

Exhibit 99.2 Mining for Tomorrow’s Cures Strategic Partnership With Taiho to Jointly Develop and Commercialize CLN-081 in the U.S. May 12, 2022

Important Notice and Disclaimers This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, express or implied statements regarding Cullinan’s beliefs and expectations regarding the milestone payments we may receive from Taiho; the anticipated development and commercialization of CLN-081/TAS6417; the development of our commercial infrastructure; potential investments in our pipeline and the potential for such product candidates; and our cash runway. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “hope,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Any forward-looking statements in this press release are based on management's current expectations and beliefs of future events, and are subject to known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any expressed or implied by the forward-looking statements. These risks include, but are not limited to, the following: uncertainty regarding the timing and results of regulatory submissions; success of our clinical trials and preclinical studies; risks related to our ability to protect and maintain our intellectual property position; risks related to manufacturing, supply, and distribution of our product candidates; risks related to the impact of COVID-19 affecting countries or regions in which we have operations or do business, including potential negative impacts on our employees, customers, supply chain and production as well as global economies and financial markets; the risk that any one or more of our product candidates, including those that are co- developed, will not be successfully developed and commercialized; the risk that the results of preclinical studies or clinical studies will not be predictive of future results in connection with future studies; and performance and results of any collaboration, partnership, license or similar agreements. These and other important risks and uncertainties discussed in our filings with the Securities and Exchange Commission (SEC), including under the caption “Risk Factors” in our most recent Annual Report on Form 10-K and subsequent filings with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. While we may elect to update such forward-looking statements in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change, except to the extent required by law. These forward- looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release. Moreover, except as required by law, neither Cullinan nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements included in this press release. Any forward-looking statement included in this press release speaks only as of the date on which it was made. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source. 2

Webcast Agenda AGENDA PRESENTERS Nadim Ahmed 1. Introduction Chief Executive Officer Corinne Savill, Ph.D. 2. Collaboration Overview Chief Business Officer 3. Financial Context Jeff Trigilio Chief Financial Officer 4. Strategic Perspective JOINING US FOR Q&A Jeffrey Jones, M.D., MPH, MBA 5. Q&A Promise of Chief Medical Officer single agent activity 3
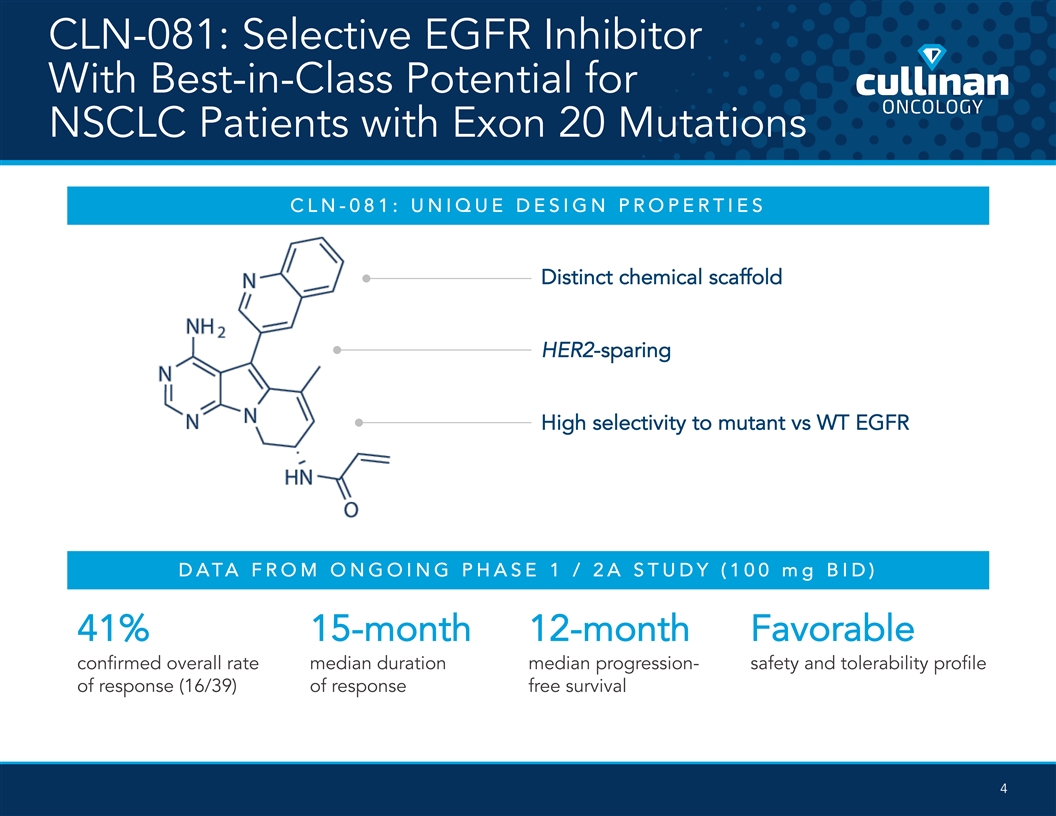
CLN-081: Selective EGFR Inhibitor With Best-in-Class Potential for NSCLC Patients with Exon 20 Mutations CLN -081: UNIQUE DESIGN PROPERTIES Distinct chemical scaffold HER2-sparing High selectivity to mutant vs WT EGFR DATA FROM ONGOING PHASE 1 / 2A STUDY (100 mg BID) 41% 15-month 12-month Favorable confirmed overall rate median duration median progression- safety and tolerability profile of response (16/39) of response free survival 4
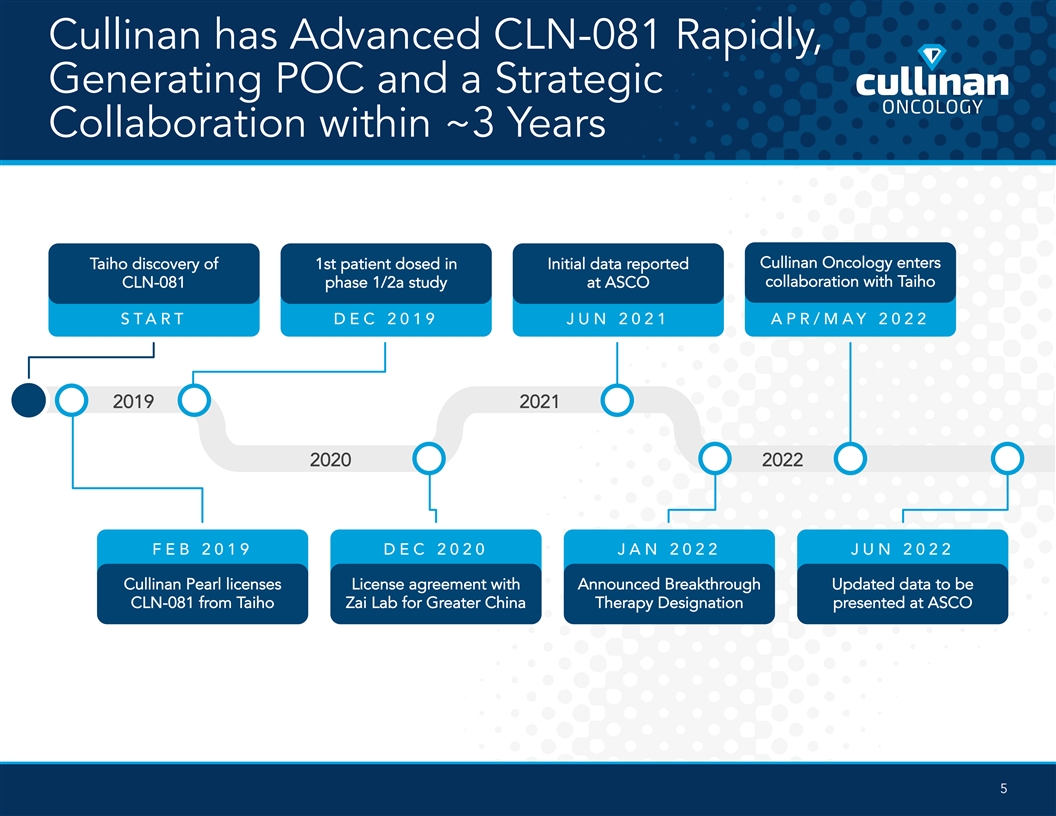
Cullinan has Advanced CLN-081 Rapidly, Generating POC and a Strategic Collaboration within ~3 Years Cullinan Oncology enters Taiho discovery of 1st patient dosed in Initial data reported collaboration with Taiho CLN-081 phase 1/2a study at ASCO START DEC 2019 JUN 2021 APR/MAY 2022 2019 2021 2020 2022 FEB 2019 DEC 2020 JAN 2022 JUN 2022 Cullinan Pearl licenses License agreement with Announced Breakthrough Updated data to be CLN-081 from Taiho Zai Lab for Greater China Therapy Designation presented at ASCO 5
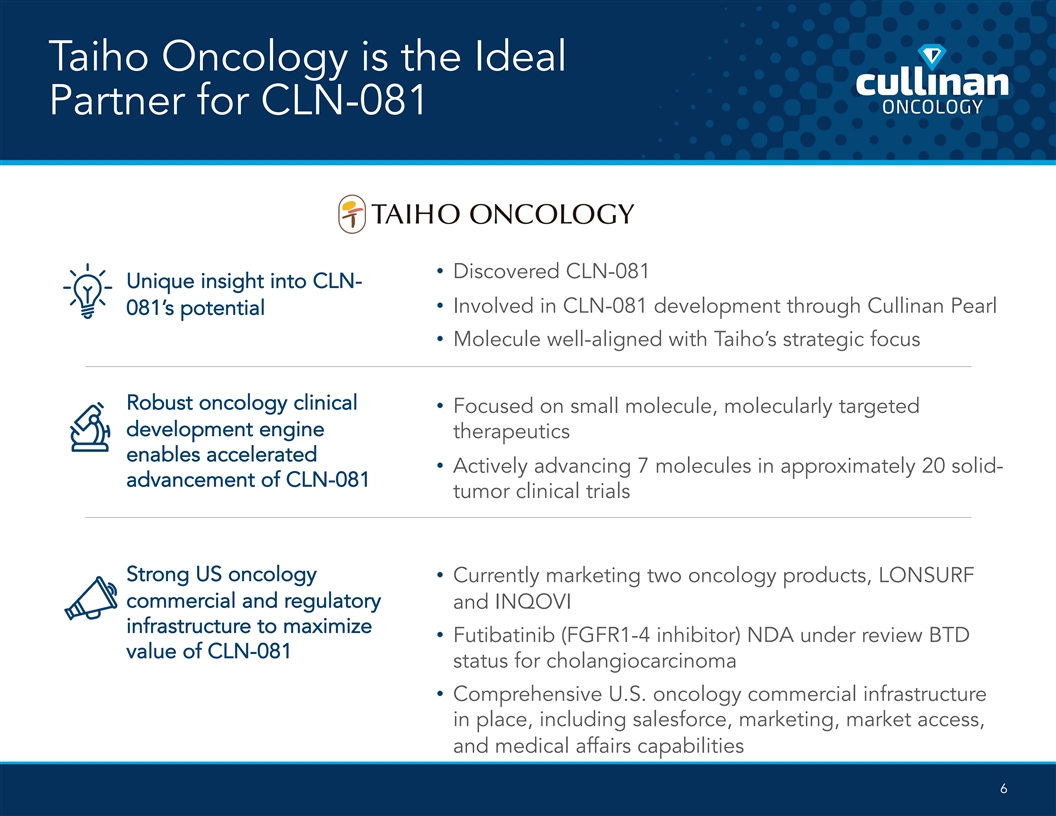
Taiho Oncology is the Ideal Partner for CLN-081 • Discovered CLN-081 Unique insight into CLN- • Involved in CLN-081 development through Cullinan Pearl 081’s potential • Molecule well-aligned with Taiho’s strategic focus Robust oncology clinical • Focused on small molecule, molecularly targeted development engine therapeutics enables accelerated • Actively advancing 7 molecules in approximately 20 solid- advancement of CLN-081 tumor clinical trials Strong US oncology • Currently marketing two oncology products, LONSURF commercial and regulatory and INQOVI infrastructure to maximize • Futibatinib (FGFR1-4 inhibitor) NDA under review BTD value of CLN-081 status for cholangiocarcinoma • Comprehensive U.S. oncology commercial infrastructure in place, including salesforce, marketing, market access, and medical affairs capabilities 6
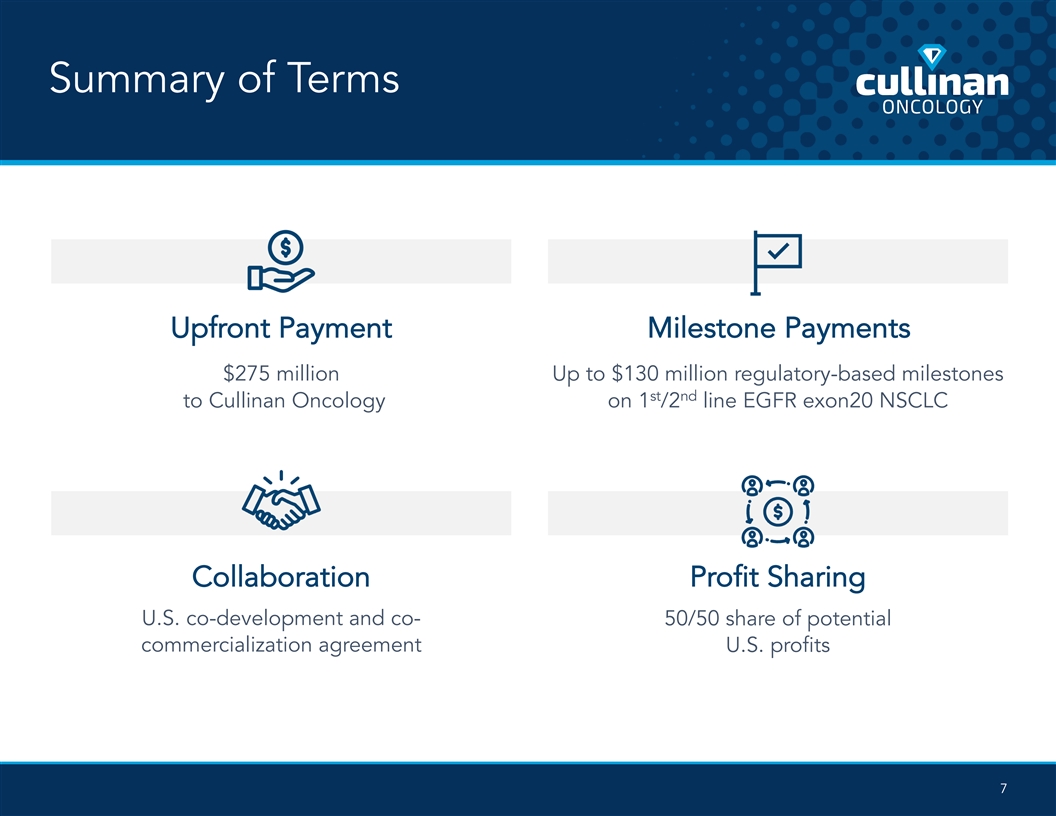
Summary of Terms Upfront Payment Milestone Payments $275 million Up to $130 million regulatory-based milestones st nd to Cullinan Oncology on 1 /2 line EGFR exon20 NSCLC Collaboration Profit Sharing U.S. co-development and co- 50/50 share of potential commercialization agreement U.S. profits 7
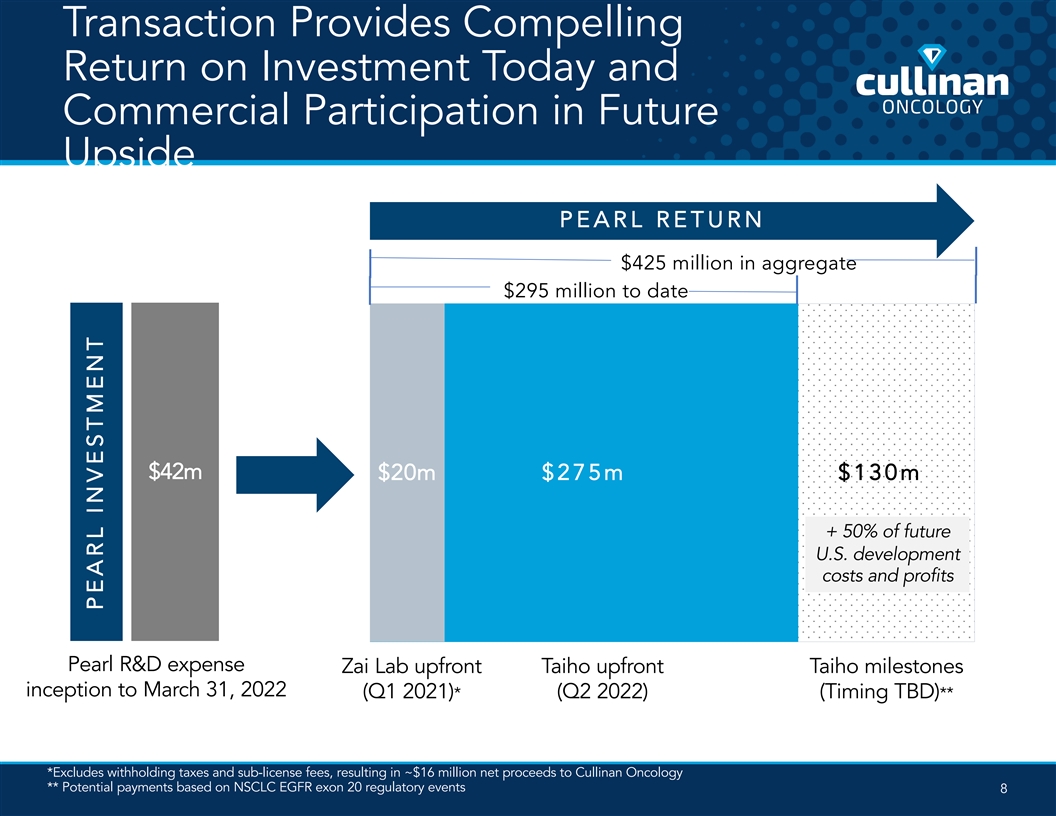
Transaction Provides Compelling Return on Investment Today and Commercial Participation in Future Upside PEARL RETURN $425 million in aggregate $295 million to date $42m $20m $275m $130m + 50% of future U.S. development costs and profits Pearl R&D expense Zai Lab upfront Taiho upfront Taiho milestones inception to March 31, 2022 (Q1 2021)* (Q2 2022) (Timing TBD)** *Excludes withholding taxes and sub-license fees, resulting in ~$16 million net proceeds to Cullinan Oncology ** Potential payments based on NSCLC EGFR exon 20 regulatory events 8 PEARL INVESTMENT
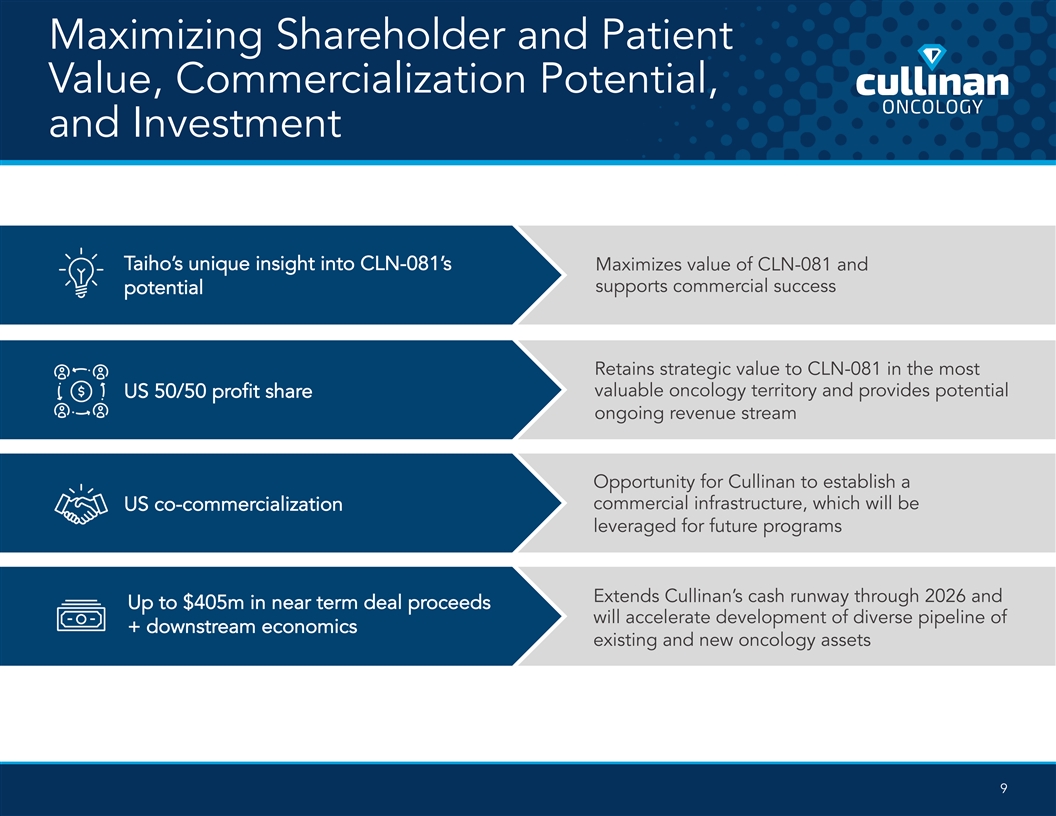
Maximizing Shareholder and Patient Value, Commercialization Potential, and Investment Taiho’s unique insight into CLN-081’s Maximizes value of CLN-081 and supports commercial success potential Retains strategic value to CLN-081 in the most valuable oncology territory and provides potential US 50/50 profit share ongoing revenue stream Opportunity for Cullinan to establish a commercial infrastructure, which will be US co-commercialization leveraged for future programs Extends Cullinan’s cash runway through 2026 and Up to $405m in near term deal proceeds will accelerate development of diverse pipeline of + downstream economics existing and new oncology assets 9

Q & A Nadim Ahmed Corinne Savill, Ph.D. Chief Executive Officer Chief Business Officer Jeffrey Jones, M.D., MPH, MBA Jeff Trigilio Chief Medical Officer Chief Financial Officer 10