00-0000000 false 0001805890 0001805890 2023-02-10 2023-02-10
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): February 10, 2023
FUSION PHARMACEUTICALS INC.
(Exact name of registrant as specified in its charter)
|
|
|
|
|
| Canada |
|
001-39344 |
|
Not Applicable |
(State or other jurisdiction of incorporation) |
|
(Commission File Number) |
|
(I.R.S. Employer Identification No.) |
270 Longwood Road South
Hamilton, Ontario, Canada, L8P 0A6
(Address of principal executive offices, including zip code)
(289) 799-0891
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ |
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Trade Symbol(s) |
|
Name of each exchange on which registered |
| Common shares, no par value per share |
|
FUSN |
|
The Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 1.01 |
Entry into a Material Definitive Agreement. |
Option and Asset Purchase Agreement
On November 14, 2022, Fusion Pharmaceuticals Inc. (the “Company”) and RadioMedix, Inc. (“RadioMedix”) entered into an option and asset purchase agreement (the “Option and Asset Purchase Agreement”), pursuant to which RadioMedix granted to Fusion the exclusive right, but not the obligation (the “Option”), to acquire certain of RadioMedix’s assets related to its on-going Phase 2 clinical trial evaluating 225-actinium PSMA I&T (the “TATCIST Study”), a small molecule targeting prostate specific membrane antigens, expressed on prostate tumors. Such assets include, among other things, the investigational new drug application for the TATCIST Study, and all governmental authorizations and materials related thereto, all know-how, intellectual property and information of RadioMedix related to 225-actinium PSMA, any third-party license held, or later acquired, by RadioMedix relating to 225-actinium PSMA, and clinical and other data for the TATCIST Study (collectively, the “Assets”). In exchange for the Option, the Company paid RadioMedix an option fee of $750,000 upon the execution of the Option Asset and Purchase Agreement. The Option has an exercise fee of $1.5 million payable to RadioMedix (the “Exercise Fee”). Pursuant to the terms of the Option and Asset Purchase Agreement, the Company must exercise the Option within a specified time period following the delivery by RadioMedix of specified data related to the TATCIST Study (the “Option Trigger”), though the Company has the right to exercise the Option prior to achievement of the Option Trigger.
On February 10, 2023 (the “Option Exercise Date”), the Company notified RadioMedix of its decision to exercise the Option and paid the Exercise Fee. The acquisition closed on February 10, 2023 (the “Closing”). Following the Closing, the alpha-emitting radiopharmaceutical being evaluated in the TATCIST Study is referred to as FPI-2265.
Pursuant to the terms of the Option and Asset Purchase Agreement, the Company will be obligated to pay RadioMedix (i) up to an additional $10.5 million upon the achievement of certain clinical and regulatory milestones, (ii) low single-digit royalties on potential future net sales, subject to specified reductions, and (iii) up to an additional $50.0 million in net sales milestones; in each case, relating to products covered by the Option and Asset Purchase Agreement. In addition, in the event RadioMedix or the Company is successful in obtaining certain intellectual property rights from a third party relating to 225-actinium PSMA I&T (the “Third Party IP Rights”), the amount of the clinical and regulatory milestone payments will be increased by up to an aggregate of $4.0 million and the royalty rates will increase but remain in the low- to mid-single digits.
Pursuant to the Option and Asset Purchase Agreement, the Company is prohibited from terminating or deprioritizing the development of 225-actinium PSMA I&T, subject to specified exceptions, including, but not limited to regulatory, safety, efficacy, market exclusivity and competition and patentability developments. If the Company terminates or deprioritizes the development of 225-actinium PSMA I&T, and does not sell, license or otherwise transfer its rights to a third-party within 12 months of such termination, the Company and RadioMedix are required to negotiate the return of 225-actinium PSMA I&T and related assets to RadioMedix in return for specified reimbursement costs to the Company.
The Option and Asset Purchase Agreement includes representations, warranties and covenants of the parties customary for a transaction of this nature. Among other things, RadioMedix has agreed, subject to certain exceptions, not to develop or research a molecule that targets PSMA I&T for a certain period of time following the Closing.
The Option and Asset Purchase Agreement also contains customary termination provisions, including termination (i) by mutual written agreement of the parties, (ii) by either party in the event that the applicable conditions for termination of the TATCIST Study set forth in the TATCIST Study protocol are met, and (iii) by either party upon a material breach of the Agreement by the other party.
To date, Excel Diagnostics and Nuclear Oncology Center (“Excel”), an affiliate of RadioMedix, has been the only clinical trial site dosing patients in the TATCIST Study. At the Closing, the Company and Excel entered into a clinical trial agreement, pursuant to which Excel shall remain a clinical trial site following the Closing. Additionally, at the Closing, the Company and RadioMedix entered into manufacturing agreements under which RadioMedix will supply FPI-2265 to the Company for use in clinical trials.
The description of the Option and Asset Purchase Agreement contained herein does not purport to be complete and is qualified in its entirety by reference to the complete text of the Option and Asset Purchase Agreement, a copy of which is attached hereto as Exhibit 10.1 and is incorporated herein by reference.
Securities Purchase Agreement
On February 13, 2023, the Company entered into a Securities Purchase Agreement (the “Purchase Agreement”) with the purchasers named therein (the “Investors”).
Pursuant to the Purchase Agreement, the Company agreed to sell an aggregate of 17,648,596 of its common shares (the “Shares”), no par value per share (the “Common Shares”), at a purchase price equal to $3.40 per share, which represents the closing price on the Nasdaq Global Select Market on February 10, 2023, to the Investors for aggregate gross proceeds of approximately $60 million (collectively, the “Offering”). The Offering is expected to close on or about February 16, 2023, subject to the satisfaction of certain customary closing conditions.
Upon the closing of the Offering, the Company anticipates having $248.0 million in cash and cash equivalents, which it believes will be sufficient to fund its planned operating expenses and capital expenditure requirements into the first quarter of 2025.
Registration Rights Agreement
On February 13, 2023, in connection with the Purchase Agreement, the Company entered into a Registration Rights Agreement (the “Registration Rights Agreement”) with the Investors. Pursuant to the Registration Rights Agreement, the Company agreed to prepare and file a registration statement with the Securities and Exchange Commission (the “SEC”) within 45 calendar days after the closing of the Offering for purposes of registering the resale of the Shares and any Common Shares as a dividend or other distribution with respect to the Shares. The Company agreed to use its commercially reasonable efforts to cause this registration statement to be declared effective by the SEC within 60 days after the filing of the registration statement.
The Company has also agreed, among other things, to indemnify the Investors, their officers, directors, members, employees and agents, successors and assigns under the registration statement from certain liabilities and to pay all fees and expenses (excluding any legal fees of the selling holder(s), and any underwriting discounts and selling commissions) incident to the Company’s obligations under the Registration Rights Agreement.
The Offering was exempt from registration pursuant to Section 4(a)(2) of the Securities Act and Regulation D promulgated thereunder, as a transaction by an issuer not involving a public offering. The Investors have acquired the securities not with a view to or for sale in connection with any distribution thereof, and appropriate legends have been affixed to the securities issued in this transaction.
The foregoing summaries of the Purchase Agreement and Registration Rights Agreement do not purport to be complete and are qualified in their entirety by reference to the Purchase Agreement and Registration Rights Agreement, which are filed as Exhibits 10.2 and 10.3, respectively, to this Current Report on Form 8-K.
| Item 3.02 |
Sale of Unregistered Securities. |
The information contained in Item 1.01 of this Current Report on Form 8-K is incorporated by reference into this Item 3.02.
| Item 7.01 |
Regulation FD Disclosure. |
On February 13, 2023, the Company issued a press release announcing, among other things, the Offering, its exercise of the Option under the Option and Asset Purchase Agreement and the Closing. A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated by reference herein.
The information set forth in this Item 7.01 is “furnished” and shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall such information be deemed incorporated by reference in any filing under the Exchange Act or the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such a filing.
On February 13, 2023, the Company filed an inter partes review with the Patent Trial and Appeal Board to invalidate an issued U.S. patent with claims directed to methods for treating PSMA expressing cancers with multiple 225Ac-PSMA targeting agents issued to the University of Heidelberg (the “IPR”).
On February 13, 2023, the Company will host an investor call regarding FPI-2265. A copy of the presentation to be presented is filed as Exhibit 99.2 to this Current Report on Form 8-K and is incorporated herein by reference.
In connection with the exercise of the Option and the IPR, the Company is filing this information with this Current Report on Form 8-K for the purpose of supplementing and updating disclosures contained in the Company’s prior filings with the SEC including those discussed under the heading “Item 1A. Risk Factors,” in the Company’s most recent Quarter Report on Form 10-Q for the quarter ended September 30, 2022. The supplemental risk factors are filed herewith as Exhibit 99.3 and are incorporated herein by reference.
Forward-Looking Statements
This Current Report on Form 8-K contains “forward-looking statements” for purposes of the safe harbor provisions of The Private Securities Litigation Reform Act of 1995, including but not limited to the statements regarding the Company’s future business and financial performance. For this purpose, any statements contained herein that are not statements of historical fact may be deemed forward-looking statements. Without limiting the foregoing, the words “expect,” “plans,” “anticipates,” “intends,” “will,” and similar expressions are also intended to identify forward-looking statements, as are expressed or implied statements with respect to the Company’s potential drug candidates, including any expressed or implied statements regarding the successful development of its product candidates. Actual results may differ materially from those indicated by such forward-looking statements as a result of risks and uncertainties, including but not limited to the following: the Company’s ability to close the Offering; the timing and advancement of current and planned clinical trials, the Company’s ability to replicate results achieved in its preclinical studies or clinical trials, or that of RadioMedix in any future studies or trials; the Company’s ability to maintain its intellectual property portfolio, including through its IPR; and the timing and success of the Company’s development and commercialization of its product candidates; risks relating to the regulatory process; unexpected patient recruitment delays or regulatory actions or delays; the Company’s ability to obtain additional funding required to conduct its research, development and commercialization activities; changes in the Company’s business plan or objectives; and the Company’s ability to obtain, maintain and enforce patent and other intellectual property protection for its product candidates and its discoveries. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from any future results, performance or achievements expressed or implied by such statements. These and other risks which may impact management’s expectations are described in greater detail under the heading “Risk Factors” in the Company’s quarterly report on Form 10-Q for the quarter ended September 30, 2022, as filed with the SEC and in any subsequent periodic or current report that the Company files with the SEC. All forward-looking statements reflect the Company’s estimates only as of the date of this release (unless another date is indicated) and should not be relied upon as reflecting the Company’s views, expectations or beliefs at any date subsequent to the date of this release. While the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so, even if the Company’s estimates change.
| Item 9.01 |
Financial Statements and Exhibits. |
(d) Exhibits.
|
|
|
| Exhibit No. |
|
Description |
|
|
| 10.1†* |
|
Option and Asset Purchase Agreement by and between Fusion Pharmaceuticals Inc. and RadioMedix, Inc., dated November 14, 2022 |
|
|
| 10.2#* |
|
Securities Purchase Agreement, dated February 13, 2023, by and among Fusion Pharmaceuticals Inc. and the Investors named therein |
|
|
| 10.3#* |
|
Registration Rights Agreement, dated February 13, 2023, by and among Fusion Pharmaceuticals Inc. and the Investors named therein |
|
|
| 99.1 |
|
Press Release, issued by Fusion Pharmaceuticals Inc., dated February 13, 2023 |
|
|
| 99.2 |
|
Investor Presentation |
|
|
| 99.3 |
|
Updated Corporate Disclosure |
|
|
| 104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document). |
| † |
Certain portions of this exhibit (indicated by asterisks) were omitted in accordance with the rules of the Securities and Exchange Commission. |
| * |
Exhibits and/or schedules to this exhibit have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The registrant agrees to furnish supplementally copies of any omitted exhibits or schedules to the U.S. Securities and Exchange Commission upon request; provided, however, that the registrant may request confidential treatment pursuant to Rule 24b-2 of the Exchange Act for any exhibits or schedules so furnished. |
| # |
The representations and warranties contained in this agreement were made only for purposes of the transactions contemplated by the agreement as of specific dates and may have been qualified by certain disclosures between the parties and a contractual standard of materiality different from those generally applicable under securities laws, among other limitations. The representations and warranties were made for purposes of allocating contractual risk between the parties to the agreement and should not be relied upon as a disclosure of factual information relating to the Company, the Investors or the transactions described in this Current Report on Form 8-K. |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
|
|
|
|
Fusion Pharmaceuticals Inc. |
|
|
|
|
| Date: February 13, 2023 |
|
|
|
By: |
|
/s/ Maria Stahl |
|
|
|
|
|
|
Maria Stahl |
|
|
|
|
|
|
Chief Legal Officer |
Exhibit 10.1
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT HAVE BEEN OMITTED AND REPLACED WITH “[***]”. SUCH IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS
EXHIBIT BECAUSE IT IS (I) NOT MATERIAL AND (II) WOULD LIKELY CAUSE COMPETITIVE HARM TO THE COMPANY IF DISCLOSED.
OPTION AND ASSET PURCHASE AGREEMENT
dated as of November 14, 2022
by and between
RADIOMEDIX, INC.
and
FUSION PHARMACEUTICALS INC.
TABLE OF CONTENTS
|
|
|
|
|
|
|
| |
|
|
|
Page |
|
| ARTICLE I DEFINITIONS |
|
|
1 |
|
|
|
|
| 1.1 |
|
Definitions |
|
|
1 |
|
| 1.2 |
|
Construction |
|
|
13 |
|
| 1.3 |
|
Performance of Obligations by Affiliates |
|
|
13 |
|
|
|
| ARTICLE II OPTION GRANT AND EXERCISE |
|
|
14 |
|
|
|
|
| 2.1 |
|
Grant of Option |
|
|
14 |
|
| 2.2 |
|
TATCIST Study |
|
|
14 |
|
| 2.3 |
|
Additional Site Payments |
|
|
16 |
|
| 2.4 |
|
Exercise of Option |
|
|
16 |
|
|
|
| ARTICLE III PURCHASE AND SALE |
|
|
17 |
|
|
|
|
| 3.1 |
|
Agreement to Purchase and Sell |
|
|
17 |
|
| 3.2 |
|
Excluded Assets |
|
|
18 |
|
| 3.3 |
|
Assumed Liabilities |
|
|
19 |
|
| 3.4 |
|
Excluded Liabilities |
|
|
19 |
|
| 3.5 |
|
Procedures for Assignments |
|
|
20 |
|
| 3.6 |
|
Wrong Pocket Assets |
|
|
21 |
|
| 3.7 |
|
Purchase Price |
|
|
21 |
|
| 3.8 |
|
Deferred Payments |
|
|
22 |
|
| 3.9 |
|
Allocation of Purchase Price |
|
|
26 |
|
| 3.10 |
|
Withholding and Other Taxes |
|
|
26 |
|
| 3.11 |
|
Third Party Patent |
|
|
27 |
|
|
|
| ARTICLE IV CLOSING |
|
|
29 |
|
|
|
|
| 4.1 |
|
Closing |
|
|
29 |
|
| 4.2 |
|
Transactions at Closing |
|
|
29 |
|
|
|
| ARTICLE V REPRESENTATIONS AND WARRANTIES OF SELLER |
|
|
30 |
|
|
|
|
| 5.1 |
|
Qualification, Organization, etc. |
|
|
30 |
|
| 5.2 |
|
Authority; Binding Effect |
|
|
30 |
|
| 5.3 |
|
No Conflicts; Consents |
|
|
31 |
|
| 5.4 |
|
Consents and Governmental Approvals |
|
|
31 |
|
| 5.5 |
|
Title to Assets |
|
|
31 |
|
| 5.6 |
|
Absence of Certain Changes or Events |
|
|
31 |
|
| 5.7 |
|
Orders; Litigation |
|
|
32 |
|
| 5.8 |
|
Compliance with Laws; Regulatory Matters |
|
|
32 |
|
| 5.9 |
|
Tax Matters |
|
|
34 |
|
-i-
|
|
|
|
|
|
|
| 5.10 |
|
Intellectual Property |
|
|
35 |
|
| 5.11 |
|
Insurance |
|
|
36 |
|
| 5.12 |
|
Brokers |
|
|
36 |
|
| 5.13 |
|
Solvency |
|
|
36 |
|
| 5.14 |
|
No Reliance |
|
|
36 |
|
|
|
| ARTICLE VI REPRESENTATIONS AND WARRANTIES OF BUYER |
|
|
37 |
|
|
|
|
| 6.1 |
|
Qualification, Organization, etc. |
|
|
37 |
|
| 6.2 |
|
Authority; Binding Effect |
|
|
37 |
|
| 6.3 |
|
No Conflicts; Consents |
|
|
38 |
|
| 6.4 |
|
Consents and Governmental Approvals |
|
|
38 |
|
| 6.5 |
|
Orders; Litigation |
|
|
38 |
|
| 6.6 |
|
Solvency |
|
|
38 |
|
| 6.7 |
|
Brokers |
|
|
38 |
|
| 6.8 |
|
No Reliance |
|
|
38 |
|
|
|
| ARTICLE VII PRE-CLOSING COVENANTS |
|
|
39 |
|
|
|
|
| 7.1 |
|
Conduct Prior to the Closing |
|
|
39 |
|
| 7.2 |
|
Efforts; Execution of Transfer Documents |
|
|
40 |
|
| 7.3 |
|
Access |
|
|
41 |
|
| 7.4 |
|
Exclusivity |
|
|
41 |
|
| 7.5 |
|
Notification of Certain Matters |
|
|
42 |
|
| 7.6 |
|
Insurance |
|
|
42 |
|
| 7.7 |
|
Manufacturing Diligence |
|
|
42 |
|
| 7.8 |
|
Manufacturing and Quality Agreements |
|
|
43 |
|
| 7.9 |
|
Manufacturing Technology Transfer |
|
|
43 |
|
| 7.10 |
|
Delivery of Data Site Copies |
|
|
44 |
|
| 7.11 |
|
Biweekly Data Reports and Pre-Closing Data License |
|
|
44 |
|
| 7.12 |
|
Clinical Trial Agreement |
|
|
44 |
|
|
|
| ARTICLE VIII OTHER AGREEMENTS |
|
|
45 |
|
|
|
|
| 8.1 |
|
Books and Records; Access; Assistance |
|
|
45 |
|
| 8.2 |
|
Privileged Matters |
|
|
46 |
|
| 8.3 |
|
Non-Competition |
|
|
46 |
|
| 8.4 |
|
TATCIST Study and Regulatory Transfers |
|
|
46 |
|
| 8.5 |
|
Additional TATCIST Transfer Services |
|
|
46 |
|
| 8.6 |
|
Further Assurances |
|
|
47 |
|
| 8.7 |
|
[***]. |
|
|
47 |
|
| 8.8 |
|
Residual Know-How License |
|
|
48 |
|
|
|
| ARTICLE IX TAX MATTERS |
|
|
48 |
|
|
|
|
| 9.1 |
|
Transfer Taxes |
|
|
48 |
|
| 9.2 |
|
VAT |
|
|
48 |
|
| 9.3 |
|
Tax Cooperation |
|
|
48 |
|
-ii-
|
|
|
|
|
|
|
| 9.4 |
|
Straddle Period Allocation |
|
|
48 |
|
| 9.5 |
|
Section 56.4 Election |
|
|
49 |
|
| 9.6 |
|
Tax Provision Priority |
|
|
49 |
|
|
|
| ARTICLE X CONDITIONS TO CLOSING |
|
|
49 |
|
|
|
|
| 10.1 |
|
Conditions to Each Party’s Obligations |
|
|
49 |
|
| 10.2 |
|
Conditions to Seller’s Obligations |
|
|
49 |
|
| 10.3 |
|
Conditions to Buyer’s Obligations |
|
|
50 |
|
| 10.4 |
|
Frustration of Closing Conditions |
|
|
50 |
|
|
|
| ARTICLE XI TERMINATION |
|
|
51 |
|
|
|
|
| 11.1 |
|
Termination Prior to the Closing |
|
|
51 |
|
| 11.2 |
|
Procedure and Effect of Termination |
|
|
52 |
|
|
|
| ARTICLE XII INDEMNIFICATION |
|
|
52 |
|
|
|
|
| 12.1 |
|
Survival; Effect of Materiality Qualifiers; Losses |
|
|
52 |
|
| 12.2 |
|
Indemnification by Seller |
|
|
53 |
|
| 12.3 |
|
Indemnification by Buyer |
|
|
54 |
|
| 12.4 |
|
Third Party Claims |
|
|
55 |
|
| 12.5 |
|
Direct Claims |
|
|
57 |
|
| 12.6 |
|
Set-Off |
|
|
57 |
|
| 12.7 |
|
Adjustment to Losses |
|
|
57 |
|
| 12.8 |
|
Characterization of Indemnification Payments |
|
|
57 |
|
| 12.9 |
|
Exclusive Remedy |
|
|
57 |
|
| 12.10 |
|
Satisfaction of Indemnification Claims |
|
|
58 |
|
|
|
| ARTICLE XIII MISCELLANEOUS |
|
|
58 |
|
|
|
|
| 13.1 |
|
Assignment |
|
|
58 |
|
| 13.2 |
|
Public Announcements |
|
|
58 |
|
| 13.3 |
|
Confidentiality |
|
|
59 |
|
| 13.4 |
|
Expenses |
|
|
61 |
|
| 13.5 |
|
Severability |
|
|
61 |
|
| 13.6 |
|
No Third-Party Beneficiaries |
|
|
61 |
|
| 13.7 |
|
Waiver |
|
|
61 |
|
| 13.8 |
|
Governing Law |
|
|
61 |
|
| 13.9 |
|
Consent to Jurisdiction; Venue; Waiver of Jury Trial |
|
|
62 |
|
| 13.10 |
|
Specific Performance |
|
|
62 |
|
| 13.11 |
|
Representation by Counsel |
|
|
62 |
|
| 13.12 |
|
English Language |
|
|
63 |
|
| 13.13 |
|
Bulk Transfers |
|
|
63 |
|
| 13.14 |
|
Notices |
|
|
63 |
|
| 13.15 |
|
Counterparts; Signature Pages |
|
|
64 |
|
| 13.16 |
|
Entire Agreement; Amendment |
|
|
64 |
|
-iii-
SCHEDULES
Schedule 1.1(A) – Expert Dispute Resolution
Schedule
1.1(B) – Permitted Liens
Schedule 2.2(a) – TATCIST Study Protocol
Schedule 3.1(d) – Transferred Regulatory Materials
Schedule
3.1(j) – Other Acquired Assets
Schedule 3.11 – Patent at Issue
Schedule 7.9(a) – Licensed Manufacturing Know-How
EXHIBITS
Exhibit A – Bill of Sale
and Assignment and Assumption Agreement
Exhibit B – Compound
Exhibit C – Data Package
-iv-
Exhibit 10.1
OPTION AND ASSET PURCHASE AGREEMENT
This Option and Asset Purchase Agreement (this “Agreement”), dated as of November 14, 2022, is entered into by and
between Fusion Pharmaceuticals Inc., a Canadian federal corporation (“Buyer”), and RadioMedix, Inc., a Texas corporation (“Seller”). Seller and Buyer are
sometimes referred to in this Agreement collectively as the “Parties” and individually as a “Party”.
WHEREAS, Seller is engaged in, among other things, the Business;
WHEREAS, upon the terms and subject to the conditions set forth herein, Buyer desires to acquire from Seller an exclusive option to purchase,
at the Closing, the Acquired Assets (the “Option”), upon the terms and conditions set forth herein, and in connection therewith, if the Option is exercised, the Parties wish to enter into the additional transactions contemplated by
this Agreement and by the Ancillary Agreements (collectively, the “Transactions”);
WHEREAS, Seller is willing to grant
the Option to Buyer for an option fee of Seven Hundred and Fifty Thousand Dollars ($750,000) (the “Option Fee”), which will be paid by Buyer to Seller in accordance with the terms and conditions set forth herein; and
WHEREAS, if the Option is exercised by Buyer, at the Closing, Seller shall sell, transfer and deliver to Buyer, and Buyer shall purchase from
Seller, the Acquired Assets, and Buyer shall assume the Assumed Liabilities, each upon the terms and conditions set forth herein.
NOW,
THEREFORE, in consideration of the premises and mutual covenants, agreements and provisions herein contained, the Parties agree as follows:
ARTICLE I
DEFINITIONS
1.1 Definitions. In addition to the terms defined above and other terms defined in other Sections of this Agreement, the
following capitalized terms have the following meanings when used herein:
“Additional Site Dosing” means the dosing of a
patient in the TATCIST Study with the Compound at a clinical site at which no TATCIST Study patient has previously received a dose of the Compound.
“Adverse Law or Order” means (a) any Law enacted, promulgated or enforced by any Governmental Authority of competent
jurisdiction which prohibits or makes illegal the consummation of the Transactions or (b) any Order preventing the consummation of the Transactions, whether preliminary or final.
“Affiliate” means, with respect to any Person, any other Person which, at
the time of determination, directly or indirectly controls, is controlled by, or is under common control with, such first Person. For purposes of this definition, “control” (including, with correlative meanings, the terms
“controlled by” and “under common control with”), as used with respect to any Person, means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such Person,
whether through the ownership of voting securities, by Contract or otherwise. Excel Diagnostics and Nuclear Oncology Center are each an “Affiliate” of Seller for all purposes of this Agreement. Notwithstanding the foregoing, as it pertains
to the representations and warranties in Article V of this Agreement and the covenants contained in Section 8.3 of this Agreement, “Affiliate” of Seller shall be limited to Excel Diagnostics and Nuclear
Oncology Center and any Subsidiaries of Seller. Notwithstanding the foregoing, during any period in which any class of Buyer’s equity securities are listed on one or more stock exchanges, no Person shall be deemed an “Affiliate” of
Buyer solely due to such Person’s ownership of securities of Buyer, except if such Person owns, directly or indirectly, a majority of the outstanding voting securities of Buyer.
“Ancillary Agreements” means the Transfer Documents, the Clinical Trial Agreement, the Manufacturing Agreement and the
Quality Agreement.
“Annual Net Sales” means the total, aggregate Net Sales of all Products by all Payment Obligors in a
particular calendar year.
“Bill of Sale and Assignment and Assumption Agreement” means a bill of sale and assignment and
assumption agreement to be entered into between Seller and Buyer at the Closing substantially in the form of Exhibit A.
“Books and Records” means original or, if originals do not exist, true and complete copies, of all books, records, files
(including data files), work papers and other documents, including (a) supplier lists, purchase orders and invoices, inventory records, quality control records and manuals, and product development, stability, clinical study and manufacturing
processes records; (b) all files relating to the filing, prosecution, issuance, maintenance, enforcement or defense of any intellectual Property; (c) records (including trial master file, case report forms and all other clinical study
documentation), files (including data files, PSMA PET, CT/MRI and bone imaging data and DICOM imaging files) and documents related to research, pre-clinical and clinical testing and studies (including in
vivo and in vitro studies), including pre-clinical data files arising out of the development of compounds or any precursors or derivatives thereof, laboratory notebooks, dosage information, safety
and efficacy and study protocols; (d) investigators brochures and (e) all pharmacovigilance and other safety records, in each case in all forms, including electronic, in which they are stored or maintained.
“Business” means that portion of the business of Seller and its Affiliates consisting of researching, developing and
manufacturing any Compound or Product.
“Business Day” means any day other than (a) a Saturday or Sunday, or
(b) a day on which commercial banks in New York, NY; Ontario, Canada; or Houston, Texas are authorized or required to remain closed.
“Cap” means the sum of $[***] and [***]% of the Deferred Fixed Payments actually paid upon achievement of the Development and
Regulatory Events.
“Claim” means any claim, demand, cause of
action, chose in action, suit, litigation, Proceeding, arbitration, hearing or investigation.
-2-
“Code” means the means the United States Internal Revenue Code of 1986, as
amended.
“Combination Product” means (a) a product that contains the Compound as a therapeutically active
ingredient and at least one (1) other therapeutically active ingredient that is not the Compound; or (b) a product consisting of one or more separate drugs, devices, tests, kits or biological products and sold together with a Product in a
single package or as a unit at a single price.
“Competing Product” means any
Ac-225 labeled PSMA ligand.
“Compound” means that which is described in
Exhibit B.
“Confidentiality Agreement” means that certain Confidentiality Agreement, dated February 15,
2022, by and between the Parties.
“Consent” means any consent, approval, ratification, authorization, waiver, grant,
agreement, license, certificate, exemption, order, registration, declaration, filing or notice of, with or to any Person.
“Contract” means any contract, agreement, lease, instrument, note, indenture, license or sublicense, or other legally binding
commitment. “Contractual” has the correlative meaning.
“Controlled” means, with respect to any Know-How and Information the possession of the right, whether directly or indirectly, and whether by license, covenant not to sue or otherwise, to grant a license, sublicense or other right (including a covenant not
to sue) to or under such Know-How and Information as provided for herein without violating the terms of any agreement with any Third Party.
“Cover”, “Covering” or “Covered” means, with respect to a Valid Claim of a patent and a
compound or product, that the manufacture, use, offer for sale, sale or importation of such compound or product would infringe a Valid Claim of such patent in the country in which such activity occurred.
“Deductible” means $[***].
“Deferred Variable Payment Term” means, subject to Section 3.11(d), the time period beginning on
the First Commercial Sale of a Product anywhere in the Territory following the Closing and ending on the [***]; provided that if FDA grants Buyer or any of its Affiliates [***] for the Compound under U.S. 21 C.F.R. § [***], the Deferred
Variable Payment Term will expire [***].
“Development and Regulatory
Events” means Deferred Fixed Payment Events (1) through (4).
-3-
“Direct Claim” has the meaning set forth in
Section 12.5.
“Distributor” means any Person appointed by a Payment Obligor to distribute,
market and sell any Product with or without packaging rights, in one or more countries in the Territory, in circumstances where such Person purchases its requirements of Product from a Payment Obligor but does not otherwise make any royalty or other
payment to such Payment Obligor with respect to its intellectual property rights with respect to such Product.
“EMA”
means the European Medicines Agency and any successor agency thereto.
“FDA” means the U.S. Food and Drug Administration
and any successor agency thereto.
“FDCA” means the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et
seq.), as amended from time to time, and any successor statute.
“Final Determination” means any final
determination of liability in respect of a Tax that, under applicable Law, is not subject to further appeal, review or modification through Proceedings or otherwise, including the expiration of a statute of limitations or a period for the filing of
claims for refunds, amended Tax Returns or appeals from adverse determinations including, for the avoidance of doubt, in the case of United States federal Taxes, a “determination” as defined in Section 1313(a) of the Code or execution
of an IRS Form 870-AD (or successor form).
“First Commercial Sale” means the
first invoiced sale by a Payment Obligor to a Third Party of any Product for therapeutic use after obtaining all Regulatory Approvals therefor in and for the country in which such sale occurred, including, where required by applicable Law, pricing
and reimbursement approval; provided, that any disposal or transfer of Product for any compassionate use or named patient access program or other similar programs prior to receipt of pricing or reimbursement approvals shall not constitute
“First Commercial Sale”.
“Fraud” means (i) actual fraud with intent to deceive (as determined under
Delaware law) by Seller perpetrated by a breach or inaccuracy of any representation or warranty set forth in Article V or in the certificate delivered by Seller pursuant to Section 10.3(d)(ii) or (ii) the actual
fraud with intent to deceive (as determined under Delaware law) by the Buyer perpetrated by a breach or inaccuracy of any representation or warranty of the Buyer set forth in Article VI or in the certificate delivered by Buyer pursuant to
Section 10.2(c)(ii) (and, in each case, for the avoidance of doubt does not include any fraud based on negligent misrepresentation, recklessness or a similar theory).
“GAAP” means generally accepted accounting principles, as applied in the United States.
“GCP” means the current standards for the design, conduct, performance, monitoring, auditing, recording, analysis and
reporting of clinical trials required by FDA or any other applicable U.S. Governmental Authority, including U.S. 21 C.F.R. §§ 11, 50, 54, 56, 312 and 314, Directive 2005/28/EC laying down principles and detailed guidelines for good
clinical practice, the ICH Guideline for Good Clinical Practice (E6), and the World Medical Association Declaration of Helsinki entitled “Ethical Principles for Medical Research Involving Human Subjects”, each as amended from time to time.
-4-
“GMP” means the current Good Manufacturing Practices officially published
and interpreted by the EMA, FDA and other applicable Regulatory Authorities that may be in effect from time to time and are applicable to the Manufacture of the Compound.
“Governmental Authority” means (a) any nation or government, including any federal, state, local or non-U.S. international or supranational organization or body thereof, municipality, principality, commonwealth, province, territory, county, district or other jurisdiction of any nature or other political
subdivision thereof in which Buyer, Seller, the Compound or any Product is subject to its jurisdiction; or (b) any entity, department, commission, bureau, agency, authority, board, court, arbitral tribunal, official or officer, U.S. or non-U.S., exercising executive, judicial, regulatory, administrative, police, military, or taxing governmental functions in which Buyer, Seller, the Compound or any Product is subject to its jurisdiction.
“Governmental Authorizations” means all licenses, consents, permits, certificates, filings, registrations, notifications,
franchises, concessions, authorizations, approvals, ratifications, permissions, clearances, confirmations, exemptions, endorsements, waivers, designations (including orphan drug designations) or qualifications issued, granted, given or otherwise
made available by or under the authority of any Governmental Authority or pursuant to any requirement under the applicable Laws of any Governmental Authority, including INDs but excluding Regulatory Approvals.
“IND” means an investigational new drug application submitted to the FDA pursuant to U.S. 21 C.F.R. § 312 or an
investigational device exemption application submitted to, or considered approved by, the FDA pursuant to U.S. 21 C.F.R. § 812, including any amendments thereto, and any comparable filing(s) with a Governmental Authority outside of the
U.S. for the purpose of obtaining permission to conduct clinical trials, including a clinical trial application.
“Independent
Accountant” means an independent accounting firm that Seller and Buyer shall mutually agree upon, and, if Seller and Buyer cannot agree, mutually chosen by their respective independent accounting firms.
“Information” means either Buyer Information or Seller Information, as applicable.
“Inventory Price” means, with respect to each unit of Inventory to be purchased by Buyer in accordance with
Section 3.1(g), an amount equal to [***].
“Intellectual Property” means intellectual property
or other proprietary rights anywhere in the world, including registered, unregistered, applied for and pending: (a) patents, patent applications, including provisional applications, statutory invention registrations, inventions, discoveries and
invention disclosures (whether or not patented), and related continuations, continuations-in-part, divisions, reissues,
re-examinations, substitutions, and extensions thereof (collectively, “Patent Rights”); (b) Know-How and Information; (c) trademarks, service
marks, trade names, trade dress, corporate names, logos, in each case whether or not registered, together with derivations and combinations thereof, and common law rights
-5-
thereto, and the goodwill associated with the foregoing, and applications (including intent to use applications), registration and renewals of the foregoing (collectively,
“Trademarks”); and (d) published and unpublished works of authorship including computer software programs, applications, source code and object code, and databases, copyrights in and to the foregoing, together with common law
rights and moral rights therein, and any applications and registrations therefor, including extensions, renewals, restorations, reversions, derivatives, translations, localizations, adaptations and combinations of the above.
“IRS” means the United States Internal Revenue Service.
“ITA” means the Income Tax Act (Canada), R.S.C., 1985, c. 1 (5th supplement).
“Know-How and Information” means data (biologic, chemical, CMC, non-clinical, pre-clinical, clinical and otherwise), information, know-how, proprietary information, trade secrets, prototypes,
techniques, research results, inventions, discoveries, other technology (including any proprietary models and any routes and methods for synthesis), procedures, tests, criteria for patient selection, and all other data and information, in each case
whether or not patentable or copyrightable.
“Law” means each provision of any national, territorial, multinational,
supranational, federal, state, provincial, local, municipal or foreign, civil and criminal law, common law, constitution, statute, regulation, legislation, ordinance, Order, code, proclamation, treaty, convention, rule, ruling, directive,
requirement, determination, decision, opinion or interpretation, promulgated, adopted, enacted, implemented, issued, passed, approved, or otherwise put into effect by or under the authority of any Governmental Authority.
“Liability” means any debt, liability, duty or obligation of such Person, whether known or unknown, absolute or contingent,
accrued or unaccrued, matured or unmatured, disputed or undisputed, liquidated or unliquidated, secured or unsecured, joint or several, due or to become due, vested or unvested, executory, determined, determinable or otherwise.
“Lien” means any lien, encumbrance, mortgage, security interest, pledge, conditional sale agreement or other title retention
agreement, or other charge or encumbrance on any property or property interest.
“Liquidation Event” means (i) a
merger or consolidation in which Seller is a constituent party or a Subsidiary of Seller is a constituent party and Seller issues shares of its capital stock pursuant to such merger or consolidation, except any such merger or consolidation involving
Seller or a Subsidiary in which the shares of capital stock of Seller outstanding immediately prior to such merger or consolidation continue to represent, or are converted into or exchanged for shares of capital stock that represent, immediately
following such merger or consolidation, at least a majority, by voting power, of the capital stock of (1) the surviving or resulting corporation; or (2) if the surviving or resulting corporation is a wholly owned subsidiary of another
corporation immediately following such merger or consolidation, the parent corporation of such surviving or resulting corporation; (ii) the sale, lease, transfer, exclusive license or other disposition, in a single transaction or series of
related transactions, by Seller or any Subsidiary of all or substantially all the assets of Seller and its subsidiaries taken as a whole; or (iii) the sale or
-6-
disposition (whether by merger, consolidation or otherwise, and whether in a single transaction or a series of related transactions) of one (1) or more subsidiaries of Seller if
substantially all of the assets of Seller and its subsidiaries taken as a whole are held by such Subsidiary or Subsidiaries, except where such sale, lease, transfer, exclusive license or other disposition is to a wholly owned Subsidiary of Seller,
in each case solely to the extent that the surviving entity in the preceding clause (i) or the acquirer, transferee or licensee, as applicable, in the case of the preceding clause (ii) or (iii), agrees in writing for the benefit of Buyer
to assume this Agreement and all of Seller’s rights and obligations hereunder.
“Litigation Matters” means all
Proceedings that have been or may be asserted by a Third Party against Seller or its Affiliates and the Compound or the TATCIST Study.
“Losses” means damages, Liabilities, losses, payments, fines, fees, penalties, interest, charges, judgments, settlements,
assessments, Taxes, costs and expenses (including reasonable attorneys’, accountants’ and other experts’ fees, and out-of-pocket disbursements, including
reasonable costs and expenses of investigation); provided, however, that Losses shall not include punitive, exemplary or consequential damages (including any such damages based on lost profit, business opportunity, diminution of value
or business multiples), except to the extent required to be paid to a Third Party not affiliated with the applicable Indemnified Party in connection with a Third Party claim.
“made available” when used in Article V means delivered (in physical or electronic form) to Buyer or its legal counsel
or made available in the electronic data room maintained by Digify for Seller in connection with the Transactions (the “Data Site”), in any case, not less than two days prior to the date of this Agreement or, with respect to any
representation or warranty in Article V made as of the Closing Date, not later than the 10th day after the date on which the Option Trigger Notice is given.
“Major European Market” means each of the following: [***].
“Manufacture” and “Manufacturing” means all activities related to the synthesis, making, production,
processing, filling, finishing, packaging, labeling, shipping, and holding of the Compound, any Product, or any intermediate thereof, including process development, process qualification and validation,
scale-up, pre-clinical, clinical and commercial production and analytic development, product characterization, stability testing, quality assurance, and quality control.
“Material Adverse Effect” means any change, effect, development, circumstance, condition, state of facts or occurrence
that [***].
“Net Sales” means [***].
“Order” means any writ, judgment, edict, decree, injunction, ruling, pronouncement, order, determination or other binding
obligation of any Governmental Authority (whether preliminary or final).
-7-
“Organizational Documents” means, with respect to any Person, collectively,
its organizational documents, including any certificate of incorporation, notarial deed of incorporation, certificate of formation, articles of organization, articles of association, business rules and regulations, bylaws, operating agreement,
certificate of limited partnership, partnership agreement, equityholders’ agreement or certificates of existence, or any non-U.S. equivalent of any of the foregoing, as applicable.
“Payment Obligor” means Buyer, its Affiliates, any of their successors, and any transferee, assignee, licensee or sublicensee
(and any of their transferees, assignees, licensees or sublicensees) of rights to develop and commercialize the Compound or any Product. Distributors are not Payment Obligors.
“Permitted Lien” means (a) statutory Liens for Taxes and other governmental charges and assessments that are not yet due
and payable or that are being contested in good faith by appropriate proceedings and for which the appropriate reserves have established in accordance with GAAP, (b) mechanics’, materialmen’s, carriers’, workers’,
repairers’ and similar statutory Liens arising or incurred in the ordinary course of Business, and (c) Liens specifically identified on Schedule 1.1(B).
“Person” means any natural person, individual, corporation (including any non-profit
corporation), partnership, joint venture, limited liability company, estate, trust, cooperative, foundation, society, political party, union, other unincorporated organization, joint stock company or Governmental Authority.
“Personally Identifiable Information” means any information that alone or in combination with other information held by
Seller or any of its Affiliates can be used to specifically identify an individual Person and any individually identifiable health information.
“Phase 2 Clinical Trial” means a human clinical trial of a compound or product that would meet the description in U.S. 21
C.F.R. § 312.21(b), as amended, or ICH Guidelines E8, and is intended to explore a variety of doses, dose response, and duration of effect, and to generate evidence of clinical safety and effectiveness for a particular indication or indications
in a target patient population.
“Pivotal Clinical Trial” means a human clinical trial of a compound or product that
would meet the description in U.S. 21 C.F.R. § 312.21(c), as amended, or ICH Guidelines E8, and is intended to (a) gather the additional information about effectiveness and safety that is needed to evaluate the overall benefit-risk
relationship of the compound or product and to provide an adequate basis for physician labeling, and (b) support Regulatory Approval for such compound or product.
“Post-Closing Tax Period” means any taxable period (or portion thereof) beginning after the Closing Date and ending on or
before [***].
“Pre-Closing Tax Period” means any taxable period (or portion
thereof) ending on or before the Closing Date and any taxable period (or portion thereof) beginning after [***].
-8-
“Privileged Information” means, with respect to each Party, information
regarding such Party or its Affiliates, or any of its operations, assets or liabilities (whether in documents or stored in any other form or known to its employees or agents) that is protected from disclosure pursuant to the attorney-client
privilege, the work product doctrine or another applicable legal privilege, in each case that the other Party or its Affiliates may come into possession of or obtain access to in connection with this Agreement or the Ancillary Agreements.
“Proceeding” or “Proceedings” means any claim, action, arbitration, audit, hearing, inquiry, prosecution,
contest, examination, proceeding, investigation, litigation, suit (whether civil, criminal, administrative, or investigative or appellate proceeding) commenced, brought, conducted, or heard by or before any Governmental Authority, arbitrator or
arbitration panel, or commenced or brought by any other Third Party.
“Product” means any product that contains or is
comprised of the Compound, including all formulations, modes of administration and dosage forms thereof.
“Regulatory
Approvals” means all permits, licenses, registrations and authorizations of the applicable Regulatory Authority, including approvals by FDA of new drug applications (as defined in U.S. 21 C.F.R. § 314.3(b)) and biologics license
applications (as described in U.S. 21 C.F.R. § 601.2), necessary for the marketing, labeling, distribution, commercialization and sale of a pharmaceutical product for a particular indication in a country or other jurisdiction in the world
(including separate pricing or reimbursement approvals, if required by applicable Law). INDs are not Regulatory Approvals.
“Regulatory Authority” means any Governmental Authority that is responsible for issuing technical, medical, scientific,
labeling and similar licenses, registrations, authorizations, permits, certifications, variances, exemptions, orders and approvals necessary for the manufacture, commercialization, labeling, distribution, use, storage, import, export, transport,
marketing or sale of any Product.
“Regulatory Materials” means all submissions, supporting materials, and related
correspondence to or from any Regulatory Authority in support of a Governmental Authorization or Regulatory Approval for the research, development, manufacture, distribution or commercialization of a biological or pharmaceutical product in a
regulatory jurisdiction, and all documents referenced in the complete regulatory chronology for each Governmental Authorization and Regulatory Approval, including all Drug Master Files (as defined in U.S. 21 C.F.R. § 314.420), INDs, pricing and
reimbursement approvals, and any non-U.S. equivalents of any of the foregoing.
“Representatives” means, with respect to any Person, any officers, directors, employees, attorneys, investment bankers,
financial advisors, agents and other representatives of such Person or such Person’s Affiliates.
“Review Board”
means all institutional review boards, privacy boards, data safety monitoring boards, or ethics committees responsible for review, oversight, or approval of any clinical trial involving a Compound or Product in any jurisdiction.
“Sales Events” means Deferred Fixed Payment Events (5) through (8).
-9-
“Seller’s Knowledge” means the actual knowledge of [***] and the
knowledge such Persons would have after performing a reasonably diligent investigation with respect to the relevant matter; provided, however, that, a reasonably diligent investigation shall not include the commissioning of a
“freedom to operate” or similar non-infringement or patent invalidity analysis.
“Subsidiary” means, with respect to any specified Person, any other Person of which such specified Person, directly or
indirectly through one or more Subsidiaries, (a) owns at least 50% of the outstanding equity interests entitled to vote generally in the election of the board of directors or similar governing body of such other Person, or (b) has the
power to generally direct the business and policies of that other Person as a general partner, managing member, manager or in similar capacity.
“TATCIST Study” means the Targeted Alpha Therapy With 225-Actinium-PSMA I&T of
Castration-resISTant Prostate Cancer (TATCIST) Phase 2 Clinical Trial, identified as NCT05219500 as the IND number assigned by the FDA and sponsored by Excel Diagnostics and Nuclear Oncology Center.
“Tax Return” means any return (including estimated returns), declaration, filing, report, claim for refund, information
return or other statement relating to Taxes (whether in tangible, electronic or other form), including any schedule, supplement, appendices and exhibits or attachment thereto and any amendment thereof made, prepared, filed or required to be made,
with any Taxing Authority.
“Taxes” means all U.S. federal, Canadian federal, state, provincial, local and non-U.S. and non-Canadian taxes, assessments, charges, duties, fees, levies or other similar amounts in the nature of a tax payable to a Taxing Authority, including all
income, franchise, profits, capital gains, capital stock, transfer, sales, use, occupation, property, excise, severance, windfall profits, stamp, stamp duty reserve, license, payroll, withholding, ad valorem, value added, alternative minimum,
environmental, customs, customs duties and import and export taxes, social security (or similar), unemployment, employment insurance, health insurance, sick pay, disability, registration, escheat, unclaimed property and other taxes, assessments,
charges, duties, fees, levies or other similar amounts and Canada, Quebec and other government pension plan premiums or contributions, together with all estimated taxes, deficiency assessments, additions to tax, penalties and interest with respect
thereto.
“Taxing Authority” means any Governmental Authority exercising any authority to impose, regulate or administer
the imposition of, or collect Taxes.
“Territory” means worldwide.
“Third Party” means any Person other than Buyer or Seller that is not an Affiliate of Buyer or Seller.
“Third Party Product” means any product containing or comprised of a 225-Ac-PSMA molecule, inclusive of any competitor molecules, that is not a Product.
“Transaction Proposal” means any inquiry, proposal or offer from any Person (other than Buyer or its Affiliates) with respect
to, or that would reasonably be expected to lead to, any direct or indirect acquisition, sale, transfer or license of the Compound or any Product or any rights (including any right to research, develop, manufacture or commercialize) to the Compound
or any Product or any material part of the Acquired Assets (other than (i) any license or grant of rights permitted to be made under Section 7.1(b), or (ii) a Liquidation Event).
-10-
“Transfer Documents” means, collectively, any and all agreements,
assignments, deeds, notarial forms, certificates and other instruments of sale, conveyance, transfer, assignment or assumption, as the case may be, between Seller or any of its Affiliates and Buyer as necessary under the Law of the relevant
jurisdiction or contemplated by this Agreement in order to transfer all right, title and interest of Seller and its Affiliates in and to the Acquired Assets, and for the Assumed Liabilities to be effectively assumed by and transferred to Buyer, in
accordance with the terms hereof, including the Bill of Sale and Assignment and Assumption Agreement.
“Transfer Taxes”
means any transfer, conveyance, documentary, real estate transfer, mortgage recording, use, stamp, registration, and other similar Taxes and fees (including any penalties and interest in respect thereof) imposed with respect to the Transactions
other than VAT.
“United States” and “U.S.” mean the United States of America (including its territories
and possessions).
“Valid Claim” means a claim of an issued patent that has not expired, lapsed, been cancelled or
abandoned, or been dedicated to the public, disclaimed, or held unenforceable, invalid, revoked or cancelled by a court or administrative agency of competent jurisdiction in an order or decision from which no appeal has been or can be taken,
including through opposition, reexamination, reissue, disclaimer, inter partes review, post grant review, other post grant procedures or similar proceedings.
“VAT” means any sales, use, value added, goods and services, and similar gross margin or turnover Tax incurred or imposed in
respect of the transfer of the Acquired Assets or the grant of the Option pursuant to this Agreement.
The following terms shall have the
meanings assigned to them in the corresponding Section below:
|
|
|
| Term |
|
Section |
| Acquired Assets |
|
3.1 |
| After Acquired Assets |
|
2.4(b) |
| Agreement |
|
Preamble |
| Allocation Schedule |
|
3.9(a) |
| Assumed Liabilities |
|
3.3 |
| Buyer |
|
Preamble |
| Buyer Disclosure Letter |
|
Article VI |
| Buyer Indemnified Parties |
|
12.2 |
| Buyer Information |
|
13.3(d) |
| Buyer Product Information |
|
13.3(d) |
| Claim Notice |
|
12.4(a) |
| Clinical Trial Agreement |
|
7.12 |
-11-
|
|
|
| Closing |
|
4.1 |
| Closing Date |
|
4.1 |
| Data Package |
|
2.4(b) |
| Data Site |
|
Definitions |
| Deferred Fixed Payment |
|
3.8(a) |
| Deferred Fixed Payment Event |
|
3.8(a) |
| Deferred Variable Payment |
|
3.8(b) |
| Development Report |
|
3.8(d) |
| Direct Claim |
|
12.5 |
| Excluded Assets |
|
3.2 |
| Excluded Liabilities |
|
3.4 |
| Fundamental Representations |
|
12.1 |
| Health Care Laws |
|
5.8 |
| Indemnified Party |
|
12.3(a) |
| Indemnifying Party |
|
12.4(a) |
| Invalidation Date |
|
3.11(d)(ii) |
| Licensed Manufacturing Know-How |
|
7.9(a) |
| Manufacturing Agreement |
|
7.8 |
| Option |
|
Recitals |
| Option Exercise Price |
|
4.2(b) |
| Option Exercise Notice |
|
2.4(c) |
| Option Fee |
|
Recitals |
| Option Period |
|
2.4(c) |
| Option Trigger |
|
2.4(b) |
| Option Trigger Notice |
|
2.4(b) |
| Other Licensed Know-How |
|
8.8 |
| Outside Date |
|
11.1(b) |
| Parties or Party |
|
Preamble |
| Patent at Issue |
|
3.11(a) |
| Permitted Uses |
|
13.3(b) |
| Pre-Closing Period |
|
7.1(a) |
| Pre-Commercial Termination |
|
8.7(b) |
| Proceeds |
|
12.7(a) |
| Prohibited Information |
|
3.2(j) |
| Purchase Price |
|
3.7 |
| Quality Agreement |
|
7.8 |
| Restrictive Covenants |
|
9.5 |
| [***] |
|
8.7(b) |
| Seller |
|
Preamble |
| Seller Disclosure Letter |
|
Article V |
| Seller Indemnified Parties |
|
12.3(a) |
| Seller Information |
|
13.3(g) |
| Straddle Period |
|
9.4 |
-12-
|
|
|
| Study Termination Event |
|
2.2(b) |
| TATCIST Study Protocol |
|
2.2(a) |
| Termination-Period Exercise |
|
11.2 |
| Third Party Claim |
|
12.4(a) |
| Third Party Grantee |
|
3.8(c) |
| Transactions |
|
Recitals |
| Transferred Books and Records |
|
3.1(e) |
| Transferred Governmental Authorizations |
|
3.1(c) |
| Transferred Know-How |
|
3.1(b) |
| Transferred Regulatory Materials |
|
3.1(d) |
1.2 Construction. The table of contents, articles, titles and headings to sections herein are inserted
for convenience of reference only and are not intended to be part of or to affect the meaning or interpretation of this Agreement. Unless expressly specified otherwise, whenever used in this Agreement, the terms “Article,”
“Exhibit,” “Schedule” and “Section” refer to articles, exhibits, schedules and sections of this Agreement (and, for the avoidance of doubt, do not refer to appendices, articles, sections, schedules and exhibits of any
Ancillary Agreement). Whenever used in this Agreement, the terms “hereby,” “hereof,” “herein” and “hereunder” and words of similar import refer to this Agreement as a whole, including all articles, sections,
schedules and exhibits hereto. Whenever used in this Agreement, the terms “include,” “includes” and “including” mean “include, without limitation,” “includes, without limitation” and “including,
without limitation,” respectively. The word “or” is not exclusive, and as used in this Agreement shall mean “and/or”. Whenever the context of this Agreement permits, the masculine, feminine or neuter gender, and the singular
or plural number, are each deemed to include the others. “Days” means calendar days unless otherwise specified. Unless expressly specified otherwise, all payments to be made in accordance with or under this Agreement (or any Ancillary
Agreement) shall be made in U.S. dollars. References in this Agreement to particular sections of a Law shall be deemed to refer to such sections or provisions as they may be amended after the date of this Agreement. Unless otherwise specified in
this Agreement, in computing any period of time described in this Agreement, the date that is the reference date in calculating such period, or the day of the act or event after which the designated period of time begins to run, will be excluded,
and the last day of the period so computed will be included. The Parties have participated jointly in the negotiation and drafting of this Agreement and in the event an ambiguity or question of intent or interpretation arises, this Agreement shall
be construed as if drafted jointly by the Parties and no presumption or burden of proof shall arise favoring or disfavoring any Party (or any Affiliate thereof) by virtue of the authorship of any of the provisions of this Agreement.
1.3 Performance of Obligations by Affiliates. Any obligation of Seller under or pursuant to this Agreement may be satisfied, met or
fulfilled, in whole or in part, at Seller’s sole and exclusive option, either by Seller directly or by any Affiliate of Seller that Seller causes to satisfy, meet or fulfill such obligation, in whole or in part; provided, that no such
action shall relieve Seller of any obligation under this Agreement. Any obligation of Buyer under or pursuant to this Agreement may be satisfied, met or fulfilled, in whole or in part, at Buyer’s sole and exclusive option, either by Buyer
directly or by any Affiliate of Buyer that Buyer causes to satisfy, meet or fulfill such obligation, in whole or in part; provided, that no such action shall relieve Buyer
-13-
of any obligation under this Agreement. With respect to any particular action, the use of the words “Seller shall” and “Seller agrees to,” and any similar variation with
respect to any action, also means “Seller shall cause” the particular action to be performed, and the use of the words “Buyer shall,” “Buyer agrees to” and any similar variation with respect to any action, also means
“Buyer shall cause” the particular action to be performed, irrespective of whether an Affiliate is referenced in that particular instance, because Seller and Buyer each understand, agree and acknowledge that they are entering into this
Agreement on behalf of themselves and certain of their respective Affiliates. In case Seller undertakes any actions, agreements and obligations that should be performed by any Affiliates of Seller under the terms and conditions of this Agreement,
Seller shall be deemed to be acting on behalf of such Affiliates.
ARTICLE II
OPTION GRANT AND EXERCISE
2.1 Grant of Option.
(a)
Seller hereby grants to Buyer, and Buyer hereby accepts the grant from Seller of, the Option, in exchange for the consideration set forth in Section 2.1(b).
(b) In consideration for the grant of the Option, Buyer shall promptly, and in any event within [***], following the execution and delivery of
this Agreement, deposit, or cause to be deposited, with Seller by wire transfer of immediately available funds to the account designated by Seller on the date hereof, an amount in cash equal to the Option Fee.
2.2 TATCIST Study.
(a)
During the Pre-Closing Period, Seller and its Affiliates shall, subject to Section 2.2(b) below, (i) use commercially reasonable efforts to execute the TATCIST Study in
accordance with the expected clinical timelines made available to Buyer prior to the date hereof and fund all aspects to the TATCIST Study, including the potential expansion of such study to additional clinical sites and the manufacturing
responsibilities for such study, and shall not decrease the level of funding below that which is required to progress the TATCIST Study in the ordinary course and in accordance in all material respects with the study protocol made available to Buyer
prior to the date hereof and attached as Schedule 2.2(a) (the “TATCIST Study Protocol”); (ii) subject to Section 7.1, retain decision-making rights and responsibilities for the TATCIST Study;
(iii) provide Buyer with biweekly updates on the progress of the TATCIST Study, including clinical and imaging data, for purposes of facilitating Buyer’s decision with respect to the exercise of the Option; (iv) enter all patient data
obtained during a clinical visit by a patient as part of the TATCIST Study into the primary database for such study within five Business Days of such visit; (v) permit representatives of Buyer to review and monitor the source documents
containing TATCIST Study patient data at the trial sites for the TATCIST Study; and (vi) subject to the second sentence of Section 2.2(b), (x) not take, or permit to be taken, any actions designed to avoid or
(y) except to the extent events beyond the control of Seller or any of its Affiliates (and that occur without the fault or as a result of the actions of Seller or any of its Affiliates) necessitate the taking of any such actions, materially
delay, achieving the Option Trigger.
-14-
(b) Seller and its Affiliates shall not have any obligation to expand the TATCIST Study
pursuant to Section 2.2(a) to more than [***] patients who have received at least the [***] dose of the Compound in such study as provided in the TATCIST Study Protocol. Furthermore, Seller and its Affiliates shall not have
any obligation to continue conducting the TATCIST Study if the applicable conditions for termination set forth in Section 6.10 of the TATCIST Study Protocol are met (a “Study Termination Event”). Seller shall notify Buyer
promptly, and in any case within five days, following the occurrence of any Study Termination Event.
(c) Seller and its Affiliates shall
maintain complete and accurate records with respect to the TATCIST Study and their other development and manufacturing activities with respect to the Compound and in accordance with GMPs and GCPs, as appropriate. Such records shall fully and
properly reflect all work done and results achieved in the development and manufacture of the Compound in sufficient detail and in a manner that would reasonably be expected to enable use of such documents to seek or challenge Patent Rights with
respect to the Compound or the manufacture, sale or use thereof or Regulatory Approval of any Product.
(d) Without limiting the generality
of Section 2.2(a), during the Pre-Closing Period, Seller shall keep Buyer informed on a biweekly basis regarding the schedule and process for any submissions to applicable
Governmental Authorities or Review Boards related to the Compound or the TATCIST Study. Seller shall furnish Buyer with drafts of all such submissions at least five Business Days in advance of being filed with or submitted to the applicable
Governmental Authority or Review Board and will consider in good faith any comments Buyer provides to such drafts. Seller will provide Buyer with at least five Business Days’ prior notice (or, such lesser period of notice if Seller is not
provided with five Business Days’ prior notice) of (i) any scheduled meeting (including any advisory committee meetings) with any Regulatory Authority relating to the Compound and (ii) any inspection by any Regulatory Authority of
Seller or any Third Party contracted by Seller relating to the Compound, or any facility at which the Compound (or any component thereof) is manufactured, and, in each case ((i) and (ii)), to the extent permitted by applicable Law, permit
representatives of Buyer to be present at, and participate in, such meeting or inspection. Seller shall also provide to Buyer, promptly, and in any event within 10 Business Days following Seller’s or any of its Affiliates’ receipt
thereof (A) written copies of any meeting minutes or other written records of any meetings, conferences, or discussions (including any advisory committee meetings) with any Governmental Authority or Review Board relating to the Compound,
(B) written inspectional observations (e.g., observations on Form FDA 483) that the FDA or any other Governmental Authority issues to Seller, any of its Affiliates or any Third Party contracted by Seller as a result of any such inspection
relating to the Compound or any facility at which the Compound, (or any component thereof) is manufactured and (C) other material correspondence or communications received from any Governmental Authority or Review Board relating to the Compound
(including, with respect to any written correspondence or communication, a copy thereof). Seller shall further provide to Buyer, within twenty-four (24) hours, following Seller’s or any of its Affiliates’ receipt thereof of a partial
or full clinical hold imposed upon the TATCIST Study by any Regulatory Authority.
-15-
(e) Except as required by Law or legal or administrative process to be disclosed or as
otherwise contemplated in Section 2.2(d), neither Seller nor any of its Affiliates shall publish or otherwise publicly disclose or present or submit for publication or other public disclosure, including by way of
manuscripts, articles, posters or abstracts, whether orally or in writing, any data arising out of the TATCIST Study or the research or development of the Compound without (i) the prior written consent of Buyer and (ii) first providing
Buyer a reasonable opportunity for not less than [***] to comment on any proposed publication or other public disclosure and implementing any reasonable comments provided by Buyer thereon.
(f) During the Pre-Closing Period, Buyer shall have the right to disclose data arising out of the
TATCIST Study to its current and potential future investors; provided, that all such disclosures shall be made pursuant to the terms of a customary nondisclosure agreement between Buyer and the investor or potential investor receiving such data.
2.3 Additional Site Payments(a) . During the Pre-Closing Period, Seller shall notify Buyer
promptly following the occurrence of each Additional Site Dosing and provide Buyer with reasonably satisfactory evidence of such Additional Site Dosing. Buyer shall pay to Seller, by wire transfer of immediately available funds to the account
designated by Seller, [***] ($[***]) in respect of each Additional Site Dosing, up to a maximum of [***] Additional Site Dosings, within [***] following Buyer’s receipt of Seller’s notice of the related Additional Site Dosing.
2.4 Exercise of Option.
(a) Buyer shall have the right to exercise the Option at any time from the date hereof through the expiration of the Option Period and as
provided in Section 11.2. Buyer’s right to exercise the Option hereunder shall not be contingent upon, and may be exercised prior to, achievement of the Option Trigger or delivery of the Option Trigger Notice by
Seller.
(b) Seller shall provide prompt written notice to Buyer following (and in any event within [***] after) the first date on which
Seller has received all of the data identified on Exhibit C (the “Data Package”) for each of [***] patients in the TATCIST Study who each have received [***] doses of the Compound (the “Option Trigger”),
together with copies of the complete Data Package for each such patient (collectively, the “Option Trigger Notice”). Within [***] after its delivery of the Option Trigger Notice, or, if Buyer delivers an Option Exercise Notice prior
to the Option Trigger, within [***] after Buyer’s delivery of such Option Exercise Notice, Seller (i) may deliver to Buyer an updated version of the Seller Disclosure Letter and (ii) shall deliver to Buyer (A) a materially
complete and correct list of all Intellectual Property, Contracts and other assets related to the Compound or the development, manufacture or commercialization thereof obtained or entered into by Seller or any of its Affiliates after the date of
this Agreement and not previously disposed of or consumed in the TATCIST Study (the “After Acquired Assets”) and (B) a complete and correct listing of all Inventory eligible for purchase by Buyer, along with the applicable
manufacturing cost for each unit of such Inventory. If Seller fails to deliver to Buyer an updated version of the Seller Disclosure Letter within [***] after its delivery of the Option Trigger Notice or, if Buyer delivers an Option Exercise Notice
prior to the Option Trigger, within [***] after Buyer’s delivery of such Option Exercise Notice, the version of the Seller Disclosure Letter delivered by Seller immediately prior to the execution of this Agreement shall be the Seller Disclosure
Letter for all purposes hereunder. If Buyer delivers an Option Exercise Notice prior to the Option Trigger, Seller shall deliver to Buyer copies of the complete Data Package (or as much of the Data Package as then exists) for each patient in the
TATCIST Study within [***] after Buyer’s delivery of such Option Exercise Notice.
-16-
(c) Buyer shall have a period of [***] after delivery of the Option Trigger Notice (such
period, as it may be extended below, the “Option Period”) to determine whether to exercise the Option. For clarity, the Option Period shall expire immediately upon expiration or termination of this Agreement. In the event that Buyer
determines to exercise the Option, Buyer shall deliver written notice of its intention to exercise the Option (the “Option Exercise Notice”) to Seller no later than the expiration of the Option Period. Buyer shall be under no
obligation to deliver an Option Exercise Notice, and if Buyer does deliver an Option Exercise Notice, its obligation to effect the Closing shall remain subject to the satisfaction of the conditions set forth in Sections 10.1 and 10.3.
During the Option Period, Seller shall provide Buyer with all information in Seller’s and its Affiliates’ possession that is reasonably requested by Buyer and that is relevant to its decision whether to exercise the Option; provided
that Buyer shall have requested any data missing from the Data Package promptly upon discovering any such data was not delivered. If Seller fails to provide material portions of any such information prior to the [***] preceding the last day of the
Option Period, the Option Period shall be extended until the date that is [***] after Buyer receives such requested information; provided, however, that in no event will the Option Exercise Period be extended beyond the [***] following
the date on which the Option Trigger Notice is delivered if all data within the Data Package for at least [***] patients in the TATCIST Study who each have received [***] doses of the Compound has been delivered to Buyer.
ARTICLE III
PURCHASE
AND SALE
3.1 Agreement to Purchase and Sell. If the Option is exercised by Buyer, at the Closing, in accordance with and
pursuant to the terms and conditions of this Agreement, for the consideration specified in Section 3.7, Seller shall sell, transfer, convey, assign and deliver to Buyer, and Buyer shall purchase and accept from Seller, all
right, title and interest of Seller or any of its Affiliates, as of the Closing, in and to the following assets, properties, rights and interests (collectively, the “Acquired Assets”) free and clear of all Liens, other than
Permitted Liens:
(a) the TATCIST Study;
(b) all Know-How and Information [***] related to 225-Ac-PSMA or the Compound, including all radiolabeled complexes thereof, but excluding the Licensed Manufacturing Know-How (the “Transferred Know-How”);
(c) all Governmental Authorizations [***] related to the Compound or the TATCIST
Study (“Transferred Governmental Authorizations”);
(d) all Regulatory Materials [***] related to the Compound, 225-Ac-PSMA or the TATCIST Study, including all materials set forth on Schedule 3.1(d) (the “Transferred Regulatory Materials”);
(e) all Books and Records [***] related to the Compound or
225-Ac-PSMA (including the Books and Records evidencing the Transferred Know-How) (the “Transferred Books and
Records”);
-17-
(f) all rights, claims or causes of action against Third Parties [***] related to the
Compound, the TATCIST Study, the Acquired Assets or the Assumed Liabilities;
(g) the inventories of the Compound (if any) designated by
Buyer in writing not less than five Business Days prior to the Closing Date (the “Inventory”);
(h) any license agreement entered into by Seller or any of its Affiliates in accordance with Section 3.11(a);
(i) all After Acquired Assets that Buyer notifies Seller, no later than the [***] prior to the Closing Date, shall be Acquired Assets; and
(j) all other assets, properties and rights set forth on Schedule 3.1(j), which Schedule may be updated by the mutual written consent of
the Parties no later than the [***] prior to the Closing Date.
Seller shall deliver to Buyer the
Acquired Assets as soon as reasonably possible, but in any event within the later of [***] after the Closing Date and [***] after the Option Exercise Notice is delivered, provided that (x) if Seller requests additional time to transfer
any specific Acquired Assets, Buyer’s approval of such request shall not be unreasonably withheld, delayed, or conditioned and (y) notwithstanding the foregoing, all INDs within the Acquired Assets shall be transferred to Buyer within the
later of (1) [***] following Buyer’s request for such transfer and (2) [***] following the Closing Date. If the Option is exercised, the Parties will discuss and agree upon reasonable security measures in compliance with all applicable Laws
prior to transmitting any Personally Identifiable Information that is included in the Acquired Assets. All Acquired Assets shall be delivered to Buyer or its designated Affiliate at [***] cost and expense. It is explicitly understood and agreed to
that nothing herein shall relieve Seller of its data retention responsibilities and obligations under relevant GCP and GMP practices.
3.2
Excluded Assets. Notwithstanding anything to the contrary in this Agreement, Seller shall not sell, transfer, convey, assign or deliver, and Buyer shall not purchase, accept or otherwise acquire, any right, title or interest in any assets,
properties, rights or interests of Seller or any of Seller’s Affiliates other than the Acquired Assets (the “Excluded Assets”), which include the following:
(a) rights of Seller arising under this Agreement or the Ancillary Agreements or from the consummation of the Transactions;
(b) all Tax refunds, credits, offsets, rebates, recoveries, credits of Taxes, Tax losses, loss and credit carry-forwards and similar benefits
related to the Acquired Assets for a Pre-Closing Tax Period or relating to the other Excluded Assets for all periods;
(c) all Tax Returns, as well as other Tax data and records, or any Tax Return of Seller or any of its Affiliates relevant in each case to Pre-Closing Tax Periods;
(d) all accounts receivable and rights of Seller or its Affiliates relating to
deposits and prepaid expenses, claims for refunds and rights to offset in respect thereof;
-18-
(e) cash, cash equivalents, bank accounts, bank deposits and marketable securities on hand
and in transit of Seller or any of its Affiliates;
(f) Trademarks owned or held for use by Seller;
(g) the corporate books and records of Seller and its Affiliates and all books and records related to the employees of Seller and its
Affiliates;
(h) all current and prior insurance policies, and all rights of any nature with respect thereto, including all insurance
recoveries thereunder and rights to assert claims with respect to any such insurance recoveries;
(i) all employees and all employee
benefit plans or programs of Seller or any of its Affiliates;
(j) all information of Seller or any of its Affiliates that is protected
from disclosure pursuant to the attorney-client privilege, the work product doctrine or another applicable legal privilege to the extent arising out of the negotiation of this Agreement and the Ancillary Agreements or the consummation of the
Transactions (“Prohibited Information”);
(k) any equity or other interest in Excel Diagnostics and Nuclear Oncology
Center; and
(l) all real property owned by Seller or its Affiliates, and all leasehold interests in real property, which interests are
held by Seller or its Affiliates.
3.3 Assumed Liabilities. At the Closing, in accordance with and pursuant to the terms and
conditions of this Agreement, Buyer shall assume and agree to satisfy and discharge the following, and only the following, Liabilities (collectively, the “Assumed Liabilities”):
(a) all Liabilities arising out of the ownership or use of, or Buyer’s or any of its Affiliates’ performance under, any Acquired
Asset, to the extent (i) first arising or accruing after the Closing and [***] and (ii) not arising out of or accruing as a result of any facts or occurrences existing prior to or at the Closing and [***], including such liabilities
arising out of the TATCIST Study, including any failure to perform or other breach, default or violation by Seller or any of its Affiliates of any Contract or applicable Law; and
(b) all Liabilities for Taxes (other than Transfer Taxes) attributable to the Acquired Assets for any Post-Closing Tax Period, to the extent
that such Liabilities do not relate to any failure to perform or other breach, default or violation by Seller or any of its Affiliates prior to or at the Closing.
3.4 Excluded Liabilities. Neither Buyer nor any of its Affiliates shall assume, nor shall they be or become responsible for, any
Liabilities of Seller or of any of Seller’s Affiliates other than the Assumed Liabilities (collectively, the “Excluded Liabilities”). Without limiting the generality of the foregoing, the following shall constitute the Excluded
Liabilities notwithstanding any other provision of this Agreement:
(a) all Liabilities for (i) Transfer Taxes and (ii) Taxes
(other than Transfer Taxes) attributable to the Acquired Assets for any Pre-Closing Tax Period or any period after [***], including any Taxes allocated to a Pre-Closing
Tax Period pursuant to Section 9.4;
-19-
(b) all Liabilities with respect to employees of Seller or any of its Affiliates or any
employee benefit plans or programs of Seller or any of its Affiliates;
(c) all Liabilities related to any Excluded Asset;
(d) all Liabilities arising out of the ownership or use of, or Seller’s or any of its Affiliates’ performance under, any Acquired
Asset, to the extent arising or occurring (i) prior to or at the Closing or [***] or (ii) as a result of any facts or occurrences existing prior to or at the Closing or [***], including any failure to perform or other breach, default or
violation by Seller or any of its Affiliates of any Contract or applicable Law prior to or at the Closing or [***]; and
(e) all
Liabilities arising out of (i) the TATCIST Study to the extent arising or occurring prior to or at the Closing or arising out of or occurring as a result of any facts or occurrences existing prior to or at the Closing or (ii) any other pre-clinical or clinical trial with respect to the Compound that was commenced prior to the Closing, including, in each case, all Liabilities for product liability claims with respect to any patient first dosed with
the Compound or any Product prior to the Closing.
3.5 Procedures for Assignments.
(a) Notwithstanding anything to the contrary contained herein, this Agreement shall not constitute an agreement to assign or transfer any
Acquired Asset (or right, benefit or obligation thereunder or resulting therefrom) if an assignment or transfer thereof, without the Consent of a Person, would constitute a breach or violation thereof and such Consent is not obtained at or prior to
the Closing. Seller shall use commercially reasonable efforts to obtain all such Consents prior to the Closing. If Seller is not successful in obtaining any such Consent at or prior to the Closing, then the Parties agree that, following the Closing,
Seller will use its commercially reasonable efforts, at the sole cost and expense of Seller to obtain such Consent. Without limiting the representations and warranties of Seller set forth in Section 5.3 and
Section 5.4, the fact of a failure to obtain any such Consent shall not result in a breach of this Agreement in any manner.
(b) If Seller is not successful in transferring or assigning any Acquired Asset or right, benefit or obligation thereunder or resulting
therefrom at the Closing in accordance with Section 3.5(a), then following the Closing and pending the receipt of such Consent, the Parties shall cooperate with each other in any mutually agreeable, reasonable and lawful
arrangements designed to provide Buyer with the benefits and burdens of any such Acquired Assets and any right, benefit or obligation thereunder or resulting therefrom as if the appropriate Consent had been obtained, including provision of the
consideration and other economic benefits to be received by Buyer in and under every Acquired Asset and right, benefit or obligation thereunder or resulting therefrom, which consideration shall be held for the benefit of, and shall be promptly
delivered to, Buyer. Once the applicable Consent for the assignment or transfer of any such Acquired Asset not
-20-
assigned or transferred at the Closing is obtained, Seller shall assign and transfer such Acquired Asset to Buyer at no additional cost. To the extent that any such Acquired Asset cannot be
transferred or the full benefits and burdens of use of any such Acquired Asset cannot be provided to Buyer following the Closing pursuant to this Section 3.5, then Buyer and Seller shall enter into such arrangements
(including subcontracting) to provide to the Parties the economic (considering Tax costs and benefits) and operational equivalent, to the extent permitted, of obtaining such Consent. Seller shall hold in trust for and pay to Buyer promptly upon
receipt thereof, all income, proceeds and other monies received by Seller or any of its Affiliates in connection with the use of any Acquired Asset in connection with the arrangements under this Section 3.5. To the extent
that Buyer is provided the benefits and burdens of any Acquired Asset or right, benefit or obligation thereunder or resulting therefrom referred to herein (whether from Seller or otherwise) as if the appropriate Consent had been obtained, Buyer
shall discharge and perform the Liabilities of Seller thereunder or in connection therewith, as applicable, to the same extent as if the appropriate Consent had been obtained.
3.6 Wrong Pocket Assets.
(a) Subject to Section 3.5, if any Acquired Asset remains vested in Seller or any of its Affiliates following
Closing, Seller shall transfer such Acquired Asset as soon as reasonably practicable to Buyer or its designee for no additional consideration. Seller shall notify Buyer as soon as reasonably practicable in writing upon becoming aware that that there
are any Acquired Assets in its possession or control or that of any Affiliate of Seller.
(b) If any Excluded Asset is vested in Buyer or
any of its Affiliates following Closing, Buyer shall transfer such Excluded Asset as soon as reasonably practicable to Seller or its designee for no consideration. Buyer shall notify Seller as soon as reasonably practicable in writing upon becoming
aware that that there are any Excluded Assets in its possession or control or that of any Affiliate of Buyer.
3.7 Purchase Price.
(a) In consideration for the sale and transfer of the Acquired Assets, and subject to the terms and conditions of this Agreement, Buyer
shall, in addition to the assumption of the Assumed Liabilities:
(i) at the Closing, pay to Seller an amount equal to the
sum of the Option Exercise Price and, if Buyer has elected to purchase any Inventory, the aggregate Inventory Price;
(ii)
upon the achievement of any Deferred Fixed Payment Event, pay to Seller the corresponding Deferred Fixed Payment in accordance with Section 3.8(a); and
(iii) to the extent payable under Section 3.8(b), pay to Seller the Deferred Variable Payments in
accordance with Section 3.8(b).
The Option Exercise Price, the aggregate Inventory Price and the amounts described in the
foregoing clauses (ii) and (iii), in the aggregate, shall be referred to as the “Purchase Price”.
-21-
3.8 Deferred Payments.
(a) Deferred Fixed Payments.
(i) Deferred Fixed Payments. Subject to the terms of this Section 3.8, upon the first
occurrence of each of the following events following the Closing (each, a “Deferred Fixed Payment Event”), Buyer shall pay to Seller the corresponding amount (each, a “Deferred Fixed Payment”); provided that
in the event [***]:
|
|
|
|
|
|
| Development and Regulatory
Events |
|
|
|
| |
|
Deferred Fixed Payment Event |
|
Deferred Fixed Payment |
|
|
|
| (1) |
|
[***] |
|
$[***] |
|
|
|
| (2) |
|
[***] |
|
$[***] |
|
|
|
| (3) |
|
[***] |
|
$[***] (or $[***] if [***], subject to Section 3.11(d) |
|
|
|
| (4) |
|
[***] |
|
$[***] (or $[***] if [***] |
|
| Sales Events |
|
|
|
| |
|
Deferred Fixed Payment Event |
|
Deferred Fixed Payment |
|
|
|
| (5) |
|
[***] |
|
$[***] |
|
|
|
| (6) |
|
[***] |
|
$[***] |
|
|
|
| (7) |
|
[***] |
|
$[***] |
|
|
|
| (8) |
|
[***] |
|
$[***] |
(ii) Each of the foregoing Deferred Fixed Payments shall be payable a maximum of one
(1) time as set forth in the foregoing charts regardless of (A) the number of Products achieving the applicable Deferred Fixed Payment Event or (B) the number of indications for which a Product achieves the applicable Deferred Fixed
Payment Event, and no Deferred Fixed Payments shall be due hereunder for subsequent or repeated achievement of such Deferred Fixed Payment Events. The Deferred Fixed Payments corresponding to the Sales Events are additive, such that if more than one
Sales Event is achieved in the same calendar year, then each corresponding Deferred Fixed Payment will be payable. The maximum [***] is [***]; provided that the maximum [***] may be increased to [***] pursuant to
Section 3.11(d). The maximum [***] is [***]. The maximum [***]in the aggregate is [***].
(b) Deferred
Variable Payments. Subject to the terms of this Section 3.8 and Section 3.11(d), Buyer shall make payments (each such payment, a “Deferred Variable Payment”) to Seller based on
a percentage of Annual Net Sales of Products in the Territory during the Deferred Variable Payment Term, which Deferred Variable Payments shall be calculated using the applicable percentage rate set forth below:
-22-
|
|
|
|
|
| Annual Net Sales |
|
Rate |
|
| For that portion of Annual Net Sales less than $[***] |
|
|
[ |
***]% |
| For that portion of Annual Net Sales equal to or greater than $[***] and less than or equal to
$[***] |
|
|
[ |
***]% |
| For that portion of Annual Net Sales greater than $[***] and less than or equal to $[***] |
|
|
[ |
***]% |
| For that portion of Annual Net Sales greater than $[***] and less than or equal to $[***] |
|
|
[ |
***]% |
| For that portion of Annual Net Sales greater than $[***] |
|
|
[ |
***]% |
(c) Deferred Variable Payment Rate Reductions. Notwithstanding
Section 3.8(b):
(i) from and after the end of the calendar quarter in which FDA first grants
Regulatory Approval to a Third Party for a Third Party Product, all Deferred Variable Payment rates shall be reduced by [***] percent ([***]%) of the original percentage rate set forth in Section 3.8(b);
(ii) from and after the end of the calendar quarter in which FDA grants Regulatory Approval to a Third Party for a second Third
Party Product (i.e., a Third Party Product in addition to the Third Party Product referenced in the preceding clause (i)), all Deferred Variable Payment rates shall be reduced by an additional [***] percent ([***]%) of the original percentage rate
set forth in Section 3.8(b) (i.e., a [***]% aggregate reduction when combined with the reduction in the preceding clause (i)); and
(iii) subject to Section 3.11(d), if any Payment Obligor in good faith (A) enters into an
agreement with a Third Party in order to obtain a license or other right to a Valid Claim that Covers the making, use or sale of the Compound or any Product in any country in the Territory in order to avoid infringing such Valid Claim, or
(B) pursues any Order that invalidates or narrows the scope of any Valid Claim of a Third Party that Covers the making, use or sale of the Compound or any Product, Buyer shall be entitled to deduct from any Deferred Variable Payments payable
under Section 3.8(b) [***] or in connection with obtaining the Order referred to in the preceding clause (B), up to a maximum of [***];
(d) Payment Terms; Reporting.
(i) Currency and Conversion. All payments under this Agreement shall be in United States Dollars ($). Whenever
calculations of Net Sales or Deferred Variable Payments require conversion from any currency into Dollars, Buyer shall convert into Dollars, using the median daily average exchange rate over the applicable period to which such Net Sales or Deferred
Variable Payments relate, of the daily Dollars foreign exchange reference rate of the Dollar against the currency of the country in which the applicable Net Sales were made.
-23-
(ii) Deferred Fixed Payments.
(A) Promptly following the first achievement of any Development and Regulatory Event, but in no event later than [***]
thereafter, Buyer shall inform Seller of the achievement of such Development and Regulatory Event and Buyer shall pay to Seller, by wire transfer of immediately available funds in accordance with written instructions provided by Seller, the
corresponding Deferred Fixed Payment.
(B) Buyer shall inform Seller of the achievement of any Sales Event and Buyer shall
pay to Seller, by wire transfer of immediately available funds in accordance with written instructions provided by Seller, the corresponding Deferred Fixed Payment, in each case within [***] following the end of the calendar year in which such Sales
Event was achieved.
(iii) Development Reports. Following the Closing, until the earlier of (i) the achievement
of all Development and Regulatory Events and (ii) Buyer’s delivery of a notice of Pre-Commercial Termination to Seller, within [***] after the end of each calendar year, Buyer shall submit to Seller
a written report, describing at a level of detail consistent with Buyer’s internal reporting standards and governance requirements, the status of the development of the Compound (each, a “Development Report”).
(iv) Deferred Variable Payments and Reporting. After the First Commercial Sale of a Product in the Territory, Buyer
shall calculate Deferred Variable Payments quarterly at the end of each calendar quarter and shall pay to Seller, by wire transfer of immediately available funds in accordance with written instructions provided by Seller, Deferred Variable Payments
on Annual Net Sales achieved during such calendar quarter within [***] after the end of each of the first three calendar quarters of the year and within [***] after the end of the fourth calendar quarter of the year. With each such payment, Buyer
shall provide in writing to Seller for the relevant calendar quarter the following information on a country-by-country and Product-by-Product basis (all of which shall constitute Buyer Information):
(A)
the amount of Product sold, the Net Sales (expressed in Dollars where gross sales are invoiced in Dollars, for gross sales invoiced in any other non-Dollar currency, the applicable conversion rates and the
resulting amount in Dollars, in accordance with the provisions of Section 3.8(d)(i)) on a Product-by-Product basis, in value, and a calculation
of aggregate Net Sales for such calendar quarter (which shall include the aggregate total for each input of such calculation, for example the aggregate deductions for such calendar quarter for credits or allowances for [***]); and
(B) the total Deferred Variable Payments payable (expressed in Dollars, in accordance with the provisions of
Section 3.8(b)) on a Product-by-Product basis.
-24-
(v) Accounting and Audit. Buyer and its Affiliates shall, and shall
use commercially reasonable efforts to cause all other Payment Obligors to, maintain complete and accurate books of account containing the information necessary for the purpose of calculating Deferred Variable Payments payable under this Agreement
and verifying all other payments to be made pursuant to this Agreement. Buyer and its Affiliates shall, and shall use commercially reasonable efforts to cause all other Payment Obligors to, permit Seller, through an Independent Accountant to examine
such books and records during normal business hours on not less than [***] advanced notice, but not later than [***] following Buyer’s payment of the Deferred Variable Payment to which such audit relates. The foregoing right of audit may be
exercised only once during any [***] period, except in the event that a previous audit during such [***] period detected any material inaccuracies in payments, in which case, Seller may exercise its right of audit once more during [***] to confirm
that such inaccuracies have been corrected. The auditing Independent Accountant may be required by Buyer or its Affiliates to enter into a reasonably acceptable confidentiality agreement, and in no event shall such Independent Accountant disclose to
Seller or its Affiliates any information other than such as relates to the accuracy of reports and payments made or due hereunder. The opinion of such Independent Accountant regarding such reports, accountings and payments shall be binding on the
Parties. Seller shall bear the cost of any such audit; provided, that if the audit shows an underpayment due by Buyer to Seller of more than [***] percent ([***]%) of the amount due, then Buyer shall promptly reimburse Seller for the costs of
the Independent Accountant incurred in connection with such audit. If the audit concludes that (A) additional amounts are owed by Buyer, Buyer shall promptly pay to Seller the amount of any such underpayment revealed by such audit, together
with simple interest accrued on such amount at the rate of [***] percent ([***]%) per annum between the date the relevant payment was due hereunder and the date on which such underpayment is paid in full and the costs of the Independent Accountant,
as applicable; or (B) excess payments were made by Buyer, Buyer shall be entitled to set-off against and deduct the amount of any such excess payment from any Deferred Fixed Payment or Deferred Variable
Payment that is then due and payable hereunder, or which becomes due and payable hereunder within [***] following such conclusion, or if no Deferred Fixed Payments or Deferred Variable Payments become due and payable hereunder within [***] following
such conclusion, Seller shall promptly reimburse Buyer for such excess payments.
(vi) Offset. The obligation of
Buyer to pay, or cause to be paid, any Deferred Fixed Payment or Deferred Variable Payment pursuant to this Section 3.8 is subject to the set-off rights of Buyer set forth in
Section 12.7.
(e) Product Transfers. [***].
-25-
(f) Deferred Payments Not Securities. The right of Seller to receive Deferred Fixed
Payments and Deferred Variable Payments if and when such payments become due and payable in accordance with this Section 3.8: (i) does not give Seller dividend rights, voting rights, liquidation rights, preemptive rights or
other rights of shareholders of Buyer; (ii) shall not be represented by any form of certificate or other instrument; (iii) does not represent any right other than the right to receive the consideration set forth in this
Section 3.8; and (iv) except as otherwise provided in Section 13.1, shall not be assignable or transferable except by operation of Law or Liquidation Event, without Buyer’s prior written
consent not to be unreasonably withheld, delayed or conditioned (and neither Buyer nor Seller shall give effect to any purported assignment or transfer made in contravention of this clause (iv) (and any such purported assignment or transfer shall be
void and of no effect)).
(g) Information. Seller (i) acknowledges that the information provided by Buyer under this
Section 3.8 may constitute material, non-public information of Buyer, (ii) is aware that United States federal and state securities Laws restrict persons with material, non-public information about a company obtained directly or indirectly from that company from purchasing or selling securities of such company, or from communicating such information to any other person under
circumstances in which it is reasonably foreseeable that such other person is likely to purchase or sell such company’s securities and (iii) agrees that Seller and its Affiliates shall not use or disclose any information provided by Buyer
under this Section 3.8 in violation of such Laws.
3.9
Allocation of Purchase Price.
(a) Buyer shall prepare a schedule (the “Allocation Schedule”) that allocates the
Purchase Price and Assumed Liabilities (plus other relevant items) to the Acquired Assets consistent with applicable Tax Laws that the Parties must satisfy with respect to the allocation of the Purchase Price for tax purposes, including for United
States tax purposes Sections 1060 and 338 of the Code and the Treasury regulations promulgated thereunder, and for Canadian tax purposes Section 68 of the ITA. Buyer shall provide a copy of such Allocation Schedule to Seller no later than [***]
after the Closing Date or, if earlier, [***] prior to the due date (taking into account any extensions) for filing a Tax Return for which the allocation is relevant for Seller’s review.
(b) Upon any adjustment to the Purchase Price, the Allocation Schedule shall be revised by Buyer and submitted to Seller as soon as practicable
for Seller’s review. Buyer and Seller shall cooperate in the preparation and filing of any forms required by applicable Law with respect to the allocation, including any amendments to such forms required as a result of any adjustment to the
Purchase Price pursuant to this Agreement.
3.10 Withholding and Other Taxes. Buyer, its Affiliates, and its Representatives shall
be entitled to deduct and withhold from any amounts payable pursuant to this Agreement any withholding Taxes required by applicable Law to be deducted and withheld. If Buyer intends to deduct or withhold any amounts pursuant to this
Section 3.10, Buyer shall use its commercially reasonable efforts (a) to notify Seller within a reasonable time period in advance thereof and (b) to provide Seller a reasonable opportunity to provide forms or
other evidence to Buyer that would reduce or eliminate such deduction or withholding to the extent permitted under applicable Law. Any amounts deducted or withheld and remitted to the appropriate Taxing Authority will be treated for all purposes of
this Agreement as having been paid to the Person in respect of which such deduction and withholding was made. Buyer shall remit any amount deducted or withheld to the appropriate Taxing Authority.
-26-
3.11 Third Party Patent.
(a) Rights to Challenge and/or In-License. Notwithstanding anything to the contrary in this
Agreement, (i) [***] the patent set forth on Schedule 3.11 or any Patent Rights with respect thereto (the “Patent At Issue”) [***], (ii) until [***], subject to Section 3.11(b), Seller
and Buyer shall each have the right to negotiate and obtain a license under the Patent At Issue for the research, development, manufacture, commercialization and other exploitation of the Compound and any Product; provided that Seller shall
not execute or otherwise enter into any agreement containing such license without Buyer’s prior written consent (which will not be unreasonably withheld, conditioned or delayed if such agreement complies with
Section 3.11(b)), (iii) from and after [***], Buyer shall have the sole right to negotiate and obtain a license under the Patent At Issue for the research, development, manufacture, commercialization and other
exploitation of the Compound and any Product. If, as of [***], Seller is engaged in ongoing negotiations with the Third Party that controls such Patent At Issue for the license described in clause (ii), at Buyer’s election, Seller shall either
(x) immediately terminate all discussions related to such license and negotiations or (y) facilitate the transition of such negotiations to Buyer and, to the extent requested by Buyer, continue to participate in such negotiations.
(b) If Seller exercises its rights under clause (ii) of Section 3.11(a) to negotiate and obtain a license to the
Patent At Issue for the research, development, manufacture, commercialization and other exploitation of the Compound and any Product, unless otherwise agreed by Buyer in writing (including by Buyer’s written consent to any agreement that does
not comply with the requirements set forth in this Section 3.11(b)), Seller shall ensure that such license:
(c) Transfer. If (i) Seller exercises its rights under clause (ii) of Section 3.11(a) to negotiate
and obtain, and thereafter does obtain, a license to the Patent At Issue for the research, development, manufacture, commercialization or other exploitation of the Compound and any Product in compliance with Section 3.11(a)
and Section 3.11(b), and (ii) Buyer exercises its Option, such license shall be included in the Acquired Assets pursuant to Section 3.1 or, if such license is entered into after the Closing,
immediately assigned to Buyer for no additional consideration. For clarity, Buyer shall assume all obligations set forth in any license to the Patent At Issue in connection with any assignment of such license to Buyer pursuant to this
Section 3.11(c), including, if applicable, any royalty or other payment obligations set forth therein to the extent not fulfilled by Seller as of the date of assignment; provided, however, that Buyer shall not assume any
such obligations or liabilities that result from or arise out of a breach by Seller or such license prior to such assignment.
-27-
(d) Financial Consequences.
(i) Subject to Section 3.11(d)(ii), if either Buyer or Seller obtains a license under the Patent At
Issue for the research, development, manufacture, commercialization and other exploitation of the Compound and any and all Products (provided that, if such license is obtained by Seller, it was entered into in compliance with
Section 3.11(a) and Section 3.11(b) and is fully assigned to Buyer in accordance with Section 3.1 or, if such license is entered into after the Closing, it is promptly
assigned to Buyer for no additional consideration other than Buyer’s reimbursement obligations pursuant to this Section 3.11(d)), then:
(A) the Deferred Fixed Payment set forth in item (3) of Section 3.8(a) shall instead equal:
$[***] (or $[***] if [***]),
(B) the Deferred Fixed Payment set forth in item (4) of
Section 3.8(a) shall instead equal: $[***] (or $[***] if a [***]),
(C) the Deferred Variable
Payments set forth in Section 3.8(b) shall instead equal:
|
|
|
|
|
| Annual Net Sales |
|
Rate |
|
| For that portion of Annual Net Sales less than $[***] |
|
|
[*** |
]% |
| For that portion of Annual Net Sales equal to or greater than $[***] and less than or equal to
$[***] |
|
|
[*** |
]% |
| For that portion of Annual Net Sales greater than $[***] and less than or equal to $[***] |
|
|
[*** |
]% |
| For that portion of Annual Net Sales greater than $[***] and less than or equal to $[***] |
|
|
[*** |
]% |
| For that portion of Annual Net Sales greater than $[***] |
|
|
[*** |
]% |
(D) [***],
(E) [***],
(F) the “Deferred Variable Payment Term” shall instead mean the time period beginning on the First Commercial
Sale of a Product anywhere in the Territory following the Closing and ending on the latest of (i) the [***] and (ii) the earlier of (A) the expiration of the last Valid Claim of the Patent At Issue that Covers such Product or the
exploitation thereof and (B) [***]; provided that if FDA grants Buyer or any of its Affiliates [***] for the Compound under U.S. 21 C.F.R. § [***], the Deferred Variable Payment Term will expire [***] at the later of [***]
(y) the earlier of (I) [***] that Covers such Product or the exploitation thereof and (II) [***], and
-28-
(G) for clarity, the provisions of Section 3.8
shall otherwise remain unaffected, including that Sections 3.8(a)(ii), 3.8(c)(1), and 3.8(c)(2) shall apply to the Deferred Fixed Payments set forth above.
(ii) [***].
ARTICLE IV
CLOSING
4.1 Closing. If the Option is exercised by Buyer, the closing of the Transactions (the “Closing”) shall take
place at 10:00 a.m., Eastern time, on the third Business Day following the satisfaction or waiver of the conditions set forth in Article X (other than satisfaction of those conditions that by their nature are to be satisfied at the Closing,
but subject to satisfaction or waiver of such conditions at the Closing), or on such later date and time (prior to any effective date of termination of this Agreement pursuant to Article XI) that may be agreed to in writing by Buyer and
Seller, by electronic exchange of executed documents by Buyer and Seller. For purposes of this Agreement, the “Closing Date” shall be the date on which the Closing occurs.
4.2 Transactions at Closing. At the Closing, subject to the terms and conditions hereof:
(a) Seller’s Actions and Deliveries. Seller shall execute and deliver, or cause to be executed and delivered, to
Buyer (or to such Affiliates of Buyer as instructed in writing by Buyer prior to the Closing Date):
(i) the Ancillary
Agreements that call for Seller’s signature; and
(ii) evidence, in form and substance reasonably satisfactory to
Buyer, that Seller has obtained each of the consents or approvals, if any, set forth in Section 5.4 of the Seller Disclosure Letter.
(b) Buyer’s Actions and Deliveries. Buyer shall:
(i) pay, or cause to be paid, an amount equal to the sum of One Million Five Hundred Thousand Dollars ($1,500,000) (the
“Option Exercise Price”) and, if Buyer has elected to purchase any Inventory, the aggregate Inventory Price, to Seller by wire transfer of immediately available funds in accordance with written instructions provided by Seller not
less than five Business Days prior to the Closing Date; and
(ii) execute and deliver, or cause to be executed and
delivered, to Seller the Ancillary Agreements that call for Buyer’s signature.
-29-
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF SELLER
Except as disclosed in the disclosure letter delivered by Seller to Buyer immediately prior to the execution of this Agreement or, with
respect to the making of the representations and warranties in this Article V as of the Closing Date, pursuant to Section 2.4(b) (the “Seller Disclosure Letter”) (it being agreed that disclosure of
any item in any section of the Seller Disclosure Letter shall be deemed disclosure with respect to any other section of this Agreement to which the relevance of such item is reasonably apparent from the face of such disclosure, notwithstanding the
absence of a cross-reference in or to any such corresponding section of this Agreement), Seller represents and warrants to Buyer, as of the date hereof and as of the Closing Date, as set forth below.
5.1 Qualification, Organization, etc. Seller is a corporation duly incorporated, validly existing and in good standing under the Laws of
Texas and has all requisite corporate power and authority to own, lease and operate its properties and assets and to carry on its business as presently conducted. Seller is qualified to do business and is in good standing as a foreign corporation or
other entity in each jurisdiction where the ownership, leasing or operation of its assets or properties or conduct of its business requires such qualification, except where the failure to be so qualified or, where relevant, in good standing, would
not reasonably be expected to, individually or in the aggregate, have a Material Adverse Effect.
5.2 Authority; Binding Effect.
(a) Seller has all requisite corporate power and authority to execute and deliver this Agreement and each Ancillary Agreement to which it
will be a party, to perform its obligations hereunder and thereunder and to consummate the Transactions. The execution and delivery by Seller of this Agreement and each such Ancillary Agreement, the performance by Seller of its obligations hereunder
and thereunder and the consummation of the Transactions, have been, or in the case of the Ancillary Agreements will have been prior to Closing, duly authorized by all requisite corporate action on the part of Seller.
(b) This Agreement has been duly executed and delivered by Seller and constitutes a legal, valid and binding obligation of Seller, and each
Ancillary Agreement will be, prior to the Closing, duly executed and delivered by Seller and will, upon the Closing, constitute a legal, valid and binding obligation of Seller, in each case enforceable against Seller in accordance with its terms,
except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is
sought in a proceeding in equity or at law).
(c) Each Affiliate of Seller that will enter into any Ancillary Agreement has all requisite
organizational power and authority to execute and deliver each Ancillary Agreement to which it will be a party, to perform its obligations thereunder and to consummate the transactions contemplated thereby. The execution and delivery by each such
Affiliate of each Ancillary Agreement to which it will be a party, the performance by such Affiliate of its obligations thereunder and the consummation of the transactions contemplated thereby, will have been prior to Closing duly authorized by all
requisite organizational action on the part of such Affiliate prior to its execution and delivery of each such Ancillary Agreement.
-30-
(d) Each Ancillary Agreement to which an Affiliate of Seller will be a party, as of the
Closing, will have been duly executed and delivered by such Affiliate and, as of the Closing, constitutes a legal, valid and binding obligation of such Affiliate, in each case enforceable against such Affiliate in accordance with its terms, except
as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is sought in
a proceeding in equity or at law).
5.3 No Conflicts; Consents. The execution, delivery and performance of this Agreement by Seller,
the execution, delivery and performance by Seller and its Affiliates of each Ancillary Agreement to which Seller or any of its Affiliates is a party and the consummation of the Transactions by Seller do not and will not: (a) violate any
provision of Seller’s or such Affiliate’s Organizational Documents; (b) subject to making or obtaining the Consents referred to in Section 5.4, violate or result in a breach of or constitute a default under
any Law to which Seller is subject or may be bound or affected; (c) conflict with, violate any provision of or result in a breach of, or require a Consent under, or result in the termination of or the acceleration of (whether after the giving
of notice or the lapse of time or both) any rights or obligations under (i) that certain Exclusive License and Collaboration Agreement, dated [***], by and between [***]) and Seller or (ii) any other Contract to which Seller or any of its
Affiliates is a party or (d) result in the creation of any Lien other than a Permitted Lien on any Acquired Asset; except, with respect to clauses (b) and (c), for any violations, breaches, conflicts, defaults, terminations, cancellations,
modifications or accelerations as would not reasonably be expected to, individually or in the aggregate, be material to the Compound, the Acquired Assets or the Assumed Liabilities.
5.4 Consents and Governmental Approvals. No Consent to, with or from any Governmental Authority is required for Seller or any of its
Affiliates to execute, deliver or perform its obligations under this Agreement or any Ancillary Agreement or to consummate the Transactions, including any approval from the Small Business Administration as a result of receiving any funds from the
Paycheck Protection Program pursuant to the Coronavirus Aid, Relief, and Economic Security Act, Pub. L. No. 116–136, 131 Stat. 281.
5.5 Title to Assets. Seller or an Affiliate thereof has good, marketable and valid title to all of the Acquired Assets, free and clear
of all Liens other than Permitted Liens. At the Closing, Buyer will acquire from Seller or its applicable Affiliate good and marketable title to all of the Acquired Assets, free and clear of all Liens (other than Permitted Liens). Neither Seller nor
any of its Affiliates is developing, Manufactures for itself or any Third Party, or is commercializing any Competing Product.
5.6
Absence of Certain Changes or Events.
(a) Since December 31, 2021, there has not occurred any Material Adverse Effect.
(b) From December 31, 2021 through the date of this Agreement, other than with respect to the Transactions, Seller and its Affiliates have
conducted the development of the Compound in the ordinary course of business.
-31-
(c) From December 31, 2021 through the date of this Agreement, neither Seller nor any
of its Affiliates has taken any actions that, if taken after the date of this Agreement, would constitute a breach of any of the covenants set forth in Section 7.1(b).
5.7 Orders; Litigation. There are no Orders issued by any Governmental Authority binding on Seller or any of its Affiliates with respect
to the Acquired Assets, the Compound, or the Transactions, and there are no Proceedings pending or, to Seller’s Knowledge, threatened, against Seller or any of its Affiliates with respect to the Acquired Assets, the Compound, or the
Transactions.
5.8 Compliance with Laws; Regulatory Matters.
(a) Neither Seller nor any of its Affiliates holds or has applied for any Regulatory Approvals for any Product. Seller and its Affiliates have
conducted the research, development and manufacture of the Compound in material compliance with all applicable Laws, including all Laws applicable to the research, nonclinical and clinical testing, development, manufacturing, ownership, operation,
storage, import, export, distribution, marketing, pricing, sale, promotion, warehousing, packaging, labeling, and handling of the Compound, including the FDCA and its implementing regulations, and any comparable applicable state and foreign Laws
(all such applicable Laws, collectively, the “Health Care Laws”). None of Seller its Affiliates or to Seller’s Knowledge, any Third Party manufacturer of any Compound engaged by Seller or any of its Affiliates has received any
written notice, including any FDA Form 483, clinical hold, warning letter, notice of adverse finding, notice of violation, untitled letter, or notice of deficiency, or similar written communication from the FDA or any other Governmental Authority,
(i) alleging that the Compound or the ownership, manufacturing, operation, storage, import, export, distribution, development, clinical trials, marketing, pricing, sale, promotion, warehousing, packaging, labeling, handling or testing thereof
is in violation of any applicable Health Care Law or Governmental Authorization, or (ii) otherwise alleging any violation of any Health Care Laws or Governmental Authorization by Seller or any of its Affiliates with respect to the research,
development or manufacture of the Compound; except, in each case, to the extent that such violation has been remedied.
(b) All clinical
studies of the Compound conducted by or on behalf of Seller and its Affiliates have been and are being conducted in compliance in all material respects with valid study protocols, GCP and all other applicable Law. Neither Seller nor any of its
Affiliates has received any written notice that FDA or any other Governmental Authority, or any Review Board or ethics committee has recommended, initiated, or threatened to initiate any action to suspend or terminate any clinical trial with respect
to the Compound sponsored by Seller and its Affiliates, or to otherwise materially restrict the preclinical research or clinical study of the Compound. All clinical trials conducted by or on behalf of Seller or any of its Affiliates, and the results
of all such clinical trials, have been registered and disclosed in accordance with Section 801 of the Food and Drug Administration Amendments Act of 2007 (Section 402(j) of the Public Health Service (PHS) Act), U.S. 42 C.F.R. § 11, and all
other applicable Laws.
-32-
(c) All material documents, reports and notices required to be filed with any Governmental
Authority by Seller and its Affiliates with respect to the Compound have been so filed on a timely basis, and were complete and accurate in all material respects as of the date of filing, or were subsequently updated, changed, corrected, or
modified. No such filing with any Governmental Authority contained any materially false, misleading or otherwise inaccurate statements or information, whether express or due to omission of material information, as of the date of filing.
(d) Section 5.8(d) of the Seller Disclosure Letter sets forth a true and complete list of all human clinical trials, previously or currently
undertaken or sponsored by or on behalf of Seller or its Affiliates and, to the Seller’s Knowledge, any third-party investigator for whom Seller and its Affiliates provides material or financial support for any such clinical trial, in each
case, with respect to the Compound. True and complete and correct copies of all material data and material reports with respect to such trials have been made available to Buyer; provided that
CMC-related Know-How and Information shall not be made available prior to receipt of the Option Fee. Seller has made available to Buyer a true and complete copy of all
material correspondence and meeting minutes between Seller or any of its Affiliates and the FDA, other Governmental Authorities, or any Review Board regarding such clinical trials; provided that
CMC-related Know-How and Information shall not be made available prior to receipt of the Option Fee.
(e) Neither Seller nor any of its Affiliates has performed any pre-clinical studies or any animal
studies with respect to the Compound.
(f) All manufacturing operations conducted by, or for the benefit of, Seller or its Affiliates with
respect to the Compound being used in human clinical trials have been and are being conducted in accordance, in all material respects, with the applicable current Good Manufacturing Practice, as set forth in Parts 210 and 211, or otherwise under 21
U.S.C. § 351, and comparable applicable state and foreign Laws. All such manufacturing operations are in material compliance with all applicable registration and listing requirements set forth in 21 U.S.C. Section 360 and U.S. 21 C.F.R.
§ 207 and all similar Laws in those jurisdictions in which human clinical trials or manufacturing activities have been or are being conducted by Seller or its Affiliates involving the Compound. No manufacturing site used for the manufacture of
the Compound is subject to a Regulatory Authority shutdown or import or export prohibitions.
(g) Seller has made available to Buyer true
and complete copies of (i) all INDs related to the Compound held by Seller or any of its Affiliates, (ii) all material correspondence in Seller’s or any of its Affiliates’ possession or control to or from FDA and any other
applicable Regulatory Authorities, in each case concerning the Compound and the likelihood or timing of, or requirements for, regulatory approval of the Compound, and (iii) except for any Prohibited Information, all material information in
Seller’s or any of its Affiliates’ possession or control concerning the safety, efficacy, side effects, toxicity, or manufacturing quality and controls of the Compound; provided that
CMC-related Know-How and Information shall not be made available prior to receipt of the Option Fee.
(h) Neither Seller nor any of its Affiliates nor, to Seller’s Knowledge, any of their Representatives has received any written notice that
the FDA or any other Governmental Authorities or Review Board has initiated, or threatened to initiate, any action to suspend or terminate any IND, in each case sponsored by Seller or any of its Affiliates with respect to the Compound, or to recall
or suspend the manufacture of the Compound.
-33-
(i) None of Seller, any of its Affiliates or any of their respective directors or employees
and, to Seller’s Knowledge, no other Representatives acting for Seller or any of its Affiliates, has committed any act, made any statement or failed to make any statement, relating to the Compound or the development or manufacturing thereof
that would reasonably be expected to provide a basis for the FDA to invoke its policy with respect to “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities” set forth in 56 Fed. Reg. 46191 (September 10, 1991) and
any amendments thereto. None of Seller any of its Affiliates or any of their respective directors or employees or, to Seller’s Knowledge, any other Representative acting for Seller or any of its Affiliates in connection with the Compound is or
ever has been debarred, excluded, or suspended from participation, or otherwise been deemed ineligible to participate, in any health care programs of any Governmental Authority, or convicted of any crime regarding health care products or services,
or engaged in any conduct that would reasonably be expected to result in any such debarment, exclusion, suspension, or ineligibility, including (i) debarment under 21 U.S.C. Section 335a or any similar applicable state or foreign Law and
(ii) exclusion under 42 U.S.C. Section 1320a–7 or any similar applicable state or foreign Law.
(j) Neither Seller nor any
of its Affiliates is a party to any corporate integrity agreement, monitoring agreement, deferred prosecution agreement, consent decree, settlement order, or similar agreement imposed by any Governmental Authority that covers the Compound or any of
the Acquired Assets.
5.9 Tax Matters.
(a) All material Tax Returns that are required to be filed by or with respect to the Acquired Assets and the research, development or
manufacture of the Compound have been timely filed (taking into account any extension of time within which to file), and all such Tax Returns are true, complete and accurate in all material respects;
(b) All Taxes due and owing with respect to the Acquired Assets and the research, development or manufacture of the Compound have been paid,
other than Taxes for which adequate reserves have been established in accordance with GAAP on the financial statements of Seller or its Affiliates and no failure, if any, of Seller to duly and timely pay all Taxes for the current year that are due
and payable by it will result in a Lien on the Acquired Assets;
(c) there are no Liens for Taxes on any of the Acquired Assets other than
Liens for Taxes not yet due and payable that arose by operation of Law;
(d) to Seller’s Knowledge, no Tax Proceedings are pending, in
progress or have been threatened in writing with respect to any Taxes relating to the Acquired Assets or the research, development or manufacture of the Compound and there are no matters under discussion, audit or appeal with any Taxing Authority
which will reasonably be expected to result in a Lien on the Acquired Assets;
(e) Seller is a
non-resident of Canada within the meaning of the ITA;
(f) the Acquired Assets are not taxable
Canadian property within the meaning of the ITA; and
-34-
(g) Seller is not a registrant for the purposes of the Excise Tax Act (Canada), and
is not registered for GST/HST or any other Canadian sales Taxes.
5.10 Intellectual Property.
(a) The Transferred Know-How and Licensed Manufacturing
Know-How constitute all of the Intellectual Property that is owned or licensed by Seller or its Affiliates and necessary for the research, development or manufacture of the Compound as currently conducted by
Seller and its Affiliates. Other than the Transferred Know-How and Licensed Manufacturing Know-How, neither Seller nor any of its Affiliates owns, licenses or otherwise
uses any Intellectual Property used in and that is material to the research, development or manufacture of the Compound.
(b) Neither the
Transferred Know-How nor the Licensed Manufacturing Know-How is subject to any outstanding Order with respect to its use.
(c) No Proceedings are pending or, to Seller’s Knowledge, threatened against Seller or any of its Affiliates alleging that the Compound,
the research, development, manufacture or commercialization of the Compound, the Transferred Know-How or Licensed Manufacturing Know-How, or any act by Seller or any of
its Affiliates related thereto or the use thereof, infringes, misappropriates or otherwise violates (or in the past infringed, misappropriated or otherwise violated) any Intellectual Property of any Person.
(d) To Seller’s Knowledge, the research, development or manufacture of the Compound or any Product, and the making, use, import, offer for
sale or other disposition of the Compound or any Product, has not, does not, and would not infringe, misappropriate or otherwise violate, even when considered in the absence of 35 U.S.C. 271(e)(1) in the United States or a similar research use
exception elsewhere, any Intellectual Property of any Person.
(e) To Seller’s Knowledge, there is no, nor has there been any,
infringement, misappropriation or other violation by any Person of any of the rights of Seller or any of its Affiliates in or to the Transferred Know-How or Licensed Manufacturing Know-How.
(f) Seller and its Affiliates have taken commercially reasonable measures to maintain the
confidentiality of the Transferred Know-How and Licensed Manufacturing Know-How. The Know-How and Information that are owned,
used or held by Seller and its Affiliates in connection with the research, development or manufacture of the Compound have not been used, disclosed to or discovered by any Person except pursuant to written
non-disclosure or license agreements which have not, to Seller’s Knowledge, been breached.
(g) No funding, Intellectual Property, facilities, personnel or other resources of any Governmental Authority or university or other academic
institution or academic research center has been used in connection with the conception, invention, reduction to practice, development or other creation by or on behalf of Seller and its Affiliates of any Intellectual Property related to the
Compound.
-35-
(h) Seller and its Affiliates may lawfully use all Personally Identifiable Information
collected or used by Seller and its Affiliates in the research and development of the Compound or included in the Acquired Assets and have complied in all material respects with all applicable Laws and contractual and fiduciary obligations relating
to the collection, storage, use, transfer and any other processing of any Personally Identifiable Information collected or used by Seller or any of its Affiliates (including Personally Identifiable Information collected during any clinical trials
conducted with respect to the Compound, or during the development, preclinical and clinical testing, manufacture, storage, testing, distribution, supply and administration of the Compound). The transfer in accordance with this Agreement of
Personally Identifiable Information included in the Acquired Assets from Seller or any of its Affiliates to Buyer in connection with the Transactions will not violate any applicable U.S. Law.
(i) To the Seller’s Knowledge, neither Seller nor any of its Affiliates has suffered any breach of security leading to the accidental or
unlawful destruction, loss, alteration, unauthorized disclosure of, or access to, any Personally Identifiable Information applicable to the research, development or manufacture of the Compound or included in the Acquired Assets.
(j) The representations set forth in the first two sentences of Section 5.5 and the representations set forth in this
Section 5.10 are the only representations given by Seller with respect to ownership of Intellectual Property, and the representations set forth in Section 5.10(c),
Section 5.10(d) and Section 5.10(e) are the only representations given by Seller with respect to non-infringement, misappropriation or violation of
Intellectual Property.
5.11 Insurance. Seller and its Affiliates have all material policies of insurance covering the Business,
including policies of property, clinical trial and products liability, and such policies are in a form and amount which are adequate for the research, development and manufacture of the Compound. All such insurance policies are in full effect, no
written notice of cancellation has been received by Seller or any of its Affiliates under such policies, and there is no existing material default under any such policies.
5.12 Brokers. No broker, investment banker, agent, finder or other Person acting on behalf of Seller or any of its Subsidiaries or under
the authority thereof is or shall be entitled to any broker’s or finder’s fee or any other commission or similar fee directly or indirectly in connection with any of the Transactions.
5.13 Solvency. Immediately after giving effect to the Transactions, Seller and each of its Affiliates shall be solvent and shall
(a) be able to pay its debts as they become due, (b) own property that has a fair saleable value greater than the amounts required to pay its debts (including a reasonable estimate of the amount of all contingent liabilities), and
(c) have adequate capital to carry on its business, including, but not limited to, in accordance with the terms of this Agreement. No transfer of property is being made and no obligation is being incurred in connection with the Transactions
with the intent to hinder, delay or defraud either present or future creditors of Seller or its Affiliates.
5.14 No Reliance.
Seller acknowledges that (i) none of Buyer, its Affiliates or Representatives is making, and Seller is not relying on, any statement, representation or warranty, oral or written, express or implied, regarding the Compound, the Acquired Assets,
the Assumed Liabilities, or Buyer and its Affiliates, except as expressly set forth in Article VI and (ii) all other representations and warranties regarding the Compound, the Acquired Assets or the Assumed Liabilities of any kind or
nature, express or implied (including any implied warranty of merchantability or fitness for a particular purpose) are specifically disclaimed.
-36-
ARTICLE VI
REPRESENTATIONS AND WARRANTIES OF BUYER
Except as disclosed in the applicable section of the disclosure letter delivered by Buyer to Seller immediately prior to the execution of this
Agreement or, with respect to the making of the representations and warranties in this Article VI as of the Closing Date, in conjunction with Buyer’s delivery of an Option Exercise Notice (the “Buyer Disclosure Letter”)
(it being agreed that disclosure of any item in any section of the Buyer Disclosure Letter shall be deemed disclosure with respect to any other section of this Agreement to which the relevance of such item is reasonably apparent from the face of
such disclosure, notwithstanding the absence of a cross-reference in or to any such corresponding section of this Agreement), Buyer represents and warrants to Seller, as of the date hereof and as of the Closing Date, as set forth below.
6.1 Qualification, Organization, etc. Buyer is a legal entity duly organized, validly existing and in good standing under the
Laws of Canada and has all requisite corporate or similar power and authority to own, lease and operate its properties and assets and to carry on its business as presently conducted. Buyer is qualified to do business and is in good standing as a
foreign corporation or other entity in each jurisdiction where the ownership, leasing or operation of its assets or properties or conduct of its business requires such qualification, except where the failure to be so qualified or, where relevant, in
good standing, would not, individually or in the aggregate, reasonably be expected to prevent or materially delay Buyer’s consummation of the Transactions.
6.2 Authority; Binding Effect.
(a) Buyer has all requisite corporate power and authority to execute and deliver this Agreement and each Ancillary Agreement to which it will
be a party to perform its obligations hereunder and thereunder and to consummate the Transactions. The execution and delivery by Buyer of this Agreement and each such Ancillary Agreement, the performance by Buyer of its obligations hereunder and
thereunder and the consummation of the Transactions, have been, or in the case of the Ancillary Agreements will have been prior to Closing, duly authorized by all requisite corporate action on the part of Buyer.
(b) This Agreement has been duly executed and delivered by Buyer and constitutes a legal, valid and binding obligation of Buyer, and each
Ancillary Agreement will be, prior to the Closing, duly executed and delivered by Buyer and will, upon the Closing, constitute a legal, valid and binding obligation of Buyer, in each case enforceable against Buyer in accordance with its terms,
except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is
sought in a proceeding in equity or law).
-37-
6.3 No Conflicts; Consents. The execution, delivery and performance of this Agreement
by Buyer and the consummation of the Transactions do not and will not: (a) violate any provision of Buyer’s Organizational Documents; (b) violate or result in a breach of or constitute a default under any Law to which Buyer is subject
or may be bound or affected; or (c) conflict with, violate any provision of or result in a breach of, or result in the termination of or the acceleration of (whether after the giving of notice or the lapse of time or both) any rights or
obligations of Buyer under any Contract to which Buyer is a party; except, with respect to clauses (b) and (c), for any violations, breaches, conflicts, defaults, terminations, cancellations, modifications or accelerations as would not
reasonably be expected to, individually or in the aggregate, prevent or materially delay Buyer’s consummation of the Transactions.
6.4 Consents and Governmental Approvals. No Consent to, with or from any Governmental Authority is required for Buyer to execute,
deliver or perform its obligations under this Agreement or any Ancillary Agreement or to consummate the Transactions.
6.5 Orders;
Litigation. There are no Orders issued by any Governmental Authority binding on Buyer or any of its Affiliates with respect to the Acquired Assets, the Compound, or the Transactions, and there are no Proceedings pending or, to Buyer’s
knowledge, threatened, against Buyer or any of its Affiliates with respect to the Acquired Assets, the Compound, or the Transactions.
6.6
Solvency. Immediately after giving effect to the Transactions, Buyer and each of its Subsidiaries shall be solvent and shall (a) be able to pay its debts as they become due, (b) own property that has a fair saleable value greater
than the amounts required to pay its debts (including a reasonable estimate of the amount of all contingent liabilities), and (c) have adequate capital to carry on its business, including, but not limited to, in accordance with the terms of
this Agreement. No transfer of property is being made and no obligation is being incurred in connection with the Transactions with the intent to hinder, delay or defraud either present or future creditors of Buyer or its Subsidiaries.
6.7 Brokers. No broker, investment banker, agent, finder or other Person acting on behalf of Buyer or any of its Subsidiaries or under
the authority thereof is or shall be entitled to any broker’s or finder’s fee or any other commission or similar fee directly or indirectly in connection with any of the Transactions.
6.8 No Reliance. Buyer acknowledges that (i) none of Seller, its Affiliates or Representatives is making, and Buyer is not relying
on, any statement, representation or warranty, oral or written, express or implied, regarding the Compound, the Acquired Assets or the Assumed Liabilities, except as expressly set forth in Article V and (ii) all other representations and
warranties regarding the Compound, the Acquired Assets or the Assumed Liabilities of any kind or nature, express or implied (including any implied warranty of merchantability or fitness for a particular purpose) are specifically disclaimed.
-38-
ARTICLE VII
PRE-CLOSING COVENANTS
7.1 Conduct Prior to the Closing.
(a) Unless Buyer consents in writing (which consent shall not be unreasonably withheld, delayed or conditioned), except as required by
applicable Law or expressly required by this Agreement, from and after the date of this Agreement until the earlier of: (i) the Closing Date, and (ii) the termination of this Agreement pursuant to Section 11.1
(the “Pre-Closing Period”), Seller shall, and shall cause its Affiliates engaged in the research, development or manufacture of the Compound or any Product to, use commercially reasonable
efforts to conduct such activities only in the ordinary course of business consistent with past practice and preserve the Acquired Assets and the existing business relationships of Seller and its Affiliates related to the research, development or
manufacture of the Compound or any Product.
(b) Without limiting the generality of Section 7.1(a), unless Buyer
consents in writing (which consent shall not be unreasonably withheld, delayed or conditioned), except as required by applicable Law or expressly required by this Agreement or as set forth in Section 7.1(b) of the Seller Disclosure Letter,
during the Pre-Closing Period, Seller shall not take any of the following actions:
(i) [***], transfer, sell, lease, enter into any sale lease back (or similar arrangement), license, mortgage, pledge, encumber
or otherwise convey or dispose of or grant any rights in, or create any Liens (other than Permitted Liens) on, the Compound, any Product or any of the Acquired Assets, other than the grant of non-exclusive
licenses to rights to the Compound for the sole purpose of continuing the TATCIST Study that are restricted to such use and the use of inventory of the Compound for purposes of the TATCIST Study;
(ii) enter into any Contract in respect of the research, development, manufacture or commercialization of the Compound or any
Product other than Contracts reasonably necessary or appropriate to continue the TATCIST Study and that otherwise comply with the restrictions set forth in this Section 7.1(b) or Section 7.4;
(iii) discharge, settle, compromise, satisfy or consent to any entry of any judgment with respect to, any Claim that
(A) results by its terms in any express restriction on the research, development, manufacture or commercialization of the Compound or any Product or (B) results in a Liability to the extent that such Liability would constitute an Assumed
Liability;
(iv) vary any inventory practices with respect to the Compound or any Product in any respect materially
inconsistent with past practice, except to the extent necessary to conduct the TATCIST Study;
(v) [***], adopt a plan of
complete or partial liquidation or dissolution, recapitalization or other reorganization affecting the Acquired Assets, the Compound or any Product;
-39-
(vi) disclose to any Third Party any trade secrets or other confidential
information included in the Acquired Assets other than pursuant to a confidentiality agreement on customary terms;
(vii)
correspond, communicate or consult with the FDA, EMA or similar Regulatory Authorities concerning the Compound or any Product without providing Buyer prior written notice and the opportunity to consult with Seller with respect to such
correspondence, communication or consultation, to the extent such written notice and consultation is not prohibited by such Regulatory Authorities; provided, that prior to the Closing Date, Seller shall retain all final decision-making
authority with respect to such correspondence, communications and consultation;
(viii) except as requested by the FDA, EMA
or similar Regulatory Authorities or in connection with a Study Termination Event, make any material modification to the TATCIST Study or the study protocol for the TATCIST Study;
(ix) commence, or submit any application for a Governmental Authorization with respect to the commencement of, any clinical
study of the Compound or any Product;
(x) make any filing with any Governmental Authority to obtain Patent Rights or
register any trademark, service mark, trade name or similar indicia of ownership with respect to the Compound or any Product or the making, use or sale thereof; or
(xi) make a binding commitment to take any of the foregoing actions.
(c) Nothing contained in this Agreement shall give Buyer, directly or indirectly, the right to control or direct Seller’s or any of its
Affiliates’ operations prior to the Closing. Prior to the Closing, the management of Seller shall exercise, consistent with and in accordance with the terms and conditions of this Agreement, complete control and supervision over the operations
of Seller and its Affiliates.
(d) Immediately prior to the Closing, Seller shall cause any of its Affiliates holding any asset(s) that
constitute Acquired Asset(s) to transfer and assign such asset(s) to Seller. Seller or its applicable Affiliates shall be solely responsible for, and shall pay, all costs and expenses, including all Taxes, resulting from such transfers.
7.2 Efforts; Execution of Transfer Documents.
(a) Subject to the terms and conditions of this Agreement and without limiting the express obligations hereunder, each of the Parties agrees to
cooperate fully with each other and to use its commercially reasonable efforts to take, or cause to be taken, all actions, and to do, or cause to be done, in each case promptly following the date of the Option Exercise Notice, all things necessary,
proper or advisable to consummate and make effective, at the time and in the manner contemplated by this Agreement, the Transactions, including to make and obtain all Consents required under applicable Laws, and to execute and deliver such documents
and other papers and any other agreements, as may be necessary to, promptly following the date of the Option Exercise Notice, carry out the provisions of this Agreement and consummate and make effective the Transactions.
-40-
(b) At the Closing, Buyer shall, and shall cause each of its applicable Affiliates to,
execute and deliver each Ancillary Agreement, and Seller shall, and shall cause each of its applicable Affiliates to, execute and deliver each Ancillary Agreement, in each case to which it is a party.
7.3 Access. During the Pre-Closing Period, not more than [***] during the period prior to
delivery of the Option Trigger Notice, in addition to the rights of Buyer under Section 2.2, Seller shall and shall cause its Subsidiaries and Representatives to, upon reasonable prior notice, give Buyer and its authorized
Representatives reasonable access during normal business hours to the Contracts, Books and Records, management and other personnel, in each case to the extent directly related to the Compound, any Product, the Acquired Assets or the Assumed
Liabilities; provided, that Buyer and its Representatives shall not interfere unreasonably with the business and operations of Seller. The terms of the Confidentiality Agreement shall apply to any information provided to Buyer and its
Representatives pursuant to this Section 7.3. Notwithstanding anything to the contrary set forth in this Section 7.3, Seller shall not be required to provide access to, or to disclose information,
where such access or disclosure would, in the opinion of outside legal counsel to Seller, (a) jeopardize the attorney client or other legal privilege of Seller, or (b) violate any applicable Law; provided, that in each case, Seller
shall: (i) give reasonable notice to Buyer of the fact that it is restricting or otherwise prohibiting access to any documents or information pursuant to this Section 7.3, (ii) inform Buyer with sufficient detail of
the reason for such restriction or prohibition, and (iii) use and cause each of its applicable Affiliates to use its commercially reasonable efforts to cause the documents or information that are subject to such restriction or prohibition to be
provided in a manner that would not reasonably be expected to violate such restriction or prohibition.
7.4 Exclusivity.
(a) During the Pre-Closing Period, Seller shall not, and Seller shall not permit any of its Affiliates
or authorize any of their respective Representatives to, directly or indirectly through another Person, (i) solicit, initiate or knowingly encourage, or take any other action designed to, or that would reasonably be expected to, facilitate or
lead to, any Transaction Proposal; (ii) enter into, continue or otherwise participate in any discussions or negotiations with, or furnish to or disclose any information regarding the Compound or any Product to, or otherwise cooperate in any way
with, any Person in connection with (or that would reasonably be expected to facilitate or lead to) any Transaction Proposal; provided, that any correspondence required to comply with the requirements of the following sentence shall not be
considered a breach of the obligations set forth in this clause (ii); or (iii) enter into any letter of intent, agreement in principle, acquisition agreement, option agreement or other similar agreement relating to a Transaction Proposal.
Seller shall, shall cause its Affiliates, and shall direct their respective Representatives to, (A) immediately cease and cause to be terminated all existing discussions or negotiations with any Person conducted heretofore with respect to any
potential Transaction Proposal and (B) promptly after the date hereof cause to be terminated all access to any electronic data room relating to any potential Transaction Proposal. The taking of any action by any Person covered by
Section 7.4(a) that violates the restrictions set forth in this Section 7.4(a) shall be deemed to be a breach of this Section 7.4(a) by Seller.
-41-
(b) If Seller or any of its Affiliates or Representatives receives any Transaction Proposal
or inquiry that would reasonably be expected to lead to a Transaction Proposal, Seller shall, shall cause its Affiliates, and shall direct their respective Representatives to, in each case, promptly advise Buyer orally and in writing of such
Transaction Proposal and the identity of the Person making any such Transaction Proposal or any such inquiry. Seller shall not, and it shall not permit any of its Affiliates or Representatives on behalf of Seller to, enter into any agreement with a
Third Party that prevents Seller from complying with its obligations under this Section 7.4.
(c) During the
period between Buyer’s exercise of the Option and the Closing, unless otherwise approved by Buyer, Seller shall not and Seller shall not permit any of its Affiliates or authorize any of their respective Representatives to, directly or
indirectly through another Person, effect or enter into an agreement with respect to any Liquidation Event. [***].
7.5 Notification of
Certain Matters. During the Pre-Closing Period, Seller shall promptly notify Buyer in writing (a) if any representation or warranty made by Seller contained in this Agreement becomes inaccurate in any
material respect, or of any failure of Seller to comply with in any material respect any covenant or agreement to be complied with by them or it under this Agreement, such that any of the conditions to Buyer’s obligations to consummate the
Transactions set forth in Section 10.1 or Section 10.3 would not be satisfied; and (b) of any written notice or other written communication received by Seller or any of its Subsidiaries from
any Governmental Authority or from any Person indicating that the Transactions may require such Person’s consent. The delivery of any notice pursuant to this Section 7.5 shall not limit or otherwise affect the remedies
available hereunder to Buyer or the conditions to Buyer’s obligations to consummate the Transactions set forth in Section 10.1 or Section 10.3.
7.6 Insurance. During the Pre-Closing Period, Seller shall maintain, at its own expense,
commercially reasonable insurance coverage provided by insurance carriers with a financial strength rating of A- or greater from A.M. Best or an equivalent rating from Standard and Poor’s. Such insurance
coverage will include clinical trial and products liability coverage, with limits of not less than $[***]. If the Closing occurs and any such insurance coverage is on a “claims-made” basis, such insurance must be maintained until at least
the [***]anniversary of the Closing Date. Maintenance of insurance coverage in accordance with this Section 7.6 will not relieve Seller of any responsibility under this Agreement for damage in excess of insurance limits.
Upon Buyer’s written request, Seller will promptly provide Buyer with a certificate from the insurer(s), or copies of policies, evidencing the insurance coverage required to be maintained by Seller under this
Section 7.6.
7.7 Manufacturing Diligence. As promptly as practicable following the date hereof and in any
event prior to the date of the Option Trigger, Seller shall, (a) provide Buyer with access to an electronic data room containing all documentation and other information reasonably necessary for Buyer to conduct full diligence on Seller as a
contract development and manufacturing company, including documentation and information with respect to the Seller’s (or as applicable, its Affiliate’s) quality management system and manufacturing process for the Compound, all
manufacturing data in Seller’s or any of its Affiliates’ possession or control with respect to the Compound, and all regulatory inspection documentation and responses relating to
-42-
Seller and its applicable Affiliates; (b) host, on a date and time mutually agreed by Seller and Buyer, an on-site
pre-qualification visit for Buyer; (c) promptly respond to Buyer’s manufacturing diligence questions and findings; (d) if requested by Buyer, complete Buyer’s vendor qualification and
quality assurance processes; and (e) provide Buyer access to Seller’s critical vendor list for the manufacture of the Compound; provided, that Seller shall not be required to disclose any Prohibited Information.
7.8 Manufacturing and Quality Agreements. If Buyer selects Seller as a contract manufacturer for the Compound following the completion
of Buyer’s due diligence described in Section 7.7, the Parties shall negotiate in good faith and use commercially reasonable efforts to agree, no later than the earlier of (i) the date that is [***] from the date
hereof and (ii) the date that is [***] after Buyer’s delivery of the Option Exercise Notice, on a form of manufacturing agreement containing terms consistent with this Section 7.8 under which Seller would have the
right to manufacture and supply to Buyer (a) up to [***]% of Buyer’s demand for the Compound related to the TATCIST Study and (b) up to [***]% of Buyer’s demand for the Compound related to the first Pivotal Clinical Trial of the
Compound initiated by Buyer (the “Manufacturing Agreement”), and a quality agreement containing customary terms with respect to the manufacture of the Compound under the Manufacturing Agreement (the “Quality
Agreement”). [***]. The supply price for the Compound supplied under the Manufacturing Agreement for use by Buyer in Phase 2 Clinical Trials and any Pivotal Clinical Trial will be $[***], subject to annual adjustments for inflation (using a
mutually agreed standard price index benchmark) and for [***]. If Seller supplies the Actinium-225 used in the manufacture of the Compound under the Manufacturing Agreement, Buyer will pay to Seller [***] for
each dose of Actinium-225 supplied. No payments for Actinium-225 will be made to Seller if Buyer supplies the Actinium-225 used
in the manufacture of the Compound under the Manufacturing Agreement. [***].
7.9 Manufacturing Technology Transfer.
(a) Effective as of the Closing, Seller, on its own behalf and on behalf of its Affiliates, hereby grants to Buyer and its Affiliates an
exclusive (except to the limited extent required for Seller to perform its obligations under the Manufacturing Agreement), perpetual, irrevocable ([***]), royalty-free, worldwide, and transferable right and license, with a right to grant sublicenses
through multiple tiers, under all Know-How and Information owned or Controlled by Seller or any of its Affiliates relating to the Manufacture of the Compound or any Product (the “Licensed Manufacturing
Know-How”) solely to Manufacture the Compound or any Product. The Licensed Manufacturing Know-How, as of the date of this Agreement, is listed on
Schedule 7.9(a).
(b) In connection with Buyer’s exercise of the Option, when and as requested by Buyer, Seller shall,
and shall cause its Affiliates to, transfer to Buyer or its designee all Licensed Manufacturing Know-How, including by providing Buyer with: (a) all Books and Records reflecting the Licensed Manufacturing
Know-How, including all documentation relating to the then-current process for the Manufacture of the Compound or any Product and any improvements or enhancements to such processes which have been made but not
yet implemented; and (b) all reasonable assistance requested by Buyer or its designee to implement the Manufacturing process at the facilities designated by Buyer; provided that such assistance shall cease on the date [***]. In order to
facilitate such transfer, Seller and its applicable Affiliates shall (i) participate in a
-43-
reasonable number of meetings (including video-conference and in person meetings) between the Parties’ experts in the critical aspects of the execution of such technology transfer;
(ii) participate in a reasonable number of post-meeting consultations among the Parties’ experts via phone or video-conference; and (iii) facilitate, at Buyer’s expense, Buyer’s entry into applicable agreements with any
Third Party suppliers who perform any Manufacturing or contracted activities relating to the Compound or any Product for Seller or any of its Affiliates as of the Closing Date. Except as expressly provided herein, each Party shall bear its own costs
and expenses in connection with any transfer made pursuant to this Section 7.9; provided that, with respect to Seller’s costs and expenses incurred under clause (b) of the immediately preceding sentence,
the first [***] of activities and support shall be at Seller’s cost and expense and thereafter shall be at Buyer’s cost and expense (with Buyer’s cost and expense being equal to [***]). Buyer shall and shall cause its Affiliates and
shall use commercially reasonable efforts to cause Payment Obligors and other sublicensees to maintain the confidentiality of all Licensed Manufacturing Know-How and to use such Licensed Manufacturing Know-How solely in connection with Manufacturing the Compound or any Product.
7.10 Delivery of Data
Site Copies(a) . Promptly, but no later than 10 days, following the date of this Agreement, Seller shall deliver to Buyer a USB flash drive or other electronic storage device containing the true and complete contents of the Data Site as of 11:59
pm, Eastern Time, on the date that is two days prior to the date of this Agreement and as of the time at which this Agreement was executed and delivered by the Parties. Promptly, but no later than 10 days, following the Closing Date, Seller shall
deliver to Buyer a USB flash drive or other electronic storage device containing the true and complete contents of the Data Site as of the Closing Date.
7.11 Biweekly Data Reports and Pre-Closing Data License. Effective upon the execution of this
Agreement, Seller shall provide Buyer with clinical reports of the status of the TATCIST Study on a [***] basis, such reports to be limited to (a) prior to Buyer’s exercise of the Option, the first [***] patients enrolled during the Pre-Closing Period and thereafter, [***] and (b) information directly related to the number of patients enrolled, clinical reports including labs, available imaging reports, the clinical condition of the
patients in regard to effectiveness of the therapy and any adverse events, in such form as shall be mutually agreed to by Seller and Buyer. Effective upon Buyer’s delivery of an Option Exercise Notice, Seller, on its own behalf and on behalf of
its Affiliates, hereby grants to Buyer and its Affiliates for the remainder of the Pre-Closing Period an exclusive, irrevocable, royalty-free, worldwide right and license, with a right to grant sublicenses
through multiple tiers, to use any and all Know-How and Information generated with respect to the Compound that is owned or Controlled by Seller or any of its Affiliates for any lawful purpose. Without
limiting Seller’s obligations under Section 2.4(b), Seller shall promptly, and in any event within [***], following Buyer’s delivery of an Option Exercise Notice deliver to Buyer copies of any Books and Records
reflecting such Know-How and Information.
7.12 Clinical Trial Agreement. Promptly following
the date hereof, the Parties shall negotiate in good faith a clinical trial agreement on customary terms under which Seller and its Affiliates would continue to conduct the TATCIST Study on behalf of Buyer following the Closing (the
“Clinical Trial Agreement”).
-44-
ARTICLE VIII
OTHER AGREEMENTS
8.1
Books and Records; Access; Assistance.
(a) Subject to Section 8.1(b), including limitations that are
required to preserve any applicable attorney-client privilege, after the Closing, as applicable, Buyer and Seller shall and Seller shall cause its applicable Affiliates to make reasonably available to each other and their respective Affiliates and
Representatives (as reasonably requested) and to any Taxing Authority or any other Governmental Authority, all books and records to the extent related to the Compound, the Acquired Assets or the Assumed Liabilities for all periods prior to the
Closing Date and shall use commercially reasonable efforts to preserve, for at least [***] after the Closing Date or, if applicable and later, the expiration of the applicable statutes of limitations or extensions thereof: (i) all such books
and records, (ii) Tax Returns solely pertaining to the research, development or manufacture of the Compound, the Acquired Assets or any Products and (iii) government Contract information, records or documents, to the extent relating to the
research, development or manufacture of the Compound, the Acquired Assets or the Assumed Liabilities; provided, that a Party and its Affiliates and Representatives shall only be entitled to access such books and records identified in clause
(ii) to the extent necessary in connection with any Tax or other Proceeding, including any Tax audit, by or before any Governmental Authority or to satisfy their respective Tax, accounting or financial reporting obligations. Buyer and Seller
shall also make available to each other for such period, during normal business hours and upon reasonable advanced request, personnel responsible for preparing or maintaining such information, records and documents; provided, that no such
personnel access shall unreasonably disrupt the other Party’s normal business operations. The right to access provided by this Section 8.1 shall include the right to make copies of accessed documents; provided,
that all such copies shall be at the sole cost and expense of the requesting Party. The Party providing access under this Section 8.1 shall have the right to reasonably redact all such documents.
(b) Notwithstanding the foregoing, this Section 8.1 shall not provide Buyer or Seller (or their respective
Representatives) any access rights to documents, information or personnel of the other Party (i) which access would violate any Laws or Contractual or other obligations regarding the confidentiality thereof (unless any such violation could be
and is avoided by the recipient’s execution and delivery of an appropriate confidentiality agreement), (ii) which access would, in the opinion of outside legal counsel to the Party receiving such request, jeopardize any attorney-client, work
product, or like privilege, or (iii) for the purpose of use in connection with potential or actual litigation, arbitration or mediation between Buyer or any Affiliate of Buyer, on the one hand, and Seller or any Affiliate of Seller, on the
other hand (nor, for the avoidance of doubt, shall Buyer or Seller or any of their respective Affiliates have any right to use any document or information obtained from the other pursuant to this Section 8.1 in any such
Proceeding); provided, that in the case of the preceding clauses (i) and (ii), the Party that is requested to provide such access shall (x) give reasonable notice to the requesting Party of the fact that it is restricting or otherwise
prohibiting access to any documents or information pursuant to this Section 8.1, (y) inform the requesting Party with sufficient detail of the reason for such restriction or prohibition and (z) use its commercially
reasonable efforts to cause the documents or information that are subject to such restrictions or prohibitions to be provided in a manner that would not reasonably be expected to violate such restrictions or prohibitions.
-45-
8.2 Privileged Matters. Each Party acknowledges that: (a) each Party and its
Affiliates has or may obtain Privileged Information; (b) there are or may be Litigation Matters affecting both of Buyer and Seller or their respective Affiliates; (c) both Buyer and Seller have a common legal interest in Litigation
Matters, in the Privileged Information and in the preservation of the confidential status of the Privileged Information, in each case to the extent relating to the Business; and (d) both Buyer and Seller intend that the Transactions and any
transfer of Privileged Information in connection therewith shall not operate as a waiver of any potentially applicable privilege.
8.3 Non-Competition. From the Closing Date through the expiration of the Deferred Variable
Payment Term, (a) neither Seller nor its Affiliates shall, directly or indirectly, research, seek to identify, develop, make, have made, use, distribute, market, sell or otherwise commercialize any Competing Product and (b) neither Seller
nor its Affiliates shall grant to any Third Party any right or license to research, seek to identify, discover, develop, make, have made, use, distribute, market, sell or otherwise commercialize any Competing Product. Seller acknowledges and agrees
that its agreements in this Section 8.3 are an integral part of the Transactions and without the agreements in this Section 8.3, Buyer would not have entered into this Agreement. [***].
8.4 TATCIST Study and Regulatory Transfers. As soon as is reasonably practicable (but, in the absence of any event described in clause
(iii) or clause (vi) of the definition of Material Adverse Effect, within (1) the later of [***] following the Buyer’s request therefor and [***] following the Closing Date, (a) Seller shall, or shall cause its applicable
Affiliate to, execute and deliver to the applicable Regulatory Authority all documents required to authorize the transfer to Buyer of all Transferred Governmental Authorizations, Transferred Regulatory Materials and the sponsorship of the TATCIST
Study, in each case, effective as of the Closing, and (b) Buyer shall execute and deliver to the applicable Regulatory Authority all documents required to accept such transfer. From and after the Closing, Buyer shall assume all operational and
financial responsibility for the TATCIST Study (subject to the allocation of Assumed Liabilities and Excluded Liabilities set forth in this Agreement) and, subject to the terms of any Contract to be entered into by Buyer and Excel Diagnostics and
Nuclear Oncology Center with respect to the TATCIST Study, Excel Diagnostics and Nuclear Oncology Center shall continue to be a primary clinical site for the TATCIST Study. Each of Seller and Buyer shall, and shall cause their respective Affiliates
to, take all actions required to facilitate the orderly transition of the TATCIST Study to Buyer, and Seller shall, or shall cause its applicable Affiliates to, provide Buyer with such regulatory assistance regarding the Compound and the TATCIST
Study as Buyer may reasonably request. [***] shall be responsible for all reasonable costs and expenses of third-party advisors or consultants engaged by Seller for the purpose of providing such regulatory assistance; provided that [***]
prior approval will be required to the extent such costs or expenses exceed $[***] in the aggregate, such approval not to be unreasonably withheld, delayed or conditioned.
8.5 Additional TATCIST Transfer Services. Upon Buyer’s reasonable request, Seller shall make available to Buyer and its Affiliates
during normal business hours those of Seller’s and its Affiliates’ personnel (to the extent such personnel continue to be employed by Seller or its Affiliates) who, prior to the Closing, were involved in the TATCIST Study and the
development of the Compound and who are knowledgeable with regard to research and development, regulatory and clinical matters regarding the TATCIST Study and the Compound, to consult with Buyer and
-46-
its Affiliates regarding such matters and provide assistance with respect to the transfer of any INDs from Seller to Buyer pursuant to this Agreement. All such access, consultation and assistance
shall be provided at no cost to Buyer from the Closing Date until the date that is [***] after the Closing Date. For any of such consultation or assistance provided after the date that is [***] after the Closing Date, Buyer shall pay to Seller
$[***] of working time spent by Seller’s or its Affiliates’ employees engaged in providing such consultation or assistance. Buyer shall pay all undisputed amounts due under this Section 8.5 within [***] following
its receipt from Seller of a reasonably detailed invoice therefor.
8.6 Further Assurances. Seller shall, and shall cause its
applicable Affiliates to, at any time and from time to time after the Closing Date, upon the request of Buyer, do, execute, acknowledge, deliver and file, or cause to be done, executed, acknowledged, delivered or filed, all such further acts, deeds,
transfers, conveyances, assignments or assurances as may be reasonably required for the better transferring, conveying, assigning and assuring to Buyer, or for the aiding and assisting in the reducing to possession by Buyer of, any of the Acquired
Assets, or for otherwise carrying out the purposes of this Agreement and the Ancillary Agreements and the consummation of the Transactions.
8.7 [***].
(a) Buyer
shall not terminate or deprioritize the development of the Compound in favor of internal Ac-PSMA-617 or Ac-PSMA I&T
development programs prior to the achievement of all Development and Regulatory Events. Buyer may, in its discretion, deprioritize or terminate the development of the Compound based on scientific, commercial and other relevant factors, including the
regulatory environment, safety and efficacy, expected and actual market exclusivity and competition, patentability, the expected and actual cost and time to develop, product life, the expected and actual pricing, reimbursement and profitability, the
expected likelihood of Regulatory Approval, and the amounts of marketing and promotional expenditures required.
(b) Buyer shall notify
Seller of the complete termination by Buyer and its Affiliates of all development activities with respect to the Compound prior to the achievement of all Development and Regulatory Events (a
“Pre-Commercial Termination”). In the event of a Pre-Commercial Termination, Buyer shall notify Seller if Buyer has not sold, licensed or otherwise
transferred the rights to develop and commercialize the Compound to a Third Party within [***] after the date on which the Pre-Commercial Termination became effective. Promptly after Seller’s receipt of
such notice, Buyer will negotiate with Seller for up to [***] the terms on which Buyer will promptly transfer to Seller, and Seller will acquire, all pre-clinical and clinical data, orphan drug designations,
INDs and other Regulatory Materials then owned by Buyer or any of its Affiliates that (i) were included in the Acquired Assets or (ii) relate exclusively to the Compound [***] Seller will not be required to pay a purchase price for the
transfer of the Reversion assets, but will be financially responsible for, and shall pay directly or reimburse Buyer for, all out-of-pocket costs incurred by Buyer and
its Affiliates in connection with a Reversion that exceed [***].
-47-
8.8 Residual Know-How License(a) . Effective
as of the Closing, Seller, on its own behalf and on behalf of its Affiliates, hereby grants to Buyer and its Affiliates an exclusive, perpetual, irrevocable, royalty-free, worldwide, and transferable right and license, with a right to grant
sublicenses through multiple tiers, under all Know-How and Information owned or Controlled by Seller or any of its Affiliates to the extent relating to 225-Ac-PSMA or the Compound, including all radiolabeled complexes thereof, that is not included in the Licensed Manufacturing Know-How or Transferred Know-How (the “Other Licensed Know-How”) solely to research, develop, Manufacture, commercialize and otherwise exploit the Compound or any Product. Upon
Buyer’s request therefor, Seller shall and shall cause its Affiliates to provide Seller and its Affiliates with access to and copies of the portions of the Books and Records related to the Compound or 225-Ac-PSMA pertaining to or reflecting the Other Licensed Know-How that are not included in the Transferred Books and Records.
ARTICLE IX
TAX MATTERS
9.1 Transfer Taxes. Transfer Taxes shall be borne by Seller. Seller and Buyer shall cooperate to timely prepare and file any
Tax Returns relating to such Transfer Taxes, including any claim for exemption or exclusion from the imposition of any Transfer Taxes. With respect to any Tax Returns relating to Transfer Taxes required to be filed by Buyer or any of its Affiliates,
Seller shall pay to Buyer, not later than [***] before the due date for payment of such Transfer Taxes, the amount of Transfer Taxes shown to be due on such Tax Return in immediately available funds in accordance with written instructions of Buyer.
The Party filing such Tax Return shall furnish to the other Party a copy of the relevant Tax Return and a copy of a receipt showing payment of any such Transfer Tax.
9.2 VAT. Each of the Option Fee payable pursuant to Section 2.1(b), the Option Exercise Price, the payments in
respect of Additional Site Dosings pursuant to Section 2.3, any Deferred Fixed Payment and any Deferred Variable Payment is exclusive of any VAT. Seller shall be responsible for any VAT imposed in connection with the
transfer of the Acquired Assets or the grant of the Option pursuant to this Agreement, and shall timely issue a valid invoice to Buyer with respect to any VAT.
9.3 Tax Cooperation. Subject to Section 8.1, Buyer and Seller agree to furnish or cause their Affiliates to
furnish, to each other, upon request, as promptly as practicable, such information and assistance relating to the Acquired Assets or the Business as is reasonably necessary for the filing of all Tax Returns and other Tax filings, the preparation for
any audit by any Taxing Authority and the defense of any Claim or Proceeding relating to Taxes of the Acquired Assets or the Business. No Party shall be required to deliver or otherwise provide cooperation, documentation or information with respect
to Taxes that is not related to the Acquired Assets or the operation of the Business or that such Party considers in good faith to be proprietary, including any documentation or information relating to any consolidated, combined or unitary Tax
Return of the Parties or any of their respective Affiliates, or any other Tax Return of the Parties or any of their respective Affiliates to the extent not related to the Acquired Assets or the Business.
9.4 Straddle Period Allocation. In the case of any Tax with respect to the Acquired Assets that is assessed with respect to a taxable
period beginning before the Closing Date and ending after the Closing Date (a “Straddle Period”), the amount of such Taxes based on or measured by income, sales, use, receipts or similar items (other than property and ad
valorem Taxes) of such Acquired Assets for the portion of the Straddle Period ending on the Closing Date shall be determined based on an interim closing of the books as of the close of business on the Closing Date, and the amount of any other
Taxes and any exemptions, allowances or deductions determined for the entire Straddle Period that, in each case, relate to the portion of the Straddle Period ending on and including the Closing Date shall be
pro-rated on a per diem basis.
-48-
9.5 Section 56.4 Election. The Parties intend that the conditions set forth in
subsection 56.4(7) of the ITA have been met such that subsection 56.4(5) of the ITA applies to any “restrictive covenants” (as defined in subsection 56.4(1) of the ITA) granted by Seller pursuant to this Agreement with respect to the
Business, including the conditions set forth in Section 8.3 (the “Restrictive Covenants”). The Parties acknowledge and agree (a) that the Restrictive Covenants are integral to this Agreement and are
being granted, executed and delivered to maintain or preserve the fair market value of the Acquired Assets, and (b) that no proceeds or other amount received or receivable under this Agreement by Seller shall be for granting any Restrictive
Covenant under this Agreement. At the request of Buyer or Seller and to the extent permitted by the ITA, the Parties shall make and file any election or amended election in prescribed form (or such other form as Buyer or Seller may reasonably
request) and within the prescribed time limits pursuant to subsection 56.4(7) of the ITA, and any analogous provision of Canadian provincial or territorial Tax Laws.
9.6 Tax Provision Priority. To the extent that this Article IX conflicts with any other provision in this Agreement, this
Article IX shall govern.
ARTICLE X
CONDITIONS TO CLOSING
10.1 Conditions to Each Party’s Obligations. The respective obligations of each Party to consummate the Transactions
are subject to the satisfaction, at or prior to the Closing, of each of the following conditions, any and all of which may be waived, in whole or in part, by Seller and Buyer, as the case may be, to the extent permitted by Law:
(a) No Adverse Law or Order shall be in effect, and no Proceedings shall be pending or threatened by any Governmental Authority seeking any
such Adverse Law or Order.
(b) Buyer shall have delivered the Option Exercise Notice to the Seller pursuant to, and in accordance with,
the provisions of Section 2.4.
10.2 Conditions to Seller’s Obligations. The obligation
of Seller to consummate the Transactions is subject to the satisfaction, at or prior to the Closing, of each of the following conditions, unless waived by Seller:
(a) The representations and warranties set forth in Article VI shall be true and correct as of the Closing Date (except that
representations and warranties that by their terms speak specifically as of the date of this Agreement or some other date shall be true and correct as of such date), except for inaccuracies that, individually or in the aggregate, would not
reasonably be expected to prevent or materially delay Buyer’s consummation of the Transactions.
-49-
(b) Buyer shall have performed and complied in all material respects with all covenants,
agreements and obligations required to be performed or complied with by Buyer under this Agreement at or prior to the Closing.
(c) Buyer
shall have delivered to Seller:
(i) each of the documents required to be delivered by Buyer or its Affiliates pursuant to
Section 4.2(b); and
(ii) a certificate dated as of the Closing Date, signed by a duly authorized
officer of Buyer, certifying that the conditions set forth in Sections 10.2(a) and 10.2(b) have been duly satisfied.
10.3
Conditions to Buyer’s Obligations. The obligation of Buyer to consummate the Transactions is subject to the satisfaction, at or prior to the Closing, of each of the following conditions, unless waived by Buyer:
(a) The representations and warranties set forth in Article V (i) that are (A) qualified by materiality or Material Adverse
Effect qualifications or (B) the Fundamental Representations shall be true and correct as of the Closing Date (except that any such representations that by their terms speak specifically as of the date of this Agreement or some other date shall
be true and correct as of such date); and (ii) other than the Fundamental Representations, that are not qualified by materiality or Material Adverse Effect qualifications shall be true and correct as of the Closing Date (except that
representations and warranties that by their terms speak specifically as of the date of this Agreement or some other date shall be true and correct as of such date), except for, in the case of clause (ii), inaccuracies that, individually or in the
aggregate, are not and would not reasonably be expected to have a Material Adverse Effect.
(b) Seller shall have performed and complied in
all material respects with all covenants, agreements and obligations required to be performed or complied with by Seller under this Agreement at or prior to the Closing.
(c) Since the date of this Agreement, there shall not have been a Material Adverse Effect.
(d) Seller shall have delivered to Buyer:
(i) each of the documents required to be delivered by Seller pursuant to Section 4.2(a); and
(ii) a certificate dated as of the Closing Date, signed by a duly authorized officer of Seller, certifying that the conditions
set forth in Sections 10.3(a) through 10.3(c) have been duly satisfied.
10.4 Frustration of Closing Conditions.
Except as required by applicable Law, neither Seller nor Buyer may rely on the failure of any condition set forth in Sections 10.1, 10.2 or 10.3, as the case may be, to be satisfied if such Party’s (or its Affiliate’s)
breach of any representation, warranty, covenant or agreement set forth in this Agreement has been the cause of, or resulted in, such failure.
-50-
ARTICLE XI
TERMINATION
11.1
Termination Prior to the Closing. This Agreement may be terminated at any time prior to the Closing:
(a) by the mutual
written agreement of Seller and Buyer;
(b) by Seller or Buyer, by written notice to the other Party, if the Closing has not occurred by
11:59 p.m., Eastern Time, on [***] (the “Outside Date”); provided, however, that the right to terminate this Agreement pursuant to this Section 11.1(b) shall not be available to any Party
whose breach of any representation, warranty, covenant or agreement set forth in this Agreement has been the cause of, or resulted in, the Closing not occurring prior to the Outside Date;
(c) subject to Section 11.2(b) below, by Seller or Buyer upon the occurrence of a Study Termination Event;
(d) by Seller, by written notice to Buyer, if, after the date on which Buyer delivers the Option Exercise Notice, Buyer shall have breached any
of its representations or warranties or failed to comply with any of its covenants or agreements contained in this Agreement, which breach or failure (i) would give rise to the failure of the conditions set forth in
Section 10.2(a) or Section 10.2(b) and (ii) is incapable of being cured, or is not cured, by Buyer within [***] following receipt of written notice of such breach or failure to comply from
Seller; provided, however, that the right to terminate this Agreement under this Section 11.1(d) shall not be available to Seller if Seller has breached any of its representations or warranties or failed to
comply with any of its covenants or agreements contained in this Agreement in any material respect;
(e) by Buyer, by written notice to
Seller, if, after the date on which Buyer delivers the Option Exercise Notice, Seller shall have breached any of its representations or warranties or failed to comply with any of its covenants or agreements contained in this Agreement, which breach
or failure (i) would give rise to the failure of the conditions set forth in Section 10.3(a) or Section 10.3(b) and (ii) is incapable of being cured, or is not cured, by Seller within
[***] following receipt of written notice of such breach or failure to comply from Buyer; provided, however, that the right to terminate this Agreement under this Section 11.1(e) shall not be available to
Buyer if Buyer has breached any of its representations or warranties or failed to comply with any of its covenants or agreements contained in this Agreement in any material respect;
(f) by either Seller or Buyer, by written notice to the other, in the event that any final and
non-appealable Adverse Law or Order is issued by a Governmental Authority;
(g) by Buyer, by
written notice to Seller, at any time prior to the delivery of the Option Exercise Notice, if Buyer shall determine in its sole discretion that it does not wish to exercise the Option; or
-51-
(h) by either Seller or Buyer, by written notice to the other, if Buyer has not delivered
the Option Exercise Notice to Seller by the end of the Option Period (as the Option Period may be extended pursuant to Section 2.4(c)).
11.2 Procedure and Effect of Termination. In the event of the termination of this Agreement and the abandonment of the Transactions
pursuant to Section 11.1, written notice thereof shall forthwith be given by the terminating Party to the other Party, each Party shall comply and cause its Affiliates and direct its Representatives to comply with its
obligations under the Confidentiality Agreement related to the destruction or return of “[Confidential Information]” (as defined in the Confidentiality Agreement), and no Party to this Agreement shall have any Liability under this
Agreement to any other Party except for any Liability of any Party for Fraud or any willful and material breach by such Party of any of its covenants or agreements set forth in this Agreement; it being understood and agreed that the Confidentiality
Agreement, this Section 11.2 and Article XIII (except for Section 13.3) shall remain in full force and effect following such termination. Notwithstanding anything to the contrary herein,
(a) this Section 11.2, Article XII and Article XIII (except for Section 13.3) shall survive any termination of this Agreement pursuant to Section 8.7
and (b) if Seller provides notice of intent to terminate this Agreement pursuant to Section 11.1(c), Buyer shall have [***] following its receipt of Seller’s notice of termination in which to deliver an Option
Exercise Notice. If Buyer delivers an Option Exercise Notice within such period (the “Termination-Period Exercise”), Seller’s notice of termination shall be deemed rescinded and this Agreement shall thereafter remain in effect
according with its terms; provided that in the event of a Termination-Period Exercise, Seller shall not be liable for any Losses related to the Study Termination Event pursuant to Article XII or otherwise.
ARTICLE XII
INDEMNIFICATION
12.1
Survival; Effect of Materiality Qualifiers; Losses. The representations, warranties and covenants of Seller and Buyer contained in this Agreement shall survive the Closing for the period set forth in this
Section 12.1. All representations and warranties contained in this Agreement and all claims with respect thereto shall terminate upon the date that is [***] after the Closing Date; provided, however, that the
representations and warranties contained in [***] (collectively, the “Fundamental Representations”), shall, other than with respect to the representations and warranties in [***], terminate upon the earlier of (i) the date that
is [***] after the Closing Date and (ii) [***], and the representations and warranties in [***] shall terminate on the date that is [***] following the [***] anniversary of Closing. All claims with respect to the covenants of Seller and Buyer
(i) which by their terms do not contemplate performance after the Closing shall terminate upon the date that is [***] after the Closing Date, and (ii) which by their terms contemplate performance after the Closing, shall terminate upon
[***]. In the event that notice of any claim for indemnification under this Article XII has been given pursuant to Section 12.4 or Section 12.5, as the case may be, within the applicable
survival period, the representations and warranties or covenants that are the subject of such indemnification claim (and the right to pursue such claim) shall survive with respect to such claim until such time as such claim is finally resolved. It
is the intention of the Parties that the survival periods and termination date set forth in this Section 12.1 supersede any statute of limitations applicable to such representations and warranties and covenants or claim
with respect thereof.
-52-
12.2 Indemnification by Seller.
(a) From and after the Closing and subject to the provisions of this Article XII, Seller shall indemnify, defend and hold harmless
Buyer, its Affiliates and their respective officers, directors, employees, agents, successors and permitted assigns (collectively, the “Buyer Indemnified Parties”) from, against and in respect of, and compensate and reimburse them
for, any and all Losses imposed on, sustained, incurred or suffered by any of the Buyer Indemnified Parties to the extent arising out of or resulting from:
(i) the breach of any representation or warranty made by Seller in this Agreement or the certificate delivered by Seller
pursuant to Section 10.3(d)(ii);
(ii) the breach of any covenant or agreement of Seller in this
Agreement; or
(iii) any Excluded Liability.
(b) Seller shall have no Liability for any claim for indemnification pursuant to Section 12.2(a)(i) unless the
aggregate amount of Losses in respect of breaches of Seller’s representations and warranties exceeds the Deductible, in which event Seller shall be liable for Losses in excess of the Deductible up to the Cap; provided,
however, that the foregoing limitations set forth in this Section 12.2(b) shall not apply to (x) breaches of the Fundamental Representations, or (y) claims based upon Fraud with respect to the
representations and warranties made by Seller in this Agreement.
(c) Other than in the case of breaches of the Fundamental Representations
or Fraud with respect to the representations and warranties made by Seller in this Agreement or the certificate delivered by Seller pursuant to Section 10.3(d)(ii), Seller’s aggregate liability for indemnification
under Section 12.2(a)(i) shall in no event exceed the Cap; provided, that to the extent that Buyer or any other Buyer Indemnified Party is not able to recover any Losses resulting from any such breach solely as a
result of the Cap in effect at such time, and the amount of the Cap subsequently increases, then the amount of any such unrecovered Losses for such breach shall be recoverable subject to such increased Cap; provided, further, that, for
clarity, the foregoing proviso shall not extend the applicable survival period under Section 12.1.
(d) Other
than in the case of Fraud with respect to the representations and warranties made by Seller in this Agreement or the certificate delivered by Seller pursuant to Section 10.3(d)(ii), the cumulative indemnification
obligations of Seller under Sections 12.2(a)(i) and 12.2(a)(ii) (other than in the case of [***]) shall in no event exceed, in the aggregate, [***]. To the extent that Buyer or any other Buyer Indemnified Party is not able to
recover any Losses resulting from any such breach solely as a result of the limitation in the immediately preceding sentence, and the aggregate of the amount actually received by and the amount due and payable (but not yet paid) to Seller under this
Agreement subsequently increases, then the amount of any such unrecovered Losses for such breach shall be recoverable up to the amount of such increase; provided, that, for clarity, this sentence shall not extend the applicable survival
period under Section 12.1.
-53-
(e) The right of Buyer to indemnification pursuant to Section 12.2
will not be affected by any investigation conducted or knowledge acquired (or capable of being acquired) at any time, whether before or after the execution and delivery of this Agreement or the Closing, with respect to any accuracy of any
representation or warranty, or performance of or compliance with any covenant or agreement herein. [***].
12.3 Indemnification by
Buyer.
(a) From and after the Closing and subject to the provisions of this Article XII, Buyer shall indemnify, defend and hold
harmless Seller, its Affiliates and their respective officers, directors, employees, agents, successors and permitted assigns (collectively, the “Seller Indemnified Parties”, and each of the Buyer Indemnified Parties and the Seller
Indemnified Parties, an “Indemnified Party”) from, against and in respect of, and compensate and reimburse them for, any and all Losses imposed on, sustained, incurred or suffered by any of the Seller Indemnified Parties to the
extent arising out of or resulting from:
(i) the breach of any representation or warranty made by Buyer in this Agreement
or the certificate delivered by Buyer pursuant to Section 10.2(c)(ii);
(ii) the breach of any
covenant or agreement of Buyer in this Agreement; or
(iii) any Assumed Liability.
(b) Buyer shall have no Liability for any claim for indemnification pursuant to Section 12.3(a)(i) unless the
aggregate amount of Losses in respect of breaches of Buyer’s representations and warranties exceeds the Deductible, in which event Buyer shall be liable solely for Losses in excess of the Deductible up to the Cap; provided,
however, that the foregoing limitations set forth in this Section 12.3(b) shall not apply to (x) breaches of Fundamental Representations, or (y) claims based upon Fraud with respect to the representations
and warranties made by Buyer in this Agreement or the certificate delivered by Buyer pursuant to Section 10.2(c)(ii).
(c) Other than in the case of breaches of the Fundamental Representations or Fraud with respect to the representations and warranties made by
Buyer in this Agreement or the certificate delivered by Buyer pursuant to Section 10.2(c)(ii), Buyer’s aggregate liability for indemnification under Section 12.3(a)(i) shall in no event exceed
the Cap; provided, that to the extent that Seller or any other Seller Indemnified Party is not able to recover any Losses resulting from any such breach solely as a result of the Cap in effect at such time, and the amount of the Cap
subsequently increases, then the amount of any such unrecovered Losses for such breach shall be recoverable subject to such increased Cap; provided, further, that, for clarity, the foregoing proviso shall not extend the applicable
survival period under Section 12.1.
-54-
(d) Other than in the case of Fraud with respect to the representations and warranties made
by Buyer in this Agreement or the certificate delivered by Buyer pursuant to Section 10.2(c)(ii), the cumulative indemnification obligations of Buyer under Sections 12.3(a)(i) and 12.3(a)(ii) (other than in
the case of [***]) shall in no event exceed, in the aggregate, [***]. To the extent that Seller or any other Seller Indemnified Party is not able to recover any Losses resulting from any such breach solely as a result of the limitation in the
immediately preceding sentence, and the aggregate of the amount actually received by and the amount due and payable (but not yet paid) to Seller under this Agreement subsequently increases, then the amount of any such unrecovered Losses for such
breach shall be recoverable up to the amount of such increase; provided, that, for clarity, this sentence shall not extend the applicable survival period under Section 12.1.
(e) The right of Seller to indemnification pursuant to Section 12.3 will not be affected by any investigation
conducted or knowledge acquired (or capable of being acquired) at any time, whether before or after the execution and delivery of this Agreement or the Closing, with respect to any accuracy of any representation or warranty, or performance of or
compliance with any covenant or agreement herein. [***].
12.4 Third Party Claims.
(a) In the event that any Claim for which Buyer or Seller (an “Indemnifying Party”) may have Liability to any
Indemnified Party hereunder is asserted against or sought to be collected from any Indemnified Party by a Third Party (a “Third Party Claim”), Buyer (on behalf of any Buyer Indemnified Party) or Seller (on behalf of any Seller
Indemnified Party), as applicable, shall promptly, but in no event more than [***] following such Indemnified Party’s receipt of a Third Party Claim, notify the Indemnifying Party in writing of such Third Party Claim, the amount or the
estimated amount of damages sought under such Third Party Claim to the extent then ascertainable (which amount shall not be conclusive of the final amount of such claim), any other remedy sought thereunder, any relevant time constraints relating
thereto and, to the extent practicable, any other material details pertaining thereto (a “Claim Notice”); provided, however, that the failure to timely give a Claim Notice shall affect the rights of an Indemnified
Party hereunder only to the extent that such failure has an actual and materially prejudicial effect on the defenses or other rights available to the Indemnifying Party with respect to such Third Party Claim.
(b) The Indemnifying Party shall have the right, at its option and expense, to defend the Indemnified Party in connection with any Third Party
Claim and direct and control such defense with counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnified Party provided, however, that the Indemnifying Party may not assume control of the defense of
any Third Party Claim (i) involving any criminal proceeding, action, indictment, allegation or investigation, or in which relief other than monetary damages is sought, (ii) involving the alleged misuse, infringement, misappropriation or
violation of any Intellectual Property, (iii) involving a purported class action, (iv) if the Indemnifying Party has not notified the Indemnified Party in writing that it will be liable to indemnify the Indemnified Party with respect to
all Losses relating to such Third Party Claim, subject to the limitations of Section 13.2 or Section 13.3, as applicable, or (v) if the Third Party Claim relates to a Proceeding by any
Regulatory Authority with respect to the Compound or any Product. In addition, the Indemnifying Party may not maintain the defense of a Third Party Claim if it has failed to defend such Third Party Claim in good faith. If the Indemnifying Party
elects to defend the Indemnified Party against a Third Party Claim, it shall within [***] after its receipt of the applicable Claim Notice (or sooner, if the nature
-55-
of the Third Party Claim so requires) notify the Indemnified Party of its intent to do so. Once the Indemnifying Party has assumed the defense of a Third Party Claim, the Indemnified Party shall
have the right, but not the obligation, to participate in any such defense and to employ separate counsel of its choosing at its own cost and expense; provided, however, that such Indemnified Party shall be entitled to participate in
any such defense with separate counsel at the expense of the Indemnifying Party if (A) so requested by the Indemnifying Party to participate, (B) in the reasonable opinion of outside counsel to the Indemnified Party a conflict or potential
conflict exists between the Indemnified Party and the Indemnifying Party that would make such separate representation advisable, or (C) in the reasonable opinion of outside counsel to the Indemnified Party there are legal defenses available to
the Indemnified Party that are different from or additional to those available to Indemnifying Party; and provided, further, that the Indemnifying Party shall not be required to pay for more than one such counsel (plus any appropriate
local counsel) for all Indemnified Parties in connection with any Third Party Claim. If after assuming the defense of a Third Party Claim the Indemnifying Party determines that it is not required to provide indemnification therefor, it shall
promptly notify the Indemnified Party, cease to control the defense of such Third Party Claim, and shall nonetheless be responsible for all costs of defense incurred by it prior to such notice. Neither the Indemnifying Party nor the Indemnified
Party shall, without the written consent of Buyer (on behalf of any Buyer Indemnified Party) or Seller (on behalf of any Seller Indemnified Party), as applicable, settle or compromise any Third Party Claim or permit a default or consent to entry of
any Order unless (1) the claimant provides to such other party an unqualified release of the Indemnified Parties and Indemnifying Parties from all liability in respect of such Third Party Claim, (2) such settlement does not involve any
injunctive relief binding upon the Indemnified Party or any of its Affiliates, (3) such settlement does not encumber any of the material assets of any Indemnified Party or impose any restriction or condition that would apply to or adversely
affect any Indemnified Party or the conduct of any Indemnified Party’s business and (4) such settlement does not involve any admission of liability or wrongdoing or violation of Law by any Indemnified Party or any of its Affiliates.
(c) If the Indemnifying Party elects not to defend the Indemnified Party against a Third Party Claim, or is not permitted to assume the defense
of a Third Party Claim pursuant to Section 12.4(b), the Indemnified Party shall have the right but not the obligation to assume its own defense, it being understood that any right of the Indemnified Party to indemnification
for a Third Party Claim shall not be adversely affected by assuming the defense of such Third Party Claim.
(d) The Indemnified Party and
the Indemnifying Party shall reasonably cooperate in order to ensure the proper and adequate defense of a Third Party Claim. The Indemnified Party and the Indemnifying Party shall keep each other fully informed with respect to the status of such
Third Party Claim.
(e) The Indemnified Party and the Indemnifying Party shall use commercially reasonable efforts to avoid production of
confidential information (consistent with applicable Law), and to cause all communications among employees, counsel and others representing any party to a Third Party Claim to be made so as to preserve any applicable attorney-client or work-product
privileges.
-56-
12.5 Direct Claims.
(a) If an Indemnified Party wishes to make a claim for indemnification hereunder for a Loss that does not result from a Third Party Claim (a
“Direct Claim”), Buyer (on behalf of any Buyer Indemnified Party) or Seller (on behalf of any Seller Indemnified Party), as applicable, shall notify the Indemnifying Party in writing of such Direct Claim, the amount or the estimated
amount of damages sought with respect to such Direct Claim to the extent then ascertainable (which amount shall not be conclusive of the final amount of such claim), any other remedy sought thereunder, any relevant time constraints relating thereto
and, to the extent practicable, any other material details pertaining thereto; provided, however, that the failure to give such a claim notice shall affect the rights of an Indemnified Party hereunder only to the extent that such
failure has an actual and materially prejudicial effect on the defenses or other rights available to the Indemnifying Party with respect to such claim.
(b) The Indemnifying Party shall have a period of [***] within which to respond to such Direct Claim. If the Indemnifying Party does not
respond within such [***] period, the Indemnified Party shall provide a second notice and if Indemnifying Party does not respond to such second notice within [***], will be deemed to have accepted the Direct Claim. If the Indemnifying Party rejects
all or any part of the Direct Claim, the Indemnified Party shall be free to seek enforcement of its rights to indemnification under this Agreement with respect to such Direct Claim.
12.6 Set-Off(a) . In addition to all other remedies contemplated herein, subject to
Section 12.2, Buyer shall have the right and option (but not the obligation) to set-off against and deduct from any Deferred Fixed Payment or Deferred Variable Payment that becomes
due and payable hereunder the amount of Losses relating to any indemnification claim made by a Buyer Indemnified Party finally determined to be recoverable under this Agreement with respect to such claim.
12.7 Adjustment to Losses.
(a) [***]. [***].
(b)
[***]. [***].
(c) [***]. [***].
12.8 Characterization of Indemnification Payments. Except as otherwise required by applicable Law, all payments made by an Indemnifying
Party to an Indemnified Party in respect of any claim pursuant to Section 12.2 or Section 12.3 shall be treated as adjustments to the Purchase Price for all applicable Tax purposes.
12.9 Exclusive Remedy. Subject to Section 13.10, and except as otherwise expressly provided in this Agreement
(including disputes under Section 1.1 (Net Sales), which are to be resolved pursuant to the procedures set forth in Schedule 1.1(A)), the Parties acknowledge that their sole and exclusive monetary remedy after
the Closing with respect to any claims relating to, arising under, or resulting from this Agreement, and the sole and exclusive monetary remedy available to any Buyer Indemnified Party or Seller Indemnified Party after the Closing with respect
-57-
to any claims relating to, arising under, or resulting from this Agreement, shall be pursuant to the indemnification provisions set forth in this Article XII. The foregoing shall in no way
limit (a) the remedies available to any Party in the case of Fraud or any Person under any Ancillary Agreement or (b) the rights of a Party to seek equitable remedies (including specific performance or injunctive relief) or any remedies
available to it under applicable Law in the event of a Party’s failure to comply with its indemnification obligations hereunder.
12.10 Satisfaction of Indemnification Claims. Subject to the rights of Buyer set forth in Section 12.6, Buyer
or Seller, as applicable, shall pay the amount of any indemnification claim for which it agrees it is liable, or that has been finally determined to be owing by a court of competent jurisdiction or an arbitral body, in each case in accordance with
this Agreement, and which has not otherwise been satisfied in accordance with this Agreement, by wire transfer of funds within 30 days after such agreement or determination.
ARTICLE XIII
MISCELLANEOUS
13.1
Assignment.
(a) Except as provided in Section 13.1(b), this Agreement may not be assigned or transferred
by either Party in whole or in part, nor may any Party assign or transfer any rights or obligations created by this Agreement, without the prior written consent of the other Party, which consent will not be unreasonably withheld, conditioned or
delayed.
(b) Buyer may assign or transfer this Agreement, or any rights or obligations hereunder in whole or in part, to (i) one or
more of its Affiliates, (ii) its successor in interest in connection with any sale, license or other disposition of all or substantially all of that portion of its business pertaining to the subject matter of this Agreement or (iii) as
contemplated under Section 3.8(e); provided, however, that in the case of the foregoing clause (i), Buyer remains liable and responsible to Seller for the performance of its obligations hereunder and in the
case of the foregoing clause (ii) such transferee assumes those obligations in writing. Seller may assign or transfer this Agreement and all of its rights and obligations hereunder in whole (but not in part) to its successor in interest in
connection with a Liquidation Event permitted hereunder; provided, however, that such transferee assumes those obligations in writing.
(c) Subject to Section 13.1(a) and Section 13.1(b), the terms of this Agreement will be
binding upon and will inure to the benefit of the successors, heirs, administrators and permitted assigns of the applicable Party. Any purported assignment in violation of this Section 13.1 will be null and void ab
initio.
13.2 Public Announcements. Neither Party nor any of their respective Affiliates shall issue any press release or make
any public announcement relating to the subject matter of this Agreement without the prior written consent of the other Party, which consent shall not be unreasonably withheld, delayed or conditioned; provided, however, that
(a) Buyer may make any public disclosure it believes in good faith, upon advice of counsel, is required by applicable Law or any listing or trading agreement concerning its publicly traded securities (in which case Buyer will provide reasonable
advance notice, to the extent reasonably practicable, to Seller prior to making the disclosure and will in good faith consider Seller’s reasonable comments on such disclosure) and (b) either Party may make any public announcement that is
substantially consistent with a prior press release or public announcement made with the consent of the other Party.
-58-
13.3 Confidentiality.
(a) Buyer and Seller agree that the Confidentiality Agreement, as it relates to Information, shall, as of the Closing Date, be terminated and
of no further force and effect.
(b) Seller agrees that, from the Closing Date until the date that is [***] years following the Closing
Date (or, with respect to any Buyer Information delivered to Seller in accordance with Section 3.8, the date that is [***] years following the date of such delivery) or with respect to any Buyer Information that constitutes
a trade secret under applicable Law, until the later of the date that is [***] years following the Closing Date and the date on which such Buyer Information no longer constitutes a trade secret under applicable Law (other than as a result of any
disclosure in violation of the Confidentiality Agreement, this Agreement or any Ancillary Agreement), Seller, any of its applicable Affiliates, and their Representatives shall (i) shall not use any Buyer Information for any purpose other than
as required to perform their respective obligations or exercise or enforce their respective rights and remedies under this Agreement or any Ancillary Agreement or as required to comply with their respective regulatory, stock exchange, Tax,
accounting or financial reporting requirements (collectively, “Permitted Uses”), and (ii) subject to Section 13.3(c), (A) not disclose any Buyer Information to any Person other than any of
Seller’s Affiliates and Representatives who need to know such Buyer Information for any purpose described in the preceding clause (i) and (B) keep confidential and exercise the same degree of care with respect to maintaining the
confidentiality of any Buyer Information in any of their possession that Seller and its Affiliates exercise with respect to similar types of their own proprietary information, but in no event less than a reasonable degree of care. [***].
(c) If any Buyer Information is required by Law or legal or administrative process to be disclosed, Seller shall promptly (and in any event
prior to making such disclosure, to the extent permitted by Law) notify Buyer of such disclosure requirement so that Buyer or its Affiliates may seek a protective Order or other appropriate remedy. Seller shall, shall cause its Affiliates to and
shall direct its respective Representatives to, reasonably cooperate, at Buyer’s expense, with Buyer or its Affiliates’ efforts to obtain such protective Order or other appropriate remedy. In the event that no such protective Order or
other remedy is obtained, or Buyer does not waive compliance with this Section 13.3(c), and any of Seller, its applicable Affiliates, or their Representatives are nonetheless legally compelled to disclose such Buyer
Information, then Seller, such Affiliate or such Representative, as the case may be, will furnish only that portion of the Buyer Information which Seller, such Affiliate or such Representative is advised by counsel is legally required to be
furnished and will give Buyer written notice of the Buyer Information to be disclosed as far in advance as reasonably practicable and exercise commercially reasonable efforts to obtain reliable assurance that confidential treatment will be accorded
the Buyer Information.
-59-
(d) The term “Buyer Information” means (i) upon consummation of
the Closing, all information, knowledge and data of Seller and its Affiliates as of immediately prior to the Closing to the extent related to the Acquired Assets, the Compound or any Product or the Assumed Liabilities (the “Buyer Product
Information”), (ii) all information, knowledge and data provided by Buyer or any of its Affiliates to Seller in connection with the Transactions other than any information contemplated by clause (i), but including all information provided
by Buyer under Section 3.8, and (iii) all analyses, compilations, forecasts, studies, interpretations, summaries, notes, data and other documents and materials (in any form or medium of communication, whether written,
oral, electronic or magnetic), whether prepared by Seller, any of its applicable Affiliates, any of their Representatives, or others, which contain, reflect or are generated from or based upon, in whole or in part, the information referred to in
clauses (i) and (ii) of this sentence, other than any such information that (A) only with respect to clause (ii) above, is known to Seller prior to receipt thereof from Buyer or any of its Affiliates, (B) is disclosed to Seller
by a Third Party which has a legal right to make such disclosure without requiring Seller to maintain the confidentiality thereof, (C) is or becomes part of the public domain through no fault of Seller or any of its Affiliates or
Representatives, or (D) is independently developed by or for Seller after the Closing Date, without reliance on or reference to any information contemplated by clauses (i) or (ii) of this sentence, in the case of the foregoing clauses
(A) and (D) as evidenced by Seller’s internal contemporaneous records.
(e) Buyer agrees that, from the Closing Date until the
date that is [***] years following the Closing Date, or with respect to any Seller Information that constitutes a trade secret under applicable Law, until the later of the date that is [***] years following the Closing Date and the date on which
such Seller Information no longer constitutes a trade secret under applicable Law (other than as a result of any disclosure in violation of the Confidentiality Agreement, this Agreement or any Ancillary Agreement), Buyer and its Representatives
shall not use any Seller Information for any purpose other than, [***], and as required to (i) perform their respective obligations or exercise or enforce their respective rights and remedies under this Agreement or any Ancillary Agreement or
(ii) to comply with their respective regulatory, stock exchange, Tax, accounting or financial reporting requirements, and shall, subject to Section 13.3(f), (A) not disclose any Seller Information to any Person other
than any of Buyer’s Affiliates and Representatives who need to know such Buyer Information for any purpose described in the preceding clause (i) or (ii) and (B) keep confidential and exercise the same degree of care with respect to
maintaining the confidentiality of any Seller Information in any of their possession that Buyer exercises with respect to similar types of its own proprietary information, but in no event less than a reasonable degree of care.
(f) If any Seller Information is required by Law or legal or administrative process to be disclosed, Buyer shall promptly (and in any event
prior to making such disclosure, to the extent permitted by Law) notify Seller of such disclosure requirement so that Seller or its Affiliates may seek a protective Order or other appropriate remedy. Buyer shall, and shall direct its Representatives
to, reasonably cooperate, at Seller’s expense, with Seller’s or Affiliate’s efforts to obtain such protective Order or other appropriate remedy. In the event that no such protective Order or other remedy is obtained, or Seller does
not waive compliance with this Section 13.3(e), and Buyer or its Representatives are nonetheless legally compelled to disclose such Seller Information, Buyer or its Representatives, as the case may be, will furnish only
that portion of the Seller Information which Buyer or its Representatives are advised by counsel is legally required to be furnished and will give Seller written notice of the Seller Information to be disclosed as far in advance as reasonably
practicable and exercise all reasonable efforts to obtain reliable assurance that confidential treatment will be accorded the Seller Information.
-60-
(g) The term “Seller Information” means (i) all information,
knowledge and data provided in connection with the Transactions by Seller or its Affiliates to Buyer unrelated to the Acquired Assets, the Compound or any Product or the Assumed Liabilities, (ii) [***] and (iii) all analyses, compilations,
forecasts, studies, interpretations, summaries, notes, data and other documents and materials (in any form or medium of communication, whether written, oral, electronic or magnetic), whether prepared by Buyer, Buyer’s Representatives or others,
which contain, reflect or are generated from or based upon, in whole or in part, the information referred to in clause (i) or, [***], other than any such information that (A) is known to Buyer prior to receipt thereof from Seller or any of
its Affiliates, (B) is disclosed to Buyer by a Third Party which has a legal right to make such disclosure without requiring Buyer to maintain the confidentiality thereof, (C) is or becomes part of the public domain through no fault of
Buyer or any of its Affiliates or Representatives, or (D) is independently developed by or for Buyer, without reliance on or reference to any information received from Seller or any of its Affiliates, in the case of the foregoing clauses
(A) and (D) as evidenced by Buyer’s or its applicable Affiliate’s internal contemporaneous records.
13.4 Expenses.
Whether or not the Transactions are consummated, and except as otherwise specified herein, each Party shall bear its own costs and expenses in connection with this Agreement and the Ancillary Agreements and with respect to the Transactions.
13.5 Severability. In the event that any provision of this Agreement or the application thereof becomes or is declared by an arbitration
panel or court of competent jurisdiction to be illegal, void or unenforceable, the remainder of this Agreement will continue in full force and effect and the application of such provision to other Persons or circumstances will be interpreted so as
reasonably to effect the intent of the Parties. Further, the Parties agree to replace such void or unenforceable provision of this Agreement with a valid and enforceable provision that will achieve, to the greatest extent possible, the economic,
business and other purposes of such void or unenforceable provision.
13.6 No Third-Party Beneficiaries. This Agreement is solely
for the benefit of the Parties and, to the extent set forth herein, their respective Affiliates and the Indemnified Parties, and no provision of this Agreement shall be deemed to otherwise confer upon any other Third Parties any remedy, Claim,
Liability, reimbursement or other right in excess of those existing without reference to this Agreement.
13.7 Waiver. The waiver by
a Party of any breach of any of the terms, covenants or conditions of this Agreement or of any right or privilege conferred by this Agreement shall not be construed as a subsequent waiver of any such terms, covenants, conditions, rights or
privileges or as a waiver of any other terms, covenants, conditions, rights or privileges. No waiver shall be effective unless it is in writing and signed by an authorized representative of the waiving Party.
13.8 Governing Law. This Agreement (and any Claim or controversy arising out of or relating to this Agreement or the Transactions) shall
be governed by and construed in accordance with the Laws of the State of Delaware without giving effect to any choice or conflict of law provision or rule that would cause the application of the Laws of any jurisdiction other than the State of
Delaware.
-61-
13.9 Consent to Jurisdiction; Venue; Waiver of Jury Trial. Except for disputes under
Section 1.1 (Net Sales) resolved pursuant to the procedures set forth in Schedule 1.1(A), each Party hereby (a) irrevocably submits to the exclusive jurisdiction of all state and federal courts sitting in
the State of Delaware for the purpose of any and all actions, suits or proceedings arising in whole or in part out of, related to, or based upon or in connection with this Agreement, the other Ancillary Agreements or the transactions contemplated
hereby or thereby, (b) waives to the extent not prohibited by applicable Law, and agrees not to assert, by way of motion, as a defense or otherwise, in any such action, any claim that it is not subject personally to the jurisdiction of the
above-named courts, that its property is exempt or immune from attachment or execution, that any such action brought in one of the above-named courts should be dismissed on grounds of forum non conveniens, should be transferred to any court
other than one of the above-named courts, or should be stayed by reason of the pendency of some other proceeding in any other court other than one of the above-named courts, or that this Agreement or the other Ancillary Agreements or the
transactions contemplated hereby or thereby may not be enforced in or by such court, and (c) agrees not to commence any such action other than before one of the above-named courts nor to make any motion or take any other action seeking or
intending to cause the transfer or removal of any such action to any court other than one of the above-named courts whether on the grounds of inconvenient forum or otherwise. Each of the Parties hereby irrevocably and unconditionally waives any
right it may have to a trial by jury in respect of any legal action arising out of or relating to this Agreement, the other Ancillary Agreements or the transactions contemplated hereby or thereby.
13.10 Specific Performance. The Parties agree that irreparable damage may occur in the event that any of the provisions of this
Agreement were not performed in accordance with their specific terms or were otherwise breached or threatened to be breached. It is accordingly agreed that the Parties shall be entitled (without proof of actual damages or otherwise or posting or
securing any bond) to an injunction or injunctions to prevent breaches of this Agreement and to seek to enforce specifically the terms and provisions of this Agreement, these being in addition to any other remedy to which they are entitled at law or
in equity. The Parties further agree not to assert that a remedy of specific performance is unenforceable, invalid, contrary to Law or inequitable for any reason, nor to assert that a remedy of monetary damages would provide adequate remedy. Each of
the Parties acknowledges and agrees that the right to specific performance and the other relief contemplated herein is an integral part of the Transactions and without such right, none of the Parties would have entered into this Agreement.
13.11 Representation by Counsel. Each Party represents and agrees with the other that it has been represented by or had the opportunity
to be represented by independent counsel of its own choosing, and that it has had the full right and opportunity to consult with its respective attorney(s), that to the extent, if any, that it desired, it availed itself of this right and
opportunity, that it or its authorized officers (as the case may be) have carefully read and fully understand this Agreement and the Ancillary Agreements in their entirety and have had them fully explained to them by such Party’s respective
counsel, that each is fully aware of the contents thereof and their meaning, intent and legal effect, and that it or its authorized officer (as the case may be) is competent to execute this Agreement and has executed this Agreement free from
coercion, duress or undue influence.
-62-
13.12 English Language. This Agreement shall be written and executed in, and all
other communications under or in connection with this Agreement shall be in, the English language. Any translation into any other language shall not be an official version thereof, and in the event of any conflict in interpretation between the
English version and such translation, the English version shall control.
13.13 Bulk Transfers. Buyer waives compliance with the
provisions of all applicable Laws relating to bulk transfers in connection with the transfer of the Acquired Assets.
13.14 Notices.
All notices and other communications required or permitted to be given or made pursuant to this Agreement shall be made in writing signed by the sender and shall be deemed duly given (a) on the date delivered, if personally delivered,
(b) the day that receipt is confirmed (with receipt confirmed by telephone or email) after being sent by email in PDF or similar electronic format, or (c) on the Business Day after being sent by Federal Express or another recognized
overnight mail service that utilizes a written form of receipt for next day or next Business Day delivery, in each case addressed to the applicable Party at the address set forth below; provided, that a Party may change its address for
receiving notice by the proper giving of notice hereunder:
If to Seller:
RadioMedix, Inc.
9701 Richmond Avenue, Suite 222
Houston, TX 77042
Attention: Dr. Ebrahim Delpassand
Email:
Telephone:
with a copy to (which shall not constitute notice):
Gunderson Dettmer Stough Villeneuve Franklin & Hachigian LLP
500 West Fifth Street, Suite 1215
Austin, Texas 78701
Attention: Wes Watts
Email:
Telephone:
If to Buyer:
Fusion Pharmaceuticals Inc.
270 Longwood Road
S. Hamilton, Ontario Canada L8P 0A6
Attention: Maria Stahl
Email:
Telephone:
-63-
with a copy to (which shall not constitute notice):
Covington & Burling LLP
One CityCenter
850 Tenth Street NW
Washington, DC 20001
Attention: Michael J. Riella
Email:
Telephone:
13.15
Counterparts; Signature Pages. The Parties may execute this Agreement in two or more counterparts, each of which will be deemed an original and all of which, when taken together, will be deemed to constitute one and the same agreement. Any
signature page hereto may be signed manually or electronically and delivered by e-mail (including in PDF or similar electronic format) and shall be binding to the same extent as an original signature page and
may be used in lieu of the original signatures for all purposes.
13.16 Entire Agreement; Amendment. This Agreement, the
Confidentiality Agreement, the Ancillary Agreements (including the Transfer Documents after their execution and delivery hereunder), the Seller Disclosure Letter, the Buyer Disclosure Letter and the Schedules and Exhibits hereto contain the entire
agreement of the Parties with respect to the Transactions, superseding all negotiations, prior discussions and preliminary agreements made prior to the date hereof. This Agreement may not be amended, supplemented or otherwise modified except by an
instrument in writing signed by both Seller and Buyer.
[remainder of page intentionally left blank]
-64-
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their
respective duly authorized officers as of the date first above written.
|
|
|
| SELLER |
|
| RADIOMEDIX, INC. |
|
|
| By: |
|
/s/ Dr. Ebrahim Delpassand |
|
|
Name: Dr. Ebrahim Delpassand |
|
|
Title: Chief Executive Officer |
|
| BUYER |
|
| FUSION PHARMACEUTICALS INC. |
|
|
| By: |
|
/s/ John Valliant |
|
|
Name: John Valliant |
|
|
Title: Chief Executive Officer |
[Option and Asset Purchase Agreement]
Exhibit A
Bill of Sale and Assignment and Assumption Agreement
[See Attached.]
A-1
EXHIBIT A
FORM OF BILL OF SALE AND
ASSIGNMENT AND ASSUMPTION AGREEMENT
This Bill of Sale and Assignment and Assumption Agreement (this “Agreement”) is made as of this [•] day of [•],
[2022], by and between RadioMedix, Inc., a Texas corporation (“Seller”), and Fusion Pharmaceuticals Inc., a Canadian federal corporation (“Buyer”). Seller and Buyer are sometimes referred to herein collectively as
the “Parties” and individually as a “Party.”
RECITALS
WHEREAS, Seller and Buyer have entered into an Option and Asset Purchase Agreement, dated as of November 14, 2022 (the
“Option and Asset Purchase Agreement”); and
WHEREAS, pursuant to the Option and Asset Purchase Agreement,
Buyer has exercised the Option, Seller has agreed to sell, transfer, convey, assign and deliver the Acquired Assets and assign the Assumed Liabilities to Buyer, and Buyer has agreed to purchase and accept the Acquired Assets and assume and satisfy
and discharge when due the Assumed Liabilities from Seller.
AGREEMENT
NOW, THEREFORE, in consideration of the premises and mutual covenants, agreements and provisions herein contained and contained in the
Option and Asset Purchase Agreement, the Parties agree as follows:
| |
1. |
Definitions. Unless otherwise specifically provided herein, capitalized terms used in this Agreement and
not otherwise defined herein shall have the respective meanings ascribed thereto in the Option and Asset Purchase Agreement. |
| |
2. |
Conveyance and Acceptance. In accordance with the provisions of the Option and Asset Purchase Agreement,
Seller hereby sells, transfers, conveys, assigns and delivers to Buyer all of Seller’s rights, title and interests in and to the Acquired Assets, and Buyer hereby purchases and accepts the Acquired Assets, in each case, free and clear of all
Liens other than Permitted Liens. |
| |
3. |
Assumption of Assumed Liabilities. Seller hereby assigns to Buyer the Assumed Liabilities and Buyer
hereby unconditionally assumes and agrees to satisfy and discharge the Assumed Liabilities. |
| |
4. |
Option and Asset Purchase Agreement Controls. This Agreement is subject to, and governed entirely in
accordance with, the terms and conditions of the Option and Asset Purchase Agreement. |
| |
5. |
Miscellaneous. Sections 13.1, 13.4, 13.5, 13.7 through 13.9, 13.15 and 13.16 of the Option and Asset
Purchase Agreement are incorporated by reference into this Agreement, mutatis mutandis, as if such provisions were set forth herein in their entirety. |
[Signature page follows]
A-2
IN WITNESS WHEREOF, the Parties have caused this Agreement to be duly executed as of
the date first written above.
|
|
|
| RADIOMEDIX, INC. |
|
|
| By: |
|
|
|
|
Name: |
|
|
Title: |
|
| FUSION PHARMACEUTICALS INC. |
|
|
| By: |
|
|
|
|
Name: |
|
|
Title: |
A-3
Exhibit B
Compound
[***]
B-1
Exhibit C
Data Package
[***]
C-1
Exhibit 10.2
SECURITIES PURCHASE AGREEMENT
This SECURITIES PURCHASE AGREEMENT (this “Agreement”) is made and entered into as of February 13, 2023 by and among
Fusion Pharmaceuticals Inc., a corporation existing under the Canada Business Corporations Act (the “Company”), and the investors identified on Exhibit A attached hereto (each an “Investor” and
collectively the “Investors”).
RECITALS
A. The Company and the Investors are, severally and not jointly, executing and delivering this Agreement in reliance upon the exemption from
securities registration afforded by the provisions of Section 4(a)(2) of the 1933 Act (as defined below), and/or Rule 506 of Regulation D (“Regulation D”) as promulgated by the SEC (as defined below) under the 1933 Act;
B. The Investors wish to purchase, severally and not jointly, from the Company, and the Company wishes to sell and issue to the Investors,
upon the terms and subject to the conditions stated in this Agreement, an aggregate of 17,648,596 shares (the “Shares”) of the Company’s Common Shares, no par value (the “Common Shares”); and
C. Contemporaneously with the sale of the Shares hereunder, at the Closing the parties hereto will execute and deliver a Registration Rights
Agreement, in the form attached hereto as Exhibit B (the “Registration Rights Agreement”), pursuant to which the Company will agree to provide certain registration rights in respect of the Shares under the 1933 Act and
applicable state securities laws.
In consideration of the mutual promises made herein and for other good and valuable consideration, the
receipt and sufficiency of which are hereby acknowledged, the parties hereto agree as follows:
1. Definitions. For the purposes of
this Agreement, the following terms shall have the meanings set forth below:
“Affiliate” means, with respect to any Person, any other
Person which directly or indirectly through one or more intermediaries Controls, is controlled by, or is under common Control with such Person.
“Announcing 8-K” has the meaning set forth in Section 4.51.
“Anti-Money Laundering Laws” has the meaning set forth in Section 4.23.
“Breach” has the meaning set forth in Section 4.29.
“Business Day” means a day, other than a Saturday or Sunday, on which banks in New York City are open for the general transaction of
business.
“Canadian Qualifying Jurisdictions” means each of the provinces of Canada, except Quebec.
“Canadian Securities Commissions” means the Ontario Securities Commission and the securities commissions or
other securities regulatory authorities in the Canadian Qualifying Jurisdictions.
“Canadian Securities Laws” means all applicable securities laws in each of the Canadian Qualifying Jurisdictions, including the respective
rules and regulations made thereunder together with applicable published national and local instruments, policy statements, notices, blanket orders and rulings of the Canadian Securities Commissions, and all discretionary rulings and orders
applicable to the Company, if any, of the Canadian Securities Commissions.
“Closing” has the meaning set forth in Section 3.1.
“Closing Date” has the meaning set forth in Section 3.1.
“Common Shares” has the meaning set forth in the recitals to this Agreement.
“Company Covered Person” means, with respect to the Company as an “issuer” for
purposes of Rule 506 promulgated under the 1933 Act, any Person listed in the first paragraph of Rule 506(d)(1).
“Company Intellectual
Property” has the meaning set forth in Section 4.27.
“Control” (including the terms “controlling,”
“controlled by” or “under common control with”) means the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting
securities, by contract or otherwise.
“Data” has the meaning set forth in Section 4.28.
“Data Protection Obligations” has the meaning set forth in Section 4.28.
“Disqualification Event” has the meaning set forth in Section 4.47.
“DTC” has the meaning set forth in Section 4.50.
“EDGAR system” means the Electronic Data Gathering, Analysis, and Retrieval system of the SEC.
“Environmental Laws” has the meaning set forth in Section 4.20.
“Government Official” has the meaning set forth in Section 4.22.
“Health Care Laws” has the meaning set forth in Section 4.37.
“Investor Questionnaire” has the meaning set forth in Section 3.1.
“Material Adverse Effect” means a material adverse effect on (i) the assets, liabilities, results of operations, financial condition or
business of the Company and its subsidiaries taken as a whole, (ii) the legality or enforceability of any of the Transaction Documents or (iii) the ability of the Company to perform its obligations under the Transaction Documents.
“Nasdaq” means the Nasdaq Global Select Market.
“Person” means an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company,
joint venture, sole proprietorship, unincorporated organization, governmental authority or any other form of entity not specifically listed here.
“Placement Agents” has the meaning set forth in Section 4.46.
“Press Release” has the meaning set forth in Section 9.8.
“Principal Trading Market” means the Trading Market on which the Common Shares are primarily listed on and quoted for trading, which, as of
the date of this Agreement and the Closing Date, shall be the Nasdaq Global Select Market.
“Registration Rights Agreement” has the
meaning set forth in the recitals to this Agreement.
“Regulation D” has the meaning set forth in the recitals to this Agreement.
“Regulatory Agencies” has the meaning set forth in Section 4.36.
“Relevant Taxing Jurisdiction” has the meaning set forth in Section 4.6.
“Required Investors” has the meaning set forth in the Registration Rights Agreement.
“Sanctions” has the meaning set forth in Section 4.24(i)(A).
“SEC” means the U.S. Securities and Exchange Commission.
“SEC Filings” means all reports, schedules, forms, statements and other documents filed or required to be filed by the Company under the 1933
Act and the 1934 Act, including pursuant to Section 13(a) or 15(d) thereof, for the one year period preceding the date hereof.
“Selling
Stockholder Questionnaire” has the meaning set forth in Section 3.1.
“Shares” has the meaning set forth in the recitals to
this Agreement.
“Short Sales” means all “short sales” as defined in Rule 200 of Regulation SHO under the 1934 Act (but shall
not be deemed to include the location and/or reservation of borrowable shares of Common Shares).
“Stamp Taxes” has the meaning set forth
in Section 4.7.
“Trading Day” means (i) a day on which the Common Shares are listed or quoted and traded on its Principal
Trading Market, or (ii) if the Common Shares are not listed on a Trading Market (other than the OTCQX Market or the OTCQB Market), a day on which the Common Shares is traded in the OTCQX Market or the OTCQB Market, or (iii) if the Common
Shares is not listed or quoted on any Trading Market, a day on which the Common Shares is quoted in the over-the-counter market as reported in the Pink Open Market of
OTC Markets Group by OTC Markets Group Inc. (or any similar organization or agency succeeding to its functions of reporting prices); provided, that in the event that the Common Shares are not listed or quoted as set forth in (i), (ii) or
(iii) hereof, then Trading Day shall mean a Business Day.
“Trading Market” means whichever of the New York Stock Exchange, the NYSE
American, the Nasdaq Global Select Market, the Nasdaq Global Market, the Nasdaq Capital Market or the OTCQX Market or the OTCQB Market (or any successors to any of the foregoing) on which the Common Shares are listed or quoted for trading on the
date in question.
“Transfer Agent” has the meaning set forth in Section 7.2(a).
“Transaction Documents” means this Agreement and the Registration Rights Agreement.
“U.S. GAAP” has the meaning set forth in Section 4.21.
“1933 Act” means the Securities Act of 1933, as amended, or any successor statute, and the rules and regulations promulgated thereunder.
“1934 Act” means the Securities Exchange Act of 1934, as amended, or any successor statute, and the rules and regulations promulgated
thereunder.
2. Purchase and Sale of the Shares. On the Closing Date (as defined below), upon the terms and subject to the
conditions set forth herein, the Company will issue and sell, and each Investor will purchase, severally and not jointly, the number of Shares set forth opposite the name of such Investor under the heading “Number of Shares” on Exhibit
A attached hereto. The purchase price per Share shall be $3.40.
3. Closing.
3.1 Closing. Upon the satisfaction of the conditions set forth in Section 6, the completion of the purchase and sale of the Shares
(the “Closing”) shall occur remotely via exchange of documents and signatures on February 16, 2023 (the “Closing Date”). At or prior to the Closing, each Investor shall execute, on or before the Closing Date,
the Transaction Documents, the Investor Questionnaire or such other similar forms or information provided to the Company by such Investor in the form attached hereto as Exhibit C (the “Investor Questionnaire”) and the Selling
Stockholder Questionnaire in the form attached to the Registration Rights Agreement (the “Selling Stockholder Questionnaire”).
3.2 Delivery of Funds. On the Closing Date, against, and concurrently with, delivery
of the Shares pursuant to Section 3.3, each Investor shall deliver or cause to be delivered to the Company, via wire transfer of immediately available funds pursuant to the wire instructions delivered to such Investor by the Company not less
than two (2) Business Days prior to the Closing Date, an amount equal to the purchase price to be paid by the Investor for the Shares to be acquired by it as set forth opposite the name of such Investor under the heading “Aggregate
Purchase Price of Shares” on Exhibit A attached hereto.
3.3 Delivery of Shares. At the Closing, the Company shall
deliver or cause to be delivered to each Investor (i) a number of Shares, registered in the name of the Investor (or its nominee in accordance with its delivery instructions), free and clear of any liens or other restrictions whatsoever (other
than those arising under state or federal securities laws), equal to the number of Shares set forth opposite the name of such Investor under the heading “Number of Shares” on Exhibit A attached hereto, and (ii) written notice
from the Company or the Transfer Agent evidencing the issuance to each Investor of such Shares on and as of the Closing Date. The Shares shall be delivered via a book entry record through the Transfer Agent (as defined below). Unless the Company and
an Investor otherwise mutually agree with respect to such Investor’s Shares, at Closing settlement shall occur on a “delivery versus payment” basis.
4. Representations and Warranties of the Company. The Company hereby represents and warrants to and agrees with each of the Investors
that:
4.1 Organization, Good Standing and Qualification. The Company has been duly incorporated, is validly existing as a
corporation in good standing under the laws of the jurisdiction of its incorporation, has the corporate power and capacity to own or lease its property and to conduct its business as described in the SEC Filings and is duly qualified to transact
business and is in good standing in each jurisdiction in which the conduct of its business or its ownership or leasing of property requires such qualification, except to the extent that the failure to be so qualified or be in good standing would
not, singly or in the aggregate, have or reasonably be expected to have a Material Adverse Effect.
4.2 Subsidiaries. Each
subsidiary of the Company has been duly incorporated, organized or formed, is validly existing as a corporation or other business entity in good standing under the laws of the jurisdiction of its incorporation, organization or formation, has the
corporate or other business entity power and authority to own or lease its property and to conduct its business as described in the SEC Filings and is duly qualified to transact business and is in good standing in each jurisdiction in which the
conduct of its business or its ownership or leasing of property requires such qualification, except to the extent that the failure to be so qualified or be in good standing would not, singly or in the aggregate, have or reasonably be expected to
have a Material Adverse Effect; all of the issued share capital or other equity interests of each subsidiary of the Company have been duly and validly authorized and issued, are fully paid and non-assessable
and are owned directly by the Company, free and clear of all liens, encumbrances, equities or claims.
4.3 Transaction Documents.
The Company has all requisite corporate power and authority to enter into the Transaction Documents to carry out and perform its obligations under the terms of the Transaction Documents. The Agreement has been duly authorized, executed and delivered
by the Company, and each of the other Transaction Documents has been duly authorized, and upon the Closing will have been duly executed and delivered by the Company. This Agreement constitutes, and the other Transaction Documents when executed and
delivered by the Company will constitute, the valid and legally binding obligations of the Company, enforceable against the Company in accordance with their respective terms, subject to bankruptcy, insolvency, fraudulent transfer, reorganization,
moratorium and similar laws of general applicability, relating to or affecting creditors’ rights generally, and general principles of equity.
4.4 Authorization. All necessary action on the part of the Company, its officers, directors and shareholders necessary for the
authorization of the Shares, the authorization, execution, delivery and performance of the Transaction Documents and the consummation of the transactions contemplated herein has been taken by the Company. No further action on the part of the
shareholders of the Company is necessary to approve the transactions contemplated by the Transaction Documents (under the rules of the Nasdaq Stock Market, the laws of Canada or otherwise).
4.5 Capitalization. The authorized capital stock of the Company consists of an
unlimited number of common shares, no par value, and an unlimited number of preferred shares, no par value, issuable in series, all of which preferred shares are undesignated. The Company’s issued and outstanding capital stock is as set forth
in the most recent SEC Filing containing such disclosure as of the date indicated in such SEC Filing (except for subsequent issuances, if any, pursuant to this Agreement or pursuant to reservations, agreements or employee benefit plans, in each
case, referred to in the SEC Filings, pursuant to the exercise of convertible securities or options or the vesting of restricted stock units referred to in the SEC Filings or pursuant to the Company’s Open Market Sale Agreement with Jefferies
LLC).
4.6 Payments. All payments to be made by or on behalf of the Company under this Agreement and all dividends and other
distributions declared and payable on the Shares, under the federal laws of Canada or the laws of any province, and of any other jurisdiction in which the Company is organized or incorporated, engaged in business or otherwise resident for tax
purposes or has a permanent establishment or any political subdivision, authority or agency in or of any of the foregoing having power to tax (each, a “Relevant Taxing Jurisdiction”), will not be subject to withholding or deduction
under the current laws and regulations of any Relevant Taxing Jurisdiction without the necessity of obtaining any governmental authorization; provided, however, that dividends and other distributions declared and payable on the Shares will be
subject to withholding tax at the applicable rate under the current laws and regulations of the Relevant Taxing Jurisdiction in Canada, subject to reduction under an applicable tax treaty.
4.7 Stamp Taxes. No stamp, documentary, capital, issuance, registration, transfer, withholding, capital gains, income or other taxes or
duties (“Stamp Taxes”) are payable by or on behalf of the Investors, the Company or any of its subsidiaries in any Relevant Taxing Jurisdiction in connection with (i) the execution and delivery of the Transaction Documents or
the consummation of the transactions contemplated by the Transaction Documents, (ii) the creation, allotment and issuance of the Shares, or (iii) the sale and delivery of the Shares to the Investors.
4.8 Common Shares. The Common Shares in the capital of the Company outstanding prior to the issuance of the Shares have been duly
authorized and are validly issued, fully paid, non-assessable and were not issued in violation of any applicable U.S. federal securities laws or Canadian Securities Laws.
4.9 Valid Issuance. The Shares have been duly authorized and, when issued, delivered and paid for in accordance with the terms of this
Agreement, will be validly issued, fully paid and non-assessable, and will be issued free and clear of any liens or other restrictions (other than those under applicable securities laws) and the issuance of
the Shares will not be subject to any preemptive or similar rights.
4.10 Non-Contravention;
Consents. The execution and delivery by the Company of, and the performance by the Company of its obligations under, the Transaction Documents will not contravene any provision of (i) applicable law, (ii) the Articles of the
Corporation or General By-laws of the Company, (iii) any agreement or other instrument binding upon the Company or any of its subsidiaries that is material to the Company and its subsidiaries, taken as a
whole, or (iv) any judgment, order or decree of any governmental body, agency or court having jurisdiction over the Company or any subsidiary, except in the case of clauses (i), (iii) and (iv), where such contravention would not, individually
or in the aggregate, reasonably be expected to have a Material Adverse Effect. No consent, approval, authorization or order of, or qualification with, any governmental body, agency or court is required for the performance by the Company of its
obligations under the Transaction Documents, except such as may be required by the Financial Industry Regulatory Authority or Nasdaq in connection with the offer and sale of the Shares.
4.11 Nasdaq Listing Requirements. The Common Shares are registered pursuant to Section 12(b) or 12(g) of the 1934 Act and are
listed on Nasdaq, and the Company has taken no action designed to, or likely to have the effect of, terminating the registration of the Common Shares under the 1934 Act or delisting the Common Shares from Nasdaq, nor has the Company received any
notification that the SEC or Nasdaq is contemplating terminating such registration or listing. To the Company’s knowledge, it is in compliance with all applicable listing requirements of Nasdaq.
4.12 Registration Rights. Except as contemplated by the Transaction Documents, there
are no contracts, agreements or understandings between the Company and any Person granting such Person the right to require the Company to file a registration statement under the 1933 Act or a prospectus under the rules and regulations made
thereunder, except for such rights as have been satisfied or duly waived.
4.13 Canadian Securities Laws. The Company has complied
with all Canadian Securities Laws required to be complied with by the Company to distribute the Shares to Investors in the Canadian Qualifying Jurisdictions on a basis exempt from the prospectus requirements of Canadian Securities Laws.
4.14 SEC Filings. The Company has timely filed all reports, schedules, forms, statements and other documents required to be filed by
the Company under the 1933 Act and the 1934 Act, including pursuant to Section 13(a) or 15(d) thereof, for the one year period preceding the date hereof. At the time of filing thereof, the SEC Filings complied in all material respects with the
requirements of the 1933 Act or the 1934 Act, as applicable, and, as of their respective dates, did not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make
the statements made therein, in the light of the circumstances under which they were made, not misleading.
4.15 Internal
Controls. The Company has established and maintains disclosure controls and procedures (as defined in Rules 13a-15 and 15d-15 under
the 1934 Act), which (i) are designed to ensure that material information relating to the Company, including its consolidated subsidiaries, is made known to the Company’s principal executive officer and its principal financial officer by
others within those entities, particularly during the periods in which the periodic reports required under the 1934 Act are being prepared; (ii) have been evaluated by management of the Company for effectiveness as of the end of the
Company’s most recent fiscal quarter; and (iii) are effective in all material respects to perform the functions for which they were established.
4.16 No Material Adverse Change. There has not occurred any material adverse change, or any development involving a prospective
material adverse change, in the condition, financial or otherwise, or in the earnings, business or operations of the Company and its subsidiaries, taken as a whole, since September 30, 2022.
4.17 Legal Proceedings. There are no legal or governmental proceedings pending or, to the Company’s knowledge, threatened to which
the Company or any of its subsidiaries is a party or to which any of the properties of the Company or any of its subsidiaries is subject other than proceedings (i) accurately described in all material respects in the SEC Filings or disclosed to
the Investors (and to be publicly disclosed in the Announcing 8-K (as defined below)) or (ii) that would not have a Material Adverse Effect.
4.18 Investment Company. The Company is not, and after the Closing will not be, required to register as an “investment
company” as such term is defined in the Investment Company Act of 1940, as amended.
4.19 Transfer Agent. American
Stock & Transfer Trust Company, LLC at its principal office in New York, New York has been duly appointed as transfer agent and registrar for the Shares.
4.20 Environmental Matters. The Company and each of its subsidiaries (i) are in compliance with any and all applicable foreign,
federal, state and local laws and regulations relating to the protection of human health and safety, the environment or hazardous or toxic substances or wastes, pollutants or contaminants (“Environmental Laws”), (ii) have received
all permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses and (iii) are in compliance with all terms and conditions of any such permit, license or approval, except where
such noncompliance with Environmental Laws, failure to receive required permits, licenses or other approvals or failure to comply with the terms and conditions of such permits, licenses or approvals would not, singly or in the aggregate, reasonably
be expected to have a Material Adverse Effect.
4.21 Costs and Liabilities with Environmental Laws. There are no costs or liabilities
associated with Environmental Laws (including, without limitation, any capital or operating expenditures required for clean-up, closure of properties or compliance with Environmental Laws or any permit,
license or approval, any related constraints on operating activities and any potential liabilities to third parties) which have or would, singly or in the aggregate, reasonably be expected to have a Material Adverse Effect, or would be required to
be disclosed under United States generally accepted accounting principles (“U.S. GAAP”) or SEC rules or regulations.
4.22 Anti-Bribery Laws. (i) None of the Company or any of its subsidiaries or controlled Affiliates, or any director, officer, or
employee thereof, or, to the Company’s knowledge, any agent or representative of the Company or of any of its subsidiaries or controlled Affiliates, has taken or will take any action in furtherance of an offer, payment, promise to pay, or
authorization or approval of the payment, giving or receipt of money, property, gifts or anything else of value, directly or indirectly, to any government official (including any officer or employee of a government or government-owned or controlled
entity or of a public international organization, or any Person acting in an official capacity for or on behalf of any of the foregoing, or any political party or party official or candidate for political office) (“Government
Official”) in order to influence official action, or to any Person in violation of any applicable anti-corruption laws; (ii) the Company and each of its subsidiaries and controlled Affiliates have conducted their businesses in
compliance with applicable anti-corruption laws and have instituted and maintained and will continue to maintain policies and procedures reasonably designed to promote and achieve compliance with such laws and with the representations and warranties
contained herein; and (iii) neither the Company nor any of its subsidiaries will use, directly or indirectly, the proceeds of the offering in furtherance of an offer, payment, promise to pay, or authorization of the payment or giving of money,
or anything else of value, to any Person in violation of any applicable anti-corruption laws.
4.23 Anti-Money Laundering Laws. The
operations of the Company and each of its subsidiaries are and have been conducted at all times in material compliance with all applicable financial recordkeeping and reporting requirements, including those of the Bank Secrecy Act, as amended by
Title III of the Uniting and Strengthening America by Providing Appropriate Tools Required to Intercept and Obstruct Terrorism Act of 2001 (USA PATRIOT Act), and the applicable anti-money laundering statutes of jurisdictions where the Company and
each of its subsidiaries conduct business, the rules and regulations thereunder and any related or similar rules, regulations or guidelines, issued, administered or enforced by any governmental agency (collectively, the “Anti-Money
Laundering Laws”), and no action, suit or proceeding by or before any court or governmental agency, authority or body or any arbitrator involving the Company or any of its subsidiaries with respect to the Anti-Money Laundering Laws is
pending or, to the best knowledge of the Company, threatened.
4.24 Sanctions. (i) None of the Company, any of its
subsidiaries, or any director, officer, or employee thereof, or, to the Company’s knowledge, any agent, controlled Affiliate or representative of the Company or any of its subsidiaries, is an individual or entity that is, or is owned or
controlled by one or more Persons that are:
| |
(A) |
the subject of any sanctions administered or enforced by the U.S. Department of the Treasury’s Office of
Foreign Assets Control, the United Nations Security Council, the European Union, His Majesty’s Treasury, or other relevant sanctions authority (collectively, “Sanctions”), or |
| |
(B) |
located, organized or resident in a country or territory that is the subject of Sanctions (including, without
limitation, Crimea, the so-called Donetsk People’s Republic or the so-called Luhansk People’s Republic, Russia, Cuba, Iran, North Korea and Syria).
|
| |
(ii) |
The Company will not, directly or indirectly, use the proceeds of the offering, or lend, contribute or
otherwise make available such proceeds to any subsidiary, joint venture partner or other Person: |
| |
(A) |
to fund or facilitate any activities or business of or with any Person or in any country or territory that, at
the time of such funding or facilitation, is the subject of Sanctions; or |
| |
(B) |
in any other manner that will result in a violation of Sanctions by any Person (including any Person
participating in the offering, whether as underwriter, advisor, investor or otherwise). |
| |
(iii) |
The Company and each of its subsidiaries have not knowingly engaged in, are not now knowingly engaged in, and
will not engage in, any dealings or transactions with any Person, or in any country or territory, that at the time of the dealing or transaction is or was the subject of Sanctions. |
4.25 Material Obligations; Share Repurchases; Material Changes. Subsequent to September 30, 2022, (i) the Company and its
subsidiaries, taken as a whole, have not incurred any material liability or obligation, direct or contingent, nor entered into any material transaction, other than as disclosed to the Investors; (ii) the Company has not purchased any of its
outstanding Common Shares (other than from its employees or other service providers in connection with the termination of their service pursuant to the terms of its equity compensation plans or agreements), nor declared, paid or otherwise made any
dividend or distribution of any kind on its Common Shares other than ordinary and customary dividends; and (iii) there has not been any material change in the share capital, short-term debt or long-term debt of the Company and its subsidiaries,
taken as a whole.
4.26 Title to Properties. The Company and each of its subsidiaries do not own any real property. The Company and
its subsidiaries have good and marketable title to all personal property (other than intellectual property which is addressed exclusively in Section 4.27 below) owned by them which is material to the business of the Company and its
subsidiaries, in each case free and clear of all liens, encumbrances and defects except such as are described in the SEC Filings, or such as do not materially affect the value of such property and do not materially interfere with the use made and
proposed to be made of such property by the Company and its subsidiaries; and any real property and buildings held under lease by the Company and its subsidiaries are held by them under valid, subsisting and enforceable leases with such exceptions
as are not material and would not reasonably be expected to materially interfere with the use made and proposed to be made of such property and buildings by the Company and its subsidiaries.
4.27 Intellectual Property. Except as disclosed to the Investors or as would not reasonably be expected, individually or in the
aggregate, to have a Material Adverse Effect, (i) the Company and its subsidiaries own, or have a license to, all patents, patent applications, patent rights, trademarks, service marks, trade names, trademark registrations, service mark
registrations, domain names and other source indicators, copyrights and copyrightable works, know-how, trade secrets, systems, procedures, proprietary or confidential information and all other worldwide
intellectual property, industrial property and proprietary rights (collectively, “Company Intellectual Property”) currently used in the conduct of their respective businesses, free and clear of all liens, security interests or
encumbrances and such Company Intellectual Property is subsisting and unexpired, and to the knowledge of the Company, valid, enforceable, and free of material defects; (ii) to the knowledge of Company, the Company’s and its
subsidiaries’ conduct of their respective businesses does not infringe, misappropriate or otherwise violate any intellectual property or contractual rights of any person; (iii) the Company and its subsidiaries have not received any written
notice of any claim relating to Company Intellectual Property; (iv) to the knowledge of the Company, the Company Intellectual Property and its subsidiaries is not being infringed, misappropriated or otherwise violated by any Person;
(v) there are no actions pending, or to the knowledge of the Company, threatened against the Company or its subsidiaries relating to Company Intellectual Property; (vi) the Company and its subsidiaries have complied in all material
respects with the terms of each agreement pursuant to which Company Intellectual Property has been licensed to the Company or its subsidiaries, as applicable, and, to the Company’s knowledge, all such agreements are in full force and effect;
and (vii) the Company and its subsidiaries have taken all reasonable steps to protect, maintain and safeguard Company Intellectual Property and to require all employees and contractors (A) with access to trade secrets and confidential
information to execute non-disclosure and confidentiality agreements with the Company or its subsidiaries, as applicable, and (B) who have been involved in the creation, invention or development of
material Company Intellectual Property for or on behalf of the Company to assign in writing to the Company or its subsidiaries, as applicable, all of their rights therein.
4.28 Data Security. (i) The Company and each of its subsidiaries have complied
in all material respects and are presently in compliance in all material respects with all internal privacy policies, contractual obligations, applicable laws, statutes and judgments, orders, rules and regulations of any court or arbitrator or other
governmental or regulatory authority, in each case, relating to the collection, use, transfer, import, export, storage, protection, disposal and disclosure by the Company or any of its subsidiaries personally identifiable or other regulated data
(“Data Protection Obligations,” and such data, “Data”); (ii) the Company has not received any written notification of or complaint regarding material non-compliance with any
Data Protection Obligation; and (iii) there is no action, suit or proceeding by or before any court or governmental agency, authority or body pending or, to the Company’s knowledge, threatened alleging
non-compliance with any Data Protection Obligation.
4.29 No Breach. The Company and each
of its subsidiaries have implemented appropriate controls, policies, procedures and technological safeguards to maintain and protect the information technology systems and Data used in connection with the operation of the Company’s and its
subsidiaries’ businesses. Without limiting the foregoing, the Company and its subsidiaries have used reasonable efforts to implement appropriate controls, policies and procedures, and technological safe guards to establish and maintain
reasonable data protection controls, policies and procedures that are designed to protect against and prevent breach, destruction, loss, unauthorized distribution, use, access, disablement, misappropriation or modification, or other compromise or
misuse of any Data used in connection with the operation of the Company’s and its subsidiaries’ businesses (“Breach”). To the Company’s knowledge, there has been no material Breach, and the Company and its
subsidiaries have not been notified of and have no knowledge of any event or condition that would reasonably be expected to result in, any such material Breach.
4.30 Labor Matters. No material labor dispute with the employees of the Company or any of its subsidiaries exists, or, to the knowledge
of the Company, is imminent; and the Company is not aware of any existing, threatened or imminent labor disturbance by the employees of any of its principal suppliers, manufacturers or contractors, in the case, that would, singly or in the
aggregate, reasonably be expected to have a Material Adverse Effect.
4.31 Insurance Coverage. The Company and each of its
subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as in the Company’s reasonable judgment are prudent and customary in the businesses in which they are engaged; neither
the Company nor any of its subsidiaries has been refused any insurance coverage sought or applied for; and neither the Company nor any of its subsidiaries has any reason to believe that it will not be able to renew its existing insurance coverage as
and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not reasonably be expected to have a Material Adverse Effect.
4.32 Financial Statements. The financial statements included in each of the SEC Filings, together with the related schedules and notes
thereto, comply as to form in all material respects with the applicable accounting requirements of the 1933 Act and present fairly the consolidated financial position of the Company and its subsidiaries as of the dates shown and its results of
operations and cash flows for the periods shown, and such financial statements have been prepared in conformity with U.S. GAAP applied on a consistent basis throughout the periods covered thereby except for any normal
year-end adjustments in the Company’s quarterly financial statements. The other financial information included in each of the SEC Filings has been derived from the accounting records of the Company and
its consolidated subsidiaries and presents fairly in all material respects the information shown thereby.
4.33 Accounting
Controls. The Company and each of its subsidiaries, taken as a whole, maintain a system of internal accounting controls sufficient to provide reasonable assurance that (i) transactions are executed in accordance with management’s
general or specific authorizations; (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with U.S. GAAP and to maintain asset accountability; (iii) access to assets is permitted only in
accordance with management’s general or specific authorization; and (iv) the recorded accountability for assets is compared with the existing assets at reasonable intervals and appropriate action is taken with respect to any differences.
Since the end of the Company’s most recent audited fiscal year, there has been (i) no significant deficiencies or material weaknesses in the Company’s internal control over financial reporting (whether or not remediated) and
(ii) no change in the Company’s internal control over financial reporting that has adversely affected, or is reasonably likely to adversely affect, the Company’s internal control over financial reporting.
4.34 No Sales of Common Shares. The Company has not sold, issued or distributed any
Common Shares during the six-month period preceding the date hereof, including any sales pursuant to Rule 144A under, or Regulation D or S of, the 1933 Act, other than Common Shares issued pursuant to employee
benefit plans, qualified stock option plans or other employee compensation plans or pursuant to outstanding options, rights or warrants and shares sold pursuant to its Open Market Sale Agreement with Jefferies LLC following the period ended
September 30, 2022.
4.35 Certificates, Authorities and Permits. The Company and its subsidiaries have operated and currently
are in compliance in all material respects with all applicable laws, rules and regulations of the jurisdictions in which they are conducting business. None of the Company or any of its subsidiaries is in violation of, or has received any notices of
violations with respect to, any applicable laws, statutes, ordinances, rules or regulations of any governmental body, court or government agency or instrumentality, except for violations which, individually or in the aggregate, have not had or would
not reasonably be expected to have a Material Adverse Effect. Except as would not reasonably be expected to have a Material Adverse Effect, the Company and each of its subsidiaries possess all certificates, authorizations and permits issued by the
appropriate federal, state or foreign regulatory authorities necessary to conduct their respective businesses as currently conducted, and neither the Company nor any of its subsidiaries has received any notice of proceedings relating to the
revocation or modification of any such certificate, authorization or permit which, singly or in the aggregate, if the subject of an unfavorable decision, ruling or finding, would reasonably be expected to have a Material Adverse Effect.
4.36 Tests and Preclinical and Clinical Trials. The preclinical tests and clinical trials conducted by or on behalf of the
Company, or in which the Company’s product candidates have participated, including those that are described in, or the results of which are referred to in, the SEC Filings, were and, if still pending, are being and have been conducted in all
material respects in accordance with all applicable laws, rules, and regulations of the U.S. Food and Drug Administration and comparable regulatory agencies outside of the United States to which they are subject, including the European Medicines
Agency (collectively, the “Regulatory Agencies”), including without limitation 21 C.F.R. Parts 50, 54, 56, 58, and 312; each description of such tests and trials, and the results thereof, contained in the SEC Filings is accurate and
complete in all material respects and fairly presents the data about and derived from such tests and trials, and the Company has no knowledge of any other studies or tests the results of which are inconsistent with, or otherwise call into question,
the results described or referred to in the SEC Filings; and the Company has not received any notices or other correspondence from any Regulatory Agency requiring the termination, suspension or material adverse modification of any preclinical tests
or clinical trials that are, or whose results of which are, described or referred to in the SEC Filings.
4.37 Health Care Laws.
The Company and their directors, officers, employees and, to the knowledge of the Company, agents, are, in compliance in all material respects with all applicable Health Care Laws (as defined below), and have not engaged in activities which are, as
applicable, cause for false claims liability, civil penalties, or mandatory or permissive exclusion from Medicare, Medicaid, or any other state, provincial, or federal health care program. “Health Care Laws” means the laws and
regulations of the Regulatory Agencies, including, without limitation, the Public Health Service Act (42 U.S.C. § 201 et. seq.), the Federal Food, Drug, and Cosmetic Act (21 U.S.C. §§ 301 et seq.), the regulations promulgated pursuant
to such laws, and any other similar local, state, provincial, or federal law. The Company has not received any FDA Form 483, notice of adverse finding, warning letter, untitled letter or other correspondence or notice from the U.S. Food and Drug
Administration or any other Regulatory Agency alleging or asserting noncompliance with any Health Care Laws applicable to the Company or its subsidiaries. Additionally, the Company is not a party to nor has any ongoing reporting obligations pursuant
to any corporate integrity agreements, deferred prosecution agreements, monitoring agreements, consent decrees, settlement orders, plans of correction or similar agreements with or imposed by any Regulatory Agency. None of the Company nor any of its
respective employees, officers or directors, or, to the knowledge of the Company, agents, has been excluded, suspended or debarred from participation in any U.S. federal health care program or human clinical research or, to the knowledge of the
Company, is subject to a governmental inquiry, investigation, proceeding, or other similar action that could reasonably be expected to result in debarment, suspension, or exclusion.
4.38 Tax Matters. The Company and each of its subsidiaries have filed all U.S.
federal, state, provincial, local and non-U.S. income tax returns and all other material tax returns required to be filed through the date of this Agreement and have paid all material taxes and other
assessments of a similar nature (whether imposed directly or indirectly or through withholding) including any interest, additions to tax, or penalties applicable thereto due or claimed to be due from such entities (whether or not reflected on such
returns), except any such taxes as are currently being contested in good faith and by appropriate proceedings and for which adequate reserves have been established in the financial statements of the Company in accordance with U.S. GAAP, and no
material tax deficiency has been, or could reasonably be expected to be, asserted against or determined adversely to the Company or any of its subsidiaries or any of their respective properties or assets, nor does the Company or any of its
subsidiaries have any notice or knowledge of any such tax deficiency.
4.39 ERISA. Except as would not, individually or in the
aggregate, reasonably be expected to have a Material Adverse Effect, (i) each employee benefit plan, within the meaning of Section 3(3) of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”), that is
sponsored, maintained, administered or contributed to by the Company has been maintained in compliance with its terms and the requirements of any applicable statutes, orders, rules and regulations, including but not limited to ERISA and the Internal
Revenue Code of 1986, as amended (the “Code”), and (ii) neither the Company nor any member of its “Controlled Group” (defined as any trade or business, whether or not incorporated, that would be regarded as a single
employer with the Company under Section 414 of the Code) (x) has ever sponsored, maintained, contributed to or has had any obligation to contribute to, any employee benefit plan that is subject to Title IV of ERISA or any
“multiemployer plan” as defined in Section 3(37) of ERISA or (y) has incurred, or reasonably expects to incur, any liability under Title IV of ERISA.
4.40 No Relationship. No relationship, direct or indirect, exists between the Company, on the one hand, and the directors, officers, or
shareholders of the Company, on the other, that is required by the 1933 Act or Canadian Securities Laws, as applicable, to be described in the SEC Filings and that is not so described in the SEC Filings.
4.41 Manipulation of Price. Neither the Company nor any Affiliate of the Company has taken, nor will the Company or any Affiliate take,
directly or indirectly, any action which is designed to or which would be expected to cause or result in the stabilization or manipulation of the price of any security of the Company to facilitate the sale or resale of the Shares.
4.42 No Integrated Offering. Neither the Company nor its subsidiaries nor any of their Affiliates has, directly or indirectly, made, or
will make, any offers or sales of any Company security or solicited any offers to buy any Company security, under circumstances that would adversely affect reliance by the Company on Section 4(a)(2) and Rule 506(b) of Regulation D thereunder
for the exemption from registration for the transactions contemplated hereby or would require registration of the Shares under the 1933 Act.
4.43 Exemption from Registration Requirements. Assuming the accuracy of the representations and warranties of the Investors set forth
in Section 5, the offer and sale of the Shares to the Investors are exempt from the registration requirements of the 1933 Act. The issuance and sale of the Shares do not contravene the rules and regulations of Nasdaq.
4.44 Sarbanes-Oxley Act. The Company is in compliance, in all material respects, with all applicable provisions of the Sarbanes-Oxley
Act of 2002, as amended, and the rules and regulations promulgated thereunder.
4.45 Shell Company Status. The Company is not
currently, and has never been, an issuer identified in Rule 144 (i)(1) under the 1933 Act.
4.46 Brokers and Finders. Other than
Morgan Stanley & Co. LLC and Jefferies LLC (the “Placement Agents”), no Person will have, as a result of the transactions contemplated by the Transaction Documents, any valid right, interest or claim against or upon the
Company or any Investor for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of the Company. No Investor shall have any obligation with respect to any fees, or with
respect to any claims made by or on behalf of other Persons for fees, in each case of the type contemplated by this Section 4.46 that may be due in connection with the transactions contemplated by the Transaction Documents.
4.47 No Bad Actors. No “bad actor” disqualifying event described in Rule
506(d)(1)(i)-(viii) of the 1933 Act (a “Disqualification Event”) is applicable to the Company or, to the Company’s knowledge, any Company Covered Person, except for a Disqualification Event as to which Rule
506(d)(2)(ii–iv) or (d)(3) is applicable.
4.48 General Instruction I.B.6. The Company meets the registration and transaction
requirements for use of Form S-3 (including pursuant to General Instruction I.B.1 and, as applicable, I.B.6 of Form S-3) for the registration of the Shares and for
resale by the Investors.
4.49 No Additional Agreements. The Company does not have any agreement or understanding with any Investor
with respect to the transactions contemplated by the Transaction Documents other than as specified in the Transaction Documents. For the avoidance of doubt, the Company has not entered into any other purchase agreement with any other Person on or
around the date hereof, or any other agreement in connection with any Person’s direct or indirect equity investment in the Company that includes terms and conditions that are materially more advantageous to such Person than to any Investor
hereunder.
4.50 DTC Eligibility. The Common Shares are eligible for clearing through the Depository Trust Company
(“DTC”), through its Deposit/Withdrawal At Custodian (DWAC) system, and the Company is eligible for and participating in the Direct Registration System (DRS) of DTC with respect to the Common Shares. The Transfer Agent is a
participant in, and the Common Shares are eligible for transfer pursuant to, DTC’s Fast Automated Securities Transfer Program. The Common Shares are not, and have not at any time been, subject to any DTC “chill,” “freeze” or
similar restriction with respect to any DTC services, including the clearing of transactions in Common Shares through DTC.
4.51
Disclosure. Except with respect to the material terms and conditions of the transactions contemplated by the Transaction Documents, the Company confirms that neither it nor any other Person acting on its behalf has provided any Investor or
its agents or counsel with any information that it believes constitutes or might constitute material, nonpublic information which will not otherwise be disclosed in the Press Release (as defined below) or in the Company’s Current Report on Form
8-K to be filed simultaneously with the Press Release (the “Announcing 8-K”). The Company understands and confirms that each Investor will rely on the foregoing representation in effecting
transactions in securities of the Company. All of the disclosure furnished by or on behalf of the Company to the Investors regarding the Company and its subsidiaries, their respective businesses and the transactions contemplated hereby is true and
correct in all material respects and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made,
not misleading. The Company acknowledges and agrees that no Investor makes or has made any representations or warranties with respect to the transaction contemplated hereby other than those specifically set forth in Section 5 hereof.
5. Representations and Warranties of the Investors. Each of the Investors hereby severally, and not jointly, represents and warrants to
the Company that:
5.1 Organization and Existence. Such Investor is a duly incorporated or organized and validly existing
corporation, limited partnership, limited liability company or other legal entity, has all requisite corporate, partnership or limited liability company power and authority to enter into and consummate the transactions contemplated by the
Transaction Documents and to carry out its obligations hereunder and thereunder, and to invest in the Shares pursuant to this Agreement, and is in good standing under the laws of the jurisdiction of its incorporation or organization.
5.2 Authorization. The execution, delivery and performance by such Investor of the Transaction Documents to which such Investor is a
party have been duly authorized and each has been duly executed and when delivered will constitute the valid and legally binding obligation of such Investor, enforceable against such Investor in accordance with their respective terms, subject to
bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and similar laws of general applicability, relating to or affecting creditors’ rights generally, and general principles of equity.
5.3 Purchase Entirely for Own Account. The Shares to be purchased by such Investor
hereunder will be acquired for such Investor’s own account, not as nominee or agent, and not with a view to the resale or distribution of any part thereof in violation of the 1933 Act or Canadian Securities Laws, and such Investor has no
present intention of selling, granting any participation in, or otherwise distributing the same in violation of the 1933 Act or Canadian Securities Laws without prejudice, however, to such Investor’s right at all times to sell or otherwise
dispose of all or any part of such Shares in compliance with applicable federal and state securities laws or Canadian Securities Laws. The Shares are being purchased by such Investor in the ordinary course of its business. Nothing contained herein
shall be deemed a representation or warranty by such Investor to hold the Shares for any period of time. Such Investor is not a broker-dealer registered with the SEC under the 1934 Act or an entity engaged in a business that would require it to be
so registered.
5.4 Investment Experience. Such Investor acknowledges that it can bear the economic risk and complete loss of its
investment in the Shares and has such knowledge and experience in financial or business matters that it is capable of evaluating the merits and risks of the investment contemplated hereby.
5.5 Disclosure of Information. Such Investor has had an opportunity to receive, review and understand all information related to the
Company requested by it and to ask questions of and receive answers from the Company regarding the Company, its business and the terms and conditions of the offering of the Shares, and has conducted and completed its own independent due diligence.
Such Investor acknowledges that copies of the SEC Filings are available on the EDGAR system. Based on the information such Investor has deemed appropriate, it has independently made its own analysis and decision to enter into the Transaction
Documents. Such Investor is relying exclusively on its own investment analysis and due diligence (including professional advice it deems appropriate) with respect to the execution, delivery and performance of the Transaction Documents, the Shares
and the business, condition (financial and otherwise), management, operations, properties and prospects of the Company, including but not limited to all business, legal, regulatory, accounting, credit and tax matters, based, in part, upon the
information provided or made available by or on behalf of the Company (on the EDGAR system or otherwise) to such Investor. Neither such inquiries nor any other due diligence investigation conducted by such Investor shall modify, limit or otherwise
affect such Investor’s right to rely on the Company’s representations and warranties contained in this Agreement.
5.6
Restricted Securities. Such Investor understands that the Shares are characterized as “restricted securities” under the U.S. federal securities laws and are subject to a restricted period under Canadian Securities Laws inasmuch as
they are being acquired from the Company in a transaction not involving a public offering and that under such laws and applicable regulations such securities may be resold without registration under the 1933 Act Laws or without a prospectus pursuant
to Canadian Securities Laws only in certain limited circumstances.
5.7 Legends. It is understood that, except as provided below,
certificates or book entry positions evidencing the Shares may bear the following or any similar legend (and will not, for the avoidance of doubt, bear any legend under Canadian Securities Laws):
“These securities represented hereby have not been registered with the Securities and Exchange Commission or the securities commission of
any state but have been issued in reliance upon an exemption from registration under the Securities Act of 1933, as amended, and, accordingly, may not be transferred unless (i) such securities have been registered for sale pursuant to the
Securities Act of 1933, as amended, (ii) such securities may be sold pursuant to Rule 144, (iii) the Company has received an opinion of counsel reasonably satisfactory to it that such transfer may lawfully be made without registration under the
Securities Act of 1933, as amended, or (iv) the securities are transferred without consideration to an affiliate of such holder or a custodial nominee (which for the avoidance of doubt shall require neither consent nor the delivery of an
opinion).”
5.8 Accredited Investor. Such Investor is an “accredited investor” within
the meaning of Rule 501(a) of Regulation D. Prior to the Closing, such Investor will execute and deliver to the Company its Investor Questionnaire, which such Investor represents and warrants will be true, correct and complete in all material
respects. Such investor is a sophisticated institutional investor with sufficient knowledge and experience in investing in private equity transactions to properly evaluate the risks and merits of its purchase of the Shares. Such Investor has
determined based on its own independent review and such professional advice as it deems appropriate that its purchase of the Shares and participation in the transactions contemplated by the Transaction Documents (i) are fully consistent with
its financial needs, objectives and condition and (ii) comply and are fully consistent with all investment policies, guidelines and other restrictions applicable to such Investor.
5.9 No General Solicitation. Such Investor did not learn of the offering of the Shares as a result of any general or public
solicitation or general advertising, or publicly disseminated advertisements or sales literature, including (a) any advertisement, article, notice or other communication published in any newspaper, magazine, website, or similar media, or
broadcast over television or radio, or (b) any seminar or meeting to which such Investor was invited by any of the foregoing means of communications.
5.10 Placement Agents. Such Investor hereby acknowledges and agrees that it has independently evaluated the merits of its
decision to purchase the Shares, and that (a) the Placement Agents are acting solely as placement agents in connection with the execution, delivery and performance of the Transaction Documents and are not acting as an underwriter or in any
other capacity and is not and shall not be construed as a fiduciary for such Investor, the Company or any other Person in connection with the execution, delivery and performance of the Transaction Documents, (b) the Placement Agents have not
made and will not make any representation or warranty, whether express or implied, of any kind or character and has not provided any advice or recommendation in connection with the execution, delivery and performance of the Transaction Documents and
(c) the Placement Agents will not have any responsibility with respect to (i) any representations, warranties or agreements made by any Person under or in connection with the execution, delivery and performance of the Transaction
Documents, or the execution, legality, validity or enforceability (with respect to any Person) thereof, or (ii) the business, affairs, financial condition, operations, properties or prospects of, or any other matter concerning the Company.
5.11 Brokers and Finders. No Person will have, as a result of the transactions contemplated by the Transaction Documents, any valid
right, interest or claim against or upon the Company or an Investor for any commission, fee or other compensation pursuant to any agreement, arrangement or understanding entered into by or on behalf of such Investor.
5.12 Short Sales and Confidentiality Prior to the Date Hereof. Other than consummating the transactions contemplated hereunder, such
Investor has not, either directly or indirectly by any Person acting on behalf of or pursuant to any understanding with such Investor, directly or indirectly executed any purchases or sales, including Short Sales, of the securities of the Company
during the period commencing as of the time that such Investor was first contacted by the Company or any other Person representing the Company regarding the transactions contemplated hereby and ending immediately prior to the date hereof.
Notwithstanding the foregoing, (i) in the case of an Investor that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Investor’s assets and the portfolio managers have no direct
knowledge of the investment decisions made by the portfolio managers managing other portions of such Investor’s assets, the representation set forth above shall only apply with respect to the portion of assets managed by the portfolio manager
that made the investment decision to purchase the Shares covered by this Agreement and (ii) and in the case of an Investor whose investment adviser utilized an information barrier with respect to the information regarding the transactions
contemplated hereunder after first being contacted by the Company or such other Person representing the Company, the representation set forth above shall only apply after the point in time when the portfolio manager who manages such Investor’s
assets was informed of the information regarding the transactions contemplated hereunder and, with respect to the Investor’s investment adviser, the representation set forth above shall only apply with respect to any purchases or sales,
including Short Sales, of the securities of the Company on behalf of other funds or investment vehicles for which the Investor’s investment adviser is also an investment adviser or sub-adviser after the
point in time when the portfolio manager who manages the assets of such other funds or investment vehicles for which the Investor’s investment adviser is also an investment adviser or sub-adviser was
informed of the information regarding the transactions contemplated hereunder. Other than to other Persons party to this Agreement and other than to such Person’s Affiliates, outside attorneys, accountants, auditors or investment advisors only
to the extent necessary to permit evaluation of the investment, and the performance of the necessary or required tax, accounting, financial, legal, or administrative tasks and services and other than as may be required by law, such Investor has
maintained the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction). Notwithstanding the foregoing, for avoidance of doubt, nothing contained herein shall
constitute a representation or warranty, or preclude any actions, with respect to the identification of the availability of, or securing of, available shares to borrow in order to effect Short Sales or similar transactions in the future.
5.13 No Government Recommendation or Approval. Such Investor understands that no
United States federal or state agency, or similar agency of any other country, has reviewed, approved, passed upon, or made any recommendation or endorsement of the Company or the purchase of the Shares.
5.14 No Intent to Effect a Change of Control. Such Investor has no present intent to effect a “change of control” of the
Company as such term is understood under the rules promulgated pursuant to Section 13(d) of the 1934 Act. Except as set forth in its Selling Stockholder Questionnaire or in filings made by the Investor or its Affiliates with the SEC prior to
the date hereof, as of the date hereof, neither the Investor nor any of its Affiliates is the owner of record or the beneficial owner of shares of Common Shares or securities convertible into or exchangeable for Common Shares.
5.15 Residency. Such Investor’s office in which its investment decision with respect to the Shares was made is located at the
address immediately below such Investor’s name on its signature page hereto.
5.16 No Conflicts. The execution, delivery and
performance by such Investor of the Transaction Documents and the consummation by such Investor of the transactions contemplated hereby and thereby will not (i) result in a violation of the organizational documents of such Investor or
(ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture
or instrument to which such Investor is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws) applicable to such Investor, except in the case of clauses
(ii) and (iii) above, for such conflicts, defaults, rights or violations which would not, individually or in the aggregate, reasonably be expected to have a material adverse effect on the ability of such Investor to perform its obligations
hereunder.
Such Investor hereby acknowledges and agrees that the Placement Agents are third party beneficiaries to the representations
and warranties set forth in this Section 5.
6. Conditions to Closing.
6.1 Conditions to the Investors’ Obligations. The obligation of each Investor to purchase Shares at the Closing is subject to the
fulfillment to such Investor’s satisfaction, on or prior to the Closing Date, of the following conditions, any of which may be waived by such Investor (as to itself only):
| |
(a) |
The representations and warranties made by the Company in Section 4 hereof, as qualified by the SEC
Filings, shall be true and correct in all respects as of such date, except to the extent any such representation or warranty expressly speaks as of an earlier date, in which case such representation or warranty shall be true and correct in all
material respects as of such earlier date. The Company shall have performed in all material respects all obligations and covenants herein required to be performed by it on or prior to the Closing Date. |
| |
(b) |
The Company shall have obtained any and all consents, permits, approvals, including to the extent applicable,
any shareholder approvals, registrations and waivers necessary for the consummation of the purchase, sale and issuance of the Shares and the consummation of the other transactions contemplated by the Transaction Documents, all of which shall be in
full force and effect. |
| |
(c) |
The Company shall have executed and delivered the Registration Rights Agreement. |
| |
(d) |
The Company shall have filed with Nasdaq a Notification Form: Listing of Additional Shares for the listing of
the Shares, and Nasdaq shall have raised no objection to the consummation of the transactions contemplated by the Transaction Documents. |
| |
(e) |
No judgment, writ, order, injunction, award or decree of or by any court, or judge, justice or magistrate,
including any bankruptcy court or judge, or any order of or by any governmental authority, shall have been issued, and no action or proceeding shall have been instituted by any governmental authority, enjoining or preventing the consummation of the
transactions contemplated hereby or in the other Transaction Documents. |
| |
(f) |
The Company shall have delivered a certificate, executed on behalf of the Company by its Chief Executive
Officer or its Chief Financial Officer, dated as of the Closing Date, certifying to the fulfillment of the conditions specified in subsections (a), (b), (d), (e), (i) and (j) of this Section 6.1. |
| |
(g) |
The Company shall have delivered a certificate, executed on behalf of the Company by its Secretary, dated as of
the Closing Date, certifying the resolutions adopted by the Board of Directors of the Company approving the transactions contemplated by this Agreement, the other Transaction Documents, the issuance of the Shares, certifying the current versions of
the Articles of Incorporation and General By-laws of the Company and certifying as to the signatures and authority of Persons signing the Transaction Documents and related documents on behalf of the Company.
|
| |
(h) |
The Investors shall have received an opinion from Goodwin Procter LLP, the Company’s counsel, dated as of
the Closing Date, in form and substance reasonably acceptable to the Investors. The Investors shall have received an opinion from Osler, Hoskin & Harcourt LLP, the Company’s Canadian counsel, dated as of the Closing Date, in form and
substance reasonably acceptable to the Investors. |
| |
(i) |
There shall have been no Material Adverse Effect since the date hereof. |
| |
(j) |
No stop order or suspension of trading shall have been imposed by Nasdaq, the SEC or any other governmental or
regulatory body with respect to public trading in the Common Shares and no proceedings for any such purposes shall have been initiated or threatened. |
6.2 Conditions to Obligations of the Company. The Company’s obligation to sell and issue Shares to each Investor at the Closing is
subject to the fulfillment to the satisfaction of the Company on or prior to the Closing Date of the following conditions, any of which may be waived by the Company:
| |
(a) |
The representations and warranties made by such Investor in Section 5 hereof shall be true and correct in
all material respects as of the date hereof, and shall be true and correct as of the Closing Date with the same force and effect as if they had been made on and as of such date, except to the extent any such representation or warranty expressly
speaks as of an earlier date, in which case such representation or warranty shall be true and correct in all material respects as of such earlier date. Such Investor shall have performed in all material respects all obligations and covenants herein
required to be performed by such Investor on or prior to the Closing Date. |
| |
(b) |
Such Investor shall have executed and delivered the Registration Rights Agreement, an Investor Questionnaire
and a Selling Stockholder Questionnaire. |
| |
(c) |
Such Investor purchasing Shares at the Closing shall have paid in full its purchase price to the Company.
|
6.3 Termination of Obligations to Effect Closing; Effects.
| |
(a) |
The obligations of the Company, on the one hand, and each Investor, on the other hand, to effect the Closing
shall terminate as follows: |
| |
(i) |
upon the mutual written consent of the Company and an Investor solely as it itself; |
| |
(ii) |
by the Company if any of the conditions set forth in Section 6.2 shall have become incapable of
fulfillment, and shall not have been waived by the Company; |
| |
(iii) |
by an Investor (with respect to itself only) if any of the conditions set forth in Section 6.1 shall have
become incapable of fulfillment, and shall not have been waived by such Investor; or |
| |
(iv) |
automatically without further action by the Company or the Investors if the Closing has not occurred on or
before 5:00 p.m. (New Yok City time) on February 21, 2023. |
provided, however, that, in the case of clauses
(ii) and (iii) above, the party seeking to terminate its obligation to effect the Closing shall not be entitled to so terminate if such party shall then be in breach of any of its representations, warranties, covenants or agreements contained
in this Agreement or the other Transaction Documents and such breach has resulted in the circumstances giving rise to such party’s seeking to terminate its obligation to effect the Closing.
| |
(b) |
In the event of termination by the Company or any Investor of its obligations to effect the Closing pursuant to
Section 6.3(a)(i), written notice thereof shall be given to the other Investors by the Company, and the other Investors shall have the right to terminate their obligations to effect the Closing upon written notice to the Company and the other
Investors. Nothing in this Section 6.3 shall be deemed to release any party from any liability for any breach by such party of the terms and provisions of this Agreement or the other Transaction Documents or to impair the right of any party to
compel specific performance by any other party of its obligations under this Agreement or the other Transaction Documents. |
7. Covenants and Agreements of the Parties
7.1 Nasdaq Listing. The Company will use commercially reasonable efforts to continue the listing and trading of its Common Shares on
Nasdaq and, in accordance therewith, will use commercially reasonable efforts to comply in all material respects with the Company’s reporting, filing and other obligations under the bylaws or rules of such market or exchange, as applicable.
7.2 Removal of Legends. Within one (1) Business Day following the date the Registration Statement (as defined in the
Registration Rights Agreement) is declared effective, the Company shall provide or cause its legal counsel to provide the transfer agent for the Common Shares (the “Transfer Agent”) one or more opinions regarding the removal of
legends in connection with sales or other permitted dispositions pursuant to the effective Registration Statement. In connection therewith, the Company shall use reasonable best efforts to have the Transfer Agent remove any restrictive legends
related to the book entry account holding such Shares and make a new, unlegended entry for such book entry Shares sold or disposed of without restrictive legends within two (2) Trading Days receipt of notice of the sale or disposition pursuant
to the Registration Statement, provided that the Transfer Agent and/or Company has timely received from the Investor a customary seller representation letter regarding such disposition. In addition, either (i) any time after the holding period
specified in Rule 144(d)(1)(ii) has been satisfied or (ii) in connection with any sale, assignment, transfer or other disposition of the Shares by an Investor pursuant to Rule 144 or pursuant to any other exemption under the 1933 Act such that
the purchaser acquires freely tradable shares and upon compliance by the Investor with the requirements of this Agreement, if requested by an Investor, the Company shall use reasonable best efforts to have provide or cause its legal counsel to
provide the Transfer Agent one or more opinions (including blanket opinions) regarding the removal of legends in reliance of such rule, and to have the Transfer Agent remove any restrictive legends related to the book entry account holding such
shares and make a new, unlegended entry for such book entry shares without restrictive legends within two (2) Trading Days of any such request, provided that the Transfer Agent and/or Company has timely received from the Investor customary
representations and other documentation reasonably acceptable to the Company in connection therewith. Shares subject to legend removal hereunder may be transmitted by the Transfer Agent to the Investor by crediting the account of the Investor’s
prime broker with the DTC’s system as directed by such Investor. The Company shall be responsible for the fees of its Transfer Agent and all DTC fees associated with such issuance.
7.3 Transfer Restrictions. Each Investor agrees that it will sell, transfer or
otherwise dispose of the Shares only pursuant to an effective registration statement under the 1933 Act or an exemption from the registration requirements thereof and that any Shares sold by such Investor pursuant to an effective registration
statement will be sold in compliance with the plan of distribution set forth therein.
7.4 Subsequent Equity Sales by the Company.
The Company shall not, and shall use its commercially reasonable efforts to ensure that no Affiliate of the Company shall, sell, offer for sale or solicit offers to buy or otherwise negotiate in respect of any security (as defined in
Section 2 of the 1933 Act) that will be integrated with the offer or sale of the Shares in a manner that would require the registration under the 1933 Act of the sale of the Shares to the Investors, or that will be integrated with the offer or
sale of the Shares for purposes of the rules and regulations of any Trading Market such that it would require shareholder approval prior to the closing of such other transaction unless stockholder approval is obtained before the closing of such
subsequent transaction. The Company shall not take any action or steps that would adversely affect reliance by the Company on Section 4(a)(2) and Rule 506(b) of Regulation D for the exemption from registration for the transactions contemplated
hereby or require registration of the Shares under the 1933 Act.
7.5 Fees. The Company shall be responsible for the payment of any
placement agent’s fees, financial advisory fees, or broker’s commissions (other than for Persons engaged by any Investor) relating to or arising out of the transactions contemplated hereby.
7.6 Short Sales and Confidentiality After the Date Hereof. Each Investor covenants that it will not, nor will it cause any Person
acting on its behalf or pursuant to any understanding with it to, execute any Short Sales during the period from the date hereof until the earlier of such time as (i) the transactions contemplated by this Agreement are first publicly announced
or (ii) this Agreement is terminated in full. Notwithstanding the foregoing, in the case of an Investor that is a multi-managed investment vehicle whereby separate portfolio managers manage separate portions of such Investor’s assets and
the portfolio managers have no direct knowledge of the investment decisions made by the portfolio managers managing other portions of such Investor’s assets, the covenant set forth above shall only apply with respect to the portion of assets
managed by the portfolio manager that made the investment decision to purchase the Shares covered by this Agreement. Each Investor covenants that until such time as the transactions contemplated by this Agreement are publicly disclosed by the
Company, such Investor will maintain the confidentiality of all disclosures made to it in connection with this transaction (including the existence and terms of this transaction), other than to such Person’s outside attorney, accountant,
auditor or investment advisor only to the extent necessary to permit evaluation of the investment, and the performance of the necessary or required tax, accounting, financial, legal, or administrative tasks and services and other than as may be
required by law.
7.7 Filings. The Company shall make all filings with the SEC and its Trading Market and Canadian Securities Laws
as required by the transactions contemplated hereby.
7.8 Nonpublic Information. Except with respect to the material terms and
conditions of the transactions contemplated by the Transaction Documents, which shall be disclosed pursuant to Section 9.8, the Company covenants and agrees that neither it, nor any other Person acting on its behalf will provide any Investor or
its agents or counsel with any information that constitutes, or the Company reasonably believes constitutes, material nonpublic information, unless prior thereto such Investor shall have consented to the receipt of such information and agreed with
the Company to keep such information confidential. The Company understands and confirms that each Investor shall be relying on the foregoing covenant in effecting transactions in securities of the Company. To the extent that any notice provided
pursuant to any Transaction Document constitutes, or contains, material, nonpublic information regarding the Company or any subsidiaries, the Company shall simultaneously file such notice with the SEC pursuant to a Current Report on Form 8-K. The Company understands and confirms that each Investor shall be relying on the foregoing covenant in effecting transactions in securities of the Company.
8. Survival and Indemnification.
8.1 Survival. The representations, warranties, covenants and agreements contained in this Agreement shall survive the Closing of the
transactions contemplated by this Agreement for the applicable statute of limitations.
8.2 Indemnification. The Company agrees to indemnify, defend and hold harmless each
Investor and its Affiliates, and their respective directors, officers, trustees, partners, members, managers, stockholders, employees, investment advisers and agents, and each person who controls the Investor (within the meaning of Section 15
of the 1933 Act or Section 20 of the 1934 Act), and the officers, directors, partners, members, managers, stockholders, agents, affiliates, employees and investment advisers of each such controlling person from and against any and all losses,
claims, damages, liabilities and expenses (including without limitation reasonable and documented attorney’s fees and disbursements and other documented
out-of-pocket expenses reasonably incurred in connection with investigating, preparing or defending any action, claim or proceeding, pending or threatened and the costs
of enforcement thereof) to which such Person may become subject as a result of any breach of representation, warranty, covenant or agreement made by or to be performed on the part of the Company under the Transaction Documents, and will reimburse
any such Person for all such amounts as they are incurred by such Person solely to the extent such amounts have been finally judicially determined not to have resulted from such Person’s fraud or willful misconduct; provided, however, that the
Company will not be liable in any such case to the extent that any such loss, claim, damage, liability or expense arises out of or is based upon (i) the material failure of such Person entitled to indemnification to comply with the covenants
and agreements contained herein, or (ii) the material inaccuracy of any representations or warranties made herein by such Person entitled to indemnification herein, or (y) has been finally judicially determined to have resulted from such
Person’s fraud or willful misconduct. The Company shall notify the Investors promptly of the institution, threat or assertion of any proceeding arising from or in connection with the transactions contemplated by the Transaction Documents of
which the Company is aware.
8.3 Conduct of Indemnification Proceedings. Any Person entitled to indemnification
hereunder shall (i) give prompt written notice to the indemnifying party of any claim with respect to which it seeks indemnification and (ii) permit such indemnifying party to assume the defense of such claim with counsel reasonably
satisfactory to the indemnified party; provided that any Person entitled to indemnification hereunder shall have the right to employ separate counsel and to participate in the defense of such claim, but the fees and expenses of such counsel
shall be at the expense of such Person unless (a) the indemnifying party has agreed in writing to pay such fees or expenses, (b) the indemnifying party shall have failed to assume the defense of such claim and employ counsel reasonably
satisfactory to such indemnified party or (c) in the reasonable judgment of any such indemnified party, based upon written advice of its counsel, a conflict of interest exists between such indemnified party and the indemnifying party with
respect to such claims (in which case, if the indemnified party notifies the indemnifying party in writing that such indemnified party elects to employ separate counsel at the expense of the indemnifying party, the indemnifying party shall not have
the right to assume the defense of such claim on behalf of such Person); and provided, further, that the failure of any indemnified party to give written notice as provided herein shall not relieve the indemnifying party of its obligations
hereunder, except to the extent that such failure to give notice shall materially adversely affect the indemnifying party in the defense of any such claim or litigation. It is understood that the indemnifying party shall not, in connection with any
proceeding in the same jurisdiction, be liable for fees or expenses of more than one separate firm of attorneys at any time for all such indemnified parties. No indemnifying party will, except with the consent of the indemnified party, which consent
shall not be unreasonably withheld, conditioned or delayed, consent to entry of any judgment or enter into any settlement that does not include as an unconditional term thereof the giving by the claimant or plaintiff to such indemnified party of a
release from all liability in respect of such claim or litigation or that includes any admission as to fault, culpability or failure to act on the part of such indemnified party. No indemnified party will, except with the consent of the indemnifying
party, which consent shall not be unreasonably withheld, conditioned or delayed, consent to entry of any judgment or enter into any settlement on any such matter.
9. Miscellaneous.
9.1
Successors and Assigns. No party hereunder may assign its rights or obligations hereunder without, in the case of any Investor, the consent of the Company and, in the case of the Company, the consent of each of the Investors, except that each
Investor may, prior to the Closing and without the consent of the Company, assign its rights hereunder to any Affiliate of such Investor and/or, after the Closing, to any assignee or transferee of the Common Shares; provided, that no such assignment
shall relieve such Investor of its obligations hereunder and provided such assignee or transferee agrees in writing to be bound by the provisions hereof that apply to Investors. The provisions of this Agreement shall inure to the benefit of and be
binding upon the respective permitted successors and assigns of the parties. Without limiting the generality of the foregoing, in the event that the Company is a party to a merger, consolidation, share exchange or similar business combination
transaction in which the
Common Shares is converted into the equity securities of another Person, from and after the effective time of such transaction, such Person shall, by virtue of such transaction, be deemed to have
assumed the obligations of the Company hereunder, the term “Company” shall be deemed to refer to such Person and the term “Shares” shall be deemed to refer to the shares received by the Investors in connection with such
transaction. Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective permitted successors and assigns any rights, remedies, obligations, or liabilities under or by reason
of this Agreement, except as expressly provided in this Agreement.
9.2 Counterparts. This Agreement may be executed in one or more
counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signatures complying with
the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
9.3 Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in
construing or interpreting this Agreement.
9.4 Notices. Unless otherwise provided, any notice required or permitted under this
Agreement shall be given in writing and shall be deemed effectively given as hereinafter described (i) if given by personal delivery, then such notice shall be deemed given upon such delivery, (ii) if given by e-mail, then such notice shall be deemed given upon receipt of confirmation of receipt of an e-mail transmission, (iii) if given by mail, then such notice shall be deemed
given upon the earlier of (A) receipt of such notice by the recipient or (B) three (3) days after such notice is deposited in first class mail, postage prepaid, and (iv) if given by an internationally recognized overnight air courier,
then such notice shall be deemed given one Business Day after delivery to such carrier. All notices shall be addressed to the party to be notified at the address as follows, or at such other address as such party may designate by ten
(10) days’ advance written notice to the other party:
If to the Company:
Fusion Pharmaceuticals Inc.
Two
International Place, Suite 2310
Boston, MA, 02110
Telephone: (617) 388-4573
Attention: Maria Stahl
Email:
With a copy (which shall not constitute notice) to:
Goodwin Procter LLP
620 Eighth
Avenue
New York, NY 10018
Telephone: (212) 459-7234
Attention: Mitchell Bloom; Siavosh Salimi
Email:
If to the Investors:
Only to the addresses set forth on the signature pages hereto.
9.5 Expenses. The parties hereto shall pay their own costs and expenses in connection herewith regardless of whether the transactions
contemplated hereby are consummated; it being understood that each of the Company and each Investor has relied on the advice of its own respective counsel.
9.6 Amendments and Waivers. Prior to Closing, no amendment or waiver of any provision
of this Agreement will be effective with respect to any party unless made in writing and signed by a duly authorized representative of such party. Following the Closing, any term of this Agreement may be amended and the observance of any term of
this Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and the Required Investors. Notwithstanding the foregoing, this Agreement may not be
amended and the observance of any term of this Agreement may not be waived with respect to any Investor without the written consent of such Investor unless such amendment or waiver applies to all Investors in the same fashion. Any amendment or
waiver effected in accordance with this paragraph shall be binding upon (i) prior to Closing, each Investor that signed such amendment or waiver and (ii) following the Closing, each holder of any Shares purchased under this Agreement at
the time outstanding, and in each case, each future holder of all such Shares and the Company.
9.7 Other Agreements. The Company
has not entered and will not enter, subsequent to the date hereof, into any side letter or similar agreement relating to the purchase of the Shares with any other Investor, other than this Agreement, which would reflect a purchase price or other
terms and conditions with respect to the purchase of the Shares that are more favorable to such Investor than the terms of this Agreement.
9.8 Publicity. Except as set forth below, no public release or announcement concerning the transactions contemplated hereby shall be
issued by the Investors without the prior consent of the Company, except as such release or announcement may be required by law or the applicable rules or regulations of any securities exchange or securities market, in which case the Investors shall
allow the Company reasonable time to comment on such release or announcement in advance of such issuance. Notwithstanding the foregoing, each Investor may identify the Company and the value of such Investor’s security holdings in the Company in
accordance with, and provide any other information that is required to be disclosed pursuant to, applicable investment reporting and disclosure regulations or internal policies without prior notice to or consent from the Company (including, for the
avoidance of doubt, filings pursuant to Sections 13 and 16 of the 1934 Act). The Company shall not include the name of any Investor or any Affiliate or investment adviser of such Investor in any press release or public announcement (which, for the
avoidance of doubt, shall not include any SEC Filing to the extent such disclosure is required by SEC rules and regulations) without the prior written consent of such Investor. No later than the 9:01 a.m. (New York City time) on the Business Day
immediately following the date this Agreement is executed (provided that, if this Agreement is executed between midnight and 9:00 a.m. (New York City time) on any Business Day, no later than 9:01 a.m. on the date the Agreement is executed), the
Company shall issue a press release disclosing all material terms of the transactions contemplated by this Agreement (the “Press Release”) and shall file with the SEC the Announcing 8-K
disclosing any other material non-public information provided or made available by or on behalf of the Company to any of the Investors on or prior to the date hereof and including as exhibits thereto the Press
Release, this Agreement and the Registration Rights Agreement (in each case without redaction). In addition, the Company will make such other legally required filings and notices in the manner and time required by the SEC or Nasdaq and Canadian
Securities Laws; provided that, to the extent such filings include the name of any Investor or any Affiliate or investment adviser of such Investor, then the Company shall provide such Investor with prompt prior notice of such filing requirement so
that the Investor may (a) seek appropriate relief to prevent or limit such disclosure, (b) furnish only that portion of the information which is legally required to be furnished or disclosed, and to the extent reasonably feasible, and
(c) consult with the Company on content and timing prior to any such disclosure. The Company represents and warrants that, from and after the issuance of the Press Release and the filing of the Announcing
8-K, no Investors shall be in possession of any material nonpublic information received from the Company, its subsidiaries or any of their respective officers, directors, employees or agents in connection with
the transactions contemplated by this Agreement. The Company hereby acknowledges and agrees that no Investor (nor any of such Investor’s Affiliates, attorneys, accountants, agents or representatives) shall have any duty of trust or confidence
(including any such obligation under any confidentiality or non-disclosure agreement entered into by such Investor in connection with the purchase of the Common Shares hereunder) with respect to, or any
obligation not to trade in any securities while aware of, any material nonpublic information (X) provided by, or on behalf of, the Company any of its Affiliates or any of their officers, directors (or equivalent persons), employees, attorneys,
agents or representatives in violation of any of the representations, warranties, covenants, provisions or agreements set forth in this Section 9.8 or elsewhere herein or (Y) otherwise possessed (or continued to be possessed) by any
Investor (or any Affiliate, attorney, accountant, agent or representative thereof) as a result of any breach or violation of any representation, warranty, covenant, provision or agreement set forth in this Section 9.8.
9.9 Severability. Any provision of this Agreement that is prohibited or unenforceable in any jurisdiction shall, as to such
jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof but shall be interpreted as if it were written so as to be enforceable to the maximum extent permitted by
applicable law, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. To the extent permitted by applicable law, the parties hereby waive any provision
of law which renders any provision hereof prohibited or unenforceable in any respect.
9.10 Entire Agreement. This Agreement, including the signature pages and Exhibits and
any confidentiality agreement between the Company and each Investor constitute the entire agreement among the parties hereof with respect to the subject matter hereof and thereof and supersede all prior agreements and understandings, both oral and
written, between the parties with respect to the subject matter hereof and thereof.
9.11 Further Assurances. On or prior to the
Closing Date, the parties shall execute and deliver all such further instruments and documents and take all such other actions as may reasonably be required to carry out the transactions contemplated hereby and to evidence the fulfillment of the
agreements herein contained.
9.12 Governing Law. This Agreement shall be governed by, and construed in accordance with, the
internal laws of the State of New York without regard to the choice of law principles thereof (other than sections 5-1401 and 5-1402 of the General Obligations Law) to
the extent that those principles would result in the application of the laws of another jurisdiction. Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the courts of the State of New York located in New York County and
the United States District Court for the Southern District of New York for the purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process in
connection with any such suit, action or proceeding may be served on each party hereto anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. Each of the parties hereto irrevocably consents to the
jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. Each party hereto irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such courts
and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum. EACH OF THE PARTIES HERETO WAIVES ANY RIGHT TO REQUEST A TRIAL BY JURY IN ANY LITIGATION WITH RESPECT TO
THIS AGREEMENT AND REPRESENTS THAT COUNSEL HAS BEEN CONSULTED SPECIFICALLY AS TO THIS WAIVER.
9.13 Independent Nature of
Investors’ Obligations and Rights. The obligations of each Investor under any Transaction Document are several and not joint with the obligations of any other Investor, and no Investor shall be responsible in any way for the performance of
the obligations of any other Investor under any Transaction Document. The decision of each Investor to purchase Shares pursuant to the Transaction Documents has been made by such Investor independently of any other Investor. Nothing contained herein
or in any Transaction Document, and no action taken by any Investor pursuant thereto, shall be deemed to constitute the Investors as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the
Investors are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by the Transaction Documents. Each Investor acknowledges that no other Investor has acted as agent for such Investor in
connection with making its investment hereunder and that no Investor will be acting as agent of such Investor in connection with monitoring its investment in the Shares or enforcing its rights under the Transaction Documents. Each Investor shall be
entitled to independently protect and enforce its rights, including, without limitation, the rights arising out of this Agreement or out of the other Transaction Documents, and it shall not be necessary for any other Investor to be joined as an
additional party in any proceeding for such purpose. The Company acknowledges that each of the Investors has been provided with the same Transaction Documents for the purpose of closing a transaction with multiple Investors and not because it was
required or requested to do so by any Investor. It is expressly understood and agreed that each provision contained in this Agreement is between the Company and an Investor, solely, and not between the Company and the Investors collectively and not
between and among the Investors.
9.14 Exculpation of the Placement Agents. Each party hereto agrees, for the express benefit of
the Placement Agents, their respective affiliates and their respective representatives, that, in connection with the Transaction Documents and the transactions contemplated thereby:
| |
(a) |
Neither of the Placement Agents nor any of their respective affiliates or any of their respective
representatives (i) shall be liable for any improper payment made in accordance with the information provided by the Company; (ii) makes any representation or warranty, or has any responsibilities as to the validity, accuracy, value or
genuineness of any information, certificates or |
| |
documentation delivered by or on behalf of the Company pursuant to this Agreement or the other Transaction Documents or in connection with any of the transactions contemplated by this Agreement
or the other Transaction Documents, including any offering or marketing materials; or (iii) shall be liable (x) for any action taken, suffered or omitted by any of them in good faith and reasonably believed to be authorized or within the
discretion or rights or powers conferred upon them by this Agreement or any other Transaction Document or (y) for anything which any of them may do or refrain from doing in connection with this Agreement or any other Transaction Document,
except for such party’s own gross negligence, willful misconduct or bad faith. |
| |
(b) |
Each of the Placement Agents, their respective affiliates and their respective representatives shall be
entitled to rely on, and shall be protected in acting upon, any certificate, instrument, opinion, notice, letter or any other document or security delivered to any of them by or on behalf of the Company. |
[Remainder of page intentionally left blank]
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized
officers to execute this Agreement as of the date first above written.
|
|
|
| COMPANY: |
|
| FUSION PHARMACEUTICALS INC. |
|
|
| By: |
|
/s/ John Crowley |
| Name: |
|
John Crowley |
| Title: |
|
Chief Financial Officer |
[Signature Page to Securities Purchase Agreement]
|
|
|
| INVESTOR:
[●] |
|
|
| By: |
|
|
|
|
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
|
|
| Address: |
|
|
|
|
|
|
|
|
[Signature Page to
Securities Purchase Agreement]
EXHIBIT B
Registration Rights Agreement
EXHIBIT C
Investor Questionnaire
Exhibit 10.3
REGISTRATION RIGHTS AGREEMENT
This REGISTRATION RIGHTS AGREEMENT (this “Agreement”) is made and entered into as of February 13, 2023 by and among
Fusion Pharmaceuticals Inc., a corporation existing under the Canada Business Corporations Act (the “Company”), and the “Investors” named in that certain Securities Purchase Agreement by and among the Company
and the Investors, dated as of February 13, 2023 (the “Purchase Agreement”). Capitalized terms used herein shall have the respective meanings ascribed thereto in the Purchase Agreement unless otherwise defined herein.
The parties hereby agree as follows:
1. Definitions. As used in this Agreement, the following terms shall have the following meanings:
“Agreement” has the meaning set forth in the first paragraph.
“Allowed Delay” has the meaning set forth in Section 2(c)(ii).
“Company” has the meaning set forth in the first paragraph.
“Constructive Primary Offering” has the meaning set forth in Section 2(e).
“Cut Back Shares” has the meaning set forth in Section 2(e).
“Effectiveness Deadline” means, with respect to the Registration Statement, the earlier of to occur of (i) the sixtieth (60th) calendar day following the Filing Deadline (or, in the event the SEC reviews and has written comments to the Registration Statement, the ninetieth
(90th) calendar day following the Filing Deadline) and (ii) five (5) Business Days after the SEC informs the Company (whether in writing or orally) that no review of the Registration
Statement will be made or that the SEC has no further comments on such Registration Statement; provided, however, that if the Effectiveness Deadline falls on a Saturday, Sunday or other day that the SEC is closed for business, the Effectiveness
Deadline shall be extended to the next Business Day on which the SEC is open for business.
“Effectiveness Period” has the meaning set
forth in Section 3(a).
“Filing Deadline” has the meaning set forth in Section 2(a)(i).
“Inspectors” has the meaning set forth in Section 3(k).
“Investors” means each of Investors identified in the Purchase Agreement and any Affiliate or permitted transferee of such Investor who is a
subsequent holder of Registrable Securities.
“Investor Information” has the meaning set forth in Section 5(a).
“Liquidated Damages” has the meaning set forth in Section 2(d)(ii).
“Losses” has the meaning set forth in Section 5(a).
“Maintenance Failure” has the meaning set forth in Section 2(d)(ii).
“Maintenance Liquidated Damages” has the meaning set forth in Section 2(d)(ii).
“Prospectus” means (i) the prospectus included in any Registration Statement, as amended or supplemented by any prospectus supplement,
with respect to the terms of the offering of any portion of the Registrable Securities covered by such Registration Statement and by all other amendments and supplements to the prospectus, including post-effective amendments and all material
incorporated by reference in such prospectus, and (ii) any “free writing prospectus” as defined in Rule 405 under the 1933 Act.
“Purchase Agreement” has the meaning set forth in the first paragraph.
“Qualification Date” has the meaning set forth in Section 2(a)(ii).
“Qualification Deadline” has the meaning set forth in Section 2(a)(ii).
“Questionnaire” has the meaning set forth in Section 4(a).
“Records” has the meaning set forth in Section 3(k).
“Register,” “registered” and “registration” refer to a registration made by preparing and filing a
Registration Statement or similar document in compliance with the 1933 Act, and the declaration or ordering of effectiveness of such Registration Statement or document.
“Registrable Securities” means (i) the Shares and (ii) any other securities issued or issuable with respect to or in exchange for
Shares, whether by merger, charter amendment or otherwise, including upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the Shares; provided, that a security shall cease to be a Registrable
Security upon (A) sale pursuant to a Registration Statement or Rule 144 under the 1933 Act, or (B) such security becoming eligible for sale without restriction by the Investor holding such security pursuant to Rule 144, including without
any manner of sale or volume limitations, and without the requirement to be in compliance with Rule 144(c)(1) (or any successor thereto) promulgated under the 1933 Act, including any additional registration statement required to be filed pursuant to
Section 2(e) to register Cut Back Shares.
“Registration Liquidated Damages” has the meaning set forth in Section 2(d)(i).
“Registration Statement” means any registration statement of the Company under the 1933 Act that covers the resale of any of the Registrable
Securities pursuant to the provisions of this Agreement, amendments and supplements to such Registration Statement, including post-effective amendments, all exhibits and all material incorporated by reference in such Registration Statement.
“Required Investors” means the Investors holding at least ninety percent (90%) of the Registrable Securities outstanding from time to time.
“Restriction Termination Date” has the meaning set forth in Section 2(e).
“SEC” means the U.S. Securities and Exchange Commission.
“SEC Restrictions” has the meaning set forth in Section 2(e).
“Shelf Registration Statement” has the meaning set forth in Section 2(a)(ii).
2. Registration.
(a) Registration Statements.
(i) By no later than forty-five (45) calendar days after the Closing Date (the “Filing Deadline”), the Company shall prepare
and file with the SEC one (1) Registration Statement covering the resale of all of the Registrable Securities. Subject to any SEC comments, such Registration Statement shall include the plan of distribution substantially in the form
attached hereto as Exhibit A; provided, however, that no Investor shall be named as an “underwriter” in such Registration Statement without the Investor’s prior written consent. Such Registration Statement also shall cover, to the
extent allowable under the 1933 Act and the rules promulgated thereunder (including Rule 416), such indeterminate number of additional Common Shares resulting from stock splits, stock dividends or similar transactions with respect to the Registrable
Securities. Such Registration Statement
2
shall not include any Common Shares or other securities for the account of any other holder. The Company shall not file any other registration statements until the Registration Statement is
declared effective by the SEC, provided that this Section 2(a)(i) shall not prohibit the Company from filing (x) amendments to registration statements filed prior to the date of this Agreement, (y) a shelf registration statement on
Form S-3 for a primary offering by the Company, provided that the Company makes no offering of securities pursuant to such shelf registration statement prior to the effective date of the Registration Statement
required hereunder, or (z) one (1) or more registration statements on Form S-8 for the registration of the securities underlying its equity incentive plans.
(ii) The Registration Statement referred to in Section 2(a)(i) shall be on Form S-3. In the event
that Form S-3 is not available for the registration of the resale of Registrable Securities hereunder, the Company shall (i) register the resale of the Registrable Securities on such other form as is
available to the Company and (ii) so long as Registrable Securities remain outstanding, promptly following the date (the “Qualification Date”) upon which the Company becomes eligible to use a registration statement on Form S-3 to register the Registrable Securities for resale, but in no event more than thirty (30) days after the Qualification Date (the “Qualification Deadline”), file a registration statement on
Form S-3 covering the Registrable Securities (or a post-effective amendment on Form S-3 to a registration statement on Form S-1)
(a “Shelf Registration Statement”) and use commercially reasonable efforts to cause such Shelf Registration Statement to be declared effective as promptly as practicable thereafter; provided that the Company shall maintain the
effectiveness of the Registration Statement then in effect until such time as a Shelf Registration Statement covering the Registrable Securities has been declared effective by the SEC.
(b) Expenses. The Company will pay all expenses associated with each Registration Statement, including filing and printing fees, fees of
the Company’s counsel, fees of the Transfer Agent, accounting fees and expenses, costs associated with clearing the Registrable Securities for sale under applicable state securities laws and listing fees, but excluding discounts, commissions,
fees of underwriters, selling brokers, dealer managers or similar securities industry professionals with respect to the Registrable Securities being sold.
(c) Effectiveness.
(i)
The Company shall use commercially reasonable efforts to have each Registration Statement declared effective as soon as practicable after such Registration Statement has been filed with the SEC, but no later than the Effectiveness Deadline. The
Company shall notify the Investors by e-mail as promptly as practicable, and in any event within twenty-four (24) hours, after any Registration Statement is declared effective and shall simultaneously
provide the Investors with copies of any related Prospectus to be used in connection with the sale or other disposition of the securities covered thereby.
(ii) On not more than two (2) occasions and for not more than thirty (30) consecutive days or for a total of not more than sixty
(60) days, in each case in any twelve (12) month period, the Company may suspend the use of any Prospectus included in any Registration Statement contemplated by this Section 2: (A) if the negotiation or consummation of a transaction
by the Company is pending or an event has occurred, which negotiation, consummation or event, the Board of Directors of the Company reasonably believes, upon the advice of legal counsel, would require additional disclosure by the Company in the
Registration Statement of material information that the Company has a bona fide business purpose for keeping confidential and the non-disclosure of which in the Registration Statement would be expected, in the
reasonable determination of the Board of Directors of the Company upon advice of legal counsel, to cause the Registration Statement to fail to comply with applicable disclosure requirements or (B) in the event the Company determines in good
faith, upon advice of legal counsel, that suspension is necessary to amend or supplement the affected Registration Statement or the related Prospectus so that such Registration Statement or Prospectus shall not include an untrue statement of a
material fact or omit to state a material fact required to be stated therein or necessary to make the statements therein, in the case of the Prospectus in light of the circumstances under which they were made, not misleading (an “Allowed
Delay”); provided, that the Company shall promptly (a) notify each Investor in writing of the commencement of an Allowed Delay, but shall not (without the prior written consent of an Investor) disclose to such Investor any material
nonpublic information giving rise to an Allowed Delay, (b) advise the Investors in writing to cease all sales under such Registration Statement until the end of the Allowed Delay and (c) use commercially reasonable efforts to terminate an
Allowed Delay as promptly as practicable.
3
(d) Effect of Failure to File and Obtain and Maintain Effectiveness of Registration
Statement.
(i) Subject to Section 2(e), if a Registration Statement covering the Registrable Securities is not filed with the
SEC on or prior to the Filing Deadline, the Company will make pro rata payments to each Investor then holding Registrable Securities, as liquidated damages and not as a penalty (the “Registration Liquidated Damages”), in an amount
equal to one percent (1.0%) of the aggregate amount invested by such Investor for the initial day of failure to file such Registration Statement by the Filing Deadline and for each thirty (30) day period (or pro rata portion thereof with
respect to a final period, if any) thereafter during which no such Registration Statement is filed with respect to the Registrable Securities. Such payments shall be made to each Investor then holding Registrable Securities in cash no later than ten
(10) Business Days after the end of the date of the initial failure to file such Registration Statement by the Filing Deadline and each subsequent thirty (30) day period (or portion thereof with respect to a final period, if any)
thereafter until such Registration Statement is filed with respect to the Registrable Securities. Interest shall accrue at the rate of one percent (1.0%) per month on any such liquidated damages payments that shall not be paid by the applicable
payment date until such amount is paid in full.
(ii) Subject to Section 2(e), if (A) a Registration Statement covering the
Registrable Securities is not declared effective by the SEC prior to the earlier of (x) five (5) Business Days after the SEC informs the Company that no review of such Registration Statement will be made or that the SEC has no further comments
on such Registration Statement or (y) the Effectiveness Deadline or (B) after a Registration Statement has been declared effective by the SEC, (1) such Registration Statement ceases for any reason (including, without limitation, by
reason of a stop order, or the Company’s failure to update the Registration Statement) to remain continuously effective as to sell all Registrable Securities for which it is required to be effective, (2) the Investors are not permitted to
utilize the Prospectus therein to resell such Registrable Securities (other than during an Allowed Delay), or (3) an Allowed Delay exceeds the permitted length of the Allowed Delay hereunder, (C) after the Filing Deadline, and only in the
event a Registration Statement is not effective or available to sell all Registrable Securities, the Company fails to file with the SEC any required reports under Section 13 or 15(d) of the Exchange Act such that it is not in compliance with
Rule 144(c)(1), as result of which holders who are not affiliates are unable to sell Registrable Securities without restriction under Rule 144 or (D) the Registration Statement is on Form S-1, for a
period of twenty (20) days following the date on which the Company files a post-effective amendment to incorporate the Company’s Annual Report on Form 10-K (each of (A), (B), (C) and (D), a
“Maintenance Failure”), then in addition to any other rights the Investors may have hereunder or under applicable law, then the Company will make pro rata payments to each Investor then holding Registrable Securities, as liquidated
damages and not as a penalty (the “Maintenance Liquidated Damages,” and together with the Registration Liquidated Damages, the “Liquidated Damages”), in an amount equal to one percent (1.0%) of the aggregate amount
invested by such Investor for the Registrable Securities then held by such Investor for the initial day of a Maintenance Failure and for each thirty (30) day period (or pro rata portion thereof with respect to a final period, if any) thereafter
until the Maintenance Failure is cured. The Maintenance Liquidated Damages shall be paid monthly within ten (10) Business Days of the date of such Maintenance Failure and the end of each subsequent thirty (30) day period (or portion
thereof with respect to a final period, if any) thereafter until the Maintenance Failure is cured. Such payments shall be made in cash to each Investor then holding Registrable Securities. Interest shall accrue at the rate of one percent (1%) per
month on any such liquidated damages payments that shall not be paid by the applicable payment date until such amount is paid in full.
(iii) The parties agree that (1) notwithstanding anything to the contrary herein or in the Purchase Agreement, no Liquidated Damages
shall be payable with respect to any period after the expiration of the Effectiveness Period (as defined below) (it being understood that this sentence shall not relieve the Company of any Liquidated Damages accruing prior to the expiration of the
Effectiveness Period), and in no event shall the aggregate amount of Liquidated Damages payable to an Investor exceed, in the aggregate, five percent (5.0%) of the aggregate purchase price paid by such Investor pursuant to the Purchase Agreement and
(2) in no event shall the Company be liable in any thirty (30) day period for Liquidated Damages under this Agreement in excess of one percent (1.0%) of the aggregate purchase price paid by the Investors pursuant to the Purchase Agreement.
(iv) Notwithstanding the foregoing, the Company and the Investors agree that the Company will not be liable for any liquidated damages
under this Section 2(d) with respect to any Registrable Securities prior to their issuance. The Liquidated Damages described in this Section 2(d) shall constitute the Investors’ exclusive monetary remedy for any failure to meet the
Filing Deadline and for any Maintenance Failure (unless any of the foregoing shall occur as a result of a willful breach by the Company of its obligations hereunder, in which case, the Investors’ monetary remedies shall not be limited to the
Liquidated Damages described herein), but shall not affect the right of the Investors to seek injunctive relief or specific performance of the Company’s obligations hereunder. The Company acknowledges that a breach by it of its obligations
hereunder will cause irreparable harm to the Investors by vitiating the intent and purpose of the transactions contemplated hereby. Accordingly, the Company acknowledges that the remedy at law for breach of its obligations hereunder may be
inadequate and agrees, in the event of a breach or threatened breach by the Company of any of the provisions hereunder, that the Investors shall be entitled, in addition to all other available remedies in law or in equity, to an injunction or
injunctions to prevent or cure breaches of the provisions of this Agreement and to enforce specifically the terms and provisions hereof, without the necessity of showing economic loss and without any bond or other security being required.
4
(v) For purposes of clarification, any failure by the Company to file the Registration
Statement by the Filing Deadline or to effect such Registration Statement by the Effectiveness Deadline shall not otherwise relieve the Company of its obligations to file or effect the Registration Statement as set forth above in this Section 2
and in Section 3.
(e) Rule 415; Cut Back. If at any time the SEC takes the position that the offering of some or all of the
Registrable Securities in a Registration Statement is a primary offering or not eligible to be made on a delayed or continuous basis under the provisions of Rule 415 under the 1933 Act, or requires any Investor to be named as an
“underwriter,” the Company shall use commercially reasonable efforts to advocate before the SEC its reasonable position that the offering contemplated by such Registration Statement is a valid secondary offering and not an offering
“by or on behalf of the issuer” as defined in Rule 415 (a “Constructive Primary Offering”) and that none of the Investors is an “underwriter.” The Investors shall have the right to review and oversee any
registration or matters pursuant to this Section 2(e), including participation in any meetings or discussions with the SEC regarding the SEC’s position and to comment on any written submission made to the SEC with respect thereto. In the
event that, despite the Company’s commercially reasonable efforts, the SEC does not alter its position, the Company shall (i) remove from such Registration Statement such portion of the Registrable Securities (the “Cut Back
Shares”) and/or (ii) agree to such restrictions and limitations on the registration and resale of the Registrable Securities as the SEC may require to assure the Company’s compliance with the requirements of Rule 415
(collectively, the “SEC Restrictions”); provided, however, that the Company shall not agree to name any Investor as an “underwriter” in such Registration Statement without the prior written consent of such Investor. Any
cut back imposed on the Investors pursuant to this Section 2(e) shall be allocated among the Investors on a pro rata basis and shall be applied first to any of the Registrable Securities of such Investor as such Investor shall designate, unless
the SEC Restrictions otherwise require or provide or the Investors otherwise agree. The parties agree that the Company’s delay or failure to have a Registration Statement declared effective due to the SEC taking the position that the offering
is a Constructive Primary Offering shall not be a breach of any provision of this Agreement and no liquidated damages shall accrue as to any Cut Back Shares. From and after such date as the Company is able to effect the registration of such Cut Back
Shares in accordance with any SEC Restrictions applicable to such Cut Back Shares (such date, the “Restriction Termination Date”), all of the provisions of this Section 2 (including the Company’s obligations with respect
to the filing of a Registration Statement and its obligations to use commercially reasonable efforts to have such Registration Statement declared effective within the time periods set forth herein and the liquidated damages provisions relating
thereto) shall again be applicable to such Cut Back Shares; provided, however, (i) that the Filing Deadline and/or the Qualification Deadline, as applicable, for such Registration Statement including such Cut Back Shares shall be ten
(10) Business Days after such Restriction Termination Date, and (ii) the date by which the Company is required to obtain effectiveness with respect to such Cut Back Shares under Section 2(c) shall be the sixtieth (60th) calendar day
immediately after the Restriction Termination Date (or the ninetieth (90th) calendar day if the SEC reviews such Registration Statement); provided, further, that if such date falls on a Saturday, Sunday or other day that the SEC is closed for
business, such date shall be extended to the next business day on which the SEC is open for business.
3. Company
Obligations. The Company will use commercially reasonable efforts to effect the registration of the Registrable Securities in accordance with the terms hereof, and pursuant thereto the Company will, as expeditiously as possible:
(a) use commercially reasonable efforts to cause such Registration Statement to become effective and to remain continuously effective for a
period that will terminate upon the earlier of (i) the date on which all Registrable Securities covered by such Registration Statement, as amended from time to time, have been sold pursuant to a Registration Statement or Rule 144, and
(ii) the date on which all Shares cease to be Registrable Securities (the “Effectiveness Period”);
(b) prepare and
file with the SEC such amendments and post-effective amendments to such Registration Statement and the related Prospectus as may be necessary to keep such Registration Statement effective for the Effectiveness Period and to comply with the
provisions of the 1933 Act and the 1934 Act with respect to the distribution of all of the Registrable Securities covered thereby;
5
(c) provide copies to and permit each Investor to review each Registration Statement and all
amendments and supplements thereto at least two (2) Business Days prior to their filing with the SEC and a reasonable opportunity to furnish comments thereon;
(d) furnish to each Investor whose Registrable Securities are included in any Registration Statement (i) promptly after the same is
prepared and filed with the SEC, if requested by the Investor, one (1) copy of any Registration Statement and any amendment thereto, each preliminary prospectus and Prospectus and each amendment or supplement thereto, and each letter written by
or on behalf of the Company to the SEC or the staff of the SEC, and each item of correspondence from the SEC or the staff of the SEC, in each case relating to such Registration Statement (other than any portion thereof which contains information for
which the Company has sought confidential treatment), and (ii) such number of copies of a Prospectus, including a preliminary prospectus, and all amendments and supplements thereto and such other documents as each Investor may reasonably
request in order to facilitate the disposition of the Registrable Securities owned by such Investor that are covered by such Registration Statement;
(e) use commercially reasonable efforts to (i) prevent the issuance of any stop order or other suspension of effectiveness and,
(ii) if such order is issued, obtain the withdrawal of any such order at the earliest practical moment;
(f) use commercially
reasonable efforts to cause all Registrable Securities covered by a Registration Statement to be listed on each securities exchange, interdealer quotation system or other market on which similar securities issued by the Company are then listed;
(g) promptly notify the Investors, at any time prior to the end of the Effectiveness Period, upon discovery that, or upon the happening of any
event as a result of which, the Prospectus includes an untrue statement of a material fact or omits to state any material fact required to be stated therein or necessary to make the statements therein not misleading in light of the circumstances
then existing (provided that such notice shall not, without the prior written consent of an Investor, disclose to such Investor any material nonpublic information regarding the Company), and promptly prepare, file with the SEC and furnish to such
holder a supplement to or an amendment of such Prospectus as may be necessary so that such Prospectus shall not include an untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary to make the
statements therein not misleading in light of the circumstances then existing;
(h) otherwise use commercially reasonable efforts to
(i) comply with all applicable rules and regulations of the SEC under the 1933 Act and the 1934 Act, including, without limitation, Rule 172 under the 1933 Act; (ii) file any final Prospectus, including any supplement or amendment thereof,
with the SEC pursuant to Rule 424 under the 1933 Act; (iii) promptly inform the Investors in writing if, at any time during the Effectiveness Period, the Company does not satisfy the conditions specified in Rule 172 under the 1933 Act and, as a
result thereof, the Investors are required to deliver a Prospectus in connection with any disposition of Registrable Securities and take such other actions as may be reasonably necessary to facilitate the registration of the Registrable Securities
hereunder; and (iv) make available to its security holders, as soon as reasonably practicable, an earnings statement satisfying the provisions of Section 11(a) of the 1933 Act;
(i) if requested by an Investor, the Company shall (i) as soon as practicable, incorporate in a prospectus supplement or post-effective
amendment such information as an Investor reasonably requests to be included therein relating to the sale and distribution of Registrable Securities, including, without limitation, information with respect to the number of Registrable Securities
being offered or sold, the purchase price being paid therefor and any other terms of the offering of the Registrable Securities to be sold in such offering; (ii) as soon as practicable, make all required filings of such prospectus supplement or
post-effective amendment after being notified of the matters to be incorporated in such prospectus supplement or post-effective amendment; and (iii) as soon as practicable, supplement or make amendments to any Registration Statement if
reasonably requested by an Investor holding any Registrable Securities;
(j) within two (2) Business Days after a Registration
Statement which covers Registrable Securities is ordered effective by the SEC, the Company shall deliver to the Transfer Agent (with copies to the Investors whose Registrable Securities are included in such Registration Statement) confirmation that
such Registration Statement has been declared effective by the SEC;
6
(k) make available upon reasonable prior notice during normal business hours and for
reasonable periods for inspection by the Investors and by any attorney, accountant or other agent retained by the Investors and who is reasonably acceptable to the Company (collectively, the “Inspectors”), all pertinent financial
and other records and pertinent corporate documents and properties of the Company (collectively, the “Records”) as may be reasonably necessary for the purpose of such review, and cause the Company’s officers, directors and
employees and the independent public accountants who have certified its financial statements to make themselves available to discuss the business of the Company and to supply all information reasonably requested by the Inspectors for the sole
purpose of conducting initial and ongoing due diligence with respect to the Company and the accuracy of the Registration Statement; provided, however, that each Investor shall agree to, and to direct its Inspectors to, hold in strict confidence and
shall not make any disclosure or use of any Record or other information which the Company determines in good faith to be confidential, and of which determination the Inspectors are so notified, unless (a) the disclosure of such Records is
necessary to avoid or correct a misstatement or omission in any Registration Statement or is otherwise required under the 1933 Act, (b) the release of such Records is ordered pursuant to a final,
non-appealable subpoena or order from a court or government body of competent jurisdiction, or (c) the information in such Records has been made generally available to the public other than by disclosure
in violation of this Section 3(k). Notwithstanding the foregoing, the Company shall not disclose material nonpublic information to the Investors, or to advisors to or representatives of the Investors, unless prior to disclosure of such
information the Company identifies such information as being material nonpublic information and provides the Investors, such advisors and such representatives with the opportunity to accept or refuse to accept such material nonpublic information for
review and any Investor wishing to obtain such information enters into an appropriate confidentiality agreement with the Company with respect thereto; and
(l) with a view to making available to the Investors the benefits of Rule 144 (or its successor rule) and any other rule or regulation of the
SEC that may at any time permit the Investors to sell shares of Common Stock to the public without registration: (i) make and keep adequate current public information available, as those terms are understood and defined in Rule 144, until the
earlier of (A) six (6) months after such date as all of the Registrable Securities may be sold without restriction by the holders thereof pursuant to Rule 144 or any other rule of similar effect (without restriction, limitation or current
information requirement) or (B) such date as all of the Registrable Securities shall have been resold; (ii) file with the SEC in a timely (without giving effect to any extensions that may be available pursuant to Rule 12b-25 under the 1934 Act or any similar provision) manner all reports and other documents required of the Company under the 1934 Act; and (iii) furnish to each Investor upon request, as long as such Investor
owns any Registrable Securities, (A) a written statement by the Company that it has complied with the reporting requirements of the 1934 Act, and (B) such other information as may be reasonably requested in order to avail such Investor of
any rule or regulation of the SEC that permits the selling of any such Registrable Securities without registration.
4. Obligations
of the Investors.
(a) Notwithstanding any other provision of the Agreement, no Investor may include any of its Registrable
Securities in the Registration Statement pursuant to this Agreement unless such Investor furnishes to the Company a completed questionnaire substantially in the form of Exhibit B or such other form as agreed upon between the Company and the
Investor (the “Questionnaire”) for use in connection with the Registration Statement at or prior to Closing if such Investor elects to have any of the Registrable Securities included in such Registration Statement. In addition to
the Questionnaire, each Investor shall furnish such other information as shall be reasonably required to effect the registration of such Registrable Securities, and shall execute such documents in connection with such registration as the Company may
reasonably request.
(b) Each Investor, by its acceptance of the Registrable Securities, agrees to cooperate with the Company as reasonably
requested by the Company in connection with the preparation and filing of a Registration Statement hereunder, unless such Investor has notified the Company in writing of its election to exclude all of its Registrable Securities from such
Registration Statement.
7
(c) Each Investor agrees that, upon receipt of any notice from the Company of either
(i) the commencement of an Allowed Delay pursuant to Section 2(c)(ii) or (ii) the happening of an event pursuant to Section 3(g) hereof, such Investor will immediately discontinue disposition of Registrable Securities pursuant to
any Registration Statement covering such Registrable Securities, until the Investor is advised by the Company that such dispositions may again be made and/or the use of the applicable Prospectus (as it may have been supplemented or amended) may be
resumed.
(d) Each Investor covenants and agrees that it will comply with the prospectus delivery requirements of the 1933 Act as
applicable to it or an exemption therefrom in connection with sales of Registrable Securities pursuant to any Registration Statement.
(e)
Each Investor resident in Canada or otherwise subject to Canadian Securities Laws acknowledges that no prospectus will be filed with Canadian Securities Commissions in order to qualify the distribution of Shares in any province or territory of
Canada, and applicable resale restrictions under Canadian Securities Laws will continue to apply to any sale of Shares by such Investor.
5. Indemnification.
(a) Indemnification by the Company. The Company will, notwithstanding any termination of this Agreement, indemnify and hold harmless
each Investor and its officers, directors, members, investment advisors, employees and agents, and each other person, if any, who controls such Investor within the meaning of the 1933 Act, against any losses, claims, damages, expenses (including
reasonable and documented attorneys’ fees) or liabilities (collectively, “Losses”), joint or several, to which they may become subject under the 1933 Act or otherwise, insofar as such Losses (or actions in respect thereof)
arise out of or are based upon (i) any untrue or alleged untrue statement of any material fact contained in any Registration Statement, any preliminary Prospectus or final Prospectus, or any amendment or supplement thereof or (ii) any
omission or alleged omission of a material fact required to be stated therein or necessary to make the statements in any preliminary Prospectus or final Prospectus, or any amendment or supplement thereof, in light of the circumstances under which
they were made not misleading, except to the extent that any such Losses arise out of or are based upon (i) an untrue statement or alleged untrue statement or omission or alleged omission so made in conformity with any information relating to
such Investor furnished in writing by such Investor to the Company specifically for inclusion in such Registration Statement or Prospectus or amendment or supplement thereto (“Investor Information”), (ii) the use by an Investor of
an outdated or defective Prospectus after the Company has notified such Investor in writing that such Prospectus is outdated or defective; or (iii) an Investor’s failure to send or give a copy of the Prospectus or supplement (as then
amended or supplemented), if required (and not exempted) to the Persons asserting an untrue statement or omission or alleged untrue statement or omission at or prior to the written confirmation of the sale of Registrable Securities.
(b) Indemnification by the Investors. Each Investor agrees, severally but not jointly, to indemnify and hold harmless, to the fullest
extent permitted by law and notwithstanding any termination of this Agreement, the Company, its directors, officers who sign the Registration Statement, employees and each other person who controls the Company (within the meaning of the 1933 Act)
against any Losses resulting from any untrue statement of a material fact or any omission of a material fact required to be stated in any Registration Statement or Prospectus or preliminary Prospectus or amendment or supplement thereto or necessary
to make the statements therein not misleading, to the extent, but only to the extent that such untrue statement or omission is contained in the Investor Information. In no event shall the liability of an Investor be greater in amount than the dollar
amount of the proceeds (net of all expenses paid by such Investor in connection with any claim relating to this Section 5 and the amount of any damages such Investor has otherwise been required to pay by reason of such untrue statement or
omission) received by such Investor upon the sale of the Registrable Securities included in such Registration Statement giving rise to such indemnification obligation.
(a) Conduct of Indemnification Proceedings. Any person entitled to indemnification hereunder shall (i) give prompt written notice
to the indemnifying party of any claim with respect to which it seeks indemnification and (ii) permit such indemnifying party to assume the defense of such claim with counsel reasonably satisfactory to the indemnified party; provided that any
person entitled to indemnification hereunder shall have the right to employ separate counsel and to participate in the defense of such claim, but the fees and expenses of such counsel shall be at the expense of such person unless (a) the
indemnifying party has agreed in writing to pay
8
such fees or expenses, (b) the indemnifying party shall have failed to assume the defense of such claim and employ counsel reasonably satisfactory to such person or (c) in the
reasonable judgment of any such person, based upon written advice of its counsel, a conflict of interest exists between such person and the indemnifying party with respect to such claims (in which case, if the person notifies the indemnifying party
in writing that such person elects to employ separate counsel at the expense of the indemnifying party, the indemnifying party shall not have the right to assume the defense of such claim on behalf of such person); and provided, further, that the
failure of any indemnified party to give written notice as provided herein shall not relieve the indemnifying party of its obligations hereunder, except to the extent that such failure to give notice shall materially adversely affect the
indemnifying party in the defense of any such claim or litigation. It is understood that the indemnifying party shall not, in connection with any proceeding in the same jurisdiction, be liable for fees or expenses of more than one separate firm of
attorneys at any time for all such indemnified parties. No indemnifying party will, except with the consent of the indemnified party, consent to entry of any judgment or enter into any settlement that does not include as an unconditional term
thereof the giving by the claimant or plaintiff to such indemnified party of a release from all liability in respect of such claim or litigation or that includes any admission as to fault, culpability or failure to act on the part of such
indemnified party.
(b) Contribution. If for any reason the indemnification provided for in the preceding paragraphs (a) and
(b) is unavailable to an indemnified party or insufficient to hold it harmless, other than as expressly specified therein, then the indemnifying party shall contribute to the amount paid or payable by the indemnified party as a result of such loss,
claim, damage or liability in such proportion as is appropriate to reflect the relative fault of the indemnified party and the indemnifying party, as well as any other relevant equitable considerations. No person guilty of fraudulent
misrepresentation within the meaning of Section 11(f) of the 1933 Act shall be entitled to contribution from any person not guilty of such fraudulent misrepresentation. In no event shall the contribution obligation of an Investor be greater in
amount than the dollar amount of the proceeds (net of all expenses paid by such holder in connection with any claim relating to this Section 5 and the amount of any damages such holder has otherwise been required to pay by reason of such untrue
or alleged untrue statement or omission or alleged omission) received by it upon the sale of the Registrable Securities giving rise to such contribution obligation.
6. Miscellaneous.
(a) Amendments and Waivers. This Agreement may be amended only by a writing signed by the Company and the Required Investors. The
Company may take any action herein prohibited, or omit to perform any act herein required to be performed by it, only if the Company shall have obtained the written consent to such amendment, action or omission to act of the Required Investors.
(b) Notices. All notices and other communications provided for or permitted hereunder shall be made as set forth in Section 9.4 of
the Purchase Agreement.
(c) Assignments and Transfers by Investors. The provisions of this Agreement shall be binding upon and
inure to the benefit of the Investors and their respective successors and assigns. An Investor or its respective successor or assign may transfer or assign, in whole or from time to time in part, to one or more persons its rights hereunder in
connection with the transfer of Registrable Securities by such holder to such person, provided that the transferring holder complies with all laws applicable thereto, and the provisions of the Purchase Agreement, and provides written notice of
assignment to the Company promptly after such assignment is effected, with all information reasonably requested by the Company, and such person agrees in writing with the Company to be bound by all of the provisions contained herein and all
applicable federal and state laws; provided further, that unless the transferee or assignee is an Affiliate of, and after such transfer or assignment continues to be an Affiliate of, such holder, the amount of Registrable Securities transferred or
assigned to such transferee or assignee represents at least $5.0 million of Registrable Securities (based on the then-current market price of the Common Shares).
(d) Assignments and Transfers by the Company. This Agreement may not be assigned by the Company (whether by operation of law or
otherwise) without the prior written consent of the Required Investors; provided, however, that in the event that the Company is a party to a merger, consolidation, share exchange or similar business combination transaction in which the Common
Shares are converted into the equity securities of another Person, from and after the effective time of such transaction, such Person shall, by virtue of such transaction, be deemed to have assumed the obligations of the Company hereunder, the term
“Company” shall be deemed to refer to such Person and the term “Registrable Securities” shall be deemed to include the securities received by the Investors in connection with such transaction unless such securities are otherwise
freely tradable by the Investors after giving effect to such transaction.
9
(e) Benefits of the Agreement. The terms and conditions of this Agreement shall inure
to the benefit of and be binding upon the respective permitted successors and assigns of the parties. Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective successors
and assigns any rights, remedies, obligations, or liabilities under or by reason of this Agreement, except as expressly provided in this Agreement.
(f) Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of
which together shall constitute one and the same instrument. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signatures complying with the U.S. federal ESIGN Act of 2000, e.g., www.docusign.com) or other
transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
(g) Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in
construing or interpreting this Agreement.
(h) Severability. Any provision of this Agreement that is prohibited or unenforceable in
any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such prohibition or unenforceability without invalidating the remaining provisions hereof but shall be interpreted as if it were written so as to be enforceable to the
maximum extent permitted by applicable law, and any such prohibition or unenforceability in any jurisdiction shall not invalidate or render unenforceable such provision in any other jurisdiction. To the extent permitted by applicable law, the
parties hereby waive any provision of law which renders any provisions hereof prohibited or unenforceable in any respect.
(i) Further
Assurances. The parties shall execute and deliver all such further instruments and documents and take all such other actions as may reasonably be required to carry out the transactions contemplated hereby and to evidence the fulfillment of the
agreements herein contained.
(j) Entire Agreement. This Agreement is intended by the parties as a final expression of their
agreement and intended to be a complete and exclusive statement of the agreement and understanding of the parties hereto in respect of the subject matter contained herein. This Agreement supersedes all prior agreements and understandings between the
parties with respect to such subject matter.
(k) Governing Law. This Agreement shall be governed by, and construed in accordance
with, the internal laws of the State of New York without regard to the choice of law principles thereof (other than sections 5-1401 and 5-1402 of the General Obligations
Law) to the extent that those principles would result in the application of the laws of another jurisdiction. Each of the parties hereto irrevocably submits to the exclusive jurisdiction of the courts of the State of New York located in New York
County and the United States District Court for the Southern District of New York for the purpose of any suit, action, proceeding or judgment relating to or arising out of this Agreement and the transactions contemplated hereby. Service of process
in connection with any such suit, action or proceeding may be served on each party hereto anywhere in the world by the same methods as are specified for the giving of notices under this Agreement. Each of the parties hereto irrevocably consents to
the jurisdiction of any such court in any such suit, action or proceeding and to the laying of venue in such court. Each party hereto irrevocably waives any objection to the laying of venue of any such suit, action or proceeding brought in such
courts and irrevocably waives any claim that any such suit, action or proceeding brought in any such court has been brought in an inconvenient forum. EACH OF THE PARTIES HERETO WAIVES ANY RIGHT TO REQUEST A TRIAL BY JURY IN ANY LITIGATION WITH
RESPECT TO THIS AGREEMENT AND REPRESENTS THAT COUNSEL HAS BEEN CONSULTED SPECIFICALLY AS TO THIS WAIVER.
(l) Cumulative Remedies.
The remedies provided herein are cumulative and not exclusive of any remedies provided by law.
[Remainder of page intentionally left
blank]
10
IN WITNESS WHEREOF, the parties have executed this Agreement or caused their duly authorized
officers to execute this Agreement as of the date first above written.
|
|
|
| COMPANY: |
|
| FUSION PHARMACEUTICALS INC. |
|
|
| By: |
|
/s/ John Crowley |
| Name: |
|
John Crowley |
| Title: |
|
Chief Financial Officer |
[Signature Page to Registration Rights Agreement]
|
|
|
| INVESTOR:
[●] |
|
|
| By: |
|
|
|
|
| By: |
|
|
| Name: |
|
|
| Title: |
|
|
[Signature Page to
Registration Rights Agreement]
EXHIBIT A
Plan of Distribution
The selling
stockholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling common shares or interests in common shares received
after the date of this prospectus from a selling stockholder as a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their common shares or interests in common
shares on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing
market price, at varying prices determined at the time of sale, or at negotiated prices.
The selling stockholders may use any one or more of the
following methods when disposing of shares or interests therein:
| |
• |
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
| |
• |
block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a
portion of the block as principal to facilitate the transaction; |
| |
• |
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
| |
• |
an exchange distribution in accordance with the rules of the applicable exchange; |
| |
• |
privately negotiated transactions; |
| |
• |
short sales effected after the date the registration statement of which this prospectus is a part is declared
effective by the SEC; |
| |
• |
through the writing or settlement of options or other hedging transactions, whether through an options exchange
or otherwise; |
| |
• |
broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a
stipulated price per share; |
| |
• |
a combination of any such methods of sale; and |
| |
• |
any other method permitted by applicable law. |
The selling stockholders may, from time to time, pledge or grant a security interest in some or all of the common shares owned by them and, if they default in
the performance of their secured obligations, the pledgees or secured parties may offer and sell the common shares, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision
of the Securities Act of 1933, as amended (the “Securities Act”), amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus. The
selling stockholders also may transfer the common shares in other circumstances, in which case the transferees, pledgees, donees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
In connection with the sale of our common shares or interests therein, the selling stockholders may enter into hedging transactions with broker-dealers or
other financial institutions, which may in turn engage in short sales of the common shares in the course of hedging the positions they assume. The selling stockholders may also sell our common shares short and deliver these securities to close out
their short positions, or loan or pledge the common shares to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or the
creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to
this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to the selling stockholders from the sale of the common
shares offered by them will be the purchase price of the common shares less discounts or commissions, if any. Each of the selling stockholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in
part, any proposed purchase of common shares to be made directly or through agents. We will not receive any of the proceeds from this offering.
The selling stockholders also may resell all or a portion of the shares in open market transactions in
reliance upon Rule 144 under the Securities Act of 1933, provided that they meet the criteria and conform to the requirements of that rule.
The selling
stockholders and any underwriters, broker-dealers or agents that participate in the sale of the common shares or interests therein may be “underwriters” within the meaning of Section 2(11) of the Securities Act. Any discounts,
commissions, concessions or profit they earn on any resale of the shares may be underwriting discounts and commissions under the Securities Act. Selling stockholders who are “underwriters” within the meaning of Section 2(11) of the
Securities Act will be subject to the prospectus delivery requirements of the Securities Act.
To the extent required, our common shares to be sold, the
names of the selling stockholders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, and any applicable commissions or discounts with respect to a particular offer will be set forth in an
accompanying prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
In order
to comply with the securities laws of some states, if applicable, the common shares may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the common shares may not be sold unless they
have been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
We have
advised the selling stockholders that the anti-manipulation rules of Regulation M under the Securities Exchange Act of 1934, as amended, may apply to sales of shares in the market and to the activities of the selling stockholders and their
affiliates. In addition, to the extent applicable, we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling stockholders for the purpose of satisfying the prospectus delivery
requirements of the Securities Act. The selling stockholders may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
We have agreed to indemnify the selling stockholders against liabilities, including liabilities under the Securities Act and state securities laws, relating
to the registration of the shares offered by this prospectus.
We have agreed with the selling stockholders to use commercially reasonable efforts to
cause the registration statement of which this prospectus constitutes a part effective and to remain continuously effective until the earlier of (1) such time as all of the shares covered by this prospectus have been disposed of pursuant to and
in accordance with such registration statement or (2) the date on which all of the shares may be sold without restriction pursuant to Rule 144 of the Securities Act.
EXHIBIT B
Form of Selling Securityholder Questionnaire
Exhibit 99.1

Fusion Pharmaceuticals to Acquire Phase 2 Program for
225Ac-PSMA I&T, a Radiopharmaceutical
Targeting Metastatic Castrate Resistant Prostate Cancer
Acquisition of Phase 2 program with established clinical proof of concept
strengthens pipeline of innovative targeted alpha therapies
In connection with the transaction, Fusion announces $60.0 million
private placement financing
Fusion to host conference call at 4:45 ET
Hamilton, ON & Boston, MA, February 13, 2023 – Fusion Pharmaceuticals Inc. (Nasdaq: FUSN) (“Fusion”), a
clinical-stage oncology company focused on developing next-generation targeted alpha therapies (“TATs”) as precision medicines, today announced the acquisition from RadioMedix, Inc. (“RadioMedix”) of the investigational new drug
application (“IND”) for an ongoing Phase 2 clinical trial (the “TATCIST” trial) evaluating 225Ac-PSMA I&T, a small molecule
targeting prostate specific membrane antigen (“PSMA”) expressed on prostate cancers. Following the closing, the alpha-emitting radiopharmaceutical being evaluated in the TATCIST trial will be known
as FPI-2265.
“We are pleased to announce this acquisition, which adds an ongoing Phase 2 program for a
validated cancer target to our pipeline of innovative TATs,” commented Fusion Chief Executive Officer John Valliant, Ph.D. “From our inception, Fusion has recognized the potential opportunity for actinium-based therapies to address unmet
needs in cancer given the power and potency of alpha radiation. We believe that with Fusion’s TAT development expertise, and early investments that provide us with our actinium supply advantage, we are uniquely positioned to be first-to-market with an actinium-based PSMA agent.”
“A
growing body of clinical data demonstrates the power of targeted alpha therapies in prostate cancer, including for patients who progress on or after lutetium-based PSMA therapies,” said
Oliver Sartor, M.D, Laborde Professor for Cancer Research and Medical Director at Tulane Cancer Center. “With more than 250 patients treated with actinium-based radiopharmaceuticals targeting PSMA in investigator
sponsored studies, this class of therapy has both the efficacy data and safety profile that supports continued development. I believe 225Ac-PSMA I&T will have the potential to target a growing patient population with significant unmet need. In addition, it has the potential to move into earlier lines of therapy as
monotherapy as well as in combination with other agents.”
The TATCIST trial is designed to evaluate patients with metastatic castration-resistant prostate
cancer (“mCRPC”) with progressive disease, including patients who are naïve to PSMA targeted radiopharmaceuticals and those who have been pre-treated with 177Lu-based PSMA radiopharmaceuticals such as PLUVICTO™. The trial is expected to evaluate
approximately 100 patients with four treatment cycles per patient occurring every eight weeks. Patients are initially dosed at 100 kBq/kg with dose de-escalation possible based on biochemical response.
Efficacy will be assessed using change in PSA levels and radiographic response.
Fusion plans to expand the Phase 2 program to additional sites and
expects to report data on 20 to 30 patients in the first quarter of 2024.
“Having treated mCRPC patients for many years, I initiated the TATCIST
trial to address the unmet needs for the many patients who are not adequately addressed with currently available therapies,” said Ebrahim Delpassand, M.D., Chairman and CEO of RadioMedix and Medical Director of Excel Diagnostics &
Nuclear Oncology Center. “Given Fusion’s radiopharmaceutical development capabilities, leadership in Actinium supply and established infrastructure, we look forward to this preeminent partnership to advance
FPI-2265 through the Phase 2 program for the benefit of our patients.”
Dr. Valliant continued,
“With one Phase 2 program, three ongoing Phase 1 programs, and an IND submission through our collaboration with AstraZeneca expected in the first quarter of 2023, Fusion continues to extend its leadership in targeted alpha therapy development.
Following the encouraging data we reported from the cold antibody sub study of the FPI-1434 trial in June, we continue to dose escalate and we look forward to reporting the preliminary Phase 1 data in the
second quarter of this year. The FPI-1434 data will be the first in what we expect will be multiple clinical updates generated from our pipeline over the next 24 months.”
Private Placement Financing
In connection with the
closing of the acquisition of the TATCIST trial and related assets, Fusion has agreed to sell an aggregate of [•] common shares to certain accredited institutional investors in a private placement in public equity financing (the
“Offering”). The Offering is expected to result in gross proceeds to Fusion of approximately $[•] million, before deducting placement agent fees and other offering expenses payable by Fusion.
Pursuant to the terms of the securities purchase agreement, at the closing of the Offering, Fusion will
issue approximately 17.6 million of its common shares at a price of $3.40 per share, equal to the closing price of Fusion’s common shares, as reported by Nasdaq on February 10, 2023. The closing of the Offering is subject to customary
closing conditions and is expected to occur on or about February 16, 2023.
Morgan Stanley and Jefferies served as
co-placement agents for the Offering. New and existing investors in the Offering include Avidity Partners, Federated Hermes Kaufmann Funds, a fund affiliated with Deerfield Management Company, L.P., Invus,
Perceptive Advisors and Woodline Master Fund LP.
Upon the closing of the Offering, Fusion anticipates having $248.0 million in cash and cash equivalents,
which it believes will be sufficient to fund its planned operating expenses and capital expenditure requirements into the first quarter of 2025.
The
offer and sale of the foregoing shares are being made in a transaction not involving a public offering and have not been registered under the Securities Act of 1933, as amended (the “Securities Act”). The shares being issued in the private
placement may not be offered or sold in the United States or Canada absent registration or pursuant to an exemption from the registration requirements of the Securities Act and applicable state securities laws or pursuant to an exemption from the
prospectus requirements of Canadian securities laws, as applicable. Fusion has agreed to file a registration statement with the Securities and Exchange Commission covering the resale of the shares acquired by the investors in the private placement.
This press release does not constitute an offer to sell or the solicitation of an offer to buy the securities, nor shall there be any sale of the
securities in any state in which such offer or sale would be unlawful prior to the registration or qualification under the securities laws of such state. Any offering of the shares under the resale registration statement will only be by means of a
prospectus.
Fusion Conference Call Information
Fusion will host a live conference call and webcast today beginning at 4:45 p.m. ET to discuss the acquisition. To access the live call, please dial
1-877-870-4263 (U.S.), 1-855-669-9657 (Canada) or 1-412-317-0790 and reference confirmation number 10175558. A webcast of the conference call will be available under “Events and Presentations” in the Investors & Media section of
Fusion’s website at https://ir.fusionpharma.com/overview. The archived webcast will be available on Fusion’s website shortly after the conclusion and will be available for 90 days following the event.
About RadioMedix
RadioMedix, Inc. is a clinical-stage biotechnology company, focused on innovative radiopharmaceuticals for diagnosis, monitoring, and Targeted Alpha Therapy
(“TAT”) of cancer. The company has also established facilities including a drug discovery center for the early probe development, a pre-clinical core facility for in vitro and in vivo evaluation of
radiopharmaceuticals, and 27,500 SQF cGMP manufacturing and analytical suite for Phase I-III clinical trials, and the large-scale post-approval commercial manufacturing, also known as the Spica Center. To
learn more, visit www.radiomedix.com
About Fusion
Fusion Pharmaceuticals is a clinical-stage oncology company focused on developing next-generation radiopharmaceuticals as precision medicines. Fusion connects
alpha particle emitting isotopes to various targeting molecules to selectively deliver the alpha emitting payloads to tumors. Fusion’s first program, FPI-1434 targeting insulin-like growth factor 1
receptor, is currently in a Phase 1 clinical trial. The pipeline includes FPI-1966, targeting the fibroblast growth factor receptor 3, currently in a Phase 1 study; and
FPI-2059, a small molecule targeting neurotensin receptor 1, which has received FDA IND clearance and will begin a Phase 1 study. In addition to a robust proprietary pipeline, Fusion has a collaboration with
AstraZeneca to jointly develop novel targeted alpha therapies and combination programs between Fusion’s TATs and AstraZeneca’s DNA Damage Response Inhibitors and immuno-oncology agents. Fusion has also entered into a collaboration with
Merck to evaluate FPI-1434 in combination with Merck’s KEYTRUDA® (pembrolizumab) in patients with solid tumors expressing IGF-1R. To support Fusion’s growing pipeline of TATs, the company has signed strategic agreements for actinium supply with TRIUMF, Niowave, Inc. and BWXT Medical Ltd.
Forward-Looking Statements
This press release contains
“forward-looking statements” for purposes of the safe harbor provisions of The Private Securities Litigation Reform Act of 1995, including but not limited to the statements regarding Fusion’s future business and financial performance.
For this purpose, any statements contained herein that are not statements of historical fact may be deemed forward-looking statements. Without limiting the foregoing, the words “expect,” “plans,” “anticipates,”
“intends,” “will,” and similar expressions are also intended to identify forward-looking statements, as are expressed or implied statements with respect to Fusion’s potential drug candidates, including any expressed or
implied statements regarding the successful development of its product candidates. Actual results may differ materially from those indicated by such forward-looking statements as a result of risks and uncertainties, including but not limited to the
following: the ability to close the Offering; the timing and advancement of current and planned clinical trials, Fusion’s ability to replicate results achieved in its preclinical studies or clinical trials, or that of RadioMedix in any future
studies or trials; Fusion’s ability to maintain its intellectual property portfolio; and the timing and success of our development and commercialization of its product candidates; risks relating to the
regulatory process; unexpected patient recruitment delays or regulatory actions or delays; Fusion’s ability to obtain additional funding required to conduct its research, development and
commercialization activities; changes in Fusion’s business plan or objectives; and Fusion’s ability to obtain, maintain and enforce patent and other intellectual property protection for its product candidates and its discoveries. Such
forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause actual results to be materially different from any future results, performance or achievements expressed or implied by such statements. These
and other risks which may impact management’s expectations are described in greater detail under the heading “Risk Factors” in Fusion’s quarterly report on Form 10-Q for the quarter ended
September 30, 2022, as filed with the U.S. Securities and Exchange Commission (the “SEC”) and in any subsequent periodic or current report that Fusion files with the SEC. All forward-looking statements reflect Fusion’s estimates
only as of the date of this release (unless another date is indicated) and should not be relied upon as reflecting Fusion’s views, expectations or beliefs at any date subsequent to the date of this release. While Fusion may elect to update
these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so, even if Fusion’s estimates change.
Investors and others should note that Fusion communicates with its investors and the public using the Fusion website, www.fusionpharma.com, including, but not
limited to, company disclosures, investor presentations, SEC filings, and press releases. The information that Fusion posts on this website could be deemed to be material information. As a result, Fusion encourages investors, media and others
interested to review the information that Fusion posts there on a regular basis.
Contact:
Amanda Cray
Senior Director of Investor Relations &
Corporate Communications
(617) 967-0207
cray@fusionpharma.com

Fusion Pharmaceuticals to Acquire Phase
2 Program for 225Ac-PSMA I&T Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved February 13, 2023 Exhibit 99.2

Forward Looking Statements This
presentation contains forward-looking statements of Fusion Pharmaceuticals, Inc. (“we,” “us,” “our,” “Fusion” or the “Company”) within the meaning of the Private Securities Litigation
Reform Act of 1995. Forward looking statements include all statements that are not historical facts, and in some cases, can be identified by terms such as “may,” “might,” “will,” “could,”
“would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,”
“potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward-looking statements contained in this presentation
include, but are not limited to, statements regarding our future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters; our current and prospective product candidates, the
timing and advancement of current and planned clinical trials, our ability to replicate results achieved in our preclinical studies or clinical trials, or that of RadioMedix, Inc. in any future studies or trials; research and development costs; the
competitive landscape and market for our product candidates; our ability to maintain our intellectual property portfolio; the success of our planned inter partes review (“IPR”) filing; and the timing and success of our development and
commercialization of our product candidates, including our ability to establish and maintain collaborations or strategic relationships. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and
uncertainties. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if
new information becomes available in the future. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements,
see the section entitled “Risk Factors” in our most recent annual report on Form 10-K filed with the Securities and Exchange Commission (the “SEC”), as well as discussions of potential risks, uncertainties, and other
important factors in our other subsequent filings with the SEC. In addition, we have not conducted any head-to-head studies comparing our product candidates to any third-party drug products or candidates, whether investigated or approved.
Information regarding other drug products in this presentation is meant to provide context for illustrative purposes only. Because there are no head-to-head studies, no conclusions should be made based on cross-study comparisons. Recipients are
cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made and should not be construed as statements of fact. This presentation includes statistical and other industry and
market data that we obtained from industry publications and research, surveys and studies conducted by third parties as well as our own estimates of potential market opportunities. All of the market data used in this prospectus involves a number of
assumptions and limitations, and you are cautioned not to give undue weight to such data. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be
reliable, although they do not guarantee the accuracy or completeness of such information. Our estimates of the potential market opportunities for our product candidates include several key assumptions based on our industry knowledge, industry
publications, third-party research and other surveys, which may be based on a small sample size and may fail to accurately reflect market opportunities. While we believe that our internal assumptions are reasonable, and management is responsible for
the accuracy of such assumptions and data, no independent source has verified such assumptions. Copyright © 2020 Fusion Pharmaceuticals Inc. All Rights Reserved
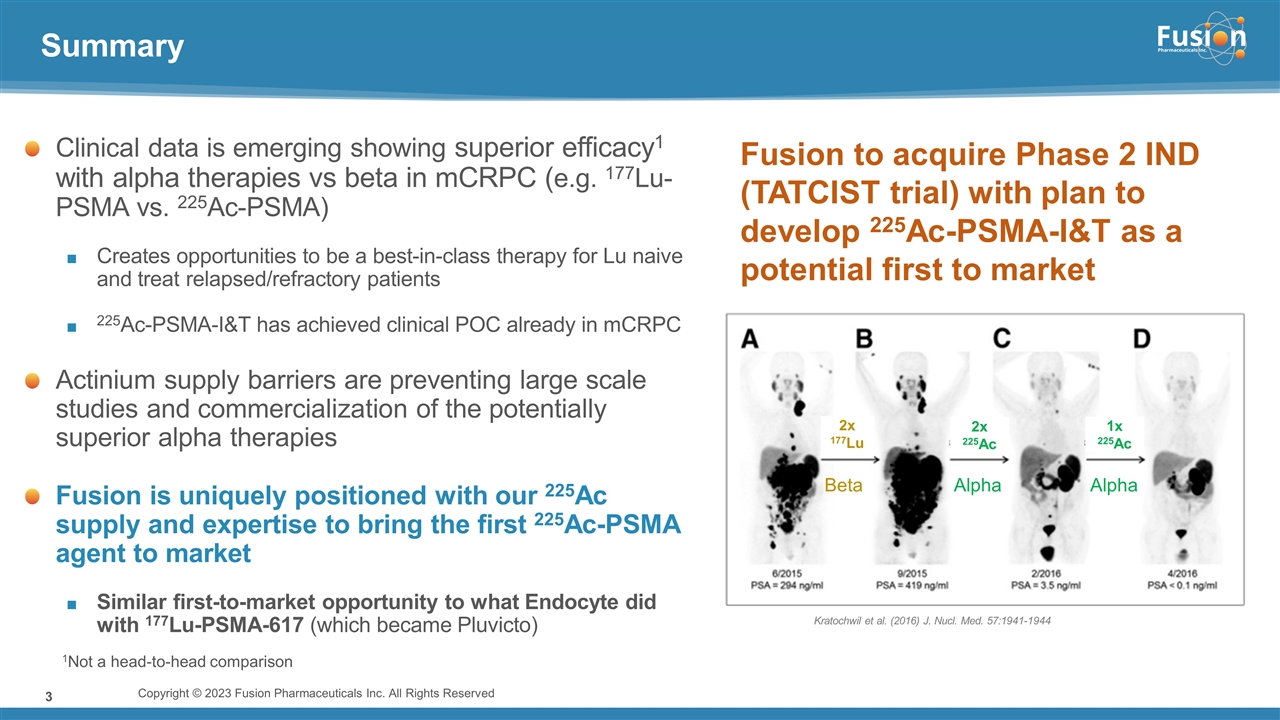
Summary Copyright © 2023 Fusion
Pharmaceuticals Inc. All Rights Reserved Clinical data is emerging showing superior efficacy1 with alpha therapies vs beta in mCRPC (e.g. 177Lu-PSMA vs. 225Ac-PSMA) Creates opportunities to be a best-in-class therapy for Lu naive and treat
relapsed/refractory patients 225Ac-PSMA-I&T has achieved clinical POC already in mCRPC Actinium supply barriers are preventing large scale studies and commercialization of the potentially superior alpha therapies Fusion is uniquely positioned
with our 225Ac supply and expertise to bring the first 225Ac-PSMA agent to market Similar first-to-market opportunity to what Endocyte did with 177Lu-PSMA-617 (which became Pluvicto) Beta Alpha Alpha 2x 177Lu 1x 225Ac 2x 225Ac Kratochwil et al.
(2016) J. Nucl. Med. 57:1941-1944 Fusion to acquire Phase 2 IND (TATCIST trial) with plan to develop 225Ac-PSMA-I&T as a potential first to market 1Not a head-to-head comparison

PSMA Radiopharmaceuticals: Current
Developments, Challenges and Opportunities Oliver Sartor, MD Laborde Professor of Cancer Research Medical Director Tulane Cancer Center Departments of Medicine and Urology Associate Dean for Oncology Tulane Medical School New Orleans, Louisiana
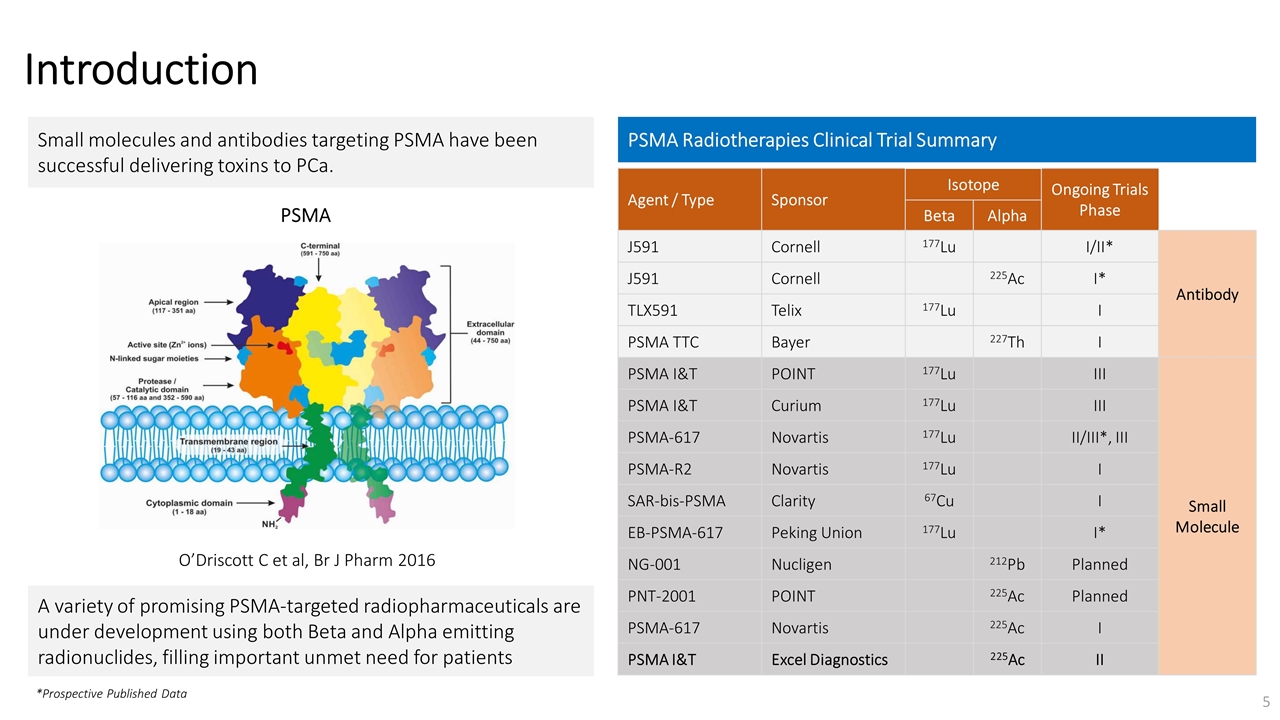
Introduction O’Driscott C et al,
Br J Pharm 2016 Agent / Type Sponsor Isotope Ongoing Trials Phase Beta Alpha J591 Cornell 177Lu I/II* Antibody J591 Cornell 225Ac I* TLX591 Telix 177Lu I PSMA TTC Bayer 227Th I PSMA I&T POINT 177Lu III Small Molecule PSMA I&T Curium 177Lu
III PSMA-617 Novartis 177Lu II/III*, III PSMA-R2 Novartis 177Lu I SAR-bis-PSMA Clarity 67Cu I EB-PSMA-617 Peking Union 177Lu I* NG-001 Nucligen 212Pb Planned PNT-2001 POINT 225Ac Planned PSMA-617 Novartis 225Ac I PSMA I&T Excel Diagnostics 225Ac
II A variety of promising PSMA-targeted radiopharmaceuticals are under development using both Beta and Alpha emitting radionuclides, filling important unmet need for patients PSMA Radiotherapies Clinical Trial Summary PSMA Small molecules and
antibodies targeting PSMA have been successful delivering toxins to PCa. *Prospective Published Data
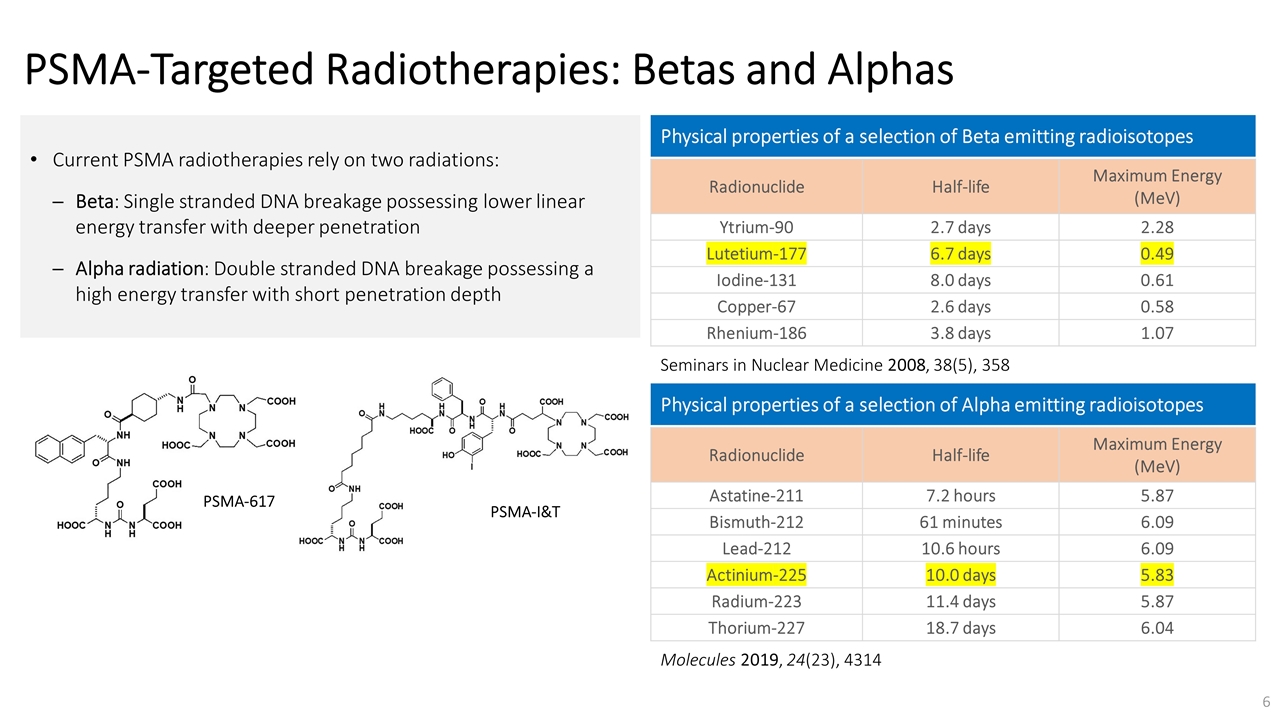
PSMA-Targeted Radiotherapies: Betas and
Alphas Current PSMA radiotherapies rely on two radiations: Beta: Single stranded DNA breakage possessing lower linear energy transfer with deeper penetration Alpha radiation: Double stranded DNA breakage possessing a high energy transfer with short
penetration depth Radionuclide Half-life Maximum Energy (MeV) Ytrium-90 2.7 days 2.28 Lutetium-177 6.7 days 0.49 Iodine-131 8.0 days 0.61 Copper-67 2.6 days 0.58 Rhenium-186 3.8 days 1.07 Physical properties of a selection of Beta emitting
radioisotopes Radionuclide Half-life Maximum Energy (MeV) Astatine-211 7.2 hours 5.87 Bismuth-212 61 minutes 6.09 Lead-212 10.6 hours 6.09 Actinium-225 10.0 days 5.83 Radium-223 11.4 days 5.87 Thorium-227 18.7 days 6.04 Physical properties of a
selection of Alpha emitting radioisotopes Molecules 2019, 24(23), 4314 Seminars in Nuclear Medicine 2008, 38(5), 358 PSMA-617 PSMA-I&T
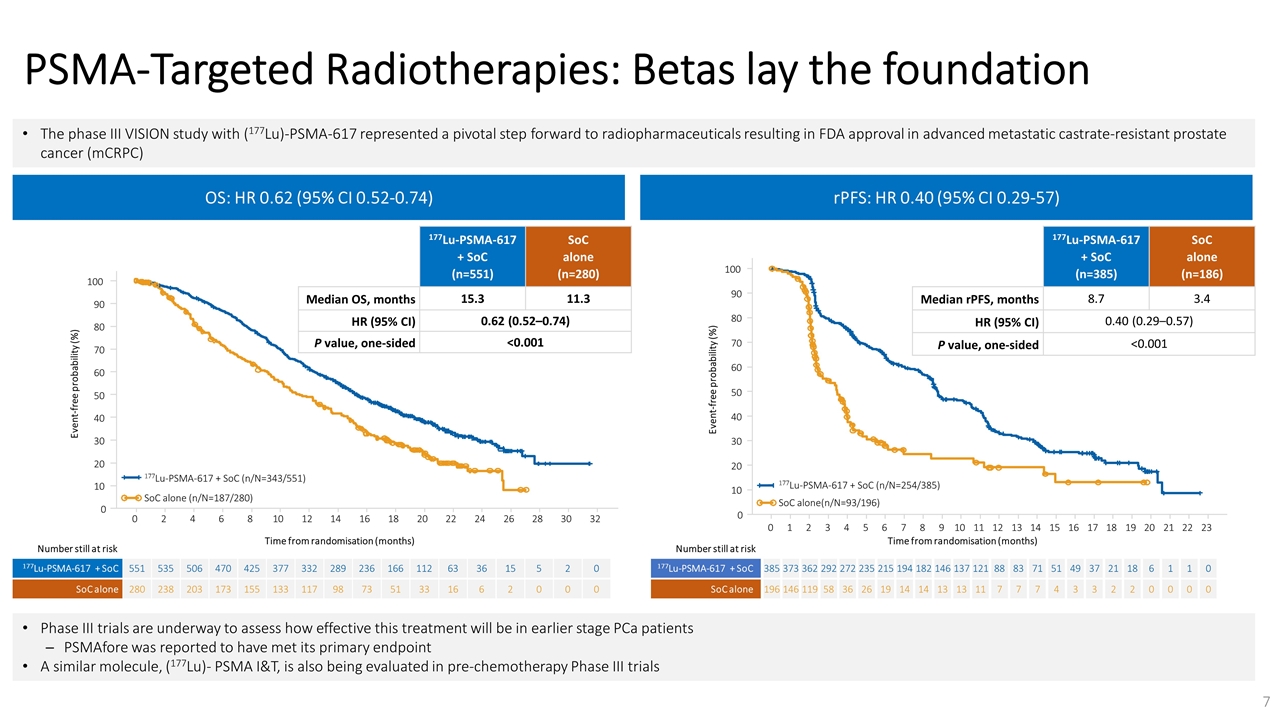
PSMA-Targeted Radiotherapies: Betas lay
the foundation The phase III VISION study with (177Lu)-PSMA-617 represented a pivotal step forward to radiopharmaceuticals resulting in FDA approval in advanced metastatic castrate-resistant prostate cancer (mCRPC) OS: HR 0.62 (95% CI 0.52-0.74)
Number still at risk 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 551 535 506 470 425 377 332 289 236 166 112 63 36 15 5 2 0 280 238 203 173 155 133 117 98 73 51 33 16 6 2 0 0 0 Time from randomisation (months) 0 10 20 30 40 50 60 70 80 90 100
Event-free probability (%) 177Lu-PSMA-617 + SoC SoC alone 177Lu-PSMA-617 + SoC (n/N=343/551) SoC alone (n/N=187/280) 177Lu-PSMA-617 + SoC (n=551) SoC alone (n=280) Median OS, months 15.3 11.3 HR (95% CI) 0.62 (0.52–0.74) P value, one-sided
<0.001 rPFS: HR 0.40 (95% CI 0.29-57) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 Event-free probability (%) 385 373 362 292 272 235 194 182 146 137 121 88 83 71 51 49 37 196 146 119 58 36 26 215 19 14 14 13 13 11 7 7 7 4 3 3 21
2 18 2 6 0 1 0 1 0 0 0 0 10 20 30 40 50 60 70 80 90 100 Time from randomisation (months) 177Lu-PSMA-617 + SoC (n/N=254/385) SoC alone(n/N=93/196) 177Lu-PSMA-617 + SoC SoC alone Number still at risk 177Lu-PSMA-617 + SoC (n=385) SoC alone (n=186)
Median rPFS, months 8.7 3.4 HR (95% CI) 0.40 (0.29–0.57) P value, one-sided <0.001 Phase III trials are underway to assess how effective this treatment will be in earlier stage PCa patients PSMAfore was reported to have met its primary
endpoint A similar molecule, (177Lu)- PSMA I&T, is also being evaluated in pre-chemotherapy Phase III trials

Betas Are Now an Important Part of the
Treatment Paradigm New unmet need exists for post-PLUVICTO® patients What do we do when patients still progress?
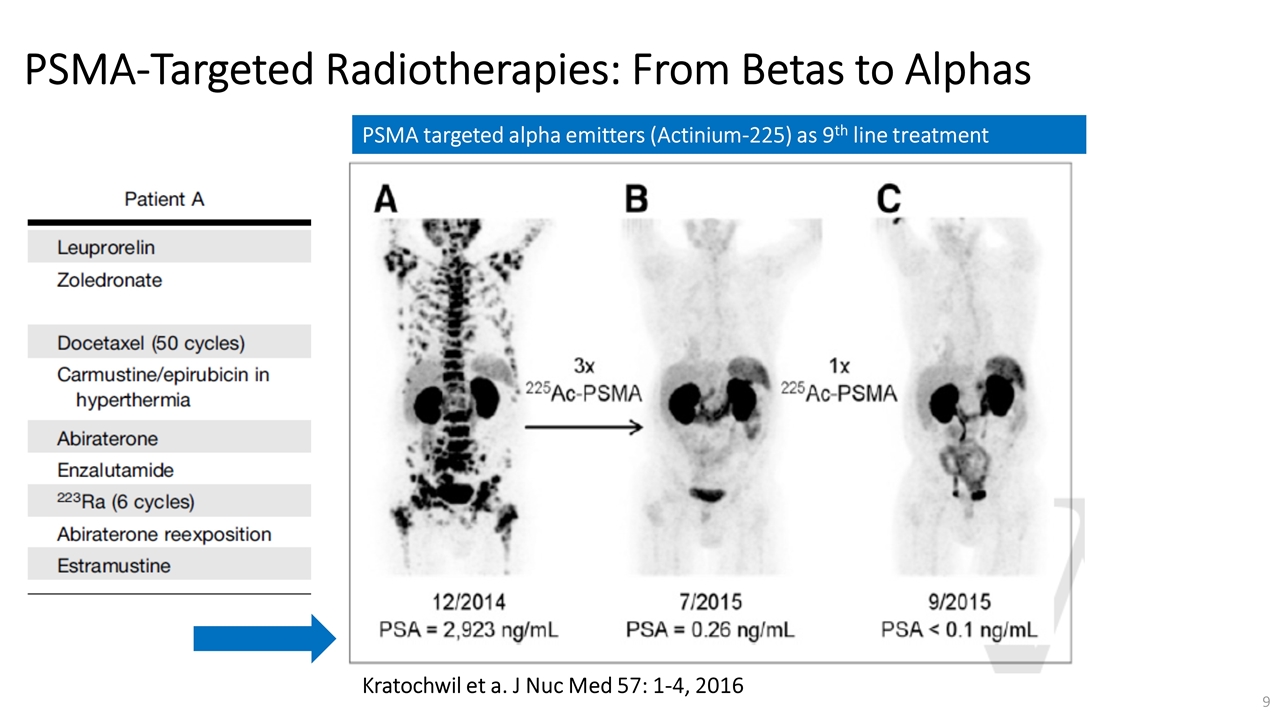
Kratochwil et a. J Nuc Med 57: 1-4,
2016 PSMA-Targeted Radiotherapies: From Betas to Alphas PSMA targeted alpha emitters (Actinium-225) as 9th line treatment
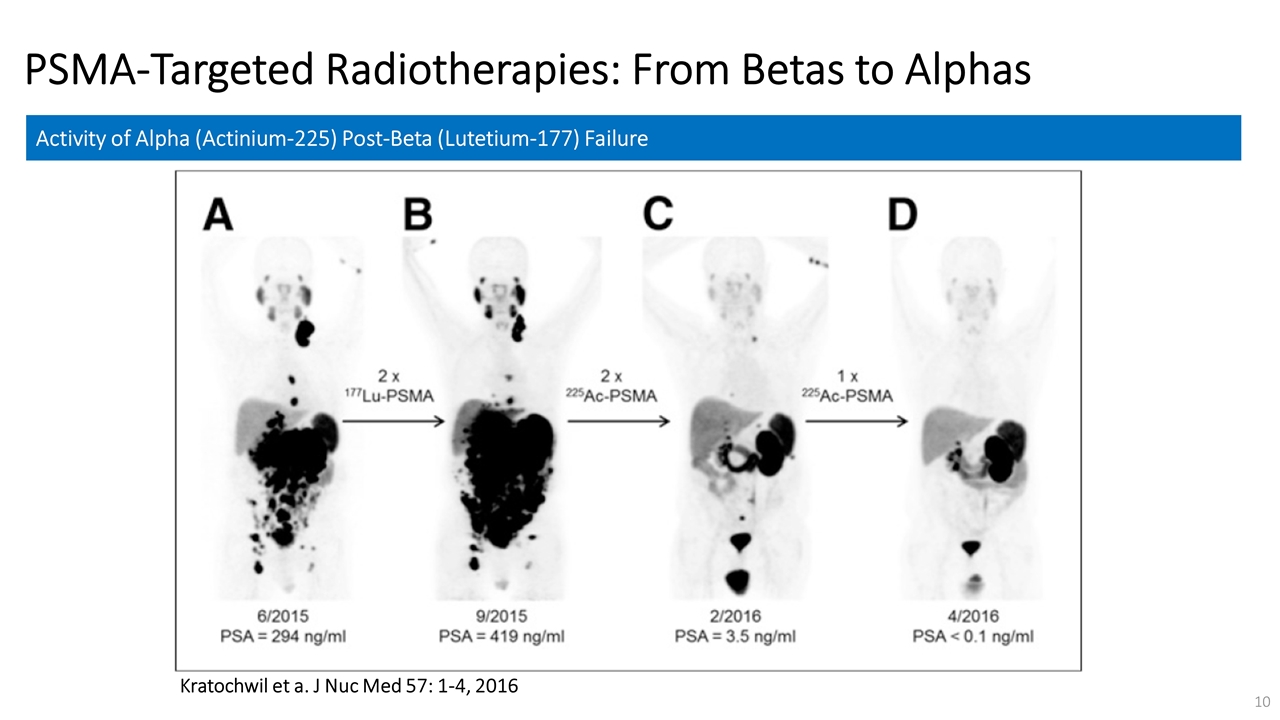
Kratochwil et a. J Nuc Med 57: 1-4,
2016 PSMA-Targeted Radiotherapies: From Betas to Alphas Activity of Alpha (Actinium-225) Post-Beta (Lutetium-177) Failure
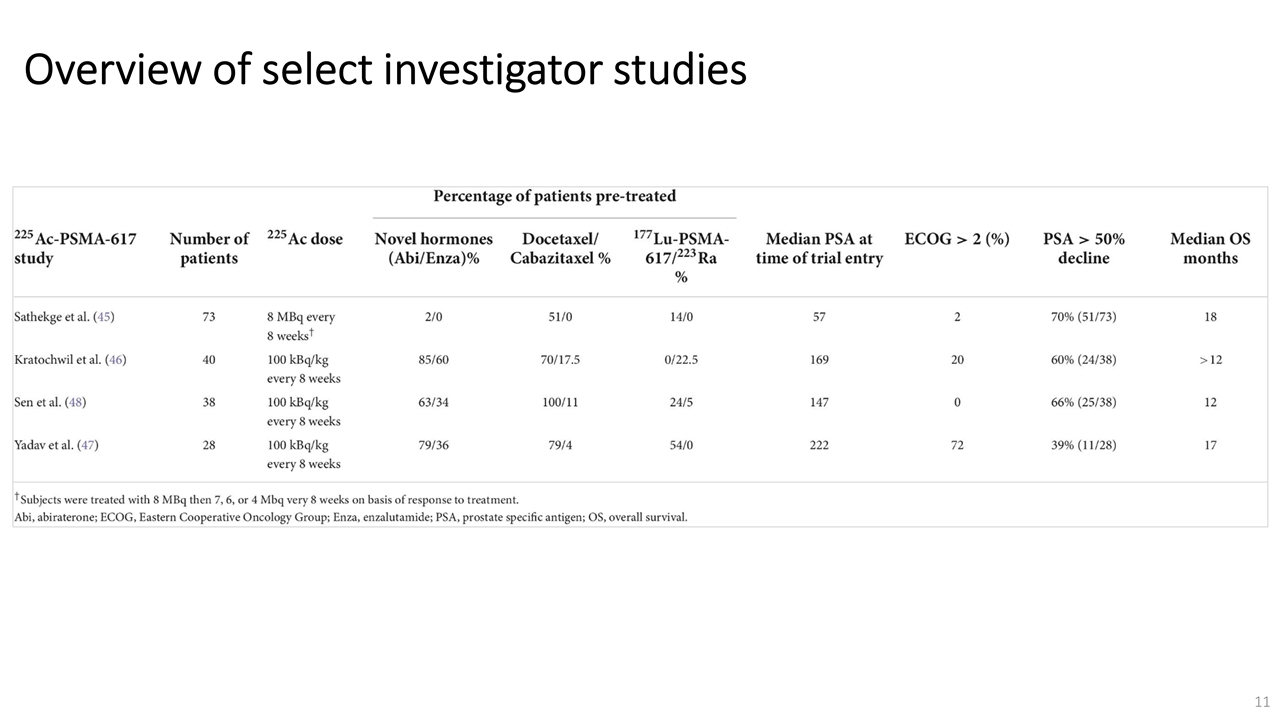
Overview of select investigator
studies

Summary PSMA represents a validated
target for prostate cancer therapeutics The Phase III VISION study with 177lutetium (177Lu)-PSMA-617 represented a pivotal step forward and the FDA has now approved this agent in advanced metastatic castrate-resistant prostate cancer (mCRPC)
Patients progressing after lutetium therapy represent a new unmet need Company-sponsored drug development, such as Fusion taking over the TATCIST trial with PSMA I&T, is needed to advance targeted alpha therapies towards Phase 3 for these
relapsed and refractory patients, and potentially eventually earlier lines of therapy
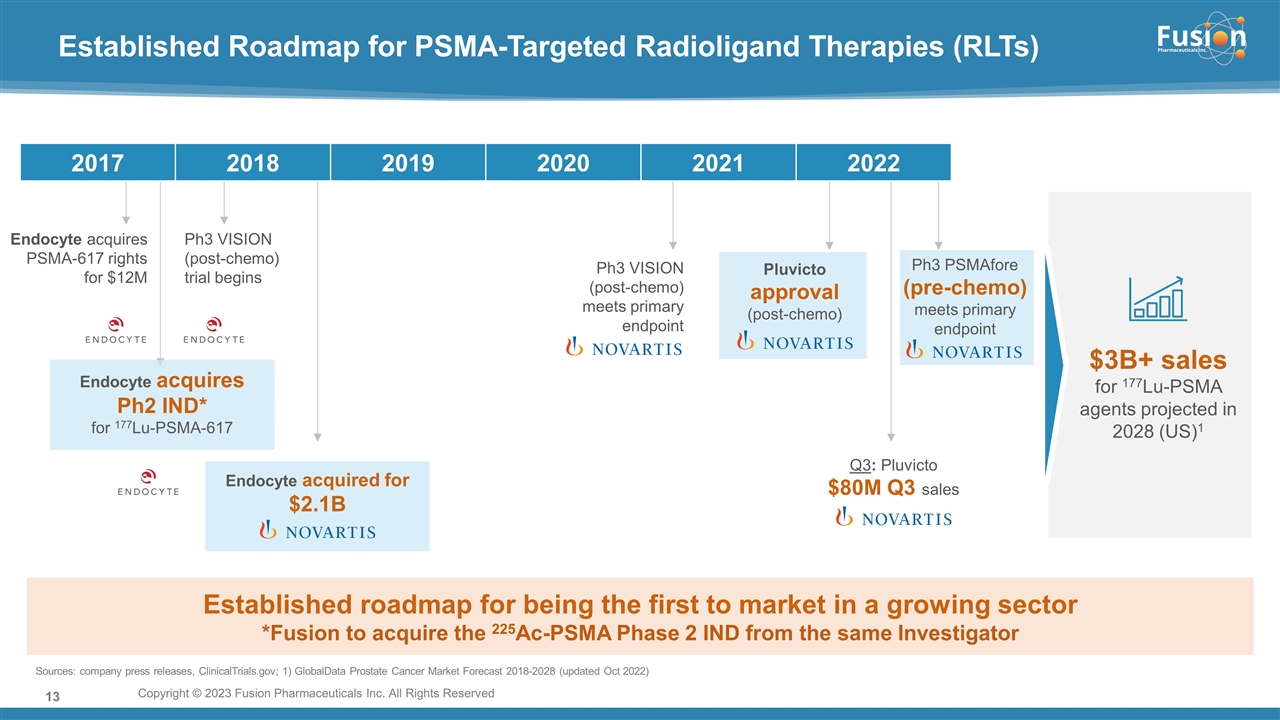
Established Roadmap for
PSMA-Targeted Radioligand Therapies (RLTs) 2017 2018 2019 2020 2021 2022 Sources: company press releases, ClinicalTrials.gov; 1) GlobalData Prostate Cancer Market Forecast 2018-2028 (updated Oct 2022) Established roadmap for being the first to
market in a growing sector *Fusion to acquire the 225Ac-PSMA Phase 2 IND from the same Investigator Endocyte acquires PSMA-617 rights for $12M Ph3 VISION (post-chemo) trial begins Ph3 VISION (post-chemo) meets primary endpoint Endocyte acquired for
$2.1B Ph3 PSMAfore (pre-chemo) meets primary endpoint Q3: Pluvicto $80M Q3 sales Pluvicto approval (post-chemo) $3B+ sales for 177Lu-PSMA agents projected in 2028 (US)1 Endocyte acquires Ph2 IND* for 177Lu-PSMA-617 Copyright © 2023 Fusion
Pharmaceuticals Inc. All Rights Reserved
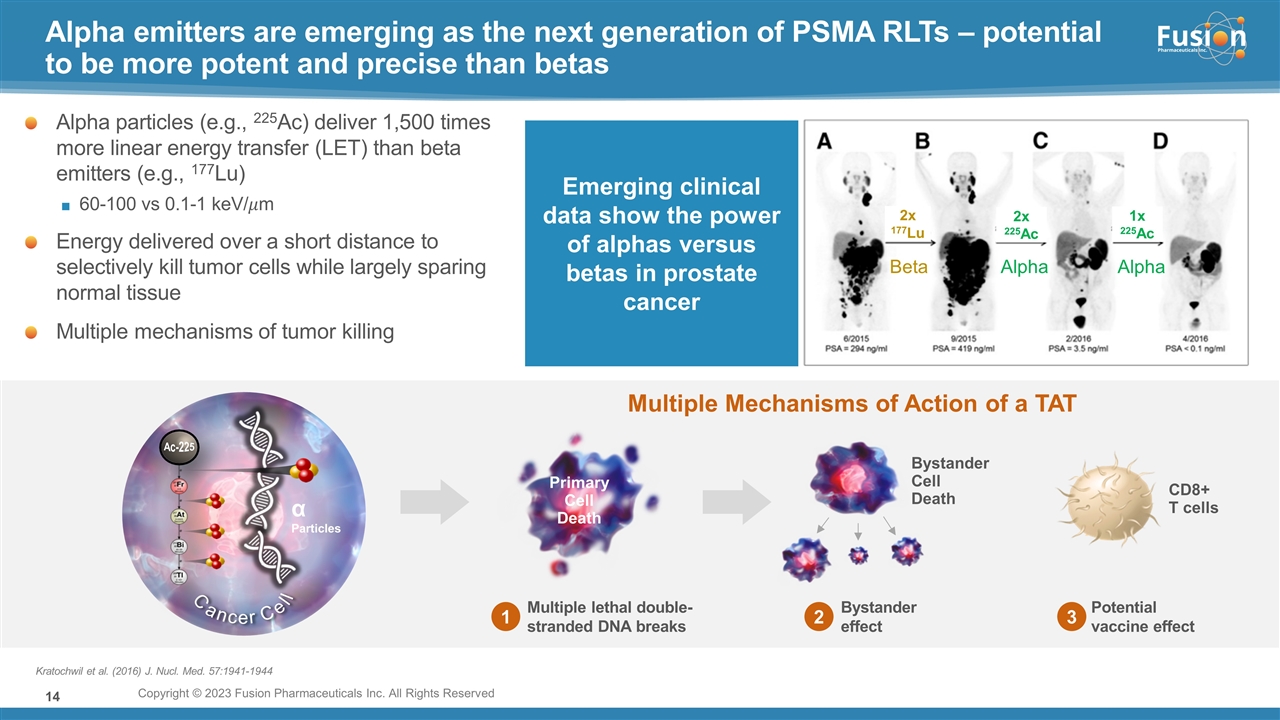
Alpha emitters are emerging as the
next generation of PSMA RLTs – potential to be more potent and precise than betas Alpha particles (e.g., 225Ac) deliver 1,500 times more linear energy transfer (LET) than beta emitters (e.g., 177Lu) 60-100 vs 0.1-1 keV/m Energy delivered over
a short distance to selectively kill tumor cells while largely sparing normal tissue Multiple mechanisms of tumor killing Kratochwil et al. (2016) J. Nucl. Med. 57:1941-1944 Primary Cell Death CD8+ T cells Cancer Cell α Particles Ac-225
Multiple lethal double-stranded DNA breaks Bystander effect Potential vaccine effect Multiple Mechanisms of Action of a TAT Beta Alpha Alpha 2x 177Lu 1x 225Ac 2x 225Ac Emerging clinical data show the power of alphas versus betas in prostate cancer
Bystander Cell Death 1 2 3 Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved
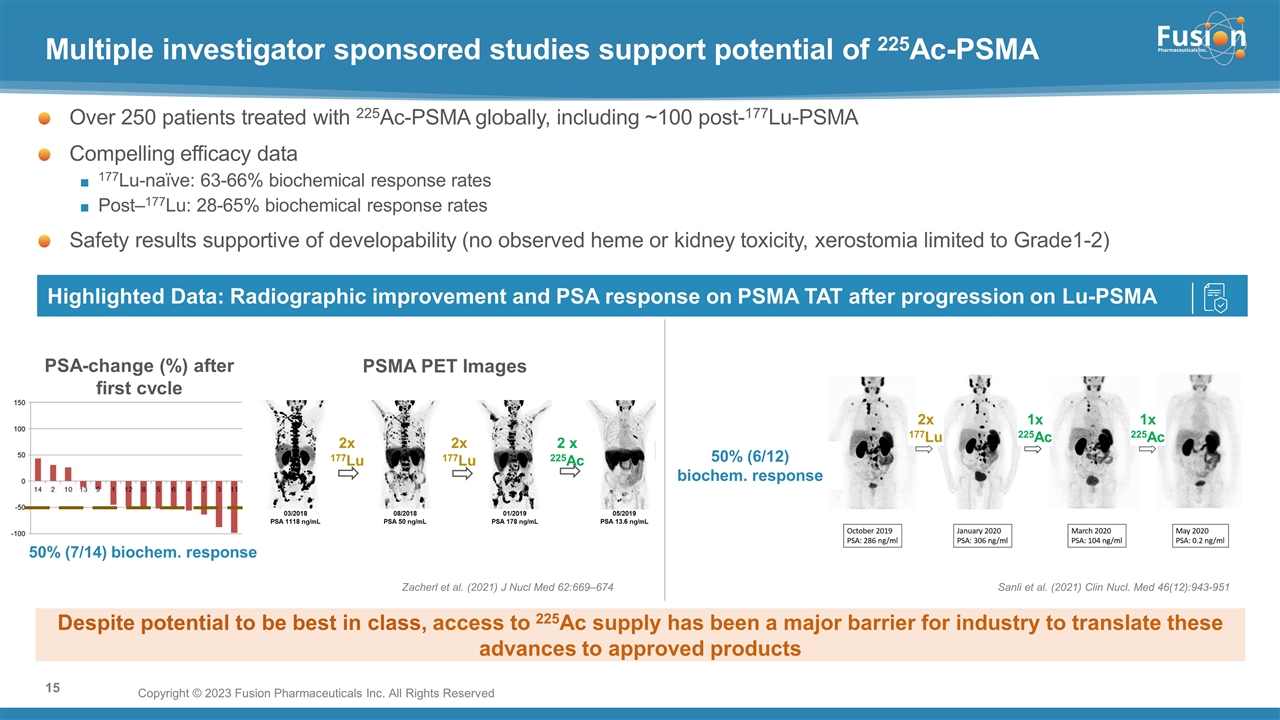
2x 177Lu 1x 225Ac 1x 225Ac 2x 177Lu
2 x 225Ac 2x 177Lu Multiple investigator sponsored studies support potential of 225Ac-PSMA Over 250 patients treated with 225Ac-PSMA globally, including ~100 post-177Lu-PSMA Compelling efficacy data 177Lu-naïve: 63-66% biochemical response
rates Post–177Lu: 28-65% biochemical response rates Safety results supportive of developability (no observed heme or kidney toxicity, xerostomia limited to Grade1-2) Zacherl et al. (2021) J Nucl Med 62:669–674 Highlighted Data:
Radiographic improvement and PSA response on PSMA TAT after progression on Lu-PSMA Sanli et al. (2021) Clin Nucl. Med 46(12):943-951 PSA-change (%) after first cycle Despite potential to be best in class, access to 225Ac supply has been a major
barrier for industry to translate these advances to approved products 50% (7/14) biochem. response 50% (6/12) biochem. response PSMA PET Images Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved

Fusion is well positioned to bring
the first 225Ac-PSMA agent to market from supply chain and alpha experience perspectives Largest dedicated TAT manufacturing facility globally Internal GMP manufacturing to be fully operational by 2024 Clinical & commercial supply capabilities
Adjacent to current R&D facility for efficiency Multiple CDMO relationships in place today to augment supply Secure Actinium Supply Global Leaders in Actinium Production Currently Producing and Shipping Material Supply Agreement: Strategic
Partnerships Ac-225 Guaranteed access to % of capacity Preferred access to excess capacity Option to invest for additional production Preferential access to supply Ability to scale to meet our needs Co-ownership of NewCo for production of Ac-225
Global commercial medical isotope producer and distributor Partnership for preferred access to actinium Skills, infrastructure, and experience – Fusion is the only company with three 225Ac-based radiopharmaceuticals currently in the clinic
Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved

Current landscape of actinium PSMA
programs Sources: GlobalData, Company websites, ClinicalTrials.gov 225Ac-PSMA programs Others [225Ac]-PSMA-617* [225Ac]-J591 (Ph1/2) [225Ac]-PSMA-I&T *ex-US only Company/Study Phase 1 Phase 2 Phase 3 Preclinical Marketed Bulk of other PSMA
development is with beta emitters TATCIST Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved
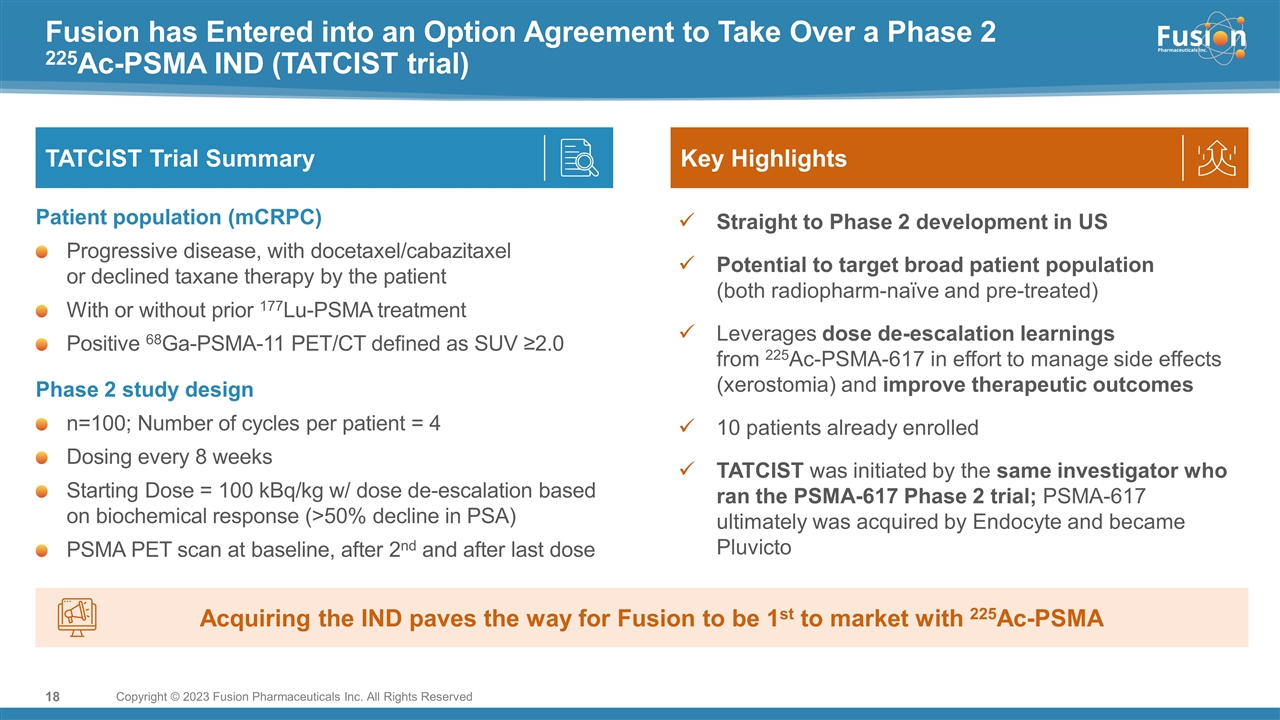
Patient population (mCRPC)
Progressive disease, with docetaxel/cabazitaxel or declined taxane therapy by the patient With or without prior 177Lu-PSMA treatment Positive 68Ga-PSMA-11 PET/CT defined as SUV ≥2.0 Phase 2 study design n=100; Number of cycles per patient = 4
Dosing every 8 weeks Starting Dose = 100 kBq/kg w/ dose de-escalation based on biochemical response (>50% decline in PSA) PSMA PET scan at baseline, after 2nd and after last dose TATCIST Trial Summary Straight to Phase 2 development in US
Potential to target broad patient population (both radiopharm-naïve and pre-treated) Leverages dose de-escalation learnings from 225Ac-PSMA-617 in effort to manage side effects (xerostomia) and improve therapeutic outcomes 10 patients already
enrolled TATCIST was initiated by the same investigator who ran the PSMA-617 Phase 2 trial; PSMA-617 ultimately was acquired by Endocyte and became Pluvicto Key Highlights Fusion has Entered into an Option Agreement to Take Over a Phase 2 225Ac-PSMA
IND (TATCIST trial) Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved Acquiring the IND paves the way for Fusion to be 1st to market with 225Ac-PSMA
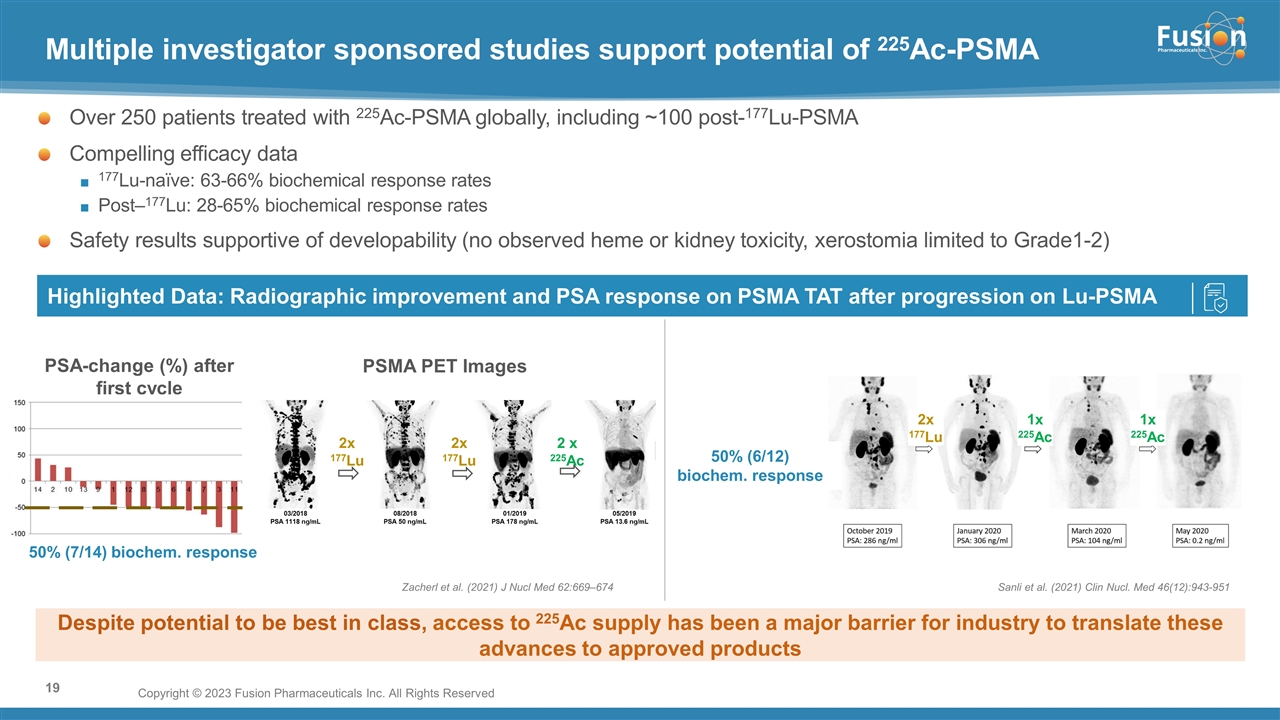
2x 177Lu 1x 225Ac 1x 225Ac 2x 177Lu
2 x 225Ac 2x 177Lu Multiple investigator sponsored studies support potential of 225Ac-PSMA Over 250 patients treated with 225Ac-PSMA globally, including ~100 post-177Lu-PSMA Compelling efficacy data 177Lu-naïve: 63-66% biochemical response
rates Post–177Lu: 28-65% biochemical response rates Safety results supportive of developability (no observed heme or kidney toxicity, xerostomia limited to Grade1-2) Zacherl et al. (2021) J Nucl Med 62:669–674 Highlighted Data:
Radiographic improvement and PSA response on PSMA TAT after progression on Lu-PSMA Sanli et al. (2021) Clin Nucl. Med 46(12):943-951 PSA-change (%) after first cycle Despite potential to be best in class, access to 225Ac supply has been a major
barrier for industry to translate these advances to approved products 50% (7/14) biochem. response 50% (6/12) biochem. response PSMA PET Images Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved
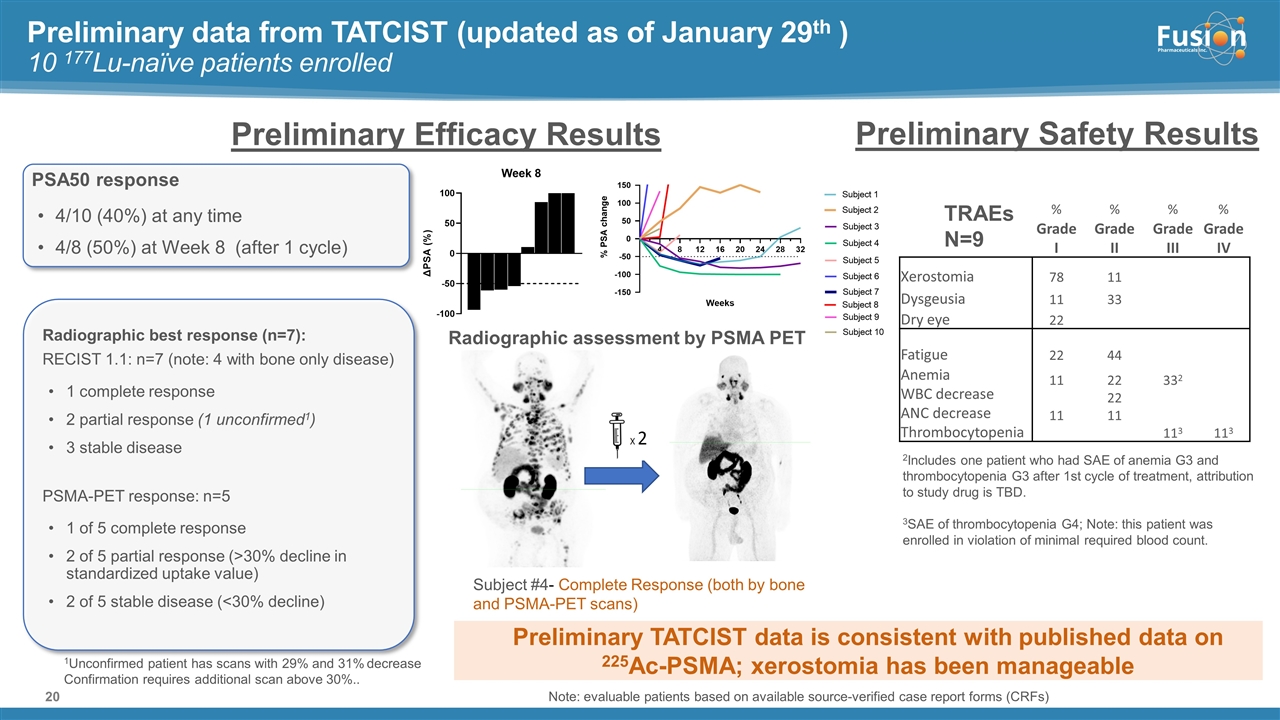
Preliminary data from TATCIST
(updated as of January 29th ) 10 177Lu-naïve patients enrolled PSA50 response 4/10 (40%) at any time 4/8 (50%) at Week 8 (after 1 cycle) Preliminary TATCIST data is consistent with published data on 225Ac-PSMA; xerostomia has been manageable
Note: evaluable patients based on available source-verified case report forms (CRFs) Subject #4- Complete Response (both by bone and PSMA-PET scans) Radiographic assessment by PSMA PET Preliminary Efficacy Results Preliminary Safety Results
TRAEs N=9 % % % % Grade I Grade II Grade III Grade IV Xerostomia 78 11 Dysgeusia 11 33 Dry eye 22 Fatigue 22 44 Anemia WBC decrease ANC decrease Thrombocytopenia 11 11 22 22 11 332 113 113 Radiographic best response (n=7): RECIST 1.1:
n=7 (note: 4 with bone only disease) 1 complete response 2 partial response (1 unconfirmed1) 3 stable disease PSMA-PET response: n=5 1 of 5 complete response 2 of 5 partial response (>30% decline in standardized uptake value) 2 of 5 stable
disease (<30% decline) 2Includes one patient who had SAE of anemia G3 and thrombocytopenia G3 after 1st cycle of treatment, attribution to study drug is TBD. 3SAE of thrombocytopenia G4; Note: this patient was enrolled in violation of minimal
required blood count. 1Unconfirmed patient has scans with 29% and 31% decrease Confirmation requires additional scan above 30%..
Results consistent with expectations
based upon study population Summary: Increase in PSA has been observed in certain patients in the study prior to 8-week post-treatment target efficacy assessment date Two of these patients had ECOG Performance Status 2, a well-known adverse
prognostic factor (signifying poor treatment outcomes) Patient with treatment-related Grade 4 thrombocytopenia and SAE of intracranial hemorrhage leading to treatment discontinuation was enrolled with Grade 2 decreased blood cell count in violation
of protocol exclusion criteria The TATCIST study protocol was amended in December 2022 and no longer allows inclusion of Performance Status 2 patients
Development strategy Copyright
© 2023 Fusion Pharmaceuticals Inc. All Rights Reserved In ~12 months Fusion will be able to report: Notes: Endocyte (acq. By Novartis) had data on just 30 patients from an IIT Ph2 trial before they commenced Ph3; POINT leveraged data from 27
patients from their lead-in study for SPLASH at ESMO 2022 to raise $225M PSMAfore trial (Ph3 chemo-naive, n=470) took 17 months to get to primary completion with actual start in June 2021 and actual primary completion in October 2022 per
clinicaltrials.gov (NCT04689828) Data for 20-30 patients, including safety and efficacy results (incl. PSA50 responses, ORR, rPFS) In 2024: Anticipated completion of Phase 2 study Initiation of Phase 3 study activities (pending alignment with FDA on
study design) Initial Approval: Potential first-to-market in 177Lu-R/R patients - area of highest unmet need; addressing the expected growth in the number of patients treated with 177Lu-PSMA agents Follow-On Opportunities: Potential to expand into
the treatment of 177Lu-naive patients and move to early lines of therapy leveraging our combination IP (I/O, DDRis) Focus in 2023 will be expanding to additional sites, expanding manufacturing capacity and reporting initial set of data We expect
high demand for access to the treatment
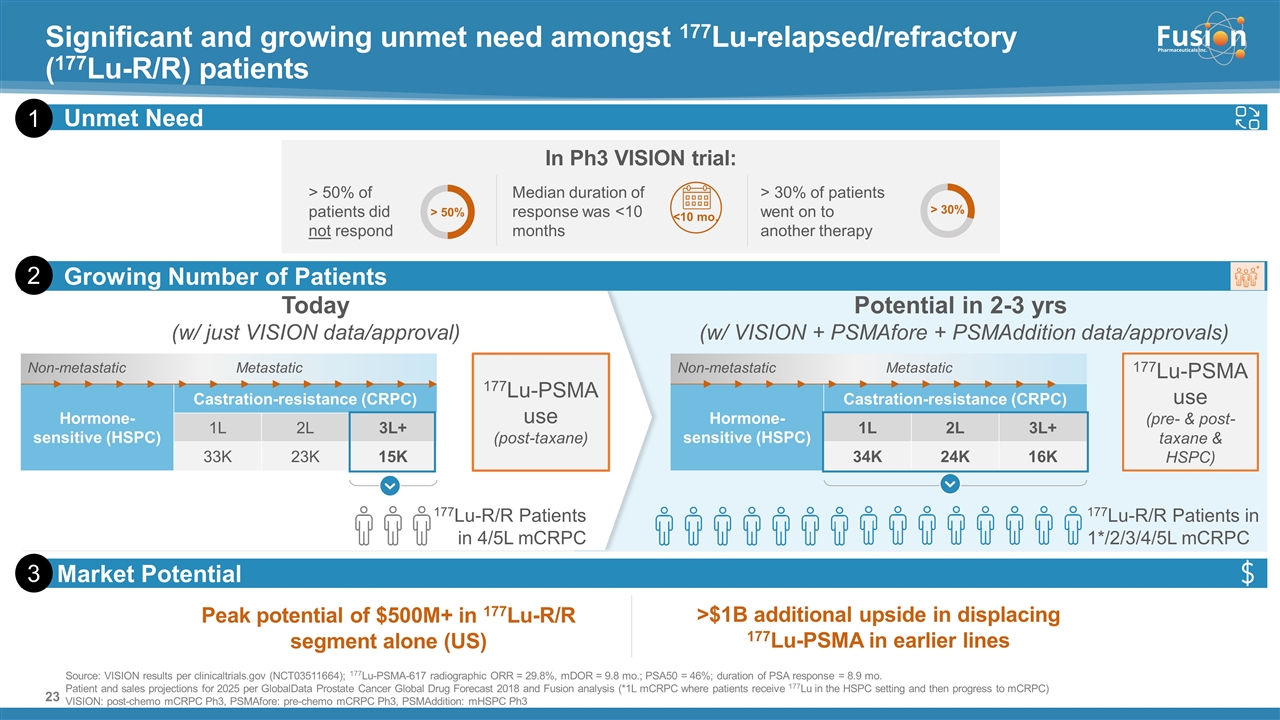
Significant and growing unmet need
amongst 177Lu-relapsed/refractory (177Lu-R/R) patients Unmet Need Today (w/ just VISION data/approval) Potential in 2-3 yrs (w/ VISION + PSMAfore + PSMAddition data/approvals) 177Lu-PSMA use (post-taxane) Hormone-sensitive (HSPC)
Castration-resistance (CRPC) Castration-sensitive (CSPC) 1L 2L 3L+ 33K 23K 15K Non-metastatic Metastatic 177Lu-PSMA use (pre- & post-taxane & HSPC) Source: VISION results per clinicaltrials.gov (NCT03511664); 177Lu-PSMA-617 radiographic ORR
= 29.8%, mDOR = 9.8 mo.; PSA50 = 46%; duration of PSA response = 8.9 mo. Patient and sales projections for 2025 per GlobalData Prostate Cancer Global Drug Forecast 2018 and Fusion analysis (*1L mCRPC where patients receive 177Lu in the HSPC setting
and then progress to mCRPC) VISION: post-chemo mCRPC Ph3, PSMAfore: pre-chemo mCRPC Ph3, PSMAddition: mHSPC Ph3 Hormone-sensitive (HSPC) Castration-resistance (CRPC) Castration-sensitive (CSPC) 1L 2L 3L+ 34K 24K 16K Non-metastatic Metastatic
177Lu-R/R Patients in 1*/2/3/4/5L mCRPC 177Lu-R/R Patients in 4/5L mCRPC > 30% In Ph3 VISION trial: > 50% of patients did not respond Median duration of response was <10 months > 30% of patients went on to another therapy > 50% >
30% <10 mo. Growing Number of Patients Peak potential of $500M+ in 177Lu-R/R segment alone (US) >$1B additional upside in displacing 177Lu-PSMA in earlier lines Market Potential 1 2 3

Strong team positioned to execute
Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved Expanded R&D team with deep PSMA, radiopharmaceutical and oncology experience Clinical Operations expertise from VISION trial Scientific co-founder of Endocyte (Pluvicto
developer acq. by Novartis) Oncol./prostate cancer drug development experience (Sanofi-Jevtana, Tesaro-Zejula) Strong Leadership Team with Radiopharmaceutical and Commercial Drug Development Expertise 30 years radiopharm experience; Founder and CEO
of commercial radiopharm manufacturer Commercial and Business Development Leader from Novartis Oncology, AbbVie and McKinsey Finance and operations experience at Merus, Charles River Labs, Ironwood, Vertex & Sunovian Chris Leamon, CSO Dmitri
Bobilev, CMO John Valliant, CEO Mohit Rawat, Pres. & CBO John Crowley, CFO CSO at CPDC (Global radiopharm manufacturer) Eric Burak, CTO Cara Ferreira R&D leadership at Nordion (Global leader in medical isotopes) Clinical advisory board with
deep prostate cancer and PSMA radiopharmaceutical expertise
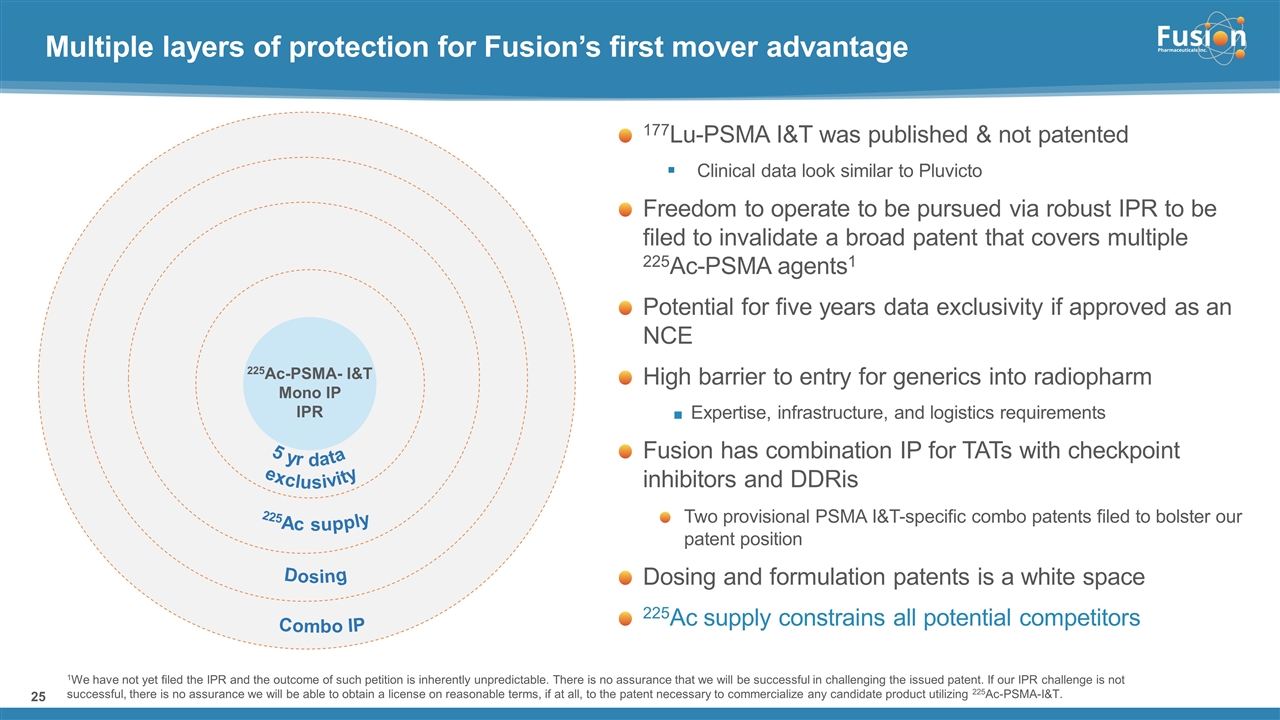
Multiple layers of protection for
Fusion’s first mover advantage 177Lu-PSMA I&T was published & not patented Clinical data look similar to Pluvicto Freedom to operate to be pursued via robust IPR to be filed to invalidate a broad patent that covers multiple 225Ac-PSMA
agents1 Potential for five years data exclusivity if approved as an NCE High barrier to entry for generics into radiopharm Expertise, infrastructure, and logistics requirements Fusion has combination IP for TATs with checkpoint inhibitors and DDRis
Two provisional PSMA I&T-specific combo patents filed to bolster our patent position Dosing and formulation patents is a white space 225Ac supply constrains all potential competitors Dosing Combo IP 225Ac supply 225Ac-PSMA- I&T Mono IP IPR 5
yr data exclusivity 1We have not yet filed the IPR and the outcome of such petition is inherently unpredictable. There is no assurance that we will be successful in challenging the issued patent. If our IPR challenge is not successful, there is no
assurance we will be able to obtain a license on reasonable terms, if at all, to the patent necessary to commercialize any candidate product utilizing 225Ac-PSMA-I&T.
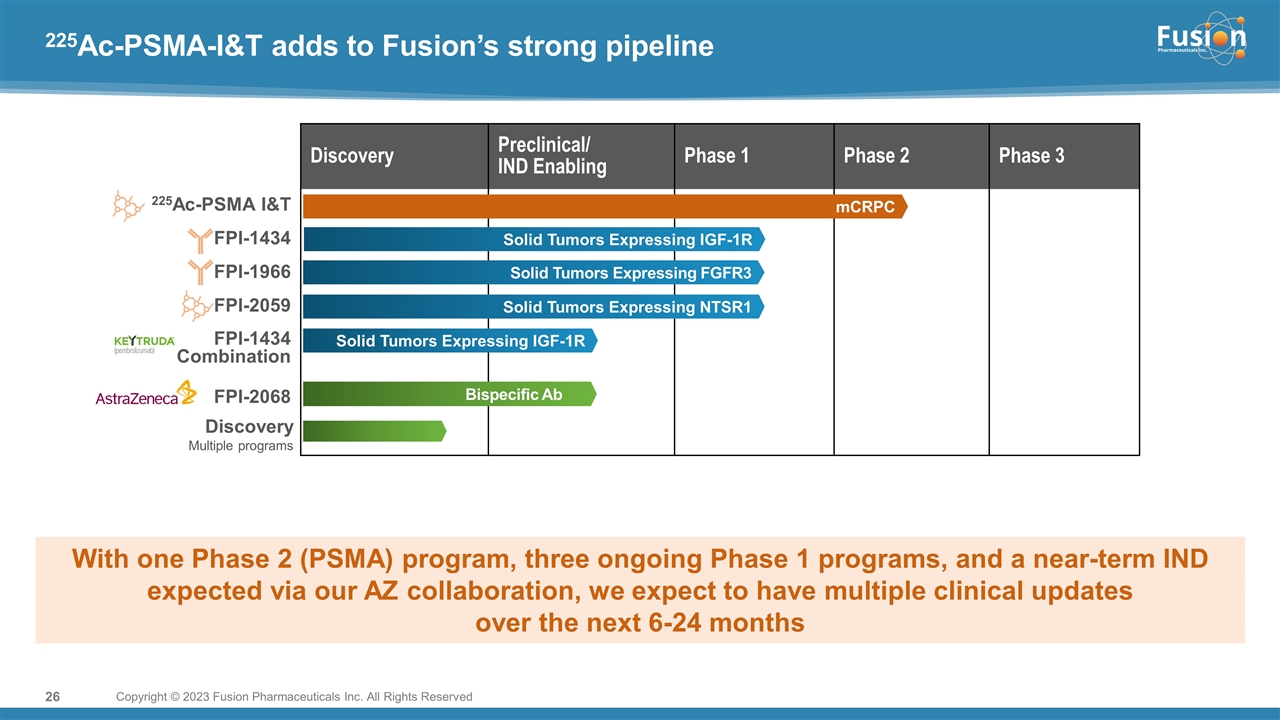
225Ac-PSMA-I&T adds to
Fusion’s strong pipeline Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved With one Phase 2 (PSMA) program, three ongoing Phase 1 programs, and a near-term IND expected via our AZ collaboration, we expect to have multiple
clinical updates over the next 6-24 months Discovery Preclinical/ IND Enabling Phase 1 Phase 2 Phase 3 225Ac-PSMA I&T FPI-1434 FPI-1966 FPI-2059 FPI-1434 Combination FPI-2068 Solid Tumors Expressing IGF-1R Solid Tumors Expressing IGF-1R Solid
Tumors Expressing NTSR1 Bispecific Ab Solid Tumors Expressing FGFR3 Discovery Multiple programs mCRPC
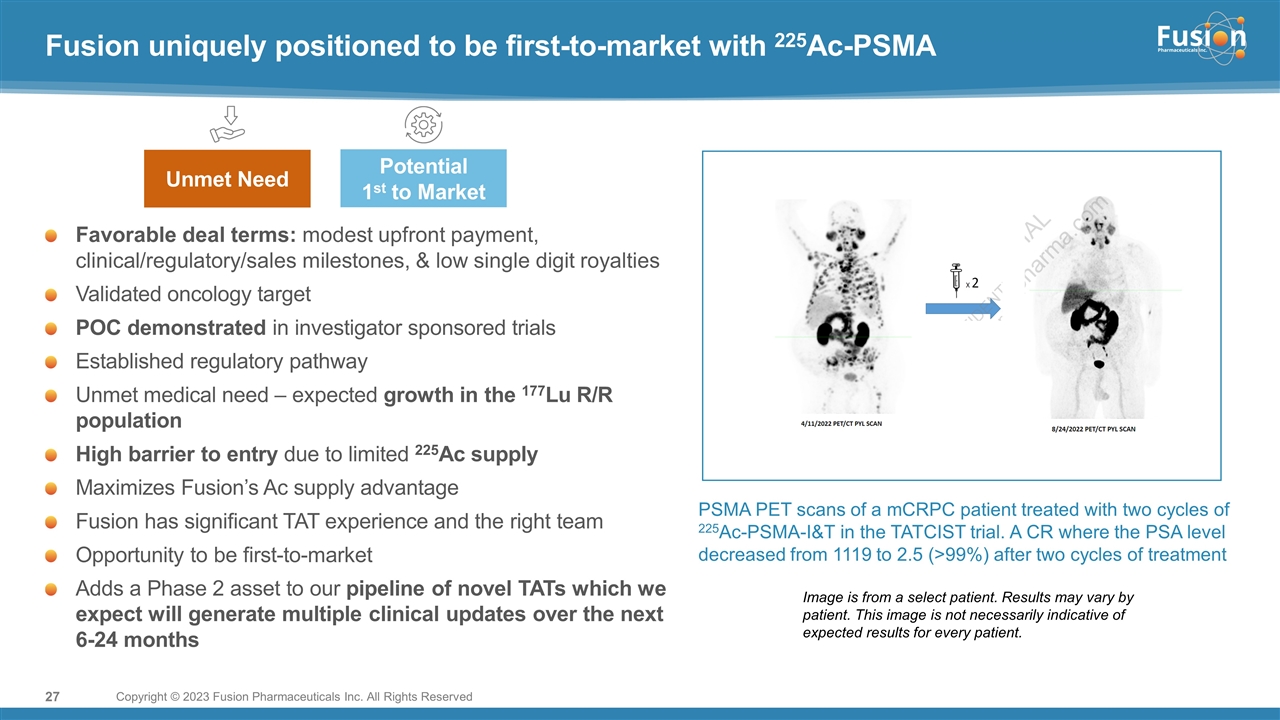
Fusion uniquely positioned to be
first-to-market with 225Ac-PSMA Copyright © 2023 Fusion Pharmaceuticals Inc. All Rights Reserved Unmet Need Potential 1st to Market PSMA PET scans of a mCRPC patient treated with two cycles of 225Ac-PSMA-I&T in the TATCIST trial. A CR where
the PSA level decreased from 1119 to 2.5 (>99%) after two cycles of treatment Favorable deal terms: modest upfront payment, clinical/regulatory/sales milestones, & low single digit royalties Validated oncology target POC demonstrated in
investigator sponsored trials Established regulatory pathway Unmet medical need – expected growth in the 177Lu R/R population High barrier to entry due to limited 225Ac supply Maximizes Fusion’s Ac supply advantage Fusion has significant
TAT experience and the right team Opportunity to be first-to-market Adds a Phase 2 asset to our pipeline of novel TATs which we expect will generate multiple clinical updates over the next 6-24 months Image is from a select patient. Results may vary
by patient. This image is not necessarily indicative of expected results for every patient.
Exhibit 99.3
As used in this Exhibit 99.3, unless the context indicates otherwise, references to “Fusion,” “the Company,” “we,”
“us,” “our” and similar references refer to Fusion Pharmaceuticals, Inc. and its wholly owned subsidiaries.
Risks
associated with the in-licensing or acquisition of drug candidates could cause substantial delays in the preclinical and clinical development of our drug candidates.
We acquired rights to FPI-2265, our newly acquired PSMA-I&T asset from
RadioMedix Inc., or RadioMedix, in February, 2023, after exercising our rights under an option and asset purchase agreement entered into in November 2022. Because we were not involved in the preclinical or clinical development of FPI-2265 prior to such date, we may experience difficulties in the transition of certain development activities from RadioMedix and its affiliates to us, which may result in delays in clinical trials, as well as
problems in our development efforts, particularly if we do not receive all of the necessary information, reports and data from RadioMedix and its affiliates in a timely manner. Further, we have had no involvement with or control over the preclinical
and clinical development of FPI-2265 to date. We have relied on RadioMedix having conducted such research and development in accordance with the applicable protocol, legal, regulatory and scientific standards,
having accurately reported the results of all clinical trials conducted prior to our agreement with RadioMedix and having correctly collected and interpreted the data from these trials. To the extent any of these has not occurred, expected
development time and costs may be increased which could adversely affect any future revenue from this drug candidate.
We may also acquire or in-license additional drug candidates for preclinical or clinical development in the future as we continue to build our pipeline. The risks associated with acquiring or
in-licensing current or future drug candidates could result in delays in the commencement or completion of our preclinical studies and clinical trials, if ever, and our ability to generate revenues from our
drug candidates may be delayed.
Third-party claims of intellectual property infringement may prevent or delay our product discovery and development
efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a
substantial amount of litigation involving the infringement of patents and other intellectual property rights in the biotechnology and pharmaceutical industries. We may be exposed to, or threatened with, future litigation by third parties having
patent or other intellectual property rights and who allege that our product candidates, uses and/or other proprietary technologies infringe their intellectual property rights. We are aware of certain third party patent rights that some may argue
cover our FPI-2265, our PSMA I&T product candidate or its use. We have filed an Inter Partes Review (IPR) petition with the United States Patent and Trademark Office to challenge the validity of a certain
issued U.S. Patent. In the event that we are unsuccessful in the IPR and that such patent has not expired at the time of approval of FPI-2265, our PSMA I&T product candidate and the patent owners were to
bring an infringement action against us, we may have to argue that FPI-2265, our PSMA I&T product candidate, its manufacture or use does not infringe a valid claim of the patent in question. Furthermore,
if we were to challenge the validity of this or any other issued U.S. patent in court, we would need to overcome a statutory presumption of validity that attaches to every U.S. patent. This means that in order to prevail, we would need to present
clear and convincing evidence as to the invalidity of the patent’s claims. There is no assurance that a court would find in our favor on questions of infringement or validity. In the event that a patent is successfully asserted against us such
that the patent is found to be valid and enforceable and infringed by FPI-2265, our PSMA I&T product candidate or its use, unless we obtain a license to such a patent, which may not be available on
commercially reasonable terms or at all, we could be prevented from continuing to develop or commercialize our product.
Numerous U.S. and foreign issued
patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk that
our product candidates may give rise to claims of infringement of the patent rights of others increases. Moreover, it is not always clear to industry participants, including us, which patents exist which may be found to cover various types of drugs,
products or their methods of use or manufacture. Thus, because of the large number of patents issued and patent applications currently pending in our fields, there may be a risk that third parties may allege they have patent rights which are
infringed by our product candidates, technologies or methods.
If a third party alleges that we infringe its intellectual property rights, we may face a number of issues,
including, but not limited to:
| |
• |
|
infringement and other intellectual property misappropriation which, regardless of merit, may be expensive and
time-consuming to litigate and may divert our management’s attention from our core business; |
| |
• |
|
substantial damages for infringement or misappropriation, which we may have to pay if a court decides that the
product candidate or technology at issue infringes on or violates the third-party’s rights, and, if the court finds we have willfully infringed intellectual property rights, we could be ordered to pay treble damages and the patent owner’s
attorneys’ fees; |
| |
• |
|
an injunction prohibiting us from manufacturing, marketing or selling our product candidates, or from using our
proprietary technologies, unless the third party agrees to license its patent rights to us; |
| |
• |
|
even if a license is available from a third party, we may have to pay substantial royalties, upfront fees and
other amounts, and/or grant cross-licenses to intellectual property rights protecting our products; and |
| |
• |
|
we may be forced to try to redesign our product candidates or processes so they do not infringe third-party
intellectual property rights, an undertaking which may not be possible or which may require substantial monetary expenditures and time. |
Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater
resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations or could otherwise have a material
adverse effect on our business, results of operations, financial condition and prospects.
Third parties may assert that we are employing their
proprietary technology without authorization. Generally, conducting preclinical and clinical trials and other development activities in the United States is not considered an act of infringement. If FPI-1434, FPI-1966, FPI-2059 or another product candidate is approved by the FDA, a third party may then seek to enforce its patent by filing a patent infringement lawsuit against us.
While we may believe that patent claims or other intellectual property rights of a third party would not have a materially adverse effect on the commercialization of our product candidates, we may be incorrect in this belief, or we may not be able
to prove it in litigation. In this regard, patents issued in the United States by law enjoy a presumption of validity that can be rebutted only with evidence that is “clear and convincing,” a heightened standard of proof. There may be
issued third-party patents of which we are currently unaware with claims to compositions, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Patent applications can take many
years to issue. There may be currently pending patent applications which may later result in issued patents that may be infringed by our product candidates. Moreover, we may fail to identify relevant patents or incorrectly conclude that a patent is
invalid, not enforceable, exhausted, or not infringed by our activities. If any third-party patents, held now or obtained in the future by a third party, were found by a court of competent jurisdiction to cover the manufacturing process of our
product candidates, constructs or molecules used in or formed during the manufacturing process, or any final product or methods use of the product, the holders of any such patents may be able to block our ability to commercialize the product
candidate unless we obtained a license under the applicable patents, or until such patents expire or they are finally determined to be held invalid or unenforceable. Similarly, if any third-party patent were held by a court of competent jurisdiction
to cover any aspect of our formulations, any combination therapies or patient selection methods, the holders of any such patent may be able to block our ability to develop and commercialize the product candidate unless we obtained a license or until
such patent expires or is finally determined to be held invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all. If we are unable to obtain a necessary license to a third-party patent
on commercially reasonable terms, or at all, our ability to commercialize our product candidates may be impaired or delayed, which could in turn significantly harm our business. Even if we obtain a license, it may be
non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In addition, if the breadth or strength of protection provided by our patents and patent applications is
threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could
effectively block our ability to further develop and commercialize our product candidates. Defense of these claims, regardless of their merit, could involve substantial litigation expense and would be a substantial diversion of employee resources
from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay
royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially
reasonable terms. Furthermore, even in the absence of litigation, we may need or may choose to obtain licenses from third parties to advance our research or allow commercialization of our product candidates. We may fail to obtain any of these
licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize our product candidates, which could harm our business significantly.
Our proprietary position depends upon patents that are manufacturing, formulation or
method-of-use patents, which may not prevent a competitor or other third party from using the same product candidate for another use.
Composition-of-matter patents on the active pharmaceutical ingredient, or API,
in prescription drug products are generally considered to be the strongest form of intellectual property protection for drug products because such patents provide protection without regard to any particular method of use or manufacture or
formulation of the API used. We currently have claims in an in-licensed issued U.S. patent that cover the antibody composition of matter incorporated in some of our product candidates. We license at least one
issued U.S. patent with claims that cover the FPI-1434 product candidate. We are pursuing claims in our owned and in-licensed pending patent applications to provide
additional compositions of matter coverage, including coverage of our product candidates. We cannot be certain that claims in any future patents issuing from our pending owned or in-licensed patent
applications or our future owned or in-licensed patent applications will cover the composition of matter of our current or future product candidates.
However, we do not own or in-license any composition of matter patents or patent applications in the United States or
any other jurisdiction with respect to our FPI-2265 product candidate. Further, we do not currently own or in-license any U.S. or foreign patents or patent applications
covering our FPI-2265 product candidate, including method of use patents or patent applications or formulation patents or patent applications. Although we intend to file patent applications in the future that
cover our FPI-2265 product candidate, we cannot be certain that our future owned or licensed patent applications will cover our FPI-2265 product candidate. As a result,
our owned patent portfolio and any patent portfolio we may license in the future may not provide us with adequate and continuing patent protection sufficient to exclude others from commercializing products similar or identical to our FPI-2265 product candidate.
Method-of-use patents protect the use of a product for the specified method
and formulation patents cover formulations of the API. These types of patents do not prevent a competitor or other third party from developing or marketing an identical product for an indication that is outside the scope of the patented method or
from developing a different formulation that is outside the scope of the patented formulation. Moreover, with respect to method-of-use patents, even if competitors or
other third parties do not actively promote their product for our targeted indications or uses for which we may obtain patents, physicians may recommend that patients use these products off-label, or patients
may do so themselves. Although off-label use may infringe or contribute to the infringement of method-of-use patents, the
practice is common, and this type of infringement is difficult to prevent or prosecute. In addition, there are numerous publications and other prior art that may be relevant to our owned or in-licensed method-of-use patents and patent applications and may be used to challenge the validity of these owned or in-licensed patents and
patent applications in litigation or other intellectual property-related proceedings. If these types of challenges are successful, our owned or in-licensed patents and patent applications may be narrowed or
found to be invalid and we may lose valuable intellectual property rights. Any of the foregoing could have a material adverse effect on our business, financial conditions, prospects and results of operations.
v3.22.4
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionISO 3166-1 alpha-2 country code.
| Name: |
dei_EntityAddressCountry |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:countryCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
{
"instance": {
"d271395d8k.htm": {
"axisCustom": 0,
"axisStandard": 0,
"baseTaxonomies": {
"http://xbrl.sec.gov/dei/2022": 24
},
"contextCount": 1,
"dts": {
"inline": {
"local": [
"d271395d8k.htm"
]
},
"labelLink": {
"local": [
"fusn-20230210_lab.xml"
]
},
"presentationLink": {
"local": [
"fusn-20230210_pre.xml"
]
},
"schema": {
"local": [
"fusn-20230210.xsd"
],
"remote": [
"http://www.xbrl.org/2003/xbrl-instance-2003-12-31.xsd",
"http://www.xbrl.org/2003/xbrl-linkbase-2003-12-31.xsd",
"http://www.xbrl.org/2003/xl-2003-12-31.xsd",
"http://www.xbrl.org/2003/xlink-2003-12-31.xsd",
"http://www.xbrl.org/2005/xbrldt-2005.xsd",
"https://www.xbrl.org/dtr/type/2020-01-21/types.xsd",
"https://xbrl.sec.gov/dei/2022/dei-2022.xsd"
]
}
},
"elementCount": 25,
"entityCount": 1,
"hidden": {
"http://xbrl.sec.gov/dei/2022": 3,
"total": 3
},
"keyCustom": 0,
"keyStandard": 24,
"memberCustom": 0,
"memberStandard": 0,
"nsprefix": "fusn",
"nsuri": "http://fusionpharma.com/20230210",
"report": {
"R1": {
"firstAnchor": {
"ancestors": [
"span",
"p",
"div",
"div",
"body",
"html"
],
"baseRef": "d271395d8k.htm",
"contextRef": "duration_2023-02-10_to_2023-02-10",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "dei:DocumentType",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
},
"groupType": "document",
"isDefault": "true",
"longName": "100000 - Document - Document and Entity Information",
"menuCat": "Cover",
"order": "1",
"role": "http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation",
"shortName": "Document and Entity Information",
"subGroupType": "",
"uniqueAnchor": {
"ancestors": [
"span",
"p",
"div",
"div",
"body",
"html"
],
"baseRef": "d271395d8k.htm",
"contextRef": "duration_2023-02-10_to_2023-02-10",
"decimals": null,
"first": true,
"lang": "en-US",
"name": "dei:DocumentType",
"reportCount": 1,
"unique": true,
"unitRef": null,
"xsiNil": "false"
}
}
},
"segmentCount": 0,
"tag": {
"dei_AmendmentFlag": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the XBRL content amends previously-filed or accepted submission.",
"label": "Amendment Flag",
"terseLabel": "Amendment Flag"
}
}
},
"localname": "AmendmentFlag",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "booleanItemType"
},
"dei_CityAreaCode": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Area code of city",
"label": "City Area Code",
"terseLabel": "City Area Code"
}
}
},
"localname": "CityAreaCode",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "normalizedStringItemType"
},
"dei_CoverAbstract": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Cover page.",
"label": "Cover [Abstract]",
"terseLabel": "Cover [Abstract]"
}
}
},
"localname": "CoverAbstract",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"xbrltype": "stringItemType"
},
"dei_DocumentPeriodEndDate": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "For the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.",
"label": "Document Period End Date",
"terseLabel": "Document Period End Date"
}
}
},
"localname": "DocumentPeriodEndDate",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "dateItemType"
},
"dei_DocumentType": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "The type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.",
"label": "Document Type",
"terseLabel": "Document Type"
}
}
},
"localname": "DocumentType",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "submissionTypeItemType"
},
"dei_EntityAddressAddressLine1": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Address Line 1 such as Attn, Building Name, Street Name",
"label": "Entity Address, Address Line One",
"terseLabel": "Entity Address, Address Line One"
}
}
},
"localname": "EntityAddressAddressLine1",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityAddressCityOrTown": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Name of the City or Town",
"label": "Entity Address, City or Town",
"terseLabel": "Entity Address, City or Town"
}
}
},
"localname": "EntityAddressCityOrTown",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityAddressCountry": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "ISO 3166-1 alpha-2 country code.",
"label": "Entity Address, Country",
"terseLabel": "Entity Address, Country"
}
}
},
"localname": "EntityAddressCountry",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "countryCodeItemType"
},
"dei_EntityAddressPostalZipCode": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Code for the postal or zip code",
"label": "Entity Address, Postal Zip Code",
"terseLabel": "Entity Address, Postal Zip Code"
}
}
},
"localname": "EntityAddressPostalZipCode",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityAddressStateOrProvince": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Name of the state or province.",
"label": "Entity Address, State or Province",
"terseLabel": "Entity Address, State or Province"
}
}
},
"localname": "EntityAddressStateOrProvince",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "stateOrProvinceItemType"
},
"dei_EntityCentralIndexKey": {
"auth_ref": [
"r1"
],
"lang": {
"en-us": {
"role": {
"documentation": "A unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK.",
"label": "Entity Central Index Key",
"terseLabel": "Entity Central Index Key"
}
}
},
"localname": "EntityCentralIndexKey",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "centralIndexKeyItemType"
},
"dei_EntityEmergingGrowthCompany": {
"auth_ref": [
"r1"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate if registrant meets the emerging growth company criteria.",
"label": "Entity Emerging Growth Company",
"terseLabel": "Entity Emerging Growth Company"
}
}
},
"localname": "EntityEmergingGrowthCompany",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "booleanItemType"
},
"dei_EntityExTransitionPeriod": {
"auth_ref": [
"r7"
],
"lang": {
"en-us": {
"role": {
"documentation": "Indicate if an emerging growth company has elected not to use the extended transition period for complying with any new or revised financial accounting standards.",
"label": "Entity Ex Transition Period",
"terseLabel": "Entity Ex Transition Period"
}
}
},
"localname": "EntityExTransitionPeriod",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "booleanItemType"
},
"dei_EntityFileNumber": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Commission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.",
"label": "Securities Act File Number",
"terseLabel": "Entity File Number"
}
}
},
"localname": "EntityFileNumber",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "fileNumberItemType"
},
"dei_EntityIncorporationStateCountryCode": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Two-character EDGAR code representing the state or country of incorporation.",
"label": "Entity Incorporation, State or Country Code",
"terseLabel": "Entity Incorporation State Country Code"
}
}
},
"localname": "EntityIncorporationStateCountryCode",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "edgarStateCountryItemType"
},
"dei_EntityRegistrantName": {
"auth_ref": [
"r1"
],
"lang": {
"en-us": {
"role": {
"documentation": "The exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC.",
"label": "Entity Registrant Name",
"terseLabel": "Entity Registrant Name"
}
}
},
"localname": "EntityRegistrantName",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "normalizedStringItemType"
},
"dei_EntityTaxIdentificationNumber": {
"auth_ref": [
"r1"
],
"lang": {
"en-us": {
"role": {
"documentation": "The Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS.",
"label": "Entity Tax Identification Number",
"terseLabel": "Entity Tax Identification Number"
}
}
},
"localname": "EntityTaxIdentificationNumber",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "employerIdItemType"
},
"dei_LocalPhoneNumber": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Local phone number for entity.",
"label": "Local Phone Number",
"terseLabel": "Local Phone Number"
}
}
},
"localname": "LocalPhoneNumber",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "normalizedStringItemType"
},
"dei_PreCommencementIssuerTenderOffer": {
"auth_ref": [
"r3"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act.",
"label": "Pre-commencement Issuer Tender Offer",
"terseLabel": "Pre Commencement Issuer Tender Offer"
}
}
},
"localname": "PreCommencementIssuerTenderOffer",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "booleanItemType"
},
"dei_PreCommencementTenderOffer": {
"auth_ref": [
"r4"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act.",
"label": "Pre-commencement Tender Offer",
"terseLabel": "Pre Commencement Tender Offer"
}
}
},
"localname": "PreCommencementTenderOffer",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "booleanItemType"
},
"dei_Security12bTitle": {
"auth_ref": [
"r0"
],
"lang": {
"en-us": {
"role": {
"documentation": "Title of a 12(b) registered security.",
"label": "Title of 12(b) Security",
"terseLabel": "Security 12b Title"
}
}
},
"localname": "Security12bTitle",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "securityTitleItemType"
},
"dei_SecurityExchangeName": {
"auth_ref": [
"r2"
],
"lang": {
"en-us": {
"role": {
"documentation": "Name of the Exchange on which a security is registered.",
"label": "Security Exchange Name",
"terseLabel": "Security Exchange Name"
}
}
},
"localname": "SecurityExchangeName",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "edgarExchangeCodeItemType"
},
"dei_SolicitingMaterial": {
"auth_ref": [
"r5"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act.",
"label": "Soliciting Material",
"terseLabel": "Soliciting Material"
}
}
},
"localname": "SolicitingMaterial",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "booleanItemType"
},
"dei_TradingSymbol": {
"auth_ref": [],
"lang": {
"en-us": {
"role": {
"documentation": "Trading symbol of an instrument as listed on an exchange.",
"label": "Trading Symbol",
"terseLabel": "Trading Symbol"
}
}
},
"localname": "TradingSymbol",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "tradingSymbolItemType"
},
"dei_WrittenCommunications": {
"auth_ref": [
"r6"
],
"lang": {
"en-us": {
"role": {
"documentation": "Boolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act.",
"label": "Written Communications",
"terseLabel": "Written Communications"
}
}
},
"localname": "WrittenCommunications",
"nsuri": "http://xbrl.sec.gov/dei/2022",
"presentation": [
"http://fusionpharma.com//20230210/taxonomy/role/DocumentDocumentAndEntityInformation"
],
"xbrltype": "booleanItemType"
}
},
"unitCount": 0
}
},
"std_ref": {
"r0": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "12",
"Subsection": "b",
"role": "http://www.xbrl.org/2003/role/presentationRef"
},
"r1": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "12",
"Subsection": "b-2",
"role": "http://www.xbrl.org/2003/role/presentationRef"
},
"r2": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "12",
"Subsection": "d1-1",
"role": "http://www.xbrl.org/2003/role/presentationRef"
},
"r3": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "13e",
"Subsection": "4c",
"role": "http://www.xbrl.org/2003/role/presentationRef"
},
"r4": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "14d",
"Subsection": "2b",
"role": "http://www.xbrl.org/2003/role/presentationRef"
},
"r5": {
"Name": "Exchange Act",
"Number": "240",
"Publisher": "SEC",
"Section": "14a",
"Subsection": "12",
"role": "http://www.xbrl.org/2003/role/presentationRef"
},
"r6": {
"Name": "Securities Act",
"Number": "230",
"Publisher": "SEC",
"Section": "425",
"role": "http://www.xbrl.org/2003/role/presentationRef"
},
"r7": {
"Name": "Securities Act",
"Number": "7A",
"Publisher": "SEC",
"Section": "B",
"Subsection": "2",
"role": "http://www.xbrl.org/2003/role/presentationRef"
}
},
"version": "2.2"
}



























