
An offering statement pursuant to Regulation A relating to these securities has been filed with the Securities and Exchange Commission. Information contained in this preliminary offering circular is subject to completion or amendment. These securities may not be sold nor may offers to buy be accepted before the offering statement filed with the Commission is qualified. This Preliminary offering circular shall not constitute an offer to sell or the solicitation of an offer to buy nor may there be any sales of these securities in any state in which such offer, solicitation or sale would be unlawful before registration or qualification under the laws of any such state. We may elect to satisfy our obligation to deliver a final offering circular by sending you a notice within two business days after the completion of our sale to you that contains the URL where the final offering circular or the offering statement in which such Final offering circular was filed may be obtained.
Preliminary Offering Circular Dated April 25, 2022

AERIS BIOTECHNOLOGIES, INC.
8105 Rasor Boulevard, Suite 129
Plano, TX 75024
1-214-436-2986
www.aerisbiotech.com
OFFERING SUMMARY
Up to 15,000,000 shares of Common Stock, par value $0.0001
Minimum investment 500 shares at $500
SEE “SECURITIES BEING OFFERED” AT PAGE 36
| Price to Public | Underwriting discount and commissions (1) | Proceeds, Before Expenses, to Issuer (2) | |||||
| Per Share | $1.00 | $0.01 | $0.99 | ||||
| Total Maximum | $15,000,000.00 | $150,000.00 | $14,850,000 |
| (1) | Aeris Biotechnologies, Inc. (the “Company”) has engaged Dalmore Group, member FINRA/SIPC (“Dalmore”), to act as the broker-dealer of record in connection with this offering, but not for underwriting or placement agent services. This includes a 1% commission, but it does not include the one-time set-up fee and consulting fee payable by the Company to Dalmore. See “Plan of Distribution” for details. |
| (2) | The Company expects that, not including state filing fees, the maximum amount of expenses of the offering that it will pay will be approximately $1.7 million assuming that the maximum number of shares are sold in this offering. |
The Company is offering shares of its common stock, par value $0.0001 per share. This offering (the “Offering”) will terminate at the earlier of: (1) the date at which the maximum offering amount has been sold, (2) one year from the qualification of this circular or ______, 2023, or (3) the date at which the Offering is earlier terminated by the Company at its sole discretion. Funds will be deposited into a segregated account maintained at Dalmore, who acts as the funds collection agent for the Offering. Dalmore is an online platform administering this Offering for the benefit of the Company. The Offering is being conducted on a best-efforts basis with the targeted maximum offering amount (the “Maximum Offering Amount”) of $15,000,000. There is no minimum offering amount in this Offering. The Company may undertake one or more closings on a rolling basis, and the proceeds of this Offering will not be placed into an escrow account. After each closing, funds tendered by investors will be made available to the Company assuming the Company has accepted the investors’ subscription for the shares. After the initial closing of this offering, we expect to hold closings on at least a monthly basis.
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION DOES NOT PASS UPON THE MERITS OR GIVE ITS APPROVAL OF ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION
GENERALLY, NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(d)(2)(i)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO www.investor.gov.
This offering is inherently risky. See “Risk Factors” on page 2.
Sales of these securities will commence on approximately , 2022.
The Company is following the “Offering Circular” format of disclosure under Regulation A.
The date of this offering circular is , 2022.
TABLE OF CONTENTS
In this offering circular, the terms “Aeris,” “we,” “us, “our” or the “Company” refer to Aeris Biotechnologies, Inc., a Delaware corporation.
Please read this offering circular carefully. It describes our business, our financial condition and results of operations. We have prepared this offering circular so that you will have the information necessary to make an informed investment decision.
You should rely only on the information contained in this offering circular. We have not authorized anyone to provide you with any information other than that contained in this offering circular. We are offering to sell, and seeking offers to buy, the securities covered hereby only in jurisdictions where offers and sales are permitted. The information in this offering circular is accurate only as of the date of this offering circular, regardless of the time of delivery of this offering circular or any sale of the securities covered hereby. Our business, financial condition, results of operations and prospects may have changed since that date.
For investors outside the United States: We have not taken any action that would permit this offering or possession or distribution of this offering circular in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this offering circular must inform themselves about, and observe any restrictions relating to, the offering of the securities covered hereby or the distribution of this offering circular outside the United States.
This offering circular includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We believe that the data obtained from these industry publications and third-party research, surveys and studies are reliable.
THIS OFFERING CIRCULAR MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS, WHICH CONSTITUTE FORWARD LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
i
This summary highlights information contained elsewhere in this offering circular. This summary is not complete and does not contain all of the information that you should consider before investing in our shares of common stock. You should carefully read the entire offering circular, especially concerning the risks associated with the investment in the securities covered by this offering circular discussed under the “Risk Factor” section beginning on page 2.
The Company
Aeris Biotechnologies, Inc. is a health and biomedical technology company based in the United States seeking to provide a novel “green” approach to the control of a key cause of and trigger for asthma, based on the acquisition of multiple awarded patents and an established technology platform.
The Offering
| Securities offered by us | Up to 15,000,000 shares of common stock, par value $0.0001 per share (the “Shares”) | |
| Common Stock outstanding before the Offering | 16,080,500 | |
| Share price | $1.00 per Share | |
| Minimum Investment | 500 Shares for $500 |
Use of Proceeds
Proceeds from this offering will be used to pay our licensor for the rights to the intellectual property underlying our planned products, support establishment of the planned core research and development facility and the progression of development work through to commercialization of our first product offering, and general corporate and operational expenses. See “Use of Proceeds” section of this offering circular.
1
Investing in our Shares involves a high degree of risk. In addition to the other information provided in this offering circular, you should carefully consider the following risk factors in evaluating our business and before purchasing any of our securities. We are subject to a number of risks, including risks that may prevent us from achieving our business objectives or that may adversely affect our business, financial condition, results of operations, cash flows and prospects.
Risks Related to Our Business
We are a development stage company with a limited operating history and no revenue, making it difficult for you to evaluate our business and your investment.
Our operations are subject to all of the risks inherent in the establishment of a new business enterprise, including but not limited to the absence of an operating history, lack of fully-developed or commercialized products, insufficient capital, expected substantial and continual losses for the foreseeable future, limited experience in dealing with regulatory issues, lack of manufacturing, distribution and marketing experience, need to rely on third parties for the development and commercialization of our proposed products, a competitive environment characterized by well-established and well-capitalized competitors and reliance on key personnel.
We may not be successful in carrying out our business objectives. The revenue and income potential of our business and operations are unproven as the lack of operating history makes it difficult to evaluate the future prospects of our business. There is nothing at this time on which to base an assumption that our business operations will prove to be successful or that we will ever be able to operate profitably. Accordingly, we have no track record of successful business activities, strategic decision-making by management, fund-raising ability, and other factors that would allow an investor to assess the likelihood that we will be successful in our business. There is a substantial risk that we will not be successful in fully implementing our business plan, or if initially successful, in thereafter generating material operating revenues or in achieving profitable operations.
Even if we successfully develop and market our proposed products, we may not generate sufficient or sustainable revenue to achieve or sustain profitability, which could cause us to cease operations and cause you to lose all of your investment. Because we are subject to these risks, you may have a difficult time evaluating our business and your investment in our Company.
We are at an early stage of marketing, and we have no sales history.
While our intent is to deliver a commercially viable product by 2025, our efforts may not lead to commercially successful products, for a number of reasons, including that:
| ● | Our products may not be accepted by the individuals or commercial customers; |
| ● | We may not have adequate financial or other resources to complete the development and commercialization of our products; and any products that are sold may not be accepted or may have significant competition in the marketplace; and |
| ● | If sales of our projects are delayed, we may have to raise additional capital or reduce or cease our operations. |
Our ability to continue our operations requires that we raise additional capital and our operations could be curtailed if we are unable to obtain the additional funding as or when needed.
The continued growth of our business, including the development and commercialization of our proposed products, will significantly increase our expenses going forward, regardless of our revenues. As a result, we will likely be required to seek substantial additional funds to continue and fully commercialize our business. Our future capital requirements will depend on many factors, including:
| ● | the cost of developing our proposed products; |
| ● | the costs associated with commercializing our proposed products; |
2
| ● | any change in our development priorities; |
| ● | the revenue generated by sales of our proposed products, if commercialization is reached; |
| ● | the costs associated with expanding our sales and marketing infrastructure for commercialization of our proposed products, if commercialization is reached; |
| ● | any change in our plans regarding the manner in which we choose to commercialize any product in the United States or internationally; |
| ● | the cost of ongoing compliance with regulatory requirements, if any; |
| ● | the costs to develop additional intellectual property; |
| ● | anticipated or unanticipated capital expenditures; and |
| ● | unanticipated general and administrative expenses. |
We may not be able to raise additional capital on terms acceptable to us, or at all. Any failure to raise additional capital could compromise our ability to execute on our business plan, and we may be forced to liquidate our assets. In such a scenario, the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements.
If we issue equity or debt securities to raise additional funds, our existing stockholders may experience dilution, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, if we raise additional funds through collaborations, licensing, joint ventures, strategic alliances, partnership arrangements or other similar arrangements, it may be necessary to relinquish valuable rights to our potential future products or proprietary technologies, or grant licenses on terms that are not favorable to us.
We may never complete the development of the any of our proposed products into marketable products.
We do not know when or whether we will successfully complete the development and commercialization of the “Aeris-Shield,” our planned biological treatment kit to address a key cause of asthma, or any other proposed or contemplated product, for any of our target markets. We continue to seek to improve our technologies before we are able to produce a commercially viable product. Failure to improve on any of our technologies could delay or prevent their successful development for any of our target markets.
Developing any technology into a marketable product is a risky, time consuming and expensive process. You should anticipate that we will encounter setbacks, discrepancies requiring time consuming and costly redesigns and changes and that there is the possibility of outright failure.
We may not meet our product development and commercialization milestones.
We have established milestones, based upon our expectations regarding our technologies at that time, which we use to assess our progress toward developing our products. These milestones relate to technology and design improvements as well as to dates for achieving development goals. If our products exhibit technical defects or are unable to meet cost or performance goals, our commercialization schedule could be delayed and potential purchasers of our initial commercial products may decline to purchase such products or may opt to pursue alternative products.
Generally, we have made technological advances meeting our milestone schedules. We can give no assurance that our commercialization schedule will continue to be met as we further develop the Aeris-Shield or any of our other proposed products.
3
Customers will be unlikely to buy any of our proposed products unless we can demonstrate that they can be produced for sale to consumers at attractive prices.
To date, we have focused primarily on research and development of the first generation version of the Aeris-Shield. Consequently, we have no experience in manufacturing these products on a commercial basis. We may manufacture our products through third-party manufacturers. We can offer no assurance that either we or our manufacturing partners will develop efficient, automated, low-cost manufacturing capabilities and processes to meet the quality, price, engineering, design and production standards or production volumes required to successfully mass market our products. Even if we or our manufacturing partners are successful in developing such manufacturing capability and processes, we do not know whether we or they will be timely in meeting our product commercialization schedule or the production and delivery requirements of potential customers. A failure to develop such manufacturing processes and capabilities could have a material adverse effect on our business and financial results.
The proposed price of our products is expected to be in part dependent on material and other manufacturing costs. We are unable to offer any assurance that either we or a manufacturing partner will be able to reduce costs to a level which will allow production of a competitive product or that any product produced using lower cost materials and manufacturing processes will not suffer from a reduction in performance, reliability and longevity. Furthermore, although we have estimated a pricing structure for our initial product, we can give no assurance that these estimates will be correct in light of any manufacturing process we adopt or distribution channels we use.
Our proposed products may not be accepted in the market.
We cannot be certain that our proposed initial product or any other products we may develop or market will achieve or maintain market acceptance. Market acceptance of our products depends on many factors, including our ability to convince key opinion leaders to provide recommendations regarding our products, convince distributors and customers that our product’s technology is an attractive alternative to alternatives, demonstrate that our products are reliable and supported by us in the field, supply and service sufficient quantities of products directly or through marketing alliances, and price products competitively in light of the current macroeconomic environment, which are becoming increasingly price sensitive.
We are subject to environmental laws and regulations and the risk of environmental liabilities, violations and litigation.
We are subject to numerous U.S. federal, state, local and non-U.S. environmental, health and safety laws and regulations concerning, among other things, the health and safety of our employees, the generation, storage, use and transportation of hazardous materials, emissions or discharges of substances into the environment, and investigation and remediation of hazardous substances or materials at various sites. Our operations involve the use of substances regulated under such laws and regulations, primarily those used in manufacturing processes. If we violate these environmental laws and regulations, we could be fined, criminally charged or otherwise sanctioned by regulators.
In addition, certain environmental laws assess liability on current or previous owners or operators of real property for the costs of investigation, removal or remediation of hazardous substances or materials at their properties or at properties which they have disposed of hazardous substances. Liability for investigative, removal and remedial costs under certain U.S. federal and state laws are retroactive, strict and joint and several. In addition to cleanup actions brought by governmental authorities, private parties could bring personal injury or other claims due to the presence of, or exposure to, hazardous substances. The ultimate cost of site cleanup and timing of future cash outflows is difficult to predict, given the uncertainties regarding the extent of the required cleanup, the interpretation of applicable laws and regulations, and alternative cleanup methods.
We may in the future be subject to additional environmental claims for personal injury or cleanup based on our past, present or future business activities (including the past activities of companies we may acquire). The costs of complying with current or future environmental protection and health and safety laws and regulations, or liabilities arising from past or future releases of, or exposures to, hazardous substances, may exceed our estimates, or have a material adverse effect on the financial condition of our business and our business operations.
4
Quality problems with, and product liability claims in connection with our proposed products could lead to recalls or safety alerts, harm to our reputation, or adverse verdicts or costly settlements, and could have a material adverse effect on our financial condition and business operations.
Quality is extremely important to us due to the serious and costly consequences of product failure and our business exposes us to potential product liability risks. Failures, defects, flaws, off-label use, or inadequate disclosure of product-related risks or product-related information with respect to our proposed products, could result in an unsafe condition or injury to, or death of, a patient or other user of our products. These problems could lead to the recall of, or issuance of a safety alert relating to, our proposed products, and could result in unfavorable judicial decisions or settlements arising out of product liability claims and lawsuits, including class actions, which could negatively affect our financial condition and business operations. In particular, a material adverse event involving one of our products could result in reduced market acceptance and demand for all products offered under our brand, and could harm our reputation and ability to market products in the future.
High quality products are critical to the success of our business. If we fail to meet the high standards we set for ourselves and which our customers expect, and our products are the subject of recalls, safety alerts, or other material adverse events, our reputation could be damaged, we could lose customers, and our revenue and results of operations could decline. In certain situations, we may undertake a voluntary recall of products or temporarily shut down product production lines if we determine, based on performance relative to our own internal safety and quality monitoring and testing data, that we have or may be in danger of failing to meet the high quality standards we have set for ourselves and which our customers expect. Such recalls or cessation of services or product manufacturing may also negatively impact our business.
Any product liability claim brought against us, with or without merit, could be costly to defend and resolve. Any of the foregoing problems, including product liability claims or product recalls in the future, regardless of their ultimate outcome, could harm our reputation and have a material adverse effect on our financial condition and business operations.
We are substantially dependent on patent and other proprietary rights and failing to protect such rights or to be successful in litigation related to our rights or the rights of others may result in our payment of significant monetary damages and/or royalty payments, negatively impact our ability to sell current or future proposed products, or prohibit us from enforcing our patent and other proprietary rights against others.
We are and will continue to be materially dependent on intellectual property protections, such as patents, trade secrets, trademarks, and/or non-disclosure and non-competition agreements, which are expected to enable us to maintain our proprietary competitiveness. Patent litigation against us can result in significant damage awards and injunctions that could prevent our manufacture and sale of affected proposed products or require us to pay significant royalties in order to continue to manufacture or sell affected proposed products. At any given time, we could potentially be involved as a plaintiff and/or as a defendant in a number of patent infringement and/or other contractual or intellectual property related actions, the outcomes of which may not be known for prolonged periods of time. While it is not possible to predict the outcome of such litigation, we acknowledge the possibility that any such litigation could result in our payment of significant monetary damages and/or royalty payments, negatively impact our ability to sell current or future proposed products, or prohibit us from enforcing our patent and proprietary rights against others, which would have a material adverse effect on the financial condition of our business and on our business operations.
In addition, the laws of certain countries in which we market, or intend to market, some or all of our proposed products do not protect our intellectual property rights to the same extent as the laws of the U.S., which could make it easier for competitors to capture market position in such countries by utilizing technologies and other intellectual property that are similar to those developed or licensed by us. Competitors may also harm our sales by designing products or offering services that mirror the capabilities of our proposed products, or the technology contained therein, without infringing our intellectual property rights. If we are unable to protect our intellectual property in these countries, it could have a material adverse effect on our financial condition and business operations.
5
The ability to commercialize and offer our proposed products, and the continuing development of proposed products, depends upon us maintaining strong relationships with our network of collaborators.
If we fail to maintain our working relationships with key professions and organizations, we may have more limited access to developing technology and research. The research, development, marketing, and sales of our proposed products is expected to be dependent upon our maintaining working relationships with such professionals, and the use of our proposed products is expected to often require the participation of these key collaborators. If we are unable to maintain our relationships with these professionals, our proposed products may not be utilized correctly or to their full potential, and our ability to develop, manufacture, and market future proposed products may be significantly stunted.
Laws and regulations governing the export of our proposed products could adversely impact our business.
The U.S. Department of the Treasury’s Office of Foreign Assets Control and the Bureau of Industry and Security at the U.S. Department of Commerce administer certain laws and regulations that restrict U.S. persons and, in some instances, non-U.S. persons, in conducting activities, transacting business with or making investments in certain countries, governments, entities and individuals subject to U.S. economic sanctions. Due to our planned international operations, we expect to be subject to such laws and regulations, which are complex, could restrict our business dealings with certain countries and individuals, and are constantly changing. Further restrictions may be enacted, amended, enforced or interpreted in a manner that adversely impacts our financial condition and business operations.
We operate in a competitive industry and we may be unable to compete effectively.
We expect to compete domestically and internationally in the biomedical and asthma markets. These markets are characterized by fragmentation among treatment approaches such as physical barriers, chemical applications, steam cleaning and vacuuming to control dust mite populations and rapid change resulting from technological advances and scientific discoveries. In the product lines and offered services in which we expect to compete, we face a mixture of competitors ranging from large manufacturers with multiple business lines to small manufacturers that offer a limited selection of niche products. Development by other companies of new or improved products, processes, technologies, or the introduction of reprocessed products or generic versions when our proprietary proposed products lose their patent protection may make our proposed products or proposed products less competitive. In addition, we expect to face competition from providers of alternative medical therapies such as pharmaceutical companies. Competitive factors include product reliability, product performance, product technology, product quality, breadth of product lines, product services, customer support, price, and reimbursement approval from health care insurance providers.
We also face competition for marketing, distribution, and collaborative development agreements, for establishing relationships with health care professionals, medical associations, and academic and research institutions, and for licenses to intellectual property. In addition, academic institutions, governmental agencies and other public and private research organizations also may conduct research, seek patient protection and establish collaborative arrangements for discovery, research, and marketing of products similar to ours. These companies, professionals, and institutions compete with us in recruiting and retaining qualified scientific and management personnel, as well as in acquiring necessary product technologies.
We could be negatively impacted if we are unable to capitalize on research and development spending.
We have and intend to continue to spend a significant amount of time and resources on research and development projects in order to develop and validate new and innovative products. We believe these projects will result in the commercialization of new products and will create additional future sales. However, factors including regulatory delays, safety concerns or patent disputes could delay the introduction or marketing of new products. We may experience an unfavorable impact on our financial condition and business operations if we are unable to capitalize on those efforts by attaining the proper approval or to successfully market new products.
We may be unable to attract and retain key employees.
Our executive, technical, scientific and other key personnel play an integral role in the development, commercialization and selling of our proposed products. If we are unable to recruit, hire, develop and retain a talented, competitive work force, we may not be able to meet our strategic business objectives.
6
Risks Related to the Investment in our Securities
The Offering price has been arbitrarily set by the Company.
We have set the offering price of the Shares at $1.00 per Share. Valuations for companies such as Aeris are purely speculative. The company’s valuation has not been validated by any independent third party and may fall precipitously. It is a question of whether you, the investor, are willing to pay this price for a percentage ownership of a start-up company. You should not invest if you disagree with this valuation.
There is no minimum Offering amount required as a condition to a first closing and using the funds raised in this Offering.
Because this is a “best efforts” offering with no Offering minimum, we will have access to any funds tendered. This means that any investment made could be the only investment in this Offering, leaving the Company without adequate capital to pursue its business plan or even to cover the expenses of this Offering.
There is no current market for our common stock.
There is no formal marketplace for the resale of our securities. Shares of our common stock may eventually be traded to the extent any demand and/or trading platform(s) exists. However, there is no guarantee there will be demand for the Shares, or a trading platform that allows you to sell them. Investors should assume that they may not be able to liquidate their investment or pledge their shares as collateral for some time.
Concentration of ownership of our common stock among our existing executive officers, directors and principal stockholders may prevent new investors from influencing significant corporate decisions.
Our executive officers, directors, significant employees and their affiliates, in the aggregate, beneficially own approximately 96% of our outstanding common stock as of April 25, 2022. As a result, these persons, acting together, would be able to significantly influence all matters requiring stockholder approval, including the election and removal of directors, any merger, consolidation, sale of all or substantially all of our assets, or other significant corporate transactions.
Some of these persons or entities may have interests different than yours. For example, they may be more interested in selling our company to an acquirer than other investors, or they may want us to pursue strategies that deviate from the interests of other stockholders.
We intend to issue more shares to raise capital, which will result in substantial dilution.
Our certificate of incorporation authorizes the issuance of a maximum of 50,000,000 shares of common stock. Any additional financings effected by us may result in the issuance of additional securities without stockholder approval and the substantial dilution in the percentage of common stock held by our then existing stockholders. Moreover, the securities issued in any such transaction may be valued on an arbitrary or non-arm’s-length basis by our management, resulting in an additional reduction in the percentage of common stock held by our current stockholders on an as converted, fully-diluted basis. Our board of directors has the power to issue any or all of such authorized but unissued shares without stockholder approval. To the extent that additional shares of common stock or other securities convertible into or exchangeable for common stock are issued in connection with a financing, dilution to the interests of our stockholders will occur and the rights of the holder of common stock might be materially and adversely affected.
7
As of the date of this Offering Statement, an aggregate of 16,080,500 shares of common stock are issued and outstanding.
If you purchase Shares in this offering, your ownership interest in our Common Stock will be diluted immediately, to the extent of the difference between the offering price for each Share in this Offering and the net tangible book value per share of our common stock after this offering.
Our net tangible book value as of October 31, 2021 was $(71,306), or $(0.0046) per share, based on 15,670,500 shares of common stock outstanding. Net tangible book value per share equals the amount of our total tangible assets less total liabilities, divided by the total number of shares of common stock outstanding, all as of the date specified.
If the Maximum Offering Amount, at an offering price of $1.00 per Share, is sold in this offering, after deducting approximately $1,670,600 at most in offering expenses payable by us, our pro forma as adjusted net tangible book value at October 31, 2021 would be approximately $13,224,852, or $0.4312 per share. This amount represents an immediate increase in pro forma net tangible book value of $0.4358 per share to our existing shareholders as of the date of this Offering Circular, and an immediate dilution in pro forma net tangible book value of approximately $0.5688 per share to new investors purchasing Shares.
The following table illustrates the approximate per share dilution to new investors discussed above, assuming the sale of, respectively, 100%, 50% and 25% of the Shares offered for sale in this Offering (after deducting our estimated offering expenses in various scenarios):
| Funding Level | $ | 15,000,000 | $ | 7,500,000 | $ | 3,750,000 | ||||||
| Net proceeds (after deducting the estimated offering expenses) | 13,329,400 | 6,202,820 | 2,937,950 | |||||||||
| Offering price per Share | 1.00 | 1.00 | 1.00 | |||||||||
| Net tangible book value per share before the Offering | (0.0044 | ) | (0.0044 | ) | (0.0044 | ) | ||||||
| Increase per share attributable to investment in this Offering | 0.4302 | 0.2653 | 0.1516 | |||||||||
| Pro forma net tangible book value per share after the Offering | 0.4258 | 0.2609 | 0.1472 | |||||||||
| Dilution to investors after the Offering | 0.5742 | 0.7391 | 0.8528 |
The following tables set forth, assuming the sale of, respectively, 100%, 50% and 25% of the Shares offered for sale in this Offering, the total number of shares previously sold to existing shareholders during the twelve months prior to the date of this Circular, including shares issued for services, the total consideration paid for the foregoing (based on cash actually received and the value of shares issued for services), and the respective percentages applicable to such purchased shares and consideration paid based on an average price of $0.0001 per share paid by our existing shareholders or as the value of shares issued for services, and $1.00 per Share paid by investors in this offering.
| Shares Purchased | Total Consideration | |||||||||||||||
| Number | Percentage | Amount | Percentage | |||||||||||||
| Assuming 100% of Shares Sold: | ||||||||||||||||
| Existing Shareholders | 16.080,500 | 51.74 | % | $ | 1,608 | 0.01 | % | |||||||||
| New Investors | 15,000,000 | 48.26 | % | $ | 15,000,000 | 99.99 | % | |||||||||
| Total | 31,080,500 | 100.00 | % | $ | 15,001,608 | 100.00 | % | |||||||||
| Shares Purchased | Total Consideration | |||||||||||||||
| Number | Percentage | Amount | Percentage | |||||||||||||
| Assuming 50% of Shares Sold: | ||||||||||||||||
| Existing Shareholders | 16,080,500 | 68.19 | % | $ | 1,608 | 0.02 | % | |||||||||
| New Investors | 7,500,000 | 31.81 | % | $ | 7,500,000 | 99.98 | % | |||||||||
| Total | 23,580,500 | 100.00 | % | $ | 7,501,608 | 100.00 | % | |||||||||
| Shares Purchased | Total Consideration | |||||||||||||||
| Number | Percentage | Amount | Percentage | |||||||||||||
| Assuming 25% of Shares Sold: | ||||||||||||||||
| Existing Shareholders | 16,080,500 | 81.09 | % | $ | 1,608 | 0.04 | % | |||||||||
| New Investors | 3,750,000 | 18.91 | % | $ | 3,750,000 | 99.96 | % | |||||||||
| Total | 19,830,500 | 100.00 | % | $ | 3,751,608 | 100.00 | % | |||||||||
8
Another important way of looking at dilution is the dilution that happens due to future actions by the Company. The investor’s stake in a company could be diluted due to the Company issuing additional shares. In other words, when the Company issues more shares, the percentage of the company that you own will go down, even though the value of the Company may go up. You will own a smaller piece of a larger company. This increase in number of shares outstanding could result from a stock offering (such as an initial public offering, another crowdfunding round, a venture capital round, angel investment), employees exercising stock options, or by conversion of certain instruments (e.g. convertible bonds, preferred shares or warrants) into stock.
If the Company decides to issue more shares, an investor could experience value dilution, with each share being worth less than before, and control dilution, with the total percentage an investor owns being less than before. There may also be earnings dilution, with a reduction in the amount earned per share (though this typically occurs only if the company offers dividends, and most early stage companies are unlikely to offer dividends, preferring to invest any earnings into the company). The tables above do not include 505,500 shares of our common stock issuable to an existing stockholder upon the sale by us of a minimum of $3,000,000 in this offering, and an additional 674,000 shares of our common stock issuable to such stockholder upon a going-public transaction of Aeris.
Dilution might also happen upon conversion of convertible notes into shares. Typically, the terms of convertible notes issued by early-stage companies provide that in the event of another round of financing, the holders of the convertible notes get to convert their notes into equity at a “discount” to the price paid by the new investors, i.e., they get more shares than the new investors would for the same price. Additionally, convertible notes may have a “price cap” on the conversion price, which effectively acts as a share price ceiling. Either way, the holders of the convertible notes get more shares for their money than new investors. In the event that the financing is a “down round” the holders of the convertible notes will dilute existing equity holders, and even more than the new investors do, because they get more shares for their money. Investors should pay careful attention to number of convertible notes that the Company has issued (and may issue in the future), and the terms of those notes. The tables above do not include or take into account the issuance of shares of our common stock that may be issued upon the conversion of outstanding convertible promissory notes.
If you are making an investment expecting to own a certain percentage of the Company or expecting each share to hold a certain amount of value, it’s important to realize how the value of those shares can decrease by actions taken by the Company. Dilution can make drastic changes to the value of each share, ownership percentage, voting control, and earnings per share.
9
The Company is offering a maximum of 15,000,000 Shares on a “best efforts” basis. There is no minimum offering amount in this Offering.
The cash price is $1.00 per Share. We will not issue fractional Shares.
The Company intends to market the Shares in this Offering both through online and offline means. Online marketing may take the form of contacting potential investors through electronic media and posting our offering circular materials on an online investment platform.
The Offering will terminate at the earliest of: (1) the date at which the Maximum Amount has been sold, (2) the date which is one year from this Offering being qualified by the Commission, and (3) the date at which the Offering is earlier terminated by the Company in its sole discretion. Funds will be deposited into a segregated account maintained at Dalmore, which will act as a funds collection agent for the offering. Dalmore is an online platform administering this Offering for the benefit of the Company. The Company will pay Dalmore the fees described below for hosting the Offering materials and be responsible for other expenses due to Dalmore, such as monthly subscription, transaction fees and tranche releases. A copy of the service agreement between the Company and Dalmore is filed herein as Exhibit 6.2.
The Company may undertake one or more closings on an ongoing basis. After each closing, funds tendered by investors will be available to the Company when and if the Company decides to accept the investors’ subscription for the Shares. After the initial closing of this Offering, the Company expects to hold closings on at least a monthly basis.
The proceeds of this Offering will not be placed into an escrow account. As there is no minimum offering, upon the approval of any subscription to this Offering Circular, the Company shall immediately deposit the proceeds into the bank account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds on Page 13.
The Company is offering its securities in all states.
The Company has engaged Dalmore Group, LLC (“Dalmore”), a New York limited liability company and broker-dealer registered with the SEC and a member of FINRA, to act as the broker-dealer of record in connection with this offering, but not for underwriting or placement agent services. Dalmore will:
| ● | Review investor information, including KYC (“Know Your Customer”) data, AML (“Anti Money Laundering”) and other compliance background checks, and provide a recommendation to the company whether or not to accept investor as a customer. |
| ● | Review each investor’s subscription agreement to confirm such investor’s participation in the offering, and provide a determination to the Company whether or not to accept the use of the subscription agreement for the investor’s participation. |
| ● | Contact and/or notify the Company, if needed, to gather additional information or clarification on an investor. |
| ● | Not provide any investment advice nor any investment recommendations to any investor. |
| ● | Keep investor details and data confidential and not disclose to any third-party except as required by regulators or pursuant to the terms of the agreement (e.g. as needed for AML and background checks). |
| ● | Coordinate with third party providers to ensure adequate review and compliance. |
As compensation for the services listed above, the Company has agreed to pay Dalmore $5,000 as a one-time set up fee, plus a commission equal to 1% of the amount raised in the offering to support the offering. In addition, the Company has agreed to engage Dalmore as a consultant to provide ongoing general consulting services relating to the Offering, such as coordination with third party vendors and general guidance with respect to the Offering. The Company will pay a one-time consulting fee of $20,000, which will be due and payable within 30 days after FINRA issues a no-objection letter and the Company receives the SEC Qualification. Assuming that the Maximum Offering Amount is sold, the Company estimates that the total fees the Company will pay to Dalmore will be approximately $175,000.
10
TAX CONSEQUENCES FOR RECIPIENT (INCLUDING FEDERAL, STATE, LOCAL AND FOREIGN INCOME TAX CONSEQUENCES) WITH RESPECT TO THE INVESTMENT BENEFIT PACKAGES ARE THE SOLE RESPONSIBILITY OF THE INVESTOR. INVESTORS MUST CONSULT WITH THEIR OWN PERSONAL ACCOUNTANT(S) AND/OR TAX ADVISOR(S) REGARDING THESE MATTERS.
The Online Platform
The Company has engaged Dalmore to host and administer the Offering of the Shares on its online platform. Dalmore will act as a funds collection agent for the Offering. Dalmore will not directly solicit or communicate with investors with respect to offerings posted on its site, although it does advertise the existence of its platform, which may include identifying issuers listed on the platform. Our offering circular will be furnished to prospective investors in this offering via download 24 hours a day, 7 days a week on a designated website.
Process of Subscribing
You will be required to complete a subscription agreement in order to invest. The subscription agreement includes a representation by the investor to the effect that, if you are not an “accredited investor” as defined under securities law, you are investing an amount that does not exceed the greater of 10% of your annual income or 10% of your net worth (excluding your principal residence).
If you decide to subscribe for the Shares in this Offering, you should complete the following steps:
| 1. | Go to a designated website, and click on the “Offering Circular” button; |
| 2. | After reviewing the Offering Circular, click on the “Invest Now” button; |
| 3. | Complete the online investment form; |
| 4. | Electronically receive, review, execute and deliver to us a subscription agreement. |
| 5. | Deliver funds directly by check, wire, credit card, debit card, or electronic funds transfer via ACH to the specified account; and |
| 6. | Once funds or documentation are received an automated AML check will be performed to verify the identity and status of the investor. |
Any potential investor will have ample time to review the subscription agreement, along with their counsel, prior to making any final investment decision. Dalmore will review all subscription agreements completed by the investor. After Dalmore has completed its review of a subscription agreement for an investment in the Company, the funds may be released from the designated account, provided that the Company has accepted the investment.
If the subscription agreement is not complete or there is other missing or incomplete information, the funds will not be released until the investor provides all required information. Dalmore will generally review all subscription agreements on the same day, but not later than the day after the submission of the subscription agreement.
All funds tendered (by check, wire, credit card, debit card, or electronic funds transfer via ACH to the specified account) by investors will be deposited into a segregated account at Dalmore for the benefit of the Company. The Company has engaged Dalmore to act as a funds collection agent for receipt of funds from investors for this Offering. There is no minimum to disburse funds from the segregated account at Dalmore and the Company will maintain a discretionary schedule for release into its operating accounts. All funds received by wire transfer will be made available immediately while funds transferred by ACH will be restricted for a minimum of three days to clear the banking system prior to deposit into the designated account.
The Company maintains the right to accept or reject subscriptions in whole or in part, for any reason or for no reason, including, but not limited to, in the event that an investor fails to provide all necessary information, even after further requests from the Company, in the event an investor fails to provide requested follow up information to complete background checks or fails background checks, and in the event the Company receives oversubscriptions in excess of the maximum offering amount.
11
In the interest of allowing interested investors as much time as possible to complete the paperwork associated with a subscription, the Company has not set a maximum period of time to decide whether to accept or reject a subscription. If a subscription is rejected, funds will not be accepted by wire transfer or ACH, and payments made by debit card, credit card or check will be returned to subscribers within 30 days of such rejection without deduction or interest. Upon acceptance of a subscription, the Company will send a confirmation of such acceptance to the subscriber.
Dalmore has not investigated the desirability or advisability of investment in the shares nor approved, endorsed or passed upon the merits of purchasing the Shares. Dalmore is not participating as an underwriter and under no circumstance will it solicit any investment in the company, recommend the Company’s securities or provide investment advice to any prospective investor, or make any securities recommendations to investors. Dalmore is not distributing any offering circulars or making any oral representations concerning this offering circular or this offering. Based upon Dalmore’s anticipated limited role in this offering, it has not and will not conduct extensive due diligence of this offering and no investor should rely on the involvement of Dalmore in this offering as any basis for a belief that it has done extensive due diligence. Dalmore does not expressly or impliedly affirm the completeness or accuracy of the offering statement and/or offering circular presented to investors by the Company. All inquiries regarding this offering should be made directly to the Company.
Transfer Agent
We have engaged DealMaker Transfer Agent LLC, a registered transfer agent with the SEC, who will serve as transfer agent to maintain shareholder information on a book-entry basis; there are no set up costs for this service, fees for this service will be limited to secondary market activity, which cannot be quantified at this time.
Upon confirmation that an investor’s funds have cleared, the Company will instruct the Transfer Agent to issue shares to the investor. The Transfer Agent will notify an investor when shares are ready to be issued and the Transfer Agent has set up an account for the investor.
12
The following discussion addresses the use of proceeds from this offering. We currently estimate that, at a per Share price of $1.00, the net proceeds from the sale of the 15,000,000 Shares will be $13,329,400 after deducting the estimated offering expenses of approximately $1,670,600.
We intend to use the net proceeds from this offering for working capital and general corporate purposes. Additional specific uses include:
| 25% | 50% | 75% | 100% | |||||||||||||
| Gross Proceeds | $ | 3,750,000 | $ | 7,500,000 | $ | 11,250,000 | $ | 15,000.000 | ||||||||
| Dalmore (1) | 67,500 | 105,000 | 142,500 | 180,000 | ||||||||||||
| Estimated offering expenses (2) | 744,550 | 1,192,108 | 1,341,390 | 1,490,600 | ||||||||||||
| Estimated net proceeds | $ | 2,937,950 | $ | 6,202,892 | $ | 9,766,110 | $ | 13,329,400 | ||||||||
| Specific use: | ||||||||||||||||
| License Fees to Evolution Limited | $ | 0 | $ | 600,000 | $ | 600,000 | $ | 2,000,000 | ||||||||
| Sales, Marketing and Business Development | 567,818 | 1,151,867 | 1,784,927 | 1,784,927 | ||||||||||||
| IP (Patents) and Legal Services | 179,882 | 366,933 | 402,531 | 402,531 | ||||||||||||
| Research and Development, Product Development | 1,564,894 | 2,114,911 | 3,958,361 | 3,958,361 | ||||||||||||
| Administrative Costs | 518,067 | 940,140 | 2,102,243 | 2,102,243 | ||||||||||||
| Accrued Salary Payments | 54,000 | 118,000 | 182,000 | 246,000 | ||||||||||||
| Working Capital | 53,289 | 911,041 | 736,048 | 2,835,338 |
| (1) | Dalmore Group expenses total an estimated $30,000 up front including FINRA fees plus 1% of Gross Proceeds. |
| (2) | Excluding fees and expenses to Dalmore. |
As of the date of this offering circular, we cannot specify with certainty all of the particular uses of the proceeds from this offering. Pending the use of the net proceeds from this offering as described above, we intend to invest the net proceeds in investment-grade, interest-bearing instruments.
Except with respect to the payment of accrued salaries to our executives as set forth above, no proceeds will be used to compensate, make loans, or otherwise make payments to officers or directors of the issuer or any of its subsidiaries.
The use of the proceeds represents management’s estimates based upon current business and economic conditions. We reserve the right to use of the net proceeds we receive in the offering in any manner we consider to be appropriate. Although we do not contemplate changes in the proposed use of proceeds, to the extent we find that adjustment is required for other uses by reason of existing business conditions, the use of proceeds may be adjusted.
13
Overview
Aeris Biotechnologies is a US-based company developing a novel “green” approach to the control of a key cause of and trigger for asthma, based on the acquisition of multiple awarded patents and an established technology platform.
Asthma is a major health problem with significant unmet need:
| ● | 339 million people worldwide are estimated to suffer from asthma. |
| ● | Approximately 17% are “difficult-to-treat” and require frequent hospitalization. |
| ● | More than 400,000 people every year die as a result of asthma, with a potentially life-threatening asthma attack occurring in the USA every two seconds. |
| ● | Asthma costs, even in 2002, exceeded $85 billion in the US and EU alone, with comparable costs in China; since 2002, asthma rates in the US have increased by 14%. |
| ● | An allergic response to house dust mite allergens is a primary cause and trigger of asthma. |
| ● | Existing control methods are of severely limited efficacy and may involve toxic chemicals. |
| ● | House dust mite control is a fragmented but multibillion dollar global market. |
| ● | An effective control could drive a major reduction in healthcare costs. |
The Aeris approach is based on biological control, building on proven efficacy in agricultural applications, and moving this into high-value biomedical markets.
The first Aeris product line is expected to be a biological control system for house dust mites:
| ● | Our approach is expected to reduce allergen load at the source to limit asthma events. |
| ● | Effective biological control systems amplify at the expense of their target and can treat large areas, including those that are difficult to access, from limited initial applications. |
| ● | The product is expected to be positioned as a biopesticide for domestic use, not as a therapeutic, bypassing the need for expensive and time-consuming clinical trials. |
| ● | Aeris anticipates a favorable commercial and regulatory environment, based on the “green” nature of biological control, with the potential for organic certification. |
| ● | The projected market for domestic sales alone for the product exceeds $2 billion annually just in markets where we currently hold awarded patents. Additional markets would add to this. |
| ● | Hospitality, travel, and commercial markets could further increase potential sales. |
| ● | Additional health-related products are anticipated based on the core platform technology. |
We have access to a unique skillset to progress the development of such technologies:
| ● | Work to date has already identified potential control agents for the house dust mite product. |
| ● | Patents covering the broad use of house dust mite control technology has been granted by the US, UK, European, Swiss, Hong Kong, Japanese and Australian patent offices, providing protection of this uniquely capable technology in major markets worldwide. |
Aeris is intending to deliver a commercially viable first product in 2025, subject to the availability of funds.
14
History
We were initially organized on July 28, 2021 as a Delaware corporation. Our principal executive office is located at 8105 Rasor Boulevard, Suite 129, Plano, TX 75024, and our telephone number is 1-214-436-2986. Our website address is www.aerisbiotech.com. The information on our website is not part of this circular.
The Problem
Asthma is a chronic and increasing problem. It is estimated that 339 million people worldwide suffer from asthma. Of those, approximately one-sixth will have asthma classed as “difficult to treat”, requiring frequent hospitalization. The number of people with asthma is growing at an average of 2.8% per annum. The U.S. Centers for Disease Control reported over 1.6 million visits annually to emergency departments from 2016 to 2018, with asthma as the primary diagnosis. The World Health Organization notes that more than 400,000 people every year will die worldwide as a result of asthma.
The house dust mite is recognized as a key source of the major allergens responsible for both sensitization (developing asthma) and for triggering asthma attacks. Between 50% and 80% of asthmatics who react to airborne material are sensitive to dust mite allergens.
We believe that current house dust mite controls simply do not work. A 2008 Cochrane systematic review of 56 trials concluded that “Chemical and physical methods aimed at reducing exposure to house dust mite allergens cannot be recommended”.
Aeris believes that there is an alternative. Biological control has been extensively proven in agricultural use, where it is the fastest growing approach, as it is both highly specific and environmentally friendly. There are also examples in other industries, including food service and the oil & gas sector. Despite this, it has not yet been commercially developed in high value biomedical applications.
The Aeris Answer
Any pest has its own diseases. These are highly specific and, if used properly, can be an effective approach to controlling their target. For targets from drug-resistant bacteria to crop destroying insects, biological control is seeing increasing use worldwide.
Biological control using natural agents that can replicate as they are needed, amplifying locally from low initial doses, has unique strengths. These include:
| ● | It is well proven in agricultural applications, including uses for crop mites; |
| ● | Low initial dosing amplifies at the expense of its target; |
| ● | Naturally spreads to (even difficult to access) areas around the initial application site; |
| ● | Can exert long lasting control; |
| ● | The control agent has the ability to adapt (or evolve) to counter any development of resistance; |
| ● | Highly specific for target, reducing risks of unwanted effects, with lower toxicity and environmental impact, making this an environmentally friendly approach; |
| ● | Once the target is cleared, the control agent also reduces, but re-application is easy should the target return; |
| ● | There is a favorable regulatory environment, with the US Environmental Protection Agency stating that “Since biopesticides tend to pose fewer risks than conventional pesticides, EPA generally requires much less data to register a biopesticide than to register a conventional pesticide;” |
| ● | This is a “green” approach with potential for organic certification, which we believe will aid uptake. |
Aeris has a unique skillset in the area of developing such approaches.
15
Progression to Market
We currently expect to enter into collaborations or joint ventures with third parties as a go-to market strategy. Examples of potential partners include biopesticide manufacturers, consumer health companies, particularly those that also have divisions dedicated to cleaning products, or companies with an interest in our technological approach. Individual partners with local expertise may be sought for specific markets.
Sales for the domestic sector alone are projected as a maximum of over $4 billion per annum in major markets, with sales of over $2 billion in markets with awarded patent protection. Maximum sales are projected within the lifetime of existing intellectual property. The commercial sector has a potential market for the Aeris-Shield, our first planned product, of over $600 million for the hospitality industry in the U.S., Canada, UK, Western European Union, China, Australia and Japan. It is intended that other future products that the Company is proposing to develop will synergize with the house dust mite product.
Valuation of individual technology streams to be brought to joint venture negotiations reflect established industry comparators which we expect will provide a basis for negotiations. For a biological control technology in the lower value agricultural sector, where margins are lower than in the biomedical fields targeted by Aeris, these ranged from $100 million to $500 million during 2012.
Milestone payments are projected for a proposed joint venture route to market as initiating in the third year of funded operations, with payments projected as $50 million (based on a 50% share of the technology) over three years. A trade sale, if undertaken, would be expected to generate initial revenues considerably higher than this, while reducing future revenue accordingly.
Alongside this, the potential exists for direct marketing through e-commerce enabled websites in approved jurisdictions.
The Strategic Position
We were established to be a long-term technology player focused on acquiring and developing health-related products using biological control methodologies. We plan to continue using a lean-burn funding model to develop multiple products based on the applied use of biological control technologies in areas important to human health. These technologies are expected to be progressed through field trials to maximize value prior to partnering and commercial exploitation. The house dust mite development stream is the first such technology, expected to be followed by additional products, initially through low cost collaborations with expert academic and commercial partners. This low-cost route is based on the development of closely related approaches, and is intended to maximize value creation and to minimize dilution of initial investors.
Technology Development to Date
Aeris has acquired, through a worldwide, exclusive license, technology developed by Evolution Limited, based in the United Kingdom (“Evolution”). Evolution received initial seed funding in 2016 and used this to advance its technology through initial lead identification to the award of multiple patents worldwide. Future developments by the Evolution team are included in the license.
The Technology
Asthma: unmet need in a high value market
Asthma is a chronic and increasing problem. The 2018 Global Asthma Report estimated that 339 million people worldwide suffer from asthma. Of those, approximately one in six will have “difficult to treat” asthma requiring frequent hospitalization. The American Academy of Allergy, Asthma, and Immunology notes that “The number of people with asthma continues to grow. One in 12 people (about 25 million, or 8% of the US population) had asthma in 2009, compared with 1 in 14 (about 20 million, or 7%) in 2001.” Figures from the US Centers for Disease Control are even higher, and report 1.7 million visits to emergency departments with asthma as the primary diagnosis. Asthma prevalence varies, ranging from 5% (e.g., China) to 25% (e.g., Australia) worldwide. The World Health Organization notes that more than 400,000 people every year will die worldwide as a result of asthma.
16
Asthma is a multi-factorial disease that includes a genetic predisposition. While many factors can contribute to symptoms, it is commonly believed that specific allergens can trigger both sensitization and attacks. The house dust mite is recognized as the source of major allergens responsible for both for initial sensitization and the triggering of asthma attacks. The actual cause is proteins present in the feces of dust mites which stimulate a potent allergic response.
Between 50% and 80% of asthmatics who react to airborne material are sensitive to dust mite allergens.
House dust mites are small, approximately 0.3mm in length. One gram of dust can contain 500 mites, while a mattress can hold more than two million. In a carpet, there can be between 1,000 and 10,000 mites per square meter. In the three months of her life, a female house dust mite will lay 25-100 eggs. An average house dust mite will produce 20 feces each day of its life. These adhere to fibers and materials in the environment. Both mites and feces are very difficult to remove, and may generate allergenic dust when such removal is attempted.
Despite the many areas affected, two species are responsible for the vast majority of the problem. These are: Dermatophagoides pteronyssinus and Dermatophagoides farinae. Additional mites are covered by existing awarded and filed patent filings, including Euroglyphus maynei, and Blomia tropicalis, which is a potential target for warmer regions such as China and India.
As well as causing problems in those already suffering from asthma, exposure to house dust mite allergens early in life is also commonly believed to be responsible for sensitizing children. Such sensitization is strongly linked to the future development of asthma. Other conditions linked to house dust mite sensitivity include allergic rhinitis, conjunctivitis, and eczema in human health, as well as related conditions in companion animals.
Since house dust mites require a humid environment, some geographic regions are not as affected as others. However, the main areas which are affected include much of the United States and almost all of western Europe. Billions of people living in these areas are at risk.
In addition, research has shown a similar frequency of sensitization to house dust mite allergens among asthmatics even in very dry environments such as the Persian Gulf States, where even higher asthma-related death rates have been reported, apparently due to humidification and soft furnishings in the indoor environment. At base, when humans make their environment comfortable for themselves, they make it comfortable for house dust mites, extending the range of these persistent pests even to dryer areas.
Asthma costs, even in 2002, exceeded $85 billion in the US and EU alone. Since 2002, asthma rates in the US have increased by 14%. Other areas such as China, Japan, India and Australia will form important targets for the agents being developed by Aeris.
The Aeris house dust mite product has the potential to save lives and improve lives worldwide, while producing a major reduction in asthma-related healthcare costs.
House Dust Mite Control Methods
We believe that control of mites by any of the currently available methods, outlined below, is of strictly limited efficacy. Current approaches only affect areas to which the treatment is directly applied, generally allowing rapid recolonization from untreated nearby areas once the treatment has ended or the active ingredient has dispersed.
Washing or steam cleaning may remove house dust mites but has no long-term preventative effect. Vacuum cleaning is of very limited efficacy and may stir up allergenic dust, as most filters are not able to catch extremely small particles. House dust mites are sensitive to long-term reductions in humidity; however, this is difficult to achieve in domestic environments.
Barrier methods that are intended to block dust mite access to mattresses and bedding are in common use, but are also of limited value. No such barrier method can be used on carpets and other soft furnishings, which are a major source of exposure to allergens, as well as providing for transfer of house dust mites from carpets back to bedding. Despite this, barrier methods are often cited as the best available option, though best used as part of a combination of approaches.
17
Chemical approaches do exist. These include denaturing solutions intended to inactivate allergens or acaricides – a substance poisonous to mites or ticks – intended to kill the mites themselves. The latter include both organophosphates and carbamates. However, use of such chemical pesticides and the persistence of their residues in the domestic environment is problematical. One pesticide used commercially for this purpose in the United Kingdom has been noted as causing “rapid poisoning” of humans. Multiple adverse effects are listed in available toxicology data sheets, and it is noted that asthmatics are at particular risk. One major product in this market was recalled after the U.S. Environmental Protection Agency documented more than 400 cases of adverse side effects among users. Even newer pesticides such as the neonicotinoids have been associated with adverse environmental effects. Additionally, work with other mites, notably storage mites in grain, shows that resistance to chemical pesticides develops rapidly and can become total at acceptable dose levels. Thus, any increased use of chemical acaricides is likely to become compromised even if these are initially effective. Consumer resistance to the use of such pesticides is also a major factor.
We believe that many of the current methods that are in wide use, including chemicals, physical barriers, steam cleaning and vacuuming, do not actually produce reductions large enough to provide any significant clinical effect. The 2008 Cochrane review concluded that “Chemical and physical methods aimed at reducing exposure to house dust mite allergens cannot be recommended.” Despite this and their high cost, in the absence of any better alternative their use is widespread, generating a multi-billion dollar market, supported by manufacturers of the range of products in current use.
A biomedical basis for a reduction in house dust mite populations as a control for asthma is well documented in the scientific literature, although the efficacy of existing controls is controversial. Such a reduction, if it can be achieved, is regarded as beneficial both to established asthmatics and in the prevention of sensitization in children and other potential asthmatics. The latter represents an important target market.
The limitation of individual treatments to specific items or areas can also allow rapid recolonization from nearby untreated areas once treatment regimen is completed. No single current treatment can be used for all infested items, whereas a replicating biological control agent will have the potential to address a broad range of domestic habitats (including hard-to-access areas) from a limited initial application, as demonstrated by agricultural uses.
The potential markets for a novel, proven and environmentally friendly approach are correspondingly large.
Current Methods
Given the fragmentation of the market, accurate estimates of overall spending on house dust mite control measures are difficult to obtain. However, given the costs of existing commercial products it is clear that a multi-billion dollar market exists worldwide. Given the very limited efficacy of existing controls, we believe the Aeris house dust mite product will have the potential to achieve significant market penetration and expansion on the basis of validated efficacy as an acaricide, able to reduce mite numbers and thus allergen production across the domestic environment. It will also benefit from the potential for the product to exert long term control of house dust mites in all domestic locations.
A comparison of “state of the art” approaches to house dust mite control and how the Aeris house dust mite product will go beyond these is described below. Prices are derived from searches of internet-based suppliers for the cited products conducted in February 2021:
18
| Current State of Art | Typical cost | Annual cost per treatment area | Mode of action | Major limitations | Advancement of the Aeris kit beyond current State of the Art |
| Biological
acaricide (Aeris dust mite kit) |
$60-$99 | $60-$396 (whole house) |
Direct killing of mites | Still under development, proprietary technology | First use of biological control for house dust mites |
| Chemical acaricides | $15-$30 | 4x treatments per year: $60-$120 | Direct killing of mites | Limited area of action, toxicities to humans, animals, and to the environment, resistance | Biological agents are able to amplify and spread, have higher specificity and extremely limited toxicity, and can adapt to counter resistance. One Aeris treatment could be equivalent to multiple doses of a chemical acaricide |
| Barrier methods | $30-$100 | Wash frequently, change annually: $30-$100 | Blocking of access for mites and allergens | Usually only protect bedding | Able to exert durable control across multiple dust mite habitats (not just bedding) |
| Steam cleaning | $30-$200 | Low | Direct killing of mites | Can only treat exposed surfaces, very limited penetration, allows immediate recolonization from nearby untreated areas | Does not require elevated temperatures, able to exert durable control across multiple dust mite habitats with far lower levels of user effort. Prevents recolonization |
| Vacuum cleaning | $45-$450 | Filter replacements every 6-24 months: $15-$70 | Removal of mites and allergens | Can only treat accessible surfaces, very limited effect, increases allergen exposure by stirring up dust, allows immediate recolonization from untreated areas | Able to exert control across multiple dust mite habitats and produce durable control with far lower levels of user effort |
| Mite feeding inhibitors | $10-$25 | As denturants | Destroy or denature materials eaten by mites | Limited effects, especially in upholstered materials | Attacks dust mites directly, able to amplify and spread |
| Allergen denaturant | $20-$30 | Repeat original dose every 1-3 months | Denatures mite-produced allergens | Requires frequent re-application, very limited effects | By eliminating the source of the allergen, load is reduced |
| Hot washing (above 60°C) | N/A | Low | Direct killing of mites | Not usable for heat or water sensitive items, allows immediate recolonization from untreated areas | Does not require elevated temperatures, able to exert durable control across most dust mite habitats |
| Anoxic fumigation | Extremely expensive, high-value items only | N/A | Direct killing of mites in treated item only | May require removal of item to specialist facility, lengthy and expensive, allows immediate recolonization | Able to exert control across multiple dust mite habitats and to produce durable control |
19
| Air treatment - dehumidifier | $60-$250 | Low | Reduces humidity in air | Difficult to maintain treated areas at effective levels in the domestic setting | Works regardless of environmental conditions where its target is present |
| Air treatment - filtration | $50-$700 | Filter replacements every 6-24 months: $15-$70 | Removes particulate allergens from air | Difficult to maintain treated areas at effective levels in the domestic setting | Works regardless of environmental conditions where its target is present |
| Ultrasonic emitters | $15-$45 | Low | Claimed to inhibit HDM activity | Very limited data on mode of action or efficacy | Verifiable activity based on validated data |
Given the limitations of existing approaches, and as noted by the World Health Organization and the Cochrane review cited above, a concerted effort to reduce house dust mite infestation must use multiple methods, which increases costs. For example, combined use of bedding protectors (for one bed), chemical acaricides, allergen denaturant, air treatment, and HEPA filtered vacuum cleaning would involve an initial outlay of $220 to $1,560, with ongoing costs for filter replacement and repeat treatments with chemical agents of $65-$200 per cycle. Despite these high costs, we believe that current house dust mite controls simply do not do the job. Although beneficial results have been reported in individual studies, the Cochrane review concluded that “Chemical and physical methods aimed at reducing exposure to house dust mite allergens cannot be recommended”.
A new, effective control method able to control house dust mites in all domestic locations could thus be expected to find a ready market, particularly given increasing concerns over the environmental effects of some existing approaches. The guideline retail price for the Aeris-Shield, our first planned product, is at least $60 for an encapsulated product both facilitating mite consumption and isolating the contents and thus expected to be suitable for use by all asthmatics. Therefore, even before allowing for the unique capabilities of the proposed product, we believe it will be superior in cost for both initial and repeat use.
It is planned that the product will be supplied initially as treatment kit suitable for home use, with a re-usable case, manual, and upholstery injector. Further purchases would then be supplied as a “refill pack” of the packaged active agents, minimizing waste and emphasizing the “green” nature of the product.
A Biological Approach
We believe that biological control provides a proven and environmentally friendlier approach than other alternatives, which has been extensively validated in the agricultural sector. A number of pests and bacterial contaminations are controlled with biological agents, known as biopesticides, and the extension of such methods into the control of infections and infestations of veterinary and medical importance has real potential for the development of commercial products.
Existing agricultural applications provide a technological and regulatory basis for such development. However, in agricultural use, biological control agents are typically limited by many factors present in outdoor settings, including wide dispersal of targets, ultraviolet light, wind, rain and other factors which are of far more limited relevance in the domestic and medical settings, where high target densities are seen in constant, controlled environments, without such limiting issues. We believe that such settings are thus highly suited to biological approaches.
Biological control is highly specific in nature, is perceived as environmentally friendly, and may achieve organic certification. All of these factors are expected to aid in supporting consumer uptake; this is an important element of the approach. The US Environmental Protection Agency states that “Since biopesticides tend to pose fewer risks than conventional pesticides, EPA generally requires much less data to register a biopesticide”. While this currently relates to the agricultural sector, we believe such attitudes are highly supportive of the extension of such technologies to the biomedical sector. An initial meeting our management had with EPA staff has produced encouraging outcomes in support of this belief.
20
Biological agents: adaptable and durable
Biopesticides also have a unique strength. A properly selected replicating biological agent amplifies itself, multiplying at the expense of its target organism and producing high levels of the agent where, and only where, its specific target is present. It will then generate multiple cycles of control. As a result of this ability they are capable of exerting effective control over far longer periods (and with less frequent applications) than is achieved with chemical agents. For example:
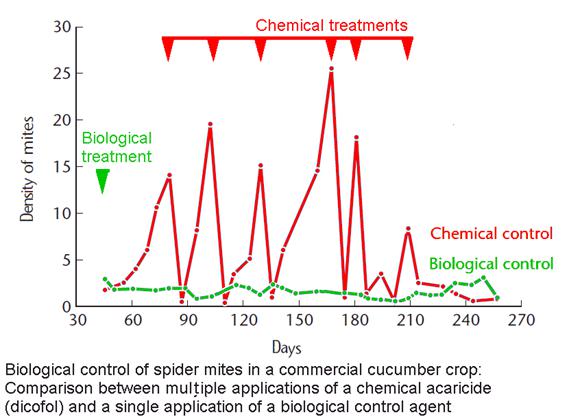
Adapted from Markkula M, Tiittanen K and MieminenM (1972). Annales Agriculturae Fenniae 11: 74–78.
Adaptation and resistance
Resistance to chemical control agents is a major and increasing problem since the chemical itself cannot change. The rapid development of resistance to such agents is a widely observed problem, including uses in storage mite control where such resistance can be complete at achievable pesticide levels. With chemical approaches, resistance then requires the development and approval of entirely new agents, which can then in turn have unexpected and damaging environmental effects (as seen with neonicotinoid insecticides). By comparison, resistance to biological control agents has been closely monitored in agricultural applications, and is believed to be limited. Such agents can themselves adapt (or be adapted) to counter the development of resistance by the target pest should it develop. While chemical pesticides cannot change, a biological agent can change far faster than its much larger target. It does this naturally as part of its adaptation to its environment.
Even if problematic resistance develops, a relatively short process of selective breeding is expected to result in the development of agents able to counter this resistance. Simpler forms of life change more rapidly than more complex forms, and mites, bedbugs, bacteria, or any other target are far more complex than the potential biological control agents under investigation by Aeris. The process of “product stewardship” involving monitoring and countering resistance also represents a continuing development and revenue opportunity. An alternative approach to preventing such (hypothetical) resistance is the simultaneous use of multiple agents, which could also aid in the development of intellectual property. These approaches will be evaluated during development of candidate agents.
The proposed development of the agent does not involve genetic manipulation. The agents to be developed are to be selected from naturally occurring agents which are already present in the environment, aiding both regulatory approval and public acceptance. Organic certification will be sought where appropriate, as has already been achieved with biological control products in other areas.
21
Bioprospecting
The basic approach to be used for the Aeris-Shield is “bioprospecting”: the identification of natural infections with potential for use as biological control (biocontrol) agents by screening environmental samples against the target pest. This has already been proven to be successful in bringing agents to and through trials, and involves the following steps:
Unlike some other approaches, a validated and supportive pathway through registration and on to market exists for biological pesticides. In agricultural applications, biological control is a mature technology with multiple marketed products. It is the expansion of this proven approach into novel areas of biomedical importance that underlies the Aeris technology.
Steps 1 through 6 have already been achieved for the house dust mite in studies to date, with resulting agents, along with the broader technology, protected by awarded patents.
Work conducted in the United Kingdom by Evolution has proven the practicality of the approach and led to multiple patent awards worldwide, all of which are subject to our worldwide exclusive license.
The viability of this approach is further supported by published work with red citrus mites infesting crops and the use of such agents for Varroa mites infesting bee colonies.
This work uses established techniques and procedures, extended and adapted by Aeris scientists collaborating with Evolution scientists under our existing License Agreement. Novel agents identified by such routes can then be formulated, prepared and stabilized for delivery. All stages provide potential for the generation of additional intellectual property and proprietary know-how.
Markets
Three potential markets are projected for the house dust mite product:
| 1. | “Difficult to treat” asthmatics (17% of treated asthmatics, according to Global Asthma Report, 2018), who are likely to provide a receptive market for an effective acaricide, of which 17 million individuals are residents in intellectual property-protected and target markets. We project 25% of this group using premium product at 4 room kits per year. |
| 2. | Other asthmatics (the remaining 83%), who may use an acaricide somewhat less (15% uptake is assumed, with less frequent use) in a preventive way or during more severe episodes: 82 million individuals in intellectual property-protected and target markets. We project 15% of this group using product at 2 room kits per year. |
| 3. | Concerned parents and others seeking to use the product in a preventive way, since sensitization to house dust mites is frequently reported to be related to the future development of asthma. Less frequent use is assumed below, along with much lower uptake (1% of the general population): 1,215 million individuals in intellectual property-protected and target markets. We project 1% of this group using product at 1 room kit per year. |
22
These projections do not include secondary markets, or travel kits, commercial or use by the hospitality industry, landlords, hospitals and health care facilities, or employers, which also are large potential markets.
Protected markets, where patent protection is awarded, are the U.S., UK, European Union, Japan, Hong Kong, and Australia, shown in green, above. Expected initial expansion markets are China (assuming 35% of the population in China as the target market based on income levels), India (uptake in India is assumed to be restricted to 20% of the total population, again based on income levels) and other areas as shown in orange. Additional areas could provide further potential markets and income.
Product sales into mature protected markets are projected as $2.35 billion per annum based on the assumptions given below, with the initially targeted expansion markets adding a further $1.89 billion.
Commercial markets are not included in this figure, which is based only on domestic use. Projections for commercial markets indicate potential sales of approximately $600 million per annum for the hospitality industry alone. Additional markets exist for other conditions, including allergic rhinitis, conjunctivitis, and eczema, as well as related conditions in companion animals.
Intellectual Property
Protection of our intellectual property is a strategic priority for our business. We rely or intend to rely on a combination of patents, license agreements, trademarks, copyrights, trade secrets as well as nondisclosure and assignment of invention agreements, material transfer agreements, confidentiality agreements and other measures to protect our intellectual property and other proprietary rights.
Patents are significant to our business to the extent that a product or an attribute of a product represents a unique design or process. Patent protection restricts competitors from duplicating unique designs and features. To protect our proprietary secrets and competitive technologies, we have obtained and are seeking to further obtain patent, trade secret, trademark and other intellectual property protection on our proposed products whenever appropriate.
The Aeris technology is based on that developed by Evolution in the United Kingdom. All patents (awarded and in process) and future biological control technologies developed by Evolution are licensed to Aeris pursuant to a worldwide exclusive license agreement, and the CEO and founder of Evolution is expected to head up the research team as Chief Scientific Officer and Chairman of Aeris.
23
The general approach to the generation of patent filings, is to maintain confidentiality of commercial information until such point as candidate commercial products are developed, and then to file for patent protection around such candidates, as well as for technologies developed later in the product stream. However, the novelty and inventive nature of the approach has allowed for earlier filing of wide-ranging patent filings protecting the broad use of any replicating biological agent or agents to target house dust mites.
Patents deriving from WO2017025732A1 “Acaricides”, have now been awarded in multiple jurisdictions (with a divisional application covering composition of matter in progress in Europe) including European Patent Office #3331366, Swiss and Liechtenstein #3331366, Hong Kong Patents Registry #18111847.2, Japan Patent Office #2018-525822, and Australian Patent Office #AU 2016305553. In 2022, notice of allowance has been authorized by the United Stated Patent and Trademark Office (USPTO; details awaited) for all fungal agents, with divisional applications for viral and bacterial agents planned.
The international (PCT) stage search and examination, conducted by the European Patent Office (EPO), confirmed the broad claim to a method of treating or preventing a house dust mite infestation using an acaricidal infectious agent as both novel and inventive. This is a key step in obtaining broad protection of technologies for controlling house dust mites, a major cause of asthma and other allergic conditions in both humans and companion animals in markets around the world. Additional applications are planned, to be based on the progression of specified agents through testing
The awarded patents, with broad claims covering the technology as a whole provides control in the protected markets until 2036. Additional markets are being evaluated.
Aeris has exclusive rights to this proven technological approach in large and expanding markets, worldwide.
The first claim of the awarded European patent protects the wide area of “A method of treating or preventing a house dust mite infestation, said method comprising applying an acaricidal infectious agent at a site of a house dust mite infestation, or at a site proximal thereto, or at a site at risk of such infestation”. It should be noted that this protects all house dust mites, including those native to the tropics (e.g., Blomia tropicalis) as well as the Dermatophagoides and other dust mites.
License Agreement with Evolution
Aeris entered into a Worldwide Exclusive License Agreement, dated October 26, 2021, with Evolution. Pursuant to the License Agreement, Evolution granted to Aeris an exclusive, unlimited license to all of Evolution’s right, title and interest, past, present and future, in and to awarded patents and its ongoing patent applications, and all intellectual property rights to such patents and patent applications, for use anywhere in the world. In return, Aeris is obligated to pay to Evolution $2,000,000, of which $600,000 shall be due and payable upon Aeris raising at least $3,000,000 in this offering, and the remainder due and payable within 30 days of Aeris entering into a going public transaction. In addition, Aeris shall be responsible for the ongoing funding of research activities as agreed to by Aeris and Evolution.
Future Projects
Once the house dust mite control product is in field trials, the technology platform used for house dust mite control is expected to be extended to identify candidate control agents for other target organisms, such as the bed bug, subject to available funding. Although not a major health issue, bed bugs represent a widespread and significant concern that affects many people, both where they live and in hotels and residential health-care facilities. It is also a market that is “adjacent” to the house dust mite market with an estimated market size of $900 million, initially targeting the hospitality industry. Domestic sales would add to this. Such a product could also help to leverage uptake of the house dust mite product into the hospitality and other commercial settings.
The product roadmap includes other potential products related to human health that are based around biological control technologies.
24
Research and Development
Our research and development programs are expected to be generally pursued by engineers and scientists employed by us on a full or part-time basis or hired as per diem consultants or through partnerships with industry leaders in manufacturing and design and researchers and academia.
The primary objective of our research and development program is to advance the development of our proposed products, to enhance the commercial value of such proposed products.
We have not incurred any research and development costs for the fiscal year ended October 31, 2021, as all such expenses had been incurred at Evolution. We expect to incur substantial research and development expenses during the fiscal year ending October 31, 2022 to perfect and commercialize our first product, and as required pursuant to the terms of our license agreement with Evolution.
Employees
As of April 25, 2022, we had 2 full-time employees and 1 part-time employee, none of whom are represented by a labor union or covered by a collective bargaining agreement. This does not include employees of Evolution, including Dr. Harper, who we expect will work on research and development matters on our behalf pursuant to the License Agreement with Evolution. We expect that additional staff will be recruited to fill out and support the team as the work progresses. We consider our relationship with our employees to be satisfactory.
Key to the recruitment of highly skilled staff are the existing contacts of both the management team, scientific collaborators, and the appointment of skilled recruits to the development team. This permits cost-effective identification of suitable staff members as noted below.
Research and development activities are expected to be undertaken at an Evolution facility in the UK that is funded by the Company, with a subsidiary research team to be based in the USA and another expected in China for environmental sampling and, potentially, product manufacture. International collaborators have been identified to provide environmental samples. We expect that employees of Evolution, including Dr. Harper, of whom we expect will work on research and development matters on our behalf pursuant to the License Agreement with Evolution, will be paid fully or partially through Evolution.
The house dust mite development stream is expected to employ seven scientific staff to be based at the UK facility, with additional input from third-party collaborators. Experienced microbiologists and entomologists have been interviewed for all planned posts. An experienced outsourcing manager is already in place.
Once the house dust mite product is finalized and in field trials, it is anticipated that appointed staff will transition to other projects as resources permit, with such transition expected to commence in 2024 as funds allow.
Legal Proceedings
As of the date of this circular, we are not party to, and our property is the subject of, any material legal proceedings.
25
Our principal executive office is located in leased premises of approximately 132 square feet at a rental cost of $850.00 per month at 8105 Rasor Boulevard, Suite 129, Plano, TX 75024. We believe that these facilities are adequate for our needs, including providing the space and infrastructure to accommodate our development work based on our current operating plan. We do not own any real estate.
26
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of financial condition and results of operations of the Company together with our financial statements and the related notes included elsewhere in this circular. Some of the information contained in this discussion and analysis or set forth elsewhere in this circular, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks and uncertainties. You should review the “Risk Factors” section of this circular for a discussion of important factors that could cause actual results to differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Forward Looking Statements
The following discussion should be read in conjunction with our financial statements and related notes included in this circular. Certain information contained in this MD&A includes “forward-looking statements.” Statements which are not historical reflect our current expectations and projections about our future results, performance, liquidity, financial condition and results of operations, prospects and opportunities and are based upon information currently available to us and our management and their interpretation of what is believed to be significant factors affecting our existing and proposed business, including many assumptions regarding future events. Actual results, performance, liquidity, financial condition and results of operations, prospects and opportunities could differ materially and perhaps substantially from those expressed in, or implied by, these forward-looking statements as a result of various risks, uncertainties and other factors, including those risks described in detail in the section entitled “Risk Factors” of this circular.
Forward-looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “should,” “would,” “will,” “could,” “scheduled,” “expect,” “anticipate,” “estimate,” “believe,” “intend,” “seek,” or “project” or the negative of these words or other variations on these words or comparable terminology.
In light of these risks and uncertainties, and especially given the nature of our existing and proposed business, there can be no assurance that the forward-looking statements contained in this section and elsewhere in this circular will in fact occur. Potential investors should not place undue reliance on any forward-looking statements. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
Overview
Aeris Biotechnologies, Inc. is a health and biomedical technology company based in the United States seeking to provide a novel “green” approach to the control of a key cause of and trigger for asthma, based on the acquisition of multiple awarded patents and an established technology platform.
We have very limited resources. To date, our primary activities have been limited to, and our limited resources have been dedicated to, performing business and financial planning, raising capital, recruiting personnel, negotiating with business partners and the licensors of our intellectual property and conducting development activities. Our first proposed product is not expected to be ready for commercialization and sale until 2025 at the earliest.
We have incurred losses since inception in 2021 and had an accumulated deficit of $72,914 as of October 31, 2021. We expect to continue to incur significant expenses and increasing operating and net losses for the foreseeable future.
Historically, our primary source of cash has been proceeds from the sale of convertible promissory notes and other borrowings. To fund our operations, from inception (July 28, 2021) to October 31, 2021, we issued convertible promissory notes for gross proceeds of $50,000. Since that date, we have issued convertible promissory notes for aggregate gross proceeds of $86,800.
We need to obtain substantial additional funding in connection with our continuing operations through public or private equity or debt financings or other sources, which may include collaborations with third parties. However, we may be unable to raise additional funds when needed on favorable terms or at all. Our failure to raise such capital as and when needed would have a negative impact on our financial condition and our ability to develop and commercialize our products and future products and our ability to pursue our business strategy. See “–Liquidity and Capital Requirements” below.
27
Financial Overview
Revenue
We have not generated any revenue to date, and do not expect to generate revenue unless or until we successfully commercialize our products.
General and Administrative
General and administrative expenses consist primarily of personnel-related costs for personnel in functions not directly associated with research and development activities. Other significant costs include legal fees relating to corporate matters, intellectual property costs, professional fees for consultants assisting with regulatory, product development and financial matters, and product costs. We anticipate that our general and administrative expenses will significantly increase in the future to support our continued research and development activities, commercialization of our products, if approved, and the increased costs of ongoing securities law compliance. These increases will include increased costs related to the hiring of additional personnel and fees for legal and professional services, as well as other related costs.
Research and Development
Research and development expenses consist of expenses incurred in performing research and development activities in developing our products. Research and development expenses include compensation and benefits for research and development employees, overhead expenses, cost of laboratory supplies, clinical trial and related clinical manufacturing expenses, costs related to regulatory operations, fees paid to consultants, and other outside expenses. Research and development costs are expensed as incurred and costs incurred by third parties are expensed as the contracted work is performed.
We expect our research and development expenses to increase as we continue to develop and commercialize our products.
Interest Expense
Interest expense primarily consists of interest costs related to the convertible notes we issued to investors. The convertible notes bear interest at 10% per annum.
Results of Operations
Inception (July 28, 2021) Through October 31, 2021
General and administrative expenses
General and administrative expenses were $5,200 for the period, relating primarily to consulting fees. In addition, we accrued compensation equal to $65,685.
Interest expense
Interest expense was $548 related to our convertible promissory notes totaling $50,000.
Liquidity and Capital Resources
We anticipate that we will continue to incur losses for the foreseeable future, as we do not expect to generate revenue until 2025 at the earliest, which is when we expect to launch our first product. We anticipate that our expenses will increase substantially as we develop our products and pursue testing and trials, seek any further regulatory approvals, contract to manufacture any products, establish our own sales, marketing and distribution infrastructure to commercialize our products, hire additional staff, add operational, financial and management systems and operate.
28
Our primary source of cash since inception has been proceeds from the sale of convertible promissory notes and related party loans. We have also issued shares of our common stock to individuals and entities as payment for services rendered to us in lieu of cash, and expect to continue to do so in the future. For instance, we are obligated to issue 505,500 shares of our common stock to an existing stockholder for services rendered upon the sale by us of a minimum of $3,000,000 in this offering, and an additional 674,000 shares of our common stock to such stockholder upon a going-public transaction of Aeris.
All of our outstanding convertible promissory notes, in the aggregate principal amount through April 25, 2022 of $86,800, are expected to convert into shares of our common stock upon the closing of at least $1,000,000 in proceeds from this Offering at a 50% discount.
Until such time, if ever, as we can generate revenue, we expect to finance our cash needs through a combination of equity and debt financings as well as collaborations, strategic alliances and licensing arrangements. We do not have any committed external source of funds. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances or licensing arrangements with third-party partners, we may have to relinquish valuable rights to our technologies, future revenue streams or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or through collaborations, strategic alliances or licensing arrangements when needed, we may be required to delay, limit, reduce or terminate our product development, future commercialization efforts, or grant rights to develop and market our intellectual property that we would otherwise prefer to develop and market ourselves.
In management’s opinion, we expect that the proceeds from this offering, assuming we raise the maximum amount of $15,000,000, will satisfy our cash requirements through our 2025 launch of our first proposed product. In the event we raise substantially less than that, we expect we will be required to raise additional funds prior to commercialization to implement our plan of operations.
Our independent registered public accounting firm included an explanatory paragraph in its report on our financial statements as of and for the period from inception (July 28, 2021) to October 31, 2021, noting the existence of substantial doubt about our ability to continue as a going concern. This uncertainty arose from management’s review of our results of operations and financial condition and its conclusion that, based on our operating plans, we did not have sufficient existing working capital to sustain operations for a period of twelve months from the date of the issuance of these financial statements.
The development of our products is subject to numerous uncertainties, and we have based these estimates on assumptions that may prove to be substantially different than we currently anticipate and could use our cash resources sooner than we expect. Additionally, the process of developing our proposed products is costly, and the timing of progress in tests and trials is uncertain. Our ability to successfully transition to profitability will be dependent upon achieving a level of product sales adequate to support our cost structure. We cannot assure you that we will ever be profitable or generate positive cash flow from operating activities.
Net cash used for operating activities
Net cash used for operating activities was $16,758 for the period from inception (July 28, 2021) to October 31, 2021, primarily due to the net loss of approximately $72,991 primarily offset by an increase in stock-based compensation of $1,685 and accounts payable and accrued expenses of $54,000.
Net cash provided by financing activities
Net cash provided by financing activities was $50,000 for the period from inception (July 28, 2021) to October 31, 2021, which consisted of the sale of the Company’s convertible promissory notes for aggregate gross proceeds of $50,000.
29
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
The following table sets forth our directors.
| Name | Position | Age | Term of Office | Approximate hours per week for part-time employees | ||||
| Aaron Michael Gunn | Director | 52 | Since July 2021 | Full Time | ||||
| David Richard Harper | Chairman | 61 | Since July 2021 | Full Time (1) |
| (1) | Dr. Harper’s day-to-day services to the Company will be provided through his positions at Evolution, pursuant to the License Agreement. |
The following table sets forth our executive officers.
| Name | Position | Age | Term of Office | Approximate hours per week for part-time employees | ||||
| Aaron Michael Gunn | President | 52 | Since July 2021 | Full Time | ||||
David Richard Harper |
Sr. Vice President, Chief Scientific Officer | 61 | Since July 2021 | Full Time (1) | ||||
Edward Charles Cappabianca |
Vice President, Chief Business Officer | 57 | Since July 2021 | 20 Hours | ||||
Steven Albert Portnoy |
Vice President, General Counsel and Secretary | 64 | Since July 2021 | Full Time |
| (1) | Dr. Harper’s day-to-day services to the Company will be provided through his positions at Evolution, pursuant to the License Agreement. |
The following tables sets forth our significant employees.
| Name | Position | Age | Term of Office | Approximate hours per week for part-time employees | ||||
| Benjamin Burrowes | Chief of U.S. Scientific Activities | 49 | Since July 2021 | 20 Hours |
The following are the biographies of our directors, executive officers and significant employees.
Aaron Michael Gunn, President and Director. Mr. Gunn, the co-founder, president and a director of Aeris since July 2021, has over twenty years of experience in the foundation and growth of many companies across multiple industries. Since February 2020, he has been the Chief Operating Officer and a director of U.S.-based Evolution Inc. and since March 2015, he has been the Chief Operating Officer and director of U.K.-based Evolution Ltd., each private biotechnology research and development companies. The UK company is the licensor of Aeris’ intellectual property and primary assets. Since 2011, he has been a co-founder and co-owner of Texas Republic Bank. From August 2012 to March 2021, Mr. Gun was the president of Mercury Towers/Bridger Wireless, a specialized wireless communications real estate development company. From 2001 to 2006, Mr. Gunn was the managing member of Elite Wireless LLC and from 2006 to 2011 was the owner and president of Elite Wireless, LP. In addition to wireless ventures, Mr. Gunn has participated in the purchase of multiple private companies in the banking and technology sectors. Throughout his career, Mr. Gunn has used his experience and contacts in both business and governmental agencies to build value in an extensive range of companies. Mr. Gunn received a BA in Economics from Princeton University and an MA in Economics from Texas Tech University.
30
Dr. David Richard Harper, Sr. Vice President, Chief Scientific Officer and Chairman. Dr. David Harper, the co-founder, Senior Vice President, Chief Scientific Officer and Chairman of the Board of Aeris since July 2021, has twenty years’ experience in managing and developing commercial biological controls. These include sourcing funding, building operations, and managing the growth of a startup company from establishment to global operations and public listing. Since February 2020, he has been the Chief Executive Officer and Chief Science Officer of U.S.-based Evolution Inc. and since March 2015, he has been the Chief Executive Officer and Chief Science Officer of U.K.-based Evolution Biotechnologies Ltd., each private biotechnology research and development companies. The UK company is the licensor of Aeris’ intellectual property and primary assets. As CEO and CSO of Evolution, he will manage the scientific work to be conducted under the licensing agreement with Aeris. From May 2012 to February 2021, Dr. Harper was the Lead Editor, “Bacteriophages: Biology, Technology, Therapy” at John Wiley Publishers, and from July 2006 to December 2018, he was the Virology section editor, Encyclopedia of Life Sciences at Springer Nature publishing. As the founder, CEO and then CSO of Biocontrol Limited, Dr. Harper conducted the first fully regulated trial to demonstrate the efficacy of a bacteriophage-based biomedical biocontrol agent, and also the first study to show the potential for biological control of the house dust mite. Dr. Harper is also a named inventor on multiple patents relating to biological control, with wide experience of the generation and management of intellectual property portfolios. He is a high profile and expert virologist and microbiologist with a wide range of relevant publications. Dr. Harper attended the University of Warwick and received his Ph.D. from the University of Newcastle-upon-Tyne.
Edward Charles Cappabianca, Vice President, Chief Business Officer. Mr. Cappabianca, the Vice President, Chief Business Officer of Aeris since July 2021, has a strong background in investment banking, cross-border mergers & acquisitions and experience as a C-suite officer of biotechnology companies. He has been the Chief Business Officer of Evolution Biotechnologies Ltd. since November 2018. Since November 2019, Mr. Cappabianca has served as the CEO of Ingenion Medical Ltd, a private medical device company developing products to address chronic urinary control issues for men & women. From October 2012 to September 2018, Mr. Cappabianca was the founder and First Chief Executive Officer at EnXray Limited, and from 2010 to 2011, was the Chief Executive Officer of AmpliPhi Biosciences Corporation, a biopharmaceutical company focused on the development of an internally generated pipeline of naturally occurring viruses called bacteriophage (phage) for the treatment of bacterial infections. In each such case, he developed operational management experience in the Biotech & Life Sciences sector. Prior to that, from 2006 to 2010, he worked at Rodman & Renshaw Ltd. as Senior Vice President, European Investment Banking. Previously, he had positions at Lion Capital Partners, William Blair International and Alex. Brown & Sons, Limited. Mr. Cappabianca received his B.A. in Chemistry from the University of Virginia and an MBA in Finance from the Wharton School of the University of Pennsylvania.
Steven Albert Portnoy, Esq., Vice President, General Counsel and Secretary. Mr. Portnoy, the Vice President, General Counsel and Secretary of Aeris since July 2021, is a highly experienced corporate lawyer with more than thirty-five years in U.S. practice, and over twenty five years’ experience in founding and operating successful companies. Since 1997, he has been president and a director of Integrity Towers, Inc., a telecommunications tower company, and since December 2014, he has been the president and sole director of Steven A, Portnoy, Esq. P.C., where he has practiced real estate, corporate and telecommunications law. Mr. Portnoy received his BSBA from the Washington University, St. Louis and his J.D. from the Southern Methodist University - School of Law.
Dr. Benjamin Burrowes, Chief of U.S. Scientific Activities. Dr. Burrowes, the Chief of U.S. Scientific Activities of Aeris since July 2021, is an experienced bacteriophage biologist specializing in therapeutic applications who has worked for startup and large, established biotechnology companies. Since 2015, he has been a consultant to Evolution Biotechnologies Ltd. From 2015 to 2019, Dr. Burrowes was Principal Scientist II at Roche Molecular Systems, Inc., a New Jersey-based pharmaceutical company. Prior to that, from 2012 to 2015, he was Senior Scientist at GeneWEAVE Biosciences and from 2011 to 2012 he was Senior Scientist at AmpliPhi Biosciences Corporation. Dr. Burrowes received his 2i B.Sc. honors in Biological Sciences from the University of Brighton, and his Ph.D. in Medical Microbiology from Texas Tech University Health Sciences Center.
Family Relationship
There are no family relationships between any of our officers and directors.
31
Involvement in Certain Legal Proceedings
To our knowledge, our directors and executive officers have not been involved in any of the following events during the past ten years:
| 1. | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; |
| 4. | being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| 5. | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| 6. | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
32
COMPENSATION OF DIRECTORS AND EXECUTIVE OFFICERS
Executive Officer Compensation
Summary Compensation Table
The following table sets forth information regarding each element of compensation that was paid or awarded to the named executive officers of the Company for the periods indicated.
| Name | Capacities In Which Compensation Was Received | Cash Compensation | Other Compensation | Total Compensation | ||||||||||
| Aaron Michael Gunn | President | $ | 20,000 | (1) | — | $ | 20,000 | (1) | ||||||
| David Richard Harper | Sr. Vice President, Chief Scientific Officer | $ | 20,000 | (2) | — | $ | 20,000 | (2) | ||||||
| Edward Charles Cappabianca | Vice President, Chief Business Officer | $ | 8,000 | (1) | — | $ | 8,000 | (1) | ||||||
| Steven Albert Portnoy | Vice President, General Counsel and Secretary | $ | 16,000 | (1) | — | $ | 16,000 | (1) | ||||||
| (1) | All of such compensation has been accrued and will be payable out of the proceeds of this offering. See “USE OF PROCEEDS” above. |
| (2) | $10,000 of such compensation has been accrued and will be payable out of the proceeds of this offering. See “USE OF PROCEEDS” above. Such amounts are payable through Evolution. |
The aggregate annual compensation of Aeris’ directors as a group for the fiscal year ended October 31, 2021 was $nil. This does not include any payments to such directors through their executive officer positions, described above.
33
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITYHOLDERS
The following table shows the beneficial ownership of our common stock as of April 25, 2022 held by (i) all directors and executive officers as a group, individually naming each director or executive officer who beneficially owns more than 10% of any class of Aeris’ common stock and (ii) any other security holder who beneficially owns more than 10% of Aeris’ common stock.
Beneficial ownership is determined in accordance with the rules of the SEC, and generally includes voting power and/or investment power with respect to the securities held. Shares of common stock subject to options and warrants currently exercisable or which may become exercisable within 60 days of April 25, 2022 are deemed outstanding and beneficially owned by the person holding such options or warrants for purposes of computing the number of shares and percentage beneficially owned by such person, but are not deemed outstanding for purposes of computing the percentage beneficially owned by any other person. Except as indicated in the footnotes to this table, the persons or entities named have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them.
The following table provides for percentage ownership assuming 16,080,500 shares are issued and outstanding as of April 25, 2022.
| Title of Class | Name and Address of Beneficial Owner(1) | Amount and Nature of Beneficial Ownership | Amount and Nature of Beneficial Ownership Acquirable | Percent of Class | ||||||||||
| Common Stock | Dr. David Harper | 8,200,000 | - | 50.99 | % | |||||||||
| Common Stock | Aaron Gunn | 5,200,000 | - | 32.34 | % | |||||||||
| Common Stock | All Directors and Officers as a Group (4 persons) | 14,950,000 | - | 92.97 | % | |||||||||
| (1) | The address of each beneficial holder of our common stock is our corporate address. |
34
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
On October 26, 2021, the Company entered into a License Agreement with Evolution Biotechnologies Limited (“Evolution”), a private company, whereas Evolution agreed to license to Aeris certain assets and intellectual property rights. Such rights included past, present and future intellectual property rights of whatever nature anywhere in the world, whether registered or unregistered, and including, without limitation, all copyrights, trademarks, trade and business names, design rights, database rights, know how, confidential information, patents and patent applications, internet rights, domain names, semi-conductor topography rights and any and all applications for any of the foregoing and any and all rights to apply for any of the foregoing in and to the assets. The payment obligations of Aeris to Evolution under the agreement are $2,000,000, with $600,000 payable upon the successful raise of a minimum of $3,000,000 in this Offering and the remaining $1.4 million upon a going public transaction of the company.
Dr. David Harper serves as the Chief Executive Officer and Chief Scientific Officer of Evolution Biotechnologies and he will manage the scientific work to be conducted under the licensing agreement. Dr. David Harper also serves as the Company’s Chairman and Chief Scientific Officer. Additionally, Mr. Gunn has been the Chief Operating Officer and director of Evolution.
35
We are offering up to 15,000,000 Shares in this Offering.
Our authorized capital stock consists of 50,000,000 shares of common stock, with a par value of $0.0001 per share, of which 16,080,500 shares are issued and outstanding.
Common Stock
Each holder of common stock is entitled to one vote for each share of common stock held of record by such holder with respect to all matters to be voted on or consented to by our stockholders, except as may otherwise be required by applicable Delaware law. A vote by the holders of a majority of the Company’s outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to the Company’s certificate of incorporation. Holders of the Company’s common stock are entitled to share in all dividends that the board of directors, in its discretion, declares from legally available funds. In the event of a liquidation, dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over the common stock. The Company’s common stock has no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to the Company’s common stock.
36
| F-1 |
Report of Independent Registered Public Accounting Firm
To the shareholders and the board of directors of Aeris Biotechnologies, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheet of Aeris Biotechnologies, Inc. (the “Company”) as of October 31, 2021, the related statement of operations, stockholders’ equity (deficit), and cash flows for the period July 28, 2021 (Inception) through October 31, 2021 and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of October 31, 2021, and the results of its operations and its cash flows for the period July 28, 2021 (Inception) through October 31, 2021, in conformity with accounting principles generally accepted in the United States.
Substantial Doubt about the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company’s significant operating losses raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/S BF Borgers CPA PC
BF Borgers CPA PC
We have served as the Company’s auditor since 2021
Lakewood, CO
April 25, 2022
| F-2 |
BALANCE SHEET
| October 31, | ||||
| 2021 | ||||
| ASSETS | ||||
| Current assets | ||||
| Cash | $ | 33,242 | ||
| Total Assets | $ | 33,242 | ||
| LIABILITIES & STOCKHOLDERS’ DEFICIT | ||||
| Current liabilities | ||||
| Accrued liabilities -related parties | $ | 54,000 | ||
| Accrued interest | 548 | |||
| Notes payable | 50,000 | |||
| Total current liabilities | 104,548 | |||
| Total liabilities | 104,548 | |||
| Stockholders’ Equity | ||||
| Common stock, Par Value $0.0001, 50,000,000 shares authorized, 15,670,500 issued and outstanding of shares as of October 31, 2021 | 1,567 | |||
| Additional paid in capital | - | |||
| Accumulated (deficit) | (72,873 | ) | ||
| Total Stockholders’ Deficit | (71,306 | ) | ||
| Total Liabilities and Stockholders’ Deficit | $ | 33,242 | ||
The accompanying notes are an integral part of these financial statements.
| F-3 |
STATEMENT OF OPERATIONS
| Period from | ||||
| July 28, 2021 to | ||||
| October 31, | ||||
| 2021 | ||||
| Revenue | $ | - | ||
| Operating Expenses: | ||||
| Administrative expenses | 5,200 | |||
| Rent | 1,558 | |||
| Officer’s compensation -related party | 65,567 | |||
| Total operating expenses | 72,325 | |||
| (Loss) from operations | (72,325 | ) | ||
| Other expense | ||||
| Interest expense | (548 | ) | ||
| Income (loss) before provision for income taxes | (548 | ) | ||
| Provision for income taxes | - | |||
| Net (Loss) | $ | (72,873 | ) | |
The accompanying notes are an integral part of these financial statements.
| F-4 |
STATEMENT OF CHANGES IN STOCKHOLDERS’ EQUITY
| Additional | Total | |||||||||||||||||||
| Common Stock | paid in | Accumulated | Stockholders’ | |||||||||||||||||
| Shares | Amount | capital | Deficit | Equity | ||||||||||||||||
| Balance, July 27, 2021 | - | $ | - | $ | - | $ | - | $ | - | |||||||||||
| Common shares issued | 15,670,500 | 1,567 | - | 1,567 | ||||||||||||||||
| Net loss | (72,873 | ) | (72,873 | ) | ||||||||||||||||
| Balance, October 31, 2021 | 15,670,500 | $ | 1,567 | $ | - | $ | (72,873 | ) | $ | (71,306 | ) | |||||||||
The accompanying notes are an integral part of these unaudited financial statements.
| F-5 |
STATEMENT OF CASH FLOWS
| Period from | ||||
| July 28, 2021 to | ||||
| October 31, | ||||
| 2021 | ||||
| Cash Flows From Operating Activities: | ||||
| Net income (loss) | $ | (72,873 | ) | |
| Stock based compensation - related party | 1,567 | |||
| Adjustments to reconcile net income to net cash provided by (used for) operating activities | ||||
| Accounts payable and accrued expenses | 54,000 | |||
| Interest payable | 548 | |||
| Net cash provided by (used for) operating activities | (16,758 | ) | ||
| Cash Flows From Financing Activities: | ||||
| Notes payable-related parties | 50,000 | |||
| Net cash provided by (used for) financing activities | 50,000 | |||
| Net Increase (Decrease) In Cash | 33,242 | |||
| Cash At The Beginning Of The Period | - | |||
| Cash At The End Of The Period | $ | 33,242 | ||
The accompanying notes are an integral part of these financial statements.
| F-6 |
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
Aeris Biotechnologies, Inc. (“Aeris,” or the “Company”) is a Delaware company that incorporated on July 28, 2021 and is developing a novel “green” approach to the control of asthma and allergy control. Asthma is a major health problem with significant unmet need.
An allergic response to house dust mite allergens is a primary cause and trigger of asthma. Existing control methods are of severely limited efficacy and many involve toxic chemicals. Aeris is developing a new “green” approach to asthma control, based around biological control of house dust mites. This builds on proven efficacy for this approach in agricultural applications, moving this into high-value biomedical markets.
Through its Worldwide License Agreement, with Evolution Biotechnologies Limited, Aeris has exclusive rights in and to patents which are exclusively awarded until 2036 in multiple jurisdictions around the world. Additional health-related products are anticipated based on the core platform technology.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying financial statements have been prepared in accordance with the Financial Accounting Standards Board (“FASB”) “FASB Accounting Standard Codification™” (the “Codification”) which is the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in conformity with generally accepted accounting principles (“GAAP”) in the United States.
Going Concern
The accompanying financial statements have been prepared assuming the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business for the twelve months following the date of these financial statements. As of October 31, 2021, the Company had an accumulated deficit of $72,873.
Because the Company does not expect that existing operational cash flow will be sufficient to fund presently anticipated operations, this raises substantial doubt about the Company’s ability to continue as a going concern. Therefore, the Company will need to raise additional funds and is currently exploring alternative sources of financing. Historically, the Company raised capital through private placements, to finance working capital needs and may attempt to raise capital through the sale of common stock or other securities and obtaining some short-term loans. The Company will be required to continue to do so until its operations become profitable. Also, the Company has, in the past, paid for consulting services with its common stock to maximize working capital, and intends to continue this practice where feasible.
Use of Estimates
The preparation of financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. The most significant estimates relate to income taxes and contingencies. The Company bases its estimates on historical experience, known or expected trends, and various other assumptions that are believed to be reasonable given the quality of information available as of the date of these financial statements. The results of these assumptions provide the basis for making estimates about the carrying amounts of assets and liabilities that are not readily apparent from other sources. Actual results could differ from these estimates.
| F-7 |
Cash and cash equivalents
The Company considers all highly liquid temporary cash investments with an original maturity of three months or less to be cash equivalents on October 31, 2021.
Income taxes
The Company accounts for income taxes under FASB ASC 740, “Accounting for Income Taxes”. Under FASB ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Under FASB ASC 740, the effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. FASB ASC 740-10-05, “Accounting for Uncertainty in Income Taxes” prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more likely than not to be sustained upon examination by taxing authorities.
The amount recognized is measured as the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. The Company assesses the validity of its conclusions regarding uncertain tax positions quarterly to determine if facts or circumstances have arisen that might cause it to change its judgment regarding the likelihood of a tax position’s sustainability under audit.
Net Loss per Share
Net loss per common share is computed by dividing net loss by the weighted average common shares outstanding during the period as defined by Financial Accounting Standards, ASC Topic 260, “Earnings per Share.” Basic earnings per common share (“EPS”) calculations are determined by dividing net income by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per common share calculations are determined by dividing net income by the weighted average number of common shares and dilutive common share equivalents outstanding.
Recent Accounting Pronouncements
There are no recent accounting pronouncements that impact the Company’s operations.
NOTE 3 – RELATED PARTY TRANSACTIONS
Notes payable
On September 11, 2021, the Company entered into an unsecured $50,000, ten percent 10% (ten percent) Promissory Note with a related party. The Promissory Note has a maturity date of September 30, 2023. As of October 31, 2021 the balance of this Promissory Note was $50,000 with $548 in accrued interest.
Accrued liabilities -compensation
As of October 31, 2021 $54,000 in accrued compensation was accrued for four company officers.
NOTE 4 – EQUITY
Common Stock
The Company has 50,000,000 shares of $0.0001 shares authorized. As of October 31, 2021, there were 15,670,500 shares outstanding. These shares were issued to founders and were not paid for. These shares were valued at par value and as a result $1,567 was charged to stock-based compensation for the period ended October 31, 2021.
| F-8 |
NOTE 5 – COMMITMENTS AND CONTINGENCIES
Pursuant to the terms of a Worldwide Exclusive License Agreement entered into on October 16, 2021 with Evolution Biotechnologies Limited(“Evolution”), a private company located in Bedfordshire, England; the Company is obligated to pay Evolution up to $2,000,000 expressly contingent upon the following terms:
| ● | The Company shall pay Evolution $600,000 if the Company raises at least $3,000,000 in a Reg. A Offering with the Securities and Exchange Commission |
| ● | The remaining $1,400,000 shall be due and payable with 30 days of the Company going public |
The Company has not accrued any liability on its financial statements because the probability of determining the likelihood of these events occurring is not currently possible.
NOTE 6 – SUBSEQUENT EVENTS
In accordance with SFAS 165 (ASC 855-10) management has performed an evaluation of subsequent events through the date that the financial statements were available to be issued and has determined that it does not have any material subsequent events to disclose in these financial statements.
| F-9 |
PART III—EXHIBITS
| Exhibit | Description | |
| 2.1 | Certificate of Incorporation | |
| 2.2 | By-Laws | |
| 4.1 | Form of the Subscription Agreement for the Offering | |
| 6.1 | Broker-Dealer Agreement with Dalmore Group, LLC | |
| 6.2 | Worldwide Exclusive License Agreement, dated October 26, 2021, by and between Evolution Biotechnologies Limited and Aeris Biotechnologies Inc. | |
| 6.3 | Form of Convertible Promissory Note | |
| 11.1 | Consent of BF Borgers CPA PC | |
| 11.2* | Consent of Ruskin Moscou Faltischek PC (included in Exhibit 12.1) | |
| 12.1* | Opinion of Ruskin Moscou Faltischek PC | |
| 13.1* | “Test the Waters” materials |
| * | To be filed by amendment. |
III-1
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this offering statement to be signed on its behalf by the undersigned, thereunto duly authorized, in City of Frisco, State of Texas, on April 25, 2022.
| Aeris Biotechnologies Corp. | ||
| By: | /s/ Aaron Gunn | |
| Name: | Aaron Gunn | |
| Title: | President | |
This offering statement has been signed by the following persons in the capacities and on the dates indicated.
| /s/ Aaron Gunn | ||||
Aaron Gunn
|
President (principal executive and financial and accounting officer) |
April 25, 2022 | ||
| /s/ David Richard Harper | ||||
Dr. David Richard Harper |
Chairman, Sr. Vice President, |
April 25, 2022 | ||
| Chief Scientific Officer and Director |
III-2
Exhibit 2.1
State of Delaware
Secretary of State
Division of Corporations
Delivered 12:39 PM 07/28/2021
FILED 12:39 PM 07/28/2021
SR 20212823627 – File Number 6122850
STATE OF DELAWARE
CERTIFICATE OF INCORPORATION
A STOCK CORPORATION
The undersigned Incorporator, desiring to form a corporation under pursuant to the General Corporation Law of the State of Delaware, hereby certifies as follows:
| 1. | The name of the Corporation is Aeris Biotechnologies, Inc. . |
| 2. | The Registered Office of the corporation in the State of Delaware is located at 1013 Centre Road, Suite 403S (street), in the City of Wilmington , County of New Castle Zip Code The name of the Registered Agent at such address upon whom process against this corporation may be served is BlumbergExcelsior Corporate Services, Inc. |
| 3. | The purpose of the corporation is to engage in any lawful act or activity for which corporations may be organized under the General Corporation Law of Delaware. |
| 4. | The total amount of stock this corporation is authorized to issue is 50,000,000 shares (number of authorized shares) with a par value of $0.0001 per share. |
| 5. | The name and mailing address of the incorporator are as follows: |
| Name | Mary Brooks, BlumbergExcelsior Corporate Services, Inc. | |||
| Mailing Address | 725 Decker Prairie Drive | |||
| Austin, Texas | Zip Code | 78748 | ||
| By: | /s/ Mary Brooks | |
| Name: | Mary Brooks | |
| Print or Type |
Exhibit 2.2
BYLAWS
OF
AERIS BIOTECHNOLOGIES, INC.
(A DELAWARE CORPORATION)
APPROVED AND ADOPTED BY THE BOARD OF DIRECTORS
EFFECTIVE MARCH 9, 2022
TABLE OF CONTENTS
| PAGE | |||
| ARTICLE I | OFFICES | 1 | |
| Section 1. | Registered Office | 1 | |
| Section 2. | Other Offices | 1 | |
| ARTICLE II | CORPORATE SEAL | 1 | |
| Section 3. | Corporate Seal | 1 | |
| ARTICLE III | STOCKHOLDERS' MEETINGS | 1 | |
| Section 4. | Place of Meetings | 1 | |
| Section 5. | Annual Meeting | 1 | |
| Section 6. | Special Meetings | 3 | |
| Section 7. | Notice of Meetings | 4 | |
| Section 8. | Quorum | 4 | |
| Section 9. | Adjournment and Notice of Adjourned Meetings | 5 | |
| Section 10. | Voting Rights | 5 | |
| Section 11. | Joint Owners of Stock | 5 | |
| Section 12. | List of Stockholders | 5 | |
| Section 13. | Action Without Meeting | 6 | |
| Section 14. | Organization | 7 | |
| ARTICLE IV | DIRECTORS | 7 | |
| Section 15. | Number and Term of Office | 7 | |
| Section 16. | Powers | 8 | |
| Section 17. | Classes of Directors | 8 | |
| Section 18. | Vacancies | 8 | |
| Section 19. | Resignation | 8 | |
| Section 20. | Removal | 9 | |
| Section 21. | Meetings | 9 | |
| (a) Regular Meetings | 9 | ||
| (b) Special Meetings | 9 | ||
| (c) Meetings by Electronic Communications Equipment | 9 | ||
| (d) Notice of Special Meetings | 9 | ||
| (e) Waiver of Notice | 9 | ||
| Section 22. | Quorum and Voting | 10 | |
| Section 23. | Action Without Meeting | 10 | |
| Section 24. | Fees and Compensation | 10 | |
| Section 25. | Committees | 10 | |
| (a) Executive Committee | 10 | ||
| (b) Other Committees | 10 | ||
| (c) Term | 11 | ||
| (d) Meetings | 11 | ||
| Section 26. | Organization | 11 | |
i
TABLE
OF CONTENTS
(CONTINUED)
| PAGE | |||
| ARTICLE V | OFFICERS | 12 | |
| Section 27. | Officers Designated | 12 | |
| Section 28. | Tenure and Duties of Officers | 12 | |
| (a) General | 12 | ||
| (b) Duties of Chairman of the Board of Directors | 12 | ||
| (c) Duties of Executive Officer | 12 | ||
| (d) Duties of President | 12 | ||
| (e) Duties of Vice Presidents | 13 | ||
| (f) Duties of Secretary | 13 | ||
| (g) Duties of Chief Financial Officer | 13 | ||
| Section 29. | Delegation of Authority | 13 | |
| Section 30. | Resignations | 13 | |
| Section 31. | Removal | 14 | |
| ARTICLE VI | EXECUTION OF CORPORATE INSTRUMENTS AND VOTING OF SECURITIES OWNED BY THE CORPORATION | 14 | |
| Section 32. | Execution of Corporate Instruments | 14 | |
| Section 33. | Voting of Securities Owned by the Corporation | 14 | |
| ARTICLE VII | SHARES OF STOCK | 14 | |
| Section 34. | Generally | 14 | |
| Section 35. | Form and Execution of Certificates | 15 | |
| Section 36. | Lost Certificates | 15 | |
| Section 37. | Transfers | 15 | |
| Section 38. | Fixing Record Dates | 16 | |
| Section 39. | Registered Stockholders | 17 | |
| ARTICLE VIII | OTHER SECURITIES OF THE CORPORATION | 17 | |
| Section 40. | Execution of Other Securities | 17 | |
| ARTICLE IX | DIVIDENDS | 17 | |
| Section 41. | Declaration of Dividends | 17 | |
| Section 42. | Dividend Reserve | 17 | |
| ARTICLE X | FISCAL YEAR | 18 | |
| Section 43. | Fiscal Year | 18 | |
| ARTICLE XI | INDEMNIFICATION | 18 | |
| Section 44. | Indemnification of Directors, Executive Officers, Other Officers, Employees and Other Agents | 18 | |
| (a) Directors and Officers | 18 | ||
| (b) Employees and Other Agents | 18 | ||
| (c) Expenses | 18 | ||
| (d) Enforcement | 19 | ||
| (e) Non-Exclusivity of Rights | 19 | ||
ii
TABLE
OF CONTENTS
(CONTINUED)
| PAGE | |||
| (f) Survival of Rights | 20 | ||
| (g) Insurance | 20 | ||
| (h) Amendments | 20 | ||
| (i) Saving Clause | 20 | ||
| (j) Certain Definitions | 20 | ||
| ARTICLE XII | NOTICES | 21 | |
| Section 45. | Notices | 21 | |
| (a) Notice to Stockholders | 21 | ||
| (b) Notice to Directors | 21 | ||
| (c) Affidavit of Mailing | 21 | ||
| (d) Methods of Notice | 21 | ||
| (e) Notice to Person with Whom Communication Is Unlawful | 22 | ||
| ARTICLE XIII | AMENDMENTS | 22 | |
| Section 46. | Amendments | 22 | |
| ARTICLE XIV | REPEALED | 22 | |
| Section 47. | Repealed | 22 | |
| ARTICLE XV | LOANS TO OFFICERS | 22 | |
| Section 48. | Loans to Officers | 22 | |
iii
BYLAWS OF AERIS BIOTECHNOLOGIES, INC.
ARTICLE I
OFFICES
Section 1. Registered Office. The registered office of the corporation in the State of Delaware shall be in the City of Wilmington, County of New Castle.
Section 2. Other Offices. The corporation shall also have and maintain an office or principal place of business at such place as may be fixed by the Board of Directors, and may also have offices at such other places, both within and without the State of Delaware, as the Board of Directors may from time to time determine or the business of the corporation may require.
ARTICLE II
CORPORATE SEAL
Section 3. Corporate Seal. The Board of Directors may adopt a corporate seal. The corporate seal shall consist of a die bearing the name of the corporation and the inscription, “Corporate Seal-Delaware.” Said seal may be used by causing it or a facsimile thereof to be impressed or affixed or reproduced or otherwise.
ARTICLE III
STOCKHOLDERS’ MEETINGS
Section 4. Place of Meetings. Meetings of the stockholders of the corporation may be held at such place, either within or without the State of Delaware, as may be determined from time to time by the Board of Directors. The Board of Directors may, in its sole discretion, determine that the meeting shall not be held at any place, but may instead be held solely by means of remote communication as provided under the Delaware General Corporation Law (“DGCL”).
Section 5. Annual Meeting.
(a) The annual meeting of the stockholders of the corporation, for the purpose of election of directors and for such other business as may lawfully come before it, shall be held on such date and at such time as may be designated from time to time by the Board of Directors. Nominations of persons for election to the Board of Directors of the corporation and the proposal of business to be considered by the stockholders may be made at an annual meeting of stockholders: (i) pursuant to the corporation’s notice of meeting of stockholders; (ii) by or at the direction of the Board of Directors; or (iii) by any stockholder of the corporation who was a stockholder of record at the time of giving of notice provided for in the following paragraph, who is entitled to vote at the meeting and who complied with the notice procedures set forth in Section 5.
1
(b) At an annual meeting of the stockholders, only such business shall be conducted as shall have been properly brought before the meeting. For nominations or other business to be properly brought before an annual meeting by a stockholder pursuant to clause (iii) of Section 5(a) of these Bylaws, (i) the stockholder must have given timely notice thereof in writing to the Secretary of the corporation, (ii) such other business must be a proper matter for stockholder action under the DGCL, (iii) if the stockholder, or the beneficial owner on whose behalf any such proposal or nomination is made, has provided the corporation with a Solicitation Notice (as defined in this Section 5(b)), such stockholder or beneficial owner must, in the case of a proposal, have delivered a proxy statement and form of proxy to holders of at least the percentage of the corporation’s voting shares required under applicable law to carry any such proposal, or, in the case of a nomination or nominations, have delivered a proxy statement and form of proxy to holders of a percentage of the corporation’s voting shares reasonably believed by such stockholder or beneficial owner to be sufficient to elect the nominee or nominees proposed to be nominated by such stockholder, and must, in either case, have included in such materials the Solicitation Notice, and (iv) if no Solicitation Notice relating thereto has been timely provided pursuant to this section, the stockholder or beneficial owner proposing such business or nomination must not have solicited a number of proxies sufficient to have required the delivery of such a Solicitation Notice under this Section 5. To be timely, a stockholder’s notice shall be delivered to the Secretary at the principal executive offices of the Corporation not later than the close of business on the ninetieth (90th) day nor earlier than the close of business on the one hundred twentieth (120th) day prior to the first anniversary of the preceding year’s annual meeting; provided, however, that in the event that the date of the annual meeting is advanced more than thirty (30) days prior to or delayed by more than thirty (30) days after the anniversary of the preceding year’s annual meeting, notice by the stockholder to be timely must be so delivered not earlier than the close of business on the one hundred twentieth (120th) day prior to such annual meeting and not later than the close of business on the later of the ninetieth (90th) day prior to such annual meeting or the tenth (10th) day following the day on which public announcement of the date of such meeting is first made. In no event shall the public announcement of an adjournment of an annual meeting commence a new time period for the giving of a stockholder’s notice as described above. Such stockholder’s notice shall set forth: (A) as to each person whom the stockholder proposed to nominate for election or reelection as a director all information relating to such person that is required to be disclosed in solicitations of proxies for election of directors in an election contest, or is otherwise required, in each case pursuant to Regulation 14A under the Securities Exchange Act of 1934, as amended (the “1934 Act”) and Rule 14a-4(d) thereunder (including such person’s written consent to being named in the proxy statement as a nominee and to serving as a director if elected); (B) as to any other business that the stockholder proposes to bring before the meeting, a brief description of the business desired to be brought before the meeting, the reasons for conducting such business at the meeting and any material interest in such business of such stockholder and the beneficial owner, if any, on whose behalf the proposal is made; and (C) as to the stockholder giving the notice and the beneficial owner, if any, on whose behalf the nomination or proposal is made (i) the name and address of such stockholder, as they appear on the corporation’s books, and of such beneficial owner, (ii) the class and number of shares of the corporation which are owned beneficially and of record by such stockholder and such beneficial owner, and (iii) whether either such stockholder or beneficial owner intends to deliver a proxy statement and form of proxy to holders of, in the case of the proposal, at least the percentage of the corporation’s voting shares required under applicable law to carry the proposal or, in the case of a nomination or nominations, a sufficient number of holders of the corporation’s voting shares to elect such nominee or nominees (an affirmative statement of such intent, a “Solicitation Notice”).
2
(c) Notwithstanding anything in the second sentence of Section 5(b) of these Bylaws to the contrary, in the event that the number of directors to be elected to the Board of Directors of the Corporation is increased and there is no public announcement naming all of the nominees for director or specifying the size of the increased Board of Directors made by the corporation at least one hundred (100) days prior to the first anniversary of the preceding year’s annual meeting, a stockholder’s notice required by this Section 5 shall also be considered timely, but only with respect to nominees for any new positions created by such increase, if it shall be delivered to the Secretary at the principal executive offices of the corporation not later than the close of business on the tenth (10th) day following the day on which such public announcement is first made by the corporation.
(d) Only such persons who are nominated in accordance with the procedures set forth in this Section 5 shall be eligible to serve as directors and only such business shall be conducted at a meeting of stockholders as shall have been brought before the meeting in accordance with the procedures set forth in this Section 5. Except as otherwise provided by law, the Chairman of the meeting shall have the power and duty to determine whether a nomination or any business proposed to be brought before the meeting was made, or proposed, as the case may be, in accordance with the procedures set forth in these Bylaws and, if any proposed nomination or business is not in compliance with these Bylaws, to declare that such defective proposal or nomination shall not be presented for stockholder action at the meeting and shall be disregarded.
(e) Notwithstanding the foregoing provisions of this Section 5, in order to include information with respect to a stockholder proposal in the proxy statement and form of proxy for a stockholders’ meeting, stockholders must provide notice as required by the regulations promulgated under the 1934 Act. Nothing in these Bylaws shall be deemed to affect any rights of stockholders to request inclusion of proposals in the corporation proxy statement pursuant to Rule 14a-8 under the 1934 Act.
(f) For purposes of this Section 5, “public announcement” shall mean disclosure in a press release reported by the Dow Jones News Service, Associated Press or comparable national news service or in a document publicly filed by the corporation with the Securities and Exchange Commission pursuant to Section 13, 14 or 15(d) of the 1934 Act.
Section 6. Special Meetings.
(a) Special meetings of the stockholders of the corporation may be called, for any purpose or purposes, by (i) the Chairman of the Board of Directors, (ii) the Chief Executive Officer, (iii) the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors (whether or not there exist any vacancies in previously authorized directorships at the time any such resolution is presented to the Board of Directors for adoption) or (iv) by the holders of shares entitled to cast not less than ten percent (10%) of the votes at the meeting, and shall be held at such place, on such date, and at such time as the Board of Directors shall fix.
3
(b) If a special meeting is properly called by any person or persons other than the Board of Directors, the request shall be in writing, specifying the general nature of the business proposed to be transacted, and shall be delivered personally or sent by certified or registered mail, return receipt requested, or by telegraphic or other facsimile transmission to the Chairman of the Board of Directors, the Chief Executive Officer, or the Secretary of the corporation. No business may be transacted at such special meeting otherwise than specified in such notice. The Board of Directors shall determine the time and place of such special meeting, which shall be held not less than thirty-five (35) nor more than one hundred twenty (120) days after the date of the receipt of the request. Upon determination of the time and place of the meeting, the officer receiving the request shall cause notice to be given to the stockholders entitled to vote, in accordance with the provisions of Section 7 of these Bylaws. Nothing contained in this paragraph (b) shall be construed as limiting, fixing, or affecting the time when a meeting of stockholders called by action of the Board of Directors may be held.
Section 7. Notice of Meetings. Except as otherwise provided by law, notice, given in writing or by electronic transmission, of each meeting of stockholders shall be given not less than ten (10) nor more than sixty (60) days before the date of the meeting to each stockholder entitled to vote at such meeting, such notice to specify the place, if any, date and hour, in the case of special meetings, the purpose or purposes of the meeting, and the means of remote communications, if any, by which stockholders and proxyholders may be deemed to be present in person and vote at any such meeting. If mailed, notice is given when deposited in the United States mail, postage prepaid, directed to the stockholder at such stockholder’s address as it appears on the records of the corporation. Notice of the time, place, if any, and purpose of any meeting of stockholders may be waived in writing, signed by the person entitled to notice thereof or by electronic transmission by such person, either before or after such meeting, and will be waived by any stockholder by his attendance thereat in person, by remote communication, if applicable, or by proxy, except when the stockholder attends a meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Any stockholder so waiving notice of such meeting shall be bound by the proceedings of any such meeting in all respects as if due notice thereof had been given.
Section 8. Quorum. At all meetings of stockholders, except where otherwise provided by statute or by the Certificate of Incorporation, or by these Bylaws, the presence, in person, by remote communication, if applicable, or by proxy duly authorized, of the holders of a majority of the outstanding shares of stock entitled to vote shall constitute a quorum for the transaction of business. In the absence of a quorum, any meeting of stockholders may be adjourned, from time to time, either by the chairman of the meeting or by vote of the holders of a majority of the shares represented thereat, but no other business shall be transacted at such meeting. The stockholders present at a duly called or convened meeting, at which a quorum is present, may continue to transact business until adjournment, notwithstanding the withdrawal of enough stockholders to leave less than a quorum. Except as otherwise provided by statute, or by the Certificate of Incorporation or these Bylaws, in all matters other than the election of directors, the affirmative vote of a majority of shares present in person, by remote communication, if applicable, or represented by proxy duly authorized at the meeting and entitled to vote generally on the subject matter shall be the act of the stockholders. Except as otherwise provided by statute, the Certificate of Incorporation or these Bylaws, directors shall be elected by a plurality of the votes of the shares present in person, by remote communication, if applicable, or represented by proxy duly authorized at the meeting and entitled to vote generally on the election of directors. Where a separate vote by a class or classes or series is required, except where otherwise provided by the statute or by the Certificate of Incorporation or these Bylaws, a majority of the outstanding shares of such class or classes or series, present in person, by remote communication, if applicable, or represented by proxy duly authorized, shall constitute a quorum entitled to take action with respect to that vote on that matter. Except where otherwise provided by statute or by the Certificate of Incorporation or these Bylaws, the affirmative vote of the majority (plurality, in the case of the election of directors) of shares of such class or classes or series present in person, by remote communication, if applicable, or represented by proxy at the meeting shall be the act of such class or classes or series.
4
Section 9. Adjournment and Notice of Adjourned Meetings. Any meeting of stockholders, whether annual or special, may be adjourned from time to time either by the chairman of the meeting or by the vote of a majority of the shares present in person, by remote communication, if applicable, or represented by proxy. When a meeting is adjourned to another time or place, if any, notice need not be given of the adjourned meeting if the time and place, if any, thereof are announced at the meeting at which the adjournment is taken. At the adjourned meeting, the corporation may transact any business which might have been transacted at the original meeting. If the adjournment is for more than thirty (30) days or if after the adjournment a new record date is fixed for the adjourned meeting, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting.
Section 10. Voting Rights. For the purpose of determining those stockholders entitled to vote at any meeting of the stockholders, except as otherwise provided by law, only persons in whose names shares stand on the stock records of the corporation on the record date, as provided in Section 12 of these Bylaws, shall be entitled to vote at any meeting of stockholders. Every person entitled to vote or execute consents shall have the right to do so either in person, by remote communication, if applicable, or by an agent or agents authorized by a proxy granted in accordance with Delaware law. An agent so appointed need not be a stockholder. No proxy shall be voted after three (3) years from its date of creation unless the proxy provides for a longer period.
Section 11. Joint Owners of Stock. If shares or other securities having voting power stand of record in the names of two (2) or more persons, whether fiduciaries, members of a partnership, joint tenants, tenants in common, tenants by the entirety, or otherwise, or if two (2) or more persons have the same fiduciary relationship respecting the same shares, unless the Secretary is given written notice to the contrary and is furnished with a copy of the instrument or order appointing them or creating the relationship wherein it is so provided, their acts with respect to voting shall have the following effect: (a) if only one (1) votes, his act binds all; (b) if more than one (1) votes, the act of the majority so voting binds all; (c) if more than one (1) votes, but the vote is evenly split on any particular matter, each faction may vote the securities in question proportionally, or may apply to the Delaware Court of Chancery for relief as provided in the DGCL, Section 217(b). If the instrument filed with the Secretary shows that any such tenancy is held in unequal interests, a majority or even-split for the purpose of subsection (c) shall be a majority or even-split in interest.
Section 12. List of Stockholders. The Secretary shall prepare and make, at least ten (10) days before every meeting of stockholders, a complete list of the stockholders entitled to vote at said meeting, arranged in alphabetical order, showing the address of each stockholder and the number of shares registered in the name of each stockholder. Such list shall be open to the examination of any stockholder, for any purpose germane to the meeting, on a reasonably accessible electronic network, provided that the information required to gain access to such list is provided with the notice of the meeting, or during ordinary business hours, at the principal place of business of the corporation. In the event that the corporation determines to make the list available on an electronic network, the corporation may take reasonable steps to ensure that such information is available only to stockholders of the corporation. The list shall be open to examination of any stockholder during the time of the meeting as provided by law.
5
Section 13. Action Without Meeting.
(a) Unless otherwise provided in the Certificate of Incorporation, any action required by statute to be taken at any annual or special meeting of the stockholders, or any action which may be taken at any annual or special meeting of the stockholders, may be taken without a meeting, without prior notice and without a vote, if a consent in writing, or by electronic transmission setting forth the action so taken, shall be signed by the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted.
(b) Every written consent or electronic transmission shall bear the date of signature of each stockholder who signs the consent, and no written consent or electronic transmission shall be effective to take the corporate action referred to therein unless, within sixty (60) days of the earliest dated consent delivered to the corporation in the manner herein required, written consents or electronic transmissions signed by a sufficient number of stockholders to take action are delivered to the corporation by delivery to its registered office in the State of Delaware, its principal place of business or an officer or agent of the corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery made to a corporation’s registered office shall be by hand or by certified or registered mail, return receipt requested.
(c) Prompt notice of the taking of the corporate action without a meeting by less than unanimous written consent shall be given to those stockholders who have not consented in writing or by electronic transmission and who, if the action had been taken at a meeting, would have been entitled to notice of the meeting if the record date for such meeting had been the date that written consents signed by a sufficient number of stockholders to take action were delivered to the corporation as provided in Section 228(c) of the DGCL. If the action which is consented to is such as would have required the filing of a certificate under any section of the DGCL if such action had been voted on by stockholders at a meeting thereof, then the certificate filed under such section shall state, in lieu of any statement required by such section concerning any vote of stockholders, that written consent has been given in accordance with Section 228 of the DGCL.
(d) A telegram, cablegram or other electronic transmission consenting to an action to be taken and transmitted by a stockholder or proxyholder, shall be deemed to be written, signed and dated for the purposes of this section, provided that any such telegram, cablegram or other electronic transmission sets forth or is delivered with information from which the corporation can determine (i) that the telegram, cablegram or other electronic transmission was transmitted by the stockholder or proxyholder or by a person or persons authorized to act for the stockholder and (ii) the date on which such stockholder or proxyholder or authorized person or persons transmitted such telegram, cablegram or electronic transmission. The date on which such telegram, cablegram or electronic transmission is transmitted shall be deemed to be the date on which such consent was signed. No consent given by telegram, cablegram or other electronic transmission shall be deemed to have been delivered until such consent is reproduced in paper form and until such paper form shall be delivered to the corporation by delivery to its registered office in the state of Delaware, its principal place of business or an officer or agent of the corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery made to a corporation’s registered office shall be made by hand or by certified or registered mail, return receipt requested. Notwithstanding the foregoing limitations on delivery, consents given by telegram, cablegram or other electronic transmission may be otherwise delivered to the principal place of business of the corporation or to an officer or agent of the corporation having custody of the book in which proceedings of meetings of stockholders are recorded if, to the extent and in the manner provided by resolution of the board of directors of the corporation. Any copy, facsimile or other reliable reproduction of a consent in writing may be substituted or used in lieu of the original writing for any and all purposes for which the original writing could be used, provided that such copy, facsimile or other reproduction shall be a complete reproduction of the entire original writing.
6
Section 14. Organization.
(a) At every meeting of stockholders, the Chairman of the Board of Directors, or, if a Chairman has not been appointed or is absent, the President, or, if the President is absent, a chairman of the meeting chosen by a majority in interest of the stockholders entitled to vote, present in person or by proxy, shall act as chairman. The Secretary, or, in his absence, an Assistant Secretary directed to do so by the President, shall act as secretary of the meeting.
(b) The Board of Directors of the corporation shall be entitled to make such rules or regulations for the conduct of meetings of stockholders as it shall deem necessary, appropriate or convenient. Subject to such rules and regulations of the Board of Directors, if any, the chairman of the meeting shall have the right and authority to prescribe such rules, regulations and procedures and to do all such acts as, in the judgment of such chairman, are necessary, appropriate or convenient for the proper conduct of the meeting, including, without limitation, establishing an agenda or order of business for the meeting, rules and procedures for maintaining order at the meeting and the safety of those present, limitations on participation in such meeting to stockholders of record of the corporation and their duly authorized and constituted proxies and such other persons as the chairman shall permit, restrictions on entry to the meeting after the time fixed for the commencement thereof, limitations on the time allotted to questions or comments by participants and regulation of the opening and closing of the polls for balloting on matters which are to be voted on by ballot. The date and time of the opening and closing of the polls for each matter upon which the stockholders will vote at the meeting shall be announced at the meeting. Unless and to the extent determined by the Board of Directors or the chairman of the meeting, meetings of stockholders shall not be required to be held in accordance with rules of parliamentary procedure.
ARTICLE IV
DIRECTORS
Section 15. Number and Term of Office. The authorized number of directors of the corporation shall be fixed by the Board of Directors from time to time. Directors need not be stockholders unless so required by the Certificate of Incorporation. If for any cause, the directors shall not have been elected at an annual meeting, they may be elected as soon thereafter as convenient.
7
Section 16. Powers. The powers of the corporation shall be exercised, its business conducted and its property controlled by the Board of Directors, except as may be otherwise provided by statute or by the Certificate of Incorporation.
Section 17. Classes of Directors. Subject to the rights of the holders of any series of Preferred Stock to elect additional directors under specified circumstances, the directors shall be divided into three classes designated as Class I, Class II and Class III, respectively. At the first annual meeting of stockholders following the initial classification of the Board of Directors, the term of office of the Class I directors shall expire and Class I directors shall be elected for a full term of three years. At the second annual meeting of stockholders following such initial classification, the term of office of the Class II directors shall expire and Class II directors shall be elected for a full term of three years. At the third annual meeting of stockholders following such initial classification, the term of office of the Class III directors shall expire and Class III directors shall be elected for a full term of three years. At each succeeding annual meeting of stockholders, directors shall be elected for a full term of three years to succeed the directors of the class whose terms expire at such annual meeting.
Notwithstanding the foregoing provisions of this section, each director shall serve until his successor is duly elected and qualified or until his earlier death, resignation or removal. No decrease in the number of directors constituting the Board of Directors shall shorten the term of any incumbent director.
Section 18. Vacancies. Unless otherwise provided in the Certificate of Incorporation, and subject to the rights of the holders of any series of Preferred Stock, any vacancies on the Board of Directors resulting from death, resignation, disqualification, removal or other causes and any newly created directorships resulting from any increase in the number of directors shall, unless the Board of Directors determines by resolution that any such vacancies or newly created directorships shall be filled by stockholders, be filled only by the affirmative vote of a majority of the directors then in office, even though less than a quorum of the Board of Directors. Any director elected in accordance with the preceding sentence shall hold office for the remainder of the full term of the director for which the vacancy was created or occurred and until such director’s successor shall have been elected and qualified. A vacancy in the Board of Directors shall be deemed to exist under this Bylaw in the case of the death, removal or resignation of any director.
Section 19. Resignation. Any director may resign at any time by delivering his or her notice in writing or by electronic transmission to the Secretary, such resignation to specify whether it will be effective at a particular time, upon receipt by the Secretary or at the pleasure of the Board of Directors. If no such specification is made, it shall be deemed effective at the pleasure of the Board of Directors. When one or more directors shall resign from the Board of Directors, effective at a future date, a majority of the directors then in office, including those who have so resigned, shall have power to fill such vacancy or vacancies, the vote thereon to take effect when such resignation or resignations shall become effective, and each Director so chosen shall hold office for the unexpired portion of the term of the Director whose place shall be vacated and until his successor shall have been duly elected and qualified.
8
Section 20. Removal. Subject to any limitations imposed by applicable law, the Board of Directors or any director may be removed from office at any time with cause by the affirmative vote of the holders of a majority of the voting power of all then-outstanding shares of capital stock of the corporation entitled to vote generally at an election of directors. For the purposes of these Bylaws “cause” shall mean fraud, dishonest conduct or gross abuse of authority or discretion.
Section 21. Meetings
(a) Regular Meetings. Unless otherwise restricted by the Certificate of Incorporation, regular meetings of the Board of Directors may be held at any time or date and at any place within or without the State of Delaware which has been designated by the Board of Directors and publicized among all directors, either orally or in writing, including a voice-messaging system or other system designated to record and communicate messages, facsimile, telegraph or telex, or by electronic mail or other electronic means. No further notice shall be required for a regular meeting of the Board of Directors.
(b) Special Meetings. Unless otherwise restricted by the Certificate of Incorporation, special meetings of the Board of Directors may be held at any time and place within or without the State of Delaware whenever called by the Chairman of the Board, the President or any director.
(c) Meetings by Electronic Communications Equipment. Any member of the Board of Directors, or of any committee thereof, may participate in a meeting by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear each other, and participation in a meeting by such means shall constitute presence in person at such meeting.
(d) Notice of Special Meetings. Notice of the time and place of all special meetings of the Board of Directors shall be orally or in writing, by telephone, including a voice messaging system or other system or technology designed to record and communicate messages, facsimile, telegraph or telex, or by electronic mail or other electronic means, during normal business hours, at least twenty-four (24) hours before the date and time of the meeting. If notice is sent by US mail, it shall be sent by first class mail, postage prepaid at least three (3) days before the date of the meeting. Notice of any meeting may be waived in writing or by electronic transmission at any time before or after the meeting and will be waived by any director by attendance thereat, except when the director attends the meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened.
(e) Waiver of Notice. The transaction of all business at any meeting of the Board of Directors, or any committee thereof, however called or noticed, or wherever held, shall be as valid as though had at a meeting duly held after regular call and notice, if a quorum be present and if, either before or after the meeting, each of the directors not present who did not receive notice shall sign a written waiver of notice or shall waive notice by electronic transmission. All such waivers shall be filed with the corporate records or made a part of the minutes of the meeting.
9
Section 22. Quorum and Voting.
(a) Unless the Certificate of Incorporation requires a greater number, a quorum of the Board of Directors shall consist of a majority of the exact number of directors fixed from time to time by the Board of Directors in accordance with the Certificate of Incorporation; provided, however, at any meeting, whether a quorum be present or otherwise, a majority of the directors present may adjourn from time to time until the time fixed for the next regular meeting of the Board of Directors, without notice other than by announcement at the meeting.
(b) At each meeting of the Board of Directors at which a quorum is present, all questions and business shall be determined by the affirmative vote of a majority of the directors present, unless a different vote be required by law, the Certificate of Incorporation or these Bylaws.
Section 23. Action Without Meeting. Unless otherwise restricted by the Certificate of Incorporation or these Bylaws, any action required or permitted to be taken at any meeting of the Board of Directors or of any committee thereof may be taken without a meeting, if all members of the Board of Directors or committee, as the case may be, consent thereto in writing or by electronic transmission, and such writing or writings or transmission or transmissions are filed with the minutes of proceedings of the Board of Directors or committee. Such filing shall be in paper form if the minutes are maintained in paper form and shall be in electronic form if the minutes are maintained in electronic form.
Section 24. Fees and Compensation. Directors shall be entitled to such compensation for their services as may be approved by the Board of Directors, including, if so approved, by resolution of the Board of Directors, a fixed sum and expenses of attendance, if any, for attendance at each regular or special meeting of the Board of Directors and at any meeting of a committee of the Board of Directors. Nothing herein contained shall be construed to preclude any director from serving the corporation in any other capacity as an officer, agent, employee, or otherwise and receiving compensation therefor.
Section 25. Committees.
(a) Executive Committee. The Board of Directors may appoint an Executive Committee to consist of one (1) or more members of the Board of Directors. The Executive Committee, to the extent permitted by law and provided in the resolution of the Board of Directors shall have and may exercise all the powers and authority of the Board of Directors in the management of the business and affairs of the corporation, and may authorize the seal of the corporation to be affixed to all papers which may require it; but no such committee shall have the power or authority in reference to (i) approving or adopting, or recommending to the stockholders, any action or matter expressly required by the DGCL to be submitted to stockholders for approval, or (ii) adopting, amending or repealing any bylaw of the corporation.
(b) Other Committees. The Board of Directors may, from time to time, appoint such other committees as may be permitted by law. Such other committees appointed by the Board of Directors shall consist of one (1) or more members of the Board of Directors and shall have such powers and perform such duties as may be prescribed by the resolution or resolutions creating such committees, but in no event shall any such committee have the powers denied to the Executive Committee in these Bylaws.
10
(c) Term. The Board of Directors, subject to any requirements of any outstanding series of Preferred Stock and the provisions of subsections (a) or (b) of this Bylaw may at any time increase or decrease the number of members of a committee or terminate the existence of a committee. The membership of a committee member shall terminate on the date of his death or voluntary resignation from the committee or from the Board of Directors. The Board of Directors may at any time for any reason remove any individual committee member and the Board of Directors may fill any committee vacancy created by death, resignation, removal or increase in the number of members of the committee. The Board of Directors may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee, and, in addition, in the absence or disqualification of any member of a committee, the member or members thereof present at any meeting and not disqualified from voting, whether or not he or they constitute a quorum, may unanimously appoint another member of the Board of Directors to act at the meeting in the place of any such absent or disqualified member.
(d) Meetings. Unless the Board of Directors shall otherwise provide, regular meetings of the Executive Committee or any other committee appointed pursuant to this Section 25 shall be held at such times and places as are determined by the Board of Directors, or by any such committee, and when notice thereof has been given to each member of such committee, no further notice of such regular meetings need be given thereafter. Special meetings of any such committee may be held at any place which has been determined from time to time by such committee, and may be called by any director who is a member of such committee, upon notice to the members of such committee of the time and place of such special meeting given in the manner provided for the giving of notice to members of the Board of Directors of the time and place of special meetings of the Board of Directors. Notice of any special meeting of any committee may be waived in writing at any time before or after the meeting and will be waived by any director by attendance thereat, except when the director attends such special meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Unless otherwise provided by the Board of Directors in the resolutions authorizing the creation of the committee, a majority of the authorized number of members of any such committee shall constitute a quorum for the transaction of business, and the act of a majority of those present at any meeting at which a quorum is present shall be the act of such committee.
Section 26. Organization. At every meeting of the directors, the Chairman of the Board of Directors, or, if a Chairman has not been appointed or is absent, the President, or if the President is absent, the most senior Vice President, (if a director) or, in the absence of any such person, a chairman of the meeting chosen by a majority of the directors present, shall preside over the meeting. The Secretary, or in his absence, any Assistant Secretary directed to do so by the President, shall act as secretary of the meeting.
11
ARTICLE V
OFFICERS
Section 27. Officers Designated. The officers of the corporation shall include, if and when designated by the Board of Directors, the Chairman of the Board of Directors, the Chief Executive Officer, the President, one or more Vice Presidents, the Secretary, the Chief Financial Officer, the Treasurer and the Controller, all of whom shall be elected at the annual organizational meeting of the Board of Directors. The Board of Directors may also appoint one or more Assistant Secretaries, Assistant Treasurers, Assistant Controllers and such other officers and agents with such powers and duties as it shall deem necessary. The Board of Directors may assign such additional titles to one or more of the officers as it shall deem appropriate. Any one person may hold any number of offices of the corporation at any one time unless specifically prohibited therefrom by law. The salaries and other compensation of the officers of the corporation shall be fixed by or in the manner designated by the Board of Directors.
Section 28. Tenure and Duties of Officers.
(a) General. All officers shall hold office at the pleasure of the Board of Directors and until their successors shall have been duly elected and qualified, unless sooner removed. Any officer elected or appointed by the Board of Directors may be removed at any time by the Board of Directors. If the office of any officer becomes vacant for any reason, the vacancy may be filled by the Board of Directors.
(b) Duties of Chairman of the Board of Directors. The Chairman of the Board of Directors, when present, shall preside at all meetings of the stockholders and the Board of Directors. The Chairman of the Board of Directors shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors shall designate from time to time. If there is no President, then the Chairman of the Board of Directors shall also serve as the Chief Executive Officer of the corporation and shall have the powers and duties prescribed in paragraph (c) of this Section 28.
(c) Duties of Chief Executive Officer. The Chief Executive shall preside at all meetings of the stockholders and at all meetings of the Board of Directors, unless the Chairman of the Board of Directors has been appointed and is present. The Chief Executive shall be the chief executive officer of the corporation and shall, subject to the control of the Board of Directors, have general supervision, direction and control of the business and officers of the corporation. The Chief Executive shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors shall designate from time to time.
(d) Duties of President. The President shall preside at all meetings of the stockholders and at all meetings of the Board of Directors, unless the Chairman of the Board of Directors or Chief Executive Officer has been appointed and is present. Unless some other officer has been elected Chief Executive Officer of the corporation, the President shall be the chief executive officer of the corporation and shall, subject to the control of the Board of Directors, have general supervision, direction and control of the business and officers of the corporation. The President shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors shall designate from time to time. In the event that the corporation has appointed a Chief Executive Officer, then the President shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or Chief Executive Officer shall designate from time to time
12
(e) Duties of Vice Presidents. The Vice Presidents may assume and perform the duties of the President in the absence or disability of the President or whenever the office of President is vacant. The Vice Presidents shall perform other duties commonly incident to their office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time.
(f) Duties of Secretary. The Secretary shall attend all meetings of the stockholders and of the Board of Directors and shall record all acts and proceedings thereof in the minute book of the corporation. The Secretary shall give notice in conformity with these Bylaws of all meetings of the stockholders and of all meetings of the Board of Directors and any committee thereof requiring notice. The Secretary shall perform all other duties provided for in these Bylaws and other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors shall designate from time to time. The President may direct any Assistant Secretary to assume and perform the duties of the Secretary in the absence or disability of the Secretary, and each Assistant Secretary shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time.
(g) Duties of Chief Financial Officer. The Chief Financial Officer shall keep or cause to be kept the books of account of the corporation in a thorough and proper manner and shall render statements of the financial affairs of the corporation in such form and as often as required by the Board of Directors or the President. The Chief Financial Officer, subject to the order of the Board of Directors, shall have the custody of all funds and securities of the corporation. The Chief Financial Officer shall perform other duties commonly incident to his office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time. The President may direct the Treasurer or any Assistant Treasurer, or the Controller or any Assistant Controller to assume and perform the duties of the Chief Financial Officer in the absence or disability of the Chief Financial Officer, and each Treasurer and Assistant Treasurer and each Controller and Assistant Controller shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time.
Section 29. Delegation of Authority. The Board of Directors may from time to time delegate the powers or duties of any officer to any other officer or agent, notwithstanding any provision hereof.
Section 30. Resignations. Any officer may resign at any time by giving notice in writing or by electronic transmission notice to the Board of Directors or to the President or to the Secretary. Any such resignation shall be effective when received by the person or persons to whom such notice is given, unless a later time is specified therein, in which event the resignation shall become effective at such later time. Unless otherwise specified in such notice, the acceptance of any such resignation shall not be necessary to make it effective. Any resignation shall be without prejudice to the rights, if any, of the corporation under any contract with the resigning officer.
13
Section 31. Removal. Any officer may be removed from office at any time, either with or without cause, by the affirmative vote of a majority of the directors in office at the time, or by the unanimous written consent of the directors in office at the time, or by any committee or superior officers upon whom such power of removal may have been conferred by the Board of Directors.
ARTICLE VI
EXECUTION OF CORPORATE INSTRUMENTS AND VOTING
OF SECURITIES OWNED BY THE CORPORATION
Section 32. Execution of Corporate Instruments. The Board of Directors may, in its discretion, determine the method and designate the signatory officer or officers, or other person or persons, to execute on behalf of the corporation any corporate instrument or document, or to sign on behalf of the corporation the corporate name without limitation, or to enter into contracts on behalf of the corporation, except where otherwise provided by law or these Bylaws, and such execution or signature shall be binding upon the corporation.
All checks and drafts drawn on banks or other depositaries on funds to the credit of the corporation or in special accounts of the corporation shall be signed by such person or persons as the Board of Directors shall authorize so to do.
Unless authorized or ratified by the Board of Directors or within the agency power of an officer, no officer, agent or employee shall have any power or authority to bind the corporation by any contract or engagement or to pledge its credit or to render it liable for any purpose or for any amount.
Section 33. Voting of Securities Owned by the Corporation. All stock and other securities of other corporations owned or held by the corporation for itself, or for other parties in any capacity, shall be voted, and all proxies with respect thereto shall be executed, by the person authorized so to do by resolution of the Board of Directors, or, in the absence of such authorization, by the Chairman of the Board of Directors, the Chief Executive Officer, the President, or any Vice President.
ARTICLE VII
SHARES OF STOCK
Section 34. Generally. The total capital of the Corporation shall consist of fifty million (50,000,000) shares of common stock, with a par value of $.0001 per share. Any and all such common or preferred shares may be issued by the Corporation for consideration consisting of any tangible or intangible property or benefit to the Corporation as authorized by the Board of Directors. All such shares shall be fully paid and nonassessable.
14
Section 35. Form and Execution of Certificates. Certificates for the shares of stock of the corporation shall be in such form as is consistent with the Certificate of Incorporation and applicable law. Every holder of stock in the corporation shall be entitled to have a certificate signed by or in the name of the corporation by the Chairman of the Board of Directors, or the President or any Vice President and by the Treasurer or Assistant Treasurer or the Secretary or Assistant Secretary, certifying the number of shares owned by him in the corporation. Any or all of the signatures on the certificate may be facsimiles. In case any officer, transfer agent, or registrar who has signed or whose facsimile signature has been placed upon a certificate shall have ceased to be such officer, transfer agent, or registrar before such certificate is issued, it may be issued with the same effect as if he were such officer, transfer agent, or registrar at the date of issue. Each certificate shall state upon the face or back thereof, in full or in summary, all of the powers, designations, preferences, and rights, and the limitations or restrictions of the shares authorized to be issued or shall, except as otherwise required by law, set forth on the face or back a statement that the corporation will furnish without charge to each stockholder who so requests the powers, designations, preferences and relative, participating, optional, or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights. Within a reasonable time after the issuance or transfer of uncertificated stock, the corporation shall send to the registered owner thereof a written notice containing the information required to be set forth or stated on certificates pursuant to this section or otherwise required by law or with respect to this section a statement that the corporation will furnish without charge to each stockholder who so requests the powers, designations, preferences and relative participating, optional or other special rights of each class of stock or series thereof and the qualifications, limitations or restrictions of such preferences and/or rights.
Section 36. Lost Certificates. A new certificate or certificates shall be issued in place of any certificate or certificates theretofore issued by the corporation alleged to have been lost, stolen, or destroyed, upon the making of an affidavit of that fact by the person claiming the certificate of stock to be lost, stolen, or destroyed. The corporation may require, as a condition precedent to the issuance of a new certificate or certificates, the owner of such lost, stolen, or destroyed certificate or certificates, or the owner’s legal representative, to agree to indemnify the corporation in such manner as it shall require or to give the corporation a surety bond in such form and amount as it may direct as indemnity against any claim that may be made against the corporation with respect to the certificate alleged to have been lost, stolen, or destroyed.
Section 37. Transfers.
(a) Transfers of record of shares of stock of the corporation shall be made only upon its books by the holders thereof, in person or by attorney duly authorized, and upon the surrender of a properly endorsed certificate or certificates for a like number of shares.
(b) The corporation shall have power to enter into and perform any agreement with any number of stockholders of any one or more classes of stock of the corporation to restrict the transfer of shares of stock of the corporation of any one or more classes owned by such stockholders in any manner not prohibited by the DGCL.
15
Section 38. Fixing Record Dates.
(a) In order that the corporation may determine the stockholders entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, the Board of Directors may fix, in advance, a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board of Directors, and which record date shall, subject to applicable law, not be more than sixty (60) nor less than ten (10) days before the date of such meeting. If no record date is fixed by the Board of Directors, the record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be at the close of business on the day next preceding the day on which notice is given, or if notice is waived, at the close of business on the day next preceding the day on which the meeting is held. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Board of Directors may fix a new record date for the adjourned meeting.
(b) In order that the corporation may determine the stockholders entitled to consent to corporate action in writing without a meeting, the Board of Directors may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board of Directors, and which date shall not be more than ten (10) days after the date upon which the resolution fixing the record date is adopted by the Board of Directors. Any stockholder of record seeking to have the stockholders authorize or take corporate action by written consent shall, by written notice to the Secretary, request the Board of Directors to fix a record date. The Board of Directors shall promptly, but in all events within ten (10) days after the date on which such a request is received, adopt a resolution fixing the record date. If no record date has been fixed by the Board of Directors within ten (10) days of the date on which such a request is received, the record date for determining stockholders entitled to consent to corporate action in writing without a meeting, when no prior action by the Board of Directors is required by applicable law, shall be the first date on which a signed written consent setting forth the action taken or proposed to be taken is delivered to the corporation by delivery to its registered office in the State of Delaware, its principal place of business or an officer or agent of the corporation having custody of the book in which proceedings of meetings of stockholders are recorded. Delivery made to the corporation’s registered office shall be by hand or by certified or registered mail, return receipt requested. If no record date has been fixed by the Board of Directors and prior action by the Board of Directors is required by law, the record date for determining stockholders entitled to consent to corporate action in writing without a meeting shall be at the close of business on the day on which the Board of Directors adopts the resolution taking such prior action.
(c) In order that the corporation may determine the stockholders entitled to receive payment of any dividend or other distribution or allotment of any rights or the stockholders entitled to exercise any rights in respect of any change, conversion or exchange of stock, or for the purpose of any other lawful action, the Board of Directors may fix, in advance, a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted, and which record date shall be not more than sixty (60) days prior to such action. If no record date is fixed, the record date for determining stockholders for any such purpose shall be at the close of business on the day on which the Board of Directors adopts the resolution relating thereto.
16
Section 39. Registered Stockholders. The corporation shall be entitled to recognize the exclusive right of a person registered on its books as the owner of shares to receive dividends, and to vote as such owner, and shall not be bound to recognize any equitable or other claim to or interest in such share or shares on the part of any other person whether or not it shall have express or other notice thereof, except as otherwise provided by the laws of Delaware.
ARTICLE VIII
OTHER SECURITIES OF THE CORPORATION
Section 40. Execution of Other Securities. All bonds, debentures and other corporate securities of the corporation, other than stock certificates (covered in Section 34), may be signed by the Chairman of the Board of Directors, the President or any Vice President, or such other person as may be authorized by the Board of Directors, and the corporate seal impressed thereon or a facsimile of such seal imprinted thereon and attested by the signature of the Secretary or an Assistant Secretary, or the Chief Financial Officer or Treasurer or an Assistant Treasurer; provided, however, that where any such bond, debenture or other corporate security shall be authenticated by the manual signature, or where permissible facsimile signature, of a trustee under an indenture pursuant to which such bond, debenture or other corporate security shall be issued, the signatures of the persons signing and attesting the corporate seal on such bond, debenture or other corporate security may be the imprinted facsimile of the signatures of such persons. Interest coupons appertaining to any such bond, debenture or other corporate security, authenticated by a trustee as aforesaid, shall be signed by the Treasurer or an Assistant Treasurer of the corporation or such other person as may be authorized by the Board of Directors, or bear imprinted thereon the facsimile signature of such person. In case any officer who shall have signed or attested any bond, debenture or other corporate security, or whose facsimile signature shall appear thereon or on any such interest coupon, shall have ceased to be such officer before the bond, debenture or other corporate security so signed or attested shall have been delivered, such bond, debenture or other corporate security nevertheless may be adopted by the corporation and issued and delivered as though the person who signed the same or whose facsimile signature shall have been used thereon had not ceased to be such officer of the corporation.
ARTICLE IX
DIVIDENDS
Section 41. Declaration of Dividends. Dividends upon the capital stock of the corporation, subject to the provisions of the Certificate of Incorporation and applicable law, if any, may be declared by the Board of Directors pursuant to law at any regular or special meeting. Dividends may be paid in cash, in property, or in shares of the capital stock, subject to the provisions of the Certificate of Incorporation and applicable law.
Section 42. Dividend Reserve. Before payment of any dividend, there may be set aside out of any funds of the corporation available for dividends such sum or sums as the Board of Directors from time to time, in their absolute discretion, think proper as a reserve or reserves to meet contingencies, or for equalizing dividends, or for repairing or maintaining any property of the corporation, or for such other purpose as the Board of Directors shall think conducive to the interests of the corporation, and the Board of Directors may modify or abolish any such reserve in the manner in which it was created.
17
ARTICLE X
FISCAL YEAR
Section 43. Fiscal Year. The fiscal year of the corporation shall be fixed by resolution of the Board of Directors.
ARTICLE XI
INDEMNIFICATION
Section 44. Indemnification of Directors, Officers, Employees and Other Agents.
(a) Directors and Officers. The corporation shall indemnify its directors and officers to the fullest extent not prohibited by the DGCL or any other applicable law; provided, however, that the corporation may modify the extent of such indemnification by individual contracts with its directors and officers; and, provided, further, that the corporation shall not be required to indemnify any director or officer in connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law, (ii) the proceeding was authorized by the Board of Directors of the corporation, (iii) such indemnification is provided by the corporation, in its sole discretion, pursuant to the powers vested in the corporation under the Delaware General Corporation Law or any other applicable law or (iv) such indemnification is required to be made under subsection (d).
(b) Employees and Other Agents. The corporation shall have power to indemnify its employees and other agents as set forth in the DGCL or any other applicable law. The Board of Directors shall have the power to delegate the determination of whether indemnification shall be given to any such person to such officers or other persons as the Board of Directors shall determine.
(c) Expenses. The corporation shall advance to any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was a director or officer, of the corporation, or is or was serving at the request of the corporation as a director or officer of another corporation, partnership, joint venture, trust or other enterprise, prior to the final disposition of the proceeding, promptly following request therefor, all expenses incurred by any director or officer in connection with such proceeding, provided, however, that, if the DGCL requires, an advancement of expenses incurred by a director or officer in his or her capacity as a director or officer (and not in any other capacity in which service was or is rendered by such indemnitee, including, without limitation, service to an employee benefit plan) shall be made only upon delivery to the corporation of an undertaking, by or on behalf of such indemnitee, to repay all amounts so advanced if it shall ultimately be determined by final judicial decision from which there is no further right to appeal that such indemnitee is not entitled to be indemnified for such expenses under this Section 44 or otherwise.
18
Notwithstanding the foregoing, unless otherwise determined pursuant to paragraph (e) of this Bylaw, no advance shall be made by the corporation to an officer of the corporation (except by reason of the fact that such officer is or was a director of the corporation, in which event this paragraph shall not apply) in any action, suit or proceeding, whether civil, criminal, administrative or investigative, if a determination is reasonably and promptly made (i) by a majority vote of a quorum consisting of directors who were not parties to the proceeding, even if not a quorum, or (ii) by a committee of such directors designated by a majority of such directors, even though less than a quorum, or (iii) if there are no such directors, or such directors so direct, by independent legal counsel in a written opinion, that the facts known to the decision-making party at the time such determination is made demonstrate clearly and convincingly that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to the best interests of the corporation.
(d) Enforcement. Without the necessity of entering into an express contract, all rights to indemnification and advances to directors and officers under this Bylaw shall be deemed to be contractual rights and be effective to the same extent and as if provided for in a contract between the corporation and the director or officer. Any right to indemnification or advances granted by this Bylaw to a director or officer shall be enforceable by or on behalf of the person holding such right in any court of competent jurisdiction if (i) the claim for indemnification or advances is denied, in whole or in part, or (ii) no disposition of such claim is made within ninety (90) days of request therefor. The claimant in such enforcement action, if successful in whole or in part, shall be entitled to be paid also the expense of prosecuting the claim. In connection with any claim for indemnification, the corporation shall be entitled to raise as a defense to any such action that the claimant has not met the standards of conduct that make it permissible under the DGCL or any other applicable law for the corporation to indemnify the claimant for the amount claimed. In connection with any claim by an officer of the corporation (except in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such officer is or was a director of the corporation) for advances, the corporation shall be entitled to raise a defense as to any such action clear and convincing evidence that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to the best interests of the corporation, or with respect to any criminal action or proceeding that such person acted without reasonable cause to believe that his conduct was lawful. Neither the failure of the corporation (including its Board of Directors, independent legal counsel or its stockholders) to have made a determination prior to the commencement of such action that indemnification of the claimant is proper in the circumstances because he has met the applicable standard of conduct set forth in the DGCL or any other applicable law, nor an actual determination by the corporation (including its Board of Directors, independent legal counsel or its stockholders) that the claimant has not met such applicable standard of conduct, shall be a defense to the action or create a presumption that claimant has not met the applicable standard of conduct. In any suit brought by a director or officer to enforce a right to indemnification or to an advancement of expenses hereunder, the burden of proving that the director or officer is not entitled to be indemnified, or to such advancement of expenses, under this Article XI or otherwise shall be on the corporation.
(e) Non-Exclusivity of Rights. The rights conferred on any person by this Bylaw shall not be exclusive of any other right which such person may have or hereafter acquire under any applicable statute, provision of the Certificate of Incorporation, Bylaws, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in his official capacity and as to action in another capacity while holding office. The corporation is specifically authorized to enter into individual contracts with any or all of its directors, officers, employees or agents respecting indemnification and advances, to the fullest extent not prohibited by the DGCL or any other applicable law.
19
(f) Survival of Rights. The rights conferred on any person by this Bylaw shall continue as to a person who has ceased to be a director, officer, employee or other agent and shall inure to the benefit of the heirs, executors and administrators of such a person.
(g) Insurance. To the fullest extent permitted by the DGCL, or any other applicable law, the corporation, upon approval by the Board of Directors, may purchase insurance on behalf of any person required or permitted to be indemnified pursuant to this Bylaw.
(h) Amendments. Any repeal or modification of this Bylaw shall only be prospective and shall not affect the rights under this Bylaw in effect at the time of the alleged occurrence of any action or omission to act that is the cause of any proceeding against any agent of the corporation.
(i) Saving Clause. If this Bylaw or any portion hereof shall be invalidated on any ground by any court of competent jurisdiction, then the corporation shall nevertheless indemnify each director and officer to the full extent not prohibited by any applicable portion of this Bylaw that shall not have been invalidated, or by any other applicable law. If this Section 44 shall be invalid due to the application of the indemnification provisions of another jurisdiction, then the corporation shall indemnify each director and officer to the full extent under applicable law.
(j) Certain Definitions. For the purposes of this Bylaw, the following definitions shall apply:
(1) The term “proceeding” shall be broadly construed and shall include, without limitation, the investigation, preparation, prosecution, defense, settlement, arbitration and appeal of, and the giving of testimony in, any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative.
(2) The term “expenses” shall be broadly construed and shall include, without limitation, court costs, attorneys’ fees, witness fees, fines, amounts paid in settlement or judgment and any other costs and expenses of any nature or kind incurred in connection with any proceeding.
(3) The term the “corporation” shall include, in addition to the resulting corporation, any constituent corporation (including any constituent of a constituent) absorbed in a consolidation or merger which, if its separate existence had continued, would have had power and authority to indemnify its directors, officers, and employees or agents, so that any person who is or was a director, officer, employee or agent of such constituent corporation, or is or was serving at the request of such constituent corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, shall stand in the same position under the provisions of this Bylaw with respect to the resulting or surviving corporation as he would have with respect to such constituent corporation if its separate existence had continued.
20
(4) References to a “director,” “officer,” “employee,” or “agent” of the corporation shall include, without limitation, situations where such person is serving at the request of the corporation as, respectively, a director, officer, employee, trustee or agent of another corporation, partnership, joint venture, trust or other enterprise.
(5) References to “other enterprises” shall include employee benefit plans; references to “fines” shall include any excise taxes assessed on a person with respect to an employee benefit plan; and references to “serving at the request of the corporation” shall include any service as a director, officer, employee or agent of the corporation which imposes duties on, or involves services by, such director, officer, employee, or agent with respect to an employee benefit plan, its participants, or beneficiaries; and a person who acted in good faith and in a manner he reasonably believed to be in the interest of the participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the corporation” as referred to in this Bylaw.
ARTICLE XII
NOTICES
Section 45. Notices.
(a) Notice to Stockholders. Written notice to stockholders of stockholder meetings shall be given as provided in Section 7 herein. Without limiting the manner by which notice may otherwise be given effectively to stockholders under any agreement or contract with such stockholder, and except as otherwise required by law, written notice to stockholders for purposes other than stockholder meetings may be sent by United States mail or nationally recognized overnight courier, or by facsimile, telegraph or telex or by electronic mail or other electronic means.
(b) Notice to Directors. Any notice required to be given to any director may be given by the method stated in subsection (a), or as provided for in Section 21 of these Bylaws. If such notice is not delivered personally, it shall be sent to such address as such director shall have filed in writing with the Secretary, or, in the absence of such filing, to the last known post office address of such director.
(c) Affidavit of Mailing. An affidavit of mailing, executed by a duly authorized and competent employee of the corporation or its transfer agent appointed with respect to the class of stock affected or other agent, specifying the name and address or the names and addresses of the stockholder or stockholders, or director or directors, to whom any such notice or notices was or were given, and the time and method of giving the same, shall in the absence of fraud, be prima facie evidence of the facts therein contained.
(d) Methods of Notice. It shall not be necessary that the same method of giving notice be employed in respect of all recipients of notice, but one permissible method may be employed in respect of any one or more, and any other permissible method or methods may be employed in respect of any other or others.
21
(e) Notice to Person with Whom Communication Is Unlawful. Whenever notice is required to be given, under any provision of law or of the Certificate of Incorporation or Bylaws of the corporation, to any person with whom communication is unlawful, the giving of such notice to such person shall not be required and there shall be no duty to apply to any governmental authority or agency for a license or permit to give such notice to such person. Any action or meeting which shall be taken or held without notice to any such person with whom communication is unlawful shall have the same force and effect as if such notice had been duly given. In the event that the action taken by the corporation is such as to require the filing of a certificate under any provision of the DGCL, the certificate shall state, if such is the fact and if notice is required, that notice was given to all persons entitled to receive notice except such persons with whom communication is unlawful.
ARTICLE XIII
AMENDMENTS
Section 46. Amendments. The Board of Directors is expressly empowered to adopt, amend or repeal Bylaws of the corporation. The stockholders shall also have power to adopt, amend or repeal the Bylaws of the corporation; provided, however, that, in addition to any vote of the holders of any class or series of stock of the corporation required by law or by the Certificate of Incorporation, the affirmative vote of the holders of at least a majority of the voting power of all of the then-outstanding shares of the capital stock of the corporation entitled to vote generally in the election of directors, voting together as a single class, shall be required to adopt, amend or repeal any provision of the Bylaws of the corporation.
ARTICLE XIV
REPEALED
Section 47. Repealed.
ARTICLE XV
LOANS TO OFFICERS
Section 48. Loans to Officers. The corporation may lend money to, or guarantee any obligation of, or otherwise assist any officer or other employee of the corporation or of its subsidiaries, including any officer or employee who is a Director of the corporation or its subsidiaries, whenever, in the judgment of the Board of Directors, such loan, guarantee or assistance may reasonably be expected to benefit the corporation. The loan, guarantee or other assistance may be with or without interest and may be unsecured, or secured in such manner as the Board of Directors shall approve, including, without limitation, a pledge of shares of stock of the corporation. Nothing in these Bylaws shall be deemed to deny, limit or restrict the powers of guaranty or warranty of the corporation at common law or under any statute.
22
Exhibit 4.1
SUBSCRIPTION AGREEMENT
THIS INVESTMENT INVOLVES A HIGH DEGREE OF RISK. THIS INVESTMENT IS SUITABLE ONLY FOR PERSONS WHO CAN BEAR THE ECONOMIC RISK FOR AN INDEFINITE PERIOD OF TIME AND WHO CAN AFFORD TO LOSE THEIR ENTIRE INVESTMENT. FURTHERMORE, INVESTORS MUST UNDERSTAND THAT SUCH INVESTMENT IS ILLIQUID AND IS EXPECTED TO CONTINUE TO BE ILLIQUID FOR AN INDEFINITE PERIOD OF TIME. NO PUBLIC MARKET EXISTS FOR THE SECURITIES, AND NO PUBLIC MARKET MAY DEVELOP FOLLOWING THIS OFFERING.
THE SECURITIES OFFERED HEREBY HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), OR ANY STATE SECURITIES OR BLUE SKY LAWS AND ARE BEING OFFERED AND SOLD IN RELIANCE ON EXEMPTIONS FROM THE REGISTRATION REQUIREMENTS OF THE ACT AND STATE SECURITIES OR BLUE SKY LAWS. ALTHOUGH AN OFFERING STATEMENT HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION (THE “SEC”), THAT OFFERING STATEMENT DOES NOT INCLUDE THE SAME INFORMATION THAT WOULD BE INCLUDED IN A REGISTRATION STATEMENT UNDER THE SECURITIES ACT. THE SECURITIES HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SEC, ANY STATE SECURITIES COMMISSION OR OTHER REGULATORY AUTHORITY, NOR HAVE ANY OF THE FOREGOING AUTHORITIES PASSED UPON THE MERITS OF THIS OFFERING OR THE ADEQUACY OR ACCURACY OF THE SUBSCRIPTION AGREEMENT OR ANY OTHER MATERIALS OR INFORMATION MADE AVAILABLE TO SUBSCRIBERS IN CONNECTION WITH THIS OFFERING.
SUBSCRIBERS WHO ARE NOT “ACCREDITED INVESTORS” (AS THAT TERM IS DEFINED IN SECTION 501 OF REGULATION D PROMULGATED UNDER THE ACT) ARE SUBJECT TO LIMITATIONS ON THE AMOUNT THEY MAY INVEST, AS SET OUT IN SECTION 4. THE COMPANY IS RELYING ON THE REPRESENTATIONS AND WARRANTIES SET FORTH BY EACH SUBSCRIBER IN THIS SUBSCRIPTION AGREEMENT AND THE OTHER INFORMATION PROVIDED BY SUBSCRIBER IN CONNECTION WITH THIS OFFERING TO DETERMINE THE APPLICABILITY TO THIS OFFERING OF EXEMPTIONS FROM THE REGISTRATION REQUIREMENTS OF THE ACT.
THE OFFERING MATERIALS MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
THE COMPANY MAY NOT BE OFFERING THE SECURITIES IN EVERY STATE. THE OFFERING MATERIALS DO NOT CONSTITUTE AN OFFER OR SOLICITATION IN ANY STATE OR JURISDICTION IN WHICH THE SECURITIES ARE NOT BEING OFFERED.
THE COMPANY RESERVES THE RIGHT IN ITS SOLE DISCRETION AND FOR ANY REASON WHATSOEVER TO MODIFY, AMEND AND/OR WITHDRAW ALL OR A PORTION OF THE OFFERING AND/OR ACCEPT OR REJECT IN WHOLE OR IN PART ANY PROSPECTIVE INVESTMENT IN THE SECURITIES OR TO ALLOT TO ANY PROSPECTIVE INVESTOR LESS THAN THE AMOUNT OF SECURITIES SUCH INVESTOR DESIRES TO PURCHASE. EXCEPT AS OTHERWISE INDICATED, THE OFFERING MATERIALS SPEAK AS OF THEIR DATE. NEITHER THE DELIVERY NOR THE PURCHASE OF THE SECURITIES SHALL, UNDER ANY CIRCUMSTANCES, CREATE ANY IMPLICATION THAT THERE HAS BEEN NO CHANGE IN THE AFFAIRS OF THE COMPANY SINCE THAT DATE.
| To: | AERIS BIOTECHNOLOGIES, INC. |
8105 Rasor Boulevard, Suite 129
Plano, TX 75024
Ladies and Gentlemen:
1. SUBSCRIPTION.
(a) The undersigned (“Subscriber”) hereby irrevocably subscribes for and agrees to purchase shares of common stock, par value $0.0001 per share (the “Securities”) of Aeris Biotechnologies, Inc., a Delaware corporation (the “Company”), at a purchase price of $1.00 per share (the “Per Security Price”), provided that a Subscriber must purchase the Securities in at least the amount of the minimum investment of $500 (500 shares of Securities), upon the terms and conditions set forth herein.
(b) Subscriber understands that the Securities are being offered pursuant to an offering circular dated ____, 2022 (the “Offering Circular”) filed with the SEC as part of the Offering Statement of the Company filed with the SEC (the “Offering Statement”). By executing this Subscription Agreement, Subscriber acknowledges that Subscriber has received this Subscription Agreement, copies of the Offering Circular and Offering Statement including exhibits thereto and any other information required by the Subscriber to make an investment decision.
(c) The Subscriber’s subscription may be accepted or rejected in whole or in part, at any time prior to a Closing Date (as hereinafter defined), by the Company at its sole discretion. In addition, the Company, at its sole discretion, may allocate to Subscriber only a portion of the number of Securities Subscriber has subscribed for. The Company will notify Subscriber whether this subscription is accepted (whether in whole or in part) or rejected. If Subscriber’s subscription is rejected, Subscriber’s payment (or portion thereof if partially rejected) will be returned to Subscriber without interest and all of Subscriber’s obligations hereunder shall terminate.
(d) The aggregate number of Securities sold shall not exceed 15,000,000 Securities (the “Maximum Offering”). The Company may accept subscriptions until ____, 2023 unless otherwise extended by the Company in its sole discretion in accordance with applicable SEC regulations for such other period required to sell the Maximum Offering (the “Termination Date”). The Company may elect at any time to close all or any portion of this offering, on various dates at or prior to the Termination Date (each a “Closing Date”).
(e) In the event of rejection of this subscription in its entirety, or in the event that the sale of the Securities (or any portion thereof) is not consummated for any reason, this Subscription Agreement shall have no force or effect, except for Section 5 hereof, which shall remain in force and effect.
(f) The terms of this Subscription Agreement shall be binding upon Subscriber and its transferees, heirs, successors and assigns (collectively, “Transferees”); provided that for any such transfer to be deemed effective, the Transferee shall have executed and delivered to the Company in advance an instrument in a form acceptable to the Company in its sole discretion, pursuant to which the proposed Transferee shall acknowledge, agree, and be bound by the representations and warranties of Subscriber, and the terms of this Subscription Agreement.
2. PURCHASE PROCEDURE.
The purchase price for the Securities shall be paid simultaneously with Subscriber’s subscription. Subscriber shall deliver payment for the aggregate purchase price of the Securities by a check for available funds made payable as directed by the Company, by ACH electronic transfer, credit card, debit card, or wire transfer to an account designated by the Company, or by any combination of such methods. Payment for the Securities shall be received as directed by the Company, from the undersigned by check, ACH electronic transfer credit card, debit card, or wire transfer of immediately available funds at least two days prior to the applicable Closing Date, in the amount as set forth on the signature page hereto. The undersigned shall receive notice and evidence of the digital entry of the number of the Securities owned by undersigned reflected on the books and records of the Company, which books and records shall bear a notation that the Securities were sold in reliance upon Regulation A.
2
3. REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
The Company represents and warrants to Subscriber that the following representations and warranties are true and complete in all material respects as of the date of each Closing Date, except as otherwise indicated. For purposes of this Agreement, an individual shall be deemed to have “knowledge” of a particular fact or other matter if such individual is actually aware of such fact. The Company will be deemed to have “knowledge” of a particular fact or other matter if one of the Company’s current officers has, or at any time had, actual knowledge of such fact or other matter.
(a) Organization and Standing. The Company is a corporation duly formed, validly existing and in good standing under the laws of the State of Delaware. The Company has all requisite power and authority to own and operate its properties and assets, to execute and deliver this Subscription Agreement, and any other agreements or instruments required hereunder. The Company is duly qualified and is authorized to do business and is in good standing as a foreign corporation in all jurisdictions in which the nature of its activities and of its properties (both owned and leased) makes such qualification necessary, except for those jurisdictions in which failure to do so would not have a material adverse effect on the Company or its business.
(b) Issuance of the Securities. The issuance, sale and delivery of the Securities in accordance with this Subscription Agreement has been duly authorized by all necessary corporate action on the part of the Company. The Securities, when so issued, sold and delivered against payment therefor in accordance with the provisions of this Subscription Agreement, will be duly and validly issued, fully paid and non-assessable.
4. REPRESENTATIONS AND WARRANTIES OF SUBSCRIBER. By executing this Subscription Agreement, Subscriber (and, if Subscriber is purchasing the Securities subscribed for hereby in a fiduciary capacity, the person or persons for whom Subscriber is so purchasing) represents and warrants, which representations and warranties are true and complete in all material respects as of such Subscriber’s respective Closing Date(s):
(a) Requisite Power and Authority. Such Subscriber has all necessary power and authority under all applicable provisions of law to execute and deliver this Subscription Agreement and other agreements required hereunder and to carry out their provisions. All action on Subscriber’s part required for the lawful execution and delivery of this Subscription Agreement and other agreements required hereunder have been or will be effectively taken prior to the Closing Date. Upon their execution and delivery, this Subscription Agreement and other agreements required hereunder will be valid and binding obligations of Subscriber, enforceable in accordance with their terms, except (i) as limited by applicable bankruptcy, insolvency, reorganization, moratorium or other laws of general application affecting enforcement of creditors’ rights, and (ii) as limited by general principles of equity that restrict the availability of equitable remedies.
(b) Investment Representations. Subscriber understands that the Securities have not been registered under the Securities Act. Subscriber also understands that the Securities are being offered and sold pursuant to an exemption from registration contained in the Securities Act based in part upon Subscriber’s representations contained in this Subscription Agreement.
(c) Illiquidity and Continued Economic Risk. Subscriber acknowledges and agrees that there is no ready public market for the Securities and that there is no guarantee that a market for their resale will ever exist. Subscriber must bear the economic risk of this investment indefinitely. The Company has no obligation to list the Securities on any market or take any steps (including registration under the Securities Act or the Securities Exchange Act of 1934, as amended) with respect to facilitating trading or resale of the Securities. Further, there can be no assurance that an active market may develop or be sustained. Subscriber acknowledges that Subscriber is able to bear the economic risk of losing Subscriber’s entire investment in the Securities. Subscriber also understands that an investment in the Company involves significant risks and has taken full cognizance of and understands all of the risks relating to the purchase of the Securities and an investment in the Company, including those risks set forth in the Offering Circular and in the Company’s filings with the SEC.
3
(d) Subscriber Determination of Suitability. Subscriber has evaluated the risks of an investment in the Securities including those described in the section of the Offering Circular captioned “Risk Factors”, and has determined that the investment is suitable for Subscriber. Subscriber has adequate financial resources for an investment of this character, and at this time Subscriber could bear a complete loss of Subscriber’s investment in the Company.
(e) Accredited Investor Status or Investment Limits. Subscriber represents that either:
(i) Subscriber is an “accredited investor” within the meaning of Rule 501 of Regulation D under the Securities Act. Subscriber represents and warrants that the information set forth in response to question (c) on the signature page hereto concerning Subscriber is true and correct; or
(ii) The purchase price set out in the signature page to this Subscription Agreement, together with any other amounts previously used to purchase Securities in this offering, does not exceed 10% of the greater of the Subscriber’s annual income or net worth.
Subscriber represents that to the extent it has any questions with respect to its status as an accredited investor, or the application of the investment limits, it has sought professional advice.
5. SURVIVAL OF REPRESENTATIONS AND INDEMNITY. The representations, warranties and covenants made by the Subscriber herein shall survive the Termination Date of this Agreement. The Subscriber agrees to indemnify and hold harmless the Company and its respective officers, directors and affiliates, and each other person, if any, who controls the Company within the meaning of Section 15 of the Securities Act against any and all loss, liability, claim, damage and expense whatsoever (including, but not limited to, any and all reasonable attorneys’ fees, including attorneys’ fees on appeal) and expenses reasonably incurred in investigating, preparing or defending against any false representation or warranty or breach of failure by the Subscriber to comply with any covenant or agreement made by the Subscriber herein or in any other document furnished by the Subscriber to any of the foregoing in connection with this transaction.
6. GOVERNING LAW. This Subscription Agreement shall be governed and construed in accordance with the laws of the State of New York.
7. NOTICES. Notice, requests, demands and other communications relating to this Subscription Agreement and the transactions contemplated herein shall be in writing and shall be deemed to have been duly given if and when (a) delivered personally, on the date of such delivery; or (b) mailed by registered or certified mail, postage prepaid, return receipt requested, in the third day after the posting thereof; or (c) emailed on the date of such delivery to the address of the respective parties as follows:
| If to the Company, to: | Aeris Biotechnologies, Inc. | |
| 8105 Rasor Boulevard, Suite 129 | ||
| Plano, TX 75024 | ||
| amg@aerisbiotech.com | ||
| Copy to: | Ruskin Moscou Faltischek PC | |
| Attn: Stephen E. Fox, Esq. | ||
| 1425 RXR Plaza | ||
| 15th Floor, East Tower | ||
| Uniondale, NY 11556 | ||
| sfox@rmfpc.com |
If to a Subscriber, to the Subscriber’s address as shown on the signature page hereto or to such other address as may be specified by written notice from time to time by the party entitled to receive such notice.
4
8. MISCELLANEOUS.
(a) All pronouns and any variations thereof shall be deemed to refer to the masculine, feminine, neuter, singular or plural, as the identity of the person or persons or entity or entities may require.
(b) This Subscription Agreement is not transferable or assignable by Subscriber.
(c) The representations, warranties and agreements contained herein shall be deemed to be made by and be binding upon Subscriber and its heirs, executors, administrators and successors and shall inure to the benefit of the Company and its successors and assigns.
(d) None of the provisions of this Subscription Agreement may be waived, changed or terminated orally or otherwise, except as specifically set forth herein or except by a writing signed by the Company and Subscriber.
(e) In the event any part of this Subscription Agreement is found to be void or unenforceable, the remaining provisions are intended to be separable and binding with the same effect as if the void or unenforceable part were never the subject of agreement.
(f) The invalidity, illegality or unenforceability of one or more of the provisions of this Subscription Agreement in any jurisdiction shall not affect the validity, legality or enforceability of the remainder of this Subscription Agreement in such jurisdiction or the validity, legality or enforceability of this Subscription Agreement, including any such provision, in any other jurisdiction, it being intended that all rights and obligations of the parties hereunder shall be enforceable to the fullest extent permitted by law.
(g) This Subscription Agreement supersedes all prior discussions and agreements between the parties with respect to the subject matter hereof and contains the sole and entire agreement between the parties hereto with respect to the subject matter hereof.
(h) The terms and provisions of this Subscription Agreement are intended solely for the benefit of each party hereto and their respective successors and assigns, and it is not the intention of the parties to confer, and no provision hereof shall confer, third-party beneficiary rights upon any other person.
(i) The headings used in this Subscription Agreement have been inserted for convenience of reference only and do not define or limit the provisions hereof.
(j) This Subscription Agreement may be executed in any number of counterparts, each of which will be deemed an original, but all of which together will constitute one and the same instrument.
(k) If any recapitalization or other transaction affecting the capital stock of the Company is effected, then any new, substituted or additional securities or other property which is distributed with respect to the Securities shall be immediately subject to this Subscription Agreement, to the same extent that the Securities, immediately prior thereto, shall have been covered by this Subscription Agreement.
(l) No failure or delay by any party in exercising any right, power or privilege under this Subscription Agreement shall operate as a waiver thereof nor shall any single or partial exercise thereof preclude any other or further exercise thereof or the exercise of any other right, power or privilege. The rights and remedies herein provided shall be cumulative and not exclusive of any rights or remedies provided by law.
9. SUBSCRIPTION PROCEDURE.
Each Subscriber, by providing his or her name and subscription amount and clicking “accept” and/or checking the appropriate box on the Platform (“Online Acceptance”), confirms such Subscriber’s investment through the Platform and confirms such Subscriber’s electronic signature to this Agreement. Subscriber agrees that his or her electronic signature as provided through Online Acceptance is the legal equivalent of his or her manual signature on this Agreement and Online Acceptance establishes such Subscriber’s acceptance of the terms and conditions of this Agreement.
[SIGNATURE PAGE FOLLOWS]
5
AERIS BIOTECHNOLOGIES, INC.
SUBSCRIPTION AGREEMENT SIGNATURE PAGE
The undersigned, desiring to: (i) enter into the Subscription Agreement, dated as of ______________ (the “Subscription Agreement”), between the undersigned, Aeris Biotechnologies, Inc., a Delaware corporation (the “Company”), and the other parties thereto, in or substantially in the form furnished to the undersigned and (ii) purchase the common stock, par value $0.0001 per share (the “Securities”) of the Company as set forth below, hereby agrees to purchase such Securities from the Company and further agrees to join the Subscription Agreement as a party thereto, with all the rights and privileges appertaining thereto, and to be bound in all respects by the terms and conditions thereof. The undersigned specifically acknowledges having read the representations section in the Subscription Agreement entitled “Representations and Warranties of Subscriber,” and hereby represents that the statements contained therein are complete and accurate with respect to the undersigned as a Subscriber.
| SUBSCRIBER (individual) | |
| Signature | |
| Print Name | |
| Signature (if Joint Tenants or Tenants in Common) | |
| This Subscription is accepted on _________ |
| AERIS BIOTECHNOLOGIES, INC. | ||
| By: | ||
| Name: | ||
| Title: | ||
6
Exhibit 6.1
 |
DALMORE |
Broker-Dealer Agreement
This agreement (together with exhibits and schedules, the “Agreement”) is entered into by and between Aeris Biotechnologies, Inc. (“Client”), a Delaware Corporation, and Dalmore Group, LLC., a New York Limited Liability Company (“Dalmore”). Client and Dalmore agree to be bound by the terms of this Agreement, effective as of March 7, 2022 (the “Effective Date”):
WHEREAS, Dalmore is a registered broker-dealer providing services in the equity and debt securities market, including offerings conducted via exemptions from registration with the Securities Exchange Commission (“SEC”);
WHEREAS, Client is offering securities directly to the public in an offering exempt from registration under Regulation A (the “Offering”); and
WHEREAS, Client recognizes the benefit of having Dalmore as a broker dealer of record and service provider for investors who participate in the Offering (collectively, the “Investors”).
NOW, THEREFORE, in consideration of the mutual promises and covenants contained herein and for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
1. Appointment, Term, and Termination.
| a. | Services. Client hereby engages Dalmore to perform the services listed on Exhibit A attached hereto and made apart hereof, in connection with the Offering (the “Services”). Unless otherwise agreed to in writing by the parties, the services to be performed by Dalmore are limited to those Services. |
| b. | Term. The Agreement will commence on the Effective Date and will remain in effect for a period of twelve (12) months and will renew automatically for successive renewal terms of twelve (12) months each unless any party provides notice to the other party of non-renewal at least sixty (60) days prior to the expiration of the current term. If Client or Dalmore defaults in performing the obligations under this Agreement, the Agreement may be terminated (i) upon thirty (30) days written notice if Client or Dalmore fails to perform or observe any material term, covenant or condition to be performed or observed by it under this Agreement and such failure continues to be unremedied, (ii) upon written notice, if any material representation or warranty made by Client proves to be incorrect at any time in any material respect, or (iii) upon thirty (30) days’ written notice if Client or Dalmore commences a voluntary proceeding seeking liquidation, reorganization or other relief, or is adjudged bankrupt or insolvent or has entered against it a final and unappealable order for relief, under any bankruptcy, insolvency or other similar law, or either party executes and delivers a general assignment for the benefit of its creditors. |
1
 |
DALMORE |
2. Compensation. As compensation for the Services, Client shall pay to Dalmore the following fees:
| a. | a fee equal to one percent (1%) on the aggregate amount raised by the Client (the “Offering Fee”). The Offering Fee shall only be payable after the Financial Industry Regulatory Authority (“FINRA”) department of Corporate Finance issues a no objection letter (the “No Objection Letter”) for the Offering. Client authorizes Dalmore to deduct the Offering Fee directly from the Client’s third-party escrow or payment account. |
| b. | a one-time expense fee of five thousand ($5,000) for out-of-pocket expenses incurred by Dalmore (the “Expense Fee”). The Expense Fee is due and payable upon execution of this Agreement. The Expense Fee shall cover expenses anticipated to be incurred by the firm such as FINRA filings and any other expenses incurred by Dalmore in connection with the Offering. Notwithstanding the foregoing, Dalmore will refund to the Client any portion of the Expense Fee that remains unused. |
| c. | A one-time consulting fee of twenty thousand ($20,000) (the “Consulting Fee”) which is due and payable within five (5) days of receipt of the No Objection Letter. In the event the Consulting Fee is not paid by the first closing, Client authorizes Dalmore to deduct the Consulting Fee directly from the Client’s third-party escrow or payment account upon the first closing. |
3. Regulatory Compliance
| a. | Client and all its third-party providers shall at all times (i) maintain all required registrations and licenses, including foreign qualification, if necessary; and (iii) pay all related fees and expenses (including all fees associated with FINRA filings), in each case that are necessary or appropriate to perform their respective obligations under this Agreement. |
FINRA Corporate Filing Fee for this $20,000,000, best efforts offering will be $3,500 and will be a pass-through fee payable to Dalmore, from the Client, who will then forward it to FINRA as payment for the filing. This fee is due and payable prior to any submission by Dalmore to FINRA.
| b. | Client and Dalmore will each be responsible for supervising the activities and training of their respective sales employees, as well as all of their other respective employees in the performance of functions specifically allocated to them pursuant to the terms of this Agreement. |
| c. | Client and Dalmore agree to promptly notify the other concerning any material communications from or with any Governmental Authority or Self Regulatory Organization with respect to this Agreement or the performance of its obligations unless such notification is expressly prohibited by the applicable Governmental Authority. |
2
 |
DALMORE |
4. Role of Dalmore. Client acknowledges and agrees that Dalmore’s sole responsibilities in connection with an Offering are set forth on Exhibit A, and that Dalmore is strictly acting in an administrative and compliance capacity as the broker dealer of record, and is not being engaged by the Client to act as an underwriter or placement agent in connection with the Offering. Dalmore will use commercially reasonable efforts to perform the Services. Dalmore (i) makes no representations with respect to the quality of any investment opportunity; (ii) does not guarantee the performance of any Investor; (iii) is not soliciting or approaching investors in connection with the Offering, (iv) is not an investment adviser, does not provide investment advice and does not recommend securities transactions, (v) in performing the Services is not making any recommendation as to the appropriateness, suitability, legality, validity or profitability of the Offering, and (vi) does not take any responsibility for any documentation created and used in connection with the Offering.
5. Indemnification. Client shall indemnify and hold Dalmore, its affiliates and their representatives and agents harmless from, any and all actual or direct losses, liabilities, judgments, arbitration awards, settlements, damages and costs (collectively, “Losses”), resulting from or arising out of any third party suits, actions, claims, demands or similar proceedings (collectively, “Proceedings”) to the extent they are based upon (i) a breach of this Agreement by Client, (ii) the wrongful acts or omissions of Client, or (iii) the Offering except to the extent caused by or attributable to the gross negligence or willful misconduct of Dalmore, its employees, representatives, agents or servants.
6. Confidentiality. For purposes of this Agreement, the term “Confidential Information” means all confidential and proprietary information of a party, including but not limited to (i) financial information, (ii) business and marketing plans, (iii) the names of employees and owners, (iv) the names and other personally-identifiable information of users of the third-party provided online fundraising platform, (v) security codes, and (vi) all documentation provided by Client or Investor, but shall not include (i) information already known or independently developed by the recipient without the use of any confidential and proprietary information, or (ii) information known to the public through no wrongful act of the recipient. During the term of this Agreement and at all times thereafter, neither party shall disclose Confidential Information of the other party or use such Confidential Information for any purpose without the prior written consent of such other party. Without limiting the preceding sentence, each party shall use at least the same degree of care in safeguarding the other party’s Confidential Information as it uses to safeguard its own Confidential Information. Notwithstanding the foregoing, a party may disclose Confidential Information (i) if required to do by order of a court of competent jurisdiction, provided that such party shall notify the other party in writing promptly upon receipt of knowledge of such order so that such other party may attempt to prevent such disclosure or seek a protective order; or (ii) to any applicable governmental authority as required by applicable law. Nothing contained herein shall be construed to prohibit the SEC, FINRA, or other government official or entities from obtaining, reviewing, and auditing any information, records, or data. Client acknowledges that regulatory record-keeping requirements, as well as securities industry best practices, require Dalmore to maintain copies of practically all data, including communications and materials, regardless of any termination of this Agreement.
3
 |
DALMORE |
7. Notices. Any notices required by this Agreement shall be in writing and shall be addressed, and delivered or mailed postage prepaid, or faxed or emailed to the other parties hereto at such addresses as such other parties may designate from time to time for the receipt of such notices. Until further notice, the address of each party to this Agreement for this purpose shall be the following:
If to the Client:
Aeris Biotechnologies, Inc.
8105 Rasor Blvd, Suite 129
Plano, TX 75024
Attn: Aaron Gunn, President
Tel: 214-436-2986
Email: amg@aerisbiotech.com
If to Dalmore:
Dalmore Group, LLC
525 Green Place
Woodmere, NY 11598
Attn: Etan Butler, Chairman
Tel: 917-319-3000
Email: etan@dalmorefg.com
8. Miscellaneous.
| a. | ANY DISPUTE OR CONTROVERSY BETWEEN THE CLIENT AND PROVIDER RELATING TO OR ARISING OUT OF THIS AGREEMENT WILL BE SETTLED BY ARBITRATION BEFORE AND UNDER THE RULES OF THE ARBITRATION COMMITIEE OF FINRA. |
| b. | This Agreement is non-exclusive and shall not be construed to prevent either party from engaging in any other business activities. |
| c. | This Agreement will be binding upon all successors, assigns or transferees of Client. No assignment of this Agreement by either party will be valid unless the other party consents to such an assignment in writing. Either party may freely assign this Agreement to any person or entity that acquires all or substantially all of its business or assets. Any assignment by the either party to any subsidiary that it may create or to a company affiliated with or controlled directly or indirectly by it will be deemed valid and enforceable in the absence of any consent from the other party. |
| d. | Neither party will, without prior written approval of the other party, reference such other party in any advertisement, website, newspaper, publication, periodical or any other communication, and shall keep the contents of this Agreement confidential in accordance with the provisions set forth herein. |
4
 |
DALMORE |
| e. | THE CONSTRUCTION AND EFFECT OF EVERY PROVISION OF THIS AGREEMENT, THE RIGHTS OF THE PARTIES UNDER THIS AGREEMENT AND ANY QUESTIONS ARISING OUT OF THE AGREEMENT, WILL BE SUBJECT TO THE LAWS OF THE STATE OF NEW YORK, WITHOUT REGARD TO CONFLICT OF LAW PRINCIPLES TO THE EXTENT SUCH APPLICATION WOULD CAUSE THE LAWS OF A DIFFERENT STATE TO APPLY. The language used in this Agreement shall be deemed to be the language chosen by the parties to express their mutual intent, and no rule of strict construction will be applied against any party. |
| f. | If any provision or condition of this Agreement is held to be invalid or unenforceable by any court, or regulatory or self-regulatory agency or body, the validity of the remaining provisions and conditions will not be affected and this Agreement will be carried out as if any such invalid or unenforceable provision or condition were not included in the Agreement. |
| g. | This Agreement sets forth the entire agreement between the parties with respect to the subject matter hereof and supersedes any prior agreement relating to the subject matter herein. The Agreement may not be modified or amended except by written agreement. |
| h. | This Agreement may be executed in multiple counterparts and by facsimile or electronic means, each of which shall be deemed an original but all of which together shall constitute one and the same agreement. |
[SIGNATURES APPEAR ON FOLLOWING PAGE(S)]
5
 |
DALMORE |
IN WITNESS WHEREOF, the parties have executed this Agreement as of the date first written above.
| CLIENT: Aeris Biotechnologies, Inc. | ||
| By: | /s/ Aaron Gunn | |
| Name: | Aaron Gunn | |
| Its: | President | |
| Dalmore Group, LLC: | ||
| By: | /s/ Etan Butler | |
| Name: | Etan Butler | |
| Its: | Chairman | |
6
 |
DALMORE |
Exhibit A
Services:
| i. | Review Investor information, including KYC (Know Your Customer) data, AML (Anti-Money Laundering), OFAC compliance background checks (it being understood that KYC and AML processes may be provided by a qualified third party); |
| ii. | Review each Investor’s subscription agreement to confirm such Investor’s participation in the Offering, and provide confirmation of completion of such subscription documents to Client; |
| iii. | Contact and/or notify the issuer, if needed, to gather additional information or clarification on an Investor; |
| iv. | Keep Investor information and data confidential and not disclose to any third-party except as required by regulatory agencies or in our performance under this Agreement (e.g. as needed for AML and background checks); |
| v. | Coordinate with third party providers to ensure adequate review and compliance; |
| vi. | Provide, or coordinate the provision by a third party, of an “invest now” payment processing mechanism, including connection to a qualified escrow agent. |
7
Exhibit 6.2
WORLDWIDE EXCLUSIVE LICENSE AGREEMENT
by and between
EVOLUTION BIOTECHNOLOGIES LIMITED
and
AERIS BIOTECHNOLOGIES INC.
2021
THIS LICENSE AGREEMENT is entered into this 26th day of October, 2021, by and between, EVOLUTION BIOTECHNOLOGIES LIMITED, a private company, incorporated under the Companies Acts in England registered number 09473027 and having its registered office at 1 Doolittle Yard, Froghall Road, Ampthill, Bedfordshire, England, MK45 2NW (hereinafter referred to as “the Licensor”)
and
AERIS BIOTECHNOLOGIES INC., a private company, incorporated under the laws of the State of Delaware having its registered office at 1013 Centre Road, suite 408S, Wilmington, Delaware, and a current place of business located at 8105 Rasor Blvd., Suite 129, Plano, Texas 75024 (hereinafter referred to as “the Licensee”)
(Jointly, The Parties)
WHEREAS
(A) The Licensor is the owner of the Assets (as hereinafter defined) and of the Intellectual Property Rights (as hereinafter defined); and Licensee in additional consideration of such License undertakes to support such Research Activities of the Licensor as shall be agreed between the Parties as necessary to foster commercial development of the Assets and the Intellectual Property Rights, as well as meeting its financial obligations as stated hereinafter.
(B) the Licensor has agreed to license to the Licensee (in consideration of the ongoing support of Research Activities) the Assets and the Intellectual Property Rights and financial consideration as outlined further herein and the Licensee has agreed to receive such license.
NOW THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, and other good and valuable consideration, the receipt of which is hereby acknowledged, the parties agree as follows:
1. DEFINITIONS AND INTERPRETATION
In this Agreement:
1.1 The following words and expressions shall bear the meanings respectively set opposite them (unless the context otherwise requires):
“Act” Shall mean under relevant UK law;
“Agreement” Shall mean this License Agreement or as amended in accordance with its terms;
“Area” Shall mean the biological control of arthropods
“Assets” Shall mean the Patents, both awarded and ongoing applications;
“License Effective Date” Shall mean the last date of execution of this Agreement;
“Associated Rights” Shall mean the rights specified in Clauses 2.1.1, 2.1.2, 2.1.3 and 2.1.4;
2
“Confidential Information” Shall mean information in whatever form which is disclosed by one party to the other in terms of this Agreement relating to the Assets or the Intellectual Property Rights;
“Intellectual Property Rights” Shall mean all past, present and future intellectual property rights of whatever nature anywhere in the world (including, without limitation or prejudice to the foregoing generality, all copyrights, trademarks (whether registered or unregistered), trade and business names, design rights, database rights, know how, confidential information, patents, patents applications, internet rights, domain names, semi-conductor topography rights and any and all applications for any of the foregoing and any and all rights to apply for any of the foregoing) in and to the Assets;
“Parties” Shall mean the Licensor and the Licensee;
“Patent and Patent Applications” Shall mean the patents and patent applications named “Acaricides”, further particulars of which are set out in Part 1 of the Schedule, which is attached hereto and made a part hereof for all purposes, and all subsequent filings in the Area, and;
Research Activities” Shall mean scientific and commercial activities relating to the Area, including reasonable staff salaries and related costs, property costs, and related and accrued professional costs (including those for accounting, insurance, legal, and intellectual property).
1.2 Reference to any statute or statutory provision shall include a reference to any statute or statutory provision which amends, extends, consolidates or replaces the same or which has been amended, extended, consolidated or replaced by the same and shall include any orders, regulations, instruments or other subordinate legislation made under the relevant statute or statutory provision but not so as to defeat the purpose of this Agreement.
1.3 The singular includes the plural and the masculine includes the feminine and vice versa.
1.4 References to persons shall include bodies corporate, unincorporated associations and partnerships.
1.5 References to any Clause, Sub-clause, Schedule or Part of a Schedule are references to such terms contained in this Agreement, unless otherwise specified.
1.6 Reference to this Agreement shall include the Recitals.
1.7 The headings to Clauses are for convenience only and shall not affect the interpretation of this Agreement.
1.8 Reference to any party in this Agreement shall be deemed to include a reference to its successors, permitted transferees and permitted Licensees.
3
2. LICENSE
2.1 In consideration of the ongoing funding of Research Activities undertaken by the Licensee as shall be agreed between the Parties and in addition thereto the payment of $2,000,000 to the Licensor with such payment being $600,000 being due and payable upon Licensee raise of $3,000,000 pursuant to its Reg A filing with the Securities and Exchange Commission and the remaining $1,400,000 due and payable within 30 days of Licensee going public, being the Licensee is listed upon a publicly traded stock exchange platform, RTO, IPO, self-listing or SPAC, etc., the Licensor hereby grants an exclusive, unlimited license to the Licensee on the License Effective Date hereof, all of the Licensor’s right, title and interest, past and present and future in and to the Assets and the Intellectual Property Rights, for use anywhere in the world, including but not limited to:
2.1.1 with regard to patents and patent applications, all rights, powers, privileges, immunities and advantages conferred on the owner thereof in respect of any past, existing or future infringements thereof and the right to apply for, prosecute and obtain patent or similar protection throughout the world (together with the right to claim priority under the International Convention for the Protection of Industrial Property) in respect of the inventions claimed in any patents or patent applications and to the intent that the grant of any patents or similar protection shall be in the name of and vest in the Licensee;
2.1.2 with regard to copyright and design right the exclusive right to do and to authorise others to do any and all acts restricted by the Act in the United Kingdom and the right to register or record such copyright and/or design right in any country in the world where such registration or recording is possible;
2.1.3 with regard to trade marks, all rights, powers, privileges, immunities and advantages conferred on the owner thereof in respect of any past, existing or future infringements thereof and the right to apply for, prosecute and obtain trademarks or similar protection throughout the world; and
2.1.4 all rights of a similar nature to those described in Clauses 2.1.1, 2.1.2 and 2.1.3 above conferred in respect of the Assets and the Intellectual Property Rights by the laws in force in all parts of the world; all for the full period thereof including all corresponding patents, registered trademarks or registered designs and all applications for the same and any re-issues, renewals or extensions of the same (and in the case of applications, all divisions and continuations of such applications) and including all rights of action accrued to each of the above rights at the License Effective Date.
2.2 On the License Effective Date the Licensor will in so far as the Licensor has the right and title to do so deliver to the Licensee all Confidential Information (which will be provided in written or any other agreed format), including any drawings, descriptions, data and prototypes, which are relevant to the Licensee’s use and exploitation of the Assets and the Intellectual Property Rights under this Agreement.
2.3 The Licensor undertakes at the request and expense of the Licensee to do all acts and execute all documents, forms and authorisations anywhere in the world which may be necessary or reasonably desirable to confirm the title of the Licensee to the Assets and the Intellectual Property Rights assigned or vested in the Licensee hereunder, whether in connection with any registration of such title or otherwise and in the event that the Licensor fails to so the Licensee is hereby irrevocably authorized and empowered to exercise and perform such acts and take such proceedings in the name and on behalf of the Licensor and as the attorney for the Licensor.
4
2.4 The Licensor will so far as necessary and at the expense of the Licensee permit and enable the Licensee to apply for and will take all reasonable steps to assist the Licensee in obtaining the grant of patent, registered trade mark, registered design or like protection in respect of the Assets and Intellectual Property Rights assigned under this Agreement in any territory as may be required by the Licensee.
2.5 The Licensor hereby acknowledges and agrees that all physical or tangible embodiments of the Assets and the Intellectual Property Rights licensed hereunder (including but not limited to all models, drawings, documents, prototypes, domain name certificates, computer printouts and discs, CD Roms, graphs, charts, substances or compounds) though the ownership of the property of the shall remain in the Licensor, shall be delivered to the Licensee on the License Effective Date (if not already in the Licensee’s possession). Notwithstanding any language to the contrary contained herein, Licensee acknowledges and agrees that Licensor shall retain in the UK what is reasonably necessary and required to conduct Research Activities.
3. CONFIDENTIALITY
3.1 The Licensor agrees and undertakes to the Licensee that it shall keep secret and confidential at all times both during and after the term of this Agreement all and any Confidential Information of the Licensee (including all rights hereby assigned) which comes into the Licensor’s possession at any time either before, during or after the term of this Agreement. The Licensor shall not (except for the performance of the Licensor’s obligations hereunder) use, copy or divulge such Confidential Information to any third party except with the express written consent of the Licensee (and that except insofar as such disclosure is permitted in terms of Clauses 3.2 and 3.3). Any such permitted disclosure shall in no way affect the ownership of such Confidential Information.
3.2 The obligations contained in Clause 3.1 shall not apply to Confidential Information which:
3.2.1 at the License Effective Date is demonstrably within the public domain; or
3.2.2 after the License Effective Date comes into the public domain, otherwise than by reason of a breach of this Clause 3 or any other undertaking or a party is required to disclose information by applicable law, regulation or order of a governmental agency or a court of competent jurisdiction.
3.3 Any announcement, press or media release or other publicity regarding this Agreement, directly or indirectly, shall only be made if it is approved in writing by the Licensee, or is required by law, and the terms of this Agreement, subject to the provisions of Clauses 3.1 and 3.2 shall otherwise be kept secret and confidential by the Licensor at all times.
4. WARRANTIES
4.1 The Licensor warrants and represents to the Licensee as at the date hereof:
5
4.1.1 that there are no liens or other encumbrances in respect of the Assets or the Intellectual Property Rights which will affect the ability of the Licensor to grant the exclusive license under this Agreement;
4.1.2 that the Licensor has not and will not grant or assign or licence any rights of any nature in and to the Assets and/or the Intellectual Property Rights to any third party;
4.1.3 that the Intellectual Property Rights are in full force and effect and each of those rights is valid and enforceable, and none of them is being used, claimed, opposed or attacked by any other person;
4.1.4 no right or licence has been granted or shall be granted to any person or body by the Licensor to use in any manner or to do anything that would or might otherwise infringe any of the rights granted hereunder; and
4.1.5 the Assets do not and are not likely to infringe any intellectual property right of any third party and the Licensor.
4.2 If the Licensor detects, suspects, or otherwise becomes aware of any infringement by a third party of the Assets and/or the Intellectual Property Rights the Licensor shall promptly notify the Licensee and provide all details within the Licensor’s knowledge with respect to the same.
4.3 If any such misuse, misappropriation or infringement of the Assets and/or the intellectual Property Rights occurs or the Assets and/or the Intellectual Property Rights infringes the rights of any third party, then the Licensee shall be entitled, at its expense, to institute such proceedings as it may deem necessary or desirable in the circumstances; the Licensor shall give such assistance in connection with any such proceedings as the Licensee shall reasonably deem necessary (subject to the Licensee paying the Licensor’s reasonable costs and expenses in connection therewith).
5. SEVERABILITY
If any provision of this Agreement shall be found by any court or administrative body of competent jurisdiction to be invalid or unenforceable, the invalidity or unenforceability of such provision shall not affect the other provisions of this Agreement and all provisions unaffected by such invalidity or unenforceability shall remain in full force and effect. The parties hereby agree to attempt to substitute for any invalid or unenforceable provision a valid or enforceable provision which achieves to the greatest extent possible the economic, legal and commercial objectives of the invalid or unenforceable provision.
6. WAIVER
The rights and powers which the parties have under this Agreement shalt not be prejudiced or restricted by any delay in the exercise of those rights or powers or by any indulgence or by any forbearance extended to another party. No failure or delay by any party hereto to exercise any such right or power shall operate as a waiver thereof nor shall any partial exercise of any such right or power preclude any other or further exercise of that or any other right or power.
6
7. ENTIRE AGREEMENT
7.1 This Agreement contains the entire and only agreement between the parties hereto in relation to the subject matter hereof and supersedes all previous negotiations, representations, undertakings and agreements both written and oral made between the parties with respect to the subject matter hereof.
7.2 Modification or amendment of this Agreement or of any of the provisions herein contained shall not be valid unless made in writing and signed on behalf of the respective parties or duly authorised agents thereof.
8. FURTHER ASSURANCES
Each of the parties agrees at the cost of the other party to perform without delay such further acts and execute and deliver such further documents as may be required by law or otherwise necessary or reasonably desirable to implement and/or perfect this Agreement.
9. COSTS AND EXPENSES
Subject to the provisions of Clauses 2.3, 2.4 and 8 each of the parties hereto shall bear their own costs and expenses incidental to the negotiation of and to the preparation and carrying into effect of this Agreement.
10. NOTICES
Any notice document consent or approval relating to this Agreement (including this Clause) shall be in writing and may be served upon or delivered to the parties hereto at their respective addresses stated in this Agreement or at such other address (if any) as may have been notified for the purpose. Notices sent by recognised courier or by recorded delivery mail shall be deemed to have been delivered forty eight hours after posting and proof of due posting shall be sufficient evidence of delivery.
11. LAW AND JURISDICTION
This Agreement shall be governed by, interpreted and construed in accordance with the laws of the State of Delaware, without regard to the conflicts of law principles thereof. The courts of the State of Delaware shall have jurisdiction over the parties hereto in all matters arising hereunder and the exclusive venue for any such action shall be a state or federal court in Wilmington Delaware.
12. ASSIGNMENT
Neither this Agreement nor any right or obligation hereunder may be assigned or delegated, in whole or in part, by either party without the expressed written consent of the other; provided however, that either party may, without the written consent of the other, assign this Agreement and its rights and delegate its obligations hereunder in connection with the sale of all or substantially all of its business, or in the event of a merger, consolidation, change of control or similar transaction, Any permitted Licensee shall assume all obligations of its Licensor under this Agreement. Any purported assignment in violation of this Section 12 shall be void ab initio.
7
13. NO CONSEQUENTIAL DAMAGES
IN NO EVENT SHALL A PARTY BE LIABLE FOR SPECIAL, INCIDENTAL OR CONSEQUENTIAL DAMAGES ARISING OUT OF THIS AGREEMENT OR THE EXERCISE OF ITS RIGHTS HEREUNDER.
14. COUNTERPARTS
This Agreement may be executed in counterparts, each of which shall be deemed to be an original and together shall be deemed to be one and the same agreement.
8
IN WITNESS WHEREOF, the parties hereto have each caused this Agreement to be executed by their duly authorized representatives as of the License Effective Date.
| EVOLUTION BIOTECHNOLOGIES LIMITED | ||
| By: | /s/ Malcolm McConville | |
| Name: | Malcolm McConville | |
| Title: | Director | |
| Date: | 16 October 2021 | |
| AERIS BIOTECHNOLOGIES INC. | ||
| By: | /s/ Aaron Gunn | |
| Name: | Aaron Gunn | |
| Title: | President | |
| Date: | October 26, 2021 | |
9
SCHEDULE TO THE LICENSE AGREEMENT
Part 1 – Existing Patents and Patent Applications
Deriving from WO2017025732A1 “Acaricides,” original filing 7th August 2015
Patent Applicant portfolio
| Our
Ref Country |
Patent
Title Instructor |
Application no. | Inventors Application Date Patent /Publ. no Status |
Next diary action | Due Date | |||||||||
| P47027 | Acaricides Evolution Biotechnologies Ltd | Harper. David | ||||||||||||
| Australia | 2016205553 | 08/08/2016 | 2016305553 | Granted | Renewal Reminder No. 1 | 07/04/2022 | ||||||||
| Switzerland & Liechtenstein | 16751329.0 | 08/08/2016 | 3331366 | Granted | Renewal Reminder No, 1 | 30/04/2022 | ||||||||
| Germany | 16751329.0 | 08/08/2016 | 3331366 | Granted | Renewal billed and completed | 28/02/2022 | ||||||||
| European Patent Office | 19153733.1 | 08/08/2016 | 3494792 | Pending | Mailing Period | 22/06/2019 | ||||||||
| European Patent Office | 16751329.0 | 08/08/2016 | 3331366 | Granted | (Grant) OA Family - 2 years | 20/12/2020 | ||||||||
| France | 16751329.0 | 08/08/2016 | 3331366 | Granted | Renewal billed and completed | 28/02/2022 | ||||||||
| United Kingdom | 1513981.9 | 07/08/2015 | Priority Only | Fie Declaration of Inventor | 07/12/2016 | |||||||||
| United Kingdom | 16751329.0 | 08/08/2016 | 3331366 | Granted | Renewal Reminder No. 1 | 30/04/2022 | ||||||||
| Hong Kong | 19126065.2 | 08/08/2016 | 40002838 | Pending | Critical date for law change | 03/04/2023 | ||||||||
| Hong Kong | 18111847.2 | 08/08/2016 | 1252537 | Granted | Renewal Reminder No. 1 | 07/04/2023 | ||||||||
| Ireland | 16751329.0 | 08/08/2016 | 3331366 | Granted | Renewal Grace Period | 28/02/2022 | ||||||||
| Japan | 2018-525822 | 08/08/2016 | 6840753 | Granted | Renewal Reminder No 1 | 18/10/2023 | ||||||||
| Netherlands | 16751329.0 | 08/08/2018 | 3331366 | Granted | Renewal billed and completed | 28/02/2022 | ||||||||
| United States of America | 15/750474 | 08/08/2016 | 2018/0235237 | Pending | Final OA - Advisory Response | 14/11/2021 | ||||||||
| Deadline (2mths) | ||||||||||||||
| Patent Cooperation Treaty | PCT/GB2016/052455 | 08/08/2016 | WO2017/025732 | See National Phase | Expiry date | 08/08/2036 | ||||||||
10
Exhibit 6.3
THIS NOTE AND THE SECURITIES ISSUABLE UPON THE CONVERSION HEREOF HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “ACT”), OR UNDER THE SECURITIES LAWS OF CERTAIN STATES. IN ADDITION TO OTHER RESTRICTIONS ON TRANSFER SET FORTH HEREIN, THESE SECURITIES MAY NOT BE OFFERED, SOLD OR OTHERWISE TRANSFERRED, PLEDGED OR HYPOTHECATED EXCEPT AS PERMITTED UNDER THE ACT AND APPLICABLE STATE SECURITIES LAWS PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT OR AN EXEMPTION THEREFROM. THE ISSUER OF THESE SECURITIES MAY REQUIRE AN OPINION OF COUNSEL REASONABLY SATISFACTORY TO THE ISSUER THAT SUCH OFFER, SALE OR TRANSFER, PLEDGE OR HYPOTHECATION OTHERWISE COMPLIES WITH THE ACT AND ANY APPLICABLE STATE SECURITIES LAWS.
AERIS BIOTECHNOLOGIES, INC.
CONVERTIBLE PROMISSORY NOTE
| $[___] | [ISSUE DATE] |
FOR VALUE RECEIVED, Aeris Biotechnologies, Inc., a Delaware corporation (the “Company”), promises to pay to [_____] (“Investor”), or its registered assigns, in lawful money of the United States of America, the principal sum of [_____] dollars ($[___]), or such lesser amount as shall equal the outstanding principal amount hereof, together with interest from the date of this Convertible Promissory Note (this “Note”) on the unpaid principal balance at a rate equal to [__]% per annum. Unless converted into equity securities pursuant to Section 5 hereof, all unpaid principal, together with any then unpaid and accrued interest and other amounts payable hereunder, shall be due and payable on the earlier of (i) [DATE] (the “Maturity Date”) or (ii) when, upon the occurrence and during the continuance of an Event of Default, such amounts are declared due and payable by Investor or made automatically due and payable, in each case, in accordance with the terms hereof.
The following is a statement of the rights of Investor and the conditions to which this Note is subject, and to which Investor, by the acceptance of this Note, agrees:
1. Payments.
(a) Interest. Unless converted into equity securities pursuant to Section 5 hereof, accrued interest on this Note shall be payable at maturity.
(b) Voluntary Prepayment. This Note may not be prepaid without the written consent of the Investor.
2. Events of Default. The occurrence of any of the following shall constitute an “Event of Default” under this Note:
(a) Failure to Pay. The Company shall fail to pay (i) when due any principal payment on the due date hereunder or (ii) any interest payment or other payment required under the terms of this Note on the date due and such payment shall not have been made within ten (10) business days of the Company’s receipt of written notice to the Company of such failure to pay; or
(b) Voluntary Bankruptcy or Insolvency Proceedings. The Company shall (i) apply for or consent to the appointment of a receiver, trustee, liquidator or custodian of itself or of all or a substantial part of its property, (ii) make a general assignment for the benefit of its or any of its creditors, (iii) be dissolved or liquidated, (iv) commence a voluntary case or other proceeding seeking liquidation, reorganization or other relief with respect to itself or its debts under any bankruptcy, insolvency or other similar law now or hereafter in effect or consent to any such relief or to the appointment of or taking possession of its property by any official in an involuntary case or other proceeding commenced against it or (v) take any action for the purpose of effecting any of the foregoing; or
(c) Involuntary Bankruptcy or Insolvency Proceedings. Proceedings for the appointment of a receiver, trustee, liquidator or custodian of the Company, or of all or a substantial part of the property thereof, or an involuntary case or other proceedings seeking liquidation, reorganization or other relief with respect to the Company or any of its subsidiaries, if any, or the debts thereof under any bankruptcy, insolvency or other similar law now or hereafter in effect shall be commenced and an order for relief entered or such proceeding shall not be dismissed or discharged within 90 days of commencement.
3. Rights of Investor upon Default. Upon the occurrence of an Event of Default described in Section 2(a) and at any time thereafter during the continuance of such Event of Default, Investor may by written notice to the Company, declare all outstanding Obligations payable by the Company hereunder to be immediately due and payable without presentment, demand, protest or any other notice of any kind, all of which are hereby expressly waived, anything contained herein to the contrary notwithstanding. Upon the occurrence of any Event of Default described in Sections 2(b) and 2(c), immediately and without notice, all outstanding Obligations payable by the Company hereunder shall automatically become immediately due and payable, without presentment, demand, protest or any other notice of any kind, all of which are hereby expressly waived, anything contained herein or to the contrary notwithstanding. In addition to the foregoing remedies, upon the occurrence and during the continuance of any Event of Default, Investor may exercise any other right, power or remedy permitted to it by law, either by suit in equity or by action at law, or both.
4. Security. This Note is a general unsecured obligation of the Company.
5. Conversion Upon Qualified Financing.
(a) Automatic Conversion Upon a Qualified Financing. If a Qualified Financing occurs prior to the Maturity Date or an earlier conversion of this Note in accordance with the terms hereof, then the entire outstanding principal amount of this Note and all accrued and unpaid interest on this Note shall automatically convert into Qualified Shares issued in such Qualified Financing at the Conversion Price.
2
(b) Conversion Procedure. Upon the conversion of this Note, Investor shall execute and deliver to the Company all transaction documents entered into by other purchasers participating in the Qualified Financing. Investor shall also deliver the original of this Note (or a notice to the effect that the original Note has been lost, stolen or destroyed and an agreement acceptable to the Company whereby the holder agrees to indemnify the Company from any loss incurred by it in connection with this Note) at the closing of the Qualified Financing for cancellation; provided, however, that upon the closing of the Qualified Financing, this Note shall be deemed converted and of no further force and effect, whether or not it is delivered for cancellation as set forth in this sentence. The Company shall, as soon as practicable thereafter, at its expense issue and deliver to such Investor a certificate or certificates for the number of Qualified Shares to which Investor shall be entitled upon such conversion.
(c) Fractional Shares; Interest; Effect of Conversion. No fractional securities shall be issued upon conversion of this Note. In lieu of the Company issuing any fractional securities to the Investor upon the conversion of this Note, the Company shall pay to Investor an amount equal to the product obtained by multiplying the Conversion Price by the fraction of a unit of the security not issued pursuant to the previous sentence. In addition, to the extent not converted into Qualified Shares, the Company shall pay to Investor any interest accrued on the amount converted and on the amount to be paid to the Company pursuant to the previous sentence. Upon conversion of this Note in full and the payment of the amounts specified in this paragraph, the Company shall be forever released from all its obligations and liabilities under this Note and this Note shall be deemed of no further force or effect, whether or not the original of this Note has been delivered to the Company for cancellation.
6. Definitions. As used in this Note, the following capitalized terms have the following meanings:
“Applicable Discount” shall mean 50%.
“Conversion Price” shall mean the share price per share determined by multiplying (i) the Applicable Discount by (ii) the per share price of the Qualified Shares sold in the Qualified Financing.
“Event of Default” has the meaning given in Section 2 hereof.
“Investor” shall mean the Person specified in the introductory paragraph of this Note or any Person who shall at the time be the registered holder of this Note.
“Obligations” shall mean and include all loans, advances, debts, liabilities and obligations, howsoever arising, owed by the Company to Investor of every kind and description, now existing or hereafter arising under or pursuant to the terms of this Note, including, all interest, fees, charges, expenses, attorneys’ fees and costs and accountants’ fees and costs chargeable to and payable by the Company hereunder and thereunder, in each case, whether direct or indirect, absolute or contingent, due or to become due, and whether or not arising after the commencement of a proceeding under Title 11 of the United States Code (11 U. S. C. Section 101 et seq.), as amended from time to time (including post-petition interest) and whether or not allowed or allowable as a claim in any such proceeding.
3
“Person” shall mean and include an individual, a partnership, a corporation (including a business trust), a joint stock company, a limited liability company, an unincorporated association, a joint venture or other entity or a governmental authority.
“Qualified Financing” is a transaction or series of transactions completed after the date hereof pursuant to which the Company issues and sells Qualified Shares for an aggregate amount of at least $[___] (excluding any and all indebtedness that is converted into equity securities, or otherwise cancelled in consideration for the issuance of such equity securities, including conversion of the Note) with the principal purpose of raising capital.
“Qualified Shares” shall mean a new or existing class of shares of capital stock of the Company (whether common, preferred or otherwise) designated or to be designated by the Company, offered, issued and sold in a Qualified Financing.
7. Miscellaneous.
(a) Successors and Assigns; Transfer of this Note or Securities Issuable on Conversion Hereof.
(i) Subject to the restrictions on transfer described in this Section 7(a), the rights and obligations of the Company and Investor shall be binding upon and benefit the successors, assigns, heirs, administrators and transferees of the parties hereto.
(ii) Neither this Note nor any of the rights, interests or obligations hereunder may be assigned, in whole or in part, by the Company without the prior written consent of the Investor.
(b) Waiver and Amendment. Any provision of this Note may be amended, waived or modified upon the written consent of the Company and the Investor.
(c) Notices. All notices, requests, demands, consents, instructions or other communications required or permitted hereunder shall be in writing and shall be faxed, mailed or delivered to each party as follows: (i) if to Investor, at such Investor’s address or facsimile number set forth in the Company’s records, or at such other address as such Investor shall have furnished to the Company in writing, or (ii) if to the Company, at 8105 Rasor Boulevard, Suite 129, Plano, TX 75024, or at such other address as the Company shall have furnished to Investor. All such notices and communications will be deemed effectively given the earlier of (i) when received if delivered personally, (ii) one business day after being deposited with an overnight courier service of recognized standing or (iii) four days after being deposited in the U.S. mail, first class with postage prepaid.
(d) Payment. Unless converted into the Company’s equity securities pursuant to the terms hereof, payment shall be made in lawful tender of the United States.
(e) Usury. In the event any interest is paid on this Note which is deemed to be in excess of the then legal maximum rate, then that portion of the interest payment representing an amount in excess of the then legal maximum rate shall be deemed a payment of principal and applied against the principal of this Note.
4
(f) Waivers. The Company hereby waives notice of default, presentment or demand for payment, protest or notice of nonpayment or dishonor and all other notices or demands relative to this instrument.
(g) Governing Law; Jurisdiction. This Note and all actions arising out of or in connection with this Note shall be governed by, and construed in accordance with, the laws of the State of Texas applicable to contracts made and to be performed wholly within said State, without giving effect to the conflict of laws principles thereof. The Company and the Investor each irrevocably and unconditionally submits to the exclusive jurisdiction of the United States District Court for the District including Plano, Texas or, if such court will not accept jurisdiction, any court of competent civil jurisdiction sitting in Collin County, Texas. In any action, suit or other proceeding, the Company and the Investor each hereto irrevocably and unconditionally waives and agrees not to assert by way of motion, as a defense or otherwise any claims that it is not subject to the jurisdiction of the above courts, that such action or suit is brought in an inconvenient forum or that the venue of such action, suit or other proceeding is improper.
(h) WAIVER OF JURY TRIAL. EACH OF THE COMPANY AND THE INVESTOR HEREBY WAIVES ANY RIGHT TO TRIAL BY JURY IN ANY ACTION OR PROCEEDING ARISING OUT OF OR RELATING TO THIS AGREEMENT, WHETHER NOW EXISTING OR HEREAFTER ARISING, AND WHETHER SOUNDING IN CONTRACT, TORT OR OTHERWISE. EACH OF THE COMPANY AND THE INVESTOR EACH AGREES THAT THE COMPANY OR THE INVESTOR MAY FILE A COPY OF THIS PARAGRAPH WITH ANY COURT AS WRITTEN EVIDENCE OF THE KNOWING, VOLUNTARY AND BARGAINED-FOR AGREEMENT BETWEEN THE COMPANY AND THE INVESTOR IRREVOCABLY TO WAIVE TRIAL BY JURY AND THAT ANY ACTION OR PROCEEDING WHATSOEVER BETWEEN THEM RELATING TO THIS NOTE SHALL INSTEAD BE TRIED IN A COURT OF COMPETENT JURISDICTION BY A JUDGE SITTING WITHOUT A JURY.
(i) Counterparts. This Note may be executed in any number of counterparts and by different parties on separate counterparts, each of which, when executed and delivered, shall be deemed to be an original, and all of which, when taken together, shall constitute but one and the same Note.
(j) No Rights as Member or Shareholder. This Note does not entitle the Investor to any voting rights or other rights as a shareholder of the Company prior to the conversion hereof.
[Remainder of Page Intentionally Left Blank; Signature Page Follows]
5
The Company has caused this Note to be issued as of the date first written above.
| Aeris Biotechnologies, Inc. | |||
| By: | |||
| Name: | |||
| Title: | |||
6
Exhibit 11.1
CONSENT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation in this Offering Statement on Form 1-A of our report dated April 25, 2022, relating to the financial statements of Aeris Biotechnologies, Inc. as of October 31, 2021 and to all references to our firm included in this Registration Statement.

Certified Public Accountants
Lakewood, CO
April 25, 2022