial; met both primary endp oints with significant reduction in fibrosis • Pre - clinical data demonstrated synergistic effect of combination of FA SN inhibitor and resmetirom • Phase 1 clinical trial to evaluate the pharmacokinetics ථ (PK) and tolerability of a combi nation of denifanstat and resmetirom planned to initiate in 2H 2025; data readout expected 1H 2026 Denifanstat in MASH TVB - 3567 in Acne • Our follow - on FASN inhibitor, TVB 3567, received Investigational New Drug (IND) clearance in March 2025 • First - in - human Phase 1 clinical trial for development of an acne indication expected to initiate 2H 2025
Cover |
May 08, 2025 |
|---|---|
| Cover [Abstract] | |
| Document Type | 8-K |
| Amendment Flag | false |
| Document Period End Date | May 08, 2025 |
| Entity File Number | 001-41742 |
| Entity Registrant Name | SAGIMET BIOSCIENCES INC. |
| Entity Central Index Key | 0001400118 |
| Entity Tax Identification Number | 20-5991472 |
| Entity Incorporation, State or Country Code | DE |
| Entity Address, Address Line One | 155 Bovet Road |
| Entity Address, Address Line Two | Suite 303 |
| Entity Address, City or Town | San Mateo |
| Entity Address, State or Province | CA |
| Entity Address, Postal Zip Code | 94402 |
| City Area Code | 650 |
| Local Phone Number | 561-8600 |
| Written Communications | false |
| Soliciting Material | false |
| Pre-commencement Tender Offer | false |
| Pre-commencement Issuer Tender Offer | false |
| Title of 12(b) Security | Series A Common Stock, $0.0001 par value per share |
| Trading Symbol | SGMT |
| Security Exchange Name | NASDAQ |
| Entity Emerging Growth Company | true |
| Elected Not To Use the Extended Transition Period | false |
{
"version": "2.2",
"instance": {
"tm2514358d1_8k.htm": {
"nsprefix": "sgmt",
"nsuri": "http://sagimet.com/20250508",
"dts": {
"schema": {
"local": [
"sgmt-20250508.xsd"
],
"remote": [
"http://www.xbrl.org/2003/xbrl-instance-2003-12-31.xsd",
"http://www.xbrl.org/2003/xbrl-linkbase-2003-12-31.xsd",
"http://www.xbrl.org/2003/xl-2003-12-31.xsd",
"http://www.xbrl.org/2003/xlink-2003-12-31.xsd",
"http://www.xbrl.org/2005/xbrldt-2005.xsd",
"http://www.xbrl.org/2006/ref-2006-02-27.xsd",
"http://www.xbrl.org/lrr/role/negated-2009-12-16.xsd",
"http://www.xbrl.org/lrr/role/net-2009-12-16.xsd",
"https://www.xbrl.org/2020/extensible-enumerations-2.0.xsd",
"https://www.xbrl.org/dtr/type/2020-01-21/types.xsd",
"https://www.xbrl.org/dtr/type/2022-03-31/types.xsd",
"https://xbrl.fasb.org/srt/2023/elts/srt-2023.xsd",
"https://xbrl.fasb.org/srt/2023/elts/srt-roles-2023.xsd",
"https://xbrl.fasb.org/srt/2023/elts/srt-types-2023.xsd",
"https://xbrl.fasb.org/us-gaap/2023/elts/us-gaap-2023.xsd",
"https://xbrl.fasb.org/us-gaap/2023/elts/us-roles-2023.xsd",
"https://xbrl.fasb.org/us-gaap/2023/elts/us-types-2023.xsd",
"https://xbrl.sec.gov/country/2023/country-2023.xsd",
"https://xbrl.sec.gov/dei/2023/dei-2023.xsd"
]
},
"labelLink": {
"local": [
"sgmt-20250508_lab.xml"
]
},
"presentationLink": {
"local": [
"sgmt-20250508_pre.xml"
]
},
"inline": {
"local": [
"tm2514358d1_8k.htm"
]
}
},
"keyStandard": 24,
"keyCustom": 0,
"axisStandard": 0,
"axisCustom": 0,
"memberStandard": 0,
"memberCustom": 0,
"hidden": {
"total": 2,
"http://xbrl.sec.gov/dei/2023": 2
},
"contextCount": 1,
"entityCount": 1,
"segmentCount": 0,
"elementCount": 59,
"unitCount": 3,
"baseTaxonomies": {
"http://xbrl.sec.gov/dei/2023": 24
},
"report": {
"R1": {
"role": "http://sagimet.com/role/Cover",
"longName": "00000001 - Document - Cover",
"shortName": "Cover",
"isDefault": "true",
"groupType": "document",
"subGroupType": "",
"menuCat": "Cover",
"order": "1",
"firstAnchor": {
"contextRef": "AsOf2025-05-08",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"b",
"p",
"body",
"html"
],
"reportCount": 1,
"baseRef": "tm2514358d1_8k.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "AsOf2025-05-08",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"b",
"p",
"body",
"html"
],
"reportCount": 1,
"baseRef": "tm2514358d1_8k.htm",
"first": true,
"unique": true
}
}
},
"tag": {
"dei_AmendmentDescription": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "AmendmentDescription",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Amendment Description",
"documentation": "Description of changes contained within amended document."
}
}
},
"auth_ref": []
},
"dei_AmendmentFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "AmendmentFlag",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Amendment Flag",
"documentation": "Boolean flag that is true when the XBRL content amends previously-filed or accepted submission."
}
}
},
"auth_ref": []
},
"dei_AnnualInformationForm": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "AnnualInformationForm",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Annual Information Form",
"documentation": "Boolean flag with value true on a form if it is an annual report containing an annual information form."
}
}
},
"auth_ref": [
"r14"
]
},
"dei_AuditedAnnualFinancialStatements": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "AuditedAnnualFinancialStatements",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Audited Annual Financial Statements",
"documentation": "Boolean flag with value true on a form if it is an annual report containing audited financial statements."
}
}
},
"auth_ref": [
"r14"
]
},
"dei_CityAreaCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "CityAreaCode",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "City Area Code",
"documentation": "Area code of city"
}
}
},
"auth_ref": []
},
"dei_CountryRegion": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "CountryRegion",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Country Region",
"documentation": "Region code of country"
}
}
},
"auth_ref": []
},
"dei_CoverAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "CoverAbstract",
"lang": {
"en-us": {
"role": {
"label": "Cover [Abstract]",
"documentation": "Cover page."
}
}
},
"auth_ref": []
},
"dei_CurrentFiscalYearEndDate": {
"xbrltype": "gMonthDayItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "CurrentFiscalYearEndDate",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Current Fiscal Year End Date",
"documentation": "End date of current fiscal year in the format --MM-DD."
}
}
},
"auth_ref": []
},
"dei_DocumentAccountingStandard": {
"xbrltype": "accountingStandardItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentAccountingStandard",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Accounting Standard",
"documentation": "The basis of accounting the registrant has used to prepare the financial statements included in this filing This can either be 'U.S. GAAP', 'International Financial Reporting Standards', or 'Other'."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_DocumentAnnualReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentAnnualReport",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Annual Report",
"documentation": "Boolean flag that is true only for a form used as an annual report."
}
}
},
"auth_ref": [
"r11",
"r13",
"r14"
]
},
"dei_DocumentFiscalPeriodFocus": {
"xbrltype": "fiscalPeriodItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentFiscalPeriodFocus",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Fiscal Period Focus",
"documentation": "Fiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY."
}
}
},
"auth_ref": []
},
"dei_DocumentFiscalYearFocus": {
"xbrltype": "gYearItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentFiscalYearFocus",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Fiscal Year Focus",
"documentation": "This is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006."
}
}
},
"auth_ref": []
},
"dei_DocumentPeriodEndDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentPeriodEndDate",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Period End Date",
"documentation": "For the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD."
}
}
},
"auth_ref": []
},
"dei_DocumentPeriodStartDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentPeriodStartDate",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Period Start Date",
"documentation": "The start date of the period covered in the document, in YYYY-MM-DD format."
}
}
},
"auth_ref": []
},
"dei_DocumentQuarterlyReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentQuarterlyReport",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Quarterly Report",
"documentation": "Boolean flag that is true only for a form used as an quarterly report."
}
}
},
"auth_ref": [
"r12"
]
},
"dei_DocumentRegistrationStatement": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentRegistrationStatement",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Registration Statement",
"documentation": "Boolean flag that is true only for a form used as a registration statement."
}
}
},
"auth_ref": [
"r0"
]
},
"dei_DocumentShellCompanyEventDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentShellCompanyEventDate",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Shell Company Event Date",
"documentation": "Date of event requiring a shell company report."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_DocumentShellCompanyReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentShellCompanyReport",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Shell Company Report",
"documentation": "Boolean flag that is true for a Shell Company Report pursuant to section 13 or 15(d) of the Exchange Act."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_DocumentTransitionReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentTransitionReport",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Transition Report",
"documentation": "Boolean flag that is true only for a form used as a transition report."
}
}
},
"auth_ref": [
"r15"
]
},
"dei_DocumentType": {
"xbrltype": "submissionTypeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentType",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Type",
"documentation": "The type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'."
}
}
},
"auth_ref": []
},
"dei_DocumentsIncorporatedByReferenceTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "DocumentsIncorporatedByReferenceTextBlock",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Documents Incorporated by Reference [Text Block]",
"documentation": "Documents incorporated by reference."
}
}
},
"auth_ref": [
"r3"
]
},
"dei_EntityAddressAddressLine1": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityAddressAddressLine1",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Address Line One",
"documentation": "Address Line 1 such as Attn, Building Name, Street Name"
}
}
},
"auth_ref": []
},
"dei_EntityAddressAddressLine2": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityAddressAddressLine2",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Address Line Two",
"documentation": "Address Line 2 such as Street or Suite number"
}
}
},
"auth_ref": []
},
"dei_EntityAddressAddressLine3": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityAddressAddressLine3",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Address Line Three",
"documentation": "Address Line 3 such as an Office Park"
}
}
},
"auth_ref": []
},
"dei_EntityAddressCityOrTown": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityAddressCityOrTown",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, City or Town",
"documentation": "Name of the City or Town"
}
}
},
"auth_ref": []
},
"dei_EntityAddressCountry": {
"xbrltype": "countryCodeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityAddressCountry",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Country",
"documentation": "ISO 3166-1 alpha-2 country code."
}
}
},
"auth_ref": []
},
"dei_EntityAddressPostalZipCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityAddressPostalZipCode",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Postal Zip Code",
"documentation": "Code for the postal or zip code"
}
}
},
"auth_ref": []
},
"dei_EntityAddressStateOrProvince": {
"xbrltype": "stateOrProvinceItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityAddressStateOrProvince",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, State or Province",
"documentation": "Name of the state or province."
}
}
},
"auth_ref": []
},
"dei_EntityBankruptcyProceedingsReportingCurrent": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityBankruptcyProceedingsReportingCurrent",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Bankruptcy Proceedings, Reporting Current",
"documentation": "For registrants involved in bankruptcy proceedings during the preceding five years, the value Yes indicates that the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court; the value No indicates the registrant has not. Registrants not involved in bankruptcy proceedings during the preceding five years should not report this element."
}
}
},
"auth_ref": [
"r6"
]
},
"dei_EntityCentralIndexKey": {
"xbrltype": "centralIndexKeyItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityCentralIndexKey",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Central Index Key",
"documentation": "A unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityCommonStockSharesOutstanding": {
"xbrltype": "sharesItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityCommonStockSharesOutstanding",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Common Stock, Shares Outstanding",
"documentation": "Indicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument."
}
}
},
"auth_ref": []
},
"dei_EntityCurrentReportingStatus": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityCurrentReportingStatus",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Current Reporting Status",
"documentation": "Indicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure."
}
}
},
"auth_ref": []
},
"dei_EntityEmergingGrowthCompany": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityEmergingGrowthCompany",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Emerging Growth Company",
"documentation": "Indicate if registrant meets the emerging growth company criteria."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityExTransitionPeriod": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityExTransitionPeriod",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Elected Not To Use the Extended Transition Period",
"documentation": "Indicate if an emerging growth company has elected not to use the extended transition period for complying with any new or revised financial accounting standards."
}
}
},
"auth_ref": [
"r19"
]
},
"dei_EntityFileNumber": {
"xbrltype": "fileNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityFileNumber",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity File Number",
"documentation": "Commission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen."
}
}
},
"auth_ref": []
},
"dei_EntityFilerCategory": {
"xbrltype": "filerCategoryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityFilerCategory",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Filer Category",
"documentation": "Indicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityIncorporationStateCountryCode": {
"xbrltype": "edgarStateCountryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityIncorporationStateCountryCode",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Incorporation, State or Country Code",
"documentation": "Two-character EDGAR code representing the state or country of incorporation."
}
}
},
"auth_ref": []
},
"dei_EntityInteractiveDataCurrent": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityInteractiveDataCurrent",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Interactive Data Current",
"documentation": "Boolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files)."
}
}
},
"auth_ref": [
"r16"
]
},
"dei_EntityPrimarySicNumber": {
"xbrltype": "sicNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityPrimarySicNumber",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Primary SIC Number",
"documentation": "Primary Standard Industrial Classification (SIC) Number for the Entity."
}
}
},
"auth_ref": [
"r14"
]
},
"dei_EntityPublicFloat": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityPublicFloat",
"crdr": "credit",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Public Float",
"documentation": "The aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter."
}
}
},
"auth_ref": []
},
"dei_EntityRegistrantName": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityRegistrantName",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Registrant Name",
"documentation": "The exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityShellCompany": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityShellCompany",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Shell Company",
"documentation": "Boolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntitySmallBusiness": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntitySmallBusiness",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Small Business",
"documentation": "Indicates that the company is a Smaller Reporting Company (SRC)."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityTaxIdentificationNumber": {
"xbrltype": "employerIdItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityTaxIdentificationNumber",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Tax Identification Number",
"documentation": "The Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityVoluntaryFilers": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityVoluntaryFilers",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Voluntary Filers",
"documentation": "Indicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act."
}
}
},
"auth_ref": []
},
"dei_EntityWellKnownSeasonedIssuer": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "EntityWellKnownSeasonedIssuer",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Well-known Seasoned Issuer",
"documentation": "Indicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A."
}
}
},
"auth_ref": [
"r17"
]
},
"dei_Extension": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "Extension",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Extension",
"documentation": "Extension number for local phone number."
}
}
},
"auth_ref": []
},
"dei_LocalPhoneNumber": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "LocalPhoneNumber",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Local Phone Number",
"documentation": "Local phone number for entity."
}
}
},
"auth_ref": []
},
"dei_NoTradingSymbolFlag": {
"xbrltype": "trueItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "NoTradingSymbolFlag",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "No Trading Symbol Flag",
"documentation": "Boolean flag that is true only for a security having no trading symbol."
}
}
},
"auth_ref": []
},
"dei_OtherReportingStandardItemNumber": {
"xbrltype": "otherReportingStandardItemNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "OtherReportingStandardItemNumber",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Other Reporting Standard Item Number",
"documentation": "\"Item 17\" or \"Item 18\" specified when the basis of accounting is neither US GAAP nor IFRS."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_PreCommencementIssuerTenderOffer": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "PreCommencementIssuerTenderOffer",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Pre-commencement Issuer Tender Offer",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act."
}
}
},
"auth_ref": [
"r7"
]
},
"dei_PreCommencementTenderOffer": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "PreCommencementTenderOffer",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Pre-commencement Tender Offer",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act."
}
}
},
"auth_ref": [
"r8"
]
},
"dei_Security12bTitle": {
"xbrltype": "securityTitleItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "Security12bTitle",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Title of 12(b) Security",
"documentation": "Title of a 12(b) registered security."
}
}
},
"auth_ref": [
"r1"
]
},
"dei_Security12gTitle": {
"xbrltype": "securityTitleItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "Security12gTitle",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Title of 12(g) Security",
"documentation": "Title of a 12(g) registered security."
}
}
},
"auth_ref": [
"r5"
]
},
"dei_SecurityExchangeName": {
"xbrltype": "edgarExchangeCodeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "SecurityExchangeName",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Security Exchange Name",
"documentation": "Name of the Exchange on which a security is registered."
}
}
},
"auth_ref": [
"r4"
]
},
"dei_SecurityReportingObligation": {
"xbrltype": "securityReportingObligationItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "SecurityReportingObligation",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Security Reporting Obligation",
"documentation": "15(d), indicating whether the security has a reporting obligation under that section of the Exchange Act."
}
}
},
"auth_ref": [
"r9"
]
},
"dei_SolicitingMaterial": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "SolicitingMaterial",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Soliciting Material",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act."
}
}
},
"auth_ref": [
"r10"
]
},
"dei_TradingSymbol": {
"xbrltype": "tradingSymbolItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "TradingSymbol",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Trading Symbol",
"documentation": "Trading symbol of an instrument as listed on an exchange."
}
}
},
"auth_ref": []
},
"dei_WrittenCommunications": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2023",
"localname": "WrittenCommunications",
"presentation": [
"http://sagimet.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Written Communications",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act."
}
}
},
"auth_ref": [
"r18"
]
}
}
}
},
"std_ref": {
"r0": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12"
},
"r1": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b"
},
"r2": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b-2"
},
"r3": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b-23"
},
"r4": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "d1-1"
},
"r5": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "g"
},
"r6": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12, 13, 15d"
},
"r7": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "13e",
"Subsection": "4c"
},
"r8": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "14d",
"Subsection": "2b"
},
"r9": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "15",
"Subsection": "d"
},
"r10": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Section": "14a",
"Number": "240",
"Subsection": "12"
},
"r11": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 10-K",
"Number": "249",
"Section": "310"
},
"r12": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 10-Q",
"Number": "240",
"Section": "308",
"Subsection": "a"
},
"r13": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Number": "249",
"Section": "220",
"Subsection": "f"
},
"r14": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Number": "249",
"Section": "240",
"Subsection": "f"
},
"r15": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Forms 10-K, 10-Q, 20-F",
"Number": "240",
"Section": "13",
"Subsection": "a-1"
},
"r16": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-T",
"Number": "232",
"Section": "405"
},
"r17": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Securities Act",
"Number": "230",
"Section": "405"
},
"r18": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Securities Act",
"Number": "230",
"Section": "425"
},
"r19": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Securities Act",
"Number": "7A",
"Section": "B",
"Subsection": "2"
}
}
}