UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported):
(Exact Name of Registrant as Specified in Charter)
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
| (Address of Principal Executive Offices) | (Zip Code) |
(
(Registrant’s telephone number, including area code)
Unite Acquisition 1 Corp.
12 E. 49th Street, 11th Floor, New York, NY 10017
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act: None.
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
EXPLANATORY NOTE
As used in this Current Report on Form 8-K (this “Report”), unless otherwise stated or the context clearly indicates otherwise, the terms the “Company,” “we,” “us” and “our” refer to Adaptin Bio, Inc., incorporated in the State of Delaware, and its subsidiaries after giving effect to the Merger (as defined below) and the company name change described herein.
The registrant was incorporated as Unite Acquisition 1 Corp. (“Unite Acquisition”) in the State of Delaware on March 10, 2022. Prior to the Merger (as defined below), the registrant was a “shell company” (as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)).
On February 11, 2025, Unite Acquisition’s wholly-owned subsidiary, Adaptin Acquisition Co., a Delaware corporation formed in the State of Delaware on January 30, 2025 (“Merger Sub”), merged with and into Adaptin Bio, Inc., a privately held Delaware corporation (“Private Adaptin”) formerly known as Centaur Bio Inc. Pursuant to this transaction (the “Merger”), Private Adaptin was the surviving corporation and became the Company’s wholly owned subsidiary, and all of the outstanding stock of Private Adaptin was converted into shares of the combined Company’s common stock, par value $0.0001 per share (the “Common Stock”). In addition, in connection with the Merger, all of Private Adaptin’s outstanding convertible promissory notes converted into shares of Common Stock at $3.30 per share and all of Private Adaptin’s outstanding warrants became exercisable for shares of Common Stock.
As a result of the Merger, we acquired the business of Private Adaptin and will continue its business operations as a public reporting company under the same name, Adaptin Bio, Inc. Concurrent with the consummation of the Merger, Private Adaptin changed its name to “Adaptin Bio Operating Corporation.”
Concurrent with the closing of the Merger, the Company also sold 1,080,814 units (the “Units”) at a purchase price of $4.40 per Unit, each consisting of (i) one share of Common Stock, (ii) a one-year warrant to purchase one share of Common Stock at an exercise price of $4.40 per share, and (iii) a five-year warrant to purchase one-half of a share of Common Stock at an exercise price of $6.60 per share. Additional information concerning the private placement is presented below under Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—The Offering” and under Item 3.02, “Unregistered Sales of Equity Securities.” This Report is not a solicitation for or an offer to purchase Units.
In accordance with “reverse merger” or “reverse acquisition” accounting treatment, our historical financial statements as of period ends, and for periods ended, prior to the Merger will be replaced with the historical financial statements of Private Adaptin prior to the Merger, in all future filings with the U.S. Securities and Exchange Commission (the “SEC”).
This Report contains summaries of the material terms of various agreements executed in connection with the transactions described herein. The summaries of these agreements are subject to, and are qualified in their entirety by, reference to these agreements, which are filed as exhibits hereto and incorporated herein by reference.
This Report responds to the following Items:
| Item 1.01 | Entry into a Material Definitive Agreement. |
| Item 2.01 | Completion of Acquisition or Disposition of Assets. |
| Item 3.02 | Unregistered Sales of Equity Securities. |
| Item 3.03 | Material Modification to Rights of Security Holders. |
| Item 4.01 | Changes in Registrant’s Certifying Accountant. |
| Item 5.01 | Changes in Control of Registrant. |
| Item 5.02 | Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers; Compensatory Arrangements of Certain Officers. |
| Item 5.03 | Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. |
| Item 5.05 | Amendments to the Registrant’s Code of Ethics, or Waiver of a Provision of the Code of Ethics. |
| Item 5.06 | Change in Shell Company Status. |
| Item 9.01 | Financial Statements and Exhibits. |
Prior to the Merger, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act). As a result of the Merger, we have ceased to be a “shell company.” The information included in this Report constitutes the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act of 1933, as amended (the “Securities Act”).
FORWARD-LOOKING STATEMENTS
This Report, including the sections entitled “Risk Factors”, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Description of Business”, includes forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. Forward-looking statements relate to, among others, our plans, objectives and expectations for our business, operations and financial performance and condition, and can be identified by terminology such as “may,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “will,” “could,” “project,” “target,” “potential,” “continue” and similar expressions that do not relate solely to historical matters. Forward-looking statements are based on management’s belief and assumptions and on information currently available to management. Although we believe that the expectations reflected in forward-looking statements are reasonable, such statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by forward-looking statements.
Forward-looking statements include, but are not limited to, statements about:
| ● | our ability to raise additional money to fund our operations for at least the next twelve months as a going concern; |
| ● | our ability to develop our current and any future product candidates; |
| ● | our ability to receive marketing approval from the FDA for our product candidates; |
| ● | our ability to maintain our license rights to our intellectual property and to adequately protect or enforce our intellectual property rights; |
| ● | our reliance on third parties to supply drug substance and drug product for our clinical trials and preclinical studies, and produce commercial supplies of product candidates; |
| ● | our ability to market and commercialize our products, if approved; |
| ● | our product candidates’ ability to achieve market acceptance, if approved; |
| ● | developments and projections relating to our competitors and our industry; |
| ● | our ability to adequately control the costs associated with our operations; |
| ● | our dependence on third-party reimbursement for commercial viability; |
| ● | the impact of current and future laws and regulations, especially those related to drug development and drug pricing controls; |
| ● | potential cybersecurity risks to our operational systems, infrastructure, and integrated software by us or third-party vendors; |
| ● | the development of a market for our Common Stock; and |
| ● | other risks and uncertainties, including those listed under the caption “Risk Factors.” |
We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, operating results, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described in the section titled “Risk Factors.” Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties, and assumptions, the future events and trends discussed in this Report may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
1
You should not rely upon forward-looking statements as predictions of future events. The events and circumstances reflected in the forward-looking statements may not be achieved or occur. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, performance, or achievements. We undertake no obligation to update any of these forward-looking statements for any reason after the date of this Report or to conform these statements to actual results or revised expectations, except as required by law.
You should read this Report and the documents that we reference in this Report as exhibits with the understanding that our actual future results, performance, and events and circumstances may be materially different from what we expect.
ITEM 1.01 ENTRY INTO A MATERIAL DEFINITIVE AGREEMENT.
The information contained in Item 2.01 below relating to the various agreements described therein is incorporated herein by reference. All descriptions of the agreements described below are qualified in their entirety by reference to the form of the relevant agreement that is filed as an exhibit to this Report and incorporated herein by reference.
ITEM 2.01 COMPLETION OF ACQUISITION OR DISPOSITION OF ASSETS.
THE MERGER AND RELATED TRANSACTIONS
Bridge Financings
Private Adaptin raised bridge financing through the offer and sale (a) in 2023 of $500,000 principal amount of its 10% Secured Promissory Notes (the “2023 Bridge Notes”) (including warrants to purchase up to 56,815 shares of our Common Stock at an exercise price of $4.40 per share) and (b) in 2024 of $1,000,000 principal amount of its 10% Secured Subordinated Convertible Promissory Notes (the “2024 Bridge Notes”, which in each case were sold to a limited number of accredited investors pursuant to Regulation D under the Securities Act. The 2023 Bridge Notes were exchanged for $500,000 principal amount of the Company’s 10% Secured Convertible Promissory Notes (the “Exchange Notes”). In connection with the note exchange, the holders of the 2023 Bridge Notes were also issued warrants to purchase up to 75,755 shares of our Common Stock at an exercise price of $3.30 per share. The Exchange Notes and the 2024 Bridge Notes are referred to herein as the “Bridge Notes.”
At the closing of the Offering (as defined below), the $1,500,000 aggregate principal amount of outstanding Bridge Notes, plus accrued interest thereon, automatically converted into shares of Company common stock, par value $0.0001 per share (“Common Stock”), at a conversion price of $3.30 per share, or 501,140 shares of Company Common Stock (the “Note Conversion Shares”), and the holders of the 2023 Bridge Notes were issued, pursuant to existing agreements, warrants to purchase up to 132,570 shares of our Common Stock at an exercise price of either $3.30 or $4.40 per share and with a term of five years, as described above (the “Pre-Merger Warrants”).
Merger Agreement
On February 11, 2025, we entered into the Merger Agreement with Merger-Sub and Private Adaptin, pursuant to which Merger Sub merged with and into Private Adaptin, with Private Adaptin continuing as the surviving corporation and as our wholly owned subsidiary.
Pursuant to the Merger Agreement, all of the outstanding capital stock of Private Adaptin was cancelled in exchange for shares of Common Stock, and all of the outstanding Private Adaptin warrants were assumed by, the Company at the, with appropriate adjustments to the per share exercise or conversion price thereof, and otherwise on their original terms and conditions. The total number of shares of Common Stock issued to pre-Merger stockholders of Private Adaptin was 3,249,999 shares.
2
Prior to the closing of the Offering (as defined below), Unite Acquisition’s board of directors adopted an equity incentive plan reserving a number of shares of Common Stock equal to 15% of the shares to be outstanding upon each closing of the Offering, up to a maximum aggregate amount of 15% of the fully diluted shares outstanding of the Company following the final closing of the Offering (assuming exercise or conversion of all then-outstanding Common Stock equivalents), for the future issuance, at the discretion of the board of directors, of options and other incentive awards to officers, key employees, consultants and directors of the Company and its subsidiaries. See “Compensation of Directors and Executive Officers—Description of the 2025 Equity Incentive Plan” below for more information about the equity incentive plan.
The sole holder of common stock of Unite Acquisition prior to the Merger, Lucius Partners, retained 3,250,000 shares of Common Stock after the Merger.
The Merger Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions.
As a condition to the Merger, we entered into a pre-Merger indemnity agreement with Unite Acquisition’s sole officer and director, Nathan P. Pereira, pursuant to which the Company agreed to indemnify Mr. Pereira for actions taken by him in his official capacity relating to the consideration, approval and consummation of the Merger and certain related transactions.
We expect the Merger to be treated as a recapitalization and reverse acquisition for us for financial reporting purposes. Private Adaptin is to be considered the acquirer for accounting purposes, and our historical financial statements before the Merger will be replaced with the historical financial statements of Private Adaptin before the Merger in future filings with the SEC. The Merger is intended to be treated as a tax-free reorganization under Section 368(a) of the Internal Revenue Code of 1986, as amended.
The Offering
Immediately following the effective time of the Merger, we sold in a closing of our private placement offering (the “Offering”), 1,080,814 Units, for an aggregate purchase price of $4,755,581.60, at a purchase price of $4.40 per Unit, with each Unit consisting of (i) one share of Common Stock, (ii) a warrant representing the right to purchase one share of Common Stock, exercisable from issuance until one year after the final closing of the Offering at an exercise price of $4.40 per share (the “A Warrant”), and (iii) a warrant, representing the right to purchase one-half of a share of Common Stock, exercisable from issuance until five years after the final closing of the Offering at an exercise price of $6.60 per whole share (the “B Warrant,” and together with the A Warrant, the “Warrants”) (such shares of Common Stock issuable upon the exercise of the Warrants, the “Warrant Shares”). The Company and Laidlaw & Company (UK) Ltd. (the “Placement Agent”) may conduct additional closings of the Offering at their discretion for up to $8.5 million (the “Maximum Offering”). Each investor in any subsequent closing will be required to represent that, as of the date of entering into the subscription agreement and the date of the applicable closing, it (i) has a substantive, pre-existing relationship with us or has direct contact with us or the Placement Agent outside of the Offering, and (ii) did not independently contact us or a Placement Agent or become interested in the Offering as a result of reading or otherwise being aware of this Current Report, any press release or any other public disclosure disclosing the terms of the Offering.
3
In connection with the Offering, the Placement Agent (a) will be paid at each closing from the Offering proceeds a total cash commission of 10.0% of the aggregate gross purchase price paid by purchasers in the Offering at that closing (the “Cash Fee”), (b) will be paid at each closing from the Offering proceeds a total non-allocable expense allowance equal to 2.0% of the aggregate gross purchase price paid by purchasers in the Offering at that Closing (the “Expense Allowance”), and (c) will receive (and/or its designees will receive) warrants to purchase a total number of shares of Common Stock equal to 10.0% of the sum of (i) the number of shares of Common Stock included in the Units sold in the Offering at that closing and (ii) the number of shares of Common Stock issuable upon exercise of the Warrants included in the Units sold in the Offering at that closing, with a term expiring seven years after the final closing date and an exercise price of $4.40 per share (the “Placement Agent Warrants”). Any sub-agent of the Placement Agent that introduces investors to the Offering will be entitled to share in the Cash Fee, Expense Allowance and Placement Agent Warrants attributable to those investors pursuant to the terms of an executed sub-agent agreement with such Placement Agent. The Company has agreed to pay certain other expenses of the Placement Agent, including the fees and expenses of its counsel, in connection with the Offering. Subject to certain customary exceptions, we will also indemnify the Placement Agent to the fullest extent permitted by law against certain liabilities that may be incurred in connection with the Offering, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the Placement Agent and its sub-agents may be required to make in respect of such liabilities.
Description of Warrants
The A Warrants will have an exercise price of $4.40 per share and a term of one year from the final closing of the Offering and will be exercisable solely for cash.
The B Warrants will have an exercise price of $6.60 per share and a term of five years from the final closing of the Offering and will be exercisable either for cash or, when there is no effective registration statement covering the shares of Common Stock issuable upon exercise of the B Warrants, on a cashless net exercise basis.
The Warrants will have “weighted average” anti-dilution protection, subject to customary exceptions, including but not limited to issuances of awards under the executive incentive plan.
The Placement Agent Warrants will have an exercise price of $4.40 per share and a term of seven years from the final closing of the Offering and will be exercisable for cash or on a cashless net exercise basis.
Lock-Up Agreements
All officers and directors of the Company following the Merger and their affiliates and associated entities entered into lock-up agreements with us for a term ending two years after the closing of the Merger, whereby they have agreed to certain restrictions on the sale or disposition (including pledge) of all of our Common Stock held by (or issuable to) them. The lock-up agreements contain customary transfer exceptions.
Registration Rights
In connection with the Merger and the Offering, the Company entered into a registration rights agreement (the “Registration Rights Agreement”), pursuant to which the Company will file a registration statement (the “Registration Statement”) within 60 calendar days after termination of the Offering with the SEC registering for resale the following: (a) the shares of Common Stock issued pursuant to the subscription agreements in the Offering (the “Offering Shares”); (b) the Warrant Shares; (c) shares of Common Stock issued or issuable upon exercise of the Pre-Merger Warrants (the “Pre-Merger Warrant Shares”); (d) the Note Conversion Shares; (e) shares of Common Stock held by stockholders of the Company prior to the Merger and remaining outstanding immediately following the effective time of the Merger (the “Registrable Pre-Merger Shares”); (f) shares of Common Stock issued or issuable upon exercise of the Placement Agent Warrants (the “Placement Agent Warrant Shares”); and (g) other shares of restricted Common Stock held by the signatories to the Registration Rights Agreement acquired or issuable in respect of the foregoing shares of Common Stock by way of conversion, dividend, stock-split, distribution or exchange, merger, consolidation, recapitalization or reclassification or similar transaction (from (a) to (g), the “Registrable Shares”).
4
The 60 day period shall be tolled as of February 14, 2025, and resume when the audited financial statements of the Company for the fiscal year ending December 31, 2024 are issued, or March 31, 2025, whichever is earlier (the number of days of the 60 day period tolled as of February 14, 2025, the “Tolling Period”), and provided further that the Tolling Period, if applicable, shall be a minimum of 14 calendar days. The Company will use its commercially reasonable efforts to cause the Registration Statement to be declared effective within 120 calendar days after the termination of the Offering, plus, if applicable, a number of days equal to the Tolling Period.
If (a) the Company is late in filing the Registration Statement, (b) the Registration Statement is not declared effective within 120 days after the final closing date of the Offering (subject to certain exceptions), (c) the Registration Statement ceases for any reason to remain effective or the holders of Registrable Shares are otherwise not permitted to utilize the prospectus therein to resell the Registrable Shares for a period of more than five consecutive trading days (subject to permitted blackout periods (as defined in the Registration Rights Agreement) and certain other exceptions), or (d) following the listing or inclusion for quotation of the Common Stock on OTC Markets, Nasdaq, NYSE or NYSE American, the Registrable Shares are not listed or included for quotation on such a market, or trading of the Common Stock is suspended or halted on the principal market for the Common Stock, for more than three consecutive trading days (subject to certain exceptions) (each, a “Registration Event”), then in any such case monetary penalties payable by the Company to the holders of the Registrable Shares that are affected by such Registration Event will commence to accrue at a rate equal to 12% per annum of the total of the following, to the extent applicable to such holder: (i) if the holder is a holder of Note Conversion Shares, the product of $3.30 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Note Conversion Shares held by or issuable to such holder as of the date of such Registration Event, (ii) if the holder is a holder of Offering Shares, A Warrant Shares, Pre-Merger Warrant Shares, Registrable Pre-Merger Shares or Placement Agent Warrant Shares, the product of $4.40 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Offering Shares, A Warrant Shares, Pre-Merger Warrant Shares, Registrable Pre-Merger Shares or Placement Agent Warrant Shares, as the case may be, held by or issuable to such holder as of the date of such Registration Event, or (iii) if the holder is a holder of B Warrant Shares, the product of $6.60 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Note Conversion Shares held by or issuable to such holder as of the date of such Registration Event, but in each case, only with respect to such holder’s Registrable Shares that are affected by such Registration Event and only for the applicable Registration Default Period (as defined in the Registration Rights Agreement); provided, however, that in no event will the aggregate of any such penalties exceed 5% of the offering price per share. Notwithstanding the foregoing, no penalties will accrue with respect to any Registrable Shares removed from the Registration Statement in response to a comment from the staff of the SEC limiting the number of shares of Common Stock which may be included in the Registration Statement (a “Cutback Comment”). Any cutback resulting from a Cutback Comment (the “Reduction Securities”) shall be in the following order: (i) first from the Placement Agent Warrant Shares, on a pro rata basis among the holders thereof; (ii) second from the Registrable Pre-Merger Shares, on a pro rata basis among the holders thereof; (iii) third from the Offering Shares, the Offering Warrant Shares, the Pre-Merger Warrant Shares and the Note Conversion Shares, on a pro rata basis among the holders thereof.
The Company will use its commercially reasonable efforts to keep the Registration Statement continuously effective until the earlier of five years from the date it is declared effective by the SEC or for such shorter period ending on the earlier to occur of (a) the sale of all Registrable Shares and (b) the availability of Rule 144 for the holders to sell all of the Registrable Shares without restrictions, including volume limitations, and without the need for current public information, required by Rule 144 (other than Rule 144(i)) or otherwise, during any 90-day period.
If fewer than all of the Registrable Shares are included in the Registration Statement when it becomes effective, the Company will use its commercially reasonable efforts within 60 calendar days after the effective date of the Registration Statement, or within ten business days after the first date that is permitted by the SEC to, register for resale as many of the Reduction Securities as the SEC will permit (pro rata among the holders of such Reduction Securities) using one or more registration statements that it is then entitled to use, and to cause such registration statement(s) to become effective as soon as practicable, until all of the Reduction Securities have been so registered; provided, however, that the Company shall not be required to register such Reduction Securities during a Blackout Period (as defined in the Registration Rights Agreement).
The holders of Registrable Shares shall have “piggyback” registration rights for Registrable Shares not registered as provided above with respect to any registration statement filed by the Company following the effectiveness of the aforementioned Registration Statement that would permit the inclusion of such underlying shares, subject, in an underwritten offering, to customary cut-back on a pro rata basis among the holders of Registrable Shares if the underwriter or the Company determines that marketing factors require a limitation on the number of shares of stock or other securities to be underwritten.
5
OTC Quotation
Our Common Stock is currently not listed on a national securities exchange or any other exchange, or quoted on an over-the-counter market. Following completion of the Offering, we intend to cause our Common Stock to be quoted on the OTC Markets QB tier as soon as practicable following the effectiveness of the Registration Statement. However, we cannot assure you that we will be able to do so and, even if we do so, there can be no assurance that our Common Stock will continue to be quoted on the OTC Markets or quoted or listed on any other market or exchange, or that an active trading market for our Common Stock will develop or continue. See “Risk Factors—There is currently no market for our Common Stock and there can be no assurance that any market will ever develop.”
Our 2025 Equity Incentive Plan
Pursuant to the Merger Agreement, Unite Acquisition adopted the 2025 Equity Incentive Plan (the “2025 Plan”), which provides for the issuance of incentive awards of stock options, restricted stock awards, restricted stock units, stock appreciation rights and performance awards. The number of shares reserved for issuance under the 2025 Plan will increase automatically on January 1 of each of 2026 through 2035 by the number of shares equal to the lesser of 4% of the total number of outstanding shares of our Common Stock as December 31 (calculated on a fully-diluted and as-converted basis), or a number as may be determined by the Company’s board of directors. See “Compensation of Directors and Executive Officers—Description of the 2025 Equity Incentive Plan” below for more information about the 2025 Plan.
Departure and Appointment of Directors and Officers
Prior to the effective time of the Merger, the Unite Acquisition board of directors consisted of one member, Mr. Pereira, who also was its President, Chief Executive Officer, Chief Financial Officer, and Secretary. As of the Effective Time, Mr. Pereira resigned from the board of directors, and Patrick Gallagher, Simon Pedder, Michael J. Roberts, J. Nick Riehle and Anthony Zook were appointed to the Company’s board of directors.
Also, as of the Effective Time, Mr. Pereira resigned from all officer positions with the Company, and Simon Pedder was appointed as our Executive Chairman, Michael J. Roberts was appointed as our President and Chief Executive Officer, Timothy L. Maness was appointed as our Chief Financial Officer, and L. Arthur Hewitt was appointed as our Chief Development Officer.
See “Management” below for information about our new directors and executive officers.
Combined Company Ownership
Following the Merger and the closing of the Offering, there are issued and outstanding 8,081,953 shares of our Common Stock, as follows:
| ● | the stockholders of Private Adaptin prior to the Merger will hold 3,249,999 shares of our Common Stock; |
| ● | investors in the Offering will hold 1,080,814 shares of our Common Stock; |
| ● | former holders of the Bridge Notes will hold 501,140 shares of our Common Stock; and |
| ● | Unite Acquisition’s sole stockholder prior to the Merger will hold 3,250,000 shares of our Common Stock. |
In addition, there are outstanding:
| ● | A Warrants to purchase 1,080,814 shares of our Common Stock; |
| ● | B Warrants to purchase 540,407 shares of our Common Stock; |
| ● | Placement Agent Warrants to purchase an aggregate of 270,204 shares of our Common Stock; and |
| ● | Pre-Merger Warrants to purchase 132,570 shares of our Common Stock. (Like the B Warrants, the Pre-Merger Warrants will be exercisable either for cash or, when there is no effective registration statement covering the shares of Common Stock issuable upon exercise of the Pre-Merger Warrants, on a cashless net exercise basis.) |
6
In addition, there are up to 2,196,390 shares of our Common Stock reserved for future issuance under the 2025 Plan. No other securities convertible into or exercisable or exchangeable for our Common Stock are outstanding as of the date of this Current Report.
Accounting Treatment; Change of Control
The Merger is being accounted for as a “reverse merger” or “reverse acquisition,” and Private Adaptin is deemed to be the acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that are reflected in our financial statements relating to periods prior to the Merger are those of Private Adaptin, and are recorded at the historical cost basis of Private Adaptin, and the consolidated financial statements after completion of the Merger will include the assets and liabilities of Private Adaptin, historical operations of Private Adaptin, and operations of the Company from the Closing Date. As a result of the issuance of the shares of our Common Stock pursuant to the Merger, a change in control of the Company occurred as of the date of consummation of the Merger.
Except as described in this Report, no arrangements or understandings exist among present or former controlling stockholders with respect to the election of members of our board of directors and, to our knowledge, no other arrangements exist that might result in a change of control of the Company.
We continue to be a “smaller reporting company,” as defined under the Exchange Act, and an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) following the Merger. We believe that as a result of the Merger, we have ceased to be a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act).
FORM 10 INFORMATION
DESCRIPTION OF BUSINESS
Formation History
The Company is a Delaware corporation initially formed in March 2022 as Unite Acquisition 1 Corp. Effective February 11, 2025, Unite Acquisition’s wholly owned subsidiary, Merger Sub, merged with an into Private Adaptin, a Delaware corporation. Private Adaptin was the surviving corporation in the transaction and became Unite Acquisition’s wholly owned subsidiary, renamed as Adaptin Operating Co. At the same time, Unite Acquisition changed its name to Adaptin Bio, Inc.
The Company’s website address is www.adaptinbio.com and we can be contacted at info@adaptinbio.com. Information contained on, or that can be accessed through, our website is not a part of this Report.
Business Overview
Adaptin is a biopharmaceutical company pioneering a transformational approach to enhance the transfer of therapeutics into the brain, facilitating the treatment of brain cancers and other unmet medical conditions. The Company’s proprietary technology harnesses the human immune system’s ability to target, recognize, destroy or deliver therapeutics to specific cells, including cancer cells. Our mission is to be the global leader and pioneer of this new treatment paradigm, integrating recombinant technology, gene therapy and cell therapy to address the challenges of targeting and delivering effective therapies, including to the brain for cancer and other central nervous system (“CNS”) indications.
The cause(s), or etiology, of many diseases can be addressed in part through manipulation of engineered cells. We view targeted manipulation of the human immune system, together with recombinant technology and/or gene therapy, as a therapeutically disruptive transformation in the way we treat brain and other diseases. Our experienced group of scientists and business leaders are developing our proprietary in vivo and ex vivo technology platforms to revolutionize treatment across a broad array of therapeutic areas with unmet treatment needs, including oncology, CNS disorders, autoimmune disease and cardiovascular diseases, among others. Our lead product candidate has been recently accepted under an investigator-led IND to begin first-in-human studies in brain cancer. Our goal is to complete preclinical studies on additional product candidates and file multiple investigational new drug applications (each, an “IND”) in 2026, if not earlier.
7
Adaptin’s novel technology was originally developed by researchers in the Department of Neurosurgery at Duke University, led by Dr. John H. Sampson, the prior Robert H. and Gloria Wilkins, Distinguished Professor and Chair of the Department of Neurosurgery and currently the Dean and Vice Chancellor at the University of Colorado School of Medicine. The group recognized that adoptive transfer of specifically activated functional human immune cells significantly increases the “hitchhiking” and intracerebral accumulation of macromolecules that are bound to their surface. While circulating naïve T cells do not typically penetrate the CNS, activated T cells are known to traffic frequently past the blood brain barrier (“BBB”) and perform routine immune surveillance in the CNS. Adaptin and its collaborators at Duke University are taking advantage of this CNS trafficking to enhance the localization of macromolecules and other agents to the CNS for cancer and other CNS disorders.
Adaptin is closely working with researchers at Duke University to translate preclinical proof of concept data of its first proprietary platform technology called BRiTE (Brain Bispecific T-cell Engager) into human clinical trials. BRiTE focuses on the transport of difficult to deliver T cell targeting agents to tumor tissue, including in the immunoprivileged brain and overcoming the challenges with other immunotherapeutic approaches. BRiTE is a translatable method to specifically target malignant glioma using a tumor-specific, fully human bispecific antibody that redirects patients’ own T cells to recognize and destroy tumor cells.
The first application of Adaptin’s technology is APTN-101, a proprietary epidermal growth factor receptor variant III, or EGFRVIII, BRiTE in order to eliminate malignant glioma tumors in a variety of aggressive preclinical tumor models where the tumor is implanted behind the BBB in the CNS (i.e., orthotopic). We designed APTN-101 to specifically redirect T cells against tumors expressing a well-characterized, mutated form of epidermal growth factor receptors (“EGFRs”) known as EGFRVIII, on a number of tumor types, including glioblastoma, breast and lung cancer. Because EGFRVIII is exclusively expressed on tumor cells, but not normal healthy cells, we believe it represents an ideal target for immunotherapy. We have made significant progress towards first-in-human clinical studies, including:
| ● | A pre-IND meeting with the U.S. Food and Drug Administration (the “FDA”) outlining a clear path to filing an IND; |
| ● | Completion of single-dose IND-enabling preclinical studies; |
| ● | Submission in April 2023 of an IND for an investigator-initiated, single-dose clinical trial, and its acceptance in May 2023 by the FDA; and |
| ● | Manufacturing of APTN-101 in more than sufficient quantities for Phase 1 trials. |
We also expect to expand our proprietary platform to other targets and indications. The Company is exploring several external opportunities to continue to advance and expand the product pipeline.
Strategy
Our goal is to become a leading biopharmaceutical company focused on the transfer of drugs across barriers and to targeted tissues, including the brain and CNS, to transform current treatment paradigms for patients and address unmet medical needs. The critical components of our strategy are as follows:
| ● | Advance the development of APTN-101 for the treatment of glioblastoma. The FDA’s acceptance in May 2023 of the IND for APTN-101 for the treatment of glioblastoma sets the stage for first-in-human clinical trials. |
| ● | Advance preclinical development of APTN-101 to support one or more additional INDs for additional kinds of cancer. We have designed APTN-101 to incorporate EGFRvIII, which is expressed on a number of tumor types, including breast and lung cancer (with or without brain metastases), so we are considering pre-clinical work to support INDs for these indications to be filed in 2026, if not earlier. |
8
| ● | Design and advance other early-stage drug product candidates for undisclosed rare and unmet needs. Because our proprietary technology enables drugs to cross barriers and target tissues, including the brain, we believe it has numerous potential applications in areas of unmet medical need. We are evaluating which of those indications would be most strategic to pursue in the near-term, and plan to initiate one or more preclinical studies in 2025 to support the filing of future INDs. |
| ● | Acquire, in-license or develop complementary delivery technologies that will allow us to produce BRiTE compounds or manipulate and activate immune cells in vivo. We continually evaluate technologies that will further enhance therapeutic effect, improve safety and manufacturability, or reduce costs of our products. |
| ● | Acquire targeted clinical compounds for conditions with unmet needs where our technology could be transformative. We continually evaluate development and in-licensing opportunities and may acquire clinical compounds for conditions with unmet medical needs where our technology’s ability to cross barriers and target specific tissues, including the brain, could be transformative of the treatment paradigm. |
| ● | Pursue a capital-efficient commercialization strategy. For products with smaller and/or orphan patient populations, our plan is to build an infrastructure to commercialize our drug products within the United Sates. Drawing upon our experience in commercializing specialty pharmaceutical products, we aim to build a specialized yet efficient infrastructure that will support the entire commercialization continuum, including stakeholder education, treatment decision and initiation, and product access throughout the patient journey. In addition, we plan to seek established companies to commercialize our drug products for larger addressable markets and outside of the United States. |
| ● | Leverage, protect and enhance our intellectual property portfolio and secure patents for additional products and indications. We intend to expand our intellectual property, grounded in securing composition of matter and method of use patents for new products and indications. We plan to enhance the intellectual property portfolio further through learnings from ongoing preclinical studies, clinical trials and manufacturing processes. |
| ● | Outsource capital-intensive operations. We plan to continue to outsource capital-intensive operations, including most clinical development and all manufacturing operations of our product candidates, and to facilitate the rapid development of our pipeline by using high quality specialist vendors and consultants in a capital efficient manner. |
Glioblastoma
Background
Glioblastoma multiforme (“GBM”), the highest grade (WHO IV) astrocytoma, is the most common and malignant brain tumor, accounting for about 50% of all gliomas and 12%–15% of all brain tumors. GBM tumor cells, which arise from stem cells or immature astrocytes due to genetic abnormalities, grow rapidly and disseminate in the brain. In addition, GBM cells can invade the intracranial blood vessels to areas away from the tumor core.
Although glioblastoma can happen anywhere in the brain, it usually forms in the frontal lobe and the temporal lobe. Glioblastoma rarely occurs in the brain stem or spinal cord. As glioblastoma grows, it spreads into the surrounding brain. This makes it difficult to remove the entire tumor with surgery. Although radiation therapy and chemotherapy can reach the tumors, glioblastoma cells can survive and regrow. Glioblastoma is very challenging to treat due to tumor-specific features, such as its rapid growth rate, the poor function of the immune system cells within the tumor, and inherent resistance of the tumor cells to many types of treatments.
There is no direct risk factor associated with most cases of glioblastoma. Certain rare genetic diseases, such as Li-Fraumeni and Lynch syndrome, are associated with gliomas. However, these affect only a small portion of patients with glioblastoma. Besides genetic syndromes, the only well-established risk factor is prior exposure to ionizing radiation that is used to treat certain head and neck cancers.
9
Brain tumor symptoms vary and depend on the tumor location. The most common glioblastoma symptoms are headaches, seizures, and progressively worsening numbness or weakness. Headaches with red flag symptoms warrant a trip to the doctor for a neurologic evaluation. Red flag symptoms include waking up due to pain, worsening pain on changing position, and continuous pain not relieved with over-the-counter headache medications.
Neurologic imaging with an MRI of the brain is often the first step in diagnosis. Brain imaging showing contrast-enhancing masses can be suggestive of glioblastoma. Most cases can be definitively diagnosed after surgery through histological testing. This takes place when a neuropathologist examines tissue or cells under a microscope to help confirm a glioblastoma diagnosis.
Incidence and Mortality
In the United States, the average annual age-adjusted incidence of glioblastoma is 3.2 per 100,000 population (or about 12,000 patients annually) with an average age of 64 at diagnosis. Glioblastoma is 1.6 times more common in males compared with females and 2.0 times higher in Caucasians compared to African Americans, with lower incidence in Asians and American Indians. Globally, glioblastoma incidence is highest in North America, Australia, and Northern and Western Europe. Veterans who served in Iraq or Afghanistan are 26% more likely to develop glioblastoma, according to the U.S. Department of Veterans Affairs and National Institutes of Health data, likely due to environmental exposures. Malignant primary brain tumors are the most frequent cause of cancer death in children, are more common than Hodgkin lymphoma, ovarian and testicular cancer and are responsible for more deaths than malignant melanoma.
Overall, the one-year relative survival rate is about 40% for patients diagnosed in the United States. The five-year survival rate is only about 5%. Treatment outcome remains poor, with a median survival rate of about 15 months.
Current Treatment/Management
A patient’s care team will take into account age, functional status, medical history and medication tolerability when planning the best treatment. For most newly diagnosed patients, the standard approach utilizes what is known as the Stupp protocol. Treatment is comprised of maximal surgical resection, which allows for accurate histological diagnosis, tumor genotyping, and a reduction in tumor volume, followed by 6 weeks of radiotherapy and concomitant daily temozolomide and a further 6 cycles of maintenance temozolomide. In patients with minimal functional impairment, the median overall survival (“OS”) is 15 months for radiotherapy plus temozolomide versus 12 months for radiotherapy alone.
Treatment options in the relapsed or recurrent setting are less well defined, with no established standard of care and little evidence for any interventions that prolong OS. Indeed, a significant proportion of patients may not even be eligible for second-line therapy. Options include further surgical resection, reirradiation, systemic therapies such as carmustine or bevacizumab, combined approaches, or supportive care alone.
Patients may also be treated with tumor treatment fields. This is a portable device placed on the scalp that uses mild electrical fields to try to interrupt cancer cell growth.
Standard-of-care treatments fail to specifically eliminate tumor cells and are limited by incapacitating damage to surrounding normal brain and systemic tissues leading to lymphopenia and many other detrimental side effects. All of this demonstrates that a more targeted immunotherapeutic approach is needed.
10
Over the last decade, emerging immunotherapies (such as monoclonal antibodies, oncolytic virus therapy, adoptive cell therapy, and cellular vaccines therapy) aimed at improving specific immune response against tumor cells have brought a glimmer of hope to patients with GBM. Adoptive cell therapy, including tumor-infiltrate lymphocytes (“TILs”) transfer and genetically engineered T cells transfer, is one of the most significant breakthroughs in the field of immune-oncology. Chimeric antigen receptor (“CAR”) engineered autologous T cells have produced sustained remissions in refractory lymphomas, but this approach needs further study in the treatment of solid tumors. While there is significant potential with targeted immunotherapy in GBM, significant challenges remain, including primarily the difficulty of crossing the BBB.
Bi-Specific Antibodies
The concept of bispecific antibodies was first introduced in 1980s as a method to target multiple antigens by a single antibody. The recombinant bispecific antibodies are classified into two types. The first are antibodies containing the crystallizable region (i.e., Fc-containing antibodies) and the second are antibody derivatives without Fc regions. The Fc region is the tail region of an antibody that interacts with cell surface receptors called Fc receptors and some proteins of the complement system. The mechanism of action of bispecific antibodies includes binding to the tumor cells on one side through the Fab (antigen-binding region) portion of the antibody against the tumor-specific antigen (such as CD19, HER2, EGFR, or GD2) and to the immune effector cells such as T cells and NK cells, which leads to activation of those immune effector cells and Fc-receptor bearing phagocytic cells such as monocytes/macrophages that can also mediate direct lysis of the tumor cells.
Bispecific antibodies termed bispecific T cell engagers (“BiTEs”) are monomeric proteins consisting of two antibody-derived single-chain variable fragments (“scFvs”) translated in tandem. These constructs possess one effector-binding arm specific for the epsilon subunit of T-cell CD3 and an opposing target-binding arm directed against an antigen that is expressed on the surface of tumor cells (e.g., EGFRvIII).
We believe EGFRvIII is an attractive target tumor specific antigen, in part because it is specific to cancer cells and is not expressed in non-tumor tissue. Importantly, antibodies directed against EGFRvIII are entirely tumor-specific and do not cross react with the wild-type receptor located on healthy cells. Therefore, by retargeting T cells against the tumor-specific EGFRvIII antigen, we believe we can avoid killing healthy tissue and the related adverse effects. EGFRs are involved in deregulated cancer signaling pathways, leading to atypical proliferation and growth of tumor cells. EGFRvIII is the most common variant not presented in a major histocompatibility complex (“MHC”) -dependent manner and is seen in approximately 31 to 50% of patients with GBM and in a broad array of other cancers including breast and lung carcinoma. Lung and breast carcinoma are the two main types of cancer that lead to secondary brain tumors (i.e., brain metastases) in about 25% of these patients. Among patients with EGFRvIII-positive GBM, 37 to 86% of tumor cells express the mutated receptor, indicating that the mutation is translated with significant consistency.
Among patients with GBM, expression of EGFRvIII is an independent, negative prognostic indicator. EGFRvIII also enhances the growth of neighboring EGFRvIII-negative tumor cells via cytokine-mediated paracrine signaling and by transferring a functionally active oncogenic receptor to EGFRvIII-negative cells through the release of lipid-raft related microvesicles. Recent research has also found that EGFRvIII is expressed in glioma stem cells, an important consideration given the paradigm that tumor stem cells represent a subpopulation of cells that give rise to all differentiated tumor cells. Altogether, the specificity, high frequency of surface expression and oncogenicity of the EGFRvIII mutation make it an ideal target for antibody-based immunotherapy.
The divalent structure of BiTEs brings T cells into close proximity to the tumor cell, creating a synapse. Following BiTE-mediated synapse formation, T cells proliferate, secrete pro-inflammatory cytokines and express surface activation markers. Following BiTE-mediated synapse formation, T cells release perforin and granzyme proteases that kill tumor cells. BiTEs are capable of mediating serial rounds of killing and can trigger specific tumor cell killing from naïve T cells at exceedingly low concentrations and effector-to-target ratios.
It is well established that certain gliomas, such as glioblastoma, are uniquely shielded from the immune system due to its location within the CNS. While this privilege is not absolute, a significant proportion of tumors have been noted to be devoid of any TILs that can be redirected by bispecific T-cell engagers. In those tumors that do demonstrate invasion by TILs, they are often induced to be dysfunctional and anergic by the suppressive tumor microenvironment. Increased numbers of intratumoral CD8+ cytotoxic T lymphocytes (“CTLs”) have been associated with favorable outcomes in patients with glioblastoma.
11
Concomitant administration of stimulated CTLs may therefore synergistically enhance the efficacy of this treatment. The migration of T-cell engagers across the BBB may also be facilitated by activated T cells which adhere to the brain microvascular endothelium and subsequently cross by diapedesis. Concurrent administration of activated functional T cells could therefore enhance the trafficking of bispecific T-cell engagers and other therapeutics into the intracranial compartment, increasing their density at the tumor site and thus the therapeutic effect.
Additionally, target cell killing with BiTE occurs in the absence of regular MHC peptide antigen recognition and costimulation and is therefore resistant to certain immune escape mechanisms affecting antigen presentation and those affecting generation of tumor-specific T cell clones. Because the CD3ε target of BiTE antibody construct is the same in CD8+ and CD4+ T cells of any phenotype, they are all engaged, leading to a polyclonal T cell activation, expansion and broad tumor cell killing.
Bispecific T-cell engagers offer immunotherapy in a manufacturing format which is both scalable and standardizable. In contrast to CAR T cells, T-cell engagers do not require initial lymphodepletion. Ex vivo manipulation of autologous cells has significant limitations, including the need for a centralized manufacturing infrastructure with extensively trained laboratory personnel to genetically modify each patient’s own T cells, use viral transduction which poses uncertain risks, are limited to the initial subset of T cells manipulated and infused, and still face uncertainty as to the optimal T cell phenotype to infuse.
Our Product Pipeline
APTN-101
We have recently reported the development of our first novel T cell engaging molecule, known as APTN-101, using our BRiTE technology. BRiTE focuses on the transport of difficult to deliver T cell targeting agents across the BBB allowing access to the immunoprivileged brain and overcoming the challenges with other immunotherapeutic approaches. This is accomplished by sequentially or simultaneously administering both BRiTE and specifically activated T cells by adoptive transfer. APTN-101 was designed to specifically redirect T cells against tumors expressing a well-characterized, mutated form of the EGFR, EGFRvIII, on a number of tumor types, including GBM. Because EGFRvIII is exclusively expressed on tumor cells, but not normal healthy cells, it represents an ideal target for immunotherapy.
APTN-101 (EGFRvIII x CD3 BRiTE) successfully activates human T cells against EGFRvIII expressing target cells, in the absence of any additional immunostimulatory signal, resulting in the secretion of Th-1-associated cytokines and tumor-cell killing. APTN-101 is similarly effective in vivo. Intravenous administration of APTN-101 induced consistent antitumor responses in mice bearing established, late-stage, aggressive, intracerebral patient-derived gliomas, rapidly achieving complete remission rates as high as 75% in the absence of apparent toxicity. Given the exquisite tumor-specificity of APTN-101, it represents a critical conceptual advance in safety contrary to target antigens having a promiscuous expression pattern.
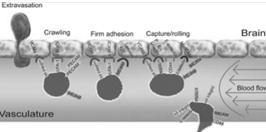
The concept of BRiTE is the combination of novel T cell targeting agents with specifically activated polyclonal T cells. In order to exert antineoplastic affects against brain tumors, both the T cell targeting agent and T cells need to efficiently access areas that have long been considered as immunoprivileged. While circulating naïve T cells do not typically penetrate the CNS, activated T cells are known to cross the BBB to perform routine immunosurveillance of the central nervous system (Figure 1).

Figure 1- The process of T cells crossing the inflamed BBB is coordinating and sequential. Briefly, activated T cells initially arresting on the endothelium is mediated by the lymphocyte-associated antigen-1 (“LFA- 1”) and α4β1-integrin expressed on the T cells, respectively binding to the intracellular cell adhesion molecule 1 (“ICAM1”) and adhesion molecules vascular cell adhesion molecule 1 (“VCAM1”) on brain endothelial cells. Subsequently, the T cell crawling and polarization exclusively involve LFA-1 and ICAM1/2 interactions. After arriving at sites where are rich in the laminin isoform α4 but not laminin β5, the T cells use α6β1-integrin to traverse the endothelial basement membrane.
12
Upon intravenous administration, the T cell targeting agent (i.e., EGFRviii x CD3) binds to circulating T cells via its CD3 receptor and carries or “hitchhikes” the agent to tumors located behind the BBB. Studies in aggressive orthotopic GBM models have revealed that adoptive transfer of activated T cells significantly increases the biodistribution of intravenously administered EGFRvIII x CD3 BRiTE to orthotopic glioma (Figure 2).
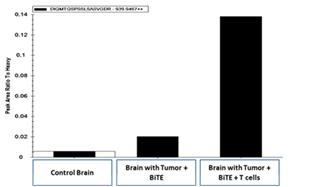
Figure 2- Mass spectroscopy demonstrates that pre-administration (four days) of ex vivo activated T cells increases the biodistribution of intravenously administered EGFRvIII x CD3 to the brain parenchyma.
BRiTE circumvents ordinary clonotypic T-cell specificity, potentially allowing any T-cell, regardless of endogenous specificity or phenotype, to exert an anti-neoplastic effect. In vivo experiments show that BRiTEs can reactivate potentially unresponsive, anergic T cells, such as those frequently encountered among TIL populations thereby enhancing the spread of T cells reactive towards other antigens (epitope spreading). Proximal contact between T cells and tumor cells could directly reactivate tumor infiltrating lymphocytes specific for cancer antigens other than directed by BRiTE, without cross-presentation. The EGFRvIII x CD3 BRiTE molecule has the potential to directly activate and expand pre-existing T cells among a polyclonal population that are specific for tumor antigens other than EGFRvIII. Indeed, others have discovered that by re-activating pre-existing T cell clones using a CD3 binding bispecific antibody specific for Wilms’ tumor protein (WT1) it is possible to induce effective and persistent epitope spreading responses to multiple antigens. If the clinical utility of this mechanism of epitope spreading is confirmed, this could provide an exciting mechanism to combat tumor heterogeneity.
Importantly, our data suggest that once APTN-101 reaches the brain, it can activate even suppressive Tregs to kill glioblastoma tumor cells by redirecting their natural granzyme-mediated cytotoxic potential and enhance a cytotoxic immune response. These findings not only highlight a new mechanism by which BRiTEs may circumvent certain aspects of Treg mediated suppression, but also have broader implications with regard to the natural functional role of activated, tumor infiltrating Tregs that ordinarily suppress and kill cytotoxic T lymphocytes in the tumor microenvironment.
By tethering cytotoxic effectors to target cells without the need for antigen presentation via the MHC, BRiTEs can furthermore overcome tumor immune escape mechanisms, such as the downregulation of MHC.
13
In addition to enhancing the biodistribution of EGFRvIII x CD3 BRiTE to the brain, the activated polyclonal T cells (compared to the addition of no activated polyclonal T cells or naïve polyclonal T cells) have the potential to restore effector T cells function at intracerebral sites (Figure 3) leading to cures in greater than 75% of animals.
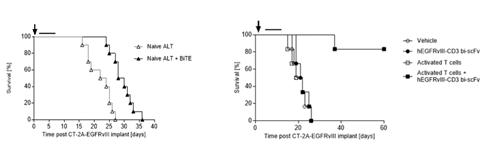
Figure 3- IV administration of activated T cells enhances hEGFRvIII-CD3 bi-scFv efficacy against syngeneic, highly-invasive, orthotopic glioma (right panel) compared to IV administration of naïve T cells when combined with hEGFRvIII-CD3 bi-scFv (left panel). The highly invasive murine glioma CT-2A-EGFRvIII was implanted orthotopically in human CD3 transgenic mice (females, 8-10 weeks old, n=10 per group).
Next, we hypothesized that the increase in efficacy observed with the pre-administration of activated polyclonal T cells would be abrogated if those T cells were to be blocked from entering the CNS parenchyma. Natalizumab, a clinically approved drug for the treatment of multiple sclerosis, functions by binding to polyclonal T cells and preventing their association with receptors involved in the process of extravasation. Remarkably, in cohorts of mice receiving adoptive transfer of activated polyclonal T cells along with treatment with the extravasation blocking molecule natalizumab, efficacy was decreased to levels observed in cohorts that did not receive adoptive transfer of activated polyclonal T cells (Figure 4).
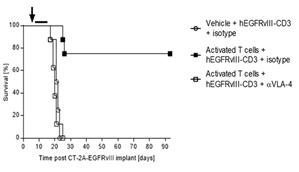
Figure 4- Blocking T cell extravasation with Natalizumab (αVLA-4) abrogates the observed increase in efficacy with adoptive cell transfer (n=8 per group). Natalizumab is a clinically approved drug for the treatment of multiple sclerosis that functions by blocking T cell extravasation.
We have also demonstrated an effective “antidote” for any potential toxicity that may result from administration of the EGFRvIII x CD3 BRiTE in a clinical setting. By administering a short peptide that spans the EGFRvIII mutation (PEPvIII), we have effectively blocked bispecific antibody function both in vitro and in vivo, providing a tool highly likely to aid in safe clinical administration of any EGFRvIII targeted bispecific antibody.
The above data demonstrates that BRiTE technology has the ability to transport difficult to deliver agents, including T cell targeting agents, across the BBB and demonstrate superior antineoplastic activity in aggressive orthotopic models of GBM while having an acceptable safety profile. This newly uncovered hitchhiking mechanism of drug delivery to the CNS provides an important tool to enhance the immunotherapy of brain tumors and has potentially far-reaching consequences for the treatment of other CNS disorders, such as Alzheimer’s or Parkinson’s disease, where issues regarding drug delivery to the CNS are relevant.
14
In summary, the results of preclinical studies demonstrate that the EGFRvIII targeting BRiTE may provide a safe, highly effective therapeutic option for GBM patients. Future studies will determine whether these results can be recapitulated in the clinical setting and whether BRiTEs favorably interact with other therapies that are currently employed as a standard-of-care for GBM patients.
APTN-101 Clinical Studies
The proposed Phase 1 study will evaluate a novel hEGFRvIII-CD3-biscFv Bispecific T cell engager (BRiTE) in patients diagnosed with pathologically documented supratentorial World Health Organization grade IV malignant glioma with an EGFR mutation (either newly diagnosed or at first progression/recurrence) at the Preston Robert Tisch Brain Tumor Center at Duke University.
The primary objective of this phase 1 study is to determine the safety and tolerability of BRiTE and recommend phase 2 dose of BRiTE injected with and without activated polyclonal T-cells in among a patient population that includes newly diagnosed patients after completion of standard of care therapy consisting of radiation and adjuvant temozolomide, and patients at first progression (defined as progression during or after standard of care radiation and adjuvant temozolomide).
Another secondary objective is to describe the PK in subjects treated with BRiTE. A population PK analysis will be performed to characterize the PK of BRiTE using the software Nonlinear Mixed Effects Modeling (NONMEM, version 7.2). Different structural PK models (e.g., 1 and 2 compartment) with linear or non-linear (e.g., Michaelis-Menten) kinetics will be fitted to the plasma concentration-time data of BRiTE.
Exploratory objectives include an evaluation of the pharmacodynamics effect of BRiTE, an evaluation of the formation and incidence of anti-BRiTE antibodies, and a description of overall survival and progression-free survival.
Our Intellectual Property
We strive to protect the proprietary technology that we believe is important to our business, including our product candidates and our processes. We seek patent protection in the United States and internationally for our product candidates, their methods of use and processes of manufacture, and any other technology to which we have rights, as appropriate. Additionally, we have licensed the rights to intellectual property related to certain of our product candidates, including patents and patent applications that cover the products or their methods of use or processes of manufacture. The terms of the licenses are described below under the heading “License Agreement.” We also rely on trade secrets that may be important to the development of our business.
We hold a world-wide exclusive license to three issued or allowed United States patents and one pending PTC patent application covering the enhanced delivery of drugs and other compounds to the brain and other tissues. The patents and patent applications that we licensed provide patent terms or anticipated patent terms ranging from 2031 to 2039 without patent term extensions.
Our success will in part depend on the ability to obtain and maintain patent and other proprietary rights in commercially important technology, inventions and know-how related to our business, the validity and enforceability of our patents, the continued confidentiality of our trade secrets, and our ability to operate without infringing the valid and enforceable patents and proprietary rights of third parties. We also rely on continuing technological innovation and in-licensing opportunities to develop and maintain our proprietary position.
We cannot be sure that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications we may own or license in the future, nor can we be sure that any of our existing patents or any patents we may own or license in the future will be useful in protecting our technology and products. For this and more comprehensive risks related to our intellectual property, please see “Risk Factors—Risks Related to Our Intellectual Property.”
15
License Agreement
Patent License Agreement with Duke University
Effective January 11, 2023, Private Adaptin entered into a patent license agreement (the “Duke License”) with Duke University, a nonprofit educational and research institution organized under the laws of North Carolina (“Duke University”), whereby Duke University granted us an exclusive license with a right to grant sublicenses to “BISPECIFIC EGFRvIII ANTIBODY ENGAGING MOLECULES,” “HUMAN BISPECIFIC EGFRvIII ANTIBODY AND CD3 ENGAGING MOLECULES,” “CERTAIN IMPROVED HUMAN BISPECIFIC EGFRvIII ANTIBODY ENGAGING MOLECULES” and “ENHANCED DELIVERY OF DRUGS AND OTHER COMPOUNDS TO THE BRAIN AND OTHER TISSUES” (together, the “precision medicine technology”). With this technology, which the Company assumed pursuant to the Merger, we intend to develop our BRiTE Platform, a combination of an immune cell engager with activated functional T-cells, which focuses on transporting difficult to deliver agents across the blood brain barrier, allowing access to the immunoprivileged central nervous system. Under the Duke License, we are required to use commercially reasonable efforts to obtain and retain the relevant governmental approvals and to commercialize the precision medicine technology. We must also use reasonable efforts to reach certain commercialization and research and development milestones as outlined in the Duke License.
On August 8, 2024, Private Adaptin entered into a Sponsored Research Agreement (the “SRA”), which the Company assumed pursuant to the Merger, whereby Duke University agreed to perform research exploring the administration methods of our BRiTE Platform for a fixed fee. The Duke License has been amended (the “First Amendment”) such that the Company has the option to add any invention conceived as a result of the performance under the SRA to the license.
As part of the consideration for the license, Private Adaptin issued Duke University shares, representing 5% of its issued and outstanding common stock on a fully diluted basis. Following the Merger, Duke University holds 2.0% of the combined Company. We also agreed to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities. These milestones include initiation of a Phase II or Phase III clinical trials, submission of applications for market approval in multiple jurisdictions including the United States, European Union and Japan and the initiation of post-approval commercial sales in the same jurisdictions. Based on an assumption that all milestones related to the current development program are met during the course of the Duke License, these milestone payments would total approximately $11.7 million. Under the terms of the Duke License, we must pay running royalties equal to low- to mid-single digit percentages of annual net sales, depending on the level of sales by us, our sublicensees and affiliates in that year, and subject to downward adjustment to low single digit percentages of our net annual sales in the event there is no valid claim of a patent for the product, with minimum annual royalty levels established. We also must pay Duke University low to mid-double-digit percentages of any sublicensing fees as set forth in the Duke License. We will be responsible for all patent expenses incurred by Duke University and must reimburse Duke University for previous patent expenses incurred by Duke University for filing and prosecution of the patent rights.
The term of the Duke License will extend until the expiration of the last to expire patent rights, subject to early termination as set forth in the Duke License. The foregoing descriptions of the Duke License, First Amendment and SRA do not purport to be complete and are qualified in their entirety by the terms and conditions of the Duke License, First Amendment and SRA, forms of which are attached hereto as Exhibits 10.1, 10.2 and 10.3, respectively, and are incorporated herein by reference.
Manufacturing and Supply
We contract with third parties for the manufacturing of all of our product candidates, and for pre-clinical and clinical studies and intend to continue to do so in the future. We do not own or operate any manufacturing facilities and we have no plans to build any owned clinical or commercial-scale manufacturing capabilities. We believe that the use of contract manufacturing organizations (“CMOs”) eliminates the need to directly invest in manufacturing facilities, equipment and additional staff. Although we rely on contract manufacturers, our personnel and consultants have extensive manufacturing experience overseeing CMOs.
16
We have produced the EGFRvIII x CD3 BRiTE in a fashion suitable for clinical translational and compatible with clinical biologic manufacturing infrastructure. This has included generating and certifying a Master Cell Bank and developing a scalable expression and tag-free purification and formulation process suitable for clinical translation. On the basis of our data and development work, we have had a CMO produce clinical-grade EGFRvIII x CD3 BRiTE suitable for clinical study.
As we further develop our product candidates, we expect to consider secondary or back-up manufacturers for both active pharmaceutical ingredient and drug product manufacturing. To date, our third-party manufacturers have met the manufacturing requirements for our product candidates in a timely manner. We expect third-party manufacturers to be capable of providing sufficient quantities of our product candidates to meet anticipated full-scale commercial demand but we have not assessed these capabilities beyond the supply of clinical materials to date. We currently engage CMOs on a “fee for services” basis based on our current development plans. We plan to identify CMOs and enter into longer-term contracts or commitments if and as we move our product candidates into Phase 3 clinical trials.
We believe alternate sources of manufacturing will be available to satisfy our clinical and potential future commercial requirements; however, we cannot guarantee that identifying and establishing alternative relationships with such sources will be successful, cost-effective, or completed on a timely basis without significant delay in the development or commercialization of our product candidates. All of the vendors we use are required to conduct their operations under current Good Manufacturing Practices (“cGMP”), a regulatory standard for the manufacture of pharmaceuticals.
Competition
The pharmaceutical industry is highly competitive and characterized by intense and rapidly changing competition to develop new technologies and proprietary products, particularly in some of the areas of high unmet medical need that we are targeting. Our potential competitors include both major and specialty pharmaceutical and biotechnology companies worldwide, many of which have far greater resources and access to capital than we do. In particular, Affimed N.V. and NexImmune, Inc. are studying the combination of immune cell engagers with T or NK cells using a different bispecific immune cell engager, and Amgen Inc. and Genentech, Inc. (a wholly-owned subsidiary of Roche Holding AG) have programs using a form of EGFRvIII x CD3 bispecific T cell engager (although neither are using them in combination with activated T cells). Our success will be based in part on our ability to identify, develop, and manage a portfolio of safe and effective product candidates that address the unmet needs of patients before our competitors.
Government Regulations
The FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of drugs, such as those we are developing. Along with our third-party contractors, we will be required to navigate the various preclinical, clinical, and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our product candidates. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources.
FDA Regulation of Drugs
Before any of our drug product candidates may be marketed in the United States, they must be approved by the FDA. The process required by the FDA before drug product candidates may be marketed in the United States generally involves the following:
| ● | completion of preclinical laboratory tests and animal studies performed in accordance with the FDA’s current Good Laboratory Practices regulations; |
17
| ● | submission to the FDA of an IND, which must become effective before human clinical trials may begin and must be updated annually or when significant changes are made; |
| ● | approval by an independent institutional review board (“IRB”) or ethics committee for each clinical site before a clinical trial can begin; |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed product candidate for its intended purpose; |
| ● | preparation of and submission to the FDA of a New Drug Application (“NDA”) after completion of all required clinical trials; |
| ● | a determination by the FDA within 60 days of its receipt of an NDA to file the application for review; |
| ● | satisfactory completion of an FDA Advisory Committee review, if required by the FDA; |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with cGMP, and to assure that the facilities, methods, and controls are adequate to preserve the product’s continued safety, purity and potency, and of selected clinical investigational sites to assess compliance with current Good Clinical Practices; and |
| ● | FDA review and approval of the NDA to permit commercial marketing of the product for particular indications for use in the United States, which must be updated annually and when significant changes are made. |
The testing and approval processes require substantial time, effort, and financial resources and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all, and we may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our product candidates. The FDA may delay or refuse approval of an NDA if applicable regulatory criteria are not satisfied, or may require additional testing, information, and/or post-marketing testing and surveillance to monitor safety or efficacy of a product candidate.
If regulatory approval of a product candidate is granted, such approval may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the NDA with a Risk Evaluation and Mitigation Strategy (“REMS”) plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing regulatory standards is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more post-market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization and may limit further marketing of the product based on the results of these post-marketing studies. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development.
FDA Programs for Expedited Review and Increased Exclusivity
A sponsor may seek approval of a product candidate under Fast Track and Breakthrough Therapy programs designed to accelerate the FDA’s review and approval of new drug candidates that meet certain criteria, and/or to receive increased exclusivity under the orphan drug program. We intend to pursue these programs where our product candidates qualify.
Fast Track. A new drug candidate is eligible for Fast Track designation if it is intended to treat a serious or life-threatening condition, fill an unmet medical need, and demonstrate a significant improvement in the safety or effectiveness in the treatment of that condition.
A drug that receives Fast Track designation is eligible for the following:
| ● | more frequent meetings with FDA to discuss the drug’s development plan and ensure collection of appropriate data needed to support drug approval; |
18
| ● | more frequent written correspondence from FDA about the design of clinical trials; |
| ● | priority review to shorten the FDA review process for a new drug from ten months to six months; and |
| ● | rolling review, which means we can submit completed sections of its NDA for review by FDA, rather than waiting until every section of the application is completed before the entire application can be reviewed. |
Under the accelerated approval program, the FDA may approve an NDA on the basis of either a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Fast Track designation and priority review do not change the standards for approval but may expedite the development or approval process.
Breakthrough Therapy. A new drug candidate is eligible for Breakthrough Therapy designation if it is intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on one or more clinically significant endpoints.
A drug that receives Breakthrough Therapy designation is eligible for the following:
| ● | All Fast Track designation features; |
| ● | Intensive guidance from the FDA on an efficient drug development program, beginning as early as Phase 1; and |
| ● | FDA organizational commitment involving senior managers. |
Orphan Drug Designation. Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug candidate intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that costs of research and development of the drug for the indication can be recovered by sales of the drug in the United States. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Although there may be some increased communication opportunities, orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a drug candidate that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications, including a full NDA, to market the same drug for the same indication for seven years, except in very limited circumstances, such as if the second applicant demonstrates the clinical superiority of its product or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA application user fee.
As in the United States, designation as an orphan drug for the treatment of a specific indication in the European Union must be made before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including up to ten years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan designated product.
The inability to obtain or failure to maintain adequate product exclusivity for our product candidates could have a material adverse effect on our business prospects, results of operations and financial condition.
19
Other Healthcare Laws and Compliance Requirements
Our sales, promotion, medical education, clinical research, and other activities following product approval will be subject to regulation by numerous regulatory and law enforcement authorities in the United States in addition to the FDA, including potentially the Federal Trade Commission, the Department of Justice, the Centers for Medicare and Medicaid Services (“CMS”), the U.S. Department of Health and Human Services (“DHHS”) Office of Inspector General, and other divisions of DHHS, and state and local governments.
Our business and our relationships with customers, physicians, and third-party payors are and will continue to be subject, directly and indirectly, to federal and state healthcare fraud and abuse laws and regulations. These laws also apply to the physicians and third-party payors who will play a primary role in the recommendation and prescription of our product candidates, if they become commercially available products. These laws may constrain the business or financial arrangements and relationships through which we might market, sell and distribute our products and will impact, among other things, any proposed sales, marketing and educational programs. There are also laws, regulations and requirements applicable to the award and performance of federal grants and contracts. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to them, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, disgorgement, the reimbursement of overpayments, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs, imprisonment, contractual damages, reputational harm, and diminished profits and earnings—any of which could adversely affect our ability to operate our business and our financial results.
Restrictions under applicable federal and state healthcare related laws and regulations include but are not limited to the following:
| ● | the federal Anti-Kickback Statute; |
| ● | the civil federal False Claims Act; |
| ● | the criminal federal False Claims Act; |
| ● | the Health Insurance Portability and Accountability Act, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and its implementing regulations (collectively, “HIPAA”); |
| ● | the civil monetary penalties statute; |
| ● | federal transparency laws, including the federal Physician Sunshine Act (“PSA”); and |
| ● | analogous or similar state, federal, and foreign laws, regulations, and requirements. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations involve substantial costs. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other laws, regulations, or other requirements that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, restitution exclusion from government funded healthcare programs, corporate integrity agreements, deferred prosecution agreements, debarment from government contracts and grants and refusal of future orders under existing contracts, contractual damages, the curtailment or restructuring of our operations and other consequences. If any of the physicians or other healthcare providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, that person or entity may be subject to criminal, civil, or administrative sanctions, including exclusions from government funded healthcare programs. Moreover, availability of any federal grant funds which we may receive or for which we may apply is subject to federal appropriations law. Such grant funding may also be withdrawn or denied due to a violation of the above laws and/or for other reasons.
20
Coverage and Reimbursement; Healthcare Reform
Sales of pharmaceutical products depend significantly on the extent to which coverage and adequate reimbursement are provided by third-party payers. Third-party payers include state and federal government health care programs, managed care providers, private health insurers, and other organizations. Although we currently believe that third-party payers will provide coverage and reimbursement for our product candidates, if approved, we cannot be certain of this. Third-party payers are increasingly challenging the price, examining the cost-effectiveness and reducing reimbursement for medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. The United States government, state legislatures, and foreign governments have continued implementing healthcare reform and cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of generic products. Adoption of price controls and cost containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. We might need to conduct expensive clinical studies to demonstrate the comparative cost-effectiveness of our product candidates. Third-party payers might not consider the product candidates that we develop to be cost-effective and not cover or sufficiently reimburse for their use. It is time-consuming and expensive for us to seek coverage and reimbursement from third-party payers, as each payer will make its own determination as to whether to cover a product and at what level of reimbursement. Thus, one payer’s decision to provide coverage and adequate reimbursement for a product does not assure that another payer will provide coverage or that the reimbursement levels will be adequate. Moreover, a payer’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our product candidates to the extent we choose to develop or sell any product candidates outside of the United States. The approval process varies from country to country and the time may be longer or shorter than that required to obtain FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement, and privacy, can vary greatly from country to country.
Employees
As of the Effective Time of the Merger, we had four employees, all of whom are located in the United States. None of our employees is represented by a labor union or covered by a collective bargaining agreement. We consider our relationship with our employees to be good.
Property
The Company owns no real property. We maintain a month-to-month membership providing mail services and shared space for meetings and other business activities at 3540, Toringdon Way, Suite 200, #250, Charlotte, North Carolina. The current rent is approximately $100 per month.
Litigation
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
We are currently not aware of any pending legal proceedings to which we are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
Available Information
Our principal offices are located at 3540 Toringdon Way, Suite 200, #250, Charlotte, NC 28277 and our telephone number is (888) 609-1498. Our website is www.adaptinbio.com, and we can be contacted at info@adaptinbio.com. We are subject to the informational requirements of the Exchange Act and file or furnish reports, proxy statements, and other information with the SEC. Such reports and other information filed by us with the SEC will be available free of charge on our website at www.adaptinbio.com when such reports are available on the SEC’s website. The SEC maintains a website that contains reports, proxy and information statements, and other information that issuers file electronically with the SEC at www.sec.gov.
The contents of the websites referred to above are not incorporated into this filing. Further, our references to the URLs for these websites are intended to be inactive textual references only.
21
RISK FACTORS
This Report contains forward-looking statements which involve risks and uncertainties. Our actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including those set forth in the following risk factors.
Risk Factors Summary
Risks Related to Our Business and Operations
| ● | We have generated no revenue from commercial sales to date and our future profitability is uncertain. |
| ● | The report of our independent registered accounting firm expresses substantial doubt about our ability to continue as a going concern. |
| ● | We have limited access to the capital markets and even if we can raise additional funding, we may be required to do so on terms that are dilutive to our stockholders. |
| ● | If we fail to obtain the additional capital necessary to fund our operations, we will be unable to continue or complete our product development and our business will be substantially harmed. |
| ● | We may expand our business through the acquisition of rights to new drug candidates that could disrupt our business, harm our financial condition and may also dilute our stockholders’ ownership. |
| ● | If any future collaborations with third parties for the discovery, development and commercialization of our product candidates fail to lead to commercial products, and we may never receive milestone payments or future royalties under these agreements. |
| ● | The marketing approval process of the FDA is lengthy, time consuming and inherently unpredictable, and if we were ultimately unable to obtain marketing approval for the product candidates we intend to develop, our business will be substantially harmed. |
| ● | Our product candidates are in the early stages of development. |
| ● | We have relied and may in the future rely on third parties to conduct investigator-sponsored trials of our products, which is cost-effective but affords the investigators the ability to retain significant control over the design and conduct of the trials, and use of the data generated from their efforts. |
| ● | We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our products. |
| ● | Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities. |
| ● | Conducting successful clinical studies may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit. |
| ● | We currently rely significantly on third parties to conduct our nonclinical testing and clinical studies and other aspects of our development programs. If those third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our products. |
| ● | The results of our current or future clinical trials may not support our product candidate claims or may result in the discovery of unexpected adverse side effects. |
| ● | Even if approved, our product candidates and future products may never achieve market acceptance. |
| ● | Our revenue stream will depend upon third-party reimbursement. |
| ● | If a product candidate is approved and we are unable to establish satisfactory sales and marketing capabilities or secure a sales and marketing partner, we may not successfully commercialize our approved product candidates. |
| ● | We will rely on the availability of specific off-the-shelf components. |
| ● | If we market any of our products in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties. |
| ● | Our products will face significant competition in the markets for such products, and if they are unable to compete successfully, our business will suffer. |
| ● | After approval, if obtained, our products will remain subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional risk and expense, including the FDA withdrawing or modifying approval for our products and product recalls that could harm our reputation, business and financial results. |
22
| ● | We may be adversely affected by a failure or compromise from a cyberattack or data breach, which could have an adverse effect on our business. |
| ● | We may undertake international operations, which will subject us to risks inherent with operations outside of the United States. |
| ● | We may not be successful in hiring and retaining key employees. |
| ● | Managing our growth as we expand operations may strain our resources. |
Risks Related to Our Intellectual Property
| ● | We could lose our license rights to our important intellectual property if we do not fulfill our contractual obligations to our current and future licensors. |
| ● | Our ability to protect and enforce any patents we may obtain does not guaranty that we will secure the right to commercialize such patents. |
| ● | If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish, and our business and competitive position would suffer. |
| ● | We rely on confidentiality agreements to protect our trade secrets. If these agreements are breached by our employees or other parties, our trade secrets may become known to our competitors. |
| ● | If we or our third-party suppliers are claimed to or found to be infringing on patents or trade secrets owned by others, we may be forced to cease or alter our product development efforts, obtain a license to continue the development or sale of our products, and/or pay damages. |
Risks Related to Ownership of our Common Stock
| ● | If we are unable to register in a timely manner the shares of Common Stock issued to stockholders in the Merger or the Offering, we may be subject to certain liquidated damages. |
| ● | There is currently no market for our Common Stock and there can be no assurance that any market will ever develop and our Common Stock may not be eligible for listing or quotation on any securities exchange or over-the-counter trading system. |
| ● | The market price and trading volume of our Common Stock may be volatile and could decline significantly. |
| ● | The designation of our Common Stock as “penny stock” or FINRA sales practice requirements may limit the liquidity of our Common Stock. |
| ● | Because we do not intend to pay dividends, stockholders will benefit from an investment in our Common Stock only if it appreciates in value. |
| ● | Because we became a reporting company under the Exchange Act by means other than a traditional underwritten initial public offering, we may not be able to attract the attention of research analysts at major brokerage firms. |
| ● | Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management. |
| ● | The issuance of our preferred stock could adversely affect the holders of our Common Stock in some circumstances. |
| ● | We are subject to the reporting requirements of federal securities laws, which can be expensive and may divert resources from other projects, thus impairing our ability to grow. |
| ● | Our principal stockholders will have significant influence over the election of our board of directors and approval of any significant corporate actions, including any sale of the Company. |
23
Risks Related to Our Business and Operations
We have generated no revenue from commercial sales to date and our future profitability is uncertain.
Private Adaptin was incorporated as Centaur Bio Inc., a Delaware corporation in 2021. The combined Company has a limited operating history and our business is subject to all of the risks inherent in the establishment of a new business enterprise, which make our prospects hard to evaluate. Any evaluation of our business and our prospects must be considered in light of the uncertainties, problems, expenses, difficulties, complications and delays frequently encountered in connection with development and expansion of a new business enterprise. Since inception, we have incurred losses and expect to continue to operate at a net loss for at least the next several years as we commence our research and development efforts, conduct clinical trials and develop manufacturing, sales, marketing and distribution capabilities. There can be no assurance that the products under development by us will be approved for sale in the United States or elsewhere. Furthermore, there can be no assurance that if such products are approved, they will be successfully commercialized, and the extent of our future losses and the timing of our profitability are highly uncertain. Many of these factors are beyond the control of our management. If we are unable to achieve profitability, we may be unable to continue our operations.
The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern.
Our auditor, KNAV CPA LLP, has indicated in their report on our financial statements for the fiscal year ended December 31, 2023, that conditions exist that raise substantial doubt about our ability to continue as a going concern due to our recurring losses from operations and significant accumulated deficit. In addition, we continue to experience negative cash flows from operations. A “going concern” opinion could impair our ability to finance our operations through the sale of equity. Our ability to continue as a going concern will depend upon the availability of equity financing which represents the primary source of cash flows that will permit us to meet our financial obligations as they come due and continue our research and development efforts.
We have limited access to the capital markets and even if we can raise additional funding, we may be required to do so on terms that are dilutive to our stockholders.
We have limited access to the capital markets to raise capital. The capital markets have been unpredictable in the recent past for unprofitable companies such as ours. In addition, it is generally difficult for development stage companies to raise capital under current market conditions. The amount of capital that a company such as ours is able to raise often depends on variables in market conditions that are beyond our control. As a result, we may not be able to secure financing on terms attractive to us, or at all. If we are able to consummate a financing arrangement, the amount raised may not be sufficient to meet our future needs. If adequate funds are not available on acceptable terms, or at all, our business, including our results of operations, financial condition and our continued viability will be materially adversely affected. If we are able to secure future financing, it may be done on terms that are potentially dilutive to our stockholders.
If we fail to obtain the additional capital necessary to fund our operations, we will be unable to continue or complete our product development and our business will be substantially harmed.
The net proceeds from our Offering are not sufficient to capitalize the development and commercialization of the product candidates we intend to develop and we will need to continue to seek capital from time to time to continue development of the BRiTE platform beyond the initial Phase 1 clinical trials and to acquire and develop other product candidates. Our first product is not expected to be commercialized until at least 2028, if at all, and we cannot provide any assurances that any revenues it may generate in the future will be sufficient to fund our ongoing operations.
We believe the Company can fund its operations through the planned Phase 1 clinical trial and into 2026 with net proceeds from the Offering, if we raise the Maximum Offering amount of approximately $8.5 million. As of the date of this Report, we have raised approximately $4.8 million in the Offering. Accordingly, we believe that we will need to raise substantial additional capital to fund our continuing operations and the development and commercialization of our product candidates in or before 2026. Our business or operations may change in a manner that would consume available funds more rapidly than anticipated and substantial additional funding may be required to maintain operations, fund expansion, develop new or enhanced products, acquire complementary products, business or technologies or otherwise respond to competitive pressures and opportunities, such as a change in the regulatory environment or a change in the global healthcare environment. In addition, if our product candidates are commercialized, we may need to accelerate the growth of our sales capabilities and distribution beyond what is currently envisioned, and this would require additional capital. However, we may not be able to secure funding when we need it or on acceptable terms. We may not be able to raise sufficient funds to commercialize the product candidates we intend to develop.
24
If we cannot raise adequate funds to satisfy our capital requirements, we will have to delay, scale back or eliminate our research and development activities, clinical studies or future operations. We may also be required to obtain funds through arrangements with collaborators, which arrangements may require us to relinquish rights to certain products that we otherwise would not consider relinquishing, including rights to future product candidates or certain major geographic markets. This could result in sharing revenues that we might otherwise retain for ourselves. Any of these actions may harm our business, financial condition and results of operations. If adequate funds cannot be secured to sustain our business operations, our board of directors may decide that it is in the best interest of our stockholders to dissolve our Company and liquidate our assets.
The amount of capital we may need depends on many factors, including the progress, timing and scope of our product development programs; the progress, timing and scope of our preclinical studies and clinical trials; the time and cost necessary to obtain regulatory approvals; the time and cost necessary to further develop manufacturing processes and arrange for contract manufacturing; our ability to enter into and maintain collaborative, licensing and other commercial relationships; and our partners’ commitment of time and resources to the development and commercialization of our products.
We may expand our business through the acquisition of rights to new drug candidates that could disrupt our business, harm our financial condition and may also dilute current stockholders’ ownership interests in us.
Our business strategy includes expanding our products and capabilities, and we may seek acquisitions of drug candidates or technologies to do so. Acquisitions involve numerous risks, including substantial cash expenditures; potentially dilutive issuance of equity securities; incurrence of debt and contingent liabilities, some of which may be difficult or impossible to identify at the time of acquisition; difficulties in assimilating the acquired technologies or the operations of the acquired companies; diverting our management’s attention away from other business concerns; risks of entering markets in which we have limited or no direct experience; and the potential loss of our key employees or key employees of the acquired companies.
We cannot assure you that any acquisition will result in short-term or long-term benefits to us. We may misjudge the value or worth of an acquired product, company or business. In addition, our future success would depend in part on our ability to manage the rapid growth associated with acquisitions. We cannot assure you that we will be able to make the combination of our business with that of acquired products, businesses or companies work or be successful. Acquisitions and the subsequent integration of new assets, businesses, key personnel, customers, vendors, and suppliers require significant attention from our management and could result in a diversion of resources from our existing business, which in turn could have an adverse effect on our operations. Furthermore, the development or expansion of our business or any acquired products, business or companies may require a substantial capital investment by us. We may not have these necessary funds, or they might not be available to us on acceptable terms or at all. We may also seek to raise funds by selling shares of our preferred or Common Stock, which could dilute each current stockholder’s ownership interest in us.
We may, in the future, seek to enter into collaborations with third parties for the discovery, development and commercialization of our product candidates. If our collaborators cease development efforts under our collaboration agreements, or if any of those agreements are terminated, these collaborations may fail to lead to commercial products, and we may never receive milestone payments or future royalties under these agreements.
We may in the future seek to enter into agreements with other third-party collaborators for research, development and commercialization of other therapeutic technologies or product candidates. Biopharmaceutical companies are our likely future collaborators for any marketing, distribution, development, licensing or broader collaboration arrangements. If we fail to enter into future collaborations on commercially reasonable terms, or at all, or such collaborations are not successful, we may not be able to execute our strategy to develop our product candidates or therapies that we believe could benefit from the resources of either larger biopharmaceutical companies or those specialized in a particular area of relevance.
25
With our existing Duke License and with any future collaboration agreements, we have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Moreover, our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. Collaborations involving our product candidates pose the following risks to us:
| ● | collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations; |
| ● | collaborators may not pursue development and commercialization of our product candidates or may elect not to continue or renew development or commercialization programs based on preclinical studies or clinical trial results, changes in the collaborators’ strategic focus or available funding, or external factors such as an acquisition that diverts resources or creates competing priorities; |
| ● | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| ● | collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than ours; |
| ● | collaborators with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and distribution of such product or products; |
| ● | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to litigation or potential liability; |
| ● | collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; |
| ● | disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and |
| ● | collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates. |
As a result of the foregoing, our current and any future collaboration agreements may not lead to development or commercialization of our product candidates in the most efficient manner or at all. If a collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated. Any failure to successfully develop or commercialize our product candidates pursuant to our current or any future collaboration agreements could have a material and adverse effect on our business, financial condition, results of operations and prospects.
Moreover, to the extent that any of our existing or future collaborators were to terminate a collaboration agreement, we may be forced to independently develop these product candidates, including funding preclinical studies or clinical trials, assuming marketing and distribution costs and defending intellectual property rights, or, in certain instances, abandon product candidates altogether, any of which could result in a change to our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
26
The marketing approval process of the FDA is lengthy, time consuming and inherently unpredictable, and if we were ultimately unable to obtain marketing approval for the product candidates we intend to develop, our business will be substantially harmed.
We cannot commercialize our product candidates in the United States without first obtaining approval from the FDA to market each product candidate. None of the product candidates we intend to develop have gained marketing approval in the United States and we cannot guarantee that we will gain the required marketing approval. Our business is substantially dependent on our ability to complete the development of, obtain marketing approval for, and successfully commercialize our product candidates in a timely manner. Our product candidates could fail to receive marketing approval for many reasons, including among others:
| ● | The FDA may disagree with the design or implementation of our clinical trials; |
| ● | Our clinical trials may not produce results demonstrating that our products are safe and effective; and |
| ● | The FDA could determine that our manufacturing processes and procedures are not sufficient to support approval. |
In addition, the process of seeking regulatory clearance or approval to market the product candidates we intend to develop is expensive and time consuming and, notwithstanding the effort and expense incurred, clearance or approval is never guaranteed. If we are not successful in obtaining timely clearance or approval of our products from the FDA, we may never be able to generate any revenue and may be forced to cease operations. The NDA process is costly, lengthy and uncertain. Any NDA application filed by the Company will have to be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the product for its intended use.
Obtaining clearances or approvals from the FDA and from the regulatory agencies in other countries could result in unexpected and significant costs for us and consume management’s time and other resources. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our product candidates under development. The FDA and other agencies could ask us to supplement our submissions, collect non-clinical data, conduct additional clinical trials or engage in other time-consuming actions, or simply deny our applications. In addition, even if we obtain an NDA approval or pre-market approvals in other countries, the approval could be revoked or other restrictions imposed if post-marketing studies and surveillance demonstrates safety issues or lack of effectiveness after commercialization. We cannot predict with certainty how, or when, the FDA will act. If we are unable to obtain the necessary regulatory approvals, our financial condition and cash flow may be adversely affected, and our ability to grow domestically and internationally may be limited. Additionally, even if cleared or approved, the Company’s products may not be approved for the specific indications that are most necessary or desirable for successful commercialization or profitability.
Our product candidates are in the early stages of development.
Extensive further laboratory and specific clinical testing will be required prior to regulatory approval of any of our product candidates. Adverse or inconclusive results from preclinical testing or clinical trials of our product candidates may substantially delay, or halt entirely, any further development of one or more of our products.
We have relied and may in the future rely on third parties to conduct investigator-sponsored trials (“ISTs”) of our products, which is cost-effective for us but affords the investigators the ability to retain significant control over the design and conduct of the trials, as well as the use of the data generated from their efforts.
We have relied and may in the future rely on third parties to conduct and sponsor clinical trials. Such ISTs may provide us with valuable clinical data that can inform our future development strategy in a cost-efficient manner, but we do not control the design or conduct of the ISTs, and it is possible that the FDA or non-United States regulatory authorities will not view these ISTs as providing adequate support for future clinical trials, whether controlled by us or third parties, for any one or more reasons, including elements of the design or execution of the trials or safety concerns or other trial results.
27
These arrangements provide us limited information rights with respect to the ISTs, including access to and the ability to use and reference the data, including for our own regulatory filings, resulting from the ISTs. However, we would not have control over the timing and reporting of the data from ISTs, nor would we own the data from the ISTs. If we are unable to confirm or replicate the results from the ISTs or if negative results are obtained, we would likely be further delayed or prevented from advancing further clinical development. Further, if investigators or institutions breach their obligations with respect to the clinical development of our product candidates, or if the data proves to be inadequate compared to the first-hand knowledge we might have gained had the ISTs been sponsored and conducted by us, then our ability to design and conduct any future clinical trials ourselves may be adversely affected.
Clinical trials necessary to support NDA approval of our product candidates will be time-consuming and expensive. Delays or failures in our clinical trials will prevent us from commercializing our products and will adversely affect our business, operating results and prospects and could cause us to cease operations.
Initiating and completing clinical trials necessary to support NDA approval of our product candidates and other new products will be time consuming and expensive, and the outcome uncertain. Moreover, the results of early clinical trials are not necessarily predictive of future results, and any product we advance into clinical trials may not have favorable results in later clinical trials.
Each modification to the protocol during a clinical trial has to be submitted to the FDA. This could result in the delay or halt of a clinical trial while the modification is evaluated. In addition, depending on the quantity and nature of the changes made, the FDA could take the position that the data generated by the clinical trial is not poolable because the same protocol was not used throughout the trial. This might require the enrollment of additional subjects, which could result in the extension of the clinical trial and the FDA delaying clearance or approval of a product. Any such delay could have a material adverse effect on our business and results of operations. Serious injury or death resulting from a failure of one of our drug candidates during current or future clinical trials could also result in the FDA delaying our clinical trials or denying or delaying clearance or approval of a product.
Even though an adverse event may not be the result of the failure of our drug candidate, the FDA or an IRB could delay or halt a clinical trial for an indefinite period of time while an adverse event is reviewed, and likely would do so in the event of multiple such events.
Any delay or termination of our current or future clinical trials as a result of the risks summarized above, including delays in obtaining or maintaining required approvals from IRBs, delays in patient enrollment, the failure of patients to continue to participate in a clinical trial, and delays or termination of clinical trials as a result of protocol modifications or adverse events during the trials, may cause an increase in costs and delays in the filing of any product submissions with the FDA, delay the approval and commercialization of our products or result in the failure of the clinical trial, which could adversely affect our business, operating results and prospects.
Modifications to our products may require new NDA approvals.
Once a particular product receives FDA approval or clearance, expanded uses or uses in new indications of our products may require additional human clinical trials and new regulatory approvals or clearances, including additional IND and NDA submissions and premarket approvals before we can begin clinical development, and/or prior to marketing and sales. If the FDA requires new clearances or approvals for a particular use or indication, we may be required to conduct additional clinical studies, which would require additional expenditures and harm our operating results. If the products are already being used for these new indications, we may also be subject to significant enforcement actions. Conducting clinical trials and obtaining clearances and approvals can be a time-consuming process, and delays in obtaining required future clearances or approvals could adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
28
We are subject to a multitude of manufacturing risks, any of which could substantially increase our costs and limit supply of our products.
We are, and will for the foreseeable future continue to be, wholly dependent on third party CMOs for the timely supply of adequate quantities of our products which meet or exceed requisite quality and production standards for use in clinical and nonclinical studies. Given the extensive risks, scope, complexity, cost, regulatory requirements and commitment of resources associated with developing the capabilities to manufacture one or more of our products, we have no present plan or intention of developing in-house manufacturing capabilities for nonclinical, clinical or commercial scale production, beyond our current supervision and management of our third-party contract manufacturers. In addition, in order to balance risk and conserve financial and human resources, we have and may continue from time to time to defer commitment to production of product, which could result in delays to the continued progress of our clinical and nonclinical testing.
Currently, we do not have alternative CMOs to back up our primary CMOs. Identification of and discussions with other CMOs may be protracted and/or unsuccessful, or these new CMOs may be unsuccessful in producing the same results as the current primary CMOs producing the material. Therefore, if our primary CMOs become unable or unwilling to perform their required activities, we could experience protracted delays or interruptions in the supply of clinical trial material and, ultimately, product for commercial sale, which would materially and adversely affect our development programs, commercial activities, operating results and financial condition. In addition, the FDA or regulatory authorities outside of the United States may require us to have an alternate manufacturer before approving any product candidate for marketing and sale in the United States or abroad and securing such alternate manufacturer, if possible, could result in considerable additional time and cost prior to approval.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of pharmaceutical products often encounter difficulties in production, particularly in scaling-up initial production. These problems include difficulties with production costs and yields, quality control, including stability of the product candidate and quality assurance testing and shortages of qualified personnel. Our product candidates have not been manufactured at the scale we believe will be necessary to maximize their commercial value, and accordingly, we may encounter difficulties in attempting to scale-up production and may not succeed in that effort on a timely basis or at all. In addition to the foregoing, the process of manufacturing our products is complex, highly regulated and subject to several risks, including but not limited to the following:
| ● | We, and our CMOs, must comply with the FDA’s cGMP, regulations and guidance. We, and our CMOs, may encounter difficulties in achieving quality control and quality assurance and may experience shortages in qualified personnel. We, and our CMOs, are subject to inspections by the FDA and comparable agencies in other jurisdictions to confirm compliance with applicable regulatory requirements. Any failure to follow cGMP or other regulatory requirements or any delay, interruption or other issues that arise in the manufacture, fill-finish, labeling, packaging, or storage of our products as a result of a failure of our facilities or the facilities or operations of third parties to comply with regulatory requirements, or a failure to pass any regulatory authority inspection, could significantly impair our ability to develop and commercialize our products, including leading to significant delays in the availability of products for our clinical studies or the termination or hold on a clinical study, or the delay or prevention of a filing or approval of marketing applications for our product candidates. Significant noncompliance could also result in the imposition of sanctions, including injunctions, civil penalties, failure of regulatory authorities to grant marketing approvals for our product candidates, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions, adverse publicity, and criminal prosecutions, any of which could damage our reputation. If we are not able to maintain regulatory compliance, we may not be permitted to market our products and/or may be subject to product recalls, seizures, injunctions, or criminal prosecution. Any adverse developments affecting manufacturing operations for our products or our CMOs generally, may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of our products. Once our product candidates are approved, we may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly manufacturing alternatives. |
| ● | The manufacturing facilities in which our products are made could be adversely affected by equipment failures, plant closures, capacity constraints, competing customer priorities or changes in corporate strategy or priorities, process changes or failures, changes in business models or operations, materials or labor shortages, natural disasters, power failures and numerous other factors. |
29
| ● | We are wholly dependent upon CMOs for the timely supply of adequate quantities of requisite quality product for our nonclinical, clinical and, if approved by regulatory authorities, commercial scale production. |
| ● | The process of manufacturing biologics is extremely susceptible to product loss due to contamination, equipment failure or improper installation or operation of equipment, or vendor or operator error. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects and other supply disruptions. If microbial, viral or other contaminations are discovered in our products or in the manufacturing facilities in which our products are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. |
We rely completely on third parties, most of which are sole source suppliers, to supply drug substance and manufacture drug product for our clinical trials and preclinical studies and intend to rely on other third parties to produce commercial supplies of product candidates, and our dependence on third parties could adversely impact our business.
We are completely dependent on third-party suppliers, most of which are sole source suppliers of the drug substance and drug product for our product candidates. We regularly evaluate potential alternate sources of supply but there can be no assurance that any such suppliers would be available, acceptable or successful. The costs of manufacturing our drug candidates are high, and we will require additional capital to ensure that we can maintain an adequate supply to conduct our contemplated development programs.
If our third-party suppliers do not supply sufficient quantities for product candidates to us on a timely basis and in accordance with applicable specifications and other regulatory requirements, there could be a significant interruption of our supplies, which would adversely affect clinical development of the product candidate, including affecting our ability to enroll in and timely progress clinical trials. Furthermore, if any of our CMOs cannot successfully manufacture material that conforms to our specifications and with regulatory requirements, we will not be able to secure and/or maintain regulatory approval for our product candidates.
We will also rely on our CMOs to purchase from third-party suppliers the materials necessary to produce our product candidates for our anticipated clinical trials. There are a small number of suppliers for certain capital equipment and raw materials used to manufacture our product candidates. We do not have any control over the process or timing of the acquisition of these raw materials by our contract manufacturers. Moreover, we currently do not have agreements in place for the commercial production of these raw materials. Any significant delay in the supply of a product candidate or the raw material components thereof for an ongoing clinical trial could considerably delay completion of that clinical trial, product candidate testing, and potential regulatory approval of that product candidate.
We do not expect to have the resources or capacity to commercially manufacture any of our proposed product candidates if approved and will likely continue to be dependent on CMOs. Our dependence on third parties to manufacture and supply us with clinical trial materials and any approved product candidates may adversely affect our ability to develop and commercialize our product candidates on a timely basis.
Governments may impose price controls, which may adversely affect our future profitability.
We intend to seek approval to market our future product candidates in the United States and potentially in foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions, we will be subject to rules and regulations in those jurisdictions relating to our product candidates. In some foreign countries, particularly in the European Union, the pricing of prescription pharmaceuticals and biologics is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product candidate. If reimbursement of our future products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability. We face potential product liability exposure and, if successful claims are brought against us, we may incur substantial liability for a product candidate and may have to limit its commercialization.
30
The use of our product candidates in clinical trials and the sale of any product candidates for which we may obtain marketing approval expose us to the risk of product liability claims. Product liability claims may be brought against us or any future development partners by participants enrolled in our clinical trials, patients, health care providers, or others using, administering, or selling our product candidates. If we cannot successfully defend ourselves against any such claims, or have insufficient insurance protection, we would incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in:
| ● | withdrawal of clinical trial participants; |
| ● | termination of clinical trial sites or entire trial programs; |
| ● | costs of related litigation; |
| ● | substantial monetary awards to trial participants or other claimants; |
| ● | decreased demand for our product candidates and loss of revenue; |
| ● | impairment of our business reputation; |
| ● | diversion of management and scientific resources from our business operations; and |
| ● | the inability to commercialize our product candidates. |
In the future, we anticipate that we will need to obtain additional or increased product liability insurance coverage and we are uncertain whether such increased or additional insurance coverage can be obtained on commercially reasonable terms, if at all.
We have obtained limited product liability insurance coverage for our clinical trials domestically and in selected foreign countries where we are conducting clinical trials. As such, our insurance coverage may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive and in the future we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. We intend to expand our insurance coverage for product candidates to include the sale of commercial products if we obtain marketing approval for our product candidates in development; however, we may be unable to obtain commercially reasonable product liability insurance for any product candidates approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our working capital and adversely affect our business.
Our insurance policies are expensive and protect us only from some business risks, which leaves us exposed to significant uninsured liabilities.
We do not carry insurance for the some of the categories of risk that our business may encounter, although we do intend to obtain customary insurance coverage. No assurance can be given that we will be able to obtain and/or maintain insurance with adequate levels of coverage. Any significant, uninsured liability may require us to pay substantial amounts, which would adversely affect our working capital and results of operations.
Conducting successful clinical studies may require the enrollment of large numbers of patients, and suitable patients may be difficult to identify and recruit.
We may not be able to commence or continue clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. Patient enrollment in clinical trials and completion of patient participation and follow-up depends on many factors, including the size of the patient population; the nature of the trial protocol; the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects; the availability of appropriate clinical trial investigators; support staff; and proximity of patients to clinical sites and ability to comply with the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of our products or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competitive products that treat the same indications.
31
We currently rely significantly on third parties to conduct our nonclinical testing and clinical studies and other aspects of our development programs. If those third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for or commercialize our products.
We do not have the ability to independently conduct our pre-clinical and clinical trials for our products and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories and others to assist in the design and conduct of non-clinical and clinical studies of our product candidates, with interpretation of the results of those studies and with regulatory activities and expect to continue to outsource all or a significant amount of such activities. As a result, many important aspects of our development programs are and will continue to be outside our direct control and our third parties may not perform their activities as required or expected, including the maintenance of Good Laboratory Practices or Good Clinical Practices compliance, which are ultimately our responsibility to ensure. Further, such third parties may not be as committed to the success of our programs as our own employees and, therefore, may not devote the same time, thoughtfulness or creativity to completing projects or problem-solving as our own employees would. To the extent we are unable to successfully manage the performance of our third parties, our business may be adversely affected. If these third parties do not successfully carry out their contractual duties or regulatory obligations, meet expected deadlines or need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize, our products on a timely basis, if at all. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control. The occurrence of any of the foregoing may adversely affect our business, operating results and prospects.
The results of our current or future clinical trials may not support our product candidate claims or may result in the discovery of unexpected adverse side effects.
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our drug candidate claims or that the FDA or foreign authorities will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our drug candidates are safe and effective for the proposed indicated uses. If the FDA concludes that the clinical trials for our product candidates, or any other product for which we might seek clearance, has failed to demonstrate safety and effectiveness, we will not receive FDA clearance to market that product in the United States for the indications sought.
In addition, such an outcome could cause us to abandon the product candidate and might delay development of others. Any delay or termination of our clinical trials will delay the filing of any product submissions with the FDA and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile. In addition, our clinical trials for our product candidates may involve a relatively small patient population in some cases. Because of the small sample size, our results may not be indicative of future results.
Even if approved, our product candidates and future products may never achieve market acceptance.
Even if approved, our product candidates and future products that we may develop may never gain market acceptance among physicians, patients, third-party payers and the medical community. The degree of market acceptance of any of our products will depend on a number of factors, including the actual and perceived effectiveness and reliability of our products; the results of any long-term clinical trials relating to use of our products; our ability to convert patients from our clinical trials into users of our commercial products, once approved; the availability, relative cost and perceived advantages and disadvantages of alternative technologies; the degree to which treatments using our products are approved for reimbursement by public and private insurers; the willingness of patients to pay out of pocket in the absence of government or third-party coverage; the strength of our marketing and distribution infrastructure; the level of education and awareness among physicians and hospitals concerning our products; and prevalence and severity of any side effects. In addition, our efforts to educate the medical community and third-party payers regarding the benefits of our products may require significant resources and may never be successful. If approved, the failure of our product candidates resulting from the BRiTE platform or any of our other products to significantly penetrate current or new markets would negatively impact our business, financial condition and results of operations.
32
To be commercially successful, physicians must be persuaded that using our products for treatment of cancer, infectious diseases and other diseases are effective alternatives to existing therapies and treatments.
We believe that physicians will not widely adopt our products unless they determine, based on experience, clinical data, and published peer-reviewed journal articles, that the use of our products provides an effective alternative to other means of treating our target indications or any other disease that our products are approved to treat. Patient studies or clinical experience may indicate that treatment with our products does not provide patients with sufficient benefits in quality of life. We believe that recommendations and support for the use of our products from influential physicians will be essential for widespread market acceptance. Our products are still in the preclinical development stage and it is premature to attempt to gain support from physicians at this time. We can provide no assurance that such support will ever be obtained. If our products do not receive such support from these physicians and from long-term data, physicians may not use or continue to use, and hospitals may not purchase or continue to purchase, our products.
Even if our products are approved by regulatory authorities, if we, our CMOs or our suppliers fail to comply with ongoing FDA regulation or if we experience unanticipated problems with our products, these products could be subject to restrictions or withdrawal from the market.
Any product for which we obtain clearance or approval, and the manufacturing processes, reporting requirements, post-approval clinical data and promotional activities for such product, will be subject to continued regulatory review, oversight and periodic inspections by the FDA. In particular, we, our CMOs and our suppliers are required to comply with the FDA’s cGMP regulations for the manufacture of our products and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval. Regulatory bodies, such as the FDA, enforce these regulations through periodic inspections. The failure by us, our CMOs or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, enforcement actions by the FDA.
If any of these actions were to occur it would harm our reputation and cause our product sales and profitability to suffer and may prevent us from generating revenue. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements, which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Even if regulatory clearance or approval of a product is granted, such clearance or approval may be subject to limitations on the intended uses for which the product may be marketed and reduce the potential to successfully commercialize the product and generate revenue from the product. If the FDA determines that the product promotional materials, labeling, training or other marketing or educational activities constitute promotion of an unapproved use, it could request that we or our commercialization partners cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider such training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with adverse event and pharmacovigilance reporting requirements, including the reporting of adverse events which occur in connection with, and whether or not directly related to, our products. For example, the FDA may approve the NDA with a REMS plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries, and other risk minimization tools. Later discovery of previously unknown problems with our products, including unanticipated adverse events or adverse events of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to recall, replace or refund the cost of any product we manufacture or distribute, fines, suspension of regulatory approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
33
Our revenue stream will depend upon third-party reimbursement.
The commercial success of our products in both domestic and international markets will be substantially dependent on whether third-party coverage and reimbursement is available for patients that use our products. However, the availability of insurance coverage and reimbursement for newly approved therapies is uncertain, and therefore, third-party coverage may be particularly difficult to obtain even if our products are approved by the FDA as safe and efficacious. Patients using existing approved therapies are generally reimbursed all or part of the product cost by Medicare or other third-party payors. Medicare, Medicaid, health maintenance organizations and other third-party payors are increasingly challenging the prices and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy, and increasingly attempting to contain healthcare costs by limiting both coverage and the level of reimbursement of new drugs, and, as a result, they may not cover or provide adequate payment for these products.
Significant uncertainty exists as to the reimbursement status for newly approved drug products, including coding, coverage and payment. There is no uniform policy requirement for coverage and reimbursement for drug products among third-party payers in the United States; therefore coverage and reimbursement for drug products can differ significantly from payer to payer. The coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payer separately, with no assurance that coverage and adequate payment will be applied consistently or obtained. The process for determining whether a payer will cover and how much it will reimburse a product may be separate from the process of seeking approval of the product or for setting the price of the product. Even if reimbursement is provided, market acceptance of our products may be adversely affected if the amount of payment for our products proves to be unprofitable for healthcare providers or less profitable than alternative treatments or if administrative burdens make our products less desirable to use. Third-party payer reimbursement to providers of our products, if approved, may be subject to a bundled payment that also includes the procedure of administering our products or third-party payers may require providers to perform additional patient testing to justify the use of our products. To the extent there is no separate payment for our products, there may be further uncertainty as to the adequacy of reimbursement amounts.
The containment of healthcare costs is a priority of federal, state and foreign governments and the prices of drug products have been a focus in this effort. The continuing efforts of government, private insurance companies and other organizations to contain or reduce costs of healthcare may adversely affect our ability to set as high a price for our products as we might otherwise and the rate and scope of adoption of our products by healthcare providers. We expect that federal, state and local governments in the United States, as well as governments in other countries, will continue to consider legislation directed at lowering the total cost of healthcare. In addition, in certain foreign markets, the pricing of drug products is subject to government control and reimbursement may in some cases be unavailable or insufficient. It is uncertain whether and how future legislation, whether domestic or abroad, could affect prospects for our product candidates or what actions governmental or private payers for healthcare treatment and services may take in response to any such healthcare reform proposals or legislation. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, may prevent or limit our ability to generate revenue, attain profitability or commercialize our product candidates.
These potential courses of action are unpredictable and the potential impact of new legislation on our operations and financial position is uncertain, but may result in more rigorous coverage criteria, lower reimbursement and additional downward pressure on the price we may receive for an approved product. Any reduction in reimbursement from Medicare or other government-funded programs may result in a similar reduction in payments from private payers. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products, if approved.
34
We currently have no sales and marketing organization. If a product candidate is approved and we are unable to establish satisfactory sales and marketing capabilities and/or secure a sales and marketing partner, we may not successfully commercialize our approved product candidates.
We do not have experience in selling or marketing. To commercialize our products, if approved, in the United States and other jurisdictions in which we may seek approvals, we must build our marketing, sales, managerial and other non-technical capabilities or make arrangements with third parties to perform these services and we may not be successful in doing so. Despite the experience of individual members of management, we have limited experience as a company in the marketing and sale of pharmaceutical products and there are significant risks involved in building and managing a sales organization, including our ability to hire, retain and incentivize qualified individuals, generate sufficient sales leads, provide adequate training to sales and marketing personnel and effectively manage a geographically dispersed sales and marketing team.
In addition, we may not be able to enter into collaboration agreements with sales and marketing partners on terms acceptable to us or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. If we elect to establish a sales and marketing infrastructure, we may not realize a positive return on this investment. In addition, we will have to compete with established and well-funded pharmaceutical and biotech companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to commercialize product candidates without strategic partners or licensees include:
| ● | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| ● | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to use our products; |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| ● | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
We will rely on the availability of specific off-the-shelf components.
We intend to use in part, off-the-shelf components in our product candidates. We may also rely on additional off- the-shelf components for our product candidates or other products. If the supply of any specific off-the-shelf components for our products is terminated due to (i) failure to obtain regulatory approval for the use of such off- the-shelf component, (ii) market demand or (iii) by choice of the manufacturer, then our clinical trials, production, and ultimately, revenue, could be negatively impacted. In addition, our resources would need to be diverted to locate a replacement component, which must then be rigorously tested and qualified. If we fail to have the ability to seamlessly switch from a non-available component to a replacement, then our business, financial condition and results of operations would be materially harmed.
We may have conflicts with our partners that could delay or prevent the development or commercialization of our product candidates.
We may have conflicts with our partners, such as conflicts concerning the interpretation of preclinical or clinical data, the achievement of milestones, the interpretation of contractual obligations, payments for services, development obligations or the ownership of intellectual property developed during our collaboration. If any conflicts arise with any of our partners, such partner may act in a manner that is adverse to our best interests. Any such disagreement could result in one or more of the following, each of which could delay or prevent the development or commercialization of our product candidates, and in turn prevent us from generating revenues: unwillingness on the part of a partner to pay us milestone payments or royalties we believe are due to us under a collaboration; uncertainty regarding ownership of intellectual property rights arising from our collaborative activities, which could prevent us from entering into additional collaborations; unwillingness by the partner to cooperate in the development or manufacture of the product, including providing us with product data or materials; unwillingness on the part of a partner to keep us informed regarding the progress of its development and commercialization activities or to permit public disclosure of the results of those activities; initiating of litigation or alternative dispute resolution options by either party to resolve the dispute; and attempts by either party to terminate the agreement.
35
If we market any of our products in a manner that violates healthcare fraud and abuse laws, or if we violate government price reporting laws, we may be subject to civil or criminal penalties and significant civil liability.
The FDA and other government authorities enforce laws and regulations that require that the promotion of pharmaceutical products be consistent with the approved prescribing information. While physicians may prescribe an approved product for a so-called “off-label” use, it is unlawful for a pharmaceutical company to promote its products in a manner that is inconsistent with its approved label and any company which engages in such conduct may be subject to significant liability. Similarly, industry codes in the European Union and other foreign jurisdictions prohibit companies from engaging in off-label promotion and regulatory agencies in various countries enforce violations of the code with civil penalties. While we intend to ensure that our promotional materials are consistent with our label, regulatory agencies may disagree with our assessment and may issue untitled letters, warning letters or may institute other civil or criminal enforcement proceedings. In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws include the U.S. Federal Healthcare Program Anti-Kickback Statute, U.S. False Claims Act and similar state laws. Because of the breadth of these laws and the narrowness of the safe harbors, it is possible that some of our business activities could be subject to challenge under one or more of these laws.
The U.S. Federal Healthcare Program Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted broadly to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are several statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not, in all cases, meet all of the criteria for safe harbor protection from anti-kickback liability. Moreover, recent health care reform legislation has strengthened these laws. For example, the Affordable Care Act, among other things, amends the intent requirement of the U.S. Federal Healthcare Program Anti-Kickback Statute and criminal health care fraud statutes; a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the U.S. Federal Healthcare Program Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the U.S. False Claims Act. Federal false claims laws, including the U.S. False Claims Act, impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government.
Over the past few years, pharmaceutical and other healthcare companies have been prosecuted under these laws for a variety of alleged promotional and marketing activities, such as: allegedly providing free trips, free goods, sham consulting fees and grants and other monetary benefits to prescribers; reporting to pricing services inflated average wholesale prices that were then used by federal programs to set reimbursement rates; engaging in off-label promotion that caused claims to be submitted to Medicare or Medicaid for non-covered, off-label uses; using a charity as an illegal conduit to cover the copays of Medicare patients; and submitting inflated best price information to the Medicaid Drug Rebate Program to reduce liability for Medicaid rebates.
Other restrictions under applicable United States federal and state healthcare laws and regulations may include the following:
| ● | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for, among other things, knowingly and willfully executing or attempting to execute a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
36
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health, or HITECH Act, and its implementing regulations, also imposes obligations, including mandatory contractual terms, on certain types of people and entities with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| ● | federal transparency laws, including the PSA created under Section 6002 of the Affordable Care Act and its implementing regulations. The PSA requires manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the Centers for Medicare and Medicaid Services, or CMS, information related to payments or other “transfers of value” made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, and requires applicable manufacturers and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by physicians (as defined above) and their immediate family members; and |
| ● | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. |
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
Our products will face significant competition in the markets for such products, and if they are unable to compete successfully, our business will suffer.
Our product candidates face, and will continue to face, intense competition from large pharmaceutical companies, as well as academic and research institutions. We compete in an industry that is characterized by: (i) rapid technological change, (ii) evolving industry standards, (iii) emerging competition and (iv) new product introductions. Our competitors have existing products and technologies that will compete with our products and technologies and may develop and commercialize additional products and technologies that will compete with our products and technologies. Because several competing companies and institutions have greater financial resources than us, they may be able to: (i) provide broader services and product lines, (ii) make greater investments in research and development and (iii) carry on larger research and development initiatives. Our competitors also have greater development capabilities than we do and have substantially greater experience in undertaking preclinical and clinical testing of products, obtaining regulatory approvals, and manufacturing and marketing pharmaceutical products. They also have greater name recognition and better access to customers than us.
Numerous treatments are already established that will compete with our planned commercial therapies, and others are under development. Our competitors may introduce new products that render all or some of our technologies and products obsolete or noncompetitive. In addition, we may not compete successfully against generic competitors. Legislation such as the 21st Century Cures Act, which was enacted in December 2016 and designed to encourage innovation and bring pharmaceutical products to market more quickly, may enable our competitors to bring competing products to market on an expedited basis. In addition, alternative approaches to treating chronic diseases, such as gene therapy, cell therapy or transplantation technologies, may make our products obsolete or noncompetitive.
After approval, if obtained, our products will remain subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional risk and expense, including the FDA withdrawing or modifying approval for our products and product recalls that could harm our reputation, business and financial results.
Drug products remain subject to FDA jurisdiction after they have been approved. Even if we obtain regulatory approval of our product candidates or other products, the FDA may impose significant restrictions on its indicated uses or marketing or the conditions of approval, or impose ongoing requirements for potentially costly and time- consuming post-approval trials, including Phase 4 clinical trials, and post-market surveillance to monitor safety and efficacy. Our product candidates, if approved, will be subject to ongoing regulatory requirements governing the manufacturing, labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, sampling, recordkeeping and reporting of adverse events and other post-market information. These requirements include registration with the FDA and continued compliance with cGMPs, and current Good Clinical Practices requirements, for any clinical trials that we conduct post approval.
37
In addition, once a product receives FDA approval, products may be subject to recall for various reasons, including adverse effects, impurities or other product contamination, manufacturing errors, design or labeling defects or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and have an adverse effect on our financial condition and results of operations. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA could take enforcement action for failing to report the recalls when they were conducted.
We may be adversely affected by a failure or compromise from a cyberattack or data breach, which could have an adverse effect on our business.
Companies are subject to a wide variety of cybersecurity attacks on their information technology systems, which we use to maintain proprietary and confidential information. We, our key business partners and third-party suppliers rely on information technology (“IT”) systems to perform business operations, including processing, transmitting and storing electronic information, and interacting with customers, suppliers, healthcare payers, and other third parties. Like other companies in the biopharmaceutical fields, the size and complexity of our IT systems will make them vulnerable to a cyber-attack, malicious intrusion, breakdown, destruction, loss of data privacy, or other significant disruption. Our IT systems will require an ongoing commitment to maintain, protect, and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards, the increasing need to protect patient and customer information, and changing customer patterns.
Any failure by us to maintain or protect our information technology systems and data integrity related to our products, including from cyber-attacks, intrusions or other breaches, could result in the unauthorized access to patient data and personally identifiable information, theft of intellectual property or other misappropriation of assets, or otherwise compromise the health of patients using our products, our confidential or proprietary information and disrupt our operations.
In the United States, federal and state privacy and security laws require certain of our operations to protect the confidentiality of personal information including patient medical records and other health information. In Europe, the Data Protection Directive will require us to manage individually identifiable information in the European Union and, the new General Data Protection Regulation may impose fines of up to 4% of our global revenue in the event of violations. Internationally, some countries have also passed laws that require individually identifiable data on their citizens to be maintained on local servers and that may restrict transfer or processing of that data. We believe that we will meet the expectations of applicable regulations and that the ongoing costs of compliance with such rules will not be material to our business. However, there is no guarantee that we will be able to comply with these regulations, or otherwise avoid the negative reputational and other affects that might ensue from a significant data breach or failure to comply with applicable data privacy regulations, each of which could have significant adverse effects on our business, financial condition or results of operations.
We may undertake international operations, which will subject us to risks inherent with operations outside of the United States.
We intend to seek to obtain market clearances in foreign markets that we deem to generate significant opportunities. However, even with the cooperation of a commercialization partner, conducting drug development in foreign countries involves inherent risks, including, but not limited to: difficulties in staffing, funding and managing foreign operations; unexpected changes in regulatory requirements; export restrictions; tariffs and other trade barriers; difficulties in protecting, acquiring, enforcing and litigating intellectual property rights; fluctuations in currency exchange rates; and potentially adverse tax consequences.
38
If we were to experience any of the difficulties listed above, or any other difficulties, any international development activities and our overall financial condition may suffer and cause us to reduce or discontinue our international development and registration efforts.
We may not be successful in hiring and retaining key employees.
Our future operations and successes depend in large part upon the continued service of Michael J. Roberts, our President and Chief Executive Officer, and Simon C. Pedder, our Executive Chairman. If these executives terminate their employment with us, such a departure may have a material adverse effect on our business.
Our future success also depends on our ability to identify and recruit prospective executives with proven experience in the biopharmaceutical industry, specifically candidates who have managed and completed FDA-required submissions and clinical trials concerning new products; however, there can be no assurance that such personnel will be available to us or, that once engaged, will be retained by us. Our management team has expertise in many different aspects of drug development and commercialization. However, we will need to hire additional personnel as we further develop the BRiTE platform and product candidates.
Competition for skilled personnel in our market is intense and competition for experienced scientists may limit our ability to hire and retain highly qualified personnel on acceptable terms. Despite our efforts to retain valuable employees, members of our management, scientific and medical teams may terminate their employment with us on short notice. The loss of the services of any of our executive officers or other key employees could potentially harm our business, operating results or financial condition. In particular, we believe that the loss of the services of these or other personnel, would have a material adverse effect on our business. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level, and senior managers as well as junior, mid-level, and senior scientific and medical personnel. Other biopharmaceutical companies with which we compete for qualified personnel have greater financial and other resources, different risk profiles, and a longer operating history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high-quality candidates than what we have to offer. If we are unable to establish, attract and retain high-quality personnel, the rate and success at which we can develop and commercialize product candidates would be limited and would have a material adverse effect on our business and results of operations.
In addition, the Sarbanes-Oxley Act and related rules implemented by the SEC impose certain requirements on the corporate governance practices of public companies. As a public company, we expect these new rules and regulations to increase our compliance costs in 2025 and beyond and to make certain activities more time consuming and costly. As a public company, we also expect that these rules and regulations may make it more difficult and expensive for us to obtain director and officer liability insurance in the future and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified persons to serve on our board of directors or as executive officers.
Managing our growth as we expand operations may strain our resources.
We intend to grow rapidly in order to support additional, larger, and potentially international, pivotal clinical trials of our drug candidates, which will place a significant strain on our financial, managerial and operational resources. In order to achieve and manage growth effectively, we must continue to improve and expand our operational and financial management capabilities. Moreover, we will need to increase staffing and to train, motivate and manage our employees. All of these activities will increase our expenses and may require us to raise additional capital sooner than expected. Failure to manage growth effectively could harm our business, financial condition or results of operations.
39
Risks Related to Our Intellectual Property
We could lose our license rights to our important intellectual property if we do not fulfill our contractual obligations to our current and future licensors.
Our rights to significant parts of the technology we use in our product candidates are licensed from a third party and are subject to termination if we do not fulfill our contractual obligations to our licensor. Termination of intellectual property rights under our current and future license agreements could adversely impact our ability to produce or protect our products or candidates. Our obligations under our current and future license agreements may include requirements that we make milestone payments to our licensors upon the achievement of clinical development and regulatory approval milestones, royalties as we sell commercial products, and reimbursement of patent filing and maintenance expenses. Should we become bankrupt or otherwise unable to fulfill our contractual obligations, our licensors could terminate our rights to critical technology that we rely upon.
Our ability to protect and enforce any patents we may obtain does not guaranty that we will secure the right to commercialize such patents.
A patent is a limited monopoly right conferred upon an inventor, and his or her successors in title, in return for the making and disclosing of a new and non-obvious invention. This monopoly is of limited duration but, while in force, allows the patent holder to prevent others from making and/or using his or her invention. While a patent gives the holder this right to exclude others, it is not a license to commercialize the invention, where other permissions may be required for permissible commercialization to occur. For example, a drug cannot be marketed without the appropriate authorization from the FDA, regardless of the existence of a patent covering the product. Further, the invention, even if patented itself, cannot be commercialized if it infringes the valid patent rights of another party.
If we fail to adequately protect or enforce our intellectual property rights or secure rights to patents of others, the value of our intellectual property rights would diminish, and our business and competitive position would suffer.
Our success, competitive position and future revenues will depend in part on our ability and the abilities of our licensor to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties. We plan to have an active patent protection program that includes filing patent applications on new products or candidates, formulations, delivery systems and methods of making and using products or candidates and prosecuting these patent applications in the United States and abroad. As patents issue, we also file continuation and provisional applications as appropriate. Although we have taken steps to build what we believe to be a strong patent portfolio, we cannot predict:
| ● | the degree and range of protection any patents will afford us against competitors, including whether third parties are able to invalidate or circumvent our licensed patents; |
| ● | if and when patents will issue in the United States or any other country; |
| ● | whether or not others will obtain patents claiming aspects similar to those covered by our licensed patents and patent applications; |
| ● | whether we will need to initiate litigation or administrative proceedings to protect our intellectual property rights, which may be costly whether we win or lose; |
| ● | whether any of the patents we have licensed or may own or license in the future will be challenged by our competitors alleging invalidity or unenforceability and, if opposed or litigated, the outcome of any administrative or court action as to patent validity, enforceability or scope; |
| ● | whether a competitor will develop similar products that are outside the scope of protection afforded by our licensed patents, for example, due to interpretation of claim scope by a court; |
| ● | whether there were activities previously undertaken by our partners that could limit the scope, validity or enforceability of licensed patents and intellectual property; or |
| ● | whether a competitor will assert infringement of its patents or intellectual property, whether or not meritorious, and what the outcome of any related litigation or challenge may be. |
Our success also depends upon the skills, knowledge and experience of our scientific and technical personnel, our consultants and advisors as well as our licensor and contractors. To help protect our proprietary know-how and our inventions for which patents may be unobtainable or difficult to obtain, we intend to rely on trade secret protection and confidentiality agreements. To this end, we require all employees, consultants and directors to enter into agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. These agreements may not provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information. If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know- how and other proprietary rights would be significantly impaired, and our business and competitive position would suffer.
40
Due to legal and factual uncertainties regarding the scope and protection afforded by patents and other proprietary rights, we may not have meaningful protection from competition.
Our long-term success will substantially depend upon our ability to protect our proprietary technologies from infringement, misappropriation, discovery and duplication and avoid infringing the proprietary rights of others. Our licensed patent rights, like the patent rights of biopharmaceutical companies in general, are highly uncertain and include complex legal and factual issues. These uncertainties also mean that any patents that we may obtain or license in the future could be subject to challenge, and even if not challenged, may not provide us with meaningful protection from competition. Our licensed patents and pending applications may become subject to dispute, and those disputes could be resolved against us.
Any inability to protect intellectual property rights in the United States and foreign countries could limit our ability to manufacture or sell products.
We will rely on patent protection, in some cases trade secrets, unpatented proprietary know-how, and continuing technological innovation to preserve our competitive position. Our patents and licensed patent rights may be challenged, invalidated, infringed or circumvented, and the rights granted in those patents may not provide proprietary protection or competitive advantages to us. We and our licensor may not be able to develop patentable products with acceptable patent protection. Even if patent claims are allowed, the claims may not issue, or in the event of issuance, may not be sufficient to protect the technology owned by or licensed to us. If patents containing competitive or conflicting claims are issued to third parties, we may be prevented from commercializing the products covered by such patents, or may be required to obtain or develop alternate technology. In addition, other parties may duplicate, design around or independently develop similar or alternative technologies.
We may not be able to prevent third parties from infringing or using our intellectual property, and the parties from whom we may license intellectual property may not be able to prevent third parties from infringing or using the licensed intellectual property. We plan to control and limit access to, and the distribution of, our product documentation and other proprietary information. Despite efforts to protect this proprietary information, unauthorized parties may obtain and use information that we may regard as proprietary. Other parties may independently develop similar know-how or may even obtain access to these technologies.
The laws of some foreign countries do not protect proprietary information to the same extent as the laws of the United States, and many companies have encountered significant problems and costs in protecting their proprietary information in these foreign countries.
Neither the U.S. Patent and Trademark Office nor the courts have established a consistent policy regarding the breadth of claims allowed in pharmaceutical and biotechnology patents. The allowance of broader claims may increase the incidence and cost of patent administrative proceedings and the risk of infringement litigation. On the other hand, the allowance of narrower claims may limit the value of our proprietary rights.
If some or all of our licensed patents expire or are invalidated or are found to be unenforceable, or if some or all of our licensed patent applications do not result in issued patents or result in patents with narrow, overbroad, or unenforceable claims, or claims that are invalidated for, for example not being supported in regard to written description or enablement by the specification, or if we are prevented from asserting that the claims of an issued patent cover a product of a third party or its use, we may be subject to competition from third parties with products in the same class of products as our product candidates or products with the same product platform as our product candidates, including in those jurisdictions in which we have no patent protection.
Our commercial success will depend in part on obtaining and maintaining patent and trade secret protection for our product candidates, as well as the methods for using these product candidates, and the methods for producing our product candidates. We will be able to protect our product candidates and the methods for using these product candidates from unauthorized use by third parties only to the extent that we or our exclusive licensor owns or controls such valid and enforceable patents or trade secrets.
41
Even if our product candidates and the methods for using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. Our and any licensor’s abilities to obtain patents can be highly uncertain and involve complex and in some cases unsettled legal issues and factual questions. Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries provide different degrees of protection against the use of a patented invention by others. Therefore, if a patent covers an invention issues in a given country and no patents covering the same invention issued in other countries, or if any judicial interpretation of the validity, enforceability, or scope of the claims in, or the utility, written description or enablement in, a patent issued in one country is not similar to the interpretation given to the corresponding patent issued in another country, our ability to protect our intellectual property in those countries may be limited. Changes in either patent laws or in interpretations of patent laws in the United States and other countries may materially diminish the value of our intellectual property or narrow the scope of our patent protection.
We may be subject to competition from third parties with products in the same class of products as our product candidates, or products with the same product platform as our product candidates in those jurisdictions in which we have no patent protection. Even if patents are issued to us or any licensor regarding our product candidates or methods of using and making them, those patents can be challenged by our competitors as invalid or unenforceable on a variety of grounds, including lack of sufficient written description or enablement, lack of utility, lack of novelty and inventiveness, or that the claims of the issued patents should be limited or narrowly construed. Patents also will not protect our product candidates if competitors devise ways of making or using these products without legally infringing our patents. The current United States regulatory environment may have the effect of encouraging companies to challenge branded drug patents or to create non-infringing versions of a patented product in order to facilitate the approval of abbreviated new drug applications for generic substitutes. These same types of incentives encourage competitors to submit new drug applications that rely on literature and clinical data not prepared for or by the drug sponsor, providing another less burdensome pathway to approval.
We rely on confidentiality agreements to protect our trade secrets. If these agreements are breached by our employees or other parties, our trade secrets may become known to our competitors.
We rely on trade secrets that we seek to protect through confidentiality agreements with our employees and other parties. If these agreements are breached, our competitors may obtain and use our trade secrets to gain a competitive advantage over us. We may not have any remedies against our competitors and any remedies that may be available to us may not be adequate to protect our business or compensate us for the damaging disclosure. In addition, we may have to expend significant resources to protect our interests from possible infringement by others.
If we or our third-party suppliers are found to be infringing on patents or trade secrets owned by others, we may be forced to cease or alter our product development efforts, obtain a license to continue the development or sale of our products, and/or pay damages.
As the pharmaceutical industry expands and more patents are issued, the risk increases that our or our third-party suppliers’ processes and potential products may give rise to claims that they infringe the patents, trademarks, copyrights, trade secrets, or other intellectual property rights of others. Although we have reviewed certain third- party patents and patent filings that we believe may be relevant to our therapeutic candidates or products, we have not conducted any freedom-to-operate search or analysis for our therapeutic candidates or products, and we may not be aware of patents or pending or future patent applications that, if issued, would block us from commercializing our therapeutic candidates or products. Thus, we cannot guarantee that our therapeutic candidates or products, or our commercialization thereof, do not and will not infringe any third party’s intellectual property. We may, from time to time, be notified of claims that we or our third party suppliers are infringing upon patents, trademarks, copyrights, trade secrets or other intellectual property rights owned by third parties, including potential competitors, and we cannot provide assurances that other companies will not, in the future, pursue such infringement claims against us or any third-party proprietary technologies we have licensed. If we or our third party suppliers were found to infringe upon a patent or other intellectual property right, or if we failed to obtain or renew a license under a patent or other intellectual property right from a third party, or if a third party that we were licensing technologies from was found to infringe upon a patent or other intellectual property rights of another third party, we may be required to pay damages. These other persons could also bring legal actions against us, or our third-party suppliers, claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the affected product or process. If any of these actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to conduct clinical tests, manufacture or market the affected product or use the affected process. Required licenses may not be available on acceptable terms, if at all, and the results of litigation are uncertain. If we become involved in litigation or other proceedings, it could consume a substantial portion of our financial resources and the efforts of our personnel. If our suppliers become involved in litigation or other proceedings, it could prevent us from continuing to develop or commercialize our products. Additionally, rights granted under licensing agreements may not provide a competitive advantage to us. Efforts to enforce patent rights can involve substantial expense and may not be successful. Furthermore, others may independently develop similar, superior, or parallel technologies to any technology developed or licensed by us, or our technology may prove to infringe on patents or rights owned by others. Thus the patents licensed to us, or in the future licensed or held by us, may not afford us any meaningful competitive advantage. Also, our confidentiality agreements may not provide meaningful protection of our proprietary information. Our inability to maintain our proprietary rights could have a material adverse effect on our business, financial condition, and results of operations.
42
Other parties may claim that we infringe their intellectual property or proprietary rights, which could cause us to incur significant expenses or prevent us from selling products.
Our success will depend in part on our ability to operate without infringing the patents and proprietary rights of third parties. The development, manufacture, use and sale of new products have been subject to substantial patent rights litigation in the pharmaceutical and biotech industry. These lawsuits generally relate to the validity and infringement of patents or proprietary rights of third parties. Infringement litigation is prevalent with respect to generic versions of products for which the patent covering the brand name product is expiring, particularly since many companies that market generic products focus their development efforts on products with expiring patents. Pharmaceutical companies, biotechnology companies, universities, research institutions or other third parties may have filed patent applications or may have been granted patents that cover aspects of our product candidates or other technologies.
Future or existing patents issued to third parties may contain patent claims that cover our products or candidates. We expect to be subject to infringement claims from time to time in the ordinary course of business, and third parties could assert infringement claims against us in the future with respect to our current product candidates or with respect to product candidates that we may develop or license. Litigation or administrative proceedings could force us to:
| ● | stop or delay selling, manufacturing or using products that incorporate, or are made using the challenged intellectual property; |
| ● | pay damages; or |
| ● | enter into licensing or royalty agreements that may not be available on acceptable terms, if at all. |
Any litigation or administrative proceedings, regardless of their outcome, would likely delay the regulatory approval process, be costly and require significant time and attention of our key management and technical personnel.
We may be subject to claims that our consultants or independent contractors have wrongfully used or disclosed alleged trade secrets of their other clients or former employers to us.
As is common in the biotechnology and pharmaceutical industry, we engage the services of consultants to assist us in the development of our product candidates. Many of these consultants were previously employed at, or may have previously or may be currently providing consulting services to, other biotechnology or pharmaceutical companies including our competitors or potential competitors. We may become subject to claims that the Company or a consultant inadvertently or otherwise used or disclosed trade secrets or other information proprietary to their former employers or their former or current clients. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management team.
43
Risks Related to Ownership of our Common Stock
If we are unable to register in a timely manner the shares of Common Stock issued to stockholders in the Merger or the Offering, then we may be subject to certain liquidated damages.
We have agreed to prepare and file with the SEC a registration statement registering the resale of shares sold in the Offering. If (a) we are late in filing the resale registration statement, (b) the resale registration statement is not declared effective within 120 days after the final closing of the Offering (subject to certain exceptions), (c) the resale registration statement ceases for any reason to remain effective or the holders of shares are otherwise not permitted to utilize the prospectus therein to resell their shares for a period of more than five consecutive trading days (subject to permitted blackout periods (as defined in the Registration Rights Agreement) and certain other exceptions), or (d) following the listing or inclusion for quotation of the Common Stock on OTC Markets, Nasdaq, NYSE or NYSE American, the shares are not listed or included for quotation on such a market, or trading of the Common Stock is suspended or halted on the principal market for the Common Stock, for more than three consecutive trading days (subject to certain exceptions), then in any such case monetary penalties payable by the Company to the affected holders of the shares will commence to accrue at a rate equal to 12% per annum of the value of the Registrable Shares, as set forth in the Registration Rights Agreement. If we are required to pay the holders of the Registrable Shares a significant cash penalty our financial condition may be harmed.
There is currently no market for our Common Stock and there can be no assurance that any market will ever develop.
Our Common Stock is not listed on a national securities exchange or any other exchange, or quoted on an over-the- counter market. Therefore, there is no trading market, active or otherwise, for our Common Stock and our Common Stock may never be included for trading on any stock exchange, automated quotation system or any over-the-counter market. Accordingly, our Common Stock is highly illiquid and our investors will likely experience difficulty in selling their shares at times and prices that they may desire.
Our Common Stock may not be eligible for listing or quotation on any securities exchange or over-the-counter trading system.
We do not currently meet the initial quantitative listing standards of any national securities exchange or over-the- counter trading system. We cannot assure you that we will be able to meet the initial listing standards of any national securities exchange or over-the-counter trading system, or, if we do meet such initial listing standards, that we will be able to maintain any such listing. Further, will be required to meet certain requirements, including prescribed periods of time trading over-the-counter and minimum filings of periodic reports with the SEC, before we are eligible to apply for listing on a national securities exchanges. We intend to contact an authorized market maker for an over-the-counter quotation system for sponsorship of our Common Stock, but we cannot guarantee that such sponsorship will be approved and our Common Stock listed and quoted for sale. Even if our Common Stock is quoted for sale on an over-the-counter quotation system, buyers may be insufficient in numbers to allow for a robust market and it may prove impossible to sell your shares. In addition, an investor may find it difficult to obtain accurate quotations as to the market value of our Common Stock. In addition, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling our Common Stock, which may further affect its liquidity. This would also make it more difficult for us to raise additional capital.
44
The market price and trading volume of our Common Stock may be volatile and could decline significantly.
The quotation systems, including the OTC Markets QB tier and QX tier, or stock exchanges, including Nasdaq, on which our Common Stock may be quoted or on which our Common Stock may be listed in the future have from time to time experienced significant price and volume fluctuations. Even if an active, liquid and orderly trading market develops and is sustained for our Common Stock following the Merger, the market price of our Common Stock may be volatile and could decline significantly. In addition, the trading volume in our Common Stock may fluctuate and cause significant price variations to occur. If the market price of our Common Stock declines significantly, our stockholders may be unable to resell their shares at or above the market price of our Common Stock as of the date they purchased them. We cannot assure you that the market price of our Common Stock will not fluctuate widely or decline significantly in the future in response to a number of factors, including, among others, the following:
| ● | the realization of any of the risk factors presented in this Report; |
| ● | future issuances, sales, resales or repurchases or anticipated issuances, sales, resales or repurchases, of our Common Stock; |
| ● | actual or anticipated differences in our estimates, or in the estimates of analysts, for our revenues, results of operations, level of indebtedness, liquidity or financial condition; |
| ● | additions and departures of key personnel; |
| ● | failure to comply with the requirements of the OTCQB market, or following our potential up listing on Nasdaq; |
| ● | failure to comply with the Sarbanes-Oxley Act or other laws or regulations; |
| ● | publication of research reports about us, or our industry; |
| ● | the performance and market valuations of other similar companies; |
| ● | broad disruptions in the financial markets, including sudden disruptions in the credit markets; |
| ● | speculation in the press or investment community; |
| ● | actual, potential or perceived control, accounting or reporting problems; and |
| ● | changes in accounting principles, policies and guidelines. |
In the past, securities class-action litigation has often been instituted against companies following periods of volatility in the market price of their shares. This type of litigation could result in substantial costs and divert our management’s attention and resources, which could have a material adverse effect on us.
The designation of our Common Stock as “penny stock” would limit the liquidity of our Common Stock.
Our Common Stock may be deemed a “penny stock” (as that term is defined under Rule 3a51-1 of the Exchange Act) in any market that may develop in the future. Generally, a “penny stock” is a common stock that is not listed on a securities exchange and trades for less than $5.00 a share. Prices often are not available to buyers and sellers and the market may be very limited. Penny stock in start-up companies is among the riskiest equity investments. Broker-dealers who sell penny stock must provide purchasers with a standardized risk-disclosure document prepared by the SEC. The document provides information about penny stock and the nature and level of risks involved in investing in the penny stock market. A broker must also provide purchasers with bid and offer quotations and information regarding broker and salesperson compensation and make a written determination that the penny stock is a suitable investment for the purchaser and obtain the purchaser’s written agreement to the purchase. Many brokers choose not to participate in penny stock transactions. If our Common Stock is deemed “penny stock”, because of penny stock rules, there may be less trading activity in any market that develops for our Common Stock in the future and stockholders are likely to have difficulty selling their shares.
FINRA sales practice requirements may limit a stockholder’s ability to buy and sell our Common Stock.
The Financial Industry Regulatory Authority, or FINRA, has adopted rules requiring that, in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative or low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA has indicated its belief that there is a high probability that speculative or low-priced securities will not be suitable for at least some customers. If these FINRA requirements are applicable to us or our securities, they may make it more difficult for broker-dealers to recommend that at least some of their customers buy our Common Stock, which may limit the ability of our stockholders to buy and sell our Common Stock and could have an adverse effect on the market for and price of our Common Stock.
45
Because we became a reporting company under the Exchange Act by means other than a traditional underwritten initial public offering, we may not be able to attract the attention of research analysts at major brokerage firms.
Because we will become a reporting company by conducting an underwritten initial public offering of our Common Stock, and because we will not be listed on a national securities exchange, security analysts of brokerage firms may not provide coverage of our Company. In addition, investment banks may be less likely to agree to underwrite secondary offerings on our behalf than they might if we became a public reporting company by means of an underwritten initial public offering, because they may be less familiar with our Company as a result of more limited coverage by analysts and the media, and because we became public at an early stage in our development. The failure to receive research coverage or support in the market for our shares will have an adverse effect on our ability to develop a liquid market for our Common Stock.
Because the Merger was a reverse merger, the Registration Statement we file with respect to the shares of Common Stock received by investors in the Merger might be subject to heightened scrutiny by the SEC, and we may not be able to attract the attention of major brokerage firms.
Additional risks may exist as a result of our becoming a public reporting company through a “reverse merger.” Certain SEC rules are more restrictive when applied to reverse merger companies, such as the ability of stockholders to resell their shares of Common Stock pursuant to Rule 144, and the SEC may subject the Registration Statement we file with respect to the shares of Common Stock received by investors in the Merger and the Offering to heightened scrutiny. In addition, securities analysts of major brokerage firms may not provide coverage of our capital stock or business. Because we became a public reporting operating company through a reverse merger, there is no incentive to brokerage firms to recommend the purchase of our Common Stock. We cannot assure you that brokerage firms will want to provide analyst coverage of our capital stock or business in the future.
We are obligated to develop and maintain proper and effective internal control over financial reporting. If we fail to develop and maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable laws and regulations could be impaired. In addition, the presence of material weaknesses increases the risk of material misstatement of the consolidated financial statements.
Pursuant to Section 404(a) of the Sarbanes-Oxley Act, we are required to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting in our Annual Report on Form 10-K. Effective internal control over financial reporting is necessary for reliable financial reports and, together with adequate disclosure controls and procedures, such internal controls are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations. Ineffective internal controls could also cause investors to lose confidence in reported financial information, which could have a negative effect on the trading price of our Common Stock.
The report by management will need to include disclosure of any material weaknesses identified in internal control over financial reporting. However, for as long as we are an “emerging growth company” under the JOBS Act following the consummation of the Merger, our independent registered public accounting firm will not be required to attest to the effectiveness of internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act.
Management’s assessment of internal controls, when implemented, could detect problems with internal controls, and an independent assessment of the effectiveness of internal controls by our auditors could detect further problems that management’s assessment might not, and could result in the identification of material weaknesses that were not otherwise identified. For example, in connection with its audit of Private Adaptin’s financial statements as of and for the years ended December 31, 2023 and 2022, Private Adaptin’s external auditor identified certain material weaknesses in its internal control over financial reporting with respect to the following items:
| ● | insufficient evidence of board preapproval before entering into debt, equity, and licensing agreements; |
| ● | errors in accounting for non-routine transactions; and |
| ● | segregation of duties. |
46
Private Adaptin’s management intends to take measures to remediate the aforementioned material weaknesses, including, but not limited to the engagement of a professional services firm to provide back-office accounting support, including the services of a corporate controller; and consulting support with respect to the application of Generally Accepted Accounting Principles and SEC financial reporting regulations. The material weaknesses will not be considered remediated until management designs and implements effective controls that operate for a sufficient period of time and management has concluded, through testing, that these controls are effective. Management will monitor the effectiveness of these remediation plans and will make changes management determines to be appropriate. There can be no assurance that any remediation plans will be successful.
Undetected material weaknesses in internal controls, or those that are detected and not timely resolved, could lead to financial statement restatements and require us to incur the expense of remediation. We are required to disclose changes made in internal control and procedures on a quarterly basis. To comply with the public company requirements, we may need to undertake various actions, such as implementing new internal controls and procedures and hiring accounting or internal audit staff.
We are in the early stages of developing the system and processing documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete its evaluation, testing, and any required remediation in a timely fashion. During the evaluation and testing process, if we cannot remedy any identified material weaknesses or if we identify additional material weaknesses in internal control over financial reporting, we will be unable to assert that internal control over financial reporting is effective.
If we are unable to assert that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion on the effectiveness of its internal control, including as a result of any new or existing material weaknesses described above, we could lose investor confidence in the accuracy and completeness of financial reports, which would cause the price of our Common Stock to decline, and we may be subject to investigation or sanctions by the SEC. In addition, if we are unable to continue to meet these requirements, we may not be able to remain quoted on any over-the-counter trading system, or following any potential listing, listed on any securities exchange.
We are an emerging growth company and a smaller reporting company, and any decision on our part to comply only with certain reduced reporting and disclosure requirements applicable to emerging growth companies and smaller reporting companies could make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and, for as long as we continue to be an emerging growth company, we may choose to take advantage of exemptions from various reporting requirements applicable to other public companies but not to emerging growth companies, including:
| ● | not being required to have our independent registered public accounting firm audit our internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act; |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports and annual report on Form 10-K; and |
| ● | exemptions from the requirements of holding non-binding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
Our status as an emerging growth company will end upon the earlier of: (a) the fifth anniversary of the first sale of our common equity securities pursuant to an effective registration statement; (b) the last day of the fiscal year in which we have more than $1.235 billion in annual gross revenues; (c) the date we qualify as a “large accelerated filer,” with at least $700 million of equity securities held by non-affiliates; and (d) the date on which we have issued, in any three-year period, more than $1 billion in non-convertible debt securities.
We cannot predict if investors will find our Common Stock less attractive if we choose to rely on any of the exemptions afforded emerging growth companies. If some investors find our Common Stock less attractive because we rely on any of these exemptions, there may be a less active trading market for our Common Stock and the market price of our Common Stock may be more volatile.
47
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to avail ourselves of this provision of the JOBS Act. As a result, we will not be subject to new or revised accounting standards at the same time as other public companies that are not emerging growth companies. Therefore, our consolidated financial statements may not be comparable to those of companies that comply with new or revised accounting pronouncements as of public company effective dates.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a “smaller reporting company” even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as our voting and non-voting Common Stock held by non-affiliates is less than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues is less than $100 million during the most recently completed fiscal year and our voting and non-voting Common Stock held by non-affiliates is less than $700 million measured on the last business day of our second fiscal quarter.
We may face risks related to securities litigation that could result in significant legal expenses and settlement or damage awards.
We may in the future become subject to claims and litigation alleging violations of the securities laws or other related claims, which could harm our business and require us to incur significant costs. Significant litigation costs could impact our ability to comply with certain financial covenants under our credit agreement. We are generally obliged, to the extent permitted by law, to indemnify our current and former directors and officers who are named as defendants in these types of lawsuits. Regardless of the outcome, litigation may require significant attention from management and could result in significant legal expenses, settlement costs or damage awards that could have a material impact on our financial position, results of operations and cash flows.
Anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Our restated certificate of incorporation and our restated bylaws contain provisions that could delay or prevent a change in control of our company. These provisions could also make it difficult for stockholders to elect directors who are not nominated by current members of our board of directors or take other corporate actions, including effecting changes in our management. These provisions:
| ● | permit only the board of directors to establish the number of directors and fill vacancies on the board; | |
| ● | require super-majority voting to amend some provisions in our restated certificate of incorporation and restated bylaws; | |
| ● | authorize the issuance of “blank check” preferred stock that our board of directors could use to implement a stockholder rights plan; | |
| ● | eliminate the ability of our stockholders to call special meetings of stockholders; | |
| ● | prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our stockholders; | |
| ● | prohibit cumulative voting; and | |
| ● | establish advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted upon by stockholders at annual stockholder meetings. |
In addition, our restated certificate of incorporation provides that the Court of Chancery of the State of Delaware is the exclusive forum for: any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law (the “DGCL”), our restated certificate of incorporation, or our restated bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine.
48
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all claims brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Our restated certificate of incorporation provides that the federal district courts of the United States is, unless we consent in writing to an alternative forum, the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act (“Federal Forum Provision”). Our decision to adopt a Federal Forum Provision followed a decision by the Supreme Court of the State of Delaware holding that such provisions are facially valid under Delaware law. While there can be no assurance that federal courts or state courts will follow the holding of the Delaware Supreme Court or determine that the Federal Forum Provision should be enforced in a particular case, application of the Federal Forum Provision means that suits brought by our stockholders to enforce any duty or liability created by the Securities Act must be brought in federal court and cannot be brought in state court. While neither the exclusive forum provision nor the Federal Forum Provision applies to suits brought to enforce any duty or liability created by the Exchange Act creates exclusive federal jurisdiction over all claims brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. Accordingly, actions by our stockholders to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder also must be brought in federal court. Our stockholders are not deemed to have waived our compliance with the federal securities laws and the regulations promulgated thereunder.
Any person or entity purchasing or otherwise acquiring or holding any interest in any of our securities is deemed to have notice of and consented to our exclusive forum provisions, including the Federal Forum Provision. These provisions may limit a stockholder’s ability to bring a claim in a judicial forum of their choosing for disputes with us or our directors, officers or other employees, which may discourage lawsuits against us and our directors, officers, and other employees.
In addition, Section 203 of the DGCL may discourage, delay or prevent a change in control of our company. Section 203 imposes certain restrictions on mergers, business combinations and other transactions between us and holders of 15% or more of our Common Stock.
The issuance of our preferred stock could adversely affect the holders of our Common Stock in some circumstances.
The issuance of some or all of our authorized preferred stock could adversely affect the holders of our Common Stock in some circumstances. Our board of directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights, which could adversely affect the voting power, or other rights of the holders of the Common Stock. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of the Company. Although we have no present intention to issue any shares of our authorized preferred stock, there can be no assurance that the Company will not do so in the future.
Because we do not intend to pay dividends, stockholders will benefit from an investment in our Common Stock only if it appreciates in value.
We have never declared or paid any cash dividends on our preferred stock or Common Stock nor do we intend to do so. For the foreseeable future, it is expected that earnings, if any, generated from our operations will be used to finance the growth of our business, and that, other than with respect to dividends we may be obligated to pay on our preferred stock, if any, no dividends will be paid to holders of our Common Stock. As a result, the success of an investment in our Common Stock will depend upon any future appreciation in its value. There is no guarantee that our Common Stock will appreciate in value.
If securities or industry analysts do not publish research or publish unfavorable or inaccurate research about our business, our stock price and trading volume could decline.
Our stock price and trading volume following our quotation on the OTC Markets QB tier, if any, or following our potential listing on a securities exchange, if any, will be heavily influenced by the way analysts and investors interpret our financial information and other disclosures. Securities and industry analysts do not currently, and may never, publish research on our business. If few securities or industry analysts commence coverage of us, our stock price could be negatively affected. If securities or industry analysts downgrade our Common Stock, or publish negative reports about our business, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our Common Stock could decrease, which might cause our stock price to decline and could decrease the trading volume of our Common Stock.
49
The future issuance of equity or of debt securities that are convertible into equity may dilute our stockholders’ investment and reduce their equity interest.
We may choose to raise additional capital in the future, depending on market conditions, strategic considerations and operational requirements. To the extent that additional capital is raised through the issuance of shares or other securities convertible into shares, our stockholders will be diluted, including to the extent there are additional closings of the Offering. Future issuances of our Common Stock or other equity securities, or the perception that such sales may occur, could adversely affect the prevailing market price of our Common Stock and impair our ability to raise capital through future offerings of equity or equity-linked securities. For example, we have agreed, at our expense, to prepare and file a registration statement with the SEC registering the resale of certain shares of our Common Stock pursuant to the Registration Rights Agreement. See “Completion of Acquisition or Disposition of Assets-The Merger and Related Transactions-Registration Rights” for more information. The resale of a substantial number of shares of our Common Stock in the public market could adversely affect the market price for our Common Stock and make it more difficult for you to sell shares of our Common Stock at times and prices that our stockholders feel are appropriate. Furthermore, we expect that, because there will be a large number of shares registered pursuant to a registration statement, selling stockholders will continue to offer shares covered by such registration statement for a significant period of time, the precise duration of which cannot be predicted. Accordingly, the adverse market and price pressures resulting from an offering pursuant to a registration statement may continue for an extended period of time and continued negative pressure on the market price of our Common Stock could have a material adverse effect on our ability to raise additional equity capital.
We are subject to the reporting requirements of federal securities laws, which can be expensive and may divert resources from other projects, thus impairing our ability to grow.
We are a public reporting company and, accordingly, subject to the information and reporting requirements of the Exchange Act and other federal securities laws, including compliance with the Sarbanes-Oxley Act. The costs of preparing and filing annual and quarterly reports, proxy statements, registration statements and other information with the SEC and furnishing audited reports to stockholders would cause our expenses to be higher than they would be if Private Adaptin remained privately held and did not consummate the Merger. In addition, we will incur substantial expenses in connection with the preparation of the Registration Statement and related documents required under the terms of the Offering.
It may be time consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance personnel in order to develop and implement appropriate internal controls and reporting procedures. If we are unable to comply with the internal controls requirements of the Sarbanes-Oxley Act, then we may not be able to obtain the independent accountant certifications required by such act, which may preclude us from keeping our filings with the SEC current.
Our principal stockholders will have significant influence over the election of our board of directors and approval of any significant corporate actions, including any sale of the Company.
The Company’s founders, executive officers, directors, and other principal stockholders, in the aggregate, beneficially own a majority of our outstanding stock. These stockholders will have significant influence with respect to the election of our board of directors and approval or disapproval of all significant corporate actions. The concentrated voting power of these stockholders could have the effect of delaying or preventing an acquisition of the company or another significant corporate transaction.
For example, the sole holder of Unite Acquisition prior to the Merger, Lucius Partners, holds 3,250,000 shares of Common Stock after the Merger, which represents 40.2% of the outstanding shares of the combined Company as of the date of this Report. Lucius Partners purchased its shares upon formation of the Company for a nominal price. Matthew Eitner, the Chief Executive Officer of the Placement Agent, James Ahern, the Managing Partner of the Placement Agent, and Patrick Gallagher, a member of our board of directors, a Managing Director of the Placement Agent, are managing members, members, and/or officers of Lucius Partners, and therefore are indirectly material stakeholders of the Company. Anthony Zook is also a member of the board of directors of the Company and is a member of Lucius Partners. The Placement Agent and/or its designees hold warrants to purchase an aggregate of 270,204 shares of our Common Stock. Therefore, following the Merger, such persons can exert substantial control over matters requiring the approval of our stockholders. Additionally, Lucius Partners has received, and will continue to receive after the Merger, fees from us for advisory and certain other services to the Company.
50
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our consolidated financial statements and the related notes and other financial information included in this Report. Some of the information contained in this discussion and analysis or set forth elsewhere in this Report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties as described under the heading “Forward-Looking Statements” elsewhere in this Report. You should review the disclosure under the heading “Risk Factors” in this Report for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
Concurrent with the closing of the Offering, Unite Acquisition, Merger Sub, and Private Adaptin entered into Merger Agreement and effected the Merger. Pursuant to the terms of the Merger Agreement, on the Closing Date, Merger Sub merged with and into Private Adaptin, with Private Adaptin continuing as the surviving corporation and the wholly- owned subsidiary of Unite Acquisition. As a result of the Merger, Unite Acquisition acquired the business of Private Adaptin and will continue the existing business operations of Private Adaptin as a public reporting company under the name “Adaptin Bio, Inc.” On the Closing Date, Private Adaptin was renamed “Adaptin Bio Operating Corporation.”
The Merger is treated as a recapitalization and reverse acquisition for Unite Acquisition for financial reporting purposes, and Private Adaptin is considered the acquirer for accounting purposes. As a result of the Merger and the change in Unite Acquisition’s business and operations, a discussion of the past financial results of Unite Acquisition is not pertinent, and under applicable accounting principles, the historical financial results of Private Adaptin, the accounting acquirer, prior to the Merger will be considered the combined Company’s historical financial results.
Company Overview
Adaptin is a biopharmaceutical company pioneering a transformational approach to enhancing the transfer of therapeutics into the brain, facilitating the treatment of brain cancers and other unmet medical conditions. The Company’s precision medicine technology, originally developed by researchers in the Department of Neurosurgery at Duke University, harnesses the human immune system’s ability to target, recognize, destroy or deliver therapeutics to specific cells, including cancer cells. Our mission is to be the global leader and pioneer of this new treatment paradigm, integrating recombinant technology, gene therapy and cell therapy to address the challenges of targeting and delivering effective therapies, including to the brain for cancer and other CNS, indications.
We are closely working with the researchers at Duke University to translate preclinical proof of concept data of our proprietary platform technology, the BRiTE Platform, into human clinical trials. BRiTE is a translatable method to specifically target malignant glioma using a tumor-specific, fully human bispecific antibody that redirects patients’ own T cells to recognize and destroy tumor cells. Our first application of BRiTE is APTN-101, a proprietary EGFRvIII x CD3 bispecific T cell engager, that is able to eliminate malignant glioma tumors in a variety of aggressive preclinical orthotopic tumor models. We designed APTN-101 to specifically redirect T cells against tumors expressing a well-characterized, mutated form of EGFR on a number of tumor types, including glioblastoma, breast and lung cancer. APTN-101 has been recently accepted under an investigator-led IND to begin first-in-human studies in brain cancer. Our goal is to complete preclinical studies on additional product candidates and file multiple INDs.
Duke University Exclusive Licensing Agreement
As described elsewhere in this Report, effective January 11, 2023, Private Adaptin entered into the Duke License, which was assumed by the Company in the Merger, whereby Duke University granted Private Adaptin an exclusive license to the patents and patent application relating to the precision medicine technology, which we intend to develop using our BRiTE Platform. As part of the consideration for the license, Private Adaptin issued Duke University 75 shares (that were valued at $175.86 per share, or $13,189), representing 5% of its then-issued and outstanding common stock on a fully diluted basis, which converted into 161,960 shares of Common Stock, representing 2.0% of the currently outstanding Common Stock. We are obligated to make milestone payments and pay royalties to Duke University, as well as to reimburse Duke University for prior patent expenses, as set forth elsewhere in this Report.
51
The Company has recorded the issuance of common stock and has accrued for the reimbursement of prior patent expenses, but has not reached any of the clinical or regulatory milestones or transacted its first commercial sale as of September 30, 2024. The Company will record corresponding liabilities as such events occur.
Recent Events
Bridge Financings
Private Adaptin raised bridge financing through the offer and sale (a) in 2023 of $500,000 principal amount of its 2023 Bridge Notes and (b) in 2024 of $1,000,000 principal amount of its 2024 Bridge Notes, which in each case were sold to a limited number of accredited investors pursuant to Regulation D under the Securities Act.
At the closing of the Offering, the $1,500,000 aggregate principal amount of outstanding Bridge Notes, plus accrued interest thereon, converted automatically into shares of Common Stock at a conversion price of $3.30 per share, or 501,140 shares of our Common Stock, and the holders of the 2023 Bridge Notes were issued, pursuant to existing agreements, Pre-Merger Warrants to purchase up to 132,570 shares of our Common Stock at an exercise price of either $3.30 or $4.40 per share and with a term of five years.
Operations Overview
Since the commencement of Private Adaptin’s operations in 2021, we have devoted substantially all of our resources to supporting our product development efforts, raising capital to support and expand such activities, and providing general and administrative support for these operations. We operate our business using a significant outsourcing model. As such, our team is composed of a small group of employees who direct a significantly large number of team members, including vendors and consultants, to enable execution of our operational plans. We do not currently have any products approved for sale, and we will continue to incur significant research and development and general administrative expenses related to our operations.
Since inception, we have incurred significant operating losses. For the nine months ended September 30, 2024 and 2023, we recorded net losses of approximately $2.3 million and $0.7 million, respectively. As of September 30, 2024, we had an accumulated deficit of approximately $3.2 million. We expect to continue to incur significant losses for the foreseeable future. We anticipate that a substantial portion of our capital resources and efforts in the foreseeable future will be focused on completing the necessary development activities required for applying for and obtaining regulatory approval for our product candidates and, subsequently, preparing for potential commercialization of our product candidates. We had approximately $115,000 of cash at September 30, 2024.
We expect to continue to incur significant expenses and operating losses for at least the next several years. Our net losses may fluctuate significantly from period to period, depending on the timing of our planned clinical trials and expenditures on other research and development activities. We expect our expenses will increase substantially over time as we:
| ● | continue our ongoing and planned development of APTN-101, including pre-clinical activity and our Phase 1 investigator-led trial for the treatment of GBM; | |
| ● | build a portfolio of product candidates through development, or the acquisition or in-license of drugs, product candidates or technologies; | |
| ● | initiate preclinical studies and clinical trials for APTN-101 for any additional indications we may pursue and for any additional product candidates that we may pursue in the future; | |
| ● | maintain, protect and expand our intellectual property portfolio; | |
| ● | hire clinical, regulatory and scientific personnel; | |
| ● | add operational, financial and management information systems and personnel, including personnel to support our product development efforts; and | |
| ● | incur additional legal, accounting, insurance and other expenses associated with operating as a public company. |
52
The Macroeconomic Climate
The recent economic trends and political changes, including the proposed implementation of a tariff structure, may materially adversely affect our business and corresponding financial position and cash flows. While inflationary factors have trended down and interest rates are beginning to trend down, they still may impact our overhead costs and may adversely affect our operating results. While interest rates have recently been trending down, they remain high and present a challenge impacting the United States and global economies. Such factors could make it more difficult for us to obtain traditional financing on acceptable terms, if at all, in the future. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, we may experience increases in the near future on our operating costs, including our labor, due to supply chain constraints, consequences associated with pandemics or public health situations, the Russia-Ukraine war, and other United States geopolitical issues, such as the proposed tariff structures, affecting other territories and employee availability and wage increases, all of which may result in additional stress on our working capital resources.
Components of Results of Operations
Research and Development Expenses
Research and development expenses consist primarily of fees paid to third-party service providers and, in future periods, we expect will include additional personnel costs and other personnel-related compensation expenses. Research and development expenses also include the cost of in-process research and development (“IPR&D”) assets purchased in an asset acquisition transaction. We have concluded that the costs associated with licensing our technology are IPR&D and we expensed those costs upon execution of the Duke License. Research and development costs after the acquisition are expensed as incurred. We expense research and development costs in the periods in which they are incurred. Costs for certain development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors, collaborators and third-party service providers.
To date, substantially all our research and development expenses have been related to the licensing and preclinical development of APTN-101. As we progress, we expect our research and development costs to increase for additional preclinical and clinical development of APTN-101 in GBM.
The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming and is subject to uncertainties and delays. As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent we will generate revenue from the commercialization and sale of our product candidates, if at all.
General and Administrative Expenses
General and administrative expenses include expenses for outside professional services and other general administrative expenses. Outside professional services consist of patent maintenance expenses, legal, accounting and audit services and other consulting fees.
We also expect to incur expenses as a public company, including expenses related to compliance with SEC rules and regulations and those of any national securities exchange on which our securities are traded, additional insurance expenses, investor relations activities, and other administrative and professional services.
Interest Expense
Interest expense primarily consists of contractual debt interest expense, the amortization of debt issuance costs and the amortization of discounts arising from derivative liabilities.
53
Results of Operations
Comparison of the Nine Months Ended September 30, 2024 and 2023
The following table summarizes our results of operations and changes for the periods indicated:
| Nine months ended September 30, | ||||||||||||||||
| 2024 | 2023 | $ Change | % Change | |||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Research and development | $ | 1,566,533 | $ | 440,299 | $ | 1,126,234 | 256 | % | ||||||||
| General and administrative | 495,272 | 212,623 | 282,649 | 133 | % | |||||||||||
| Total Operating Expenses | 2,061,805 | 652,922 | 1,408,883 | 216 | % | |||||||||||
| LOSS FROM OPERATIONS | (2,061,805 | ) | (652,922 | ) | (1,408,883 | ) | 216 | % | ||||||||
| Interest Expense | 195,161 | 66,711 | 128,450 | 193 | % | |||||||||||
| Loss on fair value of derivative liability | 8,755 | - | 8,755 | 100 | % | |||||||||||
| NET LOSS | $ | (2,265,721 | ) | $ | (719,633 | ) | $ | (1,546,088 | ) | 215 | % | |||||
Research and Development Expenses
Research and development expenses increased by $1,126,234, or 256%, for the nine months ended September 30, 2024 when compared to research and development expenses for the nine months ended September 30, 2023. During 2024, the increase in research and development costs are primarily associated with a repeat-dose toxicology study of APTN-101 that began in late 2023. Costs incurred during the nine months ended September 30, 2023 consist primarily of costs related to the execution of the Duke License that was determined to be IPR&D acquired in an asset transaction. These costs were expensed as the technology has not received regulatory approval for marketing and, absent obtaining such approval, has no established alternative future use.
General and Administrative Expenses
General and administrative expenses increased by $282,649, or 133%, for the nine months ended September 30, 2024 when compared to the nine months ended September 30, 2023. The increase was primarily related to increases in consulting costs related to management of the Company as business activities increased.
Interest Expense
Interest expense increased $128,450 for the nine months ended September 30, 2024 when compared to the nine months ended September 30, 2023. The increase in interest expense was related to issuance of $1.0 million in 2024 Notes and the interest accrued, debt issuance costs amortization, discounts related to derivative liability amortization and related costs. In addition, we recorded a loss on fair market value of the derivative liability for nine months ended September 30, 2024.
54
Comparison of the Year Ended December 31, 2023 and 2022
The following table summarizes our results of operations and changes for the periods indicated:
| Year ended December 31, | ||||||||||||||||
| 2023 | 2022 | $ Change | % Change | |||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Research and development | $ | 510,356 | $ | - | $ | 510,356 | 100 | % | ||||||||
| General and administrative | 366,960 | 11,772 | 355,188 | 3017 | % | |||||||||||
| Total Operating Expenses | 877,316 | 11,772 | 865,544 | 7353 | % | |||||||||||
| LOSS FROM OPERATIONS | (877,316 | ) | (11,772 | ) | (865,544 | ) | 7353 | % | ||||||||
| Interest Expense | 104,487 | - | 104,487 | 100 | % | |||||||||||
| NET LOSS | $ | (981,803 | ) | $ | (11,772 | ) | $ | (970,031 | ) | 8240 | % | |||||
Research and Development Expenses
Research and development expenses for the year ended December 31, 2023 were $510,356. There were no research and development expenses for the year ended December 31, 2022. During 2023, the increase in research and development costs are primarily associated with the execution of the Duke License in 2023. The expenses incurred upon execution of the Duke License of approximately $340,000 were determined to be IPR&D acquired in an asset transaction. These costs were expensed as the technology has not received regulatory approval for marketing and, absent obtaining such approval, has no established alternative future use. The remainder of the increase is primarily related to the initiation of pre-clinical activities in 2023.
General and Administrative Expenses
General and administrative expenses increased by $355,188, or 3,017%, for the year ended December 31, 2023 when compared to the year ended December 31, 2022. The increase was primarily related to increases in consulting costs related to management of the Company as business activities increased along with increases in professional fees for legal and accounting services.
Liquidity and Capital Resources
We have incurred operating losses and experienced negative operating cash flows since our inception, and we anticipate continuing to incur losses for at least the next several years as we continue to develop our product candidates. As of September 30, 2024, we had cash of $115,308 and an accumulated deficit of approximately $3.2 million.
As set forth above, in 2023 and 2024, we raised bridge financing through the offer and sale of $1,500,000 of Bridge Notes. At the closing of the Offering, the $1,500,000 aggregate principal amount of outstanding Bridge Notes, plus accrued interest thereon, converted automatically into shares of Common Stock at a conversion price of $3.30 per share, or 501,140 shares of our Common Stock, and the holders of the 2023 Bridge Notes were issued, pursuant to existing agreements, Pre-Merger Warrants to purchase up to 132,570 shares of our Common Stock at an exercise price of $3.30 or $4.40 per share and with a term of five years.
Based on our current operating plan, we anticipate that our existing cash balance will not be sufficient to fund our operating activities for the next twelve months and, as such, we will need to obtain additional funding. We plan to continue to fund our losses from operations through cash on hand, as well as through future equity offerings, debt financings, or other third-party funding. There can be no assurance that additional funds will be available when needed from any source or, if available, will be available on terms that are acceptable to us. Even if we raise additional capital, we may also be required to modify, delay or abandon some of our plans which could have a material adverse effect on our business, operating results and financial condition and our ability to achieve our intended business objectives. Any of these actions could materially harm our business, results of operations and future prospects.
55
Cash Flows
The following table shows a summary of our cash flows for the periods presented:
| Nine months ended September 30, | ||||||||||||||||
| 2024 | 2023 | $ Change | % Change | |||||||||||||
| Net cash (used in) provided by: | ||||||||||||||||
| Operating activities | (761,936 | ) | (198,047 | ) | (563,889 | ) | 285 | % | ||||||||
| Financing activities | 872,490 | 398,890 | 473,600 | 119 | % | |||||||||||
| Net increase in cash during the periods | 110,554 | 200,843 | (90,289 | ) | 45 | % | ||||||||||
| For the years ended December 31, | ||||||||||||||||
| 2023 | 2022 | $ Change | % Change | |||||||||||||
| Net cash (used in) provided by: | ||||||||||||||||
| Operating activities | (394,186 | ) | 50 | (394,236 | ) | 788472 | % | |||||||||
| Financing activities | 398,890 | - | 398,890 | 100 | % | |||||||||||
| Net increase in cash during the periods | 4,704 | 50 | 4,654 | 9308 | % | |||||||||||
Operating Activities
Net cash used in operating activities was $761,936 for the nine months ended September 30, 2024, compared to $198,047 of cash used in operating activities for the comparable prior year period. The increase of $563,889 is related to increased operating activity in 2024 consisting of a repeat-dose toxicology study that was initiated in late 2023 along with increases in consulting costs in 2024 related to management of the Company with increased business activities.
Net cash used in operating activities for the year ended December 31, 2023 was $394,186 and reflects costs associated with the execution of the Duke License in 2023 and increases in legal and professional fees. For the year ended December 31, 2022, such costs were only $50 related to the initial cash account funding by the Company’s founders.
Financing Activities
Net cash provided by financing activities was $872,490 for the nine months September 30, 2024 related to the issuance of the 2024 Notes in the amount of $1,000,000 offset by debt issuance costs. For the nine months ended September 30, 2023, net cash provided by financing activities was $398,890 related to the issuance of the 2023 Notes in the amount of $500,000 offset by debt issuance costs.
Net cash provided by financing activities was $398,890 for the year ended December 31, 2023 related to the issuance of the 2023 Notes offset by debt issuance costs. There were no financing activities during the year ended December 31, 2022.
56
Indebtedness
In 2023, we entered into Securities Purchase Agreements resulting in issuance of an aggregate of $500,000 of the 2023 Bridge Notes. We incurred $101,110 in debt issuance costs related to the issuance of this debt. For the year ended December 31, 2023, we also recorded accrued interest of $33,615 and amortization expense of the debt issuance costs of $70,872. There were no debt costs for the year ended December 31, 2022.
During the nine months ended September 30, 2024, we entered into Securities Purchase Agreements resulting in the issuance of an aggregate of $1.0 million of the 2024 Bridge Notes. We incurred approximately $128,000 in debt issuance costs related to the issuance of this debt. As of September 30, 2024, we had recorded accrued interest expense of $26,264, amortization of debt issuance costs of $28,070, amortization of discounts related to an embedded derivative liability of $73,091 and a loss on the fair value of derivative liabilities of $8,755.
Funding Requirements
We use our cash primarily to fund research and development expenditures. We expect our research and development expenses to increase as we continue the development of APTN-101. We expect to incur an increase in general and administrative expenses in 2025 primarily related to supporting our increasing research and development activities and becoming a publicly held company with the resulting professional fees, personnel and regulatory compliance related costs. We expect to incur increasing operating losses for the foreseeable future as we continue the preclinical and clinical development of our product candidate. At this time, due to the inherently unpredictable nature of clinical development, we cannot reasonably estimate the costs we will incur and the timelines that will be required to complete development, obtain marketing approval, and commercialize APTN-101 or any future product candidates, if at all. For the same reasons, we are also unable to predict when, if ever, we will generate revenue from product sales or whether, or when, if ever, we may achieve profitability. Clinical and preclinical development timelines, the probability of success, and development costs can differ materially from expectations.
The timing and amount of our operating expenditures will depend largely on:
| ● | the timing, progress and results of our ongoing and planned preclinical and clinical development activities for APTN- 101 in GBM; |
| ● | the scope, progress, results and costs of preclinical development, testing and clinical trials of APTN-101 for any additional indications; |
| ● | the ability of our vendors and third-party service providers to accurately forecast expenses and deliver on expectations; |
| ● | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; and |
| ● | the extent to which we acquire or in-license other product candidates and technologies. |
Until such time, if ever, as we can generate substantial revenue from product sales, we expect to fund our operations and capital funding needs through equity and/or debt financing. We may also consider entering into collaboration arrangements or selectively partnering for clinical development and commercialization. The sale of additional equity would result in additional dilution to our shareholders. The incurrence of debt financing would result in debt service obligations and the instruments governing such debt could provide for operating and financing covenants that restrict our operations or our ability to incur additional indebtedness, among other items. If we are not able to secure adequate additional funding, we may be forced to make reductions in spending, extend payment terms with suppliers, liquidate assets where possible, and/or suspend or curtail planned programs. Any of these actions could materially and adversely affect our business, financial condition and results of operations.
57
Contractual Obligations and Commitments
Duke University License
In January 2023, Private Adaptin entered into the Duke License with Duke University and the National Cancer Institute, under the agency of the U.S. Department of Health and Human Services for an exclusive, world-wide, sub-licensable license to the technology more fully described above, which was assumed by the Company in the Merger. As a component of the Duke License, the Company is obligated to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities. These milestones include initiation of Phase II or Phase III clinical trials, submission of applications for market approval in multiple jurisdictions including the United States, European Union and Japan and the initiation of post-approval commercial sales in the same jurisdictions. Based on an assumption that all milestones related to the current development program are met during the course of the Duke License, these milestone payments would total approximately $11.7 million. As of September 30, 2024, the Company has not met any milestones as defined in the Duke License and, accordingly, has recorded no expense or liability related to such payments.
The Company is also obligated to pay royalties equal to low- to mid- single digit percentages of annual net sales on a country-by-country and product-by-product basis subject to downward adjustment to low single digit percentages of our net annual sales in the event there is no valid claim of a patent for the product, with minimum annual royalty levels established. We also must pay Duke University low to mid-double digit percentages of any sublicensing fees as set forth in the Duke License. The Company has not recorded and does not owe any royalties or sublicensing fees for the nine months ended September 30, 2024.
Off-Balance Sheet Transactions
We did not have during the periods presented, and we do not currently have, any off-balance sheet financing arrangements or any relationships with unconsolidated entities or financial partnerships, such as structured finance or special purpose entities, that were established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Critical Accounting Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates and assumptions reflected in the financial statements relate to and include, but are not limited to, the fair value of common stock and other assumptions used to measure share-based consideration transferred for acquired assets under the Duke License, the fair value of derivative liabilities along with prepaid expenses and accrued liabilities that are measured based on progress toward completion of research and development projects.
Future events and their effects cannot be predicted with certainty; accordingly, accounting estimates require the exercise of judgment. Accounting estimates used in the preparation of these financial statements change as new events occur, as more experience is acquired, as additional information is obtained and as the operating environment changes.
JOBS Act Accounting Election
We are an “emerging growth company,” as defined in the JOBS Act. The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to either early adopt or delay the adoption of some accounting standards until those standards would otherwise apply to private companies. We have elected to use the extended transition period under the JOBS Act until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
58
MANAGEMENT
Executive Officers and Directors
The following table provides information regarding our executive officers and directors as of February 11, 2025:
| Name | Age | Positions | ||
| Executive Officers | ||||
| Michael J. Roberts, PhD | 55 | Chief Executive Officer and Director | ||
| Simon C. Pedder, PhD | 63 | Executive Chairman and Director | ||
| Timothy L. Maness, CPA | 64 | Chief Financial Officer | ||
| L. Arthur Hewitt, PhD | 71 | Chief Development Officer | ||
| Non-Employee Directors | ||||
| Patrick Gallagher | 59 | Director | ||
| Anthony Zook | 64 | Director | ||
| J. Nick Riehle | 72 | Director |
| (1) | Member of the Audit Committee. |
Executive Officers
Michael J. Roberts, PhD – Chief Executive Officer and Director
Dr. Roberts has been our Chief Executive Officer and a member of our board of directors since February 2025, and was Chief Executive Officer and a director of Private Adaptin from March 2021 until February 2025. He has over 25 years of pharmaceutical research, development, corporate development, and executive experience. He is co-founder and President and CEO of Adaptin Bio and also serves on the board of directors. Prior to Adaptin he was co-founder of Corino Therapeutics and is currently its acting CEO. Dr. Roberts led and ran all activities related to Corino and the development of a disease-modifying drug called CRX-1008, which is used to treat the protein disorder transthyretin amyloidosis. He is a pharmaceutical and biotech consultant and owner of MAC B Consulting. Prior to Corino, Dr. Roberts was VP, Business Development and Corporate Officer of publicly traded Chelsea Therapeutics International, Ltd. He was responsible for business development efforts focused primarily on licensing and M&A with consideration to pipeline management. He led the sale of Chelsea Therapeutics to H. Lundbeck A/S in 2014 for approximately $658 million. Prior to Chelsea Therapeutics, Dr. Roberts was Director of Business Development for their Nektar Therapeutics’ Molecule Engineering technology and completed a number of transactions with large and specialty pharmaceutical companies. Dr. Roberts has completed pharmaceutical transactions valued at over $1B. Prior to this he was Manager of Biopharmaceutical Research at Shearwater Corporation where he led and was successful in the development of preclinical drug candidates from initial stages of research through Phase I clinical study, including inventing the product Movantik™, a treatment for opioid-induced constipation, subsequently licensed to AstraZeneca in a transaction valued at approximately $1 billion. Shearwater was sold to Inhale Therapeutic Systems (now known as Nektar Therapeutics) in 2001 for approximately $200 million. Dr. Roberts obtained his Ph.D. in Materials Science from the University of Alabama and B.S. in Chemical Engineering from Pennsylvania State University. We believe that Dr. Roberts’ experience in the life sciences industry and in the operation of publicly traded companies as an executive qualifies him to serve on our board of directors.
Simon C. Pedder, PhD – Executive Chairman and Director
Dr. Pedder has been our Executive Chairman and a member of our board of directors since February 2025, and was Executive Chairman and a director of Private Adaptin since October 2023. He has a career of over 30 years in drug development and commercialization. He was Chief Executive Officer of Nirogy from November 2021 to November 2022. From December 2016 to November 2021, he had leadership roles as Chief Business and Strategy Officer for Athenex; President and CEO of Cellectar Biosciences from April 2014 to June 2015; President and CEO of Chelsea Therapeutics International, Ltd. from May 2004 to July 2012; and Global Vice President of Oncology Pharma Business and Executive Officer at Hoffmann-LaRoche. Previous positions at Roche included Life Cycle Leader and Global Project Leader of Pegasys/IFN and Head of Hepatitis Franchise. Prior to that, he was Clinical Leader for a number of development compounds at Roche. Dr. Pedder served on the board of directors of Cerecor, Inc. from April 2018 to June 2020, Mateon Therapeutics, Inc. from March 2016 to April 2019 and Delcath Systems, Inc. from November 2017 to April 2019.
59
Early in his career, he was a faculty member in the Department of Pharmacology in College of Medicine at the University of Saskatchewan, where he obtained his Ph.D. in Clinical Pharmacology. During his longstanding career in pharmaceutical development, Dr. Pedder played key roles in the successful development and commercialization of multiple proprietary pharmaceutical products including Tasmar®, Pegasys®, Copegus®, Northera® and Klisyri®. In addition to his Ph.D., Dr. Pedder obtained a Master of Science in Toxicology from Concordia University, a Joint Honors Bachelor degree in Environmental Studies/Biology from the University of Waterloo, and he completed the Roche-sponsored Pharmaceutical Executive Management Program at Columbia Business School. We believe Dr. Pedder’s experience in the life sciences industry and in the operation of publicly traded companies as an executive and board member qualifies him to serve on our board of directors.
Timothy L. Maness, CPA – Chief Financial Officer
Mr. Maness has been our Chief Financial Officer since February 2025, and was Chief Financial Officer of Private Adaptin from September 2024 to February 2025. Since April 2016, he has provided financial consulting services through Adamanteus, LLC. Mr. Maness has been a consultant to multiple publicly held and private-equity-backed life sciences companies, and he has held several senior financial management roles, primarily in software and technology services.
In 2018, Mr. Maness was engaged by Milestone Pharmaceuticals to prepare and lead its initial public offering, serving as its interim CFO, CAO, and Vice President of Finance. Before that, he served as the CFO of Ballantyne Therapeutics. Earlier, during his eight years as the Senior Director of Finance and Corporate Controller at Chelsea Therapeutics International, he was heavily involved in Chelsea’s reverse-public-shell merger to complete its public listing in 2005. Mr. Maness is a noted expert in forensic accounting and internal audit and has served as the internal auditor hired by public company audit committees on several occasions.
Mr. Maness received a B.S. in accounting from the University of North Carolina at Charlotte, and he is licensed as a Certified Public Accountant and as a Chartered Global Management Accountant.
L. Arthur Hewitt, PhD – Chief Development Officer
Dr. Hewitt has been our Chief Development Officer since February 2025, and was Chief Development Officer of Private Adaptin from September 2024 to February 2025. He has worked for approximately 35 years in clinical research and regulatory affairs. From December 2016 to December 2021, he served as a scientific advisor to Amneal Pharmaceuticals and Senior Scientific Advisor – Neurology at Lundbeck Pharmaceuticals. Prior to Lundbeck, Dr. Hewitt was Chief Scientific Officer for Chelsea Therapeutics International, Ltd. from January 2010 to June 2014 and Vice President, Drug Development from May 2004 to June 2014. At Chelsea, he oversaw the clinical development and regulatory approval of Northera™ (droxidopa), a novel therapeutic agent, for the treatment of symptomatic neurogenic orthostatic hypotension, or Neurogenic OH, in patients with primary autonomic failure (Parkinson’s disease, or PD, multiple systems atrophy, or MSA, and pure autonomic failure, or PAF), dopamine β-hydroxylase, or DBH, deficiency and non-diabetic autonomic neuropathy. Prior to Chelsea, Dr. Hewitt served as an independent contractor from January 2003 to May 2004, and as Director of Scientific Affairs at Shearwater Corporation, a drug delivery company, from October 2002 until January 2003.
From July 1991 until November 2000, Dr. Hewitt was Director of Scientific Affairs for Amgen Canada where he oversaw the clinical research and regulatory requirements for a wide variety of proprietary biologic treatments undergoing Phase I, II and III research. Thirteen individual investigational new drug, or IND, research programs were established covering the therapeutic domains of Hematology, Oncology, Neurology, Infectious Disease, and Inflammation. Dr. Hewitt also developed clinical research programs supporting the approval of three products including Neupogen, Stemgen and Infergen and six supplementary approvals. Prior to Amgen, Dr. Hewitt held positions at Jansen Pharmaceuticals and Park-Davis where he developed research programs for multiple neurology and oncology products. Prior to entering the pharmaceutical industry, he was a Lecturer at Concordia University in Montreal, Canada. Dr. Hewitt obtained his Ph.D. in Pharmacology from the University of Montreal. Additionally, Dr. Hewitt received a Master of Science degree in Toxicology and a B.Sc. (Hon) in Comparative Anatomy and Physiology from Concordia University.
60
Non-Employee Directors
Patrick Gallagher
Mr. Gallagher has been a member of our board of directors since February 2025. He has 20 years of healthcare experience including alternative investments, research and marketing in both the public and private markets. Since January 2018, he has served as the Chief Executive Officer and as a member of the board of directors of Voltron Therapeutics, a privately held biotechnology company. Since August 2014, Mr. Gallagher has served as a Managing Director of Laidlaw & Company (UK) Ltd. From January 2018 to September 2024, Mr. Gallagher served as the Chief Executive Officer and a member of the board of directors at PD Theranostics, Inc. He has also served as Treasurer of Aerwave Medical, Inc. since November 2020. Formerly, Mr. Gallagher was a founding partner and Chief Executive Officer of BDR Research Group, LLC (“BDR”), from July 2001 through October 2010. BDR was an independent sell-side research firm specializing in healthcare investing, financing and operations, serving the institutional investing community at large.
Mr. Gallagher served as VP of Business Development and Investor Relations as well as a strategic consultant for Kinex Pharmaceuticals, a biotechnology firm focused on next-generation therapies in oncology and immunology (now traded on NASDAQ: ATNX). He also served as an advisor to CHD Biosciences, a novel antimicrobial company from July 2012 through August 2014. Mr. Gallagher has served on the boards of directors of BioSig Technologies, Inc., NASDAQ-listed a medical device company that is developing a proprietary technology platform in the electrophysiology space since July 2014 Cingulate Therapeutics, a therapeutics company with a novel drug delivery platform since January 2014; Evermore Global, a global special situations money manager since June 2015; and Algorithm Sciences, Inc. since May 2019. Since September 2014, Mr. Gallagher has served as a Managing Partner at Laidlaw Venture Partners dba Laidlaw & Company (UK) Ltd. and is part of the Investment Banking Healthcare Team. Mr. Gallagher also is a member of Lucius Partners.
Mr. Gallagher earned his Master in Business Administration from Penn State University and his Bachelor of Science in Finance from the University of Vermont. We believe that Mr. Gallagher’s extensive experience in the life sciences industry qualifies him to serve on our board of directors.
Anthony Zook
Mr. Zook has been a member of our board of directors since February 2025. He currently is a member of Lucius Partners. Since July 2020, Mr. Zook has served as a director of BioSig Technologies, Inc. (Nasdaq: BSGM). From May 2023 to December 2024, Mr. Zook was a director of NeoGenomics (Nasdaq: NEO), an oncology laboratory service company where he also served as the chair of the compensation committee. From January 2022 to December 2024, he also served as Chairman to Voltron Therapeutics and Algorithm Sciences, respectively. Mr. Zook was executive vice president of global commercial operations of AstraZeneca Plc from 2010 until 2012. He also served as president and chief executive officer of the North American division of AstraZeneca Plc from 2006 until 2009, and president of Medimmune, the company’s wholly owned biologics division from 2009 until 2010. Mr. Zook previously served as a member of the board of directors of AltheRx from 2013-2014, InHibikase in 2014, Rib-X Pharmaceuticals in 2009, the National Pharmaceutical Council from 2007-2009, PhRMA from 2011-2012, the Pennsylvania Division of the American Cancer Society from 2005-2007 and his alma mater, Frostburg State University from 2016-2018 and re-joined in 2021, where he earned a B.S. degree. Mr. Zook also earned an A.A. degree in chemical engineering from Pennsylvania State University. We believe Mr. Zook’s extensive commercialization experience and expertise in executive leadership qualify him to serve on our board of directors.
61
J. Nick Riehle
Mr. Riehle has been a member of our board of directors since February 2025. He has over 25 years of business and management experience with both large companies and start-up ventures, and nearly 20 years of that has been as the chief financial officer of pharmaceutical companies, including taking these same companies public. Mr. Riehle formerly served as the CFO of Athenex, Inc., managing all aspects of the accounting, treasury, IT, HR, and legal functions and providing support to the company when it went public in June 2017. Prior to this, he was a freelance contractor for companies seeking his expertise in financing and related support. From 2004 – 2014, Mr. Riehle was the CFO of Chelsea Therapeutics International, Ltd. He managed all aspects of the accounting, treasury, facilities, IT, HR, IR and legal functions, including supporting the company in over $300 million in public and private equity financings and being actively involved in the sale of the company to H. Lundbeck A/S in 2014 for $658 million. Prior to Chelsea Therapeutics, Mr. Riehle was the CFO for HAHT Commerce, Inc., managing all aspects of treasury, accounting, IT, legal and inventory administration, as well as having significant involvement in sales administration, services management, HR, facilities and related operational activities, including supporting the company in transactions involving over $60 million in venture financing. Prior to HAHT Commerce, Mr. Riehle held various positions in Canada, the United States and Asia with Nortel Networks, including financial planning and analysis, accounting, marketing and sales administration, government relations, and general administration.
Mr. Riehle earned his Bachelor of Commerce from McGill University, his MBA from York University and is a Certified Management Accountant (CMA). We believe that Mr. Riehle’s experience in the life sciences industry qualifies and in the operation of publicly traded companies as a senior financial executive qualifies him to serve on our board of directors.
Corporate Governance
Appointment of Officers
Our executive officers are appointed by, and serve at the discretion of, our board of directors provided, however, that the board of directors may empower the Chief Executive Officer of the Company to appoint any officer other than the Executive Chairperson, the Chief Executive Officer, the President, the Chief Financial Officer or the Treasurer.
Board of Directors Composition
Our board of directors currently consists of five members. Dr. Roberts, Dr. Pedder, Mr. Gallagher, Mr. Zook, and Mr. Riehle have been designated to serve as members of our board of directors.
Each of our current directors will continue to serve until the election and qualification of his successor, or his earlier death, resignation, disqualification or removal.
Director Independence
Our securities are not listed on a national securities exchange or on any inter-dealer quotation system that has a requirement that a majority of directors be independent. We evaluate independence by the standards for director independence set forth in the Nasdaq Marketplace Rules. Under such rules, our board of directors has determined that all members of the board of directors except for Dr. Roberts and Dr. Pedder are independent directors under Nasdaq Listing Rule 5605(a)(2). In making such independence determination, our board of directors considered the relationships that each non-employee director has with us and all other facts and circumstances that our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Under Nasdaq Marketplace Rules, a director only qualifies as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.
62
Audit committee members must also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act. In order to be considered independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (i) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (ii) be an affiliated person of the listed company or any of its subsidiaries. We intend to satisfy the audit committee independence requirements of Rule 10A-3 as of the time we list on a national securities exchange.
Our board of directors has undertaken a review of the independence of each director and considered whether each director has a material relationship with us that could compromise his or her ability to exercise independent judgment in carrying out his or her responsibilities. As a result of this review, our board of directors determined that Messrs. Gallagher, Riehle and Zook are “independent directors” as defined under the applicable rules and regulations of the SEC and the listing requirements and rules of Nasdaq. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and current and prior relationships as they may relate to us and our management, including the beneficial ownership of our capital stock by each non-employee director and the transactions involving them described in the section titled “Certain Relationships and Related Party Transactions.”
Family Relationships
There are no family relationships by between or among the members of the board of directors or other executive officers of the Company.
Legal Proceedings
None of the Company’s directors or executive officers are involved in any legal proceedings described in Item 401(f) of SEC Regulation S-K.
Committee of the Board of Directors
Our board of directors has an Audit Committee, which has the composition and responsibilities described below. Members serve on the Audit Committee until their resignation or until otherwise determined by our board of directors.
Audit Committee
The members of our Audit Committee are Messrs. Gallagher, Riehle, and Zook. Mr. Riehle serves as the Chair of this committee. The board of directors has determined that Mr. Riehle is “independent” under the Nasdaq Marketplace Rules and standards established by the SEC rules regarding audit committee members as set forth above.
The Audit Committee oversees on behalf of the board of directors (a) the conduct of the Company’s accounting and financial reporting processes, the audits of our financial statements and the integrity of the Company’s audited financial statements and other financial reports; (b) the performance of the Company’s internal accounting, internal auditing, and financial controls function; (c) the engagement, replacement, compensation, qualifications, independence and performance of our independent auditors, and (d) the portions of our Code of Conduct and Ethics and related policies regarding our accounting, internal accounting controls or auditing matters. The Audit Committee also reviews and approves or disapproves related party transactions identified in Item 404 of SEC Regulation S-K and makes recommendations to the full board of directors regarding the same.
The Audit Committee meets privately with our independent registered public accounting firm from time to time, and such firm has unrestricted access to, and reports directly to, the Audit Committee.
63
CODE OF CONDUCT AND ETHICS
In connection with the Merger, our board of directors adopted a new Code of Conduct and Ethics (the “Code of Ethics”), which applies to all directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions) and employees.
The Code of Ethics addresses, among other matters, conflicts of interest and corporate opportunities, fair dealing, record- keeping and public disclosures, compliance with laws and corporate policies, confidentiality and corporate assets, and reporting and consequences of violations. The provisions of the Code of Ethics are intended to reflect current best practices and enhance the Company’s personnel’s understanding of the Company’s standards of ethical business practices, promote awareness of ethical issues that may be encountered in carrying out an employee’s or director’s responsibilities and improve clarity as to how to address ethical issues that may arise.
If any substantive amendments are made to our Code of Ethics, or if any waiver (including any implicit waiver) of any provision of the Code of Ethics is granted that is required to be disclosed under the rules of the SEC, such amendment or waiver will be disclosed on our website, or, if required, in a current report on Form 8-K.
The newly adopted Code of Ethics did not result in any explicit or implicit waiver of any prior code of conduct or ethics.
compensation of directors and executive officers
Information with respect to the Company’s directors and executive officers is described in the section titled “Management”.
Director Compensation
The Company did not pay any compensation to its directors since inception through September 30, 2024. Following the Merger, the Company intends to provide its non-employee directors with annual cash compensation of $25,000. The chair of the Audit Committee will receive an additional $10,000 in annual cash compensation. Non-employee directors will be eligible to receive such equity grants or awards as may be approved by the board in its discretion. The board of directors intends to grant each non-employee director equity awards equal to 0.20% of the Company’s fully diluted shares outstanding at the time of grant. Messrs. Gallagher and Zook have waived any compensation for their services as directors of the Company. Drs. Roberts and Pedder will be employees and executive officers of the Company and will not be separately compensated for the service as directors.
Executive Officer Compensation
Throughout this section, unless otherwise noted, “we,” “us,” “our,” “Company” and similar terms refer to Private Adaptin prior to the closing of the Merger, and to the Company and its subsidiaries after the closing of the Merger.
This section discusses the material components of the executive compensation program for the Company’s named executive officers who appear in the “2024 Summary Compensation Table” below. In 2024, the “named executive officers” and their positions with the Company were as follows:
| ● | Michael J. Roberts: President and Chief Executive Officer |
| ● | Simon C. Pedder: Executive Chairman |
| ● | Timothy L. Maness: Chief Financial Officer |
This discussion may contain forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from the currently planned programs summarized in this discussion.
64
2024 and 2023 Summary Compensation Table
The following table sets forth information concerning the compensation of the named executive officers for the Company’s most recent fiscal year.
| Name and principal position | Year | Salary | Bonus | Stock awards | Option awards | All other compensation | Total | |||||||||||||||||||||
| Simon
C. Pedder, PhD Executive Chairman | 2024 | -- | -- | -- | -- | $ | 40,000 | (1) | $ | 40,000 | ||||||||||||||||||
| 2023 | -- | -- | -- | -- | $ | 10,000 | (1) | $ | 10,000 | |||||||||||||||||||
| Michael
J. Roberts, PhD Chief Executive Officer | 2024 | -- | -- | -- | -- | $ | 60,000 | (2) | $ | 60,000 | ||||||||||||||||||
| 2023 | -- | -- | -- | -- | $ | 15,000 | (2) | $ | 15,000 | |||||||||||||||||||
| Timothy
L. Maness, CPA Chief Financial Officer | 2024 | -- | -- | -- | -- | $ | 20,550 | (3) | $ | 20,550 | ||||||||||||||||||
| 2023 | -- | -- | -- | -- | $ | 34,200 | (3) | $ | 34,200 | |||||||||||||||||||
| (1) | Represents amount paid to Dr. Pedder pursuant to the Compensation Agreement as described below. |
| (2) | Represents amount paid to MAC B Consulting LLC, which is owned by Dr. Roberts, pursuant to the Compensation Agreement as described below. |
| (3) | Represents amount paid to Adamanteus LLC, which is owned by Mr. Maness, pursuant to the Consulting Agreement as described below. |
Outstanding Equity Awards at Fiscal Year-End
As of the end of our most recently completed fiscal year, none of our named executive officers held any outstanding equity awards.
Pre-Merger Agreements with Named Executive Officers
We are currently party to compensation agreements with Dr. Pedder and an entity owned by Dr. Roberts, as well as a consulting agreement with an entity owned by Mr. Maness. Descriptions of these agreements appear below. Upon closing of the Merger, these agreements were terminated and replaced by employment agreements with each of Dr. Roberts, Dr. Pedder, and Mr. Maness. See “Post-Merger Employment Agreements with Named Executive Officers” below.
Compensation Agreement – MAC B Consulting LLC. On October 1, 2023, the Company and MAC B Consulting LLC, d/b/a Michael J. Roberts entered into a compensation agreement (the “Roberts Compensation Agreement”). Pursuant to the Roberts Compensation Agreement, Dr. Roberts provides business and pharmaceutical development related services to the Company. The Roberts Compensation Agreement may be terminated by the Company or Dr. Roberts upon 30 days prior written notice to the other party. Pursuant to the Roberts Compensation Agreement, Dr. Roberts is entitled to receive cash compensation at a rate of $15,000 per month so long as the Company has sufficient funding.
65
Compensation Agreement – Simon C. Pedder. On October 1, 2023, the Company and Dr. Pedder entered into a compensation agreement (the “Pedder Compensation Agreement”). Pursuant to the Pedder Compensation Agreement, Dr. Pedder provides business and pharmaceutical development related services to the Company. The Pedder Compensation Agreement may be terminated by the Company or Dr. Pedder upon 30 days prior written notice to the other party. Pursuant to the Pedder Compensation Agreement, Dr. Pedder is entitled to receive cash compensation at a rate of $10,000 per month so long as the Company has sufficient funding.
Consulting Agreement – Adamanteus LLC. On May 31, 2023, the Company and Adamanteus LLC, d/b/a Timothy Maness entered into a consulting agreement (the “Maness Consulting Agreement”). Pursuant to the Maness Consulting Agreement, Mr. Maness provides finance and accounting services to the Company. The Maness Consulting Agreement was for an initial term of six months and has continued for subsequent six-month terms in accordance with the terms of the Maness Consulting Agreement. The Maness Consulting Agreement may be terminated by the Company or Mr. Maness upon 30 days prior written notice to the other party. Pursuant to the Maness Consulting Agreement, Mr. Maness is entitled to receive cash compensation at a rate of $300 per hour.
Post-Merger Employment Agreements with Named Executive Officers
We entered into executive employment agreements with each of Dr. Roberts, Dr. Pedder, and Mr. Maness (each an “Executive” and collectively, the “Executives”), which were effective upon the closing of the Merger. The agreements include customary non-competition, non-solicitation, and confidentiality covenants; establish the Executives’ duties and compensation; and provide for their continued employment with the Company. The discussion that follows summarizes certain other anticipated terms of these agreements.
Term. The initial term of each of the employment agreements commenced upon the closing of the Merger and continue for a term of three years in the case of Dr. Roberts and Dr. Pedder, and two years in the case of Mr. Maness, unless terminated sooner in accordance with the employment agreement (see “Termination” below). After the initial term expires, the employment agreements will automatically renew for successive one-year terms unless either the Company or the Executive provides written notice of their intent not to renew at least 90 days prior to the expiration of the then-current term.
Board Service. In the case of Drs. Roberts and Pedder, the Company will use commercially reasonable efforts to cause these individuals to be elected as members of the Company’s board of directors throughout the terms of their employment agreements.
Base Salary. The Company intends to pay each Executive a base salary at the following annual rates:
Dr. Roberts – $480,000
Dr. Pedder – $240,000
Mr. Maness – $240,000
These salaries will be reviewed by the board of directors from time to time and may be increased in the board of directors’ sole discretion. Salaries may not be reduced except in connection with an across-the-board reduction of executive-level salaries in which the Executive will not be subject to a greater reduction, on a percentage basis, than any other executive- level employee.
Bonus. For each calendar year, the Executives will be eligible to receive an annual bonus based on the following target amounts of each Executive’s base salary:
Dr. Roberts – 50%
Dr. Pedder – 30%
Mr. Maness – 30%
The actual amount of each annual bonus will depend on the level of achievement of the Company’s corporate objectives and the Executive’s individual objectives, in each case, as established by the board for the calendar year with respect to which such annual bonus relates. The annual bonus for a calendar year, to the extent earned, will be paid in a lump sum in the following calendar year. The annual bonus will not be deemed earned until the date that it is paid. Accordingly, in order for the Executive to receive an annual bonus, the Executive must be actively employed by the Company at the time of such payment.
66
Equity Compensation. Each Executive will be eligible to receive such equity grants or awards as may be approved by the board of directors in its discretion.
Subject to approval by the board of directors and the terms of the Company’s equity compensation plan then in place, in the event the Company issues additional securities raising aggregate funds of $10,000,000 (in one or more transactions), occurring, if at all, within two years following the Merger (the “Additional Financing Period”), the Company will grant each Executive options to purchase a number of shares of Common Stock of the Company (the “Anti-Dilution Options”) sufficient to ensure that their respective ownership immediately following the Additional Financing Period, on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable) is equal to their respective ownership immediately following the Merger, as determined on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable). The per share exercise price of the Anti-Dilution Options will be equal to the fair market value of a share of the Company’s Common Stock on the date of grant, as determined by the board of directors. The Anti-Dilution Options, if any, will become exercisable in four equal annual installments, in each case subject to the continued employment of each Executive with the Company on the date each such vesting milestone is achieved, and will be subject to the terms of the Company’s equity incentive plan then in place and a related option grant agreement to be entered between Executive and the Company.
Other Benefits. Dr. Roberts and Dr. Pedder will each be entitled to no less than 25 paid vacation days per year. Mr. Maness will be entitled to no less than 20 paid vacation days per year. The Executives will also be entitled to such other benefits, and to participate in such benefit plans, as are generally made available to similarly situated senior executive employees of the Company. The Company will also reimburse the Executives for all reasonable business expenses they incur in connection with the performance of their duties.
Termination. The employment agreements may be terminated in the following circumstances:
| ● | Automatically effective upon the Executive’s death. |
| ● | By the Company upon notice to the Executive in the event of the Executive’s disability. “Disability” means the inability of the Executive to perform the essential functions of their job for 90 consecutive days or for 120 days in any one-year period due to the condition of the Executive’s physical, mental, or emotional health. |
| ● | By the Company for cause. “Cause” means the Executive’s (i) fraud, embezzlement or misappropriation with respect to the Company; (ii) willful or grossly negligent misconduct that has or may reasonably be expected to have a material adverse effect on the property, business, or reputation of the Company; (iii) material breach of their employment agreement; (iv) willful failure or refusal to perform their material duties or willful failure to follow any specific lawful instructions of the board of directors (in the case of Dr. Roberts and Dr. Pedder) or the chief executive officer (in the case of Mr. Maness); (v) conviction or plea of nolo contendere in respect of a felony or of a misdemeanor involving moral turpitude; or (vi) material failure to comply with the Company’s workplace rules, policies, or procedures. |
| ● | By the Company for any reason other than for cause or the Executive’s disability. |
| ● | By the Executive for good reason. “Good reason” means the occurrence of any of the following events without the Executive’s written consent: (i) the Company’s requiring the Executive to be based at any office or location more than 25 miles from their principal work location, except for travel reasonably required in the performance of the Executive’s responsibilities to the Company; (ii) a material reduction of the Executive’s base salary not in compliance with their employment agreement; (iii) a material diminution of the Executive’s authority, duties, or responsibilities; or (iv) the Company’s material breach of the Executive’s employment agreement. The Company will have an opportunity to cure any such conditions following notice by the Executive. |
| ● | By the Executive upon 30 days’ written notice to the Company at any time for any reason. |
67
Separation Benefits Not in Connection with a Change in Control. If the Company terminates the Executive’s employment without cause or if the Executive resigns for good reason, in either case not in connection with a change in control of the Company, then the Executive will be entitled to the following benefits:
| ● | 24 months of then-current base salary in the case of Dr. Roberts and Dr. Pedder, and 12 months of then-current base salary in the case of Mr. Maness. |
| ● | Continuation of health insurance coverage for the Executive and their family for 18 months in the case of Dr. Roberts and Dr. Pedder, and 12 months in the case or Mr. Maness, or until the Executive becomes eligible for coverage under another employer’s plan. |
The Company’s obligation to provide such benefits is conditioned upon the Executive executing, and not revoking, a release of claims in a form acceptable to the Company.
Separation Benefits in Connection with a Change in Control. If the Company terminates the Executive’s employment without cause, or if the Executive resigns for good reason, in either case at the time of, or within six months following a change in control of the Company, then the Executive will be entitled to the following benefits:
| ● | 24 months of then-current base salary in the case of Dr. Roberts and Dr. Pedder, and 12 months of then- current base salary in the case of Mr. Maness. |
| ● | Accelerated vesting of all equity awards such that all awards are deemed to have been vested as of the date of termination. |
| ● | Continuation of health insurance coverage for the Executive and their family for 18 months in the case of Dr. Roberts and Dr. Pedder, and 12 months in the case or Mr. Maness, or until the Executive becomes eligible for coverage under another employer’s plan. |
The Company’s obligation to provide such benefits is conditioned upon the Executive executing, and not revoking, a release of claims in a form acceptable to the Company.
A “change in control” means the occurrence of any of the following events:
| ● | Any “person,” including a “group” (as such terms are used in Sections 13(d) and 14(d) of the Exchange Act) but excluding the Company, any entity controlling, controlled by or under common control with the Company, any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any such entity, and, with respect to any particular qualified participant of any equity incentive plan of the Company (a “Participant”), the Participant and any “group” (as such term is used in Section 13(d)(3) of the Exchange Act) of which the Participant is a member), is or becomes the “beneficial owner” (as defined in Rule 13(d)(3) under the Exchange Act), directly or indirectly, of securities of the Company representing 50% or more of either (i) the combined voting power of the Company’s then-outstanding securities; or (ii) the Company’s then-outstanding equity securities (in either such case other than as a result of an acquisition of securities directly from the Company). |
| ● | Any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own, directly or indirectly, equity securities representing in the aggregate 50% or more of the combined voting power of the securities of the entity issuing cash or securities in the consolidation or merger (or of its ultimate parent entity, if any). |
| ● | Any sale, lease, exclusive license, exchange or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of the assets of the Company, other than a sale or disposition by the Company of all or substantially all of the Company’s assets to an entity, at least 50% of the combined voting power of the voting securities of which are owned by “persons” (as defined above) in substantially the same proportion as their ownership of the Company immediately prior to such sale. |
| ● | The members of the board at the beginning of any consecutive 24-calendar-month period (the “Incumbent Directors”) cease for any reason other than due to death to constitute at least a majority of the members of the board; provided that any director whose election, or nomination for election by the Company’s stockholders, was approved or ratified by a vote of at least a majority of the members of the board of directors then still in office who were members of the board of directors at the beginning of such 24-calendar-month period, will be deemed to be an Incumbent Director. |
68
Indemnification. The Company will indemnify each Executive and hold them harmless in connection with the defense of any lawsuit or other claim to which they are made a party by reason of being an officer, director, or employee of the Company, to the fullest extent permitted by Delaware law. This indemnification obligation does not extend to claims arising out of the Executives’ willful misconduct. In addition, the Company will use commercially reasonable efforts to maintain directors’ and officers’ liability insurance for each Executive for acts and omissions occurring during Executive’s employment with the Company.
Description of the 2025 Equity Incentive Plan
Following is a summary of the principal features of the Company’s 2025 Equity Incentive Plan, which we refer to as the 2025 Plan.
Key Provisions
Following are the key provisions of the 2025 Plan:
| Provisions of the 2025 Plan | Description | ||
| Share Reserve: | ● | As of the date of this Report, up to 2,196,390 shares of Common Stock are reserved under the 2025 Plan. | |
| ● | For a period of ten years commencing on February 11, 2025 and ending on (and including) February 11, 2035, the share reserve under the 2025 Plan will be increased by an amount equal to the lesser of (i) 4% of the number of shares outstanding as of December 31 of the immediately preceding calendar year or (ii) such lesser number of shares as determined by the board of directors prior to January 1 of a particular calendar year. | ||
| ● | The reserved shares will be reduced (i) by one share for each share granted pursuant to awards awarded under the 2025 Plan, and (ii) to the extent cash is delivered in lieu of shares of Common Stock upon the exercise of a stock appreciation right, we will be deemed to have issued the number of shares of Common Stock which it was entitled to issue upon such exercise. | ||
| Award Types: | ● | Incentive and nonstatutory stock options | |
| ● | Stock appreciation rights (“SARs”) | ||
| ● | Restricted stock awards | ||
| ● | Restricted stock unit awards (“RSUs”) | ||
| ● | Dividend equivalent rights | ||
| Vesting: | Determined by our board of directors or a committee designated by our board of directors. | ||
| Repricing: | Repricing of outstanding stock awards is not permitted without the approval of our stockholders, except for certain proportionate capitalization adjustments as set forth in the 2025 Plan. | ||
| Termination Date: | February 11, 2035 | ||
Administration
The 2025 Plan will be administered by our board of directors or a committee designated by our board of directors. With respect to grants of awards to our officers or directors, the 2025 Plan will be administered by our board of directors or a designated committee in a manner that permits such grants and related transactions to be exempt from Section 16(b) of the Exchange Act. The plan administrator will have the full authority to select recipients of the grants, determine the extent of the grants, establish additional terms, conditions, rules, or procedures to accommodate rules or laws of applicable non-United States jurisdictions, adjust awards, and to take any other action deemed appropriate; however, no action may be taken that is inconsistent with the terms of the 2025 Plan.
69
To the extent permitted by applicable law, the board of directors may delegate to a committee of one or more officers of the Company the authority to make awards or to take other actions pursuant to the 2025 Plan, but in no event shall an officer be delegated the authority to grant awards to, or amend awards held by, individuals who are subject to Section 16 of the Exchange Act, members of the board of directors, or officers to whom authority to grant or amend awards has been delegated. Any such delegation will be subject to the restrictions and limits that the board of directors specifies at the time of such delegation, and may be rescinded at any time by the board of directors.
Available Shares
Subject to annual adjustment and upon certain corporate transactions or events, the maximum aggregate number of shares of Common Stock which may be issued pursuant to all awards under the 2025 Plan is up to 2,196,390 shares as of the date of this Report.
For a period of ten years commencing on February 11, 2025 and ending on (and including) February 11, 2035, the share reserve described above will be increased by an amount equal to the lesser of (i) 4% of the number of shares outstanding as of December 31 of the immediately preceding calendar year or (ii) such lesser number of shares as determined by the board of directors prior to January 1 of a particular calendar year.
Any shares covered by an award that is forfeited, canceled, or expires will be deemed to have not been issued for purposes of determining the maximum aggregate number of shares which may be issued under the 2025 Plan. Shares that actually have been issued under the 2025 Plan pursuant to an award will not be returned to the 2025 Plan and will not become available for future issuance under the 2025 Plan, other than unvested shares that are forfeited or repurchased by us. In the event any option or other award granted under the 2025 Plan is exercised through the tendering of shares (either actually or through attestation), or in the event tax withholding obligations are satisfied by tendering or withholding shares, any shares so tendered or withheld are not again available for awards under the 2025 Plan. To the extent that cash is delivered in lieu of shares of Common Stock upon the exercise of an SAR, then we will be deemed, for purposes of applying the limitation on the number of shares, to have issued the number of shares of Common Stock which were otherwise issuable upon such exercise. Shares of Common Stock we reacquire on the open market or otherwise using cash proceeds from the exercise of options will not be available for awards under the 2025 Plan.
Dividends
No dividend or dividend equivalent will be paid on any unvested award, although the plan administrator may provide in an award agreement that dividends with respect to unvested portions of awards may accrue and be paid when and if the awards vest and shares are actually issued to the participant.
Eligibility and Types of Awards
The 2025 Plan will permit us to grant stock awards, including stock options, SARs, restricted stock, RSUs, and dividend equivalent rights to our employees, directors, and consultants.
Stock Options
A stock option may be an incentive stock option within the meaning of, and qualifying under, Section 422 of the Code, or a nonstatutory stock option. However, only our employees (or employees of our parent or subsidiaries, if any) may be granted incentive stock options. Incentive and nonstatutory stock options are granted pursuant to option agreements adopted by the plan administrator. The plan administrator will determine the exercise price for a stock option, within the terms and conditions of the 2025 Plan provided that the exercise price of a stock option cannot be less than 100% of the fair market value of our Common Stock on the date of grant (or 110% of the fair market value in the case of certain incentive stock options, as described below). Options granted under the 2025 Plan will become exercisable at the rate specified by the plan administrator.
70
The plan administrator will determine the term of the stock options granted under the 2025 Plan up to a maximum of ten years, except in the case of certain incentive stock options, as described below. Unless the terms of an optionholder’s stock option agreement provide otherwise, if an optionholder’s relationship with us, or any of our affiliates, ceases for any reason other than disability or death, the optionholder may exercise any options otherwise exercisable as of the date of termination, but only during the post-termination exercise period designated in the optionholder’s stock option award agreement. The optionholder’s stock option award agreement may provide that upon the termination of the optionholder’s relationship with us for cause, the optionholder’s right to exercise his or her options will terminate concurrently with the termination of the relationship. If an optionholder’s service relationship with us, or any of our affiliates, ceases due to disability or death, or an optionholder dies within a certain period following cessation of service, the optionholder or his or her estate or person who acquired the right to exercise the award by bequest or inheritance may exercise any vested options for a period of 12 months. The option term may be extended in the event that exercise of the option within the applicable time periods is prohibited by applicable securities laws or such longer period as specified in the stock option award agreement but in no event beyond the expiration of its term.
Acceptable consideration for the purchase of Common Stock issued upon the exercise of a stock option will be determined by the plan administrator and may include (i) cash or check, (ii) a broker-assisted cashless exercise, (iii) the tender of Common Stock previously owned by the optionholder, (iv) a net exercise of the option, (v) past or future services rendered, and (vi) any combination of the foregoing methods of payment.
Unless the plan administrator provides otherwise, awards generally are not transferable, except by will or the laws of descent and distribution. Incentive stock options may be granted only to our employees (or to employees of our parent company and subsidiaries, if any). To the extent that the aggregate fair market value, determined at the time of grant, of shares of our Common Stock with respect to which incentive stock options are exercisable for the first time by an optionholder during any calendar year under any of our equity plans exceeds $100,000, such options will not qualify as incentive stock options and will instead be treated as nonstatutory stock options. A stock option granted to any employee who, at the time of the grant, owns or is deemed to own stock representing more than 10% of the voting power of all classes of our stock (or that of our parent or subsidiaries, if any) may not be an incentive stock option unless (i) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and (ii) the term of the incentive stock option does not exceed five years from the date of grant.
Stock Appreciation Rights
SARs may be granted under the 2025 Plan either concurrently with the grant of an option or alone, without reference to any related stock option. The plan administrator will determine both the number of shares of Common Stock related to each SAR and the exercise price for an SAR, within the terms and conditions of the 2025 Plan, provided that the exercise price of an SAR cannot be less than 100% of the fair market value of the Common Stock subject thereto on the date of grant. In the case of an SAR granted concurrently with a stock option, the number of shares of Common Stock to which the SAR relates will be reduced in the same proportion that the holder of the stock option exercises the related option.
The plan administrator will determine whether to deliver cash in lieu of shares of Common Stock upon the exercise of an SAR. If Common Stock is issued, the number of shares of Common Stock that will be issued upon the exercise of an SAR is determined by dividing (i) the number of shares of Common Stock as to which the SAR is exercised multiplied by the amount of the appreciation in such shares, by (ii) the fair market value of a share of Common Stock on the exercise date.
If the plan administrator elects to pay the holder of the SAR cash in lieu of shares of Common Stock, the holder of the SAR will receive cash equal to the fair market value on the exercise date of any or all of the shares that would otherwise be issuable.
The exercise of an SAR related to a stock option is permissible only to the extent that the stock option is exercisable under the terms of the 2025 Plan on the date of surrender. Any incentive stock option surrendered will be deemed to have been converted into a nonstatutory stock option immediately prior to such surrender.
71
Restricted Stock
Restricted stock awards are awards of shares of our Common Stock that are subject to established terms and conditions. The plan administrator sets the terms of the restricted stock awards, including the size of the restricted stock award, the price (if any) to be paid by the recipient, and the vesting schedule and criteria (which may include continued service to us for a period of time or the achievement of performance criteria). If a participant’s service terminates before the restricted stock is fully vested, all of the unvested shares generally will be forfeited to, or repurchased by, us.
Restricted Stock Units
An RSU is a right to receive stock, cash equal to the value of a share of stock, or other securities, or a combination of the three at the end of a set period or the attainment of performance criteria. No stock is issued at the time of grant. The plan administrator sets the terms of the RSU award, including the size of the RSU award, the consideration (if any) to be paid by the recipient, vesting schedule, and criteria and form (stock or cash) in which the award will be settled. If a participant’s service terminates before the RSU is fully vested, the unvested portion of the RSU award generally will be forfeited to us.
Dividend Equivalent Rights
Dividend equivalent rights entitle the recipient to compensation measured by dividends paid with respect to a specified number of shares of Common Stock. The plan administrator sets the terms of any award of dividend equivalent rights.
Performance-Based Compensation
The 2025 Plan establishes procedures for the Company to grant performance-based awards, meaning awards structured so that they will vest only upon the achievement of performance criteria established by the plan administrator for a specified performance period. Performance criteria may be measured on an absolute (e.g., plan or budget) or relative basis, and may be established on a corporate-wide basis or with respect to one or more business units, divisions, subsidiaries or business segments, or may be established on an individual basis. Relative performance may be measured against a group of peer companies, a financial market index or other acceptable objective and quantifiable indices. The plan administrator will have the discretion to adjust the minimum level of achievement required for achievement of performance awards if the plan administrator determines that a change in our business, operations, corporate structure or capital structure, the manner in which we conduct our business, or other events or circumstances render the performance objectives unsuitable. The plan administrator will also have the discretion to adjust the performance objectives for other material events not originally contemplated when the performance objectives were established, such as extraordinary gains and losses, the effect of changes in accounting standards or principles, acquisitions or divestitures, changes in tax rules or regulations, capital transactions, restructuring, nonrecurring gains or losses or other unusual items.
The business measures that may be used to establish the performance criteria may include one of, or combination of, the following:
| ● | Net earnings or net income (before or after taxes); |
| ● | Earnings per share; |
| ● | Net sales growth; |
| ● | Net operating profit; |
| ● | Return measures (including, but not limited to, return on assets, capital, equity, or sales); |
| ● | Cash flow (including, but not limited to, operating cash flow, free cash flow, and cash flow return on capital); |
| ● | Cash flow per share; |
| ● | Earnings before or after taxes, interest, depreciation, and/or amortization; |
| ● | Gross or operating margins; |
72
| ● | Productivity ratios; |
| ● | Share price (including, but not limited to, growth measures and total stockholder return); |
| ● | Expense targets or ratios; |
| ● | Charge-off levels; |
| ● | Improvement in or attainment of revenue levels; |
| ● | Operating efficiency; |
| ● | Operating expenses; |
| ● | Economic value added; |
| ● | Improvement in or attainment of expense levels; |
| ● | Improvement in or attainment of working capital levels; |
| ● | Debt reduction; |
| ● | Capital targets; |
| ● | Consummation of acquisitions, dispositions, projects, or other specific events or transactions; or |
| ● | Other significant business milestones. |
Corporate Transactions
Effective upon the consummation of a corporate transaction, all outstanding awards under the 2025 Plan will terminate unless they are assumed in connection with the corporate transaction.
The plan administrator has the authority to determine, before or at the time of any corporate transaction, the impact that the corporate transaction will have on outstanding awards under the 2025 Plan. For example, the plan administrator may determine that (i) awards will vest and become exercisable, or that other restrictions on such awards will lapse, (ii) awards will be assumed by the surviving corporation in the corporate transaction or replaced with awards that have substantially equivalent terms, (iii) participants will receive a payment in satisfaction of outstanding awards, and (iv) in the case of options and SARs, participants will receive a payment in an amount equal to the amount, if any, by which the fair market value of the shares subject to award exceeds the exercise price. The plan administrator is not required to treat all awards in the same way.
Compensation Recovery (Clawback) Policy
All awards under the 2025 Plan will be subject to reduction, cancellation, forfeiture or recoupment to the extent necessary to comply with any applicable compensation recovery, clawback, forfeiture or other similar policy adopted by our board of directors and as in effect from time to time or applicable law. Further, to the extent that a participant receives any amount in excess of the amount that the participant should otherwise have received under the terms of an award for any reason (including, without limitation, by reason of a financial restatement, mistake in calculations or other administrative error), the participant may be required to repay any such excess amount to the Company.
Amendment and Termination
Our board of directors generally may amend, suspend, or terminate the 2025 Plan. However, it may not amend the 2025 Plan without stockholder approval for certain actions, such as an increase in the number of shares reserved under the 2025 Plan, modifications to the provisions of the 2025 Plan regarding the grant of incentive stock options, modifications to the provisions of the 2025 Plan regarding the exercise prices at which shares may be offered pursuant to options, extension of the expiration date of the 2025 Plan, and certain modifications to awards, such as reducing the exercise price per share, canceling and regranting new awards with lower prices per share than the original prices per share of the cancelled awards, or canceling any awards in exchange for cash or the grant of replacement awards with an exercise price that is less than the exercise price of the original awards.
Tax Withholding
The plan administrator may require a participant to satisfy any federal, state, local, or foreign tax withholding obligation relating to a stock award by (i) causing the participant to tender a cash payment, (ii) withholding shares of Common Stock from the shares of Common Stock issued or otherwise issuable to the participant in connection with the award, (iii) delivering to the Company already-owned shares of Common Stock, (iv) selling shares of Common Stock from the shares of Common Stock issued or otherwise issuable to the participant in connection with the award, (v) withholding cash from an award settled in cash or other amounts payable to the participant, and/or (vi) any other means that the plan administrator determines both to comply with applicable laws and be consistent with the purposes of the 2025 Plan.
73
Summary of United States Federal Income Tax Aspects Related to 2025 Plan
The following summary is intended only as a general guide to certain United States federal income tax consequences under current law of participation in the 2025 Plan and does not attempt to describe all possible federal or other tax consequences of such participation or tax consequences based on any participant’s particular circumstances. The summary does not purport to be complete, and it does not address the tax consequences of the participant’s death, any tax laws of any municipality, state or foreign country in which a participant might reside, or any other laws other than United States federal income tax laws. Furthermore, the tax consequences are complex and subject to change, and a participant’s particular situation may be such that some variation of the described rules is applicable. Recipients of awards under the 2025 Plan should consult their own tax advisors to determine the tax consequences to them as a result of their particular circumstances.
Incentive Stock Options
A participant recognizes no taxable income for regular income tax purposes as a result of the grant or exercise of an incentive stock option qualifying under Section 422 of the Code.
If a participant holds stock acquired through exercise of an incentive stock option for more than two years from the date on which the option was granted and more than one year after the date the option was exercised for those shares, any gain or loss on a disposition of those shares (a “qualifying disposition”) will be a long-term capital gain or loss. Upon such a qualifying disposition, we will not be entitled to any income tax deduction.
If a participant disposes of underlying shares within two years after the date of grant of the option or within one year after the date of exercise of the option (a “disqualifying disposition”), the difference between the fair market value of the shares on the option exercise date and the exercise price (not to exceed the gain realized on the sale if the disposition is a transaction with respect to which a loss, if sustained, would be recognized) will be taxed to the participant as ordinary income at the time of disposition. Any gain in excess of that amount will be a capital gain. If a loss is recognized, there will be no ordinary income, and such loss will be a capital loss. To the extent the participant recognizes ordinary income by reason of a disqualifying disposition, generally we will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Internal Revenue Code of 1986, as amended (“the Code”) limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation) to a corresponding income tax deduction in the tax year in which the disqualifying disposition occurs.
The difference between the option exercise price and the fair market value of the shares on the exercise date of an incentive stock option is treated as an adjustment in computing the participant’s alternative minimum taxable income and may subject the participant to alternative minimum tax liability for the year of exercise. Special rules may apply after exercise for (i) sales of the shares in a disqualifying disposition, (ii) basis adjustments for computing alternative minimum taxable income on a subsequent sale of the shares, and (iii) tax credits that may be available to participants subject to the alternative minimum tax.
Nonstatutory Stock Options
Options not qualifying as incentive stock options, along with options expressly designated as nonstatutory stock options, will be nonstatutory stock options having no special tax status. A participant generally recognizes no taxable income upon the grant of such an option so long as (i) the exercise price is not less than the fair market value of the stock on the date of grant, and (ii) the option (and not the underlying stock) at such time does not have a readily ascertainable fair market value (as defined in Treasury Regulations under the Code). Upon exercise of a nonstatutory stock option, the participant normally recognizes ordinary income in the amount of the difference between the option exercise price and the then-fair market value of the shares purchased. If the participant is an employee, such ordinary income amount will be subject to withholding of income and employment taxes. Generally, the Company will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation) to an income tax deduction in the tax year in which such ordinary income is recognized by the participant.
74
Upon the disposition of stock acquired by the exercise of a nonstatutory stock option, any recognized gain or loss, based on the difference between the sale price and the fair market value on the exercise date, will be taxed as capital gain or loss, which will be short-term or long-term gain or loss, depending on the holding period of the stock.
Stock Appreciation Rights
A participant will not normally recognize taxable income upon the receipt of an SAR. Upon the exercise of an SAR, the participant will recognize ordinary income in an amount equal to the excess of the fair market value of the underlying shares of Common Stock on the exercise date over the exercise price. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes. The Company generally will be entitled to a deduction equal to the amount of ordinary income recognized by the participant in connection with the exercise of the SAR (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation).
Restricted Stock
A participant acquiring restricted stock generally will recognize ordinary income equal to the difference between the fair market value of the shares on the “determination date” (as defined below) and their purchase price, if any. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes.
The “determination date” is the date on which the participant acquires the shares unless they are subject to a substantial risk of forfeiture and are not transferable, in which case the determination date is the earliest of (i) the date the shares become transferable, (ii) the date the shares are no longer subject to a substantial risk of forfeiture, or (iii) the date the shares are acquired if the participant makes a timely election under Code Section 83(b). If the shares are subject to a substantial risk of forfeiture and not transferable when issued, the participant may elect, pursuant to Section 83(b) of the Code, to have the date of acquisition be the determination date by filing an election with the Internal Revenue Service, and other provisions, no later than 30 days after the date the shares are acquired.
Upon the taxable disposition of shares acquired pursuant to a restricted stock award, any gain or loss, based on the difference between the sale price and the fair market value on the determination date, will generally be taxed as capital gain or loss; however, for any shares returned to the Company pursuant to a forfeiture provision, a participant’s loss may be computed based only on the purchase price (if any) of the shares and may not take into account any income recognized by reason of a Section 83(b) election. Such gain or loss will be long-term or short- term depending on whether the stock was held for more than one year.
We generally will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax reporting obligation) to a corresponding income tax deduction in the year in which the ordinary income from restricted stock is recognized by the participant.
Restricted Stock Units
A participant will not normally recognize taxable income upon receipt of an RSU award. In general, the participant will recognize ordinary income in the year in which the units vest and are settled in an amount equal to any cash received and/or the fair market value of any nonrestricted shares received. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes. We generally will be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax reporting obligation) to an income tax deduction equal to the amount of ordinary income recognized by the participant.
75
Dividend Equivalent Rights
A recipient of dividend equivalent rights generally will recognize ordinary income at the time the dividend equivalent right is paid. If the participant is an employee, such ordinary income generally is subject to withholding of income and employment taxes. We will generally be entitled (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax reporting obligation) to an income tax deduction equal to the amount of ordinary income recognized by the participant.
Other Awards
We generally will be entitled to an income tax deduction in connection with an award under the 2025 Plan in an amount equal to the ordinary income realized by the participant at the time the participant recognizes such income (subject to the requirement of reasonableness, the provisions of Section 162(m) and other provisions of the Code limiting the deduction of compensation, and the satisfaction of a tax-reporting obligation). Participants typically are subject to income (and employment) tax and recognize such tax at the time that an award is granted, exercised, vests, or becomes nonforfeitable, unless the award provides for a further deferral.
Section 409A
Section 409A of the Code (“Section 409A”) imposes certain requirements on nonqualified deferred compensation arrangements. Most awards granted under the 2025 Plan will be designed to qualify for an exemption from the requirements of Section 409A. Certain awards under the 2025 Plan, however, may be subject to the requirements of Section 409A in form and in operation. Awards that are subject to Section 409A will generally be designed to meet the conditions under Section 409A for avoiding the adverse tax consequences resulting from a failure to comply with Section 409A. If an award under the 2025 Plan is subject to Section 409A and fails to satisfy the requirements of Section 409A, the recipient of that award may recognize ordinary income on the amounts deferred under the award, to the extent vested, which may be before the compensation is actually or constructively received.
Also, if an award that is subject to Section 409A fails to comply with the requirements of Section 409A, Section 409A imposes an additional 20% tax on the participant’s compensation recognized as ordinary income, as well as interest on such deferred compensation.
Impact of Section 162(m) on Tax Deductibility of Awards Under the 2025 Plan
Section 162(m) of the Code limits the deductibility for federal income tax purposes of certain compensation paid to any of our covered employees in excess of $1 million. For purposes of Section 162(m), the term “covered employee” generally includes our chief executive officer, our chief financial officer, our three other most highly compensated officers, any individual who was a covered employee for any taxable year beginning after December 31, 2016, and, for any taxable year beginning after December 31, 2026, the next five highest-compensated employees. Compensation attributable to awards under the 2025 Plan either on its own or when combined with all other types of compensation received by a covered employee from the Company, may cause this limitation to be exceeded in any particular year. In addition, the Company’s ability to realize the benefit of any tax deductions described above depends on our generation of taxable income as well as the requirement of reasonableness, other limitations on deductions in the Code and the satisfaction of tax reporting obligations.
2025 Plan Benefits
The 2025 Plan does not provide for set benefits or amounts of awards and we have not approved any stock awards that are conditioned on stockholder approval of the 2025 Plan. We have not approved any stock awards under the 2025 Plan in connection with the Merger. All future awards to directors, executive officers, employees and consultants under the 2025 Plan are discretionary and cannot be determined at this time.
76
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transactions with Lucius Partners and Related Persons
On March 10, 2022, Unite Acquisition issued an aggregate of 5,000,000 shares of Common Stock to its sole stockholder, Lucius Partners, for an aggregate purchase price equal to $500, pursuant to the terms and conditions set forth in the Common Stock Purchase Agreement with Lucius Partners. The Company issued these shares of Common Stock under the exemption from registration provided by Section 4(a)(2) of the Securities Act.
Also on March 10, 2022, Unite Acquisition issued an unsecured promissory note to Lucius Partners, pursuant to which the Company agreed to repay Lucius Partners the sum of any and all amounts that Lucius Partners may advance to the Company on or before the date that the Company consummates a business combination with a private company or reverse takeover transaction or other transaction after which the Company would cease to be a shell company (as defined in Rule 12b-2 under the Exchange Act). The Company has used the proceeds from the note to cover its expenses. Interest did not accrue on the outstanding principal amount of the note except if an Event of Default (as defined in the note) has occurred. In the event of an Event of Default, the entire note shall automatically become due and payable (the “Default Date”), and starting from five days after the Default Date, the interest rate on the note shall accrue at the rate of 18% per annum. As of September 30, 2024 and December 31, 2023, the amount due under the note payable was $137,764 and $81,219, respectively.
Also effective March 10, 2022, Unite Acquisition entered into a services agreement with Lucius Partners, pursuant to which Unite Acquisition paid Lucius Partners a quarterly fee of $1,250 for advisory, accounting, and administrative support services. Unite Acquisition used the office space and equipment of its management under this agreement. This services agreement was terminated in connection with the Merger.
On October 28, 2024, Unite Acquisition issued an unsecured promissory note (the “October 2024 Note”) to Lucius Partners Opportunity Fund, LP (“LPOF”) and received $275,000. The annual interest rate on the promissory note is 12%. The note matures on October 28, 2025, and can be prepaid at any time without penalty. Unite Acquisition used the proceeds to pay off the note to Lucius Partners described above and the director fees owed to Nathan Pereira and other accrued expenses. The general partner of the new lender LPOF is Lucius Capital Partners LLC (“LCP”). The investment manager of LPOF is Lucius Capital Fund Management, LLC (“LCFM”). Lucius Partners, LCP and LCFM have two individuals in common as members. The October 2024 Note was repaid in connection with the Merger and Offering.
Following the closing of the Offering, pursuant to an Advisory Services Agreement dated February 11, 2025 with Lucius Partners, the combined Company will pay to Lucius Partners a cash fee of $180,000 for advisory services during the first year following such closing, and has agreed to pay Lucius Partners for advisory services, in advance for four consecutive three-month periods, commencing on the first day of the month that is the first full month 12 months or more after the closing, a cash fee of $45,000 for one year (such two-year period, the “Advisory Period”). The Advisory Period can be renewed for additional one-year periods upon written request by the Company within 60 days prior to the expiry of any Advisory Period.
Policies and Procedures for Related Person Transactions
Our Audit Committee has the primary responsibility for reviewing and approving or disapproving “related party transactions,” as defined in applicable SEC rules and regulations. As provided in the Audit Committee charter, in approving or rejecting any such transaction, our Audit Committee is to consider the relevant facts and circumstances available and deemed relevant to it, including, among other factors, whether the terms or other aspects of the transaction differ from those that would likely be negotiated with independent third parties.
All of the transactions described in this section were entered into prior to the adoption of the Audit Committee charter. Although we have not had a written policy for the review and approval of transactions with related persons, our board of directors has historically reviewed and approved any transaction where a director or officer had a financial interest, including the transactions described above. Prior to approving such a transaction, the material facts as to the relationship or interest of the relevant related person in the agreement or transaction were disclosed to our board of directors. Our board of directors took this information into account when evaluating the transaction and in determining whether such transaction was fair to us and in the best interest of all our stockholders.
77
POTENTIAL CONFLICTS OF INTEREST
The sole holder of Unite Acquisition prior to the Merger, Lucius Partners, holds 3,250,000 shares of Common Stock after the Merger, which represents 40.2% of the outstanding shares of the combined Company as of the date of this Report. Lucius Partners purchased its shares upon formation of the Company for a nominal price. Matthew Eitner, the Chief Executive Officer of the Placement Agent, James Ahern, the Managing Partner of the Placement Agent, and Patrick Gallagher, a member of our board of directors, a Managing Director of the Placement Agent, are members and managers and/or officers of Lucius Partners, and therefore are indirectly material stakeholders of the Company. Anthony Zook is also a member of the board of directors of the Company and is a member of Lucius Partners. The Placement Agent and/or its designees hold warrants to purchase an aggregate of 270,204 shares of our Common Stock. Therefore, following the Merger, such persons can exert substantial control over matters requiring the approval of our stockholders.
Additionally, Lucius Partners has received, and will continue to receive after the Merger, fees from us for advisory and certain other services to the Company, as described above under “Certain Relationships and Related Party Transactions—Transactions with Lucius Partners and Related Persons.” Lucius has acted as Unite Acquisition’s advisor from inception and has provided certain services to Unite Acquisition including, but not limited to, formation and development work, strategic advisory services, operational support services, legal and accounting referral services, working capital and financial strategy and compliance direction services, including but limited to identifying Private Adaptin as a potential merger candidate and assisting Unite Acquisition in all aspect of its development through the Merger.
See “Risk Factors - Our principal stockholders will have significant influence over the election of our board of directors and approval of any significant corporate actions, including any sale of the Company” and “Certain Relationships and Related Party Transactions—Transactions with Lucius Partners and Related Persons” above.
78
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information with respect to the beneficial ownership of our Common Stock as of February 11, 2025, immediately following the closing of the Merger and the Offering, by:
| ● | each of our named executive officers; |
| ● | each of our directors; |
| ● | all of our current directors and executive officers as a group; and |
| ● | each person, or group of affiliated persons, who beneficially owned more than 5% of our Common Stock. |
We have determined beneficial ownership in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Except as indicated by the footnotes below, we believe, based on information furnished to us, that the persons and entities named in the table below have sole voting and sole investment power with respect to all shares of Common Stock that they beneficially owned, subject to applicable community property laws.
The percentage of shares beneficially owned is computed on the basis of 8,081,953 shares of Common Stock outstanding as of February 11, 2025, after giving effect to the Merger and the closing of the Offering. Shares of Common Stock that a person has the right to acquire within 60 days of February 11, 2025 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated, the address of each beneficial owner in the table below is 3540 Toringdon Way, Suite 200, #250 Charlotte, NC 28277.
| Name | Shares
of Common Stock Beneficially Owned | Percentage of Common Stock Beneficially Owned | ||||||
| 5% Stockholders | ||||||||
| Lucius Partners LLC(1) | 3,520,204 | 42.15 | % | |||||
| Directors and Named Executive Officers | ||||||||
| Michael J. Roberts, PhD | 2,159,468 | 26.72 | % | |||||
| Simon C. Pedder, PhD | 928,571 | 11.49 | % | |||||
| Timothy L. Maness, CPA | - | - | ||||||
| L. Arthur Hewitt, PhD | - | - | ||||||
| Patrick Gallagher | - | - | ||||||
| Anthony Zook | - | - | ||||||
| J. Nick Riehle | - | - | ||||||
| All directors and executive officers as a group (7 persons) | 3,088,039 | 38.21 | % | |||||
| (1) | Includes 270,204 shares of our Common Stock issuable upon the exercise of immediately vested warrants the Placement Agent and/or its designees hold as of February 11, 2025. Matthew Eitner, the Chief Executive Officer of the Placement Agent, James Ahern, the Managing Partner of the Placement Agent, and Patrick Gallagher, a Managing Director of the Placement Agent, are managing members, members and/or officers of Lucius Partners. Mr. Eitner, but not Messrs. Ahern and Gallagher, has voting and investment control over securities held by the Placement Agent and Lucius Partners. |
79
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY
AND RELATED STOCKHOLDER MATTERS
Our Common Stock is not listed on a national securities exchange, an over-the-counter market or any other exchange. Therefore, there is no trading market, active or otherwise, for our Common Stock and our Common Stock may never be included for trading on any stock exchange, automated quotation system or any over-the-counter market.
As of the date of this Report, we have 8,081,953 shares of Common Stock outstanding held by 114 stockholders of record.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our board of directors and will be dependent upon financial condition, results of operations, capital requirements and such other factors as the board of directors deems relevant.
Shares Eligible for Future Sale
Prior to the Merger, there has been a limited public market for our Common Stock. Future sales of our Common Stock, including shares issued upon the exercise of options or warrants that we may issue, in the public market after the Merger, or the perception that those sales may occur, could cause the prevailing price for our Common Stock to fall or impair our ability to raise equity capital in the future. As described below, only a limited number of shares of our Common Stock will be available for sale in the public market for a period of several months after consummation of the Merger due to contractual and legal restrictions on resale described below. Future sales of our Common Stock in the public market either before (to the extent permitted) or after restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing price of our Common Stock at such time and our ability to raise equity capital at a time and price we deem appropriate.
Upon the closing of the Offering, we had 8,081,953 shares of Common Stock outstanding, of which our directors and executive officers beneficially own an aggregate of 3,088,039 shares. Of those outstanding shares, no shares of Common Stock are freely tradable, without restriction, as of the date of this Report. No shares issued in connection with the Merger or the Offering can be publicly sold under Rule 144 under the Securities Act until 12 months after the date of filing this Report.
Sale of Restricted Shares
All of the approximately 8,081,953 shares of Common Stock outstanding upon completion of the Merger and the initial Offering are “restricted securities” as such term is defined in Rule 144. These restricted securities were issued and sold by us in private transactions and are eligible for public sale only if registered under the Securities Act or if they qualify for an exemption from registration under the Securities Act, including the exemptions provided by Rule 144, which rules are summarized below.
Rule 144
Pursuant to Rule 144 promulgated under the Securities Act, sales of the securities of a former shell company, such as us, under that rule are not permitted (i) until at least 12 months have elapsed from the date on which this Report, reflecting our status as a non-shell company, is filed with the SEC and (ii) unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than Current Reports on Form 8-K. Our stockholders will be forced to hold their shares of our Common Stock for at least the 12-month period after this Report is filed before they are eligible to sell those shares, and even after that 12-month period, sales may not be made under Rule 144 unless we and the selling stockholders are in compliance with other requirements of Rule 144.
80
In general, Rule 144 provides that (i) any of our non-affiliates that has held restricted Common Stock for at least 12 months is thereafter entitled to sell its restricted stock freely and without restriction, provided that we remain compliant and current with our SEC reporting obligations, and (ii) any of our affiliates, which includes our directors, executive officers and other person in control of us, that has held restricted Common Stock for at least 12 months is thereafter entitled to sell its restricted stock subject to the following restrictions: (a) we are compliant and current with our SEC reporting obligations, (b) certain manner of sale provisions are satisfied, (c) a Form 144 is filed with the SEC, and (d) certain volume limitations are satisfied, which limit the sale of shares within any three-month period to a number of shares that does not exceed 1% of the total number of outstanding shares or, if our Common Stock is then listed or quoted for trading on a national securities exchange, then the greater of 1% of the total number of outstanding shares and the average weekly trading volume of our Common Stock during the four calendar weeks preceding the filing of the Form 144 with respect to the sale. A person who has ceased to be an affiliate at least three months immediately preceding the sale and who has owned such shares of Common Stock for at least one year is entitled to sell the shares under Rule 144 without regard to any of the limitations described above.
Regulation S
Regulation S under the Securities Act provides that shares owned by any person may be sold without registration in the United States, provided that the sale is effected in an offshore transaction and no directed selling efforts are made in the United States (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that our shares of Common Stock may be sold in some other manner outside the United States without requiring registration in the United States.
Stock Plans
We intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the shares of Common Stock that are outstanding or reserved for issuance under the 2025 Plan. Such registration statement is expected to be filed and become effective as soon as practicable after the consummation of the Merger (but no fewer than 60 days thereafter pursuant to SEC rules that apply to former shell companies). Accordingly, shares registered under such registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the lock-up agreements described above, if applicable.
81
DESCRIPTION OF CAPITAL STOCK
The following description summarizes the most important terms of our capital stock following the Merger and Offering. Because it is only a summary, it does not contain all the information that may be important to you and the descriptions herein are qualified by reference to our restated certificate of incorporation and restated bylaws. For a complete description, you should refer to our restated certificate of incorporation and restated bylaws, which are included as exhibits hereto, and to the applicable provisions of Delaware law.
We have authorized capital stock consisting of 50,000,000 shares of Common Stock, par value $0.0001 per share, and 10,000,000 shares of preferred stock, par value $0.0001 per share.
As of the date of this Report, we had 8,081,953 shares of Common Stock issued and outstanding, warrants exercisable for 2,023,995 shares of Common Stock outstanding, and no shares of preferred stock issued and outstanding. Unless stated otherwise, the following discussion summarizes the term and provisions of our restated certificate of incorporation and our restated bylaws.
Common Stock
Dividend Rights
Subject to applicable law and the rights and preferences, if any, of any holders of any outstanding series of preferred stock, the holders of our Common Stock are entitled to receive dividends if our board of directors, in its discretion, determines to issue dividends and then only at the times and in the amounts that our board of directors may determine, payable either in cash, in property or in shares of capital stock.
Voting Rights
Holders of our Common Stock are entitled to one vote for each share of Common Stock held on all matters submitted to a vote of stockholders. Except as otherwise required by law, holders of Common Stock are not entitled to vote on any amendment to the restated certificate of incorporation (including any certificate of designation relating to any series of preferred stock) that relates solely to the terms of one or more outstanding series of preferred stock if the holders of such affected series are entitled, either separately or together as a class with the holders of one or more other such series, to vote on such amendment pursuant to the restated certificate (including any certificate of designation relating to any series of preferred stock). We have not provided for cumulative voting for the election of directors in our restated certificate of incorporation. Accordingly, holders of a majority of the shares of our Common Stock are able to elect all of our directors.
No Preemptive or Similar Rights
Our Common Stock is not entitled to preemptive rights and is not subject to conversion, redemption or sinking fund provisions.
Right to Receive Liquidation Distributions
Upon our liquidation, dissolution, or winding-up and after payment in full of all amounts required to be paid to creditors and to any holders of preferred stock having liquidation preferences, if any, the assets legally available for distribution to our stockholders would be distributable ratably among the holders of our Common Stock.
Preferred Stock
Our board of directors is authorized, subject to limitations prescribed by Delaware law, to issue preferred stock in one or more series, to establish from time to time the number of shares to be included in each series, and to fix the designation, vesting, powers (including voting powers), preferences, and relative, participating, optional or other rights of the shares of each series and any of its qualifications, limitations, or restrictions, in each case without further vote or action by our stockholders.
82
Our board of directors can also increase or decrease the number of shares of any series of preferred stock, but not below the number of shares of that series then outstanding or above the total number of authorized shares of the class, without any further vote or action by our stockholders. Our board of directors may, without stockholder approval, authorize the issuance of preferred stock with voting or other rights that could adversely affect the voting power or other rights of the holders of our Common Stock and could have anti-takeover effects. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring, or preventing a change in our control or the removal of existing management and might adversely affect the market price of our Common Stock.
Stock Options
As of the date of this Report, we have no outstanding stock options to shares of our Common Stock under the 2025 Plan or otherwise.
Warrants
As of the date of this Report, we had outstanding the following warrants:
| (i) | Common A Warrants to purchase an aggregate of 1,080,814 shares of our Common Stock, with an exercise price of $4.40 per share; |
| (ii) | Common B Warrants to purchase an aggregate of 540,407 shares of our Common Stock, with an exercise price of $6.60 per share; |
| (iii) | Pre-Merger Warrants to purchase an aggregate of 132,570 shares of our Common Stock, with an exercise price of either $3.30 per share or $4.40 per share; and |
| (iv) | Placement Agent Warrants to purchase an aggregate of 270,204 shares of our Common Stock, with an exercise price of $4.40 per share of our Common Stock. |
Registration Rights Agreement
For a description of the Registration Rights Agreement that we entered into in connection with the Merger and the Offering, see “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Registration Rights” above. All descriptions of the Registration Rights Agreement herein are qualified in their entirety by reference to the text thereof filed as Exhibit 10.8 hereto and incorporated herein by reference.
Anti-Takeover Provisions
The provisions of the DGCL, our restated certificate of incorporation, and our restated bylaws could have the effect of delaying, deferring, or discouraging another person from acquiring control of our Company by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers and directors. These provisions, which are summarized below, are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and encourage persons seeking to acquire control of our Company to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms. However, these provisions may delay, deter or prevent a merger or acquisition of us that a stockholder might consider is in their best interest or in our best interests, including transactions that might result in a premium over the prevailing market price of our Common Stock.
83
Section 203 of the DGCL
We are subject to the provisions of Section 203 of the DGCL regulating corporate takeovers. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner as summarized below. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| ● | before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| ● | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding shares owned by persons who are directors and also officers, and employee stock plans in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| ● | at or after the time the stockholder became interested, the business combination was approved by our board and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| ● | any merger or consolidation involving the corporation and the interested stockholder; |
| ● | any sale, transfer, lease, pledge, or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| ● | subject to exceptions, any transaction that results in the issuance of transfer by the corporation of any stock of the corporation to the interested stockholder; |
| ● | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; and |
| ● | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Restated Certificate of Incorporation and Restated Bylaw Provisions
Our restated certificate of incorporation and our restated bylaws include a number of provisions that may have the effect of deterring hostile takeovers, or delaying or preventing changes in control of our management team or changes in our board of directors or our governance or policy, including the following:
| ● | Board of Director Vacancies. Our restated bylaws and restated certificate of incorporation provide, subject to the special rights of the holders of any series of preferred stock to elect directors, that any vacancy on the board of directors may be filled by the affirmative vote of a majority of the directors then in office, even if less than a quorum, or by a sole remaining director, and not by the stockholders, unless (a) the board of directors determines by resolution that any such vacancies or newly created directorships shall be filled by the stockholders or (b) as otherwise provided by law. Any director chosen to fill a vacancy will hold office until the next annual meeting of stockholders and until his or her successor is duly elected and qualified, or until his or her earlier death, resignation, disqualification or removal. In addition, the number of directors constituting the total number of authorized directors is permitted to be set only by a resolution adopted by a majority of the board of directors. These provisions prevent a stockholder from increasing the size of our board of directors and gaining control of our board of directors by filling the resulting vacancies with its own nominees. This makes it more difficult to change the composition of the board of directors, but promotes continuity of management. |
84
| ● | Supermajority Requirements for Amendments of Our Restated Certificate of Incorporation and Restated Bylaws. Our restated certificate of incorporation provides that the affirmative vote of holders of at least 66 2/3% of our capital stock entitled to vote generally in the election of directors, voting together as a single class, is required to amend certain provisions of our restated certificate of incorporation, including provisions relating to the size of the board of directors, the limitation of personal liability for the board of directors and officers, special meetings, actions by written consent, the choice of forum provision, and designation of our preferred stock. The affirmative vote of holders of at least 66 2/3% of our capital stock entitled to vote generally in the election of directors, voting together as a single class, is required to amend or repeal our restated bylaws, although our restated bylaws may be amended by the approval of a majority of the board of directors. |
| ● | Stockholder Action; Special Meetings of Stockholders. Our restated certificate of incorporation provides that our stockholders may not take action by written consent but may only take action at annual or special meetings of our stockholders. As a result, holders of our capital stock are not able to amend our restated bylaws or remove directors without holding a meeting of our stockholders called in accordance with our restated bylaws. Our restated certificate of incorporation and our restated bylaws provide that special meetings of our stockholders may be called only by the chairperson or executive chairperson of the board of directors, the lead independent director, our chief executive officer or the board of directors acting pursuant to a resolution adopted by a majority of the board of directors. Additionally, only the business as stated in the notice for a special meeting may be considered at a special meeting of stockholders. Therefore, stockholders are both prohibited from calling a special meeting and from raising additional matters for consideration at a special meeting of stockholders. These provisions might delay the ability of our stockholders to force consideration of a proposal or for stockholders to take any action, including the removal of directors. |
| ● | Advance Notice Requirements for Stockholder Proposals and Director Nominations. Our restated bylaws provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. Our restated bylaws also specify certain requirements regarding the timing, form and content of a stockholder’s notice. These provisions may preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders. These provisions might also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our Company. |
| ● | No Cumulative Voting. The DGCL provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. Our restated certificate of incorporation and restated bylaws do not provide for cumulative voting. |
| ● | Issuance of Undesignated Preferred Stock. Our board has the authority, without further action by the stockholders, to issue up to 10,000,000 shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by our board of directors. The existence of authorized but unissued shares of preferred stock enables our board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest, or otherwise. |
| ● | Choice of Forum. Our restated certificate of incorporation provides that, unless we consent in writing to the selection of an alternative forum and to the fullest extent permitted by law, that the Court of Chancery of the State of Delaware (or, if and only if the Court of Chancery of the State of Delaware lacks subject matter jurisdiction, any state court located within the State of Delaware or, if and only if all such state courts lack subject matter jurisdiction, the federal district court for the District of Delaware) and any appellate court therefrom, is the sole and exclusive forum for: (a) any derivative action, suit or proceeding brought on behalf of us; (b) any action, suit or proceeding asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, employee, or agent of ours; (c) any action, suit or proceeding asserting a claim against us or any current or former director, officer or employee of ours arising out of or pursuant to, or seeking to enforce any right, obligation or remedy under, or to interpret, apply, or determine the validity of, any provision of the DGCL, the restated certificate of incorporation or the restated bylaws (as each may be amended from time to time); (d) any action, suit or proceeding as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware; or (e) any action, suit or proceeding asserting a claim against us or any current or former director, officer or employee of ours governed by the internal affairs doctrine, in all cases subject to the court having personal jurisdiction over the indispensable parties named as defendants. However, such forum selection provisions do not apply to actions, suits or proceedings brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts of the United States have exclusive jurisdiction. The restated certificate of incorporation also provides that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States of America is the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. |
85
Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all claims brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. Accordingly, both state and federal courts have jurisdiction to entertain such claims. As noted above, the restated certificate of incorporation provides that the federal district courts of the United States have exclusive jurisdiction over any action asserting a cause of action arising under the Securities Act. Accordingly, there is uncertainty as to whether a court would enforce such provision. Our stockholders shall not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.
Section 27 of the Exchange Act creates exclusive federal jurisdiction over all claims brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder. As noted above, the restated certificate of incorporation provides that the choice of forum provision does not apply to suits brought to enforce any duty or liability created by the Exchange Act. Accordingly, actions by our stockholders to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder must be brought in federal court. Our stockholders shall not be deemed to have waived our compliance with the federal securities laws and the regulations promulgated thereunder.
Any person or entity purchasing or otherwise acquiring or holding any interest in shares of our capital stock shall be deemed to have notice of and consented to the forum selection provisions in the restated certificate of incorporation.
The choice of forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees or could result in increased costs for our stockholders to bring a claim in the chosen forum, which may discourage such lawsuits against us and our directors, officers, and other employees. Alternatively, if a court were to find the choice of forum provisions contained in the restated certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
Limitation on Liability and Indemnification of Directors and Officers
The restated bylaws provide that our directors and officers will be indemnified and advanced expenses by us to the fullest extent authorized or permitted by the DGCL as it now exists or may in the future be amended. In addition, the restated certificate of incorporation provides that our directors and officers will not be personally liable to us or our stockholders for monetary damages for breaches of their fiduciary duty as directors or officers to the fullest extent permitted by the DGCL as it now exists or may in the future be amended.
The restated bylaws also permit us to purchase and maintain insurance on behalf of any officer, director, employee or agent of ours for any liability arising out of his or her status as such, regardless of whether the DGCL would permit indemnification.
86
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against our directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against our directors and officers pursuant to these indemnification provisions.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is Vstock Transfer, LLC. The transfer agent’s address is 18 Lafayette Place, Woodmere, NY 11598, and its telephone number is (212) 828-8436.
Stock Quotation
OUR COMMON STOCK IS CURRENTLY NOT LISTED ON A NATIONAL SECURITIES EXCHANGE OR ANY OTHER EXCHANGE, OR QUOTED ON AN OVER THE COUNTER MARKET. FOLLOWING COMPLETION OF THE OFFERING, WE INTEND TO CAUSE OUR COMMON STOCK TO BE QUOTED ON THE OTC MARKETS QB TIER AS SOON AS PRACTICABLE FOLLOWING THE EFFECTIVENESS OF THE REGISTRATION STATEMENT. HOWEVER, WE CANNOT ASSURE YOU THAT WE WILL BE ABLE TO DO SO AND, EVEN IF WE DO SO, THERE CAN BE NO ASSURANCE THAT OUR COMMON STOCK WILL CONTINUE TO BE QUOTED ON THE OTC MARKETS OR QUOTED OR LISTED ON ANY OTHER MARKET OR EXCHANGE, OR THAT AN ACTIVE TRADING MARKET FOR OUR COMMON STOCK WILL DEVELOP OR CONTINUE.
LEGAL PROCEEDINGS
From time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
We are currently not aware of any pending legal proceedings to which we, or any of our officers or directors in their capacity as such, are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
ITEM 3.02 UNREGISTERED SALES OF EQUITY SECURITIES.
The Offering
The information regarding the Bridge Notes, the Pre-Merger Warrants, the securities issued in the Offering and the Placement Agent Warrants set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Bridge Financings” and “—The Offering” and “Description of Capital Stock” is incorporated herein by reference.
On February 11, 2025, in connection with the Offering, we issued 1,080,814 shares of our Common Stock to 95 accredited investors. These transactions were exempt from registration under Section 4(a)(2) of the Securities Act as not involving any public offering or Regulation D promulgated thereunder.
87
Sales of Unregistered Securities of Adaptin
The following list sets forth information as to all securities Private Adaptin sold from its incorporation as Centaur Bio Inc. on March 12, 2021 through immediately prior to the consummation of the Merger, which were not registered under the Securities Act. The following description is historical and has not been adjusted to give effect to the Merger.
| 1. | On March 17, 2021, Private Adaptin issued a total of 1,000 shares of Private Adaptin’s common stock at a price per share of $0.001 to Dr. Roberts for gross proceeds of $1.00. Private Adaptin relied upon the exemption from registration provided by Regulation 4(a)(2) under the Securities Act. |
| 2. | On August 25, 2021, Private Adaptin issued a total of 430 shares of Private Adaptin’s common stock at a price per share of $0.001 to Dr. Pedder for gross proceeds of $0.43. Private Adaptin relied upon the exemption from registration provided by Regulation 4(a)(2) under the Securities Act. |
| 3. | Private Adaptin issued a total of 75 shares of Private Adaptin’s common stock as part of the consideration for the Duke License. Private Adaptin relied upon the exemption from registration provided by Regulation 4(a)(2) under the Securities Act. |
| 4. | From March 2024 to September 2024, Private Adaptin entered into securities purchase agreements with certain accredited investors for the 2024 Bridge Notes, which provided for an aggregate of $1,000,000, 10% secured subordinated convertible promissory notes payable to investors with a term of 12 months from the issuance date. Additionally, the 2024 Bridge Notes, as amended, provided that, upon a qualified offering, the 2024 Bridge Notes, together with all accrued but unpaid interest at the date of conversion, shall automatically be converted into shares of Private Adaptin or Company common stock at a conversion price equal to 75% of the gross offering price to the investors in such qualified offering. The 2024 Bridge Notes were sold pursuant to Regulation D under the Securities Act. See Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Bridge Financings” for additional information on the 2024 Bridge Notes. |
| 5. | From April 2023 to September 2023, Private Adaptin entered into securities purchase agreements with certain accredited investors for the 2023 Bridge Notes, which provided for an aggregate of $500,000, 10% secured subordinated promissory notes payable to investors with a term of 12 months from the issuance date. The 2023 Bridge Notes were exchanged for an aggregate of $500,000 of the Exchange Notes. The Exchange Notes provided that, upon a qualified offering, the Exchange Notes, together with all accrued but unpaid interest at the date of conversion, shall automatically be converted into shares of Private Adaptin or Company common stock at a conversion price equal to 75% of the gross offering price to the investors in such qualified offering. The 2023 Bridge Notes were sold pursuant to Regulation D under the Securities Act. See Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Bridge Financings” for additional information on the 2023 Bridge Notes. |
ITEM 3.03 MATERIAL MODIFICATION TO RIGHTS OF SECURITY HOLDERS.
The information contained in Item 5.03, “Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year” is incorporated herein by reference.
ITEM 4.01 CHANGES IN REGISTRANT’S CERTIFYING ACCOUNTANT.
On February 11, 2025, the Company’s Board of Directors approved the engagement of WithumSmith+Brown, PC (“Withum”) as the Company’s independent registered public accounting firm to audit its financial statements, effective following the filing of its Form 10-K for the fiscal year ended December 31, 2024 (“Form 10-K”). Upon filing of the Company’s Form 10-K, the Company’s current independent registered public accounting firm, KNAV CPA LLP (“KNAV”), will be dismissed.
During the period from March 10, 2022 (inception) to December 31, 2022, the fiscal years ended December 31, 2024 and 2023 and the subsequent interim period through the date of this Report, there were no disagreements with KNAV on any matter of accounting principles or practices, financial statement disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of KNAV, would have caused it to make reference to the subject matter thereof in connection with its report.
KNAV’s report on the Company’s financial statements as of December 31, 2023 and 2022 and for the year ended December 31, 2023, and for the period from March 10, 2022 (inception) through December 31, 2022 did not contain any adverse opinion or disclaimer of opinion, nor were they qualified or modified as to uncertainty, audit scope or accounting principles except for an explanatory paragraph in such report regarding substantial doubt about Company’s ability to continue as a going concern.
88
During the period from March 10, 2022 (inception) to December 31, 2022, the fiscal years ended December 31, 2024 and 2023 and the subsequent interim period through the date of this Report, neither the Company nor anyone acting on its behalf consulted Withum regarding the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements.
We have provided KNAV with a copy of this Report prior to the filing hereof and have requested that KNAV furnish to us a letter addressed to the SEC stating whether KNAV agrees with the statements made by us under this Item 4.01. KNAV has furnished such letter, which letter is filed as Exhibit 16.1 hereto, as required by Item 304(a)(3) of Regulation S-K.
ITEM 5.01 CHANGES IN CONTROL OF REGISTRANT.
The information regarding change of control of the Company in connection with the Merger set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions” is incorporated herein by reference.
ITEM 5.02 DEPARTURE OF DIRECTORS OR PRINCIPAL OFFICERS; ELECTION OF DIRECTORS; APPOINTMENT OF PRINCIPAL OFFICERS; COMPENSATORY ARRANGEMENTS OF CERTAIN OFFICERS.
The information regarding departure and election of our directors and departure and appointment of our principal officers in connection with the Merger set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets— The Merger and Related Transactions” is incorporated herein by reference.
For information regarding the terms of employment of our newly appointed executive officers, see “Compensation of Directors and Executive Officers—Executive Compensation Arrangements” in Item 2.01 of this Report, which description is incorporated herein by reference. For certain biographical, related party and other information regarding our newly appointed executive officers, see the disclosure under the headings “Management” and “Certain Relationships and Related Party Transactions” in Item 2.01 of this Report, which disclosures are incorporated herein by reference.
For information about compensation to our directors, see “Management” in Item 2.01 of this Report, which description is incorporated herein by reference. For information about the committees each director serves on, see “Management—Committee of the Board of Directors” in Item 2.01 of this Report, which description is incorporated herein by reference. There are no arrangements or understandings pursuant to which any of our current directors was appointed as a director. For certain biographical, related party and other information regarding our newly appointed directors, see the disclosure under the headings “Management” and “Certain Relationships and Related Party Transactions” in Item 2.01 of this Report, which disclosures are incorporated herein by reference.
ITEM 5.03 AMENDMENTS TO ARTICLES OF INCORPORATION OR BYLAWS; CHANGE IN FISCAL YEAR.
Amendments to Certificate of Incorporation
Prior to the Merger, Unite Acquisition’s board of directors approved the amendment and restatement of our certificate of incorporation on February 11, 2025, and stockholders holding 100% of the then outstanding shares of our Common Stock approved the amendment and restatement to our certificate of incorporation on February 11, 2025. The information regarding the restated certificate of incorporation in Item 2.01, “Completion of Acquisition or Disposition of Assets— Description of Capital Stock—Anti-Takeover Provisions” is incorporated herein by reference. Our restated certificate of incorporation is filed as Exhibit 3.2 hereto and is incorporated herein by reference.
Amendments to Bylaws
Prior to the Merger, we amended and restated our bylaws in their entirety, to be effective upon closing of the Merger. The information regarding the restated bylaws in Item 2.01, “Completion of Acquisition or Disposition of Assets— Description of Capital Stock—Anti-Takeover Provisions” is incorporated herein by reference. Our restated bylaws are filed as Exhibit 3.3 hereto and is incorporated herein by reference.
89
ITEM 5.05 Amendments to the Registrant’s Code of Ethics, or Waiver of a Provision of the Code of Ethics.
In connection with the Merger, the board of directors adopted a new Code of Ethics, which applies to all directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions) and employees. The information regarding Code of Ethics of the Company is set forth in Item 2.01, “Code of Conduct and Ethics” is incorporated herein by reference. The newly adopted Code of Ethics did not result in any explicit or implicit waiver of any prior code of conduct or ethics. The foregoing description of the Code of Ethics does not purport to be complete and is qualified in its entirety by the full text of the Code of Ethics, a copy of which is attached hereto as Exhibit 14.1 and is incorporated herein by reference.
ITEM 5.06 CHANGE IN SHELL COMPANY STATUS.
Prior to the Merger, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act). As a result of the Merger, we have ceased to be a shell company. The information contained in this Report constitutes the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act.
ITEM 9.01 FINANCIAL STATEMENTS AND EXHIBITS.
| (a) | As a result of the Merger, as described in Item 2.01, the registrant is filing herewith audited financial statements for Private Adaptin as of and for the fiscal years ended December 31, 2023 and 2022 as Exhibit 99.1 to this Report. |
| As a result of the Merger, as described in Item 2.01, the registrant is filing herewith unaudited financial statements for Private Adaptin as of and for the nine-month periods ended September 30, 2024 and 2023 as Exhibit 99.2 to this Report. | |
| (b) | As a result of the Merger, as described in Item 2.01, the registrant is filing herewith unaudited pro forma condensed combined financial statements as of September 30, 2024, for the nine months ended September 30, 2024 and for the year ended December 31, 2023 as Exhibit 99.3 to this Report. |
| (c) | Shell Company Transactions. Reference is made to Items 9.01(a) and 9.01(b) and the exhibits referred to therein, which are incorporated herein by reference. |
| (d) | Exhibits. |
90
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
| * | Portions of this exhibit (indicated by asterisks) have been omitted in accordance with Item 601(b)(10) of Regulation S-K. The registrant hereby agrees to furnish supplementally copies of any of the omitted portions of this exhibit to the SEC upon its request. |
| § | Certain exhibits or schedules to this exhibit have been omitted in accordance with Item 601(a)(5) of Regulation S-K. The registrant hereby agrees to furnish supplementally a copy of any omitted exhibit or schedule to the SEC upon its request. |
91
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Adaptin Bio, Inc. | ||
| Date: February 18, 2025 | By: | /s/ Michael J. Roberts |
| Michael J. Roberts | ||
| President and Chief Executive Officer | ||
92
Exhibit 2.1
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
among
UNITE ACQUISITION 1 CORP., a Delaware corporation,
ADAPTIN ACQUISITION CO., a Delaware corporation
and
ADAPTIN BIO, INC., a Delaware corporation
February 11, 2025
TABLE OF CONTENTS
| Page | ||
| ARTICLE I. | THE MERGER | 2 |
| 1.1 | The Merger | 2 |
| 1.2 | The Closing | 3 |
| 1.3 | Actions at the Closing | 3 |
| 1.4 | Additional Actions | 3 |
| 1.5 | Conversion of Company Securities | 3 |
| 1.6 | Dissenting Shares | 4 |
| 1.7 | Fractional Shares | 5 |
| 1.8 | [Reserved] | 5 |
| 1.9 | Directors and Officers | 5 |
| 1.10 | Certificate of Incorporation and Bylaws | 6 |
| 1.11 | No Further Rights | 6 |
| 1.12 | Closing of Transfer Books | 6 |
| 1.13 | Exemption from Registration; Rule 144 | 7 |
| 1.14 | Certain Tax Matters | 8 |
| 1.15 | Withholding | 8 |
| 1.16 | Debt Payoff | 8 |
| ARTICLE II. | REPRESENTATIONS AND WARRANTIES OF THE COMPANY | 9 |
| 2.1 | Organization, Qualification and Corporate Power | 9 |
| 2.2 | Capitalization | 10 |
| 2.3 | Authorization of Transaction | 10 |
| 2.4 | Non-contravention | 11 |
| 2.5 | Subsidiaries | 11 |
| 2.6 | Compliance with Laws | 12 |
| 2.7 | Financial Statements | 12 |
| 2.8 | Absence of Certain Changes | 12 |
| 2.9 | Undisclosed Liabilities | 12 |
| 2.10 | Contracts | 13 |
| 2.11 | Litigation | 13 |
| 2.12 | Brokers’ Fees | 13 |
| 2.13 | Books and Records | 13 |
| 2.14 | Schedule 14_F-1 | 13 |
| 2.15 | No Other Representations | 13 |
| ARTICLE III. | REPRESENTATIONS AND WARRANTIES OF THE PARENT AND THE ACQUISITION SUBSIDIARY | 14 |
| 3.1 | Organization, Qualification and Corporate Power | 14 |
| 3.2 | Capitalization | 15 |
| 3.3 | Authorization of Transaction | 15 |
| 3.4 | Noncontravention | 15 |
| 3.5 | Subsidiaries | 16 |
| 3.6 | SEC Reports and Prior Registration Statement Matters | 16 |
| 3.7 | Compliance with Laws | 17 |
i
| 3.8 | Financial Statements | 17 |
| 3.9 | Absence of Certain Changes | 18 |
| 3.10 | Undisclosed Liabilities | 18 |
| 3.11 | Off-Balance Sheet Arrangements | 18 |
| 3.12 | Tax Matters | 19 |
| 3.13 | Assets | 20 |
| 3.14 | Real Property | 20 |
| 3.15 | Contracts | 20 |
| 3.16 | Powers of Attorney | 20 |
| 3.17 | Insurance | 20 |
| 3.18 | Litigation | 20 |
| 3.19 | Employees | 21 |
| 3.20 | Employee Benefits | 21 |
| 3.21 | Environmental Matters | 21 |
| 3.22 | Permits | 22 |
| 3.23 | Certain Business Relationships with Affiliates | 22 |
| 3.24 | Tax-Free Reorganization | 22 |
| 3.25 | Brokers’ Fees | 24 |
| 3.26 | Interested Party Transactions | 24 |
| 3.27 | Accountants | 24 |
| 3.28 | Minute Books | 24 |
| 3.29 | Board Action | 24 |
| 3.30 | Intellectual Property | 24 |
| 3.31 | Investment Company | 24 |
| 3.32 | Foreign Corrupt Practices Act | 25 |
| 3.33 | No Integrated Offering | 25 |
| 3.34 | No General Solicitation | 25 |
| 3.35 | Application of Takeover Provisions | 25 |
| 3.36 | No Other Representations | 25 |
| ARTICLE IV. | COVENANTS | 26 |
| 4.1 | Conduct of the Business Prior to Closing; Closing Efforts | 26 |
| 4.2 | Governmental and Third-Party Notices and Consents | 26 |
| 4.3 | Super 8-K | 26 |
| 4.4 | Access to Information | 26 |
| 4.5 | Expenses | 27 |
| 4.6 | Indemnification; Insurance | 28 |
| 4.7 | Name | 29 |
| 4.8 | Parent Board; Amendment of Charter Documents; Corporate Policies | 29 |
| 4.9 | Equity Plans | 29 |
| 4.10 | Information Provided to Stockholders | 30 |
| 4.11 | Securities Exemptions | 30 |
| 4.12 | Parent Auditor Letter | 30 |
| 4.13 | Private Placement | 30 |
| 4.14 | Failure to Fulfill Conditions | 30 |
| 4.15 | Notification of Certain Matters | 30 |
ii
| ARTICLE V. | CONDITIONS TO CONSUMMATION OF MERGER | 31 |
| 5.1 | Conditions to Each Party’s Obligations | 31 |
| 5.2 | Conditions to Obligations of the Parent and the Acquisition Subsidiary | 32 |
| 5.3 | Conditions to Obligations of the Company | 33 |
| ARTICLE VI. | DEFINITIONS | 36 |
| 7.1 | Termination | 38 |
| 7.2 | Effect of Termination | 38 |
| ARTICLE VIII. | MISCELLANEOUS | 39 |
| 8.1 | Press Releases and Announcements | 39 |
| 8.2 | No Third Party Beneficiaries | 39 |
| 8.3 | Entire Agreement | 39 |
| 8.4 | Succession and Assignment | 39 |
| 8.5 | Counterparts and Facsimile Signature | 39 |
| 8.6 | Headings | 40 |
| 8.7 | Notices | 40 |
| 8.8 | Governing Law | 40 |
| 8.9 | Amendments and Waivers | 41 |
| 8.10 | Severability | 41 |
| 8.11 | Submission to Jurisdiction | 41 |
| 8.12 | WAIVER OF JURY TRIAL | 41 |
| 8.13 | Remedies; Specific Performance | 42 |
| 8.14 | Survival | 42 |
| 8.15 | Construction | 42 |
EXHIBITS
| Exhibit A | Amended and Restated Certificate of Incorporation of the Parent |
| Exhibit B | Amended and Restated Bylaws of the Parent |
| Exhibit C | Form of Pre-Merger Indemnity Agreement |
| Exhibit D | Form of Lucius Advisory Services Agreement |
| Exhibit E | Form of Certificate of Merger |
SCHEDULES
| Schedule 1.5(a) | Conversion Ratios |
| Schedule 2 | Company Knowledge Persons |
| Company Disclosure Schedule | |
| Parent Disclosure Schedule | |
| Schedule 4.6(c) | Parent Indemnified Executives |
| Schedule 5.1(c) | Terminated Agreements |
| Schedule 5.2(a) | Company Closing Consents |
| Schedule 5.3(b) | Parent Closing Consents |
iii
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION
INTRODUCTION
AGREEMENT AND PLAN OF MERGER AND REORGANIZATION (this “Agreement”), dated as of February 11, 2025, by and among UNITE ACQUISITION 1 CORP., a Delaware corporation (the “Parent”), ADAPTIN ACQUISITION CO., a Delaware corporation (the “Acquisition Subsidiary”), and ADAPTIN BIO, INC., a Delaware corporation (the “Company”). The Parent, the Acquisition Subsidiary and the Company are each a “Party” and referred to collectively herein as the “Parties.”
RECITALS
WHEREAS, the Parties to this Agreement intend to effect a merger of the Acquisition Subsidiary, a wholly owned subsidiary of the Parent, with and into the Company, with the Company remaining as the surviving entity and wholly-owned subsidiary of the Parent after the merger (the “Merger”) in accordance with this Agreement and the Delaware General Corporation Law, as amended (the “DGCL”), whereby the stockholders of the Company as of immediately prior to the Effective Time (“Company Stockholders”) who are accredited investors as defined in Rule 501(a) of Regulation D promulgated under the Securities Act of 1933, as amended (“Securities Act”) will receive shares of Parent’s common stock, par value $0.0001 per share (the “Parent Common Stock”) in exchange for their capital stock of the Company;
WHEREAS, the board of directors of the Company, after considering the terms and conditions of this Agreement and the transactions contemplated hereby, has unanimously, unconditionally and irrevocably (i) determined that this Agreement and transactions contemplated hereby are in the best interests of the Company and the Company Stockholders, (ii) approved and declared advisable the Merger, this Agreement and the consummation of the transactions contemplated hereby, (iii) directed that this Agreement be submitted for approval to the Company Stockholders, and (iv) resolved to recommend the approval and adoption of this Agreement by the Company Stockholders; and
WHEREAS, the board of directors of each of the Parent and Acquisition Subsidiary, and the Parent, in its capacity as the sole stockholder of Acquisition Subsidiary, have unanimously, unconditionally and irrevocably approved and consented to the Merger, the execution by the Parent and Acquisition Subsidiary of this Agreement, and the consummation of the transactions contemplated hereby; and
WHEREAS, contemporaneously with the Merger, the Parent will complete a private placement offering (the “Private Placement Offering”) of a minimum of 795,455 Units of Parent’s securities, each “Unit” consisting of (i) one share of Parent Common Stock, (ii) a warrant to purchase one share of Parent Common Stock, exercisable from issuance until one year after the final closing of the Private Placement Offering at an exercise price of $4.40 per share, and (iii) a warrant, to purchase one-half share of Parent Common Stock, exercisable from issuance until five years after the final closing of the Private Placement Offering at an exercise price of $6.60 per whole share, at a purchase price of $4.40 per Unit (the “Purchase Price”), upon the terms and subject to the conditions of subscription agreements in a form reasonably acceptable to the Parent and the Company; and
WHEREAS, as a condition and inducement to the Company’s willingness to enter into this Agreement, contemporaneously with the execution and delivery of this Agreement by the Parties, certain stockholders of the Parent prior to the Merger have entered into binding share cancellation agreements with the Parent (the “Share Cancellation Agreements”), pursuant to which an aggregate of 1,750,000 shares of Parent Common Stock (the “Cancelled Shares”) will be cancelled immediately prior to the Effective Time contingent upon the effectiveness of the Merger; and
WHEREAS, for U.S. federal and applicable state and local tax purposes, the Parties intend for the Merger to qualify as a transaction described in Section 351(a) of the Code, and also as a “reorganization” within the meaning of Section 368(a) of the Internal Revenue Code of 1986, as amended (the “Code”), and this Agreement to constitute a “plan of reorganization” within the meaning of the regulations promulgated under the Code by the U.S. Department of the Treasury (the “Treasury Regulations”), specifically Treasury Regulations Section 1.368-2(g) (collectively, the “Intended Tax Treatment”);
NOW, THEREFORE, in consideration of the representations, warranties and covenants herein contained, and for other good and valuable consideration, the receipt, adequacy and sufficiency of which are hereby acknowledged, the Parties, intending legally to be bound, agree as follows:
ARTICLE I. THE MERGER
1.1 The Merger.
(a) Upon and subject to the terms and conditions set forth in this Agreement, the Acquisition Subsidiary shall merge with and into the Company at the Effective Time (as defined below). From and after the Effective Time, the separate corporate existence of the Acquisition Subsidiary shall cease, and the Company shall continue as the surviving corporation in the Merger (the “Surviving Corporation”) and will continue as the wholly owned subsidiary of the Parent.
(b) On the Closing Date (as defined below), the Parties shall cause the Merger to be consummated by filing a certificate of merger in the form attached hereto as Exhibit E (the “Certificate of Merger”) with the Secretary of State of Delaware in accordance with the DGCL. The Merger will become effective when the Certificate of Merger becomes effective with the Secretary of State of the State of Delaware (the “Effective Time”).
(c) The Merger shall have the effects set forth herein and in the applicable provisions of the DGCL including, without limiting the generality of the foregoing and subject thereto and except as otherwise provided herein, (i) all the property, rights, privileges, powers and franchises of the Company and the Acquisition Subsidiary will be vested in the Surviving Corporation, without reservation or impairment, without further action or deed, and without any conveyance, transfer or assignment having occurred and (ii) the Surviving Corporation will have all debts, liabilities and duties of the Company and Acquisition Subsidiary.
(d) The Parties shall each use their respective best efforts to take all such action as may be necessary or appropriate to effectuate the Merger in accordance with the DGCL at the Effective Time. If at any time after the Effective Time, any further action is necessary or desirable to carry out the purposes of this Agreement and to vest the Surviving Corporation with full right, title and possession to all properties, rights, privileges, immunities, powers and franchises of either the Company or the Acquisition Subsidiary, the officers of the Surviving Corporation are fully authorized in the name of Parent, the Company and Acquisition Subsidiary or otherwise to take, and shall take, all such lawful and necessary action.
2
1.2 The Closing. The closing of the transactions contemplated by this Agreement (the “Closing”) shall take place remotely, via electronic exchange of documents, on such mutually agreeable date as soon as practicable (and in any event not later than three Business Days) after the satisfaction or waiver of all conditions (excluding the delivery of any documents to be delivered at the Closing by any of the Parties) set forth in ARTICLE V hereof (the “Closing Date”). As used in this Agreement, the term “Business Day” means any day other than a Saturday, a Sunday or a day on which banks in the state of New York are required or authorized by applicable Law to close.
1.3 Actions at the Closing. At the Closing:
(a) the Company shall deliver to the Parent and the Acquisition Subsidiary the various certificates, instruments and documents to be delivered by the Company pursuant to Sections 5.1 and 5.2;
(b) the Parent and the Acquisition Subsidiary shall deliver to the Company the various certificates, instruments and documents to be delivered by the Parent and/or Acquisition Subsidiary pursuant to Sections 5.1 and 5.3; and
(c) the Company shall file the Certificate of Merger with the Secretary of State of the State of Delaware in accordance with the DGCL.
1.4 Additional Actions. If at any time after the Effective Time the Surviving Corporation or Parent shall consider or be advised that any deeds, bills of sale, assignments or assurances or any other acts or things are necessary, desirable or proper (a) to vest, perfect or confirm, of record or otherwise, in the Surviving Corporation or Parent, its right, title or interest in, to or under any of the rights, privileges, powers, franchises, properties or assets of either the Company or the Acquisition Subsidiary or (b) otherwise to carry out the purposes of this Agreement, the Surviving Corporation, Parent and its officers and directors or their designees shall be authorized (to the fullest extent allowed under applicable Law) to execute and deliver, in the name and on behalf of either the Company, Parent or the Acquisition Subsidiary, all such deeds, bills of sale, assignments and assurances and do, in the name and on behalf of the Company, Parent or the Acquisition Subsidiary, all such other acts and things necessary, desirable or proper to vest, perfect or confirm its right, title or interest in, to or under any of the rights, privileges, powers, franchises, properties or assets of the Company, Parent or the Acquisition Subsidiary, as applicable, and otherwise to carry out the purposes of this Agreement.
1.5 Conversion of Company Securities. At the Effective Time, by virtue of the Merger and without any action on the part of any Party or the holder of any of the following securities the Merger will be effected in accordance with the following terms:
(a) Each share of capital stock of the Acquisition Subsidiary issued and outstanding immediately prior to the Effective Time will be converted into one (1) validly issued, fully paid and nonassessable share of common stock of the Surviving Corporation, and the stock of the Surviving Corporation issued pursuant to such conversion will constitute all of the issued and outstanding shares of capital stock of the Surviving Corporation.
3
(b) Each share of the Company’s common stock held by the Company (or held in the Company’s treasury) or held by the Parent or Acquisition Subsidiary immediately prior to the Effective Time (such shares the “Excluded Shares”) will be cancelled and retired and will cease to exist, and no consideration will be delivered in exchange therefor.
(c) Subject to Section 1.5(b) and Section 1.6, at the Effective Time, each share of the Company’s common stock issued and outstanding immediately prior to the Effective Time (the “Company Shares” which shall not include, for the avoidance of doubt, the Excluded Shares) other than Dissenting Shares (as defined below), shall be converted into and represent the right to receive such number of shares of Parent Common Stock as is equal to the aggregate number of Company Shares (including any Dissenting Shares) multiplied by the “Conversion Ratio” for that class or series set forth on Schedule 1.5(a) hereto, rounded up to the nearest whole share. The shares of Parent Common Stock into which the Company Shares are converted pursuant to this Section shall be referred to herein as the “Merger Shares.” The Merger Shares shall be adjusted to reflect appropriately the effect of any stock split, reverse stock split, stock dividend (including any dividend or distribution of securities convertible into or exercisable or exchangeable for Parent Common Stock or Company Shares), reorganization, recapitalization, reclassification, combination, exchange of shares or other like change with respect to Parent Common Stock or Company Shares occurring or having a record date on or after the date hereof and prior to the Effective Time.
(d) After the Effective Time, the Parent shall issue to each Company Stockholder entitled to a portion of the Merger Shares pursuant to Section 1.5(a) and who shall have delivered a certificate representing the Company Shares that were owned by such Company Stockholder immediately prior to the Effective Time (the “Company Shares Certificates”) such Company Stockholder’s portion of the Merger Shares and deliver or cause to be delivered certificates (which, for all purposes in this Agreement, may be in book entry form) representing such Merger Shares (the “Merger Share Certificates”). If any Company Shares Certificate shall have been lost, stolen or destroyed, the Parent may, in its sole discretion and as a condition to the issuance of any certificates representing Merger Shares, require the owner of such lost, stolen or destroyed Company Shares Certificate to provide an appropriate affidavit with respect to such Company Shares Certificate (without the requirement to post a bond). The Parent shall issue each Company Stockholder’s portion of the Merger Shares and Merger Shares Certificates within three (3) Business Days or receiving such Stockholder’s Company Shares Certificates.
1.6 Dissenting Shares.
(a) For purposes of this Agreement, “Dissenting Shares” means Company Shares held as of the Effective Time by a Company Stockholder who has not voted such Company Shares in favor of the adoption of this Agreement and the Merger and with respect to which such Company Stockholder properly demands dissenters’ rights pursuant to, and who comply in all respects with, the provisions of Section 262 of the DGCL and the Company’s by-laws, and who have not effectively withdrawn or forfeited such rights prior to the Effective Time. Dissenting Shares shall not be converted into or represent the right to receive shares of Parent Common Stock as set forth in Section 1.5(c), but instead such Company Stockholders will be entitled to such rights (and only such rights) as are granted under Section 262 of the DGCL unless and until such Company Stockholder’s right to appraisal shall have ceased in accordance with the DGCL and the Company’s by-laws. Beginning at the Effective Time, Dissenting Shares will cease to exist, and except as otherwise provided by applicable Law, each holder of Dissenting Shares will cease to have any rights with respect thereto other than the rights granted pursuant to Section 262 of the DGCL. Notwithstanding the foregoing, if any Company Stockholder fails to validly perfect, or forfeits or withdraws his, her or its right to appraisal of Dissenting Shares under Section 262 of the DGCL, or if a court of competent jurisdiction determines that such holder is not entitled to relief provided by Section 262 of the DGCL, then (i) as of the occurrence of such event, such holder’s Dissenting Shares shall cease to be Dissenting Shares and shall be converted into and represent the right to receive the Merger Shares issuable in respect of such Company Shares pursuant to Section 1.5(c), and (ii) promptly following the occurrence of such event and, if requested by the Parent, the proper surrender of such person’s Company Shares Certificate, the Parent shall issue the portion of the Merger Shares to which such Company Stockholder is entitled pursuant to Section 1.5(c) and issue a Merger Shares Certificate representing such Merger Shares.
4
(b) The Company shall give the Parent prompt notice of any written demands for appraisal of any Company Shares, withdrawals of such demands, and any other instruments that relate to such demands received by the Company. The Company shall not, except with the prior written consent of the Parent (such consent not to be unreasonably withheld, conditioned or delayed), make any payment with respect to any demands for appraisal of Company Shares or offer to settle or settle any such demands unless required by the court of the State of Delaware having jurisdiction thereof.
1.7 Fractional Shares. No certificates or scrip representing fractional Merger Shares shall be issued to Company Stockholders on the surrender for exchange of Company Shares, and such Company Stockholders shall not be entitled to any voting rights, rights to receive any dividends or distributions or other rights as a stockholder of the Parent with respect to any fractional Merger Shares that would have otherwise been issued to such Company Stockholders. No payment shall be made with respect to any fractional Merger Shares to which the holder would otherwise be entitled, and the number thereof shall be rounded up to the nearest whole share.
1.8 Reserved.
1.9 Directors and Officers.
(a) At the Effective Time, by virtue of the Merger and without any action on the part of the Parent, Acquisition Subsidiary, the Company or the holders of any shares of capital stock of any of the foregoing, the directors and officers of the Company as of immediately prior to the Effective Time or such other persons as are designated by the Company shall be the directors and officers of the Surviving Corporation, each to hold office until the earlier of his or her resignation or removal or until his or her respective successors are duly appointed and qualified, as the case may be, and the Surviving Corporation and the Parent shall take any necessary actions (whether prior to, at or after the Effective Time) as shall be necessary or appropriate to effectuate or carry out the purpose of this Section 1.9.
(b) At or prior to the Closing, the Board of Directors of Parent shall, subject to compliance with Section 14(f) of the Exchange Act and Rule 14f-1 promulgated thereunder, take the following actions, to be effective upon the Effective Time: (i) elect to the Board of Directors of Parent those persons set forth in Section 5.3(j); (ii) appoint as the officers of Parent those persons set forth in Section 5.3(j), or, in either case with regard to clauses (i) and (ii), such other persons designated by the Company (including any replacement for a director of the Company immediately prior to the Closing who is either unwilling or unable to serve as a director of the Parent upon the Effective Time); and (iii) appoint such persons set forth in (ii) as an “officer” within the meaning of Section 16 and Rule 16a-1(f) under the Exchange Act and as an “executive officer” within the meaning of Item 401(b) of Regulation S-K, Rule 405 promulgated under the Securities Act and Rule 3b-7 promulgated under the Exchange Act. All of the persons serving as directors of the Parent immediately prior to the Closing shall resign effective upon the election of the new directors, and all of the persons serving as officers of the Parent immediately prior to the Closing shall resign effective upon the appointment of the new officers, all subject to compliance with Rule 14f-1 promulgated under the Exchange Act. Subject to applicable law, the Parent, with the assistance of the Company, has taken or shall take all action reasonably requested by the Company, but consistent with the certificate of incorporation and bylaws of the Parent, that is reasonably necessary to effect any such election or appointment of the designees of the Company to the Parent’s Board of Directors, including mailing to the Parent’s stockholders an information statement containing the information required by Section 14(f) of the Exchange Act and Rule 14f-1 promulgated thereunder at least 10 days prior to the Effective Time.
5
(c) The provisions of this Section 1.9 are in addition to and shall not limit any rights which the Company or any of its Affiliates may have as a holder or beneficial owner of shares of capital stock of the Parent as a matter of law with respect to the election of directors or otherwise. The newly-appointed directors and officers of the Parent shall hold office for the term specified in, and subject to the provisions contained in, the certificate of incorporation and bylaws of the Parent and applicable law.
1.10 Certificate of Incorporation and Bylaws. The Surviving Corporation or the Parent may make any necessary filings in the State of Delaware as shall be necessary or appropriate to effectuate or carry out fully the purpose of this Section 1.10:
(a) the certificate of incorporation of the Acquisition Subsidiary in effect immediately prior to the Effective Time shall be the certificate of incorporation of the Surviving Corporation until thereafter amended as provided by Delaware Law and such certificate of incorporation;
(b) the bylaws of the Acquisition Subsidiary in effect immediately prior to the Effective Time shall be the bylaws of the Surviving Corporation until duly amended or repealed;
(c) the certificate of incorporation of the Parent will be amended and restated at the Effective Time to read in its entirety as set forth on Exhibit A hereto, and, as so amended and restated, will be the certificate of incorporation of the Parent until thereafter amended as provided by Delaware Law and such certificate of incorporation; and
(d) the bylaws of the Parent will be amended and restated at the Effective Time to read in its entirety as set forth on Exhibit B hereto, and, as so amended and restated, will be the bylaws of the Parent until thereafter amended as provided by Delaware law and the Parent’s certificate of incorporation.
1.11 No Further Rights. From and after the Effective Time, no Company Shares shall be deemed to be outstanding, and holders of Company Shares, certificated or uncertificated, shall cease to have any rights with respect thereto, except as provided herein or by applicable Law, other than the right to receive Parent Common Stock in connection with the Merger.
1.12 Closing of Transfer Books. On the Business Day immediately preceding the Effective Time, the stock transfer books of the Company shall be closed, and no transfer of Company Shares shall thereafter be made. If, after the Effective Time, Company Shares Certificates are presented to the Parent or the Surviving Corporation, they shall be cancelled and exchanged for Merger Shares in accordance with Section 1.5, subject to the provisions hereof and applicable Law in the case of Dissenting Shares.
6
1.13 Exemption from Registration; Rule 144.
(a) The Parent and the Company intend that the shares of Parent Common Stock to be issued pursuant to Section 1.5, will be issued in a transaction exempt from registration under the Securities Act, by reason of Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated by the United States Securities and Exchange Commission (the “SEC”) thereunder and that any recipient of such shares of Parent Common Stock shall be an “accredited investor” as such term is defined in Regulation D. In addition, the Parent and the Company intend that the deemed offer and sale of Parent Common Stock to any stockholder of Parent prior to the Merger under Rule 145a under the Securities Act will be exempt from registration under the Securities Act by reason of Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated by the SEC thereunder and that each holder of such shares of Parent Common Stock shall be an “accredited investor” as such term is defined in Regulation D. The shares of Parent Common Stock to be issued pursuant to Section 1.5 hereof will be “restricted securities” within the meaning of Rule 144 under the Securities Act and may not be offered, sold, pledged, assigned or otherwise transferred unless (A) a registration statement with respect thereto is effective under the Securities Act and any applicable state securities laws, or (B) an exemption from such registration exists and either the Parent receives an opinion of counsel to the holder of such securities, which counsel and opinion are satisfactory to the Parent, that such securities may be offered, sold, pledged, assigned or transferred in the manner contemplated without an effective registration statement under the Securities Act or applicable state securities laws; and the certificates (or book-entry security entitlements) representing such shares of Parent Common Stock will bear an appropriate legend and restriction on the books of the Parent or its transfer agent to that effect.
(b) The Parent is a “shell company” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). The Company acknowledges that pursuant to Rule 144(i), securities issued by a former shell company (such as the Merger Shares) that otherwise meet the holding period and other requirements of Rule 144 nevertheless cannot be sold in reliance on Rule 144 until one year after the Parent (i) is no longer a shell company; and (ii) has filed current “Form 10 information” (as defined in Rule 144(i)) with the SEC reflecting that it is no longer a shell company, and provided that at the time of a proposed sale pursuant to Rule 144, the Parent is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and has filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months (or for such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports. As a result, the restrictive legends on certificates or book entry positions for the Merger Shares cannot be removed except in connection with an actual sale meeting the foregoing requirements or pursuant to an effective registration statement.
(c) Notwithstanding Section 1.13(a) and (b) hereto, the Parent has entered into that certain Registration Rights Agreement, on or about the date hereof, by and among the Parent, the Purchasers (as defined therein), the persons or entities holding Pre-Merger Warrants (as defined therein), the persons or entities holding Note Conversion Shares (as defined therein), the persons or entities holding Placement Agent Warrants (each as defined therein), and the persons or entities holding Registrable Pre-Merger Shares (as defined therein) (the “Registration Rights Agreement”), pursuant to which the Parent will file, subject to customary exceptions and the other terms and conditions provided therein, a registration statement with the SEC, covering the Registrable Securities (as defined therein).
7
1.14 Certain Tax Matters. The Parties hereby adopt this Agreement as a “plan of reorganization” within the meaning of Sections 1.368-2(g) and 1.368-3(a) of the Treasury Regulations. Each of the Parties shall use its reasonable best efforts to cause the transactions contemplated hereby to qualify for the Intended Tax Treatment. None of the Parties shall (and each of the Parties shall cause their respective Subsidiaries and Affiliates not to) take any action, or fail to take any action, that could reasonably be expected to cause the Merger to fail to qualify for the Intended Tax Treatment. The Parties intend to report and, except to the extent otherwise required by a “final determination” within the meaning of Section 1313(a) of the Code (or analogous provisions of state, local or other Tax Laws), shall report (including, without limitation, on all applicable United States, state, local or foreign government reports, returns, declarations, statements, or other information required to be supplied to a taxing authority, or submitted to a third party pursuant to Tax Laws, in connection with Taxes (collectively, “Tax Returns”) and in connection with any Tax audit), for all Tax purposes, the transactions contemplated hereby in accordance with the Intended Tax Treatment. For purposes of this Agreement, “Tax” or “Taxes” means any and all taxes or levies or other similar assessments or liabilities in the nature of a tax, including without limitation income, gross receipts, ad valorem, premium, value-added, excise, real property, personal property, sales, use, transfer, withholding, employment, unemployment insurance, social security, business license, business organization, environmental, workers compensation, payroll, profits, license, lease, service, service use, severance, stamp, occupation, windfall profits, customs, duties, franchise and other taxes, imposed by the United States of America or any state, local or foreign government, or any agency thereof, or other political subdivision of the United States or any such government, and any interest, fines, penalties, assessments or additions to tax resulting from, attributable to or incurred in connection with any tax or any contest or dispute thereof.
1.15 Withholding. Parent shall be entitled to deduct and withhold (or cause to be deducted and withheld) from any consideration payable or transferrable pursuant to this Agreement such amounts as are required to be deduced and withheld under applicable Tax Laws. If Parent determines that an amount is required to be deducted or withheld (other than for any payments treated as compensation for or with respect to employment under applicable Tax Law), Parent shall provide the person with respect to which the deduction or withholding is to be made with (a) reasonable advance notice of the intent to withhold, (b) sufficient opportunity to provide any forms or other documentation, and otherwise cooperation with such person to take such other steps, in order to reduce or eliminate such deduction or withholding, and (c) a statement in reasonable detail of the reasons Parent believes such withholding is required under applicable Laws. To the extent that amounts are so withheld and timely remitted to the applicable taxing authority, such withheld amounts shall be treated for all purposes of this Agreement as having been paid to the person in respect of which such deduction and withholding was made. The Parties shall cooperate in good faith to eliminate or reduce any such deduction or withholding (including through the request and provision of any statements, forms or other documents to reduce or eliminate any such deduction or withholding).
1.16 Debt Payoff. At the first closing of the Private Placement Offering, the Parent shall cause to be paid from the gross proceeds thereof to the Debt Holder (as defined below) the amount specified in the Payoff Letter.
8
ARTICLE II. REPRESENTATIONS AND WARRANTIES OF THE COMPANY
The Company represents and warrants to the Parent that the statements contained in this ARTICLE II are true and correct, except as set forth in the disclosure schedule provided by the Company to the Parent on the date hereof (the “Company Disclosure Schedule”). The Company Disclosure Schedule shall be arranged in separate schedules corresponding to the numbered and lettered paragraphs contained in this ARTICLE II; and to the extent that it is reasonably apparent from the context of any disclosure that such disclosure also applies to any other numbered paragraph contained in this ARTICLE II, such disclosure shall qualify such other corresponding numbered paragraph in this ARTICLE II. For purposes of this ARTICLE II, the phrase “to the knowledge of the Company” or any phrase of similar import shall be deemed to refer to the actual knowledge of any of the individuals identified on Schedule 2 as well as any other knowledge which such person would have possessed had such person made reasonable inquiry of officers, directors and key employees of the Company and the accountants and attorneys of the Company.
2.1 Organization, Qualification and Corporate Power. The Company is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware. The Company is duly qualified to conduct business and is in good standing under the Laws of each jurisdiction in which the nature of its businesses or the ownership or leasing of its properties requires such qualification, except where the failure to be so qualified or in good standing, individually or in the aggregate, has not had and would not reasonably be expected to have a Company Material Adverse Effect (as defined below). The Company has all requisite corporate power and authority to carry on the businesses in which it is engaged and to own and use the properties owned and used by it. The Company has furnished or made available to the Parent complete and accurate copies of its certificate of incorporation and by-laws, each as amended to date. The Company is not in default under or in violation of any provision of its certificate of incorporation, as amended to date, or its by-laws, as amended to date, or under any Material Contract (as defined below), except where such default or violation would not be reasonably expected to have a Company Material Adverse Effect. For purposes of this Agreement, “Company Material Adverse Effect” means any effect that either alone or in combination with any other effect has a material adverse effect on (i) the assets, business, financial condition or results of operations of the Company and the Company Subsidiaries (as defined below), taken as a whole or (ii) the ability of the Company to consummate the transactions contemplated by this Agreement; provided, that, in no event shall any effects (whether alone or in combination) resulting from or arising in connection with any of the following be deemed to constitute, nor shall any of the following be taken into account in determining whether there has occurred, a Company Material Adverse Effect: (a) conditions generally affecting the industries in which the Company participates or the U.S. or global economy or capital markets as a whole; (b) any failure by the Company or its Subsidiaries to meet internal projections, budgets, or forecasts or revenue or earnings predictions; (c) the execution, delivery, announcement, pendency or performance of the obligations under this Agreement or the announcement, pendency or anticipated consummation of the Merger or other transactions contemplated hereby; (d) any acts of terrorism, sabotage, military action or war (whether or not declared) or other international or national calamity or any escalation or worsening thereof; (e) earthquakes, hurricanes, tornadoes, floods, epidemics or disease outbreaks or other natural disasters or Acts of God; (f) any changes (after the date of this Agreement) in United States generally accepted accounting principles (“GAAP”), other applicable accounting rules or applicable Law, or changes or developments in political, regulatory or legislative conditions; (g) general financial, credit, capital market or regulatory conditions or any changes therein (provided, however, that such effects do not affect the Company and its Subsidiaries taken as a whole disproportionately as compared to other similarly situated participants in the industry in which the Company operates); (h) any matter disclosed in the Company Disclosure Schedule or the draft Super 8-K (as defined below) provided to Parent on February 11, 2025 (excluding any disclosures (whether contained under the heading “Risk Factors,” in any “forward-looking statements” disclaimer or in any other section) therein to the extent they are predictive or forward-looking in nature); or (i) the taking of any action required by this Agreement, the failure to take any action prohibited by this Agreement, or the taking of any action at the written request or with the prior written consent of the Parent and/or the Acquisition Subsidiary.
9
2.2 Capitalization. As of the date hereof, the authorized capital stock of the Company consists of (a) 10,000 shares of Company common stock and (b) no shares of preferred stock. As of the date of this Agreement, and without giving effect to the transactions contemplated by this Agreement or any of the other Transaction Documentation, 1,505 shares of Company common stock are issued and outstanding, and no Company Shares are held in the treasury of the Company. As of the date of this Agreement and as of immediately prior to the Effective Time, there are no outstanding options to purchase shares of the Company common stock (“Company Options”). As of the date of this Agreement and as of immediately prior to the Effective Time, there are and will be outstanding warrants to purchase Company Shares as set forth on Section 2.2 of the Company Disclosure Schedule (“Company Warrants”). As of the date of this Agreement and as of immediately prior to the Effective Time, there are and will be outstanding the promissory notes of the Company that are convertible into shares of the Company’s common stock or preferred stock as set forth on Section 2.2 of the Company Disclosure Schedule (“Company Convertible Notes”). Section 2.2 of the Company Disclosure Schedule sets forth a complete and accurate list of (a) all Company Stockholders, indicating the number and class of the Company’s shares held by each Company Stockholder, (b) all stock option plans and other stock or equity-related plans of the Company (“Company Equity Plans”) and the number of shares of company common stock or preferred stock reserved for future awards thereunder, (c) all outstanding Company Options, indicating (i) the holder thereof, (ii) the number and class of the Company’s shares subject to each Company Option, (iii) the exercise price, date of grant, vesting schedule and expiration date for each Company Option, and (iv) any terms regarding the acceleration of vesting in connection with the transactions contemplated hereby, (d) all outstanding Company Warrants, indicating (i) the holder thereof, (ii) the number of shares of Company common stock or preferred stock subject to each Company Warrant, (iii) the exercise price, date of grant, vesting schedule and expiration date for each Company Warrant, and (iv) any terms regarding the acceleration of vesting in connection with the transactions contemplated hereby, and (e) all outstanding Company Convertible Notes, indicating (i) the holder thereof, (ii) the original and outstanding principal amounts thereof, (iii) the interest rate thereon, (iv) the maturity date thereof, and (v) the conversion price per Company share therein (or the formula for determining such price). All of the issued and outstanding shares of Company capital stock are duly authorized, validly issued, fully paid, nonassessable and, effective as of the Effective Time, subject to Section 2.2 of the Company Disclosure Schedule are free of all preemptive rights and have been issued in accordance with applicable Laws, including but not limited to, the Securities Act. and not in violation of any Security Interests, purchase, option, call option, right of first refusal, preemptive right, subscription right or any similar right Other than Company Warrants listed in Section 2.2 of the Company Disclosure Schedule, or as contemplated by the Private Placement Offering, there are no outstanding or authorized options, warrants, phantom stock or similar rights, securities, rights, agreements or commitments to which the Company is a party or which are binding upon the Company providing for the issuance or redemption of any shares of Company common stock or preferred stock, or pursuant to which any outstanding shares of Company common stock or preferred stock is subject to vesting. Other than as listed in Section 2.2 of the Company Disclosure Schedule or as contemplated by this Agreement or the Private Placement Offering, there are no agreements to which the Company is a party or by which it is bound with respect to the voting (including without limitation voting trusts or proxies), registration under the Securities Act, or sale or transfer (including without limitation agreements relating to pre-emptive rights, rights of first refusal, co-sale rights or “drag-along” rights) of any securities of the Company. To the knowledge of the Company, there are no agreements among other parties, to which the Company is not a party and by which it is not bound, with respect to the voting (including without limitation voting trusts or proxies) or sale or transfer (including without limitation agreements relating to rights of first refusal, co- sale rights or “drag-along” rights) of any securities of the Company. All of the issued and outstanding shares of Company common stock or preferred stock were issued in compliance with applicable securities Laws.
2.3 Authorization of Transaction. Subject to the receipt of the Company Consents (as defined below), the Company has all requisite corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder. The execution and delivery by the Company of this Agreement and the Transaction Documentation to which it is a party, and, subject to the adoption of this Agreement and (a) the approval of the Merger by the vote of Company Stockholders required by the DGCL and the Company’s by-laws, (b) any required vote in accordance with the requirements of Section 280G(b)(5)(B) of the Code and the final and temporary regulations promulgated under the Code by the United States Department of the Treasury, and (c) the approvals and waivers set forth in Section 2.3 of the Company Disclosure Schedule (collectively, the “Company Consents”), the consummation by the Company of the transactions contemplated hereby have been duly and validly authorized by all necessary corporate action on the part of the Company. Without limiting the generality of the foregoing, the board of directors of the Company (i) determined that the Merger is fair and in the best interests of the Company and the Company Stockholders, (ii) adopted this Agreement in accordance with the provisions of the DGCL, and (iii) directed that this Agreement and the Merger be submitted to the Company Stockholders for their adoption and approval and resolved to recommend that the Company Stockholders vote in favor of the adoption of this Agreement and the approval of the Merger. This Agreement has been duly and validly executed and delivered by the Company and, assuming it is a valid and binding obligation of the Parent and the Acquisition Subsidiary, constitutes a valid and binding obligation of the Company, enforceable against the Company in accordance with its terms, except as such enforceability may be limited under applicable bankruptcy, insolvency and similar Laws, rules or regulations affecting creditors’ rights and remedies generally and to general principles of equity, whether applied in a court of Law or a court of equity.
10
2.4 Non-contravention. Subject to the receipt of Company Consents and the filing of the Certificate of Merger with the Secretary of State of the State of Delaware in accordance with the DGCL, neither the execution and delivery by the Company of this Agreement or the Transaction Documentation to which it is a party, nor the consummation by the Company of the transactions contemplated hereby or thereby will (a) conflict with or violate any provision of the certificate of incorporation or the by-laws of the Company, each as amended to date, (b) require on the part of the Company any filing with, or any permit, authorization, consent or approval of, any court, arbitrational tribunal, administrative agency or commission or other governmental or regulatory authority or agency (a “Governmental Entity”), except for such permits, authorizations, consents and approvals as to which the failure to obtain or make the same would not reasonably be expected to have a Company Material Adverse Effect and would not reasonably be expected to adversely affect the consummation of the transactions contemplated hereby, (c) except as set forth in Section 2.4 of the Company Disclosure Schedule, conflict with, result in a breach of, constitute (with or without due notice or lapse of time or both) a default under, result in the acceleration of obligations under, create in any party the right to terminate, modify or cancel, or require any notice, consent or waiver under, any Material Contract, except, in the case of the foregoing clause (c), for any conflict, breach, default, acceleration, termination, modification or cancellation which would not reasonably be expected to have a Company Material Adverse Effect or any notice, consent or waiver the absence of which would not reasonably be expected to have a Company Material Adverse Effect, (d) result in the imposition of any Security Interest upon any material assets of the Company or (e) violate any federal, state, local, municipal, foreign, international, multinational, or other constitution, law, statute, ordinance, principle of common law, rule, regulation, code, governmental determination, order, writ, injunction, decree, treaty, convention, governmental certification requirement or other public limitation, U.S. or non-U.S., including Tax and U.S. antitrust laws, in each case issued or imposed by a Governmental Entity (collectively, “Laws”) applicable to the Company, except, in the case of the foregoing clause (e), such violations that would not reasonably be expected to have a Company Material Adverse Effect. For purposes of this Agreement, “Security Interest” means any mortgage, pledge, security interest, encumbrance, charge or other lien (whether arising by contract or by operation of law), other than (i) mechanic’s, materialmen’s and similar Security Interests, (ii) Security Interests arising under worker’s compensation, unemployment insurance, social security, retirement and similar legislation, (iii) Security Interests on goods in transit incurred pursuant to documentary letters of credit, (iv) Security Interests for current Taxes not yet due and payable, and (v) restrictions pursuant to applicable securities Laws, in each case arising in the Ordinary Course of Business (as defined below) of the subject person or entity and not material to the subject person or entity, taken as a whole. For purposes of this Agreement, “Ordinary Course of Business” means the ordinary course of such person’s business, consistent with past practice (including with respect to frequency and amount).
2.5 Subsidiaries.
(a) Section 2.5(a) of the Company Disclosure Schedule sets forth: (i) the name of each Company Subsidiary; (ii) the number and type of outstanding equity securities of each Company Subsidiary and a list of the holders thereof; and (iii) the jurisdiction of organization of each Company Subsidiary. For purposes of this Agreement, a “Subsidiary” shall mean any corporation, partnership, joint venture or other entity in which a Party has, directly or indirectly, an equity interest representing 50% or more of the equity securities thereof or other equity interests therein; a “Company Subsidiary” is a Subsidiary of the Company.
(b) Each Company Subsidiary is an entity duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation. Each Company Subsidiary is duly qualified to conduct business and is in good standing under the Laws of each jurisdiction in which the nature of its businesses or the ownership or leasing of its properties requires qualification to do business, except where the failure to be so qualified or in good standing, individually or in the aggregate, has not had and would not reasonably be expected to have a Company Material Adverse Effect. Each Company Subsidiary has all requisite power and authority to carry on the businesses in which it is engaged and to own and use the properties owned and used by it. No Company Subsidiary is in default under or in violation of any provision of its charter, bylaws or other organizational documents. All of the issued and outstanding equity securities of each Company Subsidiary (i) are duly authorized, validly issued, fully paid, nonassessable and free of preemptive rights, (ii) are held of record and beneficially by either the Company or any other Company Subsidiary and (iii) are held or owned free and clear of any restrictions on transfer (other than restrictions under the Securities Act and state or other applicable securities Laws), claims, Security Interests, options, warrants, rights, contracts, calls, commitments, equities and demands. Except as set forth in Section 2.5(b) of the Company Disclosure Schedule, there are no outstanding or authorized options, warrants, rights, agreements or commitments to which the Company or any Company Subsidiary is a party or which are binding on any of them providing for the issuance, disposition or acquisition of any equity securities of any Company Subsidiary.
11
(c) Except as set forth in Section 2.5(c) of the Company Disclosure Schedule, the Company does not control directly or indirectly or have any direct or indirect equity participation or similar interest in any corporation, partnership, limited liability company, joint venture, trust or other business association which is not a Company Subsidiary.
2.6 Compliance with Laws. The Company:
(a) and the conduct and operations of its business, are in compliance with each Law applicable to the Company or any of its properties or assets, except for any violations or defaults that, individually or in the aggregate, have not had and would not reasonably be expected to have a Company Material Adverse Effect;
(b) has complied with all federal and state securities Laws and regulations, except for any violations or defaults that, individually or in the aggregate, have not had and would not reasonably be expected to have a Company Material Adverse Effect;
(c) has not been the subject of any voluntary or involuntary bankruptcy proceeding, nor has it been a party to any material litigation or, within the past two years, the subject of any threat of material litigation; and
(d) is not and has not, and to the knowledge of the Company, the officers and directors of the Company are not and have not in their capacity as an officer or director of the Company, as applicable, been the subject of any civil, criminal or administrative investigation or proceeding brought by any federal or state agency having regulatory authority over such entity or person or alleging a violation of securities Laws (in the case of an individual, that is described in Item 401(f)(1)-(3) of SEC Regulation S-K).
2.7 Financial Statements. The Company has provided or made available to the Parent: (a) the audited balance sheet of the Company at December 31, 2023, and the related statements of operations and cash flows for the years ended December 31, 2023 and 2022 (collectively, the “Company Financial Statements”) and its unaudited balance sheet (the “Company Balance Sheet”) as of September 30, 2024 (the “Company Balance Sheet Date”), and the related unaudited statements of operations and cash flows of the Company for the nine-month period then ended (the “Company Interim Statements”). The Company Financial Statements and the Company Interim Statements have been prepared in accordance with GAAP applied on a consistent basis throughout the periods covered thereby (except in each case as described in the notes thereto), and fairly present in all material respects the financial condition, results of operations and (to the extent applicable) cash flows of the Company as of the respective dates thereof and for the periods referred to therein and comply as to form in all material respects with the applicable rules and regulations of the SEC for inclusion of such Company Financial Statements and Company Interim Statements in the Parent’s filings with the SEC as required by the Exchange Act.
2.8 Absence of Certain Changes. Since the Company Balance Sheet Date, to the knowledge of the Company, there has occurred no event or development which, individually or in the aggregate, has had, or would reasonably be expected to have, a Company Material Adverse Effect.
2.9 Undisclosed Liabilities. To the knowledge of the Company, except as set forth in Section 2.9 of the Company Disclosure Schedule, the Company has no liability (whether absolute or contingent, whether liquidated or unliquidated and whether due or to become due), except for (a) liabilities shown on the Company Balance Sheet, (b) liabilities not exceeding $100,000 in the aggregate that have arisen since the Company Balance Sheet Date in the Ordinary Course of Business, (c) contractual and other liabilities incurred in the Ordinary Course of Business which are not required by GAAP to be reflected on a balance sheet, and (d) liabilities under this Agreement or reasonably incurred in connection with the negotiation and execution of this Agreement.
12
2.10 Contracts. (a) Each Material Contract (as defined below) of the Company is a legal, valid, binding and enforceable obligation of the Company and in full force and effect, except as such enforceability may be limited under applicable bankruptcy, insolvency and similar Laws affecting creditors’ rights and remedies generally and to general principles of equity whether applied in a court of Law or a court of equity, (b) neither the Company nor, to the knowledge of the Company, any other party, is in breach or violation of, or default under, any such Material Contract, except for any breach, violation or default that has not had and would not reasonably be expected to have a Company Material Adverse Effect, and (c) no event has occurred, is pending or, to the knowledge of the Company, is threatened, which, after the giving of notice, with lapse of time, or otherwise, would constitute a breach or default by the Company of such Material Contract, except for any breach, violation or default that has not had and would not reasonably be expected to have a Company Material Adverse Effect, and, to the knowledge of the Company, no such event has occurred, is pending or is threatened with respect to any other contracting party to a Material Contract which, after the giving of notice, with lapse of time, or otherwise, would constitute a breach or default by such other party of such Material Contract, except for any breach, violation or default that has not had and would not reasonably be expected to have a Company Material Adverse Effect. For purposes of this Section 2.10, a “Material Contract” is a material contract as defined by Item 601(b)(10) of Regulation S-K.
2.11 Litigation. There is no action, suit, proceeding, claim, arbitration or investigation before any Governmental Entity or before any arbitrator (a “Legal Proceeding”) which is pending or, to the Company’s knowledge, threatened against the Company in writing which (a) seeks either damages in excess of $250,000 individually or $1,000,000 in the aggregate, (b) if determined adversely to the Company, would have or be reasonably expected to have, individually or in the aggregate, a Company Material Adverse Effect or (c) in any manner challenges or seeks to prevent, enjoin, alter or delay the transactions contemplated by this Agreement.
2.12 Brokers’ Fees. Other than as set forth on Section 2.12 of the Company Disclosure Schedule, the Company has no liability or obligation to pay any fees or commissions to any broker, finder or agent with respect to the transactions contemplated by this Agreement.
2.13 Books and Records. The Company has made available to Parent its minute books and other similar records of the Company, which, to the Company’s knowledge, include records, which records are complete and accurate in all material respects, of meetings of the Company Stockholders, board of directors or any committees thereof and written consents executed in lieu of the holding of any such meetings.
2.14 Schedule 14_F-1. The Company has supplied the Parent all information with respect to it and its nominees, officers, directors and Affiliates required by such Section 14(f) and Rule 14f-1, and such information is true, correct and complete in all material respects.
2.15 No Other Representations. The representations and warranties contained in this ARTICLE II are the only representations and warranties made by the Company. The Company disclaims any and all other representations and warranties other than those contained in this ARTICLE II, whether express or implied. The Company hereby expressly disclaims any such other representation or warranty, whether by the Company, or any of its representatives or any other person, notwithstanding the delivery or disclosure to Parent, Acquisition Subsidiary or any other person of any documentation or other written or oral information by the Company or any of its representatives.
2.16 Independent Investigation; Disclaimer of Other Representations. The Company acknowledges and agrees that (a) in making its decision to enter into this Agreement and to consummate the transactions contemplated hereby, it has relied solely upon its own investigation and the express representations and warranties of the Parent and the Acquisition Subsidiary set forth in ARTICLE III of this Agreement (and as qualified by the Parent Disclosure Schedule), (b) the express representations and warranties made by the Parent and the Acquisition Subsidiary in ARTICLE III of this Agreement (and as qualified by the Parent Disclosure Schedule) are the exclusive representations and warranties made by the Parent and Acquisition Subsidiary with respect to the Parent and Acquisition Subsidiary or the subject matter of this Agreement, and (c) no other Person has made any representation or warranty as to the Parent or the Acquisition Subsidiary, except for those representations and warranties made by the Parent and Acquisition Subsidiary as expressly set forth in ARTICLE III of this Agreement (and as qualified by the Parent Disclosure Schedule). The Company specifically disclaims that it is relying upon or has relied upon any such other representations or warranties that may have been made by any Person and acknowledges that the Parent and Acquisition Subsidiary and their respective Affiliates hereby specifically disclaim any such other representation or warranty made by any Person. Except for the representations and warranties of the Parent and Acquisition Subsidiary set forth in ARTICLE III (and as qualified by the Parent Disclosure Schedule), neither the Parent nor the Acquisition Subsidiary is, directly or indirectly, and no other Person on behalf of the either the Parent or the Acquisition Subsidiary is, making, and the Company specifically disclaims that it is relying on, any representations or warranties regarding any pro-forma financial information, financial projections or other forward-looking prospects, risks or statements (financial or otherwise) of the Parent or the Acquisition Subsidiary made, communicated or furnished (orally or in writing) to the Company or its representatives (including any opinion, information, projection or advice in any management presentation), and the Parent and Acquisition Subsidiary disclaims any and all liability and responsibility for any such information and statements.
13
ARTICLE III. REPRESENTATIONS AND WARRANTIES OF THE PARENT AND THE ACQUISITION SUBSIDIARY
Each of the Parent and the Acquisition Subsidiary represents and warrants to the Company, jointly and severally, that the statements contained in this ARTICLE III are true and correct, except as set forth in the disclosure schedule provided by the Parent to the Company on the date hereof (the “Parent Disclosure Schedule”). The Parent Disclosure Schedule shall be arranged in separate schedules corresponding to the numbered and lettered paragraphs contained in this ARTICLE III; and to the extent that it is reasonably apparent from the context of any disclosure that such disclosure also applies to any other numbered paragraph contained in this ARTICLE III, such disclosure shall qualify such other corresponding numbered paragraph in this ARTICLE III. For purposes of this ARTICLE III, the phrase “to the knowledge of the Parent” or any phrase of similar import shall be deemed to refer to the actual knowledge of any director or executive officer of the Parent as well as any other knowledge which such person would have possessed had such person made reasonable inquiry of officers, directors and key employees of the Parent and the accountants and attorneys of the Parent.
3.1 Organization, Qualification and Corporate Power. The Parent is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware and the Acquisition Subsidiary is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware. The Parent is duly qualified to conduct business and is in good standing under the Laws of each jurisdiction in which the nature of its businesses or the ownership or leasing of its properties requires such qualification, except where the failure to be so qualified or in good standing, individually or in the aggregate, has not had and would not reasonably be expected to have a Parent Material Adverse Effect (as defined below). The Parent has all requisite corporate power and authority to carry on the businesses in which it is engaged and to own and use the properties owned and used by it. The Parent has furnished or made available to the Company complete and accurate copies of its certificate of incorporation and by-laws, each as amended to date. Neither the Parent nor the Acquisition Subsidiary is in default under or in violation of any provision of its certificate of incorporation, as amended to date, its by-laws, as amended to date, or any mortgage, indenture, lease, license or any other agreement or instrument referred to in Sections 3.15 or 3.16, except where such default or violation would not reasonably be expected to have a Parent Material Adverse Effect. The Parent is a “shell company,” formed as a vehicle to pursue a business combination and has no current or historical operations and only nominal assets. For purposes of this Agreement, “Parent Material Adverse Effect” means any effect that either alone or in combination with any other effect has a material adverse effect on (i) the assets, business, financial condition, or results of operations of the Parent and its Subsidiaries, taken as a whole or (ii) the ability of the Parent or the Acquisition Subsidiary to consummate the transactions contemplated by this Agreement; provided, that, in no event shall any effects (whether alone or in combination) resulting from or arising in connection with any of the following be deemed to constitute, nor shall any of the following be taken into account in determining whether there has occurred, a Parent Material Adverse Effect: (a) conditions generally affecting the industries in which the Parent participates or the U.S. or global economy or capital markets as a whole; (b) any failure by the Parent or its Subsidiaries to meet internal projections, budgets or forecasts or revenue or earnings predictions; (c) the execution, delivery, announcement, pendency or or performance of the obligations under this Agreement or the announcement, pendency or anticipated consummation of the Merger or other transactions contemplated hereby; (d) any natural disaster or any acts of terrorism, sabotage, military action or war or any escalation or worsening thereof; (e) any changes (after the date of this Agreement) in GAAP, other applicable accounting rules or applicable Law, or changes or developments in political, regulatory or legislative conditions, or (f) the taking of any action required by this Agreement, the failure to take any action prohibited by this Agreement, or the taking of any action at the written request or with the prior written consent of the Parent and/or the Acquisition Subsidiary.
14
3.2 Capitalization. As of immediately prior to the Effective Time, after giving effect to the surrender and cancellation of the Cancelled Shares, but prior to giving effect to the issuance of the Merger Shares or the shares to be issued in the Private Placement Offering, the authorized capital stock of the Parent will consist of 50,000,000 shares of Parent Common Stock, $0.0001 par value per share, of which 3,250,000 shares will be issued and outstanding (the “Pre-Merger Shares”), and 5,000,000 shares of preferred stock, $0.0001 par value per share, of which no shares will be outstanding. Section 3.2 of the Parent Disclosure Schedule sets forth a complete and accurate list of all stockholders of the Parent, indicating the number and class of Pre-Merger Shares held by each stockholder. All of the issued and outstanding shares of Parent Common Stock are duly authorized, validly issued, fully paid, nonassessable and free of all preemptive, anti-dilution and similar rights and have been issued in accordance with applicable Laws, including, but not limited to, the Securities Act, and not in violation of any Security Interests, purchase, option, call option, right of first refusal, preemptive right, subscription right or any similar right. Except in connection with the Private Placement Offering, as expressly contemplated by the Transaction Documentation, there are no outstanding or authorized options, warrants, rights, agreements or commitments to which the Parent is a party or which are binding upon the Parent providing for the issuance or redemption of any of its capital stock. There are no outstanding or authorized stock appreciation, phantom stock or similar rights with respect to the Parent. Except in connection with the Private Placement Offering or as contemplated by the Transaction Documentation, there are no agreements to which the Parent is a party or by which it is bound with respect to the voting (including without limitation voting trusts or proxies), registration under the Securities Act, or sale or transfer (including without limitation agreements relating to pre-emptive rights, rights of first refusal, co-sale rights or “drag-along” rights) of any securities of the Parent. There are no agreements among other parties, to which the Parent is not a party and by which it is not bound, with respect to the voting (including without limitation voting trusts or proxies) or sale or transfer (including without limitation agreements relating to rights of first refusal, co- sale rights or “drag-along” rights) of any securities of the Parent. All of the issued and outstanding shares of Parent Common Stock were issued in compliance with applicable federal and state securities Laws. The Merger Shares to be issued at the Closing pursuant to Section 1.5 hereof, when issued and delivered in accordance with the terms hereof and of the Certificate of Merger, shall be duly and validly issued, fully paid and nonassessable and free of all preemptive rights and Security Interests and will be issued in compliance with applicable federal and state securities Laws and not in violation of any Security Interests, purchase, option, call option, right of first refusal, preemptive right, subscription right or any similar right.
3.3 Authorization of Transaction. Each of the Parent and the Acquisition Subsidiary has all requisite corporate power and authority to execute and deliver this Agreement and to perform its obligations hereunder. The execution and delivery by the Parent and the Acquisition Subsidiary of this Agreement and the agreements and documents contemplated hereby (collectively, the “Transaction Documentation”) to which it is a party, and the consummation by the Parent and the Acquisition Subsidiary of the transactions contemplated hereby and thereby have been duly and validly authorized by all necessary corporate action on the part of the Parent and the Acquisition Subsidiary, respectively. Each of the documents included in the Transaction Documentation has been duly and validly executed and delivered by the Parent or the Acquisition Subsidiary, as the case may be, and, assuming it is a valid and binding obligation of the Company, constitutes a valid and binding obligation of the Parent or the Acquisition Subsidiary, as the case may be, enforceable against them in accordance with its terms, except as such enforceability may be limited under applicable bankruptcy, insolvency and similar Laws, rules or regulations affecting creditors’ rights and remedies generally and to general principles of equity, whether applied in a court of Law or a court of equity.
3.4 Noncontravention. Subject to the filing of the Certificate of Merger as required by the DGCL, neither the execution and delivery by the Parent or the Acquisition Subsidiary, as the case may be, of this Agreement or the Transaction Documentation to which it is a party, nor the consummation by the Parent or the Acquisition Subsidiary, as the case may be, of the transactions contemplated hereby or thereby, will (a) conflict with or violate any provision of the organizational documents or bylaws of the Parent or the Acquisition Subsidiary, as the case may be, in each case as amended to date, (b) require on the part of the Parent or the Acquisition Subsidiary, as the case may be, any filing with, or any permit, authorization, consent or approval of, any Governmental Entity, other than filing of Form D with the SEC and any applicable state securities filings with respect to the offering of the Merger Shares, which will be completed by Parent following the Effective Time in compliance with applicable Laws, (c) conflict with, result in a breach of, constitute (with or without due notice or lapse of time or both) a default under, result in the acceleration of obligations under, create in any party any right to terminate, modify or cancel, or require any notice, consent or waiver under, any contract or instrument to which the Parent or the Acquisition Subsidiary, as the case may be, is a party or by which either is bound or to which any of their assets are subject, except, in the case of the foregoing clauses (b) and (c), for (i) any conflict, breach, default, failure, acceleration, termination, modification or cancellation which would not reasonably be expected to have a Parent Material Adverse Effect and would not reasonably be expected to adversely affect the consummation of the transactions contemplated hereby or (ii) any notice, consent or waiver the absence of which would not reasonably be expected to have a Parent Material Adverse Effect and would not reasonably be expected to adversely affect the consummation of the transactions contemplated hereby, (d) result in the imposition of any Security Interest upon any assets of the Parent or the Acquisition Subsidiary or (e) violate any Laws applicable to the Parent or the Acquisition Subsidiary, except, in the case of the foregoing clause (e), such violations that would not reasonably be expected to have a Parent Material Adverse Effect.
15
3.5 Subsidiaries.
(a) The Parent has no Subsidiaries, nor does it have any direct or indirect interest in any Subsidiary, other than the Acquisition Subsidiary. The Acquisition Subsidiary is a corporation duly organized, validly existing and in corporate and Tax good standing under the Laws of the jurisdiction of its organization. The Acquisition Subsidiary was formed solely to effectuate the Merger, and has not conducted any business operations since its organization. The Parent has delivered or made available to the Company complete and accurate copies of the charter, bylaws or other organizational documents of the Acquisition Subsidiary. The Acquisition Subsidiary has no assets other than minimal paid-in capital, has no liabilities or other obligations, and is not in default under or in violation of any provision of its charter, bylaws or other organizational documents. All of the issued and outstanding shares of capital stock of the Acquisition Subsidiary are duly authorized, validly issued, fully paid, nonassessable and free of preemptive rights. All shares of the Acquisition Subsidiary are owned by the Parent free and clear of any restrictions on transfer (other than restrictions under the Securities Act and state securities Laws), claims, Security Interests, options, warrants, rights, contracts, calls, commitments, equities and demands. There are no outstanding or authorized options, warrants, rights, agreements or commitments to which the Parent or the Acquisition Subsidiary is a party or which are binding on any of them providing for the issuance, disposition or acquisition of any capital stock of the Parent or the Acquisition Subsidiary (except as contemplated by this Agreement). There are no outstanding stock appreciation, phantom stock or similar rights with respect to the Acquisition Subsidiary. There are no voting trusts, proxies or other agreements or understandings with respect to the voting of any capital stock of the Acquisition Subsidiary.
(b) At all times from January 30, 2025 (inception) through the date of this Agreement, the business and operations of the Acquisition Subsidiary have been conducted exclusively through the Parent.
(c) The Parent does not control directly or indirectly or have any direct or indirect participation or similar interest in any corporation, partnership, limited liability company, joint venture, trust or other business association which is not a Subsidiary.
3.6 SEC Reports and Prior Registration Statement Matters. Since the filing of the Parent’s Registration Statement on Form 10 on August 10, 2023 (the “Parent Form 10”), the Parent has timely filed (or has been deemed to have timely filed pursuant to Rule 12b-25 under the Exchange Act) all reports, forms and documents that it was required to file with the SEC pursuant to the Exchange Act (together with the Parent Form 10, the “Parent Previous Filings”). The Parent shall notify the Company immediately and in writing of the filing of any additional forms, reports or documents with the SEC by the Parent after the date hereof and prior to the Effective Time, provided that Company is aware that the Parent will timely file a Form 8-K Current Report with respect to the execution and delivery of this Agreement (together with the Parent Previous Filings, the “Parent SEC Filings”). The Parent has timely filed (or has been deemed to have timely filed pursuant to Rule 12b-25 under the Exchange Act) and made publicly available on the SEC’s EDGAR system, and the Company may rely upon, all certifications and statements required by (i) Rule 13a-14 or Rule 15d-14 under the Exchange Act and (ii) Section 906 of the Sarbanes Oxley Act of 2002 with respect to any documents filed with the SEC. The Parent is in compliance in all material respects with all of the provisions of the Sarbanes-Oxley Act of 2002 which are applicable to it. The Parent SEC Filings complied in all material respects with the requirements of the Exchange Act and the rules and regulations thereunder when filed. As of the date hereof, there are no outstanding or unresolved comments in comment letters received from the staff of the SEC with respect to any of the Parent SEC Filings. As of their respective dates, the Parent SEC Filings, including any financial statements, schedules or exhibits included or incorporated by reference therein, did not contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in light of the circumstances under which they were made, not misleading. None of the Subsidiaries of the Parent is required to file or furnish any forms, reports or other documents with the SEC. No order suspending the effectiveness of any registration statement of the Parent under the Securities Act or the Exchange Act has been issued by the SEC and, to the Parent’s knowledge after reasonable inquiry, no proceedings for that purpose have been initiated or threatened by the SEC. Since the most recent filing of such certifications and statements, there have been no significant changes in the Parent’s internal control over financial reporting (as such term is defined in Rule 13a-15(f) under the Exchange Act), or in other factors that could significantly affect its disclosure controls and procedures. The Parent has established and maintains disclosure controls and procedures (as defined in Rules 13a-14 and 15d-14 under the Exchange Act) and such controls and procedures are effective in ensuring that material information relating to the Parent, including its Subsidiaries, is made known to the principal executive officer and the principal financial officer.
16
3.7 Compliance with Laws. Each of the Parent and its Subsidiaries:
(a) and the conduct and operations of their respective businesses, are in compliance in all material respects with each Law applicable to the Parent, any Subsidiary of the Parent or any of their properties or assets;
(b) has complied with all federal and state securities Laws and regulations, including being current in all of its reporting obligations under such federal and state securities laws and regulations, except for any violations or defaults that, individually or in the aggregate, have not had and would not reasonably be expected to have a Parent Material Adverse Effect, and all prior issuances of its securities have been either registered under the Securities Act or exempt from registration;
(c) has not been the subject of any voluntary or involuntary bankruptcy proceeding, nor has it been a party to any material litigation or, within the past three years, the subject of any threat of material litigation;
(d) is not and has not, and the past and present officers, directors and Affiliates of the Parent are not and have not, been the subject of, nor does any officer or director of the Parent have any reason to believe that the Parent or any of its officers, directors or Affiliates are the subject of, any civil, criminal or administrative investigation or proceeding brought by any federal or state agency having regulatory authority over such entity or person or alleging a violation of securities Laws (in the case of an individual, that is described in Item 401(f)(1)-(3) of SEC Regulation S-K);
(e) except as set forth in Section 3.7(e) of the Parent Disclosure Schedule, does not and will not at the Closing, have any liabilities, contingent or otherwise, including but not limited to notes payable and accounts payable, and is not a party to any executory agreements; and
(f) is not a “blank check company” as such term is defined by Rule 419 of the Securities Act, except for Parent which is a “blank check company.”
3.8 Financial Statements. The audited financial statements and unaudited interim financial statements of the Parent included in the Parent SEC Filings (collectively, the “Parent Financial Statements”) (a) complied as to form in all material respects with applicable accounting requirements and, as appropriate, the published rules and regulations of the SEC with respect thereto when filed, (b) were prepared in accordance with GAAP applied on a consistent basis throughout the periods covered thereby (except as may be indicated therein or in the notes thereto, and in the case of quarterly financial statements, as permitted by Form 10-Q under the Exchange Act), (c) fairly present in all material respects the financial condition, results of operations and cash flows of the Parent as of the respective dates thereof and for the periods referred to therein, and (d) are consistent in all material respects with the books and records of the Parent. There has been no change in Parent accounting policies except as described in the notes to the Parent Financial Statements.
17
3.9 Absence of Certain Changes. Since the date of the most recent balance sheet contained in a Parent SEC Filing, Parent has conducted its business only in the ordinary course consistent with past practice, and there has not occurred or been entered into, as the case may be, any (a) event or development which, individually or in the aggregate, has had, or could reasonably be expected to have in the future, a Parent Material Adverse Effect, (b) event that would reasonably be expected to prevent or materially delay the performance of the Parent’s obligations pursuant to this Agreement, (c) material change by the Parent in its accounting methods, principles or practices, (d) declaration, setting aside or payment of any dividend or distribution in respect of the shares of capital stock of the Parent or any redemption, purchase or other acquisition of any of the Parent’s securities, (e) increase in the compensation or benefits payable or to become payable to any officers or directors of the Parent or the Acquisition Subsidiary or establishment or modification of any compensatory plan of the Parent, (f) issuance, grants or sale of any stock, options, warrants, notes, bonds or other securities, or entry into any agreement with respect thereto by the Parent, (g) amendment to the certificate of incorporation or bylaws of the Parent, (h) capital expenditures by the Parent, purchase, sale, assignment or transfer of any material assets by the Parent, mortgage, pledge or existence of any Security Interest, lien, encumbrance or charge on any material assets or properties, tangible or intangible of the Parent, except for liens for Taxes not yet due, or any cancellation, compromise, release or waiver by the Parent of any rights of material value or any material debts or claims, (i) incurrence by the Parent of any material liability (absolute or contingent), except for current liabilities and obligations incurred in the Ordinary Course of Business (which liabilities are not material, individually or in the aggregate), (j) damage, destruction or similar loss, whether or not covered by insurance, materially affecting the business or properties of the Parent, (k) entry by the Parent into any agreement, contract, lease or license, (l) acceleration, termination, modification or cancellation of any agreement, contract, lease or license to which the Parent is a party or by which it is bound, (m) entry by the Parent into any loan or other transaction with any officers, directors or employees of the Parent, (n) charitable or other capital contribution by the Parent or pledge therefore, (o) entry by the Parent into any transaction of a material nature, or (p) negotiation or agreement by the Parent to do any of the things described in the preceding clauses (a) through (o), other than activities in connection with the transactions contemplated by this Agreement.
3.10 Undisclosed Liabilities. None of the Parent and its Subsidiaries has any liability (whether known or unknown, whether absolute or contingent, whether liquidated or unliquidated and whether due or to become due), except for (a) liabilities shown on the most recent balance sheet contained a Parent SEC Filing, (b) liabilities which have arisen since the date of the most recent balance sheet contained a Parent SEC Filing in the Ordinary Course of Business which do not exceed $25,000 in the aggregate and (c) contractual and other liabilities incurred in the Ordinary Course of Business which are not required by GAAP to be reflected on a balance sheet.
3.11 Off-Balance Sheet Arrangements. Neither the Parent nor any of its Subsidiaries is a party to, or has any commitment to become a party to, any joint venture, off balance sheet partnership or any similar contract or arrangement (including any contract or arrangement relating to any transaction or relationship between or among the Parent and any of its Subsidiaries, on the one hand, and any unconsolidated Affiliate, including any structured finance, special purpose or limited purpose entity or person, on the other hand, or any “off balance sheet arrangements” (as defined in Item 303(a) of Regulation S-K under the Exchange Act)), where the result, purpose or intended effect of such contract is to avoid disclosure of any material transaction involving, or material liabilities of, the Parent or any of its Subsidiaries in the Parent’s or such Subsidiary’s published financial statements or other Parent SEC Filings.
18
3.12 Tax Matters.
(a) Each of the Parent and its Subsidiaries has filed on a timely basis all Tax Returns that it was required to file, and all such Tax Returns were true, complete, and accurate in all material respects. The officers of the Parent and its Subsidiaries after the Effective Time shall be responsible for preparing and filing all Tax Returns required to be filed after the Effective Time. Neither the Parent nor any of its Subsidiaries is or has ever been a member of a group of corporations with which it has filed (or been required to file) consolidated, combined or unitary Tax Returns, other than a group of which the Parent was the common parent. Each of the Parent and its Subsidiaries has paid on a timely basis all Taxes that were due and payable. The unpaid Taxes of the Parent and its Subsidiaries for Tax periods through the date of the balance sheet contained in the most recent Parent SEC Filing do not exceed the accruals and reserves for Taxes (excluding accruals and reserves for deferred Taxes established to reflect timing differences between book and Tax income) set forth on such balance sheet. Neither the Parent nor any of its Subsidiaries has any actual or potential liability for any Tax obligation of any taxpayer (including without limitation any affiliated group of corporations or other entities that included the Parent or any of its Subsidiaries during a prior period) other than the Parent and its Subsidiaries. All Taxes that the Parent or any of its Subsidiaries is or was required by Laws to withhold or collect have been duly withheld or collected and, to the extent required, have been paid to the proper Governmental Entity. There are no liens for Taxes (other than Taxes not yet due and payable) upon any of the assets of the Parent or its Subsidiaries.
(b) The Parent has delivered or made available to the Company complete and accurate copies of all federal and state income Tax Returns, Tax examination reports, and statements of Tax deficiencies assessed against or agreed to by the Parent or any of its Subsidiaries since March 10, 2022, which is the date of the Parent’s inception. No examination or audit of any Tax Return of the Parent or any of its Subsidiaries by any Governmental Entity is currently in progress or, to the knowledge of the Parent, threatened or contemplated. Neither the Parent nor any of its Subsidiaries has been informed by any jurisdiction that the jurisdiction believes that the Parent or any of its Subsidiaries was required to file any Tax Return for any Taxes generally or for any particular types of Taxes that was not filed. Neither the Parent nor any of its Subsidiaries has waived any statute of limitations with respect to Taxes or agreed to an extension of time with respect to a Tax assessment or deficiency.
(c) Neither the Parent nor any of its Subsidiaries will be required to include any item of income in, or exclude any item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for a taxable period ending on or prior to the Closing Date, including any adjustment pursuant to Code Sections 481 or 263A (or any corresponding or similar provision of state, local or foreign Laws); (ii) use of an improper method of accounting for a taxable period ending on or prior to the Closing Date; (iii) “closing agreement” as described in Section 7121 of the Code (or any corresponding or similar provision of U.S. state, local or non-U.S. Laws) executed on or prior to the Closing Date; (iv) installment sale or open transaction disposition made on or prior to the Closing Date; (v) prepaid amount or any other income eligible for deferral under the Code or Treasury Regulations promulgated thereunder (including, without limitation, pursuant to Sections 455 or 456 of the Code, Treasury Regulations Section 1.451-5 and U.S. Internal Revenue Service (“the “IRS”) Revenue Procedure 2004-34, 2004-33 I.R.B. 991) received on or prior to the Closing Date; (vi) intercompany transactions or any excess loss account described in Treasury Regulations under Section 1502 of the Code (or any corresponding or similar provision of U.S. state, local or non-U.S. income Tax Laws); (vii) election made under Section 108(i) of the Code prior to the Closing or (viii) any similar election, action, or agreement that would have the effect of deferring any liability for Taxes of the Parent or any of its Subsidiaries from any period ending on or before the Closing Date to any period ending after such date.
19
(d) Neither the Parent nor any of its Subsidiaries has participated in any “listed transaction,” as defined in Section 6706A(c)(2) of the Code and Treasury Regulations Section 1.6011- 4(b)(2).
(e) Neither the Parent nor any of its Subsidiaries has taken or agreed to take any action not contemplated by this Agreement that could reasonably be expected to prevent the Merger, together with the Private Placement Offering, from qualifying for the Intended Tax Treatment. To the knowledge of Parent, no facts or circumstances exist that could reasonably be expected to prevent the Merger, together with the Private Placement Offering, from qualifying for the Intended Tax Treatment.
3.13 Assets. Each of the Parent and the Acquisition Subsidiary owns or leases all tangible assets necessary for the conduct of its businesses as presently conducted and as presently proposed to be conducted. Each such tangible asset is free from material defects, has been maintained in accordance with normal industry practice, is in good operating condition and repair (subject to normal wear and tear) and is suitable for the purposes for which it presently is used. No asset of the Parent or the Acquisition Subsidiary (tangible or intangible) is subject to any Security Interest.
3.14 Real Property. Neither the Parent nor any of its Subsidiaries owns, leases or uses any real property, nor have they ever owned, leased or used any real property.
3.15 Contracts. Except for this Agreement, the agreements to be executed by the Parent that are included as exhibits to this Agreement or such agreements that comprise the Transaction Documentation, the agreements filed as exhibits to the Parent SEC Filings, and the agreements set forth on Section 3.15 of the Parent Disclosure Schedule, the Parent is not a party to any contract, agreement, arrangement or other understanding, whether written or oral, which is currently in effect. All agreements or commitments set forth on Section 3.15 of the Parent Disclosure Schedule shall either be cancelled or satisfied at the Effective Time except for outstanding liabilities set forth in Section 3.7(e) of the Parent Disclosure Schedule.
3.16 Powers of Attorney. There are no outstanding powers of attorney executed on behalf of the Parent or any of its Subsidiaries.
3.17 Insurance. The Parent does not own or maintain any insurance policies, nor is any insurance necessary for the operation of its business.
3.18 Litigation. There is no Legal Proceeding which is pending or, to the Parent’s knowledge, threatened against the Parent or any Subsidiary of the Parent and there is no reasonable basis for any proceeding, claim, action or governmental investigation directly or indirectly involving the Parent, the Acquisition Subsidiary, or the Parent’s officers, directors or employees, in their capacities as such, individually or in the aggregate. Neither the Parent nor the Acquisition Subsidiary are party to any inquiry, investigation, order, judgment or decree conducted or issued by any Governmental Entity.
20
3.19 Employees.
(a) Other than the sole officer of the Parent, the Parent and the Subsidiaries of the Parent have no employees.
(b) Neither the Parent nor any of its Subsidiaries is or ever has been a party to or bound by any collective bargaining agreement, nor have any of them experienced any strikes, grievances, claims of unfair labor practices or other collective bargaining disputes. There has been no organizational effort made or, to the knowledge of the Parent, threatened, either currently or since the date of organization of the Parent, by or on behalf of any labor union with respect to the service providers of the Parent or any of its Subsidiaries. Each individual providing services to the Parent or any of its Subsidiaries has been properly classified as an employee or a non-employee service provider with respect to each such entity for all purposes under applicable Laws. No current or former employee, consultant or director of the Parent or the Acquisition Subsidiary owes any indebtedness to the Parent, the Acquisition Subsidiary or their Affiliates, nor does the Parent, the Acquisition Subsidiary or their Affiliates owe any indebtedness to any current or former employee, consultant or director of the Parent or the Acquisition Subsidiary other than as set forth in the Payoff Letter.
3.20 Employee Benefits. Neither the Parent nor any of its Subsidiaries or ERISA Affiliates maintains, sponsors or contributes to or in the past has maintained, sponsored or contributed to any Employee Benefit Plan (as defined in Section 3(3) of ERISA, whether or not ERISA applies to the arrangement) or multiemployer plan (each capitalized term in this sentence as defined in Section 4001(a)(3) of ERISA). Neither the execution of this Agreement nor the consummation of the transactions contemplated by this Agreement shall, individually or in the aggregate, (a) result in any payment becoming due to any officer, employee, consultant or director of the Parent or the Acquisition Subsidiary, (b) increase or modify any benefits otherwise payable by the Parent or the Acquisition Subsidiary to any employee, consultant or director of the Parent or the Acquisition Subsidiary, or (c) result in the acceleration of time of payment or vesting of any such benefits.
3.21 Environmental Matters.
(a) Each of the Parent and its Subsidiaries has complied with all applicable Environmental Laws, except for violations of Environmental Laws that, individually or in the aggregate, have not had and would not reasonably be expected to have a Parent Material Adverse Effect. There is no pending or, to the knowledge of the Parent, threatened civil or criminal litigation, written notice of violation, formal administrative proceeding, or investigation, inquiry or information request by any Governmental Entity, relating to any Environmental Law involving the Parent or any of its Subsidiaries, except for litigation, notices of violations, formal administrative proceedings or investigations, inquiries or information requests that, individually or in the aggregate, have not had and would not reasonably be expected to have a Parent Material Adverse Effect.
21
(b) The Parent has no environmental reports, investigations or audits relating to premises currently or previously owned or operated by the Parent or any of its Subsidiaries (whether conducted by or on behalf of the Parent or its Subsidiaries or a third party, and whether done at the initiative of the Parent or any of its Subsidiaries or directed by a Governmental Entity or other third party) which were issued or conducted during the past five years and which the Parent has possession of or access to.
(c) To the knowledge of the Parent, there is no material environmental liability of any solid or hazardous waste transporter or treatment, storage or disposal facility that has been used by the Parent or any of its Subsidiaries.
(d) For purposes of this Agreement, “Environmental Law” means any Law relating to the environment, including without limitation any Law pertaining to (i) treatment, storage, disposal, generation and transportation of industrial, toxic or hazardous materials or substances or solid or hazardous waste; (ii) air, water and noise pollution; (iii) groundwater and soil contamination; (iv) the release or threatened release into the environment of industrial, toxic or hazardous materials or substances, or solid or hazardous waste, including without limitation emissions, discharges, injections, spills, escapes or dumping of pollutants, contaminants or chemicals; (v) the protection of wild life, marine life and wetlands, including without limitation all endangered and threatened species; (vi) storage tanks, vessels, containers, abandoned or discarded barrels, and other closed receptacles; (vii) the reclamation of mines; (viii) health and safety of employees and other persons; and (ix) manufacturing, processing, using, distributing, treating, storing, disposing, transporting or handling of materials regulated under any Law as pollutants, contaminants, toxic or hazardous materials or substances or oil or petroleum products or solid or hazardous waste. As used above, the terms “release” and “environment” shall have the meaning set forth in the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended.
3.22 Permits. Parent has no licenses, permits and certificates from federal, state, local and foreign authorities (including, without limitation, federal and state agencies regulating occupational health and safety), or any other Governmental Entity, and none are necessary to its operations and business.
3.23 Certain Business Relationships with Affiliates. No Affiliate of the Parent or of any of its Subsidiaries (a) owns any property or right, tangible or intangible, which is used in the business of the Parent or any of its Subsidiaries, (b) has any claim or cause of action against the Parent or any of its Subsidiaries, or (c) owes any money to, or is owed any money by, the Parent or any of its Subsidiaries except as disclosed in the Parent SEC Filings.
3.24 Tax-Free Reorganization.
(a) The Parent (i) is not an “investment company” as defined in Section 368(a)(2)(F)(iii) and (iv) of the Code; (ii) has no present plan or intention to liquidate the Surviving Corporation or to merge the Surviving Corporation with or into any other corporation or entity, or to sell or otherwise dispose of the stock of the Surviving Corporation which the Parent will acquire in the Merger, or to cause the Surviving Corporation to sell or otherwise dispose of its assets, all except in the Ordinary Course of Business of the Parent or if such liquidation, merger or disposition is described in Section 368(a)(2)(C) or Treasury Regulation Section 1.368-2(d)(4) or Section 1.368-2(k); and (iii) has no present plan or intention, following the Merger, to issue any additional shares of stock of the Surviving Corporation or to create any new class of stock of the Surviving Corporation.
22
(b) The Acquisition Subsidiary is a direct, wholly-owned Subsidiary of the Parent, formed solely for the purpose of engaging in the Merger, and will carry on no business prior to the Merger.
(c) Immediately prior to the Merger, the Parent will be in control of Acquisition Subsidiary within the meaning of Section 368(c) of the Code.
(d) Neither the Parent, nor, to the knowledge of the Parent, any person related to the Parent (within the meaning of Treasury Regulations Section 1.368-1(e)(3)) or any person acting as an intermediary for the Parent, has any present plan or intention to reacquire any of the Merger Shares.
(e) The Acquisition Subsidiary will have no liabilities assumed by the Surviving Corporation and will not transfer to the Surviving Corporation any assets subject to liabilities in the Merger.
(f) The Parent conducts no activities other than activities related to (i) maintaining its legal and/or corporate existence and its status as a “shell company” as defined in Rule 12b-2 under the Exchange Act, (ii) holding the capital stock of Acquisition Subsidiary, and (iii) any related accounting, legal, financial, administrative, Tax and other similar activities related to such matters.
(g) The Acquisition Subsidiary does not hold any property and does not have any Tax attributes immediately prior to the Merger, other than a de minimis amount of assets to facilitate its organization or maintain its legal existence and Tax attributes related to holding those assets.
(h) The Parent has not made purchases of its own stock described in Code Section 1202(c)(3)(B) during the one (1)-year period preceding the Closing Date, except for purchases that are disregarded for such purposes under Treasury Regulations Section 1.1202-2.
(i) As of the date of this Agreement it is the present intention, and as of the day of the Effective Time it will be the present intention, of the Parent to continue, either through the Parent or through a member of the Parent’s “qualified group” within the meaning of Treasury Regulations Section 1.368-1(d)(4)(ii) (the “Qualified Group”), at least one significant historic business line of the Company, or to use at least a significant portion of the Company’s historic business assets in a business, in each case within the meaning of Treasury Regulations Section 1.368-1(d). As of the date of this Agreement and as of the date of the Effective Time, neither the Parent nor any “related person” (as defined in Treasury Regulations Section 1.368-1(e)(4)) with respect to the Parent has or will have any plan or intention to redeem or reacquire, either directly or indirectly, any of the Merger Shares issued to any holder of Company Shares in connection with the Merger. As of the date of this Agreement and as of the date of the Effective Time, the Parent does not have and will not have any plan or intention to sell or otherwise dispose of any of the assets of the Company acquired in the Merger, except for dispositions made in the ordinary course of business or transfers described in Section 368(a)(2)(C) of the Code or described and permitted in Treasury Regulations Section 1.368-2(k).
23
3.25 Brokers’ Fees. Except as listed on Section 3.25 of the Parent Disclosure Schedule, neither the Parent nor any of its Subsidiaries has any liability or obligation to pay any fees or commissions to any broker, finder or agent with respect to the transactions contemplated by this Agreement.
3.26 Interested Party Transactions. To the knowledge of the Parent, no officer, director or stockholder of the Parent or any “affiliate” (as such term is defined in Rule 12b-2 under the Exchange Act) (each, an “Affiliate”) or “associate” (as such term is defined in Rule 405 under the Securities Act) of any such person currently has or has had, either directly or indirectly, (a) an interest in any person that (i) furnishes or sells services or products that are furnished or sold or are proposed to be furnished or sold by the Parent or any of its Subsidiaries or (ii) purchases from or sells or furnishes to the Parent or any of its Subsidiaries any goods or services, or (b) other than as disclosed in the Parent SEC Filings, a beneficial interest in any contract or agreement to which the Parent or any of its Subsidiaries is a party or by which it may be bound or affected. Except as set forth in the Parent SEC Filings, Parent is not indebted to any officer, director or stockholder of the Parent or any “affiliate” or “associate” of any such person (each such person, a “Parent Insider”) (except for reimbursement of ordinary business expenses) and no Parent Insider is indebted to the Parent (except for cash advances for ordinary business expenses), all of which shall be paid or cancelled immediately at or prior to the Effective Time by Parent’s stockholders. Neither the Parent nor any of its Subsidiaries has extended or maintained credit, arranged for the extension of credit, or renewed an extension of credit, in the form of a personal loan to or for any director or executive officer (or equivalent thereof) of the Parent or any of its Subsidiaries.
3.27 Accountants. Except for the preparation and filing of the Parent’s corporate Tax Returns, there have been no non-audit services performed by KNAV CPA LLP (the “Parent Auditor”) for the Parent and/or any of its Subsidiaries, and the Parent has not taken any action or failed to take any action that would reasonably be expected to impair the independence of the Parent Auditor. The report of the Parent Auditor on the financial statements of the Parent for the past fiscal year did not contain an adverse opinion or a disclaimer of opinion, or was qualified as to uncertainty, audit scope, or accounting principles, although it did express uncertainty as to the Parent’s ability to continue as a going concern. During the Parent’s most recent fiscal year and the subsequent interim periods, there were no disagreements with the Parent Auditor on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedures. None of the reportable events listed in Item 304(a)(1)(iv) or (v) of Regulation S-K occurred with respect to the Parent Auditor.
3.28 Minute Books. The minute books and other similar records of the Parent and each of its Subsidiaries contain, in all material respects, complete and accurate records of all actions taken at any meetings of directors (or committees thereof) and stockholders or actions by written consent in lieu of the holding of any such meetings since the time of organization of each such corporation through the date of this Agreement. The Parent has provided true and complete copies of all such minute books and other similar records to the Company’s representatives.
3.29 Board Action. The Parent’s board of directors (a) has unanimously determined that the Merger is advisable and in the best interests of the Parent’s stockholders and is on terms that are fair to such Parent stockholders, (b) has caused the Parent, in its capacity as the sole stockholder of the Acquisition Subsidiary, and the Board of Directors of the Acquisition Subsidiary, to approve the Merger and this Agreement by unanimous written consent, and (c) adopted this Agreement in accordance with the provisions of the DGCL.
3.30 Intellectual Property. The Parent does not own or license the right to use any patents, copyrights, trademarks, know-how or software, and none are or ever have been necessary for the operation of its business. To the Parent’s knowledge, the Parent is not infringing, and has never infringed, upon the intellectual property or proprietary rights of any person. There are no claims pending or, to the Parent’s knowledge, threatened alleging that the Parent is currently infringing upon or using in an unauthorized manner or violating the intellectual or proprietary rights of any person, and the Parent is unaware of any facts which would form a reasonable basis for any such claim. The Parent is not, nor will it be as a result of the execution and delivery of this Agreement or the performance of its obligations under this Agreement, in breach of any license, sublicense or other agreement or contract relating to intellectual property.
3.31 Investment Company. None of the Parent or Acquisition Subsidiary is as of the date of this Agreement, nor upon the Closing will be, an “investment company,” a company controlled by an “investment company,” or an “affiliated person” of, or “promoter” or “principal underwriter” for, an “investment company,” as such terms are defined in the Investment Company Act of 1940, as amended.
24
3.32 Foreign Corrupt Practices Act. Neither the Parent nor its Subsidiaries, nor to the Parent’s knowledge, any agent or other person acting on behalf of the Parent or its Subsidiaries, has: (a) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (b) made any unlawful payment to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (c) failed to disclose fully any contribution made by the Company (or made by any person acting on its behalf of which the Parent is aware) which is in violation of Law or (d) violated in any material respect any provision of the Foreign Corrupt Practices Act of 1977, as amended.
3.33 No Integrated Offering. Neither Parent nor any Affiliates of Parent, nor any person acting on the behalf of any of the foregoing, has, directly or indirectly, (a) made any offers or sales of any security or solicited any offers to purchase any security, under circumstances that would require registration of any of the shares of Parent Common Stock issuable pursuant to this Agreement under the Securities Act or cause this offering of such shares of Parent Common Stock to be integrated with prior offerings by Parent for purposes of the Securities Act or any applicable shareholder approval requirements of any authority, or (b) made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would require registration of the shares to be issued in the Private Placement Offering under the Securities Act or cause Private Placement Offering to be integrated with prior offerings by the Parent for purposes of the Securities Act.
3.34 No General Solicitation. Neither the Parent, nor any of its Affiliates, nor, to the knowledge of the Parent, any person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation D) in connection with the offer or sale of the shares to be issued in the Private Placement Offering.
3.35 Application of Takeover Provisions. The Parent and its Board of Directors have taken all necessary action, if any, in order to render inapplicable any control share acquisition, business combination, or other similar takeover, anti-takeover, moratorium, fair price, interested shareholder or similar provision under the certificate of incorporation of the Parent or the Laws of the State of Delaware to the transactions contemplated hereby, including the Merger and the Parent’s issuance of shares of Parent Common Stock to the Company Stockholders. The Parent has never adopted any shareholder rights plan or similar arrangement relating to accumulations of beneficial ownership of Parent Common Stock or a change in control of the Parent.
3.36 No Other Representations. The representations and warranties contained in this ARTICLE III are the only representations and warranties made by the Parent and Acquisition Subsidiary. The Parent disclaims any and all other representations and warranties other than those contained in this ARTICLE III, whether express or implied. The Parent hereby expressly disclaims any such other representation or warranty, whether by the Parent, Acquisition Subsidiary, or any of its representatives or any other person, notwithstanding the delivery or disclosure to the Company or any other person of any documentation or other written or oral information by the Parent, Acquisition Subsidiary or any of their respective representatives.
3.37 Independent Investigation; Disclaimer of Other Representations. The Parent and Acquisition Subsidiary have conducted their own independent investigation, review and analysis of the business, results of operations, condition (financial or otherwise) and assets of the Company and acknowledge that they have been provided reasonable access to the personnel, properties, assets, premises, books and records, and other documents and data of the Company for such purpose. The Parent and Acquisition Subsidiary each acknowledge and agree that (a) in making its decision to enter into this Agreement and to consummate the transactions contemplated hereby, it has relied solely upon its own investigation and the express representations and warranties of the Company set forth in ARTICLE II of this Agreement (and as qualified by the Company Disclosure Schedule), (b) the express representations and warranties made by the Company in ARTICLE II of this Agreement (and as qualified by the Company Disclosure Schedule) are the exclusive representations and warranties made by the Company with respect to the Company, its business, or its assets or the subject matter of this Agreement, and (c) none of the Company Stockholders or any other Person has made any representation or warranty as to the Company, its business, or its assets or the Company Stockholders, except for those representations and warranties made by the Company as expressly set forth in ARTICLE II of this Agreement (and as qualified by the Company Disclosure Schedule). Each of the Parent and Acquisition Subsidiary specifically disclaims that it is relying upon or has relied upon any such other representations or warranties that may have been made by any Person and acknowledges that the Company and each Company Stockholder and their respective Affiliates hereby specifically disclaim any such other representation or warranty made by any Person. Except for the representations and warranties of the Company set forth in ARTICLE II (and as qualified by the Company Disclosure Schedule), none of the Company or any Company Stockholder is, directly or indirectly, and no other Person on behalf of the Company is, making, and each of the Parent and Acquisition Subsidiary specifically disclaims that it is relying on, any representations or warranties regarding any pro-forma financial information, financial projections or other forward-looking prospects, risks or statements (financial or otherwise) of the Company made, communicated or furnished (orally or in writing) to the Parent or its Affiliates or their respective representatives (including any opinion, information, projection or advice in any management presentation), and the Company and each Company Stockholder disclaims any and all liability and responsibility for any such information and statements.
25
ARTICLE IV. COVENANTS
4.1 Conduct of the Business Prior to Closing; Closing Efforts.
(a) From the date hereof to the earlier of the Closing Date or the termination of this Agreement, the Parent shall not take any of the actions specified in Section 3.9 except (i) as consented to by the Company, (ii) as expressly contemplated by this Agreement or (iii) as required by applicable Laws.
(b) Each of the Parties shall use its best efforts, to the extent commercially reasonable in light of the circumstances (“Reasonable Best Efforts”), to take all actions and to do all things necessary, proper or advisable to consummate the transactions contemplated by this Agreement, including without limitation using its Reasonable Best Efforts to ensure that (a) its representations and warranties remain true and correct in all material respects through the Closing Date and (b) the conditions to the obligations of the other Parties to consummate the Merger are satisfied.
4.2 Governmental and Third-Party Notices and Consents.
(a) Each Party shall use its Reasonable Best Efforts to obtain, at its expense, all waivers, permits, consents, approvals or other authorizations from Governmental Entities, and to effect all registrations, filings and notices with or to Governmental Entities, as may be required for such Party to consummate the transactions contemplated by this Agreement and to otherwise comply with all applicable Laws in connection with the consummation of the transactions contemplated by this Agreement. The Parent will, following the Effective Time, timely complete all filings with the SEC and individual states required by Regulation D under the Securities Act with respect to the issuance of the Merger Shares and in connection with the Private Placement Offering.
(b) The Company shall use its Reasonable Best Efforts to obtain, at its expense, all such waivers, consents or approvals from third parties, and to give all such notices to third parties, if any, as are required to be listed in Section 2.4 of the Company Disclosure Schedule.
4.3 Super 8-K. Promptly after the execution of this Agreement, the Parties shall complete a Current Report on Form 8-K relating to this Agreement and the transactions contemplated hereby (including the “Form 10 information” required by Items 2.01(f) and 5.01(a)(8) of Form 8-K and the financial statements required thereby) (the “Super 8-K”). Each of the Company and the Parent shall use its Reasonable Best Efforts to cause the Super 8-K to be filed with the SEC within four Business Days after the Closing of the transactions contemplated by this Agreement and to otherwise comply with all requirements of applicable federal and state securities Laws.
4.4 Access to Information.
(a) During the period from the date of this Agreement to the Effective Time, the Company shall permit representatives of the Parent to have reasonable access (during normal business hours at reasonable times, upon reasonable notice, and in a manner so as not to interfere with the normal business operations of the Company) to all premises, properties, financial and accounting records, contracts, other records and documents, and personnel, of or pertaining to the Company; except that (i) such investigation will not include the sampling of the soil, groundwater, surface water, indoor or outdoor air, or vapors of the Company’s facilities without the Company’s prior written consent and (ii) the Company shall not be required to disclose any information that would jeopardize attorney-client privilege, contravene any applicable Law, or violate any agreement binding on the Company as of the date of this Agreement.
26
(b) During the period from the date of this Agreement to the Effective Time, the Parent and Acquisition Subsidiary shall permit representatives of the Company to have reasonable access (during normal business hours at reasonable times, upon reasonable notice, and in a manner so as not to interfere with the normal business operations of the Parent or the Acquisition Subsidiary) to all premises, properties, financial and accounting records, contracts, other records and documents, and personnel, of or pertaining to the Parent or the Acquisition Subsidiary; except that neither the Parent nor Acquisition Subsidiary shall be required to disclose any information that would jeopardize attorney-client privilege, contravene any applicable Law, or violate any agreement binding on the Parent or the Acquisition Subsidiary as of the date of this Agreement.
(c) The Parent and each of its Subsidiaries (i) shall treat and hold as confidential any Company Confidential Information (as defined below), (ii) shall not use any of the Company Confidential Information for any purpose except in connection with this Agreement, and (iii) if this Agreement is terminated for any reason whatsoever, shall return to the Company or destroy (at the option of the Company) all embodiments (and destroy all copies) thereof which are in its possession. For purposes of this Agreement, “Company Confidential Information” means any information concerning the Company, whether furnished to Parent or any of its Subsidiaries before or after the date of this Agreement, together with any and all analyses or other documents prepared by the Parent or any of its Subsidiaries or their respective directors, employees advisors, attorneys, accountants, consultants, subcontractors, or representatives (collectively “Representatives”) which contain or otherwise reflect such information; provided, however, that Company Confidential Information shall not include any information (A) which, at the time of disclosure, is available publicly other than as a result of non-permitted disclosure by the Parent, any of its Subsidiaries or their respective Representatives, (B) which, after disclosure, becomes available publicly through no fault of the Parent, any of its Subsidiaries or their respective Representatives, (C) which the Parent or any of its Subsidiaries knew or to which the Parent or any of its Subsidiaries had access prior to disclosure, as demonstrated by competent evidence, provided that the source of such information is not known by the Parent or any of its Subsidiaries to be bound by a confidentiality obligation to the Company, or (D) which the Parent or any of its Subsidiaries rightfully obtains from a source other than the Company, provided that the source of such information is not known by the Parent or any of its Subsidiaries to be bound by a confidentiality obligation to the Company. Parent and Acquisition Subsidiary shall be liable for any breach of this Section 4.4 by any of their respective Subsidiaries or Representatives.
(d) The Company (i) shall treat and hold as confidential any Parent Confidential Information (as defined below), (ii) shall not use any of the Parent Confidential Information for any purpose except in connection with this Agreement, and (iii) if this Agreement is terminated for any reason whatsoever, shall return to the Parent or destroy (at the option of Parent) all embodiments (and destroy all copies) thereof which are in its possession. For purposes of this Agreement, “Parent Confidential Information” means any information concerning the Parent or any Subsidiary of the Parent that is furnished to the Company by the Parent or its Subsidiaries in connection with this Agreement, whether before or after the date of this Agreement, together with any and all analyses or other documents prepared by the Company or any of its Representatives which contain or reflect such information; provided, however, that Parent Confidential Information shall not include any information (A) which, at the time of disclosure, is available publicly other than as a result of non-permitted disclosure by the Company or its Representatives, (B) which, after disclosure, becomes available publicly through no fault of the Company or its Representatives, (C) which the Company knew or to which the Company had access prior to disclosure, as demonstrated by competent evidence, provided that the source of such information is not known by the Company or any Company Subsidiary to be bound by a confidentiality obligation to the Parent or any Subsidiary of the Parent or (D) which the Company rightfully obtains from a source other than the Parent or a Subsidiary of the Parent, provided that the source of such information is not known by the Company or any Company Subsidiary to be bound by a confidentiality obligation to the Parent or any Subsidiary of the Parent. Company shall be liable for any breach of this Section 4.4(d) by any of its Representatives.
4.5 Expenses. The costs and expenses of each Party (including legal fees and expenses of such Party) incurred in connection with this Agreement and the transactions contemplated hereby shall be paid by the Party that incurred such costs and expenses, unless otherwise agreed to by such Parties, provided, that, the Parties agree that an aggregate of $150,000 of the fees of Sichenzia Ross Ference Carmel LLP related to the transactions contemplated by this Agreement and the Private Placement Offering, plus its reasonable and documented out-of-pocket expenses related to the transactions contemplated by this Agreement and the Private Placement Offering, shall be paid from the gross proceeds of the Private Placement Offering at the first closing thereof.
27
4.6 Indemnification; Insurance.
(a) The Parent agrees to, and agrees to cause the Surviving Corporation to, take any action necessary to ensure that all rights to indemnification and exculpation now existing in favor of any individual who is currently serving, or has previously served, as a director or officer of the Company as provided in (i) the certificate of incorporation, by-laws, or other governing documents or (ii) any written indemnification agreements to which any current or former officer or director is a party or bound as of the date of this Agreement will survive the Closing and will continue in full force and effect in accordance with their respective terms, except in each case for any changes which may be required to conform with changes in applicable Law and any changes which do not affect the application of such provisions to acts or omissions of such individuals prior to the Effective Time.
(b) From and after the Effective Time, the Parent agrees that it will, and will cause the Surviving Corporation to, indemnify and hold harmless each current and former director and officer of the Company (the “Indemnified Executives”) against any costs or expenses (including reasonable attorneys’ fees), judgments, fines, losses, claims, damages, liabilities or amounts paid in settlement or otherwise incurred in connection with any claim, action, suit, proceeding or investigation, whether civil, criminal, administrative or investigative, arising out of or pertaining to matters existing or occurring at or prior to the Effective Time, whether asserted or claimed prior to, at or after the Effective Time, to the fullest extent permitted under Delaware Law (and the Parent and the Surviving Corporation shall also advance expenses as incurred to the fullest extent permitted under Delaware Law, provided the Indemnified Executive to whom expenses are advanced provides an undertaking to repay such advances if it is ultimately determined that such Indemnified Executive is not entitled to indemnification).
(c) From and after the Effective Time, the Parent agrees that it will take any action necessary to ensure that all rights to indemnification now existing in favor of any individual who is currently serving, or has previously served, as a director or officer of the Parent and that is listed on Schedule 4.6(c) (the “Parent Indemnified Executives”) as provided in (i) the certificate of incorporation, by-laws or other governing documents of Parent or (ii) (a) any written indemnification agreements to which any Parent Indemnified Executive is a party or bound as of the date of this Agreement or (b) the indemnification agreements entered into between the Parent and each Parent Indemnified Executive prior to the Effective Time in the form attached hereto as Exhibit C (collectively, the “Pre-Merger Indemnity Agreement”), will survive the Closing and will continue in full force and effect in accordance with their respective terms, except in each case for any changes which may be required to conform with changes in applicable Law and any changes which do not affect the application of such provisions to acts or omissions of such individuals prior to the Effective Time.
(d) Effective as of 12:01 a.m. on the Closing Date, Parent will purchase or cause the Surviving Corporation to purchase, (“D&O Insurance”) to be effective as of 12:01 a.m. on the Closing Date, director and officer liability insurance covering the directors and officers of the Parent and the Surviving Corporation immediately after the Effective Time, and such D&O Insurance shall include coverage for any acts or omissions that take place on or after the Effective Time, including, without limitation, in connection with the transactions contemplated by this Agreement, and shall be maintained in effect for a period of at least six (6) years following the Effective Time. At the Closing, Parent will also purchase or cause the Surviving Corporation to purchase, to be effective as of 12:01 a.m. on the Closing Date (i) a directors’ and officers’ liability insurance “tail policy” to the policy of directors’ and officers’ liability insurance maintained by the Company as of the date of this Agreement with a claims period of six (6) years following the Effective Time, and on terms and conditions no less favorable to the Indemnified Executives than those in effect under the policy of directors’ and officers’ liability insurance maintained by the Company as of the date of this Agreement with respect to their acts and omissions as directors and officers of the Company occurring prior to the Effective Time, including, without limitation, in connection with the transactions contemplated by this Agreement (such policy, the “D&O Tail Policy”), (ii) a directors’ and officers’ liability insurance policy or policy endorsement covering the Parent Indemnified Executives with a claims period of six (6) years following the Effective Time, on terms and conditions reasonably satisfactory to the Parent Indemnified Executives with respect to their acts and omissions as directors and officers of Parent occurring prior to the Effective Time (such policy, the “Pre-Merger D&O Policy”) and (iii) “run-off” coverage as provided by the Company’s fiduciary and employee benefit policies, in each case, covering those Persons who are covered on the date of this Agreement by such policies and with terms, conditions, retentions, and limited of liability that are no less advantageous than the coverage provided under the Company’s policies which are in effect as of the date of this Agreement.
28
(e) The provisions of this Section 4.6 shall survive the Closing and are intended to be for the benefit of, and enforceable by, each Indemnified Executive and Parent Indemnified Executive, as applicable, and nothing in this Agreement shall affect any indemnification rights that any such person may have under the certificate of incorporation or the by-laws of the Company or the Parent or any contract or instrument or applicable Law, including any contract, agreement or arrangement between the Parent, the Company, the Surviving Corporation or any of their respective Subsidiaries (on the one hand) and any such Indemnified Executive, any investor or third party (on the other hand). Notwithstanding anything in this Agreement to the contrary, the obligations under this Section 4.6 shall not be terminated or modified in such a manner as to adversely affect any Indemnified Executive or Parent Indemnified Executive without the prior written consent of such person. The provisions of this Section 4.6 with respect to limitation of liabilities of any Indemnified Executive and Parent Indemnified Executive and advancement of expenses and indemnification that are set forth as of the date hereof in the governing documents of the Company and the Surviving Corporation are incorporated herein by reference and shall continue to apply after the date hereof with respect to the applicable Person(s) in their current or former capacities. The obligations of this Section 4.6 will be binding on the successors and assigns of the Parent and the Surviving Corporation.
4.7 Name. Promptly after the Effective Time, the Parent shall (a) cause the Surviving Corporation to amend its Certificate of Incorporation to change its corporate name to one other than “Adaptin Bio, Inc.” and (b) amend its Certificate of Incorporation to change its corporate name to Adaptin Bio, Inc., or such other name as specified by the Company.
4.8 Parent Board; Amendment of Charter Documents; Corporate Policies. The Parent shall take such actions as are necessary (including the solicitation of approvals by the Board of Directors and the stockholders of the Parent), effective at or prior to the Effective Time, (a) to authorize the Parent’s Board of Directors to consist of five (5) members, (b) to amend and restate its certificate of incorporation to read in its entirety as set forth on Exhibit A hereto in a manner satisfactory to the Company, (c) to amend and restate its bylaws to read in their entirety as set forth on Exhibit B hereto in a manner satisfactory to the Company; and (d) to adopt various corporate policies and charters in a manner satisfactory to the Company.
4.9 Equity Plans. As of the Effective Time, (i) the Board of Directors of Parent shall adopt the equity incentive plan provided to Parent by the Company (the “2025 Plan”) and (ii) the stockholders of the Parent shall adopt the 2025 Plan, subject to effectiveness in accordance with Regulation 14C of the Exchange Act, if applicable. Fifteen percent (15%) of the total number of shares of the Parent Common Stock to be outstanding after completion of the Merger and the final closing of the Private Placement Offering, on a fully diluted basis (assuming exercise or conversion of all then-outstanding Parent Common Stock equivalents), will be reserved for future issuance under the 2025 Plan. The 2025 Plan will provide that the shares of Parent Common Stock reserved for issuance will be subject to increase annually on the first day of each year beginning in 2027, at the discretion of the Administrator (as such term is defined in the 2025 Plan), in an amount equal to the lesser of (a) at the discretion of the Board of Directors, in an amount up to four percent (4%) of the shares of stock outstanding (on an as-converted basis) on the last day of the immediately preceding year, or (b) such number of shares as determined by the Administrator.
29
4.10 Information Provided to Stockholders. The Company shall prepare, with the cooperation of the Parent, information to be sent to the holders of Company Shares in connection with soliciting their approval of the Merger, this Agreement and the related transactions, and the Parent shall prepare, with the cooperation of the Company, information to be sent to the holders of shares of Parent Common Stock in connection with receiving their approval of the Merger, this Agreement and the related transactions. The Parent and the Company shall each use Reasonable Best Efforts to cause information provided to such party’s stockholders to comply with applicable federal and state securities Laws requirements and other applicable Laws. Each of the Parent and the Company agrees to provide promptly to the other such information concerning its business and financial statements and affairs as, in the reasonable judgment of the providing party or its counsel, may be required or appropriate for inclusion in the information sent, or in any amendments or supplements thereto, and to cause its counsel and auditors to cooperate with the other’s counsel and auditors in the preparation of the information to be sent to the stockholders of each Party. The Company will promptly advise the Parent, and the Parent will promptly advise the Company, in writing if at any time prior to the Effective Time either the Company or the Parent shall obtain knowledge of any facts that might make it necessary or appropriate to amend or supplement the information sent in order to make the statements contained or incorporated by reference therein not misleading or to comply with applicable Laws. The information sent by the Company shall contain the recommendation of the Board of Directors of the Company that the Company Stockholders approve the Merger and this Agreement and the conclusion of the Board of Directors of the Company that the terms and conditions of the Merger are advisable and fair and in the best interests of the Company and such holders. The information sent by the Parent shall contain the conclusion of the Board of Directors of the Parent that the terms and conditions of the Merger are advisable and fair and in the best interests of the Parent. Anything to the contrary contained herein notwithstanding, the Company shall not include in the information sent to the Company Stockholders any information with respect to the Parent or its Affiliates or associates, the form and content of which information shall not have been approved by such party in its reasonable discretion prior to such inclusion.
4.11 Securities Exemptions. Each of the Company and the Parent will use its commercially reasonable efforts to solicit from each Company Stockholder and each stockholder of the Parent prior to the Merger, as the case may be, a certification noting whether such stockholder is an “accredited investor” as such term is defined in Regulation D under the Securities Act.
4.12 Parent Auditor Letter. The Parent shall provide the Parent Auditor with a copy of the Super 8-K and shall request that the Parent Auditor furnish a letter (the “Auditor Letter”) addressed to the Securities and Exchange Commission stating whether the Parent Auditor agrees with the statements made about it by the Parent in the Super 8-K.
4.13 Private Placement. Each of the Company and the Parent shall use commercially reasonable efforts to ensure that the issuance of the Merger Shares to Company Stockholders and the deemed offer and sale of shares of Parent Common Stock to Parent’s stockholders prior to the Merger are each exempt from registration under the Securities Act.
4.14 Failure to Fulfill Conditions. In the event that any of the Parties hereto determines that a condition to its respective obligations to consummate the transactions contemplated hereby cannot be fulfilled on or prior to the termination of this Agreement, it will promptly notify the other party.
4.15 Notification of Certain Matters. At or prior to the Effective Time, each party shall give prompt written notice to the other party of (a) the occurrence or failure to occur of any event or the discovery of any information, which occurrence, failure or discovery would be likely to cause any representation or warranty on its part contained in this Agreement to be untrue, inaccurate or incomplete after the date hereof in any material respect or, in the case of any representation or warranty given as of a specific date, would be likely to cause any such representation or warranty on its part contained in this Agreement to be untrue, inaccurate or incomplete in any material respect as of such specific date, and (b) any material failure of such party to comply with or satisfy any covenant or agreement to be complied with or satisfied by it hereunder. Without limiting the foregoing, Parent shall give prompt written notice to the Company of the occurrence or failure to occur of any event or the discovery of any information, which occurrence, failure or discovery would be likely to cause any Parent Fundamental Representation to be untrue, inaccurate or incomplete after the date hereof in any respect.
30
4.16 Tax Matters.
(a) From and after the date of this Agreement and until the Effective Time, each Party (i) shall use its commercially reasonable efforts to ensure that the Merger will qualify for the Intended Tax Treatment, and (ii) shall not take any action, cause or permit any action to be taken, fail to take any action, or cause any action to fail to be taken, which action or failure to act could prevent the Merger from qualifying for the Intended Tax Treatment. Following the Effective Time, none of the Company, the Parent, or any of their respective Affiliates shall take any action, cause or permit any action to be taken, fail to take any action, or cause any action to fail to be taken, which action or failure to act could prevent the Intended Tax Treatment from being applicable.
(b) At and after the Effective Time, each of the Parent and the Surviving Corporation covenants and agrees that it:
(i) will maintain all books and records and file all federal, state, and local income Tax Returns and schedules thereto of the Parent, the Surviving Corporation, and the Acquisition Subsidiary in a manner consistent with the Merger’s being qualified as a reorganization and nontaxable exchange under Section 368(a)(1)(A) of the Code (and comparable provisions of any applicable state or local income Tax Laws);
(ii) will, either directly or through a member of the Parent’s Qualified Group, continue at least one significant historic business line of the Company, or use at least a significant portion of the historic business assets of the Company in a business, in each case within the meaning of Treasury Regulations Section 1.368-1(d);
(iii) in connection with the Merger, will not reacquire, and will not permit any person that is a “related person” (as defined in Treasury Regulations Section 1.368-1(e)(4)) to the Parent to acquire, any of the Merger Shares issued in connection with the Merger; and
(iv) will not sell or otherwise dispose of any of the Company assets acquired via the Merger, except for dispositions made in the ordinary course of business or transfers described in Section 368(a)(2)(C) of the Code or described and permitted in Treasury Regulations Section 1.368-2(k).
ARTICLE V. CONDITIONS TO CONSUMMATION OF MERGER
5.1 Conditions to Each Party’s Obligations. The respective obligations of each Party to consummate the Merger are subject to the satisfaction or waiver of the following conditions:
(a) the Company shall have obtained (and shall have provided copies thereof to the Parent) the written consents or approval from (i) all of the members of its Board of Directors and (ii) Company Stockholders holding Company Shares representing (x) at least a majority of the votes represented by the outstanding Company Shares entitled to vote on this Agreement and the Merger, voting as a single class on an as-converted basis and (y) at least a majority of the votes represented by the outstanding Company Shares entitled to vote on this Agreement and the Merger held by disinterested Company Stockholders, voting as a separate class on an as-converted basis, in each case to approve the execution, delivery and performance by the Company of this Agreement and the other Transaction Documentation to which the Company is a party, in form and substance reasonably satisfactory to the Parent;
31
(b) prior to the Closing, the Company and the Parent shall have in escrow in connection with the Private Placement Offering an amount of cash that equals at least $3,500,002, and the conditions to the closing of such Private Placement Offering shall have been satisfied (other than the consummation of the Merger and those other conditions that, by their nature, will be satisfied at the Closing of the Private Placement Offering) and such amount of gross proceeds shall be unencumbered cash available to the Parent and the Surviving Corporation at the Effective Time (other than as expressly contemplated by this Agreement);
(c) the Company shall have provided evidence reasonably satisfactory to the Parent and the Acquisition Subsidiary of the termination of the Company agreements set forth on Schedule 5.1(c);
(d) the Registration Rights Agreement executed by the parties thereto shall be in full force and effect and shall not have been revoked, rescinded or otherwise repudiated by such parties;
(e) the Parent and Lucius Partners LLC shall each have executed and delivered to the other the Advisory Services Agreement in the form attached hereto as Exhibit D (collectively, the “Lucius Advisory Services Agreement”); and
(f) there is no order of any court or other Governmental Entity pending or in effect restraining or prohibiting the completion of the transactions contemplated hereby.
5.2 Conditions to Obligations of the Parent and the Acquisition Subsidiary. The obligation of each of the Parent and the Acquisition Subsidiary to consummate the Merger is subject to the satisfaction (or waiver by the Parent) of the following additional conditions:
(a) the Company shall have obtained (and shall have provided copies thereof to the Parent) all waivers, permits, consents, approvals or other authorizations, and effected all of the registrations, filings and notices set forth on Schedule 5.2(a);
(b) the representations and warranties of the Company set forth in this Agreement other than the Company Fundamental Representations (when read without regard to any qualification as to materiality or Company Material Adverse Effect contained therein) shall be true and correct as of the date of this Agreement and shall be true and correct in all material respects as of the Effective Time as though made as of the Effective Time (provided, however, that to the extent such representation and warranty expressly relates to a specified date, such representation and warranty shall be true and correct as of such specified date), and (ii) the representations and warranties of the Company set forth in Sections 2.1, 2.2, 2.3, 2.4, 2.5, and 2.12 (the “Company Fundamental Representations”) (when read without regard to any qualification as to materiality or Company Material Adverse Effect contained therein) shall be true and correct in all respects as of the date of this Agreement and as of the Effective Time as if made as of the Effective Time (or in the case of representations and warranties that are made as of a specific date, shall have been true and correct as of such specified date);
32
(c) the Company shall have performed or complied with its agreements and covenants required to be performed or complied with under this Agreement as of or prior to the Effective Time, except for such non-performance or non-compliance as does not have a Company Material Adverse Effect or a material adverse effect on the ability of the Parties to consummate the transactions contemplated by this Agreement;
(d) no Legal Proceeding shall be pending wherein an unfavorable judgment, order, decree, stipulation or injunction would (i) prevent consummation of any of the transactions contemplated by this Agreement or (ii) cause any of the transactions contemplated by this Agreement to be rescinded following consummation, and no such judgment, order, decree, stipulation or injunction shall be in effect;
(e) the Company shall have delivered to the Parent and the Acquisition Subsidiary a copy of each written consent received from a Company Stockholder consenting to the Merger;
(f) the Company shall have delivered to the Parent and the Acquisition Subsidiary a certificate executed by the Chief Executive Officer of the Company (the “Company Certificate”) to the effect that each of the conditions specified in clause (a) of Section 5.1 and clauses (b) through (d) (insofar as clause (d) relates to Legal Proceedings involving the Company) of this Section 5.2 has been satisfied in all respects;
(g) the Company shall have delivered to the Parent and the Acquisition Subsidiary a certificate executed by the Secretary of the Company, certifying as to (i) true, correct and complete copies of the certificate of incorporation or the by-laws of the Company; (ii) the valid adoption of resolutions of the board of directors and Company Stockholders (whereby this Agreement, the Merger and the transactions contemplated hereunder were unanimously approved by the board of directors and the requisite vote of the Company Stockholders); and (iii) a good standing certificate from the Secretary of State of the State of Delaware dated within five (5) Business Days prior to the Closing Date; and (iv) incumbency of the officers of the Company executing this Agreement or any other agreement contemplated by this Agreement; and
(h) the Company shall have delivered to the Parent audited and interim unaudited financial statements of the Company pro forma in respect of the Merger, compliant with applicable SEC regulations for inclusion under Item 2.01(f) and/or 5.01(a)(8) of Form 8-K in substantially final form;
(i) no Company Material Adverse Effect shall have occurred since the date of this Agreement;
(j) the Company shall have delivered the Pre-Merger Indemnity Agreement to the Parent, duly executed by the Company; and
(k) the Company shall have delivered to the Parent written evidence reasonably satisfactory to the Parent of the binding of the Pre-Merger D&O Policy.
5.3 Conditions to Obligations of the Company. The obligation of the Company to consummate the Merger is subject to the satisfaction (or waiver by the Company) of the following additional conditions:
(a) the Parent shall have obtained (and shall have provided copies thereof to the Company) the written consents or approvals of (i) all of the members of its Board of Directors of Parent, (ii) all the stockholders of Parent, (iii) all of the members of the Board of Directors of Acquisition Subsidiary, and (iv) the sole stockholder of Acquisition Subsidiary, in each case to the execution, delivery and performance by each such entity of this Agreement and/or the other Transaction Documentation to which each such entity is a party, in form and substance reasonably satisfactory to the Company;
33
(b) the Parent shall have obtained (and shall have provided copies thereof to the Company) all of the other waivers, permits, consents, approvals or other authorizations, and effected all of the registrations, filings and notices set forth on Schedule 5.3(b);
(c) (i) the representations and warranties of the Parent and the Acquisition Subsidiary set forth in this Agreement other than the Parent Fundamental Representations (when read without regard to any qualification as to materiality or Parent Material Adverse Effect contained therein) shall be true and correct as of the date of this Agreement and shall be true and correct in all material respects as of the Effective Time as though made as of the Effective Time (provided, however, that to the extent such representation and warranty expressly relates to a specified date, such representation and warranty shall be true and correct as of such specified date); and (ii) the representations and warranties of the Parent and the Acquisition Subsidiary set forth in Sections 3.1, 3.2, 3.3, 3.4, 3.5, 3.20, 3.24, 3.25, 3.31, and 3.33 (the “Parent Fundamental Representations”) (when read without regard to any qualification as to materiality or Parent Material Adverse Effect contained therein) shall be true and correct in all respects as of the date of this Agreement and as of the Effective Time as if made as of the Effective Time (or in the case of representations and warranties that are made as of a specific date, shall have been true and correct as of such specified date);
(d) each of the Parent and the Acquisition Subsidiary shall have performed or complied with its agreements and covenants required to be performed or complied with under this Agreement as of or prior to the Effective Time, except for such non-performance or non-compliance as does not have a Parent Material Adverse Effect or a material adverse effect on the ability of the Parties to consummate the transactions contemplated by this Agreement;
(e) no Legal Proceeding shall be pending wherein an unfavorable judgment, order, decree, stipulation or injunction would (i) prevent consummation of any of the transactions contemplated by this Agreement or (ii) cause any of the transactions contemplated by this Agreement to be rescinded following consummation, and no such judgment, order, decree, stipulation or injunction shall be in effect;
(f) the Board of Directors of the Parent and the stockholders of the Parent shall each have adopted the 2025 Plan (such stockholder approval subject to effectiveness in accordance with Regulation 14C of the Exchange Act, if applicable);
(g) the Parent shall have delivered to the Company a certificate executed by the Chief Executive Officer or President of the Parent (the “Parent Certificate”) to the effect that each of the conditions specified in clause (b) of Section 5.1 and clauses (a) through (e) (insofar as clause (e) relates to Legal Proceedings involving the Parent or the Acquisition Subsidiary) of this Section 5.3 has been satisfied in all respects;
34
(h) Each of the Parent and Acquisition Subsidiary shall have delivered to the Company a certificate, validly executed by the Secretary of the Parent and the Secretary of the Acquisition Subsidiary, as applicable, certifying as to (i) true, correct and complete copies of its certificate of incorporation and bylaws; (ii) the valid adoption of resolutions of the board of directors and stockholders of the Parent or Acquisition Subsidiary, as applicable (whereby this Agreement, the Merger and the transactions contemplated hereunder were unanimously approved by the board of directors and, if requested, the requisite vote of the stockholders of Parent or the Acquisition Subsidiary, as applicable); (iii) a good standing certificate from the Secretary of State of the State of Delaware dated within five (5) Business Days prior to the Closing Date; (iv) incumbency of the officers of the Parent or the Acquisition Subsidiary, as applicable, executing this Agreement or any other agreement contemplated by this Agreement; and (v) a true, correct and complete list of all stockholders of Parent as of immediately prior to the Effective Time and the shares of Parent Common Stock held by each such stockholder that are then-outstanding, which shares shall equal, in the aggregate, 3,250,000 shares of Parent Common Stock;
(i) the Share Cancellation Agreement executed by the sole stockholder of the Parent concurrently with this Agreement shall be in full force and effect and shall not have been revoked, rescinded or otherwise repudiated by such stockholders of the Parent;
(j) the Parent shall have delivered to the Company (i) evidence that the Parent’s Board of Directors is, as of the Effective Time, authorized to consist of five (5) individuals, (ii) evidence of the resignations of all individuals who served as directors and/or officers of the Parent as of immediately prior to the Effective Time, which resignations shall be effective as of the Effective Time, (iii) evidence of the appointment of the following persons to serve as directors of the Parent effective as of the Effective Time: Michael J. Roberts, Simon C. Pedder, Patrick Gallagher, Anthony Zook, and J. Nick Riehle, and (iv) evidence of the appointment of such executive officers of the Parent to serve immediately following the Effective Time as shall have been designated by the Company, including Michael J. Roberts, PhD, President and Chief Executive Officer; Simon C. Pedder, PhD, Executive Chairman; Timothy L. Maness, CPA, Chief Financial Officer; and L. Arthur Hewitt, PhD, Chief Development Officer;
(k) the Auditor Letter shall have been furnished to the Parent and the Parent shall have delivered a copy of such Auditor Letter to the Company, and the Parent Auditor shall have consented to the filing of the Auditor Letter in the Super 8-K;
(l) the Parent shall be in compliance in all material respects with all requirements of applicable securities laws, including, without limitation, the filing of reports required by the Exchange Act, and shall have taken all actions with respect thereto as shall be required or reasonably requested by the Company in connection therewith;
(m) the Parent shall have delivered to the Company a payoff letter executed by Lucius Partners Opportunity Fund, LP (the “Debt Holder”) in a form reasonably acceptable to the Company and the Debt Holder (the “Payoff Letter”) setting forth (x) the amount required to pay off the indebtedness owing to the Debt Holder, (y) that upon payment of such amount, the contract with respect to such indebtedness will be terminated and the Parent released therefrom, and (z) the Debt Holder’s commitment to release all liens, if any, that the Debt Holder may hold on the Parent’s assets prior to the Closing Date or an authorization for the Parent to do so;
(n) the Parent shall have delivered the Pre-Merger Indemnity Agreements to the Company, duly executed by the Parent and the Parent Indemnified Executives; and
(o) No Parent Material Adverse Effect shall have occurred since the date of this Agreement.
35
ARTICLE VI. DEFINITIONS
For purposes of this Agreement, each of the following defined terms is defined in the Section of this Agreement indicated below.
| Definition | Section | |
| 2025 Plan | 4.9 | |
| Acquisition Subsidiary | INTRODUCTION | |
| Agreement | INTRODUCTION | |
| Auditor Letter | 4.12 | |
| Business Day | 1.2 | |
| Certificate of Merger | 1.1(b) | |
| Closing | 1.2 | |
| Closing Date | 1.2 | |
| Code | RECITALS | |
| Company | INTRODUCTION | |
| Company Balance Sheet | 2.7 | |
| Company Balance Sheet Date | 2.7 | |
| Company Certificate | 0 | |
| Company Confidential Information | 4.4(a) | |
| Company Convertible Notes | 2.2 | |
| Company Consents | 2.3 | |
| Company Disclosure Schedule | ARTICLE II | |
| Company Equity Plan(s) | 2.2 | |
| Company Financial Statements | 2.7 | |
| Company Material Adverse Effect | 2.1 | |
| Company Options | 1.8 | |
| Company Shares Certificates | 1.5(d) | |
| Conversion Ratio | 1.5(c) | |
| D&O Insurance | 4.6(d) | |
| D&O Tail Policy | 4.6(d) | |
| Debt Holder | 5.3(m) | |
| Defaulting Party | 8.13 | |
| DGCL | RECITALS | |
| Effective Time | 1.1(b) | |
| End Date | 7.1(b) | |
| Environmental Law | 3.21(d) | |
| Exchange Act | 1.13(b) | |
| Excluded Shares | 1.5(b) | |
| Fraud | 8.14 | |
| GAAP | 2.1 | |
| Governmental Entity | 2.4 |
36
| Indemnified Executives | 4.6(b) | |
| IRS | 3.12(c) | |
| Laws | 2.4 | |
| Legal Proceeding | 2.10 | |
| Lucius Advisory Services Agreement | 5.1(e) | |
| Merger | RECITALS | |
| Merger Shares | 1.5(c) | |
| Merger Shares Certificates | 1.5(d) | |
| Non-Defaulting Party | 8.13 | |
| Parent | INTRODUCTION | |
| Parent Auditor | 3.27 | |
| Parent Certificate | 5.3(g) | |
| Parent Common Stock | RECITALS | |
| Parent Confidential Information | 4.4(d) | |
| Parent Disclosure Schedule | Article III | |
| Parent Financial Statements | 3.8 | |
| Parent Form 10 | 3.6 | |
| Parent Fundamental Representations | 5.3(c) | |
| Parent Indemnified Executives | 4.6(c) | |
| Parent Material Adverse Effect | 3.1 | |
| Parent Options | 1.8(a) | |
| Parent Previous Filings | 3.6 | |
| Parent SEC Filings | 3.6 | |
| Party | INTRODUCTION | |
| Payoff Letter | 5.3(m) | |
| Pre-Merger D&O Policy | 4.6(d) | |
| Pre-Merger Indemnity Agreement | 4.6(c) | |
| Private Placement Offering | RECITALS | |
| Purchase Price | RECITALS | |
| Qualified Group | 3.24(i) | |
| Reasonable Best Efforts | 4.1(b) | |
| Registration Rights Agreement | 1.13(c) | |
| Representatives | 4.4(c) | |
| SEC | 1.13(a) | |
| Securities Act | RECITALS | |
| Subsidiary | 2.5(a) | |
| Super 8-K | 4.3 | |
| Surviving Corporation | 1.1(a) | |
| Tax Returns | 1.14 | |
| Taxes | 1.14 | |
| Transaction Documentation | 3.3 | |
| Treasury Regulations | RECITALS |
37
ARTICLE VII. TERMINATION
7.1 Termination. Except as provided in Section 7.2, this Agreement may be terminated and the Merger abandoned at any time prior to the Closing only:
(a) by the mutual agreement of the Company and the Parent:
(b) by the Company or the Parent if the Closing Date shall not have occurred within ten (10) Business Days after the date hereof (such date, the “End Date”); provided, however, that the right to terminate this Agreement under this Section 7.1(b) shall not be available to any party whose action or failure to act has been the principal cause of the Merger failing to occur on or before the End Date and such action or failure to act constitutes a breach of this Agreement;
(c) by the Company if (i) any Law shall be in effect which has the effect of making the Merger illegal or otherwise prohibits or prevents the consummation of the Merger or (ii) if the consummation of the Merger would violate any final and non-appealable order;
(d) by the Company if it is not in material breach of its obligations under this Agreement and there has been a breach of or inaccuracy in any representation, warranty, covenant or agreement of the Parent or the Acquisition Subsidiary contained in this Agreement (excluding conditions that by their nature are to be satisfied at the Closing) such that the conditions set forth in Sections 5.1 or 5.3 for the benefit of the Company would not be satisfied as of the time of such breach or inaccuracy and such breach or inaccuracy has not been cured within ten (10) calendar days after written notice thereof to the Parent (or otherwise waived in writing by the Company); provided, however, that no cure period shall be required (i) for a breach or inaccuracy which by its nature cannot be cured or (ii) if any of the conditions to Closing in Sections 5.1 or 5.3 for the benefit of the Company are incapable of being satisfied on or before the End Date; or
(e) by the Parent if it is not in material breach of its obligations under this Agreement and there has been a breach of or inaccuracy in any representation, warranty, covenant or agreement of the Company contained in this Agreement (excluding conditions that by their nature are to be satisfied at the Closing) such that the conditions set forth in Sections 5.1 or 5.2 for the benefit of the Parent or the Acquisition Subsidiary would not be satisfied as of the time of such breach or inaccuracy and such breach or inaccuracy has not been cured within ten (10) calendar days after written notice thereof to the Company (or otherwise waived in writing by the Parent and the Acquisition Subsidiary); provided, however, that no cure period shall be required (i) for a breach or inaccuracy which by its nature cannot be cured or (ii) if any of the conditions to Closing in Sections 5.2 or 5.3 for the benefit of the Parent or the Acquisition Subsidiary are incapable of being satisfied on or before the End Date.
7.2 Effect of Termination. In the event of the termination of this Agreement as provided in Section 7.1, this Agreement shall forthwith become void and there shall be no liability or obligation hereunder on the part of the Parent, the Acquisition Subsidiary or the Company, or their respective representatives, as applicable; provided, however, (i) if this Agreement is terminated by a Party because of a breach of this Agreement by another Party or because one or more of the conditions to the terminating Party’s obligations under this Agreement is not satisfied as a result of another Party’s willful or intentional failure to comply with its obligations under this Agreement, the terminating Party’s right to pursue all legal remedies in accordance with this Agreement will survive such termination (provided, however, that a terminating Party may not both (x) terminate this Agreement or pursue such legal remedies and (y) bring or maintain any claim, action, or proceeding for injunction or specific performance as provided in Section 8.13 and (ii) that the provisions of Section 4.4(c), Section 4.4(d), ARTICLE VIII (Miscellaneous) and this Section 7.2 shall remain in full force and effect and survive any termination of this Agreement pursuant to the terms of this ARTICLE VII.
38
ARTICLE VIII. MISCELLANEOUS
8.1 Press Releases and Announcements. Except as expressly contemplated by this Agreement, no Party shall issue any press release or public announcement relating to the subject matter of this Agreement without the prior written approval of the other Parties, which shall be unreasonably withheld; provided, however, that any Party may make any public disclosure it believes in good faith is required by applicable Law or stock market rules (in which case the disclosing Party shall use reasonable efforts to advise the other Parties and provide them with a copy of the proposed disclosure prior to making the disclosure).
8.2 No Third Party Beneficiaries. This Agreement shall not confer any rights or remedies upon any person other than the Parties and their respective successors and permitted assigns; provided, however, that (a) the provisions in ARTICLE I concerning issuance of the Merger Shares is intended for the benefit of the Company Stockholders and (b) the provisions in Section 4.8 concerning indemnification are intended for the benefit of the Indemnified Executives and the Parent Indemnified Executives, respectively, and their respective successors and assigns.
8.3 Entire Agreement. This Agreement (including the documents referred to herein) constitutes the entire agreement among the Parties and supersedes any prior or (other than as set forth in the Transaction Documentation) contemporaneous understandings, agreements or representations by or among the Parties, written or oral, with respect to the Merger. Notwithstanding anything to the contrary in this Agreement, the Company Disclosure Schedules, schedules and similar documents and instruments delivered pursuant to this Agreement shall not be deemed part of this Agreement for purposes of Section 268(b) of the DGCL, but shall have the effects provided in this Agreement otherwise (including with respect this Section 8.3).
8.4 Succession and Assignment. This Agreement shall be binding upon and inure to the benefit of the Parties named herein and their respective successors and permitted assigns. No Party may assign either this Agreement or any of its rights, interests or obligations hereunder without the prior written approval of the other Parties.
8.5 Counterparts and Facsimile Signature. This Agreement may be executed in one or more counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument and will become effective when one or more counterparts have been signed by each Party hereto and delivered to the other Parties hereto; it being understood and agreed that all Parties hereto need not sign the same counterpart. The delivery by facsimile or by electronic delivery in PDF format (including any electronic signature complying with the U.S. federal ESIGN Act of 2000, e.g. www.docusign.com) will be sufficient and binding as if they were originals and such delivery shall constitute valid delivery of this Agreement. All counterparts will together constitute one and the same instrument and each counterpart will constitute an original of this Agreement.
39
8.6 Headings. The section headings contained in this Agreement are inserted for convenience only and shall not affect in any way the meaning or interpretation of this Agreement.
8.7 Notices. All notices, requests, demands, claims and other communications hereunder shall be in writing. Any notice, request, demand, claim or other communication hereunder shall be deemed duly delivered and received: (a) when received if given in person with confirmation of receipt (email being sufficient), (b) upon receipt if sent by registered or certified mail or via a reputable nationwide overnight courier service, with postage or delivery fees prepaid and electronic delivery tracking information available, or (c) on the date of transmission if sent by electronic mail or other wire transmission during normal business hours (and if not, on the succeeding Business Day) with non-automated confirmation of receipt (email being sufficient). All notices, requests, demands, claims or other communication hereunder will be addressed as follows:
| If to the Company or the Company Stockholders: | Adaptin Bio, Inc. | |
| 3540 Toringdon Way, Suite 200, #250 | ||
| Charlotte, NC 28277 | ||
| Attention: Michael J. Roberts | ||
| E-mail: mroberts@adaptin.com | ||
| With copy to (which copy shall not constitute notice hereunder): | Wyrick Robbins Yates & Ponton LLP | |
| 4101 Lake Boone Trail, Suite 300 | ||
| Raleigh, NC 27607 | ||
| Attention: Donald R. Reynolds | ||
| E-mail: dreynolds@wyrick.com | ||
| If to the Parent or the Acquisition Subsidiary (prior to the Closing): | Unite Acquisition 1 Corp. | |
| 12 E. 49th Street, 11th Floor | ||
| New York, NY 10017 | ||
| Attention: Nathan Pereira, CEO | ||
| With copy to (which copy shall not constitute notice hereunder): | Sichenzia Ross Ference Carmel LLP | |
| 1185 Avenue of the Americas, 31st Floor | ||
| New York, NY 10036 | ||
| Attention: Barrett S. DiPaolo | ||
| Facsimile: 212-930-9725 | ||
| E-mail: bdipaolo@srf.law |
Any Party may give any notice, request, demand, claim or other communication hereunder using any other means (including personal delivery, expedited courier, messenger service, telecopy, telex, ordinary mail or electronic mail), but no such notice, request, demand, claim or other communication shall be deemed to have been duly given unless and until it actually is received by the Party for whom it is intended. Any Party may change the address to which notices, requests, demands, claims and other communications hereunder are to be delivered by giving the other Parties notice in the manner herein set forth.
8.8 Governing Law. This Agreement must be interpreted and construed in accordance with the internal Laws of the State of Delaware. Any and all claims, controversies, and causes of action arising out of or relating to this Agreement, whether sounding in contract, tort, or statute, shall be governed by the Laws of the State of Delaware without giving effect to any choice or conflict of Law provision or rule (whether of the State of Delaware or any other jurisdiction) that would cause the application of Laws of any jurisdictions other than those of the State of Delaware.
40
8.9 Amendments and Waivers. The Parties may mutually amend any provision of this Agreement at any time prior to the Effective Time, provided that no such amendment shall be valid unless the same shall be in writing and signed by all of the Parties. No waiver of any right or remedy hereunder shall be valid unless the same shall be in writing and signed by the Party giving such waiver. No waiver by any Party with respect to any default, misrepresentation or breach of warranty or covenant hereunder shall be deemed to extend to any prior or subsequent default, misrepresentation or breach of warranty or covenant hereunder or affect in any way any rights arising by virtue of any prior or subsequent such occurrence.
8.10 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If the final judgment of a court of competent jurisdiction declares that any term or provision hereof is invalid or unenforceable, the Parties agree that the court making the determination of invalidity or unenforceability shall have the power to limit the term or provision, to delete specific words or phrases, or to replace any invalid or unenforceable term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be enforceable as so modified.
8.11 Submission to Jurisdiction. Each of the parties hereto irrevocably consents to the exclusive jurisdiction and venue of the Delaware Court of Chancery and any state appellate court therefrom within the State of Delaware (or, if the Delaware Court of Chancery declines to accept jurisdiction over a particular matter, any state or federal court within the State of Delaware) in connection with any matter based upon or arising out of this Agreement or the matters contemplated herein, agrees that process may be served upon them in any manner authorized by the Laws of the State of Delaware for such persons and irrevocably waives, to the fullest extent permitted by applicable Law, and covenants not to assert or plead any objection it may now or hereafter have to the laying of the venue of any such suit, action or proceeding in any such court or that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum. Any Party may make service on another Party by sending or delivering a copy of the process to the Party to be served at the address and in the manner provided for the giving of notices in Section 8.7. Nothing in this Section 8.11, however, shall affect the right of any Party to serve legal process in any other manner permitted by law.
8.12 WAIVER OF JURY TRIAL. EACH OF THE PARTIES IRREVOCABLY WAIVES ANY AND ALL RIGHTS TO TRIAL BY JURY IN ANY ACTION OR PROCEEDING BETWEEN THE PARTIES ARISING OUT OF OR RELATING TO THIS AGREEMENT AND THE TRANSACTIONS CONTEMPLATED BY THIS AGREEMENT. NO PARTY TO THIS AGREEMENT OR ANY ASSIGNEE, SUCCESSOR, HEIR, OR PERSONAL REPRESENTATIVE WILL SEEK A JURY TRIAL IN ANY LAWSUIT, PROCEEDING, COUNTERCLAIM, OR ANY OTHER LITIGATION PROCEDURE BASED ON OR ARISING OUT OF THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY OR ANY OF THE OTHER AGREEMENTS OR THE DEALINGS OR THE RELATIONSHIP BETWEEN THE PARTIES CONTEMPLATED HEREBY OR REFERENCED HEREIN. NO PARTY WILL SEEK TO CONSOLIDATE ANY SUCH ACTION, IN WHICH A JURY TRIAL HAS BEEN WAIVED, WITH ANY OTHER ACTION IN WHICH A JURY TRIAL HAS NOT OR CANNOT BE WAIVED. THE PROVISIONS OF THIS SECTION 8.12 HAVE BEEN FULLY DISCUSSED BY THE PARTIES AND WILL BE SUBJECT TO NO EXCEPTIONS.
41
8.13 Remedies; Specific Performance. The Parties agree that irreparable damage would occur if any provision of this Agreement were not performed in accordance with the terms hereof, and agree that in the event that any Party shall fail or refuse to consummate the transactions contemplated by this Agreement or if any default under or breach of any representation, warranty, covenant or condition of this Agreement on the part of any Party (the “Defaulting Party”) shall have occurred that results in the failure to consummate the transactions contemplated by this Agreement, then in addition to the other remedies provided herein, the other Party or Parties (the “Non-Defaulting Party”) shall be entitled to seek enforcement of any provision of this Agreement by a decree of specific performance, temporary, preliminary and permanent injunctive relief or other equitable relief to prevent breaches or threatened breaches of any of the provisions of this Agreement from a court of competent jurisdiction. In addition, the Non-Defaulting Party shall be entitled to obtain from the Defaulting Party court costs and reasonable attorneys’ fees incurred in connection with or in pursuit of enforcing the rights and remedies provided hereunder. Each of the Parties agrees that it will not oppose the granting of an injunction, specific performance or other equitable relief on the basis that any other party has an adequate remedy at Law or that any award of specific performance is not an appropriate remedy for any reason at Law or in equity.
8.14 Survival. Except with respect to any claims for Fraud, the representations or warranties in this Agreement and in any certificate delivered pursuant to this Agreement shall not survive the Effective Time. For purposes of this Agreement, “Fraud” means, with respect to any Person, an actual and intentional common law fraud under the Laws of the State of Delaware (as interpreted by Delaware courts) by such Person in the making of such Person’s representations and warranties set forth in this Agreement, provided, that, for the avoidance of doubt, “Fraud” shall not include any equitable fraud, negligent misrepresentation, promissory fraud, unfair dealings, extra contractual fraud, or any other fraud or torts based on recklessness or negligence.
8.15 Construction.
(a) The language used in this Agreement shall be deemed to be the language chosen by the Parties to express their mutual intent, and no rule of strict construction shall be applied against any Party.
(b) Any reference to any federal, state, local or foreign statute or Law shall be deemed also to refer to all rules and regulations promulgated thereunder, unless the context requires otherwise.
(c) The use of the masculine, feminine or neuter gender or the singular or plural form of words herein will not limit any provision of this Agreement.
(d) The use of the terms “including” or “include” will in all cases herein mean “including, without limitation” or “include, without limitation,” respectively.
(e) Unless the context clearly provides otherwise, the use of the word “or” is inclusive.
(f) Reference to any Person includes such Person’s successors and assigns to the extent such successors and assigns are permitted by the terms of this Agreement. Reference to a Person in a particular capacity excludes such Person in any other capacity or individually.
42
(g) Reference to any agreement (including this Agreement), document or instrument means such agreement, document or instrument as amended or modified and in effect from time to time in accordance with the terms thereof and, if applicable, the terms hereof.
(h) References to Articles, Sections, paragraphs, clauses, Schedules, Annexes or Exhibits will refer to those portions of this Agreement.
(i) The use of the terms “hereunder,” “hereof,” “hereto” and words of similar import will refer to this Agreement as a whole and not to any particular Article, Section, paragraph or clause of, or Schedule, Annex or Exhibit to, this Agreement unless otherwise specified.
(j) The parties hereto have participated jointly in the negotiation and drafting of this Agreement and, in the event an ambiguity or question of intent or interpretation arises, this Agreement will be construed as jointly drafted by the parties hereto and no presumption or burden of proof will arise favoring or disfavoring any party by virtue of the authorship of any provision of this Agreement.
(k) Any reference in this Agreement to “dollars” or “$” means United States dollars.
(l) All references to a specific time of day refer to the specific time of day in the Eastern Time Zone of the United States of America.
(m) Unless expressly provided otherwise, the measure of a period of one month or year for purposes of this Agreement shall be that date of the following month or year corresponding to the starting date, but if no corresponding date exists, the measure will be that date of the following month or year corresponding to the next day following the starting date. For example, one month following February 18 is March 18, and one month following March 31 is May 1.
[SIGNATURE PAGE FOLLOWS]
43
IN WITNESS WHEREOF, the Parties have executed this Agreement and Plan of Merger and Reorganization as of the date first above written.
| PARENT: | ||
| UNITE ACQUISITION 1 CORP. | ||
| By: | /s/ Nathan Pereira | |
| Name: | Nathan Pereira | |
| Title: | President and Chief Executive Officer | |
| ACQUISITION SUBSIDIARY: | ||
| ADAPTIN ACQUISITION CO. | ||
| By: | /s/ Nathan Pereira | |
| Name: | Nathan Pereira | |
| Title: | President | |
| COMPANY: | ||
| ADAPTIN BIO, INC. | ||
| By: | /s/ Michael J. Roberts | |
| Name: | Michael J. Roberts | |
| Title: | President and Chief Executive Officer | |
[Signature Page to Merger Agreement]
Exhibits
EXHIBIT A
Amended and Restated Certificate of Incorporation of the Parent
EXHIBIT B
Amended and Restated Bylaws of Parent
EXHIBIT C
Form of Pre-Merger Indemnity Agreement
EXHIBIT D
Form of Lucius Advisory Agreement
EXHIBIT E
Form of Certificate of Merger
Exhibit 3.1
STATE OF DELAWARE CERTIFICATE OF MERGER
FOR THE MERGER OF
ADAPTIN ACQUISITION CO. WITH AND INTO ADAPTIN BIO, INC.
February 11, 2025
Pursuant to Section 251(c) of the
General Corporation Law of the State of Delaware
Adaptin Bio, Inc., a Delaware corporation (the “Corporation”), does hereby certify to the following facts relating to the merger (the “Merger”) of Adaptin Acquisition Co., a Delaware corporation (“Merger Sub”), with and into the Corporation, with the Corporation remaining as the surviving corporation of the Merger (the “Surviving Corporation”):
| FIRST: | The name of each constituent corporation is Adaptin Bio, Inc., a Delaware corporation, and Adaptin Acquisition Co., a Delaware corporation. |
| SECOND: | An Agreement and Plan of Merger (the “Merger Agreement”) has been approved, adopted, executed and acknowledged by each of the constituent corporations in accordance with Section 251 of the General Corporation Law of the State of Delaware (the “DGCL”). |
| THIRD: | In accordance with the Merger Agreement and upon the effectiveness of this filing, Merger Sub will merge with and into the Corporation. The name of the Surviving Corporation of the Merger shall be “Adaptin Bio Operating Corporation.” |
| FOURTH: | Upon the effectiveness of the Merger, the Certificate of Incorporation of the Surviving Corporation shall be amended and restated in its entirety to read as set forth on Exhibit A attached hereto. |
| FIFTH: | The Merger shall become effective on February 11, 2025, at 1:25 p.m. Eastern Time. |
| SIXTH: | The executed Merger Agreement is on file at the principal place of business of the Surviving Corporation at 3540 Toringdon Way, Suite 200, #250 Charlotte, NC 28277. |
| SEVENTH: | A copy of the executed Merger Agreement will be furnished by the Surviving Corporation on request and without cost, to any stockholder of any constituent corporation of the Merger. |
IN WITNESS WHEREOF, the Corporation has caused this Certificate of Merger to be executed by its duly authorized officer as of the date first above written.
|
|
Adaptin Bio, Inc. | |
| By: | /s/ Michael J. Roberts | |
| Name: | Michael J. Roberts | |
| Title: | Chief Executive Officer | |
2
Exhibit A
AMENDED AND RESTATED
CERTIFICATE OF INCORPORATION
OF
ADAPTIN BIO OPERATING CORPORATION
1. The name of the corporation is Adaptin Bio Operating Corporation (the “Corporation”).
2. The address of its registered office in the State of Delaware is 108 West 13th Street, Suite 100, Wilmington, DE 19801 in the county of New Castle. The name of its registered agent at such address is Vcorp Services, LLC.
3. The nature of the business or purposes to be conducted or promoted is to engage in any lawful act or activity for which corporations may be organized under the General Corporation Law of Delaware (the “DGCL”).
4. The Corporation is to have perpetual existence.
5. The total number of shares of capital stock which the Corporation shall have authority to issue is: ten thousand (10,000). These shares shall be of a single class designated as common stock (the “Common Stock”) with a par value of $0.0001.
Holders of shares of Common Stock shall be entitled to cast one vote for each share held at all stockholders’ meetings for all purposes, including the election of directors. The Common Stock does not have cumulative voting rights.
No holder of shares of stock of the Corporation’s capital stock shall be entitled as a matter of right to subscribe for or purchase or receive any part of any new or additional issue of shares of stock of any class, or of securities convertible into shares of stock of any class, whether now hereafter authorized or whether issued for money, for consideration other than money, or by way of dividend.
6. Effective upon the registration of any class of the Corporation’s stock under the Securities Act of 1934, as amended, any action required or permitted to be taken by the stockholders of the Corporation must be effected at a duly called annual or special meeting of the stockholders of the Corporation and may not be effected by any consent in writing by such stockholders.
7. Unless and except to the extent that the by-laws of the Corporation (the “By-laws”) shall so require, the election of directors of the Corporation need not be by written ballot.
3
8. The Board of Directors shall have the power to adopt, amend or repeal the By-laws.
9. To the fullest extent permitted by the DGCL, no director of the Corporation shall be personally liable to the Corporation or its stockholders for monetary damages for any breach of fiduciary duty by such director as a director. If the DGCL hereafter is amended to authorize the further elimination or limitation of the liability of directors, then the liability of a director of the Corporation, in addition to the limitation on personal liability provided herein, shall be limited to the fullest extent permitted by the amended DGCL. No amendment to or repeal of this Article 9 shall apply to or have any effect on the liability or alleged liability of any director of the Corporation for or with respect to any acts or omissions of such director occurring prior to such amendment.
10. The Corporation shall indemnify, to the fullest extent permitted by Section 145 of the DGCL, as amended from time to time, each person that such section grants the Corporation the power to indemnify.
11. The Corporation shall have the right, subject to any express provisions or restrictions contained in this Certificate of Incorporation or the By-laws, from time to time, to amend, alter or repeal any provision of this Certificate of Incorporation in any manner now or hereafter provided by law, and all rights and powers of any kind conferred upon a director or stockholder of this Corporation by the Certificate of Incorporation or any amendment thereof are conferred subject to such right.
12. Unless the Corporation consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for (a) any derivative action or proceeding brought on behalf of the Corporation, (b) any action asserting a claim for breach of a fiduciary duty owed by any director, officer, employee or agent of the Corporation to the Corporation or the Corporation’s stockholders, (c) any action asserting a claim arising pursuant to any provision of the DGCL, the Certificate of Incorporation or the By-laws and (d) any action asserting a claim governed by the internal affairs doctrine, in each case subject to said Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein. If any provision or provisions of this Article 12 shall be held to be invalid, illegal or unenforceable as applied to any person or entity or circumstance for any reason whatsoever, then, to the fullest extent permitted by law, the validity, legality and enforceability of such provision in any other circumstance and of the remaining provision of this Article 12 (including, without limitation, each portion of any sentence of this Article 12 containing any such provision held to be invalid, illegal or unenforceable that is not itself held to be invalid, illegal or unenforceable) and the application of such provision to other persons or entities and circumstances shall not in any way be affected or impaired thereby.
4
Exhibit 3.2
AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF
UNITE ACQUISITION 1 CORP.
Unite Acquisition 1 Corp., a corporation organized and existing under the laws of the State of Delaware, does hereby certify as follows:
A. The name of this corporation is Unite Acquisition 1 Corp., to be renamed Adaptin Bio, Inc. Its original Certificate of Incorporation was filed with the Secretary of State of the State of Delaware on March 10, 2022 under the name Unite Acquisition 1 Corp.
B. This Amended and Restated Certificate of Incorporation (this “Restated Certificate of Incorporation”) was duly adopted by the Board of Directors of this corporation and by the stockholders in accordance with Sections 242 and 245 of the General Corporation Law of the State of Delaware, with the approval of the stockholders of this corporation having been given by written consent without a meeting in accordance with Section 228 of the General Corporation Law of the State of Delaware.
C. The text of the Restated Certificate of Incorporation is hereby amended and restated in its entirety to read as follows, effective as of 1:25 p.m. Eastern Time on February 11, 2025:
Article I
The name of this corporation is Adaptin Bio, Inc. (the “Corporation”).
Article II
The address of the Corporation’s registered office in the State of Delaware is 108 W. 13th Street Suite 100 in the City of Wilmington, County of New Castle, Zip Code 19801. The name of its registered agent at such address is Vcorp Services, LLC.
Article III
The nature of the business or purposes to be conducted or promoted by the Corporation is to engage in any lawful act or activity for which corporations may be organized under the General Corporation Law of the State of Delaware (the “DGCL”).
Article IV
Section 4.1 The total number of shares of all classes of stock that the Corporation has authority to issue is 60,000,000 shares, consisting of two classes: 50,000,000 shares of Common Stock, $0.0001 par value per share (“Common Stock”), and 10,000,000 shares of Preferred Stock, $0.0001 par value per share (“Preferred Stock”).
Section 4.2 The Corporation’s Board of Directors (the “Board”) is authorized, subject to any limitations prescribed by the law of the State of Delaware, by resolution or resolutions adopted from time to time, to provide for the issuance of shares of Preferred Stock in one or more series, and, by filing a certificate of designation pursuant to the applicable law of the State of Delaware (the “Certificate of Designation”), to establish from time to time the number of shares to be included in each such series, to fix the designation, vesting, powers (including voting powers), preferences and relative, participating, optional or other rights (and the qualifications, limitations or restrictions thereof) of the shares of each such series and to increase (but not above the total number of authorized shares of the class) or decrease (but not below the number of shares of such series then outstanding) the number of shares of any such series. The number of authorized shares of Preferred Stock may also be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then-outstanding shares of capital stock of the Corporation entitled to vote thereon, without a separate vote of the holders of the Preferred Stock or any series thereof, irrespective of the provisions of Section 242(b)(2) of the DGCL, unless a vote of any such holders is required pursuant to the terms of any Certificate of Designation designating a series of Preferred Stock.
Section 4.3 Except as otherwise expressly provided in any Certificate of Designation designating any series of Preferred Stock pursuant to the foregoing provisions of this Article IV, (i) any new series of Preferred Stock may be designated, fixed and determined as provided herein by the Board without approval of the holders of Common Stock or the holders of Preferred Stock, or any series thereof, and (ii) any such new series may have powers, preferences and rights, including, without limitation, voting rights, dividend rights, liquidation rights, redemption rights and conversion rights, senior to, junior to or pari passu with the rights of the Common Stock, the Preferred Stock or any future class or series of Preferred Stock or Common Stock.
Section 4.4 Each outstanding share of Common Stock shall entitle the holder thereof to one vote on each matter properly submitted to the stockholders of the Corporation for their vote; provided, however, that, except as otherwise required by law, holders of Common Stock shall not be entitled to vote on any amendment to this Restated Certificate of Incorporation (including any Certificate of Designation relating to any series of Preferred Stock) that relates solely to the terms of one or more outstanding series of Preferred Stock if the holders of such affected series are entitled, either separately or together as a class with the holders of one or more other such series, to vote thereon pursuant to this Restated Certificate of Incorporation (including any Certificate of Designation relating to any series of Preferred Stock).
Article V
Section 5.1 The business and affairs of the Corporation shall be managed by or under the direction of the Board, except as otherwise provided by law. In addition to the powers and authority expressly conferred upon them by statute or by this Restated Certificate of Incorporation or the Amended and Restated Bylaws of the Corporation (the “Bylaws”), the directors are hereby empowered to exercise all such powers and do all such acts and things as may be exercised or done by the Corporation.
Section 5.2 Subject to the rights of the holders of any series of Preferred Stock to elect additional directors under specified circumstances, the total number of directors constituting the Board shall be fixed from time to time exclusively by resolution adopted by a majority of the Board.
Section 5.3 Directors shall be elected at each annual meeting of stockholders and may be elected at any special meeting of stockholders called for that purpose, to serve until the next annual meeting of stockholders and until their respective successors are elected, unless they shall sooner resign, become disqualified or disabled or be removed from office. A director or the entire Board of Directors may be removed with or without cause by the affirmative vote of the holders of a majority of the securities entitled to vote for each director. Any director may resign at any time upon notice to the Corporation given in writing or by any electronic transmission permitted by the Bylaws. No decrease in the authorized number of directors constituting the Board shall shorten the term of any incumbent director.
Section 5.4 Subject to the special rights of the holders of any series of Preferred Stock to elect directors, any vacancy occurring in the Board for any cause, and any newly created directorship resulting from any increase in the authorized number of directors, shall, unless the Board determines by resolution that any such vacancies or newly created directorships shall be filled by the stockholders or as otherwise provided by law, be filled only by the affirmative vote of a majority of the directors then in office, even if less than a quorum, or by a sole remaining director, and not by the stockholders. Any director elected in accordance with the preceding sentence shall hold office for a term expiring at the next annual meeting of stockholders or until such director’s successor shall have been duly elected and qualified, or until such director’s earlier death, resignation, disqualification or removal.
Section 5.5 Election of directors need not be by written ballot unless the Bylaws shall so provide.
2
Article VI
To the fullest extent permitted by the DGCL as the same exists or as may hereafter be amended, no director or officer of the Corporation shall be personally liable to the Corporation or its stockholders for monetary damages for breach of fiduciary duty as a director or officer. If the DGCL is hereafter amended to authorize corporate action further limiting or eliminating the personal liability of directors or officers, then the liability of the directors or officers of the Corporation shall be limited or eliminated to the fullest extent permitted by the DGCL, as so amended from time to time. Any amendment or repeal of this Article VI, or the adoption of any provision of this Restated Certificate of Incorporation inconsistent with this Article VI, shall not adversely affect any right or protection of a director or officer of the Corporation existing at the time of such amendment or repeal or adoption of such inconsistent provision with respect to acts or omissions occurring prior to such amendment or repeal or adoption of such inconsistent provision.
Article VII
The Board shall have the power to adopt, amend or repeal the Bylaws. Any adoption, amendment or repeal of the Bylaws by the Board shall require the approval of a majority of the Board. The stockholders shall also have power to adopt, amend or repeal the Bylaws; provided, however, that, notwithstanding any other provision of this Restated Certificate of Incorporation (including any Certificate of Designation) or any provision of law that might otherwise permit a lesser or no vote, but in addition to any vote of the holders of any class or series of stock of the Corporation required by applicable law or by this Restated Certificate of Incorporation (including any Preferred Stock issued pursuant to any Certificate of Designation), the affirmative vote of the holders of at least two-thirds (2/3) of the voting power of all of the then-outstanding shares of the capital stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class, shall be required to adopt, amend or repeal any provision of the Bylaws; provided, further, that if at least two-thirds (2/3) of the Board has approved such adoption, amendment or repeal of any provisions of the Bylaws, then only the affirmative vote of the holders of at least a majority of the voting power of all of the then-outstanding shares of the capital stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class, shall be required to adopt, amend or repeal any provision of the Bylaws.
Article VIII
Section 8.1 Subject to the rights of any series of Preferred Stock then outstanding, any action required or permitted to be taken by the stockholders of the Corporation must be effected at a duly called annual or special meeting of stockholders of the Corporation and may not be effected by any consent in writing by such stockholders.
Section 8.2 Special meetings of stockholders of the Corporation may be called only by the Chairperson or Executive Chairperson of the Board, the Chief Executive Officer, the Lead Independent Director (as defined in the Bylaws) or the Board acting pursuant to a resolution adopted by a majority of the Board, and may not be called by any other person or persons. Only such business shall be considered at a special meeting of stockholders as shall have been stated in the notice for such meeting.
Section 8.3 Advance notice of stockholder nominations for the election of directors and of business to be brought by stockholders before any meeting of the stockholders of the Corporation shall be given in the manner and to the extent provided in the Bylaws.
3
Article IX
Section 9.1 Unless the Corporation consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if and only if the Court of Chancery of the State of Delaware lacks subject matter jurisdiction, any state court located within the State of Delaware or, if and only if all such state courts lack subject matter jurisdiction, the federal district court for the District of Delaware) and any appellate court therefrom, to the fullest extent permitted by law, shall be the sole and exclusive forum for: (a) any derivative action, suit or proceeding brought on behalf of the Corporation; (b) any action, suit or proceeding asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, employee, or agent of the Corporation to the Corporation or the Corporation’s stockholders, or any action asserting a claim for aiding and abetting any such breach of fiduciary duty; (c) any action, suit or proceeding asserting a claim against the Corporation or any current or former director, officer or employee of the Corporation arising out of or pursuant to, or seeking to enforce any right, obligation or remedy under, or to interpret, apply, or determine the validity of, any provision of the DGCL, this Restated Certificate of Incorporation or the Bylaws (as each may be amended from time to time); (d) any action, suit or proceeding as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware, or (e) any action, suit or proceeding asserting a claim against the Corporation or any current or former director, officer or employee of the Corporation governed by the internal affairs doctrine, in all cases subject to the court having personal jurisdiction over the indispensable parties named as defendants. If any action, suit or proceeding the subject matter of which is within the scope of the preceding sentence is filed in a court other than the courts in the State of Delaware (a “Foreign Action”) in the name of any stockholder, such stockholder shall be deemed to have consented to (x) the personal jurisdiction of the state and federal courts in the State of Delaware in connection with any action, suit or proceeding brought in any such court to enforce the provisions of the preceding sentence and (y) having service of process made upon such stockholder in any such action by service upon such stockholder’s counsel in the Foreign Action as agent for such stockholder. This Section 9.1 shall not apply to actions, suits or proceedings brought to enforce a duty or liability created by the Securities Exchange Act of 1934, as amended, or any other claim for which the federal courts have exclusive jurisdiction.
Section 9.2 Unless the Corporation consents in writing to the selection of an alternative forum, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act of 1933, as amended (such act, and the rules and regulations promulgated thereunder, the “Securities Act”), including all causes of action asserted against any defendant to such complaint. For the avoidance of doubt, this provision is intended to benefit and may be enforced by the Corporation, its directors and officers, the underwriters to any offering giving rise to such complaint, and any other professional or entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying such offering.
Section 9.3 Any person or entity purchasing or otherwise acquiring or holding any interest in shares of capital stock of the Corporation shall be deemed to have notice of and to have consented to the provisions of this Article IX.
Section 9.4 Failure to enforce the foregoing provisions of this Article IX would cause the Corporation irreparable harm, and the Corporation shall be entitled to equitable relief, including injunctive relief and specific performance, to enforce the foregoing provisions.
Article X
If any provision of this Restated Certificate of Incorporation shall be held to be invalid, illegal or unenforceable, then such provision shall nonetheless be enforced to the maximum extent possible consistent with such holding and the remaining provisions of this Restated Certificate of Incorporation (including without limitation, all portions of any section of this Restated Certificate of Incorporation containing any such provision held to be invalid, illegal or unenforceable, that are not themselves invalid, illegal or unenforceable) shall remain in full force and effect.
Article XI
The Corporation reserves the right to amend or repeal any provision contained in this Restated Certificate of Incorporation in the manner prescribed by the laws of the State of Delaware and all rights conferred upon stockholders are granted subject to this reservation; provided, however, that, notwithstanding any other provision of this Restated Certificate of Incorporation (including any Certificate of Designation) or any provision of law that might otherwise permit a lesser vote or no vote, but in addition to any vote of the holders of any class or series of the stock of the Corporation required by law or by this Restated Certificate of Incorporation (including any Certificate of Designation), and subject to Section 4.2 and Section 4.3 of Article IV, the affirmative vote of the holders of at least two-thirds (2/3) of the voting power of all of the then-outstanding shares of the capital stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class, shall be required to amend or repeal or adopt any provision inconsistent with this Article XI, Section 4.2 and Section 4.4 of Article IV, or Article V, Article VI, Article VII, Article VIII, Article IX or Article X (the “Specified Provisions”); provided, further, that if at least two-thirds (2/3) of the Board has approved such amendment or repeal of, or any provision inconsistent with, the Specified Provisions, then only the affirmative vote of the holders of at least a majority of the voting power of all of the then-outstanding shares of the capital stock of the Corporation entitled to vote generally in the election of directors, voting together as a single class, shall be required to amend, repeal, or adopt any provision inconsistent with, the Specified Provisions.
4
IN WITNESS WHEREOF, Unite Acquisition 1 Corp., has caused this Restated Certificate of Incorporation to be signed by Nathan Pereira, a duly authorized officer of the Corporation, on this 11 day of February, 2025.
| /s/ Nathan Pereira | |
| Nathan Pereira | |
| President and Chief Executive Officer |
5
Exhibit 3.3
Adaptin Bio, Inc.
a Delaware Corporation
AMENDED AND RESTATED BYLAWS
As adopted on February 11, 2025
(Effective as of February 11, 2025)
Article I: STOCKHOLDERS
Section 1.1 Annual Meetings. If required by applicable law, an annual meeting of stockholders shall be held for the election of directors at such date and time as the Board of Directors (the “Board”) of Adaptin Bio, Inc. (the “Corporation”) shall each year fix. The meeting may be held either at a place, within or without the State of Delaware as permitted by the General Corporation Law of the State of Delaware (the “DGCL”), or by means of remote communication as the Board in its sole discretion may determine. Any proper business may be transacted at the annual meeting.
Section 1.2 Special Meetings. Special meetings of stockholders for any purpose or purposes shall be called in the manner set forth in the Restated Certificate of Incorporation of the Corporation (as the same may be amended and/or restated from time to time, the “Certificate of Incorporation”). The special meeting may be held either at a place, within or without the State of Delaware as permitted by the DGCL, or by means of remote communication as the Board in its sole discretion may determine. Business transacted at any special meeting of stockholders shall be limited to matters relating to the purpose or purposes stated in the notice of the meeting.
Section 1.3 Notice of Meetings. Notice of all meetings of stockholders shall be given in accordance with applicable law (including, without limitation, as set forth in Section 7.1.1 of these Bylaws of the Corporation (the “Bylaws”)) stating the date, time and place, if any, of the meeting, the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at such meeting, and the record date for determining the stockholders entitled to vote at the meeting (if such date is different from the record date for determining stockholders entitled to notice of the meeting). In the case of a special meeting, such notice shall also set forth the purpose or purposes for which the meeting is called. Unless otherwise required by applicable law or the Certificate of Incorporation, notice of any meeting of stockholders shall be given not less than ten (10), nor more than sixty (60), days before the date of the meeting to each stockholder of record entitled to vote at such meeting as of the record date for determining the stockholders entitled to notice of the meeting.
Section 1.4 Adjournments. Notwithstanding Section 1.5 of these Bylaws, the chairperson of the meeting shall have the power to adjourn the meeting to another time, date and place (if any) regardless of whether a quorum is present, at any time and for any reason. Any meeting of stockholders, annual or special, may be adjourned from time to time (including an adjournment taken to address a technical failure to convene or continue a meeting using remote communication), and notice need not be given of any such adjourned meeting if the time, date and place (if any) thereof and the means of remote communication (if any) by which stockholders and proxy holders may be deemed to be present in person and vote at such adjourned meeting are announced at the meeting at which the adjournment is taken, or are provided in any other manner permitted by the DGCL; provided, however, that if the adjournment is for more than thirty (30) days, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting. If, after the adjournment, a new record date for determination of stockholders entitled to vote is fixed for the adjourned meeting, the Board shall fix as the record date for determining stockholders entitled to notice of such adjourned meeting the same or an earlier date as that fixed for determination of stockholders entitled to vote at the adjourned meeting, and shall give notice of the adjourned meeting to each stockholder of record as of the record date so fixed for notice of such adjourned meeting. At the adjourned meeting, the Corporation may transact any business that might have been transacted at the original meeting. To the fullest extent permitted by law, if a quorum is present at the original meeting, it shall also be deemed present at the adjourned meeting. To the fullest extent permitted by law, the Board may postpone, reschedule or cancel at any time and for any reason any previously scheduled special or annual meeting of stockholders before it (or any adjournment) is to be held, regardless of whether any notice or public disclosure with respect to any such meeting (or adjournment) has been sent or made pursuant to Section 1.3 hereof or otherwise, in which case notice shall be provided to the stockholders of the new date, time and place, if any, of the meeting as provided in Section 1.3 above.
Section 1.5 Quorum. Except as otherwise required by applicable law or as provided in the Certificate of Incorporation or these Bylaws, at each meeting of stockholders the holders of a majority of the voting power of the shares of stock issued and outstanding and entitled to vote at the meeting, present in person or represented by proxy, shall constitute a quorum for the transaction of business; provided, however, that where a separate vote by a class or classes or series of stock is required by applicable law or the Certificate of Incorporation, the holders of a majority of the voting power of the shares of such class or classes or series of the stock issued and outstanding and entitled to vote on such matter, present in person or represented by proxy at the meeting, shall constitute a quorum entitled to take action with respect to the vote on such matter. A quorum, once established at a meeting, shall not be broken by the withdrawal of enough votes to leave less than a quorum, including, to the fullest extent permitted by law, at any adjournment thereof (unless a new record date is fixed for the adjourned meeting).
Section 1.6 Organization; Conduct of Meetings. Meetings of stockholders shall be presided over by (a) such person as the Board may designate, or (b) in such person’s absence, the Chairperson of the Board, or (c) in such person’s absence, the Lead Independent Director, or (d) in such person’s absence, the Chief Executive Officer of the Corporation or (e) in such person’s absence, the President of the Corporation, or (f) in the absence of such person, by a Vice President. Such person shall be chairperson of the meeting and, subject to Section 1.10 of these Bylaws, shall determine the order of business and the rules, regulations and procedures at the meeting, including such regulation of the manner of voting and the conduct of discussion as seems to such person to be in order. The Secretary of the Corporation shall act as secretary of the meeting, but in such person’s absence the chairperson of the meeting may appoint any person to act as secretary of the meeting.
Section 1.7 Voting; Proxies. Each stockholder of record entitled to vote at a meeting of stockholders may authorize another person or persons to act for such stockholder by proxy. Such a proxy may be prepared, transmitted and delivered in any manner permitted by applicable law. Except as may be required in the Certificate of Incorporation, directors shall be elected by a plurality of the votes cast by the holders of the shares present in person or represented by proxy at the meeting and entitled to vote on the election of directors. The stockholders shall not have the right to cumulate their votes for the election of directors of the Corporation. At all meetings of stockholders at which a quorum is present, unless a different or minimum vote is required by applicable law, the rules or regulations of any stock exchange applicable to the Corporation, the Certificate of Incorporation or these Bylaws, the vote required for stockholder action shall be majority of the votes cast affirmatively or negatively (excluding abstentions) by the holders of shares present in person or represented by proxy at the meeting and entitled to vote thereon (or if there are two or more classes or series of stock are entitled to vote as separate classes, then the majority of the votes cast affirmatively or negatively (excluding abstentions) of each class or series present in person or represented by proxy at the meeting and entitled to vote thereon). Any stockholder directly or indirectly soliciting proxies from other stockholders must use a proxy card color other than white, which shall be reserved for exclusive use by the Board.
Section 1.8 Fixing Date for Determination of Stockholders of Record. In order that the Corporation may determine the stockholders entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, the Board may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board, and which record date shall, unless otherwise required by law, not be more than sixty (60) nor less than ten (10) days before the date of such meeting. If no record date is fixed by the Board, the record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be at the close of business on the day next preceding the day on which notice is given, or, if notice is waived, at the close of business on the day next preceding the day on which the meeting is held. A determination of stockholders of record entitled to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Board may fix a new record date for determination of stockholders entitled to notice of or to vote at the adjourned meeting, and in such case shall also fix as the record date for stockholder entitled to notice of such adjourned meeting the same or an earlier date as that fixed for determination of stockholders entitled to vote in accordance herewith at the adjourned meeting.
2
In order that the Corporation may determine the stockholders entitled to receive payment of any dividend or other distribution or allotment of any rights, or entitled to exercise any rights in respect of any change, conversion or exchange of stock or for the purpose of any other lawful action, the Board may fix, in advance, a record date, which shall not precede the date upon which the resolution fixing the record date is adopted by the Board and which shall not be more than sixty (60) days prior to such action. If no such record date is fixed by the Board, then the record date for determining stockholders for any such purpose shall be at close of business on the day on which the Board adopts the resolution relating thereto.
Section 1.9 List of Stockholders Entitled to Vote. The Corporation shall prepare, no later than the tenth (10th) day before each meeting of stockholders, a complete list of stockholders entitled to vote at the meeting, arranged in alphabetical order and showing the address of each stockholder and the number of shares registered in the name of each stockholder. The Corporation shall not be required to include electronic mail addresses or other electronic contact information on such list. Such list shall be open to the examination of any stockholder, for any purpose germane to the meeting, for a period of ten (10) days ending on the day before the meeting date, (a) on a reasonably accessible electronic network as permitted by applicable law (provided that the information required to gain access to the list is provided with the notice of the meeting), or (b) during ordinary business hours, at the principal executive offices of the Corporation. In the event that the Corporation determines to make the list available on an electronic network, the Corporation may take reasonable steps to ensure that such information is available only to stockholders of the Corporation. Except as otherwise provided by law, the stock ledger of the Corporation shall be the only evidence as to the identity of the stockholders entitled to examine the list of stockholders required by this Section 1.9 or to vote in person or by proxy at any meeting. Notwithstanding the foregoing, the Corporation may maintain and authorize examination of the list of stockholders required by this Section 1.9 in any manner expressly permitted by the DGCL at the time.
Section 1.10 Inspectors of Elections.
1.10.1 Applicability. Unless otherwise required by the Certificate of Incorporation or by applicable law, the following provisions of this Section 1.10 shall apply only if and when the Corporation has a class of voting stock that is: (a) listed on a national securities exchange; (b) authorized for quotation on an interdealer quotation system of a registered national securities association; or (c) held of record by more than two thousand (2,000) stockholders. In all other cases, observance of the provisions of this Section 1.10 shall be optional, and at the discretion of the Board.
1.10.2 Appointment. The Corporation shall, in advance of any meeting of stockholders, appoint one or more inspectors of election to act at the meeting and make a written report thereof. The Corporation may designate one or more persons as alternate inspectors to replace any inspector who fails to act. If no inspector or alternate is able to act at a meeting of stockholders, the chairperson of the meeting shall appoint one or more inspectors to act at the meeting.
1.10.3 Inspector’s Oath. Each inspector of election, before entering upon the discharge of such inspector’s duties, shall take and sign an oath faithfully to execute the duties of inspector with strict impartiality and according to the best of such inspector’s ability.
1.10.4 Duties of Inspectors. At a meeting of stockholders, the inspectors of election shall (a) ascertain the number of shares outstanding and the voting power of each share, (b) determine the shares represented at a meeting and the validity of proxies and ballots, (c) count all votes and ballots, (d) determine and retain for a reasonable period of time a record of the disposition of any challenges made to any determination by the inspectors, and (e) certify their determination of the number of shares represented at the meeting, and their count of all votes and ballots. The inspectors may appoint or retain other persons or entities to assist the inspectors in the performance of the duties of the inspectors.
1.10.5 Opening and Closing of Polls. The date and time of the opening and the closing of the polls for each matter upon which the stockholders will vote at a meeting shall be announced at the meeting. No ballot, proxies or votes, nor any revocations thereof or changes thereto, shall be accepted by the inspectors after the closing of the polls unless the Court of Chancery, upon application by a stockholder, shall determine otherwise.
1.10.6 Determinations. In determining the validity and counting of proxies and ballots cast at any meeting of stockholders, the inspectors may consider such information as is permitted by applicable law.
3
Section 1.11 Notice of Stockholder Business; Nominations.
1.11.1 Annual Meeting of Stockholders.
(a) Nominations of persons for election to the Board and the proposal of other business to be considered by the stockholders may be made at an annual meeting of stockholders only: (i) pursuant to the Corporation’s notice of such meeting (or any supplement thereto), (ii) by or at the direction of the Board or any committee thereof or (iii) by any stockholder of the Corporation who was a stockholder of record at the time of giving of the notice provided for in this Section 1.11 (the “Record Stockholder”), who is entitled to vote at such meeting and who complies with the notice and other procedures set forth in this Section 1.11 in all applicable respects. For the avoidance of doubt, the foregoing clause (iii) shall be the exclusive means for a stockholder to make nominations or propose business (other than business included in the Corporation’s proxy materials pursuant to Rule 14a-8 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)), at an annual meeting of stockholders, and such stockholder must fully comply with the notice and other procedures set forth in this Section 1.11 to bring such nominations or other business properly before an annual meeting.
(b) For nominations or other business to be properly brought before an annual meeting by a Record Stockholder pursuant to Section 1.11.1(a) of these Bylaws:
(i) the Record Stockholder must have given timely notice thereof in writing to the Secretary of the Corporation and have provided any updates or supplements to such notice at the times and in the forms required by this Section 1.11;
(ii) such other business (other than the nomination of persons for election to the Board) must otherwise be a proper matter for stockholder action;
(iii) (a) if the Proposing Person (as defined below) has provided the Corporation with a Solicitation Notice (as defined below), such Proposing Person must, in the case of a proposal other than the nomination of persons for election to the Board, have delivered a proxy statement and form of proxy to holders of at least the percentage of the Corporation’s voting shares required under applicable law to carry any such proposal and must have included in such materials the Solicitation Notice, or (b) if the Proposing Person has delivered a notice of nomination or nominations of persons for election to the Board, such Proposing Person must have complied with the requirements of Rule 14a-19 under the Exchange Act, if applicable; and
(iv) in the case of a proposal other than the nomination of persons for election to the Board, if no Solicitation Notice relating thereto has been timely provided pursuant to this Section 1.11, the Proposing Person proposing such business must not have solicited a number of proxies sufficient to have required the delivery of such a Solicitation Notice under this Section 1.11.
To be timely, (a) a Record Stockholder’s notice must be delivered to the Secretary at the principal executive offices of the Corporation not later than the close of business on the ninetieth (90th) day nor earlier than the close of business on the one hundred and twentieth (120th) day prior to the first anniversary of the preceding year’s annual meeting (except in the case of the Corporation’s first annual meeting following the adoption of these Bylaws, for which such notice shall be timely if delivered in the same time period as if such meeting were a special meeting governed by Section 1.11.2 of these Bylaws); provided, however, that in the event that no annual meeting was held during the preceding year or the date of the annual meeting is more than thirty (30) days before, or more than sixty (60) days after, such anniversary date, or if no annual meeting was held in the preceding year, notice by the Record Stockholder to be timely must be so delivered (A) no earlier than close of business on the one hundred and twentieth (120th) day prior to such annual meeting and (B) no later than close of business on the later of the ninetieth (90th) day prior to such annual meeting or the tenth (10th) day following the day on which Public Announcement (as defined below) of the date of such meeting is first made by the Corporation, (b) in the case of a proposal for the nomination of persons for election to the Board, the Record Stockholder shall have complied in all respects with the requirements of Section 14 of the Exchange Act, including without limitation, if applicable, the requirements of Rule 14a-19 (as such rule and regulations may be amended from time to time by the Securities and Exchange Commission, including any Securities and Exchange Commission Staff interpretations relating thereto) and (c) in the case of a proposal for nomination of persons for election to the Board, the Board or an executive officer designated thereby shall have determined that the Record Stockholder has satisfied the requirements of Section 1.11. In no event shall an adjournment or postponement of an annual meeting commence a new time period (or extend any time period) for providing the Record Stockholder’s notice. Such Record Stockholder’s notice shall set forth:
(X) as to each person whom the Record Stockholder proposes to nominate for election or reelection as a director (in addition to the matters set forth in paragraph (Z) below):
(i) the name, age, business address and residence address of such person;
4
(ii) the principal occupation or employment of such person;
(iii) the class, series and number of any shares of stock of the Corporation that are beneficially owned or owned of record by such person or any Associated Person (as defined in Section 1.11.3(f));
(iv) the date or dates such shares were acquired and the investment intent of such acquisition;
(v) a written questionnaire with respect to the background and qualification of such person, completed and executed by such person, which questionnaire shall be provided by the Secretary upon written request of any Record Stockholder within five (5) business days of such written request;
(vi) a written representation and agreement, in the form provided by the Secretary upon written request of any Record Stockholder within five (5) business days of such written request, that such person (a) is not and will not become a party to (1) any agreement, arrangement or understanding (whether written or oral) with, and has not given any commitment or assurance to, any person or entity as to how such person, if elected as a director of the Corporation, will act or vote on any issue or question (for purposes of this Section 1.11, a “Voting Commitment”) that has not been disclosed to the Corporation or (2) any Voting Commitment that could limit or interfere with such person’s ability to comply, if elected as a director of the Corporation, with such person’s fiduciary duties under applicable law, (b) is not and will not become a party to any agreement, arrangement or understanding with any person or entity other than the Corporation with respect to any direct or indirect compensation, reimbursement or indemnification in connection with service or action as a director that has not been disclosed to the Corporation, (c) would be in compliance, if elected as a director of the Corporation, and will comply with all applicable rules or regulations of any stock exchange applicable to the Corporation and all applicable publicly disclosed corporate governance, policies and guidelines of the Corporation applicable to directors, (d) will act, if elected as a director of the Corporation, in the best interests of the Corporation and its stockholders and not in the interests of individual constituencies, (e) consents to being named as a nominee in a proxy statement and form of proxy relating to the meeting at which directors are to be elected and agrees to serve if elected as a director and (f) intends to serve a full term if elected as a director of the Corporation;
(vii) all other information relating to such person that would be required to be disclosed in solicitations of proxies for election of directors in an election contest (even if an election contest is not involved), or would be otherwise required, in each case pursuant to and in accordance with Section 14(a) (or any successor provision) under the Exchange Act and the rules and regulations thereunder (including such person’s written consent to being named as a nominee in a proxy statement and form of proxy relating to the meeting at which directors are to be elected, to the public disclosure of information regarding or related to such person provided to the Corporation by such person or otherwise pursuant to this Section 1.11 and to serving a full term as a director of the Corporation if elected);
(viii) whether such person meets the independence requirements of any stock exchange applicable to the Corporation;
5
(ix) a description of all direct and indirect compensation and other material monetary agreements, arrangements and understandings during the past three (3) years, and any other material relationships, between or among such Proposing Person or any of its respective affiliates and associates, on the one hand, and each proposed nominee, and his or her respective affiliates and associates, on the other hand, including all information that would be required to be disclosed pursuant to Item 404 under Regulation S-K if the Proposing Person or any of its respective affiliates and associates were the “registrant” for purposes of such rule and the nominee were a director or executive officer of such registrant; and
(x) a description of any position of such person as an officer or director of any principal competitor of the Corporation within the three (3) years preceding the submission of the notice.
(Y) as to any business other than the nomination of persons for election to the Board that the Record Stockholder proposes to bring before the meeting:
(i) a brief description of the business desired to be brought before the meeting, the text of the proposal or business (including the text of any resolutions proposed for consideration and in the event that such business includes a proposal to amend the Bylaws, the text of the proposed amendment), the reasons for conducting such business at the meeting and any material interest in such business of such Proposing Person, including any anticipated benefit to any Proposing Person therefrom; and
(ii) a description of all agreements, arrangements and understandings between or among any such Proposing Person and any of its respective affiliates or associates, on the one hand, and any other person or persons, on the other hand, (including their names) in connection with the proposal of such business by such Proposing Person.
(Z) as to the Proposing Person giving the notice:
(i) the current name and address of such Proposing Person, including, if applicable, their name and address as they appear on the Corporation’s stock ledger, if different;
(ii) the class or series and number of shares of stock of the Corporation that are directly or indirectly owned of record or beneficially owned by such Proposing Person, including any shares of any class or series of the Corporation as to which such Proposing Person has a right to acquire beneficial ownership at any time in the future;
(iii) whether and the extent to which any derivative interest in the Corporation’s equity securities (including without limitation any option, warrant, convertible security, stock appreciation right, or similar right with an exercise or conversion privilege or a settlement payment or mechanism at a price related to any class or series of shares of the Corporation or with a value derived in whole or in part from the value of any class or series of shares of the Corporation, whether or not such instrument or right shall be subject to settlement in the underlying class or series of shares of the Corporation or otherwise, and any cash-settled equity swap, total return swap, synthetic equity position or similar derivative arrangement (any of the foregoing, a “Derivative Instrument”), as well as any rights to dividends on the shares of any class or series of shares of the Corporation that are separated or separable from the underlying shares of the Corporation) or any short interest in any security of the Corporation (for purposes of this Bylaw a person shall be deemed to have a short interest in a security if such person directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise, has the opportunity to profit or share in any profit derived from any increase or decrease in the value of the subject security, including through performance-related fees) is held directly or indirectly by or for the benefit of such Proposing Person, including without limitation whether and the extent to which any ongoing hedging or other transaction or series of transactions has been entered into by or on behalf of, or any other agreement, arrangement or understanding (including without limitation any short position or any borrowing or lending of shares) has been made, the effect or intent of which is to mitigate loss to or manage risk or benefit of share price changes for, or to increase or decrease the voting power of, such Proposing Person with respect to any share of stock of the Corporation (any of the foregoing, a “Short Interest”);
(iv) any proportionate interest in shares of the Corporation or Derivative Instruments held, directly or indirectly, by a general or limited partnership in which such Proposing Person or any of its respective affiliates or associates is a general partner or, directly or indirectly, beneficially owns an interest in a general partner of such general or limited partnership;
(v) any direct or indirect material interest in any material contract or agreement with the Corporation, any affiliate of the Corporation or any principal competitor of the Corporation (including, in any such case, any employment agreement, collective bargaining agreement or consulting agreement);
6
(vi) any significant equity interests or any Derivative Instruments or Short Interests in any principal competitor of the Corporation held by such Proposing Person and/or any of its respective affiliates or associates;
(vii) any other material relationship between such Proposing Person, on the one hand, and the Corporation, any affiliate of the Corporation or any principal competitor of the Corporation, on the other hand;
(viii) all information that would be required to be set forth in a Schedule 13D filed pursuant to Rule 13d-1(a) or an amendment pursuant to Rule 13d-2(a) if such a statement were required to be filed under the Exchange Act and the rules and regulations thereunder by such Proposing Person and/or any of its respective affiliates or associates;
(ix) any other information relating to such Proposing Person that would be required to be disclosed in a proxy statement or other filing required to be made in connection with solicitations of proxies or consents by such Proposing Person in support of the business or nomination proposed to be brought before the meeting pursuant to Section 14(a) (or any successor provision) under the Exchange Act and the rules and regulations thereunder;
(x) such Proposing Person’s written consent to the public disclosure of information provided to the Corporation pursuant to this Section 1.11;
(xi) a complete written description of any agreement, arrangement or understanding (whether oral or in writing) (including any knowledge that another person or entity is Acting in Concert (as defined in Section 1.11.3(f)) with such Proposing Person) between or among such Proposing Person, any of its respective affiliates or associates and any other person Acting in Concert with any of the foregoing persons;
(xii) a representation that the Record Stockholder is a holder of record of stock of the Corporation entitled to vote at such meeting and intends to appear in person (including virtually in the case of a meeting conducted solely by means of remote communication) or by proxy at the meeting to propose such business or nomination;
(xiii) in the case of a proposal other than the nomination of persons for election to the Board, a representation whether such Proposing Person intends (or is part of a group that intends) to (a) deliver a proxy statement or form of proxy to holders of at least the percentage of the Corporation’s voting shares required under applicable law to carry the proposal (an affirmative statement of such intent being a “Solicitation Notice”); (b) in the case of a nomination or nominations other than the Corporation’s nominees, a representation that such Proposing Person intends to solicit proxies from holders of shares representing at least 67% of the voting power of shares entitled to vote on the election of directors in support of such nominee or nominees other than the Corporation’s nominees, in accordance with Rule 14a-19 under the Exchange Act, and the name of each participant (as defined in Item 4 of Schedule 14A under the Exchange Act) in such solicitation; and
(xiv) any proxy, contract, arrangement, or relationship pursuant to which the Proposing Person has a right to vote, directly or indirectly, any shares of any security of the Corporation.
The disclosures to be made pursuant to the foregoing clauses (Z)(ii), (Z)(iii), (Z)(iv) and (Z)(vi) shall not include any information with respect to the ordinary course business activities of any broker, dealer, commercial bank, trust company or other nominee who is a Proposing Person solely as a result of being the stockholder directed to prepare and submit the notice required by these Bylaws on behalf of a beneficial owner.
7
A stockholder providing written notice required by this Section 1.11 shall update such notice in writing (and such update shall clearly identify the information that has changed since the prior submission), if necessary, so that the information provided or required to be provided in such notice is true and correct in all material respects as of (i) the record date for determining the stockholders entitled to notice of the meeting and (ii) close of business on the tenth (10th) business day prior to the meeting or any adjournment or postponement thereof. In the case of an update pursuant to clause (i) of the foregoing sentence, such update shall be received by the Secretary of the Corporation at the principal executive office of the Corporation not later than five (5) business days after the record date for determining the stockholders entitled to notice of the meeting, and in the case of an update pursuant to clause (ii) of the foregoing sentence, such update shall be received by the Secretary of the Corporation at the principal executive office of the Corporation not later than eight (8) business days prior to the date for the meeting, and, if practicable, any adjournment or postponement thereof. Notwithstanding the foregoing, if a Proposing Person no longer plans to solicit proxies in accordance with its representation pursuant to Section 1.11.1(b)(Z)(xiii), the Record Holder shall inform the Corporation of this change by delivering a writing to the Secretary at the principal executive offices of the Corporation no later than two (2) business days after the occurrence of such change. For the avoidance of doubt, the obligation to update as set forth in this paragraph shall not limit the Corporation’s rights with respect to any deficiencies in any notice provided by a stockholder, extend any applicable deadlines hereunder or enable or be deemed to permit a stockholder who has previously submitted notice hereunder to amend or update any proposal or nomination or to submit any new proposal, including by changing or adding nominees, matters, business and/or resolutions proposed to be brought before a meeting of stockholders.
(c) Notwithstanding anything in Section 1.11 or any other provision of the Bylaws to the contrary, in the event that the number of directors to be elected to the Board is increased and there is no Public Announcement by the Corporation naming all of the nominees for director or specifying the size of the increased Board at least ninety (90) days prior to the first anniversary of the preceding year’s annual meeting (or, if the annual meeting is held more than thirty (30) days before or sixty (60) days after such anniversary date, at least ninety (90) days prior to such annual meeting), a stockholder’s notice required by this Section 1.11 shall also be considered timely, but only with respect to nominees for any new positions created by such increase, if it shall be delivered to the Secretary of the Corporation at the principal executive office of the Corporation no later than the close of business on the tenth (10th) day following the day on which such Public Announcement is first made by the Corporation.
(d) Notwithstanding anything in Section 1.11 or any other provision of the Bylaws to the contrary, any person who has been determined by a majority of the Board to have violated Section 2.11 of these Bylaws or a Board Confidentiality Policy (as defined below) while serving as a director of the Corporation in the preceding five (5) years shall be ineligible to be nominated to serve as a member of the Board, absent a prior waiver for such nomination approved by two-thirds of the Board.
1.11.2 Special Meetings of Stockholders. Only such business shall be conducted at a special meeting of stockholders as shall have been brought before the meeting pursuant to the Corporation’s notice of such meeting. Nominations of persons for election to the Board may be made at a special meeting of stockholders at which directors are to be elected pursuant to the Corporation’s notice of such meeting (a) by or at the direction of the Board or any committee thereof or (b) provided that the Board has determined that directors shall be elected at such meeting, by any stockholder of the Corporation who is a stockholder of record at the time of giving of notice of the special meeting, who shall be entitled to vote at the meeting and who complies with the notice and other procedures set forth in this Section 1.11 in all applicable respects. In the event the Corporation calls a special meeting of stockholders for the purpose of electing one or more directors to the Board, any such stockholder may nominate a person or persons (as the case may be), for election to such position(s) as specified in the Corporation’s notice of meeting, if (A) the stockholder’s notice required by Section 1.11.1(b) of these Bylaws shall be delivered to the Secretary of the Corporation at the principal executive offices of the Corporation (i) no earlier than close of business on the one hundred and twentieth (120th) day prior to such special meeting and (ii) no later than close of business on the later of the ninetieth (90th) day prior to such special meeting or the tenth (10th) day following the day on which Public Announcement is first made of the date of the special meeting and of the nominees proposed by the Board to be elected at such meeting, (B) the stockholder has complied in all respects with the requirements of Section 14 of the Exchange Act, including, without limitation, if applicable, the requirements of Rule 14a-19 (as such rule and regulations may be amended from time to time by the Securities and Exchange Commission, including any Securities and Exchange Commission Staff interpretations relating thereto) and (C) the Board or an executive officer designated thereby shall have determined that the stockholder has satisfied the requirements of Section 1.11. In no event shall an adjournment or postponement of a special meeting commence a new time period (or extend any time period) for providing such notice.
8
1.11.3 General.
(a) Except as otherwise expressly provided in any appliable rule or regulation under the Exchange Act, only such persons who are nominated in accordance with the procedures set forth in this Section 1.11 shall be eligible to be elected at a meeting of stockholders and serve as directors and only such business shall be conducted at a meeting of stockholders as shall have been brought before the meeting in accordance with the procedures set forth in this Section 1.11. The number of nominees a stockholder may nominate for election at a meeting of stockholders (or in the case of a Record Stockholder giving the notice on behalf of another Proposing Person, the number of nominees a stockholder nominate for election at the meeting on behalf of such Proposing Person) shall not exceed the number of directors to be elected at such meeting. Except as otherwise provided by law or these Bylaws, the chairperson of the meeting shall have the power and duty to determine whether a nomination or any other business proposed to be brought before the meeting was made or proposed, as the case may be, in accordance with the procedures set forth in this Section 1.11 and, if any proposed nomination or business is not in compliance herewith, to declare that such defective proposal or nomination shall be disregarded (and any such nominee shall be disqualified), including the circumstances described in Section 1.11.3(b), including the provision to the Corporation of notices required under Rule 14a-19 under the Exchange Act in a timely manner. Notwithstanding the foregoing provisions of this Section 1.11, unless otherwise required by law, if the stockholder (or a Qualified Representative of the stockholder (as defined below)) does not appear at the annual or special meeting of stockholders of the Corporation to present a nomination or proposed business, such nomination shall be disregarded and such proposed business shall not be transacted, notwithstanding that proxies in respect of such vote may have been received by the Corporation. Notwithstanding the foregoing provisions of this Section 1.11, unless otherwise required by law, no stockholder shall solicit proxies in support of director nominees other than the Corporation’s nominees unless such stockholder has complied with Rule 14a-19 under the Exchange Act in connection with the solicitation of such proxies, including the provision to the Corporation of notices required thereunder in a timely manner.
(b) If (i) any Proposing Person provides notice pursuant to Rule 14a-19(b) under the Exchange Act and (ii) such Proposing Person subsequently fails to comply with the requirements of Rule 14a-19(a)(2) or (3) under the Exchange Act, then the Corporation shall disregard any proxies for any proposed nominees on the Corporation’s proxy card other than the Corporation’s nominees (and any such proposed nominee(s) shall be disqualified), notwithstanding that proxies in favor thereof may have been received by the Corporation. If any Proposing Person provides notice pursuant to Rule 14a-19(b) under the Exchange Act, such Proposing Person shall deliver to the Secretary, no later than close of business on the fifth (5th) business day prior to the applicable meeting, reasonable evidence that the requirements of Rule 14a-19(a)(3) under the Exchange Act have been satisfied.
(c) Notwithstanding the foregoing provisions of this Section 1.11, a stockholder shall also comply with all applicable requirements of the Exchange Act and the rules and regulations thereunder with respect to the matters set forth herein. Nothing in this Section 1.11 shall be deemed to affect any rights of (i) stockholders to request inclusion of proposals in the Corporation’s proxy statement pursuant to Rule 14a-8 under the Exchange Act or (ii) the holders of any series of Common Stock or Preferred Stock to elect directors pursuant to any applicable provisions of the Certificate of Incorporation.
(d) The Corporation may also, as a condition to any nomination or business being deemed properly brought before a meeting of stockholders, require any stockholder, Proposing Person or any proposed nominee to deliver to the Secretary at the principal executive offices of the Corporation, within five (5) business days of any such request, such other information as may reasonably be requested by the Corporation, including (i) such other information as may be reasonably required by the Board, in its sole discretion, to determine (a) the eligibility of such proposed nominee to serve as a director of the Corporation, and (b) whether such proposed nominee qualifies as an “independent director” or “audit committee financial expert” under applicable law, securities exchange rule or regulation and (ii) such other information that the Board determines, in its sole discretion, could be material to a reasonable stockholder’s understanding of the independence, or lack thereof, of such proposed nominee.
(e) Any written notice, supplement, update or other information required to be delivered by a stockholder or by a Proposing Person to the Corporation pursuant to this Section 1.11 must be given by (i) hand delivery (including use of a delivery service), by registered or certified mail, postage prepaid, or by sending such notice by overnight express courier, to the Secretary at the principal executive offices of the Corporation, and (ii) facsimile, electronic mail or other form of electronic transmission to the Secretary.
9
(f) For purposes of this Section 1.11 the following definitions shall apply:
(i) a person shall be deemed to be “Acting in Concert” with another person if such person knowingly acts (whether or not pursuant to an express agreement, arrangement or understanding) in concert with, or toward a common goal relating to the management, governance or control of the Corporation in substantial parallel with, such other person where (1) each person is conscious of the other person’s conduct or intent and this awareness is an element in their decision-making processes and (2) at least one additional factor suggests that such persons intend to act in concert or in substantial parallel, which such additional factors may include, without limitation, exchanging information (whether publicly or privately), attending meetings, conducting discussions or making or soliciting invitations to act in concert or in substantial parallel; provided, that a person shall not be deemed to be Acting in Concert with any other person solely as a result of the solicitation or receipt of revocable proxies or consents from such other person in response to a solicitation made pursuant to, and in accordance with, Section 14(a) (or any successor provision) of the Exchange Act by way of a proxy or consent solicitation statement filed on Schedule 14A. A person Acting in Concert with another person shall be deemed to be Acting in Concert with any third party who is also Acting in Concert with such other person;
(ii) “affiliate” and “associate” shall have the meanings ascribed thereto in Rule 405 under the Securities Act of 1933, as amended; provided, however, that the term “partner” as used in the definition of “associate” shall not include any limited partner that is not involved in the management of the relevant partnership;
(iii) “Associated Person” shall mean with respect to any subject stockholder or other person (including any proposed nominee) (1) any person directly or indirectly controlling, controlled by or under common control with such stockholder or other person, (2) any beneficial owner of shares of stock of the Corporation owned of record or beneficially by such stockholder or other person, (3) any associate of such stockholder or other person, and (4) any person directly or indirectly controlling, controlled by or under common control or Acting in Concert with any such Associated Person;
(iv) “Proposing Person” shall mean (1) the Record Stockholder providing the notice of business proposed to be brought before an annual meeting or nomination of persons for election to the Board at a stockholder meeting, (2) the beneficial owner or beneficial owners, if different, on whose behalf the notice of business proposed to be brought before the annual meeting or nomination of persons for election to the Board at a stockholder meeting is made, and (3) any Associated Person on whose behalf the notice of business proposed to be brought before the annual meeting or nomination of persons for election to the Board at a stockholder meeting is made;
(vi) “Public Announcement” shall mean disclosure in a press release reported by a national news service or in a document publicly filed by the Corporation with the Securities and Exchange Commission pursuant to Section 13, 14 or 15(d) of the Exchange Act; and
(vii) to be considered a “Qualified Representative” of a stockholder, a person must be a duly authorized officer, manager, trustee or partner of such stockholder or must be authorized by a writing executed by such stockholder or an electronic transmission delivered by such stockholder to act for such stockholder as a proxy at the meeting of stockholders and such person must produce such writing or electronic transmission, or a reliable reproduction thereof, at the meeting. The Secretary of the Corporation, or any other person who shall be appointed to serve as secretary of the meeting, may require, on behalf of the Corporation, reasonable and appropriate documentation to verify the status of a person purporting to be a “Qualified Representative” for purposes hereof.
Article II: BOARD OF DIRECTORS
Section 2.1 Number; Qualifications. The total number of directors constituting the Board shall be fixed from time to time in the manner set forth in the Certificate of Incorporation. No decrease in the authorized number of directors constituting the Board shall shorten the term of any incumbent director. Directors need not be stockholders of the Corporation.
Section 2.2 Election; Resignation; Removal; Vacancies. Election of directors need not be by written ballot. Each director shall hold office until the annual meeting at which such director’s term expires and until such director’s successor is elected and qualified or until such director’s earlier death, resignation, disqualification or removal. Any director may resign by delivering a resignation in writing or by electronic transmission to the Corporation at its principal office or to the Chairperson of the Board, the Chief Executive Officer, or the Secretary. Such resignation shall be effective upon delivery unless it is specified to be effective at a later time or upon the happening of an event. Subject to the special rights of holders of any series of Preferred Stock to elect directors, directors may be removed only as provided by the Certificate of Incorporation and applicable law. All vacancies occurring in the Board and any newly created directorships resulting from any increase in the authorized number of directors shall be filled in the manner set forth in the Certificate of Incorporation.
10
Section 2.3 Regular Meetings. Regular meetings of the Board may be held at such places, within or without the State of Delaware, and at such times as the Board may from time to time determine. Notice of regular meetings need not be given if the date, times and places thereof are fixed by resolution of the Board.
Section 2.4 Special Meetings. Special meetings of the Board may be called by the Chairperson of the Board, the Chief Executive Officer, the Lead Independent Director or a majority of the members of the Board then in office and may be held at any time, date or place, within or without the State of Delaware, as the person or persons calling the meeting shall fix. Notice of the time, date and place of such meeting shall be given, orally, in writing or by electronic transmission (including electronic mail), by or at the direction of the person or persons calling the meeting to all directors at least four (4) days before the meeting if the notice is mailed, or at least twenty-four (24) hours before the meeting if such notice is given by telephone, hand delivery, telegram, telex, mailgram, facsimile, electronic mail or other means of electronic transmission; provided, however, that if, under the circumstances, the Chairperson of the Board, the Lead Independent Director or the Chief Executive Officer calling a special meeting deems that more immediate action is necessary or appropriate, notice may delivered on the day of such special meeting. Unless otherwise indicated in the notice, any and all business may be transacted at a special meeting.
Section 2.5 Remote Meetings Permitted. Members of the Board, or any committee of the Board, may participate in a meeting of the Board or such committee by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear each other, and participation in a meeting pursuant to conference telephone or other communications equipment shall constitute presence in person at such meeting.
Section 2.6 Quorum; Vote Required for Action. At all meetings of the Board, a majority of the Board shall constitute a quorum for the transaction of business. If a quorum shall fail to attend any meeting, a majority of those present may adjourn the meeting to another place, date or time. Except as otherwise provided herein or in the Certificate of Incorporation, or required by law, the vote of a majority of the directors present at a meeting at which a quorum is present shall be the act of the Board.
Section 2.7 Organization. Meetings of the Board shall be presided over by (a) the Chairperson of the Board, or (b) in such person’s absence, the Lead Independent Director, or (c) in such person’s absence, by the Chief Executive Officer, or (d) in such person’s absence, by a chairperson chosen by the Board at the meeting. The Secretary shall act as secretary of the meeting, but in such person’s absence the chairperson of the meeting may appoint any person to act as secretary of the meeting.
Section 2.8 Unanimous Action by Directors in Lieu of a Meeting. Any action required or permitted to be taken at any meeting of the Board, or of any committee thereof, may be taken without a meeting if all members of the Board or such committee, as the case may be, consent thereto in writing or by electronic transmission. After an action is take, the consent or consents shall be filed with the minutes of proceedings of the Board or committee, as applicable. Such filing shall be in paper form if the minutes are maintained in paper form and shall be in electronic form if the minutes are maintained in electronic form.
Section 2.9 Powers. Except as otherwise provided by the Certificate of Incorporation or the DGCL, the business and affairs of the Corporation shall be managed by or under the direction of the Board.
Section 2.10 Compensation of Directors. Members of the Board, as such, may receive, pursuant to a resolution of the Board, fees and other compensation for their services as directors, including without limitation their services as members of committees of the Board.
Section 2.11 Confidentiality. Each director shall maintain the confidentiality of, and shall not share with any third party person or entity (including third parties that originally sponsored, nominated or designated such director (the “Sponsoring Party”)), any non-public information learned in their capacities as directors, including communications among Board members in their capacities as directors. The Board may adopt a board confidentiality policy further implementing and interpreting this bylaw (a “Board Confidentiality Policy”). All directors are required to comply with this bylaw and any such Board Confidentiality Policy unless such director or the Sponsoring Party for such director has entered into a specific written agreement with the Corporation, in either case as approved by the Board, providing otherwise with respect to such confidential information.
11
Article III: COMMITTEES
Section 3.1 Committees. The Board may designate one or more committees, each committee to consist of one or more of the directors of the Corporation. The Board may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee. In the absence or disqualification of a member of the committee, the member or members thereof present at any meeting of such committee who are not disqualified from voting, whether or not such member or members constitute a quorum, may unanimously appoint another member of the Board to act at the meeting in place of any such absent or disqualified member. Any such committee, to the extent provided in a resolution of the Board, shall have and may exercise all the powers and authority of the Board in the management of the business and affairs of the Corporation and may authorize the seal of the Corporation to be affixed to all papers that may require it.; but no such committee shall have the power or authority in reference to the following matters: (a) approving, adopting or recommending to the stockholders any action or matter (other than the election or removal of members of the Board) expressly required by the DGCL to be submitted to stockholders for approval or (b) adopting, amending or repealing any bylaw of the Corporation.
Section 3.2 Committee Rules. Each committee shall keep records of its proceedings and make such reports as the Board may from time to time request. Unless the Board otherwise provides, each committee designated by the Board may make, alter and repeal rules for the conduct of its business. In the absence of such rules each committee shall conduct its business in the same manner as the Board conducts its business pursuant to Article II of these Bylaws. Except as otherwise provided in the Certificate of Incorporation, these Bylaws or the resolution of the Board designating the committee, any committee may create one or more subcommittees, each subcommittee to consist of one or more members of the committee, and may delegate to any such subcommittee any or all of the powers and authority of the committee.
Article IV: OFFICERS; Chairperson; Lead Independent Director
Section 4.1 Generally. The officers of the Corporation shall consist of a Chief Executive Officer (who may be the Chairperson of the Board or the President), a President, a Secretary and a Treasurer and may consist of such other officers, including, without limitation, an Executive Chairperson, Chief Financial Officer and one or more Vice Presidents, as may from time to time be appointed by the Board. All officers shall be elected by the Board; provided, however, that the Board may empower the Chief Executive Officer of the Corporation to appoint any officer other than the Executive Chairperson, Chief Executive Officer, the President, the Chief Financial Officer or the Treasurer. Except as otherwise provided by law, by the Certificate of Incorporation or these Bylaws, each officer shall hold office until such officer’s successor is duly elected and qualified or until such officer’s earlier resignation, death, disqualification or removal. Any number of offices may be held by the same person. Any officer may resign by delivering a resignation in writing or by electronic transmission to the Corporation at its principal office or to the Chairperson of the Board, the Chief Executive Officer or the Secretary. Such resignation shall be effective upon delivery unless it is specified to be effective at some later time or upon the happening of some later event. Any vacancy occurring in any office of the Corporation by death, resignation, removal or otherwise may be filled by the Board and the Board may, in its discretion, leave unfilled, for such period as it may determine, any offices. Each such successor shall hold office for the unexpired term of such officer’s predecessor and until a successor is duly elected and qualified or until such officer’s earlier resignation, death, disqualification or removal.
Section 4.2 Chief Executive Officer. Subject to the control of the Board and such supervisory powers, if any, as may be given by the Board, the powers and duties of the Chief Executive Officer of the Corporation are:
(a) to act as the general manager and, subject to the control of the Board, to have general supervision, direction and control of the business and affairs of the Corporation;
12
(b) to affix the signature of the Corporation to all deeds, conveyances, mortgages, guarantees, leases, obligations, bonds, certificates and other papers and instruments in writing which have been authorized by the Board or which, in the judgment of the Chief Executive Officer, should be executed on behalf of the Corporation;
(c) to sign certificates for shares of stock of the Corporation (if any); and
(d) subject to the direction of the Board, to have general charge of the property of the Corporation and to supervise and control all officers, agents and employees of the Corporation.
Section 4.3 Chairperson of the Board. Subject to the provisions of Section 2.7 of these Bylaws, the Chairperson of the Board shall have the power to preside at all meetings of the Board and shall have such other powers and duties as provided in these Bylaws and as the Board may from time to time prescribe. The Chairperson of the Board may or may not be an officer of the Corporation.
Section 4.4 Executive Chairperson. The Executive Chairperson shall have all powers and perform all duties commonly incident to such position, or which are, or from time to time may be, delegated to him or her by the Board. The powers and duties of the Executive Chairperson shall include, but will not necessarily be limited to: (i) consulting on the strategic vision and direction of the Corporation, (ii) developing, improving and maintaining the Corporation’s government relations and strategic alliances, (iii) improving the business climate for the Corporation around the world, (iv) enhancing relationships with the Corporation’s key customers, partners, investors and employees and (v) serving as a facilitator for communication between the officers of the Corporation and the Board. If the Board deems it proper to elect an Executive Chairperson, the office of the Executive Chairperson and the office of the Chairperson of the Board shall be filled by the same person.
Section 4.5 Lead Independent Director. The Board may, in its discretion, elect a lead independent director from among its members that are Independent Directors (as defined below) (such director, the “Lead Independent Director”). The Lead Independent Director shall preside at all meetings at which the Chairperson of the Board is not present and shall exercise such other powers and duties as may from time to time be assigned to such person by the Board or as prescribed by these Bylaws. For purposes of these Bylaws, “Independent Director” has the meaning ascribed to such term under the rules of the exchange upon which the Corporation’s common stock is primarily traded.
Section 4.6 President. The person holding the office of Chief Executive Officer shall be the President of the Corporation unless the Board shall have designated one individual as the President and a different individual as the Chief Executive Officer of the Corporation. Subject to the provisions of these Bylaws and to the direction of the Board, and subject to the supervisory powers of the Chief Executive Officer (if the Chief Executive Officer is an officer other than the President), and subject to such supervisory powers and authority as may be given by the Board to the Chairperson of the Board, and/or to any other officer, the President shall have the responsibility for the general management and control of the business and affairs of the Corporation and the general supervision and direction of all of the officers, employees and agents of the Corporation (other than the Chief Executive Officer, if the Chief Executive Officer is an officer other than the President) and shall perform all duties and have all powers that are commonly incident to the office of President or that are delegated to the President by the Board.
Section 4.7 Vice President. Each Vice President shall have all such powers and duties as are commonly incident to the office of Vice President or that are delegated to such person by the Board or the Chief Executive Officer. A Vice President may be designated by the Board to perform the duties and exercise the powers of the Chief Executive Officer or President in the event of the Chief Executive Officer’s or President’s absence or disability.
Section 4.8 Chief Financial Officer. The person holding the office of Chief Financial Officer shall be the Treasurer of the Corporation unless the Board shall have designated another officer as the Treasurer of the Corporation. Subject to the direction of the Board and the Chief Executive Officer, the Chief Financial Officer shall perform all duties and have all powers that are commonly incident to the office of Chief Financial Officer, or as the Board or Chief Executive Officer may from time to time prescribe.
Section 4.9 Treasurer. The person holding the office of Treasurer shall have custody of all monies and securities of the Corporation. The Treasurer shall make such disbursements of the funds of the Corporation as are authorized and shall render from time to time an account of all such transactions. The Treasurer shall also perform such other duties and have such other powers as are commonly incident to the office of Treasurer, or as the Board or the Chief Executive Officer may from time to time prescribe.
13
Section 4.10 Secretary. The Secretary shall issue or cause to be issued all authorized notices for, and shall keep, or cause to be kept, minutes of all meetings of the stockholders and the Board. The Secretary shall have charge of the corporate minute books and similar records and shall perform such other duties and have such other powers as are commonly incident to the office of Secretary, or as the Board or the Chief Executive Officer may from time to time prescribe.
Section 4.11 Delegation of Authority. The Board may from time to time delegate the powers or duties of any officer of the Corporation to any other officers or agents of the Corporation.
Section 4.12 Removal. Any officer of the Corporation shall serve at the pleasure of the Board and may be removed at any time, with or without cause, by the Board; provided, that if the Board has empowered the Chief Executive Officer to appoint any officer of the Corporation, then such officer may also be removed by the Chief Executive Officer. Such removal shall be without prejudice to the contractual rights of such officer, if any, with the Corporation.
Article V: STOCK
Section 5.1 Certificates; Uncertificated Shares. The shares of capital stock of the Corporation shall be uncertificated shares; provided, however, that the resolution of the Board that the shares of capital stock of the Corporation shall be uncertificated shares shall not apply to shares represented by a certificate until such certificate is surrendered to the Corporation (or the transfer agent or registrar, as the case may be). Notwithstanding the foregoing, the Board may provide by resolution or resolutions that some or all of any or all classes or series of its stock shall be certificated shares. Every holder of stock represented by certificates shall be entitled to have a certificate signed by, or in the name of the Corporation, by the Chairperson or Vice-Chairperson of the Board, the Chief Executive Officer or the President or a Vice President, and by the Treasurer or an Assistant Treasurer, or the Secretary or an Assistant Secretary, of the Corporation, representing the number of shares registered in certificate form. Any or all of the signatures on the certificate may be a facsimile. In case any officer, transfer agent or registrar who has signed or whose facsimile signature has been placed upon a certificate shall have ceased to be such officer, transfer agent or registrar before such certificate is issued, it may be issued by the Corporation with the same effect as if such person were an officer, transfer agent or registrar at the date of issue.
Section 5.2 Lost, Stolen or Destroyed Stock Certificates; Issuance of New Certificates or Uncertificated Shares. The Corporation may issue a new certificate of stock or uncertificated shares in the place of any certificate previously issued by it, alleged to have been lost, stolen or destroyed, upon the making of an affidavit of that fact by the person claiming the certificate of stock to be lost, stolen or destroyed, and the Corporation may require the owner of the lost, stolen or destroyed certificate, or such owner’s legal representative, to give the Corporation a bond sufficient to indemnify it, against any claim that may be made against it on account of the alleged loss, theft or destruction of any such certificate or the issuance of such new certificate or uncertificated shares.
Section 5.3 Other Regulations. Subject to applicable law, the Certificate of Incorporation and these Bylaws, the issue, transfer, conversion and registration of shares represented by certificates and of uncertificated shares shall be governed by such other regulations as the Board may establish.
14
Article VI: INDEMNIFICATION
Section 6.1 Indemnification of Officers and Directors. Each person who was or is made a party to, or is threatened to be made a party to, or is involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative, legislative, investigative, preliminary, informal or informal, or any other type whatsoever, including any arbitration or other alternative dispute resolution and including any appeal of the foregoing (a “Proceeding”), by reason of the fact that such person (or a person of whom such person is the legal representative), is or was a member of the Board or is or was an officer of the Corporation designated by the Board to be entitled to the indemnification and advancement rights set forth in this Article VI or, while serving in such capacity, is or was serving at the request of the Corporation as a director, officer, employee, agent or trustee of another corporation, or of a partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans (for purposes of this Article VI, an “Indemnitee”), shall be indemnified and held harmless by the Corporation to the fullest extent permitted by the DGCL as the same exists or may hereafter be amended (but, in the case of any such amendment, only to the extent that such amendment permits the Corporation to provide broader indemnification rights than such law permitted the Corporation to provide prior to such amendment), against all expenses, liability and loss (including attorneys’ fees, judgments, fines, ERISA excise taxes and penalties and amounts paid or to be paid in settlement) reasonably incurred or suffered by such Indemnitee in connection therewith, provided such Indemnitee acted in good faith and in a manner that the Indemnitee reasonably believed to be in or not opposed to the best interests of the Corporation, and, with respect to any criminal Proceeding, had no reasonable cause to believe the Indemnitee’s conduct was unlawful. Such indemnification shall continue as to an Indemnitee who has ceased to be a member of the Board or officer of the Corporation and shall inure to the benefit of such Indemnitees’ heirs, executors and administrators. Notwithstanding the foregoing, subject to Section 6.5 of these Bylaws, the Corporation shall indemnify any such Indemnitee seeking indemnity in connection with a Proceeding (or part thereof) initiated by such Indemnitee only if such Proceeding (or part thereof) was authorized by the Board or such indemnification is authorized by an agreement approved by the Board.
Section 6.2 Advance of Expenses. Except as otherwise provided in a written indemnification agreement between the Corporation and an Indemnitee, the Corporation shall pay all reasonable expenses (including attorneys’ fees) incurred by the Indemnitee in defending any Proceeding as they are incurred or otherwise in advance of its final disposition; provided, however, that if the DGCL then so requires, the advancement of such expenses (i.e., payment of such expenses as incurred or otherwise in advance of the final disposition of the Proceeding) shall be made only upon delivery to the Corporation of an undertaking, by or on behalf of such Indemnitee, to repay such amounts if it shall ultimately be determined by final judicial decision from which there is no appeal that such Indemnitee is not entitled to be indemnified under this Article VI or otherwise.
Section 6.3 Non-Exclusivity of Rights. The rights conferred on any person in this Article VI shall not be exclusive of any other right that such person may have or hereafter acquire under any statute, provision of the Certificate of Incorporation, Bylaws, agreement, vote or consent of stockholders or disinterested directors, or otherwise. Additionally, nothing in this Article VI shall limit the ability of the Corporation, in its discretion, to indemnify or advance expenses to persons whom the Corporation is not obligated to indemnify or advance expenses pursuant to this Article VI.
Section 6.4 Indemnification Contracts. The Board is, or as otherwise delegated by the Board to the officers of the Corporation, the officers are, authorized to cause the Corporation to enter into indemnification contracts with any member of the Board, officer, employee or agent of the Corporation, or any person serving at the request of the Corporation as a director, officer, employee, agent or trustee of another corporation, partnership, joint venture, trust or other enterprise, including employee benefit plans, providing indemnification or advancement rights to such person. Such rights may be greater than those provided in this Article VI.
Section 6.5 Right of Indemnitee to Bring Suit. The following shall apply to the extent not in conflict with any indemnification contract provided for in Section 6.4 of these Bylaws.
6.5.1 Right to Bring Suit. If a claim under Section 6.1 or Section 6.2 of these Bylaws is not paid in full by the Corporation within sixty (60) days after a written claim has been received by the Corporation, except in the case of a claim for an advancement of expenses, in which case the applicable period shall be twenty (20) days, the Indemnitee may at any time thereafter bring suit against the Corporation to recover the unpaid amount of the claim. If the Indemnitee is successful in whole or in part in any such suit, or in a suit brought by the Corporation to recover an advancement of expenses pursuant to the terms of an undertaking, the Indemnitee also shall be entitled to be paid, to the fullest extent permitted by law, the expense of prosecuting or defending such suit. In any suit brought by the Indemnitee to enforce a right to indemnification hereunder (but not in a suit brought by the Indemnitee to enforce a right to an advancement of expenses) it shall be a defense that the Indemnitee has not met any applicable standard for indemnification set forth in applicable law. In any suit brought by the Corporation to recover advancement of expenses pursuant to the terms of an undertaking, the Corporation shall be entitled to recover such expenses upon a final adjudication that the Indemnitee has not met any applicable standard for indemnification set forth in applicable law.
15
6.5.2 Effect of Determination. Neither the absence of a determination by or on behalf of the Corporation prior to the commencement of such suit that indemnification of the Indemnitee is proper in the circumstances because the Indemnitee has met the applicable standard of conduct set forth in applicable law, nor an actual determination by or on behalf of the Corporation that the Indemnitee has not met such applicable standard of conduct, shall create a presumption that the Indemnitee has not met the applicable standard of conduct or, in the case of such a suit brought by the Indemnitee, be a defense to such suit.
6.5.3 Burden of Proof. In any suit brought by the Indemnitee to enforce a right to indemnification or to an advancement of expenses hereunder, or brought by the Corporation to recover an advancement of expenses pursuant to the terms of an undertaking, the burden of proving that the Indemnitee is not entitled to be indemnified, or to such advancement of expenses, under this Article VI, or otherwise, shall be on the Corporation.
Section 6.6 Successful Defense. To the extent that an Indemnitee has been successful on the merits or otherwise in defense of any Proceeding (or in defense of any claim, issue or matter therein), such Indemnitee shall be indemnified under this Section 6.6 against expenses (including attorneys’ fees) actually and reasonably incurred in connection with such defense. Indemnification under this Section 6.6 shall not be subject to satisfaction of a standard of conduct, and the Corporation may not assert the failure to satisfy a standard of conduct as a basis to deny indemnification or recover amounts advanced, including in a suit brought pursuant to Section 6.5 of these Bylaws (notwithstanding anything to the contrary therein); provided, however, that, any Indemnitee who is not a current or former member of the Board or officer (as such term is defined in the final sentence of Section 145(c)(1) of the DGCL) shall be entitled to indemnification under Section 6.1 of these Bylaws and this Section 6.6 only if such Indemnitee has satisfied the standard of conduct required for indemnification under Section 145(a) or Section 145(b) of the DGCL.
Section 6.7 Nature of Rights. The rights conferred upon Indemnitees in this Article VI shall be contract rights and such rights shall continue as to an Indemnitee who has ceased to be a member of the Board, officer, employee or agent and shall inure to the benefit of the Indemnitee’s heirs, executors and administrators. Any amendment, repeal or modification of any provision of this Article VI that adversely affects any right of an Indemnitee or an Indemnitee’s successors shall be prospective only, and shall not adversely affect any right or protection conferred on a person pursuant to this Article VI with respect to any Proceeding involving any occurrence or alleged occurrence of any action or omission to act that took place prior to such amendment, repeal or modification.
Section 6.8 Insurance. The Corporation may purchase and maintain insurance, at its expense, to protect itself and any member of the Board, officer, employee or agent of the Corporation or another corporation, partnership, joint venture, trust or other enterprise against any expense, liability or loss, whether or not the Corporation would have the power to indemnify such person against such expense, liability or loss under the DGCL.
Article VII: NOTICES
Section 7.1 Notice.
7.1.1 Form and Delivery. Except as otherwise specifically required by law, notice may be given in writing directed to a stockholders’ mailing address as it appears on the records of the Corporation and shall be given: (a) if mailed, when notice is deposited in the U.S. mail, postage prepaid; and (b) if delivered by courier service, the earlier of when the notice is received or left at such stockholder’s address. So long as the Corporation is subject to the Securities and Exchange Commission’s proxy rules set forth in Regulation 14A under the Exchange Act, notice shall be given in the manner required by such rules. To the extent permitted by such rules, or if the Corporation is not subject to Regulation 14A, notice may be given by electronic transmission directed to the stockholder’s electronic mail address, and if so given, shall be given when directed to such stockholder’s electronic mail address unless the stockholder has notified the Corporation in writing or by electronic transmission of an objection to receiving notice by electronic mail or such notice is prohibited by Section 232(e) of the DGCL. If notice is given by electronic mail, such notice shall comply with the applicable provisions of Sections 232(a) and 232(d) of the DGCL. Notice may be given by other forms of electronic transmission with the consent of a stockholder in the manner permitted by Section 232(b) of the DGCL and shall be deemed given as provided therein.
16
7.1.2 Affidavit of Giving Notice. An affidavit of the Secretary or an Assistant Secretary or of the transfer agent or other agent of the Corporation that the notice has been given in writing or by a form of electronic transmission shall, in the absence of fraud, be prima facie evidence of the facts stated therein.
Section 7.2 Waiver of Notice. Whenever notice is required to be given under any provision of the DGCL, the Certificate of Incorporation or these Bylaws, a written waiver of notice, signed by the person entitled to notice, or waiver by electronic transmission by such person, whether before or after the time stated therein, shall be deemed equivalent to notice. Attendance of a person at a meeting shall constitute a waiver of notice of such meeting, except when the person attends a meeting for the express purpose of objecting at the beginning of the meeting to the transaction of any business because the meeting is not lawfully called or convened. Neither the business to be transacted at, nor the purpose of, any regular or special meeting of the stockholders, directors or members of a committee of directors need be specified in any waiver of notice.
Article VIII: INTERESTED DIRECTORS
Section 8.1 Interested Directors. No contract or transaction between the Corporation and one or more of its members of the Board or officers, or between the Corporation and any other corporation, partnership, association or other organization in which one or more of its directors or officers are members of the board of directors or officers, or have a financial interest, shall be void or voidable solely for this reason, or solely because the director or officer is present at or participates in the meeting of the Board or committee thereof that authorizes the contract or transaction, or solely because such director’s or officer’s votes are counted for such purpose, if: (a) the material facts as to such director’s or officer’s relationship or interest and as to the contract or transaction are disclosed or are known to the Board or the committee, and the Board or committee in good faith authorizes the contract or transaction by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors be less than a quorum; (b) the material facts as to such director’s or officer’s relationship or interest and as to the contract or transaction are disclosed or are known to the stockholders entitled to vote thereon, and the contract or transaction is specifically approved in good faith by vote of the stockholders; or (c) the contract or transaction is fair as to the Corporation as of the time it is authorized, approved or ratified by the Board, a committee thereof, or the stockholders.
Section 8.2 Quorum. Interested directors may be counted in determining the presence of a quorum at a meeting of the Board or of a committee which authorizes a contract or transaction described in Section 8.1 of these Bylaws.
Article IX: MISCELLANEOUS
Section 9.1 Fiscal Year. The fiscal year of the Corporation shall be determined by resolution of the Board.
Section 9.2 Seal. The Board may provide for a corporate seal, which may have the name of the Corporation inscribed thereon and shall otherwise be in such form as may be approved from time to time by the Board.
Section 9.3 Form of Records. Any records administered by or on behalf of the Corporation in the regular course of its business, including its stock ledger, books of account and minute books, may be kept on or by means of, or be in the form of any other information storage device, method, or one or more electronic networks or databases (including one or more distributed electronic networks or databases) electronic or otherwise, provided that the records so kept can be converted into clearly legible paper form within a reasonable time and otherwise comply with the DGCL. The Corporation shall so convert any records so kept upon the request of any person entitled to inspect such records pursuant to any provision of the DGCL.
Section 9.4 Reliance Upon Books and Records. A member of the Board, or a member of any committee designated by the Board shall, in the performance of such person’s duties, be fully protected in relying in good faith upon the books and records of the Corporation and upon such information, opinions, reports or statements presented to the Corporation by any of the Corporation’s officers or employees, or committees of the Board, or by any other person as to matters the member reasonably believes are within such other person’s professional or expert competence and who has been selected with reasonable care by or on behalf of the Corporation.
17
Section 9.5 Certificate of Incorporation Governs. In the event of any conflict between the provisions of the Certificate of Incorporation and Bylaws, the provisions of the Certificate of Incorporation shall govern.
Section 9.6 Severability. If any provision of these Bylaws shall be held to be invalid, illegal, unenforceable or in conflict with the provisions of the Certificate of Incorporation, then such provision shall nonetheless be enforced to the maximum extent possible consistent with such holding and the remaining provisions of these Bylaws (including without limitation, all portions of any section of these Bylaws containing any such provision held to be invalid, illegal, unenforceable or in conflict with the Certificate of Incorporation, that are not themselves invalid, illegal, unenforceable or in conflict with the Certificate of Incorporation) shall remain in full force and effect.
Section 9.7 Time Periods. In applying any provision of these Bylaws which requires that an act be done or not be done a specified number of days prior to an event or that an act be done during a period of a specified number of days prior to an event, calendar days shall be used (unless otherwise specified herein), the day of the doing of the act shall be excluded, and the day of the event shall be included.
Article X: AMENDMENT
Notwithstanding any other provision of these Bylaws, any alteration, amendment or repeal of these Bylaws, and any adoption of new Bylaws, shall require the approval of the Board or the stockholders of the Corporation as expressly provided in the Certificate of Incorporation.
* * * * *
18
CERTIFICATION
OF Amended and RESTATED BYLAWS
OF
Adaptin Bio, Inc.
(a Delaware corporation)
I, Nathan Pereira, certify that I am Secretary of Adaptin Bio, Inc., a Delaware corporation (the “Corporation”), that I am duly authorized to make and deliver this certification, and that the attached Bylaws are a true and complete copy of the Amended and Restated Bylaws of the Corporation in effect as of the date of this certificate.
| Dated: February 11, 2025 | /s/ Nathan Pereira |
| Secretary |
19
Exhibit 4.1
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”). THESE SECURITIES MAY BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED ONLY (A) TO THE COMPANY, (B) IN COMPLIANCE WITH RULE 144 UNDER THE SECURITIES ACT, IF AVAILABLE, AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS, (C) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT, OR (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, AND THE HOLDER HAS, PRIOR TO SUCH SALE, FURNISHED TO THE COMPANY AN OPINION OF COUNSEL OR OTHER EVIDENCE OF EXEMPTION, IN EITHER CASE REASONABLY SATISFACTORY TO THE COMPANY. HEDGING TRANSACTIONS INVOLVING THESE SECURITIES MAY NOT BE CONDUCTED UNLESS IN COMPLIANCE WITH THE SECURITIES ACT.
FORM OF COMMON STOCK PURCHASE WARRANT
ADAPTIN BIO, INC.
| Warrant No. _____ | Issue Date: _______ __, 202__ |
THIS COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, ________________________________________ (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the Initial Exercise Date (as defined below) and on or prior to the close of business on the fifth (5th) anniversary of the Initial Exercise Date (the “Termination Date”) but not thereafter, to subscribe for and purchase from Adaptin Bio, Inc., formerly known as Centaur Bio Inc., a Delaware corporation, or its Parent (as defined in the Notes issued to the initial Holder of this Warrant under the Note Exchange Agreement (the “Notes”) (collectively, the “Company”), up to a number shares of Common Stock (the “Warrant Shares”) equal to fifty percent (50%) of the number of shares of Common Stock ( or of the number of shares of Common Stock issuable upon exercise or conversion of Common Stock Equivalents sold in the Qualified Offering) that the principal amount of the Notes would purchase at the Qualified Offering Price. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1. Definitions. For the purposes hereof, in addition to the terms defined elsewhere in this Warrant, (a) capitalized terms not otherwise defined herein shall have the meanings set forth in the Purchase Agreement and (b) the following terms shall have the following meanings:
“Affiliate” means, with respect to the Company, any entity that directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, the Company.
“Business Day” means any day except any Saturday, any Sunday, any day which shall be a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
”Control” means the ownership, directly or indirectly, in the aggregate of more than fifty percent (50%) of the beneficial ownership interests of an entity and the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of an entity, whether through the ability to exercise voting power, by contract or otherwise.
“Common Stock” means shares of the Offering Stock.
”Exchange Notes” means the 10% Secured Subordinated Convertible Promissory Notes issued by the Company pursuant to the terms of the Note Exchange Agreement, dated as of ________________, 202__.
“Fair Market Value” of one share of Common Stock as of a particular date shall mean: (i) if traded on a National Securities Exchange or quoted on an over the counter market operated by OTC Markets Group, Inc., or its successor, the closing price of the Common Stock on such exchange or market on the applicable date of valuation; and (ii) if (i) does not apply, the Fair Market Value shall be the value thereof, as agreed upon by the Company and the Holder; provided, however, that if the Company and the Holder cannot agree on such value, such value shall be determined by an independent valuation firm experienced in valuing businesses such as the Company and jointly selected in good faith by the Company and the Holder. Fees and expenses of the valuation firm shall be paid for by the Company.
“Initial Exercise Date” means the closing date of a Qualified Offering.
“National Securities Exchange” means the following markets or exchanges on which the Common Stock may be listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange or the NYSE American, LLC.
“Note Exchange Agreement” means the Note Exchange Agreement, dated as of _____________, 202___, by and between the Company and the Holder.
“Purchase Agreement” means, collectively, the Securities Purchase Agreement, dated as of _______________, 2023, between the Company and the original Holder, as amended, modified or supplemented from time to time in accordance with its terms.
“Qualified Offering” means the first offering (public or private) by the Company or its Parent after November 1, 2024 of the Company’s or its Parent’s Offering Stock (as defined in the Notes) with gross proceeds to the Company or its Parent (before underwriter, placement agent, or broker discounts and commissions and expenses of the offering) of $5,000,000 or more. For purposes of calculating such gross proceeds, the principal amount of, together with all accrued but unpaid interest on, all indebtedness converted into Common Stock will be counted.
-2-
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Trading Day” means a day on which the New York Stock Exchange is open for business.
“Trading Market” means the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the NYSE American LLC, the OTCQB, the OTCQX or the OTC Pink (or any successor of the foregoing).
“Transaction Documents” shall have the meaning set forth in the Purchase Agreement.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a National Securities Exchange, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. New York City time to 4:02 p.m. New York City time); (b) if prices for the Common Stock are reported on the OTC markets, including the OTCQX, OTCQB and OTC Pink markets, or in the “Pink Sheets” published by Pink Sheets, LLC (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported; or (c) in all other cases, the Fair Market Value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Subscribers of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company; provided that in each case where Bloomberg L.P. data is being relied upon, Holder shall provide to the Company a copy of such information for the Company’s records.
Section 2. Exercise.
(a) Exercise of Warrant.
i. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed notice of exercise (“Notice of Exercise”) form attached hereto as Exhibit A; and, within two (2) Trading Days of the date said Notice of Exercise is delivered to the Company, the Company shall have received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer or cashier’s check drawn on a United States bank. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. In the event of any dispute or discrepancy, the records of the Company shall be controlling and determinative in the absence of manifest error.
-3-
ii. If at any time from the Initial Exercise Date, provided that there is no effective registration statement registering, or no current prospectus available for the resale of the Warrant Shares by the Holder, then in lieu of the payment methods set forth in Section 2(a)(i) above, the Holder may elect to exchange all or some of this Warrant for shares of Common Stock equal to the value of the amount of the Warrant being exchanged on the date of exchange. If Holder elects to exchange this Warrant as provided in this Section 2(a)(ii), Holder shall tender to the Company the Warrant for the amount being exchanged, along with written notice of Holder’s election to exchange some or all of the Warrant, and the Company shall issue to Holder the number of shares of the Common Stock computed using the following formula:
| X = | Y (A-B) | |
| A |
| Where: | X = | the number of shares of Common Stock to be issued to Holder. | |
| Y = | the number of shares of Common Stock purchasable under the amount of the Warrant being exchanged (as adjusted to the date of such calculation). | ||
| A = | the Fair Market Value of one share of the Common Stock on the date prior to the date that the notice of exercise is received by the Company. | ||
| B = | Exercise Price (as adjusted to the date of such calculation). | ||
Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant to this Section 2(a)(ii).
(b) Exercise Price. The exercise price per share of Common Stock under this Warrant shall be equal to the Qualified Offering Price, subject to adjustment as described herein (the “Exercise Price”).
-4-
(c) Exercise Limitations. The Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, if at the time of such exercise the Common Stock is registered pursuant to section 12 of the Exchange Act, and to the extent that after giving effect to such issuance after exercise, the Holder (together with the Holder’s affiliates, and any other person or entity acting as a group together with the Holder or any of the Holder’s affiliates), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of this Section, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. Holder is solely responsible for any schedules required to be filed in accordance therewith. The Company shall have no obligation to verify or confirm the accuracy of such filings. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its affiliates since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of Warrant Shares issuable upon exercise of this Warrant. The Holder may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(c), provided that any such increase shall not be effective until 61 days’ after the Holder gives notice of such increase to the Company, and the provisions of this Section 2(c) shall continue to apply, unless the Holder upon not less than 61 days’ prior notice to the Company determines to waive the Beneficial Ownership Limitation requirements described in this Section 2(c) in its entirety. Any such increase will not be effective until the 61st day after such notice is delivered to the Company. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
(d) Mechanics of Exercise.
i. Delivery of Certificates Upon Exercise. Certificates for shares purchased hereunder shall be transmitted by the Company’s transfer agent (the “Transfer Agent”) to the Holder by crediting the account of the Holder’s prime broker with the Depository Trust Company through its Deposit Withdrawal Agent Commission (“DWAC”) system if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the resale of the Warrant Shares by the Holder or (B) the shares are eligible for resale without volume or manner-of-sale limitations pursuant to Rule 144, and otherwise by physical delivery of certificates to the address specified by the Holder in the Notice of Exercise within two (2) Trading Days from the delivery to the Company of the Notice of Exercise Form, surrender of this Warrant (if required) and payment of the aggregate Exercise Price as set forth above (the “Warrant Share Delivery Date”). This Warrant shall be deemed to have been exercised on the date the Exercise Price is received by the Company. The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised by payment to the Company of the Exercise Price (except in the case of a cashless exercise pursuant to Section 2(a)(ii)) and all taxes required to be paid by the Holder, if any, pursuant to Section 2(d)(v) prior to the issuance of such shares, have been paid.
-5-
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to Holder a new Warrant evidencing the rights of Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder a certificate or the certificates representing the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Obligation Absolute; Partial Liquidated Damages. The Company’s obligations to issue and deliver the Warrant Shares upon exercise of this Warrant in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by the Holder or any other person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other person, and irrespective of any other circumstance which might otherwise limit such obligation of the Company to the Holder in connection with the issuance of such Warrant Shares; provided, however, that such delivery shall not operate as a waiver by the Company of any such action the Company may have against the Holder. The Company may not refuse exercise based on any claim that the Holder or anyone associated or affiliated with the Holder has been engaged in any violation of law, agreement or for any other reason, unless an injunction from a court, on notice to Holder, restraining and or enjoining exercise of all or part of this Warrant shall have been sought and obtained, and the Company posts a surety bond for the benefit of the Holder in the amount of 130% of the Fair Market Value of the Warrant Shares issuable upon exercise of this Warrant, which is subject to the injunction, which bond shall remain in effect until the completion of arbitration/litigation of the underlying dispute and the proceeds of which shall be payable to the Holder to the extent it obtains judgment. In the absence of such injunction, the Company shall issue Warrant Shares. If the Company fails for any reason to deliver to the Holder such certificate or certificates pursuant to Section 2(d)(i) by the third (3rd) Trading Day after the Conversion Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Fair Market Value of Warrant Shares being exercised, $10 per Trading Day for each Trading Day after such third (3rd) Trading Day until such certificates are delivered. Nothing herein shall limit a Holder’s right to pursue actual damages or declare an Event of Default under the Exchange Note for the Company’s failure to deliver Warrant Shares within the period specified herein and the Holder shall have the right to pursue all remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
-6-
v. Compensation for Buy-In on Failure to Timely Deliver Certificates upon Conversion. In addition to any other rights available to the Holder, if the Company fails for any reason to deliver to the Holder such certificate or certificates by the Share Delivery Date, and if after such Share Delivery Date the Holder is required by its brokerage firm to purchase (in an open market transaction or otherwise), or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder was entitled to receive upon the exercise relating to such Share Delivery Date (a “Buy-In”), then the Company shall (A) pay in cash to the Holder (in addition to any other remedies available to or elected by the Holder), if any, the amount by which (x) the Holder’s total purchase price (including any brokerage commissions) for the Common Stock so purchased exceeds (y) the product of (1) the aggregate number of shares of Common Stock that the Holder was entitled to receive from the exercise at issue multiplied by (2) the actual per share sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of the Holder, either reissue (if surrendered) this Warrant for a number of Warrant Shares equal to the number of Warrant Shares of the attempted exercise (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued if the Company had timely complied with its delivery requirements under Section 2(d)(i). For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of this Warrant with respect to which the actual sale price of the Warrant Shares (including any brokerage commissions) giving rise to such purchase obligation was a total of $10,000 under clause (A) of the immediately preceding sentence, the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock upon exercise of this Warrant as required pursuant to the terms hereof.
vi. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
-7-
vii. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, all of which taxes and expenses shall be paid by the Company, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the assignment form (“Assignment Form”) attached hereto as Exhibit B duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto.
viii. Closing of Books. The Company will not close its shareholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
Section 3. [Intentionally Omitted.]
Section 4. Certain Adjustments.
(a) Stock Dividends and Splits. If, at any time after the Initial Exercise Date until the Termination Date, the Company: (i) pays a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any Warrant Shares issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 4(a) shall become effective immediately after the record date for the determination of shareholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
-8-
(b) Subsequent Equity Sales. If, at any time after the Initial Exercise Date while this Warrant is outstanding, the Company sells or grants any option to purchase or sells or grants any right to reprice, or otherwise disposes of or issues (or announces any sale, grant or any option to purchase or other disposition), any Common Stock or Common Stock Equivalents at an effective price per share that is lower than the then Exercise Price (such issuances, collectively, a “Dilutive Issuance”) (if the holder of the Common Stock or Common Stock Equivalents so issued shall at any time, whether by operation of purchase price adjustments, reset provisions, floating conversion, exercise or exchange prices or otherwise, or due to warrants, options or rights per share which are issued in connection with such issuance, be entitled to receive shares of common stock at an effective price per share that is lower than the Exercise Price, such issuance shall be deemed to have occurred for less than the Exercise Price on such date of the Dilutive Issuance), then immediately upon such issuance or sale (or deemed issuance or sale) the Exercise Price in effect immediately prior to such issuance or sale (or deemed issuance or sale) shall be reduced (and in no event increased) to an Exercise Price equal to the quotient obtained by dividing:
(A) the sum of (1) the product obtained by multiplying the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) by the Exercise Price then in effect plus (2) the aggregate consideration, if any, received by the Company upon such issuance or sale (or deemed issuance or sale); by
(B) the sum of (1) the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) plus (2) the aggregate number of shares of Common Stock issued or sold (or deemed issued or sold) by the Company in such issuance or sale (or deemed issuance or sale).
“Common Stock Deemed Outstanding” means, at any given time, the sum of (a) the number of shares of Common Stock actually outstanding at such time, plus (b) the number of shares of Common Stock issuable upon exercise of any warrants or other rights or options to subscribe for or purchase Common Stock and conversion or exchange of any securities (directly or indirectly) convertible into or exchangeable for Common Stock, in each case actually outstanding at such time (treating as actually outstanding any such warrants, rights, options or other securities issuable upon exercise of other such securities actually outstanding at such time), in each case, regardless of whether such securities are actually exercisable, convertible or exchangeable at such time; provided, that Common Stock Deemed Outstanding at any given time shall not include shares owned or held by or for the account of the Company or any of its wholly-owned subsidiaries.
Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. Notwithstanding the foregoing, no adjustment will be made under this Section 4(b) in respect of an Exempt Issuance (as defined below).
The Company shall notify the Holder in writing, no later than one (1) Business Day following the issuance of any Common Stock or Common Stock Equivalents subject to this Section 4(b), indicating therein the applicable issuance price, or applicable reset price, exchange price, conversion price and other pricing terms (such notice, the “Dilutive Issuance Notice”). For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 4(b), upon the occurrence of any Dilutive Issuance, the Holder is entitled to receive a number of Conversion Shares based upon the new Conversion Price provided above on or after the date of such Dilutive Issuance, regardless of whether the Holder accurately refers to the new Conversion Price in the Notice of Conversion.
-9-
“Exempt Issuance” means the issuance of (a) shares of Common Stock, restricted stock units or options, and the underlying shares of Common Stock to consultants, employees, officers or directors of the Company pursuant to any stock or option plan duly adopted for such purpose, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose for services rendered to the Company, (b) securities issued upon the exercise or exchange of or conversion of any securities issued hereunder and/or other securities issuable pursuant to existing agreements, exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of this Warrant and disclosed in the Purchase Agreement, provided that such securities have not been amended since the date of this Warrant to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock dividends, stock splits or combinations) or to extend the term of such securities, (c) securities issued pursuant, acquisitions or strategic transactions approved by a majority of the directors of the Company, provided that any such issuance shall only be to a Person (or to the equity holders of a Person) which is, itself or through its Subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of the Company and which shall reasonably be expected to provide to the Company additional benefits, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities, (d) securities issued pursuant to any purchase money equipment loan or capital leasing arrangement, purchasing agent or debt financing from a commercial bank or similar financial institution, (e) securities issued pursuant to any presently outstanding warrants disclosed in the Purchase Agreement or this Warrant, and (f) securities upon a stock split, stock dividend or subdivision of the Common Stock and shares of common stock in a public offering.
(c) Pro Rata Distributions. If, at any time after the Initial Exercise Date until the Termination Date, the Company shall distribute to all holders of Common Stock (and not to the Holder) evidences of its indebtedness or assets (including cash and cash dividends) or rights or warrants to subscribe for or purchase any security other than the Common Stock, then in each such case the Exercise Price shall be adjusted by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the VWAP determined as of the record date mentioned above, and of which the numerator shall be such VWAP on such record date less than the per share Fair Market Value at such record date of the portion of such assets or evidence of indebtedness or rights or warrants so distributed applicable to one outstanding share of the Common Stock as determined by the Board of Directors in good faith. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
-10-
(d) Fundamental Transaction. If, at any time prior to the Termination Date, (i) the Company effects any merger or consolidation of the Company into another Person, (ii) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to tender or exchange their shares for other securities, cash or property or (iv) the Company effects any reclassification of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property (each “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction. For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. To the extent necessary to effectuate the foregoing provisions, any successor to the Company or surviving entity in such Fundamental Transaction shall issue to the Holder a new warrant consistent with the foregoing provisions and evidencing the Holder’s right to exercise such warrant into Alternate Consideration. The terms of any agreement pursuant to which a Fundamental Transaction is effected shall include terms requiring any such successor or surviving entity to comply with the provisions of this Section 4(c) and insuring that this Warrant (or any such replacement security) will be similarly adjusted upon any subsequent transaction analogous to a Fundamental Transaction.
(e) Subsequent Rights Offerings. In addition to any adjustments pursuant to this Section 4, if at any time prior to the Termination Date the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to all of the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation.
-11-
(f) Calculations. All calculations under this Section 4 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 4, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
(g) Notice to Holder.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 4, the Company shall promptly mail to the Holder a notice setting forth the Exercise Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any shareholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, of any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. The Holder is entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice.
-12-
Section 5. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws and the conditions set forth in Section 5(d) herein and to the provisions of the Purchase Agreement, this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with an Assignment Form duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. The Warrant, if properly assigned, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 5(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the Initial Exercise Date and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
d) Transfer Restrictions. If, at the time of the surrender of this Warrant in connection with any transfer of this Warrant, the transfer of this Warrant shall not be either (i) registered pursuant to an effective registration statement under the Securities Act and under applicable state securities or blue sky laws or (ii) eligible for resale without volume or manner-of-sale restrictions pursuant to Rule 144, the Company may require, as a condition of allowing such transfer, that the Holder or transferee of this Warrant, as the case may be, comply with the provisions of the Purchase Agreement.
-13-
Section 6. Miscellaneous.
a) No Rights as Shareholder Until Exercise. This Warrant does not entitle the Holder to any voting rights or other rights as a shareholder of the Company prior to the exercise hereof.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then, such action may be taken or such right may be exercised on the next succeeding Business Day.
d) Authorized Shares.
The Company covenants that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock one hundred (100%) of the number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. In case such amount of Common Stock is insufficient at any time, the Company shall call and hold a special meeting to increase the number of authorized shares of common stock. Management of the Company shall recommend to shareholders to vote in favor of increasing the number of authorized shares of common stock.
The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the National Securities Exchange upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation, as amended, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
-14-
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Purchase Agreement.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice Holder’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date. If the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be delivered in accordance with the notice provisions of the Purchase Agreement.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of Holder, shall give rise to any liability of Holder for the purchase price of any Common Stock or as a shareholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of all Holders from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company and the Holder.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
[Signature Page Follows.]
-15-
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| ADAPTIN BIO, INC. | |||
| By: | |||
| Name: | Michael J. Roberts | ||
| Title: | Chief Executive Officer | ||
[SIGNATURE PAGE TO ADAPTIN BIO WARRANT]
EXHIBIT A
NOTICE OF EXERCISE
TO: ADAPTIN BIO, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant and
☐ tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any; or
☐ elects to purchase the number of Warrant Shares stated above pursuant to the cashless exercise procedure set forth in Section 2(a) of the Warrant
(2) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number or by physical delivery of a certificate to:
_______________________________
_______________________________
_______________________________
(3) Accredited Investor. The undersigned is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended.
[SIGNATURE OF HOLDER]
Name of Investing Entity:
________________________________________________________________________
Signature of Authorized Signatory of Investing Entity:
_________________________________________________
Name of Authorized Signatory:
___________________________________________________________________
Title of Authorized Signatory:
____________________________________________________________________
Date: _______________________________________________________________
A-1
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, [____] all of or [_______] shares of the foregoing Warrant and all rights evidenced thereby are hereby assigned to
_______________________________________________ whose address is
____________________________________________________________.
_______________________________________________________________
Dated: ______________, _______
Holder’s Signature: _____________________________
Holder’s Address: ______________________________
_______________________________
Signature Guaranteed: ___________________________________________
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
B-1
Exhibit 4.2
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”). THESE SECURITIES MAY BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED ONLY (A) TO THE COMPANY, (B) IN COMPLIANCE WITH RULE 144 UNDER THE SECURITIES ACT, IF AVAILABLE, AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS, (C) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT, OR (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, AND THE HOLDER HAS, PRIOR TO SUCH SALE, FURNISHED TO THE COMPANY AN OPINION OF COUNSEL OR OTHER EVIDENCE OF EXEMPTION, IN EITHER CASE REASONABLY SATISFACTORY TO THE COMPANY. HEDGING TRANSACTIONS INVOLVING THESE SECURITIES MAY NOT BE CONDUCTED UNLESS IN COMPLIANCE WITH THE SECURITIES ACT.
FORM OF COMMON STOCK PURCHASE WARRANT
ADAPTIN BIO, INC.
| Warrant No. _____ | Issue Date: _______ __, 202__ |
THIS COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, ________________________________________ (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the Initial Exercise Date (as defined below) and on or prior to the close of business on the fifth (5th) anniversary of the Initial Exercise Date (the “Termination Date”) but not thereafter, to subscribe for and purchase from Adaptin Bio, Inc., formerly known as Centaur Bio Inc., a Delaware corporation, or its Parent (as defined in the the Notes issued to the initial Holder of this Warrant under the Note Exchange Agreement (the “Notes”)) (collectively, the “Company”), up to a number shares of Common Stock (the “Warrant Shares”) equal to fifty percent (50%) of the number of shares of Common Stock (or of the number of shares of Common Stock issuable upon exercise or conversion of Common Stock Equivalents sold in the Qualified Offering) that the principal amount of the Notes would purchase at the Qualified Offering Price. The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1. Definitions. For the purposes hereof, in addition to the terms defined elsewhere in this Warrant, (a) capitalized terms not otherwise defined herein shall have the meanings set forth in the Purchase Agreement and (b) the following terms shall have the following meanings:
“Affiliate” means, with respect to the Company, any entity that directly or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, the Company.
“Business Day” means any day except any Saturday, any Sunday, any day which shall be a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Control” means the ownership, directly or indirectly, in the aggregate of more than fifty percent (50%) of the beneficial ownership interests of an entity and the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of an entity, whether through the ability to exercise voting power, by contract or otherwise.
“Common Stock” means shares of the Offering Stock.
“Fair Market Value” of one share of Common Stock as of a particular date shall mean: (i) if traded on a National Securities Exchange or quoted on an over the counter market operated by OTC Markets Group, Inc., or its successor, the closing price of the Common Stock on such exchange or market on the applicable date of valuation; and (ii) if (i) does not apply, the Fair Market Value shall be the value thereof, as agreed upon by the Company and the Holder; provided, however, that if the Company and the Holder cannot agree on such value, such value shall be determined by an independent valuation firm experienced in valuing businesses such as the Company and jointly selected in good faith by the Company and the Holder. Fees and expenses of the valuation firm shall be paid for by the Company.
“Initial Exercise Date” means the closing date of a Qualified Offering.
“National Securities Exchange” means the following markets or exchanges on which the Common Stock may be listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange or the NYSE American, LLC.
“Note Exchange Agreement” means the Note Exchange Agreement, dated as of _____________, 202___, between the Company and the original Holder, as amended, modified or supplemented from time to time in accordance with its terms.
“Purchase Agreement” means, collectively, the Securities Purchase Agreement, dated as of _______________, 2023, between the Company and the original Holder, as amended, modified or supplemented from time to time in accordance with its terms.
“Qualified Offering” means the first offering (public or private) by the Company or its Parent after November 1, 2024 of the Company’s or its Parent’s Offering Stock (as defined in the Notes) with gross proceeds to the Company or its Parent (before underwriter, placement agent, or broker discounts and commissions and expenses of the offering) of $5,000,000 or more. For purposes of calculating such gross proceeds, the principal amount of, together with all accrued but unpaid interest on, all indebtedness converted into Common Stock will be counted.
-2-
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Trading Day” means a day on which the New York Stock Exchange is open for business.
“Trading Market” means the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the NYSE American LLC, the OTCQB, the OTCQX or the OTC Pink (or any successor of the foregoing).
“Transaction Documents” shall have the meaning set forth in the Purchase Agreement.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a National Securities Exchange, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. New York City time to 4:02 p.m. New York City time); (b) if prices for the Common Stock are reported on the OTC markets, including the OTCQX, OTCQB and OTC Pink markets, or in the “Pink Sheets” published by Pink Sheets, LLC (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported; or (c) in all other cases, the Fair Market Value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Subscribers of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company; provided that in each case where Bloomberg L.P. data is being relied upon, Holder shall provide to the Company a copy of such information for the Company’s records.
Section 2. Exercise.
(a) Exercise of Warrant.
i. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed notice of exercise (“Notice of Exercise”) form attached hereto as Exhibit A; and, within two (2) Trading Days of the date said Notice of Exercise is delivered to the Company, the Company shall have received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer or cashier’s check drawn on a United States bank. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. In the event of any dispute or discrepancy, the records of the Company shall be controlling and determinative in the absence of manifest error.
-3-
ii. If at any time from the Initial Exercise Date, provided that there is no effective registration statement registering, or no current prospectus available for the resale of the Warrant Shares by the Holder, then in lieu of the payment methods set forth in Section 2(a)(i) above, the Holder may elect to exchange all or some of this Warrant for shares of Common Stock equal to the value of the amount of the Warrant being exchanged on the date of exchange. If Holder elects to exchange this Warrant as provided in this Section 2(a)(ii), Holder shall tender to the Company the Warrant for the amount being exchanged, along with written notice of Holder’s election to exchange some or all of the Warrant, and the Company shall issue to Holder the number of shares of the Common Stock computed using the following formula:
| X = | Y (A-B) | |
| A |
| Where: | X = | the number of shares of Common Stock to be issued to Holder. | |
| Y = | the number of shares of Common Stock purchasable under the amount of the Warrant being exchanged (as adjusted to the date of such calculation). | ||
| A = | the Fair Market Value of one share of the Common Stock on the date prior to the date that the notice of exercise is received by the Company. | ||
| B = | Exercise Price (as adjusted to the date of such calculation). | ||
Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant to this Section 2(a)(ii).
(b) Exercise Price. The exercise price per share of Common Stock under this Warrant shall be equal to the Conversion Price, as such term is defined in Section 1.03(a) of the Note issued to the Holder pursuant to the Note Exchange Agreement (the “Exercise Price”).
-4-
(c) Exercise Limitations. The Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, if at the time of such exercise the Common Stock is registered pursuant to section 12 of the Exchange Act, and to the extent that after giving effect to such issuance after exercise, the Holder (together with the Holder’s affiliates, and any other person or entity acting as a group together with the Holder or any of the Holder’s affiliates), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of this Section, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. Holder is solely responsible for any schedules required to be filed in accordance therewith. The Company shall have no obligation to verify or confirm the accuracy of such filings. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its affiliates since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of Warrant Shares issuable upon exercise of this Warrant. The Holder may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(c), provided that any such increase shall not be effective until 61 days’ after the Holder gives notice of such increase to the Company, and the provisions of this Section 2(c) shall continue to apply, unless the Holder upon not less than 61 days’ prior notice to the Company determines to waive the Beneficial Ownership Limitation requirements described in this Section 2(c) in its entirety. Any such increase will not be effective until the 61st day after such notice is delivered to the Company. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
(d) Mechanics of Exercise.
i. Delivery of Certificates Upon Exercise. Certificates for shares purchased hereunder shall be transmitted by the Company’s transfer agent (the “Transfer Agent”) to the Holder by crediting the account of the Holder’s prime broker with the Depository Trust Company through its Deposit Withdrawal Agent Commission (“DWAC”) system if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the resale of the Warrant Shares by the Holder or (B) the shares are eligible for resale without volume or manner-of-sale limitations pursuant to Rule 144, and otherwise by physical delivery of certificates to the address specified by the Holder in the Notice of Exercise within two (2) Trading Days from the delivery to the Company of the Notice of Exercise Form, surrender of this Warrant (if required) and payment of the aggregate Exercise Price as set forth above (the “Warrant Share Delivery Date”). This Warrant shall be deemed to have been exercised on the date the Exercise Price is received by the Company. The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised by payment to the Company of the Exercise Price (except in the case of a cashless exercise pursuant to Section 2(a)(ii)) and all taxes required to be paid by the Holder, if any, pursuant to Section 2(d)(v) prior to the issuance of such shares, have been paid.
-5-
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to Holder a new Warrant evidencing the rights of Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder a certificate or the certificates representing the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Obligation Absolute; Partial Liquidated Damages. The Company’s obligations to issue and deliver the Warrant Shares upon exercise of this Warrant in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by the Holder or any other person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other person, and irrespective of any other circumstance which might otherwise limit such obligation of the Company to the Holder in connection with the issuance of such Warrant Shares; provided, however, that such delivery shall not operate as a waiver by the Company of any such action the Company may have against the Holder. The Company may not refuse exercise based on any claim that the Holder or anyone associated or affiliated with the Holder has been engaged in any violation of law, agreement or for any other reason, unless an injunction from a court, on notice to Holder, restraining and or enjoining exercise of all or part of this Warrant shall have been sought and obtained, and the Company posts a surety bond for the benefit of the Holder in the amount of 130% of the Fair Market Value of the Warrant Shares issuable upon exercise of this Warrant, which is subject to the injunction, which bond shall remain in effect until the completion of arbitration/litigation of the underlying dispute and the proceeds of which shall be payable to the Holder to the extent it obtains judgment. In the absence of such injunction, the Company shall issue Warrant Shares. If the Company fails for any reason to deliver to the Holder such certificate or certificates pursuant to Section 2(d)(i) by the third (3rd) Trading Day after the Conversion Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Fair Market Value of Warrant Shares being exercised, $10 per Trading Day for each Trading Day after such third (3rd) Trading Day until such certificates are delivered. Nothing herein shall limit a Holder’s right to pursue actual damages or declare an Event of Default under the Note for the Company’s failure to deliver Warrant Shares within the period specified herein and the Holder shall have the right to pursue all remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
-6-
v. Compensation for Buy-In on Failure to Timely Deliver Certificates upon Conversion. In addition to any other rights available to the Holder, if the Company fails for any reason to deliver to the Holder such certificate or certificates by the Share Delivery Date, and if after such Share Delivery Date the Holder is required by its brokerage firm to purchase (in an open market transaction or otherwise), or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder was entitled to receive upon the exercise relating to such Share Delivery Date (a “Buy-In”), then the Company shall (A) pay in cash to the Holder (in addition to any other remedies available to or elected by the Holder), if any, the amount by which (x) the Holder’s total purchase price (including any brokerage commissions) for the Common Stock so purchased exceeds (y) the product of (1) the aggregate number of shares of Common Stock that the Holder was entitled to receive from the exercise at issue multiplied by (2) the actual per share sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of the Holder, either reissue (if surrendered) this Warrant for a number of Warrant Shares equal to the number of Warrant Shares of the attempted exercise (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued if the Company had timely complied with its delivery requirements under Section 2(d)(i). For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of this Warrant with respect to which the actual sale price of the Warrant Shares (including any brokerage commissions) giving rise to such purchase obligation was a total of $10,000 under clause (A) of the immediately preceding sentence, the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock upon exercise of this Warrant as required pursuant to the terms hereof.
vi. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
-7-
vii. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, all of which taxes and expenses shall be paid by the Company, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the assignment form (“Assignment Form”) attached hereto as Exhibit B duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto.
viii. Closing of Books. The Company will not close its shareholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
Section 3. [Intentionally Omitted.]
Section 4. Certain Adjustments.
(a) Stock Dividends and Splits. If, at any time after the Initial Exercise Date until the Termination Date, the Company: (i) pays a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any Warrant Shares issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 4(a) shall become effective immediately after the record date for the determination of shareholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
(b) Subsequent Equity Sales. If, at any time after the Initial Exercise Date while this Warrant is outstanding, the Company sells or grants any option to purchase or sells or grants any right to reprice, or otherwise disposes of or issues (or announces any sale, grant or any option to purchase or other disposition), any Common Stock or Common Stock Equivalents at an effective price per share that is lower than the then Exercise Price (such issuances, collectively, a “Dilutive Issuance”) (if the holder of the Common Stock or Common Stock Equivalents so issued shall at any time, whether by operation of purchase price adjustments, reset provisions, floating conversion, exercise or exchange prices or otherwise, or due to warrants, options or rights per share which are issued in connection with such issuance, be entitled to receive shares of common stock at an effective price per share that is lower than the Exercise Price, such issuance shall be deemed to have occurred for less than the Exercise Price on such date of the Dilutive Issuance), then immediately upon such issuance or sale (or deemed issuance or sale) the Exercise Price in effect immediately prior to such issuance or sale (or deemed issuance or sale) shall be reduced (and in no event increased) to an Exercise Price equal to the quotient obtained by dividing:
(A) the sum of (1) the product obtained by multiplying the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) by the Exercise Price then in effect plus (2) the aggregate consideration, if any, received by the Company upon such issuance or sale (or deemed issuance or sale); by
-8-
(B) the sum of (1) the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) plus (2) the aggregate number of shares of Common Stock issued or sold (or deemed issued or sold) by the Company in such issuance or sale (or deemed issuance or sale).
“Common Stock Deemed Outstanding” means, at any given time, the sum of (a) the number of shares of Common Stock actually outstanding at such time, plus (b) the number of shares of Common Stock issuable upon exercise of any warrants or other rights or options to subscribe for or purchase Common Stock and conversion or exchange of any securities (directly or indirectly) convertible into or exchangeable for Common Stock, in each case actually outstanding at such time (treating as actually outstanding any such warrants, rights, options or other securities issuable upon exercise of other such securities actually outstanding at such time), in each case, regardless of whether such securities are actually exercisable, convertible or exchangeable at such time; provided, that Common Stock Deemed Outstanding at any given time shall not include shares owned or held by or for the account of the Company or any of its wholly-owned subsidiaries.
Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. Notwithstanding the foregoing, no adjustment will be made under this Section 4(b) in respect of an Exempt Issuance (as defined below).
The Company shall notify the Holder in writing, no later than one (1) Business Day following the issuance of any Common Stock or Common Stock Equivalents subject to this Section 4(b), indicating therein the applicable issuance price, or applicable reset price, exchange price, conversion price and other pricing terms (such notice, the “Dilutive Issuance Notice”). For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 4(b), upon the occurrence of any Dilutive Issuance, the Holder is entitled to receive a number of Conversion Shares based upon the new Conversion Price provided above on or after the date of such Dilutive Issuance, regardless of whether the Holder accurately refers to the new Conversion Price in the Notice of Conversion.
-9-
“Exempt Issuance” means the issuance of (a) shares of Common Stock, restricted stock units or options, and the underlying shares of Common Stock to consultants, employees, officers or directors of the Company pursuant to any stock or option plan duly adopted for such purpose, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose for services rendered to the Company, (b) securities issued upon the exercise or exchange of or conversion of any securities issued hereunder and/or other securities issuable pursuant to existing agreements, exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of this Warrant and disclosed in the Purchase Agreement, provided that such securities have not been amended since the date of this Warrant to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock dividends, stock splits or combinations) or to extend the term of such securities, (c) securities issued pursuant, acquisitions or strategic transactions approved by a majority of the directors of the Company, provided that any such issuance shall only be to a Person (or to the equity holders of a Person) which is, itself or through its Subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of the Company and which shall reasonably be expected to provide to the Company additional benefits, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities, (d) securities issued pursuant to any purchase money equipment loan or capital leasing arrangement, purchasing agent or debt financing from a commercial bank or similar financial institution, (e) securities issued pursuant to any presently outstanding warrants disclosed in the Purchase Agreement or this Warrant, and (f) securities upon a stock split, stock dividend or subdivision of the Common Stock and shares of common stock in a public offering.
(c) Pro Rata Distributions. If, at any time after the Initial Exercise Date until the Termination Date, the Company shall distribute to all holders of Common Stock (and not to the Holder) evidences of its indebtedness or assets (including cash and cash dividends) or rights or warrants to subscribe for or purchase any security other than the Common Stock, then in each such case the Exercise Price shall be adjusted by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the VWAP determined as of the record date mentioned above, and of which the numerator shall be such VWAP on such record date less than the per share Fair Market Value at such record date of the portion of such assets or evidence of indebtedness or rights or warrants so distributed applicable to one outstanding share of the Common Stock as determined by the Board of Directors in good faith. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
-10-
(d) Fundamental Transaction. If, at any time prior to the Termination Date, (i) the Company effects any merger or consolidation of the Company into another Person, (ii) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to tender or exchange their shares for other securities, cash or property or (iv) the Company effects any reclassification of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property (each “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction. For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. To the extent necessary to effectuate the foregoing provisions, any successor to the Company or surviving entity in such Fundamental Transaction shall issue to the Holder a new warrant consistent with the foregoing provisions and evidencing the Holder’s right to exercise such warrant into Alternate Consideration. The terms of any agreement pursuant to which a Fundamental Transaction is effected shall include terms requiring any such successor or surviving entity to comply with the provisions of this Section 4(c) and insuring that this Warrant (or any such replacement security) will be similarly adjusted upon any subsequent transaction analogous to a Fundamental Transaction.
(e) Subsequent Rights Offerings. In addition to any adjustments pursuant to this Section 4, if at any time prior to the Termination Date the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to all of the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation.
-11-
(f) Calculations. All calculations under this Section 4 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 4, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
(g) Notice to Holder.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 4, the Company shall promptly mail to the Holder a notice setting forth the Exercise Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any shareholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, of any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. The Holder is entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice.
-12-
Section 5. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws and the conditions set forth in Section 5(d) herein and to the provisions of the Purchase Agreement, this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with an Assignment Form duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. The Warrant, if properly assigned, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 5(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the Initial Exercise Date and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
d) Transfer Restrictions. If, at the time of the surrender of this Warrant in connection with any transfer of this Warrant, the transfer of this Warrant shall not be either (i) registered pursuant to an effective registration statement under the Securities Act and under applicable state securities or blue sky laws or (ii) eligible for resale without volume or manner-of-sale restrictions pursuant to Rule 144, the Company may require, as a condition of allowing such transfer, that the Holder or transferee of this Warrant, as the case may be, comply with the provisions of the Purchase Agreement.
-13-
Section 6. Miscellaneous.
a) No Rights as Shareholder Until Exercise. This Warrant does not entitle the Holder to any voting rights or other rights as a shareholder of the Company prior to the exercise hereof.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then, such action may be taken or such right may be exercised on the next succeeding Business Day.
d) Authorized Shares.
The Company covenants that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock one hundred (100%) of the number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. In case such amount of Common Stock is insufficient at any time, the Company shall call and hold a special meeting to increase the number of authorized shares of common stock. Management of the Company shall recommend to shareholders to vote in favor of increasing the number of authorized shares of common stock.
The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the National Securities Exchange upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
-14-
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation, as amended, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Purchase Agreement.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice Holder’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date. If the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be delivered in accordance with the notice provisions of the Purchase Agreement.
-15-
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of Holder, shall give rise to any liability of Holder for the purchase price of any Common Stock or as a shareholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of all Holders from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company and the Holder.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
[Signature Page Follows.]
-16-
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| ADAPTIN BIO, INC. | |||
| By: | |||
| Name: | Michael J. Roberts | ||
| Title: | Chief Executive Officer | ||
[SIGNATURE PAGE TO ADAPTIN BIO, INC. WARRANT]
EXHIBIT A
NOTICE OF EXERCISE
TO: ADAPTIN BIO, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant and
☐ tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any; or
☐ elects to purchase the number of Warrant Shares stated above pursuant to the cashless exercise procedure set forth in Section 2(a) of the Warrant
(2) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number or by physical delivery of a certificate to:
_______________________________
_______________________________
_______________________________
(3) Accredited Investor. The undersigned is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended.
[SIGNATURE OF HOLDER]
Name of Investing Entity:
________________________________________________________________________
Signature of Authorized Signatory of Investing Entity:
_________________________________________________
Name of Authorized Signatory:
___________________________________________________________________
Title of Authorized Signatory:
____________________________________________________________________
Date: _______________________________________________________________
A-1
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, [__] all of or ___________ shares of the foregoing Warrant and all rights evidenced thereby are hereby assigned to
_______________________________________________ whose address is
____________________________________________________________.
_______________________________________________________________
Dated: ______________, _______
Holder’s Signature: _____________________________
Holder’s Address: ______________________________
_______________________________
Signature Guaranteed: ___________________________________________
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
B-1
Exhibit 4.3
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”). THESE SECURITIES MAY BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED ONLY (A) TO THE COMPANY, (B) IN COMPLIANCE WITH RULE 144 UNDER THE SECURITIES ACT, IF AVAILABLE, AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS, (C) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT, OR (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, AND THE HOLDER HAS, PRIOR TO SUCH SALE, FURNISHED TO THE COMPANY AN OPINION OF COUNSEL OR OTHER EVIDENCE OF EXEMPTION, IN EITHER CASE REASONABLY SATISFACTORY TO THE COMPANY. HEDGING TRANSACTIONS INVOLVING THESE SECURITIES MAY NOT BE CONDUCTED UNLESS IN COMPLIANCE WITH THE SECURITIES ACT.
FORM OF COMMON STOCK PURCHASE WARRANT
ADAPTIN BIO, INC.
| Warrant No. 2025-___ | Issue Date: _______ __, 2025 |
THIS COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, Laidlaw & Company (UK) Ltd. (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the Initial Exercise Date (as defined below) and on or prior to the close of business on the seventh (7th) anniversary of the final Closing of the Offering (the “Termination Date”) but not thereafter, to subscribe for and purchase from Adaptin Bio, Inc. (formerly known as Unite Acquisition 1 Corp.), a Delaware corporation (the “Company”), up to _______________________________________ (__________) shares of Common Stock (the “Warrant Shares”). The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1. Definitions. For the purposes hereof, in addition to the terms defined elsewhere in this Warrant, (a) capitalized terms not otherwise defined herein shall have the meanings set forth in the Subscription Agreement and (b) the following terms shall have the following meanings:
“Business Day” means any day except any Saturday, any Sunday, any day which shall be a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Fair Market Value” of one share of Common Stock as of a particular date shall mean: (i) if traded on a National Securities Exchange or quoted on an over the counter market operated by OTC Markets Group, Inc., or its successor, the closing price of the Common Stock on such exchange or market on the applicable date of valuation; and (ii) if (i) does not apply, the Fair Market Value shall be the value thereof, as agreed upon by the Company and the Holder; provided, however, that if the Company and the Holder cannot agree on such value, such value shall be determined by an independent valuation firm experienced in valuing businesses such as the Company and jointly selected in good faith by the Company and the Holder. Fees and expenses of the valuation firm shall be paid for by the Company.
“Initial Exercise Date” means the Issue Date.
“National Securities Exchange” means the following markets or exchanges on which the Common Stock may be listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange or the NYSE American, LLC.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Subscription Agreement” means, collectively, the Subscription Agreement between the Company and the purchasers signatory thereto relating to the private placement offering of the Company’s “Units,” each Unit consisting of one share of Common Stock, one A Warrant and one B Warrant (each as defined therein), as amended, modified or supplemented from time to time in accordance with its terms.
“Trading Day” means a day on which the Trading Market is open for business.
“Trading Market” means the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the NYSE American LLC, the OTCQB, the OTCQX or the OTC Pink (or any successor of the foregoing).
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a National Securities Exchange, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. New York City time to 4:02 p.m. New York City time); (b) if prices for the Common Stock are reported on the OTC markets, including the OTCQX, OTCQB and OTC Pink markets, or in the “Pink Sheets” published by Pink Sheets, LLC (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported; or (c) in all other cases, the Fair Market Value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Subscribers of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company; provided that in each case where Bloomberg L.P. data is being relied upon, Holder shall provide to the Company a copy of such information for the Company's records.
-2-
Section 2. Exercise.
(a) Exercise of Warrant.
i. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed notice of exercise (“Notice of Exercise”) form attached hereto as Exhibit A; and, within two (2) Trading Days of the date said Notice of Exercise is delivered to the Company, the Company shall have received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer or cashier’s check drawn on a United States bank. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. In the event of any dispute or discrepancy, the records of the Company shall be controlling and determinative in the absence of manifest error.
ii. If at any time there is no effective registration statement registering, or no current prospectus available for, the resale of the Warrant Shares by the Holder, then in lieu of the payment methods set forth in Section 2(a)(i) above, the Holder may elect to exchange all or some of this Warrant for shares of Common Stock equal to the value of the amount of the Warrant being exchanged on the date of exchange. If Holder elects to exchange this Warrant as provided in this Section 2(a)(ii), Holder shall tender to the Company the Warrant for the amount being exchanged, along with written notice of Holder’s election to exchange some or all of the Warrant, and the Company shall issue to Holder the number of shares of the Common Stock computed using the following formula:
| X = | Y (A-B) |
| A |
| Where: X = | the number of shares of Common Stock to be issued to Holder. | |
| Y = | the number of shares of Common Stock purchasable under the amount of the Warrant being exchanged (as adjusted to the date of such calculation). | |
| A = | the Fair Market Value of one share of the Common Stock on the date prior to the date that the notice of exercise is received by the Company. | |
| B = | Exercise Price (as adjusted to the date of such calculation). |
-3-
Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant to this Section 2(a)(ii).
(b) Exercise Price. The exercise price per share of Common Stock under this Warrant shall initially be $4.40, subject to adjustment as described herein (the “Exercise Price”).
(c) Exercise Limitations. The Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, if at the time of such exercise the Common Stock is registered pursuant to section 12 of the Exchange Act, and to the extent that after giving effect to such issuance after exercise, the Holder (together with the Holder’s affiliates, and any other person or entity acting as a group together with the Holder or any of the Holder’s affiliates), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of this Section, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. Holder is solely responsible for any schedules required to be filed in accordance therewith. The Company shall have no obligation to verify or confirm the accuracy of such filings. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its affiliates since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of Warrant Shares issuable upon exercise of this Warrant. The Holder may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(c), provided that any such increase shall not be effective until 61 days’ after the Holder gives notice of such increase to the Company, and the provisions of this Section 2(c) shall continue to apply, unless the Holder upon not less than 61 days’ prior notice to the Company determines to waive the Beneficial Ownership Limitation requirements described in this Section 2(c) in its entirety. Any such increase will not be effective until the 61st day after such notice is delivered to the Company. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
(d) Mechanics of Exercise.
i. Delivery of Certificates Upon Exercise. Certificates for shares purchased hereunder shall be transmitted by the Company’s transfer agent (the “Transfer Agent”) to the Holder by crediting the account of the Holder’s prime broker with the Depository Trust Company through its Deposit Withdrawal Agent Commission (“DWAC”) system if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the resale of the Warrant Shares by the Holder or (B) the shares are eligible for resale without volume or manner-of-sale limitations pursuant to Rule 144, and otherwise by physical delivery of certificates to the address specified by the Holder in the Notice of Exercise within two (2) Trading Days from the delivery to the Company of the Notice of Exercise Form, surrender of this Warrant (if required) and payment of the aggregate Exercise Price as set forth above (the “Warrant Share Delivery Date”). This Warrant shall be deemed to have been exercised on the date the Exercise Price is received by the Company. The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised by payment to the Company of the Exercise Price (except in the case of a cashless exercise pursuant to Section 2(a)(ii)) and all taxes required to be paid by the Holder, if any, pursuant to Section 2(d)(v) prior to the issuance of such shares, have been paid.
-4-
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to Holder a new Warrant evidencing the rights of Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder a certificate or the certificates representing the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Obligation Absolute; Partial Liquidated Damages. The Company’s obligations to issue and deliver the Warrant Shares upon exercise of this Warrant in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by the Holder or any other person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other person, and irrespective of any other circumstance which might otherwise limit such obligation of the Company to the Holder in connection with the issuance of such Warrant Shares; provided, however, that such delivery shall not operate as a waiver by the Company of any such action the Company may have against the Holder. The Company may not refuse exercise based on any claim that the Holder or anyone associated or affiliated with the Holder has been engaged in any violation of law, agreement or for any other reason, unless an injunction from a court, on notice to Holder, restraining and or enjoining exercise of all or part of this Warrant shall have been sought and obtained, and the Company posts a surety bond for the benefit of the Holder in the amount of 130% of the Fair Market Value of the Warrant Shares issuable upon exercise of this Warrant, which is subject to the injunction, which bond shall remain in effect until the completion of arbitration/litigation of the underlying dispute and the proceeds of which shall be payable to the Holder to the extent it obtains judgment. In the absence of such injunction, the Company shall issue Warrant Shares. If the Company fails for any reason to deliver to the Holder such certificate or certificates pursuant to Section 2(d)(i) by the third (3rd) Trading Day after the Conversion Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Fair Market Value of Warrant Shares being exercised, $10 per Trading Day for each Trading Day after such third (3rd) Trading Day until such certificates are delivered. Nothing herein shall limit a Holder’s right to pursue actual damages or to exercise any other rights of the Holder for the Company’s failure to deliver Warrant Shares within the period specified herein and the Holder shall have the right to pursue all remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
-5-
v. Compensation for Buy-In on Failure to Timely Deliver Certificates upon Conversion. In addition to any other rights available to the Holder, if on the Share Delivery Date the Common Stock is listed or quoted on a Trading Market, and the Company fails for any reason to deliver to the Holder such certificate or certificates by the Share Delivery Date, and if after such Share Delivery Date the Holder is required by its brokerage firm to purchase (in an open market transaction or otherwise), or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder was entitled to receive upon the exercise relating to such Share Delivery Date (a “Buy-In”), then the Company shall (A) pay in cash to the Holder (in addition to any other remedies available to or elected by the Holder), if any, the amount by which (x) the Holder’s total purchase price (including any brokerage commissions) for the Common Stock so purchased exceeds (y) the product of (1) the aggregate number of shares of Common Stock that the Holder was entitled to receive from the exercise at issue multiplied by (2) the actual per share sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of the Holder, either reissue (if surrendered) this Warrant for a number of Warrant Shares equal to the number of Warrant Shares of the attempted exercise (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued if the Company had timely complied with its delivery requirements under Section 2(d)(i). For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of this Warrant with respect to which the actual sale price of the Warrant Shares (including any brokerage commissions) giving rise to such purchase obligation was a total of $10,000 under clause (A) of the immediately preceding sentence, the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock upon exercise of this Warrant as required pursuant to the terms hereof.
-6-
vi. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
v. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, all of which taxes and expenses shall be paid by the Company, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the assignment form (“Assignment Form”) attached hereto as Exhibit B duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay any Transfer Agent fees required for same-day processing of any Notice of Exercise and any fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
vi. Closing of Books. The Company will not close its shareholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
Section 3. Certain Adjustments.
(a) Stock Dividends and Splits. If, at any time after the Initial Exercise Date until the Termination Date, the Company: (i) pays a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any Warrant Shares issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of shareholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
-7-
(b) Subsequent Equity Sales. If, at any time after the Initial Exercise Date while this Warrant is outstanding, the Company sells or grants any option to purchase or sells or grants any right to reprice, or otherwise disposes of or issues (or announces any sale, grant or any option to purchase or other disposition), any Common Stock or Common Stock Equivalents at an effective price per share that is lower than the then Exercise Price (such issuances, collectively, a “Dilutive Issuance”) (if the holder of the Common Stock or Common Stock Equivalents so issued shall at any time, whether by operation of purchase price adjustments, reset provisions, floating conversion, exercise or exchange prices or otherwise, or due to warrants, options or rights per share which are issued in connection with such issuance, be entitled to receive shares of common stock at an effective price per share that is lower than the Exercise Price, such issuance shall be deemed to have occurred for less than the Exercise Price on such date of the Dilutive Issuance), then immediately upon such issuance or sale (or deemed issuance or sale) the Exercise Price in effect immediately prior to such issuance or sale (or deemed issuance or sale) shall be reduced (and in no event increased) to an Exercise Price equal to the quotient obtained by dividing:
(A) the sum of (1) the product obtained by multiplying the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) by the Exercise Price then in effect plus (2) the aggregate consideration, if any, received by the Company upon such issuance or sale (or deemed issuance or sale); by
(B) the sum of (1) the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) plus (2) the aggregate number of shares of Common Stock issued or sold (or deemed issued or sold) by the Company in such issuance or sale (or deemed issuance or sale).
“Common Stock Deemed Outstanding” means, at any given time, the sum of (a) the number of shares of Common Stock actually outstanding at such time, plus (b) the number of shares of Common Stock issuable upon exercise of any warrants or other rights or options to subscribe for or purchase Common Stock and conversion or exchange of any securities (directly or indirectly) convertible into or exchangeable for Common Stock, in each case actually outstanding at such time (treating as actually outstanding any such warrants, rights, options or other securities issuable upon exercise of other such securities actually outstanding at such time), in each case, regardless of whether such securities are actually exercisable, convertible or exchangeable at such time; provided, that Common Stock Deemed Outstanding at any given time shall not include shares owned or held by or for the account of the Company or any of its wholly-owned subsidiaries.
-8-
Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. Notwithstanding the foregoing, no adjustment will be made under this Section 3(b) in respect of an Exempt Issuance (as defined below).
The Company shall notify the Holder in writing, no later than one (1) Business Day following the issuance of any Common Stock or Common Stock Equivalents subject to this Section 3(b), indicating therein the applicable issuance price, or applicable reset price, exchange price, conversion price and other pricing terms (such notice, the “Dilutive Issuance Notice”). For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 3(b), upon the occurrence of any Dilutive Issuance, the Holder is entitled to receive a number of Conversion Shares based upon the new Conversion Price provided above on or after the date of such Dilutive Issuance, regardless of whether the Holder accurately refers to the new Conversion Price in the Notice of Conversion.
“Exempt Issuance” means the issuance of (a) shares of Common Stock, restricted stock units or options, and the underlying shares of Common Stock to consultants, employees, officers or directors of the Company pursuant to any stock or option plan duly adopted for such purpose, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose for services rendered to the Company, (b) securities issued upon the exercise or exchange of or conversion of any securities issued hereunder and/or other securities issuable pursuant to existing agreements, exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of this Warrant, provided that such securities have not been amended since the date of this Warrant to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock dividends, stock splits or combinations) or to extend the term of such securities, (c) securities issued pursuant, acquisitions or strategic transactions approved by a majority of the directors of the Company, provided that any such issuance shall only be to a Person (or to the equity holders of a Person) which is, itself or through its Subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of the Company and which shall reasonably be expected to provide to the Company additional benefits, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities, (d) securities issued pursuant to any purchase money equipment loan or capital leasing arrangement, purchasing agent or debt financing from a commercial bank or similar financial institution, (e) securities issued pursuant to any presently outstanding warrants disclosed in the Subscription Agreement or this Warrant, and (f) securities upon a stock split, stock dividend or subdivision of the Common Stock and shares of common stock in a public offering.
-9-
(c) Pro Rata Distributions. If, at any time after the Initial Exercise Date until the Termination Date, the Company shall distribute to all holders of Common Stock (and not to the Holder) evidences of its indebtedness or assets (including cash and cash dividends) or rights or warrants to subscribe for or purchase any security other than the Common Stock, then in each such case the Exercise Price shall be adjusted by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the VWAP determined as of the record date mentioned above, and of which the numerator shall be such VWAP on such record date less than the per share Fair Market Value at such record date of the portion of such assets or evidence of indebtedness or rights or warrants so distributed applicable to one outstanding share of the Common Stock as determined by the Board of Directors in good faith. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
(d) Fundamental Transaction. If, at any time prior to the Termination Date, (i) the Company effects any merger or consolidation of the Company into another Person, (ii) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to tender or exchange their shares for other securities, cash or property or (iv) the Company effects any reclassification of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property (each “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction. For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. To the extent necessary to effectuate the foregoing provisions, any successor to the Company or surviving entity in such Fundamental Transaction shall issue to the Holder a new warrant consistent with the foregoing provisions and evidencing the Holder’s right to exercise such warrant into Alternate Consideration. The terms of any agreement pursuant to which a Fundamental Transaction is effected shall include terms requiring any such successor or surviving entity to comply with the provisions of this Section 3(c) and insuring that this Warrant (or any such replacement security) will be similarly adjusted upon any subsequent transaction analogous to a Fundamental Transaction.
-10-
(e) Subsequent Rights Offerings. In addition to any adjustments pursuant to this Section 3, if at any time prior to the Termination Date the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to all of the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation.
(f) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
(g) Notice to Holder.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly mail to the Holder a notice setting forth the Exercise Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any shareholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, of any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. The Holder is entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice.
-11-
Section 4. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws and the conditions set forth in Section 4(d) herein and to the provisions of the Subscription Agreement, this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with an Assignment Form duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. The Warrant, if properly assigned, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the Initial Exercise Date and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
-12-
d) Transfer Restrictions. Prior to and as a condition to the sale or transfer of the Warrant Shares issuable upon exercise of this Warrant, the holder shall furnish to the Company such certificates, representations, agreements and other information, including an opinion of counsel, as the Company or the Company’s transfer agent reasonably may require to confirm that such sale or transfer is being made pursuant to an exemption from, or in a transaction not subject to, the registration requirements of the Securities Act, unless such Warrant Shares are being sold or transferred pursuant to an effective registration statement.
Section 5. Miscellaneous.
a) No Rights as Shareholder Until Exercise. This Warrant does not entitle the Holder to any voting rights or other rights as a shareholder of the Company prior to the exercise hereof.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then, such action may be taken or such right may be exercised on the next succeeding Business Day.
d) Authorized Shares.
The Company covenants that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock one hundred (100%) of the number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. In case such amount of Common Stock is insufficient at any time, the Company shall call and hold a special meeting to increase the number of authorized shares of common stock. Management of the Company shall recommend to shareholders to vote in favor of increasing the number of authorized shares of common stock.
-13-
The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the National Securities Exchange upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation, as amended, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be governed by and construed and enforced in accordance with the internal laws of the State of New York, without regard to the principles of conflicts of law thereof. Each party agrees that all legal proceedings concerning the interpretations, enforcement and defense of the transactions contemplated by this Warrant (whether brought against a party hereto or their respective affiliates, directors, officers, shareholders, partners, members, employees or agents) shall be commenced exclusively in the United States District Court for the Southern District of New York or the Supreme Court of the State of New York, and the appellate courts therefrom, in each case sitting in New York County, New York. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof via registered or certified mail or overnight delivery (with evidence of delivery) to such party at the address in effect for notices to it under this Warrant and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any other manner permitted by law. If either party shall commence an action, suit or proceeding to enforce any provisions of this Warrant, the prevailing party in such action, suit or proceeding shall be reimbursed by the other party for their reasonable attorneys’ fees and other costs and expenses incurred with the investigation, preparation and prosecution of such action or proceeding. Notwithstanding the foregoing, nothing in this paragraph shall limit or restrict the federal district court in which a Holder may bring a claim under the federal securities laws.
-14-
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice Holder’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date. If the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be to the address for the Holder in the Warrant Register.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of Holder, shall give rise to any liability of Holder for the purchase price of any Common Stock or as a shareholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of all Holders from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company and the Holder.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
[Signature Page Follows.]
-15-
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| Adaptin BIO, INC. | ||
| By: | ||
| Name: | Michael J. Roberts | |
| Title: | Chief Executive Officer | |
[SIGNATURE PAGE TO ADAPTIN BIO, INC. WARRANT]
EXHIBIT A
NOTICE OF EXERCISE
TO: ADAPTIN BIO, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant and
☐ tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any; or
☐ elects to purchase the number of Warrant Shares stated above pursuant to the cashless exercise procedure set forth in Section 2(a) of the Warrant
(2) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number or by physical delivery of a certificate to:
_______________________________
_______________________________
_______________________________
(3) Accredited Investor. The undersigned is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended.
[SIGNATURE OF HOLDER]
Name of Investing Entity: ________________________________________________________________________
Signature of Authorized Signatory of Investing Entity: _________________________________________________
Name of Authorized Signatory: ___________________________________________________________________
Title of Authorized Signatory: ____________________________________________________________________
Date: _______________________________________________________________
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, [____] all of or [_______] shares of the foregoing Warrant and all rights evidenced thereby are hereby assigned to
_______________________________________________ whose address is
____________________________________________________________.
_______________________________________________________________
Dated: ______________, _______
Holder’s Signature: _____________________________
Holder’s Address: _____________________________
_____________________________
Signature Guaranteed: ___________________________________________
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
Exhibit 4.4
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”). THESE SECURITIES MAY BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED ONLY (A) TO THE COMPANY, (B) IN COMPLIANCE WITH RULE 144 UNDER THE SECURITIES ACT, IF AVAILABLE, AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS, (C) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT, OR (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, AND THE HOLDER HAS, PRIOR TO SUCH SALE, FURNISHED TO THE COMPANY AN OPINION OF COUNSEL OR OTHER EVIDENCE OF EXEMPTION, IN EITHER CASE REASONABLY SATISFACTORY TO THE COMPANY. HEDGING TRANSACTIONS INVOLVING THESE SECURITIES MAY NOT BE CONDUCTED UNLESS IN COMPLIANCE WITH THE SECURITIES ACT.
FORM OF COMMON STOCK PURCHASE WARRANT
ADAPTIN BIO, INC.
| Warrant No. 2025-A-___ | Issue Date: _______ __, 2025 |
THIS COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, __________________________________________________ (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the Initial Exercise Date (as defined below) and on or prior to the close of business on the first (1st) anniversary of the final Closing of the Offering (the “Initial Termination Date”), provided that if on the Initial Termination Date or any Extended Termination Date (as defined below) the Common Stock is not admitted for trading or listed on an Approved Market, then the term of exercise of this Warrant shall be extended or further extended to the date that is six (6) months after such Initial Termination Date or Extended Termination Date, as the case may be (each such six- (6-) month extension date an “Extended Termination Date,” and the latest to occur of the Initial Termination Date or the last Extended Termination Date the “Termination Date”) but not thereafter, to subscribe for and purchase from Adaptin Bio, Inc. (formerly known as Unite Acquisition 1 Corp.), a Delaware corporation (the “Company”), up to _______________________________________ (__________) shares of Common Stock (the “Warrant Shares”). The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1. Definitions. For the purposes hereof, in addition to the terms defined elsewhere in this Warrant, (a) capitalized terms not otherwise defined herein shall have the meanings set forth in the Subscription Agreement and (b) the following terms shall have the following meanings:
“Approved Market” means any of the OTCQB or OTCQX market of OTC Markets Group, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the NYSE American (or, in each case, a successor over-the-counter trading market or national securities exchange thereto).
“Business Day” means any day except any Saturday, any Sunday, any day which shall be a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Fair Market Value” of one share of Common Stock as of a particular date shall mean: (i) if traded on a National Securities Exchange or quoted on an over the counter market operated by OTC Markets Group, Inc., or its successor, the closing price of the Common Stock on such exchange or market on the applicable date of valuation; and (ii) if (i) does not apply, the Fair Market Value shall be the value thereof, as agreed upon by the Company and the Holder; provided, however, that if the Company and the Holder cannot agree on such value, such value shall be determined by an independent valuation firm experienced in valuing businesses such as the Company and jointly selected in good faith by the Company and the Holder. Fees and expenses of the valuation firm shall be paid for by the Company.
“Initial Exercise Date” means the Issue Date.
“National Securities Exchange” means the following markets or exchanges on which the Common Stock may be listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange or the NYSE American, LLC.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Subscription Agreement” means, collectively, the Subscription Agreement, dated as of [EXECUTION DATE] between the Company and the original Holder, as amended, modified or supplemented from time to time in accordance with its terms.
“Trading Day” means a day on which the Trading Market is open for business.
“Trading Market” means the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the NYSE American LLC, the OTCQB, the OTCQX or the OTC Pink (or any successor of the foregoing).
“Transaction Documents” shall have the meaning set forth in the Subscription Agreement.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a National Securities Exchange, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. New York City time to 4:02 p.m. New York City time); (b) if prices for the Common Stock are reported on the OTC markets, including the OTCQX, OTCQB and OTC Pink markets, or in the “Pink Sheets” published by Pink Sheets, LLC (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported; or (c) in all other cases, the Fair Market Value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Subscribers of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company; provided that in each case where Bloomberg L.P. data is being relied upon, Holder shall provide to the Company a copy of such information for the Company's records.
-2-
Section 2. Exercise.
(a) Exercise of Warrant. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed notice of exercise (“Notice of Exercise”) form attached hereto as Exhibit A; and, within two (2) Trading Days of the date said Notice of Exercise is delivered to the Company, the Company shall have received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer or cashier’s check drawn on a United States bank. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. In the event of any dispute or discrepancy, the records of the Company shall be controlling and determinative in the absence of manifest error.
(b) Exercise Price. The exercise price per share of Common Stock under this Warrant shall initially be $4.40, subject to adjustment as described herein (the “Exercise Price”).
(c) Exercise Limitations. The Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, if at the time of such exercise the Common Stock is registered pursuant to section 12 of the Exchange Act, and to the extent that after giving effect to such issuance after exercise, the Holder (together with the Holder’s affiliates, and any other person or entity acting as a group together with the Holder or any of the Holder’s affiliates), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of this Section, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. Holder is solely responsible for any schedules required to be filed in accordance therewith. The Company shall have no obligation to verify or confirm the accuracy of such filings. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its affiliates since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of Warrant Shares issuable upon exercise of this Warrant. The Holder may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(c), provided that any such increase shall not be effective until 61 days’ after the Holder gives notice of such increase to the Company, and the provisions of this Section 2(c) shall continue to apply, unless the Holder upon not less than 61 days’ prior notice to the Company determines to waive the Beneficial Ownership Limitation requirements described in this Section 2(c) in its entirety. Any such increase will not be effective until the 61st day after such notice is delivered to the Company. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
-3-
(d) Mechanics of Exercise.
i. Delivery of Certificates Upon Exercise. Certificates for shares purchased hereunder shall be transmitted by the Company’s transfer agent (the “Transfer Agent”) to the Holder by crediting the account of the Holder’s prime broker with the Depository Trust Company through its Deposit Withdrawal Agent Commission (“DWAC”) system if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the resale of the Warrant Shares by the Holder or (B) the shares are eligible for resale without volume or manner-of-sale limitations pursuant to Rule 144, and otherwise by physical delivery of certificates to the address specified by the Holder in the Notice of Exercise within two (2) Trading Days from the delivery to the Company of the Notice of Exercise Form, surrender of this Warrant (if required) and payment of the aggregate Exercise Price as set forth above (the “Warrant Share Delivery Date”). This Warrant shall be deemed to have been exercised on the date the Exercise Price is received by the Company. The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised by payment to the Company of the Exercise Price and all taxes required to be paid by the Holder, if any, pursuant to Section 2(d)(v) prior to the issuance of such shares, have been paid.
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to Holder a new Warrant evidencing the rights of Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
-4-
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder a certificate or the certificates representing the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
iv. Obligation Absolute; Partial Liquidated Damages. The Company’s obligations to issue and deliver the Warrant Shares upon exercise of this Warrant in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by the Holder or any other person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other person, and irrespective of any other circumstance which might otherwise limit such obligation of the Company to the Holder in connection with the issuance of such Warrant Shares; provided, however, that such delivery shall not operate as a waiver by the Company of any such action the Company may have against the Holder. The Company may not refuse exercise based on any claim that the Holder or anyone associated or affiliated with the Holder has been engaged in any violation of law, agreement or for any other reason, unless an injunction from a court, on notice to Holder, restraining and or enjoining exercise of all or part of this Warrant shall have been sought and obtained, and the Company posts a surety bond for the benefit of the Holder in the amount of 130% of the Fair Market Value of the Warrant Shares issuable upon exercise of this Warrant, which is subject to the injunction, which bond shall remain in effect until the completion of arbitration/litigation of the underlying dispute and the proceeds of which shall be payable to the Holder to the extent it obtains judgment. In the absence of such injunction, the Company shall issue Warrant Shares. If the Company fails for any reason to deliver to the Holder such certificate or certificates pursuant to Section 2(d)(i) by the third (3rd) Trading Day after the Conversion Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Fair Market Value of Warrant Shares being exercised, $10 per Trading Day for each Trading Day after such third (3rd) Trading Day until such certificates are delivered. Nothing herein shall limit a Holder’s right to pursue actual damages or to exercise any other rights of the Holder for the Company’s failure to deliver Warrant Shares within the period specified herein and the Holder shall have the right to pursue all remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
-5-
v. Compensation for Buy-In on Failure to Timely Deliver Certificates upon Conversion. In addition to any other rights available to the Holder, if on the Share Delivery Date the Common Stock is listed or quoted on a Trading Market, and the Company fails for any reason to deliver to the Holder such certificate or certificates by the Share Delivery Date, and if after such Share Delivery Date the Holder is required by its brokerage firm to purchase (in an open market transaction or otherwise), or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder was entitled to receive upon the exercise relating to such Share Delivery Date (a “Buy-In”), then the Company shall (A) pay in cash to the Holder (in addition to any other remedies available to or elected by the Holder), if any, the amount by which (x) the Holder’s total purchase price (including any brokerage commissions) for the Common Stock so purchased exceeds (y) the product of (1) the aggregate number of shares of Common Stock that the Holder was entitled to receive from the exercise at issue multiplied by (2) the actual per share sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of the Holder, either reissue (if surrendered) this Warrant for a number of Warrant Shares equal to the number of Warrant Shares of the attempted exercise (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued if the Company had timely complied with its delivery requirements under Section 2(d)(i). For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of this Warrant with respect to which the actual sale price of the Warrant Shares (including any brokerage commissions) giving rise to such purchase obligation was a total of $10,000 under clause (A) of the immediately preceding sentence, the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock upon exercise of this Warrant as required pursuant to the terms hereof.
vi. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
v. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, all of which taxes and expenses shall be paid by the Company, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the assignment form (“Assignment Form”) attached hereto as Exhibit B duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay any Transfer Agent fees required for same-day processing of any Notice of Exercise and any fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
-6-
vi. Closing of Books. The Company will not close its shareholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
Section 3. Certain Adjustments.
(a) Stock Dividends and Splits. If, at any time after the Initial Exercise Date until the Termination Date, the Company: (i) pays a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any Warrant Shares issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of shareholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
(b) Subsequent Equity Sales. If, at any time after the Initial Exercise Date while this Warrant is outstanding, the Company sells or grants any option to purchase or sells or grants any right to reprice, or otherwise disposes of or issues (or announces any sale, grant or any option to purchase or other disposition), any Common Stock or Common Stock Equivalents at an effective price per share that is lower than the then Exercise Price (such issuances, collectively, a “Dilutive Issuance”) (if the holder of the Common Stock or Common Stock Equivalents so issued shall at any time, whether by operation of purchase price adjustments, reset provisions, floating conversion, exercise or exchange prices or otherwise, or due to warrants, options or rights per share which are issued in connection with such issuance, be entitled to receive shares of common stock at an effective price per share that is lower than the Exercise Price, such issuance shall be deemed to have occurred for less than the Exercise Price on such date of the Dilutive Issuance), then immediately upon such issuance or sale (or deemed issuance or sale) the Exercise Price in effect immediately prior to such issuance or sale (or deemed issuance or sale) shall be reduced (and in no event increased) to an Exercise Price equal to the quotient obtained by dividing:
(A) the sum of (1) the product obtained by multiplying the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) by the Exercise Price then in effect plus (2) the aggregate consideration, if any, received by the Company upon such issuance or sale (or deemed issuance or sale); by
-7-
(B) the sum of (1) the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) plus (2) the aggregate number of shares of Common Stock issued or sold (or deemed issued or sold) by the Company in such issuance or sale (or deemed issuance or sale).
“Common Stock Deemed Outstanding” means, at any given time, the sum of (a) the number of shares of Common Stock actually outstanding at such time, plus (b) the number of shares of Common Stock issuable upon exercise of any warrants or other rights or options to subscribe for or purchase Common Stock and conversion or exchange of any securities (directly or indirectly) convertible into or exchangeable for Common Stock, in each case actually outstanding at such time (treating as actually outstanding any such warrants, rights, options or other securities issuable upon exercise of other such securities actually outstanding at such time), in each case, regardless of whether such securities are actually exercisable, convertible or exchangeable at such time; provided, that Common Stock Deemed Outstanding at any given time shall not include shares owned or held by or for the account of the Company or any of its wholly-owned subsidiaries.
Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. Notwithstanding the foregoing, no adjustment will be made under this Section 3(b) in respect of an Exempt Issuance (as defined below).
The Company shall notify the Holder in writing, no later than one (1) Business Day following the issuance of any Common Stock or Common Stock Equivalents subject to this Section 3(b), indicating therein the applicable issuance price, or applicable reset price, exchange price, conversion price and other pricing terms (such notice, the “Dilutive Issuance Notice”). For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 3(b), upon the occurrence of any Dilutive Issuance, the Holder is entitled to receive a number of Conversion Shares based upon the new Conversion Price provided above on or after the date of such Dilutive Issuance, regardless of whether the Holder accurately refers to the new Conversion Price in the Notice of Conversion.
-8-
“Exempt Issuance” means the issuance of (a) shares of Common Stock, restricted stock units or options, and the underlying shares of Common Stock to consultants, employees, officers or directors of the Company pursuant to any stock or option plan duly adopted for such purpose, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose for services rendered to the Company, (b) securities issued upon the exercise or exchange of or conversion of any securities issued hereunder and/or other securities issuable pursuant to existing agreements, exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of this Warrant and disclosed in the Subscription Agreement, provided that such securities have not been amended since the date of this Warrant to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock dividends, stock splits or combinations) or to extend the term of such securities, (c) securities issued pursuant, acquisitions or strategic transactions approved by a majority of the directors of the Company, provided that any such issuance shall only be to a Person (or to the equity holders of a Person) which is, itself or through its Subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of the Company and which shall reasonably be expected to provide to the Company additional benefits, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities, (d) securities issued pursuant to any purchase money equipment loan or capital leasing arrangement, purchasing agent or debt financing from a commercial bank or similar financial institution, (e) securities issued pursuant to any presently outstanding warrants disclosed in the Subscription Agreement or this Warrant, and (f) securities upon a stock split, stock dividend or subdivision of the Common Stock and shares of common stock in a public offering.
(c) Pro Rata Distributions. If, at any time after the Initial Exercise Date until the Termination Date, the Company shall distribute to all holders of Common Stock (and not to the Holder) evidences of its indebtedness or assets (including cash and cash dividends) or rights or warrants to subscribe for or purchase any security other than the Common Stock, then in each such case the Exercise Price shall be adjusted by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the VWAP determined as of the record date mentioned above, and of which the numerator shall be such VWAP on such record date less than the per share Fair Market Value at such record date of the portion of such assets or evidence of indebtedness or rights or warrants so distributed applicable to one outstanding share of the Common Stock as determined by the Board of Directors in good faith. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
-9-
(d) Fundamental Transaction. If, at any time prior to the Termination Date, (i) the Company effects any merger or consolidation of the Company into another Person, (ii) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to tender or exchange their shares for other securities, cash or property or (iv) the Company effects any reclassification of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property (each “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction. For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. To the extent necessary to effectuate the foregoing provisions, any successor to the Company or surviving entity in such Fundamental Transaction shall issue to the Holder a new warrant consistent with the foregoing provisions and evidencing the Holder’s right to exercise such warrant into Alternate Consideration. The terms of any agreement pursuant to which a Fundamental Transaction is effected shall include terms requiring any such successor or surviving entity to comply with the provisions of this Section 3(c) and insuring that this Warrant (or any such replacement security) will be similarly adjusted upon any subsequent transaction analogous to a Fundamental Transaction.
(e) Subsequent Rights Offerings. In addition to any adjustments pursuant to this Section 3, if at any time prior to the Termination Date the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to all of the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation.
-10-
(f) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
(g) Notice to Holder.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly mail to the Holder a notice setting forth the Exercise Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any shareholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, of any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. The Holder is entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice.
-11-
Section 4. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws and the conditions set forth in Section 4(d) herein and to the provisions of the Subscription Agreement, this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with an Assignment Form duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. The Warrant, if properly assigned, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the Initial Exercise Date and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
d) Transfer Restrictions. If, at the time of the surrender of this Warrant in connection with any transfer of this Warrant, the transfer of this Warrant shall not be either (i) registered pursuant to an effective registration statement under the Securities Act and under applicable state securities or blue sky laws or (ii) eligible for resale without volume or manner-of-sale restrictions pursuant to Rule 144, the Company may require, as a condition of allowing such transfer, that the Holder or transferee of this Warrant, as the case may be, comply with the provisions of the Subscription Agreement.
Section 5. Miscellaneous.
a) No Rights as Shareholder Until Exercise. This Warrant does not entitle the Holder to any voting rights or other rights as a shareholder of the Company prior to the exercise hereof.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
-12-
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then, such action may be taken or such right may be exercised on the next succeeding Business Day.
d) Authorized Shares.
The Company covenants that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock one hundred (100%) of the number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. In case such amount of Common Stock is insufficient at any time, the Company shall call and hold a special meeting to increase the number of authorized shares of common stock. Management of the Company shall recommend to shareholders to vote in favor of increasing the number of authorized shares of common stock.
The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the National Securities Exchange upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation, as amended, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
-13-
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Subscription Agreement.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice Holder’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date. If the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
h) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be delivered in accordance with the notice provisions of the Subscription Agreement.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of Holder, shall give rise to any liability of Holder for the purchase price of any Common Stock or as a shareholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of all Holders from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company and the Holder.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
[Signature Page Follows.]
-14-
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| Adaptin BIO, INC. | ||
| By: | ||
| Name: | Michael J. Roberts | |
| Title: | Chief Executive Officer | |
[SIGNATURE PAGE TO ADAPTIN BIO, INC. WARRANT]
EXHIBIT A
NOTICE OF EXERCISE
TO: ADAPTIN BIO, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant and tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any.
(2) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number or by physical delivery of a certificate to:
_______________________________
_______________________________
_______________________________
(3) Accredited Investor. The undersigned is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended.
[SIGNATURE OF HOLDER]
Name of Investing Entity: ________________________________________________________________________
Signature of Authorized Signatory of Investing Entity: _________________________________________________
Name of Authorized Signatory: ___________________________________________________________________
Title of Authorized Signatory: ____________________________________________________________________
Date: _______________________________________________________________
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, [____] all of or [_______] shares of the foregoing Warrant and all rights evidenced thereby are hereby assigned to
_______________________________________________ whose address is
____________________________________________________________.
_______________________________________________________________
Dated: ______________, _______
Holder’s Signature: _____________________________
Holder’s Address: _____________________________
_____________________________
Signature Guaranteed: ___________________________________________
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
Exhibit 4.5
NEITHER THIS SECURITY NOR THE SECURITIES FOR WHICH THIS SECURITY IS EXERCISABLE HAVE BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”). THESE SECURITIES MAY BE OFFERED, SOLD, PLEDGED OR OTHERWISE TRANSFERRED ONLY (A) TO THE COMPANY, (B) IN COMPLIANCE WITH RULE 144 UNDER THE SECURITIES ACT, IF AVAILABLE, AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS, (C) PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT, OR (D) IN A TRANSACTION THAT DOES NOT REQUIRE REGISTRATION UNDER THE SECURITIES ACT OR ANY APPLICABLE STATE SECURITIES LAWS, AND THE HOLDER HAS, PRIOR TO SUCH SALE, FURNISHED TO THE COMPANY AN OPINION OF COUNSEL OR OTHER EVIDENCE OF EXEMPTION, IN EITHER CASE REASONABLY SATISFACTORY TO THE COMPANY. HEDGING TRANSACTIONS INVOLVING THESE SECURITIES MAY NOT BE CONDUCTED UNLESS IN COMPLIANCE WITH THE SECURITIES ACT.
FORM OF COMMON STOCK PURCHASE WARRANT
ADAPTIN BIO, INC.
| Warrant No. 2025-B-___ | Issue Date: _______ __, 2025 |
THIS COMMON STOCK PURCHASE WARRANT (the “Warrant”) certifies that, for value received, __________________________________________________ (the “Holder”) is entitled, upon the terms and subject to the limitations on exercise and the conditions hereinafter set forth, at any time on or after the Initial Exercise Date (as defined below) and on or prior to the close of business on the fifth (5th) anniversary of the final Closing of the Offering (the “Termination Date”) but not thereafter, to subscribe for and purchase from Adaptin Bio, Inc. (formerly known as Unite Acquisition 1 Corp.), a Delaware corporation (the “Company”), up to _______________________________________ (__________) shares of Common Stock (the “Warrant Shares”). The purchase price of one share of Common Stock under this Warrant shall be equal to the Exercise Price, as defined in Section 2(b).
Section 1. Definitions. For the purposes hereof, in addition to the terms defined elsewhere in this Warrant, (a) capitalized terms not otherwise defined herein shall have the meanings set forth in the Subscription Agreement and (b) the following terms shall have the following meanings:
“Business Day” means any day except any Saturday, any Sunday, any day which shall be a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Fair Market Value” of one share of Common Stock as of a particular date shall mean: (i) if traded on a National Securities Exchange or quoted on an over the counter market operated by OTC Markets Group, Inc., or its successor, the closing price of the Common Stock on such exchange or market on the applicable date of valuation; and (ii) if (i) does not apply, the Fair Market Value shall be the value thereof, as agreed upon by the Company and the Holder; provided, however, that if the Company and the Holder cannot agree on such value, such value shall be determined by an independent valuation firm experienced in valuing businesses such as the Company and jointly selected in good faith by the Company and the Holder. Fees and expenses of the valuation firm shall be paid for by the Company.
“Initial Exercise Date” means the Issue Date.
“National Securities Exchange” means the following markets or exchanges on which the Common Stock may be listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange or the NYSE American, LLC.
“Securities Act” means the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder.
“Subscription Agreement” means, collectively, the Subscription Agreement, dated as of [EXECUTION DATE] between the Company and the original Holder, as amended, modified or supplemented from time to time in accordance with its terms.
“Trading Day” means a day on which the Trading Market is open for business.
“Trading Market” means the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the NYSE American LLC, the OTCQB, the OTCQX or the OTC Pink (or any successor of the foregoing).
“Transaction Documents” shall have the meaning set forth in the Subscription Agreement.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (a) if the Common Stock is then listed or quoted on a National Securities Exchange, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the Trading Market on which the Common Stock is then listed or quoted as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. New York City time to 4:02 p.m. New York City time); (b) if prices for the Common Stock are reported on the OTC markets, including the OTCQX, OTCQB and OTC Pink markets, or in the “Pink Sheets” published by Pink Sheets, LLC (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share of the Common Stock so reported; or (c) in all other cases, the Fair Market Value of a share of Common Stock as determined by an independent appraiser selected in good faith by the Subscribers of a majority in interest of the Securities then outstanding and reasonably acceptable to the Company, the fees and expenses of which shall be paid by the Company; provided that in each case where Bloomberg L.P. data is being relied upon, Holder shall provide to the Company a copy of such information for the Company's records.
-2-
Section 2. Exercise.
(a) Exercise of Warrant.
i. Exercise of the purchase rights represented by this Warrant may be made, in whole or in part, at any time or times on or after the Initial Exercise Date and on or before the Termination Date by delivery to the Company (or such other office or agency of the Company as it may designate by notice in writing to the registered Holder at the address of the Holder appearing on the books of the Company) of a duly executed notice of exercise (“Notice of Exercise”) form attached hereto as Exhibit A; and, within two (2) Trading Days of the date said Notice of Exercise is delivered to the Company, the Company shall have received payment of the aggregate Exercise Price of the shares thereby purchased by wire transfer or cashier’s check drawn on a United States bank. Notwithstanding anything herein to the contrary, the Holder shall not be required to physically surrender this Warrant to the Company until the Holder has purchased all of the Warrant Shares available hereunder and the Warrant has been exercised in full, in which case, the Holder shall surrender this Warrant to the Company for cancellation within three (3) Trading Days of the date the final Notice of Exercise is delivered to the Company. Partial exercises of this Warrant resulting in purchases of a portion of the total number of Warrant Shares available hereunder shall have the effect of lowering the outstanding number of Warrant Shares purchasable hereunder in an amount equal to the applicable number of Warrant Shares purchased. The Holder and the Company shall maintain records showing the number of Warrant Shares purchased and the date of such purchases. In the event of any dispute or discrepancy, the records of the Company shall be controlling and determinative in the absence of manifest error.
ii. If at any time there is no effective registration statement registering, or no current prospectus available for, the resale of the Warrant Shares by the Holder, then in lieu of the payment methods set forth in Section 2(a)(i) above, the Holder may elect to exchange all or some of this Warrant for shares of Common Stock equal to the value of the amount of the Warrant being exchanged on the date of exchange. If Holder elects to exchange this Warrant as provided in this Section 2(a)(ii), Holder shall tender to the Company the Warrant for the amount being exchanged, along with written notice of Holder’s election to exchange some or all of the Warrant, and the Company shall issue to Holder the number of shares of the Common Stock computed using the following formula:
| X = | Y (A-B) |
| A |
| Where: X = | the number of shares of Common Stock to be issued to Holder. | |
| Y = | the number of shares of Common Stock purchasable under the amount of the Warrant being exchanged (as adjusted to the date of such calculation). | |
| A = | the Fair Market Value of one share of the Common Stock on the date prior to the date that the notice of exercise is received by the Company. | |
| B = | Exercise Price (as adjusted to the date of such calculation). |
-3-
Notwithstanding anything herein to the contrary, on the Termination Date, this Warrant shall be automatically exercised via cashless exercise pursuant to this Section 2(a)(ii).
(b) Exercise Price. The exercise price per share of Common Stock under this Warrant shall initially be $6.60, subject to adjustment as described herein (the “Exercise Price”).
(c) Exercise Limitations. The Holder shall not have the right to exercise any portion of this Warrant, pursuant to Section 2 or otherwise, if at the time of such exercise the Common Stock is registered pursuant to section 12 of the Exchange Act, and to the extent that after giving effect to such issuance after exercise, the Holder (together with the Holder’s affiliates, and any other person or entity acting as a group together with the Holder or any of the Holder’s affiliates), would beneficially own in excess of the Beneficial Ownership Limitation (as defined below). For purposes of this Section, beneficial ownership shall be calculated in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder. Holder is solely responsible for any schedules required to be filed in accordance therewith. The Company shall have no obligation to verify or confirm the accuracy of such filings. In any case, the number of outstanding shares of Common Stock shall be determined after giving effect to the conversion or exercise of securities of the Company, including this Warrant, by the Holder or its affiliates since the date as of which such number of outstanding shares of Common Stock was reported. The “Beneficial Ownership Limitation” shall be 4.99% of the number of shares of the Common Stock outstanding immediately after giving effect to the issuance of Warrant Shares issuable upon exercise of this Warrant. The Holder may increase or decrease the Beneficial Ownership Limitation provisions of this Section 2(c), provided that any such increase shall not be effective until 61 days’ after the Holder gives notice of such increase to the Company, and the provisions of this Section 2(c) shall continue to apply, unless the Holder upon not less than 61 days’ prior notice to the Company determines to waive the Beneficial Ownership Limitation requirements described in this Section 2(c) in its entirety. Any such increase will not be effective until the 61st day after such notice is delivered to the Company. The limitations contained in this paragraph shall apply to a successor holder of this Warrant.
-4-
(d) Mechanics of Exercise.
i. Delivery of Certificates Upon Exercise. Certificates for shares purchased hereunder shall be transmitted by the Company’s transfer agent (the “Transfer Agent”) to the Holder by crediting the account of the Holder’s prime broker with the Depository Trust Company through its Deposit Withdrawal Agent Commission (“DWAC”) system if the Company is then a participant in such system and either (A) there is an effective registration statement permitting the resale of the Warrant Shares by the Holder or (B) the shares are eligible for resale without volume or manner-of-sale limitations pursuant to Rule 144, and otherwise by physical delivery of certificates to the address specified by the Holder in the Notice of Exercise within two (2) Trading Days from the delivery to the Company of the Notice of Exercise Form, surrender of this Warrant (if required) and payment of the aggregate Exercise Price as set forth above (the “Warrant Share Delivery Date”). This Warrant shall be deemed to have been exercised on the date the Exercise Price is received by the Company. The Warrant Shares shall be deemed to have been issued, and Holder or any other person so designated to be named therein shall be deemed to have become a holder of record of such shares for all purposes, as of the date the Warrant has been exercised by payment to the Company of the Exercise Price (except in the case of a cashless exercise pursuant to Section 2(a)(ii)) and all taxes required to be paid by the Holder, if any, pursuant to Section 2(d)(v) prior to the issuance of such shares, have been paid.
ii. Delivery of New Warrants Upon Exercise. If this Warrant shall have been exercised in part, the Company shall, at the request of a Holder and upon surrender of this Warrant certificate, at the time of delivery of the certificate or certificates representing Warrant Shares, deliver to Holder a new Warrant evidencing the rights of Holder to purchase the unpurchased Warrant Shares called for by this Warrant, which new Warrant shall in all other respects be identical with this Warrant.
iii. Rescission Rights. If the Company fails to cause the Transfer Agent to transmit to the Holder a certificate or the certificates representing the Warrant Shares pursuant to Section 2(d)(i) by the Warrant Share Delivery Date, then the Holder will have the right to rescind such exercise.
-5-
iv. Obligation Absolute; Partial Liquidated Damages. The Company’s obligations to issue and deliver the Warrant Shares upon exercise of this Warrant in accordance with the terms hereof are absolute and unconditional, irrespective of any action or inaction by the Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by the Holder or any other person of any obligation to the Company or any violation or alleged violation of law by the Holder or any other person, and irrespective of any other circumstance which might otherwise limit such obligation of the Company to the Holder in connection with the issuance of such Warrant Shares; provided, however, that such delivery shall not operate as a waiver by the Company of any such action the Company may have against the Holder. The Company may not refuse exercise based on any claim that the Holder or anyone associated or affiliated with the Holder has been engaged in any violation of law, agreement or for any other reason, unless an injunction from a court, on notice to Holder, restraining and or enjoining exercise of all or part of this Warrant shall have been sought and obtained, and the Company posts a surety bond for the benefit of the Holder in the amount of 130% of the Fair Market Value of the Warrant Shares issuable upon exercise of this Warrant, which is subject to the injunction, which bond shall remain in effect until the completion of arbitration/litigation of the underlying dispute and the proceeds of which shall be payable to the Holder to the extent it obtains judgment. In the absence of such injunction, the Company shall issue Warrant Shares. If the Company fails for any reason to deliver to the Holder such certificate or certificates pursuant to Section 2(d)(i) by the third (3rd) Trading Day after the Conversion Date, the Company shall pay to the Holder, in cash, as liquidated damages and not as a penalty, for each $1,000 of Fair Market Value of Warrant Shares being exercised, $10 per Trading Day for each Trading Day after such third (3rd) Trading Day until such certificates are delivered. Nothing herein shall limit a Holder’s right to pursue actual damages or to exercise any other rights of the Holder for the Company’s failure to deliver Warrant Shares within the period specified herein and the Holder shall have the right to pursue all remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief. The exercise of any such rights shall not prohibit the Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
v. Compensation for Buy-In on Failure to Timely Deliver Certificates upon Conversion. In addition to any other rights available to the Holder, if on the Share Delivery Date the Common Stock is listed or quoted on a Trading Market, and the Company fails for any reason to deliver to the Holder such certificate or certificates by the Share Delivery Date, and if after such Share Delivery Date the Holder is required by its brokerage firm to purchase (in an open market transaction or otherwise), or the Holder’s brokerage firm otherwise purchases, shares of Common Stock to deliver in satisfaction of a sale by the Holder of the Warrant Shares which the Holder was entitled to receive upon the exercise relating to such Share Delivery Date (a “Buy-In”), then the Company shall (A) pay in cash to the Holder (in addition to any other remedies available to or elected by the Holder), if any, the amount by which (x) the Holder’s total purchase price (including any brokerage commissions) for the Common Stock so purchased exceeds (y) the product of (1) the aggregate number of shares of Common Stock that the Holder was entitled to receive from the exercise at issue multiplied by (2) the actual per share sale price at which the sell order giving rise to such purchase obligation was executed (including any brokerage commissions) and (B) at the option of the Holder, either reissue (if surrendered) this Warrant for a number of Warrant Shares equal to the number of Warrant Shares of the attempted exercise (in which case such exercise shall be deemed rescinded) or deliver to the Holder the number of shares of Common Stock that would have been issued if the Company had timely complied with its delivery requirements under Section 2(d)(i). For example, if the Holder purchases Common Stock having a total purchase price of $11,000 to cover a Buy-In with respect to an attempted exercise of this Warrant with respect to which the actual sale price of the Warrant Shares (including any brokerage commissions) giving rise to such purchase obligation was a total of $10,000 under clause (A) of the immediately preceding sentence, the Company shall be required to pay the Holder $1,000. The Holder shall provide the Company written notice indicating the amounts payable to the Holder in respect of the Buy-In and, upon request of the Company, evidence of the amount of such loss. Nothing herein shall limit a Holder’s right to pursue any other remedies available to it hereunder, at law or in equity including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock upon exercise of this Warrant as required pursuant to the terms hereof.
-6-
vi. No Fractional Shares or Scrip. No fractional shares or scrip representing fractional shares shall be issued upon the exercise of this Warrant. As to any fraction of a share which the Holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the Exercise Price or round up to the next whole share.
v. Charges, Taxes and Expenses. Issuance of certificates for Warrant Shares shall be made without charge to the Holder for any issue or transfer tax or other incidental expense in respect of the issuance of such certificate, all of which taxes and expenses shall be paid by the Company, and such certificates shall be issued in the name of the Holder or in such name or names as may be directed by the Holder; provided, however, that in the event certificates for Warrant Shares are to be issued in a name other than the name of the Holder, this Warrant when surrendered for exercise shall be accompanied by the assignment form (“Assignment Form”) attached hereto as Exhibit B duly executed by the Holder and the Company may require, as a condition thereto, the payment of a sum sufficient to reimburse it for any transfer tax incidental thereto. The Company shall pay any Transfer Agent fees required for same-day processing of any Notice of Exercise and any fees to the Depository Trust Company (or another established clearing corporation performing similar functions) required for same-day electronic delivery of the Warrant Shares.
vi. Closing of Books. The Company will not close its shareholder books or records in any manner which prevents the timely exercise of this Warrant, pursuant to the terms hereof.
Section 3. Certain Adjustments.
(a) Stock Dividends and Splits. If, at any time after the Initial Exercise Date until the Termination Date, the Company: (i) pays a stock dividend or otherwise make a distribution or distributions on shares of its Common Stock or any other equity or equity equivalent securities payable in shares of Common Stock (which, for avoidance of doubt, shall not include any Warrant Shares issued by the Company upon exercise of this Warrant), (ii) subdivides outstanding shares of Common Stock into a larger number of shares, (iii) combines (including by way of reverse stock split) outstanding shares of Common Stock into a smaller number of shares or (iv) issues by reclassification of shares of the Common Stock any shares of capital stock of the Company, then in each case the Exercise Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding treasury shares, if any) outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event and the number of shares issuable upon exercise of this Warrant shall be proportionately adjusted such that the aggregate Exercise Price of this Warrant shall remain unchanged. Any adjustment made pursuant to this Section 3(a) shall become effective immediately after the record date for the determination of shareholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or re-classification.
-7-
(b) Subsequent Equity Sales. If, at any time after the Initial Exercise Date while this Warrant is outstanding, the Company sells or grants any option to purchase or sells or grants any right to reprice, or otherwise disposes of or issues (or announces any sale, grant or any option to purchase or other disposition), any Common Stock or Common Stock Equivalents at an effective price per share that is lower than the then Exercise Price (such issuances, collectively, a “Dilutive Issuance”) (if the holder of the Common Stock or Common Stock Equivalents so issued shall at any time, whether by operation of purchase price adjustments, reset provisions, floating conversion, exercise or exchange prices or otherwise, or due to warrants, options or rights per share which are issued in connection with such issuance, be entitled to receive shares of common stock at an effective price per share that is lower than the Exercise Price, such issuance shall be deemed to have occurred for less than the Exercise Price on such date of the Dilutive Issuance), then immediately upon such issuance or sale (or deemed issuance or sale) the Exercise Price in effect immediately prior to such issuance or sale (or deemed issuance or sale) shall be reduced (and in no event increased) to an Exercise Price equal to the quotient obtained by dividing:
(A) the sum of (1) the product obtained by multiplying the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) by the Exercise Price then in effect plus (2) the aggregate consideration, if any, received by the Company upon such issuance or sale (or deemed issuance or sale); by
(B) the sum of (1) the Common Stock Deemed Outstanding immediately prior to such issuance or sale (or deemed issuance or sale) plus (2) the aggregate number of shares of Common Stock issued or sold (or deemed issued or sold) by the Company in such issuance or sale (or deemed issuance or sale).
“Common Stock Deemed Outstanding” means, at any given time, the sum of (a) the number of shares of Common Stock actually outstanding at such time, plus (b) the number of shares of Common Stock issuable upon exercise of any warrants or other rights or options to subscribe for or purchase Common Stock and conversion or exchange of any securities (directly or indirectly) convertible into or exchangeable for Common Stock, in each case actually outstanding at such time (treating as actually outstanding any such warrants, rights, options or other securities issuable upon exercise of other such securities actually outstanding at such time), in each case, regardless of whether such securities are actually exercisable, convertible or exchangeable at such time; provided, that Common Stock Deemed Outstanding at any given time shall not include shares owned or held by or for the account of the Company or any of its wholly-owned subsidiaries.
-8-
Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. Notwithstanding the foregoing, no adjustment will be made under this Section 3(b) in respect of an Exempt Issuance (as defined below).
The Company shall notify the Holder in writing, no later than one (1) Business Day following the issuance of any Common Stock or Common Stock Equivalents subject to this Section 3(b), indicating therein the applicable issuance price, or applicable reset price, exchange price, conversion price and other pricing terms (such notice, the “Dilutive Issuance Notice”). For purposes of clarification, whether or not the Company provides a Dilutive Issuance Notice pursuant to this Section 3(b), upon the occurrence of any Dilutive Issuance, the Holder is entitled to receive a number of Conversion Shares based upon the new Conversion Price provided above on or after the date of such Dilutive Issuance, regardless of whether the Holder accurately refers to the new Conversion Price in the Notice of Conversion.
“Exempt Issuance” means the issuance of (a) shares of Common Stock, restricted stock units or options, and the underlying shares of Common Stock to consultants, employees, officers or directors of the Company pursuant to any stock or option plan duly adopted for such purpose, by a majority of the non-employee members of the Board of Directors or a majority of the members of a committee of non-employee directors established for such purpose for services rendered to the Company, (b) securities issued upon the exercise or exchange of or conversion of any securities issued hereunder and/or other securities issuable pursuant to existing agreements, exercisable or exchangeable for or convertible into shares of Common Stock issued and outstanding on the date of this Warrant and disclosed in the Subscription Agreement, provided that such securities have not been amended since the date of this Warrant to increase the number of such securities or to decrease the exercise price, exchange price or conversion price of such securities (other than in connection with stock dividends, stock splits or combinations) or to extend the term of such securities, (c) securities issued pursuant, acquisitions or strategic transactions approved by a majority of the directors of the Company, provided that any such issuance shall only be to a Person (or to the equity holders of a Person) which is, itself or through its Subsidiaries, an operating company or an owner of an asset in a business synergistic with the business of the Company and which shall reasonably be expected to provide to the Company additional benefits, but shall not include a transaction in which the Company is issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities, (d) securities issued pursuant to any purchase money equipment loan or capital leasing arrangement, purchasing agent or debt financing from a commercial bank or similar financial institution, (e) securities issued pursuant to any presently outstanding warrants disclosed in the Subscription Agreement or this Warrant, and (f) securities upon a stock split, stock dividend or subdivision of the Common Stock and shares of common stock in a public offering.
-9-
(c) Pro Rata Distributions. If, at any time after the Initial Exercise Date until the Termination Date, the Company shall distribute to all holders of Common Stock (and not to the Holder) evidences of its indebtedness or assets (including cash and cash dividends) or rights or warrants to subscribe for or purchase any security other than the Common Stock, then in each such case the Exercise Price shall be adjusted by multiplying the Exercise Price in effect immediately prior to the record date fixed for determination of stockholders entitled to receive such distribution by a fraction of which the denominator shall be the VWAP determined as of the record date mentioned above, and of which the numerator shall be such VWAP on such record date less than the per share Fair Market Value at such record date of the portion of such assets or evidence of indebtedness or rights or warrants so distributed applicable to one outstanding share of the Common Stock as determined by the Board of Directors in good faith. In either case the adjustments shall be described in a statement provided to the Holder of the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above.
(d) Fundamental Transaction. If, at any time prior to the Termination Date, (i) the Company effects any merger or consolidation of the Company into another Person, (ii) the Company effects any sale of all or substantially all of its assets in one or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Company or another Person) is completed pursuant to which holders of Common Stock are permitted to tender or exchange their shares for other securities, cash or property or (iv) the Company effects any reclassification of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property (each “Fundamental Transaction”), then, upon any subsequent exercise of this Warrant, the Holder shall have the right to receive, for each Warrant Share that would have been issuable upon such exercise immediately prior to the occurrence of such Fundamental Transaction, the number of shares of Common Stock of the successor or acquiring corporation or of the Company, if it is the surviving corporation, and any additional consideration (the “Alternate Consideration”) receivable as a result of such Fundamental Transaction by a holder of the number of shares of Common Stock for which this Warrant is exercisable immediately prior to such Fundamental Transaction. For purposes of any such exercise, the determination of the Exercise Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Company shall apportion the Exercise Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holder shall be given the same choice as to the Alternate Consideration it receives upon any exercise of this Warrant following such Fundamental Transaction. To the extent necessary to effectuate the foregoing provisions, any successor to the Company or surviving entity in such Fundamental Transaction shall issue to the Holder a new warrant consistent with the foregoing provisions and evidencing the Holder’s right to exercise such warrant into Alternate Consideration. The terms of any agreement pursuant to which a Fundamental Transaction is effected shall include terms requiring any such successor or surviving entity to comply with the provisions of this Section 3(c) and insuring that this Warrant (or any such replacement security) will be similarly adjusted upon any subsequent transaction analogous to a Fundamental Transaction.
-10-
(e) Subsequent Rights Offerings. In addition to any adjustments pursuant to this Section 3, if at any time prior to the Termination Date the Company grants, issues or sells any Common Stock Equivalents or rights to purchase stock, warrants, securities or other property pro rata to all of the record holders of any class of shares of Common Stock (the “Purchase Rights”), then the Holder will be entitled to acquire, upon the terms applicable to such Purchase Rights, the aggregate Purchase Rights which the Holder could have acquired if the Holder had held the number of shares of Common Stock acquirable upon complete exercise of this Warrant (without regard to any limitations on exercise hereof, including without limitation, the Beneficial Ownership Limitation) immediately before the date on which a record is taken for the grant, issuance or sale of such Purchase Rights, or, if no such record is taken, the date as of which the record holders of shares of Common Stock are to be determined for the grant, issue or sale of such Purchase Rights (provided, however, to the extent that the Holder’s right to participate in any such Purchase Right would result in the Holder exceeding the Beneficial Ownership Limitation, then the Holder shall not be entitled to participate in such Purchase Right to such extent (or beneficial ownership of such shares of Common Stock as a result of such Purchase Right to such extent) and such Purchase Right to such extent shall be held in abeyance for the Holder until such time, if ever, as its right thereto would not result in the Holder exceeding the Beneficial Ownership Limitation.
(f) Calculations. All calculations under this Section 3 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be. For purposes of this Section 3, the number of shares of Common Stock deemed to be issued and outstanding as of a given date shall be the sum of the number of shares of Common Stock (excluding treasury shares, if any) issued and outstanding.
(g) Notice to Holder.
i. Adjustment to Exercise Price. Whenever the Exercise Price is adjusted pursuant to any provision of this Section 3, the Company shall promptly mail to the Holder a notice setting forth the Exercise Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
-11-
ii. Notice to Allow Exercise by Holder. If (A) the Company shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Company shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Company shall authorize the granting to all holders of the Common Stock rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any shareholders of the Company shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Company is a party, any sale or transfer of all or substantially all of the assets of the Company, of any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Company shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Company, then, in each case, the Company shall cause to be mailed to the Holder at its last address as it shall appear upon the Warrant Register of the Company, at least 20 calendar days prior to the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange; provided that the failure to mail such notice or any defect therein or in the mailing thereof shall not affect the validity of the corporate action required to be specified in such notice. The Holder is entitled to exercise this Warrant during the period commencing on the date of such notice to the effective date of the event triggering such notice.
Section 4. Transfer of Warrant.
a) Transferability. Subject to compliance with any applicable securities laws and the conditions set forth in Section 4(d) herein and to the provisions of the Subscription Agreement, this Warrant and all rights hereunder (including, without limitation, any registration rights) are transferable, in whole or in part, upon surrender of this Warrant at the principal office of the Company or its designated agent, together with an Assignment Form duly executed by the Holder or its agent or attorney and funds sufficient to pay any transfer taxes payable upon the making of such transfer. Upon such surrender and, if required, such payment, the Company shall execute and deliver a new Warrant or Warrants in the name of the assignee or assignees, as applicable, and in the denomination or denominations specified in such instrument of assignment, and shall issue to the assignor a new Warrant evidencing the portion of this Warrant not so assigned, and this Warrant shall promptly be cancelled. The Warrant, if properly assigned, may be exercised by a new holder for the purchase of Warrant Shares without having a new Warrant issued.
b) New Warrants. This Warrant may be divided or combined with other Warrants upon presentation hereof at the aforesaid office of the Company, together with a written notice specifying the names and denominations in which new Warrants are to be issued, signed by the Holder or its agent or attorney. Subject to compliance with Section 4(a), as to any transfer which may be involved in such division or combination, the Company shall execute and deliver a new Warrant or Warrants in exchange for the Warrant or Warrants to be divided or combined in accordance with such notice. All Warrants issued on transfers or exchanges shall be dated the Initial Exercise Date and shall be identical with this Warrant except as to the number of Warrant Shares issuable pursuant thereto.
-12-
c) Warrant Register. The Company shall register this Warrant, upon records to be maintained by the Company for that purpose (the “Warrant Register”), in the name of the record Holder hereof from time to time. The Company may deem and treat the registered Holder of this Warrant as the absolute owner hereof for the purpose of any exercise hereof or any distribution to the Holder, and for all other purposes, absent actual notice to the contrary.
d) Transfer Restrictions. If, at the time of the surrender of this Warrant in connection with any transfer of this Warrant, the transfer of this Warrant shall not be either (i) registered pursuant to an effective registration statement under the Securities Act and under applicable state securities or blue sky laws or (ii) eligible for resale without volume or manner-of-sale restrictions pursuant to Rule 144, the Company may require, as a condition of allowing such transfer, that the Holder or transferee of this Warrant, as the case may be, comply with the provisions of the Subscription Agreement.
Section 5. Miscellaneous.
a) No Rights as Shareholder Until Exercise. This Warrant does not entitle the Holder to any voting rights or other rights as a shareholder of the Company prior to the exercise hereof.
b) Loss, Theft, Destruction or Mutilation of Warrant. The Company covenants that upon receipt by the Company of evidence reasonably satisfactory to it of the loss, theft, destruction or mutilation of this Warrant or any stock certificate relating to the Warrant Shares, and in case of loss, theft or destruction, of indemnity or security reasonably satisfactory to it (which, in the case of the Warrant, shall not include the posting of any bond), and upon surrender and cancellation of such Warrant or stock certificate, if mutilated, the Company will make and deliver a new Warrant or stock certificate of like tenor and dated as of such cancellation, in lieu of such Warrant or stock certificate.
c) Saturdays, Sundays, Holidays, etc. If the last or appointed day for the taking of any action or the expiration of any right required or granted herein shall not be a Business Day, then, such action may be taken or such right may be exercised on the next succeeding Business Day.
d) Authorized Shares.
The Company covenants that, during the period the Warrant is outstanding, it will reserve from its authorized and unissued Common Stock one hundred (100%) of the number of shares to provide for the issuance of the Warrant Shares upon the exercise of any purchase rights under this Warrant. In case such amount of Common Stock is insufficient at any time, the Company shall call and hold a special meeting to increase the number of authorized shares of common stock. Management of the Company shall recommend to shareholders to vote in favor of increasing the number of authorized shares of common stock.
-13-
The Company further covenants that its issuance of this Warrant shall constitute full authority to its officers who are charged with the duty of executing stock certificates to execute and issue the necessary certificates for the Warrant Shares upon the exercise of the purchase rights under this Warrant. The Company will take all such reasonable action as may be necessary to assure that such Warrant Shares may be issued as provided herein without violation of any applicable law or regulation, or of any requirements of the National Securities Exchange upon which the Common Stock may be listed. The Company covenants that all Warrant Shares which may be issued upon the exercise of the purchase rights represented by this Warrant will, upon exercise of the purchase rights represented by this Warrant, be duly authorized, validly issued, fully paid and nonassessable and free from all taxes, liens and charges created by the Company in respect of the issue thereof (other than taxes in respect of any transfer occurring contemporaneously with such issue).
Except and to the extent as waived or consented to by the Holder, the Company shall not by any action, including, without limitation, amending its certificate of incorporation, as amended, or through any reorganization, transfer of assets, consolidation, merger, dissolution, issue or sale of securities or any other voluntary action, avoid or seek to avoid the observance or performance of any of the terms of this Warrant, but will at all times in good faith assist in the carrying out of all such terms and in the taking of all such actions as may be necessary or appropriate to protect the rights of Holder as set forth in this Warrant against impairment. Without limiting the generality of the foregoing, the Company will (i) not increase the par value of any Warrant Shares above the amount payable therefor upon such exercise immediately prior to such increase in par value, (ii) take all such action as may be necessary or appropriate in order that the Company may validly and legally issue fully paid and nonassessable Warrant Shares upon the exercise of this Warrant and (iii) use commercially reasonable efforts to obtain all such authorizations, exemptions or consents from any public regulatory body having jurisdiction thereof, as may be, necessary to enable the Company to perform its obligations under this Warrant.
Before taking any action which would result in an adjustment in the number of Warrant Shares for which this Warrant is exercisable or in the Exercise Price, the Company shall obtain all such authorizations or exemptions thereof, or consents thereto, as may be necessary from any public regulatory body or bodies having jurisdiction thereof.
e) Jurisdiction. All questions concerning the construction, validity, enforcement and interpretation of this Warrant shall be determined in accordance with the provisions of the Subscription Agreement.
f) Restrictions. The Holder acknowledges that the Warrant Shares acquired upon the exercise of this Warrant, if not registered, will have restrictions upon resale imposed by state and federal securities laws.
g) Nonwaiver and Expenses. No course of dealing or any delay or failure to exercise any right hereunder on the part of Holder shall operate as a waiver of such right or otherwise prejudice Holder’s rights, powers or remedies, notwithstanding the fact that all rights hereunder terminate on the Termination Date. If the Company willfully and knowingly fails to comply with any provision of this Warrant, which results in any material damages to the Holder, the Company shall pay to Holder such amounts as shall be sufficient to cover any costs and expenses including, but not limited to, reasonable attorneys’ fees, including those of appellate proceedings, incurred by Holder in collecting any amounts due pursuant hereto or in otherwise enforcing any of its rights, powers or remedies hereunder.
-14-
h) Notices. Any notice, request or other document required or permitted to be given or delivered to the Holder by the Company shall be delivered in accordance with the notice provisions of the Subscription Agreement.
i) Limitation of Liability. No provision hereof, in the absence of any affirmative action by Holder to exercise this Warrant to purchase Warrant Shares, and no enumeration herein of the rights or privileges of Holder, shall give rise to any liability of Holder for the purchase price of any Common Stock or as a shareholder of the Company, whether such liability is asserted by the Company or by creditors of the Company.
j) Remedies. The Holder, in addition to being entitled to exercise all rights granted by law, including recovery of damages, will be entitled to specific performance of its rights under this Warrant. The Company agrees that monetary damages would not be adequate compensation for any loss incurred by reason of a breach by it of the provisions of this Warrant and hereby agrees to waive and not to assert the defense in any action for specific performance that a remedy at law would be adequate.
k) Successors and Assigns. Subject to applicable securities laws, this Warrant and the rights and obligations evidenced hereby shall inure to the benefit of and be binding upon the successors of the Company and the successors and permitted assigns of Holder. The provisions of this Warrant are intended to be for the benefit of all Holders from time to time of this Warrant and shall be enforceable by the Holder or holder of Warrant Shares.
l) Amendment. This Warrant may be modified or amended or the provisions hereof waived with the written consent of the Company and the Holder.
m) Severability. Wherever possible, each provision of this Warrant shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Warrant shall be prohibited by or invalid under applicable law, such provision shall be ineffective to the extent of such prohibition or invalidity, without invalidating the remainder of such provisions or the remaining provisions of this Warrant.
n) Headings. The headings used in this Warrant are for the convenience of reference only and shall not, for any purpose, be deemed a part of this Warrant.
[Signature Page Follows.]
-15-
IN WITNESS WHEREOF, the Company has caused this Warrant to be executed by its officer thereunto duly authorized as of the date first above indicated.
| Adaptin BIO, INC. | |||
| By: | |||
| Name: | Michael J. Roberts | ||
| Title: | Chief Executive Officer | ||
[SIGNATURE PAGE TO ADAPTIN BIO, INC. WARRANT]
EXHIBIT A
NOTICE OF EXERCISE
TO: ADAPTIN BIO, INC.
(1) The undersigned hereby elects to purchase ________ Warrant Shares of the Company pursuant to the terms of the attached Warrant and
☐ tenders herewith payment of the exercise price in full, together with all applicable transfer taxes, if any; or
☐ elects to purchase the number of Warrant Shares stated above pursuant to the cashless exercise procedure set forth in Section 2(a) of the Warrant
(2) Please issue a certificate or certificates representing said Warrant Shares in the name of the undersigned or in such other name as is specified below:
_______________________________
The Warrant Shares shall be delivered to the following DWAC Account Number or by physical delivery of a certificate to:
_______________________________
_______________________________
_______________________________
(3) Accredited Investor. The undersigned is an “accredited investor” as defined in Regulation D promulgated under the Securities Act of 1933, as amended.
[SIGNATURE OF HOLDER]
Name of Investing Entity: ________________________________________________________________________
Signature of Authorized Signatory of Investing Entity: _________________________________________________
Name of Authorized Signatory: ___________________________________________________________________
Title of Authorized Signatory: ____________________________________________________________________
Date: _______________________________________________________________
EXHIBIT B
ASSIGNMENT FORM
(To assign the foregoing warrant, execute
this form and supply required information.
Do not use this form to exercise the warrant.)
FOR VALUE RECEIVED, [____] all of or [_______] shares of the foregoing Warrant and all rights evidenced thereby are hereby assigned to
_______________________________________________ whose address is
____________________________________________________________.
_______________________________________________________________
Dated: ______________, _______
Holder’s Signature: _____________________________
Holder’s Address: _____________________________
_____________________________
Signature Guaranteed: ___________________________________________
NOTE: The signature to this Assignment Form must correspond with the name as it appears on the face of the Warrant, without alteration or enlargement or any change whatsoever, and must be guaranteed by a bank or trust company. Officers of corporations and those acting in a fiduciary or other representative capacity should file proper evidence of authority to assign the foregoing Warrant.
Exhibit 10.1
CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THE EXHIBIT BECAUSE IT IS BOTH NOT MATERIAL AND IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. SUCH EXCLUDED INFORMATION HAS BEEN MARKED WITH “[*].”
PATENT LICENSE AGREEMENT
This Patent License Agreement (this “Agreement) is effective as of January 11, 2023 (the “EFFECTIVE DATE”), between Centaur Bio, Inc. (“LICENSEE”) having the address in Article 12 below, and Duke University, a nonprofit educational and research institution organized under the laws of North Carolina (“DUKE”). LICENSEE and DUKE hereby agree as follows:
Whereas DUKE and the National Cancer Institute (“NCI”), the agency of the U.S Department of Health and Human Services (“DHHS”) (jointly, the “LICENSORS”) have entered into Interinstitutional Agreements dated January 19, 2022 (NCI References numbers L-067-2022, L- 068-2022 and L-069-2022) granting to DUKE an exclusive license to NCI’s rights in the PATENT RIGHTS, with the right to sublicense, and the right to negotiate license terms and maintain patent protection and licenses for the commercialization of the PATENT RIGHTS as further defined below.
ARTICLE 1 – DEFINITIONS
1.1 “AFFILIATE” means any corporation or non-corporate entity that controls, is controlled by or is under the common control with a party. A corporation or a non-corporate entity, as applicable, is deemed to be in control of another corporation if: (a) it owns or directly or indirectly controls at least 50% of the voting stock of the other corporation; or (b) in the absence of ownership of at least 50% of the voting stock of a corporation, or in the case of a non- corporate entity, if it possesses directly or indirectly, the power to direct or cause the direction of the management and policies of such corporation or non-corporate entity, as applicable.
1.2 “COMBINATION PRODUCT” means any product comprising a combination: of (a) a LICENSED PRODUCT; and (b) any active ingredients (other than a LICENSED PRODUCT), devices, delivery systems, or other technology for which rights are not included in the licenses granted under this Agreement but, with respect to the item(s) in this clause (b) of this Paragraph 1.2, which may each or collectively form the basis for a separately saleable product (such separately saleable product incorporating such additional ingredient, device, delivery system or other technology, an “OTHER PRODUCT”), whether co-formulated or co-packaged.
1.3 “LICENSORS,” as used in Articles 2, 8, 9 and 10, shall include DUKE, NCI and their trustees, officers, employees, students, and agents.
1.4 “FIELD OF USE” means all uses in human health.
1.5 “FIRST COMMERCIAL SALE” means the first SALE through a bona fide arm’s length transaction of any LICENSED PRODUCT or first commercial use of any LICENSED PROCESS by LICENSEE or a SUBLICENSEE, excluding the SALE of a LICENSED PRODUCT or use of a LICENSED PROCESS for use in trials, as a sample, or that is of temporary availability.
1.6 “LICENSED PROCESS(ES)” means any process or method that, but for this Agreement, comprises an infringement of (including contributory or inducement), or is covered by, an issued, unexpired claim or a pending claim contained in the PATENT RIGHTS in the country in which such process or method is performed or employs a LICENSED PRODUCT.
1.7 “LICENSED PRODUCT(S)” means any product that: (a) but for this Agreement comprises an infringement of (including contributory or inducement), or is covered by, an issued, unexpired claim or a pending claim contained in the PATENT RIGHTS in the country in which any such product or product part is made, used, imported, offered for SALE or sold; or (b) is manufactured by using a LICENSED PROCESS or is employed to practice a LICENSED PROCESS.
1.8 “NET SALES” means [*].
If a COMBINATION PRODUCT is sold in a country, NET SALES for the purposes of calculating royalties due on such COMBINATION PRODUCT shall be calculated by [*].
1.9 “PATENT RIGHTS” means LICENSORS’ legal rights under the patent laws of the United States or relevant foreign countries for all of the following:
(a) the following United States and foreign patent(s) and/or patent application(s):
(i) US Issued Patent 9,249,217, “BISPECIFIC EGFRvIII ANTIBODY ENGAGING MOLECULES” (DUKE File 3590);
(ii) US Issued Patent 9,676,858, “HUMAN BISPECIFIC EGFRvIII ANTIBODY AND CD3 ENGAGING MOLECULES” (DUKE File 3590);
(iii) US Issued Patent 10,053,514, “CERTAIN IMPROVED HUMAN BISPECIFIC EGFRvIII ANTIBODY ENGAGING MOLECULES” (DUKE File 4008); and
(iv) US Patent Application 17/265,731, “ENHANCED DELIVERY OF DRUGS AND OTHER COMPOUNDS TO THE BRAIN AND OTHER TISSUES” (DUKE File 6386).
(b) all additions, divisionals, continuations, continuations-in-part (only to the extent that such claims are fully supported by another patent or application in the PATENT RIGHTS), substitutions, reissues, re-examinations, extensions, registrations, patent term extensions, revalidations, supplementary protection certificates, and renewals of any of the foregoing or claiming priority to any of the foregoing, any patents issuing from any of the foregoing, and all foreign applications and patents corresponding to or claiming priority to any of the foregoing.
1.10 “PHASE II STUDY” means a human clinical trial of a LICENSED PRODUCT, the principal purpose of which is to make a preliminary determination that such LICENSED PRODUCT is safe in patients for its intended use and to obtain sufficient information about such LICENSED PRODUCT's efficacy to permit the design of further clinical trials, and generally consistent with 21 CFR § 312.21(b).
2
1.11 “PHASE III STUDY” means a human clinical trial of a LICENSED PRODUCT, the principal purpose of which is to establish safety and efficacy in patients with the disease being studied in a manner sufficient to file for regulatory approval of such LICENSED PRODUCT and would satisfy the requirements of 21 C.F.R. §312.21(c), or a similar clinical study prescribed by the Regulatory Authorities in a country other than the United States.
1.12 “ROYALTY PERIOD(S)” means the six-month periods ending on the last days of June and December each year.
1.13 “ROYALTY TERM” means, on a country-by-country and LICENSED PRODUCT-by- LICENSED PRODUCT basis, the date commencing on the FIRST COMMERCIAL SALE of such LICENSED PRODUCT and continuing until the later of the last to expire PATENT RIGHTS or the fifteenth anniversary of the FIRST COMMERCIAL SALE.
1.14 “SALE” means sale, rental, or lease, however characterized, and SOLD means the past tense of SALE.
1.15 “SUBLICENSEE(S)” means any person or entity in writing sublicensed any of the rights under PATENT RIGHTS granted to LICENSEE under this Agreement, whether such sublicense is granted by LICENSEE, any AFFILIATE thereof, or any preceding SUBLICENSEE.
1.16 “SUBLICENSING FEES” means any consideration actually received by LICENSEE or any AFFILIATE thereof from a third party as consideration for the grant of rights under the PATENT RIGHTS to such third party (net of any tax or similar withholding obligations imposed by any tax or other government authority(ies) that are not reasonably recoverable by LICENSEE) (e.g., license issue fees, maintenance or annual minimum fees, milestone payments, and the like), provided that SUBLICENSING FEES shall exclude: (a) sales-based royalties; (b) purchases of equity or debt securities of LICENSEE or any AFFILIATE thereof for a price equal to or less than the fair market value thereof (as reasonably determined in good faith by LICENSEE), provided that any such amounts paid in excess of fair market value shall be deemed SUBLICENSING FEES; (c) fair market value payments made in connection with research and development agreements, joint ventures, partnerships, or collaboration agreements where LICENSEE or an AFFILIATE of LICENSEE is obligated to perform research and development of any LICENSED PRODUCT or LICENSED PROCESS; and (d) other payments made by a SUBLICENSEE as consideration for performance of services or provision of goods by LICENSEE or an AFFILIATE of LICENSEE.
1.17 “TECHNICAL INFORMATION” means any research information, technical information, technical data, Standard Operating Procedures (“SOPs”), or other information (to the extent relating in each case to the Invention or its use), that is not claimed in the PATENT RIGHTS, and was: (a) generated at DUKE by, under the direct supervision of, or for the benefit of any DUKE inventor on any PATENT RIGHTS before the EFFECTIVE DATE, including any such research information, SOPs, technical information, or technical data; or (b) otherwise owned by DUKE and necessary for practice of the LICENSED PRODUCT or LICENSED PROCESS.
3
1.18 “TERRITORY” means worldwide.
1.19 “VALID CLAIM” means any claim of a pending patent application or issued and unexpired patent, included, in either case, in the PATENT RIGHTS, that: (a) has not been held unpatentable, invalid, or unenforceable by a court or other government agency of competent jurisdiction in a decision over which no appeal can be, or has been, taken; and (b) has not been dedicated to the public, abandoned, or admitted to be invalid, unenforceable, or of a scope not covering the subject matter at issue through reissue, re-examination, disclaimer, or otherwise; provided, however, that if the relevant holding of such court or agency is later reversed by a court or agency with overriding authority, the claim shall be deemed a VALID CLAIM with respect to NET SALES made after the date of such reversal to the extent such claim otherwise remains a VALID CLAIM under this definition.
ARTICLE 2 – GRANT OF LICENSE
2.1 DUKE hereby grants to LICENSEE an exclusive license under the PATENT RIGHTS, with the right to grant sublicenses (through multiple tiers), and a non-exclusive license to TECHNICAL INFORMATION, subject to the terms and conditions of this Agreement, in the FIELD OF USE and the TERRITORY to make, have made, import, use, market, offer for sale and sell LICENSED PRODUCTS and to sell, use, or provide LICENSED PROCESSES. The rights and licenses granted by DUKE to LICENSEE under this Paragraph 2.1 include the grant of such rights and licenses to any AFFILIATES of LICENSEE if any such AFFILIATE expressly assumes, in writing, the same obligations as those of LICENSEE. LICENSEE shall be responsible for the performance of all obligations by such AFFILIATES and for such AFFILIATES’ compliance with all terms and conditions of this Agreement. References to LICENSEE under this Agreement shall be deemed to also include references to any such AFFILIATE.
2.2 LICENSORS retain the right to practice or license any invention, product, or method covered by the PATENT RIGHTS for their own noncommercial purposes (as defined below) without restriction and without payment of royalties or other fees, including without limitation the right to provide licenses to the PATENT RIGHTS to governmental laboratories and to other non-profit or not-for-profit institutions solely for non-commercial purposes. For the purposes of this agreement “noncommercial purposes” means the use of PATENT RIGHTS for academic educational, research, clinical or other not-for-profit or scholarly purposes which are undertaken at a non-profit or governmental institution that does not use PATENT RIGHTS in the production or manufacture of products for sale or the performance of services for a fee.
2.3 Notwithstanding the above, the Government of the United States (“Government”) shall have the irrevocable, royalty-free, paid-up right to practice and have practiced the PATENT RIGHTS throughout the world by or on behalf of the Government and on behalf of any foreign government or international organization pursuant to any existing or future treaty or agreement to which the Government is a signatory.
2.4 NCI reserves the right to require LICENSEE or its SUBLICENSEES, to grant SUBLICENSES to responsible applicants, on terms that are reasonable under the circumstances when necessary to fulfill health or safety needs or when necessary to meet requirements for public use specified by Federal regulations.
4
2.5 In addition to the reserved licenses of Paragraphs 2.2, 2.3 and 2.4, LICENSORS reserve the right to grant nontransferable, nonexclusive licenses to make and to use any tangible embodiment of the PATENT RIGHTS and to practice any process(es) included within the PATENT RIGHTS for purposes of internal research and not for purposes of commercial manufacture or distribution or in lieu of purchase (“Research License”) directly or to require the LICENSEE to grant Research Licenses on reasonable terms. The purpose of these Research Licenses is to encourage basic research, whether conducted at an academic or corporate facility. In order to safeguard the licensed PATENT RIGHTS, however, the LICENSORS shall consult with the LICENSEE before granting to commercial entities a Research License or providing to them research samples of materials made through the LICENSED PROCESSES.”
2.6 LICENSEE shall supply to NCI at the address in Article 12 with inert samples of the LICENSED PRODUCTS and LICENSED PROCESSES, as covered by the PATENT RIGHTS, or their packaging for educational and display purposes only.
2.7 This Agreement shall extend until expiration of the last to expire of the PATENT RIGHTS, unless sooner terminated as provided in another specific provision of this Agreement.
2.8 The licenses granted in this Agreement are subject to any rights required to be granted under applicable law as a result of prior research or sponsorship agreements, or required to be retained by the U.S. government, for example in accordance with Chapter 18 of Title 35 of U.S.C. 200-212 and the regulations thereunder (37 CFR Part 401), when applicable. LICENSEE agrees to comply in all respects, including with respect to any applicable requirement that LICENSED PRODUCTS used, leased or sold in the United States shall be manufactured substantially in the United States; and shall provide DUKE with all reasonably requested information and cooperation necessary for DUKE to comply with applicable provisions of the same and any requirements of any agreements between DUKE and any agency of the U.S. government that provided funding for the subject matter covered by the PATENT RIGHTS. LICENSEE agrees to mark the LICENSED PRODUCTS sold in the United States with all applicable United States patent numbers as necessary to meet the requirements of 35 U.S.C. 287 so that the full benefits of patent enforcement may be realized. All LICENSED PRODUCTS shipped to or sold in other countries shall be marked to comply with the patent laws and practices of the countries of manufacture, use and SALE.
2.9 Board Observation Rights. LICENSEE hereby agrees to cause DUKE to have observation rights at any of LICENSEE’s Board of Directors meetings. At such meetings, DUKE, at its option, shall be permitted to have a designee (the “OBSERVER”) attend in-person or via telephone for sessions covering matters relating to LICENSEE’s business, provided that: (a) the OBSERVER will be excused from any Board activities involving attorney-client privilege or a conflict of interest; and (b) such rights shall terminate on the earliest of (i) LICENSEE becoming subject to the reporting requirements of the Securities Exchange Act of 1934 (its “IPO”), (ii) a CHANGE OF CONTROL of LICENSEE (as defined below), or (iii) such time as DUKE’s equity interest in LICENSEE is less than [*] percent ([*]%) of TOTAL OWNERSHIP INTERESTS.
5
ARTICLE 3 - CONSIDERATION
3.1 LICENSEE shall pay the following to DUKE:
(a) During the ROYALTY TERM, running royalties according to the following schedule on a country-by-country and product-by-product basis (such royalties, “RUNNING ROYALTIES”):
(i) until the date on which there are no VALID CLAIMS in a particular country COVERING (as defined below) a particular LICENSED PRODUCT (or the manufacture of use thereof), [*] percent ([*]%) of annual NET SALES by LICENSEE, SUBLICENSEE or any AFFILIATE thereof, up to and including $[*] and [*] percent ([*]%) of annual NET SALES above $[*]; and
(ii) following the date on which there are no VALID CLAIMS in a particular country COVERING a particular LICENSED PRODUCT (or the manufacture of use thereof), [*]% of annual NET SALES up to and including $[*] and [*]% of annual NET SALES above $[*].
“COVERING” means the use, manufacture, sale, offer for sale, development, commercialization or importation of the subject matter in question by an unlicensed entity would infringe a VALID CLAIM of a PATENT RIGHT.
If LICENSEE or any SUBLICENSEE determines that it is necessary to pay consideration to any Third Party that holds a patent(s) that would, in the reasonable judgement of LICENSEE be infringed by the manufacture, use, offer for SALE or SALE of a LICENSED PRODUCT, LICENSEE shall provide written notice of such determination to DUKE. If LICENSEE or any SUBLICENSEE pays fees, milestones or royalties or other consideration to any such Third Party for any such rights (such consideration, “Third-Party Technology Payments”), then LICENSEE may deduct up to [*] percent ([*]%) of the Third-Party Technology Payments from the amounts due to DUKE hereunder. Notwithstanding the foregoing, the total amount payable to DUKE for a specific milestone payment or royalty shall not be reduced by more than [*] percent ([*]%), as a result of any such deduction.
Upon expiration of the ROYALTY TERM with respect to a particular LICENSED PRODUCT and country, (i) LICENSEE and its AFFILIATES shall have a perpetual, unrestricted, irrevocable, fully paid, royalty-free non-exclusive right and license, with rights of sublicense (through multiple tiers of sublicensees), to use TECHNICAL INFORMATION to make, have made, use, sell, offer for sale, and import such LICENSED PRODUCT in such country and (ii) LICENSEE’s obligations under this Agreement with respect to such LICENSED PRODUCT shall terminate.
6
(b) LICENSEE shall pay DUKE a share of the SUBLICENSING FEES according to the following schedule:
Date of execution of applicable SUBLICENSE | Percentage of SUBLICENSING FEES payable to DUKE | |
| Prior to initiation of the first PHASE II STUDY | [*]% | |
| After initiation of the first PHASE II STUDY but prior to initiation of the first PHASE III STUDY | [*]% | |
| After initiation of the first PHASE III STUDY | [*]% |
For the purposes of this Agreement, a clinical study shall be deemed initiated upon the first administration of LICENSED PRODUCT to a subject enrolled in such study.
(c) In addition to payment of ongoing patent expenses pursuant to Article 7 hereof, LICENSEE shall reimburse DUKE for previous patent expenses incurred by DUKE for filing and prosecution of PATENT RIGHTS as of the EFFECTIVE DATE (“PAST PATENT EXPENSES”). PAST PATENT EXPENSES shall be paid in four (4) equal installments, with each such installment payable on the last day of each calendar quarter starting on the first full calendar quarter following the EFFECTIVE DATE.
(d) Minimum Annual Royalties. Minimum annual royalties are due for the applicable calendar year on each following January 31, as set forth below (“MINIMUM ANNUAL ROYALTIES”). MINIMUM ANNUAL ROYALTIES shall only be payable with respect to a particular calendar year to the extent Running Royalties payable under Paragraph 3.1(a) with respect to NET SALES made during such calendar year do not equal or exceed such calendar year’s MINIMUM ANNUAL ROYALTY. MINIMUM ANNUAL ROYALTIES paid in excess of Running Royalties shall not be creditable to amounts due for future years. The MINIMUM ANNUAL ROYALTIES are:
| (1) | In 2025: $[*]; |
| (2) | In 2026 and in each year thereafter: $[*]. |
(e) Milestone payments are due within thirty (30) days of LICENSEE achieving the following milestones, as set forth below (“MILESTONE PAYMENTS”). In the event a SUBLICENSEE pays LICENSEE a fee for achieving one of the milestone events listed below, LICENSEE shall pay the higher of: (i) the MILESTONE PAYMENT in this Paragraph 3.1(e); or (ii) the SUBLICENSING FEE due on such payment pursuant to Paragraph 3.1(b).
7
| (1) | $[*] upon initiation of PHASE II STUDY. |
| (2) | $[*] upon initiation of PHASE III STUDY. |
| (3) | $[*] upon first submission of an application for market approval of a LICENSED PRODUCT in the United States. |
| (4) | $[*] upon first submission of an application for regulatory approval of a LICENSED PRODUCT in any one of the United Kingdom, Germany, France, Italy, or Spain. |
| (5) | $[*] upon first submission of an application for market approval of a LICENSED PRODUCT in Japan. |
| (6) | $[*] upon FIRST COMMERCIAL SALE of LICENSED PRODUCT in the United States. |
| (7) | $[*] upon FIRST COMMERCIAL SALE of LICENSED PRODUCT in any one of the United Kingdom, Germany, France, Italy, or Spain. |
| (8) | $[*] upon FIRST COMMERCIAL SALE of LICENSED PRODUCT in Japan. |
MILESTONE PAYMENTS are non-refundable and non-creditable. MILESTONE PAYMENTS are payable only one time, regardless of the number of LICENSED PRODUCTS, formulations thereof, or indications therefor.
3.2 LICENSEE is not obligated to pay multiple royalties if any LICENSED PRODUCT or LICENSED PROCESS is covered by more than one claim of PATENT RIGHTS or the same LICENSED PRODUCT is covered by claims in two or more countries.
3.3 All payments due to DUKE under this Agreement shall be made payable to “Duke University.” Payments drawn directly on a U.S. bank may be made by either check to the address in Article 12 or by wire transfer. Any payment drawn on a foreign bank or foreign branch of a U.S. bank shall be made only by wire transfer. Wire transfers shall be made in accordance with the following or any other instructions as may be specified by DUKE. If payments are made by wire, the wiring instructions below must be followed.
| Bank: | [*] | |
| ABA #: [*] | ||
| Swift Code: | [*] | |
| Beneficiary: | [*] | |
| Account #: | [*] | |
| Attention: | [*] | |
| Email: | [*] |
All payments due to DUKE under this Agreement must be paid in United States Dollars in Durham, North Carolina, or at such place as DUKE may reasonably designate consistent with the laws and regulations controlling in any foreign country. If any currency conversion is required in connection with such payments due, such conversion must be made by using the exchange rate prevailing at Wells Fargo Bank (N.A.) (or its successor, as the case may be) on the last business day of the reporting period to which such payments relate.
8
3.4 Royalty payments shall be made on a semi-annual basis with submission of the reports required by Article 4. All amounts due under this Agreement, including amounts due for the payment of patent expenses, shall, if overdue, be subject to a charge of interest compounded monthly until payment, at a per annum rate of [*] percent ([*]%) above the prime rate in effect at the JP Morgan Chase Bank, N.A. or its successor bank on the due date (or at the highest allowed rate if a lower rate is required by law). The payment of such interest shall not foreclose DUKE from exercising any other rights it may have resulting from any late payment. LICENSEE shall reimburse DUKE for its reasonable and documented out-of-pocket expenses, including reasonable attorneys’ fees, for expenses paid in order to collect any amounts overdue more than 120 days.
3.5 All payments made under this Agreement are and shall be non-refundable and, except as set forth in this Agreement, non-creditable. DUKE shall have no obligation whatsoever to pay, return, credit, or refund any amounts paid hereunder, except as may be specifically provided in this Agreement. By way of example only, notwithstanding the deductions permitted to NET SALES, DUKE shall have no obligation to pay any amounts to LICENSEE even if such deductions should result in a negative amount for NET SALES in any given ROYALTY PERIOD.
3.6 Should LICENSEE be required under any law or regulation of any government entity or authority to withhold or deduct any portion of the payments on royalties due to DUKE, then the sum payable to DUKE shall be increased by the amount necessary (if any) to ensure that DUKE receives an amount equal to the sum it would have received had no withholdings or deductions been required. DUKE shall cooperate reasonably with LICENSEE or any SUBLICENSEE in the event LICENSEE or any SUBLICENSEE elects to seek or assert, at its own expense, any exemption from, refund of, or credit with respect to any such tax or deduction. Additionally, if DUKE is issued or awarded any credit or refund, the amount of any such credit or refund shall be applied against future amounts payable to DUKE under this Agreement.
3.7.1 Equity. On the EFFECTIVE DATE, LICENSEE must issue to DUKE shares of LICENSEE’s common stock representing five percent (5%) of the TOTAL OWNERSHIP INTERESTS in LICENSEE as of the EFFECTIVE DATE, subject to DUKE’s execution, and pursuant to the terms of the Equity Transfer Agreement attached herein as Appendix A.
“TOTAL OWNERSHIP INTERESTS” shall mean the total number of issued and outstanding shares of LICENSEE on a fully diluted basis, including, for purposes of such calculation, issued and outstanding shares of LICENSEE’s common stock and other capital stock, all shares of LICENSEE’s capital stock subject to granted, unexercised stock options, all shares of LICENSEE’s capital stock reserved for issuance under LICENSEE’s stock option plan that have not been issued and are not the subject of granted, unexercised stock options, all shares of LICENSEE’s capital stock that can be issued pursuant to the exercise of issued, unexercised warrants, and any other outstanding securities issued by LICENSEE that may become convertible to LICENSEE’s capital stock (all as calculated on as as-converted-into- LICENSEE’s-common-stock-basis, but excluding debt securities).
9
3.7.2 Anti-Dilution Protection. Upon each issuance of NEW SECURITIES (as defined below in Paragraph 3.7.3), increase in the number of shares of capital stock reserved for issuance pursuant to LICENSEE’s stock option plan, and/or the issuance by LICENSEE of any other security (other than a debt security or the grant of securities pursuant to LICENSEE’s stock option plan) that can be converted into capital stock of LICENSEE, LICENSEE shall issue to DUKE that additional number of shares of common stock in LICENSEE (“ADJUSTING SHARES”) as required such that DUKE holds, in aggregate, five percent (5%) of the TOTAL OWNERSHIP INTERESTS in the LICENSEE (“ANTI-DILUTION PROTECTION”), provided that the ANTI-DILUTION PROTECTION shall automatically terminate upon the earliest of: (i) LICENSEE completing an equity financing of not less than an aggregate of ten million ($10,000,000) (including, for purposes of such calculation, the amount of any unpaid principal and interest due under any debt securities that may be converted into or used to purchase equity securities in any equity financing, and any securities issued to DUKE for cash in any such financing); (ii) any transaction that is an IPO; or (iii) a CHANGE OF CONTROL of LICENSEE.
For purposes of this Agreement, a “CHANGE OF CONTROL” means: (a) the acquisition of LICENSEE or its equity securities by another person or entity by means of any transaction or series of related transactions (including, without limitation, any reorganization, merger or consolidation) that results in the transfer of 50% or more of the outstanding voting power of LICENSEE; or (ii) a sale of all or substantially all of the assets of LICENSEE (or the portion thereof related to the subject matter of this Agreement).
3.7.3 If LICENSEE proposes to offer, issue, sell or exchange (“OFFER”) any equity securities of LICENSEE, or any securities of any type whatsoever that are, or may become, convertible or exchangeable into or exercisable for such equity securities, other than those securities that are not subject to this Paragraph 3.7.3 (collectively, “NEW SECURITIES”), then LICENSEE shall first deliver to DUKE a written notice of its intent to OFFER such NEW SECURITIES. Such notice should specify in reasonable detail the NEW SECURITIES to be offered, including the total number of securities, the applicable rights and preferences associated therewith, the purchase price, and the number of securities eligible for purchase by DUKE under this provision. For thirty days after receipt of the written notice, DUKE or its designee will have the right to purchase up to its PRO RATA SHARE of such OFFER. If at the end of the thirty-day period, DUKE elects to purchase less than all of its PRO RATA SHARE, then LICENSEE may, during the ninety day period following the expiration of such thirty day period, OFFER the remaining unsubscribed portion of such NEW SECURITIES to any person at a price not less than, and upon terms no more favorable to the offeree than, those specified in the written notice. If LICENSEE does not enter into an agreement for the sale of the NEW SECURITIES within such ninety-day period, or if such agreement is not consummated within thirty days of the execution thereof, the rights provided hereunder shall be deemed to be revived and such NEW SECURITIES shall not be OFFERED unless also reoffered to DUKE in accordance with this Paragraph 3.7.3. DUKE shall be entitled to apportion the right of first offer hereby granted to it among itself and to any entity controlled by DUKE, or any affiliate of DUKE or any other entity in which DUKE has a financial interest or investment, provided that such affiliate or entity is an “accredited investor” within the meaning of Regulation D under the Securities Act of 1933, as amended, in such proportions as it deems appropriate. This right of first offer shall not apply to the first financing of LICENSEE and it shall not apply to and shall expire immediately prior to the earliest of: (a) any transaction that is an IPO; (b) a CHANGE OF CONTROL of LICENSEE; or (c) LICENSEE’s execution and consummation of a transaction involving a sale of NEW SECURITIES in which DUKE has not purchased its PRO RATA SHARE. This right of first offer shall not be applicable to NEW SECURITIES: (t) that are issued to employees, officers or directors of, or consultants or advisors to, LICENSEE pursuant to equity compensation plans or other arrangements approved by the Board of Directors of LICENSEE; (u) that are issued upon the conversion, exercise or exchange of other securities outstanding on the date of this Agreement; or (v) that are issued in a stock split or stock split in the nature of dividend or a combination or other reclassification by LICENSEE that is paid on a proportionate non-cash basis to all holders of LICENSEE's capital stock; (w) issued pursuant to LICENSEE’s IPO; (x) issued pursuant to LICENSEE’s CHANGE OF CONTROL; (y) issued in connection with any real property or equipment leases or financings; or (z) issued in connection with a licensing, strategic, or collaborative transaction entered into by LICENSEE or an AFFILIATE of LICENSEE. For purposes of this Agreement, “PRO RATA SHARE” means the fraction, (i) the numerator of which is the number of shares of LICENSEE’s common stock that has been previously issued to DUKE pursuant to this Agreement as of the time immediately prior to the applicable sale of NEW SECURITIES and (ii) the denominator of which is the TOTAL OWNERSHIP INTERESTS as of the time immediately prior to such sale of NEW SECURITIES.
10
ARTICLE 4 - REPORTS
4.1 Until the FIRST COMMERCIAL SALE, by July 31 of each year LICENSEE shall provide to DUKE a written annual report that includes reports on progress since the prior annual report and general future plans regarding (with respect to LICENSED PRODUCTS): research and development, regulatory approvals, manufacturing, sublicensing, and marketing, including each milestone under Article 3 or Article 5 having a deadline during the ROYALTY TERM, and a specific identification of whether or not it was achieved. Further, LICENSEE shall specifically report to DUKE the FIRST COMMERCIAL SALE within seventy-five (75) days following the end of the ROYALTY PERIOD in which it occurs, and provide a brief description of the products or services subject of the SALE, and terms thereof.
4.2 After the FIRST COMMERCIAL SALE, LICENSEE shall provide semi-annual reports to DUKE. Specifically, by each July 31 and January 31 (i.e., within one month after each ROYALTY PERIOD closes, including the close of the ROYALTY PERIOD immediately following any termination of this Agreement), LICENSEE shall report the following to DUKE for the applicable ROYALTY PERIOD.
(a) number of LICENSED PRODUCTS sold, leased, or distributed, however characterized, by LICENSEE and each SUBLICENSEE.
(b) NET SALES, excluding the deductions provided therefor, of LICENSED PRODUCTS SOLD by LICENSEE and all SUBLICENSEES.
(c) a description and accounting for all LICENSED PROCESSES SOLD, by LICENSEE and all SUBLICENSEES included in NET SALES, excluding the deductions therefor.
(d) deductions applicable as provided in the definition for NET SALES above, and an explanation of the rationale(s) therefor.
(e) SUBLICENSING FEES due on payments from SUBLICENSEES under Paragraph 3.1 above, including supporting figures.
(f) foreign currency conversion rate and calculations (if applicable) and total royalties due.
(g) each milestone achieved under Article 3 or each milestone under Article 5 having a deadline during the ROYALTY PERIOD, and a specific identification of whether or not such milestone was achieved.
(h) for each sublicense or amendment thereto completed in the particular ROYALTY PERIOD (including agreements under which LICENSEE will have LICENSED PRODUCTS made by a third party): names, addresses, and U.S.P.T.O. Entity Status (as discussed in Paragraph 6.1) of such SUBLICENSEE; the date of each agreement and amendment; the territory of the sublicense; the scope of the sublicense; and the nature, timing and amounts of all fees and royalties to be paid to LICENSEE thereunder.
(i) summary of progress on research and development, regulatory approvals, manufacturing, sublicensing, marketing and SALES, and general plans for the future with respect to LICENSED PRODCUTS.
(j) the date of FIRST COMMERCIAL SALE of LICENSED PRODUCTS (or results of LICENSED PROCESSES) in each country, to the extent reasonably ascertainable by LICENSEE.
LICENSEE shall include the amount of all payments due, and the various calculations used to arrive at those amounts, including the quantity, description (nomenclature and type designation as described in Paragraph 4.3 below), country of manufacture, and country of SALE or use of LICENSED PRODUCTS and LICENSED PROCESSES.
11
If no payment is due, LICENSEE shall so report to DUKE that no payment is due. If LICENSEE does not provide reports as required under this Article 4, DUKE may notify LICENSEE of such failure, which shall be a material breach of this Agreement if not cured within fifteen (15) days of LICENSEE’s receipt of notice of such failure. LICENSEE agrees to reasonably cooperate with DUKE regarding any questions it may have relating to compliance with this Agreement, for example to discuss the information in reports.
4.3 LICENSEE shall promptly establish and consistently employ a system of specific nomenclature and type designations for LICENSED PRODUCTS and LICENSED PROCESSES to permit identification and segregation of various types where necessary, and shall require SUBLICENSEES to use the same nomenclature and type designations.
4.4 LICENSEE shall keep, and shall require SUBLICENSEES to keep, true and accurate records containing data reasonably required for the computation and verification of payments due under this Agreement. LICENSEE shall: (a) open its records for inspection upon reasonable notice during business hours, and no more than once per year, by an independent certified accountant selected by DUKE and reasonably acceptable to LICENSEE, for the purpose of verifying the amount of payments due, and shall provide information to DUKE’s accountant to facilitate such inspection; and (b) retain such records for three (3) years from date of origination. LICENSEE shall, upon DUKE’s reasonable written request, at DUKE’s expense, and subject to the applicable audit provisions of any such SUBLICENSE, exercise its audit rights with respect to royalties payable on LICENSED PRODUCTS under sublicenses granted by LICENSEE and report the results thereof to DUKE.
The terms of this Article shall survive any termination of this Agreement. DUKE is responsible for all expenses of such inspection, except that if any inspection reveals an underpayment greater than [*] percent of royalties due DUKE, then LICENSEE shall pay all reasonable and documented out-of-pocket expenses of that inspection and the amount of the underpayment and interest to DUKE within twenty-one days of written notice thereof. LICENSEE shall also reimburse DUKE for its reasonable and documented out-of-pocket expenses required to collect the amount underpaid if LICENSEE fails to pay the underpaid amount within such thirty (30)- day period. Any overpayment revealed by any such inspection shall be credited against future amounts due hereunder.
ARTICLE 5 - DILIGENCE
5.1 LICENSEE shall use commercially reasonable efforts to develop and commercialize LICENSED PRODUCTS or LICENSED PROCESSES within the TERRITORY through a thorough, vigorous and diligent program for utilizing the PATENT RIGHTS and to employ active and diligent marketing efforts for approved LICENSED PRODUCTS or LICENSED PROCESSES. LICENSEE shall use commercially reasonable efforts to obtain and retain any governmental approvals necessary to manufacture and/or sell LICENSED PRODUCTS and/or use LICENSED PROCESSES for all relevant activities of LICENSEE and SUBLICENSEES. If the commercialization of multiple LICENSED PRODUCTS or LICENSED PROCESSES is commercially reasonable, then the requirements of this Paragraph 5.1 shall apply to all such LICENSED PRODUCTS and/or LICENSED PROCESSES.
5.2 Without limiting Paragraph 5.1, LICENSEE agrees to use reasonable efforts to reach the following commercialization and research and development milestones for the LICENSED PRODUCTS and LICENSED PROCESSES (together the “MILESTONES”) by the following dates:
(a) Initiation of Phase I Study by [*].
(b) Initiation of PHASE II STUDY by [*].
(c) Initiation of PHASE III STUDY by [*].
(d) FIRST COMMERICIAL SALE by [*].
12
5.3 LICENSEE must use reasonable efforts to achieve each MILESTONE on or before the deadline dates indicated above. LICENSEE shall notify DUKE within ten days after each such deadline as to whether or not such MILESTONE was met. If LICENSEE fails to meet any MILESTONE under this Article 5 by the date of such MILESTONE deadline and such failure results from LICENSEE’s failure to use reasonable efforts to achieve such MILESTONE, DUKE may terminate the Agreement effective on thirty days’ prior written notice, unless LICENSEE achieves the MILESTONE within such thirty-day period. For clarity, the achievement of a MILESTONE by a SUBLICENSEE shall be deemed to be the achievement of such MILESTONE by LICENSEE. DUKE acknowledges that LICENSEE’s ability to timely achieve the MILESTONE may be impacted by delays outside of LICENSEE’s reasonable control. When LICENSEE identifies any such delays that will impact its ability to timely achieve the MILESTONE, LICENSEE shall promptly notify DUKE. DUKE shall not reasonably withhold or deny its consent to any revisions to milestone data for the applicable MILESTONE.
ARTICLE 6 - SUBLICENSING
6.1 LICENSEE shall notify DUKE in writing of every sublicense agreement and each amendment thereto within thirty days after their execution, and indicate the name of the SUBLICENSEE, the territory of the sublicense, the scope of the sublicense, and the nature, timing and amounts of all fees and royalties to be paid thereunder, and whether or not the SUBLICENSEE has greater or fewer than 500 employees. Upon request, LICENSEE shall provide DUKE with a copy of sublicense agreements, which LICENSEE may redact in its reasonable discretion to protect the confidentiality of any SUBLICENSEE’s proprietary or confidential information that is not necessary for DUKE to determine compliance with this Agreement.
6.2 LICENSEE shall not receive from SUBLICENSEES anything of value other than cash payments in consideration for any sublicense under this Agreement, without the express prior written permission of DUKE.
6.3 LICENSEE shall require that all sublicenses: (a) not be inconsistent with the terms and conditions of this Agreement; (b) contain the SUBLICENSEE’S acknowledgment of the disclaimer of warranty and limitation on DUKE and NCI's liability, as provided by Article 9 below; and (c) contain provisions under which the SUBLICENSEE accepts duties at least equivalent to those accepted by the LICENSEE in the following Paragraphs: 4.4 (duty to keep records), 10.1 (duty to defend, hold harmless, and indemnify DUKE and NCI), 10.3 (duty to maintain insurance), 2.4 (duty to properly mark LICENSED PRODUCTS with patent notices), and 15.5 (duty to restrict the use of DUKE and NCI's name).
6.4 Upon termination of this Agreement, any sublicenses granted by LICENSEE under the PATENT RIGHTS shall, to the extent provided in such sublicense, remain in effect and be deemed to have been assigned by LICENSEE to DUKE immediately prior to such termination provided that: (a) the sublicensing agreement requires the SUBLICENSEE to thereafter pay DUKE any consideration that would have been due to LICENSEE with respect to the rights granted under this Agreement and (b) LICENSEE remains responsible for all other obligations thereunder to the extent applicable to LICENSEE and in excess of DUKE’s obligations under this Agreement. If any terms of such sublicense agreements fail to comply with the requirements of Article 6 herein relating to sublicensing, or are otherwise inconsistent with this Agreement, and DUKE provides notice thereof to SUBLICENSEE such terms will be renegotiated between DUKE and the SUBLICENSEE; provided, such sublicense will remain in effect pending resolution and mutual agreement upon of such renegotiated terms. Any sublicense executed by LICENSEE must contain language to implement this Paragraph 6.4 in order for any SUBLICENSEE to enjoy the benefits of this Paragraph 6.4.
13
ARTICLE 7 - PATENT APPLICATIONS AND MAINTENANCE
7.1 DUKE shall have the right to control, and will use reasonable efforts to perform, all aspects of filing, prosecuting, and maintaining all of the patents and patent applications that form the basis for the PATENT RIGHTS, including (a) administrative reexaminations and reviews; and (b) disputes (including litigation) regarding inventorship and derivation, and interferences. LICENSEE shall reasonably cooperate with DUKE in activities relating to the PATENT RIGHTS, including said activities. Upon DUKE’s request, to the fullest extent permitted by law, LICENSEE shall use reasonable efforts to apply for and prosecute, or support in any reasonable way DUKE’s application for, a patent term extension for patents included in the PATENT RIGHTS.
7.2 DUKE shall: (a) promptly notify LICENSEE in writing of all information received by DUKE relating to the filing, prosecution, or maintenance of the PATENT RIGHTS; and (b) make reasonable efforts to allow LICENSEE to review, comment, and advise upon such information. LICENSEE shall hold such information confidential and use the information provided by DUKE only for the purpose of advancing DUKE’s PATENT RIGHTS.
7.3 With the exception of foreign filing fees as detailed below, LICENSEE shall reimburse DUKE for all reasonable and documented out-of-pocket fees and costs incurred by DUKE with respect to the activities described in this Article 7 for PATENT RIGHTS licensed to LICENSEE hereunder. Such reimbursement shall be made within thirty days of receipt of DUKE’s invoice and shall be subject to the interest and other requirements specified in Article 3 above. LICENSEE agrees that, to the extent its failure to comply with all Paragraphs in this Agreement relating to entity status requires DUKE to pay “Large Entity” patent fees, LICENSEE shall be obligated to reimburse DUKE for “Large Entity” patent fees. For the avoidance of doubt, this Article 7 shall not modify the rights and obligations of either DUKE or LICENSEE with respect to the payment of PAST PATENT EXPENSES.
7.4 LICENSEE must inform DUKE in writing of any foreign countries in which LICENSEE desires patent protection, and this Agreement will be amended in writing to reflect those designations. LICENSEE will pay to DUKE [*] of the foreign filing fees reasonably estimated in good faith by DUKE to be due for each requested country at least thirty (30) days in advance of any filing with respect thereto. DUKE and/or its other licensees of the PATENT RIGHTS may elect to seek patent protection in countries not so designated by LICENSEE, in which case DUKE and/or such other licensees of the PATENT RIGHTS are responsible for all expenses attendant thereto. In such instances, such patent applications in such countries will not be PATENT RIGHTS (this Agreement shall be deemed to be so amended accordingly, if necessary), and LICENSEE forfeits all rights under this Agreement to such patent applications and any resulting patents in such countries.
7.5 If LICENSEE provides DUKE with written notification that it will no longer support the filing, prosecution, or maintenance of a specified patent(s) and/or patent application(s) within the PATENT RIGHTS, then LICENSEE’s responsibility for fees and costs related to the filing, prosecution, and maintenance of such subject PATENT RIGHTS will terminate sixty (60) days after DUKE’s receipt of such written notification. At that time, such patents and/or patent applications will no longer be included in the PATENT RIGHTS (and this Agreement is deemed to be so amended accordingly), and LICENSEE surrenders all rights under this Agreement to such patents, patent applications, and any patent or patent applications arising therefrom.
7.6 LICENSEE shall notify DUKE promptly if, at any time during the term of this Agreement, LICENSEE, its AFFILIATES, or any of its SUBLICENSEES does not qualify as a “small entity” as under section 1.27, as amended, of the Consolidated Patent Rules of the United States Patent and Trademark Office.
14
ARTICLE 8 – ENFORCEMENT
8.1 Each party shall promptly advise the other in writing of any known acts of potential infringement of the PATENT RIGHTS by another party. LICENSEE is hereby granted the first right (which, for clarity, it may grant to AFFILIATES or SUBLICENSEES) to enforce the PATENT RIGHTS against other parties within the TERRITORY and the FIELD OF USE, including against those acts that occurred prior to the EFFECTIVE DATE. LICENSEE shall not file any suit without (a) first performing a thorough, diligent investigation of the merits of such suit, including with respect to the validity and enforceability of the PATENT RIGHTS; (b) there being reasonable legal and economic bases for doing so; and (c) notifying DUKE ninety (90) days before any such filing. This right to enforce includes filing, prosecuting, and settling all infringement actions at its expense, except that LICENSEE shall make any such settlement that materially and adversely affects the scope or enforceability of the PATENT RIGHTS, only with the advice and consent of LICENSORS. LICENSEE has the right to file suit using counsel of its choosing, subject to LICENSORS’ approval, which shall not be unreasonably withheld or delayed. LICENSEE may grant to SUBLICENSEES the right to enforce hereunder, within the scope of rights granted to such SUBLICENSEES.
8.2 If LICENSEE has complied with Paragraph 8.1, LICENSORS shall provide reasonable assistance to LICENSEE with respect to such actions, and LICENSEE will promptly reimburse DUKE and NCI for out-of-pocket expenses incurred in connection with any such assistance rendered at LICENSEE'S request or reasonably required by DUKE and NCI, including but not limited to expenses incurred in complying with discovery duties. LICENSORS retain the right to participate, with counsel of their own choosing and at their own expense, in any action under this Article. LICENSEE shall defend, indemnify and hold harmless LICENSORS with respect to any claims asserted by an alleged infringer reasonably related to the enforcement of the PATENT RIGHTS under this Article 8, including but not limited to antitrust counterclaims and claims for recovery of attorney fees.
8.3 DUKE and its employees have a vital interest in lawsuits relating to the validity and enforceability of the PATENT RIGHTS. If a third party files a suit, including as a counterclaim, alleging that any of the PATENT RIGHTS is invalid or unenforceable, then the parties shall jointly control the defense of such claim. Each party shall consult with the other with respect to the defense of such claim, and shall reasonably consider the other party’s input. In furtherance of such joint control, at the onset of such claim and as reasonable during the pendency of any such claim, the parties shall meet and confer in good faith to set a plan for handling the defense thereof. The parties expect that in general (a) LICENSEE will have the right to lead daily activities, including but not limited to discovery, relating to the defense and (b) the parties would make joint filings. Notwithstanding the foregoing, in the event that the parties cannot agree on how to proceed with respect to such claim, DUKE shall have the right to control the defense thereof on either a temporary or permanent basis. LICENSEE shall be responsible for the reasonable costs and fees associated with activities under this Paragraph 8.3. The parties shall consider reasonable controls on costs and fees as part of an aforementioned meet and confer with respect to the handling of the defense. Notwithstanding, if a third party asserts jurisdiction for any such action solely as the result of acts of DUKE, then DUKE shall be responsible for such reasonable costs and fees.
8.4 If LICENSEE recovers damages in patent litigation regarding the PATENT RIGHTS or settlement thereof, the award shall be applied first to satisfy LICENSEE’s and LICENSORS’ reasonable expenses and legal fees for the litigation. The remaining balance shall be treated as SUBLICENSING FEES. This provision shall control the division of revenues where a sublicense, covenant not to sue, or assignment of rights is granted as part of a settlement of such lawsuit (including prospective rights).
ARTICLE 9 - NO WARRANTIES; LIMITATION ON DUKE’S LIABILITY
9.1 LICENSORS make no representations or warranties that any claim within the PATENT RIGHTS is or will be held valid, patentable, or enforceable, or that the manufacture, importation, use, offer for SALE, SALE or other distribution of any LICENSED PRODUCTS or LICENSED PROCESSES will not infringe upon any patent or other rights.
15
9.2 LICENSORS MAKE NO REPRESENTATIONS, EXTEND NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO THE IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, AND ASSUME NO RESPONSIBILITIES WHATEVER WITH RESPECT TO DESIGN, DEVELOPMENT, MANUFACTURE, USE, SALE OR OTHER DISPOSITION BY LICENSEE OR SUBLICENSEES OF LICENSED PRODUCTS OR LICENSED PROCESSES. LICENSEE AND SUBLICENSEES ASSUME THE ENTIRE RISK AS TO PERFORMANCE OF LICENSED PRODUCTS AND LICENSED PROCESSES.
9.3 In no event shall LICENSORS be responsible for any direct, indirect, special, incidental, or consequential damages or lost profits or other economic loss or damage with respect to LICENSED PRODUCTS, LICENSED PROCESSES, or the PATENT RIGHTS to LICENSEE, SUBLICENSEES Agreement regardless of legal or equitable theory.
9.4 LICENSEE shall not make any statements, representations or warranties whatsoever to any person or entity, or accept any liabilities or responsibilities whatsoever from any person or entity that are inconsistent with any disclaimer or limitation included in this Article 9.
ARTICLE 10 - INDEMNITY; INSURANCE
10.1 LICENSEE shall defend, indemnify and hold harmless and shall require SUBLICENSEES to defend, indemnify and hold harmless LICENSORS for and against any and all claims, demands, damages, losses, and expenses of any nature (including attorneys' fees and other litigation expenses) (hereinafter, “CLAIM”), including, but not limited to, death, personal injury, illness, property damage, economic loss or products liability, or errors and omissions, arising from or in connection with, any of the following: (1) any manufacture, use, SALE or other disposition by LICENSEE, SUBLICENSEES or transferees of LICENSED PRODUCTS or LICENSED PROCESSES; (2) the use by any third party of LICENSED PRODUCTS made, used, sold or otherwise distributed by LICENSEE or SUBLICENSEES; (3) the use or practice by LICENSEE or SUBLICENSEES of any invention or computer software related to the PATENT RIGHTS; and (4) any CLAIM of infringement and/or invalidity of any claim(s) of the PATENT RIGHTS.
10.2 LICENSORS are entitled to participate at their option and expense through counsel of their own selection, and may join in any legal actions related to any such claims, demands, damages, losses and expenses under Paragraph 10.1 above. LICENSEE shall not settle any such legal action with an admission of liability of LICENSORS’ without LICENSORS’ written approval.
10.3 Prior to any distribution or commercial use of any LICENSED PRODUCT or use of any LICENSED PROCESS by LICENSEE, LICENSEE shall purchase and maintain in effect commercial general liability insurance, product liability insurance, and errors and omissions insurance which shall protect LICENSEE and LICENSORS with respect to the events covered by Paragraph 10.1, and LICENSEE shall require the same of any SUBLICENSEE. Each such insurance policy must provide reasonable coverage for all claims with respect to any LICENSED PROCESS used and any LICENSED PRODUCTS manufactured, used, sold, licensed or otherwise distributed by LICENSEE (or, in the case of a SUBLICENSEE’s policy, by said SUBLICENSEE) and must specify LICENSORS as an additional insured. LICENSEE shall furnish proof of such insurance to DUKE, upon request.
16
ARTICLE 11 - TERM AND TERMINATION
11.1 This Agreement shall immediately terminate if the LICENSEE enters liquidation, has a receiver or administrator appointed over any assets related to this Agreement, makes an assignment for the benefit of its creditors, or ceases to carry on business, or files for bankruptcy or if an involuntary petition is filed against LICENSEE and not dismissed within [*] ([*]) days, or any similar event under the law of any foreign jurisdiction. Except as set forth in Paragraph 15.8, this Agreement cannot be assumed or assigned by LICENSEE, any trustee acting on behalf of the assets of LICENSEE, or otherwise, without DUKE’s prior written consent, which may not be unreasonably withheld.
11.2 If at any time the LICENSEE i) fails to meet the MILESTONES as provided in Paragraph 5.2 or ii) shall permanently cease to pursue commercial development of the PATENT RIGHTS as contemplated herein in any country in the TERRITORY, then the license granted to LICENSEE set forth in Article 2 with respect to that country in the TERRITORY shall automatically terminate without obligation on the part of DUKE to refund any of the fees or royalties which may have been paid by LICENSEE prior to such termination. LICENSEE must provide notice to DUKE promptly in writing if LICENSEE permanently ceases to pursue commercial development of the PATENT RIGHTS in any specific country in the TERRITORY as contemplated herein.
11.3 If LICENSEE fails to make any payment due to DUKE by the applicable due date therefor, such failure shall be deemed to be a material breach of this Agreement, subject to the cure period set forth in Article 4 or Paragraph 11.4. Any termination of this Agreement stemming from such breach shall not foreclose DUKE from collection of any amounts remaining unpaid or seeking other legal relief.
11.4 Upon any material breach or default of this Agreement by LICENSEE (other than as specifically provided herein, the terms of which shall take precedence over the handling of any other material breach or default under this Paragraph 11.4), DUKE has the right to terminate this Agreement effective on [*] (or in the case of non-payment, [*]) days’ written notice to LICENSEE. Such termination shall become automatically effective upon expiration of the applicable period unless LICENSEE cures the material breach or default before the period expires. LICENSEE's right to cure a material breach will apply only to the first [*] ([*]) material breaches properly noticed under the terms of this Agreement, within any [*] ([*])- year period. Any subsequent breach or any uncured breach by that Party will entitle the DUKE to terminate this Agreement by written notice without opportunity to cure.
11.5 LICENSEE has the right to terminate this Agreement at any time on [*] ([*]) days’ written notice to DUKE. Upon termination pursuant to this Paragraph 11.5, LICENSEE shall:
(a) pay all amounts due DUKE through the effective date of the termination;
(b) submit a final report of the type described in Paragraph 4.2;
(c) return any patent documentation (including that exchanged under Article 7) and any other CONFIDENTIAL INFORMATION or remaining physical materials provided to LICENSEE by DUKE in connection with this Agreement, or, with prior approval by DUKE, destroy such materials, and certify in writing that such materials have all been returned or destroyed;
17
(d) suspend its manufacture, use and SALE of the LICENSED PROCESS(ES) and LICENSED PRODUCT(S);
(e) provides DUKE with all data and know-how developed by LICENSEE in the course of LICENSEE’s efforts to develop LICENSED PRODUCTS and LICENSED PROCESSES; DUKE shall have the right to use such data and know-how for any purpose whatsoever, including the right to transfer same to future licensees;
(f) provide DUKE with copies of any regulatory information filed with any U.S. or foreign government agency with respect to LICENSED PRODUCTS and LICENSED PROCESSES.
11.6 Upon any termination of this Agreement, and except as provided herein to the contrary, all rights and obligations of the parties hereunder shall cease, except any previously accrued rights and obligations and further as follows: (a) obligations to pay royalties and other sums, including any outstanding patent fees and costs pursuant to Article 7 up to the termination date, or to transfer equity or other consideration, accruing hereunder up to the day of such termination, whether or not this Agreement provides for a number of days before which actual payment is due and such date is after the day of termination and whether or not a required funding event or other stock transfer trigger has yet been met; (b) DUKE's rights to inspect books and records as described in Article 4, and LICENSEE's obligations to keep such records for the required time; (c) any cause of action or claim of LICENSEE or DUKE accrued or to accrue because of any breach or default by the other party hereunder; (d) the provisions of Articles 1, 3.7.3, 9, 10, 13, 15; and (e) all other terms, provisions, representations, rights and obligations contained in this Agreement that by their sense and context are intended to survive until performance thereof by either or both parties.
Termination by either party hereunder shall not alter or affect any other rights or relief that either party may be entitled to under law.
11.7 Upon termination of this Agreement, if LICENSEE has filed patent applications or obtained patents to any modification or improvement to LICENSED PRODUCTS or LICENSED PROCESSES within the scope of the PATENT RIGHTS, LICENSEE agrees upon request to enter into good faith negotiations with DUKE or DUKE’s future licensee(s) for the purpose of granting licensing rights to said modifications or improvements in a timely fashion and under commercially reasonable terms.
11.8 If LICENSEE, any AFFILIATE thereof, or any SUBLICENSEE asserts the invalidity or unenforceability of any claim included in the PATENT RIGHTS, including by way of litigation or administrative proceedings, either directly or through any other party, then DUKE shall have the right to immediately terminate this Agreement upon written notice to LICENSEE. Notwithstanding the foregoing, the Parties agree that this Paragraph 11.8 shall not apply to: (a) any such assertion by any SUBLICENSEE with respect to any PATENT RIGHT to which such SUBLICENSEE is not sublicensed rights under this Agreement; and (b) arguments and comments made by or on behalf of LICENSEE, any AFFILIATE thereof, or any SUBLICENSEE with respect to the prosecution, maintenance, or defense of LICENSEE’s or any SUBLICENSEES’ patents or patent applications in response to office actions, inter partes review, interferences, and other communications from or interactions with patent offices, agencies, or authorities or third parties in the prosecution, maintenance, or defense process.
ARTICLE 12 - NOTICES
Any notice, request, or report required or permitted to be given or made under this Agreement by either party is effective when mailed if sent by recognized overnight carrier, certified or registered mail, or electronic mail followed by confirmation by U.S. mail, to the address set forth below or such other address as such party specifies by written notice given in conformity herewith. Any notice, request, or report not so given is not effective until actually received by the other party.
18
| To DUKE: | To LICENSEE: | |
| For delivery via the U.S. Postal Service | ||
| [*] | [*] | |
| For delivery via nationally/internationally recognized courier | ||
| DUKE UNIVERSITY | ||
| [*] | ||
| For delivery via electronic mail | ||
| [*] | ||
| To NCI: | ||
| For delivery via the U.S. Postal Service | ||
| [*] | ||
| For delivery via electronic mail | ||
| [*] | ||
ARTICLE 13 - CONFIDENTIALITY
13.1 DUKE and LICENSEE will treat any CONFIDENTIAL INFORMATION disclosed to it by the other party with reasonable care and will not disclose such information to any other person, firm or corporation, except Affiliates bound by the obligations of confidentiality and restricted use set forth in this Article 13. The receiving party may not use the disclosing Party’s CONFIDENTIAL INFORMATION other than for the benefit of the Parties hereto and for the performance of this Agreement. These obligations of non-disclosure and restricted use remain in effect for each subject disclosure of CONFIDENTIAL INFORMATION for [*] ([*]) years from the date of disclosure. However, neither Party is obligated, with respect to CONFIDENTIAL INFORMATION disclosed to it, or any part thereof, which:
(a) is already known to the receiving party at the time of the disclosure;
(b) becomes publicly known without the wrongful act or breach of this Agreement by the receiving party;
(c) is rightfully received by the receiving party from a Third Party on a non- confidential basis;
(d) is subsequently and independently developed by employees of the receiving party who had no knowledge of the information, as verified by written records;
(e) is approved for release by prior written authorization of the disclosing Party; or
(f) is disclosed pursuant to the requirements of applicable law or pursuant to any judicial or government requirement or order, provided that the party so disclosing takes reasonable steps to provide the other party sufficient prior notice in order to contest such request, requirement or order and provided that such disclosed information otherwise remains subject to the obligations of confidentiality set forth in this Article 13.
19
13.2 DUKE and LICENSEE agree that any information to be treated as CONFIDENTIAL INFORMATION under this Article 13 must be disclosed in writing or in another tangible medium and must be clearly marked “CONFIDENTIAL.” CONFIDENTIAL INFORMATION disclosed orally must be summarized and reduced to writing and communicated to the other party within 30 days of such disclosure.
13.3 LICENSEE may use and disclose any CONFIDENTIAL INFORMATION of DUKE related to the PATENT RIGHTS, or the terms of this Agreement, to investors, prospective investors, banking or lending institutions, acquirors, acquisition or merger targets, employees, consultants, contractors, agents, collaborators, prospective collaborators, and other third parties in the chain of manufacturing and distribution, but if and only if LICENSEE obtains from each such recipient a written confidentiality agreement, the provisions of which are at least as protective of DUKE’s CONFIDENTIAL INFORMATION as those provided in this Article 13. However, nothing in this Article 13 shall require LICENSEE to obtain a written confidentiality agreement from actual or prospective investors in connection with disclosures made by LICENSEE in response to standard diligence requests or representations or warranties contained in financing or investment documents.
13.4 “CONFIDENTIAL INFORMATION” shall mean, with respect to a party hereto, all information regarding such party’s technology, products, business, finances, or objectives, that is disclosed to the other party and shall be marked “CONFIDENTIAL”. Additionally, all information relating to filing, prosecution, maintenance, defense, and the like regarding the PATENT RIGHTS (no matter how disclosed) is the CONFIDENTIAL INFORMATION of DUKE and subject to the provisions of this Article 13. DUKE acknowledges that other than information relating to PATENT RIGHTS, DUKE has not disclosed any CONFIDENTIAL INFORMATION to LICENSEE.
ARTICLE 14 – DISPUTE RESOLUTION
14.1 In the event of any dispute, claim, question, or disagreement arising from or relating to this agreement or the breach thereof, the Parties hereto shall use their best efforts to settle the dispute, claim, question, or disagreement. To this effect, they shall consult and negotiate with each other in good faith and, recognizing their mutual interests, attempt to reach a just and equitable solution satisfactory to both parties. If they do not reach such solution within a period of [*] ([*]) days of the first written notice of dispute by either party to the other, then, upon written request for mediation by either party to the other, the parties agree to try in good faith to settle the dispute by mediation administered by the American Arbitration Association under its Commercial Mediation Procedures and to be scheduled within [*] ([*]) days of the written notice requesting mediation. If the parties fail to reach agreement by mediation, then all disputes, claims, questions, or differences shall be finally settled by arbitration administered by the American Arbitration Association in accordance with the provisions of its Commercial Arbitration Rules. The arbitration panel shall consist of three members, selected as follows: one member to be selected by each party, and those two members are to select a third member who will chair the panel. Judgment on the award rendered by the arbitrators may be entered in any court having jurisdiction thereof and shall be binding on the parties. The negotiation, mediation, and arbitration described above shall take place at a mutually agreed upon location in Durham, North Carolina.
14.2 Either Party may seek to enforce any written agreement reached by the Parties during mediation, or to confirm and enforce any final award entered in arbitration, in any court of competent jurisdiction, provided that any Party moving to enforce, confirm or vacate any such agreement or award, as the case may be, will file such motion under seal unless prohibited under applicable court rules. Notwithstanding the agreement to such procedures, either Party may seek equitable or injunctive relief to enforce its rights in any court of competent jurisdiction.
20
ARTICLE 15 - MISCELLANEOUS PROVISIONS
15.1 This Agreement shall be governed by and construed under the laws of the state of North Carolina without regard for principles of choice of law, except that questions affecting the construction and effect of any patent shall be determined by the law of the country in which the patent was granted.
15.2 DUKE and LICENSEE agree that this Agreement sets forth their entire understanding concerning the subject matter of this Agreement. The parties may amend this Agreement from time to time, such as to add new rights, but no modification will be effective unless both DUKE and LICENSEE agree to it in writing.
15.3 If a court of competent jurisdiction finds any term of this Agreement invalid, illegal or unenforceable, that term will be curtailed, limited or deleted, but only to the extent necessary to remove the invalidity, illegality or unenforceability, and without in any way affecting or impairing the remaining terms.
15.4 No waiver by either party of any breach of this Agreement, no matter how long continuing or how often repeated, is a waiver of any subsequent breach thereof, nor is any delay or omission on the part of either party to exercise or insist on any right, power, or privilege hereunder a waiver of such right, power or privilege. In no event shall any waiver be deemed valid unless it is in writing and signed by an authorized representative of each party.
15.5 LICENSEE shall, and shall require its AFFILIATES to, refrain from using and to require SUBLICENSEES to refrain from using the name, mark, logo, image or any adaption thereof of LICENSORS or their employees in publicity or advertising without the prior written approval of LICENSORS, except as required by law, provided that reports in scientific literature and presentations of joint research and development work are not publicity or advertising for the purposes hereof. However, in no event, shall LICENSEE or SUBLICENSEE represent, either directly or indirectly, that any product or service is a product or service of LICENSORS.
15.6 LICENSEE agrees to comply with all applicable laws and regulations, including but not limited to all United States laws and regulations controlling the export of commodities and technical data. LICENSEE shall be solely responsible for any violation of such laws and regulations involving LICENSEE or its SUBLICENSEES, and to defend, indemnify and hold harmless LICENSORS if any legal action of any nature results from any such violation.
15.7 It is expressly understood and agreed that LICENSORS and LICENSEE are independent contractors. Neither party is an agent of the other in connection with the exercise of any rights hereunder, and neither has any right or authority to assume or create any obligation or responsibility on behalf of the other. Nothing in this Agreement shall be deemed to create or constitute a partnership or joint venture between LICENSORS and LICENSEE.
15.8 LICENSEE may not assign this Agreement without the prior written consent of DUKE and shall not pledge any of the license rights granted in this Agreement as security for any creditor. Any attempted pledge of any of the rights under this Agreement or assignment of this Agreement not permitted under this Paragraph 15.8 will be void from the beginning. No assignment by LICENSEE will be effective until the intended assignee agrees in writing to accept all of the terms and conditions of this Agreement, and such writing is provided to DUKE. Notwithstanding the foregoing, LICENSEE may, without DUKE’s consent, assign its rights and obligations under this Agreement to a purchaser or assignee (by operation of law, contract, or otherwise) of all or substantially all of LICENSEE’s assets or business (or that portion thereof relating to the subject matter of this Agreement), so long as: (a) LICENSEE is not in material breach of this Agreement; and (b) such assignee provides a statement in writing to DUKE that it agrees to accept all the terms and conditions of this Agreement (including obligations existing as of the time of such assignment) in the place of LICENSEE. The transfer of this Agreement by LICENSEE to an AFFILIATE shall not be considered to be an assignment subject to this Paragraph 15.8.
15.9 If the registration, recordation, or reporting to a national or supranational agency of this Agreement, its terms, or assignment thereof is or becomes required or advisable (e.g., as a prerequisite to enforceability of the Agreement in such nation), LICENSEE shall, at its expense, promptly undertake such action. LICENSEE shall provide prompt notice thereof to DUKE along with copies of relevant documentation.
21
IN WITNESS WHEREOF, the parties hereto have executed this PATENT LICENSE AGREEMENT in duplicate originals by their duly authorized officers or representatives.
| FOR CENTAUR BIO | FOR DUKE UNIVERSITY | |||
| By: | /s/ Michael J. Roberts | By: | /s/ Robin L. Rasor | |
| (authorized representative) | Robin L. Rasor | |||
| Associate VP | ||||
| Printed Name: | Michael J. Roberts |
| Title: | CEO | |||
| Date: | 2/2/2023 | Date | 1/12/2023 |
[Signature Page to Duke – Centaur Bio Patent License Agreement]
APPENDIX A. EQUITY TRANSFER AGREEMENT
THIS EQUITY TRANSFER AGREEMENT (the "Agreement") is made as of February 2nd, 2023 between CENTAUR BIO, a Delaware corporation, having offices at 7805 Pemswood Street, Charlotte, NC 28277. (the "LICENSEE"), and Duke University, a nonprofit educational and research institution organized under the laws of North Carolina ("DUKE").
RECITALS
Pursuant to that certain License Agreement (DUKE File No(s): [*], [*], [*]), dated the 11th of January, 2023 (the "License"), between LICENSEE and DUKE, DUKE licensed certain rights to LICENSEE. A copy of the License is attached to this Agreement as Schedule A.
Pursuant to Paragraph 3.7.1 of the License and in consideration thereof, the LICENSEE agreed to transfer to DUKE a specified portion of the equity interest in LICENSEE at the times and on the basis described in such Paragraph.
The obligation of LICENSEE to issue such equity to DUKE has matured.
NOW, THEREFORE, in consideration of the License and this Agreement, LICENSEE and DUKE agree as follows.
1. Issuance of Equity.
(a) In partial consideration of the License and in satisfaction of the requirements of Paragraph 3.7.1 thereof, LICENSEE shall, upon execution of this Agreement, issue DUKE a duly endorsed certificate for 75 shares of the common stock of LICENSEE (the "DUKE Equity"). The DUKE Equity is subject to the designations, powers, preferences and rights, and qualifications, limitations and restrictions set forth in LICENSEE's charter or other applicable instrument relating thereto.
(b) If the anti-dilution provisions of Paragraph 3.7.2 of the License create an obligation of the LICENSEE to issue ADJUSTING SHARES (as defined in the License) to DUKE, then this Agreement shall be deemed effective to transfer the ADJUSTING SHARES in LICENSEE to DUKE by LICENSEE required to meet the LICENSEE's obligations under Paragraph 3.7.2 of the License without additional documentation.
2. LICENSEE Representations and Warranties. LICENSEE represents and warrants to DUKE that:
(a) LICENSEE is a corporation validly existing in good standing in its state of incorporation or organization and has the power and authority to enter into this Agreement and to issue the DUKE Equity as contemplated hereby;
(b) this Agreement has been duly authorized, executed, and delivered by LICENSEE and is a valid and binding obligation of LICENSEE, enforceable in accordance with its terms, except as limited by laws relating to creditors' rights and general principals of equity;
[Appendix A to Duke – Centaur Bio Patent License Agreement]
A-1
(c) issuance of the DUKE Equity satisfies all of the requirements of Paragraph 3.7.1 of the License, including with respect to the amount or percentage of shares of LICENSEE equity that LICENSEE is obligated to transfer to DUKE under Paragraph 3.7.1 of the License;
(d) upon issuance pursuant to this Agreement, the DUKE Equity will be free of any lien, charge or other encumbrance, and will be validly issued, fully-paid and non-assessable;
(e) issuance of the DUKE Equity does not and will not violate (i) the charter, bylaws or operating agreement, as applicable, of LICENSEE (ii) any rights of preemption, first offer, first refusal, co-sale, registration, dividends or similar rights (collectively, “Equity Rights”), (iii) any agreement by which LICENSEE, its owners, property or assets are bound, or (iv) any Federal or applicable state securities law, rule or regulation;
(f) the DUKE Equity constitutes 5.0% of the TOTAL OWNERSHIP INTERESTS of LICENSEE as of the EFFECTIVE DATE. For the purposes of this provision, “TOTAL OWNERSHIP INTERESTS” shall have the same meaning as in Paragraph 3.7.1 of the License.
3. DUKE’s Representations and Warranties. DUKE represents and warrants to LICENSEE that (a) DUKE is a nonprofit educational and research institution organized under the laws of North Carolina; (b) this Agreement is a valid and binding obligation of DUKE, enforceable in accordance with its terms, except as limited by laws relating to creditors’ rights and general principals of equity; (c) DUKE has full power and authority to execute and deliver this Agreement; and (d) DUKE is an “accredited investor”, as that term is defined in Rule 501(a) of Regulation D, as promulgated under the Securities Act of 1933, as amended (the “Securities Act”).Additional Rights.
4. LICENSEE agrees DUKE shall be entitled as of the date hereof to all the contractual rights granted by LICENSEE to the holders of the same type and class of equity security issued to DUKE pursuant to Paragraph 1 hereof, including, by way of example and not limitation, Equity Rights, any cash flow priority or preference and any reporting obligations; subject, however, to any threshold limitations applied on an equal basis to all holders of such equity security. Notwithstanding any such threshold limitation, for so long as DUKE holds not less than [*]% of the issued and outstanding equity interest of LICENSEE, LICENSEE shall provide to DUKE the highest level of written financial and other information that LICENSEE provides to those holders of equity interest in LICENSEE that have contractual information rights. DUKE agrees to promptly execute and deliver to LICENSEE the documents relating to such contractual rights and to be bound by the provisions thereof; provided, however, the execution and delivery (or deemed execution and delivery) by DUKE of such documents shall in no event incur any obligation of DUKE to make a capital contribution to preserve its equity interest in LICENSEE.
[Appendix A to Duke – Centaur Bio Patent License Agreement]
A-2
5. Limited Transferability. DUKE acknowledges that (a) the DUKE Equity will not be registered under the Securities Act, (b) DUKE is taking the DUKE Equity for its own account and not with a view towards resale or redistribution thereof, and (c) the DUKE Equity may not be sold or transferred unless (i) if prior to an IPO, it is first offered to LICENSEE and LICENSEE’s other stockholders at fair market value, and (ii) in any event, unless (A) registered under the Securities Act and registered or qualified under applicable state securities laws, or (B) pursuant to an applicable exemption from such registration or qualification requirements and LICENSEE receives an opinion of counsel reasonably acceptable to LICENSEE to the effect that no such registration or qualification is required. Accordingly, until the DUKE Equity has been registered under the Securities Act or LICENSEE receives an opinion of counsel to DUKE to the foregoing effect or to the effect that the DUKE Equity can be freely transferred under Rule 144 promulgated under the Securities Act, the certificate or other instrument evidencing the DUKE Equity shall bear the following legend:
THE SECURITY REPRESENTED BY THIS CERTIFICATE HAS NOT BEEN REGISTERED PURSUANT TO THE SECURITIES ACT OF 1933 OR ANY STATE SECURITIES LAW. NEITHER THIS SECURITY NOR ANY PORTION HEREOF OR INTEREST HEREIN MAY BE SOLD, ASSIGNED, TRANSFERRED, PLEDGED OR OTHERWISE DISPOSED OF UNLESS THE SAME IS REGISTERED UNDER SUCH ACT AND ANY APPLICABLE STATE SECURITIES LAW, OR UNLESS AN EXEMPTION FROM SUCH REGISTRATION IS AVAILABLE AND THE ISSUER SHALL HAVE RECEIVED, AT THE EXPENSE OF THE HOLDER HEREOF, EVIDENCE OF SUCH EXEMPTION REASONABLY SATISFACTORY TO THE ISSUER (WHICH MAY INCLUDE, AMONG OTHER THINGS, AN OPINION OF COUNSEL SATISFACTORY TO THE ISSUER).
6. General.
(a) Assignment. This Agreement is not assignable by LICENSEE.
(b) Binding Effect. All of the covenants and provisions of this Agreement shall bind and inure to the benefit of successors and permitted assigns and transferees of LICENSEE and DUKE.
(c) Notices. Any notice, request, claim or other communication hereunder must be in writing and will be deemed to have been duly given if delivered by hand or if sent by certified mail, postage and certification prepaid, to LICENSEE and DUKE at the addresses for each set forth in the introductory paragraph of this Agreement. Either party may change such address by giving notice to the other in the manner required by this subsection.
(d) Entire Agreement; Amendments. This Agreement and the License constitute the entire agreement between LICENSEE and DUKE with respect to the subject matter of this Agreement. LICENSEE and DUKE may only amend this Agreement by a written instrument executed by them both.
(e) Governing Law. This Agreement will be construed and governed by the laws of the State of Delaware, without giving effect to principals of conflicts of laws.
(f) Counterparts. This Agreement may be executed in any number of counterparts and by facsimile, each of which will be an original, but all of which together shall constitute one and the same instrument.
LICENSEE and DUKE have executed this Agreement as of the date first written above.
[Appendix A to Duke – Centaur Bio Patent License Agreement]
A-3
| LICENSEE: | DUKE: | |||
| By: | /s/ Michael J. Roberts | By: | /s/ Robin L. Rasor | |
| Name: | Michael J. Roberts | Name: | Robin L. Rasor | |
| Title: | CEO | Title: | Associate Vice President | |
[Appendix A to Duke – Centaur Bio Patent License Agreement]
A-4
Exhibit 10.2
CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THE EXHIBIT BECAUSE IT IS BOTH NOT MATERIAL AND IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. SUCH EXCLUDED INFORMATION HAS BEEN MARKED WITH “[*].”
FIRST AMENDMENT TO LICENSE AGREEMENT
This First Amendment, dated August 9, 2024, is by and between Centaur Bio, Inc (“LICENSEE”) and Duke University ("DUKE").
WHEREAS, LICENSEE and DUKE entered into a license agreement dated January 11, 2023 (the "License Agreement"); and
WHEREAS, DUKE and LICENSEE desire to modify certain provisions of the License Agreement as provided herein.
NOW THEREFORE, DUKE and LICENSEE hereby agree as follows:
1. The following new Paragraphs 1.20 and 1.21 shall be added.
1.20 “SRA” means the Sponsored Research Agreement titled “Centaur-Duke Structured Research Agreement” entered into by DUKE and LICENSEE on August 8, 2024.
1.21 “IMPROVEMENTS” means any invention, patentable or otherwise, conceived as a direct result of the performance under the SRA, under the direction of [*] and where the invention has been disclosed in writing to DUKE’s Office for Translation & Commercialization.
2. The following new Paragraph 2.10 shall be added.
2.10 DUKE hereby grants LICENSEE an exclusive option to add IMPROVEMENTS to the definition of PATENT RIGHTS or TECHNICAL INFORMATION as applicable under the License Agreement. So long as LICENSEE reimburses DUKE for patent expenses covering such IMPROVEMENTS, LICENSEE’s OPTION shall be exercisable for [*] ([*]) months following disclosure of any such IMPROVEMENT by DUKE to LICENSEE (“OPTION PERIOD”). Prior to the expiration of the OPTION PERIOD, LICENSEE shall notify DUKE in writing if it wishes to exercise its OPTION after which time (a) patentable IMPROVEMENTS will be added to the list of PATENT RIGHTS and/or (b) non-patentable IMPROVEMENTS will be added as TECHNICAL INFORMATION, as applicable, under the terms of the License Agreement.
3. Except as specifically modified and amended above, all other terms and conditions of the License Agreement remain unchanged and in effect and are hereby ratified and adopted as though fully set forth herein.
IN WITNESS WHEREOF, the parties have entered into this First Amendment to the License Agreement as of the date and year first above-written.
| LICENSEE | DUKE UNIVERSITY | |||
| By: | /s/ Michael J. Roberts | By | /s/ Robin L. Rasor | |
| Michael J. Roberts | Robin L. Rasor | |||
| Title: | CEO | Title | Associate Vice President | |
Exhibit 10.3
CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THE EXHIBIT BECAUSE IT IS BOTH NOT MATERIAL AND IS THE TYPE THAT THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. SUCH EXCLUDED INFORMATION HAS BEEN MARKED WITH “[*].”
SPONSORED RESEARCH AGREEMENT
This sponsored research agreement (“Agreement”) is effective August 8, 2024 (“Effective Date”) and is between Duke University, a tax-exempt research and educational institution located in Durham, North Carolina, acting for and on behalf of its School of Medicine (“Duke”), and Centaur Bio a corporation with offices at 7805 Pemswood Street, Charlotte, NC 28277 (“Sponsor”). The parties represented in this Agreement shall be referred to individually as a “Party” and collectively as the “Parties”.
WHEREAS, the research contemplated by this Agreement is of mutual interest and benefit to Duke and Sponsor, and will further the instructional and research objectives of Duke in a manner consistent with its status as a non-profit research and educational institution.
NOW THEREFORE, in consideration of the promises and covenants herein, the Parties hereto agree as follows:
Definitions:
As used in this Agreement, the following terms shall have the following meanings:
Duke Investigator: [*], M.D.
Confidential Information: All proprietary information disclosed by a Party (the “Disclosing Party”) to the other Party (the “Receiving Party”) that the Disclosing Party marks as confidential. If Confidential Information is provided orally, it will be reduced to writing within [*] ([*]) days of such oral disclosure and marked confidential. Notwithstanding the previous sentence, if Confidential Information is communicated where a reasonable person knowledgeable in the field would recognize from the content of the communication or the circumstances under which the communication is made that the communication is intended to be confidential, then such communication shall be considered Confidential Information regardless of whether it is marked as confidential or reduced in writing.
Terms and Conditions of this Agreement:
| 1. | Duke and Sponsor agree to performance and payment under this Agreement as follows: |
| (a) | Duke agrees to perform a program of scientific research as described in Exhibit A of this Agreement (the “Research”). |
| (b) | The Research will be supervised by the Duke Investigator. |
| (c) | This Agreement will commence on the Effective Date and will terminate upon completion of the Research unless earlier terminated as otherwise provided in this Agreement. |
| (d) | Payment. |
| (i) | In consideration of the performance of the Research, and as detailed in the budget included as Exhibit A (the “Budget”), Sponsor shall pay Duke a fixed-price total of $[*]. |
| 2. | Duke’s relationship to Sponsor under this Agreement will be that of an independent contractor and not an agent, joint venturer or partner of Sponsor. |
| 3. | If Confidential Information is provided, the Receiving Party agrees that the Disclosing Party is the sole owner of such Confidential Information and that the Receiving Party will formulate and adopt appropriate safeguards, using the same care and discretion that the Receiving Party uses with similar information that it considers confidential that shall, at a minimum, be reasonable care and discretion, to maintain and protect the confidentiality of the Confidential Information for a period of [*] ([*]) years from the Effective Date (the “Term of Confidentiality”), and agrees not to disclose it or use it for any purpose not contemplated by this Agreement. The Receiving Party shall not be bound by confidentiality obligations hereunder with respect to the Confidential Information, or any part thereof, that: |
| (a) | is already known to the Receiving Party at the time of the disclosure; |
| (b) | is or becomes publicly known without the wrongful act or breach of this Agreement by the Receiving Party; |
| (c) | is legally received by the Receiving Party from a third party that is not subject to restrictions on disclosure; |
| (d) | is approved for release by written authorization of the Disclosing Party; or |
| (e) | is subsequently and independently developed by employees of the Receiving Party without use of or reliance upon the Confidential Information. |
Nothing herein shall prevent the Receiving Party from disclosing Confidential Information to the extent required pursuant to a judicial or government request, requirement or order. In such instances, the Receiving Party shall take reasonable steps to provide the Disclosing Party sufficient prior written notice in order for the Disclosing Party to contest such request, requirement or order.
| 4. | Results: |
| (a) | At mutually agreeable times, Duke will provide Sponsor with periodic progress reports disclosing the information and data generated from the performance of the Research (collectively, “Results”). In addition, Duke will provide Sponsor with a scientifically complete final report within [*] ([*]) days of termination of this Agreement. |
| (b) | Duke hereby grants Sponsor a perpetual, irrevocable, royalty-free license to use the Results for any lawful purpose, which shall include, without limitation, research and development, pending patent application support, and regulatory purposes, provided that such use will be in a manner consistent with preserving: (i) the patentability of any Inventions (as defined below); and (ii) Duke’s publication rights. Sponsor will first notify Duke before using the Results to support filings of new patent applications or continuing applications. |
| (c) | Duke shall be free to use the Results for all purposes subject to Article 5. |
2
| 5. | Duke agrees to submit to Sponsor for review a copy of any proposed publication disclosing the Results at least [*] ([*]) days prior to the date of submission for publication, and agrees to consider in good faith all comments received during that time. If Sponsor determines that the proposed publication contains patentable subject matter requiring protection, Sponsor may require the delay of the publication for a period of time not to exceed an additional [*] ([*]) days for the purpose of allowing the pursuit of such protection, provided that in no event shall any such publication include Confidential Information of Sponsor. If removal of such Confidential Information will materially and adversely affect Duke’s ability to support its scientific conclusions, the Parties agree to work together in good faith to find mutually acceptable, alternative information that can be published. |
| 6. | Inventions. |
| (a) | Background Intellectual Property. Ownership of inventions, technologies, know-how, works of authorship, processes, algorithms, programs, devices, biologics, compositions, designs, prototypes, databases and models, existing as of the Effective Date or otherwise not arising from the performance of the Research, and all patent rights, copyrights, and other intellectual property rights (including foreign equivalent rights) therein (collectively, “Background Intellectual Property”), are not affected by this Agreement, and neither Party shall have any claims to or rights in any Background Intellectual Property of the other Party. |
| (b) | Any new invention or discovery developed by Duke during the performance of the Research under this Agreement (an “Invention”) shall be promptly and confidentially disclosed in writing to Sponsor (an “Invention Disclosure”). Inventorship of any new Invention shall be determined in accordance with United States patent law, whether patentable or not, and ownership of each Invention shall be determined as follows. |
| (i) | Inventions made solely by inventors who are Sponsor’s personnel shall be owned by Sponsor (“Sponsor Inventions”), |
| (ii) | Inventions made solely by inventors who are Duke personnel shall be owned by Duke (“Duke Inventions”), |
| (iii) | Inventions made jointly by inventors who are Sponsor personnel and inventors who are Duke personnel shall be owned jointly by the Parties (“Joint Inventions”). |
| (c) | Patent Prosecution. |
| (i) | Sponsor shall have the first right to pursue and maintain patent protection for any Joint Inventions in the names of both Parties, at Sponsor’s expense, and will not voluntarily discontinue the pursuit and maintenance of any United States patent protection for such Joint Inventions without first notifying Duke. |
| (ii) | Sponsor will notify Duke if it requests Duke to file for patent protection on any Duke Invention and Duke shall file for such patent protection, at Sponsor’s expense. |
| (iii) | For any exercise of Sponsor’s first right to pursue and maintain patent protection of Joint Inventions or requests for Duke to file for patent protection, Sponsor shall pay for all costs in connection with the preparation, filing, prosecution, and maintenance of U.S. and foreign application(s) covering Duke Inventions or Joint Inventions. |
3
| (iv) | In both cases (c)(i) and (c)(ii), the Parties will cooperate to assure that such application(s) will cover, to the best of each Party’s knowledge, all items of commercial interest and importance. |
| (v) | While Duke will be responsible for making decisions regarding scope and content of Duke filed applications and prosecution thereof, and Sponsor will be responsible for making decisions regarding scope and content of jointly owned applications, the other Party will be given an opportunity to review and provide input thereto. |
| (vi) | Each Party will inform the other of all developments with respect to such application(s) and will promptly supply to the other copies of all papers received and filed in connection with the prosecution thereof in sufficient time for the other Party to comment thereon. |
| (vii) | Sponsor shall have the sole right, but not obligation, to pursue and maintain patent protection for Sponsor Inventions. |
| (d) | Option and License. |
| (e) | Duke hereby grants Sponsor a first option to negotiate an exclusive, worldwide, sublicensable, royalty bearing license to Duke’s rights to any Duke Invention or Joint Invention to practice such Invention (the “Option”). Provided that Sponsor has participated in bearing the patent expenses as described in Section 6(c) above, such Option shall be exercisable, on a per- Invention basis, for [*] ([*]) months after Sponsor’s receipt of the Invention Disclosure with respect to each such Invention (the “Option Period”). The Parties may extend the Option Period upon mutual agreement. Sponsor will notify Duke in writing of its exercise of the Option within the Option Period and terms and conditions of these licenses are to be negotiated in good faith and agreed upon between Duke and Sponsor. |
| (i) | If Sponsor does not exercise the Option with respect to any Duke Invention or Joint Invention, or notifies Duke that it will not exercise the Option with respect to any Duke Invention or Joint Invention, then Sponsor shall no longer have any claim or interest in Duke’s rights in the subject Duke Invention or Joint Invention. However, (i) Sponsor shall retain its rights under the non-exclusive license to practice any such Duke Invention for research purposes pursuant to Section (v) below, and (ii) each Party shall continue to have its joint ownership interest in any Joint Invention and shall be free to practice any Joint Invention without the consent of, or any obligation to account to, the other Party. |
| (ii) | Duke reserves the right to make or use any Invention licensed by Duke to Sponsor for Duke’s research, educational, clinical, regulatory, patent, and publication purposes. |
| (iii) | Duke hereby grants Sponsor a non-exclusive royalty free, fully paid-up, worldwide, non-sublicensable license to practice Duke’s rights in any Duke Invention for internal noncommercial research and development purposes. |
4
| 7. | THE CONFIDENTIAL INFORMATION, RESULTS, AND/OR INVENTIONS ARE PROVIDED “AS IS” WITHOUT ANY REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, OR THAT THE USE OF THE CONFIDENTIAL INFORMATION, RESULTS, AND/OR INVENTIONS WILL NOT INFRINGE UPON ANY PATENT, COPYRIGHT, TRADEMARK, OR OTHER RIGHTS. |
| 8. | Indemnification. |
| (a) | Each Party agrees to indemnify, hold harmless and defend the other Party, its officers, employees, trustees, staff members, and agents (and with respect to Duke, including without limitation the Duke Investigator) (collectively, “Indemnitees”) against any and all liabilities, claims, suits, losses, damages, costs, and expenses, including attorney’s fees (collectively, “Losses”), resulting from any claim, suit or proceeding made or brought by a third party, both government and non-government, against any Party’s Indemnitee to the extent arising from the other Party’s Indemnities (i) negligence, (ii) willful misconduct in the activities carried out pursuant to this Agreement, (iii) breach of this Agreement, provided, however, that the indemnification obligations of a Party shall not apply to the extent such Losses are attributed to any of the other Party’s Indemnitee’s gross negligence, willful misconduct, or violation of applicable laws, rules or regulations. In addition, Sponsor shall indemnify Duke for its use of the Results and Inventions. |
| (b) | Sponsor shall maintain in force at its sole cost and expense, with reputable insurance companies, insurance of a type and in an amount reasonably sufficient to protect against liability hereunder. Duke shall have the right to request the appropriate certificates of insurance from Sponsor for the purpose of ascertaining the sufficiency of such coverage. |
| 9. | Neither Party will, without the prior written consent of the other Party: (a) use in advertising, publicity or otherwise, the name of any employee or agent, any trade-name, trademark, trade device, service mark, symbol, or any abbreviation, contraction or simulation thereof owned by the other Party, or (b) represent, either directly or indirectly, that (i) any product or service of the other Party is a product or service of the representing Party, (ii) any product or service of the representing Party is made in accordance with or utilizes the information or documents of the other Party, or (iii) the other Party is endorsing the representing Party’s product or service. Notwithstanding the foregoing, Duke may acknowledge the contributions of the Sponsor in any academic publication prepared in accordance with Article 5 and shall have the right to post Sponsor’s name, the Research name, and the Research period on Duke’s publicly accessible lists of Research conducted at Duke and as may be required in submissions to funding agencies. |
| 10. | Any notice or other communication required or permitted under this Agreement will be in writing and will be deemed given as of the date it is: (a) delivered by hand, or (b) mailed, postage prepaid, first class, certified mail, return receipt requested, to the Party at the address listed below or subsequently specified in writing, (c) received by electronic mail notification, so long as receipt of the electronic mail notification is acknowledged by the recipient with a return electronic mail transmission by a person, with email addresses to be used as directed in advance by the Parties, or (d) sent, shipping prepaid, return receipt requested, by national courier service, to the Party at the address listed below or subsequently specified in writing: |
As to Duke: [*]
As to Sponsor: [*]
5
| 11. | Termination |
| (a) | Unless this Agreement terminates as otherwise provided for in this Article, this Agreement will terminate upon expiration of the Term as defined above. |
| (b) | The Parties agree that the provisions of this Agreement are intended to be interpreted and implemented so as to comply with all applicable laws, governmental rules and regulations; however, if it is determined that any provision of this Agreement is not in such compliance, then the Parties agree to modify that provision or this Agreement so as to be in compliance. If such modification is not possible, or practical, or if the Parties are unable to agree upon the modification to be made, then either Party may immediately terminate this Agreement. |
| (c) | Either Party may terminate this Agreement upon [*] ([*]) days written notice if the other Party materially breaches this Agreement and such breach remains uncured for such [*] ([*]) day period. Either Party is permitted to terminate this Agreement for its convenience upon [*] ([*]) days written notice to the other Party. |
| (d) | Either Party may terminate this Agreement immediately if the other Party engages in fraud or illegal conduct during the performance of the Research. |
| (e) | In the event of early termination, Sponsor will compensate Duke for i) [*], ii) [*], and iii) [*]. |
| (f) | Upon termination for any reason: |
| (i) | A Receiving Party, at the Disclosing Party’s discretion, shall return or destroy all Confidential Information received from the Disclosing Party. |
| (ii) | A Receiving Party may retain one (1) copy of the Disclosing Party’s Confidential Information as a record of obligations under this Agreement and for legal and regulatory purposes. |
| (iii) | In addition, any computer records or files containing such Confidential Information that have been created solely by the Receiving Party’s automatic archiving and back- up procedures, to the extent created and retained in a manner consistent with the Receiving Party’s standard archiving and back-up procedures, may be retained by the Receiving Party, but not for any other use or purpose. |
6
| 12. | Nonperformance by either Party shall be excused to the extent that performance is rendered impossible by strike, fire, earthquake, flood, governmental acts or orders or restrictions, failure of suppliers or any other reason where failure to perform is beyond the reasonable control of the non-performing Party. In such event the affected Party, as the case may be, shall promptly notify the other Party of such inability and of the period for which such inability is anticipated to continue. Without limiting the foregoing, the Party subject to such inability shall use reasonable efforts to minimize the duration of any force majeure event. |
| 13. | This Agreement is binding upon and will inure to the benefit of the Parties hereto and their respective successors and assigns. This Agreement shall not be assignable in whole or in part by a Party without the prior written consent of the other Party. Notwithstanding the foregoing, Sponsor may assign its rights or obligations under this Agreement in connection with a merger or similar reorganization or the sale of all or substantially all of its assets or of the Sponsor’s subject matter of this Agreement, without the prior written consent of Duke. Upon completion of such an event, Sponsor will provide Duke with written notification disclosing the name and address of the third party that has assumed Sponsor’s rights and obligations under this Agreement. |
| 14. | Any and all provisions of this Agreement which by their nature or effect are required or intended to be observed, kept or performed after termination or expiration of this Agreement will survive the termination or expiration of this Agreement, as the case may be, and remain binding upon and for the benefit of the Parties hereto. |
| 15. | This Agreement and its attached Exhibit represent the entire understanding between the Parties, and supersedes all other agreements, express or implied, between the Parties as to its subject matter. Any alteration, modification, or amendment to this Agreement must be in writing and signed by an authorized office of both Parties. |
| AGREED: | ||||
| DUKE UNIVERSITY | CENTAUR BIO | |||
| By: | /s/ Oshrat BenMoshe-Doriocourt | By: | /s/ Michael J. Roberts | |
| Name: | Oshrat BenMoshe-Doriocourt | Name: | Michael J. Roberts | |
| Title: | Director, ORC Operations | Title: | CEO | |
| Date executed: | 8/12/2024 | Date executed: | 8/8/2024 | |
7
EXHIBIT A
Research and Budget
[*]
A-1
Exhibit 10.4
Advisory Services Agreement
Advisory Services Agreement (the “Agreement”), made this 11th day of February 2025, is entered into by Unite Acquisition 1 Corp., a Delaware corporation (the “Company”), and Lucius Partners LLC, a Delaware limited liability company (the “Advisor”).
WHEREAS, the Company proposes to (a) enter into that certain Agreement and Plan of Merger and Reorganization (the “Merger Agreement”), by and among the Company, Adaptin Acquisition Co., a Delaware corporation and wholly owned subsidiary of the Corporation (“Merger Sub”), and Adaptin Bio, Inc., a privately held Delaware corporation (“Adaptin”), pursuant to which, among other things, Merger Sub would merge with and into Adaptin, with Adaptin continuing as the surviving entity and as a wholly owned subsidiary of the Corporation (the “Merger”), and all of the issued and outstanding capital stock of Adaptin will be exchanged for shares of common stock of the Company, par value $0.0001 per share (“Common Stock”); and (b) contemporaneously with the Merger, complete a private placement offering (the “Offering”) of a minimum of 795,455 Units of the Company’s securities, each “Unit” consisting of (i) one share of Company Common Stock, (ii) a warrant to purchase one share of Company Common Stock, exercisable from issuance until one year after the final closing of the Offering at an exercise price of $4.40 per share, and (iii) a warrant, to purchase one-half share of Company Common Stock, exercisable from issuance until five years after the final closing of the Offering at an exercise price of $6.60 per whole share, at a purchase price of $4.40 per Unit, upon the terms and subject to the conditions of subscription agreements in a form reasonably acceptable to the Parent and the Company; and
WHEREAS, the Company and the Advisor desire to establish the terms and conditions under which the Advisor will provide services to the Company as provided herein post consummation of the Merger and the Offering;
NOW, THEREFORE, in consideration of the mutual covenants and promises contained herein and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged by the parties hereto, the parties agree as follows:
1. Services.
1.1 The Advisor agrees to perform such consulting, advisory and related services to and for the Company as may be reasonably requested from time to time by the Company, including, but not limited to, the services specified on Schedule A to this Agreement (the “Services”) using the principals, officers, employees and Subcontractors (as defined below) of the Advisor (the “Personnel”). The Advisor shall not engage the services of third party contractors, subcontractors or consultants (each, a “Subcontractor”) in the performance of the Services without the prior written consent of the Company, which consent of the Company may be granted or withheld in its sole discretion. In the event that the Company permits the Advisor to use the services of one or more Subcontractors, each such Subcontractor shall sign a written agreement agreeing to be bound by all of the provisions of this Agreement to the same extent as the Advisor and the Personnel. The Company shall not have any responsibility or obligation to any such Subcontractor.
1.2 It is expressly understood and agreed that Advisor shall be required to perform only such tasks as may be necessary or desirable in connection with the rendering of its services hereunder and therefore may not perform all of the enumerated tasks during the Term (as defined below). Moreover, it is further understood that the Advisor need not perform each of the enumerated tasks in order to receive the fees and expense reimbursements. It is further understood that the Advisor’s tasks may not be limited to those enumerated in this Agreement, but that the Services to be rendered by the Advisor to the Company shall under no circumstances include the following: (i) any activities which could be deemed to constitute investment banking or any other activities requiring the Advisor to be registered as a broker-dealer under the Securities Exchange Act of 1934, as amended (the “Exchange Act”); and/or (ii) any activities which could be deemed to be in connection with the offer or sale of securities in a capital raising transaction. It is specifically understood and agreed by the parties hereto that all capital raising transactions associated with the Company will be processed and completed through a FINRA-registered broker-dealer, and the Advisor will have no involvement therewith.
1.3 In connection with Advisor’s activities hereunder, the Company will cooperate with Advisor and furnish Advisor upon request with all information regarding the business, operations, properties, historical and projected financials (in GAAP format), management and prospects of the Company (all such information so furnished being the “Information”) that Advisor deems appropriate and will provide Advisor with access to the Company’s officers, directors, employees, independent accountants and legal counsel. The Company represents and warrants to Advisor that all Information made available to Advisor hereunder will be complete and correct in all material respects and will not contain any untrue statement of a material fact or omit to state a material fact necessary in order to make the statements therein not misleading in light of the circumstances under which such statements are or will be made. The Company further represents and warrants that any projections and other forward-looking information provided by it to Advisor will have been prepared in good faith and will be based upon assumptions which, in light of the circumstances under which they are made, are reasonable. The Company recognizes and confirms that Advisor: (i) will use and rely primarily on the Information and on information available from generally recognized public sources in performing the Services contemplated by this Agreement without having independently verified the same; (ii) does not assume responsibility for the accuracy or completeness of the Information and such other information; and (iii) will not make an appraisal of any assets of the Company. Any advice rendered by Advisor pursuant to this Agreement may not be disclosed publicly without Advisor’s prior written consent.
1.4 The Company recognizes that in order for Advisor to perform properly its obligations in a professional manner, the Company will keep Advisor informed of and, to the extent practicable, permit Advisor to participate in, meetings and discussions between the Company and any third party relating to the matters covered by the terms of Advisor’s engagement. If at any time during the course of Advisor’s engagement, the Company becomes aware of any material change in any of the information previously furnished to Advisor, it will promptly advise Advisor of the change.
-2-
1.5 Under this Agreement, Advisor will have no authority whatsoever to assume or create any obligation, liability, or undertake and responsibility whatsoever, express or implied on behalf of or in the name of the Company or any affiliate other than those required to perform the Services identified under this Agreement.
1.6 The Company acknowledges that Advisor has been, and may in the future be, engaged to provide services to other companies in the industry in which the Company is involved. Subject to the provisions of this Agreement, the Company acknowledges and agrees that nothing contained in this Agreement shall limit or restrict the right of Advisor or of any member, manager, officer, employee, agent or representative of Advisor, to be a member, manager, partner, officer, director, employee, agent or representative of, investor in, or to engage in, any other business, whether or not of a similar nature to the Company’s business, nor to limit or restrict the right of Advisor to render services of any kind to any other corporation, firm, individual or association. Advisor may, but shall not be required to, present opportunities to the Company.
2. Term. This Agreement shall commence on the date hereof and shall continue for twenty-four (24) months from the date of closing of the Offering (the “Term”). The Term may be extended for additional one-year periods upon written request by the Company within 60 days prior to the expiry of the Term (as may be extended), or sooner terminated in accordance with the provisions of Section 4.
3. Fees.
3.1 Upon the initial closing of the Offering (the “Initial Closing”), the Company will pay to the Advisor a cash fee of $180,000 for the Services during the first year following such closing, and will agree to pay the Advisor for the Services, in advance for four consecutive three-month periods, commencing on the first day of the month that is the first full month twelve months or more after the Initial Closing, a cash fee of $45,000 for one year.
4. Termination. This Agreement may be terminated prior to the end of the Term in the following manner: (a) by the non-breaching party, upon twenty-four (24) hours prior written notice to the breaching party if one party has materially breached this Agreement; or (b) at any time upon the mutual written consent of the parties hereto. In the event of termination, the Advisor shall be entitled to payment for any expenses paid or incurred that the Company has theretofore agreed to reimburse. Notwithstanding the foregoing, the Company may terminate this Agreement effective immediately by giving written notice to the Advisor if the Advisor breaches or threatens to breach any provision of Section 5.
5. Proprietary Information and Inventions.
5.1 The Advisor acknowledges that its relationship with the Company is one of high trust and confidence and that in the course of its service to the Company it will have access to and contact with Proprietary Information. The Advisor will not disclose any Proprietary Information to any person or entity other than employees of the Company or use the same for any purposes (other than in the performance of the Services) without written approval by an officer of the Company, either during or after the Consultation Period, unless and until such Proprietary Information has become public knowledge without fault by the Advisor.
-3-
5.2 For purposes of this Agreement, Proprietary Information shall mean, by way of illustration and not limitation, all information, whether or not in writing, whether or not patentable and whether or not copyrightable, of a private, secret or confidential nature, owned, possessed or used by the Company, concerning the Company’s business, business relationships or financial affairs, including, without limitation, any Invention, formula, vendor information, customer information, apparatus, equipment, trade secret, process, research, report, technical or research data, clinical data, know-how, computer program, software, software documentation, hardware design, technology, product, processes, methods, techniques, formulas, compounds, projects, developments, marketing or business plan, forecast, unpublished financial statement, budget, license, price, cost, customer, supplier or personnel information or employee list that is communicated to, learned of, developed or otherwise acquired by the Advisor in the course of its service as a consultant to the Company.
5.3 The Advisor’s obligations under this Section 5 shall not apply to any information that (i) is or becomes known to the general public under circumstances involving no breach by the Advisor or others of the terms of this Section 5, (ii) is generally disclosed to third parties by the Company without restriction on such third parties, or (iii) is approved for release by written authorization of an officer of the Company.
5.4 The Advisor agrees that all files, documents, letters, memoranda, reports, records, data sketches, drawings, models, laboratory notebooks, program listings, computer equipment or devices, computer programs or other written, photographic, or other tangible material containing Proprietary Information, whether created by the Advisor or others, which shall come into its custody or possession, shall be and are the exclusive property of the Company to be used by the Advisor only in the performance of its duties for the Company and shall not be copied or removed from the Company’s premises except in the pursuit of the business of the Company. All such materials or copies thereof and all tangible property of the Company in the custody or possession of the Advisor shall be delivered to the Company, upon the earlier of (i) a request by the Company or (ii) the termination of this Agreement. After such delivery, the Advisor shall not retain any such materials or copies thereof or any such tangible property.
5.5 The Advisor agrees that its obligation not to disclose or to use information and materials of the types set forth in paragraphs (b) and (d) above, and its obligation to return materials and tangible property set forth in paragraph (d) above extends to such types of information, materials and tangible property of customers of the Company or suppliers to the Company or other third parties who may have disclosed or entrusted the same to the Company or to the Advisor.
6. Independent Contractor Status.
6.1 The Advisor and its Personnel shall perform all services under this Agreement as “independent contractors” and not as employees or agents of the Company. The Advisor and its Personnel are not authorized to assume or create any obligation or responsibility, express or implied, on behalf of, or in the name of, the Company or to bind the Company in any manner.
-4-
6.2 The Advisor and its Personnel shall have the right to control and determine the time, place, methods, manner and means of performing the Services. In performing the Services, the amount of time devoted by the Advisor and its Personnel on any given day will be entirely within the Advisor’s and its Personnel’s control, and the Company will rely on the Advisor and its Personnel to put in the amount of time necessary to fulfill the requirements of this Agreement. The Advisor and its Personnel will provide all equipment and supplies required to perform the Services. The Advisor and its Personnel are not required to attend regular meetings at the Company. However, upon reasonable notice, the Advisor and its Personnel shall meet with representatives of the Company at a location to be designated by the parties to this Agreement.
6.3 In the performance of the Services, the Advisor and its Personnel have the authority to control and direct the performance of the details of the Services, the Company being interested only in the results obtained.
6.4 The Advisor and its Personnel shall not use the Company’s trade names, trademarks, service names or service marks without the prior approval of the Company.
6.5 The Advisor and its Personnel shall be solely responsible for all federal, state and foreign income and other taxes relating to any compensation received hereunder.
6.6 Subject to the Advisor’s obligations in Section 5 above, the Advisor and its Personnel retain the right to contract with other companies or entities for their consulting and other services without restriction. Subject to the Company’s obligations in any other agreement, the Company retains a right to contract with other companies and/or individuals for consulting services without restriction.
7. Representations, Warranties and Covenants.
7.1 The Advisor hereby covenants that it shall be liable for the acts and omissions of the Personnel, including without limitation any breach of this Agreement or violation of law.
7.2 The Advisor hereby represents, warrants and covenants to the Company that it and its Personnel have the skills and experience necessary to perform the Services, that it and the Personnel will perform said services in a professional, competent and timely manner, that it has the power to enter into this Agreement and that its and the Personnel’ performance hereunder will not infringe upon or violate the rights of any third party or violate any federal, state or municipal laws.
7.3 The Advisor hereby represents and warrants to the Company that its and its Personnel’s performance of the terms of this Agreement and the performance of the Services hereunder as a consultant of the Company do not and will not breach any agreement with any third party to which the Advisor and/or its Personnel are a party (including, without limitation, any nondisclosure or non-competition agreement), and that the Advisor and its Personnel will not disclose to the Company or induce the Company to use any confidential or proprietary information or material belonging to any other person that the Advisor or its Personnel do not have the right to disclose.
-5-
7.4 The Company hereby represents and warrants to the Advisor that the Company’s performance of the terms of this Agreement does not and will not breach any agreement with any third party to which the Company is a party (including, without limitation, any exclusivity, right of first refusal, nondisclosure or non-competition agreement), and that the Company will not disclose to the Advisor or its Personnel or induce any of them to use any confidential or proprietary information or material belonging to any other person that the Company does not have the right to disclose.
8. Notices. All notices, consents, waivers, and other communications which are required or permitted under this Agreement shall be in writing will be deemed given to a party (a) upon receipt, when personally delivered; (b) one (1) business day after deposit with a nationally recognized overnight courier service with next day delivery specified, costs prepaid on the date of delivery, if delivered to the appropriate address by hand or by nationally recognized overnight courier service (costs prepaid); (c) the date of transmission if sent by e-mail with confirmation of transmission by the transmitting equipment, provided confirmation of email is kept on file, whether electronically or otherwise, by the sending party and the sending party does not receive an automatically generated message from the recipients email server that such e-mail could not be delivered to such recipient; (d) the date received or rejected by the addressee, if sent by certified mail, return receipt requested, postage prepaid; or (e) seven (7) days after the placement of the notice into the mails (first class postage prepaid), to the party at the address or e-mail address furnished by the such party at each party’s address or such other address as any party shall have furnished to the other parties in writing in accordance with this Section 8.
9. Entire Agreement. This Agreement constitutes the entire agreement between the parties and supersedes all prior agreements and understandings, whether written or oral, relating to the subject matter of this Agreement.
10. Amendment. This Agreement may be amended or modified only by a written instrument executed by both the Company and the Advisor.
11. Non-Assignability of Contract. Except as expressly provided herein, the Advisor shall not have the right to assign any of its rights or delegate any of its duties without the express written consent of the Company. Any non-consented-to assignment or delegation, whether express or implied or by operation of law, shall be void and shall constitute a breach and a default by the Advisor.
12. Governing Law. This Agreement shall be governed by and construed in accordance with the laws of the State of New York without giving effect to any choice or conflict of law provision or rule that would cause the application of laws of any other jurisdiction.
13. Successors and Assigns. This Agreement shall be binding upon, and inure to the benefit of, both parties and their respective permitted successors and assigns, including any corporation with which, or into which, the Company may be merged or which may succeed to its assets or business, provided, however, that the obligations of the Advisor are personal and shall not be assigned by Advisor.
-6-
14. Indemnification. The Company agrees to indemnify Advisor in accordance with the indemnification and other provisions attached to this Agreement as Schedule B (the “Indemnification Provisions”), which provisions are incorporated herein by reference and shall survive the termination or expiration of this Agreement. In addition, Advisor and the Company agree that neither Advisor nor any of its affiliates or any of their respective officers, directors, controlling persons (within the meaning of Section 15 of the Act or Section 20 of the Exchange Act), employees or agents shall have any liability to the Company, its security holders or creditors, or any person asserting claims on behalf of or in the right of the Company (whether direct, indirect, in contract, tort, for an act of negligence or otherwise) for any losses, fees, damages, liabilities, costs, expenses or equitable relief arising out of or relating to this Agreement or the Services rendered hereunder, except for losses, fees, damages, liabilities, costs or expenses that arise out of or are based on any action of or failure of Advisor and that are finally and judicially determined to have resulted solely from the gross negligence or willful misconduct of Advisor.
15. Miscellaneous.
15.1 No delay or omission by the Company in exercising any right under this Agreement shall operate as a waiver of that or any other right. A waiver or consent given by the Company on any one occasion shall be effective only in that instance and shall not be construed as a bar or waiver of any right on any other occasion.
15.2 The captions of the sections of this Agreement are for convenience of reference only and in no way define, limit or affect the scope or substance of any section of this Agreement.
15.3 In the event that any provision of this Agreement shall be invalid, illegal or otherwise unenforceable, the validity, legality and enforceability of the remaining provisions shall in no way be affected or impaired thereby.
15.4 This Agreement may be executed in any number of counterparts, each of which shall be enforceable against the parties actually executing such counterparts, and all of which together shall constitute one instrument. In the event that any signature is delivered by an e-mail, which contains a copy of an executed signature page such as a portable document format (.pdf) file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such e-mail of an executed signature page such as a .pdf signature page were an original thereof.
[Remainder of Page Intentionally Left Blank]
-7-
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date and year first above written.
| COMPANY: | ||
| Unite Acquisition 1 Corp. | ||
| By: | /s/ Nathan Pereira | |
| Name: | Nathan Pereira | |
| Title: | CEO | |
| ADVISOR: | ||
| Lucius Partners LLC | ||
| By: | /s/ Matthew Eitner | |
| Name: | Matthew Eitner | |
| Title: | Managing Member | |
SIGNATURE PAGE TO ADVISORY SERVICES AGREEMENT
-8-
Exhibit 10.5
LOCK-UP AGREEMENT
This LOCK-UP AGREEMENT (this “Agreement”) is made as of February 11, 2025, by and between the undersigned person or entity (the “Restricted Holder”) and Adaptin Bio, Inc. (formerly known as Unite Acquisition 1 Corp.), a Delaware corporation (the “Parent”). Capitalized terms used and not otherwise defined herein shall have the meanings given to such terms in the Merger Agreement (as defined below).
WHEREAS, pursuant to the transactions contemplated under that certain Agreement and Plan of Merger and Reorganization, dated as of February 11, 2025 (the “Merger Agreement”), by and among the Parent, Adaptin Acquisition Co., a Delaware corporation and wholly-owned subsidiary of the Parent (the “Merger Sub”), and Adaptin Bio, Inc., a privately held Delaware corporation (“Adaptin”), Merger Sub will merge with and into Adaptin, with Adaptin continuing as the surviving entity, and a wholly owned subsidiary of the Parent, and, among other things, all of the outstanding stock of Adaptin will be exchanged for shares of common stock of the Parent, par value $0.0001 per share (the “Parent Common Stock”) on the terms set forth in the Merger Agreement (the “Merger”); and
WHEREAS, contemporaneously with the closing of the Merger, the Parent will complete a private placement offering pursuant to Rule 506(b) of Regulation D under the Securities Act (the “Private Placement Offering”) of a minimum of 795,455 Units (as defined below) of the Parent’s securities, at a purchase price of $4.40 per Unit (the “Per Unit Purchase Price”), for an aggregate purchase price of $3,500,002 (the “Minimum Offering Amount”), and a maximum of 1,931,819 Units at the Per Unit Purchase Price for an aggregate purchase price of $8,500,003.60. Each “Unit” consists of (i) one share of the Parent Common Stock, (ii) a warrant (the “A Warrant”), representing the right to purchase one (1) share of Parent Common Stock, exercisable from issuance until one (1) year after the final closing of the Private Placement Offering at an exercise price of $4.40 per share, and (iii) a warrant (the “B Warrant,” and together with the A Warrant, the “Warrants”), representing the right to purchase one-half (1/2) share of Parent Common Stock, exercisable from issuance until five (5) years after the final Closing of the Private Placement Offering at an exercise price of $6.60 per whole share.
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereby agree as follows:
1. Definitions.
(a) “Affiliate” shall have the meaning set forth in Rule 405 under the Securities Act of 1933, as amended (the “Securities Act”).
(b) “Business Day” means any day other than a Saturday, a Sunday or a day on which banks in the state of New York are required or authorized by applicable law to close.
(c) “Change of Control” means the transfer (whether by tender offer, merger, consolidation or other similar transaction), in one transaction or a series of related transactions, to a person or group of Affiliated persons, of the Parent’s voting securities if, after such transfer, such person or group of Affiliated persons would hold more than 50% of the outstanding voting securities of the Parent (or the surviving entity).
(d) “Immediate Family” means any relationship by blood, domestic partnership, marriage or adoption, not more remote than first cousin.
(e) “Restricted Period” means the period of time commencing on the closing date of the Merger and ending two (2) years after the Parent Common Stock begins to trade on the OTCQB or OTCQX market maintained by OTC Markets Group, the Nasdaq Stock Market, the New York Stock Exchange or the NYSE American (each an “Approved Market”).
(f) “Restricted Securities” means all shares of Parent Common Stock held by the Restricted Holder and all securities held by the Restricted Holder that are convertible into or exercisable or exchangeable for shares of Parent Common Stock, in each case held immediately following the closing of the Private Placement Offering or thereafter acquired by any means (including, for the avoidance of doubt, through the receipt of equity incentive awards from the Parent), and whether held beneficially or of record, but excluding (i) any shares of Parent Common Stock purchased by the Restricted Holder in the Private Placement Offering, and (ii) any shares of Parent Common Stock received by the Restricted Holder upon the conversion of the convertible promissory notes of Adaptin.
2. Restrictions.
(a) During the Restricted Period, the Restricted Holder will not, directly or indirectly:
(i) offer, sell, assign, transfer, pledge, hypothecate, contract to sell, grant an option to purchase or otherwise dispose of, or announce the intention to so dispose of, any Restricted Securities or (ii) enter into any swap, hedge or similar agreement or arrangement that transfers, in whole or in part, the economic consequence of ownership of any Restricted Securities (the actions described in clause (i) or (ii) above being hereinafter referred to as a “Disposition”). The foregoing restrictions are expressly agreed to preclude the Restricted Holder from engaging in any hedging or other transaction which is designed to or which reasonably could be expected to lead to or result in a sale or disposition of any of the Restricted Securities of the Restricted Holder during the Restricted Period, even if such securities would be disposed of by someone other than the Restricted Holder.
(b) Notwithstanding anything contained herein to the contrary, the restrictions set forth in Section 2(a) shall not apply to:
(i) if the Restricted Holder is a natural person, any transfers made by the Restricted Holder (A) to any member of the Immediate Family of the Restricted Holder or to a trust the direct or indirect beneficiaries of which are exclusively the Restricted Holder or members of the Restricted Holder’s Immediate Family, or (B) by bona fide gift, will or intestacy;
(ii) if the Restricted Holder is a natural person, corporation, partnership, limited liability company or other business entity, any transfers to a charitable organization, or to any stockholder, partner, manager, director, officer, Affiliate, employee, trustee or member of, or owner of a similar equity interest in, the Restricted Holder or its Affiliates, or any trust for the benefit of any of the foregoing or any Affiliate of the foregoing, or any limited partnership in which the Restricted Holder or its Affiliates holds a limited partnership interest, as the case may be;
(iii) if the Restricted Holder is a corporation, partnership, limited liability company or other business entity, any transfer made by the Restricted Holder:
(A) in connection with the sale or other bona fide transfer in a single transaction of all or substantially all of the Restricted Holder’s capital stock, partnership interests, membership interests or other similar equity interests, as the case may be, or all or substantially all of the Restricted Holder’s assets, in any such case not undertaken for the purpose of avoiding the restrictions imposed by this Agreement,
2
(B) to another corporation, partnership, limited liability company or other business entity so long as the transferee is an Affiliate of the Restricted Holder, or
(C) to any investment fund or other entity controlling, controlled by, managing or managed by or under common control with the Restricted Holder (including, for the avoidance of doubt, a fund managed by the same manager or managing member or general partner or management company or by an entity controlling, controlled by, or under common control with such manager or managing member or general partner or management company as the Restricted Holder) if such transfer is not for value (for purposes of this paragraph the term control (including the terms controlling, controlled by and under common control with) shall have the meaning set forth in Rule 405 under the Securities Act);
(iv) if the Restricted Holder is a trust, to a trustor or beneficiary of the trust if such transfer is not for value;
(v) any transfers of the Restricted Securities to the Parent upon a vesting event or upon the exercise of options or warrants to purchase the Parent’s securities, in each case on a “cashless” or “net” exercise basis, including to cover tax withholding obligations of the Restricted Holder in connection with such vesting or exercise (and for the avoidance of doubt, any securities issued to the Restricted Holder upon such exercise shall be Restricted Securities subject to the restrictions set forth herein);
(vi) any transfers of the Restricted Securities pursuant to a court order or by operation of law, including pursuant to a domestic order or a negotiated divorce settlement;
(vii) any transfers of the Restricted Securities to the Parent pursuant to agreements under which the Parent has the option to repurchase such Restricted Securities or the Parent has a right of first refusal with respect to transfers of such Restricted Securities; or
(viii) any transfers of the Restricted Securities pursuant to a bona fide third-party tender offer, merger, consolidation or other similar transaction made to all holders of Restricted Securities involving a Change of Control of the Parent (it being further understood that this Agreement shall not restrict the undersigned from entering into any agreement or arrangement in connection therewith, including an agreement to vote in favor of, or tender Restricted Securities or other securities of the Parent in, any such transaction or taking any other action in connection with any such transaction); provided that the restrictions set forth herein shall continue to apply should the completion of such transaction not occur, and provided, further, that such transaction has been approved by the Board of Directors of the Parent.
provided, however, that
| (A) | in the case of any transfer described in clause (i), (ii), (iii), (iv), or (vi) above, it shall be a condition to the transfer that the transferee execute and deliver to the Parent, not later than one Business Day prior to such transfer, a written agreement in substantially the form of this Agreement covering the transferred Restricted Securities for the balance of the Restricted Period (it being understood that any references to “Immediate Family” in the agreement executed by such transferee shall expressly refer only to the Immediate Family of the Restricted Holder and not to the Immediate Family of the transferee) and otherwise reasonably satisfactory in form and substance to the Parent; |
3
| (B) | in the case of any transfer described in clause (i), (ii), (iii) or (iv) above, such transfers are not required to be reported under Section 16 of the Exchange Act, and the Restricted Holder does not otherwise voluntarily effect any public filing or report regarding such transfers during the Restricted Period (other than a filing on Form 5 or a required filing on Form 4 for bona fide gifts); |
| (C) | in the case of any transfer described in clause (v), (vi) or (vii) above, if the transfer is required to be reported under Section 16 of the Exchange Act, any filing under Section 16 of the Exchange Act related to such transfer shall clearly indicate in the footnotes thereto that (a) the filing relates to the circumstances described in clause (v), (vi) or (vii) above, as applicable, (b) no shares were sold by the reporting person and (c) with respect to a transfer described in clause (v) above, any remaining shares received upon exercise of an option or a warrant (net of any shares transferred in connection with such “cashless” or “net” exercise to cover tax withholding obligations) or the remaining vested shares are subject to a written agreement with the Parent in substantially the form of this Agreement for the balance of the Restricted Period; and |
| (D) | in the case of any transfer described in clause (viii) above, in the event that the tender offer, merger, consolidation or other such transaction is not completed, the Restricted Securities owned by the Restricted Holder shall remain subject to the restrictions contained in this Agreement. |
(c) Furthermore, during the Restricted Period, the Restricted Holder may exercise any rights to purchase, exchange or convert any stock options granted to the Restricted Holder pursuant to the Parent’s equity incentive plans or awards existing after the Closing Date or warrants or any other securities held by the Restricted Holder after the Closing Date, which securities are convertible into or exchangeable or exercisable for Parent Common Stock, and the Restricted Holder agrees that the shares of Parent Common Stock received upon such exercise, purchase, exchange or conversion shall be and remain Restricted Securities subject to the terms of this Agreement.
(d) In addition, the restrictions set forth in Section 2(a) shall not apply to the repurchase of Restricted Securities by the Parent in connection with the termination of the Restricted Holder’s employment or other service with the Parent or any of its subsidiaries.
(e) Notwithstanding anything herein to the contrary, nothing herein shall prevent the Restricted Holder from establishing a 10b5-1 trading plan that complies with Rule 10b5-1 under the Exchange Act (“10b5-1 Trading Plan”) or from amending an existing 10b5-1 Trading Plan so long as there are no sales or other Dispositions of Restricted Securities under such plans during the Restricted Period.
(f) In the event that, during the Restricted Period, the Parent waives any of the restrictions on the transfer of any Restricted Securities held by any executive officer or director of Parent or any holder of more than five percent (5.0%) of the outstanding Parent Common Stock of the Parent (on a fully-diluted basis) that is subject to a lock-up agreement similar in terms or form to this Agreement, then Parent shall be deemed to have also waived, on the same terms, the restrictions set forth in this Agreement that would otherwise have applied to the undersigned on a pro-rata basis with respect to the same proportion of the undersigned’s Restricted Securities subject to this Agreement as (x) the aggregate Restricted Securities held by such party receiving the waiver that is subject to the waiver bears to (y) the aggregate Restricted Securities held by such party that is subject to a lock-up agreement similar in terms or form to this Agreement. The provisions of this paragraph will not apply: (i) unless and until the Parent has first waived more than five percent (5.0%) of the total outstanding Parent Common Stock (determined as of immediately following the Private Placement Offering and giving effect thereto) from such prohibitions, (ii) (a) if the release or waiver is effected solely to permit a transfer not involving a disposition for value and (b) the transferee has agreed in writing to be bound by the same terms described in this Agreement to the extent and for the duration that such terms remain in effect at the time of the transfer, or (iii) if the release or waiver is granted to a holder of Restricted Securities who participates in an underwritten public offering during the Restricted Period, whether or not such offering is wholly or partially a secondary offering, of securities pursuant to a registration statement under the Securities Act of 1933, as amended, provided that the undersigned Restricted Holder is offered the opportunity to participate in the offering on a pro rata basis. In the event that any percentage of such Restricted Securities released from the restrictions set forth in this Agreement are subject to any restrictions of the type set forth in this Agreement, the same restrictions shall be applicable to the release of the same percentage of the undersigned’s Restricted Securities. In the event that, as a result of this paragraph, any Restricted Securities held by the undersigned are released from the restrictions imposed by this Agreement, Parent shall use commercially reasonable efforts to notify the undersigned within two Business Days thereafter that the same percentage of aggregate Restricted Securities held by the undersigned has been released from the restrictions set forth in this Agreement; provided that the failure to give such notice to the undersigned shall not give rise to any claim or liability against the Parent.
4
3. Legends; Stop Transfer Instructions.
(a) In addition to any legends to reflect applicable transfer restrictions under federal or state securities laws, each certificate or book entry representing Restricted Securities shall be stamped or otherwise imprinted with the following legend:
“THE SECURITIES REPRESENTED HEREBY ARE SUBJECT TO THE TERMS AND CONDITIONS OF A LOCK-UP AGREEMENT, DATED AS OF FEBRUARY 11, 2025, BETWEEN THE HOLDER HEREOF AND THE ISSUER, AND MAY ONLY BE SOLD OR TRANSFERRED IN ACCORDANCE WITH THE TERMS THEREOF.”
(b) The Restricted Holder hereby agrees and consents to the entry of stop transfer instructions with the Parent’s transfer agent and registrar against the transfer of the Restricted Securities except in compliance with this Agreement.
4. Miscellaneous.
(a) Material Inducement and Consideration. The Restricted Holder acknowledges and agrees that its entering into this Agreement with the Parent and its covenants and agreements herein are a material inducement to the Parent’s entering into the Merger Agreement and proceeding with the Merger and the Private Placement Offering, and Parent’s so doing constitute valuable consideration to the Restricted Holder. The Restricted Holder further acknowledges and understands that the Restricted Holder’s entry into this Agreement is a condition to the obligation of each of the purchasers in the Private Placement Offering to purchase the Units pursuant to a Subscription Agreement and that each of the Subscription Agreements provides that the Parent shall not amend, modify, waive or terminate any provision of this Agreement except to extend the term of the lock-up period and shall enforce the provisions of this Agreement in accordance with its terms.
(b) Specific Performance. The Restricted Holder agrees that, in the event of any breach or threatened breach by the Restricted Holder of any covenant, obligation or other provision contained in this Agreement, then money damages would be inadequate and the Parent shall be entitled (in addition to any other remedy that may be available to the Parent) to seek: (i) a decree or order of specific performance or mandamus to enforce the observance and performance of such covenant, obligation or other provision; and (ii) an injunction restraining such breach or threatened breach. The Restricted Holder further agrees that neither the Parent nor any other person or entity shall be required to obtain, furnish or post any bond or similar instrument in connection with or as a condition to obtaining any remedy referred to in this Section, and the Restricted Holder irrevocably waives any right that he, she, or it may have to require the obtaining, furnishing or posting of any such bond or similar instrument.
(c) Other Agreements. Nothing in this Agreement shall limit any of the rights or remedies of the Parent or Restricted Holder under the Merger Agreement, or any of the rights, remedies or obligations of the Parent or the Restricted Holder under any other agreement between the Restricted Holder and the Parent or any certificate or instrument executed by the Restricted Holder in favor of the Parent; and nothing in the Merger Agreement or in any other agreement, certificate or instrument shall limit any of the rights or remedies of the Parent or any of the obligations of the Restricted Holder under this Agreement.
5
(d) Notices. All notices, consents, waivers, and other communications which are required or permitted under this Agreement shall be in writing and will be deemed given to a party (i) on the date of delivery, if delivered to the appropriate address by hand or by nationally recognized overnight courier service (costs prepaid); (ii) the date of transmission if sent by facsimile or e-mail with confirmation of transmission by the transmitting equipment if such notice or communication is delivered prior to 5:00 P.M., Eastern Time, on a Business Day, or the next Business Day after the date of transmission, if such notice or communication is delivered on a day that is not a Business Day or later than 5:00 P.M., Eastern Time, on a Business Day; (iii) the date received or rejected by the addressee, if sent by certified mail, return receipt requested; or (iv) seven days after the placement of the notice into the mails (first class postage prepaid), to the party at the address, facsimile number, or e-mail address furnished by such party,
| If to the Parent: | With a copy (which copy shall not constitute notice hereunder) to: | |
| Adaptin Bio, In. | Wyrick Robbins Yates & Ponton LLP | |
| 3540 Toringdon Way, | 4101 Lake Boone Trail, Suite 300 | |
| Suite 200, #250 | Raleigh, NC 27607 | |
| Charlotte, NC 28277 | Attention: Donald R. Reynolds | |
| Attention Michael J. Roberts | Email: dreynolds@wyrick.com | |
| Email: mroberts@adaptin.com |
If to the Restricted Holder:
To the address set forth on the signature page hereto.
Any party may give any notice, request, demand, claim or other communication hereunder using any other means (including personal delivery, expedited courier, messenger service, telecopy, telex, ordinary mail or electronic mail), but no such notice, request, demand, claim or other communication shall be deemed to have been duly given unless and until it actually is received by the party for whom it is intended. Any party may change the address to which notices, requests, demands, claims, and other communications hereunder are to be delivered by giving the other party notice in the manner herein set forth.
(e) Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction. If the final judgment of a court of competent jurisdiction declares that any term or provision hereof is invalid or unenforceable, the parties hereto agree that the court making such determination shall have the power to limit the term or provision, to delete specific words or phrases, or to replace any invalid or unenforceable term or provision with a term or provision that is valid and enforceable and that comes closest to expressing the intention of the invalid or unenforceable term or provision, and this Agreement shall be enforceable as so modified. In the event such court does not exercise the power granted to it in the prior sentence, the parties hereto agree to replace such invalid or unenforceable term or provision with a valid and enforceable term or provision that will achieve, to the extent possible, the economic, business and other purposes of such invalid or unenforceable term.
6
(f) Applicable Law; Jurisdiction. THIS AGREEMENT IS MADE UNDER, AND SHALL BE CONSTRUED AND ENFORCED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW YORK APPLICABLE TO AGREEMENTS MADE AND TO BE PERFORMED SOLELY THEREIN, WITHOUT GIVING EFFECT TO PRINCIPLES OF CONFLICTS OF LAW.
(g) Waiver; Termination. No failure on the part of the Parent to exercise any power, right, privilege or remedy under this Agreement, and no delay on the part of the Parent in exercising any power, right, privilege or remedy under this Agreement, shall operate as a waiver of such power, right, privilege or remedy; and no single or partial exercise of any such power, right, privilege or remedy shall preclude any other or further exercise thereof or of any other power, right, privilege or remedy. The Parent shall not be deemed to have waived any claim arising out of this Agreement, or any power, right, privilege or remedy under this Agreement, unless the waiver of such claim, power, right, privilege or remedy is expressly set forth in a written instrument duly executed and delivered on behalf of the Parent; and any such waiver shall not be applicable or have any effect except in the specific instance in which it is given. If the Merger Agreement is terminated prior to Closing, this Agreement shall thereupon terminate.
(h) Captions. The captions contained in this Agreement are for convenience of reference only, shall not be deemed to be a part of this Agreement and shall not be referred to in connection with the construction or interpretation of this Agreement.
(i) Further Assurances. The Restricted Holder hereby represents and warrants that the Restricted Holder has full power and authority to enter into this Agreement and that this Agreement has been duly authorized (if the Restricted Holder is not a natural person), executed and delivered by the Restricted Holder and is a valid and binding agreement of the Restricted Holder.
(j) Entire Agreement. This Agreement sets forth the entire understanding of the Parent and the Restricted Holder relating to the subject matter hereof and supersedes all other prior agreements and understandings between the Parent and the Restricted Holder relating to the subject matter hereof.
(k) Non-Exclusivity. The rights and remedies of the Parent hereunder are not exclusive of or limited by any other rights or remedies which the Parent may have, whether at law, in equity, by contract or otherwise, all of which shall be cumulative (and not alternative).
(l) Amendments. This Agreement may not be amended, modified, altered or supplemented other than by means of a written instrument duly executed and delivered on behalf of the Parent and the Restricted Holder.
(m) Binding Nature. This Agreement and all authority herein conferred are irrevocable and shall survive the death or incapacity of the Restricted Holder (if a natural person) and shall be binding upon the heirs, personal representatives, successors and assigns of the Restricted Holder.
(n) Counterparts. This Agreement may be executed in separate counterparts, each of which shall be deemed an original and both of which shall constitute one and the same instrument.
[SIGNATURE PAGE FOLLOWS]
7
IN WITNESS WHEREOF, the undersigned has executed and delivered this Agreement as of the date first set forth above.
| RESTRICTED HOLDER (individual) | RESTRICTED HOLDER (entity) | ||
| Signature | Name of Entity | ||
| By: | |||
| Print Name | Signature | ||
| Print Name: | |||
| Signature (if Joint Tenants or Tenants in | |||
| Common) | Title: |
| |
| Address of Principal Residence: | Address of Executive Offices: | ||
| E-mail Address: | E-mail Address: | ||
[Signature Page to Lock-up Agreement]
8
| Acknowledged and Agreed: | |||
| ADAPTIN BIO, INC. | |||
| By: | |||
| Name: | |||
| Title: | |||
[Signature Page to Lock-up Agreement]
9
Exhibit 10.6
INDEMNITY AGREEMENT
This Indemnity Agreement (the “Agreement”), dated as of ____________, 2025 is entered into by and among Unite Acquisition 1 Corp., a Delaware corporation (the “Parent”), Adaptin Bio, Inc., a Delaware corporation (“Adaptin” and together with the Parent, the “Companies”), and the undersigned Indemnitee (the “Indemnitee”).
W I T N E S S E T H:
WHEREAS, Indemnitee is a director on the board of directors of the Parent (the “Board of Directors”) and/or an officer of the Parent, as well as a director and/or an officer of Merger Sub (hereinafter defined), and in such capacity(ies) is performing valuable services for the Parent; and
WHEREAS, the Parent, Adaptin Acquisition Co. (the “Merger Sub”), a Delaware corporation, and Adaptin plan to enter into an Agreement and Plan of Merger and Reorganization (the “Merger Agreement”) substantially concurrently with the date hereof, pursuant to which, among other things, Merger Sub shall merge with and into Adaptin, with Adaptin remaining as the surviving entity and a wholly-owned operating subsidiary of the Parent (the “Merger”); and
WHEREAS, it is intended that Indemnitee shall be paid promptly by the Companies all amounts necessary to effectuate in full the indemnity provided herein;
NOW, THEREFORE, in consideration of the premises and the covenants in this Agreement, and of Indemnitee and the Companies intending to be legally bound hereby, the parties hereto agree as follows:
1. Indemnification. Subject to the limitations set forth herein and in Section 5 hereof, the Companies hereby agree to indemnify Indemnitee as follows:
The Companies shall, from and after the Effective Time (as defined in the Merger Agreement), with respect to any Proceeding (as hereinafter defined), indemnify Indemnitee to the fullest extent permitted by (in the case of the Parent) Section 145 of the General Corporation Law of the State of Delaware (the “DGCL”) and the certificate of incorporation and by-laws of the Parent or Merger Sub in effect on the date hereof or as such law or constitutive document may from time to time be amended (but, in the case of any such amendment, only to the extent such amendment permits the relevant Company to provide broader indemnification rights than applicable law or constitutive document permitted the applicable Company to provide before such amendment). Notwithstanding the foregoing, the Companies shall not be required to indemnify Indemnitee for acts or omissions of Indemnitee constituting fraud, bad faith, gross negligence or intentional misconduct. The right to indemnification conferred herein and in the constitutive documents of the Companies shall be presumed to have been relied upon by Indemnitee in serving the Parent and shall be enforceable as a contract right. Without in any way diminishing the scope of the indemnification provided by this Section 1, the Companies will, from and after the Effective Time, indemnify Indemnitee against Expenses (as hereinafter defined) and Liabilities (as hereinafter defined) actually and reasonably incurred by Indemnitee or on their behalves in connection with the investigation, defense, settlement or appeal of such Proceeding. In addition to, and not as a limitation of, the foregoing, the rights of indemnification of Indemnitee provided under this Agreement shall include those rights set forth in Section 7 below. Notwithstanding the foregoing, from and after the Effective Time, the Companies shall be required to indemnify Indemnitee in connection with a Proceeding commenced by Indemnitee (other than a Proceeding commenced by Indemnitee to enforce Indemnitee’s rights under this Agreement) only if the commencement of such Proceeding was authorized by the Board of Directors following the Effective Time. Notwithstanding anything to the contrary contained herein, the Parent shall have no obligation to indemnify the Indemnitee to the extent such indemnification would not be permitted under Section 145 of the DGCL or the Parent’s certificate of incorporation in effect on the date hereof.
2. Presumptions and Effect of Certain Proceedings. Upon making a request for indemnification, Indemnitee shall be presumed to be entitled to indemnification under this Agreement, and the Companies shall have the burden of proof to overcome that presumption in reaching any contrary determination. Except as determined by a judgment or other final adjudication adverse to Indemnitee, the termination of any Proceeding by judgment, order, settlement, arbitration award or conviction, or upon a plea of nolo contendere or its equivalent, shall not affect this presumption or establish a presumption with regard to any factual matter relevant to determining Indemnitee’s rights to indemnification hereunder.
3. Advancement of Expenses. To the extent not prohibited by law, from and after the Effective Time, the Companies shall advance the Expenses or Liabilities incurred by Indemnitee in connection with any Proceeding, and such advancement shall be made within thirty (30) calendar days after the receipt by the Companies of a statement or statements requesting such advances (which shall include invoices received by Indemnitee in connection with such Expenses or Liabilities but, in the case of invoices in connection with legal services, any references to legal work performed or to expenditures made that would cause Indemnitee to waive any privilege accorded by applicable law shall not be included with the invoice) and upon request of the Companies, an undertaking to repay the advancement of Expenses or Liabilities if and to the extent that it is ultimately determined by a court of competent jurisdiction in a final judgment, not subject to appeal, that Indemnitee is not entitled to be indemnified by the Companies. Advances shall be unsecured, interest free and without regard to Indemnitee’s ability to repay the expenses. Advances shall include any and all Expenses and/or Liabilities actually and reasonably incurred by Indemnitee pursuing an action to enforce Indemnitee’s right to indemnification under this Agreement, or otherwise and this right of advancement, including Expenses and/or Liabilities incurred preparing and forwarding statements to the Company to support the advances claimed. Indemnitee acknowledges that the execution and delivery of this Agreement shall constitute an undertaking providing that Indemnitee shall, to the fullest extent required by law, repay the advance if and to the extent that it is ultimately determined by a court of competent jurisdiction in a final judgment, not subject to appeal, that Indemnitee is not entitled to be indemnified by the Company. The right to advances under this Section shall continue until final disposition of any proceeding, including any appeal therein. This Section 3 shall not apply to any claim made by Indemnitee for which indemnity is excluded pursuant to Section 14(d)(ii) below.
2
4. Procedure for Determination of Entitlement to Indemnification.
(a) Whenever Indemnitee believes that Indemnitee is entitled to indemnification pursuant to this Agreement, Indemnitee shall submit a written request for indemnification or advancement of expenses to the Companies. Any request for indemnification or advancement of expenses shall include sufficient documentation or information reasonably available to Indemnitee for the determination of entitlement to indemnification or advancement of expenses. In any event, Indemnitee shall submit Indemnitee’s claim for indemnification or advancement of expenses within a reasonable time, not to exceed sixty calendar (60) days after any judgment, order, settlement, dismissal, arbitration award, conviction, acceptance of a plea of nolo contendere or its equivalent, or final termination, whichever is the later date for which Indemnitee requests indemnification.
(b) Independent Legal Counsel (as hereinafter defined) shall determine whether Indemnitee is entitled to indemnification or advancement of expenses. Determination of Indemnitee’s entitlement to indemnification or advancement of expenses shall be made not later than ninety calendar (90) days after the Companies’ receipt of Indemnitee’s written request for such indemnification or advancement of expenses, provided that any request for indemnification or advancement of expenses for Liabilities, other than amounts paid in settlement, shall have been made after a determination thereof in a Proceeding.
5. Specific Limitations on Indemnification. Notwithstanding anything in this Agreement to the contrary, the Companies shall not be obligated under this Agreement to make any indemnity or payment to Indemnitee in connection with any claim against Indemnitee:
(a) to the extent that payment is actually made to Indemnitee under any insurance policy, contract, agreement or otherwise or is made to Indemnitee by either of the Companies or affiliates otherwise than pursuant to this Agreement. Notwithstanding the availability of such insurance, Indemnitee also may claim indemnification from the Companies pursuant to this Agreement by assigning to the Companies any claims under such insurance to the extent Indemnitee is paid by the Companies;
(b) for Liabilities in connection with Proceedings settled without the Companies’ consent, which consent, however, shall not be unreasonably withheld;
(c) in no event shall the Companies be liable to pay the fees and disbursements of more than one counsel in any single Proceeding except to the extent that, in the written opinion of counsel of the Indemnitee, the Indemnitee has conflicting interests in the outcome of such Proceeding;
(d) to the extent it would be otherwise prohibited by law, if so established by a judgment or other final adjudication adverse to Indemnitee;
(e) for an accounting of profits made from the purchase and sale (or sale and purchase) by Indemnitee of securities of the Companies within the meaning of Section 16(b) of the Securities Exchange Act of 1934, as amended, or similar provisions of state statutory law or common law;
3
(f) in connection with any Proceeding (or any part of any Proceeding) initiated by Indemnitee, including any Proceeding (or any part of any Proceeding) initiated by Indemnitee against the Companies or their directors, officers, employees or other indemnitees, unless (i) the commencement of such Proceeding was authorized by the Board of Directors (or any part of any Proceeding) prior to its initiation and following the Effective Time, or (ii) the Company provides the indemnification, in its sole discretion, pursuant to the powers vested in the Company under applicable law; or
(g) for any reimbursement of the Companies by Indemnitee of any bonus or other incentive-based or equity-based compensation or of any profits realized by Indemnitee from the sale of securities of the Companies, as required in each case under the Securities Exchange Act of 1934, as amended (including any such reimbursements that arise from an accounting restatement of the Company pursuant to Section 304 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), or the payment to the Companies of profits arising from the purchase and sale by Indemnitee of securities in violation of Section 306 of the Sarbanes-Oxley Act), if Indemnitee is held liable therefor.
6. Fees and Expenses of Independent Legal Counsel. The Companies agree to pay the reasonable fees and expenses of Independent Legal Counsel and to fully indemnify such Independent Legal Counsel against any and all reasonable expenses and losses incurred by any of them arising out of or relating to this Agreement or their engagement pursuant hereto.
7. Remedies of Indemnitee.
(a) In the event that (i) a determination pursuant to Section 4 hereof is made that Indemnitee is not entitled to indemnification, (ii) payment has not been timely made following a determination of entitlement to indemnification pursuant to this Agreement, (iii) the person or persons empowered to make a determination pursuant to Section 4 hereof shall have failed to make the requested determination within ninety calendar (90) days after the Companies’ receipt of Indemnitee’s written request for such indemnification or advancement of expenses, or (iv) Indemnitee otherwise seeks enforcement of this Agreement, Indemnitee shall be entitled to a final adjudication in a court of competent jurisdiction in the State of Delaware of the remedy sought.
(b) If a determination that Indemnitee is entitled to indemnification has been made pursuant to Section 4 hereof or is deemed to have been made pursuant to Section 4 hereof or otherwise pursuant to the terms of this Agreement, the Companies shall be bound by such determination in the absence of a misrepresentation or omission of a material fact by Indemnitee in connection with such determination.
(c) The Companies shall be precluded from asserting that the procedures and presumptions of this Agreement are not valid, binding and enforceable. The Companies shall stipulate in any such court or before any such arbitrator that the Companies are bound by all the provisions of this Agreement and are precluded from making any assertion to the contrary.
(d) Expenses reasonably incurred by Indemnitee in connection with Indemnitee’s request for indemnification under, seeking enforcement of or to recover damages for breach of this Agreement shall be borne by the Companies when and as incurred by Indemnitee, to the extent it is determined that Indemnitee is entitled to indemnification hereunder.
4
8. Contribution. To the fullest extent permissible under applicable law, in the event the Companies are obligated to indemnify Indemnitee under this Agreement and the indemnification provided for herein is unavailable to Indemnitee for any reason whatsoever, the Companies, in lieu of indemnifying Indemnitee, shall contribute to the amount incurred by Indemnitee, whether for judgments, fines, penalties, excise taxes, amounts paid or to be paid in settlement and/or for Expenses, in connection with any claim relating to an indemnifiable event under this Agreement, in such proportion as is deemed fair and reasonable in light of all of the circumstances of such Proceeding in order to reflect (i) the relative benefits received by the Companies and Indemnitee as a result of the event(s) and/or transaction(s) giving cause to such Proceeding; and/or (ii) the relative fault of the Companies (and their respective directors, officers, employees and agents) and Indemnitee in connection with such event(s) and/or transaction(s).
9. Modification, Waiver, Termination and Cancellation. No supplement, modification, termination, cancellation or amendment of this Agreement shall be binding unless executed in writing by all of the parties hereto. No waiver of any of the provisions of this Agreement shall be deemed or shall constitute a waiver of any other provisions hereof (whether or not similar), nor shall such waiver constitute a continuing waiver.
10. Subrogation. In the event of any payment under this Agreement, the Companies shall be subrogated to the extent of such payment to all of the rights of recovery of Indemnitee, who shall execute all papers required and shall do everything that may be necessary to secure such rights, including the execution of such documents necessary to enable the Companies effectively to bring suit to enforce such rights.
11. Notice by Indemnitee and Defense of Claim. Indemnitee shall promptly notify the Companies in writing upon being served with any summons, citation, subpoena, complaint, indictment, information or other document relating to any matter, whether civil, criminal, administrative or investigative for which such Indemnitee is entitled to indemnification or an advancement of expenses hereunder, but the omission so to notify the Companies will not relieve it from any liability that it may have to Indemnitee if such omission does not prejudice the Companies’ rights. If such omission does prejudice the Companies’ rights, the Companies will be relieved from liability only to the extent of such prejudice. No such omission shall relieve the Companies of any liability they may otherwise have to Indemnitee outside of this Agreement under applicable law, the Companies’ constitutive documents or any agreements.
5
12. Notices. All notices and other communications hereunder shall be in writing and shall be deemed to have been duly delivered and received hereunder (a) one business day after being sent for next business day delivery, fees prepaid, via a reputable international overnight courier service, (b) upon delivery in the case of delivery by hand, or (c) on the date delivered in the place of delivery if sent by email (with a written or electronic confirmation of delivery from the recipient, excluding any automated response) prior to 5:00 p.m. Eastern time, otherwise on the next succeeding business day, in each case to the intended recipient as set forth below:
| (a) | If to the Parent | Unite Acquisition 1 Corp. | |
| (prior to Merger closing): | 12 E. 49th Street, 11th Floor | ||
| New York, NY 10017 | |||
| Attention: Nathan P. Pereira, CEO | |||
| (b) | If to Adaptin: | Adaptin Bio, Inc. | |
| 3540 Toringdon Way, Suite 200, #250 | |||
| Charlotte, NC 28277 | |||
| Attention: Michael J. Roberts | |||
| Email: roberts@adaptinbio.com | |||
| With copy to (which copy shall not constitute | Wyrick Robbins Yates & Ponton LLP | ||
| notice hereunder): | 4101 Lake Boone Trail, Suite 300 | ||
| Raleigh, NC 27607 | |||
| Attention: Donald R. Reynolds | |||
| Email: dreynolds@wyrick.com | |||
| (c) | If to Indemnitee: | The address set forth on the signature page hereto. |
or any party may change the address to which notices, requests, demands, claims and other communications hereunder are to be delivered by giving the other parties notice in the manner herein set forth.
13. Non-Exclusivity. The rights of Indemnitee hereunder shall not be deemed exclusive of any other rights to which Indemnitee may be entitled under applicable law, the Companies’ constitutive documents, or any agreements, vote of stockholders, resolution of the Boards of Directors or otherwise with respect to any Proceeding (as hereinafter defined) associated with Indemnitee acting in his official capacity as an officer and director of the Parent arising out of or pertaining to actions relating to the approval of and entering into the Merger Agreement, the Transaction Documentation (as defined in the Merger Agreement), the Merger and each of the transactions contemplated thereby, whether asserted or claimed prior to, at or after the Effective Time.
14. Certain Definitions.
(a) “Expenses” shall include all direct and indirect costs (including, without limitation, reasonable attorneys’ fees, retainers, court costs, transcripts, fees of experts, witness fees, travel expenses, duplicating costs, printing and binding costs, telephone charges, postage, delivery service fees, all other disbursements or out-of-pocket expenses) actually and reasonably incurred in connection with either the investigation, defense, settlement or appeal of a Proceeding or establishing or enforcing a right to indemnification under this Agreement, applicable law or otherwise; provided, however, that “Expenses” shall not include any Liabilities.
6
(b) “Independent Legal Counsel” means a law firm or a member of a firm selected by the Companies and approved by Indemnitee (which approval shall not be unreasonably withheld). Notwithstanding the foregoing, the term “Independent Legal Counsel” shall not include any person who, under the applicable standards of professional conduct then prevailing, would have a conflict of interest in representing either the Companies or Indemnitee in an action to determine Indemnitee’s right to indemnification under this Agreement.
(c) “Liabilities” means liabilities of any type whatsoever including, but not limited to, any judgments, fines, ERISA excise taxes and penalties, penalties and amounts paid in settlement (including all interest assessments and other charges paid or payable in connection with or in respect of such judgments, fines, penalties or amounts paid in settlement) of any Proceeding.
(d) “Proceeding” means any threatened, pending or completed action, claim, suit, arbitration, alternative dispute resolution mechanism, investigation, administrative hearing or any other proceeding, whether civil, criminal, administrative or investigative, that (i) is asserted or claimed or otherwise arises after the Effective Time, (ii) is associated with Indemnitee’s actions as an officer and/or director of the Parent arising out of or pertaining to actions relating to the approval of and entering into the Merger Agreement, the Transaction Documentation (as defined in the Merger Agreement), the Merger and each of the transactions contemplated thereby, including any action brought by or in the right of the Parent or Merger Sub, and (iii) is not initiated or brought by one or more Indemnitee(s).
15. Binding Effect; Duration and Scope of Agreement. This Agreement shall be binding upon and inure to the benefit of and be enforceable by the parties hereto and their respective successors and assigns (including any direct or indirect successor by purchase, merger, consolidation or otherwise to all or substantially all of the business or assets of the Companies), spouses, heirs and personal and legal representatives. This Agreement shall continue in effect for six (6) years subsequent to the date of this Agreement, regardless of whether Indemnitee continues to serve as director or an officer of the Parent.
16. Severability. If any provision or provisions of this Agreement (or any portion thereof) shall be held to be invalid, illegal or unenforceable for any reason whatsoever:
(a) the validity, legality and enforceability of the remaining provisions of this Agreement shall not in any way be affected or impaired thereby; and
(b) to the fullest extent legally possible, the provisions of this Agreement shall be construed so as to give effect to the intent of any provision held invalid, illegal or unenforceable.
17. Governing Law. This Agreement shall be governed by and construed and enforced in accordance with the laws of the State of Delaware, as applied to contracts between Delaware residents entered into and to be performed entirely within the State of Delaware, without regard to conflict of laws rules.
18. Consent to Jurisdiction. The Companies and Indemnitee each irrevocably consent to the jurisdiction of the courts of the State of Delaware for all purposes in connection with any action or Proceeding that arises out of or relates to this Agreement and agree that any action instituted under this Agreement shall be brought only in the state courts of the State of Delaware.
19. Entire Agreement. This Agreement represents the entire agreement between the parties hereto, and there are no other agreements, contracts or understandings between the parties hereto with respect to the subject matter of this Agreement.
20. Counterparts. This Agreement may be executed in one or more counterparts, each of which shall for all purposes be deemed to be an original but all of which together shall constitute one and the same Agreement. This Agreement and any documents relating to it may be executed and transmitted to any other party by email of a PDF, which PDF shall be deemed to be, and utilized in all respects as, an original, wet-inked document.
[Signature Page Follows]
7
IN WITNESS WHEREOF, the parties hereto have caused this Agreement to be duly executed as of the date first written above.
| UNITE ACQUISITION 1 CORP. | ||
| By: | ||
| Name: | ||
| Its: | ||
| ADAPTIN BIO, INC. | ||
| By: | ||
| Name: | ||
| Its: | ||
| INDEMNITEE | ||
| By: | ||
| Name: | ||
| Address: | ||
[Signature Page to Indemnity Agreement]
8
Exhibit 10.7
SUBSCRIPTION AGREEMENT
This Subscription Agreement (this “Agreement”) has been entered into by and between the purchaser set forth on the Omnibus Signature Page hereof (the “Purchaser”) and Unite Acquisition 1 Corp, (to be renamed “Adaptin Bio, Inc.” upon consummation of the Merger (as defined below)), a Delaware corporation (the “Company”), in connection with the private placement offering (the “Offering”) by the Company.
R E C I T A L S
A. The Company is offering a minimum of 795,455 Units (as defined below) of the Company’s securities, at a purchase price of $4.40 per Unit (the “Per Unit Purchase Price”), for an aggregate purchase price of $3,500,002 (the “Minimum Offering Amount”), and a maximum of 1,931,819 Units at the Per Unit Purchase Price for an aggregate purchase price of $8,500,003.60. Each “Unit” consists of (i) one share of the Company’s common stock, par value $0.0001 per share (“Common Stock”), (ii) a warrant, substantially in the form of Exhibit A hereto (the “A Warrant”), representing the right to purchase one (1) share of Common Stock, exercisable from issuance until one (1) year after the final Closing of the Offering at an exercise price of $4.40 per share, and (iii) a warrant, substantially in the form of Exhibit B hereto (the “B Warrant,” and together with the A Warrant, the “Warrants”), representing the right to purchase one-half (1/2) share of Common Stock, exercisable from issuance until five (5) years after the final Closing of the Offering at an exercise price of $6.60 per whole share.
B. The Initial Closing (as defined below) in an amount no less than the Minimum Offering Amount is contingent upon, and shall be consummated simultaneously with, the closing of a reverse triangular merger in accordance with the terms of that certain Agreement and Plan of Merger, dated as of the Initial Closing Date (as defined below) (the “Merger Agreement”), by and among the Company, Adaptin Acquisition Co., a Delaware corporation (“Merger-Sub”) and wholly owned Subsidiary of the Company, and Adaptin Bio, Inc., a Delaware corporation (“Adaptin”), pursuant to which Merger-Sub will merge with and into Adaptin, with Adaptin surviving the merger as a wholly owned Subsidiary of the Company (the “Merger”), and pursuant to which all of the outstanding capital stock of Adaptin will be cancelled in exchange for shares of the Company’s Common Stock, and all outstanding Adaptin options and warrants (if any) will be either cancelled or assumed by, or exchanged for new securities to acquire Common Stock of, the Company, at the same ratio at which outstanding shares of capital stock of Adaptin are exchanged, with appropriate adjustments to the per share exercise or conversion price thereof, and otherwise on their original terms and conditions. The total number of shares of the Company’s Common Stock that will be issued to pre-Merger stockholders of Adaptin or reserved for issuance upon exercise of warrants, options and any other convertible securities of Adaptin is expected to be 3,883,709 (subject to rounding for fractions) shares, which includes shares of Common Stock reserved for issuance under outstanding pre-Merger Adaptin incentive options and other incentive awards to be assumed by, or exchanged for options and other incentive awards of, the Company. Before the Initial Closing, the Company’s board of directors (the “Board of Directors”) will have adopted an equity incentive plan reserving a number of shares of Common Stock equal to 15% of the shares to be outstanding after completion of the Merger and the final Closing (as defined below) of the Offering, on a fully diluted basis (assuming exercise or conversion of all then-outstanding Common Stock equivalents), for the future issuance, at the discretion of the Board, of options and other incentive awards to officers, key employees, consultants and directors of the Company and its Subsidiaries (the “EIP”). Holders of Common Stock of the Company prior to the Merger will retain in the aggregate 3,250,000 shares of Common Stock after the Merger. On or before the consummation of the Merger, the Company will change its name to “Adaptin Bio, Inc.,” and Adaptin will change its name to Adaptin Bio Operating Corporation.
C. One or more current security holders of Adaptin or its Affiliates (as defined below) or its or their officers, directors, shareholders or employees may (but are not obligated to) purchase Units in the Offering (an “Adaptin Investment”), and to the extent they do so, such purchases will be counted towards the achievement of the Minimum Offering Amount. The Placement Agent (as defined below) or its Affiliates or its or their officers, directors, shareholders or employees may (but are not obligated to) also purchase shares of Common Stock in the Offering (a “Placement Agent Investment”), and to the extent they do so, such purchases will also be counted towards the achievement of the Minimum Offering Amount.
D. None of the Units (as defined below) subscribed for pursuant to this Agreement nor the shares of Common Stock included therein (the “Shares”) nor the Warrants nor the shares of Common Stock issuable upon exercise of the Warrants (the “Warrant Shares”) (all of the foregoing sometimes referred to herein collectively as the “Securities”) have been registered under the Securities Act of 1933, as amended, and the rules and regulations promulgated thereunder (the “Securities Act”) or any state or foreign securities Law. The Offering is being made on a reasonable best efforts basis to “accredited investors,” as defined in Regulation D under the Securities Act, in reliance upon the exemption from securities registration afforded by Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D. For purposes of this Agreement, “Law” or “Laws” means any federal, state, local or foreign or provincial statute, law (including, for the avoidance of doubt, any statutory, common, or civil law), ordinance, rule, regulation, order, injunction, decree or agency requirement having the force of law or any undertaking to or agreement with any Governmental Authority (as defined below).
E. At the Initial Closing, $1,500,000 aggregate principal amount of Adaptin’s outstanding 10% convertible promissory notes (the “Bridge Notes”), plus accrued interest thereon, shall convert into shares of Common Stock at a conversion price of $3.30 per share.
F. The parties intend to treat the Merger, together with the Initial Closing and the Subsequent Closing, if relevant, as part of a transaction that is described in Section 351(a) of the Internal Revenue Code of 1986, as amended (the “Code”), to the extent property is exchanged for stock as described therein.
AGREEMENT
The Company and the Purchaser hereby agree as follows:
1. Subscription.
(a) Purchase and Sale of the Units.
(i) Subject to the terms and conditions of this Agreement, the Purchaser agrees to purchase, and the Company agrees to sell and issue to the Purchaser, that number of Units set forth on the Purchaser’s Omnibus Signature Page attached hereto at the Per Unit Purchase Price, for a total aggregate purchase price for the Units as set forth on such Omnibus Signature Page (the “Purchase Price”). The minimum subscription amount for each purchaser in the Offering is $110,000 (or 25,000 Units). The Company may accept subscriptions for less than $110,000 from any Purchaser in the Offering in its sole discretion. For the purposes of this Agreement, “Units” means the shares of Common Stock issued and sold to the Purchaser hereunder in the Offering at the Initial Closing (as defined below) and at any Subsequent Closing (as defined below).
2
(ii) In connection with the Offering, the Company has entered or will enter into other subscription agreements in the same form and containing the same terms and conditions as this Agreement for Units (“Other Units”) (each, an “Other Subscription Agreement”) with purchasers in the Offering other than the Purchaser (collectively, “Other Purchasers”).
(b) Subscription Procedure; Closing.
(i) Initial Closing. Subject to the terms and conditions of this Agreement, the initial closing of the Offering shall take place upon the satisfaction (or waiver as provided herein) of the conditions set forth in Section 5 and Section 6 of this Agreement (other than those conditions that by their nature will be satisfied at the Closing, but subject to the satisfaction (or waiver as provided herein) of such conditions) or at such other time and place as is mutually agreed to by the Company and the Placement Agent contingent upon and simultaneously with the closing of the Merger (the “Initial Closing” and the date that the Initial Closing occurs, the “Initial Closing Date”).
(ii) Subsequent Closings. At any time prior to February 28, 2025, or at such later date as the Company and Placement Agent may mutually agree, without notice to or consent from the Purchaser or any Other Purchaser, subject to the satisfaction (or waiver as provided herein) of the conditions set forth in Section 5 and Section 6 of this Agreement (other than those conditions that by their nature will be satisfied at the Closing, but subject to the satisfaction (or waiver as provided herein) of such conditions) (each a “Subsequent Closing” and collectively the “Subsequent Closings” and the date that a Subsequent Closing occurs, a “Subsequent Closing Date”), the Company may sell additional shares of Common Stock up to the Maximum Offering Amount (collectively, the “Subsequent Closing Units”) to such persons as may be approved by the Company and who are reasonably acceptable to the Placement Agent, including the Purchaser. Any Subsequent Closing Units issued and sold to the Purchaser pursuant to this Section 1(b)(ii) shall be deemed to be “Units” for all purposes under this Agreement.
The Initial Closing and the Subsequent Closings, if any, shall be known collectively herein as the “Closings” or individually as a “Closing.” The Initial Closing Date and the Subsequent Closing Dates are each referred to herein as a “Closing Date.” Closings may take place remotely via the exchange by electronic transmission of documents and signatures.
(iii) Subscription Procedure. To complete a subscription for the Units, the Purchaser must fully comply with the subscription procedure provided in subparagraphs (A) through (D) of this paragraph (iii) on or before the applicable Closing Date:
(A) Subscription Documents. At or before the applicable Closing, the Purchaser shall review, complete and execute the Omnibus Signature Page to this Agreement and the Registration Rights Agreement substantially in the form of Exhibit C hereto (the “Registration Rights Agreement”), the Selling Securityholder Questionnaire (as defined in the Registration Rights Agreement), the Purchaser Profile, and the Accredited Investor Certification, attached hereto following the Omnibus Signature Page (collectively, the “Subscription Documents”), and deliver the Subscription Documents to the party indicated thereon at the address set forth under the caption “How to subscribe for Units in the private offering of Unite Acquisition 1 Corp. (to be renamed “Adaptin Bio, Inc.” below. Executed documents may be delivered to such party by facsimile or .pdf sent by electronic mail (e-mail).
3
(B) Purchase Price. At or before the applicable Closing, the Purchaser shall deliver to Flagstar Bank, N.A., in its capacity as escrow agent (the “Escrow Agent”), under an escrow agreement among the Company, Adaptin, the Placement Agent and the Escrow Agent (the “Escrow Agreement”) the full Purchase Price set forth on the Purchaser’s Omnibus Signature Page attached hereto, by certified or other bank check or by wire transfer of immediately available funds, pursuant to the instructions set forth under the caption “How to subscribe for Units in the private offering of Unite Acquisition 1 Corp. (to be renamed “Adaptin Bio, Inc.” below. Such funds will be held for the Purchaser’s benefit in the escrow account established for the Offering (the “Escrow Account”), without interest or offset.
(C) Termination. This Agreement shall terminate automatically and be of no further force and effect, and any amounts deposited into the Escrow Account by or on behalf of the Purchaser shall be returned to the Purchaser or its designee promptly, without interest or offset, if (i) the Purchaser and the Company agree in writing to terminate this Agreement prior to the applicable Closing, (ii) the subscription has been revoked in full by the Purchaser in accordance with Section 8, (iii) prior to the applicable Closing, in the Purchaser’s sole and absolute discretion, upon written notice to the Company, if any representation or warranty of the Company set forth in Section 3 hereof shall be or shall have become inaccurate or the Company shall have breached or failed to perform any of its covenants or other agreements set forth in this Agreement, which inaccuracy, breach or failure to perform would give rise to the failure to satisfy any of the conditions set forth in Section 6(a) or Section 6(b) of this Agreement and which inaccuracy, breach or failure to perform cannot be cured by the Company or, if capable of being cured, is not cured within two (2) Business Days of the Purchaser’s notice to the Company thereof; or (iv) the Merger Agreement is terminated pursuant to its terms. For the purposes of this Agreement, “Business Day” means a day, other than a Saturday or Sunday, on which commercial banks in New York City are open for the general transaction of business. The Company shall promptly (and in any event within one (1) Business Day) provide the Purchaser with written notice of the termination of the Merger Agreement.
(D) Company Discretion. The Purchaser understands and agrees that, prior to the execution and delivery of this Agreement by the Company, the Company in its sole discretion reserves the right to accept or reject this subscription for Units, in whole or in part. The Company and the Purchaser shall have no obligation hereunder until the Company shall execute and deliver to the Purchaser an executed copy of this Agreement.
4
(iv) Deliveries at Closing. On or prior to the Closing Date, the Company shall deliver or cause to be delivered to the Purchaser the following:
(A) this Agreement duly executed by the Company;
(B) a copy of the irrevocable instructions to the Transfer Agent instructing the Transfer Agent to deliver, on an expedited basis, a certificate (or at the request of the Purchaser, book entry statement) evidencing a number of Shares included in the Units being purchased by the Purchaser at the Closing, as set forth in the Purchaser’s Omnibus Signature Page, registered in the name of the Purchaser (but notwithstanding the foregoing, upon the Closing, the Purchaser shall be deemed for all corporate purposes to have become the holder of record of such Shares, irrespective of the date of delivery of the Shares to the Purchaser;
(C) a duly executed A Warrant, registered in the name of the Purchaser, to purchase up to a number of shares of Common Stock equal to the number of Shares included in the Units being purchased by the Purchaser at the Closing;
(D) a duly executed B Warrant, registered in the name of the Purchaser, to purchase up to a number of shares of Common Stock equal to one half (1/2) (with any fraction rounded up to the nearest whole share) of the number of Shares included in the Units being purchased by the Purchaser at the Closing; and
(E) the certificates, legal opinion and other documents set forth in Section 6 below.
“Transfer Agent” means Vstock Transfer, LLC, with a mailing address of 18 Lafayette Place, Woodmere, New York 11598, and any successor transfer agent of the Company for the Common Stock.
2. Placement Agent. Laidlaw & Company (UK) Ltd. (“Laidlaw” or the “Placement Agent”), a U.S.-registered broker-dealer, has been engaged by the Company as the Company’s placement agent, on a reasonable “best efforts” basis, for the Offering. The Placement Agent (a) will be paid at each Closing from the Offering proceeds a total cash placement fee of ten percent (10.0%) of the gross Purchase Price paid by the Purchaser and the aggregate gross purchase price paid by all Other Purchasers in the Offering at that Closing (the “Cash Fee”), (b) will be paid at each Closing from the Offering proceeds a total non-allocable expense allowance equal to two percent (2.0%) of the gross Purchase Price paid by the Purchaser and the aggregate gross purchase price paid by all Other Purchasers in the Offering at that Closing (the “Expense Allowance”), (c) will be paid a cash fee of ten percent (10.0%) of the gross proceeds delivered to the Company upon any cash exercises of a Warrant, and (d) will receive warrants to purchase a total number of shares of Common Stock equal to ten percent (10.0%) of the sum of (i) the number of shares of Common Stock included in the Units sold in the Offering at that Closing and (ii) the number of shares of Common Stock issuable upon exercise of the Warrants included in the Units sold in the Offering at that Closing, with a term expiring seven (7) years after the final Closing Date and an exercise price of $4.40 per share (the “Placement Agent Warrants”). Any sub-agent of the Placement Agent that introduces investors to the Offering will be entitled to share in the Cash Fee, Expense Allowance and Placement Agent Warrants attributable to those investors pursuant to the terms of an executed sub-agent agreement with such Placement Agent. The Company has agreed to pay certain other expenses of the Placement Agent, including the fees and expenses of its counsel, in connection with the Offering.
5
3. Representations and Warranties of the Company. Except as set forth in (i) the Disclosure Schedule delivered to the Purchaser prior to or concurrently with the execution of this Agreement (the “Disclosure Schedule”), or (ii) the Confidential Private Placement Memorandum for the Offering, as the same may be supplemented or amended (the “PPM”) delivered to the Purchaser by the Company or the Placement Agent or any of their respective representatives (but excluding any disclosures (whether contained under the heading “Risk Factors,” in any “forward-looking statements” disclaimer or in any other section) to the extent they are cautionary, predictive or forward-looking in nature), the Company hereby represents and warrants to the Purchaser, as of the applicable Closing Date and after giving effect to the Merger and the other transactions contemplated by the Merger Agreement, the following (provided that, (x) as used in this Section 3, the term “Subsidiaries” shall be construed to include Adaptin as of each applicable Closing Date, and (y) any qualification as to “knowledge” shall refer to the knowledge of the officers of the Company after giving effect to the Merger and of Adaptin, in each case, both actual knowledge or knowledge that they would have had upon reasonable inquiry of the personnel of the Company or Adaptin, as applicable, responsible for the applicable subject matter):
(a) Organization and Qualification. The Company and each of its Subsidiaries is a corporation or limited liability company, as the case may be, duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation or formation, and has the requisite corporate or limited liability company power to own, lease and operate its properties and to carry on its business as currently conducted and as described in the PPM. The Company and each of its Subsidiaries is duly qualified as a foreign corporation or limited liability company, as the case may be, to do business and is in good standing in every jurisdiction in which the nature of the business as currently conducted and as described in the PPM makes such qualification necessary, except to the extent that the failure to be so qualified or be in good standing would not have a Material Adverse Effect. For purposes of this Agreement, “Material Adverse Effect” means any event, circumstance, development, condition, occurrence, state of facts, change or effect that, individually or in the aggregate with any other event, circumstance, development, condition, occurrence, state of facts, change or effect, has or would reasonably be expected to (x) prevent or materially delay or materially impair the ability of the Company or its Subsidiaries to carry out its obligations under this Agreement or (y) have any material adverse effect on the business, properties, assets, liabilities, operations or condition (financial or otherwise), results of operations or future prospects of the Company and its Subsidiaries, taken as a whole; provided, however, that for purposes of clause (y), none of the following shall be deemed in themselves, either alone or in combination, to constitute, and none of the following shall be taken into account in determining whether there has been or would reasonably be expected to have a “Material Adverse Effect”: (i) general economic, financial, credit, capital market or regulatory conditions or any changes therein (provided, however, that such effects do not affect the Company and its Subsidiaries taken as a whole disproportionately as compared to the Company’s similarly-situated competitors), (ii) any effects alone or in combination that arise out of, or result from, directly or indirectly, the announcement, pendency, execution or performance of this Agreement, the transactions contemplated hereby or any action contemplated by this Agreement, (iii) acts of God, war (whether or not declared), disease, the commencement, continuation or escalation of a war, acts of armed hostility, sabotage or terrorism or other international or national calamity or any material worsening of such conditions (provided, however, that such changes do not affect the Company or its Subsidiaries disproportionately as compared to the Company’s similarly-situated competitors), (iv) any matter disclosed in the Disclosure Schedule or the PPM (excluding any disclosures (whether contained under the heading “Risk Factors,” in any “forward looking statements” disclaimer or in any other section) to the extent they are cautionary, predictive or forward-looking in nature); (v) any failure by the Company or its Subsidiaries to meet any projections, budgets or estimates of revenue or earnings (it being understood that the facts giving rise to such failure may be taken into account in determining whether there has been a Material Adverse Effect (except to the extent such facts are otherwise excluded from being taken into account by this proviso)), (vi) changes affecting the industry generally in which the Company or its Subsidiaries operate (provided, however, that such changes do not affect the Company or its Subsidiaries disproportionately as compared to the Company’s similarly-situated competitors), or (vii) changes in Law or U.S. generally accepted accounting principles (“GAAP”) (provided, however, that such changes do not affect the Company or its Subsidiaries disproportionately as compared to the Company’s similarly-situated competitors). For purposes of this Agreement, “Subsidiary” means, with respect to the Company, any corporation, partnership, limited liability company, joint venture or other legal entity of any kind (i) of which fifty percent (50%) or more of the capital stock or other equity interests or voting power are, directly or indirectly, controlled, owned or held by, or (ii) that is, at the time any determination is made, controlled (whether by voting power, Contract (as defined below) or otherwise) by, in each case, the Company (either alone or through or together with one or more of its other Subsidiaries); provided, that for all purposes of the representations and warranties of the Company set forth in this Agreement, whether made as of the date hereof or as of the applicable Closing Date, Adaptin and its Subsidiaries shall be deemed to be Subsidiaries of the Company regardless of whether the Merger has been consummated.
6
(b) Authorization, Enforcement, Compliance with Other Instruments. (i) The Company and each of its Subsidiaries party thereto has the requisite corporate or limited liability company power and authority to enter into and perform its obligations under this Agreement, the Warrants, the Registration Rights Agreement, the Escrow Agreement and the Merger Agreement (collectively with all other documents, certificates or instruments executed and delivered in connection with the transactions contemplated hereby or thereby, the “Transaction Documents”) and to consummate the transactions contemplated thereby, including to issue the Securities, in accordance with the terms hereof and thereof; (ii) the execution and delivery by the Company and each of its Subsidiaries party thereto of each of the Transaction Documents and the consummation by it of the transactions contemplated hereby and thereby, including, without limitation, the issuance of the Securities, have been, or will be at the time of execution of such Transaction Document, duly authorized by the Board of Directors or other applicable governing body of the Company or such Subsidiary, and no further action, proceeding, consent, waiver or authorization is, or will be at the time of execution of each such Transaction Document, required by or from the Company or any such Subsidiary, its respective board of directors or other governing body or its respective stockholders or equity holders; (iii) this Agreement has been, and at the Closing each of the other Transaction Documents will be when delivered at the Closing, duly executed and delivered by the Company and each of its Subsidiaries party thereto; and (iv) this Agreement and the other Transaction Documents, when delivered at the Closing or at the closing of the Merger, as applicable, will constitute the valid and binding obligations of the Company and its Subsidiaries party thereto enforceable against the Company and its Subsidiaries party thereto in accordance with their terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of creditors’ rights and remedies and, with respect to any rights to indemnity or contribution contained in the Transaction Documents, as such rights may be limited by state or federal laws or public policy underlying such laws.
(c) Capitalization. As of the date hereof and without giving effect to the Merger, the authorized capital stock of the Company consists of 50,000,000 shares of Common Stock and 10,000,000 shares of preferred stock, par value $0.0001 per share (the “Preferred Stock”), and there are 5,000,000 shares of Common Stock outstanding and no shares of Preferred Stock outstanding. The current capitalization of the Company before and after giving effect to the Merger is set forth under Schedule 3(c). Immediately following the effective time of the Merger, but immediately before the Initial Closing, the authorized capital stock of the Company will consist of 50,000,000 shares of Common Stock and 10,000,000 shares of Preferred Stock, and the Company is expected to have 6,499,999 shares of Common Stock issued and outstanding (assuming the exchange of all Adaptin securities, as contemplated by the Merger Agreement) and will have no shares of Preferred Stock issued and outstanding. All of the outstanding shares of Common Stock and of the capital stock of each of the Company’s Subsidiaries have been duly authorized, validly issued and are fully paid and non-assessable and free of preemptive or similar rights and other Liens (as defined below) and have been issued in compliance with all applicable Laws. All of the issued and outstanding capital stock of each Subsidiary of the Company are owned, directly or indirectly, by the Company, free and clear of any Liens. Immediately after giving effect to the Merger and the Closing of the Minimum Offering Amount, the pro forma outstanding capitalization of the Company will be as set forth under “Pro Forma Capitalization” in Schedule 3(c). Immediately after giving effect to the Merger and the Closing: (i) no shares of capital stock of the Company or any of its Subsidiaries will be subject to preemptive rights or any other similar rights or any Liens suffered or permitted by the Company; (ii) except as set forth on Schedule 3(c)(ii), there will be no outstanding options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible, exercisable or exchangeable into, any shares of capital stock of the Company or any of its Subsidiaries, or any Contracts by which the Company or any of its Subsidiaries is or may become bound or pursuant to which the Company or any of its Subsidiaries is otherwise obligated to issue additional shares of capital stock of the Company or any of its Subsidiaries; (iii) there will be no outstanding debt securities of the Company or any of its Subsidiaries other than indebtedness as set forth in Schedule 3(c)(iii); (iv) other than pursuant to the Registration Rights Agreement or as set forth in Schedule 3(c)(iv), there will be no agreements or arrangements under which the Company or any of its Subsidiaries is obligated to register the sale of any of their securities under the Securities Act; (v) there will be no outstanding registration statements of the Company or any of its Subsidiaries, other than pursuant to the Registration Rights Agreement; (vi) except as set forth in Schedule 3(c)(vi), there will be no securities or instruments of the Company or any of its Subsidiaries containing anti-dilution or similar provisions, including the right to adjust the exercise, exchange or reset price under such securities, that will be triggered by the issuance of the Securities as described in this Agreement; (vii) no co-sale right, right of first refusal or other similar right will exist with respect to the Securities or the issuance and sale thereof and (viii) no shares of Common Stock shall be reserved for issuance, other than (A) up to 2,196,390 shares of Common Stock reserved for future issuances under the EIP and (B) shares of Common Stock reserved for issuance upon exercise or conversion of the securities listed in Schedule 3(c)(viii). The Company has made available to the Purchaser true and correct copies of the Company’s Certificate of Incorporation, as in effect as of the Initial Closing, and the Company’s Bylaws, as in effect as of the Initial Closing, and the terms of all securities exercisable for Common Stock and the material rights of the holders thereof in respect thereto other than stock options issued to officers, directors, employees and consultants. Except for the interests in the Company’s Subsidiaries, neither the Company nor any of its Subsidiaries owns any equity interest or other interest of any nature in, or any interest convertible, exchangeable, or exercisable for, equity interests or other interests of any nature in any other person. The Company does not have outstanding stockholder purchase rights or a “poison pill” or any similar arrangement in effect giving any person the right to purchase any equity interest in the Company upon the occurrence of certain events.
7
(d) Issuance of Securities. The Shares that are being issued to the Purchaser hereunder, when issued, sold and delivered in accordance with the terms and upon payment of the consideration set forth in this Agreement, and the Warrant Shares, when issued and delivered upon exercise of the Warrants in accordance with the terms and upon payment of the exercise price therefor as provided therein, will be duly and validly issued, fully paid and non-assessable, and free of preemptive or similar rights, Taxes and other Liens with respect to the issuance thereof, and restrictions on transfer other than restrictions on transfer under the Transaction Documents, applicable state and federal securities Laws and Liens created by or imposed by the Purchaser. Assuming the accuracy of each of the representations and warranties of the Purchaser herein, and of the Other Purchasers in each of their respective Other Subscription Agreements, the offer, issuance and sale by the Company of the Units, the Shares, the Warrants and (upon exercise of the Warrants) the Warrant Shares to the Purchaser are exempt from registration under the Securities Act and exempt from (or not subject to) registration, qualification or prospectus delivery requirements under the securities laws of any state or other jurisdiction.
(e) No Conflicts. The execution, delivery and performance of each of the Transaction Documents by the Company, and the consummation by the Company of the transactions contemplated hereby and thereby, including issuance and sale of the Securities in accordance with this Agreement and the other Transaction Documents, have not and will not (i) result in a violation of the Certificate of Incorporation or the Bylaws (or equivalent constitutive document) of the Company or any of its Subsidiaries; (ii) violate or conflict with, or result in a breach of any provision of, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any Contract to which the Company or any Subsidiary is a party, except for those which would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect, or (iii) result in a violation of any Law applicable to the Company or any Subsidiary or by which any property or asset of the Company or any Subsidiary is bound or affected, except for those which would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect. Neither the Company nor any Subsidiary is in violation of or in default under, any provision of its Certificate of Incorporation or Bylaws or any other constitutive documents. Neither the Company nor any Subsidiary is in violation of any term of or in default under any Contract, judgment, decree or order or any Law applicable to the Company or any Subsidiary, which violation or breach has been or would reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole. Except as specifically contemplated by this Agreement and as required under the Securities Act and any applicable state securities Laws, neither the Company nor any of its Subsidiaries is required to obtain any Authorization of, or provide any notice to or make any filing or registration with, any Governmental Authority in order for it to execute, deliver or perform any of its obligations under or contemplated by this Agreement or the other Transaction Documents in accordance with the terms hereof or thereof, other than (i) the filings required pursuant to Section 9(j), (ii) the filing of the registration statement contemplated by the Registration Rights Agreement and (iii) the filing of a Notice of Exempt Offering of Securities on Form D with the Securities and Exchange Commission (the “SEC”) under Regulation D. Except as set forth on Schedule 3(e), neither the execution and delivery by the Company of the Transaction Documents, nor the consummation by the Company of the transactions contemplated hereby or thereby, will require any notice, consent or waiver under any Contract to which the Company or any Subsidiary is a party or by which the Company or any Subsidiary is bound or to which any of their assets or businesses is subject, except for any notice, consent or waiver the absence of which would not reasonably be expected, individually or in the aggregate, to be material to the business of the Company and its Subsidiaries, taken as a whole. All notices, consents, authorizations, orders, filings and registrations which the Company or any of its Subsidiaries is required to deliver or obtain pursuant to the preceding two sentences have been or will be delivered or obtained or effected, and shall remain in full force and effect, on or prior to the Closing.
8
(f) Absence of Litigation. Except as set forth on Schedule 3(f), there is no, and since the date that is two (2) years prior to the date hereof (the “Lookback Date”) there has not been any, action, suit, claim, inquiry, notice of violation, arbitration, petition, charge, citation, summons, subpoena, proceeding (including any partial proceeding such as a deposition) or investigation of any nature, civil, criminal, administrative, regulatory or otherwise, whether at law or in equity, before or by any Governmental Authority (an “Action”) pending or threatened in writing or, to the knowledge of the Company, threatened orally, against or affecting the Company or any of its Subsidiaries or any of their respective officers or directors or any of their respective assets or businesses, which has or would be reasonably likely to have, a Material Adverse Effect Neither the Company nor any of its Subsidiaries is, and since the Lookback Date has not been, subject to any judgment, decree, or order which has been, or would reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole.
(g) Acknowledgment Regarding Purchaser’s Purchase of the Units. The Company acknowledges and agrees that the Purchaser is acting solely in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated hereby and thereby. The Company further acknowledges that the Purchaser is not acting as a financial advisor or fiduciary of the Company (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated thereby and any advice given by the Purchaser or any of its representatives or agents in connection with the Transaction Documents and the transactions contemplated thereby is merely incidental to the Purchaser’s purchase of the Units.
(h) No General Solicitation. Neither the Company, nor to its knowledge any of its Affiliates (as defined below), or any person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation D) in connection with the offer, sale or issuance of the Securities. “Affiliate” means, with respect to any person, any other person that, directly or indirectly through one or more intermediaries, controls, is controlled by or is under common control with such person, as such terms are used in and construed under Rule 144 under the Securities Act (“Rule 144”). With respect to the Purchaser, any investment fund or managed account that is managed on a discretionary basis by the same investment manager as the Purchaser will be deemed to be an Affiliate of the Purchaser.
(i) No Integrated Offering. Neither the Company, nor any of its Affiliates, nor to the knowledge of the Company, any person acting on its or their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would eliminate the availability of the exemption from registration under Rule 506(b) of Regulation D or afforded by Section 4(a)(2) of the Securities Act in connection with the Offering of the Units contemplated hereby or cause this Offering of the Units to be integrated with prior offerings by the Company for purposes of the Securities Act.
(j) Employee Relations. Since the Lookback Date, there has been no actual or threatened in writing, or to the knowledge of the Company, threatened orally, labor dispute, work stoppage, request for representation, union organizing activity, or unfair labor practice charges involving the employees of the Company or any of its Subsidiaries. Neither the Company nor any Subsidiary is party to any collective bargaining agreement. The Company’s and/or its Subsidiaries’ employees are not members of any union, and the Company believes that its and its Subsidiaries’ relationships with their respective employees are good.
9
(k) Intellectual Property Rights. Except as set forth on Schedule 3(k), the Company and each of its Subsidiaries exclusively owns, possesses, or has valid and enforceable rights to use, license, and exploit all Intellectual Property used in, necessary or advisable for the conduct of the Company’s and its Subsidiaries’ business as currently conducted and as described in the PPM, except for a failure to own, possess or have such rights that would not reasonably be expected to result in a Material Adverse Effect. There are no unreleased liens or security interests which have been filed, or which the Company has received notice of, against any of the Intellectual Property owned by the Company. All Intellectual Property owned by the Company or its Subsidiaries, and all Contracts pursuant to which the Company or its Subsidiaries license Intellectual Property, are valid and enforceable, and the Company and its Subsidiaries are in full compliance with all such Contracts except as would not reasonably be expected to result in a Material Adverse Effect. Furthermore, except as has not been and would not reasonably be expected to result in a Material Adverse Effect, since the Lookback Date: (A) to the Company’s knowledge, there has been no infringement, misappropriation or violation by third parties of any such Intellectual Property of the Company or its Subsidiaries; (B) there has been no Action pending or threatened in writing (or to the Company’s knowledge, threatened orally) by others challenging the Company’s or any of its Subsidiaries’ ownership of or any rights in or to any such Intellectual Property; (C) the Intellectual Property owned by the Company and its Subsidiaries and, to the Company’s knowledge, the Intellectual Property licensed to the Company and its Subsidiaries, has not been adjudged invalid or unenforceable, in whole or in part, and there has been no Action pending or threatened in writing (or to the Company’s knowledge, threatened orally) by others challenging the validity, enforceability or scope of any such Intellectual Property; (D) there has been no Action pending or threatened in writing (or to the Company’s knowledge, threatened orally) by others that the Company or any of its Subsidiaries infringes, misappropriates or otherwise violates any Intellectual Property or other proprietary rights of others, and neither the Company nor any of its Subsidiaries has received any written notice of such Action; and (E) to the Company’s knowledge, no employee of the Company or any of its Subsidiaries has violated any term of any employment Contract, patent disclosure agreement, invention assignment agreement, non-competition agreement, non-solicitation agreement, nondisclosure agreement or restrictive covenant to or with a former employer where the basis of such violation relates to such employee’s employment with the Company or any of its Subsidiaries or actions undertaken by the employee while employed with the Company or any of its Subsidiaries. Except as would not reasonably be expected to have a Material Adverse Effect, the Company and its Subsidiaries have complied in all material respects with 37 C.F.R. §1.56 (Duty to disclose information material to patentability). The consummation of the transactions contemplated hereby or by the other Transaction Documents will not result in the loss or impairment of or payment of any additional amounts with respect to, nor require the consent of any other person in respect of, the Company or any of its Subsidiaries’ right to own, use or hold for use any Intellectual Property as owned, used or held for use in the conduct of the Company’s and its Subsidiaries’ business as currently conducted and as described in the PPM, except as would not reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole. The rights of the Company and each of its Subsidiaries in their Intellectual Property are valid, subsisting and enforceable, except as would not reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole. The Company and each of its Subsidiaries has taken reasonable steps to maintain their Intellectual Property and to protect and preserve the confidentiality of all of their Trade Secrets. To the Company’s knowledge, there has not been any disclosure or access to any Trade Secrets of the Company and each of its Subsidiaries by any unauthorized person. The Company and each of its Subsidiaries have taken and continue to take commercially reasonable measures, at least consistent with prevailing industry practice, to ensure that all personal information in their possession, custody or control is protected against loss and against unauthorized, access, use, modification, disclosure or other misuse. “Intellectual Property” shall mean any and all rights title and interest in, arising out of, or associated with any intellectual or intangible property, whether protected, created or arising in any jurisdiction throughout the world, including the following: (a) issued patents and patent applications (whether provisional or non-provisional), including divisionals, continuations, continuations-in-part, substitutions, reissues, reexaminations, extensions, or restorations of any of the foregoing, and other Governmental Authority issued indicia of invention ownership (including certificates of invention, petty patents, and patent utility models) (“Patents”); (b) trademarks, service marks, brands, certification marks, logos, trade dress, slogans, trade names, and other similar indicia of source or origin, together with the goodwill connected with the use of and symbolized by, and all registrations, applications for registration, and renewals of, any of the foregoing (“Trademarks”); (c) copyrights and works of authorship, whether or not copyrightable, and all registrations, applications for registration, and renewals of any of the foregoing (“Copyrights”); (d) internet domain names and social media account or user names (including “handles”), whether or not Trademarks, all associated web addresses, URLs, websites and web pages, social media sites and pages, and all content and data thereon or relating thereto, whether or not Copyrights; (e) mask works, and all registrations, applications for registration, and renewals thereof; (f) industrial designs, and all Patents, registrations, applications for registration, and renewals thereof; (g) trade secrets, know-how, inventions (whether or not patentable), discoveries, improvements, technology, business and technical information, databases, data compilations and collections, tools, methods, processes, techniques, and other confidential and proprietary information and all rights therein (“Trade Secrets”); (h) computer programs, operating systems, applications, firmware and other code, including all source code, object code, application programming interfaces, data files, databases, protocols, specifications, and other documentation thereof; (i) rights of publicity; and (j) all other intellectual or industrial property and proprietary rights.
10
(l) Environmental Laws.
(i) Except as, individually or in the aggregate, have not had and would not reasonably be expected to have a Material Adverse Effect: (x) the Company and each Subsidiary is in compliance and has complied with all applicable Environmental Laws (as defined below); (y) the Company or its applicable Subsidiary is in possession of all Authorizations required pursuant to Environmental Laws to conduct their respective businesses as currently conducted and as described in the PPM and (z) the Company or its applicable Subsidiary is in material compliance with all terms and conditions of such Authorizations. There is no Action pending or threatened in writing (or to the Company’s knowledge, threatened orally) relating to any violation or noncompliance with any Environmental Law involving the Company or any Subsidiary. For purposes of this Agreement, “Environmental Law” means any national, state, provincial or local Law, statute, rule or regulation or the common law relating to the environment or occupational health and safety, including without limitation any statute, regulation, administrative decision or order pertaining to (A) treatment, storage, disposal, generation and transportation of Hazardous Substances; (B) air, water and noise pollution; (C) groundwater and soil contamination; (D) the release or threatened release into the environment of industrial, toxic or hazardous materials or substances, or solid or hazardous waste, including without limitation emissions, discharges, injections, spills, escapes or dumping of pollutants, contaminants or chemicals; (E) the protection of wild life, marine life and wetlands, including without limitation all endangered and threatened species; (F) storage tanks, vessels, containers, abandoned or discarded barrels, and other closed receptacles; (G) health and safety of employees and other persons; and (H) manufacturing, processing, using, distributing, treating, storing, disposing, transporting or handling of Hazardous Substances. As used above, the terms “release” and “environment” shall have the meaning set forth in the Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended.
(ii) To the knowledge of the Company, none of the Company or any of its Subsidiaries has any liability or obligation under any Environmental Law with respect to any release, spill, emission, leaking, pumping, pouring, emptying, leaching, escaping, dumping, injection, deposit, discharge or disposing of any Hazardous Substance in, onto or through the environment, except as would not reasonably be expected to have a Material Adverse Effect. “Hazardous Substances” means all materials, wastes, or substances defined by, or regulated under, any Environmental Laws, including as a hazardous waste, hazardous material, hazardous substance, extremely hazardous waste, restricted hazardous waste, contaminant, pollutant, toxic waste, or toxic substance, and specifically including petroleum and petroleum products, asbestos, radon, lead, toxic mold, radioactive materials, and polychlorinated biphenyls.
(m) Authorizations. Except as set forth on Schedule 3(m), the Company and each of its Subsidiaries holds, and is operating in compliance with, all authorizations, licenses, permits, approvals, clearances, registrations, exemptions, consents, certificates, waivers, filings, qualifications and orders of the U.S. Food and Drug Administration (“FDA”), its foreign counterparts and any other entity or body exercising executive, legislative, judicial, regulatory or administrative functions of or pertaining to United States federal, state or local government or foreign, or other governmental, including any department, commission, board, agency, bureau, official or other regulatory, administrative or judicial or arbitral authority thereto (each a “Governmental Authority”) and supplements and amendments thereto (collectively, “Authorizations”) required for the conduct of its business as currently conducted and as described in the PPM, or that are otherwise material to the business of the Company and its Subsidiaries, in all applicable jurisdictions, except as would not reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole. All Authorizations held by the Company or its Subsidiaries are valid and in full force and effect. Neither the Company nor any of its Subsidiaries is in material violation of any terms of any such Authorizations; and neither the Company nor any of its Subsidiaries has received written notice from any Governmental Authority of any revocation or modification of any such Authorization, or written notice (or to the Company’s knowledge, oral notice) that such revocation or modification is being considered, except to the extent that any such revocation or modification would not be reasonably expected to be material to the business of the Company and its Subsidiaries, taken as a whole.
11
(n) Regulatory Compliance The Company and each of its Subsidiaries is in compliance, and has since the Lookback Date been in compliance, with all applicable federal, state, local and foreign Laws, including such Laws applicable to the manufacture, distribution, import and export of regulated products and component parts and ingredients, except as would not reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole. Neither the Company nor any of its Subsidiaries has received any Form FDA 483, warning letter, untitled letter or other correspondence or written notice from any Governmental Authority, alleging or asserting noncompliance with the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq.) (“FDCA”) or comparable foreign Laws. Neither the Company nor any of its Subsidiaries has been notified, either orally or in writing, by any Governmental Authority that a clinical study has been put on hold or may be put on hold. The Company and each of its Subsidiaries, and to the Company’s knowledge, each of their respective directors, officers, employees and agents, is and has been in material compliance with applicable health care Laws, including, to the extent applicable, without limitation, the FDCA, the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)), the Health Insurance Portability and Accountability Act of 1996 (42 U.S.C. § 1320d et seq.), as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (42 U.S.C. § 17921 et seq.), and the regulations promulgated pursuant to such Laws, and comparable state Laws and foreign Laws (collectively, “Health Care Laws”). Neither the Company nor any of its Subsidiaries has received written notice (or to the Company’s knowledge, oral notice) of any ongoing claim, action, suit, proceeding, hearing, enforcement, investigation, arbitration or other action from any Governmental Authority or third party alleging that any product operation or activity is in material violation of any Health Care Laws or any Authorizations. The Company and each of its Subsidiaries has filed, obtained, maintained or submitted all material reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments thereto as required by any Health Care Laws or any Authorizations and all such reports, documents, forms, notices, applications, records, claims, submissions and supplements or amendments, to the Company’s knowledge, were complete, correct and not misleading on the date filed in all material respects (or were corrected or supplemented by a subsequent submission). Neither the Company nor any of its Subsidiaries has, either voluntarily or involuntarily, initiated, conducted, or issued or caused to be initiated, conducted or issued, any other notice or action relating to any alleged product defect or violation and, to the Company’s knowledge, no third party has initiated or conducted any such notice or action relating to any of the Company’s products in development. Neither the Company nor any of its Subsidiaries is a party to any corporate integrity agreement, deferred prosecution agreement, monitoring agreement, consent decree, settlement order, or similar agreements, or has any reporting obligations pursuant to any such agreement, plan or correction or other remedial measure entered into with any Governmental Authority.
(o) Title. Neither the Company nor any of its Subsidiaries owns any real property. Except as set forth on Schedule 3(o), each of the Company and its Subsidiaries has good and marketable title to all of its personal property and other tangible assets (i) purportedly owned or used by them as reflected in the PPM, or (ii) necessary for the conduct of their business as currently conducted and as described in the PPM, free and clear of any legal or equitable, specific or floating, lien (statutory or otherwise), restriction, mortgage, deed of trust, pledge, lien, security interest, restrictive covenant, or other adverse right, charge, claim or encumbrance of any kind or nature whatsoever (collectively, “Liens”), except for Liens which would not reasonably be expected to have a Material Adverse Effect. Except as set forth on Schedule 3(o), with respect to properties and assets it leases, each of the Company and its Subsidiaries is in compliance with such leases and holds a valid leasehold interest free of any Liens, except for such Liens which would not reasonably be expected to have a Material Adverse Effect.
12
(p) Tax Status. The Company and each Subsidiary has filed (taking into account any valid extensions) all federal, state, local and foreign income and all other material returns, declarations, reports, elections, designations, or information returns or statements made to a Governmental Authority relating to Taxes, including any schedules or attachments thereto and any amendments thereof (collectively, “Tax Returns”) required to be made or filed by it or with respect to it by any jurisdiction to which it is subject. Such Tax Returns accurately reflect, in all material respects, the Tax liabilities of the Company and its Subsidiaries (other than Taxes not yet due and payable). The Company and each Subsidiary has timely paid all income Taxes and all other material Taxes and other material governmental assessments and material charges, shown or determined to be due on such returns, reports and declarations, except those being contested in good faith and for which the Company and its Subsidiaries have adequately reserved and accrued for in accordance with GAAP. The Company has reserved and accrued on its books provisions in accordance with GAAP amounts that are reasonably adequate for the payment of all material taxes for periods subsequent to the periods to which such returns, reports or declarations apply. There are no unpaid Taxes in any material amount claimed to be due from the Company or any Subsidiary by the taxing authority of any jurisdiction. There are no, and since the Lookback Date there have been no, pending or threatened in writing (or to the Company’s knowledge, threatened orally) Actions by the taxing authority of any jurisdiction against the Company or any of its Subsidiaries. Neither the Company nor any of its Subsidiaries is a party to, or otherwise bound by, any Tax indemnity, Tax sharing or Tax allocation agreement (but not including any agreement whose primary subject matter is not Taxes) (a “Tax Agreement”). The Company is not a “United States real property holding corporation” within the meaning of Section 897(c) of the Code. For purposes of this Agreement, “Tax” or “Taxes” means (i) any and all U.S. federal, state, local, or non-U.S. taxes, assessment, levy or other charges, including net or gross income, gross receipts, net proceeds, estimated, sales, use, ad valorem, value added, franchise, license, withholding, payroll, employment, excise, property (including both real and personal), unclaimed property remittance/escheat, deed, stamp, alternative or add-on minimum, occupation, severance, unemployment, social security, workers’ compensation, capital, premium, windfall profit, environmental, custom duties, fees, transfer and registration taxes, and any governmental charges in the nature of a tax imposed by a Governmental Authority, (ii) any liability for the payment of any amounts of any of the foregoing types as a result of being a member of an Affiliated, consolidated, combined or unitary group, or being a party to any agreement or arrangement whereby liability for payment of such amounts was determined or taken into account with reference to the liability of any other person and (iii) any liability for the payment of any amounts as a result of being a party to any Tax Agreement.
(q) Certain Transactions. Except as set forth on Schedule 3(p), none of the direct or indirect equity holders, stockholders, controlling persons, partners, managers, members, officers, directors, employees, general or limited partners or assignees (each, a “Related Party”) of the Company or any Subsidiary is presently, or has since the Lookback Date been, a party to any Contract or transaction with the Company or any Subsidiary (other than for services as employees, officers and directors (including director or officer indemnification agreements) and for the purchase of shares of the Company’s capital stock and the issuance of options to purchase shares of the Company’s Common Stock), including any Contract providing for the furnishing of services to or by, providing for rental of real or personal property to or from, or otherwise requiring payments to or from any officer, director or such employee or, to the knowledge of the Company, any corporation, partnership, trust or other entity in which any officer, director, or any such employee has a substantial interest or is an officer, director, trustee or partner. All transactions that would be required to be disclosed by the Company pursuant to Item 404 of Regulation S-K promulgated under the Securities Act are disclosed in the SEC Reports (as defined below) or the PPM in accordance with Item 404 of Regulation S-K.
13
(r) Rights of First Refusal. Except as set forth on Schedule 3(r), the Company is not obligated to offer the securities offered hereunder on a right of first refusal basis or otherwise to any third parties including, but not limited to, current or former stockholders of the Company, underwriters, brokers, agents or other third parties.
(s) Insurance. The Company and its Subsidiaries have insurance policies of the type and in amounts customarily carried by organizations conducting businesses or owning assets similar to those of the Company and its Subsidiaries, and in any event maintain insurance policies in amounts as required by applicable Law or any Contract to which the Company or its Subsidiaries is a party or to which any of its assets or businesses is subject. All such insurance policies are in full force and effect and binding and enforceable in accordance with their terms, and all premiums due and payable thereon have been timely paid in full. Neither the Company nor any of its Subsidiaries is in default with respect to its obligations under any such insurance policy, nor has there been any failure to give any notice or present any claim under any such insurance policy in due and timely fashion except as would not, individually or in the aggregate, reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole. There is no material claim pending under any such policy as to which coverage has been questioned, denied or disputed by the underwriter of such policy and there has been no notice of cancellation of nonrenewal of any such insurance policy received by the Company or any of its Subsidiaries. Since the Lookback Date, no limits on any insurance policy of the Company or any of its Subsidiaries have been exhausted, materially eroded or materially reduced.
(t) SEC Reports. The Company has timely filed or furnished, as applicable, all reports, proxy statements, schedules, forms, statements, certifications and other documents (including exhibits and all other information incorporated by reference therein) required to be filed or furnished by the Company under the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder (the “Exchange Act”) (the “SEC Reports”) since the Lookback Date (or such shorter period since the Company was first required by Law or regulation to file such material). The Super 8-K (as defined below) when filed will comply, and the SEC Reports at the time they were filed complied, in all material respects with the Securities Act or the Exchange Act, as applicable. There are no Contracts (or any material change or amendment thereto, or any waiver of any material right thereunder) that are required to be described in the SEC Reports or the PPM that were or are not described, in all material respects, therein. There are no Contracts (or any material change or amendment thereto, or any waiver of any material right thereunder) that are required to be filed as exhibits to the SEC Reports or the Super 8-K that were not or will not have been filed as required in the SEC Reports or the Super 8-K. There are no outstanding or unresolved comments in comment letters received from the SEC staff with respect to the SEC Reports. To the Company’s knowledge, none of the SEC Reports is the subject of an ongoing SEC review. There are no SEC inquiries or investigations, other governmental inquiries or investigations or internal investigations pending or threatened in writing (or, to the Company’s knowledge, threatened orally), in each case regarding any accounting practice of the Company.
14
(u) Financial Statements.
(i) The (A) audited financial statements of Adaptin as of and for the fiscal years ended December 31, 2023 and 2022, and (B) the unaudited interim financial statements of Adaptin for the nine months ended September 30, 2024 (in each case consisting of the balance sheets, related statements of operations, changes in stockholders’ equity (deficit) and cash flows), each of (A) and (B) above included in the PPM, and (C) the unaudited pro forma consolidated financial statements of the Company (after taking into effect the Merger) and including, in each of (A), (B) and (C) above, the notes thereto, and each of (A), (B) and (C) above to be included in the Super 8-K, or with respect to (C) above in an amendment thereto as permitted by Law, comply or will comply, as applicable, in all material respects with GAAP and the rules and regulations of the SEC with respect thereto (the foregoing financial statements, the “Financial Statements”). The Financial Statements have been prepared in accordance with GAAP applied on a consistent basis during the periods involved and include all adjustments (consisting only of normal recurring accruals) that are necessary for a fair presentation of the consolidated financial condition of the business to which they relate as of the date thereof, subject, in the case of the unaudited interim financial statements of Adaptin for the nine months ended September 30, 2024, to normal year-end adjustments that will not, individually or in the aggregate, be material and the absence of notes, and fairly present in all material respects the financial position of Adaptin and its Subsidiaries taken as a whole, or the Company and its consolidated Subsidiaries taken as a whole, as applicable, as of and for the dates thereof and the results of operations and cash flows for the periods then ended, subject, in the case of unaudited statements, to normal, year-end audit adjustments that will not, individually or in the aggregate, be material. The pro forma financial information and the related notes, if any, to be included in the Super 8-K, have been properly compiled and prepared in accordance with the applicable requirements of the Securities Act and fairly present in all material respects the information shown therein, and the assumptions used in the preparation thereof are reasonable and the adjustments used therein are appropriate to give effect to the transactions and circumstances referred to therein.
(ii) Except as disclosed in the PPM, the Company (A) maintains a standard system of accounting established and administered in accordance with GAAP and (B) has established and maintains a system of internal controls over financial reporting designed to provide reasonable assurance regarding the reliability of the financial reporting and the preparation of the Financial Statements for external purposes in accordance with GAAP. There (x) are no significant deficiencies or material weaknesses in any system of internal accounting controls used by each of the Company’s Subsidiaries, , (y) has not since the Lookback Date been any fraud or other unlawful act on the part of any of management or other employees of the Company and each of its Subsidiaries who have a role in the preparation of Financial Statements or the internal accounting controls used by the Company and each of its Subsidiaries related to such preparation or controls and (z) has not since the Lookback Date been any claim or allegation regarding any of the foregoing.
(iii) Neither the Company nor any of its Subsidiaries has any liabilities (whether accrued, absolute, contingent or otherwise) other than (A) liabilities disclosed on the audited balance sheet (including the notes thereto) or the interim balance sheet (including the notes thereto) and (B) liabilities that have been incurred since the date of the latest balance sheet of the Company and the latest balance sheet of Adaptin included in the Financial Statements in the ordinary course of business, which liabilities, individually or in the aggregate, are not material to the business of the Company and its Subsidiaries (taken as a whole).
(iv) To the knowledge of the Company, Withum Smith+Brown, PC (the “Auditor”), whose report will be filed with the SEC in the Super 8-K, is an independent registered public accounting firm with respect to the Company as required by the Exchange Act and the rules and regulations promulgated thereunder and the rules and regulations of the Public Company Accounting Oversight Board. The Auditor has not, during the periods covered by the Financial Statements provided to the Company any non-audit services, as such term is used in Section 10A(g) of the Exchange Act.
15
(v) Material Changes. Except for the transactions contemplated hereby or in the Merger Agreement, since the date of the latest balance sheet of the Company and the latest balance sheet of Adaptin included in the financial statements contained in the PPM, except as set forth on Schedule 3(v), (i) there have been no events, occurrences or developments that have had or would reasonably be expected to have a Material Adverse Effect with respect to the Company or Adaptin, (ii) there have not been any changes in the assets, financial condition, business or operations of the Company or Adaptin from that reflected in the financial statements contained in the PPM except changes in the ordinary course of business which have not been, either individually or in the aggregate, materially adverse to the business, properties, financial condition, results of operations or future prospects of the Company or Adaptin, (iii) none of the Company or Adaptin or any of their respective Subsidiaries has altered its method of accounting or the manner in which it keeps its accounting books and records, and (iv) none of the Company or Adaptin or any of their respective Subsidiaries has declared or made any dividend or distribution of cash or other property to its stockholders or equity holders or purchased, redeemed or made any agreements to purchase or redeem any shares of its capital stock (other than in connection with repurchases of unvested stock issued to employees of the Company). The Company and its Subsidiaries, individually and on a consolidated basis, are not as of the date hereof, and after giving effect to the transactions contemplated hereby to occur at the Initial Closing, will not be Insolvent (as defined below). “Insolvent” means, with respect to the Company, on a consolidated basis with its Subsidiaries, (i) the present fair saleable value of the Company’s and its Subsidiaries’ assets is less than the amount required to pay the Company’s and its Subsidiaries’ total indebtedness, (ii) the Company and its Subsidiaries are unable to pay their debts and liabilities, subordinated, contingent or otherwise, as such debts and liabilities become absolute and matured or (iii) the Company and its Subsidiaries intend to incur or believe that they will incur debts that would be beyond their ability to pay as such debts mature.
(w) Disclosure Controls. The Company has established and maintains disclosure controls and procedures (as defined in Rules 13a-14 and 15d-15 under the Exchange Act) and except as disclosed in the PPM, such controls and procedures are effective in ensuring that material information relating to the Company, including its Subsidiaries, is made known to the principal executive officer and the principal financial officer.
(x) Sarbanes-Oxley. The Company is, and has been since the Lookback Date, to the extent applicable, in compliance in all material respects with all of the provisions of the Sarbanes-Oxley Act of 2002 which are applicable to it.
(y) Off-Balance Sheet Arrangements. There is no transaction, arrangement, or other relationship between the Company or any Subsidiary and an unconsolidated or other off-balance sheet entity that is required to be disclosed by the Company in the PPM and is not so disclosed.
(z) Foreign Corrupt Practices. Neither the Company and its Subsidiaries, nor any of their respective directors, managers, officers, agents or employees or other person acting on behalf of the Company or its Subsidiaries, has: (i) directly or indirectly, used any funds for unlawful contributions, gifts, entertainment or other unlawful expenses related to foreign or domestic political activity, (ii) made any unlawful payment or offered anything of value to foreign or domestic government officials or employees or to any foreign or domestic political parties or campaigns from corporate funds, (iii) failed to disclose fully any contribution made by the Company or any of its Subsidiaries (or, to the Company’s knowledge, made by any person acting on their behalf) which is in violation of Law or (iv) violated any applicable anti-terrorism Law or regulation, nor have any of them otherwise taken any action which would reasonably cause the Company or any of its Subsidiaries to be in violation of the Foreign Corrupt Practices Act of 1977, as amended, or any applicable Law of similar effect.
(aa) Office of Foreign Assets Control. Neither the Company nor any Subsidiary nor, to the Company’s knowledge, any director, manager, officer, agent, employee or Affiliate of the Company or any Subsidiary is, or is acting under the direction of, on behalf of or for the benefit of a person that is, or is owned or controlled by a person that is, currently subject to any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Treasury Department.
(bb) Money Laundering. The operations of the Company and its Subsidiaries are and have been conducted at all times in compliance with applicable financial record-keeping and reporting requirements of the Currency and Foreign Transactions Reporting Act of 1970, as amended, and other applicable money laundering Laws and applicable rules and regulations thereunder (collectively, the “Money Laundering Laws”), and no Action by or before any Governmental Authority involving the Company or any Subsidiary with respect to the Money Laundering Laws is pending or threatened in writing (or to the Company’s knowledge, threatened orally).
16
(cc) Regulation M Compliance. The Company has not, and to its knowledge no one acting on its behalf has, (i) taken, directly or indirectly, any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company to facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or paid any compensation for soliciting purchases of, any of the Securities, or (iii) paid or agreed to pay to any person any compensation for soliciting another to purchase any other securities of the Company, other than, in the case of clauses (ii) and (iii), compensation paid to the Placement Agent in connection with the placement of the Units.
(dd) Privacy and Data Security.
(i) “Business Privacy and Data Security Policies” means all of the Company’s or one of its Subsidiaries’ present, internal or public-facing policies, notices, and statements concerning the privacy, security, or Processing of Personal Information in the conduct of the Business. “Personal Information” means any information that identifies or, alone or in combination with any other information, could reasonably be used to identify, locate, or contact a natural person, including name, street address, telephone number, email address, identification number issued by a Governmental Authority, credit card number, bank information, customer or account number, online identifier, device identifier, IP address, browsing history, search history, or other website, application, or online activity or usage data, location data, biometric data, medical or health information, or any other information that is considered “personally identifiable information,” “personal information,” or “personal data” under applicable Law, and all data associated with any of the foregoing that are or could reasonably be used to develop a profile or record of the activities of a natural person across multiple websites or online services, to predict or infer the preferences, interests, or other characteristics of a natural person, or to target advertisements or other content to a natural person. “Privacy Laws” means all applicable Laws, orders, writs, judgments, injunctions, decrees, stipulations, determinations or awards entered by or with any Governmental Authority, and binding guidance issued by any Governmental Authority concerning the privacy, security, or Processing of Personal Information (including Laws of jurisdictions where Personal Information was collected), including, as applicable, data breach notification Laws, consumer protection Laws, Laws concerning requirements for website and mobile application privacy policies and practices, Social Security number protection Laws, data security Laws, and Laws concerning email, text message, or telephone communications. Without limiting the foregoing, Privacy Laws include the Health Insurance Portability and Accountability Act of 1996, as amended and supplemented by the Health Information Technology for Economic and Clinical Health Act of the American Recovery and Reinvestment Act of 2009 and all other similar international, federal, state, provincial, and local Laws. “Processing” means any operation performed on Personal Information, including the collection, creation, receipt, access, use, handling, compilation, analysis, monitoring, maintenance, storage, transmission, transfer, protection, disclosure, destruction, or disposal of Personal Information.
(ii) The Company and each of its Subsidiaries, and, to the Company’s knowledge, all vendors, processors, or other third parties acting for or on behalf of the Company or any of its Subsidiaries in connection with the Processing of Personal Information or that otherwise have been authorized to have access to Personal Information in the possession or control of the Company or any of its Subsidiaries, comply and at all times since the Lookback Date have complied, with all of the following in the conduct of its business as currently conducted and as disclosed in the PPM: (A) Privacy Laws; (B) rules of self-regulatory organizations; (C) industry standards, guidelines, and best practices; (D) the Business Privacy and Data Security Policies; and (E) all obligations or restrictions concerning the privacy, security, or Processing of Personal Information under any Contract to which the Company or any of its Subsidiaries is a party or otherwise bound as of the date hereof, in each case, except for violations that, individually or in the aggregate, have not been and would not reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole.
17
(iii) Neither the consummation of the Merger nor the execution, delivery and performance of this Agreement and the consummation of the transactions contemplated hereby, does or will: (A) conflict with or result in a violation or breach of any Privacy Laws or Business Privacy and Data Security Policies (as currently existing or as existing at any time during which any Personal Information was collected or Processed by or for the Company or any of its Subsidiaries in the conduct of its business as now being conducted); or (B) require the consent of or notice to any person concerning such person’s Personal Information, in each case, except as has not been and would not reasonably be expected to have a Material Adverse Effect.
(iv) Since the Lookback Date, (A) to the Company’s knowledge, no Personal Information in the possession or control of the Company or any of its Subsidiaries, or held or Processed by any vendor, processor, or other third party for or on behalf of the Company or any of its Subsidiaries, in the conduct of its business has been subject to any data or security breach or unauthorized access, disclosure, use, loss, denial or loss of use, alteration, destruction, compromise, or Processing (a “Security Incident”), and (B) neither the Company nor any of its Subsidiaries has notified and, to the Company’s knowledge, there have been no facts or circumstances that would require the Company or any of its Subsidiaries to notify, any Governmental Authority or other person of any Security Incident in the conduct of its business, in each case, except as has not been and would not reasonably be expected to have a Material Adverse Effect.
(v) Since the Lookback Date, neither the Company nor any of its Subsidiaries has received any notice, request, claim, complaint, correspondence, or other communication in writing (or to the Company’s knowledge, orally) from any Governmental Authority or other person, and to the Company’s knowledge there has not been any audit, investigation, enforcement action (including any fines or other sanctions), or other Action relating to, any actual, alleged, or suspected Security Incident or violation of any Privacy Law involving Personal Information in the possession or control of the Company or any of its Subsidiaries, or held or Processed by any vendor, processor, or other third party for or on behalf of the Company or any of its Subsidiaries, in the conduct of its business, in each case, except as has not been and would not reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole.
(vi) In the conduct of its business, the Company and each of its Subsidiaries has at all times since the Lookback Date implemented and maintained, and required all vendors, processors, and other third parties that Process any Personal Information for or on behalf of the Company or any of its Subsidiaries to implement and maintain, all security measures, plans, procedures, controls, and programs, including written information security programs, to (A) identify and address internal and external risks to the privacy and security of Personal Information in their possession or control; (B) implement, monitor, and improve adequate and effective administrative, technical, and physical safeguards to protect such Personal Information and the operation, integrity, and security of its software, systems, applications, and websites involved in the Processing of Personal Information; and (C) provide notification in compliance with applicable Privacy Laws in the case of any Security Incident, in each case, except as has not been and would not reasonably be expected to be material to the business of the Company and its Subsidiaries, taken as a whole.
(ee) Brokers’ Fees. Except as set forth on Schedule 3(ee), neither of the Company nor any of its Subsidiaries has any liability or obligation to pay any fees or commissions to any broker, finder or agent with respect to the transactions contemplated by this Agreement, except for the payment of fees and expenses to the Placement Agent and issuance of the Placement Agent Warrants as described in Section 2 above.
18
(ff) Disclosure Materials. The SEC Reports, at the time filed or furnished, and the Disclosure Materials, at the time delivered to the Purchaser, were true and correct in all material respects and did not contain an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading. For the purposes of this Agreement, “Disclosure Materials” means the PPM, the Confidential and Non-Binding Summary Term Sheet of the Company (the “Term Sheet”) previously provided to the Purchaser, and any roadshow presentation delivered to the Purchaser in connection with the contemplated purchase of the Units, each as amended from time to time, relating to the Offering and any supplement or amendment thereto, and any disclosure schedule or other information document, including the Disclosure Schedule, delivered to the Purchaser prior to its execution of this Agreement, and any such document delivered to the Purchaser after its execution of this Agreement and prior to the closing of the Purchaser’s subscription hereunder.
(gg) Investment Company. The Company is not required to be registered as, and is not an Affiliate of, and immediately following the Closing will not be required to register as, an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
(hh) Reliance. The Company acknowledges that the Purchaser is relying on the representations and warranties (as modified by the disclosures on the Disclosure Schedule or the PPM (excluding any disclosures (whether contained under the heading “Risk Factors,” in any “forward-looking statements” disclaimer or in any other section) to the extent they are cautionary, predictive or forward-looking in nature) made by the Company hereunder and that such representations and warranties (as modified by the Disclosure Schedule or the PPM (excluding any disclosures (whether contained under the heading “Risk Factors,” in any “forward-looking statements” disclaimer or in any other section) to the extent they are cautionary, predictive or forward-looking in nature) are a material inducement to the Purchaser purchasing the Units. The Company further acknowledges that without such representations and warranties of the Company made hereunder, the Purchaser would not enter into this Agreement with the Company.
(ii) Bad Actor Disqualification. No “bad actor” disqualifying event described in Rule 506(d)(1)(i)-(viii) of the Securities Act (a “Disqualification Event”) is applicable to the Company or, to the Company’s knowledge, any Company Covered Person, except for a Disqualification Event as to which Rule 506(d)(2)(ii–iv) or (d)(3), is applicable. “Company Covered Person” means, with respect to the Company as an “issuer” for purposes of Rule 506 promulgated under the Securities Act, any person listed in the first paragraph of Rule 506(d)(1). The Company represents that it has exercised reasonable care to determine the accuracy of the representation made by the Company in this paragraph.
(jj) Anti-Dilution. There are no securities or instruments issued by or to which the Company is a party as of the date hereof or as of the Closing containing anti-dilution or similar provisions that will be triggered by the issuance of shares of Common Stock in connection with the Offering or pursuant to any Other Subscription Agreement entered into in connection with the Offering that have not been or will not be validly waived on or prior to each Closing Date.
19
(kk) Other Purchasers. The Company has not entered into any side letter or similar agreement with any Other Purchaser in connection with such Other Purchaser’s direct or indirect investment in the Company other than the applicable Other Subscription Agreement. Each Other Purchaser will enter into the applicable Other Subscription Agreement and no other side letters or similar agreements with respect to its investment in the shares of Common Stock in connection with the Offering. Each Other Subscription Agreement is in the same form and contains the same terms and provisions as this Agreement.
(ll) Leased Real Property. There are no pending or, to the knowledge of the Company, any threatened condemnation proceedings, lawsuits or other Actions relating to any real property leased by the Company or any of its Subsidiaries or any of the buildings, structures and facilities located thereon (the “Leased Real Property”) or other matters affecting adversely the current use, occupancy or value thereof. The Company and its applicable Subsidiaries enjoy quiet possession under all leases for each parcel of Leased Real Property (each, a “Lease”) and no Leased Real Property under any such Lease is subject to any Lien, easement, right-of-way, building or use restriction, exception, variance, reservation or limitation, as might, in any material respect, interfere with or impair the present and continued use thereof by the Company or its Subsidiaries in the usual and normal conduct of the business of the Company and its Subsidiaries.
(mm) Material Contracts. Each Material Contract (as defined below) is the legal, valid and binding obligation of the Company or one of its Subsidiaries that is a party thereto, and is enforceable against the Company or one of its Subsidiaries, as applicable, and, to the knowledge of the Company, the counterparties, in accordance with its terms, other than, in all cases, Material Contracts that have expired, been terminated or superseded in accordance with their terms following the date hereof. Neither the Company or any of its Subsidiaries, nor to the knowledge of the Company, any counterparty, is in violation, breach or default under any such Contract or has improperly terminated, revoked or accelerated any Material Contract and no event or condition exists or has occurred which, with the giving of notice or the lapse of time or both, would, under any Material Contract, (A) constitute a breach or default by the Company or any of its Subsidiaries, or to the knowledge of the Company, a counterparty, (B) give to the counterparty any rights of termination, acceleration or cancellation of, (C) result in any obligation imposed on the Company or any of its Subsidiaries thereunder or a loss of a benefit in favor of the Company or any of its Subsidiaries thereunder, (D) allow the imposition of any fees or penalties on the Company or any of its Subsidiaries thereunder, require the offering or making of any payment or redemption by the Company or any of its Subsidiaries thereunder or (E) give rise to any increased, guaranteed, accelerated or additional rights or entitlements to the counterparty thereunder, in each case, except for (i) such breaches, defaults and events which have not had and would not reasonably be expected to have, individually or in the aggregate, a Material Adverse Effect, and (ii) any Material Contracts that will expire or terminate in accordance with their terms in connection with or as contemplated by or directly related to the Merger Agreement and the transactions contemplated thereby, including to the extent applicable, Contracts with the stockholders or investors of the Company or any of its Subsidiaries, indemnification agreements with each of their respective directors or officers, employment, consulting agreements or equity award agreements with each of their employees or other service providers. None of the Company or any of its Subsidiaries has received any written notice of the intention of any person to terminate, fail to renew or materially and adversely modify any Material Contract.
20
As used herein, “Material Contract” means any written or oral agreement, contract, commitment, arrangement, subcontract, license, sublicense, lease, sublease, sales order, purchase order, indenture, mortgage, note, bond, letter of credit, warrant, instrument, obligation, or understanding (collectively, including all amendments, supplements and modifications thereto, “Contracts”) to which the Company or any of its Subsidiaries is a party or by which any of their respective assets or businesses are bound:
(i) that is required to be filed pursuant to Item 601(b)(10) of Regulation S-K promulgated under the Securities Act);
(ii) that contains an exclusivity clause that restricts the Company or any of its Subsidiaries or a covenant not to compete in any line of business with any person in any geographical area that restricts the Company or any of its Subsidiaries or that otherwise restricts the Company or any of its Subsidiaries from freely providing products or services to any customer or potential customer, or that restricts the right of the Company or any of its Subsidiaries to sell to or purchase from any other person;
(iii) that relates to the acquisition or disposition of any business (whether by merger, sale of stock or assets or otherwise) at any time since the Lookback Date, other than those related to the Company’s efforts to seek the acquisition of an operating company prior to the acquisition of Adaptin;
(iv) that is with any Related Party of the Company or any of its Subsidiaries;
(v) that grants to the counterparty a right of first refusal, first offer or first negotiation outside of the ordinary course of business of the Company, except for any such preemptive or similar rights in favor of the equity holders of Adaptin that will be terminated or extinguished in connection with the Merger; or
(vi) that grants the other party or any third party “most favored nation” status or any similar rights.
(nn) Sanctions. Neither the Company nor any of its Subsidiaries, directors, officers, or employees, nor, to the knowledge of the Company, after due inquiry, any agent, affiliate or other person acting on behalf of the Company or any of its Subsidiaries is currently the subject or the target of any U.S. sanctions administered by the Office of Foreign Assets Control of the U.S. Department of the Treasury or the U.S. Department of State, the United Nations Security Council, the European Union, His Majesty’s Treasury of the United Kingdom, or other relevant sanctions authority (collectively, “Sanctions”); nor is the Company or any of its subsidiaries located, organized or resident in a country or territory that is the subject or the target of Sanctions, including, without limitation, the Crimea region and the Donetsk People’s Republic and Luhansk People’s Republic in Ukraine, Cuba, Iran, North Korea, and Syria (collectively, “Sanctioned Countries”); and the Company will not directly or indirectly use the proceeds of this offering, or lend, contribute or otherwise make available such proceeds to any subsidiary, or any joint venture partner or other person or entity, for the purpose of financing the activities of or business with any person, or in any country or territory, that at the time of such financing, is the subject or the target of Sanctions or in any other manner that will result in a violation by any person (including any person participating in the transaction whether as underwriter, advisor, investor or otherwise) of applicable Sanctions. For the past five years, the Company and its subsidiaries have not knowingly engaged in and are not now knowingly engaged in any dealings or transactions with any person that at the time of the dealing or transaction is or was the subject or the target of Sanctions or with any Sanctioned Country.
21
(oo) Employee Benefits.
(i) “Benefit Plan” means any plan, program, arrangement or agreement that is a pension, profit-sharing, savings, retirement, employment, consulting, severance pay, termination, executive compensation, incentive compensation, deferred compensation, bonus, stock purchase, stock option, phantom stock or other equity-based compensation, change-in-control, retention, salary continuation, vacation, sick leave, disability, death benefit, group insurance, hospitalization, medical, dental, life (including all individual life insurance policies as to which the Company is the owner, the beneficiary, or both), Code Section 125 “cafeteria” or “flexible” benefit, employee loan, educational assistance or fringe benefit plan, program, arrangement or agreement, whether written or oral, including, without limitation, any (A) “employee benefit plan” within the meaning of Section 3(3) of the Employee Retirement Income Security Act of 1974, as amended, and the rules and regulations promulgated thereunder (“ERISA”) or (B) other employee benefit plans, agreements, programs, policies, arrangements or payroll practices, whether or not subject to ERISA (including any funding mechanism therefor now in effect or required in the future as a result of the transaction contemplated by this Agreement or otherwise), which the Company or any of its Subsidiaries sponsors or maintains for the benefit of its current or former officer, director, employee, leased employee, consultant or agent (or their respective beneficiaries), or with respect to which the Company or any of its Subsidiaries has, or could reasonably be expected to have, any direct or indirect present or future liability.
(ii) Each Benefit Plan has been established, maintained and operated in all respects in accordance with its terms and in compliance with all applicable provisions of applicable Laws, including Section 409A of the Code and the regulations and other guidance issued thereunder, in each case, except as has not been and would not reasonably be expected to have, a Material Adverse Effect. There are no investigations by any Governmental Authority, termination proceedings or other claims (except routine claims for benefits payable under the Benefit Plans) or Actions pending in writing (or to the Company’s knowledge, orally) against any Benefit Plan or asserting any rights to or claims for benefits under any Benefit Plan that would reasonably be expected to give rise to any material liability. No non-exempt “prohibited transaction” (within the meaning of Section 406 of ERISA and Section 4975 of the Code) has occurred or is reasonably expected to occur with respect to any Benefit Plan. No Benefit Plan is (A) subject to Section 412 of the Code, Title IV of ERISA or Section 302 of ERISA (including a “multiemployer” plan within the meaning of Section 3(37) of ERISA), (B) a “multiple employer plan” as defined in Section 413(c) of the Code, or (C) a “multiple employer welfare arrangement” within the meaning of Section 3(40) of ERISA. No Benefit Plan is subject to the Laws of any jurisdiction other than the United States.
(iii) Neither the execution and delivery of this Agreement nor the consummation of the transactions contemplated hereby shall, in connection with any other event(s), (i) result in any payment or benefit becoming due to any current or former employee, contractor or director of the Company or its Subsidiaries or under any Benefit Plan, (ii) increase any amount of compensation or benefits otherwise payable to any current or former employee, contractor or director of the Company or its Subsidiaries or under any Benefit Plan, (iii) result in the acceleration of the time of payment, funding or vesting of any benefits to any current or former employee, contractor or director of the Company or its Subsidiaries or under any Benefit Plan, (iv) limit the right to merge, amend or terminate any Benefit Plan (except any limitations imposed by applicable Law, if any), or (v) give rise to any “excess parachute payment” as defined in Section 280G(b)(l) of the Code, any excise tax owing under Section 4999 of the Code or any other amount that would not be deductible under Section 280G of the Code.
22
4. Representations, Warranties and Agreements of the Purchaser. The Purchaser represents and warrants to, and agrees with, the Company, as of the date hereof and as of the applicable Closing Date, the following:
(a) The Purchaser has the knowledge and experience in financial and business matters necessary to evaluate the merits and risks of its prospective investment in the Company, and has carefully reviewed and understands the risks of, and other considerations relating to, the purchase of Units and the tax consequences of the investment. The Purchaser has adequate means of providing for its current and anticipated financial needs and contingencies and is able to bear the economic risks of the investment for an indefinite period of time and has no need for liquidity of the investment in the Units or the Securities included therein. The Purchaser can afford the loss of his, her or its entire investment.
(b) The Purchaser is acquiring the Units and the Securities included therein for investment for his, her or its own account and not with the view to, or for resale in connection with, any distribution thereof. The Purchaser understands and acknowledges that the Offering, sale and delivery of the Securities have not been registered under the Securities Act or any state securities Laws, by reason of a specific exemption from the registration provisions of the Securities Act and applicable state securities Laws, which depends upon, among other things, the bona fide nature of the investment intent as expressed herein. The Purchaser further represents that he, she or it does not have any contract, undertaking, agreement or arrangement with any person to sell, transfer or grant participation to any third person with respect to any of the Securities, other than with respect to an Affiliate of the Purchaser. The Purchaser understands and acknowledges that the Offering, sale and delivery of the Securities will not be registered under the Securities Act nor under the state securities laws on the ground that the sale of the Units to the Purchaser as provided for in this Agreement and the issuance of Shares and Warrants hereunder and of the Warrant Shares upon exercise of the Warrants are exempt from the registration requirements of the Securities Act and any applicable state securities laws. The Purchaser is an “accredited investor” as defined in Rule 501 of Regulation D as promulgated by the SEC under the Securities Act for the reason(s) specified on the Accredited Investor Certification attached hereto as completed by the Purchaser, and Purchaser shall submit to the Company such further assurances of such status as may be reasonably requested by the Company. The Purchaser resides in the jurisdiction set forth on the Purchaser’s Omnibus Signature Page affixed hereto. If the Purchaser is, with respect to the Company, (i) a predecessor of the Company; (ii) an Affiliated issuer; (iii) a director, executive officer, other officer participating in the offering, general partner or managing member of the Company; (iv) any beneficial owner of twenty percent (20%) or more of the Company’s outstanding voting equity securities, calculated on the basis of voting power; (v) any promoter connected with the Company in any capacity at the time of such sale; (vi) any investment manager of the Company if the Company is a pooled investment fund; (vii) any person that has been or will be paid (directly or indirectly) remuneration for solicitation of purchasers in connection with the Offering; (viii) any general partner or managing member of any such investment manager or solicitor; or (ix) any director, executive officer or other officer participating in the offering of any such investment manager or solicitor or general partner or managing member of such investment manager or solicitor (each such category, a “Covered Person”), the Purchaser has not taken any of the actions set forth in, and is not subject to, the disqualification provisions of Rule 506(d)(1) of the Securities Act.
23
(c) The Purchaser (i) if a natural person, represents that he or she is the greater of (A) 21 years of age or (B) the age of legal majority in his or her jurisdiction of residence, and has full power and authority to execute and deliver this Agreement and all other related agreements or certificates and to carry out the provisions hereof and thereof; (ii) if a corporation, partnership, limited liability company, association, joint stock company, trust, unincorporated organization or other entity, represents that such entity is duly organized, validly existing and in good standing under the Laws of the state or jurisdiction of its organization, the consummation of the transactions contemplated hereby is authorized by, and will not result in a violation of applicable Law or its charter or other organizational documents, such entity has full power and authority to execute and deliver this Agreement and all other related agreements or certificates and to carry out the provisions hereof and thereof and to purchase and hold the Securities, the execution and delivery of this Agreement has been duly authorized by all necessary action, this Agreement has been duly executed and delivered on behalf of such entity and is a legal, valid and binding obligation of such entity; or (iii) if executing this Agreement in a representative or fiduciary capacity, represents that he, she or it has full power and authority to execute and deliver this Agreement in such capacity and on behalf of the subscribing individual, ward, partnership, trust, estate, corporation, or limited liability company or partnership, or other entity for whom the Purchaser is executing this Agreement, and such individual, partnership, ward, trust, estate, corporation, or limited liability company or partnership, or other entity has full right and power to perform pursuant to this Agreement and make an investment in the Company, and represents that this Agreement constitutes a legal, valid and binding obligation of such entity. The execution and delivery of this Agreement will not violate or be in conflict with any order, judgment, injunction, agreement or controlling document to which the Purchaser is a party or by which it is bound, except for any violation or conflict that, individually or in the aggregate, has not had and would not reasonably be expected to have a material adverse effect on the ability of the Purchaser to perform its obligations under this Agreement and the other Transaction Documents or to consummate any transactions contemplated hereby or thereby.
(d) The Purchaser understands that the Units and the Securities included therein are being offered and sold to him, her or it in reliance on specific exemptions from the registration requirements of United States federal and state securities Laws and that the Company is relying in part upon the truth and accuracy of, and the Purchaser’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of the Purchaser set forth herein in order to determine the availability of such exemptions and the eligibility of the Purchaser to acquire such securities. The Purchaser further acknowledges and understands that the Company is relying on the representations and warranties made by the Purchaser hereunder and that such representations and warranties are a material inducement to the Company to sell the Units to the Purchaser and to issue the Warrant Shares upon exercise of the Warrants. The Purchaser further acknowledges that without such representations and warranties of the Purchaser made hereunder, the Company would not enter into this Agreement with the Purchaser.
(e) The Purchaser understands that, other than as expressly provided in the Registration Rights Agreement, the Company does not currently intend to register any of the Securities under the Securities Act at any time in the future; and the undersigned will not immediately be entitled to the benefits of Rule 144 with respect to any of the Securities. The Purchaser understands that no public market exists for the Company’s Common Stock and that there can be no assurance that any public market for the Common Stock will exist or continue to exist. The Company’s Common Stock is not approved for quotation on OTC Markets or any other quotation system or listed on any exchange.
24
(f) The Purchaser has received, reviewed and understood the information about the Company, including all Disclosure Materials provided to it by the Company and/or the Placement Agent (at the Company’s direction), and has had an opportunity to discuss the Company’s business, management and financial affairs with the Company’s management. The Purchaser understands that such discussions, as well as any Disclosure Materials provided by the Company and/or the Placement Agent (at the Company’s direction), were intended to describe the aspects of the Company’s business and prospects and the Offering which the Company believes to be material, but were not necessarily a thorough or exhaustive description and except as expressly set forth in this Agreement (as modified by the disclosures on the Disclosure Schedule or the PPM (excluding any disclosures contained under the heading “Risk Factors,” any disclosures of risks included in any “forward looking statements” or disclosures that are cautionary, predictive or forward-looking in nature)), the Company makes no representation or warranty with respect to the completeness of such information and makes no representation or warranty of any kind with respect to any information provided by any entity other than the Company. Some of such information may include projections as to the future performance of the Company, which projections may not be realized, may be based on assumptions which may not be correct and may be subject to numerous factors beyond the Company’s control. The Purchaser acknowledges that he, she or it is not relying upon any person or entity, other than the Company and its officers and directors, in making its investment or decision to invest in the Company. In entering into this Agreement, the Purchaser has not relied on any oral or, except as otherwise expressly set forth in the Transaction Documents or the Disclosure Materials, written information provided by the Company, Adaptin any Placement Agent, or by their respective Affiliates, agents, employees, representatives or trustees, or by any other agent or broker. No agent, employee or representative of the Company, Adaptin or any Placement Agent or other agent or broker has been authorized to make, and the Purchaser has not relied on, any statements other than those expressly set forth in the Transaction Documents or the Disclosure Materials. Without limiting or derogating from Section 3(ff), the Purchaser understands and represents that he, she or it is purchasing the Shares notwithstanding the fact that the Company may disclose in the future certain information the Purchaser has not received that is not required to be disclosed in the Super 8-K, including (without limitation) financial statements of the Company and/or Adaptin for the current fiscal period, and any subsequent period financial statements that will be filed with the SEC, that he, she or it is not relying on any such information in connection with his, her or its purchase of the Securities. The Purchaser has sought such accounting, legal and tax advice as the Purchaser has considered necessary to make an informed investment decision with respect to his, her or its acquisition of the Units.
(g) The Purchaser acknowledges that neither the Company nor any Placement Agent is acting as a financial advisor or fiduciary of the Purchaser (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated hereby and thereby, and no investment advice has been given by the Company, any Placement Agent or any of their respective representatives or agents in connection with the Transaction Documents and the transactions contemplated hereby and thereby. The Purchaser further represents to the Company that the Purchaser’s decision to enter into the Transaction Documents has been based solely on the independent evaluation by the Purchaser and the Purchaser’s representatives.
(h) As of the applicable Closing, all actions on the part of the Purchaser, and its officers, directors and partners, if applicable, necessary for the authorization, execution and delivery of this Agreement and the Registration Rights Agreement and the performance of all obligations of the Purchaser hereunder and thereunder shall have been taken, and this Agreement and the Registration Rights Agreement, assuming due execution by the parties hereto and thereto, constitute valid and legally binding obligations of the Purchaser, enforceable in accordance with their respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar Laws relating to, or affecting generally, the enforcement of creditors’ rights and remedies and, with respect to any rights to indemnity or contribution contained in the Transaction Documents, as such rights may be limited by state or federal laws or public policy underlying such laws.
25
(i) The Purchaser represents that neither it nor, to its knowledge, any person or entity controlling, controlled by or under common control with it, nor any person having a beneficial interest in the Purchaser, nor any person on whose behalf the Purchaser is acting: (i) is a person listed in the Annex to Executive Order No. 13224 (2001) issued by the President of the United States (Executive Order Blocking Property and Prohibiting Transactions with Persons Who Commit, Threaten to Commit, or Support Terrorism); (ii) is named on the List of Specially Designated Nationals and Blocked Persons maintained by the U.S. Office of Foreign Assets Control; (iii) is a non-U.S. shell bank or is providing banking services indirectly to a non-U.S. shell bank; (iv) is a senior non-U.S. political figure or an immediate family member or close associate of such figure; or (v) is otherwise prohibited from investing in the Company pursuant to applicable U.S. anti-money laundering, anti-terrorist and asset control Laws, regulations, rules or orders (categories (i) through (v), each a “Prohibited Purchaser”). The Purchaser (A) agrees to provide the Company, promptly upon request, all information that the Company reasonably deems necessary or appropriate to comply with applicable U.S. anti-money laundering, anti-terrorist and asset control Laws, regulations, rules and orders and (B) consents to the disclosure to U.S. regulators and law enforcement authorities by the Company and its Affiliates and agents of such information about the Purchaser as the Company reasonably deems necessary or appropriate to comply with applicable U.S. anti-money laundering, anti-terrorist and asset control Laws, regulations, rules and orders. If the Purchaser is a financial institution that is subject to the USA Patriot Act, the Purchaser represents that it has met all of its obligations under the USA Patriot Act. The Purchaser acknowledges that if, following its investment in the Company, the Company reasonably believes that the Purchaser is a Prohibited Purchaser or is otherwise engaged in suspicious activity or refuses to promptly provide information that the Company requests, the Company has the right or may be obligated to prohibit additional investments, segregate the assets constituting the investment in accordance with applicable regulations or immediately require the Purchaser to transfer all or any of the Securities. The Purchaser further acknowledges that neither the Purchaser nor any of the Purchaser’s Affiliates or agents will have any claim against the Company or Adaptin for any form of damages as a result of any of the foregoing actions.
(j) If the Purchaser is an Affiliate of a non-U.S. banking institution (a “Foreign Bank”), or if the Purchaser receives deposits from, makes payments on behalf of, or handles other financial transactions related to a Foreign Bank, the Purchaser represents and warrants to the Company that: (1) the Foreign Bank has a fixed address, other than solely an electronic address, in a country in which the Foreign Bank is authorized to conduct banking activities; (2) the Foreign Bank maintains operating records related to its banking activities; (3) the Foreign Bank is subject to inspection by the banking authority that licensed the Foreign Bank to conduct banking activities; and (4) the Foreign Bank does not provide banking services to any other Foreign Bank that does not have a physical presence in any country and that is not a regulated Affiliate.
(k) The Purchaser or its duly authorized representative realizes that because of the inherently speculative nature of businesses of the kind conducted and contemplated by the Company, the Company’s financial results may be expected to fluctuate from month to month and from period to period and will, generally, involve a high degree of financial and market risk that could result in substantial or, at times, even total losses for investors in securities of the Company. The Purchaser has considered the risk factors in the PPM before deciding to invest in the Units.
(l) The Purchaser is not subscribing for Units as a result of or subsequent to any advertisement, article, notice or other communication, published in any newspaper, magazine or similar media or broadcast over television, radio, or the internet, or presented at any seminar or meeting, or any solicitation of a subscription by a person not previously known to the Purchaser in connection with investments in securities generally.
(m) The Purchaser acknowledges that no U.S. federal or state agency or any other government or governmental agency has passed upon any of the Securities or made any finding or determination as to the fairness, suitability or wisdom of any investments therein.
26
(n) Other than consummating the transactions contemplated hereunder, the Purchaser has not directly or indirectly, nor has any individual or entity acting on behalf of or pursuant to any understanding with the Purchaser, executed any purchases or sales, including Short Sales (as defined below), of the securities of the Company during the period commencing at the time the Purchaser was first contacted by the Company or any other individual or entity representing the Company (including the Placement Agent) regarding the transactions contemplated hereunder. Notwithstanding the foregoing, in the case of the Purchaser being a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of the Purchaser’s assets and the portfolio managers do not communicate or share information with, and have no direct knowledge of the investment decisions made by, the portfolio managers managing other portions of the Purchaser’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by the portfolio manager that made the investment decision to purchase the Units covered by this Agreement. Other than to other individuals or entities party to this Agreement, or to the Purchaser’s representatives, agents or advisors, such Purchaser has maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction). Notwithstanding the foregoing, for avoidance of doubt, nothing contained herein shall constitute a representation or warranty, or preclude any actions, with respect to the identification of the availability of, or securing of, available shares to borrow in order to effect Short Sales or similar transactions in the future. For purposes of this Agreement, “Short Sales” means all “short sales” as defined in Rule 200 of Regulation SHO under the Exchange Act (but shall not be deemed to include the location and/or reservation of borrowable shares of Common Stock).
(o) The Purchaser is aware that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of any of the Securities and other activities with respect to the Securities by the Purchaser, and will comply with such anti-manipulation rules of Regulation M.
(p) All of the information concerning the Purchaser set forth herein, and any other information furnished by the Purchaser in writing to the Company or the Placement Agent for use in connection with the transactions contemplated by this Agreement, is true, correct and complete in all material respects as of the date of this Agreement, and, if there should be any material change in such information prior to the Purchaser’s purchase of the Units, the Purchaser will promptly furnish revised or corrected information to the Company.
(q) The Purchaser has reviewed with its own tax advisors the U.S. federal, state, local and foreign tax consequences of this investment and the transactions contemplated by the Transaction Documents. With respect to such matters, the Purchaser relies solely on such advisors and not on any statements or representations of the Company or any of its agents, written or oral. The Purchaser understands that it (and not the Company) shall be responsible for its own tax liability that may arise as a result of this investment or the transactions contemplated by the Transaction Documents.
(r) If the Purchaser is not a United States person (as defined by Section 7701(a)(30) of the Internal Revenue Code of 1986, as amended), the Purchaser hereby represents that it has satisfied itself as to the observance in all material respects of the Laws of its jurisdiction in connection with any invitation to subscribe for the Units or any use of this Agreement, including (a) the legal requirements within its jurisdiction for the purchase of the Units and the Securities included therein; (b) any foreign exchange restrictions applicable to such purchase; (c) any governmental or other consents that may need to be obtained; and (d) the income tax and other tax consequences, if any, that may be relevant to the purchase, holding, redemption, sale or transfer of any of the Securities. The Purchaser’s subscription and payment for and continued beneficial ownership of the Securities will not violate any applicable securities or other Laws of the Purchaser’s jurisdiction.
(s) The Purchaser represents that it is not a “foreign person” for purposes of Section 721 of the Defense Production Act of 1950 (as amended) or the rules or regulations promulgated thereunder (including 31 C.F.R. Part 800 and 31 C.F.R. part 801); provided, however, that if the Purchaser is a “foreign person” for such purposes, it agrees that it will not (i) obtain any control rights over the Company, including the ability to determine, direct, or decide important matters affecting the Company; (ii) have access to any material nonpublic technical information in the possession of the company; (iii) obtain membership or observer rights on the Board of Directors or the right to nominate an individual to a position on the Board of Directors; or (iv) have any involvement, other than through voting of shares, in substantive decision making of the Company regarding the use, development, acquisition or release of the Company’s technology.
27
(t) (For ERISA plans only) The fiduciary of the Employee Retirement Income Security Act of 1974 (“ERISA”) plan (the “Plan”) represents that such fiduciary has been informed of and understands the Company’s investment objectives, policies and strategies, and that the decision to invest “plan assets” (as such term is defined in ERISA) in the Company is consistent with the provisions of ERISA that require diversification of plan assets and impose other fiduciary responsibilities. The Purchaser fiduciary or Plan (a) is responsible for the decision to invest in the Company; (b) is independent of the Company or any of its Affiliates; (c) is qualified to make such investment decision; and (d) in making such decision, the Purchaser fiduciary or Plan has not relied primarily on any advice or recommendation of the Company or any of its Affiliates.
(u) If the Purchaser is a Covered Person, neither the Purchaser nor, to the Purchaser’s knowledge, any of its directors, executive officers, other officers that may Adaptin as a director or officer of any company in which it invests, general partners or managing members is subject to any Disqualification Events, except for Disqualification Events covered by Rule 506(d)(2)(ii) or (iii) under the Securities Act, and disclosed reasonably in advance of the applicable Closing in writing in reasonable detail to the Company.
(v) The Purchaser understands that there are substantial restrictions on the transferability of the Shares, the Warrants and the Warrant Shares and that the certificates or book-entry positions representing the foregoing shall bear a restrictive legend in substantially the following form (and a stop-transfer order may be placed against transfer of such certificates or other instruments):
THE SECURITIES REPRESENTED BY THIS [CERTIFICATE] [BOOK-ENTRY POSITION] [AND THE SECURITIES ISSUABLE UPON THE EXERCISE HEREOF] HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, AND THE RULES AND REGULATIONS PROMULGATED THEREUNDER (THE “SECURITIES ACT”), OR ANY STATE SECURITIES LAWS, AND NEITHER SUCH SECURITIES NOR ANY INTEREST THEREIN MAY BE OFFERED, SOLD, PLEDGED, ASSIGNED OR OTHERWISE TRANSFERRED UNLESS (1) A REGISTRATION STATEMENT WITH RESPECT THERETO IS EFFECTIVE UNDER THE SECURITIES ACT AND ANY APPLICABLE STATE SECURITIES LAWS, OR (2) AN EXEMPTION FROM SUCH REGISTRATION EXISTS AND THE COMPANY RECEIVES AN OPINION OF COUNSEL, WHICH COUNSEL AND OPINION ARE REASONABLY SATISFACTORY TO THE COMPANY, THAT SUCH SECURITIES MAY BE OFFERED, SOLD, PLEDGED, ASSIGNED OR TRANSFERRED IN THE MANNER CONTEMPLATED WITHOUT AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR APPLICABLE STATE SECURITIES LAWS OR (3) SOLD PURSUANT TO RULE 144 UNDER THE SECURITIES ACT.
In addition, if the Purchaser is an Affiliate of the Company, certificates or book-entry positions evidencing the Securities issued to the Purchaser may bear a customary “Affiliates” legend.
Any fees (with respect to the Company’s transfer agent for the Common Stock (the “Transfer Agent”), counsel or otherwise) associated with the removal of such legend(s) shall be borne by the Company.
28
Upon request from Purchaser and subject to receipt by the Company and its transfer agent from the Purchaser of customary representations and other documentation reasonably acceptable to the Company and the transfer agent in connection therewith, the Company shall be obligated to promptly reissue unlegended certificates or book entry positions at such time as the Securities evidenced by such certificates or book entry positions (x) are sold pursuant to Rule 144 or another applicable exemption from the registration requirements of the Securities Act has been satisfied, provided that Rule 144 or such other applicable exemption is available and, if the transfer agent requires an opinion, the Company shall cause its legal counsel to deliver an opinion in a form reasonably acceptable to the transfer agent, to the effect that the removal of such restrictive legends in such circumstances may be effected under the Securities Act or (y) are sold pursuant to an effective resale registration statement under the Securities Act, provided that if the transfer agent requires an opinion, the Company shall cause its legal counsel to deliver an opinion in a form reasonably acceptable to the transfer agent, to the effect that the removal of such restrictive legends in such circumstances may be effected under the Securities Act, or (z) are covered by an effective resale registration statement under the Securities Act and are Legend Removal Shares (as defined in the next sentence). If a resale registration statement under the Securities Act covering the Shares and the Warrant Shares becomes effective, then the Company shall cause legal counsel to the Company, at the Company’s expense: (a) to issue to the Transfer Agent, within one (1) Trading Day after the effective date thereof, a “blanket” legal opinion in customary form to the effect that the Securities covered by the Registration Statement have been registered for resale under the Securities Act and, if such counsel has received a signed certificate in the form attached as Exhibit A to the Registration Rights Agreement (a “Legend Removal Certificate”) from the holder of such Securities, may then be reissued without any legend or restriction relating to their status as “restricted securities” as defined in Rule 144 (“Legend Removal Shares”), or, otherwise, may then be reissued without any legend or restriction relating to their status as “restricted securities” as defined in Rule 144 upon resale pursuant to such registration statement; and (b) promptly to amend such opinion to cause such Securities to be Legend Removal Shares after later receipt of a Legend Removal Certificate from the Holder. Under the foregoing circumstances, the Company shall direct its Transfer Agent to issue unlegended shares, in the case of clause (x) above, within one (1) Trading Day after the Transfer Agent’s receipt of such opinion or, in the case of clause (y) above, within three (3) Trading Days after the Transfer Agent’s receipt of such legal opinion with respect to Legend Removal Shares or otherwise within three (3) Trading Days after the Transfer Agent’s receipt of evidence in customary form that the Securities have been sold pursuant to an effective resale registration statement under the Securities Act, in either case via DWAC or as otherwise requested by the holder.
(w) If the Purchaser is an individual, then the Purchaser resides in the state or province identified in the address of the Purchaser set forth on such Purchaser’s Omnibus Signature Page to this Agreement; if the Purchaser is a partnership, corporation, limited liability company or other entity, then the office or offices of the Purchaser in which its principal place of business is identified in the address or addresses of the Purchaser set forth on such Purchaser’s Omnibus Signature Page to this Agreement.
(x) The Purchaser understands that the Company prior to the Merger will have been a “shell company” as defined in Rule 12b-2 under the Exchange Act, and that upon filing with the SEC of the Super 8-K reporting the consummation of the Merger and related transactions and the transactions contemplated by this Agreement, and otherwise containing “Form 10 information” discussed below, the Company will reflect therein that it is no longer a shell company. Pursuant to Rule 144(i), securities issued by a current or former shell company (including the Securities) that otherwise meet the holding period and other requirements of Rule 144 nevertheless cannot be sold in reliance on Rule 144 until one (1) year after the Company (a) is no longer a shell company; and (b) has filed current “Form 10 information” (as defined in Rule 144(i)) with the SEC reflecting that it is no longer a shell company, and provided that at the time of a proposed sale pursuant to Rule 144, the Company is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and has filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding twelve (12) months (or for such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports. As a result, the restrictive legends on certificates or book-entry positions for the Securities cannot be removed except in connection with (i) an actual sale meeting the foregoing requirements or (ii) pursuant to an effective registration statement.
29
(y) The Purchaser, if and to the extent that it purchases Units in any Subsequent Closing, represents that it (i)(A) has a substantive, pre-existing relationship with the Company or (B) had direct contact by the Company or the Placement Agent outside of the Offering, and (ii) did not contact the Company or the Placement Agent or become interested in the Offering as a result of reading or otherwise being aware of the Super 8-K or any press release or any other public disclosure disclosing the terms of the Offering.
(z) To effectuate the terms and provisions hereof, the Purchaser hereby appoints the Placement Agent as its attorney-in-fact for the purpose of carrying out the provisions of the Escrow Agreement, including, without limitation, taking any action on behalf of, or at the instruction of, the Purchaser and executing any release notices required under the Escrow Agreement and taking any action and executing any instrument that the Placement Agent may deem necessary or advisable (and lawful) to accomplish the purposes hereof, in each case, subject to and in accordance with the terms of this Agreement. All lawful acts done under the foregoing authorization are hereby ratified and approved, and neither the Placement Agent nor any designee nor agent thereof shall be liable for any acts of commission or omission, for any error of judgment, for any mistake of fact or law except for acts of fraud, gross negligence or willful misconduct. This power of attorney, being coupled with an interest, is irrevocable while the Escrow Agreement remains in effect.
5. Conditions to Company’s Obligations at Closing. The Company’s obligation to complete the sale and issuance of the Units and deliver the Shares and the Warrants to the Purchaser and to consummate the other transactions contemplated hereby at the Initial Closing and, if applicable, a Subsequent Closing, shall be subject to the satisfaction or written waiver by the Company (in whole or in part) of the following conditions, to the extent such condition can be waived, in its sole discretion, on or prior to the Initial Closing Date and each Subsequent Closing Date, as applicable (provided, that any waiver by the Company of the condition set forth in Section 5(f) shall require the prior written consent of the Purchaser):
(a) Receipt of Payment. The Company shall have received payment, by certified or other bank check or by wire transfer of immediately available funds, in the full amount of the Purchase Price for the number of Units being purchased by the Purchaser at the Initial Closing and, if applicable, a Subsequent Closing.
(b) Receipt of Executed Transaction Documents. The Purchaser shall have executed and delivered to the Company the Omnibus Signature Page, the Purchaser Profile, the Accredited Investor Certification and the Selling Securityholder Questionnaire (as defined in the Registration Rights Agreement).
(c) Representations and Warranties. The representations and warranties made by the Purchaser in Section 4 hereof shall be true and correct in all respects as of the date of this Agreement and as of such Closing Date with the same force and effect as if they had been made on and as of such Closing Date (except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct in all respects as of such earlier date), except for the failure of any such representation or warranty to be so true and correct as would not, individually or in the aggregate, have a material adverse effect on the ability of the Purchaser to consummate the transactions contemplated hereby.
(d) Performance. The Purchaser shall have performed or complied with in all material respects all obligations and covenants herein required to be performed by the Purchaser on or prior to the applicable Closing.
(e) Effectiveness of the Merger Transactions. The Merger and each of the other transactions contemplated by the Merger Agreement shall have been effected and consummated.
(f) Minimum Offering. In connection with the Initial Closing only, the Company shall have received proceeds from the Offering equal to or greater than the Minimum Offering Amount (inclusive of any Adaptin Investment and any Placement Agent Investment).
30
(g) Qualifications. All Authorizations of, or notices to, any Governmental Authority that are required in connection with the transactions contemplated by this Agreement, including the lawful issuance and sale of the Securities pursuant to this Agreement at each Closing except for Blue Sky law permits and qualifications that may be properly obtained after such Closing and filing of a Notice of Exempt Offering of Securities on Form D with the SEC under Regulation D which may be filed no later than fifteen (15) calendar days after the “date of first sale” in the Offering.
6. Conditions to Purchaser’s Obligations at the applicable Closing. The Purchaser’s obligation to accept delivery of the Units and to pay for the Units to be issued to the Purchaser hereunder at the Initial Closing and, if applicable, a Subsequent Closing, and to consummate the other transactions contemplated hereby, shall be subject to the satisfaction by the Company or written waiver by the Purchaser (in whole or in part) of the following conditions, to the extent such condition can be waived, in its sole discretion, on or prior to the Initial Closing Date and each Subsequent Closing Date, as applicable:
(a) Representations and Warranties. (i) The representations and warranties made by the Company (as modified by the disclosures on the Disclosure Schedule or in the PPM (excluding any disclosures (whether contained under the heading “Risk Factors,” in any “forward-looking statements” disclaimer or in any other section) to the extent they are cautionary, predictive or forward-looking in nature) set forth in Sections 3(a), 3(b), 3(c), 3(d), 3(e), 3(h), 3(i), and 3(ee) hereof (collectively, the “Company Fundamental Representations”) shall be true and correct in all respects as of the date of this Agreement and as of such Closing Date with the same force and effect as if they had been made on and as of such Closing Date (except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct in all respects as of such earlier date) and (ii) the other representations and warranties made by the Company in Section 3 shall be true and correct in all material respects (without giving effect to any limitation as to “materiality” or “Material Adverse Effect” or similar qualifier) as of the date of this Agreement and as of such Closing Date with the same force and effect as if they had been made on and as of such Closing Date (except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct in all material respects as of such earlier date).
(b) Performance. The Company shall have performed or complied with in all material respects all obligations and covenants herein required to be performed by it on or prior to the applicable Closing.
(c) Receipt of Executed Transaction Documents. The Company shall have duly executed and delivered to the Placement Agent on behalf of the Purchaser the Registration Rights Agreement and the Escrow Agreement.
(d) Effectiveness of the Merger Transactions. The Merger and each of the other transactions contemplated by the Merger Agreement shall have been effected and consummated.
(e) Minimum Offering. In connection with the Initial Closing only, the Company shall have received proceeds from the Offering equal to or greater than the Minimum Offering Amount (inclusive of any Adaptin Investment and any Placement Agent Investment).
(f) Equity Incentive Plan. The Board of Directors and the stockholders of the Company shall have duly adopted the EIP as described in Recital B above.
(g) Certificate. At each applicable Closing, an executive officer of the Company shall have duly executed and delivered or caused to be delivered to the Placement Agent a certificate addressed to the Purchaser and the Placement Agent certifying as to the satisfaction of the conditions set forth in Section 6(a) and Section 6(b) as of the applicable Closing Date.
(h) Good Standing. The Company and each of its Subsidiaries is a corporation or other business entity duly organized, validly existing, and in good standing under the Laws of the jurisdiction of its formation.
31
(i) Judgments. No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate, including any bankruptcy court or judge, or any order of or by any Governmental Authority, shall have been issued, and no action or proceeding shall have been instituted by any Governmental Authority, enjoining or preventing the consummation of the transactions contemplated hereby.
(j) Bridge Notes. All outstanding principal amount of, and accrued but unpaid interest on, the Bridge Notes shall have converted into shares of Common Stock.
(k) Legal Opinion. Wyrick Robbins Yates & Ponton LLP (“Wyrick”), legal counsel for Adaptin and, upon closing of the Merger, for the Company, shall deliver an opinion addressed to the Purchaser and the Placement Agent, dated as of the applicable Closing Date, in form and substance reasonably acceptable to the Placement Agent.
(l) Comfort Letter. (a) The Auditor shall deliver a letter, addressed to the Purchaser and the Placement Agent, dated as of the applicable Closing Date, in form and substance reasonably satisfactory to the Placement Agent, containing statements and information of the type customarily included in auditors’ “comfort letters” with respect to the financial statements and certain financial information contained in the PPM.
(m) Lock-up Agreements. The Company delivered to the Purchaser lock-up agreements of the Company’s officers and directors after giving effect to the Merger and their Affiliates and associated entities (the “Lock-Up Parties”), duly executed by the Company and each Lock-Up Party, substantially in the form attached hereto as Exhibit D (the “Lock-Up Agreements”).
(n) Compliance with Laws. The transactions contemplated by this Agreement and the other Transaction Documents, including the sale and issuance of the Units and the Securities included therein, shall be legally permitted by all Laws and regulations to which the Company is subject or which are otherwise applicable to the transactions contemplated by the Transaction Documents.
(o) Qualifications. All Authorizations of, or notices to, any Governmental Authority that are required in connection with the transactions contemplated by this Agreement, including the lawful issuance and sale of the Units and the Securities included therein pursuant to this Agreement at each Closing, shall have been delivered or obtained and effective as of such Closing except for Blue Sky law permits and qualifications that may be properly obtained after such Closing and filing of a Notice of Exempt Offering of Securities on Form D with the SEC under Regulation D which may be filed no later than fifteen (15) calendar days after the “date of first sale” in the Offering.
(p) No Material Adverse Effect. There shall have been no Material Adverse Effect.
32
7. Indemnification.
(a) In addition to the indemnity provided to the Purchaser in the applicable Registration Rights Agreement, the Company agrees to indemnify and hold harmless the Purchaser and its Affiliates, and its and their respective directors, officers, stockholders, equity holders, members, managers, partners, employees, attorneys, consultants, representatives and agents (and any other persons with a functionally equivalent role of a person holding such titles notwithstanding a lack of such title or any other title), each person who controls the Purchaser (within the meaning of Section 15 of the Securities Act and Section 20 of the Exchange Act), and the directors, officers, stockholders, equity holders, members, managers, partners, employees, attorneys, consultants, representatives and agents (and any other persons with a functionally equivalent role of a person holding such titles notwithstanding a lack of such title or any other title) of such controlling person (collectively, the “Purchaser Indemnitees”), from and against all losses, liabilities, claims, damages, costs, fees, charges, Taxes, judgements, fines, penalties and expenses whatsoever (including, but not limited to, amounts paid in settlement and any and all reasonable out-of-pocket expenses, including attorneys’ fees and expenses, incurred in investigating, preparing or defending against any litigation commenced or threatened) (collectively, “Indemnified Liabilities”) arising out of or relating to: (i) the inaccuracy, violation or breach of any of the Company’s representations or warranties made in Section 3 of this Agreement; (ii) any breach or failure to perform by the Company of any of its covenants and obligations contained herein or (iii) any Action brought or made against such Purchaser Indemnitee by a third party (including for these purposes a derivative action brought on behalf of the Company) and arising out of, relating to or resulting from (A) the execution, delivery, performance or enforcement of the Transaction Documents or the Merger Agreement or the transactions contemplated hereby or thereby, including the issuance of the Units and the Securities included therein and the Merger or (B) the status of the Purchaser as an investor in the Company pursuant to the transactions contemplated hereby or by the other Transaction Documents, except to the extent that any such Indemnified Liabilities arise out of a violation of Law or a breach of this Agreement by the Purchaser or one of its related Purchaser Indemnitees. To the extent that the foregoing undertaking by the Company may be unenforceable for any reason, the Company shall make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities that is permissible under applicable Law. The liability of the Company under this paragraph shall not exceed the total Purchase Price paid by the Purchaser hereunder, except in the case of fraud.
(b) The Company shall have the right to control the investigation and defense of any Action for which a Purchaser Indemnitee may be entitled to indemnification hereunder with counsel reasonably satisfactory to such Purchaser Indemnitee, at the sole cost and expense of the Company, upon written notice to the applicable Purchaser Indemnitee; provided, that (i) such notice contains confirmation that the Company has agreed to indemnify the Purchaser Indemnitee (subject to the limitations on indemnification set forth herein) for the Indemnified Liabilities arising out of, relating to or resulting from such Action and (ii) the Company shall not be entitled to assume or control the investigation and defense, if (A) such claim seeks non-monetary, equitable or injunctive relief or alleges any violation of criminal Law or (B) the Company is also a party and the Purchaser Indemnitee determines in good faith after consultation with counsel that there may be one or more legal defenses available to such Purchaser Indemnitee that are different or additional to those available to the Company. If the Company assumes the investigation and defense of such Action in accordance herewith, the Purchaser Indemnitee may retain separate co-counsel at its sole cost and expense and participate in the investigation and defense of such Action.
(c) Notwithstanding anything to the contrary herein, without the prior written consent of the Purchaser Indemnitee, the Company shall not, and shall not cause or permit any of its Subsidiaries or its or their respective Related Parties to, negotiate, consent to or enter into any settlement, or consent to the entry of any judgment, with respect to any Action for which such Purchaser Indemnitee may be entitled to indemnification hereunder, unless such settlement (i) includes an unconditional release of such Purchaser Indemnitee from all liability arising out of such proceeding, (ii) does not require any admission of wrongdoing by any Purchaser Indemnitee, and (iii) does not obligate or require any Purchaser Indemnitee to take, or refrain from taking, any action.
33
(d) The Purchaser acknowledges on behalf of itself and each Purchaser Indemnitee that, other than (i) for Actions seeking specific performance of the obligations under this Agreement; or (ii) in the case of a breach or violation of this Agreement by the Company which has resulted from either (A) intentional fraud or (B) a deliberate act or failure to act with actual knowledge that the act or failure to act constituted or would result in a breach or violation, in each case, the sole and exclusive remedy of the Purchaser and the Purchaser Indemnitees with respect to any and all claims relating to this Agreement shall be pursuant to the indemnification provisions (including the limitations thereof) set forth in this Section 7.
8. Revocability; Binding Effect. The subscription hereunder may be revoked, in whole or in part, prior to the Initial Closing or any Subsequent Closing, as applicable, in the sole discretion of the Purchaser, for any reason or no reason, provided that written notice of revocation is sent and is received by the Company or the Placement Agent at least two (2) Business Days prior to the Initial Closing Date or the applicable Subsequent Closing Date. The Purchaser hereby acknowledges and agrees that this Agreement shall survive the death or disability of the Purchaser and shall be binding upon and inure to the benefit of the parties and their heirs, executors, administrators, successors, legal representatives and permitted assigns. If the Purchaser is more than one person, the obligations of the Purchaser hereunder shall be joint and several and the agreements, representations, warranties and acknowledgments herein shall be deemed to be made by and be binding upon each such person and such person’s heirs, executors, administrators, successors, legal representatives and permitted assigns.
9. Miscellaneous.
(a) Use of Proceeds. The Company currently intends use the net proceeds from the Offering as set forth in the PPM.
(b) Modification. This Agreement shall not be amended, modified or waived except by an instrument in writing signed by the Company and the holders of at least a majority of the Shares and the shares of Common Stock included in the Other Units; provided that this Agreement may not be amended and the observance of any term hereof may not be waived with respect to any Purchaser without the written consent of such Purchaser if such amendment or waiver on its face materially and adversely affects the rights of such Purchaser under this Agreement in a manner that is different than the Other Purchasers. Any amendment, modification or waiver effected in accordance with this Section (i)(b) shall be binding upon the Purchaser and each transferee of any of the Securities, each future holder of all such Securities, and the Company, its successors and assigns.
(c) Third-Party Beneficiary. The Placement Agent shall be an express third party beneficiary of the representations and warranties of the Company and the Purchaser included in Sections 3 and 4 of this Agreement. This Agreement is intended for the benefit of the parties hereto and their respective successors and permitted assigns and is not for the benefit of, nor may any provision hereof be enforced by, any other person, except as otherwise set forth in Section 7 and this Section 9(c).
34
(d) Notices. Any notice, consents, waivers or other communication required or permitted to be given hereunder shall be in writing and will be deemed to have been delivered: (i) upon receipt, when personally delivered; (ii) upon receipt when sent by certified mail, return receipt requested, postage prepaid; (iii) upon receipt, when sent by facsimile (provided confirmation of transmission is mechanically or electronically generated and kept on file by the sending party); (iv) when sent, if by e-mail (provided that such sent e-mail is kept on file (whether electronically or otherwise) by the sending party and the sending party does not receive an automatically generated message from the recipient’s e- mail server that such e-mail could not be delivered to such recipient); or (v) one (1) Business Day after deposit with a nationally recognized overnight courier service with next day delivery specified, in each case, properly addressed to the party to receive the same. The addresses, facsimile numbers and email addresses for such communications shall be:
| (i) | if to the Company, at |
Unite Acquisition 1 Corp.
12 E. 49th Street, 11th Floor
New York, NY 10017
Attention: Nathan Pereira, CEO
with copies (which shall not constitute notice) to:
Sichenzia Ross Ference Carmel LLP
1185 Avenue of the Americas, 31st Floor
New York, NY 10036
Attention: Barrett S. DiPaolo
Facsimile: 212-930-9725
E-mail: bdipaolo@srfc.law
and
Wyrick Robbins Yates & Ponton LLP
4101 Lake Boone Trail, Suite 300
Raleigh, NC 27607
Attention: Donald R. Reynolds
E-mail: dreynolds@wyrick.com
| (ii) | if to the Purchasers, at the address set forth on each such Omnibus Signature Page hereof |
(or, in either case, to such other address as the party shall have furnished in writing in accordance with the provisions of this Section).
(e) Assignability. This Agreement is binding upon, and is enforceable by, each of the Company and the Purchaser and their respective successors and permitted assigns. This Agreement and the rights, interests and obligations hereunder are not transferable or assignable by the Purchaser other than an assignment of the rights, interests and obligations hereunder in connection with any transfer of the Securities by a Purchaser to a Permitted Assignee (as such term is defined in the Registration Rights Agreement). Notwithstanding the foregoing, the Purchaser may assign its rights and obligations under this Agreement to one or more of its Affiliates or, with the Company’s prior written consent, to another person, in either case that is an accredited investor as defined in Rule 501 of Regulation D; provided, that no such assignment shall relieve the Purchaser of its obligations hereunder if any such assignee fails to perform such obligations, unless the Company has given its prior written consent to such relief. For the avoidance of doubt, nothing in this Section 9(e) is intended to, or shall have the effect of, restricting or otherwise impairing any transfer of the Securities by the Purchaser in accordance with applicable Laws and the provisions of the Transaction Documents.
(f) Applicable Law. This Agreement and the other Transaction Documents and the transactions contemplated hereby and thereby shall be governed by and construed in accordance with the Laws of the State of New York, without reference to the principles thereof relating to the conflict of Laws to the extent they would result in the application of the Laws of any other jurisdiction. Any litigation based hereon, or arising out of, under or in connection with, this Agreement or any other Transaction Document or the transactions contemplated hereby or thereby shall be brought and maintained exclusively in the United States District Court for the Southern District of New York or the Supreme Court of the State of New York, and the appellate courts therefrom, in each case sitting in New York County, New York. Each party irrevocably consents to the service of process of any of the aforementioned courts in any such suit, action or proceeding by the mailing of copies thereof by registered or certified mail, postage prepaid, return receipt requested, to such party’s address set forth in Section 9(d), such service to become effective ten (10) days after such mailing.
35
(g) WAIVER OF JURY TRIAL. EACH PARTY HERETO HEREBY IRREVOCABLY WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY ACTION OR COUNTERCLAIM (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT, ANY OTHER TRANSACTION DOCUMENT, THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY OR THE ACTIONS OF SUCH PARTY IN THE NEGOTIATION, ADMINISTRATION, PERFORMANCE AND ENFORCEMENT HEREOF.
(h) Form D; Blue Sky Qualification. The Company agrees to timely file a Form D with respect to the Units and to provide a copy thereof, promptly upon request of the Purchaser. The Company shall take such action as the Company shall reasonably determine is necessary in order to obtain an exemption for, or to qualify the Units and the Securities included therein for, sale to the Purchaser at such Closing under applicable securities or “Blue Sky” laws of the states of the United States, and shall provide evidence of such actions promptly upon request of the Purchaser.
(i) Use of Pronouns. All pronouns and any variations thereof used herein shall be deemed to refer to the masculine, feminine, neuter, singular or plural as the identity of the person or persons referred to may require.
(j) Securities Law Disclosure; Publicity. By 9:00 a.m., New York City time, on the trading day immediately following the Initial Closing, the Company shall issue a press release disclosing all material terms of the Offering. The Company will also file with the SEC, as soon as practicable following the closing date of the Merger but in no event more than four (4) Business Days following the closing date of the Merger, a Current Report on Form 8-K describing the Merger, the Offering and the related transactions, including “Form 10 information” (as defined in Rule 144(i)(3) under the Securities Act), including any audited and interim unaudited financial statements of Adaptin and, either with such Form 8-K or by amendment thereto as permitted by Law, pro forma financial information reflecting the Merger, as required by Item 9.01 of SEC Form 8-K, and including as exhibits the material Transaction Documents (including, without limitation, this Agreement, the Warrants, the Registration Rights Agreement and the Merger Agreement, in each case without redaction and including all schedules, exhibits and appendices, except as permitted by applicable SEC rules and instructions and provided that any redaction or schedule, exhibit or appendix not so filed in reliance on such rules and instructions shall not contain any material non-public information) (the “Super 8-K”). Upon request of the Purchaser, the Company will deliver to the Purchaser a copy of any exhibit to be filed or furnished with the Super 8-K, in the form intended to be filed or furnished, including, without limitation, a substantially complete draft of the Merger Agreement and each other material Transaction Document contemplated by or related to the Merger Agreement, including the disclosure schedules thereto; provided, however, that such delivery shall be deemed to have been effected to the extent such document has been filed with or furnished to the SEC pursuant to its Electronic Data Gathering and Retrieval System. Notwithstanding the foregoing, the Company shall not publicly disclose the name of the Purchaser or an Affiliate of the Purchaser, or include the name of the Purchaser or an Affiliate of the Purchaser in any press release or filing with the SEC (other than the Registration Statement) or any regulatory agency or principal trading market, without the prior written consent of the Purchaser, except (i) as required by federal securities Law in connection with (A) any registration statement contemplated by the Registration Rights Agreement and (B) the filing of final Transaction Documents with the SEC, or (ii) in connection with a request by FINRA relating to the Form 211 to be filed by a market maker on the Company’s behalf, or (iii) to the extent such disclosure is required by applicable Law, request of the staff of the SEC or of any regulatory agency or principal trading market regulations, in which case the Company shall to the extent legally permissible provide the Purchaser with prior written notice of such disclosure permitted under this sub-clause (ii). From and after the filing of the Super 8-K, no Purchaser shall be in possession of any material, non-public information received from the Company or any of its respective officers, directors, employees or agents or any other person acting on its behalf in connection with the Offering that is not disclosed in the Super 8-K unless the Purchaser shall have executed a written agreement with the Company regarding the confidentiality and use of such information or is otherwise subject to confidentiality restrictions. The Purchaser, severally and not jointly with the Other Purchasers, covenants that until such time as the transactions contemplated by this Agreement are publicly disclosed by the Company as described in this Section 9(j), the Purchaser will maintain the confidentiality of all disclosures made to it in connection with such transactions (including the existence and terms of such transactions), except to the extent such disclosure is required by applicable Law and then only after providing the Company with advance notice of such disclosure to the extent legally permissible so that the Company may seek a protective order to prevent such disclosure. In addition, the Purchaser acknowledges that it is aware that United States securities laws may restrict persons who have material, non-public information about a company from purchasing or selling any securities of such company while in possession of such information. The provisions of this Section 9(j) are in addition to and not in replacement of any other confidentiality agreement, if any, between the Company and the Purchaser.
36
(k) Non-Public Information. Except for information (including the terms of this Agreement and the transactions contemplated hereby) that will be disclosed in the Super 8-K and filed with the SEC, the Company shall not and shall cause each of its officers, directors, employees, agents and other representatives, not to, provide the Purchaser with any material, non-public information regarding the Company or any of its Subsidiaries or any of the Company’s securities without the express prior written consent of the Purchaser. The Company understands, acknowledges and agrees that (a) the Purchaser, its Affiliates and persons acting on its or their behalf will rely on the provisions of Section 9(n) and this Section 9(o) in effecting transactions in the Shares and other securities of the Company and of other persons, and (b) notwithstanding anything to the contrary contained herein, the Purchaser shall not (nor shall any of the Purchaser’s Affiliates, attorneys, agents or representatives) have, solely as a result of this Agreement or any of the other Transaction Documents or the purchase of the Shares, any duty of trust or confidence with respect to, or any obligation not to trade in any securities while aware of, any material non-public information (i) provided by, or on behalf of, the Company, any of its Subsidiaries or any of its or their officers, directors (or equivalent persons), employees, attorneys, agents or representatives in violation of any of the representations, covenants, provisions or agreements set forth in Section 9(n) or this Section 9(o) or (ii) otherwise possessed (or continued to be possessed) by the Purchaser (or any Affiliate, agent or representative thereof) as a result of any breach or violation by the Company of any representation, covenant, provision or agreement set forth in Section 9(j) or this Section 9(k)).
(l) Entire Agreement. This Agreement, together with the Registration Rights Agreement and each other Transaction Document, and all exhibits, schedules and attachments hereto and thereto, including the Disclosure Schedule and any confidentiality agreement between the Purchaser and the Company, constitute the entire agreement between the Purchaser and the Company with respect to the Offering and supersede all prior oral or written agreements and understandings, if any, relating to the subject matter hereof.
(m) Share Certificates. The Shares issued at any Closing and the Warrant Shares may be certificated or represented by book-entry positions on the books of the Transfer Agent. If the Shares or Warrant Shares are subsequently certificated and any certificate or instrument evidencing any Shares or Warrant Shares is mutilated, lost, stolen or destroyed, the Company shall issue or cause to be issued in exchange and substitution for and upon cancellation thereof, or in lieu of and substitution therefor, a new certificate or instrument, but only upon receipt of evidence reasonably satisfactory to the Company and the Transfer Agent of such loss, theft or destruction and the execution by the holder thereof of a customary lost certificate affidavit of that fact and an agreement to indemnify and hold harmless the Company and its Transfer Agent for any losses in connection therewith and/or, if required by such Transfer Agent, a bond in such form and amount as is required by the Transfer Agent. The applicants for a new certificate or instrument under such circumstances shall also pay any reasonable third-party costs associated with the issuance of such replacement certificate or instrument. If a replacement certificate or instrument evidencing any Shares or Warrant Shares is requested due to a mutilation thereof, the Company may require delivery of such mutilated certificate or instrument as a condition precedent to any issuance of a replacement.
(n) Expenses. Except as explicitly provided otherwise in this Agreement, all parties shall bear their own fees and expenses in connection with the Offering and the Merger and any due diligence activities relating thereto. Without limiting the foregoing, the Company shall pay all Transfer Agent fees, stamp taxes and other Taxes and duties levied in connection with the sale and issuance of the Offering, and the Company shall file all necessary Tax Returns and other documentation with respect to such fees, Taxes and duties, and the Company shall pay all fees and expenses of its counsel in connection with the issuance of any opinion required by Section 6(k) above and of any opinion to the Transfer Agent for the removal of any legend on the Shares or Warrant Shares. Any expenses of the Placement Agent (or any sub-agents), including fees and expenses of their legal counsel, will be paid or reimbursed as agreed by Adaptin and the Company with the Placement Agent in the Placement Agent Agreement (or similar agreement) by and between the Company and such Placement Agent. All other fees and expenses relating to the Merger and the Offering, including but not limited to the Placement Agent’s Cash Fee and Expense Allowance, legal and accounting fees of Adaptin, any expenses of the Company will be payable at each closing of the Offering from the proceeds thereof.
37
(o) Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument. The exchange of copies of this Agreement and of signature pages that contain copies of an executed signature page such as in .pdf format shall constitute effective execution and delivery of this Agreement as to the parties and may be used in lieu of the original Agreement for all purposes. Signatures of the parties transmitted by facsimile or by e-mail of a document in .pdf format shall be deemed to be their original signatures for all purposes.
(p) Severability. Each provision of this Agreement shall be considered separable and, if for any reason any provision or provisions hereof are determined to be invalid or contrary to applicable Law, such invalid or contrary provision shall be replaced with a valid provision that as closely as possible reflects the parties’ intent with respect thereto, and invalidity or illegality shall not impair the operation of or affect the remaining portions of this Agreement.
(q) Headings. Paragraph titles are for descriptive purposes only and shall not control or alter the meaning of this Agreement as set forth in the text.
(r) Multiple Closings. The Purchaser understands and acknowledges that there may be multiple Closings for the Offering.
(s) Additional Information; Further Assurances. The Purchaser hereby agrees to furnish the Company such other information as the Company may reasonably request prior to the applicable Closing with respect to its subscription hereunder. Each party hereto shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such other agreements, certificates, instruments and documents, as the other party hereto may reasonably request in order to effect the transactions contemplated hereby and to accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
(t) Survival. The parties, agree that, if the Closing occurs, (i) the Company Fundamental Representations shall survive the execution and delivery of this Agreement for a period of three (3) years from the Initial Closing Date and (ii) the other representations and warranties of the Company and the representations and warranties of the Purchaser contained in this Agreement shall survive the execution and delivery of this Agreement for a period of one (1) year from the Initial Closing Date and in each case, shall in no way be affected by any investigation or knowledge of the subject matter thereof made by or on behalf of the Purchaser or the Company. The covenants and agreements contained in this Agreement (including the covenants and agreements set forth in Section 7 hereof) shall survive the Closing and delivery of the Securities included in the Units in accordance with their terms or, if no term is specified, such covenants and agreements shall survive indefinitely. Notwithstanding anything herein to the contrary, in no event shall the Purchaser have any liability to the Company or to any other person in connection with the Offering other than pursuant to this Agreement.
(u) Omnibus Signature Page. This Agreement is intended to be read and construed in conjunction with the Registration Rights Agreement. Accordingly, pursuant to the terms and conditions of this Agreement and the Registration Rights Agreement, it is hereby agreed that the execution by the Purchaser of this Agreement, in the place set forth on the Omnibus Signature Page below, shall constitute agreement to be bound by the terms and conditions hereof and the terms and conditions of the Registration Rights Agreement, with the same effect as if each of such separate but related agreement were separately signed.
(v) Public Disclosure. Neither the Purchaser nor any officer, manager, director, member, partner, stockholder, employee, Affiliate, Affiliated person or entity of the Purchaser shall make or issue any press releases or otherwise make any public statements or make any disclosures to any third person or entity with respect to the transactions contemplated herein and will not make or issue any press releases or otherwise make any public statements of any nature whatsoever with respect to the Company without the Company’s express prior approval (which may be withheld in the Company’s sole discretion), except to the extent such disclosure is required by Law, request of the staff of the SEC or of any regulatory agency or principal trading market regulations.
38
(w) Potential Conflicts. The Placement Agent, its sub-agents, legal counsel to the Company, the Placement Agent or Adaptin and/or their respective Affiliates, principals, representatives or employees may now or hereafter own shares of the Company. Purchaser specifically acknowledges that it (a) has read the information set forth under “Certain Relationships and Related Party Transactions—Transactions with Lucius Partners and Related Persons” and “Potential Conflicts of Interest” in the PPM, (b) understands the risks of such potential or actual conflicts, (c) acknowledges that it has had an opportunity to ask for information relevant to these disclosures, and (d) gives its informed consent to the transactions and relationships described therein.
(x) Independent Nature of the Purchaser’s Obligations and Rights. For avoidance of doubt, the obligations of the Purchaser under this Agreement, the other Transaction Documents and any other agreements delivered in connection herewith are several and not joint with the obligations of any Other Purchaser in connection with the Offering, and the Purchaser shall not be responsible in any way for the performance of the obligations of any Other Purchaser in connection with the Offering. The decision of the Purchaser to purchase Shares pursuant to this Agreement has been made by the Purchaser independently of any Other Purchaser or any other investor and independently of any information, materials, statements or opinions as to the business, affairs, operations, assets, properties, liabilities, results of operations, condition (financial or otherwise) or prospects of the Company or any of its Subsidiaries which may have been made or given by any Other Purchaser or investor or by any agent or employee of any Other Purchaser or investor, and neither the Purchaser nor any of its agents or employees shall have any liability to any Other Purchaser or investor (or any other person) relating to or arising from any such information, materials, statements or opinions. Nothing contained herein and no action taken by the Purchaser shall be deemed to constitute the Purchaser as a partnership, an association, a joint venture, or any other kind of entity, or create a presumption that the Purchaser is in any way acting in concert or as a group with any Other Purchaser in connection with the Offering with respect to such obligations or the transactions contemplated by this Agreement or any other Transaction Document or any Other Subscription Agreement. Except as specifically set forth herein, the Purchaser shall be entitled to independently protect and enforce its rights, including without limitation the rights arising out of this Agreement, and it shall not be necessary for any other party to be joined as an additional party in any proceeding for such purpose.
(y) Waiver of Conflicts of Legal Counsel. Each party to this Agreement acknowledges that each of Sichenzia Ross Ference Carmel LLP (“SRFC”), counsel to the Placement Agent, and Wyrick, counsel to Adaptin, and upon the effectiveness of the Merger, to the Company, may have in the past performed and may continue to or in the future perform legal services for certain of the Purchasers in matters unrelated to the transactions described in this Agreement, including financings and other matters. Accordingly, each party to this Agreement hereby (a) acknowledges that it has had an opportunity to ask for information relevant to this disclosure; (b) acknowledges that SRFC and Wyrick represented the clients referred to above, respectively, in the transactions contemplated by this Agreement and have not represented any individual Purchaser in connection with such transactions; and (c) gives its informed consent to SRFC’s and Wyrick’ representation of certain of the Purchasers in unrelated matters and to SRFC’s and Wyrick’s representation of the clients referred to above, respectively, in connection with this Agreement and the other Transaction Documents and the transactions contemplated hereby and thereby.
39
(z) Adjustments. In the event of any stock split, subdivision, dividend or distribution payable in shares of Common Stock (or other securities or rights convertible into, or entitling the holder thereof to receive directly or indirectly shares of Common Stock), combination or other similar recapitalization or event occurring after the date hereof, each reference in any Transaction Document to a number of shares of Common Stock or the Per Unit Purchase Price shall be deemed to be amended to appropriately account for such event.
(aa) Remedies. The parties hereto agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed by them in accordance with the terms hereof and that each party hereto may be entitled to seek protective orders, injunctive relief and other remedies available at Law or in equity (including, without limitation, seeking specific performance or rescission of purchases, sales and other transfers). The parties hereto agree not to raise any objections to the availability of the equitable remedy of specific performance to prevent or restrain breaches of this Agreement by the Purchaser or the Company, as applicable, and to specifically enforce the terms and provisions of this Agreement to prevent breaches or threatened breaches of, or to enforce compliance with, the respective covenants and obligations of the Purchaser and the Company, as applicable, under this Agreement all in accordance with the terms of this Section 9(aa). Neither the Purchaser nor the Company, as applicable, shall be required to provide any bond or other security in connection with seeking an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement, all in accordance with the terms of this Section 9(aa).
(bb) Recourse. Notwithstanding anything that may be expressed or implied in this Agreement or in any other Transaction Document, and notwithstanding the fact that the Purchaser may be partnerships or limited liability companies, the Company hereto covenants, agrees and acknowledges that no recourse under this Agreement or any Transaction Document shall be had against any the Purchaser’s future, present or former Affiliates, or the Purchaser’s or its Affiliates’ respective future, present or former officers, directors, managers, employees, partners, equity holders, controlling persons, members, agents, attorneys, investment advisers, representatives, successors or permitted assigns (the “Purchaser Parties”) (other than the Purchaser and its successors and Permitted Assignees under this Agreement), whether by the enforcement of any assessment or by any legal or equitable proceeding, or by virtue of any applicable Law, it being expressly agreed and acknowledged that no personal liability whatsoever shall attach to, be imposed on or otherwise be incurred by any of the Purchaser Parties, as such, for any obligation or liability of any party under this Agreement or any other Transaction Document for any claim based on, in respect of or by reason of such obligations or liabilities or their creation; provided, however, nothing in this Section 9(bb) shall relieve or otherwise limit the liability of the Purchaser or any of its successors or Permitted Assignees, for any breach or violation of its obligations under such agreements, documents or instruments. The liability limitation provision in this Section 9(aa) shall survive termination of this Agreement. The Purchaser Parties are intended third party beneficiaries of the provisions of this Section 9(ff), entitled to enforce such provisions as if directly party to this Agreement.
(cc) Lock-Up Agreements. The Company shall not amend, modify, waive or terminate any provision of any of the Lock-Up Agreements except to extend the term of the lock-up period and shall enforce the provisions of each Lock-Up Agreement in accordance with its terms.
(dd) Rules of Construction. Unless the context otherwise requires, (i) all references to Sections, Schedules or Exhibits are to Sections, Schedules or Exhibits contained in or attached to this Agreement, (ii) each accounting term not otherwise defined in this Agreement has the meaning assigned to it in accordance with GAAP, (iii) words in the singular or plural include the singular and plural and pronouns stated in either the masculine, the feminine or neuter gender shall include the masculine, feminine and neuter, (iv) the use of the word “including” in this Agreement shall be by way of example rather than limitation, and (v) the word “or” shall not be exclusive.
[Signature page follows.]
40
IN WITNESS WHEREOF, the Company has duly executed this Agreement as of the day of , 202 .
| UNITE ACQUISITION 1 CORP. | ||
| (to be renamed “Adaptin Bio, Inc.”) | ||
| By: | ||
| Name: | ||
| Title: | ||
Unite Acquisition 1 Corp. (to be renamed “Adaptin Bio, Inc.)”)
OMNIBUS SIGNATURE PAGE TO
SUBSCRIPTION AGREEMENT AND REGISTRATION RIGHTS AGREEMENT
The undersigned, desiring to: (i) enter into the Subscription Agreement, dated as of , 202 1 (the “Subscription Agreement”), between the undersigned, Unite Acquisition 1 Corp. (to be renamed “Adaptin Bio, Inc.”, a Delaware corporation (the “Company”), and the other parties thereto, in the form furnished to the undersigned, (ii) enter into the Registration Rights Agreement (the “Registration Rights Agreement”), among the undersigned, the Company and the other parties thereto, in the form furnished to the undersigned, and (iii) purchase the Units of the Company’s securities as set forth in the Subscription Agreement and below, hereby agrees to purchase such Units from the Company and further agrees to join the Subscription Agreement and the Registration Rights Agreement as a party thereto, with all the rights and privileges appertaining thereto, and to be bound in all respects by the terms and conditions thereof. The undersigned specifically acknowledges having read the representations section in the Subscription Agreement entitled “Representations and Warranties of the Purchaser” and hereby represents that the statements contained therein are complete and accurate with respect to the undersigned as a Purchaser.
IN WITNESS WHEREOF, the Purchaser hereby executes the Subscription Agreement and the Registration Rights Agreement.
Dated: ______________, 202___
| × | $4.40 | = $ | |
| Number of Units | Purchase Price per Unit | Total Purchase Price |
| PURCHASER (individual) | PURCHASER (entity) | ||
| Signature | Name of Entity | ||
| By: | |||
| Print Name | Signature | ||
| Print Name: | |||
| Signature (if Joint Tenants or Tenants in Common) | Title: | ||
| Address of Principal Residence: | Address of Executive Offices: | ||
| Social Security Number(s): | IRS Tax Identification Number: | ||
| Telephone Number: | Telephone Number: | ||
| Facsimile Number: | Facsimile Number: | ||
| E-mail Address: | E-mail Address: | ||
| 1 | Will reflect the Closing Date. Not to be completed by Subscriber. |
EXHIBIT A
Form of A Warrant
EXHIBIT B
Form of B Warrant
EXHIBIT C
Form of Registration Rights Agreement
Exhibit 10.8
REGISTRATION RIGHTS AGREEMENT
This Registration Rights Agreement (this “Agreement”) is made and entered into effective as of , 2025, among Adaptin Bio, Inc., a Delaware corporation (f.k.a. Unite Acquisition 1 Corp.) (the “Company”), the persons who have purchased the Units (as defined below) and have executed omnibus or counterpart signature page(s) hereto (each, a “Purchaser” and collectively, the “Purchasers”), the persons or entities identified on Schedule 1 hereto holding Pre-Merger Warrants (as defined below), the persons or entities identified on Schedule 2 hereto holding Note Conversion Shares (as defined below), the persons or entities identified on Schedule 3 hereto holding Registrable Pre-Merger Shares (as defined below), and the persons or entities identified on Schedule 4 hereto holding Placement Agent Warrants (collectively, the “Brokers”). Capitalized terms used herein shall have the meanings ascribed to them in Section 1 below or in the Subscription Agreement (as defined below).
RECITALS:
WHEREAS, the Company has offered and sold in compliance with Section 4(a)(2) of the Securities Act and Rule 506(b) of Regulation D promulgated thereunder to accredited investors in a private placement offering (the “Offering”) Units (as defined below) of its securities, pursuant to certain Subscription Agreements entered into by and between the Company and each of the Purchasers of the Units set forth on the signature pages affixed thereto (the “Subscription Agreements”), each “Unit” consisting of (i) one share of the Company’s Common Stock (as defined below), (ii) an A Warrant (as defined in the Subscription Agreements), representing the right to purchase one (1) share of Common Stock, and (iii) a B Warrant (as defined in the Subscription Agreements), representing the right to purchase one-half (1/2) share of Common Stock; and
WHEREAS, the Company has outstanding (a) certain warrants identified on Schedule 1 hereto, to purchase the number of shares of Common Stock indicated next to such person’s name on Schedule 1 (the “Pre-Merger Warrants”), and (b) the Placement Agent Warrants held by the Brokers, to purchase the number of shares of Common Stock indicated next to such person’s name on Schedule 4; and
WHEREAS, the Company has agreed to enter into a registration rights agreement with each of the Purchasers, with each of the Pre-Merger Warrant Holders, with each of the holders of Note Conversion Shares, with each of the holders of Registrable Pre-Merger Shares and with each of the Brokers, or their designees; and
WHEREAS, contemporaneously with the initial closing of the Offering, pursuant to an Agreement and Plan of Merger and Reorganization by and among the Company, Adaptin Bio, Inc., a Delaware corporation (“Adaptin”), and the Acquisition Subsidiary (as defined therein), all of the outstanding capital stock of Adaptin was exchanged for shares of the Company’s Common Stock and Adaptin became a wholly owned subsidiary of the Company (the “Merger”);
NOW, THEREFORE, in consideration of the foregoing and of the mutual promises, representations, warranties, covenants and conditions set forth herein, the parties mutually agree as follows:
1. Certain Definitions. As used in this Agreement, the following terms shall have the following respective meanings:
“A Warrant Shares” means shares of Common Stock issued or issuable upon exercise of the A Warrants issued to the Purchasers pursuant to the Subscription Agreements, and any shares of Common Stock issued or issuable with respect to such shares upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
“Approved OTC Market” means the OTCQB or OTCQX market of OTC Markets Group (or, in each case, a successor over-the-counter trading market thereto).
“B Warrant Shares” means shares of Common Stock issued or issuable upon exercise of the B Warrants issued to the Purchasers pursuant to the Subscription Agreements, and any shares of Common Stock issued or issuable with respect to such shares upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
“Blackout Period” means, with respect to a distribution or registration, a period during which the Company, in the good faith judgment of its board of directors, determines (because of the existence of, or in anticipation of, any acquisition, financing activity, or other material corporate development or other material transaction involving the Company, or the unavailability for reasons beyond the Company’s control of any required financial statements, or any other event or condition of similar material significance to the Company) that the registration and/or distribution of the Registrable Securities to be covered by such registration statement, if any, or the circumstances described in Section 4(h) below, would require additional disclosure by the Company in such registration statement of material information that the Company has a bona fide business purpose for keeping confidential and the non-disclosure of which in such registration statement would be expected, in the reasonable determination of the Company’s board of directors to cause such registration statement to fail to comply with applicable disclosure requirements, in each case commencing on the day the Company notifies the Holders that they are required, because of the determination described above, to suspend offers and sales of Registrable Securities and ending on the earlier of (1) the date upon which the material non-public information resulting in the Blackout Period is disclosed to the public or ceases to be material and (2) such time as the Company notifies the selling Holders that sales pursuant to such Registration Statement or a new or amended Registration Statement or prospectus may resume; provided, however, that the aggregate of all Blackout Periods shall not exceed twenty (20) consecutive Trading Days or more than forty-five (45) Trading Days in any twelve (12) month period (except for suspension of the use of any Registration Statement on Form S-1 in connection with the filing of a post-effective amendment to the Registration Statement to update the prospectus therein in connection with the filing of the Company’s Annual Report on Form 10-K or any other fundamental change, which Blackout Period may extend for the amount of time reasonably required to respond to comments of the staff of the Commission (the “Staff”) on such amendment; provided that (and as a condition to any such extension) the Company shall file any such post-effective amendment on the date of filing of the Company’s Annual Report on Form 10-K and the Company shall use its reasonable best effort to cause any such post-effective amendment to become effective as soon as possible after the filing thereof).
“Business Day” means any day of the year, other than a Saturday, Sunday, or other day on which banks in the State of New York are required or authorized to close.
2
“Commission” means the U. S. Securities and Exchange Commission or any other federal agency at the time administering the Securities Act.
“Common Stock” means the common stock, par value $0.0001 per share, of the Company and any and all shares of capital stock or other equity securities of: (i) the Company which are added to or exchanged or substituted for the Common Stock by reason of the declaration of any stock dividend or stock split, the issuance of any distribution or the reclassification, readjustment, recapitalization or other such modification of the capital structure of the Company; and (ii) any other corporation, now or hereafter organized under the laws of any state or other governmental authority, with which the Company is merged, which results from any consolidation or reorganization to which the Company is a party, or to which is sold all or substantially all of the shares or assets of the Company, if immediately after (and as a result of) such merger, consolidation, reorganization or sale, the Holders own equity securities of such other corporation.
“Effective Date” means the date of the final closing of the Offering.
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and the rules and regulations of the Commission promulgated thereunder.
“Excluded Registrable Securities” shall have the meaning set forth in Section 3(d)(i) of this Agreement.
“Family Member” means (a) with respect to any individual, such individual’s spouse, any descendants (whether natural or adopted), any trust all of the beneficial interests of which are owned by any of such individuals or by any of such individuals together with any organization described in Section 501(c)(3) of the Internal Revenue Code of 1986, as amended, the estate of any such individual, and any corporation, association, partnership or limited liability company all of the equity interests of which are owned by those above described individuals, trusts or organizations and (b) with respect to any trust, the owners of the beneficial interests of such trust.
“Holder” means (i) each Purchaser or any of such Purchaser’s respective successors and assignees who acquire rights in accordance with this Agreement with respect to any Registrable Securities directly or indirectly from a Purchaser or from any assignee thereof; (ii) each Broker or any of such Broker’s respective successors and assignees who acquire rights in accordance with this Agreement with respect to any Registrable Securities directly or indirectly from a Broker or from any assignee; (iii) each holder of Registrable Pre-Merger Shares or its respective successors and assignees who acquire rights in accordance with this Agreement with respect to any Registrable Securities directly or indirectly from such holder or from any assignee thereof; (iv) each holder of Pre-Merger Warrant Shares or its respective successors and assignees who acquire rights in accordance with this Agreement with respect to any Registrable Securities directly or indirectly from such holder or from any assignee thereof, and (v) each holder of Note Conversion Shares or its respective successors and assignees who acquire rights in accordance with this Agreement with respect to any Registrable Securities directly or indirectly from such holder or from any assignee thereof.
3
“Majority Holders” means, at any time, Holders of a majority of the Registrable Securities then issuable and/or outstanding. For purposes of this Agreement, a person is deemed to be a holder of Shares or Registrable Securities whenever such person owns of record, or owns beneficially through a “street name” holder, such Shares or Registrable Securities or securities upon exercise, conversion or exchange of which such Shares or Registrable Securities are issuable.
“National Securities Exchange” means each of the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market, the New York Stock Exchange, the NYSE American and any other U.S. national securities exchange which the Majority Holders identify in writing as a National Securities Exchange for purposes hereof (and, in each case, a successor U.S. national securities exchange thereto).
“Note Conversion Shares” means shares of Common Stock issued at the effective time of the Merger upon conversion (or in exchange for securities of Adaptin issued upon conversion) of the outstanding Bridge Notes of Adaptin, in the number indicated next to each Registrable Adaptin Stockholder’s name on Schedule 4 hereto, and any shares of Common Stock issued or issuable with respect to such shares upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
“Offering Shares” means the shares of Common Stock issued to the Purchasers pursuant to the Subscription Agreements, and any shares of Common Stock issued or issuable with respect to such shares upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
“Offering Warrant Shares” means the A Warrant Shares and the B Warrant Shares.
“Piggyback Registration” shall have the meaning set forth in Section 3(d)(i) of this Agreement.
“Placement Agent Warrant Shares” means the shares of Common Stock issued or issuable upon exercise of the Placement Agent Warrants, and any shares of Common Stock issued or issuable with respect to such shares upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
“Placement Agent Warrants” shall have the meaning set forth in the Subscription Agreement.
“Pre-Merger Warrant Shares” means the shares of Common Stock issued or issuable upon exercise of the Pre-Merger Warrants, and any shares of Common Stock issued or issuable with respect to such shares upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
The terms “register,” “registered,” and “registration” refer to a registration effected by preparing and filing a registration statement in compliance with the Securities Act, and such registration statement becoming effective.
“Prospectus” means the prospectus included in a Registration Statement (including, without limitation, a prospectus that includes any information previously omitted from a prospectus filed as part of an effective registration statement in reliance upon Rule 430A promulgated by the Commission pursuant to the Securities Act), as amended or supplemented by any prospectus supplement, with respect to the terms of the offering of any portion of the Registrable Securities covered by a Registration Statement, and all other amendments and supplements to the Prospectus, including post-effective amendments, and all material incorporated by reference or deemed to be incorporated by reference in such Prospectus.
4
“Registrable Pre-Merger Shares” means shares of Common Stock held by stockholders of the Company prior to the Merger and remaining outstanding immediately following the effective time of the Merger in the number indicated next to each Registrable Pre-Merger Stockholder’s name on Schedule 3 hereto, and any shares of Common Stock issued or issuable with respect to such shares upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing.
“Registrable Pre-Merger Stockholder” means a person holding Registrable Pre-Merger Shares immediately prior to the effective time of the Merger.
“Registrable Securities” means the following held by a Holder: (a) the Offering Shares, (b) the Offering Warrant Shares, (c) the Pre-Merger Warrant Shares, (d) the Note Conversion Shares, (e) the Registrable Pre-Merger Shares, (f) the Placement Agent Warrant Shares and (g) other shares of Restricted Common Stock held by the Holders, hereinafter acquired or issuable in respect of the foregoing shares of Common Stock by way of conversion, dividend, stock-split, distribution or exchange, merger, consolidation, recapitalization or reclassification or similar transaction. Such securities shall cease to be Registrable Securities hereunder with respect to any Holder on the earlier of (x) the date on which they have been sold or otherwise transferred other than in a transaction in which the purchaser or other transferee acquires rights hereunder in accordance herewith, and (y) the date on which Rule 144 becomes available for a Holder, permitting such Holder to sell within a ninety (90)-day period all the Registrable Securities held by such Holder without volume or manner of sale restrictions.
“Registration Default Period” means the period beginning on the date on which any Registration Event occurs and ending on the date on which such Registration Event is cured, inclusive.
“Registration Effectiveness Date” means the date that is the earlier of (a) five (5) Trading Days after the Company is notified by the Commission that the Initial Registration Statement will not be reviewed or is no longer subject to further review and comment, and (b) one hundred twenty (120) calendar days after the Effective Date (plus, if applicable, a number of days equal to the Tolling Period).
“Registration Event” means the occurrence of any of the following events:
(a) the Company fails to file with the Commission the Initial Registration Statement on or before the Registration Filing Date;
(b) the Initial Registration Statement is not declared effective by the Commission on or before the Registration Effectiveness Date;
(c) any New Registration Statement has not been filed by the New Registration Statement Filing Deadline;
(d) any New Registration Statement has not been declared effective by the New Registration Statement Effectiveness Deadline;
5
(e) after the SEC Effective Date, any Registration Statement ceases for any reason to remain effective or the Holders of any of the Registrable Securities are otherwise not permitted to utilize the Prospectus therein to resell the Registrable Securities covered thereby for a period of more than five (5) consecutive Trading Days, except for Blackout Periods permitted herein;
(f) following the inclusion for quotation on an Approved OTC Market, the Registrable Securities, if issued and outstanding, are not listed or included for quotation on an Approved OTC Market or a National Securities Exchange, or trading of the Common Stock is suspended or halted on the Approved OTC Market, which at the time constitutes the principal market for the Common Stock, for more than three (3) full, consecutive Trading Days (other than as a result of suspension or halt of substantially all trading in equity securities (including the Common Stock)) on such Approved OTC Market; or
(g) following the inclusion for quotation on a National Securities Exchange, the Registrable Securities, if issued and outstanding, are not listed on a National Securities Exchange, or trading of the Common Stock is suspended or halted on the National Securities Exchange, which at the time constitutes the principal market for the Common Stock, for more than three (3) full, consecutive Trading Days (other than as a result of suspension or halt of substantially all trading in equity securities (including the Common Stock)) on the National Securities Exchange.
“Registration Filing Date” means the date that is sixty (60) calendar days after the Effective Date, provided that if such sixty- (60-) day period extends beyond February 14, 2025, such period shall be tolled as of February 14, 2025, and resume when the audited financial statements of the Company for the fiscal year ending December 31, 2024, are issued, or March 31, 2025, whichever is earlier (the number of days of the sixty- (60) day period tolled as of February 14, 2025, the “Tolling Period”), and provided further that the Tolling Period, if applicable, shall be a minimum of 14 calendar days.
“Registration Statement” means any registration statement that the Company is required to file or files pursuant to Section 3(a) or 3(d) of this Agreement to register the Registrable Securities and any successor registration statement.
“Restricted Common Stock” means any shares of Common Stock that are subject to resale restrictions pursuant to the Securities Act and the rules and regulations promulgated thereunder, including, but not limited to, securities: (1) acquired directly or indirectly from the issuer or an affiliate of the issuer in unregistered offerings such as private placements; (2) acquired through an employee stock benefit plan or as compensation for professional services; or (3) considered “restricted securities” under Rule 144. For purposes of clarity Restricted Common Stock does not include Common Stock that is restricted solely as a result of contractual restrictions, including but not limited to lock-up or similar contractual agreements.
“Rule 144” means Rule 144 promulgated by the Commission under the Securities Act, as such rule may be amended or supplemented from time to time, or any similar successor rule that may be promulgated by the Commission.
6
“Rule 145” means Rule 145 promulgated by the Commission under the Securities Act, as such rule may be amended or supplemented from time to time, or any similar successor rule that may be promulgated by the Commission.
“Rule 415” means Rule 415 promulgated by the Commission under the Securities Act, as such rule may be amended or supplemented from time to time, or any similar successor rule that may be promulgated by the Commission.
“SEC Effective Date” means the date the Registration Statement is declared effective by the Commission.
“Securities Act” means the Securities Act of 1933, as amended, or any similar federal statute promulgated in replacement thereof, and the rules and regulations of the Commission promulgated thereunder, all as the same shall be in effect at the time.
“Trading Day” means any day on which the Approved OTC Market or National Securities Exchange that at the time constitutes the principal securities market for the Common Stock, is open for general trading of securities (or if there is no Approved OTC Market or National Securities Exchange that at the time constitutes the principal securities market for the Common Stock, then any day on which the New York Stock Exchange is open for general trading of securities).
2. Term. This Agreement shall terminate with respect to each Holder on the earlier of: (i) the date that is five (5) years from the SEC Effective Date, and (ii) the date on which no Registrable Securities are outstanding (the “Term”). Notwithstanding the foregoing, Section 3(b), Section 6, Section 8, Section 9 and Section 10 shall survive the termination of this Agreement.
3. Registration.
(a) Registration on Form S-1. The Company shall prepare and file with the Commission a Registration Statement on Form S-1, or any other form for which the Company then qualifies or which counsel for the Company shall deem appropriate and which form shall be available for the resale by the Holders of all of the Registrable Securities on a delayed or continuous basis (including in stock exchange transactions and underwritten offerings) (the “Initial Registration Statement”), and the Company shall (i) make the initial filing of the Initial Registration Statement with the Commission no later than the Registration Filing Date, (ii) use its commercially reasonable efforts to cause the Initial Registration Statement to be declared effective no later than the Registration Effectiveness Date and (iii) use its commercially reasonable efforts to keep such Registration Statement continuously effective (including by filing a new Registration Statement if the initial Registration Statement expires) for a period of five (5) years after the SEC Effective Date or for such shorter period ending on the first date on which (a) there no longer any outstanding Registrable Securities and (b) the availability of Rule 144 for the holders to sell all of the Registrable Securities without volume limitations or other manner of sale restrictions within a 90-day period (the “Effectiveness Period”). Any Registration Statement shall contain the “Plan of Distribution” section in substantially the form thereof attached as Exhibit A hereto. Upon the Company becoming eligible to register the Registrable Securities for resale by the Holders on Form S-3, the Company shall use commercially reasonable efforts to amend the Registration Statement to a Registration Statement on Form S-3 or file a Registration Statement on Form S-3 in substitution of the Registration Statement as initially filed as soon as reasonably practicable thereafter (provided that the Company shall maintain the effectiveness of the Registration Statement then in effect until such time as a Registration Statement (or post-effective amendment) on Form S-3 covering such Registrable Securities has been declared effective by the Commission). The Company shall be entitled to suspend sales of Registrable Securities pursuant to a Registration Statement and the use of any related prospectus during a Blackout Period for the reasons and time periods set forth in the definition thereof. The Company shall use its commercially reasonable efforts within sixty (60) calendar days after the SEC Effective Date, or within ten Business Days after the first date that is permitted by the Commission to (the “New Registration Statement Filing Deadline”), register for resale as many of the Reduction Securities as the Commission will permit (pro rata among the Holders of such Reduction Securities) using one or more Registration Statements (any such Registration Statement, a “New Registration Statement”) that it is then entitled to use, and to cause such registration statement(s) to become effective as soon as practicable(the “New Registration Statement Effectiveness Deadline”), until all of the Reduction Securities have been so registered; provided, however, that the Company shall not be required to register such Reduction Securities during a Blackout Period. The Company shall use its commercially reasonable efforts to cause each Registration Statement to be declared effective under the Securities Act, as soon as possible, and shall use its commercially reasonable efforts to keep such Registration Statement continuously effective (including by filing an additional Registration Statement if the Initial Registration Statement expires), and under the Securities Act during the Effectiveness Period. Notwithstanding the foregoing, the Company shall be entitled to suspend the effectiveness of any Registration Statement at any time prior to the expiration of the Effectiveness Period for the reasons and time periods permitted during a Blackout Period. No liquidated damages shall accrue or be payable to any Holder pursuant to Section 3(b) below with respect to any Registrable Securities that are excluded by reason of (i) the Staff limiting the number of Registrable Securities that may be sold pursuant to a registration statement (provided that the Company continues to use commercially reasonable efforts to register such Reduction Securities for resale by other available means as set forth herein) or (ii) such Holder failing to provide to the Company information concerning the Holder and the manner of distribution of the Holder’s Registrable Securities that is required by the SEC or in response to SEC comments to be disclosed in a registration statement utilized in connection with the registration of Registrable Securities. Notwithstanding anything herein to the contrary, if the Commission limits the Company’s ability to file, or prohibits or delays the filing of a new registration statement, the Company’s compliance with such limitation, prohibition or delay solely to the extent of such limitation, prohibition or delay shall not be deemed a failure by the Company to use commercially reasonable efforts as set forth above or elsewhere in this Agreement and shall not require the payment of any liquidated damages by the Company under this Agreement.
7
(b) Partial Liquidated Damages. If a Registration Event occurs, then the Company will make payments to each Holder of Registrable Securities, as partial liquidated damages to such Holder by reason of the Registration Event (but without limiting the rights and remedies of such Holder, including injunctive and other equitable relief), a cash sum calculated at a rate of twelve percent (12%) per annum (for the duration of the applicable Registration Default Period) of the total of the following, to the extent applicable to such Holder: (i) if the Holder is a Holder of Note Conversion Shares, the product of $3.30 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Note Conversion Shares held by or issuable to such Holder as of the date of such Registration Event, (ii) if the Holder is a Holder of Offering Shares, A Warrant Shares, Pre-Merger Warrant Shares, Registrable Pre-Merger Shares or Placement Agent Warrant Shares, the product of $4.40 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Offering Shares, A Warrant Shares, Pre-Merger Warrant Shares, Registrable Pre-Merger Shares or Placement Agent Warrant Shares, as the case may be, held by or issuable to such Holder as of the date of such Registration Event, or (iii) if the Holder is a Holder of B Warrant Shares, the product of $6.60 (as adjusted for stock splits, stock dividends, combinations, recapitalizations or similar events) multiplied by the number of Note Conversion Shares held by or issuable to such Holder as of the date of such Registration Event, but in each case, only with respect to such Holder’s Registrable Securities that are affected by such Registration Event and only for the applicable Registration Default Period. Notwithstanding the foregoing, (i) the maximum amount of liquidated damages that may be paid by the Company to any Holder pursuant to this Section 3(b) shall be an amount equal to five percent (5%) of the applicable foregoing amounts described in clauses (i) and (ii) in the preceding sentence with respect to such Holder’s Registrable Securities that are affected by all Registration Events in the aggregate, and (ii) no penalties shall accrue with respect to any Registrable Securities removed from the Registration Statement in response to a comment from the Staff limiting the number of shares of Registrable Securities which may be included in the Registration Statement, after they cease to be Registrable Securities, or, with respect to any Registration Event defined by clause (b) or (d) of the definition of “Registration Event” set forth above, are held by any Holder who delayed or failed to provide information reasonably requested by the Company in connection with the preparation of the applicable Registration Statement. For clarity, and by way of example, if the sum of clauses (i) and (ii) for a specified Holder in the first sentence of this Section 3(b) is $10,000,000, liquidated damages payable by the Company to such Holder by reason of one or more Registration Events affecting all Registrable Securities of such Holder would accrue at a rate of twelve percent (12%) per annum (for the duration of the applicable Registration Default Period) until such time that all liquidated damages payable to such Holder reached a cap of $500,000 in the aggregate for all Registration Events. Each payment pursuant to this Section 3(b) shall be due and payable in cash in arrears within five (5) days after the end of each full 30-day period of the Registration Default Period until the termination of the Registration Default Period and within five (5) days after such termination. Until the maximum amount of liquidated damages is paid, such payments shall constitute the Holder’s sole and exclusive remedy for money damages in respect of any Registration Event; provided, for the avoidance of doubt, that the foregoing shall not affect any Holder’s right, at any time, to seek or obtain injunction or other equitable relief in respect of any Registration Event. The Registration Default Period shall terminate upon the earlier of such time as the Registrable Securities that are affected by the Registration Event cease to be Registrable Securities or (i) the filing of the Registration Statement in the case of clause (a) of the definition of Registration Event, (ii) the SEC Effective Date in the case of clause (b) of the definition of Registration Event, (iii) the ability of the Holders to effect sales pursuant to the Registration Statement in the case of clause (c) of the definition of Registration Event, and (iv) the listing or inclusion and/or trading of the Common Stock on an Approved OTC Market or National Securities Exchange, as the case may be, in the case of clause (d) or clause (e) of the definition of Registration Event; provided, that in the event of a cure of one or more of the Registration Events described in clauses (i)-(iv) above when a separate Registration Event shall be continuing, the Registration Default Period shall continue until all such Registration Events have ceased. The amounts payable as liquidated damages pursuant to this Section 3(b) shall be payable in lawful money of the United States.
8
(c) Other Limitations. Notwithstanding the registration obligations set forth in Section 2(a), if the Staff informs the Company that all of the Registrable Securities cannot, as a result of the application of Rule 415 under the Securities Act, be registered for resale as a secondary offering on a single Registration Statement, the Company agrees to promptly inform each of the Holders thereof and file and an amendment to the Registration Statement as required by the Staff, covering the maximum number of Registrable Securities permitted to be registered by the Staff as a secondary offering; provided, however, that prior to filing such amendment, the Company shall be obligated to use reasonably diligent efforts to advocate with the Staff for the registration of all of the Registrable Securities in accordance with applicable guidance of the Staff , including without limitation, Compliance and Disclosure Interpretation 612.09. Notwithstanding any other provision of this Agreement and subject to the payment of liquidated damages pursuant to Section 2(c), if the Staff limits the number of Registrable Securities permitted to be registered on a particular Registration Statement as a secondary offering (and notwithstanding that the Company used reasonably diligent efforts to advocate with the Staff for the registration of all or a greater portion of Registrable Securities), the Company shall remove from the Registration Statement such number of Registrable Securities as specified by the Commission on behalf of all of the holders of Registrable Securities from the Registrable Securities in the following order: (i) first from the Placement Agent Warrant Shares, on a pro rata basis among the holders thereof; (ii) second from the Registrable Pre-Merger Shares, on a pro rata basis among the holders thereof; (iii) third from the Offering Shares, the Offering Warrant Shares, the Pre-Merger Warrant Shares and the Note Conversion Shares, on a pro rata basis among the holders thereof (any such eliminated shares, the “Reduction Securities”). In the event of a cutback hereunder, the Company shall give the Holder at least three (3) Trading Days prior written notice along with the calculations as to such Holder’s allotment. In the event the Company amends the Initial Registration Statement in accordance with the foregoing, the Company will file with the Commission, as promptly as allowed by the Staff or applicable guidance of the Staff provided to the Company or to registrants of securities in general, one or more New Registration Statements on such other form available to register for resale the Reduction Securities.
(d) Piggyback Registrations.
(i) With respect to any Registrable Securities not otherwise included in a Registration Statement pursuant to Section 3(a) as a result of any limitation imposed by the Staff, or otherwise (the “Excluded Registrable Securities”), whenever the Company proposes to register (including, for this purpose, a registration effected by the Company for other shareholders) any of its securities under the Securities Act (other than pursuant to (i) a Registration Statement pursuant to Section 3(a) hereof or (ii) registration pursuant to a registration statement on Form S-4 or S-8 or any successor forms thereto), and the registration form to be used may be used for the registration of Registrable Securities, the Company will give written notice to each holder of Excluded Registrable Securities of its intention to effect such a registration and will, subject to the provisions of Subsection 3(d)(ii) hereof, and to the extent permitted by the Staff, include in such registration all Excluded Registrable Securities with respect to which the Company has received a written request for inclusion therein within fifteen (15) days after the receipt of the Company’s notice (a “Piggyback Registration”).
9
(ii) If a Piggyback Registration is an underwritten secondary registration on behalf of holders of the Company’s securities, and the managing underwriters advise the Company in writing that in their opinion the number of securities requested to be included in such registration exceeds the number which can be sold in such offering without adversely affecting the marketability of the offering, the Company will include in such registration a pro rata share of Excluded Registrable Securities requested to be included in such Registration Statement as calculated by dividing the number of Excluded Registrable Securities requested to be included in such Registration Statement by the number of the Company’s securities requested to be included in such Registration Statement by all selling security holders. In such event, the holder of Excluded Registrable Securities shall continue to have registration rights under this Agreement with respect to any Excluded Registrable Securities not so included in, and sold pursuant to, such Registration Statement.
(iii) Notwithstanding the foregoing, if, at any time after giving a notice of Piggyback Registration and prior to the effective date of the Registration Statement filed in connection with such registration, the Company shall determine for any reason not to register or to delay registration of such securities, the Company may, at its election, give written notice of such determination to each record holder of Excluded Registrable Securities and, following such notice, (i) in the case of a determination not to register, shall be relieved of its obligation to register any Excluded Registrable Securities in connection with such registration, and (ii) in the case of a determination to delay registering, shall be permitted to delay registering any Excluded Registrable Securities for the same period as the delay in registering such other securities.
4. Registration Procedures. The Company will keep each Holder reasonably advised as to the filing and effectiveness of any Registration Statement. At its expense with respect to any Registration Statement, the Company will:
(a) subject to compliance with Section 5(b), prepare and file with the Commission with respect to the Registrable Securities, any Registration Statement in accordance with Section 3(a) hereof, and use its reasonable efforts to cause such Registration Statement to become effective as soon as possible after the filing thereof and to remain effective (with the Prospectus included therein available for the resale of the Registrable Securities) for the Effectiveness Period and file the Prospectus pursuant to Rule 424(b) under the Securities Act within one (1) Trading Day following the date the Registration Statement is declared effective;
(b) not name any Holder in any Registration Statement as an underwriter without that Holder’s prior written consent;
(c) if any Registration Statement or any post-effective amendment thereto is subject to review by the Commission, promptly respond to all comments, diligently pursue resolution of any comments to the satisfaction of the Commission and file all amendments and supplements to such Registration Statement as may be required to respond to comments from the Commission and otherwise to enable such Registration Statement to be declared effective;
(d) during the Effectiveness Period, prepare and file with the Commission such amendments and supplements to any Registration Statement as may be necessary to keep such Registration Statement continuously effective, current and up-to-date for the applicable time period required hereunder and, if applicable, file any Registration Statement pursuant to Rule 462(b) under the Securities Act; and cause the related Prospectus to be supplemented by any required prospectus supplement, and as so supplemented to be filed pursuant to Rule 424 (or any similar provisions then in force) promulgated under the Securities Act;
10
(e) not less than four (4) Trading Days prior to filing any Registration Statement or any related prospectus or any amendment or supplement thereto, furnish to the Holders (and/or, if so specified by any Holder, legal counsel to such Holder) copies of or a link to all such documents proposed to be filed (other than those incorporated by reference and those amendments and supplements which are solely composed of a cover page and the form of one or more of the Company’s reports previously filed under the Exchange Act) and duly consider in good faith any comments timely received from the Holders (or from legal counsel to any such Holder, as applicable);
(f) furnish, without charge, to each Holder of Registrable Securities covered by such Registration Statement (i) a reasonable number of copies of such Registration Statement (including any exhibits thereto other than exhibits incorporated by reference), each amendment and supplement thereto as such Holder may reasonably request, (ii) such number of copies of the Prospectus included in such Registration Statement (including each preliminary prospectus and any prospectus filed pursuant to Rule 424 under the Securities Act) as such Holders may reasonably request, in conformity with the requirements of the Securities Act, and (iii) such other documents as such Holder may reasonably require to consummate the disposition of the Registrable Securities owned by such Holder, but only during the Effectiveness Period; provided that the Company shall have no obligation to furnish any document pursuant to this clause that is available on the Electronic Data Gathering, Analysis, and Retrieval (“EDGAR”) system;
(g) use its reasonable efforts to register or qualify the securities covered by such Registration Statement under such other applicable securities laws of such jurisdictions within the United States, including “blue sky” laws, as any Holder of Registrable Securities covered by such Registration Statement reasonably requests and as may be reasonably necessary for the marketability of the Registrable Securities and do any and all other acts and things reasonably necessary to enable such Holder to consummate the disposition in such jurisdictions of the Registrable Securities owned by such Holder; provided, that the Company shall not be required to (i) qualify generally to do business in any jurisdiction where it would not otherwise be required to qualify but for this paragraph or (ii) consent to general service of process in any such jurisdiction where it has not already done so;
(h) as promptly as practicable after becoming aware of any event, notify each Holder of Registrable Securities at any time when a Prospectus relating thereto is required to be delivered under the Securities Act, of the happening of any event that will, after the occurrence of such event, cause the Prospectus included in such Registration Statement, if not amended or supplemented, to contain an untrue statement of a material fact or an omission to state a material fact required to be stated therein or necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading and the Company shall promptly thereafter prepare and furnish to such Holder a supplement or amendment to such Prospectus (or, if a Registration Statement is on Form S-3, prepare and file appropriate reports under the Exchange Act) so that, as thereafter delivered to the purchasers of such Registrable Securities, such Prospectus shall not contain an untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading, except in the event of a Blackout Period, in which case no supplement or amendment need be furnished (or Exchange Act filing made) until the termination of such Blackout Period;
11
(i) comply, and continue to comply during the Effectiveness Period, in all material respects with the Securities Act and the Exchange Act and with all applicable rules and regulations of the Commission with respect to the disposition of all securities covered by such Registration Statement;
(j) as promptly as practicable after becoming aware of such event, notify each Holder of Registrable Securities being offered or sold pursuant to any Registration Statement of the issuance by the Commission or any other federal or state governmental authority of any stop order or other suspension of effectiveness of the Registration Statement or the initiation of any proceedings for that purpose;
(k) use its reasonable best efforts to obtain all other approvals, consents, exemptions or authorizations from such governmental agencies or authorities as may be necessary to enable the Holders and underwriters to consummate the disposition of Registrable Securities;
(l) enter into customary agreements (including any underwriting agreements in customary form, including any representations and warranties and lock-up provisions therein), and take such other actions as may be reasonably required in order to expedite or facilitate the disposition of Registrable Securities pursuant to any Piggyback Registration;
(m) use its reasonable best efforts to furnish, or cause to be furnished, on the date that such Registrable Securities are delivered to the underwriters for sale, if such securities are being sold through underwriters in a firm commitment public offering, (i) an opinion, dated as of such date, of the counsel representing the Company for the purposes of such registration, in form and substance reasonably acceptable to the managing underwriter, addressed to the underwriters and (ii) a “comfort” letter dated as of such date, from the independent certified public accountants of the Company, in form and substance reasonably acceptable to the managing underwriter, addressed to the underwriters;
(n) use its reasonable best efforts to comply with all applicable rules and regulations of the Commission and make available to its shareholders, as soon as reasonably practicable, but no later than sixteen (16) months after the effective date of any Registration Statement (as defined in Rule 168(c) under the Securities Act), an earnings statement that satisfies the provisions of Section 11(a) of the Securities Act and Rule 158 thereunder;
(o) provide officers’ certificates and other customary closing documents, if applicable;
(p) use its reasonable best efforts to cause the shares of Common Stock to be, and remain, quoted on an Approved OTC Market unless listed on a National Securities Exchange;
(q) cooperate with each Holder and each underwriter participating in the disposition of such Registrable Securities and underwriters’ counsel in connection with any filings required to be made with the Financial Industry Regulatory Authority (“FINRA”); and
12
(r) use its commercially reasonable efforts to:
(i) cause a FINRA-registered broker-dealer (the “Market Maker”) to (A) sponsor the Common Stock, (B) file with FINRA, no later than fifteen (15) days after the Registration Statement is initially filed with the Commission, a Form 211 together with the required documentation and information in connection therewith, (C) respond promptly to any requests from FINRA for additional information in connection therewith (and the Company will provide reasonable cooperation to the Market-Maker in fulfillment thereof), and (D) clear the Market Maker by FINRA to initiate quotation of the Common Stock on an Approved OTC Market at the earliest practicable date after the filing of the Form 211, and use its reasonable best efforts to cause a second Market Maker to register with FINRA in respect of the Common Stock as soon thereafter as possible; and
(ii) cause the Common Stock to be DTC-, DWAC- and DRS-eligible no later than the initiation of quotation of the Common Stock on an Approved OTC Market.
(s) in the event of an underwritten public offering by the Company, cause appropriate officers as are reasonably requested by a managing underwriter or investment bank to participate in a “road show” or similar marketing effort being conducted by such underwriter with respect to such underwritten public offering;
(t) provide a transfer agent and registrar that is registered with the Commission and a participant in DTC’s Fast Automated Securities Transfer Program, which shares may be a single entity, for the shares of Common Stock at all times, and cooperate with the Holders to facilitate the timely preparation and delivery of the Registrable Securities to be delivered to a transferee pursuant to a resale of Registrable Securities pursuant to a Registration Statement (whether electronically or in certificated form) which Registrable Securities shall be free, to the extent permitted by the applicable Subscription Agreement and applicable law, of all restrictive legends, and to enable such Registrable Securities to be in such denominations and registered in such names as any such Holders may request;
(u) cooperate with the Holders of Registrable Securities being offered pursuant to any Registration Statement to issue and deliver, or cause its transfer agent to issue and deliver, evidence of book-entry positions representing Registrable Securities to be offered pursuant to such Registration Statement within a reasonable time after the delivery of evidence of book-entry positions representing the Registrable Securities to the transfer agent or the Company, as applicable, and enable such positions to be in such denominations or amounts as the Holders may reasonably request and registered in such names as the Holders may request;
(v) notify the Holders, the Brokers and their counsel as promptly as reasonably possible and (if requested by any such person) confirm such notice in writing no later than one (1) Trading Day following the day: (i)(A) when a Prospectus or any prospectus supplement or post-effective amendment to a Registration Statement is proposed to be filed (other than those incorporated by reference and those amendments and supplements which are solely composed of a cover page and the form of one or more of the Company’s reports previously filed under the Exchange Act); (B) when the Commission notifies the Company whether there will be a “no review,” “review” or a “completion of a review” of such Registration Statement and whenever the Commission comments in writing on such Registration Statement (in which case the Company shall provide true and complete copies thereof and all written responses thereto to each of the Holders that pertain to the Holders as a selling stockholder, but not information which the Company believes would constitute material non-public information); and (C) with respect to the Registration Statement or any post-effective amendment, when the same has been declared effective, provided, however, that such notice under this clause (C) shall be delivered to each Holder; (ii) during the Effectiveness Period, of any request by the Commission or any other federal or state governmental authority for amendments or supplements to a Registration Statement or prospectus or for additional information that pertains to the Holders as selling stockholders; or (iii) during the Effectiveness Period, of the receipt by the Company of any notification with respect to the suspension of the qualification or exemption from qualification of any of the Registrable Securities for sale in any jurisdiction, or the initiation or threatening of any proceeding for such purpose;
13
(w) during the Effectiveness Period, refrain from bidding for or purchasing any Common Stock or any right to purchase Common Stock or attempting to induce any person to purchase any such security or right if such bid, purchase or attempt would in any way limit the right of the Holders to sell Registrable Securities by reason of the limitations set forth in Regulation M under the Exchange Act;
(x) use its reasonable best efforts to avoid the issuance of, or, if issued, obtain the withdrawal of (i) any order stopping or suspending the effectiveness of a Registration Statement or suspending or preventing the use of any related prospectus, or (ii) any suspension of the qualification (or exemption from qualification) of any of the Registrable Securities for sale in any jurisdiction, at the earliest practicable moment;
(y) use its reasonable best efforts to assist a Holder in facilitating any sales (including but not limited to private sales) or other transfers of Registrable Securities by, among other things, providing officers’ certificates and other customary closing documents reasonably requested by a Holder without charge to the Holder (but the Holder shall be responsible for any third-party expenses and for clarity, such certificates and other customary closing documents shall not be deemed to include opinions or negative assurance letters from Company counsel or “comfort letters” delivered by the Company’s independent registered public accounting firm);
(z) if required by the Financial Industry Regulatory Authority, Inc. Corporate Financing Department, promptly effect, or cause to have effected, a filing with the FINRA Corporate Financing Department pursuant to FINRA Rule 5110 (or successor thereto) with respect to the public offering contemplated by resales of securities under the Registration Statement (an “Issuer Filing”), pay the filing fee required by such Issuer Filing, and use its reasonable best efforts to pursue the Issuer Filing until FINRA issues a letter confirming that it does not object to the terms of the offering contemplated by the Registration Statement, and otherwise cooperate with any broker-dealer through which a Holder proposes to resell its Registrable Securities in effecting a filing with the FINRA Corporate Financing Department pursuant to FINRA Rule 5110 (or successor thereto), as requested by any such Holder, and the Company shall pay the filing fee required by such filing within two (2) Trading Days of the request therefor;
14
(aa) (i) cause legal counsel to the Company, at the Company’s expense, (a) to issue to the transfer agent for the Common Stock, within one (1) Trading Day after the SEC Effective Date, a “blanket” legal opinion in customary form to the effect that the Registrable Securities covered by the Registration Statement have been registered for resale under the Securities Act and, if such counsel has received a signed certificate in the form attached as Exhibit B hereto (a “Legend Removal Certificate”) from the holder of the Registrable Securities, may then be reissued without any legend or restriction relating to their status as “restricted securities” as defined in Rule 144 (“Legend Removal Shares”), or, otherwise, may then be reissued without any legend or restriction relating to their status as “restricted securities” as defined in Rule 144 upon resale pursuant to such registration statement; and (b) promptly to amend such opinion to cause the Registrable Securities to be Legend Removal Shares after later receipt of a Legend Removal Certificate from the Holder, and (ii) cause the transfer agent for the Common Stock to issue such Registrable Securities without any such legend within three (3) Trading Days after the transfer agent’s receipt of such legal opinion with respect to Legend Removal Shares or otherwise within three (3) Trading Days after the transfer agent’s receipt of evidence in customary form that the Registrable Securities have been sold pursuant to an effective resale registration statement under the Securities Act, in either case via DWAC or as otherwise requested by the Holder; and
(bb) take all other commercially reasonable actions necessary to enable, expedite or facilitate the Holders to dispose of the Registrable Securities by means of any Registration Statement contemplated hereby during the Term.
5. Obligations of the Holders.
(a) At any time, and from time to time, after the Registration Effectiveness Date, the Company may notify one or more of the Holders (in each case, the “Specified Holders”) in writing (each, a “Suspension Notice”) of the happening of: (i) any event of the kind described in Section 4(h) or (j); (ii) any Blackout Period; or (iii) only with respect to a Holder who is an “insider” covered by such program, any suspension by the Company, pursuant to a written insider trading policy adopted by the Company’s Board of Directors, of the ability of all “insiders” covered by such program to transact in the Company’s securities because of the existence of material non-public information (each, a “Suspension Event”). Upon receipt of any Suspension Notice, each Specified Holder shall as promptly as practicable discontinue disposition of such Holder’s Registrable Securities covered by the Registration Statement until such Specified Holder receives the supplemented or amended prospectus contemplated by Section 4(h), such Blackout Period shall have terminated or the restriction on the ability of “insiders” to transact in the Company’s securities is removed, as applicable. The foregoing right to suspend may be exercised by the Company for no longer than forty-five (45) Trading Days in any consecutive twelve (12)-month period, except, in the case of Holders that are subject to such policy by its terms, with respect to suspensions under the written insider trading policy adopted by the Company’s Board of Directors, (and for the avoidance of doubt, if the delay or suspension relates to a Blackout Period, the period of delay or suspension shall also count against the maximum number of days for Blackout Periods in the definition of such term). Notwithstanding anything to the contrary, the Company shall cause its transfer agent to deliver unlegended shares of Common Stock to a transferee of a Holder in accordance with any sale of Registrable Securities with respect to which a Holder has entered into a contract for sale prior to the Holder's receipt of a notice from the Company of the happening of a Blackout Period or other Suspension Event.
(b) The Holders of the Registrable Securities shall provide such information as may reasonably be requested by the Company in connection with the preparation of the Registration Statement, including amendments and supplements thereto, in order to effect the registration of any Registrable Securities under the Securities Act pursuant to Section 3(a) of this Agreement and in connection with the Company’s obligation to comply with federal and applicable state securities laws, including a completed questionnaire in the form attached to this Agreement as Annex A (a “Selling Securityholder Questionnaire”).
15
(c) Each Holder, by its acceptance of the Registrable Securities, agrees to cooperate with the Company as reasonably requested by the Company in connection with the preparation and filing of the Registration Statement hereunder, unless such Holder has notified the Company in writing of its election to exclude all of its Registrable Securities from such Registration Statement.
6. Registration Expenses. The Company shall pay all expenses arising from or incident to the performance of, or compliance with, this Agreement, including, without limitation, (i) the Commission, stock exchange, OTC Markets Group, FINRA and other registration and filing fees, (ii) rating agencies fees to the extent necessary to provide for blue sky qualification as required by Section 4(g) herein, (iii) all fees and expenses incurred in connection with complying with any securities or blue sky laws (including reasonable and documented fees, charges and disbursements of counsel in connection with blue sky qualifications of the Registrable Securities), (iv) all printing (including financial printer), messenger and delivery expenses, (v) the fees, charges and disbursements of counsel to the Company and of its independent registered public accounting firm and any other accounting and legal fees, charges and expenses incurred by the Company (including any expenses arising from any special audits or “comfort letters” required in connection with or incident to any registration), (vi) the fees, charges and disbursements of any special experts retained by the Company in connection with any registration pursuant to the terms of this Agreement, (vii) all internal expenses of the Company (including all salaries and expenses of its officers and employees performing legal or accounting duties), (viii) the fees and expenses incurred in connection with the listing of the Registrable Securities on any securities exchange, (ix) Securities Act liability insurance (if the Company elects to obtain such insurance), regardless of whether a Registration Statement filed in connection with such registration is declared effective and (x) reasonable and documented fees, charges and disbursements of a single counsel to the Holders selected by at least a majority of the Registrable Securities, in an amount not to exceed $35,000 in the aggregate per Registration Statement or Piggyback Registration; provided, that, in any underwritten registration, the Company shall have no obligation to pay any underwriting discounts, selling commissions or transfer taxes attributable to the Registrable Securities being sold by the Holders thereof, which underwriting discounts, selling commissions and transfer taxes shall be borne by such Holders. Except as provided in this Section 6 and Section 8 of this Agreement, the Company shall not be responsible for the expenses of any attorney or other advisor employed by a Holder or for any other fees, disbursements and expenses incurred by Holders not specifically agreed to in this Agreement.
7. Assignment of Rights. The rights under this Agreement shall be automatically assignable by each Purchaser to any transferee or assignee of all or any portion of the Registrable Securities (which Registrable Securities continue to constitute Restricted Common Stock following such transfer or assignment) as long as (i) such transfer or assignment is not a sale or transfer pursuant to a Registration Statement and is effected in accordance with applicable securities laws; (ii) such transferee or assignee agrees in writing to become bound by and subject to the terms of this Agreement; and (iii) such Holder notifies the Company in writing of such transfer or assignment, stating the name and address of the transferee or assignee and identifying the Registrable Securities with respect to which such rights are being transferred or assigned. The Company may not assign this Agreement or any rights or obligations hereunder without the prior written consent of the Majority Holders (other than by merger or consolidation or to a corporation which acquires the Company including by way of acquiring all or substantially all of the Company’s assets, if immediately after (and as a result of) such merger, consolidation, reorganization or sale, the Holders own equity securities of such other corporation, which shall not require such consent).
16
8. Indemnification.
(a) To the fullest extent permitted by applicable law, the Company shall, and hereby does, indemnify and hold harmless, to the fullest extent permitted by law, each Holder, its affiliates, directors, officers, stockholders, members, managers, partners, investment advisers, employees and agents, and each other person, if any, who controls or is under common control with such Holder within the meaning of Section 15 of the Securities Act (collectively, the “Holder Indemnified Parties”), against any and all losses, claims, damages, liabilities, costs, expenses, judgments, fines, penalties, charges and amounts paid in settlement (or actions or proceedings, whether commenced or threatened, in respect thereof) (collectively, “Losses”) that arise out of or are based upon any untrue statement or alleged untrue statement of any material fact contained in any registration statement prepared and filed by the Company under which Registrable Securities were registered under the Securities Act, any preliminary prospectus, free writing prospectus as defined under Rule 433(d) of the Securities Act (“Free Writing Prospectus”), any “testing-the-water” communication that is a written communication within the meaning of Rule 405 under the Securities Act (“Testing the Water Communication”), any road show communication as defined in Rule 433(h) under the Securities Act (“Road Show Communication”), final prospectus or summary prospectus contained therein, or any amendment or supplement thereto, or arise out of or are based upon any omission or alleged omission to state therein a material fact required to be stated or necessary to make the statements therein not misleading (in the case of any prospectus or amendment or supplement thereto, in light of the circumstances in which the statements were made), and the Company shall reimburse the Holder Indemnified Parties for any legal or any other expenses reasonably incurred by them in connection with investigating, defending or settling any such loss, claim, damage, liability, action or proceeding; provided, however, that the Company shall not be liable in any such case (i) to the extent, but only to the extent, that any such loss, claim, damage, liability (or action or proceeding in respect thereof) or expense arises out of or is based upon (x) an untrue statement in or omission from such registration statement, any such preliminary prospectus, Free Writing Prospectus, Testing the Water Communication, Road Show Communication, final prospectus, summary prospectus, amendment or supplement in reliance upon and in conformity with written information included in the Selling Securityholder Questionnaire, attached hereto as Annex A, furnished by a Holder or its representative (acting on such Holder’s behalf) to the Company expressly for use in the preparation thereof or (y) the failure of a Holder to comply with the covenants and agreements contained in Section 5 hereof respecting the sale of Registrable Securities. Such indemnity shall remain in full force and effect regardless of any investigation made by or on behalf of the Holder Indemnified Parties and shall survive the transfer of such shares by the Holder.
(b) As a condition to including Registrable Securities in the registration statement filed pursuant to this Agreement, each Holder agrees, severally and not jointly, to be bound by the terms of this Section 8 and to indemnify and hold harmless, to the fullest extent permitted by law, the Company, each of its directors, officers, partners, and each underwriter, if any, and each other person, if any, who controls the Company within the meaning of Section 15 of the Securities Act, against any Losses, insofar as such Losses arise out of or are based upon any untrue statement or alleged untrue statement of a material fact contained in any registration statement, any preliminary prospectus, Free Writing Prospectus, Testing the Water Communication, Road Show Communication, final prospectus, summary prospectus, amendment or supplement thereto, or arise out of or are based upon any omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein not misleading, to the extent, but only to the extent, that such untrue statement or omission is included or omitted in reliance upon and in conformity with written information included in the Selling Securityholder Questionnaire, attached hereto as Annex A, furnished by the Holder or its representative (acting on such Holder’s behalf) to the Company expressly for use in the preparation thereof, and such Holder shall reimburse the Company, and its directors, officers, partners, and any such controlling persons for any legal or other expenses reasonably incurred by them in connection with investigating, defending, or settling any such loss, claim, damage, liability, action, or proceeding; provided, however, that the indemnity obligation contained in this Section 8(b) shall in no event exceed the amount of the net proceeds received by such Holder as a result of the sale of such Holder’s Registrable Securities pursuant to such registration statement. Such indemnity shall remain in full force and effect, regardless of any investigation made by or on behalf of the Company or any such director, officer or controlling person and shall survive the transfer by any Holder of such shares.
17
(c) Promptly after receipt by an indemnified party of notice of the commencement of any action or proceeding involving a claim referred to in this Section 8 (including any governmental action), such indemnified party shall, if a claim in respect thereof is to be made against an indemnifying party, give written notice to the indemnifying party of the commencement of such action; provided, however, that the failure of any indemnified party to give notice as provided herein shall not relieve the indemnifying party of its obligations under this Section 8, except to the extent that the indemnifying party is actually prejudiced in defending such claim by such failure to give notice in any material respect. In case any such action is brought against an indemnified party, unless in the reasonable judgment of counsel to such indemnified party a conflict of interest between such indemnified party and indemnifying parties may exist or the indemnified party may have defenses not available to the indemnifying party in respect of such claim, the indemnifying party shall be entitled to participate in and to assume the defense thereof, with counsel reasonably satisfactory to such indemnified party and, after notice from the indemnifying party to such indemnified party of its election so to assume the defense thereof, the indemnifying party shall not be liable to such indemnified party for any legal or other expenses subsequently incurred by the latter in connection with the defense thereof, other than reasonable costs of investigation, unless in such indemnified party’s reasonable judgment a conflict of interest between such indemnified and indemnifying parties arises in respect of such claim or the indemnified party may have defenses not available to the indemnifying party in respect of such claim after the assumption of the defenses thereof or the indemnifying party fails to defend such claim in a diligent manner. Neither an indemnified party nor an indemnifying party shall be liable for any settlement of any action or proceeding effected without its consent (which shall not be unreasonably withheld or delayed). No indemnifying party shall, without the consent of the indemnified party, consent to entry of any judgment or enter into any settlement, which does not include as an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a release from all liability in respect of such claim or litigation or which includes any admission as to fault, culpability or failure to act on the part of such indemnified party. Notwithstanding anything to the contrary set forth herein, and without limiting any of the rights set forth above, in any event any party shall have the right to retain, at its own expense, counsel with respect to the defense of a claim. Each indemnified party shall furnish such information regarding itself or the claim in question as an indemnifying party may reasonably request in writing and as shall be reasonably required in connection with defense of such claim and litigation resulting therefrom.
(d) If an indemnifying party does not or is not permitted to assume the defense of an action pursuant to Section 8(c) or in the case of the expense reimbursement obligation set forth in Sections 8(a) and 8(b), the indemnification required by Sections 8(a) and 8(b) shall be made by periodic payments of the amount thereof during the course of the investigation or defense, as and when bills are received or Losses are incurred.
18
(e) If the indemnification provided for in Sections 8(a) and 8(b) is held by a court of competent jurisdiction to be unavailable to an indemnified party with respect to any loss, liability, claim, damage or expense referred to herein, the indemnifying party, in lieu of indemnifying such indemnified party hereunder, shall contribute to the amount paid or payable by such indemnified party as a result of such loss, liability, claim, damage or expense (i) in such proportion as is appropriate to reflect the proportionate relative fault of the indemnifying party on the one hand and the indemnified party on the other (determined by reference to, among other things, whether the untrue or alleged untrue statement of a material fact or omission relates to information supplied by the indemnifying party or the indemnified party and the parties’ relative intent, knowledge, access to information and opportunity to correct or prevent such untrue statement or omission), or (ii) if the allocation provided by clause (i) above is not permitted by applicable law or provides a lesser sum to the indemnified party than the amount hereinafter calculated, then in such proportion as is appropriate to reflect not only the proportionate relative fault of the indemnifying party and the indemnified party, but also the relative benefits received by the indemnifying party on the one hand and the indemnified party on the other, as well as any other relevant equitable considerations. Notwithstanding any other provision of this Section 8(e), no Holder shall be required to contribute any amount in excess of the amount by which the net proceeds received by such Holder from the sale of the Registrable Securities pursuant to the Registration Statement exceeds the amount of damages that such Holder has otherwise been required to pay by reason of such untrue or alleged untrue statement of a material fact or omission. No indemnified party guilty of fraudulent misrepresentation (within the meaning of Section 11(f) of the Securities Act) shall be entitled to contribution from any indemnifying party who was not guilty of such fraudulent misrepresentation.
(f) The indemnity and contribution agreements contained in this Section 8 are in addition to any liability that the indemnifying parties may have to the indemnified parties and are not in diminution or limitation of the indemnification provisions under the applicable Subscription Agreement.
9. (a) Rule 144. The Company hereby represents and warrants to each of the Holders that the Common Stock is, and as of the Effective Date will be, registered under Section 12(g) of the Exchange Act. The Company shall file with the Commission a current report on Form 8-K containing “Form 10 information” (as defined in Rule 144(i)(3) under the Securities Act) reflecting its status as an entity that is no longer an issuer described in Rule 144(i)(1)(i) as promptly as practicable, but in no event more than four (4) Business Days, following the closing of the Merger. At all times on and after the date of this Agreement, the Company shall timely file (or furnish, as applicable) all reports, statements and other documents required to be filed with (or furnished to) the Commission pursuant to the Exchange Act (the “SEC Documents”), and without the prior written consent of the Majority Holders, the Company shall not terminate or suspend, or allow the termination or suspension of, the registration of the Common Stock under the Exchange Act or otherwise terminate or suspend, or allow the termination or suspension of, its status as an issuer required to file reports under the Exchange Act, even if the applicable securities laws would otherwise permit any such termination or suspension, except in connection with a sale of the Company subject to approval of its stockholders. None of the SEC Documents, when filed, furnished or submitted, shall contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they are made, not misleading. Without limiting the foregoing and with a view to making available to the Holders the benefits of Rule 144, the Company hereby agrees to:
(i) make and keep public information available, as those terms are understood and defined in Rule 144; and
19
(ii) so long as any of the Holders holds any Registrable Securities, promptly upon such Holder’s request at any time on or after the date that is one (1) year following the Company’s filing of the Super 8-K, furnish to such Holder (A) a written statement by the Company that it has complied with the reporting requirements of the Exchange Act as required for applicable provisions of Rule 144, (B) a copy of the most recent annual or quarterly report of the Company and such other reports and documents so filed by the Company and (C) such other information as may be reasonably requested to permit such Holder to sell such securities pursuant to Rule 144 without registration.
(b) Stock Exchange Listing. The Company shall use its reasonable best efforts to cause the Common Stock to be registered under Section 12(b) of the Exchange Act and listed on a National Securities Exchange as soon as practicable after the Company meets all of the applicable listing criteria for any National Securities Exchange and use its reasonable best efforts to cause the Common Stock to all times thereafter remain registered under Section 12(b) of the Exchange Act and listed on a National Securities Exchange, including by ongoing compliance with all applicable listing requirements of the National Securities Exchange. The Company shall use its commercially reasonable efforts to meet the listing criteria for at least one National Securities Exchange as soon as reasonably possible after the Effective Date. Except as otherwise provided herein, all expenses in connection with the matters contemplated by this Section 9(b) shall be borne by the Company.
10. Miscellaneous.
(a) Governing Law. This Agreement and any matter related hereto shall be governed by and construed in accordance with the federal securities laws of the United States of America (as applicable) and the laws of the State of New York, both substantive and remedial, without regard to New York conflicts of law principles that would result in the application of the laws of any other jurisdiction. Any judicial proceeding brought against any of the parties to this Agreement or any dispute arising out of this Agreement or any matter related hereto shall be brought in the state or federal courts of the State of New York, New York County, and, by its execution and delivery of this Agreement, each party to this Agreement accepts the jurisdiction of such courts. The foregoing consent to jurisdiction shall not be deemed to confer rights on any person other than the parties to this Agreement. EACH PARTY HERETO HEREBY IRREVOCABLY WAIVES ALL RIGHT TO TRIAL BY JURY IN ANY ACTION OR COUNTERCLAIM (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT, ANY OTHER TRANSACTION DOCUMENT, THE TRANSACTIONS CONTEMPLATED HEREBY OR THEREBY OR THE ACTIONS OF SUCH PARTY IN THE NEGOTIATION, ADMINISTRATION, PERFORMANCE AND ENFORCEMENT HEREOF.
20
(b) Remedies. In the event of a breach by the Company or by a Holder of any of their respective obligations under this Agreement, each Holder or the Company, as the case may be, in addition to being entitled to exercise all rights granted by law and under this Agreement, including recovery of damages (except as otherwise specifically set forth herein with respect to Registration Events), shall be entitled to specific performance of its rights under this Agreement, without the necessity of posting bond or other security. Each of the Company and the Holders agree that monetary damages would not provide adequate compensation for any losses incurred by reason of a breach by it of any of the provisions of this Agreement and hereby further agrees that, in the event of any action for specific performance in respect of such breach, it shall not assert or shall waive the defense that a remedy at law would be adequate.
(c) Successors and Assigns. Except as otherwise provided herein, the provisions hereof shall inure to the benefit of, and be binding upon, the successors, permitted assigns, executors and administrators of the parties hereto.
(d) No Inconsistent Agreements. The Company has not entered, as of the date hereof, and shall not enter, on or after the date of this Agreement, into any agreement with respect to its securities that would have the effect of impairing the rights granted to the Holders in this Agreement or otherwise conflicts with the provisions hereof. Without limiting the foregoing, the Company shall not enter into any agreement that would require the inclusion, and shall not include, any securities other than the Registrable Securities in any Registration Statement required be filed hereunder without the prior written consent of the Majority Holders.
(e) Entire Agreement. This Agreement and the documents, instruments and other agreements specifically referred to herein or delivered pursuant hereto (including the Subscription Agreements) constitute the full and entire understanding and agreement between the parties with regard to the subjects hereof.
(f) Notices, etc. All notices, consents, waivers, and other communications which are required or permitted under this Agreement shall be in writing will be deemed given to a party (a) upon receipt, when personally delivered; (b) one (1) Business Day after deposit with a nationally recognized overnight courier service with next day delivery specified, costs prepaid on the date of delivery, if delivered to the appropriate address by hand or by nationally recognized overnight courier service (costs prepaid); (c) the time of transmission if sent by facsimile or e-mail with confirmation of transmission by the transmitting equipment if such notice or communication is delivered prior to 5:00 P.M., New York City time, on a Trading Day, or the next Trading Day after the date of transmission, if such notice or communication is delivered on a day that is not a Trading Day or later than 5:00 P.M., New York City time, on any Trading Day, provided confirmation of facsimile is mechanically or electronically generated and kept on file by the sending party and confirmation of email is kept on file, whether electronically or otherwise, by the sending party and the sending party does not receive an automatically generated message from the recipient’s email server that such e-mail could not be delivered to such recipient; (d) the date received or rejected by the addressee, if sent by certified mail, return receipt requested, postage prepaid; or (e) seven (7) days after the placement of the notice into the mails (first class postage prepaid), in each case, to the party at the address, facsimile number, or e-mail address furnished by the such party,
21
If to the Company, to:
Adaptin Bio, Inc.
3540 Toringdon Way, Suite 200, #250
Charlotte, NC 28277
Attention: Michael J. Roberts
E-mail: mroberts@adaptin.com
with copy to:
Wyrick Robbins Yates & Ponton LLP
4101 Lake Boone Trail, Suite 300
Raleigh, NC 27607
Attention: Donald R. Reynolds
E-mail: dreynolds@wyrick.com
if to a Holder, to:
such Holder at the address set forth on the signature page hereto or in the Company’s records;
or at such other address as any party shall have furnished to the other parties in writing in accordance with this Section 10(h).
(g) Delays or Omissions. No delay or omission to exercise any right, power or remedy accruing to any Holder, upon any breach or default of the Company under this Agreement, shall impair any such right, power or remedy of such Holder nor shall it be construed to be a waiver of any such breach or default, or an acquiescence therein, or of any similar breach or default thereunder occurring; nor shall any waiver of any single breach or default be deemed a waiver of any other breach or default theretofore or thereafter occurring. Any waiver, permit, consent or approval of any kind or character on the part of any Holder of any breach or default under this Agreement, or any waiver on the part of any Holder of any provisions or conditions of this Agreement, must be in writing and shall be effective only to the extent specifically set forth in such writing. All remedies, either under this Agreement, or by law, in equity or otherwise afforded to any holder, shall be cumulative and not alternative.
(h) Counterparts. This Agreement may be executed in any number of counterparts, and with respect to any Purchaser, by execution of an Omnibus Signature Page to this Agreement and the applicable Subscription Agreement, each of which shall be enforceable against the parties actually executing such counterparts, and all of which together shall constitute one instrument. In the event that any signature is delivered by facsimile transmission or by an e-mail, which contains a copy of an executed signature page such as a portable document format (.pdf) file, such signature shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such facsimile or e-mail of an executed signature page such as a .pdf signature page were an original thereof.
(i) Severability. In the case any provision of this Agreement shall be invalid, illegal or unenforceable, such provision shall be replaced with a valid, legal and enforceable provision that as closely as possible reflects the parties’ intent with respect thereto, and the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby.
22
(j) Amendments. Except as otherwise provided herein, the provisions of this Agreement may be amended at any time and from time to time, and particular provisions of this Agreement may be waived, with and only with an agreement or consent in writing signed by the Company and the Majority Holders; provided that this Agreement may not be amended and the observance of any term hereof may not be waived with respect to any Holder without the written consent of such Holder if such amendment or waiver adversely affects the rights of such Holder under this Agreement in a manner that is different than the other Holders. Notwithstanding the foregoing, a waiver or consent to depart from the provisions hereof with respect to a matter that relates exclusively to the rights of one or more Holder and that does not adversely directly or indirectly affect the rights of other Holder may be given by Holders holding all of the Registrable Securities to which such waiver or consent relates. Notwithstanding anything in this Agreement to the contrary, Schedule 1 may be amended by the Company from time to time to update the list of Brokers who hold Placement Agent Warrants (or the amount of Placement Agent Warrant Shares that such Broker is entitled to pursuant to the terms thereof) in compliance with the terms of this Agreement and the Subscription Agreement without the consent of the other parties hereto..
(k) Independent Nature of Holders’ Obligations and Rights. The obligations of each Holder hereunder are several and not joint with the obligations of any other Holder hereunder, and no Holder shall be responsible in any way for the performance of the obligations of any other Holder hereunder. Nothing contained herein or in any other agreement or document delivered at any closing, and no action taken by any Holder pursuant hereto or thereto, shall be deemed to constitute the Holders as a partnership, an association, a joint venture or any other kind of group or entity, or create a presumption that the Holders are in any way acting in concert or as a group or entity with respect to such obligations or the transactions contemplated by this Agreement or any other matters and the Company acknowledges that the Holders are not acting in concert or as a group, and the Company shall not assert any such claim, with respect to such obligations or transactions. Except as expressly provided herein, each Holder shall be entitled to protect and enforce its rights, including without limitation the rights arising out of this Agreement, and it shall not be necessary for any other Holder to be joined as an additional party in any proceeding for such purpose. The use of a single agreement with respect to the obligations of the Company contained herein was solely in the control of the Company, not the action or decision of any Holder, and was done solely for the convenience of the Company and not because it was required or requested to do so by any Holder. Except as expressly provided herein, it is expressly understood and agreed that each provision contained in this Agreement is between the Company and a Holder, solely, and not between the Company and the Holders collectively and not between and among Holders.
(l) Subsequent Registration Rights. Until all of the Registrable Securities have been registered for resale under an effective Registration Statement and the Common Stock is quoted on an Approved OTC Market, the Company shall not enter into any agreement granting registration rights more favorable than the registration rights set forth in this Agreement without the written consent of the Majority Holders.
(m) Rules of Construction. Unless the context otherwise requires, (i) all references to Sections, Schedules or Exhibits are to Sections, Schedules or Exhibits contained in or attached to this Agreement, (ii) each accounting term not otherwise defined in this Agreement has the meaning assigned to it in accordance with GAAP, (iii) words in the singular or plural include the singular and plural and pronouns stated in either the masculine, the feminine or neuter gender shall include the masculine, feminine and neuter, (iv) the use of the word “including” in this Agreement shall be by way of example rather than limitation, and (v) the word “or” shall not be exclusive.
[Signature page follows.]
23
This Registration Rights Agreement is hereby executed as of the date first above written.
| THE COMPANY: ADAPTIN BIO, INC. | |||
| By: | |||
| Name: | |||
| Title: | |||
| PURCHASERS: | |||
| See Omnibus Signature Pages to Subscription Agreement | |||
| HOLDER OF PRE-MERGER WARRANTS (INDIVIDUAL): |
HOLDER OF PRE-MERGER WARRANTS (ENTITY): | ||
| Print Name | Print Name of Entity | ||
| By: | |||
| Signature | Name: | ||
| Title: | |||
| HOLDER OF NOTE CONVERSION SHARES (INDIVIDUAL): | HOLDER OF NOTE CONVERSION SHARES (ENTITY): | ||
| Print Name | Print Name of Entity | ||
| By: | |||
| Signature | Name: | ||
| Title: | |||
| HOLDER OF REGISTRABLE PRE-MERGER SHARES (INDIVIDUAL): | HOLDER OF REGISTRABLE PRE-MERGER SHARES (ENTITY): | ||
| Print Name | Print Name of Entity | ||
| By: | |||
| Signature | Name: | ||
| Title: | |||
| BROKER (INDIVIDUAL): | BROKER (ENTITY): | ||
| Print Name | Print Name of Entity | ||
| By: | |||
| Signature | Name: | ||
| Title: | |||
| All Holders: Print Address | |||
[Signature Page to Registration Rights Agreement]
24
Exhibit A
Form of Plan of Distribution
PLAN OF DISTRIBUTION
The selling stockholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling shares of common stock or interests in shares of common stock received after the date of this prospectus from a selling stockholder as a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their shares of common stock or interests in shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. At and after such time, the selling stockholders may sell all or a portion of their shares through public or private transactions at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The selling stockholders may use any one or more of the following methods when disposing of shares or interests therein:
| ● | purchases by a broker-dealer as principal and resale by such broker-dealer for its own account pursuant to this prospectus; | |
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
| ● | block trades in which the broker-dealer so engaged will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; | |
| ● | an over-the-counter distribution in accordance with the rules of the applicable exchange; | |
| ● | through trading plans entered into by a selling stockholder pursuant to Rule 10b5-1 under the Exchange Act that are in place at the time of an offering pursuant to this prospectus and any applicable prospectus supplement hereto that provide for periodic sales of their securities on the basis of parameters described in such trading plans; | |
| ● | short sales; | |
| ● | distribution to employees, members, limited partners or stockholders of the selling stockholders; | |
| ● | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; | |
| ● | by pledge to secured debts and other obligations; | |
| ● | delayed delivery arrangements; | |
| ● | to or through underwriters or agents; | |
| ● | in “at the market” offerings, as defined in Rule 415 under the Securities Act, at negotiated prices, at prices prevailing at the time of sale or at prices related to such prevailing market prices, including sales made directly on a national securities exchange or sales made through a market maker other than on an exchange or other similar offerings through sales agents; |
25
| ● | in privately negotiated transactions; | |
| ● | in options transactions; and |
| ● | through a combination of any of the above methods of sale, as described below, or any other method permitted pursuant to applicable law. |
The selling stockholders may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The selling stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
There can be no assurance that the selling stockholders will sell all or any of the securities offered by this prospectus. In addition, the selling stockholders may also sell securities under Rule 144 under the Securities Act, if available, or in other transactions exempt from registration, rather than under this prospectus. The selling stockholders have the sole and absolute discretion not to accept any purchase offer or make any sale of securities if they deem the purchase price to be unsatisfactory at any particular time.
In connection with the sale of our common stock or interests therein, the selling stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The selling stockholders may also sell shares of our common stock short and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into options or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to the selling stockholders from the sale of the common stock offered by them will be the purchase price of the common stock less discounts or commissions, if any. Each of the selling stockholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents. We will not receive any of the proceeds from this sale of common stock by the selling stockholders.
The selling stockholders and any underwriters, broker-dealers or agents that are involved in selling the common stock or interests therein may be deemed to be “underwriters” within the meaning of Section 2(a)(11) of the Securities Act. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each selling stockholder in the Offering has informed us that it does not have any agreement or understanding, directly or indirectly, with any person to distribute the common stock. If a selling stockholder is deemed to be an “underwriter” within the meaning of the Securities Act, it will be subject to the prospectus delivery requirements of the Securities Act.
26
To the extent required, the shares of our common stock to be sold, the names of the selling stockholders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to this registration statement that includes this prospectus.
In order to comply with the securities laws of some states, if applicable, the common stock may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the common stock may not be sold unless it has been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
We have advised the selling stockholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the selling stockholders and their affiliates. In addition, we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling stockholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act.
The selling stockholders may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
We have agreed to indemnify the selling stockholders against certain liabilities, including liabilities under the Securities Act and state securities laws, relating to the registration of the shares offered by this prospectus.
Agents, broker-dealers and underwriters may be entitled to indemnification by us and the selling stockholders against certain civil liabilities, including liabilities under the Securities Act, or to contribution with respect to payments which the agents, broker-dealers or underwriters may be required to make in respect thereof.
We have agreed with the selling stockholders to keep this registration statement of which this prospectus constitutes a part effective for five years from the date it is declared effective by the SEC or until the date on which all of the shares of common stock required to be registered by us have been sold or otherwise transferred other than to assignees pursuant to the Registration Rights Agreement. See the section of this prospectus captioned [“Shares Eligible for Future Sale — Registration Rights.”]
Certain of our stockholders have entered into lock-up agreements. See the section of this prospectus captioned [“Shares Eligible for Future Sale — Lock-Up Agreements.”]
27
Exhibit B
Form of Legend Removal Certificate
ADAPTIN BIO, INC.
LEGEND REMOVAL CERTIFICATE
(Resale Registration Statement)
The undersigned securityholder (the “Securityholder”) of Adaptin Bio, Inc., a Delaware corporation (the “Company”), is delivering this certificate to the Company in connection with the Securityholder’s request to remove the transfer restriction legends under the Securities Act of 1933, as amended (the “Securities Act”), from certificates or book-entry notations issued in the Securityholder’s name with respect to the number of shares of common stock, par value of $0.0001 per share, of the Company set forth under the Securityholder’s name on the signature page hereof (the “Shares”).
| A. | The Securityholder hereby represents and warrants to the Company that the Securityholder is sophisticated in financial matters and is familiar with the registration requirements under the Securities Act. If the Securityholder is an investment fund, the Securityholder’s chief compliance officer (or the chief compliance officer of the general partner, manager or other entity which manages the Securityholder) has reviewed this certificate and is aware that the Securityholder will be executing and delivering this certificate to the Company and undertaking the obligations set forth herein. |
| B. | The Securityholder hereby covenants to the Company that: |
| 1. | The Securityholder will transfer the Shares only: |
| (a) | pursuant to an effective resale registration statement covering the Securityholder’s resale of the Shares, which includes a prospectus that is current, and in the manner contemplated by such registration statement, including the “Plan of Distribution” contained therein, provided that the Securityholder has not received oral or written notice from the Company that use of the prospectus is suspended or that the prospectus otherwise may not be used for transfers of the Shares; or |
| (b) | on or after the date that is one (1) year following the Company’s filing of the Super 8-K (as defined in the Subscription Agreement pursuant to which the Shares were originally issued), pursuant to Rule 144 under the Securities Act, subject to the satisfaction, as of the time of the transfer of the Shares, to the Company’s satisfaction of the “current public information” requirement of Rule 144, the holding period provisions of Rule 144(d) and, if applicable, the volume, manner-of-sale and notice provisions of Rule 144. |
| (c) | otherwise in accordance with the Securities Act, provided that the Securityholder provides the Company with advance notice of such transfer and an opinion of counsel that the proposed transfer is in compliance with the Securities Act. |
| 2. | The Securityholder will provide the Company with any update to the Securityholder’s contact information set forth on the signature page hereof for purposes of any notification to be delivered to me relating hereto. |
28
The Securityholder acknowledges and agrees that the Company’s inside and outside legal counsel are each authorized to rely on this certificate for purposes of preparing and delivering any legal opinion(s) required in connection with the removal of the transfer restriction legends from the Shares and the Company’s transfer agent is authorized to rely on this certificate in connection with the removal of the transfer restriction legends from the Shares.
| Very truly yours, | |
| Name of Securityholder: | |
| Signature: | |
| Name of Signatory: | |
| Title of Signatory: | |
| Date: | |
| Address: | |
| E-mail address: | |
| Number of Shares for Legend Removal: | |
| Share Certificate No. or Book Entry Information: |
29
Annex A
Adaptin Bio, Inc.
Selling Securityholder Notice and Questionnaire
The undersigned beneficial owner of Registrable Securities of [Adaptin __________________________], a Delaware corporation (the “Company”), understands that the Company has filed or intends to file with the U.S. Securities and Exchange Commission a registration statement (the “Registration Statement”) for the registration and resale under Rule 415 of the Securities Act of 1933, as amended, of the Registrable Securities, in accordance with the terms of the Registration Rights Agreement (the “Registration Rights Agreement”) to which this document is annexed. A copy of the Registration Rights Agreement is available from the Company upon request at the address set forth below. All capitalized terms not otherwise defined herein shall have the meanings ascribed thereto in the Registration Rights Agreement.
Certain legal consequences arise from being named as a selling security holder in the Registration Statement and the related prospectus. Accordingly, holders and beneficial owners of Registrable Securities are advised to consult their own securities law counsel regarding the consequences of being named or not being named as a selling security holder in the Registration Statement and the related prospectus.
NOTICE
The undersigned beneficial owner (the “Selling Securityholder”) of Registrable Securities hereby elects to include the Registrable Securities owned by it in the Registration Statement.
The undersigned hereby provides the following information to the Company and represents and warrants that such information is accurate:
QUESTIONNAIRE
1. Name:
| (a) | Full Legal Name of Selling Securityholder |
| (b) | Full Legal Name of Registered Holder (holder of record) (if not the same as (a) above) through which Registrable Securities are held: |
| (c) | If you are not a natural person, full Legal Name of Natural Control Person (which means a natural person who directly or indirectly alone or with others has power to vote or dispose of the securities covered by this Questionnaire): |
30
2. Address for Notices to Selling Securityholder:
| Telephone: | Fax: |
| Email: |
| Contact Person |
3. Broker-Dealer Status:
| (a) | Are you a broker-dealer? |
Yes ☐ No ☐
| (b) | If “yes” to Section 3(a), did you receive your Registrable Securities as compensation for investment banking services to the Company? |
Yes ☐ No ☐
Note: If “no” to Section 3(b), the Commission’s staff has indicated that you should be identified as an underwriter in the Registration Statement.
| (c) | Are you an affiliate of a broker-dealer? |
Yes ☐ No ☐
| (d) | If you are an affiliate of a broker-dealer, do you certify that you purchased the Registrable Securities in the ordinary course of business, and at the time of the purchase of the Registrable Securities to be resold, you had no agreements or understandings, directly or indirectly, with any person to distribute the Registrable Securities? |
Yes ☐ No ☐
Note: If “no” to Section 3(d), the Commission’s staff has indicated that you should be identified as an underwriter in the Registration Statement.
31
4. Beneficial Ownership of Securities of the Company Owned by the Selling Securityholder:
Except as set forth below in this Item 4, the undersigned is not the beneficial or registered owner of any securities of the Company.
| (a) | Please list the type (common stock, warrants, etc.) and amount of all securities of the Company (including any Registrable Securities) beneficially owned1 by the Selling Securityholder: | |
5. Relationships with the Company:
Except as set forth below, neither you nor (if you are a natural person) any member of your immediate family, nor (if you are not a natural person) any of your affiliates2, officers, directors or principal equity holders (owners of 5% of more of the equity securities of the undersigned) has held any position or office or has had any other material relationship with the Company (or its predecessors or affiliates) during the past three years.
State any exceptions here:
| 1 | Beneficially Owned: A “beneficial owner” of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship or otherwise has or shares (i) voting power, including the power to direct the voting of such security, or (ii) investment power, including the power to dispose of, or direct the disposition of, such security. In addition, a person is deemed to have “beneficial ownership” of a security of which such person has the right to acquire beneficial ownership at any time within sixty (60) days, including, but not limited to, any right to acquire such security: (i) through the exercise of any option, warrant or right, (ii) through the conversion of any security or (iii) pursuant to the power to revoke, or the automatic termination of, a trust, discretionary account or similar arrangement. |
It is possible that a security may have more than one “beneficial owner,” such as a trust, with two co-trustees sharing voting power, and the settlor or another third party having investment power, in which case each of the three would be the “beneficial owner” of the securities in the trust. The power to vote or direct the voting, or to invest or dispose of, or direct the investment or disposition of, a security may be indirect and arise from legal, economic, contractual or other rights, and the determination of beneficial ownership depends upon who ultimately possesses or shares the power to direct the voting or the disposition of the security.
The final determination of the existence of beneficial ownership depends upon the facts of each case. You may, if you believe the facts warrant it, disclaim beneficial ownership of securities that might otherwise be considered “beneficially owned” by you.
| 2 | Affiliate: An “affiliate” is a company or person that directly, or indirectly through one or more intermediaries, controls you, or is controlled by you, or is under common control with you. |
32
The undersigned agrees to promptly notify the Company of any material inaccuracies or changes in the information provided herein that may occur subsequent to the date hereof at any time while the Registration Statement remains effective; provided, that the undersigned shall not be required to notify the Company of any changes to the number of securities held or owned by the undersigned or its affiliates.
By signing below, the undersigned consents to the disclosure of the information contained herein in its answers to Items 1 through 5 and the inclusion of such information in the Registration Statement and the related prospectus and any amendments or supplements thereto. The undersigned understands that such information will be relied upon by the Company in connection with the preparation or amendment of the Registration Statement and the related prospectus and any amendments or supplements thereto.
33
IN WITNESS WHEREOF the undersigned, by authority duly given, has caused this Selling Securityholder Notice and Questionnaire to be executed and delivered either in person or by its duly authorized agent.
| BENEFICIAL OWNER (individual) | BENEFICIAL OWNER (entity) | |||
| Signature | Name of Entity | |||
| Print Name | Signature | |||
| Print Name: | ||||
| Signature (if Joint Tenants or Tenants in Common) | ||||
| Title: | ||||
PLEASE E-MAIL A COPY OF THE COMPLETED AND EXECUTED SELLING SECURITYHOLDER NOTICE AND QUESTIONNAIRE TO:
Jonathan A. Greene
Email: jgreene@wyrick.com
34
Exhibit 10.9
ADAPTIN Bio, inc.
2025 EQUITY INCENTIVE PLAN
2025 Equity Incentive Plan Approved by
the Board and Stockholders on February 11, 2025 and February 11, 2025, respectively
1. Purposes of the Plan. The purposes of this Plan are to attract and retain the best available personnel to serve as Employees, Directors or Consultants; to provide additional incentives to Employees, Directors and Consultants to contribute to the successful performance of the Company and any Related Entity; to promote the growth of the market value of the Company’s Common Stock; to align the interests of Participants with those of the Company’s stockholders; and to promote the success of the Company’s business.
2. Definitions. The following definitions will apply as used herein and in all individual Award Agreements except as a term may be otherwise defined in an individual Award Agreement. In the event a term is separately defined in an individual Award Agreement, such definition will supersede the definition contained in this Section 2.
(a) “Administrator” means the Plan Administrator as described in Section 4. With reference to the duties of the Administrator under the Plan which have been delegated to one or more persons pursuant to Section 4(c), the term “Administrator” will refer to such person(s) unless such delegation has been revoked.
(b) “Applicable Laws” means the legal requirements relating to the Plan and the Awards under applicable provisions of federal and state securities laws, the corporate laws of Delaware, and, to the extent other than Delaware, the corporate law of the state of the Company’s incorporation, the Code, the rules of any applicable stock exchange or national market system, and the rules of any non-U.S. jurisdiction applicable to Awards granted to residents therein.
(c) “Assumed” means, with respect to an Award, that pursuant to a Corporate Transaction either (i) the Award is expressly affirmed as continuing in effect by the Company, or (ii) the contractual obligations represented by the Award are expressly assumed (and not simply by operation of law) by the successor entity or its Parent in connection with the Corporate Transaction with appropriate adjustments to the number and type of securities of the successor entity or its Parent subject to the Award and the exercise or purchase price thereof which at least preserves the compensation element of the Award existing at the time of the Corporate Transaction as determined in accordance with the instruments evidencing the agreement to assume the Award.
(d) “Award” means the grant of an Option, Stock Appreciation Right, Dividend Equivalent Right, Restricted Stock, Restricted Stock Unit, or other right or benefit under the Plan.
(e) “Award Agreement” means the written agreement evidencing the grant of an Award executed by the Company and the Participant, including any amendments thereto.
(f) “Board” means the Board of Directors of the Company.
(g) “Cause” means, with respect to the termination by the Company or a Related Entity of a Participant’s Continuous Service:
(i) that such termination is for “Cause” as such term (or word of like import) is expressly defined in a then-effective written employment agreement, consulting agreement, service agreement or other similar agreement between the Participant and the Company or such Related Entity, provided, however, that with regard to any agreement that defines “Cause” on the occurrence of or in connection with a Corporate Transaction, such definition of “Cause” will not apply until a Corporate Transaction actually occurs; or
(ii) in the absence of such then-effective written agreement and definition, is based on, in the determination of the Administrator: (A) the Participant’s performance of any act, or failure to perform any act, in bad faith and to the detriment of the Company or a Related Entity; (B) the Participant’s dishonesty, intentional misconduct or material breach of any agreement with the Company or a Related Entity; (C) the Participant’s material breach of any noncompetition, confidentiality or similar agreement with the Company or a Related Entity, as determined under such agreement; (D) the Participant’s commission of a crime involving dishonesty, breach of trust, or physical or emotional harm to any person; (E) if the Participant is an Employee or Consultant, the Participant’s engaging in acts or omissions constituting gross negligence, misconduct or a willful violation of a Company or a Related Entity policy which is or is reasonably expected to be materially injurious to the Company and/or a Related Entity; or (F) if the Participant is an Employee, the Participant’s failure to follow the reasonable instructions of the Board or such Participant’s direct supervisor, which failure, if curable, is not cured within 10 days after notice to such Participant or, if cured, recurs within 180 days.
(h) “Code” means the Internal Revenue Code of 1986, as amended, or any successor statute.
(i) “Committee” means any committee appointed by the Board to administer the Plan in accordance with Section 4(a) below.
(j) “Common Stock” means the Company’s voting common stock, $0.0001 par value per share.
(k) “Company” means Adaptin Bio, Inc., a Delaware corporation, or any successor entity that adopts the Plan in connection with a Corporate Transaction.
(l) “Consultant” means any person (other than an Employee or a Director, solely with respect to rendering services in such person’s capacity as a Director) who is engaged by the Company or any Related Entity to render consulting or advisory services to the Company or such Related Entity.
2
(m) “Continuous Service” means that the provision of services to the Company or a Related Entity in any capacity of Employee, Director or Consultant is not interrupted or terminated. In jurisdictions requiring notice in advance of an effective termination as an Employee, Director or Consultant, Continuous Service will be deemed terminated upon the actual cessation of providing services to the Company or a Related Entity notwithstanding any required notice period that must be fulfilled before a termination as an Employee, Director or Consultant can be effective under Applicable Laws. A Participant’s Continuous Service will be deemed to have terminated either upon an actual termination of Continuous Service or upon the entity for which the Participant provides services ceasing to be a Related Entity. Continuous Service will not be considered interrupted in the case of (i) any approved leave of absence, (ii) transfers among the Company, any Related Entity, or any successor in any capacity of Employee, Director or Consultant, or (iii) any change in status as long as the individual remains in the service of the Company or a Related Entity in any capacity of Employee, Director or Consultant (except as otherwise provided in the Award Agreement). An approved leave of absence for purposes of this Plan will include sick leave, military leave, or any other authorized personal leave, so long as the Company or Related Entity has a reasonable expectation that the individual will return to provide services for the Company or Related Entity, and provided further that the leave does not exceed six months, unless the individual has a statutory or contractual right to re-employment following a longer leave. For purposes of each Incentive Stock Option granted under the Plan, if such leave exceeds three months, and reemployment upon expiration of such leave is not guaranteed by statute or contract, then the Incentive Stock Option will be treated as a Non-statutory Stock Option beginning on the day three months and one day following the expiration of such three month period.
(n) “Corporate Transaction” means any of the following transactions, provided, however, that the Administrator will determine under parts (ii), (iii) and (iv) whether multiple transactions are related, and its determination will be final, binding and conclusive:
(i) a merger or consolidation in which the Company is not the surviving entity, except for a transaction the principal purpose of which is to change the state in which the Company is incorporated;
(ii) the sale, transfer or other disposition of all or substantially all of the assets of the Company in one or a series of related transactions;
(iii) any reverse merger or series of related transactions culminating in a reverse merger (including, but not limited to, a tender offer followed by a reverse merger) in which the Company is the surviving entity but (A) the Shares outstanding immediately prior to such merger are converted or exchanged by virtue of the merger into other property, whether in the form of securities, cash or otherwise, or (B) in which securities possessing more than 50% of the total combined voting power of the Company’s outstanding securities are transferred to a person or persons different from those who held such securities immediately prior to such merger or the initial transaction culminating in such merger; or
(iv) acquisition in a single or series of related transactions by any person or related group of persons (other than the Company or by a Company-sponsored employee benefit plan) of beneficial ownership (within the meaning of Rule 13d-3 of the Exchange Act) of securities possessing more than 50% of the total combined voting power of the Company’s outstanding securities; or
(v) the complete liquidation or dissolution of the Company.
3
(o) “Data” has the meaning set forth in Section 21 of this Plan.
(p) “Director” means a member of the Board or the board of directors of any Related Entity.
(q) “Disability” means a “disability” (or word of like import) as defined under the long-term disability policy of the Company or the Related Entity to which the Participant provides services regardless of whether the Participant is covered by such policy. If the Company or the Related Entity to which the Participant provides service does not have a long-term disability plan in place, “Disability” means that a Participant is unable to carry out the responsibilities and functions of the position held by the Participant by reason of any medically determinable physical or mental impairment for a period of not less than 90 consecutive days. A Participant will not be considered to have incurred a Disability unless he or she furnishes proof of such impairment sufficient to satisfy the Administrator.
(r) “Disqualifying Disposition” means any disposition (including any sale) of Common Stock received upon exercise of an Incentive Stock Option before either (i) two years after the date the Employee was granted the Incentive Stock Option, or (ii) one year after the date the Employee acquired Common Stock by exercising the Incentive Stock Option. If the Employee has died before such stock is sold, these holding period requirements do not apply and no Disqualifying Disposition can occur thereafter.
(s) “Dividend Equivalent Right” means a right entitling the Participant to compensation measured by dividends paid with respect to Common Stock.
(t) “Effective Date” has the meaning set forth in Section 15 below.
(u) “Employee” means any person, including an Officer or Director, who is in the employ of the Company or any Related Entity, subject to the control and direction of the Company or any Related Entity as to both the work to be performed and the manner and method of performance. The payment of a director’s fee by the Company or a Related Entity to an individual will not be sufficient to make such individual an “Employee” of the Company or a Related Entity.
(v) “Exchange Act” means the Securities Exchange Act of 1934, as amended.
4
(w) “Fair Market Value” means, as of any date, the value of the Common Stock determined as follows.
(i) If the Common Stock is listed on one or more established stock exchanges or national market systems, including without limitation The NASDAQ Global Select Market, The NASDAQ Global Market, or The NASDAQ Capital Market of The NASDAQ Stock Market LLC, its Fair Market Value will be the closing sales price for such stock (or the closing bid, if no sales were reported) as quoted on the principal exchange or system on which the Common Stock is listed (as determined by the Administrator) on the date of determination (or, if no closing sales price or closing bid was reported on that date, as applicable, on the last trading date such closing sales price or closing bid was reported), as reported in The Wall Street Journal or such other source as the Administrator deems reliable;
(ii) If the Common Stock is regularly quoted on an automated quotation system (including the OTC markets and systems maintained by OTC Markets Group Inc.) or by a recognized securities dealer, its Fair Market Value will be the closing sales price for such stock as quoted on such system or by such securities dealer on the date of determination, but if selling prices are not reported, the Fair Market Value of a Share will be the mean between the high bid and low asked prices for the Common Stock on the date of determination (or, if no such prices were reported on that date, on the last date such prices were reported), as reported in The Wall Street Journal or such other source as the Administrator deems reliable; or
(iii) In the absence of an established market for the Common Stock of the type described in (i) and (ii), above, the Fair Market Value thereof will be determined by the Administrator in good faith by application of a reasonable valuation method consistently applied and taking into consideration all available information material to the value of the Company in a manner in compliance with Section 409A, or in the case of an Incentive Stock Option, in a manner in compliance with Section 422 of the Code.
(x) “Incentive Stock Option” means an Option intended to qualify as an incentive stock option within the meaning of Section 422 of the Code.
(y) “Non-statutory Stock Option” means an Option that either (i) is not intended to qualify as an Incentive Stock Option, or (ii) fails to qualify as an Incentive Stock Option.
(z) “Officer” means a person who is an officer of the Company or a Related Entity within the meaning of Section 16 of the Exchange Act and the rules and regulations promulgated thereunder.
(aa) “Option” means an option to purchase one or more Shares pursuant to an Award Agreement granted under the Plan.
(bb) “Parent” means a “parent corporation,” whether now or hereafter existing, as defined in Section 424(e) of the Code.
(cc) “Participant” means the holder of an outstanding Award.
(dd) “Performance Award” means an Award under the Plan in which the vesting or other realization of the Award by a Participant is subject to the achievement of certain performance criteria over the course of a Performance Period, all as determined by the Administrator in accordance with Section 8 below.
5
(ee) “Performance Period” means the time period established by the Administrator during which specified performance criteria must be met in connection with a Performance Award as described in Section 8 below.
(ff) “Plan” means this Adaptin Bio, Inc. 2025 Equity Incentive Plan, as the same may be amended from time to time.
(gg) “Post-Termination Exercise Period” means the period specified in the Award Agreement of not less than 30 days commencing on the date of termination (other than termination by the Company or any Related Entity for Cause) of the Participant’s Continuous Service, or such longer period as may be applicable upon death or Disability.
(hh) “Related Entity” means any Parent or Subsidiary of the Company.
(ii) “Restricted Stock” means Shares issued under the Plan to the Participant for such consideration, if any, and subject to such restrictions on transfer, rights of first refusal, repurchase provisions, forfeiture provisions, and other terms and conditions as established by the Administrator.
(jj) “Restricted Stock Units” means an Award which may be earned in whole or in part upon the passage of time or the attainment of performance criteria established by the Administrator and which may be settled for cash, Shares or other securities or a combination of cash, Shares or other securities as established by the Administrator.
(kk) “Rule 16b-3” means Rule 16b-3 promulgated by the Securities and Exchange Commission pursuant to the Exchange Act, as such rule may be amended from time to time, and includes any successor provisions thereto.
(ll) “Stock Appreciation Right” means an Award entitling the Participant to Shares or cash compensation, as established by the Administrator, measured by appreciation in the value of Common Stock.
(mm) “Section 409A” means Section 409A of the Code, the Treasury Regulations and other guidance issued thereunder by the United States Department of the Treasury (whether issued before or after the Effective Date), and all state laws of similar effect.
(nn) “Share” means a share of the Common Stock.
(oo) “Subsidiary” means a “subsidiary corporation,” whether now or hereafter existing, as defined in Section 424(f) of the Code.
(pp) “Tax Obligations” means all federal, state, local, and foreign income tax, social insurance, payroll tax, fringe benefits tax, or other tax-related liabilities related to a Participant’s participation in the Plan and the receipt of any benefits hereunder, as determined under the Applicable Laws.
6
3. Stock Subject to the Plan.
(a) General Share Reserve. Subject to adjustment as described in Section 13 below, the aggregate number of Shares which may be issued pursuant to all Awards under the Plan shall be equal to 15% of the fully diluted Shares outstanding upon each closing of the Offering (as such term is defined in the Subscription Agreement dated as of February 11, 2025, by and among the Company and the purchasers set forth therein), up to a maximum aggregate amount of 15% of the fully diluted Shares outstanding of the Company following the closing of the final Offering. The Shares may be authorized, but unissued, or reacquired Common Stock. During the term of the Plan, the Company will at all times reserve and keep available a sufficient number of Shares to satisfy the requirements of the Plan.
(b) Incentive Stock Option Limit. Subject to adjustment in accordance with Section 13, no more than 2,196,390 Shares may be issued in the aggregate pursuant to the exercise of Incentive Stock Options.
(c) Annual Increase in Share Reserve. For a period of 10 years commencing on February 11, 2025 and ending on (and including) February 11, 2035, the share reserve described in Section 3(a) above will be increased by an amount equal to the lesser of (i) 4% of the number of Shares outstanding as of December 31 of the immediately preceding calendar year (calculated on a fully-diluted and as-converted basis) or (ii) such lesser number of Shares as determined by the Board prior to January 1 of a particular calendar year.
(d) Reversion of Shares to Share Reserve. Any Shares covered by an Award (or portion of an Award) which is forfeited, canceled or expires (whether voluntarily or involuntarily) will be deemed not to have been issued for purposes of determining the maximum aggregate number of Shares which may be issued under the Plan, except that the maximum aggregate number of Shares which may be issued pursuant to the exercise of Incentive Stock Options will not exceed the number specified in Section 3(b). Shares that actually have been issued under the Plan pursuant to an Award will not be returned to the Plan and will not become available for future issuance under the Plan, except that if unvested Shares are forfeited or repurchased by the Company, such Shares will become available for future grant under the Plan. In the event any Option or other Award granted under the Plan is exercised through the tendering of Shares (either actually or through attestation), or in the event tax withholding obligations are satisfied by tendering or withholding Shares, any Shares so tendered or withheld will not again be available for Awards under the Plan. To the extent that cash is delivered in lieu of Shares upon the exercise of a Stock Appreciation Right pursuant to Section 6(l), the Company will be deemed, for purposes of applying the limitation on the number of shares, to have issued the total number of Shares which were otherwise issuable upon such exercise, notwithstanding that cash was issued in lieu of such Shares. Shares reacquired by the Company on the open market or otherwise using cash proceeds from the exercise of Options will not be available for Awards under the Plan.
7
4. Administration of the Plan.
(a) Plan Administrator. The Plan will be administered by (i) the Board or (ii) a Committee designated by the Board, which Committee will be constituted in such a manner as to satisfy the Applicable Laws and to permit such grants and related transactions under the Plan to be exempt from Section 16(b) of the Exchange Act in accordance with Rule 16b-3. Once appointed, such Committee will continue to serve in its designated capacity until otherwise directed by the Board.
(b) Powers of the Administrator. Subject to the Applicable Laws, the provisions of the Plan, and in the case of a Committee, subject to the specific duties delegated by the Board to such Committee, the Administrator will have the authority, in its discretion:
| (i) | to select the Employees, Directors and Consultants to whom Awards may be granted from time to time hereunder; |
| (ii) | to determine whether and to what extent Awards are granted hereunder; |
| (iii) | to determine the number of Shares or the amount of other consideration to be covered by each Award granted hereunder; |
| (iv) | determine the vesting schedule (if any) applicable to all Awards under the Plan; |
| (v) | to determine the type, terms and conditions of any Award granted hereunder; |
| (vi) | to accelerate vesting on any Award or to waive any forfeiture restrictions applicable thereto or to waive any other limitation or restriction with respect to an Award; |
| (vii) | to approve forms of Award Agreements for use under the Plan; |
| (viii) | to establish additional terms, conditions, rules or procedures to accommodate the rules or laws of applicable non-U.S. jurisdictions and to afford Participants favorable treatment under such rules or laws; provided, however, that no Award will be granted under any such additional terms, conditions, rules or procedures with terms or conditions which are inconsistent with the provisions of the Plan; |
| (ix) | to amend the terms of any outstanding Award granted under the Plan, provided that any amendment that would materially adversely affect the Participant’s rights under an outstanding Award will not be made without the Participant’s written consent; provided, however, that an amendment or modification that may cause an Incentive Stock Option to become a Non-statutory Stock Option will not be treated as adversely affecting the rights of the Participant; |
| (x) | to construe and interpret the terms of the Plan and Awards, including without limitation, any notice of Award or Award Agreement, granted pursuant to the Plan; |
| (xi) | to make other determinations as provided in this Plan; and |
| (xii) | to take such other action, not inconsistent with the terms of the Plan, as the Administrator deems appropriate. |
8
The express grant in the Plan of any specific power to the Administrator will not be construed as limiting any power or authority of the Administrator; provided that the Administrator may not exercise any right or power reserved to the Board. Any decision made, or action taken, by the Administrator or in connection with the administration of this Plan will be final, conclusive and binding on all persons having an interest in the Plan.
(c) Delegation of Authority. To the extent permitted by Applicable Law, the Board may from time to time delegate to a committee of one or more Officers of the Company the authority to grant or amend Awards or to take other actions pursuant to this Section 4; provided, however, that in no event may an Officer of the Company be delegated the authority to grant awards to, or amend Awards held by, the following individuals: (i) individuals who are subject to Section 16 of the Exchange Act, (ii) Directors, or (iii) Officers to whom authority to grant or amend Awards has been delegated hereunder; provided, further, that any delegation of authority will only be permitted to the extent it is permissible under Applicable Law. Any delegation hereunder will be subject to the restrictions and limits that the Board or Committee specifies at the time of such delegation, and the Board may at any time rescind the authority so delegated or appoint a new delegatee. At all times, the delegatee appointed under this Section 4(c) will serve in such capacity at the pleasure of the Board.
(d) Indemnification. In addition to such other rights of indemnification as they may have as members of the Board or as Officers or Employees of the Company or a Related Entity, members of the Board and any Officers or Employees of the Company or a Related Entity to whom authority to act for the Board, the Administrator or the Company is delegated will be defended and indemnified by the Company to the extent permitted by law on an after-tax basis against all reasonable expenses, including attorneys’ fees, actually and necessarily incurred in connection with the defense of any claim, investigation, action, suit or proceeding, or in connection with any appeal therein, to which they or any of them may be a party by reason of any action taken or failure to act under or in connection with the Plan, or any Award granted hereunder, and against all amounts paid by them in settlement thereof (provided such settlement is approved by the Company) or paid by them in satisfaction of a judgment in any such claim, investigation, action, suit or proceeding, except in relation to such liabilities, costs, and expenses as may arise out of, or result from, the bad faith, gross negligence, willful misconduct, or criminal acts of such persons; provided, however, that within 30 days after the institution of such claim, investigation, action, suit or proceeding, such person will offer to the Company, in writing, the opportunity at the Company’s expense to defend the same.
5. Eligibility. Awards other than Incentive Stock Options may be granted to Employees, Directors, and Consultants of the Company or any Related Entity. Incentive Stock Options may be granted only to Employees of the Company or a Related Entity. An Employee, Director, or Consultant who has been granted an Award may, if otherwise eligible, be granted additional Awards. Awards may be granted to such Employees, Directors, or Consultants who are residing in non-U.S. jurisdictions as the Administrator may determine from time to time.
9
6. Terms and Conditions of Awards.
(a) Types of Awards. The Administrator is authorized under the Plan to award any type of arrangement to an Employee, Director or Consultant that is not inconsistent with the provisions of the Plan and that by its terms involves or might involve the issuance of (i) Shares, (ii) cash or (iii) an Option, a Stock Appreciation Right, or similar right with a fixed or variable price related to the Fair Market Value of the Shares and with an exercise or conversion privilege related to the passage of time, the occurrence of one or more events, or the satisfaction of performance criteria or other conditions. Such Awards include, without limitation, Options, Stock Appreciation Rights, sales or bonuses of Restricted Stock, Restricted Stock Units, Performance Awards, and Dividend Equivalent Rights. An Award may consist of one such security or benefit, or two or more of them in any combination or alternative.
(b) Designation of Award. Each Award will be evidenced by an Award Agreement in form and substance satisfactory to the Administrator. The type of each Award will be designated in the Award Agreement. In the case of an Option, the Option will be designated as either an Incentive Stock Option or a Non-statutory Stock Option. However, notwithstanding such designation, any portion of an Option designated as an Incentive Stock Option that exceeds the $100,000 limitation of Section 422(d) of the Code will be treated as a Non-statutory Stock Option. The $100,000 limitation of Section 422(d) of the Code is calculated based on the aggregate Fair Market Value of the Shares subject to Options designated as Incentive Stock Options which become exercisable for the first time by a Participant during any calendar year (under all plans of the Company or any Related Entity). For purposes of this calculation, Incentive Stock Options will be taken into account in the order in which they were granted, and the Fair Market Value of the Shares will be determined as of the grant date of the relevant Option. Any Option granted which fails to satisfy the requirements of the Applicable Laws for treatment as an Incentive Stock Option will be a Non-statutory Stock Option.
(c) Conditions of Award. Subject to the terms of the Plan, the Administrator will determine the provisions, terms, and conditions of each Award including, but not limited to, the Award vesting schedule, repurchase provisions, rights of first refusal, forfeiture provisions, form of payment (cash, Shares, or other consideration) upon settlement of the Award, payment contingencies, and satisfaction of any performance criteria that may be established by the Administrator.
(d) Term of Award. The term of each Award will be the term stated in the Award Agreement as determined by the Administrator, provided, however, that the term of any Option will be no more than 10 years from the date of grant thereof, and provided further that in the case of an Incentive Stock Option granted to a Participant who, at the time the Option is granted, owns stock representing more than 10% of the voting power of all classes of stock of the Company or any Related Entity, the term of the Incentive Stock Option will be no more than five years from the date of grant thereof. Notwithstanding the foregoing, the specified term of any Award will not include any period for which the Participant has elected to defer the receipt of the Shares or cash issuable pursuant to the Award.
10
(e) Date of Grant. The date of grant of an Award will be, for all purposes, the date on which the Administrator makes the determination to grant such Award, or such other later date as is determined by the Administrator.
(f) Notice to Company of Disqualifying Disposition. Each Employee who receives an Incentive Stock Option must agree to notify the Company in writing immediately after the Employee makes a Disqualifying Disposition of any Common Stock acquired pursuant to the exercise of an Incentive Stock Option.
(g) Acquisitions and Other Transactions. The Administrator may issue Awards under the Plan in settlement, assumption or substitution for, outstanding awards or obligations to grant future awards in connection with the Company or a Related Entity acquiring another entity, an interest in another entity or an additional interest in a Related Entity whether by merger, stock purchase, asset purchase or other form of transaction.
(h) Deferral of Award Payment. The Administrator may establish one or more programs under the Plan to permit selected Participants the opportunity to elect to defer receipt of consideration upon exercise of an Award, satisfaction of performance criteria, or other event that absent the election would entitle the Participant to payment or receipt of Shares or other consideration under an Award. The Administrator may establish the election procedures, the timing of such elections, the mechanisms for payments of, and accrual of interest or other earnings, if any, on amounts, Shares or other consideration so deferred, and such other terms, conditions, rules and procedures that the Administrator deems advisable for the administration of any such deferral program.
(i) Separate Programs. The Administrator may establish one or more separate programs under the Plan for the purpose of issuing particular forms of Awards to one or more classes of Participants on such terms and conditions as determined by the Administrator from time to time.
(j) Early Exercise. An Award Agreement may, but need not, include a provision whereby the Participant may elect at any time while an Employee, Director or Consultant to exercise any part or all of the Award prior to full vesting of the Award. Any unvested Shares received pursuant to such exercise may be subject to a repurchase right in favor of the Company or a Related Entity or to any other restriction the Administrator determines to be appropriate.
(k) Transferability of Awards. Unless the Administrator provides otherwise, no Award may be sold, pledged, assigned, hypothecated, transferred, or disposed of in any manner other than by will or by the laws of descent or distribution and may be exercised, during the lifetime of the Participant, only by the Participant. Notwithstanding the foregoing, the Participant may designate one or more beneficiaries of the Participant’s Award in the event of the Participant’s death on a beneficiary designation form provided by the Administrator.
11
(l) Stock Appreciation Rights. Stock Appreciation Rights may be granted (i) with respect to any Option granted under this Plan, either concurrently with the grant of such Option or at such later time as determined by the Administrator (as to all or any portion of the Shares subject to the Option), or (ii) alone, without reference to any related Option. Each Stock Appreciation Right granted by the Administrator under this Plan will be subject to the following terms and conditions. Each Stock Appreciation Right granted to any Participant will relate to such number of Shares as determined by the Administrator, subject to adjustment as provided in Section 13. In the case of a Stock Appreciation Right granted with respect to an Option, the number of Shares to which the Stock Appreciation Right pertains will be reduced in the same proportion that the holder of the Option exercises the related Option. The exercise price of a Stock Appreciation Right will be determined by the Administrator at the date of grant but may not be less than 100% of the Fair Market Value of the Shares subject thereto on the date of grant. Subject to the right of the Administrator to deliver cash in lieu of Shares (which, as it pertains to Officers and Directors of the Company, will comply with all requirements of the Exchange Act), the number of Shares which issuable upon the exercise of a Stock Appreciation Right will be determined by dividing:
(i) the number of Shares as to which the Stock Appreciation Right is exercised multiplied by the amount of the appreciation in such Shares (for this purpose, the “appreciation” will be the amount by which the Fair Market Value of the Shares subject to the Stock Appreciation Right on the exercise date exceeds (A) in the case of a Stock Appreciation Right related to an Option, the exercise price of the Shares under the Option or (B) in the case of a Stock Appreciation Right granted alone, without reference to a related Option, an amount determined by the Administrator at the time of grant, subject to adjustment under Section 13); by
(ii) the Fair Market Value of a Share on the exercise date.
In lieu of issuing Shares upon the exercise of a Stock Appreciation Right, the Administrator may elect to pay the holder of the Stock Appreciation Right cash equal to the Fair Market Value on the exercise date of any or all of the Shares which would otherwise be issuable. No fractional Shares will be issued upon the exercise of a Stock Appreciation Right; instead, the holder of the Stock Appreciation Right will be entitled to receive a cash adjustment equal to the same fraction of the Fair Market Value of a Share on the exercise date or to purchase the portion necessary to make a whole share at its Fair Market Value on the date of exercise. The exercise of a Stock Appreciation Right related to an Option will be permitted only to the extent that the Option is exercisable under its terms on the date of surrender. Any Incentive Stock Option surrendered pursuant to the provisions of this Section 6(l) will be deemed to have been converted into a Non-statutory Stock Option immediately prior to such surrender.
7. Award Exercise or Purchase Price.
(a) Exercise or Purchase Price. The exercise or purchase price, if any, for an Award will be as follows.
(i) In the case of an Incentive Stock Option:
(1) granted to an Employee who, at the time of the grant of such Incentive Stock Option owns stock representing more than 10% of the voting power of all classes of stock of the Company or any Related Entity, the per Share exercise price will be not less than 110% of the Fair Market Value per Share on the date of grant; or
12
(2) granted to any Employee other than an Employee described in the preceding paragraph, the per Share exercise price will be not less than 100% of the Fair Market Value per Share on the date of grant.
(ii) In the case of a Non-statutory Stock Option, the per Share exercise price will be not less than 100% of the Fair Market Value per Share on the date of grant.
(iii) In the case of other Awards, such price as is determined by the Administrator.
(iv) Notwithstanding the foregoing provisions of this Section 7(a), in the case of an Award issued pursuant to Section 6(g) above, the exercise or purchase price for the Award will be determined in accordance with the provisions of the relevant instrument evidencing the agreement to issue such Award and the Applicable Laws.
(b) Consideration. Subject to Applicable Laws, the consideration to be paid for the Shares to be issued upon exercise or purchase of an Award, including the method of payment, will be determined by the Administrator. In addition to any other types of consideration the Administrator may determine, the Administrator is authorized to accept as consideration for Shares issued under the Plan the following:
(i) cash;
(ii) check;
(iii) surrender of Shares or delivery of a properly executed form of attestation of ownership of Shares as the Administrator may require which have a Fair Market Value on the date of surrender or attestation equal to the aggregate exercise price of the Shares as to which said Award is exercised;
(iv) with respect to Options, payment through a broker-dealer sale and remittance procedure pursuant to which the Participant (A) provides written instructions to a broker-dealer acceptable to the Company to effect the immediate sale of some or all of the purchased Shares and remit to the Company sufficient funds to cover the aggregate exercise price payable for the purchased Shares and (B) provides written directives to the Company to deliver the certificates (or other evidence satisfactory to the Company to the extent that the Shares are uncertificated) for the purchased Shares directly to such broker-dealer in order to complete the sale transaction;
(v) with respect to Options, payment through a “net exercise” such that, without the payment of any funds, the Participant may exercise the Option and receive the net number of Shares equal to (i) the number of Shares as to which the Option is being exercised, multiplied by (ii) a fraction, the numerator of which is the Fair Market Value per Share (on such date as is determined by the Administrator) less the Exercise Price per Share, and the denominator of which is such Fair Market Value per Share;
(vi) past or future services actually or to be rendered to the Company or a Related Entity; or
(vii) any combination of the foregoing methods of payment.
13
The Administrator may at any time or from time to time, by adoption of or by amendment to the standard forms of Award Agreement described in Section 4(b)(vii), or by other means, grant Awards which do not permit all of the foregoing forms of consideration to be used in payment for the Shares or which otherwise restrict one or more forms of consideration.
8. Performance Awards.
(a) Grant of Performance Awards. The Administrator may issue Performance Awards under the Plan in accordance with this Section 8. Performance Awards may be Options, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units, or any other form of Award permitted pursuant to the Plan.
(b) Award Agreement for Performance Awards. Any Award intended to be a Performance Award pursuant to this Section 8 will be evidenced by an Award Agreement that will specify the number of Shares covered by the Award, the applicable performance criteria, the duration of the Performance Period, and such other terms and conditions as the Administrator, in its sole discretion, will determine. Unless otherwise determined by the Administrator, payment of the Award to a Participant will occur following the end of the Performance Period, or if later, the date on which any applicable contingency or restriction has ended.
(c) Performance Criteria. The performance criteria for any Performance Awards will be established by the Administrator and may include, but are not limited to, any one of, or combination of, the following criteria:
| (i) | Net earnings or net income (before or after taxes); |
| (ii) | Earnings per share; |
| (iii) | Net sales growth; |
| (iv) | Net operating profit; |
| (v) | Return measures (including, but not limited to, return on assets, capital, equity, or sales); |
| (vi) | Cash flow (including, but not limited to, operating cash flow, free cash flow, and cash flow return on capital); |
| (vii) | Cash flow per share; |
| (viii) | Earnings before or after taxes, interest, depreciation, and/or amortization; |
| (ix) | Gross or operating margins; |
| (x) | Productivity ratios; |
14
| (xi) | Share price (including, but not limited to, growth measures and total shareholder return); |
| (xii) | Expense targets or ratios; |
| (xiii) | Charge-off levels; |
| (xiv) | Improvement in or attainment of revenue levels; |
| (xv) | Operating efficiency; |
| (xvi) | Operating expenses; |
| (xvii) | Economic value added; |
| (xviii) | Improvement in or attainment of expense levels; |
| (xix) | Improvement in or attainment of working capital levels; |
| (xx) | Debt reduction; |
| (xxi) | Capital targets; |
| (xxii) | Consummation of acquisitions, dispositions, projects or other specific events or transactions; or |
| (xxiii) | Other significant operational or business milestones. |
(d) Determination of Performance Criteria. Performance criteria may be measured on an absolute (e.g., plan or budget) or relative basis, and may be established on a corporate-wide basis or with respect to one or more business units, divisions, subsidiaries or business segments, or may be established on an individual basis. Relative performance may be measured against a group of peer companies, a financial market index or other acceptable objective and quantifiable indices. If the Administrator determines that a change in the business, operations, corporate structure or capital structure of the Company, or the manner in which the Company conducts its business, or other events or circumstances render the performance objectives unsuitable, the Administrator may modify the minimum acceptable level of achievement, in whole or in part, as the Administrator deems appropriate and equitable. Performance objectives may be adjusted for material items not originally contemplated in establishing the performance target for items resulting from discontinued operations, extraordinary gains and losses, the effect of changes in accounting standards or principles, acquisitions or divestitures, changes in tax rules or regulations, capital transactions, restructuring, nonrecurring gains or losses or unusual items. Performance measures may vary from Performance Award to Performance Award, and from Participant to Participant, and may be established on a stand-alone basis, in tandem or in the alternative. The Administrator will have the authority to impose such other restrictions on as it may deem necessary or appropriate to ensure that Performance Awards satisfy all requirements of the Applicable Laws.
15
(e) Administrator Certification of Achievement of Performance Criteria. Following the completion of each Performance Period, the Administrator will determine whether the applicable performance criteria have been achieved for the Performance Awards for such Performance Period. In determining the amounts earned by a Participant pursuant to an Award issued pursuant to this Section 8, the Administrator will have the right to (i) adjust the amount payable at a given level of performance to take into account additional factors that the Administrator may deem relevant to the assessment of individual or corporate performance for the Performance Period, (ii) determine what actual Award, if any, will be paid in the event of a Corporate Transaction or in the event of a termination of employment following a Corporate Transaction prior to the end of the Performance Period, and (iii) determine what actual Award, if any, will be paid in the event of a termination of employment other than as the result of a Participant’s death or Disability prior to a Corporate Transaction and prior to the end of the Performance Period.
9. Tax Withholding.
(a) Prior to the delivery of any Shares or cash pursuant to an Award (or the exercise thereof), or at such other time as the Tax Obligations are due, the Company, in accordance with the Code and any Applicable Laws, will have the power and the right to deduct or withhold, or require a Participant to remit to the Company, an amount sufficient to satisfy all Tax Obligations. The Administrator may condition such delivery, payment, or other event pursuant to an Award on the payment by the Participant of any such Tax Obligations.
(b) The Administrator, pursuant to such procedures as it may specify from time to time, may designate the method or methods by which a Participant may satisfy the Tax Obligations. As determined by the Administrator in its sole discretion from time to time, these methods may include one or more of the following:
(i) paying cash;
(ii) directing the Company withhold cash or Shares deliverable to the Participant having a Fair Market Value equal to the amount required to be withheld;
(iii) delivering to the Company already-owned Shares having a Fair Market Value equal to the amount required to be withheld or remitted, provided the delivery of such Shares will not result in any adverse accounting consequences as the Administrator determines;
(iv) selling a sufficient number of Shares otherwise deliverable to the Participant through such means as the Administrator may determine (whether through a broker or otherwise) equal to the Tax Obligations required to be withheld;
(v) retaining from salary or other amounts payable to the Participant cash having a sufficient value to satisfy the Tax Obligations; or
(vi) any other means which the Administrator determines to both comply with Applicable Laws, and to be consistent with the purposes of the Plan.
16
The amount of Tax Obligations will be deemed to include any amount that the Administrator determines may be withheld at the time the election is made, not to exceed the amount determined by using the maximum federal, state, local and foreign marginal income tax rates applicable to the Participant or the Company, as applicable, with respect to the Award on the date that the amount of tax or social insurance liability to be withheld or remitted is to be determined. The Fair Market Value of the Shares to be withheld or delivered will be determined as of the date that the Tax Obligations are required to be withheld.
10. Rights As a Stockholder.
(a) Restricted Stock. Except as otherwise provided in any Award Agreement, a Participant will not have any rights of a stockholder with respect to any of the Shares granted to the Participant under an Award of Restricted Stock (including the right to vote or receive dividends and other distributions paid or made with respect thereto). No dividends or Dividend Equivalent Rights will be paid in respect of any unvested Award of Restricted Stock, unless and until such Shares vest.
(b) Other Awards. In the case of Awards other than Restricted Stock, a Participant will not have any rights of a stockholder, nor will dividends or Dividend Equivalent Rights accrue or be paid, with respect to any of the Shares granted pursuant to such Award until the Award is exercised or settled and the Shares are delivered (as evidenced by the appropriate entry on the books of the Company or of a duly authorized transfer agent of the Company).
11. Exercise of Award.
(a) Procedure for Exercise.
(i) Any Award granted hereunder will be exercisable at such times and under such conditions as determined by the Administrator under the terms of the Plan and as specified in the Award Agreement.
(ii) An Award will be deemed to be exercised when written notice of such exercise has been given to the Company in accordance with the terms of the Award Agreement by the person entitled to exercise the Award and full payment for the Shares with respect to which the Award is exercised has been made in compliance with the terms of the Award Agreement and the Plan.
(b) Exercise of Award Following Termination of Continuous Service. In the event of termination of a Participant’s Continuous Service for any reason other than Disability or death, such Participant may, but only during the Post-Termination Exercise Period (but in no event later than the expiration date of the term of such Award), exercise the portion of the Participant’s Award that was vested at the date of such termination (or such greater portion of the Participant’s Award as may be determined by the Administrator). Unless otherwise provided in the applicable Award Agreement, the Participant’s right to exercise the Award will terminate concurrently with the termination of Participant’s Continuous Service for Cause. In the event of a Participant’s change of status from Employee to Consultant, an Employee’s Incentive Stock Option will convert automatically to a Non-statutory Stock Option on the day three months and one day following such change of status. Unless otherwise determined by the Administrator, the unvested portion of a Participant’s Award will terminate as of the date of termination. In addition, if the Participant does not exercise the vested portion of the Participant’s Award within the Post-Termination Exercise Period, the Award will terminate upon the conclusion of the Post-Termination Exercise Period.
17
(c) Disability of Participant. In the event of termination of a Participant’s Continuous Service as a result of his or her Disability, such Participant may, but only within 12 months from the date of such termination (or such longer period as specified in the Award Agreement but in no event later than the expiration date of the term of such Award as set forth in the Award Agreement), exercise the portion of the Participant’s Award that was vested at the date of such termination; provided, however, that if such Disability is not a “disability” as such term is defined in Section 22(e)(3) of the Code, in the case of an Incentive Stock Option such Incentive Stock Option will automatically convert to a Non-statutory Stock Option on the day three months and one day following such termination. Unless otherwise determined by the Administrator, the unvested portion of a Participant’s Award will terminate as of the date of such termination. In addition, if the Participant does not exercise the vested portion of the Participant’s Award within the period specified in the Award Agreement following such termination, the Award will terminate upon the conclusion of such period.
(d) Death of Participant. In the event of a termination of the Participant’s Continuous Service as a result of his or her death, or in the event of the death of the Participant during the Post-Termination Exercise Period or during the 12 month period following the Participant’s termination of Continuous Service as a result of his or her Disability, the Participant’s estate or a person who acquired the right to exercise the Award by bequest or inheritance may exercise the portion of the Participant’s Award that was vested as of the date of termination, within 12 months from the date of death (or such longer period as specified in the Award Agreement but in no event later than the expiration of the term of such Award as set forth in the Award Agreement). Unless otherwise determined by the Administrator, the unvested portion of a Participant’s Award will terminate as of the date of the Participant’s death. In addition, if the Participant’s estate or a person who acquired the right to exercise the Award by bequest or inheritance does not exercise the vested portion of the Participant’s Award within the period specified in the Award Agreement following the Participant’s death, the Award will terminate upon the conclusion of such period.
(e) Extension if Exercise Prevented by Law. Notwithstanding the foregoing, if the exercise of an Award within the applicable time periods set forth in this Section 11 is prevented by the provisions of Section 12 below, the Award will remain exercisable until one month after the date the Participant is notified by the Company that the Award is exercisable, but in any event no later than the expiration of the term of such Award as set forth in the Award Agreement.
18
12. Conditions Upon Issuance of Shares; Manner of Issuance of Shares.
(a) Legal Compliance. Shares will not be issued pursuant to the exercise or vesting of an Award unless the issuance and delivery of such Shares will comply with Applicable Laws. If at any time the Administrator determines that the delivery of Shares pursuant to the exercise, vesting or any other provision of an Award is or may be unlawful under Applicable Laws, the vesting or right to exercise an Award or to otherwise receive Shares pursuant to the terms of an Award will be suspended until the Administrator determines that such delivery is lawful and will be further subject to the approval of counsel for the Company with respect to such compliance. The Company will have no obligation to effect any registration or qualification of the Shares under any Applicable Law.
(b) Investment Representations. As a condition to the exercise of an Award, the Company may require the person exercising such Award to represent and warrant at the time of any such exercise that the Shares are being purchased only for investment and without any present intention to sell or distribute such Shares if, in the opinion of counsel for the Company, such a representation is required by any Applicable Laws.
(c) Inability to Obtain Authority. The inability of the Company to obtain authority from any regulatory body having jurisdiction, which authority is deemed by the Company’s counsel to be necessary to the lawful issuance and sale of any Shares hereunder, will relieve the Company of any liability in respect of the failure to issue or sell such Shares as to which such requisite authority has not been obtained.
(d) Form of Issuance of Shares. Subject to the Applicable Laws and any governing rules or regulations, the Company will issue or cause to be issued the Shares acquired pursuant to an Award and will deliver such Shares to or for the benefit of the Participant by means of one or more of the following as determined by the Administrator: (i) by delivering to the Participant evidence of book entry Shares credited to the account of the Participant, (ii) by depositing such Shares for the benefit of the Participant with any broker with which the Participant has an account relationship, or (iii) by delivering such Shares to the Participant in certificate form.
(e) Fractional Shares. No fractional Shares will be issued pursuant to any Award under the Plan; any Participant who would otherwise be entitled to receive a fraction of a Share upon exercise or vesting of an Award will receive from the Company cash in lieu of such fractional Shares in an amount equal to the Fair Market Value of such fractional Shares, as determined by the Administrator.
13. Adjustments. Subject to any required action by the stockholders of the Company, the number of Shares covered by each outstanding Award, and the number of Shares which have been authorized for issuance under the Plan but as to which no Awards have yet been granted or which have been returned to the Plan, the exercise or purchase price of each such outstanding Award, as well as any other terms that the Administrator determines require adjustment will be proportionately adjusted for (i) any increase or decrease in the number of issued and outstanding Shares resulting from a stock split, reverse stock split, stock dividend, combination or reclassification of the Shares, or similar transaction affecting the Shares, (ii) any other increase or decrease in the number of issued and outstanding Shares effected without receipt of consideration by the Company, or (iii) any other transaction with respect to the Company’s Common Stock including a corporate merger, consolidation, acquisition of property or stock, separation (including a spin-off or other distribution of stock or property), reorganization, liquidation (whether partial or complete) or any similar transaction; provided, however that conversion of any convertible securities of the Company will not be deemed to have been “effected without receipt of consideration.” Such adjustment will be made by the Administrator and its determination will be final, binding and conclusive. Except as the Administrator determines, no issuance by the Company of shares of stock of any class, or securities convertible into shares of stock of any class, will affect, and no adjustment by reason hereof will be made with respect to, the number or price of Shares subject to an Award. No adjustments will be made for dividends paid in cash or in property other than Common Stock of the Company, nor will cash dividends or dividend equivalents accrue or be paid in respect of unexercised Options or unvested Awards hereunder.
19
14. Corporate Transactions.
(a) Termination of Awards to Extent Not Assumed in Corporate Transaction. Effective upon the consummation of a Corporate Transaction, all outstanding Awards under the Plan will terminate, except that Awards will not terminate to the extent they are Assumed in connection with the Corporate Transaction.
(b) Effect of a Corporate Transaction. The Administrator may establish such terms and conditions relating to the effect of a Corporate Transaction on Awards as the Administrator deems appropriate, including, but not limited to, determining that: (i) one or more Awards will vest and become exercisable, realizable, or payable, or restrictions applicable to an Award will lapse, in whole or in part prior to or upon consummation of such Corporate Transaction, and, to the extent the Administrator determines, terminate upon or immediately prior to the effectiveness of such Corporate Transaction; (ii) Awards will be Assumed by, or replaced with awards that have substantially equivalent terms by, the surviving corporation (or a parent or subsidiary of the surviving corporation); (iii) Participants will receive a payment in satisfaction of outstanding Awards of Restricted Stock Units or of other rights or benefits, in such amount and form as may be determined by the Administrator; (iv) outstanding Options and Stock Appreciation Rights be terminated or surrendered, in exchange for a payment by the Company, in cash or other property as determined by the Administrator, in an amount equal to the amount, if any, by which the then Fair Market Value of the Shares subject to the Participant’s unexercised Options and Stock Appreciation Rights exceeds the exercise price (and, for the avoidance of doubt, if the Administrator determines in good faith that as of the date of the occurrence of the Corporate Transaction the per share Fair Market Value of the Shares does not exceed the per share exercise price of a given Award, then such Award may be terminated by the Company without payment), and (v) after giving Participants an opportunity to exercise all of their outstanding Options and Stock Appreciation Rights, the Administrator may terminate any or all unexercised Options and Stock Appreciation Rights at such time as the Administrator deems appropriate. In taking any of the actions permitted under this Section 14(b), the Administrator will not be obligated to treat all Awards, all Awards held by a Participant, or all Awards of the same type, similarly. Such action(s) by the Administrator will take place as of the date of the Corporate Transaction or such other date as the Administrator may specify.
(c) Effect of Acceleration on Incentive Stock Options. Any Incentive Stock Option accelerated under this Section 14 in connection with a Corporate Transaction will remain exercisable as an Incentive Stock Option under the Code only to the extent the $100,000 limitation of Section 422(d) of the Code is not exceeded.
20
15. Effective Date and Term of Plan; Stockholder Approval.
(a) This Plan became effective upon its adoption by the Board on February 11, 2025 (the “Effective Date”). The Plan will continue in effect for a period of 10 years from the Effective Date unless sooner terminated, subject to the approval of the Plan by the stockholders of the Company as described in Section 15(c) below.
(b) The expiration of the Plan will not have the effect of terminating any Awards outstanding on such date, except as otherwise provided in the applicable Award Agreement.
(c) The Plan will be subject to approval by the stockholders of the Company. Such stockholder approval will be obtained in the manner and to the degree required under Applicable Laws.
16. Amendment, Suspension or Termination of the Plan.
(a) The Board may at any time suspend or terminate the Plan, or amend the Plan in any respect, except that it may not, without the approval of the stockholders obtained within 12 months before or after the Board adopts a resolution authorizing any of the following actions, do any of the following:
(i) increase the total number of shares that may be issued under the Plan (except by adjustment pursuant to Section 13 or by operation of Section 3(c));
(ii) modify the provisions of Section 6 regarding eligibility for grants of Incentive Stock Options;
(iii) modify the provisions of Section 7(a) regarding the exercise price at which shares may be offered pursuant to Options (except by adjustment pursuant to Section 13); or
(iv) extend the expiration date of the Plan.
(b) Except as provided in Section 13 (including, without limitation, any stock dividend, stock split, extraordinary cash dividend, recapitalization, reorganization, merger, consolidation, split-up, spin-off, combination, or exchange of shares), the Company may not, without approval by the Company’s stockholders, (i) lower the exercise price of an Option or Stock Appreciation Right, (ii) cancel an Option or Stock Appreciation Right when the exercise price per Share exceeds the Fair Market Value of a Share in exchange for cash or another Award, or (iii) take any other action with respect to an Option or Stock Appreciation Right that would be treated as a repricing under the rules and regulations of the principal U.S. national securities exchange on which the Shares are listed.
(c) No Award may be granted during any suspension of the Plan or after termination of the Plan. No suspension or termination of the Plan will adversely affect any rights under Awards already granted to a Participant without his or her consent.
21
17. No Effect on Terms of Employment/Consulting Relationship. Neither the Plan nor any Award will confer upon any Participant any right with respect to the Participant’s Continuous Service, nor will either interfere in any way with the Participant’s right or the right of the Company or a Related Entity to terminate the Participant’s Continuous Service at any time, with or without Cause, and with or without notice. The ability of the Company or any Related Entity to terminate the employment of a Participant who is employed on an “at will” basis is in no way affected by its determination that the Participant’s Continuous Service has been terminated for Cause for the purposes of this Plan.
18. No Effect on Retirement and Other Benefit Plans. Except as specifically provided in a retirement or other benefit plan of the Company or a Related Entity, Awards will not be deemed compensation for purposes of computing benefits or contributions under any retirement plan of the Company or a Related Entity, and will not affect any benefits under any other benefit plan of any kind or any benefit plan subsequently instituted under which the availability or amount of benefits is related to level of compensation. The Plan is not a “Retirement Plan” or “Welfare Plan” under the Employee Retirement Income Security Act of 1974, as amended.
19. Information to Participants. The Company will provide to each Participant, during the period for which such Participant has one or more Awards outstanding, such information as required by Applicable Laws.
20. Electronic Delivery. The Administrator may decide to deliver any documents related to any Award granted under the Plan through an online or electronic system established and maintained by the Company or another third party designated by the Company or to request a Participant’s consent to participate in the Plan by electronic means. By accepting an Award, each Participant consents to receive such documents by electronic delivery and agrees to participate in the Plan through an online or electronic system established and maintained by the Company or another third party designated by the Company, and such consent will remain in effect throughout Participant’s Continuous Service with the Company and any Related Entity and thereafter until withdrawn in writing by Participant.
21. Data Privacy. The Administrator may decide to collect, use and transfer, in electronic or other form, personal data as described in this Plan or any Award for the exclusive purpose of implementing, administering and managing participation in the Plan. By accepting an Award, each Participant acknowledges that the Company holds certain personal information about Participant, including, but not limited to, name, home address and telephone number, date of birth, social security number or other identification number, salary, nationality, job title, details of all Awards awarded, cancelled, exercised, vested or unvested, for the purpose of implementing, administering and managing the Plan (the “Data”). Each Participant further acknowledges that Data may be transferred to any third parties assisting in the implementation, administration and management of the Plan and that these third parties may be located in jurisdictions that may have different data privacy laws and protections, and Participant authorizes such third parties to receive, possess, use, retain and transfer the Data, in electronic or other form, for the purposes of implementing, administering and managing the Plan, including any requisite transfer of such Data as may be required to a broker or other third party with whom the recipient or the Company may elect to deposit any Shares acquired upon any Award.
22
22. Application of Section 409A. This Plan and the Awards granted hereunder will be construed and administered such that the Awards either qualify for an exemption from the application of Section 409A or satisfy the requirements of Section 409A. If an Award is subject to Section 409A: (i) distributions will only be made in a manner and upon an event permitted under Section 409A, (ii) payments to be made upon a termination of employment will only be made upon a “separation from service” under Section 409A, (iii) payments to be made upon a Corporate Transaction will only be made upon an event that qualifies as a “change in control event” under Section 409A (without giving effect to any elective provisions permitted thereunder), and (iv) in no event will a Participant, directly or indirectly, designate the calendar year in which a distribution is made, except in accordance with Section 409A. Each payment in any series of installment payments under an Award will be treated as a separate payment for purposes of Section 409A. Any Award granted under this Plan that is subject to Section 409A and that is to be distributed to a “specified employee” (as defined in Section 409A) upon a separation from service will be administered so that any distribution with respect to such Award will be postponed for six months following the date of the Participant’s separation from service, if required by Section 409A. If a distribution is so delayed pursuant to Section 409A, the distribution will be paid within 30 days after the end of the six-month period or the Participant’s death, if earlier. Notwithstanding any provision of the Plan to the contrary, in the event that following the Effective Date the Administrator determines that any Award may be subject to Section 409A, the Administrator may adopt such amendments to the Plan and the applicable Award Agreement or adopt other policies and procedures, or take any other actions, that the Administrator determines are necessary or appropriate to (A) exempt the Award from Section 409A and/or preserve the intended tax treatment of the benefits provided with respect to the Award, or (B) comply with the requirements of Section 409A. Notwithstanding anything in the Plan or any Award Agreement to the contrary, each Participant will be solely responsible for the tax consequences of Awards, and in no event will the Company have any responsibility or liability if an Award does not meet any applicable requirements of Section 409A. Although the Company intends to administer the Plan to avoid taxation under Section 409A, the Company does not represent or warrant that the Plan or any Award is exempt from, or compliant with, Section 409A.
23. Clawback/Repayment. All Awards will be subject to reduction, cancellation, forfeiture or recoupment to the extent necessary to comply with (i) any applicable compensation recovery, clawback, forfeiture or other similar policy adopted by the Board and as in effect from time to time or (ii) applicable law. Further, and without limiting the foregoing, to the extent that the Participant receives any amount in excess of the amount that the Participant should otherwise have received under the terms of the Award for any reason (including, without limitation, by reason of a financial restatement, mistake in calculations or other administrative error), the Participant may be required to repay any such excess amount to the Company.
23
24. Unfunded Obligation. Participants will have the status of general unsecured creditors of the Company. Any amounts payable to Participants pursuant to the Plan will be unfunded and unsecured obligations for all purposes, including, without limitation, Title I of the Employee Retirement Income Security Act of 1974, as amended. Neither the Company nor any Related Entity will be required to segregate any monies from its general funds, or to create any trusts, or establish any special accounts with respect to such obligations. The Company will retain at all times beneficial ownership of any investments, including trust investments, which the Company may make to fulfill its payment obligations hereunder. Any investments or the creation or maintenance of any trust or any Participant account will not create or constitute a trust or fiduciary relationship between the Administrator, the Company or any Related Entity and a Participant, or otherwise create any vested or beneficial interest in any Participant or the Participant’s creditors in any assets of the Company or a Related Entity. The Participants will have no claim against the Company or any Related Entity for any changes in the value of any assets that may be invested or reinvested by the Company with respect to the Plan.
25. Construction. Captions and titles contained herein are for convenience only and will not affect the meaning or interpretation of any provision of the Plan. Except when otherwise indicated by the context, the singular includes the plural and the plural includes the singular. Use of the term “or” is not intended to be exclusive, unless the context clearly requires otherwise.
24
ADAPTIN BIO, INC.
2025 EQUITY INCENTIVE PLAN
RESTRICTED STOCK UNIT GRANT NOTICE
Adaptin Bio, Inc., a Delaware corporation (the “Company”), pursuant to its 2025 Equity Incentive Plan, as amended from time to time (the “Plan”), hereby grants to the individual listed below (“Participant”) the number of Restricted Stock Units set forth below (the “Units”). The Units are subject to the terms and conditions set forth in this Restricted Stock Unit Grant Notice (the “Grant Notice”), the Restricted Stock Unit Award Agreement attached hereto as Exhibit A (the “Agreement”), and the Plan, each of which is incorporated herein by reference. Capitalized terms used in this Grant Notice or in the Agreement which are not explicitly defined herein will have the meaning ascribed to them in the Plan.
| Participant: | [●] |
| Number of Units: | [●] |
| Date of Grant: | [●] |
| Vesting Commencement Date: | [●] |
| Vesting Schedule: | [●] |
By signing below, Participant agrees to be bound by the terms and conditions of the Plan, the Agreement and the Grant Notice. Participant has reviewed the Agreement, the Plan and the Grant Notice in their entirety, has had an opportunity to obtain the advice of counsel prior to executing the Grant Notice and fully understands all provisions of the Grant Notice, the Agreement, and the Plan. Participant hereby agrees to accept as binding, conclusive and final all decisions and interpretations of the Administrator upon any questions arising under the Plan, the Grant Notice or the Agreement.
| COMPANY: | PARTICIPANT: | |||
| Adaptin Bio, Inc. | [Name] | |||
| By: | ||||
| [Name] | ||||
| [Title] | ||||
[Exhibit A immediately follows]
25
EXHIBIT A
ADAPTIN BIO, INC.
2025 EQUITY INCENTIVE PLAN
RESTRICTED STOCK UNIT AWARD AGREEMENT
This Restricted Stock Unit Award Agreement (this “Agreement”) governs the award of Restricted Stock Units to the Participant identified on the accompanying Grant Notice.
1. Grant of Units. Effective as of the Date of Grant, the Company grants the Participant the number of Units listed on the Grant Notice. The Units are subject to the vesting, payment, and other provisions of this Agreement and the Plan. Each Unit is subject to settlement into one share of Common Stock of the Company that will be delivered to the Participant when and if such Unit becomes vested subject to the terms of this Agreement.
2. Vesting; Forfeiture. The Units are unvested when granted and will vest in accordance with the vesting schedule set forth on the Grant Notice, subject to Participant’s Continuous Service through the vesting date(s). Vesting will cease upon the termination of Participant’s Continuous Service. All Units that are not vested upon the termination of Participant’s Continuous Service for any reason will be immediately forfeited.
3. Delivery of Shares to Settle Vested Units. Units that become vested as provided in Section 2 will be settled by delivering to Participant a number of Shares equal to the number of vested Units as soon as practicable after the date on which the Units vest, provided that the Company may provide a reasonable delay in the issuance or delivery of the Shares to address tax withholding and other administrative matters and provided further that delivery of the Shares will occur no later than two and one-half months following the conclusion of the year in which the vesting occurs. At the time of settlement, the Company will, at its election, either: (a) issue a certificate representing the Shares deliverable pursuant to this Agreement; or (b) not issue any certificate representing the Shares deliverable pursuant to this Agreement and instead document the Participant’s interest in the Shares by registering such Shares with the Company’s transfer agent (or another custodian selected by the Company) in bookentry form in the Participant’s name.
4. Capitalization Changes. The number of Units convertible to Shares subject to this Award may be adjusted from time to time by the Administrator to account for changes in capitalization as described in Section 13 of the Plan.
5. Rights as a Stockholder. The Units represent a right to payment from the Company if the conditions of the Agreement are met and do not give the Participant ownership of any Common Stock prior to delivery as provided in Section 3. Participant will not have any rights and/or privileges of a stockholder of the Company with respect to the Units prior to such delivery. If Participant becomes vested in Units as provided in Section 2, any Shares to which Participant becomes entitled will be delivered to Participant as provided in Section 3, and Participant will have full ownership of the Shares upon such delivery.
26
6. Non-Transferability of the Award. The Units and the right to payment under this Agreement are not transferable, may not be sold, exchanged, transferred, pledged, hypothecated, encumbered or otherwise disposed of except as provided in the Plan. Any purported transfer of the Units or the right to payment under this Agreement is null and void and will not be given effect.
7. Award Not A Service Contract. Neither the Award nor this Agreement is an employment or service contract, and nothing this Agreement confers or will be construed as conferring upon the Participant any right to continue in the employment or service of the Company or a Related Entity, or as interfering with or restricting in any way the right of either party to terminate such employment or service at any time.
8. Tax Consequences. Participant acknowledges that he/she understands the federal, state, and local tax consequences of the Award and the issuance, vesting, forfeiture, and delivery provisions hereof relating to the Units. Participant will rely solely on the advice of his/her own tax advisors and not on any statements or representations of the Company or any of its agents. Participant understands that Participant (and not the Company) will be responsible for his/her own tax liability that may arise as a result of the Award or the transactions contemplated by this Agreement. The Company has no duty or obligation to minimize the tax consequences associated with this Award to the Participant and will not be liable to the Participant for any adverse tax consequences arising in connection with this Award.
9. Withholding Obligations. Participant understands that, at the time that Participant becomes vested and/or receives payment for any Units (including through the delivery of Shares), the Company may be required to withhold federal, state and local income and employment taxes. Participant hereby authorizes the Company to satisfy any required withholding to satisfy federal, state, local, payroll, and foreign tax withholding obligations of the Company or any Related Entity that arise in connection with the Units through any method authorized in the Plan. Unless otherwise determined by the Administrator in its sole discretion, Participant acknowledges that the Company will satisfy such tax withholding obligation by arranging for the sale, by a broker of the Company’s choosing, of such number of Shares otherwise deliverable to the Participant equal in value to the tax obligation required to be withheld (plus any applicable broker commission). Participant understands that all matters with respect to the total amount of taxes to be withheld in respect of such compensation income will be determined by the Company in its reasonable discretion. Participant further understands that, although the Company will pay withheld amounts to the applicable taxing authorities, Participant remains responsible for payment of all taxes due as a result of income arising under the Agreement.
10. Application of Section 409A.
(a) The parties intend that this Agreement and the delivery of Shares or other consideration in respect of the Units provided under this Agreement satisfies, to the greatest extent possible, the exemption from the application of Section 409A provided under Treasury Regulations section 1.409A-1(b)(4) (or any other applicable exemption), and this Agreement will be construed to the greatest extent possible as consistent with those provisions. To the extent not so exempt, the delivery of Shares in respect of the Units provided under this Agreement will be conducted, and this Agreement will be construed, in a manner that complies with Section 409A and is consistent with the requirements for avoiding taxes or penalties under Section 409A. The parties further intend that each installment of any payments provided for in this Agreement is a separate “payment” for purposes of Section 409A.
27
(b) To the extent any payment hereunder due upon the termination of the Participant’s Continuous Service is deferred compensation that is subject to Section 409A, and is not otherwise exempt from complying with the provisions of Section 409A, then such payment will not be made unless and until Participant has also incurred a “separation from service” (as such term is defined in Treasury Regulation section 1.409A-1(h)). To the extent that (i) one or more of the payments received or to be received by the Participant pursuant to this Agreement would constitute deferred compensation subject to the requirements of Section 409A, and (ii) the Participant is a “specified employee” within the meaning of Section 409A, then solely to the extent necessary to avoid the imposition of any additional taxes or penalties under Section 409A, the commencement of any payments under this Agreement will be deferred until the date that is six months and one day following the Participant’s termination of Continuous Service (or, if earlier, the date of death of the Participant) and will instead be paid on the date that immediately follows the end of such period (or death) or as soon as administratively practicable within 30 days thereafter.
(c) The Company makes no representations to Participant regarding the compliance of this Agreement or the Units with Section 409A, and Participant is solely responsible for the payment of any taxes or penalties arising under Section 409A(a)(1), or any state law of similar effect, with respect to the grant or vesting of the Units or the delivery of the Shares subject to this Award.
11. Notices. Any notice or request required or permitted hereunder must be given in writing to each of the other parties hereto and will be deemed effectively given on the earlier of (a) the date of personal delivery, (b) one business day after deposit in the custody of a reputable overnight delivery service with next business day charges prepaid, or (c) three business days after the date of deposit in the United States Mail by registered or certified mail, postage prepaid, return receipt requested, addressed in the case of the Company to the Company’s Chief Executive Officer at the Company’s primary business address and in the case of the Participant to the most recent address shown in the Company’s records.
12. Incorporation of the Plan; Entire Agreement; Modification. The Award is subject to all the provisions of the Plan, the provisions of which are hereby made a part of this Agreement, and is further subject to all interpretations, amendments, rules and regulations which may from time to time be promulgated and adopted pursuant to the Plan. In the event of any conflict between the provisions of this Agreement and those of the Plan, the provisions of the Plan will control. This Agreement (including the Plan and the Grant Notice) sets forth all of the promises, agreements, conditions and understandings between the parties hereto with respect to the Award, and there are no promises, agreements, conditions, understandings, warranties or representations, oral or written, express or implied, between them with respect to the Award other than as set forth therein or herein. This Agreement (including the Plan and the Grant Notice) supersedes and replaces any and all prior agreements between the parties hereto with respect to the Units granted under this Award. Except as provided by the Plan, no modification, amendment or waiver of any of the provisions of this Agreement will be effective unless approved in writing by both parties.
28
13. Choice of Law. The interpretation, performance and enforcement of this Agreement and the Grant Notice will be governed by the law of the State of Delaware without regard to principles of choice or conflict of laws (whether of the State of Delaware or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of Delaware.
14. Miscellaneous.
(a) The headings of the Sections in this Agreement are inserted for convenience only and will not be deemed to constitute a part of this Agreement or to affect the meaning of this Agreement.
(b) If all or any part of this Agreement or the Plan is declared by any court or governmental authority to be unlawful or invalid, such unlawfulness or invalidity will not invalidate any portion of this Agreement or the Plan not declared to be unlawful or invalid. Any Section of this Agreement (or part of such a Section) so declared to be unlawful or invalid will, if possible, be construed in a manner which will give effect to the terms of such Section or part of a Section to the fullest extent possible while remaining lawful and valid.
(c) This Agreement will inure to the benefit of and be binding upon the parties hereto and their respective heirs, executors, administrators, successors and assigns. The rights and obligations of the Company under this Agreement will be transferable by the Company to any one or more persons or entities, and all covenants and agreements hereunder will inure to the benefit of, and be enforceable by, the Company’s successors and assigns.
(d) The waiver by either party of compliance with any provision of this Agreement by the other party will not operate or be construed as a waiver of any other provision of this Agreement, or of any subsequent breach by such party of a provision of this Agreement.
(e) Participant agrees upon request to execute any further documents or instruments necessary or desirable in the sole determination of the Company to carry out the purposes or intent of the Award.
(f) Participant further acknowledges receipt or the right to receive a document providing the information required by Rule 428(b)(1) promulgated under the Securities Act of 1933, as amended.
(g) This Agreement will be subject to all applicable laws, rules, and regulations, and to such approvals by any governmental agencies or national securities exchanges as may be required.
(h) All obligations of the Company under the Plan and this Agreement will be binding on any successor to the Company, whether the existence of such successor is the result of a direct or indirect purchase, merger, consolidation, or otherwise, of all or substantially all of the business and/or assets of the Company.
29
ADAPTIN BIO, INC.
2025 EQUITY INCENTIVE PLAN
NOTICE OF STOCK OPTION GRANT
Participant:
You have been granted an option to purchase shares of the Common Stock of Adaptin Bio, Inc. (the “Company”) as follows, subject to the terms of the Adaptin Bio, Inc. 2025 Equity Incentive Plan (the “Plan”) and the attached Stock Option Award Agreement.
| Date of Grant: [●] | Vesting Commencement Date: [●] |
| Exercise Price per Share: [●] | Expiration Date: [●] |
| Total Shares Subject to Option: [●] | Total Exercise Price: [●] |
| Vesting Schedule: [●] | Type of Option: [●]
Note: If the Option is designated a Non-statutory Stock Option above, or if the Option otherwise fails to qualify as an incentive stock option pursuant to Section 422 of the Code, then this Option will not be treated as an incentive stock option within the meaning of Section 422 of the Code.
|
Exercise Period: The Option may be exercised for up to three months after the termination of Continuous Service to the Company or a Related Entity, except as set out in Section 4 of the Stock Option Award Agreement (but in no event later than the Expiration Date); provided that upon a termination for Cause the Option will be immediately terminated.
In no event may this Option be exercised after the Expiration Date as provided above. | |
[SIGNATURE PAGE FOLLOWS]
30
By your signature and the signature of the Company’s representative below, you and the Company agree that this Option is granted under and governed by the terms and conditions of the Plan and the attached Stock Option Agreement, all of which are made a part of this document.
| COMPANY: | ||
| Adaptin Bio, Inc. | ||
| By: | ||
| Name: | ||
| Title: | ||
| PARTICIPANT: | ||
| [NAME] | ||
| Address: | ||
31
ADAPTIN BIO, INC.
2025 EQUITY INCENTIVE PLAN
STOCK OPTION Award Agreement
This Stock Option Award Agreement (this “Agreement”) is made by and between Adaptin Bio, Inc. (the “Company”) and _____________________ (“Participant”) effective as of the Date of Grant shown on the accompanying Notice of Stock Option Grant (the “Grant Notice”). Capitalized terms not explicitly defined in this Agreement but defined in the Company’s 2025 Equity Incentive Plan (the “Plan”) will have the same definition and meaning as in the Plan.
1. Grant of Option. The Company has granted to Participant an option to purchase, on the terms and conditions set forth in the Plan and this Agreement, all or any part of the number of Shares described in the Grant Notice, at the Exercise Price set forth in the Grant Notice (the “Option”), subject to adjustment as set forth in Section 13 of the Plan.
2. Vesting. Subject to the terms and conditions set forth in the Plan and this Agreement, the Option will vest as provided in the Grant Notice, provided that vesting will cease upon the termination of Participant’s Continuous Service.
3. Forfeiture; Expiration. Any unvested portion of the Option will be forfeited immediately, automatically, and without consideration upon a termination of Participant’s Continuous Service for any reason. In the event Participant’s Continuous Service is terminated for Cause, the vested portion of the Option will also be forfeited immediately, automatically, and without consideration upon that termination for Cause. Any unexercised vested portion of the Option will expire on the Expiration Date set forth in the Grant Notice.
4. Period of Exercise. Subject to the terms and conditions set forth in the Plan and this Agreement, Participant may exercise all or any part of the vested portion of the Option at any time prior to the earliest to occur of:
(a) the Expiration Date indicated in the Grant Notice;
(b) the effective date of the termination of Participant’s Continuous Service for Cause;
(c) the date that is 12 months after the termination of Participant’s Continuous Service due to his or her death or Disability, provided, however, that in the event Participant dies within such 12 month period after the termination of Participant’s Continuous Service due to his or her Disability, the period for exercise will be extended until the date 12 months after his or her death (but in no event later than the Expiration Date); or
(d) the date that is three months after the termination of Participant’s Continuous Service for any reason other than Cause, Disability or death; provided however, that in the event that Participant dies within such three month period, the period for exercise will be extended until the date 12 months after his or her death (but in no event later than the Expiration Date).
32
5. Exercise of Option. Participant or, in the case of Participant’s death or Disability, Participant’s representative, may exercise all or any part of the vested portion of the Option by delivering to the Company at its principal office a written notice of exercise in the form attached as Exhibit A or any other form that the Administrator may permit (such notice, a “Notice of Exercise”), signed by the person exercising the Option. In the event that the Option is being exercised by Participant’s representative, the Notice of Exercise must be accompanied by proof (satisfactory to the Administrator) of the representative’s right to exercise the Option. In addition, any exercise of the Option, whether in whole or in part, is subject to the following conditions:
(a) Participant (or Participant’s representative, if applicable) will deliver to the Company, at the time of giving the Notice of Exercise, payment in a form permissible under Section 6 below for the full amount of the Purchase Price.
(b) Participant (or Participant’s representative, if applicable) may exercise the Option only for whole Shares.
(c) Participant (or Participant’s representative, if applicable) may not exercise the Option unless the tax withholding obligations of the Company and/or any Related Entity, as described in Section 9 below, are satisfied.
(d) In the event that Participant is an employee eligible for overtime compensation under the Fair Labor Standards Act of 1938, as amended (sometimes referred to as a “non-exempt employee”), then he or she may not exercise the Option until he or she has completed at least six months of Continuous Service measured from the Date of Grant specified in the Grant Notice, notwithstanding any other provision of the Option.
6. Payment for Shares. The “Purchase Price” will be the Exercise Price multiplied by the number of Shares with respect to which the Option is being exercised. The Purchase Price may be paid as follows:
(a) in cash;
(b) by check or money order;
(c) by surrender to the Company (either by actual delivery or attestation) of already-owned shares of Common Stock that are owned by Participant free and clear of any liens, claims, encumbrances or security interests, with a Fair Market Value on the date of surrender or attestation equal to the Purchase Price (provided that Participant may not exercise the Option by tender to the Company of Common Stock to the extent such tender would violate the provisions of any law, regulation or agreement restricting the redemption of the Company’s stock);
(d) through a formal “net exercise” arrangement adopted by the Company pursuant to which Participant may exercise the Option and receive the net number of Shares equal to (i) the number of Shares as to which the Option is being exercised, multiplied by (ii) a fraction, the numerator of which is the Fair Market Value per Share (on such date as is determined by the Administrator) less the Exercise Price per Share, and the denominator of which is such Fair Market Value per Share;
33
(e) through a broker-dealer sale and remittance procedure pursuant to which Participant (i) provides written instructions to a Company designated brokerage firm to effect the immediate sale of some or all of the purchased Shares and remit to the Company sufficient funds to cover the aggregate Exercise Price payable for the purchased Shares and (ii) provides written directives to the Company to deliver the certificates (or other evidence satisfactory to the Company to the extent that the Shares are uncertificated) for the purchased Shares directly to such brokerage firm in order to complete the sale transaction; or
(f) any combination of the foregoing methods of payment.
7. Securities Law Compliance. No Shares will be issued pursuant to this Agreement unless and until all then applicable requirements imposed by federal and state securities and other laws, rules and regulations and by any regulatory agencies having jurisdiction, and by any exchanges upon which the Shares may be listed, have been fully met. The Company may impose such conditions on any Shares issuable pursuant to this Agreement as it may deem advisable, including, without limitation, restrictions under the Securities Act of 1933, as amended (the “Securities Act”), under the requirements of any exchange upon which shares of the same class are then listed and under any blue sky or other securities laws applicable to those Shares.
8. Notice of Disqualifying Disposition. If the Option is designated as an Incentive Stock Option, then in the event of a Disqualifying Disposition, Participant will immediately, and in any event not later than 15 days after such disposition, notify the Company in writing of such disposition.
9. Withholding Obligations. Participant may incur Tax Obligations under federal, state, local, and/or foreign law, in connection with the grant, vesting, or exercise of the Option, the ownership of the Shares, and other actions taken pursuant to this Agreement, and the Company may be required to satisfy by withholding from Participant’s compensation or otherwise collect from Participant. Participant agrees that the exercise of the Option is conditioned upon the satisfaction of such withholding tax obligations, and the Company may satisfy such withholding obligations by any of the following means or by a combination of such means, in the Administrator’s discretion: (i) withholding from any compensation otherwise payable to Participant by the Company; (ii) causing Participant to tender a cash payment; or (iii) withholding from the Shares otherwise issuable to Participant upon exercise of the Option the number of Shares with a Fair Market Value (measured as of the date the tax withholding obligations are to be determined) sufficient to satisfy such tax withholding obligations; provided, however, that the number of such Shares so withheld will not exceed the amount necessary to satisfy the Company’s required tax withholding obligations using the statutory withholding rates for federal, state, local and foreign tax purposes, including payroll taxes as determined by the Administrator. Participant understands that all matters with respect to the total amount of taxes to be withheld in respect of such compensation income will be determined by the Administrator in its reasonable discretion. Participant further understands that, although the Company will pay withheld amounts to the applicable taxing authorities, Participant remains responsible for payment of all taxes due as a result of income arising under the Agreement.
34
10. Rights as a Stockholder. Neither Participant nor anyone claiming through him/her will have any rights as a stockholder of the Company with respect to any Shares subject to the Option until the Participant has exercised the Option as described herein and the Shares are delivered (as evidenced by delivery of a certificate for such Shares or the appropriate entry on the books of the Company or of a duly authorized transfer agent of the Company).
11. Transferability. The Option may not be sold, pledged, assigned, hypothecated, transferred, except by will or by the laws of descent and distribution and in accordance with the Applicable Laws, and is exercisable during Participant’s life only by Participant. Notwithstanding the foregoing, by delivering written notice to the Company, in a form satisfactory to the Administrator, Participant may designate a third party who, in the event of Participant’s death, will thereafter be entitled to exercise the Option.
12. Option Not a Service Contract. Neither the Option nor this Agreement is an employment or service contract, and nothing in this Agreement or the Grant Notice creates or will be deemed to create in any way whatsoever any obligation on Participant’s part to continue in the service of the Company or a Related Entity, or of the Company or a Related Entity to continue Participant’s service.
13. Governing Plan Document. This Option is subject to all the provisions of the Plan, the provisions of which are hereby made a part of this Agreement, and is further subject to all interpretations, amendments, rules and regulations, which may from time to time be promulgated and adopted pursuant to the Plan. Participant acknowledges receipt of a copy of the Plan. In the event of any conflict between the provisions of this Agreement and those of the Plan, the provisions of the Plan will control.
14. Miscellaneous.
(a) Notices. Any notice, demand or request required or permitted to be given pursuant to the terms of this Agreement will be in writing and will be deemed given when delivered personally, one day after deposit with a recognized international delivery service (such as FedEx), or three days after deposit in the U.S. mail, first class, certified or registered, return receipt requested, with postage prepaid, in each case addressed to the parties at the addresses of the parties set forth in the Grant Notice or such other address as a party may designate by notifying the other in writing.
(b) Successors and Assigns. The provisions of this Agreement will inure to the benefit of, and be binding upon, the Company and its successors and assigns and upon the Participant, Participant’s executor, personal representative(s), distributees, administrators, permitted transferees, permitted assignees, beneficiaries, and legatee(s), as applicable, whether or not any such person will have become a party to this Agreement and have agreed in writing to be joined herein and be bound by the terms hereof.
(c) Severability. The provisions of this Agreement are severable, and if any one or more provisions are determined to be illegal or otherwise unenforceable, in whole or in part, then the remaining provisions will nevertheless be binding and enforceable.
(d) Amendment. Except as otherwise provided in the Plan, this Agreement will not be amended unless the amendment is agreed to in writing by both Participant and the Company.
(e) Choice of Law. This Agreement will be construed and enforced in accordance with and governed by the laws of the State of Delaware, without giving effect to the choice of law rules of any jurisdiction.
(f) Entire Agreement. This Agreement, along with the Grant Notice and the Plan, constitutes the entire agreement between the parties hereto with regard to the subject matter hereof, and supersedes any other agreements, representations or understandings (whether oral or written and whether express or implied) that relate to such subject matter.
35
EXHIBIT A
ADAPTIN BIO, INC.
2025 EQUITY INCENTIVE PLAN
NOTICE OF EXERCISE
Adaptin Bio, Inc.
____________________________
____________________________
Attention: [President]
Date of Exercise: _________________
1. Exercise of Option. This constitutes notice to Adaptin Bio, Inc. (the “Company”) that, pursuant to the Adaptin Bio, Inc. 2025 Equity Incentive Plan (the “Plan”) and the Stock Option Award Agreement, dated _________, 20 _ (the “Award Agreement”), I elect to purchase the number of Shares set forth below for the price set forth below.
| Number of Shares as to which Option is exercised (the “Optioned Shares”): | |||
| Exercise Price per Share: | |||
| Total Purchase Price: |
2. Delivery of Payment. With this notice, I hereby deliver to the Company the full Purchase Price for the Optioned Shares, in a form permitted by the Award Agreement.
3. Representations. By signing and delivering this notice to the Company, I acknowledge that I am the holder of the Option exercised by this notice and have full power and authority to exercise the Option. I further represent that I have received, read, and understood the Plan and the Award Agreement, and I confirm my agreement to abide by and be bound by their terms and conditions. Capitalized terms used and not otherwise defined in this notice will have the meanings ascribed to those terms in the Award Agreement.
4. Compliance with Securities Laws. Notwithstanding any other provision of the Award Agreement to the contrary, the exercise of any rights to purchase any Optioned Shares is expressly conditioned upon compliance with the Securities Act, all applicable state securities laws and all applicable requirements of any stock exchange or over the counter market on which the Company’s Common Stock may be listed or traded at the time of exercise and transfer. I agree to cooperate with the Company to ensure compliance with such laws. I further understand that the Optioned Shares cannot be resold and must be held indefinitely unless they are registered under the Securities Act or unless an exemption from such registration is available and that the certificate(s) representing the Optioned Shares may bear a legend to that effect. I understand that the Company is under no obligation to register the Optioned Shares and that an exemption may not be available or may not permit me to transfer Optioned Shares in the amounts or at the times I may desire.
36
5. Tax Withholding. I acknowledge that my exercise of the Option may result in Tax Obligations which require the Company to withhold certain amounts to satisfy federal, state, local, and/or foreign taxes. I agree to satisfy such tax withholding obligations as described in Section 9 of the Award Agreement.
6. Rights as a Stockholder. While the Company will endeavor to process this notice in a timely manner, I acknowledge that, until the issuance of the Optioned Shares (or, in the Company’s discretion, in un-certificated form, upon the books of the Company’s transfer agent) and my satisfaction of any other conditions imposed by the Company pursuant to the Plan or as set forth in the Award Agreement, no right to vote or receive dividends or any other rights as a stockholder will exist with respect to the Optioned Shares, notwithstanding the exercise of my Option. No adjustment will be made for a dividend or other right for which the record date is prior to the date of issuance of the Optioned Shares.
7. Tax Consultation. I understand that I may experience adverse tax consequences as a result of my exercise of the Option or my disposition of the Optioned Shares. I represent that I have consulted with any tax consultants I deem advisable in connection with the exercise of the Option and/or the disposition of the Optioned Shares and that I am not relying on the Company or its agents for any tax advice.
8. Interpretation. Any dispute regarding the interpretation of this notice will be resolved by the Administrator in its discretion, and the Administrator’s determination will be final and binding on all parties.
9. Entire Agreement. The Plan and the Award Agreement under which the Option was originally granted are incorporated herein by reference and, together with this notice, constitute the entire agreement of the parties with respect to the subject matter of this notice.
| PARTICIPANT: | ||
| Print Name: | ||
| Address: | ||
37
Exhibit 10.10
EXECUTIVE EMPLOYMENT AGREEMENT
This Executive Employment Agreement (this “Agreement”) is entered into as of February 11, 2025, by and between Adaptin Bio, Inc. (f/k/a Unite Acquisition 1 Corp.), a Delaware corporation (the “Company”), and Simon Pedder (the “Executive”).
WHEREAS, in connection with, and conditioned upon the completion of, the merger (the “Merger”) between the wholly-owned subsidiary of Unite Acquisition 1 Corp. and private company Adaptin Bio, Inc. (“Adaptin”), the Company wishes to employ Executive as its Executive Chairman, and Executive wishes to accept such employment, on the terms set forth in this Agreement;
WHEREAS Executive acknowledges and agrees that through Executive’s association with Adaptin as an employee, Executive will acquire a considerable amount of knowledge and goodwill with respect to the business of the Company, which knowledge and goodwill are highly valuable to the Company and which would be detrimental to the Company if used by Executive to compete with the Company; and
WHEREAS the Company wishes to protect its investment in its business, employees, customer relationships, and confidential information, by requiring Executive to abide by certain restrictive covenants regarding confidentiality, non-competition, and non-solicitation, each of which is an inducement to the Company to enter into this Agreement;
NOW, THEREFORE, in consideration of the foregoing, the mutual agreements contained herein, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1. Employment. Subject to the terms and conditions of this Agreement, the Company hereby employs Executive, and Executive accepts such employment. The employment relationship hereunder will be for the period commencing on the closing of the Merger (such date, the “Effective Date”) and will continue for a term of three years (the “Initial Term”) unless terminated sooner in accordance with Section 5 below. After the Initial Term expires, this Agreement will automatically renew for successive one-year terms (subject, in any event, to termination in accordance with Section 5 below), unless either party provides written notice of its intent not to renew the Agreement at least 90 days prior to the expiration of the then-current term. The period from the Effective Date through the date of the termination of Executive’s employment hereunder is referred to herein as the “Term.”
2. Position and Duties.
(a) Position. Executive will serve as the Company’s Executive Chairman, reporting to the Company’s Board of Directors (the “Board”). Executive will perform such services for the Company and have such powers, responsibilities and authority as are customarily associated with the position of Executive Chairman in similar companies, and will perform such additional duties as may otherwise be reasonably assigned to Executive from time to time by the Board.
(b) Part-Time Employment. Executive will devote approximately 50% of Executive’s business time, attention, and efforts to the affairs of the Company and to the duties hereunder, and will perform such duties diligently and to the best of Executive’s ability, in compliance with the Company’s policies and procedures and the laws and regulations that apply to the Company’s business. Notwithstanding the foregoing, Executive may (i) participate in charitable, civic, educational, professional, community or industry affairs, (ii) continue to serve as a director for Voltron Therapeutics, and (iii) manage Executive’s passive personal investments, so long as, in each case, such activities individually or in the aggregate do not materially interfere or conflict with the Executive’s duties hereunder or create a potential business or fiduciary conflict (in each case, as determined by the Board).
- 1 -
(c) Board Service. The Company will use commercially reasonable efforts to cause the Executive to be elected as a member of the Board throughout the Term and will include him in the management slate for election as a director at every stockholders meeting during the Term at which his term as a director would otherwise expire. Executive agrees to accept election, and to serve during the Term, as director of the Company, without any compensation therefor other than as specified in this Agreement.
(d) Principal Work Location. Executive’s principal workplace for the performance of his duties under this Agreement will be at the Company’s headquarters or the Executive’s residence. Notwithstanding the foregoing, the Executive will be required to travel as necessary to perform his duties hereunder.
3. Compensation and Benefits. As compensation for the services to be rendered by Executive under this Agreement, the Company will provide the following compensation and benefits during Executive’s employment hereunder.
(a) Base Salary. During the Term, the Company will pay Executive a base salary at an annual rate of $240,000 (the “Base Salary”). The Base Salary will be payable in equal installments in accordance with the Company’s payroll practices as in effect from time to time. The Base Salary will be reviewed by the Board from time to time, and may be increased in the sole discretion of the Board. Executive’s salary may not be reduced except in connection with an across-the-board reduction of executive level salaries in which Executive will not be subject to a greater reduction, on a percentage basis, than any other executive-level employee.
(b) Bonus Opportunity. For each calendar year ending during the Term, Executive will be eligible to receive an annual bonus (the “Annual Bonus”) with a target amount of 30% of Executive’s Base Salary. The actual amount of each Annual Bonus will be based upon the level of achievement of the Company’s corporate objectives and the Executive’s individual objectives, in each case, as established by the Board for the calendar year with respect to which such Annual Bonus relates. The Board has the authority to increase the Annual Bonus to up to 100% of the Executive Base Salary if the fiscal year corporate goals are exceeded or based on exemplary performance during the fiscal year by the Executive. The determination of the level of achievement of the corporate objectives and the Executive’s individual performance objectives for a year will be made by the Board in its reasonable discretion. The Annual Bonus for a calendar year, to the extent earned, will be paid in a lump sum in the following calendar year. The Annual Bonus will not be deemed earned until the date that it is paid. Accordingly, in order for the Executive to receive an Annual Bonus, the Executive must be actively employed by the Company at the time of such payment.
(c) Equity Compensation.
(i) Subject to approval by the Board and subject to the terms of the Company’s equity incentive plan then in place, in the event the Company issues additional securities raising aggregate funds of $10,000,000 (in one or more transactions), occurring, if at all, within two years following the Merger (the “Additional Financing Period”), the Company will grant Executive options to purchase a number of shares of common stock of the Company (the “Anti-Dilution Options”) sufficient to ensure that Executive’s ownership immediately following the Additional Financing Period, on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable) is equal to Executive’s ownership immediately following the Merger, as determined on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable). The per share exercise price of the Anti-Dilution Options will be equal to the fair market value of a share of the Company’s common stock on the date of grant, as determined by the Board. The Anti-Dilution Options, if any, will become exercisable in four equal annual installments, in each case subject to Executive’s continued employment with the Company on the date each such vesting milestone is achieved. The Anti-Dilution Options will be subject to the terms of the Company’s equity incentive plan then in place and a related option grant agreement to be entered between Executive and the Company.
- 2 -
(ii) During the Term, Executive will be eligible to receive from time to time such equity grants or awards, if any, pursuant to the terms of any equity incentive plan of the Company (or any successor plan as may be in place from time to time) as may be approved by the Board in its discretion. Such grants or awards will be subject to the terms and conditions of such plan (or any successor plan) and such other terms and conditions as the Board in its discretion may establish.
(d) Paid Vacation. Executive will be entitled to paid vacation days in accordance with the Company’s vacation policies in effect from time to time for its executive team; provided, however that the Executive will be entitled to no less than 25 paid vacation days per calendar year during the Term.
(e) General Benefits. Executive will be entitled to such other benefits, and to participate in such benefit plans, as are generally made available to similarly situated senior executive employees of the Company from time to time, subject to Company policy and the terms and conditions of any applicable benefit plans. Nothing in this Agreement will be deemed to alter the Company’s rights to modify or terminate any such plans or programs in its sole discretion.
(f) Withholdings. The Company will withhold from any amounts payable under this Agreement such federal, state, and local taxes as the Company determines are required to be withheld pursuant to applicable law.
(g) Recoupment of Compensation. Any incentive-based or other compensation paid to Executive under this Agreement or any other agreement or arrangement with the Company which is subject to recovery under any law, government regulation, stock exchange listing requirement or any compensation recovery or clawback policy adopted by the Company from time to time will be subject to reduction, cancellation, forfeiture or recoupment as may be required by such law, government regulation, stock exchange listing requirement or policy. In addition, if Executive is or becomes an executive officer subject to the incentive compensation repayment requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), then if required by the Dodd-Frank Act or any of its regulations Executive will enter into an amendment to this Agreement or a separate written agreement with the Company to comply with the Dodd-Frank Act and any of its regulations.
4. Reimbursement of Expenses. The Company will reimburse Executive for all reasonable business expenses incurred by Executive in connection with the performance of Executive’s duties hereunder, subject to Executive’s compliance with the Company’s reimbursement policies in effect from time to time.
5. Termination. This Agreement, and Executive’s employment hereunder, are subject to termination as follows.
(a) Death. Automatically effective upon Executive’s death.
(b) Disability. By the Company upon notice to Executive in the event of Executive’s Disability. As used herein, “Disability” means the inability of Executive, due to the condition of Executive’s physical, mental or emotional health, effectively to perform the essential functions of Executive’s job with or without reasonable accommodation for a continuous period of at least 90 consecutive days or for 120 days in any period of 12 consecutive months, as determined by the Board in good faith. In any event, the Company will comply fully with the applicable provisions of the Americans with Disabilities Act, as amended, the Family and Medical Leave Act, and any similar applicable law. For purposes of making a determination as to whether a Disability exists, at the Company’s request Executive agrees to make himself available and to cooperate in a reasonable examination by a reputable independent physician retained by the Company and to authorize the disclosure and release to the Company of all medical records related to such examination.
(c) For Cause. By the Company effective upon written notice to Executive for Cause. For purposes of this Agreement, “Cause” means: (i) Executive’s fraud, embezzlement or misappropriation with respect to the Company; (ii) Executive’s willful or grossly negligent misconduct that has or may reasonably be expected to have a material adverse effect on the property, business, or reputation of the Company; (iii) Executive’s material breach of this Agreement; (iv) Executive’s willful failure or refusal to perform Executive’s material duties under this Agreement or willful failure to follow any specific lawful instructions of the Board; (v) Executive’s conviction or plea of nolo contendere in respect of a felony or of a misdemeanor involving moral turpitude; or (vi) Executive’s material failure to comply with the Company’s workplace rules, policies, or procedures. In the event that the Company concludes that Executive has engaged in acts constituting in Cause as defined in clause (iii) or (iv) above, prior to terminating this Agreement for Cause the Company will provide Executive with at least 15 days’ advance notice of the circumstances constituting such Cause, and an opportunity to correct such circumstances, to the extent such circumstances are susceptible of being corrected.
- 3 -
(d) Without Cause. By the Company effective upon 90 days advance written notice to Executive at any time for any reason other than for Cause or Executive’s Disability.
(e) Resignation for Good Reason. Executive may resign his employment for Good Reason by complying with the Good Reason Procedure as described herein. The “Good Reason Procedure” means (i) Executive reasonably determines in good faith that a Good Reason condition has occurred; (ii) Executive notifies the Company in writing of the first occurrence of the Good Reason condition within 60 days of the first occurrence of such condition; (iii) Executive cooperates in good faith with the Company’s efforts, for a period not less than 30 days following such notice (the “Good Reason Cure Period”), to remedy the condition; (iv) notwithstanding such efforts, the Good Reason condition continues to exist; and (v) Executive terminates Executive’s employment within 60 days after the end of the Good Reason Cure Period. If the Company cures the Good Reason condition during the Good Reason Cure Period, Good Reason will be deemed not to have occurred. For purposes of this Agreement, “Good Reason” means the occurrence of any of the following events without Executive’s written consent: (i) the Company’s requiring Executive to be based at any office or location more than 25 miles from his principal work location as of the Effective Date, except for travel reasonably required in the performance of Executive’s responsibilities to the Company; (ii) a material reduction of Executive’s Base Salary not in compliance with Section 3(a) above; (iii) a material diminution of the Executive’s authority, duties, or responsibilities; or (iv) the Company’s material breach of this Agreement.
(f) Other Resignation. By Executive effective upon 30 days’ written notice to the Company at any time for any reason.
(g) Employment During Notice Periods. During any notice period under Sections 5(c), 5(e), or 5(f) the Company may, in its sole discretion, relieve Executive of some or all of his duties during the notice period, but the Company will continue to provide Executive with all required salary and benefits during such period.
6. Effect of Termination.
(a) Generally. When Executive’s employment with the Company is terminated for any reason, Executive, or Executive’s estate, as the case may be, will be entitled to receive the compensation and benefits earned through the effective date of termination, along with reimbursement for any unreimbursed business expenses incurred through the date of termination that Executive has timely submitted (or does timely submit) for reimbursement in accordance with the Company’s expense reimbursement policy or practice.
(b) Resignation from Positions. Immediately upon the termination of Executive’s employment with the Company for any reason, Executive will be deemed to have resigned from all positions as an officer or director of the Company, along with any other positions he may hold with or for the benefit of the Company and/or its affiliates. In furtherance of the preceding sentence, Executive will execute and return to the Company all letters and documents that the Company may reasonably require in order to evidence such resignation(s), but Executive’s failure to execute and return such documents will not have the effect of delaying or in any way invalidating the resignation(s) provided for by the preceding sentence.
(c) Separation Benefits upon Certain Terminations not in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 24 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination; and
(ii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for 18 months or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Internal Revenue Code of 1986, as amended (the “Code”).
- 4 -
(d) Separation Benefits upon Certain Terminations in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), in either case at the time of, or within 6 months following, a Change in Control (as defined below) then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “CIC Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 24 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination;
(ii) the vesting of all stock options, restricted stock units, and other equity awards will be accelerated such that all such awards are deemed to have been vested as of the date of termination; and
(iii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for 18 months or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Code.
(e) Change in Control Defined. As used in this Agreement, the term “Change in Control” means the occurrence of any one of the following events:
(i) any “person,” including a “group” (as such terms are used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), but excluding the Company, any entity controlling, controlled by or under common control with the Company, any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any such entity, and, with respect to any particular qualified participant of any equity incentive plan of the Company (a “Participant”), the Participant and any “group” (as such term is used in Section 13(d)(3) of the Exchange Act) of which the Participant is a member), is or becomes the “beneficial owner” (as defined in Rule 13(d)(3) under the Exchange Act), directly or indirectly, of securities of the Company representing 50% or more of either (A) the combined voting power of the Company’s then outstanding securities; or (B) the Company’s then outstanding equity securities (in either such case other than as a result of an acquisition of securities directly from the Company);
(ii) any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own (as such term is defined in Rule 13d-3 under the Exchange Act), directly or indirectly, equity securities representing in the aggregate 50% or more of the combined voting power of the securities of the entity issuing cash or securities in the consolidation or merger (or of its ultimate parent entity, if any);
(iii) any sale, lease, exclusive license, exchange or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of the assets of the Company, other than a sale or disposition by the Company of all or substantially all of the Company’s assets to an entity, at least 50% of the combined voting power of the voting securities of which are owned by “persons” (as defined above) in substantially the same proportion as their ownership of the Company immediately prior to such sale; or
(iv) the members of the Board at the beginning of any consecutive 24-calendar-month period (the “Incumbent Directors”) cease for any reason other than due to death to constitute at least a majority of the members of the Board; provided that any director whose election, or nomination for election by the Company’s stockholders, was approved or ratified by a vote of at least a majority of the members of the Board then still in office who were members of the Board at the beginning of such 24-calendar-month period, will be deemed to be an Incumbent Director.
- 5 -
Notwithstanding the foregoing, no event or condition will constitute a Change in Control to the extent that, if it were, a penalty tax would be imposed under Section 409A; provided that, in such a case, the event or condition will continue to constitute a Change in Control to the maximum extent possible (e.g., if applicable, in respect of vesting without an acceleration of distribution) without causing the imposition of such penalty tax.
(f) Release. The Company’s obligation to provide the Separation Benefits or the CIC Separation Benefits, as applicable, is conditioned upon Executive executing and returning a release of claims in a form acceptable to the Company (the “Release”) within the time specified therein, which Release is not revoked within any time period allowed for revocation under applicable law.
(g) Post-Termination Breach. If Executive is entitled to receive the Separation Benefits or the CIC Separation Benefits but violates any provisions of Sections 8 through 10 hereof after termination of employment, the Company will be entitled to immediately stop paying any further installments of the Separation Benefits or the CIC Separation Benefits as applicable, in addition to any other remedies that may be available to the Company in law or at equity.
(h) No Further Obligations. Except as expressly provided above or as otherwise required by law, the Company will have no obligations to Executive in the event of the termination of this Agreement for any reason. For the avoidance of doubt, in no event will Executive be entitled to benefits under both Section 6(c) and 6(d).
7. Representations of Executive. Executive represents and warrants that he is not obligated or restricted under any agreement (including any non-competition or confidentiality agreement), judgment, decree, order or other restraint of any kind that could impair Executive’s ability to perform the duties and obligations required hereunder. Executive further agrees that Executive will not divulge to the Company any confidential information and/or trade secrets belonging to others, including Executive’s former employers. Consistent with the foregoing, Executive will not provide to the Company any documents or copies of documents containing such information.
8. Confidential Information.
(a) Executive acknowledges that the Company has and will give Executive access to certain highly-sensitive, confidential, and proprietary information belonging to the Company (or third parties who may have furnished such information under obligations of confidentiality), relating to and used in the Company’s business (collectively, “Confidential Information”). Executive acknowledges that Confidential Information includes, but is not limited to, the following categories of Company related confidential or proprietary information and material: financial statements and information; budgets, forecasts, and projections; business and strategic plans; marketing, sales, and business development strategies; research and development projects; records relating to any intellectual property developed by, owned by, controlled, or maintained by the Company; information related to the Company’s inventions, research, products, designs, methods, formulae, techniques, systems, processes; customer lists; non-public information relating to the Company’s customers, suppliers, distributors, or investors; the specific terms of the Company’s agreements or arrangements, whether oral or written, with any customer, supplier, vendor, or contractor with which the Company may be associated from time to time; and any and all information relating to the operation of the Company’s business which the Company may from time to time designate as confidential or proprietary or that Executive reasonably knows should be, or has been, treated by the Company as confidential or proprietary. Executive further understands that Company will receive confidential business information from third parties, including but not limited to the Company’s clients (the “Third Party Information”), under a duty to maintain the confidentiality of such Third Party Information and to use it only for limited purposes. Confidential Information and Third Party Information encompass all formats in which information is preserved, whether electronic, print, or any other form, including all originals, copies, notes, or other reproductions or replicas thereof.
(b) Confidential Information does not include any information that: (i) at the time of disclosure is generally known to, or readily ascertainable by, the public; (ii) becomes known to the public through no fault of Executive or other violation of this Agreement; or (iii) is disclosed to Executive by a third party under no obligation to maintain the confidentiality of the information.
(c) Executive acknowledges that the Confidential Information is owned or licensed by the Company (and Third Party Information is possessed by the Company with the permission of its owner); is unique, valuable, proprietary and confidential; derives independent actual or potential commercial value from not being generally known or available to the public; and is subject to reasonable efforts to maintain its secrecy. Executive hereby relinquishes and agrees that Executive will not at any time claim, any right, title or interest of any kind in or to any Confidential Information or Third Party Information.
- 6 -
(d) During and after Executive’s employment with the Company, Executive will hold in trust and confidence all Confidential Information and Third Party Information, and will not disclose any Confidential Information or Third Party Information to any person or entity, except in the course of performing duties assigned by the Company or as authorized in writing by the Company. Executive further agrees that during and after Executive’s employment with the Company, Executive will not use any Confidential Information or Third Party Information for any purpose, except in the course of performing duties assigned by the Company or as authorized in writing by the Company.
(e) Notwithstanding the covenants contained in Section 8(d) above, Executive may disclose Confidential Information or Third Party Information solely to the extent that Executive is required to do so by law, provided that Executive (i) notifies the Company of the existence and terms of such obligation, (ii) gives the Company a reasonable opportunity to seek a protective or similar order to prevent or limit such disclosure, and (iii) only discloses that information actually required to be disclosed.
(f) Nothing in this Agreement is intended to or will prohibit Executive from communicating with any governmental authority, or making a report in good faith and with a reasonable belief of any violations of law or regulation to a governmental authority, or from filing, testifying or participating in a legal proceeding relating to such violations, including making disclosures protected or required by any whistleblower law or regulation to the Securities and Exchange Commission, the Department of Labor, or any other appropriate government authority charged with the enforcement of any applicable laws. In addition, nothing in this Agreement is intended to or will limit any employee’s right to discuss the terms, wages, and working conditions of their employment, as protected by applicable law. Pursuant to the Defend Trade Secrets Act of 2016, Executive is hereby notified that an individual will not be held criminally or civilly liable under any federal or state trade secret law for the disclosure of a trade secret that (i) is made (A) in confidence to a federal, state or local government official, either directly or indirectly, or to an attorney; and (B) solely for the purpose of reporting or investigating a suspected violation of law; or (ii) is made in a complaint or other document filed in a lawsuit or other proceeding, if such filing is made under seal. An individual who files a lawsuit for retaliation by an employer for reporting a suspected violation of law may disclose the trade secret to his or her attorney and use the trade secret information in the court proceeding, if the individual files any document containing the trade secret under seal, and does not disclose the trade secret, except pursuant to court order.
(g) Return of Property. Upon request during employment and immediately at the termination of Executive’s employment, Executive will return to the Company all Confidential Information and Third Party Information in any form (including all copies and reproductions thereof) and all other property whatsoever of the Company in Executive’s possession or under Executive’s control. If requested by the Company, Executive will certify in writing that all such materials have been returned to the Company. Upon request by the Company, Executive will cooperate with the Company in transferring all logins, passwords, and other access information for accounts and systems previously used by Executive in connection with her employment with the Company. Executive also expressly agrees that immediately upon the termination of Executive’s employment with the Company for any reason, Executive will cease using any secure website, computer systems, e-mail system, or phone system or voicemail service provided by the Company for the use of its employees.
9. Assignment of Inventions.
(a) Executive agrees that all developments or inventions (including without limitation any and all software programs (source and object code), algorithms and applications, concepts, designs, discoveries, improvements, processes, techniques, know-how and data) that are initiated, conceived, discovered, reduced to practice, or made by Executive, either alone or in conjunction with others, during the Term and relate to or result from the Executive’s work for the Company or which relate directly to the Company’s businesses, whether or not patentable or registrable under copyright or similar statutes or subject to analogous protection (“Inventions”), will be the sole and exclusive property of the Company or its nominees. Executive will and hereby does assign to the Company all rights in and to such Inventions upon the creation of any such Invention, including, without limitation: (i) patents, patent applications and patent rights throughout the world; (ii) rights associated with works of authorship throughout the world, including copyrights, copyright applications, copyright registrations, mask work rights, mask work applications and mask work registrations; (iii) rights relating to the protection of trade secrets and confidential information throughout the world; (iv) rights analogous to those set forth herein and any other proprietary rights relating to intangible property; and (v) divisions, continuations, renewals, reissues and extensions of the foregoing (as applicable), now existing or hereafter filed, issued or acquired (collectively, the “IP Rights”).
- 7 -
(b) For avoidance of doubt, if any Invention falls within the definition of “work made for hire” as such term is defined in 17 U.S.C. § 101, such Invention(s) will be considered “work made for hire” and the copyright of such Invention(s) will be owned solely and exclusively by the Company. If any Invention does not fall within such definition of “work made for hire” then Executive’s right, title and interest in and to such Invention(s) will be assigned to the Company pursuant to Section 9(a) above.
(c) The Company and its nominees will have the right to use and/or to apply for statutory or common law protections for such Inventions in any and all countries. Executive further agrees, at the Company’s expense, to: (i) reasonably assist the Company in obtaining and from time to time enforcing such IP Rights relating to Inventions, and (ii) execute and deliver to the Company or its nominee upon reasonable request all such documents as the Company or its nominee may reasonably determine are necessary or appropriate to effect the purposes of this Section 9, including assignments of inventions. Such documents may be necessary to: (1) vest in the Company or its nominee clear and marketable title in and to Inventions; (2) apply for, prosecute and obtain patents, copyrights, mask works rights and other rights and protections relating to Inventions; or (3) enforce patents, copyrights, mask works rights and other rights and protections relating to Inventions. Executive’s obligations pursuant to this Section 9 will continue beyond the termination of Executive’s employment with the Company. Executive hereby irrevocably designates and appoints the Company and its then-current Chief Executive Officer as Executive’s agent and attorney-in-fact to act for and in behalf and instead of Executive, to execute and file any such application and to do all other lawfully permitted acts to further the prosecution and issuance of patents, trademarks, copyrights or other rights thereon with the same legal force and effect as if executed by Executive, if the Company is unable after reasonable effort to secure Executive’s signature to any lawful and necessary document required to apply for or execute any patent, trademark, copyright or other applications with respect to any Inventions (including renewals, extensions, continuations, divisions or continuations in part thereof).
(d) The obligations of Executive under Section 9(a) above will not apply to any Invention that Executive developed or conceived prior to the Effective Date or entirely on Executive’s own time after the Effective Date without using the Company’s equipment, supplies, facility or trade secret information, except for those Inventions that (i) relate directly to the Company’s business or actual or demonstrably anticipated research or development or (ii) result from any work performed by Executive for Company. Executive will bear the burden of proof in establishing the applicability of this subsection to a particular circumstance.
10. Restrictive Covenants.
(a) Purpose. Executive understands and agrees that the purpose of this Section 10 is solely to protect the Company’s legitimate business interests, including, but not limited to its confidential and proprietary information, customer relationships and goodwill, and the Company’s competitive advantage, and will not unduly impair Executive’s ability or right to work or earn a living. Therefore, Executive agrees to be subject to restrictive covenants under the following terms.
(b) Definitions. As used in this Agreement, the following terms have the meanings given to such terms below.
(i) “Business” means (A) the development or commercialization of treatments for diseases of the central nervous system, including cancers, and (B) the business(es) in which the Company was actively engaged at the time of, or during the 12-month period immediately preceding the Termination Date, provided that this clause (B) will only apply if Executive is involved with such other business.
(ii) “Company Employee” means any person who is or was an employee or independent contractor of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(iii) ”Customer” means any person or entity who is or was a customer or client of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date, and in either case with whom Executive had dealings on behalf of the Company in the course of his employment with the Company during such period, or about whom Executive learned or received confidential and proprietary information in the course of his employment with Company during such period.
- 8 -
(iv) “Prospective Customer” means any person or entity to whom, within the 12 months immediately prior to the Termination Date the Company had submitted a proposal for products or services of which Executive has knowledge, provided that Executive has had material business contacts with such person or entity on behalf of the Company during such 12-month period, whether such contact was initiated by the person or entity or by Executive.
(v) “Restricted Period” means the period commencing on the Termination Date and ending 12 months after such date, provided, however, that this period will not run during any time Executive is in violation of this Section 10, it being the intent of the parties that the Restricted Period will be extended for any period of time in which Executive is in violation of this Section 10.
(vi) “Restricted Territory” means the United States of America, it being understood that the Company’s business is nationwide in scope. In the event that the preceding definition of Restricted Territory is deemed by a court of competent jurisdiction to be too broad to be enforced under the circumstances, then “Restricted Territory” will mean the following severable and distinct territories:
(A) the State of North Carolina;
(B) each state, province, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
(C) each city, county, township, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
(D) each state, province, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date; or
(E) each city, county, township, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(vii) “Termination Date” means the effective date of the termination of Executive’s employment for any reason.
(c) Non-Competition. During Executive’s employment with the Company, Executive will not, on Executive’s own behalf or on behalf of any other person, engage in any business competitive with or adverse to that of the Company. In addition, during Executive’s employment with the Company and during the Restricted Period, Executive will not hold a position based in or with responsibility for all or part of the Restricted Territory, with any person or entity engaging in the Business, whether as employee, consultant, or otherwise, in which Executive will have duties, or will perform or be expected to perform services for such person or entity, that is or are the same as or substantially similar to the position held by Executive or those duties or services actually performed by Executive for the Company within the 12-month period immediately preceding (i) the conduct in question, if such conduct occurs prior to the Termination Date, or (ii) the Termination Date, if the conduct in question occurs on or after the Termination Date, or in which Executive will use or disclose or be reasonably expected to use or disclose any confidential or proprietary information of the Company for the purpose of providing, or attempting to provide, such person or entity with a competitive advantage with respect to the Business. The Parties agree that nothing in this Section 10(c) will prohibit Executive from owning, as a passive investment, not more than 1% of any class of securities of a business entity which is engaged, directly or indirectly, in a business which competes with the Business, or restrict or limit Executive’s ability to work or provide services to a distinct department, division, or other segment of a business entity that does not engage in the Business, even though other departments, divisions, or segments of the business entity may engage in the Business.
- 9 -
(d) Non-Solicitation. During Executive’s employment with the Company and during the Restricted Period, Executive will not, directly or indirectly, on Executive’s own behalf or on behalf of any other party:
(i) Call upon, solicit, divert, encourage or attempt to call upon, solicit, divert, or encourage any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
(ii) Accept as a customer any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
(iii) Induce, encourage, or attempt to induce or encourage any Customer or Prospective Customer to purchase or accept products or services that are competitive with those offered by the Company from any person or entity (other than the Company) engaging in the Business;
(iv) Induce, encourage, or attempt to induce or encourage any Customer to reduce, limit, or cancel its business with the Company;
(v) Solicit, induce, or attempt to solicit or induce any Company Employee to terminate his or her employment or engagement with the Company; or
(vi) Otherwise interfere with or engage in any conduct that would have the effect of interfering with the business relationship between the Company and any of its vendors, suppliers, consultants, or contractors.
(e) Reasonableness of Restrictions. Executive acknowledges and agrees that the restrictive covenants in this Agreement (i) are essential elements of Executive’s employment by the Company and are reasonable given Executive’s access to the Company’s Confidential Information and the substantial knowledge and goodwill Executive will acquire with respect to the business of the Company as a result of Executive’s employment with the Company, and the unique and extraordinary services to be provided by Executive to the Company; and (ii) are reasonable in time, territory, and scope, and in all other respects. Provisions in this Section 10 calling for a “look back” prior to a given date are intended solely as a means of identifying the individuals, entities, and/or place to which the restrictions described in this Section 10 apply and are not intended to extend the length of the restrictions. The existence or assertion of any claim of or by the Executive, whether predicated on this Agreement or otherwise, will not constitute a defense to the enforcement by the Company of the covenants contained in this Section 10.
(f) Consideration. Executive acknowledges and agrees that the covenants set forth in this Agreement are supported by adequate consideration, including, but not limited to the Company’s promise to provide the Separation Benefits or the CIC Separation Benefits, as applicable, and the Company’s other promises as described in this Agreement. The Company would not have agreed to enter into this Agreement but for Executive’s agreement to the restrictions imposed by this Section 10.
(g) Judicial Modification. Should any part or provision of this Section 10 be held invalid, void, or unenforceable in any court of competent jurisdiction, such invalidity, voidness, or unenforceability will not render invalid, void, or unenforceable any other part or provision of this Agreement. The parties further agree that if any portion of this Section 10 is found to be invalid or unenforceable by a court of competent jurisdiction because its duration, territory, or other restrictions are deemed to be invalid or unreasonable in scope, the parties intend that the court will, to the maximum extent permitted by law, replace the invalid or unreasonable terms with terms that are valid and enforceable and that come closest to expressing the intention of such invalid or unenforceable terms.
11. Enforcement. Executive acknowledges and agrees that the Company will suffer irreparable harm in the event that Executive breaches any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement and that monetary damages would be inadequate to compensate the Company for such breach. Accordingly, Executive agrees that, in the event of a breach by Executive of any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement, the Company will be entitled to obtain from any court of competent jurisdiction preliminary and permanent injunctive relief in order to prevent or to restrain any such breach, without the need for posting bond or other security.
- 10 -
12. Cooperation. Upon the receipt of reasonable notice from the Company (including its outside counsel), Executive agrees that while employed by, or providing services to, the Company and thereafter, Executive will respond and provide information with regard to matters in which the Executive has knowledge as a result of the Executive’s employment or service with the Company, and will, at the Company’s sole expense, provide reasonable assistance to the Company, its affiliates and their respective representatives in defense of all claims that may be made against the Company (but excluding claims by Executive), and will assist the Company in the prosecution of all claims that may be made by the Company or its affiliates, to the extent that such claims may relate to the period of the Executive’s employment or service with the Company. Following the Term, the Company will reasonably cooperate with Executive regarding the scheduling of such instances of cooperation under this Section 12, taking into account Executive’s professional and personal commitments. Executive agrees to promptly inform the Company if Executive becomes aware of any lawsuit involving such claims that is likely to be filed or threatened against the Company. Executive also agrees to promptly inform the Company (to the extent that Executive is legally permitted to do so) if Executive is asked to assist in any investigation of the Company (or its actions), regardless of whether a lawsuit or other proceeding has then been filed against the Company with respect to such investigation, and will not do so unless legally required. Upon presentation of appropriate documentation, the Company will reimburse Executive for any reasonable expenses Executive incurs in connection with Executive’s cooperation under this provision.
13. Indemnification. The Company will indemnify Executive and hold Executive harmless in connection with the defense of any lawsuit or other claim, but excluding claims arising out of Executive’s willful misconduct, to which he is made a party by reason of being an officer, director or employee of the Company, to the fullest extent permitted by the laws of the State of Delaware, as in effect at the time of the subject act or omission. The Company will use commercially reasonable efforts to maintain directors’ and officers’ liability insurance for acts and omissions occurring during Executive’s employment with the Company, in amounts and on terms reasonable and customary for similarly situated companies, and Executive will be covered by such insurance on the same basis as other executive officers of the Company.
14. Certain Tax Matters.
(a) Application of Section 409A of the Code. The parties intend that this Agreement and the payments made hereunder will be exempt from, or comply with, the requirements of Section 409A of the Code and the regulations and other guidance thereunder and any state law of similar effect (collectively “Section 409A”), and this Agreement will be interpreted and applied to the greatest extent possible in a manner that is consistent with the requirements for avoiding taxes or penalties under Section 409A. In no event, however, will the Company be liable for any additional tax, interest, or penalty that may be imposed on Executive by Section 409A or damages for failing to comply with Section 409A. Notwithstanding anything to the contrary set forth herein, any payments and benefits provided under Section 6 above that constitute “deferred compensation” within the meaning of Section 409A will not commence in connection with Executive’s termination of employment unless and until Executive has also incurred a “separation from service” (as such term is defined in Treasury Regulation Section 1.409A-1(h)), unless the Company reasonably determines that such amounts may be provided to Executive without causing Executive to incur the additional 20% tax under Section 409A. The parties intend that each installment of the Separation Benefits or the CIC Separation Benefits, as applicable, is a separate “payment” for purposes of Section 409A. For the avoidance of doubt, the parties intend that the Separation Benefits or the CIC Separation Benefits, as applicable, satisfy, to the greatest extent possible, the exemptions from the application of Section 409A provided under Treasury Regulation Sections 1.409A-1(b)(4) and 1.409A-1(b)(9). However, if the Company determines that the Separation Benefits or the CIC Separation Benefits, as applicable, constitute “deferred compensation” under Section 409A and Executive is, as of the separation from service, a “specified employee” of the Company or any successor entity thereto, as such term is defined in Section 409A, then, solely to the extent necessary to avoid the incurrence of the adverse personal tax consequences under Section 409A, the timing of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, will be delayed until the earlier to occur of: (i) the date that is six months and one day after Executive’s separation from service, or (ii) the date of Executive’s death (such applicable date, the “Specified Employee Initial Payment Date”), and the Company (or the successor entity thereto, as applicable) will (A) pay to Executive a lump sum amount equal to the sum of the Separation Benefits payments or the CIC Separation Benefits payments, as applicable, that Executive would otherwise have received through the Specified Employee Initial Payment Date if the commencement of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, had not been so delayed pursuant to this Section, and (B) commence paying the balance of the Separation Benefits or the CIC Separation Benefits, as applicable, in accordance with the applicable payment schedules set forth in this Agreement.
- 11 -
(b) Application of Section 280G of the Code. If any payment, benefit or distribution of any type to or for the benefit of Executive, whether paid or payable, provided or to be provided, or distributed or distributable pursuant to the terms of this Agreement or otherwise (collectively, the “Parachute Payments”) would subject the Executive to the excise tax imposed under Section 4999 of the Code (the “Excise Tax”), the Parachute Payments will be reduced so that the maximum amount of the Parachute Payments (after reduction) will be $1.00 less than the amount which would cause the Parachute Payments to be subject to the Excise Tax; provided that the Parachute Payments will only be reduced to the extent the after-tax value of amounts received by the Executive after application of the above reduction would exceed the after-tax value of the amounts received without application of such reduction. For this purpose, the after-tax value of an amount will be determined taking into account all federal, state, and local income, employment and excise taxes applicable to such amount. If a reduction the Parachute Payments as described in this Section is necessary, the Company will reduce or eliminate the Parachute Payments by first reducing or eliminating any cash payments (with the payments to be made furthest in the future being reduced first), then by reducing or eliminating accelerated vesting of stock options or similar awards, and then by reducing or eliminating any other remaining Parachute Payments; provided, that no such reduction or elimination will apply to any non-qualified deferred compensation amounts (within the meaning of Section 409A) to the extent such reduction or elimination would accelerate or defer the timing of such payment in manner that does not comply with Section 409A. The determination as to whether (x) any of the Parachute Payments received by the Executive in connection with the occurrence of a change in the ownership or control of the Company or in the ownership of a substantial portion of the assets of the Company will be subject to the Excise Tax, and (y) the amount of any reduction, if any, that may be required pursuant to the previous paragraph, will be made by an independent accounting firm selected by the Company (the “Accounting Firm”) prior to the consummation of such change in the ownership or effective control of the Company or in the ownership of a substantial portion of the assets of the Company. For purposes of making the calculations required by this Section 14(b), the Accounting Firm may make reasonable assumptions and approximations concerning applicable taxes and may rely on reasonable, good faith interpretations concerning the application of the Code (including but not limited to Sections 280G and 4999). The parties agree to furnish to the Accounting Firm such information and documents as the Accounting Firm may reasonably request in order to make a determination under this Section 14(b). The Company will bear all costs the Accounting Firm may reasonably incur in connection with any calculations contemplated by this Section 14(b). Executive will be furnished with notice of all determinations made as to the Excise Tax payable with respect to the Executive’s Parachute Payments, together with the related calculations of the Accounting Firm, promptly after such determinations and calculations have been received by the Company.
15. Miscellaneous.
(a) Entire Agreement. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements (whether written or oral and whether express or implied) between the parties to the extent related to such subject matter.
(b) Successors and Assigns. This Agreement will be binding upon and inure to the benefit of the parties and their respective successors, permitted assigns and, in the case of Executive, heirs, executors, and/or personal representatives. The Company may freely assign or transfer this Agreement to an affiliated company or to a successor following a merger, consolidation, sale of assets, or other business transaction. Executive may not assign, delegate or otherwise transfer any of Executive’s rights, interests or obligations in this Agreement without the prior written approval of the Company.
(c) Counterparts; Electronic Delivery. This Agreement may be executed in one or more counterparts, each of which will be deemed an original but all of which together will constitute one instrument reflecting the terms of the Agreement. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature complying with the U.S. ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered will be deemed to have been duly and validly delivered and be valid and effective for all purposes.
(d) Notices. Any notice pursuant to this Agreement must be in writing and will be deemed effectively given to the other party (i) on the date it is actually delivered by personal delivery of such notice, (ii) one business day after its deposit in the custody of a reputable overnight courier service (such as FedEx) next business day delivery charges prepaid; or (iii) three business days after its deposit in the custody of the U.S. mail, certified or registered postage prepaid, return receipt requested; in each case to the appropriate address shown below (or to such other address as a party may designate by notice to the other party):
| If to Executive: | Simon Pedder | |
| If to Company: | Adaptin Bio, Inc. 3540 Toringdon Way, Suite 200 #250 Charlotte, North Carolina 28277 Attention: Board of Directors |
- 12 -
(e) Amendments and Waivers. No amendment of any provision of this Agreement will be valid unless the amendment is in writing and signed by the Company and Executive. No waiver of any provision of this Agreement will be valid unless the waiver is in writing and signed by the waiving party. The failure of a party at any time to require performance of any provision of this Agreement will not affect such party’s rights at a later time to enforce such provision. No waiver by a party of any breach of this Agreement will be deemed to extend to any other breach hereunder or affect in any way any rights arising by virtue of any other breach.
(f) Severability. Each provision of this Agreement is severable from every other provision of this Agreement. Any provision of this Agreement that is determined by any court of competent jurisdiction to be invalid or unenforceable will not affect the validity or enforceability of any other provision. Any provision of this Agreement held invalid or unenforceable only in part or degree will remain in full force and effect to the extent not held invalid or unenforceable.
(g) Construction. The section headings in this Agreement are inserted for convenience only and are not intended to affect the interpretation of this Agreement. The word “including” in this Agreement means “including without limitation.” This Agreement will be construed as if drafted jointly by the Company and Executive and no presumption or burden of proof will arise favoring or disfavoring the Company or Executive by virtue of the authorship of any provision in this Agreement. All words in this Agreement will be construed to be of such gender or number as the circumstances require.
(h) Survival. This Agreement, and all obligations of the Company and Executive hereunder, will terminate upon the termination of Executive’s employment, with the following exceptions: (i) Executive’s continuing obligations under Sections 6, 8, 9, 10, 12, and 14; (ii) the Company’s obligations under Section 6 (subject to Executive’s obligations thereunder); (c) Executive’s right to indemnification under Section 13; and (d) the relevant provisions of Sections 11 and 15.
(i) Remedies Cumulative. The rights and remedies of the parties under this Agreement are cumulative (not alternative) and in addition to all other rights and remedies available to such parties at law, in equity, by contract or otherwise.
(j) Governing Law. This Agreement will be governed by the laws of the State of North Carolina without regard to principles of choice or conflict of laws (whether of the State of North Carolina or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of North Carolina.
(k) Venue. The parties agree that any litigation arising out of or related to this Agreement or Executive’s employment by Company will be brought exclusively in any state or federal court in Mecklenburg County, North Carolina. Each party (i) consents to the personal jurisdiction of said courts, (ii) waives any venue or inconvenient forum defense to any proceeding maintained in such courts, and (iii) except as expressly provided above, agrees not to bring any proceeding arising out of or relating to this Agreement or Executive’s employment by Company in any other court.
(l) Legal Counsel. This Agreement was prepared by Wyrick Robbins Yates & Ponton LLP in its capacity as legal counsel to the Company. Both Parties acknowledge that they have had the opportunity to seek and obtain the advice of counsel before entering into this Agreement and have done so to the extent desired, and have fully read the Agreement and understand the meaning and import of all the terms hereof.
[Signature Page Immediately Follows]
- 13 -
IN WITNESS WHEREOF, the parties hereto have executed and delivered this Agreement as of the date first written above.
| EXECUTIVE: | COMPANY: | ||
| Adaptin Bio, Inc. | |||
| /s/ Simon Pedder | By: | /s/ Michael J. Roberts | |
| Simon Pedder | Name: | Michael J. Roberts | |
| Title: | Chief Executive Officer | ||
[Signature Page to Executive Employment Agreement]
Exhibit 10.11
EXECUTIVE EMPLOYMENT AGREEMENT
This Executive Employment Agreement (this “Agreement”) is entered into as of February 11, 2025, by and between Adaptin Bio, Inc. (f/k/a Unite Acquisition 1 Corp.), a Delaware corporation (the “Company”), and Michael J. Roberts (the “Executive”).
WHEREAS, in connection with, and conditioned upon the completion of, the merger (the “Merger”) between the wholly-owned subsidiary of Unite Acquisition 1 Corp. and private company Adaptin Bio, Inc. (“Adaptin”), the Company wishes to employ Executive as its President and Chief Executive Officer, and Executive wishes to accept such employment, on the terms set forth in this Agreement;
WHEREAS Executive acknowledges and agrees that through Executive’s association with Adaptin as an employee, Executive will acquire a considerable amount of knowledge and goodwill with respect to the business of the Company, which knowledge and goodwill are highly valuable to the Company and which would be detrimental to the Company if used by Executive to compete with the Company; and
WHEREAS the Company wishes to protect its investment in its business, employees, customer relationships, and confidential information, by requiring Executive to abide by certain restrictive covenants regarding confidentiality, non-competition, and non-solicitation, each of which is an inducement to the Company to enter into this Agreement;
NOW, THEREFORE, in consideration of the foregoing, the mutual agreements contained herein, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1. Employment. Subject to the terms and conditions of this Agreement, the Company hereby employs Executive, and Executive accepts such employment. The employment relationship hereunder will be for the period commencing on the closing of the Merger (such date, the “Effective Date”) and will continue for a term of three years (the “Initial Term”) unless terminated sooner in accordance with Section 5 below. After the Initial Term expires, this Agreement will automatically renew for successive one-year terms (subject, in any event, to termination in accordance with Section 5 below), unless either party provides written notice of its intent not to renew the Agreement at least 90 days prior to the expiration of the then-current term. The period from the Effective Date through the date of the termination of Executive’s employment hereunder is referred to herein as the “Term.”
2. Position and Duties.
(a) Position. Executive will serve as the Company’s President and Chief Executive Officer, reporting to the Company’s Board of Directors (the “Board”). Executive will perform such services for the Company and have such powers, responsibilities and authority as are customarily associated with the position of Chief Executive Officer in similar companies, and will perform such additional duties as may otherwise be reasonably assigned to Executive from time to time by the Board.
- 1 -
(b) Full-Time Employment. Executive will devote Executive’s full business time, attention, and efforts to the affairs of the Company and to the duties hereunder, and will perform such duties diligently and to the best of Executive’s ability, in compliance with the Company’s policies and procedures and the laws and regulations that apply to the Company’s business. Notwithstanding the foregoing, Executive may (i) participate in charitable, civic, educational, professional, community or industry affairs, (ii) continue to serve as advisor to Corino Therapeutics, Inc., (iii) continue to serve as President of MAC B Consulting, (iv) serve on the board of directors of a company formed to advance any of the inventions listed in Exhibit A attached hereto, and (iv) manage Executive’s passive personal investments, so long as, in each case, such activities individually or in the aggregate do not materially interfere or conflict with the Executive’s duties hereunder or create a potential business or fiduciary conflict (in each case, as determined by the Board).
(c) Board Service. The Company will use commercially reasonable efforts to cause the Executive to be elected as a member of the Board throughout the Term and will include him in the management slate for election as a director at every stockholders meeting during the Term at which his term as a director would otherwise expire. Executive agrees to accept election, and to serve during the Term, as director of the Company, without any compensation therefor other than as specified in this Agreement.
(d) Principal Work Location. Executive’s principal workplace for the performance of his duties under this Agreement will be at the Company’s headquarters or the Executive’s residence in the state of North Carolina. Notwithstanding the foregoing, the Executive will be required to travel as necessary to perform his duties hereunder.
3. Compensation and Benefits. As compensation for the services to be rendered by Executive under this Agreement, the Company will provide the following compensation and benefits during Executive’s employment hereunder.
(a) Base Salary. During the Term, the Company will pay Executive a base salary at an annual rate of $480,000 (the “Base Salary”). The Base Salary will be payable in equal installments in accordance with the Company’s payroll practices as in effect from time to time. The Base Salary will be reviewed by the Board from time to time, and may be increased in the sole discretion of the Board. Executive’s salary may not be reduced except in connection with an across-the-board reduction of executive level salaries in which Executive will not be subject to a greater reduction, on a percentage basis, than any other executive-level employee.
(b) Bonus Opportunity. For each calendar year ending during the Term, Executive will be eligible to receive an annual bonus (the “Annual Bonus”) with a target amount of 50% of Executive’s Base Salary. The actual amount of each Annual Bonus will be based upon the level of achievement of the Company’s corporate objectives and the Executive’s individual objectives, in each case, as established by the Board for the calendar year with respect to which such Annual Bonus relates. The Board has the authority to increase the Annual Bonus to up to 100% of the Executive Base Salary if the fiscal year corporate goals are exceeded or based on exemplary performance during the fiscal year by the Executive. The determination of the level of achievement of the corporate objectives and the Executive’s individual performance objectives for a year will be made by the Board in its reasonable discretion. The Annual Bonus for a calendar year, to the extent earned, will be paid in a lump sum in the following calendar year. The Annual Bonus will not be deemed earned until the date that it is paid. Accordingly, in order for the Executive to receive an Annual Bonus, the Executive must be actively employed by the Company at the time of such payment.
- 2 -
(c) Equity Compensation.
(i) Subject to approval by the Board and subject to the terms of the Company’s equity incentive plan then in place, in the event the Company issues additional securities raising aggregate funds of $10,000,000 (in one or more transactions), occurring, if at all, within two years following the Merger (the “Additional Financing Period”), the Company will grant Executive options to purchase a number of shares of common stock of the Company (the “Anti-Dilution Options”) sufficient to ensure that Executive’s ownership immediately following the Additional Financing Period, on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable) is equal to Executive’s ownership immediately following the Merger, as determined on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable). The per share exercise price of the Anti-Dilution Options will be equal to the fair market value of a share of the Company’s common stock on the date of grant, as determined by the Board. The Anti-Dilution Options, if any, will become exercisable in four equal annual installments, in each case subject to Executive’s continued employment with the Company on the date each such vesting milestone is achieved. The Anti-Dilution Options will be subject to the terms of the Company’s equity incentive plan then in place and a related option grant agreement to be entered between Executive and the Company.
(ii) During the Term, Executive will be eligible to receive from time to time such equity grants or awards, if any, pursuant to the terms of any equity incentive plan of the Company (or any successor plan as may be in place from time to time) as may be approved by the Board in its discretion. Such grants or awards will be subject to the terms and conditions of such plan (or any successor plan) and such other terms and conditions as the Board in its discretion may establish.
(d) Paid Vacation. Executive will be entitled to paid vacation days in accordance with the Company’s vacation policies in effect from time to time for its executive team; provided, however that the Executive will be entitled to no less than 25 paid vacation days per calendar year during the Term.
(e) General Benefits. Executive will be entitled to such other benefits, and to participate in such benefit plans, as are generally made available to similarly situated senior executive employees of the Company from time to time, subject to Company policy and the terms and conditions of any applicable benefit plans. Nothing in this Agreement will be deemed to alter the Company’s rights to modify or terminate any such plans or programs in its sole discretion.
- 3 -
(f) Withholdings. The Company will withhold from any amounts payable under this Agreement such federal, state, and local taxes as the Company determines are required to be withheld pursuant to applicable law.
(g) Recoupment of Compensation. Any incentive-based or other compensation paid to Executive under this Agreement or any other agreement or arrangement with the Company which is subject to recovery under any law, government regulation, stock exchange listing requirement or any compensation recovery or clawback policy adopted by the Company from time to time will be subject to reduction, cancellation, forfeiture or recoupment as may be required by such law, government regulation, stock exchange listing requirement or policy. In addition, if Executive is or becomes an executive officer subject to the incentive compensation repayment requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), then if required by the Dodd-Frank Act or any of its regulations Executive will enter into an amendment to this Agreement or a separate written agreement with the Company to comply with the Dodd-Frank Act and any of its regulations.
4. Reimbursement of Expenses. The Company will reimburse Executive for all reasonable business expenses incurred by Executive in connection with the performance of Executive’s duties hereunder, subject to Executive’s compliance with the Company’s reimbursement policies in effect from time to time.
5. Termination. This Agreement, and Executive’s employment hereunder, are subject to termination as follows.
(a) Death. Automatically effective upon Executive’s death.
(b) Disability. By the Company upon notice to Executive in the event of Executive’s Disability. As used herein, “Disability” means the inability of Executive, due to the condition of Executive’s physical, mental or emotional health, effectively to perform the essential functions of Executive’s job with or without reasonable accommodation for a continuous period of at least 90 consecutive days or for 120 days in any period of 12 consecutive months, as determined by the Board in good faith. In any event, the Company will comply fully with the applicable provisions of the Americans with Disabilities Act, as amended, the Family and Medical Leave Act, and any similar applicable law. For purposes of making a determination as to whether a Disability exists, at the Company’s request Executive agrees to make himself available and to cooperate in a reasonable examination by a reputable independent physician retained by the Company and to authorize the disclosure and release to the Company of all medical records related to such examination.
(c) For Cause. By the Company effective upon written notice to Executive for Cause. For purposes of this Agreement, “Cause” means: (i) Executive’s fraud, embezzlement or misappropriation with respect to the Company; (ii) Executive’s willful or grossly negligent misconduct that has or may reasonably be expected to have a material adverse effect on the property, business, or reputation of the Company; (iii) Executive’s material breach of this Agreement; (iv) Executive’s willful failure or refusal to perform Executive’s material duties under this Agreement or willful failure to follow any specific lawful instructions of the Board; (v) Executive’s conviction or plea of nolo contendere in respect of a felony or of a misdemeanor involving moral turpitude; or (vi) Executive’s material failure to comply with the Company’s workplace rules, policies, or procedures. In the event that the Company concludes that Executive has engaged in acts constituting in Cause as defined in clause (iii) or (iv) above, prior to terminating this Agreement for Cause the Company will provide Executive with at least 15 days’ advance notice of the circumstances constituting such Cause, and an opportunity to correct such circumstances, to the extent such circumstances are susceptible of being corrected.
- 4 -
(d) Without Cause. By the Company effective upon 90 days advance written notice to Executive at any time for any reason other than for Cause or Executive’s Disability.
(e) Resignation for Good Reason. Executive may resign his employment for Good Reason by complying with the Good Reason Procedure as described herein. The “Good Reason Procedure” means (i) Executive reasonably determines in good faith that a Good Reason condition has occurred; (ii) Executive notifies the Company in writing of the first occurrence of the Good Reason condition within 60 days of the first occurrence of such condition; (iii) Executive cooperates in good faith with the Company’s efforts, for a period not less than 30 days following such notice (the “Good Reason Cure Period”), to remedy the condition; (iv) notwithstanding such efforts, the Good Reason condition continues to exist; and (v) Executive terminates Executive’s employment within 60 days after the end of the Good Reason Cure Period. If the Company cures the Good Reason condition during the Good Reason Cure Period, Good Reason will be deemed not to have occurred. For purposes of this Agreement, “Good Reason” means the occurrence of any of the following events without Executive’s written consent: (i) the Company’s requiring Executive to be based at any office or location more than 25 miles from his principal work location as of the Effective Date, except for travel reasonably required in the performance of Executive’s responsibilities to the Company; (ii) a material reduction of Executive’s Base Salary not in compliance with Section 3(a) above; (iii) a material diminution of the Executive’s authority, duties, or responsibilities; or (iv) the Company’s material breach of this Agreement.
(f) Other Resignation. By Executive effective upon 30 days’ written notice to the Company at any time for any reason.
(g) Employment During Notice Periods. During any notice period under Sections 5(c), 5(e), or 5(f) the Company may, in its sole discretion, relieve Executive of some or all of his duties during the notice period, but the Company will continue to provide Executive with all required salary and benefits during such period.
6. Effect of Termination.
(a) Generally. When Executive’s employment with the Company is terminated for any reason, Executive, or Executive’s estate, as the case may be, will be entitled to receive the compensation and benefits earned through the effective date of termination, along with reimbursement for any unreimbursed business expenses incurred through the date of termination that Executive has timely submitted (or does timely submit) for reimbursement in accordance with the Company’s expense reimbursement policy or practice.
- 5 -
(b) Resignation from Positions. Immediately upon the termination of Executive’s employment with the Company for any reason, Executive will be deemed to have resigned from all positions as an officer or director of the Company, along with any other positions he may hold with or for the benefit of the Company and/or its affiliates. In furtherance of the preceding sentence, Executive will execute and return to the Company all letters and documents that the Company may reasonably require in order to evidence such resignation(s), but Executive’s failure to execute and return such documents will not have the effect of delaying or in any way invalidating the resignation(s) provided for by the preceding sentence.
(c) Separation Benefits upon Certain Terminations not in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 24 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination; and
(ii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for 18 months or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Internal Revenue Code of 1986, as amended (the “Code”).
(d) Separation Benefits upon Certain Terminations in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), in either case at the time of, or within 6 months following, a Change in Control (as defined below) then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “CIC Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 24 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination;
- 6 -
(ii) the vesting of all stock options, restricted stock units, and other equity awards will be accelerated such that all such awards are deemed to have been vested as of the date of termination; and
(iii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for 18 months or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Code.
(e) Change in Control Defined. As used in this Agreement, the term “Change in Control” means the occurrence of any one of the following events:
(i) any “person,” including a “group” (as such terms are used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), but excluding the Company, any entity controlling, controlled by or under common control with the Company, any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any such entity, and, with respect to any particular qualified participant of any equity incentive plan of the Company (a “Participant”), the Participant and any “group” (as such term is used in Section 13(d)(3) of the Exchange Act) of which the Participant is a member), is or becomes the “beneficial owner” (as defined in Rule 13(d)(3) under the Exchange Act), directly or indirectly, of securities of the Company representing 50% or more of either (A) the combined voting power of the Company’s then outstanding securities; or (B) the Company’s then outstanding equity securities (in either such case other than as a result of an acquisition of securities directly from the Company);
(ii) any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own (as such term is defined in Rule 13d-3 under the Exchange Act), directly or indirectly, equity securities representing in the aggregate 50% or more of the combined voting power of the securities of the entity issuing cash or securities in the consolidation or merger (or of its ultimate parent entity, if any);
(iii) any sale, lease, exclusive license, exchange or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of the assets of the Company, other than a sale or disposition by the Company of all or substantially all of the Company’s assets to an entity, at least 50% of the combined voting power of the voting securities of which are owned by “persons” (as defined above) in substantially the same proportion as their ownership of the Company immediately prior to such sale; or
- 7 -
(iv) the members of the Board at the beginning of any consecutive 24-calendar-month period (the “Incumbent Directors”) cease for any reason other than due to death to constitute at least a majority of the members of the Board; provided that any director whose election, or nomination for election by the Company’s stockholders, was approved or ratified by a vote of at least a majority of the members of the Board then still in office who were members of the Board at the beginning of such 24-calendar-month period, will be deemed to be an Incumbent Director.
Notwithstanding the foregoing, no event or condition will constitute a Change in Control to the extent that, if it were, a penalty tax would be imposed under Section 409A; provided that, in such a case, the event or condition will continue to constitute a Change in Control to the maximum extent possible (e.g., if applicable, in respect of vesting without an acceleration of distribution) without causing the imposition of such penalty tax.
(f) Release. The Company’s obligation to provide the Separation Benefits or the CIC Separation Benefits, as applicable, is conditioned upon Executive executing and returning a release of claims in a form acceptable to the Company (the “Release”) within the time specified therein, which Release is not revoked within any time period allowed for revocation under applicable law.
(g) Post-Termination Breach. If Executive is entitled to receive the Separation Benefits or the CIC Separation Benefits but violates any provisions of Sections 8 through 10 hereof after termination of employment, the Company will be entitled to immediately stop paying any further installments of the Separation Benefits or the CIC Separation Benefits as applicable, in addition to any other remedies that may be available to the Company in law or at equity.
(h) No Further Obligations. Except as expressly provided above or as otherwise required by law, the Company will have no obligations to Executive in the event of the termination of this Agreement for any reason. For the avoidance of doubt, in no event will Executive be entitled to benefits under both Section 6(c) and 6(d).
7. Representations of Executive. Executive represents and warrants that he is not obligated or restricted under any agreement (including any non-competition or confidentiality agreement), judgment, decree, order or other restraint of any kind that could impair Executive’s ability to perform the duties and obligations required hereunder. Executive further agrees that Executive will not divulge to the Company any confidential information and/or trade secrets belonging to others, including Executive’s former employers. Consistent with the foregoing, Executive will not provide to the Company any documents or copies of documents containing such information.
- 8 -
8. Confidential Information.
(a) Executive acknowledges that the Company has and will give Executive access to certain highly-sensitive, confidential, and proprietary information belonging to the Company (or third parties who may have furnished such information under obligations of confidentiality), relating to and used in the Company’s business (collectively, “Confidential Information”). Executive acknowledges that Confidential Information includes, but is not limited to, the following categories of Company related confidential or proprietary information and material: financial statements and information; budgets, forecasts, and projections; business and strategic plans; marketing, sales, and business development strategies; research and development projects; records relating to any intellectual property developed by, owned by, controlled, or maintained by the Company; information related to the Company’s inventions, research, products, designs, methods, formulae, techniques, systems, processes; customer lists; non-public information relating to the Company’s customers, suppliers, distributors, or investors; the specific terms of the Company’s agreements or arrangements, whether oral or written, with any customer, supplier, vendor, or contractor with which the Company may be associated from time to time; and any and all information relating to the operation of the Company’s business which the Company may from time to time designate as confidential or proprietary or that Executive reasonably knows should be, or has been, treated by the Company as confidential or proprietary. Executive further understands that Company will receive confidential business information from third parties, including but not limited to the Company’s clients (the “Third Party Information”), under a duty to maintain the confidentiality of such Third Party Information and to use it only for limited purposes. Confidential Information and Third Party Information encompass all formats in which information is preserved, whether electronic, print, or any other form, including all originals, copies, notes, or other reproductions or replicas thereof.
(b) Confidential Information does not include any information that: (i) at the time of disclosure is generally known to, or readily ascertainable by, the public; (ii) becomes known to the public through no fault of Executive or other violation of this Agreement; or (iii) is disclosed to Executive by a third party under no obligation to maintain the confidentiality of the information.
(c) Executive acknowledges that the Confidential Information is owned or licensed by the Company (and Third Party Information is possessed by the Company with the permission of its owner); is unique, valuable, proprietary and confidential; derives independent actual or potential commercial value from not being generally known or available to the public; and is subject to reasonable efforts to maintain its secrecy. Executive hereby relinquishes and agrees that Executive will not at any time claim, any right, title or interest of any kind in or to any Confidential Information or Third Party Information.
(d) During and after Executive’s employment with the Company, Executive will hold in trust and confidence all Confidential Information and Third Party Information, and will not disclose any Confidential Information or Third Party Information to any person or entity, except in the course of performing duties assigned by the Company or as authorized in writing by the Company. Executive further agrees that during and after Executive’s employment with the Company, Executive will not use any Confidential Information or Third Party Information for any purpose, except in the course of performing duties assigned by the Company or as authorized in writing by the Company.
- 9 -
(e) Notwithstanding the covenants contained in Section 8(d) above, Executive may disclose Confidential Information or Third Party Information solely to the extent that Executive is required to do so by law, provided that Executive (i) notifies the Company of the existence and terms of such obligation, (ii) gives the Company a reasonable opportunity to seek a protective or similar order to prevent or limit such disclosure, and (iii) only discloses that information actually required to be disclosed.
(f) Nothing in this Agreement is intended to or will prohibit Executive from communicating with any governmental authority, or making a report in good faith and with a reasonable belief of any violations of law or regulation to a governmental authority, or from filing, testifying or participating in a legal proceeding relating to such violations, including making disclosures protected or required by any whistleblower law or regulation to the Securities and Exchange Commission, the Department of Labor, or any other appropriate government authority charged with the enforcement of any applicable laws. In addition, nothing in this Agreement is intended to or will limit any employee’s right to discuss the terms, wages, and working conditions of their employment, as protected by applicable law. Pursuant to the Defend Trade Secrets Act of 2016, Executive is hereby notified that an individual will not be held criminally or civilly liable under any federal or state trade secret law for the disclosure of a trade secret that (i) is made (A) in confidence to a federal, state or local government official, either directly or indirectly, or to an attorney; and (B) solely for the purpose of reporting or investigating a suspected violation of law; or (ii) is made in a complaint or other document filed in a lawsuit or other proceeding, if such filing is made under seal. An individual who files a lawsuit for retaliation by an employer for reporting a suspected violation of law may disclose the trade secret to his or her attorney and use the trade secret information in the court proceeding, if the individual files any document containing the trade secret under seal, and does not disclose the trade secret, except pursuant to court order.
(g) Return of Property. Upon request during employment and immediately at the termination of Executive’s employment, Executive will return to the Company all Confidential Information and Third Party Information in any form (including all copies and reproductions thereof) and all other property whatsoever of the Company in Executive’s possession or under Executive’s control. If requested by the Company, Executive will certify in writing that all such materials have been returned to the Company. Upon request by the Company, Executive will cooperate with the Company in transferring all logins, passwords, and other access information for accounts and systems previously used by Executive in connection with her employment with the Company. Executive also expressly agrees that immediately upon the termination of Executive’s employment with the Company for any reason, Executive will cease using any secure website, computer systems, e-mail system, or phone system or voicemail service provided by the Company for the use of its employees.
9. Assignment of Inventions.
(a) Executive agrees that all developments or inventions (including without limitation any and all software programs (source and object code), algorithms and applications, concepts, designs, discoveries, improvements, processes, techniques, know-how and data) that are initiated, conceived, discovered, reduced to practice, or made by Executive, either alone or in conjunction with others, during the Term and relate to or result from the Executive’s work for the Company or which relate directly to the Company’s businesses, whether or not patentable or registrable under copyright or similar statutes or subject to analogous protection (“Inventions”), will be the sole and exclusive property of the Company or its nominees. Executive will and hereby does assign to the Company all rights in and to such Inventions upon the creation of any such Invention, including, without limitation: (i) patents, patent applications and patent rights throughout the world; (ii) rights associated with works of authorship throughout the world, including copyrights, copyright applications, copyright registrations, mask work rights, mask work applications and mask work registrations; (iii) rights relating to the protection of trade secrets and confidential information throughout the world; (iv) rights analogous to those set forth herein and any other proprietary rights relating to intangible property; and (v) divisions, continuations, renewals, reissues and extensions of the foregoing (as applicable), now existing or hereafter filed, issued or acquired (collectively, the “IP Rights”).
- 10 -
(b) For avoidance of doubt, if any Invention falls within the definition of “work made for hire” as such term is defined in 17 U.S.C. § 101, such Invention(s) will be considered “work made for hire” and the copyright of such Invention(s) will be owned solely and exclusively by the Company. If any Invention does not fall within such definition of “work made for hire” then Executive’s right, title and interest in and to such Invention(s) will be assigned to the Company pursuant to Section 9(a) above.
(c) The Company and its nominees will have the right to use and/or to apply for statutory or common law protections for such Inventions in any and all countries. Executive further agrees, at the Company’s expense, to: (i) reasonably assist the Company in obtaining and from time to time enforcing such IP Rights relating to Inventions, and (ii) execute and deliver to the Company or its nominee upon reasonable request all such documents as the Company or its nominee may reasonably determine are necessary or appropriate to effect the purposes of this Section 9, including assignments of inventions. Such documents may be necessary to: (1) vest in the Company or its nominee clear and marketable title in and to Inventions; (2) apply for, prosecute and obtain patents, copyrights, mask works rights and other rights and protections relating to Inventions; or (3) enforce patents, copyrights, mask works rights and other rights and protections relating to Inventions. Executive’s obligations pursuant to this Section 9 will continue beyond the termination of Executive’s employment with the Company. Executive hereby irrevocably designates and appoints the Company and its then-current Chief Executive Officer as Executive’s agent and attorney-in-fact to act for and in behalf and instead of Executive, to execute and file any such application and to do all other lawfully permitted acts to further the prosecution and issuance of patents, trademarks, copyrights or other rights thereon with the same legal force and effect as if executed by Executive, if the Company is unable after reasonable effort to secure Executive’s signature to any lawful and necessary document required to apply for or execute any patent, trademark, copyright or other applications with respect to any Inventions (including renewals, extensions, continuations, divisions or continuations in part thereof).
(d) The obligations of Executive under Section 9(a) above will not apply to any Invention that Executive developed or conceived prior to the Effective Date or entirely on Executive’s own time after the Effective Date without using the Company’s equipment, supplies, facility or trade secret information, except for those Inventions that (i) relate directly to the Company’s business or actual or demonstrably anticipated research or development or (ii) result from any work performed by Executive for Company. Obligations of Executive under Section 9(a) above do not apply to and are not limited to Inventions listed in Exhibit A attached hereto. Executive will bear the burden of proof in establishing the applicability of this subsection to a particular circumstance.
- 11 -
10. Restrictive Covenants.
(a) Purpose. Executive understands and agrees that the purpose of this Section 10 is solely to protect the Company’s legitimate business interests, including, but not limited to its confidential and proprietary information, customer relationships and goodwill, and the Company’s competitive advantage, and will not unduly impair Executive’s ability or right to work or earn a living. Therefore, Executive agrees to be subject to restrictive covenants under the following terms.
(b) Definitions. As used in this Agreement, the following terms have the meanings given to such terms below.
(i) “Business” means (A) the development or commercialization of treatments for diseases of the central nervous system, including cancers, and (B) the business(es) in which the Company was actively engaged at the time of, or during the 12-month period immediately preceding the Termination Date, provided that this clause (B) will only apply if Executive is involved with such other business.
(ii) “Company Employee” means any person who is or was an employee or independent contractor of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(iii) “Customer” means any person or entity who is or was a customer or client of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date, and in either case with whom Executive had dealings on behalf of the Company in the course of his employment with the Company during such period, or about whom Executive learned or received confidential and proprietary information in the course of his employment with Company during such period.
(iv) “Prospective Customer” means any person or entity to whom, within the 12 months immediately prior to the Termination Date the Company had submitted a proposal for products or services of which Executive has knowledge, provided that Executive has had material business contacts with such person or entity on behalf of the Company during such 12-month period, whether such contact was initiated by the person or entity or by Executive.
(v) “Restricted Period” means the period commencing on the Termination Date and ending 12 months after such date, provided, however, that this period will not run during any time Executive is in violation of this Section 10, it being the intent of the parties that the Restricted Period will be extended for any period of time in which Executive is in violation of this Section 10.
- 12 -
(vi) “Restricted Territory” means the United States of America, it being understood that the Company’s business is nationwide in scope. In the event that the preceding definition of Restricted Territory is deemed by a court of competent jurisdiction to be too broad to be enforced under the circumstances, then “Restricted Territory” will mean the following severable and distinct territories:
(A) the State of North Carolina;
(B) each state, province, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
(C) each city, county, township, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
(D) each state, province, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date; or
(E) each city, county, township, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(vii) “Termination Date” means the effective date of the termination of Executive’s employment for any reason.
(c) Non-Competition. During Executive’s employment with the Company, Executive will not, on Executive’s own behalf or on behalf of any other person, engage in any business competitive with or adverse to that of the Company. In addition, during Executive’s employment with the Company and during the Restricted Period, Executive will not hold a position based in or with responsibility for all or part of the Restricted Territory, with any person or entity engaging in the Business, whether as employee, consultant, or otherwise, in which Executive will have duties, or will perform or be expected to perform services for such person or entity, that is or are the same as or substantially similar to the position held by Executive or those duties or services actually performed by Executive for the Company within the 12-month period immediately preceding (i) the conduct in question, if such conduct occurs prior to the Termination Date, or (ii) the Termination Date, if the conduct in question occurs on or after the Termination Date, or in which Executive will use or disclose or be reasonably expected to use or disclose any confidential or proprietary information of the Company for the purpose of providing, or attempting to provide, such person or entity with a competitive advantage with respect to the Business. The Parties agree that nothing in this Section 10(c) will prohibit Executive from owning, as a passive investment, not more than 1% of any class of securities of a business entity which is engaged, directly or indirectly, in a business which competes with the Business, or restrict or limit Executive’s ability to work or provide services to a distinct department, division, or other segment of a business entity that does not engage in the Business, even though other departments, divisions, or segments of the business entity may engage in the Business.
- 13 -
(d) Non-Solicitation. During Executive’s employment with the Company and during the Restricted Period, Executive will not, directly or indirectly, on Executive’s own behalf or on behalf of any other party:
(i) Call upon, solicit, divert, encourage or attempt to call upon, solicit, divert, or encourage any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
(ii) Accept as a customer any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
(iii) Induce, encourage, or attempt to induce or encourage any Customer or Prospective Customer to purchase or accept products or services that are competitive with those offered by the Company from any person or entity (other than the Company) engaging in the Business;
(iv) Induce, encourage, or attempt to induce or encourage any Customer to reduce, limit, or cancel its business with the Company;
(v) Solicit, induce, or attempt to solicit or induce any Company Employee to terminate his or her employment or engagement with the Company; or
(vi) Otherwise interfere with or engage in any conduct that would have the effect of interfering with the business relationship between the Company and any of its vendors, suppliers, consultants, or contractors.
(e) Reasonableness of Restrictions. Executive acknowledges and agrees that the restrictive covenants in this Agreement (i) are essential elements of Executive’s employment by the Company and are reasonable given Executive’s access to the Company’s Confidential Information and the substantial knowledge and goodwill Executive will acquire with respect to the business of the Company as a result of Executive’s employment with the Company, and the unique and extraordinary services to be provided by Executive to the Company; and (ii) are reasonable in time, territory, and scope, and in all other respects. Provisions in this Section 10 calling for a “look back” prior to a given date are intended solely as a means of identifying the individuals, entities, and/or place to which the restrictions described in this Section 10 apply and are not intended to extend the length of the restrictions. The existence or assertion of any claim of or by the Executive, whether predicated on this Agreement or otherwise, will not constitute a defense to the enforcement by the Company of the covenants contained in this Section 10.
- 14 -
(f) Consideration. Executive acknowledges and agrees that the covenants set forth in this Agreement are supported by adequate consideration, including, but not limited to the Company’s promise to provide the Separation Benefits or the CIC Separation Benefits, as applicable, and the Company’s other promises as described in this Agreement. The Company would not have agreed to enter into this Agreement but for Executive’s agreement to the restrictions imposed by this Section 10.
(g) Judicial Modification. Should any part or provision of this Section 10 be held invalid, void, or unenforceable in any court of competent jurisdiction, such invalidity, voidness, or unenforceability will not render invalid, void, or unenforceable any other part or provision of this Agreement. The parties further agree that if any portion of this Section 10 is found to be invalid or unenforceable by a court of competent jurisdiction because its duration, territory, or other restrictions are deemed to be invalid or unreasonable in scope, the parties intend that the court will, to the maximum extent permitted by law, replace the invalid or unreasonable terms with terms that are valid and enforceable and that come closest to expressing the intention of such invalid or unenforceable terms.
11. Enforcement. Executive acknowledges and agrees that the Company will suffer irreparable harm in the event that Executive breaches any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement and that monetary damages would be inadequate to compensate the Company for such breach. Accordingly, Executive agrees that, in the event of a breach by Executive of any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement, the Company will be entitled to obtain from any court of competent jurisdiction preliminary and permanent injunctive relief in order to prevent or to restrain any such breach, without the need for posting bond or other security.
12. Cooperation. Upon the receipt of reasonable notice from the Company (including its outside counsel), Executive agrees that while employed by, or providing services to, the Company and thereafter, Executive will respond and provide information with regard to matters in which the Executive has knowledge as a result of the Executive’s employment or service with the Company, and will, at the Company’s sole expense, provide reasonable assistance to the Company, its affiliates and their respective representatives in defense of all claims that may be made against the Company (but excluding claims by Executive), and will assist the Company in the prosecution of all claims that may be made by the Company or its affiliates, to the extent that such claims may relate to the period of the Executive’s employment or service with the Company. Following the Term, the Company will reasonably cooperate with Executive regarding the scheduling of such instances of cooperation under this Section 12, taking into account Executive’s professional and personal commitments. Executive agrees to promptly inform the Company if Executive becomes aware of any lawsuit involving such claims that is likely to be filed or threatened against the Company. Executive also agrees to promptly inform the Company (to the extent that Executive is legally permitted to do so) if Executive is asked to assist in any investigation of the Company (or its actions), regardless of whether a lawsuit or other proceeding has then been filed against the Company with respect to such investigation, and will not do so unless legally required. Upon presentation of appropriate documentation, the Company will reimburse Executive for any reasonable expenses Executive incurs in connection with Executive’s cooperation under this provision.
- 15 -
13. Indemnification. The Company will indemnify Executive and hold Executive harmless in connection with the defense of any lawsuit or other claim, but excluding claims arising out of Executive’s willful misconduct, to which he is made a party by reason of being an officer, director or employee of the Company, to the fullest extent permitted by the laws of the State of Delaware, as in effect at the time of the subject act or omission. The Company will use commercially reasonable efforts to maintain directors’ and officers’ liability insurance for acts and omissions occurring during Executive’s employment with the Company, in amounts and on terms reasonable and customary for similarly situated companies, and Executive will be covered by such insurance on the same basis as other executive officers of the Company.
14. Certain Tax Matters.
(a) Application of Section 409A of the Code. The parties intend that this Agreement and the payments made hereunder will be exempt from, or comply with, the requirements of Section 409A of the Code and the regulations and other guidance thereunder and any state law of similar effect (collectively “Section 409A”), and this Agreement will be interpreted and applied to the greatest extent possible in a manner that is consistent with the requirements for avoiding taxes or penalties under Section 409A. In no event, however, will the Company be liable for any additional tax, interest, or penalty that may be imposed on Executive by Section 409A or damages for failing to comply with Section 409A. Notwithstanding anything to the contrary set forth herein, any payments and benefits provided under Section 6 above that constitute “deferred compensation” within the meaning of Section 409A will not commence in connection with Executive’s termination of employment unless and until Executive has also incurred a “separation from service” (as such term is defined in Treasury Regulation Section 1.409A-1(h)), unless the Company reasonably determines that such amounts may be provided to Executive without causing Executive to incur the additional 20% tax under Section 409A. The parties intend that each installment of the Separation Benefits or the CIC Separation Benefits, as applicable, is a separate “payment” for purposes of Section 409A. For the avoidance of doubt, the parties intend that the Separation Benefits or the CIC Separation Benefits, as applicable, satisfy, to the greatest extent possible, the exemptions from the application of Section 409A provided under Treasury Regulation Sections 1.409A-1(b)(4) and 1.409A-1(b)(9). However, if the Company determines that the Separation Benefits or the CIC Separation Benefits, as applicable, constitute “deferred compensation” under Section 409A and Executive is, as of the separation from service, a “specified employee” of the Company or any successor entity thereto, as such term is defined in Section 409A, then, solely to the extent necessary to avoid the incurrence of the adverse personal tax consequences under Section 409A, the timing of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, will be delayed until the earlier to occur of: (i) the date that is six months and one day after Executive’s separation from service, or (ii) the date of Executive’s death (such applicable date, the “Specified Employee Initial Payment Date”), and the Company (or the successor entity thereto, as applicable) will (A) pay to Executive a lump sum amount equal to the sum of the Separation Benefits payments or the CIC Separation Benefits payments, as applicable, that Executive would otherwise have received through the Specified Employee Initial Payment Date if the commencement of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, had not been so delayed pursuant to this Section, and (B) commence paying the balance of the Separation Benefits or the CIC Separation Benefits, as applicable, in accordance with the applicable payment schedules set forth in this Agreement.
- 16 -
(b) Application of Section 280G of the Code. If any payment, benefit or distribution of any type to or for the benefit of Executive, whether paid or payable, provided or to be provided, or distributed or distributable pursuant to the terms of this Agreement or otherwise (collectively, the “Parachute Payments”) would subject the Executive to the excise tax imposed under Section 4999 of the Code (the “Excise Tax”), the Parachute Payments will be reduced so that the maximum amount of the Parachute Payments (after reduction) will be $1.00 less than the amount which would cause the Parachute Payments to be subject to the Excise Tax; provided that the Parachute Payments will only be reduced to the extent the after-tax value of amounts received by the Executive after application of the above reduction would exceed the after-tax value of the amounts received without application of such reduction. For this purpose, the after-tax value of an amount will be determined taking into account all federal, state, and local income, employment and excise taxes applicable to such amount. If a reduction the Parachute Payments as described in this Section is necessary, the Company will reduce or eliminate the Parachute Payments by first reducing or eliminating any cash payments (with the payments to be made furthest in the future being reduced first), then by reducing or eliminating accelerated vesting of stock options or similar awards, and then by reducing or eliminating any other remaining Parachute Payments; provided, that no such reduction or elimination will apply to any non-qualified deferred compensation amounts (within the meaning of Section 409A) to the extent such reduction or elimination would accelerate or defer the timing of such payment in manner that does not comply with Section 409A. The determination as to whether (x) any of the Parachute Payments received by the Executive in connection with the occurrence of a change in the ownership or control of the Company or in the ownership of a substantial portion of the assets of the Company will be subject to the Excise Tax, and (y) the amount of any reduction, if any, that may be required pursuant to the previous paragraph, will be made by an independent accounting firm selected by the Company (the “Accounting Firm”) prior to the consummation of such change in the ownership or effective control of the Company or in the ownership of a substantial portion of the assets of the Company. For purposes of making the calculations required by this Section 14(b), the Accounting Firm may make reasonable assumptions and approximations concerning applicable taxes and may rely on reasonable, good faith interpretations concerning the application of the Code (including but not limited to Sections 280G and 4999). The parties agree to furnish to the Accounting Firm such information and documents as the Accounting Firm may reasonably request in order to make a determination under this Section 14(b). The Company will bear all costs the Accounting Firm may reasonably incur in connection with any calculations contemplated by this Section 14(b). Executive will be furnished with notice of all determinations made as to the Excise Tax payable with respect to the Executive’s Parachute Payments, together with the related calculations of the Accounting Firm, promptly after such determinations and calculations have been received by the Company.
15. Miscellaneous.
(a) Entire Agreement. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements (whether written or oral and whether express or implied) between the parties to the extent related to such subject matter.
- 17 -
(b) Successors and Assigns. This Agreement will be binding upon and inure to the benefit of the parties and their respective successors, permitted assigns and, in the case of Executive, heirs, executors, and/or personal representatives. The Company may freely assign or transfer this Agreement to an affiliated company or to a successor following a merger, consolidation, sale of assets, or other business transaction. Executive may not assign, delegate or otherwise transfer any of Executive’s rights, interests or obligations in this Agreement without the prior written approval of the Company.
(c) Counterparts; Electronic Delivery. This Agreement may be executed in one or more counterparts, each of which will be deemed an original but all of which together will constitute one instrument reflecting the terms of the Agreement. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature complying with the U.S. ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered will be deemed to have been duly and validly delivered and be valid and effective for all purposes.
(d) Notices. Any notice pursuant to this Agreement must be in writing and will be deemed effectively given to the other party (i) on the date it is actually delivered by personal delivery of such notice, (ii) one business day after its deposit in the custody of a reputable overnight courier service (such as FedEx) next business day delivery charges prepaid; or (iii) three business days after its deposit in the custody of the U.S. mail, certified or registered postage prepaid, return receipt requested; in each case to the appropriate address shown below (or to such other address as a party may designate by notice to the other party):
| If to Executive: | Michael J. Roberts | |
| If to Company: | Adaptin Bio, Inc. | |
3540 Toringdon Way, Suite 200 #250 | ||
Charlotte, North Carolina 28277 | ||
Attention: Board of Directors |
(e) Amendments and Waivers. No amendment of any provision of this Agreement will be valid unless the amendment is in writing and signed by the Company and Executive. No waiver of any provision of this Agreement will be valid unless the waiver is in writing and signed by the waiving party. The failure of a party at any time to require performance of any provision of this Agreement will not affect such party’s rights at a later time to enforce such provision. No waiver by a party of any breach of this Agreement will be deemed to extend to any other breach hereunder or affect in any way any rights arising by virtue of any other breach.
(f) Severability. Each provision of this Agreement is severable from every other provision of this Agreement. Any provision of this Agreement that is determined by any court of competent jurisdiction to be invalid or unenforceable will not affect the validity or enforceability of any other provision. Any provision of this Agreement held invalid or unenforceable only in part or degree will remain in full force and effect to the extent not held invalid or unenforceable.
- 18 -
(g) Construction. The section headings in this Agreement are inserted for convenience only and are not intended to affect the interpretation of this Agreement. The word “including” in this Agreement means “including without limitation.” This Agreement will be construed as if drafted jointly by the Company and Executive and no presumption or burden of proof will arise favoring or disfavoring the Company or Executive by virtue of the authorship of any provision in this Agreement. All words in this Agreement will be construed to be of such gender or number as the circumstances require.
(h) Survival. This Agreement, and all obligations of the Company and Executive hereunder, will terminate upon the termination of Executive’s employment, with the following exceptions: (i) Executive’s continuing obligations under Sections 6, 8, 9, 10, 12, and 14; (ii) the Company’s obligations under Section 6 (subject to Executive’s obligations thereunder); (c) Executive’s right to indemnification under Section 13; and (d) the relevant provisions of Sections 11 and 15.
(i) Remedies Cumulative. The rights and remedies of the parties under this Agreement are cumulative (not alternative) and in addition to all other rights and remedies available to such parties at law, in equity, by contract or otherwise.
(j) Governing Law. This Agreement will be governed by the laws of the State of North Carolina without regard to principles of choice or conflict of laws (whether of the State of North Carolina or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of North Carolina.
(k) Venue. The parties agree that any litigation arising out of or related to this Agreement or Executive’s employment by Company will be brought exclusively in any state or federal court in Mecklenburg County, North Carolina. Each party (i) consents to the personal jurisdiction of said courts, (ii) waives any venue or inconvenient forum defense to any proceeding maintained in such courts, and (iii) except as expressly provided above, agrees not to bring any proceeding arising out of or relating to this Agreement or Executive’s employment by Company in any other court.
(l) Legal Counsel. This Agreement was prepared by Wyrick Robbins Yates & Ponton LLP in its capacity as legal counsel to the Company. Both Parties acknowledge that they have had the opportunity to seek and obtain the advice of counsel before entering into this Agreement and have done so to the extent desired, and have fully read the Agreement and understand the meaning and import of all the terms hereof.
[Signature Page Immediately Follows]
- 19 -
IN WITNESS WHEREOF, the parties hereto have executed and delivered this Agreement as of the date first written above.
| EXECUTIVE: | COMPANY: | ||
| Adaptin Bio, Inc. | |||
| /s/ Michael J. Roberts | By: | /s/ Simon Pedder | |
| Michael J. Roberts | Name: | Simon Pedder | |
| Title: | Executive Chairman and Director | ||
[Signature Page to Executive Employment Agreement]
Exhibit 10.12
EXECUTIVE EMPLOYMENT AGREEMENT
This Executive Employment Agreement (this “Agreement”) is entered into as of February 11, 2025, by and between Adaptin Bio, Inc. (f/k/a Unite Acquisition 1 Corp.), a Delaware corporation (the “Company”), and Timothy L. Maness (the “Executive”).
WHEREAS, in connection with, and conditioned upon the completion of, the merger (the “Merger”) between the wholly-owned subsidiary of Unite Acquisition 1 Corp. and private company Adaptin Bio, Inc. (“Adaptin”), the Company wishes to employ Executive as its Chief Financial Officer, and Executive wishes to accept such employment, on the terms set forth in this Agreement;
WHEREAS Executive acknowledges and agrees that through Executive’s association with Adaptin as an employee, Executive will acquire a considerable amount of knowledge and goodwill with respect to the business of the Company, which knowledge and goodwill are highly valuable to the Company and which would be detrimental to the Company if used by Executive to compete with the Company; and
WHEREAS the Company wishes to protect its investment in its business, employees, customer relationships, and confidential information, by requiring Executive to abide by certain restrictive covenants regarding confidentiality, non-competition, and non-solicitation, each of which is an inducement to the Company to enter into this Agreement;
NOW, THEREFORE, in consideration of the foregoing, the mutual agreements contained herein, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1. Employment. Subject to the terms and conditions of this Agreement, the Company hereby employs Executive, and Executive accepts such employment. The employment relationship hereunder will be for the period commencing on the closing of the Merger (such date, the “Effective Date”) and will continue for a term of two years (the “Initial Term”) unless terminated sooner in accordance with Section 5 below. After the Initial Term expires, this Agreement will automatically renew for successive one-year terms (subject, in any event, to termination in accordance with Section 5 below), unless either party provides written notice of its intent not to renew the Agreement at least 90 days prior to the expiration of the then-current term. The period from the Effective Date through the date of the termination of Executive’s employment hereunder is referred to herein as the “Term.”
2. Position and Duties.
(a) Position. Executive will serve as the Company’s Chief Financial Officer, reporting to the Company’s Chief Executive Officer (the “CEO”). Executive will perform such services for the Company and have such powers, responsibilities and authority as are customarily associated with the position of Chief Financial Officer in similar companies, and will perform such additional duties as may otherwise be reasonably assigned to Executive from time to time by the CEO.
- 1 -
(b) Part-Time Employment. Executive will devote approximately 80% of Executive’s business time, attention, and efforts to the affairs of the Company and to the duties hereunder, and will perform such duties diligently and to the best of Executive’s ability, in compliance with the Company’s policies and procedures and the laws and regulations that apply to the Company’s business. Notwithstanding the foregoing, Executive may (i) participate in charitable, civic, educational, professional, community or industry affairs, and (ii) manage Executive’s passive personal investments, so long as, in each case, such activities individually or in the aggregate do not materially interfere or conflict with the Executive’s duties hereunder or create a potential business or fiduciary conflict (in each case, as determined by the Company’s Board of Directors (the “Board”)).
(c) Principal Work Location. Executive’s principal workplace for the performance of his duties under this Agreement will be at the Company’s headquarters or the Executive’s residence. Notwithstanding the foregoing, the Executive will be required to travel as necessary to perform his duties hereunder.
3. Compensation and Benefits. As compensation for the services to be rendered by Executive under this Agreement, the Company will provide the following compensation and benefits during Executive’s employment hereunder.
(a) Base Salary. During the Term, the Company will pay Executive a base salary at an annual rate of $240,000 (the “Base Salary”). The Base Salary will be payable in equal installments in accordance with the Company’s payroll practices as in effect from time to time. The Base Salary will be reviewed by the Board from time to time, and may be increased in the sole discretion of the Board. Executive’s salary may not be reduced except in connection with an across-the-board reduction of executive level salaries in which Executive will not be subject to a greater reduction, on a percentage basis, than any other executive-level employee.
(b) Bonus Opportunity. For each calendar year ending during the Term, Executive will be eligible to receive an annual bonus (the “Annual Bonus”) with a target amount of 30% of Executive’s Base Salary. The actual amount of each Annual Bonus will be based upon the level of achievement of the Company’s corporate objectives and the Executive’s individual objectives, in each case, as established by the Board for the calendar year with respect to which such Annual Bonus relates. The determination of the level of achievement of the corporate objectives and the Executive’s individual performance objectives for a year will be made by the Board in its reasonable discretion. The Annual Bonus for a calendar year, to the extent earned, will be paid in a lump sum in the following calendar year. The Annual Bonus will not be deemed earned until the date that it is paid. Accordingly, in order for the Executive to receive an Annual Bonus, the Executive must be actively employed by the Company at the time of such payment.
- 2 -
(c) Equity Compensation.
(i) Subject to approval by the Board and subject to the terms of the Company’s equity incentive plan then in place, in the event the Company issues additional securities raising aggregate funds of $10,000,000 (in one or more transactions), occurring, if at all, within two years following the Merger (the “Additional Financing Period”), the Company will grant Executive options to purchase a number of shares of common stock of the Company (the “Anti-Dilution Options”) sufficient to ensure that Executive’s ownership immediately following the Additional Financing Period, on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable) is equal to Executive’s ownership immediately following the Merger, as determined on a fully diluted basis and assuming the exercise of all outstanding options (whether or not then exercisable). The per share exercise price of the Anti-Dilution Options will be equal to the fair market value of a share of the Company’s common stock on the date of grant, as determined by the Board. The Anti-Dilution Options, if any, will become exercisable in four equal annual installments, in each case subject to Executive’s continued employment with the Company on the date each such vesting milestone is achieved. The Anti-Dilution Options will be subject to the terms of the Company’s equity incentive plan then in place and a related option grant agreement to be entered between Executive and the Company.
(ii) During the Term, Executive will be eligible to receive from time to time such equity grants or awards, if any, pursuant to the terms of any equity incentive plan of the Company (or any successor plan as may be in place from time to time) as may be approved by the Board in its discretion. Such grants or awards will be subject to the terms and conditions of such plan (or any successor plan) and such other terms and conditions as the Board in its discretion may establish.
(d) Paid Vacation. Executive will be entitled to paid vacation days in accordance with the Company’s vacation policies in effect from time to time for its executive team; provided, however that the Executive will be entitled to no less than 20 paid vacation days per calendar year during the Term.
(e) General Benefits. Executive will be entitled to such other benefits, and to participate in such benefit plans, as are generally made available to similarly situated senior executive employees of the Company from time to time, subject to Company policy and the terms and conditions of any applicable benefit plans. Nothing in this Agreement will be deemed to alter the Company’s rights to modify or terminate any such plans or programs in its sole discretion.
(f) Withholdings. The Company will withhold from any amounts payable under this Agreement such federal, state, and local taxes as the Company determines are required to be withheld pursuant to applicable law.
(g) Recoupment of Compensation. Any incentive-based or other compensation paid to Executive under this Agreement or any other agreement or arrangement with the Company which is subject to recovery under any law, government regulation, stock exchange listing requirement or any compensation recovery or clawback policy adopted by the Company from time to time will be subject to reduction, cancellation, forfeiture or recoupment as may be required by such law, government regulation, stock exchange listing requirement or policy. In addition, if Executive is or becomes an executive officer subject to the incentive compensation repayment requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), then if required by the Dodd-Frank Act or any of its regulations Executive will enter into an amendment to this Agreement or a separate written agreement with the Company to comply with the Dodd-Frank Act and any of its regulations.
- 3 -
4. Reimbursement of Expenses. The Company will reimburse Executive for all reasonable business expenses incurred by Executive in connection with the performance of Executive’s duties hereunder, subject to Executive’s compliance with the Company’s reimbursement policies in effect from time to time.
5. Termination. This Agreement, and Executive’s employment hereunder, are subject to termination as follows.
(a) Death. Automatically effective upon Executive’s death.
(b) Disability. By the Company upon notice to Executive in the event of Executive’s Disability. As used herein, “Disability” means the inability of Executive, due to the condition of Executive’s physical, mental or emotional health, effectively to perform the essential functions of Executive’s job with or without reasonable accommodation for a continuous period of at least 90 consecutive days or for 120 days in any period of 12 consecutive months, as determined by the Board in good faith. In any event, the Company will comply fully with the applicable provisions of the Americans with Disabilities Act, as amended, the Family and Medical Leave Act, and any similar applicable law. For purposes of making a determination as to whether a Disability exists, at the Company’s request Executive agrees to make himself available and to cooperate in a reasonable examination by a reputable independent physician retained by the Company and to authorize the disclosure and release to the Company of all medical records related to such examination.
(c) For Cause. By the Company effective upon written notice to Executive for Cause. For purposes of this Agreement, “Cause” means: (i) Executive’s fraud, embezzlement or misappropriation with respect to the Company; (ii) Executive’s willful or grossly negligent misconduct that has or may reasonably be expected to have a material adverse effect on the property, business, or reputation of the Company; (iii) Executive’s material breach of this Agreement; (iv) Executive’s willful failure or refusal to perform Executive’s material duties under this Agreement or willful failure to follow any specific lawful instructions of the CEO; (v) Executive’s conviction or plea of nolo contendere in respect of a felony or of a misdemeanor involving moral turpitude; or (vi) Executive’s material failure to comply with the Company’s workplace rules, policies, or procedures. In the event that the Company concludes that Executive has engaged in acts constituting in Cause as defined in clause (iii) or (iv) above, prior to terminating this Agreement for Cause the Company will provide Executive with at least 15 days’ advance notice of the circumstances constituting such Cause, and an opportunity to correct such circumstances, to the extent such circumstances are susceptible of being corrected.
(d) Without Cause. By the Company effective upon written notice to Executive at any time for any reason other than for Cause or Executive’s Disability.
- 4 -
(e) Resignation for Good Reason. Executive may resign his employment for Good Reason by complying with the Good Reason Procedure as described herein. The “Good Reason Procedure” means (i) Executive reasonably determines in good faith that a Good Reason condition has occurred; (ii) Executive notifies the Company in writing of the first occurrence of the Good Reason condition within 60 days of the first occurrence of such condition; (iii) Executive cooperates in good faith with the Company’s efforts, for a period not less than 30 days following such notice (the “Good Reason Cure Period”), to remedy the condition; (iv) notwithstanding such efforts, the Good Reason condition continues to exist; and (v) Executive terminates Executive’s employment within 60 days after the end of the Good Reason Cure Period. If the Company cures the Good Reason condition during the Good Reason Cure Period, Good Reason will be deemed not to have occurred. For purposes of this Agreement, “Good Reason” means the occurrence of any of the following events without Executive’s written consent: (i) the Company’s requiring Executive to be based at any office or location more than 25 miles from his principal work location as of the Effective Date, except for travel reasonably required in the performance of Executive’s responsibilities to the Company; (ii) a material reduction of Executive’s Base Salary not in compliance with Section 3(a) above; (iii) a material diminution of the Executive’s authority, duties, or responsibilities; or (iv) the Company’s material breach of this Agreement.
(f) Other Resignation. By Executive effective upon 30 days’ written notice to the Company at any time for any reason.
(g) Employment During Notice Periods. During any notice period under Sections 5(c), 5(e), or 5(f) the Company may, in its sole discretion, relieve Executive of some or all of his duties during the notice period, but the Company will continue to provide Executive with all required salary and benefits during such period.
6. Effect of Termination.
(a) Generally. When Executive’s employment with the Company is terminated for any reason, Executive, or Executive’s estate, as the case may be, will be entitled to receive the compensation and benefits earned through the effective date of termination, along with reimbursement for any unreimbursed business expenses incurred through the date of termination that Executive has timely submitted (or does timely submit) for reimbursement in accordance with the Company’s expense reimbursement policy or practice.
(b) Resignation from Positions. Immediately upon the termination of Executive’s employment with the Company for any reason, Executive will be deemed to have resigned from all positions as an officer or director of the Company, along with any other positions he may hold with or for the benefit of the Company and/or its affiliates. In furtherance of the preceding sentence, Executive will execute and return to the Company all letters and documents that the Company may reasonably require in order to evidence such resignation(s), but Executive’s failure to execute and return such documents will not have the effect of delaying or in any way invalidating the resignation(s) provided for by the preceding sentence.
- 5 -
(c) Separation Benefits upon Certain Terminations not in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 12 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination; and
(ii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for the same period over which severance is paid or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Internal Revenue Code of 1986, as amended (the “Code”).
(d) Separation Benefits upon Certain Terminations in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), in either case at the time of, or within six months following, a Change in Control (as defined below) then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “CIC Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 12 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination;
(ii) the vesting of all stock options, restricted stock units, and other equity awards will be accelerated such that all such awards are deemed to have been vested as of the date of termination; and
(iii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for the same period over which severance is paid or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Code.
- 6 -
(e) Change in Control Defined. As used in this Agreement, the term “Change in Control” means the occurrence of any one of the following events:
(i) any “person,” including a “group” (as such terms are used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), but excluding the Company, any entity controlling, controlled by or under common control with the Company, any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any such entity, and, with respect to any particular qualified participant of any equity incentive plan of the Company (a “Participant”), the Participant and any “group” (as such term is used in Section 13(d)(3) of the Exchange Act) of which the Participant is a member), is or becomes the “beneficial owner” (as defined in Rule 13(d)(3) under the Exchange Act), directly or indirectly, of securities of the Company representing 50% or more of either (A) the combined voting power of the Company’s then outstanding securities; or (B) the Company’s then outstanding equity securities (in either such case other than as a result of an acquisition of securities directly from the Company);
(ii) any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own (as such term is defined in Rule 13d-3 under the Exchange Act), directly or indirectly, equity securities representing in the aggregate 50% or more of the combined voting power of the securities of the entity issuing cash or securities in the consolidation or merger (or of its ultimate parent entity, if any);
(iii) any sale, lease, exclusive license, exchange or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of the assets of the Company, other than a sale or disposition by the Company of all or substantially all of the Company’s assets to an entity, at least 50% of the combined voting power of the voting securities of which are owned by “persons” (as defined above) in substantially the same proportion as their ownership of the Company immediately prior to such sale; or
(iv) the members of the Board at the beginning of any consecutive 24-calendar-month period (the “Incumbent Directors”) cease for any reason other than due to death to constitute at least a majority of the members of the Board; provided that any director whose election, or nomination for election by the Company’s stockholders, was approved or ratified by a vote of at least a majority of the members of the Board then still in office who were members of the Board at the beginning of such 24-calendar-month period, will be deemed to be an Incumbent Director.
Notwithstanding the foregoing, no event or condition will constitute a Change in Control to the extent that, if it were, a penalty tax would be imposed under Section 409A; provided that, in such a case, the event or condition will continue to constitute a Change in Control to the maximum extent possible (e.g., if applicable, in respect of vesting without an acceleration of distribution) without causing the imposition of such penalty tax.
- 7 -
(f) Release. The Company’s obligation to provide the Separation Benefits or the CIC Separation Benefits, as applicable, is conditioned upon Executive executing and returning a release of claims in a form acceptable to the Company (the “Release”) within the time specified therein, which Release is not revoked within any time period allowed for revocation under applicable law.
(g) Post-Termination Breach. If Executive is entitled to receive the Separation Benefits or the CIC Separation Benefits but violates any provisions of Sections 8 through 10 hereof after termination of employment, the Company will be entitled to immediately stop paying any further installments of the Separation Benefits or the CIC Separation Benefits as applicable, in addition to any other remedies that may be available to the Company in law or at equity.
(h) No Further Obligations. Except as expressly provided above or as otherwise required by law, the Company will have no obligations to Executive in the event of the termination of this Agreement for any reason. For the avoidance of doubt, in no event will Executive be entitled to benefits under both Section 6(c) and 6(d).
7. Representations of Executive. Executive represents and warrants that he is not obligated or restricted under any agreement (including any non-competition or confidentiality agreement), judgment, decree, order or other restraint of any kind that could impair Executive’s ability to perform the duties and obligations required hereunder. Executive further agrees that Executive will not divulge to the Company any confidential information and/or trade secrets belonging to others, including Executive’s former employers. Consistent with the foregoing, Executive will not provide to the Company any documents or copies of documents containing such information.
8. Confidential Information.
(a) Executive acknowledges that the Company has and will give Executive access to certain highly-sensitive, confidential, and proprietary information belonging to the Company (or third parties who may have furnished such information under obligations of confidentiality), relating to and used in the Company’s business (collectively, “Confidential Information”). Executive acknowledges that Confidential Information includes, but is not limited to, the following categories of Company related confidential or proprietary information and material: financial statements and information; budgets, forecasts, and projections; business and strategic plans; marketing, sales, and business development strategies; research and development projects; records relating to any intellectual property developed by, owned by, controlled, or maintained by the Company; information related to the Company’s inventions, research, products, designs, methods, formulae, techniques, systems, processes; customer lists; non-public information relating to the Company’s customers, suppliers, distributors, or investors; the specific terms of the Company’s agreements or arrangements, whether oral or written, with any customer, supplier, vendor, or contractor with which the Company may be associated from time to time; and any and all information relating to the operation of the Company’s business which the Company may from time to time designate as confidential or proprietary or that Executive reasonably knows should be, or has been, treated by the Company as confidential or proprietary. Executive further understands that Company will receive confidential business information from third parties, including but not limited to the Company’s clients (the “Third Party Information”), under a duty to maintain the confidentiality of such Third Party Information and to use it only for limited purposes. Confidential Information and Third Party Information encompass all formats in which information is preserved, whether electronic, print, or any other form, including all originals, copies, notes, or other reproductions or replicas thereof.
- 8 -
(b) Confidential Information does not include any information that: (i) at the time of disclosure is generally known to, or readily ascertainable by, the public; (ii) becomes known to the public through no fault of Executive or other violation of this Agreement; or (iii) is disclosed to Executive by a third party under no obligation to maintain the confidentiality of the information.
(c) Executive acknowledges that the Confidential Information is owned or licensed by the Company (and Third Party Information is possessed by the Company with the permission of its owner); is unique, valuable, proprietary and confidential; derives independent actual or potential commercial value from not being generally known or available to the public; and is subject to reasonable efforts to maintain its secrecy. Executive hereby relinquishes and agrees that Executive will not at any time claim, any right, title or interest of any kind in or to any Confidential Information or Third Party Information.
(d) During and after Executive’s employment with the Company, Executive will hold in trust and confidence all Confidential Information and Third Party Information, and will not disclose any Confidential Information or Third Party Information to any person or entity, except in the course of performing duties assigned by the Company or as authorized in writing by the Company. Executive further agrees that during and after Executive’s employment with the Company, Executive will not use any Confidential Information or Third Party Information for any purpose, except in the course of performing duties assigned by the Company or as authorized in writing by the Company.
(e) Notwithstanding the covenants contained in Section 8(d) above, Executive may disclose Confidential Information or Third Party Information solely to the extent that Executive is required to do so by law, provided that Executive (i) notifies the Company of the existence and terms of such obligation, (ii) gives the Company a reasonable opportunity to seek a protective or similar order to prevent or limit such disclosure, and (iii) only discloses that information actually required to be disclosed.
(f) Nothing in this Agreement is intended to or will prohibit Executive from communicating with any governmental authority, or making a report in good faith and with a reasonable belief of any violations of law or regulation to a governmental authority, or from filing, testifying or participating in a legal proceeding relating to such violations, including making disclosures protected or required by any whistleblower law or regulation to the Securities and Exchange Commission, the Department of Labor, or any other appropriate government authority charged with the enforcement of any applicable laws. In addition, nothing in this Agreement is intended to or will limit any employee’s right to discuss the terms, wages, and working conditions of their employment, as protected by applicable law. Pursuant to the Defend Trade Secrets Act of 2016, Executive is hereby notified that an individual will not be held criminally or civilly liable under any federal or state trade secret law for the disclosure of a trade secret that (i) is made (A) in confidence to a federal, state or local government official, either directly or indirectly, or to an attorney; and (B) solely for the purpose of reporting or investigating a suspected violation of law; or (ii) is made in a complaint or other document filed in a lawsuit or other proceeding, if such filing is made under seal. An individual who files a lawsuit for retaliation by an employer for reporting a suspected violation of law may disclose the trade secret to his or her attorney and use the trade secret information in the court proceeding, if the individual files any document containing the trade secret under seal, and does not disclose the trade secret, except pursuant to court order.
- 9 -
(g) Return of Property. Upon request during employment and immediately at the termination of Executive’s employment, Executive will return to the Company all Confidential Information and Third Party Information in any form (including all copies and reproductions thereof) and all other property whatsoever of the Company in Executive’s possession or under Executive’s control. If requested by the Company, Executive will certify in writing that all such materials have been returned to the Company. Upon request by the Company, Executive will cooperate with the Company in transferring all logins, passwords, and other access information for accounts and systems previously used by Executive in connection with her employment with the Company. Executive also expressly agrees that immediately upon the termination of Executive’s employment with the Company for any reason, Executive will cease using any secure website, computer systems, e-mail system, or phone system or voicemail service provided by the Company for the use of its employees.
9. Assignment of Inventions.
(a) Executive agrees that all developments or inventions (including without limitation any and all software programs (source and object code), algorithms and applications, concepts, designs, discoveries, improvements, processes, techniques, know-how and data) that are initiated, conceived, discovered, reduced to practice, or made by Executive, either alone or in conjunction with others, during the Term and relate to or result from the Executive’s work for the Company or which relate directly to the Company’s businesses, whether or not patentable or registrable under copyright or similar statutes or subject to analogous protection (“Inventions”), will be the sole and exclusive property of the Company or its nominees. Executive will and hereby does assign to the Company all rights in and to such Inventions upon the creation of any such Invention, including, without limitation: (i) patents, patent applications and patent rights throughout the world; (ii) rights associated with works of authorship throughout the world, including copyrights, copyright applications, copyright registrations, mask work rights, mask work applications and mask work registrations; (iii) rights relating to the protection of trade secrets and confidential information throughout the world; (iv) rights analogous to those set forth herein and any other proprietary rights relating to intangible property; and (v) divisions, continuations, renewals, reissues and extensions of the foregoing (as applicable), now existing or hereafter filed, issued or acquired (collectively, the “IP Rights”).
(b) For avoidance of doubt, if any Invention falls within the definition of “work made for hire” as such term is defined in 17 U.S.C. § 101, such Invention(s) will be considered “work made for hire” and the copyright of such Invention(s) will be owned solely and exclusively by the Company. If any Invention does not fall within such definition of “work made for hire” then Executive’s right, title and interest in and to such Invention(s) will be assigned to the Company pursuant to Section 9(a) above.
- 10 -
(c) The Company and its nominees will have the right to use and/or to apply for statutory or common law protections for such Inventions in any and all countries. Executive further agrees, at the Company’s expense, to: (i) reasonably assist the Company in obtaining and from time to time enforcing such IP Rights relating to Inventions, and (ii) execute and deliver to the Company or its nominee upon reasonable request all such documents as the Company or its nominee may reasonably determine are necessary or appropriate to effect the purposes of this Section 9, including assignments of inventions. Such documents may be necessary to: (1) vest in the Company or its nominee clear and marketable title in and to Inventions; (2) apply for, prosecute and obtain patents, copyrights, mask works rights and other rights and protections relating to Inventions; or (3) enforce patents, copyrights, mask works rights and other rights and protections relating to Inventions. Executive’s obligations pursuant to this Section 9 will continue beyond the termination of Executive’s employment with the Company. Executive hereby irrevocably designates and appoints the Company and its then-current Chief Executive Officer as Executive’s agent and attorney-in-fact to act for and in behalf and instead of Executive, to execute and file any such application and to do all other lawfully permitted acts to further the prosecution and issuance of patents, trademarks, copyrights or other rights thereon with the same legal force and effect as if executed by Executive, if the Company is unable after reasonable effort to secure Executive’s signature to any lawful and necessary document required to apply for or execute any patent, trademark, copyright or other applications with respect to any Inventions (including renewals, extensions, continuations, divisions or continuations in part thereof).
(d) The obligations of Executive under Section 9(a) above will not apply to any Invention that Executive developed or conceived prior to the Effective Date or entirely on Executive’s own time after the Effective Date without using the Company’s equipment, supplies, facility or trade secret information, except for those Inventions that (i) relate directly to the Company’s business or actual or demonstrably anticipated research or development or (ii) result from any work performed by Executive for Company. Executive will bear the burden of proof in establishing the applicability of this subsection to a particular circumstance.
10. Restrictive Covenants.
(a) Purpose. Executive understands and agrees that the purpose of this Section 10 is solely to protect the Company’s legitimate business interests, including, but not limited to its confidential and proprietary information, customer relationships and goodwill, and the Company’s competitive advantage, and will not unduly impair Executive’s ability or right to work or earn a living. Therefore, Executive agrees to be subject to restrictive covenants under the following terms.
(b) Definitions. As used in this Agreement, the following terms have the meanings given to such terms below.
(i) “Business” means (A) the development or commercialization of treatments for diseases of the central nervous system, including cancers, and (B) the business(es) in which the Company was actively engaged at the time of, or during the 12-month period immediately preceding the Termination Date, provided that this clause (B) will only apply if Executive is involved with such other business.
- 11 -
(ii) “Company Employee” means any person who is or was an employee or independent contractor of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(iii) “Customer” means any person or entity who is or was a customer or client of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date, and in either case with whom Executive had dealings on behalf of the Company in the course of his employment with the Company during such period, or about whom Executive learned or received confidential and proprietary information in the course of his employment with Company during such period.
(iv) “Prospective Customer” means any person or entity to whom, within the 12 months immediately prior to the Termination Date the Company had submitted a proposal for products or services of which Executive has knowledge, provided that Executive has had material business contacts with such person or entity on behalf of the Company during such 12-month period, whether such contact was initiated by the person or entity or by Executive.
(v) “Restricted Period” means the period commencing on the Termination Date and ending 12 months after such date, provided, however, that this period will not run during any time Executive is in violation of this Section 10, it being the intent of the parties that the Restricted Period will be extended for any period of time in which Executive is in violation of this Section 10.
(vi) “Restricted Territory” means the United States of America, it being understood that the Company’s business is nationwide in scope. In the event that the preceding definition of Restricted Territory is deemed by a court of competent jurisdiction to be too broad to be enforced under the circumstances, then “Restricted Territory” will mean the following severable and distinct territories:
(A) the State of North Carolina;
(B) each state, province, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
(C) each city, county, township, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
- 12 -
(D) each state, province, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date; or
(E) each city, county, township, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(vii) “Termination Date” means the effective date of the termination of Executive’s employment for any reason.
(c) Non-Competition. During Executive’s employment with the Company, Executive will not, on Executive’s own behalf or on behalf of any other person, engage in any business competitive with or adverse to that of the Company. In addition, during Executive’s employment with the Company and during the Restricted Period, Executive will not hold a position based in or with responsibility for all or part of the Restricted Territory, with any person or entity engaging in the Business, whether as employee, consultant, or otherwise, in which Executive will have duties, or will perform or be expected to perform services for such person or entity, that is or are the same as or substantially similar to the position held by Executive or those duties or services actually performed by Executive for the Company within the 12-month period immediately preceding (i) the conduct in question, if such conduct occurs prior to the Termination Date, or (ii) the Termination Date, if the conduct in question occurs on or after the Termination Date, or in which Executive will use or disclose or be reasonably expected to use or disclose any confidential or proprietary information of the Company for the purpose of providing, or attempting to provide, such person or entity with a competitive advantage with respect to the Business. The Parties agree that nothing in this Section 10(c) will prohibit Executive from owning, as a passive investment, not more than 1% of any class of securities of a business entity which is engaged, directly or indirectly, in a business which competes with the Business, or restrict or limit Executive’s ability to work or provide services to a distinct department, division, or other segment of a business entity that does not engage in the Business, even though other departments, divisions, or segments of the business entity may engage in the Business.
(d) Non-Solicitation. During Executive’s employment with the Company and during the Restricted Period, Executive will not, directly or indirectly, on Executive’s own behalf or on behalf of any other party:
(i) Call upon, solicit, divert, encourage or attempt to call upon, solicit, divert, or encourage any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
- 13 -
(ii) Accept as a customer any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
(iii) Induce, encourage, or attempt to induce or encourage any Customer or Prospective Customer to purchase or accept products or services that are competitive with those offered by the Company from any person or entity (other than the Company) engaging in the Business;
(iv) Induce, encourage, or attempt to induce or encourage any Customer to reduce, limit, or cancel its business with the Company;
(v) Solicit, induce, or attempt to solicit or induce any Company Employee to terminate his or her employment or engagement with the Company; or
(vi) Otherwise interfere with or engage in any conduct that would have the effect of interfering with the business relationship between the Company and any of its vendors, suppliers, consultants, or contractors.
(e) Reasonableness of Restrictions. Executive acknowledges and agrees that the restrictive covenants in this Agreement (i) are essential elements of Executive’s employment by the Company and are reasonable given Executive’s access to the Company’s Confidential Information and the substantial knowledge and goodwill Executive will acquire with respect to the business of the Company as a result of Executive’s employment with the Company, and the unique and extraordinary services to be provided by Executive to the Company; and (ii) are reasonable in time, territory, and scope, and in all other respects. Provisions in this Section 10 calling for a “look back” prior to a given date are intended solely as a means of identifying the individuals, entities, and/or place to which the restrictions described in this Section 10 apply and are not intended to extend the length of the restrictions. The existence or assertion of any claim of or by the Executive, whether predicated on this Agreement or otherwise, will not constitute a defense to the enforcement by the Company of the covenants contained in this Section 10.
(f) Consideration. Executive acknowledges and agrees that the covenants set forth in this Agreement are supported by adequate consideration, including, but not limited to the Company’s promise to provide the Separation Benefits or the CIC Separation Benefits, as applicable, and the Company’s other promises as described in this Agreement. The Company would not have agreed to enter into this Agreement but for Executive’s agreement to the restrictions imposed by this Section 10.
(g) Judicial Modification. Should any part or provision of this Section 10 be held invalid, void, or unenforceable in any court of competent jurisdiction, such invalidity, voidness, or unenforceability will not render invalid, void, or unenforceable any other part or provision of this Agreement. The parties further agree that if any portion of this Section 10 is found to be invalid or unenforceable by a court of competent jurisdiction because its duration, territory, or other restrictions are deemed to be invalid or unreasonable in scope, the parties intend that the court will, to the maximum extent permitted by law, replace the invalid or unreasonable terms with terms that are valid and enforceable and that come closest to expressing the intention of such invalid or unenforceable terms.
- 14 -
11. Enforcement. Executive acknowledges and agrees that the Company will suffer irreparable harm in the event that Executive breaches any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement and that monetary damages would be inadequate to compensate the Company for such breach. Accordingly, Executive agrees that, in the event of a breach by Executive of any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement, the Company will be entitled to obtain from any court of competent jurisdiction preliminary and permanent injunctive relief in order to prevent or to restrain any such breach, without the need for posting bond or other security.
12. Cooperation. Upon the receipt of reasonable notice from the Company (including its outside counsel), Executive agrees that while employed by, or providing services to, the Company and thereafter, Executive will respond and provide information with regard to matters in which the Executive has knowledge as a result of the Executive’s employment or service with the Company, and will, at the Company’s sole expense, provide reasonable assistance to the Company, its affiliates and their respective representatives in defense of all claims that may be made against the Company (but excluding claims by Executive), and will assist the Company in the prosecution of all claims that may be made by the Company or its affiliates, to the extent that such claims may relate to the period of the Executive’s employment or service with the Company. Following the Term, the Company will reasonably cooperate with Executive regarding the scheduling of such instances of cooperation under this Section 12, taking into account Executive’s professional and personal commitments. Executive agrees to promptly inform the Company if Executive becomes aware of any lawsuit involving such claims that is likely to be filed or threatened against the Company. Executive also agrees to promptly inform the Company (to the extent that Executive is legally permitted to do so) if Executive is asked to assist in any investigation of the Company (or its actions), regardless of whether a lawsuit or other proceeding has then been filed against the Company with respect to such investigation, and will not do so unless legally required. Upon presentation of appropriate documentation, the Company will reimburse Executive for any reasonable expenses Executive incurs in connection with Executive’s cooperation under this provision.
13. Indemnification. The Company will indemnify Executive and hold Executive harmless in connection with the defense of any lawsuit or other claim, but excluding claims arising out of Executive’s willful misconduct, to which he is made a party by reason of being an officer, director or employee of the Company, to the fullest extent permitted by the laws of the State of Delaware, as in effect at the time of the subject act or omission. The Company will use commercially reasonable efforts to maintain directors’ and officers’ liability insurance for acts and omissions occurring during Executive’s employment with the Company, in amounts and on terms reasonable and customary for similarly situated companies, and Executive will be covered by such insurance on the same basis as other executive officers of the Company.
- 15 -
14. Certain Tax Matters.
(a) Application of Section 409A of the Code. The parties intend that this Agreement and the payments made hereunder will be exempt from, or comply with, the requirements of Section 409A of the Code and the regulations and other guidance thereunder and any state law of similar effect (collectively “Section 409A”), and this Agreement will be interpreted and applied to the greatest extent possible in a manner that is consistent with the requirements for avoiding taxes or penalties under Section 409A. In no event, however, will the Company be liable for any additional tax, interest, or penalty that may be imposed on Executive by Section 409A or damages for failing to comply with Section 409A. Notwithstanding anything to the contrary set forth herein, any payments and benefits provided under Section 6 above that constitute “deferred compensation” within the meaning of Section 409A will not commence in connection with Executive’s termination of employment unless and until Executive has also incurred a “separation from service” (as such term is defined in Treasury Regulation Section 1.409A-1(h)), unless the Company reasonably determines that such amounts may be provided to Executive without causing Executive to incur the additional 20% tax under Section 409A. The parties intend that each installment of the Separation Benefits or the CIC Separation Benefits, as applicable, is a separate “payment” for purposes of Section 409A. For the avoidance of doubt, the parties intend that the Separation Benefits or the CIC Separation Benefits, as applicable, satisfy, to the greatest extent possible, the exemptions from the application of Section 409A provided under Treasury Regulation Sections 1.409A-1(b)(4) and 1.409A-1(b)(9). However, if the Company determines that the Separation Benefits or the CIC Separation Benefits, as applicable, constitute “deferred compensation” under Section 409A and Executive is, as of the separation from service, a “specified employee” of the Company or any successor entity thereto, as such term is defined in Section 409A, then, solely to the extent necessary to avoid the incurrence of the adverse personal tax consequences under Section 409A, the timing of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, will be delayed until the earlier to occur of: (i) the date that is six months and one day after Executive’s separation from service, or (ii) the date of Executive’s death (such applicable date, the “Specified Employee Initial Payment Date”), and the Company (or the successor entity thereto, as applicable) will (A) pay to Executive a lump sum amount equal to the sum of the Separation Benefits payments or the CIC Separation Benefits payments, as applicable, that Executive would otherwise have received through the Specified Employee Initial Payment Date if the commencement of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, had not been so delayed pursuant to this Section, and (B) commence paying the balance of the Separation Benefits or the CIC Separation Benefits, as applicable, in accordance with the applicable payment schedules set forth in this Agreement.
(b) Application of Section 280G of the Code. If any payment, benefit or distribution of any type to or for the benefit of Executive, whether paid or payable, provided or to be provided, or distributed or distributable pursuant to the terms of this Agreement or otherwise (collectively, the “Parachute Payments”) would subject the Executive to the excise tax imposed under Section 4999 of the Code (the “Excise Tax”), the Parachute Payments will be reduced so that the maximum amount of the Parachute Payments (after reduction) will be $1.00 less than the amount which would cause the Parachute Payments to be subject to the Excise Tax; provided that the Parachute Payments will only be reduced to the extent the after-tax value of amounts received by the Executive after application of the above reduction would exceed the after-tax value of the amounts received without application of such reduction. For this purpose, the after-tax value of an amount will be determined taking into account all federal, state, and local income, employment and excise taxes applicable to such amount. If a reduction the Parachute Payments as described in this Section is necessary, the Company will reduce or eliminate the Parachute Payments by first reducing or eliminating any cash payments (with the payments to be made furthest in the future being reduced first), then by reducing or eliminating accelerated vesting of stock options or similar awards, and then by reducing or eliminating any other remaining Parachute Payments; provided, that no such reduction or elimination will apply to any non-qualified deferred compensation amounts (within the meaning of Section 409A) to the extent such reduction or elimination would accelerate or defer the timing of such payment in manner that does not comply with Section 409A. The determination as to whether (x) any of the Parachute Payments received by the Executive in connection with the occurrence of a change in the ownership or control of the Company or in the ownership of a substantial portion of the assets of the Company will be subject to the Excise Tax, and (y) the amount of any reduction, if any, that may be required pursuant to the previous paragraph, will be made by an independent accounting firm selected by the Company (the “Accounting Firm”) prior to the consummation of such change in the ownership or effective control of the Company or in the ownership of a substantial portion of the assets of the Company. For purposes of making the calculations required by this Section 14(b), the Accounting Firm may make reasonable assumptions and approximations concerning applicable taxes and may rely on reasonable, good faith interpretations concerning the application of the Code (including but not limited to Sections 280G and 4999). The parties agree to furnish to the Accounting Firm such information and documents as the Accounting Firm may reasonably request in order to make a determination under this Section 14(b). The Company will bear all costs the Accounting Firm may reasonably incur in connection with any calculations contemplated by this Section 14(b). Executive will be furnished with notice of all determinations made as to the Excise Tax payable with respect to the Executive’s Parachute Payments, together with the related calculations of the Accounting Firm, promptly after such determinations and calculations have been received by the Company.
- 16 -
15. Miscellaneous.
(a) Entire Agreement. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements (whether written or oral and whether express or implied) between the parties to the extent related to such subject matter.
(b) Successors and Assigns. This Agreement will be binding upon and inure to the benefit of the parties and their respective successors, permitted assigns and, in the case of Executive, heirs, executors, and/or personal representatives. The Company may freely assign or transfer this Agreement to an affiliated company or to a successor following a merger, consolidation, sale of assets, or other business transaction. Executive may not assign, delegate or otherwise transfer any of Executive’s rights, interests or obligations in this Agreement without the prior written approval of the Company.
(c) Counterparts; Electronic Delivery. This Agreement may be executed in one or more counterparts, each of which will be deemed an original but all of which together will constitute one instrument reflecting the terms of the Agreement. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature complying with the U.S. ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered will be deemed to have been duly and validly delivered and be valid and effective for all purposes.
(d) Notices. Any notice pursuant to this Agreement must be in writing and will be deemed effectively given to the other party (i) on the date it is actually delivered by personal delivery of such notice, (ii) one business day after its deposit in the custody of a reputable overnight courier service (such as FedEx) next business day delivery charges prepaid; or (iii) three business days after its deposit in the custody of the U.S. mail, certified or registered postage prepaid, return receipt requested; in each case to the appropriate address shown below (or to such other address as a party may designate by notice to the other party):
| If to Executive: | Timothy L. Maness | |
| If to Company: | Adaptin Bio, Inc. 3540 Toringdon Way, Suite 200 #250 Charlotte, North Carolina 28277 Attention: Board of Directors |
(e) Amendments and Waivers. No amendment of any provision of this Agreement will be valid unless the amendment is in writing and signed by the Company and Executive. No waiver of any provision of this Agreement will be valid unless the waiver is in writing and signed by the waiving party. The failure of a party at any time to require performance of any provision of this Agreement will not affect such party’s rights at a later time to enforce such provision. No waiver by a party of any breach of this Agreement will be deemed to extend to any other breach hereunder or affect in any way any rights arising by virtue of any other breach.
- 17 -
(f) Severability. Each provision of this Agreement is severable from every other provision of this Agreement. Any provision of this Agreement that is determined by any court of competent jurisdiction to be invalid or unenforceable will not affect the validity or enforceability of any other provision. Any provision of this Agreement held invalid or unenforceable only in part or degree will remain in full force and effect to the extent not held invalid or unenforceable.
(g) Construction. The section headings in this Agreement are inserted for convenience only and are not intended to affect the interpretation of this Agreement. The word “including” in this Agreement means “including without limitation.” This Agreement will be construed as if drafted jointly by the Company and Executive and no presumption or burden of proof will arise favoring or disfavoring the Company or Executive by virtue of the authorship of any provision in this Agreement. All words in this Agreement will be construed to be of such gender or number as the circumstances require.
(h) Survival. This Agreement, and all obligations of the Company and Executive hereunder, will terminate upon the termination of Executive’s employment, with the following exceptions: (i) Executive’s continuing obligations under Sections 6, 8, 9, 10, 12, and 14; (ii) the Company’s obligations under Section 6 (subject to Executive’s obligations thereunder); (c) Executive’s right to indemnification under Section 13; and (d) the relevant provisions of Sections 11 and 15.
(i) Remedies Cumulative. The rights and remedies of the parties under this Agreement are cumulative (not alternative) and in addition to all other rights and remedies available to such parties at law, in equity, by contract or otherwise.
(j) Governing Law. This Agreement will be governed by the laws of the State of North Carolina without regard to principles of choice or conflict of laws (whether of the State of North Carolina or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of North Carolina.
(k) Venue. The parties agree that any litigation arising out of or related to this Agreement or Executive’s employment by Company will be brought exclusively in any state or federal court in Mecklenburg County, North Carolina. Each party (i) consents to the personal jurisdiction of said courts, (ii) waives any venue or inconvenient forum defense to any proceeding maintained in such courts, and (iii) except as expressly provided above, agrees not to bring any proceeding arising out of or relating to this Agreement or Executive’s employment by Company in any other court.
(l) Legal Counsel. This Agreement was prepared by Wyrick Robbins Yates & Ponton LLP in its capacity as legal counsel to the Company. Both Parties acknowledge that they have had the opportunity to seek and obtain the advice of counsel before entering into this Agreement and have done so to the extent desired, and have fully read the Agreement and understand the meaning and import of all the terms hereof.
[Signature Page Immediately Follows]
- 18 -
IN WITNESS WHEREOF, the parties hereto have executed and delivered this Agreement as of the date first written above.
| EXECUTIVE: | COMPANY: | ||
| Adaptin Bio, Inc. | |||
| /s/ Timothy L. Maness | By: | /s/ Michael J. Roberts | |
| Timothy L. Maness | Name: | Michael J. Roberts | |
| Title: | Chief Executive Officer | ||
[Signature Page to Executive Employment Agreement]
Exhibit 10.13
EXECUTIVE EMPLOYMENT AGREEMENT
This Executive Employment Agreement (this “Agreement”) is entered into as of February 11, 2025, by and between Adaptin Bio, Inc. (f/k/a Unite Acquisition 1 Corp.), a Delaware corporation (the “Company”), and L. Arthur Hewitt (the “Executive”).
WHEREAS, in connection with, and conditioned upon the completion of, the merger (the “Merger”) between the wholly-owned subsidiary of Unite Acquisition 1 Corp. and private company Adaptin Bio, Inc. (“Adaptin”), the Company wishes to employ Executive as its Chief Development Officer, and Executive wishes to accept such employment, on the terms set forth in this Agreement;
WHEREAS Executive acknowledges and agrees that through Executive’s association with Adaptin as an employee, Executive will acquire a considerable amount of knowledge and goodwill with respect to the business of the Company, which knowledge and goodwill are highly valuable to the Company and which would be detrimental to the Company if used by Executive to compete with the Company; and
WHEREAS the Company wishes to protect its investment in its business, employees, customer relationships, and confidential information, by requiring Executive to abide by certain restrictive covenants regarding confidentiality, non-competition, and non-solicitation, each of which is an inducement to the Company to enter into this Agreement;
NOW, THEREFORE, in consideration of the foregoing, the mutual agreements contained herein, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties agree as follows:
1. Employment. Subject to the terms and conditions of this Agreement, the Company hereby employs Executive, and Executive accepts such employment. The employment relationship hereunder will be for the period commencing on the closing of the Merger (such date, the “Effective Date”) and will continue for a term of one year (the “Initial Term”) unless terminated sooner in accordance with Section 5 below. After the Initial Term expires, this Agreement will automatically renew for successive one-year terms (subject, in any event, to termination in accordance with Section 5 below), unless either party provides written notice of its intent not to renew the Agreement at least 90 days prior to the expiration of the then-current term. The period from the Effective Date through the date of the termination of Executive’s employment hereunder is referred to herein as the “Term.”
2. Position and Duties.
(a) Position. Executive will serve as the Company’s Chief Development Officer, reporting to the Company’s Chief Executive Officer (the “CEO”). Executive will perform such services for the Company and have such powers, responsibilities and authority as are customarily associated with the position of Chief Development Officer in similar companies, and will perform such additional duties as may otherwise be reasonably assigned to Executive from time to time by the CEO.
- 1 -
(b) Part-Time Employment. Executive will devote approximately 25% of Executive’s business time, attention, and efforts to the affairs of the Company and to the duties hereunder, and will perform such duties diligently and to the best of Executive’s ability, in compliance with the Company’s policies and procedures and the laws and regulations that apply to the Company’s business. Notwithstanding the foregoing, Executive may (i) participate in charitable, civic, educational, professional, community or industry affairs and (ii) manage Executive’s passive personal investments, so long as, in each case, such activities individually or in the aggregate do not materially interfere or conflict with the Executive’s duties hereunder or create a potential business or fiduciary conflict (in each case, as determined by the Company’s Board of Directors (the “Board”)).
(c) Principal Work Location. Executive’s principal workplace for the performance of his duties under this Agreement will be at the Company’s headquarters or the Executive’s residence. Notwithstanding the foregoing, the Executive will be required to travel as necessary to perform his duties hereunder.
3. Compensation and Benefits. As compensation for the services to be rendered by Executive under this Agreement, the Company will provide the following compensation and benefits during Executive’s employment hereunder.
(a) Base Salary. During the Term, the Company will pay Executive a base salary at an annual rate of $75,000 (the “Base Salary”). The Base Salary will be payable in equal installments in accordance with the Company’s payroll practices as in effect from time to time. The Base Salary will be reviewed by the Board from time to time, and may be increased in the sole discretion of the Board. Executive’s salary may not be reduced except in connection with an across-the-board reduction of executive level salaries in which Executive will not be subject to a greater reduction, on a percentage basis, than any other executive-level employee.
(b) Bonus Opportunity. For each calendar year ending during the Term, Executive will be eligible to receive an annual bonus (the “Annual Bonus”) with a target amount of 25% of Executive’s Base Salary. The actual amount of each Annual Bonus will be based upon the level of achievement of the Company’s corporate objectives and the Executive’s individual objectives, in each case, as established by the Board for the calendar year with respect to which such Annual Bonus relates. The determination of the level of achievement of the corporate objectives and the Executive’s individual performance objectives for a year will be made by the Board in its reasonable discretion. The Annual Bonus for a calendar year, to the extent earned, will be paid in a lump sum in the following calendar year. The Annual Bonus will not be deemed earned until the date that it is paid. Accordingly, in order for the Executive to receive an Annual Bonus, the Executive must be actively employed by the Company at the time of such payment.
- 2 -
(c) Equity Compensation. During the Term, Executive will be eligible to receive from time to time such equity grants or awards, if any, pursuant to the terms of any equity incentive plan of the Company (or any successor plan as may be in place from time to time) as may be approved by the Board in its discretion. Such grants or awards will be subject to the terms and conditions of such plan (or any successor plan) and such other terms and conditions as the Board in its discretion may establish.
(d) Paid Vacation. Executive will be entitled to paid vacation days in accordance with the Company’s vacation policies in effect from time to time for its executive team; provided, however that the Executive will be entitled to no less than 20 paid vacation days per calendar year during the Term.
(e) General Benefits. Executive will be entitled to such other benefits, and to participate in such benefit plans, as are generally made available to similarly situated senior executive employees of the Company from time to time, subject to Company policy and the terms and conditions of any applicable benefit plans. Nothing in this Agreement will be deemed to alter the Company’s rights to modify or terminate any such plans or programs in its sole discretion.
(f) Withholdings. The Company will withhold from any amounts payable under this Agreement such federal, state, and local taxes as the Company determines are required to be withheld pursuant to applicable law.
(g) Recoupment of Compensation. Any incentive-based or other compensation paid to Executive under this Agreement or any other agreement or arrangement with the Company which is subject to recovery under any law, government regulation, stock exchange listing requirement or any compensation recovery or clawback policy adopted by the Company from time to time will be subject to reduction, cancellation, forfeiture or recoupment as may be required by such law, government regulation, stock exchange listing requirement or policy. In addition, if Executive is or becomes an executive officer subject to the incentive compensation repayment requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act (the “Dodd-Frank Act”), then if required by the Dodd-Frank Act or any of its regulations Executive will enter into an amendment to this Agreement or a separate written agreement with the Company to comply with the Dodd-Frank Act and any of its regulations.
- 3 -
4. Reimbursement of Expenses. The Company will reimburse Executive for all reasonable business expenses incurred by Executive in connection with the performance of Executive’s duties hereunder, subject to Executive’s compliance with the Company’s reimbursement policies in effect from time to time.
5. Termination. This Agreement, and Executive’s employment hereunder, are subject to termination as follows.
(a) Death. Automatically effective upon Executive’s death.
(b) Disability. By the Company upon notice to Executive in the event of Executive’s Disability. As used herein, “Disability” means the inability of Executive, due to the condition of Executive’s physical, mental or emotional health, effectively to perform the essential functions of Executive’s job with or without reasonable accommodation for a continuous period of at least 90 consecutive days or for 120 days in any period of 12 consecutive months, as determined by the Board in good faith. In any event, the Company will comply fully with the applicable provisions of the Americans with Disabilities Act, as amended, the Family and Medical Leave Act, and any similar applicable law. For purposes of making a determination as to whether a Disability exists, at the Company’s request Executive agrees to make himself available and to cooperate in a reasonable examination by a reputable independent physician retained by the Company and to authorize the disclosure and release to the Company of all medical records related to such examination.
(c) For Cause. By the Company effective upon written notice to Executive for Cause. For purposes of this Agreement, “Cause” means: (i) Executive’s fraud, embezzlement or misappropriation with respect to the Company; (ii) Executive’s willful or grossly negligent misconduct that has or may reasonably be expected to have a material adverse effect on the property, business, or reputation of the Company; (iii) Executive’s material breach of this Agreement; (iv) Executive’s willful failure or refusal to perform Executive’s material duties under this Agreement or willful failure to follow any specific lawful instructions of the CEO; (v) Executive’s conviction or plea of nolo contendere in respect of a felony or of a misdemeanor involving moral turpitude; or (vi) Executive’s material failure to comply with the Company’s workplace rules, policies, or procedures. In the event that the Company concludes that Executive has engaged in acts constituting in Cause as defined in clause (iii) or (iv) above, prior to terminating this Agreement for Cause the Company will provide Executive with at least 15 days’ advance notice of the circumstances constituting such Cause, and an opportunity to correct such circumstances, to the extent such circumstances are susceptible of being corrected.
(d) Without Cause. By the Company effective upon written notice to Executive at any time for any reason other than for Cause or Executive’s Disability.
- 4 -
(e) Resignation for Good Reason. Executive may resign his employment for Good Reason by complying with the Good Reason Procedure as described herein. The “Good Reason Procedure” means (i) Executive reasonably determines in good faith that a Good Reason condition has occurred; (ii) Executive notifies the Company in writing of the first occurrence of the Good Reason condition within 60 days of the first occurrence of such condition; (iii) Executive cooperates in good faith with the Company’s efforts, for a period not less than 30 days following such notice (the “Good Reason Cure Period”), to remedy the condition; (iv) notwithstanding such efforts, the Good Reason condition continues to exist; and (v) Executive terminates Executive’s employment within 60 days after the end of the Good Reason Cure Period. If the Company cures the Good Reason condition during the Good Reason Cure Period, Good Reason will be deemed not to have occurred. For purposes of this Agreement, “Good Reason” means the occurrence of any of the following events without Executive’s written consent: (i) the Company’s requiring Executive to be based at any office or location more than 25 miles from his principal work location as of the Effective Date, except for travel reasonably required in the performance of Executive’s responsibilities to the Company; (ii) a material reduction of Executive’s Base Salary not in compliance with Section 3(a) above; (iii) a material diminution of the Executive’s authority, duties, or responsibilities; or (iv) the Company’s material breach of this Agreement.
(f) Other Resignation. By Executive effective upon 30 days’ written notice to the Company at any time for any reason.
(g) Employment During Notice Periods. During any notice period under Sections 5(c), 5(e), or 5(f) the Company may, in its sole discretion, relieve Executive of some or all of his duties during the notice period, but the Company will continue to provide Executive with all required salary and benefits during such period.
6. Effect of Termination.
(a) Generally. When Executive’s employment with the Company is terminated for any reason, Executive, or Executive’s estate, as the case may be, will be entitled to receive the compensation and benefits earned through the effective date of termination, along with reimbursement for any unreimbursed business expenses incurred through the date of termination that Executive has timely submitted (or does timely submit) for reimbursement in accordance with the Company’s expense reimbursement policy or practice.
(b) Resignation from Positions. Immediately upon the termination of Executive’s employment with the Company for any reason, Executive will be deemed to have resigned from all positions as an officer or director of the Company, along with any other positions he may hold with or for the benefit of the Company and/or its affiliates. In furtherance of the preceding sentence, Executive will execute and return to the Company all letters and documents that the Company may reasonably require in order to evidence such resignation(s), but Executive’s failure to execute and return such documents will not have the effect of delaying or in any way invalidating the resignation(s) provided for by the preceding sentence.
- 5 -
(c) Separation Benefits upon Certain Terminations not in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 12 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination; and
(ii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for the same period over which severance is paid or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Internal Revenue Code of 1986, as amended (the “Code”).
(d) Separation Benefits upon Certain Terminations in Connection with a Change in Control. If the Company terminates Executive’s employment without Cause pursuant to Section 5(d), or if Executive resigns for Good Reason pursuant to Section 5(e), in either case at the time of, or within six months following, a Change in Control (as defined below) then conditioned upon Executive executing and not revoking a Release (as described below) following such termination, the Company will provide Executive with the following benefits (together, the “CIC Separation Benefits”):
(i) the Company will pay Executive an amount of severance equal to 12 months of Executive’s then-current Base Salary, such amounts payable to Executive over time in accordance with the Company’s payroll practices and procedures beginning on the 60th day following the termination of Executive’s employment with the Company, provided that the first installment will include all amounts that would have been paid if such payments had commenced effective on the date of termination;
(ii) the vesting of all stock options, restricted stock units, and other equity awards will be accelerated such that all such awards are deemed to have been vested as of the date of termination; and
(iii) if Executive timely elects continued health insurance coverage pursuant to COBRA, the Company will contribute toward the premium necessary to continue such coverage for Executive and Executive’s family at the same rate as the Company had contributed for such coverage as of the date of termination, for the same period over which severance is paid or until Executive becomes eligible for group health insurance coverage under another employer’s plan, whichever occurs first, provided however, that the Company will have the right to terminate such payment of COBRA premiums on behalf of Executive and instead pay Executive a lump sum amount equal to the COBRA premium times the number of months remaining in the specified period if the Company determines in its discretion that continued payment of the COBRA premiums is or may be discriminatory under Section 105(h) of the Code.
- 6 -
(e) Change in Control Defined. As used in this Agreement, the term “Change in Control” means the occurrence of any one of the following events:
(i) any “person,” including a “group” (as such terms are used in Sections 13(d) and 14(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), but excluding the Company, any entity controlling, controlled by or under common control with the Company, any trustee, fiduciary or other person or entity holding securities under any employee benefit plan or trust of the Company or any such entity, and, with respect to any particular qualified participant of any equity incentive plan of the Company (a “Participant”), the Participant and any “group” (as such term is used in Section 13(d)(3) of the Exchange Act) of which the Participant is a member), is or becomes the “beneficial owner” (as defined in Rule 13(d)(3) under the Exchange Act), directly or indirectly, of securities of the Company representing 50% or more of either (A) the combined voting power of the Company’s then outstanding securities; or (B) the Company’s then outstanding equity securities (in either such case other than as a result of an acquisition of securities directly from the Company);
(ii) any consolidation or merger of the Company where the stockholders of the Company, immediately prior to the consolidation or merger, would not, immediately after the consolidation or merger, beneficially own (as such term is defined in Rule 13d-3 under the Exchange Act), directly or indirectly, equity securities representing in the aggregate 50% or more of the combined voting power of the securities of the entity issuing cash or securities in the consolidation or merger (or of its ultimate parent entity, if any);
(iii) any sale, lease, exclusive license, exchange or other transfer (in one transaction or a series of transactions contemplated or arranged by any party as a single plan) of all or substantially all of the assets of the Company, other than a sale or disposition by the Company of all or substantially all of the Company’s assets to an entity, at least 50% of the combined voting power of the voting securities of which are owned by “persons” (as defined above) in substantially the same proportion as their ownership of the Company immediately prior to such sale; or
(iv) the members of the Board at the beginning of any consecutive 24-calendar-month period (the “Incumbent Directors”) cease for any reason other than due to death to constitute at least a majority of the members of the Board; provided that any director whose election, or nomination for election by the Company’s stockholders, was approved or ratified by a vote of at least a majority of the members of the Board then still in office who were members of the Board at the beginning of such 24-calendar-month period, will be deemed to be an Incumbent Director.
Notwithstanding the foregoing, no event or condition will constitute a Change in Control to the extent that, if it were, a penalty tax would be imposed under Section 409A; provided that, in such a case, the event or condition will continue to constitute a Change in Control to the maximum extent possible (e.g., if applicable, in respect of vesting without an acceleration of distribution) without causing the imposition of such penalty tax.
- 7 -
(f) Release. The Company’s obligation to provide the Separation Benefits or the CIC Separation Benefits, as applicable, is conditioned upon Executive executing and returning a release of claims in a form acceptable to the Company (the “Release”) within the time specified therein, which Release is not revoked within any time period allowed for revocation under applicable law.
(g) Post-Termination Breach. If Executive is entitled to receive the Separation Benefits or the CIC Separation Benefits but violates any provisions of Sections 8 through 10 hereof after termination of employment, the Company will be entitled to immediately stop paying any further installments of the Separation Benefits or the CIC Separation Benefits as applicable, in addition to any other remedies that may be available to the Company in law or at equity.
(h) No Further Obligations. Except as expressly provided above or as otherwise required by law, the Company will have no obligations to Executive in the event of the termination of this Agreement for any reason. For the avoidance of doubt, in no event will Executive be entitled to benefits under both Section 6(c) and 6(d).
7. Representations of Executive. Executive represents and warrants that he is not obligated or restricted under any agreement (including any non-competition or confidentiality agreement), judgment, decree, order or other restraint of any kind that could impair Executive’s ability to perform the duties and obligations required hereunder. Executive further agrees that Executive will not divulge to the Company any confidential information and/or trade secrets belonging to others, including Executive’s former employers. Consistent with the foregoing, Executive will not provide to the Company any documents or copies of documents containing such information.
8. Confidential Information.
(a) Executive acknowledges that the Company has and will give Executive access to certain highly-sensitive, confidential, and proprietary information belonging to the Company (or third parties who may have furnished such information under obligations of confidentiality), relating to and used in the Company’s business (collectively, “Confidential Information”). Executive acknowledges that Confidential Information includes, but is not limited to, the following categories of Company related confidential or proprietary information and material: financial statements and information; budgets, forecasts, and projections; business and strategic plans; marketing, sales, and business development strategies; research and development projects; records relating to any intellectual property developed by, owned by, controlled, or maintained by the Company; information related to the Company’s inventions, research, products, designs, methods, formulae, techniques, systems, processes; customer lists; non-public information relating to the Company’s customers, suppliers, distributors, or investors; the specific terms of the Company’s agreements or arrangements, whether oral or written, with any customer, supplier, vendor, or contractor with which the Company may be associated from time to time; and any and all information relating to the operation of the Company’s business which the Company may from time to time designate as confidential or proprietary or that Executive reasonably knows should be, or has been, treated by the Company as confidential or proprietary. Executive further understands that Company will receive confidential business information from third parties, including but not limited to the Company’s clients (the “Third Party Information”), under a duty to maintain the confidentiality of such Third Party Information and to use it only for limited purposes. Confidential Information and Third Party Information encompass all formats in which information is preserved, whether electronic, print, or any other form, including all originals, copies, notes, or other reproductions or replicas thereof.
- 8 -
(b) Confidential Information does not include any information that: (i) at the time of disclosure is generally known to, or readily ascertainable by, the public; (ii) becomes known to the public through no fault of Executive or other violation of this Agreement; or (iii) is disclosed to Executive by a third party under no obligation to maintain the confidentiality of the information.
(c) Executive acknowledges that the Confidential Information is owned or licensed by the Company (and Third Party Information is possessed by the Company with the permission of its owner); is unique, valuable, proprietary and confidential; derives independent actual or potential commercial value from not being generally known or available to the public; and is subject to reasonable efforts to maintain its secrecy. Executive hereby relinquishes and agrees that Executive will not at any time claim, any right, title or interest of any kind in or to any Confidential Information or Third Party Information.
(d) During and after Executive’s employment with the Company, Executive will hold in trust and confidence all Confidential Information and Third Party Information, and will not disclose any Confidential Information or Third Party Information to any person or entity, except in the course of performing duties assigned by the Company or as authorized in writing by the Company. Executive further agrees that during and after Executive’s employment with the Company, Executive will not use any Confidential Information or Third Party Information for any purpose, except in the course of performing duties assigned by the Company or as authorized in writing by the Company.
(e) Notwithstanding the covenants contained in Section 8(d) above, Executive may disclose Confidential Information or Third Party Information solely to the extent that Executive is required to do so by law, provided that Executive (i) notifies the Company of the existence and terms of such obligation, (ii) gives the Company a reasonable opportunity to seek a protective or similar order to prevent or limit such disclosure, and (iii) only discloses that information actually required to be disclosed.
(f) Nothing in this Agreement is intended to or will prohibit Executive from communicating with any governmental authority, or making a report in good faith and with a reasonable belief of any violations of law or regulation to a governmental authority, or from filing, testifying or participating in a legal proceeding relating to such violations, including making disclosures protected or required by any whistleblower law or regulation to the Securities and Exchange Commission, the Department of Labor, or any other appropriate government authority charged with the enforcement of any applicable laws. In addition, nothing in this Agreement is intended to or will limit any employee’s right to discuss the terms, wages, and working conditions of their employment, as protected by applicable law. Pursuant to the Defend Trade Secrets Act of 2016, Executive is hereby notified that an individual will not be held criminally or civilly liable under any federal or state trade secret law for the disclosure of a trade secret that (i) is made (A) in confidence to a federal, state or local government official, either directly or indirectly, or to an attorney; and (B) solely for the purpose of reporting or investigating a suspected violation of law; or (ii) is made in a complaint or other document filed in a lawsuit or other proceeding, if such filing is made under seal. An individual who files a lawsuit for retaliation by an employer for reporting a suspected violation of law may disclose the trade secret to his or her attorney and use the trade secret information in the court proceeding, if the individual files any document containing the trade secret under seal, and does not disclose the trade secret, except pursuant to court order.
- 9 -
(g) Return of Property. Upon request during employment and immediately at the termination of Executive’s employment, Executive will return to the Company all Confidential Information and Third Party Information in any form (including all copies and reproductions thereof) and all other property whatsoever of the Company in Executive’s possession or under Executive’s control. If requested by the Company, Executive will certify in writing that all such materials have been returned to the Company. Upon request by the Company, Executive will cooperate with the Company in transferring all logins, passwords, and other access information for accounts and systems previously used by Executive in connection with her employment with the Company. Executive also expressly agrees that immediately upon the termination of Executive’s employment with the Company for any reason, Executive will cease using any secure website, computer systems, e-mail system, or phone system or voicemail service provided by the Company for the use of its employees.
9. Assignment of Inventions.
(a) Executive agrees that all developments or inventions (including without limitation any and all software programs (source and object code), algorithms and applications, concepts, designs, discoveries, improvements, processes, techniques, know-how and data) that are initiated, conceived, discovered, reduced to practice, or made by Executive, either alone or in conjunction with others, during the Term and relate to or result from the Executive’s work for the Company or which relate directly to the Company’s businesses, whether or not patentable or registrable under copyright or similar statutes or subject to analogous protection (“Inventions”), will be the sole and exclusive property of the Company or its nominees. Executive will and hereby does assign to the Company all rights in and to such Inventions upon the creation of any such Invention, including, without limitation: (i) patents, patent applications and patent rights throughout the world; (ii) rights associated with works of authorship throughout the world, including copyrights, copyright applications, copyright registrations, mask work rights, mask work applications and mask work registrations; (iii) rights relating to the protection of trade secrets and confidential information throughout the world; (iv) rights analogous to those set forth herein and any other proprietary rights relating to intangible property; and (v) divisions, continuations, renewals, reissues and extensions of the foregoing (as applicable), now existing or hereafter filed, issued or acquired (collectively, the “IP Rights”).
(b) For avoidance of doubt, if any Invention falls within the definition of “work made for hire” as such term is defined in 17 U.S.C. § 101, such Invention(s) will be considered “work made for hire” and the copyright of such Invention(s) will be owned solely and exclusively by the Company. If any Invention does not fall within such definition of “work made for hire” then Executive’s right, title and interest in and to such Invention(s) will be assigned to the Company pursuant to Section 9(a) above.
- 10 -
(c) The Company and its nominees will have the right to use and/or to apply for statutory or common law protections for such Inventions in any and all countries. Executive further agrees, at the Company’s expense, to: (i) reasonably assist the Company in obtaining and from time to time enforcing such IP Rights relating to Inventions, and (ii) execute and deliver to the Company or its nominee upon reasonable request all such documents as the Company or its nominee may reasonably determine are necessary or appropriate to effect the purposes of this Section 9, including assignments of inventions. Such documents may be necessary to: (1) vest in the Company or its nominee clear and marketable title in and to Inventions; (2) apply for, prosecute and obtain patents, copyrights, mask works rights and other rights and protections relating to Inventions; or (3) enforce patents, copyrights, mask works rights and other rights and protections relating to Inventions. Executive’s obligations pursuant to this Section 9 will continue beyond the termination of Executive’s employment with the Company. Executive hereby irrevocably designates and appoints the Company and its then-current Chief Executive Officer as Executive’s agent and attorney-in-fact to act for and in behalf and instead of Executive, to execute and file any such application and to do all other lawfully permitted acts to further the prosecution and issuance of patents, trademarks, copyrights or other rights thereon with the same legal force and effect as if executed by Executive, if the Company is unable after reasonable effort to secure Executive’s signature to any lawful and necessary document required to apply for or execute any patent, trademark, copyright or other applications with respect to any Inventions (including renewals, extensions, continuations, divisions or continuations in part thereof).
(d) The obligations of Executive under Section 9(a) above will not apply to any Invention that Executive developed or conceived prior to the Effective Date or entirely on Executive’s own time after the Effective Date without using the Company’s equipment, supplies, facility or trade secret information, except for those Inventions that (i) relate directly to the Company’s business or actual or demonstrably anticipated research or development or (ii) result from any work performed by Executive for Company. Executive will bear the burden of proof in establishing the applicability of this subsection to a particular circumstance.
10. Restrictive Covenants.
(a) Purpose. Executive understands and agrees that the purpose of this Section 10 is solely to protect the Company’s legitimate business interests, including, but not limited to its confidential and proprietary information, customer relationships and goodwill, and the Company’s competitive advantage, and will not unduly impair Executive’s ability or right to work or earn a living. Therefore, Executive agrees to be subject to restrictive covenants under the following terms.
(b) Definitions. As used in this Agreement, the following terms have the meanings given to such terms below.
(i) “Business” means (A) the development or commercialization of treatments for diseases of the central nervous system, including cancers, and (B) the business(es) in which the Company was actively engaged at the time of, or during the 12-month period immediately preceding the Termination Date, provided that this clause (B) will only apply if Executive is involved with such other business.
- 11 -
(ii) “Company Employee” means any person who is or was an employee or independent contractor of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(iii) “Customer” means any person or entity who is or was a customer or client of the Company at the time of, or during the 12-month period immediately preceding, (A) the conduct in question, if such conduct occurs prior to the Termination Date, or (B) the Termination Date, if the conduct in question occurs on or after the Termination Date, and in either case with whom Executive had dealings on behalf of the Company in the course of his employment with the Company during such period, or about whom Executive learned or received confidential and proprietary information in the course of his employment with Company during such period.
(iv) “Prospective Customer” means any person or entity to whom, within the 12 months immediately prior to the Termination Date the Company had submitted a proposal for products or services of which Executive has knowledge, provided that Executive has had material business contacts with such person or entity on behalf of the Company during such 12-month period, whether such contact was initiated by the person or entity or by Executive.
(v) “Restricted Period” means the period commencing on the Termination Date and ending 12 months after such date, provided, however, that this period will not run during any time Executive is in violation of this Section 10, it being the intent of the parties that the Restricted Period will be extended for any period of time in which Executive is in violation of this Section 10.
(vi) “Restricted Territory” means the United States of America, it being understood that the Company’s business is nationwide in scope. In the event that the preceding definition of Restricted Territory is deemed by a court of competent jurisdiction to be too broad to be enforced under the circumstances, then “Restricted Territory” will mean the following severable and distinct territories:
(A) the State of North Carolina;
(B) each state, province, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
(C) each city, county, township, or similar political subdivision in which the Company engaged in material business at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date;
- 12 -
(D) each state, province, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date; or
(E) each city, county, township, or similar political subdivision in which the Company engaged in material business with respect to which Executive provided material services on behalf of the Company at the time of, or during the 12-month period immediately prior to, (y) the conduct in question, if such conduct occurs prior to the Termination Date, or (z) the Termination Date, if the conduct in question occurs on or after the Termination Date.
(vii) “Termination Date” means the effective date of the termination of Executive’s employment for any reason.
(c) Non-Competition. During Executive’s employment with the Company, Executive will not, on Executive’s own behalf or on behalf of any other person, engage in any business competitive with or adverse to that of the Company. In addition, during Executive’s employment with the Company and during the Restricted Period, Executive will not hold a position based in or with responsibility for all or part of the Restricted Territory, with any person or entity engaging in the Business, whether as employee, consultant, or otherwise, in which Executive will have duties, or will perform or be expected to perform services for such person or entity, that is or are the same as or substantially similar to the position held by Executive or those duties or services actually performed by Executive for the Company within the 12-month period immediately preceding (i) the conduct in question, if such conduct occurs prior to the Termination Date, or (ii) the Termination Date, if the conduct in question occurs on or after the Termination Date, or in which Executive will use or disclose or be reasonably expected to use or disclose any confidential or proprietary information of the Company for the purpose of providing, or attempting to provide, such person or entity with a competitive advantage with respect to the Business. The Parties agree that nothing in this Section 10(c) will prohibit Executive from owning, as a passive investment, not more than 1% of any class of securities of a business entity which is engaged, directly or indirectly, in a business which competes with the Business, or restrict or limit Executive’s ability to work or provide services to a distinct department, division, or other segment of a business entity that does not engage in the Business, even though other departments, divisions, or segments of the business entity may engage in the Business.
(d) Non-Solicitation. During Executive’s employment with the Company and during the Restricted Period, Executive will not, directly or indirectly, on Executive’s own behalf or on behalf of any other party:
(i) Call upon, solicit, divert, encourage or attempt to call upon, solicit, divert, or encourage any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
- 13 -
(ii) Accept as a customer any Customer or Prospective Customer for purposes of marketing, selling, or providing products or services to such Customer or Prospective Customer that are competitive with those offered by the Company;
(iii) Induce, encourage, or attempt to induce or encourage any Customer or Prospective Customer to purchase or accept products or services that are competitive with those offered by the Company from any person or entity (other than the Company) engaging in the Business;
(iv) Induce, encourage, or attempt to induce or encourage any Customer to reduce, limit, or cancel its business with the Company;
(v) Solicit, induce, or attempt to solicit or induce any Company Employee to terminate his or her employment or engagement with the Company; or
(vi) Otherwise interfere with or engage in any conduct that would have the effect of interfering with the business relationship between the Company and any of its vendors, suppliers, consultants, or contractors.
(e) Reasonableness of Restrictions. Executive acknowledges and agrees that the restrictive covenants in this Agreement (i) are essential elements of Executive’s employment by the Company and are reasonable given Executive’s access to the Company’s Confidential Information and the substantial knowledge and goodwill Executive will acquire with respect to the business of the Company as a result of Executive’s employment with the Company, and the unique and extraordinary services to be provided by Executive to the Company; and (ii) are reasonable in time, territory, and scope, and in all other respects. Provisions in this Section 10 calling for a “look back” prior to a given date are intended solely as a means of identifying the individuals, entities, and/or place to which the restrictions described in this Section 10 apply and are not intended to extend the length of the restrictions. The existence or assertion of any claim of or by the Executive, whether predicated on this Agreement or otherwise, will not constitute a defense to the enforcement by the Company of the covenants contained in this Section 10.
(f) Consideration. Executive acknowledges and agrees that the covenants set forth in this Agreement are supported by adequate consideration, including, but not limited to the Company’s promise to provide the Separation Benefits or the CIC Separation Benefits, as applicable, and the Company’s other promises as described in this Agreement. The Company would not have agreed to enter into this Agreement but for Executive’s agreement to the restrictions imposed by this Section 10.
(g) Judicial Modification. Should any part or provision of this Section 10 be held invalid, void, or unenforceable in any court of competent jurisdiction, such invalidity, voidness, or unenforceability will not render invalid, void, or unenforceable any other part or provision of this Agreement. The parties further agree that if any portion of this Section 10 is found to be invalid or unenforceable by a court of competent jurisdiction because its duration, territory, or other restrictions are deemed to be invalid or unreasonable in scope, the parties intend that the court will, to the maximum extent permitted by law, replace the invalid or unreasonable terms with terms that are valid and enforceable and that come closest to expressing the intention of such invalid or unenforceable terms.
- 14 -
11. Enforcement. Executive acknowledges and agrees that the Company will suffer irreparable harm in the event that Executive breaches any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement and that monetary damages would be inadequate to compensate the Company for such breach. Accordingly, Executive agrees that, in the event of a breach by Executive of any of Executive’s obligations under Sections 8, 9, or 10 of this Agreement, the Company will be entitled to obtain from any court of competent jurisdiction preliminary and permanent injunctive relief in order to prevent or to restrain any such breach, without the need for posting bond or other security.
12. Cooperation. Upon the receipt of reasonable notice from the Company (including its outside counsel), Executive agrees that while employed by, or providing services to, the Company and thereafter, Executive will respond and provide information with regard to matters in which the Executive has knowledge as a result of the Executive’s employment or service with the Company, and will, at the Company’s sole expense, provide reasonable assistance to the Company, its affiliates and their respective representatives in defense of all claims that may be made against the Company (but excluding claims by Executive), and will assist the Company in the prosecution of all claims that may be made by the Company or its affiliates, to the extent that such claims may relate to the period of the Executive’s employment or service with the Company. Following the Term, the Company will reasonably cooperate with Executive regarding the scheduling of such instances of cooperation under this Section 12, taking into account Executive’s professional and personal commitments. Executive agrees to promptly inform the Company if Executive becomes aware of any lawsuit involving such claims that is likely to be filed or threatened against the Company. Executive also agrees to promptly inform the Company (to the extent that Executive is legally permitted to do so) if Executive is asked to assist in any investigation of the Company (or its actions), regardless of whether a lawsuit or other proceeding has then been filed against the Company with respect to such investigation, and will not do so unless legally required. Upon presentation of appropriate documentation, the Company will reimburse Executive for any reasonable expenses Executive incurs in connection with Executive’s cooperation under this provision.
13. Indemnification. The Company will indemnify Executive and hold Executive harmless in connection with the defense of any lawsuit or other claim, but excluding claims arising out of Executive’s willful misconduct, to which he is made a party by reason of being an officer, director or employee of the Company, to the fullest extent permitted by the laws of the State of Delaware, as in effect at the time of the subject act or omission. The Company will use commercially reasonable efforts to maintain directors’ and officers’ liability insurance for acts and omissions occurring during Executive’s employment with the Company, in amounts and on terms reasonable and customary for similarly situated companies, and Executive will be covered by such insurance on the same basis as other executive officers of the Company.
- 15 -
14. Certain Tax Matters.
(a) Application of Section 409A of the Code. The parties intend that this Agreement and the payments made hereunder will be exempt from, or comply with, the requirements of Section 409A of the Code and the regulations and other guidance thereunder and any state law of similar effect (collectively “Section 409A”), and this Agreement will be interpreted and applied to the greatest extent possible in a manner that is consistent with the requirements for avoiding taxes or penalties under Section 409A. In no event, however, will the Company be liable for any additional tax, interest, or penalty that may be imposed on Executive by Section 409A or damages for failing to comply with Section 409A. Notwithstanding anything to the contrary set forth herein, any payments and benefits provided under Section 6 above that constitute “deferred compensation” within the meaning of Section 409A will not commence in connection with Executive’s termination of employment unless and until Executive has also incurred a “separation from service” (as such term is defined in Treasury Regulation Section 1.409A-1(h)), unless the Company reasonably determines that such amounts may be provided to Executive without causing Executive to incur the additional 20% tax under Section 409A. The parties intend that each installment of the Separation Benefits or the CIC Separation Benefits, as applicable, is a separate “payment” for purposes of Section 409A. For the avoidance of doubt, the parties intend that the Separation Benefits or the CIC Separation Benefits, as applicable, satisfy, to the greatest extent possible, the exemptions from the application of Section 409A provided under Treasury Regulation Sections 1.409A-1(b)(4) and 1.409A-1(b)(9). However, if the Company determines that the Separation Benefits or the CIC Separation Benefits, as applicable, constitute “deferred compensation” under Section 409A and Executive is, as of the separation from service, a “specified employee” of the Company or any successor entity thereto, as such term is defined in Section 409A, then, solely to the extent necessary to avoid the incurrence of the adverse personal tax consequences under Section 409A, the timing of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, will be delayed until the earlier to occur of: (i) the date that is six months and one day after Executive’s separation from service, or (ii) the date of Executive’s death (such applicable date, the “Specified Employee Initial Payment Date”), and the Company (or the successor entity thereto, as applicable) will (A) pay to Executive a lump sum amount equal to the sum of the Separation Benefits payments or the CIC Separation Benefits payments, as applicable, that Executive would otherwise have received through the Specified Employee Initial Payment Date if the commencement of the payment of the Separation Benefits or the CIC Separation Benefits, as applicable, had not been so delayed pursuant to this Section, and (B) commence paying the balance of the Separation Benefits or the CIC Separation Benefits, as applicable, in accordance with the applicable payment schedules set forth in this Agreement.
(b) Application of Section 280G of the Code. If any payment, benefit or distribution of any type to or for the benefit of Executive, whether paid or payable, provided or to be provided, or distributed or distributable pursuant to the terms of this Agreement or otherwise (collectively, the “Parachute Payments”) would subject the Executive to the excise tax imposed under Section 4999 of the Code (the “Excise Tax”), the Parachute Payments will be reduced so that the maximum amount of the Parachute Payments (after reduction) will be $1.00 less than the amount which would cause the Parachute Payments to be subject to the Excise Tax; provided that the Parachute Payments will only be reduced to the extent the after-tax value of amounts received by the Executive after application of the above reduction would exceed the after-tax value of the amounts received without application of such reduction. For this purpose, the after-tax value of an amount will be determined taking into account all federal, state, and local income, employment and excise taxes applicable to such amount. If a reduction the Parachute Payments as described in this Section is necessary, the Company will reduce or eliminate the Parachute Payments by first reducing or eliminating any cash payments (with the payments to be made furthest in the future being reduced first), then by reducing or eliminating accelerated vesting of stock options or similar awards, and then by reducing or eliminating any other remaining Parachute Payments; provided, that no such reduction or elimination will apply to any non-qualified deferred compensation amounts (within the meaning of Section 409A) to the extent such reduction or elimination would accelerate or defer the timing of such payment in manner that does not comply with Section 409A. The determination as to whether (x) any of the Parachute Payments received by the Executive in connection with the occurrence of a change in the ownership or control of the Company or in the ownership of a substantial portion of the assets of the Company will be subject to the Excise Tax, and (y) the amount of any reduction, if any, that may be required pursuant to the previous paragraph, will be made by an independent accounting firm selected by the Company (the “Accounting Firm”) prior to the consummation of such change in the ownership or effective control of the Company or in the ownership of a substantial portion of the assets of the Company. For purposes of making the calculations required by this Section 14(b), the Accounting Firm may make reasonable assumptions and approximations concerning applicable taxes and may rely on reasonable, good faith interpretations concerning the application of the Code (including but not limited to Sections 280G and 4999). The parties agree to furnish to the Accounting Firm such information and documents as the Accounting Firm may reasonably request in order to make a determination under this Section 14(b). The Company will bear all costs the Accounting Firm may reasonably incur in connection with any calculations contemplated by this Section 14(b). Executive will be furnished with notice of all determinations made as to the Excise Tax payable with respect to the Executive’s Parachute Payments, together with the related calculations of the Accounting Firm, promptly after such determinations and calculations have been received by the Company.
- 16 -
15. Miscellaneous.
(a) Entire Agreement. This Agreement constitutes the entire agreement between the parties with respect to the subject matter hereof and supersedes all prior agreements (whether written or oral and whether express or implied) between the parties to the extent related to such subject matter.
(b) Successors and Assigns. This Agreement will be binding upon and inure to the benefit of the parties and their respective successors, permitted assigns and, in the case of Executive, heirs, executors, and/or personal representatives. The Company may freely assign or transfer this Agreement to an affiliated company or to a successor following a merger, consolidation, sale of assets, or other business transaction. Executive may not assign, delegate or otherwise transfer any of Executive’s rights, interests or obligations in this Agreement without the prior written approval of the Company.
(c) Counterparts; Electronic Delivery. This Agreement may be executed in one or more counterparts, each of which will be deemed an original but all of which together will constitute one instrument reflecting the terms of the Agreement. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature complying with the U.S. ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered will be deemed to have been duly and validly delivered and be valid and effective for all purposes.
(d) Notices. Any notice pursuant to this Agreement must be in writing and will be deemed effectively given to the other party (i) on the date it is actually delivered by personal delivery of such notice, (ii) one business day after its deposit in the custody of a reputable overnight courier service (such as FedEx) next business day delivery charges prepaid; or (iii) three business days after its deposit in the custody of the U.S. mail, certified or registered postage prepaid, return receipt requested; in each case to the appropriate address shown below (or to such other address as a party may designate by notice to the other party):
| If to Executive: | L. Arthur Hewitt | |
| If to Company: | Adaptin Bio, Inc. 3540 Toringdon Way, Suite 200 #250 Charlotte, North Carolina 28277 Attention: Board of Directors |
(e) Amendments and Waivers. No amendment of any provision of this Agreement will be valid unless the amendment is in writing and signed by the Company and Executive. No waiver of any provision of this Agreement will be valid unless the waiver is in writing and signed by the waiving party. The failure of a party at any time to require performance of any provision of this Agreement will not affect such party’s rights at a later time to enforce such provision. No waiver by a party of any breach of this Agreement will be deemed to extend to any other breach hereunder or affect in any way any rights arising by virtue of any other breach.
- 17 -
(f) Severability. Each provision of this Agreement is severable from every other provision of this Agreement. Any provision of this Agreement that is determined by any court of competent jurisdiction to be invalid or unenforceable will not affect the validity or enforceability of any other provision. Any provision of this Agreement held invalid or unenforceable only in part or degree will remain in full force and effect to the extent not held invalid or unenforceable.
(g) Construction. The section headings in this Agreement are inserted for convenience only and are not intended to affect the interpretation of this Agreement. The word “including” in this Agreement means “including without limitation.” This Agreement will be construed as if drafted jointly by the Company and Executive and no presumption or burden of proof will arise favoring or disfavoring the Company or Executive by virtue of the authorship of any provision in this Agreement. All words in this Agreement will be construed to be of such gender or number as the circumstances require.
(h) Survival. This Agreement, and all obligations of the Company and Executive hereunder, will terminate upon the termination of Executive’s employment, with the following exceptions: (i) Executive’s continuing obligations under Sections 6, 8, 9, 10, 12, and 14; (ii) the Company’s obligations under Section 6 (subject to Executive’s obligations thereunder); (c) Executive’s right to indemnification under Section 13; and (d) the relevant provisions of Sections 11 and 15.
(i) Remedies Cumulative. The rights and remedies of the parties under this Agreement are cumulative (not alternative) and in addition to all other rights and remedies available to such parties at law, in equity, by contract or otherwise.
(j) Governing Law. This Agreement will be governed by the laws of the State of North Carolina without regard to principles of choice or conflict of laws (whether of the State of North Carolina or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of North Carolina.
(k) Venue. The parties agree that any litigation arising out of or related to this Agreement or Executive’s employment by Company will be brought exclusively in any state or federal court in Mecklenburg County, North Carolina. Each party (i) consents to the personal jurisdiction of said courts, (ii) waives any venue or inconvenient forum defense to any proceeding maintained in such courts, and (iii) except as expressly provided above, agrees not to bring any proceeding arising out of or relating to this Agreement or Executive’s employment by Company in any other court.
(l) Legal Counsel. This Agreement was prepared by Wyrick Robbins Yates & Ponton LLP in its capacity as legal counsel to the Company. Both Parties acknowledge that they have had the opportunity to seek and obtain the advice of counsel before entering into this Agreement and have done so to the extent desired, and have fully read the Agreement and understand the meaning and import of all the terms hereof.
[Signature Page Immediately Follows]
- 18 -
IN WITNESS WHEREOF, the parties hereto have executed and delivered this Agreement as of the date first written above.
| EXECUTIVE: | COMPANY: | ||
| /s/ L. Arthur Hewitt | Adaptin Bio, Inc. | ||
| L. Arthur Hewitt | |||
| By: | /s/ Michael J. Roberts | ||
| Name: | Michael J. Roberts | ||
| Title: | Chief Executive Officer | ||
[Signature Page to Executive Employment Agreement]
Exhibit 10.14
COMPENSATION AGREEMENT
THIS COMPENSATION AGREEMENT, dated as of October 1, 2023 (this “Agreement”), is by and between Simon C. Pedder with an address of 845 Jim Wilson Road, Indian Land, SC 29707 (“Executive Chairman”), and CENTAUR BIO, INC, with principal executive offices at 7805 Pemswood Street, Charlotte, NC 28277 (“Company”).
W I T N E S S E T H:
IN CONSIDERATION of the mutual covenants and agreements hereinafter set forth, the parties hereto agree as follows:
Section 1. Services. Executive Chairman agrees to provide business and pharmaceutical development related services to Company (the “Services”). Executive Chairman hereby agrees that the Services shall be provided at such times and at such places as the Company shall reasonably request, and in accordance with the highest prevailing industry standards and practices for the performance of similar services.
Section 2. Term of Agreement. The retention of the Executive Chairman by the Company as provided in Section I above shall be until a formal Employment Agreement has been executed between the Company and Executive Chairman (the “Term”). Notwithstanding anything to the contrary contained herein, the Agreement may be terminated by Executive Chairman or the Company upon thirty (30) days prior written notice to the other party.
Section 3. Compensation.
(a) So long as Company has sufficient funding and as full compensation for the performance by Executive Chairman of his/her duties under this Agreement, the Company shall pay Executive Chairman $10,000 per month (the “Fee”) that Executive Chairman spends performing his/her duties pursuant to the terms hereof.
(b) The Company agrees that payment shall be made to Executive Chairman within fifteen (15) business days from the beginning of each month. Executive Chairman will be solely responsible for all taxes, withholding and other similar statutory obligations.
Section 4. Expenses. The Company shall reimburse Executive Chairman for all reasonable and necessary expenses incurred by Executive Chairman in connection with the Services provided hereunder; provided, however, that such expenses are pre-approved in writing by the Company.
Section 5. Miscellaneous.
This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the State of North Carolina, without giving effect to its principles of conflicts of laws.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above by proper person thereunto duly authorized.
| CENTAUR BIO, INC. | |
| By: /s/ Michael J. Roberts | |
| Name: Michael J. Roberts | |
| Title: CEO | |
| EXECUTIVE CHAIRMAN | |
| /s/ Simon C. Pedder | |
| Simon C. Pedder |
Exhibit 10.15
COMPENSATION AGREEMENT
THIS COMPENSATION AGREEMENT, dated as of October 1, 2023 (this “Agreement”), is by and between MAC B Consulting LLC, a North Carolina Limited Liability Company d/b/a Michael J. Roberts with an address of 7805 Pemswood Street, Charlotte, NC 28277 (“CEO”), and CENTAUR BIO, INC, with principal executive offices at 7805 Pemswood Street, Charlotte, NC 28277 (“Company”).
W I T N E S S E T H:
IN CONSIDERATION of the mutual covenants and agreements hereinafter set forth, the parties hereto agree as follows:
Section 1. Services. CEO agrees to provide business and pharmaceutical development related services to Company (the “Services”). CEO hereby agrees that the Services shall be provided at such times and at such places as the Company shall reasonably request, and in accordance with the highest prevailing industry standards and practices for the performance of similar services.
Section 2. Term of Agreement. The retention of the CEO by the Company as provided in Section 1 above shall be until a formal Employment Agreement has been executed between the Company and CEO (the “Term”). Notwithstanding anything to the contrary contained herein, the Agreement may be terminated by CEO or the Company upon thirty (30) days prior written notice to the other party.
Section 3. Compensation.
(a) So long as Company has sufficient funding and as full compensation for the performance by CEO of his/her duties under this Agreement, the Company shall pay CEO $15,000 per month (the “Fee”) that CEO spends performing his/her duties pursuant to the terms hereof.
(b) The Company agrees that payment shall be made to CEO within fifteen (15) business days from the beginning of each month. CEO will be solely responsible for all taxes, withholding and other similar statutory obligations.
Section 4. Expenses. The Company shall reimburse CEO for all reasonable and necessary expenses incurred by CEO in connection with the Services provided hereunder; provided, however, that such expenses are pre-approved in writing by the Company.
Section 5. Miscellaneous.
This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the State of North Carolina, without giving effect to its principles of conflicts of laws.
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above by proper person thereunto duly authorized.
| CENTAUR BIO, INC. | |
| By: /s/ Simon C. Pedder | |
| Name: Simon C. Pedder | |
| Title: Executive Chairman | |
| CEO | |
| /s/ Michael J. Roberts | |
| Michael J. Roberts |
Exhibit 10.16
CONSULTING AGREEMENT
THIS CONSULTING AGREEMENT, dated as of May 31, 2023 (this “Agreement”), is by and between Adamanteus LLC, a North Carolina Limited Liability Company d/b/a Timothy Maness, CPA with a principal place of business at 2519 Roundabout Lane, Charlotte, NC 28210 (“Consultant”), and CENTAUR BIO, INC, with principal executive offices at 7805 Pemswood Street, Charlotte, NC 28277 (“Company”).
W I T N E S S E T H:
WHEREAS, Company is a development stage biomedical and pharmaceutical company;
WHEREAS, Consultant provides expertise in finance and accounting and other matters related to the pharmaceutical, biotechnology and life science industries;
WHEREAS, Company desires that it be able to call upon the knowledge and experience of Consultant for consultation services; and
WHEREAS, Consultant is willing to render such services to Company on the terms and conditions hereinafter set forth in this Agreement.
NOW, THEREFORE, in consideration of the mutual covenants and agreements hereinafter set forth, the parties hereto agree as follows:
Section 1. Services. Consultant agrees to provide financial and accounting services to Company. Consultant’s services to the Company shall include assisting with financial audits, regulatory filings and other projects assigned to him by the Company (the “Services”). Consultant hereby agrees that the Services shall be provided at such times and at such places as the Company shall reasonably request, and in accordance with the highest prevailing industry standards and practices for the performance of similar services.
Section 2. Term of Agreement. The retention of the Consultant by the Company as provided in Section 1 above shall be for a period of six (6) month from the date hereof, unless sooner terminated in accordance herewith (the “Term”); provided, however, that the Term shall be extended automatically for an additional six-month period upon mutual written agreement of both Consultant and Company. Notwithstanding anything to the contrary contained herein, the Agreement may be terminated by Consultant or the Company upon thirty (30) days prior written notice to the other party. Immediately upon receipt of such notice from the Company, Consultant shall institute such termination procedures as may be specified in the notice and shall use his/her best efforts to minimize the cost to Company resulting from such termination. Sections 5, 6, 7, 8, 9 and 10 shall survive the expiration or termination of this Agreement.
Section 3. Compensation.
(a) As full compensation for the performance by Consultant of his/her duties under this Agreement, the Company shall pay Consultant $300 per hour (the “Fee”) that Consultant spends performing his/her duties pursuant to the terms hereof, provided, however that the amount of time Consultant spends performing his/her duties pursuant to this Agreement shall not exceed 20 hours per month unless otherwise agreed in writing by Company and Consultant.
(b) Consultant shall provide the Company with written invoices on a monthly basis for services rendered for such month, which shall set forth the actual number of hours Consultant expends performing the Services, a description of the activities undertaken by Consultant, and the itemization of all expenses incurred that are reimbursable pursuant to Section 4 hereof. The Company agrees that payment shall be made to Consultant within thirty (30) business days of its receipt of each undisputed monthly invoice.
(c) Consultant and Company acknowledge and agree that the compensation set forth herein represents the fair market value of the services provided to Company by Consultant, negotiated in an arms-length transaction, and has not been determined in a manner which takes into account the volume or value of any current or future referrals or business otherwise generated between the Company and the Consultant. Nothing contained in this Agreement constitutes or shall be construed in any manner as an obligation or inducement for Consultant to recommend the prescribing, purchase, use, or preferential formulary status or dispensing of any of the Company’s products or services or those of any organizations affiliated with the Company.
Section 4. Expenses. The Company shall reimburse Consultant for all reasonable and necessary expenses incurred by Consultant in connection with the Services provided hereunder; provided, however, that such expenses are pre-approved in writing by the Company.
Section 5. Confidential Information and Inventions.
(a) Consultant recognizes and acknowledges that in the course of his duties Consultant is likely to receive confidential or proprietary information owned by the Company, its affiliates or third parties with whom the Company or any such affiliates has an obligation of confidentiality. Accordingly, during and after the Term, Consultant shall use his best efforts to protect the confidentiality of the Confidential and Proprietary Information and agrees to keep confidential and not disclose or make accessible to any other person or use for any other purpose other than in connection with the fulfillment of his duties under this Agreement, any Confidential and Proprietary Information (as defined below) owned by, or received by or on behalf of, the Company or any of its affiliates. “Confidential and Proprietary Information” shall include, but shall not be limited to, confidential or proprietary scientific or technical information, data, formulas and related concepts, business plans (both current and under development), client lists, promotion and marketing programs, trade secrets, or any other confidential or proprietary business information relating to development programs, costs, revenues, marketing, investments, sales activities, promotions, credit and financial data, manufacturing processes, financing methods, plans or the business and affairs of the Company or of any affiliate or client of the Company. Consultant expressly acknowledges the trade secret status of the Confidential and Proprietary Information and that the Confidential and Proprietary Information constitutes a protectable business interest of the Company. Consultant agrees: (i) not to use any such Confidential and Proprietary Information for himself/herself or others; and (ii) not to take any Company material or reproductions (including but not limited to writings, correspondence, notes, drafts, records, invoices, technical and business policies, computer programs or disks) thereof from the Company’s offices at any time during the Term, except as required in the execution of Consultant’s duties to the Company. Consultant agrees to return immediately all Company material and reproductions (including but not limited, to writings, correspondence, notes, drafts, records, invoices, technical and business policies, computer programs or disks) thereof in his possession to the Company upon request and in any event immediately upon termination or expiration of the Term.
2
(b) Except with prior written authorization by the Company or unless otherwise required by law, Consultant agrees not to disclose or publish any of the Confidential and Proprietary Information, or any confidential, scientific, technical or business information of any other party to whom the Company or any of its affiliates owes an obligation of confidence, at any time during or after the Term.
(c) Consultant agrees that all inventions, discoveries, improvements and patentable or copyrightable works (“Inventions”) initiated, conceived or made by him/her, either alone or in conjunction with others, in connection with or as a result of performance of Services by Consultant during the Term shall be the sole property of the Company to the maximum extent permitted by applicable law and, to the extent permitted by law, shall be “works made for hire” as that term is defined in the United States Copyright Act (17 U.S.C.A., Section 101). The Company shall be the sole owner of all patents, copyrights, trade secret rights, and other intellectual property or other rights in connection therewith. Consultant hereby assigns to the Company all right, title and interest he may have or acquire in all such Inventions. Consultant further agrees to assist the Company in every proper way (but at the Company’s expense) to obtain and from time to time enforce patents, copyrights or other rights on such Inventions in any and all countries, and to that end Consultant will execute all documents necessary:
(i) to apply for, obtain and vest in the name of the Company alone (unless the Company otherwise directs) letters patent, copyrights or other analogous protection in any country throughout the world and when so obtained or vested to renew and restore the same; and
(ii) to defend any opposition proceedings in respect of such applications and any opposition proceedings or petitions or applications for revocation of such letters patent, copyright or other analogous protection.
(d) Consultant acknowledges that while performing the Services under this Agreement Consultant may locate, identify and/or evaluate patented or patentable inventions having commercial potential in the fields of pharmacy, pharmaceutical, biotechnology, healthcare, technology and other fields which may be of potential interest to the Company or one of its affiliates (the “Third Party Inventions”). Consultant understands, acknowledges and agrees that all rights to, interests in or opportunities regarding, all Third-Party Inventions identified by the Company, any of its affiliates or either of the foregoing persons’ officers, directors, employees, agents or consultants (including the Consultant) during the Term shall be and remain the sole and exclusive property of the Company or such affiliate and Consultant shall have no rights whatsoever to such Third-Party Inventions and will not pursue for himself or for others any transaction relating to the Third-Party Inventions which is not on behalf of the Company.
(e) Consultant agrees that he will promptly disclose to the Company, or any persons designated by the Company, all improvements and Inventions relating to, involving or based on Company’s information, technologies or the Services that are made or conceived or reduced to practice or learned by him/her, either alone or jointly with others, during the Term.
(f) Consultant agrees that the Company shall be entitled to enjoin any breach of the confidentiality and other obligations hereunder without having to post a bond in addition to all other remedies it may have under applicable law. Consultant will notify the Company in writing immediately upon the occurrence of any unauthorized release of any Confidential and Proprietary Information or other breach of any of the obligations under this Section 5 of which it is or becomes aware.
Section 6. Insider Trading. Consultant recognizes that in the course of his duties hereunder, Consultant may receive from the Company or others information that may be considered “material, nonpublic information” concerning a public company that is subject to the reporting requirements of the Securities and Exchange Act of 1934, as amended. Consultant agrees NOT to: (a) purchase or sell, directly or indirectly, any securities of any company while in possession of relevant material, nonpublic information relating to such company received from the Company or others in connection herewith; (b) provide Company with information with respect to any public company that may be considered material, nonpublic information; or (c) communicate any material, nonpublic information to any other person in which it is reasonably foreseeable that such person is likely to (i) purchase or sell securities of any company with respect to which such information relates, or (ii) otherwise directly or indirectly benefit from such information. Without limiting any of the confidentiality and insider trading obligations included in this Agreement, Consultant shall not discuss any information concerning Company obtained by Consultant in the course of performing the Services with any financial, securities or industry analyst or with the media without the written agreement of Company.
3
Section 7. Representations, Warranties and Covenants of Consultant. The Consultant hereby represents, warrants and covenants to the Company as follows:
(a) Neither the execution or delivery of this Agreement nor the performance by Consultant of his duties and other obligations hereunder violate or will violate any statute, law, determination or award, or conflict with or constitute a default or breach of any covenant or obligation under (whether immediately, upon the giving of notice or lapse of time or both) any prior employment agreement, contract, or other instrument to which Consultant is a party or by which he is bound.
(b) Consultant has the full right, power and legal capacity to enter and deliver this Agreement, as applicable, and to perform his duties and other obligations hereunder. This Agreement constitutes the legal, valid and binding obligation of Consultant enforceable against him/her in accordance with its terms. No approvals or consents of any persons or entities are required for Consultant to execute and deliver this Agreement, as applicable, or perform his duties and other obligations hereunder.
(c) Consultant represents that his performance of all the terms of this Agreement will not breach any agreement to keep in confidence any confidential information or trade secrets acquired by Consultant from any third party, and Consultant agrees not to use any confidential information or trade secrets of any third party in connection with the provision of the Services in violation of the agreements under which he had access to or knowledge of such confidential information or trade secrets.
(d) Consultant hereby represents that he (i) has not been debarred and (ii) to the best of Consultant’s knowledge, is not under consideration to be disbarred by the Food and Drug Administration (the “FDA”) or any other government agency from working in or providing services to any pharmaceutical or biotechnology company. Consultant shall notify the Company immediately if, during the Term, Consultant comes under investigation by the FDA for debarment or disqualification or is debarred or disqualified. Consultant shall notify the Company immediately if the FDA or any other regulatory authority requests permission to or does inspect Consultant’s records in connection with the Services provided under this Agreement, and Consultant will deliver to the Company promptly all materials, correspondence, statements, forms, and records which Consultant receives, obtains or generates pursuant to any such inspection.
(e) Consultant will not use any confidential information or trade secrets of any third party in his employment by Company in violation of the terms of the agreements under which he had access to or knowledge of such confidential information or trade secrets.
(f) During the Term of this Agreement and for a period of one-year thereafter, if Consultant uses, recommends, or comments upon the attributes of any Company product or service in connection with the treatment of a patient, a scientific or educational presentation or publication, a media interview, or any other third-party communication or interaction, Consultant shall disclose that Consultant is or has been a paid consultant of Company and the fact of any other of Consultant’s financial relationships with Company.
4
Section 8. Non-Circumvention. Consultant acknowledges that the Company regards the identity of the technologies, potential products and business partners evaluated by it to be a trade secret. Consultant agrees during the Term and for a period of one year thereafter not to contact any such business partners or parties (including any of their officers, directors, employees, agents, affiliates and/or consultants) who hold rights to such technologies and potential products without the presence of an officer of the Company or without the prior written consent of an officer of the Company, and Consultant further agrees not to take any action which would adversely affect or otherwise hinder the Company’s ability to ultimately execute any agreements with such parties. In addition, Consultant agrees not to circumvent, avoid, bypass or obviate the Company directly or indirectly regarding the Confidential and Proprietary Information disclosed in connection with the Services and any possible business arrangement envisioned thereby.
Section 9. Consultant not an Employee. Company and Consultant hereby acknowledge and agree that Consultant shall perform the services hereunder as an independent contractor and not as an employee or agent of Company or any Company affiliate. Consultant will be solely responsible for all taxes, withholding and other similar statutory obligations. Consultant shall not represent that he is an employee of Company or any Company affiliate under any circumstance. In addition, nothing in this Agreement shall be construed as establishing any joint venture, partnership or other business relationship between the parties hereto or representing any commitment by either party to enter into any other agreement by implication or otherwise except as specifically stated herein. Consultant shall not have any authority, express or implied, to bind Company or any Company affiliate to any agreement, contract, or other commitment. Consultant further understands and agrees that this Agreement is entered into by Company on a non-exclusive basis and that Company and its affiliates remain free to deal with others and retain other consultants, employees, brokers, finders and other agents in the same or similar capacity as Consultant has been retained at any time at their own option.
Section 10. Miscellaneous.
This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the State of North Carolina, without giving effect to its principles of conflicts of laws.
Any dispute arising out of, or relating to, this Agreement or the breach thereof (other than Section 5 hereof), or regarding the interpretation thereof, shall be finally settled by arbitration conducted in Charlotte, North Carolina in accordance with the rules of the American Arbitration Association then in effect before a single arbitrator appointed in accordance with such rules. Judgment upon any award rendered therein may be entered and enforcement obtained thereon in any court having jurisdiction. The arbitrator shall have authority to grant any form of appropriate relief, whether legal or equitable in nature, including specific performance. For the purpose of any judicial proceeding to enforce such award or incidental to such arbitration or to compel arbitration and for purposes of Section 5 hereof, the parties hereby submit to the exclusive venue and personal jurisdiction of the state and federal courts located in Charlotte, North Carolina, and agree that service of process in such arbitration or court proceedings shall be satisfactorily made upon it if sent by registered mail addressed to it at the address referred to in paragraph (g) below. The costs of such arbitration shall be borne proportionate to the finding of fault as determined by the arbitrator.
5
This Agreement shall be binding upon and inure to the benefit of the parties hereto, and their respective heirs, legal representatives, successors and permitted assigns.
This Agreement, and Consultant’s rights and obligations hereunder, may not be assigned, delegated or otherwise subcontracted by Consultant. The Company may assign its rights, together with its obligations, hereunder in connection with any sale, transfer or other disposition of all or substantially all of its business or assets.
This Agreement cannot be amended orally, or by any course of conduct or dealing, but only by a written agreement signed by the parties hereto.
The failure of either party to insist upon the strict performance of any of the terms, conditions and provisions of this Agreement shall not be construed as a waiver or relinquishment of future compliance therewith, and such terms, conditions and provisions shall remain in full force and effect. No waiver of any term or condition of this Agreement on the part of either party shall be effective for any purpose whatsoever unless such waiver is in writing and signed by such party.
All notices, demands or other communications desired or required to be given by any party to any other party hereto shall be in writing and shall be deemed effectively given upon (i) personal delivery to the party to be notified, (ii) upon confirmation of receipt of telecopy or other electronic facsimile transmission, (iii) one business day after deposit with a reputable overnight courier, prepaid for priority overnight delivery, or (iv) five days after deposit with the United States Post Office, postage prepaid, in each case to such party at the address set forth above, or to such other addresses and to the attention of such other individuals as any party shall have designated to the other parties by notice given in the foregoing manner.
This Agreement sets forth the entire agreement and understanding of the parties relating to the subject matter hereof, and supersedes all prior agreements, arrangements and understandings, written or oral, relating to the subject matter hereof. No representation, promise or inducement has been made by either party that is not embodied in this Agreement, and neither party shall be bound by or liable for any alleged representation, promise or inducement not so set forth.
As used in this Agreement, “affiliate” of a specified person or entity shall mean and include any person or entity controlling, controlled by or under common control with the specified Person.
The section headings contained herein are for reference purposes only and shall not in any way affect the meaning or interpretation of this Agreement.
This Agreement may be executed in any number of counterparts, each of which shall constitute an original, but all of which together shall constitute one and the same instrument.
(l) As used in this Agreement, the masculine, feminine or neuter gender, and the singular or plural, shall be deemed to include the others whenever and wherever the context so requires. Additionally, unless the context requires otherwise, “or” is not exclusive.
[Remainder of Page Intentionally Left Blank – Signature Page Follows]
6
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above by proper person thereunto duly authorized.
| CENTAUR BIO, INC. | ||
| By: | /s/ Michael J. Roberts | |
| Name: | Michael J. Roberts | |
| Title: | CEO | |
| CONSULTANT | ||
| /s/ Timothy Maness | ||
| Timothy Maness | ||
| Managing Member | ||
| Adamanteus LLC | ||
7
Exhibit 10.17
CONSULTING AGREEMENT
THIS CONSULTING AGREEMENT, dated as of September 5, 2024 (this “Agreement”), is by and between L. Arthur Hewitt, having an address of 8002 Pemswood Street, Charlotte, NC 28277 (“Consultant”), and Adaptin Bio, Inc, with principal executive offices at 3540 Toringdon Way, Suite 200, Charlotte, NC 28277 (“Company”).
W I T N E S S E T H:
WHEREAS, Company is a development stage biomedical and pharmaceutical company;
WHEREAS, Consultant provides expertise in research, development and regulatory matters related to the pharmaceutical, biotechnology and life science industries;
WHEREAS, Company desires that it be able to call upon the knowledge and experience of Consultant for consultation services; and
WHEREAS, Consultant is willing to render such services to Company on the terms and conditions hereinafter set forth in this Agreement.
NOW, THEREFORE, in consideration of the mutual covenants and agreements hereinafter set forth, the parties hereto agree as follows:
Section 1. Services. Consultant agrees to provide research, development, and regulatory services to Company. Consultant’s services to the Company shall include assisting with the development of Company’s pharmaceutical products and other projects assigned to him by the Company (the “Services”). Consultant hereby agrees that the Services shall be provided at such times and at such places as the Company shall reasonably request, and in accordance with the highest prevailing industry standards and practices for the performance of similar services.
Section 2. Term of Agreement. The retention of the Consultant by the Company as provided in Section 1 above shall be for a period of six (6) month from the date hereof, unless sooner terminated in accordance herewith (the “Term”); provided, however, that the Term shall be extended automatically for an additional six-month period upon mutual written agreement of both Consultant and Company. Notwithstanding anything to the contrary contained herein, the Agreement may be terminated by Consultant or the Company upon thirty (30) days prior written notice to the other party. Immediately upon receipt of such notice from the Company, Consultant shall institute such termination procedures as may be specified in the notice and shall use his/her best efforts to minimize the cost to Company resulting from such termination. Sections 5, 6, 7, 8, 9 and 10 shall survive the expiration or termination of this Agreement.
Section 3. Compensation.
(a) As full compensation for the performance by Consultant of his/her duties under this Agreement, the Company shall pay Consultant $350 per hour (the “Fee”) that Consultant spends performing his/her duties pursuant to the terms hereof, provided, however that the amount of time Consultant spends performing his/her duties pursuant to this Agreement shall not exceed 40 hours per month unless otherwise agreed in writing by Company and Consultant.
(b) Consultant shall provide the Company with written invoices on a monthly basis for services rendered for such month, which shall set forth the actual number of hours Consultant expends performing the Services, a description of the activities undertaken by Consultant, and the itemization of all expenses incurred that are reimbursable pursuant to Section 4 hereof. The Company agrees that payment shall be made to Consultant within thirty (30) business days of its receipt of each undisputed monthly invoice.
(c) Consultant and Company acknowledge and agree that the compensation set forth herein represents the fair market value of the services provided to Company by Consultant, negotiated in an arms-length transaction, and has not been determined in a manner which takes into account the volume or value of any current or future referrals or business otherwise generated between the Company and the Consultant. Nothing contained in this Agreement constitutes or shall be construed in any manner as an obligation or inducement for Consultant to recommend the prescribing, purchase, use, or preferential formulary status or dispensing of any of the Company’s products or services or those of any organizations affiliated with the Company.
Section 4. Expenses. The Company shall reimburse Consultant for all reasonable and necessary expenses incurred by Consultant in connection with the Services provided hereunder; provided, however, that such expenses are pre-approved in writing by the Company.
Section 5. Confidential Information and Inventions.
(a) Consultant recognizes and acknowledges that in the course of his duties Consultant is likely to receive confidential or proprietary information owned by the Company, its affiliates or third parties with whom the Company or any such affiliates has an obligation of confidentiality. Accordingly, during and after the Term, Consultant shall use his best efforts to protect the confidentiality of the Confidential and Proprietary Information and agrees to keep confidential and not disclose or make accessible to any other person or use for any other purpose other than in connection with the fulfillment of his duties under this Agreement, any Confidential and Proprietary Information (as defined below) owned by, or received by or on behalf of, the Company or any of its affiliates. “Confidential and Proprietary Information” shall include, but shall not be limited to, confidential or proprietary scientific or technical information, data, formulas and related concepts, business plans (both current and under development), client lists, promotion and marketing programs, trade secrets, or any other confidential or proprietary business information relating to development programs, costs, revenues, marketing, investments, sales activities, promotions, credit and financial data, manufacturing processes, financing methods, plans or the business and affairs of the Company or of any affiliate or client of the Company. Consultant expressly acknowledges the trade secret status of the Confidential and Proprietary Information and that the Confidential and Proprietary Information constitutes a protectable business interest of the Company. Consultant agrees: (i) not to use any such Confidential and Proprietary Information for himself/herself or others; and (ii) not to take any Company material or reproductions (including but not limited to writings, correspondence, notes, drafts, records, invoices, technical and business policies, computer programs or disks) thereof from the Company’s offices at any time during the Term, except as required in the execution of Consultant’s duties to the Company. Consultant agrees to return immediately all Company material and reproductions (including but not limited, to writings, correspondence, notes, drafts, records, invoices, technical and business policies, computer programs or disks) thereof in his possession to the Company upon request and in any event immediately upon termination or expiration of the Term.
2
(b) Except with prior written authorization by the Company or unless otherwise required by law, Consultant agrees not to disclose or publish any of the Confidential and Proprietary Information, or any confidential, scientific, technical or business information of any other party to whom the Company or any of its affiliates owes an obligation of confidence, at any time during or after the Term.
(c) Consultant agrees that all inventions, discoveries, improvements and patentable or copyrightable works (“Inventions”) initiated, conceived or made by him/her, either alone or in conjunction with others, in connection with or as a result of performance of Services by Consultant during the Term shall be the sole property of the Company to the maximum extent permitted by applicable law and, to the extent permitted by law, shall be “works made for hire” as that term is defined in the United States Copyright Act (17 U.S.C.A., Section 101). The Company shall be the sole owner of all patents, copyrights, trade secret rights, and other intellectual property or other rights in connection therewith. Consultant hereby assigns to the Company all right, title and interest he may have or acquire in all such Inventions. Consultant further agrees to assist the Company in every proper way (but at the Company’s expense) to obtain and from time to time enforce patents, copyrights or other rights on such Inventions in any and all countries, and to that end Consultant will execute all documents necessary:
(i) to apply for, obtain and vest in the name of the Company alone (unless the Company otherwise directs) letters patent, copyrights or other analogous protection in any country throughout the world and when so obtained or vested to renew and restore the same; and
(ii) to defend any opposition proceedings in respect of such applications and any opposition proceedings or petitions or applications for revocation of such letters patent, copyright or other analogous protection.
(d) Consultant acknowledges that while performing the Services under this Agreement Consultant may locate, identify and/or evaluate patented or patentable inventions having commercial potential in the fields of pharmacy, pharmaceutical, biotechnology, healthcare, technology and other fields which may be of potential interest to the Company or one of its affiliates (the “Third Party Inventions”). Consultant understands, acknowledges and agrees that all rights to, interests in or opportunities regarding, all Third-Party Inventions identified by the Company, any of its affiliates or either of the foregoing persons’ officers, directors, employees, agents or consultants (including the Consultant) during the Term shall be and remain the sole and exclusive property of the Company or such affiliate and Consultant shall have no rights whatsoever to such Third-Party Inventions and will not pursue for himself or for others any transaction relating to the Third-Party Inventions which is not on behalf of the Company.
3
(e) Consultant agrees that he will promptly disclose to the Company, or any persons designated by the Company, all improvements and Inventions relating to, involving or based on Company’s information, technologies or the Services that are made or conceived or reduced to practice or learned by him/her, either alone or jointly with others, during the Term.
(f) Consultant agrees that the Company shall be entitled to enjoin any breach of the confidentiality and other obligations hereunder without having to post a bond in addition to all other remedies it may have under applicable law. Consultant will notify the Company in writing immediately upon the occurrence of any unauthorized release of any Confidential and Proprietary Information or other breach of any of the obligations under this Section 5 of which it is or becomes aware.
Section 6. Insider Trading. Consultant recognizes that in the course of his duties hereunder, Consultant may receive from the Company or others information that may be considered “material, nonpublic information” concerning a public company that is subject to the reporting requirements of the Securities and Exchange Act of 1934, as amended. Consultant agrees NOT to: (a) purchase or sell, directly or indirectly, any securities of any company while in possession of relevant material, nonpublic information relating to such company received from the Company or others in connection herewith; (b) provide Company with information with respect to any public company that may be considered material, nonpublic information; or (c) communicate any material, nonpublic information to any other person in which it is reasonably foreseeable that such person is likely to (i) purchase or sell securities of any company with respect to which such information relates, or (ii) otherwise directly or indirectly benefit from such information. Without limiting any of the confidentiality and insider trading obligations included in this Agreement, Consultant shall not discuss any information concerning Company obtained by Consultant in the course of performing the Services with any financial, securities or industry analyst or with the media without the written agreement of Company.
Section 7. Representations, Warranties and Covenants of Consultant. The Consultant hereby represents, warrants and covenants to the Company as follows:
(a) Neither the execution or delivery of this Agreement nor the performance by Consultant of his duties and other obligations hereunder violate or will violate any statute, law, determination or award, or conflict with or constitute a default or breach of any covenant or obligation under (whether immediately, upon the giving of notice or lapse of time or both) any prior employment agreement, contract, or other instrument to which Consultant is a party or by which he is bound.
(b) Consultant has the full right, power and legal capacity to enter and deliver this Agreement, as applicable, and to perform his duties and other obligations hereunder. This Agreement constitutes the legal, valid and binding obligation of Consultant enforceable against him/her in accordance with its terms. No approvals or consents of any persons or entities are required for Consultant to execute and deliver this Agreement, as applicable, or perform his duties and other obligations hereunder.
4
(c) Consultant represents that his performance of all the terms of this Agreement will not breach any agreement to keep in confidence any confidential information or trade secrets acquired by Consultant from any third party, and Consultant agrees not to use any confidential information or trade secrets of any third party in connection with the provision of the Services in violation of the agreements under which he had access to or knowledge of such confidential information or trade secrets.
(d) Consultant hereby represents that he (i) has not been debarred and (ii) to the best of Consultant’s knowledge, is not under consideration to be disbarred by the Food and Drug Administration (the “FDA”) or any other government agency from working in or providing services to any pharmaceutical or biotechnology company. Consultant shall notify the Company immediately if, during the Term, Consultant comes under investigation by the FDA for debarment or disqualification or is debarred or disqualified. Consultant shall notify the Company immediately if the FDA or any other regulatory authority requests permission to or does inspect Consultant’s records in connection with the Services provided under this Agreement, and Consultant will deliver to the Company promptly all materials, correspondence, statements, forms, and records which Consultant receives, obtains or generates pursuant to any such inspection.
(e) Consultant will not use any confidential information or trade secrets of any third party in his employment by Company in violation of the terms of the agreements under which he had access to or knowledge of such confidential information or trade secrets.
(f) During the Term of this Agreement and for a period of one-year thereafter, if Consultant uses, recommends, or comments upon the attributes of any Company product or service in connection with the treatment of a patient, a scientific or educational presentation or publication, a media interview, or any other third-party communication or interaction, Consultant shall disclose that Consultant is or has been a paid consultant of Company and the fact of any other of Consultant’s financial relationships with Company.
Section 8. Non-Circumvention. Consultant acknowledges that the Company regards the identity of the technologies, potential products and business partners evaluated by it to be a trade secret. Consultant agrees during the Term and for a period of one year thereafter not to contact any such business partners or parties (including any of their officers, directors, employees, agents, affiliates and/or consultants) who hold rights to such technologies and potential products without the presence of an officer of the Company or without the prior written consent of an officer of the Company, and Consultant further agrees not to take any action which would adversely affect or otherwise hinder the Company’s ability to ultimately execute any agreements with such parties. In addition, Consultant agrees not to circumvent, avoid, bypass or obviate the Company directly or indirectly regarding the Confidential and Proprietary Information disclosed in connection with the Services and any possible business arrangement envisioned thereby.
5
Section 9. Consultant not an Employee. Company and Consultant hereby acknowledge and agree that Consultant shall perform the services hereunder as an independent contractor and not as an employee or agent of Company or any Company affiliate. Consultant will be solely responsible for all taxes, withholding and other similar statutory obligations. Consultant shall not represent that he is an employee of Company or any Company affiliate under any circumstance. In addition, nothing in this Agreement shall be construed as establishing any joint venture, partnership or other business relationship between the parties hereto or representing any commitment by either party to enter into any other agreement by implication or otherwise except as specifically stated herein. Consultant shall not have any authority, express or implied, to bind Company or any Company affiliate to any agreement, contract, or other commitment. Consultant further understands and agrees that this Agreement is entered into by Company on a non-exclusive basis and that Company and its affiliates remain free to deal with others and retain other consultants, employees, brokers, finders and other agents in the same or similar capacity as Consultant has been retained at any time at their own option.
Section 10. Miscellaneous.
This Agreement shall be governed by, and construed and interpreted in accordance with, the laws of the State of North Carolina, without giving effect to its principles of conflicts of laws.
Any dispute arising out of, or relating to, this Agreement or the breach thereof (other than Section 5 hereof), or regarding the interpretation thereof, shall be finally settled by arbitration conducted in Charlotte, North Carolina in accordance with the rules of the American Arbitration Association then in effect before a single arbitrator appointed in accordance with such rules. Judgment upon any award rendered therein may be entered and enforcement obtained thereon in any court having jurisdiction. The arbitrator shall have authority to grant any form of appropriate relief, whether legal or equitable in nature, including specific performance. For the purpose of any judicial proceeding to enforce such award or incidental to such arbitration or to compel arbitration and for purposes of Section 5 hereof, the parties hereby submit to the exclusive venue and personal jurisdiction of the state and federal courts located in Charlotte, North Carolina, and agree that service of process in such arbitration or court proceedings shall be satisfactorily made upon it if sent by registered mail addressed to it at the address referred to in paragraph (g) below. The costs of such arbitration shall be borne proportionate to the finding of fault as determined by the arbitrator.
This Agreement shall be binding upon and inure to the benefit of the parties hereto, and their respective heirs, legal representatives, successors and permitted assigns.
This Agreement, and Consultant’s rights and obligations hereunder, may not be assigned, delegated or otherwise subcontracted by Consultant. The Company may assign its rights, together with its obligations, hereunder in connection with any sale, transfer or other disposition of all or substantially all of its business or assets.
This Agreement cannot be amended orally, or by any course of conduct or dealing, but only by a written agreement signed by the parties hereto.
The failure of either party to insist upon the strict performance of any of the terms, conditions and provisions of this Agreement shall not be construed as a waiver or relinquishment of future compliance therewith, and such terms, conditions and provisions shall remain in full force and effect. No waiver of any term or condition of this Agreement on the part of either party shall be effective for any purpose whatsoever unless such waiver is in writing and signed by such party.
6
All notices, demands or other communications desired or required to be given by any party to any other party hereto shall be in writing and shall be deemed effectively given upon (i) personal delivery to the party to be notified, (ii) upon confirmation of receipt of telecopy or other electronic facsimile transmission, (iii) one business day after deposit with a reputable overnight courier, prepaid for priority overnight delivery, or (iv) five days after deposit with the United States Post Office, postage prepaid, in each case to such party at the address set forth above, or to such other addresses and to the attention of such other individuals as any party shall have designated to the other parties by notice given in the foregoing manner.
This Agreement sets forth the entire agreement and understanding of the parties relating to the subject matter hereof, and supersedes all prior agreements, arrangements and understandings, written or oral, relating to the subject matter hereof. No representation, promise or inducement has been made by either party that is not embodied in this Agreement, and neither party shall be bound by or liable for any alleged representation, promise or inducement not so set forth.
As used in this Agreement, “affiliate” of a specified person or entity shall mean and include any person or entity controlling, controlled by or under common control with the specified Person.
The section headings contained herein are for reference purposes only and shall not in any way affect the meaning or interpretation of this Agreement.
This Agreement may be executed in any number of counterparts, each of which shall constitute an original, but all of which together shall constitute one and the same instrument.
(l) As used in this Agreement, the masculine, feminine or neuter gender, and the singular or plural, shall be deemed to include the others whenever and wherever the context so requires. Additionally, unless the context requires otherwise, “or” is not exclusive.
[Remainder of Page Intentionally Left Blank – Signature Page Follows]
7
IN WITNESS WHEREOF, the parties hereto have executed this Agreement as of the date first written above by proper person thereunto duly authorized.
| ADAPTIN BIO, INC. | ||
| By: | /s/ Michael J. Roberts | |
| Name: Michael J. Roberts | ||
| Title: CEO | ||
| CONSULTANT | ||
| /s/ L. Arthur Hewitt | ||
| L. Arthur Hewitt | ||
8
Exhibit 14.1
Code of Ethics and Business Conduct of
Adaptin Bio, Inc.
Adopted as of February 11, 2025
I. INTRODUCTION AND GENERAL POLICY
Adaptin Bio, Inc. (the “Company”) is committed to the highest standards of legal and ethical business conduct, and seeks to foster an environment of awareness where the prompt reporting of any unethical or illegal behavior or any violations of our corporate policies is encouraged and dealt with fairly. Ethical conduct is an inherent obligation of our directors, officers and employees. Accordingly, we have adopted this Code of Ethics and Business Conduct (the “Code”) to promote the high standards of ethical conduct we value.
This Code does not cover every issue that may arise, but is intended to provide a basic summary of the legal, ethical and regulatory principles that should guide the conduct of all our directors, officers and employees.
We expect each of our directors, officers and employees to read and become familiar with and agree to follow the ethical standards described in this Code. A director’s, officer’s or employee’s failure to fulfill his or her responsibilities under this Code may result in disciplinary action, up to and possibly including immediate termination.
This Code requires at a minimum:
| (1) | Honest, prudent and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships; | |
| (2) | Full, fair, accurate, timely and understandable disclosures in reports and documents that we file with, or submit to, the Securities and Exchange Commission and in any other of our public communications; | |
| (3) | Compliance with our other corporate policies and with applicable governmental laws, rules and regulations; | |
| (4) | The prompt internal reporting of violations of this Code, including any illegal activity, to the appropriate person or persons identified in this Code; and | |
| (5) | Accountability for adherence to this Code. |
II. CONFLICTS OF INTEREST AND CORPORATE OPPORTUNITIES
Our directors, officers and employees should not be involved in any activity that creates or gives the appearance of a conflict of interest. A “conflict of interest” exists when a person’s private interest interferes, or appears to interfere, in any way with the interests of the Company. A conflict situation can arise when a director, officer or employee takes actions or has interests that may make it difficult to perform his or her work for the Company objectively and effectively. Conflicts of interest also arise when an employee, officer or director (or a member of their family) receives improper personal benefits as a result of their position in the Company.
Accordingly, directors, officers and employees are prohibited from taking for their own personal gain opportunities that are discovered through the use of the Company’s property, information or position, without the written consent of our Board of Directors.
A conflict situation may arise when a director, officer or employee has a financial interest, including significant stock ownership, in any entity with which we do business. Conflicts of interest also may arise when a director, officer or employee, or members of his or her family, receives improper personal benefits as a result of his or her position in the Company. Loans to, or guarantees of obligations of, directors, officers or employees, or their family members, by the Company or any entity with which we do business, may create conflicts of interest. Loans by the Company to, or guarantees by the Company of obligations of, any director or executive officer (or their family members) are expressly prohibited.
It is almost always a conflict of interest for a director, officer or employee to have other duties, responsibilities or obligations that run counter to his or her duty to the Company, such as working or providing service simultaneously for a competitor, customer, supplier or other business. The best policy is to avoid any direct or indirect business connection with the Company’s customers, suppliers or competitors, or with any other outside business, except on behalf of the Company.
Directors, officers and employees should notify in writing the appropriate person or persons identified in Section VII of this Code of the existence of any actual or potential conflict of interest.
III. FAIR DEALING
We require our directors, officers and employees to deal honestly and fairly with, and respect the rights of, our customers, suppliers, competitors and other third parties. Stealing proprietary information, possessing trade secret information that was obtained without the owner’s consent or inducing such disclosures by past or present employees of other companies is prohibited. Each director, officer and employee should endeavor to make our contracts, advertising, literature and other public statements clear and precise and to eliminate any misstatement of fact or misleading impressions. No director, officer or employee should take unfair advantage of anyone through manipulation, concealment, abuse of privileged information, misrepresentation of material facts or any other unfair-dealing practice.
No bribes, kickbacks or any other form of improper payment, direct or indirect, should ever be offered, given, provided or accepted by any director, officer or employee, their family members or agents. In addition, no gifts, favors or business entertainment should ever be offered, given, provided or accepted by any director, officer or employee, their family members or agents, unless it:
| (1) | is not a cash gift; | |
| (2) | is consistent with customary business practices; | |
| (3) | is of nominal value; | |
| (4) | cannot be construed as a bribe or payoff; and | |
| (5) | does not otherwise violate our corporate policies or any laws or regulations. |
IV. RECORD-KEEPING AND PUBLIC DISCLOSURES
We require honest and accurate recording and reporting of information. All of our books, records, accounts and financial statements must be maintained in reasonable detail, accurately and fairly reflect our transactions, not contain false or misleading entries, comply with generally accepted accounting principles at all times and conform both to applicable legal requirements and to our system of internal accounting controls. Unrecorded or “off the books” funds, work, or assets should not be maintained unless permitted by applicable law or regulation.
We expect our directors, officers and employees to notify our Chief Financial Officer in writing of any:
| (1) | material information or unreported transactions that affect the disclosures made in our public filings; | |
| (2) | information concerning significant deficiencies and material weaknesses in the design or operation of our internal control over financial reporting which are reasonably likely to adversely affect our ability to record, process, summarize and report financial information; and | |
| (3) | fraud, whether or not material, that involves management or other employees who have a significant role in our internal control over financial reporting. |
Directors, officers and employees should avoid exaggeration, derogatory remarks, guesswork, and inappropriate characterizations of people and companies in their e-mail, correspondence, internal memos, reports and other records and communications, as these things often become public and can be easily misunderstood. Records always should be retained or destroyed according to our record retention policies. No director, officer or employee should communicate to the public any material, nonpublic information except through our Chief Executive Officer or Chief Financial Officer.
2
V. COMPLIANCE WITH LAWS AND CORPORATE POLICIES
We expect our directors, officers and employees to respect and obey the law, both in letter and spirit. We expect our directors, officers and employees to be committed to, among other things:
| (1) | maintaining a safe and healthy work environment; | |
| (2) | promoting a workplace that is free from discrimination or harassment based on race, color, religion, sex, age, national origin, disability or other factors that are unrelated to our business interests; | |
| (3) | supporting fair competition and laws prohibiting restraints of trade and other unfair trade practices; | |
| (4) | conducting our activities in full compliance with all applicable environmental laws; | |
| (5) | keeping the political activities of our directors, officers and employees separate from our business; | |
| (6) | prohibiting any direct or indirect illegal payments, gifts, favors or gratuities to any government officials, candidates or political parties; | |
| (7) | prohibiting the unauthorized use, reproduction, or distribution of any third party’s trade secrets, copyrighted information or confidential information; | |
| (8) | prohibiting the sale or export, either directly or through our representatives, of our products to countries where technology related goods such as ours may not be sold; and | |
| (9) | complying with all applicable state and federal securities laws. |
Our directors, officers and employees are prohibited from trading our securities while in possession of material, nonpublic (“inside”) information about the Company.
We encourage our directors, officers and employees to seek advice regarding the details of the policies, laws, rules and regulations with which they must comply, by submitting a written request to our Chief Financial Officer.
VI. CONFIDENTIALITY AND CORPORATE ASSETS
Our directors, officers and employees are entrusted with our confidential information and with the confidential information of our suppliers, customers or other business partners. This information may include without limitation:
| (1) | trade secrets, patents, trademarks, copyrights, and other proprietary information and ideas; | |
| (2) | technical or scientific information about current and future products, services or research; | |
| (3) | business, marketing or service plans or projections; | |
| (4) | earnings and other internal financial data; | |
| (5) | personnel information; | |
| (6) | supply and customer lists; and | |
| (7) | other non-public information that, if disclosed, might be of use to our competitors, or harmful to our suppliers, customers or other business partners. |
This information is our property, or the property of our suppliers, customers or business partners, and in many cases was developed at great expense. Our directors, officers and employees must not discuss or disclose confidential information with, in the presence of, or to any unauthorized persons, including family members and friends, and must not use confidential information or other Company property or resources for personal gain, for the personal benefit of anyone else, or for anything other than our legitimate business purposes.
These obligations are described in our proprietary information and inventions agreement that we require every director, officer and employee to execute upon commencement of service to the Company.
3
VII. REPORTING AND CONSEQUENCES OF VIOLATIONS
Reporting Violations and Asking Questions
We hold all directors, officers and employees individually responsible for carrying out and monitoring compliance with this Code. Except as provided in the paragraph below, directors and officers immediately should report in writing any known or suspected illegal or unethical behavior to the Chair of our Audit Committee, and employees who are not directors or officers immediately should report in writing any known or suspected illegal or unethical behavior to our Board of Directors or appropriate management representative. When in doubt, we encourage directors, officers and employees to seek counseling about the best course of action to take in any particular situation.
If your complaint, concern or question pertains to accounting, internal accounting controls or auditing matters, or financial fraud, securities fraud or other securities law violations, you should submit the complaint, concern, or question, anonymously if you wish, to our Board of Directors or to members of our Audit Committee.
Investigations and Non-Retaliation
The person or persons to whom a potential or actual violation is reported or forwarded will promptly investigate any such violation and will oversee an appropriate response, including corrective action and preventative measures. The Chair of our Audit Committee or the Chief Executive Officer will be involved when appropriate. All reports will be treated confidentially to the extent possible.
It is our policy to not allow reprisal or retaliation of any kind against a director, officer or employee who acts in good faith in reporting any known or suspected illegal or unethical behavior, or who asks any questions regarding this Code or appropriate actions in light of the Code. All directors, officers and employees must fully cooperate in internal investigations of misconduct.
Consequences of a Violation
Directors, officers and employees who violate any laws, governmental regulations, or any provisions of this Code will face appropriate, case-specific disciplinary action, which may include demotion or immediate discharge. Any director, officer or employee who engages in illegal activity may be reported to the appropriate governmental authorities.
Administration
Our Board of Directors has established the standards of business conduct contained in this Code and generally oversee compliance with this Code. Our Board of Directors or Audit Committee is responsible for updating these standards as they deem appropriate to reflect changes in the legal and regulatory framework applicable to the Company, the business practices within our industry, our own business practices and the prevailing ethical standards of the communities in which we operate. Our Audit Committee will oversee the procedures designed to implement this Code to ensure that they are operating effectively.
VIII. CHANGES IN OR WAIVERS OF THE CODE
Any change in or waiver of this Code for directors or officers (including our president, principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions, any vice-president in charge of a principal business unit, division or function, or any other officer who performs a policy-making function) may be made only in writing by the Company’s Board of Directors. The fact of and reasons for such change or waiver will be publicly disclosed will be posted on our website at adaptinbio.com, or if required, will be publicly disclosed in a Form 8-K filed by the Company with the Securities and Exchange Commission within four business days of such change or waiver. No waiver shall be granted except where necessary and warranted, and where such waiver is limited and qualified so as to protect the Company to the greatest extent possible.
The text of this Code, and any changes in or waivers of this Code, will be posted on our website at adaptinbio.com.
4
COMPLIANCE CERTIFICATE
I have read and understand the Code of Ethics and Business Conduct of Adaptin Bio, Inc. (the “Code”). I will adhere in all respects to the ethical standards described in the Code. I further confirm my understanding that any violation of the Code will subject me to appropriate disciplinary action, which may include demotion or discharge.
I certify to Adaptin Bio, Inc. that I am not in violation of the Code, unless I have noted such violation in a signed Statement of Exceptions attached to this Compliance Certificate.
| Unless indicated below, | ||||
| Date: | ||||
| (Signature) | ||||
| Print Name: | ||||
| Title/Position: | ||||
| Check one of the following: | ||
| ☐ | A Statement of Exceptions is attached. | |
| ☐ | No Statement of Exceptions is attached. | |
| Check one of the following: | ||
| ☐ | I am not aware of any violations of the Code. | |
| ☐ | I wish to report the following violations of this Code: | |
5
Exhibit 16.1
February 18, 2025
Securities and Exchange Commission
100 F Street, N.E.
Washington, DC 20549
Commission File Number: 000-56583
Dear Sirs,
We have received a copy of, and are in agreement with, the statements being made by Adaptin Bio, Inc. (formerly Unite Acquisition 1 Corp.) under Item 4.01 of its Form 8-K dated February 18, 2025, insofar as it relates to our firm. We are not in a position to agree or disagree with other statements of Adaptin Bio, Inc. (formerly Unite Acquisition 1 Corp.) contained therein.
We hereby consent to the filing of this letter as an exhibit to the foregoing report on Form 8-K.
| Very truly yours, | |
| /s/ KNAV CPA LLP | |
| KNAV CPA LLP | |
| Atlanta, Georgia | |
| February 18, 2025 |
Exhibit 21.1
List of Subsidiaries of Adaptin Bio, Inc.
| Name of Subsidiary | Jurisdiction of Incorporation | |
| Adaptin Acquisition Co. | Delaware |
Exhibit 99.1
ADAPTIN bio, Inc.
FINANCIAL STATEMENTS
For the years ended december 31, 2023 and 2022
ADAPTIN
BIO, INC.
TABLE OF CONTENTS
| Financial Statements | |
| report of independent registered public accounting firm | F-3 |
| Balance Sheets | F-4 |
| Statements of OPERATIONS | f-5 |
| Statements of Changes in stockholders’ DEFICIT | f-6 |
| Statements of Cash Flows | f-7 |
| Notes to Financial Statements | f-8 |
F-2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Adaptin Bio, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Adaptin Bio, Inc. as of December 31, 2023 and 2022, and the related statements of operations, changes in stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023, in conformity with accounting principles generally accepted in the United States of America.
Substantial Doubt Regarding Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company has suffered recurring losses from operations and has a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty. Our opinion is not modified with respect to this matter.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ WithumSmith+Brown, PC
We have served as the Company's auditor since 2023.
East Brunswick, New Jersey
January 8, 2025
F-3
BALANCE SHEETS
AS OF DECEMBER 31, 2023 AND 2022
| 2023 | 2022 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 4,754 | $ | 50 | ||||
| Total assets | $ | 4,754 | $ | 50 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable - trade | $ | 280,337 | $ | 7,402 | ||||
| Accrued expenses | 198,599 | 2,100 | ||||||
| Notes Payable, net of debt issuance costs | 469,762 | - | ||||||
| Accrued interest | 33,615 | - | ||||||
| Total current liabilities | 982,313 | 9,502 | ||||||
| Total liabilities | 982,313 | 9,502 | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| STOCKHOLDERS’ DEFICIT | ||||||||
| Common Stock, $0.001 par value, 10,000 shares authorized, 1,505 shares issued and oustanding as of December 31, 2023 and 1,430 shares issued and outstanding as of December 31, 2022 | 1 | 1 | ||||||
| Additional Paid-in Capital | 24,115 | 10,419 | ||||||
| Accumulated deficit | (1,001,675 | ) | (19,872 | ) | ||||
| Total stockholders’ deficit | (977,559 | ) | (9,452 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 4,754 | $ | 50 | ||||
The accompanying notes are an integral part of these financial statements.
F-4
ADAPTIN BIO, INC.
STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| 2023 | 2022 | |||||||
| OPERATING EXPENSES | ||||||||
| Research and development | $ | 510,356 | $ | - | ||||
| General and administrative | 366,960 | 11,772 | ||||||
| Total Operating Expenses | 877,316 | 11,772 | ||||||
| LOSS FROM OPERATIONS | (877,316 | ) | (11,772 | ) | ||||
| Interest Expense | 104,487 | - | ||||||
| Net loss before provision for income taxes | (981,803 | ) | (11,772 | ) | ||||
| Provision for income taxes | - | - | ||||||
| NET LOSS | $ | (981,803 | ) | $ | (11,772 | ) | ||
| NET LOSS PER SHARE | ||||||||
| Net loss per common share, basic and diluted | $ | (655.41 | ) | $ | (8.23 | ) | ||
| Weighted average number of common shares outstanding | 1,498 | 1,430 | ||||||
The accompanying notes are an integral part of these financial statements.
F-5
ADAPTIN
BIO, INC.
STATEMENTS OF STOCKHOLDERS’ DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| Common Stock | Additional Paid-In | Accumulated | Total Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||
| Balance as of December 31, 2021 | 1,430 | $ | 1 | $ | 6,836 | $ | (8,100 | ) | $ | (1,263 | ) | |||||||||
| Other Capital Contributions | - | - | 3,583 | - | 3,583 | |||||||||||||||
| Net loss | - | - | - | (11,772 | ) | (11,772 | ) | |||||||||||||
| Balance as of December 31, 2022 | 1,430 | 1 | 10,419 | (19,872 | ) | (9,452 | ) | |||||||||||||
| Common stock issued | 75 | - | 13,189 | - | 13,189 | |||||||||||||||
| Other Capital Contributions | - | - | 507 | - | 507 | |||||||||||||||
| Net loss | - | - | - | (981,803 | ) | (981,803 | ) | |||||||||||||
| Balance as of December 31, 2023 | 1,505 | $ | 1 | $ | 24,115 | $ | (1,001,675 | ) | $ | (977,559 | ) | |||||||||
The accompanying notes are an integral part of these financial statements.
F-6
ADAPTIN BIO, INC.
STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| 2023 | 2022 | |||||||
| CASH FLOWS USED BY OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (981,803 | ) | $ | (11,772 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Issuance of common stock for license agreement | 13,189 | - | ||||||
| Amortization of debt issuance costs | 70,872 | - | ||||||
| Payment of operating expenses as additional paid-in capital by founders | 507 | 3,583 | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts payable - trade | 272,935 | 6,391 | ||||||
| Accrued expenses | 230,114 | 1,848 | ||||||
| Net cash provided by (used by) operating activities | (394,186 | ) | 50 | |||||
| CASH FLOWS PROVIDED BY FINANCING ACTIVITIES | ||||||||
| Proceeds from Notes Payable | 500,000 | - | ||||||
| Payment of debt issuance costs | (101,110 | ) | - | |||||
| Net cash provided by financing activities | 398,890 | - | ||||||
| NET INCREASE IN CASH | 4,704 | 50 | ||||||
| Cash - Beginning of Year | 50 | - | ||||||
| CASH - END OF YEAR | $ | 4,754 | $ | 50 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for income taxes | $ | - | $ | - | ||||
| Cash paid for interest expense | $ | - | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
F-7
ADAPTIN BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| NOTE 1 | NATURE OF OPERATIONS |
Adaptin Bio, Inc., a Delaware corporation, was founded as Centaur Bio, Inc. in 2021, (the “Company”) and changed its name in 2024. The Company is dedicated to the development and commercialization of products utilizing novel technology that enhances the delivery of drugs and other compounds to the brain and other tissues for a variety of indications. The Company’s novel technology was originally developed by researchers in the Department of Neurosurgery at Duke University and licensed by the Company in 2023. (See Note 8).
For many drugs and diagnostic agents, no readily available technology permits effective delivery of a broad range of difficult-to-deliver drugs to specific areas of the body. The Company’s technology is engineered to facilitate the transport of therapeutics to tissues of interest, including the brain, potentially generating improved treatments for solid tumors and central nervous system (“CNS”) disorders.
The first application of the Company’s precision medicine technology is a Brain Bispecific T Cell Engager (“BRiTE”). BRiTE focuses on the transport of difficult-to-deliver T cell targeting agents across the blood brain barrier, allowing access to the brain. BRiTE is a translatable method to specifically target malignant glioma using a tumor-specific, fully human bispecific antibody that redirects patients’ own T cells to recognize and destroy tumor cells. The lead product, APTN-101, a proprietary EGFRvIII x CD3 BRiTE, has shown the ability to eliminate malignant glioma tumors in a variety of aggressive preclinical orthotopic tumor models and the Company intends to complete IND-enabling pre-clinical studies and initiate the first-in-human studies in 2025.
GOING CONCERN
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. As of December 31, 2023, the Company had cash on hand of $4,754 and an accumulated deficit of $1,001,675. The Company intends to continue the conduct of significant development activities that began in 2023 which, together with expenses incurred for general and administrative expenses, are expected to result in continuing operating losses for the foreseeable future. The amount of future losses and when, if ever, the Company will achieve profitability are uncertain. The Company’s ability to achieve profitability will depend, among other things, on successfully completing clinical studies, obtaining requisite regulatory approvals, establishing appropriate pricing for its product with payers, and raising sufficient funds to finance the Company’s activities. No assurance can be given that the Company’s clinical development efforts will be successful, that regulatory approvals will be obtained, or that the Company will be able to achieve appropriate pricing and market access or that profitability, if achieved, can be sustained. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments related to the outcome of this uncertainty.
Management has evaluated the Company’s operating plan against its existing cash and determined that the Company expects to be unable to support its operations and fund its obligations for the next twelve months from the date of issuance of these financial statements. The Company’s ability to execute its operating plan depends on the Company’s ability to obtain additional funding through equity offerings and debt financings. The Company plans to continue to fund its losses from operations through cash and cash equivalents on hand, as well as through future equity offerings, debt financings, or other third-party funding. There can be no assurance that additional funds will be available when needed from any source or, if available, will be available on terms that are acceptable to the Company. Even if the Company raises additional capital, it may also be required to modify, delay or abandon some of its plans which could have a material adverse effect on the business, operating results and financial condition and its ability to achieve its intended business objectives. Any of these actions could materially harm the business, results of operations and future prospects.
F-8
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| NOTE 2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation
The accompanying financial statements of the Company have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”). Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”). These financial statements are presented in U.S. Dollars as that is the Company’s functional currency.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates and assumptions reflected in the financial statements relate to and include, but are not limited to, the fair value of common stock and other assumptions used to measure share-based consideration transferred for acquired assets under the Duke License (See Note 5 and Note 8), and prepaid expenses and accrued liabilities that are measured based on progress toward completion of research and development projects.
Future events and their effects cannot be predicted with certainty; accordingly, accounting estimates require the exercise of judgment. Accounting estimates used in the preparation of these financial statements change as new events occur, as more experience is acquired, as additional information is obtained and as the operating environment changes.
Segments
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business as a single segment.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of 90 days or less on the date of purchase to be cash equivalents. The carrying value of cash and cash equivalents approximates fair value due to the short-term nature of these items.
Currently, all cash is deposited in one financial institution. The balances are insured by the Federal Deposit Insurance Corporation (FDIC) up to certain limits. In the event there is cash in the bank, it may, at times, exceed FDIC insurable limits. Any loss incurred or a lack of access to such funds could have a significant adverse impact on the Company’s financial position.
F-9
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
Derivative Liability
The Company does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. The Company analyzes all financial instruments, including its convertible debt under the FASB ASC Topic No. 815 Derivatives and Hedging (“ASC 815”) to determine if such instruments contain features that qualify as embedded derivatives. The accounting treatment of derivative financial instruments requires that the Company record embedded conversion options and any related freestanding instruments at their fair values as of the inception date of the agreement and at fair value as of each subsequent balance sheet date. Any increase or decrease in the fair value would be recorded in the results of operations within other income/expense as change in fair value of derivative liabilities. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is reassessed at the end of each reporting period.
Upon conversion or repayment of a debt or equity instrument in exchange for equity shares, where the embedded conversion option has been bifurcated and accounted for as a derivative liability (generally convertible debt), the Company would record the equity shares at fair value on the date of conversion, relieve all related debt, derivative liabilities, and unamortized debt discounts, and recognize a net gain or loss on debt extinguishment, if any.
Accrued Expense
Accrued expenses consist primarily of research and development, legal, consulting and license-related fees.
Research and Development
Research and development costs are charged to expense as costs are incurred in performing research and development activities. To date, the Company’s research and development costs consist primarily of costs related to obtaining the license to its technology (see Notes 1 and 8) and conducting pre-clinical IND-enabling activities. It is anticipated that additional expenses in future periods for both pre-clinical and clinical activities will consist primarily of fees paid to contract research organizations (CROs) and to contract manufacturing organizations (CMOs).
Research and development expenses include direct costs associated with pre-clinical activities and will, in future periods, include direct costs associated with CROs, direct CMO costs for the formulation and packaging of trial material, as well as investigator and patient related costs at sites at which the Company’s clinical trials will be conducted. Direct costs associated with the Company’s CROs and CMOs are generally payable on a time and materials basis, or when milestones are achieved. The invoicing from third-party vendors can be for work performed on a time and material basis or reflecting milestones outlined in the initial contract for services and may not reflect actual work performed as of a specific measurement date. The Company records expenses for its pre-clinical and clinical activities performed by third parties based upon estimates of the percentage of work completed of the total work over the life of the individual study in accordance with agreements established with the third-party vendors. The Company determines the estimates through discussions with development personnel, third-party vendors as to the progress or stage of completion of trials or services and the agreed upon fee to be paid for such services based on facts and circumstances known to the Company as of each balance sheet date. The actual costs and timing of clinical trials are highly uncertain, subject to risks and may change depending upon a number of factors, including the Company’s clinical development plan. If the actual timing of the performance of services of the level of effort varies from the estimate, the Company will adjust the accrual accordingly. If the receipt of invoices is in advance of estimated work performed, the Company will record a prepaid expense as of the measurement date.
F-10
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
Research and development expenses also include the cost of in-process research and development (“IPR&D”) assets purchased in an asset acquisition transaction. IPR&D assets are expensed unless the assets acquired are deemed to have an alternative future use, provided that the acquired assets did not also include processes or activities that would constitute a business as defined under US GAAP, the technology has not received regulatory approval for marketing and, absent obtaining such approval, has no established alternative future use. Acquired IPR&D payments are immediately expensed in the period in which they are incurred and include upfront payments, as well as subsequent pre-commercial milestone payments. Research and development costs after the acquisition are expensed as incurred. The costs associated with maintaining the patents after the acquisition are the responsibility of the Company and will be expensed as incurred as a general and administrative expense.
Income Taxes
Deferred income taxes are provided on temporary differences between financial statement and income tax reporting. Temporary differences are differences between the amounts of assets and liabilities reported for financial statement and income tax purposes.
Deferred tax assets are recognized for temporary differences that will be deductible in future years’ tax returns and for operating loss and tax credit carryforwards. Deferred tax assets are recognized only if it is more likely than not that a tax position will be realized or sustained upon examination by the relevant taxing authority. A tax position that meets the more-likely-than-not recognition threshold is initially and subsequently measured as the largest amount of tax benefit that has a greater than 50% likelihood of being realized upon settlement with a taxing authority that has full knowledge of all relevant information.
Deferred tax assets are reduced by a valuation allowance if it is deemed more likely than not that some or all of the deferred tax assets will not be realized. Deferred tax liabilities are recognized for temporary differences that will be taxable in future years.
Net Loss per Share
Basic net loss per common share is calculated by dividing net loss by the weighted-average number of common shares outstanding for the period, without consideration for potentially dilutive securities. For the periods presented, basic and diluted net loss per common share are identical as there are currently no potentially dilutive securities issued by the Company.
F-11
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
Recently Adopted Accounting Pronouncements
In November 2023, the FASB issued ASU 2023-07, “Improvements to Reportable Segment Disclosures”, effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. While the Company operates as a single segment, it anticipates that there may be additional disclosures required under ASU 2023-07. The Company continues to evaluate the effect of adopting this new accounting guidance on its financial statements but does not intend to early adopt.
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures (“ASU 2023-09”). The amendments in this update require that public business entities on an annual basis (1) disclose specific categories in the rate reconciliation and (2) provide additional information for reconciling items that meet a quantitative threshold (if the effect of those reconciling items is equal to or greater than 5 percent of the amount computed by multiplying pretax income [or loss] by the applicable statutory income tax rate). The amendments also require entities on an annual basis to disclose disaggregated amounts of income taxes paid. ASU 2023-09 is effective for annual periods beginning after December 15, 2024. Early adoption is permitted for annual financial statements that have not yet been issued or made available for issuance. The Company is evaluating the effect of adopting this new accounting guidance on its financial statements but does not intend to early adopt. The Company does not currently believe that adoption will have a material impact on its financial statements.
The Company has also considered other recent accounting pronouncements and concluded that they are either not applicable to the business or that the effect is not expected to be material to the financial statements as a result of future adoption.
Significant Risks and Uncertainties
The Company is subject to challenges and risks specific to its business and its ability to execute on its strategy, as well as risks and uncertainties common to companies in the pharmaceutical industry, including, without limitation, risks and uncertainties associated with: obtaining regulatory approval of its product candidate; delays or problems in the supply of its study drug or failure to comply with manufacturing regulations; identifying, acquiring or in-licensing product candidates; pharmaceutical product development and the inherent uncertainty of clinical success; and the challenges of protecting and enhancing its intellectual property rights; and complying with applicable regulatory requirements.
Further, the Company may be impacted by general economic, political, and market conditions, including overall fluctuations in the financial markets in the U.S. and abroad.
F-12
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| NOTE 3 | ACCRUED LIABILITIES |
Accrued liabilities comprised the following as of December 31, 2023 and 2022:
| 2023 | 2022 | |||||||
| Accrued professional fees | $ | 3,596 | $ | - | ||||
| Accrued consulting fees | 3,604 | 1,800 | ||||||
| Accrued legal fees | 629 | - | ||||||
| Accrued fees of license agreement | 163,565 | - | ||||||
| Accrued research and development | 26,905 | - | ||||||
| Accrued franchise taxes | 300 | 300 | ||||||
| $ | 198,599 | $ | 2,100 | |||||
| NOTE 4 | NOTES PAYABLE |
In April 2023, the Company entered into Securities Purchase Agreements with accredited investors that provides for an aggregate of up to $500,000, 10% secured promissory notes payable to the investors with a term of twelve months from the issuance date (or prepayable in the event of a Qualified Offering) (the “2023 Senior Debt”). A Qualified Offering is defined under these agreements as the closing of the first offering (public or private) by the Company after the original issue date of its Common Stock or Common Stock Equivalents with gross proceeds to the Company (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more. Additionally, the Securities Purchase Agreement provides the buyers to be issued Warrants, exercisable beginning on the closing of a Qualified Offering. These Warrants have an exercise price of the price of a Common Share equal to the Qualified Offering price and have a term of five years from the closing of a Qualified Offering. As of December 31, 2023, the Company has issued an aggregate of $500,000 in 2023 Senior Debt. In addition, the Company incurred debt issuance costs of $101,110 which will be amortized over the life of the loans with such amortization recorded as interest expense. As of December 31, 2023, the notes, net of unamortized debt issuance costs, total $469,762. Interest expense for the year ended December 31, 2023 was $104,487 including $33,615 of interest expense and $70,872 of amortization of debt issuance costs.
As the terms of the Qualified Offering are not yet determined the Company is unable to determine the number or pricing of the warrants. At issuance, these warrants are generally not a liability within the scope of ASC 480 if it is within the reporting entity’s control to decide whether it will enter into a Qualified Offering and, if so, at what terms. Accordingly, the Company has determined that there will be no accounting recognition of these warrants until the terms of the Qualified Offering are determined and the Qualified Offering has closed. Once the terms of the Qualified Offering are known, the Company may be able to estimate the value and begin to evaluate the accounting implications (liability vs equity), however, that will not become final until the Qualified Offering closes.
| NOTE 5 | COMMITMENTS AND CONTINGENCIES |
Litigation
While there is currently no ongoing litigation, the Company may, from time to time, be involved in various legal matters that arise in the ordinary course of business. Should matters arise, management will then make a determination as to the ultimate disposition of these matters and measure if it could have a material adverse effect on the Company’s financial position, results of operations or liquidity.
F-13
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
License Agreement
In January 2023, the Company entered into a patent license agreement with Duke University and the National Cancer Institute, under the agency of the U.S. Department of Health and Human Services (the “Duke License”) for an exclusive, world-wide, sub-licensable license to the technology more fully described in Note 1 and Note 8. The Duke License was amended in August 2024 to include improvements within the definitions of patent rights and technical information. As a component of the Duke License, the Company agreed to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities. These milestones include initiation of Phase II or Phase III clinical trials, submission of applications for market approval in multiple jurisdictions including the US, EU and Japan and the initiation of post-approval commercial sales in the same jurisdictions. Based on an assumption that all milestones related to the current development program are met during the course of the Duke License, these milestone payments would total approximately $11.7 million. As of December 31, 2023, the Company has not met any milestones as defined in the agreement and, accordingly, has recorded no expense or liability related to such payments.
The Company also agreed to pay royalties equal to low- to mid- single digit percentages of annual net sales on a country-by-country and product-by-product basis subject to downward adjustment to low single digit percentages of our net annual sales in the event there is no valid claim of a patent for the product, with minimum annual royalty levels established. We also must pay Duke low to mid-double digit percentages of any sublicensing fees as set forth in the Duke License. The Company has not recorded and does not owe any royalties or sublicensing fees for the year ended December 31, 2023.
| NOTE 6 | INCOME TAXES |
The Company believes that there are no uncertain tax positions for which a liability (unrecognized tax benefit) should be recognized. The federal and state income tax returns of the Company are subject to examination by the Internal Revenue Service and state taxing authorities, generally for three years after they were filed.
The Company has incurred net operating losses (“NOL”) for U.S. tax purposes. As of December 31, 2023, the Company has $1,001,674 related to U.S. NOLs that may be carried forward and are available to reduce future taxable income. At December 31, 2022, the U.S. NOLs were $19,872.
The NOL carryforwards are subject to review and possible adjustment by the U.S. and state tax authorities. NOL carryforwards may become subject to an annual limitation in the event of certain cumulative changes in the ownership interest of significant shareholders, as defined under Section 382 Internal Revenue Code. This could limit the amount of NOLs that the Company can utilize annually to offset future taxable income or tax liabilities. As of December 31, 2023, the Company has not performed such an analysis evaluating the potential limitation of the Company’s net operating loss carryforwards due to the “change in ownership” provisions as defined under Sections 382 and 383 of the Internal Revenue Code. Subsequent ownership changes and proposed future changes to tax rules in respect of the utilization of losses carried forward may further affect the limitation in future years.
F-14
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The net deferred tax assets have not been recognized in these financial statements because the criteria for recognition of these assets were not met. Accordingly, the Company has recorded a valuation allowance against its deferred tax assets for the year ended December 31, 2022 of $4,670 and subsequently increased that valuation allowance by $230,724 to $235,394 for the year ended December 31, 2023 related to the increase in the net operating loss carryforwards as the realization of the deferred tax assets cannot occur until there is future taxable income, the certainty of which cannot be determined.
The components of the deferred tax assets and the valuation allowance are shown below. The state carryforwards are shown net of federal tax benefit.
| 2023 | 2022 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforward - Federal | $ | 210,352 | $ | 4,173 | ||||
| Net operating loss carryforward - State | 25,042 | 497 | ||||||
| Total deferred tax assets | 235,394 | 4,670 | ||||||
| Less-Valuation allowance | (235,394 | ) | (4,670 | ) | ||||
| $ | - | $ | - | |||||
The reasons for the difference between actual income tax benefit and the amount computed by applying the statutory federal income tax rate to the losses before income tax benefit are as follows:
| 2023 | 2022 | |||||||
| Rate Reconciliation: | ||||||||
| Statutory federal rate | 21.00 | % | 21.00 | % | ||||
| Statutory state rate (net of federal benefit) | 2.00 | % | 2.00 | % | ||||
| Effect of increase in valuation allowance | -23.00 | % | -23.00 | % | ||||
| Effective tax rate | 0.00 | % | 0.00 | % | ||||
| NOTE 7 | SHAREHOLDERS’ EQUITY |
Common Stock
The Company’s Certificate of Incorporation provides the authority to issue common stock, fully voting and participating, with a $0.001 par value, of which 10,000 shares were authorized. In 2021, a total of 1,430 shares were issued to the founding shareholders.
In February 2023, 75 shares were issued to Duke University pursuant to the Duke License (see Note 8). A total of 1,430 shares were outstanding as of December 31, 2022 and 1,505 shares are outstanding as of December 31, 2023.
F-15
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| NOTE 8 | LICENSE AGREEMENT |
In January 2023, the Company entered into the Duke License for an exclusive, world-wide, sub-licensable license to the technology more fully described in Note 1 and Note 5. Under the terms of this agreement, the Company issued a five percent (5%) common stock ownership interest, or 75 common shares, in the Company at a value of $175.86 per share.
The estimated fair value of the Company’s common stock was determined by a third-party independent valuation firm in accordance with the guidelines outlined in the American Institute of Certified Public Accountants Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation”. These valuations took into account numerous factors, including developments at the Company and market conditions.
Consistent with FASB ASC 820-10-35-24, if multiple valuation techniques are used to measure fair value, the results (respective indications of fair value) should be evaluated and weighted, as appropriate, considering the reasonableness of the range indicated by those results. A fair value measurement is the point within that range that is most representative of fair value in the circumstances. The February 2, 2023 valuation utilized multiple valuation techniques that considered a set of discrete potential liquidity scenarios for the Company, the value common stock would receive in each scenario, and the time required and risk inherent in achieving those values. The third-party valuation expert examined the following scenarios for the Company: (i) the net assets of the Company or the Asset Approach; (ii) a recently executed term sheet related to a proposed merger transaction and capital raise or the Market Approach; and (iii) dissolution or the failure scenario. The Asset Approach was assumed to be appropriate given the Company’s limited operations and expenditures to date as of the valuation date. The Asset Approach was weighted at 80% assuming continued operations as the funding proposed in the term sheet is non-binding and the Company has no assurance such funding will occur. The Market Approach utilized a back-solve methodology to determine the value of the common stock based on the terms outlined in the term sheet assuming the contemplated transaction is consummated and was weighted at 10% as there is limited or no assurance such transaction will be completed. Finally, the failure scenario was weighted at 10% assuming that the Company is unable to obtain suitable financing and would discontinue operations.
In accordance with ASC 805, the license agreement was evaluated and the Company concluded that substantially all of the value acquired is concentrated in a group of similar identifiable IPR&D assets. Accordingly, it has been accounted for as an asset acquisition as the technology transferred to the Company had not yet received regulatory approval and the IPR&D did not have an alternative use. The Company recorded research and development expense related to the issuance of the common stock of $13,189 during the year ended December 31, 2023. In addition, the Company agreed to reimburse the licensor for all patent related costs previously incurred for the technology prior to the license execution date. The total of these costs was approximately $327,000. The Company expensed these costs in their entirety upon execution of the Duke License as a research and development expense as they were deemed to be part of the acquisition price of the IPR&D. Any unpaid amounts are reflected in accrued expenses. Reimbursement for these costs will be made in four equal installments with the first two payments having been made in 2023. At December 31, 2023, the Company had a remaining liability of $163,565 included in accrued expenses on the balance sheet. The Company further agreed to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities (See Note 5).
F-16
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
| NOTE 9 | RELATED PARTIES |
Dr. Michael Roberts and Dr. Simon Pedder are both shareholders of the Company and the only members of the Company’s Board of Directors.
Dr. Roberts currently owns 1,000 shares of the common stock of the Company, or 66% of outstanding shares. During the year ended December, 31, 2022, Dr. Roberts has directly paid operating expenses of the Company of approximately $2,508 and such payments were recorded as additional paid-in capital. During the year ended December 31, 2023, Dr. Roberts paid $507 of such expenses on behalf of the Company. Additionally, the Company paid Dr. Roberts $15,000 and recorded additional accounts payable of $30,000 in consulting fees in 2023.
Dr. Pedder currently owns 430 shares of the common stock of the Company, or 29% of outstanding shares. During the year ended December 31, 2022, Dr. Pedder has directly paid operating expenses totaling approximately $1,075 and such payments were recorded as additional paid-in capital. During the year ended December 31, 2023, Dr. Pedder made no such payments on behalf of the Company. Additionally, the Company paid Dr. Pedder $10,000 and recorded additional accounts payable of $20,000 in consulting fees in 2023.
| NOTE 10 | SUBSEQUENT EVENTS |
Convertible Notes
In February 2024, the Company entered into Securities Purchase Agreements with certain accredited investors that provide for an aggregate of up to $1,000,000, 10% secured subordinated convertible promissory notes payable to investors with a term of twelve months from the issuance date (the “2024 Convertible Debt”). Additionally, the Securities Purchase Agreements provide that, upon a Qualified Offering, the debt together with all accrued but unpaid interest at the date of conversion shall automatically be converted into shares of the Company’s $0.0001 per share par value common stock (the “Conversion Shares”) at a conversion price equal to 75% of the gross offering price to the investors in the Qualified Offering. A Qualified Offering is defined under these agreements as the closing of the first offering (public or private) by the Company after the original issue date of its Common Stock or Common Stock Equivalents with gross proceeds to the Company (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more inclusive of the 2023 Senior Debt of $500,000 and the 2024 Convertible Debt of $1.0 million. As of September 30, 2024, the Company has issued an aggregate of $1,000,000 in 2024 Convertible Debt with maturities ranging from March 2025 to September 2025. In addition, the Company incurred debt issuance costs of approximately $127,510 which will be amortized over the life of the loans with such amortization recorded as interest expense.
F-17
ADAPTIN BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
Derivative Liability
The Company’s 2024 Convertible Debt, together with all accrued but unpaid interest at the date of conversion, shall automatically be converted into shares of the Company’s $0.0001 per share par value common stock (the “Conversion Shares”) at a conversion price equal to 75% of the gross offering price to the investors in the Qualified Offering. The Company evaluated this embedded feature under the guidance of ASC 815 and determined that it required bifurcation of the embedded derivative liability from the underlying debt at its fair value. While the number of common shares to be issued under these agreements is undetermined, this feature results in a fixed monetary amount of the settlement with a variable number of Conversion Shares issued. As the price per share under a Qualified Offering is unknown and the number of shares to be issued is variable, the resulting intrinsic value would be the same regardless of the Conversion Price. Accounting standards require that the Company recognize the derivative as a liability, based on its underlying instrument, in the balance sheet and measure the instrument at fair value at each balance sheet date. A change in the market value of the financial instrument would be recognized as a gain or loss in the results of operations in the period of change.
2024 Convertible Debt Amendment
At issuance, the 2024 Convertible Debt was convertible into Conversion Shares as more fully detailed above. With the agreement of the holders of the 2024 Convertible Debt, the Company has amended the 2024 Convertible Debt to be convertible into shares of common stock of either the Company or any entity that directly or indirectly through one or more intermediaries, controls the Company. The terms of the conversion remain as detailed above. A “Qualified Offering” , as amended, means the closing of the first offering (public or private) by the Company or its parent after the original issue date of the Company’s or its parent’s common stock with gross proceeds to the Company or its parent (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more inclusive of the 2023 Senior Debt of $500,000 and the 2024 Convertible Debt of $1.0 million.
2023 Senior Debt Modification
In November 2024, the Company entered into Note Exchange Agreements with the holders of the 2023 Senior Debt (see Note 4) whereby these 10% secured promissory notes payable to holders with a term of twelve months from the issuance date shall be cancelled and exchanged for the Company’s 10% Secured Convertible Promissory Notes (the “Exchange Notes”). The Exchange Notes will be issued in the aggregate principal amount of $500,000 and provide that, upon the closing of a Qualified Offering, the outstanding principal amount, together with all accrued but unpaid interest, at the date of conversion shall automatically be converted into shares of common stock of the Company or its parent (the “Conversion Shares”) at a conversion price equal to 75% of the gross offering price to the investors in the Qualified Offering (the “Conversion Price”). A Qualified Offering is defined in the Exchange Notes as the closing of the first offering (public or private) by the Company or its Parent after the Original Issue Date of the Company’s or the Parent’s common stock (the “Offering Stock”)with gross proceeds to the Company or its Parent (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more, including the aggregate principal amount of, together with all accrued but unpaid interest on, this Note and the other Notes that will convert thereupon.
F-18
ADAPTIN
BIO, INC.
NOTES TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2023 AND 2022
Additionally, as contemplated in connection with the issuance of the 2023 Senior Debt, the holders will be issued warrants to purchase up to a number of shares of Common Stock equal to fifty percent (50%) of the number of shares of Common Stock that the principal amount of the Notes issued to the initial Holder would purchase at the Qualified Offering Price. These warrants are exercisable beginning on the closing of a Qualified Offering, have an exercise price of the price of a Common Share equal to the Qualified Offering price and have a term of five years from the closing of a Qualified Offering.
Lastly, as an inducement to enter into the Note Exchange Agreements, the holders are to be issued additional warrants to purchase up to a number of shares of Common Stock equal to fifty percent (50%) of the number of shares of Common Stock that the principal amount of the Notes issued to the initial Holder would purchase at the Conversion Price. The purchase price of one share of Common Stock under this Warrant shall be equal to the Conversion Price. These warrants are exercisable beginning on the closing of a Qualified Offering and have a term of five years from the closing of a Qualified Offering.
As the terms of the Qualified Offering are not yet finalized, the Company is unable to determine the number or pricing of these warrants. At issuance, these warrants are generally not a liability within the scope of ASC 480 if it is within the reporting entity’s control to decide whether it will enter into a Qualified Offering and, if so, at what terms. Accordingly, the Company has determined that there will be no accounting recognition of these warrants until the terms of the Qualified Offering are determined and the Qualified Offering has closed. Once the terms of the Qualified Offering are known, the Company may be able to estimate the value and begin to evaluate the accounting implications (liability vs equity), however, that will not become final until the Qualified Offering closes.
F-19
Exhibit 99.2
adaptin bio Inc.
FINANCIAL STATEMENTS
For the NINE months ended SEPTEMBER 30, 2024 and 2023
ADAPTIN
BIO, INC.
TABLE OF CONTENTS
| Financial Statements | |
| Balance Sheets | f-3 |
| Statements of OPERATIONS | f-4 |
| Statements of Changes in stockholders’ DEFICIT | f-5 |
| Statements of Cash Flows | f-6 |
| Notes to Financial Statements | f-7 |
F-2
ADAPTIN
BIO, INC.
BALANCE SHEETS
AS OF SEPTEMBER 30, 2024 (UNAUDITED) AND DECEMBER 31, 2023
| (Unaudited) | ||||||||
| September 30,
2024 | December 31,
2023 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 115,308 | $ | 4,754 | ||||
| Total assets | $ | 115,308 | $ | 4,754 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable - trade | $ | 931,182 | $ | 280,337 | ||||
| Accrued expenses | 847,623 | 198,599 | ||||||
| Notes Payable | 500,000 | 469,762 | ||||||
| Convertible Notes Payable, net of debt issuance costs and discounts | 640,319 | - | ||||||
| Derivative obligation arising from Convertible Notes Payable | 342,088 | - | ||||||
| Accrued interest | 97,376 | 33,615 | ||||||
| Total current liabilities | 3,358,588 | 982,313 | ||||||
| Total liabilities | 3,358,588 | 982,313 | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| STOCKHOLDERS’ DEFICIT | ||||||||
| Common Stock, $0.001 par value, 10,000 shares authorized, 1,505 shares issued and outstanding as of September 30, 2024 and December 31, 2023 | 1 | 1 | ||||||
| Additional Paid-in Capital | 24,115 | 24,115 | ||||||
| Accumulated deficit | (3,267,396 | ) | (1,001,675 | ) | ||||
| Total stockholders’ deficit | (3,243,280 | ) | (977,559 | ) | ||||
| Total liabilities and stockholders’ deficit | $ | 115,308 | $ | 4,754 | ||||
The accompanying notes are an integral part of these condensed financial statements.
F-3
ADAPTIN
BIO INC.
STATEMENTS OF OPERATIONS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
| Nine
months ended September 30, | ||||||||
| 2024 | 2023 | |||||||
| OPERATING EXPENSES | ||||||||
| Research and development | $ | 1,566,533 | $ | 440,299 | ||||
| General and administrative | 495,272 | 212,623 | ||||||
| Total Operating Expenses | 2,061,805 | 652,922 | ||||||
| LOSS FROM OPERATIONS | (2,061,805 | ) | (652,922 | ) | ||||
| Interest Expense | 195,161 | 66,711 | ||||||
| Loss on fair value of derivative liability | 8,755 | - | ||||||
| NET LOSS | $ | (2,265,721 | ) | $ | (719,633 | ) | ||
| NET LOSS PER SHARE | ||||||||
| Net loss per common share, basic and diluted | $ | (1,505.46 | ) | $ | (481.04 | ) | ||
| Weighted average number of common shares outstanding | 1,505 | 1,496 | ||||||
The accompanying notes are an integral part of these condensed financial statements.
F-4
ADAPTIN
BIO, INC.
STATEMENT OF STOCKHOLDERS’ DEFICIT (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
| Common Stock | Additional Paid-In | Accumulated | Total Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Deficit | ||||||||||||||||
| Balance as of December 31, 2022 | 1,430 | $ | 1 | $ | 10,419 | $ | (19,872 | ) | $ | (9,452 | ) | |||||||||
| Common stock issued | 75 | - | 13,189 | - | 13,189 | |||||||||||||||
| Other Capital Contributions | - | - | 507 | - | 507 | |||||||||||||||
| Net loss | - | - | - | (719,633 | ) | (719,633 | ) | |||||||||||||
| Balance as of September 30, 2023 | 1,505 | $ | 1 | $ | 24,115 | $ | (739,505 | ) | $ | (715,389 | ) | |||||||||
| Balance as of December 31, 2023 | 1,505 | $ | 1 | $ | 24,115 | $ | (1,001,675 | ) | $ | (977,559 | ) | |||||||||
| Net loss | - | - | - | (2,265,721 | ) | (2,265,721 | ) | |||||||||||||
| Balance as of September 30, 2024 | 1,505 | $ | 1 | $ | 24,115 | $ | (3,267,396 | ) | $ | (3,243,280 | ) | |||||||||
The accompanying notes are an integral part of these condensed financial statements.
F-5
ADAPTIN
BIO, INC.
STATEMENT OF CASH FLOWS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
| Nine months ended September 30, | ||||||||
| 2024 | 2023 | |||||||
| CASH FLOWS USED BY OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (2,265,721 | ) | $ | (719,633 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Issuance of common stock for license agreement | - | 13,190 | ||||||
| Amortization of debt issuance costs and discounts | 131,400 | 45,595 | ||||||
| Payment of operating expenses as additional paid-in capital by founders | - | 507 | ||||||
| Loss on derivative liabilities | 8,755 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts payable - Trade | 650,845 | 220,261 | ||||||
| Accrued expenses | 712,785 | 242,033 | ||||||
| Net cash used by operating activities | (761,936 | ) | (198,047 | ) | ||||
| CASH FLOWS PROVIDED BY FINANCING ACTIVITIES | ||||||||
| Proceeds from Notes Payable | - | 500,000 | ||||||
| Proceeds from Convertible Notes Payable | 1,000,000 | - | ||||||
| Debt Issuance Costs | (127,510 | ) | (101,110 | ) | ||||
| Net cash provided by financing activities | 872,490 | 398,890 | ||||||
| NET INCREASE IN CASH | 110,554 | 200,843 | ||||||
| Cash - Beginning of Period | 4,754 | 50 | ||||||
| CASH - END OF PERIOD | $ | 115,308 | $ | 200,893 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid for income taxes | $ | - | $ | - | ||||
| Cash paid for interest expense | $ | - | $ | - | ||||
The accompanying notes are an integral part of these condensed financial statements.
F-6
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
| Note 1 | Nature of operation |
Adaptin Bio, Inc., a Delaware corporation, was founded as Centaur Bio, Inc. in 2021 (the “Company”) and changed its name in 2024. The Company is dedicated to the development and commercialization of products utilizing novel technology that enhances the delivery of drugs and other compounds to the brain and other tissues for a variety of indications. The Company’s novel technology was originally developed by researchers in the Department of Neurosurgery at Duke University and licensed by the Company in 2023. (See Note 8 and Note 10).
For many drugs and diagnostic agents, no readily available technology permits effective delivery of a broad range of difficult-to-deliver drugs to specific areas of the body. The Company’s technology is engineered to facilitate the transport of therapeutics to tissues of interest, including the brain, potentially generating improved treatments for solid tumors and central nervous system (“CNS”) disorders.
GOING CONCERN
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. As of September 30, 2024, the Company had cash on hand of approximately $0.1 million and an accumulated deficit of approximately $3.3 million. The Company intends to continue conduct of significant development activities that began in 2023 which, together with expenses incurred for general and administrative expenses, are expected to result in continuing operating losses for the foreseeable future. The amount of future losses and when, if ever, the Company will achieve profitability are uncertain. The Company’s ability to achieve profitability will depend, among other things, on successfully completing pre-clinical and clinical studies, obtaining requisite regulatory approvals, establishing appropriate pricing for its product with payers, and raising sufficient funds to finance the Company’s activities. No assurance can be given that the Company’s development efforts will be successful, that regulatory approvals will be obtained, or that the Company will be able to achieve appropriate pricing and market access or that profitability, if achieved, can be sustained. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments related to the outcome of this uncertainty.
Management has evaluated the Company’s operating plan against its existing cash and determined that the Company expects to be unable to support its operations and fund its obligations for the next twelve months from the date of issuance of these financial statements. The Company’s ability to execute its operating plan depends on the Company’s ability to obtain additional funding through equity offerings and debt financings. The Company plans to continue to fund its losses from operations through cash and cash equivalents on hand, as well as through future equity offerings, debt financings, or other third-party funding. There can be no assurance that additional funds will be available when needed from any source or, if available, will be available on terms that are acceptable to the Company. Even if the Company raises additional capital, it may also be required to modify, delay or abandon some of its plans which could have a material adverse effect on the business, operating results and financial condition and its ability to achieve its intended business objectives. Any of these actions could materially harm the business, results of operations and future prospects.
F-7
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
| NOTE 2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation
The accompanying unaudited condensed financial statements of the Company have been prepared in conformity with accounting principles generally accepted in the United States of America (“GAAP”) applicable to interim statements. Any reference in these notes to applicable guidance is meant to refer to the authoritative GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Update (“ASU”) of the Financial Accounting Standards Board (“FASB”). These financial statements are presented in U.S. Dollars.
These condensed financial statements are presented in accordance with the rules and regulations of the U.S. Securities and Exchange Commission (“SEC”) and do not include all disclosures normally required in annual financial statements prepared in accordance with U.S. GAAP. As such, the information included herein should be read in conjunction with the Company’s financial statements and accompanying notes as of and for the year ended December 31, 2023 (the “audited financial statements”). In management’s opinion, these unaudited condensed financial statements have been prepared on the same basis as the annual financial statements and reflect all adjustments, which include normal recurring adjustments, necessary for the fair statement of the Company’s financial position as of September 30, 2024 and the results of operations for the nine months ended September 30, 2024 and 2023. The results of operations for the nine months ended September 30, 2024 are not necessarily indicative of the results to be expected for the full year ending December 31, 2024 or any other future interim or annual period.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates and assumptions reflected in the financial statements relate to and include, but are not limited to, the fair value of common stock and other assumptions used to measure share-based consideration transferred for acquired assets under the Duke License (as defined below), the fair value of derivative liabilities along with prepaid expenses and accrued liabilities that are measured based on progress toward completion of research and development projects.
Future events and their effects cannot be predicted with certainty; accordingly, accounting estimates require the exercise of judgment. Accounting estimates used in the preparation of these financial statements change as new events occur, as more experience is acquired, as additional information is obtained and as the operating environment changes.
Segments
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision-maker in making decisions regarding resource allocation and assessing performance. The Company views its operations and manages its business as a single segment.
F-8
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of 90 days or less on the date of purchase to be cash equivalents. The carrying value of cash and cash equivalents approximates fair value due to the short-term nature of these items.
Currently, all cash is deposited in one financial institution. The balances are insured by the Federal Deposit Insurance Corporation (“FDIC”) up to certain limits. In the event there is cash in the bank, it may, at times, exceed FDIC insurable limits. Any loss incurred or a lack of access to such funds could have a significant adverse impact on the Company’s financial position.
Derivative Liability
The Company does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. The Company analyzes all financial instruments, including its convertible debt under the FASB ASC Topic No. 815 Derivatives and Hedging (“ASC 815”) to determine if such instruments contain features that qualify as embedded derivatives. The accounting treatment of derivative financial instruments requires that the Company record embedded conversion options and any related freestanding instruments at their fair values as of the inception date of the agreement and at fair value as of each subsequent balance sheet date. Any increase or decrease in the fair value would be recorded in the results of operations within other income or expense as change in fair value of derivative liabilities. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is reassessed at the end of each reporting period.
Upon conversion or repayment of a debt or equity instrument in exchange for equity shares, where the embedded conversion option has been bifurcated and accounted for as a derivative liability (generally convertible debt), the Company would record the equity shares at fair value on the date of conversion, relieve all related debt, derivative liabilities, and unamortized debt discounts, and recognize a net gain or loss on debt extinguishment, if any.
Accrued Expense
Accrued expenses consist primarily of legal, accounting, research and development and consulting.
Research and Development
Research and development costs are charged to expense as costs are incurred in performing research and development activities. To date, the Company’s research and development costs consist primarily of costs related to obtaining the license to its technology (see Note 1, Note 8 and Note 10) and conducting pre-clinical activities. It is anticipated that additional expenses in future periods will consist primarily of fees paid to contract research organizations (“CROs”) and to contract manufacturing organizations (“CMOs”) related to the Company’s pre-clinical and clinical activities.
F-9
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
Research and development expenses will include direct costs associated with CROs, direct CMO costs for the formulation and packaging of trial material, as well as investigator and patient related costs at sites at which the Company’s trials will be conducted. Direct costs associated with the Company’s CROs and CMOs will be generally payable on a time and materials basis, or when milestones are achieved. The invoicing from clinical trial sites can lag several months. The Company will record expenses for its clinical trial activities performed by third parties based upon estimates of the percentage of work completed of the total work over the life of the individual study in accordance with agreements established with CROs and CMOs. The Company determines the estimates through discussions with development personnel, CROs and CMOs as to the progress or stage of completion of trials or services and the agreed upon fee to be paid for such services based on facts and circumstances known to the Company as of each consolidated balance sheet date. The actual costs and timing of clinical trials are highly uncertain, subject to risks and may change depending upon a number of factors, including the Company’s clinical development plan. If the actual timing of the performance of services of the level of effort varies from the estimate, the Company will adjust the accrual accordingly.
Research and development expenses also include the cost of in-process research and development (“IPR&D”) assets purchased in an asset acquisition transaction. IPR&D assets are expensed unless the assets acquired are deemed to have an alternative future use, provided that the acquired assets did not also include processes or activities that would constitute a business as defined under US GAAP, the technology has not received regulatory approval for marketing and, absent obtaining such approval, has no established alternative future use. Acquired IPR&D payments are immediately expensed in the period in which they are incurred and include upfront payments, as well as subsequent pre-commercial milestone payments. Research and development costs after the acquisition are expensed as incurred. The costs associated with maintaining the patents after the acquisition are the responsibility of the Company and will be expensed as incurred as a general and administrative expense.
Net Loss per Share
Basic net loss per common share is calculated by dividing net loss by the weighted-average number of common shares outstanding for the period, without consideration for potentially dilutive securities. For the periods presented, basic and diluted net loss per common share are identical as the Company has incurred losses for both periods. While the Company has entered into convertible debt agreements during 2024 that are a potentially dilutive security, the number of shares available under a conversion cannot be determined until a Qualifying Offering occurs as defined in the debt agreements (See Note 5 and Note 6).
Fair Value of Financial Instruments
ASC Topic 820 defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements.
Included in the ASC Topic 820 framework is a three level valuation inputs hierarchy with Level 1 being inputs and transactions that can be effectively fully observed by market participants spanning to Level 3 where estimates are unobservable by market participants outside of the Company and must be estimated using assumptions developed by the Company. The Company discloses the lowest level input significant to each category of asset or liability valued within the scope of ASC Topic 820 and the valuation method as exchange, income or use. The Company uses inputs which are as observable as possible and the methods most applicable to the specific situation of each company or valued item.
F-10
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
Interest rate risk is the risk that the value of a financial instrument might be adversely affected by a change in the interest rates. The Company’s outstanding notes payable and convertible notes payable issued in 2023 and 2024 have fixed interest rates therefore the Company is exposed to interest rate risk in that they could not benefit from a decrease in market interest rates. In seeking to minimize the risks from interest rate fluctuations, the Company manages exposure through its normal operating and financing activities.
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40). This update amends the guidance on convertible instruments and the derivatives scope exception for contracts in an entity’s own equity and improves and amends the related EPS guidance for both Subtopics. This standard is effective for fiscal years and interim periods within those fiscal years beginning after December 15, 2023, which means it was effective for our fiscal year beginning January 1, 2024. Early adoption was permitted but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years.
In November 2023, the FASB issued ASU 2023-07, “Improvements to Reportable Segment Disclosures”, effective for fiscal years beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. Early adoption is permitted. While the Company operates as a single segment, it anticipates that there may be additional disclosures required under ASU 2023-07. The Company continues to evaluate the effect of adopting this new accounting guidance on its financial statements but does not intend to early adopt.
Other recent accounting pronouncements issued by the FASB, including its Emerging Issues Task Force, the American Institute of Certified Public Accountants, and the Securities and Exchange Commission did not or are not believed by management to have a material impact on the Company’s present or future financial statements.
Significant Risks and Uncertainties
The Company is subject to challenges and risks specific to its business and its ability to execute on its strategy, as well as risks and uncertainties common to companies in the pharmaceutical industry, including, without limitation, risks and uncertainties associated with: obtaining regulatory approval of its product candidate; delays or problems in the supply of its study drug or failure to comply with manufacturing regulations; identifying, acquiring or in-licensing product candidates; pharmaceutical product development and the inherent uncertainty of clinical success; and the challenges of protecting and enhancing its intellectual property rights; and complying with applicable regulatory requirements.
Further, the Company may be impacted by general economic, political, and market conditions, including overall fluctuations in the financial markets in the U.S. and abroad.
F-11
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
| NOTE 3 | FAIR VALUE |
FASB ASC 820 - Fair Value Measurements and Disclosures, defines fair value as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. FASB ASC 820 requires disclosures about the fair value of all financial instruments, whether or not recognized, for financial statement purposes. Disclosures about the fair value of financial instruments are based on pertinent information available to the Company on September 30, 2024. Accordingly, the estimates presented in these unaudited financial statements are not necessarily indicative of the amounts that could be realized on disposition of the financial instruments. FASB ASC 820 specifies a hierarchy of valuation techniques based on whether the inputs to those valuation techniques are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurement) and the lowest priority to unobservable inputs (Level 3 measurement).
The three levels of the fair value hierarchy are as follows:
Level 1:
Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2:
Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other than quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3:
Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the balance sheets for cash, prepaid expenses, accounts payable, accrued liabilities, accrued research and development costs and notes payable approximate their fair market value based on the short-term maturity of these instruments.
Liabilities measured at fair value on a recurring basis include embedded conversion liability in convertible debt (see Note 6 and Note 7) that did not exist at December 31, 2023 and were as follows on September 30, 2024:
| September 30, 2024 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Derivative Liabilities | $ | - | $ | - | $ | 342,088 | $ | 342,088 | ||||||||
F-12
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
A roll forward of the level 3 valuation financial instrument is as follows:
| For
the nine months ended September 30, 2024 | ||||
| Balance at beginning of year | $ | - | ||
| Initial valuations of derivative liabilites for debt conversion | 333,333 | |||
| Change in fair value | 8,755 | |||
| Balance at end of period | $ | 342,088 | ||
Given the short-term maturities of these debts, in order to determine the fair value of the embedded derivative, the intrinsic value of the conversion feature would be further adjusted for the time value of money based on a risk-free rate and the Company’s current estimate of the timing of a Qualified Offering, which is a Level 3 input in the fair value hierarchy. As of September 30, 2024, the effect of the time value of money on the recognized fair value of the derivative obligation is not considered material given the Company’s expectation that a Qualified Offering should occur in the fourth quarter of 2024.
ASC 825-10 “Financial Instruments” allows entities to voluntarily choose to measure certain financial assets and liabilities at fair value (fair value option). The fair value option may be elected on an instrument-by-instrument basis and is irrevocable unless a new election date occurs. If the fair value option is elected for an instrument, unrealized gains and losses for that instrument should be reported in earnings at each subsequent reporting date. The Company did not elect to apply the fair value option to any outstanding debt instruments.
| NOTE 4 | ACCRUED LIABILITIES |
Accrued liabilities comprised the following as of September 30, 2024 and December 31, 2023:
| September 30,
2024 | December 31,
2023 | |||||||
| Accrued professional fees | $ | - | $ | 3,596 | ||||
| Accrued research and development | 795,997 | 26,905 | ||||||
| Accrued consulting fees | 13,901 | 3,604 | ||||||
| Accrued legal fees | 37,725 | 629 | ||||||
| Accrued fees of license agreement | - | 163,565 | ||||||
| Accrued franchise taxes | - | 300 | ||||||
| $ | 847,623 | $ | 198,599 | |||||
F-13
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
| NOTE 5 | NOTES PAYABLE |
In April 2023, the Company entered into Securities Purchase Agreements with certain accredited investors that provides for an aggregate of up to $500,000, 10% secured promissory notes payable to investors with a term of twelve months from the issuance date (or prepayable in the event of a Qualified Offering) (the “2023 Senior Debt”). A Qualified offering is defined under these agreements as the closing of the first offering (public or private) by the Company after the original issue date of its Common Stock or Common Stock Equivalents with gross proceeds to the Company (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more. Additionally, the Securities Purchase Agreement provides for the buyer(s) to be issued Warrants, exercisable beginning on the closing of a Qualified Offering. These Warrants have an exercise price of the price of a Common Share equal to the Qualified Offering price and have a term of five years from the closing of a Qualified Offering. As of September 30, 2024, the Company has issued an aggregate of $500,000 in secured promissory notes under these agreements with maturities ranging from April 2024 to September 2024. In addition, the Company incurred debt issuance costs of $101,110 which were amortized over the life of the loans with such amortization recorded as interest expense. As of September 30, 2024, the notes total $500,000 and have related accrued interest of $71,113.
As the terms of the Qualified Offering are not yet determined the Company is unable to determine the number or pricing of the warrants. At issuance, these warrants are generally not a liability within the scope of ASC 480 if it is within the reporting entity’s control to decide whether it will enter into a Qualified Offering and, if so, at what terms. Accordingly, the Company has determined that there will be no accounting recognition of these warrants until the terms of the Qualified Offering are determined and the Qualified Offering has closed. Once the terms of the Qualified Offering are known, the Company may be able to estimate the value and begin to evaluate the accounting implications (liability vs equity), however, that will not become final until the Qualified Offering closes.
Interest expense for the nine months ended September 30, 2024 related to the 2023 Senior Debt totals $67,736 and consists of accrued interest of $37,498 and amortized debt issuance costs of $30,238. Interest expense for the nine months ended September 30, 2023 of $66,711 consists of amortized debt issuance costs of $45,595 and accrued interest of $21,116, all of which was related to the 2023 Senior Debt.
| NOTE 6 | CONVERTIBLE NOTES PAYABLE |
In March 2024, the Company entered into Securities Purchase Agreements with certain accredited investors that provides for an aggregate of up to $1.0 million, 10% secured subordinated convertible promissory notes payable to investors with a term of twelve months from the issuance date (the “2024 Convertible Debt”). Additionally, the Securities Purchase Agreement provides that, upon a Qualified Offering, the debt together with all accrued but unpaid interest at the date of conversion shall automatically be converted into shares of the Company’s $0.0001 per share par value common stock (the “Conversion Shares”) at a conversion price equal to 75% of the gross offering price to the investors in the Qualified Offering. A Qualified Offering is defined under these agreements as the closing of the first offering (public or private) by the Company after the original issue date of its Common Stock or Common Stock Equivalents with gross proceeds to the Company (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more inclusive of the 2023 Senior Debt of $500,000 and the 2024 Convertible Debt of $1.0 million.
F-14
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
As of September 30, 2024, the Company has issued an aggregate of $1,000,000 in secured promissory notes under these agreements with maturities ranging from March 2025 to September 2025. In addition, the Company incurred debt issuance costs of $127,510 which will be amortized over the life of the loans with such amortization recorded as interest expense. As of September 30, 2024, the notes, net of unamortized debt issuance costs and discounts related to an embedded derivative, totaled $640,319 and related accrued interest totaled $26,263. Interest expense for the nine months ended September 30, 2024 related to the 2024 Convertible Debt totaled $127,424 and consists of accrued interest of $26,263, amortized debt issuance costs of $28,070 and amortized discounts of $73,091.
| NOTE 7 | DERIVATIVE LIABILITY |
The Company’s 2024 Convertible Debt, together with all accrued but unpaid interest at the date of conversion, shall automatically be converted into shares of the Company’s $0.0001 per share par value common stock (the “Conversion Shares”) at a conversion price equal to 75% of the gross offering price to the investors in the Qualified Offering. The Company evaluated this embedded feature under the guidance of ASC 815 and determined that it required bifurcation of the embedded derivative liability from the underlying debt at its fair value. While the number of common shares to be issued under these agreements is undetermined, this feature results in a fixed monetary amount of the settlement with a variable number of Conversion Shares issued. As the price per share under a Qualified Offering is unknown and the number of shares to be issued is variable, the resulting intrinsic value would be the same regardless of the Conversion Price. Accounting standards require that the Company recognize the derivative as a liability, based on its underlying instrument, in the statement of financial position and measure the instrument at fair value at each balance sheet date. A change in the market value of the financial instrument would be recognized as a gain or loss in the results of operations in the period of change. Upon initiation, the Company recognized a derivative liability of $333,333. At September 30, 2024, the derivative liability totals $342,088, after giving effect for the change in fair value.
| NOTE 8 | COMMITMENTS AND CONTINGENCIES |
Litigation
While there is currently no ongoing litigation, the Company may, from time to time, be involved in various legal matters that arise in the ordinary course of business. Should matters arise, management will then make a determination as to the ultimate disposition of these matters and measure if it could have a material adverse effect on the Company’s financial position, results of operations or liquidity.
License Agreement
In January 2023, the Company entered into a patent license agreement with Duke University and the National Cancer Institute, under the agency of the U.S. Department of Health and Human Services (the “Duke License”) for an exclusive, world-wide, sub-licensable license to the technology more fully described in Note 1 and Note 10. The Duke License was amended in August 2024 to include improvements within the definitions of patent rights and technical information. As a component of the Duke License, the Company agreed to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities. These milestones include initiation of Phase II or Phase III clinical trials, submission of applications for market approval in multiple jurisdictions including the US, EU and Japan and the initiation of post-approval commercial sales in the same jurisdictions. Based on an assumption that all milestones related to the current development program are met during the course of the Duke License, these milestone payments would total approximately $11.7 million. As of September 30, 2024, the Company has not met any milestones as defined in the agreement and, accordingly, has recorded no expense or liability related to such payments.
F-15
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
The Company also agreed to pay royalties equal to low- to mid- single digit percentages of annual net sales on a country-by-country and product-by-product basis subject to downward adjustment to low single digit percentages of our net annual sales in the event there is no valid claim of a patent for the product, with minimum annual royalty levels established. We also must pay Duke low to mid-double digit percentages of any sublicensing fees as set forth in the Duke License. The Company has not recorded and does not owe any royalties or sublicensing fees for the nine months ended September 30, 2024.
| NOTE 9 | SHAREHOLDERS’ EQUITY |
Common Stock
The Company’s Certificate of Incorporation provides the authority to issue common stock, fully voting and participating, with a $0.001 par value, of which 10,000 shares were authorized. In 2021, a total of 1,430 shares were issued to the founding shareholders. In February 2023, 75 shares were issued to Duke University pursuant to the Duke License (see Note 10). A total of 1,505 shares are outstanding as of September 30, 2024 and December 31, 2023.
| NOTE 10 | LICENSE AGREEMENT |
In January 2023, the Company entered into the Duke License for an exclusive, world-wide, sub-licensable license to the technology more fully described in Note 1 and Note 8. Under the terms of this agreement, the Company issued a five percent (5%) common stock ownership interest, or 75 common shares, in the Company at a value of $175.86 per share.
The estimated fair value of the Company’s common stock was determined by a third-party independent valuation firm in accordance with the guidelines outlined in the American Institute of Certified Public Accountants Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation”. These valuations took into account numerous factors, including developments at the Company and market conditions.
Consistent with FASB ASC 820-10-35-24, if multiple valuation techniques are used to measure fair value, the results (respective indications of fair value) should be evaluated and weighted, as appropriate, considering the reasonableness of the range indicated by those results. A fair value measurement is the point within that range that is most representative of fair value in the circumstances. The February 2, 2023 valuation utilized multiple valuation techniques that considered a set of discrete potential liquidity scenarios for the Company, the value common stock would receive in each scenario, and the time required and risk inherent in achieving those values. The third-party valuation expert examined the following scenarios for the Company: (i) the net assets of the Company or the Asset Approach; (ii) a recently executed term sheet related to a proposed merger transaction and capital raise or the Market Approach; and (iii) dissolution or the failure scenario. The Asset Approach was assumed to be appropriate given the Company’s limited operations and expenditures to date as of the valuation date. The Asset Approach was weighted at 80% assuming continued operations as the funding proposed in the term sheet is non-binding and the Company has no assurance such funding will occur. The Market Approach utilized a back-solve methodology to determine the value of the common stock based on the terms outlined in the term sheet assuming the contemplated transaction is consummated and was weighted at 10% as there is limited or no assurance such transaction will be completed. Finally, the failure scenario was weighted at 10% assuming that the Company is unable to obtain suitable financing and would discontinue operations.
F-16
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
In accordance with ASC 805, the license agreement was evaluated and the Company concluded that substantially all of the value acquired is concentrated in a group of similar identifiable IPR&D assets. Accordingly, it has been accounted for as an asset acquisition as the technology transferred to the Company had not yet received regulatory approval and the IPR&D did not have an alternative use. The Company recorded research and development expense related to the issuance of the common stock of $13,189 during the year ended December 31, 2023. In addition, the Company agreed to reimburse the licensor for all patent related costs previously incurred for the technology prior to the license execution date. The total of these costs was approximately $327,000. The Company expensed these costs in their entirety upon execution of the Duke License as a research and development expense as they were deemed to be part of the acquisition price of the IPR&D. Any unpaid amounts are reflected in accrued expenses. Reimbursement for these costs will be made in four equal installments with the first two payments having been made in 2023 and an additional payment made in 2024. At September 30, 2024, the Company had a remaining liability of $81,783 included in accounts payable on the balance sheet. The Company further agreed to make payments based on clinical and commercial milestones and continuing royalty payments on any sales made after approval by regulatory authorities (See Note 8).
| NOTE 11 | SUBSEQUENT EVENTS |
2023 Senior Debt Modification
In November 2024, the Company entered into Note Exchange Agreements with the holders of the 2023 Senior Debt (see Note 5) whereby these 10% secured promissory notes payable to holders with a term of twelve months from the issuance date shall be cancelled and exchanged for the Company’s 10% Secured Convertible Promissory Notes (the “Exchange Notes”). The Exchange Notes will be issued in the aggregate principal amount of $500,000 and provide that, upon the closing of a Qualified Offering, the outstanding principal amount, together with all accrued but unpaid interest, at the date of conversion shall automatically be converted into shares of common stock of the Company or its parent (the “Conversion Shares”) at a conversion price equal to 75% of the gross offering price to the investors in the Qualified Offering (the “Conversion Price”). A Qualified Offering is defined in the Exchange Notes as the closing of the first offering (public or private) by the Company or its Parent after the Original Issue Date of the Company’s or the Parent’s common stock (the “Offering Stock”)with gross proceeds to the Company or its Parent (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more, including the aggregate principal amount of, together with all accrued but unpaid interest on, this Note and the other Notes that will convert thereupon.
Additionally, as contemplated in connection with the issuance of the 2023 Senior Debt, the holders will be issued warrants to purchase up to a number of shares of Common Stock equal to fifty percent (50%) of the number of shares of Common Stock that the principal amount of the Notes issued to the initial Holder would purchase at the Qualified Offering Price. These warrants are exercisable beginning on the closing of a Qualified Offering, have an exercise price of the price of a Common Share equal to the Qualified Offering price and have a term of five years from the closing of a Qualified Offering.
F-17
ADAPTIN
BIO INC.
CONDENSED NOTES TO FINANCIAL STATEMENTS (UNAUDITED)
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2024 AND 2023
Lastly, as an inducement to enter into the Note Exchange Agreements, the holders are to be issued additional warrants to purchase up to a number of shares of Common Stock equal to fifty percent (50%) of the number of shares of Common Stock that the principal amount of the Notes issued to the initial Holder would purchase at the Conversion Price. The purchase price of one share of Common Stock under this Warrant shall be equal to the Conversion Price. These warrants are exercisable beginning on the closing of a Qualified Offering and have a term of five years from the closing of a Qualified Offering.
As the terms of the Qualified Offering are not yet finalized, the Company is unable to determine the number or pricing of these warrants. At issuance, these warrants are generally not a liability within the scope of ASC 480 if it is within the reporting entity’s control to decide whether it will enter into a Qualified Offering and, if so, at what terms. Accordingly, the Company has determined that there will be no accounting recognition of these warrants until the terms of the Qualified Offering are determined and the Qualified Offering has closed. Once the terms of the Qualified Offering are known, the Company may be able to estimate the value and begin to evaluate the accounting implications (liability vs equity), however, that will not become final until the Qualified Offering closes.
2024 Convertible Debt Amendment
At issuance, the 2024 Convertible Debt was convertible into Conversion Shares as more fully detailed in Note 6. With the agreement of the holders of the 2024 Convertible Debt, the Company has amended the 2024 Convertible Debt to be convertible into shares of common stock of either the Company or any entity that directly or indirectly through one or more intermediaries, controls the Company. The terms of the conversion remain as described in Note 6. A “Qualified Offering” , as amended, means the closing of the first offering (public or private) by the Company or its parent after the original issue date of the Company’s or its parent’s common stock with gross proceeds to the Company or its parent (before underwriter, placement agent or broker discounts and commissions and expenses of the offering) of $5,000,000 or more inclusive of the 2023 Senior Debt of $500,000 and the 2024 Convertible Debt of $1.0 million.
F-18
Exhibit 99.3
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
Unless the context requires otherwise, references to “Private Adaptin” in this section are to the business and operations of Adaptin Bio, Inc. prior to the Merger, references to “Unite Acquisition” in this section are to the public company shell, Unite Acquisition 1 Corp. prior to the Merger, references to “Merger Sub” refer to Adaptin Acquisition Co., a wholly-owned subsidiary of Unite Acquisition, prior to the Merger, and references to “Adaptin Bio”, “we,” “us,” “our” and “the Company” in this section are to the combined and renamed public company following the consummation of the Merger.
Introduction
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X, as amended by the final rule, Release No. 33-10786, “Amendments to the Financial Disclosures about Acquired and Disposed Businesses.” The unaudited pro forma condensed combined financial information presents the pro forma effects of the Merger, the Offering and the related material transactions that have occurred, or are probable of occurring, subsequent to the latest balance sheet date that are material to investors. The Merger, the Offering and the related transactions, as further described elsewhere in the unaudited pro forma financial information, were completed on February 11, 2025 (the “Closing”).
Unite Acquisition was a blank check company incorporated in Delaware in 2022. The business purpose of Unite Acquisition was to seek the acquisition of or merger with, an existing company. Since inception, Unite Acquisition has been engaged in organizational efforts and obtaining initial financing. Unite Acquisition was formed as a vehicle to pursue a business combination.
Private Adaptin was incorporated in Delaware in 2021 and is a biopharmaceutical company pioneering a transformational approach to enhance the transfer of therapeutics into the brain, facilitating the treatment of brain cancers and other unmet medical conditions. The Company’s proprietary technology harnesses the human immune system’s ability to target, recognize, destroy or deliver therapeutics to specific cells, including cancer cells. Our mission is to be the global leader and pioneer of this new treatment paradigm, integrating recombinant technology, gene therapy and cell therapy to address the challenges of targeting and delivering effective therapies, including to the brain for cancer and other central nervous system (“CNS”) indications.
Bridge Financings
Private Adaptin raised bridge financing through the offer and sale (a) in 2023 of $500,000 principal amount of its 10% Secured Promissory Notes (the “2023 Bridge Notes”) (including an obligation to issue five-year warrants to purchase up to 56,815 shares of our Common Stock at an exercise price of $4.40 per share) and (b) in 2024 of $1,000,000 principal amount of its 10% Secured Subordinated Convertible Promissory Notes (the “2024 Bridge Notes”), which in each case were sold to a limited number of accredited investors pursuant to Regulation D under the Securities Act. The 2023 Bridge Notes were exchanged for $500,000 principal amount of the Company’s 10% Secured Convertible Promissory Notes (the “Exchange Notes”). In connection with the note exchange, the holders of the 2023 Bridge Notes were also issued warrants to purchase up to 75,755 shares of our Common Stock at an exercise price of $3.30 per share (the “Exchange Warrants”). The Exchange Notes and the 2024 Bridge Notes are referred to herein as the “Bridge Notes.”
At the initial closing of the Offering (as defined below), the $1,500,000 aggregate principal amount of outstanding Bridge Notes, plus accrued interest thereon, automatically converted into shares of Company common stock, par value $0.0001 per share (“Common Stock”), at a conversion price of $3.30 per share, or 484,054 shares of Common Stock (the “Note Conversion Shares”) (as a result of the accrued interest being computed as of September 30, 2024 for the purposes of the pro forma financial statements, the number of Note Conversion Shares used for the pro forma financial statements is less than the actual number of Note Conversion Shares issued at Closing, which includes accrued interest computed through the date of the Closing), and the holders of the 2023 Bridge Notes were issued, pursuant to existing agreements, warrants to purchase up to 132,570 shares of our Common Stock at an exercise price of either $3.30 or $4.40 per share and with a term of five years, as described above (the “Pre-Merger Warrants”).
Merger Agreement
On February 11, 2025, Unite Acquisition entered into the Merger Agreement with Merger Sub and Private Adaptin, pursuant to which Merger Sub merged with and into Private Adaptin, with Private Adaptin continuing as the surviving corporation and as our wholly owned subsidiary.
Pursuant to the Merger Agreement, all of the outstanding capital stock of Private Adaptin was cancelled in exchange for shares of Common Stock, and all of the outstanding Private Adaptin warrants were assumed by the Company with appropriate adjustments to the per share exercise or conversion price thereof, and otherwise on their original terms and conditions. The total number of shares of Common Stock issued to Pre-Merger stockholders of Private Adaptin was 3,249,999 shares.
Prior to the initial closing of the Offering (as defined below), Unite Acquisition’s board of directors adopted an equity incentive plan reserving a number of shares of Common Stock equal to 15% of the shares to be outstanding after completion of the Merger and the final closing of the Offering, on a fully diluted basis (assuming exercise or conversion of all then-outstanding Common Stock equivalents), for the future issuance, at the discretion of the board of directors, of options and other incentive awards to officers, key employees, consultants and directors of the Company and its subsidiaries.
The sole holder of common stock of Unite Acquisition prior to the Merger, Lucius Partners, retained 3,250,000 shares of Common Stock after the Merger, following cancellation of 1,750,000 shares of Common Stock. The Merger Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions.
As a condition to the Merger, we entered into a Pre-Merger indemnity agreement with Unite Acquisition’s sole officer and director, Nathan P. Pereira, pursuant to which the Company agreed to indemnify Mr. Pereira for actions taken by him in his official capacity relating to the consideration, approval and consummation of the Merger and certain related transactions.
The Offering
Immediately following the effective time of the Merger, we issued, in the initial closing of the private placement offering, 1,080,814 Units, for an aggregate purchase price of $4,755,581.60, at a purchase price of $4.40 per Unit, with each Unit consisting of (i) one share of Common Stock, (ii) a warrant representing the right to purchase one share of Common Stock, exercisable from issuance until one year after the final Closing of the Offering at an exercise price of $4.40 per share (the “A Warrant”), and (iii) a warrant, representing the right to purchase one-half of a share of Common Stock, exercisable from issuance until five years after the final Closing of the Offering at an exercise price of $6.60 per whole share (the “B Warrant,” and together with the A Warrant, the “Warrants”) (such shares of Common Stock issuable upon the exercise of the Warrants, the “Warrant Shares”). The private placement offering is referred to herein as the “Offering.”
The offering period commenced on January 8, 2025 and will continue until the later of (i) February 28, 2025, unless extended by Company and the placement agent, Laidlaw & Company (UK) Ltd. (the “Placement Agent”); (ii) the date on which the maximum offering amount of approximately $8.5 million (the “Maximum Offering”) is sold by the Company; or (iii) on a date mutually agreed upon in writing by the Company and the Placement Agent (the “Offering Period”). The final day of the Offering Period is referred to as the “Termination Date.” The initial closing of this Offering occurred immediately following the closing of the Merger (the “Initial Closing”). The Company and Placement Agent may conduct additional closings of the Offering at their discretion to the extent that subscriptions for additional Units, up to the Maximum Offering, are received before the Termination Date.
In connection with the Offering, the Placement Agent (a) will be paid at each closing from the Offering proceeds a total cash commission of 10.0% of the aggregate gross purchase price paid by purchasers in the Offering at that closing (the “Cash Fee”), (b) will be paid at each closing from the Offering proceeds a total non-allocable expense allowance equal to 2.0% of the aggregate gross purchase price paid by purchasers in the Offering at that Closing (the “Expense Allowance”), and (c) will receive (and/or its designees will receive) warrants to purchase a total number of shares of Common Stock equal to 10.0% of the sum of (i) the number of shares of Common Stock included in the Units sold in the Offering at that closing and (ii) the number of shares of Common Stock issuable upon exercise of the Warrants included in the Units sold in the Offering at that closing, with a term expiring seven years after the final closing date and an exercise price of $4.40 per share (the “Placement Agent Warrants”). Any sub-agent of the Placement Agent that introduces investors to the Offering will be entitled to share in the Cash Fee, Expense Allowance and Placement Agent Warrants attributable to those investors pursuant to the terms of an executed sub-agent agreement with such Placement Agent. The Company has agreed to pay certain other expenses of the Placement Agent, including the fees and expenses of its counsel, in connection with the Offering. Subject to certain customary exceptions, we will also indemnify the Placement Agent to the fullest extent permitted by law against certain liabilities that may be incurred in connection with the Offering, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the Placement Agent and its sub-agents may be required to make in respect of such liabilities.
Accounting for the Merger
The Merger is expected to be accounted for as a reverse recapitalization in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Under this method of accounting, Unite Acquisition, which is the legal acquirer, is treated as the “acquired” company for financial reporting purposes and Private Adaptin is treated as the accounting acquirer. This determination was primarily due to Unite Acquisition being determined to be a shell company in that it did not meet the U.S. GAAP definition of a business, did not have more than nominal assets, and does not have more than nominal operations at the time of the merger. Accordingly, for accounting purposes, the Merger will be treated as the equivalent of a capital transaction in which Private Adaptin is issuing stock for the net assets of Unite Acquisition. The net assets of Unite Acquisition will be stated at historical cost, with no goodwill or other intangible assets recorded.
2
Basis for Pro Forma Presentation
The following unaudited pro forma condensed combined financial information has been prepared in accordance with Article 11 of Regulation S-X, as amended by the final rule, Release No. 33-10786, “Amendments to Financial Disclosures about Acquired and Disposed Businesses.” Release No. 33-10786 replaces the existing pro forma adjustment criteria with simplified requirements to depict the accounting for the Merger, Offering, and related transactions (“Transaction Accounting Adjustments”) and are permitted to present the reasonably estimable synergies and other transaction effects that have occurred or reasonably expected to occur (“Management’s Adjustments”). Management of the Company has elected not to present Management’s Adjustments and has only presented Transaction Accounting Adjustments in the unaudited pro forma condensed combined financial statements.
The unaudited pro forma condensed combined financial statements are provided for informational purposes as required by Form 8-K and do not purport to represent what the results of operations or financial position of the Company would actually have been had the Merger and Offering occurred on the dates noted above, or to project the results of operations or financial position of the Company for any future periods. In the opinion of management, all necessary adjustments to the unaudited pro forma condensed combined financial statements have been made.
The following unaudited pro forma condensed combined balance sheet as of September 30, 2024 combines the historical condensed balance sheet of Unite Acquisition as of September 30, 2024 with the historical balance sheet of Private Adaptin as of September 30, 2024 giving further effect to the Transaction Accounting Adjustments, as if they had been consummated as of September 30, 2024.
The following unaudited pro forma condensed combined statements of operations for the nine months ended September 30, 2024 combine the historical condensed statement of operations of Unite Acquisition for the nine months ended September 30, 2024 and the historical statements of operations of Private Adaptin for the nine months ended September 30, 2024, giving effect to the Pro Forma Adjustments as if they had been consummated on January 1, 2023, the beginning of the earliest period presented.
The following unaudited pro forma condensed combined statements of operations for the year ended December 31, 2023 combine the historical condensed statement of operations of Unite Acquisition for the year ended December 31, 2023 and the historical statements of operations of Private Adaptin for the year ended December 31, 2023, giving effect to the Transaction Accounting Adjustments as if they had been consummated on January 1, 2023, the beginning of the earliest period presented.
Assumptions underlying the Transaction Accounting Adjustments are described in the accompanying notes, which should be read in conjunction with the unaudited pro forma condensed combined financial statements. The Transaction Accounting Adjustments and other adjustments are based on available information and assumptions that the Company’s management believes are reasonable. Such adjustments are estimates and actual experience may differ from expectations.
The financial statements of Private Adaptin and Unite Acquisition were prepared in accordance with U.S. GAAP.
The unaudited pro forma condensed combined financial information has been derived from, and should be read in conjunction with:
| ● | Unite Acquisition’s audited financial statements included in its Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on April 1, 2024 and incorporated herein by reference; |
| ● | Unite Acquisition’s unaudited condensed financial statements included in its Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2024, filed with the Securities and Exchange Commission (“SEC”) on November 14, 2024 and incorporated herein by reference; |
| ● | Private Adaptin’s audited financial statements for the year ended December 31, 2023, that are included as Exhibit 99.1 in the Company’s Current Report on Form 8-K to which these unaudited pro forma condensed combined financial statements are being filed as an exhibit; and |
| ● | Private Adaptin’s unaudited condensed financial statements for the nine months ended September 30, 2024, that are included as Exhibit 99.2 in the Company’s Current Report on Form 8-K to which these unaudited pro forma condensed combined financial statements are being filed as an exhibit. |
3
ADAPTIN BIO, INC.
Unaudited Pro Forma Condensed Combined Balance Sheet
As of September 30, 2024
| Historical | Transaction | Pro Forma | ||||||||||||||||
| Private Adaptin | Unite Acquisition | Accounting Adjustments | Notes | Combined Total | ||||||||||||||
| Note A | Note B | Note C | ||||||||||||||||
| ASSETS | ||||||||||||||||||
| CURRENT ASSETS: | ||||||||||||||||||
| Cash | $ | 115,308 | $ | 322 | $ | 3,194,772 | (a), (f) | $ | 3,310,402 | |||||||||
| Total Assets | $ | 115,308 | $ | 322 | $ | 3,194,772 | $ | 3,310,402 | ||||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | ||||||||||||||||||
| CURRENT LIABILITIES: | ||||||||||||||||||
| Accounts payable and accrued expenses | $ | 1,778,805 | $ | 53,126 | $ | 188,624 | (e), (f) | $ | 2,020,555 | |||||||||
| Related party payables | - | 44,750 | - | 44,750 | ||||||||||||||
| Notes payable | 500,000 | - | (500,000 | ) | (c) | - | ||||||||||||
| Note payable - stockholder | - | 137,764 | (137,764 | ) | (f) | - | ||||||||||||
| Convertible notes payable, net | 640,319 | - | (640,319 | ) | (c) | - | ||||||||||||
| Derivative liability | 342,088 | - | (342,088 | ) | (b) | - | ||||||||||||
| Accrued interest | 97,376 | - | (97,376 | ) | (c) | - | ||||||||||||
| Total Current Liabilities | 3,358,588 | 235,640 | (1,528,923 | ) | 2,065,305 | |||||||||||||
| Total Liabilities | 3,358,588 | 235,640 | (1,528,923 | ) | 2,065,305 | |||||||||||||
| STOCKHOLDERS’ EQUITY (DEFICIENCY) | ||||||||||||||||||
| Common stock | $ | 1 | $ | 500 | $ | 305 | (a), (c), (d) | $ | 806 | |||||||||
| Additional paid-in capital | 24,115 | - | 4,785,354 | (a), (c), (d), (f) | 4,809,469 | |||||||||||||
| Accumulated deficit | (3,267,396 | ) | (235,818 | ) | (61,964 | ) | (b), (c), (d), (e) | (3,565,178 | ) | |||||||||
| Total Stockholders’ Equity (Deficiency) | (3,243,280 | ) | (235,318 | ) | 4,723,695 | 1,245,097 | ||||||||||||
| Total Liabilities and Stockholders’ Equity (Deficiency) | $ | 115,308 | $ | 322 | $ | 3,194,772 | $ | 3,310,402 | ||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial statements
4
ADAPTIN BIO, INC.
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Nine Months Ended September 30, 2024
| Historical | Transaction | Pro Forma | ||||||||||||||||
| Private Adaptin | Unite Acquisition | Accounting Adjustments | Notes | Combined Total | ||||||||||||||
| Note A | Note B | Note C | ||||||||||||||||
| Operating Expenses | ||||||||||||||||||
| Research and development | $ | 1,566,533 | $ | - | $ | - | $ | 1,566,533 | ||||||||||
| General and administrative | 495,272 | 122,421 | - | 617,693 | ||||||||||||||
| Total operating expenses | 2,061,805 | 122,421 | - | 2,184,226 | ||||||||||||||
| Loss from operations | (2,061,805 | ) | (122,421 | ) | - | (2,184,226 | ) | |||||||||||
| Other expense (income): | ||||||||||||||||||
| Interest expense | 195,161 | - | (195,161 | ) | (a) | - | ||||||||||||
| Loss on fair value of derivative liability | 8,755 | - | (8,755 | ) | (a) | - | ||||||||||||
| Total other expense (income) | 203,916 | - | (203,916 | ) | - | |||||||||||||
| Net loss | $ | (2,265,721 | ) | $ | (122,421 | ) | $ | 203,916 | $ | (2,184,226 | ) | |||||||
| Net loss per common share - basic and diluted | $ | (1,505.46 | ) | $ | (0.02 | ) | $ | (0.27 | ) | |||||||||
| Weighted average number of common shares outstanding - basic and diluted | 1,505 | 5,000,000 | 3,063,362 | (b) | 8,064,867 | |||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial statements
5
ADAPTIN BIO, INC.
Unaudited Pro Forma Condensed Combined Statement of Operations
For the Year Ended December 31, 2023
| Historical | Transaction | Pro Forma | ||||||||||||||||
| Private Adaptin | Unite Acquisition | Accounting Adjustments | Notes | Combined Total | ||||||||||||||
| Note A | Note B | Note C | ||||||||||||||||
| Operating Expenses | ||||||||||||||||||
| Research and development | $ | 510,356 | $ | - | $ | - | $ | 510,356 | ||||||||||
| General and administrative | 366,960 | 85,593 | 241,751 | (a) | 694,304 | |||||||||||||
| Total operating expenses | 877,316 | 85,593 | 241,751 | 1,204,660 | ||||||||||||||
| Loss from operations | (877,316 | ) | (85,593 | ) | (241,751 | ) | (1,204,660 | ) | ||||||||||
| Other expense (income): | ||||||||||||||||||
| Interest expense | 104,487 | - | (104,487 | ) | (d) | - | ||||||||||||
| Loss on extinguishment | - | - | 398,119 | (c) | 398,119 | |||||||||||||
| Gain on change in fair value | - | - | (342,088 | ) | (b) | (342,088 | ) | |||||||||||
| Total other expense (income) | 104,487 | - | (48,456 | ) | 56,031 | |||||||||||||
| Net loss | $ | (981,803 | ) | $ | (85,593 | ) | $ | (193,295 | ) | $ | (1,260,691 | ) | ||||||
| Net loss per common share - basic and diluted | $ | (655.41 | ) | $ | (0.02 | ) | $ | (0.16 | ) | |||||||||
| Weighted average number of common shares outstanding - basic and diluted | 1,498 | 5,000,000 | 3,063,369 | (e) | 8,064,867 | |||||||||||||
See accompanying notes to the unaudited pro forma condensed combined financial statements
6
Notes to Unaudited Pro Forma Condensed Combined Financial Statements
1. Basis of Pro Forma Presentation
The unaudited pro forma condensed combined financial statements have been prepared for illustrative and informational purposes only and were prepared from the respective historical information of Unite Acquisition and Private Adaptin, and reflect adjustments to the historical information in accordance with the SEC Final Rule Release No. 33-10786 and in accordance with Article 11 of Regulation S-X of the Securities Exchange Act of 1934. We have accounted for the Merger in these unaudited pro forma condensed combined financial statements as a reverse recapitalization, in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification Topic 805 “Business Combinations” (“ASC 805”). In accordance with ASC 805, this is a capital transaction of Private Adaptin (the legal acquiree) and is the equivalent to the issuance of shares by Private Adaptin for the net monetary assets of Unite Acquisition, accompanied by a recapitalization.
Transaction Accounting Adjustments reflected in the unaudited pro forma condensed combined balance sheet are based on items that are factually supportable and directly attributable to the business combination. Transaction Accounting Adjustments reflected in the pro forma condensed combined statements of income are based on items that are factually supportable, directly attributable to the business combination and are expected to have a continuing impact on the combined results. The unaudited pro forma condensed combined financial information does not reflect the cost of any integration activities or benefits from the business combination, including potential synergies that may be generated in future periods.
2. Transaction Accounting Adjustments
The following Transaction Accounting Adjustments give effect to the Merger.
Unaudited Pro Forma Condensed Combined Balance Sheets as of September 30, 2024
| Note A | Derived from the condensed balance sheet of Private Adaptin as of the nine months ended September 30, 2024, included elsewhere in this Current Report on form 8-K. |
| Note B | Derived from the condensed balance sheet of Unite Acquisition as of the nine months ended September 30, 2024, filed with the SEC. |
Transaction Accounting Adjustments:
Note C
| (a) | To record the $4,755,582 of gross proceeds received in connection with the initial closing of the Offering for which 1,080,814 Units are to be issued, with each Unit consisting of (i) one share of Common Stock, (ii) an A Warrant representing the right to purchase one share of Common Stock, and (iii) a B Warrant, representing the right to purchase one-half of a share of Common Stock. |
| (b) | To record the remeasurement of the derivative liability associated with the 2024 Bridge Notes immediately prior to the extinguishment of the liabilities, by reducing the derivative liability by $342,088. |
| (c) | To record the loss on extinguishment of $398,119 associated with the (i) exchange of 2023 Bridge Notes for the Exchange Notes and related issuance of Pre-Merger Warrants with aggregate fair value of $246,581 and (ii) conversion of the Bridge Notes with aggregate principal of $1,500,000, debt discount of $359,681 and accrued interest of $97,376 into an aggregate of 484,054 shares of Unite Acquisition common stock with aggregate fair value of $1,389,233. |
7
| (d) | To give effect to the reverse recapitalization whereby Unite Acquisition will issue 3,249,999 shares of Unite Acquisition common stock to the shareholders of Private Adaptin in exchange for Private Adaptin’s historical common stock and additional paid-in capital. In connection with the Merger, the Unite Acquisition shareholder has agreed to cancel and retire 1,750,000 shares of common stock. Furthermore, to eliminate the historical accumulated deficit of Unite Acquisition of $235,818 as it has been deemed to be the accounting acquiree. |
| (e) | To give effect to an additional estimated $241,751 of Merger expenses to be incurred subsequent to September 30, 2024 with an offset to accrued expenses. |
| (f) | To record Offering costs in aggregate of $1,369,920 deducted against proceeds from the Offering to additional paid-in capital. Furthermore, to record the repayment of $190,890 of related-party liabilities pursuant to the Merger agreement consisting of accounts payable and a note payable held by a stockholder of $53,126 and $137,764, respectively. |
Unaudited Pro Forma Condensed Combined Statement of Operations For The Nine Months Ended September 30, 2024
| Note A | Derived from the condensed statement of operations of Private Adaptin for the nine months ended September 30, 2024, included elsewhere in this Current Report on Form 8-K. |
| Note B | Derived from the condensed statement of operations of Unite Acquisition for the nine months ended September 30, 2024, filed with the SEC. |
Transaction Accounting Adjustments:
Note C
| (a) | To eliminate interest expense and amortization of debt discount related to the Bridge Notes extinguished in the transaction. Furthermore, to eliminate the loss on change in fair value of derivative liability. |
| (b) | The below table illustrates the adjustment to the weighted average shares outstanding used in the earnings per share calculations for the additional shares of Unite Acquisition common stock issued as consideration to the Private Adaptin stockholders, less the cancellation shares, plus the additional shares of Unite Acquisition common stock issued in connection with the Offering, shares of Unite Acquisition common stock issued in connection with the conversion of Bridge Notes less the historical outstanding Private Adaptin common stock. |
| For the Nine Months Ended September 30, 2024 | Weighted Average Number of Shares | |||
| Shares issued to shareholders of Private Adaptin | 3,249,999 | |||
| Unite Acquisition shares eliminated under share cancellation agreement | (1,750,000 | ) | ||
| Shares issued in connection with the initial closing of the Offering | 1,080,814 | |||
| Conversion of Bridge Notes | 484,054 | |||
| Private Adaptin historical shares exchanged | (1,505 | ) | ||
| 3,063,362 | ||||
8
Unaudited Pro Forma Condensed Combined Statement of Operations For The Year Ended December 31, 2023
| Note A | Derived from the statement of operations of Private Adaptin for the year ended December 31, 2023, included elsewhere in this Current Report on Form 8-K. |
| Note B | Derived from the statement of operations of Unite Acquisition for the year ended December 31, 2023, filed with the SEC. |
Transaction Accounting Adjustments:
Note C
| (a) | To record the $241,751 of estimated Merger expenses incurred subsequent to September 30, 2024. |
| (b) | To record the gain on the change in fair value of the derivative liability associated with the 2024 Bridge Notes immediately prior to the extinguishment of the liabilities, by recording a gain on change in fair value of $342,088. |
| (c) | To record the loss on extinguishment of $398,119 associated with the (i) exchange of the 2023 Bridge Notes for the Exchange Notes and related issuance of the Pre-Merger Warrants with aggregate fair value of $246,581 and (ii) conversion of the Bridge Notes with aggregate principal of $1,500,000, debt discount of $359,681 and accrued interest of $97,376 into an aggregate of 484,054 shares of Unite Acquisition common stock with aggregate fair value of $1,389,233. |
| (d) | To eliminate interest expense and amortization of debt discount related to the Bridge Notes extinguished in the transaction. |
| (e) | The below table illustrates the adjustment to the weighted average shares outstanding used in the earnings per share calculations for the additional shares of Unite Acquisition common stock issued as consideration to the Private Adaptin stockholders, less the cancellation shares, plus the additional shares of Unite Acquisition common stock issued in connection with the Offering, shares of Unite Acquisition common stock issued in connection with the conversion of the Bridge Notes less the outstanding Private Adaptin common stock. |
| For the Year Ended December 31, 2023 | Weighted Average Number of Shares | |||
| Shares issued to shareholders of Private Adaptin | 3,249,999 | |||
| Unite Acquisition shares eliminated under share cancellation agreement | (1,750,000 | ) | ||
| Shares issued in connection with the initial closing of the Offering | 1,080,814 | |||
| Conversion of Bridge Notes | 484,054 | |||
| Private Adaptin historical shares exchanged | (1,498 | ) | ||
| 3,063,369 | ||||
9
Cover |
Feb. 11, 2025 |
|---|---|
| Cover [Abstract] | |
| Document Type | 8-K |
| Amendment Flag | false |
| Document Period End Date | Feb. 11, 2025 |
| Current Fiscal Year End Date | --12-31 |
| Entity File Number | 000-56583 |
| Entity Registrant Name | ADAPTIN BIO, INC. |
| Entity Central Index Key | 0001938571 |
| Entity Tax Identification Number | 88-1566415 |
| Entity Incorporation, State or Country Code | DE |
| Entity Address, Address Line One | 3540 Toringdon Way |
| Entity Address, Address Line Two | Suite 200 |
| Entity Address, Address Line Three | #250 |
| Entity Address, City or Town | Charlotte |
| Entity Address, State or Province | NC |
| Entity Address, Postal Zip Code | 28277 |
| City Area Code | 888 |
| Local Phone Number | 609-1498 |
| Written Communications | false |
| Soliciting Material | false |
| Pre-commencement Tender Offer | false |
| Pre-commencement Issuer Tender Offer | false |
| Entity Emerging Growth Company | true |
| Elected Not To Use the Extended Transition Period | false |
{
"version": "2.2",
"instance": {
"ea0230169-8k_adaptin.htm": {
"nsprefix": "cik0001938571",
"nsuri": "http://uniteacquisition1corp.com/20250211",
"dts": {
"schema": {
"local": [
"cik0001938571-20250211.xsd"
],
"remote": [
"http://www.xbrl.org/2003/xbrl-instance-2003-12-31.xsd",
"http://www.xbrl.org/2003/xbrl-linkbase-2003-12-31.xsd",
"http://www.xbrl.org/2003/xl-2003-12-31.xsd",
"http://www.xbrl.org/2003/xlink-2003-12-31.xsd",
"http://www.xbrl.org/2005/xbrldt-2005.xsd",
"http://www.xbrl.org/2006/ref-2006-02-27.xsd",
"http://www.xbrl.org/lrr/role/negated-2009-12-16.xsd",
"http://www.xbrl.org/lrr/role/net-2009-12-16.xsd",
"https://www.xbrl.org/2020/extensible-enumerations-2.0.xsd",
"https://www.xbrl.org/dtr/type/2020-01-21/types.xsd",
"https://www.xbrl.org/dtr/type/2022-03-31/types.xsd",
"https://xbrl.fasb.org/srt/2024/elts/srt-2024.xsd",
"https://xbrl.fasb.org/srt/2024/elts/srt-roles-2024.xsd",
"https://xbrl.fasb.org/srt/2024/elts/srt-types-2024.xsd",
"https://xbrl.fasb.org/us-gaap/2024/elts/us-gaap-2024.xsd",
"https://xbrl.fasb.org/us-gaap/2024/elts/us-roles-2024.xsd",
"https://xbrl.fasb.org/us-gaap/2024/elts/us-types-2024.xsd",
"https://xbrl.sec.gov/country/2024/country-2024.xsd",
"https://xbrl.sec.gov/dei/2024/dei-2024.xsd",
"https://xbrl.sec.gov/stpr/2024/stpr-2024.xsd"
]
},
"labelLink": {
"local": [
"cik0001938571-20250211_lab.xml"
]
},
"presentationLink": {
"local": [
"cik0001938571-20250211_pre.xml"
]
},
"inline": {
"local": [
"ea0230169-8k_adaptin.htm"
]
}
},
"keyStandard": 23,
"keyCustom": 0,
"axisStandard": 0,
"axisCustom": 0,
"memberStandard": 0,
"memberCustom": 0,
"hidden": {
"total": 3,
"http://xbrl.sec.gov/dei/2024": 3
},
"contextCount": 1,
"entityCount": 1,
"segmentCount": 0,
"elementCount": 59,
"unitCount": 3,
"baseTaxonomies": {
"http://xbrl.sec.gov/dei/2024": 23
},
"report": {
"R1": {
"role": "http://uniteacquisition1corp.com/role/Cover",
"longName": "00000001 - Document - Cover",
"shortName": "Cover",
"isDefault": "true",
"groupType": "document",
"subGroupType": "",
"menuCat": "Cover",
"order": "1",
"firstAnchor": {
"contextRef": "AsOf2025-02-11",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"b",
"span",
"p",
"body",
"html"
],
"reportCount": 1,
"baseRef": "ea0230169-8k_adaptin.htm",
"first": true,
"unique": true
},
"uniqueAnchor": {
"contextRef": "AsOf2025-02-11",
"name": "dei:DocumentType",
"unitRef": null,
"xsiNil": "false",
"lang": "en-US",
"decimals": null,
"ancestors": [
"span",
"b",
"span",
"p",
"body",
"html"
],
"reportCount": 1,
"baseRef": "ea0230169-8k_adaptin.htm",
"first": true,
"unique": true
}
}
},
"tag": {
"dei_AmendmentDescription": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "AmendmentDescription",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Amendment Description",
"documentation": "Description of changes contained within amended document."
}
}
},
"auth_ref": []
},
"dei_AmendmentFlag": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "AmendmentFlag",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Amendment Flag",
"documentation": "Boolean flag that is true when the XBRL content amends previously-filed or accepted submission."
}
}
},
"auth_ref": []
},
"dei_AnnualInformationForm": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "AnnualInformationForm",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Annual Information Form",
"documentation": "Boolean flag with value true on a form if it is an annual report containing an annual information form."
}
}
},
"auth_ref": [
"r14"
]
},
"dei_AuditedAnnualFinancialStatements": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "AuditedAnnualFinancialStatements",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Audited Annual Financial Statements",
"documentation": "Boolean flag with value true on a form if it is an annual report containing audited financial statements."
}
}
},
"auth_ref": [
"r14"
]
},
"dei_CityAreaCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "CityAreaCode",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "City Area Code",
"documentation": "Area code of city"
}
}
},
"auth_ref": []
},
"dei_CountryRegion": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "CountryRegion",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Country Region",
"documentation": "Region code of country"
}
}
},
"auth_ref": []
},
"dei_CoverAbstract": {
"xbrltype": "stringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "CoverAbstract",
"lang": {
"en-us": {
"role": {
"label": "Cover [Abstract]",
"documentation": "Cover page."
}
}
},
"auth_ref": []
},
"dei_CurrentFiscalYearEndDate": {
"xbrltype": "gMonthDayItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "CurrentFiscalYearEndDate",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Current Fiscal Year End Date",
"documentation": "End date of current fiscal year in the format --MM-DD."
}
}
},
"auth_ref": []
},
"dei_DocumentAccountingStandard": {
"xbrltype": "accountingStandardItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentAccountingStandard",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Accounting Standard",
"documentation": "The basis of accounting the registrant has used to prepare the financial statements included in this filing This can either be 'U.S. GAAP', 'International Financial Reporting Standards', or 'Other'."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_DocumentAnnualReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentAnnualReport",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Annual Report",
"documentation": "Boolean flag that is true only for a form used as an annual report."
}
}
},
"auth_ref": [
"r11",
"r13",
"r14"
]
},
"dei_DocumentFiscalPeriodFocus": {
"xbrltype": "fiscalPeriodItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentFiscalPeriodFocus",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Fiscal Period Focus",
"documentation": "Fiscal period values are FY, Q1, Q2, and Q3. 1st, 2nd and 3rd quarter 10-Q or 10-QT statements have value Q1, Q2, and Q3 respectively, with 10-K, 10-KT or other fiscal year statements having FY."
}
}
},
"auth_ref": []
},
"dei_DocumentFiscalYearFocus": {
"xbrltype": "gYearItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentFiscalYearFocus",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Fiscal Year Focus",
"documentation": "This is focus fiscal year of the document report in YYYY format. For a 2006 annual report, which may also provide financial information from prior periods, fiscal 2006 should be given as the fiscal year focus. Example: 2006."
}
}
},
"auth_ref": []
},
"dei_DocumentPeriodEndDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentPeriodEndDate",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Period End Date",
"documentation": "For the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD."
}
}
},
"auth_ref": []
},
"dei_DocumentPeriodStartDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentPeriodStartDate",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Period Start Date",
"documentation": "The start date of the period covered in the document, in YYYY-MM-DD format."
}
}
},
"auth_ref": []
},
"dei_DocumentQuarterlyReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentQuarterlyReport",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Quarterly Report",
"documentation": "Boolean flag that is true only for a form used as an quarterly report."
}
}
},
"auth_ref": [
"r12"
]
},
"dei_DocumentRegistrationStatement": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentRegistrationStatement",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Registration Statement",
"documentation": "Boolean flag that is true only for a form used as a registration statement."
}
}
},
"auth_ref": [
"r0"
]
},
"dei_DocumentShellCompanyEventDate": {
"xbrltype": "dateItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentShellCompanyEventDate",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Shell Company Event Date",
"documentation": "Date of event requiring a shell company report."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_DocumentShellCompanyReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentShellCompanyReport",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Shell Company Report",
"documentation": "Boolean flag that is true for a Shell Company Report pursuant to section 13 or 15(d) of the Exchange Act."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_DocumentTransitionReport": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentTransitionReport",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Transition Report",
"documentation": "Boolean flag that is true only for a form used as a transition report."
}
}
},
"auth_ref": [
"r15"
]
},
"dei_DocumentType": {
"xbrltype": "submissionTypeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentType",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Document Type",
"documentation": "The type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'."
}
}
},
"auth_ref": []
},
"dei_DocumentsIncorporatedByReferenceTextBlock": {
"xbrltype": "textBlockItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "DocumentsIncorporatedByReferenceTextBlock",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Documents Incorporated by Reference [Text Block]",
"documentation": "Documents incorporated by reference."
}
}
},
"auth_ref": [
"r3"
]
},
"dei_EntityAddressAddressLine1": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressAddressLine1",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Address Line One",
"documentation": "Address Line 1 such as Attn, Building Name, Street Name"
}
}
},
"auth_ref": []
},
"dei_EntityAddressAddressLine2": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressAddressLine2",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Address Line Two",
"documentation": "Address Line 2 such as Street or Suite number"
}
}
},
"auth_ref": []
},
"dei_EntityAddressAddressLine3": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressAddressLine3",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Address Line Three",
"documentation": "Address Line 3 such as an Office Park"
}
}
},
"auth_ref": []
},
"dei_EntityAddressCityOrTown": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressCityOrTown",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, City or Town",
"documentation": "Name of the City or Town"
}
}
},
"auth_ref": []
},
"dei_EntityAddressCountry": {
"xbrltype": "countryCodeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressCountry",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Country",
"documentation": "ISO 3166-1 alpha-2 country code."
}
}
},
"auth_ref": []
},
"dei_EntityAddressPostalZipCode": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressPostalZipCode",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, Postal Zip Code",
"documentation": "Code for the postal or zip code"
}
}
},
"auth_ref": []
},
"dei_EntityAddressStateOrProvince": {
"xbrltype": "stateOrProvinceItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityAddressStateOrProvince",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Address, State or Province",
"documentation": "Name of the state or province."
}
}
},
"auth_ref": []
},
"dei_EntityBankruptcyProceedingsReportingCurrent": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityBankruptcyProceedingsReportingCurrent",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Bankruptcy Proceedings, Reporting Current",
"documentation": "For registrants involved in bankruptcy proceedings during the preceding five years, the value Yes indicates that the registrant has filed all documents and reports required to be filed by Section 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court; the value No indicates the registrant has not. Registrants not involved in bankruptcy proceedings during the preceding five years should not report this element."
}
}
},
"auth_ref": [
"r6"
]
},
"dei_EntityCentralIndexKey": {
"xbrltype": "centralIndexKeyItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityCentralIndexKey",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Central Index Key",
"documentation": "A unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityCommonStockSharesOutstanding": {
"xbrltype": "sharesItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityCommonStockSharesOutstanding",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Common Stock, Shares Outstanding",
"documentation": "Indicate number of shares or other units outstanding of each of registrant's classes of capital or common stock or other ownership interests, if and as stated on cover of related periodic report. Where multiple classes or units exist define each class/interest by adding class of stock items such as Common Class A [Member], Common Class B [Member] or Partnership Interest [Member] onto the Instrument [Domain] of the Entity Listings, Instrument."
}
}
},
"auth_ref": []
},
"dei_EntityCurrentReportingStatus": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityCurrentReportingStatus",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Current Reporting Status",
"documentation": "Indicate 'Yes' or 'No' whether registrants (1) have filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that registrants were required to file such reports), and (2) have been subject to such filing requirements for the past 90 days. This information should be based on the registrant's current or most recent filing containing the related disclosure."
}
}
},
"auth_ref": []
},
"dei_EntityEmergingGrowthCompany": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityEmergingGrowthCompany",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Emerging Growth Company",
"documentation": "Indicate if registrant meets the emerging growth company criteria."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityExTransitionPeriod": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityExTransitionPeriod",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Elected Not To Use the Extended Transition Period",
"documentation": "Indicate if an emerging growth company has elected not to use the extended transition period for complying with any new or revised financial accounting standards."
}
}
},
"auth_ref": [
"r19"
]
},
"dei_EntityFileNumber": {
"xbrltype": "fileNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityFileNumber",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity File Number",
"documentation": "Commission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen."
}
}
},
"auth_ref": []
},
"dei_EntityFilerCategory": {
"xbrltype": "filerCategoryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityFilerCategory",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Filer Category",
"documentation": "Indicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityIncorporationStateCountryCode": {
"xbrltype": "edgarStateCountryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityIncorporationStateCountryCode",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Incorporation, State or Country Code",
"documentation": "Two-character EDGAR code representing the state or country of incorporation."
}
}
},
"auth_ref": []
},
"dei_EntityInteractiveDataCurrent": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityInteractiveDataCurrent",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Interactive Data Current",
"documentation": "Boolean flag that is true when the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files)."
}
}
},
"auth_ref": [
"r16"
]
},
"dei_EntityPrimarySicNumber": {
"xbrltype": "sicNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityPrimarySicNumber",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Primary SIC Number",
"documentation": "Primary Standard Industrial Classification (SIC) Number for the Entity."
}
}
},
"auth_ref": [
"r14"
]
},
"dei_EntityPublicFloat": {
"xbrltype": "monetaryItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityPublicFloat",
"crdr": "credit",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Public Float",
"documentation": "The aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter."
}
}
},
"auth_ref": []
},
"dei_EntityRegistrantName": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityRegistrantName",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Registrant Name",
"documentation": "The exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityShellCompany": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityShellCompany",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Shell Company",
"documentation": "Boolean flag that is true when the registrant is a shell company as defined in Rule 12b-2 of the Exchange Act."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntitySmallBusiness": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntitySmallBusiness",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Small Business",
"documentation": "Indicates that the company is a Smaller Reporting Company (SRC)."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityTaxIdentificationNumber": {
"xbrltype": "employerIdItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityTaxIdentificationNumber",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Tax Identification Number",
"documentation": "The Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS."
}
}
},
"auth_ref": [
"r2"
]
},
"dei_EntityVoluntaryFilers": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityVoluntaryFilers",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Voluntary Filers",
"documentation": "Indicate 'Yes' or 'No' if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act."
}
}
},
"auth_ref": []
},
"dei_EntityWellKnownSeasonedIssuer": {
"xbrltype": "yesNoItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "EntityWellKnownSeasonedIssuer",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Entity Well-known Seasoned Issuer",
"documentation": "Indicate 'Yes' or 'No' if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Is used on Form Type: 10-K, 10-Q, 8-K, 20-F, 6-K, 10-K/A, 10-Q/A, 20-F/A, 6-K/A, N-CSR, N-Q, N-1A."
}
}
},
"auth_ref": [
"r17"
]
},
"dei_Extension": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "Extension",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Extension",
"documentation": "Extension number for local phone number."
}
}
},
"auth_ref": []
},
"dei_LocalPhoneNumber": {
"xbrltype": "normalizedStringItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "LocalPhoneNumber",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Local Phone Number",
"documentation": "Local phone number for entity."
}
}
},
"auth_ref": []
},
"dei_NoTradingSymbolFlag": {
"xbrltype": "trueItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "NoTradingSymbolFlag",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "No Trading Symbol Flag",
"documentation": "Boolean flag that is true only for a security having no trading symbol."
}
}
},
"auth_ref": []
},
"dei_OtherReportingStandardItemNumber": {
"xbrltype": "otherReportingStandardItemNumberItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "OtherReportingStandardItemNumber",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Other Reporting Standard Item Number",
"documentation": "\"Item 17\" or \"Item 18\" specified when the basis of accounting is neither US GAAP nor IFRS."
}
}
},
"auth_ref": [
"r13"
]
},
"dei_PreCommencementIssuerTenderOffer": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "PreCommencementIssuerTenderOffer",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Pre-commencement Issuer Tender Offer",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act."
}
}
},
"auth_ref": [
"r7"
]
},
"dei_PreCommencementTenderOffer": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "PreCommencementTenderOffer",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Pre-commencement Tender Offer",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act."
}
}
},
"auth_ref": [
"r9"
]
},
"dei_Security12bTitle": {
"xbrltype": "securityTitleItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "Security12bTitle",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Title of 12(b) Security",
"documentation": "Title of a 12(b) registered security."
}
}
},
"auth_ref": [
"r1"
]
},
"dei_Security12gTitle": {
"xbrltype": "securityTitleItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "Security12gTitle",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Title of 12(g) Security",
"documentation": "Title of a 12(g) registered security."
}
}
},
"auth_ref": [
"r5"
]
},
"dei_SecurityExchangeName": {
"xbrltype": "edgarExchangeCodeItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "SecurityExchangeName",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Security Exchange Name",
"documentation": "Name of the Exchange on which a security is registered."
}
}
},
"auth_ref": [
"r4"
]
},
"dei_SecurityReportingObligation": {
"xbrltype": "securityReportingObligationItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "SecurityReportingObligation",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Security Reporting Obligation",
"documentation": "15(d), indicating whether the security has a reporting obligation under that section of the Exchange Act."
}
}
},
"auth_ref": [
"r10"
]
},
"dei_SolicitingMaterial": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "SolicitingMaterial",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Soliciting Material",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act."
}
}
},
"auth_ref": [
"r8"
]
},
"dei_TradingSymbol": {
"xbrltype": "tradingSymbolItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "TradingSymbol",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Trading Symbol",
"documentation": "Trading symbol of an instrument as listed on an exchange."
}
}
},
"auth_ref": []
},
"dei_WrittenCommunications": {
"xbrltype": "booleanItemType",
"nsuri": "http://xbrl.sec.gov/dei/2024",
"localname": "WrittenCommunications",
"presentation": [
"http://uniteacquisition1corp.com/role/Cover"
],
"lang": {
"en-us": {
"role": {
"label": "Written Communications",
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act."
}
}
},
"auth_ref": [
"r18"
]
}
}
}
},
"std_ref": {
"r0": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12"
},
"r1": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b"
},
"r2": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b-2"
},
"r3": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "b-23"
},
"r4": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "d1-1"
},
"r5": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12",
"Subsection": "g"
},
"r6": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "12, 13, 15d"
},
"r7": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "13e",
"Subsection": "4c"
},
"r8": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "14a",
"Subsection": "12"
},
"r9": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "14d",
"Subsection": "2b"
},
"r10": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Exchange Act",
"Number": "240",
"Section": "15",
"Subsection": "d"
},
"r11": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 10-K",
"Number": "249",
"Section": "310"
},
"r12": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 10-Q",
"Number": "240",
"Section": "308",
"Subsection": "a"
},
"r13": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 20-F",
"Number": "249",
"Section": "220",
"Subsection": "f"
},
"r14": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Form 40-F",
"Number": "249",
"Section": "240",
"Subsection": "f"
},
"r15": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Forms 10-K, 10-Q, 20-F",
"Number": "240",
"Section": "13",
"Subsection": "a-1"
},
"r16": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Regulation S-T",
"Number": "232",
"Section": "405"
},
"r17": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Securities Act",
"Number": "230",
"Section": "405"
},
"r18": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Securities Act",
"Number": "230",
"Section": "425"
},
"r19": {
"role": "http://www.xbrl.org/2003/role/presentationRef",
"Publisher": "SEC",
"Name": "Securities Act",
"Number": "7A",
"Section": "B",
"Subsection": "2"
}
}
}