| Quarterly Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |||||
| Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) | |||||||
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
| Large Accelerated Filer | ☐ | Accelerated Filer | ☐ | |||||||||||||||||
| ☒ | Smaller Reporting Company | |||||||||||||||||||
| Emerging Growth Company | ||||||||||||||||||||
| Page Number | ||||||||
| PART I. FINANCIAL INFORMATION | ||||||||
| PART II. OTHER INFORMATION | ||||||||
| June 30, 2025 | December 31, 2024 | ||||||||||
| ASSETS | |||||||||||
| Current assets: | |||||||||||
| Cash and cash equivalents | $ | $ | |||||||||
| Receivables from collaborative partners | |||||||||||
| Short-term investments | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Property and equipment, net | |||||||||||
| Operating lease right-of-use assets | |||||||||||
| Long-term investments | |||||||||||
| Other long-term assets | |||||||||||
| Total assets | $ | $ | |||||||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued expenses | |||||||||||
| Current portion of operating lease liability | |||||||||||
| Total current liabilities | |||||||||||
| Liability related to sale of future royalties | |||||||||||
| Operating lease liability, net of current portion | |||||||||||
| Stockholders’ equity: | |||||||||||
Preferred stock, $ | |||||||||||
Common stock, $ | |||||||||||
| Additional paid in capital | |||||||||||
| Accumulated other comprehensive (loss) gain | ( | ||||||||||
| Accumulated deficit | ( | ( | |||||||||
| Total stockholders’ (deficit) equity | ( | ||||||||||
| Total liabilities and stockholders’ equity | $ | $ | |||||||||
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2025 | 2024 | 2025 | 2024 | ||||||||||||||||||||
| $ | $ | $ | $ | ||||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| Research and development | |||||||||||||||||||||||
| General and administrative | |||||||||||||||||||||||
| Total operating expenses | |||||||||||||||||||||||
| Loss from operations | ( | ( | ( | ( | |||||||||||||||||||
| Other income (expense), net: | |||||||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Non-cash interest expense for the sale of future royalties | ( | ( | ( | ( | |||||||||||||||||||
| Other income (expense), net | ( | ||||||||||||||||||||||
| Total other expense, net | ( | ( | ( | ( | |||||||||||||||||||
| Loss before income taxes | ( | ( | ( | ( | |||||||||||||||||||
| Provision for income taxes | ( | ( | ( | ( | |||||||||||||||||||
| Net loss | ( | ( | ( | ( | |||||||||||||||||||
Unrealized (loss) gain on available for sale securities | ( | ( | |||||||||||||||||||||
| Comprehensive loss | $ | ( | $ | ( | $ | ( | $ | ( | |||||||||||||||
| Net loss per common share: | |||||||||||||||||||||||
| Basic and diluted | $ | ( | $ | ( | $ | ( | $ | ( | |||||||||||||||
| Weighted-average number of shares outstanding: | |||||||||||||||||||||||
| Basic and diluted | |||||||||||||||||||||||
| Common Stock | Additional Paid-in Capital | Accumulated Other Comprehensive Gain (Loss) | Accumulated Deficit | Total Stockholders’ (Deficit) Equity | |||||||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||||||||
| Balance, December 31, 2024 | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||||
| Issuance of common stock from exercises of options and employee stock purchase plan | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Net share settlement of restricted stock units | ( | — | ( | — | — | ( | |||||||||||||||||||||||||||||
| Repurchases and retirements of common stock | ( | — | ( | — | — | ( | |||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Comprehensive loss, net | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
| Balance, March 31, 2025 | $ | $ | $ | $ | ( | $ | |||||||||||||||||||||||||||||
| Issuance of common stock from exercises of options and employee stock purchase plan | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Repurchases and retirements of common stock | ( | ( | ( | — | — | ( | |||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Comprehensive loss, net | — | — | — | ( | — | ( | |||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
| Balance, June 30, 2025 | $ | $ | $ | ( | $ | ( | $ | ( | |||||||||||||||||||||||||||
| Common Stock | Additional Paid-in Capital | Accumulated Other Comprehensive Gain (Loss) | Accumulated Deficit | Total Stockholders’ Equity | |||||||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||
| Issuance of common stock from exercises of options and employee stock purchase plan | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Net share settlement of restricted stock units | ( | — | ( | — | — | ( | |||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Comprehensive gain, net | — | — | — | — | |||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
| Balance, March 31, 2024 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||
| Issuance of common stock from exercises of options and employee stock purchase plan | — | — | — | ||||||||||||||||||||||||||||||||
| Issuance of common stock upon vesting of restricted stock units | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | — | |||||||||||||||||||||||||||||||
| Comprehensive gain, net | — | — | — | — | |||||||||||||||||||||||||||||||
| Net loss | — | — | — | — | ( | ( | |||||||||||||||||||||||||||||
| Balance, June 30, 2024 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||||||||
| Six Months Ended June 30, | |||||||||||
| 2025 | 2024 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | |||||||||||
| Net loss | $ | ( | $ | ( | |||||||
Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
| Depreciation and amortization | |||||||||||
| Stock-based compensation | |||||||||||
| Accretion/amortization of investments, net | ( | ( | |||||||||
Amortization of right-of-use assets – operating | |||||||||||
| Non-cash interest expense | |||||||||||
| Changes in operating assets and liabilities: | |||||||||||
| Receivables from collaborative partners | ( | ||||||||||
| Prepaid expenses and other assets | |||||||||||
| Accounts payable and other liabilities | ( | ||||||||||
| Operating lease liabilities | ( | ( | |||||||||
| Net cash used in operating activities | ( | ( | |||||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | |||||||||||
| Purchases of investments | ( | ( | |||||||||
| Sales and maturities of investments | |||||||||||
| Purchases of property and equipment | ( | ( | |||||||||
| Net cash provided by investing activities | |||||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | |||||||||||
| Proceeds from issuance of common stock | |||||||||||
| Repurchase and retirements of common stock | ( | ||||||||||
| Proceeds from the sale of future royalties | |||||||||||
| Payment for net share settlement of equity awards | ( | ( | |||||||||
| Principal repayment of liability for sale of future royalties | ( | ( | |||||||||
| Payments for debt issuance costs | ( | ||||||||||
| Net cash (used in) provided by financing activities | ( | ||||||||||
| Net (decrease) increase in cash and cash equivalents | ( | ||||||||||
| Cash and cash equivalents, beginning of period | |||||||||||
| Cash and cash equivalents, end of period | $ | $ | |||||||||
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION | |||||||||||
| Interest portion of repayment for sale of future royalties | $ | $ | |||||||||
| Income taxes paid | $ | $ | |||||||||
| Non-cash investing and financing activities: | |||||||||||
| Amounts accrued for issuance costs related to the sale of future royalties | $ | $ | |||||||||
| Amounts accrued for repurchases of common stock | $ | $ | |||||||||
| Receivable related to issuance of common stock, upon exercise of stock options | $ | $ | |||||||||
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| (in thousands) | 2025 | 2024 | 2025 | 2024 | |||||||||||||||||||
| Options to purchase common stock | |||||||||||||||||||||||
Stock awards | |||||||||||||||||||||||
| Total | |||||||||||||||||||||||
| (in thousands) | June 30, 2025 | December 31, 2024 | |||||||||
| Laboratory equipment | $ | $ | |||||||||
| Office furniture and equipment | |||||||||||
| Leasehold improvements | |||||||||||
| Property and equipment, gross | |||||||||||
| Less: accumulated depreciation and amortization | ( | ( | |||||||||
| Total property and equipment, net | $ | $ | |||||||||
| (in thousands) | June 30, 2025 | December 31, 2024 | |||||||||
| Accrued compensation and related expenses | $ | $ | |||||||||
| Accrued professional fees and other expenses | |||||||||||
| Accrued research, development and manufacturing expenses | |||||||||||
| Accrued for repurchases of common stock | |||||||||||
| Total accrued expenses | $ | $ | |||||||||
PD-1 (Jemperli/Dostarlimab) | TIM-3 (GSK4069889/Cobolimab) | ||||||||||||||||
| Milestone Event | Amount | Quarter Recognized | Amount | Quarter Recognized | |||||||||||||
Initiated in vivo toxicology studies using good laboratory practices (GLPs) | $ | Q2'15 | $ | Q4'15 | |||||||||||||
| IND clearance from the FDA | $ | Q1'16 | $ | Q2'16 | |||||||||||||
| Phase 2 clinical trial initiation | $ | Q2'17 | $ | Q4'17 | |||||||||||||
| Phase 3 clinical trial initiation - first indication | $ | Q3'18 | $ | Q4'22 | |||||||||||||
| Phase 3 clinical trial initiation - second indication | $ | Q2'19 | $ | — | |||||||||||||
Filing of the first BLA(1) - first indication | $ | Q1'20 | $ | — | |||||||||||||
Filing of the first MAA(2) - first indication | $ | Q1'20 | $ | — | |||||||||||||
Filing of the first BLA - second indication | $ | Q1'21 | $ | — | |||||||||||||
| First BLA approval - first indication | $ | Q2'21 | $ | — | |||||||||||||
First MAA approval - first indication | $ | Q2'21 | $ | — | |||||||||||||
| First BLA approval - second indication | $ | Q3'21 | $ | — | |||||||||||||
Filing of the first MAA - second indication(3) | $ | — | $ | — | |||||||||||||
First MAA approval - second indication(3) | $ | — | $ | — | |||||||||||||
First commercial sales milestone(3) | $ | Q3'24 | $ | — | |||||||||||||
Second commercial sales milestone(3) | $ | Q4'24 | $ | — | |||||||||||||
Third commercial sales milestone(3) | $ | — | $ | — | |||||||||||||
Fourth commercial sales milestone (4) | $ | — | $ | — | |||||||||||||
| Milestones recognized through June 30, 2025 | $ | — | $ | — | |||||||||||||
| Milestones that may be recognized in the future | $ | — | $ | — | |||||||||||||
Milestone Event | Amount | Quarter Recognized | ||||||
FDA regulatory approval for marketing of first licensed product in the USA for the treatment of active flares in GPP | $ | — | ||||||
Regulatory approval for marketing of the first licensed product in the EU | $ | — | ||||||
Commercial sales first exceed $ | $ | — | ||||||
| Milestones recognized through June 30, 2025 | — | |||||||
Milestones that may be recognized in the future | $ | — | ||||||
| (in thousands) | June 30, 2025 | |||||||
| Liability related to sale of future Jemperli royalties and milestones – balance at 12/31/2024 | $ | |||||||
| Amortization of issuance costs | ||||||||
| Royalty and milestone payments to Sagard | ( | |||||||
Non-cash interest expense recognized(1) | ||||||||
Liability related to sale of future royalties and milestones – ending balance | $ | |||||||
| (in thousands) | June 30, 2025 | |||||||
| Liability related to sale of future Zejula royalties and milestones – balance at 12/31/2024 | $ | |||||||
| Amortization of issuance costs | ||||||||
| Royalty and milestone payments to DRI | ( | |||||||
Non-cash interest expense recognized(1) | ||||||||
| Liability related to sale of future royalties and milestones – ending balance | $ | |||||||
| Fair Value Measurements at End of Period Using: | |||||||||||||||||||||||
| (in thousands) | Fair Value | Quoted Market Prices for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||||||||
| At June 30, 2025 | |||||||||||||||||||||||
Money market funds(1) | $ | $ | $ | $ | |||||||||||||||||||
Mutual funds(1) | |||||||||||||||||||||||
U.S. Treasury securities(2) | |||||||||||||||||||||||
Agency securities(2) | |||||||||||||||||||||||
Commercial and corporate obligations(2) | |||||||||||||||||||||||
| At December 31, 2024 | |||||||||||||||||||||||
Money market funds(1) | $ | $ | $ | $ | |||||||||||||||||||
Mutual funds(1) | |||||||||||||||||||||||
U.S. Treasury securities(1)(2) | |||||||||||||||||||||||
Agency securities(2) | |||||||||||||||||||||||
Commercial and corporate obligations(2) | |||||||||||||||||||||||
| (in thousands) | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Total Fair Value | |||||||||||||||||||
Agency securities(1) | $ | $ | $ | ( | $ | ||||||||||||||||||
Commercial and corporate obligations(2) | ( | ||||||||||||||||||||||
U.S. Treasury securities(3) | ( | ||||||||||||||||||||||
| Total available for sale investments | $ | $ | $ | ( | $ | ||||||||||||||||||
| (in thousands) | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Total Fair Value | |||||||||||||||||||
Agency securities(1) | $ | $ | $ | ( | $ | ||||||||||||||||||
Commercial and corporate obligations(2) | |||||||||||||||||||||||
U.S. Treasury securities(3) | ( | ||||||||||||||||||||||
| Total available for sale investments | $ | $ | $ | ( | $ | ||||||||||||||||||
| June 30, 2025 | |||||||||||||||||||||||||||||||||||
| Less than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||||||||||||||
| (in thousands) | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | |||||||||||||||||||||||||||||
| Agency securities | $ | $ | ( | $ | $ | $ | $ | ( | |||||||||||||||||||||||||||
| Commercial and corporate obligations | ( | ( | |||||||||||||||||||||||||||||||||
| U.S. Treasury Securities | ( | ( | |||||||||||||||||||||||||||||||||
Total | $ | $ | ( | $ | $ | $ | $ | ( | |||||||||||||||||||||||||||
| December 31, 2024 | |||||||||||||||||||||||||||||||||||
| Less than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||||||||||||||
| (in thousands) | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | |||||||||||||||||||||||||||||
| Agency securities | $ | $ | ( | $ | $ | $ | $ | ( | |||||||||||||||||||||||||||
| U.S. Treasury Securities | ( | ( | |||||||||||||||||||||||||||||||||
Total | $ | $ | ( | $ | $ | $ | $ | ( | |||||||||||||||||||||||||||
| Total number of shares purchased | Average price paid per share | Approximate dollar value of shares purchased (in thousands) | |||||||||||||||
| First Quarter 2025 | $ | $ | |||||||||||||||
| Second Quarter 2025 | |||||||||||||||||
| Total | $ | ||||||||||||||||
Shares Subject to Options | Weighted-Average Exercise Price per Share | Weighted-Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||||||
Outstanding at January 1, 2025 | $ | $ | |||||||||||||||||||||
| Granted | $ | ||||||||||||||||||||||
| Exercised | ( | $ | |||||||||||||||||||||
| Forfeitures and cancellations | ( | $ | |||||||||||||||||||||
| Outstanding at June 30, 2025 | $ | $ | |||||||||||||||||||||
| Exercisable at June 30, 2025 | $ | $ | |||||||||||||||||||||
| Number of Restricted Stock Units | Weighted-Average Grant Date Fair Value | Weighted-Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value (in thousands) | ||||||||||||||||||||
| Outstanding at January 1, 2025 | $ | $ | |||||||||||||||||||||
| Granted | $ | ||||||||||||||||||||||
| Released | ( | $ | |||||||||||||||||||||
| Forfeitures and cancellations | ( | $ | |||||||||||||||||||||
| Outstanding at June 30, 2025 | $ | $ | |||||||||||||||||||||
| RSU expected to vest at June 30, 2025 | $ | $ | |||||||||||||||||||||
| Six months ended June 30, | ||||||||
| 2025 | ||||||||
| Volatility of common stock | % | |||||||
| Risk-free interest rate | % | |||||||
| Contract term (in years) | ||||||||
| Number of Performance Stock Units | Weighted-Average Grant Date Fair Value | Weighted-Average Remaining Contractual Term (in years) | |||||||||||||||
| Outstanding at January 1, 2025 | $ | ||||||||||||||||
| Granted | $ | ||||||||||||||||
| Released | $ | ||||||||||||||||
| Forfeitures | ( | $ | |||||||||||||||
| Outstanding at June 30, 2025 | $ | ||||||||||||||||
| Six Months Ended June 30, | |||||||||||||||||||||||
| 2025 | 2024 | ||||||||||||||||||||||
| Risk-free interest rate | % | % | |||||||||||||||||||||
| Expected volatility | % | % | |||||||||||||||||||||
| Expected dividend yield | % | % | |||||||||||||||||||||
| Expected term (in years) | |||||||||||||||||||||||
| Weighted-average grant date fair value per share | $ | $ | |||||||||||||||||||||
| Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||||||||||||
| (in thousands) | 2025 | 2024 | 2025 | 2024 | ||||||||||||||||||||||
| Research and development | $ | $ | $ | $ | ||||||||||||||||||||||
| General and administrative | (1) | |||||||||||||||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||||||||||||
| Six Months Ended June 30, | ||||||||||||||||||||
| Leases | Classification on the Cash Flow | 2025 | 2024 | |||||||||||||||||
| Operating lease cost | Operating | $ | $ | |||||||||||||||||
| Cash paid for amounts included in the measurement of lease liabilities | Operating | |||||||||||||||||||
| Years Ending December 31, | |||||
| 2025 | $ | ||||
| 2026 | |||||
| 2027 | |||||
| 2028 | |||||
| 2029 | |||||
| Thereafter | |||||
| Total minimum payments required | |||||
| Less imputed interest | ( | ||||
| Total | $ | ||||
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2025 | 2024 | 2025 | 2024 | ||||||||||||||||||||
| Collaboration revenue | $ | $ | $ | $ | |||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| External R&D | |||||||||||||||||||||||
| Rosnilimab | |||||||||||||||||||||||
| ANB033 | |||||||||||||||||||||||
| ANB101 | |||||||||||||||||||||||
| ANB032 | |||||||||||||||||||||||
| Imsidolimab | ( | ( | |||||||||||||||||||||
| Preclinical and other unallocated costs | |||||||||||||||||||||||
Total External R&D(1) | |||||||||||||||||||||||
Total Internal R&D(2) | |||||||||||||||||||||||
| Total R&D | |||||||||||||||||||||||
External G&A(3) | |||||||||||||||||||||||
Internal G&A(2) | |||||||||||||||||||||||
| Total G&A | |||||||||||||||||||||||
| Total operating expenses | |||||||||||||||||||||||
| Loss from operations | ( | ( | ( | ( | |||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Non-cash interest expense | ( | ( | ( | ( | |||||||||||||||||||
| Other income (expense), net | ( | ||||||||||||||||||||||
Total other expense, net | ( | ( | ( | ( | |||||||||||||||||||
| Loss before income taxes | ( | ( | ( | ( | |||||||||||||||||||
Provision for income taxes | ( | ( | ( | ( | |||||||||||||||||||
| Segment net loss | $ | ( | $ | ( | $ | ( | $ | ( | |||||||||||||||

| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||||||||||||||
| (in thousands) | 2025 | 2024 | Increase/(Decrease) | 2025 | 2024 | Increase/(Decrease) | |||||||||||||||||||||||||||||
| External Costs | |||||||||||||||||||||||||||||||||||
| Rosnilimab | $ | 15,614 | $ | 12,926 | $ | 2,688 | $ | 30,639 | $ | 22,965 | $ | 7,674 | |||||||||||||||||||||||
| ANB033 | 3,925 | 2,982 | 943 | 7,729 | 5,645 | 2,084 | |||||||||||||||||||||||||||||
| ANB101 | 2,040 | 544 | 1,496 | 3,521 | 944 | 2,577 | |||||||||||||||||||||||||||||
| ANB032 | 108 | 7,584 | (7,476) | 3,644 | 12,451 | (8,807) | |||||||||||||||||||||||||||||
| Imsidolimab | (967) | 1,867 | (2,834) | (1,156) | 6,363 | (7,519) | |||||||||||||||||||||||||||||
| Preclinical and other unallocated costs | 4,456 | 4,693 | (237) | 8,388 | 8,037 | 351 | |||||||||||||||||||||||||||||
| Total External Costs | 25,176 | 30,596 | (5,420) | 52,765 | 56,405 | (3,640) | |||||||||||||||||||||||||||||
| Internal Costs | 12,648 | 11,401 | 1,247 | 26,239 | 22,634 | 3,605 | |||||||||||||||||||||||||||||
| Total Costs | $ | 37,824 | $ | 41,997 | $ | (4,173) | $ | 79,004 | $ | 79,039 | $ | (35) | |||||||||||||||||||||||
| Six Months Ended June 30, | |||||||||||
| (in thousands) | 2025 | 2024 | |||||||||
| Net cash (used in) provided by: | |||||||||||
| Operating activities | $ | (50,944) | $ | (58,575) | |||||||
| Investing activities | 51,250 | 66,174 | |||||||||
| Financing activities | (79,088) | 28,257 | |||||||||
| Net (decrease) increase in cash and cash equivalents | $ | (78,782) | $ | 35,856 | |||||||
| Period | (a) Total number of shares repurchased | (b) Average price paid per share | (c) Total number of shares repurchased as part of publicly announced plans or programs | (d) Maximum approximate dollar value of shares that may yet be purchased under the plans or programs (in thousands) | ||||||||||||||||||||||
| April 1, 2025 - April 30, 2025 | 1,007,397 | $ | 18.19 | 1,007,397 | $ | 51,303 | ||||||||||||||||||||
| May 1, 2025 - May 31, 2025 | 1,456,709 | $ | 20.42 | 1,456,709 | $ | 21,555 | ||||||||||||||||||||
| June 1, 2025 - June 30, 2025 | 96,832 | $ | 21.45 | 96,832 | $ | 19,478 | ||||||||||||||||||||
| 2,560,938 | 2,560,938 | |||||||||||||||||||||||||
Exhibit Number | Exhibit Description | |||||||
| 31.1 | ||||||||
| 31.2 | ||||||||
32.1** | ||||||||
32.2** | ||||||||
101.INS | Inline XBRL Report Instance Document - The Instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | |||||||
101.SCH | Inline XBRL Taxonomy Extension Schema Document | |||||||
101.CAL | Inline XBRL Taxonomy Calculation Linkbase Document | |||||||
101.LAB | Inline XBRL Taxonomy Label Linkbase Document | |||||||
101.PRE | Inline XBRL Presentation Linkbase Document | |||||||
101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | |||||||
| 104 | Cover Page Interactive Data File - (formatted in Inline XBRL and contained in Exhibit 101) | |||||||
| AnaptysBio, Inc. | |||||||||||
| Date: | August 6, 2025 | By: | /s/ Daniel Faga | ||||||||
| Daniel Faga | |||||||||||
| President and Chief Executive Officer | |||||||||||
| (Principal Executive Officer) | |||||||||||
| Date: | August 6, 2025 | By: | /s/ Dennis Mulroy | ||||||||
| Dennis Mulroy | |||||||||||
| Chief Financial Officer | |||||||||||
| (Principal Financial and Accounting Officer) | |||||||||||
| /s/ Daniel Faga | ||
| Daniel Faga | ||
| President and Chief Executive Officer | ||
(Principal Executive Officer) | ||
| /s/ Dennis Mulroy | ||
| Dennis Mulroy | ||
| Chief Financial Officer | ||
| (Principal Financial Officer) | ||
| /s/ Daniel Faga | ||
| Daniel Faga | ||
| President and Chief Executive Officer | ||
(Principal Executive Officer) | ||
| /s/ Dennis Mulroy | ||
| Dennis Mulroy | ||
| Chief Financial Officer | ||
| (Principal Financial Officer) | ||
Consolidated Balance Sheets (Parenthetical) - $ / shares |
Jun. 30, 2025 |
Dec. 31, 2024 |
|---|---|---|
| Statement of Financial Position [Abstract] | ||
| Preferred stock, par value (in dollars per share) | $ 0.001 | $ 0.001 |
| Preferred stock, shares authorized (in shares) | 10,000,000 | 10,000,000 |
| Preferred stock, shares issued (in shares) | 0 | 0 |
| Preferred stock, shares outstanding (in shares) | 0 | 0 |
| Common stock, par value (in dollars per share) | $ 0.001 | $ 0.001 |
| Common stock, shares authorized (in shares) | 500,000,000 | 500,000,000 |
| Common stock, shares issued (in shares) | 27,972,997 | 30,473,000 |
| Common stock, shares outstanding (in shares) | 27,972,997 | 30,473,000 |
Consolidated Statements of Operations and Comprehensive Loss - USD ($) shares in Thousands, $ in Thousands |
3 Months Ended | 6 Months Ended | ||
|---|---|---|---|---|
Jun. 30, 2025 |
Jun. 30, 2024 |
Jun. 30, 2025 |
Jun. 30, 2024 |
|
| Income Statement [Abstract] | ||||
| Revenue from Contract with Customer, Product and Service [Extensible Enumeration] | Collaboration Revenue [Member] | Collaboration Revenue [Member] | Collaboration Revenue [Member] | Collaboration Revenue [Member] |
| Collaboration revenue | $ 22,263 | $ 10,971 | $ 50,034 | $ 18,150 |
| Operating expenses: | ||||
| Research and development | 37,824 | 41,997 | 79,004 | 79,039 |
| General and administrative | 10,609 | 9,295 | 24,739 | 21,633 |
| Total operating expenses | 48,433 | 51,292 | 103,743 | 100,672 |
| Loss from operations | (26,170) | (40,321) | (53,709) | (82,522) |
| Other income (expense), net: | ||||
| Interest income | 3,654 | 4,623 | 8,067 | 9,207 |
| Non-cash interest expense for the sale of future royalties | (19,606) | (10,953) | (37,667) | (17,270) |
| Other income (expense), net | 3,531 | 0 | 5,433 | (2) |
| Total other expense, net | (12,421) | (6,330) | (24,167) | (8,065) |
| Loss before income taxes | (38,591) | (46,651) | (77,876) | (90,587) |
| Provision for income taxes | (39) | (9) | (83) | (9) |
| Net loss | (38,630) | (46,660) | (77,959) | (90,596) |
| Unrealized (loss) gain on available for sale securities | (167) | 209 | (311) | 382 |
| Comprehensive loss | $ (38,797) | $ (46,451) | $ (78,270) | $ (90,214) |
| Net loss per common share: | ||||
| Basic (in dollars per share) | $ (1.34) | $ (1.71) | $ (2.62) | $ (3.35) |
| Diluted (in dollars per share) | $ (1.34) | $ (1.71) | $ (2.62) | $ (3.35) |
| Weighted-average number of shares outstanding: | ||||
| Basic (in shares) | 28,810 | 27,356 | 29,722 | 27,079 |
| Diluted (in shares) | 28,810 | 27,356 | 29,722 | 27,079 |
Description of the Business |
6 Months Ended |
|---|---|
Jun. 30, 2025 | |
| Organization, Consolidation and Presentation of Financial Statements [Abstract] | |
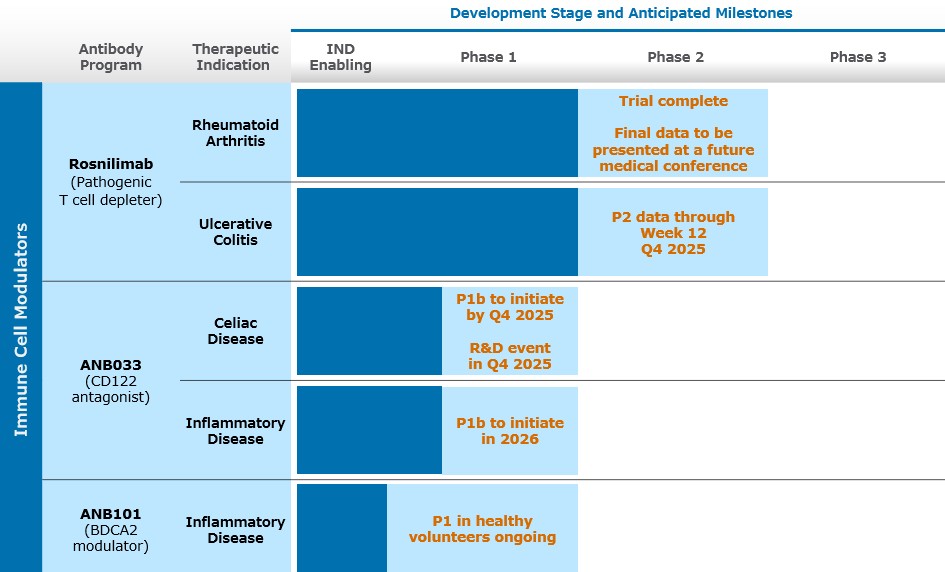
| Description of the Business | Description of the Business AnaptysBio, Inc. (“we,” “us,” “our,” or the “company”) was incorporated in the state of Delaware in November 2005. We are a clinical-stage biotechnology company focused on delivering innovative immunology therapeutics for autoimmune and inflammatory diseases. Our pipeline includes our lead program, rosnilimab, a selective and potent pathogenic T cell depleter, that completed a Phase 2b trial for the treatment of moderate-to-severe rheumatoid arthritis (“RA”) and is in a Phase 2 trial for the treatment of moderate-to-severe ulcerative colitis (“UC”). We also have other antibodies in our portfolio, including ANB033, a CD122 antagonist, in celiac disease (“CeD”), and ANB101, a BDCA2 modulator, both in Phase 1 trials. We have also discovered and out-licensed in financial collaborations multiple therapeutic antibodies, including a PD-1 antagonist (Jemperli (dostarlimab-gxly) or “Jemperli”) to GSK and an IL-36R antagonist (imsidolimab) to Vanda Pharmaceuticals Inc. (“Vanda”). We currently recognize revenue from milestones and royalties achieved under our immuno-oncology collaboration with GSK and license and transition services revenue from our collaboration with Vanda . Since our inception, we have devoted our primary effort to research and development activities. Our financial support has been provided primarily from the sale of our common stock and royalty monetizations, as well as through funds received under our collaborative research and development agreements. Going forward, as we continue our expansion, we may seek additional financing and/or strategic investments. However, there can be no assurance that any additional financing or strategic investments will be available to us on acceptable terms, if at all. If events or circumstances occur such that we do not obtain additional funding, we will most likely be required to reduce our plans and/or certain discretionary spending, which could have a material adverse effect on our ability to achieve our intended business objectives. Our management believes our currently available resources will provide sufficient funds to enable us to meet our operating plans for at least the next 12 months from the issuance of our consolidated financial statements. The accompanying consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern.
|
Summary of Significant Accounting Policies |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accounting Policies [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Summary of Significant Accounting Policies | Summary of Significant Accounting Policies Basis of Presentation The accompanying unaudited consolidated financial statements have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). Certain information and note disclosures normally included in annual financial statements prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) have been omitted. The accompanying unaudited consolidated financial statements include all known adjustments necessary for a fair presentation of the results of interim periods as required by U.S. GAAP. These adjustments consist primarily of normal recurring accruals and estimates that impact the carrying value of assets and liabilities. Operating results for the six months ended June 30, 2025 are not necessarily indicative of the results that may be expected for the year ending December 31, 2025. The financial statements should be read in conjunction with our audited financial statements for the year ended December 31, 2024 included in our Annual Report on Form 10-K. Basis of Consolidation The accompanying consolidated financial statements include us and our wholly owned Australian subsidiary, which was deregistered with the Australian Securities & Investments Commission as of June 30, 2025. The deregistration did not have a material impact on our consolidated financial statements. All intercompany accounts and transactions have been eliminated in consolidation. We operate in one reportable segment, and our functional and reporting currency is the U.S. dollar. Use of Estimates The preparation of the accompanying consolidated financial statements in conformity with U.S. GAAP requires our management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. We base our estimates and assumptions on historical experience when available and on various factors that we believe to be reasonable under the circumstances. Significant estimates relied upon in preparing these financial statements include estimates related to revenue recognition, accrued research and development expenses, stock-based compensation, and the liability related to the sale of future royalties. We evaluate our estimates and assumptions on an ongoing basis. Our actual results could differ from these estimates under different assumptions or conditions. Net Loss Per Common Share Basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing net loss by the weighted-average number of common equivalent shares outstanding for the period, as well as any dilutive effect from outstanding stock options and warrants using the treasury stock method. For each period presented, there is no difference in the number of shares used to calculate basic and diluted net loss per share. The following table sets forth the weighted-average outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per share because to do so would be anti-dilutive (in common stock equivalent shares):
Accounting Pronouncements We have implemented all new accounting pronouncements that are in effect and may have an impact on our consolidated financial statements. Unless otherwise discussed, we believe the impact of any recently issued and not yet effective pronouncements will not have a material impact on our consolidated financial statements. Recent Accounting Pronouncements Not Yet Adopted In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures Income Taxes, which improves the transparency of income tax disclosures by requiring consistent categories and greater disaggregation of information in the effective tax rate reconciliation and income taxes paid disaggregated by jurisdiction. It also includes certain other amendments to improve the effectiveness of income tax disclosures. This guidance will be effective for the annual periods beginning after December 15, 2024. Early adoption is permitted. Upon adoption, the guidance can be applied prospectively or retrospectively. We are currently assessing the impact that this standard will have on our consolidated financial statements. In November 2024, the FASB issued ASU No. 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses, which requires disclosure about the types of costs and expenses included in certain expense captions presented on the income statement. The new disclosure requirements are effective for annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027. Early adoption is permitted. We are currently evaluating the provisions of this guidance and assessing the potential impact on our financial statement disclosures.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Balance Sheet Accounts and Supplemental Disclosures |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance Sheet Related Disclosures [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance Sheet Accounts and Supplemental Disclosures | Balance Sheet Accounts and Supplemental Disclosures Property and Equipment, Net Property and equipment, net consist of the following:
Accrued Expenses Accrued expenses consist of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Collaborative Research and Development Agreements |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenue from Contract with Customer [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Collaborative Research and Development Agreements | Collaborative Research and Development Agreements GSK Collaboration In March 2014, we entered into a Collaboration and Exclusive License Agreement (the “GSK Agreement”) with TESARO, Inc. (“Tesaro”), an oncology-focused biopharmaceutical company now a part of GSK (Tesaro and GSK are hereinafter referred to, collectively, as “GSK”). Currently, under the GSK Agreement, GSK is developing Jemperli (dostarlimab) as a monotherapy, and in combination with additional therapies, for various solid tumor indications. For Jemperli and cobolimab, a TIM-3 antibody, each remaining development programs under the GSK Agreement, we are eligible to receive milestone payments if certain clinical trial events are achieved by GSK, if certain U.S. and European regulatory submissions and approvals in multiple indications are achieved, and upon the achievement of specified levels of annual worldwide net sales. We will also be eligible to receive tiered 4-8% royalties related to worldwide net sales of products developed under the collaboration. On October 23, 2020, Amendment No. 3 to the GSK Agreement (the “GSK Amendment No. 3”) was agreed to by both parties to permit GSK to conduct development and commercialization in combination with any third party molecules of Zejula, an oral, once-daily poly (ADP-ribose) polymerase (PARP) inhibitor (“Zejula”). Under GSK Amendment No. 3, we were granted increased royalties upon sales of Jemperli, equal to 8% of net sales (as defined in the GSK Agreement) below $1.0 billion, 12% of net sales between $1.0 billion and $1.5 billion, 20% of net sales between $1.5 billion and $2.5 billion and 25% of net sales above $2.5 billion. Unless earlier terminated by either party upon specified circumstances, the GSK Agreement will terminate, with respect to each specific developed product, upon the later of the 12th anniversary of the first commercial sale of the product or the expiration of the last to expire of any patent. We assessed these arrangements in accordance with Accounting Standards Codification (“ASC”) 606 and concluded that the contract counterparty, GSK, is a customer. We identified the following material promises under the GSK Agreement: (1) the licenses under certain patent rights and transfer of certain development and regulatory information, (2) research and development (“R&D”) services, and (3) joint steering committee meetings. We considered the research and discovery capabilities of GSK for these specific programs and the fact that the discovery and optimization of these antibodies is proprietary and could not, at the time of contract inception, be provided by other vendors, to conclude that the license does not have stand-alone functionality and is therefore not distinct. Additionally, we determined that the joint steering committee participation would not have been provided without the R&D services and GSK Agreement. Based on these assessments, we identified all services to be interrelated and therefore concluded that the promises should be combined into a single performance obligation at the inception of the arrangement. As of June 30, 2025, the transaction price for the GSK Agreement and its associated amendments includes the upfront payment, research reimbursement revenue and milestones and royalties earned to date, which are allocated in their entirety to the single performance obligation. We recognized $22.2 million and $40.4 million in royalty revenue during the three and six months ended June 30, 2025, respectively, related to GSK’s net sales of Jemperli and Zejula during the period, which we estimate based on either GSK’s prior sales experience or actuals. Of the royalty revenue recognized during the three and six months ended June 30, 2025, $21.1 million and $38.4 million, respectively, is Jemperli non-cash revenue related to the Jemperli Royalty Monetization Agreement (as amended) and $1.1 million and $2.0 million, respectively, is Zejula non-cash revenue related to the Zejula Royalty Monetization Agreement, each of such agreements as described in Note 5. We recognized $11.0 million and $18.2 million, in royalty revenue during the three and six months ended June 30, 2024, respectively, related to GSK’s net sales of Jemperli and Zejula during the period based on GSK’s prior sales experience or actuals. Of the royalty revenue recognized during the three and six months ended June 30, 2024, $10.1 million and $16.3 million, respectively, is Jemperli non-cash revenue related to the Jemperli Royalty Monetization Agreement (as amended) and $0.9 million and $1.9 million, respectively, is Zejula non-cash revenue related to the Zejula Royalty Monetization Agreement. GSK reports sales information to us on a one quarter lag and differences between actual and estimated royalty revenues will be adjusted in the following quarter. No clinical milestones were recognized during the three and six months ended June 30, 2025 and 2024. No other future clinical or regulatory milestones have been included in the transaction price, as all milestone amounts were subject to the revenue constraint. As part of the constraint evaluation, we considered numerous factors including the fact that the receipt of milestones is outside of our control and contingent upon success in future clinical trials, an outcome that is difficult to predict, and GSK’s efforts. Any consideration related to sales-based milestones, including royalties, will be recognized when the related sales occur as they were determined to relate predominantly to the intellectual property license granted to GSK and therefore have also been excluded from the transaction price. We will re-evaluate the variable transaction price in each reporting period and as uncertain events are resolved or other changes in circumstances occur. Milestones under the GSK Agreement are as follows:
(1)Biologics License Application (“BLA”) (2)Marketing Authorization Application (“MAA”) (3)For Jemperli, the filing and approval of the first MAA for a second indication and first three commercial sales milestones are included as part of the royalty monetization agreement with Sagard (as defined below), see Note 5. Cash is generally received within 30 days of milestone achievement. (4)For Jemperli, we retained the rights to a $75.0 million sales milestone when Jemperli annual net sales exceed $1.0 billion. Vanda Collaboration On January 31, 2025, we entered into an Exclusive License Agreement (the “Vanda License Agreement”) with Vanda pursuant to which we granted to Vanda an exclusive, global license for the development and commercialization of imsidolimab (IL-36R antagonist mAb), which has completed two registration-enabling global Phase 3 trials, GEMINI-1 and GEMINI-2, evaluating the safety and efficacy of imsidolimab in patients with Generalized Pustular Psoriasis (GPP). Pursuant to the terms of the Vanda License Agreement, we received an upfront payment of $10.0 million for the license and a $5.0 million payment for existing drug supply. We allocated the total transaction price of $15.0 million on a relative standalone selling price in accordance with ASC 606. During the six months ended June 30, 2025, we recognized $9.6 million of license revenue under ASC 606 and recognized no license revenue under ASC 606 during the three months ended June 30, 2025. During the three and six months ended June 30, 2025, we recognized less than $0.1 million and $0.1 million, respectively, of transition services revenue under ASC 606 and $3.5 million and $5.4 million, respectively, related to existing drug supply transferred to Vanda as other income under ASC 610. We expensed the $2.5 million of related transaction costs within general and administrative expenses, during the six months ended June 30, 2025, as we elected the practical expedient to expense the transaction costs as incurred as the expected amortization period was less than a year. We are also eligible to receive a 10% royalty on net sales, as well as the following milestones under the Vanda License Agreement:
Centessa On November 24, 2023, we entered into an exclusive license agreement (as amended, the “Centessa Agreement”) with Centessa Pharmaceuticals (UK) Limited (“Centessa”), pursuant to which we acquired the exclusive global development and commercialization rights to a blood dendritic cell antigen 2 (BDCA2) modulator antibody portfolio, including lead asset CBS004 (renamed ANB101), CBS008 (renamed ANB102) and the related family of backup antibodies, for the treatment of autoimmune and inflammatory diseases. In connection with the Centessa Agreement, we paid Centessa an upfront cash payment of $4.0 million and an additional cash payment of $3.0 million as reimbursement to Centessa for manufacturing costs incurred. There were $0.3 million in transaction costs incurred. The total transaction amount of $7.3 million was expensed as in-process research and development and classified as an operating activity in the statement of cash flows. We accounted for the transaction as an asset acquisition as the set of acquired assets did not constitute a business. Under the terms of the agreement, Centessa may be entitled to receive potential future payments of up to $10.0 million upon the achievement of a certain event-based milestone and would be entitled to receive on a product-by-product and country-by-country basis, a royalty of low single digits on annual net sales of any product in the territory in each calendar year. As of June 30, 2025, achievement of the milestone is not probable and, therefore, we have not recognized a liability for the associated $10.0 million contingent consideration.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sale of Future Royalties |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Liabilities Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sale of Future Royalties | Sale of Future Royalties Jemperli Royalty Monetization Agreement In October 2021, we signed a royalty monetization agreement (“Jemperli Royalty Monetization Agreement”) with Sagard Healthcare Royalty Partners, LP (“Sagard”). Under the terms of the Jemperli Royalty Monetization Agreement, we received $250.0 million in exchange for royalties and milestones payable to us under our GSK collaboration on annual global net sales of Jemperli. In May 2024, we entered into an amendment to the Jemperli Royalty Monetization Agreement, Amendment No. 1 (the “Jemperli Amendment”) under which we sold additional receivables to Sagard in exchange for $50.0 million. The Jemperli Amendment includes all Jemperli sales, including any product containing Jemperli, whether or not such product constitutes a combination product, and the threshold amounts of aggregate Jemperli royalties and milestones to be received by Sagard under the Jemperli Amendment is either $600.0 million if received by the end of March 31, 2031, or $675.0 million if received thereafter. Once either of these thresholds are met, the Jemperli Royalty Monetization Agreement and the Jemperli Amendment will expire, resulting in us regaining all subsequent Jemperli royalties and milestones. As of June 30, 2025, Sagard has received a total of $121.5 million in royalties and milestones. We retained the rights to a $75.0 million sales milestone when Jemperli annual net sales exceed $1.0 billion. The proceeds received from Sagard of $250.0 million and $50.0 million were recorded as a nonrecourse liability, net of transaction costs of $0.4 million and $0.1 million, which will be amortized over the estimated life of the arrangement using the effective interest rate method. The aggregate future estimated payments, less the $299.5 million, net of proceeds, will be recognized as non-cash interest expense over the life of the agreement. Royalty and milestone revenue will be recognized as earned on net sales of Jemperli, and these payments to Sagard will be recorded as a reduction of the liability when paid. As such payments are made to Sagard, the balance of the liability will be effectively repaid over the life of the Jemperli Royalty Monetization Agreement. We estimate the effective interest rate used to record non-cash interest expense under the Jemperli Royalty Monetization Agreement based on the estimate of future royalty payments to be received by Sagard. As of June 30, 2025, the estimated effective rate under the agreement was 28.9%. Over the life of the arrangement, the actual effective interest rate will be affected by the amount and the timing of the royalty payments received by Sagard and changes in our forecasted royalties. At each reporting date, we will reassess our estimate of total future royalty payments to be received and if such payments are materially different than our prior estimates, we will prospectively adjust the imputed interest rate and the related amortization of the royalty obligation. We recognized Jemperli non-cash royalty revenue of approximately $21.1 million and $38.4 million during the three and six months ended June 30, 2025, respectively and approximately $10.1 million and $16.3 million during the three and six months ended June 30, 2024, respectively. We recognized non-cash interest expense of approximately $19.3 million and $37.2 million during the three and six months ended June 30, 2025, respectively, and $10.7 million and $16.8 million during the three and six months ended June 30, 2024. The interest and amortization of issuance costs are reflected as non-cash interest expense for the sale of future royalties in the Consolidated Statements of Operations. The following table shows the activity within the liability account for the six months ended June 30, 2025:
(1) Of the non-cash interest expense recognized, $0.6 million was negative amortization for the six months ended June 30, 2025. Zejula Royalty Monetization Agreement In October 2020, in connection with GSK Amendment No. 3, GSK agreed, under the terms of a settlement agreement (the “GSK Settlement Agreement”), to pay us a royalty of 0.5% on all GSK net sales of Zejula starting January 1, 2021. In September 2022, we signed a purchase and sale agreement (the “Zejula Royalty Monetization Agreement”) with a wholly owned subsidiary of DRI to monetize all of our future royalties on global net sales of Zejula under the GSK Settlement Agreement. Under the terms of the Zejula Royalty Monetization Agreement, we received $35.0 million in exchange for all royalties payable by GSK to us under the GSK Settlement Agreement on global net sales of Zejula starting in July 2022. In addition, under the Zejula Royalty Monetization Agreement, we are entitled to receive an additional $10.0 million payment from DRI if Zejula is approved by the U.S. Food and Drug Administration for the treatment of endometrial cancer on or prior to December 31, 2025. The proceeds received from DRI of $35.0 million were recorded as a nonrecourse liability, net of transaction costs of $0.2 million, which will be amortized over the estimated life of the arrangement using the effective interest rate method. Royalty revenue will be recognized as earned on net sales of Zejula, and these royalty payments to DRI will be recorded as a reduction of the liability when paid. The aggregate future estimated payments, less the $34.8 million, of net proceeds, will be recorded as non-cash interest expense over the life of the agreement. As such payments are made to DRI, the balance of the liability will be effectively repaid over the life of the Zejula Royalty Monetization Agreement. We recognized Zejula non-cash royalty revenue of approximately $1.1 million and $2.0 million during the three and six months ended June 30, 2025, respectively, and $0.9 million and $1.9 million during the three and six months ended June 30, 2024, respectively. We recognized non-cash interest expense of approximately $0.3 million and $0.5 million during the three and six months ended June 30, 2025, respectively, and $0.2 million and $0.4 million during the three and six months ended June 30, 2024, respectively. The interest and amortization of issuance costs is reflected as non-cash interest expense for the sale of future royalties in the Consolidated Statements of Operations. The following table shows the activity within the liability account for the six months ended June 30, 2025:
(1) Of the non-cash interest expense recognized, none was negative amortization for the six months ended June 30, 2025.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fair Value Measurements and Available for Sale Investments |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value Disclosures [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value Measurements and Available for Sale Investments | Fair Value Measurements and Available for Sale Investments Fair Value Measurements Our financial instruments consist principally of cash, cash equivalents, short-term and long-term investments, receivables, and accounts payable. Certain of our financial assets and liabilities have been recorded at fair value in the consolidated balance sheet in accordance with the accounting standards for fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants on the measurement date. Accounting guidance also establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The standard describes three levels of inputs that may be used to measure fair value: Level 1 – Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets. Level 2 – Inputs are observable, unadjusted quoted prices in active markets for similar assets or liabilities, unadjusted quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the related assets or liabilities; and Level 3 – Unobservable inputs that are supported by little or no market activities, therefore requiring an entity to develop its own assumptions. Assets and Liabilities Measured at Fair Value on a Recurring Basis The following table summarizes our assets and liabilities that require fair value measurements on a recurring basis and their respective input levels based on the fair value hierarchy:
(1) Included in cash and cash equivalents in the accompanying consolidated balance sheets. (2) Included in short-term or long-term investments in the accompanying consolidated balance sheets depending on the respective maturity date. The following methods and assumptions were used to estimate the fair value of our financial instruments for which it is practicable to estimate that value: Marketable Securities. For fair values determined by Level 1 inputs, which utilize quoted prices in active markets for identical assets, the level of judgment required to estimate fair value is relatively low. For fair values determined by Level 2 inputs, which utilize quoted prices in less active markets for similar assets, the level of judgment required to estimate fair value is also considered relatively low. Fair Value of Other Financial Instruments The carrying amounts of certain of our financial instruments, including cash and cash equivalents, receivables, accounts payable, and accrued expenses approximate fair value due to their short-term nature. Available for Sale Investments We invest our excess cash in agency securities, debt instruments of financial institutions and corporations, commercial obligations, and U.S. Treasury securities, which we classify as available-for-sale investments. These investments are carried at fair value and are included in the tables above. The aggregate market value, cost basis, and gross unrealized gains and losses of available-for-sale investments by security type, classified in short-term and long-term investments as of June 30, 2025 are as follows:
(1) Of our outstanding agency securities, $5.1 million have maturity dates of less than one year and $2.5 million have maturity dates between to two years as of June 30, 2025. (2) Of our outstanding commercial and corporate obligations, $11.6 million have maturity dates of less than one year and $5.1 million have a maturity date of between to two years as of June 30, 2025. (3) Of our outstanding U.S. Treasury securities, $204.7 million have maturity dates of less than one year and $20.4 million have a maturity date of between to two years as of June 30, 2025. The aggregate market value, cost basis, and gross unrealized gains and losses of available for sale investments by security type, classified in short-term and long-term investments as of December 31, 2024 are as follows:
(1) Of our outstanding agency securities, $7.6 million have maturity dates of less than one year and $0.0 million have a maturity date of between to two years as of December 31, 2024. (2) Of our outstanding commercial and corporate obligations, $10.7 million have maturity dates of less than one year and $0.0 million have a maturity date of between to two years as of December 31, 2024. (3) Of our outstanding U.S. Treasury securities, $249.0 million have maturity dates of less than one year and $35.5 million have a maturity date of between to two years as of December 31, 2024. The following tables present gross unrealized losses and fair values for those investments that were in an unrealized loss position as of June 30, 2025 and December 31, 2024, aggregated by investment category and the length of time that individual securities have been in a continuous loss position:
As of June 30, 2025 and December 31, 2024, unrealized losses on available-for-sale investments were less than $0.1 million for both periods, with no available-for-sale investments that were in an unrealized loss position for greater than 12 months as of June 30, 2025. We do not intend to sell the investments and it is not more likely than not that we will be required to sell the investments before recovery of their amortized cost basis, accordingly, no allowance for credit losses was recorded.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stockholders' Equity |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Equity [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stockholders' Equity | Stockholders’ Equity Common Stock Of the 500,000,000 shares of common stock authorized, 27,972,997 shares were issued and outstanding as of June 30, 2025. Stock Repurchase Program In March 2025, our Board of Directors authorized a stock repurchase program (the “2025 Repurchase Program”) to repurchase up to $75.0 million of our outstanding common stock. The following table presents the repurchase activity through June 30, 2025:
The repurchased common stock was subsequently retired after the repurchase and the par value of the shares was charged to common stock. The excess of the repurchase price over the par value was applied against additional paid in capital. As of June 30, 2025, $19.5 million remained available for future shares of common stock to be repurchased under the 2025 Repurchase Program. Open Market Sales Agreement In November 2024, we entered into a sales agreement with TD Securities (USA) LLC (“TD Cowen”), through which we may offer and sell shares of our common stock, having an aggregate offering of up to $100.0 million through TD Cowen as our sales agent. Our prior sales agreement with Cowen terminated upon effectiveness of the registration statement on the Form S-3 we filed in connection with our sales agreement with TD Cowen. As of June 30, 2025, we had sold no shares under this agreement. Underwriting Agreement In August 2024, we entered into an underwriting agreement with TD Securities (USA) LLC and Leerink Partners LLC as representatives to the several underwriters listed therein (the “Underwriters”), pursuant to which we issued and sold an aggregate of 2,750,498 shares of our common stock to the Underwriters, at an offering price of $36.50 per share. The net proceeds from these sales were approximately $93.9 million, net of underwriting discounts and commissions and offering expenses of $6.5 million, in the aggregate.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Equity Incentive Plans |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Share-Based Payment Arrangement [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Equity Incentive Plans | Equity Incentive Plans 2017 Equity Incentive Plan In January 2017, our Board of Directors and stockholders approved and adopted the 2017 Equity Incentive Plan (the “2017 Plan”). Under the 2017 Plan, we may grant stock options, stock appreciation rights, restricted stock, restricted stock units and other awards to individuals who are then our employees, officers, directors or consultants. In addition, the number of shares of stock available for issuance under the 2017 Plan were to be automatically increased each January 1, beginning on January 1, 2018, by 4% of the aggregate number of outstanding shares of our common stock as of the immediately preceding December 31 or such lesser number as determined by our Board of Directors. At our annual stockholder meeting on June 12, 2024, the 2017 Plan was amended, eliminating the automatic annual share increase and the number of shares available for issuance was increased by 2,700,000 shares. At our annual stockholder meeting on June 17, 2025, the 2017 Plan was amended and restated to further increase the number of shares available for issuance by 1,650,000 shares. All future share increases will require stockholder approval. As of June 30, 2025, 2,504,515 shares were available for future issuance. Employee Stock Purchase Plan In January 2017, our Board of Directors and stockholders approved and adopted the 2017 Employee Stock Purchase Plan (“ESPP”). In addition, the number shares of stock available for issuance under the ESPP will be automatically increased each January 1, beginning on January 1, 2018, by 1% of the aggregate number of outstanding shares of our common stock as of the immediately preceding December 31 or such lesser number as determined by our Board of Directors. The Board of Directors determined that due to sufficient shares being available in the ESPP, the number of shares available as of January 1, 2025 would not increase. As of June 30, 2025, 241,606 shares have been issued under the ESPP and 1,857,201 shares were available for future issuance under the ESPP. Stock Options Stock options granted to employees and non-employees generally vest over a four-year period while stock options granted to directors generally vest over a one-year period. Each stock option award has a maximum term of 10 years from the date of grant, subject to earlier cancellation prior to vesting upon cessation of service to us. A summary of the activity related to stock option awards during the six months ended June 30, 2025 is as follows:
Total cash received from the exercise of stock options was approximately $0.7 million during the six months ended June 30, 2025. Time-Based Restricted Stock Units Each Restricted Stock Unit (“RSU”) represents one equivalent share of our common stock to be issued after satisfying the applicable continued service-based vesting criteria over a specified period. The fair value of these RSUs is based on the closing price of our common stock on the date of the grant. We measure compensation expense over the expected vesting period on a straight-line basis. The RSUs do not entitle the participants to the rights of holders of common stock, such as voting rights, until the shares are issued.
Performance Stock Units A Performance Stock Unit (“PSU”) represents one equivalent share of our common stock to be issued after achievement of the performance metrics specified in the grant. The fair value of our PSUs is estimated as of the grant date of July 22, 2024, based upon the expected achievement of the performance metrics specified in the grant and the closing market price of our common stock on the date of grant. The grant date fair value is estimated using a Monte Carlo simulation using the following assumptions:
The compensation expense for the awards is recognized over the requisite service period regardless of whether the market conditions are achieved and will only be adjusted for pre-vesting forfeitures due to the termination of the recipient’s employment with the company prior to the expiration of the requisite service period. The requisite service period over which the compensation expense will be recognized is July 22, 2024 through July 1, 2028. The following table presents a summary of activity with respect to our PSUs:
Stock-Based Compensation Expense We recognize stock-based compensation expense for awards issued to employees and non-employees over the requisite service period based on the estimated grant-date fair value of such awards. We record the expense for stock-based compensation awards subject to performance-based milestone vesting over the requisite service period when management determines that achievement of the milestone is probable. Management evaluates when the achievement of a performance-based milestone is probable based on the expected satisfaction of the performance conditions at each reporting date. The estimated fair values of stock option awards granted were determined on the date of grant using the Black-Scholes option valuation model with the following weighted-average assumptions:
We determine the appropriate risk-free interest rate, expected term for employee stock-based awards, contractual term for non-employee stock-based awards, and volatility assumptions. The weighted-average expected option term for employee and non-employee stock-based awards reflects the historical option term. Expected volatility incorporates the historical volatility of our stock price. The risk-free interest rate is based upon U.S. Treasury securities with remaining terms similar to the expected or contractual term of the stock-based payment awards. The assumed dividend yield is based on our expectation of not paying dividends in the foreseeable future. Total non-cash stock-based compensation expense for all stock awards that was recognized in the consolidated statements of operations and comprehensive loss is as follows:
(1) Includes $2.6 million related to two year RSU initially issued to our Chief Executive Officer in March 2022 and now fully recognized. At June 30, 2025, there was $49.4 million of unrecognized compensation cost related to unvested stock option awards, which is expected to be recognized over a remaining weighted-average vesting period of 2.75 years, $23.1 million of unrecognized cost related to unvested RSU awards, which is expected to be recognized over a period of 2.75 years, $9.4 million of unrecognized cost related to unvested PSU awards, which is expected to be recognized over a period of 3.03 years, and $0.3 million of unrecognized compensation cost related to the ESPP, which is expected to be recognized over a remaining weighted-average vesting period of 0.38 years.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Commitments and Contingencies |
6 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Commitments and Contingencies Disclosure [Abstract] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Commitments and Contingencies | Commitments and Contingencies Operating Leases On May 4, 2020, we entered into a lease agreement with Wateridge Property Owner, LP, with respect to facilities in the building at 10770 Wateridge Circle, San Diego, California 92121 (the “Lease Agreement”). Under the Lease Agreement, we agreed to lease approximately 45,000 square feet of space for a term of 124 months, beginning on April 5, 2021. The terms of the Lease Agreement provide us with an option to extend the term of the lease for an additional five years, as well as a one-time option to terminate the lease after seven years with the payment of a termination fee. The exercise of the lease option is at our sole discretion, which we currently do not anticipate exercising and as such was not recognized as part of the right-of-use asset (the “ROU asset”) and lease liability. The monthly base rent was initially $4.20 per rentable square foot and is increased by 3% annually. Under the Lease Agreement, we are also responsible for our pro rata share of real estate taxes, building insurance, maintenance, direct expenses, and utilities. Upon lease commencement, on April 5, 2021, we recognized an ROU asset of $20.6 million, with a corresponding lease liability of $20.7 million on the consolidated balance sheets. The ROU asset includes adjustments for prepayments, initial direct costs, and lease incentives. As of June 30, 2025, we have recorded $0.3 million as a security deposit in accordance with the terms of the Lease Agreement. Our lease payments are fixed, and we recognize lease expense for leases on a straight-line basis over the lease term. Operating lease ROU assets and lease liabilities are recorded based on the present value of the future minimum lease payments over the lease term at commencement date. As our lease does not provide an implicit rate, we used our incremental borrowing rate based on the information available at the lease commencement date in determining the present value of future payments. The weighted-average discount rate used was 4.0% and the weighted-average remaining lease term is approximately 6.2 years. The following non-cancellable office lease costs are included in our consolidated statements of cash flow (in thousands):
At June 30, 2025, the future minimum annual obligations for our operating lease liabilities are as follows (in thousands):
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Segment Reporting |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segment Reporting [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segment Reporting | Segment Reporting We operate as one reportable segment focused on research and development activities to deliver immunology therapeutics for autoimmune and inflammatory diseases. Segment profit or loss is measured as the net loss reported on our Consolidated Statements of Operations and Comprehensive Loss and net loss is used to monitor results. Our segment revenue consists of milestone payments, royalty payments, upfront license fees and transition services provided under our collaboration agreements, see Note 4. The measure of segment assets is reported on our Consolidated Balance Sheets as total assets. The CODM is our Chief Executive Officer. The CODM manages the business activities on a consolidated basis in making decisions regarding resource allocation and performance assessment. The following table is a summary of segment revenue, loss and our significant expenses (in thousands):
(1) External R&D consists of costs associated with our research and development activities, including drug discovery efforts, preclinical and clinical development of our programs, manufacturing, and allocated facility-related costs. (2) Internal R&D and G&A consist of salaries and wages, stock-based compensation, recruiting and other employee benefits. (3) External G&A consists of general and administrative expenses including transaction costs, legal services, insurance, professional fees for auditing, tax, and market research, and allocated facility-related costs not otherwise included in research and development expenses.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Subsequent Event |
6 Months Ended |
|---|---|
Jun. 30, 2025 | |
| Subsequent Events [Abstract] | |
| Subsequent Event | Subsequent Event In July 2025, the One Big Beautiful Bill Act (“OBBBA”) was signed into law, which enacts significant changes to U.S. tax and related laws. Some of the provisions of the new tax law affecting corporations include, but are not limited to, current deduction of domestic research expenses, increasing the limit of the interest expense deduction to thirty percent of EBITDA, and one hundred percent bonus depreciation on eligible property acquired after January 19, 2025. The Company is currently evaluating the impact the new tax law will have on its financial condition and results of operations. Preliminarily, the Company does not anticipate a change to its effective income tax rate and its net deferred federal income tax assets as the Company maintains a full valuation allowance. The impact of the tax law changes from the OBBBA will be included in the Company’s financial statements beginning in the quarter ending September 30, 2025.
|
Insider Trading Arrangements |
3 Months Ended |
|---|---|
|
Jun. 30, 2025
shares
| |
| Trading Arrangements, by Individual | |
| Non-Rule 10b5-1 Arrangement Adopted | false |
| Rule 10b5-1 Arrangement Terminated | false |
| Non-Rule 10b5-1 Arrangement Terminated | false |
| Daniel Faga [Member] | |
| Trading Arrangements, by Individual | |
| Material Terms of Trading Arrangement | On April 11, 2025, Daniel Faga, chief executive officer of the company, entered into a written plan for the potential sale of common stock. The plan is intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) under the Exchange Act. No shares were sold under the plan and it was subsequently terminated on July 15, 2025.
|
| Name | Daniel Faga |
| Title | chief executive officer of the company |
| Rule 10b5-1 Arrangement Adopted | true |
| Adoption Date | April 11, 2025 |
| Expiration Date | July 15, 2025 |
| Arrangement Duration | 95 days |
| Aggregate Available | 0 |
| Dennis Mulroy [Member] | |
| Trading Arrangements, by Individual | |
| Material Terms of Trading Arrangement | On April 11, 2025, Dennis Mulroy, chief financial officer of the company, entered into a written plan for the potential sale of up to an aggregate 25,725 shares of common stock. The plan is intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) under the Exchange Act and is scheduled to terminate no later than December 31, 2025.
|
| Name | Dennis Mulroy |
| Title | chief financial officer of the company |
| Rule 10b5-1 Arrangement Adopted | true |
| Adoption Date | April 11, 2025 |
| Expiration Date | December 31, 2025 |
| Arrangement Duration | 264 days |
| Aggregate Available | 25,725 |
| Eric Loumeau [Member] | |
| Trading Arrangements, by Individual | |
| Material Terms of Trading Arrangement | On April 11, 2025, Eric Loumeau, chief legal officer of the company, entered into a written plan for the potential sale of up to an aggregate 59,828 shares of common stock. The plan is intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) under the Exchange Act and is scheduled to terminate no later than May 20, 2026.
|
| Name | Eric Loumeau |
| Title | chief legal officer of the company |
| Rule 10b5-1 Arrangement Adopted | true |
| Adoption Date | April 11, 2025 |
| Expiration Date | May 20, 2026 |
| Arrangement Duration | 404 days |
| Aggregate Available | 59,828 |
| Paul Lizzul [Member] | |
| Trading Arrangements, by Individual | |
| Material Terms of Trading Arrangement | On April 14, 2025, Paul Lizzul, chief medical officer of the company, entered into a written plan for the potential sale of up to an aggregate 36,500 shares of common stock. The plan is intended to satisfy the affirmative defense conditions of Rule 10b5-1(c) under the Exchange Act and is scheduled to terminate no later than April 14, 2026.
|
| Name | Paul Lizzul |
| Title | chief medical officer of the company |
| Rule 10b5-1 Arrangement Adopted | true |
| Adoption Date | April 14, 2025 |
| Expiration Date | April 14, 2026 |
| Arrangement Duration | 365 days |
| Aggregate Available | 36,500 |
Summary of Significant Accounting Policies (Policies) |
6 Months Ended |
|---|---|
Jun. 30, 2025 | |
| Accounting Policies [Abstract] | |
| Basis of Presentation | Basis of Presentation The accompanying unaudited consolidated financial statements have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). Certain information and note disclosures normally included in annual financial statements prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”) have been omitted. The accompanying unaudited consolidated financial statements include all known adjustments necessary for a fair presentation of the results of interim periods as required by U.S. GAAP. These adjustments consist primarily of normal recurring accruals and estimates that impact the carrying value of assets and liabilities. Operating results for the six months ended June 30, 2025 are not necessarily indicative of the results that may be expected for the year ending December 31, 2025. The financial statements should be read in conjunction with our audited financial statements for the year ended December 31, 2024 included in our Annual Report on Form 10-K.
|
| Basis of Consolidation | Basis of Consolidation The accompanying consolidated financial statements include us and our wholly owned Australian subsidiary, which was deregistered with the Australian Securities & Investments Commission as of June 30, 2025. The deregistration did not have a material impact on our consolidated financial statements. All intercompany accounts and transactions have been eliminated in consolidation. We operate in one reportable segment, and our functional and reporting currency is the U.S. dollar.
|
| Use of Estimates | Use of Estimates The preparation of the accompanying consolidated financial statements in conformity with U.S. GAAP requires our management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates. We base our estimates and assumptions on historical experience when available and on various factors that we believe to be reasonable under the circumstances. Significant estimates relied upon in preparing these financial statements include estimates related to revenue recognition, accrued research and development expenses, stock-based compensation, and the liability related to the sale of future royalties. We evaluate our estimates and assumptions on an ongoing basis. Our actual results could differ from these estimates under different assumptions or conditions.
|
| Net Loss Per Common Share | Net Loss Per Common Share Basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed by dividing net loss by the weighted-average number of common equivalent shares outstanding for the period, as well as any dilutive effect from outstanding stock options and warrants using the treasury stock method. For each period presented, there is no difference in the number of shares used to calculate basic and diluted net loss per share.
|
| Accounting Pronouncements and Recent Accounting Pronouncements Not Yet Adopted | Accounting Pronouncements We have implemented all new accounting pronouncements that are in effect and may have an impact on our consolidated financial statements. Unless otherwise discussed, we believe the impact of any recently issued and not yet effective pronouncements will not have a material impact on our consolidated financial statements. Recent Accounting Pronouncements Not Yet Adopted In December 2023, the FASB issued ASU No. 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures Income Taxes, which improves the transparency of income tax disclosures by requiring consistent categories and greater disaggregation of information in the effective tax rate reconciliation and income taxes paid disaggregated by jurisdiction. It also includes certain other amendments to improve the effectiveness of income tax disclosures. This guidance will be effective for the annual periods beginning after December 15, 2024. Early adoption is permitted. Upon adoption, the guidance can be applied prospectively or retrospectively. We are currently assessing the impact that this standard will have on our consolidated financial statements. In November 2024, the FASB issued ASU No. 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses, which requires disclosure about the types of costs and expenses included in certain expense captions presented on the income statement. The new disclosure requirements are effective for annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027. Early adoption is permitted. We are currently evaluating the provisions of this guidance and assessing the potential impact on our financial statement disclosures.
|
Summary of Significant Accounting Policies (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Accounting Policies [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Outstanding Potentially Dilutive Securities Excluded in the Calculation of Diluted Net Loss Per Share | The following table sets forth the weighted-average outstanding potentially dilutive securities that have been excluded in the calculation of diluted net loss per share because to do so would be anti-dilutive (in common stock equivalent shares):
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Balance Sheet Accounts and Supplemental Disclosures (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance Sheet Related Disclosures [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Property and Equipment, Net | Property and equipment, net consist of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Accrued Expenses | Accrued expenses consist of the following:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Collaborative Research and Development Agreements (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenue from Contract with Customer [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Milestones Achieved | Milestones under the GSK Agreement are as follows:
(1)Biologics License Application (“BLA”) (2)Marketing Authorization Application (“MAA”) (3)For Jemperli, the filing and approval of the first MAA for a second indication and first three commercial sales milestones are included as part of the royalty monetization agreement with Sagard (as defined below), see Note 5. Cash is generally received within 30 days of milestone achievement. (4)For Jemperli, we retained the rights to a $75.0 million sales milestone when Jemperli annual net sales exceed $1.0 billion. We are also eligible to receive a 10% royalty on net sales, as well as the following milestones under the Vanda License Agreement:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Sale of Future Royalties (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Liabilities Disclosure [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Royalty Transaction Activity | The following table shows the activity within the liability account for the six months ended June 30, 2025:
(1) Of the non-cash interest expense recognized, $0.6 million was negative amortization for the six months ended June 30, 2025. The following table shows the activity within the liability account for the six months ended June 30, 2025:
(1) Of the non-cash interest expense recognized, none was negative amortization for the six months ended June 30, 2025.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fair Value Measurements and Available for Sale Investments (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Fair Value Disclosures [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Assets and Liabilities that Require Fair Value Measurements on a Recurring Basis | The following table summarizes our assets and liabilities that require fair value measurements on a recurring basis and their respective input levels based on the fair value hierarchy:
(1) Included in cash and cash equivalents in the accompanying consolidated balance sheets. (2) Included in short-term or long-term investments in the accompanying consolidated balance sheets depending on the respective maturity date.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Available-for-sale Investments | The aggregate market value, cost basis, and gross unrealized gains and losses of available-for-sale investments by security type, classified in short-term and long-term investments as of June 30, 2025 are as follows:
(1) Of our outstanding agency securities, $5.1 million have maturity dates of less than one year and $2.5 million have maturity dates between to two years as of June 30, 2025. (2) Of our outstanding commercial and corporate obligations, $11.6 million have maturity dates of less than one year and $5.1 million have a maturity date of between to two years as of June 30, 2025. (3) Of our outstanding U.S. Treasury securities, $204.7 million have maturity dates of less than one year and $20.4 million have a maturity date of between to two years as of June 30, 2025. The aggregate market value, cost basis, and gross unrealized gains and losses of available for sale investments by security type, classified in short-term and long-term investments as of December 31, 2024 are as follows:
(1) Of our outstanding agency securities, $7.6 million have maturity dates of less than one year and $0.0 million have a maturity date of between to two years as of December 31, 2024. (2) Of our outstanding commercial and corporate obligations, $10.7 million have maturity dates of less than one year and $0.0 million have a maturity date of between to two years as of December 31, 2024. (3) Of our outstanding U.S. Treasury securities, $249.0 million have maturity dates of less than one year and $35.5 million have a maturity date of between to two years as of December 31, 2024.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Unrealized Loss and Fair Values in a Loss Position | The following tables present gross unrealized losses and fair values for those investments that were in an unrealized loss position as of June 30, 2025 and December 31, 2024, aggregated by investment category and the length of time that individual securities have been in a continuous loss position:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stockholders' Equity (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Equity [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Stock Repurchase Activity | The following table presents the repurchase activity through June 30, 2025:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Equity Incentive Plans (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Share-Based Payment Arrangement [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Activity Related to Stock Option Awards | A summary of the activity related to stock option awards during the six months ended June 30, 2025 is as follows:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Time-based Restricted Stock Units Activity |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Grant Date Fair Value Assumptions | The grant date fair value is estimated using a Monte Carlo simulation using the following assumptions:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Performance Shares, Outstanding Activity | The following table presents a summary of activity with respect to our PSUs:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Weighted Average Assumptions used to Estimate Fair Value | The estimated fair values of stock option awards granted were determined on the date of grant using the Black-Scholes option valuation model with the following weighted-average assumptions:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Non-cash Stock-based Compensation Expense | Total non-cash stock-based compensation expense for all stock awards that was recognized in the consolidated statements of operations and comprehensive loss is as follows:
(1) Includes $2.6 million related to two year RSU initially issued to our Chief Executive Officer in March 2022 and now fully recognized.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Commitments and Contingencies (Tables) |
6 Months Ended | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Commitments and Contingencies Disclosure [Abstract] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Non-Cancellable Office Lease Costs | The following non-cancellable office lease costs are included in our consolidated statements of cash flow (in thousands):
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Future Minimum Annual Obligations | At June 30, 2025, the future minimum annual obligations for our operating lease liabilities are as follows (in thousands):
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Segment Reporting (Tables) |
6 Months Ended | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Jun. 30, 2025 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Segment Reporting [Abstract] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Schedule of Segment Revenue, Loss and Significant Expenses | The following table is a summary of segment revenue, loss and our significant expenses (in thousands):
(1) External R&D consists of costs associated with our research and development activities, including drug discovery efforts, preclinical and clinical development of our programs, manufacturing, and allocated facility-related costs. (2) Internal R&D and G&A consist of salaries and wages, stock-based compensation, recruiting and other employee benefits. (3) External G&A consists of general and administrative expenses including transaction costs, legal services, insurance, professional fees for auditing, tax, and market research, and allocated facility-related costs not otherwise included in research and development expenses.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Summary of Significant Accounting Policies - Narrative (Details) |
6 Months Ended |
|---|---|
|
Jun. 30, 2025
segment
| |
| Accounting Policies [Abstract] | |
| Number of reportable segments | 1 |
Summary of Significant Accounting Policies - Schedule of Outstanding Potentially Dilutive Securities Excluded in the Calculation of Diluted Net Loss Per Share (Details) - shares shares in Thousands |
3 Months Ended | 6 Months Ended | ||
|---|---|---|---|---|
Jun. 30, 2025 |
Jun. 30, 2024 |
Jun. 30, 2025 |
Jun. 30, 2024 |
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] | ||||
| Options to purchase common stock (in shares) | 8,475 | 6,397 | 8,517 | 6,768 |
| Options to purchase common stock | ||||
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] | ||||
| Options to purchase common stock (in shares) | 7,621 | 6,148 | 7,644 | 6,113 |
| Stock awards | ||||
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] | ||||
| Options to purchase common stock (in shares) | 854 | 249 | 873 | 655 |
Balance Sheet Accounts and Supplemental Disclosures - Schedule of Property and Equipment, net (Details) - USD ($) $ in Thousands |
Jun. 30, 2025 |
Dec. 31, 2024 |
|---|---|---|
| Property, Plant and Equipment [Line Items] | ||
| Property and equipment, gross | $ 8,503 | $ 8,448 |
| Less: accumulated depreciation and amortization | (6,875) | (6,599) |
| Total property and equipment, net | 1,628 | 1,849 |
| Laboratory equipment | ||
| Property, Plant and Equipment [Line Items] | ||
| Property and equipment, gross | 6,719 | 6,715 |
| Office furniture and equipment | ||
| Property, Plant and Equipment [Line Items] | ||
| Property and equipment, gross | 1,581 | 1,530 |
| Leasehold improvements | ||
| Property, Plant and Equipment [Line Items] | ||
| Property and equipment, gross | $ 203 | $ 203 |
Balance Sheet Accounts and Supplemental Disclosures -Schedule of Accrued Expenses (Details) - USD ($) $ in Thousands |
Jun. 30, 2025 |
Dec. 31, 2024 |
|---|---|---|
| Balance Sheet Related Disclosures [Abstract] | ||
| Accrued compensation and related expenses | $ 6,400 | $ 8,449 |
| Accrued professional fees and other expenses | 982 | 839 |
| Accrued research, development and manufacturing expenses | 22,335 | 30,213 |
| Accrued for repurchases of common stock | 480 | 0 |
| Total accrued expenses | $ 30,197 | $ 39,501 |
Fair Value Measurements and Available for Sale Investments - Narrative (Details) - USD ($) |
Jun. 30, 2025 |
Dec. 31, 2024 |
|---|---|---|
| Fair Value Disclosures [Abstract] | ||
| Unrealized losses on available for sale investments in unrealized loss position for greater than 12 months | $ 0 | $ 0 |
| Allowance for credit losses | $ 0 |
Stockholders' Equity - Narrative (Details) - USD ($) |
1 Months Ended | 6 Months Ended | ||||
|---|---|---|---|---|---|---|
Aug. 31, 2024 |
Jun. 30, 2025 |
Jun. 30, 2024 |
Mar. 31, 2025 |
Dec. 31, 2024 |
Nov. 30, 2024 |
|
| Class of Stock [Line Items] | ||||||
| Common stock, shares authorized (in shares) | 500,000,000 | 500,000,000 | ||||
| Common stock, shares issued (in shares) | 27,972,997 | 30,473,000 | ||||
| Common stock, shares outstanding (in shares) | 27,972,997 | 30,473,000 | ||||
| Authorized repurchase amount | $ 75,000,000 | |||||
| Remaining repurchase amount | $ 19,500,000 | |||||
| Proceeds from issuance of common stock | $ 1,713,000 | $ 1,790,000 | ||||
| Underwriting discounts and commissions and offering expenses | $ 6,500,000 | |||||
| Open Market Sales Agreement | ||||||
| Class of Stock [Line Items] | ||||||
| Aggregate offering of (up to) | $ 100,000,000 | |||||
| Shares sold (in shares) | 0 | |||||
| Underwritings | ||||||
| Class of Stock [Line Items] | ||||||
| Shares sold (in shares) | 2,750,498 | |||||
| Share price (in dollars per share) | $ 36.50 | |||||
| Proceeds from issuance of common stock | $ 93,900,000 | |||||
Stockholders' Equity - Schedule of Stock Repurchase Activity (Details) - USD ($) $ / shares in Units, $ in Thousands |
3 Months Ended | 6 Months Ended | |
|---|---|---|---|
Jun. 30, 2025 |
Mar. 31, 2025 |
Jun. 30, 2025 |
|
| Equity [Abstract] | |||
| Total number of shares purchased (in shares) | 2,560,938 | 292,898 | 2,853,836 |
| Average price paid per share (in dollars per share) | $ 19.58 | $ 18.35 | |
| Approximate dollar value of shares purchased (in dollars per share) | $ 50,147 | $ 5,375 | $ 55,522 |
Equity Incentive Plans - Schedule of Grant Date Fair Value Assumptions (Details) - PSU |
6 Months Ended |
|---|---|
Jun. 30, 2025 | |
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | |
| Expected volatility | 59.00% |
| Risk-free interest rate | 4.10% |
| Expected term (in years) | 3 years 10 months 24 days |
Equity Incentive Plans - Schedule of Weighted Average Assumptions used to Estimate Fair Value (Details) - Stock Options - $ / shares |
6 Months Ended | |
|---|---|---|
Jun. 30, 2025 |
Jun. 30, 2024 |
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||
| Risk-free interest rate | 4.50% | 4.00% |
| Expected volatility | 81.80% | 78.30% |
| Expected dividend yield | 0.00% | 0.00% |
| Expected term (in years) | 6 years 4 months 6 days | 6 years 3 months 10 days |
| Weighted average grant date fair value per share (in dollars per share) | $ 10.95 | $ 15.28 |
Equity Incentive Plans -Schedule of Non-cash Stock-based Compensation Expense (Details) - USD ($) $ in Thousands |
3 Months Ended | 6 Months Ended | ||
|---|---|---|---|---|
Jun. 30, 2025 |
Jun. 30, 2024 |
Jun. 30, 2025 |
Jun. 30, 2024 |
|
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||||
| Total | $ 9,239 | $ 7,544 | $ 18,409 | $ 17,675 |
| Research and development | ||||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||||
| Total | 4,456 | 3,534 | 8,863 | 6,988 |
| General and administrative | ||||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||||
| Total | $ 4,783 | 4,010 | $ 9,546 | $ 10,687 |
| General and administrative | CEO | RSUs | ||||
| Share-based Compensation Arrangement by Share-based Payment Award [Line Items] | ||||
| Total | $ 2,600 | |||
| Award vesting period | 2 years | |||
Commitments and Contingencies - Narrative (Details) ft² in Thousands, $ in Thousands |
May 04, 2020
ft²
$ / ft²
|
Jun. 30, 2025
USD ($)
|
Dec. 31, 2024
USD ($)
|
Apr. 05, 2021
USD ($)
|
|---|---|---|---|---|
| Lessee, Lease, Description [Line Items] | ||||
| Operating lease right-of-use assets | $ 13,464 | $ 14,383 | ||
| Lease liability | $ 15,096 | |||
| Weighted-average discount rate (as a percent) | 4.00% | |||
| Weighted average remaining lease term (in years) | 6 years 2 months 12 days | |||
| Lease Agreement | ||||
| Lessee, Lease, Description [Line Items] | ||||
| Area of leased property (sqft) | ft² | 45 | |||
| Lease term (in months) | 124 months | |||
| Lease renewal term (in years) | 5 years | |||
| Lease termination term (in years) | 7 years | |||
| Monthly base rate (in dollars per sqft) | $ / ft² | 4.20 | |||
| Increase in annual rent (as a percent) | 3.00% | |||
| Operating lease right-of-use assets | $ 20,600 | |||
| Lease liability | $ 20,700 | |||
| Security deposit | $ 300 |
Commitments and Contingencies - Schedule of Non-Cancellable Office Lease Costs (Details) - USD ($) $ in Thousands |
6 Months Ended | |
|---|---|---|
Jun. 30, 2025 |
Jun. 30, 2024 |
|
| Commitments and Contingencies Disclosure [Abstract] | ||
| Operating lease cost | $ 1,239 | $ 1,239 |
| Cash paid for amounts included in the measurement of lease liabilities | $ 1,253 | $ 1,217 |
Commitments and Contingencies - Schedule of Future Minimum Annual Obligations (Details) $ in Thousands |
Jun. 30, 2025
USD ($)
|
|---|---|
| Commitments and Contingencies Disclosure [Abstract] | |
| 2025 | $ 1,278 |
| 2026 | 2,607 |
| 2027 | 2,685 |
| 2028 | 2,766 |
| 2029 | 2,849 |
| Thereafter | 4,939 |
| Total minimum payments required | 17,124 |
| Less imputed interest | (2,028) |
| Total | $ 15,096 |
Segment Reporting - Narrative (Details) |
6 Months Ended |
|---|---|
|
Jun. 30, 2025
segment
| |
| Segment Reporting [Abstract] | |
| Number of reportable segments | 1 |
{
"version": "2.2",
"instance": {
"anab-20250630.htm": {
"nsprefix": "anab",
"nsuri": "http://www.anaptysbio.com/20250630",
"dts": {
"inline": {
"local": [
"anab-20250630.htm"
]
},
"schema": {
"local": [
"anab-20250630.xsd"
],
"remote": [
"http://www.xbrl.org/2003/xbrl-instance-2003-12-31.xsd",
"http://www.xbrl.org/2003/xbrl-linkbase-2003-12-31.xsd",
"http://www.xbrl.org/2003/xl-2003-12-31.xsd",
"http://www.xbrl.org/2003/xlink-2003-12-31.xsd",
"http://www.xbrl.org/2005/xbrldt-2005.xsd",
"http://www.xbrl.org/2006/ref-2006-02-27.xsd",
"http://www.xbrl.org/lrr/role/negated-2009-12-16.xsd",
"http://www.xbrl.org/lrr/role/net-2009-12-16.xsd",
"http://www.xbrl.org/lrr/role/reference-2009-12-16.xsd",
"https://www.xbrl.org/2020/extensible-enumerations-2.0.xsd",
"https://www.xbrl.org/dtr/type/2020-01-21/types.xsd",
"https://www.xbrl.org/dtr/type/2024-01-31/types.xsd",
"https://xbrl.fasb.org/srt/2025/elts/srt-2025.xsd",
"https://xbrl.fasb.org/srt/2025/elts/srt-roles-2025.xsd",
"https://xbrl.fasb.org/srt/2025/elts/srt-types-2025.xsd",
"https://xbrl.fasb.org/us-gaap/2025/elts/us-gaap-2025.xsd",
"https://xbrl.fasb.org/us-gaap/2025/elts/us-roles-2025.xsd",
"https://xbrl.fasb.org/us-gaap/2025/elts/us-types-2025.xsd",
"https://xbrl.sec.gov/country/2025/country-2025.xsd",
"https://xbrl.sec.gov/dei/2025/dei-2025.xsd",
"https://xbrl.sec.gov/ecd/2025/ecd-2025.xsd",
"https://xbrl.sec.gov/stpr/2025/stpr-2025.xsd"
]
},
"calculationLink": {
"local": [
"anab-20250630_cal.xml"
]
},
"definitionLink": {
"local": [
"anab-20250630_def.xml"
]
},
"labelLink": {
"local": [
"anab-20250630_lab.xml"
]
},
"presentationLink": {
"local": [
"anab-20250630_pre.xml"
]
}
},
"keyStandard": 235,
"keyCustom": 48,
"axisStandard": 19,
"axisCustom": 1,
"memberStandard": 27,
"memberCustom": 65,
"hidden": {
"total": 19,
"http://www.anaptysbio.com/20250630": 6,
"http://xbrl.sec.gov/ecd/2025": 4,
"http://xbrl.sec.gov/dei/2025": 5,
"http://fasb.org/us-gaap/2025": 4
},
"contextCount": 308,
"entityCount": 1,
"segmentCount": 93,
"elementCount": 569,
"unitCount": 7,
"baseTaxonomies": {
"http://fasb.org/us-gaap/2025": 709,
"http://xbrl.sec.gov/ecd/2025": 35,
"http://xbrl.sec.gov/dei/2025": 30,
"http://fasb.org/srt/2025": 1
},
"report": {
"R1": {
"role": "http://www.anaptysbio.com/role/CoverPage",
"longName": "0000001 - Document - Cover Page",
"shortName": "Cover Page",
"isDefault": "true",
"groupType": "document",
"subGroupType": "",
"menuCat": "Cover",
"order": "1",
"firstAnchor": {
"contextRef": "c-1",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R2": {
"role": "http://www.anaptysbio.com/role/ConsolidatedBalanceSheets",
"longName": "9952151 - Statement - Consolidated Balance Sheets",
"shortName": "Consolidated Balance Sheets",
"isDefault": "false",
"groupType": "statement",
"subGroupType": "",
"menuCat": "Statements",
"order": "2",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:CashAndCashEquivalentsAtCarryingValue",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:CashAndCashEquivalentsAtCarryingValue",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R3": {
"role": "http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical",
"longName": "9952152 - Statement - Consolidated Balance Sheets (Parenthetical)",
"shortName": "Consolidated Balance Sheets (Parenthetical)",
"isDefault": "false",
"groupType": "statement",
"subGroupType": "parenthetical",
"menuCat": "Statements",
"order": "3",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:PreferredStockParOrStatedValuePerShare",
"unitRef": "usdPerShare",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"us-gaap:PreferredStockParOrStatedValuePerShare",
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:PreferredStockParOrStatedValuePerShare",
"unitRef": "usdPerShare",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"us-gaap:PreferredStockParOrStatedValuePerShare",
"span",
"div",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R4": {
"role": "http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"longName": "9952153 - Statement - Consolidated Statements of Operations and Comprehensive Loss",
"shortName": "Consolidated Statements of Operations and Comprehensive Loss",
"isDefault": "false",
"groupType": "statement",
"subGroupType": "",
"menuCat": "Statements",
"order": "4",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-5",
"name": "us-gaap:ResearchAndDevelopmentExpense",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R5": {
"role": "http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity",
"longName": "9952154 - Statement - Consolidated Statement of Stockholders\u2019 Equity",
"shortName": "Consolidated Statement of Stockholders\u2019 Equity",
"isDefault": "false",
"groupType": "statement",
"subGroupType": "",
"menuCat": "Statements",
"order": "5",
"firstAnchor": {
"contextRef": "c-30",
"name": "us-gaap:CommonStockSharesOutstanding",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-35",
"name": "anab:ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAndEmployeeStockPurchasePlan",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R6": {
"role": "http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows",
"longName": "9952155 - Statement - Consolidated Statements of Cash Flows",
"shortName": "Consolidated Statements of Cash Flows",
"isDefault": "false",
"groupType": "statement",
"subGroupType": "",
"menuCat": "Statements",
"order": "6",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:NetIncomeLoss",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:DepreciationDepletionAndAmortization",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R7": {
"role": "http://www.anaptysbio.com/role/DescriptionoftheBusiness",
"longName": "9952156 - Disclosure - Description of the Business",
"shortName": "Description of the Business",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "7",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:NatureOfOperations",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:NatureOfOperations",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R8": {
"role": "http://www.anaptysbio.com/role/SummaryofSignificantAccountingPolicies",
"longName": "9952157 - Disclosure - Summary of Significant Accounting Policies",
"shortName": "Summary of Significant Accounting Policies",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "8",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SignificantAccountingPoliciesTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SignificantAccountingPoliciesTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R9": {
"role": "http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosures",
"longName": "9952158 - Disclosure - Balance Sheet Accounts and Supplemental Disclosures",
"shortName": "Balance Sheet Accounts and Supplemental Disclosures",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "9",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SupplementalBalanceSheetDisclosuresTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SupplementalBalanceSheetDisclosuresTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R10": {
"role": "http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreements",
"longName": "9952159 - Disclosure - Collaborative Research and Development Agreements",
"shortName": "Collaborative Research and Development Agreements",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "10",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:CollaborativeArrangementDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:CollaborativeArrangementDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R11": {
"role": "http://www.anaptysbio.com/role/SaleofFutureRoyalties",
"longName": "9952160 - Disclosure - Sale of Future Royalties",
"shortName": "Sale of Future Royalties",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "11",
"firstAnchor": {
"contextRef": "c-1",
"name": "anab:SaleOfFutureRoyaltiesTextBlockTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "anab:SaleOfFutureRoyaltiesTextBlockTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R12": {
"role": "http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestments",
"longName": "9952161 - Disclosure - Fair Value Measurements and Available for Sale Investments",
"shortName": "Fair Value Measurements and Available for Sale Investments",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "12",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:FairValueDisclosuresTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:FairValueDisclosuresTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R13": {
"role": "http://www.anaptysbio.com/role/StockholdersEquity",
"longName": "9952162 - Disclosure - Stockholders' Equity",
"shortName": "Stockholders' Equity",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "13",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:StockholdersEquityNoteDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:StockholdersEquityNoteDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R14": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlans",
"longName": "9952163 - Disclosure - Equity Incentive Plans",
"shortName": "Equity Incentive Plans",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "14",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R15": {
"role": "http://www.anaptysbio.com/role/CommitmentsandContingencies",
"longName": "9952164 - Disclosure - Commitments and Contingencies",
"shortName": "Commitments and Contingencies",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "15",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:CommitmentsAndContingenciesDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:CommitmentsAndContingenciesDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R16": {
"role": "http://www.anaptysbio.com/role/SegmentReporting",
"longName": "9952165 - Disclosure - Segment Reporting",
"shortName": "Segment Reporting",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "16",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SegmentReportingDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SegmentReportingDisclosureTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R17": {
"role": "http://www.anaptysbio.com/role/SubsequentEvent",
"longName": "9952166 - Disclosure - Subsequent Event",
"shortName": "Subsequent Event",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "17",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SubsequentEventsTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:SubsequentEventsTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R18": {
"role": "http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements",
"longName": "995445 - Disclosure - Insider Trading Arrangements",
"shortName": "Insider Trading Arrangements",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "",
"menuCat": "Notes",
"order": "18",
"firstAnchor": {
"contextRef": "c-5",
"name": "ecd:NonRule10b51ArrAdoptedFlag",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-5",
"name": "ecd:NonRule10b51ArrAdoptedFlag",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R19": {
"role": "http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesPolicies",
"longName": "9955511 - Disclosure - Summary of Significant Accounting Policies (Policies)",
"shortName": "Summary of Significant Accounting Policies (Policies)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "policies",
"menuCat": "Policies",
"order": "19",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:BasisOfAccountingPolicyPolicyTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:BasisOfAccountingPolicyPolicyTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R20": {
"role": "http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesTables",
"longName": "9955512 - Disclosure - Summary of Significant Accounting Policies (Tables)",
"shortName": "Summary of Significant Accounting Policies (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "20",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R21": {
"role": "http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresTables",
"longName": "9955513 - Disclosure - Balance Sheet Accounts and Supplemental Disclosures (Tables)",
"shortName": "Balance Sheet Accounts and Supplemental Disclosures (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "21",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:PropertyPlantAndEquipmentTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:PropertyPlantAndEquipmentTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R22": {
"role": "http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsTables",
"longName": "9955514 - Disclosure - Collaborative Research and Development Agreements (Tables)",
"shortName": "Collaborative Research and Development Agreements (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "22",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfCollaborativeArrangementsAndNoncollaborativeArrangementTransactionsTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfCollaborativeArrangementsAndNoncollaborativeArrangementTransactionsTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R23": {
"role": "http://www.anaptysbio.com/role/SaleofFutureRoyaltiesTables",
"longName": "9955515 - Disclosure - Sale of Future Royalties (Tables)",
"shortName": "Sale of Future Royalties (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "23",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ContractWithCustomerAssetAndLiabilityTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ContractWithCustomerAssetAndLiabilityTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R24": {
"role": "http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsTables",
"longName": "9955516 - Disclosure - Fair Value Measurements and Available for Sale Investments (Tables)",
"shortName": "Fair Value Measurements and Available for Sale Investments (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "24",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R25": {
"role": "http://www.anaptysbio.com/role/StockholdersEquityTables",
"longName": "9955517 - Disclosure - Stockholders' Equity (Tables)",
"shortName": "Stockholders' Equity (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "25",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfTreasuryStockByClassTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfTreasuryStockByClassTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R26": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlansTables",
"longName": "9955518 - Disclosure - Equity Incentive Plans (Tables)",
"shortName": "Equity Incentive Plans (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "26",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R27": {
"role": "http://www.anaptysbio.com/role/CommitmentsandContingenciesTables",
"longName": "9955519 - Disclosure - Commitments and Contingencies (Tables)",
"shortName": "Commitments and Contingencies (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "27",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:LeaseCostTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:LeaseCostTableTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R28": {
"role": "http://www.anaptysbio.com/role/SegmentReportingTables",
"longName": "9955520 - Disclosure - Segment Reporting (Tables)",
"shortName": "Segment Reporting (Tables)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "tables",
"menuCat": "Tables",
"order": "28",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfSegmentReportingInformationBySegmentTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ScheduleOfSegmentReportingInformationBySegmentTextBlock",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R29": {
"role": "http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesNarrativeDetails",
"longName": "9955521 - Disclosure - Summary of Significant Accounting Policies - Narrative (Details)",
"shortName": "Summary of Significant Accounting Policies - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "29",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:NumberOfReportableSegments",
"unitRef": "segment",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"div",
"us-gaap:ConsolidationPolicyTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": null
},
"R30": {
"role": "http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails",
"longName": "9955522 - Disclosure - Summary of Significant Accounting Policies - Schedule of Outstanding Potentially Dilutive Securities Excluded in the Calculation of Diluted Net Loss Per Share (Details)",
"shortName": "Summary of Significant Accounting Policies - Schedule of Outstanding Potentially Dilutive Securities Excluded in the Calculation of Diluted Net Loss Per Share (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "30",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-5",
"name": "us-gaap:AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R31": {
"role": "http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails",
"longName": "9955523 - Disclosure - Balance Sheet Accounts and Supplemental Disclosures - Schedule of Property and Equipment, net (Details)",
"shortName": "Balance Sheet Accounts and Supplemental Disclosures - Schedule of Property and Equipment, net (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "31",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:PropertyPlantAndEquipmentGross",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:PropertyPlantAndEquipmentTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:PropertyPlantAndEquipmentGross",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:PropertyPlantAndEquipmentTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R32": {
"role": "http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails",
"longName": "9955524 - Disclosure - Balance Sheet Accounts and Supplemental Disclosures -Schedule of Accrued Expenses (Details)",
"shortName": "Balance Sheet Accounts and Supplemental Disclosures -Schedule of Accrued Expenses (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "32",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:EmployeeRelatedLiabilitiesCurrent",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfAccruedLiabilitiesTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:EmployeeRelatedLiabilitiesCurrent",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfAccruedLiabilitiesTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R33": {
"role": "http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"longName": "9955525 - Disclosure - Collaborative Research and Development Agreements - Narrative (Details)",
"shortName": "Collaborative Research and Development Agreements - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "33",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-78",
"name": "anab:RevenueRecognitionMilestoneMethodCommercialSaleOrExpirationOfPatentsAgreementTerm",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R34": {
"role": "http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"longName": "9955526 - Disclosure - Collaborative Research and Development Agreements - Schedule of Milestone Achieved (Details)",
"shortName": "Collaborative Research and Development Agreements - Schedule of Milestone Achieved (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "34",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ContractWithCustomerTimingOfSatisfactionOfPerformanceObligationAndPayment",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"div",
"us-gaap:ScheduleOfCollaborativeArrangementsAndNoncollaborativeArrangementTransactionsTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R35": {
"role": "http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"longName": "9955527 - Disclosure - Sale of Future Royalties - Narrative (Details)",
"shortName": "Sale of Future Royalties - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "35",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-151",
"name": "anab:AdvanceFutureRoyaltiesLiabilityNoncurrentNet",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-5",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R36": {
"role": "http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails",
"longName": "9955528 - Disclosure - Sale of Future Royalties - Schedule of Royalty Transaction Activity (Details)",
"shortName": "Sale of Future Royalties - Schedule of Royalty Transaction Activity (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "36",
"firstAnchor": {
"contextRef": "c-1",
"name": "anab:RepaymentOfAdvanceFutureRoyaltyAndMilestones",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-157",
"name": "anab:AdvanceFutureRoyaltiesLiabilities",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ContractWithCustomerAssetAndLiabilityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R37": {
"role": "http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails",
"longName": "9955529 - Disclosure - Fair Value Measurements and Available for Sale Investments - Schedule of Assets and Liabilities that Require Fair Value Measurements on a Recurring Basis (Details)",
"shortName": "Fair Value Measurements and Available for Sale Investments - Schedule of Assets and Liabilities that Require Fair Value Measurements on a Recurring Basis (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "37",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:AvailableForSaleSecuritiesDebtSecurities",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-167",
"name": "us-gaap:CashAndCashEquivalentsFairValueDisclosure",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R38": {
"role": "http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"longName": "9955530 - Disclosure - Fair Value Measurements and Available for Sale Investments -Schedule of Available-for-sale Investments (Details)",
"shortName": "Fair Value Measurements and Available for Sale Investments -Schedule of Available-for-sale Investments (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "38",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:AvailableForSaleDebtSecuritiesAmortizedCostBasis",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:AvailableForSaleDebtSecuritiesAmortizedCostBasis",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R39": {
"role": "http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails",
"longName": "9955531 - Disclosure - Fair Value Measurements and Available for Sale Investments - Schedule of Unrealized Loss and Fair Values in a Loss Position (Details)",
"shortName": "Fair Value Measurements and Available for Sale Investments - Schedule of Unrealized Loss and Fair Values in a Loss Position (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "39",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R40": {
"role": "http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsNarrativeDetails",
"longName": "9955532 - Disclosure - Fair Value Measurements and Available for Sale Investments - Narrative (Details)",
"shortName": "Fair Value Measurements and Available for Sale Investments - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "40",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerAccumulatedLoss",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:DebtSecuritiesAvailableForSaleAllowanceForCreditLoss",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R41": {
"role": "http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails",
"longName": "9955533 - Disclosure - Stockholders' Equity - Narrative (Details)",
"shortName": "Stockholders' Equity - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "41",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:CommonStockSharesAuthorized",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-21",
"name": "srt:StockRepurchaseProgramAuthorizedAmount1",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R42": {
"role": "http://www.anaptysbio.com/role/StockholdersEquityScheduleofStockRepurchaseActivityDetails",
"longName": "9955534 - Disclosure - Stockholders' Equity - Schedule of Stock Repurchase Activity (Details)",
"shortName": "Stockholders' Equity - Schedule of Stock Repurchase Activity (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "42",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:TreasuryStockSharesAcquired",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfTreasuryStockByClassTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-5",
"name": "us-gaap:TreasuryStockSharesAcquired",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfTreasuryStockByClassTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R43": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"longName": "9955535 - Disclosure - Equity Incentive Plans - Narrative (Details)",
"shortName": "Equity Incentive Plans - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "43",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ProceedsFromStockOptionsExercised",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-5",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ProceedsFromStockOptionsExercised",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-5",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R44": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails",
"longName": "9955536 - Disclosure - Equity Incentive Plans -Schedule of Option Activity (Details)",
"shortName": "Equity Incentive Plans -Schedule of Option Activity (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "44",
"firstAnchor": {
"contextRef": "c-4",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R45": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails",
"longName": "9955537 - Disclosure - Equity Incentive Plans - Schedule of Time-based Restricted Stock Units & PSU's (Details)",
"shortName": "Equity Incentive Plans - Schedule of Time-based Restricted Stock Units & PSU's (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "45",
"firstAnchor": {
"contextRef": "c-241",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-240",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod",
"unitRef": "shares",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R46": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"longName": "9955538 - Disclosure - Equity Incentive Plans - Schedule of Grant Date Fair Value Assumptions (Details)",
"shortName": "Equity Incentive Plans - Schedule of Grant Date Fair Value Assumptions (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "46",
"firstAnchor": {
"contextRef": "c-244",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate",
"unitRef": "number",
"xsiNil": "false",
"lang": "en-US",
"decimals": "3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-244",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate",
"unitRef": "number",
"xsiNil": "false",
"lang": "en-US",
"decimals": "3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R47": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails",
"longName": "9955539 - Disclosure - Equity Incentive Plans - Schedule of Weighted Average Assumptions used to Estimate Fair Value (Details)",
"shortName": "Equity Incentive Plans - Schedule of Weighted Average Assumptions used to Estimate Fair Value (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "47",
"firstAnchor": {
"contextRef": "c-237",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate",
"unitRef": "number",
"xsiNil": "false",
"lang": "en-US",
"decimals": "3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-237",
"name": "us-gaap:ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate",
"unitRef": "number",
"xsiNil": "false",
"lang": "en-US",
"decimals": "3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R48": {
"role": "http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails",
"longName": "9955540 - Disclosure - Equity Incentive Plans -Schedule of Non-cash Stock-based Compensation Expense (Details)",
"shortName": "Equity Incentive Plans -Schedule of Non-cash Stock-based Compensation Expense (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "48",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:AllocatedShareBasedCompensationExpense",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-5",
"name": "us-gaap:AllocatedShareBasedCompensationExpense",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R49": {
"role": "http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails",
"longName": "9955541 - Disclosure - Commitments and Contingencies - Narrative (Details)",
"shortName": "Commitments and Contingencies - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "49",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:OperatingLeaseRightOfUseAsset",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:OperatingLeaseWeightedAverageDiscountRatePercent",
"unitRef": "number",
"xsiNil": "false",
"lang": "en-US",
"decimals": "3",
"ancestors": [
"span",
"div",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
},
"R50": {
"role": "http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofNonCancellableOfficeLeaseCostsDetails",
"longName": "9955542 - Disclosure - Commitments and Contingencies - Schedule of Non-Cancellable Office Lease Costs (Details)",
"shortName": "Commitments and Contingencies - Schedule of Non-Cancellable Office Lease Costs (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "50",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:OperatingLeaseCost",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-1",
"name": "us-gaap:OperatingLeaseCost",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"us-gaap:LeaseCostTableTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R51": {
"role": "http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails",
"longName": "9955543 - Disclosure - Commitments and Contingencies - Schedule of Future Minimum Annual Obligations (Details)",
"shortName": "Commitments and Contingencies - Schedule of Future Minimum Annual Obligations (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "51",
"firstAnchor": {
"contextRef": "c-3",
"name": "us-gaap:LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"us-gaap:LesseeOperatingLeaseLiabilityMaturityTableTextBlock",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "c-3",
"name": "us-gaap:LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"us-gaap:LesseeOperatingLeaseLiabilityMaturityTableTextBlock",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true,
"unique": true
}
},
"R52": {
"role": "http://www.anaptysbio.com/role/SegmentReportingNarrativeDetails",
"longName": "9955544 - Disclosure - Segment Reporting - Narrative (Details)",
"shortName": "Segment Reporting - Narrative (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "52",
"firstAnchor": {
"contextRef": "c-1",
"name": "us-gaap:NumberOfReportableSegments",
"unitRef": "segment",
"xsiNil": "false",
"lang": "en-US",
"decimals": "INF",
"ancestors": [
"span",
"div",
"us-gaap:ConsolidationPolicyTextBlock",
"ix:continuation",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": null
},
"R53": {
"role": "http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails",
"longName": "9955545 - Disclosure - Segment Reporting - Schedule of Segment Revenue, Loss and Significant Expenses (Details)",
"shortName": "Segment Reporting - Schedule of Segment Revenue, Loss and Significant Expenses (Details)",
"isDefault": "false",
"groupType": "disclosure",
"subGroupType": "details",
"menuCat": "Details",
"order": "53",
"firstAnchor": {
"contextRef": "c-5",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"first": true
},
"uniqueAnchor": {
"contextRef": "c-265",
"name": "us-gaap:RevenueFromContractWithCustomerExcludingAssessedTax",
"unitRef": "usd",
"xsiNil": "false",
"lang": "en-US",
"decimals": "-3",
"ancestors": [
"span",
"td",
"tr",
"table",
"div",
"ix:continuation",
"us-gaap:ScheduleOfSegmentReportingInformationBySegmentTextBlock",
"body",
"html"
],
"reportCount": 1,
"baseRef": "anab-20250630.htm",
"unique": true
}
}
},
"tag": {
"anab_A10770WateridgeCircleSanDiegoCalifornia92121Member": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "A10770WateridgeCircleSanDiegoCalifornia92121Member",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Lease Agreement",
"label": "10770 Wateridge Circle, San Diego, California 92121 [Member]",
"documentation": "10770 Wateridge Circle, San Diego, California 92121"
}
}
},
"auth_ref": []
},
"anab_ANB032Member": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ANB032Member",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "ANB032",
"label": "ANB032 [Member]",
"documentation": "ANB032"
}
}
},
"auth_ref": []
},
"anab_ANB033Member": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ANB033Member",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "ANB033",
"label": "ANB033 [Member]",
"documentation": "ANB033"
}
}
},
"auth_ref": []
},
"anab_ANB101Member": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ANB101Member",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "ANB101",
"label": "ANB101 [Member]",
"documentation": "ANB101"
}
}
},
"auth_ref": []
},
"us-gaap_AccountingPoliciesAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AccountingPoliciesAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Accounting Policies [Abstract]",
"label": "Accounting Policies [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_AccountsPayableCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AccountsPayableCurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accounts payable",
"label": "Accounts Payable, Current",
"documentation": "Carrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer)."
}
}
},
"auth_ref": [
"r39",
"r734"
]
},
"anab_AccruedCommonStockRepurchased": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AccruedCommonStockRepurchased",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails": {
"parentTag": "us-gaap_AccruedLiabilitiesCurrent",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accrued for repurchases of common stock",
"label": "Accrued Common Stock Repurchased",
"documentation": "Accrued Common Stock Repurchased"
}
}
},
"auth_ref": []
},
"us-gaap_AccruedLiabilitiesCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AccruedLiabilitiesCurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0,
"order": 3.0
},
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails",
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accrued expenses",
"totalLabel": "Total accrued expenses",
"label": "Accrued Liabilities, Current",
"documentation": "Carrying value as of the balance sheet date of obligations incurred and payable, pertaining to costs that are statutory in nature, are incurred on contractual obligations, or accumulate over time and for which invoices have not yet been received or will not be rendered. Examples include taxes, interest, rent and utilities. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer)."
}
}
},
"auth_ref": [
"r40"
]
},
"us-gaap_AccruedProfessionalFeesCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AccruedProfessionalFeesCurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails": {
"parentTag": "us-gaap_AccruedLiabilitiesCurrent",
"weight": 1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accrued professional fees and other expenses",
"label": "Accrued Professional Fees, Current",
"documentation": "Carrying value as of the balance sheet date of obligations incurred through that date and payable for professional fees, such as for legal and accounting services received. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer)."
}
}
},
"auth_ref": [
"r40"
]
},
"anab_AccruedResearchAndDevelopmentExpensesCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AccruedResearchAndDevelopmentExpensesCurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails": {
"parentTag": "us-gaap_AccruedLiabilitiesCurrent",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accrued research, development and manufacturing expenses",
"label": "Accrued Research And Development Expenses, Current",
"documentation": "Accrued Research And Development Expenses, Current"
}
}
},
"auth_ref": []
},
"us-gaap_AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AccumulatedDepreciationDepletionAndAmortizationPropertyPlantAndEquipment",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails": {
"parentTag": "us-gaap_PropertyPlantAndEquipmentNet",
"weight": -1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Less: accumulated depreciation and amortization",
"label": "Accumulated Depreciation, Depletion and Amortization, Property, Plant, and Equipment",
"documentation": "Amount of accumulated depreciation, depletion and amortization for physical assets used in the normal conduct of business to produce goods and services."
}
}
},
"auth_ref": [
"r25",
"r122",
"r546"
]
},
"us-gaap_AccumulatedOtherComprehensiveIncomeLossNetOfTax": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AccumulatedOtherComprehensiveIncomeLossNetOfTax",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0,
"order": 5.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accumulated other comprehensive (loss) gain",
"label": "Accumulated Other Comprehensive Income (Loss), Net of Tax",
"documentation": "Amount, after tax, of accumulated increase (decrease) in equity from transaction and other event and circumstance from nonowner source."
}
}
},
"auth_ref": [
"r13",
"r14",
"r49",
"r128",
"r543",
"r582",
"r583",
"r1005"
]
},
"us-gaap_AccumulatedOtherComprehensiveIncomeMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AccumulatedOtherComprehensiveIncomeMember",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accumulated Other Comprehensive Gain (Loss)",
"label": "AOCI Attributable to Parent [Member]",
"documentation": "Accumulated increase (decrease) in equity from transactions and other events and circumstances from non-owner sources, attributable to the parent. Excludes net income (loss), and accumulated changes in equity from transactions resulting from investments by owners and distributions to owners."
}
}
},
"auth_ref": [
"r1",
"r7",
"r14",
"r429",
"r432",
"r491",
"r578",
"r579",
"r872",
"r873",
"r874",
"r926",
"r927",
"r928",
"r929"
]
},
"ecd_Additional402vDisclosureTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "Additional402vDisclosureTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Additional 402(v) Disclosure",
"label": "Additional 402(v) Disclosure [Text Block]"
}
}
},
"auth_ref": [
"r790"
]
},
"us-gaap_AdditionalPaidInCapital": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AdditionalPaidInCapital",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Additional paid in capital",
"label": "Additional Paid in Capital",
"documentation": "Amount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock."
}
}
},
"auth_ref": [
"r44",
"r734",
"r1065"
]
},
"us-gaap_AdditionalPaidInCapitalMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AdditionalPaidInCapitalMember",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Additional Paid-in Capital",
"label": "Additional Paid-in Capital [Member]",
"documentation": "Excess of issue price over par or stated value of the entity's capital stock and amounts received from other transactions involving the entity's stock or stockholders."
}
}
},
"auth_ref": [
"r592",
"r926",
"r927",
"r928",
"r929",
"r1006",
"r1067"
]
},
"ecd_AdjToCompAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AdjToCompAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Adjustment to Compensation, Amount",
"label": "Adjustment to Compensation Amount"
}
}
},
"auth_ref": [
"r803"
]
},
"ecd_AdjToCompAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AdjToCompAxis",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Adjustment to Compensation:",
"label": "Adjustment to Compensation [Axis]"
}
}
},
"auth_ref": [
"r803"
]
},
"ecd_AdjToNonPeoNeoCompFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AdjToNonPeoNeoCompFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Adjustment to Non-PEO NEO Compensation Footnote",
"label": "Adjustment to Non-PEO NEO Compensation Footnote [Text Block]"
}
}
},
"auth_ref": [
"r803"
]
},
"ecd_AdjToPeoCompFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AdjToPeoCompFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Adjustment To PEO Compensation, Footnote",
"label": "Adjustment To PEO Compensation, Footnote [Text Block]"
}
}
},
"auth_ref": [
"r803"
]
},
"us-gaap_AdjustmentsRelatedToTaxWithholdingForShareBasedCompensation": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AdjustmentsRelatedToTaxWithholdingForShareBasedCompensation",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Net share settlement of restricted stock units",
"label": "Share-Based Payment Arrangement, Decrease for Tax Withholding Obligation",
"documentation": "Amount of decrease to equity for grantee's tax withholding obligation for award under share-based payment arrangement."
}
}
},
"auth_ref": [
"r990"
]
},
"us-gaap_AdjustmentsToAdditionalPaidInCapitalShareBasedCompensationStockOptionsRequisiteServicePeriodRecognition": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AdjustmentsToAdditionalPaidInCapitalShareBasedCompensationStockOptionsRequisiteServicePeriodRecognition",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Stock-based compensation",
"label": "APIC, Share-Based Payment Arrangement, Option, Increase for Cost Recognition",
"documentation": "Amount of increase to additional paid-in capital (APIC) for recognition of cost for option under share-based payment arrangement."
}
}
},
"auth_ref": [
"r954",
"r961"
]
},
"us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Adjustments to reconcile net loss to net cash used in operating activities:",
"label": "Adjustment to Reconcile Net Income to Cash Provided by (Used in) Operating Activity [Abstract]"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesAggregateAdditionalPayment": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesAggregateAdditionalPayment",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalties and milestones, agreement amount to be received",
"label": "Advance Future Royalties, Aggregate Additional Payment",
"documentation": "Advance Future Royalties, Aggregate Additional Payment"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesAnnualSalesThreshold": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesAnnualSalesThreshold",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalties agreement, maximum annual royalty payout capacity",
"label": "Advance Future Royalties, Annual Sales Threshold",
"documentation": "Advance Future Royalties, Annual Sales Threshold"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesInterestRateEffectivePercentage": {
"xbrltype": "percentItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesInterestRateEffectivePercentage",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Effective interest rate (as a percent)",
"label": "Advance Future Royalties, Interest Rate, Effective Percentage",
"documentation": "Advance Future Royalties, Interest Rate, Effective Percentage"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesIssuanceCosts": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesIssuanceCosts",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Issuance costs related to the sale of future royalties",
"label": "Advance Future Royalties, Issuance Costs",
"documentation": "Advance Future Royalties, Issuance Costs"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesLiabilities": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesLiabilities",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"periodStartLabel": "Beginning balance",
"periodEndLabel": "Ending balance",
"label": "Advance Future Royalties, Liabilities",
"documentation": "Advance Future Royalties, Liabilities"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesLiabilityNoncurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesLiabilityNoncurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_LiabilitiesAndStockholdersEquity",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Liability related to sale of future royalties",
"label": "Advance Future Royalties, Liability, Noncurrent",
"documentation": "Advance Future Royalties, Liability, Noncurrent"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesLiabilityNoncurrentNet": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesLiabilityNoncurrentNet",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Net of proceeds",
"label": "Advance Future Royalties, Liability, Noncurrent, Net",
"documentation": "Advance Future Royalties, Liability, Noncurrent, Net"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesMinimumGlobalNetSalesRate": {
"xbrltype": "percentItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesMinimumGlobalNetSalesRate",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Settlement agreement rate (as a percent)",
"label": "Advance Future Royalties, Minimum Global Net Sales, Rate",
"documentation": "Advance Future Royalties, Minimum Global Net Sales, Rate"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesPaymentAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesPaymentAxis",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Advance Future Royalties, Payment [Axis]",
"label": "Advance Future Royalties, Payment [Axis]",
"documentation": "Advance Future Royalties, Payment"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesPaymentDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesPaymentDomain",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Advance Future Royalties, Payment [Domain]",
"label": "Advance Future Royalties, Payment [Domain]",
"documentation": "Advance Future Royalties, Payment [Domain]"
}
}
},
"auth_ref": []
},
"anab_AdvanceFutureRoyaltiesRetainedRightsAfterAnnualSalesThresholdExceeded": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AdvanceFutureRoyaltiesRetainedRightsAfterAnnualSalesThresholdExceeded",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Rights retained",
"label": "Advance Future Royalties, Retained Rights After Annual Sales Threshold Exceeded",
"documentation": "Advance Future Royalties, Retained Rights After Annual Sales Threshold Exceeded"
}
}
},
"auth_ref": []
},
"ecd_AggtChngPnsnValInSummryCompstnTblForAplblYrMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AggtChngPnsnValInSummryCompstnTblForAplblYrMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate Change in Present Value of Accumulated Benefit for All Pension Plans Reported in Summary Compensation Table",
"label": "Aggregate Change in Present Value of Accumulated Benefit for All Pension Plans Reported in Summary Compensation Table [Member]"
}
}
},
"auth_ref": [
"r836"
]
},
"ecd_AggtErrCompAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AggtErrCompAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate Erroneous Compensation Amount",
"label": "Aggregate Erroneous Compensation Amount"
}
}
},
"auth_ref": [
"r762",
"r772",
"r782",
"r814"
]
},
"ecd_AggtErrCompNotYetDeterminedTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AggtErrCompNotYetDeterminedTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate Erroneous Compensation Not Yet Determined",
"label": "Aggregate Erroneous Compensation Not Yet Determined [Text Block]"
}
}
},
"auth_ref": [
"r765",
"r775",
"r785",
"r817"
]
},
"ecd_AggtPnsnAdjsSvcCstMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AggtPnsnAdjsSvcCstMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate Pension Adjustments Service Cost",
"label": "Aggregate Pension Adjustments Service Cost [Member]"
}
}
},
"auth_ref": [
"r837"
]
},
"ecd_AllAdjToCompMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AllAdjToCompMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "All Adjustments to Compensation",
"label": "All Adjustments to Compensation [Member]"
}
}
},
"auth_ref": [
"r803"
]
},
"ecd_AllExecutiveCategoriesMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AllExecutiveCategoriesMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "All Executive Categories",
"label": "All Executive Categories [Member]"
}
}
},
"auth_ref": [
"r810"
]
},
"ecd_AllIndividualsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AllIndividualsMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure",
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure",
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements",
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "All Individuals",
"label": "All Individuals [Member]"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r810",
"r818",
"r822",
"r830"
]
},
"ecd_AllTradingArrangementsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AllTradingArrangementsMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "All Trading Arrangements",
"label": "All Trading Arrangements [Member]"
}
}
},
"auth_ref": [
"r828"
]
},
"us-gaap_AllocatedShareBasedCompensationExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AllocatedShareBasedCompensationExpense",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Total",
"label": "Share-Based Payment Arrangement, Expense",
"documentation": "Amount of expense for award under share-based payment arrangement. Excludes amount capitalized."
}
}
},
"auth_ref": [
"r392",
"r398",
"r399"
]
},
"dei_AmendmentFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "AmendmentFlag",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Amendment Flag",
"label": "Amendment Flag",
"documentation": "Boolean flag that is true when the XBRL content amends previously-filed or accepted submission."
}
}
},
"auth_ref": []
},
"anab_AmortizationOfAdvanceFutureRoyaltiesIssuanceCosts": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AmortizationOfAdvanceFutureRoyaltiesIssuanceCosts",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Amortization of issuance costs",
"label": "Amortization Of Advance Future Royalties Issuance Costs",
"documentation": "Amortization Of Advance Future Royalties Issuance Costs"
}
}
},
"auth_ref": []
},
"anab_AnaptysBioGeneratedAntiPD1JemperliDostarlimabMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AnaptysBioGeneratedAntiPD1JemperliDostarlimabMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "PD-1 (Jemperli/Dostarlimab)",
"label": "AnaptysBio-generated Anti-PD-1 (Jemperli/Dostarlimab) [Member]",
"documentation": "AnaptysBio-generated Anti-PD-1 (Jemperli/Dostarlimab)"
}
}
},
"auth_ref": []
},
"anab_AnaptysBioGeneratedAntiTIM3GSK4069889ACobolimabMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AnaptysBioGeneratedAntiTIM3GSK4069889ACobolimabMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "TIM-3 (GSK4069889/Cobolimab)",
"label": "AnaptysBio Generated Anti-TIM-3 (GSK4069889A/Cobolimab) [Member]",
"documentation": "AnaptysBio Generated Anti-TIM-3 (GSK4069889A/Cobolimab)"
}
}
},
"auth_ref": []
},
"anab_AnnualRentIncreasePercent": {
"xbrltype": "percentItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AnnualRentIncreasePercent",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Increase in annual rent (as a percent)",
"label": "Annual Rent Increase, Percent",
"documentation": "Annual Rent Increase, Percent"
}
}
},
"auth_ref": []
},
"us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Options to purchase common stock (in shares)",
"label": "Antidilutive Securities Excluded from Computation of Earnings Per Share, Amount",
"documentation": "Securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented."
}
}
},
"auth_ref": [
"r183"
]
},
"us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Antidilutive Securities [Axis]",
"label": "Antidilutive Securities [Axis]",
"documentation": "Information by type of antidilutive security."
}
}
},
"auth_ref": [
"r183"
]
},
"us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items]",
"label": "Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r183"
]
},
"us-gaap_AntidilutiveSecuritiesNameDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AntidilutiveSecuritiesNameDomain",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Antidilutive Securities, Name [Domain]",
"label": "Antidilutive Securities, Name [Domain]",
"documentation": "Incremental common shares attributable to securities that were not included in diluted earnings per share (EPS) because to do so would increase EPS amounts or decrease loss per share amounts for the period presented."
}
}
},
"auth_ref": [
"r183"
]
},
"us-gaap_AreaOfRealEstateProperty": {
"xbrltype": "areaItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AreaOfRealEstateProperty",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Area of leased property (sqft)",
"label": "Area of Real Estate Property",
"documentation": "Area of a real estate property."
}
}
},
"auth_ref": []
},
"us-gaap_ArrangementsAndNonarrangementTransactionsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ArrangementsAndNonarrangementTransactionsMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Collaborative Arrangement and Arrangement Other than Collaborative [Domain]",
"label": "Collaborative Arrangement and Arrangement Other than Collaborative [Domain]",
"documentation": "Collaborative arrangement and arrangement other than collaborative applicable to revenue-generating activity or operations."
}
}
},
"auth_ref": [
"r418"
]
},
"us-gaap_AssetAcquisitionAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AssetAcquisitionAxis",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Asset Acquisition [Axis]",
"label": "Asset Acquisition [Axis]",
"documentation": "Information by asset acquisition."
}
}
},
"auth_ref": [
"r286",
"r287",
"r288",
"r289",
"r290",
"r291",
"r585",
"r996"
]
},
"us-gaap_AssetAcquisitionConsiderationTransferred": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AssetAcquisitionConsiderationTransferred",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Total transferred",
"label": "Asset Acquisition, Consideration Transferred",
"documentation": "Amount of consideration transferred in asset acquisition. Includes, but is not limited to, cash, liability incurred by acquirer, and equity interest issued by acquirer."
}
}
},
"auth_ref": [
"r718",
"r997",
"r998",
"r999"
]
},
"us-gaap_AssetAcquisitionConsiderationTransferredTransactionCost": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AssetAcquisitionConsiderationTransferredTransactionCost",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Transaction costs incurred",
"label": "Asset Acquisition, Consideration Transferred, Transaction Cost",
"documentation": "Amount of transaction cost incurred as part of consideration transferred in asset acquisition."
}
}
},
"auth_ref": [
"r718",
"r997",
"r998",
"r999"
]
},
"anab_AssetAcquisitionContingentConsiderationArrangementsRangeOfOutcomesValueHigh": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AssetAcquisitionContingentConsiderationArrangementsRangeOfOutcomesValueHigh",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Potential future payments",
"label": "Asset Acquisition, Contingent Consideration Arrangements, Range of Outcomes, Value, High",
"documentation": "Asset Acquisition, Contingent Consideration Arrangements, Range of Outcomes, Value, High"
}
}
},
"auth_ref": []
},
"us-gaap_AssetAcquisitionDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AssetAcquisitionDomain",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Asset Acquisition [Domain]",
"label": "Asset Acquisition [Domain]",
"documentation": "Asset acquisition."
}
}
},
"auth_ref": [
"r286",
"r287",
"r288",
"r289",
"r290",
"r291",
"r585",
"r996"
]
},
"us-gaap_Assets": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "Assets",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total assets",
"label": "Assets",
"documentation": "Amount of asset recognized for present right to economic benefit."
}
}
},
"auth_ref": [
"r72",
"r79",
"r124",
"r154",
"r155",
"r156",
"r186",
"r198",
"r216",
"r220",
"r260",
"r306",
"r307",
"r308",
"r309",
"r310",
"r311",
"r312",
"r313",
"r314",
"r419",
"r421",
"r468",
"r533",
"r534",
"r540",
"r615",
"r685",
"r686",
"r695",
"r734",
"r741",
"r742",
"r753",
"r951",
"r952",
"r1022"
]
},
"us-gaap_AssetsAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AssetsAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "ASSETS",
"label": "Assets [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_AssetsCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AssetsCurrent",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_Assets",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total current assets",
"label": "Assets, Current",
"documentation": "Amount of asset recognized for present right to economic benefit, classified as current."
}
}
},
"auth_ref": [
"r117",
"r129",
"r154",
"r155",
"r156",
"r260",
"r306",
"r307",
"r308",
"r309",
"r310",
"r311",
"r312",
"r313",
"r314",
"r419",
"r421",
"r468",
"r734",
"r951",
"r952",
"r1022"
]
},
"us-gaap_AssetsCurrentAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AssetsCurrentAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Current assets:",
"label": "Assets, Current [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedGainBeforeTax",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails": {
"parentTag": "us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis",
"weight": -1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Gross Unrealized Gains",
"label": "Debt Securities, Available-for-Sale, Accumulated Gross Unrealized Gain, before Tax",
"documentation": "Amount, before tax, of unrealized gain in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r234"
]
},
"us-gaap_AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AvailableForSaleDebtSecuritiesAccumulatedGrossUnrealizedLossBeforeTax",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails": {
"parentTag": "us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Gross Unrealized Losses",
"label": "Debt Securities, Available-for-Sale, Accumulated Gross Unrealized Loss, before Tax",
"documentation": "Amount, before tax, of unrealized loss in accumulated other comprehensive income (AOCI) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r235"
]
},
"us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AvailableForSaleDebtSecuritiesAmortizedCostBasis",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Amortized Cost",
"label": "Debt Securities, Available-for-Sale, Amortized Cost",
"documentation": "Amortized cost of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r231",
"r268",
"r539"
]
},
"us-gaap_AvailableForSaleSecuritiesDebtSecurities": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AvailableForSaleSecuritiesDebtSecurities",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails": {
"parentTag": "us-gaap_AvailableForSaleDebtSecuritiesAmortizedCostBasis",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"verboseLabel": "Short and long-term investments",
"terseLabel": "Total Fair Value",
"label": "Debt Securities, Available-for-Sale",
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r232",
"r268",
"r444",
"r463",
"r464",
"r465",
"r466",
"r530",
"r661",
"r722",
"r725",
"r732",
"r938",
"r1010",
"r1011",
"r1012"
]
},
"us-gaap_AvailableForSaleSecuritiesDebtSecuritiesCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AvailableForSaleSecuritiesDebtSecuritiesCurrent",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Short-term investments",
"label": "Debt Securities, Available-for-Sale, Current",
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), classified as current."
}
}
},
"auth_ref": [
"r229",
"r268"
]
},
"us-gaap_AvailableForSaleSecuritiesDebtSecuritiesNoncurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AvailableForSaleSecuritiesDebtSecuritiesNoncurrent",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_Assets",
"weight": 1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Long-term investments",
"label": "Debt Securities, Available-for-Sale, Noncurrent",
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), classified as noncurrent."
}
}
},
"auth_ref": [
"r120",
"r229",
"r268"
]
},
"anab_AvailableforsaleDebtSecuritiesRemainingMaturity": {
"xbrltype": "durationItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "AvailableforsaleDebtSecuritiesRemainingMaturity",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Remaining maturity (in years)",
"label": "Available-for-sale Debt Securities, Remaining Maturity",
"documentation": "Available-for-sale Debt Securities, Remaining Maturity"
}
}
},
"auth_ref": []
},
"ecd_AwardExrcPrice": {
"xbrltype": "perShareItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardExrcPrice",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Exercise Price",
"label": "Award Exercise Price"
}
}
},
"auth_ref": [
"r825"
]
},
"ecd_AwardGrantDateFairValue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardGrantDateFairValue",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Fair Value as of Grant Date",
"label": "Award Grant Date Fair Value"
}
}
},
"auth_ref": [
"r826"
]
},
"ecd_AwardTmgDiscLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardTmgDiscLineItems",
"lang": {
"en-us": {
"role": {
"label": "Award Timing Disclosures [Line Items]"
}
}
},
"auth_ref": [
"r821"
]
},
"ecd_AwardTmgHowMnpiCnsdrdTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardTmgHowMnpiCnsdrdTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award Timing, How MNPI Considered",
"label": "Award Timing, How MNPI Considered [Text Block]"
}
}
},
"auth_ref": [
"r821"
]
},
"ecd_AwardTmgMethodTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardTmgMethodTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award Timing Method",
"label": "Award Timing Method [Text Block]"
}
}
},
"auth_ref": [
"r821"
]
},
"ecd_AwardTmgMnpiCnsdrdFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardTmgMnpiCnsdrdFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award Timing MNPI Considered",
"label": "Award Timing MNPI Considered [Flag]"
}
}
},
"auth_ref": [
"r821"
]
},
"ecd_AwardTmgMnpiDiscTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardTmgMnpiDiscTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award Timing MNPI Disclosure",
"label": "Award Timing MNPI Disclosure [Text Block]"
}
}
},
"auth_ref": [
"r821"
]
},
"ecd_AwardTmgPredtrmndFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardTmgPredtrmndFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award Timing Predetermined",
"label": "Award Timing Predetermined [Flag]"
}
}
},
"auth_ref": [
"r821"
]
},
"us-gaap_AwardTypeAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "AwardTypeAxis",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails",
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award Type [Axis]",
"label": "Award Type [Axis]",
"documentation": "Information by type of award under share-based payment arrangement."
}
}
},
"auth_ref": [
"r365",
"r366",
"r367",
"r368",
"r369",
"r370",
"r371",
"r372",
"r373",
"r374",
"r375",
"r376",
"r377",
"r378",
"r379",
"r380",
"r381",
"r382",
"r383",
"r384",
"r385",
"r387",
"r388",
"r389",
"r390",
"r391"
]
},
"ecd_AwardUndrlygSecuritiesAmt": {
"xbrltype": "decimalItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardUndrlygSecuritiesAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Underlying Securities",
"label": "Award Underlying Securities Amount"
}
}
},
"auth_ref": [
"r824"
]
},
"ecd_AwardsCloseToMnpiDiscIndName": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardsCloseToMnpiDiscIndName",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Name",
"label": "Awards Close in Time to MNPI Disclosures, Individual Name"
}
}
},
"auth_ref": [
"r823"
]
},
"ecd_AwardsCloseToMnpiDiscTable": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardsCloseToMnpiDiscTable",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Awards Close in Time to MNPI Disclosures",
"label": "Awards Close in Time to MNPI Disclosures [Table]"
}
}
},
"auth_ref": [
"r822"
]
},
"ecd_AwardsCloseToMnpiDiscTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "AwardsCloseToMnpiDiscTableTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Awards Close in Time to MNPI Disclosures, Table",
"label": "Awards Close in Time to MNPI Disclosures [Table Text Block]"
}
}
},
"auth_ref": [
"r822"
]
},
"us-gaap_BalanceSheetRelatedDisclosuresAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "BalanceSheetRelatedDisclosuresAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Balance Sheet Related Disclosures [Abstract]",
"label": "Balance Sheet Related Disclosures [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_BasisOfAccountingPolicyPolicyTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "BasisOfAccountingPolicyPolicyTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Basis of Presentation",
"label": "Basis of Accounting, Policy [Policy Text Block]",
"documentation": "Disclosure of accounting policy for basis of accounting, or basis of presentation, used to prepare the financial statements (for example, US Generally Accepted Accounting Principles, Other Comprehensive Basis of Accounting, IFRS)."
}
}
},
"auth_ref": [
"r919"
]
},
"us-gaap_CashAndCashEquivalentsAtCarryingValue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CashAndCashEquivalentsAtCarryingValue",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Cash and cash equivalents",
"label": "Cash and Cash Equivalent",
"documentation": "Amount of cash and cash equivalent. Cash includes, but is not limited to, currency on hand, demand deposit with financial institution, and account with general characteristic of demand deposit. Cash equivalent includes, but is not limited to, short-term, highly liquid investment that is both readily convertible to known amount of cash and so near maturity that it presents insignificant risk of change in value because of change in interest rate."
}
}
},
"auth_ref": [
"r18",
"r119",
"r663"
]
},
"us-gaap_CashAndCashEquivalentsFairValueDisclosure": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CashAndCashEquivalentsFairValueDisclosure",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Investments at fair value",
"label": "Cash and Cash Equivalents, Fair Value Disclosure",
"documentation": "Fair value portion of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates."
}
}
},
"auth_ref": [
"r464",
"r465",
"r466",
"r1008",
"r1009"
]
},
"us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalents",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"periodStartLabel": "Cash and cash equivalents, beginning of period",
"periodEndLabel": "Cash and cash equivalents, end of period",
"label": "Cash, Cash Equivalent, Restricted Cash, and Restricted Cash Equivalent, Continuing Operation",
"documentation": "Amount of cash and cash equivalent, and cash and cash equivalent restricted to withdrawal or usage; attributable to continuing operation. Cash includes, but is not limited to, currency on hand, demand deposit with financial institution, and account with general characteristic of demand deposit. Cash equivalent includes, but is not limited to, short-term, highly liquid investment that is both readily convertible to known amount of cash and so near maturity that it presents insignificant risk of change in value because of change in interest rate."
}
}
},
"auth_ref": [
"r18",
"r57",
"r151"
]
},
"us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Net (decrease) increase in cash and cash equivalents",
"label": "Cash, Cash Equivalent, Restricted Cash, and Restricted Cash Equivalent, Period Increase (Decrease), Including Exchange Rate Effect and Discontinued Operation",
"documentation": "Amount of increase (decrease) in cash and cash equivalent, and cash and cash equivalent restricted to withdrawal or usage; including effect from exchange rate change and including, but not limited to, discontinued operation. Cash includes, but is not limited to, currency on hand, demand deposit with financial institution, and account with general characteristic of demand deposit. Cash equivalent includes, but is not limited to, short-term, highly liquid investment that is both readily convertible to known amount of cash and so near maturity that it presents insignificant risk of change in value because of change in interest rate."
}
}
},
"auth_ref": [
"r0",
"r57"
]
},
"us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-cash investing and financing activities:",
"label": "Cash Flow, Noncash Investing and Financing Activities Disclosure [Abstract]"
}
}
},
"auth_ref": []
},
"anab_CentessaPharmaceuticalsUKLimitedCentessaMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CentessaPharmaceuticalsUKLimitedCentessaMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Centessa Pharmaceuticals (UK) Limited (Centessa)",
"label": "Centessa Pharmaceuticals (UK) Limited (Centessa) [Member]",
"documentation": "Centessa Pharmaceuticals (UK) Limited (Centessa)"
}
}
},
"auth_ref": []
},
"ecd_ChangedPeerGroupFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ChangedPeerGroupFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Changed Peer Group, Footnote",
"label": "Changed Peer Group, Footnote [Text Block]"
}
}
},
"auth_ref": [
"r801"
]
},
"srt_ChiefExecutiveOfficerMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "ChiefExecutiveOfficerMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "CEO",
"label": "Chief Executive Officer [Member]",
"documentation": "Person with designation of chief executive officer."
}
}
},
"auth_ref": [
"r937"
]
},
"ecd_ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ChngInFrValAsOfVstngDtOfPrrYrEqtyAwrdsVstdInCvrdYrMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Change in Fair Value as of Vesting Date of Prior Year Equity Awards Vested in Covered Year",
"label": "Change in Fair Value as of Vesting Date of Prior Year Equity Awards Vested in Covered Year [Member]"
}
}
},
"auth_ref": [
"r798"
]
},
"ecd_ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ChngInFrValOfOutsdngAndUnvstdEqtyAwrdsGrntdInPrrYrsMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Year-over-Year Change in Fair Value of Equity Awards Granted in Prior Years That are Outstanding and Unvested",
"label": "Year-over-Year Change in Fair Value of Equity Awards Granted in Prior Years That are Outstanding and Unvested [Member]"
}
}
},
"auth_ref": [
"r796"
]
},
"dei_CityAreaCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "CityAreaCode",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "City Area Code",
"label": "City Area Code",
"documentation": "Area code of city"
}
}
},
"auth_ref": []
},
"us-gaap_ClassOfStockLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ClassOfStockLineItems",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Class of Stock [Line Items]",
"label": "Class of Stock [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r125",
"r126",
"r127",
"r188",
"r323",
"r330",
"r331",
"r332",
"r334",
"r337",
"r342",
"r344",
"r427",
"r587",
"r588",
"r589",
"r590",
"r696",
"r840",
"r920",
"r922"
]
},
"anab_ClearanceOfInvestigationalNewDrugFromFDAMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ClearanceOfInvestigationalNewDrugFromFDAMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "IND clearance from the FDA",
"label": "Clearance Of Investigational New Drug From FDA [Member]",
"documentation": "Clearance Of Investigational New Drug From FDA [Member]"
}
}
},
"auth_ref": []
},
"ecd_CoSelectedMeasureAmt": {
"xbrltype": "decimalItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "CoSelectedMeasureAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Company Selected Measure Amount",
"label": "Company Selected Measure Amount"
}
}
},
"auth_ref": [
"r802"
]
},
"ecd_CoSelectedMeasureName": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "CoSelectedMeasureName",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Company Selected Measure Name",
"label": "Company Selected Measure Name"
}
}
},
"auth_ref": [
"r802"
]
},
"anab_CollaborativeArrangementCosts": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CollaborativeArrangementCosts",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Transaction costs expensed",
"label": "Collaborative Arrangement Costs",
"documentation": "Collaborative Arrangement Costs"
}
}
},
"auth_ref": []
},
"us-gaap_CollaborativeArrangementDisclosureTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CollaborativeArrangementDisclosureTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Collaborative Research and Development Agreements",
"label": "Collaborative Arrangement Disclosure [Text Block]",
"documentation": "The entire disclosure for collaborative arrangements in which the entity is a participant, including a) information about the nature and purpose of such arrangements; b) its rights and obligations thereunder; c) the accounting policy for collaborative arrangements; and d) the income statement classification and amounts attributable to transactions arising from the collaborative arrangement between participants."
}
}
},
"auth_ref": [
"r90",
"r416",
"r417"
]
},
"us-gaap_CollaborativeArrangementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CollaborativeArrangementMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "GlaxoSmithKline Collaboration",
"label": "Collaborative Arrangement [Member]",
"documentation": "Contractual arrangement that involves two or more parties that both: (i) actively participate in a joint operating activity and (ii) are exposed to significant risks and rewards that depend on the commercial success of the joint operating activity."
}
}
},
"auth_ref": [
"r418"
]
},
"anab_CollaborativeArrangementPayment": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CollaborativeArrangementPayment",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Upfront payment",
"label": "Collaborative Arrangement, Payment",
"documentation": "Collaborative Arrangement, Payment"
}
}
},
"auth_ref": []
},
"anab_CommercialAndCorporateObligationsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CommercialAndCorporateObligationsMember",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"verboseLabel": "Commercial and corporate obligations",
"label": "Commercial And Corporate Obligations [Member]",
"documentation": "Commercial And Corporate Obligations [Member]"
}
}
},
"auth_ref": []
},
"anab_CommercialSalesMilestoneMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CommercialSalesMilestoneMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Commercial sales first exceed $100.0 million",
"label": "Commercial Sales Milestone [Member]",
"documentation": "Commercial Sales Milestone"
}
}
},
"auth_ref": []
},
"us-gaap_CommitmentsAndContingenciesDisclosureAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommitmentsAndContingenciesDisclosureAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Commitments and Contingencies Disclosure [Abstract]",
"label": "Commitments and Contingencies Disclosure [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_CommitmentsAndContingenciesDisclosureTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommitmentsAndContingenciesDisclosureTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingencies"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Commitments and Contingencies",
"label": "Commitments and Contingencies Disclosure [Text Block]",
"documentation": "The entire disclosure for commitments and contingencies."
}
}
},
"auth_ref": [
"r63",
"r300",
"r301",
"r655",
"r946",
"r948"
]
},
"anab_CommonStockCapitalSharesReservedForFutureIssuanceAnnualIncreasePercentOfAggregateSharesOutstanding": {
"xbrltype": "percentItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CommonStockCapitalSharesReservedForFutureIssuanceAnnualIncreasePercentOfAggregateSharesOutstanding",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Annual increase in number of shares available for issuance percentage (as a percent)",
"label": "Common Stock, Capital Shares Reserved For Future Issuance, Annual Increase, Percent Of Aggregate Shares Outstanding",
"documentation": "Common Stock, Capital Shares Reserved For Future Issuance, Annual Increase, Percent Of Aggregate Shares Outstanding"
}
}
},
"auth_ref": []
},
"us-gaap_CommonStockMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommonStockMember",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Common Stock",
"label": "Common Stock [Member]",
"documentation": "Stock that is subordinate to all other stock of the issuer."
}
}
},
"auth_ref": [
"r744",
"r745",
"r746",
"r748",
"r749",
"r750",
"r751",
"r926",
"r927",
"r929",
"r1006",
"r1063",
"r1067"
]
},
"us-gaap_CommonStockParOrStatedValuePerShare": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommonStockParOrStatedValuePerShare",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Common stock, par value (in dollars per share)",
"label": "Common Stock, Par or Stated Value Per Share",
"documentation": "Face amount or stated value per share of common stock."
}
}
},
"auth_ref": [
"r43"
]
},
"us-gaap_CommonStockSharesAuthorized": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommonStockSharesAuthorized",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical",
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Common stock, shares authorized (in shares)",
"label": "Common Stock, Shares Authorized",
"documentation": "The maximum number of common shares permitted to be issued by an entity's charter and bylaws."
}
}
},
"auth_ref": [
"r43",
"r603"
]
},
"us-gaap_CommonStockSharesIssued": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommonStockSharesIssued",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical",
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Common stock, shares issued (in shares)",
"label": "Common Stock, Shares, Issued",
"documentation": "Total number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury."
}
}
},
"auth_ref": [
"r43"
]
},
"us-gaap_CommonStockSharesOutstanding": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommonStockSharesOutstanding",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical",
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity",
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Common stock, shares outstanding (in shares)",
"periodStartLabel": "Stockholders' equity, beginning balance (in shares)",
"periodEndLabel": "Stockholders' equity, ending balance (in shares)",
"label": "Common Stock, Shares, Outstanding",
"documentation": "Number of shares of common stock outstanding. Common stock represent the ownership interest in a corporation."
}
}
},
"auth_ref": [
"r8",
"r43",
"r603",
"r621",
"r1067",
"r1068"
]
},
"us-gaap_CommonStockValue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "CommonStockValue",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Common stock, $0.001 par value, 500,000 shares authorized, 27,973 shares and 30,473 shares issued and outstanding at June\u00a030, 2025 and December\u00a031, 2024, respectively",
"label": "Common Stock, Value, Issued",
"documentation": "Aggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity."
}
}
},
"auth_ref": [
"r43",
"r321",
"r327",
"r542",
"r734"
]
},
"ecd_CompActuallyPaidVsCoSelectedMeasureTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "CompActuallyPaidVsCoSelectedMeasureTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Compensation Actually Paid vs. Company Selected Measure",
"label": "Compensation Actually Paid vs. Company Selected Measure [Text Block]"
}
}
},
"auth_ref": [
"r807"
]
},
"ecd_CompActuallyPaidVsNetIncomeTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "CompActuallyPaidVsNetIncomeTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Compensation Actually Paid vs. Net Income",
"label": "Compensation Actually Paid vs. Net Income [Text Block]"
}
}
},
"auth_ref": [
"r806"
]
},
"ecd_CompActuallyPaidVsOtherMeasureTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "CompActuallyPaidVsOtherMeasureTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Compensation Actually Paid vs. Other Measure",
"label": "Compensation Actually Paid vs. Other Measure [Text Block]"
}
}
},
"auth_ref": [
"r808"
]
},
"ecd_CompActuallyPaidVsTotalShareholderRtnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "CompActuallyPaidVsTotalShareholderRtnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Compensation Actually Paid vs. Total Shareholder Return",
"label": "Compensation Actually Paid vs. Total Shareholder Return [Text Block]"
}
}
},
"auth_ref": [
"r805"
]
},
"us-gaap_ComprehensiveIncomeNetOfTax": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ComprehensiveIncomeNetOfTax",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Comprehensive loss",
"label": "Comprehensive Income (Loss), Net of Tax, Attributable to Parent",
"documentation": "Amount after tax of increase (decrease) in equity from transactions and other events and circumstances from net income and other comprehensive income, attributable to parent entity. Excludes changes in equity resulting from investments by owners and distributions to owners."
}
}
},
"auth_ref": [
"r15",
"r135",
"r137",
"r141",
"r531",
"r552",
"r553"
]
},
"us-gaap_ConsolidationPolicyTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ConsolidationPolicyTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Basis of Consolidation",
"label": "Consolidation, Policy [Policy Text Block]",
"documentation": "Disclosure of accounting policy regarding (1) the principles it follows in consolidating or combining the separate financial statements, including the principles followed in determining the inclusion or exclusion of subsidiaries or other entities in the consolidated or combined financial statements and (2) its treatment of interests (for example, common stock, a partnership interest or other means of exerting influence) in other entities, for example consolidation or use of the equity or cost methods of accounting. The accounting policy may also address the accounting treatment for intercompany accounts and transactions, noncontrolling interest, and the income statement treatment in consolidation for issuances of stock by a subsidiary."
}
}
},
"auth_ref": [
"r38",
"r673"
]
},
"us-gaap_ContractWithCustomerAssetAndLiabilityTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ContractWithCustomerAssetAndLiabilityTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Royalty Transaction Activity",
"label": "Contract with Customer, Contract Asset, Contract Liability, and Receivable [Table Text Block]",
"documentation": "Tabular disclosure of receivable, contract asset, and contract liability from contract with customer. Includes, but is not limited to, change in contract asset and contract liability."
}
}
},
"auth_ref": [
"r956"
]
},
"us-gaap_ContractWithCustomerTimingOfSatisfactionOfPerformanceObligationAndPayment": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ContractWithCustomerTimingOfSatisfactionOfPerformanceObligationAndPayment",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Milestone revenue recognition",
"label": "Contract with Customer, Timing of Satisfaction of Performance Obligation and Payment",
"documentation": "Description of effect, from relationship that timing of satisfaction of performance obligation has on timing of payment, on right to consideration in exchange for good or service transferred to customer when right is conditioned on something other than passage of time and on obligation to transfer good or service to customer for which consideration from customer has been received or is due."
}
}
},
"auth_ref": [
"r349"
]
},
"srt_CounterpartyNameAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "CounterpartyNameAxis",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Counterparty Name [Axis]",
"label": "Counterparty Name [Axis]",
"documentation": "Information by name of counterparty. A counterparty is the other party that participates in a financial transaction. Examples include, but not limited to, the name of the financial institution."
}
}
},
"auth_ref": [
"r107",
"r108",
"r154",
"r158",
"r159",
"r315",
"r332",
"r492",
"r511",
"r538",
"r664",
"r665",
"r666",
"r863",
"r864",
"r865",
"r866",
"r867",
"r868",
"r869",
"r870",
"r871",
"r1001",
"r1002",
"r1003",
"r1004"
]
},
"dei_CoverAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "CoverAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Cover [Abstract]",
"label": "Cover [Abstract]",
"documentation": "Cover page."
}
}
},
"auth_ref": []
},
"anab_CumulativeMilestonesRecognizedMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CumulativeMilestonesRecognizedMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Milestones recognized through June 30, 2025",
"label": "Cumulative Milestones Recognized [Member]",
"documentation": "Cumulative Milestones Recognized"
}
}
},
"auth_ref": []
},
"anab_CumulativeRepaymentOfAdvanceFutureRoyaltyAndMilestones": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "CumulativeRepaymentOfAdvanceFutureRoyaltyAndMilestones",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalties and milestones received",
"label": "Cumulative Repayment Of Advance Future Royalty And Milestones",
"documentation": "Cumulative Repayment Of Advance Future Royalty And Milestones"
}
}
},
"auth_ref": []
},
"dei_CurrentFiscalYearEndDate": {
"xbrltype": "gMonthDayItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "CurrentFiscalYearEndDate",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Current Fiscal Year End Date",
"label": "Current Fiscal Year End Date",
"documentation": "End date of current fiscal year in the format --MM-DD."
}
}
},
"auth_ref": []
},
"anab_DRIHealthcareTrustMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "DRIHealthcareTrustMember",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "DRI Healthcare Trust",
"label": "DRI Healthcare Trust [Member]",
"documentation": "DRI Healthcare Trust"
}
}
},
"auth_ref": []
},
"anab_DanielFagaMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "DanielFagaMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"label": "Daniel Faga [Member]",
"documentation": "Daniel Faga"
}
}
},
"auth_ref": []
},
"us-gaap_DebtSecuritiesAvailableForSaleAllowanceForCreditLoss": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleAllowanceForCreditLoss",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Allowance for credit losses",
"label": "Debt Securities, Available-for-Sale, Allowance for Credit Loss",
"documentation": "Amount of allowance for credit loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r233",
"r268",
"r275",
"r276"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLonger": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLonger",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails": {
"parentTag": "us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPosition",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "12 months or greater, Fair value",
"label": "Debt Securities, Available-for-Sale, Continuous Unrealized Loss Position, 12 Months or Longer",
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for more than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset."
}
}
},
"auth_ref": [
"r83",
"r272",
"r691"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerAccumulatedLoss": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPosition12MonthsOrLongerAccumulatedLoss",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails": {
"parentTag": "us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPositionAccumulatedLoss",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsNarrativeDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "12 months or greater, Gross unrealized losses",
"terseLabel": "Unrealized losses on available for sale investments in unrealized loss position for greater than 12 months",
"label": "Debt Securities, Available-for-Sale, Continuous Unrealized Loss Position, 12 Months or Longer, Accumulated Loss",
"documentation": "Amount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for 12 months or longer, without allowance for credit loss. Includes beneficial interest in securitized financial asset."
}
}
},
"auth_ref": [
"r83",
"r272"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12Months",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails": {
"parentTag": "us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPosition",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Less than 12 months, Fair value",
"label": "Debt Securities, Available-for-Sale, Continuous Unrealized Loss Position, Less than 12 Months",
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset."
}
}
},
"auth_ref": [
"r83",
"r272",
"r691"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12MonthsAccumulatedLoss": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleContinuousUnrealizedLossPositionLessThan12MonthsAccumulatedLoss",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails": {
"parentTag": "us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPositionAccumulatedLoss",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Less than 12 months, Gross unrealized losses",
"label": "Debt Securities, Available-for-Sale, Continuous Unrealized Loss Position, Less than 12 Months, Accumulated Loss",
"documentation": "Amount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in continuous unrealized loss position for less than 12 months, without allowance for credit loss. Includes beneficial interest in securitized financial asset."
}
}
},
"auth_ref": [
"r83",
"r272"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleTable",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Debt Securities, Available-for-sale [Table]",
"label": "Debt Securities, Available-for-Sale [Table]",
"documentation": "Disclosure of information about investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r230",
"r231",
"r232",
"r233",
"r234",
"r235",
"r236",
"r237",
"r238",
"r239",
"r240",
"r241"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPosition": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleUnrealizedLossPosition",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Fair Value",
"label": "Debt Securities, Available-for-Sale, Unrealized Loss Position",
"documentation": "Amount of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position without allowance for credit loss."
}
}
},
"auth_ref": [
"r81",
"r270",
"r691"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPositionAccumulatedLoss": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleUnrealizedLossPositionAccumulatedLoss",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTotalLabel": "Gross\u2028Unrealized Losses",
"label": "Debt Securities, Available-for-Sale, Unrealized Loss Position, Accumulated Loss",
"documentation": "Amount of accumulated unrealized loss on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position, without allowance for credit loss. Includes beneficial interest in securitized financial asset."
}
}
},
"auth_ref": [
"r82",
"r271"
]
},
"us-gaap_DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DebtSecuritiesAvailableForSaleUnrealizedLossPositionFairValueTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Unrealized Loss and Fair Values in a Loss Position",
"label": "Debt Securities, Available-for-Sale, Unrealized Loss Position, Fair Value [Table Text Block]",
"documentation": "Tabular disclosure of fair value of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale), in unrealized loss position, without allowance for credit loss. Includes beneficial interest in securitized financial asset."
}
}
},
"auth_ref": [
"r80",
"r691",
"r945"
]
},
"anab_DennisMulroyMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "DennisMulroyMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"label": "Dennis Mulroy [Member]",
"documentation": "Dennis Mulroy"
}
}
},
"auth_ref": []
},
"us-gaap_DepreciationDepletionAndAmortization": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DepreciationDepletionAndAmortization",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Depreciation and amortization",
"label": "Depreciation, Depletion and Amortization",
"documentation": "The aggregate expense recognized in the current period that allocates the cost of tangible assets, intangible assets, or depleting assets to periods that benefit from use of the assets."
}
}
},
"auth_ref": [
"r5",
"r143",
"r186",
"r203",
"r220",
"r667",
"r685",
"r686"
]
},
"srt_DirectorMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "DirectorMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Directors",
"label": "Director [Member]",
"documentation": "Person serving on board of directors."
}
}
},
"auth_ref": [
"r858",
"r937",
"r1064"
]
},
"us-gaap_DisaggregationOfRevenueLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DisaggregationOfRevenueLineItems",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Disaggregation of Revenue [Line Items]",
"label": "Disaggregation of Revenue [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r347",
"r348",
"r698",
"r699",
"r700",
"r701",
"r702",
"r703",
"r704"
]
},
"us-gaap_DisaggregationOfRevenueTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DisaggregationOfRevenueTable",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Disaggregation of Revenue [Table]",
"label": "Disaggregation of Revenue [Table]",
"documentation": "Disclosure of information about disaggregation of revenue into categories depicting how nature, amount, timing, and uncertainty of revenue and cash flows are affected by economic factor."
}
}
},
"auth_ref": [
"r347",
"r348",
"r698",
"r699",
"r700",
"r701",
"r702",
"r703",
"r704"
]
},
"us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlans"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Incentive Plans",
"label": "Share-Based Payment Arrangement [Text Block]",
"documentation": "The entire disclosure for share-based payment arrangement."
}
}
},
"auth_ref": [
"r361",
"r364",
"r393",
"r394",
"r396",
"r711"
]
},
"us-gaap_DisclosureOfCompensationRelatedCostsSharebasedPaymentsAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DisclosureOfCompensationRelatedCostsSharebasedPaymentsAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Share-based Payment Arrangement [Abstract]",
"label": "Share-Based Payment Arrangement [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_DisclosureOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "DisclosureOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Grant Date Fair Value Assumptions",
"label": "Disclosure of Share-Based Compensation Arrangements by Share-Based Payment Award [Table Text Block]",
"documentation": "Tabular disclosure of share-based payment arrangement."
}
}
},
"auth_ref": [
"r962"
]
},
"dei_DocumentFiscalPeriodFocus": {
"xbrltype": "fiscalPeriodItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "DocumentFiscalPeriodFocus",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Fiscal Period Focus",
"label": "Document Fiscal Period Focus",
"documentation": "Fiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY."
}
}
},
"auth_ref": []
},
"dei_DocumentFiscalYearFocus": {
"xbrltype": "gYearItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "DocumentFiscalYearFocus",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Fiscal Year Focus",
"label": "Document Fiscal Year Focus",
"documentation": "This is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006."
}
}
},
"auth_ref": []
},
"dei_DocumentPeriodEndDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "DocumentPeriodEndDate",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Period End Date",
"label": "Document Period End Date",
"documentation": "For the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD."
}
}
},
"auth_ref": []
},
"dei_DocumentQuarterlyReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "DocumentQuarterlyReport",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Quarterly Report",
"label": "Document Quarterly Report",
"documentation": "Boolean flag that is true only for a form used as an quarterly report."
}
}
},
"auth_ref": [
"r757"
]
},
"dei_DocumentTransitionReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "DocumentTransitionReport",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Transition Report",
"label": "Document Transition Report",
"documentation": "Boolean flag that is true only for a form used as a transition report."
}
}
},
"auth_ref": [
"r789"
]
},
"dei_DocumentType": {
"xbrltype": "submissionTypeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "DocumentType",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Document Type",
"label": "Document Type",
"documentation": "The type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'."
}
}
},
"auth_ref": []
},
"anab_DrugSupplyMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "DrugSupplyMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Existing drug supply",
"label": "Drug Supply [Member]",
"documentation": "Drug Supply"
}
}
},
"auth_ref": []
},
"ecd_DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "DvddsOrOthrErngsPdOnEqtyAwrdsNtOthrwsRflctdInTtlCompForCvrdYrMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Dividends or Other Earnings Paid on Equity Awards not Otherwise Reflected in Total Compensation for Covered Year",
"label": "Dividends or Other Earnings Paid on Equity Awards not Otherwise Reflected in Total Compensation for Covered Year [Member]"
}
}
},
"auth_ref": [
"r800"
]
},
"us-gaap_EarningsPerShareAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EarningsPerShareAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Net loss per common share:",
"label": "Earnings Per Share [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_EarningsPerShareBasic": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EarningsPerShareBasic",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Basic (in dollars per share)",
"label": "Earnings Per Share, Basic",
"documentation": "The amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period."
}
}
},
"auth_ref": [
"r114",
"r142",
"r165",
"r166",
"r167",
"r168",
"r169",
"r170",
"r171",
"r172",
"r176",
"r178",
"r180",
"r181",
"r182",
"r185",
"r319",
"r397",
"r410",
"r415",
"r441",
"r442",
"r532",
"r554",
"r676"
]
},
"us-gaap_EarningsPerShareDiluted": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EarningsPerShareDiluted",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Diluted (in dollars per share)",
"label": "Earnings Per Share, Diluted",
"documentation": "The amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period."
}
}
},
"auth_ref": [
"r114",
"r142",
"r165",
"r166",
"r167",
"r168",
"r169",
"r170",
"r171",
"r172",
"r178",
"r180",
"r181",
"r182",
"r185",
"r319",
"r397",
"r410",
"r415",
"r441",
"r442",
"r532",
"r554",
"r676"
]
},
"us-gaap_EarningsPerSharePolicyTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EarningsPerSharePolicyTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Net Loss Per Common Share",
"label": "Earnings Per Share, Policy [Policy Text Block]",
"documentation": "Disclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements."
}
}
},
"auth_ref": [
"r20",
"r21",
"r184"
]
},
"us-gaap_EmployeeRelatedLiabilitiesCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EmployeeRelatedLiabilitiesCurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails": {
"parentTag": "us-gaap_AccruedLiabilitiesCurrent",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofAccruedExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accrued compensation and related expenses",
"label": "Employee-related Liabilities, Current",
"documentation": "Total of the carrying values as of the balance sheet date of obligations incurred through that date and payable for obligations related to services received from employees, such as accrued salaries and bonuses, payroll taxes and fringe benefits. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer)."
}
}
},
"auth_ref": [
"r40"
]
},
"us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedPeriodForRecognition1",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted average period remaining for amortization of unrecognized compensation cost (in years)",
"label": "Share-Based Payment Arrangement, Nonvested Award, Cost Not yet Recognized, Period for Recognition",
"documentation": "Weighted-average period over which cost not yet recognized is expected to be recognized for award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r395"
]
},
"us-gaap_EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EmployeeServiceShareBasedCompensationNonvestedAwardsTotalCompensationCostNotYetRecognizedStockOptions",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Unrecognized compensation cost",
"label": "Share-Based Payment Arrangement, Nonvested Award, Option, Cost Not yet Recognized, Amount",
"documentation": "Amount of cost to be recognized for option under share-based payment arrangement."
}
}
},
"auth_ref": [
"r989"
]
},
"us-gaap_EmployeeStockMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EmployeeStockMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "ESPP",
"label": "Employee Stock [Member]",
"documentation": "An Employee Stock Purchase Plan is a tax-efficient means by which employees of a corporation can purchase the corporation's stock."
}
}
},
"auth_ref": []
},
"us-gaap_EmployeeStockOptionMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EmployeeStockOptionMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails",
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails",
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Options to purchase common stock",
"verboseLabel": "Stock Options",
"label": "Share-Based Payment Arrangement, Option [Member]",
"documentation": "Share-based payment arrangement granting right, subject to vesting and other restrictions, to purchase or sell certain number of shares at predetermined price for specified period of time."
}
}
},
"auth_ref": [
"r963",
"r964",
"r965",
"r966",
"r967",
"r968",
"r969",
"r970",
"r971",
"r972",
"r973",
"r974",
"r975",
"r976",
"r977",
"r978",
"r979",
"r980",
"r981",
"r982",
"r983",
"r984",
"r985",
"r986",
"r987",
"r988"
]
},
"anab_EmployeeStockPurchasePlan2017Member": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "EmployeeStockPurchasePlan2017Member",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Employee Stock Purchase Plan, 2017",
"label": "Employee Stock Purchase Plan, 2017 [Member]",
"documentation": "Employee Stock Purchase Plan, 2017 [Member]"
}
}
},
"auth_ref": []
},
"dei_EntityAddressAddressLine1": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityAddressAddressLine1",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, Address Line One",
"label": "Entity Address, Address Line One",
"documentation": "Address Line 1 such as Attn, Building Name, Street Name"
}
}
},
"auth_ref": []
},
"dei_EntityAddressAddressLine2": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityAddressAddressLine2",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, Address Line Two",
"label": "Entity Address, Address Line Two",
"documentation": "Address Line 2 such as Street or Suite number"
}
}
},
"auth_ref": []
},
"dei_EntityAddressCityOrTown": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityAddressCityOrTown",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, City or Town",
"label": "Entity Address, City or Town",
"documentation": "Name of the City or Town"
}
}
},
"auth_ref": []
},
"dei_EntityAddressPostalZipCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityAddressPostalZipCode",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, Postal Zip Code",
"label": "Entity Address, Postal Zip Code",
"documentation": "Code for the postal or zip code"
}
}
},
"auth_ref": []
},
"dei_EntityAddressStateOrProvince": {
"xbrltype": "stateOrProvinceItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityAddressStateOrProvince",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Address, State or Province",
"label": "Entity Address, State or Province",
"documentation": "Name of the state or province."
}
}
},
"auth_ref": []
},
"dei_EntityCentralIndexKey": {
"xbrltype": "centralIndexKeyItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityCentralIndexKey",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Central Index Key",
"label": "Entity Central Index Key",
"documentation": "A unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK."
}
}
},
"auth_ref": [
"r755"
]
},
"dei_EntityCommonStockSharesOutstanding": {
"xbrltype": "sharesItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityCommonStockSharesOutstanding",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Common Stock, Shares Outstanding",
"label": "Entity Common Stock, Shares Outstanding",
"documentation": "Indicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument."
}
}
},
"auth_ref": []
},
"dei_EntityCurrentReportingStatus": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityCurrentReportingStatus",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Current Reporting Status",
"label": "Entity Current Reporting Status",
"documentation": "Indicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure."
}
}
},
"auth_ref": []
},
"dei_EntityEmergingGrowthCompany": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityEmergingGrowthCompany",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Emerging Growth Company",
"label": "Entity Emerging Growth Company",
"documentation": "Indicate if registrant meets the emerging growth company criteria."
}
}
},
"auth_ref": [
"r755"
]
},
"dei_EntityFileNumber": {
"xbrltype": "fileNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityFileNumber",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity File Number",
"label": "Entity File Number",
"documentation": "Commission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen."
}
}
},
"auth_ref": []
},
"dei_EntityFilerCategory": {
"xbrltype": "filerCategoryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityFilerCategory",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Filer Category",
"label": "Entity Filer Category",
"documentation": "Indicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure."
}
}
},
"auth_ref": [
"r755"
]
},
"dei_EntityIncorporationStateCountryCode": {
"xbrltype": "edgarStateCountryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityIncorporationStateCountryCode",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Incorporation, State or Country Code",
"label": "Entity Incorporation, State or Country Code",
"documentation": "Two-character EDGAR code representing the state or country of incorporation."
}
}
},
"auth_ref": []
},
"dei_EntityInteractiveDataCurrent": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityInteractiveDataCurrent",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Interactive Data Current",
"label": "Entity Interactive Data Current",
"documentation": "Boolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files)."
}
}
},
"auth_ref": [
"r839"
]
},
"dei_EntityRegistrantName": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityRegistrantName",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Registrant Name",
"label": "Entity Registrant Name",
"documentation": "The exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC."
}
}
},
"auth_ref": [
"r755"
]
},
"dei_EntityShellCompany": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityShellCompany",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Shell Company",
"label": "Entity Shell Company",
"documentation": "Boolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act."
}
}
},
"auth_ref": [
"r755"
]
},
"dei_EntitySmallBusiness": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntitySmallBusiness",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Small Business",
"label": "Entity Small Business",
"documentation": "Indicates that the company is a Smaller Reporting Company (SRC)."
}
}
},
"auth_ref": [
"r755"
]
},
"dei_EntityTaxIdentificationNumber": {
"xbrltype": "employerIdItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "EntityTaxIdentificationNumber",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Entity Tax Identification Number",
"label": "Entity Tax Identification Number",
"documentation": "The Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS."
}
}
},
"auth_ref": [
"r755"
]
},
"ecd_EqtyAwrdsAdjFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "EqtyAwrdsAdjFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Awards Adjustments, Footnote",
"label": "Equity Awards Adjustments, Footnote [Text Block]"
}
}
},
"auth_ref": [
"r794"
]
},
"ecd_EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "EqtyAwrdsAdjsExclgValRprtdInSummryCompstnTblMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Awards Adjustments, Excluding Value Reported in Compensation Table",
"label": "Equity Awards Adjustments, Excluding Value Reported in the Compensation Table [Member]"
}
}
},
"auth_ref": [
"r835"
]
},
"ecd_EqtyAwrdsAdjsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "EqtyAwrdsAdjsMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Awards Adjustments",
"label": "Equity Awards Adjustments [Member]"
}
}
},
"auth_ref": [
"r835"
]
},
"ecd_EqtyAwrdsInSummryCompstnTblForAplblYrMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "EqtyAwrdsInSummryCompstnTblForAplblYrMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate Grant Date Fair Value of Equity Award Amounts Reported in Summary Compensation Table",
"label": "Aggregate Grant Date Fair Value of Equity Award Amounts Reported in Summary Compensation Table [Member]"
}
}
},
"auth_ref": [
"r835"
]
},
"us-gaap_EquityAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EquityAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity [Abstract]",
"label": "Equity [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_EquityComponentDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "EquityComponentDomain",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Component [Domain]",
"label": "Equity Component [Domain]",
"documentation": "Components of equity are the parts of the total Equity balance including that which is allocated to common, preferred, treasury stock, retained earnings, etc."
}
}
},
"auth_ref": [
"r8",
"r112",
"r113",
"r114",
"r138",
"r139",
"r140",
"r160",
"r161",
"r162",
"r164",
"r171",
"r173",
"r175",
"r187",
"r261",
"r262",
"r293",
"r318",
"r345",
"r397",
"r404",
"r405",
"r407",
"r408",
"r409",
"r411",
"r414",
"r415",
"r428",
"r429",
"r430",
"r431",
"r432",
"r433",
"r434",
"r435",
"r436",
"r437",
"r440",
"r472",
"r473",
"r474",
"r475",
"r476",
"r477",
"r478",
"r479",
"r491",
"r550",
"r578",
"r579",
"r580",
"r592",
"r640"
]
},
"anab_EquityIncentivePlan2017Member": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "EquityIncentivePlan2017Member",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Incentive Plan, 2017",
"label": "Equity Incentive Plan, 2017 [Member]",
"documentation": "Equity Incentive Plan, 2017 [Member]"
}
}
},
"auth_ref": []
},
"ecd_EquityValuationAssumptionDifferenceFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "EquityValuationAssumptionDifferenceFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Valuation Assumption Difference, Footnote",
"label": "Equity Valuation Assumption Difference, Footnote [Text Block]"
}
}
},
"auth_ref": [
"r804"
]
},
"anab_EricLoumeauMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "EricLoumeauMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"label": "Eric Loumeau [Member]",
"documentation": "Eric Loumeau"
}
}
},
"auth_ref": []
},
"ecd_ErrCompAnalysisTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ErrCompAnalysisTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Erroneous Compensation Analysis",
"label": "Erroneous Compensation Analysis [Text Block]"
}
}
},
"auth_ref": [
"r762",
"r772",
"r782",
"r814"
]
},
"ecd_ErrCompRecoveryTable": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ErrCompRecoveryTable",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Erroneously Awarded Compensation Recovery",
"label": "Erroneously Awarded Compensation Recovery [Table]"
}
}
},
"auth_ref": [
"r759",
"r769",
"r779",
"r811"
]
},
"anab_ExclusiveLicenseAgreementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ExclusiveLicenseAgreementMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Exclusive License Agreement",
"label": "Exclusive License Agreement [Member]",
"documentation": "Exclusive License Agreement"
}
}
},
"auth_ref": []
},
"ecd_ExecutiveCategoryAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ExecutiveCategoryAxis",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Executive Category:",
"label": "Executive Category [Axis]"
}
}
},
"auth_ref": [
"r810"
]
},
"anab_ExternalExpensesMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ExternalExpensesMember",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "External",
"label": "External Expenses [Member]",
"documentation": "External Expenses"
}
}
},
"auth_ref": []
},
"anab_FDARegulatoryApprovalForMarketingOfFirstLicensedProductInTheUSAForTheTreatmentOfActiveFlaresInGPPMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FDARegulatoryApprovalForMarketingOfFirstLicensedProductInTheUSAForTheTreatmentOfActiveFlaresInGPPMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "FDA regulatory approval for marketing of first licensed product in the USA for the treatment of active flares in GPP",
"label": "FDA Regulatory Approval For Marketing Of First Licensed Product In The USA For The Treatment Of Active Flares In GPP [Member]",
"documentation": "FDA Regulatory Approval For Marketing Of First Licensed Product In The USA For The Treatment Of Active Flares In GPP"
}
}
},
"auth_ref": []
},
"us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisLineItems",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items]",
"label": "Fair Value, Assets and Liabilities Measured on Recurring and Nonrecurring Basis [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r444",
"r445",
"r455",
"r722"
]
},
"us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisTable",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Fair Value, Recurring and Nonrecurring [Table]",
"label": "Fair Value, Recurring and Nonrecurring [Table]",
"documentation": "Disclosure of information about asset and liability measured at fair value on recurring and nonrecurring basis."
}
}
},
"auth_ref": [
"r444",
"r445",
"r455",
"r722"
]
},
"us-gaap_FairValueByFairValueHierarchyLevelAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueByFairValueHierarchyLevelAxis",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Fair Value Hierarchy and NAV [Axis]",
"label": "Fair Value Hierarchy and NAV [Axis]",
"documentation": "Information by level within fair value hierarchy and fair value measured at net asset value per share as practical expedient."
}
}
},
"auth_ref": [
"r316",
"r351",
"r352",
"r353",
"r354",
"r355",
"r356",
"r357",
"r358",
"r443",
"r445",
"r446",
"r447",
"r448",
"r454",
"r455",
"r457",
"r464",
"r498",
"r499",
"r500",
"r661",
"r693",
"r694",
"r705",
"r706",
"r707",
"r708",
"r709",
"r722",
"r725",
"r732"
]
},
"us-gaap_FairValueByMeasurementFrequencyAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueByMeasurementFrequencyAxis",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Measurement Frequency [Axis]",
"label": "Measurement Frequency [Axis]",
"documentation": "Information by measurement frequency."
}
}
},
"auth_ref": [
"r444",
"r445",
"r446",
"r448",
"r722",
"r1011",
"r1014"
]
},
"us-gaap_FairValueDisclosuresAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueDisclosuresAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Fair Value Disclosures [Abstract]",
"label": "Fair Value Disclosures [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_FairValueDisclosuresTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueDisclosuresTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestments"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Fair Value Measurements and Available for Sale Investments",
"label": "Fair Value Disclosures [Text Block]",
"documentation": "The entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information."
}
}
},
"auth_ref": [
"r446",
"r450",
"r452",
"r453",
"r454",
"r457",
"r458",
"r459",
"r460",
"r461",
"r529",
"r722",
"r726"
]
},
"us-gaap_FairValueInputsLevel1Member": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueInputsLevel1Member",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Quoted\u00a0Market Prices for Identical\u00a0Assets (Level 1)",
"label": "Fair Value, Inputs, Level 1 [Member]",
"documentation": "Quoted prices in active markets for identical assets or liabilities that the reporting entity can access at the measurement date."
}
}
},
"auth_ref": [
"r316",
"r351",
"r356",
"r357",
"r445",
"r455",
"r464",
"r498",
"r661",
"r705",
"r706",
"r707",
"r708",
"r709",
"r722",
"r732"
]
},
"us-gaap_FairValueInputsLevel2Member": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueInputsLevel2Member",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Significant Other\u00a0Observable Inputs (Level 2)",
"label": "Fair Value, Inputs, Level 2 [Member]",
"documentation": "Inputs other than quoted prices included within level 1 that are observable for an asset or liability, either directly or indirectly, including, but not limited to, quoted prices for similar assets or liabilities in active markets, or quoted prices for identical or similar assets or liabilities in inactive markets."
}
}
},
"auth_ref": [
"r316",
"r351",
"r356",
"r357",
"r359",
"r445",
"r446",
"r455",
"r464",
"r499",
"r661",
"r693",
"r694",
"r705",
"r706",
"r707",
"r708",
"r709",
"r722",
"r732"
]
},
"us-gaap_FairValueInputsLevel3Member": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueInputsLevel3Member",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Significant Unobservable Inputs (Level 3)",
"label": "Fair Value, Inputs, Level 3 [Member]",
"documentation": "Unobservable inputs that reflect the entity's own assumption about the assumptions market participants would use in pricing."
}
}
},
"auth_ref": [
"r316",
"r351",
"r352",
"r353",
"r354",
"r355",
"r356",
"r357",
"r358",
"r445",
"r446",
"r447",
"r448",
"r455",
"r464",
"r500",
"r661",
"r693",
"r694",
"r705",
"r706",
"r707",
"r708",
"r709",
"r722",
"r725",
"r732"
]
},
"us-gaap_FairValueMeasurementFrequencyDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueMeasurementFrequencyDomain",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Measurement Frequency [Domain]",
"label": "Measurement Frequency [Domain]",
"documentation": "Measurement frequency."
}
}
},
"auth_ref": [
"r444",
"r445",
"r446",
"r448",
"r722",
"r1011",
"r1014"
]
},
"us-gaap_FairValueMeasurementsFairValueHierarchyDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueMeasurementsFairValueHierarchyDomain",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Fair Value Hierarchy and NAV [Domain]",
"label": "Fair Value Hierarchy and NAV [Domain]",
"documentation": "Categories used to prioritize the inputs to valuation techniques to measure fair value."
}
}
},
"auth_ref": [
"r316",
"r351",
"r352",
"r353",
"r354",
"r355",
"r356",
"r357",
"r358",
"r443",
"r445",
"r446",
"r447",
"r448",
"r454",
"r455",
"r457",
"r464",
"r498",
"r499",
"r500",
"r661",
"r693",
"r694",
"r705",
"r706",
"r707",
"r708",
"r709",
"r722",
"r725",
"r732"
]
},
"us-gaap_FairValueMeasurementsRecurringMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FairValueMeasurementsRecurringMember",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Recurring",
"label": "Fair Value, Recurring [Member]",
"documentation": "Frequent fair value measurement. Includes, but is not limited to, fair value adjustment for impairment of asset, liability or equity, frequently measured at fair value."
}
}
},
"auth_ref": [
"r722",
"r1008",
"r1009",
"r1010",
"r1011",
"r1012",
"r1014"
]
},
"anab_FilingOfBiologicsLicenseApplicationFirstIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FilingOfBiologicsLicenseApplicationFirstIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Filing of the first BLA - first indication",
"label": "Filing of Biologics License Application - First Indication [Member]",
"documentation": "Filing of Biologics License Application - First Indication"
}
}
},
"auth_ref": []
},
"anab_FilingOfBiologicsLicenseApplicationSecondIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FilingOfBiologicsLicenseApplicationSecondIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Filing of the first BLA - second indication",
"label": "Filing of Biologics License Application - Second Indication [Member]",
"documentation": "Filing of Biologics License Application - Second Indication"
}
}
},
"auth_ref": []
},
"anab_FilingOfMMAFirstIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FilingOfMMAFirstIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Filing of the first MAA - first indication",
"label": "Filing of MMA - First Indication [Member]",
"documentation": "Filing of MMA - First Indication"
}
}
},
"auth_ref": []
},
"anab_FilingOfMMASecondIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FilingOfMMASecondIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Filing of the first MAA - second indication",
"label": "Filing of MMA - Second Indication [Member]",
"documentation": "Filing of MMA - Second Indication"
}
}
},
"auth_ref": []
},
"us-gaap_FinancialInstrumentAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "FinancialInstrumentAxis",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Financial Instrument [Axis]",
"label": "Financial Instrument [Axis]",
"documentation": "Information by type of financial instrument."
}
}
},
"auth_ref": [
"r230",
"r231",
"r232",
"r233",
"r234",
"r235",
"r236",
"r237",
"r238",
"r239",
"r240",
"r241",
"r242",
"r243",
"r244",
"r245",
"r246",
"r247",
"r248",
"r249",
"r250",
"r251",
"r252",
"r253",
"r254",
"r255",
"r256",
"r257",
"r258",
"r259",
"r263",
"r264",
"r265",
"r266",
"r267",
"r269",
"r273",
"r274",
"r317",
"r342",
"r427",
"r438",
"r462",
"r467",
"r470",
"r495",
"r496",
"r497",
"r498",
"r499",
"r500",
"r501",
"r502",
"r503",
"r504",
"r505",
"r506",
"r507",
"r508",
"r509",
"r512",
"r513",
"r514",
"r515",
"r516",
"r517",
"r518",
"r519",
"r520",
"r521",
"r522",
"r523",
"r524",
"r525",
"r526",
"r537",
"r551",
"r691",
"r722",
"r723",
"r725",
"r726",
"r727",
"r728",
"r729",
"r730",
"r731",
"r735",
"r843",
"r844",
"r845",
"r846",
"r847",
"r848",
"r849",
"r940",
"r941",
"r942",
"r943",
"r1007",
"r1010",
"r1011",
"r1012",
"r1013",
"r1014",
"r1015",
"r1016"
]
},
"anab_FirstBiologicsLicenseApplicationApprovalDMMRECMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FirstBiologicsLicenseApplicationApprovalDMMRECMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "First BLA approval - first indication",
"label": "First Biologics License Application Approval - dMMREC [Member]",
"documentation": "First Biologics License Application Approval - dMMREC"
}
}
},
"auth_ref": []
},
"anab_FirstBiologicsLicenseApplicationApprovalSecondIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FirstBiologicsLicenseApplicationApprovalSecondIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "First BLA approval - second indication",
"label": "First Biologics License Application Approval - Second Indication [Member]",
"documentation": "First Biologics License Application Approval - Second Indication"
}
}
},
"auth_ref": []
},
"anab_FirstCommercialSalesMilestoneMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FirstCommercialSalesMilestoneMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "First commercial sales milestone",
"label": "First Commercial Sales Milestone [Member]",
"documentation": "First Commercial Sales Milestone"
}
}
},
"auth_ref": []
},
"anab_FirstInstallmentMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FirstInstallmentMember",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "By the end of 2026",
"label": "First Installment [Member]",
"documentation": "First Installment"
}
}
},
"auth_ref": []
},
"anab_FirstMarketingAuthorizationApplicationApprovalFirstIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FirstMarketingAuthorizationApplicationApprovalFirstIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "First MAA approval - first indication",
"label": "First Marketing Authorization Application Approval - First Indication [Member]",
"documentation": "First Marketing Authorization Application Approval - First Indication"
}
}
},
"auth_ref": []
},
"anab_FirstMarketingAuthorizationApplicationApprovalSecondIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FirstMarketingAuthorizationApplicationApprovalSecondIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "First MAA approval - second indication",
"label": "First Marketing Authorization Application Approval - Second Indication [Member]",
"documentation": "First Marketing Authorization Application Approval - Second Indication"
}
}
},
"auth_ref": []
},
"ecd_ForgoneRecoveryDueToDisqualificationOfTaxBenefitsAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ForgoneRecoveryDueToDisqualificationOfTaxBenefitsAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Forgone Recovery due to Disqualification of Tax Benefits, Amount",
"label": "Forgone Recovery due to Disqualification of Tax Benefits, Amount"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r818"
]
},
"ecd_ForgoneRecoveryDueToExpenseOfEnforcementAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ForgoneRecoveryDueToExpenseOfEnforcementAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Forgone Recovery due to Expense of Enforcement, Amount",
"label": "Forgone Recovery due to Expense of Enforcement, Amount"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r818"
]
},
"ecd_ForgoneRecoveryDueToViolationOfHomeCountryLawAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ForgoneRecoveryDueToViolationOfHomeCountryLawAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Forgone Recovery due to Violation of Home Country Law, Amount",
"label": "Forgone Recovery due to Violation of Home Country Law, Amount"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r818"
]
},
"ecd_ForgoneRecoveryExplanationOfImpracticabilityTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ForgoneRecoveryExplanationOfImpracticabilityTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Forgone Recovery, Explanation of Impracticability",
"label": "Forgone Recovery, Explanation of Impracticability [Text Block]"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r818"
]
},
"ecd_ForgoneRecoveryIndName": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "ForgoneRecoveryIndName",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Name",
"label": "Forgone Recovery, Individual Name"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r818"
]
},
"anab_FourthCommercialSalesMilestoneMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "FourthCommercialSalesMilestoneMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Fourth commercial sales milestone",
"label": "Fourth Commercial Sales Milestone [Member]",
"documentation": "Fourth Commercial Sales Milestone"
}
}
},
"auth_ref": []
},
"ecd_FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "FrValAsOfPrrYrEndOfEqtyAwrdsGrntdInPrrYrsFldVstngCondsDrngCvrdYrMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Prior Year End Fair Value of Equity Awards Granted in Any Prior Year that Fail to Meet Applicable Vesting Conditions During Covered Year",
"label": "Prior Year End Fair Value of Equity Awards Granted in Any Prior Year that Fail to Meet Applicable Vesting Conditions During Covered Year [Member]"
}
}
},
"auth_ref": [
"r799"
]
},
"us-gaap_GeneralAndAdministrativeExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "GeneralAndAdministrativeExpense",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_OperatingExpenses",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "General and administrative",
"label": "General and Administrative Expense",
"documentation": "The aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line."
}
}
},
"auth_ref": [
"r52",
"r624"
]
},
"us-gaap_GeneralAndAdministrativeExpenseMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "GeneralAndAdministrativeExpenseMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "General and administrative",
"label": "General and Administrative Expense [Member]",
"documentation": "Primary financial statement caption encompassing general and administrative expense."
}
}
},
"auth_ref": [
"r52"
]
},
"anab_GlaxoSmithKlineMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "GlaxoSmithKlineMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "GSK",
"label": "GlaxoSmithKline [Member]",
"documentation": "GlaxoSmithKline"
}
}
},
"auth_ref": []
},
"anab_ImsidolimabMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ImsidolimabMember",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Imsidolimab",
"label": "Imsidolimab [Member]",
"documentation": "Imsidolimab"
}
}
},
"auth_ref": []
},
"anab_InVivoToxicologyStudiesMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "InVivoToxicologyStudiesMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Initiated in vivo toxicology studies using good laboratory practices (GLPs)",
"label": "In Vivo Toxicology Studies [Member]",
"documentation": "In Vivo Toxicology Studies [Member]"
}
}
},
"auth_ref": []
},
"us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_NetIncomeLoss",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Loss before income taxes",
"terseLabel": "Loss before income taxes",
"label": "Income (Loss) from Continuing Operations before Income Taxes, Noncontrolling Interest",
"documentation": "Amount of income (loss) from continuing operations, including income (loss) from equity method investments, before deduction of income tax expense (benefit), and income (loss) attributable to noncontrolling interest."
}
}
},
"auth_ref": [
"r50",
"r74",
"r78",
"r533",
"r535",
"r548",
"r669",
"r671",
"r672",
"r680",
"r685",
"r931",
"r933",
"r934",
"r935",
"r936"
]
},
"us-gaap_IncomeStatementAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncomeStatementAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Income Statement [Abstract]",
"label": "Income Statement [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_IncomeStatementLocationAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncomeStatementLocationAxis",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Income Statement Location [Axis]",
"label": "Statement of Income Location, Balance [Axis]",
"documentation": "Information by location in statement of income where disaggregated amount is reported."
}
}
},
"auth_ref": [
"r294",
"r296",
"r297",
"r423",
"r424",
"r425",
"r426",
"r449",
"r451",
"r456",
"r469",
"r470",
"r471",
"r575",
"r577",
"r625",
"r660",
"r661",
"r714",
"r715",
"r720",
"r721",
"r724",
"r732",
"r994",
"r995",
"r1034"
]
},
"us-gaap_IncomeStatementLocationDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncomeStatementLocationDomain",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Income Statement Location [Domain]",
"label": "Statement of Income Location, Balance [Domain]",
"documentation": "Location in statement of income where disaggregated amount is reported."
}
}
},
"auth_ref": [
"r296",
"r297",
"r423",
"r424",
"r425",
"r426",
"r449",
"r451",
"r456",
"r469",
"r470",
"r471",
"r575",
"r577",
"r625",
"r660",
"r661",
"r714",
"r715",
"r720",
"r721",
"r724",
"r732",
"r994",
"r995",
"r1034"
]
},
"us-gaap_IncomeTaxExpenseBenefit": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncomeTaxExpenseBenefit",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_NetIncomeLoss",
"weight": -1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Provision for income taxes",
"negatedLabel": "Provision for income taxes",
"label": "Income Tax Expense (Benefit)",
"documentation": "Amount of current income tax expense (benefit) and deferred income tax expense (benefit) pertaining to continuing operations."
}
}
},
"auth_ref": [
"r85",
"r89",
"r154",
"r174",
"r175",
"r186",
"r206",
"r220",
"r402",
"r403",
"r406",
"r555",
"r669",
"r671",
"r672",
"r713"
]
},
"us-gaap_IncomeTaxesPaid": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncomeTaxesPaid",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Income taxes paid",
"label": "Income Taxes Paid",
"documentation": "Amount, before refund, of cash paid to foreign, federal, state, and local jurisdictions as income tax."
}
}
},
"auth_ref": [
"r19",
"r59",
"r918",
"r992",
"r993"
]
},
"us-gaap_IncreaseDecreaseInAccountsPayableAndAccruedLiabilities": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncreaseDecreaseInAccountsPayableAndAccruedLiabilities",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0,
"order": 9.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accounts payable and other liabilities",
"label": "Increase (Decrease) in Accounts Payable and Accrued Liabilities",
"documentation": "The increase (decrease) during the reporting period in the amounts payable to vendors for goods and services received and the amount of obligations and expenses incurred but not paid."
}
}
},
"auth_ref": [
"r4"
]
},
"us-gaap_IncreaseDecreaseInContractWithCustomerAsset": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncreaseDecreaseInContractWithCustomerAsset",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0,
"order": 7.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Receivables from collaborative partners",
"label": "Increase (Decrease) in Contract with Customer, Asset",
"documentation": "Amount of increase (decrease) in right to consideration in exchange for good or service transferred to customer when right is conditioned on something other than passage of time."
}
}
},
"auth_ref": [
"r916"
]
},
"us-gaap_IncreaseDecreaseInOperatingCapitalAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncreaseDecreaseInOperatingCapitalAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Changes in operating assets and liabilities:",
"label": "Adjustment to Reconcile Net Income to Cash Provided by (Used in) Operating Activity, Increase (Decrease) in Operating Capital [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_IncreaseDecreaseInOperatingLeaseLiability": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncreaseDecreaseInOperatingLeaseLiability",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0,
"order": 10.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Operating lease liabilities",
"label": "Increase (Decrease) in Operating Lease Liability",
"documentation": "Amount of increase (decrease) in obligation for operating lease."
}
}
},
"auth_ref": [
"r842",
"r916"
]
},
"us-gaap_IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncreaseDecreaseInPrepaidDeferredExpenseAndOtherAssets",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0,
"order": 8.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Prepaid expenses and other assets",
"label": "Increase (Decrease) in Prepaid Expense and Other Assets",
"documentation": "Amount of increase (decrease) in prepaid expenses, and assets classified as other."
}
}
},
"auth_ref": [
"r4"
]
},
"us-gaap_IncreaseDecreaseInStockholdersEquityRollForward": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "IncreaseDecreaseInStockholdersEquityRollForward",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Increase (Decrease) in Stockholders' Equity [Roll Forward]",
"label": "Increase (Decrease) in Stockholders' Equity [Roll Forward]",
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period."
}
}
},
"auth_ref": []
},
"ecd_IndividualAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "IndividualAxis",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure",
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure",
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements",
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Individual:",
"label": "Individual [Axis]"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r810",
"r818",
"r822",
"r830"
]
},
"ecd_InsiderTradingArrLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "InsiderTradingArrLineItems",
"lang": {
"en-us": {
"role": {
"label": "Insider Trading Arrangements [Line Items]"
}
}
},
"auth_ref": [
"r828"
]
},
"ecd_InsiderTradingPoliciesProcLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "InsiderTradingPoliciesProcLineItems",
"lang": {
"en-us": {
"role": {
"label": "Insider Trading Policies and Procedures [Line Items]"
}
}
},
"auth_ref": [
"r758",
"r834"
]
},
"ecd_InsiderTrdPoliciesProcAdoptedFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "InsiderTrdPoliciesProcAdoptedFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingPoliciesProc"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Insider Trading Policies and Procedures Adopted",
"label": "Insider Trading Policies and Procedures Adopted [Flag]"
}
}
},
"auth_ref": [
"r758",
"r834"
]
},
"ecd_InsiderTrdPoliciesProcNotAdoptedTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "InsiderTrdPoliciesProcNotAdoptedTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingPoliciesProc"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Insider Trading Policies and Procedures Not Adopted",
"label": "Insider Trading Policies and Procedures Not Adopted [Text Block]"
}
}
},
"auth_ref": [
"r758",
"r834"
]
},
"us-gaap_InterestIncomeOther": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "InterestIncomeOther",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_NonoperatingIncomeExpense",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Interest income",
"label": "Interest Income, Other",
"documentation": "Amount of interest income earned from interest bearing assets classified as other."
}
}
},
"auth_ref": [
"r671"
]
},
"us-gaap_InterestPaidNet": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "InterestPaidNet",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Interest portion of repayment for sale of future royalties",
"label": "Interest Paid, Excluding Capitalized Interest, Operating Activity",
"documentation": "Amount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount."
}
}
},
"auth_ref": [
"r147",
"r149",
"r150"
]
},
"anab_InternalExpensesMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "InternalExpensesMember",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Internal",
"label": "Internal Expenses [Member]",
"documentation": "Internal Expenses"
}
}
},
"auth_ref": []
},
"anab_JEMPERLIRoyaltyMonetizationAgreementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "JEMPERLIRoyaltyMonetizationAgreementMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "JEMPERLI Royalty Monetization Agreement",
"label": "JEMPERLI Royalty Monetization Agreement [Member]",
"documentation": "JEMPERLI Royalty Monetization Agreement"
}
}
},
"auth_ref": []
},
"anab_JemperliRoyaltyMonetizationAgreementAmendmentOneMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "JemperliRoyaltyMonetizationAgreementAmendmentOneMember",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Jemperli Royalty Monetization Agreement Amendment 1",
"label": "Jemperli Royalty Monetization Agreement Amendment One [Member]",
"documentation": "Jemperli Royalty Monetization Agreement Amendment One"
}
}
},
"auth_ref": []
},
"anab_JemperliRoyaltyMonetizationAgreementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "JemperliRoyaltyMonetizationAgreementMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Jemperli Royalty Monetization Agreement",
"label": "Jemperli Royalty Monetization Agreement [Member]",
"documentation": "Jemperli Royalty Monetization Agreement"
}
}
},
"auth_ref": []
},
"anab_LaboratoryEquipmentMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "LaboratoryEquipmentMember",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Laboratory equipment",
"label": "Laboratory Equipment [Member]",
"documentation": "Laboratory Equipment [Member]"
}
}
},
"auth_ref": []
},
"us-gaap_LeaseCostTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LeaseCostTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Non-Cancellable Office Lease Costs",
"label": "Lease, Cost [Table Text Block]",
"documentation": "Tabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income."
}
}
},
"auth_ref": [
"r1019"
]
},
"us-gaap_LeaseholdImprovementsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LeaseholdImprovementsMember",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Leasehold improvements",
"label": "Leasehold Improvements [Member]",
"documentation": "Additions or improvements to assets held under a lease arrangement."
}
}
},
"auth_ref": [
"r62",
"r489"
]
},
"us-gaap_LesseeLeaseDescriptionLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeLeaseDescriptionLineItems",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Lessee, Lease, Description [Line Items]",
"label": "Lessee, Lease, Description [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r483",
"r490"
]
},
"us-gaap_LesseeLeaseDescriptionTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeLeaseDescriptionTable",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Lessee, Lease, Description [Table]",
"label": "Lessee, Lease, Description [Table]",
"documentation": "Disclosure of information about lessee's leases."
}
}
},
"auth_ref": [
"r483",
"r490"
]
},
"anab_LesseeOperatingLeaseExpirationPeriod": {
"xbrltype": "durationItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "LesseeOperatingLeaseExpirationPeriod",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Lease termination term (in years)",
"label": "Lessee, Operating Lease, Expiration Period",
"documentation": "Lessee, Operating Lease, Expiration Period"
}
}
},
"auth_ref": []
},
"us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityMaturityTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Future Minimum Annual Obligations",
"label": "Lessee, Operating Lease, Liability, to be Paid, Maturity [Table Text Block]",
"documentation": "Tabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position."
}
}
},
"auth_ref": [
"r1020"
]
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityPaymentsDue",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
},
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails_1": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total minimum payments required",
"label": "Lessee, Operating Lease, Liability, to be Paid",
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease."
}
}
},
"auth_ref": [
"r488",
"r921",
"r925",
"r1031"
]
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails_1": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "2026",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year One",
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach)."
}
}
},
"auth_ref": [
"r488",
"r921",
"r925",
"r1031"
]
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearFour": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueYearFour",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails_1": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "2029",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year Four",
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in fourth fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach)."
}
}
},
"auth_ref": [
"r488",
"r921",
"r925",
"r1031"
]
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueYearThree",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails_1": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 6.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "2028",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year Three",
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach)."
}
}
},
"auth_ref": [
"r488",
"r921",
"r925",
"r1031"
]
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityPaymentsDueYearTwo",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails_1": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "2027",
"label": "Lessee, Operating Lease, Liability, to be Paid, Year Two",
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach)."
}
}
},
"auth_ref": [
"r488",
"r921",
"r925",
"r1031"
]
},
"us-gaap_LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityPaymentsRemainderOfFiscalYear",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails_1": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "2025",
"label": "Lessee, Operating Lease, Liability, to be Paid, Remainder of Fiscal Year",
"documentation": "Amount of lessee's undiscounted obligation for lease payment for operating lease having initial or remaining lease term in excess of one year to be paid in remainder of current fiscal year."
}
}
},
"auth_ref": [
"r1020"
]
},
"anab_LesseeOperatingLeaseLiabilityToBePaidAfterYearFour": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "LesseeOperatingLeaseLiabilityToBePaidAfterYearFour",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails_1": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 5.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Thereafter",
"label": "Lessee, Operating Lease, Liability, to be Paid, after Year Four",
"documentation": "Lessee, Operating Lease, Liability, to be Paid, after Year Four"
}
}
},
"auth_ref": []
},
"us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseLiabilityUndiscountedExcessAmount",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Less imputed interest",
"label": "Lessee, Operating Lease, Liability, Undiscounted Excess Amount",
"documentation": "Amount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease."
}
}
},
"auth_ref": [
"r488"
]
},
"us-gaap_LesseeOperatingLeaseRenewalTerm": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseRenewalTerm",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Lease renewal term (in years)",
"label": "Lessee, Operating Lease, Renewal Term",
"documentation": "Term of lessee's operating lease renewal, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r1018"
]
},
"us-gaap_LesseeOperatingLeaseTermOfContract": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LesseeOperatingLeaseTermOfContract",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Lease term (in months)",
"label": "Lessee, Operating Lease, Term of Contract",
"documentation": "Term of lessee's operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r1018"
]
},
"us-gaap_LiabilitiesAndStockholdersEquity": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LiabilitiesAndStockholdersEquity",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total liabilities and stockholders\u2019 equity",
"label": "Liabilities and Equity",
"documentation": "Amount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any."
}
}
},
"auth_ref": [
"r48",
"r73",
"r545",
"r734",
"r741",
"r742",
"r920",
"r924",
"r944",
"r1017"
]
},
"us-gaap_LiabilitiesAndStockholdersEquityAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LiabilitiesAndStockholdersEquityAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "LIABILITIES AND STOCKHOLDERS\u2019 EQUITY",
"label": "Liabilities and Equity [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_LiabilitiesCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LiabilitiesCurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_LiabilitiesAndStockholdersEquity",
"weight": 1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total current liabilities",
"label": "Liabilities, Current",
"documentation": "Total obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer."
}
}
},
"auth_ref": [
"r41",
"r118",
"r154",
"r155",
"r156",
"r260",
"r306",
"r307",
"r308",
"r309",
"r310",
"r311",
"r312",
"r313",
"r314",
"r420",
"r421",
"r422",
"r468",
"r734",
"r951",
"r1022",
"r1023"
]
},
"us-gaap_LiabilitiesCurrentAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LiabilitiesCurrentAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Current liabilities:",
"label": "Liabilities, Current [Abstract]"
}
}
},
"auth_ref": []
},
"anab_LiabilitiesRelatedToSaleOfFutureRoyaltiesLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "LiabilitiesRelatedToSaleOfFutureRoyaltiesLineItems",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Liabilities Related To Sale Of Future Royalties [Line Items]",
"label": "Liabilities Related To Sale Of Future Royalties [Line Items]",
"documentation": "Liabilities Related To Sale Of Future Royalties"
}
}
},
"auth_ref": []
},
"anab_LiabilitiesRelatedToSaleOfFutureRoyaltiesRollForward": {
"xbrltype": "stringItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "LiabilitiesRelatedToSaleOfFutureRoyaltiesRollForward",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Liabilities Related To Sale Of Future Royalties [Roll Forward]",
"label": "Liabilities Related To Sale Of Future Royalties [Roll Forward]",
"documentation": "Liabilities Related To Sale Of Future Royalties"
}
}
},
"auth_ref": []
},
"anab_LiabilitiesRelatedToSaleOfFutureRoyaltiesTable": {
"xbrltype": "stringItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "LiabilitiesRelatedToSaleOfFutureRoyaltiesTable",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Liabilities Related To Sale Of Future Royalties [Table]",
"label": "Liabilities Related To Sale Of Future Royalties [Table]",
"documentation": "Liabilities Related To Sale Of Future Royalties"
}
}
},
"auth_ref": []
},
"anab_LicenseAgreementCentessaPharmaceuticalsUKLimitedMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "LicenseAgreementCentessaPharmaceuticalsUKLimitedMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "License Agreement, Centessa Pharmaceuticals (UK) Limited",
"label": "License Agreement, Centessa Pharmaceuticals (UK) Limited [Member]",
"documentation": "License Agreement, Centessa Pharmaceuticals (UK) Limited"
}
}
},
"auth_ref": []
},
"us-gaap_LicenseMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "LicenseMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "License",
"label": "License [Member]",
"documentation": "Right to use intangible asset. Intangible asset includes, but is not limited to, patent, copyright, technology, manufacturing process, software or trademark."
}
}
},
"auth_ref": [
"r957",
"r958"
]
},
"dei_LocalPhoneNumber": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "LocalPhoneNumber",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Local Phone Number",
"label": "Local Phone Number",
"documentation": "Local phone number for entity."
}
}
},
"auth_ref": []
},
"srt_MaximumMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "MaximumMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Maximum",
"label": "Maximum [Member]",
"documentation": "Upper limit of the provided range."
}
}
},
"auth_ref": [
"r91",
"r92",
"r93",
"r94",
"r95",
"r104",
"r105",
"r106",
"r110",
"r111",
"r193",
"r302",
"r303",
"r304",
"r305",
"r360",
"r388",
"r389",
"r390",
"r400",
"r448",
"r527",
"r574",
"r576",
"r584",
"r595",
"r596",
"r645",
"r646",
"r647",
"r648",
"r649",
"r651",
"r652",
"r653",
"r654",
"r658",
"r659",
"r690",
"r696",
"r710",
"r714",
"r716",
"r717",
"r725",
"r726",
"r730",
"r731",
"r737",
"r953",
"r1024",
"r1025",
"r1026",
"r1027",
"r1028",
"r1029"
]
},
"ecd_MeasureAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "MeasureAxis",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Measure:",
"label": "Measure [Axis]"
}
}
},
"auth_ref": [
"r802"
]
},
"ecd_MeasureName": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "MeasureName",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Name",
"label": "Measure Name"
}
}
},
"auth_ref": [
"r802"
]
},
"anab_MilestonesYetToBeRecognizedMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "MilestonesYetToBeRecognizedMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Milestones that may be recognized in the future",
"label": "Milestones Yet To Be Recognized [Member]",
"documentation": "Milestones Yet To Be Recognized"
}
}
},
"auth_ref": []
},
"srt_MinimumMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "MinimumMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Minimum",
"label": "Minimum [Member]",
"documentation": "Lower limit of the provided range."
}
}
},
"auth_ref": [
"r91",
"r92",
"r93",
"r94",
"r95",
"r104",
"r105",
"r106",
"r110",
"r111",
"r193",
"r302",
"r303",
"r304",
"r305",
"r360",
"r388",
"r389",
"r390",
"r400",
"r448",
"r527",
"r574",
"r576",
"r584",
"r595",
"r596",
"r645",
"r646",
"r647",
"r648",
"r649",
"r651",
"r652",
"r653",
"r654",
"r658",
"r659",
"r690",
"r696",
"r710",
"r714",
"r716",
"r717",
"r725",
"r726",
"r730",
"r737",
"r953",
"r1024",
"r1025",
"r1026",
"r1027",
"r1028",
"r1029"
]
},
"ecd_MnpiDiscTimedForCompValFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "MnpiDiscTimedForCompValFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "MNPI Disclosure Timed for Compensation Value",
"label": "MNPI Disclosure Timed for Compensation Value [Flag]"
}
}
},
"auth_ref": [
"r821"
]
},
"us-gaap_MoneyMarketFundsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "MoneyMarketFundsMember",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Money market funds",
"label": "Money Market Funds [Member]",
"documentation": "Fund that invests in short-term money-market instruments, for example, but not limited to, commercial paper, banker's acceptances, repurchase agreements, government securities, certificates of deposit, and other highly liquid securities."
}
}
},
"auth_ref": [
"r959",
"r960"
]
},
"ecd_MtrlTermsOfTrdArrTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "MtrlTermsOfTrdArrTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Material Terms of Trading Arrangement",
"label": "Material Terms of Trading Arrangement [Text Block]"
}
}
},
"auth_ref": [
"r829"
]
},
"us-gaap_MutualFundMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "MutualFundMember",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Mutual funds",
"label": "Mutual Fund [Member]",
"documentation": "Regulated investment instrument that pools funds from multiple investors to invest principally in a portfolio of securities and money market instruments to match the investment objective."
}
}
},
"auth_ref": [
"r959",
"r960"
]
},
"ecd_NamedExecutiveOfficersFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NamedExecutiveOfficersFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Named Executive Officers, Footnote",
"label": "Named Executive Officers, Footnote [Text Block]"
}
}
},
"auth_ref": [
"r803"
]
},
"us-gaap_NatureOfOperations": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NatureOfOperations",
"presentation": [
"http://www.anaptysbio.com/role/DescriptionoftheBusiness"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Description of the Business",
"label": "Nature of Operations [Text Block]",
"documentation": "The entire disclosure for the nature of an entity's business, major products or services, principal markets including location, and the relative importance of its operations in each business and the basis for the determination, including but not limited to, assets, revenues, or earnings. For an entity that has not commenced principal operations, disclosures about the risks and uncertainties related to the activities in which the entity is currently engaged and an understanding of what those activities are being directed toward."
}
}
},
"auth_ref": [
"r86",
"r190",
"r677",
"r678"
]
},
"us-gaap_NetCashProvidedByUsedInFinancingActivities": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NetCashProvidedByUsedInFinancingActivities",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Net cash (used in) provided by financing activities",
"label": "Cash Provided by (Used in) Financing Activity, Including Discontinued Operation",
"documentation": "Amount of cash inflow (outflow) from financing activity, including, but not limited to, discontinued operation. Financing activity includes, but is not limited to, obtaining resource from owner and providing return on, and return of, their investment; borrowing money and repaying amount borrowed, or settling obligation; and obtaining and paying for other resource obtained from creditor on long-term credit."
}
}
},
"auth_ref": [
"r148"
]
},
"us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NetCashProvidedByUsedInFinancingActivitiesAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "CASH FLOWS FROM FINANCING ACTIVITIES:",
"label": "Cash Provided by (Used in) Financing Activity, Including Discontinued Operation [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_NetCashProvidedByUsedInInvestingActivities": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NetCashProvidedByUsedInInvestingActivities",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Net cash provided by investing activities",
"label": "Cash Provided by (Used in) Investing Activity, Including Discontinued Operation",
"documentation": "Amount of cash inflow (outflow) from investing activity, including, but not limited to, discontinued operation. Investing activity includes, but is not limited to, making and collecting loan, acquiring and disposing of debt and equity instruments, property, plant, and equipment, and other productive assets."
}
}
},
"auth_ref": [
"r148"
]
},
"us-gaap_NetCashProvidedByUsedInInvestingActivitiesAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NetCashProvidedByUsedInInvestingActivitiesAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "CASH FLOWS FROM INVESTING ACTIVITIES:",
"label": "Cash Provided by (Used in) Investing Activity, Including Discontinued Operation [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_NetCashProvidedByUsedInOperatingActivities": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NetCashProvidedByUsedInOperatingActivities",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Net cash used in operating activities",
"label": "Cash Provided by (Used in) Operating Activity, Including Discontinued Operation",
"documentation": "Amount of cash inflow (outflow) from operating activity, including, but not limited to, discontinued operation. Operating activity includes, but is not limited to, transaction, adjustment, and change in value not defined as investing or financing activity."
}
}
},
"auth_ref": [
"r57",
"r58",
"r60"
]
},
"us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NetCashProvidedByUsedInOperatingActivitiesAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "CASH FLOWS FROM OPERATING ACTIVITIES:",
"label": "Cash Provided by (Used in) Operating Activity, Including Discontinued Operation [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_NetIncomeLoss": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NetIncomeLoss",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_ComprehensiveIncomeNetOfTax",
"weight": 1.0,
"order": 1.0
},
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity",
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows",
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Net loss",
"terseLabel": "Net loss",
"verboseLabel": "Net loss",
"netLabel": "Segment net loss",
"label": "Net Income (Loss) Attributable to Parent",
"documentation": "The portion of profit or loss for the period, net of income taxes, which is attributable to the parent."
}
}
},
"auth_ref": [
"r51",
"r60",
"r75",
"r114",
"r116",
"r133",
"r136",
"r140",
"r154",
"r155",
"r156",
"r157",
"r163",
"r167",
"r168",
"r169",
"r170",
"r171",
"r174",
"r175",
"r179",
"r260",
"r306",
"r307",
"r308",
"r309",
"r310",
"r311",
"r312",
"r313",
"r314",
"r319",
"r322",
"r324",
"r328",
"r397",
"r410",
"r415",
"r442",
"r468",
"r549",
"r622",
"r638",
"r639",
"r669",
"r671",
"r672",
"r752",
"r951"
]
},
"anab_NetSalesRoyaltyPercent": {
"xbrltype": "percentItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "NetSalesRoyaltyPercent",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalty percent on net sales (as a percent)",
"label": "Net Sales Royalty, Percent",
"documentation": "Net Sales Royalty, Percent"
}
}
},
"auth_ref": []
},
"anab_NetSalesRoyaltyThreshold": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "NetSalesRoyaltyThreshold",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Net sales royalty",
"verboseLabel": "Commercial sales",
"label": "Net Sales Royalty Threshold",
"documentation": "Net Sales Royalty Threshold"
}
}
},
"auth_ref": []
},
"us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NewAccountingPronouncementsPolicyPolicyTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accounting Pronouncements and Recent Accounting Pronouncements Not Yet Adopted",
"label": "New Accounting Pronouncements, Policy [Policy Text Block]",
"documentation": "Disclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact."
}
}
},
"auth_ref": []
},
"ecd_NonGaapMeasureDescriptionTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NonGaapMeasureDescriptionTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-GAAP Measure Description",
"label": "Non-GAAP Measure Description [Text Block]"
}
}
},
"auth_ref": [
"r802"
]
},
"ecd_NonNeosMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NonNeosMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-NEOs",
"label": "Non-NEOs [Member]"
}
}
},
"auth_ref": [
"r766",
"r776",
"r786",
"r810",
"r818"
]
},
"ecd_NonPeoNeoAvgCompActuallyPaidAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NonPeoNeoAvgCompActuallyPaidAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-PEO NEO Average Compensation Actually Paid Amount",
"label": "Non-PEO NEO Average Compensation Actually Paid Amount"
}
}
},
"auth_ref": [
"r793"
]
},
"ecd_NonPeoNeoAvgTotalCompAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NonPeoNeoAvgTotalCompAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-PEO NEO Average Total Compensation Amount",
"label": "Non-PEO NEO Average Total Compensation Amount"
}
}
},
"auth_ref": [
"r792"
]
},
"ecd_NonPeoNeoMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NonPeoNeoMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-PEO NEO",
"label": "Non-PEO NEO [Member]"
}
}
},
"auth_ref": [
"r810"
]
},
"ecd_NonRule10b51ArrAdoptedFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NonRule10b51ArrAdoptedFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-Rule 10b5-1 Arrangement Adopted",
"label": "Non-Rule 10b5-1 Arrangement Adopted [Flag]"
}
}
},
"auth_ref": [
"r829"
]
},
"ecd_NonRule10b51ArrTrmntdFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "NonRule10b51ArrTrmntdFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-Rule 10b5-1 Arrangement Terminated",
"label": "Non-Rule 10b5-1 Arrangement Terminated [Flag]"
}
}
},
"auth_ref": [
"r829"
]
},
"anab_NoncashAdvanceFutureRoyaltiesAndAmortizationOfAdvanceFutureRoyaltiesInterestExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "NoncashAdvanceFutureRoyaltiesAndAmortizationOfAdvanceFutureRoyaltiesInterestExpense",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-cash interest expense and amortization of issuance costs",
"label": "Noncash Advance Future Royalties And Amortization Of Advance Future Royalties, Interest Expense",
"documentation": "Noncash Advance Future Royalties And Amortization Of Advance Future Royalties, Interest Expense"
}
}
},
"auth_ref": []
},
"anab_NoncashAdvanceFutureRoyaltiesInterestExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "NoncashAdvanceFutureRoyaltiesInterestExpense",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_NonoperatingIncomeExpense",
"weight": -1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Non-cash interest expense for the sale of future royalties",
"terseLabel": "Non-cash interest expense",
"verboseLabel": "Non-cash interest expense recognized",
"negatedLabel": "Non-cash interest expense",
"label": "Noncash Advance Future Royalties, Interest Expense",
"documentation": "Noncash Advance Future Royalties, Interest Expense"
}
}
},
"auth_ref": []
},
"anab_NoncashAdvanceFutureRoyaltiesIssuanceCostsIncurredButNotYetPaid": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "NoncashAdvanceFutureRoyaltiesIssuanceCostsIncurredButNotYetPaid",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Amounts accrued for issuance costs related to the sale of future royalties",
"label": "Noncash Advance Future Royalties, Issuance Costs, Incurred but Not yet Paid",
"documentation": "Noncash Advance Future Royalties, Issuance Costs, Incurred but Not yet Paid"
}
}
},
"auth_ref": []
},
"anab_NoncashInterestExpenseNegativeAmortization": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "NoncashInterestExpenseNegativeAmortization",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Non-cash interest expense capitalized",
"label": "Noncash Interest Expense, Negative Amortization",
"documentation": "Noncash Interest Expense, Negative Amortization"
}
}
},
"auth_ref": []
},
"us-gaap_NonoperatingIncomeExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NonoperatingIncomeExpense",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total other expense, net",
"terseLabel": "Total other expense, net",
"label": "Nonoperating Income (Expense)",
"documentation": "The aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business)."
}
}
},
"auth_ref": [
"r53"
]
},
"us-gaap_NonoperatingIncomeExpenseAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NonoperatingIncomeExpenseAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Other income (expense), net:",
"label": "Nonoperating Income (Expense) [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_NumberOfReportableSegments": {
"xbrltype": "integerItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "NumberOfReportableSegments",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingNarrativeDetails",
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Number of reportable segments",
"label": "Number of Reportable Segments",
"documentation": "Number of segments reported by the entity. A reportable segment is a component of an entity for which there is an accounting requirement to report separate financial information on that component in the entity's financial statements."
}
}
},
"auth_ref": [
"r681",
"r689",
"r932"
]
},
"us-gaap_OfficeEquipmentMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OfficeEquipmentMember",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Office furniture and equipment",
"label": "Office Equipment [Member]",
"documentation": "Tangible personal property used in an office setting. Examples include, but are not limited to, computers, copiers and fax machine."
}
}
},
"auth_ref": []
},
"anab_OpenMarketSalesAgreementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "OpenMarketSalesAgreementMember",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Open Market Sales Agreement",
"label": "Open Market Sales Agreement [Member]",
"documentation": "Open Market Sales Agreement"
}
}
},
"auth_ref": []
},
"us-gaap_OperatingExpenses": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingExpenses",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": -1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total operating expenses",
"terseLabel": "Total operating expenses",
"label": "Operating Expenses",
"documentation": "Generally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense."
}
}
},
"auth_ref": [
"r672"
]
},
"us-gaap_OperatingExpensesAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingExpensesAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Operating expenses:",
"label": "Operating Expenses [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_OperatingIncomeLoss": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingIncomeLoss",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_IncomeLossFromContinuingOperationsBeforeIncomeTaxesExtraordinaryItemsNoncontrollingInterest",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Loss from operations",
"terseLabel": "Loss from operations",
"label": "Operating Income (Loss)",
"documentation": "The net result for the period of deducting operating expenses from operating revenues."
}
}
},
"auth_ref": [
"r78",
"r669",
"r672",
"r680",
"r931",
"r933",
"r934",
"r935",
"r936"
]
},
"us-gaap_OperatingLeaseCost": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseCost",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofNonCancellableOfficeLeaseCostsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Operating lease cost",
"label": "Operating Lease, Cost",
"documentation": "Amount of single lease cost, calculated by allocation of remaining cost of lease over remaining lease term. Includes, but is not limited to, single lease cost, after impairment of right-of-use asset, calculated by amortization of remaining right-of-use asset and accretion of lease liability."
}
}
},
"auth_ref": [
"r484",
"r733"
]
},
"us-gaap_OperatingLeaseLiability": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseLiability",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails": {
"parentTag": "us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails",
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofFutureMinimumAnnualObligationsDetails"
],
"lang": {
"en-us": {
"role": {
"verboseLabel": "Lease liability",
"terseLabel": "Total",
"label": "Operating Lease, Liability",
"documentation": "Present value of lessee's discounted obligation for lease payments from operating lease."
}
}
},
"auth_ref": [
"r481"
]
},
"us-gaap_OperatingLeaseLiabilityCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseLiabilityCurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_LiabilitiesCurrent",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Current portion of operating lease liability",
"label": "Operating Lease, Liability, Current",
"documentation": "Present value of lessee's discounted obligation for lease payments from operating lease, classified as current."
}
}
},
"auth_ref": [
"r481"
]
},
"us-gaap_OperatingLeaseLiabilityNoncurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseLiabilityNoncurrent",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_LiabilitiesAndStockholdersEquity",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Operating lease liability, net of current portion",
"label": "Operating Lease, Liability, Noncurrent",
"documentation": "Present value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent."
}
}
},
"auth_ref": [
"r481"
]
},
"us-gaap_OperatingLeasePayments": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeasePayments",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesScheduleofNonCancellableOfficeLeaseCostsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Cash paid for amounts included in the measurement of lease liabilities",
"label": "Operating Lease, Payments",
"documentation": "Amount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use."
}
}
},
"auth_ref": [
"r482",
"r485"
]
},
"us-gaap_OperatingLeaseRightOfUseAsset": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseRightOfUseAsset",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_Assets",
"weight": 1.0,
"order": 5.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails",
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Operating lease right-of-use assets",
"label": "Operating Lease, Right-of-Use Asset",
"documentation": "Amount of lessee's right to use underlying asset under operating lease."
}
}
},
"auth_ref": [
"r480"
]
},
"us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseRightOfUseAssetAmortizationExpense",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0,
"order": 5.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Amortization of right-of-use assets \u2013 operating",
"label": "Operating Lease, Right-of-Use Asset, Periodic Reduction",
"documentation": "Amount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease."
}
}
},
"auth_ref": [
"r917"
]
},
"us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent": {
"xbrltype": "percentItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseWeightedAverageDiscountRatePercent",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted-average discount rate (as a percent)",
"label": "Operating Lease, Weighted Average Discount Rate, Percent",
"documentation": "Weighted average discount rate for operating lease calculated at point in time."
}
}
},
"auth_ref": [
"r487",
"r733"
]
},
"us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OperatingLeaseWeightedAverageRemainingLeaseTerm1",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted average remaining lease term (in years)",
"label": "Operating Lease, Weighted Average Remaining Lease Term",
"documentation": "Weighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r486",
"r733"
]
},
"us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OrganizationConsolidationAndPresentationOfFinancialStatementsAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Organization, Consolidation and Presentation of Financial Statements [Abstract]",
"label": "Organization, Consolidation and Presentation of Financial Statements [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_OtherAssetsNoncurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OtherAssetsNoncurrent",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_Assets",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Other long-term assets",
"label": "Other Assets, Noncurrent",
"documentation": "Amount of noncurrent assets classified as other."
}
}
},
"auth_ref": [
"r123"
]
},
"us-gaap_OtherComprehensiveIncomeLossAvailableForSaleSecuritiesAdjustmentNetOfTax": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OtherComprehensiveIncomeLossAvailableForSaleSecuritiesAdjustmentNetOfTax",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_ComprehensiveIncomeNetOfTax",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Unrealized (loss) gain on available for sale securities",
"label": "OCI, Debt Securities, Available-for-Sale, Gain (Loss), after Adjustment and Tax",
"documentation": "Amount, after tax and adjustment, of unrealized gain (loss) on investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale) and unrealized gain (loss) on investment in debt security measured at amortized cost (held-to-maturity) from transfer to available-for-sale."
}
}
},
"auth_ref": [
"r130",
"r131",
"r132",
"r550"
]
},
"us-gaap_OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OtherComprehensiveIncomeLossNetOfTaxPortionAttributableToParent",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Comprehensive gain (loss), net",
"label": "Other Comprehensive Income (Loss), Net of Tax, Portion Attributable to Parent",
"documentation": "Amount after tax of other comprehensive income (loss) attributable to parent entity."
}
}
},
"auth_ref": [
"r3",
"r7",
"r71",
"r134",
"r137",
"r171"
]
},
"us-gaap_OtherLiabilitiesDisclosureAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OtherLiabilitiesDisclosureAbstract",
"lang": {
"en-us": {
"role": {
"label": "Other Liabilities Disclosure [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_OtherNoncashIncomeExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OtherNoncashIncomeExpense",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0,
"order": 6.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Non-cash interest expense",
"label": "Other Noncash Income (Expense)",
"documentation": "Amount of income (expense) included in net income that results in no cash inflow (outflow), classified as other."
}
}
},
"auth_ref": [
"r60"
]
},
"us-gaap_OtherNonoperatingIncomeExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "OtherNonoperatingIncomeExpense",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_NonoperatingIncomeExpense",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Other income (expense), net",
"label": "Other Nonoperating Income (Expense)",
"documentation": "Amount of income (expense) related to nonoperating activities, classified as other."
}
}
},
"auth_ref": [
"r54",
"r719"
]
},
"ecd_OtherPerfMeasureAmt": {
"xbrltype": "decimalItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "OtherPerfMeasureAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Other Performance Measure, Amount",
"label": "Other Performance Measure, Amount"
}
}
},
"auth_ref": [
"r802"
]
},
"ecd_OutstandingAggtErrCompAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "OutstandingAggtErrCompAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Outstanding Aggregate Erroneous Compensation Amount",
"label": "Outstanding Aggregate Erroneous Compensation Amount"
}
}
},
"auth_ref": [
"r764",
"r774",
"r784",
"r816"
]
},
"ecd_OutstandingRecoveryCompAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "OutstandingRecoveryCompAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Compensation Amount",
"label": "Outstanding Recovery Compensation Amount"
}
}
},
"auth_ref": [
"r767",
"r777",
"r787",
"r819"
]
},
"ecd_OutstandingRecoveryIndName": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "OutstandingRecoveryIndName",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Name",
"label": "Outstanding Recovery, Individual Name"
}
}
},
"auth_ref": [
"r767",
"r777",
"r787",
"r819"
]
},
"anab_PaulLizzulMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "PaulLizzulMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"label": "Paul Lizzul [Member]",
"documentation": "Paul Lizzul"
}
}
},
"auth_ref": []
},
"ecd_PayVsPerformanceDisclosureLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PayVsPerformanceDisclosureLineItems",
"lang": {
"en-us": {
"role": {
"label": "Pay vs Performance Disclosure [Line Items]"
}
}
},
"auth_ref": [
"r791"
]
},
"us-gaap_PaymentsForRepurchaseOfCommonStock": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PaymentsForRepurchaseOfCommonStock",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Repurchase and retirements of common stock",
"label": "Payments for Repurchase of Common Stock",
"documentation": "The cash outflow to reacquire common stock during the period."
}
}
},
"auth_ref": [
"r56"
]
},
"us-gaap_PaymentsOfDebtIssuanceCosts": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PaymentsOfDebtIssuanceCosts",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0,
"order": 6.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Payments for debt issuance costs",
"label": "Payments of Debt Issuance Costs",
"documentation": "The cash outflow paid to third parties in connection with debt origination, which will be amortized over the remaining maturity period of the associated long-term debt."
}
}
},
"auth_ref": [
"r17"
]
},
"anab_PaymentsOfStockIssuanceCostsAndUnderwritingDiscountsAndCommissions": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "PaymentsOfStockIssuanceCostsAndUnderwritingDiscountsAndCommissions",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Underwriting discounts and commissions and offering expenses",
"label": "Payments Of Stock Issuance Costs And Underwriting Discounts And Commissions",
"documentation": "Payments Of Stock Issuance Costs And Underwriting Discounts And Commissions"
}
}
},
"auth_ref": []
},
"us-gaap_PaymentsRelatedToTaxWithholdingForShareBasedCompensation": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PaymentsRelatedToTaxWithholdingForShareBasedCompensation",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Payment for net share settlement of equity awards",
"label": "Payment, Tax Withholding, Share-Based Payment Arrangement",
"documentation": "Amount of cash outflow to satisfy grantee's tax withholding obligation for award under share-based payment arrangement."
}
}
},
"auth_ref": [
"r146"
]
},
"us-gaap_PaymentsToAcquireAvailableForSaleSecuritiesDebt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PaymentsToAcquireAvailableForSaleSecuritiesDebt",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": -1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedLabel": "Purchases of investments",
"label": "Payments to Acquire Debt Securities, Available-for-Sale",
"documentation": "Amount of cash outflow to acquire investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r16",
"r144",
"r228"
]
},
"us-gaap_PaymentsToAcquireProductiveAssets": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PaymentsToAcquireProductiveAssets",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Cash payment",
"label": "Payments to Acquire Productive Assets",
"documentation": "The cash outflow for purchases of and capital improvements on property, plant and equipment (capital expenditures), software, and other intangible assets."
}
}
},
"auth_ref": [
"r84",
"r997",
"r998",
"r999"
]
},
"us-gaap_PaymentsToAcquirePropertyPlantAndEquipment": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PaymentsToAcquirePropertyPlantAndEquipment",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": -1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Purchases of property and equipment",
"label": "Payments to Acquire Property, Plant, and Equipment",
"documentation": "The cash outflow associated with the acquisition of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale; includes cash outflows to pay for construction of self-constructed assets."
}
}
},
"auth_ref": [
"r55"
]
},
"ecd_PeerGroupIssuersFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PeerGroupIssuersFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Peer Group Issuers, Footnote",
"label": "Peer Group Issuers, Footnote [Text Block]"
}
}
},
"auth_ref": [
"r801"
]
},
"ecd_PeerGroupTotalShareholderRtnAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PeerGroupTotalShareholderRtnAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Peer Group Total Shareholder Return Amount",
"label": "Peer Group Total Shareholder Return Amount"
}
}
},
"auth_ref": [
"r801"
]
},
"ecd_PeoActuallyPaidCompAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PeoActuallyPaidCompAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "PEO Actually Paid Compensation Amount",
"label": "PEO Actually Paid Compensation Amount"
}
}
},
"auth_ref": [
"r793"
]
},
"ecd_PeoMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PeoMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "PEO",
"label": "PEO [Member]"
}
}
},
"auth_ref": [
"r810"
]
},
"ecd_PeoName": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PeoName",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "PEO Name",
"label": "PEO Name"
}
}
},
"auth_ref": [
"r803"
]
},
"ecd_PeoTotalCompAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PeoTotalCompAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "PEO Total Compensation Amount",
"label": "PEO Total Compensation Amount"
}
}
},
"auth_ref": [
"r792"
]
},
"us-gaap_PerformanceSharesMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PerformanceSharesMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "PSU",
"label": "Performance Shares [Member]",
"documentation": "Share-based payment arrangement awarded for meeting performance target."
}
}
},
"auth_ref": [
"r963",
"r964",
"r965",
"r966",
"r967",
"r968",
"r969",
"r970",
"r971",
"r972",
"r973",
"r974",
"r975",
"r976",
"r977",
"r978",
"r979",
"r980",
"r981",
"r982",
"r983",
"r984",
"r985",
"r986",
"r987",
"r988"
]
},
"anab_Phase2ClinicalTrialInitiationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "Phase2ClinicalTrialInitiationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Phase 2 clinical trial initiation",
"label": "Phase 2 Clinical Trial Initiation [Member]",
"documentation": "Phase 2 Clinical Trial Initiation [Member]"
}
}
},
"auth_ref": []
},
"anab_Phase3ClinicalTrialInitiationFirstIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "Phase3ClinicalTrialInitiationFirstIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Phase 3 clinical trial initiation - first indication",
"label": "Phase 3 Clinical Trial Initiation - First Indication [Member]",
"documentation": "Phase 3 Clinical Trial Initiation - First Indication"
}
}
},
"auth_ref": []
},
"anab_Phase3ClinicalTrialInitiationSecondIndicationMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "Phase3ClinicalTrialInitiationSecondIndicationMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Phase 3 clinical trial initiation - second indication",
"label": "Phase 3 clinical Trial Initiation - Second Indication [Member]",
"documentation": "Phase 3 clinical Trial Initiation - Second Indication [Member]"
}
}
},
"auth_ref": []
},
"us-gaap_PlanNameAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PlanNameAxis",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Plan Name [Axis]",
"label": "Plan Name [Axis]",
"documentation": "Information by plan name for share-based payment arrangement."
}
}
},
"auth_ref": [
"r963",
"r964",
"r965",
"r966",
"r967",
"r968",
"r969",
"r970",
"r971",
"r972",
"r973",
"r974",
"r975",
"r976",
"r977",
"r978",
"r979",
"r980",
"r981",
"r982",
"r983",
"r984",
"r985",
"r986",
"r987",
"r988"
]
},
"us-gaap_PlanNameDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PlanNameDomain",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Plan Name [Domain]",
"label": "Plan Name [Domain]",
"documentation": "Plan name for share-based payment arrangement."
}
}
},
"auth_ref": [
"r963",
"r964",
"r965",
"r966",
"r967",
"r968",
"r969",
"r970",
"r971",
"r972",
"r973",
"r974",
"r975",
"r976",
"r977",
"r978",
"r979",
"r980",
"r981",
"r982",
"r983",
"r984",
"r985",
"r986",
"r987",
"r988"
]
},
"ecd_PnsnAdjsPrrSvcCstMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PnsnAdjsPrrSvcCstMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Pension Adjustments Prior Service Cost",
"label": "Pension Adjustments Prior Service Cost [Member]"
}
}
},
"auth_ref": [
"r794"
]
},
"ecd_PnsnAdjsSvcCstMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PnsnAdjsSvcCstMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Pension Adjustments Service Cost",
"label": "Pension Adjustments Service Cost [Member]"
}
}
},
"auth_ref": [
"r838"
]
},
"ecd_PnsnBnftsAdjFnTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PnsnBnftsAdjFnTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Pension Benefits Adjustments, Footnote",
"label": "Pension Benefits Adjustments, Footnote [Text Block]"
}
}
},
"auth_ref": [
"r793"
]
},
"anab_PreclinicalAndOtherUnallocatedCostsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "PreclinicalAndOtherUnallocatedCostsMember",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Preclinical and other unallocated costs",
"label": "Preclinical And Other Unallocated Costs [Member]",
"documentation": "Preclinical And Other Unallocated Costs"
}
}
},
"auth_ref": []
},
"us-gaap_PreferredStockParOrStatedValuePerShare": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PreferredStockParOrStatedValuePerShare",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Preferred stock, par value (in dollars per share)",
"label": "Preferred Stock, Par or Stated Value Per Share",
"documentation": "Face amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer."
}
}
},
"auth_ref": [
"r42",
"r330"
]
},
"us-gaap_PreferredStockSharesAuthorized": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PreferredStockSharesAuthorized",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Preferred stock, shares authorized (in shares)",
"label": "Preferred Stock, Shares Authorized",
"documentation": "The maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws."
}
}
},
"auth_ref": [
"r42",
"r603"
]
},
"us-gaap_PreferredStockSharesIssued": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PreferredStockSharesIssued",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Preferred stock, shares issued (in shares)",
"label": "Preferred Stock, Shares Issued",
"documentation": "Number of shares issued for nonredeemable preferred shares and preferred shares redeemable solely at option of issuer. Includes, but is not limited to, preferred shares issued, repurchased, and held as treasury shares. Excludes preferred shares classified as debt."
}
}
},
"auth_ref": [
"r42",
"r330"
]
},
"us-gaap_PreferredStockSharesOutstanding": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PreferredStockSharesOutstanding",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheetsParenthetical"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Preferred stock, shares outstanding (in shares)",
"label": "Preferred Stock, Shares Outstanding",
"documentation": "Aggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased."
}
}
},
"auth_ref": [
"r42",
"r603",
"r621",
"r1067",
"r1068"
]
},
"us-gaap_PreferredStockValue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PreferredStockValue",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Preferred stock, $0.001 par value, 10,000 shares authorized and no shares, issued or outstanding at June\u00a030, 2025 and December\u00a031, 2024, respectively",
"label": "Preferred Stock, Value, Issued",
"documentation": "Aggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity."
}
}
},
"auth_ref": [
"r42",
"r321",
"r326",
"r541",
"r734"
]
},
"us-gaap_PrepaidExpenseAndOtherAssetsCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PrepaidExpenseAndOtherAssetsCurrent",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Prepaid expenses and other current assets",
"label": "Prepaid Expense and Other Assets, Current",
"documentation": "Amount of asset related to consideration paid in advance for costs that provide economic benefits in future periods, and amount of other assets that are expected to be realized or consumed within one year or the normal operating cycle, if longer."
}
}
},
"auth_ref": [
"r862"
]
},
"anab_ProceedsFromAdvanceFutureRoyalties": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ProceedsFromAdvanceFutureRoyalties",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Proceeds from sale of future royalties",
"label": "Proceeds From Advance Future Royalties",
"documentation": "Proceeds From Advance Future Royalties"
}
}
},
"auth_ref": []
},
"us-gaap_ProceedsFromIssuanceOfCommonStock": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ProceedsFromIssuanceOfCommonStock",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows",
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Proceeds from issuance of common stock",
"label": "Proceeds from Issuance of Common Stock",
"documentation": "The cash inflow from the additional capital contribution to the entity."
}
}
},
"auth_ref": [
"r2"
]
},
"us-gaap_ProceedsFromSaleAndMaturityOfAvailableForSaleSecurities": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ProceedsFromSaleAndMaturityOfAvailableForSaleSecurities",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInInvestingActivities",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Sales and maturities of investments",
"label": "Proceeds from Sale and Maturity of Debt Securities, Available-for-Sale",
"documentation": "Amount of cash inflow from sale, maturity, prepayment and call of investment in debt security measured at fair value with change in fair value recognized in other comprehensive income (available-for-sale)."
}
}
},
"auth_ref": [
"r144",
"r145",
"r939"
]
},
"anab_ProceedsFromSaleOfAdvanceFutureRoyalties": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ProceedsFromSaleOfAdvanceFutureRoyalties",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Proceeds from the sale of future royalties",
"label": "Proceeds From Sale Of Advance Future Royalties",
"documentation": "Proceeds From Sale Of Advance Future Royalties"
}
}
},
"auth_ref": []
},
"us-gaap_ProceedsFromStockOptionsExercised": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ProceedsFromStockOptionsExercised",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"verboseLabel": "Proceeds from stock options exercised",
"label": "Proceeds from Stock Options Exercised",
"documentation": "Amount of cash inflow from exercise of option under share-based payment arrangement."
}
}
},
"auth_ref": [
"r2",
"r11"
]
},
"srt_ProductOrServiceAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "ProductOrServiceAxis",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Product and Service [Axis]",
"label": "Product and Service [Axis]",
"documentation": "Information by product and service, or group of similar products and similar services."
}
}
},
"auth_ref": [
"r96",
"r97",
"r98",
"r99",
"r100",
"r101",
"r109",
"r223",
"r528",
"r567",
"r568",
"r569",
"r570",
"r571",
"r572",
"r573",
"r662",
"r668",
"r671",
"r672",
"r697",
"r698",
"r736",
"r737",
"r738",
"r740",
"r743",
"r841",
"r859",
"r875",
"r876",
"r877",
"r878",
"r879",
"r880",
"r881",
"r882",
"r883",
"r884",
"r885",
"r886",
"r887",
"r888",
"r889",
"r890",
"r891",
"r892",
"r893",
"r894",
"r895",
"r896",
"r897",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r904",
"r905",
"r906",
"r907",
"r908",
"r909",
"r910",
"r911",
"r912",
"r913",
"r914",
"r915",
"r949",
"r950",
"r1033",
"r1035",
"r1036",
"r1037",
"r1038",
"r1039",
"r1040",
"r1041",
"r1042",
"r1043",
"r1044",
"r1045",
"r1046",
"r1047",
"r1048",
"r1049",
"r1050",
"r1051",
"r1052",
"r1053",
"r1054",
"r1055",
"r1056",
"r1057",
"r1058",
"r1059",
"r1060",
"r1061",
"r1062"
]
},
"srt_ProductsAndServicesDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "ProductsAndServicesDomain",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Product and Service [Domain]",
"label": "Product and Service [Domain]",
"documentation": "Product or service, or a group of similar products or similar services."
}
}
},
"auth_ref": [
"r96",
"r97",
"r98",
"r99",
"r100",
"r101",
"r109",
"r223",
"r528",
"r567",
"r568",
"r569",
"r570",
"r571",
"r572",
"r573",
"r662",
"r668",
"r671",
"r672",
"r697",
"r698",
"r736",
"r737",
"r738",
"r740",
"r743",
"r841",
"r859",
"r875",
"r876",
"r877",
"r878",
"r879",
"r880",
"r881",
"r882",
"r883",
"r884",
"r885",
"r886",
"r887",
"r888",
"r889",
"r890",
"r891",
"r892",
"r893",
"r894",
"r895",
"r896",
"r897",
"r898",
"r899",
"r900",
"r901",
"r902",
"r903",
"r904",
"r905",
"r906",
"r907",
"r908",
"r909",
"r910",
"r911",
"r912",
"r913",
"r914",
"r915",
"r949",
"r950",
"r1033",
"r1035",
"r1036",
"r1037",
"r1038",
"r1039",
"r1040",
"r1041",
"r1042",
"r1043",
"r1044",
"r1045",
"r1046",
"r1047",
"r1048",
"r1049",
"r1050",
"r1051",
"r1052",
"r1053",
"r1054",
"r1055",
"r1056",
"r1057",
"r1058",
"r1059",
"r1060",
"r1061",
"r1062"
]
},
"us-gaap_PropertyPlantAndEquipmentByTypeAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PropertyPlantAndEquipmentByTypeAxis",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Property, Plant and Equipment, Type [Axis]",
"label": "Long-Lived Tangible Asset [Axis]",
"documentation": "Information by type of long-lived, physical assets used to produce goods and services and not intended for resale."
}
}
},
"auth_ref": [
"r6",
"r489"
]
},
"us-gaap_PropertyPlantAndEquipmentGross": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PropertyPlantAndEquipmentGross",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails": {
"parentTag": "us-gaap_PropertyPlantAndEquipmentNet",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Property and equipment, gross",
"label": "Property, Plant and Equipment, Gross",
"documentation": "Amount before accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures."
}
}
},
"auth_ref": [
"r62",
"r121",
"r547"
]
},
"us-gaap_PropertyPlantAndEquipmentLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PropertyPlantAndEquipmentLineItems",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Property, Plant and Equipment [Line Items]",
"label": "Property, Plant and Equipment [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r489"
]
},
"us-gaap_PropertyPlantAndEquipmentNet": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PropertyPlantAndEquipmentNet",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_Assets",
"weight": 1.0,
"order": 2.0
},
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails": {
"parentTag": null,
"weight": null,
"order": null,
"root": true
}
},
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails",
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Property and equipment, net",
"totalLabel": "Total property and equipment, net",
"label": "Property, Plant and Equipment, Net",
"documentation": "Amount after accumulated depreciation, depletion and amortization of physical assets used in the normal conduct of business to produce goods and services and not intended for resale. Examples include, but are not limited to, land, buildings, machinery and equipment, office equipment, and furniture and fixtures."
}
}
},
"auth_ref": [
"r6",
"r489",
"r536",
"r547",
"r734"
]
},
"us-gaap_PropertyPlantAndEquipmentTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PropertyPlantAndEquipmentTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Property and Equipment, Net",
"label": "Property, Plant and Equipment [Table Text Block]",
"documentation": "Tabular disclosure of physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation."
}
}
},
"auth_ref": [
"r6"
]
},
"us-gaap_PropertyPlantAndEquipmentTypeDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "PropertyPlantAndEquipmentTypeDomain",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Property, Plant and Equipment, Type [Domain]",
"label": "Long-Lived Tangible Asset [Domain]",
"documentation": "Listing of long-lived, physical assets that are used in the normal conduct of business to produce goods and services and not intended for resale. Examples include land, buildings, machinery and equipment, and other types of furniture and equipment including, but not limited to, office equipment, furniture and fixtures, and computer equipment and software."
}
}
},
"auth_ref": [
"r62",
"r489"
]
},
"ecd_PvpTable": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PvpTable",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Pay vs Performance Disclosure",
"label": "Pay vs Performance Disclosure [Table]"
}
}
},
"auth_ref": [
"r791"
]
},
"ecd_PvpTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "PvpTableTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Pay vs Performance Disclosure, Table",
"label": "Pay vs Performance [Table Text Block]"
}
}
},
"auth_ref": [
"r791"
]
},
"srt_RangeAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "RangeAxis",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Statistical Measurement [Axis]",
"label": "Statistical Measurement [Axis]",
"documentation": "Information by statistical measurement. Includes, but is not limited to, minimum, maximum, weighted average, arithmetic average, and median."
}
}
},
"auth_ref": [
"r91",
"r92",
"r93",
"r94",
"r95",
"r104",
"r105",
"r106",
"r110",
"r111",
"r193",
"r302",
"r303",
"r304",
"r305",
"r350",
"r360",
"r388",
"r389",
"r390",
"r396",
"r400",
"r448",
"r501",
"r510",
"r527",
"r574",
"r576",
"r584",
"r595",
"r596",
"r645",
"r646",
"r647",
"r648",
"r649",
"r651",
"r652",
"r653",
"r654",
"r658",
"r659",
"r690",
"r696",
"r710",
"r714",
"r716",
"r717",
"r725",
"r726",
"r730",
"r731",
"r737",
"r746",
"r947",
"r953",
"r1011",
"r1025",
"r1026",
"r1027",
"r1028",
"r1029"
]
},
"srt_RangeMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "RangeMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Statistical Measurement [Domain]",
"label": "Statistical Measurement [Domain]",
"documentation": "Statistical measurement. Includes, but is not limited to, minimum, maximum, weighted average, arithmetic average, and median."
}
}
},
"auth_ref": [
"r91",
"r92",
"r93",
"r94",
"r95",
"r104",
"r105",
"r106",
"r110",
"r111",
"r193",
"r302",
"r303",
"r304",
"r305",
"r350",
"r360",
"r388",
"r389",
"r390",
"r396",
"r400",
"r448",
"r501",
"r510",
"r527",
"r574",
"r576",
"r584",
"r595",
"r596",
"r645",
"r646",
"r647",
"r648",
"r649",
"r651",
"r652",
"r653",
"r654",
"r658",
"r659",
"r690",
"r696",
"r710",
"r714",
"r716",
"r717",
"r725",
"r726",
"r730",
"r731",
"r737",
"r746",
"r947",
"r953",
"r1011",
"r1025",
"r1026",
"r1027",
"r1028",
"r1029"
]
},
"srt_RealEstateAndAccumulatedDepreciationDescriptionOfPropertyAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "RealEstateAndAccumulatedDepreciationDescriptionOfPropertyAxis",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Name of Property [Axis]",
"label": "Name of Property [Axis]",
"documentation": "Information by name of property."
}
}
},
"auth_ref": [
"r102",
"r103",
"r656",
"r657",
"r850",
"r851",
"r852",
"r853",
"r854",
"r855",
"r856",
"r857",
"r1069",
"r1070",
"r1071",
"r1072",
"r1073",
"r1074",
"r1075",
"r1076"
]
},
"srt_RealEstateAndAccumulatedDepreciationNameOfPropertyDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "RealEstateAndAccumulatedDepreciationNameOfPropertyDomain",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Name of Property [Domain]",
"label": "Name of Property [Domain]",
"documentation": "Name of the property, for example, but not limited to, ABC Shopping Center."
}
}
},
"auth_ref": [
"r102",
"r103",
"r656",
"r657",
"r850",
"r851",
"r852",
"r853",
"r854",
"r855",
"r856",
"r857",
"r1069",
"r1070",
"r1071",
"r1072",
"r1073",
"r1074",
"r1075",
"r1076"
]
},
"anab_ReceivablesForStockOptionsExercised": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ReceivablesForStockOptionsExercised",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Receivable related to issuance of common stock, upon exercise of stock options",
"label": "Receivables for Stock Options Exercised",
"documentation": "Receivables for Stock Options Exercised"
}
}
},
"auth_ref": []
},
"us-gaap_ReceivablesNetCurrent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ReceivablesNetCurrent",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_AssetsCurrent",
"weight": 1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Receivables from collaborative partners",
"label": "Receivables, Net, Current",
"documentation": "The total amount due to the entity within one year of the balance sheet date (or one operating cycle, if longer) from outside sources, including trade accounts receivable, notes and loans receivable, as well as any other types of receivables, net of allowances established for the purpose of reducing such receivables to an amount that approximates their net realizable value."
}
}
},
"auth_ref": [
"r734"
]
},
"ecd_RecoveryOfErrCompDisclosureLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "RecoveryOfErrCompDisclosureLineItems",
"lang": {
"en-us": {
"role": {
"label": "Recovery of Erroneously Awarded Compensation Disclosure [Line Items]"
}
}
},
"auth_ref": [
"r759",
"r769",
"r779",
"r811"
]
},
"anab_RegulatoryApprovalForMarketingOfTheFirstLicensedProductInTheEUMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RegulatoryApprovalForMarketingOfTheFirstLicensedProductInTheEUMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Regulatory approval for marketing of the first licensed product in the EU",
"label": "Regulatory Approval For Marketing Of The First Licensed Product In The EU [Member]",
"documentation": "Regulatory Approval For Marketing Of The First Licensed Product In The EU"
}
}
},
"auth_ref": []
},
"anab_ReimbursementPaymentsToAcquireProductiveAssets": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ReimbursementPaymentsToAcquireProductiveAssets",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Reimbursement",
"label": "Reimbursement Payments to Acquire Productive Assets",
"documentation": "Reimbursement Payments to Acquire Productive Assets"
}
}
},
"auth_ref": []
},
"anab_RentPerSquareFoot": {
"xbrltype": "perUnitItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RentPerSquareFoot",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Monthly base rate (in dollars per sqft)",
"label": "Rent Per Square Foot",
"documentation": "Rent Per Square Foot"
}
}
},
"auth_ref": []
},
"anab_RepaymentOfAdvanceFutureRoyaltyAndMilestones": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RepaymentOfAdvanceFutureRoyaltyAndMilestones",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInFinancingActivities",
"weight": -1.0,
"order": 5.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"negatedLabel": "Principal repayment of liability for sale of future royalties",
"negatedTerseLabel": "Royalty and milestone payments to Sagard",
"label": "Repayment Of Advance Future Royalty And Milestones",
"documentation": "Repayment Of Advance Future Royalty And Milestones"
}
}
},
"auth_ref": []
},
"srt_RepurchaseAgreementCounterpartyNameDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "RepurchaseAgreementCounterpartyNameDomain",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Counterparty Name [Domain]",
"label": "Counterparty Name [Domain]",
"documentation": "Named other party that participates in a financial transaction. Examples include, but not limited to, the name of the financial institution."
}
}
},
"auth_ref": [
"r107",
"r108",
"r154",
"r158",
"r159",
"r315",
"r332",
"r492",
"r511",
"r538",
"r664",
"r665",
"r666",
"r863",
"r864",
"r865",
"r866",
"r867",
"r868",
"r869",
"r870",
"r871",
"r1001",
"r1002",
"r1003",
"r1004"
]
},
"anab_RepurchaseOfCommonStockIncurredButNotYetPaid": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RepurchaseOfCommonStockIncurredButNotYetPaid",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Amounts accrued for repurchases of common stock",
"label": "Repurchase of Common Stock Incurred But Not Yet Paid",
"documentation": "Repurchase of Common Stock Incurred But Not Yet Paid"
}
}
},
"auth_ref": []
},
"us-gaap_ResearchAndDevelopmentExpense": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ResearchAndDevelopmentExpense",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_OperatingExpenses",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Research and development",
"label": "Research and Development Expense",
"documentation": "Amount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity."
}
}
},
"auth_ref": [
"r401",
"r660",
"r669",
"r670",
"r685",
"r1030"
]
},
"us-gaap_ResearchAndDevelopmentExpenseExcludingAcquiredInProcessCost": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ResearchAndDevelopmentExpenseExcludingAcquiredInProcessCost",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Research and development",
"label": "Research and Development Expense (Excluding Acquired in Process Cost)",
"documentation": "Amount of expense for research and development. Excludes cost for computer software product to be sold, leased, or otherwise marketed, writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both, and write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity."
}
}
},
"auth_ref": [
"r991"
]
},
"us-gaap_ResearchAndDevelopmentExpenseMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ResearchAndDevelopmentExpenseMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Research and development",
"label": "Research and Development Expense [Member]",
"documentation": "Primary financial statement caption in which the reported facts about research and development expense have been included."
}
}
},
"auth_ref": []
},
"anab_ResearchAndDevelopmentSegmentMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ResearchAndDevelopmentSegmentMember",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Reportable Segment",
"label": "Research and Development Segment [Member]",
"documentation": "Research and Development Segment"
}
}
},
"auth_ref": []
},
"ecd_RestatementDateAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "RestatementDateAxis",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Restatement Determination Date:",
"label": "Restatement Determination Date [Axis]"
}
}
},
"auth_ref": [
"r760",
"r770",
"r780",
"r812"
]
},
"ecd_RestatementDeterminationDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "RestatementDeterminationDate",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Restatement Determination Date",
"label": "Restatement Determination Date"
}
}
},
"auth_ref": [
"r761",
"r771",
"r781",
"r813"
]
},
"ecd_RestatementDoesNotRequireRecoveryTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "RestatementDoesNotRequireRecoveryTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Restatement does not require Recovery",
"label": "Restatement Does Not Require Recovery [Text Block]"
}
}
},
"auth_ref": [
"r768",
"r778",
"r788",
"r820"
]
},
"us-gaap_RestrictedStockUnitsRSUMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "RestrictedStockUnitsRSUMember",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "RSUs",
"label": "Restricted Stock Units (RSUs) [Member]",
"documentation": "Share instrument which is convertible to stock or an equivalent amount of cash, after a specified period of time or when specified performance conditions are met."
}
}
},
"auth_ref": [
"r963",
"r964",
"r965",
"r966",
"r967",
"r968",
"r969",
"r970",
"r971",
"r972",
"r973",
"r974",
"r975",
"r976",
"r977",
"r978",
"r979",
"r980",
"r981",
"r982",
"r983",
"r984",
"r985",
"r986",
"r987",
"r988"
]
},
"us-gaap_RetainedEarningsAccumulatedDeficit": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "RetainedEarningsAccumulatedDeficit",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_StockholdersEquity",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accumulated deficit",
"label": "Retained Earnings (Accumulated Deficit)",
"documentation": "Amount of accumulated undistributed earnings (deficit)."
}
}
},
"auth_ref": [
"r45",
"r67",
"r544",
"r581",
"r583",
"r591",
"r604",
"r734"
]
},
"us-gaap_RetainedEarningsMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "RetainedEarningsMember",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Accumulated Deficit",
"label": "Retained Earnings [Member]",
"documentation": "Accumulated undistributed earnings (deficit)."
}
}
},
"auth_ref": [
"r112",
"r113",
"r114",
"r160",
"r161",
"r162",
"r164",
"r171",
"r173",
"r175",
"r261",
"r262",
"r293",
"r318",
"r397",
"r404",
"r405",
"r407",
"r408",
"r409",
"r411",
"r414",
"r415",
"r428",
"r430",
"r431",
"r433",
"r440",
"r478",
"r479",
"r578",
"r580",
"r592",
"r1067"
]
},
"us-gaap_RevenueFromContractWithCustomerAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "RevenueFromContractWithCustomerAbstract",
"lang": {
"en-us": {
"role": {
"label": "Revenue from Contract with Customer [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "RevenueFromContractWithCustomerExcludingAssessedTax",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss": {
"parentTag": "us-gaap_OperatingIncomeLoss",
"weight": 1.0,
"order": 2.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Collaboration revenue",
"label": "Revenue from Contract with Customer, Excluding Assessed Tax",
"documentation": "Amount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise."
}
}
},
"auth_ref": [
"r76",
"r77",
"r186",
"r199",
"r200",
"r214",
"r220",
"r223",
"r225",
"r226",
"r346",
"r347",
"r348",
"r528",
"r669",
"r672"
]
},
"us-gaap_RevenueFromContractWithCustomerProductAndServiceExtensibleList": {
"xbrltype": "enumerationSetItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "RevenueFromContractWithCustomerProductAndServiceExtensibleList",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Revenue from Contract with Customer, Product and Service [Extensible Enumeration]",
"label": "Revenue from Contract with Customer, Product and Service [Extensible Enumeration]",
"documentation": "Indicates product and service for revenue from satisfaction of performance obligation by transferring promised product and service to customer."
}
}
},
"auth_ref": [
"r697",
"r698"
]
},
"anab_RevenueRecognitionMilestoneMethodCommercialSaleOrExpirationOfPatentsAgreementTerm": {
"xbrltype": "durationItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RevenueRecognitionMilestoneMethodCommercialSaleOrExpirationOfPatentsAgreementTerm",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Agreement term following first commercial sale or expiration of the last to expire patent (in years)",
"label": "Revenue Recognition, Milestone Method, Commercial Sale Or Expiration Of Patents, Agreement Term",
"documentation": "Revenue Recognition, Milestone Method, Commercial Sale Or Expiration Of Patents, Agreement Term"
}
}
},
"auth_ref": []
},
"anab_RevenueRecognitionMilestoneMethodContingent": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RevenueRecognitionMilestoneMethodContingent",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Milestones that may be recognized in the future",
"label": "Revenue Recognition, Milestone Method, Contingent",
"documentation": "Revenue Recognition, Milestone Method, Contingent"
}
}
},
"auth_ref": []
},
"anab_RosnilimabMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RosnilimabMember",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Rosnilimab",
"label": "Rosnilimab [Member]",
"documentation": "Rosnilimab"
}
}
},
"auth_ref": []
},
"anab_RoyaltyAgreementAbove1.0BillionBelow1.5BillionMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RoyaltyAgreementAbove1.0BillionBelow1.5BillionMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalty Agreement, Above $1. 0 billion Below $1.5 Billion",
"label": "Royalty Agreement, Above $1. 0 billion Below $1.5 Billion [Member]",
"documentation": "Royalty Agreement, Above $1. 0 billion Below $1.5 Billion"
}
}
},
"auth_ref": []
},
"anab_RoyaltyAgreementAbove1.5BillionBelow2.5BillionMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RoyaltyAgreementAbove1.5BillionBelow2.5BillionMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalty Agreement, Above $1. 5 billion Below $2.5 Billion",
"label": "Royalty Agreement, Above $1.5 billion Below $2.5 Billion [Member]",
"documentation": "Royalty Agreement, Above $1.5 billion Below $2.5 Billion"
}
}
},
"auth_ref": []
},
"anab_RoyaltyAgreementAbove2.5BillionMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RoyaltyAgreementAbove2.5BillionMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalty Agreement, Above $2.5 billion",
"label": "Royalty Agreement, Above $2.5 billion [Member]",
"documentation": "Royalty Agreement, Above $2.5 billion"
}
}
},
"auth_ref": []
},
"anab_RoyaltyAgreementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RoyaltyAgreementMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalties",
"label": "Royalty Agreement [Member]",
"documentation": "Royalty Agreement"
}
}
},
"auth_ref": []
},
"anab_RoyaltyAgreementUpTo10BillionMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "RoyaltyAgreementUpTo10BillionMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Royalty Agreement, Up To $1.0 billion",
"label": "Royalty Agreement, Up To $1. 0 billion [Member]",
"documentation": "Royalty Agreement, Up To $1. 0 billion"
}
}
},
"auth_ref": []
},
"ecd_Rule10b51ArrAdoptedFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "Rule10b51ArrAdoptedFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Rule 10b5-1 Arrangement Adopted",
"label": "Rule 10b5-1 Arrangement Adopted [Flag]"
}
}
},
"auth_ref": [
"r829"
]
},
"ecd_Rule10b51ArrTrmntdFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "Rule10b51ArrTrmntdFlag",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Rule 10b5-1 Arrangement Terminated",
"label": "Rule 10b5-1 Arrangement Terminated [Flag]"
}
}
},
"auth_ref": [
"r829"
]
},
"anab_SagardHealthcareRoyaltyPartnersLPMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "SagardHealthcareRoyaltyPartnersLPMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Sagard",
"label": "Sagard Healthcare Royalty Partners, LP [Member]",
"documentation": "Sagard Healthcare Royalty Partners, LP"
}
}
},
"auth_ref": []
},
"anab_SaleOfFutureRoyaltiesTextBlockTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "SaleOfFutureRoyaltiesTextBlockTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyalties"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Sale of Future Royalties",
"label": "Sale Of Future Royalties Text Block [Text Block]",
"documentation": "Sale Of Future Royalties Text Block"
}
}
},
"auth_ref": []
},
"anab_SaleOfStockAuthorizedAggregateOfferingMaximum": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "SaleOfStockAuthorizedAggregateOfferingMaximum",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate offering of (up to)",
"label": "Sale of Stock, Authorized Aggregate Offering, Maximum",
"documentation": "Sale of Stock, Authorized Aggregate Offering, Maximum"
}
}
},
"auth_ref": []
},
"us-gaap_SaleOfStockNameOfTransactionDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SaleOfStockNameOfTransactionDomain",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Sale of Stock [Domain]",
"label": "Sale of Stock [Domain]",
"documentation": "Sale of the entity's stock, including, but not limited to, initial public offering (IPO) and private placement."
}
}
},
"auth_ref": []
},
"us-gaap_SaleOfStockNumberOfSharesIssuedInTransaction": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SaleOfStockNumberOfSharesIssuedInTransaction",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Shares sold (in shares)",
"label": "Sale of Stock, Number of Shares Issued in Transaction",
"documentation": "The number of shares issued or sold by the subsidiary or equity method investee per stock transaction."
}
}
},
"auth_ref": []
},
"us-gaap_ScheduleOfAccruedLiabilitiesTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfAccruedLiabilitiesTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Accrued Expenses",
"label": "Schedule of Accrued Liabilities [Table Text Block]",
"documentation": "Tabular disclosure of the components of accrued liabilities."
}
}
},
"auth_ref": []
},
"us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTable",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share [Table]",
"label": "Antidilutive Security, Excluded EPS Calculation [Table]",
"documentation": "Disclosure of information about security that could potentially dilute basic earnings per share (EPS) in future that was not included in calculation of diluted EPS."
}
}
},
"auth_ref": [
"r183"
]
},
"us-gaap_ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfAntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Outstanding Potentially Dilutive Securities Excluded in the Calculation of Diluted Net Loss Per Share",
"label": "Schedule of Antidilutive Securities Excluded from Computation of Earnings Per Share [Table Text Block]",
"documentation": "Tabular disclosure of securities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) in the future that were not included in the computation of diluted EPS because to do so would increase EPS amounts or decrease loss per share amounts for the period presented, by antidilutive securities."
}
}
},
"auth_ref": [
"r20"
]
},
"us-gaap_ScheduleOfAvailableForSaleSecuritiesLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfAvailableForSaleSecuritiesLineItems",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Debt Securities, Available-for-sale [Line Items]",
"label": "Debt Securities, Available-for-Sale [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r230",
"r231",
"r232",
"r233",
"r234",
"r235",
"r236",
"r237",
"r238",
"r239",
"r240",
"r241"
]
},
"us-gaap_ScheduleOfAvailableForSaleSecuritiesReconciliationTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfAvailableForSaleSecuritiesReconciliationTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Available-for-sale Investments",
"label": "Schedule of Available-for-Sale Securities Reconciliation [Table Text Block]",
"documentation": "Tabular disclosure of the reconciliation of available-for-sale securities from cost basis to fair value."
}
}
},
"auth_ref": []
},
"us-gaap_ScheduleOfCollaborativeArrangementsAndNoncollaborativeArrangementTransactionsTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfCollaborativeArrangementsAndNoncollaborativeArrangementTransactionsTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Milestones Achieved",
"label": "Collaborative Arrangement and Arrangement Other than Collaborative [Table Text Block]",
"documentation": "Tabular disclosure of collaborative arrangement and arrangement other than collaborative applicable to revenue-generating activity or operations."
}
}
},
"auth_ref": [
"r1000"
]
},
"us-gaap_ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfCompensationCostForShareBasedPaymentArrangementsAllocationOfShareBasedCompensationCostsByPlanTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Non-cash Stock-based Compensation Expense",
"label": "Share-Based Payment Arrangement, Cost by Plan [Table Text Block]",
"documentation": "Tabular disclosure of cost recognized for award under share-based payment arrangement by plan. Includes, but is not limited to, related tax benefit."
}
}
},
"auth_ref": [
"r37"
]
},
"us-gaap_ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfFairValueAssetsAndLiabilitiesMeasuredOnRecurringBasisTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Assets and Liabilities that Require Fair Value Measurements on a Recurring Basis",
"label": "Schedule of Fair Value, Assets and Liabilities Measured on Recurring Basis [Table Text Block]",
"documentation": "Tabular disclosure of assets and liabilities, including [financial] instruments measured at fair value that are classified in stockholders' equity, if any, that are measured at fair value on a recurring basis. The disclosures contemplated herein include the fair value measurements at the reporting date by the level within the fair value hierarchy in which the fair value measurements in their entirety fall, segregating fair value measurements using quoted prices in active markets for identical assets (Level 1), significant other observable inputs (Level 2), and significant unobservable inputs (Level 3)."
}
}
},
"auth_ref": [
"r1008",
"r1009"
]
},
"us-gaap_ScheduleOfPropertyPlantAndEquipmentTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfPropertyPlantAndEquipmentTable",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosuresScheduleofPropertyandEquipmentnetDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Property, Plant and Equipment [Table]",
"label": "Property, Plant and Equipment [Table]",
"documentation": "Disclosure of information about physical assets used in the normal conduct of business and not intended for resale. Includes, but is not limited to, balances by class of assets, depreciation and depletion expense and method used, including composite depreciation, and accumulated deprecation."
}
}
},
"auth_ref": [
"r6",
"r489"
]
},
"us-gaap_ScheduleOfSegmentReportingInformationBySegmentTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfSegmentReportingInformationBySegmentTable",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Segment Reporting Information, by Segment [Table]",
"label": "Schedule of Segment Reporting Information, by Segment [Table]",
"documentation": "Disclosure of information about profit (loss) and total assets by reportable segment."
}
}
},
"auth_ref": [
"r22",
"r23",
"r24"
]
},
"us-gaap_ScheduleOfSegmentReportingInformationBySegmentTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfSegmentReportingInformationBySegmentTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Segment Revenue, Loss and Significant Expenses",
"label": "Schedule of Segment Reporting Information, by Segment [Table Text Block]",
"documentation": "Tabular disclosure of the profit or loss and total assets for each reportable segment. An entity discloses certain information on each reportable segment if the amounts (a) are included in the measure of segment profit or loss reviewed by the chief operating decision maker or (b) are otherwise regularly provided to the chief operating decision maker, even if not included in that measure of segment profit or loss."
}
}
},
"auth_ref": [
"r22",
"r23",
"r24"
]
},
"us-gaap_ScheduleOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTable",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Share-based Compensation Arrangements by Share-based Payment Award [Table]",
"label": "Schedule of Share-Based Compensation Arrangements by Share-Based Payment Award [Table]",
"documentation": "Disclosure of information about share-based payment arrangement."
}
}
},
"auth_ref": [
"r362",
"r363",
"r365",
"r366",
"r367",
"r368",
"r369",
"r370",
"r371",
"r372",
"r373",
"r374",
"r375",
"r376",
"r377",
"r378",
"r379",
"r380",
"r381",
"r382",
"r383",
"r384",
"r385",
"r386",
"r387",
"r388",
"r389",
"r390",
"r391",
"r396"
]
},
"us-gaap_ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfShareBasedCompensationRestrictedStockUnitsAwardActivityTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Time-based Restricted Stock Units Activity",
"label": "Share-Based Payment Arrangement, Restricted Stock Unit, Activity [Table Text Block]",
"documentation": "Tabular disclosure of the number and weighted-average grant date fair value for restricted stock units that were outstanding at the beginning and end of the year, and the number of restricted stock units that were granted, vested, or forfeited during the year."
}
}
},
"auth_ref": [
"r68"
]
},
"us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Activity Related to Stock Option Awards",
"label": "Share-Based Payment Arrangement, Option, Activity [Table Text Block]",
"documentation": "Tabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value."
}
}
},
"auth_ref": [
"r9",
"r10",
"r68"
]
},
"us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Weighted Average Assumptions used to Estimate Fair Value",
"label": "Schedule of Share-Based Payment Award, Stock Options, Valuation Assumptions [Table Text Block]",
"documentation": "Tabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions."
}
}
},
"auth_ref": [
"r70"
]
},
"us-gaap_ScheduleOfStockByClassTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfStockByClassTable",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Stock by Class [Table]",
"label": "Stock, Class of Stock [Table]",
"documentation": "Disclosure of information about stock by class. Includes, but is not limited to, common, convertible, and preferred stocks."
}
}
},
"auth_ref": [
"r26",
"r27",
"r28",
"r29",
"r30",
"r31",
"r65",
"r66",
"r67",
"r125",
"r126",
"r127",
"r188",
"r330",
"r331",
"r332",
"r334",
"r337",
"r342",
"r344",
"r427",
"r587",
"r588",
"r589",
"r590",
"r696",
"r840",
"r920",
"r922"
]
},
"us-gaap_ScheduleOfTreasuryStockByClassTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ScheduleOfTreasuryStockByClassTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Stock Repurchase Activity",
"label": "Class of Treasury Stock [Table Text Block]",
"documentation": "Tabular disclosure of treasury stock, including, but not limited to, average cost per share, description of share repurchase program, shares repurchased, shares held for each class of treasury stock."
}
}
},
"auth_ref": [
"r32",
"r33",
"r34",
"r35"
]
},
"anab_SecondCommercialSalesMilestoneMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "SecondCommercialSalesMilestoneMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Second commercial sales milestone",
"label": "Second Commercial Sales Milestone [Member]",
"documentation": "Second Commercial Sales Milestone"
}
}
},
"auth_ref": []
},
"anab_SecondInstallmentMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "SecondInstallmentMember",
"presentation": [
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "During 2027",
"label": "Second Installment [Member]",
"documentation": "Second Installment"
}
}
},
"auth_ref": []
},
"dei_Security12bTitle": {
"xbrltype": "securityTitleItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "Security12bTitle",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Title of 12(b) Security",
"label": "Title of 12(b) Security",
"documentation": "Title of a 12(b) registered security."
}
}
},
"auth_ref": [
"r754"
]
},
"us-gaap_SecurityDeposit": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SecurityDeposit",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/CommitmentsandContingenciesNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Security deposit",
"label": "Security Deposit",
"documentation": "The amount of an asset, typically cash, provided to a counterparty to provide certain assurance of performance by the entity pursuant to the terms of a written or oral agreement, such as a lease."
}
}
},
"auth_ref": [
"r861"
]
},
"dei_SecurityExchangeName": {
"xbrltype": "edgarExchangeCodeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "SecurityExchangeName",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Security Exchange Name",
"label": "Security Exchange Name",
"documentation": "Name of the Exchange on which a security is registered."
}
}
},
"auth_ref": [
"r756"
]
},
"us-gaap_SegmentDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SegmentDomain",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Segments [Domain]",
"label": "Segments [Domain]",
"documentation": "Components of an entity that engage in business activities from which they may earn revenue and incur expenses, including transactions with other components of the same entity."
}
}
},
"auth_ref": [
"r76",
"r77",
"r78",
"r79",
"r186",
"r195",
"r198",
"r199",
"r200",
"r201",
"r202",
"r203",
"r204",
"r205",
"r206",
"r207",
"r208",
"r209",
"r211",
"r212",
"r213",
"r214",
"r215",
"r216",
"r217",
"r218",
"r220",
"r221",
"r222",
"r226",
"r277",
"r278",
"r279",
"r280",
"r281",
"r282",
"r283",
"r284",
"r285",
"r292",
"r298",
"r299",
"r412",
"r413",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r682",
"r685",
"r686",
"r692",
"r739",
"r1033",
"r1035",
"r1036",
"r1037",
"r1038",
"r1039",
"r1040",
"r1041",
"r1042",
"r1043",
"r1044",
"r1045",
"r1046",
"r1047",
"r1048",
"r1049",
"r1050",
"r1051",
"r1052",
"r1053",
"r1054",
"r1055",
"r1056",
"r1057",
"r1058",
"r1059",
"r1060",
"r1061",
"r1062"
]
},
"us-gaap_SegmentReportingAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SegmentReportingAbstract",
"lang": {
"en-us": {
"role": {
"label": "Segment Reporting [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_SegmentReportingDisclosureTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SegmentReportingDisclosureTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReporting"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Segment Reporting",
"label": "Segment Reporting Disclosure [Text Block]",
"documentation": "The entire disclosure for reporting segments including data and tables. Reportable segments include those that meet any of the following quantitative thresholds a) it's reported revenue, including sales to external customers and intersegment sales or transfers is 10 percent or more of the combined revenue, internal and external, of all operating segments b) the absolute amount of its reported profit or loss is 10 percent or more of the greater, in absolute amount of 1) the combined reported profit of all operating segments that did not report a loss or 2) the combined reported loss of all operating segments that did report a loss c) its assets are 10 percent or more of the combined assets of all operating segments."
}
}
},
"auth_ref": [
"r79",
"r186",
"r194",
"r195",
"r196",
"r197",
"r198",
"r210",
"r212",
"r213",
"r218",
"r219",
"r220",
"r221",
"r222",
"r223",
"r224",
"r226",
"r681",
"r683",
"r684",
"r685",
"r687",
"r688",
"r689"
]
},
"us-gaap_SegmentReportingInformationLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SegmentReportingInformationLineItems",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Segment Reporting Information [Line Items]",
"label": "Segment Reporting Information [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensation": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensation",
"crdr": "debit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": 1.0,
"order": 3.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Stock-based compensation",
"label": "Share-Based Payment Arrangement, Noncash Expense",
"documentation": "Amount of noncash expense for share-based payment arrangement."
}
}
},
"auth_ref": [
"r4"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardAwardVestingPeriod1",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"verboseLabel": "Award vesting period (in years)",
"terseLabel": "Award vesting period",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Award Vesting Period",
"documentation": "Period over which grantee's right to exercise award under share-based payment arrangement is no longer contingent on satisfaction of service or performance condition, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Includes, but is not limited to, combination of market, performance or service condition."
}
}
},
"auth_ref": [
"r711"
]
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardCommonShareEquivalent": {
"xbrltype": "pureItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardCommonShareEquivalent",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award equivalent shares",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Common Share Equivalent",
"documentation": "Share-based Compensation Arrangement by Share-based Payment Award, Common Share Equivalent"
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeitedInPeriod": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeitedInPeriod",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Forfeitures and cancellations (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Forfeited in Period",
"documentation": "The number of equity-based payment instruments, excluding stock (or unit) options, that were forfeited during the reporting period."
}
}
},
"auth_ref": [
"r381"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsForfeituresWeightedAverageGrantDateFairValue",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Forfeitures and cancellations (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Forfeitures, Weighted Average Grant Date Fair Value",
"documentation": "Weighted average fair value as of the grant date of equity-based award plans other than stock (unit) option plans that were not exercised or put into effect as a result of the occurrence of a terminating event."
}
}
},
"auth_ref": [
"r381"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriod",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Granted (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Grants in Period",
"documentation": "The number of grants made during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan)."
}
}
},
"auth_ref": [
"r379"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsGrantsInPeriodWeightedAverageGrantDateFairValue",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Granted (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Grants in Period, Weighted Average Grant Date Fair Value",
"documentation": "The weighted average fair value at grant date for nonvested equity-based awards issued during the period on other than stock (or unit) option plans (for example, phantom stock or unit plan, stock or unit appreciation rights plan, performance target plan)."
}
}
},
"auth_ref": [
"r379"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedNumber",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"periodStartLabel": "Number of shares, beginning balance (in shares)",
"periodEndLabel": "Number of shares, ending balance (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Nonvested, Number",
"documentation": "The number of non-vested equity-based payment instruments, excluding stock (or unit) options, that validly exist and are outstanding as of the balance sheet date."
}
}
},
"auth_ref": [
"r376",
"r377"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedRollForward": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedRollForward",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Number of Restricted Stock Units",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Nonvested, Number of Shares [Roll Forward]",
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period."
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValue",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"periodStartLabel": "Weighted average grant date fair value, beginning balance (in dollars per share)",
"periodEndLabel": "Weighted average grant date fair value, ending balance (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Nonvested, Weighted Average Grant Date Fair Value",
"documentation": "Per share or unit weighted-average fair value of nonvested award under share-based payment arrangement. Excludes share and unit options."
}
}
},
"auth_ref": [
"r376",
"r377"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValueRollForward": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsNonvestedWeightedAverageGrantDateFairValueRollForward",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted-Average Grant Date Fair Value",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Nonvested, Weighted Average Grant Date Fair Value [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsOutstandingWeightedAverageRemainingContractualTerms": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsOutstandingWeightedAverageRemainingContractualTerms",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted average remaining contractual term, other than options outstanding (in years)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Outstanding, Weighted Average Remaining Contractual Terms",
"documentation": "Weighted average remaining contractual term for equity-based awards excluding options, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r69"
]
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingAggregateIntrinsicValue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingAggregateIntrinsicValue",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate intrinsic value, restricted stock units expected to vest",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Vested and Expected to Vest, Outstanding, Aggregate Intrinsic Value",
"documentation": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Vested and Expected to Vest, Outstanding, Aggregate Intrinsic Value"
}
}
},
"auth_ref": []
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingNumber": {
"xbrltype": "sharesItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingNumber",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Number of shares, Restricted stock units expected to vest (in shares)",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Vested and Expected to Vest, Outstanding, Number",
"documentation": "Share-based Compensation Arrangement by Share-based Payment Award, Equity Instruments Other than Options, Vested and Expected to Vest, Outstanding, Number"
}
}
},
"auth_ref": []
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingWeightedAverageGrantDateFairValue": {
"xbrltype": "perShareItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingWeightedAverageGrantDateFairValue",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Number of shares, Restricted stock units expected to vest (in dollars per share)",
"label": "Share-Based Compensation Arrangement By Share-based Payment Award, Equity Instruments Other Than Options, Vested And Expected To Vest, Outstanding, Weighted Average Grant Date Fair Value",
"documentation": "Share-Based Compensation Arrangement By Share-based Payment Award, Equity Instruments Other Than Options, Vested And Expected To Vest, Outstanding, Weighted Average Grant Date Fair Value"
}
}
},
"auth_ref": []
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingWeightedAverageRemainingContractualTerms": {
"xbrltype": "durationItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedAndExpectedToVestOutstandingWeightedAverageRemainingContractualTerms",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted average remaining contractual term, other than options vested and expected to vest (in years)",
"label": "Share-Based Compensation Arrangement By Share-Based Payment Award, Equity Instruments Other Than Options, Vested And Expected To Vest, Outstanding, Weighted Average Remaining Contractual Terms",
"documentation": "Share-Based Compensation Arrangement By Share-Based Payment Award, Equity Instruments Other Than Options, Vested And Expected To Vest, Outstanding, Weighted Average Remaining Contractual Terms"
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriod",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Released (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Vested in Period",
"documentation": "The number of equity-based payment instruments, excluding stock (or unit) options, that vested during the reporting period."
}
}
},
"auth_ref": [
"r380"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardEquityInstrumentsOtherThanOptionsVestedInPeriodWeightedAverageGrantDateFairValue",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Released (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Vested in Period, Weighted Average Grant Date Fair Value",
"documentation": "The weighted average fair value as of grant date pertaining to an equity-based award plan other than a stock (or unit) option plan for which the grantee gained the right during the reporting period, by satisfying service and performance requirements, to receive or retain shares or units, other instruments, or cash in accordance with the terms of the arrangement."
}
}
},
"auth_ref": [
"r380"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate": {
"xbrltype": "percentItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Expected dividend yield",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Fair Value Assumptions, Expected Dividend Rate",
"documentation": "The estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term."
}
}
},
"auth_ref": [
"r389"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate": {
"xbrltype": "percentItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Expected volatility",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Fair Value Assumptions, Expected Volatility Rate",
"documentation": "The estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period."
}
}
},
"auth_ref": [
"r388"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate": {
"xbrltype": "percentItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Risk-free interest rate",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Fair Value Assumptions, Risk Free Interest Rate",
"documentation": "The risk-free interest rate assumption that is used in valuing an option on its own shares."
}
}
},
"auth_ref": [
"r390"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Share-based Compensation Arrangement by Share-based Payment Award [Line Items]",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r362",
"r363",
"r365",
"r366",
"r367",
"r368",
"r369",
"r370",
"r371",
"r372",
"r373",
"r374",
"r375",
"r376",
"r377",
"r378",
"r379",
"r380",
"r381",
"r382",
"r383",
"r384",
"r385",
"r386",
"r387",
"r388",
"r389",
"r390",
"r391",
"r396"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfAdditionalSharesAuthorized",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Number of additional shares authorized",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Number of Additional Shares Authorized",
"documentation": "Number of additional shares authorized for issuance under share-based payment arrangement."
}
}
},
"auth_ref": [
"r990"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesAvailableForGrant",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Shares reserved for future award grants (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Number of Shares Available for Grant",
"documentation": "The difference between the maximum number of shares (or other type of equity) authorized for issuance under the plan (including the effects of amendments and adjustments), and the sum of: 1) the number of shares (or other type of equity) already issued upon exercise of options or other equity-based awards under the plan; and 2) shares (or other type of equity) reserved for issuance on granting of outstanding awards, net of cancellations and forfeitures, if applicable."
}
}
},
"auth_ref": [
"r36"
]
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesIssuable": {
"xbrltype": "pureItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardNumberOfSharesIssuable",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Number of shares issuable (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Number Of Shares Issuable",
"documentation": "Share-Based Compensation Arrangement by Share-Based Payment Award, Number Of Shares Issuable"
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAdditionalDisclosuresAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAdditionalDisclosuresAbstract",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted-Average Remaining Contractual Term and Aggregate Intrinsic Value",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Additional Disclosures [Abstract]"
}
}
},
"auth_ref": []
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAndEmployeeStockPurchasePlan": {
"xbrltype": "sharesItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsAndEmployeeStockPurchasePlan",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Issuance of common stock from exercises of options and employee stock purchase plan (in shares)",
"label": "Share-based Compensation Arrangement by Share-based Payment Award, Options And Employee Stock Purchase Plan",
"documentation": "Share-based Compensation Arrangement by Share-based Payment Award, Options And Employee Stock Purchase Plan"
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Shares subject to options, exercisable, ending balance (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Exercisable, Number",
"documentation": "The number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan."
}
}
},
"auth_ref": [
"r370"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"verboseLabel": "Weighted-average exercise price per share, Stock options exercisable, ending balance (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Exercisable, Weighted Average Exercise Price",
"documentation": "The weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan."
}
}
},
"auth_ref": [
"r370"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriod": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriod",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Forfeitures and cancellations (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Forfeitures and Expirations in Period",
"documentation": "For presentations that combine terminations, the number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan or that expired."
}
}
},
"auth_ref": [
"r969"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriodWeightedAverageExercisePrice": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresAndExpirationsInPeriodWeightedAverageExercisePrice",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Forfeitures and cancellations (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Forfeitures and Expirations in Period, Weighted Average Exercise Price",
"documentation": "Weighted average price of options that were either forfeited or expired."
}
}
},
"auth_ref": [
"r969"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Granted (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Gross",
"documentation": "Gross number of share options (or share units) granted during the period."
}
}
},
"auth_ref": [
"r372"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageGrantDateFairValue",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted average grant date fair value per share (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Grants in Period, Weighted Average Grant Date Fair Value",
"documentation": "The weighted average grant-date fair value of options granted during the reporting period as calculated by applying the disclosed option pricing methodology."
}
}
},
"auth_ref": [
"r382"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingIntrinsicValue",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate intrinsic value, options outstanding",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding, Intrinsic Value",
"documentation": "Amount by which the current fair value of the underlying stock exceeds the exercise price of options outstanding."
}
}
},
"auth_ref": [
"r36"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"periodStartLabel": "Stock options outstanding, beginning balance (in shares)",
"periodEndLabel": "Stock options outstanding, ending balance (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding, Number",
"documentation": "Number of options outstanding, including both vested and non-vested options."
}
}
},
"auth_ref": [
"r368",
"r369"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingRollForward",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Shares Subject to Options",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding [Roll Forward]",
"documentation": "A roll forward is a reconciliation of a concept from the beginning of a period to the end of a period."
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"periodStartLabel": "Stock options outstanding, beginning balance (in dollars per share)",
"periodEndLabel": "Stock options outstanding, ending balance (in dollars per share)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding, Weighted Average Exercise Price",
"documentation": "Weighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan."
}
}
},
"auth_ref": [
"r368",
"r369"
]
},
"us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePriceRollforward",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted-Average Exercise Price per Share",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding, Weighted Average Exercise Price [Abstract]"
}
}
},
"auth_ref": []
},
"anab_ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesIssued": {
"xbrltype": "sharesItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ShareBasedCompensationArrangementByShareBasedPaymentAwardSharesIssued",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Issued under ESPP (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Shares Issued",
"documentation": "Share-Based Compensation Arrangement by Share-Based Payment Award, Shares Issued"
}
}
},
"auth_ref": []
},
"us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardAwardTypeAndPlanNameDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementsByShareBasedPaymentAwardAwardTypeAndPlanNameDomain",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails",
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award Type [Domain]",
"label": "Award Type [Domain]",
"documentation": "Award under share-based payment arrangement."
}
}
},
"auth_ref": [
"r365",
"r366",
"r367",
"r368",
"r369",
"r370",
"r371",
"r372",
"r373",
"r374",
"r375",
"r376",
"r377",
"r378",
"r379",
"r380",
"r381",
"r382",
"r383",
"r384",
"r385",
"r387",
"r388",
"r389",
"r390",
"r391"
]
},
"us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Exercised (in dollars per share)",
"label": "Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Exercises in Period, Weighted Average Exercise Price",
"documentation": "Weighted average price at which option holders acquired shares when converting their stock options into shares."
}
}
},
"auth_ref": [
"r373"
]
},
"us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Granted (in dollars per share)",
"label": "Share-Based Compensation Arrangements by Share-Based Payment Award, Options, Grants in Period, Weighted Average Exercise Price",
"documentation": "Weighted average per share amount at which grantees can acquire shares of common stock by exercise of options."
}
}
},
"auth_ref": [
"r372"
]
},
"us-gaap_ShareBasedCompensationPerformanceSharesAwardOutstandingActivityTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "ShareBasedCompensationPerformanceSharesAwardOutstandingActivityTableTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansTables"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Schedule of Performance Shares, Outstanding Activity",
"label": "Share-Based Payment Arrangement, Performance Shares, Outstanding Activity [Table Text Block]",
"documentation": "Tabular disclosure of the number and weighted-average grant date fair value for outstanding performance shares."
}
}
},
"auth_ref": [
"r12"
]
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueAbstract",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted-Average Remaining Contractual Term and Aggregate Intrinsic Value",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Aggregate Intrinsic Value [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueNonvested": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardEquityInstrumentsOtherThanOptionsAggregateIntrinsicValueNonvested",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofTimebasedRestrictedStockUnitsPSUsDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate intrinsic value, outstanding",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Equity Instruments Other than Options, Aggregate Intrinsic Value, Nonvested",
"documentation": "Intrinsic value of nonvested award under share-based payment arrangement. Excludes share and unit options."
}
}
},
"auth_ref": [
"r990"
]
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardExpirationPeriod",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Award expiration period (in years)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Expiration Period",
"documentation": "Period from grant date that an equity-based award expires, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r712"
]
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofGrantDateFairValueAssumptionsDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofWeightedAverageAssumptionsusedtoEstimateFairValueDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Expected term (in years)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Fair Value Assumptions, Expected Term",
"documentation": "Expected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r387"
]
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableIntrinsicValue1",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate intrinsic value, options exercisable",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Exercisable, Intrinsic Value",
"documentation": "Amount of difference between fair value of the underlying shares reserved for issuance and exercise price of vested portions of options outstanding and currently exercisable."
}
}
},
"auth_ref": [
"r36"
]
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted average remaining contractual term, options exercisable (in years)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Exercisable, Weighted Average Remaining Contractual Term",
"documentation": "Weighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r36"
]
},
"us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2": {
"xbrltype": "durationItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted average remaining contractual term, options outstanding (in years)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Outstanding, Weighted Average Remaining Contractual Term",
"documentation": "Weighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days."
}
}
},
"auth_ref": [
"r69"
]
},
"us-gaap_SharesIssuedPricePerShare": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharesIssuedPricePerShare",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Share price (in dollars per share)",
"label": "Shares Issued, Price Per Share",
"documentation": "Per share or per unit amount of equity securities issued."
}
}
},
"auth_ref": []
},
"us-gaap_SharesPaidForTaxWithholdingForShareBasedCompensation": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SharesPaidForTaxWithholdingForShareBasedCompensation",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Net share settlement of restricted stock units (in shares)",
"label": "Share-Based Payment Arrangement, Shares Withheld for Tax Withholding Obligation",
"documentation": "Number of shares used to settle grantee's tax withholding obligation for award under share-based payment arrangement."
}
}
},
"auth_ref": [
"r990"
]
},
"us-gaap_SignificantAccountingPoliciesTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SignificantAccountingPoliciesTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPolicies"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Summary of Significant Accounting Policies",
"label": "Significant Accounting Policies [Text Block]",
"documentation": "The entire disclosure for all significant accounting policies of the reporting entity."
}
}
},
"auth_ref": [
"r152",
"r153"
]
},
"us-gaap_StatementBusinessSegmentsAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StatementBusinessSegmentsAxis",
"presentation": [
"http://www.anaptysbio.com/role/SegmentReportingScheduleofSegmentRevenueLossandSignificantExpensesDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Segments [Axis]",
"label": "Segments [Axis]",
"documentation": "Information by business segments."
}
}
},
"auth_ref": [
"r76",
"r77",
"r78",
"r79",
"r115",
"r186",
"r195",
"r198",
"r199",
"r200",
"r201",
"r202",
"r203",
"r204",
"r205",
"r206",
"r207",
"r208",
"r209",
"r211",
"r212",
"r213",
"r214",
"r215",
"r216",
"r217",
"r218",
"r220",
"r221",
"r222",
"r226",
"r277",
"r278",
"r279",
"r280",
"r281",
"r282",
"r283",
"r284",
"r285",
"r292",
"r295",
"r298",
"r299",
"r412",
"r413",
"r556",
"r557",
"r558",
"r559",
"r560",
"r561",
"r562",
"r563",
"r564",
"r565",
"r566",
"r682",
"r685",
"r686",
"r692",
"r739",
"r1033",
"r1035",
"r1036",
"r1037",
"r1038",
"r1039",
"r1040",
"r1041",
"r1042",
"r1043",
"r1044",
"r1045",
"r1046",
"r1047",
"r1048",
"r1049",
"r1050",
"r1051",
"r1052",
"r1053",
"r1054",
"r1055",
"r1056",
"r1057",
"r1058",
"r1059",
"r1060",
"r1061",
"r1062"
]
},
"us-gaap_StatementEquityComponentsAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StatementEquityComponentsAxis",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Equity Components [Axis]",
"label": "Equity Components [Axis]",
"documentation": "Information by component of equity."
}
}
},
"auth_ref": [
"r8",
"r43",
"r46",
"r47",
"r112",
"r113",
"r114",
"r138",
"r139",
"r140",
"r160",
"r161",
"r162",
"r164",
"r171",
"r173",
"r175",
"r187",
"r261",
"r262",
"r293",
"r318",
"r345",
"r397",
"r404",
"r405",
"r407",
"r408",
"r409",
"r411",
"r414",
"r415",
"r428",
"r429",
"r430",
"r431",
"r432",
"r433",
"r434",
"r435",
"r436",
"r437",
"r440",
"r472",
"r473",
"r474",
"r475",
"r476",
"r477",
"r478",
"r479",
"r491",
"r550",
"r578",
"r579",
"r580",
"r592",
"r640"
]
},
"us-gaap_StatementLineItems": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StatementLineItems",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Statement [Line Items]",
"label": "Statement [Line Items]",
"documentation": "Line items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table."
}
}
},
"auth_ref": [
"r160",
"r161",
"r162",
"r187",
"r320",
"r321",
"r323",
"r325",
"r479",
"r528",
"r586",
"r593",
"r594",
"r597",
"r598",
"r599",
"r600",
"r601",
"r602",
"r603",
"r606",
"r607",
"r608",
"r609",
"r610",
"r611",
"r612",
"r613",
"r614",
"r616",
"r617",
"r618",
"r619",
"r620",
"r623",
"r624",
"r626",
"r627",
"r628",
"r629",
"r630",
"r631",
"r632",
"r633",
"r634",
"r635",
"r636",
"r637",
"r640",
"r671",
"r672",
"r747",
"r1066"
]
},
"us-gaap_StatementOfCashFlowsAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StatementOfCashFlowsAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Statement of Cash Flows [Abstract]",
"label": "Statement of Cash Flows [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_StatementOfFinancialPositionAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StatementOfFinancialPositionAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Statement of Financial Position [Abstract]",
"label": "Statement of Financial Position [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_StatementOfStockholdersEquityAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StatementOfStockholdersEquityAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Statement of Stockholders' Equity [Abstract]",
"label": "Statement of Stockholders' Equity [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_StatementTable": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StatementTable",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Statement [Table]",
"label": "Statement [Table]",
"documentation": "Presentation of information about comprehensive income, income, other comprehensive income, financial position, cash flows, and shareholders' equity."
}
}
},
"auth_ref": [
"r160",
"r161",
"r162",
"r187",
"r227",
"r320",
"r321",
"r323",
"r325",
"r479",
"r528",
"r586",
"r593",
"r594",
"r597",
"r598",
"r599",
"r600",
"r601",
"r602",
"r603",
"r606",
"r607",
"r608",
"r609",
"r610",
"r611",
"r612",
"r613",
"r614",
"r616",
"r617",
"r618",
"r619",
"r620",
"r623",
"r624",
"r626",
"r627",
"r628",
"r629",
"r630",
"r631",
"r632",
"r633",
"r634",
"r635",
"r636",
"r637",
"r640",
"r671",
"r672",
"r747",
"r1066"
]
},
"ecd_StkPrcOrTsrEstimationMethodTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "StkPrcOrTsrEstimationMethodTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/ErrCompDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Stock Price or TSR Estimation Method",
"label": "Stock Price or TSR Estimation Method [Text Block]"
}
}
},
"auth_ref": [
"r763",
"r773",
"r783",
"r815"
]
},
"us-gaap_StockAppreciationRightsSARSMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockAppreciationRightsSARSMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Stock Appreciation Rights (SARs)",
"label": "Stock Appreciation Rights (SARs) [Member]",
"documentation": "Right to receive cash or shares equal to appreciation of predetermined number of grantor's shares during predetermined time period."
}
}
},
"auth_ref": [
"r963",
"r964",
"r965",
"r966",
"r967",
"r968",
"r969",
"r970",
"r971",
"r972",
"r973",
"r974",
"r975",
"r976",
"r977",
"r978",
"r979",
"r980",
"r981",
"r982",
"r983",
"r984",
"r985",
"r986",
"r987",
"r988"
]
},
"us-gaap_StockCompensationPlanMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockCompensationPlanMember",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesScheduleofOutstandingPotentiallyDilutiveSecuritiesExcludedintheCalculationofDilutedNetLossPerShareDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Stock awards",
"label": "Share-Based Payment Arrangement [Member]",
"documentation": "Share-based payment arrangement in which award of equity shares are granted. Arrangement includes, but is not limited to, grantor incurring liability for product and service based on price of its shares."
}
}
},
"auth_ref": [
"r930"
]
},
"us-gaap_StockIssuedDuringPeriodSharesRestrictedStockAwardNetOfForfeitures": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockIssuedDuringPeriodSharesRestrictedStockAwardNetOfForfeitures",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Issuance of common stock upon vesting of restricted stock units (in shares)",
"label": "Stock Issued During Period, Shares, Restricted Stock Award, Net of Forfeitures",
"documentation": "Number of shares issued during the period related to Restricted Stock Awards, net of any shares forfeited."
}
}
},
"auth_ref": [
"r8",
"r42",
"r43",
"r67"
]
},
"us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockIssuedDuringPeriodSharesStockOptionsExercised",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofOptionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Exercised (in shares)",
"label": "Share-Based Compensation Arrangement by Share-Based Payment Award, Options, Exercises in Period",
"documentation": "Number of share options (or share units) exercised during the current period."
}
}
},
"auth_ref": [
"r8",
"r42",
"r43",
"r67",
"r373"
]
},
"anab_StockIssuedDuringPeriodValueOptionsAndEmployeeStockPurchasePlan": {
"xbrltype": "monetaryItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "StockIssuedDuringPeriodValueOptionsAndEmployeeStockPurchasePlan",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Issuance of common stock from exercises of options and employee stock purchase plan",
"label": "Stock Issued During Period, Value, Options and Employee Stock Purchase Plan",
"documentation": "Stock Issued During Period, Value, Options and Employee Stock Purchase Plan"
}
}
},
"auth_ref": []
},
"srt_StockRepurchaseProgramAuthorizedAmount1": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "StockRepurchaseProgramAuthorizedAmount1",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Authorized repurchase amount",
"label": "Share Repurchase Program, Authorized, Amount",
"documentation": "Amount authorized for purchase of share under share repurchase plan. Includes, but is not limited to, repurchase of stock and unit of ownership."
}
}
},
"auth_ref": [
"r955"
]
},
"us-gaap_StockRepurchaseProgramRemainingAuthorizedRepurchaseAmount1": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockRepurchaseProgramRemainingAuthorizedRepurchaseAmount1",
"crdr": "credit",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Remaining repurchase amount",
"label": "Share Repurchase Program, Remaining Authorized, Amount",
"documentation": "Amount remaining authorized for purchase of share under share repurchase plan. Includes, but is not limited to, repurchase of stock and unit of ownership."
}
}
},
"auth_ref": []
},
"us-gaap_StockRepurchasedAndRetiredDuringPeriodShares": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockRepurchasedAndRetiredDuringPeriodShares",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Repurchases and retirements of common stock (in shares)",
"label": "Stock Repurchased and Retired During Period, Shares",
"documentation": "Number of shares that have been repurchased and retired during the period."
}
}
},
"auth_ref": [
"r8",
"r42",
"r43",
"r67"
]
},
"us-gaap_StockRepurchasedAndRetiredDuringPeriodValue": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockRepurchasedAndRetiredDuringPeriodValue",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"negatedTerseLabel": "Repurchases and retirements of common stock",
"label": "Stock Repurchased and Retired During Period, Value",
"documentation": "Equity impact of the value of stock that has been repurchased and retired during the period. The excess of the purchase price over par value can be charged against retained earnings (once the excess is fully allocated to additional paid in capital)."
}
}
},
"auth_ref": [
"r8",
"r42",
"r43",
"r67"
]
},
"us-gaap_StockholdersEquity": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockholdersEquity",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets": {
"parentTag": "us-gaap_LiabilitiesAndStockholdersEquity",
"weight": 1.0,
"order": 1.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets",
"http://www.anaptysbio.com/role/ConsolidatedStatementofStockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"totalLabel": "Total stockholders\u2019 (deficit) equity",
"periodStartLabel": "Stockholders' equity, beginning balance",
"periodEndLabel": "Stockholders' equity, ending balance",
"label": "Equity, Attributable to Parent",
"documentation": "Amount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest."
}
}
},
"auth_ref": [
"r43",
"r46",
"r47",
"r61",
"r605",
"r621",
"r641",
"r642",
"r734",
"r753",
"r920",
"r923",
"r924",
"r944",
"r1017",
"r1067"
]
},
"us-gaap_StockholdersEquityAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockholdersEquityAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedBalanceSheets"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Stockholders\u2019 equity:",
"label": "Equity, Attributable to Parent [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_StockholdersEquityNoteDisclosureTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "StockholdersEquityNoteDisclosureTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquity"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Stockholders' Equity",
"label": "Equity [Text Block]",
"documentation": "The entire disclosure for equity."
}
}
},
"auth_ref": [
"r64",
"r329",
"r331",
"r333",
"r334",
"r335",
"r336",
"r337",
"r338",
"r339",
"r340",
"r341",
"r343",
"r345",
"r427",
"r439",
"r643",
"r644",
"r650"
]
},
"us-gaap_SubsequentEventsAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SubsequentEventsAbstract",
"lang": {
"en-us": {
"role": {
"terseLabel": "Subsequent Events [Abstract]",
"label": "Subsequent Events [Abstract]"
}
}
},
"auth_ref": []
},
"us-gaap_SubsequentEventsTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SubsequentEventsTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/SubsequentEvent"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Subsequent Event",
"label": "Subsequent Events [Text Block]",
"documentation": "The entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business."
}
}
},
"auth_ref": [
"r493",
"r494"
]
},
"us-gaap_SubsidiarySaleOfStockAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SubsidiarySaleOfStockAxis",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Sale of Stock [Axis]",
"label": "Sale of Stock [Axis]",
"documentation": "Information by type of sale of the entity's stock."
}
}
},
"auth_ref": []
},
"us-gaap_SupplementalBalanceSheetDisclosuresTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SupplementalBalanceSheetDisclosuresTextBlock",
"presentation": [
"http://www.anaptysbio.com/role/BalanceSheetAccountsandSupplementalDisclosures"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Balance Sheet Accounts and Supplemental Disclosures",
"label": "Supplemental Balance Sheet Disclosures [Text Block]",
"documentation": "The entire disclosure for supplemental balance sheet disclosures, including descriptions and amounts for assets, liabilities, and equity."
}
}
},
"auth_ref": [
"r860"
]
},
"us-gaap_SupplementalCashFlowInformationAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "SupplementalCashFlowInformationAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION",
"label": "Supplemental Cash Flow Information [Abstract]"
}
}
},
"auth_ref": []
},
"ecd_TabularListTableTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TabularListTableTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Tabular List, Table",
"label": "Tabular List [Table Text Block]"
}
}
},
"auth_ref": [
"r809"
]
},
"anab_ThirdCommercialSalesMilestoneMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ThirdCommercialSalesMilestoneMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Third commercial sales milestone",
"label": "Third Commercial Sales Milestone [Member]",
"documentation": "Third Commercial Sales Milestone"
}
}
},
"auth_ref": []
},
"srt_TitleOfIndividualAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "TitleOfIndividualAxis",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Title of Individual [Axis]",
"label": "Title and Position [Axis]",
"documentation": "Information by title and position of individual or group within organization."
}
}
},
"auth_ref": [
"r937",
"r1021"
]
},
"srt_TitleOfIndividualWithRelationshipToEntityDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/srt/2025",
"localname": "TitleOfIndividualWithRelationshipToEntityDomain",
"presentation": [
"http://www.anaptysbio.com/role/EquityIncentivePlansNarrativeDetails",
"http://www.anaptysbio.com/role/EquityIncentivePlansScheduleofNoncashStockbasedCompensationExpenseDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Title of Individual [Domain]",
"label": "Title and Position [Domain]",
"documentation": "Title and position of individual or group within organization."
}
}
},
"auth_ref": []
},
"ecd_TotalShareholderRtnAmt": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TotalShareholderRtnAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Total Shareholder Return Amount",
"label": "Total Shareholder Return Amount"
}
}
},
"auth_ref": [
"r801"
]
},
"ecd_TotalShareholderRtnVsPeerGroupTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TotalShareholderRtnVsPeerGroupTextBlock",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Total Shareholder Return Vs Peer Group",
"label": "Total Shareholder Return Vs Peer Group [Text Block]"
}
}
},
"auth_ref": [
"r808"
]
},
"ecd_TradingArrAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TradingArrAxis",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Trading Arrangement:",
"label": "Trading Arrangement [Axis]"
}
}
},
"auth_ref": [
"r828"
]
},
"ecd_TradingArrByIndTable": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TradingArrByIndTable",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Trading Arrangements, by Individual",
"label": "Trading Arrangements, by Individual [Table]"
}
}
},
"auth_ref": [
"r830"
]
},
"dei_TradingSymbol": {
"xbrltype": "tradingSymbolItemType",
"nsuri": "http://xbrl.sec.gov/dei/2025",
"localname": "TradingSymbol",
"presentation": [
"http://www.anaptysbio.com/role/CoverPage"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Trading Symbol",
"label": "Trading Symbol",
"documentation": "Trading symbol of an instrument as listed on an exchange."
}
}
},
"auth_ref": []
},
"anab_TransactionServiceMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "TransactionServiceMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Transition services revenue",
"label": "Transaction Service [Member]",
"documentation": "Transaction Service"
}
}
},
"auth_ref": []
},
"us-gaap_TransfersAndServicingOfFinancialInstrumentsTypesOfFinancialInstrumentsDomain": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "TransfersAndServicingOfFinancialInstrumentsTypesOfFinancialInstrumentsDomain",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Financial Instruments [Domain]",
"label": "Financial Instruments [Domain]",
"documentation": "Instrument or contract that imposes a contractual obligation to deliver cash or another financial instrument or to exchange other financial instruments on potentially unfavorable terms and conveys a contractual right to receive cash or another financial instrument or to exchange other financial instruments on potentially favorable terms."
}
}
},
"auth_ref": [
"r230",
"r231",
"r232",
"r233",
"r234",
"r235",
"r236",
"r237",
"r238",
"r239",
"r240",
"r241",
"r242",
"r243",
"r244",
"r245",
"r246",
"r247",
"r248",
"r249",
"r250",
"r251",
"r252",
"r253",
"r254",
"r255",
"r256",
"r257",
"r258",
"r259",
"r317",
"r342",
"r427",
"r438",
"r462",
"r467",
"r470",
"r495",
"r496",
"r497",
"r498",
"r499",
"r500",
"r501",
"r502",
"r503",
"r504",
"r505",
"r506",
"r507",
"r508",
"r509",
"r512",
"r513",
"r514",
"r515",
"r516",
"r517",
"r518",
"r519",
"r520",
"r521",
"r522",
"r523",
"r524",
"r525",
"r526",
"r537",
"r551",
"r722",
"r723",
"r725",
"r726",
"r727",
"r728",
"r729",
"r730",
"r731",
"r735",
"r843",
"r844",
"r845",
"r846",
"r847",
"r848",
"r849",
"r940",
"r941",
"r942",
"r943",
"r1007",
"r1010",
"r1011",
"r1012",
"r1013",
"r1014",
"r1015",
"r1016"
]
},
"ecd_TrdArrAdoptionDate": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TrdArrAdoptionDate",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Adoption Date",
"label": "Trading Arrangement Adoption Date"
}
}
},
"auth_ref": [
"r831"
]
},
"ecd_TrdArrDuration": {
"xbrltype": "durationItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TrdArrDuration",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Arrangement Duration",
"label": "Trading Arrangement Duration"
}
}
},
"auth_ref": [
"r832"
]
},
"ecd_TrdArrExpirationDate": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TrdArrExpirationDate",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Expiration Date",
"label": "Trading Arrangement Expiration Date"
}
}
},
"auth_ref": [
"r832"
]
},
"ecd_TrdArrIndName": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TrdArrIndName",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Name",
"label": "Trading Arrangement, Individual Name"
}
}
},
"auth_ref": [
"r830"
]
},
"ecd_TrdArrIndTitle": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TrdArrIndTitle",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Title",
"label": "Trading Arrangement, Individual Title"
}
}
},
"auth_ref": [
"r830"
]
},
"ecd_TrdArrSecuritiesAggAvailAmt": {
"xbrltype": "sharesItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TrdArrSecuritiesAggAvailAmt",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Aggregate Available",
"label": "Trading Arrangement, Securities Aggregate Available Amount"
}
}
},
"auth_ref": [
"r833"
]
},
"ecd_TrdArrTerminationDate": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "TrdArrTerminationDate",
"presentation": [
"http://xbrl.sec.gov/ecd/role/InsiderTradingArrangements"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Termination Date",
"label": "Trading Arrangement Termination Date"
}
}
},
"auth_ref": [
"r831"
]
},
"us-gaap_TreasuryStockAcquiredAverageCostPerShare": {
"xbrltype": "perShareItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "TreasuryStockAcquiredAverageCostPerShare",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityScheduleofStockRepurchaseActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Average price paid per share (in dollars per share)",
"label": "Shares Acquired, Average Cost Per Share",
"documentation": "Total cost of shares repurchased divided by the total number of shares repurchased."
}
}
},
"auth_ref": [
"r32"
]
},
"us-gaap_TreasuryStockSharesAcquired": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "TreasuryStockSharesAcquired",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityScheduleofStockRepurchaseActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Total number of shares purchased (in shares)",
"label": "Treasury Stock, Shares, Acquired",
"documentation": "Number of shares that have been repurchased during the period and are being held in treasury."
}
}
},
"auth_ref": [
"r8",
"r43",
"r67"
]
},
"us-gaap_TreasuryStockValueAcquiredCostMethod": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "TreasuryStockValueAcquiredCostMethod",
"crdr": "debit",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityScheduleofStockRepurchaseActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Approximate dollar value of shares purchased (in dollars per share)",
"label": "Treasury Stock, Value, Acquired, Cost Method",
"documentation": "Equity impact of the cost of common and preferred stock that were repurchased during the period. Recorded using the cost method."
}
}
},
"auth_ref": [
"r8",
"r32",
"r67"
]
},
"us-gaap_TypeOfArrangementAxis": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "TypeOfArrangementAxis",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Collaborative Arrangement and Arrangement Other than Collaborative [Axis]",
"label": "Collaborative Arrangement and Arrangement Other than Collaborative [Axis]",
"documentation": "Information by collaborative arrangement and arrangement other than collaborative applicable to revenue-generating activity or operations."
}
}
},
"auth_ref": [
"r418"
]
},
"us-gaap_USGovernmentAgenciesDebtSecuritiesMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "USGovernmentAgenciesDebtSecuritiesMember",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Agency securities",
"label": "US Government Agencies Debt Securities [Member]",
"documentation": "Debentures, notes, and other debt securities issued by US government agencies, for example, but not limited to, Government National Mortgage Association (GNMA or Ginnie Mae). Excludes US treasury securities and debt issued by government-sponsored Enterprises (GSEs), for example, but is not limited to, Federal Home Loan Mortgage Corporation (FHLMC or Freddie Mac), Federal National Mortgage Association (FNMA or Fannie Mae), and the Federal Home Loan Bank (FHLB)."
}
}
},
"auth_ref": [
"r674",
"r675",
"r705",
"r707",
"r1032"
]
},
"us-gaap_USTreasurySecuritiesMember": {
"xbrltype": "domainItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "USTreasurySecuritiesMember",
"presentation": [
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAssetsandLiabilitiesthatRequireFairValueMeasurementsonaRecurringBasisDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofAvailableforsaleInvestmentsDetails",
"http://www.anaptysbio.com/role/FairValueMeasurementsandAvailableforSaleInvestmentsScheduleofUnrealizedLossandFairValuesinaLossPositionDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "U.S. Treasury Securities",
"label": "US Treasury Securities [Member]",
"documentation": "This category includes information about debt securities issued by the United States Department of the Treasury and backed by the United States government. Such securities primarily consist of treasury bills (short-term maturities - one year or less), treasury notes (intermediate term maturities - two to ten years), and treasury bonds (long-term maturities - ten to thirty years)."
}
}
},
"auth_ref": [
"r674",
"r675",
"r705",
"r707",
"r709",
"r722",
"r1032"
]
},
"anab_UnderwritingAgreementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "UnderwritingAgreementMember",
"presentation": [
"http://www.anaptysbio.com/role/StockholdersEquityNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Underwritings",
"label": "Underwriting Agreement [Member]",
"documentation": "Underwriting Agreement"
}
}
},
"auth_ref": []
},
"ecd_UndrlygSecurityMktPriceChngPct": {
"xbrltype": "pureItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "UndrlygSecurityMktPriceChngPct",
"presentation": [
"http://xbrl.sec.gov/ecd/role/AwardTimingDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Underlying Security Market Price Change",
"label": "Underlying Security Market Price Change, Percent"
}
}
},
"auth_ref": [
"r827"
]
},
"us-gaap_UnrealizedGainLossOnInvestments": {
"xbrltype": "monetaryItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "UnrealizedGainLossOnInvestments",
"crdr": "credit",
"calculation": {
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows": {
"parentTag": "us-gaap_NetCashProvidedByUsedInOperatingActivities",
"weight": -1.0,
"order": 4.0
}
},
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofCashFlows"
],
"lang": {
"en-us": {
"role": {
"negatedLabel": "Accretion/amortization of investments, net",
"label": "Unrealized Gain (Loss) on Investments",
"documentation": "Amount of unrealized gain (loss) on investment."
}
}
},
"auth_ref": [
"r5"
]
},
"us-gaap_UseOfEstimates": {
"xbrltype": "textBlockItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "UseOfEstimates",
"presentation": [
"http://www.anaptysbio.com/role/SummaryofSignificantAccountingPoliciesPolicies"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Use of Estimates",
"label": "Use of Estimates, Policy [Policy Text Block]",
"documentation": "Disclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles."
}
}
},
"auth_ref": [
"r87",
"r88",
"r189",
"r191",
"r192",
"r193",
"r533",
"r535",
"r679"
]
},
"anab_VandaPharmaceuticalsInc.Member": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "VandaPharmaceuticalsInc.Member",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsScheduleofMilestoneAchievedDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Vanda",
"label": "Vanda Pharmaceuticals Inc. [Member]",
"documentation": "Vanda Pharmaceuticals Inc."
}
}
},
"auth_ref": []
},
"ecd_VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "VstngDtFrValOfEqtyAwrdsGrntdAndVstdInCvrdYrMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Vesting Date Fair Value of Equity Awards Granted and Vested in Covered Year",
"label": "Vesting Date Fair Value of Equity Awards Granted and Vested in Covered Year [Member]"
}
}
},
"auth_ref": [
"r797"
]
},
"us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "WeightedAverageNumberOfDilutedSharesOutstanding",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Diluted (in shares)",
"label": "Weighted Average Number of Shares Outstanding, Diluted",
"documentation": "The average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period."
}
}
},
"auth_ref": [
"r177",
"r182"
]
},
"us-gaap_WeightedAverageNumberOfSharesOutstandingBasic": {
"xbrltype": "sharesItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "WeightedAverageNumberOfSharesOutstandingBasic",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Basic (in shares)",
"label": "Weighted Average Number of Shares Outstanding, Basic",
"documentation": "Number of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period."
}
}
},
"auth_ref": [
"r176",
"r182"
]
},
"us-gaap_WeightedAverageNumberOfSharesOutstandingDilutedDisclosureItemsAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://fasb.org/us-gaap/2025",
"localname": "WeightedAverageNumberOfSharesOutstandingDilutedDisclosureItemsAbstract",
"presentation": [
"http://www.anaptysbio.com/role/ConsolidatedStatementsofOperationsandComprehensiveLoss"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Weighted-average number of shares outstanding:",
"label": "Weighted Average Number of Shares Outstanding Reconciliation [Abstract]"
}
}
},
"auth_ref": []
},
"ecd_YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMember": {
"xbrltype": "domainItemType",
"nsuri": "http://xbrl.sec.gov/ecd/2025",
"localname": "YrEndFrValOfEqtyAwrdsGrntdInCvrdYrOutsdngAndUnvstdMember",
"presentation": [
"http://xbrl.sec.gov/ecd/role/PvpDisclosure"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Year-end Fair Value of Equity Awards Granted in Covered Year that are Outstanding and Unvested",
"label": "Year-end Fair Value of Equity Awards Granted in Covered Year that are Outstanding and Unvested [Member]"
}
}
},
"auth_ref": [
"r795"
]
},
"anab_ZejulaRevenueMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ZejulaRevenueMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Zejula revenue prior to the Zejula Royalty Monetization Agreement",
"label": "Zejula Revenue [Member]",
"documentation": "Zejula Revenue"
}
}
},
"auth_ref": []
},
"anab_ZejulaRoyaltyMonetizationAgreementMember": {
"xbrltype": "domainItemType",
"nsuri": "http://www.anaptysbio.com/20250630",
"localname": "ZejulaRoyaltyMonetizationAgreementMember",
"presentation": [
"http://www.anaptysbio.com/role/CollaborativeResearchandDevelopmentAgreementsNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesNarrativeDetails",
"http://www.anaptysbio.com/role/SaleofFutureRoyaltiesScheduleofRoyaltyTransactionActivityDetails"
],
"lang": {
"en-us": {
"role": {
"terseLabel": "Zejula Royalty Monetization Agreement",
"label": "Zejula Royalty Monetization Agreement [Member]",
"documentation": "Zejula Royalty Monetization Agreement"
}
}
},
"auth_ref": []
}
}
}
},
"std_ref": {
"r0": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"SubTopic": "230",
"Topic": "830",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477401/830-230-45-1"
},
"r1": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "10A",
"SubTopic": "10",
"Topic": "220",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-10A"
},
"r2": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "14",
"Subparagraph": "(a)",
"SubTopic": "10",
"Topic": "230",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-14"
},
"r3": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "20",
"SubTopic": "10",
"Topic": "810",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481231/810-10-45-20"
},
"r4": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "28",
"Subparagraph": "(a)",
"SubTopic": "10",
"Topic": "230",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-28"
},
"r5": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "28",
"Subparagraph": "(b)",
"SubTopic": "10",
"Topic": "230",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-28"
},
"r6": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"SubTopic": "10",
"Topic": "360",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482099/360-10-50-1"
},
"r7": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1A",
"Subparagraph": "(c)(3)",
"SubTopic": "10",
"Topic": "810",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481203/810-10-50-1A"
},
"r8": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"SubTopic": "10",
"Topic": "505",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-2"
},
"r9": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)",
"SubTopic": "10",
"Topic": "718",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r10": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(e)",
"SubTopic": "10",
"Topic": "718",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r11": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2A",
"Subparagraph": "(a)",
"SubTopic": "10",
"Topic": "718",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2A"
},
"r12": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Subparagraph": "(c)",
"Paragraph": "2",
"SubTopic": "10",
"Topic": "718",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r13": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "220",
"SubTopic": "10",
"Section": "45",
"Paragraph": "14",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-14"
},
"r14": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "220",
"SubTopic": "10",
"Section": "45",
"Paragraph": "14A",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-14A"
},
"r15": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "220",
"SubTopic": "10",
"Section": "45",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-5"
},
"r16": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "230",
"SubTopic": "10",
"Section": "45",
"Paragraph": "13",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-13"
},
"r17": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "230",
"SubTopic": "10",
"Section": "45",
"Paragraph": "15",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-15"
},
"r18": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "230",
"SubTopic": "10",
"Section": "45",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-4"
},
"r19": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "230",
"SubTopic": "10",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482913/230-10-50-2"
},
"r20": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "260",
"SubTopic": "10",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482662/260-10-50-1"
},
"r21": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "260",
"SubTopic": "10",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482662/260-10-50-2"
},
"r22": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "280",
"SubTopic": "10",
"Section": "50",
"Paragraph": "22",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r23": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "280",
"SubTopic": "10",
"Section": "50",
"Paragraph": "25",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-25"
},
"r24": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "280",
"SubTopic": "10",
"Section": "50",
"Paragraph": "30",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-30"
},
"r25": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "360",
"SubTopic": "10",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482099/360-10-50-1"
},
"r26": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "10",
"Section": "45",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481142/505-10-45-2"
},
"r27": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "10",
"Section": "50",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-10"
},
"r28": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "10",
"Section": "50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-3"
},
"r29": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "10",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-4"
},
"r30": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "10",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-5"
},
"r31": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "10",
"Section": "50",
"Paragraph": "8",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-8"
},
"r32": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "30",
"Section": "45",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481549/505-30-45-1"
},
"r33": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "30",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481520/505-30-50-2"
},
"r34": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "30",
"Section": "50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481520/505-30-50-3"
},
"r35": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "505",
"SubTopic": "30",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481520/505-30-50-4"
},
"r36": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "718",
"SubTopic": "10",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r37": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "718",
"SubTopic": "10",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(h)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r38": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Name": "Accounting Standards Codification",
"Topic": "810",
"SubTopic": "10",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481203/810-10-50-1"
},
"r39": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(19)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r40": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(20))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r41": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(21))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r42": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(28))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r43": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(29))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r44": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(30)(a)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r45": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(30)(a)(3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r46": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(30))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r47": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(31))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r48": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(32))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r49": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-11"
},
"r50": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.5-03(10))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483621/220-10-S99-2"
},
"r51": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.5-03(20))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483621/220-10-S99-2"
},
"r52": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.5-03(4))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483621/220-10-S99-2"
},
"r53": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.5-03(7))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483621/220-10-S99-2"
},
"r54": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.5-03(9))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483621/220-10-S99-2"
},
"r55": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "13",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-13"
},
"r56": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "15",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-15"
},
"r57": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "24",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-24"
},
"r58": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "25",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-25"
},
"r59": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "25",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-25"
},
"r60": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "28",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-28"
},
"r61": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "310",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SAB Topic 4.E)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480418/310-10-S99-2"
},
"r62": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "360",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482099/360-10-50-1"
},
"r63": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "440",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/440/tableOfContent"
},
"r64": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "505",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/505/tableOfContent"
},
"r65": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-6"
},
"r66": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-7"
},
"r67": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.3-04)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480008/505-10-S99-1"
},
"r68": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r69": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "718",
"SubTopic": "10",
"Subparagraph": "(e)(1)",
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Section": "50",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r70": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "718",
"SubTopic": "10",
"Subparagraph": "(f)(2)",
"Name": "Accounting Standards Codification",
"Paragraph": "2",
"Section": "50",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r71": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "810",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "19",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481231/810-10-45-19"
},
"r72": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "942",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-03(11))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478546/942-210-S99-1"
},
"r73": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "942",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-03(23))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478546/942-210-S99-1"
},
"r74": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "942",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-04(15))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478524/942-220-S99-1"
},
"r75": {
"role": "http://fasb.org/us-gaap/role/ref/legacyRef",
"Topic": "942",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-04(22))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478524/942-220-S99-1"
},
"r76": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r77": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r78": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r79": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r80": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-6"
},
"r81": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-6"
},
"r82": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-6"
},
"r83": {
"role": "http://fasb.org/us-gaap/role/ref/otherTransitionRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-7"
},
"r84": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "13",
"Subparagraph": "(c)",
"SubTopic": "10",
"Topic": "230",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-13"
},
"r85": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "2",
"Subparagraph": "(a)",
"SubTopic": "20",
"Topic": "740",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482659/740-20-45-2"
},
"r86": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)",
"SubTopic": "10",
"Topic": "275",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-1"
},
"r87": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)",
"SubTopic": "10",
"Topic": "275",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-1"
},
"r88": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"SubTopic": "10",
"Topic": "275",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-1"
},
"r89": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(h))",
"SubTopic": "10",
"Topic": "235",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r90": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Accounting Standards Codification",
"Topic": "808",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/808/tableOfContent"
},
"r91": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "12",
"Paragraph": "Column A",
"Footnote": "2",
"Publisher": "SEC"
},
"r92": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "12A",
"Paragraph": "Column A",
"Footnote": "2",
"Publisher": "SEC"
},
"r93": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "12B",
"Paragraph": "Column A",
"Subparagraph": "(a)",
"Footnote": "4",
"Publisher": "SEC"
},
"r94": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "12B",
"Paragraph": "Column A",
"Subparagraph": "(b)",
"Footnote": "4",
"Publisher": "SEC"
},
"r95": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "14",
"Paragraph": "Column A",
"Footnote": "2",
"Publisher": "SEC"
},
"r96": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "17",
"Paragraph": "Column A",
"Publisher": "SEC"
},
"r97": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "17",
"Paragraph": "Column B",
"Publisher": "SEC"
},
"r98": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "17",
"Paragraph": "Column C",
"Publisher": "SEC"
},
"r99": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "17",
"Paragraph": "Column D",
"Publisher": "SEC"
},
"r100": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "17",
"Paragraph": "Column E",
"Publisher": "SEC"
},
"r101": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "17",
"Paragraph": "Column F",
"Publisher": "SEC"
},
"r102": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column A",
"Footnote": "2",
"Publisher": "SEC"
},
"r103": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column E",
"Footnote": "5",
"Publisher": "SEC"
},
"r104": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "29",
"Paragraph": "Column A",
"Footnote": "3",
"Publisher": "SEC"
},
"r105": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "29",
"Paragraph": "Column B",
"Publisher": "SEC"
},
"r106": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "29",
"Paragraph": "Column C",
"Publisher": "SEC"
},
"r107": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "4",
"Subsection": "08",
"Paragraph": "m",
"Subparagraph": "(1)(iii)",
"Publisher": "SEC"
},
"r108": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "4",
"Subsection": "08",
"Paragraph": "m",
"Subparagraph": "(2)(ii)",
"Publisher": "SEC"
},
"r109": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Staff Accounting Bulletin (SAB)",
"Number": "Topic 11",
"Section": "L",
"Publisher": "SEC"
},
"r110": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Staff Accounting Bulletin (SAB)",
"Number": "Topic 5",
"Section": "Y",
"Paragraph": "Question 2",
"Publisher": "SEC"
},
"r111": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Name": "Staff Accounting Bulletin (SAB)",
"Number": "Topic 5",
"Section": "Y",
"Paragraph": "Question 4",
"Publisher": "SEC"
},
"r112": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "105",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "6",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479343/105-10-65-6"
},
"r113": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "105",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "9",
"Subparagraph": "(d)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479343/105-10-65-9"
},
"r114": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "105",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "9",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479343/105-10-65-9"
},
"r115": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "205",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483499/205-20-50-1"
},
"r116": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "205",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483499/205-20-50-7"
},
"r117": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483467/210-10-45-1"
},
"r118": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483467/210-10-45-5"
},
"r119": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r120": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(12))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r121": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(13))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r122": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(14))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r123": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(17))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r124": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(18))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r125": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(27)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r126": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(28))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r127": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(29))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r128": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(30)(a)(4))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r129": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(9))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r130": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "10A",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-10A"
},
"r131": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "10A",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-10A"
},
"r132": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-11"
},
"r133": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1A",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-1A"
},
"r134": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1A",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-1A"
},
"r135": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1A",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-1A"
},
"r136": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1B",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-1B"
},
"r137": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1B",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482790/220-10-45-1B"
},
"r138": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482765/220-10-50-4"
},
"r139": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482765/220-10-50-5"
},
"r140": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482765/220-10-50-6"
},
"r141": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.5-03(24))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483621/220-10-S99-2"
},
"r142": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.5-03(25))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483621/220-10-S99-2"
},
"r143": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-6"
},
"r144": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-11"
},
"r145": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "12",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-12"
},
"r146": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "15",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-15"
},
"r147": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "17",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-17"
},
"r148": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "24",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-24"
},
"r149": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "25",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-25"
},
"r150": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482913/230-10-50-2"
},
"r151": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "8",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482913/230-10-50-8"
},
"r152": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/235/tableOfContent"
},
"r153": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483426/235-10-50-1"
},
"r154": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480738/235-10-S50-1"
},
"r155": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(g)(1)(i))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r156": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(g)(1)(ii))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r157": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(k)(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r158": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(m)(1)(iii))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r159": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(m)(2)(ii))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r160": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "23",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483421/250-10-45-23"
},
"r161": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "24",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483421/250-10-45-24"
},
"r162": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "5",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483421/250-10-45-5"
},
"r163": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-1"
},
"r164": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-1"
},
"r165": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-1"
},
"r166": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-1"
},
"r167": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "11",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-11"
},
"r168": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "11",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-11"
},
"r169": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-3"
},
"r170": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-4"
},
"r171": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-6"
},
"r172": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-7"
},
"r173": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-7"
},
"r174": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "8",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-8"
},
"r175": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-9"
},
"r176": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482689/260-10-45-10"
},
"r177": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "16",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482689/260-10-45-16"
},
"r178": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482689/260-10-45-2"
},
"r179": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "60B",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482689/260-10-45-60B"
},
"r180": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "60B",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482689/260-10-45-60B"
},
"r181": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "7",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482689/260-10-45-7"
},
"r182": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482662/260-10-50-1"
},
"r183": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482662/260-10-50-1"
},
"r184": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482662/260-10-50-1"
},
"r185": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "15",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482635/260-10-55-15"
},
"r186": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "270",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482964/270-10-50-1"
},
"r187": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "272",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483014/272-10-45-1"
},
"r188": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "272",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482987/272-10-50-1"
},
"r189": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "12",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-12"
},
"r190": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-2"
},
"r191": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-4"
},
"r192": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-6"
},
"r193": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482861/275-10-50-9"
},
"r194": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/280/tableOfContent"
},
"r195": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "15",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-15"
},
"r196": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-21"
},
"r197": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-21"
},
"r198": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r199": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r200": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r201": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r202": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r203": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r204": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r205": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r206": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(h)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r207": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(j)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r208": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "25",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-25"
},
"r209": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "25",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-25"
},
"r210": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "26",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-26"
},
"r211": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "26A",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-26A"
},
"r212": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "26B",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-26B"
},
"r213": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "26C",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-26C"
},
"r214": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "30",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-30"
},
"r215": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "30",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-30"
},
"r216": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "30",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-30"
},
"r217": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "30",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-30"
},
"r218": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "31",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-31"
},
"r219": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r220": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(ee)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r221": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r222": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "34",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-34"
},
"r223": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "40",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-40"
},
"r224": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "41",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-41"
},
"r225": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "41",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-41"
},
"r226": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "42",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-42"
},
"r227": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "310",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "13",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481990/310-10-45-13"
},
"r228": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481830/320-10-45-11"
},
"r229": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481830/320-10-45-2"
},
"r230": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-2"
},
"r231": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-2"
},
"r232": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(aa)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-2"
},
"r233": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(aaa)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-2"
},
"r234": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-2"
},
"r235": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-2"
},
"r236": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-2"
},
"r237": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-3"
},
"r238": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-3"
},
"r239": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-3"
},
"r240": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-3"
},
"r241": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-3"
},
"r242": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r243": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r244": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(aaa)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r245": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r246": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r247": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r248": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(f)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r249": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(f)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r250": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(f)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r251": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(f)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5"
},
"r252": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5A",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5A"
},
"r253": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5A",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5A"
},
"r254": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5A",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5A"
},
"r255": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5B",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5B"
},
"r256": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5B",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5B"
},
"r257": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5B",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5B"
},
"r258": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5B",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5B"
},
"r259": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5B",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-5B"
},
"r260": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "323",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481687/323-10-50-3"
},
"r261": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "4",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479654/326-10-65-4"
},
"r262": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "5",
"Subparagraph": "(c)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479654/326-10-65-5"
},
"r263": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479319/326-20-50-11"
},
"r264": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479319/326-20-50-13"
},
"r265": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "14",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479319/326-20-50-14"
},
"r266": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "16",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479319/326-20-50-16"
},
"r267": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479319/326-20-50-5"
},
"r268": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479130/326-30-45-1"
},
"r269": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-4"
},
"r270": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-4"
},
"r271": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-4"
},
"r272": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-5"
},
"r273": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-7"
},
"r274": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-9"
},
"r275": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-9"
},
"r276": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-9"
},
"r277": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r278": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r279": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r280": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r281": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r282": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r283": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r284": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r285": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(h)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-1"
},
"r286": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482573/350-20-50-4"
},
"r287": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482665/350-30-50-1"
},
"r288": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482665/350-30-50-1"
},
"r289": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482665/350-30-50-1"
},
"r290": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482665/350-30-50-1"
},
"r291": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482665/350-30-50-1"
},
"r292": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482665/350-30-50-3"
},
"r293": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "350",
"SubTopic": "60",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476166/350-60-65-1"
},
"r294": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "360",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482099/360-10-50-3"
},
"r295": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "360",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482099/360-10-50-3"
},
"r296": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "405",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(e)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477092/405-40-50-1"
},
"r297": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "420",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482017/420-10-50-1"
},
"r298": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "420",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482017/420-10-50-1"
},
"r299": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "420",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SAB Topic 5.P.4.d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479823/420-10-S99-2"
},
"r300": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "440",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482648/440-10-50-4"
},
"r301": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "440",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482648/440-10-50-4"
},
"r302": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "450",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483076/450-20-50-4"
},
"r303": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "450",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483076/450-20-50-9"
},
"r304": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "450",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SAB Topic 5.Y.Q2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480102/450-20-S99-1"
},
"r305": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "450",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SAB Topic 5.Y.Q4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480102/450-20-S99-1"
},
"r306": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1A",
"Subparagraph": "(SX 210.13-01(a)(4)(i))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1A"
},
"r307": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1A",
"Subparagraph": "(SX 210.13-01(a)(4)(iii)(A))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1A"
},
"r308": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1A",
"Subparagraph": "(SX 210.13-01(a)(4)(iv))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1A"
},
"r309": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1A",
"Subparagraph": "(SX 210.13-01(a)(5))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1A"
},
"r310": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1B",
"Subparagraph": "(SX 210.13-02(a)(4)(i))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1B"
},
"r311": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1B",
"Subparagraph": "(SX 210.13-02(a)(4)(iii)(A))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1B"
},
"r312": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1B",
"Subparagraph": "(SX 210.13-02(a)(4)(iii)(B))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1B"
},
"r313": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1B",
"Subparagraph": "(SX 210.13-02(a)(4)(iv))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1B"
},
"r314": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1B",
"Subparagraph": "(SX 210.13-02(a)(5))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1B"
},
"r315": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1B",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481139/470-20-50-1B"
},
"r316": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1D",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481139/470-20-50-1D"
},
"r317": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1I",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481139/470-20-50-1I"
},
"r318": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "4",
"Subparagraph": "(f)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481538/470-20-65-4"
},
"r319": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "4",
"Subparagraph": "(f)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481538/470-20-65-4"
},
"r320": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S45",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479887/480-10-S45-1"
},
"r321": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S45",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479887/480-10-S45-2"
},
"r322": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S45",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479887/480-10-S45-3"
},
"r323": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479857/480-10-S50-1"
},
"r324": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479857/480-10-S50-3"
},
"r325": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480244/480-10-S99-1"
},
"r326": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(01)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480244/480-10-S99-1"
},
"r327": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(01)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480244/480-10-S99-1"
},
"r328": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3A",
"Subparagraph": "(24)(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480244/480-10-S99-3A"
},
"r329": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r330": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r331": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r332": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r333": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r334": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Subparagraph": "(h)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r335": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r336": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "14",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-14"
},
"r337": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "14",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-14"
},
"r338": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "14",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-14"
},
"r339": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "16",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-16"
},
"r340": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "18",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-18"
},
"r341": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "18",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-18"
},
"r342": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "18",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-18"
},
"r343": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "18",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-18"
},
"r344": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-2"
},
"r345": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.3-04)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480008/505-10-S99-1"
},
"r346": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479806/606-10-50-4"
},
"r347": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479806/606-10-50-5"
},
"r348": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479806/606-10-50-7"
},
"r349": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479806/606-10-50-9"
},
"r350": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r351": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(iv)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r352": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(iv)(02)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r353": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(iv)(02)(A)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r354": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(iv)(02)(B)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r355": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(iv)(02)(C)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r356": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(iv)(03)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r357": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(c)(iv)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-5"
},
"r358": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(c)(iv)(02)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-5"
},
"r359": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(c)(iv)(03)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-5"
},
"r360": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "715",
"SubTopic": "80",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480576/715-80-50-5"
},
"r361": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/718/tableOfContent"
},
"r362": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "35",
"Paragraph": "1D",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480483/718-10-35-1D"
},
"r363": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "35",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480483/718-10-35-3"
},
"r364": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r365": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r366": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r367": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r368": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r369": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r370": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r371": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r372": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r373": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(02)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r374": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(03)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r375": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(04)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r376": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r377": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r378": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r379": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r380": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)(02)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r381": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)(03)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r382": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r383": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r384": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(e)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r385": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(e)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r386": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r387": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r388": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r389": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r390": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(iv)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r391": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(v)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r392": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(h)(1)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r393": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(h)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r394": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(h)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r395": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r396": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(l)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r397": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "17",
"Subparagraph": "(d)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480336/718-10-65-17"
},
"r398": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S45",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479983/718-10-S45-1"
},
"r399": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SAB Topic 14.F)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479830/718-10-S99-1"
},
"r400": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "720",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483359/720-20-50-1"
},
"r401": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "730",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482916/730-10-50-1"
},
"r402": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482685/740-10-50-10"
},
"r403": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "12",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482685/740-10-50-12"
},
"r404": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "8",
"Subparagraph": "(d)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482615/740-10-65-8"
},
"r405": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "8",
"Subparagraph": "(d)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482615/740-10-65-8"
},
"r406": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SAB Topic 6.I.7)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479360/740-10-S99-1"
},
"r407": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "323",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(d)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478666/740-323-65-2"
},
"r408": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "323",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(d)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478666/740-323-65-2"
},
"r409": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "323",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478666/740-323-65-2"
},
"r410": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "323",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(g)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478666/740-323-65-2"
},
"r411": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "740",
"SubTopic": "323",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(g)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478666/740-323-65-2"
},
"r412": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "805",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479581/805-30-50-1"
},
"r413": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "805",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479581/805-30-50-2"
},
"r414": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "805",
"SubTopic": "60",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "1",
"Subparagraph": "(d)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476176/805-60-65-1"
},
"r415": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "805",
"SubTopic": "60",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "1",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476176/805-60-65-1"
},
"r416": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "808",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479402/808-10-50-1"
},
"r417": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "808",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479402/808-10-50-1"
},
"r418": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "808",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479402/808-10-50-1"
},
"r419": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "810",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "25",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481231/810-10-45-25"
},
"r420": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "810",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "25",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481231/810-10-45-25"
},
"r421": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "810",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(bb)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481203/810-10-50-3"
},
"r422": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "810",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481203/810-10-50-3"
},
"r423": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4A",
"Subparagraph": "(b)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-4A"
},
"r424": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4C",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-4C"
},
"r425": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4D",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-4D"
},
"r426": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4F",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-4F"
},
"r427": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "8A",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-8A"
},
"r428": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "6",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480528/815-20-65-6"
},
"r429": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "6",
"Subparagraph": "(h)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480528/815-20-65-6"
},
"r430": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "6",
"Subparagraph": "(h)(1)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480528/815-20-65-6"
},
"r431": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "6",
"Subparagraph": "(h)(1)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480528/815-20-65-6"
},
"r432": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "6",
"Subparagraph": "(h)(1)(iv)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480528/815-20-65-6"
},
"r433": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "6",
"Subparagraph": "(i)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480528/815-20-65-6"
},
"r434": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480870/815-30-50-2"
},
"r435": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480870/815-30-50-2"
},
"r436": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480870/815-30-50-2"
},
"r437": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480870/815-30-50-2"
},
"r438": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480237/815-40-50-5"
},
"r439": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480237/815-40-50-6"
},
"r440": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "1",
"Subparagraph": "(e)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480175/815-40-65-1"
},
"r441": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "1",
"Subparagraph": "(e)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480175/815-40-65-1"
},
"r442": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "815",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "1",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480175/815-40-65-1"
},
"r443": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "35",
"Paragraph": "54B",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482134/820-10-35-54B"
},
"r444": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r445": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r446": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(bbb)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r447": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(bbb)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r448": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(bbb)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r449": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r450": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r451": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r452": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r453": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(h)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r454": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2E",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2E"
},
"r455": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-3"
},
"r456": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-3"
},
"r457": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6A",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-6A"
},
"r458": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6A",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-6A"
},
"r459": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6A",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-6A"
},
"r460": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6A",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-6A"
},
"r461": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6A",
"Subparagraph": "(h)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-6A"
},
"r462": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6B",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-6B"
},
"r463": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1A",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482736/825-10-45-1A"
},
"r464": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-10"
},
"r465": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "11",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-11"
},
"r466": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "11",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-11"
},
"r467": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "28",
"Subparagraph": "(c)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-28"
},
"r468": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "28",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-28"
},
"r469": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "30",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-30"
},
"r470": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-32"
},
"r471": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "825",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482804/825-20-50-1"
},
"r472": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "830",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "17",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481694/830-30-45-17"
},
"r473": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "830",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "20",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481694/830-30-45-20"
},
"r474": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "830",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "20",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481694/830-30-45-20"
},
"r475": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "830",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "20",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481694/830-30-45-20"
},
"r476": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "830",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "20",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481694/830-30-45-20"
},
"r477": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "830",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481674/830-30-50-1"
},
"r478": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "8",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479832/842-10-65-8"
},
"r479": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "35",
"Paragraph": "12A",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479165/842-20-35-12A"
},
"r480": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479041/842-20-45-1"
},
"r481": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479041/842-20-45-1"
},
"r482": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "5",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479041/842-20-45-5"
},
"r483": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-3"
},
"r484": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-4"
},
"r485": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(g)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-4"
},
"r486": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(g)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-4"
},
"r487": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(g)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-4"
},
"r488": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-6"
},
"r489": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-7A"
},
"r490": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-7A"
},
"r491": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "848",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(a)(3)(iii)(03)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483550/848-10-65-2"
},
"r492": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "850",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483326/850-10-50-3"
},
"r493": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "855",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/855/tableOfContent"
},
"r494": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "855",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483399/855-10-50-2"
},
"r495": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(b)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r496": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(b)(2)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r497": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(b)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r498": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(bb)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r499": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(bb)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r500": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(bb)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r501": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r502": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r503": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r504": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-4"
},
"r505": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-4"
},
"r506": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-4"
},
"r507": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-4"
},
"r508": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481444/860-30-45-1"
},
"r509": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481420/860-30-50-7"
},
"r510": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481420/860-30-50-7"
},
"r511": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481420/860-30-50-9"
},
"r512": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-3"
},
"r513": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-3"
},
"r514": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-3"
},
"r515": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)(4)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-3"
},
"r516": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r517": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r518": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r519": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r520": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(5)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r521": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(6)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r522": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)(7)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r523": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r524": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(e)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r525": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(e)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r526": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "860",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(e)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481229/860-50-50-4"
},
"r527": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "910",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482546/910-10-50-6"
},
"r528": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "924",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SAB Topic 11.L)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479941/924-10-S99-1"
},
"r529": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "940",
"SubTopic": "820",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478119/940-820-50-1"
},
"r530": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-03(6))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478546/942-210-S99-1"
},
"r531": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-04(26))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478524/942-220-S99-1"
},
"r532": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-04(27))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478524/942-220-S99-1"
},
"r533": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478671/942-235-S50-1"
},
"r534": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-05(b)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477314/942-235-S99-1"
},
"r535": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.9-05(b)(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477314/942-235-S99-1"
},
"r536": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478451/942-360-50-1"
},
"r537": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "942",
"SubTopic": "825",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478898/942-825-50-1"
},
"r538": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(1)(6))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r539": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r540": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(12))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r541": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(21))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r542": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(22))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r543": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(23)(a)(3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r544": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(23)(a)(4))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r545": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(25))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r546": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(8)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r547": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-03(a)(8))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478777/944-210-S99-1"
},
"r548": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(11))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r549": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(18))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r550": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(19))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r551": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(2)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r552": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(20))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r553": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(22))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r554": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(23))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r555": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.7-04(9))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477250/944-220-S99-1"
},
"r556": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column A))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r557": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column B))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r558": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column C))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r559": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column D))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r560": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column E))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r561": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column F))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r562": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column G))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r563": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column H))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r564": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column I))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r565": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column J))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r566": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-16(Column K))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-1"
},
"r567": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-17(Column A))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-2"
},
"r568": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-17(Column B))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-2"
},
"r569": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-17(Column C))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-2"
},
"r570": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-17(Column D))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-2"
},
"r571": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-17(Column E))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-2"
},
"r572": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-17(Column F))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477965/944-235-S99-2"
},
"r573": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4E",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-4E"
},
"r574": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-5"
},
"r575": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-5"
},
"r576": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7A"
},
"r577": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Subparagraph": "(a)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-9"
},
"r578": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480016/944-40-65-2"
},
"r579": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(f)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480016/944-40-65-2"
},
"r580": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(f)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480016/944-40-65-2"
},
"r581": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(g)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480016/944-40-65-2"
},
"r582": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(g)(2)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480016/944-40-65-2"
},
"r583": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "2",
"Subparagraph": "(h)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480016/944-40-65-2"
},
"r584": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SAB Topic 5.W.Q2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479583/944-40-S99-1"
},
"r585": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "944",
"SubTopic": "805",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478072/944-805-50-1"
},
"r586": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-03(d))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479886/946-10-S99-3"
},
"r587": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-03(i)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479886/946-10-S99-3"
},
"r588": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-03(i)(2)(i))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479886/946-10-S99-3"
},
"r589": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-03(i)(2)(ii))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479886/946-10-S99-3"
},
"r590": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-03(i)(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479886/946-10-S99-3"
},
"r591": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480990/946-20-50-11"
},
"r592": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "205",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "4",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478009/946-205-45-4"
},
"r593": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "205",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478009/946-205-45-6"
},
"r594": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477796/946-210-45-4"
},
"r595": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478795/946-210-50-6"
},
"r596": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478795/946-210-50-6"
},
"r597": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r598": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(12)(b)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r599": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(12)(b)(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r600": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(12)(b)(3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r601": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(13)(a)(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r602": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(13)(a)(3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r603": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(16)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r604": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(17))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r605": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(19))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r606": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(2)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r607": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(2)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r608": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(3)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r609": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(3)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r610": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(3)(c))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r611": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(6)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r612": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(6)(c))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r613": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(6)(d))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r614": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(6)(e))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r615": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(8))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r616": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(9)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r617": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(9)(c))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r618": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(9)(d))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r619": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(9)(e))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r620": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.6-05(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-2"
},
"r621": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.6-05(4))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-2"
},
"r622": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "7",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479105/946-220-45-7"
},
"r623": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r624": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(2)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r625": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(2)(g)(3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r626": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(a)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r627": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(a)(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r628": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(a)(3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r629": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(a)(5))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r630": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(a)(6))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r631": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(a)(7))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r632": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(c)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r633": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(c)(2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r634": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(c)(3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r635": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(c)(5))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r636": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(c)(6))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r637": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(7)(c)(7))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r638": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-07(9))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-1"
},
"r639": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-09(1)(d))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-3"
},
"r640": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-09(4)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-3"
},
"r641": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-09(6))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-3"
},
"r642": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-09(7))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-3"
},
"r643": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477968/946-235-50-2"
},
"r644": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477968/946-235-50-2"
},
"r645": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-12(Column A)(Footnote 2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-1"
},
"r646": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-12A(Column A)(Footnote 2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-2"
},
"r647": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.12-12B(Column A)(Footnote 4)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-3"
},
"r648": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.12-12B(Column A)(Footnote 4)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-3"
},
"r649": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "6",
"Subparagraph": "(SX 210.12-14(Column A)(Footnote 2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-6"
},
"r650": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "946",
"SubTopic": "505",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478448/946-505-50-6"
},
"r651": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "948",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478322/948-310-S50-2"
},
"r652": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "948",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-29(Column A)(Footnote 3))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479628/948-310-S99-1"
},
"r653": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "948",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-29(Column B))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479628/948-310-S99-1"
},
"r654": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "948",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-29(Column C))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479628/948-310-S99-1"
},
"r655": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "954",
"SubTopic": "440",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478522/954-440-50-1"
},
"r656": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column A)(Footnote 2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r657": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column E)(Footnote 5))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r658": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "976",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477332/976-310-50-1"
},
"r659": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "978",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479230/978-310-50-1"
},
"r660": {
"role": "http://www.xbrl.org/2003/role/disclosureRef",
"Topic": "985",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481283/985-20-50-2"
},
"r661": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "10",
"SubTopic": "10",
"Topic": "825",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482881/825-10-55-10"
},
"r662": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "13H",
"Subparagraph": "(a)",
"SubTopic": "40",
"Topic": "944",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480046/944-40-55-13H"
},
"r663": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483467/210-10-45-1"
},
"r664": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "16",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483444/210-20-55-16"
},
"r665": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "21",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483444/210-20-55-21"
},
"r666": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "22",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483444/210-20-55-22"
},
"r667": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "12",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-12"
},
"r668": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476153/220-40-55-11"
},
"r669": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "14",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476153/220-40-55-14"
},
"r670": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "18",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476153/220-40-55-18"
},
"r671": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "21",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476153/220-40-55-21"
},
"r672": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476153/220-40-55-4"
},
"r673": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483426/235-10-50-4"
},
"r674": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480738/235-10-S50-1"
},
"r675": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(m)(1)(ii)(A))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r676": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "52",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482635/260-10-55-52"
},
"r677": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482836/275-10-55-2"
},
"r678": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482836/275-10-55-4"
},
"r679": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "275",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482836/275-10-55-6"
},
"r680": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "31",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-31"
},
"r681": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "47",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-47"
},
"r682": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "47",
"Subparagraph": "(bb)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-47"
},
"r683": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "47",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-47"
},
"r684": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "47",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-47"
},
"r685": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "48",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-48"
},
"r686": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "49",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-49"
},
"r687": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "54",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-54"
},
"r688": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "54",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-54"
},
"r689": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "54",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482785/280-10-55-54"
},
"r690": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "310",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "12A",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481933/310-10-55-12A"
},
"r691": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "8",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479081/326-30-55-8"
},
"r692": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "350",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "24",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482548/350-20-55-24"
},
"r693": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "69B",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481568/470-20-55-69B"
},
"r694": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "69C",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481568/470-20-55-69C"
},
"r695": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "480",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "64",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481620/480-10-55-64"
},
"r696": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "13",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-13"
},
"r697": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479806/606-10-50-5"
},
"r698": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r699": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r700": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r701": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r702": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r703": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r704": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r705": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r706": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(iv)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r707": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(c)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-5"
},
"r708": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(c)(iv)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-5"
},
"r709": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "17",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480482/715-20-55-17"
},
"r710": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "715",
"SubTopic": "80",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "8",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480547/715-80-55-8"
},
"r711": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r712": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r713": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "231",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482663/740-10-55-231"
},
"r714": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "805",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "8",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479328/805-10-50-8"
},
"r715": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "805",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "41",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479303/805-10-55-41"
},
"r716": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "805",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "43",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479303/805-10-55-43"
},
"r717": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "805",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "47",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479303/805-10-55-47"
},
"r718": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "805",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479908/805-50-55-1"
},
"r719": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4A",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-4A"
},
"r720": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "182",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480401/815-10-55-182"
},
"r721": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "184",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480401/815-10-55-184"
},
"r722": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "100",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-100"
},
"r723": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "101",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-101"
},
"r724": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "102",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-102"
},
"r725": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "103",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-103"
},
"r726": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "107",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-107"
},
"r727": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "107",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-107"
},
"r728": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "107",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-107"
},
"r729": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "107",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-107"
},
"r730": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "107",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-107"
},
"r731": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "107",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482078/820-10-55-107"
},
"r732": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "12",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482881/825-10-55-12"
},
"r733": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "53",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479589/842-20-55-53"
},
"r734": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "852",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481372/852-10-55-10"
},
"r735": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "860",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481395/860-30-55-4"
},
"r736": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479401/944-30-55-2"
},
"r737": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "29F",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480046/944-40-55-29F"
},
"r738": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "9C",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480046/944-40-55-9C"
},
"r739": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "9C",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480046/944-40-55-9C"
},
"r740": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "9E",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480046/944-40-55-9E"
},
"r741": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "605",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477548/944-605-55-11"
},
"r742": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "605",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "14",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477548/944-605-55-14"
},
"r743": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "944",
"SubTopic": "80",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "18",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480078/944-80-55-18"
},
"r744": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(b)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478795/946-210-50-1"
},
"r745": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478795/946-210-50-6"
},
"r746": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477439/946-210-55-1"
},
"r747": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477802/946-310-45-1"
},
"r748": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-12(Column A)(Footnote 2)(i))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-1"
},
"r749": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Subparagraph": "(SX 210.12-12A(Column A)(Footnote 2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-2"
},
"r750": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.12-12B(Column A)(Footnote 1)(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-3"
},
"r751": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "6",
"Subparagraph": "(SX 210.12-14(Column A)(Footnote 2))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477271/946-320-S99-6"
},
"r752": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "830",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479168/946-830-55-10"
},
"r753": {
"role": "http://www.xbrl.org/2003/role/exampleRef",
"Topic": "946",
"SubTopic": "830",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "12",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479168/946-830-55-12"
},
"r754": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b"
},
"r755": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b-2"
},
"r756": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "d1-1"
},
"r757": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 10-Q",
"Number": "240",
"Section": "308",
"Subsection": "a"
},
"r758": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "16",
"Subsection": "J",
"Paragraph": "a"
},
"r759": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1"
},
"r760": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "i"
},
"r761": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "A"
},
"r762": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "B"
},
"r763": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "C"
},
"r764": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "D"
},
"r765": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "E"
},
"r766": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "ii"
},
"r767": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "1",
"Subparagraph": "iii"
},
"r768": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Section": "6",
"Subsection": "F",
"Paragraph": "2"
},
"r769": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a"
},
"r770": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "1"
},
"r771": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "i"
},
"r772": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "ii"
},
"r773": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "iii"
},
"r774": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "iv"
},
"r775": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "v"
},
"r776": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "2"
},
"r777": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "a",
"Subparagraph": "3"
},
"r778": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Section": "19",
"Paragraph": "b"
},
"r779": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a"
},
"r780": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "1"
},
"r781": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "i"
},
"r782": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "ii"
},
"r783": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "iii"
},
"r784": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "iv"
},
"r785": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "1",
"Sentence": "v"
},
"r786": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "2"
},
"r787": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "a",
"Subparagraph": "3"
},
"r788": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form N-CSR",
"Section": "18",
"Paragraph": "b"
},
"r789": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Forms 10-K, 10-Q, 20-F",
"Number": "240",
"Section": "13",
"Subsection": "a-1"
},
"r790": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v"
},
"r791": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "1"
},
"r792": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "ii"
},
"r793": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii"
},
"r794": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "B",
"Clause": "1",
"Subclause": "ii"
},
"r795": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "C",
"Clause": "1",
"Subclause": "i"
},
"r796": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "C",
"Clause": "1",
"Subclause": "ii"
},
"r797": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "C",
"Clause": "1",
"Subclause": "iii"
},
"r798": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "C",
"Clause": "1",
"Subclause": "iv"
},
"r799": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "C",
"Clause": "1",
"Subclause": "v"
},
"r800": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "C",
"Clause": "1",
"Subclause": "vi"
},
"r801": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iv"
},
"r802": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "vi"
},
"r803": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "3"
},
"r804": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "4"
},
"r805": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "5",
"Subparagraph": "i"
},
"r806": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "5",
"Subparagraph": "ii"
},
"r807": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "5",
"Subparagraph": "iii"
},
"r808": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "5",
"Subparagraph": "iv"
},
"r809": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "6"
},
"r810": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "v",
"Paragraph": "6",
"Subparagraph": "i"
},
"r811": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1"
},
"r812": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "i"
},
"r813": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "A"
},
"r814": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "B"
},
"r815": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "C"
},
"r816": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "D"
},
"r817": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "i",
"Sentence": "E"
},
"r818": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "ii"
},
"r819": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "1",
"Subparagraph": "iii"
},
"r820": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "w",
"Paragraph": "2"
},
"r821": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "x",
"Paragraph": "1"
},
"r822": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "x",
"Paragraph": "2"
},
"r823": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "x",
"Paragraph": "2",
"Subparagraph": "ii",
"Sentence": "A"
},
"r824": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "x",
"Paragraph": "2",
"Subparagraph": "ii",
"Sentence": "C"
},
"r825": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "x",
"Paragraph": "2",
"Subparagraph": "ii",
"Sentence": "D"
},
"r826": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "x",
"Paragraph": "2",
"Subparagraph": "ii",
"Sentence": "E"
},
"r827": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "402",
"Subsection": "x",
"Paragraph": "2",
"Subparagraph": "ii",
"Sentence": "F"
},
"r828": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "408",
"Subsection": "a"
},
"r829": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "408",
"Subsection": "a",
"Paragraph": "1"
},
"r830": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "408",
"Subsection": "a",
"Paragraph": "2",
"Subparagraph": "A"
},
"r831": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "408",
"Subsection": "a",
"Paragraph": "2",
"Subparagraph": "B"
},
"r832": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "408",
"Subsection": "a",
"Paragraph": "2",
"Subparagraph": "C"
},
"r833": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "408",
"Subsection": "a",
"Paragraph": "2",
"Subparagraph": "D"
},
"r834": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Number": "229",
"Section": "408",
"Subsection": "b",
"Paragraph": "1"
},
"r835": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Section": "402",
"Number": "229",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "C",
"Clause": "1"
},
"r836": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "A",
"Number": "229"
},
"r837": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "B",
"Clause": "1",
"Number": "229"
},
"r838": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-K",
"Section": "402",
"Subsection": "v",
"Paragraph": "2",
"Subparagraph": "iii",
"Sentence": "B",
"Clause": "1",
"Subclause": "i",
"Number": "229"
},
"r839": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-T",
"Number": "232",
"Section": "405"
},
"r840": {
"role": "http://www.xbrl.org/2003/role/recommendedDisclosureRef",
"Topic": "272",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483014/272-10-45-3"
},
"r841": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(a)",
"SubTopic": "40",
"Topic": "220",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r842": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(g)(1)",
"SubTopic": "20",
"Topic": "842",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-4"
},
"r843": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-K (SK)",
"Number": "229",
"Section": "1402",
"Paragraph": "a",
"Publisher": "SEC"
},
"r844": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-K (SK)",
"Number": "229",
"Section": "1402",
"Paragraph": "b",
"Subparagraph": "(1)",
"Publisher": "SEC"
},
"r845": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-K (SK)",
"Number": "229",
"Section": "1402",
"Paragraph": "b",
"Subparagraph": "(2)",
"Publisher": "SEC"
},
"r846": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-K (SK)",
"Number": "229",
"Section": "1402",
"Paragraph": "b",
"Subparagraph": "(3)",
"Publisher": "SEC"
},
"r847": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-K (SK)",
"Number": "229",
"Section": "1402",
"Paragraph": "c",
"Subparagraph": "(2)(i)",
"Publisher": "SEC"
},
"r848": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-K (SK)",
"Number": "229",
"Section": "1402",
"Paragraph": "c",
"Subparagraph": "(2)(ii)",
"Publisher": "SEC"
},
"r849": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-K (SK)",
"Number": "229",
"Section": "1402",
"Paragraph": "c",
"Subparagraph": "(2)(iii)",
"Publisher": "SEC"
},
"r850": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column B",
"Publisher": "SEC"
},
"r851": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column C",
"Publisher": "SEC"
},
"r852": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column D",
"Publisher": "SEC"
},
"r853": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column E",
"Publisher": "SEC"
},
"r854": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column F",
"Publisher": "SEC"
},
"r855": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column G",
"Publisher": "SEC"
},
"r856": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column H",
"Publisher": "SEC"
},
"r857": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "12",
"Subsection": "28",
"Paragraph": "Column I",
"Publisher": "SEC"
},
"r858": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Regulation S-X (SX)",
"Number": "210",
"Section": "6",
"Subsection": "04",
"Paragraph": "12",
"Subparagraph": "(b)(1)",
"Publisher": "SEC"
},
"r859": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Name": "Staff Accounting Bulletin (SAB)",
"Number": "Topic 5",
"Section": "Y",
"Paragraph": "Question 2",
"Publisher": "SEC"
},
"r860": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/210/tableOfContent"
},
"r861": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(17))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r862": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.5-02(9))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480566/210-10-S99-1"
},
"r863": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483466/210-20-50-3"
},
"r864": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483466/210-20-50-3"
},
"r865": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483466/210-20-50-3"
},
"r866": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(d)(1)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483466/210-20-50-3"
},
"r867": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(d)(1)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483466/210-20-50-3"
},
"r868": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(d)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483466/210-20-50-3"
},
"r869": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483466/210-20-50-3"
},
"r870": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483444/210-20-55-10"
},
"r871": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "210",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "12",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483444/210-20-55-12"
},
"r872": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482765/220-10-50-4"
},
"r873": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482765/220-10-50-5"
},
"r874": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482765/220-10-50-6"
},
"r875": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r876": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r877": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r878": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r879": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r880": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r881": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r882": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(h)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r883": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r884": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(j)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r885": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(k)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r886": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(l)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r887": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "21",
"Subparagraph": "(m)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-21"
},
"r888": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r889": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r890": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r891": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r892": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r893": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(g)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r894": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(h)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r895": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r896": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(j)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r897": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(k)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r898": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(l)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r899": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(m)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r900": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(n)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r901": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(o)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r902": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(p)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r903": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(q)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r904": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(r)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r905": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(s)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r906": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(t)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r907": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Subparagraph": "(u)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-22"
},
"r908": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "30",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-30"
},
"r909": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "31",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-31"
},
"r910": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-32"
},
"r911": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "33",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-33"
},
"r912": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-6"
},
"r913": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-6"
},
"r914": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-6"
},
"r915": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "220",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(e)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147476148/220-40-50-6"
},
"r916": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "28",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-28"
},
"r917": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "28",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482740/230-10-45-28"
},
"r918": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "230",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2A",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482913/230-10-50-2A"
},
"r919": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483426/235-10-50-4"
},
"r920": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480738/235-10-S50-1"
},
"r921": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480738/235-10-S50-4"
},
"r922": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(d))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r923": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(g)(1)(i))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r924": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.4-08(g)(1)(ii))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-1"
},
"r925": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "235",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.12-04(a))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480678/235-10-S99-3"
},
"r926": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "23",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483421/250-10-45-23"
},
"r927": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "24",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483421/250-10-45-24"
},
"r928": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "5",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483421/250-10-45-5"
},
"r929": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "250",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483443/250-10-50-6"
},
"r930": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "260",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482662/260-10-50-1"
},
"r931": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "270",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482964/270-10-50-1"
},
"r932": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "18",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-18"
},
"r933": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-22"
},
"r934": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "30",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-30"
},
"r935": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(ee)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r936": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "280",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "32",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482810/280-10-50-32"
},
"r937": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "310",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "13",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481990/310-10-45-13"
},
"r938": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "1",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481830/320-10-45-1"
},
"r939": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "11",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481830/320-10-45-11"
},
"r940": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "320",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481800/320-10-50-9"
},
"r941": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "321",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479536/321-10-50-3"
},
"r942": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "321",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479536/321-10-50-3"
},
"r943": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "321",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479536/321-10-50-3"
},
"r944": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "323",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481687/323-10-50-3"
},
"r945": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "326",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479106/326-30-50-4"
},
"r946": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "405",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/405-30/tableOfContent"
},
"r947": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "410",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "10",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481931/410-30-50-10"
},
"r948": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "450",
"Name": "Accounting Standards Codification",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/450/tableOfContent"
},
"r949": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "450",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "9",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483076/450-20-50-9"
},
"r950": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "450",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SAB Topic 5.Y.Q2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480102/450-20-S99-1"
},
"r951": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1A",
"Subparagraph": "(SX 210.13-01(a)(4)(ii))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1A"
},
"r952": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "470",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1A",
"Subparagraph": "(SX 210.13-01(a)(4)(iii))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480097/470-10-S99-1A"
},
"r953": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "470",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1B",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481139/470-20-50-1B"
},
"r954": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "505",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481112/505-10-50-2"
},
"r955": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "505",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481520/505-30-50-4"
},
"r956": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479806/606-10-50-10"
},
"r957": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479806/606-10-50-5"
},
"r958": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "606",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "55",
"Paragraph": "91",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479777/606-10-55-91"
},
"r959": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-1"
},
"r960": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "715",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(c)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480506/715-20-50-5"
},
"r961": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "35",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480483/718-10-35-2"
},
"r962": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r963": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r964": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r965": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r966": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r967": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r968": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r969": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r970": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r971": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(02)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r972": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(03)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r973": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(1)(iv)(04)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r974": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r975": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r976": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r977": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)(01)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r978": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)(02)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r979": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)(2)(iii)(03)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r980": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r981": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(d)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r982": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(e)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r983": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(e)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r984": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r985": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(ii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r986": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(iii)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r987": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(iv)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r988": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(f)(2)(v)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r989": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-2"
},
"r990": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "718",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480429/718-10-50-4"
},
"r991": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "730",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482916/730-10-50-1"
},
"r992": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "22",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482685/740-10-50-22"
},
"r993": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "740",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "23",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482685/740-10-50-23"
},
"r994": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "805",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(e)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479328/805-10-50-2"
},
"r995": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "805",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479328/805-10-50-3"
},
"r996": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "805",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "15",
"Paragraph": "3",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480123/805-50-15-3"
},
"r997": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "805",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "25",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480060/805-50-25-1"
},
"r998": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "805",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "30",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480027/805-50-30-1"
},
"r999": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "805",
"SubTopic": "50",
"Name": "Accounting Standards Codification",
"Section": "30",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480027/805-50-30-2"
},
"r1000": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "808",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "1",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479402/808-10-50-1"
},
"r1001": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-7A"
},
"r1002": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "8",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-8"
},
"r1003": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "8",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-8"
},
"r1004": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "815",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "8",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480434/815-10-50-8"
},
"r1005": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "815",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480870/815-30-50-2"
},
"r1006": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "815",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "65",
"Paragraph": "1",
"Subparagraph": "(e)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480175/815-40-65-1"
},
"r1007": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "35",
"Paragraph": "54B",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482134/820-10-35-54B"
},
"r1008": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r1009": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r1010": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(bbb)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r1011": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(bbb)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r1012": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(bbb)(2)(i)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2"
},
"r1013": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2E",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-2E"
},
"r1014": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "820",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6A",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482106/820-10-50-6A"
},
"r1015": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "10",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-10"
},
"r1016": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "11",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-11"
},
"r1017": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "825",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "28",
"Subparagraph": "(f)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147482907/825-10-50-28"
},
"r1018": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-3"
},
"r1019": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-4"
},
"r1020": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "842",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478964/842-20-50-6"
},
"r1021": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "850",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147483326/850-10-50-2"
},
"r1022": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "852",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481404/852-10-50-7"
},
"r1023": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "852",
"SubTopic": "10",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481404/852-10-50-7"
},
"r1024": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r1025": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r1026": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(c)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-3"
},
"r1027": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-4"
},
"r1028": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-4"
},
"r1029": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "860",
"SubTopic": "20",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4",
"Subparagraph": "(b)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147481326/860-20-50-4"
},
"r1030": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "912",
"SubTopic": "730",
"Name": "Accounting Standards Codification",
"Section": "25",
"Paragraph": "1",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479532/912-730-25-1"
},
"r1031": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "942",
"SubTopic": "235",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "2",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477314/942-235-S99-2"
},
"r1032": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "942",
"SubTopic": "320",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477268/942-320-50-2"
},
"r1033": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "30",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2B",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479432/944-30-50-2B"
},
"r1034": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "310",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "3",
"Subparagraph": "(a)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147477363/944-310-50-3"
},
"r1035": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4B",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-4B"
},
"r1036": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4B",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-4B"
},
"r1037": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4C",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-4C"
},
"r1038": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4D",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-4D"
},
"r1039": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "4G",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-4G"
},
"r1040": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-5"
},
"r1041": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-5"
},
"r1042": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-5"
},
"r1043": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "5",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-5"
},
"r1044": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1045": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1046": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1047": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1048": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)(4)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1049": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)(5)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1050": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(b)(6)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1051": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "6",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-6"
},
"r1052": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7A"
},
"r1053": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(b)(1)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7A"
},
"r1054": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(b)(2)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7A"
},
"r1055": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(b)(3)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7A"
},
"r1056": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7A"
},
"r1057": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7A",
"Subparagraph": "(d)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7A"
},
"r1058": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7B",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7B"
},
"r1059": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7B",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7B"
},
"r1060": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "40",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "7B",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480081/944-40-50-7B"
},
"r1061": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "80",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480109/944-80-50-2"
},
"r1062": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "944",
"SubTopic": "80",
"Name": "Accounting Standards Codification",
"Section": "50",
"Paragraph": "2",
"Subparagraph": "(c)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147480109/944-80-50-2"
},
"r1063": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "946",
"SubTopic": "205",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "4",
"Subparagraph": "(a)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478009/946-205-45-4"
},
"r1064": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(12)(b)(1))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r1065": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "946",
"SubTopic": "210",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.6-04(18))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479170/946-210-S99-1"
},
"r1066": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "45",
"Paragraph": "3",
"Subparagraph": "(b)",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479105/946-220-45-3"
},
"r1067": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-09(4)(b))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-3"
},
"r1068": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "946",
"SubTopic": "220",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "3",
"Subparagraph": "(SX 210.6-09(7))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147479134/946-220-S99-3"
},
"r1069": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column B))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r1070": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column C))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r1071": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column D))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r1072": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column E))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r1073": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column F))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r1074": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column G))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r1075": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column H))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
},
"r1076": {
"role": "http://www.xbrl.org/2009/role/commonPracticeRef",
"Topic": "970",
"SubTopic": "360",
"Name": "Accounting Standards Codification",
"Section": "S99",
"Paragraph": "1",
"Subparagraph": "(SX 210.12-28(Column I))",
"Publisher": "FASB",
"URI": "https://asc.fasb.org/1943274/2147478979/970-360-S99-1"
}
}
}